
DEPARTMENT OF HEALTH AND HUMAN SERVICES
Centers for Medicare & Medicaid Services
42 CFR Parts 401, 405, 410, 411, 414, 423, 424, 425, 427, 428, and 491
[CMS-1807-P]
RIN 0938-AV33
Medicare and Medicaid Programs; CY 2025 Payment Policies under the Physician Fee
Schedule and Other Changes to Part B Payment and Coverage Policies; Medicare Shared
Savings Program Requirements; Medicare Prescription Drug Inflation Rebate Program;
and Medicare Overpayments
AGENCY: Centers for Medicare & Medicaid Services (CMS), Health and Human Services
(HHS).
ACTION: Proposed rule.
SUMMARY: This major proposed rule addresses: changes to the physician fee schedule (PFS);
other changes to Medicare Part B payment policies to ensure that payment systems are updated
to reflect changes in medical practice, relative value of services, and changes in the statute;
codification of, and proposing policies for, the Medicare Prescription Drug Inflation Rebate
Program under the Inflation Reduction Act of 2022; updates to the Medicare Diabetes Prevention
Program expanded model; payment for dental services inextricably linked to specific covered
medical services; updates to drugs and biological products paid under Part B including
immunosuppressive drugs and clotting factors; Medicare Shared Savings Program requirements;
updates to the Quality Payment Program; Medicare coverage of opioid use disorder services
furnished by opioid treatment programs; updates to policies for Rural Health Clinics and
Federally Qualified Health Centers; electronic prescribing for controlled substances for a
covered Part D drug under a prescription drug plan or a Medicare Advantage Prescription Drug
(MA-PD) plan under the Substance Use-Disorder Prevention that Promotes Opioid Recovery and
Treatment for Patients and Communities Act (SUPPORT Act); update to the Ambulance Fee
This document is scheduled to be published in the
Federal Register on 07/31/2024 and available online at
https://federalregister.gov/d/2024-14828, and on https://govinfo.gov
Schedule regulations; codification of the Inflation Reduction Act and Consolidated
Appropriations Act, 2023 provisions; updates to Clinical Laboratory Fee Schedule regulations;
updates to the diabetes payment structure and PHE flexibilities; expansion of colorectal cancer
screening and Hepatitis B vaccine coverage and payment; establishing payment for drugs
covered as additional preventive services; Medicare Parts A and B Overpayment Provisions of
the Affordable Care Act.
DATES: To be assured consideration, comments must be received at one of the addresses
provided below, no later than 5 p.m. on September 9, 2024.
ADDRESSES: In commenting, please refer to file code CMS-1807-P.
Comments, including mass comment submissions, must be submitted in one of the
following three ways (please choose only one of the ways listed):
1. Electronically. You may submit electronic comments on this regulation to
https://www.regulations.gov. Follow the “Submit a comment” instructions.
2. By regular mail. You may mail written comments to the following address ONLY:
Centers for Medicare & Medicaid Services,
Department of Health and Human Services,
Attention: CMS-1807-P,
P.O. Box 8016,
Baltimore, MD 21244-8016.
Please allow sufficient time for mailed comments to be received before the close of the comment
period.
3. By express or overnight mail. You may send written comments to the following
address ONLY:
Centers for Medicare & Medicaid Services,
Department of Health and Human Services,
Attention: CMS-1807-P,
Mail Stop C4-26-05,
7500 Security Boulevard,
Baltimore, MD 21244-1850.
FOR FURTHER INFORMATION CONTACT:
[email protected], for any issues not identified below. Please
indicate the specific issue in the subject line of the email.
Michael Soracoe, (410) 786-6312, Morgan Kitzmiller, (410) 786-1623, or
[email protected], for issues related to practice expense, work
RVUs, conversion factor, and PFS specialty-specific impacts.
Kris Corwin, (410) 786-8864, or [email protected], for
issues related to strategies for updates to practice expense data collection and methodology.
Hannah Ahn, (814) 769-0143, or [email protected], for
issues related to potentially misvalued services under the PFS.
Kris Corwin, (410) 786-8864, Patrick Sartini, (410) 786-9252, Mikayla Murphy, (667)
414-0093, or [email protected], for issues related to direct
supervision using two-way audio/video communication technology, telehealth, and other
services involving communications technology.
Tamika Brock, (312) 886-7904, or [email protected], for
issues related to teaching physician billing for services involving residents in teaching settings.
Sarah Leipnik, (410) 786-3933, Mikayla Murphy, (667) 414-0093, Regina Walker-Wren,
(410) 786-9160, or [email protected], for issues related to payment
for caregiver training services and addressing health-related social needs(community health
integration, principal illness navigation, and social determinants of health risk assessment).
Erick Carrera, (410) 786-8949, or [email protected], for
issues related to office/outpatient evaluation and management visit inherent complexity add-one.
Sarah Irie, (410) 786-1348, Emily Parris (667) 414-0418, or
[email protected], for issues related to payment for advanced
primary care management service.
Sarah Leipnik, (410) 786-3933, or [email protected], for
issues related to global surgery payment accuracy.
Pamela West, (410) 786-2302, for issues related to supervision of outpatient therapy
services in private practices, certification of therapy plans of care, and KX modifier threshold.
Lindsey Baldwin, (410) 786-1694, Regina Walker-Wren, (410) 786-9160, Erick Carrera,
(410) 786-8949, Mikayla Murphy, (667) 414-0093, or
[email protected], for issues related to advancing access to
behavioral health services.
Laura Ashbaugh, (410) 786-1113, and Erick Carrera, (410) 786-8949, Zehra Hussain,
(214) 767-4463, or [email protected], for issues related to dental
services inextricably linked to specific covered medical services.
Zehra Hussain, (214) 767-4463, or [email protected], for
issues related to payment of skin substitutes.
Laura Kennedy, (410) 786-3377, Adam Brooks, (202) 205-0671, Rachel Radzyner, (410)
786-8215, Rebecca Ray, (667) 414-0879, and Jae Ryu, (667) 414-0765 for issues related to
Drugs and Biological Products Paid Under Medicare Part B.
[email protected], for issues related to complex drug
administration.
Glenn McGuirk, (410) 786-5723, or [email protected] for issues related to
Clinical Laboratory Fee Schedule.
Lisa Parker, (410) 786-4949, or [email protected], for issues related to FQHC
payments.
Heidi Oumarou, (410) 786-7942, for issues related to the FQHC market basket.

Michele Franklin, (410) 786-9226, or [email protected], for issues related to RHC
payments.
Kianna Banks (410) 786-3498 and Cara Meyer (667) 290-9856, for issues related to
RHCs and FQHCs and Conditions for Certification or Coverage.
Colleen Barbero (667) 290-8794, for issues related to Medicare Diabetes Prevention
Program.
Ariana Pitcher, (667) 290- 8840, or [email protected], for issues related to
Medicare coverage of opioid use disorder treatment services furnished by opioid treatment
programs.
Sabrina Ahmed, (410) 786-7499, or [email protected], for issues
related to the Medicare Shared Savings Program (Shared Savings Program) Quality performance
standard and quality reporting requirements.
Janae James, (410) 786-0801, or [email protected], for issues related
to Shared Savings Program beneficiary assignment and benchmarking methodology.
Richard (Chase) Kendall, (410) 786-1000, or [email protected], for
issues related to reopening ACO payment determinations, and mitigating the impact of
significant, anomalous, and highly suspect billing activity on Shared Savings Program financial
calculations.
Lucy Bertocci, (410) 786-3776, or [email protected], for issues
related to Shared Savings Program prepaid shared savings, advance investment payments,
beneficiary notice and eligibility requirements.
Rachel Radzyner, (410) 786-8215, for issues related to payment for preventative services,
including preventive vaccine administration and drugs covered as additional preventive services.
Elisabeth Daniel, (667) 290-8793, for issues related to the Medicare Prescription Drug
Inflation Rebate Program.
Genevieve Kehoe, [email protected], or 1-844-711-2664 (Option
4) for issues related to the Request for Information: Building upon the MIPS Value Pathways
(MVPs) Framework to Improve Ambulatory Specialty Care.
Kimberly Long, (410) 786-5702, for issues related to expanding colorectal cancer
screening.
Rachel Katonak, (410) 786-8564, for issues related to expanding Hepatitis B vaccine
coverage.
Mei Zhang, (410) 786-7837, for issues related to requirement for electronic prescribing
for controlled substances for a covered Part D drug under a prescription drug plan or an MA-PD
plan (section 2003 of the SUPPORT Act).
Katie Parker, (410) 786-0537, for issues related to Parts A and B overpayment provisions
of the Affordable Care Act.
Amy Gruber, (410) 786-1542, for issues related to low titer O+ whole blood transfusion
therapy during ground ambulance transport.
Renee O’Neill, (410) 786-8821, or Sophia Sugumar, (410) 786-1648, for inquiries related
to Merit-based Incentive Payment System (MIPS) track of the Quality Payment Program.
Danielle Drayer, (516) 965-6630, for inquiries related to Alternative Payment Models
(APMs).
SUPPLEMENTARY INFORMATION:
Addenda Available Only Through the Internet on the CMS Website: The PFS Addenda
along with other supporting documents and tables referenced in this proposed rule are available
on the CMS website at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-
Payment/PhysicianFeeSched/index.html. Click on the link on the left side of the screen titled,
“PFS Federal Regulations Notices” for a chronological list of PFS Federal Register and other
related documents. For the CY 2025 PFS proposed rule, refer to item CMS-1807-P. Readers
with questions related to accessing any of the Addenda or other supporting documents referenced
in this proposed rule and posted on the CMS website identified above should contact
CPT (Current Procedural Terminology) Copyright Notice: Throughout this proposed
rule, we use CPT codes and descriptions to refer to a variety of services. We note that CPT
codes and descriptions are copyright 2020 American Medical Association. All Rights Reserved.
CPT is a registered trademark of the American Medical Association (AMA). Applicable Federal
Acquisition Regulations (FAR) and Defense Federal Acquisition Regulations (DFAR) apply.
I. Executive Summary
A. Purpose
This major annual rule proposes to revise payment policies under the Medicare PFS and
makes other policy changes, including proposals to implement certain provisions of the Further
Continuing Appropriations and Other Extensions Act of 2024 (Pub. L. 118-22, November 16,
2023), Consolidated Appropriations Act, 2023 (Pub. L. 117-328, September 29, 2022), Inflation
Reduction Act of 2022 (IRA) (Pub. L. 117-169, August 16, 2022), Consolidated Appropriations
Act, 2022 (Pub. L. 117-103, March 15, 2022), Consolidated Appropriations Act, 2021 (CAA,
2021) (Pub. L. 116-260, December 27, 2020), Bipartisan Budget Act of 2018 (BBA of 2018)
(Pub. L. 115-123, February 9, 2018) and the Substance Use-Disorder Prevention that Promotes
Opioid Recovery and Treatment for Patients and Communities Act (SUPPORT Act) (Pub. L.
115-271, October 24, 2018), related to Medicare Part B payment. In addition, this major
proposed rule includes proposals regarding other Medicare payment policies described in
sections III. and IV.
This rulemaking also proposes to codify policies previously established in guidance for
the Medicare Prescription Drug Inflation Rebate Program at new parts 427 and 428, including
clarifications to certain existing policies, consistent with sections 1847A(i) and 1860D-14B of
the Act. This rulemaking also proposes new policies for the Medicare Prescription Drug Inflation
Rebate Program, including removal of units of drugs subject to discarded drug refunds from the
Part B rebate amounts, exclusion of units for which a manufacturer provides a discount under the
340B Program from the Part D inflation rebate amount starting on January 1, 2026, the process
for reconciliation of a Part B or Part D rebate amount to incorporate certain revised information,
and procedures for imposing civil money penalties on manufacturers that do not pay Part B or
Part D inflation rebate amounts within a specified period of time.
This rulemaking proposes to update the Rural Health Clinic (RHC) and Federally
Qualified Health Clinic (FQHC) Conditions for Certification and Conditions for Coverage
(CfCs), respectively, by clarifying the requirements and intent of the program regarding the
provision of services. We also aim to ensure RHCs are provided flexibility in the services they
offer, including specialty and laboratory services.
This rulemaking also proposes to further advance Medicare’s overall value-based care
strategy of growth, alignment, and equity through the Medicare Shared Savings Program (Shared
Savings Program) and the Quality Payment Program. The structure of the programs enables us to
develop a set of tools for measuring and encouraging improvements in care, which may support a
shift to clinician payment over time into Advanced Alternative Payment Models (APMs) and
accountable care arrangements which reduce care fragmentation and unnecessary costs for
patients and the health system.
This rulemaking also proposes changes to Medicare regulations regarding requirements
for reporting and returning Parts A and B overpayments.
B. Summary of the Major Provisions
Please note, some sections of this proposed rule contain a request for information (RFI).
In accordance with the implementing regulations of the Paperwork Reduction Act of 1995
(PRA), specifically 5 CFR 1320.3(h)(4), these general solicitations are exempt from the PRA.
Facts or opinions submitted in response to general solicitations of comments from the public,
published in the Federal Register or other publications, regardless of the form or format thereof,
provided that no person is required to supply specific information pertaining to the commenter,
other than that necessary for self-identification, as a condition of the agency's full consideration,
are not generally considered information collections and therefore not subject to the PRA.
Respondents are encouraged to provide complete but concise responses. These RFIs are issued
solely for information and planning purposes; they do not constitute a Request for Proposal
(RFP), applications, proposal abstracts, or quotations. These RFIs do not commit the U.S.
Government to contract for any supplies or services or make a grant award. Further, CMS is not
seeking proposals through these RFIs and will not accept unsolicited proposals. Responders are
advised that the U.S. Government will not pay for any information or administrative costs
incurred in response to these RFIs; all costs associated with responding to these RFIs will be
solely at the interested party’s expense. Not responding to these RFIs does not preclude
participation in any future procurement, if conducted. It is the responsibility of the potential
responders to monitor these RFI announcements for additional information pertaining to these
requests. Please note that CMS will not respond to questions about the policy issues raised in
these RFIs. CMS may or may not choose to contact individual responders. Such communications
would only serve to further clarify written responses. Contractor support personnel may be used
to review RFI responses. Responses to this notice are not offers and cannot be accepted by the
U.S. Government to form a binding contract or issue a grant. Information obtained as a result of
these RFIs may be used by the U.S. Government for program planning on a non-attribution basis.
Respondents should not include any information that might be considered proprietary or
confidential. These RFIs should not be construed as a commitment or authorization to incur cost
for which reimbursement would be required or sought. All submissions become U.S.
Government property and will not be returned. CMS may publicly post the comments received,
or a summary thereof.
Section 1848 of the Social Security Act (the Act) requires us to establish payments under
the PFS, based on national uniform relative value units (RVUs) that account for the relative
resources used in furnishing a service. The statute requires that RVUs be established for three
categories of resources: work, practice expense (PE), and malpractice (MP) expense. In
addition, the statute requires that each year we establish, by regulation, the payment amounts for
physicians’ services paid under the PFS, including geographic adjustments to reflect the
variations in the costs of furnishing services in different geographic areas.
In this major proposed rule, we are proposing to establish RVUs for CY 2025 for the PFS
to ensure that our payment systems are updated to reflect changes in medical practice and the
relative value of services, as well as changes in the statute. This proposed rule also includes
discussions and provisions regarding several other Medicare Part B payment policies, Medicare
and Medicaid provider and supplier enrollment policies, and other policies regarding programs
administered by CMS.
Specifically, this proposed rule addresses:
● Background (section II.A.)
● Determination of PE RVUs (section II.B.)
● Potentially Misvalued Services Under the PFS (section II.C.)
● Payment for Medicare Telehealth Services Under Section 1834(m) of the Act (section
II.D.)
● Valuation of Specific Codes (section II.E.)
● Evaluation and Management (E/M) Visits (section II.F.)
● Enhanced Care Management (section II.G.)
● Supervision of Outpatient Therapy Services in Private Practices, Certification of
Therapy Plans of Care with a Physician or NPP Order, and KX Modifier Thresholds (section
II.H.)
● Advancing Access to Behavioral Health Services (section II.I.)
● Proposals on Medicare Parts A and B Payment for Dental Services Inextricably Linked
to Specific Covered Services (section II.J.)
● Payment for Skin Substitutes (section II.K.)
● Drugs and Biological Products Paid Under Medicare Part B (section III.A.)
● Rural Health Clinics (RHCs) and Federally Qualified Health Centers (FQHCs)
(section III.B.)
● Rural Health Clinic (RHC) and Federally Qualified Health Center (FQHC) Conditions
for Certification and Conditions for Coverage (CfCs) (section III.C.)
● Clinical Laboratory Fee Schedule: Revised Data Reporting Period and Phase-in of
Payment Reductions (section III.D.)
● Medicare Diabetes Prevention Program (MDPP) (section III.E.)
● Modifications Related to Medicare Coverage for Opioid Use Disorder (OUD)
Treatment Services Furnished by Opioid Treatment Programs (OTPs ) (section III.F.)
● Medicare Shared Savings Program (section III.G.)
● Medicare Part B Payment for Preventive Services (§§ 410.10, 410.57, 410.64,
410.152) (section III.H.)
● Medicare Prescription Drug Inflation Rebate Program (section III.I.)
● Request for Information: Building upon the MIPS Value Pathways (MVPs)
Framework to Improve Ambulatory Specialty Care (section III.J.)
● Expand Colorectal Cancer Screening (section III.K.)
● Requirements for Electronic Prescribing for Controlled Substances for a Covered
Part D Drug under a Prescription Drug Plan or an MA-PD Plan (section III.L.)
● Expand Hepatitis B Vaccine Coverage (section III.M.)
● Low Titer O+ Whole Blood Transfusion Therapy During Ground Ambulance
Transport (section III.N.)
● Medicare Parts A and B Overpayment Provisions of the Affordable Care Act
(section III.O.)
● Updates to the Quality Payment Program (section IV.)
● Collection of Information Requirements (section V.)
● Response to Comments (section VI.)
● Regulatory Impact Analysis (section VII.)
C. Summary of Costs and Benefits
We have determined that this proposed rule is economically significant. We estimate the
CY 2025 PFS conversion factor to be 32.3562 which reflects a 0.05 percent positive budget
neutrality adjustment required under section 1848(c)(2)(B)(ii)(II) of the Act, the 0.00 percent
update adjustment factor specified under section 1848(d)(19) of the Act, and the removal of the
temporary 2.93 percent payment increase for services furnished from March 9, 2024, through
December 31, 2024, as provided in the CAA, 2024. For a detailed discussion of the economic
impacts, see section VII., Regulatory Impact Analysis, of this proposed rule.
II. Provisions of the Proposed Rule for the PFS
A. Background
In accordance with section 1848 of the Social Security Act (the Act), CMS has paid for
physicians’ services under the Medicare physician fee schedule (PFS) since January 1, 1992.
The PFS relies on national relative values that are established for work, practice expense (PE),
and malpractice (MP), which are adjusted for geographic cost variations. These values are
multiplied by a conversion factor (CF) to convert the relative value units (RVUs) into payment
rates. The concepts and methodology underlying the PFS were enacted as part of the Omnibus
Budget Reconciliation Act of 1989 (OBRA ’89) (Pub. L. 101-239, December 19, 1989), and the
Omnibus Budget Reconciliation Act of 1990 (OBRA ’90) (Pub. L. 101-508, November 5, 1990).
The final rule published in the November 25, 1991 Federal Register (56 FR 59502) set forth the
first fee schedule used for Medicare payment for physicians’ services.
We note that throughout this proposed rule, unless otherwise noted, the term
“practitioner” is used to describe both physicians and nonphysician practitioners (NPPs) who are
permitted to bill Medicare under the PFS for the services they furnish to Medicare beneficiaries.
B. Determination of PE RVUs
1. Overview
Practice expense (PE) is the portion of the resources used in furnishing a service that
reflects the general categories of physician and practitioner expenses, such as office rent and
personnel wages, but excluding malpractice (MP) expenses, as specified in section 1848(c)(1)(B)
of the Act. As required by section 1848(c)(2)(C)(ii) of the Act, we use a resource-based system
for determining PE RVUs for each physicians’ service. We develop PE RVUs by considering
the direct and indirect practice resources involved in furnishing each service. Direct expense
categories include clinical labor, medical supplies, and medical equipment. Indirect expenses
include administrative labor, office expense, and all other expenses. The sections that follow
provide more detailed information about the methodology for translating the resources involved
in furnishing each service into service specific PE RVUs. We refer readers to the CY 2010
Physician Fee Schedule (PFS) final rule with comment period (74 FR 61743 through 61748) for
a more detailed explanation of the PE methodology.
2. Practice Expense Methodology
a. Direct Practice Expense
We determine the direct PE for a specific service by adding the costs of the direct
resources (that is, the clinical staff, medical supplies, and medical equipment) typically involved
with furnishing that service. The costs of the resources are calculated using the refined direct PE
inputs assigned to each CPT code in our PE database, which are generally based on our review of
recommendations received from the American Medical Association (AMA) Relative Value Scale
Update Committee (RUC) and those provided in response to public comment periods. For a
detailed explanation of the direct PE methodology, including examples, we refer readers to the 5-
year review of work RVUs under the PFS and proposed changes to the PE methodology in the
CY 2007 PFS proposed rule (71 FR 37242) and the CY 2007 PFS final rule with comment
period (71 FR 69629).
b. Indirect Practice Expense per Hour Data
We use survey data on indirect PEs incurred per hour worked to develop the indirect
portion of the PE RVUs. Prior to CY 2010, we primarily used the PE/HR by specialty obtained
from the AMA’s Socioeconomic Monitoring System (SMS). The AMA administered a new
survey in CY 2007 and CY 2008, the Physician Practice Information Survey (PPIS). The PPIS is
a multispecialty, nationally representative, PE survey of physicians and NPPs paid under the PFS
using a survey instrument and methods highly consistent with those used for the SMS and the
supplemental surveys. The PPIS gathered information from 3,656 respondents across 51
physician specialty and health care professional groups. We believe the PPIS is the most
comprehensive source of PE survey information available. We used the PPIS data to update the
PE/HR data for the CY 2010 PFS for almost all of the Medicare-recognized specialties that
participated in the survey.
When we began using the PPIS data in CY 2010, we did not change the PE RVU
methodology or how the PE/HR data are used. We only updated the PE/HR data based on the
new survey. Furthermore, as we explained in the CY 2010 PFS final rule with comment period
(74 FR 61751), because of the magnitude of payment reductions for some specialties resulting
from the use of the PPIS data, we transitioned its use over a 4-year period from the previous PE
RVUs to the PE RVUs developed using the new PPIS data. As provided in the CY 2010 PFS
final rule with comment period (74 FR 61751), the transition to the PPIS data was complete for
CY 2013. Therefore, PE RVUs from CY 2013 forward are developed based entirely on the PPIS
data, except as noted in this section.
Section 1848(c)(2)(H)(i) of the Act requires us to use the medical oncology supplemental
survey data submitted in 2003 for oncology drug administration services. Therefore, the PE/HR
for medical oncology, hematology, and hematology/oncology reflects the continued use of these
supplemental survey data.
Supplemental survey data on independent labs from the College of American
Pathologists were implemented for payments beginning in CY 2005. Supplemental survey data

from the National Coalition of Quality Diagnostic Imaging Services (NCQDIS), representing
independent diagnostic testing facilities (IDTFs), were blended with supplementary survey data
from the American College of Radiology (ACR) and implemented for payments beginning in
CY 2007. Neither IDTFs nor independent labs participated in the PPIS. Therefore, we continue
to use the PE/HR that was developed from their supplemental survey data.
Consistent with our past practice, the previous indirect PE/HR values from the
supplemental surveys for these specialties were updated to CY 2006 using the Medicare
Economic Index (MEI) to put them on a comparable basis with the PPIS data.
We also do not use the PPIS data for reproductive endocrinology and spine surgery since
these specialties are not separately recognized by Medicare, nor do we have a method to blend
the PPIS data with Medicare-recognized specialty data.
Previously, we established PE/HR values for various specialties without SMS or
supplemental survey data by crosswalking them to other similar specialties to estimate a proxy
PE/HR. For specialties that were part of the PPIS for which we previously used a crosswalked
PE/HR, we instead used the PPIS based PE/HR. We use crosswalks for specialties that did not
participate in the PPIS. These crosswalks have been generally established through notice and
comment rulemaking and are available in the file titled “CY 2025 PFS proposed rule PE/HR” on
the CMS website under downloads for the CY 2025 PFS proposed rule at
https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-
Federal-Regulation-Notices.html.
For CY 2025, we have incorporated the available utilization data for two new specialties,
Marriage and Family Therapist (MFT) and Mental Health Counselor (MHC), which we
recognized effective January 1, 2024, in accordance with section 4121 of the CAA, 2023. We
are proposing to use proxy PE/HR values for these new specialties, as there are no PPIS data for
these specialties, by crosswalking the PE/HR as follows from specialties that furnish similar
services in the Medicare claims data:
● Marriage and Family Therapist (MFT) from Licensed Clinical Social Workers; and
● Mental Health Counselor (MHC) from Licensed Clinical Social Workers
These updates are reflected in the “CY 2025 PFS proposed rule PE/HR” file available on
the CMS website under the supporting data files for the CY 2025 PFS proposed rule at
https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-
Federal-Regulation-Notices.html.
c. Allocation of PE to Services
To establish PE RVUs for specific services, it is necessary to establish the direct and
indirect PE associated with each service.
(1) Direct Costs
The relative relationship between the direct cost portions of the PE RVUs for any two
services is determined by the relative relationship between the sum of the direct cost resources
(that is, the clinical staff, medical supplies, and medical equipment) typically involved with
furnishing each of the services. The costs of these resources are calculated from the refined
direct PE inputs in our PE database. For example, if one service has a direct cost sum of $400
from our PE database and another service has a direct cost sum of $200, the direct portion of the
PE RVUs of the first service would be twice as much as the direct portion of the PE RVUs for
the second service.
(2) Indirect Costs
We allocate the indirect costs at the code level based on the direct costs specifically
associated with a code and the greater of either the clinical labor costs or the work RVUs. We
also incorporate the survey data described earlier in the PE/HR discussion. The general
approach to developing the indirect portion of the PE RVUs is as follows:
● For a given service, we use the direct portion of the PE RVUs calculated as previously
described and the average percentage that direct costs represent of total costs (based on survey
data) across the specialties that furnish the service to determine an initial indirect allocator. That
is, the initial indirect allocator is calculated so that the direct costs equal the average percentage
of direct costs of those specialties furnishing the service. For example, if the direct portion of the
PE RVUs for a given service is 2.00 and direct costs, on average, represent 25 percent of total
costs for the specialties that furnish the service, the initial indirect allocator would be calculated
so that it equals 75 percent of the total PE RVUs. Thus, in this example, the initial indirect
allocator would equal 6.00, resulting in a total PE RVU of 8.00 (2.00 is 25 percent of 8.00 and
6.00 is 75 percent of 8.00).
● Next, we add the greater of the work RVUs or clinical labor portion of the direct
portion of the PE RVUs to this initial indirect allocator. In our example, if this service had a
work RVU of 4.00 and the clinical labor portion of the direct PE RVU was 1.50, we would add
4.00 (since the 4.00 work RVUs are greater than the 1.50 clinical labor portion) to the initial
indirect allocator of 6.00 to get an indirect allocator of 10.00. In the absence of any further use
of the survey data, the relative relationship between the indirect cost portions of the PE RVUs for
any two services would be determined by the relative relationship between these indirect cost
allocators. For example, if one service had an indirect cost allocator of 10.00 and another service
had an indirect cost allocator of 5.00, the indirect portion of the PE RVUs of the first service
would be twice as great as the indirect portion of the PE RVUs for the second service.
● Then, we incorporate the specialty specific indirect PE/HR data into the calculation.
In our example, if, based on the survey data, the average indirect cost of the specialties
furnishing the first service with an allocator of 10.00 was half of the average indirect cost of the
specialties furnishing the second service with an indirect allocator of 5.00, the indirect portion of
the PE RVUs of the first service would be equal to that of the second service.
(3) Facility and Nonfacility Costs
For procedures that can be furnished in a physician’s office, as well as in a facility
setting, where Medicare makes a separate payment to the facility for its costs in furnishing a
service, we establish two PE RVUs: facility and nonfacility. The methodology for calculating
PE RVUs is the same for both the facility and nonfacility RVUs but is applied independently to
yield two separate PE RVUs. In calculating the PE RVUs for services furnished in a facility, we
do not include resources that would generally not be provided by physicians when furnishing the
service. For this reason, the facility PE RVUs are generally lower than the nonfacility PE RVUs.
(4) Services with Technical Components and Professional Components
Diagnostic services are generally comprised of two components: a professional
component (PC); and a technical component (TC). The PC and TC may be furnished
independently or by different healthcare providers, or they may be furnished together as a global
service. When services have separately billable PC and TC components, the payment for the
global service equals the sum of the payment for the TC and PC. To achieve this, we use a
weighted average of the ratio of indirect to direct costs across all the specialties that furnish the
global service, TCs, and PCs; that is, we apply the same weighted average indirect percentage
factor to allocate indirect expenses to the global service, PCs, and TCs for a service. (The direct
PE RVUs for the TC and PC sum to the global.)
(5) PE RVU Methodology
For a more detailed description of the PE RVU methodology, we direct readers to the CY
2010 PFS final rule with comment period (74 FR 61745 through 61746). We also direct readers
to the file titled “Calculation of PE RVUs under Methodology for Selected Codes” which is
available on our website under downloads for the CY 2025 PFS proposed rule at
https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-
Federal-Regulation-Notices.html. This file contains a table that illustrates the calculation of PE
RVUs as described in this proposed rule for individual codes.
(a) Setup File
First, we create a setup file for the PE methodology. The setup file contains the direct
cost inputs, the utilization for each procedure code at the specialty and facility/nonfacility place
of service level, and the specialty specific PE/HR data calculated from the surveys.
(b) Calculate the Direct Cost PE RVUs
Sum the costs of each direct input.
Step 1: Sum the direct costs of the inputs for each service.
Step 2: Calculate the aggregate pool of direct PE costs for the current year. We set the
aggregate pool of PE costs equal to the product of the ratio of the current aggregate PE RVUs to
current aggregate work RVUs and the projected aggregate work RVUs.
Step 3: Calculate the aggregate pool of direct PE costs for use in ratesetting. This is the
product of the aggregate direct costs for all services from Step 1 and the utilization data for that
service.
Step 4: Using the results of Step 2 and Step 3, use the CF to calculate a direct PE scaling
adjustment to ensure that the aggregate pool of direct PE costs calculated in Step 3 does not vary
from the aggregate pool of direct PE costs for the current year. Apply the scaling adjustment to
the direct costs for each service (as calculated in Step 1).
Step 5: Convert the results of Step 4 to an RVU scale for each service. To do this, divide
the results of Step 4 by the CF. Note that the actual value of the CF used in this calculation does
not influence the final direct cost PE RVUs as long as the same CF is used in Step 4 and Step 5.
Different CFs would result in different direct PE scaling adjustments, but this has no effect on
the final direct cost PE RVUs since changes in the CFs and the associated direct scaling
adjustments offset one another.
(c) Create the Indirect Cost PE RVUs
Create indirect allocators.
Step 6: Based on the survey data, calculate direct and indirect PE percentages for each
physician specialty.
Step 7: Calculate direct and indirect PE percentages at the service level by taking a
weighted average of the results of Step 6 for the specialties that furnish the service. Note that for
services with TCs and PCs, the direct and indirect percentages for a given service do not vary by
the PC, TC, and global service.
We generally use an average of the three most recent years of available Medicare claims
data to determine the specialty mix assigned to each code. Codes with low Medicare service
volume require special attention since billing or enrollment irregularities for a given year can
result in significant changes in specialty mix assignment. We finalized a policy in the CY 2018
PFS final rule (82 FR 52982 through 59283) to use the most recent year of claims data to
determine which codes are low volume for the coming year (those that have fewer than 100
allowed services in the Medicare claims data). For codes that fall into this category, instead of
assigning a specialty mix based on the specialties of the practitioners reporting the services in the
claims data, we use the expected specialty that we identify on a list developed based on medical
review and input from expert interested parties. We display this list of expected specialty
assignments as part of the annual set of data files we make available as part of notice and
comment rulemaking and consider recommendations from the RUC and other interested parties
on changes to this list annually. Services for which the specialty is automatically assigned based
on previously finalized policies under our established methodology (for example, “always
therapy” services) are unaffected by the list of expected specialty assignments. We also finalized
in the CY 2018 PFS final rule (82 FR 52982 through 52983) a policy to apply these service-level
overrides for both PE and MP, rather than one or the other category.
Step 8: Calculate the service level allocators for the indirect PEs based on the
percentages calculated in Step 7. The indirect PEs are allocated based on the three components:
the direct PE RVUs; the clinical labor PE RVUs; and the work RVUs.
For most services the indirect allocator is: indirect PE percentage * (direct PE
RVUs/direct percentage) + work RVUs.
There are two situations where this formula is modified:
● If the service is a global service (that is, a service with global, professional, and
technical components), then the indirect PE allocator is: indirect percentage (direct PE
RVUs/direct percentage) + clinical labor PE RVUs + work RVUs.
● If the clinical labor PE RVUs exceed the work RVUs (and the service is not a global
service), then the indirect allocator is: indirect PE percentage (direct PE RVUs/direct percentage)
+ clinical labor PE RVUs.
(Note: For global services, the indirect PE allocator is based on both the work RVUs and
the clinical labor PE RVUs. We do this to recognize that, for the PC service, indirect PEs would
be allocated using the work RVUs, and for the TC service, indirect PEs would be allocated using
the direct PE RVUs and the clinical labor PE RVUs. This also allows the global component
RVUs to equal the sum of the PC and TC RVUs.)
For presentation purposes, in the examples in the download file titled “Calculation of PE
RVUs under Methodology for Selected Codes”, the formulas were divided into two parts for
each service.
● The first part does not vary by service and is the indirect percentage (direct PE
RVUs/direct percentage).
● The second part is either the work RVU, clinical labor PE RVU, or both depending on
whether the service is a global service and whether the clinical PE RVUs exceed the work RVUs
(as described earlier in this step).
Apply a scaling adjustment to the indirect allocators.
Step 9: Calculate the current aggregate pool of indirect PE RVUs by multiplying the
result of step 8 by the average indirect PE percentage from the survey data.
Step 10: Calculate an aggregate pool of indirect PE RVUs for all PFS services by adding
the product of the indirect PE allocators for a service from Step 8 and the utilization data for that
service.
Step 11: Using the results of Step 9 and Step 10, calculate an indirect PE adjustment so
that the aggregate indirect allocation does not exceed the available aggregate indirect PE RVUs
and apply it to indirect allocators calculated in Step 8.
Calculate the indirect practice cost index.
Step 12: Using the results of Step 11, calculate aggregate pools of specialty specific
adjusted indirect PE allocators for all PFS services for a specialty by adding the product of the
adjusted indirect PE allocator for each service and the utilization data for that service.
Step 13: Using the specialty specific indirect PE/HR data, calculate specialty specific
aggregate pools of indirect PE for all PFS services for that specialty by adding the product of the
indirect PE/HR for the specialty, the work time for the service, and the specialty’s utilization for
the service across all services furnished by the specialty.
Step 14: Using the results of Step 12 and Step 13, calculate the specialty specific indirect
PE scaling factors.
Step 15: Using the results of Step 14, calculate an indirect practice cost index at the
specialty level by dividing each specialty specific indirect scaling factor by the average indirect
scaling factor for the entire PFS.
Step 16: Calculate the indirect practice cost index at the service level to ensure the
capture of all indirect costs. Calculate a weighted average of the practice cost index values for
the specialties that furnish the service. (Note: For services with TCs and PCs, we calculate the
indirect practice cost index across the global service, PCs, and TCs. Under this method, the
indirect practice cost index for a given service (for example, echocardiogram) does not vary by
the PC, TC, and global service.)
Step 17: Apply the service level indirect practice cost index calculated in Step 16 to the
service level adjusted indirect allocators calculated in Step 11 to get the indirect PE RVUs.
(d) Calculate the Final PE RVUs
Step 18: Add the direct PE RVUs from Step 5 to the indirect PE RVUs from Step 17 and
apply the final PE budget neutrality (BN) adjustment. The final PE BN adjustment is calculated
by comparing the sum of steps 5 and 17 to the aggregate work RVUs scaled by the ratio of
current aggregate PE and work RVUs. This adjustment ensures that all PE RVUs in the PFS
account for the fact that certain specialties are excluded from the calculation of PE RVUs but
included in maintaining overall PFS BN. (See “Specialties excluded from ratesetting
calculation” later in this proposed rule.)
Step 19: Apply the phase-in of significant RVU reductions and its associated adjustment.
Section 1848(c)(7) of the Act specifies that for services that are not new or revised codes, if the
total RVUs for a service for a year would otherwise be decreased by an estimated 20 percent or
more as compared to the total RVUs for the previous year, the applicable adjustments in work,
PE, and MP RVUs shall be phased in over a 2-year period. In implementing the phase-in, we
consider a 19 percent reduction as the maximum 1-year reduction for any service not described
by a new or revised code. This approach limits the year one reduction for the service to the
maximum allowed amount (that is, 19 percent), and then phases in the remainder of the
reduction. To comply with section 1848(c)(7) of the Act, we adjust the PE RVUs to ensure that
the total RVUs for all services that are not new or revised codes decrease by no more than 19
percent, and then apply a relativity adjustment to ensure that the total pool of aggregate PE
RVUs remains relative to the pool of work and MP RVUs. For a more detailed description of
the methodology for the phase-in of significant RVU changes, we refer readers to the CY 2016
PFS final rule with comment period (80 FR 70927 through 70931).
(e) Setup File Information
● Specialties excluded from ratesetting calculation: To calculate the PE and MP RVUs,
we exclude certain specialties, such as NPPs paid at a percentage of the PFS and low volume
specialties, from the calculation. These specialties are included to calculate the BN adjustment.
They are displayed in Table 1.

TABLE 1: Specialties Excluded from Ratesetting Calculation
Specialty
Code
Specialty Description
49
Ambulatory surgical center
50
Nurse practitioner
51
Medical supply company with certified orthotist
52
Medical supply company with certified prosthetist
53
Medical supply company with certified prosthetist-orthotist
54
Medical supply company not included in 51, 52, or 53.
55
Individual certified orthotist
56
Individual certified prosthetist
57
Individual certified prosthetist-orthotist
58
Medical supply company with registered pharmacist
59
Ambulance service supplier, e.g., private ambulance companies, funeral homes, etc.
60
Public health or welfare agencies
61
Voluntary health or charitable agencies
73
Mass immunization roster biller
74
Radiation therapy centers
87
All other suppliers (e.g., drug and department stores)
88
Unknown supplier/provider specialty
89
Certified clinical nurse specialist
96
Optician
97
Physician assistant
A0
Hospital
A1
SNF
A2
Intermediate care nursing facility
A3
Nursing facility, other
A4
HHA
A5
Pharmacy
A6
Medical supply company with respiratory therapist
A7
Department store
A8
Grocery store
B1
Supplier of oxygen and/or oxygen related equipment (eff. 10/2/2007)
B2
Pedorthic personnel
B3
Medical supply company with pedorthic personnel
B4
Rehabilitation Agency
B5
Ocularist
C1
Centralized Flu
C2
Indirect Payment Procedure
C5
Dentistry
● Crosswalk certain low volume physician specialties: Crosswalk the utilization of
certain specialties with relatively low PFS utilization to the associated specialties.
● Physical therapy utilization: Crosswalk the utilization associated with all physical
therapy services to the specialty of physical therapy.
● Identify professional and technical services not identified under the usual TC and 26
modifiers: Flag the services that are PC and TC services but do not use TC and 26 modifiers (for
example, electrocardiograms). This flag associates the PC and TC with the associated global

code for use in creating the indirect PE RVUs. For example, the professional service, CPT code
93010 (Electrocardiogram, routine ECG with at least 12 leads; interpretation and report only), is
associated with the global service, CPT code 93000 (Electrocardiogram, routine ECG with at
least 12 leads; with interpretation and report).
● Payment modifiers: Payment modifiers are accounted for in creating the file consistent
with the current payment policy as implemented in claims processing. For example, services
billed with the assistant at surgery modifier are paid 16 percent of the PFS amount for that
service; therefore, the utilization file is modified to only account for 16 percent of any service
that contains the assistant at surgery modifier. Similarly, for those services to which volume
adjustments are made to account for the payment modifiers, time adjustments are applied as well.
For time adjustments to surgical services, the intraoperative portion in the work time file is used;
where it is not present, the intraoperative percentage from the payment files used by contractors
to process Medicare claims is used instead. Where neither is available, we use the payment
adjustment ratio to adjust the time accordingly. Table 2 details the manner in which the
modifiers are applied.
TABLE 2: Application of Payment Modifiers to Utilization Files
Modifier
Description
Volume Adjustment
Time Adjustment
80,81,82
Assistant at Surgery
16%
Intraoperative portion
AS
Assistant at Surgery –
Physician Assistant
14% (85% * 16%)
Intraoperative portion
50 or
LT and RT
Bilateral Surgery
150%
150% of work time
51
Multiple Procedure
50%
Intraoperative portion
52
Reduced Services
50%
50%
53
Discontinued Procedure
50%
50%
54
Intraoperative Care only
Preoperative + Intraoperative
Percentages on the payment files used
by Medicare contractors to process
Medicare claims
Preoperative + Intraoperative
portion
55
Postoperative Care only
Postoperative Percentage on the
payment files used by Medicare
contractors to process Medicare claims
Postoperative portion
62
Co-surgeons
62.5%
50%
66
Team Surgeons
33%
33%
CO, CQ
Physical and Occupational
Therapy Assistant Services
88%
88%
We also adjust volume and time that correspond to other payment rules, including special
multiple procedure endoscopy rules and multiple procedure payment reductions (MPPRs). We
note that section 1848(c)(2)(B)(v) of the Act exempts certain reduced payments for multiple
imaging procedures and multiple therapy services from the BN calculation under section
1848(c)(2)(B)(ii)(II) of the Act. These MPPRs are not included in the development of the
RVUs.
Beginning in CY 2022, section 1834(v)(1) of the Act required that we apply a 15 percent
payment reduction for outpatient occupational therapy services and outpatient physical therapy
services that are provided, in whole or in part, by a physical therapist assistant (PTA) or
occupational therapy assistant (OTA). Section 1834(v)(2)(A) of the Act required CMS to
establish modifiers to identify these services, which we did in the CY 2019 PFS final rule (83 FR
59654 through 59661), creating the CQ and CO payment modifiers for services provided in
whole or in part by PTAs and OTAs, respectively. These payment modifiers are required to be
used on claims for services with dates of service beginning January 1, 2020, as specified in the
CY 2020 PFS final rule (84 FR 62702 through 62708). We applied the 15 percent payment
reduction to therapy services provided by PTAs (using the CQ modifier) or OTAs (using the CO
modifier), as required by statute. Under sections 1834(k) and 1848 of the Act, payment is made
for outpatient therapy services at 80 percent of the lesser of the actual charge or applicable fee
schedule amount (the allowed charge). The remaining 20 percent is the beneficiary copayment.
For therapy services to which the new discount applies, payment will be made at 85 percent of
the 80 percent of allowed charges. Therefore, the volume discount factor for therapy services to
which the CQ and CO modifiers apply is: (0.20 + (0.80* 0.85), which equals 88 percent.
We note that for CY 2025, we are proposing mandatory use of the 54 and 55 modifiers
when practitioners furnishing global surgery procedures share in patient care and intend only to
furnish preoperative/intraoperative or postoperative portions of the total global procedure. If
finalized, this proposal will likely increase the number of claims subject to the adjustment
described in the discussion above. We discuss this proposal in section II.G. of this proposed
rule.
For anesthesia services, we do not apply adjustments to volume since we use the average
allowed charge when simulating RVUs; therefore, the RVUs as calculated already reflect the
payments as adjusted by modifiers, and no volume adjustments are necessary. However, a time
adjustment of 33 percent is made only for medical direction of two to four cases since that is the
only situation where a single practitioner is involved with multiple beneficiaries concurrently, so
that counting each service without regard to the overlap with other services would overstate the
amount of time spent by the practitioner furnishing these services.
● Work RVUs: The setup file contains the work RVUs from this proposed rule.
(6) Equipment Cost per Minute
The equipment cost per minute is calculated as:
(1/ (minutes per year * usage)) * price * ((interest rate/(1 (1/((1 + interest rate)^ life of
equipment)))) + maintenance)
Where:
minutes per year = maximum minutes per year if usage were continuous (that is,
usage=1); generally, 150,000 minutes.
usage = variable, see discussion below in this proposed rule.
price = price of the particular piece of equipment.
life of equipment = useful life of the particular piece of equipment.
maintenance = factor for maintenance; 0.05.
interest rate = variable, see discussion below in this proposed rule.
Usage: We currently use an equipment utilization rate assumption of 50 percent for most
equipment, with the exception of expensive diagnostic imaging equipment, for which we use a
90 percent assumption as required by section 1848(b)(4)(C) of the Act.
Useful Life: In the CY 2005 PFS final rule we stated that we updated the useful life for
equipment items primarily based on the AHA’s “Estimated Useful Lives of Depreciable Hospital
Assets” guidelines (69 FR 66246). The most recent edition of these guidelines was published in
2018. This reference material provides an estimated useful life for hundreds of different types of
equipment, the vast majority of which fall in the range of 5 to 10 years, and none of which are
lower than two years in duration. We believe that the updated editions of this reference material
remain the most accurate source for estimating the useful life of depreciable medical equipment.
In the CY 2021 PFS final rule, we finalized a proposal to treat equipment life durations of
less than 1 year as having a duration of 1 year for the purpose of our equipment price per minute
formula. In the rare cases where items are replaced every few months, we noted that we believe
it is more accurate to treat these items as disposable supplies with a fractional supply quantity as
opposed to equipment items with very short equipment life durations. For a more detailed
discussion of the methodology associated with very short equipment life durations, we refer
readers to the CY 2021 PFS final rule (85 FR 84482 through 84483).
● Maintenance: We finalized the 5 percent factor for annual maintenance in the CY
1998 PFS final rule with comment period (62 FR 33164). As we previously stated in the CY
2016 PFS final rule with comment period (80 FR 70897), we do not believe the annual
maintenance factor for all equipment is precisely 5 percent, and we concur that the current rate
likely understates the true cost of maintaining some equipment. We also noted that we believe it
likely overstates the maintenance costs for other equipment. When we solicited comments
regarding data sources containing equipment maintenance rates, commenters could not identify
an auditable, robust data source that CMS could use on a wide scale. We noted that we did not
believe voluntary submissions regarding the maintenance costs of individual equipment items
would be an appropriate methodology for determining costs. As a result, in the absence of
publicly available datasets regarding equipment maintenance costs or another systematic data
collection methodology for determining a different maintenance factor, we did not propose a

variable maintenance factor for equipment cost per minute pricing as we did not believe that we
have sufficient information at present. We noted that we would continue to investigate potential
avenues for determining equipment maintenance costs across a broad range of equipment items.
● Interest Rate: In the CY 2013 PFS final rule with comment period (77 FR 68902), we
updated the interest rates used in developing an equipment cost per minute calculation (see 77
FR 68902 for a thorough discussion of this issue). The interest rate was based on the Small
Business Administration (SBA) maximum interest rates for different categories of loan size
(equipment cost) and maturity (useful life). The Interest rates are listed in Table 3.
TABLE 3: SBA Maximum Interest Rates
Price
Useful Life
Interest Rate
<$25K
<7 Years
7.50%
$25K to $50K
<7 Years
6.50%
>$50K
<7 Years
5.50%
<$25K
7+ Years
8.00%
$25K to $50K
7+ Years
7.00%
>$50K
7+ Years
6.00%
We are not proposing any changes to the equipment interest rates for CY 2025.
3. Adjusting RVUs To Match the PE Share of the Medicare Economic Index (MEI)
In the past, we have stated that we believe that the MEI is the best measure available of
the relative weights of the three components in payments under the PFS—work, practice expense
(PE), and malpractice (MP). Accordingly, we believe that to ensure that the PFS payments
reflect the relative resources in each of these PFS components as required by section 1848(c)(3)
of the Act, the RVUs used in developing rates should reflect the same weights in each
component as the cost share weights in the Medicare Economic Index (MEI). In the past, we
have proposed (and subsequently finalized) to accomplish this by holding the work RVUs
constant and adjusting the PE RVUs, MP RVUs, and CF to produce the appropriate balance in
RVUs among the three PFS components and payment rates for individual services, that is, that
the total RVUs on the PFS are proportioned to approximately 51 percent work RVUs, 45 percent
PE RVUs, and 4 percent MP RVUs. As the MEI cost shares are updated, we would typically
propose to modify steps 3 and 10 to adjust the aggregate pools of PE costs (direct PE in step 3
and indirect PE in step 10) in proportion to the change in the PE share in the rebased and revised
MEI cost share weights, and to recalibrate the relativity adjustment that we apply in step 18 as
described in the CY 2023 PFS final rule (87 FR 69414 and 69415) and CY 2014 PFS final rule
(78 FR 74236 and 74237). The most recent recalibration was done for the CY 2014 RVUs.
In the CY 2014 PFS proposed rule (78 FR 43287 through 43288) and final rule (78 FR
74236 through 74237), we detailed the steps necessary to accomplish this result (see steps 3, 10,
and 18). The CY 2014 proposed and final adjustments were consistent with our longstanding
practice to make adjustments to match the RVUs for the PFS components with the MEI cost
share weights for the components, including the adjustments described in the CY 1999 PFS final
rule (63 FR 58829), CY 2004 PFS final rule (68 FR 63246 and 63247), and CY 2011 PFS final
rule (75 FR 73275).
In the CY 2023 PFS final rule (87 FR 69688 through 69711), we finalized to rebase and
revise the MEI to reflect more current market conditions faced by physicians in furnishing
physicians' services (referred to as the “2017-based MEI”). We also finalized a delay of the
adjustments to the PE pools in steps 3 and 10 and the recalibration of the relativity adjustment in
step 18 until the public had an opportunity to comment on the rebased and revised MEI (87 FR
69414 through 69416). Because we finalized significant methodological and data source
changes to the MEI in the CY 2023 PFS final rule and significant time has elapsed since the last
rebasing and revision of the MEI in CY 2014, we believed that delaying the implementation of
the finalized CY 2023 rebased and revised MEI was consistent with our efforts to balance
payment stability and predictability with incorporating new data through more routine updates.
We refer readers to the discussion of our comment solicitation in the CY 2023 PFS final rule (87
FR 69429 through 69432), where we reviewed our ongoing efforts to update data inputs for PE
to aid stability, transparency, efficiency, and data adequacy. We also solicited comment in the
CY 2023 PFS proposed rule on when and how to best incorporate the CY 2023 rebased and
revised MEI into PFS ratesetting, and whether it would be appropriate to consider a transition to
full implementation for potential future rulemaking. We presented the impacts of implementing
the rebased and revised MEI in PFS ratesetting through a 4-year transition and through full
immediate implementation, that is, with no transition period in the CY 2023 PFS proposed rule.
We also solicited comment on other implementation strategies for potential future rulemaking in
the CY 2023 PFS proposed rule. In the CY 2023 PFS final rule, we discussed that many
commenters supported our proposed delayed implementation, and many commenters expressed
concerns with the redistributive impacts of the implementation of the rebased and revised MEI in
PFS ratesetting. Many commenters also noted the AMA’s intent to collect practice cost data
from physician practices, which could be used to derive cost share weights for the MEI and RVU
shares.
In light of the AMA’s current data collection efforts and because the methodological and
data source changes to the MEI finalized in the CY 2023 PFS final rule would have significant
impacts on PFS payments, similar to our discussion of this topic in the CY 2024 PFS rulemaking
cycle (88 FR 78829 through 78831), we continue to believe that delaying the implementation of
the finalized 2017-based MEI cost share weights for the RVUs is consistent with our efforts to
balance payment stability and predictability with incorporating new data through more routine
updates. For these reasons, we did not propose to incorporate the 2017-based MEI in PFS
ratesetting for CY 2024. As we noted in the CY 2024 PFS final rule, many commenters on the
CY 2024 PFS proposed rule supported our continued delayed implementation of the 2017-based
MEI in PFS ratesetting (88 FR 78830). Most of these commenters urged us to pause
consideration of other sources for the MEI until the AMA’s efforts to collect practice cost data
from physician practices have concluded, although a few commenters recommended that we
implement the MEI for PFS ratesetting as soon as possible. We agree with the commenters that
it would be prudent, and avoid potential duplication of effort, to wait to consider other data
sources for the MEI while the AMA’s data collection activities are ongoing. As we discussed in
the CY 2024 PFS final rule, we continue to monitor the data available related to physician
services' input expenses, but we are not proposing to update the data underlying the MEI cost
weights at this time. Given our previously described policy goal to balance PFS payment
stability and predictability with incorporating new data through more routine updates to the MEI,
we are not proposing to incorporate the 2017-based MEI in PFS ratesetting for CY 2025. We
invite comments on this approach as well as any information on the timing of the AMA’s
practice cost data collection efforts and other sources of data we could consider for updating the
MEI.
4. Changes to Direct PE Inputs for Specific Services
This section focuses on specific PE inputs. The direct PE inputs are included in the CY
2025 direct PE input public use files, which are available on the CMS website under downloads
for the CY 2025 PFS proposed rule at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-
Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices.html.
a. Standardization of Clinical Labor Tasks
As we noted in the CY 2015 PFS final rule with comment period (79 FR 67640 through
67641), we continue to make improvements to the direct PE input database to provide the
number of clinical labor minutes assigned for each task for every code in the database instead of
only including the number of clinical labor minutes for the preservice, service, and post service
periods for each code. In addition to increasing the transparency of the information used to set
PE RVUs, this level of detail would allow us to compare clinical labor times for activities
associated with services across the PFS, which we believe is important to maintaining the
relativity of the direct PE inputs. This information would facilitate the identification of the usual
numbers of minutes for clinical labor tasks and the identification of exceptions to the usual
values. It would also allow for greater transparency and consistency in the assignment of
equipment minutes based on clinical labor times. Finally, we believe that the detailed
information can be useful in maintaining standard times for particular clinical labor tasks that can
be applied consistently to many codes as they are valued over several years, similar in principle
to physician preservice time packages. We believe that setting and maintaining such standards
would provide greater consistency among codes that share the same clinical labor tasks and
could improve the relativity of values among codes. For example, as medical practice and
technologies change over time, standards could be updated simultaneously for all codes with the
applicable clinical labor tasks instead of waiting for individual codes to be reviewed.
In the CY 2016 PFS final rule with comment period (80 FR 70901), we solicited
comments on the appropriate standard minutes for the clinical labor tasks associated with
services that use digital technology. After consideration of comments received, we finalized
standard times for clinical labor tasks associated with digital imaging at 2 minutes for
“Availability of prior images confirmed”, 2 minutes for “Patient clinical information and
questionnaire reviewed by technologist, order from physician confirmed and exam protocoled by
radiologist”, 2 minutes for “Review examination with interpreting MD”, and 1 minute for “Exam
documents scanned into PACS” and “Exam completed in RIS system to generate billing process
and to populate images into Radiologist work queue.” In the CY 2017 PFS final rule (81 FR
80184 through 80186), we finalized a policy to establish a range of appropriate standard minutes
for the clinical labor activity, “Technologist QCs images in PACS, checking for all images,
reformats, and dose page.” These standard minutes will be applied to new and revised codes that
make use of this clinical labor activity when they are reviewed by us for valuation. We finalized
a policy to establish 2 minutes as the standard for the simple case, 3 minutes as the standard for
the intermediate case, 4 minutes as the standard for the complex case, and 5 minutes as the
standard for the highly complex case. These values were based upon a review of the existing
minutes assigned for this clinical labor activity; we determined that 2 minutes is the duration for
most services and a small number of codes with more complex forms of digital imaging have
higher values. We also finalized standard times for a series of clinical labor tasks associated
with pathology services in the CY 2016 PFS final rule with comment period (80 FR 70902). We
do not believe these activities would be dependent on number of blocks or batch size, and we
believe that the finalized standard values accurately reflect the typical time it takes to perform
these clinical labor tasks.
In reviewing the RUC-recommended direct PE inputs for CY 2019, we noticed that the 3
minutes of clinical labor time traditionally assigned to the “Prepare room, equipment and
supplies” (CA013) clinical labor activity were split into 2 minutes for the “Prepare room,
equipment and supplies” activity and 1 minute for the “Confirm order, protocol exam” (CA014)
activity. We proposed to maintain the 3 minutes of clinical labor time for the “Prepare room,
equipment and supplies” activity and remove the clinical labor time for the “Confirm order,
protocol exam” activity wherever we observed this pattern in the RUC-recommended direct PE
inputs. Commenters explained in response that when the new version of the PE worksheet
introduced the activity codes for clinical labor, there was a need to translate old clinical labor
tasks into the new activity codes, and that a prior clinical labor task was split into two of the new
clinical labor activity codes: CA007 (Review patient clinical extant information and
questionnaire) in the preservice period, and CA014 (Confirm order, protocol exam) in the
service period. Commenters stated that the same clinical labor from the old PE worksheet was
now divided into the CA007 and CA014 activity codes, with a standard of 1 minute for each
activity. We agreed with commenters that we would finalize the RUC-recommended 2 minutes
of clinical labor time for the CA007 activity code and 1 minute for the CA014 activity code in
situations where this was the case. However, when reviewing the clinical labor for the reviewed
codes affected by this issue, we found that several of the codes did not include this old clinical
labor task, and we also noted that several of the reviewed codes that contained the CA014
clinical labor activity code did not contain any clinical labor for the CA007 activity. In these
situations, we believe that the three total minutes of clinical staff time would be more accurately
described by the CA013 “Prepare room, equipment and supplies” activity code, and we finalized
these clinical labor refinements. We direct readers to the discussion in the CY 2019 PFS final
rule (83 FR 59463 through 59464) for additional details.
Following the publication of the CY 2020 PFS proposed rule, one commenter expressed
concern with the published list of common refinements to equipment time. The commenter
stated that these refinements were the formulaic result of applying refinements to the clinical
labor time and did not constitute separate refinements; the commenter requested that CMS no
longer include these refinements in the table published each year. In the CY 2020 PFS final rule,
we agreed with the commenter that these equipment time refinements did not reflect errors in the
equipment recommendations or policy discrepancies with the RUC’s equipment time
recommendations. However, we believed it was important to publish the specific equipment
times that we were proposing (or finalizing in the case of the final rule) when they differed from
the recommended values due to the effect these changes can have on the direct costs associated
with equipment time. Therefore, we finalized the separation of the equipment time refinements
associated with changes in clinical labor into a separate table of refinements. We direct readers
to the discussion in the CY 2020 PFS final rule (84 FR 62584) for additional details.
Historically, the RUC has submitted a “PE worksheet” that details the recommended
direct PE inputs for our use in developing PE RVUs. The format of the PE worksheet has varied
over time, and among the medical specialties developing the recommendations. These variations
have made it difficult for the RUC’s development and our review of code values for individual
codes. Beginning with its recommendations for CY 2019, the RUC mandated the use of a new
PE worksheet for its recommendation development process that standardizes the clinical labor
tasks and assigns them a clinical labor activity code. We believe the RUC’s use of the new PE
worksheet in developing and submitting recommendations helps us simplify and standardize the
hundreds of clinical labor tasks currently listed in our direct PE database. As in previous
calendar years, to facilitate rulemaking for CY 2025, we are continuing to display two versions
of the Labor Task Detail public use file: one version with the old listing of clinical labor tasks
and one with the same tasks crosswalked to the new listing of clinical labor activity codes.
These lists are available on the CMS website under downloads for the CY 2025 PFS proposed
rule at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-
Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices.html.
b. Updates to Prices for Existing Direct PE Inputs
In the CY 2011 PFS final rule with comment period (75 FR 73205), we finalized a
process to act on public requests to update equipment and supply price and equipment useful life
inputs through annual rulemaking, beginning with the CY 2012 PFS proposed rule. Beginning in
CY 2019 and continuing through CY 2022, we conducted a market-based supply and equipment
pricing update using information developed by our contractor, StrategyGen, which updated
pricing recommendations for approximately 1300 supplies and 750 equipment items currently
used as direct PE inputs. Given the potentially significant changes in payment that would occur,
in the CY 2019 PFS final rule, we finalized a policy to phase in our use of the new direct PE
input pricing over a 4-year period using a 25/75 percent (CY 2019), 50/50 percent (CY 2020),
75/25 percent (CY 2021), and 100/0 percent (CY 2022) split between new and old pricing. We
believed that implementing the proposed updated prices with a 4-year phase-in would improve
payment accuracy while maintaining stability and allowing interested parties to address potential
concerns about changes in payment for particular items. This 4-year transition period to update
supply and equipment pricing concluded in CY 2022; for a more detailed discussion, we refer
readers to the CY 2019 PFS final rule with comment period (83 FR 59473 through 59480).
For CY 2025, we are proposing to update the price of 17 supplies and one equipment
item in response to the public submission of invoices following the publication of the CY 2024
PFS final rule. The 18 supply and equipment items with proposed updated prices are listed in the
valuation of specific codes section of the preamble under Table 16, CY 2025 Invoices Received
for Existing Direct PE Inputs.
An interested party submitted 30 invoices to update pricing for the human amniotic
membrane allograft mounted on a non-absorbable self-retaining ring (SD248) supply. We
previously updated the price of this supply in the CY 2024 final rule (88 FR 78901) based on
averaging together the price of the Prokera Slim, Prokera Classic, and Prokera Plus devices. The
interested party submitted new invoices for all three of these devices which averaged to a new
price of $1149.00 which we are proposing for the SD248 supply. We are soliciting additional
comments from interested parties regarding the price of the SD248 supply as well as any
information as far as whether one of these three devices (the Prokera Slim, Prokera Classic, and
Prokera Plus) would be more typical than the other two for use as a supply in CPT code 65778.
In the case of the indocyanine green (25ml uou) (SL083) supply, we noticed that there
was a clear bimodal distribution of prices on the eight submitted invoices, clustered around
$91.00 and $141.67, respectively, with no pricing in between $100 and $140. We are proposing
the updated total average price of $125.11 based on the eight submitted invoices for the SL083
supply, however, we are soliciting comments on why there was such divergence in the pricing on
the submitted invoices, as well as whether these may represent pricing for two different supplies.
Regarding the Reaction buffer 10X (Ventana 950-300) (SL478) supply, we are proposing
to update the price from $0.037 to $0.045, which is less than the $0.075 contained on the invoice
submitted by interested parties. We were able to find this product readily available for purchase
online at a quantity of 10 liters for $453 or a price of $0.045. We do not believe that it would be
typical for providers to pay a higher price based on smaller unit quantities; therefore, we are
proposing to update the price of the SL478 supply but only to $0.045, which is the price to
purchase this supply online, as stated above.
Interested parties also alerted CMS to a technical correction for pricing the Atomizer tips
(disposable) (SL464) supply. We previously finalized a price of $2.66 for the SL464 supply,
which was included in the table of Invoices Received for Existing Direct PE Inputs in the CY
2018 final rule (82 FR 53162). However, due to a technical error, the updated pricing for the
SL464 supply was never implemented. We are proposing to make this correction for CY 2025;
the corrected price of $2.66 for the SL464 supply is included in Table 16.
We are not proposing to update the price of another ten supplies, which were the subject
of public submission of invoices. Our reasons for not proposing updates to these prices are
detailed below, and we are seeking additional information from interested parties for assistance
in pricing these supplies:
● Liposorber supplies: Tubing set (SC083), Plasma LDL adsorption column (SD186),
and Plasma separator (SD188): We received invoices for these three Liposorber supplies from
an interested party. However, it was unclear from the invoice submissions what the unit quantity
size is for each product. We require additional information regarding the unit size of each supply
included on these invoices to establish updated pricing, and therefore, we are not proposing
updates to the prices for these supplies. We are seeking additional comments regarding the
pricing of these supplies and whether the pricing has increased so dramatically, as it seems
unlikely that prices have tripled in the five years since we most recently updated the pricing for
these supplies.
● Congo Red kits (SA110): We received three invoices from interested parties requesting
an increase in the price of the SA110 supply from $6.80 to $18.78. However, we were able to
find Congo Red staining kits readily available online at a price of 100 for $410 or $4.10 per kit.
The unit size of these kits was also unclear, which made price comparisons with the submitted
invoices difficult. Based on the three invoices and the online price of 100 for $410 or $4.10 per
kit, we do not believe there is enough pricing data to support an increase in the price of the
SA110 supply from $6.80 to $18.78, and we are not proposing an increase in the price of this
supply.
● Gauze, non-sterile 4in x 4in (SG051): We received one invoice from interested parties
requesting an increase in the price of the SG051 supply from $0.03 to $0.04. However, the
submitted invoice price appeared to be for surgical gauze, not non-sterile gauze. We were able
to find the 4x4 non-sterile gauze readily available online at less than the invoice price. Based on
this information, we do not believe there is enough pricing data to support an increase in the
price of the SG051 supply from $0.03 to $0.04, and we are not proposing an increase in the price
of this supply.
● Permanent marking pen (SL477): We received one invoice from interested parties
requesting an increase in the price of the SL477 supply from $2.81 to $4.62. However, we found
black marking pens, such as Sharpies, widely available at unit prices around $2.00 when
purchased in larger quantities. Based on this information, we do not believe there is enough
pricing data to support an increase in the price of the SL477 supply from $2.81 to $4.62, and we
are not proposing an increase in the price of this supply.
● Hematoxylin II (Ventana 790-2208) (SL483): We received four invoices from
interested parties requesting an increase in the price of the SL483 supply from $0.780 to $2.722.
However, we were able to find hematoxylin II stains readily available online at cheaper prices,
such as $52.00 for 500 ml ($0.104 per ml). Based on this information, we do not believe there is
enough pricing data to support an increase in the price of the SL483 supply from $0.780 to
$2.722, and we are not proposing an increase in the price of this supply.
● Bluing reagent (Ventana 760-2037) (SL484): We received three invoices from
interested parties requesting an increase in the price of the SL484 supply from $4.247 to $6.130.
While researching the pricing of the SL484 supply, we were unable to determine the unit
quantity size on invoices, which made it difficult to evaluate if the requested price accurately
reflected market pricing. As best we could tell, the requested price increase to $6.130 was more
expensive than comparable online bluing reagents available for purchase. Based on this
information, we do not believe there is enough pricing data to support an increase in the price of
the SL484 supply from $4.247 to $6.130, and we are not proposing an increase in the price of
this supply.
● EZ Prep (10X) (Ventana 950-102) (SL481) and 250 Test Prep Kit # 78 (Ventana 786-
3034) (SL486): In each of these cases, we received invoices from interested parties requesting
substantial increases in the price of the associated supplies, from $0.034 to $0.509 for the SL481
supply and from $0.309 to $2.134 for the SL486 supply. We do not believe that it is reasonable
to expect that the typical market prices for these supplies have increased by 1400 percent and
600 percent, respectively, in the 5 years since we most recently updated the pricing for these
supplies. The limited pricing information we could find online for each product also failed to
support these drastic increases in pricing. Based on this information, we do not believe there is
enough pricing data to support the requested increases for the SL481 and SL486 supplies, and we
are not proposing increases to the prices for these supplies.
(1) Invoice Submission
We remind readers that we routinely accept public submissions of invoices as part of our
process for developing payment rates for new, revised, and potentially misvalued codes. Often,
these invoices are submitted in conjunction with the RUC-recommended values for the codes.
To be included in a given year’s proposed rule, we generally need to receive invoices by the
same February 10th deadline we noted for consideration of RUC recommendations. However,
we will consider invoices submitted as public comments during the comment period following
the publication of the PFS proposed rule and would consider any invoices received after
February 10th or outside of the public comment process as part of our established annual process
for requests to update supply and equipment prices. Interested parties are encouraged to submit
invoices with their public comments or, if outside the notice and comment rulemaking process,
via email at [email protected].
In recent years, we have noticed a growing number of invoice submissions for use in
updating supply and equipment pricing. Although we continue to believe in the importance of
using the most recent and accurate invoice data to reflect current market pricing, we do have
some concerns that the increased use of these submissions may distort relativity across the fee
schedule. Relying on voluntary invoice submissions to update pricing for a small subset of the
total number of supply and equipment items in our database, while leaving the overwhelming
majority of prices untouched, could be distorting pricing in favor of the most recent submissions.
We believe that it may be more efficient, and more accurate, to update supply and equipment
pricing in a more comprehensive fashion similar to the pricing update that took place from CY
2019 to CY 2022. For example, future updates to supply and equipment pricing could take place
in tandem with updates to clinical labor pricing after the current clinical labor update concludes
in CY 2025. We welcome public comments on this general topic of more comprehensive updates
to supply and equipment pricing, and we may consider comments we receive to inform future
rulemaking.
(2) Supply Pack Pricing Update
Interested parties previously notified CMS that they identified numerous discrepancies
between the aggregated cost of some supply packs and the individual item components contained
within. The interested parties indicated that CMS should rectify these mathematical errors as
soon as possible to ensure that the sum correctly matches the totals from the individual items,
and they recommended that we resolve these pricing discrepancies in the supply packs during
CY 2024 rulemaking. The AMA RUC convened a workgroup on this subject and submitted
recommendations to update pricing for a series of supply packs along with the RUC’s comment
letter for the CY 2024 rule cycle.
We appreciated the additional information and RUC workgroup recommendations
regarding discrepancies in the aggregated cost of some supply packs. However, due to the
projected significant cost revisions in the pricing of supply packs and because we did not
propose to address supply pack pricing in the CY 2024 proposed rule, we stated that this issue
would be better addressed in future rulemaking. For example, the cleaning and disinfecting
endoscope pack (SA042) is included as a supply input in more than 300 HCPCS codes, which
could have a sizable impact on the overall valuation of these services, and which was not
incorporated into the proposed RVUs published for the CY 2024 proposed rule. We stated that
interested parties would be better served if we comprehensively addressed this topic during
future rulemaking in which commenters could provide feedback in response to proposed pricing
updates (88 FR 78833 through 78834).
For CY 2025, we are proposing to implement the supply pack pricing update and
associated revisions as recommended by the RUC’s workgroup. We are proposing to update the
pricing of the “pack, cleaning and disinfecting, endoscope” (SA042) supply from $19.43 to
$31.29, to update the pricing of the “pack, drapes, cystoscopy” (SA045) supply from $17.33 to
$14.99, to update the pricing of the “pack, ocular photodynamic therapy” (SA049) supply from
$16.35 to $26.35, to update the pricing of the “pack, urology cystoscopy visit” (SA058) supply
from $113.70 to $37.63, and to update the pricing of the “pack, ophthalmology visit (w-
dilation)” (SA082) supply from $3.91 to $2.33. As recommended by the RUC workgroup, we
are also proposing to delete the “pack, drapes, laparotomy (chest-abdomen)” (SA046) supply
entirely. The proposed updated prices for these supply packs are listed in the valuation of
specific codes section of the preamble under Table 16, CY 2025 Invoices Received for Existing
Direct PE Inputs.
In accordance with the RUC workgroup’s recommendations, we are also proposing to
create eight new supply codes, including components contained within previously existing
supply packs. Aside from the SB056 supply, which is a replacement in several HCPCS codes for
the deleted SA046 supply pack, all of these new supplies are not included as standalone direct
PE inputs in any current HCPCS codes, as they are, again, components contained within
previously existing supply packs. We are proposing to add:
● The kit, ocular photodynamic therapy (PDT) (SA137) supply at a price of $26.00 as a
component of the SA049 supply pack;
● The Abdominal Drape Laparotomy Drape Sterile (100 in x 72 in x 124 in) (SB056)
supply at a price of $8.049 as a replacement for the SA046 supply pack;
● The drape, surgical, legging (SB057) supply at a price of $3.284 as a component of the
SA045 supply pack;
● The drape, surgical, split, impervious, absorbent (SB058) supply at a price of $8.424
as a component of the SA045 supply pack;
● The post-mydriatic spectacles (SB059) supply at a price of $0.328 as a component of
the SA082 supply pack;
● The y-adapter cap (SD367) supply at a price of $0.352 as a component of the SA049
supply pack;
● The ortho-phthalaldehyde 0.55% (eg, Cidex OPA) (SM030) supply at a price of
$0.554 as a component of the SA042 supply pack; and
● The ortho-phthalaldehyde test strips (SM031) supply at a price of $1.556 as a
component of the SA042 supply pack.
The proposed new supply pack component items are listed in the valuation of specific
codes section of the preamble under Table 17, CY 2025 New Invoices.
We are also proposing the following additional supply substitutions based on the
recommendations of the RUC workgroup. We are proposing to remove the deleted SA046
supply pack and replace it with the drape, sterile, fenestrated 16in x 29in (SB011) supply for
CPT codes 19020, 19101, 19110, 19112, 20101, and 20102. We are proposing to remove the
deleted SA046 supply pack and replace it with two supplies – the drape, sterile, three-quarter
sheet (SB014) and the drape, towel, sterile 18in x 26in (SB019) – for CPT codes 19000 and
60300. We are proposing to remove the deleted SA046 supply pack and replace it with two
supplies – the drape, towel, sterile 18in x 26in (SB019) and the newly created Abdominal Drape
Laparotomy Drape Sterile (100 in x 72 in x 124 in) (SB056) supply – for CPT codes 22510,
22511, 22513, and 22514. We are proposing to remove the deleted SA046 supply pack without
replacing it with anything for CPT code 22526; the RUC workgroup did not make a
recommendation on what to do with CPT code 27278, which also previously contained the
SA046 supply pack. Therefore, we are also proposing not to replace the SA046 supply pack with
any supplies for this code. The RUC workgroup also recommended removing the SA046 supply
pack from CPT code 64595 with no replacement; however, this code was recently reviewed at
the April 2022 RUC meeting and it no longer includes the SA046 supply.
The RUC workgroup also reviewed the issue of skin adhesives and identified several
generic alternatives to using the skin adhesive (Dermabond) (SG007) supply. The workgroup
stated that there are multiple skin adhesive products, at different price points, available that work
similarly to Dermabond and requested that generic alternatives be used overall in place of brand
names in the CMS direct PE database. The workgroup made a series of suggestions for CMS to
create new medical supply item codes to encompass the generic formulations of cyanoacrylate
skin adhesive in multidose form and single use sterile application.
We appreciate the recommendations from the RUC workgroup and concur that generic
alternatives be used in place of brand names, where appropriate, in the CMS direct PE database.
However, we have no pricing information or submitted invoices for the four generic formulations
of cyanoacrylate skin adhesive requested by the RUC workgroup (2-Octyl-cyanoacrylate, n-
Butyl-2-cyanoacrylate, Combined n-Butyl and 2-Octylcyanoacrylate, and Ethyl-2-
cyanoacrylate). Since these four potential new supplies have no pricing information and are not
currently included as direct PE inputs for any HCPCS codes, we have not added them to our
direct PE database for the CY 2025 proposed rule due to lack of available information.
c. Clinical Labor Pricing Update
Section 220(a) of the PAMA provides that the Secretary may collect or obtain
information from any eligible professional or any other source on the resources directly or
indirectly related to furnishing services for which payment is made under the PFS and that such
information may be used in the determination of relative values for services under the PFS. Such
information may include the time involved in furnishing services; the amounts, types, and prices
of PE inputs; overhead and accounting information for practices of physicians and other
suppliers, and any other elements that would improve the valuation of services under the PFS.
Beginning in CY 2019, we updated the supply and equipment prices used for PE as part
of a market-based pricing transition; CY 2022 was the final year of this 4-year transition. We
initiated a market research contract with StrategyGen to conduct an in-depth and robust market
research study to update the supply and equipment pricing for CY 2019, and we finalized a
policy in CY 2019 to phase in the new pricing over a period of 4 years. However, we did not
propose to update the clinical labor pricing, and the pricing for clinical labor has remained
unchanged during this pricing transition. Clinical labor rates were last updated for CY 2002
using Bureau of Labor Statistics (BLS) data and other supplementary sources where BLS data
were not available; we refer readers to the full discussion in the CY 2002 PFS final rule for
additional details (66 FR 55257 through 55262).
Interested parties raised concerns that the long delay since clinical labor pricing was last
updated created a significant disparity between CMS’ clinical wage data and the market average
for clinical labor. In recent years, several interested parties suggested that certain wage rates
were inadequate because they did not reflect current labor rate information. Some interested
parties also stated that updating the supply and equipment pricing without updating the clinical
labor pricing could create distortions in the allocation of direct PE. They argued that since the
pool of aggregated direct PE inputs is budget neutral, if these rates are not routinely updated,
clinical labor may become undervalued over time relative to equipment and supplies, especially
since the supply and equipment prices are in the process of being updated. There was
considerable interest among interested parties in updating the clinical labor rates, and when we
solicited comment on this topic in past rules, such as in the CY 2019 PFS final rule (83 FR
59480), interested parties supported the idea.
Therefore, we proposed to update the clinical labor pricing for CY 2022, in conjunction
with the final year of the supply and equipment pricing update (86 FR 39118 through 39123).
We believed updating the clinical labor pricing was important to maintain relativity with the
recent supply and equipment pricing updates. We proposed to use the methodology outlined in
the CY 2002 PFS final rule (66 FR 55257), which draws primarily from BLS wage data, to
calculate updated clinical labor pricing. As we stated in the CY 2002 PFS final rule, the BLS’
reputation for publishing valid estimates that are nationally representative led to the choice to use
the BLS data as the main source. We believe that the BLS wage data continues to be the most
accurate source to use as a basis for clinical labor pricing and this data will appropriately reflect
changes in clinical labor resource inputs for setting PE RVUs under the PFS. We used the most
current BLS survey data (2019) as the main source of wage data for our CY 2022 clinical labor
proposal.
We recognized that the BLS survey of wage data does not cover all the staff types
contained in our direct PE database. Therefore, we crosswalked or extrapolated the wages for
several staff types using supplementary data sources for verification whenever possible. In
situations where the price wages of clinical labor types were not referenced in the BLS data, we
used the national salary data from the Salary Expert, an online project of the Economic Research
Institute that surveys national and local salary ranges and averages for thousands of job titles
using mainly government sources. (A detailed explanation of the methodology used by Salary
Expert to estimate specific job salaries can be found at www.salaryexpert.com.) We previously
used Salary Expert information as the primary backup source of wage data during the last update
of clinical labor pricing in CY 2002. If we did not have direct BLS wage data available for a
clinical labor type, we used the wage data from Salary Expert as a reference for pricing, then
crosswalked these clinical labor types to a proxy BLS labor category rate that most closely
matched the reference wage data, similar to the crosswalks used in our PE/HR allocation. For
example, there is no direct BLS wage data for the Mammography Technologist (L043) clinical
labor type; we used the wage data from Salary Expert as a reference and identified the BLS wage
data for Respiratory Therapists as the best proxy category. We calculated rates for the “blend”
clinical labor categories by combining the rates for each labor type in the blend and then dividing
by the total number of labor types in the blend.
As in the CY 2002 clinical labor pricing update, the proposed cost per minute for each
clinical staff type was derived by dividing the average hourly wage rate by 60 to arrive at the per
minute cost. In cases where an hourly wage rate was not available for a clinical staff type, the
proposed cost per minute for the clinical staff type was derived by dividing the annual salary
(converted to 2021 dollars using the Medicare Economic Index) by 2080 (the number of hours in
a typical work year) to arrive at the hourly wage rate and then again by 60 to arrive at the per
minute cost. We ultimately finalized the use of median BLS wage data instead of mean BLS
wage data in response to comments in the CY 2022 PFS final rule. To account for the
employers’ cost of providing fringe benefits, such as sick leave, we finalized a benefits multiplier
of 1.296 based on a BLS release from June 17, 2021 (USDL-21-1094). As an example of this
process, for the Physical Therapy Aide (L023A) clinical labor type, the BLS data reflected a
median hourly wage rate of $12.98, which we multiplied by the 1.296 benefits modifier and then
divided by 60 minutes to arrive at the finalized per-minute rate of $0.28.
After considering the comments on our CY 2022 proposals, we agreed with commenters
that the use of a multi-year transition would help smooth out the changes in payment resulting
from the clinical labor pricing update, avoiding potentially disruptive changes in payment for
affected interested parties, and promoting payment stability from year-to-year. We believed it
would be appropriate to use a 4-year transition, as we have for several other broad-based updates
or methodological changes. While we recognized that using a 4-year transition to implement the
update means that we will continue to rely in part on outdated data for clinical labor pricing until
the change is fully completed in CY 2025, we agreed with the commenters that these significant
updates to PE valuation should be implemented in the same way, and for the same reasons, as for
other major updates to pricing such as the recent supply and equipment update. Therefore, we
finalized the clinical labor pricing update implementation over four years to transition from

current prices to the final updated prices in CY 2025. We finalized the implementation of this
pricing transition over 4 years, such that one-quarter of the difference between the current price
and the fully phased-in price is implemented for CY 2022, one-third of the difference between
the CY 2022 price and the final price is implemented for CY 2023, and one-half of the difference
between the CY 2023 price and the final price is implemented for CY 2024, with the new direct
PE prices fully implemented for CY 2025. (86 FR 65025) An example of the transition from the
current to the fully-implemented new pricing that we finalized in the CY 2022 PFS final rule is
provided in Table 4.
TABLE 4: Example of Clinical Labor Pricing Transition
Current Price
$1.00
Final Price
$2.00
Year 1 (CY 2022) Price
$1.25
1/4 difference between $1.00 and $2.00
Year 2 (CY 2023) Price
$1.50
1/3 difference between $1.25 and $2.00
Year 3 (CY 2024) Price
$1.75
1/2 difference between $1.50 and $2.00
Final (CY 2025) Price
$2.00
(1) CY 2023 Clinical Labor Pricing Updates
For CY 2023, we received information from one interested party regarding the pricing of
the Histotechnologist (L037B) clinical labor type. The interested party provided data from the
2019 Wage Survey of Medical Laboratories which supported an increase in the per-minute rate
from the $0.55 finalized in the CY 2022 PFS final rule to $0.64. This rate of $0.64 for the
L037B clinical labor type is a close match to the online salary data that we had for the
Histotechnologist and matches the $0.64 rate that we initially proposed for L037B in the CY
2022 PFS proposed rule. Based on the wage data provided by the commenter, we proposed this
$0.64 rate for the L037B clinical labor type for CY 2023; we also proposed a slight increase in
the pricing for the Lab Tech/Histotechnologist (L035A) clinical labor type from $0.55 to $0.60
as it is a blend of the wage rate for the Lab Technician (L033A) and Histotechnologist clinical
labor types. We also proposed the same increase to $0.60 for the Angio Technician (L041A)
clinical labor type, as we previously established a policy in the CY 2022 PFS final rule that the
pricing for the L041A clinical labor type would match the rate for the L035A clinical labor type
(86 FR 65032).
Based on comments received on the CY 2023 proposed rule, we finalized a change in the
descriptive text of the L041A clinical labor type from “Angio Technician” to “Vascular
Interventional Technologist”. We also finalized an update in the pricing of three clinical labor
types: from $0.60 to $0.84 for the Vascular Interventional Technologist (L041A), from $0.63 to
$0.79 for the Mammography Technologist (L043A), and from $0.76 to $0.78 for the CT
Technologist (L046A) based on submitted wage data from the 2022 Radiologic Technologist
Wage and Salary Survey (87 FR 69422 through 69425).
(2) CY 2024 Clinical Labor Pricing Updates
We did not receive new wage data or other additional information for use in clinical labor
pricing from interested parties prior to the publication of the CY 2024 PFS proposed rule.
Therefore, our proposed clinical labor pricing for CY 2024 was based on the clinical labor
pricing that we finalized in the CY 2023 PFS final rule, incremented an additional step for Year
3 of the update. Based on comments received on the CY 2024 proposed rule, we finalized an
update in the clinical labor pricing of the cytotechnologist (L045A) clinical labor type from
$0.76 to $0.85 based on submitted data from the 2021 American Society of Clinical Pathologists
(ASCP) Wage Survey of Medical Laboratories (88 FR 78838).
(3) CY 2025 Clinical Labor Pricing Update Proposals
We did not receive new wage data or other additional information for use in clinical labor
pricing from interested parties prior to the publication of the CY 2025 PFS proposed rule.
Therefore, our proposed clinical labor pricing for CY 2025 in Table 5 is based on the clinical
labor pricing that we finalized in the CY 2024 PFS final rule, incremented an additional step for
the final Year 4 of the update:

TABLE 5: Proposed CY 2025 Clinical Labor Pricing
Labor
Code
Labor Description
Source
CY 2021
Rate Per
Minute
Final Y4
Rate Per
Minute
Total %
Change
L023A
Physical Therapy Aide
BLS 31-2022
0.23
0.28
22%
L026A
Medical/Technical Assistant
BLS 31-9092
0.26
0.36
38%
L030A
Lab Tech/MTA
L033A, L026A
0.30
0.46
53%
L032B
EEG Technician
BLS 29-2098
0.32
0.44
38%
L033A
Lab Technician
BLS 29-2010
0.33
0.55
67%
L033B
Optician/COMT
BLS 29-2081, BLS 29-2057
0.33
0.39
18%
L035A
Lab Tech/Histotechnologist
L033A, L037B
0.35
0.60
70%
L037A
Electrodiagnostic Technologist
BLS 29-2098
0.37
0.44
19%
L037B
Histotechnologist
BLS 29-2010
0.37
0.64
73%
L037C
Orthoptist
BLS 29-1141
0.37
0.76
105%
L037D
RN/LPN/MTA
L051A, BLS 29-2061, L026A
0.37
0.54
46%
L037E
Child Life Specialist
BLS 21-1021
0.37
0.49
32%
L038A
COMT/COT/RN/CST
BLS 29-2057, BLS 29-2055,
L051A, BLS 19-4010
0.38
0.52
37%
L038B
Cardiovascular Technician
BLS 29-2031
0.38
0.60
58%
L038C
Medical Photographer
BLS 29-2050
0.38
0.38
0%
L039A
Certified Retinal Angiographer
BLS 29-9000
0.39
0.52
33%
L039B
Physical Therapy Assistant
BLS 31-2021
0.39
0.61
56%
L039C
Psychometrist
BLS 21-1029
0.39
0.64
62%
L041A
Vascular Interventional Technologist
ASRT Wage Data
0.41
0.84
104%
L041B
Radiologic Technologist
BLS 29-2034
0.41
0.63
54%
L041C
Second Radiologic Technologist for
Vertebroplasty
BLS 29-2034
0.41
0.63
54%
L042A
RN/LPN
L051A, BLS 29-2061
0.42
0.63
50%
L042B
Respiratory Therapist
BLS 29-1126
0.42
0.64
52%
L043A
Mammography Technologist
ASRT Wage Data
0.43
0.79
84%
L045A
Cytotechnologist
BLS 29-9092
0.45
0.85
89%
L045B
Electron Microscopy Technologist
BLS 29-1124
0.45
0.89
98%
L045C
CORF social worker/psychologist
BLS 21-1022, BLS 19-3031
0.45
0.70
56%
L046A
CT Technologist*
ASRT Wage Data
0.46
0.78
70%
L047A
MRI Technologist
BLS 29-2035
0.47
0.76
62%
L047B
REEGT (Electroencephalographic
Tech)
BLS 29-2035
0.47
0.76
62%
L047C
RN/Respiratory Therapist
L051A, L042B
0.47
0.70
49%
L047D
RN/Registered Dietician
L051A, BLS 29-1031
0.47
0.70
49%
L049A
Nuclear Medicine Technologist
BLS 29-2033
0.62
0.81
32%
L050A
Cardiac Sonographer
BLS 29-2032
0.50
0.77
54%
L050B
Diagnostic Medical Sonographer
BLS 29-2032
0.50
0.77
54%
L050C
Radiation Therapist
BLS 29-1124
0.50
0.89
78%
L050D
Second Radiation Therapist for IMRT
BLS 29-1124
0.50
0.89
78%
L051A
RN
BLS 29-1141
0.51
0.76
49%
L051B
RN/Diagnostic Medical Sonographer
L051A, BLS 29-2032
0.51
0.77
51%
L051C
RN/CORF
L051A
0.51
0.76
49%
L052A
Audiologist
BLS 29-1181
0.52
0.81
56%
L053A
RN/Speech Pathologist
L051A, L055A
0.53
0.79
49%
L054A
Vascular Technologist
BLS 19-1040
0.54
0.91
69%
L055A
Speech Pathologist
BLS 29-1127
0.55
0.82
49%
L056A
RN/OCN
BLS 29-2033
0.79
0.81
3%
L057A
Genetics Counselor
BLS 29-9092
0.57
0.85
50%
L057B
Behavioral Health Care Manager
BLS 21-1018
0.57
0.57
0%
L063A
Medical Dosimetrist
BLS 19-1040
0.63
0.91
44%
L107A
Medical Dosimetrist/Medical
Physicist
L063A, L152A
1.08
1.52
41%
L152A
Medical Physicist
AAPM Wage Data
1.52
2.14
41%
As was the case for the market-based supply and equipment pricing update, the clinical
labor rates will remain open for public comment during the 60-day comment period for this CY
2025 PFS proposed rule. We expect to set the updated clinical labor rates for CY 2025 in the
final rule. We updated the pricing of some clinical labor types in the CY 2022, CY 2023, and
CY 2024 PFS final rules in response to information provided by commenters. For the full
discussion of the clinical labor pricing update, we direct readers to the CY 2022 PFS final rule
(86 FR 65020 through 65037).
5. Development of Strategies for Updates to Practice Expense Data Collection and Methodology
a. Background
The AMA PPIS was first introduced in 2007 as a means to collect comprehensive and
reliable data on the direct and indirect PEs incurred by physicians (72 FR 66222). In considering
the use of PPIS data, the goal was to improve the accuracy and consistency of PE RVUs used in
the PFS. The data collection process included a stratified random sample of physicians across
various specialties, and the survey was administered between August 2007 and March 2008.
Data points from that period of time are integrated into PFS calculations today. In the CY 2009
PFS proposed rule (73 FR 38507 through 3850), we discussed the indirect PE methodology that
used data from the AMA's survey that predated the PPIS. In CY 2010 PFS rulemaking, we
announced our intent to incorporate the AMA PPIS data into the PFS ratesetting process, which
would first affect the PE RVU. In the CY 2010 PFS proposed rule, we outlined a 4-year
transition period, during which we would phase in the AMA PPIS data, replacing the existing PE
data sources (74 FR 33554). We also explained that our proposals intended to update survey
data only (74 FR 33530 through 33531). In our CY 2010 final rule, we finalized our proposal,
with minor adjustments based on public comments (74 FR 61749 through 61750). We
responded to the comments we received about the transition to using the PPIS to inform indirect
PE allocations (74 FR 61750). In the responses, we acknowledged concerns about potential gaps
in the data, which could impact the allocation of indirect PE for certain physician specialties and
suppliers, which are issues that remain important today. The CY 2010 PFS final rule explains
that section 212 of the Balanced Budget Refinement Act of 1999 (Pub. L. 106-113, November
29, 1999) (BBRA) directed the Secretary to establish a process under which we accept and use,
to the maximum extent practicable and consistent with sound data practices, data collected or
developed by entities and organizations to supplement the data we normally collect in
determining the PE component. BBRA required us to establish criteria for accepting
supplemental survey data. Since the supplemental surveys were specific to individual specialties
and not part of a comprehensive multispecialty survey, we had required that certain precision
levels be met in order to ensure that the supplemental data was sufficiently valid, and acceptable
for use in the development of the PE RVUs. At the time, our rationale included the assumption
that because the PPIS is a contemporaneous, consistently collected, and comprehensive
multispecialty survey, we do not believe similar precision requirements are necessary, and we
did not propose to establish them for the use of the PPIS data (74 FR 61742). We noted potential
gaps in the data, which could impact the allocation of indirect PE for certain physician and
suppliers. The CY 2010 final rule adopted the proposal, with minor adjustments based on public
comments, and explained that these minor adjustments were in part due to non-response bias that
results when the characteristics of survey respondents differ in meaningful ways, such as in the
mix of practices sizes, from the general population (74 FR 61749 through 61750).
Throughout the 4-year transition period, from CY 2010 to CY 2013, we gradually
incorporated the AMA PPIS data into the PFS rates, replacing the previous data sources. The
process involved addressing concerns and making adjustments as necessary, such as refining the
PFS ratesetting methodology in consideration of interested party feedback. For background on
the refinements that we considered after the transition began, we refer readers to discussions in
the CY 2011 through 2014 final rules (75 FR 73178 through 73179; 76 FR 73033 through
73034; 77 FR 98892; 78 FR 74272 through 74276).
In the CY 2011 PFS proposed rule, we requested comments on the methodology for
calculating indirect PE RVUs, explicitly seeking input on using survey data, allocation methods,
and potential improvements (75 FR 40050). In our CY 2011 PFS final rule, we addressed
comments regarding the methodology for indirect PE calculations, focusing on using survey
data, allocation methods, and potential improvements (75 FR 73178 through 73179). We
recognized some limitations of the current PFS ratesetting methodology but maintained that the
approach was the most appropriate at the time. In the CY 2012 PFS final rule, we responded to
comments related to indirect PE methodology, including concerns about allocating indirect PE to
specific services and using the AMA PPIS data for certain specialties (76 FR 73033 through
73034). We indicated that CMS would continue to review and refine the methodology and work
with interested parties to address their concerns. In the CY PFS 2014 final rule, we responded to
comments about fully implementing the AMA PPIS data. By 2014, the AMA PPIS data had
been fully integrated into the PFS, serving as the primary source for determining indirect PE
inputs (78 FR 74235). We continued to review data and the PE methodology annually,
considering interested party feedback and evaluating the need for updates or refinements to
ensure the accuracy and relevance of PE RVUs (79 FR 67548). In the years following the full
implementation of the AMA PPIS data, we further engaged with interested parties, thought
leaders and subject matter experts to improve our PE inputs' accuracy and reliability. For further
background, we refer readers to our discussions in final rules for CY 2016 through 2022 (80 FR
70892; 81 FR 80175; 82 FR 52980 through 52981; 83 FR 59455 through 59456; 84 FR 62572;
85 FR 84476 through 84478; 86 FR 62572).
In our CY 2023 PFS final rule, we issued an RFI to solicit public comment on strategies
to update PE data collection and methodology (87 FR 69429 through 69432). We solicited
comments on current and evolving trends in health care business arrangements, the use of
technology, or similar topics that might affect or factor into PE calculations. We reminded
readers that we have worked with interested parties and CMS contractors for years to study the
landscape and identify possible strategies to reshape the PE portion of physician payments. The
fundamental issues are clear but thought leaders and subject matter experts have advocated for
more than one tenable approach to updating our PE methodology.
As described in previous rulemaking, we have continued interest in developing a
roadmap for updates to our PE methodology that account for changes in the health care
landscape. Of various considerations necessary to form a roadmap for updates, we reiterate that
allocations of indirect PE continue to present a wide range of challenges and opportunities. As
discussed in multiple cycles of previous rulemaking, our PE methodology relies on AMA PPIS
data, which may represent the best aggregated available source of information at this time.
However, we acknowledge the limitations and challenges interested parties have raised about
using the current data for indirect PE allocations, which we have also examined in related
ongoing research. We noted in our CY 2023 and CY 2024 rules that there are several competing
concerns that CMS must take into account when considering updated data sources, which also
should support and enable ongoing refinements to our PE methodology.
b. Preparation for Incorporating Refreshed Data and Request for Information on Timing to
Effectuate Routine Updates
In the CY 2024 PFS proposed rule, we continued to encourage interested parties to
provide feedback and suggestions to CMS that give an evidentiary basis to shape optimal PE
data collection and methodological adjustments over time. Considering our ratesetting
methodology and prior experiences implementing new data, we issued a follow-up from the CY
2023 comment solicitation for general information. We solicited comments from interested
parties on strategies to incorporate information that could address known challenges we
experienced in implementing the initial AMA PPIS data. Our current methodology relies on the
AMA PPIS data, legislatively mandated supplemental data sources (for, example, we use
supplemental survey data collected in 2003, as required by section 1848(c)(2)(H)(i) of the Act to
set rates for oncology and hematology specialties), and in some cases crosswalks to allocate
indirect PE as necessary for certain specialties and provider types. We also sought to understand
whether, upon completion of the updated PPIS data collection effort by the AMA, contingencies
or alternatives may be necessary and available to address the lack of data availability or response
rates for a given specialty, set of specialties, or specific service suppliers who are paid under the
PFS.
In response to last year’s RFI, most commenters stated that CMS should defer significant
changes until the AMA PPIS results become available. For further background, refer to 88 FR
78841 to 78843. In responding to our RFI, the AMA RUC provided a set of responses, which
many other commenters repeated in their separate, individual comments. In summary, the AMA
RUC letter submission from CY 2024 suggested that CMS should not consider further changes
until PPIS data collection and analysis is complete. Overall, the AMA comments generally do
not support any change to the methodology and stated that CMS should wait to consider any
further changes until PPIS updates become available. Further, we noted that through its
contractor, Mathematica, the AMA secured an endorsement for the PPIS updates from each State
society, national medical specialty society, and others prior to fielding the survey (88 FR 78843).
Refer to the AMA’s summary of the PPIS, available at https://www.ama-
assn.org/system/files/physician-practice-information-survey-summary.pdf. The AMA expects
analysis, reporting, and documentation to complete by the end of CY 2024, and the AMA would
share data with CMS when results become available.
We believe the AMA’s approach may possibly mitigate nonresponse bias, which created
challenges using previous PPIS data. However, we remain uncertain about whether
endorsements prior to fielding the survey may inject other types of bias in the validity and
reliability of the information collected. We believe it remains important to reflect on the
challenges with our current methodology, and to continue to consider alternatives that improve
the stability and accuracy of our overall PE methodology. We reiterate our discussion
summarizing the responses to previous years’ RFIs in each of the CY 2023 and CY 2024 final
rules (refer to 87 FR 69429 through 69432 and 88 FR 78841 to 78843). We have started new
work under contract with the RAND Corporation to analyze and develop alternative methods for
measuring PE and related inputs for implementation of updates to payment under the PFS. We
continue to study possible alternatives, and would include analysis of updated PPIS data, as part
of our ongoing work. In the meantime, we request general information from the public on ways
that CMS may continue work to improve the stability and predictability of any future updates.
Specifically, we request feedback from interested parties regarding scheduled, recurring updates
to PE inputs for supply and equipment costs.
We believe that establishing a cycle of timing to update supply and equipment cost inputs
every 4 years may be one means of advancing shared goals of stability and predictability. CMS
would collect available data, including, but not limited to, submissions and independent third-
party data sources, and propose a phase-in period over the following 4 years. The phase-in
approach maps to our experience with previous updates. Additionally, we believe that more
frequent updates may have the unintended consequence of disproportionate effects of various
supplies and equipment that have newly updated costs.
Further, we seek feedback on possible mechanisms to establish a balance whereby our
methodology would account for inflation and deflation in supply and equipment costs. We
remain uncertain how economies of scale (meaning a general principle that cost per unit of
production decreases as the scale of production increases) should or should not factor into future
adjustments to our methodology. There remains a diversity of perspectives among interested
parties about such effects. We seek information about specific mechanisms that may be
appropriate, and in particular, approaches that would leverage verifiable and independent, third-
party data that is not managed or controlled by active market participants.
C. Potentially Misvalued Services Under the PFS
1. Background
Section 1848(c)(2)(B) of the Act directs the Secretary to conduct a periodic review, not
less often than every 5 years, of the relative value units (RVUs) established under the PFS.
Section 1848(c)(2)(K) of the Act requires the Secretary to periodically identify potentially
misvalued services using certain criteria and to review and make appropriate adjustments to the
relative values for those services. Section 1848(c)(2)(L) of the Act also requires the Secretary to
develop a process to validate the RVUs of certain potentially misvalued codes under the PFS,
using the same criteria used to identify potentially misvalued codes, and to make appropriate
adjustments.
As discussed in section II.E. of this proposed rule, under Valuation of Specific Codes,
each year we develop appropriate adjustments to the RVUs taking into account recommendations
provided by the American Medical Association (AMA) Resource-Based Relative Value Scale
(RBRVS) Update Committee (RUC), MedPAC, and other interested parties. For many years,
the RUC has provided us with recommendations on the appropriate relative values for new,
revised, and potentially misvalued PFS services. We review these recommendations on a code-
by-code basis and consider these recommendations in conjunction with analyses of other data,
such as claims data, to inform the decision-making process as authorized by statute. We may
also consider analyses of work time, work RVUs, or direct PE inputs using other data sources,
such as the Veterans Health Administration (VHA), National Surgical Quality Improvement
Program (NSQIP), the Society for Thoracic Surgeons (STS), and the Merit-based Incentive
Payment System (MIPS) data. In addition to considering the most recently available data, we
assess the results of physician surveys and specialty recommendations submitted to us by the
RUC for our review. We also consider information provided by other interested parties such as
from the general medical-related community and the public. We conduct a review to assess the
appropriate RVUs in the context of contemporary medical practice. We note that section
1848(c)(2)(A)(ii) of the Act authorizes the use of extrapolation and other techniques to determine
the RVUs for physicians' services for which specific data are not available and requires us to take
into account the results of consultations with organizations representing physicians who provide
the services. In accordance with section 1848(c) of the Act, we determine and make appropriate
adjustments to the RVUs.
In its March 2006 Report to the Congress (https://www.medpac.gov/document/report-to-
the-congress-2006-medicare-payment-policy/), MedPAC discussed the importance of
appropriately valuing physicians’ services, noting that misvalued services can distort the market
for physicians’ services, as well as for other health care services that physicians order, such as
hospital services. In that same report, MedPAC postulated that physicians’ services under the
PFS can become misvalued over time. MedPAC stated, “When a new service is added to the
physician fee schedule, it may be assigned a relatively high value because of the time, technical
skill, and psychological stress that are often required to furnish that service. Over time, the work
required for certain services would be expected to decline as physicians become more familiar
with the service and more efficient in furnishing it.” We believe services can also become
overvalued when PE costs decline. This can happen when the costs of equipment and supplies
fall, or when equipment is used more frequently than is estimated in the PE methodology,
reducing its cost per use. Likewise, services can become undervalued when physician work
increases, or PE costs rise.
As MedPAC noted in its March 2009 Report to Congress
(https://www.medpac.gov/docs/default-source/reports/march-2009-report-to-congress-medicare-
payment-policy.pdf), in the intervening years since MedPAC made the initial recommendations,
CMS and the RUC have taken several steps to improve the review process. Also, section
1848(c)(2)(K)(ii) of the Act augments our efforts by directing the Secretary to specifically
examine, as determined appropriate, potentially misvalued services in the following categories:
● Codes that have experienced the fastest growth.
● Codes that have experienced substantial changes in PE.
● Codes that describe new technologies or services within an appropriate time-period
(such as 3 years) after the relative values are initially established for such codes.
● Codes which are multiple codes that are frequently billed in conjunction with
furnishing a single service.
● Codes with low relative values, particularly those that are often billed multiple times
for a single treatment.
● Codes that have not been subject to review since implementation of the fee schedule.
● Codes that account for the majority of spending under the PFS.
● Codes for services that have experienced a substantial change in the hospital length of
stay or procedure time.
● Codes for which there may be a change in the typical site of service since the code was
last valued.
● Codes for which there is a significant difference in payment for the same service
between different sites of service.
● Codes for which there may be anomalies in relative values within a family of codes.
● Codes for services where there may be efficiencies when a service is furnished at the
same time as other services.
● Codes with high intraservice work per unit of time.
● Codes with high PE RVUs.
● Codes with high cost supplies.
● Codes as determined appropriate by the Secretary.
Section 1848(c)(2)(K)(iii) of the Act also specifies that the Secretary may use existing
processes to receive recommendations on the review and appropriate adjustment of potentially
misvalued services. In addition, the Secretary may conduct surveys, other data collection
activities, studies, or other analyses, as the Secretary determines to be appropriate, to facilitate
the review and appropriate adjustment of potentially misvalued services. This section also
authorizes the use of analytic contractors to identify and analyze potentially misvalued codes,
conduct surveys or collect data, and make recommendations on the review and appropriate
adjustment of potentially misvalued services. Additionally, this section provides that the
Secretary may coordinate the review and adjustment of any RVU with the periodic review
described in section 1848(c)(2)(B) of the Act. Section 1848(c)(2)(K)(iii)(V) of the Act specifies
that the Secretary may make appropriate coding revisions (including using current processes for
consideration of coding changes), which may involve consolidating individual services into
bundled codes for payment under the PFS.
2. Progress in Identifying and Reviewing Potentially Misvalued Codes
To fulfill our statutory mandate, we have identified and reviewed numerous potentially
misvalued codes as specified in section 1848(c)(2)(K)(ii) of the Act, and we intend to continue
our work examining potentially misvalued codes in these areas over the upcoming years. As part
of our current process, we identify potentially misvalued codes for review, and request
recommendations from the RUC and other public commenters on revised work RVUs and direct
PE inputs for those codes. The RUC, through its own processes, also identifies potentially
misvalued codes for review. Through our public nomination process for potentially misvalued
codes established in the CY 2012 PFS final rule with comment period (76 FR 73026, 73058
through 73059), other individuals and groups submit nominations for review of potentially
misvalued codes as well. Individuals and groups may submit codes for review under the
potentially misvalued codes initiative to CMS in one of two ways. Nominations may be
submitted to CMS via email or through postal mail. Email submissions should be sent to the
CMS e-mailbox at [email protected], with the phrase “Potentially
Misvalued Codes” and the referencing CPT code number(s) and/or the CPT descriptor(s) in the
subject line. Physical letters for nominations should be sent via the U.S. Postal Service to the
Centers for Medicare & Medicaid Services, Mail Stop: C4-01-26, 7500 Security Blvd,
Baltimore, Maryland 21244. Envelopes containing the nomination letters must be labeled

“Attention: Division of Practitioner Services, Potentially Misvalued Codes.” Nominations for
consideration in our next annual rule cycle should be received by our February 10th deadline.
Since CY 2009, as a part of the annual potentially misvalued code review and Five-Year Review
process, we have reviewed over 1,700 potentially misvalued codes to refine work RVUs and
direct PE inputs. We have assigned appropriate work RVUs and direct PE inputs for these
services as a result of these reviews. A more detailed discussion of the extensive prior reviews
of potentially misvalued codes is included in the CY 2012 PFS final rule with comment period
(76 FR 73052 through 73055). In the same CY 2012 PFS final rule with comment period, we
finalized our policy to consolidate the review of physician work and PE at the same time and
established a process for the annual public nomination of potentially misvalued services.
In the CY 2013 PFS final rule with comment period (77 FR 68892, 68896 through
68897), we built upon the work we began in CY 2009 to review potentially misvalued codes that
have not been reviewed since the implementation of the PFS (so-called “Harvard-valued
codes”
1
). In the CY 2019 PFS proposed rule (73 FR 38589), we requested recommendations
from the RUC to aid in our review of Harvard-valued codes that had not yet been reviewed,
focusing first on high-volume, low intensity codes. In the fourth Five-Year Review of Work
RVUs proposed rule (76 FR 32410, 32419), we requested recommendations from the RUC to aid
in our review of Harvard-valued codes with annual utilization of greater than 30,000 services. In
the CY 2013 PFS final rule with comment period, we identified specific Harvard-valued services
with annual allowed charges that total at least $10,000,000 as potentially misvalued. In addition
to the Harvard-valued codes, in the CY 2013 PFS final rule with comment period we finalized
for review a list of potentially misvalued codes that have stand-alone PE (codes with physician
work and no listed work time and codes with no physician work that have listed work time). We
1
The research team and panels of experts at the Harvard School of Public Health developed the original work RVUs
for most CPT codes, in a cooperative agreement with the Department of Health and Human Services (HHS).
Experts from both inside and outside the Federal Government obtained input from numerous physician specialty
groups. This input was incorporated into the initial PFS, which was implemented on January 1, 1992.
continue each year to consider and finalize a list of potentially misvalued codes that have or will
be reviewed and revised as appropriate in future rulemaking.
3. CY 2025 Identification and Review of Potentially Misvalued Services
In the CY 2012 PFS final rule with comment period (76 FR 73058), we finalized a
process for the public to nominate potentially misvalued codes. In the CY 2015 PFS final rule
with comment period (79 FR 67548, 67606 through 67608), we modified this process whereby
the public and interested parties may nominate potentially misvalued codes for review by
submitting the code with supporting documentation by February 10
th
of each year. Supporting
documentation for codes nominated for the annual review of potentially misvalued codes may
include the following:
● Documentation in peer reviewed medical literature or other reliable data that
demonstrate changes in physician work due to one or more of the following: technique,
knowledge and technology, patient population, site-of-service, length of hospital stay, and work
time.
● An anomalous relationship between the code being proposed for review and other
codes.
● Evidence that technology has changed physician work.
● Analysis of other data on time and effort measures, such as operating room logs or
national and other representative databases.
● Evidence that incorrect assumptions were made in the previous valuation of the
service, such as a misleading vignette, survey, or flawed crosswalk assumptions in a previous
evaluation.
● Prices for certain high cost supplies or other direct PE inputs that are used to determine
PE RVUs are inaccurate and do not reflect current information.
● Analyses of work time, work RVU, or direct PE inputs using other data sources (for
example, VA, NSQIP, the STS National Database, and the MIPS data).
● National surveys of work time and intensity from professional and management
societies and organizations, such as hospital associations.
We evaluate the supporting documentation submitted with the nominated codes and
assess whether the nominated codes appear to be potentially misvalued codes appropriate for
review under the annual process. In the following year’s PFS proposed rule, we publish the list
of nominated codes and indicate for each nominated code whether we agree with its inclusion as
a potentially misvalued code. The public has the opportunity to comment on these and all other
proposed potentially misvalued codes. In each year’s final rule, we finalize our list of potentially
misvalued codes.
a. Public Nominations
In each proposed rule, we seek nominations from the public and from interested parties of
codes that they believe we should consider as potentially misvalued. We receive public
nominations for potentially misvalued codes by February 10
th
and we display these nominations
on our public website, where we include the submitter’s name, their associated organization, and
the submitted studies for full transparency. We sometimes receive submissions for specific, PE-
related inputs for codes, and discuss these PE-related submissions, as necessary under the
Determination of PE RVUs section of the rule. We summarize below this year’s submissions
under the potentially misvalued code initiative. For CY 2025, we received 5 nominations
concerning various codes. The nominations are as follows:
1) CPT codes 22210, 22212, 22214, 22216
An interested party nominated CPT codes 22210 (Osteotomy of spine, posterior or
posterolateral approach, 1 vertebral segment; cervical) (090 day global code), 22212
(Osteotomy of spine, posterior or posterolateral approach, 1 vertebral segment; thoracic) (090
day global code), 22214 (Osteotomy of spine, posterior or posterolateral approach, 1 vertebral
segment; lumbar) (090 day global code), and 22216 (Osteotomy of spine, posterior or
posterolateral approach, 1 vertebral segment; each additional vertebral segment (List separately

in addition to primary procedure) (add-on ZZZ) as potentially misvalued for six reasons: (1)
incorrect global period; (2) incorrect inpatient days; (3) incorrect intraservice work description;
(4) overvalued intraservice times; (5) changed surgical practice; and (6) incorrect use of posterior
osteotomy codes. The posterior osteotomy codes were last valued by the RUC in 1995.
Currently, CPT code 22210 has a work RVU of 25.38, CPT code 22212 has a work RVU of
20.99, CPT code 22214 has a work RVU of 21.02, and CPT code 22216 has a work RVU of
6.03. CPT codes 22210, 22212, and 22214 have 7 inpatient days each, while CPT code 22216
has 0 inpatient days, and it is an add-on code.
First, the nominator stated that these posterior osteotomies are always performed as an
optional addition to a spinal fusion and should be valued as add-on services and not as 90-day
global services. We note that no references are provided to support the statement that the service
is always performed as an optional addition to a spinal fusion. Second, the nominator explained
that the average hospital stay for scoliosis fusion with osteotomy is 4 to 5 days according to the
current literature,
2,3,4
in contrast with the currently included 7 inpatient days. We note that the
majority of the medical literature submitted by the nominator presented outcome information on
adolescent patients, which may be different from the Medicare population. Furthermore, the
nominator stated that the intraservice work description for CPT code 22216 describes removal of
the pedicle, which is not a typical part of a Ponte/Schwab II osteotomy. Among the posterior
osteotomy codes, only CPT code 22216 had vignettes and we do not have information to decide
whether the code descriptor is correct. We believe this issue would benefit from further review
by the medical community and welcome comments and considerations, including from the AMA
CPT.
2
Halanski, Matthew Aaron, and Jeffrey A Cassidy. “Do multilevel Ponte osteotomies in thoracic idiopathic scoliosis
surgery improve curve correction and restore thoracic kyphosis?” Journal of spinal disorders & techniques vol. 26,5
(2013): 252-5. doi:10.1097/BSD.0b013e318241e3cf.
3
Floccari, Lorena V et al. “Ponte osteotomies in a matched series of large AIS curves increase surgical risk without
improving outcomes.” Spine deformity vol. 9,5 (2021): 1411-1418. doi:10.1007/s43390-021-00339-x.
4
Buckland, Aaron J et al. “Ponte Osteotomies Increase the Risk of Neuromonitoring Alerts in Adolescent Idiopathic
Scoliosis Correction Surgery.” Spine vol. 44,3 (2019): E175-E180. doi:10.1097/BRS.0000000000002784.

The nominator also asserted that intraservice times were too high, particularly for these
osteotomy services furnished with scoliosis fusion procedures. The nominator explained that a
typical scoliosis fusion would be billed with an intraservice time of up to 840 minutes for
pediatric scoliosis fusion and 915 minutes for adult cases. However, referencing current
literature, they observed that a typical scoliosis fusion in a child requires approximately 278
minutes (243-296 minutes),
2,3,5,6,7
which contrasts significantly with the durations indicated for
the current codes. The nominator provided no studies to support a typical scoliosis fusion time in
adults. Drawing from the literature, the nominators assert that intraservice times are overvalued
for these services and propose that these times should be adjusted to align more closely with
average and/or typical surgery times.
The nominator further asserted that this code family is potentially misvalued because
surgical practice for these procedures has evolved since 1995. Approximately 30 years ago,
osteotomies were infrequently performed and usually reserved for addressing completely
ankylosed or fused spinal segments.
8
However, according to the nominator, contemporary
surgical techniques often involve posterior osteotomies to release multiple stiff vertebral
segments, thereby enhancing coronal correction and reducing thoracic hypokyphosis. In addition
to changes in surgical techniques over time, there are notable shifts in the trends regarding the
utilization of osteotomies. For instance, between 2007 and 2015, the use of posterior osteotomies
5
Samdani, Amer F et al. “Do Ponte Osteotomies Enhance Correction in Adolescent Idiopathic Scoliosis? An
Analysis of 191 Lenke 1A and 1B Curves.” Spine deformity vol. 3,5 (2015): 483-488.
doi:10.1016/j.jspd.2015.03.002.
6
Pizones, Javier et al. “Ponte osteotomies to treat major thoracic adolescent idiopathic scoliosis curves allow more
effective corrective maneuvers.” European spine journal : official publication of the European Spine Society, the
European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society vol. 24,7
(2015): 1540-6. doi:10.1007/s00586-014-3749-1.
7
Feng, Jing et al. “Clinical and radiological outcomes of the multilevel Ponte osteotomy with posterior selective
segmental pedicle screw constructs to treat adolescent thoracic idiopathic scoliosis.” Journal of orthopaedic surgery
and research vol. 13,1 305. 29 Nov. 2018, doi:10.1186/s13018-018-1001-0.
8
Ponte, Alberto et al. “The True Ponte Osteotomy: By the One Who Developed It.” Spine deformity vol. 6,1 (2018):
2-11. doi:10.1016/j.jspd.2017.06.006.

in scoliosis cases nearly doubled, increasing from 17 percent to 35 percent.
9
Additionally, 73
percent of patients undergoing scoliosis surgery received posterior osteotomies.
4
This
information supports the nominator’s assertion that there have been notable changes in the
surgical practice for these codes over time.
Lastly, the nominator highlighted incorrect usage of posterior osteotomy codes. They
noted instances where facet/soft tissue releases, such as Schwab type I osteotomies, are
inaccurately reported with these codes. According to the nominator, isolated partial facetectomy
and soft tissue release are already included in spinal fusion procedures and should not be
separately billed with an osteotomy code. Additionally, CMS in reviewing data for these services
identified potential bundling of services within this code family. For instance, CPT code 22210 is
frequently billed alongside CPT code 22600 (Arthrodesis, posterior or posterolateral technique,
single interspace; cervical below C2 segment) (090-day global code), approximately 83 percent
of the time. This indicates a common billing pattern, suggesting potential for coding revisions,
including the consideration of consolidating individual services into bundled codes.
Overall, based on the six reasons provided by the nominator, along with the fact that
these codes were last valued almost 30 years ago, and given the identified billing practices, we
concur that CPT codes 22210, 22212, 22214, and 22216 are potentially misvalued. The
nominator suggested two options to address this concern: (1) developing add-on codes to
differentiate between the number of vertebral segments involved in the osteotomy procedure and
whether it occurs in the cervical, thoracic, or lumbar regions; and (2) removing the current
posterior osteotomy codes and incorporating osteotomies into new deformity fusion codes, both
with and without osteotomy. We are proposing to consider this code family as potentially
misvalued and we appreciate the detailed information submitted by the nominator with sufficient
9
Shaheen, Mohammed et al. “Complication risks and costs associated with Ponte osteotomies in surgical treatment
of adolescent idiopathic scoliosis: insights from a national database.” Spine deformity vol. 10,6 (2022): 1339-1348.
doi:10.1007/s43390-022-00534-4.

supporting evidence. We believe that this code family would benefit from a comprehensive
review by the RUC, and we welcome comments on a broader understanding of these codes.
Additionally, we seek input on current standard billing practices. For example, information on
whether the standard of practice has evolved over time, and if so, how it has evolved, could aid
in identifying potential coding issues related to this matter.
2) CPT code 27279
CPT code 27279 (Arthrodesis, sacroiliac joint, percutaneous or minimally invasive
(indirect visualization), with image guidance, includes obtaining bone graft when performed,
and placement of transfixing device) (090 day global code) has been re-nominated as potentially
misvalued based on the absence of separate direct PE inputs for this 090 day global code in the
nonfacility setting. Currently, CPT code 27279 is only priced under the PFS in the facility
setting, but the nominator is requesting that we establish separate direct PE inputs for this service
to value the service when performed in the nonfacility/office setting (for example, in an office-
based lab). The nominator stated that establishing payment for direct PE inputs in the
nonfacility/office setting would increase access to this service for Medicare patients.
We did not nominate CPT code 27279 as potentially misvalued in the CY 2024 PFS final
rule, mainly due to a lack of consensus on whether these services may be safely and effectively
furnished in the nonfacility/office setting. In this year’s submission, the nominator provided three
post-market surveillance publications and two independent reviews of minimally invasive
sacroiliac (SI) joint fusion procedures to support their assertion that this 90-day surgical service
could be safely and effectively furnished in the nonfacility/office setting. Based on the studies,
the nominator stated that the current medical literature provides evidence supporting the
conclusion that percutaneous or minimally invasive SI joint arthrodesis (CPT code 27279)
carries a complication rate that is acceptably low, comparable to other spinal procedures
commonly performed in the office-based lab (OBL). For instance, the risk of major
complications during lateral trans iliac (LTI) SI joint fusion (CPT code 27279) is lower than the

risks associated with other OBL procedures. These include the risk of iliac perforation during
angioplasty, the risk of death, myocardial infarction (MI), and stroke during diagnostic cardiac
catheterization. The nominator did not reference literature regarding the rates of major
complications for other OBL procedures in their submission.
Based on the information submitted we recognize the possibility that CPT code 27279
may be potentially misvalued, given the nominator’s assertion that its complication rate is
acceptably low based on the five studies they submitted. The results of the studies may suggest
that CPT code 27279 can be safely performed in the office-based lab setting, as asserted by the
nominator, with a relatively low complication rate. However, upon reviewing the submitted
information, we also note that these studies collectively report heterogeneous safety outcomes.
The large variabilities in safety outcomes reported in the studies, coupled with several unreported
outcomes, may indicate that we have little knowledge about the effect of the service on safety
outcomes, prompting the need for further investigation. Therefore, we are not proposing to
consider this code as potentially misvalued, and we are instead seeking comments and additional
studies from the broader medical community regarding whether this code should be priced under
the PFS for the non-facility/office setting.
3) CPT code 95800
An interested party re-nominated CPT code 95800 (Sleep study, unattended,
simultaneous recording; heart rate, oxygen saturation, respiratory analysis (e.g., by airflow or
peripheral arterial tone), and sleep time) to update practice expenses that were last reviewed in
2017. This code was nominated as potentially misvalued in the CY 2024 PFS proposed rule (88
FR 52283). For the CY 2024 final rule, we stated that we were unable to properly assess whether
CPT code 95800 is potentially misvalued based on the evidence submitted with the original
nominations and subsequent comments that CMS received (88 FR 78849–78850). This year, an
interested party re-nominated CPT code 59800 noting two significant changes: (1) in the
technologies available to perform home sleep apnea testing (HSAT) services; and (2) in clinical

practice that leads to the typical procedure reported with the CPT code 95800. According to the
nominator, the current practice utilizes disposable HSAT technology, such as the WatchPat One
device, more often than the reusable equipment currently included in the procedure’s direct
practice expense (PE) inputs.
To account for these changes, the nominator requested the deletion of three direct PE input
codes: (1) equipment code EQ335 (WatchPAT 200 Unit with strap, cables, charger, booklet, and
patient video); (2) equipment code EQ336 (Oximetry and Airflow Device); and (3) supply code
SD263 (WatchPAT pneumo-opt sleep probes), which are WatchPAT probes used with the
reusable WatchPAT unit. Instead, the nominator requested the addition of a supply code SD362
(the WatchPAT ONE device), a disposable HSAT technology, as a replacement. According to our
PE supply list, the combined price of the items that the nominator requested to delete (EQ335,
EQ336, and SD263) is $4.71 + $4.55 + $73.32 = $82.58, which is $15.62 less than the price of
the item that the nominator requested to add (SD362), priced at $98.20. The price of $98.20 is
mentioned in the nomination letter without an accompanying specific invoice. Last year, the
nominator submitted invoices, showing a price of $99.00 each (a case of 12 totaling $1,188.00)
for the WatchPat One Device (SD362) (see Table 6).
TABLE 6: Listing of Nominator’s Practice Expense items for addition or deletion to CPT
code 95800
Current
Equipment/Supply Code
Equipment/Supply
Description
Non-Facility/ Office
Equipment/Supply
PE Cost
Recommended
Equipment/
Supply Status
EQ335
WatchPAT 200 Unit with strap,
cables, charger, booklet and
patient video
$4.71
Delete
EQ336
Oximetry and Airflow Device
$4.55
Delete
SD263
WatchPAT pneumo-opt slp
probes (reusable)
$73.32
Delete
SD362
WatchPAT ONE device
(disposable)
$98.20
Add
The nominator asserted that testing trends have shifted away from traditional airflow-
based tests, with a noticeable rise in peripheral arterial tone (PAT)-based (non-airflow) tests. The
traditional airflow-based tests use the reusable supplies and equipment, whereas the PAT-based
non-airflow tests use the disposable HSAT device. While describing these changes in trends, the
nominator did not provide us with their internal data, thus we are unable to verify its validity.
The nominator also stated that disposable HSAT devices were used for nearly 50 percent of CPT
code 95800 services in 2023 and attributed the increased use of disposable devices to the
COVID-19 public health emergency (PHE). Furthermore, the nominator projected that over 50
percent of CPT code 95800 services will be furnished using disposable devices in 2024 and
2025. Explaining the patterns and predictions, the nominator concluded that the pandemic
significantly altered the delivery of HSAT services, with many sleep physicians transitioning to
single-use, disposable sleep tests as an alternative to the reusable testing equipment that is
shipped from patient-to-patient after post-use cleaning. The nominator believes that, going
forward, the typical procedure described by CPT code 95800 in CY 2024 and beyond will be
furnished using disposable HSAT devices rather than reusable equipment.
Since the COVID-19 PHE ended in 2023, we are still unclear as to whether the typical
procedure reported with CPT code 95800 involves the use of a reusable or disposable HSAT
device. Given that we only have access to the nominator’s summary of their internal data to
observe changes in usage trends, which may not be generalizable, we propose to maintain the
current direct PE supply and equipment inputs for CPT code 95800. While we are not currently
proposing to review CPT code 95800 as potentially misvalued for CY 2025, we seek public
comments on this nomination. In particular, we seek comments on whether the typical procedure
described by CPT code 95800 now involves the use of a disposable HSAT device rather than
reusable equipment.
4) CPT codes 10021, 10004, 10005, 10006
An interested party nominated the CPT code 10021 (Fine needle aspiration biopsy,
without imaging guidance; first lesion), CPT code 10004 (Fine needle aspiration biopsy, without
imaging guidance; each additional lesion), CPT code 10005 (Fine needle aspiration biopsy,

including ultrasound guidance; first lesion) and CPT code 10006 (Fine needle aspiration biopsy,
including ultrasound guidance; each additional lesion) as potentially misvalued. We note that
this code family has been nominated several times in recent years. We discussed our review of
these codes and our rationale for finalizing the current values extensively in the CY 2019 PFS
final rule (83 FR 59517), and CY 2021 PFS final rule (85 FR 84602). Furthermore, this code
family was nominated as potentially misvalued and discussed in the CY 2020 PFS final rule (84
FR 62625). For more information we encourage the interested parties to go to our previous PFS
final rules.
The nominator specifically requested that we revisit our work RVU decisions for these
codes, stating that the underpinnings of the reduction in work RVUs from the RUC-
recommended values were flawed. The nominator suggested that CMS should adopt the RUC-
recommended work RVUs. For CPT code 10021, the RUC recommended a work RVU of 1.20,
but we adopted a lower value of 1.03. Similarly, for CPT code 10005, the RUC recommended a
work RVU of 1.63, but we adopted 1.46. The nominator disagreed with these reductions from
the RUC-recommended values by CMS, raising particular concerns about our choice for the
RVU crosswalk for CPT code 36440 (Push blood transfusion, patient 2 years or younger).
According to the nominator, the CPT code we chose is not comparable to fine needle aspiration
in any respect other than service time. The nominator raised several points, including that CPT
code 36440 is rarely utilized and is almost never billed to Medicare because it pertains to a
pediatric procedure conducted on neonates, while CPT code 10021 is never performed on
neonates. They further asserted that the training and experience levels required to properly
perform these procedures differ significantly; neonatal transfusions can be conducted by less
experienced personnel, while performing a thyroid fine needle aspiration demands more
experience. Specifically, they argued that there is a notable difference in the work intensity
between the two procedures. The thyroid is closely positioned to vital structures such as the
carotid artery, jugular vein, lymphatic vessels, nerves, trachea, and esophagus. When sampling

thyroid nodules, they are often in proximity to the carotid artery, jugular vein, or both. According
to the nominator, even a slight deviation of 1-2 millimeters during the sampling procedure can
result in accidental puncture of these critical blood vessels or other nearby structures. Factors
such as respiratory movements, patient swallowing, or anxiety may cause the thyroid to move,
further increasing the risk during the procedure. In contrast, neonatal phlebotomy does not
require such measures. Also, the CPT code 36440 is designated as facility-only, meaning it does
not include any clinical staff pre-service time and has no associated practice expense inputs.
According to the nominator, fine needle aspiration is a very complex and high-risk procedure
that may require significant physician work and a higher level of clinical expertise to furnish the
service, which is very different from CPT code 36440. We appreciated the survey (N=74) results
that the nominator submitted to support their statements. The nominator-conducted survey, and
their survey questions aimed to gather information on the practitioners' experiences, opinions,
and practices related to fine needle aspiration procedures. However, no other references such as
peer reviewed medical literature or other nationally representative survey data were provided to
reinforce their argument.
The nominator further stated that thyroid fine needle aspiration should exclusively be
performed as an outpatient procedure and does not require hospitalization. The nominator
emphasized that the reduction in payment for the code family due to the reduction in work RVUs
from the RUC-recommended values has led endocrinologists in office-based practices, those
who are not affiliated with facilities, to discontinue furnishing this service. According to the
nominator, as a consequence of this payment decrease, patients are now being referred to
hospital-based radiology practices, despite the fact that thyroid fine needle aspiration should
ideally be conducted exclusively in nonfacility outpatient settings. The nominator asserted that
radiologists in hospital settings are often unfamiliar with the patient's medical history and risk
factors for suspected thyroid cancer. The nominator further noted that radiologists' training in
thyroid cancer primarily emphasizes imaging and procedures, rather than considering the
patient's overall health perspective. This result may further lead to an increase in medically
unnecessary procedures. Additionally, the nominator believes that the payment reduction for this
code family has the potential to diminish the specialist workforce trained to perform these
procedures, thereby presenting future challenges in patient care and access to specialized
services.
Overall, we appreciate the comprehensive information and level of detail provided by the
nominator. The nominator disagreed with the choice of crosswalk CPT code 36440 made by
CMS, emphasizing the differences in provider training, procedure risk, and patient population.
They noted the rarity of Medicare billing for this code. Additionally, they emphasized the
importance of outpatient thyroid fine needle aspiration being performed by endocrinologists. The
shift to facility settings, prompted by reduced work RVUs, could raise Medicare costs. This,
along with a potential decline in specialist workforce, may hinder patient access. However, in
discussing this group of codes, we must note that these codes have been recently reviewed
multiple times through the annual PFS rulemaking process. We would like to clarify once again
that we disagree with the nominator that this code family is potentially misvalued. We
acknowledge the possibility that there could be significant changes in the practice of delivering
services described by these codes that were not fully reflected in the current work RVU. In such
cases, it would be appropriate to refer the codes to the RUC to conduct a new survey to capture
these changes accurately. However, we note that these codes underwent thorough RUC survey
and review processes during the October 2017 and January 2018 RUC meetings. Based on these
considerations, we disagree with the assertion that this code family is potentially misvalued.
Nevertheless, we welcome comments on whether these codes should be re-reviewed in light of
the arguments made by the nominator.
5) Tympanostomy codes
CMS routinely interacts with interested parties, and in our most recent review, we have
observed several new devices that could be beneficial for populations but are not currently
included in our coding system. While there are variations in the described devices, they
commonly share the following descriptions. This device uses an innovative surgical technology
that combines the separate functions of creating a myringotomy (incision in the eardrum), and
positioning and placing a ventilation tube across the tympanic membrane. The new device is
intended to deliver a tympanostomy tube (also referred to as a ventilation tube) through the
tympanic membrane of the patient and is indicated to be used in office settings for pediatric
patients 6 months and older. This device allows the tympanostomy service to be furnished to
patients without general anesthesia and the service could therefore be performed in the office
setting.
Regarding the delivery of this service using innovative surgical technology, CMS
recognizes that CPT code 69433 (Tympanostomy (requiring insertion of ventilating tube), local
or topical anesthesia) (010-day global code) may serve as a sufficient base code, adequately
describing the majority of the surgeon’s work and facility resources. However, a practitioner
may incur additional resources, due to the higher expected intraservice work driven by both time
and intensity factors, especially when furnishing a service to a child, and the cost of the device
when using these devices as part of the performed procedure. While the existing CPT code
69433 is not age-specific, both the vignette and the RVU associated with this procedure are
established for adult patients who can respond to surgeon direction, and do not have risk of
movement during the procedure. We believe that potentially establishing additional coding and
payment for tympanostomy services may enable the provision of these services utilizing new
technologies to a broader patient population who may benefit from innovative surgical
technology. To improve the accuracy of the payment for these services, we are soliciting
comments on several alternatives that we are considering for adoption in the CY 2025 PFS final
rule or future rulemaking. First, we are seeking comment on whether to establish a new G code
that accounts for the work and practice expense for a procedure involving the positioning and
placement of a ventilation tube across the tympanic membrane using an innovative surgical
technology that combines the separate functions of creating a myringotomy (incision in the
eardrum). We could assign contractor pricing to this potential G code for generalizable
innovative tympanostomy tube delivery devices and/or systems falling under emerging
technology and services categories. Alternatively, we are seeking comment on whether we
should establish an add-on payment for the service using inputs from CPT code 69433 as a
crosswalk reference, plus direct costs from invoices for the surgical devices referenced above.
We are seeking comments regarding these potential approaches, particularly on whether there is
additional information we should consider if we were to establish additional coding and payment
for these services.
D. Payment for Medicare Telehealth Services Under Section 1834(m) of the Act
As discussed in prior rulemaking, several conditions must be met for Medicare to make
payment for telehealth services under the PFS. See further details and full discussion of the scope
of Medicare telehealth services in the CY 2018 PFS final rule (82 FR 53006), the CY 2021 PFS
final rule (85 FR 84502) and the CY 2024 PFS final rule (88 FR 78861 through 78866) and in 42
CFR 410.78 and 414.65. For a discussion of Telemedicine Evaluation and Management (E/M)
Services, we refer readers to section II.E.4.18 of this proposed rule.
1. Payment for Medicare Telehealth Services Under Section 1834(m) of the Act
a. Changes to the Medicare Telehealth Services List
In the CY 2003 PFS final rule with comment period (67 FR 79988), we established a
regulatory process for adding services to or deleting services from the Medicare Telehealth
Services List in accordance with section 1834(m)(4)(F)(ii) of the Act. This process provides the
public with an ongoing opportunity to submit requests for adding services, which are then
reviewed by us and assigned to categories established through notice and comment rulemaking.
Under the process we established beginning in CY 2003, we evaluated whether a service meets
the following criteria:
● Category 1: Services similar to professional consultations, office visits, and office
psychiatry services currently on the Medicare Telehealth Services List. In reviewing these
requests, we looked for similarities between the requested and existing telehealth services for the
roles of, and interactions among, the beneficiary, the physician (or other practitioner) at the
distant site, and, if necessary, the telepresenter, a practitioner who was present with the
beneficiary in the originating site. We also looked for similarities in the telecommunications
system used to deliver the service, for example, the use of interactive audio and video equipment.
● Category 2: Services that are not similar to those on the current Medicare Telehealth
Services List. Our review of these requests included assessing whether the service was accurately
described by the corresponding code when furnished via telehealth and whether using a
telecommunications system to furnish the service produces demonstrated clinical benefit to the
patient. Submitted evidence should have included both a description of relevant clinical studies
that demonstrated the service furnished by telehealth to a Medicare beneficiary improves the
diagnosis or treatment of an illness or injury or improves the functioning of a malformed body
part, including dates and findings, and a list and copies of published peer-reviewed articles
relevant to the service when furnished via telehealth. Our evidentiary standard of clinical benefit
did not include minor or incidental benefits. Some examples of other clinical benefits that we
considered include the following:
● Ability to diagnose a medical condition in a patient population without access to
clinically appropriate in-person diagnostic services.
● Treatment option for a patient population without access to clinically appropriate in-
person treatment options.
● Reduced rate of complications.
● Decreased rate of subsequent diagnostic or therapeutic interventions (for example, due
to reduced rate of recurrence of the disease process).
● Decreased number of future hospitalizations or physician visits.
● More rapid beneficial resolution of the disease process treatment.
● Decreased pain, bleeding, or other quantifiable signs or symptoms.
● Reduced recovery time.
In the CY 2021 PFS final rule (85 FR 84507), we created a third category of criteria for adding
services to the Medicare Telehealth Services List on a temporary basis following the end of the
PHE for the COVID-19 pandemic. This new category described services that were added to the
Medicare Telehealth Services List during the PHE, for which there was likely to be clinical
benefit when furnished via telehealth, but there was not yet sufficient evidence available to
consider the services for permanent addition under the Category 1 or Category 2 criteria.
Services added on a temporary, Category 3 basis ultimately needed to meet the criteria under
Category 1 or 2 in order to be permanently added to the Medicare Telehealth Services List. To
add specific services on a Category 3 basis, we would conduct a clinical assessment to identify
those services for which we could foresee a reasonable potential likelihood of clinical benefit
when furnished via telehealth.
In the CY 2024 PFS final rule (88 FR 78861 through 78866), we consolidated these three
categories and implemented a revised 5-step process for making additions, deletions, and
changes to the Medicare Telehealth Services List (5-step process), beginning for the CY 2025
Medicare Telehealth Services List. Rather than categorizing a service as “Category 1” or
“Category 2,” each service is now assigned a “permanent” or “provisional” status. As described
further below, a service is assigned a “provisional” status if there is not enough evidence to
demonstrate that the service is of clinical benefit, but there is enough evidence to suggest that
further study may demonstrate such benefit. The 5-step process review criteria are set forth in the
CY 2024 PFS final rule (88 FR 78861 through 78866), listed at
https://www.cms.gov/medicare/coverage/telehealth/criteria-request, and summarized below.
Consistent with the deadline for our receipt of code valuation recommendations from the
American Medical Association’s Relative Value Scale Update Committee (AMA RUC) and
other interested parties (83 FR 59491) and with the process set forth in prior calendar years, for
CY 2025, requests to add services to the Medicare Telehealth Services List must have been
submitted to and received by CMS by February 10, 2024. Each request to add a service to the
Medicare Telehealth Services List must have included any supporting documentation the
requester wishes us to consider as we review the request. Because we use the annual PFS
rulemaking process to make changes to the Medicare Telehealth Services List, requesters are
advised that any information submitted as part of a request is subject to public disclosure for this
purpose. For more information on submitting a request to add services to the Medicare
Telehealth Services List, including where to send these requests, and to view the current
Medicare Telehealth Service List, see our website at https://www.cms.gov/Medicare/Medicare-
General-Information/Telehealth/index.html.
Step 1. Determine whether the service is separately payable under the PFS.
When considering whether to add, remove, or change the status of a service on the
Medicare Telehealth Services List, we first determine whether the service, as described by the
individual HCPCS code, is separately payable under the PFS because, as further discussed in CY
2024 PFS final rule (88 FR 78861 through 78866), Medicare telehealth services are limited to
those services for which separate Medicare payments can be made under the PFS. Before
gathering evidence and preparing to submit a request to add a service to the Medicare Telehealth
Services List, the submitter should therefore first check the payment status for a given service
and ensure that the service (as identified by a HCPCS code), is a covered and separately payable
service under the PFS (as identified by payment status indicators A, C, T, or R on our public use
files).
Step 2. Determine whether the service is subject to the provisions of section 1834(m) of the Act.
If we determine at Step 1 that a service is separately payable under the PFS, we apply
Step 2 under which we determine whether the service at issue is subject to the provisions of
section 1834(m) of the Act. Section 1834(m) of the Act provides for payment to a physician (or
other practitioner) for a service furnished via an interactive telecommunications system,
notwithstanding that the furnishing practitioner and patient are not in the same location, at the
same amount that would have been paid if the service was furnished without the
telecommunications system. We have historically interpreted this to mean that only services that
are ordinarily furnished with the furnishing practitioner and patient in the same location can be
classified as a “telehealth service” for which payment can be made under section 1834(m) of the
Act. Given that there may be a range of services delivered using certain telecommunications
technology that, though they are separately payable under the PFS, do not fall within the
definition of telehealth service set forth in section 1834(m) of the Act, the aim of Step 2 is
therefore to determine whether the service at issue is, in whole or in part, inherently a face-to-
face service. Such services generally include services that do not require the presence of, or
involve interaction with, the patient (for example, remote interpretation of diagnostic imaging
tests, and certain care management services). Other examples include virtual check-ins, e-visits,
and remote patient monitoring services which involve the use of telecommunications technology
to facilitate interactions between the patient and practitioner, but do not serve as a substitute for
an in-person encounter, for example, to assess whether an in-person or telehealth visit is needed
or to transmit health information to the practitioner.
In determining whether a service is subject to the provisions of section 1834(m) of the
Act, we therefore review during this Step 2 whether one or more of the elements of the service,
as described by the particular HCPCS code at issue, ordinarily involve direct, face-to-face
interaction between the patient and practitioner such that the use of an interactive
telecommunications system to deliver the service would be a substitute for an in-person visit.
Step 3. Review the elements of the service as described by the HCPCS code and determine
whether each of them is capable of being furnished using an interactive telecommunications
system as defined in § 410.78(a)(3).
Step 3 is corollary to Step 2, and is used to determine whether one or more elements of a
service are capable of being delivered via an interactive telecommunication system as defined in
§ 410.78(a)(3). In Step 3, we consider whether one or more face-to-face component(s) of the
service, if furnished via audio-video communications technology, would be equivalent to the
service being furnished in-person, and we seek information from requesters to demonstrate
evidence of substantial clinical improvement in different beneficiary populations that may
benefit from the requested service when furnished via telehealth, including, for example, in rural
populations. The services are not equivalent when the clinical actions, or patient interaction,
would not be of similar content as an in-person visit, or could not be completed.
Step 4. Consider whether the service elements of the requested service map to the service
elements of a service on the list that has a permanent status described in previous final
rulemaking.
The purpose of Step 4 is to simplify and reduce the administrative burden of submission
and review. For Step 4, we review whether the service elements of a code that we are
considering for addition to, or removal from, the Medicare Telehealth Services List map to the
service elements of a service that is already on the list and is assigned permanent status. Any
code that satisfies this criterion would require no further analysis. If the service elements of a
code maps to the service elements of a code that is already included on the Medicare Telehealth
Services List and is assigned permanent basis, we will add the code to the Medicare Telehealth
Services List and assign it permanent status. While we have not previously found that the service
elements of a code we are considering for addition to the list map to the elements of a service
that was previously added to the list and assigned permanent basis, we believe that it is
appropriate to apply this step 4 analysis to compare the candidate service with any permanent
code that is on the list on a permanent basis. When Step 4 is met, further evidence review is not
necessary. We continue to Step 5 if Step 4 is not met.
Step 5. Consider whether there is evidence of clinical benefit analogous to the clinical benefit of
the in-person service when the patient, who is located at a telehealth originating site, receives a
service furnished by a physician or practitioner located at a distant site using an interactive
telecommunications system.
Similar to Steps 3, 4, and 5 above, the purpose of the proposed step 5 is to simplify and
reduce the administrative burden. Under Step 5, we review the evidence provided with a
submission to determine the clinical benefit of a service. We then compare the clinical benefit of
that service, when provided via telehealth, to the clinical benefit of the service if it were to be
furnished in person. If there is enough evidence to suggest that further study may demonstrate
that the service, when provided via telehealth, is of clinical benefit, CMS will assign the code a
‘‘provisional’’ status on the Medicare Telehealth Services List. Where the clinical benefit of a
service, when provided via telehealth, is clearly analogous to the clinical benefit of the service
when provided in person, CMS will assign the code ‘‘permanent’’ status on the Medicare
Telehealth Services List, even if the code’s service elements do not map to the service elements
of a service that already has permanent status. We reminded readers that our evidentiary standard
of demonstrated clinical benefit does not include minor or incidental benefits (81 FR 80194).
We review the evidence submitted by interested parties, and other evidence that CMS has on
hand. The evidence should indicate that the service can be safely delivered using two-way
interactive audio-video communications technology. Clinical practice guidelines, peer-reviewed
literature, and similar materials, should illustrate specifically how the methods and findings
within the material establish a foundation of support that each element of the defined, individual
service described by the existing face-to-face service code has been studied in the typical setting
of care, typical population of beneficiaries, and typical clinical scenarios that practitioners would
encounter when furnishing the service using only interactive, two-way audio-video
communications technology to complete the visit or encounter with Medicare beneficiaries.
General evidence may also answer the question of whether a certain beneficiary population
requiring care for a specific illness or injury may benefit from receiving a service via telehealth
versus receiving no service at all, but must establish that the service is a substitute for an
equivalent in-person service. Evidence should demonstrate how all elements described by the
individual service code can be met when two-way, interactive audio-video communications
technology is used as a complete substitute for any face-to-face interaction required between the
patient and practitioner that are described in the individual code descriptor. We further remind
readers that submissions reflecting practitioner services furnished to Medicare beneficiaries are
helpful in our considerations.
b. Requests to Add Services to the Medicare Telehealth Services List for CY 2025
We received several requests to permanently add various services to the Medicare
Telehealth Services List, effective for CY 2025. The requested services are listed in Table 7.

TABLE 7: CY 2025 Requests for Permanent Addition to the Medicare Telehealth Services
List
Category
HCPCS
Short Descriptor
Radiation
Treatment
Mgmt
77427
Radiation tx management x5
96130
Psycl tst eval phys/qhp 1st
96136
Psycl/nrpsyc tst phy/qhp 1st
Psych Testing
96137
Psycl/nrpsyc tst phy/qhp ea
G0422
Intens cardiac rehab w/exerc
Intensive
Cardiac Rehab
G0423
Intens cardiac rehab no exer
96112
Devel tst phys/qhp 1st hr
Developmental
Testing
96113
Devel tst phys/qhp ea addl
0591T
Hlth&wb coaching indiv 1st
0592T
Hlth&wb coaching indiv f-up
Health and Well
Being Coaching
0593T
Hlth&wb coaching group
94625
Phy/qhp op pulm rhb w/o mntr
Outpatient
Pulmonary
Rehab
94626
Phy/qhp op pulm rhb w/mntr
93797
Cardiac rehab
Cardiac Rehab
93798
Cardiac rehab/monitor
97550
Caregiver traing 1st 30 min
Caregiver
Training
97551
Caregiver traing ea addl 15
97161
Physical therapy evaluation, low complexity
97162
Physical therapy evaluation, moderate complexity
97163
Physical therapy evaluation, high complexity
97164
Physical therapy re-evaluation
97110
Therapeutic exercises, each 15 mins
97112
Neuromuscular re-education, each 15 mins
97116
Gait training, each 15 mins
97530
Therapeutic activities, each 15 mins
Physical
Therapy
97535
Self-care home management
97165
Ot eval low complex 30 min
97166
Ot eval mod complex 45 min
97167
Ot eval high complex 60 min
OT Evaluation
97168
Ot re-eval est plan care
92507
Speech/hearing therapy
92508
Speech/hearing therapy
92521
Evaluation of speech fluency
92522
Evaluate speech production
92523
Speech sound lang comprehen
92524
Behavral qualit analys voice
96105
Assessment of aphasia
92626
Eval aud funcj 1st hour
92627
Eval aud funcj ea addl 15
96125
Cognitive test by hc pro
97129
Ther ivntj 1st 15 min
Speech,
Language, and
Voice
Evaluation and
Treatment
97130
Ther ivntj ea addl 15 min
92607
Ex for speech device rx 1hr
92608
Ex for speech device rx addl
SGD Evaluation
and Treatment
92609
Use of speech device service
92526
Oral function therapy
Swallowing
Evaluation and
Treatment
92610
Evaluate swallowing function
92550
Tympanometry & reflex thresh
92552
Pure tone audiometry air
92553
Audiometry air & bone
92555
Speech threshold audiometry
Diagnostic
Audiologic
Testing
92556
Speech audiometry complete

Category
HCPCS
Short Descriptor
92557
Comprehensive hearing test
92563
Tone decay hearing test
92565
Stenger test pure tone
92567
Tympanometry
92568
Acoustic refl threshold tst
92570
Acoustic immitance testing
92587
Evoked auditory test limited
92588
Evoked auditory tst complete
92625
Tinnitus assessment
92626
Eval aud funcj 1st hour
92627
Eval aud funcj ea addl 15
92601
Cochlear implt f/up exam <7
92602
Reprogram cochlear implt <7
92603
Cochlear implt f/up exam 7/>
Diagnostic CI
Testing
92604
Reprogram cochlear implt 7/>
Many services were added to the Medicare Telehealth Services List on a temporary basis
as discussed in the March 31st COVID–19 interim final rule with comment period (IFC) (85 FR
19235 through 19237) for the PHE for Covid-19, and we subsequently retained these services on
a provisional basis. All of the received submissions were requests for addition on a permanent
basis. We believe that, rather than selectively adjudicating only those services for which we
received requests for potential permanent status, it would be appropriate to complete a
comprehensive analysis of all provisional codes currently on the Medicare Telehealth Services
List before determining which codes should be made permanent. We are therefore not making
determinations to recategorize provisional codes as permanent until such time as CMS can
complete a comprehensive analysis of all such provisional codes which we expect to address in
future rulemaking.
The following is a discussion of the requests received for addition of services to the
Medicare Telehealth Services List:
(1) Continuous Glucose Monitoring
We received a request to add CPT code 95251 (Ambulatory continuous glucose
monitoring of interstitial tissue fluid via a subcutaneous sensor for a minimum of 72 hours;
analysis, interpretation and report) to the Medicare Telehealth Services List and assign it
permanent status. This code is not on the Medicare Telehealth Services List, nor had it been
previously added and removed. The requester stated that the ability of the practitioner to interpret
continuous glucose monitoring data and communicate changes in the diabetes care plan to our
patients is enhanced by the availability of video visits, and the code should therefore be added to
the Medicare Telehealth Services List. This service does not meet the criteria described by Step 2
of the 5-step process: determination of whether the service is subject to the provisions of section
1834(m) of the Act. Section 1834(m) of the Act limits the definition of Medicare telehealth
services to those services that would ordinarily be furnished with the furnishing practitioner and
patient in the same location (88 78863). In other words, as stated above, for a service to be
considered a Medicare telehealth service subject to and payable under section 1834(m) of the
Act, the service must be so analogous to in-person care such that the telehealth service, as
defined in § 410.78, is essentially a substitute for a face-to-face encounter. We do not consider
this service a Medicare telehealth service because it is not an inherently face-to-face service; the
patient does not need to be present for the service to be furnished in its entirety. CPT code 95251
describes sensor placement and monitoring over a 72-hour period. We do not consider CPT code
95251 a telehealth service under section 1834(m) of the Act or our regulation at § 410.78.
Therefore, we are not proposing to add this service to the Medicare Telehealth Services List.
(2) Cardiovascular and Pulmonary Rehabilitation
We received requests to permanently add cardiovascular rehabilitation services (CPT
codes 93797 and 93798) and pulmonary rehabilitation services (CPT codes 94625 and 94626) to
the Medicare Telehealth Services List. These services are currently on the Medicare Telehealth
List and are assigned provisional status. We had originally added CPT codes 93797 and 93798
and HCPCS codes G0422 and G0423 on a temporary basis in the CY 2022 PFS final rule (FR 86
65054 through 65055). A requester cited studies that they say demonstrate that the availability of
these services via telehealth enhances access and patient equity. Another requester cited evidence
of improved outcomes for patients that had access to these services via telehealth. As explained
previously, we are not proposing to revise the status of codes from provisional to permanent in
this proposed rule because we intend to conduct a comprehensive review. While considering
these issues for future rulemaking, we are not proposing to assign CPT codes 93797 and 93798
or CPT codes 94625 and 94626 permanent status on the Medicare Telehealth Services List and
would instead maintain the services on the Medicare Telehealth Services List on a provisional
basis for CY 2025.
(3) Health and Well Being-Coaching
We received a request to add Health and Well-Being Coaching (CPT codes 0591T -
0593T) to the Medicare Telehealth Services List with permanent status. These services are
currently on the Medicare Telehealth Services List and are assigned a provisional status. We
originally added these codes on a provisional basis in the CY 2024 PFS final rule (FR 88 78859
and 78860). One requester stated that health and well-being coaching, including content
education, delivered in a telehealth modality is an evidence-based, cost-effective, sustainable,
and common sense approach to facilitating lifestyle/behavioral intervention and treating the
Medicare population with or at heightened risk for chronic diseases. As explained previously, we
are not proposing to revise the status of codes from provisional to permanent in this proposed
rule because we intend to conduct a comprehensive review. Therefore, we are not proposing to
assign them to the Medicare Telehealth Services List with permanent status.
(4) Psychological Testing and Developmental Testing
We received a request to add Psychological Testing and Developmental Testing (CPT
codes 96112, 96113, 96130, 96136, and 96137) to the Medicare Telehealth Services List on a
permanent basis. These services are currently on the Medicare Telehealth Services List and are
assigned provisional status. In the March 31, 2020 interim final rule with comment period (IFC–
1) (85 FR 19239), we originally added CPT codes 96130, 96136, and 96137 to the Medicare
Telehealth Services List for the duration of the PHE for COVID–19, and in the CY 2021 PFS
final rule (85 FR 85003), we stated we were retaining them on the list on a category 3 basis. In
the CY 2023 PFS final rule (87 FR 69460), we added CPT codes 96112 and 96113 on a
temporary basis.
As explained previously, we are not proposing to revise the status of codes from
provisional to permanent in this proposed rule because we intend to conduct a comprehensive
review. Therefore, we are not proposing to either remove these services from or to assign them
permanent status on the Medicare Telehealth Services List.
(5) Therapy/Audiology/Speech Language Pathology
We received multiple requests to add the Therapy services described by CPT codes
97110, 97112, 97116, 97161 through 97164, 97530 and 97535, 97165 through 97168, and
Audiology and Speech Language Pathology services CPT codes 92507, 92508, 92521 through
92524, 92526, 92607 through 92610, 96105 92626, 92627, 96125, 97129, 97130, 92607 through
92609 92550 through 92557, 92563, 92565 92567, 92568, 92570, 92587, 92588, 92601 through
92604, 92625 through 92627, and 92651 and 92652 to the Medicare Telehealth Services List on
a permanent basis stating that continuing Telehealth flexibilities for these services could lead to
reduced health care expenditures, increased patient access, and improved management of chronic
disease and quality of life. These services are currently available on the Medicare Telehealth
Services List and are assigned provisional status, and we refer readers to section II.D.1. for
further discussion of these services. In the CY 2023 PFS final rule (87 FR 69451), we originally
added CPT codes 90901, 97150, 97530, 97537, 97542, 97763, and 98960–98962 to the Medicare
Telehealth Services List on a Category 3 basis. As explained previously, we are not proposing to
revise the status of codes from provisional to permanent in this proposed rule because we intend
to conduct a comprehensive review. Therefore, we are not proposing to assign them permanent
status on the Medicare Telehealth Services List.
(6) Care Management
We received a request to permanently add General Behavioral Health Integration (CPT
code 99484) and Principal Care Management (CPT codes 99424 – 99427) to the Medicare
Telehealth Services List. These services are not on the Medicare Telehealth Services List, nor
have they been previously added and removed. These services do not meet the criteria described
by Step 2 of the 5-step process: determination of whether the service is subject to the provisions
of section 1834(m) of the Act. As stated above, the scope of section 1834(m) of the Act is
limited to services that would ordinarily be furnished with the furnishing practitioner and patient
in the same location (88 78863), and for a service to be considered a telehealth service subject to
and payable under section 1834(m) of the Act, the service must be so analogous to in-person care
such that the telehealth service, as defined in § 410.78, is essentially a substitute for a face-to-
face encounter. We do not consider these services to be Medicare telehealth services because
they are not inherently face-to-face services, and the patient need not be present for the services
to be furnished in its entirety. Therefore, we do not consider CPT codes 99484 and 99424 –
99427 to be telehealth services under section 1834(m) of the Act or our regulation at § 410.78.
Therefore, we are not proposing to add this service to the Medicare Telehealth Services List.
(7) Posterior Tibial Nerve Stimulation for Voiding Dysfunction
We received a request to permanently add Posterior tibial neurostimulation (CPT code
64566) to the Medicare Telehealth Services List. This code is not on the Medicare Telehealth
Services List, nor had it been previously added and removed. This service does not meet the
criteria for addition described by Step 3 of the 5-step process, namely the review the elements of
the service as described by the HCPCS code and determine whether each of them is capable of
being furnished using an interactive telecommunications system as defined in § 410.78(a)(3).
The requestor describes the services underlying CPT code 64566 as the continual or recurring
treatments over a period of time consisting of the remote monitoring of device utilization and
bladder diary for the generation of reports for review by the care provider. Based on our review,
this description does not align with the elements of the service as described by CPT code 64566.
CPT code 64566 describes a single treatment provided by a clinician who has direct contact with
the patient and inserts an electrode into the skin overlying the posterior tibial nerve. Upon
conclusion of the treatment, the clinician removes the electrode and examines and dresses the
puncture wound. Providing these services would require in-person interaction. We are therefore
not proposing to add the service to the Medicare Telehealth Services List because we do not
believe the service elements can be met in full using two-way audio-video telecommunications
technology.
(8) Radiation Treatment Management
We received requests to permanently add Radiation Treatment Management (CPT code
77427) to the Medicare Telehealth Services List. The code is currently on the Medicare
Telehealth List with provisional status. In the March 31, 2020 IFC (85 FR 9240), we originally
added CPT code 77427 on the Medicare Telehealth Services List for the duration of the PHE for
Covid-19. A requester stated that data collected during the PHE demonstrates that the telehealth
option is as safe as the in-person equivalent. We also received a request that we remove this code
from the Medicare Telehealth Services List, citing the importance of in-person physical
examination to ensure quality of care and stating that a telehealth modality presents patient safety
concerns such as those related to the ability of the practitioner to address side effects of radiation
therapy. Given the safety concerns raised by members of the practitioner community, we believe
this service may not be safely and effectively furnished, and therefore believe that such concerns
merit removing this item from the telehealth list We are therefore proposing to remove this code
from the Medicare Telehealth Services List, and we are soliciting comment on these quality of
care concerns.
(9) Home International Normalized Ratio (INR) Monitoring
We received a request to permanently add Home INR Monitoring (HCPCS code G0248)
to the Medicare Telehealth Services List. This service is not on the Medicare Telehealth Services
List, nor had it been previously added and removed. We are proposing to add HCPS code G0248
to the Medicare Telehealth Services List with provision status because our clinical analyses of
these services indicate that they can be furnished in full using two-way, audio and video
technology, and information provided by requesters indicates that there may be clinical benefit;
however, there is not yet sufficient evidence available to consider the services for permanent
status. This service as described by the HCPCS code describes face-to-face demonstration of use
and care of the INR monitor, obtaining at least one blood sample, provision of instructions for
reporting home INR test results, and documentation of patient’s ability to perform testing and
report results, and we believe each of these service elements the elements is capable of being
furnished using an interactive telecommunications system. Adding this service on a provisional
basis will allow additional time for the development of evidence of clinical benefit when this
service is furnished via telehealth for CMS to consider when evaluating this service for potential
permanent addition to the Medicare Telehealth Services List.
(10) Caregiver Training
We received a request to permanently add Caregiver Training services, as described by
HCPCS codes 97550 (Caregiver training in strategies and techniques to facilitate the patient’s
functional performance in the home or community (eg, activities of daily living [ADLs],
instrumental ADLs [iADLs], transfers, mobility, communication, swallowing, feeding, problem
solving, safety practices) (without the patient present), face to face; initial 30 minutes) and CPT
code 97551 (Caregiver training in strategies and techniques to facilitate the patient’s functional
performance in the home or community (eg, activities of daily living [ADLs], instrumental ADLs
[iADLs], transfers, mobility, communication, swallowing, feeding, problem solving, safety
practices) (without the patient present), face to face; each additional 15 minutes (List separately
in addition to code for primary service)) to the Medicare Telehealth Services List. These codes
do not currently appear on the Medicare Telehealth Services List nor had they previously been
added or removed. We are proposing to add these services to the Medicare Telehealth List with
provisional status for CY 2025, in addition to the other currently payable caregiver training
service codes (CPT codes 97550, 97551, 97552, 96202, 96203). These codes are new services
that were added to the PFS beginning in 2024. Given the limited utilization of those codes added
for 2024, there are not peer-reviewed studies supporting these codes’ ability to be furnished
remotely. Adding these services on a provisional basis will allow additional time for the
development of evidence of clinical benefit when these services are furnished via telehealth for
CMS to consider when evaluating these services for potential permanent addition to the
Medicare Telehealth Services List. Contingent upon finalizing the service code descriptions that
we propose in section II.E. of this proposed rule, we also propose that HCPCS code GCTD1-3
and GCTB1-2 be added to the Medicare Telehealth Services list for CY 2025 on a provisional
basis. We believe that these codes are similar to other services already available on the Medicare
Telehealth Services List, including education and training for patient self-management (CPT
codes 98960-98962), self-care/home management training (CPT codes 97535), and caregiver-
focused health risk assessment (CPT code 96161). Further, it appears that all elements of these
services may be furnished when using two-way interactive communications technology. Adding
these services on a provisional basis will allow additional time for the development of evidence
of clinical benefit when this service is furnished via telehealth for CMS to consider when
evaluating these services for potential permanent addition to the Medicare Telehealth Services
List.
c. Other Services Proposed for Addition to the Medicare Telehealth Services List
(1) Preexposure Prophylaxis (PrEP) of Human Immunodeficiency Virus (HIV)
As discussed in Section II.E. of this proposed rule, we are proposing national rates for
HCPCS codes G0011 (Individual counseling for pre-exposure prophylaxis (PrEP) by physician
or QHP to prevent human immunodeficiency virus (HIV), includes: HIV risk assessment (initial
or continued assessment of risk), HIV risk reduction and medication adherence, 15-30 minutes)
and G0013 (Individual counseling for pre-exposure prophylaxis (PrEP) by clinical staff to
prevent human immunodeficiency virus (HIV), includes: HIV risk assessment (initial or
continued assessment of risk), HIV risk reduction and medication adherence) pending the future
finalization of the NCD for Pre-Exposure Prophylaxis (PrEP) for Human Immunodeficiency
Virus (HIV) Infection. We believe these services are similar to services currently on the
Medicare Telehealth Services list, specifically HCPCS codes G0445 (High intensity behavioral
counseling to prevent sexually transmitted infection; face-to-face, individual, includes:
education, skills training and guidance on how to change sexual behavior; performed semi-
annually, 30 minutes) and CPT code 99211 (Office or other outpatient visit for the evaluation
and management of an established patient that may not require the presence of a physician or
other qualified health care professional) as these codes are the codes from which HCPCS codes
G0011 and G0013 were unbundled, respectively. As similarity to services currently on the
Medicare telehealth list is one of our criteria for permanent addition, we are proposing to add
HCPCS codes G0011 and G0013 to the Medicare Telehealth Services List with a permanent
status.
The services that we are proposing to add to the Medicare Telehealth Services List are
listed in Table 8.

TABLE 8: Services Proposed for Addition to the Medicare Telehealth Services List for CY
2025
Category
HCPCS
Long Description
Proposed Status
G0011
Individual counseling for pre-
exposure prophylaxis (PrEP) by
physician or QHP to prevent human
immunodeficiency virus (HIV),
includes: HIV risk assessment (initial
or continued assessment of risk), HIV
risk reduction and medication
adherence, 15-30 minutes
Permanent
PrEP for
HIV
G0013
Individual counseling for pre-
exposure prophylaxis (PrEP) by
clinical staff to prevent human
immunodeficiency virus (HIV),
includes: HIV risk assessment (initial
or continued assessment of risk), HIV
risk reduction and medication
adherence
Permanent
Home INR
Monitoring
G0248
Demonstration, prior to initiation of
home inr monitoring, for patient with
either mechanical heart valve(s),
chronic atrial fibrillation, or venous
thromboembolism who meets
medicare coverage criteria, under the
direction of a physician; includes:
face-to-face demonstration of use and
care of the inr monitor, obtaining at
least one blood sample, provision of
instructions for reporting home inr
test results, and documentation of
patient's ability to perform testing and
report results
Provisional
97550
Caregiver training in strategies and
techniques to facilitate the patient's
functional performance in the home
or community (eg, activities of daily
living [adls], instrumental adls [iadls],
transfers, mobility, communication,
swallowing, feeding, problem
solving, safety practices) (without the
patient present), face to face; initial
30 minutes
Provisional
97551
Caregiver training in strategies and
techniques to facilitate the patient's
functional performance in the home
or community (eg, activities of daily
living [adls], instrumental adls [iadls],
transfers, mobility, communication,
swallowing, feeding, problem
solving, safety practices) (without the
patient present), face to face; each
additional 15 minutes (list separately
in addition to code for primary
service)
Provisional
Caregiver
Training
97552
Group caregiver training in strategies
and techniques to facilitate the
patient's functional performance in
the home or community (eg, activities
of daily living [adls], instrumental
adls [iadls], transfers, mobility,
Provisional

Category
HCPCS
Long Description
Proposed Status
communication, swallowing, feeding,
problem solving, safety practices)
(without the patient present), face to
face with multiple sets of caregivers

Category
HCPCS
Long Description
Proposed Status
96202
Multiple-family group behavior
management/modification training for
parent(s)/guardian(s)/caregiver(s) of
patients with a mental or physical
health diagnosis, administered by
physician or other qualified health
care professional (without the patient
present), face-to-face with multiple
sets of
parent(s)/guardian(s)/caregiver(s);
initial 60 minutes
Provisional
96203
Multiple-family group behavior
management/modification training for
parent(s)/guardian(s)/caregiver(s) of
patients with a mental or physical
health diagnosis, administered by
physician or other qualified health
care professional (without the patient
present), face-to-face with multiple
sets of
parent(s)/guardian(s)/caregiver(s);
each additional 15 minutes (List
separately in addition to code for
primary service)
Provisional
GCTD1
Caregiver training in direct care
strategies and techniques to support
care for patients with an ongoing
condition or illness and to reduce
complications (without the patient
present), face-to-face; initial 30
minutes
Provisional
GCTD2
Caregiver training in direct care
strategies and techniques to support
care for patients with an ongoing
condition or illness and to reduce
complications (without the patient
present), face-to-face; each additional
15 minutes (List separately in
addition to code for primary service)
(Use GCTD2 in conjunction with
GCTD1)
Provisional
GCTD3
Group caregiver training in direct
care strategies and techniques to
support care for patients with an
ongoing condition or illness and to
reduce complications (without the
patient present), face-to-face with
multiple sets of caregivers
Provisional
GCTB1
Caregiver training in behavior
management/modification for
caregiver(s) of patients with a mental
or physical health diagnosis,
administered by physician or other
qualified health care professional
(without the patient present), face-to-
face; initial 30 minutes
Provisional

Category
HCPCS
Long Description
Proposed Status
GCTB2
Caregiver training in behavior
management/modification for
parent(s)/guardian(s)/caregiver(s) of
patients with a mental or physical
health diagnosis, administered by
physician or other qualified health
care professional (without the patient
present), face-to-face; each additional
15 minutes (List separately in
addition to code for primary service)
(Use GCTB2 in conjunction with
GCTB1)
Provisional
d. Frequency Limitations on Medicare Telehealth Subsequent Care Services in Inpatient and
Nursing Facility Settings, and Critical Care Consultations
When adding some services to the Medicare Telehealth Services List in the past, we have
included certain frequency restrictions on how often practitioners may furnish the service via
Medicare telehealth. These include a limitation of one subsequent hospital care service furnished
through telehealth every 3 days, added in the CY 2011 PFS final rule (75 FR 73317 through
73318), one subsequent nursing facility visit furnished through telehealth every 14 days, added
in the CY 2011 PFS final rule (75 FR73318), and one critical care consultation service furnished
through telehealth per day, added in the CY 2017 final rule (81 FR 80198). In establishing these
limits, we cited concerns regarding the potential acuity and complexity of these patients.
We temporarily removed these frequency restrictions during the PHE for COVID-19. In
the March 31, 2020 COVID-19 interim final rule with comment period (IFC) (85 FR 19241), we
stated that we did not believe the frequency limitations for certain subsequent inpatient visits,
subsequent NF visits, and critical care consultations furnished via Medicare telehealth were
appropriate or necessary for the duration of the PHE because this would have been a patient
population who would have otherwise not had access to clinically appropriate in-person
treatment. Although the frequency limitations resumed effect on May 12, 2023 (upon expiration
of the PHE), through enforcement discretion during the remainder of CY 2023 and notice-and-
comment rulemaking for CY 2024, Medicare telehealth frequency limitations have been
suspended for CY 2024 (88 FR 78876 through 78878) for the following codes relating to
Subsequent Inpatient Visits, Subsequent Nursing Facility Visits, and Critical Care Consultation
Services:
1. Subsequent Inpatient Visit CPT Codes:
● 99231 (Subsequent hospital inpatient or observation care, per day, for the evaluation
and management of a patient, which requires a medically appropriate history and/or
examination and straightforward or low level of medical decision making. when using total time
on the date of the encounter for code selection, 25 minutes must be met or exceeded.);
● 99232 (Subsequent hospital inpatient or observation care, per day, for the evaluation
and management of a patient, which requires a medically appropriate history and/or
examination and moderate level of medical decision making. when using total time on the date of
the encounter for code selection, 35 minutes must be met or exceeded.); and
● 99233 (Subsequent hospital inpatient or observation care, per day, for the evaluation
and management of a patient, which requires a medically appropriate history and/or
examination and high level of medical decision making. when using total time on the date of the
encounter for code selection, 50 minutes must be met or exceeded.)
2. Subsequent Nursing Facility Visit CPT Codes:
● 99307 (Subsequent nursing facility care, per day, for the evaluation and management
of a patient, which requires a medically appropriate history and/or examination and
straightforward medical decision making. when using total time on the date of the encounter for
code selection, 10 minutes must be met or exceeded.);
● 99308 (Subsequent nursing facility care, per day, for the evaluation and management
of a patient, which requires a medically appropriate history and/or examination and low level of
medical decision making. when using total time on the date of the encounter for code selection,
15 minutes must be met or exceeded.);
● 99309 (Subsequent nursing facility care, per day, for the evaluation and management
of a patient, which requires a medically appropriate history and/or examination and moderate
level of medical decision making. when using total time on the date of the encounter for code
selection, 30 minutes must be met or exceeded.); and
● 99310 (Subsequent nursing facility care, per day, for the evaluation and management
of a patient, which requires a medically appropriate history and/or examination and high level
of medical decision making. when using total time on the date of the encounter for code
selection, 45 minutes must be met or exceeded.)
3. Critical Care Consultation Services: HCPCS Codes
● G0508 (Telehealth consultation, critical care, initial, physicians typically spend 60
minutes communicating with the patient and providers via telehealth.); and
● G0509 (Telehealth consultation, critical care, subsequent, physicians typically spend
50 minutes communicating with the patient and providers via telehealth.)
We are proposing to remove the frequency limitations for these codes for CY 2025.
In the CY 2024 PFS final rule (88 FR 78877), we solicited comments from interested
parties on how practitioners have been ensuring that Medicare beneficiaries receive subsequent
inpatient and nursing facility visits, as well as critical care consultation services since the
expiration of the PHE. As discussed in that final rule, many commenters supported permanently
removing these frequency limitations, stating that they are arbitrary and re-imposing the
limitations would result in decreased access to care; that practitioners should be allowed to use
their clinical judgment to determine the type of visit, how many visits, and the type of treatment
that is the best fit for the patient so long as the standard of care is met; and that lifting these
limitations during the PHE has been instructive and demonstrates the value of continuing such
flexibilities. Many commenters urged us to permanently remove them. That said, some
commenters did not support removing these frequency limitations citing patient acuity and
safety, some commenters cited the importance of in-person care for patients in acute care
settings. Some commenters stated that telehealth patient assessments and evaluations are never
the same as in-person, hands on visits and should not be considered a viable replacement with no
limitations for in-person care. We are continuing to consider what changes we should be making
to how telehealth services are reimbursed under Medicare in light of the way practice patterns
may have changed following the PHE for COVID–19. Taking into account the information
received from commenters in the CY 2024 PFS final rule, we believe it is reasonable to continue
to pause certain pre-pandemic restrictions, such as the frequency limitations for the
abovementioned codes for CY 2025. Removing such restrictions for CY 2025 will allow us to
gather an additional year of data to determine how practice patterns are evolving and what
changes, if any, to frequency limitations should be made. We do not believe pausing such
frequency limitations for another year presents a level of safety risk requiring us to immediately
reinstate the limitations. Our analysis of claims data indicates that the volume of services that
would be affected by implementing these limitations is relatively low; in other words, these
services are not being furnished via telehealth with such frequency that frequency limits are
being met or exceeded very often or for many beneficiaries (claims data from 2020 - 2023
suggest that less than five percent received one or more of these services as a telehealth service).
Therefore, while claims data does not suggest that lifting these limitations during the PHE has
led to an increase in utilization, we continue to be interested in information from interested
parties on our concerns regarding the potential acuity and complexity of these patients and how
such acuity and complexity should complexity should influence our implementation of frequency
limitations.
e. Audio-Only Communication Technology to Meet the Definition of “Telecommunications
System”
Through our regulation at § 410.78(a)(3), we define “interactive telecommunications
system” as multimedia communications equipment that includes, at a minimum, audio and video
equipment permitting two-way, real-time interactive communication between the patient and
distant site physician or practitioner. Through emergency regulations and waiver authority under
section 1135(b)(8) of the Act, in response to the PHE for COVID–19, we allowed the use of
audio-only communications technology to furnish services described by the codes for audio-only
telephone evaluation and management services and behavioral health counseling and educational
services. Section 4113 of the CAA, 2023, extended the availability of telehealth services that can
be furnished using audio-only technology and provided for the extension of other PHE-related
flexibilities including removal of the geographic and location limitations under section 1834(m)
of the Act through December 31, 2024.
In the CY 2022 PFS final rule (86 FR 65060), in part to recognize the changes made by
section 123 of the CAA, 2021 that removed the geographic restrictions for Medicare telehealth
services for the diagnosis, evaluation, or treatment of a mental health disorder and the addition of
the patient’s home as a permissible originating site for these services, we revisited our regulatory
definition of ‘‘interactive telecommunications system’’ beyond the circumstances of the PHE.
Specifically, we finalized a policy to allow for audio-only services under certain circumstances
and revised the regulation at § 410.78(a)(3) to permit the use of audio-only equipment for
telehealth services furnished to established patients in their homes for purposes of diagnosis,
evaluation, or treatment of a mental health disorder (including substance use disorders) if the
distant site physician or practitioner is technically capable of using an interactive
telecommunications system as defined previously, but the patient is not capable of, or does not
consent to, the use of video technology. We also established this policy in part because mental
health services are different from most other services on the Medicare telehealth services list in
that many of the services primarily involve verbal conversation where visualization between the
patient and furnishing physician or practitioner may be less critical to the provision of the
service.
However, with the successive statutory extensions of the telehealth flexibilities
implemented in response to the PHE for COVID-19, most recently by the CAA, 2023, and our
adoption of other extensions where we have had authority to do so, we have come to believe that
it would be appropriate to allow interactive audio-only telecommunications technology when any
telehealth service is furnished to a beneficiary in their home (when the patient’s home is a
permissible originating site) and when the distant site physician or practitioner is technically
capable of using an interactive telecommunications system as defined previously, but the patient
is not capable of, or does not consent to, the use of video technology. While practitioners should
always use their clinical judgment as to whether the use of interactive audio-only technology is
sufficient to furnish a Medicare telehealth service, we recognize that there is variable broadband
access in patients’ homes, and that even when technologically feasible, patients simply may not
always wish to engage with their practitioner in their home using interactive audio and video.
Under current statute, with the expiration of the PHE-related telehealth flexibilities on December
31, 2024, the patient’s home is a permissible originating site only for services for the diagnosis,
evaluation, or treatment of a mental health or substance use disorder, and for the monthly ESRD-
related clinical assessments described in section 1881(b)(3)(B) of the Act.
We are proposing to revise the regulation at § 410.78(a)(3) to state that an interactive
telecommunications system may also include two-way, real-time audio-only communication
technology for any telehealth service furnished to a beneficiary in their home if the distant site
physician or practitioner is technically capable of using an interactive telecommunications
system as defined as multimedia communications equipment that includes, at a minimum, audio
and video equipment permitting two-way, real-time interactive communication, but the patient is
not capable of, or does not consent to, the use of video technology. Additionally, a modifier
designated by CMS must be appended to the claim for services described in this paragraph to
verify that these conditions have been met. These are CPT modifier “93” and, for RHCs and
FQHCs, Medicare modifier “FQ” (Medicare telehealth service was furnished using audio-only
communication technology). Practitioners have the option to use the “FQ” or the “93” modifiers
or both where appropriate and true, since they are identical in meaning.
f. Distant Site Requirements
In the CY 2024 PFS final rule (88 FR 78873 through 78874) we discussed that many
commenters expressed concerns regarding the expiring flexibility for telehealth practitioners to
bill from their currently enrolled location instead of their home address when providing
telehealth services from their home. CMS issued an FAQ, available at
https://www.cms.gov/files/document/physicians-and-other-clinicians-cms-flexibilities-fight-
covid-19.pdf, which extended the flexibility for telehealth practitioners to bill from their
currently enrolled location instead of their home address when providing telehealth services from
their home through December 31, 2023. Interested parties suggested that the expiration of this
flexibility poses a potential and imminent threat to the safety and privacy of health professionals
who work from home and furnish telehealth services. Commenters cited recent examples of
workplace violence in health care facilities, where direct harm to nurses and other medical staff
occurred. In addition to safety and privacy concerns, interested parties explained that a
significant number of practitioners would need to change their billing practices or add their home
address to the Medicare enrollment file, coordinating with the appropriate Medicare
Administrative Contractor in their jurisdiction, and this would present administrative burden. To
address these concerns, commenters requested that CMS take steps to protect telehealth
practitioners by adjusting enrollment requirements so that individual practitioners do not have to
list their home addresses on enrollment forms.
In response, CMS finalized, through CY 2024, that we would continue to permit a distant
site practitioner to use their currently enrolled practice location instead of their home address
when providing telehealth services from their home.
We have continued to hear from interested parties who have stressed the importance of
continuing this flexibility for the safety and privacy of health care professionals. Given the shift
in practice patterns toward models of care that include the practitioner’s home as the distant site,
we believe it would be appropriate to continue this flexibility as CMS considers various
proposals that may better protect the safety and privacy of practitioners. We are therefore
proposing that through CY 2025 we will continue to permit the distant site practitioner to use
their currently enrolled practice location instead of their home address when providing telehealth
services from their home.
2. Other Non-Face-to-Face Services Involving Communications Technology under the PFS
a. Direct Supervision via Use of Two-way Audio/Video Communications Technology
Under Medicare Part B, certain types of services, including diagnostic tests described
under § 410.32 and services incident to a physician’s (or other practitioner’s) professional
service described under § 410.26 (incident-to services), are required to be furnished under
specific minimum levels of supervision by a physician or other practitioner. We define three
levels of supervision in our regulation at § 410.32(b)(3): General Supervision, Direct
Supervision, and Personal Supervision. Notwithstanding the temporary measures implemented in
response to the PHE for COVID-19, direct supervision requires the physician (or other
supervising practitioner) to be present in the office suite and immediately available to furnish
assistance and direction throughout the performance of the service. It does not mean that the
physician (or other supervising practitioner) must be present in the room when the service is
performed. Again, notwithstanding the temporary measures implemented in response to the PHE
for COVID-19, we have established this “immediate availability” requirement to mean in-
person, physical, not virtual, availability (please see the April 6, 2020 IFC (85 FR 19245) and the
CY 2022 PFS final rule (86 FR 65062)).
Direct supervision is required for various types of services, including most incident-to
services under § 410.26, many diagnostic tests under § 410.32, pulmonary rehabilitation services
under § 410.47, cardiac rehabilitation and intensive cardiac rehabilitation services under §
410.49, and certain hospital outpatient services as provided under § 410.27(a)(1)(iv). In the
March 31, 2020 COVID-19 IFC, we amended the definition of “direct supervision” for the
duration of the PHE for COVID-19 (85 FR 19245 through 19246) at § 410.32(b)(3)(ii) to state
that the necessary presence of the physician (or other practitioner) for direct supervision includes
virtual presence through audio/video real-time communications technology. Instead of requiring
the supervising physician’s (or other practitioner’s) physical presence, the amendment permitted
a supervising physician (or other practitioner) to be considered “immediately available” through
virtual presence using two-way, real-time audio/visual technology for diagnostic tests, incident-
to services, pulmonary rehabilitation services, and cardiac and intensive cardiac rehabilitation
services. We made similar amendments at § 410.27(a)(1)(iv) to specify that direct supervision for
certain hospital outpatient services may include virtual presence through audio/video real-time
communications. The CY 2021 PFS final rule (85 FR 84538 through 84540) and the CY 2024
PFS final rule (88 FR 78878) subsequently extended these policies through December 31, 2024.
As stated in the CY 2024 PFS final rule, we extended this definition of direct supervision
through December 31, 2024, in order to align the timeframe of the policy with other PHE-related
telehealth policies that were extended most recently under the provisions of the CAA, 2023.
We note that in the CY 2021 PFS final rule (85 FR 84539) we clarified that, to the extent
our policy allows direct supervision through virtual presence using audio/video real-time
communications technology, the requirement could be met by the supervising physician (or other
practitioner) being immediately available to engage via audio/video technology (excluding
audio-only), and would not require real-time presence or observation of the service via
interactive audio and video technology throughout the performance of the service. We noted that
this was the case during the PHE and would continue to be the case following the PHE. While
flexibility to provide direct supervision through audio/video real-time communications
technology was adopted to be responsive to critical needs during the PHE for COVID–19 to
ensure beneficiary access to care, reduce exposure risk and to increase the capacity of
practitioners and physicians to respond to COVID–19, we expressed concern that direct
supervision through virtual presence may not be sufficient to support PFS payment on a
permanent basis, beyond the PHE for COVID–19, due to issues of patient safety. For instance, in
complex, high-risk, surgical, interventional, or endoscopic procedures, or anesthesia procedures,
a patient's clinical status can quickly change; in-person supervision would be necessary for such
services to allow for rapid on-site decision-making in the event of an adverse clinical situation.
In addition to soliciting comment in the CY 2021 PFS proposed rule on whether there should be
any additional ‘‘guardrails’’ or limitations to ensure patient safety/clinical appropriateness,
beyond typical clinical standards, as well as restrictions to prevent fraud or inappropriate use, we
solicited comment in the CY 2024 PFS proposed rule on whether we should consider extending
the definition of direct supervision to permit virtual presence beyond December 31, 2024.
Specifically, we stated that we were interested in input from interested parties on potential
patient safety or quality concerns when direct supervision occurs virtually; for instance, if direct
supervision of certain types of services with virtual presence of the supervising practitioner is
more or less likely to present patient safety concerns, or if this flexibility would be more
appropriate for certain types of services, or when certain types of auxiliary personnel are
performing the supervised service. We were also interested in potential program integrity
concerns that interested parties may have regarding this policy, such as overutilization or fraud
and abuse.
(1) Proposal to Extend Definition of “Direct Supervision” to Include Audio-Video
Communications Technology through 2025
As discussed in the CY 2024 PFS final rule (88 FR 78878), in the absence of evidence
that patient safety is compromised by virtual direct supervision, we are concerned about an
abrupt transition to our pre-PHE policy that defines direct supervision to require the physical
presence of the supervising practitioner. We noted that an immediate reversion to the pre-PHE
definition of direct supervision would prohibit virtual direct supervision, which may present a
barrier to access to many services, such as incident-to services, and that physicians and/or other
supervising practitioners, in certain instances, would need time to reorganize their practice
patterns established during the PHE to reimplement the pre-PHE approach to direct supervision
without the use of audio/video technology. We acknowledge the utilization of this flexibility and
recognize that many practitioners have stressed the importance of maintaining it, however we
seek additional information regarding potential patient safety and quality of care concerns. This
flexibility has been available and widely utilized since the beginning of the PHE, and we
recognize that may enhance patient access. However, given the importance of certain services
being furnished under direct supervision in ensuring quality of care and patient safety, and in
particular the ability of the supervising practitioner to intervene if complications arise, we
believe an incremental approach is warranted, particularly in instances where unexpected or
adverse events may arise for procedures which may be riskier or more intense. In light of these
potential safety and quality of care implications, and exercising an abundance of caution, we are
extending this flexibility for all services on a temporary basis only. We are therefore proposing
to continue to define direct supervision to permit the presence and “immediate availability” of
the supervising practitioner through real-time audio and visual interactive telecommunications
through December 31, 2025.
(2) Proposal to Permanently Define “Direct Supervision” to Include Audio-Video
Communications Technology for a Subset of Services
In the CY 2024 PFS proposed rule, we solicited comment on extending or permanently
establishing the virtual presence flexibility for certain services valued under the PFS given that
these services typically are performed in their entirety by auxiliary personnel as defined at §
410.26(a)(1). We stated such services would include incident-to services wholly furnished by
auxiliary personnel, Level I office or other outpatient E/M visits for established patients. We also
mentioned Level I Emergency Department (ED) visits in this list, but have since concluded that
ED services would not be wholly furnished by auxiliary personnel and, for that reason, have
excluded them from the discussion in this proposed rule. Based on our review, these specific
services present less of a patient safety concern than services for which there may be a need for
immediate intervention of the supervising practitioner; as noted in the CY 2024 PFS proposed
rule, allowing virtual presence for direct supervision of these services could balance patient

safety concerns with the interest of supporting access and preserving workforce capacity for
medical professionals while considering potential quality and program integrity concerns. After
reviewing the various comments in response to this solicitation, additional feedback provided by
interested parties, and conducting our own independent review, we believe these services are low
risk by their nature, do not often demand in-person supervision, are typically furnished entirely
by the supervised personnel, and allowing virtual presence for direct supervision of these
services would balance patient safety concerns with the interest of supporting access and
preserving workforce capacity.
We are proposing to adopt a definition of direct supervision that allows "immediate
availability” of the supervising practitioner using audio/video real-time communications
technology (excluding audio-only), but only for the following subset of incident-to services
described under § 410.26: (1) services furnished incident to a physician or other practitioner’s
service when provided by auxiliary personnel employed by the billing practitioner and working
under their direct supervision, and for which the underlying HCPCS code has been assigned a
PC/TC indicator of ‘5’;
10
and (2) services described by CPT code 99211 (Office or other
outpatient visit for the evaluation and management of an established patient that may not require
the presence of a physician or other qualified health care professional). As provided in the code
descriptor for CPT code 99211, an office or other outpatient visit for the evaluation and
management of an established patient may not require the presence of a physician or other
practitioner and may be furnished incident to a physicians’ service by a nonphysician employee
of the physician under direct supervision. The service described by CPT code 99211 and the
services that are identified with a PC/TC indicator of ‘5’ as listed in the PFS Relative Value Files
are services that are nearly always performed in entirety by auxiliary personnel. The vignette for
10
For a full list of all PFS payment status indicators and descriptions, see the Medicare Claims Processing Manual
(IOM Pub. 100–04, chapter 23, sections 30.2.2). For a full list of all PFS payment status indicators and descriptions,
see the Medicare Claims Processing Manual (IOM Pub. 100–04, chapter 23, sections 30.2.2 and 50.6). Specific
indicators by service are listed in the PFS Relative Value files at https://www.cms.gov/medicare/payment/fee-
schedules/physician/pfs-relative-value-files).
CPT code 99211 describes the provision of supervision and guidance to the clinical staff as
necessary. The code descriptor for this service specifies an E/M service that may not require the
presence of a physician or other professional; and the current valuation, which is relatively low
compared to other office and outpatient E/M services, suggests that this service would primarily
be provided by auxiliary personnel.
We are proposing an incremental approach whereby we will adopt without any time
limitation the definition of direct supervision permitting virtual presence for services that are
inherently lower risk: that is, services that do not ordinarily require the presence of the billing
practitioner, do not require direction by the supervising practitioner to the same degree as other
services furnished under direct supervision, and are not services typically performed directly by
the supervising practitioner.
For all other services required to be furnished under the direct supervision of the
supervising physician or other practitioner, we are proposing, as described above, to continue to
define "immediate availability” to include real-time audio and visual interactive
telecommunications technology only through December 31, 2025.
We are proposing to revise the regulations at § 410.32(b)(3)(ii) to state that through
December 31, 2025, the presence of the physician (or other practitioner) would include virtual
presence through audio/video real-time communications technology (excluding audio-only).
We are proposing to revise the regulation at § 410.26(a)(2) to state that for the following
services furnished after December 31, 2025, the presence of the physician (or other practitioner)
required for direct supervision shall continue to include virtual presence through audio/video
real-time communications technology (excluding audio-only): services furnished incident to a
physician’s service when they are provided by auxiliary personnel employed by the physician
and working under his or her direct supervision and for which the underlying HCPCS code has
been assigned a PC/TC indicator of ‘5’; and office and other outpatient visits for the evaluation
and management of an established patient that may not require the presence of a physician or
other qualified health care professional.
(3) Teaching Physician Billing for Services Involving Residents with Virtual Presence
In the CY 2021 PFS final rule (85 FR 84577 through 84584), we established a policy
that, after the end of the PHE for COVID-19, teaching physicians may meet the requirements to
be present for the key or critical portions of services when furnished involving residents through
audio/video real-time communications technology (virtual presence), but only for services
furnished in residency training sites located outside of an Office of Management and Budget
(OMB)-defined metropolitan statistical area (MSA). We made this location distinction consistent
with our longstanding interest in increasing beneficiary access to Medicare-covered services in
rural areas. We noted the ability to expand training opportunities for residents in rural settings.
For all other locations, we expressed concerns that continuing to permit teaching physicians to
bill for services furnished involving residents when they are virtually present, outside the
conditions of the PHE for COVID-19, may not allow the teaching physician to have personal
oversight and involvement over the management of the portion of the case for which the
payment is sought, under section 1842(b)(7)(A)(i)(I) of the Act. In addition, we stated concerns
about patient populations that may require a teaching physician’s experience and skill to
recognize specialized needs or testing and whether it is possible for the teaching physician to
meet these clinical needs while having a virtual presence for the key portion of the service. We
refer readers to the CY 2021 PFS final rule (85 FR 84577 through 84584) for a more detailed
description of our specific concerns. At the end of the PHE for COVID-19, and as finalized in
the CY 2021 PFS final rule, we intended for the teaching physician to have a physical presence
during the key portion of the service personally provided by residents in order to be paid for the
service under the PFS, in locations that were within a MSA. This policy applied to all services,
regardless of whether the patient was co-located with the resident or only present virtually (for
example, the service was furnished as a 3-way telehealth visit, with the teaching physician,
resident, and patient in different locations). However, interested parties expressed concerns
regarding the requirement that the teaching physician be physically present with the resident
when a service is furnished virtually (as a Medicare telehealth service) within an MSA. Some
interested parties stated that during the PHE for COVID-19, when residents provided telehealth
services, and the teaching physician was virtually present, the same safe and high-quality
oversight was provided as when the teaching physician and resident were physically co-located.
In addition, these interested parties stated that during telehealth visits, the teaching physician was
virtually present during the key and critical portions of the telehealth service, available
immediately in real-time, and had access to the electronic health record. After review of the
public comments, we finalized a policy that allowed the teaching physician to have a virtual
presence in all teaching settings, only in clinical instances when the service was furnished
virtually (for example, a 3-way telehealth visit, with all parties in separate locations). This
permitted teaching physicians to have a virtual presence during the key portion of the Medicare
telehealth service for which payment was sought, through audio/video real-time communications
technology, in all residency training locations through December 31, 2024.
As stated in the CY 2024 PFS final rule (88 FR 78880), we are concerned that an abrupt
transition to our pre-PHE policy may present a barrier to access to many services. We also
understand that teaching physicians have gained clinical experience providing services involving
residents with virtual presence during the PHE for COVID–19 and could help us to identify
circumstances where the teaching physician can routinely provide sufficient personal and
identifiable services to the patient through their virtual presence during the key portion of the
Medicare telehealth service. We sought comment and information to help us consider other
clinical treatment situations where it may be appropriate to continue to permit the virtual
presence of the teaching physician, while continuing to support patient safety, meeting the
clinical needs for all patients, and ensuring burden reduction without creating risks to patient
care or increasing opportunities for fraud. As summarized in the CY 2024 PFS final rule (88 FR
78881 through 78882), commenters encouraged us to establish this policy permanently and
include in-person services to promote access to care, stated that teaching physicians should be
allowed to determine when their virtual presence would be clinically appropriate, based on their
assessment of the patient’s needs and the competency level of the resident. While we continue to
consider clinical scenarios where it may be appropriate to permit the virtual presence of the
teaching physician, we are proposing to continue our current policy to allow teaching physicians
to have a virtual presence for purposes of billing for services furnished involving residents in all
teaching settings, but only when the service is furnished virtually (for example, a 3-way
telehealth visit, with the patient, resident, and teaching physician in separate locations). This
would permit teaching physicians to have a virtual presence during the key portion of the
Medicare telehealth service for which payment is sought in any residency training location
through December 31, 2025. The teaching physician’s virtual presence would continue to require
real-time observation (not mere availability) and excludes audio-only technology. The
documentation in the medical record must continue to demonstrate whether the teaching
physician was physically present or present through audio/video real-time communications
technology at the time of the Medicare telehealth service, which includes documenting the
specific portion of the service for which the teaching physician was present through audio/video
real-time communications technology.
(a) Request for Information for Teaching Physician Services Furnished under the Primary Care
Exception
The so-called primary care exception set forth at § 415.174 permits the teaching
physician to bill for certain lower and mid-level complexity physicians’ services furnished by
residents in certain types of residency training settings even when the teaching physician is not
present with the resident during the services as long as certain conditions are met, including that
the services are furnished by residents with more than 6 months of training in the approved
residency program; and that the teaching physician directs the care of no more than four
residents at a time, remains immediately available and has no other responsibilities while
directing the care, assumes management responsibility for beneficiaries seen by the residents,
ensures that the services furnished are appropriate, and reviews certain elements of the services
with each resident during or immediately after each visit. For a more detailed description of the
list of services currently allowed under the primary care exception policy, we refer readers to the
CY 2021 PFS final rule (85 FR 84585 through 84590).
We have received feedback from interested parties requesting that we permanently
expand the list of services that can be furnished under the primary care exception to include all
levels of E/M services and additional preventive services. These interested parties have stated
that the fact that high-value primary care and preventive services are not included in the scope of
the primary care exception discourages their integration in residency training in these primary
care settings, which has a negative impact on physician training, patient access, and longer-term
outcomes. Additionally, these interested parties have suggested that including all levels of E/M
services under the primary care exception could support primary care workforce development
and improve patient continuity of care without compromising patient safety; furthermore,
including additional preventive services within the primary care exception would increase the
utilization of high-value services.
We believe the primary care exception was intended to broaden opportunities for
teaching physicians to involve residents in furnishing services under circumstances that preserve
the direction of the care by the teaching physician and promote safe, high-quality patient care. As
such, we are requesting information to help us consider whether and how best to expand the
array of services included under the primary care exception in future rulemaking. We are
interested in hearing more about the types of services that could be allowed under the primary
care exception, specifically preventive services, and whether the currently required six months of
training in an approved program is sufficient for residents to furnish these types of services
without the presence of a teaching physician. We are seeking comment to help us consider
whether adding certain preventive services or higher level E/M services to the primary care
exception would hinder the teaching physician from maintaining sufficient personal involvement
in the care to warrant PFS payment for the services being furnished by up to four residents at any
given time. Similarly, we are requesting information on whether the inclusion in the primary care
exception of specific higher-level or preventive services would impede the teaching physician’s
ability to remain immediately available for up to four residents at any given time, while directing
and managing the care furnished by these residents.
3. Telehealth Originating Site Facility Fee Payment Amount Update
Section 1834(m)(2)(B) of the Act established the Medicare telehealth originating site
facility fee for telehealth services furnished from October 1, 2001, through December 31, 2002 at
$20.00, and specifies that, for telehealth services furnished on or after January 1 of each
subsequent calendar year, the telehealth originating site facility fee is increased by the percentage
increase in the Medicare Economic Index (MEI) as defined in section 1842(i)(3) of the Act. The
proposed MEI increase for CY 2025 is 3.6 percent and is based on the expected historical
percentage increase of the 2017-based MEI. For the final rule, we propose to update the MEI
increase for CY 2025 based on historical data through the second quarter of 2024. Therefore, for
CY 2025, the proposed payment amount for HCPCS code Q3014 (Telehealth originating site
facility fee) is $31.04. Table 9 shows the Medicare telehealth originating site facility fee and the
corresponding MEI percentage increase for each applicable time period.

TABLE 9: The Medicare Telehealth Originating Site Facility Fee
Time Period
MEI (%)
Facility Fee for Q3014
Oct. 1, 2001 to Dec. 31, 2002
NA
$ 20.00
2003
3.0
$ 20.60
2004
2.9
$ 21.20
2005
3.1
$ 21.86
2006
2.8
$ 22.47
2007
2.1
$ 22.94
2008
1.8
$ 23.35
2009
1.6
$ 23.72
2010
1.2
$ 24.00
2011
0.4
$ 24.10
2012
0.6
$ 24.24
2013
0.8
$ 24.43
2014
0.8
$ 24.63
2015
0.8
$ 24.83
2016
1.1
$ 25.10
2017
1.2
$ 25.40
2018
1.4
$ 25.76
2019
1.5
$ 26.15
2020
1.9
$ 26.65
2021
1.4
$ 27.02
2022
2.1
$ 27.59
2023
3.8
$ 28.64
2024
4.6
$ 29.96
2025*
3.6
$ 31.04
*Reflects the most recent estimate of the CY 2025 MEI percentage increase and will be updated in the final rule
based on historical data through the second quarter of 2024.
4. Payment for Outpatient Therapy Services, Diabetes Self-Management Training, and Medical
Nutrition Therapy when Furnished by Institutional Staff to Beneficiaries in Their Homes
Through Communication Technology
For information related to outpatient physical therapy, occupational therapy, speech-
language pathology, diabetes self-management training (DSMT) and medical nutritional therapy
(MNT) services furnished by institutional staff in hospitals and other institutional settings to
beneficiaries in their homes through communication technology, please refer to section X.A. in
the CY 2025 Hospital Outpatient Prospective Payment System (OPPS) proposed rule (FR Doc.
2024-15087), on public inspection July 10, 2024, and publishing in the Federal Register of July
22, 2024.
E. Valuation of Specific Codes
1. Background: Process for Valuing New, Revised, and Potentially Misvalued Codes
Establishing valuations for newly created and revised CPT codes is a routine part of
maintaining the PFS. Since the inception of the PFS, it has also been a priority to revalue
services regularly to make sure that the payment rates reflect the changing trends in the practice
of medicine and current prices for inputs used in the PE calculations. Initially, this was
accomplished primarily through the 5-year review process, which resulted in revised work RVUs
for CY 1997, CY 2002, CY 2007, and CY 2012, and revised PE RVUs in CY 2001, CY 2006,
and CY 2011, and revised MP RVUs in CY 2010, CY 2015, and CY 2020. Under the 5-year
review process, revisions in RVUs were proposed and finalized via rulemaking. In addition to
the 5-year reviews, beginning with CY 2009, CMS and the RUC identified a number of
potentially misvalued codes each year using various identification screens, as outlined in section
II.C. of this proposed rule, Potentially Misvalued Services under the PFS. Historically, when we
received RUC recommendations, our process had been to establish interim final RVUs for the
potentially misvalued codes, new codes, and any other codes for which there were coding
changes in the final rule with comment period for a year. Then, during the 60-day period
following the publication of the final rule with comment period, we accepted public comment
about those valuations. For services furnished during the calendar year following the publication
of interim final rates, we paid for services based upon the interim final values established in the
final rule. In the final rule with comment period for the subsequent year, we considered and
responded to public comments received on the interim final values, and typically made any
appropriate adjustments and finalized those values.
In the CY 2015 PFS final rule with comment period (79 FR 67547), we finalized a new
process for establishing values for new, revised and potentially misvalued codes. Under the new
process, we include proposed values for these services in the proposed rule, rather than
establishing them as interim final in the final rule with comment period. Beginning with the CY
2017 PFS proposed rule (81 FR 46162), the new process was applicable to all codes, except for
new codes that describe truly new services. For CY 2017, we proposed new values in the CY
2017 PFS proposed rule for the vast majority of new, revised, and potentially misvalued codes
for which we received complete RUC recommendations by February 10, 2016. To complete the
transition to this new process, for codes for which we established interim final values in the CY
2016 PFS final rule with comment period (81 FR 80170), we reviewed the comments received
during the 60-day public comment period following release of the CY 2016 PFS final rule with
comment period (80 FR 70886), and re-proposed values for those codes in the CY 2017 PFS
proposed rule. We considered public comments received during the 60-day public comment
period for the proposed rule before establishing final values in the CY 2017 PFS final rule. As
part of our established process, we will adopt interim final values only in the case of wholly new
services for which there are no predecessor codes or values and for which we do not receive
recommendations in time to propose values.
As part of our obligation to establish RVUs for the PFS, we thoroughly review and
consider available information including recommendations and supporting information from the
RUC, the Health Care Professionals Advisory Committee (HCPAC), public commenters,
medical literature, Medicare claims data, comparative databases, comparison with other codes
within the PFS, as well as consultation with other physicians and healthcare professionals within
CMS and the Federal Government as part of our process for establishing valuations. Where we
concur that the RUC’s recommendations, or recommendations from other commenters, are
reasonable and appropriate and are consistent with the time and intensity paradigm of physician
work, we proposed those values as recommended. Additionally, we continually engage with
interested parties, including the RUC, with regard to our approach for accurately valuing codes,
and as we prioritize our obligation to value new, revised, and potentially misvalued codes. We
continue to welcome feedback from all interested parties regarding valuation of services for
consideration through our rulemaking process.
2. Methodology for Establishing Work RVUs
For each code identified in this section, we conduct a review that includes the current
work RVU (if any), RUC-recommended work RVU, intensity, time to furnish the preservice,
intraservice, and postservice activities, as well as other components of the service that contribute
to the value. Our reviews of recommended work RVUs and time inputs generally include, but
have not been limited to, a review of information provided by the RUC, the HCPAC, and other
public commenters, medical literature, and comparative databases, as well as a comparison with
other codes within the PFS, consultation with other physicians and health care professionals
within CMS and the Federal Government, as well as Medicare claims data. We also assess the
methodology and data used to develop the recommendations submitted to us by the RUC and
other public commenters and the rationale for the recommendations. In the CY 2011 PFS final
rule with comment period (75 FR 73328 through 73329), we discussed a variety of
methodologies and approaches used to develop work RVUs, including survey data, building
blocks, crosswalks to key reference or similar codes, and magnitude estimation (see the CY 2011
PFS final rule with comment period (75 FR 73328 through 73329) for more information). When
referring to a survey, unless otherwise noted, we mean the surveys conducted by specialty
societies as part of the formal RUC process.
Components that we use in the building block approach may include preservice,
intraservice, or postservice time and post-procedure visits. When referring to a bundled CPT
code, the building block components could include the CPT codes that make up the bundled code
and the inputs associated with those codes. We use the building block methodology to construct,
or deconstruct, the work RVU for a CPT code based on component pieces of the code.
Magnitude estimation refers to a methodology for valuing work that determines the appropriate
work RVU for a service by gauging the total amount of work for that service relative to the work
for a similar service across the PFS without explicitly valuing the components of that work. In
addition to these methodologies, we frequently utilize an incremental methodology in which we
value a code based upon its incremental difference between another code and another family of
codes. Section 1848(c)(1)(A) of the Act specifically defines the work component as the
resources that reflect time and intensity in furnishing the service. Also, the published literature
on valuing work has recognized the key role of time in overall work. For particular codes, we
refine the work RVUs in direct proportion to the changes in the best information regarding the
time resources involved in furnishing particular services, either considering the total time or the
intraservice time.
Several years ago, to aid in the development of preservice time recommendations for new
and revised CPT codes, the RUC created standardized preservice time packages. The packages
include preservice evaluation time, preservice positioning time, and preservice scrub, dress and
wait time. Currently, there are preservice time packages for services typically furnished in the
facility setting (for example, preservice time packages reflecting the different combinations of
straightforward or difficult procedure, and straightforward or difficult patient). Currently, there
are three preservice time packages for services typically furnished in the nonfacility setting.
We developed several standard building block methodologies to value services
appropriately when they have common billing patterns. In cases where a service is typically
furnished to a beneficiary on the same day as an E/M service, we believe that there is overlap
between the two services in some of the activities furnished during the preservice evaluation and
postservice time. Our longstanding adjustments have reflected a broad assumption that at least
one-third of the work time in both the preservice evaluation and postservice period is duplicative
of work furnished during the E/M visit.
Accordingly, in cases where we believe that the RUC has not adequately accounted for
the overlapping activities in the recommended work RVU and/or times, we adjust the work RVU
and/or times to account for the overlap. The work RVU for a service is the product of the time
involved in furnishing the service multiplied by the intensity of the work. Preservice evaluation
time and postservice time both have a long-established intensity of work per unit of time
(IWPUT) of 0.0224, which means that 1 minute of preservice evaluation or postservice time
equates to 0.0224 of a work RVU.
Therefore, in many cases when we remove 2 minutes of preservice time and 2 minutes of
postservice time from a procedure to account for the overlap with the same day E/M service, we
also remove a work RVU of 0.09 (4 minutes × 0.0224 IWPUT) if we do not believe the overlap
in time had already been accounted for in the work RVU. The RUC has recognized this
valuation policy and, in many cases, now addresses the overlap in time and work when a service
is typically furnished on the same day as an E/M service.
The following paragraphs discuss our approach to reviewing RUC recommendations and
developing proposed values for specific codes. When they exist, we also include a summary of
interested party reactions to our approach. We noted that many commenters and interested
parties have expressed concerns over the years with our ongoing adjustment of work RVUs
based on changes in the best information we had regarding the time resources involved in
furnishing individual services. We have been particularly concerned with the RUC’s and various
specialty societies’ objections to our approach given the significance of their recommendations
to our process for valuing services and since much of the information we used to make the
adjustments is derived from their survey process. We note that we are obligated under the statute
to consider both time and intensity in establishing work RVUs for PFS services. As explained in
the CY 2016 PFS final rule with comment period (80 FR 70933), we recognize that adjusting
work RVUs for changes in time is not always a straightforward process, so we have applied
various methodologies to identify several potential work values for individual codes.
We have observed that for many codes reviewed by the RUC, recommended work RVUs
have appeared to be incongruous with recommended assumptions regarding the resource costs in
time. This has been the case for a significant portion of codes for which we recently established
or proposed work RVUs that are based on refinements to the RUC-recommended values. When
we have adjusted work RVUs to account for significant changes in time, we have started by
looking at the change in the time in the context of the RUC-recommended work RVU. When the
recommended work RVUs do not appear to account for significant changes in time, we have
employed the different approaches to identify potential values that reconcile the recommended
work RVUs with the recommended time values. Many of these methodologies, such as survey
data, building block, crosswalks to key reference or similar codes, and magnitude estimation
have long been used in developing work RVUs under the PFS. In addition to these, we
sometimes use the relationship between the old time values and the new time values for
particular services to identify alternative work RVUs based on changes in time components.
In so doing, rather than ignoring the RUC-recommended value, we have used the
recommended values as a starting reference and then applied one of these several methodologies
to account for the reductions in time that we believe were not otherwise reflected in the RUC-
recommended value. If we believe that such changes in time are already accounted for in the
RUC’s recommendation, then we do not make such adjustments. Likewise, we do not arbitrarily
apply time ratios to current work RVUs to calculate proposed work RVUs. We use the ratios to
identify potential work RVUs and consider these work RVUs as potential options relative to the
values developed through other options.
We do not imply that the decrease in time as reflected in survey values should always
equate to a one-to-one or linear decrease in newly valued work RVUs. Instead, we believe that,
since the two components of work are time and intensity, absent an obvious or explicitly stated
rationale for why the relative intensity of a given procedure has increased, significant decreases
in time should be reflected in decreases to work RVUs. If the RUC’s recommendation has
appeared to disregard or dismiss the changes in time, without a persuasive explanation of why
such a change should not be accounted for in the overall work of the service, then we have
generally used one of the aforementioned methodologies to identify potential work RVUs,
including the methodologies intended to account for the changes in the resources involved in
furnishing the procedure.
Several interested parties, including the RUC, have expressed general objections to our
use of these methodologies and suggested that our actions in adjusting the recommended work
RVUs are inappropriate; other interested parties have also expressed general concerns with CMS
refinements to RUC-recommended values in general. In the CY 2017 PFS final rule (81 FR
80272 through 80277), we responded in detail to several comments that we received regarding
this issue. In the CY 2017 PFS proposed rule (81 FR 46162), we requested comments regarding
potential alternatives to making adjustments that would recognize overall estimates of work in
the context of changes in the resource of time for particular services; however, we did not
receive any specific potential alternatives. As described earlier in this section, crosswalks to key
reference or similar codes are one of the many methodological approaches we have employed to
identify potential values that reconcile the RUC-recommend work RVUs with the recommended
time values when the RUC-recommended work RVUs did not appear to account for significant
changes in time.
In response to comments, in the CY 2019 PFS final rule (83 FR 59515), we clarified that
terms “reference services”, “key reference services”, and “crosswalks” as described by the
commenters are part of the RUC’s process for code valuation. These are not terms that we
created, and we do not agree that we necessarily must employ them in the identical fashion for
the purposes of discussing our valuation of individual services that come up for review.
However, in the interest of minimizing confusion and providing clear language to facilitate
feedback from interested parties, we stated that we would seek to limit the use of the term,
“crosswalk,” to those cases where we are making a comparison to a CPT code with the identical
work RVU. (83 FR 59515) We note that we also occasionally make use of a “bracket” for code
valuation. A “bracket” refers to when a work RVU falls between the values of two CPT codes,
one at a higher work RVU and one at a lower work RVU.
We look forward to continuing to engage with interested parties and commenters,
including the RUC, as we prioritize our obligation to value new, revised, and potentially
misvalued codes; and we will continue to welcome feedback from all interested parties regarding
valuation of services for consideration through our rulemaking process. We refer readers to the
detailed discussion in this section of the valuation considered for specific codes. Table 13
contains a list of codes and descriptors for which we are proposing work RVUs for CY 2025;
this includes all codes for which we received RUC recommendations by February 10, 2024. The
proposed work RVUs, work time and other payment information for all CY 2025 payable codes
are available on the CMS website under downloads for the CY 2025 PFS proposed rule at
https://www.cms.gov/Medicare/Medicare-Fee-for-Service-
Payment/PhysicianFeeSched/index.html).
3. Methodology for the Direct PE Inputs to Develop PE RVUs
a. Background
On an annual basis, the RUC provides us with recommendations regarding PE inputs for
new, revised, and potentially misvalued codes. We review the RUC-recommended direct PE
inputs on a code-by-code basis. Like our review of recommended work RVUs, our review of
recommended direct PE inputs generally includes, but is not limited to, a review of information
provided by the RUC, HCPAC, and other public commenters, medical literature, and
comparative databases, as well as a comparison with other codes within the PFS, and
consultation with physicians and health care professionals within CMS and the Federal
Government, as well as Medicare claims data. We also assess the methodology and data used to
develop the recommendations submitted to us by the RUC and other public commenters and the
rationale for the recommendations. When we determine that the RUC’s recommendations
appropriately estimate the direct PE inputs (clinical labor, disposable supplies, and medical
equipment) required for the typical service, are consistent with the principles of relativity, and
reflect our payment policies, we use those direct PE inputs to value a service. If not, we refine
the recommended PE inputs to better reflect our estimate of the PE resources required for the
service. We also confirm whether CPT codes should have facility and/or nonfacility direct PE
inputs and refine the inputs accordingly.
Our review and refinement of the RUC-recommended direct PE inputs includes many
refinements that are common across codes, as well as refinements that are specific to particular
services. Table 14 details our refinements of the RUC’s direct PE recommendations at the code-
specific level. In section II.B. of this proposed rule, Determination of Practice Expense Relative
Value Units (PE RVUs), we address certain refinements that will be common across codes.
Refinements to particular codes are addressed in the portions of that section that are dedicated to
particular codes. We note that for each refinement, we indicate the impact on direct costs for
that service. We note that, on average, in any case where the impact on the direct cost for a
particular refinement is $0.35 or less, the refinement has no impact on the PE RVUs. This
calculation considers both the impact on the direct portion of the PE RVU, as well as the impact
on the indirect allocator for the average service. In this proposed rule, we also note that many of
the refinements listed in Table 14 result in changes under the $0.35 threshold and would be
unlikely to result in a change to the RVUs.
We note that the direct PE inputs for CY 2025 are displayed in the CY 2025 direct PE
input files, available on the CMS website under the downloads for the CY 2025 PFS proposed
rule at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-
Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices.html. The inputs displayed there
have been used in developing the CY 2025 PE RVUs as displayed in Addendum B (see
https://www.cms.gov/medicare/payment/prospective-payment-systems/hospital-
outpatient/addendum-a-b-updates).
b. Common Refinements
(1) Changes in Work Time
Some direct PE inputs are directly affected by revisions in work time. Specifically,
changes in the intraservice portions of the work time and changes in the number or level of
postoperative visits associated with the global periods result in corresponding changes to direct
PE inputs. The direct PE input recommendations generally correspond to the work time values
associated with services. We believe that inadvertent discrepancies between work time values
and direct PE inputs should be refined or adjusted in the establishment of proposed direct PE
inputs to resolve the discrepancies.
(2) Equipment Time
Prior to CY 2010, the RUC did not generally provide CMS with recommendations
regarding equipment time inputs. In CY 2010, in the interest of ensuring the greatest possible
degree of accuracy in allocating equipment minutes, we requested that the RUC provide
equipment times along with the other direct PE recommendations, and we provided the RUC
with general guidelines regarding appropriate equipment time inputs. We appreciate the RUC’s
willingness to provide us with these additional inputs as part of its PE recommendations.
In general, the equipment time inputs correspond to the service period portion of the
clinical labor times. We clarified this principle over several years of rulemaking, indicating that
we consider equipment time as the time within the intraservice period when a clinician is using
the piece of equipment plus any additional time that the piece of equipment is not available for
use for another patient due to its use during the designated procedure. For those services for
which we allocate cleaning time to portable equipment items, because the portable equipment
does not need to be cleaned in the room where the service is furnished, we do not include that
cleaning time for the remaining equipment items, as those items and the room are both available
for use for other patients during that time. In addition, when a piece of equipment is typically
used during follow-up postoperative visits included in the global period for a service, the
equipment time will also reflect that use.
We believe that certain highly technical pieces of equipment and equipment rooms are
less likely to be used during all of the preservice or postservice tasks performed by clinical labor
staff on the day of the procedure (the clinical labor service period) and are typically available for
other patients even when one member of the clinical staff may be occupied with a preservice or
postservice task related to the procedure. We also noted that we believe these same assumptions
will apply to inexpensive equipment items that are used in conjunction with and located in a
room with non-portable highly technical equipment items since any items in the room in question
will be available if the room is not being occupied by a particular patient. For additional
information, we referred readers to our discussion of these issues in the CY 2012 PFS final rule
with comment period (76 FR 73182) and the CY 2015 PFS final rule with comment period
(79 FR 67639).
(3) Standard Tasks and Minutes for Clinical Labor Tasks
In general, the preservice, intraservice, and postservice clinical labor minutes associated
with clinical labor inputs in the direct PE input database reflect the sum of particular tasks
described in the information that accompanies the RUC-recommended direct PE inputs,
commonly called the “PE worksheets.” For most of these described tasks, there is a standardized
number of minutes, depending on the type of procedure, its typical setting, its global period, and
the other procedures with which it is typically reported. The RUC sometimes recommends a
number of minutes either greater than or less than the time typically allotted for certain tasks. In
those cases, we review the deviations from the standards and any rationale provided for the
deviations. When we do not accept the RUC-recommended exceptions, we refine the proposed
direct PE inputs to conform to the standard times for those tasks. In addition, in cases when a
service is typically billed with an E/M service, we remove the preservice clinical labor tasks to
avoid duplicative inputs and to reflect the resource costs of furnishing the typical service.
We refer readers to section II.B. of this proposed rule, Determination of Practice Expense
Relative Value Units (PE RVUs), for more information regarding the collaborative work of CMS
and the RUC in improvements in standardizing clinical labor tasks.
(4) Recommended Items that are not Direct PE Inputs
In some cases, the PE worksheets included with the RUC’s recommendations include
items that are not clinical labor, disposable supplies, or medical equipment or that cannot be
allocated to individual services or patients. We addressed these kinds of recommendations in
previous rulemaking (78 FR 74242), and we do not use items included in these recommendations
as direct PE inputs in the calculation of PE RVUs.
(5) New Supply and Equipment Items
The RUC generally recommends the use of supply and equipment items that already exist
in the direct PE input database for new, revised, and potentially misvalued codes. However,
some recommendations include supply or equipment items that are not currently in the direct PE
input database. In these cases, the RUC has historically recommended that a new item be created
and has facilitated our pricing of that item by working with the specialty societies to provide us
copies of sales invoices. For CY 2025 we received invoices for several new supply and
equipment items. Tables 17 and 18 detail the invoices received for new and existing items in the
direct PE database. As discussed in section II.B. of this proposed rule, Determination of Practice
Expense Relative Value Units, we encourage interested parties to review the prices associated
with these new and existing items to determine whether these prices appear to be accurate.
Where prices appear inaccurate, we encourage interested parties to submit invoices or other
information to improve the accuracy of pricing for these items in the direct PE database by
February 10th of the following year for consideration in future rulemaking, similar to our process
for consideration of RUC recommendations.
We remind interested parties that due to the relativity inherent in the development of
RVUs, reductions in existing prices for any items in the direct PE database increase the pool of
direct PE RVUs available to all other PFS services. Tables 17 and 18 also include the number of
invoices received and the number of nonfacility allowed services for procedures that use these
equipment items. We provide the nonfacility allowed services so that interested parties will note
the impact the particular price might have on PE relativity, as well as to identify items that are
used frequently, since we believe that interested parties are more likely to have better pricing
information for items used more frequently. A single invoice may not be reflective of typical
costs, and we encourage interested parties to provide additional invoices so that we might
identify and use accurate prices in the development of PE RVUs.
In some cases, we do not use the price listed on the invoice that accompanies the
recommendation because we identify publicly available alternative prices or information that
suggests a different price is more accurate. In these cases, we include this in the discussion of
these codes. In other cases, we cannot adequately price a newly recommended item due to
inadequate information. Sometimes, no supporting information regarding the price of the item
has been included in the recommendation. In other cases, the supporting information does not
demonstrate that the item has been purchased at the listed price (for example, vendor price
quotes instead of paid invoices). In cases where the information provided on the item allows us
to identify clinically appropriate proxy items, we might use existing items as proxies for the
newly recommended items. In other cases, we include the item in the direct PE input database
without any associated price. Although including the item without an associated price means
that the item does not contribute to the calculation of the final PE RVU for particular services, it
facilitates our ability to incorporate a price once we obtain information and are able to do so.
(6) Service Period Clinical Labor Time in the Facility Setting
Generally speaking, our direct PE inputs do not include clinical labor minutes assigned to
the service period because the cost of clinical labor during the service period for a procedure in
the facility setting is not considered a resource cost to the practitioner since Medicare makes
separate payment to the facility for these costs. We address code-specific refinements to clinical
labor in the individual code sections.
(7) Procedures Subject to the Multiple Procedure Payment Reduction (MPPR) and the OPPS
Cap
We note that the list of services for the upcoming calendar year that are subject to the
MPPR on diagnostic cardiovascular services, diagnostic imaging services, diagnostic
ophthalmology services, and therapy services; and the list of procedures that meet the definition
of imaging under section 1848(b)(4)(B) of the Act, and therefore, are subject to the OPPS cap;
are displayed in the public use files for the PFS proposed and final rules for each year. The
public use files for CY 2025 are available on the CMS website under downloads for the CY 2025
PFS proposed rule at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-
Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices.html. For more information
regarding the history of the MPPR policy, we refer readers to the CY 2014 PFS final rule with
comment period (78 FR 74261 through 74263).
Effective January 1, 2007, section 5102(b)(1) of the Deficit Reduction Act of 2005 (Pub.
L. 109–171) (DRA) amended section 1848(b)(4) of the Act to require that, for imaging services,
if— (i) The TC (including the TC portion of a global fee) of the service established for a year
under the fee schedule without application of the geographic adjustment factor, exceeds (ii) The
Medicare OPD fee schedule amount established under the prospective payment system (PPS) for
HOPD services under section 1833(t)(3)(D) of the Act for such service for such year, determined
without regard to geographic adjustment under section 1833(t)(2)(D), the Secretary shall
substitute the amount described in clause (ii), adjusted by the geographic adjustment factor under
the PFS, for the fee schedule amount for such TC for such year. As required by section
1848(b)(4)(A) of the Act, for imaging services furnished on or after January 1, 2007, we cap the
TC of the PFS payment amount for the year (prior to geographic adjustment) by the Outpatient
Prospective Payment System (OPPS) payment amount for the service (prior to geographic
adjustment). We then apply the PFS geographic adjustment to the capped payment amount.
Section 1848(b)(4)(B) of the Act defines imaging services as “imaging and computer-assisted
imaging services, including X-ray, ultrasound (including echocardiography), nuclear medicine
(including PET), magnetic resonance imaging (MRI), computed tomography (CT), and
fluoroscopy, but excluding diagnostic and screening mammography.” For more information
regarding the history of the cap on the TC of the PFS payment amount under the DRA (the
“OPPS cap”), we refer readers to the CY 2007 PFS final rule with comment period (71 FR 69659
through 69662).
For CY 2025, we identified new and revised codes to determine which services meet the
definition of “imaging services” as defined at section 1848(b)(4)(B) of the Act for purposes of
this cap. Beginning for CY 2025, we are proposing to include the following services on the list
of codes to which the OPPS cap applies: CPT codes 0868T (High-resolution gastric
electrophysiology mapping with simultaneous patient-symptom profiling, with interpretation and
report), 0876T (Duplex scan of hemodialysis fistula, computer-aided, limited (volume flow,
diameter, and depth, including only body of fistula)), 74263 (Computed tomographic (ct)
colonography, screening, including image postprocessing), 9X059 (Computerized ophthalmic
diagnostic imaging (eg, optical coherence tomography [OCT]), posterior segment, with
interpretation and report, unilateral or bilateral; retina including OCT angiography), 93X94
(Vasoreactivity study performed with transcranial Doppler study of intracranial arteries,
complete (List separately in addition to code for primary procedure)), 93X95 (Emboli detection
without intravenous microbubble injection performed with transcranial Doppler study of
intracranial arteries, complete (List separately in addition to code for primary procedure)), and
93X96 (Venous-arterial shunt detection with intravenous microbubble injection performed with
transcranial Doppler study of intracranial arteries, complete (List separately in addition to code
for primary procedure)). We believe that these codes meet the definition of imaging services
under section 1848(b)(4)(B) of the Act, and thus, should be subject to the OPPS cap.
In the CY 2024 PFS final rule (88 FR 78894), we noted that in response to the CY 2024
PFS proposed rule, commenters requested that CMS remove CPT code 92229 (Imaging of retina
for detection or monitoring of disease; point-of-care autonomous analysis and report, unilateral
or bilateral) from the OPPS cap list because it does not include an associated PC or physician
interpretation and it is primarily utilized in the physician office setting. We are soliciting
comment on the appropriateness of applying the OPPS cap to services such as this for which the
interpretation component is not captured by work RVUs, and the service is not split into
technical and professional components. We are more broadly evaluating how services involving
assistive technologies are most accurately valued. We note that the OPPS rate for this service is
currently higher than what would be paid in a physician office setting, and therefore the OPPS
cap does not currently apply to CPT code 92229 as of 2024.
4. Valuation of Specific Codes for CY 2025
(1) Skin Cell Suspension Autograft (CPT codes 15XX1, 15XX2, 15XX3, 15XX4, 15XX5,
15XX6, 15XX7, and 15XX8)
In September 2023, the CPT Editorial Panel approved the creation of eight new CPT
codes to describe skin cell suspension autograft (SCSA) procedures. The code set includes a 000-
day global base code (CPT code 15XX1 (Harvest of skin for skin cell suspension autograft; first
25 sq cm or less)) and an add-on code (CPT code 15XX2 (Harvest of skin for skin cell
suspension autograft; each additional 25 sq cm or part thereof (List separately in addition to
code for primary procedure))) describing the harvesting component of the procedure, an XXX
global base code (CPT code 15XX3 (Preparation of skin cell suspension autograft, requiring
enzymatic processing, manual mechanical disaggregation of skin cells, and filtration; first 25 sq
cm or less of harvested skin) and an add-on code (CPT code 15XX4 (Preparation of skin cell
suspension autograft, requiring enzymatic processing, manual mechanical disaggregation of
skin cells, and filtration; each additional 25 sq cm of harvested skin or part thereof (List
separately in addition to code for primary procedure))) describing the preparation component of
the procedure, and two 090-day global base codes and two add-on codes for the application
component to distinguish between body areas: trunk, arms, and legs with CPT codes 15XX5
(Application of skin cell suspension autograft to wound and donor sites, including application of
primary dressing, trunk, arms, legs; first 480 sq cm or less) and 15XX6 (Application of skin cell
suspension autograft to wound and donor sites, including application of primary dressing, trunk,
arms, legs; each additional 480 sq cm or part thereof (List separately in addition to code for
primary procedure)); and face, scalp, eyelids, mouth, neck, ears, orbits, genitalia, hands, feet, or
multiple digits with CPT codes 15XX7 (Application of skin cell suspension autograft to wound
and donor sites, including application of primary dressing, face, scalp, eyelids, mouth, neck,
ears, orbits, genitalia, hands, feet, and/or multiple digits; first 480 sq cm or less) and 15XX8
(Application of skin cell suspension autograft to wound and donor sites, including application of
primary dressing, face, scalp, eyelids, mouth, neck, ears, orbits, genitalia, hands, feet, and/or
multiple digits; each additional 480 sq cm or part thereof (List separately in addition to code for
primary procedure)).
We disagree with the RUC-recommended work RVUs of 3.00, 2.00, 2.51, 2.00, 10.97,
2.50, 12.50, and 3.00 for CPT codes 15XX1 through 15XX8, respectively, and are proposing
contractor-pricing for these CPT codes due to concerns with the coding structure of the code
family and the total physician time that results when these codes are billed multiple times on the
same date of service for the typical patient.
We note that our concerns with these CPT codes are expansive. Firstly, we note that these
CPT codes represent a segmentation of a single service that is performed sequentially on the
same date of service. We are seeking comment on whether the segmentation of the harvest,
preparation, and application is necessary when these are sequential service parts of one episode
of care, and could be simplified by having just two codes that encompass all three service parts
(harvest, preparation, and application), to differentiate the two different application areas. We
also are soliciting comment on the base and add-on codes’ incremental square centimeters,
considering that the typical size treatment area described in the vignettes could result in the add-
on codes being billed multiple times, particularly for the base application CPT code 15XX5 and
add-on CPT code 15XX6. Based on the meeting notes from the September 2023 CPT Editorial
Panel meeting, the specialty society initially structured their coding request to “bundle” the

service components into fewer codes, but it is unclear to us why these codes were further
segmented. We believe that the very large range of intraservice times from the 33 burn surgeons
may have been exacerbated by the harvest, preparation, and application components of the
service being segmented in this manner. Most notably, CPT code 15XX1, which describes the
first 25 sq cm of harvest, base code, had an intraservice survey time range of 5 to 480 minutes,
and CPT code 15XX7, which describes the first 480 sq cm of application to the face, scalp,
eyelids, mouth, neck, ears, orbits, genitalia, hands, feet, and/or multiple digits, had an
intraservice survey time range of 10 to 360 minutes.
We note that the survey median intraservice times for CPT codes 15XX1 through 15XX8
contradict numerous publicly available sources that describe much lower times for this service or
specific service parts. Most notably, the manufacturer of the RECELL Autologous Cell
Harvesting Device (RECELL® System) used in this service, indicates that a suspension of
Spray-On Skin™ Cells using a small sample of the patient’s own skin for the treatment of
thermal burn wounds and full-thickness skin defects is “prepared and applied at the point of care
in as little as 30 minutes.”
11
Additionally, Temple University Hospital published a news article
on December 20, 2019, just 11 months after the U.S. Food and Drug Administration (FDA)
approval of the RECELL® System for the treatment of acute thermal second and third-degree
burns in adult patients in January 2019, stating that the entire process of skin sample collection,
enzyme solution preparation, and suspension spraying/application “can take as little as 30
minutes” and “treat a wound up to 80 times the size of the donor skin sample.”
12
Additionally, an
article published in Europe PubMed Central states that the procedure takes approximately 30
minutes and is performed by a burn surgeon trained in how to use RECELL® System, and does
11
https://avitamedical.com/.
12
Temple Burn Center Using Spray-On SkinTM Cells Technology to Offer Patients a New, Less Invasive Option
for the Treatment of Severe Burns. (2019, December 20). https://medicine.temple.edu/news/temple-burn-center-
using-spray-skin-cells-technology-offer-patients-new-less-invasive-option.

not require specialized laboratory staff.
13
Additionally, a 2007 study aimed at comparing the
results from the RECELL® System and the classic skin grafting for epidermal replacement in
deep partial thickness burns showed a total procedure time of 59±4 minutes for the RECELL®
System group.
14
More granularly, the FDA’s Instructions for Use of the RECELL® Autologous Cell
Harvesting Device state that “if a skin sample is harvested and processed according to these
instructions, it should require between 15 and 30 minutes of contact with the Enzyme”.
15
Additionally, the National Institute for Health and Care Excellence (NICE) produced guidance
on using the RECELL® System based on the consideration of evidence submitted and the views
of expert advisers, and stated that the harvested skin is added to the proprietary enzyme solution
in a processing unit and heated for 15 to 30 minutes to disaggregate the cells. The skin is then
removed and scraped with a scalpel to develop a plume of cells. These cells are added to a buffer
solution, aspirated and filtered to create a cell suspension that contains keratinocytes,
melanocytes, fibroblasts and Langerhans cells.
16
This correlates to the preparation component of
the service described by CPT codes 15XX3 and 15XX4, for which the RUC recommended the
survey median time of 33 and 28 minutes, respectively.
We believe that the publicly available sources that make representations about the total
service and preparation times contradict the RUC-recommended median times based on the
survey of 33 burn surgeons. Moreover, when we considered how the add-on CPT codes 15XX2,
15XX4, 15XX6, and 15XX8 would be billed based on the typical patient described in the
13
Cooper-Jones B, Visintini S. A Noncultured Autologous Skin Cell Spray Graft for the Treatment of Burns. In:
CADTH Issues in Emerging Health Technologies. Canadian Agency for Drugs and Technologies in Health, Ottawa
(ON); 2016. PMID: 30855772.
14
G. Gravante, M.C. Di Fede, A. Araco, M. Grimaldi, B. De Angelis, A. Arpino, V. Cervelli, A. Montone, A
randomized trial comparing ReCell® system of epidermal cells delivery versus classic skin grafts for the treatment
of deep partial thickness burns, Burns, Volume 33, Issue 8, 2007, Pages 966-972, ISSN 0305-4179,
https://doi.org/10.1016/j.burns.2007.04.011.
15
https://www.fda.gov/media/169630/download.
16
National Institute for Health and Care Excellence. The ReCell Spray-On Skin system for treating skin loss,
scarring and depigmentation after burn injury. Medical technologies guidance [MTG21] [Internet]. 2014. [Accessed
16 Nov 2017]. https://www.nice.org.uk/guidance/mtg21/documents/the-recell-sprayon-skin-system-for-treating-
skin-loss-scarring-and-depigmentation-after-burn-injury-medical-technology-consultation-document.
vignettes, we believe the survey times are inflated compared to the publicly available sources,
likely due to how the survey respondents considered the service given the segmentation of the
code set. For example, the vignette for CPT code 15XX5 describing the application to the trunk,
arms, and legs says “A 35-year-old male sustained partial-thickness thermal burns on his trunk
and arms measuring 3,600 sq cm. A skin cell suspension autograft is applied to 480 sq cm of the
wound bed.” Of the 33 burn surgeons surveyed, 96 percent found this vignette to be typical.
Given the typical sq cm application area of 3,600 sq cm and the expansion ratio of harvested and
prepared skin to treatment skin for application of 1:80, the typical episode of care would
constitute 1 unit of both CPT codes 15XX1 and 15XX2 for harvesting, 1 unit of both CPT codes
15XX3 and 15XX4 for preparation, 1 unit of CPT code 15XX5 for the first 480 sq cm of
application, and 7 units of CPT code 15XX6 for the remaining 3,120 sq cm of application area.
When the RUC-recommended intraservice and total times (not including the post-operative visit
time for CPT code 15XX5) for all the units billed on the same date of service as sequential
service parts are summed, the intraservice time totals to 399 minutes and total time (not
including the post-operative visit time included in the global period for CPT code 15XX5) totals
to 529 minutes. The intraservice time total alone is nearly 6 and 2/3 hours.
We note the RUC recommended that CPT codes 15XX1 through 15XX8 be placed on the
New Technology list to be re-reviewed by the RUC for both work and PE for the September
2026 or January 2027 RUC meeting when 2025 Medicare utilization data is available, and at that
time, the RUC would consider if other specialties were performing the service and if the service
was performed in the non-facility setting. We look forward to re-reviewing these CPT codes
when recommendations are re-submitted with more robust and inclusive survey data. In the
meantime, we encourage the reconsideration of the family’s coding structure by the CPT
Editorial Panel given the challenging aspects of this service, including the fact that the current
coding structure represents a severely segmented single episode of care with troublesome billing
patterns for the typical patient, particularly for the add-on CPT code 15XX6 describing the
additional 480 sq cm increments of application on the trunk, arms, and legs. This code is
particularly concerning because the coding structure of the family requires 7 units of add-on CPT
code 15XX6 to be billed for the typical patient. Similarly, the typical patient described in the
vignettes for this family of codes would require 3 units of add-on CPT code 15XX8 due to the
coding structure.
We are also seeking feedback on the recommended global period for CPT code 15XX3.
The RUC recommended an XXX global period, which indicates that the global concept does not
apply, but we believe a 000-day global period, indicating an endoscopic or minor procedure with
related preoperative and postoperative relative values on the day of the procedure only in the fee
schedule payment amount, may be more appropriate given the nature of the service (which is
intertwined with the other codes in the series) and that the entire service cannot be completed
without 15XX3. This would allow the entire service to run within a surgical global period.
We note that we believe contractor-pricing is appropriate for CPT codes 15XX1 through
15XX8 until reconsideration of the coding structure and re-survey is complete, given the
concerning aspects of the CPT codes. We note that this service is currently billed for using
contractor-priced CPT code 17999 (Unlisted procedure, skin, mucous membrane and
subcutaneous tissue) and the eight new codes are expected to be a very low utilization.
(2) Hand, Wrist, & Forearm Repair & Recon (CPT codes 25310, 25447, 2X005, and 26480)
In September 2022, the RUC referred CPT codes 26480 and 25447 to the CPT Editorial
Panel for a code bundling solution. In May 2023, the CPT Editorial Panel approved a new
bundled code (CPT code 2X005) to report intercarpal or carpometacarpal joint suspension
arthroplasty, including transfer or transplant of tendon, with interposition when performed while
CPT code 25447 was revised to clarify that the code only included interposition of a tendon and
not suspension. This family of codes was surveyed for the September 2023 RUC meeting.
We disagree with the RUC-recommended work RVU of 9.50 for CPT code 25310
(Tendon transplantation or transfer, flexor or extensor, forearm and/or wrist, single; each
tendon) and we are instead proposing a work RVU of 9.00 based on the survey 25
th
percentile
result. In reviewing CPT code 25310, we noted that the recommended intraservice time was
unchanged at 60 minutes in the new survey; however, the RUC-recommended work RVU is
increasing from the current 8.08 to 9.50. Although we do not imply that changes in work time as
reflected in survey values must equate to a one-to-one or linear change in the valuation of work
RVUs, we believe that since the two components of work are time and intensity, increases in the
recommended work RVU should typically be reflected in increases in the surveyed work time.
We recognize that the total time for CPT code 25310 is increasing from 235 minutes to 263
minutes (an increase of 12 percent) due to changes in the code’s post-operative office visits
which will now take place at a higher level. However, this again does not match the increase in
the recommended work RVU, which is increasing from 8.08 to 9.50 (approximately 18 percent).
We believe that it would be more accurate to propose the survey 25
th
percentile work RVU of
9.00 for CPT code 25310 which matches this increase in the total work time. We also note that
the intensity of CPT code 25310 is decreasing, not increasing, as recommended by the RUC
which further suggests that a work RVU of 9.50 would not be appropriate for this code given the
surveyed work times.
We disagree with the RUC-recommended work RVU of 11.14 for CPT code 25447
(Arthroplasty, intercarpal or carpometacarpal joints; interposition (eg, tendon)) and we are
instead proposing a work RVU of 10.50 based on the survey 25
th
percentile result. In reviewing
CPT code 25447, we noted that the recommended intraservice time was decreasing from 100
minutes to 75 minutes in the new survey; however, the RUC recommended maintaining the
current work RVU of 11.14. Although we do not imply that changes in work time as reflected in
survey values must equate to a one-to-one or linear change in the valuation of work RVUs, we
believe that since the two components of work are time and intensity, decreases in the surveyed
work time should typically be reflected in decreases to the work RVU. We recognize that the
total time for CPT code 25447 is slightly increasing from 278 minutes to 281 minutes (an
increase of about 1 percent) due to changes in the code’s post-operative office visits which will
now take place at a higher level. However, we believe that the sizable decrease in surveyed
intraservice work time (a reduction of approximately 33 percent) better supports proposing the
survey 25
th
percentile work RVU of 10.50 instead of maintaining the current work RVU of
11.14. We also disagree with the RUC that the intensity of CPT code 25447 is unchanged due to
increases in the post-operative work; we believe that the sizable decrease in surveyed
intraservice work time indicates a modest decrease in intensity. We note again that the intensity
of CPT code 25310 is decreasing, not increasing, as recommended by the RUC which suggests
that a similar pattern is likely taking place with clinically similar procedures elsewhere in the
same code family.
We disagree with the RUC-recommended work RVU of 13.90 for CPT code 2X005
(Arthroplasty, intercarpal or carpometacarpal joints; suspension, including transfer or
transplant of tendon, with interposition, when performed) and we are instead proposing a work
RVU of 11.85 based on the survey 25
th
percentile result. We note that the RUC typically values
new codes such as CPT code 2X005 using this survey 25
th
percentile work RVU as opposed to
the survey median work RVU that it recommended. The RUC’s recommendations stated that
CPT code 2X005 should be valued higher than CPT code 25447 due to having higher intensity, a
relationship which is preserved at our proposed work RVUs of 11.85 and 10.50 respectively. The
RUC also stated in its recommendations that CPT code 2X005 should be valued higher than
reference CPT code 29828 (Arthroscopy, shoulder, surgical; biceps tenodesis) because it has
more intraservice time and total work time. However, the RUC also stated elsewhere in its
recommendations that the arthroscopy described by CPT code 29828 is more intense than the
arthroplasty procedures described by this family of codes, which we believe supports CPT code
29828 having a higher work RVU despite its lower work times. Based on this information, we
believe that proposing the survey 25
th
percentile work RVU of 11.85 is the most accurate
valuation for CPT code 2X005.
We disagree with the RUC-recommended work RVU of 9.50 for CPT code 26480
(Transfer or transplant of tendon, carpometacarpal area or dorsum of hand; without free graft,
each tendon) and we are instead proposing a work RVU of 9.00 based on the survey 25
th
percentile result. In reviewing CPT code 26480, we noted that the recommended intraservice
time was unchanged at 60 minutes in the new survey; however, the RUC-recommended work
RVU is increasing from the current 6.90 to 9.50. Although we do not imply that changes in work
time as reflected in survey values must equate to a one-to-one or linear change in the valuation of
work RVUs, we believe that since the two components of work are time and intensity, increases
in the recommended work RVU should typically be reflected in increases in the surveyed work
time. We recognize that the total time for CPT code 26480 is increasing from 227 minutes to 263
minutes (an increase of 16 percent) due to changes in the code’s post-operative office visits
which will now take place at a higher level. However, this again does not match the increase in
the recommended work RVU, which is increasing from 6.90 to 9.50 (approximately 38 percent).
We believe that it would be more accurate to propose the survey 25
th
percentile work RVU of
9.00 for CPT code 26480 which more closely matches this increase in the total work time. We
also note that CPT codes 25310 and 26480 were surveyed as having identical work times and
identical survey 25
th
percentile and survey median work RVUs. We concur with the RUC that
these two codes should be valued at the same work RVU, however we continue to believe that
the survey 25
th
percentile work RVU of 9.00 is a more accurate choice in both cases.
We are proposing the RUC-recommended direct PE inputs for all four codes in the family
without refinement.
(3) CAR-T Therapy Services (CPT codes 3X018, 3X019, 3X020, and 3X021)
In September 2023, the CPT Editorial Panel deleted four category III codes (0537T-
0540T) and approved the addition of four new codes (3X018-3X021) that describe only steps of
the complex CAR-T Therapy process performed and supervised by physicians. The RUC
recommended four different work RVUs for codes 3X018, 3X019, 3X020, and 3X021 and only
recommended direct PE values for code 3X021.
For CPT code 3X018 (Chimeric antigen receptor T-cell (CAR-T) therapy; harvesting of
blood-derived T lymphocytes for development of genetically modified autologous CAR-T cells,
per day) the RUC recommended a work RVU of 1.94. For CPT code 3X019 (Chimeric antigen
receptor T-cell (CAR-T) therapy; preparation of blood-derived T lymphocytes for transportation
(eg, cryopreservation, storage)) the RUC recommended a work RVU of 0.79. For CPT code
3X021 (Chimeric antigen receptor T-cell (CAR-T) therapy; CAR-T cell administration,
autologous) the RUC recommended a work RVU of 3.00. For CPT code 3X020 (Chimeric
antigen receptor T-cell (CAR-T) therapy; receipt and preparation of CAR-T cells for
administration) the RUC recommended a work RVU of 0.80 and for CPT code 3X020, we are
proposing the RUC-recommended work RVU of 0.80. We are proposing the RUC-recommended
work RVUs for CPT codes 3X018, 3X019, and 3X021 respectively.
As mentioned previously, the RUC recommended direct PE values for only one code,
CPT code 3X021, and the RUC recommended that the non-facility PE RVU for CPT codes
3X018-3X020 should be contractor-priced. However, contractor pricing can only be applied at
the whole code level, not to a single component of the valuation. Therefore, for CPT codes
3X018-3X020 we are treating these codes as having no recommended direct PE values and are
seeking comment on direct PE values for these codes. We are proposing the RUC-recommended
direct PE inputs for CPT code 3X021.
(4) Therapeutic Apheresis and Photopheresis (CPT codes 36514, 36516, and 36522)
In the CY 2024 PFS final rule, we finalized CPT codes 36514 (Therapeutic apheresis;
for plasma pheresis), 36516 (Therapeutic apheresis; with extracorporeal immunoadsorption,
selective adsorption or selective filtration and plasma reinfusion), and 36522 (Photopheresis,
extracorporeal) as potentially misvalued, as we believed there may have been a possible
disparity with the clinical labor type (88 FR 78848). As a result, the PE clinical labor type was
reviewed for these three codes at the January 2024 RUC meeting, with no work review. The PE
Subcommittee and the RUC agreed that clinical staff code L042A (RN/LPN) did not
appropriately represent the work of an Apheresis Nurse Specialist. There is not a clinical staff
code for an Apheresis Nurse Specialist; however, the RUC agreed with the specialty societies’
recommendation that the training and experience of an oncology nurse (clinical staff code
L056A, RN/OCN) would more accurately reflect the work of an apheresis nurse for these CPT
codes. The RUC submitted new PE recommendations for these three codes based on the use of
the L056A clinical labor type.
We are proposing the RUC-recommended direct PE inputs for CPT codes 36514, 36516,
and 36522 without refinement. The RUC did not make recommendations and we are not
proposing any changes to the work RVU for CPT codes 36514, 36516, and 36522.
(5) Intra-Abdominal Tumor Excision or Destruction (CPT codes 4X015, 4X016, 4X017, 4X018,
and 4X019)
In May 2023, the CPT Editorial Panel created five new codes to describe the sum of the
maximum length of intra-abdominal (that is, peritoneal, mesenteric, retroperitoneal), primary or
secondary tumor(s) or cyst(s) excised or destroyed: CPT code 4X015 (Excision or destruction,
open, intra-abdominal (i.e., peritoneal, mesenteric, retroperitoneal), primary or secondary
tumor(s) or cyst(s), sum of the maximum length of tumor(s) or cyst(s); 5 cm or less), CPT code
4X016 (Excision or destruction, open, intra-abdominal (i.e., peritoneal, mesenteric,
retroperitoneal), primary or secondary tumor(s) or cyst(s), sum of the maximum length of
tumor(s) or cyst(s); 5.1 to 10 cm), CPT code 4X017 (Excision or destruction, open, intra-
abdominal (i.e., peritoneal, mesenteric, retroperitoneal), primary or secondary tumor(s) or
cyst(s), sum of the maximum length of tumor(s) or cyst(s); 10.1 to 20 cm), CPT code 4X018
(Excision or destruction, open, intra-abdominal (i.e., peritoneal, mesenteric, retroperitoneal),
primary or secondary tumor(s) or cyst(s), sum of the maximum length of tumor(s) or cyst(s); 20.1
to 30 cm), and CPT code 4X019 (Excision or destruction, open, intra-abdominal (i.e., peritoneal,
mesenteric, retroperitoneal), primary or secondary tumor(s) or cyst(s), sum of the maximum
length of tumor(s) or cyst(s); greater than 30 cm). These new CPT codes will replace existing
CPT codes 49203 (Excision or destruction, open, intra-abdominal tumors, cysts or
endometriomas, 1 or more peritoneal, mesenteric, or retroperitoneal primary or secondary
tumors; largest tumor 5 cm diameter or less), 49204 (Excision or destruction, open, intra-
abdominal tumors, cysts or endometriomas, 1 or more peritoneal, mesenteric, or retroperitoneal
primary or secondary tumors; largest tumor 5.1-10.0 cm diameter), and 49205 (Excision or
destruction, open, intra-abdominal tumors, cysts or endometriomas, 1 or more peritoneal,
mesenteric, or retroperitoneal primary or secondary tumors; largest tumor greater than 10.0 cm
diameter) that described tumor excision or destruction based on the size of the single largest
tumor, cyst, or endometrioma removed, no matter the number of tumors. For CY 2025, the RUC
recommended a work RVU of 22.00 for CPT code 4X015, a work RVU of 28.65 for CPT code
4X016, a work RVU of 34.00 for CPT code 4X017, a work RVU of 45.00 for CPT code 4X018,
and a work RVU of 55.00 for CPT code 4X019.
We are proposing the RUC-recommended work RVUs of 22.00 for CPT code 4X015,
28.65 for CPT code 4X016, and 34.00 for CPT code 4X017.
We disagree with the RUC-recommended work RVU of 45.00 for CPT code 4X018 and
we are proposing a work RVU of 40.00 based on the survey 25
th
percentile. Compared to the
predecessor CPT code 49205, the intra-service time ratio for CPT code 4X018 suggests a work
RVU of 41.51 and the total time ratio suggests a work RVU of 38.02. These changes in surveyed
work time as compared with predecessor CPT code 49205 suggest that the recommended work
RVU of 45.00 is inappropriately high. We also note that the RUC recommended the survey 25
th
percentile work RVU for CPT codes 4X015, 4X016, and 4X017. Therefore, we believe that
proposing a work RVU of 40.00 for CPT code 4X018 keeps the valuation consistent with the
other CPT codes in this family. Our proposed work RVU of 40.00 for CPT code 4X018 is
supported by the following reference CPT codes with similar intra-service time (310 minutes)

and similar total time (814 minutes): reference CPT code 69970 (Removal of tumor, temporal
bone) with a work RVU of 32.41 with 330 minutes intra-service time and 793 minutes of total
time, and reference CPT code 33864 (Ascending aorta graft, with cardiopulmonary bypass with
valve suspension, with coronary reconstruction and valve-sparing aortic root remodeling (e.g.,
David Procedure, Yacoub Procedure)) with a work RVU of 60.80 with 300 minutes of intra-
service time and 838 minutes of total time. We believe the proposed work RVU of 40.00 is a
more appropriate value overall than 45.00 when compared to the range of codes with similar
intra-service time and similar total time.
We disagree with the RUC-recommended work RVU of 55.00 for CPT code 4X019 and
we are proposing a work RVU of 50.00 based on the survey 25
th
percentile. Compared to the
predecessor CPT code 49205, the intra-service time ratio for CPT code 4X019 suggests a work
RVU of 48.21 and the total time ratio suggests a work RVU of 48.86. These changes in surveyed
work time as compared with predecessor CPT code 49205 suggest that the recommended work
RVU of 55.00 is inappropriately high. We also note again that the RUC recommended the survey
25
th
percentile work RVU for CPT codes 4X015, 4X016, and 4X017. Therefore, we believe that
proposing a work RVU of 50.00 for CPT code 4X019 keeps the valuation consistent with the
other CPT codes in this family. Our proposed work RVU of 50.00 for CPT code 4X019 is
supported by the following reference CPT codes with similar intra-service time (360 minutes)
and similar total time (1,046 minutes): reference CPT code 61598 (Transpetrosal approach to
posterior cranial fossa, clivus or foramen magnum, including ligation of superior petrosal sinus
and/or sigmoid sinus) with a work RVU of 36.53 with 377.7 minutes intra-service time and
1,048.1 minutes of total time, and reference CPT code 47140 (Donor hepatectomy (including
cold preservation), from living donor; left lateral segment only (segments II and III)) with a
work RVU of 59.40 with 355 minutes of intra-service time and 1,073 minutes of total time. We
believe the proposed RVU of 50.00 is a more appropriate value overall than 55.00 when
compared to the range of codes with similar intra-service time and similar total time.
We also note that the RUC’s recommendations for the first three codes in the family
(CPT codes 4X015-4X017) maintained the same amount of intensity as their respective
predecessor codes, and in fact slightly decreased in intensity in the case of CPT codes 4X015 and
4X016. However, the RUC recommended a notable increase in intensity for CPT codes 4X018
and 4X019 over predecessor code 49205 due to its selection of the survey median work RVU in
both cases. We do not believe that this increase in intensity for CPT codes 4X018 and 4X019 is
warranted due to their clinical similarities to the previous coding in the family, especially given
that CPT code 49205 had the lowest intensity in the family. We believe that this intensity
argument further supports our choice to propose the survey 25
th
percentile work RVU for these
two codes, matching the RUC recommendations for CPT code 4X015-4X017.
We are proposing the RUC-recommended direct PE inputs for CPT codes 4X015, 4X016,
4X017, 4X018, and 4X019 without refinement.
(6) Bladder Neck and Prostate Procedures (CPT codes 5XX05 and 5XX06)
In September 2023, the CPT Editorial Panel created two Category I CPT codes to
describe the insertion or removal of a temporary device to remodel the bladder neck and prostate
using pressure to create necrosis and relieve lower urinary tract symptoms (LUTS) secondary to
benign prostate hyperplasia (BPH). These two new 000-day global Category I codes were
surveyed and reviewed for the January 2024 RUC meeting.
At the January 2024 RUC meeting, the specialty society indicated that CPT code
5XX05’s survey 25th percentile work RVU of 3.91 was too high for this procedure compared to
other services in the physician fee schedule with similar intra-service time. The specialty society
recommended, and the RUC agreed that the recommended work RVU for CPT code 5XX05
should be crosswalked to CPT code 52284 (Cystourethroscopy, with mechanical urethral
dilation and urethral therapeutic drug delivery by drug-coated balloon catheter for urethral
stricture or stenosis, male, including fluoroscopy, when performed). Because these procedures
are similar in intensity and both require precise placement of an intraurethral device, we concur
with the RUC and we are proposing the RUC recommended work RVU of 3.10 for CPT code
5XX05.
At the January 2024 RUC meeting, the specialty society indicated that CPT code
5XX06’s survey 25th percentile work RVU of 2.00 was too high for this procedure compared to
other services in the physician fee schedule with similar intra-service time. The specialty society
recommended, and the RUC agreed, that CPT code 5XX06 should have a direct work RVU
crosswalk to CPT code 27096 (Injection procedure for sacroiliac joint, anesthetic/steroid, with
image guidance (fluoroscopy or CT) including arthrography when performed). We are proposing
the RUC recommended work RVU of 1.48 for CPT code 5XX06.
We are also proposing the RUC-recommended direct PE inputs for CPT codes 5XX05
and 5XX06 without refinement. However, we note possible duplications in two of the supply
items within CPT code 5XX05. Specifically, supply item SB027 (gown, staff, impervious) is
already included in supply item SA042 (pack, cleaning and disinfecting, endoscope), and supply
item SB024 (gloves, sterile) is included in supply items SA058 (pack, urology cystoscopy visit).
We are seeking comments on whether a total of three SB027 impervious staff gowns and two
SB024 pairs of sterile gloves would be typical and necessary when providing this procedure.
(7) MRI-Monitored Transurethral Ultrasound Ablation of Prostate (CPT codes 5X006, 5X007,
and 5X008)
At the April 2023 CPT Editorial Panel meeting, three new CPT codes were approved for
MRI-monitored transurethral ultrasound ablation (TULSA). These codes were surveyed for the
September 2023 RUC meeting and recommendations submitted to CMS for inclusion in the CY
2025 PFS proposed rule.
For CY 2025, we are proposing the RUC-recommended work RVUs for all three CPT
codes. However, we note that interested parties may have concerns regarding the experience of
the survey respondents and the intra-service times provided in the survey data. We welcome
commenters to provide additional data that we could consider in the valuation of the work and
direct PE inputs for these CPT codes. We are proposing a work RVU of 4.05 for CPT code
5X006 (Insertion of transurethral ablation transducers for delivery of thermal ultrasound for
prostate tissue ablation, including suprapubic tube placement during the same session and
placement of an endorectal cooling device, when performed), a work RVU of 9.80 for CPT code
5X007 (Ablation of prostate tissue, transurethral, using thermal ultrasound, including magnetic
resonance imaging guidance for, and monitoring of, tissue ablation), and a work RVU of 11.50
for CPT code 5X008 (Ablation of prostate tissue, transurethral, using thermal ultrasound,
including magnetic resonance imaging guidance for, and monitoring of, tissue ablation; with
insertion of transurethral ultrasound transducers for delivery of the thermal ultrasound,
including suprapubic tube placement and placement of an endorectal cooling device, when
performed). We are also proposing the RUC-recommended direct PE inputs for CPT codes
5X006, 5X007, and 5X008 without refinement.
(8) Insertion of Cervical Dilator (CPT code 59200)
In the CY 2024 PFS final rule, we finalized CPT Code 59200 (Insertion of cervical
dilator (e.g., laminaria, prostaglandin) (separate procedure)) as potentially misvalued. The code
is to be used to report the total duration of time spent on a patient history and physical, reviewing
lab resulting, discussing risk and benefits of the procedure, obtaining consent, performing the
procedure, and assessing the patient post-procedure. The RUC reviewed the work RVU and PE
inputs for CPT code 59200 at their January 2024 meeting. We are proposing the RUC-
recommended work RVU of 1.20 for CPT code 59200. We are also proposing the RUC-
recommended direct PE inputs for CPT code 59200 without refinements.
(9) Guided High Intensity Focused Ultrasound (CPT code 6XX00)
In September 2023, the CPT Editorial Panel created a new Category I code to describe
magnetic resonance image guided high intensity focused ultrasound intracranial ablation for
treatment of a severe central tremor that is recalcitrant to other medical treatments. This service
is typically performed by a neurosurgeon without the involvement of a separate radiologist. This
new code replaces the existing Category III code 0398T.
We are not proposing the RUC-recommended work RVU of 18.95 for CPT code 6XX00
and are instead proposing a work RVU of 16.60 based on a crosswalk to CPT code 61626
(Transcatheter permanent occlusion or embolization (eg, for tumor destruction, to achieve
hemostasis, to occlude a vascular malformation), percutaneous, any method; non-central
nervous system, head or neck (extracranial, brachiocephalic branch)), which describes a similar
tumor destruction service that has similar time and intensity values to this service, and we
support this value by referencing CPT code 33889 (Open subclavian to carotid artery
transposition performed in conjunction with endovascular repair of descending thoracic aorta,
by neck incision, unilateral) and 33894 (Endovascular stent repair of coarctation of the
ascending, transverse, or descending thoracic or abdominal aorta, involving stent placement;
across major side branches). We do not believe that this service is significantly more intense
than the key reference codes, CPT codes 61736 (Laser interstitial thermal therapy (LITT) of
lesion, intracranial, including burr hole(s), with magnetic resonance imaging guidance, when
performed; single trajectory for 1 simple lesion) and 61737 (Laser interstitial thermal therapy
(LITT) of lesion, intracranial, including burr hole(s), with magnetic resonance imaging
guidance, when performed; multiple trajectories for multiple or complex lesion(s)), as the RUC-
recommended work value implies. Our proposed work RVU of 16.60 for CPT code 6XX00
largely matches the intensity of CPT code 61736 which we believe is a more accurate valuation
for this service, as opposed to the RUC recommendation which would have significantly more
intensity.
We are proposing the RUC-recommended direct PE inputs for CPT code 6XX00 without
refinement.
(10) Percutaneous Radiofrequency Ablation of Thyroid (CPT codes 6XX01 and 6XX02)
In January 2024, the RUC surveyed codes 6XX01 (Ablation of 1 or more thyroid
nodule(s), one lobe or the isthmus, percutaneous, including imaging guidance, radiofrequency)
and its respective add-on code 6XX02 (Ablation of 1 or more thyroid nodule(s), additional lobe,
percutaneous, with imaging guidance, radiofrequency (List separately in addition to code for
primary service) and recommended both work RVUs and PE values for this code family.
For CPT code 6XX01, the RUC recommended a work RVU of 5.75 and we are
proposing the RUC-recommended work RVU of 5.75.
For add-on code CPT 6XX02, the RUC recommended a work RVU of 4.25 and we are
proposing the RUC-recommended work RVU for this code. We are also proposing the RUC-
recommended direct PE values for both codes 6XX01 and 6XX02.
(11) Fascial Plane Blocks (CPT codes 6XX07, 6XX08, 6XX09, 6XX10, 6XX11, 6XX12, 64486,
64487, 64488, and 64489)
In September 2023, the CPT Editorial Panel created six new Category I CPT codes, CPT
code 6XX07 (Thoracic fascial plane block, unilateral; by injection(s), including imaging
guidance, when performed), 6XX08 (Thoracic fascial plane block, unilateral; by continuous
infusion(s), including imaging guidance, when performed), 6XX09 (Thoracic fascial plane
block, bilateral; by injection(s), including imaging guidance, when performed), 6XX10
(Thoracic fascial plane block, bilateral; by continuous infusion(s), including imaging guidance,
when performed), 6XX11 (Lower extremity fascial plane block, unilateral; by injection(s),
including imaging guidance, when performed), and 6XX12 (Lower extremity fascial plane block,
unilateral; by continuous infusion(s), including imaging guidance, when performed) to report
thoracic or lower extremity fascial plane blocks, typically used for post-operative pain
management. Four existing CPT codes describing transversus abdominis plane (TAP) blocks,
64486 (Transversus abdominis plane (TAP) block (abdominal plane block, rectus sheath block)
unilateral; by injection(s) (includes imaging guidance, when performed)), 64487 (Transversus
abdominis plane (TAP) block (abdominal plane block, rectus sheath block) unilateral; by
continuous infusion(s) (includes imaging guidance, when performed)), 64488 (Transversus
abdominis plane (TAP) block (abdominal plane block, rectus sheath block) bilateral; by
injections (includes imaging guidance, when performed)) 64489 (Transversus abdominis plane
(TAP) block (abdominal plane block, rectus sheath block) bilateral; by continuous infusions
(includes imaging guidance, when performed)), were included as part of this code family for
RUC review in January 2024.
We are proposing the RUC-recommended work RVU for all ten codes in this family. We
are proposing a work RVU of 1.50 for CPT code 6XX07, 1.74 for CPT code 6XX08, 1.67 for
CPT code 6XX09, 1.83 for CPT code 6XX10, 1.34 for CPT code 6XX11, 1.67 for CPT code
6XX12, 1.20 for CPT code 64486, 1.39 for CPT code 64487, 1.40 for CPT code 64488, and 1.75
for CPT code 64489.
We are also proposing the RUC recommended direct PE inputs for CPT codes 6XX08,
6XX09, 6XX10, 6XX12, 64487, 64488, and 64489. We disagree with one of the RUC
recommended direct PE inputs for CPT codes 6XX07, 6XX11, and 64486. The RUC stated they
believe that there is a rounding error in the CA019 clinical labor time, “Assist physician or other
qualified healthcare professional--directly related to physician work time (67%)”, for these three
codes. We disagree with the RUC that there are rounding errors in these codes and we are
proposing to maintain the current 7 minutes of CA019 clinical labor time for CPT codes 6XX07,
6XX11, and 64486. We note that this matches the pattern of CA019 clinical labor time for the
rest of the codes in the family, which remained the same or slightly decreased in each case. This
refinement to the CA019 clinical labor time also means that we are proposing a decrease of 0.5
minutes to the equipment time for the stretcher (EF018) and 3-channel ECG (EQ011) which
decreases from 25.5 to 25 minutes for these three codes. We are proposing all of the other RUC-
recommended direct PE inputs for CPT codes 6XX07, 6XX11, and 64486 without refinement.
(12) Skin Adhesives (CPT codes 64590 and 64595 and HCPCS codes G0168, G0516, G0517,
and G0518)
In April 2022, the RUC approved the use of SG007 (adhesive, skin (Dermabond)) for
CPT code 64590 (insertion or replacement of peripheral, sacral, or gastric neurostimulator
pulse generator or receiver, requiring pocket creation and connection between electrode array
and pulse generator or receiver) and 64595 (revision or removal of peripheral, sacral, or gastric
neurostimulator pulse generator or receiver, with detachable connection to electrode array). In
April 2023, the PE Subcommittee reviewed the following six codes on the Medicare Physician
Fee Schedule 64590, 64595, G0168, G0516, G0517, G0518 that utilize Dermabond (supply code
S6007) in order to identify justification for its use versus the generic version and present its
findings to the RUC for approval. The RUC reviewed all six codes for PE only and did not
submit work recommendations.
For CPT codes 64590 and 64595 and HCPCS code G0168 (Wound closure utilizing
tissue adhesive(s) only), the RUC recommends that CMS remove the supply input SG007
adhesive, skin (Dermabond) and add one unit of SH076 adhesive, cyanoacrylate (2ml uou). We
are proposing the RUC-recommended direct PE inputs for CPT codes 64590 and 64595 and
HCPCS code G0168. Similarly, for HCPCS codes G0516 (Insertion of non-biodegradable drug
delivery implants, 4 or more (services for subdermal rod implant), G0517 (Removal of non-
biodegradable drug delivery implants, 4 or more (services for subdermal implants), and G0518
(Removal with reinsertion, non-biodegradable drug delivery implants, 4 or more (services for
subdermal implants), the RUC recommends that CMS remove the supply input SG007 adhesive,
skin (Dermabond) and add one unit of SH076 adhesive, cyanoacrylate (2ml uou). We are
proposing the RUC-recommended direct PE inputs for HCPCS codes G0516-G0518.
(13) Iris Procedures (CPT codes 66680, 66682, and 6X004)
In April 2023, the CPT Editorial Panel deleted three related Category III CPT codes, CPT
code 0616T (Insertion of iris prosthesis, including suture fixation and repair or removal of iris,
when performed; without removal of crystalline lens or intraocular lens, without insertion of
intraocular lens), CPT code 0617T (with removal of crystalline lens and insertion of intraocular
lens), and CPT code 0618T (with secondary intraocular lens placement or intraocular lens
exchange). At the same time, CPT created a new Category I code 6X004 (Implantation of iris
prosthesis, including suture fixation and repair or removal of iris, when performed) which
describes insertion of an artificial iris into an eye with a partial or complete iris defect due to a
congenital defect or surgical or non-surgical trauma. The new Category I CPT code 6X004
replaced the three Category III codes to simplify reporting. Concurrent with these updates, the
RUC surveyed the two other 90-day global iris repair codes, CPT code 66680 (Repair of iris,
ciliary body (as for iridodialysis)) and CPT code 66682 (Suture of iris, ciliary body (separate
procedure) with retrieval of suture through small incision (eg, McCannel suture)).
We disagree with the RUC-recommended work RVU of 10.25 for CPT code 66680. We
are proposing a work RVU of 7.97 for CPT code 66680 based on a crosswalk to CPT code
67904 (Repair of blepharoptosis; (tarso) levator resection or advancement, external approach).
When we reviewed CPT code 66680, we found that the RUC recommended work RVU does not
maintain relativity with other 90-day global period codes with the same intraservice time of 45
minutes and similar total time around 182 minutes. The total time ratio between the current time
of 159 minutes and the recommended time established by the RUC survey of 182 minutes equals
1.145 percent. This ratio, 1.145 percent, when applied to the current work RVU of 6.39 would
suggest a work RVU of 7.31 which is far below the RUC’s recommended work RVU of 10.25.
Based on this total time ratio, we believe a more appropriate work valuation for CPT code 66680
is 7.97 based on a crosswalk to CPT code 67904.
We disagree with the RUC-recommended work RVU of 10.87 for CPT code 66682. We
are proposing a work RVU of 8.74 based on the total time ratio between the current time of
169.5 minutes and the recommended time established by the RUC survey of 202 minutes. This
ratio equals 1.192 percent, and 1.192 percent of the current work RVU of 7.33 suggests a work
RVU of 8.74 for CPT code 66682. When we reviewed CPT code 66682, we found that the
recommended work RVU was higher than nearly all of the other 90-day global codes with
similar time values. The RUC’s recommended work RVU does not maintain relativity with other
90-day global period codes with the same intraservice time value of 45 minutes and similar total
time of 202. We found that work RVU crosswalks to CPT codes of similar intraservice and total
time were too low, such as CPT code 45171 with a work RVU of 8.13. A more appropriate work
RVU for CPT code 66682 is 8.74 based on the total time ratio.
The RUC recommended a work RVU of 12.80 for CPT 6X004, the RUC survey 25th
percentile result, with an intraservice time of 60 minutes and a total time of 224 minutes. We
disagree with the RUC-recommended work RVU of 12.80 for CPT code 6X004. Although we
disagree with the RUC-recommended work RVU, we concur that the relative difference in work
between CPT codes 66682 and 6X004 is equivalent to the recommended interval of 1.93 RVUs.
Therefore, we are proposing a work RVU of 10.67 for CPT code 6X004, based on the
recommended interval of 1.93 additional RVUs above our proposed work RVU of 8.74 for CPT
code 66682. This proposed work RVU of 10.67 falls between the work RVU values of existing
codes with similar intraservice and total time values. For example, CPT code 65850 (60 minutes
of intraservice time and 233 minutes of total time) has a work RVU of 11.39 and CPT code
24164 with the same intraservice time and 228 minutes of total time has a work RVU of 10.00.
We believe that the work valuation of these CPT codes, which bracket our proposed work RVU
of 10.67, provide additional support for our proposed valuation.
We also disagree with the RUC’s recommended work RVUs for the codes in this family
because they suggest that there has been a tremendous increase in intensity as compared to how
these services have historically been valued. CPT code 66680 is more than doubling in intensity
at the RUC’s recommended work RVU of 10.25, which we do not believe to be the case given
that the code descriptor remains unchanged and the surveyed intraservice work time is
unchanged at 45 minutes. This same pattern holds true for CPT code 66682, which would be
increasing in intensity by more than 50% at the RUC’s recommended work RVU of 10.87, and
which similarly has no change in its code descriptor and a modest increase in its surveyed work
time. We concur that the intensity of these services has likely gone up over time, which is why
we are proposing modest intensity increases for both codes, however we continue to disagree
that the very substantial intensity increases recommended by the RUC would be accurate for this
code family. We believe that our proposed work RVUs are more in line with how these services
have historically been valued and better maintain relativity with the rest of the fee schedule.
We are proposing the direct PE inputs as recommended by the RUC for all three codes in
the family without refinement.
(14) Magnetic Resonance Examination Safety Procedures (CPT codes 7XX00, 7XX01, 7XX02,
7XX03, 7XX04, and 7XX05)
In September 2023, the CPT Editorial Panel created a new code family to describe
magnetic resonance (MR) examination safety procedures and capture the physician work
involving patients with implanted medical devices that require access to MR diagnostic
procedures: CPT code 7XX00 (MR safety implant and/or foreign body assessment by trained
clinical staff, including identification and verification of implant components from appropriate
sources (e.g., surgical reports, imaging reports, medical device databases, device vendors,
review of prior imaging), analyzing current MR conditional status of individual components and
systems, and consulting published professional guidance with written report; initial 15 minutes),
CPT code 7XX01 (MR safety implant and/or foreign body assessment by trained clinical staff,
including identification and verification of implant components from appropriate sources (e.g.,
surgical reports, imaging reports, medical device databases, device vendors, review of prior
imaging), analyzing current MR conditional status of individual components and systems, and
consulting published professional guidance with written report; each additional 30 minutes (List
separately in addition to code for primary procedure)), CPT code 7XX02 (MR safety
determination by a physician or other qualified health care professional responsible for the
safety of the MR procedure, including review of implant MR conditions for indicated MR exam,
analysis of risk versus clinical benefit of performing MR exam, and determination of MR
equipment, accessory equipment, and expertise required to perform examination with written
report), CPT code 7XX03 (MR safety medical physics examination customization, planning and
performance monitoring by medical physicist or MR safety expert, with review and analysis by
physician or qualified health care professional to prioritize and select views and imaging
sequences, to tailor MR acquisition specific to restrictive requirements or artifacts associated
with MR conditional implants or to mitigate risk of non-conditional implants or foreign bodies
with written report), CPT code 7XX04 (MR safety implant electronics preparation under
supervision of physician or other qualified health care professional, including MR-specific
programming of pulse generator and/or transmitter to verify device integrity, protection of
device internal circuitry from MR electromagnetic fields, and protection of patient from risks of
unintended stimulation or heating while in the MR room with written report), and CPT code
7XX05 (MR safety implant positioning and/or immobilization under supervision of physician or
qualified health care professional, including application of physical protections to secure
implanted medical device from MR-induced translational or vibrational forces, magnetically
induced functional changes, and/or prevention of radiofrequency burns from inadvertent tissue
contact while in the MR room with written report). For CY 2025, new CPT codes 7XX00 and
7XX01 are PE only services that represent the preparatory research and review completed by
clinical staff (that is, MRI technologist and/or a medical physicist) that will be utilized by the
physician or qualified health professional for the other four services (CPT codes 7XX02, 7XX03,
7XX04, and 7XX05) in this code family.
We are proposing the RUC-recommended work RVU of 0.60 for CPT code 7XX02, the
work RVU of 0.76 for CPT code 7XX03, the work RVU of 0.75 for CPT code 7XX04, and the
work RVU of 0.60 for CPT code 7XX05.
We are proposing the following refinements to the direct PE inputs. For CPT codes
7XX00, 7XX01, 7XX02, 7XX04, and 7XX05, we are proposing to refine the clinical labor for
the CA034 activity (Document procedure (nonPACS) (e.g. mandated reporting, registry logs,

EEG file, etc.)) performed by the MRI Technologist from 2 minutes to 1 minute. We note that
the clinical labor for the CA032 activity (Scan exam documents into PACS. Complete exam in
RIS system to populate images into work queue.) included in the direct PE inputs for reference
CPT code 70543 (Magnetic resonance (e.g., proton) imaging, orbit, face, and/or neck; without
contrast material(s), followed by contrast material(s) and further sequences) is a similar clinical
labor activity and has 1 minute of time. We also note that the Medical Physicist has 1 minute of
recommended clinical labor time for the CA034 activity for CPT code 7XX03. Therefore, we
believe that the MRI Technologist should have the same time (1 minute) for the CA034 activity
for the remaining codes in the family to maintain consistency across these services.
For CPT code 7XX01, we are proposing to refine the clinical labor for the CA021
activity (Perform procedure/service---NOT directly related to physician work time) from 27
minutes to 14 minutes. We believe this clinical labor time should be double the 7 minutes
assigned to the CA021 activity for CPT code 7XX00. The description for CPT code 7XX00 is
for the “initial 15 minutes” and CPT code 7XX01 is for “each additional 30 minutes,” that is,
double the time of CPT code 7XX00. We believe that the clinical labor associated with the
CA021 activity should match this pattern in which CPT code 7XX01 contains double the time of
CPT code 7XX00. This proposed refinement to the CA021 clinical labor also results in a
proposed decrease to the equipment time for the Technologist PACS workstation (ED050) from
45 minutes to 32 minutes.
For CPT code 7XX03, the RUC recommended 13 minutes of equipment time for the
Professional PACS Workstation (ED053) listed as a Facility PE input. We believe this was an
unintended technical error and we are proposing to remove this time from the direct PE inputs
for CPT code 7XX03.
For CPT codes 7XX04 and 7XX05, we proposing to refine the clinical labor time for the
CA024 activity (Clean room/equipment by clinical staff) from 2 minutes to 1 minute. According
to the PE recommendations, only the new equipment code EQ412 (Vitals monitoring system (MR
Conditional)) is being cleaned and not the entire room. We believe that 1 minute of clinical labor
time would be typical for cleaning the EQ412 equipment. Our proposed clinical labor refinement
also results in a proposed decrease to the equipment time for EL008 (room, MR) and EQ412 by 1
minute for these two codes.
For CPT code 7XX05, we are proposing to remove supply item SL082 (impression
material, dental putty (per bite block)). We believe this was an error since the PE
recommendations did not list SL082 as one of the included supplies for CPT code 7XX05 and it
does not appear as a supply input for any of the other codes in the family.
(15) Screening Virtual Colonoscopy (CPT code 74263)
As discussed in section III.K. of this proposed rule, we are proposing to exercise our
authority at section 1861(pp)(1)(D) of the Act to update and expand coverage for colorectal
cancer screening and adding coverage for the computed tomography colonography procedure.
Accordingly, we are assigning an active payment status for CPT code 74263 (Computed
tomographic (ct) colonography, screening, including image postprocessing). We note that, as
proposed above, the OPPS cap would apply to this code, and payment for the TC of this service
would be capped at the OPPS payment rate.
(16) Ultrasound Elastography (CPT codes 76981, 76982, and 76983)
This code family was flagged for re-review at the April 2023 RUC meeting by the new
technology/new services screen. Due to increased utilization of CPT code 76981 (Ultrasound,
elastography; parenchyma (eg, organ)), the entire code family was resurveyed for the September
2023 RUC meeting. We are proposing the RUC-recommended work RVUs of 0.59, 0.59, and
0.47 for CPT codes 76981, 76982 (Ultrasound, elastography; first target lesion), and 76983
(Ultrasound, elastography; each additional target lesion (List separately in addition to code for
primary procedure)), respectively. We are proposing the RUC-recommended direct PE inputs
for CPT codes 76981, 76982, and 76983 without refinement.
(17) CT Guidance Needle Placement (CPT code 77012)
CPT code 77012 (Computed tomography guidance for needle placement (eg, biopsy,
aspiration, injection, localization device), radiological supervision and interpretation) was
reviewed at the September 2023 RUC meeting to account for deferred updates to the vignette to
reflect the typical patient until updated utilization data was available to reflect coding changes
that occurred in 2019. We are proposing the RUC-recommended work RVU of 1.50 for CPT
code 77012.
We are proposing to refine the equipment time for the CT room (EL007) to maintain the
current time of 9 minutes. CPT code 77012 is a radiological supervision and interpretation
(RS&I) procedure and there has been a longstanding convention in the direct PE inputs, shared
by 38 other codes, to assign an equipment time of 9 minutes for the equipment room in these
procedures. We made the same refinement in the CY 2019 PFS final rule (83 FR 59553 through
59554) and continue to believe that it would not serve the interests of relativity to increase the
equipment time for the CT room in CPT code 77012 without also addressing the equipment
room time for the other radiological supervision and interpretation procedures. In response to the
CY 2019 proposal, several commenters stated that they agreed with CMS that other RS&I codes
use the 9 minutes for room time as a precedent, but that it is specific to angiographic rooms. We
agreed with the commenters that at least some portion of the procedure is performed in the CT
room, but we continue to believe that it would not serve the interests of relativity to increase the
equipment time for the CT room in CPT code 77012 without also addressing the equipment
room time for the other radiological supervision and interpretation procedures in a more
comprehensive fashion. We also disagreed with the commenters that this policy is specific to
angiography rooms, as CPT codes 75989 (Radiological guidance (ie, fluoroscopy, ultrasound, or
computed tomography), for percutaneous drainage (eg, abscess, specimen collection), with
placement of catheter, radiological supervision and interpretation) and 77012 both employ CT
rooms and currently utilize the standardized 9 minutes of equipment time, and CPT code 76080
(Radiologic examination, abscess, fistula or sinus tract study, radiological supervision and
interpretation) employs a radiographic-fluoroscopic room with the 9 minute standard equipment
time. We continue to believe that 9 minutes for EL007 is appropriate for this RS&I code,
therefore, we are proposing to maintain the current equipment room time of 9 minutes for EL007
until this group of procedures can be subject to a more comprehensive review. We are proposing
all other RUC-recommended direct PE inputs for CPT code 77012.
(18) Telemedicine Evaluation and Management (E/M) Services (CPT codes 9X075, 9X076,
9X077, 9X078, 9X079, 9X080, 9X081, 9X082, 9X083, 9X084, 9X085, 9X086, 9X087, 9X088,
9X089, 9X090, and 9X091)
In February 2023, the CPT Editorial Panel added a new Evaluation and Management
(E/M) subsection to the draft CPT codebook for Telemedicine Services. The Panel added 17
codes for reporting telemedicine E/M services: CPT code 9X075 (Synchronous audio-video visit
for the evaluation and management of a new patient, which requires a medically appropriate
history and/or examination and straightforward medical decision making. When using total time
on the date of the encounter for code selection, 15 minutes must be met or exceeded.); CPT code
9X076 (Synchronous audio-video visit for the evaluation and management of a new patient,
which requires a medically appropriate history and/or examination and low medical decision
making. When using total time on the date of the encounter for code selection, 30 minutes must
be met or exceeded.); CPT code 9X077 (Synchronous audio-video visit for the evaluation and
management of a new patient, which requires a medically appropriate history and/or
examination and moderate medical decision making. When using total time on the date of the
encounter for code selection, 45 minutes must be met or exceeded.); CPT code 9X078
(Synchronous audio-video visit for the evaluation and management of a new patient, which
requires a medically appropriate history and/or examination and high medical decision making.
When using total time on the date of the encounter for code selection, 60 minutes must be met or
exceeded. (For services 75 minutes or longer, use prolonged services code 99417)); CPT code
9X079 (Synchronous audio-video visit for the evaluation and management of an established
patient, which requires a medically appropriate history and/or examination and straightforward
medical decision making. When using total time on the date of the encounter for code selection,
10 minutes must be met or exceeded.); CPT code 9X080 (Synchronous audio-video visit for the
evaluation and management of an established patient, which requires a medically appropriate
history and/or examination and low medical decision making. When using total time on the date
of the encounter for code selection, 20 minutes must be met or exceeded.); CPT code 9X081
(Synchronous audio-video visit for the evaluation and management of an established patient,
which requires a medically appropriate history and/or examination and moderate medical
decision making. When using total time on the date of the encounter for code selection, 30
minutes must be met or exceeded.); CPT code 9X082 (Synchronous audio-video visit for the
evaluation and management of an established patient, which requires a medically appropriate
history and/or examination and high medical decision making. When using total time on the date
of the encounter for code selection, 40 minutes must be met or exceeded.); CPT code 9X083
(Synchronous audio-only visit for the evaluation and management of a new patient, which
requires a medically appropriate history and/or examination, straightforward medical decision
making, and more than 10 minutes of medical discussion. When using total time on the date of
the encounter for code selection, 15 minutes must be met or exceeded.)); CPT code 9X084
(Synchronous audio-only visit for the evaluation and management of a new patient, which
requires a medically appropriate history and/or examination, low medical decision making, and
more than 10 minutes of medical discussion. When using total time on the date of the encounter
for code selection, 30 minutes must be met or exceeded.)); CPT code 9X085 (Synchronous
audio-only visit for the evaluation and management of a new patient, which requires a medically
appropriate history and/or examination, moderate medical decision making, and more than 10
minutes of medical discussion. When using total time on the date of the encounter for code
selection, 45 minutes must be met or exceeded.); CPT code 9X086 (Synchronous audio-only visit
for the evaluation and management of a new patient, which requires a medically appropriate
history and/or examination, high medical decision making, and more than 10 minutes of medical
discussion. When using total time on the date of the encounter for code selection, 60 minutes
must be met or exceeded. (For services 75 minutes or longer, use prolonged services code
99417)); CPT code 9X087 (Synchronous audio-only visit for the evaluation and management of
an established patient, which requires a medically appropriate history and/or examination,
straightforward medical decision making, and more than 10 minutes of medical discussion.
When using total time on the date of the encounter for code selection, 10 minutes must be
exceeded.)); CPT code 9X088 (Synchronous audio-only visit for the evaluation and management
of an established patient, which requires a medically appropriate history and/or examination,
low medical decision making, and more than 10 minutes of medical discussion. When using total
time on the date of the encounter for code selection, 20 minutes must be met or exceeded.)); CPT
code 9X089 (Synchronous audio-only visit for the evaluation and management of an established
patient, which requires a medically appropriate history and/or examination, moderate medical
decision making, and more than 10 minutes of medical discussion. When using total time on the
date of the encounter for code selection, 30 minutes must be met or exceeded.)) CPT code 9X090
(Synchronous audio-only visit for the evaluation and management of an established patient,
which requires a medically appropriate history and/or examination, high medical decision
making, and more than 10 minutes of medical discussion. When using total time on the date of
the encounter for code selection, 40 minutes must be met or exceeded. (For services 55 minutes
or longer, use prolonged services code 99417)); CPT code 9X091 (Brief communication
technology-based service (eg, virtual check-in) by a physician or other qualified health care
professional who can report evaluation and management services, provided to an established
patient, not originating from a related evaluation and management service provided within the
previous 7 days nor leading to an evaluation and management service or procedure within the
next 24 hours or soonest available appointment, 5-10 minutes of medical discussion)).
In April 2023, the AMA-RUC noted that the survey instrument they used to develop
valuation recommendations for the telemedicine E/M codes did not include the time (when time
is used for code selection) in the new telemedicine E/M services descriptors, or the E/M services
displayed on the reference service list. The AMA-RUC made interim valuation recommendations
and conducted a new survey for September 2023, which included the minimum required times in
the code descriptors, and those minimum times were the same as appear in existing O/O E/M
services code descriptors (CPT codes 99202-99205, 99212-99215); the new survey in September
2023 included code descriptors and times approved by the CPT Editorial Panel in May 2023.
Also, additional specialties who perform E/M services participated in the second round of this
survey. For CY 2025, the RUC recommended the following work RVUs: a work RVU of 0.93
for CPT code 9X075, a work RVU of 1.6 for CPT code 9X076, a work RVU of 2.6 for CPT code
9X077, a work RVU of 3.50 for CPT code 9X078, a work RVU of 0.70 for CPT code 9X079, a
work RVU of 1.30 for CPT code 9X080, a work RVU of 1.92 for CPT code 9X081, a work
RVU of 2.60 for CPT code 9X082, a work RVU of 0.90 for CPT code 9X083, a work RVU of
1.60 for CPT code 9X084, a work RVU of 2.42 for CPT code 9X085, a work RVU of 3.20 for
CPT code 9X086, a work RVU of 0.65. for CPT code 9X087, a work RVU of 1.20 for CPT code
9X088.
In April 2023, the AMA-RUC Practice Expense Subcommittee approved the direct
practice expense inputs as recommended by the specialty societies without modification, and
CMS received these inputs as recommendations from the RUC. The specialty societies detailed
their methodology for making some changes to specific clinical activity codes to adapt those
clinical activity codes for telemedicine. The AMA edited both CA009 and CA013. The AMA
revision to CA009 deletes, “greet patient, provide gowning”; the AMA revision to CA013
deletes, “Prepare room, equipment and supplies”. CA009 now reads, “Ensure appropriate
medical records are available” and CA013 now reads, “Prepare patient for the visit (i.e. check
audio and/or visual”. The RUC, using the Practice Expense subcommittee recommendations,
also recommended to CMS that a camera and microphone “should be considered typical in the
computer contained in the indirect overhead expense.” This determination is consistent with
CMS’ longstanding position that items that are not specifically attributable to the individual
services should not be included for valuation of specific codes.
The AMA-RUC recommended the direct practice expense inputs as submitted by the
AMA-member specialty societies, and as affirmed by the AMA-RUC Practice Expense
Subcommittee. All supply and equipment costs were zeroed out from the reference services, and
as a result, the new telemedicine E/M codes did not include any supply or equipment costs in the
recommended direct practice expense inputs that the AMA submitted to CMS. The direct PE
inputs removed from the reference services to create the new telemedicine E/M codes are:
CA010 (obtain vital signs), CA024 (clean room/equipment by clinical staff), SA047 (pack, EM
visit), SM022 sanitizing cloth-wipe (surface, instruments, equipment), EQ189 (otoscope-
ophthalmoscope [wall unit]), EF048 (Portable stand-on scale), and EF023 (table, exam).
Sixteen of the telemedicine E/M codes describe use of either audio-video or audio-only
telecommunications technology to furnish the individual service. The CPT Editorial Panel
finalized eight codes for synchronous audio-video services (CPT codes 9X075 to 9X082), and
eight codes for synchronous audio-only services (CPT codes 9X083 to 9X0890), and one code
for an asynchronous service (CPT code 9X091). The audio-video and audio-only code family
subsets have parallel codes for new patients and established patients. Like other E/M codes, these
codes may be reported based on the level of medical decision making (MDM) or total time on
the date of the encounter. For each set of four codes, there is a code that may be reported for a
straightforward, low, moderate and high level of MDM.
The CPT Editorial Panel also established new CPT code 9X091 describing a brief virtual
check-in encounter that is intended to evaluate the need for a more extensive visit (that is, a visit
described by one of the office/outpatient E/M codes). The code descriptor for CPT code 9X091
mirrors existing HCPCS code G2012 (Brief communication technology-based service, e.g.
virtual check-in, by a physician or other qualified health care professional who can report
evaluation and management services, provided to an established patient, not originating from a
related e/m service provided within the previous 7 days nor leading to an e/m service or
procedure within the next 24 hours or soonest available appointment; 5-10 minutes of medical
discussion) and, per the CPT Editorial Panel materials, is intended to replace that code. As
described in CPT Editorial Panel final edits, CPT code 9X091 does not require the use of audio
or video technology and is expected to be patient-initiated. Furnishing the complete service
described by CPT code 9X091 must involve 5-10 minutes of medical discussion (and the code
descriptor does not include MDM as means of code selection). CPT code 9X091 should not be
reported if it originates from a related E/M service furnished within the previous 7 days, or, if the
clinical interaction leads to another E/M or procedure within the next 24 hours or the soonest
available appointment. The final CPT Editorial Panel draft language explains that if the virtual
check-in described by CPT 9X091 leads to an E/M visit in the next 24 hours, and if that E/M is
reported based on time, then the time from the virtual check-in may be added to the time of the
resulting E/M visit to determine the total time on the date of encounter for the resulting E/M. The
RUC recommended a work RVU of 0.30 for 9X091.
The CPT Editorial Panel also deleted three codes (99441-99443) for reporting telephone
E/M services. We note that CPT codes 99441, 99442, and 99443, each are assigned provisional
status on the Medicare telehealth services list, and would return to bundled status when the
telehealth flexibilities expire on December 31, 2024. For further background, we refer readers to
our discussions in previous rulemaking, where CMS explains the rationale for this policy (88 FR
78871-78878).
CMS has a long-standing interpretation of section 1834(m) of the Act as specifying the
circumstances under which Medicare makes payment for services that would otherwise be
furnished in person but are instead furnished via telecommunications technology. Specifically,
section 1834(m)(2)(A) of the Act expressly requires payment to the distant site physician or
practitioner of an amount equal to the amount that such physician or practitioner would have
been paid had such service been furnished without the use of a telecommunications system. This
means that we must pay an equal amount for a service furnished using a “telecommunications
system” as for a service furnished in person (without the use of a telecommunications system). In
the CY 2019 PFS final rule, we stated that “[w]e have come to believe that section 1834(m) of
the Act does not apply to all kinds of physicians’ services whereby a medical professional
interacts with a patient via remote communication technology. Instead, we believe that section
1834(m) of the Act applies to a discrete set of physicians’ services that ordinarily involve, and
are defined, coded, and paid for as if they were furnished during an in-person encounter between
a patient and a health care professional” (83 FR 59483). Under this interpretation, services that
are coded and valued based on the understanding that they are not ordinarily furnished in person,
such as remote monitoring services and communication technology-based services, are not
considered Medicare telehealth services under section 1834(m) of the Act and thus not subject to
the geographic, site of service, and practitioner restrictions included therein.
Information provided to CMS from the RUC indicates that CPT codes 9X075-9X090
describe services that would otherwise be furnished in person, and as such the services described
by these codes are subject to section 1834(m) of the Act. In the summary of the coding changes,
the AMA states that these services are “patterned after the in-person office visit codes.” The draft
CPT prefatory language states that “[t]elemedicine services are used in lieu of an in-person
service when medically appropriate to address the care of the patient and when the patient and/or
family/caregiver agree to this format of care.” The draft CPT prefatory language likewise states
that when a telemedicine E/M is billed on the same day as another E/M service “the elements
and time of these services are summed and reported in aggregate, ensuring that any overlapping
time is only counted once,” which indicates that the work of the telemedicine E/M service is
identical to the work associated with an in-person, non-telehealth E/M. The code descriptors and
requirements for billing the codes generally mirror the existing office/outpatient E/M codes with
the exception of the technological modality used to furnish the service. The audio-video
telemedicine E/M codes have nearly identical recommended work RVUs to parallel
office/outpatient E/M codes. In general, the audio-only telemedicine E/M codes have lower
recommended work RVUs than parallel office/outpatient E/M codes. The RUC stated that this is
because, when surveyed, specialty societies indicated that “the audio-video and in-person office
visits require more physician work than the audio-only office visits.”
Table 10 describes the similarities between 16 of 17 telemedicine E/M codes and the
parallel office/outpatient E/M codes. The table shows that except for the element of “modality”
(that is, audio-video or audio-only), the service elements of the new telemedicine E/M code
family are no different than the O/O E/M codes (for each enumerated row 1 through 16 the
columns display the analogous elements). When comparing code descriptors, as described at the
start of this section,, the only difference (as represented in Table 10 when comparing the
elements of E/M services represented by columns C, D, E, and F) is that these new telemedicine
E/M code descriptors lead with the phrase “synchronous audio-video” or “synchronous audio
only” before describing the visit in full exactly as the existing office/outpatient E/M visit codes
describe a visit in the long descriptor of the analogous service.

TABLE 10: Comparison of Elements and Work RVU between Telemedicine E/M
Codes (9X075 through 9X090) and Office/Outpatient E/M Codes (99202 through 99215)
A
B
C
D
E
F
G
H
Telemedicine
E/M HCPCS
RUC-
recommended
Work RVU
Modality
Level of Medical
Decision-
Making
Time
Threshold
(minutes)
New or
Established
Patient?
Analogous
Current
Office/Out
patient
E/M Code
Current
Work
RVU
1
9X075
0.93
Audio/Vi
deo (A/V)
Straightforward
15
new
99202
0.93
2
9X076
1.60
A/V
Low
30
new
99203
1.60
3
9X077
2.60
A/V
Moderate
45
new
99204
2.60
4
9X078
3.50
A/V
High
60
new
99205
3.50
5
9X079
0.70
A/V
Straightforward
10
established
99212
0.70
6
9X080
1.30
A/V
Low
20
established
99213
1.30
7
9X081
1.92
A/V
Moderate
30
established
99214
1.92
8
9X082
2.60
A/V
High
40
established
99215
2.80
9
9X083
0.90
Audio-
only
Straightforward
15
new
99202
0.93
10
9X084
1.60
Audio-
only
Low
30
new
99203
1.60
11
9X085
2.42
Audio-
only
Moderate
45
new
99204
2.60
12
9X086
3.20
Audio-
only
High
60
new
99205
3.50
13
9X087
0.65
Audio-
only
Straightforward
10
established
99212
0.70
14
9X088
1.20
Audio-
only
Low
20
established
99213
1.30
15
9X089
1.75
Audio-
only
Moderate
30
established
99214
1.92
16
9X090
2.60
Audio-
only
High
40
established
99215
2.80
There are services already describing audio-video and audio-only telemedicine E/M
codes on the Medicare telehealth services list—the office/outpatient E/M code set—that can be
furnished via synchronous two-way, audio/video communication technology generally and via
audio-only communication technology under certain circumstances to furnish Medicare
telehealth services in the patient’s home for the purpose of diagnosis and treatment of a mental
health disorder or SUD. Additionally, as stated above, section 1834(m)(2)(A) of the Act requires
us to pay an equal amount for a service furnished using a “telecommunications system” as for a
service furnished in person (without the use of a telecommunications system). Were we to accept
the AMA’s recommendations and add the telemedicine E/M codes to the Medicare telehealth
services list, we would need to establish RVUs for the telemedicine E/M codes to equal the
corresponding non-telehealth services to satisfy the requirements for payment under section
1834(m)(2)(A) of the Act.
We do not believe that there is a programmatic need to recognize the audio/video and
audio-only telemedicine E/M codes for payment under Medicare. We are proposing to assign
CPT codes 9X075-9X090 a Procedure Status indicator of “I”, meaning that there is a more
specific code that should be used for purposes of Medicare, which in this case would be the
existing office/outpatient E/M codes currently on the Medicare telehealth services list when
billed with the appropriate POS code to identify the location of the beneficiary and, when
applicable, the appropriate modifier to identify the service as being furnished via audio-only
communication technology.
Section 4113 of the Consolidated Appropriations Act (CAA), 2023 extended the
availability of Medicare telehealth services to beneficiaries regardless of geographic location or
site of service by temporarily removing such statutory restrictions under section 1834(m) of the
Act until the end of 2024. Under the current statute, the geographic location and site of service
restrictions on Medicare telehealth services will once again take effect for services furnished
beginning January 1, 2025. Although there are some important exceptions, including for
behavioral health services and ESRD-related clinical assessments, most Medicare telehealth
services will once again, in general, be available only to beneficiaries in rural areas and only
when the patient is located in certain types of medical settings. As previously discussed, the
introduction of new CPT coding to describe telemedicine E/M services does not change our
authority to pay for visits furnished through interactive communications technology in
accordance with section 1834(m) of the Act. We recognize that there are significant concerns
about maintaining access to care through the use of Medicare telehealth services with the
expiration of the statutory flexibilities that were successively extended by legislation following
the PHE for COVID-19. We understand that millions of Medicare beneficiaries have utilized
interactive communications technology for visits with practitioners for a broad range of health
care needs for almost 5 years. We are seeking comment from interested parties on our
understanding of the applicability of section 1834(m) of the Act to the new telemedicine E/M
codes, and how we might potentially mitigate negative impact from the expiring telehealth
flexibilities, preserve some access, and assess the magnitude of potential reductions in access and
utilization. On the latter point, we note that we have developed proposed PFS payment rates for
CY 2025, including the statutory budget neutrality adjustment, based on the presumption that
changes in telehealth utilization will not affect overall service utilization. We also note that
historically we have not considered changes in the Medicare telehealth policies to result in
significant impact on utilization such that a budget neutrality adjustment would be
warranted. However, we are unsure of the continuing validity of that premise under the current
circumstances where patients have grown accustomed over several years to broad access to
services via telehealth. We are seeking comment on what impact, if any, the expiration of the
current flexibilities would be expected to have on overall service utilization for CY 2025. We
refer readers to section IV.B. of this proposed rule for our discussion of budget neutrality
adjustments.
Given the similarity between CPT code 9X091 and HCPCS code G2012, we are
proposing to accept the RUC-recommended values for CPT code 9X091, and we are proposing
to delete HCPCS code G2012. For CPT code 9X091, we propose to accept the RUC-
recommended work RVU of 0.30, and are proposing the RUC-recommended direct PE inputs.
We note that our proposal does maintain the same direct PE inputs, which the RUC
recommendations leave unchanged from the current G2012 in total amount, and allocate the
same 3 minutes of time to the same level of staff (Clinical Staff code L037D, RN/LPN/MTA).
We believe that the coding and payment recommendations for CPT code 9X091, submitted to
CMS by the AMA RUC, accurately reflect the resources associated with this service and believe
that maintaining separate coding for purposes of Medicare payment could create confusion. We
note that, similar to our current policy for payment of HCPCS code G2012, CPT code 9X091
would be considered a communication technology-based service that is not subject to the
requirements in section 1834(m) of the Act applicable to Medicare telehealth services.
(19) Genetic Counseling Services (CPT code 9X100)
In September 2023, the CPT Editorial Panel deleted CPT code 96040 (Medical genetics
and genetic counseling services, each 30 minutes face-to-face with patient/family) and created
CPT code 9X100 (Medical genetics and genetic counseling services, each 30 minutes of total
time provided by the genetic counselor on the date of the encounter) for medical genetics and
genetic counseling services to be provided by the genetic counselor. Prior to its deletion, CPT
code 96040 would only be reported by genetic counselors for genetic counseling services, though
genetic counselors are not among the practitioners who can bill Medicare directly for their
professional services. As we stated in the CY 2012 PFS final rule (76 FR 73096 through 73097),
physicians and NPPs who may independently bill Medicare for their services and who are
counseling individuals would generally report office or other outpatient E/M CPT codes for
office visits that involve significant counseling, including genetic counseling; therefore CPT
code 96040 was considered bundled into O/O E/M visits.
For CPT code 9X100, we are proposing the RUC-recommended direct PE inputs. We
note that the code descriptor now specifies that the service is provided by a genetic counselor,
therefore we considered assigning Procedure Status “X” to CPT code 9X100. Because the PE
RVUs would not display for the code with that assignment and that may impact access to the
service with other payors, we are instead proposing bundled status (Procedure Status “B”) for
CPT code 9X100 to maintain the status of predecessor CPT code 96040, and we are seeking
feedback from interested parties regarding the appropriate procedure status for this code. CPT
guidelines for CPT code 9X100 state that a physician or other qualified healthcare professional
(QHP) who may report evaluation and management services would not be able to report CPT
code 9X100. Instead, these physicians and QHPs would use the appropriate evaluation and
management code.
(20) COVID Immunization Administration (CPT code 90480)
On August 14, 2023, new CPT codes were created to consolidate over 50 previously
implemented codes and streamline the reporting of immunizations for the novel coronavirus
(SARS-CoV-2, also known as COVID-19). The CPT Editorial Panel approved the addition of a
single administration code (CPT code 90480) for administration of new and existing COVID-19
vaccine products. The RUC reviewed the specialty societies’ recommendations for this code at
the September 2023 RUC meeting.
We are proposing the RUC-recommended work RVU of 0.25 for CPT code 90480
(Immunization administration by intramuscular injection of severe acute respiratory syndrome
coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, single dose). We are
also proposing the RUC-recommended direct PE inputs for CPT code 90480 without refinement.
(21) Optical Coherence Tomography (CPT codes 92132, 92133, 92134, and 9X059)
At the February 2023 CPT Editorial Panel meeting, CPT code 9X059 (Computerized
ophthalmic diagnostic imaging (eg, optical coherence tomography [OCT]), posterior segment,
with interpretation and report, unilateral or bilateral; retina including OCT angiography) was
created in response to new technology that allows imaging of the retina using optical coherence
tomography (OCT) with and without non-dye OCT angiography (OCT-A). This code family also
includes CPT code 92132 (Computerized ophthalmic diagnostic imaging (eg, optical coherence
tomography [OCT]), anterior segment, with interpretation and report, unilateral or bilateral),
CPT code 92133 (Computerized ophthalmic diagnostic imaging (eg, optical coherence
tomography [OCT]), posterior segment, with interpretation and report, unilateral or bilateral;
optic nerve) , and CPT code 92134 (Computerized ophthalmic diagnostic imaging (eg, optical
coherence tomography [OCT]), posterior segment, with interpretation and report, unilateral or
bilateral; retina). These codes were reviewed at the April 2023 RUC meeting. The RUC
determined the survey results were inaccurate due to underestimation of time, so the entire code
family was re-surveyed and reviewed at the September 2023 RUC meeting.
We are proposing the RUC-recommended work RVUs for all codes within the Optical
Coherence Tomography code family. We are proposing a work RVU of 0.29 for CPT code
92132, a work RVU of 0.31 for CPT code 92133, a work RVU of 0.32 for CPT code 92134, and
a work RVU of 0.64 for CPT code 9X059. We are also proposing the RUC-recommended direct
PE inputs for all four codes in the family.
(22) Transcranial Doppler Studies (CPT codes 93886, 93888, 93892, 93893, 93X94, 93X95,
93X96, and 93890)
The RUC’s Relativity Assessment Workgroup (RAW) requested action plans in
September 2022 to determine if specific code bundling solutions should occur for CPT codes
93890/93886, 93890/93892, 93892/93886, and 93892/93890. The RAW referred this issue to the
CPT Editorial Panel which created three new add-on codes to report when additional studies are
performed on the same date of services as a complete transcranial Doppler study. The RUC
reviewed these three new add-on codes, as well as CPT codes 93886, 93888, 93892 and 93893
for the September 2023 RUC meeting.
We are proposing the RUC-recommended work RVU for all seven codes in the
Transcranial Doppler Studies code family. We are proposing a work RVU of 0.90 for CPT code
93886 (Transcranial Doppler study of the intracranial arteries; complete study), a work RVU of
0.73 for CPT code 93888 (Transcranial Doppler study of the intracranial arteries; limited
study), a work RVU of 1.15 for CPT code 93892 (Transcranial Doppler study of the intracranial
arteries; emboli detection without intravenous microbubble injection), a work RVU of 1.15 for
CPT code 93893 (Transcranial Doppler study of the intracranial arteries; venous-arterial shunt
detection with intravenous microbubble injection), a work RVU of 0.81 for CPT code 93X94
(Vasoreactivity study performed with transcranial Doppler study of intracranial arteries,
complete), a work RVU of 0.73 for CPT code 93X95 (Emboli detection without intravenous
microbubble injection performed with transcranial Doppler study of intracranial arteries,
complete), and a work RVU of 0.85 for CPT code 93X96 (Venous-arterial shunt detection with
intravenous microbubble injection performed with transcranial Doppler study of intracranial
arteries, complete). We are also proposing the direct PE inputs as recommended by the RUC for
all seven codes in this family.
We note that the billing instructions for this code family specify that the three new add-
on codes should be used in conjunction with CPT code 93886, and that CPT code 93888 should
not be used in conjunction with CPT codes 93886, 93892, 93893, 93X94, 93X95, and 93X96.
However, we believe that it would be beneficial for the CPT Editorial Panel to state more
explicitly that CPT code 93X95 should not be used in conjunction with CPT code 93892 and that
CPT code 93X96 should not be used in conjunction with CPT code 93893. The work performed
in the add-on codes would be duplicative of the base codes in these situations and result in
unnecessary overbilling of services.
(23) RSV Monoclonal Antibody Administration (CPT codes 96380 and 96381)
At the September 2023 CPT meeting, the CPT Editorial Panel created two codes to report
passive administration of respiratory syncytial virus, monoclonal antibody, seasonal dose, with
and without counseling. CPT codes 96380 and 96381 were reviewed the following week at the
September 2023 RUC meeting and the RUC submitted recommendations to CMS.
We are proposing the RUC-recommended work RVU of 0.24 for CPT code 96380
(Administration of respiratory syncytial virus, monoclonal antibody, seasonal dose by
intramuscular injection, with counseling by physician or other qualified health care
professional) and the RUC-recommended work RVU of 0.17 for CPT code 96381
(Administration of respiratory syncytial virus, monoclonal antibody, seasonal dose by
intramuscular injection). We understand that these are interim work recommendations from the
RUC, and that the RUC intends to conduct a more complete review at a future RUC meeting
which we would then consider in future rulemaking. We are also proposing the direct PE inputs
as recommended by the RUC for both codes.
(24) Hyperthermic Intraperitoneal Chemotherapy (CPT codes 96547 and 96548)
In September 2022, the CPT Editorial Panel created two time-based add-on Category I
codes, CPT code 96547 (Intraoperative hyperthermic intraperitoneal chemotherapy (HIPEC)
procedure, including separate incision(s) and closure, when performed; first 60 minutes (List
separately in addition to code for primary procedure)) and CPT code 96548 (Intraoperative
hyperthermic intraperitoneal chemotherapy (HIPEC) procedure, including separate incision(s)
and closure, when performed; each additional 30 minutes (List separately in addition to code for
primary procedure)), to report HIPEC procedures for 2024. At the January 2023 RUC meeting,
the RUC reached the conclusion that the survey data was flawed due to a lack of work definition
and guidelines, and the RUC recommended contractor pricing for CPT codes 96547 and 96548
for CY 2024 with further clarification from the CPT editorial panel. CMS proposed and finalized
contractor pricing for CPT codes 96547 and 96548 for 2024. At the May 2023 CPT Editorial
Panel meeting, new guidelines and descriptions of work activities were approved and the codes
were resurveyed for the September 2023 RUC meeting with recommendations for national
pricing.
We are proposing the RUC-recommended work RVU of 6.53 for CPT code 96547 and
the RUC-recommended work RVU of 3.00 for CPT code 96548. The RUC did not recommend,
and we are not proposing, any direct PE inputs for the Hyperthermic Intraperitoneal
Chemotherapy codes (CPT codes 96547 and 96548).
(25) Laser Treatment - Skin (CPT codes 96920, 96921, and 96922)
In April 2022, the RUC referred CPT codes 96920 (Excimer laser treatment for
psoriasis; total area less than 250 sq cm), 96921 (Excimer laser treatment for psoriasis; 250 sq
cm to 500 sq cm), and 96922 (Excimer laser treatment for psoriasis; over 500 sq cm) to the CPT
Editorial Panel to capture expanded indications beyond what was currently noted in the codes’
descriptions to include laser treatment for other inflammatory skin disorders such as vitiligo,
atopic dermatitis, and alopecia areata, which could result in changed physician work based on the
expanded indications. The coding change application was subsequently withdrawn from the
September 2023 CPT Editorial meeting when it was determined that existing literature was
insufficient and did not support expanded indications at that time. Therefore, these CPT codes
were re-surveyed and reviewed at the April 2023 RUC meeting without any revisions to their
code descriptors.
We disagree with the RUC-recommended work RVUs for CPT codes 96920, 96921, and
96922 of 1.00, 1.07, and 1.32, respectively. The RUC noted that there have been multiple
reviews of these CPT codes, and the valuation of the codes is currently based on the original
valuation over two decades ago in 2002 where the physician time values were lower than the
current times. A subsequent review in 2012 adopted new survey times while maintaining the
work RVUs from 2002 for CPT codes 96920 and 96922. The RUC noted that, for both CPT code
96920 and 96922 with the largest treatment area, the total times have not changed since first
implemented more than 20 years ago. While we understand that the physician times have
fluctuated over the course of several years and several reviews, yet the work RVUs have
remained mostly constant as shown in Table 11, this was not addressed in the 2012
recommendations, and we believe that our operating assumption regarding the validity of the
existing values as a point of comparison is critical to the integrity of the relative value system as
currently constructed. The work times currently associated with codes play a very important role
in PFS ratesetting, both as points of comparison in establishing work RVUs and in the allocation
of indirect PE RVUs by specialty. If we were to operate under the assumption that previously
recommended work times had been routinely over or underestimated, this would undermine the
relativity of the work RVUs on the PFS in general, in light of the fact that codes are often valued
based on comparisons to other codes with similar work times. We also believe that, since the two
components of work are time and intensity, absent an obvious or explicitly stated rationale for

why the relative intensity of a given procedure has increased, significant decreases in time
should be reflected in decreases to work RVUs.
TABLE 11: Physician Time and RVUs for CPT Codes 96920, 96921, and 96922
CPT Code
Intraservice
Time
Total Time
RUC
Recommended Work
RVU
2002
17
27
1.15
Current (from 2012)
23
35
1.15
96920
Recommended
10
23
1.00
2002
20
30
1.17
Current (from 2012)
30
42
1.30
96921
Recommended
12
25
1.07
2002
30
40
2.10
Current (from 2012)
45
57
2.10
96922
Recommended
18
31
1.32
For CPT code 96920, we are proposing a work RVU of 0.83 based on a crosswalk to
CPT code 11104 (Punch biopsy of skin (including simple closure, when performed); single
lesion), which has the same 10 minutes of intraservice time and 23 minutes of total time as CPT
code 96920. We note that of the 15 other 000-day global codes with a total time of 20 to 25
minutes, only four codes fall above the RUC-recommended work RVU of 1.00. While we
understand that commenters will dispute the validity of the current time values, we note that the
2002 intraservice time was 17 minutes, which yields an intraservice time ratio between the 2002
intraservice time and the recommended intraservice time of 10 minutes of 0.68 work RVUs ((10
minutes/17 minutes) * 1.15). We note our proposed work RVU of 0.83 maintains the intensity
associated with the 2002 review of CPT code 96920, which we believe to be more appropriate
than the significant increase in intensity that results from the RUC-recommended work RVU of
1.00 which nearly doubles the current intensity of the code. We have no evidence to indicate that
the intensity of CPT code 96920 is increasing to this degree given how the surveyed work time is
substantially decreasing.
For CPT code 96921, we are proposing a work RVU of 0.90 based on a total time ratio to
CPT code 96920 ((25/23)*0.83) and a crosswalk to CPT code 11301 (Shaving of epidermal or
dermal lesion, single lesion, trunk, arms or legs; lesion diameter 0.6 to 1.0 cm), which has 3
additional minutes of intraservice time and 1 additional minute of total time compared to CPT
code 96921. We also note that our proposed work RVU of 0.90 for CPT code 96921 maintains
the RUC-recommended incremental difference between CPT codes 96920 and 96921 of 0.07
work RVUs. Like CPT code 96920, we understand that commenters will dispute the validity of
the current time values, but we note that the 2002 intraservice time was 20 minutes, which yields
an intraservice time ratio between the 2002 intraservice time and the recommended intraservice
time of 12 minutes of 0.70 work RVUs ((12 minutes/20 minutes) * 1.17). Like CPT code 96920,
we note that proposed work RVU of 0.90 for CPT code 96921 maintains the intensity associated
with the 2002 review of CPT code 96921, which we believe is more appropriate than the
intensity increase that results from the RUC-recommended work RVU of 1.07 which again
nearly doubles the current intensity of the code.
For CPT code 96922, we are proposing a work RVU of 1.15 based on the RUC-
recommended incremental difference between CPT codes 96921 and 96922 of 0.25 work RVUs.
Like CPT code 96920 and 96921, we understand that commenters will dispute the validity of the
current time values, but we note that the 2002 intraservice time was 30 minutes, which yields an
intraservice time ratio between the 2002 intraservice time and the recommended intraservice
time of 18 minutes of 1.26 work RVUs ((18 minutes/30 minutes) * 2.10). We note that the RUC
recommended CPT code 96922 as having the lowest intensity of the three codes in this family
and that our proposed work RVU of 1.15 maintains in relationship to the other codes.
For the direct PE inputs, we are proposing to refine the clinical staff time for the CA024
activity “Clean room/equipment by clinical staff” to the standard of 3 minutes for CPT codes
96920, 96921, and 96922. We note that 3 minutes is the current CA024 time for these three CPT
codes. A rationale for extending clinical staff beyond the standard 3 minutes for the CA024
activity was absent from the PE Summary of Recommendations, therefore we believe the current
and standard 3 minutes is more appropriate than the RUC-recommended 5 minutes. We are also
proposing equipment times of 36, 38, and 44 minutes for the power table (EF031) and exam light
(EQ168) equipment for CPT codes 96920, 96921, and 96922, respectively, to account for the
proposed refinement for CA024 to the standard 3 minutes.
We also disagree with the RUC-recommended creation of new supply items for the
excimer laser and are proposing to re-include the equipment time for the excimer laser (EQ161)
using the current methodology where its cost is accounted for in the equipment of these CPT
codes’ direct PE. The RUC submitted recommendations to change this equipment item to new
supply items to account for the per-use cost to rent the equipment, stating that the business model
has changed from the standard equipment ownership that CMS recognizes using standardized
equipment formulas to a per-use rental or subscription model. While we understand that there
may have been a change in business model, we do not believe a rental, subscription, or per-use
fee of an equipment item that is still available to be purchased and is already accounted for with
our equipment methodology is appropriate, especially given its implications for direct PE costs
for these CPT codes. Therefore, we are proposing reincorporating equipment times of 36, 38, and
44 minutes for the EQ161 equipment for CPT codes 96920, 96921, and 96922, respectively,
based on the refined service period clinical labor times. We are proposing to remove the three
pay-per-use excimer lasers listed as supplies and recommended by the RUC for these three
codes.
We have repeatedly stated in past rulemaking that rental and licensing fees are typically
considered forms of indirect PE under our methodology. In the CY 2020 PFS final rule, we
omitted the inclusion of several invoices for the monthly rental price of a PET infusion cart
(ER109), and only accounted for the four purchase invoices for the equipment. We noted as well
for future reference that although we appreciated the submission of the rental invoices, we were
unable to use invoices for a monthly rental fee to determine the typical purchase price for
equipment. We believe that invoices for a monthly rental fee would not be representative of the
purchase price for equipment, in the same fashion that the rental fee for a car differs from its
purchase price (84 FR 62771). Similarly, while we appreciate the submission of per-use, rental,
and partnership invoices for the excimer laser, we believe that the excimer laser is appropriately
and adequately accounted for in the equipment formula, and note that EQ161 has a very high
cost per minute of $0.5895/minute. Compared to the nearly 700 other equipment items in our
database, only 55 equipment items have higher costs per minute (based on our standardized
formula which accounts for years of useful life, utilization rate, purchase price, and minutes per
year of use, discussed in detail in section II.B. of this proposed rule, Determination of PE RVUs)
and only 53 equipment items have higher purchase prices than the excimer laser at $151,200. We
do not believe that CPT codes 96920 through 96922 should be valued based on a significantly
more expensive pay-per-use rental version of the excimer laser when the same treatment is
cheaper and available as a purchasable form of equipment.
Therefore, we are seeking comment on the difference in direct PE costs between the
purchase and per-use rental of the laser. We note that using the equipment cost per minute
formula, discussed in detail of section II.B. of this proposed rule, Determination of PE RVUs,
yields direct PE costs of about $21.22, $22.40, and $25.94 for CPT codes 96920, 96921, 96922,
respectively. Alternatively, the new supply items for the per-use fee of the laser yielded direct PE
costs of $80, $83, and $100 for CPT codes 96920, 96921, 96922, respectively. These direct PE
disparities represent a 277 percent, 270.5 percent, and 285.5 percent increase for CPT codes
96920, 96921, 96922, respectively. Given this, we are interested in feedback from interested
parties on the payment disparity between this equipment as a per-use or rental versus how we
currently account for the purchase of equipment using the standard equipment formula, as we
understand that both manufacturers and physicians may be inclined to shift to a per-use or rental
business models to limit overhead for purchase and maintenance of expensive equipment.
(26) Physical Medicine and Rehabilitation (CPT codes 97012, 97014, 97016, 97018, 97022,
97032, 97033, 97034, 97035, 97110, 97112, 97113, 97116, 97140, 97530, 97533, 97535, 97537,
and 97542 and HCPCS code G0283)
The RUC’s Health Care Professionals Advisory Committee (HCPAC) previously
reviewed 19 physical medicine and rehabilitation codes in February 2017. In the CY 2024 PFS
proposed rule, CMS received public nominations on these same 19 therapy codes as potentially
misvalued (88 FR 78851-78852). An interested party asserted that the direct PE clinical labor
minutes reflected inappropriate multiple procedure payment reductions (MPPR), which were
duplicative of the CMS MPPR policy implemented in CMS’ claims processing systems. CMS
reviewed the clinical labor time entries for these 19 therapy codes and concluded that a payment
reduction should not have been applied in some instances to the 19 nominated therapy codes’
clinical labor time entries since the payment valuation reduction would be duplicative of the
MPPR applied during claims processing. CMS indicated that the valuation of these services
would benefit from additional review through the RUC’s HCPAC valuation process; they were
therefore reviewed by the HCPAC for PE only, with no work review, at the January 2024 RUC
meeting for inclusion in the CY 2025 PFS proposed rule.
The HCPAC’s direct PE recommendations were based on the typical number of services
reported per session, which was 3.5 units according to CMS data, to ensure that there was no
duplication in the standard inputs for preservice and postservice time. To account for the MPPR,
the HCPAC determined that 3.5 codes are billed per session, with the first paid at 100% and the
second and subsequent units paid at half and so forth for PE (for example, 1.00 + 0.5 + 0.5 +
0.25 = 2.25). This resulted in the HCPAC recommending that many of the standard clinical labor
times be divided by 2.25 to account for the MPPR, such as taking the standard 3 minutes for
greeting and gowning the patient and dividing it by 2.25 to arrive at the recommended time of
1.33 minutes (1.33 + 0.67 + 0.67 + 0.34 = 3 minutes). In most cases, the HCPAC recommended
using the standard equipment time formula aside from a few exceptions such as the use of the
whirlpool in CPT code 97022 which would require additional time for the cleaning of the
equipment.

Following the January 2024 RUC meeting, representatives from the American Physical
Therapy Association (APTA) and the American Occupational Therapy Association (AOTA) met
with CMS to express concern with the HCPAC’s recommended direct PE inputs for this family
of codes. Representatives from these trade associations stated that the HCPAC had
inappropriately recommended too few equipment minutes for these procedures. These interested
parties requested utilizing an alternate equipment time formula for the 19 reviewed therapy codes
based on adding together the intraservice work time together with the clinical labor for the
preservice and postservice portion of the service period. For 17 of the 19 reviewed therapy
codes, this alternate equipment time formula would result in an increase over the HCPAC’s
equipment time recommendations. Table 12 lists the direct PE costs of each HCPCS code under
their current pricing, under the HCPAC recommendations, and the alternate APTA and AOTA
recommendations:
TABLE 12: Direct PE Costs for Physical Medicine and Rehabilitation Codes
HCPCS
Current
HCPAC
APTA/AOTA
Utilization
97012
2.62
3.22
3.30
434,921
97014
3.60
4.16
4.33
ProcStat "I"
97016
2.94
3.50
3.67
876,440
97018
2.29
2.92
2.96
146,909
97022
8.04
7.27
7.18
135,480
97032
2.61
3.17
3.34
621,599
97033
6.61
6.74
6.90
33,953
97034
4.08
4.17
4.17
6,964
97035
4.03
4.41
4.65
1,358,936
97110
8.42
8.63
9.11
61,204,041
97112
10.23
9.87
11.08
24,990,205
97113
13.89
14.65
14.61
1,588,852
97116
8.35
8.58
9.03
4,011,592
97140
7.25
8.09
8.21
28,413,744
97530
15.01
14.38
16.40
29,187,934
97533
35.72
36.56
36.69
60,507
97535
11.50
11.64
12.67
3,118,258
97537
9.69
10.09
10.78
15,556
97542
9.26
9.41
10.42
98,989
G0283
3.60
4.16
4.33
5,721,078
After consideration of these recommendations, we are proposing the direct PE inputs as
recommended by the HCPAC for all 19 codes in the Physical Medicine and Rehabilitation code
family. We believe that the HCPAC’s equipment time recommendations better maintain
relativity with the rest of the fee schedule through primarily using standard equipment time
formulas, along with limited exceptions for additional equipment time in cases where more time
for equipment cleaning or patient positioning would be typical. We also believe that the alternate
equipment time formula recommended by APTA and AOTA leads to inconsistent equipment
times for many of these procedures, such as recommending 23.98 equipment minutes for CPT
code 97110 which is a timed code billed in 15-minute increments. Although we agree that some
additional equipment time beyond the timed 15 minutes would be typical for setup and cleaning,
9 additional minutes for each billing of CPT code 97110 would not appear to reflect typical
equipment usage.
Given the complexity of determining appropriate direct PE inputs across multiple billings
of these therapy codes, and the need to factor in the MPPR, we believe that this code family may
benefit from additional review, specifically review focused on the subject of appropriate
equipment minutes. The HCPAC review of these codes was primarily focused on the clinical
labor portion of the PE inputs and the equipment times did not receive the same degree of
scrutiny as the clinical labor. We believe that the HCPAC’s recommended direct PE inputs are
the most accurate values based on the current information that we have available, however this is
a topic that may warrant additional review to ensure that this family of codes is properly valued.
(27) Acupuncture - Electroacupuncture (CPT codes 97810, 97811, 97813, and 97814)
In September 2022, the RUC’s Relativity Assessment Workgroup identified the
acupuncture codes with 2020 Medicare utilization over 10,000 where the service was surveyed
by one specialty but is now performed by a different specialty. CPT codes 97810-97814 were
selected and surveyed for the April 2023 RUC meeting.
For CY 2025, we are proposing the RUC-recommended work RVUs for all four CPT
codes. We are proposing a work RVU of 0.61 for CPT code 97810 (Acupuncture, 1 or more
needles; without electrical stimulation, initial 15 minutes of personal one-on-one contact with
the patient), a work RVU of 0.46 for CPT code 97811 (Acupuncture, 1 or more needles; without
electrical stimulation, each additional 15 minutes of personal one-on-one contact with the
patient, with re-insertion of needle(s) (List separately in addition to code for primary
procedure)), a work RVU of 0.74 for CPT Code 97813 (Acupuncture, 1 or more needles; with
electrical stimulation, initial 15 minutes of personal one-on-one contact with the patient), and a
work RVU of 0.47 for CPT code 97814 (Acupuncture, 1 or more needles; with electrical
stimulation, each additional 15 minutes of personal one-on-one contact with the patient, with re-
insertion of needle(s) (List separately in addition to code for primary procedure)). We are also
proposing the RUC-recommended direct PE inputs for CPT codes 97810, 97811, 97813 and
97814 without refinement.
(28) Annual Alcohol Screening (HCPCS codes G0442 and G0443)
In April 2022, the Relativity Assessment Workgroup identified services with Medicare
utilization of 10,000 or more that have increased by at least 100 percent from 2015 through 2020,
including HCPCS codes G0442 (Annual alcohol misuse screening, 5 to 15 minutes) and G0443
(Brief face-to-face behavioral counseling for alcohol misuse, 15 minutes). In September 2022,
the RUC recommended that these services be surveyed for April 2023 after CMS published the
revised code descriptor for HCPCS code G0442 in the CY 2023 PFS final rule (87 FR 69523).
We are proposing the RUC-recommended work RVU of 0.18 for HCPCS code G0442
(Annual alcohol misuse screening, 5 to 15 minutes). We are also proposing the RUC-
recommended work RVU of 0.60 for HCPCS code G0443 (Brief face-to-face behavioral
counseling for alcohol misuse, 15 minutes).
The RUC recommended an increase in the work RVU for HCPCS code G0443 from 0.45
to 0.60 which we believe is warranted based on time and intensity of the service in preventing
alcohol misuse. In valuing this code, the time and work valuation is for separate and distinct
services from same-day E/M services since HCPCS codes G0442 and G0443 are typically billed
with an annual wellness visit (AWV) or office visit. We believe that the codes in the adjacent
Behavioral Counseling & Therapy family, which includes HCPCS codes G0445 (High intensity
behavioral counseling to prevent sexually transmitted infection; face-to-face, individual,
includes: education, skills training and guidance on how to change sexual behavior; performed
semi-annually, 30 minutes), G0446 (Annual, face-to-face intensive behavioral therapy for
cardiovascular disease, individual, 15 minutes), and G0447 (Face-to-face behavioral counseling
for obesity, 15 minutes), may be undervalued as their respective intensities may be lower than
what is warranted for these services. We believe that the intensity for these G-codes may be
more in line with the intensity of HCPCS code G0443 which we noted had an increase in
intensity as recommended by the RUC. As such, we believe that the Behavioral Counseling &
Therapy codes may benefit from additional review in the future to recognize the intensity of
these services.
We are proposing to maintain the current 15 minutes of clinical labor time for the CA021
“Perform procedure/service---NOT directly related to physician work time” activity for HCPCS
code G0442. This clinical labor activity is specifically noted as not corresponding to the
surveyed work time of 5 minutes, and we do not believe that it would be typical for the clinical
staff to administer the questionnaire, clarify questions as needed, and record the answers in the
patient’s electronic medical record in the RUC-recommended 5 minutes. We believe that the
current 15 minutes of clinical labor time would be more typical to ensure the accuracy of this
screening procedure. We are also proposing to maintain 15 minutes of corresponding equipment
time for the EF023 exam table as a result of our proposed clinical labor time refinement. We are
proposing the RUC-recommended direct PE inputs for HCPCS code G0443 without refinement.
We thank the RUC for their review of this code family and for highlighting an important
consideration specifically for services that fall under the Medicare preventive services benefit.
We are now considering how best to implement and maintain payment for preventive services
and may develop new payment policies in future rulemaking to address this issue more
comprehensively to ensure consistent access to these services. We considered the recommended
PE inputs for this code family, as well as for the Annual Depression Screening (HCPCS code
G0444) and Behavioral Counseling & Therapy services (HCPCS codes G0445, G0446, and
G0447) within this context, as noted below.
(29) Annual Depression Screening (HCPCS code G0444)
In 2012, HCPCS code G0444 (Annual depression screening, 5 to 15 minutes) was added
to the PFS (77 FR 68955 and 68956) to report annual depression screening for adults in primary
care settings that have staff-assisted depression care supports in place to assure accurate
diagnosis, treatment and follow up. In April 2022, the Relativity Assessment Workgroup
identified this service with Medicare utilization of 10,000 or more that have increased by at least
100 percent from 2015 through 2020. In September 2022, the RUC recommended that this
service be surveyed for April 2023 after CMS published the revised code descriptor in the CY
2023 PFS final rule (87 FR 69523).
We are proposing the RUC-recommended work RVU of 0.18 for HCPCS code G0444.
We are proposing to maintain the current 15 minutes of clinical labor time for the CA021
“Perform procedure/service---NOT directly related to physician work time” activity for HCPCS
code G0444. This clinical labor activity is specifically noted as not corresponding to the
surveyed work time of 5 minutes, and we do not believe that it would be typical for the clinical
staff to administer the questionnaire, clarify questions as needed, and record the answers in the
patient’s electronic medical record in the RUC- recommended 5 minutes. We believe that the
current 15 minutes of clinical labor time would be more typical to ensure the accuracy of this
screening procedure. We are also proposing to maintain 15 minutes of corresponding equipment
time for the EF023 exam table as a result of our proposed clinical labor time refinement.
(30) Behavioral Counseling & Therapy (HCPCS codes G0445, G0446, and G0447)
CMS created HCPCS codes G0445 (High intensity behavioral counseling to prevent
sexually transmitted infection; face-to-face, individual, includes education, skills training and
guidance on how to change sexual behavior; performed semi-annually, 30 minutes), G0446
(Annual, face-to-face intensive behavioral therapy for cardiovascular disease, individual, 15
minutes), and G0447 (Face-to-face behavioral counseling for obesity, 15 minutes) effective with
the 2012 Medicare PFS (77 FR 68892). HCPCS codes G0445-G0447 were identified to be
reviewed at the April 2023 RUC meeting because they were services with Medicare utilization of
10,000 or more that had increased by at least 100% from 2015 through 2020.
The specialty societies surveyed HCPCS codes G0445-G0447 for the April 2023 RUC
meeting but did not obtain the required number of survey responses. After the resurvey, which
occurred after the April 2023 RUC meeting, the specialty societies were again unable to achieve
the required minimum number of survey responses for any of the codes in this family for the
September 2023 RUC meeting. The RUC reviewed HCPCS codes G0445-G0447 at the
September 2023 RUC meeting. Given the insufficient number of survey responses and
considering that these are CMS-created time-based codes, the RUC determined it would be most
appropriate to maintain the current work values and flagged these codes for review in 3 years.
We are proposing the RUC-recommended work RVU of 0.45 for each of these three HCPCS
codes, G0445-G0447.
We are not proposing the RUC-recommended direct PE inputs for these codes because of
the insufficient number of survey responses, and further, we do not agree with some of the
RUC’s refinements to the direct PE inputs for this service. We are not proposing the RUC-
recommended direct PE inputs for G0445, G0446, and G0447, which include the SK062 patient
education booklet being eliminated in favor of the SK057 paper, laser printing (each sheet) in the
amount of 10 sheets and the equipment minutes being modified to equal the sum of clinical staff
time plus the physician/QHP time as reflected by the survey median. We do not agree that these
changes are substantiated given the insufficient number of survey responses and we are
proposing to maintain the current values for each of these direct PE inputs.
We are proposing the RUC recommended refinements to clinical staff time for HCPCS
code G0445. We are proposing to move two minutes from CA021 Perform procedure/service---
NOT directly related to physician work time to CA035 Review home care instructions,
coordinate visits/prescriptions. We agree with the RUC that this more accurately reflects the
clinical work involved in arranging follow-up and/or referrals with clinical and community
resources and providing educational materials. Currently, for HCPCS code G0445, PE includes a
whip mixer (EP086) and biohazard hood (EP016) among the equipment assigned to the code.
We are also proposing the RUC recommendations to eliminate both of these pieces of equipment
from the PE for HCPCS code G0445.
We note that the Behavioral Counseling & Therapy code family (HCPCS codes G0445-
G0447) should be reviewed in the future by the RUC and we anticipate the recommendations
that will come from the review for this family.
(31) Autologous Platelet Rich Plasma (HCPCS code G0465)
HCPCS code G0465 (Autologous platelet rich plasma (prp) or other blood-derived
product for diabetic chronic wounds/ulcers, using an fda-cleared device for this indication,
(includes as applicable administration, dressings, phlebotomy, centrifugation or mixing, and all
other preparatory procedures, per treatment)) was created for CY 2022 (retroactively dated back
to the effective date of the policy, April 13, 2021) and assigned contractor pricing (NCD 270.3,
CR 12403).
Following the publication of the CY 2023 PFS proposed rule, we received two comments
on the pricing of HCPCS code G0465, and the 3C patch system supply which is topically applied
for the management of exuding cutaneous wounds, such as leg ulcers, pressure ulcers, and
diabetic ulcers and mechanically or surgically debrided wounds (87 FR 69420). One commenter
submitted invoices associated with the pricing of the 3C patch system (SD343) supply for which
we established a price of $625.00 in the CY 2021 PFS final rule (85 FR 84498). The commenter
requested that CMS update its supply database based on invoices submitted for SD343 to reflect
an updated price of $750.00 per unit. The commenter also requested national pricing for HCPCS
code G0465, expressing concern that insufficient payment disproportionately impacts vulnerable
populations. The commenter requested a payment rate of $1,408.90 for HCPCS G0465 in the
office setting, stating that this rate would appropriately account for the purchase of the 3C patch,
as well as the other related costs and supply inputs required for point of care creation and
administration.
In response, we stated in the CY 2023 PFS final rule that we did not have enough
information to establish national pricing at this time for HCPCS code G0465 (87 FR 69420). We
stated that we would consider the commenters’ feedback for future rulemaking while
maintaining contractor pricing for CY 2023, which would allow for more flexibility for
contractors to establish appropriate pricing using available information. We appreciated the
invoice submission with additional pricing information for the SD343 supply and we updated our
supply database for supply code SD343 at a price of $678.57 based on an average of the
submitted invoices.
Since the publication of the CY 2023 PFS final rule, interested parties have continued to
request national pricing for HCPCS code G0465 due to their perception of inconsistent and
insufficient payment for this service by the MACs. CMS has asked the interested parties to
engage with the MACs to establish adequate payment for HCPCS code G0465. The interested
parties have continued to state that most MACs have not established consistent payment rates
and the rates are heterogeneous; some are significantly below the cost of performing this service,
leading to an unpredictable process and inadequate rates, creating barriers to access this service.
Due to these concerns, we are therefore proposing to establish national pricing for
HCPCS code G0465 for CY 2025. We are proposing to value HCPCS code G0465 using a
crosswalk to CPT code 15271 (Application of skin substitute graft to trunk, arms, legs, total
wound surface area up to 100 sq cm; first 25 sq cm or less wound surface area), drawing from a

selection of relevant studies.
17,18,19,20
We are proposing a work RVU of 1.50 for HCPCS code
G0465 based on the crosswalk to CPT code 15271 because wound surface area sizes in current
literature appear to be less than 100 sq cm for patients with diabetes and/or chronic ulcers. We
are also proposing to use the direct PE inputs included with CPT code 15271 for valuing HCPCS
code G0465, with the additional inclusion of the 3C patch system (SD343) supply that we priced
in CY 2023. We note that the payment includes debridement, which may involve a wound
reaching the bone. Therefore, debridement may not be billed separately. In addition, we are
currently seeking comments on other available crosswalks from the broader medical community.
For example, CPT code 15277 (Application of skin substitute graft to face, scalp, eyelids, mouth,
neck, ears, orbits, genitalia, hands, feet, and/or multiple digits, total wound surface area greater
than or equal to 100 sq cm; first 100 sq cm wound surface area, or 1% of body area of infants
and children) with a work RVU of 4.00 and CPT code 15273 (Application of skin substitute graft
to trunk, arms, legs, total wound surface area greater than or equal to 100 sq cm; first 100 sq cm
wound surface area, or 1% of body area of infants and children) with a work RVU of 3.50 could
also be viable crosswalk options. We are soliciting comments regarding our selection of CPT
code 15271 as a crosswalk code, as well as general comments and available studies regarding the
appropriate valuation of HCPCS code G0465.
(32) Temporary Female Intraurethral Valve-Pump (CPT codes 0596T and 0597T)
In the CY 2024 PFS proposed rule, an interested party nominated two Category III CPT
codes, CPT codes 0596T (Initial insertion of temporary valve-pump in female urethra) and
0597T (Replacement of temporary valve-pump in female urethra), as potentially misvalued. The
17
Gethin, G et al. “The profile of patients with venous leg ulcers: A systematic review and global
perspective.” Journal of tissue viability vol. 30,1 (2021): 78-88. doi:10.1016/j.jtv.2020.08.003.
18
Sheehan, Peter et al. “Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust
predictor of complete healing in a 12-week prospective trial.” Plastic and reconstructive surgery vol. 117,7 Suppl
(2006): 239S-244S. doi:10.1097/01.prs.0000222891.74489.33.
19
Oyibo, S O et al. “The effects of ulcer size and site, patient's age, sex and type and duration of diabetes on the
outcome of diabetic foot ulcers.” Diabetic medicine : a journal of the British Diabetic Association vol. 18,2 (2001):
133-8. doi:10.1046/j.1464-5491.2001.00422.x.
20
Patry, Jérôme et al. “Outcomes and prognosis of diabetic foot ulcers treated by an interdisciplinary team in
Canada.” International wound journal vol. 18,2 (2021): 134-146. doi:10.1111/iwj.13505.
nominator expressed concern about variability in MAC pricing for the contractor-priced service.
Additionally, the nominator highlighted that the payment amounts determined by MACs were
inadequately low and did not account for the time and effort required to furnish the services. In
their submission, the nominator discussed their anticipated inputs for both codes. For CPT code
0596T, the nominator stated that a physician typically spends 60 minutes inserting the Vesiflo
inFlow System. The nominator stated that CPT code 0596T included various supplies,
equipment, and clinical labor time totaling $1,902.76, with the inflow supply items making up
about 99 percent of the total cost of supplies. For CPT code 0597T, the nominator stated that a
physician spends 25 minutes replacing the Vesiflo inFlow System and PE items were similar,
with supplies, equipment and clinical labor time costing $505.30, with the inflow supply items
making up about 98 percent of the total cost of supplies. We direct interested parties to the CY
2024 PFS final rule (88 FR 78850) for more detailed submission information regarding CPT
codes 0596T and 0597T. After reviewing, we concluded that these codes were not potentially
misvalued because they are Category III codes describing relatively new and low-volume
services. Category III codes are contractor priced under the PFS, meaning that each MAC can
establish pricing for the code within its jurisdiction, resulting in variability in payments.
This year, the nominator newly informed CMS that their analysis of national payment
rates showed that in most CMS jurisdictions, not only are these codes misvalued, but in most
cases, they are not valued at all, with fee schedule amounts in most CMS jurisdictions at or near
zero dollars. The nominator further emphasized that three physician experts, all employed in
major university medical centers, have highlighted the challenges posed by the combination of
high supply costs and inadequate fee schedule payments, which have hindered their ability to
provide services covered by these codes over several years. According to the nominator, these
selected physicians also expressed frustration with the reluctance of MACs to address or discuss
this issue. Moreover, the nominator highlighted high access barriers as a significant concern.
These barriers primarily affect Medicare's most vulnerable beneficiaries, particularly women
experiencing permanent urinary retention (PUR), although we note that no quantifiable evidence
was provided to support these statements. We acknowledge and appreciate the nominator's
efforts in reaching out to experts in the field and patients who rely on these services to elucidate
their significant needs.
Since these two Category III CPT codes were not identified as potentially misvalued and
were consequently priced by contractors, each MAC can set pricing for the code within its
jurisdiction. This could result in inevitable variability in MAC pricings until they receive a
higher number of claims, as stated by the nominator. Through our engagement with MACs, we
found that claims for the two Category III CPT codes are reviewed on a case-by-case basis for
medical necessity. If the claim is payable, the price will be determined at that time by the MAC.
Additionally, these codes were a topic of discussion within the MAC pricing workgroup, and we
observed that there was not a significant difference among the MACs in terms of allowances
based on the proposed pricing methodologies. However, there is variance in how MACs load
pricing for Category III codes. For instance, some MACs publish the price for the service before
they receive any claims, while others set the price only after they receive claims that help
determine the appropriate pricing. If a MAC does not load a price for a code before receiving any
claims, the service can still be paid, but the allowance has not been published.
We continue to hear concerns about these payment inconsistencies for CPT codes 0596T
and 0597T. As a result, we are recommending that the MACs establish more consistency in
pricing, enabling the appropriate inclusion of the Vesiflo system in the code's PE valuation.
Therefore, for CY 2025, we encourage interested parties to provide more accurate and
appropriate cost data, along with additional information regarding work RVU, work time,
indicators, and utilization estimates for the MACs. This should complement the information
provided by the nominator in the CY 2024 final rule (88 FR 78850) and will facilitate the
process. To aid in this process, we are adding three new supplies to our direct PE database based
on invoices submitted by interested parties: the inFlow Measuring Device at a price of $140
(SD370), the inFlow Valve-Pump Device at a price of $495 (SD371), and the inFlow Activator
Kit at a price of $1,250 (SD372). Although we are not proposing national pricing for these two
Category III codes, we do note for the benefit of the MACs that CPT code 0596T would
typically include one of each of these supplies, whereas CPT code 0597T would typically
include only one of the supplies (SD371).
We encourage the MACs to continue to engage with interested parties by providing
information on how they price these services. We welcome additional comments from the
broader medical community regarding the usage of this service, particularly concerning its safety
and effectiveness, as well as potential factors contributing to its low utilization.
(33) PE-only replacement code for Heart Failure System
Interested parties have expressed concern about the lack of coding and a billing
mechanism when practitioners incur costs replacing identified components of the
CardioMEMS™ Heart Failure System used in the physician service described by CPT code
33289 (Transcatheter implantation of wireless pulmonary artery pressure sensor for long-term
hemodynamic monitoring, including deployment and calibration of the sensor, right heart
catheterization, selective pulmonary catheterization, radiological supervision and interpretation,
and pulmonary artery angiography, when performed).
The CardioMEMS™ Heart Failure System furnished during this service allows
practitioners treating heart failure patients to wirelessly monitor and measure pulmonary artery
pressure and heart rate in patients with heart failure and transmit the information to the physician
to inform the treatment plan for the patient. The system includes two critical components: first, a
miniaturized, wireless monitor, which is implanted into a patient’s pulmonary artery, and second,
a smart pillow (the CardioMEMS™ Patient Electronics System), which captures and transmits
readings via safe radio frequency from the patient’s implanted CardioMEMS™ Heart Failure
System. Overall, the CardioMEMS™ Heart Failure System enables patients to transmit critical
heart failure status information to clinicians regularly, potentially eliminating the need for
frequent clinic or hospital visits.
Interested parties have highlighted the critical importance of the device for heart failure
patients who require close monitoring of weight and blood pressure to prevent fluid buildup
around the heart, and have requested that CMS establish coding to describe when practitioners
incur costs during clinical scenarios when crucial components of the system require replacement.
Given that these components are crucial for system functionality and there is no existing coding
framework to address their replacement, we believe that establishing appropriate coding and
payment mechanisms can facilitate the provision of these services more effectively in the office
and hospital settings. Given provided information, we propose assigning contractor pricing to
this PE-only code for CY 2025. We are proposing a new code, HCPCS code GMEM1 (Provision
of replacement patient electronics system (for example, system pillow) for home pulmonary
artery pressure monitoring including provision of materials for use in the home and reporting of
test results to physician or qualified health care professional). We are seeking feedback from
interested parties on our contractor pricing approach with the aim of establishing national pricing
through future rulemaking that can be billed under the OPPS and PFS specifying an ongoing care
visit for the CardioMEMS™ Heart Failure System along with the provision of the replacement
part. We are specifically looking for information from the broader medical community regarding
direct costs from invoices for the replacement component referenced above, utilization estimates,
and potential indicators. Additionally, we solicit comments on additional direct PE inputs that we
should consider.
(34) Portable X-Ray (HCPCS codes R0070-R0075)
Several Portable X-Ray (PXR) suppliers and trade organizations continue to express
longstanding concerns with how payment is established for transportation related to these
services (HCPCS codes R0070-R0075). CMS has worked with interested parties over the past
several years to understand the costs of these services while taking into consideration the MACs
perspective on pricing of these costs. Through recent ongoing discussions with interested parties,
we learned that interested parties are concerned with the recognition of costs incurred from PXR
services and are wanting more consistency in the pricing of these services, including the
application of an inflation factor.
We acknowledge the interested parties’ concerns and clarify that interested parties may
best engage with the MACs through appropriate reporting of cost data in the MAC requested
format. This information provided by interested parties can help MACs establish payment rates
that are more reflective of costs incurred. MACs are then able to consider this cost information
and apply an inflation factor to update changes in costs year over year.
However, CMS recognizes that we should maintain consistency in pricing these services
that are more indicative of changes in costs that occur yearly. While still preserving MAC
discretion, CMS highlights the usage of an ambulance inflation factor (AIF) that is typically used
to adjust ambulance services, which include transportation costs. The AIF is updated annually,
and we believe MACs may consider using the AIF to price PXR services when establishing
payment rates that are more consistent and reflective of costs incurred.
Additionally, interested parties highlighted inconsistency with language found in our
manual and program memoranda policies related to transportation costs. Therefore, to remain
consistent and transparent in the pricing of PXR services, we are proposing to revise language in
our Medicare Claims Processing manual (Chapter 13, 90.3 and Chapter 23, 30.5) to reflect any
updates to our guidance for these services.
(35) Non-chemotherapy Administration
CMS received inquiries from several external parties with concerns that MACs have
developed local coverage determinations (LCDs) and local coverage articles (LCAs) that down
code or restrict payment for complex and non-chemotherapeutic drug administration for CPT
code series 96401-96549, when used for the administration of several biologic and infusion
drugs, including drugs furnished to treat, for example, rheumatology related conditions.
CMS requested information in the CY 2024 PFS proposed rule (88 FR 52837) seeking
public feedback regarding the concerns of down coding or denials for the administration of non-
chemotherapeutic infusion drugs. We received comments that asked for additional clarification
from CMS regarding the payment guidelines for the complex non-chemotherapeutic
administration code series and updates to the IOM. Commenters urged CMS to provide
additional guidance clarifying the conditions under which these complex infusion drugs should
be payable.
In response to the comments received, and in response to continuing inquiries on
downcoding and or restrictions on payment for non-chemotherapy complex infusion services, we
are proposing an updated policy based largely on the IOM Medicare Claims Processing Manual,
Chapter 12, section 30.5, to include language currently consistent with CPT code definitions for
the complex non-chemotherapy infusion code series stating that the administration of infusion
for particular kinds of drugs and biologics can be considered complex and may be appropriately
reported using the chemotherapy administration CPT codes 96401-96549. We note that CPT
guidance describes requirements for these non-chemotherapy complex drugs or biologic agents
to include the need for staff with advanced practice training and competency, such as, a
physician or other qualified health care professional to monitor the patient during these infusions
due to the incidence of severe adverse reactions. There are also special considerations for
preparation, dosage, or disposal for these infusion drugs. These services do involve serious
patient risk which requires frequent consults with a physician or other qualified healthcare
professional. Based on these facts and comments, we are proposing to update our subregulatory
guidance accordingly.
This will also provide complex clinical characteristics for the MACs to consider as
criteria when determining payment of claims for these services. The current IOM language does
not include the unique characteristics of the administration of these drugs that could provide
additional context to the MACs when they are determining appropriate payment. Updating the
IOM with the increased detail of these codes would be responsive to the concerns and requests of
external parties and will ensure the IOM is consistent with published guidance.
Therefore, we are soliciting and welcome comments on our proposal to revise the IOM to
better reflect how complex non-chemotherapeutic drug administration infusion services are
furnished and billed.
(36) Hospital Inpatient or Observation (I/O) Evaluation and Management (E/M) Add-on for
Infectious Diseases (HCPCS code GIDXX)
Interested parties have continued to engage with CMS and provide recommendations to
recognize the increased work associated with diagnosis, management, and treatment of infectious
diseases that may not be adequately accounted for in current hospital inpatient or observation
E/M codes. Infectious diseases are unique in that they present infection control risks for the
patient and close contacts, including healthcare staff, that require attention to safely care for the
patient. They present unique challenges in diagnosis in that any previous healthcare interaction
could affect the individual resistance patterns of pathogens infecting the individual patient and
require close contact with public health agencies since resistance patterns are constantly
changing, so a much more extensive medical review is required. Additionally, individual
decisions regarding treatment are unique in that use in one patient affects resistance patterns of
the entire population, which requires additional expertise to inform antimicrobial selection and
management.
We believe that the timing is appropriate for establishing a payment rate for infectious
disease physician services since the COVID-19 PHE has ignited a hypervigilance for infectious
diseases. Therefore, for CY 2025, we are proposing a new HCPCS code to describe intensity and
complexity inherent to hospital inpatient or observation care associated with a confirmed or
suspected infectious disease performed by a physician with specialized training in infectious
diseases. The full proposed descriptor for the hospital I/O E/M visit complexity add-on code is
HCPCS code GIDXX (Visit complexity inherent to hospital inpatient or observation care
associated with a confirmed or suspected infectious disease by an infectious diseases consultant,
including disease transmission risk assessment and mitigation, public health investigation,
analysis, and testing, and complex antimicrobial therapy counseling and treatment. (add-on
code, list separately in addition to hospital inpatient or observation evaluation and management
visit, initial, same day discharge, or subsequent). We anticipate that HCPCS code GIDXX would
be reported by physicians with specialized infectious disease training.
We do not believe we should limit the scope of codes with which this proposed add-on
HCPCS code could be billed based on visit level; or initial, same day discharge, or subsequent
hospital inpatient or observation codes. We are proposing HCPCS code GIDXX as an add-on
code (ZZZ global period) separately reportable in addition to CPT codes 99221 (Initial hospital
inpatient or observation care, per day, for the evaluation and management of a patient, which
requires a medically appropriate history and/or examination and straightforward or low level
medical decision making. When using total time on the date of the encounter for code selection,
40 minutes must be met or exceeded.), 99222 (Initial hospital inpatient or observation care, per
day, for the evaluation and management of a patient, which requires a medically appropriate
history and/or examination and moderate level of medical decision making. When using total
time on the date of the encounter for code selection, 55 minutes must be met or exceeded.),
99223 (Initial hospital inpatient or observation care, per day, for the evaluation and
management of a patient, which requires a medically appropriate history and/or examination
and high level of medical decision making. When using total time on the date of the encounter for
code selection, 75 minutes must be met or exceeded.), 99231 (Subsequent hospital inpatient or
observation care, per day, for the evaluation and management of a patient, which requires a
medically appropriate history and/or examination and straightforward or low level of medical
decision making. When using total time on the date of the encounter for code selection, 25
minutes must be met or exceeded.), 99232 (Subsequent hospital inpatient or observation care,
per day, for the evaluation and management of a patient, which requires a medically appropriate
history and/or examination and moderate level of medical decision making. When using total
time on the date of the encounter for code selection, 35 minutes must be met or exceeded.),
99233 (Subsequent hospital inpatient or observation care, per day, for the evaluation and
management of a patient, which requires a medically appropriate history and/or examination
and high level of medical decision making. When using total time on the date of the encounter for
code selection, 50 minutes must be met or exceeded.), 99234 (Hospital inpatient or observation
care, for the evaluation and management of a patient including admission and discharge on the
same date, which requires a medically appropriate history and/or examination and
straightforward or low level of medical decision making. When using total time on the date of the
encounter for code selection, 45 minutes must be met or exceeded.), 99235 (Hospital inpatient or
observation care, for the evaluation and management of a patient including admission and
discharge on the same date, which requires a medically appropriate history and/or examination
and moderate level of medical decision making. When using total time on the date of the
encounter for code selection, 70 minutes must be met or exceeded.), and 99236 (Hospital
inpatient or observation care, for the evaluation and management of a patient including
admission and discharge on the same date, which requires a medically appropriate history
and/or examination and high level of medical decision making. When using total time on the date
of the encounter for code selection, 85 minutes must be met or exceeded.). Based on feedback
from commenters on the CY 2022 PFS proposed rule comment solicitation regarding infectious
diseases (86 FR 65125 through 65126) and feedback from interested parties, HCPCS code
GIDXX would include the following proposed service elements:
1. Disease Transmission Risk Assessment and Mitigation
● Developing, following, and supervising specialized, individualized infection control
protocols for an individual patient based on their diagnosis and risks in order to reduce risk of
disease transmission.
● Coordinating with human resources regarding infection prevention and control
measures to enable healthcare facility staff to safely care for patient.
● Counseling patients, family members and caregivers regarding infection prevention.
● Managing infection prevention and treatment protocols associated with transitions of
care for complex patients.
2. Public Health Investigation, analysis, and testing
● In-depth patient chart review that entails going back farther in time and assessing the
complete breadth of all health care interactions, with higher-level synthesis for complex
diagnoses.
● Communicating with the clinical microbiology lab and directly reviewing specimens.
● Coordinating specialized diagnostic evaluations (for example, identifying and
facilitating diagnostic laboratory tests only available at specialized laboratories, the state health
department, and/or the Centers for Disease Control & Prevention).
● Coordinating with Federal, State and local public health agencies and laboratories to
assist with contact tracing, obtaining specimens for specialized testing, and/or identifying prior
testing and treatment for communicable diseases in other jurisdictions.
3. Complex Antimicrobial Therapy Counseling & Treatment
● Counseling patients, family members, and caregivers regarding antimicrobial
stewardship and resistance for the patient.
● Engaging in complex medical decision-making associated with antimicrobial
prescribing including considerations such as antimicrobial resistance patterns, emergence of new
variants/strains, recent antibiotic exposure, interactions/complications from comorbidities
including concurrent infections, public health considerations to minimize development of
antimicrobial resistance, and emerging and re-emerging infections.
For HCPCS code GIDXX, we are proposing a work RVU of 0.89 based on the work
RVU for HCPCS code G2211 (Visit complexity inherent to evaluation and management
associated with medical care services that serve as the continuing focal point for all needed
health care services and/or with medical care services that are part of ongoing care related to a
patient's single, serious condition or a complex condition. (add-on code, list separately in
addition to office/outpatient evaluation and management visit, new or established)), which is
0.33, multiplied by a ratio of the work RVUs for CPT codes 99223 and 99213 (Office or other
outpatient visit for the evaluation and management of an established patient, which requires a
medically appropriate history and/or examination and low level of medical decision making.
When using total time on the date of the encounter for code selection, 20 minutes must be met or
exceeded.), 3.50 and 1.30, respectively. (This ratio is the work RVU of CPT code 99223 divided
by the work RVU of CPT code 99213, 3.50 divided by 1.30, which equals 2.69. Multiplying the
0.33 work RVU of HCPCS code G2211 times 2.69 results in our proposed work RVU of 0.89.)
We believe the relationship between the complexity add-on HCPCS code G2211 and a common
base code for the add-on code, CPT code 99213, would strike the correct balance to estimate the
time and complexity associated with the new HCPCS code GIDXX, compared to what we
believe will be a common base code for this new add-on code, CPT code 99223. HCPCS code
G2211 has a total time of 11 minutes, therefore, we are proposing a total time of 30 minutes for
HCPCS code GIDXX based on the same ratio (11 minutes times the same 2.69 ratio equals 30
minutes). HCPCS code G2211 has no direct PE inputs, and we are proposing the same for
HCPCS code GIDXX.
We believe that the proposed work RVU appropriately falls between the following
bracket add-on codes: HCPCS code G0316 (Prolonged hospital inpatient or observation care
evaluation and management service(s) beyond the total time for the primary service (when the
primary service has been selected using time on the date of the primary service); each additional
15 minutes by the physician or qualified healthcare professional, with or without direct patient
contact (list separately in addition to CPT codes 99223, 99233, and 99236 for hospital inpatient
or observation care evaluation and management services). (do not report g0316 on the same
date of service as other prolonged services for evaluation and management 99358, 99359,
99418, 99415, 99416). (do not report g0316 for any time unit less than 15 minutes)) with a work
RVU of 0.61 and the professional principal care management, chronic care management, and
complex chronic care management CPT codes 99425 (Principal care management services, for a
single high-risk disease, with the following required elements: one complex chronic condition
expected to last at least 3 months, and that places the patient at significant risk of
hospitalization, acute exacerbation/decompensation, functional decline, or death, the condition
requires development, monitoring, or revision of disease-specific care plan, the condition
requires frequent adjustments in the medication regimen and/or the management of the condition
is unusually complex due to comorbidities, ongoing communication and care coordination
between relevant practitioners furnishing care; each additional 30 minutes provided personally
by a physician or other qualified health care professional, per calendar month (List separately in
addition to code for primary procedure)), 99437 (Chronic care management services with the
following required elements: multiple (two or more) chronic conditions expected to last at least
12 months, or until the death of the patient, chronic conditions that place the patient at
significant risk of death, acute exacerbation/decompensation, or functional decline,
comprehensive care plan established, implemented, revised, or monitored; each additional 30
minutes by a physician or other qualified health care professional, per calendar month (List
separately in addition to code for primary procedure)), and 99489 (Complex chronic care
management services with the following required elements: multiple (two or more) chronic
conditions expected to last at least 12 months, or until the death of the patient, chronic
conditions that place the patient at significant risk of death, acute exacerbation/decompensation,
or functional decline, comprehensive care plan established, implemented, revised, or monitored,
moderate or high complexity medical decision making; each additional 30 minutes of clinical
staff time directed by a physician or other qualified health care professional, per calendar month
(List separately in addition to code for primary procedure)) with work RVUs of 1.00.
To help inform whether our proposed descriptor is appropriate and reflects the typical
service, we are seeking comment on the typical amount of time infectious disease physicians
spend on the proposed service elements and the relative intensity compared to similar service
elements of other CPT codes. We note that the valuation of HCPCS code GIDXX is meant to
capture the visit complexity inherent to hospital inpatient or observation care associated with a
confirmed or suspected infectious disease by an infectious diseases consultant that is not
accounted for in the appropriate hospital inpatient or observation E/M base code billed by the
infectious disease physician.
Interested parties have stated that consultations are a common E/M service performed by
infectious disease clinicians, particularly in the inpatient setting, but stated that these services are
no longer recognized by Medicare. Interested parties have also stated that this has resulted in a
significant reduction in reporting and payment for infectious disease physician services. We note
that we address this in the CMS Claims Processing Manual, Chapter 12, section 30.6.9 F, stating
that “Physicians may bill initial hospital care service codes (99221-99223), for services that were
reported with CPT consultation codes (99241 – 99255) prior to January 1, 2010, when the
furnished service and documentation meet the minimum key component work and/or medical
necessity requirements. Physicians may report a subsequent hospital care CPT code for services
that were reported as CPT consultation codes (99241 – 99255) prior to January 1, 2010, where
the medical record appropriately demonstrates that the work and medical necessity requirements
are met for reporting a subsequent hospital care code (under the level selected), even though the
reported code is for the provider’s first E/M service to the inpatient during the hospital stay.”
Accordingly, we are seeking comment on any potential barriers for infectious disease physicians
to use the initial and subsequent day hospital inpatient or observation codes, CPT codes 99221
through 99223 and 99231 through 99233, for consultations if they meet the coding requirements
for time and/or medical decision making (MDM). We note that understanding the barriers to
utilizing these codes is important, as these codes will serve as the base codes for HCPCS code
GIDXX, and will need to be billed by the infectious disease physician prior to billing HCPCS
code GIDXX.
Finally, we recognize that historically, the CPT Editorial Panel has frequently created
CPT codes describing services that we originally established using G codes and adopted them
through the CPT Editorial Panel process. We note that we would consider using any newly
available CPT coding to describe services similar to those described here in future rulemaking.
(37) Preexposure Prophylaxis (PrEP) of Human Immunodeficiency Virus (HIV)
To facilitate prompt beneficiary access to PrEP for CY 2024, we established 3 HCPCS G
codes that describe the service of counseling and administration of Human Immunodeficiency
Virus (HIV) pre-exposure prophylaxis drugs. Specifically, HCPCS codes G0011 (Individual
counseling for pre-exposure prophylaxis (PrEP) by physician or QHP to prevent human
immunodeficiency virus (HIV), includes: HIV risk assessment (initial or continued assessment of
risk), HIV risk reduction and medication adherence, 15-30 minutes) and G0013 (Individual
counseling for pre-exposure prophylaxis (PrEP) by clinical staff to prevent human
immunodeficiency virus (HIV), includes: HIV risk assessment (initial or continued assessment of
risk), HIV risk reduction and medication adherence) describe the counseling portion of the
service, and G0012 (Injection of pre-exposure prophylaxis (PrEP) drug for HIV prevention,
under skin or into muscle) describes the injection of the medication.
CMS released a Proposed NCD for Pre-Exposure Prophylaxis (PrEP) for Human
Immunodeficiency Virus (HIV) Infection Prevention on July 12, 2023. This proposed NCD
announced CMS’ intent to cover and pay for those drugs under the 1861(ddd) additional
preventive services authority, and a final decision on the NCD is forthcoming. For CY 2025, we
are proposing national rates for these HCPCS codes that reflect the relative resource costs
associated with the counseling and drug administration portions of the service, pending
finalization of the NCD. For HCPCS code G0011, we are proposing a work RVU of 0.45 based
off work and direct PE inputs crosswalked from HCPCS code G0445 (High intensity behavioral

counseling to prevent sexually transmitted infection; face-to-face, individual, includes:
education, skills training and guidance on how to change sexual behavior; performed semi-
annually, 30 minutes). For HCPCS code G0012, we are proposing a work RVU of 0.17 based on
the work and direct PE crosswalked from CPT code 96372 (Therapeutic, prophylactic, or
diagnostic injection (specify substance or drug); subcutaneous or intramuscular), and for
HCPCS code G0013 we are proposing a work RVU of 0.18 based on the work and direct PE
inputs crosswalked from CPT code 99211 (Office or other outpatient visit for the evaluation and
management of an established patient that may not require the presence of a physician or other
qualified health care professional). We appreciate having this opportunity for interested parties
to provide feedback on the most accurate way to value these services.
(38) Opfolda
For CY 2024, to facilitate beneficiary access to treatment of late-onset Pompe disease
with miglustat in combination with cipaglucosidae alfa-atga, we created a new HCPCS code,
G0138, describing the service of administration of cipaglucosidase alfa-atga (Pombiliti), which
includes the intravenous administration of cipaglucosidase alfa-atga, the provider or supplier’s
acquisition cost of miglustat, clinical supervision, and oral administration of miglustat. HCPCS
code G0138 (Intravenous infusion of cipaglucosidase alfaatga, including provider/supplier
acquisition and clinical supervision of oral administration of miglustat in preparation of receipt
of cipaglucosidase alfa-atga) was added to the PFS effective April 1, 2024, as a contractor
priced service. More information regarding the creation of HCPCS code G0138 can be found at
https://www.cms.gov/files/document/2023-hcpcs-application-summary-quarter-4-2023-drugs-
and-biologicals-updated-1/30/2024.pdf.
For CY 2025, we are proposing national pricing for this service that reflects the relative
resource costs associated with the infusion administration of Cipaglucosidae alfa-atga and
clinical supervision and provision of Miglustat oral with acquisition costs. We are proposing a
work RVU of 0.21 for HCPCS code G0138 based on a crosswalk from CPT code 96365
(Intravenous infusion, for therapy, prophylaxis, or diagnosis (specify substance or drug); initial,
up to 1 hour). This includes a crosswalked total time of 9 minutes and an intraservice time of 5
minutes. We are also proposing to crosswalk the direct PE inputs from CPT code 96365 for use
in valuing HCPCS code G0138. However, we are adding 1 minute of L056A clinical staff time
during the preservice portion of the service period to capture the RN/OCN observation of the
patient during administration of the Opfolda pill. In addition, to account for the cost of the
provision of the self-administered Opfolda as a direct PE input, we are incorporating the
wholesale acquisition cost (WAC) data from the most recent available quarter. We are proposing
a price of $32.50 for the supply input that describes a 65mg capsule of Opfolda (supply code
SH111). We are seeking feedback from interested parties on our proposal of national pricing, as
well as our proposed work RVU and direct PE inputs for HCPCS code G0138 to ensure proper
payment for this service.
(39) Payment for Caregiver Training Services
a. Background
In the CY 2017 PFS final rule (81 FR 80330 through 80331), we finalized payment for
new CPT code(s) describing administration of a patient-focused health risk assessment
instrument as well as administration of a caregiver-focused health risk assessment instrument. In
the CY 2024 PFS final rule (88 FR 78914), we finalized the assignment of a payable status for
caregiver training services (CTS) for therapy and behavior management/modification services
(without the patient present) and finalized the RUC-recommended valuations for these services
to better recognize the role that caregivers play in reasonable and necessary care for Medicare
beneficiaries. These codes allow treating practitioners to report the training furnished to a
caregiver, in tandem with the diagnostic and treatment services furnished directly to the patient,
in strategies and specific activities to assist the patient in carrying out the treatment plan.
We finalized in the CY 2024 PFS final rule that payment may be made for CTS services
when the treating practitioner identifies a need to involve and train one or more caregivers to
assist the patient in carrying out a patient-centered treatment plan. We also finalized that because
CTS services are furnished outside the patient’s presence, the treating practitioner must obtain
the patient’s (or representative’s) consent for the caregiver to receive the CTS. Additionally, we
finalized that the identified need for CTS and the patient’s (or representative’s) consent for one
or more specific caregivers to receive CTS must be documented in the patient’s medical record.
These finalized policies apply to current CTS coding and we are also proposing for them to apply
to the newly proposed CTS coding that follows. We continue to receive questions and requests
from interested parties about how we can refine payment for these services.
b. Caregiver Assessment
In response to interested parties’ requests for assessment of a caregiver’s knowledge to be
included in caregiver training, we are clarifying that when reasonable and necessary, assessing
the caregiver’s skills and knowledge for the purposes of caregiver training services could be
included in the service described by CPT code 96161 (Administration of caregiver-focused
health risk assessment instrument (eg, depression inventory) for the benefit of the patient, with
scoring and documentation, per standardized instrument) to determine if caregiver training
services are needed. We also note that CPT code 96161 is currently on the Medicare Telehealth
list.
We note that, as specified in the CY 2017 PFS final rule (81 FR 80330), in particular
cases, caregiver-focused health risk assessments can be necessary components of services
furnished to Medicare beneficiaries. Examples where this service may be reasonable and
necessary may include assessment of maternal depression in the active care of infants,
assessment of parental mental health as part of evaluating a child’s functioning, assessment of
caretaker conditions as indicated where atypical parent/child interactions are observed during
care, and assessment of caregivers as part of care management for adults whose physical or
cognitive status renders them incapable of independent living and dependent on another adult
caregiver. Commenters cited that some examples of such individuals might include intellectually
disabled adults, seriously disabled military veterans, and adults with significant musculoskeletal
or central nervous system impairments (81 FR 80331).
We are proposing that because the caregiver-focused health risk assessment may be
furnished outside the patient’s presence, the treating practitioner must obtain the patient’s (or
representative’s) consent for the caregiver to receive the assessment. We are also proposing that
the definition of “caregiver” specified in the CY 2024 PFS final rule (88 FR 78917) will be the
same for caregiver training services and the caregiver-focused health risk assessment.
We are seeking comment on these proposals and clarifications.
c. Proposals and New Coding
(A) Proposed Direct Care Caregiver Training Services
i. Coding
We are proposing to establish new coding and payment for caregiver training for direct
care services and supports. The topics of training could include, but would not be limited to,
techniques to prevent decubitus ulcer formation, wound dressing changes, and infection control.
Unlike other caregiver training codes that are currently paid under the PFS, the caregiver training
codes for direct care services and support focus on specific clinical skills aimed at the caregiver
effectuating hands-on treatment, reducing complications, and monitoring the patient. For
example, in the direct care CTS codes, a caregiver could be taught how to properly change
wound dressings to promote healing and prevent infection. This skill, among other direct care
services, would not fall into the categories of CTS codes that currently exist (behavior
management/modification or strategies and techniques to facilitate the patient’s functional
performance in the home or community) but is integral in effectuating the patient’s treatment
plan. Like other codes describing caregiver training services, these proposed new codes would
reflect the training furnished to a caregiver, in tandem with the diagnostic and treatment services
furnished directly to the patient, in strategies and specific activities to assist the patient to carry
out the treatment plan. We believe that CTS may be reasonable and necessary when they are
integral to a patient's overall treatment and furnished after the treatment plan is established. The
CTS themselves need to be congruent with the treatment plan and designed to effectuate the
desired patient outcomes. We believe this is especially the case in medical treatment scenarios
where assistance by the caregiver receiving the CTS is necessary to ensure a successful treatment
outcome for the patient--for example, when the patient cannot follow through with the treatment
plan for themselves.
We are proposing three new HCPCS codes: GCTD1 (Caregiver training in direct care
strategies and techniques to support care for patients with an ongoing condition or illness and to
reduce complications (including, but not limited to, techniques to prevent decubitus ulcer
formation, wound dressing changes, and infection control) (without the patient present), face-to-
face; initial 30 minutes), GCTD2 (Caregiver training in direct care strategies and techniques to
support care for patients with an ongoing condition or illness and to reduce complications
(including, but not limited to, techniques to prevent decubitus ulcer formation, wound dressing
changes, and infection control) (without the patient present), face-to-face; each additional 15
minutes (List separately in addition to code for primary service) (Use GCTD2 in conjunction
with GCTD1)), and GCTD3 (Group caregiver training in direct care strategies and techniques
to support care for patients with an ongoing condition or illness and to reduce complications
(including, but not limited to, techniques to prevent decubitus ulcer formation, wound dressing
changes, and infection control) (without the patient present), face-to-face with multiple sets of
caregivers)).
We continue to believe that CTS may be reasonable and necessary when they are integral
to a patient’s overall treatment and furnished after the treatment plan is established. The medical
or direct care CTS themselves need to be congruent with the treatment plan and designed to
effectuate the desired patient outcomes. We believe this is especially the case in medical
treatment scenarios where assistance by the caregiver receiving the CTS is necessary to ensure a
successful treatment outcome for the patient—for example when the patient cannot follow

through with the treatment plan for themselves. Direct care training for caregivers of Medicare
beneficiaries should be directly relevant to the person-centered treatment plan for the patient in
order for the services to be considered reasonable and necessary under the Medicare program.
Each training activity should be clearly identified and documented in the treatment plan.
Additionally, this would not be billable for patients under home health plan of care, receiving at-
home therapy, or receiving DME services for involved medical equipment and supplies.
We are seeking additional information from commenters about potential service overlaps
and potential examples of direct care services to receive caregiver training to inform our final
policy. We are soliciting public comment on each of our proposals for direct care CTS.
ii. Valuation
For GCTM1, we propose a direct crosswalk to CPT Code 97550 (Caregiver training in
strategies and techniques to facilitate the patient’s functional performance in the home or
community (eg, activities of daily living [ADLs], instrumental ADLs [iADLs], transfers, mobility,
communication, swallowing, feeding, problem-solving, safety practices) (without the patient
present), face to face; initial 30 minutes), with a work RVU of 1.00 as we believe this service
reflects the resource costs associated when the billing practitioner performs HCPCS code
GCTM1. CPT code 97550 has an intraservice time of 30 minutes, and the physician work is of
similar intensity to our proposed HCPCS code GCTM1. Therefore, we are proposing a work
time of 30 minutes intraservice time (40 minutes of total time) for HCPCS code GCTM1 based
on this same crosswalk to CPT 97550. We are also proposing to use this crosswalk to establish
the direct PE inputs for HCPCS code GCTM1.
For GCTM2, we are proposing a direct crosswalk to CPT Code 97551 (Caregiver
training in strategies and techniques to facilitate the patient’s functional performance in the
home or community (eg, activities of daily living [ADLs], instrumental ADLs [iADLs], transfers,
mobility, communication, swallowing, feeding, problem solving, safety practices) (without the
patient present), face to face; each additional 15 minutes (List separately in addition to code for
primary service)), with a work RVU of 0.54 as we believe this service reflects the resource costs
associated when the billing practitioner performs HCPCS code GCTM2. CPT code 97551 has an
intraservice time of 17 minutes, and the physician work is of similar intensity to our proposed
HCPCS code GCTM2. Therefore, we are proposing a work time of 17 minutes for HCPCS code
GCTM2 based on this same crosswalk to CPT 97551. We also propose to use this crosswalk to
establish the direct PE inputs for HCPCS code GCTM2.
For GCTM3, we propose a direct crosswalk to CPT Code 97552 (Group caregiver
training in strategies and techniques to facilitate the patient's functional performance in the
home or community (eg, activities of daily living [ADLs], instrumental ADLs [iADLs], transfers,
mobility, communication, swallowing, feeding, problem solving, safety practices) (without the
patient present), face to face with multiple sets of caregivers), with a work RVU of 0.23 as we
believe this service reflects the resource costs associated when the billing practitioner performs
HCPCS code GCTM3. CPT code 97552 has an intraservice time of 9 minutes, and the physician
work is of similar intensity to our proposed HCPCS code GCTM1. Therefore, we are proposing
a work time of 9 minutes intraservice time (14 minutes total time) for HCPCS code GCTM3
based on this same crosswalk to CPT 97552. We are also proposing to use this crosswalk to
establish the direct PE inputs for HCPCS code GCTM3.
We are seeking comment on supplies/equipment that would be typical for the newly
created direct care strategies and techniques CTS codes.
We believe these services would largely involve contact between the billing practitioner
and the caregiver through in-person interactions, which could be conducted via
telecommunications, as appropriate. Therefore, we are proposing to add these codes to the
Medicare Telehealth Services List to accommodate a scenario in which the practitioner
completes the caregiver training service via telehealth. Please see section II.D. for more
information on Medicare Telehealth Services.
We are seeking comments on these proposals.
(B). Individual Behavior Management/ Modification Caregiver Training Services
i. Coding
We are proposing to establish new coding and payment for caregiver behavior
management and modification training that could be furnished to the caregiver(s) of an
individual patient. Current CPT coding (CPT 96202 and 96203) allows for “multiple-family
group behavior management/modification training services,” meaning that this caregiver training
service can only be furnished in a group setting with multiple sets of caregivers of multiple
beneficiaries (please reference 88 FR 78818 for discussion of CPT 96202 and 96203). We are
proposing two new HCPCS codes: GCTB1 (Caregiver training in behavior
management/modification for caregiver(s) of a patient with a mental or physical health
diagnosis, administered by physician or other qualified health care professional (without the
patient present), face-to-face; initial 30 minutes) and GCTB2 (Caregiver training in behavior
management/modification for caregiver(s) of a patient with a mental or physical health
diagnosis, administered by physician or other qualified health care professional (without the
patient present), face-to-face; each additional 15 minutes (List separately in addition to code for
primary service) (Use GCTB2 in conjunction with GCTB1)).
We continue to believe that CTS may be reasonable and necessary when they are integral
to a patient’s overall treatment and furnished after the treatment plan is established. The behavior
management/modification CTS themselves need to be congruent with the treatment plan and
designed to effectuate the desired patient outcomes. We believe this is especially the case in
medical treatment scenarios where assistance by the caregiver receiving the CTS is necessary to
ensure a successful treatment outcome for the patient—for example when the patient cannot
follow through with the treatment plan for themselves. Behavior management/modification
training for caregivers of Medicare beneficiaries should be directly relevant to the person-
centered treatment plan for the patient in order for the services to be considered reasonable and
necessary under the Medicare program. Each training activity should be clearly identified and
documented in the treatment plan. All other policies and procedures surrounding CPT 96202 and
96203 will also apply to these services (88 FR 78914-78920).
We are seeking comment on these proposals.
ii. Valuation
For GCTB1, we propose a direct crosswalk to CPT Code 97550 (Caregiver training in
strategies and techniques to facilitate the patient’s functional performance in the home or
community (eg, activities of daily living [ADLs], instrumental ADLs [iADLs], transfers, mobility,
communication, swallowing, feeding, problem solving, safety practices) (without the patient
present), face to face; initial 30 minutes), with a work RVU of 1.00 as we believe this service
reflects the resource costs associated when the billing practitioner performs HCPCS code
GCTB1. CPT code 97550 has an intraservice time of 30 minutes, and the physician work is of
similar intensity to our proposed HCPCS code GCTB1. Therefore, we are proposing a work time
of 30 minutes intraservice time (40 minutes of total time) for HCPCS code GCTB1 based on this
same crosswalk to CPT 97550. We also propose to use this crosswalk to establish the direct PE
inputs for HCPCS code GCTB1. We are seeking comment on supplies/equipment that would be
typical for the newly created individual behavior management/ modification CTS codes.
For GCTB2, we propose a direct crosswalk to CPT Code 97551 (Caregiver training in
strategies and techniques to facilitate the patient’s functional performance in the home or
community (eg, activities of daily living [ADLs], instrumental ADLs [iADLs], transfers, mobility,
communication, swallowing, feeding, problem solving, safety practices) (without the patient
present), face to face; each additional 15 minutes (List separately in addition to code for primary
service)), with a work RVU of 0.54 as we believe this service reflects the resource costs
associated when the billing practitioner performs HCPCS code GCTB2. CPT code 97551 has an
intraservice time of 17 minutes, and the physician work is of similar intensity to our proposed
HCPCS code GCTB2. Therefore, we are proposing a work time of 17 minutes for HCPCS code
GCTB2 based on this same crosswalk to CPT 97551. We also propose to use this crosswalk to
establish the direct PE inputs for HCPCS code GCTB2.
We are seeking comment on supplies/equipment that would be typical for the newly
created individual behavior management/ modification CTS codes.
We believe these services would largely involve contact between the billing practitioner
and the caregiver through in-person interactions, which could be conducted via
telecommunications as appropriate. Therefore, we are proposing to add these codes to the
Medicare Telehealth Services List to accommodate a scenario in which the practitioner
completes the caregiver training service via telehealth. Please see section II.D. for more
information on Medicare Telehealth Services.
We are seeking comments on these proposals.
(C). Patient Consent
In the CY 2024 PFS final rule (88 FR 78916), we finalized a requirement that the treating
practitioner must obtain the patient’s (or representative’s) consent for the caregiver to receive the
CTS and that the identified need for CTS and the patient’s (or representative’s) consent for one
or more specific caregivers to receive CTS be documented in the patient’s medical record.
We are proposing that consent for CTS can be provided verbally by the patient (or
representative). This would align consent requirements with other services paid under the PFS
that may be furnished without the patient present, such as certain care management services. This
proposal would apply to CPT codes 97550, 97551, 97552, 96202, and 96203, as well as any
caregiver training services HCPCS codes finalized in this year’s rule, and any subsequently
created caregiver training service codes. We are seeking comment on this proposal.
(D). Addition to Telehealth List
Please see section II.D. of this proposed rule, Payment for Medicare Telehealth Services,
for the discussion related to proposals to add CTS to the Medicare Telehealth list.
(40) Request for Information for Services Addressing Health-Related Social Needs (Community
Health Integration (G0019, G0022), Principal Illness Navigation (G0023, G0024), Principal
Illness Navigation-Peer Support (G0140, G0146), and Social Determinants of Health Risk
Assessment (G0136))
a. Background
In the CY 2024 PFS final rule (88 FR 78920), we finalized G-codes to reflect new coding
and payment for services describing Community Health Integration (CHI), G0019 (Community
health integration services performed by certified or trained auxiliary personnel, including a
community health worker, under the direction of a physician or other practitioner; 60 minutes per
calendar month), and G0022 (Community health integration services, each additional 30 minutes
per calendar month), which may include a community health worker (CHW), incident to the
professional services and under the general supervision of the billing practitioner. We finalized a
new stand-alone G code describing a SDOH Risk Assessment, G0136 (Administration of a
standardized, evidence-based Social Determinants of Health Risk Assessment, 5–15 minutes, not
more often than every 6 months). SDOH risk assessment refers to a review of the individual’s
SDOH or identified social risk factors that influence the diagnosis and treatment of medical
conditions. We also finalized PIN services, described by HCPCS code G0023 (Principal Illness
Navigation services by certified or trained auxiliary personnel under the direction of a physician
or other practitioner, including a patient navigator or certified peer specialist; 60 minutes per
calendar month) and G0024 (Principal Illness Navigation services, additional 30 minutes per
calendar month); G0140 (Principal Illness Navigation—Peer Support by certified or trained
auxiliary personnel under the direction of a physician or other practitioner, including a certified
peer specialist; 60 minutes per calendar month) and G0146 (Principal Illness Navigation—Peer
Support, additional 30 minutes per calendar month), to better recognize through coding and
payment policies when certified or trained auxiliary personnel under the direction of a billing
practitioner, which may include a patient navigator or certified peer support specialist, are
involved in the patient’s health care navigation as part of the treatment plan for a serious, high-
risk disease expected to last at least 3 months, that places the patient at significant risk of
hospitalization or nursing home placement, acute exacerbation/decompensation, functional
decline, or death.
b. Request for Information on Services Addressing Health-Related Social Needs
For CY 2025 we are issuing a broad request for information (RFI) on the newly
implemented Community Health Integration (CHI) (HPCCS codes G0019, G0022), Principal
Illness Navigation (PIN) (HCPCS codes G0023, G0024), Principal Illness Navigation- Peer
Support (PIN-PS) (HCPCS codes G0140, G0146), and Social Determinants of Health Risk
Assessment (SDOH RA) (HCPCS code G0136) services to engage interested parties on
additional policy refinements for CMS to consider in future rulemaking.
We are interested in better addressing the social needs of beneficiaries and requesting
information on the codes we created and finalized beginning in CY 2024 to fully encompass
what interested parties and commenters believe should be included in the coding and payment
we recently established. We are seeking comment on any related services that may not be
described by the current coding that we finalized in the CY 2024 PFS final rule and that are
medically reasonable and necessary “for the diagnosis or treatment of illness or injury” under
section 1862(a)(1)(A) of the Act. We believe we can work within the current coding framework
and explore additional opportunities to create codes that describe reasonable and necessary
services furnished by billing practitioners and the auxiliary personnel under their general
supervision. We are interested in feedback regarding any barriers to furnishing the services
addressing health-related social needs, and if the service described by the codes we established
are allowing practitioners to better address unmet social needs that interfere with the
practitioners’ ability to diagnose and treat the patient. This could include barriers specific to
certain populations, including rural and tribal communities, residents of the U.S. Territories,
individuals with disabilities, individuals with limited English proficiency, or other populations
who experience specific unmet social needs.
In response to the CY 2024 PFS proposed rule, we heard from commenters that CSWs
often connect individuals with community-based resources to address unmet social needs that
affect the diagnosis and treatment of medical problems. CSWs can bill Medicare directly for
services they personally perform for the diagnosis or treatment of mental illness but are not
authorized by statute to bill for services that are provided by auxiliary personnel incident to their
professional services. Since CHI and PIN codes are typically provided by auxiliary personnel
supervised by the billing practitioner, CSWs could serve as the auxiliary personnel. CSWs could
not directly bill Medicare for CHI and PIN services if they were provided by auxiliary personnel,
as they are not authorized to supervise, bill, and be paid directly by Medicare for services that are
provided by auxiliary personnel incident to their professional services. We believe the current
CHI and PIN coding accurately captures the services CSWs currently provide, including the
work involved in connecting beneficiaries with community-based resources for unmet social
needs that affect the diagnosis or treatment of medical problems. As we stated previously in the
CY 2024 PFS final rule (88 FR 78926), “the codes do not limit the types of other health care
professionals, such as registered nurses and social workers, that can perform CHI services (and
PIN services, as we discuss in the next section) incident to the billing practitioner’s professional
services, so long as they meet the requirements to provide all elements of the service included in
the code, consistent with the definition of auxiliary personnel at § 410.26(a)(1).” We are
clarifying that when we refer to “certified or trained auxiliary personnel” in the following codes:
G0019, G0022, G0023, G0024, G0140, G0146, this also includes CSWs.
We are requesting information if there are other types of auxiliary personnel, other
certifications, and/or training requirements that are not adequately captured in current coding and
payment for these services. We are also interested in hearing more about what types of auxiliary
personnel are typically furnishing these services, including the certifications and/or licensure that
they have. We are also interested in whether there are nuances or considerations that CMS
should understand related to auxiliary personnel and training, certifications or licensure barriers
or requirements that are specifically experienced by practitioners serving underserved
communities. This could include settings such as community mental health centers, community
health clinics including FQHCs and RHCs, tribal health centers, migrant farmworker clinics, or
facilities located in and serving rural and geographically isolated communities including the U.S.
Territories.
As noted in the CY 2023 PFS final rule (87 FR 69790) and explained in the CY 2023
PFS proposed rule (87 FR 46102), when we refer to community-based organizations, we mean
public or private not-for-profit entities that provide specific services to the community or
targeted populations in the community to address the health and social needs of those
populations. They may include community-action agencies, housing agencies, area agencies on
aging, centers for independent living, aging and disability resource centers or other non-profits
that apply for grants or contract with healthcare entities to perform social services. They may
receive grants from other agencies in the U.S. Department of Health and Human Services,
including Federal grants administered by the Administration for Children and Families (ACF),
Administration for Community Living (ACL), the Centers for Disease Control and Prevention
(CDC), the Substance Abuse and Mental Health Services Administration (SAMHSA), or State-
funded grants to provide social services. We stated that, generally, we believe such organizations
know the populations and communities they serve and may have the infrastructure or systems in
place to assist practitioners to provide CHI and PIN services. We stated that we understood that
many community-based organizations (CBOs) provide social services and do other work that is
beyond the scope of CHI and PIN services, but we believed they are well-positioned to develop
relationships with practitioners for providing reasonable and necessary CHI and PIN services.
We are interested in hearing more about CBOs and their collaborative relationships with
billing practitioners. The new codes for CHI and PIN services recognized CBOs and their role in
providing auxiliary personnel under the general supervision of the billing practitioners. We are
seeking comment regarding the extent to which practitioners are contracting with CBOs
(including current or planned contracting arrangements) for auxiliary personnel purposes, and if
there is anything else CMS should do to clarify services where auxiliary personnel can be
employed by the CBO, so long as they are under the general supervision of the billing
practitioner. Given that the CHI and PIN services may be provided incident to the billing
practitioner’s professional services, we are also seeking comment on whether the incident to
billing construct is appropriate for CBOs to supplement pre-existing staffing arrangements and
the CBO/provider interface. We are also seeking comment on CBOs' roles, the extent to which
practitioners are contracting with CBOs, incident to billing, and auxiliary personnel employed by
CBOs under general supervision of practitioners serving and located in rural, tribal and
geographically isolated communities, including the U.S. Territories.
We are also interested in any comments from interested parties across provider types and
from practitioners in geographically isolated communities (for example, rural, tribal, and island
communities) and otherwise underserved communities about coding Z codes on claims
associated with billing for CHI, PIN, and SDOH risk assessment codes. We recognize that when
screening for social needs, such needs may be identified and are interested in learning whether
practitioners are also capturing unmet social needs on claims using Z codes for social risk factors
or in some other way, and any barriers or opportunities to increase coding of Z codes when social
risk factors screen positive.
Over the past several years, we have worked to develop payment mechanisms under the
PFS to improve the accuracy of valuation and payment for the services furnished by physicians
and other health care professionals, especially in the context of evolving models of care and
addressing unmet social needs that affect the diagnosis and treatment of medical problems.
Given the Agency’s broader policy goals of increasing access to care, we are requesting
information from interested parties and commenters on anything else that we should consider in
the context of these codes and what else we could consider to be included in this newly
established code set.
We are seeking comments on ways to identify specific services and to recognize possible
barriers to improved access to these kinds of high-value, potentially underutilized services by
Medicare beneficiaries.
We are seeking public comment to understand more clearly how often evidence-based
care for persons with fractures, for example, is not provided and the reasons for this, and how
recent or new PFS codes, or their revaluation, might help resolve specific barriers to its
provision. The PFS currently includes many codes that pay for various components of care to
manage patients with fractures over a course of treatment, such as transitional care management
(TCM) and other care management services, evaluation and management visits (including the
inherent complexity add-on for office/outpatient visits), principal illness navigation services,
community health integration services, and the social determinants of health risk assessment. We
refer readers to our recent guidance on these services on the CMS website at
https://www.cms.gov/files/document/health-related-social-needs-faq.pdf. Medicare also pays for
bone mass measurement/density tests (MLN006559 –
https://www.cms.gov/medicare/prevention/prevntiongeninfo/medicare-preventive-services/mps-
quickreferencechart-1.html#BONE_MASS, and for outpatient osteoporosis medication under Part
D and, in some cases, Part B (https://www.medicare.gov/coverage/osteoporosis-drugs). These
services can be billed on their own, or in combination, where applicable. We note that in the CY
2020 PFS final rule (84 FR 62685) and CY 2021 PFS final rule (85 FR 84547), CMS indicated
that TCM may be billed concurrently with other care management codes when relevant,
medically necessary, and not duplicative.
We are proposing new coding in other sections of this CY 2025 proposed rule that might
be used to bill for managing fractures under a treatment plan, including the global post-operative
add-on code, HCPCS code GPOC1, in section II.G.5 of this proposed rule and the advanced
primary care management codes in section II.G.2 of this proposed rule. Interested parties have
indicated that orthopedic surgeons, skilled nursing facilities (SNFs), and other practitioners and
providers may not be providing comprehensive patient centered fracture management care for
quality, payment, or administrative reasons, and that there is inadequate “hand-off” when post-
discharge fracture care is transferred to practitioners in the community. They indicate a systemic
disconnect on which provider and/or specialty is responsible for osteoporosis diagnosis and
treatment, and that global surgical periods focus on acute fracture recovery rather than
addressing osteoporosis. We are interested in hearing if the proposed global postop add-on code
could help resolve these issues.

TABLE 13: CY 2025 Work RVUs for New, Revised, and Potentially Misvalued Codes
HCPCS
Descriptor
Current
work
RVU
RUC
work
RVU
CMS
work
RVU
CMS time
refinement
15XX1
Harvest of skin for skin cell suspension autograft; first 25
sq cm or less
NEW
3.00
C
Yes
15XX2
Harvest of skin for skin cell suspension autograft; each
additional 25 sq cm or part thereof (List separately in
addition to code for primary procedure)
NEW
2.00
C
Yes
15XX3
Preparation of skin cell suspension autograft, requiring
enzymatic processing, manual mechanical disaggregation
of skin cells, and filtration; first 25 sq cm or less of
harvested skin
NEW
2.51
C
Yes
15XX4
Preparation of skin cell suspension autograft, requiring
enzymatic processing, manual mechanical disaggregation
of skin cells, and filtration; each additional 25 sq cm of
harvested skin or part thereof (List separately in addition to
code for primary procedure)
NEW
2.00
C
Yes
15XX5
Application of skin cell suspension autograft to wound and
donor sites, including application of primary dressing,
trunk, arms, legs; first 480 sq cm or less
NEW
10.97
C
Yes
15XX6
Application of skin cell suspension autograft to wound and
donor sites, including application of primary dressing,
trunk, arms, legs; each additional 480 sq cm or part thereof
(List separately in addition to code for primary procedure)
NEW
2.50
C
Yes
15XX7
Application of skin cell suspension autograft to wound and
donor sites, including application of primary dressing, face,
scalp, eyelids, mouth, neck, ears, orbits, genitalia, hands,
feet, and/or multiple digits; first 480 sq cm or less
NEW
12.50
C
Yes
15XX8
Application of skin cell suspension autograft to wound and
donor sites, including application of primary dressing, face,
scalp, eyelids, mouth, neck, ears, orbits, genitalia, hands,
feet, and/or multiple digits; each additional 480 sq cm or
part thereof (List separately in addition to code for primary
procedure)
NEW
3.00
C
Yes
25310
Tendon transplantation or transfer, flexor or extensor,
forearm and/or wrist, single; each tendon
8.08
9.50
9.00
No
25447
Arthroplasty, intercarpal or carpometacarpal joints;
interposition (eg, tendon)
11.14
11.14
10.50
No
26480
Transfer or transplant of tendon, carpometacarpal area or
dorsum of hand; without free graft, each tendon
6.90
9.50
9.00
No
2X005
Arthroplasty, intercarpal or carpometacarpal joints;
suspension, including transfer or transplant of tendon, with
interposition, when performed
NEW
13.90
11.85
No
36514
Therapeutic apheresis; for plasma pheresis
1.81
1.81
1.81
No
36516
Therapeutic apheresis; with extracorporeal
immunoadsorption, selective adsorption or selective
filtration and plasma reinfusion
1.56
1.56
1.56
No
36522
Photopheresis, extracorporeal
1.75
1.75
1.75
No
3X018
Chimeric antigen receptor T-cell (CAR-T) therapy;
harvesting of blood-derived T lymphocytes for
development of genetically modified autologous CAR-T
cells, per day
NEW
1.94
1.94
No
3X019
Chimeric antigen receptor T-cell (CAR-T) therapy;
preparation of blood-derived T lymphocytes for
transportation (eg, cryopreservation, storage)
NEW
0.79
0.79
No
3X020
Chimeric antigen receptor T-cell (CAR-T) therapy; receipt
and preparation of CAR-T cells for administration
NEW
0.80
0.80
No

HCPCS
Descriptor
Current
work
RVU
RUC
work
RVU
CMS
work
RVU
CMS time
refinement
3X021
Chimeric antigen receptor T-cell (CAR-T) therapy; CAR-T
cell administration, autologous
NEW
3.00
3.00
No
4X015
Excision or destruction, open, intra-abdominal (ie,
peritoneal, mesenteric, retroperitoneal), primary or
secondary tumor(s) or cyst(s), sum of the maximum length
of tumor(s) or cyst(s); 5 cm or less
NEW
22.00
22.00
No
4X016
Excision or destruction, open, intra-abdominal (ie,
peritoneal, mesenteric, retroperitoneal), primary or
secondary tumor(s) or cyst(s), sum of the maximum length
of tumor(s) or cyst(s); 5.1 to 10 cm
NEW
28.65
28.65
No
4X017
Excision or destruction, open, intra-abdominal (ie,
peritoneal, mesenteric, retroperitoneal), primary or
secondary tumor(s) or cyst(s), sum of the maximum length
of tumor(s) or cyst(s); 10.1 to 20 cm
NEW
34.00
34.00
No
4X018
Excision or destruction, open, intra-abdominal (ie,
peritoneal, mesenteric, retroperitoneal), primary or
secondary tumor(s) or cyst(s), sum of the maximum length
of tumor(s) or cyst(s); 20.1 to 30 cm
NEW
45.00
40.00
No
4X019
Excision or destruction, open, intra-abdominal (ie,
peritoneal, mesenteric, retroperitoneal), primary or
secondary tumor(s) or cyst(s), sum of the maximum length
of tumor(s) or cyst(s); greater than 30 cm
NEW
55.00
50.00
No
59200
Insertion of cervical dilator (eg, laminaria, prostaglandin)
(separate procedure)
0.79
1.20
1.20
No
5X006
Insertion of transurethral ablation transducer for delivery of
thermal ultrasound for prostate tissue ablation, including
suprapubic tube placement during the same session and
placement of an endorectal cooling device, when
performed
NEW
4.05
4.05
No
5X007
Ablation of prostate tissue, transurethral, using thermal
ultrasound, including magnetic resonance imaging
guidance for, and monitoring of, tissue ablation;
NEW
9.80
9.80
No
5X008
Ablation of prostate tissue, transurethral, using thermal
ultrasound, including magnetic resonance imaging
guidance for, and monitoring of, tissue ablation; with
insertion of transurethral ultrasound transducer for delivery
of thermal ultrasound, including suprapubic tube placement
and placement of an endorectal cooling device, when
performed
NEW
11.50
11.50
No
5XX05
Cystourethroscopy with insertion of temporary device for
ischemic remodeling (ie, pressure necrosis) of bladder neck
and prostate
NEW
3.10
3.10
No
5XX06
Catheterization with removal of temporary device for
ischemic remodeling (ie, pressure necrosis) of bladder neck
and prostate
NEW
1.48
1.48
No
64486
Transversus abdominis plane (TAP) block (abdominal
plane block, rectus sheath block) unilateral; by injection(s)
(includes imaging guidance, when performed)
1.27
1.20
1.20
No
64487
Transversus abdominis plane (TAP) block (abdominal
plane block, rectus sheath block) unilateral; by continuous
infusion(s) (includes imaging guidance, when performed)
1.48
1.39
1.39
No
64488
Transversus abdominis plane (TAP) block (abdominal
plane block, rectus sheath block) bilateral; by injections
(includes imaging guidance, when performed)
1.60
1.40
1.40
No
64489
Transversus abdominis plane (TAP) block (abdominal
plane block, rectus sheath block) bilateral; by continuous
infusions (includes imaging guidance, when performed)
1.80
1.75
1.75
No
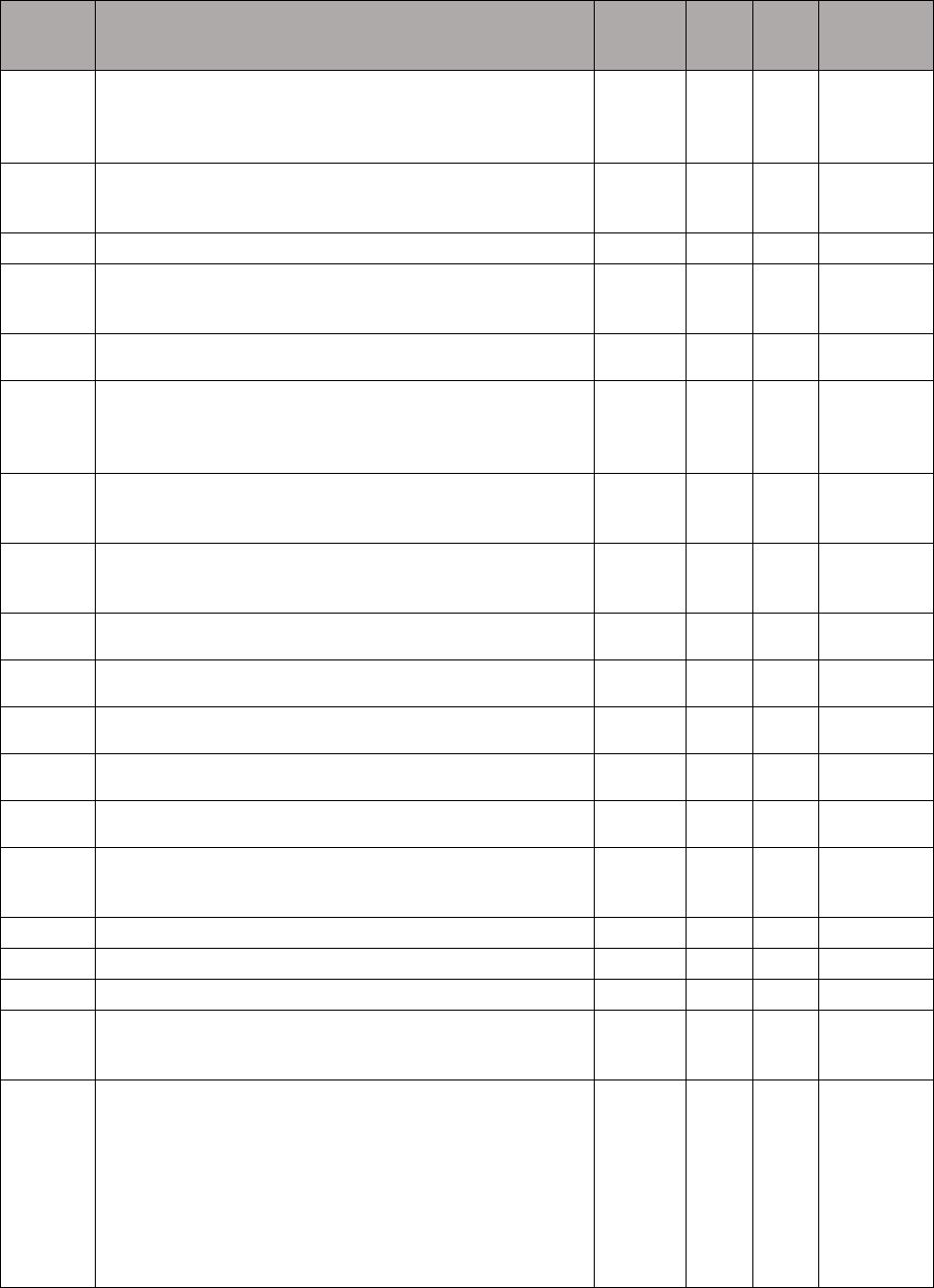
HCPCS
Descriptor
Current
work
RVU
RUC
work
RVU
CMS
work
RVU
CMS time
refinement
64590
Insertion or replacement of peripheral, sacral, or gastric
neurostimulator pulse generator or receiver, requiring
pocket creation and connection between electrode array
and pulse generator or receiver
5.10
5.10
5.10
No
64595
Revision or removal of peripheral, sacral, or gastric
neurostimulator pulse generator or receiver, with
detachable connection to electrode array
3.79
3.79
3.79
No
66680
Repair of iris, ciliary body (as for iridodialysis)
6.39
10.25
7.97
No
66682
Suture of iris, ciliary body (separate procedure) with
retrieval of suture through small incision (eg, McCannel
suture)
7.33
10.87
8.74
No
6X004
Implantation of iris prosthesis, including suture fixation
and repair or removal of iris, when performed
NEW
12.80
10.67
No
6XX00
Magnetic resonance image guided high intensity focused
ultrasound (MRgFUS), stereotactic ablation of target,
intracranial, including stereotactic navigation and frame
placement, when performed
NEW
18.95
16.60
No
6XX01
Ablation of 1 or more thyroid nodule(s), one lobe or the
isthmus, percutaneous, including imaging guidance,
radiofrequency
NEW
5.75
5.75
No
6XX02
Ablation of 1 or more thyroid nodule(s), additional lobe,
percutaneous, including imaging guidance, radiofrequency
(List separately in addition to code for primary procedure)
NEW
4.25
4.25
No
6XX07
Thoracic fascial plane block, unilateral; by injection(s),
including imaging guidance, when performed
NEW
1.50
1.50
No
6XX08
Thoracic fascial plane block, unilateral; by continuous
infusion(s), including imaging guidance, when performed
NEW
1.74
1.74
No
6XX09
Thoracic fascial plane block, bilateral; by injection(s),
including imaging guidance, when performed
NEW
1.67
1.67
No
6XX10
Thoracic fascial plane block, bilateral; by continuous
infusion(s), including imaging guidance, when performed
NEW
1.83
1.83
No
6XX11
Lower extremity fascial plane block, unilateral; by
injection(s), including imaging guidance, when performed
NEW
1.34
1.34
No
6XX12
Lower extremity fascial plane block, unilateral; by
continuous infusion(s), including imaging guidance, when
performed
NEW
1.67
1.67
No
76981
Ultrasound, elastography; parenchyma (eg, organ)
0.59
0.59
0.59
No
76982
Ultrasound, elastography; first target lesion
0.59
0.59
0.59
No
76983
Ultrasound, elastography; each additional target lesion
0.50
0.47
0.47
No
77012
Computed tomography guidance for needle placement (eg,
biopsy, aspiration, injection, localization device),
radiological supervision and interpretation
1.50
1.50
1.50
No
7XX00
MR safety implant and/or foreign body assessment by
trained clinical staff, including identification and
verification of implant components from appropriate
sources (eg, surgical reports, imaging reports, medical
device databases, device vendors, review of prior imaging),
analyzing current MR conditional status of individual
components and systems, and consulting published
professional guidance with written report; initial 15
minutes
NEW
0.00
0.00
No

HCPCS
Descriptor
Current
work
RVU
RUC
work
RVU
CMS
work
RVU
CMS time
refinement
7XX01
MR safety implant and/or foreign body assessment by
trained clinical staff, including identification and
verification of implant components from appropriate
sources (eg, surgical reports, imaging reports, medical
device databases, device vendors, review of prior imaging),
analyzing current MR conditional status of individual
components and systems, and consulting published
professional guidance with written report; each additional
30 minutes (List separately in addition to code for primary
procedure)
NEW
0.00
0.00
No
7XX02
MR safety determination by a physician or other qualified
health care professional responsible for the safety of the
MR procedure, including review of implant MR conditions
for indicated MR examination, analysis of risk vs clinical
benefit of performing MR examination, and determination
of MR equipment, accessory equipment, and expertise
required to perform examination, with written report
NEW
0.60
0.60
No
7XX03
MR safety medical physics examination customization,
planning and performance monitoring by medical physicist
or MR safety expert, with review and analysis by physician
or other qualified health care professional to prioritize and
select views and imaging sequences, to tailor MR
acquisition specific to restrictive requirements or artifacts
associated with MR conditional implants or to mitigate risk
of non-conditional implants or foreign bodies, with written
report
NEW
0.76
0.76
No
7XX04
MR safety implant electronics preparation under
supervision of physician or other qualified health care
professional, including MR-specific programming of pulse
generator and/or transmitter to verify device integrity,
protection of device internal circuitry from MR
electromagnetic fields, and protection of patient from risks
of unintended stimulation or heating while in the MR
room, with written report
NEW
0.75
0.75
No
7XX05
MR safety implant positioning and/or immobilization
under supervision of physician or other qualified health
care professional, including application of physical
protections to secure implanted medical device from MR-
induced translational or vibrational forces, magnetically
induced functional changes, and/or prevention of
radiofrequency burns from inadvertent tissue contact while
in the MR room, with written report
NEW
0.60
0.60
No
90480
Immunization administration by intramuscular injection of
severe acute respiratory syndrome coronavirus 2 (SARS-
CoV-2) (coronavirus disease [COVID-19]) vaccine, single
dose
X
0.25
0.25
No
92132
Computerized ophthalmic diagnostic imaging (eg, optical
coherence tomography [OCT]), anterior segment, with
interpretation and report, unilateral or bilateral
0.30
0.29
0.29
No
92133
Computerized ophthalmic diagnostic imaging (eg, optical
coherence tomography [OCT]), posterior segment, with
interpretation and report, unilateral or bilateral; optic nerve
0.40
0.31
0.31
No
92134
Computerized ophthalmic diagnostic imaging (eg, optical
coherence tomography [OCT]), posterior segment, with
interpretation and report, unilateral or bilateral; retina
0.45
0.32
0.32
No
93886
Transcranial Doppler study of the intracranial arteries;
complete study
0.91
0.90
0.90
No
93888
Transcranial Doppler study of the intracranial arteries;
limited study
0.50
0.73
0.73
No

HCPCS
Descriptor
Current
work
RVU
RUC
work
RVU
CMS
work
RVU
CMS time
refinement
93892
Transcranial Doppler study of the intracranial arteries;
emboli detection without intravenous microbubble
injection
1.15
1.15
1.15
No
93893
Transcranial Doppler study of the intracranial arteries;
venous-arterial shunt detection with intravenous
microbubble injection
1.15
1.15
1.15
No
93X94
Vasoreactivity study performed with transcranial Doppler
study of intracranial arteries, complete (List separately in
addition to code for primary procedure)
NEW
0.81
0.81
No
93X95
Emboli detection without intravenous microbubble
injection performed with transcranial Doppler study of
intracranial arteries, complete (List separately in addition to
code for primary procedure)
NEW
0.73
0.73
No
93X96
Venous-arterial shunt detection with intravenous
microbubble injection performed with transcranial Doppler
study of intracranial arteries, complete (List separately in
addition to code for primary procedure)
NEW
0.85
0.85
No
96380
Administration of respiratory syncytial virus, monoclonal
antibody, seasonal dose by intramuscular injection, with
counseling by physician or other qualified health care
professional
0.24
0.24
0.24
No
96381
Administration of respiratory syncytial virus, monoclonal
antibody, seasonal dose by intramuscular injection
0.17
0.17
0.17
No
96547
Intraoperative hyperthermic intraperitoneal chemotherapy
(HIPEC) procedure, including separate incision(s) and
closure, when performed; first 60 minutes (List separately
in addition to code for primary procedure)
C
6.53
6.53
No
96548
Intraoperative hyperthermic intraperitoneal chemotherapy
(HIPEC) procedure, including separate incision(s) and
closure, when performed; each additional 30 minutes
C
3.00
3.00
No
96920
Excimer laser treatment for psoriasis; total area less than
250 sq cm
1.15
1.00
0.83
No
96921
Excimer laser treatment for psoriasis; 250 sq cm to 500 sq
cm
1.30
1.07
0.90
No
96922
Excimer laser treatment for psoriasis; over 500 sq cm
2.10
1.32
1.15
No
97012
Application of a modality to 1 or more areas; traction,
mechanical
0.25
0.25
0.25
No
97014
Application of a modality to 1 or more areas; electrical
stimulation (unattended)
0.18
0.18
0.18
No
97016
Application of a modality to 1 or more areas;
vasopneumatic devices
0.18
0.18
0.18
No
97018
Application of a modality to 1 or more areas; paraffin bath
0.06
0.06
0.06
No
97022
Application of a modality to 1 or more areas; whirlpool
0.17
0.17
0.17
No
97032
Application of a modality to 1 or more areas; electrical
stimulation (manual), each 15 minutes
0.25
0.25
0.25
No
97033
Application of a modality to 1 or more areas;
iontophoresis, each 15 minutes
0.26
0.26
0.26
No
97034
Application of a modality to 1 or more areas; contrast
baths, each 15 minutes
0.21
0.21
0.21
No
97035
Application of a modality to 1 or more areas; ultrasound,
each 15 minutes
0.21
0.21
0.21
No
97110
Therapeutic procedure, 1 or more areas, each 15 minutes;
therapeutic exercises to develop strength and endurance,
range of motion and flexibility
0.45
0.45
0.45
No
97112
Therapeutic procedure, 1 or more areas, each 15 minutes;
neuromuscular reeducation of movement, balance,
0.50
0.50
0.50
No

HCPCS
Descriptor
Current
work
RVU
RUC
work
RVU
CMS
work
RVU
CMS time
refinement
coordination, kinesthetic sense, posture, and/or
proprioception for sitting and/or standing activities
97113
Therapeutic procedure, 1 or more areas, each 15 minutes;
aquatic therapy with therapeutic exercises
0.48
0.48
0.48
No
97116
Therapeutic procedure, 1 or more areas, each 15 minutes;
gait training (includes stair climbing)
0.45
0.45
0.45
No
97140
Manual therapy techniques (eg, mobilization/
manipulation, manual lymphatic drainage, manual
traction), 1 or more regions, each 15 minutes
0.43
0.43
0.43
No
97530
Therapeutic activities, direct (one-on-one) patient contact
(use of dynamic activities to improve functional
performance), each 15 minutes
0.44
0.44
0.44
No
97533
Sensory integrative techniques to enhance sensory
processing and promote adaptive responses to
environmental demands, direct (one-on-one) patient
contact, each 15 minutes
0.48
0.48
0.48
No
97535
Self-care/home management training (eg, activities of daily
living (ADL) and compensatory training, meal preparation,
safety procedures, and instructions in use of assistive
technology devices/adaptive equipment) direct one-on-one
contact, each 15 minutes
0.45
0.45
0.45
No
97537
Community/work reintegration training (eg, shopping,
transportation, money management, avocational activities
and/or work environment/modification analysis, work task
analysis, use of assistive technology device/adaptive
equipment), direct one-on-one contact, each 15 minutes
0.48
0.48
0.48
No
97542
Wheelchair management (eg, assessment, fitting, training),
each 15 minutes
0.48
0.48
0.48
No
97810
Acupuncture, 1 or more needles; without electrical
stimulation, initial 15 minutes of personal one-on-one
contact with the patient
0.60
0.61
0.61
No
97811
Acupuncture, 1 or more needles; without electrical
stimulation, each additional 15 minutes of personal one-on-
one contact with the patient, with insertion of needle(s)
(List separately in addition to code for primary procedure)
0.50
0.46
0.46
No
97813
Acupuncture, 1 or more needles; with electrical
stimulation, initial 15 minutes of personal one-on-one
contact with the patient
0.65
0.74
0.74
No
97814
Acupuncture, 1 or more needles; with electrical
stimulation, each additional 15 minutes of personal one-on-
one contact with the patient, with insertion of needle(s)
(List separately in addition to code for primary procedure)
0.55
0.47
0.47
No
9X059
Computerized ophthalmic diagnostic imaging (eg, optical
coherence tomography [OCT]), posterior segment, with
interpretation and report, unilateral or bilateral; retina,
including OCT angiography
NEW
0.64
0.64
No
9X075
Synchronous audio-video visit for the evaluation and
management of a new patient, which requires a medically
appropriate history and/or examination and straightforward
medical decision making. When using total time on the
date of the encounter for code selection, 15 minutes must
be met or exceeded.
NEW
0.93
I
Yes
9X076
Synchronous audio-video visit for the evaluation and
management of a new patient, which requires a medically
appropriate history and/or examination and low medical
decision making. When using total time on the date of the
encounter for code selection, 30 minutes must be met or
exceeded.
NEW
1.60
I
Yes

HCPCS
Descriptor
Current
work
RVU
RUC
work
RVU
CMS
work
RVU
CMS time
refinement
9X077
Synchronous audio-video visit for the evaluation and
management of a new patient, which requires a medically
appropriate history and/or examination and moderate
medical decision making. When using total time on the
date of the encounter for code selection, 45 minutes must
be met or exceeded.
NEW
2.60
I
Yes
9X078
Synchronous audio-video visit for the evaluation and
management of a new patient, which requires a medically
appropriate history and/or examination and high medical
decision making. When using total time on the date of the
encounter for code selection, 60 minutes must be met or
exceeded.
NEW
3.50
I
Yes
9X079
Synchronous audio-video visit for the evaluation and
management of an established patient, which requires a
medically appropriate history and/or examination and
straightforward medical decision making. When using total
time on the date of the encounter for code selection, 10
minutes must be met or exceeded.
NEW
0.70
I
Yes
9X080
Synchronous audio-video visit for the evaluation and
management of an established patient, which requires a
medically appropriate history and/or examination and low
medical decision making. When using total time on the
date of the encounter for code selection, 20 minutes must
be met or exceeded.
NEW
1.30
I
Yes
9X081
Synchronous audio-video visit for the evaluation and
management of an established patient, which requires a
medically appropriate history and/or examination and
moderate medical decision making. When using total time
on the date of the encounter for code selection, 30 minutes
must be met or exceeded.
NEW
1.92
I
Yes
9X082
Synchronous audio-video visit for the evaluation and
management of an established patient, which requires a
medically appropriate history and/or examination and high
medical decision making. When using total time on the
date of the encounter for code selection, 40 minutes must
be met or exceeded.
NEW
2.60
I
Yes
9X083
Synchronous audio-only visit for the evaluation and
management of a new patient, which requires a medically
appropriate history and/or examination, straightforward
medical decision making, and more than 10 minutes of
medical discussion. When using total time on the date of
the encounter for code selection, 15 minutes must be met or
exceeded.
NEW
0.90
I
Yes
9X084
Synchronous audio-only visit for the evaluation and
management of a new patient, which requires a medically
appropriate history and/or examination, low medical
decision making, and more than 10 minutes of medical
discussion. When using total time on the date of the
encounter for code selection, 30 minutes must be met or
exceeded.
NEW
1.55
I
Yes
9X085
Synchronous audio-only visit for the evaluation and
management of a new patient, which requires a medically
appropriate history and/or examination, moderate medical
decision making, and more than 10 minutes of medical
discussion. When using total time on the date of the
encounter for code selection, 45 minutes must be met or
exceeded.
NEW
2.42
I
Yes

HCPCS
Descriptor
Current
work
RVU
RUC
work
RVU
CMS
work
RVU
CMS time
refinement
9X086
Synchronous audio-only visit for the evaluation and
management of a new patient, which requires a medically
appropriate history and/or examination, high medical
decision making, and more than 10 minutes of medical
discussion. When using total time on the date of the
encounter for code selection, 60 minutes must be met or
exceeded.
NEW
3.20
I
Yes
9X087
Synchronous audio-only visit for the evaluation and
management of an established patient, which requires a
medically appropriate history and/or examination,
straightforward medical decision making, and more than 10
minutes of medical discussion. When using total time on
the date of the encounter for code selection, 10 minutes
must be exceeded.
NEW
0.65
I
Yes
9X088
Synchronous audio-only visit for the evaluation and
management of an established patient, which requires a
medically appropriate history and/or examination, low
medical decision making, and more than 10 minutes of
medical discussion. When using total time on the date of
the encounter for code selection, 20 minutes must be met or
exceeded.
NEW
1.20
I
Yes
9X089
Synchronous audio-only visit for the evaluation and
management of an established patient, which requires a
medically appropriate history and/or examination,
moderate medical decision making, and more than 10
minutes of medical discussion. When using total time on
the date of the encounter for code selection, 30 minutes
must be met or exceeded.
NEW
1.75
I
Yes
9X090
Synchronous audio-only visit for the evaluation and
management of an established patient, which requires a
medically appropriate history and/or examination, high
medical decision making, and more than 10 minutes of
medical discussion. When using total time on the date of
the encounter for code selection, 40 minutes must be met or
exceeded.
NEW
2.60
I
Yes
9X091
Brief communication technology-based service (eg, virtual
check-in) by a physician or other qualified health care
professional who can report evaluation and management
services, provided to an established patient, not originating
from a related evaluation and management service
provided within the previous 7 days nor leading to an
evaluation and management service or procedure within the
next 24 hours or soonest available appointment, 5-10
minutes of medical discussion
NEW
0.30
0.30
No
9X100
Medical genetics and genetic counseling services, each 30
minutes of total time provided by the genetic counselor on
the date of the encounter
NEW
0.00
0.00
No
G0138
Intravenous infusion of cipaglucosidase alfaatga, including
provider/supplier acquisition and clinical supervision of
oral administration of miglustat in preparation of receipt of
cipaglucosidase alfa-atga
C
-
0.21
Yes
G0168
Wound closure utilizing tissue adhesive(s) only
0.31
0.31
0.31
No
G0283
Electrical stimulation (unattended), to one or more areas
for indication(s) other than wound care, as part of a therapy
plan of care
0.18
0.18
0.18
No
G0442
Annual alcohol misuse screening, 5 to 15 minutes
0.18
0.18
0.18
No
G0443
Brief face-to-face behavioral counseling for alcohol
misuse, 15 minutes
0.45
0.60
0.60
No

HCPCS
Descriptor
Current
work
RVU
RUC
work
RVU
CMS
work
RVU
CMS time
refinement
G0444
Annual depression screening, 5 to 15 minutes
0.18
0.18
0.18
No
G0445
High intensity behavioral counseling to prevent sexually
transmitted infection; face-to-face, individual, includes:
education, skills training and guidance on how to change
sexual behavior; performed semi-annually, 30 minutes
0.45
0.45
0.45
No
G0446
Annual, face-to-face intensive behavioral therapy for
cardiovascular disease, individual, 15 minutes
0.45
0.45
0.45
No
G0447
Face-to-face behavioral counseling for obesity, 15 minutes
0.45
0.45
0.45
No
G0465
Autologous platelet rich plasma (prp) or other blood-
derived product for diabetic chronic wounds/ulcers, using
an fda-cleared device for this indication, (includes as
applicable administration, dressings, phlebotomy,
centrifugation or mixing, and all other preparatory
procedures, per treatment)
C
-
1.50
Yes
G0516
Insertion of non-biodegradable drug delivery implants, 4 or
more (services for subdermal rod implant)
1.82
1.82
1.82
No
G0517
Removal of non-biodegradable drug delivery implants, 4 or
more (services for subdermal implants)
2.10
2.10
2.10
No
G0518
Removal with reinsertion, non-biodegradable drug delivery
implants, 4 or more (services for subdermal implants)
3.55
3.55
3.55
No
GMBT1
Supply of digital mental health treatment device and initial
education and onboarding, per course of treatment that
augments a behavioral therapy plan
NEW
-
C
No
GMBT2
First 20 minutes of monthly treatment management
services directly related to the patient’s therapeutic use of
the digital mental health treatment (DMHT) device that
augments a behavioral therapy plan, physician/other
qualified health care professional time reviewing data
generated from the DMHT device from patient
observations and patient specific inputs in a calendar
month and requiring at least one interactive communication
with the patient/caregiver during the calendar month
NEW
-
0.62
No
GMBT3
Each additional 20 minutes of monthly treatment
management services directly related to the patient’s
therapeutic use of the digital mental health treatment
(DMHT) device that augments a behavioral therapy plan,
physician/other qualified health care professional time
reviewing data generated from the DMHT device from
patient observations and patient specific inputs in a
calendar month and requiring at least one interactive
communication with the patient/caregiver during the
calendar month
NEW
-
0.61
No
GCDRA
Administration of a standardized, evidence-based
Atherosclerotic Cardiovascular Disease (ASCVD) Risk
Assessment, 5-15 minutes, not more often than every 12
months
NEW
-
0.18
No
GCDRM
Atherosclerotic Cardiovascular Disease (ASCVD) risk
management services; clinical staff time; per calendar
month
NEW
-
0.18
No
GCTB1
Caregiver training in behavior management/modification
for caregiver(s) of patients with a mental or physical health
diagnosis, administered by physician or other qualified
health care professional (without the patient present), face-
to-face; initial 30 minutes
NEW
-
1.00
No

HCPCS
Descriptor
Current
work
RVU
RUC
work
RVU
CMS
work
RVU
CMS time
refinement
GCTB2
Caregiver training in behavior management/modification
for parent(s)/guardian(s)/caregiver(s) of patients with a
mental or physical health diagnosis, administered by
physician or other qualified health care professional
(without the patient present), face-to-face; each additional
15 minutes
NEW
-
0.54
No
GCTD1
Caregiver training in direct care strategies and techniques
to support care for patients with an ongoing condition or
illness and to reduce complications (without the patient
present), face-to-face; initial 30 minutes
NEW
-
1.00
No
GCTD2
Caregiver training in direct care strategies and techniques
to support care for patients with an ongoing condition or
illness and to reduce complications (without the patient
present), face-to-face; each additional 15 minutes
NEW
-
0.54
No
GCTD3
Group caregiver training in direct care strategies and
techniques to support care for patients with an ongoing
condition or illness and to reduce complications (without
the patient present), face-to-face with multiple sets of
caregivers
NEW
-
0.23
No
GFCI1
Post discharge telephonic follow-up contacts performed in
conjunction with a discharge from the emergency
department for behavioral health or other crisis encounter,
4 calls per calendar month.
NEW
-
1.00
No
GIDXX
Visit complexity inherent to hospital inpatient or
observation care associated with a confirmed or suspected
infectious disease by an infectious diseases consultant,
including disease transmission risk assessment and
mitigation, public health investigation, analysis, and
testing, and complex antimicrobial therapy counseling and
treatment. (add-on code, list separately in addition to
hospital inpatient or observation evaluation and
management visit, initial, same day discharge, or
subsequent
NEW
-
0.89
No
GIPC1
Interprofessional telephone/Internet/electronic health
record assessment and management service provided by a
practitioner in a specialty whose covered services are
limited by statute to services for the diagnosis and
treatment of mental illness, including a verbal and written
report to the patient’s treating/requesting practitioner; 5-10
minutes of medical consultative discussion and review
NEW
-
0.35
No
GIPC2
Interprofessional telephone/Internet/electronic health
record assessment and management service provided by a
practitioner in a specialty whose covered services are
limited by statute to services for the diagnosis and
treatment of mental illness, including a verbal and written
report to the patient’s treating/requesting practitioner; 11-
20 minutes of medical consultative discussion and review
NEW
-
0.70
No
GIPC3
Interprofessional telephone/Internet/electronic health
record assessment and management service provided by a
practitioner in a specialty whose covered services are
limited by statute to services for the diagnosis and
treatment of mental illness, including a verbal and written
report to the patient’s treating/requesting practitioner; 21-
30 minutes of medical consultative discussion and review
NEW
-
1.05
No

HCPCS
Descriptor
Current
work
RVU
RUC
work
RVU
CMS
work
RVU
CMS time
refinement
GIPC4
Interprofessional telephone/Internet/electronic health
record assessment and management service provided by a
practitioner in a specialty whose covered services are
limited by statute to services for the diagnosis and
treatment of mental illness, including a verbal and written
report to the patient’s treating/requesting practitioner; 31 or
more minutes of medical consultative discussion and
review
NEW
-
1.40
No
GIPC5
Interprofessional telephone/Internet/electronic health
record assessment and management service provided by a
practitioner in a specialty whose covered services are
limited by statute to services for the diagnosis and
treatment of mental illness, including a written report to the
patient’s treating/requesting practitioner, 5 minutes or more
of medical consultative time
NEW
-
0.70
No
GIPC6
Interprofessional telephone/Internet/electronic health
record referral service(s) provided by a treating/requesting
practitioner in a specialty whose covered services are
limited by statute to services for the diagnosis and
treatment of mental illness, 30 minutes
NEW
-
0.70
No
GPCM1
Advanced primary care management services furnished to
patients requiring primary care provided by clinical
staff/physician/other qualified health care professionals
who are responsible for all primary care and serve as the
continuing focal point for all needed health care services,
per calendar month, with the following elements:
• Development, implementation, revision, and maintenance
of a patient-centered electronic care plan;
• Overall comprehensive care management;
o Systematic needs assessment (medical and psychosocial)
o Ensure receipt of preventive services
o Medication reconciliation, management and oversight of
self-management
• Ongoing communication and care coordination between
relevant practitioners furnishing care (e.g., community-
based care);
o Coordinate with any home- and community-based
clinical service providers, and document communication
with them regarding psychosocial needs and functional
deficits
• Timely management of care transitions (e.g., ED visit and
hospital discharges) and referrals;
o Create/exchange continuity of care document(s) timely
(format not prescribed)
o Communication (direct contact, telephone, electronic)
with the patient and/or caregiver within 7 calendar days of
discharge
• Enhanced communications opportunities and digital
access
o Offer asynchronous non-face-to-face methods other than
telephone, such as secure email or patient portal
o Ensure access to virtual visits and consultations and
digital health communication technology-based services,
such as interprofessional consultations with other
practitioners, virtual check-ins, e-visits, and remote
evaluation of images, to maintain ongoing communication
with patient, as appropriate.).
NEW
-
0.17
No

HCPCS
Descriptor
Current
work
RVU
RUC
work
RVU
CMS
work
RVU
CMS time
refinement
GPCM2
Advanced primary care management services furnished to
patients with multiple (two or more) chronic conditions
expected to last at least 12 months, or until the death of the
patient, which place the patient at significant risk of death,
acute exacerbation/decompensation, or functional decline,
provided by clinical staff/physician/other qualified health
care professionals who are responsible for all primary care
and serve as the continuing focal point for all needed health
care services, per calendar month, with the elements
included in GPCM1
NEW
-
0.77
No
GPCM3
Advanced primary care management services furnished to
Qualified Medicare Beneficiaries with multiple (two or
more) chronic conditions expected to last at least 12
months, or until the death of the patient, which place the
patient at significant risk of death, acute
exacerbation/decompensation, or functional decline,
provided by clinical staff/physician/other qualified health
care professionals who are responsible for all primary care
and serve as the continuing focal point for all needed health
care services, per calendar month, with the elements
included in GPCM1
NEW
-
1.67
No
GPOC1
Post-operative follow-up visit complexity inherent to
evaluation and management services addressing surgical
procedure(s), provided by a physician or qualified health
care professional who is not the practitioner who
performed the procedure (or in the same group practice),
and is of a different specialty than the practitioner who
performed the procedure, within the 090-day global period
of the procedure(s), once per 090-day global period, when
there has not been a formal transfer of care and requires the
following required elements, when possible and applicable:
● Reading available surgical note to understand the
relative success of the procedure, the anatomy that was
affected, and potential complications that could have arisen
due to the unique circumstances of the patient’s operation.
● Research the procedure to determine expected post-
operative course and potential complications (in the case of
doing a post-op for a procedure outside the specialty).
● Evaluate and physically examine the patient to
determine whether the post-operative course is progressing
appropriately.
● Communicate with the practitioner who performed the
procedure if any questions or concerns arise.
(List separately in addition to office/outpatient evaluation
and management visit, new or established)
NEW
-
0.16
No
GSPI1
Safety planning interventions, including the following
elements: recognizing warning signs of an impending
suicidal crisis; employing internal coping strategies;
utilizing social contacts and social settings as a means of
distraction from suicidal thoughts; utilizing family
members or friends to help resolve the crisis; contacting
mental health professionals or agencies; and restricting
access to lethal means; (List separately in addition to an
E/M visit or psychotherapy).
NEW
-
1.09
No

TABLE 14: CY 2025 Direct PE Refinements
HCPCS
code
HCPCS code
description
Input
Code
Input code description
Nonfacility (NF)
/ Facility (F)
Labor activity
(where applicable)
RUC
recommendation
or current value
(min or qty)
CMS
refinement
(min or
qty)
Comment
Direct
costs
change
(in
dollars)
64486
Tap block unil by
injection
L037D
RN/LPN/MTA
NF
Assist physician or
other qualified
healthcare
professional---
directly related to
physician work
time (67% of
physician intra-
service time)
7.5
7
L13: Refined to correct
rounding error in clinical
labor calculation
-0.27
6XX07
Thrc fascial pln blk
uni njx
L037D
RN/LPN/MTA
NF
Assist physician or
other qualified
healthcare
professional---
directly related to
physician work
time (67% of
physician intra-
service time)
7.5
7
L13: Refined to correct
rounding error in clinical
labor calculation
-0.27
6XX11
Lwr xtr fscl pln blk
uni njx
L037D
RN/LPN/MTA
NF
Assist physician or
other qualified
healthcare
professional---
directly related to
physician work
time (67% of
physician intra-
service time)
7.5
7
L13: Refined to correct
rounding error in clinical
labor calculation
-0.27
77012
Ct scan for needle
biopsy
EL007
room, CT
NF
26
9
E11: Refined equipment
time to conform with
other codes in the family
-51.17
7XX00
Mr sfty implt&/fb
asmt stf 1
L047A
MRI Technologist
NF
Document
procedure
(nonPACS) (e.g.
mandated
reporting, registry
logs, EEG file, etc.)
2
1
L3: Refined clinical labor
time to conform with
identical labor activity in
other codes in the family
-0.76

HCPCS
code
HCPCS code
description
Input
Code
Input code description
Nonfacility (NF)
/ Facility (F)
Labor activity
(where applicable)
RUC
recommendation
or current value
(min or qty)
CMS
refinement
(min or
qty)
Comment
Direct
costs
change
(in
dollars)
7XX01
Mr sfty mplt&/fb
asmt stf ea
ED050
Technologist PACS
workstation
NF
45
32
E16: No equipment times
were included; aligned
equipment time with
assist physician time
-0.29
7XX01
Mr sfty mplt&/fb
asmt stf ea
L047A
MRI Technologist
NF
Document
procedure
(nonPACS) (e.g.
mandated
reporting, registry
logs, EEG file, etc.)
2
1
L3: Refined clinical labor
time to conform with
identical labor activity in
other codes in the family
-0.76
7XX01
Mr sfty mplt&/fb
asmt stf ea
L047A
MRI Technologist
NF
Perform
procedure/service--
-NOT directly
related to physician
work time
27
14
G1: See preamble text
-9.88
7XX02
Mr safety deter
phys/qhp
L047A
MRI Technologist
NF
Document
procedure
(nonPACS) (e.g.
mandated
reporting, registry
logs, EEG file, etc.)
2
1
L3: Refined clinical labor
time to conform with
identical labor activity in
other codes in the family
-0.76
7XX03
Mr sfty med physics
xm cstmz
ED053
Professional PACS
Workstation
F
13
0
G1: See preamble text
-0.80
7XX04
Mr safety implant
elec prepj
L047A
MRI Technologist
NF
Clean
room/equipment by
clinical staff
2
1
G1: See preamble text
-0.76
7XX04
Mr safety implant
elec prepj
L047A
MRI Technologist
NF
Document
procedure
(nonPACS) (e.g.
mandated
reporting, registry
logs, EEG file, etc.)
2
1
L3: Refined clinical labor
time to conform with
identical labor activity in
other codes in the family
-0.76
7XX05
Mr safety implt
pos&/immoblj
L047A
MRI Technologist
NF
Document
procedure
(nonPACS) (e.g.
mandated
2
1
L3: Refined clinical labor
time to conform with
identical labor activity in
other codes in the family
-0.76

HCPCS
code
HCPCS code
description
Input
Code
Input code description
Nonfacility (NF)
/ Facility (F)
Labor activity
(where applicable)
RUC
recommendation
or current value
(min or qty)
CMS
refinement
(min or
qty)
Comment
Direct
costs
change
(in
dollars)
reporting, registry
logs, EEG file, etc.)
7XX05
Mr safety implt
pos&/immoblj
L047A
MRI Technologist
NF
Clean
room/equipment by
clinical staff
2
1
G1: See preamble text
-0.76
7XX05
Mr safety implt
pos&/immoblj
SL082
impression material,
dental putty (per bite
block)
NF
1
0
S4: Supply not mentioned
in SOR work description
-2.14
96920
Excimer lsr
psriasis<250sqcm
EQ161
laser, excimer
NF
0
36
E13: Equipment item
replaces another item; see
preamble text
21.22
96920
Excimer lsr
psriasis<250sqcm
L037D
RN/LPN/MTA
NF
Clean
room/equipment by
clinical staff
5
3
L1: Refined time to
standard for this clinical
labor task
-1.08
96920
Excimer lsr
psriasis<250sqcm
SD363
laser, excimer, pay per
use (under 250 cm2 )
NF
1
0
S7: Supply item replaced
by another item; see
preamble
-80.00
96921
Excimer lsr psriasis
250-500
EQ161
laser, excimer
NF
0
38
E13: Equipment item
replaces another item; see
preamble text
22.40
96921
Excimer lsr psriasis
250-500
L037D
RN/LPN/MTA
NF
Clean
room/equipment by
clinical staff
5
3
L1: Refined time to
standard for this clinical
labor task
-1.08
96921
Excimer lsr psriasis
250-500
SD364
laser, excimer, pay per
use (250-500 cm2 )
NF
1
0
S7: Supply item replaced
by another item; see
preamble
-83.00
96922
Excimer lsr
psriasis>500sqcm
EQ161
laser, excimer
NF
0
44
E13: Equipment item
replaces another item; see
preamble text
25.94
96922
Excimer lsr
psriasis>500sqcm
L037D
RN/LPN/MTA
NF
Clean
room/equipment by
clinical staff
5
3
L1: Refined time to
standard for this clinical
labor task
-1.08
96922
Excimer lsr
psriasis>500sqcm
SD365
laser, excimer, pay per
use (> 500cm2)
NF
1
0
S7: Supply item replaced
by another item; see
preamble
-100.00
G0442
Annual alcohol
screen 15 min
L037D
RN/LPN/MTA
NF
Perform
procedure/service--
-NOT directly
related to physician
work time
5
15
G1: See preamble text
5.40

HCPCS
code
HCPCS code
description
Input
Code
Input code description
Nonfacility (NF)
/ Facility (F)
Labor activity
(where applicable)
RUC
recommendation
or current value
(min or qty)
CMS
refinement
(min or
qty)
Comment
Direct
costs
change
(in
dollars)
G0444
Depression screen
annual
L037D
RN/LPN/MTA
NF
Perform
procedure/service--
-NOT directly
related to physician
work time
5
15
G1: See preamble text
G0445
High inten beh couns
std 30m
EF023
table, exam
NF
17
20
G1: See preamble text
0.03
G0445
High inten beh couns
std 30m
SK057
paper, laser printing
(each sheet)
NF
10
0
S7: Supply item replaced
by another item; see
preamble
-0.20
G0445
High inten beh couns
std 30m
SK062
patient education booklet
NF
0
0.5
S8: Supply item replaces
another item; see
preamble
1.40
G0446
Intens behave ther
cardio dx
EF023
table, exam
NF
12
15
G1: See preamble text
0.03
G0446
Intens behave ther
cardio dx
SK057
paper, laser printing
(each sheet)
NF
10
0
S7: Supply item replaced
by another item; see
preamble
-0.20
G0446
Intens behave ther
cardio dx
SK062
patient education booklet
NF
0
0.5
S8: Supply item replaces
another item; see
preamble
1.40
G0447
Behavior counsel
obesity 15m
EF023
table, exam
NF
17
15
G1: See preamble text
-0.02
G0447
Behavior counsel
obesity 15m
SK057
paper, laser printing
(each sheet)
NF
10
0
S7: Supply item replaced
by another item; see
preamble
-0.20
G0447
Behavior counsel
obesity 15m
SK062
patient education booklet
NF
0
0.5
S8: Supply item replaces
another item; see
preamble
1.40

TABLE 15: CY 2025 Direct PE Refinements – Equipment Refinements Conforming to Changes in Clinical Labor Time
HCPCS
code
HCPCS code
description
Input
Code
Input code
description
Nonfacility
(NF) /
Facility (F)
Labor
activity
(where
applicable)
RUC
recommendation
or current value
(min or qty)
CMS
refinement
(min or
qty)
Comment
Direct
costs
change
(in
dollars)
64486
Tap block
unil by
injection
EF018
stretcher
NF
25.5
25
E15: Refined
equipment time to
conform to changes
in clinical labor time
-0.01
64486
Tap block
unil by
injection
EQ011
ECG, 3-
channel
(with SpO2,
NIBP, temp,
resp)
NF
25.5
25
E15: Refined
equipment time to
conform to changes
in clinical labor time
-0.01
6XX07
Thrc fascial
pln blk uni
njx
EF018
stretcher
NF
25.5
25
E15: Refined
equipment time to
conform to changes
in clinical labor time
-0.01
6XX07
Thrc fascial
pln blk uni
njx
EQ011
ECG, 3-
channel
(with SpO2,
NIBP, temp,
resp)
NF
25.5
25
E15: Refined
equipment time to
conform to changes
in clinical labor time
-0.01
6XX11
Lwr xtr fscl
pln blk uni
njx
EF018
stretcher
NF
25.5
25
E15: Refined
equipment time to
conform to changes
in clinical labor time
-0.01
6XX11
Lwr xtr fscl
pln blk uni
njx
EQ011
ECG, 3-
channel
(with SpO2,
NIBP, temp,
resp)
NF
25.5
25
E15: Refined
equipment time to
conform to changes
in clinical labor time
-0.01
7XX04
Mr safety
implant elec
prepj
EL008
room, MR
NF
21
20
E15: Refined
equipment time to
conform to changes
in clinical labor time
-3.28
7XX04
Mr safety
implant elec
prepj
EQ412
Vitals
monitoring
system (MR
Conditional)
NF
21
20
E15: Refined
equipment time to
conform to changes
in clinical labor time
-0.32
7XX05
Mr safety
implt
pos&/immobl
j
EL008
room, MR
NF
24
23
E15: Refined
equipment time to
conform to changes
in clinical labor time
-3.28

HCPCS
code
HCPCS code
description
Input
Code
Input code
description
Nonfacility
(NF) /
Facility (F)
Labor
activity
(where
applicable)
RUC
recommendation
or current value
(min or qty)
CMS
refinement
(min or
qty)
Comment
Direct
costs
change
(in
dollars)
7XX05
Mr safety
implt
pos&/immobl
j
EQ412
Vitals
monitoring
system (MR
Conditional)
NF
24
23
E15: Refined
equipment time to
conform to changes
in clinical labor time
-0.32
96920
Excimer lsr
psriasis<250s
qcm
EF031
table, power
NF
38
36
E15: Refined
equipment time to
conform to changes
in clinical labor time
-0.03
96920
Excimer lsr
psriasis<250s
qcm
EQ168
light, exam
NF
38
36
E15: Refined
equipment time to
conform to changes
in clinical labor time
-0.01
96921
Excimer lsr
psriasis 250-
500
EF031
table, power
NF
40
38
E15: Refined
equipment time to
conform to changes
in clinical labor time
-0.03
96921
Excimer lsr
psriasis 250-
500
EQ168
light, exam
NF
40
38
E15: Refined
equipment time to
conform to changes
in clinical labor time
-0.01
96922
Excimer lsr
psriasis>500s
qcm
EF031
table, power
NF
46
44
E15: Refined
equipment time to
conform to changes
in clinical labor time
-0.03
96922
Excimer lsr
psriasis>500s
qcm
EQ168
light, exam
NF
46
44
E15: Refined
equipment time to
conform to changes
in clinical labor time
-0.01
G0442
Annual
alcohol screen
15 min
EF023
table, exam
NF
10
15
E15: Refined
equipment time to
conform to changes
in clinical labor time
0.05
G0444
Depression
screen annual
EF023
table, exam
NF
10
15
E15: Refined
equipment time to
conform to changes
in clinical labor time
0.05

TABLE 16: CY 2025 Invoices Received for Existing Direct PE Inputs
CPT/HCPCS
codes
Item Name
CMS
code
Current
price
Updated
price
Percent
change
Number
of
invoices
Estimated non-
facility allowed
services for
HCPCS codes
using this item
30140,
30901,
30903,30905,
30906,
31231,
31237,
31238,
43197, 43198
Atomizer tips
(disposable)
SL464
$0.00
2.66
-
1
625,876
65778
human
amniotic
membrane
allograft
mounted on a
non-
absorbable
self-retaining
ring
SD248
$931.33
$1,149.00
23%
30
52,203
88341
Anti CD45
Monoclonal
Antibody
SL495
$5.15
$8.73
70%
3
1,094,158
88341,
88342,
88344,
88360, 88361
Benchmark
ULTRA
automated
slide
preparation
system
EP112
$125,040.59
$130,000.00
4%
2
2,683,605
88341,
88342,
88344,
88360, 88361
Reaction
buffer 10X
(Ventana 950-
300)
SL478
$0.037
$0.045
22%
3
2,683,605
88341,
88342,
88344,
88360, 88361
Liquid
coverslip
(Ventana 650-
010)
SL479
$0.051
$0.084
65%
3
2,683,605
88341,
88342,
88344,
88360, 88361
SSC (10X)
(Ventana 950-
110)
SL480
$0.051
$0.069
35%
2
2,683,605
88341,
88342,
88344,
88360, 88361
Cell
Conditioning 1
(Ventana 950-
124)
SL482
$0.560
$0.937
67%
3
2,683,605
88342
Confirm anti-
CD15 Mouse
Monoclonal
Antibody
(Ventana 760-
2504)
SL474
$4.90
$9.24
89%
3
1,157,793
92240, 92242
indocyanine
green (25ml
uou)
SL083
$76.94
$125.11
63%
8
36,974
93241,
93243,
93245, 93247
extended
external ECG
patch, medical
SD339
$260.35
$292.50
12%
20
510,943

CPT/HCPCS
codes
Item Name
CMS
code
Current
price
Updated
price
Percent
change
Number
of
invoices
Estimated non-
facility allowed
services for
HCPCS codes
using this item
magnetic tape
recorder
97810,
97811,
97813, 97814
needle,
acupuncture
SC075
$0.10
$0.199
99%
1
263,591
306 codes
pack, cleaning
and
disinfecting,
endoscope
SA042
$19.43
31.29
61%
2
-
7 codes
pack, drapes,
cystoscopy
SA045
$17.33
$14.99
-14%
2
-
Deleted from
all codes
pack, drapes,
laparotomy
(chest-
abdomen)
SA046
$7.26
-
-
-
-
67221
pack, ocular
photodynamic
therapy
SA049
$16.35
$26.35
61%
2
1,062
38 codes
pack, urology
cystoscopy
visit
SA058
$113.70
$37.63
-67%
2
-
145 codes
pack,
ophthalmology
visit (w-
dilation)
SA082
$3.91
$2.33
-40%
1
-

TABLE 17: CY 2025 New Invoices
CPT/HCPCS
codes
Item Name
CMS
code
Average
price
No. of
Invoices
NF Allowed
Services
5X006, 5X007,
5X008
TULSA-PRO TDC Cart
EQ41
0
1,638.60
1
2,300
5X007, 5X008
TULSA-PRO Disposable Kit
SA13
6
8,967.00
2
847
5XX05
iTIND device
SD36
6
2,695.00
2
295
6XX01
RF Electrodes 18 Gauge 70 mm Length
SD36
8
1,995.00
3
10
6XX01, 6XX02
RF Ablation System V1000 and RF
Pump
EQ41
1
49,950.0
0
2
11
7XX04, 7XX05
Disposable oximeter probe and clip (MR
Conditional)
SD36
9
6.40
1
19,215
7XX04, 7XX05
Vitals monitoring system (MR
Conditional)
EQ41
2
85,182.6
0
1
19,215
7XX05
Thermoplastic splint material 6”x9” (MR
Safe)
SG10
0
21.75
1
76
96920, 96921,
96922
Mupirocin 2% Topical Ointment 22
grams
SJ095
0.139
1
108,634
96920
laser, excimer, pay per use (under 250
cm2 )
SD36
3
80.00
5
73,369
96921
laser, excimer, pay per use (250-500 cm2
)
SD36
4
83.00
4
21,696
96922
laser, excimer, pay per use (> 500cm2)
SD36
5
100.00
3
13,569
9X059
tomographic device, optical coherence
angiography (OCTA)
EQ40
9
164,500.
00
2
360,890
G0138
Opfolda (65 mg capsule)
SH11
1
32.50
0
3,955
No codes
inFlow Measuring Device
SD37
0
140.00
1
-
No codes
inFlow Valve-Pump Device
SD37
1
495.00
1
-
No codes
inFlow Activator Kit
SD37
2
1,250.00
1
-
306 codes
(component of
SA042)
ortho-phthalaldehyde 0.55% (eg, Cidex
OPA)
SM03
0
0.554
1
-
306 codes
(component of
SA042)
ortho-phthalaldehyde test strips
SM03
1
1.556
1
-
7 codes (component
of SA045)
drape, surgical, legging
SB05
7
3.284
1
-
7 codes (component
of SA045)
drape, surgical, split, impervious,
absorbent
SB05
8
8.424
1
-
22510, 22511,
22513, 22514
Abdominal Drape Laparotomy Drape
Sterile (100 in x 72 in x 124 in)
SB05
6
8.049
1
12,721
67221 (component
of SA049)
kit, ocular photodynamic therapy (PDT)
SA13
7
26.00
1
1,062
67221 (component
of SA049)
y-adapter cap
SD36
7
0.352
1
1,062
145 codes
(component of
SA082)
post-mydriatic spectacles
SB05
9
0.328
1
-

TABLE 18: CY 2025 No PE Refinements
HCPCS
Description
15XX1
Hrv skn cll ssp agrft 1st 25
15XX2
Hrv skn cll ssp agrft ea add
15XX3
Prepj skn cll ssp agrft 1st
15XX4
Prepj skn cll ssp agrft ea
15XX5
App skn cl ssp agrft t/a/l 1
15XX6
App skn cl ssp agrf t/a/l ea
15XX7
App skn cll ssp f/n/g/hf 1st
15XX8
App skn cll ssp f/n/g/hf ea
25310
Transplant forearm tendon
25447
Repair wrist joints
26480
Transplant hand tendon
2X005
Arthrp ntrcrpl/crp/mtcrp ssp
36514
Apheresis plasma
36516
Apheresis immunoads slctv
36522
Photopheresis
3X018
Car-t hrv bld-drv t lymphcyt
3X019
Car-t prep t lymphcyt f/trns
3X020
Car-t receipt&prepj admn
3X021
Car-t admn autologous
4X015
Opn exc/dstr ntra-abd 5 cm/<
4X016
Opn exc/dstr ntra-abd 5.1-10
4X017
Opn exc/dst ntra-abd 10.1-20
4X018
Opn exc/dst ntra-abd 20.1-30
4X019
Opn exc/dstr ntra-abd >30 cm
59200
Insert cervical dilator
5X006
Ins trurl ablt trnsdc thr us
5X007
Ablt trurl prst8 tis thrm us
5X008
Ablt trurl prst8 tis trnsdcr
5XX05
Cysto insj dev ischmc rmdlg
5XX06
Cathj rmvl dev ischmc rmdlg
64487
Tap block uni by infusion
64488
Tap block bi injection
64489
Tap block bi by infusion
64590
Ins/rpl prph sac/gstr npg/r
64595
Rev/rmv prph sac/gstr npg/r
66680
Repair iris & ciliary body
66682
Repair iris & ciliary body
6X004
Implantation iris prosthesis
6XX00
Mrgfus strtctc ablt trgt icr
6XX01
Abltj 1/+thyr ndul 1lobe prq
6XX02
Abltj 1/+thyr ndul addl prq
6XX08
Thrc fascial pln blk uni nfs
6XX09
Thrc fascial pln blk bi njx

HCPCS
Description
6XX10
Thrc fascial pln blk bi nfs
6XX12
Lwr xtr fscl pln blk uni nfs
76981
Use parenchyma
76982
Use 1st target lesion
76983
Use ea addl target lesion
90480
Admn sarscov2 vacc 1 dose
92132
Cmptr ophth dx img ant segmt
92133
Cmptr ophth img optic nerve
92134
Cptr ophth dx img post segmt
93886
Intracranial complete study
93888
Intracranial limited study
93892
Tcd emboli detect w/o inj
93893
Tcd emboli detect w/inj
93X94
Vsrctv std tcd icr art compl
93X95
Emboli detcj wo iv mbubb njx
93X96
Ven-artl shunt det mbubb njx
96380
Admn rsv monoc antb im cnsl
96381
Admn rsv monoc antb im njx
97012
Mechanical traction therapy
97014
Electric stimulation therapy
97016
Vasopneumatic device therapy
97018
Paraffin bath therapy
97022
Whirlpool therapy
97032
Appl modality 1+estim ea 15
97033
App mdlty 1+iontphrsis ea 15
97034
App mdlty 1+cntrst bth ea 15
97035
App mdlty 1+ultrasound ea 15
97110
Therapeutic exercises
97112
Neuromuscular reeducation
97113
Aquatic therapy/exercises
97116
Gait training therapy
97140
Manual therapy 1/> regions
97530
Therapeutic activities
97533
Sensory integration
97535
Self care mngment training
97537
Community/work reintegration
97542
Wheelchair mngment training
97810
Acupunct w/o stimul 15 min
97811
Acupunct w/o stimul addl 15m
97813
Acupunct w/stimul 15 min
97814
Acupunct w/stimul addl 15m
9X059
Cptrz oph img pst sg rta oct
9X075
Synch audio-video new sf 15
9X076
Synch audio-video new low 30
9X077
Synch audio-video new mod 45

HCPCS
Description
9X078
Synch audio-video new hi 60
9X079
Synch audio-video est sf 10
9X080
Synch audio-video est low 20
9X081
Synch audio-video est mod 30
9X082
Synch audio-video est hi 40
9X083
Synch audio-only new sf 15
9X084
Synch audio-only new low 30
9X085
Synch audio-only new mod 45
9X086
Synch audio-only new high 60
9X087
Synch audio-only est sf 10
9X088
Synch audio-only est low 20
9X089
Synch audio-only est mod 30
9X090
Synch audio-only est high 40
9X091
Brief comunicaj tech-bsd svc
9X100
Genetic counseling svc ea 30
G0168
Wound closure by adhesive
G0283
Elec stim other than wound
G0443
Brief alcohol misuse counsel
G0516
Insert drug del implant, >=4
G0517
Remove drug implant
G0518
Remove w insert drug implant
F. Evaluation and Management (E/M) Visits
1. Office/Outpatient (O/O) Evaluation and Management (E/M) Visit Complexity Add-on
In the CY 2024 PFS final rule (88 FR 78970 through 78982), we finalized separate
payment for the O/O E/M visit complexity add-on code. The full descriptor for the O/O E/M
visit complexity add-on code, HCPCS code G2211, is (Visit complexity inherent to evaluation
and management associated with medical care services that serve as the continuing focal point
for all needed health care services and/or with medical care services that are part of ongoing
care related to a patient's single, serious condition or a complex condition. (Add-on code, list
separately in addition to office/outpatient evaluation and management visit, new or
established)).
The O/O E/M visit complexity add-on code “reflects the time, intensity, and PE resources
involved when practitioners furnish the kinds of O/O E/M visit services that enable them to build
longitudinal relationships with all patients (that is, not only those patients who have a chronic
condition or single high-risk disease) and to address the majority of a patient’s health care needs
with consistency and continuity over longer periods of time.” (88 FR 78970 through 78971). We
explained in the CY 2024 PFS final rule that it is the relationship between the patient and the
practitioner that is the determining factor for when the add-on code should be billed. The add-on
code captures the inherent complexity of the visit that is derived from the longitudinal nature of
the practitioner and patient relationship. The first part of the code descriptor, the “continuing
focal point for all needed health care services,” describes a relationship between the patient and
the practitioner when the practitioner is the continuing focal point for all health care services that
the patient needs. The second part of the add-on code also describes a relationship involving
medical services that are part of ongoing care related to a patient’s single, serious condition or a
complex condition. There is previously unrecognized but important cognitive effort of utilizing
the longitudinal relationship in making a diagnosis, developing a treatment plan, and weighing
the factors that affect a longitudinal doctor-patient relationship. The practitioner must decide
what course of action and choice of words in the visit itself would lead to the best health
outcome in the single visit while simultaneously building up an effective, trusting longitudinal
relationship with the patient. Weighing these various factors, even for a seemingly simple
condition, makes the entire visit inherently complex, which is what this add-on code is intended
to capture (88 FR 78973 through 78974).
We responded to concerns raised by commenters about potential duplicative payment and
potential misreporting of the code, noting that when procedures or other services are reported on
the same day by the same billing practitioner as a significant, separately identifiable O/O E/M
visit (the base codes that the visit complexity add-on code can be billed with), we believed that
the services involve resources that are sufficiently distinct from the costs associated with
furnishing stand-alone O/O E/M visits to warrant a different payment policy (88 FR 78971). We
finalized our proposal that the O/O E/M visit complexity add-on code is not payable when the
O/O E/M visit is reported with CPT Modifier -25, which denotes a significant, separately
identifiable O/O E/M visit by the same physician or other qualified health care professional on
the same day as a procedure or other service (88 FR 78974).
Some commenters expressed concern about our proposal to exclude payment for the visit
complexity add-on code when the O/O E/M base code is reported with Modifier -25 because
some preventive services such as the annual wellness visit (AWV) or a preventive vaccine are
often provided on the same day as a separately identifiable O/O E/M visit, appropriately billed
with Modifier -25. The commenters were concerned that practitioners might avoid the policy by
not providing a preventive service on the same day as another O/O E/M service. We
acknowledged that immunizations and AWVs were sometimes furnished on the same day as an
O/O E/M visit and that our policy would prevent payment of the add-on code with such office
visits billed with Modifier -25 and indicated that we would monitor utilization of the visit
complexity add-on code and continue engagement with interested parties as the policy is
implemented (88 FR 78975).
We have begun to monitor utilization of HCPCS code G2211 and are continuing to
engage with interested parties. We continue to hear from some practitioners that our non-
payment of the O/O E/M visit complexity add-on code when the O/O E/M base code is reported
on the same day as a preventive immunization or other Medicare preventive service is disruptive
to the way such care is usually furnished and contrary to our policy objective for establishing the
add-on payment. An early analysis of practitioner claims from the first few months of 2024
shows relatively few Medicare preventive services being billed on the day preceding or
following an O/O E/M visit. We cannot conclude from this analysis that our policy to deny
payment of the O/O E/M visit complexity add-on code when the O/O E/M base code is reported
on the same day as a preventive immunization or other Medicare preventive service is disruptive
to the way such care is usually furnished. However, we do agree with practitioners expressing
concerns that the current policy is not well-aligned with our policy objective for establishing the
add-on payment.
In response to these concerns, we are proposing to refine our current policy for services
furnished beginning in CY 2025. We are proposing to allow payment of the O/O E/M visit
complexity add-on code when the O/O E/M base code is reported by the same practitioner on the
same day as an AWV, vaccine administration, or any Medicare Part B preventive service
furnished in the office or outpatient setting. Allowing payment for the O/O E/M visit complexity
add-on code in this scenario as proposed would support our policy aims, which include paying
for previously unaccounted resources inherent in the complexity of all longitudinal primary care
office visits. In part, the O/O E/M visit complexity add-on code recognizes the inherent costs of
building trust in the practitioner-patient relationship. We believe that trust-building in the
longitudinal relationship is more significant than ever in making decisions about the
administration of immunizations and other Medicare Part B preventive services. We welcome
comments on this proposal.

G. Enhanced Care Management
1. Background
The CMS Center for Medicare and Medicaid Innovation (CMS Innovation Center) tests
innovative payment and service delivery models to reduce program expenditures while
preserving or enhancing quality of care. CMS Innovation Center models are assessed for their
impact on quality of care and expenditures under Medicare, Medicaid, and the Children’s Health
Insurance Program (CHIP) and the scope and duration of the model test may be expanded
through rulemaking if expected to either reduce spending without compromising quality of care
or enhance quality of care without increasing spending (section 1115A of the Act).
After more
than a decade of testing over 50 innovative payment and service delivery models, the CMS
Innovation Center has enabled broad transformative changes to service delivery and payment in
the Medicare, Medicaid, and CHIP programs which inspire additional transformation throughout
the health care delivery system. Participants in CMS Innovation Center models have
demonstrated improvements in care delivery and patient experience. The CMS Innovation Center
undertook a retrospective review and synthesis of select model evaluations where care delivery
changes have been observed, and the review indicated demonstrable evidence of enhanced care
delivery in several areas, such as care coordination, team-based care, and leveraging data to risk-
stratify patients.
21
Under the Medicare Physician Fee Schedule (PFS) statute at section 1848 of the Act, we
establish payment amounts for covered physicians’ services, and update our payment policies to
address changes, including changes in medical practice. In this proposed rule, we are proposing
to incorporate key payment and service delivery elements from CMS Innovation Center models
tested and evaluated over the prior decade into permanent coding and payment under the PFS.
21
Fowler, PhD, JD, E., Rudolph, MPH, N., Davidson, LCSW, MSW, K., Finke, MD, B., Flood, S., Bernheim, MD,
PhD, S. M., & Rawal, PhD, P. (2023). Accelerating Care Delivery Transformation — The CMS Innovation Center’s
Role in the Next Decade. New England Journal of Medicine, 4(11). https://doi.org/10.1056/cat.23.0228.
CMS. Synthesis of Evaluation Results across 21 Medicare Models, 2012-2020. Fowler, PhD. 2022.
https://www.cms.gov/priorities/innovation/data-and-reports/2022/wp-eval-synthesis-21models.

Specifically, we are proposing to recognize a primary care practice delivery model trend which
we will refer to as “advanced primary care” and which we propose to define using the 2021
National Academies of Sciences, Engineering, and Medicine (NASEM) report on Implementing
High-Quality Care as: “whole-person, integrated, accessible, and equitable health care by
interprofessional teams that are accountable for addressing the majority of an individual’s health
and wellness needs across settings and through sustained relationships with patients, families,
and communities.”
22
Using this definition, we are proposing to recognize the resources involved
in furnishing services using an “advanced primary care” approach to care under the PFS.
23
Under
this approach, the delivery of care is supported by a team-based care structure and involves a
restructuring of the primary care team, which includes the billing practitioner and the auxiliary
personnel under their general supervision, within practices. This restructuring creates several
advantages for patients, and provides more broad accessibility and alternative methods for
patients to communicate with their care team/practitioner about their care outside of in-person
visits (for example, virtual, asynchronous interactions, such as online chat), which can lead to
more timely and efficient identification of, and responses to, health care needs (for example,
practitioners can route patients to the optimal clinician and setting—to a synchronous visit, an
asynchronous chat, or a direct referral to the optimal site of care).
24
Practitioners using an
advanced primary care delivery model can more easily collaborate across clinical disciplines
through remote interprofessional consultations with specialists as well as standardize condition
management into evidence-based clinical workflows, which allow for closed-loop follow-up and
more real-time management for patients with acute or evolving complex issues. Practitioners can
22
National Academies of Sciences, Engineering, and Medicine. 2021. Implementing high-quality primary care:
Rebuilding the foundation of health care. Washington, DC: The National Academies Press.
https://doi.org/10.17226/25983.
23
Team-based approaches to care can achieve improved provider and care team satisfaction, improved team
communication, improved patient safety, and improved patient and family engagement in care. Coleman, M. Dexter.
D., & Nankivill, N. (2015, August). Factors affecting physician satisfaction and Wisconsin Medical Society
strategies to drive change. Wisconsin Medical Journal. 114(4), 135-142. Retrieved from
https://www.wisconsinmedicalsociety.org/professional/wmj/archives/volume-114-issue-4-august-2015/.
24
Ellner, A., Basu, N. & Phillips, R.S. From Revolution to Evolution: Early Experience with Virtual-First,
Outcomes-Based Primary Care. J GEN INTERN MED 38, 1975–1979 (2023). https://doi.org/10.1007/s11606-023-
08151-1.

then use synchronous interactions to build rapport with patients and families, partner on complex
decisions, and personalize their patients’ care plans.
Specifically, we are proposing to adopt coding and payment policies to recognize
advanced primary care management (APCM) services for use by practitioners who are providing
services under this specific model of advanced primary care, when the practitioner is the
continuing focal point for all needed health care services and responsible for all primary care
services. This new proposed coding and payment makes use of lessons learned from the CMS
Innovation Center’s testing of a series of advanced primary care models, such as Comprehensive
Primary Care (CPC),
25
Comprehensive Primary Care Plus (CPC+),
26
and Primary Care First
(PCF)
27,28
(see discussion in section II.G.2.a.(1) in this proposed rule) to inform the elements
upon which the delivery of APCM services under an advanced primary care delivery model
depend. As detailed below in sections II.G.2.b. through II.G.2.d., this proposed coding and
payment will incorporate elements of several specific, existing care management and
communication technology-based services (CTBS) into a bundle of services, that reflects the
essential elements of the delivery of advanced primary care, for payment under the PFS starting
in 2025.
In the context of the proposal, we are also interested in feedback on other related policies
for our consideration in future rulemaking. To gather information from interested parties to
inform potential future proposals, we have included an Advanced Primary Care Hybrid Payment
Request for Information (RFI) (Advanced Primary Care RFI) in this proposed rule. The
Advanced Primary Care RFI seeks feedback on whether and how we should consider additional
payment policies that reflect our efforts to recognize the delivery of advanced primary care,
including bundling of additional individual services, which may currently be furnished together
25
https://www.cms.gov/priorities/innovation/innovation-models/comprehensive-primary-care-initiative.
26
https://www.cms.gov/priorities/innovation/innovation-models/comprehensive-primary-care-plus.
27
https://www.cms.gov/priorities/innovation/innovation-models/primary-care-first-model-options.
28
Finke, Bruce, et al. “Addressing Challenges in Primary Care—Lessons to Guide Innovation.” JAMA Health
Forum, vol. 3, no. 8, 19 Aug. 2022, p. e222690, https://doi.org/10.1001/jamahealthforum.2022.2690.

as primary care services but paid separately. This focused approach to seeking feedback on
advanced primary care payment policies is an important step in our ongoing efforts to emphasize
accountable care and supports CMS’ goal of having 100 percent of Traditional Medicare
beneficiaries in accountable care relationships by 2030.
29
In addition to recognizing advanced primary care, this proposed rule also recognizes
physician and practitioner work that draws from evidence generated by the CMS Innovation
Center’s Million Hearts® model.
30
The Million Hearts® model found that quantitative
assessment of patients’ atherosclerotic cardiovascular disease (ASCVD) risk and providing high-
risk beneficiaries with cardiovascular-focused care management services improved quality of
care, including mortality.
31
We discuss a proposal in this section to establish coding and PFS
payment for these services based in part on the evidence generated by the Million Hearts®
model.
2. Advanced Primary Care Management (APCM) Services (HCPCS codes GPCM1, GPCM2,
and GPCM3)
a. Background
We have been analyzing opportunities to strengthen and invest in primary care in
alignment with the goals of the U.S. Department of Health and Human Services (HHS) Initiative
to Strengthen Primary Care.
32
Research has demonstrated that greater primary care physician
supply is associated with improved population-level mortality and reduced disparities,
33
and also,
that establishing a long-term relationship with a primary care provider leads to reduced
29
CMS White Paper on CMS Innovation Center’s Strategy: Driving Health System Transformation—A Strategy for
the CMS Innovation Center’s Second Decade (https://www.cms.gov/priorities/innovation/strategic-direction-
whitepaper).
30
https://www.cms.gov/priorities/innovation/innovation-models/million-hearts-cvdrrm.
31
Peterson G, Steiner A, Powell R, et al. Evaluation of the Million Hearts® Cardiovascular Disease Risk Reduction
Model: Fourth Annual Report. Mathematica. February 2022. https://www.cms.gov/priorities/innovation/data-and-
reports/2022/mhcvdrrm-fourthannevalrpt.
32
U.S. Department of Health and Human Services. (2023). Primary Care: Our First Line of Defense.
https://www.hhs.gov/sites/default/files/primary-care-issue-brief.pdf.
33
Basu S, Berkowitz SA, Phillips RL, Bitton A, Landon BE, Phillips RS. Association of Primary Care Physician
Supply With Population Mortality in the United States, 2005-2015. JAMA Intern Med. 2019;179(4):506–514.
doi:10.1001/jamainternmed.2018.7624.
https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2724393.

emergency department (ED) visits,
34
improved care coordination, and increased patient
satisfaction.
35
HHS recognizes that effective primary care is essential for improving access to
healthcare, for the health and wellbeing of individuals, families, and communities, and for
achieving health equity. The first coordinated set of HHS-wide actions to strengthen primary
care, as part of the Initiative, is in primary care payment; for example, adjusting payment to
ensure it supports delivery of advanced primary care. CMS Innovation Center models, described
in section II.G.2.a.(1) in this proposed rule, reflect the ongoing work within HHS and the unified,
comprehensive approach to HHS primary care activities that we are accomplishing through our
current statutory authorities and funding.
Over the last decade, we have updated PFS payment policies as appropriate, and we
remain committed to improving how Medicare payment recognizes the resources involved in
furnishing covered services that encompass aspects of advanced primary care furnished by
interprofessional care teams and typically concentrating on the delivery of appropriate preventive
care to patients and the management of individuals’ chronic conditions as they progress over
time. As part of the CY 2014 PFS final rule, we reaffirmed our support of primary care and
recognized care management as one of the critical components of primary care that contributes to
better health outcomes for individuals and reduced expenditure growth, and explained our
prioritization of the development and implementation of several initiatives (such as those
discussed in section II.G.2.a.(1) in this proposed rule) (77 FR 68978). Since then, we have
implemented coding and payment for many care management services to better recognize the
resources involved in furnishing medically necessary care management activities that generally
are performed outside the context of a face-to-face, in-person visit—most often by the billing
practitioner’s clinical staff on behalf of patients with complex health care needs, including
34
Willemijn L.A. Schäfer et al, “Are People’s Health Care Needs Better Met When Primary Care Is Strong? A
Synthesis of the Results of the QUALICOPC Study in 34 Countries,” Primary Health Care Research and
Development 20 (2019): e104. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6609545/.
35
Michael J. van den Berg, Tessa van Loenen, and Gert P Westert, “Accessible and Continuous Primary Care May
Help Reduce Rates of Emergency Department Use: An International Survey in 34 Countries,” Family Practice 33,
no. 1 (Feb. 2016): 42–50. https://academic.oup.com/fampra/article/33/1/42/2450446.
transitional care management in the CY 2013 PFS final rule (77 FR 68979); non-complex and
complex chronic care management (CCM) in the CY 2015, 2017, and 2019 PFS final rules (78
FR 74414, 83 FR 58577, and 81 FR 80244); and principal care management (PCM) in the CY
2020 PFS final rule (84 FR 62962). The CCM and PCM code families now include 5 sets of
codes which are reported monthly on a timed basis, each set with a base code of 20 to 60 minutes
and an add-on code for each additional 30 minutes. The code sets vary by the degree of
complexity of patient conditions (that is, non-complex and complex CCM for multiple chronic
conditions or PCM for a single high-risk condition), and whether the number of minutes spent by
clinical staff or the physician or non-physician practitioner (NPP) is used to meet time thresholds
for billing.
Additionally, we have established coding and payment for certain services where a
medical professional evaluates a patient’s medical information remotely using communication
technology. As discussed in the CY 2019 PFS final rule, this set of services is defined by and
inherently involves the use of communications technology, and includes certain remote patient
monitoring services, virtual check-in services, remote evaluation of pre-recorded patient
information, remote interpretations of diagnostic imaging tests, and interprofessional
consultations. We recognize that technological advances have changed and continue to change
the practitioner-patient care delivery interaction. We have recognized these technology-enabled
interactions through separately billable CTBS over the last several years. However, we
acknowledge, as we learn more about how advanced primary care services are furnished to
patients, that in some clinical care delivery scenarios, practitioners furnishing the type of care
highlighted in this discussion may furnish certain aspects of the CTBS services in complement to
care management services (for example, by allowing interprofessional care teams to answer
patient questions, refer patients to higher levels of care, view and interpret patient images, order
needed treatments, and offer reassurance or advice), in an effort to more efficiently manage the
quantity and quality of medical information that is necessary to support effective patient-centered
treatment plans.
Despite these important steps to pay separately for these care management services, there
has been limited uptake of care management services and Medicare still overwhelmingly pays
for primary care through traditional office/outpatient (O/O) Evaluation and Management (E/M)
visit codes, which describe a broad range of physicians’ services but do not fully distinguish and
account for the resources associated with primary care and other longitudinal care. As we stated
in the CY 2024 PFS final rule, we believe that because E/M visit codes are intended to be used
very broadly, the complexity of services required to provide this type of care is not fully
incorporated as part of the valuation of the work RVUs when the E/M code itself is used as the
primary way to report the work of the professional (88 FR 78972). In the CY 2024 PFS final
rule, we took steps to better recognize the inherent complexity of visits associated with primary
and longitudinal care of patients by finalizing a new add-on code (HCPCS code G2211) for use
by practitioners furnishing services as the continuing focal point for all the patient’s needed
health care services, such as a primary care practitioner (88 FR 78969). When furnishing primary
and longitudinal care, practitioners must be attuned to the factors that develop and maintain
trusting practitioner-patient relationships that enable effective diagnosis, management, and
treatment on an ongoing basis. In finalizing the O/O E/M visit complexity add-on code, we
recognized the feedback from interested parties indicating that the care management codes alone
may not have mitigated the deficiencies in the ability of existing E/M codes to reflect the time
and resources involved in furnishing visits in the context of longitudinal care—of which,
advanced primary care is one model. Many commenters responded, as reflected in the CY 2024
PFS final rule, that they did not view the coding and payment currently available under the PFS
as capable of recognizing the broad range of elements that define primary care (88 FR 52326).
Other commenters responded that they did not believe that the existing E/M service codes alone
reflect the work and resources involved in furnishing non-procedural care to Medicare
beneficiaries (88 FR 78976).
Over the years, interested parties have focused attention on the ongoing need to improve
how practitioners are paid, in and outside of payment bundles, including but not limited to the
possibility of E/M codes designed specifically to be billed in conjunction with care management
codes and the elimination of multiple disparities between the payment for E/M services in global
periods and those furnished individually. Based on feedback from the physician and practitioner
community, we understand that advanced primary care encompasses the work of
interprofessional teams who are accountable for addressing the majority of an individual’s health
and wellness needs across settings and through sustained relationships, which necessarily
involves time spent by primary care practitioners and their clinical staff outside of individual
E/M visits.
As with many services paid under the PFS, we balance making payment that recognizes
and supports technological developments in healthcare and the resources involved in evolving
medical practice to allow for appropriate and expanded access to innovative technologies and
newer services with promoting stability and efficiency in coding and billing rules for
practitioners and institutions. We recognize the important role of gathering input and information
from the CMS Innovation Center models (described in more detail in section II.G.2.a.(1) in this
proposed rule), comment solicitations, research from other public and private entities, the work
of all parties involved in furnishing primary care, and from the public at large. As previously
noted, interested parties have given ample feedback over the years to inform our recognition of
care management services; for example, as part of the CY 2022 PFS rulemaking, interested
parties specifically requested our consideration of a “30-day global period bundling care
management services” and we responded that we would consider this suggestion for future
rulemaking (86 FR 65118). We have continued to incorporate feedback into our rulemaking and
strengthen our care management code sets with the goal of better recognizing the elements of

advanced primary care as part of a multi-year strategy. Based on this feedback, we are proposing
to establish a set of codes to better describe advanced primary care management services
broadly, to provide more stability in payment and coding for practitioners in the context of
continued evolution in advanced primary care, as well as to provide us with a mechanism for
continued and intentional improvements to advanced primary care payment.
(1) Key Care Delivery Methods in Select CMS Innovation Center Models
We have prioritized the implementation or testing of a series of initiatives designed to
improve payment for, and encourage long-term investment in, primary care and care
management services. By supporting enhanced care management and coordination, these
initiatives contributed to the growing practice of advanced primary care and have also provided
valuable lessons learned that we are incorporating into our proposals.
Several CMS Innovation Center models address payment for care management services
and CTBS. The CPC initiative,
36
the CPC+ model,
37
and the PCF model
38
all included payments
for care management services that closely aligned with the care management services included in
the PFS. In these initiatives, primary care practices received risk-adjusted, per beneficiary per
month (PBPM) payments for care management services furnished to Medicare FFS beneficiaries
attributed to their practices. These model payments were designed to offer practices a stable,
predictable revenue stream that supported required infrastructure and appropriately compensated
practices for the enhanced services they would provide. Practices participating in CPC+
consistently cited these payments as the most useful type of model payment support they
received; these stable, prospectively paid payments typically served as the main funding source
for compensating care managers, behavioral health providers, and other staff hired to improve
36
https://downloads.cms.gov/files/cmmi/CPC-initiative-fourth-annual-report.pdf.
37
https://www.cms.gov/priorities/innovation/data-and-reports/2023/cpc-plus-fifth-annual-eval-report.
38
Evaluation of the Primary Care First Model. February 2024. https://www.cms.gov/priorities/innovation/data-and-
reports/2024/pcf-second-eval-rpt.

care delivery.
39
Because these payments were paid prospectively and could be used to support a
range of care management and coordination activities, they provided participants with greater
financial stability and flexibility to develop and expand capabilities to meet patients’ care
needs.
40
Table 19 identifies a number of CMS Innovation Center models and key care delivery
methods from each.
41
TABLE 19: Key Care Delivery Methods from Select CMS Innovation Center
Models
Model
Key Care Delivery Methods
Citation
ACO Investment Model (AIM)
AIM provided an opportunity for
participants to invest in care
transformation activities.
Specifically, AIM was an
opportunity for independent
primary care practices in rural
communities to hire population
health staff, such as care managers
or outreach coordinators. Care
managers conducted outreach,
scheduling, and patient education.
Care managers did this through a
variety of mechanisms including
phone, in the physician office, and
via home visits.
Evaluation of the Accountable Care
Organization Investment Model,
Final Report, September 2020,
available at:
https://www.cms.gov/priorities/inno
vation/data-and-reports/2020/aim-
final-annrpt
Comprehensive Primary Care
(CPC)
CPC practices provided
longitudinal and episodic care
management services for patients at
high or rapidly increasing risk
whom the practices believed were
most likely to benefit from
intensive support. By 2016, CPC
practices risk stratified 95% of their
empaneled patients, and provided
care management to 20% of those
patients. CPC practices also greatly
increased their use of dedicated care
managers over time. By 2016, 89%
of practices reported that, “care
managers who were members of the
[primary care] practice team
systematically provided care
management services to high-risk
patients” – an increase from 20% in
2012. Beneficiaries attributed to
CPC practices had slower growth in
hospitalizations and emergency
Evaluation of the Comprehensive
Primary Care Initiative, Fourth
Annual Report, May 2018,
available at:
https://downloads.cms.gov/files/cm
mi/CPC-initiative-fourth-annual-
report.pdfhttps://downloads.cms.go
v/files/cmmi/CPC-initiative-fourth-
annual-report.pdf
Long-Term Effects of the
Comprehensive Primary Care
Model on Health Care Spending
and Utilization. May 2022.
Available at:
https://www.ncbi.nlm.nih.gov/pmc/a
rticles/PMC9130381/.
39
O’Malley A, Singh P, Fu N, et al. Independent Evaluation of the Comprehensive Primary Care Plus (CPC+): Final
Report. Mathematica. December 2023. https://www.cms.gov/priorities/innovation/data-and-reports/2023/cpc-plus-
fifth-annual-eval-report.
40
O’Malley A, Singh P, Fu N, et al. Independent Evaluation of the Comprehensive Primary Care Plus (CPC+): Final
Report. Mathematica. December 2023. https://www.cms.gov/priorities/innovation/data-and-reports/2023/cpc-plus-
fifth-annual-eval-report.
41
For more information on how the Innovation Center is supporting primary care,
https://www.cms.gov/files/document/primary-care-infographic.pdf.

Model
Key Care Delivery Methods
Citation
department (ED) visits than those
being managed by practices not in
the model.
Comprehensive Primary Care
Plus (CPC+)
CPC+ practices used data and team-
based care to proactively identify
the needs of their patients and
efficiently manage their care.
Additionally, by the final year of
the model, about 97% of physicians
in CPC+ and comparison practices
reported the use of scheduled
phone, video, or e-visits for at least
some of their patients. Finally,
CPC+ had small favorable effects
on some claims-based, quality-of-
care measures of planned care and
population health and patient and
caregiver engagement.
Independent Evaluation of
Comprehensive Primary Care Plus.
Final Report, December 2023,
available at:
https://www.cms.gov/priorities/inno
vation/data-and-reports/2023/cpc-
plus-fifth-annual-eval-report.
Primary Care First (PCF)
All PCF practices provide 24/7
access to a care team practitioner
with real-time access to an
electronic health record (EHR).
Practices also provide risk-stratified
care management for all empaneled
patients and ensure beneficiaries
receive timely follow-up contact
from the practice after ED visits
and hospitalizations. Practices
commonly report expanding their
practice care team by hiring
additional clinical and non-clinical
staff to bolster longitudinal care
management services.
Evaluation of the Primary Care
First Model, Second Annual
Report, February 2024, available at:
https://www.cms.gov/priorities/inno
vation/data-and-reports/2024/pcf-
second-eval-rpt.
b. Proposed HCPCS G-Codes for Advanced Primary Care Management (APCM)
We are proposing to establish coding and make payment under the PFS for a newly
defined set of APCM services described and defined by three new HCPCS G-codes. To
recognize the resource costs associated with furnishing APCM services to Medicare
beneficiaries, we are proposing to establish and pay for three new G-codes that describe a set of
care management services and CTBS furnished under a broader application of advanced primary
care. This new coding and payment would reflect the recognized effectiveness and growing
adoption of the advanced primary care approach to care.
42
It would also encompass a broader
42
National Academies of Sciences, Engineering, and Medicine (NASEM). 2021. Implementing high-quality
primary care: Rebuilding the foundation of health care. Washington, DC: The National Academies Press.
https://doi.org/10.17226/25983.; Maeng DD et al. Reducing long-term cost by transforming primary care: evidence
from Geisinger's medical home model. Am J Manag Care. 2012 Mar;18(3):149-55. PMID: 22435908. Available

range of services and simplify the billing and documentation requirements, as compared to
existing care management and CTBS codes, for clinicians who care for their patients using an
advanced primary care model. We recognize that there are primary care physicians,
practitioners, and practices beyond those that have participated in CMS Innovation Center
primary care models (such as those discussed in section II.G.2.a.(1) in this proposed rule), that
may incur resource costs associated with their treatment of patients based on the advanced
primary care delivery model. Providing care using an advanced primary care delivery model
involves resource costs associated with maintaining certain practice capabilities and continuous
readiness and monitoring activities to support a team-based approach to care, where significant
resources are used on virtual, asynchronous patient interactions, collaboration across clinical
disciplines, and real-time management of patients with acute and complex concerns, that are not
fully recognized or paid for by the existing care management codes. We have observed medical
practice trends in primary care for several years. We note that in prior rulemaking, for example,
in the CY 2013 PFS final rule, we stated, “we further consider[ed] how advanced primary care
practices can fit within a fee-for-service model” (77 FR 68987), and in the CY 2015 PFS final
rule, we stated our commitment “to supporting advanced primary care, including the recognition
of care management as one of the critical components of primary care that contributes to better
health for individuals and reduced expenditure growth” (79 FR 67715). In the CY 2017 PFS final
rule, we discussed changes to retain elements of the CCM service that are “most characteristic of
the changes in medical practice toward advanced primary care” (81 FR 80251). As the delivery
of primary care has evolved to embrace advanced primary care more fully, we believe that it is
prudent to now adopt specific coding and payment policy to better recognize the resources
involved in care management under an advanced primary care delivery model.
here: https://pubmed.ncbi.nlm.nih.gov/22435908/.; Jones C et al. Vermont’s Community-Oriented All-Payer
Medical Home Model Reduces Expenditures and Utilization While Delivering High-Quality Care. Popul Health
Manag. 2016;19(3):196–205. Available here: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4913508/.
Below, we explain the proposed new codes and their descriptors. In the next section, we
propose to define the elements of the scope of service for APCM that would be required for a
practitioner to bill Medicare for the APCM service, and we explain the proposed standards for
practices that furnish APCM services to ensure that the physicians and practitioners who bill for
these services have the capability to fully furnish advanced primary care, including APCM
services (see section II.G.2.c. of this proposed rule). At this time, we are proposing to identify
specific care management and CTBS services that are a part of advanced primary care delivery
and would be bundled into the PFS payment for the APCM services. As such, we will identify
the services that we are proposing would overlap substantially with the new codes and which
would not be separately billable with the APCM codes under our proposal (see section II.G.2.d.
of this proposed rule). Finally, we propose to establish relative values for these codes for
purposes of payment under the PFS (see section II.G.2.e. of this proposed rule).
We are proposing to establish the following G-codes and descriptors for APCM services, and as
explained in the next section, due to the similar scope of APCM and other care management and CTBS
services, we are proposing to include some of the same language from the CCM and PCM service
elements in the APCM code descriptors, as well as emphasize that certain practice capabilities and
requirements are inherent in these elements and must be met in order to bill for APCM services:
HCPCS code GPCM1 (Advanced primary care management services provided by clinical
staff and directed by a physician or other qualified health care professional who is responsible
for all primary care and serves as the continuing focal point for all needed health care services,
per calendar month, with the following elements, as appropriate:
● Consent;
++ Inform the patient of the availability of the service; that only one practitioner
can furnish and be paid for the service during a calendar month; of the right to
stop the services at any time (effective at the end of the calendar month); and that
cost sharing may apply.
++ Document in patient’s medical record that consent was obtained.
● Initiation during a qualifying visit for new patients or patients not seen within 3 years;
● Provide 24/7 access for urgent needs to care team/practitioner, including providing
patients/caregivers with a way to contact health care professionals in the practice to
discuss urgent needs regardless of the time of day or day of week;
● Continuity of care with a designated member of the care team with whom the patient is
able to schedule successive routine appointments;
● Deliver care in alternative ways to traditional office visits to best meet the patient’s
needs, such as home visits and/or expanded hours;
● Overall comprehensive care management;
++ Systematic needs assessment (medical and psychosocial).
++ System-based approaches to ensure receipt of preventive services.
++ Medication reconciliation, management and oversight of self-management.
● Development, implementation, revision, and maintenance of an electronic patient-
centered comprehensive care plan;
++ Care plan is available timely within and outside the billing practice as appropriate
to individuals involved in the beneficiary’s care, can be routinely accessed and updated
by care team/practitioner, and copy of care plan to patient/caregiver;
● Coordination of care transitions between and among health care providers and
settings, including referrals to other clinicians and follow-up after an emergency department
visit and discharges from hospitals, skilled nursing facilities or other health care facilities as
applicable;
++ Ensure timely exchange of electronic health information with other
practitioners and providers to support continuity of care.
++ Ensure timely follow-up communication (direct contact, telephone,
electronic) with the patient and/or caregiver after an emergency department visit and
discharges from hospitals, skilled nursing facilities, or other health care facilities, within
7 calendar days of discharge, as clinically indicated.
● Ongoing communication and coordinating receipt of needed services from
practitioners, home- and community-based service providers, community-based social
service providers, hospitals, and skilled nursing facilities (or other health care facilities),
and document communication regarding the patient’s psychosocial strengths and needs,
functional deficits, goals, preferences, and desired outcomes, including cultural and
linguistic factors, in the patient’s medical record;
● Enhanced opportunities for the beneficiary and any caregiver to communicate
with the care team/practitioner regarding the beneficiary’s care through the use of
asynchronous non-face-to-face consultation methods other than telephone, such as
secure messaging, email, internet, or patient portal, and other communication-
technology based services, including remote evaluation of pre-recorded patient
information and interprofessional telephone/internet/EHR referral service(s), to maintain
ongoing communication with patients, as appropriate;
++ Ensure access to patient-initiated digital communications that require a
clinical decision, such as virtual check-ins and digital online assessment and
management and E/M visits (or e-visits).
● Analyze patient population data to identify gaps in care and offer additional
interventions, as appropriate;
● Risk stratify the practice population based on defined diagnoses, claims, or other
electronic data to identify and target services to patients;
● Be assessed through performance measurement of primary care quality, total cost of
care, and meaningful use of Certified EHR Technology.).
HCPCS code GPCM2 (Advanced primary care management services for a patient with
multiple (two or more) chronic conditions expected to last at least 12 months, or until the death
of the patient, which place the patient at significant risk of death, acute
exacerbation/decompensation, or functional decline, provided by clinical staff and directed by a
physician or other qualified health care professional who is responsible for all primary care and
serves as the continuing focal point for all needed health care services, per calendar month, with
the elements included in GPCM1, as appropriate) and HCPCS code GPCM3 (Advanced primary
care management services for a patient that is a Qualified Medicare Beneficiary with multiple
(two or more) chronic conditions expected to last at least 12 months, or until the death of the
patient, which place the patient at significant risk of death, acute exacerbation/decompensation,
or functional decline, provided by clinical staff and directed by a physician or other qualified
health care professional who is responsible for all primary care and serves as the continuing
focal point for all needed health care services, per calendar month, with the elements included in
GPCM1, as appropriate).
HCPCS codes GPCM1 through GPCM3 would describe APCM services furnished per
calendar month, following the initial qualifying visit (see section II.G.2.c.(1) for more on the
initiating visit). Physicians and NPPs, including nurse practitioners (NPs), physician assistants
(PAs), certified nurse midwives (CNMs) and clinical nurse specialists (CNSs), could bill for
APCM services. As we will describe in more detail in section II.G.2.c., within the code
descriptors for GPCM1, GPCM2, and GPCM3, we are including the elements of the scope of
service for APCM as well as the practice capabilities and requirements that we believe to be
inherent to care delivery by the care team/practitioner who is billing under a practice using an
advanced primary care delivery model, and necessary to fully furnish and, therefore, bill for
APCM services.
As described in more detail below, within the code descriptors for GPCM1, GPCM2, and
GPCM3, we are proposing that the practitioner who bills for APCM services intends to be
responsible for the patient’s primary care and serves as the continuing focal point for all needed
health care services. We anticipate that most practitioners furnishing APCM services will be
managing all the patient’s health care services over the month and have either already been
providing ongoing care for the beneficiary or have the intention of being responsible for the
patient’s primary care and serving as the continuing focal point for all the patient’s health care
services. We anticipate that these codes will mostly be used by the primary care specialties, such
as general medicine, geriatric medicine, family medicine, internal medicine, and pediatrics, but
we are also aware that, in some instances, certain specialists function as primary care
practitioners – for example, an OB/GYN or a cardiologist. In contrast to situations where the
patient’s overall, ongoing care is being managed, monitored, and/or observed by a practitioner,
we believe that there are situations when care is provided by a practitioner who would not serve
as “the continuing focal point for all needed health care services.”
Similarly, there are some time- or condition-limited practitioner-patient relationships that
are clearly not indicative of the ongoing care that we anticipate practitioners would be
responsible for when furnishing APCM services. As we stated in the CY 2021 PFS proposed rule
and CY 2024 PFS final rule in the context of our policies for the O/O E/M visit complexity add-
on code (HCPCS code G2211), a practitioner whose “relationship with the patient is of a
discrete, routine, or time-limited nature; such as, but not limited to, a mole removal or referral to
a physician for removal of a mole; for treatment of a simple virus, for counseling related to
seasonal allergies, initial onset of gastroesophageal reflux disease; treatment for a fracture; and
where comorbidities are either not present or not addressed, and/or when the billing practitioner
has not taken responsibility for ongoing medical care for that particular patient with consistency
and continuity over time, or does not plan to take responsibility for subsequent, ongoing medical
care for that particular patient with consistency and continuity over time” (85 FR 84570 and
84571, 88 FR 78971). For example, a patient who spends one month of the year in another
location could require physicians’ services in that location if they experience exacerbation of one
of their chronic conditions, but the practitioner who treats them would not intend to manage or
monitor that patient’s overall, ongoing care. Finally, HCPCS code G2211 can also be billed
when medical services are “part of ongoing care related to a patient’s single, serious condition or
complex condition,” but this is different from the APCM requirement. A practitioner’s
management of one or more serious conditions (as is often the case with specialty care), without
more, does not mean that the practitioner is also responsible for all primary care services and the
focal point for all needed care (the requirement for APCM), and thus would not necessarily mean
that the practitioner could bill for APCM.
As is our current policy for other care management services, and consistent with both
CPT guidance and Medicare rules for CPT codes 99487, 99489, 99490, we are proposing that
HCPCS codes GPCM1, GPCM2, and GPCM3 may only be reported once per service period
(calendar month) and only by the single practitioner who assumes the care management role with
a particular beneficiary for the service period. That is, based on a patient’s status, a physician or
practitioner would identify the patient to receive Level 1, Level 2, or Level 3 APCM services
during a given service period (calendar month), and we would make payment for only one claim
for APCM services for that service period. At this time, we do not see the need or value of
proposing restrictions or complex operational mechanisms to identify a single physician or NPP
who may bill for APCM services for a specific beneficiary. However, we recognize that other
initiatives, such as the Medicare Shared Savings program, have operational mechanisms in place
to attribute patients to certain ACOs (§ 425.400). While a similar approach could be used to
attribute patients for APCM services, we are reluctant to introduce unnecessary complexity for
these services. As we continue to develop our policies in this area, we are seeking feedback from
interested parties on methodologies that could allow for identification of the beneficiary’s
primary care practitioner. We are also seeking comment on whether there should be additional
requirements to prevent potential care fragmentation or service duplication.
We anticipate that APCM services would ordinarily be provided by clinical staff incident
to the professional services of the billing practitioner in accordance with our regulation at §
410.26. We are proposing that APCM services would be considered a “designated care
management service” under § 410.26(b)(5) and, as such, could be provided by auxiliary
personnel under the general supervision of the billing practitioner.
Unlike the current coding to describe care management services, we are further proposing
that the code descriptors for GPCM1, GPCM2, and GPCM3 would not be time-based. Based on
feedback from the physician and practitioner community, we understand that ongoing care
management and coordination services are standard parts of advanced primary care, even in
months when documented clinical staff or billing professional minutes may not reach the
required thresholds for billing or the patient’s condition does not meet the clinical conditions for
care management services under the existing code set. In consideration of the extensive feedback
from interested parties, we have learned that practitioners who currently furnish monthly care
management services may already be providing APCM services in a variety of clinical
circumstances, documenting all necessary aspects of the patient-centered care furnished monthly
to the patient without meeting the requirements to bill for care management services, such as
satisfying the administrative requirement to count clinical staff minutes to reach specific time-
based thresholds. As we stated in the CY 2024 PFS final rule in the context of the O/O E/M visit
complexity add-on code (HCPCS code G2211), primary care physicians may diagnose and treat
a condition in an O/O E/M visit that is not expected to last as long as three months or would not
reasonably be expected to result in a risk of hospitalization, and the practitioner’s clinical staff
may provide significant care management and coordination services relating to that condition.
For example, COVID–19 cases are clinical circumstances that generally do not last three months
but may require significant acute management, care coordination, and follow-up within a given
month, particularly for patients with comorbidities (88 FR 78973). Practitioners may also
provide care management and coordination services to a patient whose condition meets the
criteria in one or more care management codes, but the documented minutes of service may not
reach the minimum time threshold to bill for a care management service. For example, the
practitioner might provide care coordination for a month that includes 20 minutes of consulting
with the patient’s other healthcare providers and modifying medications to address an acute
exacerbation of hypertension, but would not meet the requirements for billing the PCM service.
We also note that, unlike the current coding to describe certain CTBS services, we are proposing
that the code descriptors for GPCM1, GPCM2, and GPCM3 would not include the timeframe
restrictions for billing certain CTBS (for example, the restriction for virtual check-in services
that there is not a related E/M service provided within the previous 7 days or an E/M service or
procedure within the next 24 hours or the soonest available appointment). As addressed in the
CY2019 Final Rule, we have heard from interested parties that the timeframe restrictions for
billing certain CTBS are administratively burdensome (83 FR 59686).
We are also proposing that not all elements included in the code descriptors for APCM
services must be furnished during any given calendar month for which the service is billed.
APCM services are largely designed to be person-centered and focused on the individual patient,
such that the elements that are provided depend on medical necessity and individual patient need.
Therefore, we anticipate that all the APCM scope of service elements (for example,
comprehensive care management and care coordination) will be routinely provided, as deemed
appropriate for each patient, acknowledging that not all elements may be necessary for every
patient during each month (for example, the beneficiary may have no hospital admissions that
month, so there is no management of a care transition after hospital discharge). We also
anticipate that there may be some months where it may be appropriate for some service elements
to be performed more than once for the patient. For example, in one month a patient with heart
failure and chronic kidney disease receiving APCM Level 2 services (GPCM2) may be on a
stable medication regimen, receive communication about their care plan, but no virtual check-
ins. The next month, the patient may experience a heart failure exacerbation requiring inpatient
admission, and then receive as part of their APCM service timely communication and follow-up
with new labs ordered, multiple virtual check-ins ensuring that the patient understands their new
medications, a phone call to help the patient understand the lab results, and an interprofessional
consultation with the patient’s cardiologist to help decide if the patient’s diuretic dosage should
be changed.
However, even if not all elements of the APCM service are furnished each month for
which APCM is billed, we propose that billing practitioners and auxiliary personnel must have
the ability to furnish every service element and furnish these elements as is appropriate for any
individual patient during any calendar month. As described in more detail in section II.G.2.c. of
this proposed rule, we believe that maintaining certain advanced primary care practice
capabilities and requirements is inherent in these elements and must be met to fully furnish and
bill APCM. For example, if in our previous example, the patient with heart failure and chronic
kidney disease receiving Level 2 APCM experiences swollen legs, the patient should be able to
submit a photo or video to the practitioner via a secure communications system, and the
practitioner must be able to interpret and communicate remotely with the patient about those
images.
While we are proposing that specific minutes spent furnishing APCM services for
purposes of billing HCPCS codes GPCM1 – GPCM3 need not be documented in the patient’s
medical record, we would expect that any actions or communications that fall within the APCM
elements of service would be described in the medical record and, as appropriate, its relationship
to the clinical problem(s) they are intended to resolve and the treatment plan, just as all clinical
care is documented in the medical record.
We are seeking feedback on these service descriptions, and on whether there are elements
of other care management services that should be removed or altered for purposes of APCM
services.
Finally, while the service descriptors above are consistent across all three APCM levels
because the scope of service elements are consistent across all levels of APCM and the elements
that are provided depend on medical necessity and individual patient need, we are proposing that
the APCM codes would be stratified into three levels based on certain patient characteristics that

are broadly indicative of patient complexity and the consequent resource intensity involved in
the provision of these services in the context of advanced primary care. We are proposing that
the new APCM coding schema would be stratified based on APCM services being furnished
using the advanced primary care model to patients with one or fewer chronic conditions (“Level
1”); patients with two or more chronic conditions (“Level 2”); and Qualified Medicare
Beneficiaries (QMBs)
43
with two or more chronic conditions (“Level 3”) (see Table 20 for the
three APCM code levels).
This stratification of APCM into three levels allows us to distinguish
among different levels of patient complexity and more accurately reflect the resources required
to furnish APCM services for certain categories of beneficiaries. We anticipate that a practitioner
using the advanced primary care model would bill for APCM services for all or nearly all the
patients for whom they intend to assume responsibility for primary care.
Furthermore, we recognize the ways in which this new APCM coding intersects with
current care management codes around number of chronic conditions. We note that the current
care management codes are generally stratified in a similar, though more granular way, by the
degree of complexity of care based on the presence of chronic conditions and complexity of
medical decision making, who directly performs the service, and the time spent furnishing the
service. In establishing separate payment for CCM services in the CY 2014 PFS final rule, we
recognized that the resources involved in furnishing comprehensive, coordinated care
management services to patients with multiple (two or more) chronic conditions were greater
than those included in a typical non-face-to-face care management service, which we continued
to consider as bundled into the payment for face-to-face E/M visits (78 FR 43337). In the CY
2017 PFS final rule, based on robust feedback from interested parties indicating that the new
CCM codes did not fully capture the service time required to furnish care to beneficiaries with
43
See 42 CFR 435.123. The proposal includes both individuals in the QMB eligibility group who also have full
scope Medicaid coverage (“QMB-plus”) and individuals in the QMB eligibility group who do not have Medicaid
eligibility under any other Medicaid coverage group (“QMB-only”). However, this proposal would not include those
QMBs who are in the Medicare Part B Immunosuppressive Drug benefit, which provides coverage of
immunosuppressive drugs based on eligibility requirements described in § 407.55, because such individuals would
not qualify for Medicare coverage of the services described in this rulemaking. See 42 CFR 435.123(c)(2).

more complex conditions, we finalized new codes for patients with complex care management
needs. In the CY 2016 PFS final rule, in considering how to improve the accuracy of our
payments for care coordination, particularly for patients requiring more extensive care
management, we stated that we believe the care coordination and management for Medicare
beneficiaries with multiple chronic conditions, a particularly complicated disease or acute
condition, or certain behavioral health conditions often requires extensive discussion,
information-sharing, and planning between a primary care physician and a specialist (for
example, with a neurologist for a patient with Alzheimer’s disease plus other chronic diseases)
(80 FR 70919).
TABLE 20: Patient-Centered Risk Stratification for Billing APCM Codes
Level 1 [GPCM1]
Level 2 [GPCM2]
Level 3 [GPCM3]
Patients with one or fewer
chronic conditions.
Patients with two or more chronic
conditions.
Patients with two or more chronic
conditions and who are Qualified
Medicare Beneficiaries.
(1) Level 1 APCM
We are proposing the Level 1 APCM code for patients with one or fewer chronic
conditions because of the increased import and use of non-face-to-face interactions in advanced
primary care even for patients with relatively fewer health needs, which has increased over time
for several observable reasons, including broad evolution in information and communication
technology in everyday life, diffusion of practices first adopted for higher-acuity patients, and
continuing practices widely adopted during the COVID-19 pandemic that reduce reliance on in-
person interactions. We believe APCM services for a patient diagnosed with one or fewer
chronic conditions will require significantly less time and resources than one with two or more
chronic conditions since, in general, there would be fewer ongoing health needs and other health
care resources to coordinate, a lower risk of drug interactions, and less complicated physiology.
Based on CY 2010 Medicare claims data, the difference in annual expenditures per beneficiary
between patients with one or fewer chronic conditions and those with two or three chronic

conditions was $3,673.
44
Our current care management coding similarly delineates patient
complexity between patients with a single serious chronic condition (PCM codes) and those with
two or more serious chronic conditions (CCM codes). We anticipate that practitioners who
would furnish APCM services may have already had experience with care management services
coding and payment for much of this population. The Level 1 APCM code would also address
the current gap in coding and payment for care management services furnished using an
advanced primary care model for patients without multiple chronic conditions.
(2) Level 2 APCM
We are proposing the Level 2 APCM code for patients with two or more chronic
conditions because of the frequency of chronic conditions in the Medicare population. In fact,
nearly four in five Medicare beneficiaries have two or more chronic conditions.
45
Furthermore,
as noted previously, our current care management coding delineates patient complexity for the
CCM codes for patients with two or more serious chronic conditions, and we anticipate that
practitioners who would furnish APCM services may have already had experience with care
management services coding and payment for much of this population.
For example, someone with chronic kidney disease and heart failure requires regular
check-ins, coordination with specialty care, follow-up after hospital admissions for heart failure
exacerbations, regular modifications of the care plan, and more. These services are typically
described by the existing CCM services. The patient may also typically need to reach out more
often to their primary care practitioner with questions or new symptoms via the patient portal.
For instance, the person sends a message through the patient portal to ask whether or not they
should come into the primary care office after gaining ten pounds in the last week—which could
be a sign of increased fluid retention and the need for increased diuretic dosages to avoid pleural
44
Centers for Medicare and Medicaid Services. Chronic Conditions among Medicare Beneficiaries, Chartbook,
2012 Edition. Baltimore, MD. 2012. https://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-
reports/chronic-conditions/downloads/2012chartbook.pdf.
45
Lochner, K., Goodman, R., Posner, S., & Parekh, A. (n.d.). Multiple Chronic Conditions Among Medicare
Beneficiaries. CMS. https://www.cms.gov/mmrr/Downloads/MMRR2013_003_03_b02.pdf.

edema (an accumulation of fluid in the lungs). The primary care team books the patient for a
same-day urgent care appointment to assess for signs of swelling and pleural edema. Again, this
on-demand access to their primary care team can help treat the patient’s chronic conditions in a
patient-centered way and avoid unnecessary complications.
(3) Level 3 APCM
We are proposing the Level 3 APCM code for patients with QMB status and two or more
chronic conditions based on our understanding that people with both multiple chronic conditions
and social risk factors generally require even more time and resources from primary care
practitioners and their teams to ensure that the patient’s chronic conditions are managed
appropriately and effectively. We are proposing to use a patient’s QMB status as a method to
identify beneficiaries with social risk factors that generally necessitate relatively greater resource
requirements to effectively furnish advanced primary care than people without such risk factors.
There is significant evidence that such dually eligible beneficiaries, on average, are more
medically complex and have higher healthcare needs;
46
for example, dually eligible
beneficiaries are more likely to have poor functional status
47
and recent expenditure data found
that the difference in Medicare spending on a per person per year basis between dually eligible
and non-dually eligible Medicare beneficiaries was $13,198 in CY 2021.
48
QMBs are the largest eligibility group within the Medicare-Medicaid dually eligible
enrollee population, comprising of 66 percent of the 12.8 million individuals per the most recent
available data.
49
For the approximately 8.5 million dually eligible beneficiaries who are QMBs,
46
Kaiser Family Foundation. (n.d.). A primer on Medicare: What is the role of Medicare for dual-eligible
beneficiaries? Retrieved June 24, 2024, from https://www.kff.org/report-section/a-primer-on-medicare-what-is-the-
role-of-medicare-for-dual-eligible-
beneficiaries/#:~:text=A%20larger%20share%20of%20dual,beneficiaries%3B%20and%20more%20than%20half
%20(.
47
ASPE. Report to Congress: Social Risk Factors and Performance Under Medicare’s Value-Based Purchasing
Programs. December 2016. https://aspe.hhs.gov/reports/report-congress-social-risk-factors-performance-under-
medicares-value-based-purchasing-programs.
48
https://www.macpac.gov/wp-content/uploads/2024/01/Jan24_MedPAC_MACPAC_DualsDataBook-508.pdf.
49
Beneficiaries Dually Eligible for Medicare and Medicaid. Data from CY 2021. (January 2024). Medpac and
Macpac. https://www.macpac.gov/wp-content/uploads/2024/01/Jan24_MedPAC_MACPAC_DualsDataBook-
508.pdf.

Medicaid provides assistance for patients to meet Medicare’s cost-sharing requirements. The
QMB eligibility group helps to ensure full access to the Medicare benefit for the lowest income
enrollees by covering these costs. Individuals can qualify for QMB status if their income is
below 100 percent of the Federal Poverty Level ($15,300/year in 2024) and assets are no more
than $9,430/$14,130 (one person/married couple in 2024), although states can request CMS
approval to disregard certain income and assets.
50
Beneficiaries apply for this benefit with their
State’s Medicaid program and must be redetermined eligible at least annually.
There is growing recognition that social risk factors – such as income, education, access
to food and housing, and employment status – play a major role in health,
51
such that social risk
factors may affect a person’s ability to reach their health goals, as well as the diagnosis and
treatment of their medical problems. A report submitted to Congress by the Office of the
Assistant Secretary for Planning and Evaluation (ASPE) in response to the Improving Medicare
Post-Acute Care Transformation (IMPACT) Act of 2014 (Pub. L. 113-185) found that dual
Medicare-Medicaid enrollment as a marker for low income was typically the most powerful
predictor of poor outcomes on quality measures among social risk factors examined.
52
Beneficiaries with social risk factors may have worse health outcomes due to a host of factors,
including higher levels of medical risk, worse living environments (for example, availability of
community services, pollution, safety), greater challenges in adherence to medication regimens
and medical recommendations (for example, diet/lifestyle), and/or bias or discrimination.
Evidence suggests that many health outcomes are related more to social, environmental, and
50
Access to Care Issues Among Qualified Medicare Beneficiaries (QMB). (2015). Centers for Medicare & Medicaid
Services. https://www.cms.gov/Medicare-Medicaid-Coordination/Medicare-and-Medicaid-Coordination/Medicare-
Medicaid-Coordination-Office/Downloads/Access_to_Care_Issues_Among_Qualified_Medicare_Beneficiaries.pdf.
51
Long P, Abrams M, Milstein A, Anderson G, Apton KL, Dahlberg M, Whicher D. Effective care for high-need
patients. Washington, DC: National Academy of Medicine. 2017. https://nam.edu/wp-
content/uploads/2017/06/Effective-Care-for-High-Need-Patients.pdf; Schroeder, S. (2007, September 20). We Can
Do Better—Improving the Health of the American People. New England Journal of Medicine, 357(12), 1221–1228.
https://www.nejm.org/doi/full/10.1056/NEJMsa073350.
52
ASPE. Report to Congress: Social Risk Factors and Performance Under Medicare’s Value-Based Purchasing
Programs. December 2016. https://aspe.hhs.gov/reports/report-congress-social-risk-factors-performance-under-
medicares-value-based-purchasing-programs.

economic factors (which may be beyond practitioners’ control) than to clinical interventions.
53
Dual enrollees, and more specifically, QMBs, are therefore a category of Medicare beneficiaries
who we believe to be the most socially at-risk of poorer clinical outcomes. As stated in the ASPE
report, “Some of the observed relationship between social risk factors and outcomes may be the
result of underlying differences in medical complexity, frailty, disability, and/or functional
status. For example, dually-enrolled beneficiaries are more likely to have poor functional status,
and therefore, may be more likely to be readmitted after a hospitalization.” As another example,
a patient with diabetes, heart failure, and QMB status may experience food, transportation, or
housing insecurity that contributes to difficulty maintaining blood glucose control which can
contribute to medical complications including potentially preventable heart failure exacerbations.
The primary care practitioner’s team may need to check-in regularly to ensure, for example, that
the patient gets needed specialty care such as an ophthalmologic examination to avoid the ocular
manifestations of diabetes; and consider the availability of transportation vouchers so the patient
can attend the ophthalmology appointment. We are proposing the Level 3 APCM code to
recognize the unique characteristics of QMBs as beneficiaries with social risk factors for whom
significantly more resources are involved in comprehensive care management by practitioners
that furnish advanced primary care services to them.
Additionally, we note that patients with QMB status are not responsible for the Medicare
cost-sharing associated with covered Medicare Part A or B services, including for any APCM
services. Generally, States cover such cost-sharing on behalf of QMBs, although many states use
a “lesser-of” policy through which states pay less than the full cost sharing amounts.
54
We solicit
comments from States on how they would cover cost sharing for the proposed APCM bundle,
considering lesser-of policies.
53
World Health Organization. (2018). Health Impact Assessment (HIA): The determinants of health.
http://www.who.int/hia/evidence/doh/en/.
54
Under the ‘‘lesser of’’ policy, a State caps its payment of Medicare cost-sharing at the Medicaid rate for a
particular service. For example, if the Medicare rate for a service is $100, of which $20 is beneficiary coinsurance,
and the Medicaid rate for the service is $90, the state would only pay $10. If the Medicaid rate is $80 or lower, the
state would make no payment.
We are also seeking feedback on the use of QMB status and multiple (two or more)
chronic conditions as the basis to bill for APCM Level 3 (GPCM3), whether QMB status is an
appropriate indicator to identify beneficiaries with added social risk, and whether there is an
equivalent marker of social deprivation for use in commercial markets that might be a possible
alternative identifier.
c. APCM Service Elements and Practice-Level Capabilities
All the elements within the scope of APCM services are included in the service
descriptors for GPCM1, GPCM2, and GPCM3, listed in Table 22, and described in this section.
As described above, we are proposing that APCM services would include nearly the same scope
of service elements and conditions we established for CCM and PCM services (including
elements of 24/7 access and care continuity, care management and care plan, care coordination,
management of care transitions, and enhanced communication). We believe this is appropriate
because care management is a key component of care delivery using an advanced primary care
model. The proposed phrasing in the code descriptors for APCM services generally tracks the
code descriptors for CCM and PCM services, except for references to “time spent” or “minutes”
of service.
We are seeking to ensure that the APCM codes would fully and appropriately capture the
care management and CTBS services that are characteristic of the changes in medical practice
toward advanced primary care, as demonstrated in select CMS Innovation Center models. As we
do for CCM and PCM services, we propose to require for APCM services that the practitioner
provide an initiating visit and obtain beneficiary consent (see section II.G.2.c.(1) and II.G.2.c.(2)
of this proposed rule). As described in more detail below, we are proposing to incorporate as
elements of APCM services “Management of Care Transitions” and “Enhanced Communications
Opportunities.” For the “Management of Care Transitions” APCM service element, we are
proposing to specify timely follow-up during care transitions (see section II.G.2.c.(6) of this
proposed rule). For the “Enhanced Communications Opportunities” APCM service element, we
are proposing to incorporate access to CTBS services, including remote evaluation of pre-
recorded patient information and interprofessional telephone/internet/EHR referral service(s), to
maintain ongoing communication with the patient, as well as access to patient-initiated digital
communications that require a clinical decision, such as virtual check-ins and digital online
assessment and management and E/M visits (or e-visits) (see section II.G.2.c.(8) of this proposed
rule).
We are also proposing to specify for APCM services the practice-level characteristics and
capabilities that are inherent to, and necessarily present when a practitioner is providing covered
services using an advanced primary care delivery model. As described in more detail below,
included in the service descriptors for GPCM1, GPCM2, and GPCM3, and listed in Table 22,
our proposed practice-level capabilities that reflect care delivery using an advanced primary care
model are focused around 24/7 access and continuity of care (see section II.G.2.c.(3) of this
proposed rule), patient population-level management (see section II.G.2.c.(9) of this proposed
rule), and performance measurement (see section II.G.2.c.(10) of this proposed rule). These
practice capabilities are indicative of, and necessary to, care delivery using an advanced primary
care model. Further, APCM services, as we propose to define them, could not be fully
performed in the absence of these practice capabilities; and, in such cases, APCM services
should not be billed.
We are proposing that practitioners participating in the ACO REACH model, the Making
Care Primary model, and the Primary Care First model would satisfy the proposed initiating
visit, patient population-level management, and performance measurement APCM service
elements and practice-level capabilities by virtue of their model participation. These CMS
Innovation Center models promote advanced primary care delivery consistent with the proposed
APCM service elements and practice-level capabilities described in Table 21. The models all
utilize attribution methods that review the most recently available two years of Medicare claims
to identify whether a model participant is responsible for a Medicare beneficiary’s primary care,

aligning with the initiating visit requirements for APCM services. Additionally, these three
models also include risk stratification and quality and cost performance metrics that are aligned
or overlap with the Value in Primary Care MVP.
55
Around-the-clock access and
continuity of care, patient population-level management, and performance measurement are
indicative of, and necessary to, care delivery using an advanced primary care model. We are
also considering whether certain practitioners in other types of CMS Innovation Center models
also satisfy the proposed service elements and requirements and seek comments on this question.
55
See, e.g., ACO Realizing Equity, Access, and Community Health (REACH) Model Request for Applications.
Available at https://www.cms.gov/priorities/innovation/media/document/aco-reach-rfa, ACO Realizing Equity,
Access, and Community Health (REACH) Model PY 2024 Quality Measurement Methodology. Available at
https://www.cms.gov/files/document/aco-reach-quality-msr-meth-py24.pdf; Making Care Primary Payment and
Attribution Methodologies. Available at https://www.cms.gov/files/document/mcp-pymt-att-methodologies.pdf,
Primary Care First Payment and Attribution Methodologies PY 2024. Available at
https://www.cms.gov/files/document/pcf-py24-payment-meth.pdf.

TABLE 21: APCM Service Elements* and Practice-Level Capabilities
Consent
• Inform the patient of the availability of APCM services; that only one practitioner can furnish and be paid for
these services during a calendar month; of the right to stop services at any time (effective at the end of the calendar
month); and that cost sharing may apply* (may be covered by supplemental health coverage)
• Document in patient’s medical record that consent was obtained
Initiating Visit for New Patients (separately paid)
• Initiation during a qualifying visit for new patients
• An initiating visit is not needed: (1) if the beneficiary is not a new patient (has been seen by the practitioner or
another practitioner in the same practice within the past three years) or (2) if the beneficiary received another care
management service (APCM, CCM, or PCM) within the previous year with the practitioner or another practitioner in
the same practice.
24/7 Access to Care and Care Continuity
• Provide 24/7 access for urgent needs to care team/practitioner with real-time access to patient’s medical
information, including providing patients/caregivers with a way to contact health care professionals in the practice to
discuss urgent needs regardless of the time of day or day of week
• Continuity of care with a designated member of the care team with whom the patient is able to schedule
successive routine appointments
• Deliver care in alternative ways to traditional office visits to best meet the patient’s needs, such as home visits
and/or expanded hours, as appropriate
Comprehensive Care Management
• Overall comprehensive care management may include, as applicable
• Systematic needs assessment (medical and psychosocial)
• System-based approaches to ensure receipt of preventive services
• Medication reconciliation, management and oversight of self-management
Patient-Centered Comprehensive Care Plan
• Development, implementation, revision, and maintenance of an electronic patient-centered comprehensive care
plan which is available timely within and outside the billing practice as appropriate to individuals involved in the
beneficiary’s care, can be routinely accessed and updated by care team/practitioner, and copy of care plan to
patient/caregiver
Management of Care Transitions (for example, discharges, ED visit follow-up, referrals, as applicable)
• Coordination of care transitions between and among health care providers and settings, including transitions
involving referrals to other clinicians, follow-up after an emergency department visit, or follow-up after discharges
from hospitals, skilled nursing facilities or other health care facilities, as applicable
• Ensure timely exchange of electronic health information with other practitioners and providers to support
continuity of care.
• Ensure timely follow-up communication (direct contact, telephone, electronic) with the patient and/or
caregiver after ED visits and discharges from hospitals, skilled nursing facilities, or other health care facilities, within
7 calendar days of discharge, as clinically indicated
Practitioner, Home-, and Community-Based Care Coordination
• Ongoing communication and coordinating receipt of needed services from practitioners, home- and community-
based service providers, community-based social service providers, hospitals, and skilled nursing facilities (or other
health care facilities), as applicable, and document communication regarding the patient’s psychosocial strengths and
needs, functional deficits, goals, preferences, and desired outcomes, including cultural and linguistic factors in the
patient’s medical record

Enhanced Communication Opportunities
• Enhanced opportunities for the beneficiary and any caregiver to communicate with the care team/practitioner
regarding the beneficiary’s care through the use of asynchronous non-face-to-face consultation methods other than
telephone, such as secure messaging, email, internet, or patient portal, and other communication technology-based
services, including remote evaluation of pre-recorded patient information and interprofessional
telephone/internet/EHR referral service(s), to maintain ongoing communication with patients, as appropriate
• Ensure access to patient-initiated digital communications that require a clinical decision, such as virtual check-ins
and digital online assessment and management and E/M visits (or e-visits)
Patient Population-Level Management
• Analyze patient population data to identify gaps in care and offer additional interventions, as appropriate
• Risk stratify the practice population based on defined diagnoses, claims, or other electronic data to identify and
target services to patients
• A practitioners who is participating in a Shared Savings Program ACO, REACH ACO, Making Care Primary, or
Primary Care First satisfies this requirement
Performance Measurement
Be assessed on primary care quality, total cost of care, and meaningful use of CEHRT, which can be met in several
ways:
• For MIPS-eligible clinicians, by registering for and reporting the Value in Primary Care MVP**
• A practitioner who is part of a TIN participating in a Shared Savings Program ACO satisfies this requirement
through the ACO’s reporting of the APM Performance Pathway
• A practitioner who is participating in a Shared Savings Program ACO, REACH ACO, Making Care Primary, or
Primary Care First satisfies this requirement through the quality reporting, assessment and performance requirement
and other program and model requirements.
* Medicare beneficiaries who are enrolled in the QMB eligibility group do not have any Medicare cost-sharing
responsibility for copays, deductibles, and coinsurance.
** See discussion in section II.G.2.c.(10) of this proposed rule for a description of the timeline of MIPS reporting.
For APCM services billed in 2025, practitioners would register to report the MVP in 2025, and report the MVP in
2026 for the 2025 performance year/2027 MIPS payment year. For more details, see the 2024 MIPS Quick Start
Guide, available at https://qpp.cms.gov/mips/reporting-options-overview.
We are seeking comment on whether the proposed elements and requirements are
appropriately reflective of care management services for advanced primary care, and whether
there are proposed elements of APCM services or proposed practice capabilities that should be
modified or removed. We are also seeking feedback on ways to align the APCM services with
other Medicare programs and initiatives, such as the Shared Savings Program, ACO REACH,
and advanced primary care models, and the Quality Payment Program, including MIPS and
Advanced Alternative Payment Models (Advanced APMs). We seek to create a low burden way
for practitioners to furnish APCM services by appropriately recognizing ways in which they may
meet APCM billing requirements as part of these programs and initiatives. We note that under
the Quality Payment Program, practitioners who are MIPS eligible clinicians would report
measures and activities as specified by CMS under the four MIPS performance categories:
quality, cost, improvement activities, and Promoting Interoperability (PI). To report to MIPS for
a performance period (§ 414.1320(i)) for the PI performance category, a MIPS eligible clinician
must use Certified EHR Technology (CEHRT), as defined at paragraph (2) under CEHRT at §
414.1305, report on the objectives and associated measures as specified by CMS, and submit
required attestations as specified in § 414.1375(b)(3) (§ 414.1375(b)). Eligible clinicians who
participate in Advanced APMs under the Quality Payment Program are required under the terms
of those APMs to use CEHRT as specified in §414.1415(a)(1)(iii); and are paid under the terms
of those APMs based on MIPS-comparable quality measures as specified in §414.1415(b).
As described in the sections below, we are proposing that a billing practitioner who is
part of a Shared Saving Program ACO, or CMS Innovation Center ACO or participating in
Making Care Primary or Primary Care First would already satisfy the proposed practice-level
requirements for patient population-level management (see section II.G.2.c.(9) of this proposed
rule), and performance measurement (see section II.G.2.c.(10) of this proposed rule) by meeting
separately applicable participation requirements within the Shared Savings Programs and
models. As noted previously, we are considering whether practitioners in other types of CMS
Innovation Center models also satisfy certain proposed service elements and practice-level
requirements through their participation in the models, and seek comments on this question.
(1) Beneficiary Consent
Consistent with other care management services, we are proposing that the beneficiary’s
consent to receive APCM services must be documented in the medical record as a condition of
payment for APCM services, as not all Medicare beneficiaries for whom APCM services would
be medically necessary may want to receive these services. As we do for CCM and PCM
services, we are proposing to require billing practitioners to inform the beneficiary of the
availability of APCM services, and ensure the beneficiary is aware that Medicare cost-sharing
usually applies (though these costs may be covered through supplemental health coverage). The
practitioner should also inform the beneficiary that, by providing APCM services, they intend to
assume responsibility for all of the patient’s primary care services and serve as the continuing
focal point for all needed health care services; and that only one practitioner can furnish and be
paid for APCM services during a calendar month, but that their consent to receive APCM
services does not limit their option to receive Medicare covered health care services from other
practitioners. The practitioner should inform the beneficiary that APCM is an ongoing, monthly
service and of their right to stop APCM services at any time (effective at the end of the calendar
month), and that they only need to provide consent once to receive APCM services from the
practitioner. We are proposing that the practitioner would document in the beneficiary’s medical
record that this information was explained and note whether the beneficiary accepted or declined
APCM services. We note that practitioners can still elect to obtain written consent rather than
verbal consent.
Practitioners have informed us that beneficiary cost sharing is a significant barrier to
provision of similar care management services, such as CCM services. The proposed patient
consent requirement is intended to ensure that patients do not incur unexpected expenses for care
that is largely, or in significant part, non-face-to-face in nature. The proposed requirement for
patient consent would also help to avoid duplicative practitioner billing, as the patient would
understand that the practitioner intends to serve as the focal point for all their care, and that only
one practitioner can furnish and be paid for APCM services in any particular month.
We seek feedback on these proposed requirements, including how best to effectively
educate both practitioners and beneficiaries on the benefits of APCM, especially as it reflects a
new bundle of services that may have previously been separately billed, and whether a CMS-
provided template to facilitate patient consent would be helpful. We also seek feedback on
whether CMS should require practitioners to revisit consent for APCM services on an ongoing
basis with patients.
(2) Initiating Visit
Consistent with CCM services (CPT codes 99437, 99439, 99487, and 99489 – 99491)
and PCM services (CPT codes 99424 – 99427), we are proposing to require an initiating visit for
APCM services only for new patients instead of for all beneficiaries receiving APCM services.

Consistent with the definition of “new patient” as described in the CPT® 2024 Professional
Edition Code Book on page 4, we are proposing to define a “new patient” as a person who did
not receive any professional services from the physician or other qualified health care
professional or another practitioner in the same group practice within the previous 3 years.
56
The
initiating visit furnished in advance of APCM services establishes the beneficiary’s relationship
with the billing practitioner, ensures the billing practitioner assesses the beneficiary prior to
initiating APCM services, facilitates collection of comprehensive health information to inform
the care plan, and provides an opportunity to obtain beneficiary consent (although beneficiary
consent can be obtained outside of the initiating visit). We are proposing that the same services
that can serve as the initiating visit for CCM services could serve as the initiating visit for
APCM, including a Level 2 through 5 E/M visit, initial preventive physician exam (IPPE), or
TCM service, and we propose that the initiating visit could be provided in person or as a
Medicare telehealth service.
We are proposing that an initiating visit would not be required for “established patients”
based on certain circumstances that we believe demonstrate an established patient-practitioner
relationship in advance of furnishing APCM services: (1) if the beneficiary is not a new patient
(has been seen by the practitioner or another practitioner in the same practice within the past
three years) or (2) if the beneficiary received another care management service (including an
APCM service, non-complex or complex CCM service (CPT codes 99487, 99489, 99490, 99491,
99439, 99437), or PCM service (CPT codes 99424, 99425, 99426, 99427)) within the previous
year with the practitioner or another practitioner in the same practice. We do not believe there
necessarily is a need for an initiating visit for APCM services for patients with whom the
practitioner (or another in the same practice) has an established relationship; and we would not
want to require an initiating visit under circumstances where a visit may not be medically
56
American Medical Association. CPT Professional 2024. American Medical Association, 2023.
necessary. The proposed policy not to require an initiating visit for beneficiaries who have
received any professional service from the physician or other qualified health care professional
or another practitioner in the same group practice within the previous 3 years is consistent with
CPT’s definition of the term “established patient,” such that we believe this captures patients
who have been seen relatively recently and who have an existing relationship with the practice.
In the case of beneficiaries who have received care management services from a practitioner
within the practice in the past year, we believe this indicates that the patient is also an
“established patient” in that the patient has an existing relationship with the practice, and the
patient previously has consented to the receipt of care management services, which have
overlapping service elements with APCM services.
We note that these standards would be consistent with applicable Shared Savings
Program and CMS Innovation Center patient attribution standards in ACO REACH, Making
Care Primary, and Primary Care First. Any beneficiary eligible to be assigned to an ACO
because of an established care relationship between the beneficiary and a billing practitioner who
would be billing for APCM services under the ACO participant’s TIN, including beneficiaries
who voluntarily aligned to a practitioner in the ACO, would not be considered a new patient and
would not require an initiating visit. Medicare rules governing patient attribution to an ACO on
the basis of care provided by an ACO-participating clinician similarly establishes where an
existing care relationship exists. Similarly, beneficiaries eligible to be assigned to a REACH
ACO, or a Making Care Primary or Primary Care First practice because of an established care
relationship between the beneficiary and a billing practitioner who would be billing for APCM
services under the model participant’s TIN, including beneficiaries who voluntarily aligned to a
practitioner participating in one of these three models would not be considered a new patient and
would not require an initiating visit. While we are proposing certain exceptions to the initiating
visit requirement for APCM services, we note that an initiating visit may still be needed even
when not required, and the billing practitioner can always furnish and bill for medically
necessary visits, including before initiating APCM services.
We seek feedback on these proposed requirements, including whether additional services
could serve as the initiating visit and whether a different period of time (for example, patients not
seen within one or two years) would be more appropriate.
(3) 24/7 Access and Continuity of Care
Access and continuity build on the patient-practitioner relationship to ensure patients
receive the right care at the right time from the right care team member. We are proposing to
include for APCM services the same scope of service elements we established for CCM and
PCM services for 24/7 Access and Continuity of Care with some modifications. For 24/7 Access
to Care, the scope of the service element we propose for APCM services would be to provide
24/7 access for urgent needs to the care team/practitioner with real-time access to patient’s
medical records, including providing patients/caregivers with a way to contact health care
professionals in the practice to discuss urgent needs regardless of the time of day or day of week.
As described in the CY 2017 PFS final rule, this accurately reflects the potential role of
clinical staff or call-sharing services in addressing after-hours care needs, and that after-hours
services typically would and should address any urgent needs and not only those explicitly
related to the beneficiary’s chronic conditions (79 FR 67722). In advanced primary care models
of care, primary care practices should be at the center of that care—providing an effective “first
contact” for patients, supporting patients in their management of care, and coordinating across
different settings of care. Achieving this level of access to primary care requires timeliness and
an effective relationship with those in the practice who are providing that care. True access is
fully informed by knowledge about the patient and their care, which is only possible through
real-time access to the patient’s electronic health information. Access to primary care, informed
by health information technology (IT), makes the right care at the right time possible, potentially
avoiding costly urgent and emergent care. Practices can achieve 24/7 access to care informed by

health IT through call coverage by a practitioner with health IT system access. This can be a
practitioner from the practice or a covering practitioner who has system access. Many practices
and systems use nurse call lines or answering services working with standard protocols to
provide the initial point of contact after hours and effectively address common problems. In this
situation, an escalation protocol will engage a practitioner with system access when needed for
decision making. Other successful practices expand hours, add urgent care services or partner
with other practices to provide these services, or contract with existing urgent care providers to
manage and coordinate care after regular office hours.
For Continuity of Care, the scope of service element would be to provide continuity of
care with a designated member of the care team with whom the patient is able to schedule
successive routine appointments. Continuity of care refers to the ability of patients to receive
care from practitioners who know them and are known by them. This continuity builds and
reinforces a relationship based in trust and shared experience that is highly valued by both
practitioners and patients. Practice focus on continuity of care can translate to improved
preventive and chronic care, patient and practitioner satisfaction, lower hospital utilization, and
lower costs.
57
Depending on the type and setting of care, there are three components of
continuity that improve patient outcomes and experience:
58
relational continuity (defined as the
“ongoing therapeutic relationship between a patient (and often their family/caregiver)” which is
foundational in advanced primary care), informational continuity (where practitioners have
57
Hussey, P. S., Schneider, E. C., Rudin, R. S., Fox, D. S., Lai, J., & Pollack, C. E. (2014). Continuity and the costs
of care for chronic disease. JAMA Internal Medicine, 174(5), 742-748.; Bayliss, E. A., Ellis, J. L., Shoup, J. A.,
Zeng, C., McQuillan, D. B., & Steiner, J. F. (2015). Effect of continuity of care on hospital utilization for seniors
with multiple medical conditions in an integrated health care system. The Annals of Family Medicine, 13(2), 123-
129.; Nyweide, D. J., Anthony, D. L., Bynum, J. P., Strawderman, R. L., Weeks, W. B., Casalino, L. P., & Fisher E.
S. (2013). Continuity of care and the risk of preventable hospitalization in older adults. JAMA Internal Medicine,
173(20), 1879–1885.; Haggerty, J. L., Reid, R. J., Freeman, G. K., Starfield, B. H., & Adair, C. E. (2003).
Continuity of care: a multidisciplinary review. BMJ, 327, 1219. doi:10.1136/bmj.327.7425.1219; Gupta, R., &
Bodenheimer, T. (2013). How primary care practices can improve continuity of care. JAMA Internal Medicine,
173(20), 1885–1886. doi:10.1001/jamainternmed.2013.7341.; Willard R., & Bodenheimer T. (2012, April). The
building blocks of high-performing primary care: Lessons from the field. California Healthcare Foundation.
http://www.chcf.org/publications/2012/04/building-blocks-primary-care.
58
Haggerty, J. L., Reid, R. J., Freeman, G. K., Starfield, B. H., & Adair, C. E. (2003). Continuity of care: a
multidisciplinary review. BMJ, 327, 1219. doi:10.1136/bmj.327.7425.1219.

access to information on patients’ past events and personal circumstances to inform current care
decisions); and longitudinal continuity (which refers to ongoing patterns of healthcare visits that
occur with the same practice over time). A key strategy to optimize continuity is ensuring that all
practitioners and/or the care team have access to the same patient information to guide care
within health IT, and successful practices start with a review and discussion of the practice-level
data developed through measurement of continuity.
59
Practices can develop the capability to
measure continuity of care between the patient and the practitioner/care team using health IT,
practice management software, or other tracking mechanisms, allowing them to track
improvements over time.
As included in the APCM code descriptors, we are proposing to specify for the “24/7
Access to Care” APCM service element that the practice would maintain the capability to deliver
care in alternative ways to traditional office visits to best meet the patient population’s needs,
such as e-visits, phone visits, home visits, and/or expanded hours. This proposed standard for
alternatives to office visits is similar to several requirements tested in CMS Innovation Center
models (such as the CPC+ model’s requirement that participating practices regularly offer at
least one alternative to traditional office visits
60
), and reflects the understanding that providing
alternatives to traditional office visits is an essential element of the delivery of care under an
advanced primary care model of care. Moving care out of traditional office visits can reduce
demand and open supply for prioritized visits. By changing where and how care is delivered,
practices may have increased availability for patients with complex needs who may be better
served by more time-intensive visits in the office, at home, or in a nursing home. We are not
proposing that a practice would need to regularly deliver care in all these alternative ways—for
example, a practice may routinely offer e-visits and phone visits, but not regularly furnish home
59
Gupta, R., & Bodenheimer, T. (2013). How primary care practices can improve continuity of care. JAMA Internal
Medicine, 173(20), 1885–1886. doi:10.1001/jamainternmed.2013.7341.; Willard R., & Bodenheimer T. (2012,
April). The building blocks of high-performing primary care: Lessons from the field. California Healthcare
Foundation. http://www.chcf.org/publications/2012/04/building-blocks-primary-care.
60
https://www.cms.gov/priorities/innovation/innovation-models/comprehensive-primary-care-plus.

visits, and still demonstrate this primary care practice capability. Another practice might offer
extended hours on certain days to help patients who may find it hard to take off work to see their
clinician, and this would satisfy this practice requirement.
We seek feedback on these proposed requirements.
(4) Comprehensive Care Management
We are proposing for APCM services the “Comprehensive Care Management” service
element we established for CCM and PCM services with some modifications. Rather than “care
management for chronic conditions,” the APCM service element would be “overall
comprehensive care management” which, like the element for CCM and PCM services, may
include, as applicable, “systematic assessment of the patient’s medical, functional, and
psychosocial needs; system-based approaches to ensure timely receipt of all recommended
preventive care services; medication reconciliation with review of adherence and potential
interactions; and oversight of patient self-management of medications.” This care management
standard is similar to several requirements tested in CMS Innovation Center models (such as the
CPC+ model’s requirement that participating practices provide targeted, proactive, relationship-
based care management to all patients identified as at increased risk and likely to benefit from
intensive care management and provide short-term care management, including medication
reconciliation, to patients following hospital admission/discharge/transfer, including observation
stays, and, as appropriate, following an ED discharge)
61
and is an essential element of the
delivery of care under an advanced primary care model of care. Care management is a resource-
intensive process of working with patients, generally outside of face-to-face office visits, to help
them understand and manage their health, navigate the health system, and meet their health
goals. Practices working with patients who have complex care needs have found care
management to be an effective and necessary strategy for mitigating risk and improving health
outcomes. Practices have found it valuable to think in terms of two broad types of patients who
61
https://www.cms.gov/priorities/innovation/innovation-models/comprehensive-primary-care-plus.

might benefit from different approaches to care management: patients with some combination of
multiple comorbidities, complex treatment regimens, frailty and functional impairment,
behavioral and social risks, and serious mental illness who would often benefit from long-term,
proactive, and relationship-based longitudinal care management; and patients who are otherwise
stable and will benefit from short-term, goal-oriented episodic care management during periods
of increased risk like transitions of care; diagnosis of a new, serious illness or injury involving
complex treatment regimens; or newly unstable chronic illness.
Successful practices use on-site, non-physician, practice-based, or integrated shared care
managers to provide longitudinal care management for the highest risk cohort of patients, with
assistance from other practice staff, as needed. Multiple team members may engage in care
management, but each patient identified as eligible should have a clinically trained individual in
the practice who is accountable for active, ongoing care management that goes beyond office-
based clinical diagnosis and treatment.
62
Longitudinal care management is captured in health IT
and includes providing proactive care that moves beyond traditional office visits or crisis-driven
care (for example, ED care or hospitalization) and is not primarily visit-based. Although office
visits are opportunities to define goals, plan patient care, engage in shared decision making, and
build a trusting relationship, most care management activities take place by phone, patient portal,
e-mail, mail, or home visits (and through visits to skilled nursing facilities or hospitals to support
transitional care).
Practices use the concept of episodic care management to identify patients who have
acute or urgent needs using “triggering events” (for example, hospital discharge, new diagnoses,
medical crisis, major life event, decompensation in otherwise controlled chronic condition) for
short-term, problem-focused care management services. Episodic care management is generally
62
Taylor, E. F., Machta, R. M., Meyers, D. S., Genevro, J., & Peikes, D. N. (2013). Enhancing the primary care
team to provide redesigned care: The roles of practice facilitators and care managers. Annals of Family Medicine,
11(1), 80–83. doi:10.1370/afm.1462.

time-limited and problem focused and most often includes coordination of services and follow-
up, patient education and support for self-management, and medication reconciliation.
We seek feedback on these proposed requirements.
(5) Patient-Centered Comprehensive Care Plan
We are proposing for APCM services the “Comprehensive Electronic Care Plan” service
element we established for CCM and PCM services with some modifications. As included in the
APCM code descriptors, we are proposing to specify that the care plan is “patient-centered”
which, as for CCM and PCM services, “is available timely within and outside the billing
practice” as appropriate to individuals involved in the beneficiary’s care, can be routinely
accessed and updated by care team/practitioner, and “copy of care plan to patient/caregiver.”
Providing longitudinal care management, which is an essential element of the delivery of
care under an advanced primary care model of care, includes the process of personalized care
planning. The personalized care planning process helps practices engage and collaborate with
patients to ensure that their care aligns with patient preferences, goals, and values.
63
A care plan
is a mutually agreed-upon document that outlines the patient’s health goals, needs, and self-
management activities and is accessible to all team members providing care for the patient. The
care plan should be patient-friendly, accessible to the patient, and should limit use of unfamiliar
medical jargon and acronyms. The care plan should also be structured and standardized,
documented in health IT to enable sharing among patient, caregivers, and care team members.
All high-risk patients receiving longitudinal care management should have a personalized care
plan developed in a joint, open-ended conversation between the patient and care team.
Personalized care planning is a dynamic process; therefore, the care plan document should be
63
Coulter A., Entwistle, V. A., Eccles, A., Ryan, S., Shepperd, S., & Perera, R. (2015). Personalised care planning
for adults with chronic or long-term health conditions. Cochrane Database System Review, 3, CD010523.; Edwards,
S. T., Dorr, D. A., & Landon, B. E. (2017). Can personalized care planning improve primary care? JAMA, 318(1),
25–26.
updated at regularly defined intervals by the care team and patient. In addition, when patients’
health status, preferences, goals, and values change, their plans of care should, too.
As described in the CY 2020 final rule, we proposed language to describe the “typical”
care plan elements which do not comprise a set of strict requirements that must be included in a
care plan for purpose of billing but are intended to reflect those that are typically included in a
care plan as medically appropriate for a particular beneficiary. The comprehensive care plan for
all health issues typically includes, but is not limited to, the following elements: problem list;
expected outcome and prognosis; measurable treatment goals; cognitive and functional
assessment; symptom management; planned interventions; medical management; environmental
evaluation; caregiver assessment; interaction and coordination with outside resources and
practitioners and providers; requirements for periodic review; and when applicable, revision of
the care plan.
We seek feedback on these proposed requirements.
(6) Management of Care Transitions
We are proposing to adopt for APCM services the “Management of Care Transitions”
service element we established for CCM and PCM services with some modifications. Rather
than requiring that the practice must facilitate communication of relevant patient information
through electronic exchange of continuity of care documents with other health care providers
regarding these transitions, we are proposing more simply to require the billing practitioner to
“ensure timely exchange of electronic health information” with other practitioners and providers.
As included in the APCM code descriptors, we are also proposing to specify for the
“Management of Care Transitions” APCM service element that the care team/practitioner would
follow up with the patient and/or caregiver within 7 days after each ED visit and hospital
discharge. This proposed timely follow-up standard is similar to several requirements tested in
CMS Innovation Center models (such as the CPC+ model’s requirement that participating
practices ensure patients with ED visits received a follow-up interaction within one week of

discharge
64
and the MCP model’s requirement that participating practices implement episodic
care management to provide timely follow-ups for high-risk patients post ED visit and
hospitalization
65
), and we patterned the timely follow-up element after our policy for TCM
services which requires, for example, “communication (direct contact, telephone, electronic)
with the patient and/or caregiver with 2 business days of discharge” and a “face-to-face visit
within 7 calendar days of discharge.” Providing timely follow-ups for patients is an essential
element of the delivery of care under an advanced primary care model of care, and we believe
this will help achieve timely, seamless care across settings especially after discharge from a
facility. Key aspects of follow-up after ED visits and hospitalizations include identifying and
partnering with target hospitals and EDs where the majority of a practice’s patients receive
services to achieve timely notification and transfer of information following hospital discharge
and ED visits.
66
When developing a standardized process for data exchange and timely follow-
up, successful practices include the following processes: information and data exchange about
patients seen in an ED or admitted to/discharged from a hospital (for example, via HIE, hospital
portal, hospital-generated report, EHR, or additional health IT system); definition for “timely”
follow-up after discharge (for example, no later than within 2 days of discharge from hospital
admission or observation stay and within 1 week of discharge from the ED); protocols for when
follow-up will be done (for example, before discharge or following a standardized follow-up
protocol); process of incorporating into the patient’s medical record so the information is
available at the time of the follow-up visit or other patient contact; and standardized processes
and protocols for data exchange and formalized partnerships to develop an efficient workflow to
ensure timely follow-up and facilitate efficient and safe transitions of care.
64
https://www.cms.gov/priorities/innovation/files/x/cpcplus-practicecaredlvreqs.pdf.
65
https://www.cms.gov/priorities/innovation/innovation-models/making-care-primary.
66
Carrier, E., Yee, T., & Holzwart, R. A. (2011). Coordination Between Emergency and Primary Care Physicians
(NIHCR Research Brief No. 3). National Institute for Health Care Reform. http://nihcr.org/analysis/improving-care-
delivery/prevention-improving-health/ed-coordination/; Ventura, T., Brown, D., Archibald, T., et al. (2010,
January–February). Improving care transitions and reducing hospital readmissions: establishing the evidence for
community-based implementation strategies through the care transitions theme.
http://www.communitysolutions.com/assets/2012_Institute_Presentations/ caretransitioninterventions051812.pdf.

Practices use a variety of scheduling strategies to prioritize same-day or next-day access
for acutely ill patients and to provide timely follow-up for patients experiencing care transitions.
Successful practices are those that can strike the right balance between timely access to visits and
the offering patients a provider of their choice (Continuity of Care). Establishing standardized
protocols and pathways to improve and ensure responsiveness and timely callbacks to patients is
an effective way to impact patient–practitioner/care team communication and to ensure a
safeguard for addressing emergent and urgent patient phone calls. Successful practices routinely
evaluate the degree to which patients’ phone calls are answered promptly or returned within a
practices’ established guidelines (for example, non-urgent, emergent, urgent) and routed to the
appropriate practitioner or care team member, incorporating patients’ clinical needs and
preferences.
67
Such strategies are paramount for practices whose patients may be contacting the
practice with care needs that require care team prioritization and urgent reply.
We seek feedback on these proposed requirements.
(7) Practitioner, Home-, and Community-Based Care Coordination
We are proposing to adopt for APCM services the “Home- and Community-Based Care
Coordination” service element we established for CCM and PCM services with some
modifications. As included in the APCM code descriptors, we are proposing to specify that the
“ongoing communication and coordinating receipt of needed services” is with not only with
home- and community-based service providers, but also with “practitioners,” “community-based
social service providers, hospitals, and skilled nursing facilities (or other health care facilities), as
applicable.” We are also proposing to add more detail about the communication documented in
the patient’s medical record in that it would include “the patient’s psychosocial strengths and
67
Hempel, S., Stockdale, S., Danz, M., Rose, D. E., Kirsh, S., Curtis, I., & Rubenstein, L. V. (2018). Access
management in primary care: Perspectives from an expert panel (Research Report No. RR-2536-DVA). Rand
Corporation. https://www.rand.org/content/dam/rand/pubs/research_reports/RR2500/RR2536/RAND_RR2536.pdf.;
O’Brien, L. K., Drobnick, P., Gehman, M., Hollenbeak, C., Iantosca, M. R., Luchs, S., Manning, M., Palm, S. K.,
Potochny, J., Ritzman, A., Tetro-Viozzi, J., Trauger, M., & Armstrong, A. D. (2017). Improving responsiveness to
patient phone calls: A pilot study. Journal of Patient Experience, 4(3), 101–107. doi:10.1177/2374373517706611.

needs, and functional deficits, goals, preferences, and desired outcomes, including cultural and
linguistic factors.”
Coordinated referral management with specialty groups and other community or
healthcare organizations ensures referrals are properly managed, coordinated, and
communicated. These efforts help practices achieve goals of enhancing the quality of patient
care, improving the patient’s care experience, and lowering cost, particularly for practices
serving high-risk patient populations. Evidence suggests that the development of formal
relationships (for example, collaborative care agreements) between the primary care practice and
referred groups/organizations that define shared goals and responsibilities, facilitate the
coordinated referral management process.
68
The foundation of successful coordinated referral
management with specialty groups and other community or healthcare organizations is the
development of processes and procedures to ensure high-value referrals, such as collaborative
care agreements and electronic consultations (e-Consults). Establishing clear and agreed-upon
expectations regarding communication and clinical responsibilities with specialty practices and
other care organizations, through a collaborative care agreement, improves the process.
Collaborative care agreements often include the following elements: defining the types of
referrals, consultation, and co-management arrangements available; specifying who is
accountable for which processes and outcomes for care within the referral, consultation, or co-
management arrangement; and specifying what clinical and other information should be
provided, how the information is transferred, and timeliness expectations. The electronic e-
Consults process is typically conducted through a system-wide EHR or a secure, web-based
system by which a practice receives guidance from a specialty provider or other care
organization.
69
In this process, a practitioner sends a clinical question and relevant clinical
68
Medicare Payment Advisory Commission (MedPAC). (2012, June). Report to the Congress: Medicare and the
Health Care Delivery System. http://medpac.gov/docs/default-
source/reports/jun18_medpacreporttocongress_sec.pdf?sfvrsn=0.
69
Vimalananda, V., Gupte, G., Seraj, S., Orlander, J., Berlowitz, D., Fincke, B., & Simon, S. (2015, September).
Electronic consultations (e-consults) to improve access to specialty care: A systematic review and narrative

information to the specialist (or other care organization), who responds by providing a clinical
opinion and guidance and/or confirms the need for a face-to-face appointment with the patient.
This tool and process has the potential to streamline consultations, reduce cost and burden for
patients, and improve access to specialty care for high-value referrals. As part of the CY 2019
PFS final rule, we finalized interprofessional consultation services codes, which support payment
both to the treating, requesting (primary care) practitioner (CPT code 99452) and the receiving,
consultative specialist (CPT codes 99446–99449 and 99451) who engage in e-Consults, and so
some practitioners have already become accustomed to providing and billing for these services.
Strategies for addressing common health-related social needs (HRSNs) for a practice’s
high-risk patients include conducting needs assessments at regular intervals, creating a resource
inventory for the most pressing needs of the patient population, and establishing relationships
with key community organizations. Practices can focus on developing relationships with
community-based organizations that support patients’ most significant HRSNs. Practices can
also seek to find common ground with community and social service organizations, focus on the
structure and process of referrals, and develop a bidirectional flow of information. Successful
practices work with their patients to ensure there is a shared understanding of the purpose of the
referral and aim to understand bottlenecks and barriers to meeting their needs through the
process. Many practices identify a care team member to be a community referral resource for
their patients. Successful referrals can help practices determine the most useful and available
resources in their community.
We seek feedback on these proposed requirements.
(8) Enhanced Communications Opportunities
We are proposing to include for APCM services the element of “Enhanced
Communications Opportunities” we established for CCM and PCM services with some
synthesis. Journal of Telemedicine and Telecare 21(6), 323–330.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4561452/.

modifications. Specifically, we would add “internet and patient portal” as examples of
asynchronous non-face-to-face consultation methods and specify that the practitioner would
provide “other communication technology-based services, including remote evaluation of pre-
recorded patient information and interprofessional telephone/internet/EHR referral service(s), to
maintain ongoing communication with patients, as appropriate” as well as specify “access to
patient-initiated digital communications that require a clinical decision, such as virtual check-ins
and digital online assessment and management and E/M visits (or e-visits).” Providing
asynchronous non-face-to-face consultation methods and other CTBS services is an essential
element of the delivery of care under an advanced primary care model of care, and we believe
this will allow patients to access their usual source of care more conveniently (see section
II.G.2.c.(3) of this proposed rule). There is growing consensus that incorporating telehealth into
primary care will allow patients to access their usual source of care more conveniently.
70
Patients
using telehealth visits have reported high satisfaction, identifying convenience and perceived
high quality of care as contributors,
71
such that these may be a good alternative and, in some
cases, preferable to in-person communication.
72
Expansion of telehealth to address episodic and
chronic conditions has been a significant trend in the evolution of telehealth applications, and
there is some evidence that video visits may enable more timely communication of test results
than in-person appointments.
As noted in section II.G.2.b. of this proposed rule, we are not proposing timeframe
restrictions for this proposed element, which includes access to certain CTBS (for example, the
restriction for virtual check-in services that there is not a related E/M service provided within the
70
Levine DM, Linder JA. Retail Clinics Shine a Harsh Light on the Failure of Primary Care Access. J Gen Intern
Med. 2016;31(3):260-262.; Dorsey ER, Topol EJ. State of Telehealth. N Engl J Med. 2016;375(2): 154-161.;
Powell, Rhea E., et al. "Patient perceptions of telehealth primary care video visits." The Annals of Family Medicine
15.3 (2017): 225-229.
71
Polinski JM, Barker T, Gagliano N, Sussman A, Brennan TA, Shrank WH. Patients’ Satisfaction with and
Preference for Telehealth Visits. J Gen Intern Med. 2016;31(3):269-275.
72
Krishnan N, Fagerlin A, Skolarus TA. Rethinking Patient-Physician Communication of Biopsy Results—The
Waiting Game. JAMA Oncol. 2015;1(8):1025-1026.; Cusack CM, Pan E, Hook JM, Vincent A, Kaelber DC,
Middleton B. The value proposition in the widespread use of telehealth. J Telemed Telecare. 2008;14(4):167-168.

previous 7 days or an E/M service or procedure within the next 24 hours or the soonest available
appointment).
We seek feedback on these proposed requirements.
(9) Patient Population-Level Management
As specified in the proposed APCM code descriptors, we are proposing to establish an
APCM service element for Patient Population-Level Management that would include practice
capabilities for population-based, data-driven approaches to manage preventive and chronic care
for their patient population and to plan and implement strategies to improve care and outcomes.
We are proposing that all practices would use data to develop clear improvement strategies and
analytic processes to proactively manage population health, including analyzing patient
population data to identify gaps in care and risk-stratifying the practice population based on
defined diagnoses, claims, or other electronic data to identify and target services to patients (such
as those at risk for poor health outcomes), and then would offer additional interventions, as
appropriate.
These proposed patient population-level management standards are similar to several
requirements tested in CMS Innovation Center models, including CPC+, which found that model
participants used data to identify and resolve gaps in care. We have modeled the proposed
patient population-level management standards on the CPC+ care delivery requirements. In the
CPC+ Model, participating practices were required, for example, to “use a two-step risk
stratification process for all empaneled patients, addressing medical need, behavioral diagnoses,
and health-related social needs” and “define at least one subpopulation of patients with specific
complex needs, develop capabilities necessary to better address those needs, and measure and
improve the quality of care and utilization of this subpopulation.”
73
Central to the delivery of
advanced primary care is the organization of the practice into care teams that have the data they
need to manage their patient populations and that have time allocated to plan and implement
73
CPC+ Care Delivery Resource. January 2019.

practice improvement strategies.
74
Using evidence-based protocols, registries, and the registry
functionality of the EHR, reminders and outreach help practices deliver appropriate preventive
care and consistent evidence-based management of chronic conditions for the entire patient
population.
75
Measurement of clinically relevant data at the practice-level guides testing and
implementing strategies to improve care and outcomes. Patient population-level management
capabilities are essential to the delivery of care under an advanced primary care model of care
and enable practices to meet the preventive and chronic care needs of their entire patient
population. Regular use of data to identify populations or groups of patients with similar needs
allows practices and care teams to use streamlined strategies, including setting goals with
measurable outcomes, to positively impact their patient populations. Evidence shows that
primary care teams supported with real-time, population-level clinical outcomes data effectively
manage population health and address care gaps which eliminates external costs to close gaps in
care.
76
More specifically, risk stratification allows practitioners to identify beneficiaries for
longitudinal care management, track beneficiaries with higher levels of need and manage their
conditions, and prevent beneficiaries from falling through the cracks, while developing strategies
to address those patients who are at increased and rising risk and most likely to benefit from
targeted, proactive, relationship-based care management and other strategies essential to
APCM.
77
Empanelment, which assigns each active patient to a practitioner and/or care team with
consideration of patient and caregiver preferences, allows practices to build responsive care
74
CPC+ Care Delivery Resource. January 2019.
75
O'Malley AS, Draper K, Gourevitch R, Cross DA, Scholle SH. Electronic health records and support for primary
care teamwork. J Am Med Inform Assoc. 2015 Mar;22(2):426-34. doi: 10.1093/jamia/ocu029. Epub 2015 Jan 27.
PMID: 25627278; PMCID: PMC4394968.
76
https://www.cms.gov/priorities/innovation/files/x/cpcplus-practicecaredlvreqs.pdf.
77
Hayes, S. L., & McCarthy, D. (2016, December 7). Care Management Plus: Strengthening Primary Care for
Patients with Multiple Chronic Conditions. The Commonwealth Fund.
http://www.commonwealthfund.org/publications/case-studies/2016/dec/care-management-plus; Hong, C. S., Siegel,
A. L., & Ferris, T. G. (2014, August). Caring for High-Need, High-Cost Patients: What Makes for a Successful Care
Management Program? The Commonwealth Fund.
http://www.commonwealthfund.org/~/media/files/publications/issue-
brief/2014/aug/1764_hong_caring_for_high_need_high_cost_patients_ccm_ib.pdf; Lakin, J. R., Robinson, M. G.,
Obermeyer, Z., Powers, B. W., Block, S. D., Cunningham, R., Tumblin, J. M.m Vogeli, c., & Bernacki, R. E. (2019).
Prioritizing primary care patients for a communication intervention using the "Surprise Question": A prospective
cohort study. Journal of General Internal Medicine, 8.

teams to optimize patient care and to address the preventive, chronic, and acute needs of all
patients, and provides a way for practices to identify care gaps and proactively reach out to
patients who have not been seen or contacted in a while.
78
For example, we believe these
elements of advanced primary care management could increase screening rates and ultimately
improve care of chronic conditions, such as hypertension and diabetes.
We note that this Patient Population-Level Management requirement of the APCM
services would be met for practitioners billing for APCM services through a TIN that is
participating in an ACO in the Shared Savings Program by virtue of the practitioner’s
participation in the ACO which must meet eligibility requirements for population management,
care coordination and quality improvement, including required processes and patient-
centeredness criteria in § 425.112. We note that ACOs in the Shared Savings Program and their
practitioners are already engaged in analyzing the patient population for care gaps, risk-
stratifying patients to further identify those at risk for poor health outcomes, and identifying
patients for whom additional interventions are appropriate. Similarly, the ACO REACH, Making
Care Primary, and Primary Care First CMS Innovation Center Models all require their participants to
deploy population health strategies to identify and offer interventions to mitigate health risks.
79
Participants in these models and their practitioners are already engaged in population health
management as described in Table 21.
We seek feedback on these proposed requirements.
78
Grumbach, K., & Olayiwola, N. J. (2015). Patient empanelment: The importance of understanding who is at home
in the medical home. Journal of the American Board of Family Medicine, 28(2), 170–272.; Altschuler, J.,
Margolius, D., Bodenheimer, T., & Grumbach, K. (2012). Estimating a reasonable patient panel size for primary
care physicians with team-based task delegation. Annals of Family Medicine, 10(5), 396–400.
doi:10.1370/afm.1400.
79
ACO Realizing Equity, Access, and Community Health (REACH) Model Request for Applications. Available a:
https://www.cms.gov/priorities/innovation/media/document/aco-reach-rfa, Making Care Primary Request for
Applications. Available at https://www.cms.gov/files/document/mcp-rfa.pdf, Primary Care First Request for
Applications Cohort 2. Available at https://www.cms.gov/priorities/innovation/media/document/pcf-cohort2-rfa.

(10) Performance Measurement
We are proposing for the APCM services a practice-level requirement of performance
measurement of primary care quality, total cost of care, and meaningful use of CEHRT.
Performance measurement is a critical element of care management services delivered in the
context of advanced primary care, and it forms the basis for practice improvement efforts by
enabling practices to identify key measures for improvement activities (for example, cost and
utilization data, clinical quality measures, patient experience of care data). Quality measurement
improves care delivery, including prevention of heart attacks, increasing vaccination rates, and
improving patient safety,
80
and quality measures are also effective tools to ensure that high-
quality advanced primary care, including care management, is being delivered. Several
performance measurement requirements were tested in CMS Innovation Center models (such as
the CPC+ model’s requirement that participating practices use data at both the practice- and
panel-level to set goals to improve population health management and to continuously improve
patients’ health, experience, and quality of care, and decrease cost). Using data resources and
developing workflows and analytics to guide practice changes can help practices achieve
reductions in total utilization and cost of care, and improvements in patient experience and
quality of care. Improving upon key outcome measures requires engaged clinical and
administrative leadership and a commitment to continuous, data-driven improvement.
81
In the
context of the PFS, performance management through quality measurement as a practice-level
requirement also ensures integrity to the provision of advanced primary care because it holds
billing practitioners accountable to factors that are affected by several service elements of APCM
coding. For example, effective patient-population level management can mean the practices
close care gaps in diabetes management, and the billing practitioner would perform better on
diabetes quality measures that assess for a patient’s control of hemoglobin A1c.
80
https://www.ahrq.gov/patient-safety/quality-measures/21st-century/challenges.html.
81
https://www.cms.gov/priorities/innovation/innovation-models/comprehensive-primary-care-plus.

We are proposing that this performance measurement practice-level requirement can be
met in several ways. For MIPS-eligible clinicians, the requirement would be met by registering
for and reporting the “Value in Primary Care” MIPS Value Pathway (MVP). A practitioner who
is part of a TIN that is participating as a Shared Savings Program ACO or a REACH ACO, or a
Primary Care First or Making Care Primary practice would meet these requirements by virtue of
the Shared Savings Program and CMS Innovation Center quality reporting, assessment of quality
performance, accountability for total cost of care, and other program and model requirements.
In the CY 2024 PFS final rule (88 FR 80042 through 80047), we finalized “The Value in
Primary Care” Merit-based Incentive Payment System (MIPS) Value Pathway (MVP), which
focuses on the clinical theme of promoting quality care for patients in order to reduce the risk of
diseases, disabilities, and death; and it includes cost measures, Promoting Interoperability (PI)
measures, improvement activities, and quality measures for common chronic conditions (for
example, hypertension, diabetes, depression).
82
The Value in Primary Care MVP contains the
Adult Universal Foundation quality measure set, which is consistent with the National Quality
Strategy goal of using the Universal Foundation measures across as many programs as is
feasible.
83
This MVP is especially well-suited to reflect the delivery of care using the advanced
primary care model as it was developed to include quality metrics that reflect clinical actions that
should be considered the foundations of primary care. The quality measures include key
elements such as cancer screening, immunization, blood pressure management, behavioral
health, care coordination, person-centered care, and screening for social drivers of health. The
improvement activities include engaging community resources to address drivers of health,
implementing changes in the practice’s patient portal to improve communication and patient
engagement, reviewing practices in place on targeted patient population needs, and chronic care
82
Value in Primary Care. Quality Payment Program. https://qpp.cms.gov/mips/explore-mips-value-
pathways/2024/M0005.
83
https://www.cms.gov/medicare/quality/cms-national-quality-strategy/aligning-quality-measures-across-cms-
universal-foundation.

and preventive care management for empaneled patients, aspects of advanced primary care
already discussed in this proposal. The cost measures include costs for common chronic
conditions, such as asthma/chronic obstructive pulmonary disease (COPD), diabetes, depression,
and heart failure, as well as the Total Per Capita Cost (TPCC) measure, which assesses the
overall cost of care delivered to a patient with a focus on the primary care they receive from their
provider(s) and captures the broader healthcare costs influenced by primary care.
84
The Value in
Primary Care MVP serves to demonstrate performance measurement that is reflective of the care
furnished using advanced primary care delivery. To ensure performance measurement consistent
with the delivery of advanced primary care services, we are proposing as an element of the
APCM services that a practitioner who is a MIPS eligible clinician as defined in § 414.1305 can
satisfy the performance measurement requirement by registering for and reporting the Value in
Primary Care MVP for the performance year in which they bill for APCM services. A MIPS
eligible clinician can report to MIPS as an individual, subgroup, group, APM Entity, or in any
combination of these four participation options, and can participate in multiple ways to report
MVPs.
85
MIPS-eligible clinicians who report the MVP are also required to report the PI
performance category measures and attestations throughout the performance period in which
they bill for APCM services,
86
as required under § 414.1375(b) (§ 414.1365(c)(4)(i)) (see section
IV of this proposed rule for details on reporting the objectives and measures for the MIPS PI
performance category for CY 2025 performance period/2027 MIPS payment year). The
measures in the MIPS PI performance category include measures such as electronic referral
loops, receiving and reconciling health information, and providing patients with electronic access
to their health information, all of which are reflective of important communication and
84
https://qpp.cms.gov/docs/cost_specifications/2023-12-13-mif-tpcc.pdf.
85
https://qpp.cms.gov/mips/mvps/learn-about-mvp-reporting-option?option=Group.
86
The MIPS PI performance period is a minimum of 180 consecutive days in the calendar year that occurs 2 years
prior to the MIPS payment year (see 42 CFR 414.1320(i)).

coordination channels between primary care, other specialist practitioners caring for the patient,
and the patient themselves. In addition, as set forth in 42 CFR 414.1375(b)(3), the MIPS PI
performance category also requires submission of affirmative attestations: (1) regarding their
cooperation in good faith with ONC direct review of their CEHRT; and (2) that they did not
knowingly and willfully take action (such as to disable functionality) to limit or restrict the
compatibility or interoperability of CEHRT.
87
For CCM services (CPT codes 99437, 99439, 99487, and 99489 – 99491) and PCM
services (CPT codes 99424 – 99427), we established that practitioners must use CEHRT to
record certain patient health information in a structured format, provide patients with access to
their health information, and exchange all relevant patient health information, including in
providing the “Management of Care Transitions” element of CCM services. For the APCM
services, which are furnished as part of a practitioner’s care delivery using the advanced primary
care model, we are proposing for practitioners who are MIPS eligible clinicians a practice-level
requirement to register for and report the MVP, including but not limited to the PI performance
category measures which focus on meaningful use of CEHRT, ensuring that patients/caregivers
and physicians or other qualified practitioners or clinical staff have real-time access to patient’s
medical information. We believe that comprehensive CEHRT use is a critical element of care
management services delivered in the context of advanced primary care.
As we stated in adopting the CEHRT use element for CCM and PCM services, we
believe that the meaningful use of CEHRT is vital to ensure that practitioners are capable of
providing the full scope of services, such as timely care coordination and continuity of care (see
our prior discussion of this issue at 79 FR 67723 and 84 FR 62696), and we believe that
flexibility in how practices can provide the requisite 24/7 access to care, continuity of care, and
87
Note that, under the Quality Payment Program, CMS may reweight the MIPS PI performance category to zero
percent of the MIPS final score, and not require an individual, group, or virtual group to use CEHRT and
demonstrate whether they are a meaningful user of CEHRT, by granting a significant hardship exception or other
type of exception based on certain circumstances as set forth in 42 CFR 414.1380(c)(2)(i)(C).

management of care transitions, can facilitate appropriate access to these services for Medicare
beneficiaries. The meaningful use of CEHRT helps ensure that members of the care team have
timely access to the patient’s most updated health information and offer an integrated view of a
patient’s clinical history from different points of care, supporting continuing, quality, and
integrated healthcare while avoiding duplication of efforts and costs, such as repeated exams.
88
For example, practices use EHRs to identify high-risk patients with chronic conditions to better
coordinate care and can supplement the practice's EHR data with data from external sources (for
example, State-level quality organizations) to obtain a more comprehensive view of patients.
Practices can also integrate clinical data from EHRs, health plan claims data, and county-level
social services data to evaluate population needs, stratify by risk, and assess what programs
would be most effective for supporting at-risk patients.
89
Standardized communication methods,
enabled by CEHRT, are a significant part of the advanced primary care delivery model. Health
IT systems that include remote access to the care plan or the full EHR after hours, or a feedback
loop that communicates back to the primary care physician and others involved in the
beneficiary’s care regarding after-hours care or advice provided, are extremely helpful.
90
They
help ensure that the beneficiary receives necessary follow up, particularly if the patient is
referred to the ED, and follow up after an ED visit is required under the element of
“Management of Care Transitions.” Accordingly, we believe the use of CEHRT or remote access
to the care plan is fundamental to providing the APCM service elements of 24/7 Access to Care,
Continuity of Care, and Management of Care Transitions under an advanced primary care
delivery model. Requiring performance of the requirements in these measures and attestations to
the meaningful use of CEHRT is similar to several requirements tested in CMS Innovation
Center models (such as the PCF model’s requirement that participating practices adopt and
88
McDonald, C. J., Tang, P. C., Hripcsak, G. and In: (eds) Biomedical Informatics. Springer, L. (2014), "Electronic
Health Record Systems," in Biomedical Informatics, Shortliffe, E.H. and Cimino, J.J., eds. London: Springer, pp.
391-421.
89
Harvey, Jillian B., et al. "Understanding how health systems facilitate primary care redesign." Health Services
Research 55 (2020): 1144-1154.
90
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3475839/#CR25.

maintain CEHRT for electronic clinical quality measure reporting, support data exchange with
other providers and health systems, and connect to their regional health information exchange
(HIE),
91
and the MCP model’s requirement that participating practices use EHR technology that
has been certified under the ONC Health IT Certification Program
92
). Furthermore, the Shared
Savings Program requires practitioners (that is, MIPS eligible clinicians, QPs and Partial QPs) in
all ACOs to demonstrate meaningful CEHRT use through the reporting of the MIPS Promoting
Interoperability annually beginning in 2025.
We recognize that many practitioners who are not MIPS eligible clinicians for a year
would be excluded from MIPS by achieving Qualifying APM Participant (QP) status based on
their levels of participation in an Advanced APM. Based on the characteristics of Advanced
APMs detailed in § 414.1415, including the requirement that payment is based on MIPS or
MIPS-comparable quality measures, practitioners who with QP status are necessarily engaging in
performance measurement through the Advanced APMs in which they participate in a way that
is consistent with advanced primary care. We also recognize there are other practitioners who are
not MIPS eligible clinicians for other reasons, such as practitioners who are newly enrolled in
Medicare or bill a low volume of Medicare services. These practitioners technically could bill for
APCM services. However, newly enrolled practitioners are only excluded from MIPS for one
year, after which the practitioner would either be a MIPS eligible clinician who would need to
report the MVP in order to bill for APCM services, or excluded from MIPS on another basis
such as QP status. In the case of practitioners with low Medicare volume, we anticipate that they
would be unlikely to bill for APCM services since the delivery of advanced primary care
generally involves time and resources to establish practice-level infrastructure, and the
economies of scale usually make this a more likely investment if the infrastructure can be
utilized across a larger patient panel.
91
https://www.cms.gov/priorities/innovation/innovation-models/primary-care-first-model-options.
92
https://www.cms.gov/priorities/innovation/innovation-models/making-care-primary.
We are also proposing that the performance measurement element of the APCM services
would be satisfied for practitioners billing for APCM services through a TIN that is participating
in a Shared Savings Program ACO for a performance year in which they furnish APCM services.
ACOs are currently required to report the APP quality measure set on behalf of their
practitioners, and would be required to report the APP Plus quality measure set as proposed in
Section III.G. of this proposed rule. Practitioners in ACOs are also already being held
accountable for reporting and performance and outcomes on many of the Universal Foundation
measures already, which are used in the Value in Primary Care MVP, and the APP Plus quality
measure set would fully align the Shared Savings Program’s quality performance standard with
the Universal Foundation measures upon the complete implementation of the APP Plus measure
set.
We propose to include the performance measurement requirement as an element of
APCM services furnished by practitioners. MIPS eligible clinicians who intend to report on the
Value in Primary Care MVP for the CY 2025 must register to report the Value in Primary Care
MVP as described under § 414.1365(b), a MIPS eligible clinician must register for an MVP
during between April 1 and November 30 of the applicable CY performance period to report the
MVP. MIPS eligible clinicians submit data on measures and activities in the first quarter of the
year following (CY 2026) the MIPS performance period. Under this proposal, a MIPS eligible
clinician billing for APCM services furnished in 2025 and who is satisfying the performance
measurement requirement through reporting the Value in Primary Care MVP, would need to
register for the MVP between April and November of 2025 and report data between January and
March 2026 on measures and activities in the Value in Primary Care MVP relating to services
furnished in 2025. A MIPS eligible clinician billing for APCM services furnished in 2026 and
who is satisfying the performance measurement requirement through reporting the Value in
Primary Care MVP, would need to register for the Value in Primary Care MVP between April
and November of 2026, and report data between January and March of 2027 on measures and
activities in the Value in Primary Care MVP relating to services furnished in 2026, and so on in
subsequent years.
As described above, we are seeking feedback on ways to align the APCM services with
other Medicare programs and initiatives, such as the Shared Savings Program and the Quality
Payment Program, including MIPS and Advanced APMs. We seek to create a low burden way
for practitioners to furnish APCM services by appropriately recognizing ways in which they may
meet APCM billing requirements as part of these programs and initiatives, including other ways
that practitioners may be fulfilling these performance measurement requirements. We are
seeking feedback on whether there are areas of duplication within the APCM service elements
and practice capabilities that we should consider addressing. We are also seeking comment on
how to appropriately align the time period for which the practitioner bills the monthly APCM
code with the calendar year reporting period covered by the MVP, and how we would verify and
enforce the performance measurement requirement of the APCM service.
We seek feedback on these proposed requirements.
d. Duplicative Services and Concurrent Billing Restrictions
In this section, we identify the services that would overlap substantially with APCM
services based on the proposed elements of the scope of service for APCM which we have built
into the service descriptors for GPCM1, GPCM2, and GPCM3 (see sections II.G.2.b. and
II.G.2.c. of this proposed rule). As such, we are proposing that APCM services could not be
billed by the same practitioner or another practitioner within the same practice for the same
patient concurrent with these other services: CCM, PCM, TCM, interprofessional consultation,
remote evaluation of patient videos/images, virtual check-in, and e-visits. Given that we have
intentionally designed the proposed elements of APCM services to track closely with the
elements of several other care management service and CTBS codes, these services are
substantially duplicative of APCM services. Further, these specific services (shown in Table 19)
are duplicative with APCM services because there is significant overlap in the patient

populations included in the code descriptors for these services and APCM services, such as
patients who have chronic conditions, high-risk conditions, or both complex and chronic
conditions.
TABLE 22: Care Management and CTBS which CMS Proposes are Substantially
Duplicative of APCM Services
Service
Description
Care Management Services (12 CPT Codes)
Chronic Care
Management
(CCM) (CPT
Codes 99487,
99489, 99490,
99491, 99439,
99437)
Management of all care for patients with two or more serious chronic conditions, timed, per
month
Principal Care
Management
(PCM) (CPT
Codes 99424,
99425, 99426,
99427)
Management of all care for patients with one serious chronic condition, timed, per month
Transitional
Care
Management
(TCM) (CPT
Codes 99495,
99496)
Management of transition from acute care or certain outpatient stays to a community setting,
with face-to-face visit (bundled into payment for the code), once per patient within 30 days
post-discharge
Communication Technology-Based Services (15 CPT Codes)
Interprofessional
Internet
Consultation
(IPC) (CPT
Codes 99446,
99447, 99448,
99449, 99451,
99452)
Consultations between or among certain kinds of medical practitioners.
Remote
Evaluation of
Patient
Videos/Images
(HCPCS code
G2250)
Remote evaluation of recorded video and/or images submitted by patient
Virtual Check-
In (HCPCS
codes G2251,
G2252)
Virtual check-in service to decide whether an office visit or other service is needed
Online Digital
E/M (e-Visit)
(CPT codes
98970, 98971,
98972, 99421,
99422, 99423)
Communication between patient and their provider through an online patient portal
As we have described in the sections above, comprehensive care management services
are essential to providing advanced primary care in the context of this proposal, and many of the

service elements for CCM/PCM/TCM shown in Table 19 are substantially the same as the
elements we are proposing for APCM services.
Also described above, providing CTBS is an essential element of the delivery of care
under an advanced primary care model of care. Recognizing this, we designed the proposed
APCM service elements to substantially overlap with the elements of the CTBS (for example,
interprofessional consultation and e-Visits) shown in Table 22. CTBS are used in delivery of
advanced primary care to maintain ongoing communication with patients and enable
interprofessional care teams to provide comprehensive support to manage chronic conditions
over time, which we believe will allow patients to access their usual source of care more
conveniently.
93
We also believe that interprofessional consultation can help promote integration
of behavioral health and primary care.
94
We also considered whether other care management services (such as Behavioral Health
Integration (BHI)), services addressing HRSNs (Community Health Integration (CHI), Social
Determinants of Health Risk Assessment, and Principal Illness Navigation (PIN)), and/or other
CTBS (Remote Physiologic Monitoring (RPM) and Remote Therapeutic Monitoring (RTM))
would be duplicative of the proposed APCM services. We believe that these services, when
appropriate, may complement APCM services rather than substantially overlap or duplicate
services, and that these other services are sufficiently different from the APCM services in the
nature and extent of the interventions and the qualifications of individuals providing the services,
to allow concurrent billing for services when appropriate. While these may be services that a
practitioner using the advanced primary care model would be likely to furnish, when appropriate,
they are not part of the core, routinely and universally essential elements of the advanced
primary care model. We also believe that several of these other services (such as BHI, CHI,
93
Levine DM, Linder JA. Retail Clinics Shine a Harsh Light on the Failure of Primary Care Access. J Gen Intern
Med. 2016;31(3):260-262.; Dorsey ER, Topol EJ. State of Telehealth. N Engl J Med. 2016;375(2): 154-161.;
Powell, Rhea E., et al. "Patient perceptions of telehealth primary care video visits." The Annals of Family Medicine
15.3 (2017): 225-229.
94
We are planning a separate proposal on expanding who can bill for IPC, including clinical psychologists, LCSWs,
marriage and family therapists (MFTs), and MHCs; see further discussion in section II.I of this proposed rule.
SDOH Risk Assessment, and PIN) could be supplemental to APCM for patients that have very
specific identified health care needs.
We are seeking more information from interested parties through our Advanced Primary
Care RFI about whether to consider incorporating additional service elements into the APCM
service elements and valuation for APCM codes; and whether and, if so, how to best incorporate
E/M services into future coding (see section II.G.3. of this proposed rule). We note that, for BHI
services, there is an established evidence base for approaches to caring for beneficiaries with
behavioral health conditions which involve integration in the primary care setting, are typically
provided by a primary care team, and include structured care management with regular
assessments of clinical status and modification of treatment. BHI is a term that refers broadly to
collaborative care that integrates behavioral health services with primary care. BHI is a team-
based approach to care that focuses on integrative treatment of patients with medical and mental
or behavioral health conditions. For BHI in particular, including CPT codes 99492, 99493,
99494, and 99484 and HCPCS code G0323, we are also seeking information regarding how
evolving changes in practice may warrant reconsideration of payment and coding policies.
We propose that the care management and CTBS codes that are identified in Table 19
could not be separately billed with the APCM codes for the same beneficiary by the same
practitioner, or a different one within the same practice, for the same service period. This would
prevent duplicative payments for substantially similar services and is consistent with how we
have paid for potentially overlapping care management services in the past.
As we refine our APCM policies, we note that we are not currently proposing to make
changes to the coding and payment policies for the existing care management and CTBS
services, other than to prohibit concurrent billing for the same patient during the same month by
the same practitioner or another in the same practice. For CY 2025, those codes would still be
available for practitioners who do not furnish care using the advanced primary care model or
who may prefer to document the existing care management and CTBS codes rather than use the
new proposed APCM codes.
We are also seeking comment on potential overlap between APCM services and other
services currently paid under the PFS, including but not limited to care management and care
coordination and other CTBS. If interested parties identify overlaps between APCM and other
services, we are seeking comment on whether the degree of overlap would warrant a policy to
restrict the services from being billed concurrently with APCM. We also seek comment on
whether any overlap would depend upon whether the same or a different practitioner reports the
services.
As we test new CMS Innovation Center models that include payments for the services
defined above, including CCM, PCM, TCM, interprofessional consultation, remote evaluation of
patient videos/images, virtual check-in, and e-visits, or as changes in the advanced primary care
model of care or more general changes to Medicare payment policy take place that affect
existing CMS Innovation Center models, consistent with existing policy, we will address
potential overlaps between payments made to model participants with our proposed payment for
APCM, elements of the proposed APCM service, and these duplicative services, and seek to
implement appropriate payment policies.
e. Valuation of APCM Services—GPCM1, GPCM2, and GPCM3
To improve the accuracy of payment for the kinds of services furnished as part of
advanced primary care and reduce the administrative burden associated with current coding and
billing rules, we are proposing to create three HCPCS codes to use for reporting the proposed
APCM service (GPCM1, GPCM2, and GPCM3) (sections II.G.2.b. and II.G.2.c. of this proposed
rule). Although these codes are unique in that they would be created to differentially pay for
advanced primary care management, the proposed APCM services incorporate elements of
existing services with the understanding that some patients will require more resources and some
fewer based on variability in patient complexity and needs (see section II.G.2.b of this proposed
rule). As we ordinarily do, we are proposing to base the PFS valuation for APCM codes on the
resources involved in furnishing the typical case of the service which may not necessarily reflect
the actual resources involved in furnishing every individual service.
In this section, we detail our proposed methods to identify a typical case and set of
resources involved in furnishing APCM, and the proposed valuation of these codes. To value
APCM, we compared the service elements described by the proposed APCM codes to the values
we have established for the specific care management and CTBS codes on which we modeled
the proposed the service elements of the APCM codes and which we built into the service
descriptors for GPCM1, GPCM2, and GPCM3 (see Table 19 and sections II.G.2.b. through
II.G.2.d. of this proposed rule). As stated above, we believe that the proposed elements of
APCM services reflect the comprehensive approach to care management involved in care
delivery using the advanced primary care model. This is a model of primary care that is being
integrated into current medical practice. As such, we believe that it would be appropriate to use
the current valuation and uptake of the codes on which we modeled the APCM codes to inform
our valuation of APCM services. Using Medicare FFS claims data and evidence from our
primary care models, we sought to understand how these different services have been used
historically and relate that information to the way we are thinking about the service elements for
APCM and the valuation of the three APCM code levels. We know that for Medicare
beneficiaries who receive care management services during a year, the non-complex CCM base
code is billed on average for five months and with three add-on codes during those five months.
We also know that initial information from practitioner interviews conducted as part of our CCM
evaluation efforts indicates that practitioners overwhelmingly meet and exceed the 20-minute
threshold time for billing the non-complex CCM base code; typically, these practitioners
reported spending between 45 minutes and an hour per month on CCM services for each patient,
with times ranging between 20 minutes and several hours per month (81 FR 80244). However,
this does not account for the care management services that are provided beyond one time-based
billing interval and without reaching the next; nor does it account for the resources involved in
maintaining certain advanced primary care practice capabilities and continuous readiness and
monitoring activities, including patient population monitoring and care needs assessment, to
fully furnish and bill APCM services as is medically reasonable and necessary for any individual
patient during any calendar month. Finally, this does not account for changes to utilization of
APCM that may occur as a result of the billing and documentation requirements for APCM
services when compared to the current coding and payment for care management and CTBS
services. While our aim is to value APCM services based on refined assumptions that we believe
better recognize likely utilization of the new proposed codes and the work required to furnish
APCM services, this is challenging without more information. We welcome comments on ideas
for other sources of data that would help us to assess APCM services valuation.
We considered various alternatives for valuing the APCM services and how these may
impact the broader health care landscape given that primary care is of such import across the
country. We are proposing to set baseline APCM code values for this first year based on
historical utilization of the care management services we have drawn upon in designing the
APCM codes. We note that utilization of the care management services has been significantly
higher than CTBS, and we found that CTBS are not typically billed for a patient in the same
month as care management services. It is unclear whether the kinds of services described by the
CTBS are not typically provided during these months or whether they are being provided but not
separately reported. We will continue to seek information, including from public comments on
this proposed rule, to help us identify the best approach to reflecting the proposed CTBS
elements incorporated into the APCM monthly bundle, and we are particularly interested in data
that could illuminate differences between what services are furnished and what is being reported
separately. We will continue to consider refinements to the valuation of APCM codes to reflect
available information about changes in the volume and mix of care management and

communication activities being furnished as part of APCM services in the delivery of advanced
primary care.
For APCM Level 1, we assumed the typical case would involve fewer resources than the
current care management services based upon the proposed GPCM1 code descriptor and a broad
eligible population that would require limited monthly APCM services; however, it would also
involve certain resources inherent to maintaining advanced primary care practice capabilities and
continuous readiness and monitoring activities, including patient population monitoring and care
needs assessment, to fully furnish and bill APCM. As described in sections II.G.2.b. and II.G.2.c.
above, certain elements of the APCM service require resources to maintain continuous readiness
and monitoring activities to furnish covered services consistent with the advanced primary care
model of care. We concluded that the APCM Level 1 services would be similar in work to that of
two billing units of the non-complex code for CCM services (CPT code 99490 (CCM services
provided by clinical staff per calendar month)) over the course of a year, and therefore based the
proposed inputs on CPT code 99490 multiplied by 1/6 (or 2 units over 12 months). Specifically,
we proposed a work RVU for GPCM1 of 0.17, which is the work RVU for CPT code 99490
multiplied by 1/6. The resulting proposed PE and MP RVUs are proportionately similar to those
for CPT code 99490 and are available in Addendum B (see
https://www.cms.gov/medicare/payment/prospective-payment-systems/hospital-
outpatient/addendum-a-b-updates).
95
Table 23 displays payment amount estimates using the
2024 PFS Conversion Factor.
For APCM Level 2, which describes APCM services to patients with two or more
chronic conditions we assumed the typical, higher intensity work associated with managing a
patient with multiple chronic conditions would involve significantly more resources and require
more, and more frequent, APCM service elements. We concluded that the APCM Level 2
95
https://www.cms.gov/medicare/payment/fee-schedules/physician/federal-regulation-
notices?DLSort=2&DLEntries=10&DLPage=1&DLSortDir=descending.

services would be similar to current utilization assumptions of five billing units of the non-
complex CCM code (CPT codes 99490) (CCM services provided by clinical staff per calendar
month) and three billing units of add-on codes annually, given that, for Medicare beneficiaries
who receive these CCM services during a year, the non-complex CCM base code is billed on
average for five months and with three add-on codes during those 5 months. Additionally, we are
proposing to account for what we believe to be continued underutilization of CCM services in
this patient population by adding one billing unit of the complex CCM code (CPT code 99490
(CCM services provided by clinical staff per calendar month) annually. As we noted in the CY
2020 PFS final rule, “utilization [of CCM services] has reached approximately 75 percent of the
level we initially assumed under the PFS when we began paying for CCM services separately
under the PFS; while these are positive results, we believe that CCM services (especially
complex CCM services) continue to be underutilized”, 81 FR 80244 and 84 FR 62688,
considering the number of eligible Medicare beneficiaries. In 2019, approximately 22.6 million
FFS beneficiaries were identified as being potentially eligible for CCM (or 63.4 percent of the
35.6 million Medicare FFS beneficiaries); however, the use of CCM services was low among
potentially eligible beneficiaries, such that just 4.0 percent of beneficiaries potentially eligible for
CCM received any CCM services.
96
Therefore, we based the proposed inputs on CPT code
99490 multiplied by 5/12 (or, five units over 12 months), plus CPT add-on code 99439 (CCM
services each additional 30 minutes by clinical staff directed by a physician or other qualified
health care professional, per calendar month) multiplied by 1/6 (or two units), plus CPT add-on
code 99489 (Complex CCM services each additional 30 minutes by clinical staff directed by a
physician or other qualified health care professional, per calendar month) multiplied by 1/12
96
The determination of potential eligibility for CCM was based on presence of two or more Chronic Condition
Warehouse (CCW) chronic condition flags, one of which was hypertension, hyperlipidemia, or diabetes.
Beneficiaries on Medicare Advantage, with end stage renal disease (ESRD) or using the hospice benefit were
excluded. ASPE. Analysis of 2019 Medicare Fee-for-Service (FFS) Claims for Chronic Care Management (CCM)
and Transitional Care Management (TCM) Services. March 2022.
https://aspe.hhs.gov/sites/default/files/documents/31b7d0eeb7decf52f95d569ada0733b4/CCM-TCM-Descriptive-
Analysis.pdf.

(one unit), plus CPT code 99487 (Complex CCM services provided by clinical staff directed by a
physician or other qualified health care professional, per calendar month) multiplied by 1/12
(one unit). Specifically, we proposed a work RVU for GPCM2 of 0.77, which is the sum of the
work RVU for CPT codes 99490, 99439, 99489, and 99487 multiplied by their respective
proportions above. The resulting proposed PE and MP RVUs are proportionately similar and are
available in Addendum B (see https://www.cms.gov/medicare/payment/prospective-payment-
systems/hospital-outpatient/addendum-a-b-updates).
97
Table 23 displays payment amount
estimates using the 2024 PFS Conversion Factor.
For APCM Level 3 (HCPCS code GPCM3), which describes APCM services to patients
with QMB status and two or more chronic conditions, we are proposing to value the service as a
relative increase to the valuation of APCM Level 2 based on recent Medicare expenditure data
for dually eligible Medicare beneficiaries. In CY 2021, per person per year spending on dually
eligible beneficiaries was $24,370 and for non-dually eligible beneficiaries was $11,172. The
difference between these two amounts is 218 percent. We have considered the likely resource
demands and intensity of the practitioner-patient interaction for this patient population,
consistent with our coding and valuation policies that reflect variations in resource cost and
patient-centered care delivery policies.
98
By taking into consideration the difference in Medicare
spending on a per person per year basis between dually eligible and non-dually eligible Medicare
beneficiaries, we believe that we can capture the increased resources involved in furnishing
APCM services to patients with QMB status and multiple chronic conditions. Therefore, we
based the proposed inputs for the APCM Level 3 code on the APCM Level 2 inputs multiplied
by 218 percent. Specifically, we proposed a work RVU for GPCM3 of 1.67, which is the
proposed work RVU for GPCM2 multiplied by 218 percent. The resulting proposed PE and MP
RVUs are proportionately similar to those and are available in Addendum B (see
97
https://www.cms.gov/medicare/payment/fee-schedules/physician/federal-regulation-
notices?DLSort=2&DLEntries=10&DLPage=1&DLSortDir=descending.
98
https://www.macpac.gov/wp-content/uploads/2024/01/Jan24_MedPAC_MACPAC_DualsDataBook-508.pdf.

https://www.cms.gov/medicare/payment/prospective-payment-systems/hospital-
outpatient/addendum-a-b-updates).
99
Table 23 displays payment amount estimates using the
2024 PFS Conversion Factor.
Table 23 includes the proposed placeholder codes (which, if finalized, will be replaced
with numeric G-codes announced in the final rule), short descriptors, crosswalk codes, proposed
RVUs (work, PE, and MP), and approximate payment rate. For illustration purposes, we
multiplied the proposed APCM relative values for work, practice expense (PE), and malpractice
(MP), without geographic adjustment, by the CY 2024 conversion factor (CF) ($32.7442) to
convert the proposed relative value units (RVUs) into approximate national payment rates.
TABLE 23: Proposed APCM Bundled Codes and Valuation
Code
Short Descriptor
Crosswalk Codes
CMS
Proposed
Work
RVU
CMS
Proposed
PE RVU
CMS
Proposed
MP RVU
CMS
Proposed
Full
RVU
Approximate
National
Payment Rate
GPCM1
APCM for patients with
up to one chronic
condition
99490
0.17
0.14
0.01
0.31
$10
GPCM2
APCM for patients with
multiple (two or more)
chronic conditions
99490, 99439, 99487,
99489
0.77
0.72
0.05
1.54
$50
GPCM3
APCM for QMBs
enrollees with multiple
chronic conditions
Calculated as a relative
increase from GPCM2
1.67
1.57
0.12
3.36
$110
We are seeking feedback on whether these proposed values appropriately reflect the
resource costs involved in furnishing these services, or whether adjustments to the proposed
values or additional coding may be needed. We are broadly interested in public comments and
input from interested parties on potential refinements in code and service definitions, including
how we might refine our utilization assumptions for these codes, and other important information
involving coding and payment for APCM services to better reflect the current practice of
advanced primary care, including elements of CTBS and care management services. We are
interested in developing a better understanding of the resource costs involved in furnishing
99
https://www.cms.gov/medicare/payment/fee-schedules/physician/federal-regulation-
notices?DLSort=2&DLEntries=10&DLPage=1&DLSortDir=descending.

comprehensive care management as part of advanced primary care to various patient
populations, including specifically QMBs.
We intend to engage in further discussions with the public over the next several years to
potentially refine our policies for 2025 and future years, and we expect that having APCM
utilization data, once the proposed codes are established, would inform future refinement of the
valuations for these codes.
Finally, as described in the Advanced Primary Care RFI that follows, we note that there
is potential for the valuation of these codes and future related codes to change and/or scale into
larger units if we expand them to incorporate more service elements (see section II.G.3. of this
proposed rule). As we receive more information about how these codes are being used and
implemented in medical practice, we anticipate that these codes and future related codes will be
refined over time. We note that the development of payment and coding policies for these and
other kinds of services under the PFS is typically an iterative process that responds to changes in
medical practice and may be best refined over several years through annual rulemaking for the
PFS, and through the development of CPT codes by the AMA’s CPT Editorial Committee.
As described in the next section, we believe that this new proposed APCM code set could
serve as a chassis to incorporate primary care model learnings over time under the PFS and an
additional pathway to accountable care for primary care practitioners.
3. Request for Information: Advanced Primary Care Hybrid Payment
a. Background
Recent evidence reviews show that while primary care is the only part of the health
system in which investments routinely result in not only improved outcomes but also increased
equity,
100
the practice and sustainability of the primary care sector is under significant strain.
101
100
National Academies of Sciences, Engineering, and Medicine (NASEM); Implementing High-Quality Primary
Care (https://nap.nationalacademies.org/read/25983).
101
Milbank Memorial Fund, The Health of US Primary Care: 2024 Scorecard (https://www.milbank.org/wp-
content/uploads/2024/02/Milbank-Scorecard-2024-ACCESS_v06.pdf).

The NASEM found that many of these challenges relate to a primary care payment system that
principally rewards visit volume versus creation and maintenance of longitudinal
102
care
relationships over time.
103
We have set a goal of having 100 percent of traditional Medicare
beneficiaries and the vast majority of Medicaid beneficiaries in accountable care relationships by
2030. Accountable care occurs when a person-centered care team takes responsibility for
improving quality of care, care coordination and health outcomes for a defined group of
individuals, to reduce care fragmentation and avoid unnecessary costs for individuals and the
health system.
104
Advanced primary care is a core mechanism for achieving this goal. With this
goal, we acknowledge the need to increase the capability of primary care clinicians to engage,
maintain, and promote longitudinal and accountable relationships with beneficiaries through
incentives and flexibilities to manage quality and total cost of care.
Over the past 11 years, the CMS Innovation Center has tested a number of primary care
models: CPC, CPC+, Maryland Primary Care Program, PCF, as well as the upcoming MCP and
ACO Primary Care Flex. Each of these primary care models has focused on testing what happens
when we pay for primary care services with hybrid payments (a mix of fee-for-service and
population-based payments), as described earlier. While these models have not met the criteria
for expansion to date, the findings suggest advanced primary care may reduce unnecessary
utilization and improve diabetes care and cancer screening rates.
In addition to testing new approaches to improve care for beneficiaries by supporting
primary care, we have focused on approaches to incorporating these innovations into Medicare
programs. For example, lessons learned from the CMS Innovation Center’s ACO models may be
incorporated into the Shared Savings Program. As such, part of the intent of our proposal to
102
Longitudinal care management is long-term, proactive, relationship-based care management that augments
routine and acute visits with intentional, proactive outreach, especially during times of illness and transitions of care.
103
NASEM, Implementing High-Quality Primary Care (https://nap.nationalacademies.org/read/25983).
104
https://www.cms.gov/priorities/innovation/key-concepts/accountable-care-and-accountable-care-organizations.

create new APCM payment and coding is that we would have a similar foundation to scale
advanced primary care model learnings over time.
Previous Innovation Center primary care model tests have helped us learn lessons to
inform our current and future work. For example, participants in primary care models have
indicated difficulty investing in and maintaining primary care redesign activities due to a range
of challenges. First, additional non-visit-based primary care payments have been generally
layered upon base payments still predominantly FFS in structure. As such, the incentives and
abilities of practices to focus on proactive, population-based non-visit activities may be limited if
the funding stream for these activities is limited in scope and duration.
105,106
(Examples of non-
visit-based activities include, but are not limited to: activities to improve care coordination,
implement data-driven quality improvement, or enhance targeted care management for
beneficiaries identified as high-risk.) Further, model funding for the clinical and administrative
staff needed to accomplish advanced primary care coordination and population health functions
is contingent on continued participation in these models.
107
Once the models end, practices are
left without the funding that they received under the models for the clinical and administrative
staff that had supported population health functions under the model. Moreover, because these
models involve additional payments tied to performance rather than changes to base primary care
payment, practices report that the funding they use to support non-visit activities is sometimes
received well after the non-visit services have occurred, leading to further challenges sustaining
these efforts fiscally. Solving these challenges is a key goal of future Innovation Center model
work.
108
105
Independent Evaluation of Comprehensive Primary Care Plus (CPC+): Final Report.
https://www.cms.gov/priorities/innovation/data-and-reports/2023/cpc-plus-fifth-annual-eval-report.
106
Schurrer J, Timmins L, Gruszczynski M, et al. Evaluation of the Primary Care First Model: Second Annual
Report. Mathematica. February 2024. https://www.cms.gov/priorities/innovation/data-and-reports/2024/pcf-second-
eval-rpt.
107
CMS defines population health as health behaviors and outcomes of a broad group of individuals, including the
distribution of such outcomes affected by the contextual factors within the group.
108
https://www.cms.gov/about-cms/what-we-do/cms-strategic-plan.
To strengthen the primary care infrastructure within FFS Medicare, we are exploring
opportunities to create new sustainable pathways to support advanced primary care, equitable
access to high-quality primary care, and continued transformation among a wide variety of
practices. One potential strategy to increase access to advanced primary care and prepare
practitioners in traditional Medicare to engage in more accountable care is through the creation
and ongoing refinement of specific billing and coding under the PFS that better recognizes
advanced primary care and incorporates the resources involved in furnishing longitudinal care
and maintaining relationships with patients over time. In section II.G.2. of this proposed rule, we
are proposing a set of APCM services that make use of lessons learned from the CMS Innovation
Center’s primary care models, grouping existing care management and CTBS service elements
into a bundle for use starting in CY 2025.
We are seeking feedback regarding potential further evolution in coding and payment
policies to better recognize advanced primary care. Through this Advanced Primary Care RFI,
we are committed to collaborating with interested parties to lay the path for a more transparent
movement to value-based care. Specifically, we are requesting input on a broader set of
questions related to care delivery and incentive structure alignment and five foundational
components:
● Streamlined Value-Based Care Opportunities
● Billing Requirements
● Person-Centered Care
● Health Equity, Clinical, and Social Risk
● Quality Improvement and Accountability
We encourage input on the questions below from diverse voices, including beneficiaries
and advocates, community-based organizations, providers, clinicians, researchers, unions, and all
other interested parties.
b. Solicitation of Public Comments
We are seeking feedback regarding potential changes to coding and payment policies for
advanced primary care services to be incorporated in traditional Medicare. For example, in the
future, coding for APCM services (proposed in section II.G.2. of this proposed rule) could be
revised to include additional service elements, including traditional E/M services. This
Advanced Primary Care RFI is designed to solicit feedback on how we can further the goals of
reducing administrative burden to refocus time on patient care; better recognizing the relative
resources involved in furnishing care; recognizing interdisciplinary, team-based primary care;
and supporting primary care sustainability and stability (especially for underserved
communities). Whenever possible, respondents are requested to draw their responses from
objective, empirical, and actionable evidence and to cite this evidence within their responses. We
anticipate potential changes to primary care coding and payment policies, such as use of coding
that recognizes groups of services furnished over a fixed time period, that would offer a new
opportunity within the PFS for primary care clinicians to move to payment structures that are not
fully dependent on billing for each discrete component of overall care and act as a step toward
accountability for the cost and quality of patient care. Therefore, we are seeking feedback on
building advanced primary care payment mechanisms that create pathways to recognize how
primary care practice has moved away from an encounter-based orientation toward population-
based care. This Advanced Primary Care RFI is the first step in ensuring ample opportunity for
public input, followed by notice and comment rulemaking in subsequent years.
(1) Streamlined Value-Based Care Opportunities
We are seeking to create a steppingstone for primary care clinicians, including those new
to value-based care, to move away from either encounters or other discrete components of
overall care as the dominant method of primary care payment and toward payments in larger
units that are better tied to the relative resource costs involved in population-based, longitudinal
care. Feedback from interested parties has been helpful when considering how to scale the
availability of payments into larger units, and incorporate population-based variability in
resources, all while driving toward accountability, and person-centered care. Ultimately, to
create more opportunities for beneficiaries to receive high-quality, accountable primary care, we
are focused on creating multiple pathways to recognize delivery of integrated care across
settings, and engagement in comprehensive, team-based, longitudinal care.
When considering the evolution of a hybrid payment system within the PFS, we seek
input on the following questions:
● How can CMS better support primary care clinicians and practices who may be new to
population-based and longitudinal care management?
● What are the primary barriers to providing particular strategies or supports needed for
pediatric clinicians and practices?
● How can CMS ensure that potential future advanced primary care payment will not
induce clinicians to leave effective accountable care relationships and clinician networks that
already produce positive results? Additionally, how can CMS support growth over time in
existing effective accountable care relationships and clinician networks?
● Should CMS evolve the proposed APCM services into an advanced primary care
payment that includes E/M and other relevant services, or maintain a separate code set for
APCM?
● If E/M services are bundled together for advanced primary care payments, how can
CMS ensure that there is not a disincentive for primary care clinicians to continue to provide
E/M visits, or increase accountability to E/M visits as warranted?
● As many codes depend on E/M visits (for example, as the base code for an add-on
code, or to initiate specific care management activities), how should CMS consider the
downstream impacts of incorporating E/M visits into advanced primary care payments?

● Should CMS consider incorporating other CTBS services into advanced primary care
hybrid payments, such as Remote Physiologic Monitoring and/or Remote Therapeutic
Monitoring?
● Should CMS consider incorporating other services that involve comprehensive care
management and care coordination, such as Behavioral Health Integration, End-Stage Renal
Disease Monthly Capitation Payment (ESRD MCP), Assessment/Care Planning for Cognitive
Impairment, and/or Advance Care Planning?
● Should CMS consider incorporating other services while the patient is under care of
home health agencies or hospices, such as Care Plan Oversight?
● Newly finalized HCPCS codes are eligible for use by other payers, including
commercial insurers, state Medicaid agencies, and others. We note that value-based alignment is
a key goal of CMS. If the APCM codes are finalized, they would be eligible for use by these
other payers as well. To what extent are other payers interested in adopting the APCM codes?
Are there any other changes that would be necessary for other payers to adopt the codes?
• CMS has historically used information presented by the Relative Value Scale Update
Committee to determine PFS payment rates. Are there other sources of data on the
relative value of primary care services that CMS should consider when setting hybrid
payment rates?
(2) Billing Requirements
Previous CMS Innovation Center primary care models have provided key lessons learned
about how to increase comfort with population-based payments, the importance of reducing the
administrative burden of billing, and how to begin addressing gaps in equitable access to
population-based payments.
109
Specifically, we have learned through Innovation Center
109
Independent Evaluation of Comprehensive Primary Care Plus (CPC+): Final Report.
https://www.cms.gov/priorities/innovation/data-and-reports/2023/cpc-plus-fifth-annual-eval-report; Independent
Evaluation of Primary Care First: Second Annual Report. https://www.cms.gov/priorities/innovation/data-and-
reports/2024/pcf-second-eval-rpt.

initiatives that retrospective reconciliation or adjustment of payments for services rendered can
be especially frustrating for practitioners, as it reduces the predictability and stability of
payments.
110
For these reasons, we are seeking to understand how advanced primary care hybrid
payments can balance program integrity, high-quality care, payment stability, and clinician
burden.
We seek input on the following questions:
● How can CMS reduce the potential burden of billing for population-based and
longitudinal care services?
● Are there particular types of items or services that should be excluded from the
advanced primary care bundle?
● Are there particular services paid under the PFS today that should be included in the
advanced primary care bundle?
● Care management activities are currently billed monthly. What episode lengths should
CMS consider when thinking about an advanced primary care bundle of services for hybrid
payment? Include evidence to support the proposed episode length.
● Should CMS attribute the advanced primary care clinical episode to a single clinician,
or consider weighted attribution and payment for multiple entities or clinicians? How could
weighted attribution and payment work? What rules or processes should CMS consider to
attribute the episode?
● Care management coding and payment have historically required an initiating visit
prior to starting monthly billing, to ensure that the services are medically reasonable and
necessary and consistent with the plan of care. Are there other ways that CMS could ensure the
clinician billing APCM is responsible for the primary care of the Medicare beneficiary?
110
Independent Evaluation of Primary Care First: Second Annual Report.
https://www.cms.gov/priorities/innovation/data-and-reports/2024/pcf-second-eval-rpt.

● Care management coding and payment require beneficiary cost sharing. Has
beneficiary cost sharing been a barrier to practitioners providing such services?
● Consistent with the initiating visit requirement in the APCM proposal, should CMS
require the billing of specific qualifying services for billing of an advanced primary care bundle
that is larger in scale and scope than APCM?
● Are there Health IT functions beyond what is proposed for APCM services that
clinicians should be required to have to bill for an advanced primary care bundle? What should
CMS consider in the design of the advanced primary care bundle to effectively incorporate
Health IT standards and functionality, to support interoperability and the aims of advanced
primary care?
● Should CMS limit the types of non-physician clinicians that can bill for an advanced
primary care bundle that is larger in scale and scope than APCM? If so, include evidence to
support the restriction.
● How should CMS reconcile instances where an advanced primary care bundle is
billed, but primary care services are then billed for and provided by separate entities?
(3) Person-Centered Care
Person-centered care integrates individuals’ clinical needs across providers and settings,
while addressing their social needs.
111
We strive for better, more affordable care and improved
health outcomes. Key to this mission are care innovations that empower beneficiaries and
clinicians, while reducing the administrative burden of providing episode-based and longitudinal
111
CMS White Paper on CMS Innovation Center’s Strategy: Driving Health System Transformation—A Strategy
for the CMS Innovation Center’s Second Decade (https://www.cms.gov/priorities/innovation/strategic-direction-
whitepaper).

care management. We are seeking comment on how an advanced primary care code(s) could be
structured to both increase efficiency and promote the use of high-value services.
We seek input on the following questions:
● What activities that support the delivery of care that is coordinated across clinicians,
support systems, and time should be considered for payment in an advanced primary care bundle
that are not currently captured in the PFS?
● How can CMS structure advanced primary care hybrid payments to improve patient
experience and outcomes?
● How can CMS structure advanced primary care hybrid payments to ensure appropriate
access to telephonic and messaging primary care services?
● What is the best reporting structure to ensure that targeted services are delivered
without causing undue or excessive documentation?
● How can CMS facilitate coordination between primary care clinicians that bill for
advanced primary care bundles and specialists to reduce costs and improve patient outcomes?
(4) Health Equity, Social and Clinical Risk
We define health equity as, “the attainment of the highest level of health for all people,
where everyone has a fair and just opportunity to attain their optimal health regardless of race,
ethnicity, disability, sexual orientation, gender identity, socioeconomic status, geography,
preferred language, or other factors that affect access to care and health outcomes.”
112
The CMS
Framework for Health Equity lays out how we are working to advance health equity by
designing, implementing, and operationalizing policies and programs that support health for all
the people served by our programs, eliminating avoidable differences in health outcomes
experienced by people who are disadvantaged or underserved, and providing the care and
support that our beneficiaries need to thrive.
113
For advanced primary care hybrid payments, this
112
https://www.cms.gov/pillar/health-equity.
113
Centers for Medicare & Medicaid Services, The CMS Framework for Health Equity (2022-2032). April 2022.
https://www.cms.gov/files/document/cms-framework-health-equity-2022.pdf.

may mean incorporating different types of social and clinical risk into the payment than have
typically been considered in traditional E/M or care management codes.
Recent models such as ACO REACH
114
and Making Care Primary
115
have incorporated
risk adjustment for social risk factors, such as Part D Low Income Subsidy enrollment status and
Area Deprivation Index, to better capture factors relevant to care of the patient. We seek input on
how advanced primary care billing and payment policy could be used to reduce health disparities
and social risk. Furthermore, we are seeking to balance a simple payment structure that
encourages the uptake of advanced primary care services, while ensuring that the risk adjustment
method used to develop the payment rates incentivizes the appropriate coding of patient
conditions and needs, including those that have previously been under-documented, such as
dementia and patient frailty.
116
We seek input on the following questions:
● What non-claims-based indicators could be used to improve payment accuracy and
reduce health disparities, and how can CMS ensure that they are collected uniformly and
documented consistently without unduly increasing administrative burden?
● What risk factors, including clinical or social, should be considered in developing
payment for advanced primary care services?
● How can CMS account for apparent changes in risk that are due to changes in coding
patterns rather than changes in health status?
● What risk adjustments should be made to proposed payments to account for higher
costs of traditionally underserved populations?
114
https://www.cms.gov/priorities/innovation/innovation-models/aco-reach.
115
https://www.cms.gov/priorities/innovation/innovation-models/making-care-primary.
116
National Academies of Sciences, Engineering, and Medicine (NASEM); Committee on the Decadal Survey of
Behavioral and Social Science Research on Alzheimer's Disease and Alzheimer's Disease-Related Dementias.
Reducing the Impact of Dementia in America: A Decadal Survey of the Behavioral and Social Sciences. National
Academies Press. July 26, 2021. https://nap.nationalacademies.org/catalog/26175/reducing-the-impact-of-
dementia-in-america-a-decadal-survey.
● What indicators are used to capture added social risk in commercial insurance? Should
CMS consider using these?
● What metrics should be used or monitored to adjust payment to ensure that health
disparities are not worsened as an unintended consequence?
● How can CMS ensure that advanced primary care hybrid payment increases access to
health care services for patients without a usual source of primary care?
● Are there steps CMS can take to ensure advanced primary care billing and coding is
utilized for dually eligible beneficiaries, and by safety net providers?
● Should CMS incorporate Community Health Integration and/or Principal Illness
Navigation services and payment into an advanced primary care bundle?
(5) Quality Improvement and Accountability
We are committed to affordable quality health care for all people with Medicare. We seek
feedback regarding how we can continue to strengthen beneficiary access to high-quality health
services within FFS Medicare. One goal of the CMS Innovation Center Strategy Refresh is to
increase the capability of practitioners furnishing advanced primary care to engage in
accountable care relationships with beneficiaries through incentives and flexibilities to manage
clinical quality, outcomes, patient experience, and total cost of care. As such, part of the intent of
evolving and creating over time advanced primary care hybrid payments is that the practitioners
who bill for these services are engaged in a relationship where they are responsible for the
quality and cost of care for the beneficiary, counting toward the overall 2030 goal of every
person with Traditional Medicare being in an accountable care relationship. This Advanced
Primary Care RFI seeks input from beneficiaries and their caregivers, primary care and other
clinicians, and health plans on how advanced primary care bundles could support that goal.
We seek input on the following questions:
● How can CMS ensure clinicians will remain engaged and accountable for their
contributions to managing the beneficiary's care?
● What are key patient-centered measures of quality, outcomes and experience that
would help ensure that hybrid payment enhances outcome and experience for patients?
● How could measures of quality, outcomes, and experience guard against and
decrement in access or quality?
● As described in the APCM proposal, reporting of the “Value in Primary Care” MVP
would be an APCM service element for MIPS eligible clinicians beginning in 2026. Since this
MVP contains measures focused on both the total cost and quality of care, would its inclusion as
an APCM service element be sufficient to count as “accountable care?” If not, what other service
delivery or quality reporting would be expected in “accountable care?”
● What should CMS consider so that that advanced primary care bundles could be used
to promote accountable care across payers, both commercial and Medicaid?
● What quality measures are other payers using to drive improvements in primary care?
● What utilization measures are other payers using to drive improvements in primary
care?
● What patient experience measures are other payers using to drive improvements in
primary care?
● Should CMS consider flexibilities for smaller practices to bill the advanced primary
care bundle? Should CMS consider flexibilities for entities exempt from MIPS to bill the
advanced primary care bundle?
● Would clinicians be willing to take on more accountability to further reduce the
frequency and/or administrative burden of billing?
● For APCM services, are there other key practice-level elements of the service that
should be considered for advanced primary care practices to bill for advanced primary care?

4. Cardiovascular Risk Assessment and Risk Management
a. Background
Cardiovascular disease (CVD) is a leading cause of death, disability, and health care
expenditures in the U.S.
117
The burden of CVD is unequal, with black Americans experiencing
higher rates of CVD-related morbidity than white Americans.
118
Atherosclerotic CVD
119
is also
distinct among leading causes of death for Americans in the proportion of CVD attributable to
behavioral causes,
120
making improvement in modifiable CVD risk factors (for example, diet,
exercise, smoking cessation) is a key treatment target to reduce the burden of CVD across
populations.
121
The CMS Innovation Center’s Million Hearts® Cardiovascular Disease (CVD) Risk Reduction
model
122
(hereafter referred to as Million Hearts® model) was launched in 2017 as part of the
ongoing HHS Million Hearts® Initiative.
123
The model's goals were to decrease the incidence of
first-time heart attacks and strokes among medium and high-risk Medicare beneficiaries over
five years and reduce Medicare spending on cardiovascular events. The model was implemented
as a randomized design where participant organizations in the intervention group agreed to (1)
calculate traditional Medicare beneficiaries’ risk of having a heart attack or stroke over 10 years,
and (2) provide cardiovascular care management services to high-risk patients (defined as a risk
of a cardiovascular event in the next decade of greater than thirty percent). The model also
identified medium-risk patients (more than fifteen percent risk of an event in the next decade) in
117
Heart Disease and Stroke Statistics—2023 Update: A Report from the American Heart Association Connie W.
Tsao, MD, MPH, FAHA et. al. Circulation. 2023;147:e93–e621.
118
Cardiovascular Health in African Americans: A Scientific Statement From the American Heart Association
Mercedes R. Carnethon, PhD, FAHA et al. Circulation. 2017;136:e393–e423.
119
What is Atherosclerosis? NIH NHLBI. https://www.nhlbi.nih.gov/health/atherosclerosis
120
Libby, P., Buring, J.E., Badimon, L. et al. Atherosclerosis. Nat Rev Dis Primers 5, 56 (2019).
https://doi.org/10.1038/s41572-019-0106-z.
121
Ebrahim S, Taylor F, Ward K, Beswick A, Burke M, Davey Smith G. Multiple risk factor interventions for
primary prevention of coronary heart disease. Cochrane Database Syst Rev. 2011;(1):CD001561
https://pubmed.ncbi.nlm.nih.gov/21249647/.
122
Sanghavi DM, Conway PH. Paying for prevention: a novel test of Medicare value-based payment for
cardiovascular risk reduction. JAMA. 2015;314(2):123-124.
https://jamanetwork.com/journals/jama/fullarticle/2300705.
123
Frieden TR, Berwick DM. The “Million Hearts” initiative: preventing heart attacks and strokes. N Engl J Med.
2011;365(13):e27. https://pubmed.ncbi.nlm.nih.gov/21913835/.

its evaluation. In exchange for doing so, CMS paid participant organizations $10 for each
eligible traditional Medicare beneficiary for whom the organizations assessed risk, and in the
first year of the model, $10 for each high-risk beneficiary during each month when
cardiovascular care management services were provided.
124
In subsequent years of the model
(2018 to 2022) participants were expected to reassess cardiovascular risk and were paid based on
cardiovascular risk reduction ($0 to $10 per beneficiary per month) for high-risk beneficiaries.
All CMS Innovation Center models are independently evaluated
125
and the evaluation of
the Million Hearts® model found the model reduced the rate of death from any cause for
medium and high-risk beneficiaries by four percent, as well as reduced the risk of death from a
cardiovascular event (that is, heart attack or stroke) by eleven percent.
126
We consider this to be
due to increased rates of cardiovascular risk assessment, discussion of cardiovascular risk by
participants’ clinicians, and the use of appropriate medications to reduce cardiovascular risk (for
example, aspirin and statins).
127
During the Million Hearts® (MH) model (which was tested from 2017-2022), there was a
recently-introduced ASCVD risk assessment tool to incorporate demographic (age, sex, race),
clinical (blood pressure, cholesterol, history of diabetes), and risk behavior (smoking status, use
of anti-hypertensives, use of statins, use of aspirin) established by the American College of
Cardiology (ACC),
128
as well as a longitudinal re-assessment tool used within the model.
129
This
124
Blue L, Kranker K, Markovitz AR, et al. Effects of the Million Hearts Model on Myocardial Infarctions, Strokes,
and Medicare Spending: A Randomized Clinical Trial. JAMA. 2023;330(15):1437–1447.
doi:10.1001/jama.2023.19597.
125
Evaluation of the Million Hearts Cardiovascular Disease Risk Reduction Model. Final Report. August 2023.
Mathematica. https://www.cms.gov/priorities/innovation/data-and-reports/2023/mhcvdrrm-finalannevalrpt.
126
Evaluation of the Million Hearts Cardiovascular Disease Risk Reduction Model, p. 43. Final Report. August
2023. Mathematica. https://www.cms.gov/priorities/innovation/data-and-reports/2023/mhcvdrrm-finalannevalrpt.
127
Evaluation of the Million Hearts Cardiovascular Disease Risk Reduction Model, p. 26. Final Report. August
2023. Mathematica. https://www.cms.gov/priorities/innovation/data-and-reports/2023/mhcvdrrm-finalannevalrpt.
128
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-
Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N,
Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC, Sperling L, Virani SS, Yeboah J. 2018 ACC guideline
on the management of blood cholesterol: a report of the American College of Cardiology Foundation/American
Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018.
https://tools.acc.org/ldl/ascvd_risk_estimator/index.html#!/calulate/estimator/.
129
Lloyd-Jones DM, Huffman MD, Karmali KN, Sanghavi DM, Wright JS, Pelser C, Gulati M, Masoudi FA, Goff
DC Jr. Estimating Longitudinal Risks and Benefits From Cardiovascular Preventive Therapies Among Medicare

tool calculated the 10-year risk of a cardiovascular event for beneficiaries ages 40-79.
Subsequently, additional ASCVD risk assessment tools have been developed.
130
Today in clinical practice, ASCVD risk is generally calculated using a tool combining
demographic data, personal history (risk behaviors and medical history), and laboratory data
(lipid panel).
131
This information is used to calculate into a 10-year estimate of a patient’s
ASCVD risk for use in determining treatment advice provided by the treating practitioner. This
determination requires both data collection at a visit and laboratory data, which may not be
available at an initial visit. This change in clinical practice occurred over time after a series of
guidelines from the American Heart Association (AHA) recommended using ASCVD risk in
determining treatment decisions for patients without a prior history of CVD.
132
This treatment
guideline also includes recommendations for lifestyle modifications for all patients. The CMS
Innovation Center Million Hearts® model contributed to this change in clinical practice by
demonstrating through a rigorous randomized control trial that the quantitative assessment of 10-
year cardiovascular risk improves quality of care, including mortality, compared to prior
practice.
133
In the Million Hearts® model, cardiovascular-focused care management services
included an initiating visit where an ASCVD risk assessment is performed, structured recording
of patient health information using CEHRT, and a comprehensive care plan focused on
cardiovascular risk reduction (including the ABCS focused on in the Million Hearts® model),
Patients: The Million Hearts Longitudinal ASCVD Risk Assessment Tool: A Special Report From the American
Heart Association and American College of Cardiology. Circulation. 2017 Mar 28;135(13):e793-e813.
130
Leading Cardiologists reveal new cardiovascular disease prevention risk calculator.
https://newsroom.heart.org/news/leading-cardiologists-reveal-new-heart-disease-risk-
calculator#:~:text=The%20American%20Heart%20Association%20PREVENT,CKM%20syndrome%20into%20CV
D%20prevention.
131
2019 ACC/AHA Primary Prevention of Cardiovascular Disease.
https://www.ahajournals.org/doi/pdf/10.1161/CIR.0000000000000678.
132
Arnett DK et. al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of
the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines.
Circulation. 2019 Sep 10;140(11):e596-e646. doi: 10.1161/CIR.0000000000000678.
133
Blue L, Kranker K, Markovitz AR, et al. Effects of the Million Hearts Model on Myocardial Infarctions, Strokes,
and Medicare Spending: A Randomized Clinical Trial. JAMA. 2023;330(15):1437–1447.
doi:10.1001/jama.2023.19597.
but did not require 24/7 access to care, management of care transitions, or home and community-
based coordination because these services are necessary for the management of complex
conditions placing a beneficiary at high risk of death, acute exacerbation/decompensation, or
functional decline, and these services are provided to prevent the development of these complex
chronic conditions. In the Million Hearts® model, cardiovascular-focused risk management
services were provided to beneficiaries at high risk for CVD (more than a thirty percent risk of a
cardiovascular event in the next 10 years).
We interpret the findings of the Million Hearts® model to be both reflective of and
perhaps augmenting an evolution in clinical practice toward quantitative ASCVD risk
assessment. We also do not believe the resources involved in these activities are appropriately
reflected in current coding and payment policies. As such, we are proposing to establish codes to
describe a separately billable cardiovascular disease risk assessment that is furnished in
conjunction with an E/M visit and cardiovascular-focused risk management, when reasonable
and necessary due to the presence of increased cardiovascular risk factors identified for the
individual patient.
b. ASCVD Risk Assessment
We are proposing a new stand-alone G-code, HCPCS code GCDRA, Administration of a
standardized, evidence-based Atherosclerotic Cardiovascular Disease (ASCVD) Risk Assessment
for patients with ASCVD risk factors on the same date as an E/M visit, 5-15 minutes, not more often
than every 12 months. Atherosclerotic Cardiovascular Disease (ASCVD) Risk Assessment refers
to a review of the individual’s demographic factors, modifiable risk factors for CVD, and risk
enhancers for CVD. We are proposing this new code to identify and value the work involved in
administering an ASCVD risk assessment when medically reasonable and necessary in relation
to an E/M visit.
We further propose that the ASCVD risk assessment must be furnished by the
practitioner on the same date they furnish an E/M visit, as the ASCVD risk assessment would be
reasonable and necessary when used to inform the patient’s diagnosis, and treatment plan
established during the visit. ASCVD risk assessment is reasonable and necessary for a patient
who has at least one predisposing condition to cardiovascular disease that may put them at
increased risk for future ASCVD diagnosis. These conditions could include but are not limited
to, obesity, a family history of CVD, a history of high blood pressure, a history of high
cholesterol, a history of smoking/alcohol/drug use, pre-diabetes, or diabetes. We further propose
that the ASCVD risk assessment would not be separately billable for patients with a
cardiovascular disease diagnosis or those who have history of a heart attack or stroke.
We are not proposing any specific tool that would have to be used for the ASCVD risk
assessment, although the assessment tool must be standardized and evidence-based. Proposed
elements of the ASCVD risk assessment service would include:
● Current (from the last 12 months) laboratory data (lipid panel) for inputs needed for the
risk assessment tool.
● Administration of a standardized, evidence-based ASCVD risk assessment tool that has
been tested and validated through research, and includes the following domains:
++ The output of the tool must include a 10-year estimate of the patient’s ASCVD risk.
This output must be documented in the patient’s medical record.
++ Demographic factors (such as age, sex).
++ Modifiable risk factors for CVD (such as blood pressure & cholesterol control,
smoking status/history, alcohol and other drug use, physical activity and nutrition,
obesity).
++ Possible risk enhancers (such as pre-eclampsia, pre-diabetes, family history of CVD).
++ Billing practitioners may choose to assess for additional domains beyond those listed
above if the tool used requires additional domains. Examples of tools include but are not limited

to, the ACC ASCVD Risk Estimator
134
and the ACC PREVENT tool.
135
CMS expects that the
tool that is used would not introduce discriminatory bias, consistent with Section 1557 final rule.
We are proposing for HCPCS code GCDRA to have a duration of 5–15 minutes for the
administration of an ASCVD risk assessment tool, billed no more often than once every 12
months.
We are requesting comments on these proposals, as well as information pertaining to potential
clinician education for these proposed codes.
(1) Proposed Valuation for ASCVD Risk Assessment GCDRA
We propose a direct crosswalk to HCPCS Code G0136 (Administration of a
standardized, evidence-based SDOH assessment, 5–15 minutes, not more often than every 6
months), with a work RVU of 0.18 as we believe this service reflects the resource costs
associated when the billing practitioner performs the service described. HCPCS code G0136 has
an intra-service time of 15 minutes, and the physician work is of similar intensity to proposed
HCPCS code GCDRA. Therefore, we are proposing a work time of 15 minutes for HCPCS code
GCDRA based on this same crosswalk to G0136. We are also proposing to use this crosswalk to
establish the direct PE inputs for HCPCS code GCDRA.
We are seeking comments on these proposals.
c. Atherosclerotic Cardiovascular Disease Risk Management Services (GCDRM)
Over the past several years, we have worked to develop payment mechanisms under the PFS to
improve the accuracy of valuation and payment for the services furnished by physicians and
other healthcare professionals, especially in the context of evolving changes in medical practice
using evidence-based models of care, such as the Million Hearts® model. We are proposing to
134
Lloyd-Jones DM, Huffman MD, Karmali KN, Sanghavi DM, Wright JS, Pelser C, Gulati M, Masoudi FA, Goff
DC Jr. Estimating Longitudinal Risks and Benefits From Cardiovascular Preventive Therapies Among Medicare
Patients: The Million Hearts Longitudinal ASCVD Risk Assessment Tool: A Special Report From the American
Heart Association and American College of Cardiology. Circulation. 2017 Mar 28;135(13):e793-e813.
135
Leading Cardiologists reveal new cardiovascular disease prevention risk calculator.
https://newsroom.heart.org/news/leading-cardiologists-reveal-new-heart-disease-risk-
calculator#:~:text=The%20American%20Heart%20Association%20PREVENT,CKM%20syndrome%20into%20CV
D%20prevention.
establish a G-code to describe ASCVD risk management services that incorporate the “ABCS”
of CVD risk reduction (aspirin, blood pressure management, cholesterol management, and
smoking cessation) for beneficiaries at medium or high risk for ASCVD (>15 percent in the next
10 years) as previously identified through an ASCVD risk assessment. We believe that ASCVD
risk management services include continuous care and coordination to reduce or eliminate
further elevation of ASCVD risk over time, and potentially prevent the development of future
cardiovascular disease diagnoses or first-time heart attacks or strokes.
We are proposing new G-code, GCDRM, Atherosclerotic Cardiovascular Disease (ASCVD) risk
management services with the following required elements: patient is without a current
diagnosis of ASCVD, but is determined to be at medium or high risk for CVD (>15 percent in the
next 10 years) as previously determined by the ASCVD risk assessment; ASCVD-Specific care
plan established, implemented, revised, or monitored that addresses risk factors and risk
enhancers and must incorporate shared decision-making between the practitioner and the
patient; clinical staff time directed by physician or other qualified health care professional; per
calendar month. Atherosclerotic Cardiovascular Disease (ASCVD) risk management services
refer to the development, implementation, and monitoring of individualized care plans for
reducing cardiovascular risk, including shared decision-making and the use of the ABCS of
cardiovascular risk reduction, as well as counseling and monitoring to improve diet and exercise.
We propose that the elements of the Atherosclerotic Cardiovascular Disease (ASCVD) risk
management service would include:
● ASCVD Specific Risk Management, which may include:
++ Promoting receipt of preventive services (including tobacco cessation
counseling, diabetes screening, diabetes self-management training)
++
Medication management (including aspirin or statins to maintain or decrease
risk of CVD)
++ Ongoing communication and care coordination via certified electronic health
record (EHR) technology
-- Synchronous, non-face-to-face communication methods must be offered
● ASCVD-Specific, Individualized, Electronic Care Plan
++ Must address modifiable risk factors and risk enhancers specific to CVD, as
applicable, such as:
--blood pressure and cholesterol control
-- smoking, alcohol, and other drug use status, history, and cessation
-- physical activity and nutrition
-- obesity
++ Plan must be established, implemented, and monitored and must incorporate
shared decision-making between the practitioner and the patient
Although there is no minimum service time requirement for ASCVD risk management
services in a month, each of the proposed elements must be addressed to bill for the service,
unless a particular element is not medically indicated or necessary at that time for that specific
patient. For example, the element of smoking cessation would not be addressed for a patient who
does not use tobacco. Documentation of each service element in the patient’s medical record is
required.
Physicians and non-physician practitioners (NPPs) who can furnish E/M services could
bill for ASCVD risk management services. We anticipate that ASCVD risk management services
would ordinarily be provided by clinical staff incident to the professional services of the billing
practitioner in accordance with our regulation at § 410.26. We are proposing that ASCVD risk
management services would be considered a “designated care management service” under §
410.26(b)(5) and, as such, could be provided by auxiliary personnel under the general
supervision of the billing practitioner.

We are proposing that patient consent must be obtained before starting ASCVD risk
management services. Like other care management services, ASCVD risk management services
would typically be provided by clinical staff outside of face-to-face patient visits. Consent can be
written or verbal and must be documented in the medical record. Consent should also include
informing the patient about these services, as well as potentially applicable Medicare cost-
sharing.
We are proposing that ASCVD risk management services could be billed no more often
than once per calendar month, and that payment is limited to one practitioner per beneficiary per
month. Patients must be determined to be at medium or high risk for CVD (>15 percent in the
next 10 years) as previously determined by the ASCVD risk assessment and must not have a
current diagnosis of cardiovascular disease or have a history of heart attack or stroke.
We are seeking comments on each of these proposals.
(1) Proposed Valuation for ASCVD risk management services (GCDRM)
We propose a direct crosswalk to CPT Code 99211 (Office or other outpatient visit for
the evaluation and management of an established patient that may not require the presence of a
physician or other qualified health care professional), with a work RVU of 0.18 as we believe
this service reflects the resource costs associated when the billing practitioner performs HCPCS
code GCDRM. CPT code 99211 has a physician intraservice time of 5 minutes, and the
physician work is of similar intensity to our proposed HCPCS code GCDRM. Therefore, we are
proposing a work time of 5 minutes for HCPCS code GCDRM based on this same crosswalk to
CPT 99211. We are also proposing to use this crosswalk to establish the direct PE inputs for
HCPCS code GCDRM, with modifications to reflect non-face-to-face services. These
modifications include eliminating PE inputs used in face-to-face services such as preparing and
cleaning the room. We are seeking comments on these proposals.

5. Strategies for Improving Global Surgery Payment Accuracy
a. Background
Currently, there are approximately 4,100 physicians’ services that are coded and valued
under the PFS as global surgical packages (herein “global packages”). Global packages are
single codes that are valued to include all services provided during a specified period of days (0-
day, 10-day, or 90-day global packages) by a physician (or another practitioner in the same
group practice (as defined at 42 CFR 411.352)) for a specific surgical procedure. The Medicare
Physician Fee Schedule (MPFS) look-up tool provides information on each procedure code,
including the global surgery indicator. This tool is available at
https://www.cms.gov/medicare/physician-fee-schedule/search/overview.
The global packages include:
● The surgical procedure itself, including day-of pre-service activities and day-of
recovery care;
● Post-operative evaluation and management (E/M) visits and discharge services
provided during specified post-operative periods (10- or 90-day periods for most minor and
major procedures, respectively; 0-day global packages do not include post-operative visits);
● Pre-operative visits on the day of the procedure (for services with 10- and 90-day
periods) and pre-operative visits on the day prior to the procedure (for major procedures with 90-
day periods only);
● Services provided during the post-operative period (for services with 10- and 90-day
periods) related to the procedure (for example, treatment of complications, pain management).
Any medical care that requires a return to the operating room during the global period is
paid separately and starts a new global period. Like other services paid under the PFS, post-
operative visits that are part of the global packages can vary by level and site of service. Global
packages, including the pre-operative, day-of, and post-operative visits associated with the
surgical procedure, are valued using our annual PFS rulemaking process.

As we discussed in the CY 2015 PFS final rule, we have identified and articulated several
concerns with the global packages related to the accuracy of valuation and payment under the
PFS. Foremost, we have longstanding concerns regarding whether the packages are valued based
on estimates consistent with the number and kind of services actually being furnished. Findings
from multiple OIG reports suggest that practitioners perform fewer post-operative visits than are
expected and accounted for in the valuation of the global packages. We provided a detailed
discussion of these concerns in the CY 2015 PFS final rule (79 FR 67582 through 67591).
Similarly, we described concerns that global packages as currently constructed may cause
potential distortions in valuation among PFS services, and that the structure of the current
packages assumes a single model of care delivery (a single practitioner or other practitioners in
the same group practice furnishing the surgical procedure and all associated care) and does not
appropriately address scenarios where the surgical procedure and follow-up care are provided by
different practitioners in different group practices. Taking these findings and concerns into
account, we finalized a policy to transition all 10-day and 90-day global packages to 0-day global
packages, which would allow any post-operative visits furnished after the day of the procedure to
be billed separately as standalone visits by any practitioner who furnishes them. However, in
2015, through amendments made by section 523 of the Medicare Access and CHIP
Reauthorization Act of 2015 (MACRA; Pub. L. 114-10, enacted April 16, 2015), we were
prohibited under section 1848(c)(8)(A) of the Act from implementing this finalized policy.
Further, under section 1848(c)(8)(B), we were required to collect data beginning in 2017 on the
number and level of post-operative visits typically provided to patients during 10- and 90-day
global periods and to use this newly collected data and other data beginning in 2019 to improve
the accuracy of global package valuation.
In response to these requirements, over the past 9 years, we have:
● Initiated research contracts and implemented a data-collection process to analyze data
to understand the extent to which post-operative visits are furnished to patients and improve the
accuracy of payment rates for the global surgical packages. This research contract was funded by
CMS (HHSM-500-2014-00036I) and carried out within the Payment, Cost, and Coverage
Program in RAND Health Care.
● Released three reports (located at https://www.cms.gov/medicare/payment/fee-
schedules/physician/global-surgery-data-collection) on the number of E/M visits furnished
during post-operative periods, the most recent finding that only 4 percent of expected post-
operative visits in 10-day global packages and 38 percent of expected post-operative visits
following 90-day global packages were furnished to patients.
● Fielded and released a report on a survey of selected global packages, collecting
information related to the level and complexity of medical visits furnished during post-operative
periods. This research contract was funded by CMS (HHSM-500-2014-00036I) and carried out
within the Payment, Cost, and Coverage Program in RAND Health Care.
● Released two reports on potential approaches for revaluing the global packages based
on these findings.
● Analyzed the prevalence of transfer of care modifiers (-54 for surgical care only; -55
for post-operative management only; and -56 pre-operative management only) applied to global
packages.
More recently, in the CY 2023 PFS proposed and final rules, we reviewed the prior work
and conversations around the accuracy of global package valuations and solicited comments
from the public on (1) suggested strategies for revaluing these services, (2) information on how
changes to healthcare delivery and payment may be impacting the relevance or accuracy of
global package payments, and (3) possible impact of changes to global packages on health care
access for beneficiaries (see 87 FR 69432 through 69437). In response to the comment
solicitation in the CY 2023 PFS proposed rule, some commenters generally disagreed with our
findings that the post-operative visits in the global packages are not performed as frequently as
assumed in our valuation of global surgical packages. However, opposition from commenters
was based on anecdotal assertions rather than alternative data. Many of these commenters’
specific points restated earlier comments submitted in response to our request for feedback in the
CY 2020 PFS proposed rule on claims-based reporting of post-operative visits, survey findings
on the level of visits, and potential revaluation approaches. Some commenters supported
eliminating 10-day global package periods and requested that the AMA RUC review these
services. However, these commenters also acknowledged that the AMA RUC review process
could take years. In addition to the comments we received in response to the CY 2023 PFS
proposed rule, we have received feedback over several years from many interested parties
regarding the findings from claims-based reporting of post-operative visits and considered
revaluation methodologies presented in our prior reports.
Overall, we have continued exploring ways to improve the accuracy of valuation and
payment for global packages to ensure appropriate payments to the practitioners providing pre-
operative, surgery, and post-operative care to Medicare beneficiaries while considering feedback
from interested parties. In addition, commenters have not proposed specific alternative strategies
to revalue global surgical packages.
Separately, we continue to review approaches to better describe physicians’ services in
the context of the evolving care delivery landscape and to allow practitioners to furnish patient-
centered care. Our review work includes considering care delivery models discussed with
interested parties (and developed though our CMS Innovation Center work), reviewing our
policies and billing requirements, identifying care elements that could serve as the building
blocks for describing newer, impactful services, and seeking opportunities to reduce
administrative burdens for practitioners while ensuring accurate payment. Through this lens, we
have also recently reviewed our billing requirements and payment policies for the global
packages, concurrent with continued analysis of the Medicare claims data.
While ongoing, our review highlights opportunities for us to clarify or revise
longstanding policy and billing instructions for global packages, using data and experience
gathered over the last several years, consistent with our overall objectives to pay more accurately
for services and to right-size the valuation of PFS services based on how practitioners currently
furnish these services. In this proposed rule, we discuss proposals (1) to revise our transfer of
care policy for global packages to address instances where one practitioner furnishes the surgical
procedure and another practitioner furnishes related post-operative E/M visits during the global
period, and (2) to develop a new add-on code that would account for resources involved in post-
operative care provided by a practitioner who did not furnish the surgical procedure. We believe
that addressing the use of transfer of care modifiers, and the resources involved when
practitioners who do not furnish the surgical procedure provide post-operative care, are essential
steps in aligning payment with the way in which surgical procedures are currently furnished as
evidenced in our data, and would make meaningful progress toward more accurate payment for
these services in particular and improve relative valuation for PFS services overall.
b. Clarifying the Scope of Global Surgical Packages
We have valued global packages to include the surgical procedure and services furnished
during the specified global period related to the surgical procedure when furnished by the
practitioner who performs the surgery (hereafter in this section, the proceduralist) or by another
practitioner in the same group practice as the proceduralist.
Under current Medicare payment policy, certain services furnished during the global
period by the proceduralist or by another practitioner in the same group practice may be
separately billed with an appropriate modifier:
● Initial decision for surgery: E/M service billed with modifier -57 (Decision for
Surgery).
● E/M services unrelated to the procedure: billed with modifier -24 (Unrelated E/M
Service During a Global Period).
● Other services unrelated to the procedure (including underlying condition treatment,
diagnostic tests, distinct procedures) not including care for complications/returns to the operating
room: no modifier required.
● Failure of a less extensive procedure requiring a more extensive procedure: no
modifier required.
● Organ transplant immunosuppressive therapy: no modifier required.
● Critical care services unrelated to surgery: billed with modifier -FT if in the post-
operative period.
In general, except where a formal transfer of care modifier applies, a practitioner other than the
proceduralist or a practitioner in the same group practice as the proceduralist can bill separately
for an E/M visit for services they furnish during the global period for a global package, including
post-operative E/M visits related to the procedure. We established formal transfer of care
modifiers to apply in cases where the work, time, and resources involved in furnishing services
included in the global packages are split between the proceduralist (or another practitioner in the
same group practice) and other practitioners providing related post-operative visits during the
global period. Under our current transfer of care policy, transfer of care modifiers must be
reported when a formal transfer of care arrangement is documented by both the proceduralist and
another practitioner providing the related post-operative visits. Based on our analysis of
Medicare fee-for-service claims data, these formal transfer of care modifiers are rarely used and,
when they are, it is often with respect to certain ophthalmologic procedures (for example,
cataract surgery).
Based on our analysis of claims data, we believe that it may be helpful to review our
current policy regarding the applicability of transfer of care modifiers for the medical and billing
communities. Under our current policy, the scope of the global package extends to services
furnished by the entire group practice of the proceduralist, including services furnished by
practitioners in the group practice who are a different specialty from the proceduralist. In other
words, the PFS payment for post-operative visits and other services furnished during the global
period that are related to the surgical procedure and provided by the proceduralist or a
practitioner in the same group practice as the proceduralist is bundled into the global package,
and those services are not separately billable. If the proceduralist or a practitioner in the same
group practice as the proceduralist wants to bill during the global period for a service furnished
to the surgical patient, but unrelated to the global package, the correct modifier must be used to
indicate that the service is not related to the global package. Without a modifier to indicate
otherwise, during the global period for a global package, all E/M services furnished to the patient
by the proceduralist or another practitioner in the same group practice as the proceduralist are
presumed to be related to, and included in the payment for, the global package. Modifiers for
separate payment (such as modifier -24) are required when services unrelated to the global
package are billed by the proceduralist or a practitioner in the same group practice as the
proceduralist during the global period.
c. Strategies to Address Global Package Valuation
We recognize that we are precluded under section 1848(c)(8)(A) of the Act from
revisiting the policy we established in the CY 2015 PFS final rule to revalue all 10-day and 90-
day global packages to 0-day global packages (79 FR 67582-67591). Further, we note that
transitioning all global packages to 0-day global periods could take several years and require
substantial CMS resources (see CY 2014 PFS final rule (77 FR 44737 through 44738) for
previous discussion). We have also considered revaluing 10-day and 90-day global packages to
reflect the observed number of post-operative visits furnished to patients based on data we have
collected over nearly a decade and note that this approach would be quicker to implement,
assuming there would be straightforward ways to revalue the services with the data. However,
interested parties have continued to express uncertainty about the validity of claims-based counts
of post-operative visits. This uncertainty stems primarily from CMS not having complete
information surrounding the use of the transfer of care modifiers since they are not currently
routinely used. The same interested parties also object conceptually to revaluing the 10-day and
90-day global packages using the “building block” framework, where each component of a
service, including bundled post-operative visits, contributes to total valuation to align valuation
with the number of post-operative visits typically provided to patients. Some interested parties
have expressed larger concerns about the redistributive impacts across the PFS among specialties
if we were to implement and revalue all global packages.
We acknowledge the practical challenges involved in revaluing 10-day and 90-day global
packages, whether they remain as 10-day and 90-day periods with fewer post-operative visits or
are transitioned to 0-day global packages, and continue to carefully consider how to best improve
global package valuation given access to administrative claims data and other inputs that help us
understand the scope of services provided to patients within global packages. Ultimately, we
want to ensure payments to practitioners and the relative values assigned to global surgical
packages are accurate and, to the extent possible, driven by real-world objective and updatable
information regarding the relative resources involved in furnishing the services.
For CY 2025, we are focused on different aspects of our policy objectives for global
packages and propose the following policies, which are not mutually exclusive, to obtain
information and allow for more accurate payment to reflect time and resources spent on post-
operative care associated with the current global packages. We will continue to assess and
monitor for potential future opportunities to improve our payment approach for the global
packages more broadly.
Additionally, in developing our proposed policies to pay more accurately for the global
packages, we also considered whether, when, or how our policies may be affected when services
are provided by the proceduralist, versus another practitioner who did not perform the procedure
but is providing follow up care. We also recognize that there may be multiple practitioners in the
same or different specialties in the same group practice and considered how our policies should
apply to practitioners in a range of specialties within the same group practice. We are seeking
comment on these considerations in the context of our proposed policies and welcome feedback
that may further inform our payment policy for global packages. Additionally, as we continue to
better understand what services are being furnished in the global period, by whom, and how the
global surgical packages are valued and billed, we are seeking comment on how remote
monitoring and other types of new technologies represent new resource costs and/or produce
efficiencies and effectiveness of post-operative care. This information could be useful both for
purposes of valuation for surgical and post-operative care, as well as for policies regarding when
specific PFS codes should be reported during global periods for global packages.
d. Expand Applicability of Transfer of Care Modifiers
We created transfer of care payment modifiers at the inception of the PFS. Under our
current policy, these modifiers are required to be appended to the relevant global package code
when billing for services that are within the scope of the global package (within the global period
and related to the surgical procedure) only when the proceduralist and one or more other
practitioners who are not in the same group practice as the proceduralist formally document their
agreement to provide distinct portions of the global package.
The following transfer of care modifiers describe the different portions of the global
surgical package that could be provided by different practitioners:
● Modifier -54 Surgical Care Only: this modifier is appended to the relevant global
package code to indicate that the proceduralist performed only the surgical procedure portion of
the global package.
● Modifier -55 Post-operative Management Only: this modifier is appended to the
relevant global package code to indicate that the practitioner performed only the post-operative
management portion of the global package.
● Modifier -56 Pre-operative Management Only: this modifier is appended to the
relevant global package code to indicate that the practitioner performed only the pre-operative
portion of the global package.
For each of these modifiers, the payment for the global package is adjusted based on the
applicable percentage noted in the PFS Relative Value files
(https://www.cms.gov/medicare/payment/fee-schedules/physician/pfs-relative-value-files).
As previously noted, we currently require the transfer of care modifiers (-56 pre-
operative care, -54 for procedures, and -55 for post-operative care) to be appended in cases
where there is a formal documented transfer of care agreement, that is, “in the form of a letter or
an annotation in the discharge summary, hospital record, or Ambulatory Surgical Center (ASC)
record” (CMS Manual System, Pub 100-04 Medicare Claims Processing, Transmittal 11287). In
our recent analyses of 2022 Medicare claims data, we identified that these modifiers were rarely
used other than for certain ophthalmology global packages. We found over 99 percent of claim
lines for 90-day surgical procedures billed with modifier –54 were ophthalmology services
(primarily cataract-related procedures). We also identified a difference in the number of claim
lines annually for a given 90-day global package with modifier –54 and with modifier –55. In
other words, there are sometimes more claim lines billed with modifier –54 than there are
corresponding lines with modifier –55 and vice versa during a year. We note that modifier –56
(pre-operative management only) is only very rarely used in practice. These recent observations
suggest (1) the overwhelming concentration of reported transfer of care modifiers is in
ophthalmology procedures, and (2) a potential mismatch in billing for formal transfer of care
cases between proceduralists and other practitioners providing post-operative care.
While we recognize the benefits to continuity of care when the proceduralist also
provides pre-operative and follow-up care for the procedure, we also recognize that it is not
always feasible, or even perhaps typical practice for the same practitioner to furnish all portions
of the global package; for example, in instances when the practitioner furnishing the procedure
does not schedule a post-operative visit(s) on the day of the procedure or plans for the patient to
follow up with their primary care provider, or when the practitioner performing the surgery
arranges alternative follow-up care because it would be difficult for the beneficiary to travel to
return for follow-up care. Because our current policies require use of the transfer of care
modifiers only where there is a formal documented agreement between practitioners to provide
specific portions of the global package, we believe there are many practical and potentially
common circumstances under which the transfer of care modifiers would not be required or used.
Beginning for services furnished in 2025, we are proposing to broaden the applicability
of the transfer of care modifiers for the 90-day global packages. We are proposing to require the
use of the appropriate transfer of care modifier (modifier -54, -55, or -56) for all 90-day global
surgical packages in any case when a practitioner plans to furnish only a portion of a global
package (including but not limited to when there is a formal, documented transfer of care as
under current policy, or an informal, non-documented but expected, transfer of care).
Practitioners billing for a global package procedure code with modifier -54 and other
practitioners in the same group practice as that practitioner would still be able to bill during the
global period for any separately identifiable E/M services they furnish to the patient that are
unrelated to the global package procedure. To do so, the practitioner would append modifier -24
to the claim line for the E/M service.
This proposed policy, which would be a first step toward improved valuation and
payment while retaining the fundamental structure of the global packages, would provide us with
more accurate information on the resources involved in furnishing components of global surgical
packages. This proposal would prevent duplicative Medicare payment for post-operative care
because the global surgical package payment would be adjusted based on the appended modifier,
and payment for post-operative care would not be made both as part of a global surgical package
and through separately billed E/M visits. We also anticipate that the proposed policy would
provide us with insight into changes in standards of practice and post-operative patient care for
services that are not billed with transfer of care modifiers pursuant to our current policy (that is,
services other than certain ophthalmology procedures).
We acknowledge the potential challenge associated with anticipating whether other
practitioners will furnish portions of the global package and, accordingly, appending the
appropriate modifier when billing global package services.
We are interested in understanding and are seeking comment on the circumstances under
which practitioners in separate group practices furnish different portions of the care included in
global packages, and what that means for reporting the transfer of care modifiers. While we are
making proposals related to the 90-day global periods beginning for services furnished in 2025,
we are also seeking comment on whether we should consider proposing these changes for the 10-
day global packages in future rulemaking.
e. Payment for Global Packages
Under our current policy for global packages where the transfer of care modifiers are
used (required only where there is a formal transfer of care arrangement), the total combined PFS
payment made for the global package during the global period does not exceed the total global
surgical package payment established for the procedure when billed without any transfer of care
modifier. In general, we continue to believe this is the appropriate result when more than one
practitioner furnishes portions of a global package. Under our proposal, we would require that
practitioners performing the surgical procedure but not intending to furnish the post-operative
portions of a 90-day global service would appropriately append the -54 modifier that we have
previously discussed, which would adjust the portion of payment received to accurately reflect
the service furnished.
More specifically, as noted in the discussion above, the transfer of care modifiers
correspond to three distinct portions of the global package (pre-operative services, the surgical
procedure itself, and post-operative care). We have assigned a proportion of the global package
payment to each portion of the service based on longstanding assumptions. Under our current
policy, the payment for the entire global package is paid to the billing practitioner unless a
transfer of care modifier is included on the claim. Payment is only adjusted if a transfer of care
modifier is included on the claim. We are requesting comments, as we further develop our
payment policies for global packages, on how best to determine the appropriate payment
proportions for the three portions of the global package, which impact payment to the different
practitioners who furnish the different portions of the service.
We are continuing to consider approaches to establishing the payment allocations for
portions of the global package when the transfer of care modifiers are used, and anticipate
revising the allocations through future rulemaking. We are seeking comment on potential
approaches to revise these payment allocations and how they could be established to better
reflect current medical practice and conventions for post-operative follow-up care. We seek to
identify a procedure-specific, data-driven method for assigning shares to portions of the global
package payment to more appropriately reflect the resources involved in each portion. We
would appreciate and carefully consider recommendations from interested parties, including the
AMA RUC, on what those allocation percentages should be, based on how the global package
codes are valued and any other relevant information.
We have contracted with RAND to support data collection and analysis in the past and, as
identified in RAND’s prior reports and described in detail, we surmise that the policy to
apportion and pay for the three portions of the global package based on the presence of transfer
of care modifiers does not always work smoothly. In considering RAND’s reports, one reason
for this is that fewer post-operative visits are provided to patients compared to the number of
visits reflected in the valuation of global packages. The global packages reflect a certain number
of post operative visits (noted in the Physician Time File) that typically occur during the post-
operative global period. However, there is no easy way within a global package to separate the
RVUs for the procedure itself from the RVUs for post-operative visits that are not typically
provided to patients. If our allocation of the global package payment based on the presence of
transfer of care modifiers were to undervalue the surgical procedure portion or the post-operative
care portion of the global package, we are concerned that we could unintentionally introduce
incentives that influence current medical practice for transfers of care. This gets at RAND’s prior
recommendation that we revalue global packages to reflect the actual number of post-operative
visits provided to patients. After revaluation, separating the procedure and post-operative
payments would reflect observed data and mitigate any possible inappropriate incentives in place
for practitioners to initiate transfers of care and support use of transfer of care modifiers as
medically appropriate. This approach has the advantage of anchoring the valuation of separate -
54 and -55 components using real-world information on post-operative visits reported to CMS
rather than on historical assumptions or current survey data reflecting estimates of the typical
number and level of visits.
In our internal review of the percentages assigned for the pre-operative, surgical care, and
post-operative portions of the global package, we found that there are a small number of codes
that do not have any assigned percentages in our files even though these codes are identified as
global packages. HCPCS codes 77750 (Infusion or instillation of radioelement solution (includes
3-month follow-up care)), HCPCS code 77761 (Intracavitary radiation source applic simple),
HCPCS code 77762 (Intracavitary radiation source applic intermed), and HCPCS code 77763
(Intracavitary radiation source applic complex) do not have assigned percentages in our RVU
files. It is our understanding, however, that the MACs have local edits in place to ensure
appropriate payment for these services when billed with the transfer of care modifiers. We are
seeking comment on whether we should consider, first, whether these codes are appropriately
categorized as 90-day global package codes. If these are appropriately considered to be 90-day
global package codes, we are seeking comment on what the assigned percentages should be for
the pre-operative, surgical care, and post-operative portions of the service.
f. Post-operative Care Services Add-on Code
We recognize the importance of continuity in surgical and post-operative care. However,
we recognize that there are instances where post-operative care is not furnished by the
proceduralist or another practitioner in the same group practice, or even by a practitioner who is
in the same specialty as the proceduralist, despite there being no formal transfer of care. We also
recognize that there is an extra level of complexity involved when a practitioner sees a patient
post-operatively after a surgical procedure performed by another practitioner in those
circumstances. The practitioner providing the post-operative care may not be involved in
creating the surgical plan, and may not have access to the operative notes to know how the
surgery went or be abreast of any particular considerations related to the procedure that may
factor in medical care decisions for the post-operative care. As such, we recognize that there are
comparatively more resource costs incurred when a practitioner who did not furnish the surgical
procedure in a global package provides the follow-up care. We are proposing to address these
scenarios, which can occur in a few different ways, by establishing a new add-on code that
would account for resources involved in post-operative care for a global package provided by a
practitioner who did not furnish the surgical procedure and does not have the benefit of a formal
transfer of care. However, we note that when a patient is seen by practitioners in the same group
practice or specialty as the surgeon, the same resources are not incurred during follow-up and
therefore, the add-on code should not be billed by another practitioner in the same group
practice as the practitioner who performed the surgical procedure, or in the same specialty as the
practitioner who performed the surgical procedure. In the case of a practitioner providing follow
up care who is of a different specialty and not within the same group practice as the
proceduralist, researching the procedure to determine expected post-operative course and
potential complications may be needed, which would warrant using the add-on code. We also
acknowledge that sometimes the proceduralist does not schedule the patient to follow up with
them post-operatively and directs the patient to follow up with other practitioners as needed,
such as with the patient’s primary care provider. The patient may independently choose to
follow up with their primary care provider or another practitioner based on other considerations
such as convenience of the practice location or ease of scheduling. We understand and
acknowledge that the patient can choose to see another practitioner without the knowledge of the
practitioner who performed the procedure.
To more appropriately reflect the time and resources involved in these kinds of visits, we are
proposing to make payment using a new add-on code to be billed with an office/outpatient E/M
visit for post-operative follow-up care during the global period of a global package to capture
additional resources associated with practitioners who were not involved in furnishing the
surgical procedure. This follow-up care may include, but is not limited to, obtaining and
reviewing the surgical notes and surgical history, monitoring for signs and symptoms of
infection, taking into account any considerations from the surgical procedure that may affect the
medical care, and monitoring for any potential post-operative complications that may arise. It is
often difficult in these circumstances for the practitioner who did not perform the surgical
procedure to know how the wound looked after the procedure, and so it is more challenging to
recognize possible changes that may have occurred since the time of the procedure (when this is
something the operating surgeon would have been able to know). This new code would be billed
by the practitioner who furnishes the post-operative office/outpatient E/M visits when that
practitioner is not the proceduralist and is not in the same specialty or group practice as the
proceduralist. Documentation in the medical record must justify use of the add-on code and that
the E/M visit was, as clinically understood by the reporting practitioner, related to a post-
operative visit furnished during the 90-day post operative period. As noted earlier, we are
proposing to expand the required use of the transfer of care modifiers as a first step toward
improving payment for the global packages to promote improved valuation and payment for
these services. Instituting an add-on code to capture the time and intensity of post-operative work
absent a formal transfer of care, would be an essential step in recognizing how the services are
currently furnished and make meaningful progress toward ‘right-sizing’ the structure of the
global packages.
Given the history of the global packages since data collection began, as specified in
section 1848(c)(8) of the Act, and in consideration of our policies for post-operative care and our
proposal requiring the use of the transfer of care modifiers in a broader set of circumstances, we
believe that the timing is appropriate to establish an add-on code and payment for post-operative
care provided in the office/outpatient setting by a practitioner other than the proceduralist (or
another practitioner in the same specialty) to account for the additional time, intensity, and
resources that are involved in post-operative care. We are proposing a new HCPCS code,
GPOC1, to capture the additional time and resources spent in providing follow up post-operative
care by a practitioner who did not perform the surgical procedure and who has not been involved
in a formal transfer of care agreement.
We propose the following code and descriptor for the proposed add-on code:
GPOC1 (Post-operative follow-up visit complexity inherent to evaluation and management
services addressing surgical procedure(s), provided by a physician or qualified health care
professional who is not the practitioner who performed the procedure (or in the same group
practice), and is of a different specialty than the practitioner who performed the procedure,
within the 090-day global period of the procedure(s), once per 090-day global period, when
there has not been a formal transfer of care and requires the following required elements, when
possible and applicable:
● Reading available surgical note to understand the relative success of the procedure,
the anatomy that was affected, and potential complications that could have arisen due to the
unique circumstances of the patient’s operation.
● Research the procedure to determine expected post-operative course and potential
complications (in the case of doing a post-op for a procedure outside the specialty).
● Evaluate and physically examine the patient to determine whether the post-operative
course is progressing appropriately.
● Communicate with the practitioner who performed the procedure if any questions or concerns
arise. (List separately in addition to office/outpatient evaluation and management visit, new or
established)).
We are proposing that HCPCS code GPOC1 would be reported by a physician or other
practitioner who did not perform the surgical procedure for a global package and provides related
post-operative visits during the global period despite the absence of a formal transfer of care.
We are proposing that the add-on code (HCPCS code GPOC1) would only be reported with an
office or other outpatient E/M visit for the evaluation and management of a new or established
patient. We would expect the documentation in the medical record to indicate the relevant
surgical procedure, to the extent the billing practitioner can readily identify it, in order to aid in
our understanding of the post-operative care being furnished and when there is no transfer of
care modifier appended on the claim.
We are proposing that this code could be billed only once during the 90-day global period
for the global package because we believe the practitioner would only have additional resource
costs upon the first visit following the procedure. We are proposing to assign a ZZZ global
period payment indicator for HCPCS code GPOC1, as this allows the add-on code to be billed
during the post-operative time frame that applies to payment for each surgical procedure and,
under our proposed policy, this code would be reportable with an E/M visit. The ZZZ global
period payment indicator would identify this code as a service that is related to another service
paid under the PFS and is always included in the global period of the other service.
g. Proposed Valuation for GPOC1 Add-On Code
We note that the proposed valuation of HCPCS code GPOC1 is meant to capture the
additional resource costs, including for visit complexity inherent to office/outpatient care
associated with a post-operative visit that is not accounted for in the appropriate office/outpatient
E/M base code billed by the physician or practitioner. Therefore, we believe that CPT code
90785 (Interactive complexity (List separately in addition to the code for primary procedure))
serves as an appropriate reference for the purposes of valuing HCPCS code GPOC1. CPT code
90785 was created to capture additional work that occurs during diagnostic psychiatric
evaluation, psychotherapy, psychotherapy performed with an E/M service and group
psychotherapy sessions, and the service refers to specific communication factors that complicate
the delivery of a psychiatric/psychotherapy procedure. However, we believe there may be
relatively less work involved for GPOC1 when compared to the work of CPT code 90785,
considering the amount of time needed to gather the operative history and conduct the elements
discussed above. Therefore, we are proposing a work RVU of 0.16, which represents
approximately half of the assigned work for minutes of CPT code 90785. Additionally, we are
proposing a work time of 5.5 minutes (or half of the 11 minutes established for CPT code
90785), personally performed by the billing practitioner including the elements discussed above
during the post-operative E/M visit furnished during the global period, that is, no later than 90-
days following a 90-day global code, respectively. CPT code 90785 has no direct PE inputs, and
we are proposing the same for HCPCS code GPOC1.
To help inform whether our proposed valuation reflects the typical service, we are
seeking comment the typical time and intensity physicians and practitioners spend over and
above a separately billed E/M visit when providing post-operative care to a patient when they did
not perform the surgical procedure, gathering the surgical history as well as the pre-operative,
intra-operative, and post-operative, and on the proposed service elements and the relative
intensity compared to similar service elements of other CPT codes. For the individual
practitioner, not having an intimate knowledge of the procedure itself and not having a
before/after comparison to look at for the wound can all complicate their E/M visit. The
proposed work RVUs are intended to account for the additional relative resource costs in time
and intensity in addition to those involved in the E/M visit.
Finally, we recognize that historically, the CPT Editorial Panel has frequently created
CPT codes describing services for which we originally established G-codes and adopted them

through the CPT Editorial Panel process. We note that we would consider using any newly
available CPT coding to describe services similar to those described here in future rulemaking.
For discussion of our expected utilization assumptions for this service, see the discussion
in the Regulatory Impact Analysis section of this proposed rule.
H. Supervision of Outpatient Therapy Services in Private Practices, Certification of Therapy
Plans of Care with a Physician or NPP Order, and KX Modifier Thresholds
1. Supervision of Outpatient Therapy Services in Private Practices
In the CY 2024 PFS final rule, we finalized our proposal to allow remote therapeutic
monitoring (RTM) services to be furnished by occupational therapy assistants (OTAs) and
physical therapy assistants (PTAs) under the general supervision of occupational therapists
(OTs) and physical therapists (PTs) in private practice, in an effort to align with the general
supervision policy for these services for physicians and other practitioners described in the CY
2023 final rule (88 FR 78990). We also noted that we would consider for possible future
rulemaking the commenters’ responses to our request for information (RFI) on changing the
supervision of therapy assistants in the private practice setting to general supervision for all
therapy services (88 FR 78990 through 78992).
In the CY 2024 PFS proposed rule, we reviewed the statutory provisions at sections
1861(p) and 1861(g) (by cross-reference to section 1861(p)) of the Act that describe outpatient
physical therapy and occupational therapy services furnished to individuals by physical
therapists (PTs) and occupational therapists (OTs) meeting licensing and other standards
prescribed by the Secretary if the services meet the necessary conditions for health and safety.
These statutory provisions refer separately to outpatient therapy services furnished by a provider
of services (such as a rehabilitation agency) and those services furnished in the therapist’s office
or the individual’s home, thus distinguishing therapists who work for an institutional provider of
therapy services from therapists who furnish and bill independently for these outpatient therapy
services (88 FR 52358 through 52359). In regulations, we have addressed these therapists as

physical or occupational therapists in private practice (PTPPs and OTPPs) (63 FR 58868 through
58870). The regulations specific to services furnished by occupational or physical therapists in
private practice are found at §§ 410.59(c) and 410.60(c), respectively.
We also summarized a history of related regulatory provisions in the CY 2024 PFS
proposed rule. In the CY 2005 PFS final rule with comment period (69 FR 66236, 66351 through
66354), we explained that the personnel requirements that are applicable for Home Health
Agencies (HHAs) at 42 CFR part 484 for therapists, therapy assistants and speech-language
pathologists (SLPs) apply to all outpatient physical therapy, occupational therapy, and speech-
language pathology services. In the CY 2005 PFS final rule, we also added a basic rule at §§
410.59(a) and 410.60(a), respectively, by cross-referencing the qualifications for OTs and their
OTAs and PTs and their PTAs for all occupational therapy and physical therapy services,
respectively, including those who work in private practices, to 42 CFR part 484. Later, in the CY
2008 PFS final rule (72 FR 66328 through 66332), we updated the qualification standards at 42
CFR part 484 for OTs, OTAs, PTs, PTAs, and SLPs.
In the CY 2024 PFS proposed rule, through our RFI on general supervision of OTAs and
PTAs by OTPPs and PTPPs, respectively, we solicited public comment, along with supporting
data, for our consideration for possible future rulemaking about the following: (a) the questions
and concerns we highlighted related to access, patient safety, and utilization; (b) revising
§§ 410.59(a)(3)(ii) and (c)(2) and 410.60(a)(3)(ii) and (c)(2) to permit general supervision of
OTAs and PTAs by the OTPP and PTPP, respectively, when furnishing therapy services; and (c)
any appropriate exceptions to allowing general supervision in the furnishing of therapy services
(88 FR 52358 through 52359).
In the CY 2024 PFS final rule, we reviewed the comments we received in response to the
proposed rule (please refer to (88 FR 78990 through 78992)). We noted that we would consider
these comments for possible future rulemaking – see our review of comments on the RFI in the
CY 2024 PFS final rule (88 FR 78992).

Over the past several years and again more recently, we have heard from interested
parties that the direct supervision requirements in the private practice setting are problematic for
OTPPs and PTPPs who must remain on-site and immediately available when Medicare patients
are treated in order to bill for therapy services furnished by their supervised OTAs and PTAs. As
a remedy to this situation, interested parties have requested that we revise our requirement for
PTPPs and OTPPs to provide direct supervision of OTAs and PTAs to align with the general
supervision policies for OTs and PTs that work in Medicare institutional settings that provide
therapy services (for example, rehabilitation agencies, outpatient hospitals, SNFs and
comprehensive outpatient rehabilitation facilities (CORFs), etc.), to allow for the general
supervision of their therapy assistants. These interested parties tell us that their respective State
laws and policies allow general supervision of therapy assistants (most often requiring the OT or
PT to be in touch with their therapy assistants via telecommunication) in at least 44 States for
PTAs,
136
and all but one State for OTAs.
Some interested parties have reported that allowing for general supervision of OTAs and
PTAs by OTPPs and PTPPs, respectively, would allow for patients to have increased access to
outpatient therapy services, even with ongoing healthcare workforce shortages. The shortages of
OTs
137
and OTAs,
138
PTs,
139
and PTAs,
140
are noted by the United States Bureau of Labor
Statistics, which shows thousands of open positions in all of these fields. Interested parties noted
that over 22,000 PTs left the workforce in 2021.
141
Additionally, these interested parties noted
136
Federation of State Boards of Physical Therapy Jurisdiction Licensure Reference Guide
https://www.fsbpt.net/lrg/Home/SupervisionRequirementLevelsBySetting.
137
Bureau of Labor Statistics, U.S. Department of Labor, Occupational Outlook Handbook, Occupational
Therapists,
at https://www.bls.gov/ooh/healthcare/occupational-therapists.htm (visited April 17, 2024).
138
Bureau of Labor Statistics, U.S. Department of Labor, Occupational Outlook Handbook, Occupational Therapy
Assistants and Aides,
at https://www.bls.gov/ooh/healthcare/occupational-therapy-assistants-and-aides.htm (visited April 17, 2024).
139
Bureau of Labor Statistics, U.S. Department of Labor, Occupational Outlook Handbook, Physical Therapists,
at https://www.bls.gov/ooh/healthcare/physical-therapists.htm (visited April 17, 2024).
140
Bureau of Labor Statistics, U.S. Department of Labor, Occupational Outlook Handbook, Physical Therapist
Assistants and Aides, at https://www.bls.gov/ooh/healthcare/physical-therapist-assistants-and-
aides.htm (visited April 17, 2024).
141
See the report by Definitive Healthcare dated October 2022 at
https://www.definitivehc.com/sites/default/files/resources/pdfs/Addressing-the-healthcare-staffing-shortage.pdf.
that workforce shortages have greater impact on private practices in rural and underserved areas
where hourly wages are lower, and the OTPPs and PTPPs in these areas tend to have small
practices. The interested parties stated that Medicare’s direct supervision policy, which requires
the PTPP and the PTA to both be present when a Medicare patient is treated, does not allow
small practices with one PT and one or two PTAs, for example, to work different or overlapping
schedules in order to accommodate all patients’ availability by allowing the OTA/PTA to work
before or after the OTPP/PTPP normal hours. The interested parties also stated that the direct
supervision requirement can unfairly delay care for Medicare patients when, for example, a
PTPP or OTPP is out sick, the practice does not have alternative coverage, and appointments for
Medicare patients must be canceled.
In light of this input, we believe that the direct supervision requirement for OTPPs and
PTPPs of OTAs and PTAs, respectively, may have had an unintended consequence of limiting
access to needed therapy services. As noted by interested parties, both the OTPP/PTPP and their
respective OTA/PTA must be present in the office in order to bill and receive Medicare payment
for therapy services furnished by OTAs and PTAs. This means, for example, that an OTPP/PTPP
cannot bill and receive payment for therapy services furnished to a Medicare patient in their
home when furnished by an OTA/PTA, without the presence of the OTPP/PTPP. The direct
supervision requirement for OTAs and PTAs in the private practice setting is more stringent than
the supervision requirements for OTAs and PTAs in institutional settings. For example, as we
noted in the CY 2024 PFS proposed rule, 42 CFR 485.713 specifies that when an OTA or PTA
provides services at a location that is off the premises of a clinic, rehabilitation agency, or public
health agency, those services are supervised by a qualified occupational or physical therapist
who makes an onsite supervisory visit at least once every 30 days. We also cited Table 4 in our
Report to Congress, titled “Standards for Supervision of PTAs and the Effects of Eliminating the
Personal PTA Supervision Requirement on the Financial Caps for Medicare Therapy

Services,”
142
in the CY 2024 PFS proposed rule to demonstrate that the minimum level of
supervision by PTs and OTs for services performed by PTAs and OTAs working in institutional
settings is a general level of supervision, in accordance with various regulations (88 FR 52359).
Therefore, we believe that a change from direct to general supervision would allow OTPPs and
PTPPs the flexibility to better accommodate patients’ availability and act to ensure access to
necessary therapy services. A change from direct to general supervision would also allow OTPPs
and PTPPs to bill and receive Medicare payment for therapy services furnished by their OTAs
and PTAs when they are not in the office or patient’s home at the same time.
We also believe that it is important to better align our supervision policies for OTPPs and
PTPPs with the majority of state-established supervision levels for therapy assistants providing
occupational therapy and physical therapy services. We note that the majority of states allow
OTs and PTs to provide general supervision of their respective OTAs and PTAs when furnishing
occupational therapy and physical therapy services. We believe that States are well aware of the
health and safety needs for their residents who receive therapy services from OTs and their
supervised OTAs, and PTs and their supervised PTAs. Given these beliefs and the input from
interested parties, we are proposing to revise our regulations at §§ 410.59(a)(3)(ii) and (c)(2) and
410.60(a)(3)(ii) and (c)(2) to allow for general supervision of OTAs and PTAs by OTPPs and
PTPPs, when the OTAs and PTAs are furnishing outpatient occupational and physical therapy
services, respectively. We expect that this proposal would both increase access to therapy
services and more closely align Medicare policy with the majority of State practice acts for
occupational therapy and physical therapy. This will parallel the 44 States that allow general
supervision of PTAs and the 49 States that allow general supervision of OTAs (most often
described as requiring the PT or OT to be in touch via telecommunication). For the States with
more restrictive supervision levels, such as direct supervision, those therapy services are always
142
See Table 4 of the Report to Congress titled Standards for Supervision of PTAs and the Effects of Eliminating the
Personal PTA Supervision Requirement on the Financial Caps for Medicare Therapy Services at
https://www.cms.gov/Medicare/Billing/TherapyServices/Downloads/61004ptartc.pdf.
furnished to the extent that is permitted under State law. We note that while we are proposing to
allow for general supervision by OTPPs and PTPPs of their OTAs/PTAs, an OTPP or PTPP
would still be required to provide direct supervision to unenrolled OTs and PTs, respectively, in
accordance with §§ 410.59(c)(2) and 410.60(c)(2).
We are soliciting comment on our proposals.
2. Certification of Therapy Plans of Care with a Physician or NPP Order
Sections 1861(p), (g), and (ll)(2) of the Act require that an individual outpatient is under
the care of a physician and for whom a plan for the physical therapy, occupational therapy, or
speech-language pathology services that are to be furnished has been established by a physician
or by a qualified PT, OT, or SLP and is periodically reviewed by a physician. Sections
1835(a)(2)(C) and 1835(a)(2)(D) of the Act require that payment for Medicare therapy services
may be made for outpatient physical therapy, occupational therapy, and speech-language
pathology services only if a physician certifies (and recertifies, where such services are furnished
over a period of time) that: (a) the services are or were required because the patient needs or
needed therapy services; (b) a plan for furnishing such services was established by a physician or
qualified therapist providing such services, and is periodically reviewed by the physician; and (c)
the services are or were furnished while the individual was under the care of a physician.
In accordance with the statute and § 424.24(b), Medicare Part B pays for outpatient
physical therapy and speech-language pathology services furnished by providers only if a
physician certifies the content specified in § 424.24(c)(1) or (4). We recognize that it may not be
clear that § 424.24(c) applies to the occupational therapy services furnished by providers, since
occupational therapy services are currently only explicitly mentioned in the recertification
requirements at § 424.24(c)(4).
We note that there are multiple references to § 424.24(c) in the Medicare Benefit Policy
Manual, Pub. 100-02, chapter 15, sections 220.1 - Conditions of Coverage and Payment for
Outpatient Physical Therapy, Occupational Therapy, or Speech-Language Pathology Services,
220.1.2 - Plans of Care for Outpatient Physical Therapy, Occupational Therapy, or Speech-
Language Pathology Services, and 220.1.3 - Certification and Recertification of Need for
Treatment and Therapy Plans of Care, which convey our current policy that all outpatient
physical therapy, occupational therapy, and speech-language pathology services are subject to
requirements for certification and recertification at § 424.24, whether furnished by providers or
by suppliers such as therapists in private practice (TPPs). We note that while section 1835 of the
Act explicitly refers to services furnished by providers of services, which would include
hospitals and other institutional providers as defined in section 1861(u) of the Act, and clinics,
rehabilitation agencies, or public health agencies as further described in section 1835(a) of the
Act, we have interpreted the requirements of section 1835(a)(2)(C) and 1835(a)(2)(D) as
applying to therapy services furnished by both providers and suppliers. See Medicare Benefit
Policy Manual, Pub. 100-02, chapter 15, sections 220.1, 220.1.2, and 220.1.3. We believe that
this interpretation is based on the certification and recertification requirements under section
1835(a) of the Act as a way to effectuate the requirement in sections 1861(p), (g), and (ll)(2) of
the Act that the patient is under the care of a physician, and that the plan of treatment/care for the
physical therapy, occupational therapy, or speech-language pathology services has been
established by a physician or by a qualified PT, OT, or SLP and is periodically reviewed by a
physician. Additionally, we thought it was important to establish conforming policies for these
therapy services in both the outpatient provider and private practice settings.
Due to the foregoing concerns, we are proposing to revise the headings of paragraphs (c)
introductory text and (c)(1)(i) to include the term “occupational therapy” after physical therapy.
We propose to replace the term speech pathology with the accepted term speech-language
pathology in 42 CFR 424.24(c)(1)(i). We are also proposing to add the term “occupational
therapist” to 42 CFR 424.24(c)(3)(ii) between physical therapist and speech-language
pathologist.
The regulations at 42 CFR 424.24(c) require that a physician, nurse practitioner (NP),
physician assistant (PA), or clinical nurse specialist (CNS) who has knowledge of the case sign
the initial certification for the patient’s plan of treatment. We remind readers that plan of
treatment is synonymous with the “plan of care” mentioned above. This terminology appears in
several sections of Pub. 100-02, chapter 15, and both terms may be used interchangeably. In
accordance with § 424.24(c)(2), the initial certification must be obtained as soon as possible after
the plan is established by a PT, OT, or SLP. In Pub. 100-02, chapter 15, section 220.1.3 for
Certification and Recertification of Need for Treatment and Therapy Plans of Care, we specify
that the physician or nonphysician practitioner (NPP) must sign the initial plan of care (POC)
with a dated signature or verbal order within 30 days from the first day of treatment, including
evaluation (or 14 days if a verbal order), in order for the PT, OT, or SLP to be paid for the
services. For this reason, the manual also states that the therapist should forward the treatment
plan to the physician/NPP as soon as it is established rather than waiting to do so. The manual
allows for a delayed certification when the physician or NPP completes certification and includes
a reason for the delay, and delayed certifications are accepted without justification up to 30 days
after the due date.
The regulations at § 424.24(c)(4) require recertification at least every 90 days, and the
plan or other documentation in the patient's medical record must indicate the continuing need for
physical therapy, occupational therapy, or speech-language pathology services. The physician,
nurse practitioner, clinical nurse specialist, or physician assistant who reviews the plan must
recertify the plan by signing the medical record. Pub. 100-02, chapter 15, section 220.1.4.C
clarifies that payment and coverage conditions require that the plan of care be reviewed as often
as necessary but at least whenever it is certified or recertified, in order to meet the certification
requirements. We explained in the CY 2008 PFS final rule, when changing the plan of care
recertification interval from 30 to 90 days, this was done in order to allow more flexibility to the
physician/NPP to order the appropriate amount of therapy for each patient’s needs (72 FR
66333). Thus, a physician or non-physician practitioner (NPP) may certify or recertify a plan of
care at an interval the physician or NPP determines is appropriate, as long as the amount of time
between each recertification does not exceed 90 calendar days. As many episodes of therapy
treatment are completed in less than 30 calendar days, we expect that physicians and NPPs will
continue to certify plans of care that appropriately estimate the duration of needed therapy
treatment for a patient, even if the duration is less than 90 days.
Over the past two years, representatives of several therapy-related organizations have
requested that CMS reduce the administrative burden involved with attempting to obtain signed
plans of treatment from the physician/NPP. They expressed concern that therapists are held
accountable for the action or inaction of physicians/NPPs who may be overwhelmed with
paperwork. These interested parties report that therapists make exhaustive efforts to obtain the
physician/NPP’s signature – some reporting that they contact physician offices (via phone, email,
or fax, etc.) more than 30 times. Without the required signature, the therapist will not meet the
conditions to be paid for the services they deliver. These interested parties recommend that
payment for therapy services should be determined by the medical necessity of the service and
whether the therapist has met their statutory and regulatory requirements. Some of these
interested parties have noted that Pub. 100-02, chapter 15, section 220.1.1, states that the
physician/NPP order provides evidence that the patient is under the care of a physician and that
the services are medically necessary. Interested parties told us that while CMS allows treatment
to begin before the physician’s/NPP’s signature is obtained, PTs, OTs, and SLPs in private
practice do so at their own risk, knowing that they might not be paid for the services if the
physician’s office does not send back the signed plan of treatment. Accordingly, such interested
parties have said that care is delayed while awaiting a physician's signature, which could place
the beneficiary’s health at risk due to the delay in obtaining outpatient therapy services.
While we do not require an order or referral for a Medicare patient to see a PT, OT, or
SLP, we have explained that the presence of a signed order from the treating physician satisfies

statutory requirements that therapy is/was medically necessary and the patient is/was under the
care of a physician (Pub. 100-02, chapter 15, section 220.1.1). However, with this order
documented in the medical record, after the therapist evaluates the patient and establishes the
plan of treatment, based on the evaluation’s findings, the therapist forwards the patient’s plan of
treatment back to the referring physician/NPP to obtain a dated signature for the same patient
with the same diagnosis to meet coverage and payment conditions to satisfy the initial
certification requirement ─ creating an administrative burden for both the physician/NPP and the
therapist. Interested parties have reported to us that most patients seeking outpatient therapy
services have written orders from their physician, not to be confused with a written plan of
treatment. These interested parties have suggested that we amend the regulation at § 424.24(c) to
permit the presumption of a physician/NPP signature for purposes of certification and
recertification in cases where a signed written order or referral from the patient’s physician/NPP
is on file and there is written documentation in the patient’s medical record to substantiate the
method and date (such as a fax, email, etc.) that the therapist forwarded the plan of care to the
physician/NPP.
Additionally, interested parties representing all therapy disciplines requested that CMS
allot time for plan of treatment changes. Interested parties requested that when a physician/NPP
orders the therapy services, the physician/NPP be allotted ten business days to modify the plan of
treatment by contacting the therapist directly after receiving it from the therapist. For patients
without a physician/NPP order, interested parties requested that physician/NPPs be given 30
days after receipt of the plan of treatment to modify the treatment plan.
After reviewing our current regulatory requirements and considering the suggestions of
interested parties, we believe it would be appropriate to propose to amend the regulation at §
424.24(c) for those cases when a patient has a signed and dated order/referral from a
physician/NPP for outpatient therapy services. Since our policy has been to accept the physician
or NPP’s signature on the plan of treatment to be their certification of the treatment plan’s

conditions in the content requirements of § 424.24(c)(1) ─ that the patient needs or needed
physical therapy, occupational therapy or speech-language pathology services, the services were
furnished while the individual was under the care of a physician, NP, PA, or CNS, and the
services were furnished under a plan of treatment that meets the requirements of § 410.61 ─ we
propose that a signed and dated order/referral from a physician/NPP combined with
documentation of such order/referral in the patient’s medical record along with further evidence
in the medical record that the therapy plan of treatment was transmitted/submitted to the
ordering/referring physician or NPP is sufficient to demonstrate the physician or NPP’s
certification of these required conditions. Rather than characterizing this proposal as a
“presumption,” we are taking the view that when the patient’s medical record includes a signed
and dated written order or referral indicating the type of therapy needed, CMS (and our
contractors) would treat the signature on the order or referral as equivalent to a signature on the
plan of treatment. We believe our proposal would be reflective of the intent of the
ordering/referring physician/NPP when that order/referral is on file in the patient’s medical
record. We further believe that this would still be consistent with the initial certification required
under section 1835(a) of the Act for providers of therapy services and our current policy for
therapy in the private practice setting. When the ordering/referring physician writes the referral
for the type of therapy services they determine their patient needs or needed, they also review the
treatment plan the therapist established at the time it is forwarded to them, and they verify that
the services are or were furnished while the patient is or was under their care. As such, we
propose to carve out an exception to the physician signature requirement at § 424.24(c) by
adding a new paragraph (c)(5). The proposed policy would be an exception to the physician
signature requirement for purposes of an initial certification in cases where a signed and dated
order/referral from a physician, NP, PA, or CNS is on file and the therapist has documented
evidence that the plan of treatment has been delivered to the physician, NP, PA, or CNS within
30 days of completion of the initial evaluation. However, at this time, we are not proposing and

do not intend to establish an exception to the signature requirement for purposes of
recertification of the therapy plan of treatment. We believe that physicians and NPPs should still
be required to sign a patient’s medical record to recertify their therapy treatment plans, in
accordance with § 424.24(c)(4), to ensure that a patient does not receive unlimited therapy
services without a treatment plan signed and dated by the patient’s physician/NPP.
Under our proposal, CMS or its contractors would be able to treat the physician/NPP
signature on the order or referral as equivalent to a signature on the plan of treatment for
purposes of the initial certification if that physician/NPP has not signed and returned the patient’s
plan of treatment to the therapist within 30 days of the initial evaluation, but only in cases where
the patient’s physician/NPP has signed and dated the written order or referral and indicated the
type of therapy needed, and that written order or referral is on file in the medical record. This
proposed policy would not affect a contractor’s ability or authority to determine whether therapy
services are reasonable and necessary for a given beneficiary. Lastly, because there is no
requirement for a physician/NPP order or referral for patients to obtain outpatient therapy
services, we propose to make clear in proposed § 424.24(c)(5) that the references to an order or
referral in § 424.24(c)(5) shall not be construed to require an order or referral for outpatient
physical therapy, occupational therapy, or speech-language pathology services. We welcome
comments on this proposal.
In addition, we are soliciting comments to gather more information about the need for a
regulation that would address the amount of time for changes to plans of treatment. Our
regulations at 42 CFR 410.61(d), which are further clarified in our manual provisions in Pub.
100-02, chapter 15, section 220.1.2.C, currently allow for changes to the treatment plan by the
physician/NPP without time restrictions. Interested parties have suggested that CMS allow
physicians/NPPs to have just ten business days from the date of receipt of a plan of care to
modify that plan of care (in the case of a patient with an order for the therapy services).
Additionally, we are also soliciting comment as to whether there should be a 90 calendar day

time limit on the order/referral for outpatient therapy services in cases where the order/referral is
intended to be used in relation to the proposed regulatory amendment for the initial certification
of the treatment plan at § 424.24(c)(5) discussed above ─ that 90-day limit would span from the
order/referral date until the initial treatment of the patient, including the evaluation furnished by
the PT, OT, or SLP. We also seek feedback about whether this limit, or one of a different
duration, should be incorporated into the regulatory provision we proposed above for §
424.24(c)(5).
We want to clarify that we are not proposing to amend § 424.27 for CORF physical
therapy, occupational therapy, and speech-language pathology treatment plans to align with our
proposed amendments at § 424.24 because section 1861(cc) of the Act and regulation at 42 CFR
410.105(c) require these treatment plans to be established by a physician.
We are soliciting comments on these proposals and comment solicitations.
3. KX Modifier Thresholds
The KX modifier thresholds were established through section 50202 of the Bipartisan
Budget Act of 2018 (Pub. L. 115-123, February 9, 2018) (BBA) and were formerly referred to as
the therapy cap amounts. These per-beneficiary amounts under section 1833(g) of the Act (as
amended by section 4541 of the Balanced Budget Act of 1997) (Pub. L. 105–33, August 5, 1997)
are updated each year based on the percentage increase in the Medicare Economic Index (MEI).
Specifically, these amounts are calculated by updating the previous year’s amount by the
percentage increase in the MEI for the upcoming calendar year and rounding to the nearest
$10.00. Thus, for CY 2025, we propose to increase the CY 2024 KX modifier threshold amount
by the most recent forecast of the 2017-based MEI. For CY 2025, the proposed MEI increase is
estimated to be 3.6 percent and is based on the expected historical percentage increase of the
2017-based MEI. Multiplying the CY 2024 KX modifier threshold amount of $2,330 by the
proposed CY 2025 percentage increase in the MEI of 3.6 percent ($2,330 x 1.036) and rounding
to the nearest $10.00 results in a proposed CY 2025 KX modifier threshold amount of $2,410 for
physical therapy and speech-language pathology services combined and $2,410 for occupational
therapy services. We propose to update the MEI increase for CY 2025 based on historical data
through the second quarter of 2024, and we would use such data, if appropriate, to determine the
final MEI percentage increase and the CY 2025 KX modifier threshold amounts in the CY 2025
PFS final rule.
Section 1833(g)(7)(B) of the Act describes the targeted medical review (MR) process for
services of physical therapy, speech-language pathology, and occupational therapy services. The
threshold for targeted MR is $3,000 through CY 2027. Effective beginning with CY 2028, the
MR threshold levels would be annually updated by the percentage increase in the MEI, per
section 1833(g)(7)(B) of the Act. Consequently, for CY 2025, the MR threshold is $3,000 for
physical therapy and speech-language pathology services combined and $3,000 for occupational
therapy services. Section 1833(g)(5)(E) of the Act states that CMS shall identify and conduct
targeted medical review using factors that may include the following:
(1) The therapy provider has had a high claims denial percentage for therapy services
under this part or is less compliant with applicable requirements under this title.
(2) The therapy provider has a billing pattern for therapy services under this part that is
aberrant compared to peers or otherwise has questionable billing practices for such services, such
as billing medically unlikely units of services in a day.
(3) The therapy provider is newly enrolled under this title or has not previously furnished
therapy services under this part.
(4) The services are furnished to treat a type of medical condition.
(5) The therapy provider is part of a group that includes another therapy provider
identified using the factors described previously in this section.
We track each beneficiary’s incurred expenses for therapy services annually and count
them towards the KX modifier and MR thresholds by applying the PFS rate for each service less
any applicable MPPR amount for services of CMS-designated “always therapy” services (see the
CY 2011 PFS final rule at 75 FR 73236). We also track therapy services furnished by critical
access hospitals (CAHs), applying the same PFS-rate accrual process, even though they are not
paid for their therapy services under the PFS and may be paid on a cost basis (effective January
1, 2014) (see the CY 2014 PFS final rule at 78 FR 74406 through 74410).
When the beneficiary’s incurred expenses for the year for outpatient therapy services
exceed one or both of the KX modifier thresholds, therapy suppliers and providers use the KX
modifier on claims for subsequent medically necessary services. Using the KX modifier, the
therapist and therapy provider attest that the services above the KX modifier thresholds are
reasonable and necessary and that documentation of the medical necessity for the services is in
the beneficiary’s medical record. Claims for outpatient therapy services exceeding the KX
modifier thresholds without the KX modifier included are denied.
I. Advancing Access to Behavioral Health Services
1. Safety Planning Interventions and Post-Discharge Telephonic Follow-up Contacts
a. Background
In the CY 2024 PFS proposed rule, we sought comment on whether there is a need for
potential separate coding and payment for interventions initiated or furnished in the emergency
department (ED) or other crisis settings for patients with suicidality or at risk of suicide, such as
safety planning interventions and/or telephonic post-discharge follow-up contacts after an
emergency department visit or crisis encounter, or whether existing payment mechanisms are
sufficient to support furnishing such interventions when indicated. Several commenters
encouraged CMS to enable wider implementation under Medicare of the Safety Planning
Intervention (SPI) and the Post-Discharge Telephonic Follow-up Contacts Intervention (FCI) and
expressed that the current payment mechanisms are not sufficient, noting that the lack of
adequate payment mechanisms and suitable billing codes for these interventions are barriers that
are essential to address. The commenters noted that EDs are not the only care setting where
there is need and opportunity to enhance suicide prevention, but that elevated suicide risk is

particularly prevalent among ED patients. One commenter noted that a designated code for SPI
would make it significantly easier to document that SPI was furnished, including in quality
reporting and value-based payment programs.
More than 49,000 people died by suicide in 2022 and death by suicide is growing
significantly in older adults, who comprise most of the Medicare population. We recognize data
showing that suicide by intentional overdose is a growing concern, particularly among young
people, older people, and Black women, although researchers acknowledge the complexities of
distinguishing intentional from unintentional death.
143
b. Safety Planning Interventions (SPI)
Safety planning interventions involve a patient working with a clinician to develop a
personalized list of coping strategies and sources of support that the person can use in the event
of experiencing thoughts of harm to themselves or others. This is not a suicide risk assessment,
but rather, an intervention provided to people determined to have elevated risk. Safety planning
interventions have also been used to reduce the risk of overdose. The basic components of a
safety plan include the following: (1) recognizing warning signs of an impending suicidal crisis
or actions that increase the risk of suicide; (2) employing internal coping strategies; (3) utilizing
social contacts and social settings as a means of distraction from suicidal thoughts and/or taking
steps to reduce the risk of suicide; (4) utilizing family members, significant others, caregivers,
and/or friends to help resolve the crisis; (5) contacting mental health professionals, crisis
services, or agencies; and (6) making the environment safe, including restricting access to lethal
means, as applicable.
144
One important aspect of making an environment safe could be, for
example, addressing a person’s access to lethal means, such as firearms.
143
https://www.nih.gov/news-events/news-releases/suicides-drug-overdose-increased-among-young-people-elderly-
people-black-women-despite-overall-downward-tren.
144
Barbara Stanley, Gregory K. Brown, Safety Planning Intervention: A Brief Intervention to Mitigate Suicide Risk,
Cognitive and Behavioral Practice, Volume 19, Issue 2, 2012, Pages 256-264, ISSN 1077-7229,
https://doi.org/10.1016/j.cbpra.2011.01.001.

We understand that safety planning is consistent with current practice standards and that
many hospitals and clinicians in other settings are already providing some or all of these services
to the people who need them, including through the Department of Veterans Affairs (VA).
145146
However, in one survey of EDs, only 15.3 percent could confirm routinely implementing safety
planning with all of the structured elements mentioned above. Provision of individual safety
planning elements ranged from 24.8 percent (n = 492) to 79.2 percent (n = 1710), with 2 of 6
elements being routinely provided more than 50 percent of the time: lists of professionals or
agencies to contact in a crisis (1710 [79.2 percent]) and helping patients to recognize warning
signs of suicide (1075 [52.2 percent]).
147
Suicide risk among people with substance use
disorders who also are at high risk for or may have experienced an intentional overdose is not
well recognized.
148
Therefore, we are proposing to establish separate coding and payment under the PFS
describing safety planning interventions. Specifically, we are proposing to create an add-on G-
code that would be billed along with an E/M visit or psychotherapy when safety planning
interventions are personally performed by the billing practitioner in a variety of settings. We
recognize that training and expertise are needed to perform these interventions safely and
appropriately and are seeking comment regarding whether clinical staff who meet the definition
of auxiliary personnel defined at 42 CFR 410.26(a)(1) or who are employed by a hospital could
participate in furnishing this service under the supervision of the billing practitioner in certain
settings with the relevant training needed to perform the service as well as what sort of training
would be needed.
145
https://www.mentalhealth.va.gov/docs/vasafetyplancolor.pdf.
146
https://www.mirecc.va.gov/visn19/research/our-research/implementation.asp.
147
Bridge JA, Olfson M, Caterino JM, Cullen SW, Diana A, Frankel M, Marcus SC. Emergency Department
Management of Deliberate Self-harm: A National Survey. JAMA Psychiatry. 2019 Jun 1;76(6):652-654. doi:
10.1001/jamapsychiatry.2019.0063. PMID: 30865243; PMCID: PMC6552299.
148
Ries RK, Livengood AL, Huh D, et al. Effectiveness of a Suicide Prevention Module for Adults in Substance Use
Disorder Treatment: A Stepped-Wedge Cluster-Randomized Clinical Trial. JAMA Netw Open. 2022;5(4):e222945.
doi:10.1001/jamanetworkopen.2022.2945.

The proposed G-code is HCPCS code GSPI1: Safety planning interventions, including
assisting the patient in the identification of the following personalized elements of a safety plan:
recognizing warning signs of an impending suicidal crisis; employing internal coping strategies;
utilizing social contacts and social settings as a means of distraction from suicidal thoughts;
utilizing family members, significant others, caregivers, and/or friends to help resolve the crisis;
contacting mental health professionals or agencies; and making the environment safe; (List
separately in addition to an E/M visit or psychotherapy). We welcome comments on the
proposed elements of the safety planning code.
We are proposing to value HCPCS code GSPI1 based on the valuation for CPT code
90839 (Psychotherapy for crisis), which describes 60 minutes, and which we believe describes a
similar level of intensity as HCPCS code GSPI1. For HCPCS code GSPI1, we are assuming a
typical time of 20 minutes, resulting in a proposed work RVU of 1.09 (based on one third of the
work value currently assigned to CPT code 90839, which is 3.28). We welcome comments on
whether 20 minutes accurately captures the typical amount of time spent with a patient on safety
planning interventions, including all six elements enumerated in this section. Additionally, we
welcome comments on whether these interventions typically occur in the context of an
encounter, such as an E/M visit or psychotherapy, or whether there may be times when they may
be furnished as a standalone service and whether we should consider allowing this code to be
billed on its own. We also welcome comments regarding which clinician types might be most
likely to bill such a code on its own.
c. Post-Discharge Telephonic Follow-up Contacts Intervention (FCI)
Some research suggests that patients seen in the ED with deliberate self-harm, intentional
overdose, and/or suicidal ideation have been associated with substantially increased risk of
suicide and other mortality during the year following their visit to the ED.
149
FCI is a specific
149
Goldman-Mellor S, Olfson M, Lidon-Moyano C, Schoenbaum M. Association of Suicide and Other Mortality
With Emergency Department Presentation. JAMA Netw Open. 2019 Dec 2;2(12):e1917571. doi:
10.1001/jamanetworkopen.2019.17571. PMID: 31834399; PMCID: PMC6991205.

protocol of services for individuals with suicide risk involving a series of telephone contacts
between a provider and patient in the weeks and sometimes months following discharge from the
emergency department and other relevant care settings, that occurs when the person is in the
community and is designed to reduce the risk for subsequent adverse outcomes. FCI calls are
typically 10-20 minutes in duration and aim to encourage use of the Safety Plan (as needed in a
crisis) and updating it to optimize effectiveness, expressing psychosocial support, and helping to
facilitate engagement in any indicated follow-up care and services. We note that this service
would not be within the scope of Medicare telehealth services and not subject to the restrictions
described in Section 1834(m) because these services are specifically structured to be delivered
via audio-only phone calls and are not a substitute for an in-person service.
In a recent study led by the Joint Commission, which surveyed a national sample of
hospitals to assess the prevalence of SPI and several other recommended suicide prevention
services, fewer than half of responding hospitals reported furnishing any post-discharge follow-
up contacts. Of these, only 33 percent (16 percent of responding hospitals overall) reported
reaching discharged patients “most of the time.” Further, among hospitals that furnish follow-up
contacts, fewer than half reported covering any of the main aims of FCI, for example, 41 percent
review the Safety Plan, 49 percent provide psychosocial support, and 38 percent facilitate
outpatient care.
150
However, some studies have demonstrated that SPI and other services may be able to
reduce suicidal behaviors. For example, in the ED-SAFE trial for emergency department (ED)
patients identified with elevated suicide risk, the intervention included SPI and up to seven post-
discharge follow-up calls with the patient “focused on identifying suicide risk factors, clarifying
values and goals, safety and future planning, facilitating treatment engagement/adherence, and
150
https://www.sciencedirect.com/science/article/pii/S1553725024000679?via%3Dihub.

facilitating patient-significant other problem-solving.”
151
In the SAFE VET study
152
of ED
patients identified with elevated suicide risk, the intervention included SPI and at least two
follow-up calls with patients “to monitor suicide risk, review and revise the SPI, and support
treatment engagement.”
153
Each of these studies reported significantly lower suicide behaviors –
attempts and/or deaths – among intervention patients compared to the respective control
conditions.
In light of this, we are proposing to create a monthly billing code to describe the specific
protocols involved in furnishing post-discharge follow-up contacts that are performed in
conjunction with a discharge from the emergency department for a crisis encounter, as a bundled
service describing four calls in a month, each lasting between 10-20 minutes. The proposed G-
code is HCPCS code GFCI1: Post discharge telephonic follow-up contacts performed in
conjunction with a discharge from the emergency department for behavioral health or other
crisis encounter, per calendar month. We seek comment on whether we should consider
finalizing a specified duration that HCPCS code GFCI1 could be billed) following discharge, for
example, allowing this code to be billed for up to two months following discharge or whether a
longer duration would be appropriate, the number of calls per month, the billing structure (for
example, four calls for each discharged patient), and any other relevant feedback.
We are proposing to price this service based on a direct crosswalk to CPT code 99426
(Principal care management; first 30 minutes of clinical staff time directed by a physician or
other qualified healthcare professional), which is assigned a work value of 1.00 work RVUs.
Since CPT code 99426 describes care management for a single condition, we believe the work
151
Miller IW, Camargo CA Jr, Arias SA, Sullivan AF, Allen MH, Goldstein AB, Manton AP, Espinola JA, Jones R,
Hasegawa K, Boudreaux ED; ED-SAFE Investigators. Suicide Prevention in an Emergency Department Population:
The ED-SAFE Study. JAMA Psychiatry. 2017 Jun 1;74(6):563-570. doi: 10.1001/jamapsychiatry.2017.0678.
PMID: 28456130; PMCID: PMC5539839.
152
https://pubmed.ncbi.nlm.nih.gov/29998307/.
153
Stanley B, Brown GK, Brenner LA, Galfalvy HC, Currier GW, Knox KL, Chaudhury SR, Bush AL, Green KL.
Comparison of the Safety Planning Intervention With Follow-up vs Usual Care of Suicidal Patients Treated in the
Emergency Department. JAMA Psychiatry. 2018 Sep 1;75(9):894-900. doi: 10.1001/jamapsychiatry.2018.1776.
PMID: 29998307; PMCID: PMC6142908.
would be similar in nature and intensity. We note that under this proposal, proposed HCPCS
code GFCI1 could be billed regardless of whether proposed HCPCS code GSPI1 was also
furnished and billed for the same patient. We propose that the billing practitioner would need to
meet a threshold of at least one real-time telephone interaction with the patient in order to bill
HCPCS code GFCI1, and that unsuccessful attempts to reach the patient would not qualify as a
real-time telephone interaction. We welcome comments on this proposed threshold to bill
HCPCS code GFCI1, recognizing that while practitioners may attempt to reach the patient, there
may be times when the patient cannot be reached. We are also proposing that the billing
practitioner could not count time or effort more than once for the purposes of billing this code
and another service.
Additionally, as we recognize that behavioral health practitioners, training programs, and
institutions have worked conscientiously to have risk assessment and safety planning for high-
risk patients integrated into their workflows for many years and that discharge instructions and
after visit planning may represent one of many final products from the synthesis of all the steps
involved in these encounters, we note that we do not intend to unnecessarily disaggregate aspects
of streamlined clinical workflows that providers are successfully using to treat high risk patients.
Moreover, we also recognize that practitioners may currently be billing for safety planning
activities using existing coding, such as E/M visits, psychotherapy, and crisis management codes
or potentially for follow-up calls using existing care management services. However, to the
extent that this intervention is part of the standard of care, we believe that Medicare payment
should accurately reflect the additional resource costs involved in furnishing this service.
Lastly, as applicable Part B cost sharing would apply for HCPCS code GFCI1, we are
proposing to require the treating practitioner to obtain verbal (or written) beneficiary consent in
advance of furnishing the services described by GFCI1, which would be documented by the
treating practitioner in the medical record, similar to the conditions of payment associated with
care management and other non-face-to-face services paid under the PFS. We note that under
this proposal, obtaining advance consent would include: (1) ensuring that the patient is aware
that Medicare cost sharing applies to these services; (2) furnishing and receiving the necessary
information to enable the patient to receive these services (for example, obtaining the patient’s
telephone number(s)); and (3) confirming that the patient consents to the contacts.
2. Digital Mental Health Treatment (DMHT)
We are proposing Medicare payment to billing practitioners for digital mental health
treatment (DMHT) devices furnished incident to or integral to professional behavioral health
services used in conjunction with ongoing behavioral health care treatment under a behavioral
health treatment plan of care. We are refining the digital cognitive behavioral therapy “digital
CBT” terminology that we have used previously (88 FR 52262, 52370 through 52371, 88 FR
78818, 79012 and 79013). We are proposing Medicare payment to billing practitioners for digital
mental health treatment (DMHT) devices furnished incident to or integral to professional
behavioral health services used in conjunction with ongoing. In this proposed rule we use the
term “digital mental health treatment (DMHT) device” to include the term “digital CBT” we
used in prior rulemaking and in general to refer to software devices cleared by the Food and
Drug Administration (FDA) that are intended to treat or alleviate a mental health condition, in
conjunction with ongoing behavioral health care treatment under a behavioral health treatment
plan of care, by generating and delivering a mental health treatment intervention that has a
demonstrable positive therapeutic impact on a patient’s health. We note first that the Diagnostic
and Statistical Manual of Mental Disorders-5 (DSM) does not refer to psychiatric disorders but
to mental disorders. In this section, following the DSM, we use the term behavioral health
conditions and mental disorders interchangeably and to mean psychiatric disorders as referenced
in FDA regulation, 21 CFR 882.5801. Second, we note that FDA guidance refers to
computerized behavioral therapy by the acronym CBT. We aim to both provide access to vital
behavioral health services and gather further information about the delivery of digital behavioral
health therapies, their effectiveness, their adoption by practitioners as complements in the care
they furnish, and their use by patients for the treatment of behavioral health conditions. We also
recognize that there are certain statutory limitations on payment for products under the broader
category of “digital health interventions.” We acknowledge that the field of digital therapeutics is
evolving and are open to feedback from the public on this topic, including the CPT Editorial
Panel. Additionally, we recognize that historically, the CPT Editorial Panel has frequently
created CPT codes describing services that we originally established using G codes and adopted
them through the CPT Editorial Panel process. We note that we would consider using any newly
available CPT coding to describe services similar to those described here in future rulemaking.
a. Background
Over the last 5 years the AMA CPT Editorial Panel and CMS have developed coding and
separate payment for monitoring physiologic status using software enabled devices that capture
and record or transmit data that may be reported to and interpreted by practitioners to manage a
patient under a specific treatment plan. (83 FR 59452, 59574) Medicare payment has long been
available for practitioner provision of monitoring equipment and other kinds of devices provided
incident to or integral to the practitioner’s professional services. Most recently we have finalized
payment for devices which record data related to signs, symptoms, and functions of a therapeutic
response (typically for use in association with physical or occupational therapy care) (86 FR
64996, 65114-65116).
However, technologies that rely primarily on software, licensing, and analysis fees, with
minimal costs in equipment and hardware may not have been typical and are not well accounted
for in our practice expense (PE) methodology. PE resources involved in furnishing services are
characterized as either direct or indirect costs. Direct costs of the PE resources involved in
furnishing a service are estimated for each HCPCS code and include clinical labor, medical
supplies, and medical equipment. Indirect costs include administrative labor, office expenses,
and all other expenses. Indirect PE is allocated to each service based on physician work, direct
costs, and a specialty-specific indirect percentage. The source of the specialty specific indirect
percentage is the Physician Practice Information Survey (PPIS), last administered in 2007 and
2008, prior to the adoption of digital therapy technologies (86 FR 65037). Nevertheless, in past
rulemaking, we have recognized that in some cases practitioners do incur resource costs for the
purchase and ongoing use of software (86 FR 65038).
In the CY 2023 PFS final rule, we finalized our proposal to accept the RUC
recommendation to contractor price CPT code 98978 (Remote therapeutic monitoring (e.g.,
therapy adherence, therapy response); device(s) supply with scheduled (e.g., daily) recording(s)
and/or programmed alert(s) transmission to monitor cognitive behavior therapy, each 30 days),
a PE-only device code (86 FR 69523, 69646). At the time, specialty societies indicated that the
technologies for this service are still evolving, and that as a result, there were no invoices for
devices specific to the cognitive behavioral therapy monitoring services described by the code
that could be shared. There was no professional work associated with the code.
In the CY 2024 PFS proposed rule, we requested information on digital therapeutics for
behavioral health. Among many questions, we asked how practitioners determine which patients
might be best served by digital therapeutics and how practitioners monitor the effectiveness of
prescribed interventions on an ongoing basis once the intervention has begun. We also asked
how the treating clinician was involved in the services received. We asked what scientific and
clinical evidence of effectiveness CMS should consider when determining whether digital
therapeutics for behavioral health, including care for substance use disorders, depression, sleep
disorders and other conditions are reasonable and necessary. We asked whether DMHT devices
were used as incident to supplies or independent of a patient visit with a practitioner and if
practitioners in such cases issued an order for such devices (88 FR 52262, 52370 through
52371). These factors related to the nature of this treatment compared to other PFS services pose
challenges for fitting DMHT services into the existing benefit structure under the PFS.
Setting appropriate pricing under the PFS has also presented challenges. As noted
previously, technologies that rely primarily on software, licensing, and analysis fees, with
minimal costs in equipment and hardware are not well accounted for in our practice expense
(PE) methodology, even though these items may be appropriately considered practice expenses.
Consequently, over the past several years, we have relied on a crosswalk methodology to
approximate relative resource costs for these kinds of services relative to other PFS services, or
contractor pricing.
Interested parties requested that we adopt coding specifically for DMHT devices, where
the digital software device is the actual therapy/intervention (the algorithm software is the
DMHT) as opposed to a therapeutic monitoring device that transmits patient data as described by
CPT code 98978 for which we finalized contractor pricing in CY 2023. Interested parties have
also asked us to set national pricing for the service to supply the DMHT device and
education/onboarding that reflects the direct practice expense incurred by practitioners when
furnishing DMHT. One of the interested parties submitted invoices to provide data we could use
as the basis to set payments for DMHT coding. The interested party submitted four invoices
reflecting considerable variation in the cost of the DMHT treatment over 30-day and 90-day
periods. There is still more variation in pricing for comparable products that are available for
considerably lower prices in various markets, with prices ranging from free to $140 per year,
based on an online search (https://www.carepatron.com/app/cbt-therapy-apps, accessed March
20, 2024).
As the field of innovative products including digital therapeutics and computerized
behavioral therapy devices for psychiatric or mental disorders develops and expands, the FDA
and Substance Abuse and Mental Health Services Administration (SAMHSA) among other
agencies such as the Veterans Health Administration (VHA) are also monitoring the
development of the field of digital therapeutic devices, including for behavioral health purposes.
For example, VHA is providing digital behavioral health applications as self-help tools, not
independent treatment interventions. The FDA has a regulatory framework, discussed below in

this section, to classify devices and review computerized behavioral therapy devices for
psychiatric disorders.
b. Payment for Digital Mental Health Treatment (DMHT) Devices
We recognize that digital therapeutics may offer innovative means to access certain
behavioral health care services. The FDA definition of devices encompasses software intended
by the manufacturer to be used, alone or in combination for the specific medical purpose of
diagnosis, prevention monitoring treatment or alleviation of disease and does not achieve its
primary intended action by pharmacological, immunological or metabolic means.
154
SAMHSA
has defined digital therapeutics (DTx) in the behavioral health context as “health software
intended to treat or alleviate a disease, disorder, condition, or injury by generating and delivering
a medical intervention that has a demonstrable positive therapeutic impact on a patient’s
health.”
155
SAMHSA also notes that “DTx may be used independently or in concert with
medications, devices, or other therapies to optimize patient care and health outcomes.” Given
nationwide behavioral health workforce shortages combined with increasing demand for
behavioral health care services, some Medicare beneficiaries may have limited access to these
services.
156
This proposal encompasses only part of what may be a spectrum of broadly similar
products, most of which might require a new statutory Medicare benefit category. Our proposed
coding and payment policy only applies to DMHT devices that have been cleared by the FDA.
Many digital platforms and applications are marketed as behavioral health and wellness
interventions; this proposal does not extend to such platforms and applications in part because
other than some DTx, few have evidence demonstrating improved behavioral health outcomes.
157
154
https://www.imdrf.org/sites/default/files/docs/imdrf/final/technical/imdrf-tech-131209-samd-key-definitions-
140901.pdf.
155
https://store.samhsa.gov/product/advisory-digital-therapeutics-management-and-treatment-behavioral-
health/pep23-06-00-001.
156
https://bhw.hrsa.gov/sites/default/files/bureau-health-workforce/data-research/behavioral-health-2013-2025.pdf.
157
https://store.samhsa.gov/product/advisory-digital-therapeutics-management-and-treatment-behavioral-
health/pep23-06-00-001.
We propose to create three new HCPCS codes for DMHT devices modeled on coding for
RTM services. Effective beginning in CY 2025, we propose that physicians and practitioners
who are authorized to furnish services for the diagnosis and treatment of mental illness would be
able to bill a new HCPCS code: GMBT1 (Supply of digital mental health treatment device and
initial education and onboarding, per course of treatment that augments a behavioral therapy
plan) for furnishing a DMHT device. GMBT1 would be payable only if the DMHT device has
been FDA cleared and the billing practitioner is incurring the cost of furnishing the DMHT
device to the beneficiary. Furnishing of the DMHT device must be incident to the billing
practitioner’s professional services in association with ongoing treatment under a plan of care by
the billing practitioner. The billing practitioner must diagnose the patient and prescribe or order
the DMHT device. The patient could then use the DMHT device at home or perhaps in an office
or other outpatient setting, if that is how the device has been cleared by FDA for use under 21
CFR 882.5801. The DMHT device furnished must have demonstrated a reasonable assurance of
safety and effectiveness. The FDA makes a determination of safety and effectiveness under 21
CFR 860.7. When making this determination, the FDA will consider a variety of factors
including users, conditions of use, probable benefit to health weighed against probable injury,
and reliability. The regulation at 21 CFR 860.7, states that “[t]here is reasonable assurance that a
device is safe when it can be determined, based upon valid scientific evidence, that the probable
benefits to health from use of the device for its intended uses and conditions of use, when
accompanied by adequate directions and warnings against unsafe use, outweigh any probable
risks.” GMBT1 would not be payable in cases where the billing practitioner incurs no cost in
acquiring and furnishing the DMHT device, or a patient procures the DMHT device independent
of the practitioner. We will continue to monitor how DMHT devices are used as part of overall
care.
We seek comment about other parameters that we should consider regarding the services
described by GMBT1:
● Whether payment should be made if the practitioner furnishes a digital device that has
not been cleared by FDA for mental health treatment for a specific use, even if the digital device
has been cleared by the FDA for another specific use;
● Whether payment should be made for DMHT devices cleared by the FDA not only
under 21 CFR 882.5801 but also under other regulations;
● Whether and how payment might be limited if a patient discontinues use of the DMHT
device before completing a course of treatment; and
● Whether and how payment might be limited to a set number of DMHT devices per
calendar month per patient.
In light of the pricing variability, as discussed above, we are proposing contractor pricing
for code GMBT1. We seek comment regarding what national pricing methodology we might
consider, including what potential crosswalks would be appropriate.
We are also proposing to establish payment for two additional new codes. These
proposed codes are GMBT2 (First 20 minutes of monthly treatment management services
directly related to the patient’s therapeutic use of the digital mental health treatment (DMHT)
device that augments a behavioral therapy plan, physician/other qualified health care
professional time reviewing data generated from the DMHT device from patient observations
and patient specific inputs in a calendar month and requiring at least one interactive
communication with the patient/caregiver during the calendar month) and GMBT3 (Each
additional 20 minutes of monthly treatment management services directly related to the patient’s
therapeutic use of the digital mental health treatment (DMHT) device that augments a
behavioral therapy plan, physician/other qualified health care professional time reviewing data
generated from the DMHT device from patient observations and patient specific inputs in a
calendar month and requiring at least one interactive communication with the patient/caregiver
during the calendar month). Under this proposal, GMBT1 requires that the billing practitioner
who diagnosed the patient and prescribed or ordered the DMHT device or that billing
practitioner’s clinical staff must monitor the patient’s therapeutic response to the DMHT device
and adjust the behavioral health therapy plan as needed. GMBT2 and GMBT3 should only be
billed when there is ongoing use of the DMHT device and should not be billed in cases where the
patient discontinues use of the DMHT device.
For GMBT2 (first 20 minutes of monthly treatment management services directly related
to use of the DMHT device), we propose valuing the first 20 minutes of treatment management
services based on a direct crosswalk to CPT code 98980 (remote therapeutic monitoring first 20
minutes), which is assigned a work RVU of .62. For GMBT3 (each additional 20 minutes of
monthly treatment management services directly related to DMHT device), we propose to value
this code based on a crosswalk to CPT code 98981 (remote therapeutic monitoring each
additional 20 minutes), which is assigned a work RVU of .61. We believe that the work and PE
described by these crosswalk codes are analogous to the services described in GMBT2 and
GMBT3, respectively, because they include similar physician/other qualified health care
professional time in a calendar month requiring at least one interactive communication with the
patient/caregiver during the calendar month. We welcome comments on the proposed RVUs.
3. Interprofessional Consultation Billed by Practitioners Authorized by Statute to Treat
Behavioral Health Conditions
a. Background
In the CY 2019 PFS final rule (83 FR 59489), we finalized payment for six CPT codes
regarding interprofessional consultations (99451, 99452, 99446, 99447, 99448, 99449). The six
codes describe assessment and management services conducted through telephone, internet, or
electronic health record consultations furnished when a patient’s treating physician or other
qualified healthcare professional requests the opinion and/or treatment advice of a consulting
physician or qualified healthcare professional with specific specialty expertise to assist with the
diagnosis and/or management of the patient’s condition without the need for the patient’s face-
to-face contact with the consulting physician or qualified healthcare professional. We established
coding and payment for these services to reflect changing healthcare practices, technology, and
the shift to treatment of chronic conditions in the Medicare population. In the CY 2019 PFS final
rule (83 FR 59491), we established a policy to limit billing of these codes to the types of
practitioners who can independently bill Medicare for E/M visits. We did not finalize the
expansion of practitioners beyond those who can furnish E/M visits in the CY 2019 PFS final
rule due to our belief that interprofessional consultations are primarily for the ongoing evaluation
and management of the patient, including collaborative medical decision making among
practitioners (83 FR 59491).
In the CY 2024 PFS proposed rule (88 FR 52369), we sought comment on expanding
access to behavioral health services, including whether we should consider new coding to allow
interprofessional consultation to be billed by practitioners in specialties whose covered services
are limited by statute (Clinical psychologists at section 1861(ii) of the Act, Clinical social
workers at section1861(hh) of the Act, Marriage and Family Therapists and Mental Health
Counselors at sections 1861(lll)(1) and 1861(lll)(3)of the Act, respectively) to services for the
diagnosis and treatment of mental illness (which includes substance use disorders). The CPT
codes describing interprofessional consultation (CPT codes 99451, 99452, 99446, 99447, 99448,
99449) are currently limited to being billed by practitioners who can independently bill Medicare
for E/M visits. As such, they cannot be billed by clinical psychologists, clinical social workers,
marriage and family therapists, or mental health counselors because these practitioners cannot
independently bill Medicare for E/M visits. We are proposing new codes that would allow
clinical psychologists, clinical social workers, marriage and family therapists, and mental health
counselors to bill for interprofessional consultations with other practitioners whose practice is
similarly limited, as well as with physicians and practitioners who can bill Medicare for E/M
services and would use the current CPT codes to bill for interpersonal consultations. These new
codes would facilitate interprofessional consultations between treating/requesting practitioners
and consultant practitioners, whether one or both of the practitioners is in a specialty whose
practice is limited to the diagnosis and treatment of mental illness. When the treating/requesting
practitioner or consultant practitioner is a physician or practitioner authorized to bill Medicare
for E/M services, the practitioner would continue to bill using the current CPT codes that
describe interprofessional consultation, listed previously in this section. Depending on which
practitioner type is billing, and assuming all service requirements of the code descriptors are met,
the consulting practitioner could bill the applicable codes, either HCPCS code (GIPC1-5) or CPT
code (99451, 99452, 99446, 99447), determined by the amount of time spent on the consultation
and whether a written and verbal consultation is provided or only a written consultation is
provided. Similarly, depending on which practitioner type is billing, and assuming all service
requirements of the code descriptors are met, the treating/requesting practitioner could bill either
HCPCS code GIPC6 or CPT code 99449 for the time spent on their referral service.
We believe that proposing payment for these interprofessional consultations performed
via communications technology such as telephone or internet (including videoconference) is
consistent with our ongoing efforts to appropriately recognize and reflect behavioral health care
within the PFS. Currently, there is no payment mechanism to recognize the time and effort of
performing these services by clinical psychologists, clinical social workers, marriage and family
therapists, or mental health counselors. We have also previously received comments from
interested parties that by not making separate payment for these services, CMS would not be
accurately paying for the work of both the treating and consulting practitioner in a consultative
scenario. With the proliferation of team-based approaches to care that are often facilitated by
electronic medical record technology, we believe that making separate payment for
interprofessional consultations undertaken for the benefit of treating a patient will contribute to
payment accuracy under the PFS for behavioral health services.
b. Coding
To further expand access to behavioral health services, we are proposing payment for six
new G codes: GIPC1 (Interprofessional telephone/Internet/electronic health record assessment
and management service provided by a practitioner in a specialty whose covered services are
limited by statute to services for the diagnosis and treatment of mental illness, including a verbal
and written report to the patient’s treating/requesting practitioner; 5-10 minutes of medical
consultative discussion and review), GIPC2 (Interprofessional telephone/Internet/electronic
health record assessment and management service provided by a practitioner in a specialty
whose covered services are limited by statute to services for the diagnosis and treatment of
mental illness, including a verbal and written report to the patient’s treating/requesting
practitioner; 11-20 minutes of medical consultative discussion and review), GIPC3
(Interprofessional telephone/Internet/electronic health record assessment and management
service provided by a practitioner in a specialty whose covered services are limited by statute to
services for the diagnosis and treatment of mental illness, including a verbal and written report
to the patient’s treating/requesting practitioner; 21-30 minutes of medical consultative
discussion and review), GIPC4 (Interprofessional telephone/Internet/electronic health record
assessment and management service provided by a practitioner in a specialty whose covered
services are limited by statute to services for the diagnosis and treatment of mental illness,
including a verbal and written report to the patient’s treating/requesting practitioner; 31 or
more minutes of medical consultative discussion and review), GIPC5 (Interprofessional
telephone/Internet/electronic health record assessment and management service provided by a
practitioner in a specialty whose covered services are limited by statute to services for the
diagnosis and treatment of mental illness, including a written report to the patient’s
treating/requesting practitioner, 5 minutes or more of medical consultative time), and GIPC6
(Interprofessional telephone/Internet/electronic health record referral service(s) provided by a
treating/requesting practitioner in a specialty whose covered services are limited by statute to
services for the diagnosis and treatment of mental illness, 30 minutes). We welcome comments
on this proposal.
Additionally, since these codes describe services that are furnished by the
treating/requesting practitioner and the consultant practitioner without the involvement of the
patient, we are proposing to require the treating practitioner to obtain the patient’s consent in
advance of these services, which would be documented by the treating practitioner in the medical
record, similar to the conditions of payment associated with the CPT interprofessional
consultation codes and certain other non-face-to-face services paid under the PFS. Obtaining
advance patient consent includes ensuring that the patient is aware that Medicare cost sharing
applies to these services, including informing the patient that there may be cost sharing for two
services (one for the treating/requesting practitioner’s service and another for the consultant
practitioner’s service). We welcome comments on this proposal.
c. Valuation
We are proposing to value the six proposed new G codes based on crosswalks to the six
CPT codes for interprofessional consultations for practitioners who can independently bill
Medicare for E/M visits (CPT codes 99451, 99452, 99446, 99447, 99448, 99449). We are
proposing a work RVU of 0.35 for GIPC1 based on a crosswalk to CPT code 99446, a work
RVU of 0.70 for GIPC2 based on a crosswalk to CPT code 99447, a work RVU of 1.05 for
GIPC3 based on a crosswalk to CPT code 99448), a work RVU of 1.40 for GIPC4 based on a
crosswalk to CPT code 99449, a work RVU of 0.70 for GIPC5 based on a crosswalk to CPT
code 99451, and a work RVU of 0.70 for GIPC6 based on a crosswalk to 99452. Since there are
no direct PE inputs assigned to the six CPT codes describing interprofessional consultation
services on which we are basing the proposed valuation for the new HCPCS codes GIPC1
through GIPC6, we are not proposing any direct PE inputs for these codes. We welcome
comments on this proposal.
4. Comment Solicitation on Payment for Services Furnished in Additional Settings, including
Freestanding SUD Treatment Facilities, Crisis Stabilization Units, Urgent Care Centers, and
Certified Community Behavioral Health Clinics (CCBHCs)
In the CY 2024 OPPS final rule (88 FR 81809 through 81858), we finalized payment for
IOP services furnished in hospital outpatient departments (HOPDs), Community Mental Health
Centers (CMHCs), Federally Qualified Health Centers (FQHCs), and Rural Health Clinics
(RHCs), and Opioid Treatment Programs (OTPs). We note that Section 4124 of the
Consolidated Appropriations Act (CAA), 2023, authorized payment for IOP services in HOPDs,
CMHCs, FQHCs, RHCs, and that we additionally used existing statutory authority to propose
and finalize payment for IOP services furnished in OTPs. CMS is monitoring utilization and
uptake of IOP services in these settings. We have heard from other treatment settings that
furnish IOP services that do not fall into the categories of HOPDs, CMHCs, FQHCs, RHCs, or
OTPs, such as freestanding SUD facilities, that have an interest in billing Medicare for these
services. In light of this, we are seeking comment on whether IOP services are furnished in other
settings in order to determine whether potential coding and payment for IOP services under the
PFS would facilitate these services being billed in additional settings.
In particular, we are interested in feedback on the following questions, as well as any
other relevant feedback:
● To what extent do freestanding SUD facilities or other entities that furnish IOP
services employ practitioner types who can supervise auxiliary personnel and bill Medicare for
their services? For example, do they typically employ physicians, clinical psychologists, nurse
practitioners, clinical nurse specialists, certified nurse midwives and physician assistants who are
eligible to provide general supervision to auxiliary personnel who furnish behavioral health
services?
● Would bundled payments under the PFS similar to those finalized in the CY 2024
OPPS final rule (88 FR 81809-81858) better facilitate billing for IOP services in a broader range
of settings?
● If CMS outlined how freestanding SUD facilities could bill Medicare under the PFS,
would there be an impact in underserved areas?
● To what extent do freestanding SUD facilities see patients with Medicare or who are
dually eligible for Medicare and Medicaid?
Additionally, we are seeking comment on entities that offer community-based crisis
stabilization, including 24/7 receiving and short-term stabilization centers, that provide
immediate access to voluntary and/or involuntary care, without the need for a referral. Regarding
such crisis stabilization units, we are interested in feedback on the following questions, as well as
any other relevant feedback:
++ What kind of services do crisis stabilization units provide? Do crisis stabilization
units provide services similar to those described by the psychotherapy for crisis codes (CPT
codes 90839 and 90840)?
++ Does the definition of crisis stabilization unit vary by State? If so, what are the
variations and similarities across States?
++ If CMS outlined how crisis stabilization units could bill Medicare under the PFS,
would there be an impact in underserved areas?
++ To what extent do crisis stabilization units see patients with Medicare or who are
dually eligible for Medicare and Medicaid?
++ To what extent do crisis stabilization units employ practitioner types who can
supervise auxiliary personnel and bill Medicare for their services. For example, do crisis
stabilization units typically employ physicians, clinical psychologists, nurse practitioners,
clinical nurse specialists, certified nurse midwives and physician assistants who are eligible to
provide general to auxiliary personnel who furnish behavioral health services?
Additionally, as a separate example, we've received information from interested parties
that there is a similar concern regarding urgent care centers more broadly. These interested
parties note that hospital emergency departments are often used by beneficiaries to address non-
emergent urgent care needs that could be appropriately served in less acute settings, but where
other settings, such as physician offices, urgent care centers or other clinics, are not available or
readily accessible. Patients enter EDs to treat common conditions like allergic reactions,
lacerations, sprains and fractures, common respiratory illnesses (for example, flu or RSV), and
bacterial infections (for example, strep throat, urinary tract infections or foodborne illness).
Conditions like these often can be treated in less acute settings. We are interested in system
capacity and workforce issues broadly and are interested in hearing more on those issues,
including how entities such as urgent care centers can play a role in addressing some of the
capacity issues in emergency departments. In particular, we are interested in feedback on the
following questions, as well as any other relevant feedback:
● What types of services would alternative settings to EDs need to offer to meet
beneficiaries’ non-emergent, urgent care needs?
● Does the current “Urgent Care Facility” Place of Service code (POS 20) adequately
identify and define the scope of services furnished in such settings? Is this place of service code
sufficiently distinct from others such as “Walk-in Retail Health Clinic (POS 17) and “Office”
(POS 11)? If not, how might these Place of Service code definitions be modified?
● Does the existing code set accurately describe and value services personally performed
by professionals and costs incurred by the facility in these settings?
● How might potential strategies to reduce overcrowding and wait times in EDs advance
equity in access to health care services?
Lastly, we are seeking comment regarding Certified Community Behavioral Health
Clinics (CCBHCs). Specifically, we are interested in feedback on the following questions:
++ What kind of services do CCBHCs provide? Do they provide IOP services, services
for the treatment of substance use disorders, psychotherapy, behavioral health integration,
community health integration, or principal illness navigation services to patients with either
Medicare or another payer?
++ If CMS outlined how CCBHCs could bill Medicare under the PFS, would there be an
impact in underserved areas?
++ To what extent do CCBHCs see patients with Medicare or who are dually eligible for
Medicare and Medicaid?
++ To what extent do CCBHCs employ practitioner types who can supervise auxiliary
personnel and bill Medicare for their services? For example, do CCBHCs employ physicians,
clinical psychologists, nurse practitioners, clinical nurse specialists, certified nurse midwives and
physician assistants who are eligible to provide general supervision to auxiliary personnel who
furnish behavioral health services?
J. Proposals on Medicare Parts A and B Payment for Dental Services Inextricably Linked to
Specific Covered Services
1. Medicare Payment for Dental Services
a. Overview
Section 1862(a)(12) of the Act generally precludes payment under Medicare Parts A or B
for any expenses incurred for services in connection with the care, treatment, filling, removal, or
replacement of teeth or structures directly supporting teeth. (Collectively here, we will refer to
“the care, treatment, filling, removal, or replacement of teeth or structures directly supporting
teeth” as “dental services.”) That section of the statute also includes an exception to allow
payment to be made for inpatient hospital services in connection with the provision of such
dental services if the individual, because of their underlying medical condition and clinical status
or because of the severity of the dental procedure, requires hospitalization in connection with the
provision of such services. Our regulation at § 411.15(i) similarly excludes payment for dental
services except for inpatient hospital services in connection with dental services when
hospitalization is required because of: (1) the individual’s underlying medical condition and
clinical status; or (2) the severity of the dental procedure.
Fee for service (FFS) Medicare Parts A and B also make payment for certain dental
services in circumstances where the services are not considered to be in connection with dental
services within the meaning of section 1862(a)(12) of the Act. In the CY 2023 PFS final rule (87
FR 69663 through 69688), we clarified and codified at § 411.15(i)(3) that Medicare payment
under Parts A and B could be made when dental services are furnished in either the inpatient or
outpatient setting when the dental services are inextricably linked to, and substantially related
and integral to the clinical success of, other covered services. We also added several examples
of clinical scenarios that are considered to meet that standard under § 411.15(i)(3) and amended
that regulation to add more examples in the CY 2024 PFS final rule (88 FR 79022 through
79029).
In the CY 2023 PFS final rule, we also established a process whereby we accept and
consider submissions from the public (the “public submission process”) to assist us to identify
additional dental services that are inextricably linked to, and substantially related and integral to
the clinical success of, other covered services (87 FR 69663 through 69688). Hereafter in this
section we will refer to these services as dental services that are “inextricably linked to other
covered services.”
We also note that the examples provided in our regulation at § 411.15(i)(3)(i) are not
exclusive. Medicare administrative contractors (MACs) retain discretion to determine on a
claim-by-claim basis whether a patient’s circumstances do or do not fit within the terms of the
preclusion or exceptions specified in section 1862(a)(12) of the Act and § 411.15(i).
In the CY 2024 PFS final rule, we discussed our plans to issue educational and outreach
materials to inform billing and payment for finalized policies for dental services. We reiterated
our commitment to review submissions we receive through the public submissions process. We
also expressed our intention to continue to engage in discussions with the public on a wide
spectrum of issues relating to Medicare payment for dental services that may be inextricably
linked to other covered services. We also described our partnership with the Agency for
Healthcare Research and Quality (AHRQ) to assist us to review available clinical evidence and
consider the relationship between dental services and specific covered medical services and to
identify other potential clinical circumstances in which dental services are inextricably linked to
other covered services (88 FR 79029).
In this proposed rule, we (1) describe recent literature review conducted by our partner
agency, AHRQ, on the potential connection between sickle cell disease and hemophilia and
dental services; (2) summarize submissions we received through the public submission process
for consideration in CY 2025 rulemaking; (3) propose to codify in section § 411.15(i)(3)(i)(A)
additional policies to permit payment for certain dental services that are inextricably linked to
other covered services (certain dental services for patients receiving dialysis services to treat
end-stage renal disease (ESRD)); (4) request public comment and information related to other
clinical scenarios that may involve dental services that are inextricably linked to other covered
services; and (5) include proposals related to Medicare billing and payment policy for dental
services. We also include a request for information regarding oral sleep apnea appliances.
b. Consideration of Dental Services that may be Inextricably Linked to Other Covered Services
We have partnered with AHRQ to help us consider the relationship between dental
services and other specific covered services. Specifically, AHRQ reviews available clinical
evidence regarding this relationship, and provides analysis of clinical scenarios where dental
services may be inextricably linked to other covered services. To better address the public's
immediate dental needs, AHRQ conducted rapid response reports instead of comprehensive
assessments. With these rapid response reports, we can better specify which payments can be
made under Medicare Parts A and B for certain dental services that are inextricably linked to
other covered services.
Through the public submissions process for consideration in CY 2024 rulemaking,
interested parties nominated dental services for individuals living with sickle cell disease (SCD)
or hemophilia, urging us to consider adding payment for these services (88 FR 52374). The
submissions included information and references supporting the inclusion of dental services that
are inextricably linked to, and substantially related and integral to the clinical success of, the

covered services used in the treatment of SCD or hemophilia, because such dental services serve
to mitigate the substantial risk to the success of the medical services. Submissions supported the
importance of preventing dental infections among individuals with SCD to reduce the need for
the extensive procedures that may lead to bleeding complications and hospitalization. They also
provided information detailing increased dental caries and periodontal disease in people with
SCD,
158
many of whom lose a number of teeth, which greatly limits nutrition, general well-
being, and overall quality of life. Interested parties recommended that we adopt a policy to allow
payment for dental services for individuals with hemophilia (88 FR 79032). They emphasized
that periodic dental care reduces the risks of dental complications that may require hemostatic
therapy or oral surgeries requiring clotting factor replacement treatment.
159,160,161
Many
submitters suggested that maintaining good oral health leads to improved outcomes for patients
with these two conditions.
Acknowledging the importance of dental health to overall well-being of patients with
these two types of diseases, in the CY 2024 proposed rule, we summarized information provided
by submitters utilizing the public submission process and solicited comment on whether certain
dental services are inextricably linked to covered services in the treatment of SCD (88 FR
52374). In the CY 2024 PFS final rule, we noted that several commenters suggested expanding
dental service coverage for individuals with SCD. They supported covering dental services
essential for treating SCD, including hydroxyurea therapy. Another commenter advocated for
158
Kakkar M, Holderle K, ShethM, Arany S, Schiff L, Planerova A. Orofacial Manifestation and Dental Management
of Sickle Cell Disease: A Scoping Review. Anemia. 2021 Oct22; 2021:5556708. Doi: 10.1155/2021/5556708. PMID:
34721900; PMCID: PMC8556080.
159
Raso S, Napolitano M, Sirocchi D, Siragusa S, Hermans C. The important impact of dental care on haemostatic
treatment burden in patients with mild haemophilia. Haemophilia. 2022 Nov;28(6):996–999.
doi:10.1111/hae.14626. Epub 2022 Jul 25. PMID: 35879819.
160
Srivastava A, Santagostino E, Dougall A, Kitchen S, Sutherland M, Pipe SW, Carcao M, Mahlangu J, Ragni MV,
Windyga J, Llina´s A, Goddard NJ, Mohan R, Poonnoose PM, Feldman BM, Lewis SZ, van den Berg HM, Pierce GF;
WFH Guidelines for the Management of Hemophilia panelists and co-authors. WFH Guidelines for the
Management of Hemophilia, 3rd edition. Haemophilia. 2020 Aug;26 Suppl 6:1–158. doi: 10.1111/hae.14046. Epub
2020 Aug 3. Erratum in: Haemophilia. 2021 Jul;27(4):699. PMID: 32744769.
161
Peisker A, Raschke GF, Schultze-Mosgau S. Management of dental extraction in patients with Haemophilia A
and B: a report of 58 extractions. Med Oral Patol Oral Cir Bucal. 2014 Jan 1;19(1):e55–60. doi:
10.4317/medoral.19191. PMID:24121912; PMCID: PMC3909433.
expanding coverage for dental services in cases of pain crises or dental abscesses in individuals
with SCD. They emphasized that improved access to dental care could improve the quality of
life for individuals with SCD and reduce healthcare costs by enabling more suitable treatment
options and decreasing hospital stays. One commenter questioned the clinical basis for
providing dental benefits for SCD or hemophilia due to their low prevalence in the Medicare
population. Ultimately, after consideration of public comments, we did not expand the examples
under § 411.15(i)(3)(i) to include additional covered medical services for SCD. We concluded
that the information provided by commenters did not sufficiently demonstrate that dental
services are essential to the clinical success of hydroxyurea therapy or other treatments for SCD
(88 FR 79032). Please refer to CY 2024 final rule (88 FR 79031 through 79032) for more
detailed information.
In the CY 2024 PFS proposed rule, we similarly solicited comments on hemophilia
regarding whether certain dental services are considered so integral to the primary covered
services that the necessary dental interventions are inextricably linked to, and substantially
related and integral to clinical success of, the primary covered services for individuals with
hemophilia (88 FR 52382). Several commenters advocated for allowing Medicare Part A and
Part B payment for dental services for individuals with hemophilia citing guidelines from
Hemophilia Treatment Centers (HTCs), the Centers for Disease Control and Prevention (CDC),
and the World Federation of Hemophilia (WFH). They emphasized the importance of good oral
health to prevent complications like gum bleeding and major dental surgeries, especially in those
with severe/moderate hemophilia. Another commenter supported this perspective, noting that
regular dental care reduces the need for clotting factor replacement therapy. While we
acknowledged the importance of maintaining oral health to prevent complications such as serious
gum bleeding, especially problematic for those with hemophilia, we also reiterated that for the
purposes of the PFS payment policy for dental services inextricably linked to covered medical
services, our statute and regulations require that specific evidence supports the integral

connection between dental services and clinical success in managing hemophilia-related medical
services, and, therefore, we did not expand the examples under § 411.15(i)(3)(i) to include
additional covered medical services for hemophilia. Please refer to CY 2024 final rule (88 FR
79032 through 79033) for more detailed information.
While interested parties have suggested the interaction of oral health care for SCD or
hemophilia, we noted that further research is necessary to find specific evidence supporting
specific medical services for which dental services are inextricably linked to their clinical
success. To gain further understanding of any potential relationship between dental services and
specific covered SCD or hemophilia medical services, we again partnered with researchers at the
Agency for Healthcare Research and Quality (AHRQ) to review available clinical evidence
regarding the relationship between dental services and covered SCD or hemophilia medical
services. AHRQ created two rapid response reports, which summarized recent evidence, aiming
to inform CMS policy development related to the possible linkage between dental services and
treatment modalities and services for SCD or hemophilia patients. For more detailed information
about the search strategies and findings, please refer to the two AHRQ rapid response reports
available at https://effectivehealthcare.ahrq.gov/products/sickle-cell-dental/research and
https://effectivehealthcare.ahrq.hgov/products/hemophilia-dental/research.
1. Consideration of Dental Services that may be Inextricably Linked to Covered Services for the
Treatment of Sickle Cell Disease
As stated in the AHRQ rapid response report, SCD is a genetic hematologic disorder
affecting approximately 100,000 individuals in the U.S.,
162
characterized by abnormal
hemoglobin formation in red blood cells, leading to complications such as pain, infection, acute
chest syndrome, and stroke. Hydroxyurea, a commonly used therapeutic measure,
163
which
162
Prevention CDC. Learn more about sickle cell disease, Centers for Disease Control and Prevention. (Available at
https://www.cdc.gov/ncbddd/sicklecell/index.html).
163
U.S. Food and Drug Administration. FDA approves hydroxyurea for treatment of pediatric patients with sickle
cell anemia, U.S. Food and Drug Administration. (Available at https://www.fda.gov/drugs/resources-information-
approved-drugs/fda-approves-hydroxyurea-treatment-pediatric-patients-sickle-cell-anemia).

increases fetal hemoglobin levels, which has a higher affinity for oxygen than adult hemoglobin.
This therapy can prevent sickling or curvature of red blood cells and help SCD patients. SCD
patients can experience dental complications due to ischemia,
164
including delayed tooth
eruption, enamel disorders, and tongue cell changes. Ischemic and inflammation affects enamel,
gums, and jawbones that can cause paresthesia and tooth pain. In response to a producing more
red blood cells to make up for anemia, bone marrow expansion can lead to malocclusion and
reduced bone density.
165,166
The AHRQ’s rapid response report highlights that despite
hydroxyurea's long-term use to treat SCD, uncertainty remains regarding whether dental care
before, during, or after treatment of SCD with hydroxyurea (and other lesser prescribed
treatments) influences clinical outcomes in patients.
In their rapid response, the Preferred Reporting Items for Systematic Reviews and Meta-
Analyses (PRISMA) flow diagram, a flow diagram used in systematic reviews and meta-
analyses intended to describe findings of the review, revealed that initially, 762 records were
identified from large databases, and 934 records were identified from grey literature sources
covering 57 years. Following the exclusion of ineligible studies, a total of five unique records
were included, extracted, and evaluated in the rapid response report. The rapid response report
found that no studies examined the specific impact of dental care on SCD treatment outcomes;
however, the rapid response identified three relevant clinical practice guidelines and two reviews
with recommendations for dental care for patients with SCD. According to the rapid response,
all guidelines and reviews highlighted the importance of preventive dental measures, with the
Sickle Cell Society in the UK recommending the inclusion of a hematologist in the dental care
team for SCD patients. However, these guidelines primarily focused on managing SCD during
dental services rather than the impact of dental care on the improvement of SCD treatment
164
da Fonseca M, Oueis HS, Casamassimo PS. Sickle cell anemia: a review for the pediatric dentist. Pediatr Dent
2007;29(2):159-69. (In eng).
165
Hsu LL, Fan-Hsu J. Evidence-based dental management in the new era of sickle cell disease: A scoping review. J
Am Dent Assoc 2020;151(9):668-677.e9. (In eng). DOI: 10.1016/j.adaj.2020.05.023.
166
Kane SF. The effects of oral health on systemic health. Gen Dent 2017;65(6):30-34. (In eng).

outcomes, which differs from the regulatory standard in § 411.15(i)(3)(i) that the dental services
are inextricably linked to, and substantially related and integral to the clinical success of, other
covered services.
The two rapid reviews with recommendations highlighted several key areas of dental
management for SCD patients. They emphasized the importance of preventive dental care to
reduce the risk of oral infections, periodontal diseases, and major dental procedures.
Additionally, the studies recommended that a complete blood count should be evaluated prior to
invasive dental procedures to mitigate infection risks in SCD patients.
167,168
Overall, the findings
from the AHRQ rapid response reports underscore the gaps in the current literature concerning
the management of oral health care for individuals with SCD. The body of evidence evaluating
dental services before, during, or after the treatment of SCD is lacking in primary clinical data
and is limited to available clinical practice guidelines and reviews with recommendations. While
guidelines and reviews have addressed particular aspects of dental care, such as early
intervention and the importance of collaboration between dentists and hematologists, there is an
absence of primary evidence that informed the potential effect of dental care on SCD treatment
outcomes.
2. Consideration of Dental Services that may be Inextricably Linked to Covered Services for the
Treatment of Hemophilia
As stated in their report, hemophilia is a rare inherited disorder characterized by the
deficiency or absence of blood-clotting proteins, affecting approximately 20,000-33,000 males in
the U.S.
169
There are three distinct types of hemophilia (A, B, and C) according to the specific
clotting factor that is deficient or missing (VIII, IX, and XI, respectively). Treatment for all
167
Kawar N, Alrayyes S, Yang B, Aljewari H. Oral health management considerations for patients with sickle cell
disease. Dis Mon 2018;64(6):296-301. (In eng). DOI: 10.1016/j.disamonth.2017.12.005.
168
Kakkar M, Holderle K, Sheth M, Arany S, Schiff L, Planerova A. Orofacial Manifestation and Dental
Management of Sickle Cell Disease: A Scoping Review. Anemia 2021;2021:5556708. (In eng). DOI:
10.1155/021/5556708.
169
Prevention CfDCa. Hemophilia Treatment. (https://www.cdc.gov
/ncbddd/hemophilia/treatment.html#:~:text=The%
20best%20way%20to%20treat,concentrates%2C%20into%20a%20person's%20vein.).

three types of hemophilia is similar but may vary based on the severity of disease, and typically
involves replacing the deficient or missing clotting factor.
170
Anticoagulation issues in
hemophilia patients can pose challenges for dental specialists due to the increased risk of
secondary bleeding after oral surgery.
171
Severe hemophilia patients face spontaneous bleeding
in joints, muscles, or soft tissues, sometimes life-threatening, while mild to moderate cases may
experience excessive bleeding, including after dental extractions.
172
According to the rapid response reports provided by AHRQ, clotting factor replacement
therapy is the standard treatment for individuals with hemophilia. Replacement therapy, which
can be self-administered or given at a hemophilia treatment center, can be used to stop a
spontaneous bleeding episode (episodic care), or to prevent bleeding from occurring
(prophylactic care). However, consistent/prolonged factor replacement can cause antibody
inhibitor development.
173
While clotting factor replacement therapy remains the standard
treatment for hemophilia, uncertainty persists regarding whether dental care before, during, or
after treatment of hemophilia improves clinical outcomes in patients. Excessive bleeding, often
associated with invasive or traumatic dental procedures, poses challenges when treating patients
with hemophilia. Hence, the timing of dental treatment in relation to factor replacement therapy
may influence outcomes.
In AHRQ's rapid response, a total of 1,414 records from large databases and 2,238
records from grey literature, spanning 73 years, were identified. Strictly following the exclusion
criteria depicted in the PRISMA diagram, the response included, extracted, and evaluated a total
of four unique publications in this rapid response. The rapid response report identified four
170
Ibid.
171
Givol N, Hirschhorn A, Lubetsky A, Bashari D, Kenet G. Oral surgery-associated postoperative bleeding in
haemophilia patients - a tertiary centre's two decade experience. Haemophilia 2015;21(2):234-240. (In eng). DOI:
10.1111/hae.12573.
172
Oomen I, Camelo RM, Rezende SM, et al. Determinants of successful immune tolerance induction in hemophilia
A: systematic review and meta-analysis. Res Pract Thromb Haemost 2023;7(1):100020. (In eng). DOI:
10.1016/j.rpth.2022.100020.
173
Castaman G, Matino D. Hemophilia A and B: molecular and clinical similarities and differences. Haematologica
2019;104(9):1702-1709. (In eng). DOI: 10.3324/haematol.2019.221093.

publications: two practice guidelines and two reviews with recommendations. The included
publications advised hemophilia patients to consult hematologists or hemophilia treatment
centers before dental procedures. They emphasized interdisciplinary collaboration between
dentists and hematologists for treatment planning. Included guidelines recommended
performing dental services during hemophilia treatment with coagulation factor replacement
therapy. However, all four reviewed publications mainly addressed hemophilia A and B, with
little mention of hemophilia C. Similar to the findings related to SCD, the body of evidence
evaluating dental services before, during, or after the treatment of hemophilia is lacking in
primary clinical data and is currently limited to available guidelines. These guidelines
emphasize the importance of professional dental care during hemophilia treatment with clotting
factor replacement therapy to reduce bleeding complications, rather than the inextricable link
between certain dental services and covered services for hemophilia, which is the regulatory
standard in § 411.15(i)(3)(i) for clinical scenarios under which payment can be made for dental
services under Medicare Parts A and B.
After reviewing AHRQ’s comprehensive rapid reviews for both SCD and hemophilia, we
found the evidence related to the linkage between dental services and outcomes for covered
medical services for both SCD and hemophilia lacking in the current research and literature.
Both rapid responses noted a limited number of studies examining the impact of dental care on
outcomes for individuals with SCD or hemophilia. Currently, the evidence base does not appear
to support that dental services may be inextricably linked to covered services for SCD or
hemophilia. Also, the body of evidence evaluating dental services before, during, or after the
treatment of SCD and hemophilia lacks primary clinical data and relies on available guidelines
and reviews. Given limited information, however, both the SCD and hemophilia rapid responses
support the need for preventive care and patient education as essential practices for both SCD
and hemophilia patients to minimize the likelihood of oral infections, periodontal disease, and
major dental procedures. In addition, both rapid response reports recommend collaborative

efforts between dentists, hematologists, and specialized clinics as crucial for improved patient
care, despite the lack of primary evidence informing the potential effect of dental care on
treatment. While both rapid response reports discuss their findings on the importance of a
multidisciplinary approach, both rapid response reports also found that the current reviews and
guidelines do not address dental care as a standard of care that is inextricably linked to
hemophilia or SCD treatment. Instead, their focus was on managing the respective conditions
during dental services, not on the inextricable linkage between the dental and medical services.
In conclusion, interested parties, including industry organizations, public commenters,
and organizations submitting through the public submissions process, requested that CMS
consider the conditions of SCD and hemophilia for the purposes of the Medicare Parts A and B
payment policy for dental services that are inextricably linked to other covered services. As part
of our commitment to exploring the inextricable link between dental and covered services
associated with SCD and hemophilia, we partnered with AHRQ to generate comprehensive rapid
responses on these topics. However, the AHRQ’s rapid response reports show that the current
evidence base does not appear to support that dental services may be inextricably linked to
services for SCD or hemophilia within the meaning of the standard at § 411.15(i)(3), and we are
not proposing to add these conditions to our regulation. Moreover, the findings of the AHRQ
rapid response reports highlight that this area merits further study by researchers and industry in
order to further explore potential connections between dental services and improved outcomes
for individuals with SCD or hemophilia. Given the new and evolving therapies and treatments in
this space, we will consider conducting additional evaluations as new studies are carried out to
examining the impact of dental services on SCD and hemophilia outcomes and will take any
future studies into consideration.
We continue to seek clinical evidence demonstrating the integral connection between
dental services and other covered services for SCD and hemophilia, and we welcome any
comments or literature regarding these two conditions. We are not proposing to amend §

411.15(i)(3)(i) as we have not identified additional dental services that are inextricably linked to
certain services associated with SCD or hemophilia. However, we remain open to considering
any such services identified by public commenters, and, if sufficient evidence is presented, we
may consider adding such services to our regulations in the final rule. In addition, we encourage
interested parties to supply additional submissions for consideration in future PFS rulemaking
through the public submission process, which may include relevant medical evidence, peer-
reviewed literature, clinical guidelines, or supporting documentation as described in section
II.J.1.c. of this proposed rule.
c. Submissions Received Through Public Submission Process
As we have in the CY 2023 and CY 2024 PFS final rules, we continue to encourage
interested parties to engage with us regularly and to submit recommendations through our public
submissions process for our consideration of additional clinical scenarios where dental services
may be inextricably linked to covered services under § 411.15(i)(3)(i). Through our annual
public submissions process, interested parties should provide clinical evidence and other
documentation to support their recommendations (87 FR 69685). We are using the PFS annual
rulemaking process to discuss public submissions and to consider whether the clinical scenario
described in the submissions should be added to § 411.15(i)(3)(i) as an example of a
circumstance where payment can be made for dental services inextricably linked to other
covered services. Using our annual notice and comment rulemaking process to discuss
submitted recommendations allows the public to comment and submit further medical evidence
and important feedback to assist us in evaluating whether certain dental services furnished in
certain clinical scenarios would meet the standard to permit Medicare payment for the dental
services.
We review clinical evidence included in submissions and public comments in
rulemaking, as well as information and analysis provided by AHRQ in rapid response reports, to
assess whether there is an inextricable link between certain dental services and certain covered
services. We would find that there is an inextricable link where the standard of care for a service
is such that the practitioner would not proceed with the procedure or service without performing
the dental service(s), for example, because the covered services would or could be significantly
and materially compromised absent the provision of the inextricably-linked dental services, or
where dental services are a clinical prerequisite to proceeding with the primary medical
procedure and/or treatment. As such, documentation accompanying recommendations should
include medical evidence to support that certain dental services are inextricably linked to certain
covered services. Specifically, as we specified in the CY 2023 PFS final rule, we request that the
medical evidence included in submissions through the public submissions process should:
(1) Provide support that the provision of certain dental services leads to improved
healing, improved quality of surgery outcomes, and the reduced likelihood of readmission and/or
surgical revisions because an infection has interfered with the integration of the medical implant
and/or interfered with the medical implant to the skeletal structure;
(2) Be clinically meaningful and demonstrate that the dental services result in a material
difference in terms of the clinical outcomes and success of the procedure such that the dental
services are inextricably linked to other covered services; and,
(3) Be compelling to support that certain dental services would result in clinically
significant improvements in quality and safety outcomes (for example, fewer revisions, fewer
readmissions, more rapid healing, quicker discharge, and quicker rehabilitation for the patient)
(87 FR 69686).
This evidence should include at least one of the following:
(1) Relevant peer-reviewed medical literature and research/studies regarding the medical
scenarios requiring medically necessary dental care;
(2) Evidence of clinical guidelines or generally accepted standards of care for the
suggested clinical scenario;
(3) Other ancillary services that may be integral to the covered services; and/or
(4) Other supporting documentation to justify the inclusion of the proposed medical
clinical scenario requiring dental services (87 FR 69686).
Submissions should focus on the inextricably linked relationship between dental services
and other services necessary to diagnose and treat the individual’s underlying medical condition
and clinical status, and whether it would not be clinically advisable to move forward with the
other covered services without performing certain dental services. To be considered for purposes
of CY 2026 PFS rulemaking, submissions through our public submissions process should be
received by February 10, 2025, via email at [email protected]. To
facilitate processing, interested parties should include the words “dental recommendations for
CY 2026 review” in the subject line of their email submission. We continue to stress to
submitters that recommendations must include at least one of the types of evidence listed earlier.
We further note that we may also consider recommendations that are submitted as public
comments during the comment period following the annual publication of the PFS proposed rule.
We thank all those who submitted recommendations for additional clinical scenarios for
which they believe Medicare payment for dental services will be consistent with the policies we
codified at § 411.15(i)(3)(i), under which Medicare payment may be made for dental services
when they are inextricably linked to other covered services. We received thirteen submissions
from various organizations and individuals on or before February 10, 2024. Several submitters
represented dozens or hundreds of other organizations in making these recommendations. We
received one submission after the deadline that presented nominations for clinical scenarios
addressed by other submitters, and a proposal outside the scope of clinical scenarios where
dental services may be inextricably linked to covered medical services under § 411.15(i)(3)(i).
One submitter recommended that oral health care is essential to the success of treatments
for individuals with sickle cell disease and other hematologic disorders. The submitter asserted
that providing appropriate and timely dental care is a crucial component for the successful
treatment of many hematologic diseases, including SCD, hemophilia, and many blood cancers,
such as acute myeloid leukemia, acute lymphoblastic leukemia, chronic lymphocytic leukemia,
chronic myeloid leukemia, and multiple myeloma. The submitter noted that we finalized that
payment can be made for certain dental services prior to or contemporaneously with
chemotherapy, CAR T-Cell therapy, and the administration of high-dose bone-modifying agents
(anti-resorptive therapy), when used in the treatment of cancer. The evidence submitted showed
that among individuals with a sickle cell crisis, those with dental infections were 72% more
likely to be admitted to the hospital than those without dental infections. The same submitter also
requested that we consider payment of dental services following organ transplantations
(including bone marrow or hematopoietic stem cell transplantation (HSTC)) because
maintenance of oral hygiene after HSCT minimizes the severity of oral and dental infections,
which is important because chronic graft versus host disease (cGVHD) is common following
allogeneic HSCT. The submitter stated that frequent dental evaluations of patients with cGVHD
are critical because of the increased rate of dental caries associated with this disease;
furthermore, gingivitis and periodontal disease should be monitored and managed appropriately
to avoid additional infection. Finally, the submitter also stated that multiple tooth extractions
without replacement of dentition leave patients with a poor capacity to eat and may negatively
impact the success of the transplant and quality of life. This submitter also recommended
including cell and gene therapy for SCD as a clinical treatment scenario for future consideration
of potentially inextricably linked dental services.
Another submitter asserted that patients frequently present with complications extending
three years or more following the direct treatment of not only head and neck cancers but also
other cancer types. The submitter also asserted that a comparable trend may be seen in patients
experiencing complications following anti-resorptive drug therapy, for non-cancer-related
conditions. They recommended that payment be available for specific dental services during a
minimum of 2 years post-treatment for head and neck cancer and up to 5 years for those who
have received radiotherapy. They emphasized the current evidence and literature as supporting
the provision of Medicare payment for a minimum of 2 years, with the understanding that some
patients may require dental or oral healthcare beyond this period due to delayed or late-onset
complications, and that the timeline for the emergence of oral or dental complications post-
treatment is not uniformly linear and can significantly vary among patients. They also stated that
literature supports the extension of Medicare payment to include certain dental services furnished
post-anti-resorptive therapy, when used in the treatment of cancer for at least two years
following treatment. Lastly, this submitter stated there is an inextricable risk of the development
of severe dental and oral complications, such as osteoradionecrosis/ medication-related
osteonecrosis of the jaw, in patients who have undergone the specific treatments for which we
have identified certain inextricably linked dental services. They suggested that once patients
receive these treatments, patients are perpetually at risk for developing such complications and
recommended that current Medicare payment for dental services should align with the long-term
healthcare needs of these patients.
We are not accepting the commenters’ suggestion to include specific time limits within
the exception in § 411.15(i)(3). We note again that MACs have the flexibility to determine on a
claim-by-claim basis whether payment can be made for certain dental services for beneficiaries,
such as those receiving other immunotherapies that may involve a lymphodepleting component,
consistent with § 411.15(i)(3). That regulation states the general rule that Medicare Parts A and
B payment can be made for certain dental services that are inextricably linked to, and
substantially related and integral to the clinical success of, covered services; and then provides a
non-exclusive list of examples of clinical scenarios under which payment can be made. Thus, a
MAC has discretion to decide on a case-by-case basis that payment can be made for certain
dental services in other circumstances not specifically addressed under § 411.15(i)(3)(i)
Several submitters recommended that we provide for payment of medically necessary
dental services for individuals with autoimmune diseases who are initiating or undergoing
immunosuppressive or immunomodulator therapy (“immunosuppressive therapy”). They stated
that immunosuppressive therapy can be severely complicated and compromised by oral/dental
disease and conditions. The submitters provided references to numerous clinical studies and
other supporting documentation in support of dental services in these clinical circumstances
being inextricably linked to immunosuppressive therapies for which payment may be made in
accordance with § 411.15(i)(3). They noted that while higher dosing is used for cancer
chemotherapy and organ transplant rejection prevention, the therapy’s duration is generally much
shorter than when used in autoimmune disease therapy and that the longer-term duration of use
for managing symptoms of autoimmune disease can expose patients to ongoing serious risk of
complicating infections for decades. They relayed that the American College of Rheumatology
states it is vital for patients to receive appropriate dental evaluation and prompt treatment so they
can continue their immune suppressant medications. The submitter explained that dental
infections could spread more easily, and therefore faster, when host immunity is compromised
by immunosuppressing/immunomodulating drugs via three pathways for the bacteria to spread:
locally through facial spaces, through the bloodstream, and by aspiration. They stated that
outcomes similar to systemic infection or sepsis and other complications can follow for those
receiving immunosuppressive therapy to treat autoimmune diseases. They recommended
immunosuppressive therapy to treat autoimmune diseases should not proceed until a dental or oral
exam is performed to address the oral complications and/or clear the patient of an oral or dental
infection. We discuss these recommendations and supporting evidence in section II.J.4. of this
proposed rule.
Several submitters recommended that dental treatments can be integral to the clinical
success of covered nephrology-related medical services including services received by
beneficiaries who are immunocompromised by end-stage renal disease (ESRD), chronic kidney
disease (CKD), other renal diseases, as well as kidney transplant candidates maintained on
immunosuppressive medications. They stated that all of these patients are at increased risk of
infection, complications, and malnutrition from dentally sourced pathogens. Submitters stated
that Medicare ESRD beneficiaries on dialysis are at greater risk for developing complications
such as cardiovascular conditions, malnutrition, anemia, and infections, making dental services
more critical to the success of kidney care treatments. They further stated that pre-
transplantation dental care involves eliminating possible sources of oral infection that can lead to
a systemic infection following transplant and that without access to dental services, individuals
on dialysis may not qualify for kidney transplantation or may have severe complications after the
transplant. One of the submitters noted that dialysis clinicians report that many bloodstream
infections (BSI) begin with bacteria in the mouth and that regular dental visits could have a
positive impact on reducing BSI. They added that the Society for Vascular Surgery has noted
that transient bacteremia from dental infections can seed hemodialysis access grafts. Among
strategies to prevent infection of vascular grafts, recommended preoperative measures include
identifying and treating remote site infections, including dental sites. We discuss these
recommendations and supporting evidence in section II.J.2. of this proposed rule.
More than half of the submissions expressed support for almost identical proposals from
a couple of other submitters, recommending that dental services are inextricably linked to
covered medical services used for the treatment of Medicare beneficiaries with diabetes. They
agreed that the delivery of appropriate dental services in accordance with clinical guidelines and
standard of care is substantially related and integral to the optimal outcome of these covered
medical services. They offered clinical studies documenting that treatment of oral infections,
such as periodontitis and its related inflammation, meaningfully improves the treatment and
management of diabetes. They stated, by contrast, the absence of treatment of chronic dental
infections complicates covered medical treatment for the management of diabetes and
exacerbates insulin resistance, worsens glycemic control, and other diabetes related
complications. They noted that the relationship between oral diseases and diabetes mellitus is
complex. Diabetes is known to increase the risk and severity of oral diseases, such as
periodontitis (gum inflammation and bone loss), tooth loss, dry mouth, and oral fungal
infections. Additionally, oral diseases are documented as affecting blood glucose control and
contributing to the development of diabetes complications, such as retinopathy, neuropathy,
cardiovascular disease, and kidney disease. They believe reciprocal management of glycemic
control and periodontal disease decreases risk for and actual cases and severity of diabetes and
periodontal disease. We discuss these recommendations and the supporting clinical evidence in
section II.J.3 of this proposed rule.
2. Proposed Additions to Current Policies Permitting Payment for Dental Services Inextricably
Linked to Other Covered Services
We have received information and requests from interested parties, including entities
submitting information through the public submissions process as well as organizations
providing comments in response to prior rulemaking efforts, that an inextricable linkage exists
between dental services and dialysis treatment services for individuals diagnosed with end-stage
renal disease (ESRD) who are receiving dialysis services, particularly those experiencing
comorbidities. Commenters and submitters have stated that dental treatment is inextricably
linked and integral, and substantially related to the clinical success and outcomes of covered
dialysis medical services.
In the CY 2024 PFS final rule, we stated that commenters had provided comments in
response to the CY 2024 PFS proposed rule supporting the coverage of annual dental
examinations, and treatment as clinically indicated, for individuals with chronic kidney disease
and ESRD. The commenters stated that chronic immunosuppression increases the risk of dental
infections leading to potentially deadly complications including BSI, peritoneal dialysis-
associated peritonitis, and the exacerbation of chronic cardiovascular conditions. They also
stated that when established by patient-specific medical and dental parameters, dental services
can be unquestionably integral to the outcome of covered medical procedures. We thanked the
commenters for the information they submitted regarding these suggestions; however, at that
time, commenters did not provide sufficient evidence to support an inextricable link between

certain dental services and certain covered services for chronic kidney disease and ESRD (88 FR
79034).
Additionally, submitters provided information through the public submissions process as
described in section II.J.1.c. of this proposed rule for our consideration in CY 2025 rulemaking.
The submitters stated that there is a connection between dental services to identify and address
dental or oral infections and covered medical services for individuals receiving dialysis in the
treatment of ESRD.
ESRD is a medical condition in which a person's kidneys successively experience loss of
functionality on a permanent basis, leading to the need for a regular course of long-term dialysis
or a kidney transplant to maintain life.
174
Chronic kidney disease (CKD) is a progressively debilitating disease and is marked by
the presence of kidney damage or reduction in the kidneys’ filtration rate. CKD is a state of
progressive loss of kidney function, in that the disease worsens over time and cannot be reversed,
ultimately resulting in the need for renal replacement therapy, generally dialysis or
transplantation.
175
The Kidney Disease Improving Global Outcomes (KDIGO) Foundation
established guidelines that define five stages of CKD using kidney damage markers, including
factors that determine proteinuria (level of protein in the urine) and glomerular filtration rate
(level of kidney function/filtration) in its KDIGO 2012 Clinical Practice Guideline for the
Evaluation and Management of Chronic Kidney Disease.
176
Chronic kidney disease is generally
defined as the presence of two factors (glomerular filtration rate [GFR] less than 60 mL/min and
albumin greater than 30 mg per gram of creatinine) along with abnormalities of kidney structure
or function for greater than three months. Stage 5 of CKD is labeled end-stage renal disease
174
https://www.cms.gov/medicare/coordination-benefits-recovery/overview/end-stage-renal-disease-esrd.
175
https://www.ncbi.nlm.nih.gov/books/NBK535404/.
176
https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pd.f

(ESRD) with a GFR of less than 15 mL/min.
177
According to the NIH, more than 500,000
people in the United States live with ESRD.
178
Per the American Academy of Family Physicians, individuals with ESRD are typically
referred to nephrologists for the development of treatment plans. Collectively the various
modalities utilized to replicate kidney function are referred to as renal replacement therapy
(RRT). Most ESRD patients are treated with dialysis, regardless of whether transplantation
ultimately occurs. Generally, kidney transplantation typically yields the best patient outcomes;
however, not all patients with ESRD are eligible for or able to undergo transplantation, and
therefore continue dialysis treatment.
179
Standards of medical care for CKD outline the need for
monitoring for signs of progression of the disease and early referral to specialists for RRT.
180
Dialysis is generally supplied via two primary modes: hemodialysis or peritoneal dialysis. In
hemodialysis, blood is filtered through a dialyzer, outside of the body. A dialyzer is sometimes
referred to as an “artificial kidney.”
181
To access the circulatory system, several access points
may be placed and utilized, including an arteriovenous (AV) fistula, AV graft, and in some cases
a central venous catheter.
182,183,184
In peritoneal dialysis, a fixed catheter is placed in the
abdomen, and dialysis solution is administered into the abdomen. The solution absorbs wastes
and excess fluid from the patient’s body.
185,186
Submissions we received through the public submissions process for consideration in CY
2025 rulemaking provided information regarding the potential linkage between dental services
and specific covered medical services associated with ESRD and dialysis including:
● CPT codes 36901-36906: Dialysis circuit procedures;
177
https://www.ncbi.nlm.nih.gov/books/NBK499861/.
178
https://www.ncbi.nlm.nih.gov/books/NBK499861/.
179
Am Fam Physician. 2021;104(5):493-499. https://www.aafp.org/pubs/afp/issues/2021/1100/p493.html.
180
https://pubmed.ncbi.nlm.nih.gov/29763036/.
181
https://www.niddk.nih.gov/health-information/kidney-disease/kidney-failure/hemodialysis.
182
https://www.ncbi.nlm.nih.gov/books/NBK563296/.
183
https://www.mayoclinic.org/tests-procedures/hemodialysis/about/pac-20384824.
184
https://www.cdc.gov/dialysis/patient/.
185
https://www.mayoclinic.org/tests-procedures/peritoneal-dialysis/about/pac-20384725
186
https://www.niddk.nih.gov/health-information/kidney-disease/kidney-failure/peritoneal-dialysis.

● CPT codes 90935, 90937, 90940: Hemodialysis procedures;
● CPT code 90961: Physician or other qualified healthcare professional visits for ESRD;
● CPT codes 90989-90999: Other dialysis procedures; and,
● DRG code 872: Hospitalization for septicemia or severe sepsis.
We note that Medicare provides coverage for individuals with ESRD, regardless of age,
when certain requirements are met.
187
We also note that dialysis procedures may be utilized for individuals who do not have
ESRD in the treatment of acute intoxication or poisoning. For example, in the case of a patient
experiencing poisoning, dialysis hemoperfusion may be employed, which passes the blood
through a column packed with granules that include a resin that act as absorbents. In this
procedure, physicochemical properties of an absorbent are used to remove toxins. Conversely, in
hemodialysis utilized in the treatment of ESRD, there is a concentration gradient between the
blood and the solvent across the dialysis membrane.
188
We note that the patient accessing
dialysis treatment for the treatment of acute intoxication or poisoning would not present with the
same diagnostic profile, treatment needs, nor face the same risks of immunodeficiency and
infection as individuals with ESRD as described below.
189
Periodontal diseases and dental caries are the main chronic infectious diseases of the oral
cavity. Periodontal diseases include a group of chronic inflammatory diseases that affect the
periodontal supporting tissues of teeth and encompass destructive and nondestructive diseases.
Gingivitis is inflammation of the soft tissue without apical migration of the junctional
epithelium. It is a reversible, nondestructive disease that does not involve loss of periodontal
tissues. Periodontitis is inflammation of the periodontium that is accompanied by apical
187
https://www.cms.gov/medicare/coordination-benefits-recovery/overview/end-stage-renal-disease-esrd.
188
Durakovic Z. Combined hemoperfusion and hemodialysis treatment of poisoning with cholinesterase inhibitors.
Korean J Intern Med. 1993 Jul;8(2):99-102. doi: 10.3904/kjim.1993.8.2.99. PMID: 8031730; PMCID:
PMC4532091. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4532091.
189
Ouellet G, Bouchard J, Ghannoum M, Decker BS. Available extracorporeal treatments for poisoning: overview
and limitations. Semin Dial. 2014 Jul;27(4):342-9. https://pubmed.ncbi.nlm.nih.gov/24697909/.

migration of the junctional epithelium, leading to destruction of the connective tissue attachment
and alveolar bone loss.
190
Periodontitis serves as a prime example of a disrupted balance between the local
microbiome and the host's inflammatory response, a condition known as dysbiosis. Although the
inflammatory response is ostensibly triggered to manage the microbial threat, it proves to be
ineffective and inadequately regulated in individuals prone to the condition. This leads to the
inflammatory destruction of the periodontium, which encompasses the tissues that encase and
support the teeth, including the gingiva, periodontal ligament, and alveolar bone. Without
appropriate treatment, this disease can progress to tooth loss, adversely affecting chewing,
appearance, and overall quality of life.
191
In 2017, the American Academy of Periodontology (AAP) and the European Federation
of Periodontology (EFP) co-presented the 2017 Classification of Periodontal and Peri-Implant
Diseases and Conditions. This disease classification framework serves to guide treatment
planning for periodontitis and aims to support customized approaches to patient care. The
revised classification includes a multi-dimensional staging and grading system for periodontitis
classification, a recategorization of various forms of periodontitis, and a classification for peri-
implant diseases and conditions.
192
Individuals with ESRD experience compromised immune systems as the immune system
and the kidneys are closely integrated and interdependent. In healthy individuals, the kidneys
contribute to immune homeostasis and regulation, while components of the immune system
mediate many acute forms of renal disease and play a central role in the progression of chronic
kidney disease. A dysregulated immune system can have either direct or indirect renal effects.
193
190
Albandar, J. M. (2005). Epidemiology and risk factors of periodontal diseases. Dent Clin North Am, 49(3), 517-
532, v-vi. doi:10.1016/j.cden.2005.03.003.
191
Hajishengallis, G., & Chavakis, T. (2021). Local and systemic mechanisms linking periodontal disease and
inflammatory comorbidities. Nature Reviews Immunology, 21(7), 426-440. doi:10.1038/s41577-020-00488-6.
192
https://www.perio.org/wp-content/uploads/2019/08/Staging-and-Grading-Periodontitis.pdf.
193
Tecklenborg J, Clayton D, Siebert S, Coley SM. The role of the immune system in kidney disease. Clin Exp
Immunol. 2018 May;192(2):142-150. doi: 10.1111/cei.13119. Epub 2018 Mar 24. PMID: 29453850; PMCID:
PMC5904695.

Moreover, uremia, the buildup of waste products in the blood that occurs as a result of declining
or decreasing kidney function, can lead to inflammation and reduction in the immune system’s
ability to function as evidenced by an increased risk of viral-associated cancers, increased
susceptibility to infections, and decreased vaccination responses in patients with ESRD.
194
ESRD is also characterized by diminished endocrine and metabolic functions of the kidney with
subsequent retention and accumulation of toxic metabolites.
195
Additionally, the presence of
indwelling catheters and grafts utilized for the administration of dialysis, malnutrition,
dysregulated inflammation, and acquired immune dysfunction due to uremia contribute to the
immune deficiency in ESRD and increase susceptibility to infection.
196
Notably, infection is the
second leading cause of death in hemodialysis patients.
197,198
Several submitters providing information through the public submissions process stated
that comorbidities frequently occur in the ESRD patient population and can cause complications
for the patient, potentially jeopardizing the outcomes of the dialysis treatment. For example,
submitters stated that comorbid diabetes can result in clinical complications for individuals
receiving dialysis services in the treatment of ESRD, stating that periodontitis can worsen blood
glucose control in diabetics by increasing levels of inflammatory mediators and may interfere
with insulin, resulting in clinical complications. Additionally, periodontitis is associated with
oral health-related quality of life in individuals with ESRD. One study evaluated whether
periodontitis may be independently associated with oral health-related quality of life (OHRQoL)
in individuals with ESRD. Researchers assessed 180 adults with ESRD and evaluated for
194
Betjes MG. Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol. 2013
May;9(5):255-65. doi: 10.1038/nrneph.2013.44. Epub 2013 Mar 19. PMID: 23507826.
https://pubmed.ncbi.nlm.nih.gov/23507826/.
195
Costantinides F, Castronovo G, Vettori E, Frattini C, Artero ML, Bevilacqua L, Berton F, Nicolin V, Di Lenarda
R. Dental Care for Patients with End-Stage Renal Disease and Undergoing Hemodialysis. Int J Dent. 2018 Nov
13;2018:9610892. doi: 10.1155/2018/9610892. PMID: 30538746; PMCID: PMC6258100.
196
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7404977/.
197
U.S. Renal Data System. USRDS 2015 Annual Data Report: Atlas of End-Stage Renal Disease in the United
States, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases,
2015.
198
Dalrymple LS, et al. Infection-related hospitalizations in older patients with ESRD. Am. J. Kidney Dis.
2010;56:522–530. doi: 10.1053/j.ajkd.2010.04.016.

impacts on various domains, and found that periodontitis exerts an influence on OHRQoL in
individuals with ESRD, with a more severe condition impacting different domains.
199
Moreover,
a prospective cohort study aimed to determine the association between an index of
radiographically assessed oral health, Panoramic Tomographic Index (PTI), and cardiovascular
and all-cause mortality, major adverse cardiovascular events (MACEs) and episodes of
bacteremia and laboratory measurements during a three-year prospective follow-up in chronic
kidney disease (CKD) stage 4–5 patients not on maintenance dialysis at baseline. The study
showed that radiographically assessed and indexed dental health is independently associated with
all-cause and cardiovascular mortality and MACEs in CKD stage 4–5 patients transitioning to
maintenance dialysis and renal transplantation during follow-up (but not with the incidence of
bacteremia).
200
Submitters providing information through the public process also stated that BSI, poor
glycemic control, and other complications arising from dental infection can jeopardize the
clinical success of medical therapies employed to manage ESRD. Research provided by
submitters described that issues and changes in the mouth and oral cavity, such as periodontitis
and other consequences of poor oral health, frequently occur in patients with CKD and may
contribute to increased morbidity and mortality because of systemic consequences such as
inflammation, infections, protein-energy wasting, and atherosclerotic complications.
201
Several submitters also stated that addressing oral health issues, including identifying and
resolving dental infections through the provision of dental and oral services, can be inextricably
linked and integral and related to the clinical success of Medicare covered dialysis services for
199
Oliveira, L. M., Sari, D., Schoffer, C., Santi, S. S., Antoniazzi, R. P., & Zanatta, F. B. (2020). Periodontitis is
associated with oral health-related quality of life in individuals with end-stage renal disease. Journal of Clinical
Periodontology, 47(3), 319-329. doi:10.1111/jcpe.13233.
200
Jarvisalo, M. J., Jokihaka, V., Hakamaki, M., Lankinen, R., Helin, H., Koivuviita, N. S., . . . Metsarinne, K.
(2021). Dental health assessed using panoramic radiograph and adverse events in chronic kidney disease stage 4-5
patients transitioning to dialysis and transplantation-A prospective cohort study. PLOS ONE, 16(9), e0258055.
doi:10.1371/journal.pone.0258055.
201
Harun Akar, Gulcan Coskun Akar, Juan Jesus Carrero, Peter Stenvinkel, and Bengt Lindholm. Systemic
Consequences of Poor Oral Health in Chronic Kidney Disease Patients, Clin J Am Soc Nephrol 6: 218–226, 2011.
doi: 10.2215/CJN.05470610.

the treatment of ESRD. The submitters stated that the consequences of poor oral health are
worse for ESRD patients than the general population due to ESRD patient characteristics such as
advanced age, higher prevalence of comorbid diabetes, polypharmacy, and impaired immune
function, and that medically necessary dental care may improve the clinical success of the
dialysis services.
A few submitters supplied a general position paper on the need for dental care and
services in the ESRD patient population receiving dialysis services, describing the unique risks
for individuals with ESRD and the increased risk of infection from oral sources. Specifically,
the position paper states that “oral diseases represent a potential and preventable cause of poor
health outcomes in people with ESRD due to their relation to infection, inflammation, and
malnutrition. Oral health represents a potential determinant of health outcomes in patients with
end-stage renal diseases (ESRD).”
202
Several submitters also provided a cohort outcomes study
of 675 randomly selected individuals receiving peritoneal dialysis services.
203
The study
outcomes described that “poor oral health was associated with lower educational levels, diabetes,
older age, marriage, and worse nutritional indicators (including lower time-averaged serum
albumin and phosphate concentrations).”
204
The research further isolated that poor oral health is independently associated with an
increased risk of peritonitis, an infection of the peritoneum where the peritoneal access graft is
placed, and mortality in patients receiving peritoneal dialysis. The authors describe that “after
adjusting for age, sex, comorbidities, serum albumin, shared frailty by study sites, and PD
vintage, poor oral health was associated with increased risks of peritonitis (adjusted hazard ratio
[HR] = 1.45, 95 percent confidence interval [CI]: 1.06–2.00) and all-cause mortality (adjusted
202
Costantinides F, Castronovo G, Vettori E, Frattini C, Artero ML, Bevilacqua L, Berton F, Nicolin V, Di Lenarda
R. Dental Care for Patients with End-Stage Renal Disease and Undergoing Hemodialysis. Int J Dent. 2018 Nov
13;2018:9610892. doi: 10.1155/2018/9610892. PMID: 30538746; PMCID: PMC6258100.
203
Sirirat Purisinsith, Patnarin Kanjanabuch, Jeerath Phannajit, Bruce Robinson, Kriang Tungsanga, et al. “Oral
Health-Related Quality of Life, A Proxy of Poor Outcomes in Patients on Peritoneal Dialysis.” doi:
https://doi.org/10.1016/j.ekir.2022.07.008 (August 5, 2022).
204
Ibid.

HR = 1.55, 95 percent CI: 1.04–2.32) but not hemodialysis (HD) transfer (adjusted HR = 1.89,
95 percent CI: 0.87–4.10) compared to participants with good oral health.” Furthermore, the
study explained that “poor oral health status was present in one-fourth of peritoneal dialysis
patients and was independently associated with a higher risk of peritonitis and death.”
205
Moreover, submitters provided information that suggests that patients with ESRD receiving
hemodialysis services and receiving preventive oral and dental services experience increased
survival while those not receiving dental services were associated with increased mortality. A
prospective cohort outcomes study of 4,205 hemodialysis patients assessed the impact of dental
health on mortality from 2010 to 2012. The study described that “in adults treated with
hemodialysis, poorer dental health was associated with early death, whereas preventive dental
health practices were associated with longer survival.”
206
Additionally, in a systematic review supplied by several submitters, studies show that
patients on RRT (for example hemodialysis, peritoneal dialysis, and/or transplantation)
experience a high prevalence of dental caries, common chronic infection resulting from tooth-
adherent cariogenic bacteria.
207
The observational data presented in the review suggests a link
between oral health and mortality in patients on RRT.
208
The review highlighted the need for
further research in this area but also stated that improved, multidisciplinary, patient-centered
dental care concepts are required to support dental and overall oral health in individuals on RRT.
Several submitters also noted that the Society for Vascular Surgery has stated that
transient bacteremia from dental infections can seed hemodialysis access grafts. Among
strategies to prevent infection of vascular grafts, recommended preoperative measures include
205
Ibid.
206
See, e.g., Palmer S. C., Ruospo M., Wong G., et al. Oral-D study investigators. Dental health and mortality in
people with end-stage kidney disease treated with hemodialysis: a multinational cohort study. American Journal of
Kidney Diseases. 2015;66:666–676.
207
https://www.ncbi.nlm.nih.gov/books/NBK551699/.
208
Deborah Kreher et.al., Prevalence of Dental Caries in Patients on Renal Replacement Therapy—A Systematic
Review J. Clin. Med. 2023, 12, 1507. https://doi.org/10.3390/jcm12041507.

identifying and treating remote site infections, including dental or oral sites of infection.
209, 210
Statements regarding best practices for managing infection control advise that sources of
infection, including those within the oral cavity, should be addressed in order to minimize the
risk of broader infection in the ESRD patient receiving hemodialysis.
211
In conclusion, the evidence base indicates that evaluation for and treatment of oral
infection leads to improved outcomes and reduced risk of mortality for individuals with ESRD
receiving covered dialysis services.
In the CY 2023 PFS final rule, we agreed with commenters that there is clinical evidence
to support that medically necessary dental care may advance the clinical success of organ
transplants and finalized that payment can be made under Medicare Parts A and B for dental
services such as dental examinations, including necessary treatment, performed as part of a
comprehensive workup prior to organ transplant surgery and medically necessary diagnostic and
treatment services immediately necessary to eliminate or eradicate the infection or its source that
are provided before transplantation because such services are inextricably linked to, and
substantially related and integral to the clinical success of, the organ transplant procedure (87 FR
69676).
Furthermore, we stated that we appreciated commenters’ feedback regarding those
individuals who are awaiting organ transplantation and the commenters’ request that Medicare
provide payment for medically necessary dental services prior to transplantation. We described
that in a case where an individual is awaiting organ transplantation, we believe that it is
appropriate for Medicare to provide payment for, including but not limited to, an oral or dental
examination, and medically necessary diagnosis and treatment for only those services that are
209
Surgical Site Infection Toolkit, CDC, SSI Toolkit Activity C: ELC Prevention Collaboratives (cdc.gov).
210
Pear S, Patient Risk Factors and Best Practices for Surgical Site Infection Prevention,
https://www.halyardhealth.com/wp-content/uploads/patient_risk_factors_best_practices_ssi.pdf.
211
Ibid.
considered immediately necessary to eliminate or eradicate the infection or its source prior to the
organ transplant (87 FR 69676).
In consideration of research and recommendations provided by the public and our
analyses of the studies and research available regarding the connection between dental services
and the clinical success of dialysis services for individuals with ESRD, we believe that dental
services to diagnose and treat infection prior to dialysis services in the treatment of ESRD
represent a clinically analogous scenario to dental services for which Medicare payment under
Parts A and B is currently permitted when furnished in the inpatient or outpatient setting, such as
prior to organ transplant. The clinical evidence supports that the medically necessary dental care
may similarly advance the clinical success of dialysis services in the treatment of ESRD because
an oral or dental infection can present substantial risk to the success and outcomes of these
procedures (including the risk of systemic infection, BSI, sepsis, and death).
As such, we believe that if a patient requiring dialysis services in the treatment of ESRD
has an oral infection, the success of those dialysis services could be compromised if the infection
is not properly diagnosed and treated prior to the covered medical services. Without an oral or
dental examination to identify such an infection and the provision of necessary treatment, such as
restorative dental services, to eradicate the infection prior to the dialysis procedure, the patient’s
ability to complete the dialysis services could be seriously complicated or compromised and the
risk of infection would further increase the risk of mortality for the patient.
Examples of restorative dental services to eradicate infection could include: extractions
(removal of the entire infection, such as pulling of teeth - for example, CDT D7140, D7210),
restorations (removal of the infection from tooth/actual structure, such as fillings - for example,
CDT D2000-2999), periodontal therapy (removal of the infection that is surrounding the tooth,
such as scaling and root planning - for example, CDT D4000-4999, more specifically D4341,
D4342, D4335 and D4910), or endodontic therapy (removal of infection from the inside of the
tooth and surrounding structures, such as root canal - for example, CDT D3000-3999).
If such an infection is not treated prior to dialysis services in the treatment of ESRD, then
there is an increased likelihood for morbidity and mortality resulting from spreading of the local
infection to BSI and sepsis. Likewise, we believe that infections occurring during the course of
dialysis treatment should similarly be addressed and resolved in order to minimize the risk of
infection and death for the patient with ESRD receiving dialysis services.
Because an oral or dental infection can present substantial risk to the success of dialysis
treatment for ESRD, we believe dental services furnished to identify, diagnose, and treat oral or
dental infections prior to or contemporaneously with dialysis services in the treatment of ESRD
are not in connection with the care, treatment, filling, removal, or replacement of teeth or
structures directly supporting teeth, but instead are inextricably linked to, and substantially
related and integral to the clinical success of, these other covered medical services. We note
that, in these circumstances, the necessary treatment to eradicate an infection may not be the
totality of recommended dental services for a given patient. For example, if an infected tooth is
identified in a patient requiring dialysis services in the treatment of ESRD, the necessary
treatment would be to eradicate the infection, which could result in the tooth being extracted.
Additional dental services, such as a dental implant or crown, may not be considered
immediately necessary to eliminate or eradicate the infection or its source prior to surgery.
Therefore, such additional services would not be inextricably linked to, and substantially related
and integral to the clinical success of, Medicare-covered dialysis services when used in the
treatment of ESRD. As such, no Medicare payment would be made for the additional services
that are not immediately necessary prior to or contemporaneously with dialysis for ESRD to
eliminate or eradicate the infection.
In conclusion, we are proposing to add this clinical scenario to the examples of clinical
scenarios under which payment can be made for certain dental services in our regulation at §
411.15(i)(3)(i)(A). Specifically, we propose to amend the regulation at paragraph A to include
dental or oral examination performed as part of a comprehensive workup in either the inpatient
or outpatient setting prior to Medicare-covered dialysis services when used in the treatment of
ESRD; and medically necessary diagnostic and treatment services to eliminate an oral or dental
infection prior to, or contemporaneously with Medicare-covered dialysis services when used in
the treatment of ESRD. We seek comments on all aspects of this proposal.
3. Request for Comment on Dental Services Integral to Specific Covered Services to Treat
Diabetes
As described in section II.J.1.c. of this proposed rule, we have received information from
interested parties, including submitters providing evidence through the public submissions
process as well as commenters on prior proposed rules suggesting that dental services are
inextricably linked to treatment services for individuals with diabetes mellitus. Several
interested parties using the public submissions process have urged us to provide Medicare
payment for dental services for individuals diagnosed with diabetes for consideration in CY 2025
rule making. These submissions included information and references supporting oral and dental
treatment of advanced periodontitis among individuals with diabetes to improve markers related
to management of the diabetes.
Submitters stated that clinical studies demonstrate that dental treatments for oral
infections, such as advanced periodontitis and related inflammation, meaningfully advance and
improve the treatment of, management of, and outcomes for patients with diabetes. Submitters
also stated that conversely, the absence of treatment of chronic dental infections in turn
complicates covered medical treatment for the management of diabetes and potentially
exacerbates insulin resistance, worsens glycemic control, and other diabetes-related
complications, leading to poor outcomes for the individuals with diabetes. Submitters also noted
that studies demonstrate cost savings when dental services are employed in the treatment of
individuals with diabetes and also serve to advance health equity among vulnerable populations.
Submitters provided information detailing the increased risk of dental caries and
periodontal disease in people with diabetes, many of whom lose teeth, which greatly limits

nutrition, general well-being, and overall quality of life. Submitted studies demonstrated the
bidirectional nature of periodontal disease and diabetes, suggesting that both conditions influence
each other and can worsen or conversely improve outcomes.
As described by submitters, numerous basic and clinical studies describe the relationship
between oral diseases and inflammation in persons with diabetes, which increases risks for
micro- and macrovascular complications including retinopathy, nephropathy, neuropathy,
cardiovascular diseases, and stroke. Several submitters stated that there is a documented
reduction in hospitalizations in persons with diabetes who receive conservative periodontal
treatment. Consequently, submitters stated that periodontal treatment is recommended for
patients with diabetes by American Diabetes Association Clinical Guidelines and is also
promoted by the American Association of Clinical Endocrinologists and others.
212
Diabetes mellitus is a chronic, metabolic disease characterized by elevated levels of
blood glucose (or blood sugar), which, over time, may lead to serious damage to the heart, blood
vessels, eyes, kidneys, and nerves. Type 2 diabetes, which usually occurs in adults, causes the
body to become resistant to insulin or not to make enough insulin. Type 1 diabetes, previously
referred to as juvenile diabetes or insulin-dependent diabetes, is a chronic condition in which the
pancreas produces little or no insulin.
213
A primary goal for the treatment of diabetes is glycemic control and requires accurate
individualization and customization of available treatment options. Interventions to address
lipoproteins, blood pressure, weight control, mental health, and lifestyle are important factors
that contribute to quality of life and the frequency of diabetes-associated complications.
214
212
Nuha A. El Sayed, Grazia Aleppo, Vanita R. Aroda, Raveendhara R. Bannuru, Florence M. Brown, Dennis
Bruemmer, Billy S. Collins, Kenneth Cusi, Sandeep R. Das, Christopher H. Gibbons, John M. Giurini, Marisa E.
Hilliard, Diana Isaacs, Eric L. Johnson, Scott Kahan, Kamlesh Khunti, Mikhail Kosiborod, Jose Leon, Sarah K.
Lyons, Lisa Murdock, Mary Lou Perry, Priya Prahalad, Richard E. Pratley, Jane Jeffrie Seley, Robert C. Stanton,
Jennifer K. Sun, Crystal C. Woodward, Deborah Young-Hyman, Robert A. Gabbay; on behalf of the American
Diabetes Association, Summary of Revisions: Standards of Care in Diabetes—2023. Diabetes Care 1 January 2023;
46 (Supplement_1): S5–S9. https://doi.org/10.2337/dc23-Srev.
213
https://www.who.int/health-topics/diabetes.
214
Melmer A, Laimer M. Treatment Goals in Diabetes. Endocr Dev. 2016;31:1-27. doi: 10.1159/000439364. Epub
2016 Jan 19. PMID: 26824869. https://pubmed.ncbi.nlm.nih.gov/26824869/.

According to recent statistics from the Centers for Disease Control and Prevention,
approximately 38 million people in the United States may have diabetes, and the CDC estimates
that 1 in 5 of them do not know they have the condition. Approximately 98 million U.S. adults
likely have prediabetes, and more than 8 in 10 of them may not know they have prediabetes.
Notably, diabetes is the eighth leading cause of death in the United States (and may be
underreported). Type 2 diabetes accounts for approximately 90 to 95 percent of all diagnosed
cases of diabetes, while Type 1 diabetes accounts for approximately 5-10 percent. The CDC
reports that over the last 20 years, the number of adults diagnosed with diabetes has more than
doubled as the American population has aged and become more overweight or obese.
215
One key marker for the measurement of glycemic control, a key goal in the treatment of
diabetes, in individuals with diabetes is the hemoglobin A1c test. The hemoglobin A1c (also
referred to as glycated hemoglobin, glycosylated hemoglobin, HbA1c, or A1c) test is used to
evaluate a person's level of glucose control and shows an average of the blood sugar level over
the past 90 days and represents a percentage.
216
Submitters through the public submissions process provided multiple research studies
regarding the interaction between dental services and outcomes for medical services to treat
diabetes. The Cochrane Library (ISSN 1465-1858) is a collection of databases that contain high-
quality, independent evidence to inform healthcare decision-making. The Cochrane Library is
owned by Cochrane and published by Wiley.
217
In the Cochrane Review entitled Treatment of
periodontitis for glycaemic control in people with diabetes mellitus, evidence from 30 trials
(results from 2,443 participants) showed that periodontitis treatment reduces blood sugar levels
(measured by HbA1c) in diabetic patients on average by 0.43 percentage points (for example,
from 7.43 to 7 percent; 4.7 mmol/mol) 3 to 4 months after receiving the treatment compared with
no active treatment or usual care. A difference of 0.30 percent (3.3 mmol/mol) was seen after 6
215
https://www.cdc.gov/diabetes/basics/quick-facts.html.
216
https://www.ncbi.nlm.nih.gov/books/NBK549816/.
217
https://www.cochranelibrary.com/about/about-cochrane-library.

months (12 studies), and 0.50 percent (5.4 mmol/mol) at 12 months (one study).
218
All studies in
the review used a parallel randomized controlled trials (RCT) design and followed participants
for between 3 and 12 months. The studies generally focused on people with type 2 diabetes,
save one study that included participants with type 1 or type 2 diabetes. Most studies were
mixed in terms of whether metabolic control of participants at baseline was good, fair, or poor
and were carried out in secondary care. Researchers compared periodontitis treatment with
control, which could be no (or delayed) treatment or usual care (oral hygiene instruction (OHI)
or supragingival scaling with or without OHI). The degree and nature of advanced periodontitis
were not specifically defined in the context of the studies. Additionally, the studies did not
control for other types of interventions deployed in the treatment of diabetes (that is, strategies
used to manage glycemic control), so patients may have been receiving other types of treatment
during the study periods.
The types of periodontal treatment provided covered a wide range of oral services:
subgingival instrumentation, surgical periodontitis treatment ‐ flap surgery or gingivectomy;
antimicrobial therapy (encompassing antibacterials and antibiotics), either locally applied
(including mouth rinses, gels, or dentifrices) or systemically administered; other drug therapy
with a possible benefit of improving the periodontal condition of the participant; other novel
interventions to manage periodontitis; supragingival scaling (also known as professional
mechanical plaque removal (PMPR)); oral hygiene instruction; and/or, education or support
sessions to improve self‐help or self‐awareness of oral hygiene.
In summary, the Cochrane review demonstrated that individuals with diabetes who have
periodontitis who receive dental services for the treatment of the periodontitis experience a
statistically significant reduction of HbA1c. Again, measurement of HbA1c is a metric for
gauging glycemic control and is a primary goal of treatment for all individuals with diabetes.
218
Simpson TC, et al. Treatment of periodontitis for glycaemic control in people with diabetes mellitus. Cochrane
Database Syst Rev. 2022;4:CD004714 https://www.ncbi.nlm.nih.gov/pubmed/35420698.

The study suggests that individuals with diabetes who also have a diagnosis of periodontitis who
receive treatment to address the periodontitis subsequently experience a reduction in HbA1c.
The study authors described the clinical outcomes related to preventive dental care, conservative
periodontal treatment, and reduction in HbA1c as statistically and clinically significant.
Moreover, the authors of the research stated that “further trials evaluating no treatment vs usual
care are unlikely to change this conclusion.”
219
Submitters providing information through the public submissions process suggested that
dental services could be inextricably linked to the following specific medical services in the
treatment of diabetes:
● CPT 36901-36906: Dialysis circuit procedures.
● CPT 82947: Chemistry procedures, blood glucose testing.
● CPT 83036: Hemoglobin A1C testing.
● CPT 90935, 90937, 90940: Hemodialysis procedures.
● CPT 90961: Physician or other qualified healthcare professional visits for ESRD.
● CPT 90989-90999: Other dialysis procedures.
● CPT 92227-92229: Diabetic retinopathy screening.
● CPT 99091: Collection and interpretation of physiologic data.
● CPT 99202-99215: Evaluation and Management (E/M) Services.
● CPT 99211: Office visit for an established patient.
● CPT 99487: Complex chronic care management services.
● CPT 99490-99491: Chronic care management services.
● CPT 99497: Remote physiologic monitoring services.
● CPT 99605-99607: Medication Management.
● CPT 99802-99804: Assessment, Intervention, Face to Face (F2F).
219
Simpson TC, et al. Treatment of periodontitis for glycaemic control in people with diabetes mellitus. Cochrane
Database Syst Rev. 2022;4:CD004714 https://www.ncbi.nlm.nih.gov/pubmed/35420698.
● DRG 637: Hospitalization for diabetes with major complications.
● G0108: Diabetes Self-Management Training.
● G0109: Group Diabetes Self-Management Training.
● G0270: Nutrition Therapy.
● G0466: FQHC visit new patient.
● G0467: FQHC visit established patient.
As described in this section, research provided by submitters suggests that periodontal
treatment for an individual with both a diagnosis of diabetes and periodontitis led to improved
HbA1c measures.
We have explained that there are instances where dental services are so integral to other
medically necessary services that they are inextricably linked to the clinical success of that
medical service(s), and, as such, they are not in connection with the care, treatment, filling,
removal, or replacement of teeth or structures directly supporting teeth within the meaning of
section 1862(a)(12) of the Act. Rather, these dental services are inextricably linked to the
clinical success of an otherwise covered medical service and are payable under Medicare Parts A
and B.
In the case of an individual with diabetes who also has a diagnosis of periodontitis, oral
services and treatment to address the periodontitis potentially lead to a reduction in HbA1c, a
marker of glycemic control that may be used to determine the effectiveness of interventions for
treatment of diabetes. In the description of the studies submitted, the research seems to indicate
that the improvement of glycemic control as evidenced by the HbA1c is due to the provision of
treatment for the periodontitis. The dental and oral services may not be integral to other specific
medically necessary, covered services, but rather the dental and oral services may serve to
influence clinical outcomes directly. The studies compare the impact of the treatment for the
periodontitis to the impact of pharmacological interventions.
We recognize that evidence submitted by interested parties demonstrates that an
individual with both a diagnosis of diabetes and a diagnosis of periodontitis who in turn receives
periodontal treatment services may experience improvements in markers for HbA1c, which is a
key target outcome for the patient population with diabetes. However, the interaction between
these diagnoses and the potential improvements due to periodontal treatment services does not
appear to align with the framework we have established to pay for dental services inextricably
linked to covered services; in our framework, the delivery of certain dental services are integral
to the successful completion of or outcomes related to the covered services.
Under § 411.15(i)(3), we have specified that payment can be made for certain dental
services that are inextricably linked to other services when the specific covered services with
which the dental services are inextricably linked are identified. The studies that have been
provided to CMS through submissions have not identified any specific covered services for the
treatment of diabetes to which dental services are inextricably linked. Rather, the studies
indicate that the primary treatment of periodontal disease in patients with diabetes generally
leads to better outcomes in the management of the patients’ diabetes. While the research makes
the case that the dental services are medically necessary for patients with diabetes, medical
necessity alone does not permit payment for dental services given the broad statutory prohibition
under section 1862(a)(12) on payment for services “in connection with the care, treatment,
filling, removal, or replacement of teeth or structures directly supporting teeth.” In the case of
patients with diabetes, the research does not appear to show that certain dental services are
inextricably linked with certain other covered services for the treatment of diabetes, in
accordance with our regulation at § 411.15(i)(3) such that the statutory prohibition under section
1862(a)(12) does not apply.
We note that some of the examples of medical services for diabetes treatment provided
by submitters are general in nature and not specific to patients with diabetes who may also have
periodontal disease, including CPT codes 99202-99215: Evaluation and Management (E/M)
Services that broadly describe outpatient office visits for the diagnosis and medical management
of practically any illness, disease, or condition.
Additionally, submitters providing evidence for our consideration suggested that the
services described by codes for diabetes self-management training (for example, G0108:
Diabetes Self-Management Training, and G0109: Group Diabetes Self-Management Training)
are services with which dental services may be inextricably linked. However, we were not
persuaded by this evidence and do not believe that dental services would be inextricably linked
to improved outcomes for services for DSMT. Therefore, we seek comment from the public
regarding specific covered services for management of patients with diabetes with which dental
services may be inextricably linked. At this time, we are not proposing to amend §
411.15(i)(3)(i) as we have not identified additional dental services that are inextricably linked to
certain services in the treatment of diabetes. However, we note that we remain open to
considering any such services identified by public commenters, and, if sufficient evidence is
presented, we may consider adding such services to our regulations in the final rule.
In the context of payment for dental services for an individual with diabetes, we seek
information from the public regarding what the coordination between a medical and dental
professional would entail in the scenario where an individual with a diagnosis of diabetes
presents with suspected periodontitis. In the CY 2023 PFS final rule, we explained that we
would make payment when a doctor of dental medicine or dental surgery (referred to as a
dentist) furnishes dental services that are an integral part of the covered primary procedure or
service furnished by another physician, or non-physician practitioner, treating the primary
medical illness. However, if there is no exchange of information, or integration, between the
medical professional (physician or other non-physician practitioner) in regard to the primary
medical service and the dentist in regard to the dental services, then there would not be an
inextricable link between the dental and covered medical service within the meaning of our
regulation at § 411.15(i)(3). Without both integration between the Medicare enrolled medical
and dental professional, and the inextricable link between the dental and covered services,
Medicare payment for dental services would be prohibited under section 1862(a)(12) because the
services are in connection with the care, treatment, filling, removal, or replacement of teeth or
structures directly supporting teeth; though they may be covered by types of supplemental health
or dental coverage (87 FR 69687 through 69688).
In a situation where a medical professional believes that an individual with a diagnosis of
diabetes may also have a diagnosis of periodontitis, how are recommendations conveyed
between the medical and dental professionals? What coordination, if any, occurs between the
medical and dental professionals? We expect that inextricably linked services related to the
treatment of periodontitis in an individual with diabetes would require significant
communication between the medical and dental professionals.
Additionally, we have stated that an inextricable linkage may exist between dental
services and covered services when the standard of care for the medical service is such that the
practitioner would not proceed with the medical procedure or service without performing the
dental services, because the covered medical services would or could be significantly and
materially compromised, or where dental services are a clinical prerequisite to proceeding with
the primary medical procedure and/or treatment (87 FR 69669). While evidence supports that
individuals with diabetes and periodontitis who receive periodontal treatment experience
improvements in their HbA1c markers, dental services do not appear to serve as a precondition
to overall treatment for the diabetes. We seek information from the public on how oral treatment
services may be a clinical prerequisite in the treatment protocol for the care of individuals with
diabetes.
We note that there does not appear to be a clear or singular definitional framework for
categorizing the state of diabetes, such as “controlled” or “uncontrolled” diabetes. Research
submitted by the public discusses improvements in glycemic control as evidence by HbA1c
markers, but does not delineate the characteristics of a patient that would require direct clinical

intervention (pharmacological, behavioral, usage of DME such as insulin pumps, etc.) versus a
patient that would not require interventions given that their disease state is not within a
concerning range requiring direct medical treatment.
In the current literature, there are two types of severity measures that can help
categorizing the state of diabetes: the severity of diabetes itself and the severity of periodontal
disease among individuals with diabetes. With respect to the severity of diabetes, the American
Diabetes Association recommends that most adults with diabetes aim for a HbA1c level below
7.0% (<53 mmol/mol), along with other recommended targets such as blood pressure below
130/80 mmHg and LDL cholesterol below 100 mg/dL.
220
In the current literature, uncontrolled
hyperglycemia is typically defined as a HbA1c level above 8.0% (>64 mmol/mol), according to
guidelines from various medical organizations including the ADA, American College of
Physicians, Association of Clinical Endocrinologists, and American College of
Endocrinology.
221,222,223,224
Based on the literature, this threshold serves as a "take action" point
in managing diabetes and has been used in previous studies to indicate poor glycemic control.
Achieving and maintaining target HbA1c levels is essential for individuals with diabetes (as well
as the general population) and is a key goal of treatment. Moreover, we note that for the
purposes of Quality Payment Program (QPP) measures, CMS has issued measures for diabetes
(e.g. Quality ID #1 (NQF 0059): Diabetes: Hemoglobin A1c (HbA1c) Poor Control (>9%)).
225
220
American Diabetes Association. “Standards of medical care in diabetes--2011.” Diabetes care vol. 34 Suppl
1,Suppl 1 (2011): S11-61. doi:10.2337/dc11-S011.
221
Liu, Longjian et al. “Burden of Uncontrolled Hyperglycemia and Its Association with Patients Characteristics
and Socioeconomic Status in Philadelphia, USA.” Health equity vol. 4,1 525-532. 30 Dec. 2020,
doi:10.1089/heq.2020.0076.
222
Qaseem, Amir et al. “Glycemic control and type 2 diabetes mellitus: the optimal hemoglobin A1c targets. A
guidance statement from the American College of Physicians.” Annals of internal medicine vol. 147,6 (2007): 417-
22. doi:10.7326/0003-4819-147-6-200709180-00012.
223
Cortez-Espinosa, Nancy et al. “Abnormal expression and function of Dectin-1 receptor in type 2 diabetes mellitus
patients with poor glycemic control (HbA1c>8%).” Metabolism: clinical and experimental vol. 61,11 (2012): 1538-
46. doi:10.1016/j.metabol.2012.03.020.
224
Hu, Huanhuan et al. “Hba1c, Blood Pressure, and Lipid Control in People with Diabetes: Japan Epidemiology
Collaboration on Occupational Health Study.” PloS one vol. 11,7 e0159071. 20 Jul. 2016,
doi:10.1371/journal.pone.0159071.
225
https://qpp.cms.gov/docs/QPP_quality_measure_specifications/CQM-
Measures/2023_Measure_001_MIPSCQM.pdf.

The measure is described as “Percentage of patients 18-75 years of age with diabetes who had
hemoglobin A1c > 9.0% during the measurement period.” Furthermore, measures of HbA1c
may fluctuate over time; therefore, a strict threshold could lead to incentives for multiple rounds
of testing in order to aim for the levels established. In general, guidelines exist, but standards
vary for defining diabetes states based on multiple severity measures.
In addition, the severity of periodontal disease is not uniformly defined. ICD-10 codes, such as
K05.2 for Aggressive Periodontitis and K05.3 for Chronic Periodontitis may be utilized to
describe more severe instances of periodontitis (and in this instance when such diagnosis codes
are also partnered with diagnoses related to diabetes for a particular individual). Another
approach involves using the Armitage criteria for periodontal diagnosis.
226,227
Severity
assessment can be based on the clinical attachment level (CAL), with CAL between 1 mm and 2
mm classified as slight, 3 mm and 4 mm as moderate, and ≥5 mm as severe.
228
Again, some
standards exist relative to the staging of periodontitis, but such criteria vary. Additionally, we
believe that the current practice of medicine would allow for variation in clinical attributes as
well as judgment and discernment by the referring practitioner regarding the clinical status of the
individual when determining the need for consultation with other practitioner types, including
the dentist. We seek comment on whether clinical standards exist that describe and define the
disease state of diabetes that would serve to inform the selection of treatment modalities,
including potential referrals to dental professionals with respect to concerns related to oral
health. We also seek comment from the public regarding the ways that CMS could ensure that
practitioners do not decrease the quality of diabetes treatment in an effort to maintain a
beneficiary’s potential access to Medicare payment for dental services.
226
Armitage, G C. “Development of a classification system for periodontal diseases and conditions.” Annals of
periodontology vol. 4,1 (1999): 1-6. doi:10.1902/annals.1999.4.1.1.
227
Caton, Jack G et al. “A new classification scheme for periodontal and peri-implant diseases and conditions -
Introduction and key changes from the 1999 classification.” Journal of clinical periodontology vol. 45 Suppl 20
(2018): S1-S8. doi:10.1111/jcpe.12935.
228
Pinho, M Morado et al. “Periodontitis and atherosclerosis: an observational study.” Journal of periodontal
research vol. 48,4 (2013): 452-7. doi:10.1111/jre.12026.

Evidence supplied by submitters also described periodontitis but without clear and
consistent definitional structure. The 2017 World Workshop on the Classification of Periodontal
and Peri-Implant Diseases and Conditions resulted in a new classification of periodontitis
characterized by a multidimensional staging and grading system. The staging considers the
aspects of severity, complexity, extent, and distribution while the grading contemplates primary
criteria such as progression and grade modifiers, including risk factors such as smoking and
diabetes.
229
For the purposes of our consideration of medical services for the treatment of diabetes for
individuals with diabetes who have periodontitis, we seek comment from the public on clinical
criteria that would determine eligibility for effectiveness of periodontal treatment as described in
the Cochrane review and other studies. We do not believe that a condition such as gingivitis or
early stages of periodontitis would require oral treatment that in turn would influence the
outcomes for an individual with diabetes. However, we seek information to address the
following questions. At what stages and grading would the periodontitis be considered advanced
and/or requiring dental and oral treatment intervention? What types of practitioners are able to
make determinations regarding the staging of periodontitis? We also seek comment on patient
eligibility. What determines patient eligibility for treatment for advanced periodontitis? Are
there other criteria for consideration?
Additionally, we seek comment on the duration of potential periodontal treatment. How
is the length of treatment determined? If a patient’s clinical status improves with respect to the
periodontal disease, what factors determine when periodontal treatment comes to an end? What
does maintenance treatment entail? What services are provided in treatment of advanced
periodontal disease? What is the service definition? Are services bundled? If yes, what is
included in the bundle? When are the services provided and over what period? Is it provided
229
Tables from Tonetti, Greenwell, Kornman. J Periodontol 2018;89 (Suppl 1): S159-S172.
https://www.perio.org/wp-content/uploads/2019/08/Staging-and-Grading-Periodontitis.pdf.
over a calendar month period? A single day? Multiple days? Are services timed? Who
provides the services? What specific terminology is involved? Are these services ever provided
under supervision? Or “incident to” by other clinical staff?
We also seek information on how services for advanced periodontal disease are provided.
Where and how are services for treatment of advanced periodontal disease provided? Are there
any special rules, such as obtaining advance consent or performance of an initiating visit?
We also seek information regarding coding and billing of periodontal services. What
coding is utilized for the treatment services for advanced periodontal disease? What claims
format is employed for the submission of claims with related oral and dental services (for
example, 837D and/or 837P)?
Additionally, we seek comment from the public regarding the risk of recurrence of
periodontal disease for this patient population. What is the level of risk for re-development of
advanced periodontitis and likelihood of recurrence?
We also seek information regarding the role of caries in management of diabetes. What
is the prevalence of caries in this patient population? What is the impact of caries on
management of diabetes?
We also seek information regarding the disease state of the diabetes itself and its
interaction with dental services. Does evidence exist to support that certain characteristics
related to diabetes management (for example, maintenance of HbA1c) are more closely tied to
certain oral interventions’ ability to yield clinical improvements?
We reiterate that section 1862(a)(12) of the Act generally precludes payment under
Medicare Parts A or B for any expenses incurred for services in connection with the care,
treatment, filling, removal, or replacement of teeth or structures directly supporting teeth. Thus,
payment is permitted only where the dental services are inextricably linked to covered medical
services. We believe that general maintenance and management of oral disease processes clearly
falls within the statutory exclusion and therefore Medicare would not permit payment for routine
dental and oral services.
We note that many submitters stated that good dental and oral health benefits a patient’s
overall health in general. Several commenters responding to the CY 2023 PFS proposed rule
also expressed that good oral hygiene, along with routine dental services, contributes to better
outcomes for patients. We recognized in the CY 2023 PFS final rule in response to those
comments that there is a great deal of evidence suggesting that dental health is generally an
important component of overall health; however, we are interested in comments on whether
certain dental services are considered so integral to the primary covered services that the
necessary dental interventions are inextricably linked to, and substantially related and integral to
clinical success of, the primary covered services such that they are not subject to the statutory
preclusion on Medicare payment for dental services under section 1862(a)(12) of the Act (88 FR
79033).
In summary, we seek comment on whether certain dental services are inextricably linked
to certain other covered services for diabetes, supported by clinical evidence as described in
section II.J.1.c. of this proposed rule. We also seek comment specifically on whether dental
services such as prophylaxis are a standard of care in the management of diabetes. We are
committed to continuing to explore the potential inextricable relationship between dental
services and covered medical services utilized in treatment for individuals with diabetes. We
thank submitters for the information they provided through the public submissions process and
may consider revisions to the clinical examples codified in our regulations at § 411.15(i)(3)(i)
based upon additional data and information received in response to this proposed rule.
4. Request for Comment on Dental Services Integral to Specific Covered Services to Treat
Systemic Autoimmune Disease Requiring Immunosuppressive Therapies
We have received information from submitters suggesting that certain dental services are
inextricably linked to immunosuppressive therapies for individuals with autoimmune disorders.

According to the NIH’s National Institute of Environmental Health Sciences, a healthy
immune system is able to defend the body against disease and infection. However, if the
immune system malfunctions, it may mistakenly attack healthy cells, tissues, and organs. This
scenario is called autoimmune disease, and these attacks can affect any part of the body, weaken
bodily function, and in some cases become life-threatening.
230
There are over 100 autoimmune
diseases, including Type 1 diabetes, multiple sclerosis, lupus, rheumatoid arthritis, and
inflammatory bowel disease. There are also other autoimmune diseases that are rare and difficult
to diagnose. In some cases, patients may suffer for years before receiving a proper diagnosis,
and most of these diseases have no cure. Additionally, some autoimmune diseases require
lifelong treatment for system management.
231
Autoimmune diseases are continuously affecting more people. Estimates indicate that as
many as 50 million people in the U.S. have an autoimmune disease, making it the third most
prevalent disease category, surpassed only by cancer and cardiac disease. Generally speaking, a
person’s genes in combination with infections and other environmental exposures likely play a
significant role in disease development, though in some instances pathology may be unknown.
Additionally, nearly 80 percent of people with a chronic autoimmune condition are women.
232
Symptoms of autoimmune diseases can include: fatigue, pain, dermatologic manifestations,
weight loss or gain, insomnia, fever, and myriad other symptoms.
233
Many treatment modalities are employed in the management of autoimmune diseases.
Treatments could include use of oral medications, including steroids, anti-inflammatory
medications, as well as infusion immunotherapy. Some autoimmune conditions may present in a
localized fashion, such as Sjogren’s, and many of the independent organ inflammations, require
immunosuppressive therapies, and may progress to a more systemic involvement. Conversely,
230
https://www.niehs.nih.gov/health/topics/conditions/autoimmune.
231
Ibid.
232
Ibid.
233
https://www.womenshealth.gov/a-z-topics/autoimmune-diseases.
some systemic autoimmune diseases, like sarcoidosis, may not require immunosuppression in
mild cases.
Submissions through the public submissions process urged us to provide that payment
can be made for dental services for individuals with autoimmune diseases receiving
immunosuppressive therapy. In submissions, several interested parties have asserted that
immunosuppressive therapies utilized in the treatment of autoimmune disease have similar
immunosuppressive effects as those of toxic chemotherapy utilized in the treatment of cancer
and that these treatments are analogous to the clinical examples finalized in CY 2024 PFS
rulemaking for dental services inextricably linked to covered medical services in the treatment of
cancer.
Submitters stated that oral and dental treatment is also often integral to the successful
care and management of beneficiaries with autoimmune diseases who are initiating or
undergoing immunosuppressive or immunomodulator therapy because the absence of medically
necessary oral and dental treatment can pose serious complications to those beneficiaries and the
covered medical services they receive. Submitters state that, for example, dental infections can
spread quickly when host immunity is compromised by immunosuppressing or
immunomodulating drugs utilized in treatment. As such, submitters note that the American
College of Physicians has described that the implications of dental disease in patients who are
undergoing immunosuppressive therapy extend beyond their oral disease, with potentially life-
threatening complications if the dental problems are not treated. For these reasons, submitters
state that the covered services upon which immunocompromised patients depend (for example,
immunosuppressive therapy) should not proceed until a dental or oral exam is performed to
address the oral complications and/or clear the patient of an oral or dental infection.
Submitters provided information regarding specific covered services that they believe
could be associated with treatments for immunosuppressive therapy for the treatment of
autoimmune disease and that may increase infection risk, such as:
● CPT codes 99212-99215: Evaluation and Management (E/M) Services.
● CPT codes 96365-96368: Infusion services.
Submitters also provided coding information related to drug therapies, such as CPT codes
for immunosuppressant drugs, including:
● J0129: Abatacept (Orencia) for Rheumatoid Arthritis.
● J0135: Adalimumad (Humira) for Crohn’s, Ulcerative Colitis, Rheumatoid Arthritis.
● J0490: Belimumab (Benlysta) for systemic lupus erythematosus (SLE), Lupus
Nephritis, and Sjögren’s.
● J0491: Anifrolumab-fnia (Saphnelo) for systemic lupus erythematosus (SLE).
● J1303: Ravulizumab-cwvz (Ultomiris) for Generalized Myasthenia Gravis.
● J1438: Etanercept (Enbrel) for Rheumatoid Arthritis, Ankylosing Spondylitis.
● J1595: Glatiramer (Copaxone) for Multiple Sclerosis.
● J1602: Golimumab (Simponi) for Rheumatoid Arthritis, UC, Ankylosing Spondylitis.
● J1745: Infliximab (Remicade) for Crohn’s, Ulcerative Colitis, Rheumatoid Arthritis.
● J2250: Upadacitinib (Rinvoq) for Rheumatoid Arthritis, Ulcerative Colitis, Crohn’s.
● J2323: Natalizumab (Tysabri) for Multiple Sclerosis.
● J2350: Ocrelizumab (Ocrevus) for Multiple Sclerosis.
● J3262: Tocilizumab (Actemra) for Scleroderma-associated lung fibrosis.
● J3357: Ustekinumab (Stelara) for Crohn’s, Ulcerative Colitis, Psoriatic Arthritis.
● J3380: Vedolizumab (Entyvio) for Crohn’s, Ulcerative Colitis.
● J3590: Secukinumab (Cosentyx) for Plaque Psoriasis.
● J7500: Azathioprine (Imuran) for Lupus, Crohn’s, Sjögren’s.
● J7517: Mycophenolate (Cellcept) for Lupus, Sjögren’s.
● J9070: Cyclophosphamide (Cytoxan) for Sjögren’s, Vasculitis.
● J9250: Methotrexate for Sjögren’s, Rheumatoid Arthritis (unresponsive to other
treatment).
● J9302: Ofatumumab (Kesimpta) for Multiple Sclerosis.
● J9312: Rituximab (Rituxan) for Rheumatoid Arthritis, Sjögren’s.
● J9332: Efgartigimod (Vyvgart) for Myasthenia Gravis.
Submitters also provided coding information for potential medical services for medical
treatment for pulmonary diseases when aspiration of dental pathogens risk or cause the initiation
and/or recurrence of complications, such as:
● CPT codes 99212-99215: Evaluation and Management (E/M) Services.
● CPT code 99291: Critical Care Services.
● DRG code 177: Hospitalization for respiratory infections and inflammation.
● DRG code 190: COPD with complications.
Submitters also provided coding regarding medical treatment for dentally sourced
dissecting maxillofacial space infections:
● CPT 41000: Intraoral incision and drainage of abscess.
● CPT 87181: Antibiotic susceptibility study.
● CPT 96365: Infusion of antibiotic.
● CPT codes 99281-99285: Emergency department services.
● CPT codes 99291-99292: Critical care services.
● DRG code 135: Sinus procedures with CC/MCC.
● DRG code 141: Major head and neck procedures with CC.
● DRG code 872: Hospitalization for septicemia or severe sepsis.
Submitters providing information through the public submissions process stated that if
dental or oral infections are left undetected or untreated in the population of individuals
undergoing immunosuppressive therapy for autoimmune disease, serious complications may
occur and negatively impact the course and outcome of the covered medical procedures, which
submitters state is analogous to previously finalized policies for dental services inextricably
linked to covered cancer treatment for the patient. Several submitters pointed out that we stated
in the CY 2024 PFS final rule that proceeding without a dental or oral exam of the mouth prior to
chemotherapy could lead to systemic infection or sepsis, among other complications, and that
similar outcomes can follow for those receiving immunosuppressive therapy to treat autoimmune
diseases.
The submitters noted that in the CY 2024 PFS final rule, we described that AHRQ
identified evidence to support that dental evaluation/treatment prior to cancer treatment led to
decreased incidence and/or less severity of serious oral infections and complications like oral
mucositis and encouraged CMS to explore this connection to confirm that dental evaluations and
treatment prior to immunosuppressive therapy would lead to decreased incidence of serious oral
infections in a similar fashion. The submitters also stated that they believe it is critical that
beneficiaries with an autoimmune disease that requires immunosuppressive therapy have access
to necessary dental services, as proper dental care for this population can reduce the incidence of
serious infection and improve overall patient outcomes for the covered service.
We appreciate the evidence and information provided by submitters and agree that we
should continue to research whether there is a connection between dental and oral evaluations
and treatment prior to immunosuppressive therapy and outcomes for said therapies, including the
potential decreased incidence of serious oral infections.
However, we seek comment on whether the level of immunosuppression utilized in the
treatment of autoimmune diseases is analogous to the immunosuppression levels employed in the
treatment of cancer. We believe that the level of immunosuppression for systemic autoimmune
disease has different characteristics versus therapies utilized in chemotherapy used in the
treatment of cancer. For example, the usage of monoclonal antibodies in the treatment of
autoimmune disease may not render the same level of immunosuppression and subsequent
susceptibility to infection as chemotherapy used in the treatment of cancer.
We also seek information on the connection between immunosuppressive therapy in the
treatment of autoimmune disease and the likelihood of systemic infection and sepsis.
Specifically, we seek information regarding the likelihood of dental and oral sources as the locus
of the seeding of infection in this patient population. Additionally, we seek information
regarding standards of care or clinical guidelines that recommend that a dental infection be
addressed before proceeding with the immunosuppressive treatment or the administration of
drugs or whether oral antibiotics would be prescribed to resolve the infection and that the therapy
would advance without direct dental or oral services to address the infection.
We also seek information regarding whether there is differential impact between drugs
that are administered in an office setting or similar versus those medications that are taken in an
oral fashion.
We thank submitters for the information they provided through the public submissions
process. We believe that additional information is necessary to consider whether there is an
inextricable link between dental services and covered services to treat systemic autoimmune
disease requiring immunosuppressive therapies and seek comment from the public. We remain
open to considering any such services identified by public commenters, and, if sufficient
evidence is presented, we may consider adding such services to § 411.15(i)(3) in the final rule.
5. Implementation of Payment for Dental Services Inextricably Linked to Other Specific
Covered Services
In the CY 2024 PFS final rule (88 FR 79035 through 79039), we solicited comments on
whether we should provide additional guidance that would aid in processing claims for dental
services that are inextricably linked to a Medicare-covered medical service. Some commenters
suggested the usage of a modifier on the dental claim format that would better identify when
dental services are inextricably linked to specific covered medical services. As we continue to
consider improvements to our payment policies and have gained experience around the provision
of dental services inextricably linked to covered medical services, we have explored tools and
resources that may help to facilitate the implementation and coordination of dental services that
are currently covered under Medicare, including the possible usage of modifiers and diagnosis
codes. The usage of modifiers on a dental claim would seek to identify the dental service as a
service the billing practitioner identifies as inextricably linked to a specific covered medical
service and for which there was an exchange of information, or integration, between the medical
and dental professional (physician, including a dentist, or other non-physician practitioner) as
specified in the CY 2023 PFS final rule (87 FR 69663 through 69688). We have explained that
if there is no exchange of information, or integration, between the medical professional
(physician or other non-physician practitioner) regarding the primary medical service and the
practitioner furnishing the dental services, then there would not be an inextricable link between
the dental and covered medical service within the meaning of our regulation at § 411.15(i)(3).
Furthermore, integration between medical and dental professionals can occur when these
professionals coordinate care. This level of coordination can occur in various forms such as, but
not limited to, a referral or exchange of information between the medical professional (physician
or non-physician practitioner) and the dentist. This coordination should occur between a dentist
and another medical professional (physician or other non-physician practitioner) regardless of
whether both individuals are affiliated with or employed by the same entity.
Currently, the KX modifier is submitted on a Medicare Part B claim to indicate that the
service or item is medically necessary, and that the healthcare provider has included appropriate
documentation in the medical record to support or justify the medical necessity of the service or
item. We believe that usage of the KX modifier in the context of claims for dental services
inextricably linked to covered services would be appropriate and support claims processing and
program integrity efforts.
Based on comments received and summarized in the CY 2024 PFS final rule (88 FR
79037), interested parties requested that we provide more guidance on how a practitioner
submitting claims for dental services can attest that the dental and medical services are
inextricably linked, and that the criteria have been met to support payment. We believe that the
use of the KX modifier would allow practitioners to signal that the dental services meet the
criteria to support payment. Also, use of the KX modifier may improve the MACs’ ability to
ascertain the volume of claims that are being submitted for dental services inextricably linked to
covered services.
Therefore we are proposing that, effective January 1, 2025, the KX modifier would be
required for claims submission for dental services inextricably linked to covered medical
services on both the dental claim format 837D and the professional claim format 837P. We are
proposing that practitioners who bill for dental services for which they seek payment in
accordance with § 411.15(i)(3) must include the KX modifier on the 837D or 837P claim to
indicate that they believe that the dental service meets the established payment criteria; that the
practitioner has included appropriate documentation in the medical record to support or justify
the medical necessity of the service or item and that demonstrates the inextricable linkage to
covered medical services; and that coordination of care between the medical and dental
practitioners has occurred. To help with this transition to potentially requiring use of the KX
modifier for claims submission for dental services inextricably linked to covered medical
services beginning in 2025, practitioners now have the option to utilize the KX modifier as
proposed, for services with dates of service in CY 2024. This optional usage in CY 2024 would
not be mandatory and would serve to support both clinician and MAC claims processing
activities. We intend to provide additional instruction and education through subregulatory
guidance regarding this voluntary phase of the usage of the KX modifier on claims submitted for
dental services inextricably linked to covered medical services. We seek comment on all aspects
of this proposal.
While the KX modifier indicates that the services are medically necessary, the GY
modifier (along with three other HCPCS denial modifiers) serves to indicate that a service is not
covered because it is outside of the scope of Medicare coverage authorized by the statute. We
reiterate that denial modifiers should be used when physicians, practitioners, or suppliers want to
indicate that the item or service is statutorily non-covered.
Use of the GY modifier could support MAC efforts to adjudicate claims and remove from
the claims processing pipeline those claims that do not require further processing. We are
seeking comment on whether we should recommend the usage of the GY modifier on the 837D
or 837P dental claim format in instances where a Medicare claim denial is sought for purposes of
submission to third party payers or when the service does not fit within a Medicare benefit
category and is statutorily excluded from coverage.
Additionally, in general, the Act and our regulations mandate the submission of
diagnostic coding (for example, ICD-10 codes) on Medicare claims. Section 1842(p)(1) of the
Act states that “each request for payment, or bill submitted, for an item or service furnished by a
physician or practitioner specified in subsection (b)(18)(C) for which payment may be made
under this part shall include the appropriate diagnosis code (or codes) as established by the
Secretary for such item or service.” Under this section, each bill or request for payment for
physicians’ services under Medicare Part B must include the appropriate diagnostic code “as
established by the Secretary” for each item or service for which the Medicare beneficiary
received treatment. In the March 4, 1994 final rule entitled Medicare Program; Diagnosis
Codes on Physician Bills, we codified that each bill or request for payment for a service
furnished by a physician under Medicare Part B must include appropriate diagnostic coding for
the diagnosis or the symptoms of the illness or injury for which the Medicare beneficiary
received care and revised our regulations at § 424.32, Basic requirements for all claims, to state
specifically that a claim for physician services must include appropriate diagnostic coding using
diagnostic information (59 FR 10290).
Additionally, in the CY 2023 PFS final rule, we stated that dentists are included in the
statutory definition of physician at section 1861(r)(2) of the Act and would generally be
considered and treated as a physician for purposes of enrollment, compliance, and other
administrative programs (87 FR 69673). Therefore, dentists, who are physicians for the purposes
of the Medicare program, are required to submit diagnosis codes on claims for physician services
as described in the statute and regulations. Furthermore, we note that diagnosis code information
is currently required on the submission of the professional claim form 837P; professional claims
lacking such information are returned to the healthcare provider and are not processed.
In the CY 2023 PFS final rule (87 FR 69679 through 69680), we acknowledged the need
to address and clarify certain operational issues related to Medicare payment for dental services
inextricably linked to covered services and noted that we were working to address these issues,
including claims processing questions raised by the commenters. We stated that we anticipated
resolving many of the additional operational issues raised by commenters potentially as soon as
CY 2024, including efforts to adopt the dental claim form (837D). Similarly, in the CY 2024
PFS final rule (88 FR 79036), we stated that we continue to work to address issues raised by
commenters, such as questions related to claims processing and efforts to accommodate the
dental claim form within our claims processing systems, effective 2024. The efforts related to
adopting the dental claim form are ongoing, and as efforts advance to address the
implementation and functionality of claims processing systems for the dental claim form, we
intend to provide appropriate guidance and education to interested parties.
We anticipate that our systems will be able to process claims submitted using the dental
claim form 837D (OMB Control No. 0938-1471) by January 1, 2025. Consistent with the
statutory and regulatory requirements discussed above, we intend to require a diagnosis code to
be included on claims submitted for physicians’ services for dental services inextricably linked
to covered medical services on both the 837P and 837D formats, beginning on January 1, 2025.
However, given the complexities related to the operational launch of and transition to the 837D
dental claims format, we are also considering further delaying the requirement to include a
diagnosis code on the 837D form. For example, interested parties have indicated that in current
dental practice, claims processing systems may not require the submission of a diagnosis code on
claims for dental services, and therefore dental practices may need time to adjust to this
requirement for the 837D form. We believe that it may be appropriate to delay this requirement

for a limited time to support clinicians and billing entities as they seek to change their workflows
and transition to using the 837D form. We seek comment on our intention to require the
inclusion of a diagnosis code on the 837D form beginning on January 1, 2025. We are
particularly interested in any operational challenges that interested parties may face in attempting
to comply, as well as other considerations that we should take into account with regard to the
timing of this requirement.
In the CY 2023 PFS final rule, we stated that we believed that MACs are appropriately
situated to establish contractor prices for dental services inextricably linked to covered services
until we have additional pricing data that could enable national pricing (87 FR 69680).
Therefore, dental services inextricably linked to covered services are currently contractor priced.
However, we have received feedback from the MACs regarding pricing information for dental
services inextricably linked to covered services, and the MACs have requested information that
would support their efforts to assign payment amounts for such dental services. The MACs
retain broad flexibility with respect to assigning payment amounts to claims for dental services
inextricably linked to covered services; however, we seek to facilitate the sharing of available
pricing information with the MACs for these purposes. Thus, we seek comment from the public
on potential sources of payment information for the pricing of dental services inextricably linked
to covered services. We note, for example, that publicly available data (such as Fair Health cost
data) are available for purchase; however, we understand that this information may not directly
inform payment amounts in a manner useful for the payment of Medicare claims for dental
services. According to Fair Health’s website, cost estimate information is based on claims for
medical and dental services paid for by private insurance plans, including the country's largest
insurers.
234
We are also aware of other fee schedules, such as those used by state governments
for state employees, or discount fee schedules, such as discount dental programs (for example,
https://www.dentalbenefitprogram.com/groupfees.php?id=NEV). We aim to support the
234
https://www.fairhealthconsumer.org/#answer2; Accessed May 22, 2024.

ongoing efforts by the MACs to price these services and seek any information from the public
that may serve to support and inform the MAC development of payment amounts for dental
services inextricably linked to covered services.
6. Request for Information: Services Associated with Furnishing Oral Appliances Used for the
Treatment of Obstructive Sleep Apnea
Section 1861(s) of the Act defines the term “medical and other health services” that may
be covered under Medicare, including physicians’ services under section 1861(s)(1), services and
supplies furnished incident to a physician’s professional service under section 1861(s)(2)(A), and
durable medical equipment (DME) under section 1861(s)(6) of the Act.
235
In addition to the statutory definition of DME, § 414.202 of our regulations defines DME
as equipment furnished by a supplier or a home health agency that meets the following
conditions:
• Can withstand repeated use.
• Effective with respect to items classified as DME after January 1, 2012, has an
expected life of at least 3 years.
• Is primarily and customarily used to serve a medical purpose.
• Generally is not useful to an individual in the absence of an illness or injury.
• Is appropriate for use in the home.
For items to be considered DME, all five criteria of the definition must be met. The CMS
policies for determining whether an item meets the definition of DME are further outlined in the
Medicare Benefit Policy Manual (CMS Pub. 100-02), Chapter 15, section 110.1. With respect to
the first and second criteria, DME items must be able to withstand repeated use in the home,
potentially by successive patients.
Different types of oral appliances are fabricated and furnished by licensed dentists (DDS
or DMD) to reposition oral and pharyngeal tissues in an effort to create and maintain a patient's
airway during sleep for treatment of obstructive sleep apnea. Obstructive sleep apnea is a
medical disorder in which a person frequently stops breathing during sleep. It results from an
235
https://www.ssa.gov/OP_Home/ssact/title18/1861.htm.
obstruction of the upper airway during sleep that occurs because of inadequate motor tone of the
tongue and/or airway dilator muscles. A treating practitioner may prescribe use of an oral
appliance as the first line of treatment for obstructive sleep apnea, or in cases where other
treatments, such as use of a continuous positive airway pressure (CPAP) device and/or bi-level
positive airway pressure device have been tried and failed.
The following two HCPCS Level II codes were established effective January 1, 2006, for
these devices:
● E0485 “Oral device/appliance used to reduce upper airway collapsibility, adjustable or
non-adjustable, prefabricated, includes fitting and adjustment”.
● E0486 “Oral device/appliance used to reduce upper airway collapsibility, adjustable or
non-adjustable, custom fabricated, includes fitting and adjustment”.
A very limited subset of custom fabricated oral appliances used to treat obstructive sleep
apnea (HCPCS code E0486) have been covered for Medicare beneficiaries by the Durable
Medical Equipment Medicare Administrative Contractors (DME MACs) under the DME benefit.
These devices are considered durable equipment by the DME MACs because of the presence of
a fixed mechanical hinge.
An additional HCPCS code was established effective April 1, 2022, to describe custom
fabricated oral appliances without a fixed mechanical hinge:
● K1027 “Oral device/appliance used to reduce upper airway collapsibility, without
fixed mechanical hinge, custom fabricated, includes fitting and adjustment”.
The DME MACs have not covered any of the prefabricated devices (HCPCS code
E0485) based on a determination that there is insufficient evidence to support that these items are
effective therapy for obstructive sleep apnea. The DME MAC Local Coverage Determination
for the devices is available at https://www.cms.gov/medicare-coverage-
database/view/lcd.aspx?lcdId=33611&ver=25.

For several years, manufacturers of products without fixed mechanical hinges that
dentists use in custom fabricating oral appliances have raised concerns regarding why their
versions of the custom fabricated oral appliances have not been classified as DME. There have
been an increasing number of requests for HCPCS codes and Medicare benefit category
determinations from several different manufacturers of these products. Using the public meeting
consultation process outlined in regulations at § 414.240, we obtained public consultation
regarding these various products. National Medicare benefit category determinations have not
been established for these appliances because we have not been able to confirm that any of the
custom fabricated devices can withstand repeated use. As indicated above, equipment must be
able to withstand repeated use in order to be classified as DME, which means they are items that
can normally be rented and used by successive patients (repeated use).
We are not aware of any oral appliances used to treat obstructive sleep apnea, regardless
of whether they include a fixed mechanical hinge, that are prefabricated, or custom fabricated,
and can withstand repeated use. These are oral appliances that do not seem to be the kind of
equipment that could potentially be rented and used by successive patients. In addition, national
fee schedule amounts have not been established for the devices with the mechanical hinge
because of complexities related to pricing of the professional services of the dentist that are
integral to the fabrication and furnishing of the device. The DME MACs have been paying
claims for devices they have been covering as DME under code E0486 using local fee schedule
amounts. Before making a final determination regarding whether these devices could be
classified as DME, we are requesting information that would help us determine if oral appliances
used to treat obstructive sleep apnea can withstand repeated use (furnished as rental equipment
for use by successive patients).
As described in section II.J.1.a. of this proposed rule, section 1862(a)(12) of the Act
generally precludes payment under Medicare Parts A or B for any expenses incurred for services
in connection with the care, treatment, filling, removal, or replacement of teeth or structures
directly supporting teeth. Oral sleep apnea appliances and similar appliances are used in the
direct treatment of sleep apnea. A treating practitioner may prescribe the use of an oral sleep
apnea appliance as the first line of treatment for obstructive sleep apnea, a medical condition, or
in instances where other treatments, such as use of a CPAP device and/or bi-level positive airway
pressure device have been tried and failed. Therefore, we believe that these appliances and
related services, while utilized in the mouth and situated on the teeth, would not be considered
services in connection with the care, treatment, filling, removal, or replacement of teeth or
structures directly supporting the teeth. Rather, we believe that oral sleep apnea appliances,
when prescribed by a practitioner for the treatment of a medical condition such as obstructive
sleep apnea, as medically reasonable and necessary and therefore may be payable by Medicare.
Further, we believe that payment for oral sleep apnea appliances would not fall within the
payment policy for dental services inextricably linked to covered medical services, as the
appliances are not services for dental care, but are instead services and supplies for the purposes
of directly treating a medical condition unrelated to teeth or structures directly supporting the
teeth (for example, sleep apnea).
The statutory definition of physician includes a doctor of dental surgery or of dental
medicine in section 1861(r)(2) of the Act, and, to the extent payment for dental services is not
precluded under section 1862(a)(12) of the Act, medically necessary services furnished by a
dentist may be covered and paid as physicians’ services under section 1861(s)(1) and (2), and
section 1848 of the Act.
We are requesting information regarding the types of services furnished by a dentist or
other practitioner related to oral sleep apnea appliances. Specifically, we are seeking
information regarding details that may inform or support a future proposal regarding a code
assignment for services related to oral sleep apnea appliances under the Medicare physician fee
schedule.
We are requesting details and information regarding the services related to the
furnishment of oral appliances used to treat obstructive sleep apnea, as described in the questions
below:
● Patient eligibility criteria: to whom is the service related to the furnishing of oral
appliances being provided? How is the nuisance snorer differentiated from the medical
condition of sleep apnea? What criteria are used to determine whether the provision of these
services may be medically reasonable and necessary?
● Services provided: What is the service definition for oral sleep apnea appliances? Is a
bundle of services provided? If yes, what services are included in the bundle? What does the
process for evaluating, furnishing, and fitting of oral sleep apnea appliances involve? What work
is involved? Which elements of the services are essential (particularly in terms of what work is
essential to the effectiveness of the device)? What are the costs associated with providing these
services? What are the payment amounts for these services?
● Timing of services: What is the service period? Are the services provided over a
period of time? Are services timed in any way?
● Practitioner type: Who provides these services? What credentialing is required? Is
supervision required? Who would be billing for these services? Would incident to payment
policy rules apply?
● Location of services: Where and how are services for oral sleep apnea appliances
provided? Are all services provided in an in-person setting? Does the patient have to be present
for all elements of this service? Does the service involve direct contact with the patient in each
instance?
● Considerations for Telehealth: Are any of these services provided, or capable of being
provided, via telehealth?
● Site of service: Can services related to oral sleep apnea appliances be provided by
hospital-based physicians?
● Other considerations: What billing procedures exist for these oral sleep apnea
appliances? What existing and/or additional coding may currently describe these types of
services? In addition to the HCPCS Level II codes described above (for example, E0485 and
E0486), we believe that the CDT codes D9947 through D9957 may describe both the fabrication
of the appliance as well as services provided during and after fabrication.
We will review any information provided by the public and consider potential
refinements to PFS payment policy accordingly. We reiterate that we do not believe the
evaluation services or fabrication and fitting of oral sleep apnea appliances by dentists or other
physicians or non-physician practitioners would be subject to the prohibition on payment for
dental services under section 1862(a)(12) or the payment policy for dental services inextricably
linked to covered services. However, we seek information regarding the services associated with
the provision of these appliances and may consider code creation or other refinements in the
context of the Medicare physician fee schedule. Additionally, we note that as described in
section II.C. of this proposed rule, individuals and groups may nominate codes that they believe
may be potentially misvalued codes through our public nomination process for potentially
misvalued codes, which was established in the CY 2012 PFS final rule with comment period (76
FR 73026, 73058 through 73059). Individuals could consider nominating codes related to oral
sleep apnea appliances for consideration under this process. Nominations may be submitted to
CMS in one of two ways: via email or through postal mail. Email submissions should be sent to
the CMS email box at [email protected], with the phrase
“Potentially Misvalued Codes” and the referencing CPT code number(s) and/or the CPT
descriptor(s) in the subject line. Physical letters for nominations should be sent via the U.S.
Postal Service to the Centers for Medicare & Medicaid Services, Mail Stop: C4–01–26, 7500
Security Blvd., Baltimore, Maryland 21244. Envelopes containing the nomination letters must
be labeled “Attention: Division of Practitioner Services, Potentially Misvalued Codes.”
Nominations for consideration in our next annual rule cycle should be received by our February
10th deadline.
K. Payment for Skin Substitutes
In the CY 2023 PFS proposed rule (87 FR 46027 through 46029), we outlined several
objectives related to refining skin substitute policies under Medicare, including: (1) ensuring a
consistent payment approach for skin substitute products across the physician office and hospital
outpatient department settings; (2) ensuring that appropriate HCPCS codes describe skin
substitute products; (3) using a uniform benefit category across products within the physician
office setting, regardless of whether the product is synthetic or comprised of human or animal-
based material, to incorporate more consistent payment methodologies; and (4) maintaining
clarity for interested parties on CMS skin substitutes policies and procedures. When considering
potential changes to policies involving skin substitutes, we noted that we believe it would be
appropriate to take a phased approach over multiple rulemaking cycles to examine how we could
appropriately incorporate skin substitutes as supplies under the PFS ratesetting methodology.
After receiving feedback from commenters requesting more information on how CMS intends to
achieve a consistent payment approach for skin substitute products, we did not finalize any
policies in the CY 2023 PFS final rule.
In alignment with our objectives, in the CY 2024 PFS final rule, we solicited comments
on different approaches CMS could use to identify appropriate practice expense (PE) direct costs
for skin substitute products, such as reviewing various sources for price information, including
performing market research, reviewing invoices submitted by interested parties, or cost
information on Medicare claims. Discussing these approaches in the CY 2024 PFS final rule
provided interested parties with more details about payment mechanisms CMS is considering
under our PFS ratesetting methodology.
The CY 2024 PFS proposed rule did not contain a specific proposal for changing how
skin substitute products are paid under the PFS; however, we continue to pursue our objectives
for refining skin substitute payment policies under Medicare, as mentioned above. More
specifically, we continue examining ways to treat skin substitute products as incident-to supplies
under the PFS ratesetting methodology. Additionally, we believe continuing this dialogue with
interested parties on payment for skin substitute products will help inform potential policy
changes for future rulemaking.
We recognize that skin substitute products may vary in composition, size, and
applicability and will continue to consider these distinct characteristics in proposing a consistent
payment approach and policy. We also note an increase in HCPCS Level II coding request
applications for newly developed skin substitute products and are considering broadly all of our
relevant payment policies. Such policies, for example, include the discarded drug refund policy
and the Part B drug inflation rebate policy and how these policies may align with the usage and
payment for skin substitute products. In the CY 2024 PFS final rule (88 FR 79060 through
79061), we finalized that billing and payment codes that describe products currently referred to
as skin substitutes are not counted for identifying refundable drugs for calendar quarters during
2023 and 2024. While we continue to consider making changes to the Medicare Part B payment
policies for such products, similar to last year, for CY 2025, we are proposing that billing and
payment codes that describe products currently referred to as skin substitutes would not be
counted for purposes of identifying refundable drugs for calendar quarters in 2025. We plan to
revisit discarded drug refund obligations for skin substitutes in future rulemaking. In section
III.I. of this proposed rule, CMS is proposing to codify existing policy by including products
currently referred to as skin substitutes on the list of product categories that are not considered
Part B rebatable drugs in proposed § 427.101(b)(5).
III. Other Provisions of the Proposed Rule
A. Drugs and Biological Products Paid Under Medicare Part B

1. Requiring Manufacturers of Certain Single-Dose Container or Single-Use Package Drugs to
Provide Refunds with Respect to Discarded Amounts (§§ 414.902 and 414.940)
a. Background
Section 90004 of the Infrastructure Investment and Jobs Act (Pub. L. 117-58, November
15, 2021) (hereinafter referred to as “the Infrastructure Act”) amended section 1847A of the Act
to redesignate subsection (h) as subsection (i) and insert a new subsection (h), which requires
manufacturers to provide a refund to CMS for certain discarded amounts from a refundable
single-dose container or single-use package drug (hereinafter referred to as “refundable drug”)
for calendar quarters beginning January 1, 2023.
In the CY 2023 PFS final rule (87 FR 69710 through 69734), we finalized many policies
to implement this provision. First, we finalized the requirement that billing providers and
suppliers report the JW modifier for all separately payable drugs and biologicals (hereinafter
referred to as “drugs”) with discarded drug amounts from single use vials or single use packages
payable under Part B, beginning January 1, 2023 (87 FR 69719). We also finalized the
requirement that billing providers and suppliers report the JZ modifier for all such drugs with no
discarded amounts beginning no later than July 1, 2023, and we stated that we would begin
claims edits for both the JW and JZ modifiers beginning October 1, 2023 (87 FR 69718 through
69719). After the issuance of the CY 2023 PFS final rule, CMS published a JW Modifier and JZ
Modifier Policy Frequently Asked Questions (FAQ) document
236
to provide further guidance on
the correct use of these modifiers.
Second, we adopted a definition of “refundable single-dose container or single-use
package drug” at § 414.902, which also specifies exclusions from this definition (87 FR 69724).
These three exclusions are: radiopharmaceutical or imaging agents, certain drugs requiring
236
https://www.cms.gov/medicare/medicare-fee-for-service-payment/hospitaloutpatientpps/downloads/jw-modifier-
faqs.pdf.
filtration, and drugs approved by FDA on or after November 15, 2021 for which payment has
been made under Part B for fewer than 18 months.
Third, regarding reports to manufacturers, we specified that we would send reports
(including information described in section 1847A(h)(1) of the Act) for each calendar quarter, on
an annual basis, to each manufacturer of a refundable drug (87 FR 69726).
Fourth, we finalized how the refund amount will be calculated, which is specified in
regulation at § 414.940 (87 FR 69731). We stated we would issue a preliminary report based on
available claims data from the first two quarters of CY 2023 to provide manufacturers
information regarding discarded amounts of refundable drugs prior to the initial refund report (87
FR 69725). In these reports, which were sent in December of 2023, we included preliminary
information on estimated discarded amounts of refundable drugs for each labeler code based on
available claims data from the first 2 quarters of CY 2023 for any refundable drug for which
discarded units were billed using the JW modifier. More information about discarded drugs,
including the discarded drug refund and the JW and JZ modifier policy, can be found at
https://www.cms.gov/medicare/payment/part-b-drugs/discarded-drugs.
Fifth, we addressed drugs with unique circumstances for which we can, through notice-
and-comment rulemaking, increase the applicable percentage otherwise applicable for
determining the refund. Section 1847A(h)(3)(B)(ii) of the Act provides that, in the case of a
refundable drug that has unique circumstances involving similar loss of product as that described
in section 1847A(h)(8)(B)(ii) of the Act, the Secretary may increase the applicable percentage
otherwise applicable as determined appropriate by the Secretary. We adopted an increased
applicable percentage of 35 percent for drugs reconstituted with a hydrogel and with variable
dosing based on patient-specific characteristics (87 FR 69731). Lastly, we adopted a dispute
resolution process through which manufacturers can challenge refund calculations, and we
established enforcement provisions, including manufacturer audits, provider audits, and civil
money penalties required by statute (87 FR 69732 through 69734).
In the CY 2024 PFS final rule (88 FR 79047 through 79064), we finalized the date of the
initial refund report to manufacturers, the date for subsequent reports, method of calculating
refunds for discarded amounts in lagged claims data, method of calculating refunds when there
are multiple manufacturers for a refundable drug, increased applicable percentages for certain
drugs with unique circumstances, and a future application process by which manufacturers may
apply for an increased applicable percentage for a drug, which would precede proposals to
increase applicable percentages in rulemaking.
We also finalized that drugs separately payable under Part B from single-dose containers
that are furnished by a supplier who is not administering the drug are required to be billed with
the JZ modifier, since we believe it is unreasonable to collect discarded drug data from
beneficiaries. We were concerned that claim rejections may occur in the absence of a claims
modifier to designate that a drug was dispensed, but not administered, by the billing supplier.
b. Application for increased applicable percentage
Section 1847A(h)(3)(B)(ii) of the Act permits the Secretary to increase the applicable
percentage for a refundable drug that has unique circumstances through notice and comment
rulemaking. In the CY 2024 PFS final rule (88 FR 79057 through 79060), we finalized an
application process (CMS-10835, OMB 0938-1435) by which manufacturers could apply for an
increased applicable percentage for a drug and may request that we consider an individual drug
to have unique circumstances for which an increased applicable percentage is appropriate. We
explained that manufacturers could benefit from a formal process through which they can
provide information, including that which may not be publicly available, in order to request an
increase in their refundable drug’s applicable percentage and provide justification for why the
drug has unique circumstances for which such an increase is appropriate, including in the case of
a drug with an applicable percentage that has already been increased by virtue of its unique
circumstances. We finalized the application deadline of February 1 of each year, adopted a
deadline of August 1 for the FDA-approval of the drug and the deadline for notifying and

submitting the FDA-approved label to CMS of September 1 of the year before the year in which
the increased applicable percentages would apply. We codified this process in regulation at §
414.940(e). The application process requires the applicant to provide a written request
comprising FDA-approved labeling for the drug; justification for the consideration of an
increased applicable percentage based on such unique circumstances; and justification for the
requested increase in the applicable percentage.
We received one application for increased applicable percentage for CY 2025 from the
manufacturer of Leukine
®
(sargramostim). Leukine
®
is a leukocyte growth factor that is
primarily used in hematological malignancies to increase white blood cell counts. The applicant
submitted the information required under § 414.940(e)(1), including its justification for
consideration for increased applicable percentage, and justification for the requested applicable
percentage of 72 percent. The applicant did not submit FDA-approved labeling for the drug for
the particular adjuvant uses described in the application (further described below in this
paragraph) due to ongoing cancer vaccine adjuvant trials. The applicant states that there are
several manufacturers in late-stage (Phase II and Phase III) development using Leukine
®
as a
vaccine adjuvant in oncology indications, specifically in stimulating the immune response of
dendritic cells when used alongside these vaccines. Cancer treatment vaccines are different from
the vaccines that work against viruses (for example, influenza). These vaccines try to get the
immune system to mount an attack against cancer cells in the body. Instead of preventing
disease, they are meant to get the immune system to attack a disease that already exists.
237
The
applicant stated that it has no ownership stake in the development of these vaccines and does not
possess control or influence over the design and execution of the clinical trials. The estimated
completion dates for Phase III clinical trials vary, with the earliest expected in March 2025
238
and the latest in March 2029.
239
The adjuvant use of Leukine
®
in predetermined dosage
237
https://www.cancer.org/cancer/managing-cancer/treatment-types/immunotherapy/cancer-vaccines.html.
238
https://clinicaltrials.gov/study/NCT04229979.
239
https://clinicaltrials.gov/study/NCT05100641.

introduces a new use of the drug that is distinct from its six FDA-approved indications, all of
which have dosages that are based on body weight or body surface area (BSA). The adjuvant
use dosages of Leukine
®
in clinical trials are generally much smaller than dosages for indications
in the FDA-approved labeling. The smallest dose of Leukine
®
used for vaccine adjuvant
purposes of which the applicant is aware (that is, 70 mcg) would lead to as much as 72 percent of
the drug being discarded from a single-dose 250 mcg lyophilized vial, which is the only size
available commercially. The applicant suggested that if use of these small doses were to become
more common, the percentage of discarded units could increase the discarded drug refund
amount that could be owed by the applicant, even though the applicant lacks control or
knowledge of the potential variability of the discarded amounts that may occur if Leukine
®
were
used for such purposes. If an FDA approval for adjuvant use of Leukine
®
were to occur by
another manufacturer, the available presentations of Leukine
®
would likely not be optimized for
the small doses being studied in these trials.
As part of CMS’s review of the application, we analyzed existing claims data from the
first quarter of 2023 through the first quarter of 2024 and found the percentage of units discarded
for the Healthcare Common Procedure Coding System (HCPCS) code for Leukine
®
(J2820)
ranged from 1.2 percent to 3.8 percent, which is below the applicable percentage of 10 percent.
Since we do not yet know the impact of a new adjuvant indication with a type of immunotherapy
commonly referred to as cancer vaccines
240
on the current percentage of units discarded, we are
not proposing an increased applicable percentage without additional information. Because it is
not yet known whether Leukine
®
will be approved for additional indications with varying dosage
requirements and the information provided by the applicant; and available data does not provide
enough information for CMS to determine whether Leukine
®
has unique circumstances that
would prompt an increase in the applicable percentage, we are not proposing an increase in the
applicable percentage for the drug at this time.
240
https://www.cancerresearch.org/treatment-types/cancer-vaccines.
The applicant may reapply in a future application cycle when more information becomes
available.
c. Clarifications for the definition of refundable single-dose container or single-use package drug
(1) Exclusions for drugs for which payment has been made under Part B for fewer than 18
months
Section 1847A(h)(8)(B)(iii) of the Act excludes from the definition of refundable drug a
drug approved or licensed by FDA on or after November 15, 2021, and for which payment has
been made under Part B for fewer than 18 months. This is codified in the definition of
refundable single-dose container or single-use package drug in § 414.902. In the CY 2023 PFS
final rule (87 FR 69720 through 69731), we finalized that the 18-month period begins on the first
day of the calendar quarter following the date of first sale as reported to CMS for the first
National Drug Code (NDC) assigned to the HCPCS code. We expected that the first date of sale
would approximate the date of payment of the first Part B claim, and we finalized that we would
use the first date of sale because it is more operationally feasible than identifying the date when
the first Part B claim was paid for a new drug. We did not receive any opposing comments to
this approach when the policy was proposed (87 FR 69719 through 69724). Since then,
however, we have found one instance where the date of first sale for a drug, as reported to CMS,
does not adequately approximate the first date for which payment was made under Part B.
We propose that, while we would continue to use the first date of sale reported to CMS
for most refundable drugs, we would use the date on which the drug is first paid under Part B if
the date of first sale as reported to CMS does not adequately approximate the first date of
payment under Part B due to an applicable National Coverage Determination (NCD). Under the
exception, the first date for which the drug is actually paid under Part B (not the date of first sale)
would be used to determine the beginning of the 18-month exclusion period.
For example, in the case of Leqembi
®
(lecanemab-irmb), the second drug targeting
cerebral amyloid-beta plaques in Alzheimer’s disease to receive FDA approval, the first date of

sale reported to CMS via the Average Sales Price (ASP) portal was in January 2023, as it was
marketed and sold under accelerated approval granted on January 6, 2023. However, because
Leqembi
®
is subject to the NCD for Monoclonal Antibodies Directed Against Amyloid for the
Treatment of Alzheimer’s Disease under coverage with evidence development (CED),
241
and
because Leqembi
®
was initially marketed and sold under accelerated approval, Leqembi
®
coverage under Part B required the product to be furnished in a randomized controlled trial
(RCT) conducted under an investigational new drug (IND) application.
242
In public comments
on the CY 2024 proposed rule, the manufacturer of Leqembi
®
explained that Leqembi
®
’s Phase
III confirmatory trial was already fully enrolled and complete prior to FDA granting accelerated
approval, and as such, there was no RCT in which to enroll Medicare beneficiaries. Leqembi
®
received traditional approval on July 6, 2023. The first Part B payments for Leqembi
®
did not
occur until after traditional FDA approval of the drug on July 6, 2023, and Medicare paid for the
drug beginning that month in CED studies using a registry.
243
Under policies finalized in the CY
2023 PFS final rule, the 18-month exclusion period for Leqembi
®
would begin on April 1, 2023,
which marks the first day of the calendar quarter after the drug’s first date of sale as reported to
CMS in January 2023. We believe that, in this situation, our current policy of using the date of
first sale as reported in ASP data does not adequately approximate the beginning of the 18-month
period for which payment has been made for the drug under Part B.
Under this proposed rule, the 18-month exclusion for Leqembi
®
would be October 1,
2023, through March 31, 2025 (that is, six full calendar quarters following the date that the drug
was first paid under Medicare Part B).
To maintain operational feasibility of this provision and better align the policy with
statutory language when the date of first sale reported to CMS does not adequately approximate
241
Section 200.3 of the Medicare National Coverage Determinations Manual.
242
https://www.cms.gov/medicare/coverage/coverage-evidence-development/monoclonal-antibodies-directed-
against-amyloid-treatment-alzheimers-disease-ad.
243
https://www.cms.gov/newsroom/press-releases/statement-broader-medicare-coverage-leqembi-available-
following-fda-traditional-approval.

the date of first payment under Medicare Part B, we are proposing to amend the exclusions in the
definition of refundable single-dose container or single-use package drug at § 414.902. We note
that we are also proposing to revise the structure of the definition of Refundable single-dose
container or single-use package drug and as part of that restructuring, we are proposing that
exclusions would be defined at paragraph (2) of the definition. Moreover, we are proposing to
add a fourth exclusion to paragraph (2) to address drugs for which the date of first sale does not
adequately approximate the first date of payment under Part B due to an applicable NCD. We
anticipate that instances of inadequately approximating the date of first payment under Medicare
Part B based on the date of first sale due to an applicable NCD will be rare, as coverage of a drug
under Part B is not often restricted by an NCD.
(2) Clarification for identifying single-dose containers
In the CY 2023 PFS final rule (87 FR 69719), we finalized that the definition of
refundable drug would apply to drugs paid under Medicare Part B (that is, under any payment
methodology) that are described in FDA-approved labeling as being supplied in a “single-dose”
container or “single-use” package. This definition also includes drugs described in FDA-
approved labeling as a part of a “kit” that is intended for a single dose or single use. We also
finalized that for a drug to meet the definition of refundable drug, all NDCs assigned to the
drug's billing and payment code must be single-dose containers, as described in each product's
labeling.
During our analysis in identifying refundable drugs for the preliminary reports (which are
based on available JW modifier data from the first and second quarters of 2023), we learned that
some product labeling
244
does not specify the package type terms (for example, whether the
product is supplied in a single-dose or single-use package or a multiple-dose preparation). This
may occur in drugs that were approved prior to October 2018 because at that time, FDA issued
244
“Product labeling” in this document means the container label, carton labeling, or prescribing information.

guidance
245
regarding the selection of the appropriate package terms to address bacterial and
viral infections among patients resulting from improper use of single-dose containers such as
vials, ampules, and prefilled syringes. The guidance defines a single-dose container as a
container of a sterile medication for parenteral administration (injection or infusion) that is not
required to meet the antimicrobial effectiveness testing requirements. The guidance further
states a single-dose container is designed for use with a single patient as a single
injection/infusion and, when space permits, the label should include the correct package type
term and appropriate discard statements. Discard statements include instruction for discarding
or, if appropriate, storage guidance for drugs remaining after preparation. The guidance defines
a multiple-dose container as a container of sterile medication for parenteral administration that
has met antimicrobial effectiveness testing requirements or is excluded from such testing
requirements. In addition, the guidance defines the term “single-patient-use” container, which
describes a package that contains multiple doses of an injectable medical product that is intended
to be used in a single patient.
Some drugs approved prior to the release of this guidance (that is, those prior to October
2018) and some Orphan drugs do not include the package type terms and explicit discard
statements. Examples of drugs without the package type terms and discard statements include
certain manufacturers of digoxin (approved in 1954), oxytocin (approved in 1980),
diphenhydramine (approved in 1982), phenobarbital (orphan drug without FDA approval).
Several of these drugs are available in small containers with only a few mL of labeled drug in the
containers.
In this proposed rule, we are proposing to include injectable drugs with a labeled volume
of 2 mL or less and that lack the package type terms and explicit discard statements in their
product labeling to be single-dose containers in the definition of refundable single-dose container
or single-use package drugs. We identified 2 mL as a threshold for this proposal for several
245
https://www.fda.gov/media/117883/download.

reasons. For intramuscular administration, the maximum volume administered at one time for
diphenhydramine and digoxin is less than or equal to 2 mL. We also note that for adults, the
maximum volume
246
for intramuscular administration is typically limited to 3 mL. For drugs
administered intravenously and supplied in containers containing 2 mL or less, like digoxin and
phenobarbital, dosages are calculated based on body weight, potentially leading to discarded
amounts. We believe that preparation of these drugs would likely be used for a single dose based
on the range of dose sizes for these drugs and the amount of drug in the container. In other
words, it is unlikely that more than one dose could be prepared from the amount of drug in the
container.
Another category of drugs approved before 2018 that lack discard statements is drugs
contained in ampules (also spelled as ampoules or ampuls, hereinafter referred to as “ampules”).
The term ampule is an airtight vial made of glass, plastic, metal, or any combination of these
materials.
247
Examples of drugs currently contained in ampules include epinephrine (approved
in 1939), lidocaine hydrochloride (1948), dicyclomine (1950), digoxin (1954), chlorpromazine
(1957), fentanyl citrate (1968), promethazine (1973), alprostadil (1981), nalbuphine (1993), and
tacrolimus (1994). Drugs contained in ampules are accessed by breaking the concaved part (“the
neck”), and the content should be passed through a sterile filter to remove any residual glass
particles.
248
Therefore, we are proposing to amend the definition of refundable single-dose container
or single-use package drug to include drugs contained in ampules and for which there is no
discard statement. We are proposing to classify drugs supplied in ampules to be drugs in single-
dose containers for purposes of this discarded drug policy because this approach would be
consistent with the description of single-dose container in the October 2018 FDA guidance. We
246
Open Resources for Nursing (Open RN); Ernstmeyer K, Christman E, editors. Nursing Skills [Internet]. 2nd
edition. Eau Claire (WI): Chippewa Valley Technical College; 2023. Chapter 18 Administration of Parenteral
Medications. Available from https://www.ncbi.nlm.nih.gov/books/NBK596739/.
247
40 CFR 273.9.
248
Pharmaceutical Compounding—Sterile Preparations. USP-NF 2023. November 1, 2023.
note that some drugs contained in ampules may be excluded from the definition of refundable
drug under section 1847A(h)(8)(B)(ii) of the Act because dosage and administration instructions
included in the product labeling require filtration during the drug preparation process, prior to
dilution and administration, and require that any unused portion of such drug after the filtration
process be discarded after the completion of such filtration process. This exclusion would still
be applicable for ampules that can demonstrate that they meet that exclusion. However, this is
not the case for the product labeling of all drugs contained in ampules.
In summary, we are proposing to amend the definition of Refundable single-dose
container or single-use package drug at § 414.902 by including “single-patient-use container” as
a package type term and adding three types of products that may be considered refundable
single-dose container or single-use package drugs under paragraph (1). These are:
(1) Product furnished from a single-dose container or single-use package based on FDA-
approved labeling or product information.
(2) Product furnished from an ampule for which product labeling does not have discard
statement or language indicating the package type term, like “single-dose container,” “single-use
package,” “multiple-dose container,” or “single-patient-use container”.
(3) Product furnished from a container with a total labeled volume 2 ml or less for which
product labeling does not have language indicating the package type term, like “single-dose
container,” “single-use package,” “multiple-dose container,” or “single-patient-use container”.
As noted above, we are also revising the organization of this definition in the regulatory text.
We welcome comments on these proposals.
(3) Skin substitutes
As discussed in the CY 2023 PFS final rule (87 FR 69650 through 69655), CMS aims to
create a consistent coding and payment approach for the suite of products currently referred to as
skin substitutes. In the CY 2024 PFS final rule (88 FR 79060 through 79061), we finalized that
billing and payment codes that describe products currently referred to as skin substitutes are not
counted for purposes of identifying refundable drugs for calendar quarters during 2023 and 2024.
While we continue to consider making changes to the Medicare Part B payment policies for such
products, similar to last year, for CY 2025, we are proposing again that billing and payment
codes that describe products currently referred to as skin substitutes not be counted for purposes
of identifying refundable drugs for calendar quarters in 2025. We plan to revisit discarded drug
refund obligations for skin substitutes in future rulemaking. More information regarding the
payment of skin substitutes is available in section II.K. of this proposed rule.
d. Discarded amounts
Effective January 1, 2017, providers and suppliers were required to report the JW
modifier on all claims that bill for drugs separately payable under Medicare Part B with unused
and discarded amounts (that is, discarded amounts) from single-dose containers or single-use
packages. In the CY 2023 PFS, we finalized the requirement to use the JW modifier for single-
dose container drugs that are separately payable under Part B, and we finalized the use of the JW
modifier (or any successor modifier that includes the same data) to identify discarded billing
units of a billing and payment code for the purpose of calculating the refund amount as described
in section 1847A(h)(3) of the Act. In that final rule, to align with the JW modifier policy, we
also finalized the requirement that, beginning July 1, 2023, the JZ modifier is required when
there are no discarded amounts of a single-dose container drug for which the JW modifier would
be required if there were discarded amounts.
In the CY 2023 PFS final rule (87 FR 69723), we discussed the applicability of the JW
and JZ modifiers to drugs that are not administered by the billing supplier, including drugs
furnished through a covered item of DME that may be administered by the beneficiary. In such
cases, we stated that the reporting requirement does not apply to drugs that are self-administered
by a patient or caregiver in the patient's home. In the JW Modifier and JZ Modifier Policy FAQ

document
249
released on January 5, 2023, we reiterated that suppliers who dispense but do not
actually administer a separately payable drug are not expected to report the JW or JZ modifier.
Then, in the CY 2024 PFS final rule (88 FR 79062), we finalized a change to this policy,
such that drugs separately payable under Part B from single-dose containers that are furnished by
a supplier who is not administering the drug be billed with the JZ modifier. This meant that the
JW modifier would not be used on these claims. As we stated in that rule, in the absence of a
claims modifier to designate that a drug was dispensed, but not administered, by the billing
supplier (as finalized in the CY 2023 PFS), we were concerned that claims rejections may occur.
Therefore, this change in policy required the JZ modifier on all such claims to ensure claims
rejections did not occur unnecessarily. On October 16, 2023, we updated the JW Modifier and JZ
Modifier Policy FAQ document to include the requirement of the JZ modifier by the supplier.
However, after this policy was finalized, interested parties have requested further clarification on
how to appropriately bill for discarded amounts from single-dose containers when there are
amounts discarded during preparation by the billing supplier who is not administering the drug.
To provide additional clarity, we are now proposing to require the JW modifier if a billing
supplier is not administering a drug, but there are amounts discarded during the preparation
process before supplying the drug to the patient. Such a supplier would report the JZ modifier if
no amounts were discarded during the preparation process before supplying the drug to the
patient.
We believe this proposal is appropriate because drug preparation occurs before supplying
a drug to the beneficiary and the billing supplier can determine the discarded amount at the site
of drug preparation. These discarded units should be billed using the JW modifier in the same
way as a drug that is administered incident-to physician service. In addition, suppliers and other
interested parties have expressed that suppliers are accustomed to using the JW modifier in this
249
https://www.cms.gov/medicare/medicare-fee-for-service-payment/hospitaloutpatientpps/downloads/jw-modifier-
faqs.pdf.
context already. Therefore, we propose to require the JW modifier if a billing supplier is not
administering a drug, but there are amounts discarded during the preparation process before
supplying the drug to the patient. For example, if a billing supplier prepares a dose from a
single-dose vial labeled as containing a total of 50 billing units such that 45 billing units of the
drug are used in the prepared dose and 5 billing units are discarded during preparation, and then
the drug is supplied to the patient (but not administered by the supplier), the claim should be
submitted on two lines: 45 units (without a modifier) and 5 units with the JW modifier. We
reiterate that suppliers who dispense a drug, but do not actually administer the drug, are not
expected to monitor or bill for discarded amounts that are discarded after the drug is supplied
because they are not at the site of administration to measure discarded amounts. For example, if
the patient who was supplied the above dose with 45 billing units subsequently only receives 35
of those billing units, the above billing supplier would not be expected to account for the 10
subsequently discarded billing units on the claim.
We welcome comments on this proposal.
2. Payment Limit Calculation When Manufacturers Report Negative or Zero Average Sales
Price (ASP) Data (§ 414.904)
a. Background
Drugs payable under Medicare Part B fall into three general categories: those furnished
incident to a physician's service (hereinafter referred to as “incident to”) (section 1861(s)(2) of
the Act), those furnished via a covered item of durable medical equipment (DME) (section
1861(s)(6) of the Act), and other drugs for which coverage is specified by statute (for example,
certain vaccines described in sections 1861(s)(10)(A) and (B) of the Act). Payment limits for
most drugs separately payable under Medicare Part B are determined using the methodology in
section 1847A of the Act, and in many cases, payment is based on the average sales price (ASP)
plus a statutorily mandated 6 percent add-on. If CMS determines a payment limit for a drug, it is

published in the ASP pricing file or Not Otherwise Classified (NOC) pricing file,
250
which are
both updated quarterly.
CMS generally calculates the payment limits for drugs payable under Part B on a
quarterly basis using the manufacturer’s ASP (as defined in § 414.902). Manufacturers are
required to report ASP to CMS pursuant to sections 1847A(f)(2) and 1927(b)(3) of the Act.
Manufacturers are instructed to calculate ASP in accordance with section 1847A(c) of the Act
and § 414.804(a).
For each NDC, in most cases, the manufacturer’s ASP is a positive dollar value, along
with a positive number of units sold (hereinafter referred to as “positive manufacturer’s ASP
data”). However, sometimes the reported data is not positive manufacturer’s ASP data.
Specifically, a manufacturer could report that an NDC has a negative or zero-dollar value for the
manufacturer’s ASP with a positive, negative, or zero number of units sold, or a positive dollar
value for the manufacturer’s ASP with a negative or zero number of units sold (each of these
scenarios is hereinafter referred to as “negative or zero manufacturer’s ASP data”). Such
negative or zero manufacturer’s ASP data could occur because of lagged discounts, units
returned to the manufacturer, drug shortages, discontinuation of a drug, or other reasons that are
not known to CMS. Negative or zero manufacturer’s ASP data can occur when a manufacturer
calculates its ASP in accordance with section 1847A of the Act.
First, section 1847A(c)(3) of the Act requires that the manufacturer's calculation of its
ASP for an NDC must include volume discounts, prompt pay discounts, cash discounts, free
goods that are contingent on any purchase requirement, chargebacks, and rebates (other than
rebates under the Medicaid drug rebate program or the Medicare Prescription Drug Inflation
Rebate Program) (hereinafter referred to as “price concessions”). Second, section
1847A(c)(5)(A) of the Act requires each manufacturer to apply a methodology based on a 12-
month rolling average for the manufacturer to estimate costs attributable to price concessions if
250
https://www.cms.gov/medicare/payment/part-b-drugs/asp-pricing-files.

there is a lag in the reporting of the information on rebates and chargebacks under section
1847A(c)(3) of the Act. These provisions may result in the inclusion of large price concessions
from a quarter or quarters with a higher sales price prior to price concessions in the ASP
calculation for a subsequent quarter with a much lower sales price, which can result in negative
dollar value ASP. The same situation could happen in a quarter if more units were returned to
the manufacturer than are sold, which can result in a negative dollar value ASP as well as a
negative number of units sold. The requirement to use a rolling average for lagged price
concessions is codified at § 414.804(a)(3), which states that, to the extent data on price
concessions are available on a lagged basis, the manufacturer must estimate its ASP in
accordance with the described methodology in that paragraph. In certain instances, as stated
above, lagged price concessions can lead to negative or zero manufacturer’s ASP data.
In 2022, the U.S. Department of Health and Human Services Office of Inspector General
(OIG) issued a report assessing potential inaccuracies in manufacturer reporting of ASP and
noted that manufacturers believe additional guidance may be needed to reduce distortions and
inconsistencies in the calculation of payment limits.
251
The report found that several
manufacturers would like additional guidance regarding reporting of negative ASP data and how
CMS uses negative ASP data in payment limit calculations. CMS concurred with the OIG’s
recommendation to actively review current guidance and determine whether additional guidance
would ensure more accurate and consistent ASP calculations.
Accordingly, we reviewed our current guidance and determined that it is appropriate for
us to provide additional guidance regarding how CMS will handle payment for drugs separately
payable under Part B when the manufacturer’s ASP for at least one NDC within the billing and
payment code (that is, HCPCS code) of the drug is negative or zero. Currently, when all NDCs
assigned to a HCPCS code have negative or zero manufacturer's ASP data, CMS establishes the
payment limit in other ways. As appropriate given the data available for a drug, CMS will either
251
OEI-BL-21-00330. https://oig.hhs.gov/oei/reports/OEI-BL-21-00330.asp.

calculate a payment limit for a billing and payment code based on other applicable and available
pricing data or not include a payment limit for the billing and payment code on the ASP pricing
file. When a payment limit for a drug separately payable under Part B is not included in the ASP
pricing file, the payment limit is based on either the published or Wholesale Acquisition Cost
(WAC) or invoice pricing, as described in section 20.1.3, Chapter 17 of the Medicare Claims
Processing Manual.
252
CMS previously contemplated how to set a payment limit in certain situations in which
ASP data is “not available” for multiple source drugs. In the CY 2011 PFS final rule (75 FR
73461 through 73465), CMS addressed situations in which ASP data for some, but not all, NDCs
in a multiple source drug billing and payment code are not available for the calculation of an
ASP payment limit (for example, if a manufacturer's entire submission of data was not received
or manufacturer’s ASP data for specific NDCs was not reported).
253
In that rule, we finalized a
process, consistent with authority in section 1847A(c)(5)(B) of the Act, to update payment limits
based on the manufacturer’s ASP reported for the most recent quarter for which data is available.
We specified that if manufacturer’s ASP data is not available for some but not all NDCs in a
multiple source drug billing and payment code prior to the publication deadline for quarterly
payment limits and such unavailability of manufacturer’s ASP data significantly changes the
quarterly payment limit for the billing and payment code when compared to the prior quarter’s
payment limit, CMS will calculate the payment limit by carrying over the most recent available
manufacturer’s ASP price from a previous quarter for an NDC, adjusted by the weighted average
of the change in the manufacturer’s ASPs for the NDCs that were reported for both the most
recently available previous quarter and the current quarter, and codified this policy in §
414.904(i).
254
In that final rule, we explained that such circumstances are limited to when a
252
https://www.cms.gov/%E2%80%8BRegulations-and-
Guidance/%E2%80%8BGuidance/%E2%80%8BManuals/%E2%80%8BDownloads/%E2%80%8Bclm104c17.pdf.
253
https://www.govinfo.gov/content/pkg/FR-2010-11-29/pdf/2010-27969.pdf.
254
https://www.ecfr.gov/current/title-42/part-414/section-414.904#p-414.904(i).
manufacturer's data for a multiple source drug product with sales during a quarter is missing, and
efforts to obtain manufacturer reported ASP data before Medicare ASP payment limits
publication deadlines have not been successful. We continue to believe that this process, which
we apply in cases ASP data is “not available” for some but not all NDCs associated with a
multiple source billing and payment code, is appropriate.
b. Approach to payment limit calculations when manufacturer’s ASP data is not available
As described in the previous section, we determined that it is appropriate for CMS to
provide additional guidance regarding how we will handle payment for drugs separately payable
under Part B when the reported manufacturer’s ASP for at least one NDC within the billing and
payment code (that is, HCPCS code) of the drug is negative or zero (that is, has negative or zero
manufacturer’s ASP data). As detailed below, we are proposing to consider ASP data to be not
“available” for the purposes of calculating a payment limit in circumstances in which negative or
zero manufacturer’s ASP data is reported, consistent with section 1847A(c)(5)(B) of the Act.
We are also proposing how CMS would calculate a payment limit in these circumstances,
consistent with section 1847A(c)(5)(B) of the Act.
Our current policy does not address how payment limits are calculated for several
situations in which a drug separately payable under Part B does not have available ASP data.
The set of situations in which this might occur include circumstances in which either some or all
NDCs for a billing and payment code have a negative or zero manufacturer’s ASP data; in which
negative or zero manufacturer’s ASP data is reported for a drug which has been discontinued;
and vary further depending on whether a drug is multiple source or single source (both as defined
in § 414.902). In each of these circumstances, there are various other pricing data available that
we believe can appropriately be used to calculate a payment limit.
Therefore, we are proposing, consistent with section 1847A(c)(5)(B) of the Act, a
methodology for calculating payment limits in certain circumstances based on manufacturer’s
ASP for the most recent quarter for which data is available. Specifically, we propose to specify
that positive manufacturer’s ASP data are considered “available” and that negative or zero
manufacturer’s ASP data are considered “not available” for purpose of CMS calculating a
payment limit under the statute. We believe it is appropriate to consider negative or zero
manufacturer’s ASP data to be not available because if used to calculate a payment limit, this
data can result in a negative or zero payment limit, which would require CMS to collect payment
from providers and suppliers for a drug, rather than make payment for a drug. Negative or zero
payment limits for a drug are not reasonable because Medicare does not expect to collect
payment from providers and suppliers for their provision of separately payable drugs. Therefore,
we are proposing to specify the methodology we will use for calculating the payment limit in
such circumstances to ensure reasonable payment amounts based on the best available data for
separately payable drugs. Below, we propose how payment limits would be determined using
available ASP data for each scenario.
c. Single and multiple source drugs when negative or zero manufacturer’s ASP data is reported
for some, but not all NDCs
In the case that a drug separately payable under Part B has negative or zero
manufacturer’s ASP data reported for some, but not all, NDCs associated with a billing and
payment code for that drug, we are proposing to calculate a payment limit using only NDCs with
positive manufacturer’s ASP data (and omitting NDCs with negative or zero manufacturer’s ASP
data) for that drug and propose to codify this at § 414.904(i). We are proposing this policy to
apply to both single source drugs, including biosimilar biological products, and multiple source
drugs. We believe this is appropriate because it would result in payment limits based on the most
recent positive manufacturer’s ASP data reported by manufacturers with NDCs associated with a
billing and payment code.
However, we note that, as discussed in section III.A.2.a of this proposed rule, CMS
already has a policy in place for multiple source drugs for which the absence of ASP data would
result in a significant change (that is, a 10 percent or greater change) in the ASP payment limit

compared to the payment limit of the previous quarter, as finalized in the CY 2011 PFS final rule
(75 FR 73461 through 73465). In that discussion (75 FR 73462), we noted several examples of
situations in which data is not available to be included in the calculation of a payment limit, such
as when a manufacturer's entire submission was not received or when the manufacturer’s ASP
data for specific NDCs has not been reported. We do not intend for our proposed policy to
override that existing policy; rather, we intend for the proposed policy described above to
address circumstances not addressed in that rulemaking (that is, we intend to address
circumstances of single source drugs when negative or zero manufacturer’s ASP data is reported
for some, but not all NDCs, and of multiple source drugs when negative or zero manufacturer’s
ASP data is reported for some, but not all NDCs and the absence of such data from the
calculation of the payment limit does not result in a significant change in the payment limit
compared to the payment limit of the previous quarter) and thus fill a policy gap. In addition, the
circumstances we provided as examples in which ASP data is not available in the CY 2011 PFS
final rule continue to be circumstances we consider manufacturer’s ASP data not available under
current § 414.904(i) (which we propose to move within § 414.904(i) to fit within the structure of
the proposed new set of payment limit methodologies); but, as noted in section III.A.2.b, we are
expanding what we consider to be not available to include circumstances in which negative or
zero manufacturer’s ASP data is reported.
d. Multiple source drugs with only negative or zero manufacturer’s ASP data
In the case of a multiple source drug (as defined in § 414.902) separately payable under
Part B that has negative or zero manufacturer’s ASP data reported for all NDCs associated with a
billing and payment code for that drug (and at least one NDC for the is actively being marketed
(that is, not discontinued)), we are proposing to carry over all positive manufacturer’s ASP data
from the most recently available previous quarter with positive manufacturer’s ASP data for at
least one NDC until at least one NDC for the drug has positive manufacturer’s ASP data for a
quarter. Specifically, we are proposing to calculate the payment limit for the applicable quarter
using data from the most recent calendar quarter for which data is available, i.e., for which there
is positive manufacturer’s ASP data. We believe this is appropriate because, similar to the
methodology described in section III.A.2.c, it would result in payment limits based on the most
recent positive manufacturer’s ASP data reported by manufacturers with NDCs associated with a
billing and payment code. Similarly, we believe the most recent available positive
manufacturer’s ASP data from NDCs associated with a billing and payment code are more likely
to be reflective of providers’ acquisition costs for drugs associated with that billing and payment
code in a given quarter than other pricing data, and unlikely to result in challenges to access for
these drugs for providers and beneficiaries.
We note that because section 1847A of the Act provides for payment limit calculations
that differ between single-source drugs (as defined in section 1847A(c)(6)(D) of the Act and §
414.902) and multiple source drugs (as defined in section 1847A(c)(6)(C) of the Act and §
414.902), we are proposing different ways to determine payment limits for each, in cases in
which only negative or zero manufacturer’s ASP data is reported, to reflect these differences.
Specifically, the payment limit for single source drugs is described in section 1847A(b)(1)(B) of
the Act; for multiple source drugs, the payment limit is described in section 1847A(b)(1)(A) of
the Act. The payment limit for single source drugs is determined using the lesser of ASP or
WAC; but WAC is not used for multiple source drugs whose ASP exceeds WAC. Nonetheless,
our proposals for the calculation of the payment limit for single source and multiple source drugs
with only negative or zero manufacturer’s ASP data are consistent in that, where ASP is used, we
propose to use the most recent available positive manufacturer’s ASP data from at least one
NDC for the drug. We believe using similar input data in our calculation of the payment limit is
consistent with our goal to ensure reasonable payment amounts based on the best available data
for separately payable drugs.
We are proposing to amend § 414.904(i) to include the above proposal regarding how
CMS would calculate the payment limit in circumstances in which only negative or zero
manufacturer’s ASP data is reported for a multiple source drug.
e. Single source drugs with only negative or zero manufacturer’s ASP data, excluding biosimilar
biological products
In the case of a single source drug, excluding biosimilar biological products (both as
defined in §414.902), separately payable under Part B that has negative or zero manufacturer’s
ASP data reported for all NDCs associated with a billing and payment code for that drug (and at
least one NDC for the drug is actively being marketed (that is, not discontinued)), we are
proposing to set the payment limit for the given quarter for the single source drug at the lesser of
the following until at least one NDC for the drug has positive manufacturer’s ASP data for a
quarter:
● 106 percent of the volume-weighted average of the most recent available positive
manufacturer’s ASP data from a previous quarter in which at least one NDC for the drug has
positive manufacturer’s ASP data for a quarter. If the payment limit from the quarter with the
most recent available positive manufacturer’s ASP data was based on 106 percent of the WAC
because of the application of § 414.904(d)(1), that payment limit would be carried over; or
● 106 percent of the WAC for the given quarter. If there is more than one WAC per
billing unit for the drug, the payment limit would be set using the lowest WAC per billing unit.
We would only use the lesser of the positive manufacturer’s ASP or WAC data from that
previous quarter or the WAC data from the given quarter until positive manufacturer’s ASP data
is available for a future quarter. Once positive manufacturer’s ASP data for a drug is available
again in a future quarter, CMS will have data available to input into the routinely used
methodologies described in section 1847A(b) of the Act and § 414.904.
As discussed above, we believe it is appropriate to propose different policies for
determining payment limits for single and multiple source drugs when negative or zero
manufacturer’s ASP data is reported because of statutory differences in the payment limit
calculations.
f. Biosimilars with only negative or zero manufacturer’s ASP data
In circumstances in which negative or zero manufacturer’s ASP data is reported for all
NDCs for a biosimilar biological product (defined at § 414.902) (hereinafter referred to as a
“biosimilar”) for a given quarter (and at least one NDC for the biosimilar is actively being
marketed (that is, not discontinued)), and positive manufacturer’s ASP data is available for
another biosimilar(s) with the same reference biological product (hereinafter referred to as a
“reference product”) for the given quarter, we are proposing to set the payment limit for the
given quarter equal to the sum of the following until at least one NDC for the particular
biosimilar for which all NDCs report negative or zero manufacturer’s ASP data has positive
manufacturer’s ASP data for a quarter:
● The volume-weighted average of the positive manufacturer’s ASP data from all other
biosimilars with the same reference product, and
● 6 percent (or 8 percent for qualifying biosimilar biologicals as defined in § 414.902, as
appropriate) of the amount determined under section 1847A(b)(4) of the Act for the reference
biological product (as defined in § 414.902) for the given quarter.
We believe this is appropriate because Section 351(i)(2) of the Public Health Service Act
defines the terms biosimilar and biosimilarity to mean that a biosimilar is highly similar to its
reference product, notwithstanding minor differences in clinically inactive components, and that
there are no clinically meaningful differences between the biosimilar and the reference product
in terms of the safety, purity, and potency of the product. In addition, biosimilars with the same
reference product likely compete in the marketplace since both rely on FDA’s previous
determination of safety, purity, and potency for the reference product clinical data for approval.
For these reasons, we believe that when a biosimilar has only negative or zero manufacturer’s
ASP data, the volume-weighted average of positive manufacturer’s ASP data of biosimilars with
the same reference product would be an appropriate payment limit for a biosimilar that, under
this proposal, would be considered to have ASP data that is not available. As such, we are
proposing to calculate the payment limit for a biosimilar with only negative or zero
manufacturer’s ASP data based on the positive manufacturer’s ASP data of other biosimilars
with the same reference product.
We note that in the CY 2016 PFS final rule (80 FR 71096 through 71101), we finalized
that we would group all biosimilars with a common reference product in a single billing and
payment code with a single payment rate, in a manner similar to how we price multiple source or
generic drugs because of the significant similarities between each biosimilar and its reference
product. In the CY 2018 PFS final rule (82 FR 53182 through 53187), we changed the initial
policy and finalized separate coding and payment for biosimilars. In that final rule, we stated that
that there is a program need for assigning Part B biosimilars into separate billing and payment
codes; specifically, that this policy change addressed concerns about the public interest in a
stronger marketplace, access to these drugs in the United States marketplace, and provider and
patient choice and competition. Our proposal for biosimilars with negative or zero
manufacturer’s ASP data reported for all NDCs is consistent with the CY 2018 PFS rulemaking,
as it would not result in grouping biosimilars with a shared reference product in a single billing
and payment code. Rather, it would allow CMS to calculate an operationally reasonable payment
limit using positive manufacturer’s ASP data for highly similar products in limited instances.
This proposal would also provide payment limit stability that could help avoid potential
access issues for providers and beneficiaries that could otherwise occur if we were to calculate a
payment limit for a drug with negative or zero manufacturer’s ASP data that is far below the
provider’s cost for acquiring the drug. If a biosimilar’s ASP falls below zero only after several
quarters of declining but still positive manufacturer’s ASP data, the most recent positive
manufacturer’s ASP data from a previous quarter for a drug may be significantly lower than the
volume-weighted average of the biosimilars with the same reference product as the biosimilar
with negative ASP data. In such a case, the payment limit based on the ASPs of competitor
biosimilars would be higher than if we were constrained to use ASP data only from the
biosimilar that has most recently reported negative or zero manufacturer’s ASP data. We note
that under the methodology proposed in section III.A.2.c, in circumstances in which some, but
not all NDCs of a single or multiple source drug are negative or zero, we would similarly
calculate the payment limit using only NDCs with positive manufacturer’s ASP data from the
given quarter and omitting those that had declined to zero or a negative value in ASP or sales.
Likewise, we believe that such an approach would likely result in a payment limit reflective of
providers’ acquisition costs of biosimilars and be helpful in avoiding access issues for providers
and beneficiaries.
In circumstances in which negative or zero manufacturer’s ASP data is reported for all
NDCs for a biosimilar for a given quarter and either no other biosimilars have been approved for
the same reference product or no other biosimilars with the same reference product report
positive manufacturer’s ASP data for the given quarter, we propose that we would set the
payment limit for the given quarter equal to the sum of the following until at least one NDC for
the biosimilar has positive manufacturer’s ASP data for a quarter:
● The volume-weighted average of the most recent available positive manufacturer’s
ASP data from a previous quarter, and
● 6 percent (or 8 percent for qualifying biosimilar biologicals, as appropriate) of the
amount determined under section 1847A(b)(4) of the Act for the reference biological product (as
defined in § 414.902) for the given quarter.
In situations in which CMS would use the volume-weighted average of the most recent
available positive manufacturer’s ASP data from a previous quarter, we would only use positive
manufacturer’s ASP data from that previous quarter until positive manufacturer’s ASP data is
available for a future quarter. This proposed methodology is similar to the proposed

methodology for multiple source drugs and single source drugs that are not biosimilars when
manufacturers report negative or zero manufacturer’s ASP for all NDCs.
In addition to the payment approaches we are proposing for biosimilars with only
negative or zero manufacturer’s ASP data, we considered two alternatives for which we are
seeking public comment. Under the first alternative, the volume-weighted ASP calculation
would include the ASP data and billing units sold of its reference product for a given quarter
along with those of the other biosimilars that reference the same reference product in the
volume-weighted average calculation. We believe including the reference product’s data in the
blended calculation for a biosimilar’s payment limit in the limited circumstance described could
be appropriate in determining an operationally reasonable payment limit because the FDA
approval for the biosimilar relies in part on FDA’s previous determination of safety, purity, and
potency for the reference product, and the biosimilar and reference product are necessarily
approved for at least one shared condition of use, as required under the 351(k) approval
pathway;
255
therefore, the case that the two are comparable is at least as strong as that for any
two biosimilars with the same reference product. If it is preferable, as we propose, to base the
payment limit on the available positive manufacturer’s ASP data submitted by manufacturers of
market competitor biosimilars (in this context, biosimilars that reference the same reference
product), then including the ASP data and billing units sold of the reference product would also
increase the likelihood that positive data in such a group is available, particularly in the case that
a reference product only has one biosimilar. Under this alternative, the payment limit would be
set equal to the sum of the volume-weighted average of the positive manufacturer’s ASP data
from all other biosimilars with the same reference product and the reference product plus 6 or 8
percent, as appropriate, of the amount determined under section 1847A(b)(4) of the Act for the
reference biological product for the given quarter. We are seeking public comments about
255
https://www.fda.gov/science-research/fda-stem-outreach-education-and-engagement/development-biosimilar-
351k-bla-clinical-pharmacology-study-database.

whether including ASP data from the reference product in a variant of the proposed calculation
would produce a more appropriate payment limit for a biosimilar with only negative or zero
manufacturer’s ASP data.
Under the second alternative, we would calculate payment limits for all biosimilars with
only negative or zero manufacturer’s ASP data in the manner described above for biosimilars
when either no other biosimilars have been approved for the same reference product or no other
biosimilars with the same reference product report positive manufacturer’s ASP data for the
given quarter. That is, under this alternative we would not consider the manufacturer’s ASP data
of other biosimilars with the same reference product; rather, we would base the payment limit of
the biosimilar on the volume-weighted average of the its own most recent available positive
manufacturer’s ASP data from a previous quarter and either 6 or 8 percent, as appropriate, of the
amount determined under section 1847A(b)(4) of the Act for the reference biological product for
the given quarter. We are interested in comments from interested parties about whether, and if
so, why, it is preferable for the payment limit to be calculated only using manufacturer’s ASP
data from the biosimilar that reports negative or zero manufacturer’s ASP data in a given quarter.
g. Discontinued drugs
Generally, for single source drugs and multiple source drugs for which negative or zero
manufacturer’s ASP data is reported for all NDCs and for which all relevant applications (for
example, new drug applications (NDAs), biologics license applications (BLAs), or abbreviated
new drug applications (ANDAs)) have a marketing status of “discontinued” on the FDA
website,
256, 257
we propose that the drug be priced by MACs consistent with section 20.1.3 in
Chapter 17 of the Medicare Claims Processing Manual for developing payment limits for
256
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm.
257
https://purplebooksearch.fda.gov/.

covered drugs when CMS does not supply the payment allowance limit on the ASP drug pricing
file.
258
Once a drug is discontinued, as indicated by the marketing status on the FDA website
(either at Drugs@FDA
259
for drugs or the Purple Book
260
for biologicals), the manufacturer
might not have sales to calculate an ASP and, therefore, the manufacturer often reports zero sales
for the drug or a negative number for its calculated ASP or number of sales. However, even if a
drug has a marketing status of discontinued on the FDA website, there may theoretically be
available product that could be billed by the provider until the expiration date of the last lot sold
for the drug. Relatedly, we have observed that very few claims are paid for drugs following their
discontinuation. For these reasons, setting a payment limit for drugs with a marketing status of
discontinued on the FDA website is not expected to be practically useful for claims processing
and is not a prudent use of CMS resources.
h. Summary
We are proposing to amend § 414.904(i) to reflect CMS’s approach to setting a payment
limit in circumstances in which negative or zero manufacturer’s ASP data is reported by a
manufacturer for a drug. Specifically, we are proposing to codify that in cases where negative or
zero manufacturer’s ASP data is reported for some, but not all, NDCs of a multiple source drug,
we would calculate the payment limit using the positive manufacturer’s ASP data reported for
the drug, except for the existing carryover policy for multiple source drugs that we would apply
when unavailable data results in a significant change in the ASP payment limit. We are
proposing to move this carryover policy for multiple source drugs within § 414.904(i) to fit
within the structure of the proposed new set of payment limit methodologies. We are also
proposing to codify that in the case of a multiple source drug for which negative or zero
258
Medicare Claims Processing Manual Chapter 17, section 20.1.3: https://www.cms.gov/regulations-and-
guidance/guidance/manuals/downloads/clm104c17.pdf.
259
https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm.
260
https://www.fda.gov/drugs/therapeutic-biologics-applications-bla/purple-book-lists-licensed-biological-
products-reference-product-exclusivity-and-biosimilarity-or.
manufacturer’s ASP data is reported for all NDCs, we would set the payment limit using the
most recent available positive manufacturer’s ASP data from a previous quarter until at least one
NDC for the drug has positive manufacturer’s ASP data for a quarter.
We are proposing to codify that in cases where negative or zero manufacturer’s ASP data
is reported for some, but not all, NDCs of a single source drug that is not a biosimilar, we would
calculate the payment limit using the positive manufacturer’s ASP data reported for the drug. We
are proposing to codify that for single source drugs that are not biosimilars with all negative or
zero manufacturer’s ASP data for a given quarter, the payment limit would be, until at least one
NDC for the drug has positive manufacturer’s ASP data for a quarter, the lesser of 106 percent of
the volume-weighted average of the most recent available positive manufacturer’s ASP data for
at least one NDC from a previous quarter and 106 percent of the WAC, and we would use 106
percent of the lowest WAC per billing unit if there is more than one WAC per billing unit
available.
We are also proposing to codify that in cases where negative or zero manufacturer’s ASP
data is reported for some, but not all, NDCs of a biosimilar, we would calculate the payment
limit using the positive manufacturer’s ASP data reported for the biosimilar. Lastly, we are
proposing to codify two scenarios when the manufacturer reports negative or zero
manufacturer’s ASP for all NDCs for a biosimilar for a given quarter:
● When positive manufacturer’s ASP data is available for another biosimilar(s) with the
same reference product for the given quarter, we are proposing to set the payment limit equal to
the sum of the volume-weighted average of the positive manufacturer’s ASP data from all other
biosimilars with the same reference product plus 6 percent (or 8 percent for a qualifying
biosimilar biological) of the amount determined under section 1847A(b)(4) of the Act for the
reference biological product for the given quarter; and
● When either no other biosimilars have been approved for the same reference product
or no other biosimilars with the same reference product report positive manufacturer’s ASP data
for the given quarter, we are proposing to set the payment limit equal to the sum of the volume-
weighted average of the most recent available positive manufacturer’s ASP data from a previous
quarter plus 6 percent (or 8 percent for a qualifying biosimilar biological) of the amount
determined under section 1847A(b)(4) of the Act for the reference biological product for the
given quarter.
We welcome comments on these proposals.
3. Payment of radiopharmaceuticals in the physician office
Section 303(c) of the Medicare Prescription Drug, Improvement, and Modernization Act
of 2003 (MMA) (Pub. L. 108–173, enacted December 8, 2003) revised the payment
methodology for most Medicare-covered Part B drugs by adding section 1847A to the Act,
which established a new average sales price (ASP) drug payment methodology for separately
payable Medicare Part B drugs, beginning January 1, 2005. Specifically, section 303(h) of the
MMA states, “Nothing in the amendments made by this section [303 of the MMA] shall be
construed as changing the payment methodology under [Medicare] Part B…for
radiopharmaceuticals, including the use by carriers of invoice pricing methodology.”
In accordance with the law, radiopharmaceuticals are not required to be paid using payment
methodology under section 1847A of the Act, as currently described in the Medicare Claims
Processing Manual (MCPM) Chapter 17, section 20.1.3. The manual instructs MACs to
determine payment limits for radiopharmaceuticals based on the methodology in place as of
November 2003, before the passage of the MMA, in the case of radiopharmaceuticals furnished
in settings other than the hospital outpatient department. Currently, payment can vary by MAC.
For example, payment can be based on 95 percent of Average Wholesale Price (AWP), invoices,

or other reasonable payment methods/data made available when the product is contractor
priced.
261, 262, 263, 264, 265, 266
CMS has heard from MACs and other interested parties that there is confusion about
which exact methodologies are available to MACs for pricing of radiopharmaceuticals in the
physician office setting, as different MACs had different methodologies in place as of November
2003. MACs are uncertain whether they can use any of these payment policies that were in
place, or only the policy that was in place for their jurisdiction as of November 2003.
Accordingly, while we evaluate our broader policies in this space for future rulemaking,
we are proposing to clarify that any payment methodology that was being used by any MAC
prior to the enactment of the MMA can continue to be used by any MAC, including the use of
invoice pricing. That is, we are proposing to clarify that any methodology that was in place to set
pricing of radiopharmaceuticals in the physician office setting prior to November 2003 can be
used by any MAC, whether or not that specific MAC used the methodology prior to November
2003.
Thus, we are proposing to codify in regulations at § 414.904(e)(6) that, for
radiopharmaceuticals furnished in a setting other than the hospital outpatient department, MACs
shall determine payment limits for radiopharmaceuticals based on any methodology used to
determine payment limits for radiopharmaceuticals in place on or prior to November 2003. Such
methodology may include, but is not limited to, the use of invoice-based pricing.
We welcome comments on these proposals.
261
How Does Palmetto GBA Price Drugs and Biologics?, Palmetto GBA.
https://www.palmettogba.com/palmetto/jjb.nsf/DIDC/8EELKH2211~Specialties~Drugs%20and%20Biologicals.
262
Radiopharmaceutical Fee Schedule 2024 Update, Noridian. https://med.noridianmedicare.com/web/jeb/fees-
news/fee-schedules/radiopharmaceutical-fees.
263
Radiopharmaceutical Drugs – Billing Instructions, A Celerian Group Company.
https://www.cgsmedicare.com/partb/pubs/news/2013/0313/cope21543.html.
264
Reimbursement Guidelines for Radiopharmaceuticals HCPCS Level II Codes, Novitas Solutions.
https://www.novitas-solutions.com/webcenter/portal/MedicareJL/pagebyid?contentId=00231502.
265
Reimbursement Guidelines for Radiopharmaceuticals Procedure Codes (Prior to January 2023), First Coast
Service Options, Inc. https://medicare.fcso.com/Coverage_News/0494780.asp.
266
Radiopharmaceutical Reimbursement, National Government Services.
https://www.ngsmedicare.com/web/ngs/fee-schedule-lookup-
details?lob=93617&state=97256&rgion=93623&selectedArticleId=4920515.
4. Immunosuppressive therapy (§§ 410.30 and 414.1001)
a. Background
Medicare Part B coverage of drugs used in immunosuppressive therapy was established
by section 9335(c) of the Omnibus Budget Reconciliation Act of 1986 (Pub. L. 99–509) (OBRA
'86). OBRA '86 added subparagraph (J) to section 1861(s)(2) of the Act to provide Medicare Part
B coverage for immunosuppressive drugs, furnished to an individual who receives an organ
transplant for which Medicare payment is made, for a period not to exceed 1 year after the
transplant procedure. Coverage of these drugs under Medicare Part B began January 1, 1987.
Section 4075 of the Omnibus Budget Reconciliation Act of 1987 (Pub. L. 100-203) (OBRA ’87)
revised section 1861(s)(2)(J) of the Act so that the scope of coverage was expanded from
coverage of ‘‘immunosuppressive drugs’’ to coverage of ‘‘prescription drugs used in
immunosuppressive therapy.’’ For the purposes of this proposed rule, we refer to this benefit as
the immunosuppressive drug benefit.
In the Medicare Coverage of Prescription Drugs Used in Immunosuppressive Therapy
final rule (60 FR 8951 through 8955), we finalized policies for the scope of drugs for which
payment may be made under this benefit. We finalized that payment may be made for
prescription drugs used in immunosuppressive therapy that have been approved for marketing by
the U.S. Food and Drug Administration (FDA) and meet one of the following conditions:
(1) The approved labeling includes the indication for preventing or treating the rejection
of a transplanted organ or tissue.
(2) The approved labeling includes the indication for use in conjunction with
immunosuppressive drugs to prevent or treat rejection of a transplanted organ or tissue.
(3) Have been determined by a Part B carrier, in processing a Medicare claim, to be
reasonable and necessary for the specific purpose of preventing or treating the rejection of a
patient's transplanted organ or tissue, or for use in conjunction with immunosuppressive drugs
for the purpose of preventing or treating the rejection of a patient's transplanted organ or tissue.

(In making these determinations, the carriers may consider factors such as authoritative drug
compendia, current medical literature, recognized standards of medical practice, and professional
medical publications.)
We also finalized the period of coverage eligibility for a transplant patient.
267
Lastly, we
established the policy that drugs are covered under this provision irrespective of whether they
can be self-administered. We codified these policies at § 410.31 (later redesignated as § 410.30).
We note that we do not maintain a list of drugs covered under this benefit; rather, MACs
are expected to maintain a list of these drugs, as stated in section 80.3, Chapter 17 of the
Medicare Claims Processing Manual. MACs are expected to keep informed of FDA approvals
of immunosuppressive drugs and update guidance as applicable.
While the eligibility timeframe has been extended and eligibility has been expanded since
the immunosuppressive drug benefit under Medicare Part B was revised by OBRA ’87, the scope
of drugs payable under this benefit has not changed. Some examples of how the benefit has been
extended and expanded include: section 13565 of the Omnibus Reconciliation Act of 1993
(OBRA ’93) (Pub. L. 103–66), amended section 1861(s)(2)(J) of the Act to extend the duration
of coverage for the immunosuppressive drug benefit to 36 months from the hospital discharge
date following a covered transplant procedure for drugs furnished after CY 1997; section 113 of
the Medicare, Medicaid and SCHIP Benefits Improvement and Protection Act of 2000 (Pub. L.
106–554) (BIPA) revised section 1861(s)(2)(J) of the Act to eliminate the time limits for
coverage of prescription drugs used in immunosuppressive therapy under the Medicare program;
and most recently, section 402 of the Consolidated Appropriations Act, 2021 (Pub. L. 116-260)
267
Since the establishment of the benefit by the enactment of OBRA ’86, the period of coverage for a transplant
patient under section 1861(s)(2)(J) of the Social Security Act has been subsequently amended by section 202 of the
Medicare Catastrophic Coverage Act of 1988 (Pub. L. 100–360), the Medicare Catastrophic Coverage Repeal Act of
1989 (Pub. L. 101–234), section 13565 of the Omnibus Reconciliation Act of 1993 (OBRA ’93) (Pub. L. 103–66),
section 160 of the Social Security Act Amendments of 1994 (Pub. L. 103–432), section 113 of the Medicare,
Medicaid and SCHIP Benefits Improvement and Protection Act of 2000 (Pub. L. 106–554) (BIPA 2000). The last of
these statutory changes eliminates the time limits for coverage of prescription drugs used in immunosuppressive
therapy under the Medicare program, effective with immunosuppressive drugs furnished on or after December 21,
2000.

amended section 226A(b)(2) to allow certain individuals whose Medicare entitlement based on
ESRD would otherwise end 36 months after a kidney transplant to continue enrollment under
Medicare Part B only for the coverage of immunosuppressive drugs described in section
1861(s)(2)(J) of the Act.
After reviewing our longstanding policies for the immunosuppressive drug benefit and
engaging with interested parties about current practices and challenges, we are proposing
policies aimed to reduce barriers faced by beneficiaries receiving immunosuppressive drugs
under this benefit, as described below.
b. Compounded Immunosuppressive Drugs with Oral or Enteral Routes of Administration
As discussed in the previous section, the immunosuppressive drug benefit currently
includes immunosuppressive therapies that have been approved for marketing by the FDA (and
meet other regulatory requirements at § 410.30). Interested parties have expressed concern that
compounded formulations of immunosuppressive drugs (for example, a liquid formulation of an
immunosuppressive drug not commercially available from a manufacturer but prepared by a
pharmacist) are not included in the immunosuppressive therapy benefit because these
formulations are not approved by the FDA (that is, FDA does not review these drugs to evaluate
their safety, effectiveness, or quality before they reach patients
268
), which is a regulatory
requirement under the current benefit. These interested parties communicated that compounded
formulations are frequently used in the treatment of transplant recipients who cannot swallow
oral capsules or tablets due to age or oral-motor dysfunction. Some examples of drugs
compounded for preventing or treating the rejection of a transplanted organ or tissue include, but
are not limited to, azathioprine,
269
cyclophosphamide,
270
and tacrolimus.
271
268
https://www.fda.gov/drugs/human-drug-compounding/compounding-laws-and-policies.
269
United States Pharmacopeia (2024). USP Monographs, Azathioprine Compounded Oral Suspension. USP-NF.
Rockville, MD: United States Pharmacopeia.
270
United States Pharmacopeia (2024). USP Monographs, Cyclophosphamide Compounded Oral Suspension. USP-
NF. Rockville, MD: United States Pharmacopeia.
271
United States Pharmacopeia (2024). USP Monographs, Tacrolimus Compounded Oral Suspension. USP-NF.
Rockville, MD: United States Pharmacopeia.

We recognize certain patient groups, such as those with dysphagia, those with enteral
feeding tubes (for example, a nasogastric feeding tube or a percutaneous endoscopic gastrostomy
(PEG) tube), and many pediatric patients
272,273
covered under Medicare rely on compounded
immunosuppressive drugs for maintenance therapy and believe that their inclusion in the
immunosuppressive drug benefit would help to ensure that each beneficiary is able to access the
most clinically appropriate formulation of an immunosuppressive drug.
274,275,276
Nonadherence
to lifelong maintenance immunosuppressive therapy contributes to unfavorable post-transplant
outcomes, with obstacles to accessing medication being a prominent risk factor for such
nonadherence.
277
Therefore, we are proposing revisions at § 410.30 to include orally and
enterally administered compounded formulations with active ingredients derived only from
FDA-approved drugs where approved labeling includes an indication for preventing or treating
the rejection of a transplanted organ or tissue, or for use in conjunction with immunosuppressive
drugs to prevent or treat rejection of a transplanted organ or tissue, or have been determined by a
MAC, in processing a Medicare claim, to be reasonable and necessary for this specific purpose
as outlined in the immunosuppressive drug benefit. As we intend this proposal to enhance access
and address adherence concerns for patients who are not able to swallow capsules or tablets and
we do not believe there are access concerns with other types of formulations, we propose to limit
the included compounded formulations to those products with oral and enteral routes of
administration (for example, oral suspensions or solutions).
272
In the United States, children under 18 years of age comprise only 0.14 percent of the total Medicare ESRD
population. Source: CY 2024 End-Stage Renal Disease Prospective Payment System final rule (88 FR 76374)
273
Lentine, K, Smith, JM, Lyden, GR, Miller, JM, Dolan, TG, Bradbrook, K, Larkin, L, Temple, K, Handarova, DK,
Weiss, S, Israni, AK, Snyder, JJ (2024). OPTN/SRTR 2022 Annual Data Report: Kidney. American Journal of
Transplantation, 24(2), S19–S118. https://doi.org/10.1016/j.ajt.2024.01.012
274
Silva RME, Portela RDP, da Costa IHF, et al. Immunosuppressives and enteral feeding tubes: An integrative
review. J Clin Pharm Ther. 2020;45:408–418. https://doi.org/10.1111/jcpt.13093
275
Goorhuis JF, Scheenstra R, Peeters PM, Albers MJ. Buccal vs. nasogastric tube administration of tacrolimus after
pediatric liver transplantation. Pediatr Transplant. 2006 Feb;10(1):74-7. doi: 10.1111/j.1399-3046.2005.00402.x.
PMID: 16499591
276
Liverman, R, Chandran, MM, Crowther, B. Considerations and controversies of pharmacologic management of
the pediatric kidney transplant recipient. Pharmacotherapy. 2021 Jan;41(1): 77-102.
https://doi.org/10.1002/phar.2483.
277
Fine RN, Becker Y, De Geest S, et al. Nonadherence consensus conference summary report. Am J
Transplant. 2009; 9(1): 35-41. doi: 10.1111/j.1600-6143.2008.02495.x.

We solicit comment on these proposals.
c. Immunosuppressive Refill Policy and Supplying Fee
Section 303(e)(2) of the MMA added section 1842(o)(6) of the Act which requires the
Secretary to pay a supplying fee (less applicable deductible and coinsurance) to pharmacies for
certain Medicare Part B drugs and biologicals, as determined appropriate by the Secretary,
including for immunosuppressive drugs described in section 1861(s)(2)(J) of the Act.
In the CY 2005 PFS, we established a supplying fee of $50 for the initial oral
immunosuppressive prescription supplied in the first month after a transplant (69 FR 66312
through 66313). In the CY 2006 Physician Fee Schedule, we established a supplying fee of $16
for all subsequent prescriptions after the initial prescription supplied during a 30-day period (70
FR 70233 through 70236).
Following the CY 2006 rulemaking, we issued program instruction
278
to the MACs that
prohibits payment for refills of immunosuppressive drug prescriptions in most circumstances and
limits payment for prescriptions to 30-day supplies. We state in Chapter 17 of the Medicare
Claims Processing Manual that contractors should limit payment for prescriptions to those of 30-
day supplies, except in special circumstances, because dosage frequently diminishes over time; it
is not uncommon for the provider to change the prescription from one drug to another; and
coinsurance liability on unused drugs could be a financial burden to the beneficiary.
We have heard from interested parties that both the 30-day limit on supplies and
prohibition on payment for refills no longer align with current practice for treating patients on
maintenance immunosuppression regimens who are prescribed a stable dosage for months or
years and receive refillable supplies for several months’ use at a time. Frequent dosage
adjustments for some immunosuppressive drugs that require therapeutic drug monitoring and
dose titration based on blood concentrations, such as tacrolimus, tend to occur more often in
278
Section 80.3, Chapter 17 of the Medicare Claims Processing Manual

newly transplanted recipients, and less frequently once patients are on stable regimens.
279
Other
immunosuppressive drugs, such as mycophenolate mofetil, do not require routine therapeutic
drug monitoring and have fixed recommended dosages per labeling where patients may be
maintained on stable dosages for several months unless patients experience complications.
280
Transplant recipients must take immunosuppressive drugs on a lifelong basis to prevent
rejection, maintain allograft function, and, for some transplanted organs, prevent death. Most
patients are eventually prescribed stable maintenance immunosuppressive drug dosages post-
transplant for extended periods of time. For example, liver transplant guidelines recommend
review of the immunosuppressive drug regimen at least every 6 months.
281
For transplant
beneficiaries, we believe that the limitation on payment to a maximum 30-day supply of
immunosuppressive therapy by our program instruction is an unnecessary burden that poses a
greater risk to adherence than does the potential for a sudden change in dosage needs. There is
considerable concern among providers and advocates that interrupted access to
immunosuppressive drugs caused by running out of or having insufficient medication supply can
decrease medication adherence, increase risk of organ transplant rejection, and ultimately
decrease the rate of survival of transplant recipients.
282,283
We agree with interested parties that it
would be beneficial to patients to reduce barriers that complicate access to immunosuppressive
medication and reasonable for CMS to make programmatic changes consistent with this
objective.
279
Tacrolimus [package insert]. Northbrook, IL: Astellas Pharma, Inc.; 2022.
https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/050708s055,010115s007lbl.pdf.
280
Cellcept [package insert]. San Francisco, CA: Genentech USA, Inc.; 2022.
https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/050722s050,050723s050,050758s048,050759s055lbl.p
df.
281
Lucey MR, Terrault N, Ojo L, et al. Long-term management of the successful adult liver transplant: 2012 practice guideline
by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl. 2013
Jan;19(1):3-26. doi: 10.1002/lt.23566.
282
Nelson J, Alvey N, Bowman L, et al. Consensus recommendations for use of maintenance immunosuppression in
solid organ transplantation: Endorsed by the American College of Clinical Pharmacy, American Society of
Transplantation, and the International Society for Heart and Lung Transplantation. Pharmacotherapy. 2022; 42:599-
633. doi: 10.1002/phar.2716.
283
Chisholm MA, Lance CE, Williamson GM, Mulloy LL. Development and validation of an immunosuppressant
therapy adherence barrier instrument. Nephrol Dial Transplant. 2005 Jan;20(1): 181–188.
https://doi.org/10.1093/ndt/gfh576.

Accordingly, we are proposing two changes regarding supplying fees and refills for
immunosuppressive drugs. First, we are proposing to allow payment of a supplying fee for a
prescription of a supply of up to 90 days. To reflect this proposal, we are proposing to revise §
414.1001 to allow payment of a supplying fee to a pharmacy for first prescriptions and for
prescriptions following the first prescription for greater than a 30-day supply. We are proposing
additional modifications at § 414.1001 to combine paragraphs (a) (for supplying fees) and (b)
(for supplying fees following a transplant). Accordingly, we are also proposing to remove
paragraph (b) and redesignate paragraphs (c) and (d) as paragraphs (b) and (c), respectively. We
intend to further study the supplying fee schedule for immunosuppressive drugs and are not
proposing to make any changes to the supplying fee amounts at this time (meaning the current
30-day supplying fees would apply to any amount of days’ supply). The dispensing and
supplying fees under Part B (§ 414.1001) have been shown to be higher than dispensing fees
paid in the commercial market.
284
So, until additional study is done regarding input costs for
dispensing drugs billed to Medicare Part B and subsequent notice-and-comment rulemaking can
be done, if appropriate, in response to such information, we aim to continue the current fee
schedule regardless of the days’ supply dispensed. Second, we are proposing to allow payment
of refills for these immunosuppressive drugs. Under our proposal, if finalized, the prescribing
healthcare provider may adjust the days’ supply up to 90 days and allow refills for an
immunosuppressive drug based on the individual circumstance of the beneficiary in accordance
with applicable state laws.
We welcome comments on these proposals.
5. Blood clotting factors (§ 410.63)
a. Background
284
https://www.pcmanet.org/rx-research-corner/mandating-pharmacy-reimbursement-increase-
spending/08/31/2021/#:~:text=The%20average%20dispensing%20fee%20in,the%20state's%20Medicaid%20FFS%
20rate.

Hemophilia is a genetic bleeding disorder resulting in a deficiency of coagulation Factor
VIII (hemophilia A) or coagulation Factor IX (hemophilia B) due to mutations in the respective
clotting factor genes.
285,286
Prophylactic use of clotting factors has been proven to improve
quality of life by preventing joint bleeds but requires maintenance therapy, usually throughout
the life of the patient. Preventing joint damage early is crucial because the initial damage will
progress, irrespective of whether further bleeds occur in the affected joints.
287
Currently, clotting
factor treatments include: plasma-derived products, which are virally inactivated and made from
human donor plasma; recombinant products, such as recombinant Factors VIIa, VIII, IX, X,
XIII, which are created using genetically engineered cells and recombinant technology; and a
monoclonal antibody product that binds to specific receptor sites of missing clotting factor,
which is needed for effective hemostasis.
288,289
Individuals with hemophilia generally self-infuse
clotting factor at home, often learning to do so in childhood.
290,291,292
Section 2324 of the Deficit Reduction Act of 1984 (Pub. L. 98-369) added subparagraph
(I) to section 1861(s)(2) of the Act to provide Medicare Part B coverage of blood clotting factor
treatments for hemophilia patients who are competent to use such factors to control bleeding
without medical supervision (that is, self-administered), and items related to the administration
of such factors; this is codified at § 410.63(b). As set forth in 1842(o)(1)(C) of the Act, payment
for clotting factor product is the amount provided for under section 1847A of the Act.
In January of 2003, the Comptroller General of the United States published a report
entitled “Payment for Blood Clotting Factor Exceeds Providers Acquisition Cost”
293
(hereinafter
285
https://www.hemophilia.org/bleeding-disorders-a-z/types/hemophilia-a, accessed April 9, 2024.
286
https://www.hemophilia.org/bleeding-disorders-a-z/types/hemophilia-b, accessed April 9, 2024.
287
Aledort LM, Haschmeyer RH, Pettersson H. A longitudinal study of orthopaedic outcomes for severe factor-VIII-
deficient haemophiliacs. The Orthopaedic Outcome Study Group. J Intern Med. 1994 Oct;236(4):391-9.
288
Srivastava A, et al. Haemophilia. 2020;26(suppl 6):1-158.
289
https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2483adba-fab6-4d1b-96c5-c195577ed071.
290
GAO-03-184 Medicare: Payment for Blood Clotting Factor. www.gao.gov/assets/gao-03-184.pdf.
291
Valentino, L. A., Baker, J. R., Butler, R., Escobar, M., Frick, N., Karp, S., … Skinner, M. (2021). Integrated
Hemophilia Patient Care via a National Network of Care Centers in the United States: A Model for Rare
Coagulation Disorders. Journal of Blood Medicine, 12, 897–911. https://doi.org/10.2147/JBM.S325031.
292
https://www.hemophilia.org/bleeding-disorders-a-z/treatment/current-treatments, accessed April 9, 2024.
293
https://www.gao.gov/assets/gao-03-184.pdf.

referred to the January 2003 report). Among other things, the report found that “providers incur
additional costs associated with delivering clotting factor that are not separately reimbursed by
Medicare.” Specifically, the report cited delivery costs generated in inventory management,
specialized refrigerated storage, shipping, and the provision of ancillary supplies such as needles,
syringes, and tourniquets to patients that were not accounted for by Medicare payment for the
clotting factor product alone.
After the release of the January 2003 report, section 303I(1) of the MMA amended
section 1842(o) of the Act by adding a new paragraph (5), requiring the Secretary to establish a
furnishing fee for the items and services associated with the furnishing of blood clotting factor.
Specifically, section 1842(o)(5) of the Act requires that for clotting factors furnished on or after
January 1, 2005, the Secretary shall provide for a separate payment to the entity which furnishes
blood clotting factors for items and services related to the furnishing of such factors in an amount
that the Secretary determines to be appropriate. Accordingly, the clotting factor furnishing fee
was codified at § 410.63(c), which states that the furnishing fee is added on a per unit basis to the
clotting factor.
In 2005, CMS established a furnishing fee of $0.14 per unit of clotting factor. The
clotting factor furnishing fee is increased by the percentage increase in the Consumer Price Index
(CPI) for Medical Care for the 12-month period ending with June of the previous year, as
required by Section 1842(o)(5)(C) of the Act, and updated annually in chapter 17, section 80.4.1
of the Medicare Claims Processing Manual. For 2024, the clotting factor furnishing fee is $0.250
per unit. Chapter 17 of the Medicare Claims Processing Manual, section 80.4.1 indicates that
“CMS includes this clotting factor furnishing fee in the nationally published payment limit for
clotting factor billing codes” along with the pricing file, which denotes which HCPCS codes
have the furnishing fee added. The payment limit in the pricing file includes the payment limit
for the clotting factor product (under methodology in section 1847A of the Act) plus furnishing
fee.
As was the case at the time the clotting factor furnishing fee regulations were originally
finalized, we continue to believe the products eligible for payment of the clotting factor
furnishing fee and those eligible for payment as clotting factor products are the same subset of
products: that is, self-administered clotting factor products, as described above. Similar to
section 1861(s)(2)(I) of the Act, section 1842(o)(5) of the Act specifically contemplates that
clotting factors are self-administered. In particular, section 1842(o)(5)(A)(ii) of the Act specifies
that the furnishing fee can take into account “ancillary supplies and patient training for the self-
administration of such factors.” As stated in the CY 2005 PFS final rule, the furnishing fee
accounts for the costs associated with supplying the clotting factor, including patient training
necessary for self-administration of such factors (69 FR 47523; 69 FR 66311). Thus, the clotting
factor furnishing fee, as implemented, pays for services and supplies in connection with the
patient’s self-administration of the product.
We note that section 1842(o)(5)(A) of the Act directed the Secretary to “review[…] the
January 2003 report” when establishing the separate payment for entities which furnish blood
clotting factors to the patient. The January 2003 report refers to self-administration of clotting
factor and the benefits beneficiaries receive from home-use of the product throughout the report.
For example, the report states, “Individuals with hemophilia generally self-infuse clotting factor.
Clotting factor can be infused on demand, when a bleeding episode occurs, or for prevention,
known as prophylactic use. By self-infusing, individuals can avoid waiting for care at a medical
facility.”
Most notably, for purposes of understanding the Medicare clotting factor payment
inadequacy that was addressed by Congress by adding the furnishing fee, the report states “[t]he
method of delivery of clotting factor has implications for Medicare payment. Most outpatient
drugs covered by Medicare are administered in a physician’s office. When a beneficiary visits a
physician in order to receive a drug, the physician receives one payment from Medicare for the
drug and another payment through the physician fee schedule for administering the drug.

Clotting factor, however, is generally not administered in a physician’s office.” That is, the
report highlighted that Medicare payment for clotting factor, in particular, was inadequate
because there are costs associated with supplying the clotting factor, but because it is self-
administered, the furnishing of clotting factor was generally not eligible for the administration
fee. Generally, the report noted that payment for supplying other outpatient drugs covered by
Medicare Part B were adequate because they are eligible for the administration fee. Again, as
stated above, Congress addressed this issue by creating the furnishing fee for these self-
administered clotting factor products in the MMA.
More recently, gene therapies have been FDA-approved for the treatment of hemophilia.
These gene therapies introduce a functional gene to the patient, which provides the genetic
information needed for the patient to produce the missing or nonfunctional protein. A viral
vector in the gene therapies, engineered with adeno-associate virus, delivers the functional copy
of the clotting factor gene into the patient's liver cells. The viral vector then releases the
functional gene which integrates into the cell's DNA and starts producing the missing clotting
factor protein (that is, Factor VIII or Factor IX) to restore normal clotting function.
In the case of hemophilia A or B, the gene therapy introduces the functional gene that
enables the patient to produce Factor VIII or Factor IX, respectively, on their own. Unlike
clotting factors, which promptly restore balance in the coagulation cascade at the point of
deficiency or bridge activated Factor IX and Factor X to restore the function of missing activated
Factor VIII,
294
allowing for stable blood clot formation and hemostasis, the gene therapies do not
directly integrate into the coagulation cascade.
295,296
In the coagulation cascade, clotting factors
become activated in response to damaged tissues or exposure to collagen at the injury site. This
activation initiates the conversion of prothrombin to thrombin. Thrombin then converts
294
Genentech, Inc. Hemlibra (emicizumab-kxwh) injection, for subcutaneous use. South San Francisco, CA:
Genentech, Inc.; 2023. Package insert.
295
Hoffman, M., & Monroe, D. M. (2001). A cell-based model of hemostasis. Thrombosis and Haemostasis, 85(6),
958-965
296
Schenone M, Furie BC, Furie B. The blood coagulation cascade. Curr Opin Hematol. 2004 Jul;11(4):272-7.

fibrinogen into fibrin strands, forming the blood clot. Clotting factors restore normal clotting
function by replacing deficient factors through repeated, dose-adjustable infusions or injections.
In contrast, a single administration of gene therapies maintains a consistent and adequate level of
clotting factors over the long term by enabling the self-production of the clotting factor
proteins—an indirect method that relies on the patient’s cells to increase clotting factor levels.
However, as the self-production of clotting factor proteins takes time, the sustained outcomes of
gene therapies may take several weeks to fully manifest. Interested parties have asked if CMS
considers these gene therapies to be clotting factors for which the clotting factor furnishing fee
would be paid.
Gene therapies for hemophilia are administered via a one-time, single-dose intravenous
infusion in a setting where personnel and equipment are immediately available to treat infusion-
related reactions. They are not typically administered by the patient in his or her home, and close
monitoring is required for at least three hours after the end of the infusion.
297
While these gene
therapy products may have a similar goal to clotting factor products, in that both products are
designed to improve outcomes for patients with hemophilia, gene therapy products prompt the
body to make clotting factors, but are not clotting factors themselves. Given that the
administration would occur incident to a physician service (that is, the product is not self-
administered), the differing mechanism of action from replacing deficient factors (that is,
triggering the body to make clotting factors rather than infusing clotting factors into the body),
and the requirement of close monitoring by a healthcare professional post-infusion, these gene
therapies do not have the characteristics described in the January 2003 report that is referenced in
section 1842(o)(5) of the Act, which the Secretary relied on in drafting § 410.63(c). Therefore,
they do not constitute “clotting factors” for purposes of Medicare payment.
297
Carvalho M, Sepodes B, Martins APPatient access to gene therapy medicinal products: a comprehensive
reviewBMJ Innovations 2021;7:123-134.

Accordingly, gene therapies for hemophilia are eligible for payment as drugs or
biologicals under Part B as part of (or incident to) a physician’s service. The “incident to”
coverage is limited to drugs that are not usually self-administered and the physician generally
must incur a cost for the drug and must bill for it. Furnishing entities will bill for its
administration, and the administration fees will reflect the resources necessary to furnish the
drug. For example, certain CPT codes for administering drugs include preparation of the dose
and patient monitoring. Specifically, CPT codes 96401 - 96549 (chemotherapy administration
and nonchemotherapy injections and infusions) include clinical labor activities such as clinical
staff preparation of chemotherapy agent(s) as well as evaluation and management services.
298
For the reasons explained above, we do not believe gene therapies for hemophilia meet
the definition of a clotting factor for purposes of Medicare payment, but even if they did, they
still would not be eligible for the furnishing fee because the costs associated with furnishing
these gene therapies would already be reflected in applicable administration codes paid under the
Physician Fee Schedule. In accordance with § 410.63(c)(1), a clotting factor furnishing fee is not
payable when the costs associated with furnishing a clotting factor are paid through another
payment system. In this case, the payment system is the payment system established under the
Physician Fee Schedule. Furnishing fees for drugs that are physician administered would result
in physicians being paid twice for incidental costs of administering the drug because the
furnishing fee is intended to compensate for supplies like needles, syringes, and tourniquets as
well as storage costs, and so is the Part B payment for administering the drug. We do not believe
this double payment is appropriate, nor do we believe this is what Congress intended in directing
CMS to establish a clotting factor furnishing fee.
Accordingly, in this proposed rule, we are proposing to update § 410.63(b) to clarify
existing CMS policy that blood clotting factors must be self-administered to be considered
clotting factors for which the furnishing fee applies. Additionally, we are proposing to clarify at
298
Section 30.5, Chapter 12 of the Medicare Claims Processing Manual.
§ 410.63(c) that the furnishing fee is only available to entities that furnish blood clotting factors,
unless the costs associated with furnishing the clotting factor are paid though another payment
system, including the Physician Fee Schedule. That is, we are proposing to clarify through
revisions to § 410.63 that clotting factors (as specified in section 1861(s)(2)(I) of the Act) and
those eligible to receive the clotting factor furnishing fee (as specified in section 1842(o)(5) of
the Act) are the same subset of products.
B. Rural Health Clinics (RHCs) and Federally Qualified Health Centers (FQHCs)
1. Background on RHC and FQHC Payment Methodologies
As provided in 42 CFR part 405, subpart X, of our regulations, RHC and FQHC visits
generally are defined as face-to-face encounters between a patient and one or more RHC or
FQHC practitioners during which one or more RHC or FQHC qualifying services are furnished.
RHC and FQHC practitioners are physicians, NPs, PAs, CNMs, clinical psychologists (CPs),
licensed marriage and family therapists, mental health counselors, and clinical social workers,
and under certain conditions, a registered nurse or licensed practical nurse furnishing care to a
homebound RHC or FQHC patient in an area verified as having shortage of home health
agencies. Transitional Care Management (TCM) services can also be paid by Medicare as an
RHC or FQHC visit. In addition, Diabetes Self-Management Training (DSMT) or Medical
Nutrition Therapy (MNT) sessions furnished by a certified DSMT or MNT program may also be
considered FQHC visits for Medicare payment purposes. Only medically necessary medical,
mental health, or qualified preventive health services that require the skill level of an RHC or
FQHC practitioner are RHC or FQHC billable visits. Services furnished by auxiliary personnel
(for example, nurses, medical assistants, or other clinical personnel acting under the supervision
of the RHC or FQHC practitioner) are considered incident to the visit and are included in the
per-visit payment.
RHCs generally are paid an all-inclusive rate (AIR) for all medically necessary medical
and mental health services and qualified preventive health services furnished on the same day
(with some exceptions). The AIR is subject to a payment limit, meaning that an RHC will not
receive any payment beyond the specified limit amount. As of April 1, 2021, all RHCs are
subject to upper payment limits determined in accordance with section 1833(f) of the Act.
FQHCs were paid under the same AIR methodology until October 1, 2014. Beginning on
that date, in accordance with section 1834(o) of the Act (as added by section 10501(i)(3) of the
Patient Protection and Affordable Care Act (Pub. L. 111-148)), FQHCs began to transition to the
FQHC PPS system, in which they are paid based on the lesser of the FQHC PPS rate or their
actual charges. The FQHC PPS rate is adjusted for geographic differences in the cost of services
by the FQHC PPS geographic adjustment factor (GAF). The rate is increased by 34 percent
when an FQHC furnishes care to a patient that is new to the FQHC, or to a beneficiary receiving
an initial preventive physical examination (IPPE) or has an annual wellness visit (AWV).
Both the RHC AIR and FQHC PPS payment rates were designed to reflect the cost of all
services and supplies that an RHC or FQHC furnishes to a patient in a single day. The rates are
not adjusted at the individual level for the complexity of individual patient health care needs, the
length of an individual visit, or the number or type of practitioners involved in the patient’s care.
Instead for RHCs, all costs for the facility over the course of the year are aggregated and an AIR
is derived from these aggregate expenditures. The FQHC PPS base rate is updated annually by
the percentage increase in the FQHC market basket reduced by a productivity adjustment. For
CY 2025, CMS is proposing to rebase and revise the FQHC market basket to reflect a 2022 base
year; see section III.B.7 of this proposed rule.
2. General Care Management Services in RHCs and FQHCs
a. Background
We have been engaged in a multi-year examination of coordinated and collaborative care
services in professional settings, and as a result established codes and separate payment in the
PFS to independently recognize and pay for these important services. The care coordination
included in services, such as office visits, does not always adequately describe the non-face-to-
face care management work involved in primary care and similar care relationships. Payment for
office visits may not reflect all the services and resources required to furnish comprehensive,
coordinated care management for certain categories of beneficiaries, such as those who are
returning to a community setting following discharge from a hospital or skilled nursing facility
(SNF) stay.
Before we get into the detailed background of our RHC and FQHC payment policies for
care coordination services, we want to acknowledge that we have used several terms to describe
these services and are providing clarification. We use the terms “care coordination” services
interchangeably with the term “care management” services in preamble and manual guidance to
describe the type of work discussed above. We began to use the term “general care
management” when we established the HCPCS code G0511 for CY 2018. Use of “general care
management” is meant to describe certain non-face-to-face care management work involved in
primary care that we have identified as appropriate for separate payment as discussed in the
following paragraphs.
As we discussed in the CY 2016 PFS final rule (80 FR 71081 through 71088), to address
the concern that the non-face-to-face care management work involved in furnishing
comprehensive, coordinated care management for certain categories of beneficiaries is not
adequately paid for as part of an office visit, beginning on January 1, 2015, practitioners billing
under the PFS are paid separately for chronic care management (CCM) services when CCM
service requirements are met. We explained that RHCs and FQHCs cannot bill under the PFS for
RHC or FQHC services and individual practitioners working at RHCs and FQHCs cannot bill
under the PFS for RHC or FQHC services while working at the RHC or FQHC. Although many
RHCs and FQHCs pay for coordination of services within their own facilities and may
sometimes help to coordinate services outside their facilities, the type of structured care
management services that are now payable under the PFS for patients with multiple chronic
conditions, particularly for those who are transitioning from a hospital or SNF back into their
communities, are generally not included in the RHC or FQHC payment. Therefore, separate
payment was established in the CY 2016 PFS final rule (80 FR 71080 through 71088) for RHCs
and FQHCs that furnish CCM services. We believe the non-face-to-face time required to
coordinate care is not captured in the RHC AIR or the FQHC PPS payment, particularly for the
rural and/or low-income populations served by RHCs and FQHCs. Allowing separate payment
for CCM services in RHCs and FQHCs is intended to reflect the additional resources necessary
for the unique components of CCM services.
In the CY 2018 PFS final rule (82 FR 53169 through 53180), we finalized revisions to
the payment methodology for CCM services furnished by RHCs and FQHCs and established
requirements for general behavioral health integration (BHI) and psychiatric collaborative care
management (CoCM) services furnished in RHCs and FQHCs, beginning on January 1, 2018.
We also initiated the use of HCPCS codes G0511 and G0512. HCPCS code G0511 is a general
care management code for use by RHCs or FQHCs when at least 20 minutes of qualified CCM
or general BHI services are furnished to a patient in a calendar month. HCPCS code G0512 is
for psychiatric CoCM and can be billed by RHCs or FQHCs when at least 60 minutes of
qualified psychiatric CoCM services are furnished to a patient in a calendar month.
For CY 2018 the payment amount for HCPCS code G0511 was set at the average of the 3
national non-facility PFS payment rates for the CCM and general BHI codes and updated
annually based on the PFS amounts. That is, for CY 2018 the 3 codes that comprised HCPCS
code G0511 were CPT code 99490 (20 minutes or more of CCM services), CPT code 99487 (60
minutes or more of complex CCM services), and CPT code 99484 (20 minutes or more of BHI
services).
In the CY 2019 PFS final rule (83 FR 59683), we explained that another CCM code was
introduced for practitioners billing under the PFS, CPT code 99491, which would correspond to
30 minutes or more of CCM furnished by a physician or other qualified health care professional
and is similar to CPT codes 99490 and 99487. Therefore, for RHCs and FQHCs, we added CPT
code 99491 as a general care management service and included it in the calculation of HCPCS
code G0511. Starting on January 1, 2019, RHCs and FQHCs were paid for HCPCS code G0511
based on the average of the national non-facility PFS payment rates for CPT codes 99490,
99487, 99484, and 99491 (83 FR 59687).
In the CY 2021 PFS final rule (85 FR 84697 through 84699), we explained that the
requirements described by the codes for principal care management (PCM) services were similar
to the requirements for the services described by HCPCS code G0511; therefore, we added
HCPCS codes G2064 and G2065 to HCPCS code G0511 as general care management services
for RHCs and FQHCs. Consequently, effective January 1, 2021, RHCs and FQHCs are paid
when a minimum of 30 minutes of qualifying PCM services are furnished during a calendar
month. The payment rate for HCPCS code G0511 for CY 2021 was the average of the national
non-facility PFS payment rate for the RHC and FQHC care management and general behavioral
health codes (CPT codes 99490, 99487, 99484, and 99491), and PCM codes (HCPCS codes
G2064 and G2065). We noted that in the CY 2022 PFS final rule (86 FR 65118), HCPCS codes
G2064 and G2065 were replaced by CPT codes 99424 and 99435. Therefore, for CY 2022 the
payment rate for HCPCS code G0511 was the average of the national non-facility PFS payment
rate for CPT codes 99490, 99487, 99484, 99491, 99424, and 99425).
In the CY 2023 PFS final rule (87 FR 69735 through 69737), we included chronic pain
management (CPM) services described by HCPCS code G3002 in the general care management
HCPCS code G0511 when at least 30 minutes of qualifying non-face-to-face CPM services are
furnished during a calendar month. We explained since HCPCS code G3002 is valued using a
crosswalk to the PCM CPT code 99424, which is currently one of the CPT codes that comprise
HCPCS code G0511, there was no change made to the average used to calculate the HCPCS
code G0511 payment rate to reflect CPM services.
Most recently, in the CY 2024 PFS final rule (88 FR 79071 through 79073) we included
the CPT codes that are associated with the suite of services that comprise remote physiologic

monitoring (RPM) and remote therapeutic monitoring (RTM) in the general care management
HCPCS code G0511 when these services are furnished by RHCs and FQHCs. In addition, we
included community heath integration (CHI), principal illness navigation (PIN), and PIN – peer
support services in HCPCS code G0511 when these services are furnished by RHCs and FQHCs
(88 FR 79073 through 79081). We noted that for each of these newly included services that they
must be medically reasonable and necessary, meet all requirements, and not be duplicative of
services paid to RHCs and FQHCs under the general care management code for an episode of
care in a given calendar month. We also clarified RHCs and FQHCs may bill HCPCS code
G0511 multiple times in a calendar month, as long as all of the requirements are met and
resource costs are not counted more than once (88 FR 79075).
Additional information on care management requirements is available on the CMS Care
Management webpage and on the CMS RHC and FQHC webpages.
299,300,301
b. Proposed Regulatory Update (§ 405.2464(c))
During our development of the proposals discussed in section III.B.2.c. and III.B.2.d. of
this proposed rule, we determined that the language located in § 405.2464(c) could use additional
information to streamline and provide clarity on our payment policy for care coordination
services. For example, using consistent terms, effective dates, and the description of the basis of
payment. Therefore, we propose technical changes to § 405.2464(c) to accurately reflect the
iterations of our payment policy for care coordination services as detailed in this background
section.
c. Proposed Payment Policy for General Care Management Services
As discussed previously, in the last few years of PFS payment rules we have expanded
the scope of care management services billable using HCPCS code G0511. Prior to CY 2024,
299
https://www.cms.gov/medicare/payment/fee-schedules/physician/care-management.
300
https://www.cms.gov/center/provider-type/rural-health-clinics-center.
301
https://www.cms.gov/medicare/payment/prospective-payment-systems/federally-qualified-health-centers-fqhc-
center.
HCPCS code G0511 was based on the national average non-facility PFS payment rate for each
base code identified as billable general care management services. That is, we added each
payment rate divided by the total number of codes to arrive at the payment amount for HCPCS
code G0511. This payment amount was a flat rate that was not subsequently adjusted for locality.
In the CY 2024 PFS final rule (88 FR 79076 through 79079), we explained continuing to
calculate the value of HCPCS code G0511 using an approach based on an average may no longer
be appropriate payment for those services since we are simply dividing by the number of codes
that comprise HCPCS code G0511 and as that number of services with lower payment rates
increases, the payment rate decreases. We noted that while the policy may address providing a
payment for furnishing non-face-to-face services, the magnitude of the value may not
appropriately account for the costs. Therefore, we finalized a revised methodology for the
calculation of HCPCS code G0511 by looking at the actual utilization of the services. We used a
weighted average of the services that comprise HCPCS code G0511. For the utilization data of
the services, we used the most recently available utilization data from the services paid under the
PFS in the physician office setting. We explained that the physician office setting may provide
an appropriate proxy for utilization of these services in the absence of actual data because this
setting most closely aligns with the types of services furnished in RHCs and FQHCs since they
typically furnish primary care.
To ensure we accounted for payments accurately, we explained that we looked at PFS
utilization of the base code for the service and any applicable add-on codes used in the same
month as well as any base codes reported alone in a month for all of the services comprising
general care management (that is, the array of services that made up HCPCS code G0511). We
believed we needed to account for the payment associated with the base code along with an
applicable add-on code in our calculation as this demonstrates a complete encounter. Then to
arrive at the payment rate for HCPCS code G0511 for CY 2024, we took the weighted average of
the base code and add-on code pairs, in addition to the individual base codes for all of the
services that comprise HCPCS code G0511 by using the CY 2021 PFS utilization.
We determined that this approach was a more accurate representation of the payment
since it is consistent with practitioners billing under the PFS, and it accounts for the additional
time spent in care coordination.
Subsequent to the issuance of the CY 2024 PFS final rule, interested parties have
requested that CMS give them the ability to bill Medicare for each of the care management
services that comprise HCPCS code G0511 when they are furnished in RHCs and FQHCs.
RHCs, FQHCs, and associations supporting access to health care for rural populations have
expressed concerns regarding the transparency of the services being billed with HCPCS code
G0511. We note, in the CY2024 PFS final rule we stated that HCPCS code G0511 could be
billed multiple times in a calendar month for each care management code that comprised HCPCS
code G0511 as long as all requirements were met, there was no overlapping of resource time and
services were furnished in accordance with CPT coding guidelines and conventions. However,
providing this guidance triggered questions on how CMS tracks which general care management
service is being furnished if the bundled code is reported so they would know when it was
appropriate to bill multiple care management services on a single claim. RHCs and FQHCs have
also requested the ability to bill the add-on codes that describe additional minutes spent on
furnishing care management services and often ask for guidance on how to account for additional
time spent.
We have also heard from interested parties that RHCs and FQHCs would not find it
burdensome to report the actual HCPCS code that describes the care management service
furnished, which was the main concern we had when we implemented HCPCS code G0511 (82
FR 53172). We understand that RHCs and FQHCs have become more sophisticated with billing
and therefore reporting multiple codes has become less burdensome than in CY 2018 when we
implemented G0511. In addition, we have heard that RHCs and FQHCs are interested in having
more exposure and recognition in playing their part the delivery of quality primary care and
believe that this could be achieved with data that shows their utilization of services which could
also be used in future payment refinements.
Due to these concerns, we have reevaluated our payment policy for care management
services. We agree with interested parties that it is important to identify the actual services being
furnished and understand the utilization of these services, especially given our strong interest in
their volume and their contribution to initiatives on health equity and social needs of services in
the care coordination space. Therefore, we are proposing to require RHCs and FQHCs to bill the
individual codes that make up the general care management HCPCS code, G0511. The current
list of base and add-on codes that make-up G0511 are listed in Table 24, titled “General Care
Management HCPCS Codes and Descriptors.” Under this proposal, HCPCS code G0511 would
no longer be payable when billed by RHCs and FQHCs. We note that the payment amounts for
some services that made up G0511 are less than the payment amount for G0511 and if an RHC
or FQHC mostly furnishes these services, they could see a potential decline in payment. We are
also proposing to allow RHCs and FQHCs to bill the add-on codes for additional time spent once
the minimum threshold of time was met to account for a complete encounter. This could
potentially offset any decrease in payments. Payment for these services would be the national
non-facility PFS payment rate when the individual code is on an RHC or FQHC claim, either
alone or with other payable services and the payment rates are updated annually based on the
PFS amounts for these codes. We believe that these proposals promote transparency in billing
and payment and allowing RHCs and FQHCs to bill the individual care management codes
would take into account the complexity of the service and the time spent furnishing the service.

TABLE 24: General Care Management HCPCS Codes and Descriptors
HCPCS
code
Short Descriptors
Long Descriptors
99091
Collj & interpj data ea 30 d
Collection and interpretation of physiologic data (e.g. Blood
pressure, glucose monitoring) digitally stored and/or
transmitted by the patient and/or caregiver to the physician or
other qualified health professional, qualified by education,
training, licensure/regulation (when applicable) requiring a
minimum of 30 minutes of time, each 30 days
99424
Prin care mgmt phys 1st 30
Principal care management services, for a single high-risk
disease, with the following required elements: one complex
chronic condition expected to last at least 3 months, and that
places the patient at significant risk of hospitalization, acute
exacerbation/ decompensation, functional decline, or death, the
condition requires development, monitoring, or revision of
disease-specific care plan, the condition requires frequent
adjustments in the medication regimen, and/or the management
of the condition is unusually complex due to comorbidities,
ongoing communication and care coordination between
relevant practitioners furnishing care; first 30 minutes provided
personally by a physician or other qualified health care
professional, per calendar month
99425
Prin care mgmt phys ea addl
Principal care management services, for a single high-risk
disease, with the following required elements: one complex
chronic condition expected to last at least 3 months, and that
places the patient at significant risk of hospitalization, acute
exacerbation/ decompensation, functional decline, or death, the
condition requires development, monitoring, or revision of
disease-specific care plan, the condition requires frequent
adjustments in the medication regimen and/or the management
of the condition is unusually complex due to comorbidities,
ongoing communication and care coordination between
relevant practitioners furnishing care; each additional 30
minutes provided personally by a physician or other qualified
health care professional, per calendar month (List separately in
addition to code for primary procedure)
99426
Prin care mgmt staff 1st 30
Principal care management services, for a single high-risk
disease, with the following required elements: one complex
chronic condition expected to last at least 3 months, and that
places the patient at significant risk of hospitalization, acute
exacerbation/ decompensation, functional decline, or death, the
condition requires development, monitoring, or revision of
disease-specific care plan, the condition requires frequent
adjustments in the medication regimen and/or the management
of the condition is unusually complex due to comorbidities,
ongoing communication and care coordination between
relevant practitioners furnishing care; first 30 minutes of
clinical staff time directed by physician or other qualified
health care professional, per calendar month
99427
Prin care mgmt staff ea addl
Principal care management services, for a single high-risk
disease, with the following required elements: one complex
chronic condition expected to last at least 3 months, and that
places the patient at significant risk of hospitalization, acute
exacerbation/ decompensation, functional decline, or death, the
condition requires development, monitoring, or revision of
disease-specific care plan, the condition requires frequent
adjustments in the medication regimen and/or the management
of the condition is unusually complex due to comorbidities,
ongoing communication and care coordination between
relevant practitioners furnishing care; each additional 30

HCPCS
code
Short Descriptors
Long Descriptors
minutes of clinical staff time directed by a physician or other
qualified health care professional per calendar month (List
separately in addition to code for primary procedure)
99437
Chrnc care mgmt phys ea addl
Chronic care management services, provided personally by a
physician or other qualified health care professional, with the
following required elements: multiple (two or more) chronic
conditions expected to last at least 12 months, or until the death
of the patient, chronic conditions place the patient at significant
risk of death, acute exacerbation/ decompensation, or
functional decline, comprehensive care plan established,
implemented, revised or monitored; each additional 30 minutes
by a physician or other qualified health care professional, per
calendar month (List separately in addition to code for primary
procedure)
99439
Chrnc care mgmt staf ea addl
Chronic care management services, each additional 20 minutes
of clinical staff time directed by a physician or other qualified
health care professional, per calendar month
99453
Rem mntr physiol param setup
Remote monitoring of physiologic parameter(s) (e.g. Weight,
blood pressure, pulse oximetry, respiratory flow rate) initial
set-up and patient education on use of equipment
99454
Rem mntr physiol param dev
Remote monitoring of physiologic parameter(s) (e.g. Weight,
blood pressure, pulse oximetry, respiratory flow rate) initial
device(s) supply with daily recording(s) or programmed alert(s)
transmission, each 30 days
99457
Rem physiol mntr 1
st
20 min
Remote physiologic monitoring treatment services, clinical
staff/physician/other qualified health care professional time in a
calendar month requiring interactive communication with the
patient/caregiver during the month; first 20 minutes
99458
Rem physiol mntr ea addl 20
Remote physiologic monitoring treatment services, clinical
staff/physician/other qualified health care professional time in a
calendar month requiring interactive communication with the
patient/caregiver during the month; each additional 20 minutes
(list separately in addition to code for primary procedure)
99474
Self-meas bp 2 readg bid 30d
Self-measured blood pressure using a device validated for
clinical accuracy; separate self-measurements of two readings
one minute apart, twice daily over a 30-day period (minimum
of 12 readings), collection of data reported by the patient
and/or caregiver to the physician or other qualified health care
professional, with report of average systolic and diastolic
pressures and subsequent communication of a treatment plan to
the patient
99484
Care mgmt svc bhvl hlth cond
Care management services for behavioral health conditions, at
least 20 minutes of clinical staff time, directed by a physician
or other qualified health care professional time, per calendar
month, with the following required element: initial assessment
or follow-up monitoring, including using applicable validated
rating scales, behavioral health care planning about behavioral
or psychiatric health problems, including revision for patients
not progressing or whose status changes, facilitating and
coordinating treatment such as psychotherapy,
pharmacotherapy, counseling, or psychiatric consultation,
continuity of care with an appointed member of the care team
99487
Cplx chrnc care 1st 60 min
Complex chronic care management services, with the following
required elements: multiple (two or more) chronic conditions
expected to last at least 12 months, or until the death of the
patient, chronic conditions place the patient at significant risk
of death, acute exacerbation/decompensation, or functional
decline, establishment or substantial revision of comprehensive
care plan, moderate or high complexity medical decision
making; first 60 minutes of clinical staff time directed by a

HCPCS
code
Short Descriptors
Long Descriptors
physician or other qualified health care professional, per
calendar month
99489
Cplx chrnc care ea addl 30
Complex chronic care management services, with the following
required elements: multiple (two or more) chronic conditions
expected to last at least 12 months, or until the death of the
patient, chronic conditions place the patient at significant risk
of death, acute exacerbation/decompensation, or functional
decline, establishment or significant revision of comprehensive
care plan, moderate or high complexity medical decision
making; each additional 30 minutes of clinical staff time
directed by a physician or other qualified health care
professional, per calendar month (List separately in addition to
code for primary procedure)
99490
Chrnc care mgmt staff 1st 20
Chronic care management services with the following required
elements: multiple (two or more) chronic conditions expected
to last at least 12 months, or until the death of the patient,
chronic conditions place the patient at significant risk of death,
acute exacerbation/decompensation, or functional decline,
comprehensive care plan established, implemented, revised, or
monitored; first 20 minutes of clinical staff time directed by a
physician or other qualified health care professional, per
calendar month
99491
Chrnc care mgmt phys 1st 30
Chronic care management services, provided personally by a
physician or other qualified healthcare professional, at least 30
minutes of physician or other qualified healthcare professional
time, per calendar month, with the following required elements:
multiple (two or more) chronic conditions expected to last at
least 12 months, or until the death of the patient, chronic
conditions place the patient at significant risk of death, acute
exacerbation/decompensation, or functional decline,
comprehensive care plan established, implemented, revised, or
monitored
98975
Rem ther mntr 1st setup&edu
Remote therapeutic monitoring (e.g. therapy adherence,
therapy response); initial set-up and patient education on use of
equipment
98976
Rem ther mntr dev sply resp
Remote therapeutic monitoring (e.g. therapy adherence,
therapy response); device(s) supply with scheduled (e.g. daily)
recording(s) and/or programmed alert(s) transmission to
monitor respiratory system, each 30 days
98977
Rem ther mntr dv sply mscskl
Remote therapeutic monitoring (e.g. therapy adherence,
therapy response); device(s) supply with scheduled (e.g. daily)
recording(s) and/or programmed alert(s) transmission to
monitor musculoskeletal system, each 30 days
98980
Rem ther mntr 1st 20 min
Remote therapeutic monitoring treatment management
services, physician or other qualified health care professional
time in a calendar month
requiring at least one interactive communication with the
patient or caregiver during the calendar month; first 20 minutes
98981
Rem ther mntr ea addl 20 min
Remote therapeutic monitoring treatment management
services, physician or other qualified health care professional
time in a calendar month requiring at least one interactive
communication with the patient or caregiver during the
calendar month; each additional 20 minutes (list separately in
addition to code for primary procedure)
G0140
Nav srv peer sup 60 min pr m
Principal Illness Navigation – Peer Support by certified or
trained auxiliary personnel under the direction of a physician or
other practitioner, including a certified peer specialist; 60
minutes per calendar month, for specified activities

HCPCS
code
Short Descriptors
Long Descriptors
G0146
Nav srv peer sup add 30 pr m
Principal Illness Navigation—Peer Support, additional 30
minutes per calendar month (List separately in addition to
G0140)
G3002
Chronic pain mgmt 30 mins
Chronic pain management and treatment, monthly bundle
including, diagnosis; assessment and monitoring;
administration of a validated pain rating scale or tool; the
development, implementation, revision, and maintenance of a
person-centered care plan that includes strengths, goals, clinical
needs, and desired outcomes; overall treatment management;
facilitation and coordination of any necessary behavioral health
treatment; medication management; pain and health literacy
counseling; any necessary chronic pain related crisis care; and
ongoing communication and care coordination between
relevant practitioners furnishing care, e.g. physical therapy and
occupational therapy, and community-based care, as
appropriate. Required initial face-to- face visit at least 30
minutes provided by a physician or other qualified health
professional; first 30 minutes personally provided by physician
or other qualified health care professional, per calendar month.
(when using G3002, 30 minutes must be met or exceeded)
G3003
Chronic pain mgmt addl 15m
Each additional 15 minutes of chronic pain management and
treatment by a physician or other qualified health care
professional, per calenda month. (List separately in addition to
G3002. When using G3003, 15 minutes mut be met or
exceeded).
G0323
Care manage beh svs 20mins
Care management services for behavioral health conditions, at
least 20 minutes of clinical psychologist or clinical social
worker time, per calendar month, with the following required
elements: initial assessment or follow-up monitoring, including
the use of applicable validated rating scales; behavioral health
care planning in relation to behavioral/psychiatric health
problems, including revision for patients who are not
progressing or whose status changes; facilitating and
coordinating treatment such as psychotherapy, coordination
with an/or referral to physicians and practitioners who are
authorized by Medicare law to prescribe medications and
furnish E/M services counseling and/or psychiatric
consultation; and continuity of care with a designated member
of the care team)
G0019
Comm hlth intg svs sdoh 60
mn
Community health integration services performed by certified
or trained auxiliary personnel, including a community health
worker, under the direction of a physician or other practitioner;
60 minutes per calendar month, in the following activities to
address social determinants of health (SDOH) need(s) that are
significantly limiting the ability to diagnose or treat problem(s)
addressed in an initiating visit.
G0022
Comm hlth intg svs add 30 m
Community health integration services, each additional 30
minutes per calendar month (List separately in addition to
G0019)
G0023
Pin srv 60 min pr m
Principal Illness Navigation services by certified or trained
auxiliary personnel under the direction of a physician or other
practitioner, including a patient navigator or certified peer
specialist; 60 minutes per calendar month, in the following
activities: • Person-centered assessment, performed to better
understand the individual context of the serious, high risk
condition. ++ Conducting a person-centered assessment to
understand the patient’s life story, strengths, needs, goals,
preferences, and desired outcomes, including understanding
cultural and linguistic factors. ++ Facilitating patient-driven
goal setting and establishing an action plan. ++ Providing

HCPCS
code
Short Descriptors
Long Descriptors
tailored support as needed to accomplish the practitioner’s
treatment plan. • Identifying or referring patient (and caregiver
or family, if applicable) to appropriate supportive services.
G0024
Pin srv add 30 min pr m
Principal Illness Navigation services, additional 30 minutes per
calendar month (List separately in addition to G0023).
We are proposing revisions at § 405.2464(c) to reflect the proposed payment method for
care management services furnished in RHCs and FQHCs beginning January 1, 2025. We
welcome comments on this proposed payment methodology.
d. New Codes for Advanced Primary Care Management (APCM) Services
As discussed in section II.G of this proposed rule, HHS and CMS have been analyzing
opportunities to strengthen and invest in primary care in alignment with the goals of the HHS
Initiative to Strengthen Primary Care.
302
Research has demonstrated that greater primary care
physician supply is associated with improved population-level mortality and reduced
disparities,
303
and also that establishing a long-term relationship with a primary care provider
leads to reduced emergency department (ED) visits,
304
improved care coordination, and
increased patient satisfaction.
305
HHS recognizes that effective primary care is essential for
improving access to healthcare, for the health and wellbeing of individuals, families, and
communities, and for achieving health equity. The first coordinated set of HHS-wide actions to
strengthen primary care, as part of the Initiative, is in primary care payment; for example,
adjusting payment to ensure it supports delivery of advanced primary care. CMS Innovation
Center models, described in section II.G.2.a.(1) of this proposed rule, reflect the ongoing work
302
U.S. Department of Health and Human Services. (2023). Primary Care: Our First Line of Defense.
https://www.hhs.gov/sites/default/files/primary-care-issue-brief.pdf.
303
Basu S, Berkowitz SA, Phillips RL, Bitton A, Landon BE, Phillips RS. Association of Primary Care Physician
Supply With Population Mortality in the United States, 2005-2015. JAMA Intern Med. 2019;179(4):506–514.
doi:10.1001/jamainternmed.2018.7624.
https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2724393.
304
Willemijn L.A. Schäfer et al, “Are People’s Health Care Needs Better Met When Primary Care Is Strong? A
Synthesis of the Results of the QUALICOPC Study in 34 Countries,” Primary Health Care Research and
Development 20 (2019): e104. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6609545/.
305
Michael J. van den Berg, Tessa van Loenen, and Gert P Westert, “Accessible and Continuous Primary Care May
Help Reduce Rates of Emergency Department Use: An International Survey in 34 Countries,” Family Practice 33,
no. 1 (Feb. 2016): 42–50. https://academic.oup.com/fampra/article/33/1/42/2450446.
within HHS and the unified, comprehensive approach to HHS primary care activities that we are
accomplishing through our current statutory authorities and funding.
In recent years, CMS has implemented significant changes aimed at better capturing the
resources required for care management services, including chronic care management (CCM),
principal care management (PCM), and transitional care management (TCM) and more recently,
community health integration (CHI), principal illness navigation (PIN) and PIN-peer support
services. For RHCs and FQHCs, we have established payment for these suites of care
management services outside of the RHC AIR and FQHC PPS. The policy requirements for
RHCs and FQHCs furnishing these various care coordination services are consistent with those
finalized under the PFS.
In section II.G.2.b. of this proposed rule, we are proposing to establish coding and make
payment under the PFS for a newly defined set of APCM services described and defined by three
new HCPCS G-codes. This new coding would reflect the recognized effectiveness and growing
adoption of the advanced primary care approach to care. It would also encompass a broader
range of services and simplify the billing and documentation requirements, as compared to
existing care management codes. The proposed coding for APCM incorporates elements of
several existing care management services into a bundle that we have already considered to be
care coordination services paid separately to RHCs and FQHCs using HCPCS code G0511. For
example, CCM and PCM. In addition, the coding for APCM incorporates elements of
communication technology-based services (CTBS) into a bundle that we have already considered
to be virtual communications paid separately to RHCs and FQHCs using HCPCS code G0071.
For example, remote evaluation of patient videos/images, virtual check-in, and e-visits.
Therefore, to allow RHCs and FQHCs the ability to simplify the billing and documentation
requirements associated with furnishing APCM services we are proposing to allow RHCs and
FQHCs to bill for these services and receive separate payment. Consistent with section II.G.2.b.
of this proposed rule, the APCM code sets vary by the degree of complexity of patient conditions
(that is, non-complex and complex CCM for multiple chronic conditions or PCM for a single
high-risk condition), and whether the number of minutes spent by clinical staff or the physician
or non-physician practitioner (NPP) is used to meet time thresholds for billing. The proposed
HCPCS codes are described below:
HCPCS code GPCM1 (Advanced primary care management services provided by clinical
staff and directed by a physician or other qualified health care professional who is responsible
for all primary care and serves as the continuing focal point for all needed health care services,
per calendar month, with the following elements, as appropriate:
● Consent;
++ Inform the patient of the availability of the service; that only one practitioner
can furnish and be paid for the service during a calendar month; of the right to
stop the services at any time (effective at the end of the calendar month); and that
cost sharing may apply.
++ Document in patient’s medical record that consent was obtained.
● Initiation during a qualifying visit for new patients or patients not seen within 3 years;
● Provide 24/7 access for urgent needs to care team/practitioner, including providing
patients/caregivers with a way to contact health care professionals in the practice to
discuss urgent needs regardless of the time of day or day of week;
● Continuity of care with a designated member of the care team with whom the patient is
able to schedule successive routine appointments;
● Deliver care in alternative ways to traditional office visits to best meet the patient’s
needs, such as home visits and/or expanded hours;
● Overall comprehensive care management;
++ Systematic needs assessment (medical and psychosocial).
++ System-based approaches to ensure receipt of preventive services.
++ Medication reconciliation, management and oversight of self-management.
● Development, implementation, revision, and maintenance of an electronic patient-
centered comprehensive care plan;
++ Care plan is available timely within and outside the billing practice as
appropriate to individuals involved in the beneficiary’s care, can be routinely
accessed and updated by care team/practitioner, and copy of care plan to
patient/caregiver;
● Coordination of care transitions between and among health care providers and
settings, including referrals to other clinicians and follow-up after an emergency
department visit and discharges from hospitals, skilled nursing facilities or other health
care facilities as applicable;
++ Ensure timely exchange of electronic health information with other
practitioners and providers to support continuity of care.
++ Ensure timely follow-up communication (direct contact, telephone,
electronic) with the patient and/or caregiver after an emergency department visit
and discharges from hospitals, skilled nursing facilities, or other health care
facilities, within 7 calendar days of discharge, as clinically indicated.
● Ongoing communication and coordinating receipt of needed services from
practitioners, home- and community-based service providers, community-based social
service providers, hospitals, and skilled nursing facilities (or other health care facilities),
and document communication regarding the patient’s psychosocial strengths and needs,
functional deficits, goals, preferences, and desired outcomes, including cultural and
linguistic factors, in the patient’s medical record;
● Enhanced opportunities for the beneficiary and any caregiver to communicate with the
care team/practitioner regarding the beneficiary’s care through the use of asynchronous
non-face-to-face consultation methods other than telephone, such as secure messaging,
email, internet, or patient portal, and other communication-technology based services,
including remote evaluation of pre-recorded patient information and interprofessional
telephone/internet/EHR referral service(s), to maintain ongoing communication with
patients, as appropriate;
++ Ensure access to patient-initiated digital communications that require a
clinical decision, such as virtual check-ins and digital online assessment and
management and E/M visits (or e-visits).
● Analyze patient population data to identify gaps in care and offer additional
interventions, as appropriate;
● Risk stratify the practice population based on defined diagnoses, claims, or other
electronic data to identify and target services to patients;
● Be assessed through performance measurement of primary care quality, total cost of
care, and meaningful use of Certified EHR Technology.).
HCPCS code GPCM2 (Advanced primary care management services for a patient with
multiple (two or more) chronic conditions expected to last at least 12 months, or until the death
of the patient, which place the patient at significant risk of death, acute exacerbation/
decompensation, or functional decline, provided by clinical staff and directed by a physician or
other qualified health care professional who is responsible for all primary care and serves as the
continuing focal point for all needed health care services, per calendar month, with the elements
included in GPCM1, as appropriate) and HCPCS code GPCM3 (Advanced primary care
management services for a patient that is a Qualified Medicare Beneficiary with multiple (two or
more) chronic conditions expected to last at least 12 months, or until the death of the patient,
which place the patient at significant risk of death, acute exacerbation/decompensation, or
functional decline, provided by clinical staff and directed by a physician or other qualified
health care professional who is responsible for all primary care and serves as the continuing
focal point for all needed health care services, per calendar month, with the elements included in
GPCM1, as appropriate).
As we’ve established previously, care coordination services are RHC/FQHC services and
as such, we are proposing to align once again with the PFS and adopt the new codes for APCM
services. Additionally, allowing separate payment for APCM services in RHCs and FQHCs is
intended to reflect the additional time and resources necessary for the unique components of care
coordination services.
Further, in alignment with our proposal earlier in this section to require RHCs and
FQHCs to utilize the same coding as when billing under the PFS and no longer use HCPCS code
G0511, which described many care coordination services, we are proposing to require RHCs and
FQHCs when furnishing APCM to use the codes created for the PFS, that is, the three HCPCS
G-codes described above. We would pay for these services in addition to the RHC AIR or FQHC
PPS because we consider these services as non-face-to-face services and similar to other care
management services such as chronic care management, principal care management, and remote
physiological monitoring, where these services are not captured in the RHC AIR or FQHC PPS
payment. Similarly, we are proposing that payment for these services would be paid at the PFS
non-facility rate.
It is important to note that if RHCs and FQHCs report these new codes, they are per
calendar month bundles. If the RHC/FQHC decides to bill for APCM then they would not bill
for individual services. For further discussion on duplicative services and concurrent billing
restrictions with regard to APCM policies, we refer readers to section II.G.2.d. of this proposed
rule.
e. Request for Information – Aligning with Services Paid Under the PFS
As we discuss in section III.B.2.a. of this proposed rule, over the last several years we
have been increasing our focus on care coordination. These services have evolved to focus on
preventing and managing chronic disease, improving a beneficiary’s transition from the hospital
to the community setting, or on integrative treatment of patients with behavioral health
conditions. We have acknowledged that the care coordination included in services such as office
visits does not always describe adequately the non-face-to-face care management work involved
and may not reflect all the services and resources required to furnish comprehensive, coordinated
care management for certain categories of beneficiaries. Therefore, under the PFS we have
proposed new services over the years that practitioners billing under the PFS can be paid
separately under the PFS. We have noted previously that RHCs and FQHCs cannot bill under the
PFS for RHC or FQHC services and individual practitioners working at RHCs and FQHCs
cannot bill under the PFS for RHC or FQHC services while working at the RHC or FQHC.
Therefore, we have proposed payment policies for RHCs and FQHCs that complement the new
services for care coordination under the PFS to align the RHC and FQHC resource cost for those
services with payment.
The increase in frequency of this complementary rulemaking has triggered us to consider
operational efficiencies internally that we believe could result in more transparency and clarity
for interested parties. Since RHCs and FQHCs are generally paid under encounter-based
payment systems, we have not systematically analyzed all services paid under the PFS (nor do
we analyze all new services proposed) to determine if they are included as a part of the visit
versus are eligible for additional payment. Another reason that we do not analyze every code is
because frequently codes created under the PFS for billing practitioners are to more
appropriately account for resources paid under the PFS. Codes for these purposes are not
applicable for RHCs and FQHCs since they are not paid under the PFS.
Generally, for PFS services that are a part of the office visit, there is no separate payment
under the RHC AIR or FQHC PPS methodologies. On the contrary, care coordination services
where the focus is on care management, coordination, or certain activities needed to manage
chronic illnesses or adapt to new models of care, we have allowed separate payment for RHCs
and FQHCs.
We are seeking comment on how we can improve the transparency and predictability
regarding which HCPCS codes are considered care coordination services. Our goal is to classify
care coordination services on the PFS in a way that makes it automated in the downstream effect
on RHCs and FQHCs. We believe establishing a streamlined policy regarding which services are
separately paid for RHCs and FQHCs versus included as part of the visit is more transparent. In
addition, a policy where codes are communicated and updated through sub-regulatory guidance
may be more efficient.
3. Telecommunication Services
a. Background
Section 3704 of the Coronavirus Aid, Relief, and Economic Security Act (the CARES
Act) (Pub. L. 116-136, March 27, 2020) directed the Secretary to establish Medicare payment for
telehealth services provided by RHCs and FQHCs serving as a distant site (that is, where the
practitioner is located) during the PHE for COVID-19. Separately, section 3703 of the CARES
Act expanded CMS' emergency waiver authority to allow for a waiver of any of the statutory
telehealth payment requirements under section 1834(m) of the Act for telehealth services
furnished during the PHE. Specifically, section 1834(m)(8)(B) of the Act, as added by the
CARES Act, requires that the Secretary develop and implement payment methods for FQHCs
and RHCs that serve as a distant site during the PHE for the COVID-19 pandemic. The payment
methodology outlined in the CARES Act requires that rates shall be based on rates that are
similar to the national average payment rates for comparable telehealth services under the
Medicare PFS. CMS established rates based on the average amount for all PFS telehealth
services on the telehealth list, weighted by volume.
In the CY 2022 PFS final rule with comment (86 FR 65211), we revised the regulatory
requirement that an RHC or FQHC mental health visit must be a face-to-face (that is, in-person)
encounter between an RHC or FQHC patient and an RHC or FQHC practitioner. We revised the
regulations under § 405.2463 to state that an RHC or FQHC mental health visit can also include
encounters furnished through interactive, real-time, audio/video telecommunications technology
or audio-only interactions in cases where beneficiaries are not capable of, or do not consent to,

the use of devices that permit a two-way, audio/video interaction for the purposes of diagnosis,
evaluation or treatment of a mental health disorder. We noted that these changes aligned with
similar mental health services furnished under the PFS. We also noted that this change allows
RHCs and FQHCs to report and be paid for mental health visits furnished via real-time,
telecommunication technology in the same way they currently do when these services are
furnished in-person.
In addition, we revised the regulation under § 405.2463 to state that there must be an in-
person mental health service furnished within 6 months prior to the furnishing of the
telecommunications service and that an in-person mental health service (without the use of
telecommunications technology) must be provided at least every 12 months while the beneficiary
is receiving services furnished via telecommunications technology for diagnosis, evaluation, or
treatment of mental health disorders, unless, for a particular 12-month period, the physician or
practitioner and patient agree that the risks and burdens outweigh the benefits associated with
furnishing the in-person item or service, and the practitioner documents the reasons for this
decision in the patient's medical record (86 FR 65210 and 65211).
As discussed in the CY 2023 PFS final rule (87 FR 69738), the Consolidated
Appropriations Act, 2022 (CAA, 2022) (Pub. L. 117-103, March 15, 2022) included the
extension of several Medicare telehealth flexibilities established during the public health
emergency (PHE) for COVID-19 for a limited 151-day period beginning on the first day after the
end of the PHE for COVID-19. Specifically, Division P, Title III, section 304 of the CAA, 2022,
delayed the in-person requirements under Medicare for mental health services furnished through
telehealth under the PFS and for mental health visits furnished by RHCs and FQHCs via
telecommunications technology until the 152nd day after the end of the PHE for COVID–19.
Therefore, in the CY 2023 PFS final rule (87 FR 69737), we revised the regulations under
§§ 405.2463 and 405.2469 again to reflect these provisions.

Further, in the CY 2024 PFS final rule (88 FR 79065), we discussed that the CAA, 2023
(Pub. L. 117-328, December 29, 2022) further extended the Medicare telehealth flexibilities
enacted in the CAA, 2022 for a period beginning on the first day after the end of the PHE for
COVID-19 and ending on December 31, 2024, if the PHE ends prior to that date. Specifically
related to RHCs and FQHCs, section 4113(c) of the CAA, 2023 amended section 1834(m)(8) of
the Act to extend payment for telehealth services furnished by FQHCs and RHCs for the period
beginning on the first day after the end of the COVID-19 PHE and ending on December 31,
2024, if the PHE ends prior to that date. We noted that payment continued to be made under the
methodology established for telehealth services furnished by FQHCs and RHCs during the PHE,
which is based on payment rates that are similar to the national average payment rates for
comparable telehealth services under the PFS.
We explained that section 4113(d) of the CAA, 2023 continues to delay the in-person
requirements under Medicare for mental health services furnished through telehealth under the
PFS and for mental health visits furnished by RHCs and FQHCs via telecommunications
technology. That is, for RHCs and FQHCs, in-person visits will not be required until January 1,
2025, or, if later, the first day after the end of the PHE for COVID-19. Therefore, we stated that
we will continue to apply the delay of the in-person requirements under Medicare for mental
health services furnished by RHCs and FQHCs. We noted that the Department of Health and
Human Services declared an end to the Federal PHE for COVID-19 under section 319 of the
Public Health Service Act on May 11, 2023.
306
Therefore, we revised the regulations under
§§ 405.2463 and 405.2469 again to reflect these provisions (88 FR 79066 through 79067).
b. Direct Supervision via Use of Two-way Audio/Video Communications Technology
Under Medicare Part B, certain types of services are required to be furnished under specific
minimum levels of supervision by a physician or practitioner. See section II.D.2.a. for the
306
https://www.hhs.gov/coronavirus/covid-19-public-health-emergency/index.html.
discussion regarding direct supervision for services provided using telecommunications
technologies under the PFS.
In the CY 2024 PFS final rule (88 FR 79067), we explained that extending this definition
of direct supervision for RHCs and FQHCs under our regulations at §§405.2413, 405.2415,
405.2448, and 405.2452 through December 31, 2024, would align the timeframe of this policy
with many of the previously discussed PHE-related telehealth policies that were extended under
provisions of the CAA, 2023. In addition, we were concerned about an abrupt transition to the
pre-PHE policy of requiring the physical presence of the supervising practitioner beginning after
December 31, 2023, given that RHCs and FQHCs have established new patterns of practice
during the PHE for COVID-19. We also believed that RHCs and FQHCs would need time to
reorganize their practices established during the PHE to reimplement the pre-PHE approach to
direct supervision without the use of audio/video technology. Similar to services furnished in
physician office setting, RHC and FQHC services and supplies furnished incident to physician’s
services are limited to situations in which there is direct physician supervision of the person
performing the service, except for certain care coordination services which may be furnished
under general supervision. For CY 2024, we continued to define “immediate availability” as
including real-time audio and visual interactive telecommunications through December 31, 2024,
and solicited comment on whether we should consider extending the definition of ‘‘direct
supervision’’ to permit virtual presence beyond December 31, 2024.
(1) Proposal for CY 2025
In the CY 2024 PFS proposed rule, we solicited comment on potential patient safety or
quality concerns when direct supervision occurs virtually in RHCs and FQHCs; for instance, if
certain types of services are more or less likely to present patient safety concerns, or if this
flexibility would be more appropriate when certain types of auxiliary personnel are performing
the supervised service. We were also interested in potential program integrity concerns such as
overutilization or fraud and abuse that interested parties may have in regard to this policy.
Comments provided were overall supportive of our proposal to continue to define
“immediate availability” to include availability through virtual means, stating that it will benefit
healthcare providers while greatly enhancing patient access to quality care, particularly in
underserved areas. Commenters also noted that direct supervision has become increasingly
challenging and the option to provide virtual direct supervision has enhanced the quality and
provision of healthcare services beneficiaries have received in medically underserved, rural
communities.
We note that in section II.D.2.a. of this proposed rule, there is a proposal to permanently
adopt a definition of direct supervision that allows "immediate availability” of the supervising
practitioner using audio/video real-time communications technology (excluding audio-only), but
only for the following subset of incident-to services described under § 410.26, (1) services
furnished incident to a physician or other practitioner’s service when provided by auxiliary
personnel employed by the billing practitioner and working under their direct supervision, and
for which the underlying HCPCS code has been assigned a Professional Component/Technical
Component indicator of ‘5’; and (2) services described by CPT code 99211 (Office or other
outpatient visit for the evaluation and management of an established patient that may not require
the presence of a physician or other qualified health care professional). In addition, under the
PFS we are proposing for all other services required to be furnished under the direct supervision
of the supervising physician or other practitioner, to continue to define "immediate availability”
to include real-time audio and visual interactive telecommunications technology only through
December 31, 2025.
After evaluating the information gathered through the comment solicitation, we believe
that we should maintain the current flexibility in RHCs and FQHCs as it continues to support
access and preserve workforce capacity. We believe that there is value in allowing RHC and
FQHC services to be furnished under direct supervision where virtual presence meets the
definition of “immediately available” as status quo, so that we may further evaluate the services
along with the analysis occurring for the remaining services that we are contemplating under the
PFS. We note that there may be nuances in the RHC and FQHC settings since generally payment
is at the AIR or PPS rate and not at the individual service code level to carve out services
limited/obvious services from other services. We could seek to establish a final policy in RHCs
and FQHCs once a final policy is determined under the PFS, to avoid confusion since they are
taking an incremental approach at the code level for CY 2025.
Therefore, we are proposing to maintain the virtual presence flexibility on a temporary
basis, that is, the presence of the physician (or other practitioner) would include virtual presence
through audio/video real-time communications technology (excluding audio-only) through
December 31, 2025.
c. Telecommunications Technology
As discussed above, section 3704 of the Coronavirus Aid, Relief, and Economic Security
Act (the CARES Act) (Pub. L. 116-136, March 27, 2020) directed the Secretary to establish
Medicare payment for telehealth services provided by RHCs and FQHCs serving as a distant site
(that is, where the practitioner is located) during the PHE for COVID-19. Separately, section
3703 of the CARES Act expanded CMS' emergency waiver authority to allow for a waiver of
any of the statutory telehealth payment requirements under section 1834(m) of the Act for
telehealth services furnished during the PHE. Specifically, section 1834(m)(8)(B) of the Act, as
added by the CARES Act, required that the Secretary develop and implement payment methods
for FQHCs and RHCs that serve as a distant site during the PHE for the COVID-19 pandemic.
The payment methodology outlined in the CARES Act requires that rates shall be based on rates
that are similar to the national average payment rates for comparable telehealth services under
the Medicare PFS. Therefore, CMS established rates based on the average amount for all PFS
telehealth services on the telehealth list, weighted by volume. RHCs and FQHCs bill for these
Medicare telehealth services using HCPCS code G2025.
In the CY 2022 PFS final rule with comment (86 FR 65211), we revised the regulatory
requirement that an RHC or FQHC mental health visit must be a face-to-face (that is, in person)
encounter between an RHC or FQHC patient and an RHC or FQHC practitioner. We revised the
regulations under § 405.2463 to state that an RHC or FQHC mental health visit can also include
encounters furnished through interactive, real-time, audio/video telecommunications technology
or audio-only interactions in cases where beneficiaries are not capable of, or do not consent to,
the use of devices that permit a two-way, audio/video interaction for the purposes of diagnosis,
evaluation or treatment of a mental health disorder. We noted that these changes aligned with
similar mental health services furnished under the PFS. We also noted that this change allows
RHCs and FQHCs to report and be paid for mental health visits furnished via real-time,
telecommunication technology in the same way they currently do when these services are
furnished in-person.
The temporary authority under section 1834(m)(8) of the Act was extended by statute
through the end of CY 2024, meaning under current law, and absent additional changes in
regulation, RHCs and FQHCs could not be paid for non-behavioral health services furnished
remotely to beneficiaries enrolled in fee-for-service Medicare after December 31, 2024.
(1) Payment Proposal for Non-Behavioral Health Telecommunication Technology Services
Widespread use of telecommunications technology to furnish services during the PHE
has illustrated interest within the medical community and among Medicare beneficiaries in
furnishing and receiving care through the use of technology beyond the PHE. During the PHE,
RHCs and FQHCs, much like other provider types, have had to change how they furnish care in
order to meet the needs of their patients, and use of the temporary authority to bill Medicare for
PFS telehealth services has been widely utilized by RHCs and FQHCs during the PHE.
Eliminating flexibilities under which RHC and FQHC services have been furnished to
beneficiaries via telecommunication technologies for over four years and resuming solely in-
person, face-to-face medical visits after December 31, 2024, would cause disruptions in access to
services from RHC and FQHC practitioners. This would be particularly problematic for the
underserved populations that these settings furnish services to since it could fragment care. We
believe that we need to preserve the flexibilities under which RHC and FQHC services have
been furnished to beneficiaries via telecommunication technologies temporarily and to do so
through an approach that these settings are familiar with in order to mitigate burden.
Technologies used in this space and the quality of care associated with them continue to evolve.
We believe that it would be prudent to allow time to engage interested parties while we consider
how to incorporate telecommunication technology services on a more permanent basis.
For these reasons, we propose, on a temporary basis, to allow payment for non-behavioral
health visits furnished via telecommunication technology. We propose to facilitate payments
using an approach that closely aligns with the mechanisms mandated by the statute but ends
December 31, 2024. That is, RHCs and FQHCs would continue to bill for RHC and FQHC
services furnished using telecommunication technologies by reporting HCPCS code G2025 on
the claim, including services furnished using audio-only communications technology. Since the
costs associated with non-behavioral health visits furnished via telecommunication technology
are not included in the calculations for the RHC AIR methodology and FQHC PPS, we believe
that we need to propose a proxy that would represent such resources used when furnishing these
services. Therefore, we propose to continue to calculate the payment amount based on the
average amount for all PFS telehealth services on the telehealth list, weighted by volume for
those services reported under the PFS. We believe that using this weighted average is
appropriate during this interim while we contemplate permanent policies for these services since
there is a wide range of values for the telehealth services under the PFS. We believe that RHCs
and FQHCs generally furnish services that are similar to and at a frequency the same as
physicians and other practitioners paid under the PFS. While we do not have actual cost
information, we believe that this weighted average is an appropriate proxy since it addresses
certain resource costs experienced by professionals and would mitigate any potential over or
under payments. Costs associated with these services would continue to not be used in
determining payments under the RHC AIR methodology or the FQHC PPS.
We believe that these proposals would preserve the telecommunication technology
flexibility under which RHC and FQHC services have been furnished for over 4 years and would
not impact access to care for Medicare beneficiaries who currently benefit from these services
while CMS contemplates next steps. We solicit comment on whether there may be other
payment methodologies that may be a proxy for costs associated with non-behavioral health
visits furnished via telecommunication technology and why those payment methodology may be
more appropriate than the weighted average of the telehealth services list under PFS.
We propose to amend § 405.2464 by adding new paragraph (g) to reflect our proposed
payment policy for non-behavioral health services furnished in RHCs and FQHCs via
telecommunication technology for CY 2025.
(2) Alternative Proposal Considered for Payment of Medical Visits Furnished Via
Telecommunication Technology
We considered reevaluating the regulations regarding face-to-face visit requirements for
encounters between a beneficiary and an RHC or FQHC practitioner in light of contemporary
medical practices. That is, we considered proposing a revision to the regulatory requirement that
an RHC or FQHC medical visit must be a face-to-face (that is, in-person) encounter between a
beneficiary and an RHC or FQHC practitioner to also include encounters furnished through
interactive, real-time, audio and video telecommunications technology. This would result in
payment for services furnished via telecommunication technology to be made under the RHC
AIR methodology and under the FQHC PPS, similar to how we revised the regulations for
mental health visits. We believe interested parties may prefer the per visit payment that aligns
with the RHC AIR or FQHC PPS. However, we did not propose this alternative because we
determined that it would have unintended consequences, especially in cases where the RHC AIR

or FQHC PPS per-visit rates would be significantly higher than the PFS rate that would apply if
other entities furnished the same service to the same beneficiary in the same location.
We believe that continuing to pay temporarily for RHC and FQHC services furnished via
telecommunication technologies in the same manner as we have done over the past several years
preserves the flexibility for RHCs and FQHCs to continue access to care, mitigates
administrative burden, and mitigates potential program integrity concerns. However, we solicit
comment on the alternative proposal we considered. That is, revising the definition of a visit to
include interactive, real-time, audio/video telecommunication technology which would result in a
capitated payment under the RHC AIR methodology or FQHC PPS.
d. In-person Visit Requirements for Remote Mental Health Services Furnished by RHC and
FQHCs
The CAA, 2021, stipulated that the beneficiary needed to receive an in person, non-
telehealth service 6 months prior to initiation of the telehealth mental health services and that the
Secretary should determine an appropriate frequency for provision of subsequent periodic in
person, non-telehealth services.
In the CY 2022 PFS final rule with comment (86 FR 65210), we revised the regulation
under § 405.2463 to state that there must be an in-person mental health service furnished within
6 months prior to the furnishing of the telecommunications service and that an in-person mental
health service (without the use of telecommunications technology) must be provided at least
every 12 months while the beneficiary is receiving services furnished via telecommunications
technology for diagnosis, evaluation, or treatment of mental health disorders, unless, for a
particular 12-month period, the physician or practitioner and patient agree that the risks and
burdens outweigh the benefits associated with furnishing the in-person item or service, and the
practitioner documents the reasons for this decision in the patient's medical record.
As discussed in the CY 2023 PFS final rule (87 FR 69738), the CAA, 2022 included the
extension of a number of Medicare telehealth flexibilities established during the PHE for

COVID-19 for a limited 151-day period beginning on the first day after the end of the PHE.
Division P, Title III, section 304 of the CAA, 2022, delayed the in-person requirements under
Medicare for mental health services furnished through telehealth under the PFS and for mental
health visits furnished by RHCs and FQHCs via telecommunications technology until the 152nd
day after the end of the PHE for COVID–19.
The CAA, 2023 (Pub. L. 117-328, December 29, 2022) extended the Medicare telehealth
flexibilities enacted in the CAA, 2022 for a period beginning on the first day after the end of the
PHE for COVID-19 and ending on December 31, 2024, if the PHE ended prior to that date.
While the CAA, 2021 only applied to the PFS, we implemented similar policies for RHCs,
FQHCs, and hospital outpatient departments. The in-person visit requirements are currently set
to take effect for services furnished on or after January 1, 2025.
However, given concerns from interested parties on the impact of enforcing these
requirements after multiple years of delay, we are proposing an additional extension. We are
proposing to continue to delay the in-person visit requirement for mental health services
furnished via communication technology by RHCs and FQHCs to beneficiaries in their homes
until January 1, 2026.
4. Intensive Outpatient Program Services (IOP)
a. Background
As we discussed in the CY 2024 OPPS final rule (88 FR 81838) section 4124 of Division
FF of the CAA, 2023 established Medicare coverage for intensive outpatient program (IOP)
services furnished by a hospital to its outpatients, or by a community mental health center
(CMHC), a FQHC or a RHC, as a distinct and organized intensive ambulatory treatment service
offering less than 24-hour daily care in a location other than an individual’s home or inpatient or
residential setting, effective January 1, 2024.
IOP is a distinct and organized outpatient program of psychiatric services provided for
individuals who have an acute mental illness, which includes, but is not limited to conditions
such as depression, schizophrenia, and substance use disorders. We noted an IOP is thought to
be less intensive than a partial hospitalization program (PHP).
This new provision mandated several areas of policy to implement an IOP program,
including scope of benefits and services, certification and plan of care requirements, and special
payment rules for IOP services in RHCs and FQHCs, all of which are discussed in the CY2024
OPPS final rule (88 FR 81838 through 81845). We made corresponding regulation changes for
IOP services at §§ 405.2400, 405.2401, 405.2410, 405.2411, 405.2446, 405.2462, 405.2463,
405.2464, 405.2468, and 405.2469.
b. Update to Special Payment Rules for Intensive Outpatient Services
As we discussed in the CY 2024 OPPS final rule (88 FR 81841), section 4124(c) of the
CAA, 2023 further amended section 1834(o) of the Act and section 1834(y) of the Act, to
provide special payment rules for both FQHCs and RHCs, respectively, for furnishing IOP
services. Section 4124(c)(1) of the CAA, 2023 amended section 1834(o) of the Act to add a new
paragraph (5)(A) to require that payment for IOP services furnished by FQHCs be equal to the
amount that would have been paid under Medicare for IOP services had they been covered
outpatient department services furnished by a hospital. In addition, section 4124(c)(2) of the
CAA, 2023 amended section 1834(y) of the Act to add a new paragraph (3)(A) to require that
payment for IOP services furnished by RHCs be equal to the amount that would have been paid
under Medicare for IOP services had they been covered outpatient department services furnished
by a hospital.
In the CY 2024 OPPS final rule (88 FR 81841), we provided a detailed discussion of the
final CY 2024 payment rate methodology for IOP. CMS finalized two payment rates, a 3- and a
4- or more services per day, for IOP services for hospitals and CMHCs. However, for RHCs and
FQHCs we established a 3-service per day payment rate. We stated that we believed it was
appropriate to establish the payment rate where the utilization is typically structured to be days
with 3 or fewer services and solicited comment on whether the hospital-based IOP payment rate
for 4-service days would be appropriate for RHCs and FQHCs. Although we previously stated
that we would review the data and consider a 4 or more services per day for future rulemaking,
we have since considered this further. We believe that we should provide parity for IOP services
across the various settings with site neutral payments while continuing to monitor access to these
services. Therefore, we are proposing to provide a payment rate for 4 or more services per day in
an RHC/FQHC setting. Additionally, as required in section 4124(c)(2) of the CAA, 2023 we
would align with the 4 or more-services per day payment rate for hospital outpatient
departments. As with the 3-services per day, the 4 or more services per day payment rates will be
updated annually.
c. Technical Correction (§§ 405.2410 and 405.2462)
In the CY 2024 Hospital Outpatient Prospective Payment (OPPS) and Ambulatory
Surgical Center (ASC) Payment Systems final rule with comment (88 FR 81844) we finalized
revisions to §§ 405.2410, 405.2462, and 405.2464 in the regulations to reflect the payment
amount for IOP services and how the Medicare Part B deductible and coinsurance are applied in
RHC’s and FQHC’s. For RHCs, the beneficiary is responsible for the Medicare Part B deductible
and coinsurance amounts at an amount not to exceed 20 percent of the clinic’s reasonable
charges for IOP services. For FQHC’s, the beneficiary is responsible for a coinsurance amount of
20 percent of the lesser of the FQHC’s actual charge for the service or the IOP rate. We revised
the regulatory requirements at §405.2410, Application of Part B deductible and coinsurance and
§405.2462, Application of deductible and coinsurance for RHCs and FQHCs paid on the basis of
the special payment rule under §405.2462(j) to reflect how the Medicare Part B deductible and
coinsurance are applied.
During a recent review of our regulations at §§ 405.2410(c) and 405.2462(j), we noticed
that both sections had errors. That is, § 405.2410(c) does not reflect the correct policy that is
applicable for beneficiary coinsurance when they receive IOP services in RHCs and FQHCs.
With regard to the error at § 405.2462(j), we inadvertently left language specific to RHCs in the
introductory text when it should have been its own paragraph. Therefore, we propose revisions to
§ 405.2410 to reflect the correct policy applicable for beneficiary coinsurance as described above
in the previous paragraph. We also propose revisions to § 405.2462(j) to accommodate the new
paragraph (j)(1).
5. Payment for Preventive Vaccine Costs in RHCs and FQHCs
a. Background
Section 1833(a)(3)(A) of the Act specifies that services described in section
1861(s)(10)(A) – pneumococcal, influenza and COVID-19 vaccines and their administration –
are exempt from the RHC and FQHC payment limit of 80 percent of reasonable costs.
Therefore, payment for pneumococcal, influenza and COVID-19 vaccines and their
administration in RHCs and FQHCs is governed by the statute at Section 1833(b) of the Act,
which requires payment at 100 percent of reasonable cost. For RHCs, this means that costs
associated with these vaccines and their administration are not included in determining the AIR
or subject to the payment limit, and for FQHCs, these costs are not included under the FQHC
PPS. We note that the hepatitis B vaccine, which is described at section 1861(s)(10)(B) of the
Act, is not specified at 1833(a)(3)(A) as exempt from the RHC/FQHC payment limit of 80
percent of reasonable costs, and therefore, payment for a hepatitis B vaccine and its
administration is included in the FQHCs PPS rate and the RHC AIR. However, since hepatitis B
vaccines and their administration are considered a Part B preventive service, no coinsurance or
deductible is charged when a hepatitis B vaccine is administered in an RHC or FQHC. Please see
section III.H.2.c. of this proposed rule for more information on hepatitis B vaccines in RHCs and
FQHCs.
In the April 3, 1996 FQHC final rule (61 FR 14657), we codified at § 405.2466(b)(1)(iv)
that, for RHCs and FQHCs, payment for pneumococcal and influenza vaccine and their
administration is 100 percent of Medicare reasonable cost, which is paid as part of the annual
reconciliation through the cost report. In the CY 2022 PFS final rule (86 FR 65207), we made
conforming changes in that section to include the COVID-19 vaccine and its administration.
b. Revisions to Current Policy
In the May 2, 2014 RHC/FQHC PPS final rule (79 FR 25449), we addressed
commenters’ recommendations that CMS apply a consistent approach to payment for Part B
vaccines. One commenter specifically recommended that CMS allow RHCs and FQHCs to bill
for Part B vaccines at the time of service, either with or without an encounter for a visit. The
commenter stated that those bills could be paid using national Part B rates, to be followed by an
annual reconciliation on the cost report between the payments and the reasonable costs of the
vaccines and their administration. This commenter wished to reduce the time between vaccine
administration and payment, and to enable the documentation on individual patient claims that
these vaccines were furnished. Commenters generally asserted that streamlining Part B vaccine
payment would help ensure broad vaccine access for Medicare beneficiaries.
In response to these comments, we responded that we did not believe that any changes in
our billing policies were necessary. We stated that RHCs and FQHCs are accustomed to
reporting and receiving payment for the reasonable costs of Part B vaccines and their
administration through the annual cost report, and we believed that an annual reconciliation
between vaccine payments and reasonable costs would create an additional administrative
burden for FQHCs and MACs. We also noted that as of January 1, 2011, FQHCs have been
required to report pneumococcal and influenza vaccines and their administration on a patient
claim with the appropriate HCPCS and revenue codes when furnished during a billable visit.
Please note that this is not a requirement for RHCs.
In the CY 2022 PFS final rule (86 FR 65207), in which we made conforming regulatory
changes at § 405.2466(b)(1)(iv) to include the COVID-19 vaccine, we received several
comments regarding the timing of vaccine payments for RHCs and FQHCs. These comments
echoed the sentiments expressed by the commenters on the same topic in the 2014 final rule

mentioned above, and while they were out of the scope of our proposals for CY2022, we will
elaborate on them here. These commenters expressed appreciation for measures taken by CMS in
April 2021 to make lump-sum payments for COVID-19 vaccine administration available to
RHCs and FQHCs in advance of cost report settlement, but commenters emphasized that those
payments were only a temporary solution. Commenters urged CMS to update the RHC and
FQHC cost report to ensure adequate, permanent reimbursement for COVID-19 vaccines.
Commenters added that RHCs and FQHCs have experienced challenges with burdensome
reporting requirements and data collection, as well as slow distribution of payments from MACs.
Another commenter stated that RHCs and FQHCs should not have to wait until settlement of
cost report to be reimbursed for other preventive vaccines, and that delayed payment may hinder
them from immunizing Medicare beneficiaries.
While we did not respond directly to those comments in the CY 2022 PFS final rule, as
they were out of scope of the policies that were finalized at the time, we did make clarifications
regarding payment for preventive vaccines and their administration in the RHC and FQHC
Frequently Asked Questions (FAQs) that accompanied the publication of the CY 2022 PFS final
rule.
307
In those FAQs, we clarified that the conforming change made to § 405.2466(b)(1)(iv) to
reflect coverage and payment for COVID-19 vaccines in RHCs and FQHCs did not reflect any
other payment policy changes regarding payment for Part B vaccines and administration in those
settings. We reiterated that RHCs and FQHCs are paid 100 percent of reasonable cost through
their cost report for Part B vaccines and their administration. Since there is a gap in time from
when costs are incurred in RHCs and FQHCs for furnishing vaccines and when payment is
received, the Medicare Administrative Contractors (MACs) could provide early payments in the
form of lump sum payments to RHCs and FQHCs in March of 2021 to facilitate COVID-19
307
RHCs CY 2022 PFS Final Rule Fact Sheet: https://www.cms.gov/files/document/rhcs-pfs-faqs.pdf; FQHCs CY
2022 PFS Final Rule Fact Sheet: https://www.cms.gov/files/document/fqhcs-pfs-faqs.pdf.
vaccinations. RHCs and FQHCs can request additional lump sum payments from their MAC at
any time.
Since the publication of the CY 2022 PFS final rule, we have given additional
consideration to the comments discussed above. During and since the COVID-19 PHE, CMS has
especially promoted efforts aimed at facilitating increased access to vaccinations for both
Medicare enrollees and all Americans. Vaccination promotion efforts also dovetail with CMS’
overarching strategic priorities of expanding health care access and advancing health equity. For
CY 2025, CMS has identified the issue of vaccination in RHCs and FQHCs as an area where
payment policy can be updated to improve access to preventive vaccines for Medicare enrollees.
We are proposing to allow RHCs and FQHCs to bill for the administration of Part B
preventive vaccines at the time of service. Based on the proposed changes in section III.M. and
III.H.2.c. of this proposed rule, this revision in policy would include all four Part B preventive
vaccines: pneumococcal, influenza, hepatitis B, and COVID-19 vaccines. These claims would
initially be paid like other Part B vaccine and vaccine administration claims, whose payments are
discussed at section III.H.1. of this proposed rule: vaccine products would be paid at 95 percent
of their Average Wholesale Price (AWP), and vaccine administration would be paid according to
the National Fee Schedule for Medicare Part B Vaccine Administration. The fee schedule’s
locality-adjusted payment rate files for CY 2024 can be found on the CMS Vaccine Pricing
website at https://www.cms.gov/medicare/payment/all-fee-service-providers/medicare-part-b-
drug-average-sales-price/vaccine-pricing. Payment rate files for influenza, pneumococcal and
hepatitis B vaccine administration can be found under the “Seasonal Flu Vaccine” tab, and
payment rate files for COVID-19 vaccines can be found under the “COVID-19 Vaccines &
Monoclonal Antibodies” tab. The CY 2025 payment rates for Part B vaccine HCPCS codes
G0008, G0009, G0010 and 90480, with the annual update applied for CY 2025, will be made
available at the time of publication of the CY 2025 PFS final rule, and Tables 45 and 46 in
section III.H.1.f. of this proposed rule provide the CY 2025 projected payment rates for those
amounts.
We are also clarifying that RHC or FQHC providers are eligible to bill HCPCS code
M0201 for an in-home additional payment for Part B preventive vaccine administration,
provided that a home visit meets all the requirements of both part 405, subpart X, for RHCs and
FQHCs services provided in the home, and § 410.152(h)(3)(iii) for the in-home additional
payment for Part B preventive vaccine administration. More information regarding the in-home
additional payment can be found at section III.H.1.d of this proposed rule, and current and
projected payment rates for M0201 can be found together with Part B vaccine administration
payment rates mentioned above.
We emphasize that the statute at section 1833(b) of the Act requires that RHCs and
FQHCs be paid at 100 percent of reasonable cost for Part B vaccines and their administration.
Therefore, payments for these services received at the time they are furnished in RHCs and
FQHCs will need to be annually reconciled with the facilities’ actual vaccine and vaccine
administration costs, including the in-home additional costs, on their cost reports. Due to the
operational systems changes needed to facilitate payment through claims, we propose that RHCs
and FQHCs begin billing for preventive vaccines and their administration at the time of service,
for dates of service beginning on or after July 1, 2025. This will also allow ample time for CMS
to release cost reporting instructions and sub-regulatory guidance with additional billing
instructions for RHCs and FQHCs to bill Medicare Part B for preventive vaccines and their
administration at the time of service.
We believe that this proposal addresses the comments and requests of stakeholders who
have suggested this payment approach over the last several years. We note that this payment
approach was mentioned in the Senate Appropriations Committee’s “Explanatory Statement For
Departments Of Labor, Health And Human Services, And Education, And Related Agencies

Appropriations Bill, 2021.”
308
That report referenced a December 2019 white paper by the
National Association of Community Health Centers, which noted that “FQHCs can face
significant delays in reimbursement for influenza and pneumococcal vaccines.”
309
The
Committee thus encouraged CMS to promote the ability of FQHCs to bill Part B directly for
vaccinations at the time of service, with reconciliation of payments at the time of cost report
settlement.
We invite public comment on these proposals. We would especially appreciate comments
on the benefits of payments for vaccine costs billed at the time of service, weighed against the
potential additional burdens of annual reconciliation of vaccine claims payments against actual
vaccine costs.
6. Productivity Standards
a. Background
Productivity standards for RHCs were first established on March 1, 1978 (43 FR 8260),
and updated on December 1, 1982 (47 FR 54163 - 54165), to help determine the average cost per
patient for Medicare reimbursement in RHCs. These productivity screening guidelines were
intended to identify situations where costs would not be allowed without acceptable justification
by the clinic and limits on the amount of payment (57 FR 24967). Physicians, nurse practitioners
(NPs), physician assistants (PAs), and certified nurse midwives (CNMs) are held to a minimum
number of visits per full time employee (FTE), as discussed in section 80.4, chapter 13 of the
Medicare Benefit Policy Manual. The productivity standards policy requires 4,200 visits per full-
time equivalent (FTE) physician and 2,100 visits per FTE non-physician practitioner (e.g., nurse
practitioner, physician assistant, or certified nurse midwife). Physician and non-physician
practitioner productivity may be combined and if so, the number of visits per full-time equivalent
team is 6,300. If actual visits are less than the productivity standards, the average cost per visit
308
https://www.appropriations.senate.gov/imo/media/doc/LHHSRept.pdf.
309
https://www.nachc.org/wp-content/uploads/2023/10/adult-imm-fqhc-white-paper-11-01-2019.pdf.
will be computed based on the productivity standards rather than actual visits, which would
result in the cost per visit to be lower than if actual visits were used. In other words, if the current
productivity standards are not met, the results would be a reduction in the cost per visit, which
could negatively impact the RHC AIR and reduce payments. There are exceptions to the
productivity standards that can be made based on individual circumstances that is at the
discretion of the MAC. We note that these standards of 4,200 visits per FTE physician and 2,100
visit per FTE nonphysician practitioner and 6,300 visits per combined FTE have not been
updated since 1982. We also note similar requirements to contain costs in this way were not
required in FQHCs or other settings paid on reasonable cost.
Interested parties have requested that CMS re-evaluate or remove the productivity
standards policy for RHCs because they believe that the environment today is very different than
when the RHC benefit began and that the “visit per FTE” is too high for practitioners to meet and
results in reducing the AIR. They also shared that the productivity standards matter even less
now since the implementation of the CAA, 2021 established payment limits for all RHCs.
During the COVID-19 PHE, CMS issued a combination of emergency authority waivers,
regulations, enforcement discretion, and subregulatory guidance to ensure and expand access to
care and to give health care providers the flexibilities needed to help keep people safe. RHCs
expressed concerns about how the productivity standards might impact them during the PHE. For
example, many RHCs had trouble meeting the productivity standards due to a change in the way
they staffed their clinics and billed for RHC services with increased telecommunications
services. RHCs claimed that they were negatively impacted even more so than other health care
settings because of these requirements. We have long standing guidance in the Medicare Benefit
Policy Manual, chapter 13, section 80.4 that describes the MAC’s role in providing flexibility to
grant productivity exceptions to RHCs who experienced disruptions in staffing and services to
minimize the burden on RHCs. During the PHE we reminded RHCs of the exception process in

FAQs,
310
and provided instructions to MACs to proactively reach out to RHCs reminding them
of the exception process and to proactively grant exceptions as necessary.
Section 130 of the CAA, 2021 restructured the payment limits for RHCs beginning April
1, 2021. That is, independent RHCs, provider-based RHCs in a hospital with 50 or more beds,
and RHCs enrolled under Medicare on or after January 1, 2021, will receive a prescribed
national statutory payment limit per visit increase over an 8-year period for each year from 2021
through 2028. This provision also established payment limits for provider-based RHCs in a
hospital with less than 50 beds. See CY 2022 PFS final rule (86 FR 65199 through 65202) for
more detailed discussion.
Since the CAA, 2021 restructured the payment limits for RHCs, and in some cases
established payment limits for RHCs beginning April 1, 2021, we believe that applying
productivity standards may no longer be necessary with the payment limits set out by Congress.
We believe that the productivity standards are outdated and redundant with the CAA, 2021
provisions. Therefore, we are proposing to remove productivity standards for RHCs.
7. Proposed Rebasing of the FQHC Market Basket
a. Background
Section 10501(i)(3)(A) of the Affordable Care Act added section 1834(o) of the Act to
establish a payment system for the costs of FQHC services under Medicare Part B based on
prospectively set rates. In the Prospective Payment System (PPS) for FQHC final rule published
in the May 2, 2014 Federal Register (79 FR 25436), we implemented a methodology and
payment rates for the FQHC PPS. Beginning on October 1, 2014, FQHCs began to transition to
the FQHC PPS based on their cost reporting periods, and as of January 1, 2016, all FQHCs have
been paid under the FQHC PPS.
Section 1834(o)(2)(B)(ii) of the Act requires that the payment for the first year after the
implementation year be increased by the percentage increase in the Medicare Economic Index
310
https://www.cms.gov/files/document/03092020-covid-19-faqs-508.pdf.
(MEI). Therefore, in CY 2016, the FQHC PPS base payment rate was increased by the MEI.
The MEI at that time was based on 2006 data from the American Medical Association (AMA)
for self-employed physicians and was used in the PFS sustainable growth rate (SGR) formula to
determine the conversion factor for physician service payments. (See the CY 2014 PFS final
rule (78 FR 74264) for a complete discussion of the 2006-based MEI.) Section 1834(o)(2)(B)(ii)
of the Act also requires that beginning in CY 2017, the FQHC PPS base payment rate will be
increased by the percentage increase in a market basket of FQHC goods and services, or if such
an index is not available, by the percentage increase in the MEI.
Beginning with CY 2017, FQHC PPS payments were updated using a 2013-based market
basket reflecting the operating and capital cost structures for freestanding FQHC facilities
(hereafter referred to as the FQHC market basket). A complete discussion of the 2013-based
FQHC market basket can be found in the CY 2017 PFS final rule (81 FR 80393 through 80403).
In the CY 2021 PFS final rule (85 FR 84699 through 84710), we rebased and revised the FQHC
market basket to reflect a 2017 base year.
For this CY 2025 PFS proposed rule, we are proposing to rebase and revise the 2017-
based FQHC market basket to reflect a 2022 base year, which would maintain our historical
frequency of rebasing the market basket every four years. The proposed 2022-based FQHC
market basket is primarily based on Medicare cost report data for FQHCs for 2022, which are for
cost reporting periods beginning on and after October 1, 2021, and prior to October 1, 2022. We
propose to use data from cost reports beginning in FY 2022 because these data are the latest
available complete set of Medicare cost report data for purposes of calculating cost weights for
the FQHC market basket at the time of rulemaking.
In the following discussion, we provide an overview of the proposed FQHC market
basket, describe the methodologies for developing the proposed 2022-based FQHC market
basket, and provide information on the proposed price proxies. We then present the CY 2025
FQHC market basket update based on the proposed 2022-based FQHC market basket.
b. Overview of the Proposed 2022-Based FQHC Market Basket
Similar to the 2017-based FQHC market basket, the proposed 2022-based FQHC market
basket is a fixed-weight, Laspeyres-type price index. A Laspeyres price index measures the
change in price, over time, of the same mix of goods and services purchased in the base period.
Any changes in the quantity or mix (that is, intensity) of goods and services purchased over time
are not measured. The index itself is constructed using three steps. First, a base period is
selected (we propose to use 2022 as the base period) and total base period expenditures are
estimated for a set of mutually exclusive and exhaustive expenditure categories, with the
proportion of total costs that each category represents being calculated. These proportions are
called cost weights. Second, each cost category is matched to an appropriate price or wage
variable, referred to as a “price proxy.” In almost every instance, these price proxies are derived
from publicly available statistical series that are published on a consistent schedule (preferably at
least on a quarterly basis). Finally, the cost weight for each cost category is multiplied by the
level of its respective price proxy. The sum of these products (that is, the cost weights multiplied
by their price index levels) for all cost categories yields the composite index level of the market
basket in a given period. Repeating this step for other periods produces a series of market basket
index levels over time. Dividing an index level for a given period by an index level for an earlier
period produces a rate of growth in the input price index over that timeframe. As previously
noted, the market basket is described as a fixed-weight index because it represents the change in
price over time of a constant mix (quantity and intensity) of goods and services needed to furnish
FQHC services. The effects on total expenditures resulting from changes in the mix of goods
and services purchased subsequent to the base period are not measured. For example, a FQHC
hiring more nurse practitioners to accommodate the needs of patients would increase the volume
of goods and services purchased by the FQHC but would not be factored into the price change
measured by a fixed-weight FQHC market basket. Only when the index is rebased would
changes in the quantity and intensity be captured, with those changes being reflected in the cost
weights. Therefore, we rebase the market basket periodically so that the cost weights reflect
recent changes in the mix of goods and services that FQHCs purchase (FQHC inputs) to furnish
care between base periods.
c. Development of the Proposed 2022-Based FQHC Market Basket Cost Categories and
Weights
We are inviting public comments on our proposed methodology, discussed in this section
of this rulemaking, for deriving the proposed 2022-based FQHC market basket.
(1) Use of Medicare Cost Report Data
The major types of costs underlying the proposed 2022-based FQHC market basket are
derived from the Medicare cost reports (CMS Form 224–14, OMB Control Number 0938-1298)
for freestanding FQHCs. Specifically, we use the Medicare cost reports for eleven specific costs:
FQHC Practitioner Wages and Salaries, FQHC Practitioner Employee Benefits, FQHC
Practitioner Contract Labor, Clinical Staff Wages and Salaries, Clinical Staff Employee Benefits,
Clinical Staff Contract Labor, Non-Health Staff Compensation, Medical Supplies,
Pharmaceuticals, Fixed Assets, and Movable Equipment. A residual category is then estimated
and reflects all remaining costs not captured in the 11 types of costs identified previously (such
as non-medical supplies and utilities).
The resulting proposed 2022-based FQHC market basket cost weights reflect Medicare
allowable costs. We propose to define Medicare allowable costs centers for freestanding FQHC
facilities as the expenses reported on: Worksheet A, lines 1 through 7, lines 9 through 12, lines
23 through 36, and line 66. For the proposed 2022-based FQHC market basket, we are
proposing to include data from the cost center from Worksheet A, line 66 (Telehealth) as
effective for CY 2022 since CMS finalized a proposal to revise the current regulatory language
for RHC or FQHC mental health visits to include visits furnished using interactive, real-time
telecommunications technology and for RHCs and FQHCs to report and be paid for mental
health visits furnished via real-time, telecommunication technology in the same way they
currently do when these services are furnished in-person (86 FR 65208 through 62511). As done
with the 2017-based FQHC market basket, we are proposing to continue to exclude Professional
Liability Insurance (PLI) costs from the total Medicare allowable costs because FQHCs that
receive section 330 grant funds also are eligible to apply for medical malpractice coverage under
Federally Supported Health Centers Assistance Act (FSHCAA) of 1992 (Pub. L. 102–501) and
FSHCAA of 1995 (Pub. L. 104–73 amending section 224 of the Public Health Service Act).
Later in this section, we explain in more detail how the costs for each of the 11 categories
are derived. Prior to estimating any costs, we apply three basic edits. First, we only include the
last submitted cost report so there is no double counting of a FQHC provider. Second, we
exclude providers that have less than half a year of reported cost data; this edit excludes 175
FQHC providers for 2022. Finally, we remove any providers that did not report net direct patient
care expenses on the FQHC cost report Worksheet A, line 37, column 7; this edit excludes 717
FQHC cost reports, or about 29 percent of FQHC providers. If a provider does not have reported
costs, then we are unable to use that provider’s costs to calculate cost weights. We encourage
providers to report net direct patient care expenses when reporting the data. After the three edits,
there are 1,713 remaining FQHC providers in the 2022 data set that we use to estimate cost
expenditures for, or roughly two-thirds of the total FQHCs in the original Medicare cost report
data set.
(a) FQHC Practitioner Wages and Salaries Costs
A FQHC practitioner is defined as one of the following occupations: physicians; nurse
practitioners (NPs); physician assistants (PAs); certified-nurse midwife (CNMs); clinical
psychologist (CPs); and clinical social workers (CSWs). We propose to calculate FQHC
Practitioner Wages and Salaries Costs using three steps. First, we propose to calculate FQHC
Practitioner Compensation Costs as equal to the net expenses (that is, costs after reclassifications
and adjustments) as reported on Worksheet A, column 7, lines 23, 25, 26, 29, 30, and 31. These
lines represent the total net costs (after reclassifications and adjustments) for physicians, PAs,
NPs, CNMs, CPs, and CSWs.
Second, we propose to further divide the FQHC Practitioner Compensation Costs for
these occupations into wages and salaries, employee benefits, and contract labor costs based on
the ratios of practitioner wages and salaries, practitioner employee benefits, and practitioner
contract labor costs to the sum of these three groups of costs. We do this by applying the ratios
of practitioner wages and salaries, practitioner employee benefits, and practitioner contract labor
to the net expense FQHC Practitioner Compensation Costs, and the determination of these ratios
is described below. We propose to derive the practitioner wages and salaries costs as the sum of
direct care wages and salaries reported on Worksheet A, column 1, lines 23, 25, 26, 29, 30, and
31. These lines represent the wages and salaries costs for physicians, PAs, NPs, CNMs, CPs,
and CSWs. We propose to derive the practitioner employee benefits costs for these occupations
as the sum of costs reported on Worksheet S-3, part II, column 2, lines 2, 3, 4, 7, 8, and 9. These
lines represent the employee benefits costs for physicians, PAs, NPs, CNMs, CPs, and CSWs.
We propose to derive the practitioner contract labor costs for these occupations as the costs
reported on Worksheet S-3, part II, column 1, lines 2, 3, 4, 7, 8, and 9. These lines represent the
contract labor costs for physicians, PAs, NPs, CNMs, CPs, and CSWs. This was the same
method used to calculate the ratios to split the FQHC Practitioner Compensation Costs as was
done for the 2017-based FQHC market basket. Approximately 56 percent of FQHCs that
reported direct patient care wages and salaries costs also reported employee benefits costs data
and approximately 99 percent of FQHCs that reported direct patient care wages and salaries costs
also reported contract labor cost data on Worksheet S–3, part II for 2022. This is higher
reporting than the percent of FQHCs reporting the same data compared to the 2017-based FQHC
market basket, which had a 45 percent and 66 percent reporting incidence for the 2017 cost
report data. We are encouraged by this improvement in the data and continue to encourage all
providers to report these data on the Medicare cost report.
The final step in the process to derive the FQHC Practitioner Wages and Salaries costs is
to apply the ratio of practitioner wages and salaries to the sum of practitioner wages and salaries
costs, practitioner employee benefits costs, and practitioner contract labor costs times the FQHC
Practitioner Compensation costs (representing the net expenses for each occupation as reported
on Worksheet A column 7) as described above. This calculation is done for each occupation
individually (physicians, PAs, NPs, CNMs, CPs, and CSWs). The resulting proposed FQHC
Practitioner Wages and Salaries costs are equal to the sum of each occupation’s wages and salary
costs. This is the same methodology that was used for the 2017-based FQHC market basket.
As stated in the CY 2022 PFS final rule (86 FR 65209), effective for CY 2022 FQHC
mental health visits furnished using interactive, real-time telecommunications technology are
paid for at the same rate as other FQHC visits when these services are furnished in-person;
therefore, we propose to include telehealth wages and salaries costs in the FQHC Practitioner
Wages and Salaries cost category. We propose to derive telehealth wages and salaries by
multiplying the net telehealth costs (as reported on Worksheet A, column 7, line 66) times the
ratio of telehealth wages and salaries (as reported on Worksheet A, column 1, line 66) to the sum
of telehealth costs (the sum of Worksheet A, column 1 and 2, line 66).
(b) FQHC Practitioner Employee Benefits Costs
To calculate FQHC Practitioner Employee Benefits costs, we propose to use a similar
methodology as used to calculate the FQHC Practitioner Wages and Salaries costs. We propose
to apply the ratio of practitioner employee benefits as described above to the FQHC Practitioner
Compensation costs (representing the net expenses for each occupation as reported on Worksheet
A column 7) as defined in the section III.B.7.(c)(1)(a). This calculation is done for each
occupation individually (physicians, PAs, NPs, CNMs, CPs, and CSWs). The FQHC Practitioner
Employee Benefits costs are equal to the sum of each occupation’s employee benefits costs. This
is the same methodology that was used for the 2017-based FQHC market basket. As stated
previously, effective for CY 2022, telehealth services are covered under the FQHC PPS;
therefore, we propose to also include in the FQHC Practitioner Employee Benefits the telehealth
employee benefits. We propose to estimate telehealth employee benefits by multiplying
telehealth wages and salaries (as described in section III.B.7.(c)(1)(a)) times the ratio of total
direct patient care facility benefits (Worksheet S3 Part II, column 2, line 1) to total facility direct
patient care salaries (the sum of Worksheet A, columns 1 and 2, lines 23 and 25 through 36),
which is estimated to be 21 percent on average. This ratio is referred to as the overall employee
benefit share and represents the ratio of employee benefits to wages and salaries for all patient
care costs reported by FQHCs.
(c) FQHC Practitioner Contract Labor Costs
To calculate FQHC Practitioner Contract Labor Costs, we propose to use a similar
methodology as used to calculate FQHC Practitioner Wages and Salaries and FQHC Practitioner
Employee Benefit Costs. We propose to multiply the ratio of practitioner contract labor, as
described above, by the FQHC Practitioner Compensation costs (representing the net expenses
for each occupation as reported on Worksheet A column 7) as defined in section III.B.7.(c)(1)(a).
This calculation is done for each occupation individually (physicians, PAs, NPs, CNMs, CPs,
and CSWs). The FQHC Practitioner Contract Labor costs are equal to the sum of each
occupation’s contract labor costs plus all net expenses reported for Physicians Services Under
Agreement from Worksheet A, column 7, line 24. This is the same methodology used for the
2017-based FQHC market basket.
(d) Clinical Staff Wages and Salaries Costs
We propose to calculate Clinical Staff Wages and Salaries Costs using three steps. First,
we propose to define Clinical Staff Compensation costs as the sum of net expenses (that is, costs
after reclassifications and adjustments) as reported on Worksheet A, column 7, lines 27, 28, 32,
33, 34, 35, and 36. Clinical Staff Compensation includes any health-related clinical staff who do
not fall under the definition of a FQHC Practitioner. These lines represent the net expenses for
visiting RNs, visiting LPNs, laboratory technicians, registered dietician/Certified DSMT/MNT
educators, PTs, OTs, and other allied health personnel.
Second, we propose to further divide the Clinical Staff Compensation costs for these
occupations into wages and salaries, employee benefits, and contract labor costs based on the
ratio of clinical staff wages and salaries, clinical staff employee benefits, and clinical staff
contract labor costs to the sum of these three groups of costs. We do this by applying the ratio of
clinical staff wages and salaries, clinical staff employee benefits, and clinical staff contract labor
to the net expense Clinical Staff Compensation costs, and the determination of these ratios is
described later in this section. We propose to derive clinical staff wages and salaries costs as the
sum of direct care cost salaries as reported on Worksheet A, column 1, lines 27, 28, 32, 33, 34,
35, and 36. These lines represent the wages and salaries costs for visiting registered nurses
(RNs), visiting licensed practical nurses (LPNs), laboratory technicians, registered
dietician/Certified DSMT/MNT educators, physical therapists (PTs), occupational therapists
(OTs), and other allied health personnel. We propose to derive the clinical staff employee
benefits costs for these occupations as the sum of costs reported on Worksheet S-3, part II,
column 2, lines 5, 6, 10, 11, 12, 13, and 14. These lines represent the employee benefits costs for
visiting RNs, visiting LPNs, laboratory technicians, registered dietician/Certified DSMT/MNT
educators, PTs, OTs, and other allied health personnel. Similarly, we propose to calculate
clinical staff contract labor costs for these occupations as the costs reported on Worksheet S-3,
part II, column 1, lines 5, 6, 10, 11, 12, 13, and 14. These lines represent the contract labor costs
for visiting RNs, visiting LPNs, laboratory technicians, registered dietician/Certified
DSMT/MNT educators, PTs, OTs, and other allied health personnel. This was the same method
used to calculate the ratios to split the Clinical Staff Compensation net expenses as was done for
the 2017-based FQHC market basket.
The final step in the process to derive the Clinical Staff Wages and Salaries costs is to
apply the ratio of clinical staff wages and salaries calculated in the prior step to the Clinical Staff
Compensation costs (representing the net expenses for each occupation as reported on Worksheet
A column 7) as described above. This calculation is done for each occupation individually
(visiting RNs, visiting LPNs, laboratory technicians, registered dietician/Certified DSMT/MNT
educators, PTs, OTs, and other allied health personnel). The Clinical Staff Wages and Salaries
costs is equal to the sum of each occupation’s wages and salary costs. This is the same
methodology that was used for the 2017-based FQHC market basket.
(e) Clinical Staff Employee Benefits Costs
To calculate Clinical Staff Employee Benefit costs, we propose to use a similar
methodology as used to calculate the Clinical Staff Wages and Salaries costs. We propose to
multiply the ratio of clinical staff employee benefits, as described above by the Clinical Staff
Compensation costs (representing the net expenses for each occupation as reported on Worksheet
A column 7) as defined in the section III.B.7.(c)(1)(d). This calculation is done for each
occupation individually (visiting RNs, visiting LPNs, laboratory technicians, registered
dietician/Certified DSMT/MNT educators, PTs, OTs, and other allied health personnel). The
Clinical Staff Employee Benefits costs are equal to the sum of each occupation’s Employee
Benefits costs. This is the same methodology that was used for the 2017-based FQHC market
basket.
(f) Clinical Staff Contract Labor Costs
To calculate Clinical Staff Contract Labor costs, we propose to use a similar
methodology as used to calculate Clinical Staff Wages and Salaries Costs and Clinical Staff
Benefit Costs. We propose to multiply the ratio of clinical staff contract labor costs, as described
above, by the Clinical Staff Compensation costs (representing the net expenses for each
occupation as reported on Worksheet A column 7) as defined in the section III.B.7.(c)(1)(d).
This calculation is done for each occupation individually (visiting RNs, visiting LPNs, laboratory
technicians, registered dietician/Certified DSMT/MNT educators, PTs, OTs, and other allied
health personnel). The Clinical Staff Contract Labor costs are equal to the sum of each
occupation’s contract labor costs. This is the same methodology that was used for the 2017-
based FQHC market basket.
(g) Non-Health Staff Compensation Costs
We propose to define Non-Health Staff Compensation costs using net expenses (that is,
costs after reclassifications and adjustments) as the estimated share of compensation costs from
Worksheet A, column 7 for lines 3, 4, 5, 6, 7, 9, 10, 11, and 12. These lines represent the net
expenses for the employee benefits department, administrative & general services, plant
operations & maintenance, janitorial, medical records, pharmacy, medical supplies,
transportation, and other general services. Since the net expenses for the General Service Cost
centers include both compensation and other costs, we estimate the share of net expenses for
each general service cost center that reflects compensation costs. First, we estimate a share of
non-health staff wages and salaries costs for each general service cost center as reported on
Worksheet A, column 1 for lines 3, 4, 5, 6, 7, 9, 10, 11, and 12 divided by Worksheet A, column
1 and 2 for lines 3, 4, 5, 6, 7, 9, 10, 11, and 12. Then, we multiply the Non-Health Staff net
expenses (that is, costs after reclassifications and adjustments) by the non-health staff wages and
salaries share to derive estimated Non-Health Staff Wages and Salaries costs for each general
service cost center (lines 3-7 and lines 9-12). Second, we estimate Non-Health Staff Employee
Benefit costs by multiplying the Non-Health Staff Wages and Salaries costs (step one) by the
overall employee benefit share as described in section III.B.7.(c)(1)(b), or 21 percent. Finally,
we sum the derived Non-Health Staff Wages and Salaries costs and the derived Non-Health Staff
Employee Benefits costs for each general service cost center (lines 3-7 and lines 9-12) to
calculate Non-Health Staff Compensation costs. This is the same methodology used for the
2017-based FQHC market basket.
(h) Pharmaceutical Costs
We propose to calculate Pharmaceutical costs as the non-compensation costs for the
Pharmacy cost center. We define this as Worksheet A, column 7, line 9 less derived pharmacy
compensation costs. Derived pharmacy compensation costs are included in the Non-health Staff
Compensation costs described in section III.B.7.(c)(1)(g). We note that the only pharmaceutical
costs eligible for inclusion in the FQHC PPS market basket are those reported on line 9 of
Worksheet A. These pharmaceutical costs would include only the costs of routine drugs (both
prescription and over the counter), pharmacy supplies, and pharmacy services, provided incident to
an FQHC visit. Other types of drugs and pharmacy supplies costs not included in this category are
those reported on line 67 (drugs charged to patients), line 77 (retail pharmacy), line 48
(pneumococcal vaccine), and line 49 (influenza vaccine, COVID-19, and monoclonal antibody
products for treatment of COVID-19), as these costs are reimbursed to FQHC providers outside of
the FQHC PPS payment. The derived pharmacy compensation costs are equal to the sum of the
estimated pharmacy wages and salaries and pharmacy employee benefits costs. This is the same
methodology used for the 2017-based FQHC market basket.
(i) Medical Supplies Costs
We propose to calculate Medical Supplies costs as the non-compensation costs for the
Medical Supplies costs center. We define this as Worksheet A, column 7, line 10 less derived
medical supplies compensation costs. Derived medical supplies compensation costs are included
in the Non-health Staff Compensation costs described in section III.B.7.(c)(1)(g). The derived
medical supplies compensation costs are equal to the sum of the estimated medical supplies
wages and salaries and medical supplies benefits costs. This is the same methodology used for
the 2017-based FQHC market basket.
(j) Fixed Assets Costs
We propose to define Fixed Asset costs to be equal to costs reported on Worksheet A,
line 1, column 7 of the Medicare cost report. This is the same methodology used for the 2017-
based FQHC market basket.
(k) Movable Equipment Costs
We propose to define Movable Equipment costs to be equal to the capital costs as
reported on Worksheet A, line 2, column 7. This is the same methodology used for the 2017-
based FQHC market basket.
(2) Proposed Major Cost Category Computation
After we derive costs for the major cost categories for each provider using the Medicare
cost report data as previously described, we propose to trim the data for outliers. For each of the
11 major cost categories, we propose to divide the calculated costs for the category by total
Medicare allowable costs calculated for the provider to obtain cost weights for the universe of
FQHC providers after basic trims described in section III.B.7.(c). For the proposed 2022-based
FQHC market basket, total Medicare allowable costs would be equal to total net expenses (after
reclassifications and adjustments) reported on: Worksheet A, column 7, for lines 1 through 7,
lines 9 through 12; lines 23 through 36, and line 66. This is the same method used to derive total
Medicare allowable costs for the 2017-based FQHC market basket with the only difference being
that we now include the net expenses for line 66, telehealth because as previously described,
effective for CY 2022 CMS finalized the policy for mental health visits furnished using
interactive, real-time telecommunications technology to be paid in the same way they currently
do when these services are furnished in-person (86 FR 65208 through 62511).
For the FQHC Practitioner Wages and Salaries, FQHC Practitioner Employee Benefits,
FQHC Practitioner Contract Labor, Clinical Staff Wages and Salaries, Clinical Staff Employee
Benefits, Clinical Staff Contract Labor, Non-Health Staff Compensation, Pharmaceuticals,
Medical Supplies, Fixed Assets, and Movable Equipment cost weights, after excluding cost
weights that are less than or equal to zero, we propose to then remove those providers whose
derived cost weights fall in the top and bottom 5 percent of provider-specific derived cost
weights to ensure the exclusion of outliers. A 5 percent trim is the standard trim applied to the
mean cost weights in most CMS market baskets and is consistent with the trimming used in the
2017-based FQHC market basket. After the outliers have been excluded, we sum the costs for

each category across all remaining providers. We propose to then divide this by the sum of total
Medicare allowable costs across all remaining providers to obtain a cost weight for the proposed
2022-based FQHC market basket for the given category. This trimming process is done for each
cost weight separately.
Finally, we propose to calculate the residual “All Other” cost weight that reflects all
remaining costs that are not captured in the 11 major cost categories listed. Table 25 provides
the resulting cost weights for these major cost categories derived from the Medicare cost reports.
Table 25 displays the proposed 2022-based FQHC market basket cost weights compared
to the 2017-based FQHC market basket cost weights.
TABLE 25: Major Cost Categories as Derived from Medicare Cost Reports
Major Cost Categories
Proposed 2022-Based
FQHC Cost Report
Weights
(Percent)
2017-Based
FQHC Cost Report
Weights
(Percent)
FQHC Practitioner Compensation
24.8
28.4
FQHC Practitioner Wages and Salaries
17.1
19.4
FQHC Practitioner Employee Benefits
3.6
4.5
FQHC Practitioner Contract Labor
4.1
4.6
Clinical Staff Compensation
15.3
16.8
Clinical Staff Wages and Salaries
11.8
12.9
Clinical Staff Employee Benefits
2.8
3.1
Clinical Staff Contract Labor
0.6
0.9
Non-Health Staff Compensation
28.4
27.2
Pharmaceuticals
3.2
2.4
Medical Supplies
2.4
2.2
Fixed Assets
5.0
4.4
Movable Equipment
2.2
2.0
All Other (Residual)
18.7
16.5
Note: Totals may not sum to 100.0 due to rounding
As we did for the 2017-based FQHC market basket, we propose to allocate the Contract
Labor cost weight to the Wages and Salaries and Employee Benefits cost weights based on their
relative proportions under the assumption that contract labor costs comprise both wages and
salaries and employee benefits for both FQHC Practitioners and Clinical Staff. The contract
labor allocation proportion for Wages and Salaries is equal to the Wages and Salaries cost weight
as a percent of the sum of the Wages and Salaries cost weight and the Employee Benefits cost
weight. This percentage based on the proposed 2022-based FQHC cost weights is 82.5 percent

for FQHC practitioners and 80.8 percent for clinical staff. Therefore, we propose to allocate 82.5
percent of the FQHC Practitioner Contract Labor cost weight to the FQHC Practitioner Wages
and Salaries cost weight and 17.5 percent to the FQHC Practitioner Employee Benefits cost
weight. Similarly, we propose to allocate 80.8 percent of the Clinical Staff Contract Labor cost
weight to the Clinical Staff Wages and Salaries cost weight and 19.2 percent to the Clinical Staff
Employee Benefits cost weight. Table 26 shows the FQHC Practitioner and Clinical Staff
Wages and Salaries and Employee Benefits proposed 2022-based cost weights after the contract
labor cost weight has been allocated. Table 26 also includes the comparison of the weights to the
2017-based cost weights for the same categories.
TABLE 26: Wages and Salaries and Employee Benefits Cost Weights After Contract
Labor Allocation
Major Cost Categories
Proposed 2022-
Based FQHC
Practitioner
Proposed 2022-
Based Clinical
Staff
2017-Based
FQHC
Practitioner
2017-Based
Clinical Staff
Compensation
24.8
15.3
28.4
16.8
Wages and Salaries
20.5
12.4
23.1
13.6
Employee Benefits
4.3
2.9
5.4
3.3
*Totals may not sum due to rounding
(3) Derivation of the Detailed Operating Cost Weights
To further divide the “All Other” residual cost weight estimated from the 2022 Medicare
cost report data into more detailed cost categories, we propose to use the 2017 Benchmark Input-
Output (I–O) “Use Tables/Before Redefinitions/Purchaser Value” for NAICS 621100, Offices of
Physicians, published by the Bureau of Economic Analysis (BEA). We note that the BEA
benchmark I-O data is used to further disaggregate residual costs in other CMS market baskets.
Therefore, we note that we believe the data from this industry are the most technically
appropriate for disaggregation of the residual net expenses since both physician offices and
FQHCs provide similar types of care. These data are publicly available at
https://www.bea.gov/industry/input-output-accounts-data. For the 2017-based FQHC market
basket, we used the 2012 Benchmark Input-Output (I–O) “Use Tables/Before

Redefinitions/Purchaser Value” for NAICS 621100, Offices of Physicians, published by the
BEA.
The BEA Benchmark I–O data are scheduled for publication every 5 years with the most
recent data available for 2017. The 2017 Benchmark I–O data are derived from the 2017
Economic Census and are the building blocks for BEA’s economic accounts. Therefore, they
represent the most comprehensive and complete set of data on the economic processes or
mechanisms by which output is produced and distributed.
311
BEA also produces Annual I–O
estimates. However, while based on a similar methodology, these estimates reflect less
comprehensive and less detailed data sources and are subject to revision when benchmark data
become available. Instead of using the less detailed Annual I–O data, we propose to inflate the
2017 Benchmark I–O data forward to 2022 by applying the annual price changes from the
respective price proxies to the appropriate market basket cost categories that are obtained from
the 2017 Benchmark I–O data. We repeat this practice for each year. We then calculate the cost
shares that each cost category represents of the 2017 data inflated to 2022. These resulting 2022
cost shares were applied to the “All Other” residual cost weight to obtain the detailed cost
weights for the proposed 2022-based FQHC market basket. For example, the cost for Medical
Equipment represents 7.8 percent of the sum of the “All Other” 2017 Benchmark I–O Offices of
Physicians Expenditures inflated to 2022. Therefore, the proposed Medical Equipment cost
weight represents 7.8 percent of the proposed 2022-based FQHC market basket’s “All Other”
cost category (18.7 percent), yielding a Medical Equipment cost weight of 1.5 percent in the
proposed 2022-based FQHC market basket (0.078 × 18.7 percent = 1.5 percent).
Using this methodology, we propose to derive six detailed FQHC market basket cost
category weights from the proposed 2022-based FQHC market basket residual cost weight (18.7
percent). These categories are: (1) Utilities; (2) Medical Equipment; (3) Miscellaneous Products;
311
http://www.bea.gov/papers/pdf/IOmanual_092906.pdf.
(4) Professional, Scientific, and Technical Services; (5) Administrative and Facilities Support
Services; and (6) All Other Services.
(4) Proposed 2022-Based FQHC Market Basket Cost Categories and Weights
Table 27 shows the cost categories and cost weights for the proposed 2022-based FQHC
market basket compared to the 2017-based FQHC market basket. The Total Compensation cost
weight of 68.5 percent (sum of FQHC Practitioner Compensation, Clinical Staff Compensation,
and Non-health Staff Compensation) calculated from the Medicare cost reports for the proposed
2022-based FQHC market basket is 4.1 percentage points lower than the total compensation cost
weight for the 2017-based FQHC market basket (72.6 percent). The decrease in the
compensation cost weight between the 2017-based and the proposed 2022-based market basket is
stemming from the decreasing FQHC Practitioner and Clinical Staff Compensation cost weights.
The proposed 2022-based cost weights for FQHC Practitioner and Clinical Staff Compensation
are 5.3 percentage points lower compared to the 2017-based FQHC market basket, while the
Non-Health Staff Compensation cost weight is 1.2 percentage points higher. Analysis of the cost
report data shows that the decline in the health-related compensation cost weights is stemming
from a change in the mix of health-related workers from higher-paid to lower-paid occupations.
Specifically, there has been a shift in full time equivalents (FTEs) from physicians to nurse
practitioners and a shift from registered and licensed practical nurses to other allied health
personnel. Additionally, the proposed 2022-based Pharmaceuticals cost weight, Non-Health
Staff Compensation costs weight, and the Capital cost weight, are each roughly 1 percentage
point higher than the cost weight in the 2017-based FQHC market basket.
We note that our analysis of the Medicare cost report data over time shows the general
trends in these cost weights (particularly for the Total Compensation and Pharmaceuticals cost
weights) began after 2017 with about half of the cost weight changes occurring between 2017
and 2019. Consistent with our historical frequency of rebasing the other CMS market baskets,
we believe it is important to rebase the FQHC market basket every four to five years to reflect

the more recent data and changing cost structure. We are soliciting comments on our proposal to
rebase and revise the market basket to reflect a 2022 base year.
TABLE 27: Proposed 2022-Based FQHC Market Basket Cost Weights Compared to 2017-
Based FQHC Market Basket Cost Weights
Cost Category
Proposed 2022-
based FQHC
Market Basket
Cost Weight
2017-based
FQHC
Market
Basket Cost
Weight
Total
100.0
100.0
Compensation
68.5
72.6
FQHC Practitioner Compensation
24.8
28.5
FQHC Practitioner Wages and Salaries
20.5
23.1
FQHC Practitioner Employee Benefits
4.3
5.4
Clinical Staff Compensation
15.3
16.9
Clinical Staff Wages and Salaries
12.4
13.6
Clinical Staff Employee Benefits
2.9
3.3
Non-Health Staff Compensation
28.4
27.2
All Other Products
9.8
8.5
Pharmaceuticals
3.2
2.4
Utilities
0.5
0.6
Medical Equipment
1.5
1.2
Medical Supplies
2.4
2.2
Miscellaneous Products
2.3
2.2
All Other Services
14.5
12.6
Professional, Scientific, and Technical Services
8.6
6.4
Administrative and Facilities Support Services
1.5
1.7
All Other Services
4.4
4.5
Capital-Related Costs
7.2
6.4
Fixed Assets
5.0
4.4
Movable Equipment
2.2
2.0
Note: Totals may not sum due to rounding.
d. Selection of Price Proxies
After developing the cost weights for the proposed 2022-based FQHC market basket, we
selected the most appropriate wage and price proxies currently available to represent the rate of
price change for each expenditure category. For most of the cost categories, we rely on using the
price proxies based on U.S. Bureau of Labor Statistics (BLS) data, as they produce indexes that
best meet the criteria of reliability, timeliness, availability, and relevance, and group them into
one of the following BLS categories:
● Employment Cost Indexes. Employment Cost Indexes (ECIs) measure the rate of
change in employment wage rates and employer costs for employee benefits per hour worked.
These indexes are fixed-weight indexes and strictly measure the change in wage rates and
employee benefits per hour. ECIs are superior to Average Hourly Earnings (AHE) as price
proxies for input price indexes because they are not affected by shifts in occupation or industry
mix, and because they measure pure price change and are available by both occupational group
and by industry. The industry ECIs are based on the North American Industry Classification
System (NAICS) and the occupational ECIs are based on the Standard Occupational
Classification System (SOC).
● Producer Price Indexes. Producer Price Indexes (PPIs) measure the average change
over time in the selling prices received by domestic producers for their output. The prices
included in the PPI are from the first commercial transaction for many products and some
services (https://www.bls.gov/ppi/).
● Consumer Price Indexes. Consumer Price Indexes (CPIs) measure the average change
over time in the prices paid by urban consumers for a market basket of consumer goods and
services (https://www.bls.gov/cpi/). CPIs are only used when the purchases are similar to those
of retail consumers rather than purchases at the producer level, or if no appropriate PPIs are
available.
We evaluate the price proxies using the criteria of reliability, timeliness, availability, and
relevance:
● Reliability. Reliability indicates that the index is based on valid statistical methods
and has low sampling variability. Widely accepted statistical methods ensure that the data were
collected and aggregated in a way that can be replicated. Low sampling variability is desirable
because it indicates that the sample reflects the typical members of the population. (Sampling
variability is variation that occurs by chance because only a sample was surveyed rather than the
entire population.)
● Timeliness. Timeliness implies that the proxy is published regularly, preferably at least
once a quarter. The market baskets are updated quarterly, and therefore, it is important for the
underlying price proxies to be up-to-date, reflecting the most recent data available. We believe
that using proxies that are published regularly (at least quarterly, whenever possible) helps to
ensure that we are using the most recent data available to update the market basket. We strive to
use publications that are disseminated frequently, because we believe that this is an optimal way
to stay abreast of the most current data available.
● Availability. Availability means that the proxy is publicly available. We prefer that
our proxies are publicly available because this will help ensure that our market basket updates
are as transparent to the public as possible. In addition, this enables the public to be able to
obtain the price proxy data on a regular basis.
● Relevance. Relevance means that the proxy is applicable and representative of the cost
category weight to which it is applied.
The CPIs, PPIs, and ECIs that we have selected to use in the proposed 2022-based FQHC
market basket meet these criteria. Therefore, we believe that they continue to be the best
measures of price changes for the cost categories to which they would be applied.
Table 28 lists all price proxies we propose to use in the proposed 2022-based FQHC
market basket. Below is a detailed explanation of the price proxies we propose for each cost
category.
(1) Price Proxies for the Proposed 2022-Based FQHC Market Basket
(a) FQHC Practitioner Wages and Salaries
We propose to use the ECI for Wages and Salaries for Private Industry Workers in
Professional and Related (BLS series code CIU2010000120000I) to measure price growth of this
category. There is no specific ECI for physicians or FQHC Practitioners, and therefore, we
propose to use an index that is based on professionals that receive advanced training similar to
those performing at the FQHC Practitioner level of care. This index is consistent with the price
proxy used to measure wages and salaries inflation pressure for physicians own time in the
Medicare Economic Index (MEI) and is based on the MEI technical panel recommendation from
2012 for more details see the CY 2014 PFS final rule (78 FR 74266 through 74271).
Additionally, this is the same price proxy used for the FQHC Practitioner Wages and Salaries
cost category in the 2017-based FQHC market basket (85 FR 84708).
(b) FQHC Practitioner Employee Benefits
We propose to use the ECI for Total Benefits for Private Industry Workers in
Professional and Related to measure price growth of this category. This ECI is calculated using
the ECI for Total Compensation for Private Industry Workers in Professional and Related (BLS
series code CIU1016220000000I) and the relative importance of wages and salaries within total
compensation. This is the same price proxy used for the FQHC Practitioner Employee Benefits
cost category in the 2017-based FQHC market basket (85 FR 84708).
(c) Clinical Staff Wages and Salaries
We propose to use the ECI for Wages and Salaries for all Civilian Workers in Health
Care and Social Assistance (BLS series code CIU1026200000000I) to measure the price growth
of this cost category. This cost category consists of wage and salary costs for Nurses, Laboratory
Technicians, and all other healthcare staff not included in the FQHC Practitioner compensation
categories. Based on the clinical staff composition of these workers, we believe that the ECI for
health-related workers is an appropriate proxy to measure wage and salary price pressures for
these workers. This is the same price proxy used for the Clinical Staff Wages and Salaries cost
category in the 2017-based FQHC market basket (85 FR 84708).
(d) Clinical Staff Employee Benefits
We propose to use the ECI for Total Benefits for all Civilian Workers in Health Care and
Social Assistance to measure price growth of this category. This ECI is calculated using the ECI
for Total Compensation for all Civilian Workers in Health Care and Social Assistance (BLS
series code CIU1016220000000I) and the relative importance of wages and salaries within total
compensation. This is the same price proxy used for the Clinical Staff Employee Benefits cost
category in the 2017-based FQHC market basket (85 FR 84708).
(e) Non-Health Staff Compensation
We propose to use the ECI for Total Compensation for Private Industry Workers in
Office and Administrative Support (BLS series code CIU2010000220000I) to measure the price
growth of this cost category. The Non-health Staff Compensation cost weight is predominately
attributable to administrative and facility type occupations, as reported in the data from the
Medicare cost reports. This is the same price proxy used for the Non-Health Staff Compensation
cost category in the 2017-based FQHC market basket (85 FR 84708).
(f) Pharmaceuticals
We propose to use the PPI Commodities for Pharmaceuticals for Human Use,
Prescription (BLS series code WPUSI07003) to measure the price growth of this cost category.
This price proxy is used to measure prices of Pharmaceuticals in other CMS market baskets, such
as the 2018-based Inpatient Prospective Payment System market basket and is the same price
proxy used for the Pharmaceuticals cost category in the 2017-based FQHC market basket (85 FR
84708).
(g) Utilities
We propose to use the CPI for Fuel and Utilities (BLS series code CUUR0000SAH2) to
measure the price growth of this cost category. This is the same price proxy used for the Utilities
cost category in the 2017-based FQHC market basket (85 FR 84708).
(h) Medical Equipment
We propose to use the PPI Commodities for Surgical and Medical Instruments (BLS
series code WPU1562) as the price proxy for this category. This is the same price proxy used for
the Medical Equipment cost category in the 2017-based FQHC market basket (85 FR 84708).
(i) Medical Supplies
We propose to use a 50/50 blended index that comprises the PPI Commodities for
Medical and Surgical Appliances and Supplies (BLS series code WPU156301) and the CPI–U
for Medical Equipment and Supplies (BLS series code CUUR0000SEMG). The 50/50 blend is
used in all market baskets where we do not have an accurate split available. We note that we
believe FQHCs purchase the types of supplies contained within these proxies, including such
items as bandages, dressings, catheters, intravenous equipment, syringes, and other general
disposable medical supplies, via wholesale purchase, as well as at the retail level. Consequently,
we propose to combine the two aforementioned indexes to reflect those modes of purchase. This
is the same price proxy used for the Medical Supplies cost category in the 2017-based FQHC
market basket (85 FR 84708 through 84709).
(j) Miscellaneous Products
We propose to use the CPI for All Items Less Food and Energy (BLS series code
CUUR0000SA0L1E) to measure the price growth of this cost category. We believe that using
the CPI for All Items Less Food and Energy is appropriate as it reflects a general level of
inflation. This is the same price proxy used for the Miscellaneous cost category in the 2017-
based FQHC market basket (85 FR 84709).
(k) Professional, Scientific, and Technical Services
We propose to use the ECI for Total Compensation for Private Industry Workers in
Professional, Scientific, and Technical Services (BLS series code CIU2015400000000I) to
measure the price growth of this cost category. This is the same price proxy used for the
Professional, Scientific, and Technical Services cost category in the 2017-based FQHC market
basket (85 FR 84709).
(l) Administrative and Facilities Support Services
We propose to use the ECI Total Compensation for Private Industry Workers in Office
and Administrative Support (BLS series code CIU2010000220000I) to measure the price growth
of this cost category. This is the same price proxy used for the Administrative and Facilities
Support Services cost category in the 2017-based FQHC market basket (85 FR 84709).
(m) All Other Services
We propose to use the ECI for Total Compensation for Private Industry Workers in
Service Occupations (BLS series code CIU2010000300000I) to measure the price growth of this
cost category. This is the same price proxy used for the All Other Services cost category in the
2017-based FQHC market basket (85 FR 84709).
(n) Fixed Assets
We propose to use the PPI Industry for Lessors of Nonresidential Buildings (BLS series
code PCU531120531120) to measure the price growth of this cost category (81 FR 80398). We
believe this continues to be the most appropriate price proxy since fixed asset costs in FQHCs
should reflect inflation for the rental and purchase of business office space. This is the same
price proxy used for the Fixed Assets cost category in the 2017-based FQHC market basket (85
FR 84709).
(o) Movable Equipment
We propose to continue to use the PPI Commodities for Machinery and Equipment (BLS
series code WPU11) to measure the price growth of this cost category as this cost category
represents nonmedical movable equipment. This is the same price proxy used for the Movable
Equipment cost category in the 2017-based FQHC market basket (85 FR 84709).
(2) Summary of Price Proxies of the Proposed 2022-Based FQHC Market Basket
Table 28 shows the cost categories and associated price proxies for the proposed 2022-
based FQHC market basket.

TABLE 28: Cost Categories and Price Proxies for the Proposed 2022-based FQHC Market
Basket
Cost Description
Price Proxies
FQHC Practitioner Wages and Salaries
ECI for Wages and Salaries for Private Industry Workers in Professional and
Related
FQHC Practitioner Employee Benefits
ECI for Total Benefits for Private Industry Workers in Professional and
Related
Clinical Staff Wages and Salaries
ECI for Wages and Salaries for All Civilian Workers in Health Care and
Social Assistance
Clinical Staff Employee Benefits
ECI for Total Benefits for All Civilian Workers in Health Care and Social
Assistance
Non-Health Staff Compensation
ECI for Total Compensation for Private Industry Workers in Office and
Administrative Support
Pharmaceuticals
PPI Special Index for Pharmaceuticals for Human Use, Prescription
Utilities
CPI-U for Fuels and Utilities
Medical Equipment
PPI Commodity Index for Surgical and Medical Instruments
Medical Supplies
Composite: PPI Commodity Index for Medical and Surgical Appliances and
Supplies (50%) and CPI for Medical Equipment and Supplies (50%)
Miscellaneous Products
CPI-U for All Items Less Food and Energy
Professional, Scientific, and Technical Services
ECI for Total compensation for Private industry workers in Professional,
Scientific, and Technical Services
Administrative and Facilities Support Services
ECI for Total Compensation for Private Industry Workers in Office and
Administrative Support
All Other Services
ECI for Total Compensation for Private Industry Workers in Service
Occupations
Fixed Assets
PPI Industry Index for Lessors of Nonresidential Buildings
Movable Equipment
PPI Commodity Index for Machinery and Equipment
We are soliciting comments on our proposal to rebase and revise the FQHC market basket to
reflect a 2022 base year.
e. Proposed CY 2025 Productivity-Adjusted Market Basket Update for FQHCs
For CY 2025 (that is, January 1, 2025, through December 31, 2025), we propose to use an
estimate of the proposed 2022-based FQHC market basket to update payments to FQHCs based on the
best available data. We propose to use the update based on the most recent historical data available at
the time of publication of the final rule. For example, the final CY 2025 FQHC update would be based
on the four-quarter moving-average percent change of the proposed 2022-based FQHC market basket
through the second quarter of 2024 (based on the final rule’s statutory publication schedule). At the
time of this proposed rule, we do not have the second quarter of 2024 historical data, and therefore, we
propose to use the most recent projection available at the time. Consistent with CMS practice, we
estimate the market basket update for the FQHC PPS based on the most recent forecast from IHS

Global, Inc. (IGI). IGI is a nationally recognized economic and financial forecasting firm with which
CMS contracts to forecast the components of the market baskets and total factor productivity (TFP).
Based on IGI’s first quarter 2024 forecast with historical data through the fourth quarter of 2023,
the proposed 2022-based FQHC market basket increase factor for CY 2025 is 4.0 percent. For
comparison, the 2017-based FQHC market basket percentage increase is also projected to be 4.0
percent in CY 2025; this estimate is based on IGI’s first quarter 2024 forecast (with historical data
through the fourth quarter of 2023). Table 29 compares the proposed 2022-based FQHC market
basket and the 2017-based FQHC market basket annual percent changes from 2021 through 2028.
TABLE 29: Proposed 2022-Based FQHC Market Basket and 2017-Based FQHC Market
Basket Percent Changes, CYs 2021 through 2028
Calendar Year
(CY)
1
Proposed
2022-Based FQHC Market Basket
Index
Percent Change
2017-Based FQHC Market
Basket Index Percent
Change
CY 2021
2.4
2.4
CY 2022
2.2
2.3
CY 2023
4.3
4.3
CY 2024
5.0
5.1
Historical Data
Average 2021-2024
3.5
3.5
CY 2025
4.0
4.0
CY 2026
3.3
3.3
CY 2027
2.9
2.9
CY 2028
2.8
2.9
Forecast
Average 2025-2028
3.3
3.3
1/ CY index percent changes are based on data through the 2
nd
quarter of the year prior to the update year.
Note that these market basket percent changes do not include any further adjustments as may be statutorily required.
Source: IHS Global Inc. 1st quarter 2024 forecast
Over the historical update time period covering CY 2021 through CY 2024, the average
growth rate of the proposed 2022-based FQHC market basket is the same as the average growth
rate of the 2017-based FQHC market basket. Over the forecasted time period covering CY 2025
through CY 2028, the average growth rate of the proposed 2022-based FQHC market basket is
also the same as the average growth rate of the 2017-based FQHC market basket. So, although
the compensation cost weights in the proposed 2022-based FQHC market basket is lower than in
the 2017-based FQHC market basket, and the weights for other products and services is higher,
there is little impact on the historical or projected FQHC market basket percentage increase.
Section 1834(o)(2)(B)(ii) of the Act describes the methods for determining updates to
FQHC PPS payment. We have included a productivity adjustment to the FQHC PPS annual
payment update since implementation of the FQHC PPS (81 FR 80393) and we propose to
continue to include a productivity adjustment to the proposed 2022-based FQHC market basket.
We propose to use the most recent estimate of the 10-year moving average of changes in annual
private nonfarm business (economy-wide) total factor productivity (TFP), which is the same
measure of TFP applied to other CMS market basket updates including the MEI. The U.S.
Department of Labor’s Bureau of Labor Statistics (BLS) publishes the official measures of
productivity for the U.S. economy. We note that previously the productivity published by BLS
was referred to as multifactor productivity. Beginning with the November 18, 2021, release of
productivity data, BLS replaced the term “multifactor productivity” (MFP) with “TFP.” Please
see https://www.bls.gov/productivity/data.htm for the BLS historical published TFP data. For the
final FQHC market basket update, we propose to use the most recent historical estimate of
annual TFP as published by the BLS. Generally, the most recent historical TFP estimate is
lagged two years from the payment year.
Therefore, we propose to use the 10-year moving average percent change in annual
private nonfarm business TFP through 2023 as published by BLS in the CY 2025 FQHC market
basket update. We note that TFP is derived by subtracting the contribution of labor and capital
input growth from output growth. Since at the time of development of this proposed rule the
measure of TFP for 2023 had not yet been published by BLS, we propose to use IGI’s first
quarter 2024 forecast of TFP. A complete description of IGI’s TFP projection methodology is
available on the CMS website at https://www.cms.gov/data-research/statistics-trends-and-
reports/medicare-program-rates-statistics/market-basket-research-and-information.
Using IGI’s first quarter 2024 forecast, the productivity adjustment for CY 2025 (the 10-
year moving average of TFP for the period ending CY 2023) is projected to be 0.5 percent.
Therefore, the proposed CY 2025 productivity-adjusted proposed 2022-based FQHC market
basket update is 3.5 percent, based on IGI’s first quarter 2024 forecast with historical data
through the fourth quarter of 2023. This reflects a 4.0 percent increase in the proposed 2022-
based FQHC market basket reduced by a 0.5 percentage point productivity adjustment. Finally,
we propose that the CY 2025 market basket update and the productivity adjustment will be
updated to reflect the most recent historical data available for the final rule.
8. Clarification for Dental Services Furnished in FQHCs
a. Payment for Dental Services Furnished in FQHCs
Section 1862(a)(12) of the Act generally precludes payment under Medicare Parts A or B
for any expenses incurred for services in connection with the care, treatment, filling, removal, or
replacement of teeth or structures directly supporting teeth. (Collectively here, we will refer to
“the care, treatment, filling, removal, or replacement of teeth or structures directly supporting
teeth” as “dental services.”) That section of the statute also includes an exception to allow
payment to be made for inpatient hospital services in connection with the provision of such
dental services if the individual, because of their underlying medical condition and clinical status
or because of the severity of the dental procedure, requires hospitalization in connection with the
provision of such services. Our regulation at 42 CFR 411.15(i) similarly excludes payment for
dental services except for inpatient hospital services in connection with dental services when
hospitalization is required because of: (1) the individual’s underlying medical condition and
clinical status; or (2) the severity of the dental procedure.
Fee for service (FFS) Medicare Parts A and B also make payment for certain dental
services in circumstances where the services are not considered to be in connection with dental
services within the meaning of section 1862(a)(12) of the Act. In the CY 2023 PFS final rule (87
FR 69663 through 69688), we clarified and codified at § 411.15(i)(3) that Medicare payment
under Parts A and B could be made when dental services are furnished in either the inpatient or
outpatient setting when the dental services are inextricably linked to, and substantially related
and integral to the clinical success of, other covered services. We also added several examples
of clinical scenarios that are considered to meet that standard under § 411.15(i)(3) and amended
that regulation to add more examples in the CY 2024 PFS final rule (88 FR 79022 through
79029).
In the CY 2024 PFS final rule (88 FR 79038), we received comments requesting we
provide payment for inextricably linked dental services in the FQHC setting. Commenters stated
that it is critical that CMS consider FQHCs’ unique Medicare payment structure and that CMS
ensure that policy changes for FQHCs are analogous to any changes made under the PFS.
Commenters noted that many FQHCs provide dental services on-site, and health center patients
could benefit from the payment policies for dental services inextricably linked to other covered
services and suggested that the FQHC billing codes should be edited in tandem. Commenters
further noted that ‘‘physicians’ services’’ component of the Medicare FQHC benefit includes
services furnished by dentists. Several commenters urged that the list of billable dental visit
codes modified in the proposed rule be added to the list of codes that may be billed in the FQHC
setting and requested that any expansion in codes recognized under the PFS for dental-related
services also be applied to FQHCs. We acknowledged the commenters concerns and noted our
intention to modify operational procedures in the FQHC setting to reflect the expansion of this
PFS policy, including updates to billable code lists.
We agree that RHC and FQHC Medicare beneficiaries could benefit from the payment
policies established under the PFS for dental services that are inextricably linked to specific
medical services. Dentists are defined as physicians in Medicare statute (42 CFR 491.2).
Services furnished by physicians are billable visits in RHCs and FQHCs and they could bill for a
face-to-face, medically necessary visit furnished by a dentist within their scope of practice.
Therefore, we are clarifying in this proposed rule that dental services exactly as described in
section II.J and furnished in an RHC or FQHC are RHC and FQHC visits and as such can be
paid under the RHC AIR methodology or FQHC PPS.
We would apply and operationalize the dental policies finalized in the CY 2023 and 2024
PFS final rules as applicable also to RHCs and FQHCs and update the FQHC qualifying visit list
as appropriate. Consistent with the discussion in section II.J of this proposed rule, if an RHC or
FQHC practitioner believes the dental services for which they submit Medicare claims are
inextricably linked to a covered service, a modifier may be reported on an RHC or FQHC claim
for payment purposes. The KX modifier is reported on an RHC or FQHC claim to indicate that
the service is medically necessary, and that the provider has included appropriate documentation
in the medical record to support or justify the medical necessity of the service or item. We
believe that usage of the KX modifier in the context of claims for dental services inextricably
linked to covered services to indicate that the clinician attests that the service is medically
necessary, and that the provider has included appropriate documentation is appropriate and will
support claims processing and program integrity efforts.
We are clarifying that when RHCs and FQHCs furnish dental services that align with the
policies and operational requirements in the physician setting, we would consider those services
to be a qualifying visit and the RHC would be paid at the RHC AIR methodology and the FQHC
would be paid under the FQHC PPS.
b. Medical and Dental Visits Furnished on the Same Day
If an RHC or FQHC patient has a medically-necessary face-to-face visit with an RHC or
FQHC practitioner, and is then seen by another RHC or FQHC practitioner, including a
specialist, for further evaluation of the same condition on the same day, or is then seen by
another RHC or FQHC practitioner, including a specialist, for evaluation of a different condition
on the same day, the multiple encounters would constitute a single RHC or FQHC visit and be
payable as one visit regardless of the length or complexity of the visit, whether the second visit is
a scheduled or unscheduled appointment, or whether the first visit is related or unrelated to the
subsequent visit.

If the RHC or FQHC patient suffers an illness or injury that requires additional diagnosis
or treatment on the same day subsequent to the first visit, or has a medical and a mental health
visit on the same day, or an RHC patient has an initial preventive physical exam (IPPE) and a
separate medical and/or mental health visit on the same day, then the RHC or FQHC would be
paid separately for each visit.
We are seeking comment on whether the multiple visits policy should apply to patients
who have an encounter with an RHC or FQHC practitioner and a dentist on the same day or
should a subsequent encounter with a dentist be considered an exception to this policy and be
paid as a separate billable visit. We are interested in understanding when these situations could
occur.
9. “Grandfathered” Technical Refinement
a. Background
We have conducted a review of our regulations and guidance to determine where
preferred terms may be used. We found several sections in part 405, subpart X, that use the term
“grandfathered.” For example, in § 405.2462(f)(1) a “grandfathered tribal FQHC” is a FQHC
that is operated by a tribe or tribal organization under the Indian Self-Determination and
Education Assistance Act (ISDEAA); was billing as if it were provider-based to an IHS hospital
on or before April 7, 2000, and is not currently operating as a provider-based department of an
IHS hospital.
b. Technical Refinement
We believe language in communication products should reflect and speak to the needs of
people in the audience of focus. In an effort to represent an ongoing shift to non-stigmatizing
language, we are proposing to make a technical change to remove the term “grandfathered” from
the regulation text in §§ 405.2462, 405.2463, 405.2464, and 405.2469 and replace it with
“historically excepted” to describe a level of protection provided to certain tribal FQHCs that
predates applicable restrictions.
C. Rural Health Clinic (RHC) and Federally Qualified Health Center (FQHC) Conditions for
Certification and Conditions for Coverage (CfCs)
1. Background and Statutory Authority
The Rural Health Clinic Services Act of 1977 (Pub. L. 95–210 enacted December 13,
1977) amended the Social Security Act (the Act) by enacting section 1861(aa) to extend
Medicare and Medicaid entitlement and payment for outpatient services and emergency care
services furnished at a rural health clinic (RHC) by physicians and certain other practitioners,
and for services and supplies incidental to their services. Other practitioners include nurse
practitioners and physician assistants, and subsequent legislation extended the definition of
covered RHC services to include the services of clinical psychologists, clinical social workers,
certified nurse midwives, marriage and family therapists, and mental health counselors.
We have broad statutory authority to establish health and safety standards for most
Medicare and Medicaid participating provider and supplier types. Section 1861(aa) of the Act
authorizes the Secretary to establish the requirements that an RHC and Federally Qualified
Health Center (FQHC) must meet to participate in the Medicare Program. As required by
subparagraph (iv) of the flush material set out after section 1861(aa)(2)(K) of the Act, Medicare
certified RHCs must not be a rehabilitation agency or a facility which is primarily for the care or
treatment of mental diseases. These statutory CfC requirements are codified in regulations at 42
CFR part 491. RHCs and FQHCs must meet these requirements to receive Medicare payment for
services. These regulations are intended to protect the health and safety of patients receiving care
from these facilities. We note that there are approximately 5,462 Medicare-certified RHCs and
11,853 Medicare-certified FQHCs.
In this proposed rule, we also aim to ensure RHCs are provided flexibility in the services
they offer, including specialty and laboratory services.
2. Proposed Changes to the RHC and FQHC Conditions for Certification and Conditions for
Coverage (CfCs)

a. Provision of Services (42 CFR 491.9)
In accordance with section 1861(aa) of the Act, § 491.9, Provision of services, establishes
the basic requirements for services RHCs and FQHCs must provide in accordance with
applicable Federal, State, and local laws. This CfC also outlines patient care policies, including
the development of written policies and the establishment of guidelines for medical management,
record-keeping, and drug administration. Additionally, this section specifies the diagnostic,
therapeutic, laboratory, and emergency services that RHCs and FQHCs must offer, as well as the
necessary agreements or arrangements with other healthcare providers to furnish additional
services not available onsite.
RHCs and FQHCs play a crucial role in providing accessible and comprehensive
healthcare services in underserved areas, often serving as the primary point of contact for
healthcare services for residents living in these communities. Approximately 20 percent of the
U.S. population lives in rural areas.
312
When compared to their urban counterparts, rural
residents are more likely to be living in poverty, unhealthy, older adults, uninsured or
underinsured, and medically underserved.
313
Accounting for the unique circumstances of
individuals living in rural areas and the broad array of services RHCs and FQHCs provide to
their communities, it is imperative to support rural facilities so they may continue to provide
healthcare services to medically underserved populations.
Research shows that accessing primary care is associated with positive health
outcomes.
314
Individuals who receive primary care services are more likely to receive preventive
312
U.S. Census Bureau. (2023, March 10). Nation’s Urban and Rural Populations Shift Following 2020 Census. U.S.
Department of Commerce. Retrieved April 12, 2024, from https://www.census.gov/newsroom/press-
releases/2022/urban-rural-populations.html.
313
Report to the Congress: Medicare and the Health Care Delivery System (June 2012) (medpac.gov).
Medicare Payment Advisory Commission. (2012, June). Medicare and the Health Care Delivery System.
https://www.medpac.gov/wp-content/uploads/import_data/scrape_files/docs/default-
source/reports/jun12_entirereport.pdf.
314
Shi L. (2012). The impact of primary care: a focused review. Scientifica, 2012, 432892.
https://doi.org/10.6064/2012/432892.

care, which results in lower rates of illness and premature death.
315
Furthermore, without access
to primary care services, an individual may delay seeking treatment until their illness has
worsened. In such cases, they may end up receiving care in the emergency department, which
costs more and leads to poorer health outcomes.
316,317,318
Rural residents also report difficulty
accessing specialty care due to having to travel considerable distances, exacerbated by rural
hospitals closing and provider shortages.
319,320
RHC practitioners have also reported additional
barriers to receiving specialized care, which include a shortage of specialty providers, limited
availability of the specialty providers, lack of transportation, and not accepting patients who are
uninsured or specialists not accepting insurance coverage (Medicare, Medicaid, or private
insurance).
321
Based on feedback from interested parties, including RHC providers and rural health
associations, we identified a discrepancy in the guidance, statute, and regulations. Specifically,
interested parties questioned the language in the CMS State Operations Manual RHC
interpretative guidance as it relates to § 491.9(a)(2), which states that “RHCs may not be
primarily engaged in specialized services.”
322
The guidance goes on to state that, in this context,
315
Hostetter, J., Schwarz, N., Klug, M., Wynne, J., & Basson, M. D. (2020). Primary care visits increase utilization
of evidence-based preventative health measures. BMC family practice, 21(1), 151. https://doi.org/10.1186/s12875-
020-01216-8.
316
Tang, N., Stein, J., Hsia, R. Y., Maselli, J. H., & Gonzales, R. (2010). Trends and characteristics of US emergency
department visits, 1997-2007. JAMA, 304(6), 664–670. https://doi.org/10.1001/jama.2010.1112.
317
Gallagher, A., Liu, J., Probst, J. C., Martin, A. B., & Hall, J. W. (2013). Maternal obesity and gestational weight
gain in rural versus urban dwelling women in South Carolina. The Journal of rural health: official journal of the
American Rural Health Association and the National Rural Health Care Association, 29(1), 1–11.
https://doi.org/10.1111/j.1748-0361.2012.00421.x.
318
Bailey, B. A., & Cole, L. K. (2009). Rurality and birth outcomes: findings from southern appalachia and the
potential role of pregnancy smoking. The Journal of rural health: official journal of the American Rural Health
Association and the National Rural Health Care Association, 25(2), 141–149. https://doi.org/10.1111/j.1748-
0361.2009.00210.x
319
Akinlotan, M., Primm, K., Khodakarami, N., Bolin, J., Ferdinand, A. (2021). Rural-Urban Variations in Travel
Burdens for Care: Findings from the 2017 National Household Travel Survey [Policy brief]. Southwest Rural
Health Research Center. https://srhrc.tamu.edu/publications/travel-burdens-07.2021.pdf.
320
Douthit, N., Kiv, S., Dwolatzky, T., & Biswas, S. (2015). Exposing some important barriers to health care access
in the Rural USA. Public Health, 129(6), 611–620. https://doi.org/10.1016/j.puhe.2015.04.001.
321
Lahr, M., Neprash, H., Henning-Smith, C., Tuttle, M., Hernandez, A. (2019). Access to Specialty Care for
Medicare Beneficiaries in Rural Communities [Policy brief]. University of Minnesota Rural Health Research Center.
https://rhrc.umn.edu/wp-content/uploads/2019/12/UMN-Access-to-Specialty-Care_12.4.pdf.
322
Centers for Medicare & Medicaid Services. (2020, February 21). State Operations Manual Appendix G -
Guidance for Surveyors: Rural Health Clinics (RHCs) (pp. 63-64). https://www.cms.gov/Regulations-and-
Guidance/Guidance/Manuals/downloads/som107ap_g_rhc.pdf.
“primarily engaged” is determined by considering the total hours of an RHC’s operation and
whether a majority, i.e., more than 50 percent, of those hours involve the provision of RHC
services, whereas section 1861(aa)(2)(A) of the Act only reference being primarily engaged in
“furnishing to outpatients” physician services and services furnished by a physician assistant or a
nurse practitioner, clinical psychologist or by a clinical social worker, as cross-referenced by
sections 1861(aa)(1)(A) and (B) of the Act. This is codified in the CfCs at § 491.9(a)(2),
requiring RHCs and FQHCs to be primarily engaged in “providing outpatient health services.”
We have enforced a standard requiring that RHCs be primarily engaged in providing primary
care services based on the interpretive guidance.
To clarify the requirements and intent of the program regarding the services provided by
RHCs and FQHCs, we are proposing changes to the Provision of services CfCs. We also aim to
ensure RHCs are provided flexibility in the services they offer, including specialty services. To
align the requirements and preserve access to primary care services in rural areas, we are
proposing to add standards to § 491.9(a)(2) explicitly requiring RHCs and FQHCs to provide
primary care services (at § 491.9(a)(2)(i)) and explicitly noting that RHCs cannot be a
rehabilitation agency or a facility which is primarily for the care and treatment of mental diseases
(at § 491.9(a)(2)(ii)). Under our proposal, RHCs and FQHCs would continue to be required to
provide primary care services to their patient populations, but CMS would no longer determine
or enforce the standard of RHCs “being primarily engaged in furnishing primary care services”
and would no longer consider the total hours of an RHC’s operation and whether a majority, that
is, more than 50 percent, of those hours involve primary care services through the survey
process. These proposed requirements, as well as the subsequent proposal discussed in section
III.C.2.b. of this proposed rule, will be enforced in accordance with section 1864 of the Act,
which requires that CMS use state surveyors to determine whether a provider or supplier subject
to certification qualifies for an agreement to participate in Medicare. However, under section
1865 of the Act, providers and suppliers subject to certification may instead elect to be

accredited by private accrediting organizations whose Medicare accreditation programs have
been approved by CMS as having standards and survey procedures that meet or exceed all
applicable Medicare requirements.
This proposal would allow RHCs to provide more outpatient-specialty services within the
practitioner’s scope of practice to meet the needs of the patient population—for example, internal
medicine, pediatrics, geriatrics, obstetrics and gynecology, dermatology, cardiology, neurology,
endocrinology, and ear, nose and throat. Section 1861(aa) of the Act does not explicitly state that
RHCs must provide primary care services, though we believe this is the intended purpose of the
statute. Clause (i) of the second sentence of the flush material after section 1861(aa)(2)(K) of the
Act, requires RHCs to be located in an area designated as either an area with a shortage of
personal health services under section 330(b)(3) or 1302(7) of the Public Health Service Act or
as a health professional shortage area described in section 332(a)(1)(A) of that Act because of its
shortage of primary medical care manpower. Additionally, we use the phrase “the entry point
into the health care system” in the RHC and FQHC CfCs at § 491.9(c)(1). We note that this
language is consistent with language used in the Rural Emergency Hospital Conditions of
Participation (CoPs) under the requirement for Additional outpatient medical and health services
(§ 485.524(a)), and the Critical Access Hospital CoPs under the requirement for Provision of
services (§ 485.635(b)(1)(i)). The American Academy of Family Physicians (AAFP) defines
primary care practice as “A primary care practice serves as the patient's entry point into the
health care system and as the continuing focal point for all needed health care services.”
Therefore, we expect RHCs and FQHCs to offer a range of primary health care services to
ensure that patients receive the necessary care at the earliest possible point of contact. Primary
care services are critical in promoting health, preventing illness, and managing chronic
conditions.
323
323
Primary Care. (n.d.). https://www.cms.gov/priorities/innovation/key-concepts/primary-care.

This proposal also provides clarification regarding the requirement that RHCs cannot be a
rehabilitation agency or a facility which is primarily for the care and treatment of mental
diseases. While this requirement is included under § 491.2, Definitions – Rural health clinic or
clinic, including this requirement under the Provision of services CfC at § 491.9(a)(2)(ii) as a
separate standard more clearly cites the requirement and allows for a clearer evaluation of
compliance with the specific requirement. CMS defines a rehabilitation agency as “[a]n agency
that provides an integrated, multidisciplinary program designed to upgrade the physical functions
of handicapped, disabled individuals by bringing together, as a team, specialized rehabilitation
personnel.”
324
Rehabilitation services may include physical therapy, occupational therapy, and
speech-language pathology services.
CMS acknowledges that the term “mental diseases” is outdated and not often used in the
field of mental health today. This term can cause stigma against people with mental health
conditions and can be a major barrier to seeking help or treatment and can be a cause of reduced
likelihood of engaging in ongoing treatment. As discussed in section III.C.1 of this proposed
rule, the U.S. Congress passed Pub. L. 95-210 (enacted December 13, 1977) that established
criteria for the establishment of Medicare-certified RHCs and has included the term “mental
diseases” since that time. As we are bound by the current statutory requirements, we have
included the term in the RHC CfCs as it appears in the statute. We note that the statute is
enacted by Congress and it would take an Act of Congress to make statutory changes.
For the purpose of this proposed rule, the term “mental diseases” refers to behavioral
health conditions (that is, mental and substance use disorders). As part of the Coverage to Care
(C2C) initiative, CMS published the Roadmap to Behavioral Health, which defines behavioral
324
Centers for Medicare & Medicaid Services. (2023, September 6). Outpatient Rehabilitation Providers. CMS.gov.
https://www.cms.gov/medicare/health-safety-standards/certification-compliance/outpatient-rehabilitation-
providers#:~:text=Rehabilitation%20Agency%20%2D%20An%20agency%20that,a%20team%2C%20specialized%
20rehabilitation%20personnel.

health conditions to include mental and substance use disorders.
325
The guide defines mental
disorders as changes in a person’s thinking, mood, and/or behavior and can only be diagnosed
based on the person’s ability to function as a result of their symptoms. The guide defines
“substance use disorder” as when a person’s repeated use of alcohol and/or drugs causes
clinically significant impairment.
326
This may include health problems, disability, and failure to
meet major responsibilities at home, school, or work.
These proposed changes to the RHC CfCs should not be construed as CMS prohibiting or
discouraging the provision of RHC specialty services or behavioral health services. An RHC or
FQHC may offer such specialty services and behavioral health services to its patients in addition
to the primary care services it already provides. However, RHCs cannot be a rehabilitation
agency or a facility, which is primarily for the care and treatment of mental diseases. As noted,
the term "mental diseases" is outdated; therefore, this proposed rule aims to define "mental
diseases" for the purpose of the RHC and FQHC health and safety standards and future guidance
will clarify how CMS will determine whether an RHC is primarily providing care and treatment
of mental diseases. CMS would assess a facility's compliance with this proposed requirement
based on policies published in subsequent interpretative guidance, which we would publish if
this revision is adopted in a final rule.
As we continue to focus on improved access to care for the rural communities served by
RHCs, and to ensure that the proposed provisions do not have unintended consequences, we are
soliciting public comment on the following questions to gain insight on the anticipated impact of
this proposed policy on access to primary care services, behavioral health services, and specialty
care services:
325
Centers for Medicare & Medicaid Services, and the Substance Abuse and Mental Health Services
Administration. (2023, October). Roadmap to Behavioral Health: Guide to Mental Health and Substance Use
Disorder Services. https://www.cms.gov/files/document/roadmap-behavioral-health-english.pdf.
326
SAMHSA. (2023, June 09). Mental Health and Substance Use Disorders. Retrieved June 17, 2024, from
https://www.samhsa.gov/find-help/disorders.
● What types of behavioral health services are currently offered by RHCs (that is,
therapy, counseling, medication management, substance use disorder treatment, etc.), and how
often are these services provided?
● For those RHCs that are currently providing behavioral health services, who provides
those services (that is, physician, psychologist, social worker, marriage and family therapist, or
mental health counselor)? What is the clinic’s capacity to accept new behavioral health patients?
What potential impacts do you anticipate for RHCs and the community if they were able to
provide more behavioral health services? How would these impacts be addressed?
● Are there specific behavioral health conditions that your clinic is better equipped to
treat than others, and if so, what are those behavioral health conditions?
● For those RHCs that are not currently providing behavioral health services, what
barriers or challenges does the RHC face that limit the ability to furnish behavioral health
services (that is, geographic location, transportation issues, service area size, staffing issues,
stigma, regulatory or survey concerns)?
● What standards or criteria should surveyors use to evaluate whether a RHC is operating
as a “facility which is primarily for the care and treatment of mental diseases”?
b. Laboratory Requirements (42 CFR 491.9)
Section 1861(aa)(2)(G) of the Act requires RHCs to provide routine diagnostic services
directly (that is, they must be furnished at the RHC, by RHC personnel), including clinical
laboratory services. We have implemented this statutory provision through regulations at
§ 491.9(c)(2), which require RHCs to provide six specific diagnostic laboratory tests directly;
chemical examinations of urine by stick or tablet method or both (including urine ketones);
hemoglobin or hematocrit; blood glucose; examination of stool specimens for occult blood;
pregnancy tests; and primary culturing for transmittal to a certified laboratory. This list reflects
the same tests that have been required since RHCs were established in 1978 (43 FR 30529) and
were chosen because they were commonly performed in physicians’ offices at that time.

The Clinical Laboratory Improvement Amendments of 1988 (CLIA) (Pub. L. 100-578),
revised section 353 of the Public Health Service Act to require all clinical laboratories that test
human specimens for health purposes to meet certain requirements. CLIA requires that the
complexity of tests and the skill in interpreting results determine the extent of supervision and
review. Tests are categorized from “waived” (those needing minimal supervision) to “high
complexity” (those requiring the highest level of supervision and review). Regulations
implementing CLIA are contained in 42 CFR part 493. Under these regulations, laboratories
performing specified tests categorized as waived, physician-performed microscopy moderate or
high complexity must obtain a certificate that shows they meet specific standards. All tests RHCs
are required to perform are classified as waived under CLIA, as published in the 1992 Federal
Register and listed at 42 CFR 493.15(c).
We are proposing to remove hemoglobin and hematocrit (H&H) from the listed
laboratory services in § 491.9(c)(2) that RHCs must perform directly. In addition, we are
proposing to revise paragraph (c)(2)(vi) to reflect current practice for microbiology specimens.
Interested parties have expressed concerns with the existing laboratory requirements, citing the
financial and physical burdens associated with maintaining the lab equipment that is used for
laboratory tests that are ordered infrequently for patients receiving services at the RHC. RHC
providers have reported that the H&H laboratory test in particular is overly burdensome. We are
therefore proposing to remove the H&H laboratory test from the list of required laboratory tests
to increase flexibility in the services offered by RHCs.
A hemoglobin test evaluates the amount of hemoglobin in the blood, which is responsible
for carrying oxygen to a person’s organs and tissues and transporting carbon dioxide back to the
lungs.
327
Hematocrit is the percentage of red blood cells in a person's blood and is tested to
327
Hemoglobin test: What it is, procedure & results. Hemoglobin Test. (n.d.). Retrieved May 7, 2024, from
https://my.clevelandclinic.org/health/diagnostics/17790-hemoglobin-test.

evaluate red blood cells.
328
Doctors test hemoglobin and hematocrit levels to evaluate overall
health, diagnose a medical condition, or monitor an existing medical condition. While RHCs are
currently required to have and maintain the equipment and supplies for these tests onsite, H&H
blood tests are typically performed as part of a comprehensive blood count (CBC) and are not
ordered separately in accordance with current clinical standards of practice. RHCs report that
when laboratory tests are ordered that are not provided by the RHC, such as a CBC, their patients
are often sent to the nearest hospital that would have a full-service laboratory available to
perform the test. As a result, some RHCs located near hospitals may not be utilizing their
laboratory equipment and supplies, or they may be utilizing them on a limited basis. This is
because when laboratory tests are ordered by the RHC, and the order includes hemoglobin and
hematocrit, and other tests that are not provided by the RHC, the patient will receive all of their
ordered laboratory tests at the nearby hospital. Additionally, removing the requirement for these
tests to be performed at the RHC limits the number of venipunctures the patient must experience.
In 2021, 66 percent of all RHCs were designated as provider-based RHCs, meaning they
are owned and operated as an integral part of a hospital, nursing home, or home health agency.
329
Provider-based RHCs are governed, supervised, and licensed by the parent organization, and
most provider-based RHCs are hospital-owned. The remaining 34 percent of RHCs are
independent clinics owned by a healthcare provider or entity and though not as common, may be
owned and/or operated by a healthcare system. Therefore, we believe a large majority of
provider-based - and some independent - RHCs have access to a full-service laboratory, thereby
maintaining access to the H&H laboratory test, even if it is no longer required to be provided
directly by RHCs.
328
Hematocrit test: What it is, levels, high & low range. Hematocrit Test. (n.d.). Retrieved May 7, 2024,
fromhttps://my.clevelandclinic.org/health/diagnostics/17683-hematocrit.
329
Gale, J. A., Croll, Z., Croom, J., Munk, L., & Jonk, Y. (2022). Community Characteristics and Financial and
Operational Performance of Rural Health Clinics in the United States: A Chartbook. University of Southern Maine,
Muskie School of Public Service, Maine Rural Health Research Center.
https://digitalcommons.usm.maine.edu/cgi/viewcontent.cgi?article=1016&context=clinics.
We have also received feedback that the current requirement at § 491.9(c)(2)(vi) does not
reflect current clinical laboratory standards of practice and laboratory techniques. This standard
currently lists the laboratory services that must be provided by RHCs, with the list specifically
including “primary culturing for transmittal to a certified laboratory.” Primary culturing is an
outdated microbiology practice that is no longer performed due to modern lab techniques.
Instead, RHCs collect specimens using appropriate collection and storage techniques and send
them to a certified laboratory without initial culturing. Therefore, we are proposing to update the
language in this standard such that the laboratory services RHCs would be required to provide
include the “collection of patient specimens for transmittal to a certified laboratory for
culturing.” This proposal, along with the proposal removing the H&H from the list of required
labs, would require that an RHC provide the remaining following laboratory services at §
491.9(c)(2): (1) chemical examinations of urine by stick or tablet methods or both (including
urine ketones); (2) blood glucose; (3) examination of stool specimens for occult blood; (4)
pregnancy tests; and (5) collection of patient specimens for transmittal to a certified laboratory
for culturing. RHCs would still be required under § 491.9(d)(1)(iii) to provide prompt access to a
Medicare or Medicaid participating provider or supplier that can furnish an H&H laboratory test
and any additional and specialized diagnostic and laboratory services the RHC is not equipped to
perform.
This proposal does not prevent RHCs from providing tests not listed in § 491.9. An RHC
is free to provide tests consistent with its CLIA certification and can choose a higher level CLIA
certification than the certificate of waiver if it wishes to provide tests of higher complexity and
comply with all CLIA requirements.
Little information on RHC laboratory utilization is available, so while we are proposing
to remove the H&H laboratory test from the list of diagnostic services RHCs are required to
provide, we solicit public comment on how this may impact access to H&H laboratory tests in
rural areas. We also are soliciting comments on data, evidence, and experience related to
laboratory services in RHCs and alternative basic lab services that would be appropriate for
RHCs to provide to meet the needs of underserved rural communities.
D. Clinical Laboratory Fee Schedule: Revised Data Reporting Period and Phase-in of Payment
Reductions
1. Background on the Clinical Laboratory Fee Schedule
Prior to January 1, 2018, Medicare paid for clinical diagnostic laboratory tests (CDLTs)
on the Clinical Laboratory Fee Schedule (CLFS) under section 1833(a), (b), and (h) of the Act.
Under the previous payment system, CDLTs were paid based on the lesser of: (1) the amount
billed; (2) the local fee schedule amount established by the Medicare Administrative Contractor
(MAC); or (3) a national limitation amount (NLA), which is a percentage of the median of all the
local fee schedule amounts (or 100 percent of the median for new tests furnished on or after
January 1, 2001). In practice, most tests were paid at the NLA. Under the previous payment
system, the CLFS amounts were updated for inflation based on the percentage change in the
Consumer Price Index for All Urban Consumers (CPI-U) and reduced by a productivity
adjustment and other statutory adjustments but were not otherwise updated or changed.
Coinsurance and deductibles generally do not apply to CDLTs paid under the CLFS.
Section 1834A of the Act, as established by section 216(a) of the Protecting Access to
Medicare Act of 2014 (PAMA), required significant changes to how Medicare pays for CDLTs
under the CLFS. A final rule entitled “Medicare Clinical Diagnostic Laboratory Tests Payment
System” (CLFS final rule), which appeared in the Federal Register on June 23, 2016 (81 FR
41036), implemented section 1834A of the Act at 42 CFR part 414, subpart G.
Under the CLFS final rule, “reporting entities” must report to CMS during a “data
reporting period” “applicable information” collected during a “data collection period” for their
component “applicable laboratories.” The first data collection period occurred from January 1,
2016, through June 30, 2016. The first data reporting period occurred from January 1, 2017,
through March 31, 2017. On March 30, 2017, we announced a 60-day period of enforcement

discretion for the application of the Secretary’s potential assessment of civil monetary penalties
for failure to report applicable information with respect to the initial data reporting period.
330
In the CY 2018 PFS proposed rule (82 FR 34089 through 34090), we solicited public
comments from applicable laboratories and reporting entities to better understand the applicable
laboratories’ experiences with data reporting, data collection, and other compliance requirements
for the first data collection and reporting periods. We discussed these comments in the CY 2018
PFS final rule (82 FR 53181 through 53182) and stated that we would consider the comments for
potential future rulemaking or guidance.
As part of the CY 2019 Medicare PFS rulemaking, we finalized two changes to the
definition of “applicable laboratory” at § 414.502 (see 83 FR 59667 through 59681, 60074; 83
FR 35849 through 35850, 35855 through 35862). First, we excluded Medicare Advantage plan
payments under Part C from the denominator of the Medicare revenues threshold calculation to
broaden the types of laboratories qualifying as an applicable laboratory. Second, consistent with
our goal of obtaining a broader representation of laboratories that could potentially qualify as an
applicable laboratory and report data, we also amended the definition of applicable laboratory to
include hospital outreach laboratories that bill Medicare Part B using the CMS-1450 14x Type of
Bill.
2. Payment Requirements for Clinical Diagnostic Laboratory Tests
In general, under section 1834A of the Act, the payment amount for each CDLT on the
CLFS furnished beginning January 1, 2018, is based on the applicable information collected
during the data collection period and reported to CMS during the data reporting period and is
equal to the weighted median of the private payor rates for the test. The weighted median is
calculated by arraying the distribution of all private payor rates, weighted by the volume for each
payor and each laboratory. The payment amounts established under the CLFS are not subject to
330
https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Downloads/2017-
March-Announcement.pdf.
any other adjustment, such as geographic, budget neutrality, or annual update, as required by
section 1834A(b)(4)(B) of the Act. Additionally, section 1834A(b)(3) of the Act, implemented
at § 414.507(d), provides for a phase-in of payment reductions, limiting the amounts the CLFS
rates for each CDLT (that is not a new advanced diagnostic laboratory test (ADLT) or new
CDLT) can be reduced as compared to the payment rates for the preceding year. Under the
original provisions enacted by section 216(a) of PAMA, for the first 3 years after implementation
(CY 2018 through CY 2020), the reduction could not be more than 10 percent per year. For the
next 3 years after implementation (CY 2021 through CY 2023), section 216(a) of PAMA stated
that the reduction could not be more than 15 percent per year. Under sections 1834A(a)(1) and
(b) of the Act, as enacted by PAMA, for CDLTs that are not ADLTs, the data collection period,
data reporting period, and payment rate update were to occur every 3 years. As such, the second
data collection period for CDLTs that are not ADLTs occurred from January 1, 2019, through
June 30, 2019, and the next data reporting period was originally scheduled to take place from
January 1, 2020, through March 31, 2020, with the next update to the Medicare payment rates for
those tests based on that reported applicable information scheduled to take effect on January 1,
2021.
Section 216(a) of PAMA established a new subcategory of CDLTs known as ADLTs,
with separate reporting and payment requirements under section 1834A of the Act. The
definition of an ADLT is set forth in section 1834A(d)(5) of the Act and implemented at
§ 414.502. Generally, under section 1834A(d) of the Act, the Medicare payment rate for a new
ADLT is equal to its actual list charge during an initial period of 3 calendar quarters. After the
new ADLT initial period, ADLTs are paid using the same methodology based on the weighted
median of private payor rates as other CDLTs. However, under section 1834A(d)(3) of the Act,
updates to the Medicare payment rates for ADLTs occur annually instead of every 3 years.

Additional information on the private payor rate-based CLFS is detailed in the CLFS
final rule, which implemented section 1834A of the Act as required by PAMA (81 FR 41036
through 41101), and this information is also available on the CMS website.
331
3. Previous Statutory Revisions to the Data Reporting Period and Phase-In of Payment
Reductions
Beginning in 2019, Congress passed a series of legislation to modify the statutory
requirements for the data reporting period and phase-in of payment reductions under the CLFS.
First, section 105(a)(1) of the Further Consolidated Appropriations Act, 2020 (FCAA) (Pub. L.
116-94, December 20, 2019) amended the data reporting requirements in section 1834A(a) of the
Act to delay the next data reporting period for CDLTs that are not ADLTs by 1 year so that data
reporting would be required during the period of January 1, 2021, through March 31, 2021,
instead of January 1, 2020, through March 30, 2020. The 3-year data reporting cycle for CDLTs
that are not ADLTs would resume after that data reporting period. Section 105(a)(1) of the
FCAA also specified that the data collection period that applied to the data reporting period of
January 1, 2021, through March 30, 2021, would be the period of January 1, 2019, through June
30, 2019, which was the same data collection period that would have applied absent the
amendments. In addition, section 105(a)(2) of the FCAA amended section 1834A(b)(3) of the
Act regarding the phase-in of payment reductions to provide that payments may not be reduced
by more than 10 percent as compared to the amount established for the preceding year through
CY 2020, and for CYs 2021 through 2023, payment may not be reduced by more than 15 percent
as compared to the amount established for the preceding year. These statutory changes were
consistent with our regulations implementing the private payor rate-based CLFS at § 414.507(d)
(81 FR 41036).
331
https://www.cms.gov/medicare/payment/fee-schedules/clinical-laboratory-fee-schedule-clfs/pama-educational-
resources.
Subsequently, section 3718 of the Coronavirus Aid, Relief, and Economic Security Act,
2020 (CARES Act) (Pub. L. 116-136, March 27, 2020) further amended the data reporting
requirements for CDLTs that are not ADLTs and the phase-in of payment reductions under the
CLFS. Specifically, section 3718(a) of the CARES Act amended section 1834A(a)(1)(B) of the
Act to delay the next data reporting period for CDLTs that are not ADLTs by one additional
year, to require data reporting during the period of January 1, 2022, through March 31, 2022.
The CARES Act did not modify the data collection period that applied to the next data reporting
period for these tests. Thus, under section 1834A(a)(4)(B) of the Act, as amended by section
105(a)(1) of the FCAA, the next data reporting period for CDLTs that are not ADLTs would
have been based on the data collection period of January 1, 2019, through June 30, 2019.
Section 3718(b) of the CARES Act further amended the provisions in section
1834A(b)(3) of the Act regarding the phase-in of payment reductions under the CLFS. First, it
extended the statutory phase-in of payment reductions resulting from private payor rate
implementation by an additional year, that is, through CY 2024 instead of CY 2023. It further
amended section 1834A(b)(3)(B)(ii) of the Act to specify that the applicable percent for CY
2021 is 0 percent, meaning that the payment amount determined for a CDLT for CY 2021 shall
not result in any reduction in payment as compared to the payment amount for that test for CY
2020. Section 3718(b) of the CARES Act further amended section 1834A(b)(3)(B)(iii) of the
Act to state that the applicable percent of 15 percent would apply for CYs 2022 through 2024,
instead of CYs 2021 through 2023. In the CY 2021 PFS rulemaking (85 FR 50210 through
50211; 85 FR 84693 through 84694), in accordance with section 105(a) of the FCAA and section
3718 of the CARES Act, we proposed and finalized conforming changes to the data reporting
and payment requirements at 42 CFR part 414, subpart G.
Section 4 of the Protecting Medicare and American Farmers from Sequester Cuts Act
(PMAFSCA) (Pub. L. 117-71, December 10, 2021) made additional revisions to the CLFS
requirements for the next data reporting period for CDLTs that are not ADLTs and to the phase-
in of payment reductions under section 1834A of the Act. Specifically, section 4(b) of
PMAFSCA amended the data reporting requirements in section 1834A(a) of the Act to delay the
next data reporting period for CDLTs that are not ADLTs by 1 year, so that data reporting would
be required during the period of January 1, 2023, through March 31, 2023. The 3-year data
reporting cycle for CDLTs that are not ADLTs would resume after that data reporting period. As
amended by section 4 of PMAFSCA, section 1834A(a)(1)(B) of the Act provided that in the case
of reporting with respect to CDLTs that are not ADLTs, the Secretary shall revise the reporting
period under subparagraph (A) such that—(i) no reporting is required during the period
beginning January 1, 2020, and ending December 31, 2022; (ii) reporting is required during the
period beginning January 1, 2023, and ending March 31, 2023; and (iii) reporting is required
every 3 years after the period described in clause (ii).
Section 4 of PMAFSCA did not modify the data collection period that applies to the next
data reporting period for these tests. Thus, under section 1834A(a)(4)(B) of the Act, as amended
by section 105(a)(1) of the FCAA, the next data reporting period for CDLTs that are not ADLTs
(January 1, 2023, through March 31, 2023) would continue to be based on the data collection
period of January 1, 2019, through June 30, 2019, as defined in § 414.502.
Section 4 of PMAFSCA further amended the provisions in section 1834A(b)(3) of the
Act regarding the phase-in of payment reductions under the CLFS. First, it extended the
statutory phase-in of payment reductions resulting from private payor rate implementation by an
additional year, that is, through CY 2025. It further amended section 1834A(b)(3)(B)(ii) of the
Act to specify that the applicable percent for each of CY 2021 and 2022 is 0 percent, meaning
that the payment amount determined for a CDLT for CY 2021 and 2022 shall not result in any
reduction in payment as compared to the payment amount for that test for CY 2020. Section 4(a)
of PMAFSCA further amended section 1834A(b)(3)(B)(iii) of the Act to state that the applicable
percent of 15 percent would apply for CYs 2023 through 2025, instead of CYs 2022 through
2024. In the CY 2023 PFS rulemaking (87 FR 46068 through 46070; 87 FR 69741 through
69744, 70225), in accordance with section 4 of PMAFSCA, we proposed and finalized
conforming changes to the data reporting and payment requirements at 42 CFR part 414, subpart
G.
Section 4114 of the Consolidated Appropriations Act, 2023 (CAA, 2023) (Pub. L. 117-
328, December 29, 2022) made further revisions to the CLFS requirements for the next data
reporting period for CDLTs that are not ADLTs and to the phase-in of payment reductions under
section 1834A of the Act. Specifically, section 4114(b) of the CAA, 2023 amended the data
reporting requirements in section 1834A(a)(1)(B) of the Act to delay the next data reporting
period for CDLTs that are not ADLTs by 1 year, so that data reporting would be required during
the period of January 1, 2024, through March 31, 2024, instead of the data reporting period of
January 1, 2023, through March 31, 2023. The 3-year data reporting cycle for CDLTs that are
not ADLTs would resume after that data reporting period. As amended by section 4114(b) of the
CAA, 2023, section 1834A(a)(1)(B) of the Act now provides that in the case of reporting with
respect to CDLTs that are not ADLTs, the Secretary shall revise the reporting period under
subparagraph (A) such that—(i) no reporting is required during the period beginning January 1,
2020, and ending December 31, 2023; (ii) reporting is required during the period beginning
January 1, 2024, and ending March 31, 2024; and (iii) reporting is required every 3 years after
the period described in clause (ii).
Section 4114 of the CAA, 2023 did not modify the data collection period that applies to
the next data reporting period for CDLTs. Thus, under section 1834A(a)(4)(B) of the Act, the
next data reporting period for CDLTs that are not ADLTs (January 1, 2024, through March 31,
2024) would continue to be based on the data collection period of January 1, 2019, through June
30, 2019, as reflected in the definitions of data collection period and data reporting period at §
414.502.
Section 4114(a) of the CAA, 2023 further amended the provisions in section 1834A(b)(3)
of the Act regarding the phase-in of payment reductions under the CLFS. First, it extended the
statutory phase-in of payment reductions resulting from private payor rate implementation by an
additional year, that is, through CY 2026. It further amended section 1834A(b)(3)(B)(ii) of the
Act to specify that the applicable percent for CY 2023 is 0 percent, meaning that the payment
amount determined for a CDLT for CY 2023 shall not result in any reduction in payment as
compared to the payment amount for that test for CY 2022. Section 4114(a) of the CAA, 2023
further amended section 1834A(b)(3)(B)(iii) of the Act to state that the applicable percent of 15
percent will apply for CYs 2024 through 2026, instead of CYs 2023 through 2025. In the CY
2024 PFS rulemaking (88 FR 79083 through 79087; 88 FR 79531), in accordance with section
4114 of the CAA, 2023, we proposed and finalized conforming changes to the data reporting and
payment requirements at 42 CFR part 414, subpart G.
4. Additional Statutory Revisions to the Data Reporting Period and Phase-In of Payment
Reductions
On November 17, 2023, section 502 of the Further Continuing Appropriations and Other
Extensions Act, 2024 (Pub. L. 118-22) (FCAOEA, 2024) was passed and delayed data reporting
requirements for CDLTs that are not ADLTs, and it also delayed the phase-in of payment
reductions under the CLFS from private payor rate implementation under section 1834A of the
Act. Specifically, section 502(b) of the FCAOEA, 2024 amended the data reporting
requirements in section 1834A(a)(1)(B) of the Act to delay the next data reporting period for
CDLTs that are not ADLTs by one year, so that data reporting would be required during the
period of January 1, 2025, through March 31, 2025, instead of the data reporting period of
January 1, 2024, through March 31, 2024. The 3-year data reporting cycle for CDLTs that are
not ADLTs would resume after that data reporting period. As amended by section 502(b) of the
FCAOEA, 2024, section 1834A(a)(1)(B) of the Act now provides that in the case of reporting
with respect to CDLTs that are not ADLTs, the Secretary shall revise the reporting period under
subparagraph (A) such that—(i) no reporting is required during the period beginning January 1,
2020, and ending December 31, 2024; (ii) reporting is required during the period beginning
January 1, 2025, and ending March 31, 2025; and (iii) reporting is required every 3 years after
the period described in clause (ii).
Section 502 of the FCAOEA, 2024 does not modify the data collection period that
applies to the next data reporting period for these tests. Thus, under section 1834A(a)(4)(B) of
the Act, the next data reporting period for CDLTs that are not ADLTs (January 1, 2025, through
March 31, 2025) will continue to be based on the data collection period of January 1, 2019,
through June 30, 2019, as reflected in the definitions of data collection period and data reporting
period at § 414.502.
Section 502(a) of the FCAOEA, 2024 further amends the provisions in section
1834A(b)(3) of the Act regarding the phase-in of payment reductions under the CLFS. First, it
extends the statutory phase-in of payment reductions resulting from private payor rate
implementation by an additional year, that is, through CY 2027. It further amends section
1834A(b)(3)(B)(ii) of the Act to specify that the applicable percent for CY 2024 is 0 percent,
meaning that the payment amount determined for a CDLT for CY 2024 shall not result in any
reduction in payment as compared to the payment amount for that test for CY 2023. Section
502(a) of the FCAOEA, 2024 further amends section 1834A(b)(3)(B)(iii) of the Act to state that
the applicable percent of 15 percent will apply for CYs 2025 through 2027.
As a result of the statutory revisions under the FCAA, CARES Act, PMAFSCA, the
CAA, 2023, and the FCAOEA, 2024, there have only been two data collection periods for
CDLTs that are not ADLTs to date. The first data collection period for these tests occurred from
January 1, 2016, through June 30, 2016, and the second occurred from January 1, 2019, through
June 30, 2019. Thus far, there has been only one data reporting period for these tests, which took
place from January 1, 2017, through March 31, 2017. We have established CLFS payment rates
for these tests using the methodology established in PAMA only one time, effective January 1,
2018, based on the applicable information collected by applicable laboratories during the 2016
data collection period and reported to CMS during the 2017 data reporting period.
Additionally, we have applied the phase-in of payment reductions for the first 3 years of
PAMA implementation, CY 2018 through CY 2020, whereby reduction of payment rates could
not be more than 10 percent per year as compared to the amount established the prior year.
However, the phase-in of payment reductions set forth in PAMA for years 4 through 6 after
PAMA implementation, whereby payment cannot exceed 15 percent per year as compared to the
amount established the prior year, has not yet occurred.
5. Proposed Conforming Regulatory Changes
In accordance with section 502 of the FCAOEA, 2024, we are proposing to make
conforming changes to the data reporting and payment requirements at 42 CFR part 414, subpart
G. Specifically, we are proposing to revise the definitions of both the “data collection period”
and “data reporting period” at § 414.502 to specify that for the data reporting period of January
1, 2025, through March 31, 2025, the data collection period is January 1, 2019, through June 30,
2019. We are also proposing to revise § 414.504(a)(1) to indicate that initially, data reporting
begins January 1, 2017, and is required every 3 years beginning January 2025. In addition, we
are proposing to make conforming changes to our requirements for the phase-in of payment
reductions to reflect the amendments in section 502(a) of the FCAOEA, 2024. Specifically, we
are proposing to revise § 414.507(d) to indicate that for CY 2024, payment may not be reduced
by more than 0.0 percent as compared to the amount established for CY 2023, and for CYs 2025
through 2027, payment may not be reduced by more than 15 percent as compared to the amount
established for the preceding year.
We note that the CYs 2024 and 2025 CLFS payment rates for CDLTs that are not ADLTs
are based on applicable information collected in the data collection period of January 1, 2016,
through June 30, 2016. Under current law, the CLFS payment rates for CY 2026 through CY
2028 will be based on applicable information collected during the data collection period of
January 1, 2019, through June 30, 2019, and reported to CMS during the data reporting period of
January 1, 2025, through March 31, 2025.

E. Medicare Diabetes Prevention Program (MDPP)
The Centers for Medicare & Medicaid Services’ (CMS) Medicare Diabetes Prevention
Program Expanded Model (hereafter, “MDPP” or “MDPP expanded model”) is an evidence-
based behavioral intervention that aims to prevent or delay the onset of type 2 diabetes for
eligible Medicare beneficiaries diagnosed with prediabetes. MDPP is an expansion in duration
and scope of the Diabetes Prevention Program (DPP) model test, which was initially tested by
CMS through a Round One Health Care Innovation Award (2012-2016).
332
MDPP was
established in 2017 as an “additional preventive service,”
333
covered by Medicare and not subject
to beneficiary cost-sharing, in addition to being available once per lifetime to eligible
beneficiaries. To facilitate delivery of MDPP in a non-clinical community setting (to align with
the certified DPP model tested by The CMS Innovation Center), CMS created a new MDPP
supplier type through rulemaking in the CY 2017 PFS final rule (81 FR 80471), in addition to
requiring organizations that wish to participate in MDPP to enroll in Medicare separately, even if
they are already enrolled in Medicare for other purposes.
MDPP is a non-pharmacological behavioral intervention consisting of up to 22 intensive
sessions furnished over 12 months by a trained Coach who provides training on topics that
include long-term dietary change, increased physical activity, and behavior change strategies for
weight control and diabetes risk reduction. MDPP sessions must be one hour in length and
adhere to a Centers for Disease Control and Prevention (CDC) approved National Diabetes
Prevention Recognition Program (National DPP) curriculum.
334
The primary goal of the MDPP
expanded model is to help Medicare beneficiaries reduce their risk for developing type 2 diabetes
by achieving at least 5 percent weight loss from the first core session (81 FR 80465).
332
The Health Care Innovation Awards funds awards to organizations that implemented the most compelling new
ideas to deliver better health, improved care, and lower costs to people enrolled in Medicare, Medicaid and
Children's Health Insurance Program (CHIP), particularly those with the highest health care needs. The CMS
Innovation Center announced the first batch of awardees for the Health Care Innovation Awards on May 8, 2012,
and the second (final) batch on June 15, 2012. For more, see https://www.cms.gov/priorities/innovation/innovation-
models/health-care-innovation-awards.
333
42 CFR 410.64 - Additional preventive services.
334
https://www.cdc.gov/diabetes/prevention/resources/curriculum.html.

Eligible organizations seeking to furnish MDPP began enrolling in Medicare as MDPP
suppliers on January 1, 2018, and began furnishing MDPP on April 1, 2018. As of May 13,
2024, there were 301 approved MDPP suppliers.
335
The most recent MDPP evaluation report,
reflected that between April 2018 and December 31, 2021, 4,848 Medicare beneficiaries
participated in MDPP, including 2,325 FFS beneficiaries and 2,523 MA beneficiaries.
336
Through the Diabetes Prevention Recognition Program (DPRP), CDC administers a national
quality assurance program recognizing eligible organizations that furnish the National DPP
through its evidence based DPRP Standards,
337
which are updated every 3 years. The CDC
established the DPRP in 2012 and possesses significant experience assessing the quality of
program delivery by organizations throughout the United States, applying a comprehensive set of
national quality standards. For further information on the DPP model test,
338
the CDC’s National
DPP,
339
and DPRP Standards,
340
please refer to the CY 2017 (81 FR 80471) and CY 2018 PFS
(82 FR 52976) final rules and related websites.
The Public Health Emergency (PHE) for COVID-19 prompted changes to allow virtual
delivery of the MDPP, among other changes (85 FR 84830 through 84841). Changes to MDPP
in the CY 2024 PFS final rule (88 FR 78818) included a simplified payment structure to allow
for fee-for-service (FFS) payments for beneficiary attendance while retaining the performance-
based payments for diabetes risk reduction (that is, weight loss). Beginning January 1, 2024,
payments are made to an MDPP supplier if an MDPP beneficiary attends any core session in the
first 6 months or core maintenance session in the second 6 months, allowing payment for up to
22 sessions in a 12-month timeframe. The CY 2024 PFS final rule also extended certain PHE
335
Medicare Provider Enrollment, Chain, and Ownership System (PECOS). Unpublished data.
336
RTI International. Evaluation of the Medicare Diabetes Prevention Program. November 2022.
https://www.cms.gov/priorities/innovation/data-and-reports/2022/mdpp-2ndannevalrpt.
337
Centers for Disease Control and Prevention Diabetes Prevention Recognition Program. Standards and Operating
Procedures. Requirements for CDC Recognition. June 2024. https://nationaldppcsc.cdc.gov/s/article/DPRP-
Standards-and-Operating-Procedures.
338
Health Care Innovation Awards. https://www.cms.gov/priorities/innovation/innovation-models/health-care-
innovation-awards.
339
https://www.cdc.gov/diabetes/prevention/index.html.
340
https://www.cdc.gov/diabetes/prevention/pdf/dprp-standards.pdf.

flexibilities, including the option to deliver some or all MDPP sessions via distance learning and
for beneficiaries to virtually self-report weight for MDPP distance learning sessions, until
December 31, 2027 (88 FR 79241).
CDC released the 2024 DPRP Standards
341
to replace the 2021 DPRP Standards in June
2024. To align MDPP with the 2024 CDC DPRP Standards, we are proposing conforming
changes to align with CDC delivery modes. These changes are expected to reduce administrative
burden, ensure compliance with existing MDPP regulations, and streamline data reporting for
MDPP suppliers. In this year’s rule, we are also proposing an additional option for self-reporting
weight in an MDPP distance learning session, removing the MDPP bridge payment, and making
minor edits to align current rule language pertaining to MDPP with previous rulemaking.
1. Proposed Changes to § 410.79 by amending paragraphs (b) and (d)(1)
We established MDPP as an expanded model in 2018 based on a Health Care Innovation
Award (HCIA) to the National Young Men’s Christian Association (YMCA) of the USA (Y-
USA), who tested the CDC’s National DPP in the Medicare population through their network of
YMCAs in multiple U.S. markets (DPP model test).
342
The DPP model test successfully met
statutory criteria for model expansion,
343
demonstrating 5 percent weight loss from their starting
weight by participants (a key metric of the program’s success) along with statistically significant
reductions in Medicare spending, emergency department (ED) visits, and inpatient stays.
344
The
MDPP expanded model was implemented through the rulemaking process in two phases, in the
341
Centers for Disease Control and Prevention Diabetes Prevention Recognition Program. Standards and Operating
Procedures. Requirements for CDC Recognition. June 2024. https://nationaldppcsc.cdc.gov/s/article/DPRP-
Standards-and-Operating-Procedures.
342
L Hinnant, S Razi, R Lewis, A Sun, M Alva, T Hoerger et al. Evaluation of the Health Care Innovation Awards:
Community Resource Planning, Prevention, and Monitoring, Annual Report 2015. RTI International. March 2016;
https://www.cms.gov/priorities/innovation/files/reports/hcia-ymcadpp-evalrpt.pdf.
343
Paul Spitalnic. Certification of Medicare Diabetes Prevention Program. Mar. 14, 2016.
https://www.cms.gov/Research-Statistics-Data-and-Systems/Research/ActuarialStudies/Downloads/Diabetes-
Prevention-Certification-2016-03-14.pdf.
344
Rojas Smith. L., Amico, P., Hoerger, T. J., Jacobs, S., Payne. J., & Renaud, J.: Evaluation of the Health Care
Innovation Awards: Community Resource Planning, Prevention, and Monitoring Third Annual Report Addendum
— August 2017 https://downloads.cms.gov/files/cmmi/hcia-crppm-thirdannrptaddendum.pdf (pp. 858-914).

CY 2017 PFS (81 FR 80459 through 80483) and CY 2018 PFS final rules (82 FR 53234 through
53339).
MDPP went into effect in 2018, with supplier enrollment starting January 1, 2018, and
beneficiary enrollment starting April 1, 2018 (82 FR 53237). After nearly 6 years of
implementation, through the CY 2024 PFS final rule, we finalized updates to MDPP based on
lessons learned since the expanded model’s launch, including updates to definitions and the core
services period and extended the flexibilities allowed under the PHE for COVID-19 for a period
of 4 years (88 FR 79241).
This year we propose to make conforming changes to § 410.79(b), Conditions of
Coverage, to align with the 2024 CDC DPRP Standards.
345
In the CY 2018 PFS final rule,
we
stated our intention to align MDPP with CDC DPRP Standards whenever possible (82 FR
53245). Several commenters encouraged CMS to consider adopting the same definitions for
MDPP as CDC uses for the National DPP, including distance learning, online, and combination
modalities to better align MDPP and the National DPP. Commenters indicated that the addition
of definitions that are consistent with the CDC’s definitions will reduce confusion about MDPP
(88 FR 79247). To increase this alignment, we worked closely with CDC to update the National
DPP and MDPP for CY 2024 final rule (88 FR 79240 through 79256), as well as the 2024 DPRP
Standards.
346
We agree in aligning terminology where applicable.
The CY 2024 PFS final rule introduced and defined “distance learning” and
“combination delivery” for MDPP and provided a definition for “online delivery” (88 FR
79243). The 2024 CDC DPRP Standards include the following delivery modes with definitions:
"in-person,” “distance learning (live),” “in-person with a distance learning component,” “online
345
Centers for Disease Control and Prevention Diabetes Prevention Recognition Program. Standards and Operating
Procedures. Requirements for CDC Recognition. June 2024. https://nationaldppcsc.cdc.gov/s/article/DPRP-
Standards-and-Operating-Procedures.
346
Centers for Disease Control and Prevention Diabetes Prevention Recognition Program. Standards and Operating
Procedures. Requirements for CDC Recognition. June 2024. https://nationaldppcsc.cdc.gov/s/article/DPRP-
Standards-and-Operating-Procedures.

(non-live),” and “combination with an online component.”
347
These delivery modes also serve as
organization codes for CDC DPRP recognition. Through this proposed rule, we are proposing to
amend § 410.79(b) to add a new term for MDPP, “in-person with a distance learning
component,” defined as “MDPP sessions that are delivered in person by trained Coaches where
participants have the option of attending sessions via MDPP distance learning. These sessions
must be furnished in a manner consistent with DPRP Standards for in-person and distance
learning sessions.” The following examples of an acceptable delivery model for the “in-person
with a distance learning component” delivery mode are provided in the 2024 CDC DPRP
Standards: a combination of in-person and distance learning during the core (first 6 months) and
core maintenance (second 6 months) phases; some participants within a cohort using the in-
person delivery mode and some participants using the distance learning delivery mode; or
participants choosing from session to session which mode (in-person or distance learning) they
wish to use.
348
To further align with 2024 CDC DPRP Standards, we also propose to add a new term at
§ 410.79(b), “combination with an online component,” defined as “sessions that are delivered as
a combination of online (non-live) with in-person or distance learning. These sessions must be
furnished in a manner consistent with the DPRP Standards for the modality being used.”
Furthermore, we propose to remove the “combination delivery” term from § 410.79(b), which
was added in the CY 2024 PFS final rule (88 FR 79241) and is defined as “MDPP sessions that
are delivered by trained Coaches and are furnished in a manner consistent with the DPRP
Standards for distance learning and in-person sessions for each individual participant.” We
believe that the MDPP “combination delivery” term and definition are no longer needed with the
347
Centers for Disease Control and Prevention Diabetes Prevention Recognition Program. Standards and Operating
Procedures. Requirements for CDC Recognition. June 2024. https://nationaldppcsc.cdc.gov/s/article/DPRP-
Standards-and-Operating-Procedures.
348
Centers for Disease Control and Prevention Diabetes Prevention Recognition Program. Standards and Operating
Procedures. Requirements for CDC Recognition. June 2024. https://nationaldppcsc.cdc.gov/s/article/DPRP-
Standards-and-Operating-Procedures.

addition of “in-person with a distance learning component,” which includes any combination of
in-person and distance learning sessions.
Lastly, we are proposing to modify the current term and definition for “online delivery”
at § 410.79(b), also added by the CY 2024 PFS final rule (88 FR 79241), to align with the 2024
CDC DPRP Standards.
349
First, we are proposing to update the term from “online delivery” to
“online” to align with both the MDPP “distance learning” term and CDC DPRP “online (non-
live)” term. We propose to revise the definition for the MDPP “online” delivery mode to provide
that sessions that are delivered one hundred percent (100%) through the internet via phone,
tablet, or laptop in an asynchronous (non-live) classroom where participants are experiencing the
content on their own time without a live (including non-artificial intelligence (AI)) Coach
teaching the content. These sessions must be furnished in a manner consistent with the DPRP
Standards for online sessions. Live Coach interaction must be offered to each participant during
weeks when the participant has engaged with content. E-mails and text messages can count
toward the requirement for live Coach interaction if there is bi-directional communication
between the Coach and participant. Chat bots and AI forums do not count as live Coach
interaction. This modified definition adds the term “non-live” and further clarifies that Chat bots
and AI forums do not constitute live interaction.
In summary, we are revising the “online” definition and adding the “combination with an
online component” term and definition to help align terminology between MDPP and DPRP and
prevent confusion about acceptable CDC delivery modes for MDPP. We are confirming that
only MDPP “in-person,” “distance learning,” and “in-person with a distance learning
component” delivery modes, can be used during the extension of the flexibilities allowed under
the COVID–19 PHE, as finalized in the CY 2024 PFS final rule (88 FR 79241), not “online” nor
“combination with an online component” delivery modes.
349
Centers for Disease Control and Prevention Diabetes Prevention Recognition Program. Standards and Operating
Procedures. Requirements for CDC Recognition. June 2024. https://nationaldppcsc.cdc.gov/s/article/DPRP-
Standards-and-Operating-Procedures.

Furthermore, in the CY 2021 PFS final rule,
we established that virtual sessions
performed under flexibilities finalized in that rule could only be performed by MDPP suppliers
who offered in-person services (85 FR 84830). For the MDPP Extended flexibilities period, we
finalized in the CY 2024 PFS final rule to limit virtual delivery to the CDC DPRP definition of
‘‘distance learning’’ (88 FR 79243). We stated that the MDPP Extended flexibilities do not
include online delivery (or asynchronous virtual), as defined in the CDC DPRP Standards
through the ‘‘online’’ modality, including virtual make-up sessions (88 FR 79244). A make-up
session in MDPP was described in CY 2018 PFS final rule (82 FR 53241) and at § 410.79(a) as
“a core session or a core maintenance session furnished to an MDPP beneficiary when the
MDPP beneficiary misses a regularly scheduled core session or core maintenance session.” The
2024 CDC DPRP Standards allow for National DPP make-up sessions to be furnished using any
delivery mode, including online.
350
In alignment with the CY 2024 final rule, we are proposing
to amend § 410.79(d)(1) to clarify that MDPP make-up sessions can only be furnished using the
modalities permitted by the CY 2024 final rule for MDPP sessions: distance learning and in-
person delivery (88 FR 79243 through 79246). Specifically, we propose to add the following:
“MDPP make-up sessions may only use in-person or distance learning delivery.”
We are proposing to amend § 410.79(b) and (d)(1) and seek comment on these proposals.
2. Proposed Changes to § 410.79(e)(3)(iii)
As part of MDPP’s Emergency Policy finalized in the CY 2021 PFS final rule,
we
allowed for virtual weight collection (88 FR 79249). We summarized our policies for alternatives
to the requirement for in-person weight collection at Alternatives to the requirement for in-
person weight measurement (§ 410.79(e)(3)(iii)), which permit an MDPP supplier to obtain
weight measurements for MDPP beneficiaries for the baseline weight and any weight loss-based
performance achievement goals in the following manner: (1) via digital technology, such as
350
Centers for Disease Control and Prevention Diabetes Prevention Recognition Program. Standards and Operating
Procedures. Requirements for CDC Recognition. June 2024. https://nationaldppcsc.cdc.gov/s/article/DPRP-
Standards-and-Operating-Procedures.
scales that transmit weights securely via wireless or cellular transmission; or (2) via self-reported
weight measurements from the at-home digital scale of the MDPP beneficiary (88 FR 79243).
We stated that self-reported weights must be obtained during live, synchronous online video
technology, such as video chatting or video conferencing, wherein the MDPP Coach observes
the beneficiary weighing themselves and views the weight indicated on the at-home digital scale.
Alternatively, the MDPP beneficiary may self-report their weight by submitting to the MDPP
supplier a date-stamped photo or video recording of the beneficiary’s weight, with the
beneficiary visible in their home. The photo or video must clearly document the weight of the
MDPP beneficiary as it appears on the digital scale on the date associated with the billable
MDPP session. This flexibility has allowed suppliers to bill for MDPP beneficiaries achieving
weight loss performance goals.
Overall, commenters on the proposed MDPP Extended flexibilities in the CY 2024 PFS
rule were very supportive of CMS continuing to allow virtual weight collection (88 FR 79240
through 79256). However, we received several comments regarding barriers suppliers
experienced relating to virtual weight collection during the PHE for COVID-19. For example,
several commenters recommended that CMS no longer require date-stamped photos to document
the self-reported beneficiary weights (88 FR 79249). The commenters also reported that many of
their beneficiaries are unable to take a picture while standing on their home scales due to risk of
injury and physical health limitations. Commenters stated that this risk has prevented
organizations from submitting claims accurately, since they have several participants who live
alone and attend sessions via distance learning (88 FR 79249). We acknowledged in our
responses to these comments that some MDPP beneficiaries may lack the technology or capacity
to provide a date-stamped photograph to document their body weight measurements. We stated
that in situations in which beneficiaries may be unable to self-report their weight according to the
MDPP conditions of coverage, suppliers may want to consider collecting weight measurements
from the MDPP beneficiary in person.
We have continued to hear from MDPP suppliers and interested parties that the
requirement to submit a photo with both the beneficiary’s weight on the scale and the beneficiary
visible is not physically possible. This problem has become even more relevant in CY 2024 as
suppliers continue to expand distance learning to help reach beneficiaries in rural and
underserved areas, sometimes across state lines. We previously responded that for situations in
which beneficiaries may be unable to self-report their weight according to the MDPP conditions
of coverage, suppliers may want to consider collecting weight measurements from the MDPP
beneficiary in person (88 FR 79249). However, this may not be a practical option for
beneficiaries who have chosen distance learning based on not living within driving distance from
an MDPP supplier location. Therefore, we propose revising § 410.79(e)(3)(iii)(C) to provide that
self-reported weights must be obtained during live, synchronous online video technology, such
as video chatting or video conferencing, wherein the MDPP Coach observes the beneficiary
weighing themselves and views the weight indicated on the at-home digital scale, or the MDPP
supplier receives 2 (two) date-stamped photos or a video recording of the beneficiary's weight,
with the beneficiary visible on the scale, submitted by the MDPP beneficiary to the MDPP
supplier. Photo or video must clearly document the weight of the MDPP beneficiary as it appears
on their digital scale on the date associated with the billable MDPP session. If choosing to
submit 2 photos, one photo must show the beneficiary’s weight on the digital scale, the second
photo must show the beneficiary visible in their home, and both photos must be date-stamped.
Similar to options in paragraphs (e)(3)(iii)(A) and (B) in § 410.79, this revised option in
paragraph (e)(30(iii)(C) is only available for MDPP beneficiaries reporting their weight for an
MDPP distance learning session. We are continuing to require the date-stamp on both photos to
ensure program integrity in the virtual setting. We are proposing to amend § 410.79(e)(3)(iii).
We seek comment on these proposals.
Lastly, we finalized in the CY 2021 PFS final rule that the flexibilities under
§ 410.79(e)(3)(iii) and (iv) would only apply only to MDPP suppliers that have and maintain

CDC DPRP “in-person” recognition (85 FR 84831). In the CY 2024 PFS final rule, we extended
flexibilities allowed during the PHE for COVID-19 or 4 years, or through December 31, 2027
(88 FR 79241). We also confirmed that that the Extended flexibilities would continue to only
apply to MDPP suppliers that have and maintain CDC DPRP “in-person” recognition, and that
virtual only suppliers were not permitted to furnish the Set of MDPP services because MDPP
beneficiaries may elect to return to in-person services, and MDPP suppliers need to be able to
accommodate their request (88 FR 79248).
To reduce confusion as MDPP suppliers transition to the new CDC DPRP recognition for
“in-person with a distance learning component,” we are clarifying that MDPP suppliers can have
and maintain either CDC’s “in-person” or the new “in-person with a distance learning
component” CDC DPRP code. The 2024 CDC DPRP Standards, implemented in June 2024,
introduced and defined the new “in-person with a distance learning component” modality and
associated code.
351
This new modality and code for recognition include a combination of in-
person and distance learning delivery, which are both modalities currently permitted until
December 31, 2027 (88 FR 79241). The new MDPP term and definition for “in person with a
distance learning component” that we are proposing to align with the 2024 CDC DPRP
Standards will replace the current MDPP “combination delivery” term, which we are proposing
to remove in this rulemaking. Aligning terminology for delivery of MDPP that involves a
combination of in-person and distance learning delivery with the 2024 CDC DPRP Standards
would reduce administrative burden to MDPP suppliers and allow them to streamline CDC
DPRP data submission (that is, they will not have to submit data for two CDC organization
codes). MDPP suppliers will not be required to switch to this new code if they already have an
in-person code; it is only being made available for their convenience.
3. Proposed Changes to § 414.84(a), (c), (d), and (e)
351
Centers for Disease Control and Prevention Diabetes Prevention Recognition Program. Standards and Operating
Procedures. Requirements for CDC Recognition. June 2024. https://nationaldppcsc.cdc.gov/s/article/DPRP-
Standards-and-Operating-Procedures.
We further propose to amend § 414.84(a), (d), and (e) to remove the MDPP bridge
payment. This payment is no longer necessary in MDPP’s CY 2024 FFS payment structure for
attendance and could introduce the potential for fraud, waste, or abuse.
The CY 2017 PFS final rule confirmed that a beneficiary may change MDPP suppliers at
any time (81 FR 80470). The MDPP bridge payment was introduced in the CY 2018 PFS final
rule at § 414.84(a) and is defined as follows: “Bridge payment means a one-time payment to an
MDPP supplier for furnishing its first MDPP session to an MDPP beneficiary who has
previously received one or more MDPP services from a different MDPP supplier” (81 FR
80470). The CY 2018 PFS final rule specified that an MDPP supplier that had previously been
paid either a bridge payment or a performance payment for an MDPP beneficiary was not
eligible to be paid a bridge payment for that beneficiary, along with other conditions. An MDPP
supplier may only receive one bridge payment per MDPP beneficiary, however, there is no limit
on how many MDPP suppliers can receive a bridge payment for the same beneficiary (82 FR
53361).
The CY 2018 PFS final rule also noted that the MDPP bridge payment was intended to be
similar (that is, the same amount) to the payment for the first core session furnished by the
previous supplier and would be received only if the subsequent supplier did not furnish the first
core session to the MDPP beneficiary (82 FR 53361). In the performance-based payment
structure, the bridge payment was intended to prevent scenarios where subsequent MDPP
suppliers would receive no payment for sessions furnished to MDPP beneficiaries who changed
suppliers during the MDPP services period in the absence of the bridge payment. We stated that
the bridge payment was not intended to be a performance payment; rather, it would account for
the financial risk a subsequent MDPP supplier took on by furnishing services to a beneficiary
changing MDPP suppliers during the MDPP services period (82 FR 53293). However, such risk
is not applicable in an FFS payment structure.
Along with the performance payments for weight loss, the MDPP bridge payment was
retained in the CY 2024 Fee Schedule for MDPP (88 FR 79252). Currently, a subsequent MDPP
supplier can receive both an attendance payment and a bridge payment for the first session
attended by an MDPP beneficiary who switches suppliers. For example, in CY 2024, if a
beneficiary changed suppliers on MDPP session 8, the subsequent supplier could receive both
the attendance payment for session 8 ($25) and the bridge payment ($25). The bridge payment
for this beneficiary could only be received by this supplier once, but if the beneficiary changed
suppliers again (for example, on session 17), the new (second) subsequent supplier could also
receive the bridge payment in addition to the payment for session 17 ($25). This could continue
as many times as the beneficiary changed suppliers until they have the maximum of 22 sessions
paid, across all suppliers, with no maximum on the total number of bridge payments. In the CY
2018 PFS final rule, we noted some program integrity risk that organizations could coordinate to
bill multiple bridge payments that would ultimately increase total MDPP payments to separately
enrolled MDPP suppliers to serve the financial interests of the umbrella organization. This
scenario could occur if MDPP suppliers systematically encouraged beneficiaries to change
suppliers for the purpose of being paid the bridge payment (82 FR 53294). Due to these reasons,
we propose to amend § 414.84(a), (d), and (e) to remove reference to, and requirements of the
MDPP bridge payment. Per our Regulatory Impact Analysis, we expect removal of the MDPP
bridge payment to be budget neutral for the Medicare program. We seek comment on these
proposals.
Additionally, at § 414.84(c), facilitate Medicare Administrative Contractors (MACs) in
processing claims for same day make-up sessions in MDPP, we are proposing to require MDPP
suppliers to append an existing claim modifier to any claim for G9886 or G9887 that indicates a
make-up session that was held on the same day as a regularly scheduled MDPP session. The CY
2018 PFS final rule
permits an MDPP beneficiary to have one make-up session on the same day
as a regularly scheduled session and for a beneficiary to have one make-up session per week (82

FR 53360),
consistent with CDC DPRP Standards.
352
In the CY 2024 PFS final rule, we stated
that we wanted to encourage suppliers to schedule make-up sessions on days other than the same
day of a regularly scheduled session to avoid claims being rejected or denied under the new CY
2024 FFS payment schedule and to allow beneficiaries to receive the benefit as intended by
having access to the full 12 months MDPP service period to build the skills needed to reduce
their risk for diabetes (88 FR 79250).
However, since then, we have heard from MDPP suppliers that same day make-up
sessions are an essential flexibility that assist an MDPP beneficiary in staying on track with the
curriculum and their cohort after an MDPP beneficiary needs to miss a regularly scheduled
session. To help prevent potential claim rejections for duplicate services, we are proposing to
require MDPP suppliers to append a modifier to the applicable G-code for the second session
held on the same day as a regularly scheduled MDPP session. Specifically, we are proposing to
add § 414.84(c)(4), which states that “Current Procedural Terminology (CPT) Modifier 79
(repeat services by same physician) must be appended to any claim for G9886 or G9887 to
identify a MDPP make-up session that was held on the same day as a regularly scheduled MDPP
session.” We believe this new requirement would contribute minimal additional complexity to
the payment structure while creating a flexibility that would have value for the program,
particularly for beneficiaries in the core phase of MDPP who may not have transportation to 2 in-
person sessions in one week or have the flexibility to make time on more than one day per week
for a distance learning session. Additionally, we believe the existing limitation on one make-up
session per week would be sufficient to ensure program benefit because whether the make-up
session is held on the same day, or the next day would likely have minimal impact on program
duration and intensity. To clarify, we are proposing that the CPT Modifier 79 would only need to
be appended to the HCPCS code (G9886 or G9887) that identifies the session that included
352
Centers for Disease Control and Prevention Diabetes Prevention Recognition Program. Standards and Operating
Procedures. Requirements for CDC Recognition. June 2024. https://nationaldppcsc.cdc.gov/s/article/DPRP-
Standards-and-Operating-Procedures.
content from a previously held session that serves as a makeup session for the session the MDPP
beneficiary missed, which was held on the same day as a regularly scheduled MDPP session.
This modifier would not need to be included on claims for make-up sessions held on different
days than regularly scheduled MDPP sessions.
Lastly, with the removal of § 414.84(d), we are proposing to amend the current §
414.84(e) to be the new § 414.84(d). We are also removing from the new § 414.84(d) the
reference to updating the MDPP bridge payment, as the bridge payment has been proposed to be
removed from this CY 2025 Physician Fee Schedule rulemaking.
We also seek comment on these proposals.
4. Aligning language with previous rulemaking in §§ 410.79, 424.205, and 414.84
We are proposing minor edits throughout §§ 410.79, 424.205, and 414.84 to update
outdated references and align with previous rulemaking pertaining to MDPP terminology,
payment structure, and requirements. This includes updating references to the performance-based
payments for attendance and ongoing maintenance sessions, which were both removed from the
2024 MDPP Fee Schedule by the CY 2024 PFS final rule (88 FR 79252), as well as including
the clarification that suppliers can offer MDPP sessions via distance learning, a flexibility
extended by the CY 2024 PFS final rule (88 FR 79241), where applicable.
At § 410.79(b), we propose to update the definition for the “Set of MDPP services” to
remove the reference to “ongoing maintenance” sessions. All references to and requirements for
the MDPP “ongoing maintenance” phase were removed by the CY 2024 PFS finale rule (88 FR
79256). We are revising this definition to read: “Set of MDPP services means the series of
MDPP sessions, composed of core sessions and core maintenance sessions, and subject to
paragraph (c)(3) of this section offered over the course of the MDPP services period.”
We also propose § 410.79(e)(3)(iv)(F)(3) to state that no more than 12 virtual sessions
offered monthly during the ongoing maintenance session intervals, months 13 through 24 for
beneficiaries enrolled before January 1, 2022.

This proposed revision adds the date that the CY 2022 PFS final rule was effective,
which is the date when no more MDPP beneficiaries could enroll in ongoing maintenance
sessions (86 FR 65317).
At § 410.79(e)(3)(v)(F)(2), we propose to remove the reference to weight measurement at
an ongoing maintenance session, so the paragraph provides that for an MDPP beneficiary who
began receiving the Set of MDPP services on or after January 1, 2021, has suspended services
during an applicable 1135 waiver event, the MDPP supplier must use the baseline weight
recorded at the beneficiary's first core session.
At § 424.205(c)(10), we propose revision to specify in-person and distance learning
delivery for MDPP core and core maintenance sessions, to provide that, except as allowed under
§ 424.205(d)(8), the MDPP supplier must offer an MDPP beneficiary no fewer than all of the
following:
● 16 in-person or distance learning core sessions no more frequently than weekly for the
first 6 months of the MDPP services period, which begins on the date of attendance at the first
such core session.
● 1 in-person or distance learning core maintenance session each month during months 7
through 12 (6 months total) of the MDPP services period.
At § 424.205(f)(1)(ii), we propose to remove reference to the HICN, as Medicare is now
using Medicare Beneficiary Identifiers (MBIs),
353
to state: Basic beneficiary information for
each MDPP beneficiary in attendance, including but not limited to beneficiary name, MBI, and
age.
At § 424.205(f)(2)(i), we propose to replace “whether a core session, a core maintenance
session, an in-person make-up session, or a virtual make-up session” with the two currently
353
https://www.cms.gov/training-education/partner-outreach-resources/new-medicare-card/medical-beneficiary-
identifiers-mbis.
permitted types of sessions (that is, in-person and distance learning), to state: Documentation of
the type of session (in-person or distance learning).
At § 424.205(f)(5), we propose to remove the references to the MDPP performance-based
payments for attendance in paragraphs (f)(5)(i) and (ii) because these payments were removed in
the CY 2024 Fee Schedule for MDPP (88 FR 79252). In their place, we are adding references to
the performance payment for the required minimum 5 percent weight loss (82 FR 53289). We
also propose to correct the references to § 414.84(b), and also to remove the reference to the
ongoing maintenance sessions from 424.205(f)(5)(iv).
At § 414.84(b)(1), we propose to clarify that the performance payment for the required
minimum weight loss is made for 5 percent weight loss, as reflected in the CY 2024 Fee
Schedule (88 FR 79252), and can be made for a distance learning, as well as an in-person MDPP
session, as allowed by the COVID-19 PHE flexibilities (85 FR 84830 through 84841) and their
extension (88 FR 79241). Performance Goal 1 provides that it achieves the required minimum 5-
percent weight loss. CMS makes a performance payment to an MDPP supplier for an MDPP
beneficiary who achieves the required minimum weight loss as measured in-person or during a
distance learning session during a core session or core maintenance session furnished by that
supplier.
Similarly, we propose to revise § 414.84(b)(2) for 9 percent weight loss. Performance
Goal 2 provides that it achieves 9-percent weight loss. CMS makes a performance payment to an
MDPP supplier for an MDPP beneficiary who achieves at least a 9-percent weight loss as
measured in-person or in a distance learning session during a core session or core maintenance
session furnished by that supplier.
We seek comment on these proposals.
F. Modifications Related to Medicare Coverage for Opioid Use Disorder (OUD) Treatment
Services Furnished by Opioid Treatment Programs (OTPs)
1. Background
Section 2005 of the Substance Use-Disorder Prevention that Promotes Opioid Recovery
and Treatment (SUPPORT) for Patients and Communities Act (SUPPORT Act) (Pub. L. 115-
271, October 24, 2018) established a new Medicare Part B benefit for OUD treatment services
furnished by OTPs during an episode of care beginning on or after January 1, 2020. In the CY
2020 PFS final rule (84 FR 62630 through 62677 and 84 FR 62919 through 62926), we
implemented Medicare coverage and provider enrollment requirements and established a
methodology for determining the bundled payments for episodes of care for the treatment of
OUD furnished by OTPs. We also established in the CY 2020 PFS final rule new codes and
finalized bundled payments for weekly episodes of care that include methadone, oral
buprenorphine, implantable buprenorphine, injectable buprenorphine or naltrexone, and non-
drug episodes of care, as well as add-on codes for intake and periodic assessments, take-home
dosages for methadone and oral buprenorphine, and additional counseling.
Since the CY 2020 PFS final rule, we have made several refinements and expansions to
services covered under the Medicare OTP benefit. Specifically, we adopted new add-on codes
for take home supplies of nasal naloxone and injectable naloxone (85 FR 84683 through 84692)
in the CY 2021 PFS final rule, and a new add-on code and payment for a higher dose of nasal
naloxone (86 FR 65340 and 65341) in the CY 2022 PFS final rule. We have also finalized
various telecommunications flexibilities, including: to allow OTPs to furnish individual and
group therapy and substance use counseling via two-way interactive audio-video
telecommunications (84 FR 62630 through 62677 and 84 FR 62919 through 62926) in the CY
2020 PFS final rule, and via audio-only telephone calls when audio-video telecommunications
are not available to the beneficiary (86 FR 65342) in the CY 2022 PFS final rule; to allow the
OTP intake add-on code to be furnished via two-way interactive audio-video
telecommunications when billed for the initiation of treatment with buprenorphine, and via
audio-only telecommunications when audio-video telecommunications are not available to the
beneficiary, to the extent that these technologies are authorized by the Drug Enforcement

Administration (DEA) and the Substance Abuse and Mental Health Services Administration
(SAMHSA) at the time the service is furnished (87 FR 69775 through 69777) in the CY 2024
final rule; and to allow periodic assessments to be furnished via two-way interactive audio-video
telecommunications as clinically appropriate (85 FR 84690) in the CY 2021 final rule. OTPs
may furnish these aforementioned services via telecommunications systems provided all other
applicable requirements are met. Additionally, for the purposes of the geographic adjustment, we
have clarified, in the CY 2023 final rule, that services furnished via OTP mobile units will be
treated as if the services were furnished in the physical location of the OTP for purposes of
determining payments to OTPs under the Medicare OTP bundled payment codes and/or add-on
codes, as long as services are medically reasonable and necessary and comply with SAMHSA
and DEA guidance (87 FR 69768 through 69777). Lastly, we have made a few changes to
various pricing methodologies under the OTP benefit in the 2023 PFS final rule, including:
revising our methodology for pricing the drug component of the methadone weekly bundle and
the add-on code for take-home supplies of methadone by using the Producer Price Index (PPI)
for Pharmaceuticals for Human Use (Prescription) to better reflect the changes in methadone
costs for OTPs over time (87 FR 69768 through 69777); and modifying the payment rate for
individual therapy in the non-drug component of the bundled payment to base the payment rate
on the rate for longer therapy sessions that better account for the greater severity of needs for
patients with an OUD (87 FR 69768 through 69777).
More recently, for CY 2024, CMS made further modifications and expansions to covered
services for the treatment of OUD by OTPs. In the CY 2024 PFS final rule (88 FR 79089
through 79093), CMS finalized an extension to allow periodic assessments to be furnished audio-
only through the end of CY 2024 when video is not available to the extent that use of audio-only
communications technology is permitted under the applicable SAMHSA and DEA requirements
at the time the service is furnished, and all other applicable requirements are met. In the final
rule, we noted that extending these flexibilities another year would allow CMS time to further

consider this issue, including whether periodic assessments should continue to be furnished using
audio-only communication technology following the end of CY 2024. Lastly, in the CY 2024
Outpatient Prospective Payment System (OPPS) final rule (88 FR 81845 through 81858), CMS
finalized an add-on code for intensive outpatient program (IOP) services furnished by OTPs for
the treatment of OUD and added a new paragraph (ix) in the definition of “Opioid disorder
treatment service” at § 410.67(b) to describe such services. We stated that Medicare would pay
for IOP services provided by OTPs if each service is medically reasonable and necessary and not
duplicative of any service paid for under any bundled payments billed for an episode of care in a
given week, and other applicable requirements are met. We believe that payment for IOP
services will improve continuity of care between different treatment settings and levels of care,
and further promote health equity for Medicare beneficiaries that may face barriers to accessing
treatment, such as racial/ethnic minorities and/or beneficiaries aged 65 or older. We continue to
monitor utilization of OUD treatment services furnished by OTPs to ensure that Medicare
beneficiaries have appropriate access to care. For CY 2025, we are proposing several
modifications to the policies governing Medicare coverage and payment for OUD treatment
services furnished by OTPs.
2. Telecommunication Flexibilities for Periodic Assessments and Initiation of Treatment with
Methadone
We have finalized several flexibilities for OTPs regarding the use of telecommunications,
both during the Public Health Emergency (PHE) for the Coronavirus Disease 2019 (COVID-19)
and outside of the PHE. In the CY 2020 PFS final rule, we finalized a policy allowing OTPs to
furnish substance use counseling and individual and group therapy via two-way interactive
audio-video communication technology. In the interim final rule with comment period (IFC)
entitled “Medicare and Medicaid Programs: Policy and Regulatory Revisions in Response to the
COVID-19 Public Health Emergency,” which appeared in the April 6, 2020 Federal Register
(85 FR 19258), we revised paragraphs (iii) and (iv) in the definition of opioid use disorder
treatment service at § 410.67(b) on an interim final basis to allow the therapy and counseling
portions of the weekly bundles, as well as the add-on code for additional counseling or therapy,
to be furnished using audio-only telephone calls rather than via two-way interactive audio-video
communication technology during the PHE for the COVID-19 if beneficiaries do not have access
to two-way audio-video communications technology, provided all other applicable requirements
are met. In the CY 2022 PFS final rule (86 FR 65341 through 65343), we finalized that after the
conclusion of the PHE for COVID-19, OTPs are permitted to furnish substance use counseling
and individual and group therapy via audio-only telephone calls when audio and video
communication technology is not available to the beneficiary. As we explained in the CY 2022
PFS final rule (86 FR 65342), we interpret the requirement that audio/video technology is “not
available to the beneficiary” to include circumstances in which the beneficiary is not capable of
or has not consented to the use of devices that permit a two-way, audio/video interaction because
in each of these instances audio/video communication technology is not able to be used in
furnishing services to the beneficiary. In the CY 2023 PFS final rule (87 FR 69775 through
69777), we further extended telecommunication flexibilities for the initiation of treatment with
buprenorphine outside of the PHE for COVID-19 in paragraph (vi) in the definition of opioid use
disorder treatment service at § 410.67(b). Specifically, we allowed the OTP intake add-on code
to be furnished via two-way, audio-video communications technology when billed for the
initiation of treatment with buprenorphine, to the extent that the use of audio-video
telecommunications technology to initiate treatment with buprenorphine is authorized by DEA
and SAMHSA at the time the service is furnished. We also permitted the use of audio-only
communication technology to initiate treatment with buprenorphine in cases where audio-video
technology is not available to the beneficiary, provided all other applicable requirements are met.
a. Proposal to Allow Periodic Assessments to be Furnished via Audio-only Telecommunications
on a Permanent Basis
In recent years, we have finalized several telecommunication flexibilities for periodic
assessments furnished by OTPs. In the IFC entitled “Medicare and Medicaid Programs, Basic
Health Program, and Exchanges; Additional Policy and Regulatory Revisions in Response to the
COVID-19 Public Health Emergency and Delay of Certain Reporting Requirements for the
Skilled Nursing Facility Quality Reporting Program,” which appeared in the May 8, 2020
Federal Register (85 FR 27558), we revised paragraph (vii) in the definition of “Opioid use
disorder treatment service” at § 410.67(b) on an interim final basis to allow periodic assessments
to be furnished during the PHE for COVID-19 via two-way interactive audio-video
telecommunication technology and, in cases where beneficiaries do not have access to two-way
audio-video communication technology, to permit the periodic assessments to be furnished using
audio-only telephone calls rather than via two-way interactive audio-video communication
technology, provided all other applicable requirements are met. In the CY 2021 PFS final rule
(85 FR 84690), we finalized our proposal to revise paragraph (vii) in the definition of “Opioid
use disorder treatment service” at § 410.67(b) to provide that periodic assessments (HCPCS code
G2077) must be furnished during a face-to-face encounter, which includes services furnished via
two-way interactive audio-video communication technology, as clinically appropriate, provided
all other applicable requirements are met, on a permanent basis.
Furthermore, in the CY 2023 PFS proposed rule (87 FR 46093), we sought comment on
whether we should allow periodic assessments to continue to be furnished using audio-only
communication technology following the end of the PHE for COVID-19 for patients who are
receiving treatment via buprenorphine, and if this flexibility should also continue to apply to
patients receiving methadone or naltrexone. In response, several commenters advocated for CMS
to continue to allow periodic assessments to be furnished audio-only when video is not available
after the end of the PHE. Commenters highlighted that allowing audio-only flexibilities would
further promote health equity for individuals who are economically disadvantaged, live in rural
areas, are members of racial and ethnic minorities, lack access to reliable broadband or internet

access, or do not possess devices with video capability. Commenters also indicated that periodic
assessments are no less complex than intake/initial assessments, and thus are equally appropriate
for audio-video and audio-only care, and that permitting audio-only flexibilities would allow an
opportunity for both the provider and patient to jointly determine that the patient would
individually benefit from telehealth services. After considering these comments, we determined
that it would be appropriate to allow periodic assessments to be furnished audio-only when video
is not available through the end of CY 2023, to the extent that it is authorized by SAMHSA and
DEA at the time the service is furnished and, in a manner consistent with all applicable
requirements. We stated our belief that this modification would allow continued beneficiary
access to these services for the duration of CY 2023 in the event the PHE terminated before the
end of 2023 and that it would also grant additional time for CMS to further consider
telecommunication flexibilities associated with periodic assessments.
Moreover, section 4113 of Division FF, Title IV, Subtitle A of the Consolidated
Appropriations Act, 2023 (CAA, 2023) (Pub. L. 117-328, December 29, 2022) extended the
telehealth flexibilities enacted in the Consolidated Appropriations Act, 2022 (CAA, 2022) (Pub.
L. 117-103, March 15, 2022). Specifically, it amended sections 1834(m), 1834(o), and 1834(y)
of the Act to delay the requirement for an in-person visit prior to furnishing certain mental health
services via telecommunications technology by physicians and other practitioners, Rural Health
Clinics (RHCs), and Federally Qualified Health Centers (FQHCs) until dates of service on or
after January 1, 2025, if the PHE for COVID-19 had ended prior to that date. Additionally, it
extended the flexibilities that were available during the PHE that allowed for certain Medicare
telehealth services defined in section 1834(m)(4)(F)(i) of the Act to be furnished via an audio-
only telecommunications system through December 31, 2024, if the PHE for COVID-19 had
ended prior to that date. The PHE for COVID-19, which was declared under section 319 of the
Public Health Service Act, expired at the end of the day on May 11, 2023, so the aforementioned
flexibilities were extended through the end of CY 2024.

To better align coverage for periodic assessments furnished by OTPs with the telehealth
flexibilities described in section 4113 of the CAA, 2023 for other settings under Medicare, in the
CY 2024 PFS final rule (88 FR 79089 through 79093; 79528), we finalized extending the audio-
only flexibilities for periodic assessments furnished by OTPs through the end of CY 2024 in
paragraph (vii) in the definition of “Opioid use disorder treatment service” at § 410.67(b). We
finalized to allow periodic assessments to be furnished audio-only when video is not available to
the extent that use of audio-only communications technology is permitted under the applicable
SAMHSA and DEA requirements at the time the service is furnished, and all other applicable
requirements are met. In submitted comments supporting the proposal, commenters reiterated
evidence showing that audio-only telehealth encounters are more prominent among individuals
who are older, Black, Hispanic, American Indian/Alaska Native, Spanish-speaking, living in
areas with low broadband access, low-income, and with public insurance, suggesting that the
proposal would have positive health equity implications for these populations.
354
Several other
commenters raised that audio-only flexibilities are important since many underserved
populations may experience challenges in partaking in video-based telehealth services, due to not
possessing the needed technological proficiencies to operate video-based services, not having a
caregiver able to assist them with appointments, feeling discomfort with the use of video, and
because of the cost of high-speed internet and data required for video technologies. Several other
commenters shared evidence that audio-only visits produce many of the same benefits as video-
based visits,
355
and that patients often report that audio-only visits left them feeling supported
and with greater privacy, provided increased access to behavioral health professionals, and
354
J.A. Rodriguez et al., “Differences in the Use of Telephone and Video Telemedicine Visits During the COVID-19 Pandemic,”
The American Journal of Managed Care 27, no. 1 (2021), https://www.ajmc.com/view/differences-in- the-use-of-telephone-and-
video-telemedicine- visits-during-the-covid-19-pandemic; R.P. Pierce and J.J. Stevermer, “Disparities in Use of Telehealth at the
Onset of the COVID-19 Public Health Emergency,” Journal of Telemedicine and Telecare (2020): 1-7,
https://doi.org/10.1177/1357633X20963893; J.E. Chang et al., “Patient Characteristics Associated with Phone Versus Video
Telemedicine Visits for Substance Use Treatment During COVID-19,” J Addict Med 16, no. 6 (2022): 659-65; C. Shoff, T-C
Yang, B.A. Shaw, “Trends in Opioid Use Disorder Among Older Adults: Analyzing Medicare Data, 2013–2018,” American
Journal of Preventive Medicine 60, no.6 (2021): 850-855, https://doi.org/10.1016/j.amepre.2021.01.010.
355
Danila, M.I., Sun, D., Jackson, L.E., Cutter, G., Jackson, E.A., Ford, E.W., DeLaney, E., Mudano, A., Foster, P.J., Rosas, G.,
Melnick, J.A, Curtis, J.R., & Saag, K.G. (2022, November). “Satisfaction with modes of telemedicine delivery during COVID-
19: A randomized, single-blind, parallel group, noninferiority trial.” The American Journal of the Medical Sciences, 364 (5).

helped reduce transportation barriers.
356
Lastly, a large number of commenters requested that
CMS make the extension for audio-only periodic assessments permanent beyond CY 2024.
Commenters stated that extending this policy permanently would retain a beneficiary’s right to
decide with their provider how best to receive their care and would curtail existing barriers that
Medicare beneficiaries with an OUD may face in accessing care. In response to these comments
that requested indefinitely extending these audio-only flexibilities for periodic assessments, CMS
stated that extending these flexibilities for one additional year at the time would allow the agency
time to further examine the issue, including to understand if a permanent extension would be
appropriate for patients who are receiving treatment via buprenorphine, methadone, and/or
naltrexone at OTPs, and whether proper safeguards are in place so these services can be
delivered in a way that would not diminish safety or quality of care for Medicare beneficiaries
with an OUD.
We continue to monitor the services provided under the OTP benefit to ensure
flexibilities for OUD treatment services are consistent with flexibilities authorized in other
settings under Medicare, as medically reasonable and necessary for the diagnosis and treatment
of OUD. In the CY 2022 PFS final rule, we revised the regulatory definition of “interactive
telecommunications system” at § 410.78(a)(3) for Medicare Telehealth services paid under the
PFS beyond the termination of the PHE for COVID-19 to allow for inclusion of audio-only
services under certain circumstances. Specifically, we redefined “interactive telecommunications
system” to include audio-only communications technology when used for telehealth services for
the diagnosis, evaluation, or treatment of mental health disorders furnished to a patient in their
home. We also finalized to limit payment for audio-only services to services furnished by a
physician or practitioner that has the technical capability at the time of the service to use two-
way audio-video telecommunications, but where the patient is not capable of, or does not consent
356
Kang AW, Walton M, Hoadley A, DelaCuesta C, Hurley L, Martin R. “Patient Experiences with the Transition to Telephone
Counseling during the COVID-19 Pandemic.” Healthcare (Basel). 2021;9(6):663. Published 2021 Jun 2.
doi:10.3390/healthcare9060663.

to, the use video technology for the service, and the patient is located at their home at the time of
service. Lastly, we clarified that SUD services are considered mental health services for purposes
of the expanded definition of “interactive telecommunications system” to include audio-only
services under §410.78(a)(3). In short, these flexibilities and policy clarifications that permit
audio-only telecommunication flexibilities for the treatment of a SUD, which can include an
OUD, already exist under other payment systems in Medicare.
Therefore, to better align coverage for periodic assessments furnished by OTPs with
other telehealth services furnished under the PFS for the diagnosis, evaluation, or treatment of a
mental health disorder including SUDs, and in response to many supportive comments received
in response to the CY 2024 PFS proposed rule that advocated for allowing OTPs to furnish
periodic assessments via audio-only telecommunications on a permanent basis, we are proposing
to allow OTPs to furnish periodic assessments using audio-only communications technology
when video is not available on a permanent basis beginning January 1, 2025. Under this
proposal, we would allow periodic assessments to be furnished via audio-only when video is not
available to the extent that use of audio-only communications technology is permitted under the
applicable SAMHSA and DEA requirements at the time the service is furnished, and all other
applicable requirements are met.
We believe permanently extending this flexibility would meaningfully promote access to
care for the Medicare population, as supported by our analysis of claims data showing the
proportion of telephonic audio-only visits increases with the age of the patient, with 17-percent
of visits delivered via audio-only interaction for patients 41-60 years of age, 30-percent for
patients 61 to 80 years of age, and 47 percent of visits for patients over 81 years of age.
357
Evidence further reveals that Medicare beneficiaries who are older than 65 years old,
racial/ethnic minorities, dual-enrollees in Medicare and Medicaid, or living in rural areas, or who
357
Lee, G., & Stewart, K. (n.d.). “2021 Medicare coverage and payment for audio only services (Telephone e/m).” AAMC.
https://www.aamc.org/media/55296/download.

experience low broadband access, low-income, and/or for whom English in not their primary
language, are more likely to be offered and use audio-only telemedicine services than audio-
video services.
358
Other evidence also suggests that while Tribal populations, including
American Indian and Alaska Natives, have the highest rates of OUD prevalence among Medicare
beneficiaries, one-third of these populations do not have adequate access to high-speed
broadband and continue to rely on audio-only visits.
359
Telemedicine flexibilities have been
shown to be feasible and effective for rural patients with an OUD with data supporting that
telemedicine flexibilities have helped improve treatment retention in OUD treatment, especially
for rural patients who are older and covered by Medicare.
360
Lastly, these audio-only flexibilities
would be meaningful for OTPs and their patients because telehealth services have become
widely used among SUD treatment facilities as regular service offerings. During the COVID-19
pandemic, SUD treatment facilities increased telemedicine offerings by 143 percent, and as of
2021, almost 60 percent of SUD treatment facilities offer telehealth.
361
Now, telephone-based
(that is, audio-only) therapy provided by SUD programs has been found to be one of the most
common modes of telehealth for treatment of OUD.
362
Given the prevalence of audio-only
modalities of care for the treatment of OUD, permanently extending this flexibility could help
prevent disruptions to care in OTP settings that may regularly provide periodic assessments via
358
Rodriguez, J. A., Betancourt, J. R., Sequist, T. D., & Ganguli, I. (2021). “Differences in the use of telephone and video
telemedicine visits during the COVID-19 pandemic.” The American Journal of Managed Care, 27(1), 21–26.
https://doi.org/10.37765/ajmc.2021.88573;Koma, W., Cubanski, J., & Published, T. N. (2021, May 19). “Medicare and
telehealth: Coverage and use during the covid-19 pandemic and options for the future.” KFF. https://www.kff.org/medicare/issue-
brief/medicare-and-telehealth-coverage-and-use-during-the-covid-19-pandemic-and-options-for-the-future/; ;Benjenk, I.,
Franzini, L., Roby, D., & Chen, J. (2021). “Disparities in Audio-Only Telemedicine use among Medicare beneficiaries during the
coronavirus disease 2019 pandemic.” Medical Care, 59(11), 1014. https://doi.org/10.1097/MLR.0000000000001631.
359
Federal Communications Commission. (2020). “2020 Broadband Deployment Report” (FCC 20-50).
https://docs.fcc.gov/public/attachments/FCC-20-50A1.pdf;Centers for Medicare and Medicaid Services, Division of Tribal
Affairs. (n.d.). Telehealth and COVID-19. https://www.cms.gov/files/document/aian-telehealthwebinar.pdf; Shoff, C., Yang, T.-
C., & Shaw, B. A. (2021). “Trends in opioid use disorder among older adults: Analyzing Medicare data, 2013–2018.” American
Journal of Preventive Medicine, 60(6), 850–855. https://doi.org/10.1016/j.amepre.2021.01.010.
360
Lira, M. C., Jimes, C., & Coffey, M. J. (2023). “Retention in telehealth treatment for opioid use disorder among rural
populations: A retrospective cohort study.” Telemedicine Journal and E-Health, 29(12), 1890–1896.
https://doi.org/10.1089/tmj.2023.0044.
361
Cantor, J., McBain, R. K., Kofner, A., Hanson, R., Stein, B. D., & Yu, H. (2022). “Telehealth adoption by mental health and
substance use disorder treatment facilities in the covid-19 pandemic.” Psychiatric Services (Washington, D.C.), 73(4), 411–417.
https://doi.org/10.1176/appi.ps.202100191;
362
Hughes, P. M., Verrastro, G., Fusco, C. W., Wilson, C. G., & Ostrach, B. (2021). “An examination of telehealth policy impacts
on initial rural opioid use disorder treatment patterns during the COVID‐19 pandemic.” The Journal of Rural Health, 37(3), 467–
472. https://doi.org/10.1111/jrh.12570.

audio-only telehealth to Medicare beneficiaries. For these reasons, we believe a permanent
extension would be appropriate for patients who are receiving buprenorphine, methadone, and/or
naltrexone at OTPs, and that proper safeguards are in place so these services can be delivered in
a way that would not diminish safety or quality of care for Medicare beneficiaries with an OUD.
Accordingly, we are proposing to revise paragraph (vii) of the definition of “Opioid
treatment services” at § 410.67(b) of the regulations to remove the references to the “Public
Health Emergency, as defined in § 400.200 of this chapter” and “through the end of CY 2024,”
in order to reflect that this flexibility would be implemented on a permanent basis. We would
continue to state that “in cases where a beneficiary does not have access to two-way audio-video
communications technology, periodic assessments can be furnished using audio-only telephone
calls if all other applicable requirements are met.” We welcome comments on this proposal to
permanently extend this audio-only flexibility for periodic assessments.
b. Proposal to Allow OTPs to Use Audio-Visual Telecommunications for Initiation of Treatment
with Methadone
Prior to the PHE for COVID-19, the Ryan Haight Online Pharmacy Consumer Protection
Act of 2008 (Pub. L. 110-425) amended the Controlled Substances Act and instructed the DEA
to promulgate regulations that required healthcare providers to conduct an in-person examination
in the presence of a practitioner prior to prescribing controlled substances (for example,
methadone, buprenorphine, etc.) to patients, with certain exceptions. These statutory provisions
prevented the distribution and dispensing of controlled substances by means of the internet
without at least one in-person medical evaluation before writing a prescription. Similarly,
SAMHSA regulations under 42 CFR 8.12(f)(2) have historically required a complete physical
evaluation before a patient begins treatment at an OTP. However, after the declaration of the
PHE for COVID-19, the DEA and SAMHSA jointly issued flexibilities for prescribing of
controlled substances via telehealth to ensure patient therapies would remain accessible.
Consequently, OTPs were exempted from the requirement to perform an in-person physical

evaluation for any patient who would be treated by the OTP with buprenorphine if a program
physician, primary care physician, or an authorized healthcare professional under the supervision
of a program physician, determines that an adequate evaluation of the patient can be
accomplished via telehealth through an audio-video or audio-only evaluation.
363
At the time, this
exemption applied exclusively to patients with an OUD being treated at an OTP with
buprenorphine, and it did not apply to new patients initiating treatment with methadone. This
meant that new OTP patients starting treatment with methadone would need to still receive an in-
person physical evaluation prior to the OTP prescribing methadone. Accordingly, in the CY
2023 PFS final rule (87 FR 69775 through 69777), we revised the regulation in paragraph (vi) of
the definition of “Opioid treatment services” at § 410.67(b) to allow the OTP intake add-on code
to be furnished via two-way audio-video communications technology when billed for the
initiation of treatment with buprenorphine, to the extent that the use of audio-video
telecommunications technology to initiate treatment with buprenorphine is authorized by DEA
and SAMHSA at the time the service is furnished. We also permitted the use of audio-only
communication technology to initiate treatment with buprenorphine in cases where audio-video
technology is not available to the beneficiary. We stated that section 1834(m)(7) of the Act
allows telehealth services for the treatment of a diagnosed SUD or co-occurring mental health
disorder to be furnished to individuals at any telehealth originating site, including in a patient’s
home, and that some codes describing new patient office/outpatient visits are already under the
Medicare Telehealth list (CPT codes 99202 through 99205). Therefore, we believed that these
changes for the initiation of treatment with buprenorphine via audio-only or audio-video
telecommunications would also be consistent with existing flexibilities under the PFS. Consistent
with SAMHSA and DEA requirements at the time of CY 2023 PFS rulemaking, we also noted
that this exemption applied exclusively to OTP patients treated with buprenorphine and did not
363
https://www.deadiversion.usdoj.gov/GDP/(DEA-DC-
022)(DEA068)%20DEA%20SAMHSA%20buprenorphine%20telemedicine%20%20(Final)%20+Esign.pdf;
https://www.samhsa.gov/medications-substance-use-disorders/statutes-regulations-guidelines/buprenorphine-at-opioid-
treatment-programs.

apply to new patients treated with methadone. Notably, SAMHSA recently finalized and codified
this flexibility at 42 CFR 8.12(f)(2)(v)(B),
364
so that OTPs may use audio-visual or audio-only
platforms when evaluating patients who are being admitted for treatment at the OTP with
schedule III medications (such as buprenorphine) on a permanent basis.
Furthermore, in their recent final rule published in the Federal Register in February of
2024 (89 FR 7528), SAMHSA made reforms to full examination requirements for initiation of
treatment with methadone at § 8.12(f)(2)(v)(A). Specifically, SAMHSA now allows for audio-
visual telehealth initiation for any new patient who will be treated by the OTP with methadone if
a practitioner or primary care provider determines that an adequate evaluation of the patient can
be accomplished via an audio-visual telehealth platform. When audio-visual technologies are not
available or their use is not feasible for a patient, it is acceptable to use audio-only devices, but
only when the patient is in the presence of a licensed practitioner who is registered to prescribe
(including dispense) controlled medications. In finalizing this new flexibility, SAMHSA
reasoned that “evidence underlying the initiation of buprenorphine using telehealth also is
applicable to the treatment of OUD with methadone, and warrants expanding access to
methadone therapy by applying some of the buprenorphine in-person examination flexibilities to
treatment with methadone in OTPs (89 FR 7533).”
365
They also noted that video-based telehealth
was overwhelmingly supported by commenters for medical intake, periodic medical assessments,
and methadone or buprenorphine initiation by OTP practitioners. SAMHSA did not extend the
flexibility to allow the use of audio-only telehealth platforms in assessing new patients who will
be treated by the OTP with methadone due to safety concerns, as they stated “methadone, in
comparison to buprenorphine, holds a higher risk profile for sedation in patients presenting with
364
89 FR 7528, February 2, 2024 (https://www.federalregister.gov/documents/2024/02/02/2024-01693/medications-
for-the-treatment-of-opioid-use-disorder).
365
Chan, B., Bougatsos, C., Priest, K. C., McCarty, D., Grusing, S., & Chou, R. (2022).” Opioid treatment programs,
telemedicine and COVID-19: A scoping review.” Substance Abuse, 43(1), 539–546.
https://doi.org/10.1080/08897077.2021.1967836.

mild somnolence which may be easier to identify through an audio-visual telehealth platform (89
FR 7533).”
366
These new flexibilities to allow new patients to initiate treatment with methadone via
audio-visual telehealth is significant, as the majority of patients who are being treated at an OTP
receive methadone.
367
Methadone is used to treat those with a confirmed diagnosis of OUD, and
is a synthetic opioid agonist that eliminates withdrawal symptoms and relieves drug cravings by
acting on opioid receptors in the brain.
368
Methadone has been associated with reducing the risk
of drug overdose, opioid-related acute care, all-cause mortality, and opioid-related mortality.
369
It
has also been shown to retain patients in treatment, reduce consequences of injection drug use
such as HIV/Hepatitis C transmission, and contribute to quality of life improvements for
patients.
370
However, many barriers currently exist for patients seeking to receive methadone
treatment. Currently, only SAMHSA-certified OTPs can dispense and administer methadone for
the treatment of OUD as provided under section 303(g)(1) of the Controlled Substances Act (21
U.S.C 823(g)(1)) and 42 CFR part 8. This often means that daily travel might be necessary if it is
determined that the risks of giving take-home doses outweigh the benefits, unless patients are
eligible to receive take-home doses after meeting certain conditions. Most adults in methadone
366
https://www.govinfo.gov/content/pkg/FR-2024-02-02/pdf/2024-01693.pdf.
367
American Association for the Treatment of Opioid Dependence, National Association of State Alcohol and Drug Abuse
Directors, & Opioid Response Network. (2022). “Technical Brief: Census of Opioid Treatment Programs.”
https://nasadad.org/wp-content/uploads/2022/12/OTP-Patient-Census-Technical-Brief-Final-for-Release.pdf.
368
National Institute on Drug Abuse. (2021, December). “How do medications to treat opioid use disorder work?”
https://nida.nih.gov/publications/research-reports/medications-to-treat-opioid-addiction/how-do-medications-to-treat-opioid-
addiction-work.
369
Wakeman, S. E., Larochelle, M. R., Ameli, O., Chaisson, C. E., McPheeters, J. T., Crown, W. H., Azocar, F., & Sanghavi, D.
M. (2020). “Comparative effectiveness of different treatment pathways for opioid use disorder.” JAMA Network Open, 3(2),
e1920622.https://doi.org/10.1001/jamanetworkopen.2019.20622;Sordo, L., Barrio, G., Bravo, M. J., Indave, B. I., Degenhardt,
L., Wiessing, L., Ferri, M., & Pastor-Barriuso, R. (2017). “Mortality risk during and after opioid substitution treatment:
Systematic review and meta-analysis of cohort studies.” The BMJ, 357, j1550. https://doi.org/10.1136/bmj.j1550; Larochelle, M.
R., Bernson, D., Land, T., Stopka, T. J., Wang, N., Xuan, Z., Bagley, S. M., Liebschutz, J. M., & Walley, A. Y. (2018).
“Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: A cohort study.” Annals of
Internal Medicine, 169(3), 137. https://doi.org/10.7326/M17-3107.
370
Mattick, R. P., Breen, C., Kimber, J., & Davoli, M. (2009). “Methadone maintenance therapy versus no opioid replacement
therapy for opioid dependence.” Cochrane Database of Systematic Reviews, 3.
https://doi.org/10.1002/14651858.CD002209.pub2;Bruce, R. D. (2010). “Methadone as HIV prevention: High volume
methadone sites to decrease HIV incidence rates in resource limited settings. The International Journal on Drug Policy, 21(2),
122–124. https://doi.org/10.1016/j.drugpo.2009.10.004; Alavian, S. M., Mirahmadizadeh, A., Javanbakht, M., Keshtkaran, A.,
Heidari, A., Mashayekhi, A., Salimi, S., & Hadian, M. (2013). “Effectiveness of methadone maintenance treatment in prevention
of hepatitis c virus transmission among injecting drug users.” Hepatitis Monthly, 13(8), e12411.
https://doi.org/10.5812/hepatmon.12411;Carlsen, S.-E. L., Lunde, L.-H., & Torsheim, T. (2019). “Predictors of quality of life of
patients in opioid maintenance treatment in the first year in treatment.” Cogent Psychology, 6(1), 1565624.
https://doi.org/10.1080/23311908.2019.1565624.

treatment report at least one barrier to accessing treatment, including lack of reliable
transportation, distance from home to treatment, and work schedule conflicts.
371
Frequent travel
to an OTP also disproportionately impacts rural residents who already face lower odds of finding
an OTP in their area, and therefore, must spend nearly 2-5 times the amount of average drive
time to access the closest OTP compared to their urban counterparts.
372
Research has also shown
that the number of missed doses of methadone increases for residents living longer distances
from an OTP. Additionally, people living with disabilities are less likely to receive Medications
for Opioid Use Disorder (MOUDs), and some data also shows that many SUD treatment
programs are not physically accessible for these populations.
373
The existence of these physical
barriers to accessing methadone and treatment at OTP facilities, especially among historically
underserved populations, warrants additional considerations to the extent that telehealth
flexibilities can mitigate these barriers to accessing care, as long as these flexibilities are
medically appropriate and reasonable for the diagnosis and treatment of OUD.
To be consistent with SAMHSA’s reforms to telehealth flexibilities for initiation of
treatment with methadone at § 8.12(f)(2)(B)(v), past conforming regulations under the Medicare
OTP benefit to allow telecommunication flexibilities for initiation of treatment with
buprenorphine, and to contribute towards efforts to reduce barriers in accessing care for
Medicare beneficiaries seeking treatment with methadone, we are proposing to make similar
telecommunication flexibilities under the Medicare OTP benefit. Specifically, we are proposing
to allow the OTP intake add-on code (HCPCS code G2076) to be furnished via two-way audio-
371
Pasman, E., Kollin, R., Broman, M., Lee, G., Agius, E., Lister, J. J., Brown, S., & Resko, S. M. (2022). “Cumulative barriers to
retention in methadone treatment among adults from rural and small urban communities.” Addiction Science & Clinical Practice,
17(1), 1–10. https://doi.org/10.1186/s13722-022-00316-3.
372
Calcaterra, S. L., Bach, P., Chadi, A., Chadi, N., Kimmel, S. D., Morford, K. L., Roy, P., & Samet, J. H. (2019). “Methadone
matters: What the United States can learn from the global effort to treat opioid addiction.” Journal of General Internal Medicine,
34(6), 1039–1042. https://doi.org/10.1007/s11606-018-4801-3;Jehan, S., Zahnd, W. E., Wooten, N. R., & Seay, K. D. (2024).
“Geographic variation in availability of opioid treatment programs across U.S. communities.” Journal of Addictive Diseases,
42(2), 136–146. https://doi.org/10.1080/10550887.2023.2165869.
373
Thomas, C. P., Stewart, M. T., Ledingham, E., Adams, R. S., Panas, L., & Reif, S. (2023). “Quality of opioid use disorder
treatment for persons with and without disabling conditions.” JAMA Network Open, 6(3), e232052.
https://doi.org/10.1001/jamanetworkopen.2023.2052;West, S. L. (2007). “The accessibility of substance abuse treatment
facilities in the United States for persons with disabilities.” Journal of Substance Abuse Treatment, 33(1), 1–5.
https://doi.org/10.1016/j.jsat.2006.11.001.

video communications technology when billed for the initiation of treatment with methadone, to
the extent that the use of audio-video telecommunications technology to initiate treatment with
methadone is authorized by DEA and SAMHSA at the time the service is furnished. We note
that under this proposal, the initiation of treatment with methadone using telecommunications
technology would be considered an intake activity for purposes of paragraph (vi) of the
definition of “Opioid treatment services” at § 410.67(b) only to the extent that the use of such
telecommunications technology is permitted under the applicable DEA and SAMHSA
regulations and guidance at the time the services are furnished. However, at this time, we are not
proposing to extend the flexibility to allow the use of audio-only telecommunications for intake
activities described in paragraph (vi) of the definition of “Opioid treatment services” at §
410.67(b) for initiation of treatment with methadone, as these flexibilities are not currently
permitted by SAMHSA and the DEA. We recognize that methadone is characterized as a
schedule II controlled substance, which means that it still has higher potential for misuse with
potential physical dependence.
374
Unlike buprenorphine that is a schedule III controlled
substance, methadone is a full agonist and does not have a “ceiling effect,” which provides more
protective overdose factors when taking additional doses of the drug.
375
Thus, use of audio-visual
telecommunications for initiation of treatment with methadone would balance potential safety
concerns associated with methadone, such as its higher potential for misuse and risk for sedation
in patients presenting with mild somnolence which may be easier to identify via a audio-visual
telehealth platform, while still allowing patients the flexibility of initiating treatment via (audio-
visual) telehealth at an OTP.
We believe that this proposal may meaningfully improve access to care, promote positive
health outcomes, and advance health equity among Medicare beneficiaries. Data indicate that
expanded use of telehealth and flexibilities for the provision of MOUD during the COVID-19
374
https://www.dea.gov/drug-information/drug-scheduling.
375
Whelan, P. J., & Remski, K. (2012). “Buprenorphine vs methadone treatment: A review of evidence in both developed and
developing worlds.” Journal of Neurosciences in Rural Practice, 3(1), 45–50. https://doi.org/10.4103/0976-3147.91934.

pandemic was associated with improved care retention and a reduction in medically treated
overdoses among Medicare beneficiaries.
376
Similarly, telehealth initiation for buprenorphine to
treat OUD was associated with improved treatment retention in a subset of U.S states.
377
Other
research has not found significant differences in clinical severity and complexity markers (for
example, OUD-related emergency department visits) between patients receiving telemedicine
inductions into treatment versus in-person examinations,
378
suggesting that quality of care can be
maintained through initiation of treatments via telehealth. Thus, many of these benefits
associated with telehealth flexibilities for initiating treatment with other MOUDs can be
potentially replicated by allowing initiation of treatment with methadone via audio-visual
telecommunications. Additionally, we believe this proposal would meaningfully impact health
equity. Individuals from Black, American Indian and Alaska Native, and Hispanic populations
are significantly less likely to initiate treatment for a SUD, as well as individuals from
economically disadvantaged communities.
379
Despite these disparities, during the COVID-19
pandemic, the odds of initiating treatment for a SUD increased for most age, race, ethnicity, and
socioeconomic status subgroups, which may have been explained by increases in treatment
initiation occurring through telehealth.
380
Thus, promoting flexibilities for telecommunication
modalities of treatment initiation in regards to methadone may provide additional options for
accessing treatment, especially for populations who often experience barriers in beginning
treatment. Lastly, we believe this proposal is in alignment with the HHS Overdose Prevention
376
Jones, C. M., Shoff, C., Hodges, K., Blanco, C., Losby, J. L., Ling, S. M., & Compton, W. M. (2022). “Receipt of telehealth
services, receipt and retention of medications for opioid use disorder, and medically treated overdose among Medicare
beneficiaries before and during the covid-19 pandemic.” JAMA Psychiatry, 79(10), 981–992.
https://doi.org/10.1001/jamapsychiatry.2022.2284.
377
Hammerslag, L. R., Mack, A., Chandler, R. K., Fanucchi, L. C., Feaster, D. J., LaRochelle, M. R., Lofwall, M. R., Nau, M.,
Villani, J., Walsh, S. L., Westgate, P. M., Slavova, S., & Talbert, J. C. (2023). "Telemedicine buprenorphine initiation and
retention in opioid use disorder treatment for Medicaid enrollees.” JAMA Network Open, 6(10), e2336914.
https://doi.org/10.1001/jamanetworkopen.2023.36914.
378
Barsky, B. A., Busch, A. B., Patel, S. Y., Mehrotra, A., & Huskamp, H. A. (2022). “Use of telemedicine for buprenorphine
inductions in patients with commercial insurance or Medicare advantage.” JAMA Network Open, 5(1), e2142531.
https://doi.org/10.1001/jamanetworkopen.2021.42531.
379
Acevedo, A., Panas, L., Garnick, D., Acevedo-Garcia, D., Miles, J., Ritter, G., & Campbell, K. (2018). “Disparities in the
treatment of substance use disorders: Does where you live matter?”. The Journal of Behavioral Health Services & Research,
45(4), 533–549. https://doi.org/10.1007/s11414-018-9586-y.
380
Palzes, V. A., Chi, F. W., Metz, V. E., Sterling, S., Asyyed, A., Ridout, K. K., & Campbell, C. I. (2023). “Overall and
telehealth addiction treatment utilization by age, race, ethnicity, and socioeconomic status in California after covid-19 policy
changes.” JAMA Health Forum, 4(5), e231018. https://doi.org/10.1001/jamahealthforum.2023.1018.

Strategy, which aims to broaden access to evidence-based care that increases willingness to
engage and remain in treatment.
381
Similarly, this proposal would further the goals of the
National Drug Control Strategy, which strives to expand policies that improve SUD treatment
engagement by lowering various barriers to enter and participate in treatment, such as through
telemedicine treatment initiation.
382
Accordingly, we are proposing to revise the regulations for intake activities at paragraph
(vi) within the definition of “opioid use disorder treatment service” at § 410.67(b). We are
proposing to add a new paragraph (vi)(A) within the description of intake activities to separately
list flexibilities for intake activities furnished via communications technology, and we are
proposing to add and reserve a new paragraph (vi)(B). We are proposing to move the language
related to the existing flexibilities for the initiation of treatment with buprenorphine to paragraph
(vi)(A)(1). Additionally, in the definition of “opioid use disorder treatment service” at §
410.67(b), we are proposing to codify telecommunications flexibilities for initiation of treatment
with methadone at paragraph (vi)(A)(2). Specifically, we propose that services to initiate
treatment with methadone may be furnished via two-way interactive audio-video communication
technology, as clinically appropriate, and in compliance with all applicable requirements, if an
OTP determines that an adequate evaluation of the patient can be accomplished through audio-
video communication technology. We look forward to comments related to this proposal.
3. Proposals Related to Reforms to 42 CFR Part 8
In the CY 2020 PFS final rule, we implemented payment and coverage for opioid use
disorder treatment services, including services such as substance use counseling by a
professional to the extent authorized under State law to furnish such services, individual and
group therapy with a physician, psychologist (or other mental health professional to the extent
authorized under State law), and other items and services that the Secretary determines are
381
https://www.hhs.gov/overdose-prevention/
382
https://www.whitehouse.gov/wp-content/uploads/2022/04/National-Drug-Control-2022Strategy.pdf.

appropriate (but in no event to include meals or transportation), as authorized by section 1861 of
the Act (84 FR 62630 through 62677 and 84 FR 62919 through 62926). Consequently, we
included these services within the definition of OUD treatment services at § 410.67(b) and
incorporated payment for these services as part of the non-drug component at § 410.67(d)(2)(ii).
We also created an add-on code described by HCPCS code G2080 to reflect an additional 30
minutes of counseling or individual or group therapy provided in a week. We further finalized
additional adjustments to the bundled payment for an episode of care, such as intake activities
and periodic assessments. At the time, we noted that both initial and periodic assessments are
required under SAMHSA regulations, and that they were integral services for the establishment
and maintenance of OUD treatment for a beneficiary at an OTP (84 FR 62634). We codified
definitions of these services within the definition of OUD treatment services at § 410.67(b); at
paragraph (vi), we stated that intake activities include initial medical examination services
required under 42 CFR 8.12(f)(2) and initial assessment services required under § 8.12(f)(4); at
paragraph (vii) we stated that periodic assessment services include those required under §
8.12(f)(4). Services under § 8.12(f) are required services as part of Federal opioid treatment
standards for OTPs, as regulated by SAMHSA. Accordingly, we created HCPCS code G2076
[Intake activities, including initial medical examination that is a complete, fully documented
physical evaluation and initial assessment conducted by a program physician or a primary care
physician, or an authorized healthcare professional under the supervision of a program
physician or qualified personnel that includes preparation of a treatment plan that includes the
patient’s short-term goals and the tasks the patient must perform to complete the short-term
goals; the patient’s requirements for education, vocational rehabilitation, and employment; and
the medical, psycho-social, economic, legal, or other supportive services that a patient needs,
conducted by qualified personnel (provision of the services by a Medicare-enrolled Opioid
Treatment Program); List separately in addition to code for primary procedure], and code
G2077 [Periodic assessment; assessing periodically by qualified personnel to determine the most

appropriate combination of services and treatment (provision of the services by a Medicare-
enrolled Opioid Treatment Program); List separately in addition to code for primary procedure]
in order to have a mechanism to make payment under Medicare to OTPs for these required
services. In the CY 2021 and CY 2022 PFS final rules (85 FR 84682 through 84690; 86 FR
65338 through 65341), we also established payment for take-home supplies of naloxone and
overdose education furnished in conjunction with providing an opioid antagonist medication.
Additionally, in the CY 2020 PFS final rule, we codified requirements specified in the
section 1861(jjj)(2) of the Act for OTPs. Specifically, we defined an “opioid treatment program”
at § 410.67(b) as an entity that is an OTP as defined in § 8.2 (or any successor regulation) that
meets the applicable requirements for an OTP. For an OTP to participate and receive payment
under the Medicare program, the OTP must be enrolled in Medicare under section 1866(j) of the
Act, have in effect a certification by SAMHSA for such a program, and be accredited by an
accrediting body approved by SAMHSA. Lastly, an OTP must meet additional conditions as the
Secretary may find necessary to ensure the health and safety of individuals being furnished
services under such program and the effective and efficient furnishing of such services.
Recently, SAMHSA issued a new final rule (89 FR 7528), which made major reforms to
42 CFR part 8, governing requirements for OTPs in providing medications for the treatment of
OUD and many other services. The rule provides significant refinements, as 42 CFR part 8 was
published over 21 years ago, by reflecting new paradigms of care for OUD that have become
increasingly patient-centered and evidence-based. The regulatory reforms for opioid treatment
standards reflect an understanding that OUD is a chronic condition, and to be successful,
treatment interventions should be individualized and include harm reduction and recovery
support services, among other services.
383
Consequently, SAMHSA redefined comprehensive
treatment at §8.2 to specify that treatment at OTPs includes “the continued use of MOUD
383
89 FR 7528, February 2, 2024 (https://www.federalregister.gov/documents/2024/02/02/2024-01693/medications-for-the-
treatment-of-opioid-use-disorder).

provided in conjunction with an individualized range of appropriate harm reduction, medical,
behavioral health, and recovery support services.” At the same time, SAMHSA constructed a
new definition of harm reduction services at § 8.2. In particular, harm reduction “refers to
practical, evidence-based strategies, including: overdose education; testing and intervention for
infectious diseases including counseling and risk mitigation activities forming part of a
comprehensive, integrated approach to address human immunodeficiency virus (HIV), viral
hepatitis, sexually transmitted infections, and bacterial and fungal infections; distribution of
opioid overdose reversal medications; linkage to other public health services; and connecting
those who have expressed interest in additional support to peer services.” Harm reduction
approaches are especially important to reduce certain health and safety issues associated with
drug use through care that is intended to be free of stigma and centered on the needs of people
who use drugs. Decades of research have shown that harm reduction strategies provide
significant benefits in preventing drug overdose deaths and transmission of infectious diseases
among those who use drugs, educate individuals and community members about reducing the
negative consequences associated with drug use, and link individuals to SUD treatment and other
recovery resources.
384
Harm reduction is also a crucial component of the HHS Overdose
Prevention Strategy, which aims to promote evidence-based harm reduction services, including
those that are integrated within healthcare delivery, and to expand sustainable funding strategies
for harm reduction services.
385
Besides defining harm reduction, SAMHSA also finalized a new
definition for “recovery support services” at § 8.2. Specifically, recovery is the process of change
through which people improve their health and wellness, live self-directed lives, and strive to
reach their full potential. Recovery support services can include, but are not limited to,
community-based recovery housing, peer recovery support services, social support, linkage to
and coordination among allied service providers and a full range of human services that facilitate
384
https://www.cdc.gov/overdose-prevention/php/od2a/harm-reduction.html.
https://nida.nih.gov/research-topics/harm-reduction.
385
https://www.hhs.gov/overdose-prevention/harm-reduction.

recovery and wellness contributing to an improved quality of life. The services extend the
continuum of care by strengthening and complementing substance use disorder (SUD) treatment
interventions in different settings and stages. Recovery support services are a vital part SUD
treatment, as they take into account the relapsing and chronic nature of a SUD, and emphasize
the need for continuous care to keep individuals engaged in treatment, especially along different
stages of recovery.
386
Recovery support services are also a component of the HHS Overdose
Prevention Strategy, which recognizes that treatment alone may not be enough to support long-
term recovery, and that enabling access to quality integrated and coordinated recovery support
services is important to prevent drug overdoses.
387
Furthermore, SAMHSA made updates to existing definitions that include some of the
services currently covered under the Medicare OTP benefit. For example, a psychoeducational
service element was added to the definition of counseling services at § 8.12(f)(5), so that both
counseling and psychoeducational services would also include harm reduction education and
recovery-oriented counseling. New guidelines on counseling related to preventing exposure to
and transmission of various infectious diseases were also added. As part of these services, at
§ 8.12(f)(5)(iii), OTPs also must continue to provide directly, or through referral to adequate and
reasonably accessible community resources, vocational training, education, and employment
services for patients who request such services or for whom these needs have been identified and
mutually agreed-upon as beneficial by the patient and program staff. Notably, SAMHSA also
made updates to their descriptions of initial and periodic assessment activities at § 8.12(f)(4),
which initially informed the definitions of intake activities and periodic assessments in the
definition of “OUD treatment services” at § 410.67(b) and the creation of codes describing these
services (HCPCS codes G2076 and G2077) when CMS first implemented the Medicare OTP
benefit in the CY 2020 PFS final rule. When introducing these changes in their proposed rule in
386
Stanojlović, M., & Davidson, L. (2021). “Targeting the barriers in the substance use disorder continuum of care with peer
recovery support.” Substance Abuse: Research and Treatment, 15, 117822182097698.
https://doi.org/10.1177/1178221820976988; https://www.samhsa.gov/find-help/recovery.
387
https://www.hhs.gov/overdose-prevention/recovery-support.
December 2022 (87 FR 77330), SAMHSA noted that “changes to the initial and periodic
medical services sections are intended to promote key issues for OTP medical practitioners and
the OTP multi-disciplinary team to address with a patient as part of treatment. This includes
areas that may increase the risk of a patient leaving care prematurely, such as unmet mental
health or other disability, medical and oral health needs, the need for culturally supportive care
that addresses race, ethnicity, sexual orientation, religion or gender identity, and social
determinants of health, such as housing and transportation, that may pose barriers to treatment
engagement, or harm reduction and recovery support service needs.” New changes to the
definition of initial assessments now include patient-centered language regarding the need for
care plans to include the patient’s goals and mutually agreed-upon actions for the patient to meet
those goals, and new references are added for harm reduction interventions and recovery support
services to be included as components of care plans if a patient needs and wishes to pursue these
services. For example, patient-centered care plans developed during initial assessments may
reflect a “patient’s goals and mutually agreed-upon actions for the patient to meet those goals,
including harm reduction interventions; the patient’s needs and goals in the areas of education,
vocational training, and employment; and the medical and psychiatric, psychosocial, economic,
legal, housing, and other recovery support services that a patient needs and wishes to pursue (89
FR 73558).” Lastly, regarding periodic assessment services at § 8.12(f)(4)(ii), SAMHSA
requires that these examinations should occur not less than one time each year and be conducted
by an OTP practitioner. The periodic physical examination should include review of MOUD
dosing, treatment response, other SUD treatment needs, responses and patient-identified goals,
and other relevant physical and psychiatric treatment needs and goals. The periodic physical
examination should be documented in the patient’s clinical record. In whole, these regulatory
changes largely reflect significant changes in evidence-based practice and towards patient-
centered care in the treatment of OUD that have occurred in the past couple of decades,
including considerations of the need to address unmet health related social needs (HRSN) that

impose barriers on a patient's ability to initiate, engage, and remain in treatment, including in
areas of education, employment, and housing as well as in harm reduction strategies that
decrease the negative consequences associated with a patient’s use or abuse of opioids, and
recovery support services that address the chronic nature of OUD and the need for supports
across the full continuum of care.
In addition to these reforms to opioid treatment standards at 42 CFR part 8, there have been
recent activities under the PFS, and through other CMS programs, that have addressed the social
determinants of health (SDOH), which often affect the diagnosis and treatment of a patient’s
medical problem. Healthy People 2030, which is a 10-year HHS initiative to identify public
health priorities that help individuals, organizations, and communities across the U.S improve
health and well-being,
388
defines the SDOH, as the “conditions in the environments where people
are born, live, learn, work, play, worship, and age that affect a wide range of health, functioning,
and quality-of-life outcomes and risks.”
389
SDOH include many domains that largely impact
health, including economic stability, education, healthcare, the neighborhood and built
environment, and social and community context. Some studies have estimated that SDOH can
affect as much as 50 percent of the variation in health outcomes compared to clinical care
impacting only 20 percent.
390
For example, individuals with a higher income have been found to
exhibit lower mortality, higher life expectancy, and slower declines in physical mobility;
individuals who lack insurance are less likely to obtain necessary medical care and prescription
medications; and, food insecurity is associated with higher rates of birth defects, cognitive
problems, hospitalization rates, asthma, and behavioral health problems.
391
Moreover, SDOH act
388
https://health.gov/healthypeople/about#:~:text=What%20is%20Healthy%20People%202030,over%20the%20first%204%20de
cades.
389
https://health.gov/healthypeople/priority-areas/social-determinants-health.
390
Whitman, A., Chapell, A., Aysola, V., Zuckerman, R., & Sommers, B. (2022). “Addressing Social Determinants of Health:
Examples of Successful Evidence-Based Strategies and Current Federal Efforts” (ASPE, Office of Health Policy HP-2022-12).
https://aspe.hhs.gov/sites/default/files/documents/e2b650cd64cf84aae8ff0fae7474af82/SDOH-Evidence-Review.pdf;Hood, C. M.,
Gennuso, K. P., Swain, G. R., & Catlin, B. B. (2016). “County health rankings: Relationships between determinant factors and
health outcomes.” American Journal of Preventive Medicine, 50(2), 129–135. https://doi.org/10.1016/j.amepre.2015.08.024.
391
National Academies of Sciences, E., Medicine, N. A. of, Nursing 2020–2030, C. on the F. of, Flaubert, J. L., Menestrel, S. L.,
Williams, D. R., & Wakefield, M. K. (2021). “Social determinants of health and health equity. In The Future of Nursing 2020-
2030: Charting a Path to Achieve Health Equity.” National Academies Press (U.S.).
https://www.ncbi.nlm.nih.gov/books/NBK573923/.

as structural and contextual factors that shape the conditions impacting health, and their unequal
distribution impacts the development of HRSNs at the individual level, which refer to an
individual’s needs that might include housing, healthy foods, transportation, financial assistance,
etc. An inability to address these HRSNs put individuals at a higher risk for exacerbating health
conditions, and it is a major driver of health inequities.
392
Health equity is the attainment of the
highest level of health for all people, where everyone has a fair and just opportunity to attain
their optimal health regardless of race, ethnicity, disability, sexual orientation, gender identity,
socioeconomic status, geography, preferred language, or other factors that affect access to care
and health outcomes, which is complicated by SDOH such as poverty, unequal access to
healthcare, lack of education or employment, stigma, and discrimination.
393
Therefore, in light of
decades of research showing that these upstream factors drive health outcomes, and evidence
suggesting interventions in healthcare settings that address social needs can improve the
treatment of an individual’s condition, CMS recently finalized coding and payment for SDOH
risk assessments in the CY 2024 PFS final rule (88 FR 78932). HCPCS code G0136 describes
SDOH risk assessments (Administration of a standardized, evidence-based Social Determinants
of Health Risk Assessment, 5–15 minutes, not more often than every 6 months) that may be billed
when practitioners spend time and resources assessing HRSNs that interfere with the
practitioner’s ability to diagnose or treat the patient. These assessments, which may also be
provided during a behavioral health visit, are often administered as part of an assessment of
patient histories, risk, and in informing medical decision-making around the care and treatment
of the disease or illness. They are often accomplished through the use of a standardized
evidence-based tool that include the domains of food insecurity, housing insecurity,
transportation needs, and utility difficulties. Besides establishing standalone payment for SDOH
392
Whitman, A., Chapell, A., Aysola, V., Zuckerman, R., & Sommers, B. (2022). “Addressing Social Determinants of Health:
Examples of Successful Evidence-Based Strategies and Current Federal Efforts” (ASPE, Office of Health Policy HP-2022-12).
https://aspe.hhs.gov/sites/default/files/documents/e2b650cd64cf84aae8ff0fae7474af82/SDOH-Evidence-Review.pdf;
https://www.whitehouse.gov/wp-content/uploads/2023/11/SDOH-Playbook-3.pdf.
393
https://www.cms.gov/pillar/health-equity.

risk assessments, in the CY 2024 PFS final rule, CMS also created coding and payment for
community health integration (CHI) (HCPCS codes G0019 & G0022) and principal illness
navigation services (PIN) (HCPCS codes G0023, G0024, G0140, and G0146). Both CHI and
PIN services involve a person-centered assessment to better understand the patient’s life story,
care coordination, contextualizing health education, building patient self-advocacy skills, health
system navigation, facilitating behavioral change, providing social and emotional support, and
facilitating access to community-based social services (for example, housing, utilities,
transportation, food assistance) to address unmet SDOH needs. The services described by the
CHI codes address unmet SDOH needs that affect the diagnosis and treatment of the patient’s
medical problems. PIN services focus on Medicare beneficiaries diagnosed with high-risk
conditions (for example, dementia, HIV/AIDS, and cancer) in order to identify and connect them
with appropriate clinical and support resources.
Moreover, many of these aforementioned services, including harm reduction
interventions, recovery support services, addressing HRSN, and facilitating access to
community-based social services to address these needs, ordinarily occur in OTP settings. In
2022, approximately 92 percent of OTP facilities offered various recovery support services,
including peer support (59.6 percent), assistance locating housing for clients (75.0 percent),
employment counseling (49.5 percent), and assistance helping patients obtain social services
(81.2 percent). The majority of OTPs also offered various types of harm reduction services,
including testing for various types of infectious diseases (> 55 percent), health education
(>77%), and naloxone and overdose education (92.3 percent). Many OTPs also conduct
community outreach services to those in need of OUD treatment (76.1 percent) and case
management services (87.8 percent).
394
Additionally, as part of initial and periodic assessment
services at § 8.12(f)(4), OTPs must designate in the care plan a patient’s needs and goals in the
394
Table SU17b: Substance use treatment facilities, by services provided and facility type: Number and column percent, 2022:
https://www.samhsa.gov/data/sites/default/files/reports/rpt42714/NSUMHSS-Annual-Detailed-Tables-22.pdf.
areas of harm reduction interventions, education, vocational training, and employment, along
with the medical and psychiatric, psychosocial, economic, legal, housing, and other recovery
support services that a patient needs and wishes to pursue, which all reflect consideration to
various health-related social needs. The new definitions of harm reduction and recovery support
services at § 8.2 are also inclusive of activities that involve linkage to and coordination with
providers that address a full range of human and public health services to facilitate recovery and
wellness for a SUD. Lastly, in the CY 2020 PFS final rule we responded to public comments
pertaining to the above-mentioned activities. Specifically, several commenters stated that OTPs
often provide case management and/or care management services and requested that CMS
consider reimbursing for these services either as part of the standard bundle or as an adjustment
to the bundled payment, as applicable. A few commenters stated that OTPs serve as a fixed point
of responsibility in the provision of whole person-centered care and improving health outcomes
through collaborative arrangements with health care providers outside of the OTP and that the
goal of care management is to reduce health care costs, specifically preventable hospital
admissions, readmissions, and avoidable emergency room visits. The commenters also stated in
the CY 2020 final rule that OTP staff also help patients with accessing food benefits, housing,
and employment searches, which are critical components for sustained recovery, as part of the
goal of complete case management (84 FR 62648). At the time, CMS stated that we would
consider making payment for these type of care management activities for future rulemaking,
including activities whereby OTPs collaborate with providers outside the OTPs to help patients
access social services. We believed it was appropriate to work with OTPs to better understand
how these services are furnished in an OTP setting, as well as to continue to look at data on
specific items and services that may fit within the scope of OUD treatment services.
a. Proposal to Establish Payment for Social Determinants of Health Risk Assessments
The recent refinements to initial assessments under § 8.12(f)(4)(i) likely necessitate
additional resource costs for OTPs in order to comply with the opioid treatment standards for

assessing various SDOHs (for example, education, vocational training, employment, economic,
legal, housing, etc.) that impact a patient’s HRSNs, and to identify a patient’s goals for harm
reduction interventions and needs for recovery support services as they relate to the treatment of
an OUD. We recognize that the paradigm for OUD treatment and care has evolved rapidly since
the implementation of the Medicare OTP benefit in CY 2020, and that providers have
increasingly incorporated interventions to address HRSNs that increase the risk of a patient
leaving OUD treatment prematurely or that pose barriers to treatment engagement. We
additionally acknowledge that coding already exists under the PFS that accounts for the
resources involved in conducting these types of assessments. For these reasons, we are proposing
to establish payment for SDOH risk assessments as part of intake activities within OUD
treatment services, as long as these assessments are medically reasonable and necessary for the
diagnosis or treatment of an OUD, and OTPs have a reason to believe unmet HRSNs or the need
for harm reduction intervention or recovery support services identified during such an
assessment could interfere with the OTP’s ability to diagnose or treat the patient’s OUD. As
previously stated, the SDOH include broad structural and contextual domains that may impact
health (for example, economic stability, education, healthcare, neighborhood and built
environment, and social and community context) and the development of HRSNs at the
individual-level (for example, housing and utilities assistance, transportation assistance, financial
assistance, healthy foods, personal safety, employment, recovery support and harm reduction
services). We understand that there are multiple standardized, evidence-based SDOH risk-
assessment tools utilized across the healthcare system that are structured to assess a patient
across various SDOH domains.
395
If an OTP furnishes SDOH risk assessments as part of initial
assessments under § 8.12(f)(4)(i), we would expect that the assessment tools used would allow
395
https://prapare.org/wp-content/uploads/2021/10/What-is-PRAPARE_2.1.21-1.pdf;
https://www.cms.gov/priorities/innovation/media/document/ahcm-screeningtool-companion

the OTP to identify more specific individual-level HRSNs as part of the care plan, including
giving consideration to potential harm reduction and recovery support services needs.
Specifically, we are proposing to update the payment rate for intake activities described
by HCPCS code G2076 by adding in the value of the non-facility rate for SDOH risk
assessments described by HCPCS code (G0136). We believe HCPCS code G0136 may serve as
a reasonable proxy to reflect the value and resources required for the type of assessment service
activities that OTPs are required to provide according to SAMHSA requirements under
§ 8.12(f)(4)(i), including an assessment to identify a patient’s unmet HRSNs or the need for
harm reduction intervention and recovery support services that are critical to the treatment of an
OUD. We understand that OTPs have been involved in collaborative agreements with
organizations who address HRSN sand offer various recovery support services (84 FR 62648),
and we believe that for OTPs to appropriately identify these types of organizations that target a
specific need, identifying these HRSNs as part of SDOH risk assessments is likely needed prior
to engaging in activities to coordinate service delivery. However, we seek comment on whether
these types of SDOH assessments ordinarily complement the type of community coordination
activities that OTPs perform.
Establishing payment to account for SDOH risk assessments as part of intake activities
under the OTP benefit is important, as unmet HRSNs identified as part of such assessments
significantly impact outcomes for OUD treatment. Evidence shows that healthcare providers who
screen for SDOH in their settings have found that patients who screen positive for a HRSN were
significantly more likely to have a history of substance use or mental illness compared to patients
who did not have an HRSN.
396
For example, one review found that between 50 to 90 percent of
patients in publicly funded OTPs were unemployed, and that older adults identified to have
396
Chukmaitov, A., Dahman, B., Garland, S. L., Dow, A., Parsons, P. L., Harris, K. A., & Sheppard, V. B. (2022). “Addressing
social risk factors in the inpatient setting: Initial findings from a screening and referral pilot at an urban safety-net academic
medical center in Virginia, USA.” Preventive Medicine Reports, 29, 101935. https://doi.org/10.1016/j.pmedr.2022.101935.

misused opioids were 22-percent less likely to be employed.
397
Patients with an OUD are also
more likely to have a lower educational attainment, encounter financial hardship, and housing
instability.
398
Even more, food insecurity has been indicated to be a strong predictor of
prescription opioid misuse and abuse.
399
The SDOH and their contribution to unmet HRSNs have
also heavily impacted the rates of drug overdoses. For example, one study examined 28 different
SDOH measures that collectively explained 89-percent of the variance in drug-overdose
mortality across states.
400
Housing insecurity, in particular, negatively affects the population with
an OUD, as this risk factor has been increasing over time among those seeking treatment with an
OUD.
401
One analysis conducted by the State of Massachusetts has revealed alarming evidence
that the risk of death from an opioid overdose is 30-times higher for those who have experienced
homelessness.
402
Lower median household income and unemployment have also been associated
with an increase in opioid death rates.
403
Moreover, unmet HRSNs have also hampered access to
treatment among Medicare beneficiaries with a SUD, as evidence has shown that among
Medicare beneficiaries with an SUD who were not receiving treatment, one-third reported
financial barriers and one-fifth reported logistical barriers such as lack of access to transportation
397
Zanis, D. A., & Coviello, D. (2001). “A case study of employment case management with chronically unemployed methadone
maintained clients.” Journal of Psychoactive Drugs, 33(1), 67–73. https://doi.org/10.1080/02791072.2001.10400470; Albright,
D. L., Johnson, K., Laha-Walsh, K., McDaniel, J., & McIntosh, S. (2021). “Social determinants of opioid use among patients in
rural primary care settings.” Social Work in Public Health, 36(6), 723–731. https://doi.org/10.1080/19371918.2021.1939831.
398
Albright, D. L., Johnson, K., Laha-Walsh, K., McDaniel, J., & McIntosh, S. (2021). “Social determinants of opioid use among
patients in rural primary care settings.” Social Work in Public Health, 36(6), 723–731.
https://doi.org/10.1080/19371918.2021.1939831; Arsene, C., Na, L., Patel, P., Vaidya, V., Williamson, A. A., & Singh, S.
(2023). “The importance of social risk factors for patients diagnosed with opioid use disorder.” Journal of the American
Pharmacists Association, 63(3), 925–932. https://doi.org/10.1016/j.japh.2023.02.016.
399
Men, F., Fischer, B., Urquia, M. L., & Tarasuk, V. (2021). “Food insecurity, chronic pain, and use of prescription opioids.”
SSM - Population Health, 14, 100768. https://doi.org/10.1016/j.ssmph.2021.100768.
400
Cesare, N., Lines, L. M., Chandler, R., Gibson, E. B., Vickers-Smith, R., Jackson, R., Bazzi, A. R., Goddard-Eckrich, D.,
Sabounchi, N., Chisolm, D. J., Vandergrift, N., & Oga, E. (2024). “Development and validation of a community-level social
determinants of health index for drug overdose deaths in the HEALing Communities Study.” Journal of Substance Use and
Addiction Treatment, 157, 209186. https://doi.org/10.1016/j.josat.2023.209186.
401
Sulley, S., & Ndanga, M. (n.d.). “Inpatient opioid use disorder and social determinants of health: A nationwide analysis of the
national inpatient sample (2012-2014 and 2016-2017).” Cureus, 12(11), e11311. https://doi.org/10.7759/cureus.11311.
402
https://www.mass.gov/files/documents/2017/08/31/legislative-report-chapter-55-aug-2017.pdf.
403
Rangachari, P., Govindarajan, A., Mehta, R., Seehusen, D., & Rethemeyer, R. K. (2022). “The relationship between Social
Determinants of Health (Sdoh) and death from cardiovascular disease or opioid use in counties across the United States (2009–
2018).” BMC Public Health, 22(1), 236. https://doi.org/10.1186/s12889-022-12653-8; Hollingsworth, A., Ruhm, C. J., & Simon,
K. (2017). Macroeconomic conditions and opioid abuse (Working Paper 23192). National Bureau of Economic Research.
https://doi.org/10.3386/w23192.

as rationales for not receiving treatment.
404
Lastly, many of these SDOH factors have impaired
treatment retention and completion rates. Those with lower levels of educational attainment and
who are unemployed are less likely to complete SUD treatment, and individuals who are
experiencing homelessness are significantly less likely to remain in treatment.
405
Therefore,
screening for the SDOH and identifying these unmet HRSNs as part of intake assessments may
help OTPs link patients with an identified social need to appropriate resources that can impact
the diagnosis of an OUD or address barriers to treating an OUD.
As previously stated above, we are proposing to update the adjustment to the bundled
payment for an episode of care for intake activities (G2076) by adding in the value of the non-
facility rate for SDOH risk assessments (G0136: Administration of a standardized, evidence-
based Social Determinants of Health Risk Assessment, 5–15 minutes, not more often than every 6
months), which is currently assigned a non-facility rate of $18.66 under the PFS. Currently, the
CY 2024 payment rate for the intake add-on code (G0276) is $201.73 and adding the value of a
crosswalk to the CY 2024 non-facility rate of $18.66 would result in a payment rate of
approximately $220.39. We believe that incorporating the value of G0136 into the intake
activities adjustment would be the most appropriate, as we believe assessment activities related
to SDOH are more likely to occur during intake assessments when a new patient is admitted to
an OTP. SAMHSA treatment guidelines recommend that during initial screenings, OTPs should
identify barriers and medical and psychosocial risk-factors that may hinder a patient’s ability to
meet treatment requirements, including co-occurring health conditions, and vocational, legal,
financial, transportation, and family concerns.
406
We note that intake activities (G2076) should
404
Parish, W. J., Mark, T. L., Weber, E. M., & Steinberg, D. G. (2022). “Substance use disorders among Medicare beneficiaries:
Prevalence, mental and physical comorbidities, and treatment barriers.” American Journal of Preventive Medicine, 63(2), 225–
232. https://doi.org/10.1016/j.amepre.2022.01.021.
405
Mennis, J., & Stahler, G. J. (2016). Racial and ethnic disparities in outpatient substance use disorder treatment episode
completion for different substances. Journal of Substance Abuse Treatment, 63, 25–33.
https://doi.org/10.1016/j.jsat.2015.12.007; Gaeta Gazzola, M., Carmichael, I. D., Christian, N. J., Zheng, X., Madden, L. M., &
Barry, D. T. (2023). “A national study of homelessness, social determinants of health, and treatment engagement among
outpatient medication for opioid use disorder-seeking individuals in the United States.” Substance Abuse, 44(1–2), 62–72.
https://doi.org/10.1177/0889707723116729.
406
SAMHSA. (2012). Medication-assisted treatment for opioid addiction in opioid treatment programs.
https://www.ncbi.nlm.nih.gov/books/NBK64164/pdf/Bookshelf_NBK64164.pdf.

only be billed for new patients (that is, patients starting treatment at the OTP), and since SDOH
risk assessments would be bundled into the code describing intake activities, this billing
requirement would similarly apply. However, we seek comment on the frequency with which
these SDOH risk assessments occur, and whether it would be more appropriate if these
assessments occur when OTPs furnish periodic assessments described by HCPCS code G2077.
When OTPs bill the intake add-on code (G2076), we are not proposing to require that
OTPs performed SDOH risk assessments in a specific manner, but rather that OTPs continued to
perform initial assessment services consistent with SAMHSA certification requirements at §
8.12(f)(4)(i) that already largely reflect these type of SDOH risk assessment activities; and, that
OTPs abided by other applicable requirements under the Medicare OTP benefit at § 410.67,
including those listed in the definition of intake activities at paragraph (vi) within the definition
of “OUD treatment service” at § 410.67(b). This also means that for the purposes of Medicare
payment, if SDOH risk assessments are furnished, they must be related to the diagnosis or
treatment of OUD, and any HRSNs identified through SDOH risk assessments performed should
be documented in the patient’s medical record to indicate how assessing and addressing the
HRSN relates to the treatment and diagnosis of an OUD. We reiterate that our proposal to
incorporate the value of HCPCS code G0136 into the OTP intake add-on code (G2076) is meant
to serve as a reasonable proxy to reflect the value and resources of the type of initial assessment
service activities that OTPs are required to provide under SAMHSA requirements, which now
include more specific updates to a patient’s care plan with considerations of a patient’s goals
related to harm reduction interventions, needs for recovery support services, and other HRSNs.
However, if OTPs utilize SDOH risk assessments during intake activities, CMS is not proposing
to require OTPs to utilize a specific type of SDOH risk assessment tool, consistent with similar
existing requirements under the PFS for these services. If OTPs do furnish these assessment
services, CMS encourages OTPs to adopt evidence-based, validated tools that are already
available (such as the CMS Accountable Health Communities tool, the Protocol for Responding

to and Assessing Patients Assets, Risks and Experiences (PRAPARE), and instruments identified
for Medicare Advantage Special Needs Population Health Risk Assessment);
407
that include the
domains of food insecurity, housing insecurity, transportation needs, and utility difficulties, and
that can be furnished in a manner appropriate for the patient’s educational, developmental, and
health literacy level, and that are culturally and linguistically appropriate. We understand that
there is not a national consensus around one specific tool, and OTPs should choose the tool that
fits their needs and allows them to appropriately detect unmet HRSNs, as well as other needs for
harm reduction interventions and recovery support services that are integral to the treatment of
an OUD.
Lastly, in light of these proposed changes, we are proposing to revise the current
descriptor for the intake add-on code for consistency with revisions to § 8.12(f)(4)(i) and to
reflect furnishing an SDOH risk assessment: G2076 (Intake activities, including initial medical
examination that is a complete, fully documented physical evaluation and initial assessment
conducted by a program physician or a primary care physician, or an authorized healthcare
professional under the supervision of a program physician or qualified personnel that includes
preparation of a care plan, which may be informed by administration of a standardized,
evidence-based Social Determinants of Health Risk Assessment to identify unmet health-related
social needs, and that includes the patient’s goals and mutually agreed-upon actions for the
patient to meet those goals, including harm reduction interventions; the patient’s needs and
goals in the areas of education, vocational training, and employment; and the medical and
psychiatric, psychosocial, economic, legal, housing, and other recovery support services that a
patient needs and wishes to pursue, conducted by qualified personnel (provision of the services
by a Medicare-enrolled Opioid Treatment Program); List separately in addition to code for
primary procedure). We welcome public comments related to this proposal.
407
https://innovation.cms.gov/files/worksheets/ahcm-screeningtool.pdf.; https://www.nachc.org/research-and-data/prapare/;
CMS–10825.
b. Request for Information on Payment for Coordinated Care and Referrals to Community-
Based Organizations that Address Unmet Health-Related Social Needs, Provide Harm Reduction
Services, and/or Provide Recovery Support Services
In the discussion above, we noted that SAMHSA’s recent reforms to 42 CFR part 8
finalized new definitions for harm reduction and recovery support services, which are included
as components of the type of services that OTPs may provide. Some examples of harm reduction
strategies include overdose education, distribution of opioid overdose reversal medications, and
linkage to other public health services. Recovery support services can include, but are not limited
to, community-based recovery housing, social support, and linkage to and coordination among
allied service providers and a full range of human services that facilitate recovery and wellness.
Under the Medicare OTP benefit, we have already established payment for some of these
services, including take-home supplies of opioid antagonist medications for emergency treatment
of known or suspected opioid overdose (for example, naloxone), overdose education furnished in
conjunction with opioid antagonist medications, and social support via group therapy. However,
we do not currently have specific coding for activities that OTPs may conduct to coordinate care
and make referrals or “link” to community-based organizations (CBOs) that help facilitate a
patient’s needs and goals related to harm reduction and recovery support services, as well as to
address unmet HRSNs. We understand that a referral is an important aspect of following up on
unmet HRSNs identified during an initial assessment service and/or SDOH risk assessment so
that a patient can be connected to resources or services that may help address their unmet HRSN
that interferes with treatment of their OUD. Additionally, we have received previous comments
that OTPs often have collaborative agreements with providers outside of the OTP. For these
reasons, we are seeking comment to understand how OTPs are currently coordinating care and
making referrals to CBOs that address unmet HRSNs, provide harm reduction services, and/or
provide recovery support services.

Some evidence has indicated that providers who coordinate care with CBOs to address
HRSNs (for example, housing, transportation, care management, etc.) can positively influence
health outcomes,
408
and that SUD treatment facilities establishing relationships with community-
based peer support services, educational and employment agencies, housing agencies, and other
organizations have been able to better support a patient’s engagement in SUD treatment.
409
Additionally, harm reduction organizations, including syringe service programs, function as
important facilitators of entry to treatment, as individuals who partake in these programs are five
times more likely to enter treatment, more likely to remain engaged in treatment, and more likely
to reduce their injection drug use.
410
Additionally, recovery support services, such as those
linking individuals in SUD treatment who are also experiencing homelessness with supportive or
transitional housing, have resulted in improved uptake of behavioral health visits;
411
and,
recovery support services facilitated by peers who have recovered from a SUD have been shown
to reduce relapse rates, improve treatment retention, enhance the provider and patient
relationship, and boost overall treatment experience.
412
Therefore, there is evidence to suggest
that linkage to these types of community-based resources may contribute to improved outcomes
related to OUD treatment; however, we seek comment on additional evidence that demonstrates
how this type of services would directly help OTPs address the diagnosis or treatment of an
OUD. CMS would also be interested in additional evidence describing how these community-
based resources and coordination of these services with MOUD provided by OTPs would impact
408
McCarthy, D., Lewis, C., Horstman, C., Bryan, A., & Shah, T. (2022). “Guide to Evidence for Health-Related Social Needs
Interventions: 2022 Update” [ROI Calculator for Partnerships to Address the Social Determinants of Health]. The
Commonwealth Fund. https://www.commonwealthfund.org/sites/default/files/2022-
09/ROI_calculator_evidence_review_2022_update_Sept_2022.pdf.
409
O’Brien, P., Crable, E., Fullerton, C., & Hughey, L. (2019). “Best Practices and Barriers to Engaging People with Substance Use
Disorders in Treatment.” ASPE. https://aspe.hhs.gov/sites/default/files/private/pdf/260791/BestSUD.pdf f.
410
Hagan, H., McGough, J. P., Thiede, H., Hopkins, S., Duchin, J., & Alexander, E. R. (2000). “Reduced injection frequency and
increased entry and retention in drug treatment associated with needle-exchange participation in Seattle drug injectors.” Journal
of Substance Abuse Treatment, 19(3), 247–252. https://doi.org/10.1016/s0740-5472(00)00104-5.
411
Brennan, K., Buggs, K., Zuckerman, P., Muyeba, S., Henry, A., Gettens, J., & Kunte, P. (2020). “The Preventive Effect of
Housing First on Health Care Utilization and Costs among Chronically Homeless Individuals.”
https://www.bluecrossmafoundation.org/sites/g/files/csphws2101/files/2020-12/Housing%20First_summary_Final.pdf.
412
Reif, S., Braude, L., Lyman, D. R., Dougherty, R. H., Daniels, A. S., Ghose, S. S., Salim, O., & Delphin-Rittmon, M. E.
(2014). “Peer recovery support for individuals with substance use disorders: Assessing the evidence.” Psychiatric Services, 65(7),
853–861. https://doi.org/10.1176/appi.ps.201400047.
access to treatment for Medicare beneficiaries who may face barriers in accessing treatment ,
such as those who are residents of rural areas, racial/ethnic minorities, living with a disability,
dual-enrollees in Medicare and Medicaid, and low-income, or other populations who may face
barriers in accessing treatment. Additionally, we seek to identify the types of entities, service
providers, and organizations that OTPs may interact with on a regular basis to address a patient’s
unmet HRSNs and needs or goals related to harm reduction and recovery support services. For
example, we seek to understand if these entities would typically include housing or
transportation agencies, local support groups, syringe service programs, non-profits that provide
financial assistance, etc. We are also seeking information on the types of collaborative
arrangements that OTPs typically have with these CBOs, including how frequently (for example,
weekly, monthly, annually, etc.) OTPs coordinate care or make referrals to these CBOs for
patients with an OUD, the types of circumstances that warrant an OTP interacting with these
CBOs, and the workflows originating from the initial SDOH assessment to identify these HRSNs
to a beneficiary successfully receiving referred services. We are also interested in learning to
what extent some of these programs are already integrated into OTP settings.
Moreover, we are also interested in learning when these coordinated activities and/or
referrals occur in the process of furnishing care to a beneficiary. For example, a component of
SAMHSA’s new revised standards for MOUD treatment under counseling and
psychoeducational services at § 8.12(f)(5)(iii) suggests that OTPs must provide directly, or
through referral to adequate and reasonably accessible community resources, vocational training,
education, and employment services for patients who request such services or for whom these
needs have been identified and mutually agreed upon as beneficial by the patient and program
staff. Thus, we are soliciting comment on whether these coordination and referral services
typically occur during SUD counseling session services, or if they may occur during initial or
periodic assessments, therapy sessions, or as part of other services. We are also interested in
understanding if, when billing for intake activities (G2076), periodic assessments (G2077),

additional therapy/counseling (G2080), and/or the non-drug component code (G2074) under the
Medicare OTP benefit, OTPs are already accounting for these coordinated care and referral
services as part of those codes.
We are also interested in additional information related to payment for these types of
coordinated care or referral services. Specifically, we seek comment on the resource costs that
OTPs must expend to coordinate or make referrals to community-based services that address
HRSNs, harm reduction, or recovery support needs. We are also especially interested in whether
there is existing coding that properly describes these types of coordinated care or referral
services, or whether there are elements to these types of services that are unique to OTPs and
require new coding. We seek comment on if any of the following codes below may describe the
type of coordinated care or referral activities that OTPs may provide, or if there are other codes
that more precisely match the type of coordinated care or referral activities at OTPs: community
health integration (G0019& G0022), principal illness navigation (G0023, G0024, G0140,
G0146), chronic care management (99437, 99439, 99490, 99491), complex chronic care
management (99487, 99489), principal care management (99424, 99425, 99426, 99427), or other
codes, including any other relevant codes used by other payers.
Lastly, we are seeking information on whether OTPs already receive funding for these
types of coordinated care or referral services from other public or private sources, and if
additional payment would be duplicative or unnecessary. We are interested in learning, for
example, if OTPs already receive state or Federal grants for these types of activities (for
example, the SAMHSA Harm Reduction Grant Program, Rural Communities Opioid Response
Program, State Opioid Response Grants, Building Communities of Recovery, Substance Use
Prevention, Treatment, and Recovery Services Block Grant, etc.).
413
Additionally, CMS would
like to understand if OTPs already receive payment from states who might already cover these
413
https://www.samhsa.gov/grants/grant-announcements/sp-22-001;
https://grants.hrsa.gov/2010/Web2External/Interface/FundingCycle/ExternalView.aspx?fCycleID=af0c3bac-6d99-4314-ab7b-
c1602e6c471c; https://www.samhsa.gov/grants/grants-dashboard; https://nashp.org/funding-options-for-states/.

services under state Medicaid programs, including through section 1115 waiver demonstrations
and delivery system reform incentive payments, state plan amendments, managed care contracts,
or other service benefits and payment arrangements,
414
and if new coding under the Medicare
OTP benefit may unintentionally supplant coverage for dually eligible beneficiaries. We
appreciate any feedback submitted by the public on these questions and issues to better
understand activities that OTPs conduct to coordinate care and make referrals to CBOs that
address unmet health-related social needs, provide harm reduction services, and/or provide
recovery support services.
4. Establishing Payment for New FDA-approved Opioid Agonist and Antagonist Medications
Section 1861(jjj)(1)(A) of the Act establishes Medicare payment for opioid agonist and
antagonist treatment medications (including oral, injected, or implanted versions) that are
approved by the Food and Drug Administration under section 505 of the Federal Food, Drug,
and Cosmetic Act (FFDCA) for use in the treatment of OUD and as part of OUD treatment
services under the OTP benefit. Additionally, section 1834(w)(2) of the Act granted CMS the
authority to establish multiple bundled payments in stating that the “Secretary may implement
this subsection through one or more bundles based on the type of medication provided (such as
buprenorphine, methadone, naltrexone, or a new innovative drug), the frequency of services, the
scope of services furnished, characteristics of the individuals furnished such services, or other
factors as the Secretary determine appropriate.” In the CY 2020 PFS final rule, we finalized
basing the OTP bundled payments, in part, on the type of medication used for treatment that
reflect those drugs currently approved by the FDA under section 505 of the FFDCA for use in
treatment of OUD. Accordingly, at § 410.67(d)(1) we specified that CMS would establish
categories of bundled payments for OTPs for an episode of care, including categories for each
type of opioid agonist and antagonist treatment medication, a category for medications not
414
Artiga, S., & Published, E. H. (2018, May 10). “Beyond health care: The role of social determinants in promoting health and
health equity.” KFF. https://www.kff.org/racial-equity-and-health-policy/issue-brief/beyond-health-care-the-role-of-social-
determinants-in-promoting-health-and-health-equity/;
https://www.health.ny.gov/diseases/aids/consumers/prevention/medicaid_harm_reduction.htm.

otherwise specified, and a category for episodes of care in which no medication is provided. At
§ 410.67(d)(2) we finalized that the bundled payment amounts for an episode of care would be
based on both a drug and non-drug component, and we codified the payment methodology for
determining these components. At § 410.67(d)(4), we described various adjustments that could
be made to the bundled payment. Since the implementation of the Medicare OTP benefit on
January 1, 2020, we have established bundled payments and/or add-on codes for the following
medications: methadone (G2067 & G2078), oral buprenorphine (G2068 & G2079), injectable
buprenorphine (G2069), buprenorphine implants (G2070 through G2072), naltrexone (G2073),
nasal naloxone (G2215 & G1028), injectable naloxone (G2216), and medication not otherwise
specified (G2075) (for new FDA-approved opioid agonist or antagonist medications for OUD
treatment that is not specified in one of our existing codes). In this CY 2025 PFS proposed rule,
we are proposing new payment for injectable buprenorphine and nalmefene hydrochloride
products furnished by OTPs.
a. Coding and Payment for a New Nalmefene Hydrochloride Product, Opvee®
In May of 2023, the FDA approved the first nalmefene hydrochloride (nalmefene) nasal
spray (under the brand name Opvee®), which is indicated for the emergency treatment of known
or suspected opioid overdose induced by natural or synthetic opioids. This is the first FDA
approval of a nasal spray for nalmefene hydrochloride for health care and community use, and it
is intended for immediate administration as emergency therapy in settings where opioids may be
present. Nalmefene acts as an opioid receptor antagonist and when administered quickly, it can
reverse the effects of an opioid overdose including respiratory depression, sedation, and low
blood pressure.
415
Newly approved Opvee® delivers 2.7 milligrams (mg) of nalmefene in a
single spray into the nasal cavity. After the first dose is administered, if the patient does not
respond, or responds and then relapses into respiratory depression, additional doses of the
Opvee® nasal spray may be administered with an additional spray every 2 to 5 minutes until
415
https://www.fda.gov/news-events/press-announcements/fda-approves-prescription-nasal-spray-reverse-opioid-overdose.

emergency medical assistance arrives.
416
Compared to naloxone which has a half-life of
approximately 2 hours and also rapidly reverses the effects of an opioid overdose, nalmefene has
a half-life of 11 hours which means that it remains in the body much longer than other overdose
reversal drugs.
417
The rise of dangerous synthetic opioids, such as fentanyl and its analogs
sufentanil and carfentanil, have made it increasingly difficult for first responders to reverse the
effects of an overdose. These synthetics have a high potency and longer half-lives than naloxone
(7-8 hours for fentanyl; 6-9 hours for sufentanil; 5-6 hours for carfentanil; and 1.3-2.4 hours for
naloxone), which means that very high doses of naloxone are often required to treat opioid
overdose and prevent recurring overdose symptoms,
418
which further demonstrates the necessity
for new overdose reversal products that can counter these highly potent opioid synthetics. In one
study, 2.7 mg of Opvee® reversed respiratory depression to baseline levels within 5 minutes,
whereas 4 mg of intranasal naloxone did so within 20 minutes,
419
demonstrating the effectiveness
of Opvee® in quickly reversing symptoms of opioid overdose.
In the CY 2021 PFS final rule (85 FR 84683 through 84692), we adopted new add-on
codes for take-home supplies of nasal naloxone (G2215) and injectable naloxone (G2216).
Additionally, we used our discretionary authority in section 1861(jjj)(1)(F) of the Act (which
generally authorizes us to include as an OTP treatment service other items and services we
determine are appropriate) to extend the definition of OUD treatment services to include short-
acting opioid antagonist medications for the emergency treatment of known or suspected opioid
overdose, such as naloxone, and overdose education furnished in conjunction with opioid
antagonist medication. We also established an adjustment at § 410.67(d)(4)(i)(E) to the weekly
bundled payments when the OTP furnishes take-home supplies of these medications. This
416
https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217470Orig1s000.pdf.
417
Harris, E. (2023). “FDA approves nalmefene, a longer-lasting opioid reversal nasal spray.” JAMA, 329(23), 2012.
https://doi.org/10.1001/jama.2023.9608.
418
Krieter, P., Gyaw, S., Crystal, R., & Skolnick, P. (2019). “Fighting fire with fire: Development of intranasal nalmefene to treat
synthetic opioid overdose.” The Journal of Pharmacology and Experimental Therapeutics, 371(2), 409–415.
https://doi.org/10.1124/jpet.118.256115.
419
Ellison, M., Hutton, E., Webster, L., & Skolnick, P. (2024). “Reversal of opioid‐induced respiratory depression in healthy
volunteers: Comparison of intranasal nalmefene and intranasal naloxone.” The Journal of Clinical Pharmacology, jcph.2421.
https://doi.org/10.1002/jcph.2421.
adjustment includes both a drug component and a non-drug component for overdose education.
The payment methodology for the drug component of the adjustment was finalized at
§ 410.67(d)(2)(i) and is updated annually using the most recent data available at the time of
ratesetting. The amount of the non-drug component of the adjustment, which includes overdose
education, is based on the CY 2020 Medicare payment rate for CPT code 96161 (Administration
of caregiver focused health risk assessment instrument (e.g., depression inventory) for the benefit
of the patient, with scoring and documentation, per standardized instrument). We also finalized
that any payment to an OTP for naloxone would be duplicative if a claim for the same
medication is separately paid under Medicare Part B or Part D for the same beneficiary on the
same date of service, and that we would recoup any duplicative payment made to an OTP for
naloxone.
Furthermore, in the CY 2022 PFS final rule (86 FR 65340 and 65341), we established a
new add-on code and payment for a higher dose of nasal naloxone (G0128). We also finalized
that the adjustment includes take-home supplies of opioid antagonist medications in the list of
items for which the non-drug component will be geographically adjusted using the Geographic
Adjustment Factor (GAF) and the payment amount will be updated annually by the growth in the
Medicare Economic Index (MEI). Lastly, we revised our regulations at § 410.67(d)(5) to state
explicitly that payments for medications that are delivered, administered or dispensed to a
beneficiary as part of an adjustment to the bundled payment are considered a duplicative
payment if a claim for delivery, administration or dispensing of the same medication(s) for the
same beneficiary on the same date of service was also separately paid under Medicare Part B or
Part D. We clarified that this revision would apply not only to duplicative payments for take-
home supplies of naloxone, but also to duplicative payments for additional take-home supplies of
other medications that are made under § 410.67(d)(4)(i)(D).
In light of a novel nalmefene product, Opvee®, receiving FDA approval as an opioid
antagonist medication for the emergency treatment of known or suspected opioid overdose, we

are proposing to make payment for this new drug under the Medicare OTP benefit. Expanding
access to overdose reversal medications, such as nalmefene, is a critical component to
confronting the opioid crisis. The number of drug overdose deaths involving prescription opioids
has grown by nearly five-fold in the past two decades. In 2021, almost 81,000 opioid overdose
deaths occurred in the U.S, and nearly 88 percent of opioid-involved deaths involved synthetic
opioids like fentanyl and fentanyl analogs (acetylfentanyl, furanylfentanyl, and carfentanil). Due
to the high potency of drugs in the nation’s drug supply, this has often meant that higher doses,
or even multiple doses, of overdose reversal medications are needed per overdose event to revive
a patient.
420
These increasing rates of drug overdose deaths has also been seen among the
Medicare-eligible population with adults aged 65 and over experiencing the largest percentage
increase (28 percent) in drug overdose deaths rates between 2020 and 2021,
421
and the rate of
drug overdose deaths involving synthetic opioids among this age group increased by over 53
percent in only one year (between 2019 and 2020).
422
Over 50,000 Medicare Part D beneficiaries
were estimated to have experienced an opioid overdose in 2021, and the number of these
beneficiaries receiving naloxone has grown.
423
Not only has the opioid crisis impacted the
Medicare-eligible population, but health disparities in drug overdose deaths have persisted. Non-
Hispanic Black men aged 65 and over have experienced drug overdose death rates that are more
than four times higher than Hispanics and non-Hispanic whites.
424
In addition, death rates from
drug overdoses among people aged 65 and over have increased at faster rates for men than
women.
425
Expanding access to overdose reversal medications is important, including for
populations at a greater risk for drug overdose, as overdose reversal medications have been
regarded as an evidence-based strategy to help individuals quickly respond to an overdose to
reduce drug overdose deaths, increase survival rates, and reduce opioid-related emergency
420
https://www.cdc.gov/drugoverdose/deaths/index.html.
421
https://www.cdc.gov/nchs/products/databriefs/db457.htm.
422
https://blogs.cdc.gov/nchs/2023/06/30/7408/.
423
https://oig.hhs.gov/oei/reports/OEI-02-22-00390.pdf.
424
https://www.cdc.gov/nchs/products/databriefs/db455.htm.
425
https://blogs.cdc.gov/nchs/2022/11/30/7193/.

department visits.
426
Lastly, we believe this proposal to pay for Opvee® under the OTP benefit
would further the objectives of the HHS Overdose Prevention Strategy and the National Drug
Control Strategy, which both aim to widen availability and access to opioid overdose reversal
treatments.
427
Section 1861(jjj)(1)(A) of the Act recognizes opioid agonist and antagonist treatment
medications (including oral, injected, or implanted versions) that are approved by the FDA under
section 505 of the FFDCA for the use in treatment of OUD, but nalmefene is not on the list of
drugs for the treatment of OUD.
428
When CMS first finalized payment for nasal and injectable
naloxone under the OTP benefit in the CY 2021 PFS final rule (85 FR 84682 through 84689 and
85026 through 85027), we used our discretionary authority under section 1861(jjj)(1)(F) of the
Act to finalize and extend the definition of OUD treatment services to include short acting opioid
antagonist medications (e.g naloxone) that are approved by the FDA under section 505 of the
FFDCA for the emergency treatment of known or suspected opioid overdose. Since nalmefene
was approved by the FDA under section 505(b)(2) authority,
429
and is an opioid antagonist and
on the list of overdose reversal drugs approved by the FDA,
430
we believe nalmefene is
consistent with our definition of OUD treatment service at § 410.67(d), which describes opioid
antagonist medications that are approved by the FDA under section 505 of the FFDCA for the
emergency treatment of known or suspected opioid overdose at paragraph (viii). Therefore, we
believe it is appropriate to propose new payment for nalmefene as it would align with existing
authority under § 410.67(b) that recognizes opioid antagonist medications which treat known or
suspected opioid overdose as an OUD treatment service.
426
https://www.cdc.gov/drugoverdose/pdf/pubs/2018-evidence-based-strategies.pdf.
427
https://www.hhs.gov/overdose-prevention/; https://www.whitehouse.gov/wp-content/uploads/2022/04/National-Drug-Control-
2022Strategy.pdf.
428
https://www.fda.gov/drugs/information-drug-class/information-about-medication-assisted-treatment-mat.
429
https://www.fda.gov/media/171605/download.
430
https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/information-about-naloxone-and-
nalmefene.
We are proposing to create a new adjustment to the bundled payment for nalmefene
hydrochloride (Opvee®) described by GOTP1 [Take-home supply of nasal nalmefene
hydrochloride; one carton of two, 2.7 mg per 0.1 mL nasal sprays (provision of the services by a
Medicare-enrolled Opioid Treatment Program); (List separately in addition to each primary
code)]. We would price this new add-on code based on the established methodology under the
OTP benefit for determining the adjustment for take-home supplies of opioid antagonist
medications at § 410.67(d)(4)(i)(E). This adjustment would include both a drug component and a
non-drug component. The amount of the drug component would be determined using the
methodology for pricing the drug component of an episode of care at § 410.67(d)(2)(i), which
tends to use ASP data when available (with certain exceptions). Accordingly, consistent with the
approach used to price the drug component for nasal naloxone (HCPCS code G2215 & G1028),
we would apply the average sales price (ASP) payment methodology set forth in section 1847A
of the Act to determine the payment for the new naloxone hydrochloride nasal spray product,
except that payment amounts would not include any add-on percentages if either ASP or
wholesale acquisition cost (WAC) is used. As stated in the CY 2021 PFS final rule (85 FR
84685), we continue to believe that using ASP provides a transparent and public benchmark for
manufacturers’ actual pricing as it reflects the manufacturers’ actual sales prices to all purchasers
(with limited exceptions as noted in section 1847A(c)(2) of the Act) and is the only pricing
methodology that includes off-invoice rebates and discounts as described in section 1847A(c)(3)
of the Act. Therefore, we believe ASP to be the most market-based approach to set drug prices,
including for the new nalmefene nasal product. As we stated in the CY 2020 PFS final rule, we
also continue to believe that limiting the payment amount to 100-percent of the volume-weighted
ASP for a HCPCS code will incentivize the use of the most clinically appropriate drug for a
given patient (84 FR 62651 through 62656). We understand that many OTPs purchase
medications directly from manufacturers, thereby limiting the markup from distribution
channels.

Furthermore, as stated in the CY 2020 PFS final rule (84 FR 62650), we usually use the
typical maintenance dose to calculate the drug component for the OTP benefit. As part of
determining a payment rate for the proposed bundles for OUD treatment services, a dosage of the
applicable medication is often selected to calculate the costs of the drug component of the
bundle. According to the product information for Opvee®, each unit-dose nasal spray device
delivers 2.7 mg of nalmefene in 0.1 mL.
431
Each unit-dose device contains a single dose of
nalmefene, so it delivers its entire contents automatically and cannot be reused. Each carton
contains two unit-dose nasal spray devices to allow for an additional repeat dose if needed. Thus,
we are proposing to price the drug component of the code for nalmefene based on an assumption
of a typical dosage for this new product to be a carton containing two 2.7 mg nasal sprays. We
would, therefore, multiply the payment amount of 100-percent of the volume-weighted ASP
reported for 2.7 mg of nalmefene by two in order to reflect a carton of two nasal spray devices.
We seek comment on whether this amount (a carton of two, 2.7 mg nasal sprays) reflects the
typical maintenance dosage for this drug when administered. The payment limit for Opvee® in
the April 2024 ASP pricing file is $92.033, which reflects a carton of two, 2.7mg nasal sprays
and would be used to price the drug component of GOTP1.
Additionally, consistent with the methodology established in § 410.67(d)(4)(i)(E), we
propose to include a non-drug component for GOTP1 that would include payment for overdose
education. Overdose education is an important component of overdose prevention and includes
educating patients and caregivers on how to recognize respiratory depression, the signs and
symptoms of a possible opioid overdose, how to administer overdose reversal medications, and
the importance of calling 911 or getting emergency medical help right away, even after the
overdose reversal medication is administered.
432
Additionally, overdose education paired with
distribution of overdose reversal medications has been found to be effective in improving
431
https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217470Orig1s000.pdf.
432
https://www.fda.gov/media/140360/download#.

knowledge about opioid overdose, improving attitudes toward using overdose reversal
medications, training individuals to safely and effectively manage overdoses, and reducing
opioid-related mortality.
433
For these reasons, we are proposing to include a non-drug component
to GOTP1 based on the CY 2020 Medicare payment rate for CPT code 96161 (Administration of
caregiver-focused health risk assessment instrument (e.g., depression inventory) for the benefit
of the patient, with scoring and documentation, per standardized instrument) and updated to
reflect the MEI updates that have been applied since that time. This is consistent with the
payment methodology for naloxone and the language in § 410.67(d)(4)(i)(E). In addition, the
language at § 410.67(d)(4)(ii) currently states that the non-drug component of the adjustments
for take-home supplies of opioid antagonist medications will be geographically adjusted using
the geographic adjustment factor described in § 414.26. Separately, § 410.67(d)(4)(iii) states that
the non-drug component of the adjustments for take-home supplies of opioid antagonist
medications will be updated annually using the Medicare Economic Index described in
§ 405.504. Since we are proposing to establish payment for Opvee® through an adjustment to
the bundled payment, and since Opvee® is also considered an opioid antagonist medication, we
are also proposing to update the non-drug component for the adjustment of GOTP1 annually
based on the GAF and MEI.
Furthermore, consistent with our established criteria for opioid antagonist medications at
§ 410.67(d)(4)(i)(E), we are also proposing to limit payment for Opvee® to one add-on code
(GOTP1) every 30 days. However, we believe that access to Opvee® should not be limited when
it is medically reasonable and necessary as part of the treatment for OUD and known or
suspected opioid overdose. Therefore, similar to flexibilities established for frequency limits for
naloxone, we are proposing to allow exceptions to this limit in the case where the beneficiary
overdoses and uses the initial supply of nalmefene dispensed by the OTP to the extent that it is
433
Razaghizad, A., Windle, S. B., Filion, K. B., Gore, G., Kudrina, I., Paraskevopoulos, E., Kimmelman, J., Martel, M. O., &
Eisenberg, M. J. (2021). “The effect of overdose education and naloxone distribution: An umbrella review of systematic
reviews.” American Journal of Public Health, 111(8), e1–e12. https://doi.org/10.2105/AJPH.2021.306306.
medically reasonable and necessary to furnish additional nalmefene. We note that section
1862(a)(1)(A) of the Act requires that for payment to be made for most Part A and Part B
services furnished to Medicare beneficiaries, those services must be reasonable and necessary for
the diagnosis or treatment of illness or injury or to improve the malfunctioning of a malformed
body member. If an additional supply of Opvee® is needed within 30 days of the original supply
being provided, we propose that OTPs must document in the medical record the reason for the
exception. Moreover, section 1834(w)(1) of the Act, added by section 2005(c) of the SUPPORT
Act, requires the Secretary to ensure, as determined appropriate by the Secretary, that no
duplicative payments are made under Medicare Part B or Part D for items and services furnished
by an OTP. Similar to naloxone, we recognize that nalmefene may also be appropriately
available to beneficiaries through other Medicare benefits, including under Medicare Part D. At
§ 410.67(d)(5), we define duplicative payment to involve circumstances when medications are
delivered, administered or dispensed to a beneficiary are paid as part of the OTP bundled
payment, and where the delivery, administration or dispensing of the same medication (that is,
same drug, dosage and formulation) is also separately paid under Medicare Part B or Part D for
the same beneficiary on the same date of service. Consistent with § 410.67(d)(5), we propose
that CMS recoup duplicative payments made to an OTP for Opvee®. CMS expects that if the
OTP provides reasonable and necessary medications for an OUD as part of an episode of care,
the OTP will take measures to ensure that there is no claim for payment for these drugs other
than as part of the OTP bundled payments. Thus, Opvee® billed by an OTP as an add-on to the
bundled payment should not be reported to or paid under a Medicare Part D plan.
We welcome comments related to this proposal to establish an adjustment to the bundled
payment for Opvee® [(GOTP1: Take-home supply of nasal nalmefene hydrochloride; one
carton of two, 2.7 mg per 0.1 mL nasal sprays (provision of the services by a Medicare-enrolled
Opioid Treatment Program); (List separately in addition to each primary code)], as well as
comments related to applicable requirements and criteria for billing this code.

b. Coding and Payment for New Injectable Buprenorphine Product Brixadi®
Another medication for the treatment of OUD for which the Secretary may establish
payment is buprenorphine, which is a partial opioid agonist that is FDA approved to treat OUD.
Buprenorphine is a schedule III substance, meaning it has low to moderate potential for physical
dependence.
434
When taken as prescribed, it can diminish the effects of opioid withdrawal
symptoms and cravings.
435
In the CY 2020 PFS final rule (84 FR 62630 through 62677 and 84
FR 62919 through 62926), we established a weekly bundled payment under the Medicare OTP
benefit for injectable buprenorphine (HCPCS G2069: Medication assisted treatment,
buprenorphine (injectable); weekly bundle including dispensing and/or administration,
substance use counseling, individual and group therapy, and toxicology testing if performed
(provision of the services by a Medicare-enrolled Opioid Treatment Program)). CMS also
established payment for other formulations of buprenorphine, including weekly bundles for oral
buprenorphine (G0268), buprenorphine implants (G2070 through G2072), and take-home
supplies of oral buprenorphine (G2079), as well as other medications like methadone and
naltrexone. At §410.67(d)(2), we codified that the bundled payment for episodes of care in which
a medication is provided will consist of a payment for a drug component, reflecting payment for
the applicable FDA-approved opioid agonist or antagonist medication in the patient's treatment
plan, and a non-drug component, reflecting payment for all other OUD treatment services
reflected in the patient's treatment plan (including dispensing/administration of the medication, if
applicable). The payments for the drug component and non-drug component are added together
to create the bundled payment amount. In the CY 2020 PFS final rule, CMS finalized a payment
methodology for the drug component related to implantable and injectable medications at 42
CFR 410.67(d)(2)(i)(A), which applied to the bundled payment for injectable buprenorphine
(G2069).
434
https://www.dea.gov/drug-information/drug-scheduling.
435
National Academies of Sciences, Engineering, and Medicine. (2019). “The effectiveness of medication-based treatment for
opioid use disorder.” In M. Mancher & A. I. Leshner (Eds.), Medications for Opioid Use Disorder Save Lives. National
Academies Press (U.S.). https://www.ncbi.nlm.nih.gov/books/NBK541393/.

For implantable and injectable medications paid under the OTP benefit, the payment is
determined using the methodology set forth in section 1847A of the Act, except that the payment
amount must be 100 percent of the ASP, if ASP is used; and the payment must be 100 percent of
the WAC, if WAC is used. We also stated in the CY 2020 PFS final rule that the typical
maintenance dose to calculate the drug component for payment under the OTP benefit, as dosing
for some, but not all, of the drugs varies considerably (84 FR 62650). As part of determining a
payment rate for the proposed bundles for OUD treatment services, a dosage of the applicable
medication must be selected to calculate the costs of the drug component of the bundle. In the
CY 2020 PFS final rule, we finalized using a 100 mg monthly dose for the extended-release
buprenorphine injection to use as the typical or average maintenance dose to calculate the drug
component of the bundle for injectable buprenorphine (G2069). At the time of ratesetting for the
CY 2020 PFS rule, the only injectable extended-release buprenorphine drug available and
approved by the FDA under section 505 of the FFDCA for the treatment of OUD was
Sublocade®;
436
and, the drug component for the bundle was based on a crosswalk to its
respective HCPCS codes Q9991 (Buprenorphine XR 100 mg or less) and Q9992 (Buprenorphine
XR over 100 mg) using the methodology set forth in section 1874A of the Act, except that the
payment amount was 100-percent of the ASP. In the CY 2020 PFS final rule, we noted that the
HCPCS codes for extended-release buprenorphine injection had the same payment rate, thus we
did not believe it was necessary to establish a second typical maintenance dose to calculate the
payment rate for the drug. For the non-drug component of the weekly bundle for injectable
buprenorphine (G2069), we finalized that in addition to services for substance use counseling,
individual and group therapy, and toxicology testing, we would include the Medicare non-facility
rate for administration of an injection in our determination of the payment rate based on CPT
code 96372 (Therapeutic, prophylactic, or diagnostic injection (specify substance or drug);
subcutaneous or intramuscular).
436
https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/209819s001lbl.pdf.

In May of 2023, the FDA approved a new drug application (NDA) under section 505(b)(2)
of the FFDCA for another extended-release buprenorphine injection (Brixadi®) for subcutaneous
use to treat moderate to severe OUD.
437
Clinical data suggest that Brixadi® likely contributes to
high rates of treatment retention, reductions in opioid withdrawal and cravings, and fewer levels
of illicit opioid use.
438
Brixadi® is available in two formulations, a weekly injection (containing
50 mg of buprenorphine per mL) that can be used in patients who have started treatment with a
single dose of a transmucosal buprenorphine product or who are already being treated with
buprenorphine-containing products, and a monthly injection (containing 356 mg of
buprenorphine per mL) for patients already being treated with buprenorphine. The weekly and
monthly formulations of the drug are available at varying doses, including lower doses that may
be appropriate for those who do not tolerate higher doses of extended-release buprenorphine that
are currently available.
439
The weekly doses are 8 mg, 16 mg, 24 mg, and 32 mg, and should be
administered in 7-day intervals; and the monthly doses are 64 mg, 96 mg, and 128 mg, and
should be administered in 28-day intervals.
440
Buprenorphine is associated with decreasing the risk for overdose, opioid-related
mortality, and all-cause mortality.
441
Data also shows that buprenorphine helps retain individuals
in treatment, lowers illicit opioid use, and reduces drug-related behaviors that increase the risk
for HIV transmission.
442
In particular, long-acting (for example, extended-release) injectable
437
https://www.fda.gov/news-events/press-announcements/fda-approves-new-buprenorphine-treatment-option-opioid-use-
disorder.
438
Frost, M., Bailey, G. L., Lintzeris, N., Strang, J., Dunlop, A., Nunes, E. V., Jansen, J. B., Frey, L. C., Weber, B., Haber, P.,
Oosman, S., Kim, S., & Tiberg, F. (2019). “Long‐term safety of a weekly and monthly subcutaneous buprenorphine depot in the
treatment of adult out‐patients with opioid use disorder.” Addiction, 114(8), 1416–1426. https://doi.org/10.1111/add.14636.
439
https://www.fda.gov/news-events/press-announcements/fda-approves-new-buprenorphine-treatment-option-opioid-use-
disorder.
440
https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/210136Orig1s000lbl.pdf.
441
Larochelle, M. R., Bernson, D., Land, T., Stopka, T. J., Wang, N., Xuan, Z., Bagley, S. M., Liebschutz, J. M., & Walley, A.
Y. (2018). “Medication for opioid use disorder after nonfatal opioid overdose and association with mortality: A cohort study.”
Annals of Internal Medicine, 169(3), 137. https://doi.org/10.7326/M17-3107; Wakeman, S. E., Larochelle, M. R., Ameli, O.,
Chaisson, C. E., McPheeters, J. T., Crown, W. H., Azocar, F., & Sanghavi, D. M. (2020). “Comparative effectiveness of different
treatment pathways for opioid use disorder.” JAMA Network Open, 3(2), e1920622.
https://doi.org/10.1001/jamanetworkopen.2019.20622.
442
Shulman, M., Wai, J. M., & Nunes, E. V. (2019). Buprenorphine treatment for opioid use disorder: An overview. CNS Drugs,
33(6), 567–580. https://doi.org/10.1007/s40263-019-00637-z; Thomas, C. P., Fullerton, C. A., Kim, M., Montejano, L., Lyman,
D. R., Dougherty, R. H., Daniels, A. S., Ghose, S. S., & Delphin-Rittmon, M. E. (2014). Medication-assisted treatment with
buprenorphine: Assessing the evidence. Psychiatric Services (Washington, D.C.), 65(2), 158–170.

forms of buprenorphine have been shown to promote adherence to treatment while reducing the
need for daily dosing, and to enhance patient-reported outcomes through improvements in
quality of life, accessibility, social relationships, participation in employment, more flexible
personal and professional schedules, and other treatment satisfaction measures.
443
Finally, a large
percentage of Medicare beneficiaries with an OUD continue to face challenges in accessing
medication, especially enrollees who are older, female, and who identify as racial/ethnic
minorities.
444
The most common reasons for not receiving SUD treatment include financial
barriers in affordability and coverage.
445
Establishing coverage and payment for a new
medication to treat OUD may provide more MOUD treatment options, reduce financial barriers
to accessing medication, and aid health equity efforts among Medicare beneficiaries.
Accordingly, for these reasons and because sections 1861(s)(2), 1861(jjj)(1)(A), and 1833(a)(1)
of the Act provide that the Secretary is to provide coverage and payment for OUD treatment
services including opioid agonist and antagonist medications that are FDA approved for use in
the treatment of OUD, we are proposing to establish payment for the weekly and monthly
formulations for this new FDA-approved injectable buprenorphine product, Brixadi®, which we
believe would further efforts to address the opioid crisis and expand access to evidence-based
treatment for OUD.
https://doi.org/10.1176/appi.ps.201300256; Gowing, L., Farrell, M. F., Bornemann, R., Sullivan, L. E., & Ali, R. (2011). Oral
substitution treatment of injecting opioid users for prevention of HIV infection. The Cochrane Database of Systematic Reviews,
8, CD004145. https://doi.org/10.1002/14651858.CD004145.pub4.
443
Maremmani, I., Dematteis, M., Gorzelanczyk, E. J., Mugelli, A., Walcher, S., & Torrens, M. (2023). Long-acting
buprenorphine formulations as a new strategy for the treatment of opioid use disorder. Journal of Clinical Medicine, 12(17),
5575. https://doi.org/10.3390/jcm12175575; Farrell, M., Shahbazi, J., Byrne, M., Grebely, J., Lintzeris, N., Chambers, M.,
Larance, B., Ali, R., Nielsen, S., Dunlop, A., Dore, G. J., McDonough, M., Montebello, M., Nicholas, T., Weiss, R., Rodgers, C.,
Cook, J., & Degenhardt, L. (2022). Outcomes of a single-arm implementation trial of extended-release subcutaneous
buprenorphine depot injections in people with opioid dependence. International Journal of Drug Policy, 100, 103492.
https://doi.org/10.1016/j.drugpo.2021.103492; Lintzeris, N., Dunlop, A. J., Haber, P. S., Lubman, D. I., Graham, R., Hutchinson,
S., Arunogiri, S., Hayes, V., Hjelmström, P., Svedberg, A., Peterson, S., & Tiberg, F. (2021). Patient-reported outcomes of
treatment of opioid dependence with weekly and monthly subcutaneous depot vs daily sublingual buprenorphine: A randomized
clinical trial. JAMA Network Open, 4(5), e219041. https://doi.org/10.1001/jamanetworkopen.2021.9041; Martin, E., Maher, H.,
McKeon, G., Patterson, S., Blake, J., & Chen, K. Y. (2022). Long-acting injectable buprenorphine for opioid use disorder: A
systematic review of impact of use on social determinants of health. Journal of Substance Abuse Treatment, 139, 108776.
https://doi.org/10.1016/j.jsat.2022.108776.
444
https://oig.hhs.gov/oei/reports/OEI-02-23-00250.pdf.
445
Parish, W. J., Mark, T. L., Weber, E. M., & Steinberg, D. G. (2022). Substance use disorders among Medicare beneficiaries:
Prevalence, mental and physical comorbidities, and treatment barriers. American Journal of Preventive Medicine, 63(2), 225–
232. https://doi.org/10.1016/j.amepre.2022.01.021.

We are proposing to establish two different payments, one for each of the weekly and
monthly injectable formulations of Brixadi®. To establish payment for the weekly and monthly
formulations of Brixadi®, we are proposing to use the existing payment methodology for
implantable and injectable medications codified at § 410.67(d)(2)(i)(A). This regulation specifies
that payment is determined using the methodology set forth in section 1847A of the Act, except
that the payment amount must be 100 percent of the ASP, if ASP is used; and the payment must
be 100 percent of the WAC, if WAC is used.
Payment limits
446
for most drugs and biologicals separately payable under Medicare Part
B are determined using the methodology in section 1847A of the Act, and in many cases,
payment is based on the ASP plus a statutorily mandated 6 percent add-on. Most drugs payable
under Part B are paid under the “incident to” benefit under section 1861(s)(2) of the Act, which
includes drugs and biologicals not usually self-administered by the patient. The ASP payment
limit determined under section 1847A of the Act reflects a volume-weighted ASP for all national
drug codes (NDCs) that are assigned to a HCPCS code. The ASP is calculated quarterly using
manufacturer-submitted data on sales to all purchasers (with limited exceptions as articulated in
section 1847A(c)(2) of the Act, such as for sales at nominal charge and sales exempt from best
price) with manufacturers’ rebates, discounts, and price concessions reflected in the
manufacturer’s determination of ASP.
Paragraphs (4)(A) and (6) of sections 1847A(b) of the Act require that the Medicare Part
B payment limit for a single-source drug or biological be determined using all of the NDCs
assigned to it. Section 1847A(b)(5) of the Act further states that the payment limit shall be
determined without regard to any special packaging, labeling, or identifiers on the dosage form
or product or package. In 2007, CMS issued a program instruction,
447
as permitted under section
446
In general, CMS establishes a single, national payment limit to Medicare Administrative Contractors (MACs) for payment of
some Part B-covered drugs and biologicals whose payment is determined based on the methodology described in section 1847A
of the Act. CMS provides an ASP pricing file to MACs, which is updated quarterly.
https://www.cms.gov/medicare/payment/part-b-drugs/asp-pricing-files.
447
https://www.cms.gov/Medicare/Coding/MedHCPCSGenInfo/Downloads/051807_coding_annoucement.pdf.

1847A(c)(5)(C) of the Act, stating that the payment limit for a single source drug or biological
will be based on the pricing information for products produced or distributed under the
applicable FDA approval (such as a New Drug Application (NDA) or Biologics License
Application (BLA)). Therefore, all versions of a single source drug or biological product (or
NDCs) marketed under the same FDA approval number (for example, NDA or BLA, including
supplements) are considered the same drug or biological for purposes of payments made under
section 1847A of the Act and are crosswalked to the same billing and payment code.
We continue to believe that use of ASP provides a transparent and public benchmark for
manufacturers’ pricing as it reflects the manufacturers’ actual sales prices to all purchasers (with
limited exceptions) and is the only pricing methodology that includes off invoice rebates and
discounts as described in section 1847A(c)(3) of the Act. Additionally, since many other
injectable drugs are paid for under Medicare part B through the ASP payment methodology in
1847A, we presume that this methodology is appropriate for pricing Brixadi®. We also propose
to limit the payment amount to 100-percent of ASP without a 6-percent add-on percentage since,
as we have previously noted, it is our understanding that many OTPs purchase directly from drug
manufacturers, thereby limiting the markup from distribution channels.
As we stated in our discussion above, we use the typical or average maintenance dose of
a drug to determine the drug costs for each of the bundles. In the CY 2020 PFS final rule, we
noted that there are often variations in the dosage and frequency of administration of
medications, but that “payment based on the typical dose means that, across the Medicare
beneficiaries served by the OTP, the payment amount should be reasonable and represent the
average costs incurred in furnishing the drug component of the OUD treatment services.” (84 FR
62650). Therefore, in the CY 2020 PFS final rule, we finalized using the typical maintenance
dose to establish the drug costs for each of the bundles as our approach to addressing variable
dosing of medications. (84 FR 62650).

In the CY 2020 PFS final rule, we finalized a 100 mg monthly dose for the extended-
release buprenorphine injection as the typical maintenance dose, which we used to calculate the
drug component of the weekly bundle for injectable buprenorphine (G2069). At the time, we did
not establish a second typical maintenance dose because both HCPCS codes for the extended
release buprenorphine injection, that is, Sublocade® [Q9991 (Buprenorphine XR 100 mg or less)
and Q9992 (Buprenorphine XR over 100 mg)] had the same payment limit because, as explained
above in this section, all NDCs marketed under the same FDA approval number are considered
the same drug or biological for purposes of payments made under section 1847A of the Act and
are crosswalked to the same billing and payment code. The weekly and monthly formulations of
Brixadi® are described by HCPCS codes J0577 (Injection, buprenorphine extended release
(brixadi), less than or equal to 7 days of therapy) and J0578 (Injection, buprenorphine extended
release (brixadi), greater than 7 days and up to 28 days of therapy). In the same manner as
Sublocade®, and as explained in the coding announcement for HCPCS codes J0577 and
J0578,
448
because all versions of a single source drug or biological product (or NDCs) marketed
under the same FDA approval number are considered the same drug or biological for purposes of
payments made under section 1847A of the Act, the payment limits for both J0577 and J0578 are
calculated using all the NDCs marketed under the applicable FDA approval. However, since the
dose descriptions for these codes are based on days of therapy (and not a measurement of the
amount of drug, like per 1 mg, as is the case with Sublocade®), the payment limits for the two
codes are different; in the April 2024 ASP pricing file, the payment limit for J0577 is $404.086
and the payment limit for J0578 is $1616.346. Therefore, we do not believe it is appropriate to
bundle the weekly and monthly formulations into a single bundled payment since, unlike the
drug Sublocade®, the Brixadi® formulations have different payment limits, and pricing them
under the same bundle would not adequately represent the average costs incurred in furnishing
448
https://www.cms.gov/files/document/2023-hcpcs-application-summary-quarter-4-2023-drugs-and-biologicals-updated-
03/04/2024.pdf.

these different formulations in an OTP setting. Additionally, creating a single bundled payment
rate that does not reflect the type and cost of the drug used could result in access issues for
beneficiaries, especially in the event that the bundled payment amount for one drug significantly
drops and unintentionally incentivizes treatment towards a drug with a higher bundled payment
amount.
In establishing the two different payments for the weekly and monthly injectable
buprenorphine formulations of Brixadi®, first, we propose to crosswalk the monthly formulation
of Brixadi® (J0578: Injection, buprenorphine extended release (brixadi), greater than 7 days
and up to 28 days of therapy) to the drug component of our existing bundled payment for
injectable buprenorphine described by HCPCS code G2069 (Medication assisted treatment,
buprenorphine (injectable); weekly bundle including dispensing and/or administration,
substance use counseling, individual and group therapy, and toxicology testing if performed
(provision of the services by a Medicare-enrolled Opioid Treatment Program). We propose to
average the payment limits of Sublocade® and monthly Brixadi® by adding their two payment
limits together and dividing the sum by two, in order to update the payment for the drug
component of HCPCS code G2069. We believe including the average of the payment limits of
Sublocade® and Brixadi® in the drug component of G2069 rather than the sum of their
respective individual payment limits is appropriate because we do not expect that a beneficiary
would receive two different types of buprenorphine monthly medication injections
simultaneously from an OTP (for example, both Sublocade® and Brixadi® during the same
episode of care and date of service). We believe that averaging the price of the two types of
buprenorphine monthly medication injections would be further be appropriate because the
individual payment limits for each of the drug codes (Q9991, Q9992, and J0578) would both be
informed by ASP data and comparable as they would be priced by the same ASP payment
methodology (ASP+0). We also note that bundling the monthly formulation of Brixadi® into the
existing HCPCS code (G2069) for injectable buprenorphine would be appropriate and no more

administratively complex for OTPs since G2069 is already billed on a monthly basis;
Sublocade®, which is already reflected in the drug component of G2069 is administered on a
monthly basis to beneficiaries as would be the monthly formulation of Brixadi®, so OTPs could
continue to bill G2069 once each month when either monthly Brixadi® or Sublocade® is
administered, as appropriate.
449
Additionally, the average typical dose of G2069 is 100mg of buprenorphine
administered monthly, as finalized in the CY 2020 PFS final rule (84 FR 62651). The monthly
formulations of Brixadi® can range from 64 mg, to 96 mg, to 128 mg. The median of these
different doses for the monthly formulation of Brixadi® (96 mg) would approximate the average
typical dose of the current injectable buprenorphine bundle (100 mg). We note that the different
monthly doses of Brixadi® are assigned to the same HCPCS code J0578 (Injection,
buprenorphine extended release (brixadi), greater than 7 days and up to 28 days of therapy) and
have the same payment limit regardless of the monthly dose (64 mg, 96 mg, or 128 mg), so
selecting a typical dose of monthly Brixadi® to potentially adjust the drug component of G2069
would not meaningfully change the payment rate. Therefore, we are not proposing to establish an
average typical dose different than 100 mg for injectable buprenorphine administered on a
monthly basis for purposes of calculating the drug component under the OTP benefit, though we
seek comment on whether this average typical dose (100 mg) is close to the dose for the monthly
formulation of Brixadi® that patients receive on average.
In all, we believe that bundling the monthly formulation of Brixadi® into our current
injectable buprenorphine coding under the OTP benefit would be appropriate for several reasons,
including: the costs for furnishing these drugs, as shown by similar ASP payment limits for
monthly Brixadi® (J0578) and the two HCPCS codes for Sublocade® (Q9991 and Q9992)
($1616.346 and $1874.902, respectively, are comparable as reflected in the April 2024 ASP file);
the average maintenance dosage for Sublocade® (100 mg) is comparable to the median monthly
449
https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/209819s001lbl.pdf.

dosage for Brixadi ® (96 mg) and; both drugs have similar frequencies and costs of
administration (on a monthly basis) with a fee paid to the OTP for one administration of an
injection once a month. We believe that our proposed payment methodology would be consistent
with section 1834(w)(2) of the Act, which allows the Secretary to implement bundled payments
for OUD treatment services with considerations to the type of medication provided and the
frequency of the services, and thus permit multiple bundles that represent injectable
buprenorphine (proposed GOTP2 and G2069) and the frequency with which injectable
buprenorphine is administered (weekly versus monthly). We propose to still calculate the non-
drug component of HCPCS code G2069 consistent with the methodology we use to calculate the
non-drug component, which is specified at § 410.67(d)(2)(ii). We are proposing to change the
code descriptor for HCPCS code G2069 to take out references to a “weekly bundle” to make it
clear that the code is to be billed on a monthly basis. Specifically, we would revise the code
descriptor to state the following: HCPCS code G2069 (Medication assisted treatment,
buprenorphine (injectable) administered on a monthly basis; bundle including dispensing and/or
administration, substance use counseling, individual and group therapy, and toxicology testing if
performed (provision of the services by a Medicare-enrolled Opioid Treatment Program)).
Lastly, consistent with current guidance in Chapter 39 of the Medicare Claims Processing
Manual, we would still expect that HCPCS code G2069 “would be billed for the week during
which the injection was administered and that HCPCS code G2074, which describes a bundle not
including the drug, would be billed during any subsequent weeks that at least one non-drug
service is furnished until the injection is administered again, at which time HCPCS code G2069
would be billed again for that week.”
450
For the weekly formulation of Brixadi®, we propose to calculate a new bundled payment
described by GOTP2 (Medication assisted treatment, buprenorphine (injectable) administered
on a weekly basis; weekly bundle including dispensing and/or administration, substance use
450
https://www.cms.gov/files/document/chapter-39-opioid-treatment-programs-otps.pdf.

counseling, individual and group therapy, and toxicology testing if performed (provision of the
services by a Medicare-enrolled Opioid Treatment Program). For the drug component of
HCPCS code GOTP2, we propose to base the payment on a crosswalk to the weekly Brixadi®
formulation described by HCPCS code J0577 (Injection, buprenorphine extended release
(brixadi), less than or equal to 7 days of therapy), which would also be based on the payment
methodology specified at § 410.67(d)(2)(i)(A) for implantable and injectable medications,
consistent with the existing monthly injectable buprenorphine bundle. We believe that
establishing a separate weekly bundled payment reflecting the weekly formulation of Brixadi®
would more appropriately pay for the subset of beneficiaries who receive less than a monthly
dosage of injectable buprenorphine on average, or who choose to discontinue treatment for the
drug before the end of the month. Additionally, establishing a separate weekly bundled payment
would contribute to stabilizing the payment of the drug component for the monthly bundle of
injectable buprenorphine (G2069) since the ASP payment limit for weekly Brixadi® costs less
than the payment for the drug component of G2069 ($404.086 April 2024 ASP payment limit
versus $1,780.167 for the CY 2024 payment rate of the drug component of G2069) and may
decrease payment after the weekly Brixadi payment limit is averaged into the drug component of
G2069®. Establishing a separate weekly bundled payment is also more appropriate because
weekly Brixadi® requires more frequent administration costs than monthly injectable
buprenorphine (weekly Brixadi® must be injected at least once every 7 days compared to once a
month for Sublocade® and monthly Brixadi®). Thus, a different bundle for weekly Brixadi®
may more closely reflect the costs incurred by OTPs. Furthermore, as noted above in this section,
different weekly doses are assigned to the same HCPCS code J0577 (Injection, buprenorphine
extended release (brixadi), less than or equal to 7 days of therapy) and have the same payment
limit regardless of the weekly dose. Therefore, we do not believe it is appropriate to propose an
average typical dose for the weekly formulation of Brixadi® for purposes of calculating the drug
component of GOTP2 under the OTP benefit.

Second, we propose to also establish payment for the non-drug component of GOTP2
consistent with the methodology utilized for the monthly bundle of injectable buprenorphine
(G2069). Specifically, we would continue to pay for substance use counseling, individual and
group therapy, and toxicology testing that are included in the non-drug components for each of
the bundled payments reflecting an episode of care, but we would include the Medicare non-
facility rate for administration of an injection in our determination of the non-drug component
payment rate based on CPT code 96372 (Therapeutic, prophylactic, or diagnostic injection
(specify substance or drug); subcutaneous or intramuscular). Consistent with the payment
amounts for the non-drug component of other bundled payments for an episode of care, we also
propose to continue to update the value of this non-drug component for GOTP2 by the GAF as
described in § 410.67(d)(4)(ii), and by the MEI as described in § 410.67(d)(4)(iii).
We welcome comments on these proposals to establish payment for the weekly and
monthly formulations of the new injectable buprenorphine drug, Brixadi®.
5. Clarification to require an opioid use disorder diagnosis on claims for OUD treatment services
Section 1861(s)(2)(HH) of the Act, as amended by section 2005 of the SUPPORT Act,
implemented Medicare coverage for “opioid use disorder treatment services.” Section
1861(jjj)(1) of the Act describes opioid use disorder treatment services as items and services that
are furnished by an opioid treatment program for the treatment of opioid use disorder. Section
1834 of the Act specifies payments to OTPs for providing opioid use disorder treatment services.
We interpreted these statutory provisions to mean that services paid to OTPs under Medicare
Part B must be for the treatment of opioid use disorder. Consequently, at § 410.67(a) we reflect
that those statutory provisions provide for coverage and payment to OTPs for OUD treatment
services, which we define at § 410.67(b).
In August of 2023, an Office of Inspector General (OIG) report (A-09-22-03005) found
that Medicare made over $1.3 million in payments to 70 OTPs for OUD treatment services that

were claimed without an OUD diagnosis.
451
Of the claims paid without an OUD diagnosis code,
39 percent were for alcohol dependence, uncomplicated (F1020), 7 percent were for cocaine
dependence, uncomplicated (F1420), and 5 percent were for generalized anxiety disorder (F411).
As a result of these findings, OIG recommended that CMS “develop billing requirements for
OTPs to include OUD diagnosis codes on claims for OUD treatment services to indicate that
enrollees have OUD diagnoses and consider working with MACs to implement a system edit to
ensure that OTP payments are made for enrollees only when OUD diagnosis codes are included
on claims.” OIG also stated that “requiring OTPs to include OUD diagnosis codes on claims
could be a way for CMS to monitor whether OTPs furnished OUD treatment services to
enrollees who had an OUD.” In our response to the OIG report, we raised that the lack of an
OUD diagnosis code on a claim is not conclusive evidence of an improper claim because an
OUD diagnosis code is not required for payment when an OTP submits a claim for OUD
treatment services. However, we agreed to explore ways to educate providers about including an
OUD diagnosis on claims.
We continue to monitor claims paid by Medicare to OTPs for furnishing OUD treatment
services, including for potential fraud and abuse. In analyzing our claims data at the beginning of
CY 2024, we found data indicating that the majority of claims paid to OTPs have an OUD
diagnosis code appended, meaning that only a small number of OTPs do not append an OUD
diagnosis code to claims. However, we do intend to ensure that payments made to OTPs are in
alignment with statutory requirements, which is that payments made must be for services
furnished for the treatment of an OUD.
Therefore, we are clarifying that all claims submitted to Medicare, on Form CMS-1450
for institutional providers, and on Form CMS-1500 for professional providers, or the electronic
equivalents, under the OTP benefit must include an OUD diagnosis. These diagnosis codes must
apply to HCPCS G-codes representing both the bundled payments (G2067 through G2075) and
451
https://oig.hhs.gov/oas/reports/region9/92203005.asp.

add-on codes to the bundled payments (G2076-G2080, G2215-G2216, G1028, and G0137).
Applicable diagnosis codes for an OUD that must be submitted on claims include ICD-10-CM
codes in the F11 range for “disorders related or resulting from abuse or misuse of opioids.”
452
CMS plans to issue additional guidance on appending these diagnosis codes to claims. We
believe clarifying these billing requirements is consistent with CMS’s strategic pillars to be a
responsible steward of public funds,
453
and that these requirements are consistent with statutory
provisions under sections 1861(s)(2)(HH), 1861(jjj)(1), and 1834 of the Act.
G. Medicare Shared Savings Program
1. Executive Summary and Background
a. Purpose
Eligible groups of providers and suppliers, including physicians, hospitals, and other
healthcare providers, may participate in the Medicare Shared Savings Program (Shared Savings
Program) by forming or joining an accountable care organization (ACO) and in so doing agree to
become accountable for the total cost and quality of care provided under Traditional Medicare to
an assigned population of Medicare fee-for-service (FFS) beneficiaries. Under the Shared
Savings Program, providers and suppliers that participate in an ACO continue to receive
Traditional Medicare FFS payments under Parts A and B, and the ACO may be eligible to
receive a shared savings payment if it meets specified quality and savings requirements, and in
some instances may be required to share in losses if it increases health care spending.
As of January 1, 2024, the Shared Savings Program has 480 ACOs with over 634,000
health care providers and organizations providing care to over 10.8 million assigned
beneficiaries, making it the largest value-based care program in the country.
454,455
The policy
452
https://www.icd10data.com/ICD10CM/Codes/F01-F99/F10-F19/F11-.
453
https://www.cms.gov/about-cms/what-we-do/cms-strategic-plan.
454
Refer to CMS, Shared Savings Program Fast Facts – As of January 1, 2024, available at
https://www.cms.gov/files/document/2024-shared-savings-program-fast-facts.pdf.
455
See CMS Press Release, “Participation Continues to Grow in CMS’ Accountable Care Organization Initiatives in
2024”, January 29, 2024, available at https://www.cms.gov/newsroom/press-releases/participation-continues-grow-
cms-accountable-care-organization-initiatives-2024.

changes to the Shared Savings Program finalized in the CY 2023 PFS final rule (87 FR 69777
through 69979) and CY 2024 PFS final rule (88 FR 79093 through 79232) are expected to grow
participation in the program and increase the number of beneficiaries assigned to ACOs by up to
four million in the next 10 years (that is, between 2024–2034).
456
These policies are expected to
drive growth in participation, particularly in rural and underserved areas, promote equity, and
advance alignment across accountable care initiatives, and are central to achieving CMS’ goal of
having 100 percent of people with Traditional Medicare in a care relationship with accountability
for quality and total cost of care by 2030.
457
Of note, 19 newly formed ACOs in the Shared
Savings Program are participating in a new, permanent payment option beginning in 2024 that is
enabling these ACOs to receive more than $20 million in advance investment payments (AIPs)
for caring for underserved communities.
458
ACOs are now delivering care to people with
Traditional Medicare in 9,032 Federally Qualified Health Centers, Rural Health Clinics, and
critical access hospitals, an increase of 27 percent from 2023.
459
Section III.G. of this proposed rule addresses changes to the Shared Savings Program
regulations to further advance Medicare’s value-based care strategy of growth, alignment, and
equity and includes changes to allow for timely improvements to program policies and
operations.
We are proposing modifications to the Shared Savings Program to require ACOs,
beginning in performance year 2025 and subsequent performance years, to report the APM
456
Refer to 87 FR 69889. See also, CMS Press Release, “CMS Announces Increase in 2023 in Organizations and
Beneficiaries Benefiting from Coordinated Care in Accountable Care Relationship”, January 17, 2023, available at
https://www.cms.gov/newsroom/press-releases/cms-announces-increase-2023-organizations-and-beneficiaries-
benefiting-coordinated-care-accountable.
457
For a description of CMS’ strategic vision and objectives, see Seshamani M, Fowler E, Brooks-LaSure C.
“Building On The CMS Strategic Vision: Working Together For A Stronger Medicare”. Health Affairs. January 11,
2022. Available at https://www.healthaffairs.org/content/forefront/building-cms-strategic-vision-working-together-
stronger-medicare. See also, CMS, Innovation Center Strategy Refresh, available at
https://innovation.cms.gov/strategic-direction-whitepaper (Innovation Center Strategic Objective 1: Drive
Accountable Care, pages 13 - 17).
458
Refer to CMS Press Release, “Participation Continues to Grow in CMS’ Accountable Care Organization
Initiatives in 2024”, January 29, 2024, available at https://www.cms.gov/newsroom/press-releases/participation-
continues-grow-cms-accountable-care-organization-initiatives-2024.
459
Ibid.
Performance Pathway (APP) Plus quality measure set proposed in section III.G.4. of this
proposed rule. The APP Plus quality measure set would incrementally grow to comprise of
eleven measures, consisting of the six measures in the existing APP quality measure set and five
newly proposed measures from the Adult Universal Foundation measure set that would be
incrementally incorporated into the APP Plus quality measure set over performance years 2025
through 2028. We are also proposing to focus the collection types available to Shared Savings
Program ACOs for reporting the APP Plus quality measure set to eCQMs and Medicare CQMs.
In adopting the APP Plus quality measure set, we are also proposing several changes to the
methodology for calculating the MIPS Quality performance category score for Shared Savings
Program ACOs reporting the APP Plus quality measure set. These include proposing that Shared
Savings Program ACOs that report the APP Plus quality measure set and MIPS eligible
clinicians, groups, and APM Entities that choose to report the APP Plus quality measure set, will
be required to report on all measures in the APP Plus quality measure set, as applicable;
establishing a Complex Organization Adjustment for Virtual Groups and APM Entities,
including Shared Savings Program ACOs, when reporting eCQMs; and scoring Medicare CQMs
using flat benchmarks in their first 2 performance periods in MIPS. Additionally, we are
proposing to extend the eCQM reporting incentive for meeting the Shared Savings Program
quality performance standard to performance year 2025 and subsequent performance years.
Collectively, these proposals aim to align the quality measures that Shared Savings Program
ACOs would be required to report with the quality measures under the Adult Universal
Foundation measure set incrementally beginning in performance year 2025 and prioritize the
eCQM collection type as the gold standard collection type that underlies CMS’ Digital Quality
Measurement Strategic Roadmap while using Medicare CQMs as the transition step on our
building block approach for ACOs’ progress to adopt digital quality measurement.
We propose to establish a new “prepaid shared savings” option to assist eligible ACOs
with a history of earning shared savings. Eligible ACOs that apply and are approved to receive

prepaid shared savings will receive advances of earned shared savings that they can use to make
investments that would aid beneficiaries, such as investments in direct beneficiary services and
investments to improve care coordination through staffing or healthcare infrastructure. At least
50 percent of prepaid shared savings would be reserved to be spent on direct beneficiary services
not otherwise payable in Traditional Medicare, such as meals, dental, vision, hearing, and Part B
cost-sharing support. Additionally, up to 50 percent of the prepaid shared savings can be spent
on staffing and infrastructure. Further, we propose refinements to advance investment payment
policies to allow ACOs receiving advance investment payments to voluntarily terminate from
the payment option while remaining in the Shared Savings Program, and to specify that if CMS
terminates an ACO’s participation agreement, the ACO must repay any outstanding advance
investment payments it received.
We propose modifications to the Shared Savings Program’s financial methodology to
encourage ACO participation in the Shared Savings Program by removing barriers for ACOs
serving underserved communities, and by providing greater specificity and clarification on how
CMS would perform certain financial calculations in the Shared Savings Program. We would
ensure the benchmarking methodology includes sufficient incentive for ACOs serving
underserved communities
460
to enter and remain in the program through the application of a
proposed health equity benchmark adjustment. We propose to specify a calculation methodology
to account for the impact of improper payments in recalculating expenditures and payment
amounts used in Shared Savings Program financial calculations, upon reopening a payment
determination pursuant to § 425.315(a). We propose to establish a methodology for excluding
payment amounts for HCPCS and CPT codes exhibiting significant, anomalous, and highly
suspect billing activity during CY 2024 or subsequent calendar years that warrant adjustment.
460
As described in the CMS Framework for Health Equity and consistent with Executive Order 13985 on Advancing
Racial Equity and Support for Underserved Communities Through the Federal Government (86 FR 7009), the term
“underserved communities” refers to populations sharing a particular characteristic, including geographic
communities that have been systematically denied a full opportunity to participate in aspects of economic, social,
and civic life, as exemplified in the definition of “equity.”

We also propose modifications to provide clarity on the methodology for capping the ACO’s risk
score growth and regional risk score growth. Additionally, building off of a comment solicitation
in CY 2024 PFS rulemaking, through a new request for information in this proposed rule, we are
seeking comments on financial arrangements that could allow for higher risk and potential
reward under a revised ENHANCED track within the Shared Savings Program, including the
designs of and trade-offs between financial model features.
We are proposing changes in connection with Shared Savings Program eligibility
requirements and application procedures. To better align program policies with our goal of
increasing the number of beneficiaries in an accountable care relationship with a health care
provider, we propose to sunset the requirement that CMS terminates the participation agreement
if the ACO’s population is not at least 5,000 by the end of the performance year specified by
CMS in its request for a Corrective Action Plan (CAP) while continuing to require ACOs to meet
the minimum threshold of 5,000 assigned beneficiaries to begin a new agreement. We are
proposing to update provisions of the Shared Savings Program regulations on application
procedures to reflect the latest approach Antitrust Agencies (the Department of Justice and the
Federal Trade Commission
461
) use to evaluate ACOs and enforce the antitrust laws. We are
proposing changes to the Shared Savings Program beneficiary assignment methodology, to
revise the definition of primary care services to align with payment policy proposals described
elsewhere in this proposed rule, and to broaden the existing exception to the program’s voluntary
alignment policy to allow for beneficiaries to be assigned to entities participating in certain
Innovation Center ACO models.
We propose modifications to the beneficiary information notification requirements under
§ 425.312(a)(2)(v)(A) to reduce administrative burden on ACOs while maintaining beneficiary
461
Refer to Withdrawn Final Policy Statement, “Statement of Antitrust Enforcement Policy Regarding Accountable
Care Organizations Participating in the Medicare Shared Savings Program,” available at
https://www.justice.gov/sites/default/files/atr/legacy/2011/10/20/276458.pdf. See also, FTC Press Release, “Federal
Trade Commission Withdraws Health Care Enforcement Policy Statements”, July 14, 2023, available at
https://www.ftc.gov/news-events/news/press-releases/2023/07/federal-trade-commission-withdraws-health-care-
enforcement-policy-statements.
protections. First, we propose to modify the requirements for the timing of the follow-up
communication to a beneficiary who has received the standardized written notice under §
425.312(a)(2)(iii) or (iv). Under the proposed approach, an ACO would be required to provide
the follow-up communication within 180 days from the date the standardized written notice was
provided, as opposed to no later than the earlier of the beneficiary's next primary care service
visit or 180 days from the date the standardized written notice was provided. In addition, we
propose to modify § 425.312(a)(2)(iii) to require ACOs under preliminary prospective
assignment with retrospective reconciliation to provide the standardized written notification to a
subset of the Medicare FFS beneficiary population that is more likely to be assigned to the ACO,
when compared to the population of beneficiaries who must receive the written notification
under current § 425.312(a)(2)(iii). If finalized, this proposal would reduce the burden on ACOs
and confusion for beneficiaries resulting from the current requirement under which ACOs are
required to send this notification to a greater number of beneficiaries who may not ultimately be
assigned to the ACO.
b. Statutory and Regulatory Background on the Shared Savings Program
On March 23, 2010, the Patient Protection and Affordable Care Act (Pub. L. 111–148)
was enacted, followed by enactment of the Health Care and Education Reconciliation Act of
2010 (Pub. L. 111–152) on March 30, 2010, which amended certain provisions of the Patient
Protection and Affordable Care Act (hereinafter collectively referred to as “the Affordable Care
Act”). Section 3022 of the Affordable Care Act amended Title XVIII of the Act (42 U.S.C. 1395
et seq.) by adding section 1899 of the Act to establish the Medicare Shared Savings Program to
facilitate coordination and cooperation among healthcare providers to improve the quality of care
for Medicare FFS beneficiaries and reduce the rate of growth in expenditures under Medicare
Parts A and B. (See 42 U.S.C. 1395jjj.)
Section 1899 of the Act has been amended through subsequent legislation. The
requirements for assignment of Medicare FFS beneficiaries to ACOs participating under the
program were amended by the 21st Century Cures Act (the CURES Act) (Pub. L. 114–255). The
Bipartisan Budget Act of 2018 (Pub. L. 115–123), further amended section 1899 of the Act to
provide for the following: expanded use of telehealth services by physicians or practitioners
participating in an applicable ACO to furnish services to prospectively assigned beneficiaries;
greater flexibility in the assignment of Medicare FFS beneficiaries to ACOs by allowing ACOs
in tracks under retrospective beneficiary assignment a choice of prospective assignment for the
agreement period; permitting Medicare FFS beneficiaries to voluntarily identify an ACO
professional as their primary care provider and requiring that such beneficiaries be notified of the
ability to make and change such identification, and mandating that any such voluntary
identification will supersede claims-based assignment; and allowing ACOs under certain two-
sided models to establish CMS-approved beneficiary incentive programs.
The Shared Savings Program regulations are codified at 42 CFR part 425. The final rule
establishing the Shared Savings Program appeared in the November 2, 2011 Federal Register
(Medicare Program; Medicare Shared Savings Program: Accountable Care Organizations; final
rule (76 FR 67802) (hereinafter referred to as the “November 2011 final rule”)). A subsequent
major update to the program rules appeared in the June 9, 2015 Federal Register (Medicare
Program; Medicare Shared Savings Program: Accountable Care Organizations; final rule (80 FR
32692) (hereinafter referred to as the “June 2015 final rule”)). The final rule entitled “Medicare
Program; Medicare Shared Savings Program; Accountable Care Organizations—Revised
Benchmark Rebasing Methodology, Facilitating Transition to Performance-Based Risk, and
Administrative Finality of Financial Calculations,” which addressed changes related to the
program’s financial benchmark methodology, appeared in the June 10, 2016 Federal Register
(81 FR 37950) (hereinafter referred to as the “June 2016 final rule”). A final rule, “Medicare
Program; Revisions to Payment Policies Under the Physician Fee Schedule and Other Revisions
to Part B for CY 2019; Medicare Shared Savings Program Requirements; Quality Payment
Program; Medicaid Promoting Interoperability Program; Quality Payment Program—Extreme
and Uncontrollable Circumstance Policy for the 2019 MIPS Payment Year; Provisions From the
Medicare Shared Savings Program—Accountable Care Organizations—Pathways to Success;
and Expanding the Use of Telehealth Services for the Treatment of Opioid Use Disorder Under
the Substance Use-Disorder Prevention That Promotes Opioid Recovery and Treatment
(SUPPORT) for Patients and Communities Act”, appeared in the November 23, 2018 Federal
Register (83 FR 59452) (hereinafter referred to as the “November 2018 final rule” or the “CY
2019 PFS final rule”). In the November 2018 final rule, we finalized a voluntary 6-month
extension for existing ACOs whose participation agreements would otherwise expire on
December 31, 2018; allowed beneficiaries greater flexibility in designating their primary care
provider and in the use of that designation for purposes of assigning the beneficiary to an ACO if
the clinician they align with is participating in an ACO; revised the definition of primary care
services used in beneficiary assignment; provided relief for ACOs and their clinicians impacted
by extreme and uncontrollable circumstances in performance year 2018 and subsequent years;
established a new Certified Electronic Health Record Technology (CEHRT) use threshold
requirement; and reduced the Shared Savings Program quality measure set from 31 to 23
measures (83 FR 59940 through 59990 and 59707 through 59715).
A final rule redesigning the Shared Savings Program appeared in the December 31, 2018
Federal Register (Medicare Program: Medicare Shared Savings Program; Accountable Care
Organizations—Pathways to Success and Uncontrollable Circumstances Policies for
Performance Year 2017; final rule (83 FR 67816) (hereinafter referred to as the “December 2018
final rule”)). In the December 2018 final rule, we finalized a number of policies for the Shared
Savings Program, including a redesign of the participation options available under the program
to encourage ACOs to transition to two-sided models; new tools to support coordination of care
across settings and strengthen beneficiary engagement; and revisions to ensure rigorous
benchmarking.
In the interim final rule with comment period (IFC) entitled “Medicare and Medicaid
Programs; Policy and Regulatory Revisions in Response to the COVID-19 Public Health
Emergency,” which was effective on the March 31, 2020 date of display and appeared in the
April 6, 2020 Federal Register (85 FR 19230), we removed the restriction that prevented the
application of the Shared Savings Program extreme and uncontrollable circumstances policy for
disasters that occur during the quality reporting period if the reporting period is extended to offer
relief under the Shared Savings Program to all ACOs that may be unable to completely and
accurately report quality data for 2019 due to the PHE for COVID-19 (85 FR 19267 and 19268).
In the IFC entitled “Medicare and Medicaid Programs; Basic Health Program, and
Exchanges; Additional Policy and Regulatory Revisions in Response to the COVID-19 Public
Health Emergency and Delay of Certain Reporting Requirements for the Skilled Nursing Facility
Quality Reporting Program,” which was effective on May 8, 2020, and appeared in the May 8,
2020 Federal Register (85 FR 27573 through 27587) (hereinafter referred to as the “May 8,
2020 COVID-19 IFC”), we modified Shared Savings Program policies to: (1) allow ACOs
whose agreement periods expired on December 31, 2020, the option to extend their existing
agreement period by 1-year, and allow ACOs in the BASIC track’s glide path the option to elect
to maintain their current level of participation for performance year 2021; (2) adjust program
calculations to remove payment amounts for episodes of care for treatment of COVID-19; and
(3) expand the definition of primary care services for purposes of determining beneficiary
assignment to include telehealth codes for virtual check-ins, e-visits, and telephonic
communication. We also clarified the applicability of the program’s extreme and uncontrollable
circumstances policy to mitigate shared losses for the period of the PHE for COVID-19 starting
in January 2020.
We have also made use of the annual CY PFS rules to address quality reporting for the
Shared Savings Program and certain other issues. For summaries of certain policies finalized in
prior PFS rules, refer to the CY 2020 PFS proposed rule (84 FR 40705), the CY 2021 PFS final
rule (85 FR 84717), the CY 2022 PFS final rule (86 FR 65253 and 65254), the CY 2023 PFS
final rule (87 FR 69779 and 69780), and the CY 2024 PFS final rule (88 FR 79094 and 79095).
In the CY 2024 PFS final rule (88 FR 79093 through 79232), we finalized changes to Shared
Savings Program policies, including to: continue to move ACOs toward digital measurement of
quality by revising the quality performance standard and reporting requirements under the APP
within the Quality Payment Program (QPP); add a third step to the step-wise beneficiary
assignment methodology under which we use an expanded period of time to identify whether a
beneficiary has met the requirement for having received a primary care service from a physician
who is an ACO professional in the ACO to allow additional beneficiaries to be eligible for
assignment, as well as related changes to how we identify assignable beneficiaries used in certain
Shared Savings Program calculations; update the definition of primary care services used for
purposes of beneficiary assignment to remain consistent with billing and coding guidelines;
refine the financial benchmarking methodology for ACOs in agreement periods beginning on
January 1, 2024, and in subsequent years to (1) cap the risk score growth in an ACO’s regional
service area when calculating regional trends used to update the historical benchmark at the time
of financial reconciliation for symmetry with the cap on ACO risk score growth, (2) apply the
same CMS–HCC risk adjustment methodology applicable to the calendar year corresponding to
the performance year in calculating risk scores for Medicare FFS beneficiaries for each
benchmark year, (3) further mitigate the impact of the negative regional adjustment on the
benchmark to encourage participation by ACOs caring for medically complex, high-cost
beneficiaries, and (4) specify the circumstances in which CMS would recalculate the prior
savings adjustment for changes in values used in benchmark calculations due to compliance
action taken to address avoidance of at-risk beneficiaries, or as a result of the issuance of a
revised initial determination of financial performance for a previous performance year following
a reopening of ACO shared savings and shared losses calculations; refine our policies for the
newly established advance investment payments (AIP); make updates to other programmatic
areas including the program's eligibility requirements; and make timely technical changes to the
regulations for clarity and consistency. Further, we also summarized comments received in
response to a comment solicitation on potential future developments to Shared Savings Program
policies, including incorporating a track with higher risk and potential reward than the
ENHANCED track.
Policies applicable to Shared Savings Program ACOs for purposes of quality reporting
for other programs have also continued to evolve based on changes in the statute. For instance,
the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) (Pub. L. 114–10)
established the Quality Payment Program. In the CY 2017 Quality Payment Program final rule
with comment period (81 FR 77008), we established regulations for the MIPS and Advanced
APMs and related policies applicable to eligible clinicians who participate in APMs, including
the Shared Savings Program. We have also made updates to policies under the Quality Payment
Program through the annual CY PFS rules.
c. Summary of Shared Savings Program Proposals
In sections III.G.2. through III.G.8. of this proposed rule, we propose modifications to the
Shared Savings Program’s policies, and describe comment solicitations. As a general summary,
we are proposing the following changes to Shared Savings Program policies to:
● Update Shared Savings Program eligibility requirements and application procedures,
including the following (section III.G.2 of this proposed rule):
++ Update compliance obligations for the requirement that ACOs maintain at least 5,000
assigned beneficiaries by the end of the performance year specified by CMS in its request for a
CAP (section III.G.2.b of this proposed rule).
++ Revise the requirement that newly formed ACOs must agree to allow CMS to share a
copy of their application with the Antitrust Agencies (section III.G.2.c of this proposed rule).
● Revise the policies for determining beneficiary assignment, including the following
(section III.G.3 of this proposed rule):

++ Update the definition of primary care services used in beneficiary assignment at
§ 425.400(c) (section III.G.3.a of this proposed rule).
++ Revise the Shared Savings Program regulations to broaden a limited exception to the
program’s voluntary alignment policy and allow a voluntarily aligned Shared Savings Program
beneficiary to be claims-based assigned to an entity participating in a disease- or condition-
specific CMS Innovation Center model when that model uses claims-based assignment that is
based on primary care and/or other services and the Secretary has determined that a waiver is
necessary solely for purposes of testing the model, in order for beneficiaries with certain diseases
or conditions to benefit from the focused attention and care coordination related to the disease or
condition that an entity participating in such a model can offer (section III.G.3.b of this proposed
rule).
● Revise the quality reporting and the quality performance standard requirements,
including the following (section III.G.4. of this proposed rule):
++ Propose to require Shared Savings Program ACOs to report the APP Plus quality
measure set (section III.G.4.b.(2)(a) of this proposed rule).
++ Propose to focus the collection types available to Shared Savings Program ACOs for
reporting the APP Plus quality measure set to eCQMs and Medicare CQMs (section
III.G.4.b.(2)(b) of this proposed rule).
++ Propose that Shared Savings Program ACOs that report the APP Plus quality
measure set and MIPS eligible clinicians, groups, and APM Entities that choose to report the
APP Plus quality measure set, will be required to report on all measures in the APP Plus quality
measure set, as applicable (section III.G.4.c.(2)(a) of this proposed rule).
++ Propose to establish a Complex Organization Adjustment for Virtual Groups and
APM Entities, including Shared Savings Program ACOs, when reporting eCQMs (section
III.G.4.c.(2)(b) of this proposed rule).
++ Propose to score Medicare CQMs using flat benchmarks in their first two
performance periods in MIPS (section III.G.4.c.(2)(c) of this proposed rule).
++ Propose to extend the eCQM reporting incentive for meeting the Shared Savings
Program quality performance standard to performance year 2025 and subsequent performance
years (section III.G.4.d of this proposed rule).
● Allow eligible ACOs to receive prepaid shared savings (section III.G.5 of this
proposed rule).
● Refine AIP policies, including the following (section III.G.6 of this proposed rule):
++ Allow ACOs receiving advance investment payments to voluntarily terminate from
the payment option while remaining in the Shared Savings Program (section III.G.6.a of this
proposed rule).
++ Codify a policy for recouping advance investment payments from ACOs whose
participation agreements are terminated by CMS (section III.G.6.b of this proposed rule).
● Revise the policies on the Shared Savings Program’s financial methodology, including
the following (section III.G.7 of this proposed rule):
++ Apply a health equity benchmark adjustment (HEBA) which would adjust upward an
ACO’s historical benchmark, based on the number of beneficiaries they serve who are dually
eligible or enrolled in the Medicare Part D Low-Income Subsidy (LIS). This would encourage
and sustain participation by ACOs serving underserved populations that do not benefit from
existing benchmark adjustments for regional efficiency or from generating prior savings (section
III.G.7.b of this proposed rule).
++ Establish a calculation methodology to account for the impact of improper payments
in recalculating expenditures and payment amounts used in Shared Savings Program financial
calculations upon reopening a payment determination pursuant to § 425.315(a) (section III.G.7.c
of this proposed rule).
++ Establish an approach to identify significant, anomalous, and highly suspect (SAHS)
billing activity occurring in CY 2024 or subsequent calendar years, and specify approaches to
mitigating the impact of the SAHS billing activity on Shared Savings Program financial
calculations in CY 2024 or subsequent calendar years. Under the proposed approach we would
exclude payment amounts from expenditure and revenue calculations for the relevant calendar
year for which the SAHS billing activity is identified, as well as from historical benchmarks used
to reconcile the ACO for a performance year corresponding to the calendar year for which the
SAHS billing activity is identified (section III.G.7.d of this proposed rule).
++ Seek comment on establishing a higher risk / reward option than the current
ENHANCED track (section III.G.7.e of this proposed rule).
++ Propose technical changes, for consistency and clarity in provisions of the Shared
Savings Program regulations on financial calculations, to align and clarify the language used to
describe weights applied to the growth in ACO and regional risk scores for each Medicare
enrollment type, as part of the calculation for capping ACO and regional risk score growth,
respectively. The weight for a given enrollment type would be equal to the product of the ACO's
historical benchmark expenditures after the application any adjustment applied under §
425.652(a)(8) of the regulations (that is, the regional adjustment, prior savings adjustment or
proposed HEBA (if finalized), or no adjustment) for that enrollment type and the ACO's
performance year assigned beneficiary person years for that enrollment type (section III.G.7.f of
this proposed rule).
● Modify beneficiary notification requirements, including the following (section III.G.8
of this proposed rule):
++ ACOs must provide the follow-up beneficiary communication no later than 180 days
after the date that the ACO provided the standardized written notice to the beneficiary (section
III.G.8.a of this proposed rule).
++ For ACOs that select preliminary prospective assignment with retrospective
reconciliation, limit the distribution of the standardized written beneficiary information
notification to beneficiaries who are more likely to be assigned to the ACO, when compared to
the beneficiaries who must receive the written notification under current regulations (section
III.G.8.b of this proposed rule).
Taken together, the Shared Savings Program proposals in this proposed rule are
anticipated to improve ACOs’ incentives to join the program and continue participating in future
years and earn shared savings. The proposals are projected to reduce program spending by $260
million in total over the 10-year period 2025 through 2034. These changes are anticipated to
support the goals outlined in the CY 2023 PFS final rule (87 FR 69777 through 69978) and CY
2024 PFS final rule (88 FR 79093 through 79232) for growing the program, with a particular
focus on including underserved communities.
Certain policies, including both existing policies and the proposed new policies described
in this proposed rule, rely upon the authority granted in section 1899(i)(3) of the Act to use other
payment models that the Secretary determines will improve the quality and efficiency of items
and services furnished under the Medicare program, and that do not result in program
expenditures greater than those that would result under the statutory payment model. The
following proposals require the use of our authority under section 1899(i) of the Act: the
proposal to allow eligible ACOs to receive prepaid shared savings; the proposal to use a
calculation methodology to account for the impact of improper payments in recalculating
expenditures and payment amounts for certain Shared Savings Program financial calculations,
upon reopening an ACO’s payment determination and issuing a revised initial determination
pursuant to § 425.315(a); the proposal to use a methodology for certain Shared Savings Program
financial calculations to mitigate the impact of SAHS billing activity occurring in CY 2024 or
subsequent calendar years; and the proposed technical changes to the provision describing how
we calculate the weights applied when capping growth in regional risk scores as part of the
regional component of the three-way blended benchmark update factor. As described in the
Regulatory Impact Analysis in section VII. and elsewhere in this proposed rule, these proposed
changes to our payment methodology are expected to improve the quality and efficiency of care
and are not expected to result in a situation in which the payment methodology under the Shared
Savings Program, including all policies adopted under the authority of section 1899(i) of the Act,
results in more spending under the program than would have resulted under the statutory
payment methodology in section 1899(d) of the Act. We will continue to reexamine this
projection in the future to ensure that the requirement under section 1899(i)(3)(B) of the Act that
an alternative payment model not result in additional program expenditures continues to be
satisfied. In the event that we later determine that the payment model that includes policies
established under section 1899(i)(3) of the Act no longer meets this requirement, we would
undertake additional notice and comment rulemaking to make adjustments to the payment model
to assure continued compliance with the statutory requirements.
2. Eligibility Requirements and Application Procedures
a. Overview
We are proposing two modifications to the Shared Savings Program eligibility and
application procedures that will be implemented for performance years beginning on or after
January 1, 2025. Specifically, we propose the following, which are discussed in more detail in
sections (b) and (c) below:
● Sunset the requirement after January 1, 2025, at § 425.110(b)(2) that CMS terminates
the participation agreement and the ACO is not eligible to share in savings for that performance
year if the ACO’s assigned population is not at least 5,000 by the end of the performance year
specified by CMS in its request for a CAP and
● Revise the antitrust language in the application procedures at §§ 425.202(a)(3) and
425.224(a)(3) for the Shared Savings Program.
b. Monitoring Compliance with the Requirement that ACOs Maintain at least 5,000 Assigned
Beneficiaries
Section 1899(b)(2)(D) of the Act requires participating ACOs to include primary care
ACO professionals that are sufficient for the number of Medicare FFS beneficiaries assigned to
the ACO and that at a minimum, the ACO shall have at least 5,000 such beneficiaries assigned to
it. In the November 2011 final rule (76 FR 67808), in alignment with the statutory requirement at
section 1899(b)(2)(D) of the Act, CMS established that, at a minimum, an ACO shall have at
least 5,000 such beneficiaries assigned to it to be eligible to participate in the Shared Savings
Program under § 425.110. We described the importance of maintaining at least 5,000 assigned
beneficiaries with respect to both eligibility of the ACO to participate in the program and the
statistical stability for purposes of calculating per capita expenditures and assessing financial and
quality performance. We noted, however, that we understood circumstances may change during
the agreement period, and that an ACO’s assigned population may vary accordingly.
To enforce program requirements under § 425.110, while still recognizing that variations
may occur for an ACO’s assigned population, CMS generally issues a warning notice and
request the ACO submit a Corrective Action Plan (CAP) should the ACO’s assigned population
fall below 5,000 beneficiaries. Few ACOs have had a beneficiary population that fell below
5,000. Between calendar year 2020 and 2023, based on the program's compliance monitoring
review, 24 ACOs have been below this assignment threshold at the start of one or more
performance years within an agreement period, which led CMS to issue compliance actions.
Approximately 55 percent of these ACOs opted to voluntarily terminate ahead of the CAP
deadline imposed by CMS, while approximately 40 percent were able to increase their
beneficiary assignment over the threshold and remain in the program. Given additional time,
more ACOs likely would be able to increase their beneficiary assignment, keeping more
beneficiaries in accountable care relationships, and maintain their participation in the Shared
Savings Program.
Separately, we had established a policy in the December 2018 final rule (83 FR 67925)
providing for an ACO to select the Minimum Savings Rate (MSR)/Minimum Loss Rate (MLR)
that CMS would use when performing shared savings and shared losses calculations for the
ACO. As we have previously discussed, the MSR/MLR protects against an ACO earning shared
savings or being liable for shared losses when the change in expenditures represents normal, or
random, variation rather than an actual change in performance (see, for example, 83 FR 67923
through 67926).
In the December 2018 final rule (83 FR 67925 through 67929), we revised § 425.110(b)
to provide for the use of a variable MSR/MLR when performing shared savings and shared
losses calculations if an ACO’s assigned beneficiary population fell below 5,000 for the
performance year regardless of whether the ACO had previously selected a fixed or variable
MSR/MLR. This policy protects the statistical stability of the program’s expenditure
calculations. As an ACO’s assigned beneficiary population decreases, variability in the
population’s expenditures increases. We thus expressed concern that the reduction in the size of
the ACO's assigned beneficiary population would cause shared savings payments made to the
ACO to not reflect true cost savings, but normal expenditure fluctuations (83 FR 67926). The use
of a variable MSR/MLR thus made it more difficult for an ACO under performance-based risk
that falls below the 5,000-beneficiary threshold to earn shared savings or be responsible for
shared losses to ensure that the savings or losses reflected the ACO’s actual performance and not
merely statistical noise. This policy provided additional protection to the Medicare Trust Funds
and greater protection for ACOs against owing shared losses.
As described above, an ACO’s failure to maintain at least 5,000 assigned beneficiaries
may result in compliance actions, up to and including termination of the ACO from the Shared
Savings Program. When originally developed, this program policy was intended in part to protect
both CMS and the ACO from variability in the expenditure calculations caused by a small
assigned beneficiary population. With the MSR and MLR adjustments finalized in the December
2018 final rule, we developed protections against issues with the benchmark calculation for
ACOs with fewer assigned beneficiaries, which provide adequate protection against variability in
the short term. The MSR and MLR sliding scale varies based on the number of beneficiaries
assigned to the ACO from 1 up to 60,000. Currently, this adjustment to the MSR/MLR protects
both CMS and the ACO from inappropriate over or underpayments, reducing the financial risk of
allowing ACOs to continue to participate in the Shared Savings Program if they experience a
reduction in assigned beneficiaries.
In light of the effectiveness of the variable MSR/MLR policy described above, we are
proposing to sunset the requirement at § 425.110(b)(2) that CMS will terminate an ACO’s
participation agreement and determine that an ACO is not eligible to share in savings for that
performance year if an ACO’s assigned population is not at least 5,000 by the end of the
performance year specified by CMS in its request for a CAP. Specifically, we propose to revise §
425.110(b)(2) to limit its application to performance years starting before January 1, 2025. Thus,
for performance years beginning on or after January 1, 2025, if the ACO’s assigned population is
not at least 5,000 by the end of the performance year specified by CMS in its request for a CAP,
CMS will not be required to terminate the participation agreement.
This proposal would not modify the requirement at § 425.110(a), which implements the
statutory requirement at section 1899(b)(2)(D) of the Act that ACOs have 5,000 beneficiaries at
critical points in CMS’s determination of the ACO’s eligibility to participate in the Shared
Savings Program, including: at the time of application in order to be eligible for the Shared
Savings Program, and at any point when an ACO elects to renew its participation in the program.
As discussed in the November 2011 final rule (76 RF 67808), CMS has found “[a] minimum
threshold is important with respect to both the eligibility of the ACO to participate in the
program and to the statistical stability for purposes of calculating per capita expenditures and
assessing quality performance”. A 5,000 beneficiary minimum, paired with a variable
MSR/MLR, enables ACOs to have their work of improving beneficiary care best reflected in

their financial performance and shared savings results. Additionally, we would retain §
425.110(b), which states that an ACO may be subject to actions under §§ 425.216 and 425.218 if
its assigned population falls below 5,000 at any time during the performance year. This proposed
approach provides CMS with additional flexibility in the compliance actions that we take in
working with ACOs to help them return to the 5,000 beneficiary threshold.
The proposed modification aligns with CMS’s broader goals to expand the number of
beneficiaries in accountable care relationships. We anticipate this flexibility will provide ACOs
with additional time and opportunities to recruit additional providers and suppliers to increase
their assigned beneficiary population rather than being required to exit the Shared Savings
Program due to their beneficiary attribution. We seek comment on this proposal. If finalized, this
proposed change would be effective beginning on January 1, 2025.
c. Update Antitrust Language
Section 425.202(a)(3) requires that ACOs that are newly formed after March 23, 2010,
agree to allow CMS to share a copy of their application with the Antitrust Agencies (the Federal
Trade Commission (FTC) and the Department of Justice (DOJ), as defined in the Statement of
Antitrust Enforcement Policy Regarding Accountable Care Organizations Participating in the
Medicare Shared Savings Program). This policy has been in effect since the enactment of the
November 2011 final rule (76 FR 67822). We stated at the time that this policy was in the public
interest to harmonize the eligibility criteria for ACOs that wished to participate in the Shared
Savings Program with similar antitrust criteria on clinical integration, because competition
among ACOs was expected to have significant benefits for Medicare beneficiaries.
In 2023, both the DOJ and the FTC withdrew the outdated Antitrust Enforcement Policy
Statement because the policy no longer served its intended purpose of providing useful guidance
to market participants.
462
Instead, both Antitrust Agencies have stated that they will continue to
462
U.S Department of Justice, Press Release, Justice Department Withdraws Outdated Enforcement Policy
Statements (Feb. 3, 2023), available at https://www.justice.gov/opa/pr/justice-department-withdraws-outdated-

vigorously enforce the antitrust laws in the health care markets by evaluating mergers and
conduct that harm competition on a case-by-case basis.
As a result, we propose to modify the Shared Savings Program eligibility requirements
that will be implemented on January 1, 2025, by removing the reference to the Antitrust
Enforcement Policy Statement in § 425.202(a)(3), and also in § 425.224(a)(3). This proposal
aligns the Shared Savings Program with the Antitrust Agencies’ decisions to withdraw the
Antitrust Enforcement Policy Statement. We propose to edit § 425.202(a)(3) to state, “An ACO
that seeks to participate in the Shared Savings Program must agree that CMS can share a copy of
their application with the Antitrust Agencies.” Similarly, we propose to edit § 425.224(a)(3) to
state, “An ACO that seeks to enter a new participation agreement under the Shared
Savings Program must agree that CMS can share a copy of its application with the Antitrust
Agencies.” We also plan to remove guidance from the Shared Savings Program website detailing
how an ACO could calculate their share of services in each applicable Primary Service Area
(PSA), as described in the Antitrust Policy Statement, as this is no longer useful to ACOs.
As we stated in 76 FR 67822, we intend to coordinate closely with the Antitrust Agencies
throughout the application process and the operation of the Shared Savings Program to ensure
there are no detrimental impacts to competition. CMS will share application and participation
information including aggregate claims data regarding allowed charges and fee-for-service
payments for all ACOs accepted in the Shared Savings Program, with the Antitrust Agencies
needed to further any investigations or support their enforcement of the antitrust laws.
We seek comment on this proposal. If finalized, this proposed change would be effective
beginning on January 1, 2025.
enforcement-policy-statements; Federal Trade Commission, Press Release, Federal Trade Commission Withdraws
Health Care Enforcement Policy Statements (July 14, 2023), available at https://www.ftc.gov/news-
events/news/press-releases/2023/07/federal-trade-commission-withdraws-health-care-enforcement-policy-
statements.
3. Beneficiary Assignment Methodology
a. Proposed Revisions to the Definition of Primary Care Services
(1) Background
Section 1899(c)(1) of the Act, as amended by the CURES Act and the Bipartisan Budget
Act of 2018, provides that for performance years beginning on or after January 1, 2019, the
Secretary shall assign beneficiaries to an ACO based on their utilization of primary care services
provided by a physician who is an ACO professional and all services furnished by Rural Health
Clinics (RHCs) and Federally Qualified Health Centers (FQHCs). However, the statute does not
specify a list of services considered to be primary care services for purposes of beneficiary
assignment.
In the November 2011 final rule (76 FR 67853), we established the initial list of services,
identified by Current Procedural Terminology (CPT) and Healthcare Common Procedure Coding
System (HCPCS) codes, that we considered to be primary care services. In that final rule, we
indicated that we intended to monitor CPT and HCPCS codes and would consider making
changes to the definition of primary care services to add or delete codes used to identify primary
care services if there were sufficient evidence that revisions were warranted. We have updated
the list of primary care service codes in subsequent rulemaking (refer to 80 FR 32746 through
32748; 80 FR 71270 through 71273; 82 FR 53212 and 53213; 83 FR 59964 through 59968; 85
FR 27582 through 27586; 85 FR 84747 through 84756; 85 FR 84785 through 84793; 86 FR
65273 through 65279; 87 FR 69821 through 69825; 88 FR 79163 through 79174) to reflect
additions or modifications to the codes that have been recognized for payment under the PFS and
to incorporate other changes to the definition of primary care services for purposes of the Shared
Savings Program. For the performance year beginning on January 1, 2024, and subsequent
performance years, we defined primary care services for purposes of assigning beneficiaries to
ACOs under § 425.402 in § 425.400(c)(1)(viii).
(2) Proposed Revisions
Based on feedback from ACOs and our further review of the HCPCS and CPT codes that
are currently recognized for payment under the PFS or that we are proposing to recognize for
payment starting in CY 2025, we believe it would be appropriate to amend the definition of
primary care services used in the Shared Savings Program assignment methodology to include
certain additional codes for the performance year starting on January 1, 2025, and subsequent
performance years, in order to remain consistent with billing and coding under the PFS.
We propose to specify a revised definition of primary care services used for assignment
in a new provision of the Shared Savings Program regulations at § 425.400(c)(1)(ix) to include
the list of HCPCS and CPT codes specified in § 425.400(c)(1)(viii), as well as the following
additions: (1) Safety Planning Interventions (HCPCS code GSPI1) when the base code is also a
primary care service code, if finalized under Medicare FFS payment policy; (2) Post-Discharge
Telephonic Follow-up Contacts Intervention (HCPCS code GFCI1), if finalized under Medicare
FFS payment policy; (3) Virtual Check-in Service (CPT code 9X091), if finalized under
Medicare FFS payment policy; (4) Advanced Primary Care Management Services (HCPCS
GPCM1, GPCM2, and GPCM3), if finalized under Medicare FFS payment policy; (5)
Cardiovascular Risk Assessment and Risk Management Services (HCPCS codes GCDRA and
GCDRM), if finalized under Medicare FFS payment policy; (6) Interprofessional Consultation
Services (CPT codes 99446, 99447, 99448, 99449, 99451, 99452); (7) Direct Care Caregiver
Training Services (HCPCS codes GCTD1, GCTD2 and GCTD3), if finalized under Medicare
FFS payment policy; and (8) Individual Behavior Management/Modification Caregiver Training
Services (HCPCS codes GCTB1 and GCTB2), if finalized under Medicare FFS payment policy.
We propose that the new provision at § 425.400(c)(1)(ix) would be applicable for use in
determining beneficiary assignment for the performance year starting on January 1, 2025, and
subsequent performance years.
The following provides additional information about the CPT and HCPCS codes that we
are proposing to add to the definition of primary care services used for purposes of beneficiary
assignment:
● Safety Planning Interventions (SPI) (HCPCS code GSPI1 (Safety planning
interventions, including assisting the patient in the identification of the following personalized
elements of a safety plan: recognizing warning signs of an impending suicidal crisis; employing
internal coping strategies; utilizing social contacts and social settings as a means of distraction
from suicidal thoughts; utilizing family members, significant others, caregivers, and/or friends to
help resolve the crisis; contacting mental health professionals or agencies; and making the
environment safe; (List separately in addition to an E/M visit or psychotherapy)): In section II.I
of this proposed rule, we are proposing under the PFS to create an add-on G-code that would be
billed along with an E/M visit or psychotherapy visit when safety planning interventions are
personally performed by the billing practitioner in a variety of settings. Safety planning
interventions involve a person working with a clinician to develop a personalized list of coping
strategies and sources of support that the person could use in the event of experiencing thoughts
of harm to themselves or others. This is not a suicide risk assessment, but rather, an intervention
provided to people determined to have elevated risk. Safety planning interventions have also
been used to reduce the risk of suicide. The basic components of a safety plan include the
following: (1) recognizing warning signs of an impending suicidal crisis or actions that increase
the risk of overdose; (2) employing internal coping strategies; (3) utilizing social contacts and
social settings as a means of distraction from suicidal thoughts and/or taking steps to reduce the
risk of suicide; (4) utilizing family members or friends to help resolve the crisis; (5) contacting
mental health professionals, crisis services, or agencies; and (6) making the environment safe,
including restricting access to lethal means, if applicable.
Refer to section II.I of this proposed rule for detailed, technical discussion regarding the
proposed description, payment, and utilization of this HCPCS code.
In the CY 2019 PFS final rule (83 FR 59965 through 59966), we finalized the addition of
prolonged evaluation and management or psychotherapy service(s) beyond the typical service
time of the primary procedure (CPT codes 99354 and 99355) to the definition of primary care
services used for purposes of assignment because these two codes are “add-on codes” that
describe additional resource components of a broader service furnished in the office or other
outpatient setting that are not accounted for in the valuation of the base codes. For the same
reason, we believe it would be appropriate to also include HCPCS code GSPI1, if finalized under
Medicare FFS policy since GSPI1 is being proposed as an add-on service to an E/M or
psychotherapy visit. Evaluation and management visits are included in the definition of primary
care services used for purposes of assignment and so we believe it would be appropriate to also
include GSPI1, when billed with an E/M visit, in the definition of primary care services used for
purposes of assignment to assign beneficiaries more accurately to ACOs participating in the
Shared Savings Program. We further believe the services billed under this code reflect the types
of services we expect primary care providers to provide in order to improve continuity of care.
Including Safety Planning Intervention services in the definition of primary care services used
for purposes of assignment would also align with the CMS Behavioral Health Strategy, the
mission of which is to ensure that high-quality behavioral health services and supports are
accessible to Medicare beneficiaries.
We note that, as proposed, HCPCS code GSPI1 could also be billed with psychotherapy
services, which are not considered for purposes of beneficiary assignment under § 425.400(c).
Therefore, we propose to include the allowed charges for HCPCS code GSPI1, for purposes of
assigning beneficiaries to ACOs, only when billed with a service which is also included in the
definition of primary care services.
● Post-Discharge Telephonic Follow-up Contacts Intervention (FCI) (HCPCS code
GFCI1: Post discharge telephonic follow-up contacts performed in conjunction with a discharge
from the emergency department for behavioral health or other crisis encounter, per calendar
month). In section II.I of this proposed rule, we describe FCI as a specific protocol of services for
individuals with suicide risk involving a series of telephone contacts between a provider and
person in the weeks and sometimes months following discharge from the emergency department
and other relevant care settings, that occurs when the person is in the community and is designed
to reduce the risk of subsequent adverse outcomes. FCI calls are typically 10-20 minutes in
duration and aim to encourage use of the Safety Plan (as needed in a crisis) and updating it to
optimize effectiveness, expressing psychosocial support, and helping to facilitate engagement in
any indicated follow-up care and services. We are proposing to create a monthly billing code to
describe the specific protocols involved in furnishing post-discharge telephonic follow-up
contacts that are performed in conjunction with a discharge from the emergency department for a
crisis encounter, as a bundled service describing four calls in a month, each lasting between 10-
20 minutes. We are proposing to price this service based on a direct crosswalk to CPT code
99426 (Principal care management; first 30 minutes of clinical staff time directed by a physician
or other qualified healthcare professional) because we believe the work would be similar in
nature and intensity.
Refer to section II.I. of this proposed rule for detailed, technical discussion regarding the
proposed description, payment, and utilization of this HCPCS code.
These services are similar to TCM services (CPT codes 99495 and 99496), which are
included in the definition of primary care services used for purposes of assignment under §
425.400(c), in that these services help eligible people transition back to a community setting after
a stay at certain facility types like TCM. Similar to the rationale described December 2014
proposed rule (79 FR 72792) and later finalized in the June 2015 final rule (80 FR 32746 through
32748) where we finalized the inclusion of TCM services in the definition of primary care
services used for purposes of assignment, providing separate payment for the work of
community physicians and practitioners in treating a patient following discharge from a hospital
or nursing facility would ensure better continuity of care for these patients and help reduce
avoidable readmissions. Therefore, we believe that FCI services should also be included in the
definition of primary care services used for beneficiary assignment since FCI services are
designed to assist in the transition from the emergency department into the community. We
believe the services billed under this code reflect the types of services we expect primary care
providers to provide in order to improve care coordination and care management. Thus, we
believe that FCI services should also be included.
Further, in determining the recommended pricing for HCPCS code GFCI1, we
recommend pricing this service based on a direct crosswalk to Principal Care Management
(PCM) service (CPT code 99426) because we believe the work would be similar in nature, as
well as time and intensity. In the CY 2021 PFS final rule (85 FR 84749), we finalized the
inclusion of HCPCS codes G2064 and G2065 in the definition of primary care services used for
purposes of assignment since we expect that most services billed under these codes will be billed
by specialists who are focused on managing patients with a single complex chronic condition
requiring substantial care management. These HCPCS codes were replaced by CPT codes 99424,
99425, 99426, and 99427 in the CY 2022 PFS final rule (86 FR 65275). PCM services (CPT
codes 99424, 99425, 99426, and 99427 and HCPCS codes G2064 and G2065) are included in the
definition of primary care services used for purposes of assignment and since FCI services are
similar in nature, time, and intensity to PCM services, we believe it would be appropriate to
include these services in the definition of primary care services used for purposes of assignment.
Including FCI services in the definition of primary care services used for purposes of assignment
would also align with the CMS Behavioral Health Strategy as the FCI services are designed to
support beneficiaries with follow-up care related to suicide risk.
● Virtual Check-in Service (CPT code 9X091):
++ CPT code 9X091 (Brief communication technology-based service (e.g., virtual check-
in) by a physician or other qualified health care professional who can report evaluation and
management services, provided to an established patient, not originating from a related
evaluation and management service provided within the previous 7 days nor leading to an

evaluation and management service or procedure within the next 24 hours or soonest available
appointment, 5-10 minutes of medical discussion).
The CPT Editorial Panel established a new CPT code 9X091 describing a brief virtual
check-in encounter that is intended to evaluate the need for a more extensive visit. The code
descriptor for CPT code 9X091 mirrors that of existing HCPCS code G2012 (Brief
communication technology-based service, e.g., virtual check-in, by a physician or other qualified
health care professional who can report evaluation and management services, provided to an
established patient, not originating from a related E/M service provided within the previous 7
days nor leading to an E/M service or procedure within the next 24 hours or soonest available
appointment; 5-10 minutes of medical discussion) and, per the CPT Editorial Panel materials, is
intended to replace that code. HCPCS code G2012 is included in the Shared Savings Program
definition of primary care services used for purposes of assignment.
In section II.E of this proposed rule, we are proposing separate payment for CPT code 9X091.
Because the code description for CPT code 9X091 mirrors HCPCS code G2012 and because, per
CPT Editorial Panel materials, is intended to replace HCPCS code G2012, we are proposing to
make CPT code 9X091 separately payable under Medicare. We note we are proposing to delete
HCPCS code G2012 for purposes of Medicare PFS payment policy, however, HCPCS code
G2012 will continue to be included in the definition of primary care services used for purposes
of assignment, consistent with how deleted CPT and HCPCS codes have been handled
historically and to allow for consistency with calculating historical benchmarks.
We propose that we would include CPT code 9X091 in the definition of primary care
services used for purposes of assignment as the code description of brief communication
technology-based service mirrors the description of HCPCS code G2012, which is included in
the definition of primary care services used for purposes of assignment since these services are
furnished to established patients by physicians or qualified health care professionals that can
report E/M services in lieu of an in person primary care visit (85 FR 84753). Since CPT code
9X091 is a direct replacement of HCPCS code G2012, 9X091 would be included in the
definition of primary care services used for purposes of assignment, under proposed §
425.400(c)(1)(ix)(C). In the CY 2022 Physician Fee Schedule file rule (86 FR 65277 through
65279), we finalized a policy wherein we would incorporate into the definition of primary care
services a permanent CPT code when it directly replaces another CPT code or a temporary
HCPCS code (for example, a G-code) that is already included in the definition of primary care
services for purposes of determining beneficiary assignment under the Shared Savings Program.
Additionally, CPT code 9X091, per the CPT Editorial Panel materials, is intended to be reported
instead of HCPCS code G2012, which is already included in the definition of primary care
services used for purposes of assignment. We further believe the services billed under this code
reflect the types of services we expect primary care providers to provide in order to improve care
coordination and care management.
We explained that this approach would help to ensure the appropriate identification of
primary care services used in the Shared Savings Program's assignment methodology by
allowing for the immediate inclusion of replacement CPT codes in the determination of
beneficiary assignment and lead to continuity in the assignment of beneficiaries receiving those
services based on current coding. This continuity would improve predictability for ACOs, while
also increasing the consistency of care coordination for their assigned beneficiaries. We further
finalized that such replacement codes would be incorporated into the definition of the primary
care services for purposes of determining beneficiary assignment for the performance year, when
the assignment window for a benchmark or performance year (as defined in § 425.20) includes
any day on or after the effective date of the replacement code for payment purposes under FFS
Medicare. CPT code 9X091 has an effective date of January 1, 2025.
● Advanced Primary Care Management (HCPCS codes GPCM1, GPCM2, and GPCM3);
1) HCPCS code GPCM1: (Advanced primary care management services provided by
clinical staff and directed by a physician or other qualified health care professional who is
responsible for all primary care and serves as the continuing focal point for all needed health
care services, per calendar month, with the following elements, as appropriate:
● Consent;
++ Inform the patient regarding availability of the service; that only one practitioner
can furnish and be paid for the service during a calendar month; of the right to stop the
services at any time (effective at the end of the calendar month); and that cost sharing
may apply.
++ Document in patient’s medical record that consent was obtained.
● Initiation during a qualifying visit for new patients or patients not seen within 3 years;
● Provide 24/7 access for urgent needs to care team/ practitioners, including providing
patients/caregivers with a way to contact health care professionals in the practice to discuss
urgent needs regardless of the time of day or day of week;
● Continuity of care with a designated member of the care team with whom the patient is
able to schedule successive routine appointments;
● Deliver care in alternative ways to traditional office visits to best meet the patient’s
needs, such as home visits, and/or expanded hours;
● Overall comprehensive care management;
++ Systematic needs assessment (medical and psychosocial).
++ System-based approaches to ensure receipt of preventive services.
++ Medication reconciliation, management and oversight of self-management.
● Development, implementation, revision, and maintenance of an electronic patient-
centered comprehensive care plan;
++ Care plan is available timely within and outside the billing practice as
appropriate to individuals involved in the beneficiary’s care, can be routinely accessed
and updated by care team/practitioner, and copy of care plan to patient/caregiver.
● Coordination of care transitions between and among health care providers and
settings, including referrals to other clinicians and follow-up after an emergency department
visit and discharges from hospitals, skilled nursing facilities or other health care facilities as
applicable;
++ Ensure timely exchange of electronic health information with other
practitioners and providers to support continuity of care.
++ Ensure timely follow-up communication (direct contact, telephone,
electronic) with the patient and/or caregiver after an emergency department visit and
discharges from hospitals, skilled nursing facilities, or other health care facilities, within
7 calendar days of discharge, as clinically indicated.
● Ongoing communication and coordinating receipt of needed services from
practitioners, home- and community-based service providers, community-based social
service providers, hospitals, and skilled nursing facilities (or other health care facilities),
and document communication regarding the patient’s psychosocial strengths and needs,
functional deficits, goals, preferences, and desired outcomes, including cultural and
linguistic factors, in the patient’s medical record;
● Enhanced opportunities for the beneficiary and any caregiver to communicate
with the care team/practitioner regarding the beneficiary’s care through the use of
asynchronous non-face-to-face consultation methods other than telephone, such as
secure messaging, email, internet, or patient portal, and other communication-
technology based services, including remote evaluation of pre-recorded patient
information and interprofessional telephone/internet/EHR referral service(s), to maintain
ongoing communication with patients, as appropriate;
++ Ensure access to patient-initiated digital communications that require a clinical
decision, such as virtual check-ins and digital online assessment and management and
E/M visits (or e-visits).
● Analyze patient population data to identify gaps in care and offer additional
interventions, as appropriate;
● Risk stratify the practice population based on defined diagnoses, claims, or other
electronic data to identify and target services to patients;
● Be assessed through performance measurement of primary care quality, total cost of
care, and meaningful use of Certified EHR Technology.
2) HCPCS code GPCM2 (Advanced primary care management services for a patient with
multiple (two or more) chronic conditions expected to last at least 12 months, or until the death
of the patient, which place the patient at significant risk of death, acute
exacerbation/decompensation, or functional decline, provided by clinical staff and directed by a
physician or other qualified health care professional who is responsible for all primary care and
serves as the continuing focal point for all needed health care services, per calendar month, with
the elements included in GPCM1, as appropriate), and
3) HCPCS code GPCM3 (Advanced primary care management services for a
patient who is a Qualified Medicare Beneficiary with multiple (two or more) chronic
conditions expected to last at least 12 months, or until the death of the patient, which
place the patient at significant risk of death, acute exacerbation/decompensation, or
functional decline, provided by clinical staff and directed by a physician or other
qualified health care professional who is responsible for all primary care and serves as
the continuing focal point for all needed health care services, per calendar month, with
the elements included in GPCM1, as appropriate).
In section II.G. of this proposed rule, we are proposing to establish coding and make
payment under the PFS for a newly defined set of APCM services as described and defined by
three HCPCS codes (GPCM1, GPCM2, and GPCM3) to recognize the resource costs associated
with furnishing services using an “advanced primary care approach” supported by a team-based
care structure under the PFS.
Delivery of care using an advanced primary care model involves
restructuring of the primary care team, which includes the billing practitioner and the auxiliary
personnel under their general supervision, within practices. This restructuring creates several
advantages for patients, and provides more broad accessibility and alternative methods for
patients to communicate with their care team/practitioner about their care outside of in-person
visits (for example, virtual, asynchronous interactions, such as online chat), which can lead to
more timely and efficient identification of, and responses to, health care needs (for example,
practitioners can route patients to the optimal clinician and setting—to a synchronous visit, an
asynchronous chat, or a direct referral to the optimal site of care). Practitioners using an
advanced primary care delivery model can more easily collaborate across clinical disciplines
through remote interprofessional consultations with specialists, as well as standardize condition
management into evidence-based clinical workflows, which allow for closed-loop follow-up and
more real-time management for patients with acute or evolving complex issues, partner on
complex decisions, and personalize their patients’ care plans.
Specifically, we are proposing to adopt specific coding and payment policies for APCM
services for use by practitioners who are providing services under this specific model of
advanced primary care, when the practitioner is the continuing focal point for all needed health
care services and responsible for all primary care services.
Providing care using an advanced primary care delivery model involves resource costs
associated with maintaining certain practice capabilities and continuous readiness and
monitoring activities to support a team-based approach to care, where significant resources are
used on virtual, asynchronous patient interactions, collaboration across clinical disciplines, and
real-time management of patients with acute and complex concerns that are not fully recognized
or paid for by the existing care management codes. As the delivery of primary care has evolved
to embrace advanced primary care more fully, we believe that it is prudent to now adopt specific
coding and payment policies to better recognize the resources involved in care management
under an advanced primary care delivery model.
We are seeking to ensure that the APCM codes would fully and appropriately capture the
care management and CTBS services that are characteristic of the changes in medical practice
toward advanced primary care, as demonstrated in select CMS Innovation Center models. As we
do for CCM and PCM services, we propose to require for APCM services that the practitioner
provide an initiating visit and obtain beneficiary consent (see section II.G.2.c.(1) and II.G.2.c.(2)
of this proposed rule). We are proposing to incorporate as elements of APCM services
“Management of Care Transitions” and “Enhanced Communications Opportunities.” For the
“Management of Care Transitions” APCM service element, we are proposing to specify timely
follow-up during care transitions (see section II.G.2.c.(6) of this proposed rule). For the
“Enhanced Communications Opportunities” APCM service element, we are proposing to
incorporate digital access through CTBS services, such as virtual check-ins and remote
evaluation of images, to maintain ongoing communication with the patient (see section
II.G.2.c.(8) of this proposed rule). We are also proposing to specify for APCM services the
practice-level characteristics and capabilities that we believe to be inherent to, and necessarily
present when a practitioner is providing covered services using, the “advanced primary care”
model. Included in the service descriptors for GPCM1, GPCM2, and GPCM3 are proposed
practice-level capabilities that reflect care delivery using an advanced primary care model that
focused around 24/7 access and continuity of care (see section II.G.2.c.(3) of this proposed rule),
patient population-level management (see section II.G.2.c.(9) of this proposed rule), and
performance measurement (see section II.G.2.c.(10) of this proposed rule). We believe these
practice capabilities are indicative of, and necessary to, care delivery using the advanced primary
care model.
Refer to section II.G. of this proposed rule for detailed, technical discussion regarding the
proposed description, payment and utilization of these HCPCS codes as well as information
about requirements for billing providers participating in ACOs.
As described in section II.G., HCPCS codes GPCM1 through GPCM3 would describe
APCM services furnished per calendar month, following the initial qualifying visit (see section
II.G.2.c.(1) for more on the initiating visit). Physicians and NPPs, including nurse practitioners
(NPs), physician assistants (PAs), certified nurse midwives (CNMs) and clinical nurse specialists
(CNSs), could bill for APCM services. As we describe in more detail in section II.G.2.c., within
the code descriptors for GPCM1, GPCM2, and GPCM3, we are including the elements of the
scope of service for APCM as well as the capabilities and requirements that we believe to be
inherent to care delivery by the practitioner using an advanced primary care model, and
necessary to fully furnish and, therefore, bill for APCM services.
We are proposing that the practitioner who bills for APCM services must intend to be
responsible for the patient’s primary care and serve as the continuing focal point for all needed
health care services. We anticipate that most practitioners furnishing APCM services would be
managing all the patient’s health care services over the month and have either already been
providing ongoing care for the patient or have the intention of being responsible for the patient’s
primary care and serving as the continuing focal point for all of the patient’s health care services.
As detailed in sections II.G.2.b. through II.G.2.d., this proposed coding and payment will
incorporate elements of several specific, existing care management and communication
technology-based services (CTBS) into a bundle of services, that reflects the essential elements
of the delivery of advanced primary care, for payment under the PFS starting in 2025.
These new codes are designed to bundle the individual utilization of codes that are
already included in the definition of primary care services used for purposes of assignment,
specifically CCM (CPT codes 99437, 99487, 99489, 99490, 99491, and 99439 and HCPCS
codes G0506 and G2058), PCM (CPT codes 99424, 99425, 99426, and 99427 and HCPCS codes
G2064 and G2065), TCM (CPT codes 99495 and 99496), remote evaluation of patient
videos/images (HCPCS code G2010), and virtual check-in and e-visits (HCPCS codes G2012
and G2252). These new codes also bundle IPC (CPT Codes 99446, 99447, 99448, 99449, 99451,
99452), which we are proposing to include in the definition of primary care services used for

purposes of assignment in this section of the rule. Further, as proposed, this new APCM bundle
represents a broader application of advanced primary care and incorporates elements included in
care management and CTBS services. We believe the services billed under these codes reflect
the types of services we expect primary care providers to provide in order to improve care
coordination and care management and so it would be appropriate to include HCPCS codes
GPCM1, GPCM2, and GPCM3 in the definition of primary care services used for purposes of
assignment since these HCPCS codes bundle services furnished under CPT and HCPCS codes
already included in the definition of primary care services used for purposes of assignment.
As described in section II.G, we anticipate that these codes would mostly be used by
primary care specialties, such as general medicine, geriatric medicine, family medicine, internal
medicine, and pediatrics, or in some instances, certain specialists functioning as primary care
practitioners – for example, an OB/GYN or a cardiologist. Since primary care specialties, such as
general medicine, geriatric medicine, family medicine, internal medicine, and pediatrics are
primary care physicians (as defined in § 425.20) and OB/GYN or a cardiologist are two of the
specialty designations (as described in § 425.402(c)) used for purposes of assignment we believe
it would be appropriate to include HCPCS codes GPCM1, GPCM2, and GPCM3 in the
definition of primary care services used for purposes of assignment. Inclusion of these APCM
services in the definition of primary care services used for purposes of assignment would also
strengthen and invest in primary care in alignment with the goals of the U.S. Department of
Health and Human Services (HHS) Initiative to Strengthen Primary Care.
463
We also believe that
updating the definition of primary care services used for purposes of assignment to include the
APCM bundle would increase the accuracy of assignment based on the provision of primary
care.
● Cardiovascular Risk Assessment and Risk Management –
463
Refer to: U.S. Department of Health and Human Services. (2023). HHS is Taking Action to Strengthen Primary
Care. https://www.hhs.gov/sites/default/files/primary-care-issue-brief.pdf.
++ Cardiovascular Disease Risk Assessment HCPCS code GCDRA (Administration of a
standardized, evidence-based Atherosclerotic Cardiovascular Disease (ASCVD) Risk Assessment
for patients with ASCVD risk factors on the same date as an E/M visit, 5-15 minutes, not more
often than every 12 months): As described in section II.G. of this proposed rule, we are
proposing a new stand-alone HCPCS code, GCDRA, to identify and value the work involved in
administering an ASCVD risk assessment when medically reasonable and necessary in relation to
an E/M visit. Atherosclerotic Cardiovascular Disease (ASCVD) Risk Assessment refers to a
review of the individual’s demographic factors, modifiable risk factors for CVD, and risk
enhancers for CVD.
We are proposing that the ASCVD risk assessment must be furnished by the practitioner
on the same date they furnish an E/M visit, as the ASCVD risk assessment would be reasonable
and necessary when used to inform the patient’s diagnosis, and treatment plan established during
the visit. ASCVD risk assessment is reasonable and necessary for a patient who has at least one
predisposing condition to cardiovascular disease that may put them at increased risk for future
ASCVD diagnosis.
++ Atherosclerotic Cardiovascular Disease Prevention Risk Management Services
HCPCS code GCDRM (Atherosclerotic Cardiovascular Disease (ASCVD) risk management
services with the following required elements: patient is without a current diagnosis of ASCVD,
but is determined to be at medium or high risk for CVD (>15 percent in the next 10 years) as previously
determined by the ASCVD risk assessment; ASCVD-Specific care plan established, implemented, revised, or
monitored that addresses risk factors and risk enhancers and must incorporate shared decision-
making between the practitioner and the patient; clinical staff time directed by physician or
other qualified health care professional; per calendar month). As described in section II.G of
this proposed rule, over the past several years, we have worked to develop payment mechanisms
under the PFS to improve the accuracy of valuation and payment for the services furnished by
physicians and other healthcare professionals, especially in the context of evolving changes in
medical practice using evidence-based models of care, such as the Million Hearts® model. We
are proposing to establish a G-code, GCDRM, for ASCVD risk management services which
refers to the development, implementation, and monitoring of individualized care plans for
reducing cardiovascular risk, including shared decision-making and the use of the “ABCS” of
cardiovascular risk reduction, as well as counseling and monitoring to improve diet and exercise.
We believe that ASCVD risk management services include continuous care and
coordination to reduce or eliminate further elevation of ASCVD risk over time, and potentially
prevent the development of future cardiovascular disease diagnoses or first-time heart attacks or
strokes. Physicians and Non-Physician Practitioners (NPPs) who can furnish E/M services could
bill for ASCVD risk management services. We anticipate that ASCVD risk management services
would ordinarily be provided by clinical staff incident to the professional services of the billing
practitioner in accordance with our regulation at § 410.26. We are proposing that ASCVD risk
management services would be considered a “designated care management service” under §
410.26(b)(5) and, as such, could be provided by auxiliary personnel under the general
supervision of the billing practitioner.
Refer to section II.G of this proposed rule for detailed, technical discussion regarding the
proposed description, payment and utilization of HCPCS codes GCDRA and GCDRM.
Because HCPCS codes GCDRA and GCDRM are proposed to be care management
services similar to CCM (CPT codes 99437, 99439, 99487, 99489, 99490, and 99491) which are
included in the Shared Savings Program definition of primary care services used for purposes of
assignment, we believe it would be consistent and appropriate to include GCDRA and GCDRM
in the definition of primary care services used for purposes of assignment. In earlier rulemaking,
we finalized the inclusion of CCM CPT codes 99487, 99489, 99490, and 99491 (codes for
chronic care management) in the definition of primary care services for the Shared Savings
Program. Refer to the June 2015 final rule (80 FR 32746 through 32748), CY 2018 PFS final
rule (82 FR 53212 through 53213), and CY 2021 PFS final rule (85 FR 84749 through 84750
and 84754). “Complex” CCM services (CPT codes 99487 and 99489) and “non-complex” CCM
services (CPT codes 99490 and 99491) share a common set of service elements, including the
following: (1) Initiating visit, (2) structured recording of patient information using certified
electronic health record technology (EHR), (3) 24/7 access to physicians or other qualified health
care professionals or clinical staff and continuity of care, (4) comprehensive care management
including systematic assessment of the patient's medical, functional, and psychosocial needs, (5)
comprehensive care plan including a comprehensive care plan for all health issues with particular
focus on the chronic conditions being managed, and (6) management of care transitions.
Elements of care management services include: (1) an initial visit, which can be an E/M
service, Annual Wellness Visit (AWV) or initial preventive physical exam (IPPE or “Welcome
to Medicare”); (2) continuity of care with a designated practitioner; (3) comprehensive care
management; (4) comprehensive care plan; (5) management of care transitions; and (6) care
coordination. In the November 2011 final rule (76 FR 67852 through 67853), we finalized the
inclusion of E/M services, the AWV, and the IPPE since those services aligns the definition of
primary care services with the definition in section 5501 of the Affordable Care Act. Because
care management, E/M services, the AWV, and the IPPE are all included in the definition of
primary care services used for purposes of assignment, we believe GCDRA and GCDRM reflect
the types of services we expect primary care providers to provide in order to improve care
coordination and care management. Additionally, GCDRA and GCDRM are care and risk
management services that include elements of continuous and coordinated care, which the
Shared Savings Program is intended to promote.
● Interprofessional Consultation (IPC) (CPT codes 99446, 99447, 99448, 99449, 99451,
99452): In the CY 2019 PFS final rule (83 FR 59489), CMS finalized six codes:
++ 99446 (Interprofessional telephone/internet assessment and management service
provided by a consultative physician including a verbal and written report to the patient’s
treating/requesting physician or other qualified health care professional; 5–10 minutes of
medical consultative discussion and review);
++ 99447 (Interprofessional telephone/internet assessment and management service
provided by a consultative physician including a verbal and written report to the patient’s
treating/requesting physician or other qualified health care professional; 11–20 minutes of
medical consultative discussion and review);
++ 99448 (Interprofessional telephone/internet assessment and management service
provided by a consultative physician including a verbal and written report to the patient’s
treating/requesting physician or other qualified health care professional; 21–30 minutes of
medical consultative discussion and review);
++ 99449 (Interprofessional telephone/internet assessment and management service
provided by a consultative physician including a verbal and written report to the patient’s
treating/requesting physician or other qualified health care professional; 31 minutes or more of
medical consultative discussion and review);
++ 99451 (Interprofessional telephone/internet/electronic health record assessment and
management service provided by a consultative physician including a written report to the
patient's treating/requesting physician or other qualified health care professional, 5 or more
minutes of medical consultative time); and
++ 99452 (Interprofessional telephone/internet/electronic health record referral
service(s) provided by a treating/requesting physician or qualified health care professional, 30
minutes).
These CPT codes describe assessment and management services conducted through
telephone, internet, or electronic health record consultations furnished when a patient's treating
physician or other qualified healthcare professional requests the opinion and/or treatment advice
of a consulting physician or qualified healthcare professional with specific specialty expertise to
assist with the diagnosis and/or management of the patient's problem without the need for the
patient's face-to-face, in-person contact with the consulting physician or qualified healthcare
professional. We believe that payment for these interprofessional consultations performed via
communications technology such as telephone or internet is consistent with our ongoing efforts
to recognize and reflect medical practice trends in primary care and patient-centered care
management within the PFS. Accordingly, because these CPT codes 99446, 99447, 99448,
99449, 99451, and 99452 recognize and reflect medical practice trends in primary care and
patient-centered care, we believe they should be included in the definition of primary care
services used for purposes of assignment.
Beginning in the CY 2012 PFS proposed rule (76 FR 42793), we recognized the
changing focus in medical practice toward managing patients' chronic conditions, many of which
particularly challenge the Medicare population, including heart disease, diabetes, respiratory
disease, breast cancer, allergies, Alzheimer's disease, and factors associated with obesity. Current
E/M coding does not adequately reflect the changes that have occurred in medical practice, and
the activities and resource costs associated with the treatment of these complex patients in the
primary care setting In the years since 2012, we have acknowledged the shift in medical practice
away from an episodic treatment-based approach to one that involves comprehensive patient-
centered care management, and have taken steps through rulemaking to better reflect that
approach in payment under the PFS. In the CY 2013 PFS final rule (77 FR 68979), we
established new codes to pay separately for TCM services. Next, in the CY 2015 PFS final rule
(79 FR 67715), we finalized new coding and separate payment beginning in CY 2015 for CCM
services provided by clinical staff. In the CY 2017 PFS final rule (81 FR 80225), we established
separate payment for complex CCM services, an add-on code to the visit during which CCM is
initiated to reflect the work of the billing practitioner in assessing the beneficiary and
establishing the CCM care plan and established separate payment for Behavioral Health
Integration (BHI) services (81 FR 80226 through 80227). As part of this shift in medical
practice, and with the proliferation of team-based approaches to care that are often facilitated by
electronic medical record technology, we believe that making separate payment for
interprofessional consultations undertaken for the benefit of treating a patient would contribute to
payment accuracy for primary care and care management services. Refer to the CY 2019 PFS
Final rule (83 FR 59489) for detailed, technical discussion regarding the description, payment
and utilization of these CPT codes.
Since the services associated with CPT codes 99446, 99447, 99448, 99449, 99451, and
99452 include TCM, CCM, and BHI services, which are included in our definition of primary
care services and are included in the proposed APCM bundle that we propose to be included in
the definition of primary care services used for purposes of assignment, we believe that the
services associated with CPT codes 99446, 99447, 99448, 99449, 99451, and 99452 should be
included in the definition of primary care services for purposes of assignment. We additionally
believe the services billed under this code reflect the types of services we expect primary care
providers to provide in order to improve care coordination and care management. These IPC
services were also designed to reimburse for comprehensive patient-centered care management
and primary care, which the Shared Savings Program is intended to promote.
● Direct Care Caregiver Training Services (HCPCS codes GCTD1, GCTD2, and
GCTD3): GCTD1 (Caregiver training in direct care strategies and techniques to support care
for patients with an ongoing condition or illness and to reduce complications (including, but not
limited to, techniques to prevent decubitus ulcer formation, wound dressing changes, and
infection control) (without the patient present), face-to-face; initial 30 minutes)), GCTD2
(Caregiver training in direct care strategies and techniques to support care for patients with an
ongoing condition or illness and to reduce complications (including, but not limited to,
techniques to prevent decubitus ulcer formation, wound dressing changes, and infection control)
(without the patient present), face-to-face; each additional 15 minutes (List separately in
addition to code for primary service) (Use GCTD2 in conjunction with GCTD1)), and GCTD3
(Group caregiver training in direct care strategies and techniques to support care for patients
with an ongoing condition or illness and to reduce complications including, but not limited to,
techniques to prevent decubitus ulcer formation, wound dressing changes, and infection control)
(without the patient present), face-to-face with multiple sets of caregivers). In section II.E. of this
proposed rule, we are proposing to establish new coding and payment for caregiver training
services (CTS) for direct care services and supports. The topics of training could include, but
would not be limited to, techniques to prevent decubitus ulcer formation, wound dressing
changes, and infection control. Refer to section II.E. of this proposed rule for detailed, technical
discussion regarding the proposed description, payment, and utilization of this HCPCS code.
Unlike other caregiver training codes that are currently paid under the PFS, the caregiver
training codes for direct care services and support focus on specific clinical skills aimed at the
caregiver effectuating hands-on treatment, reducing complications, and monitoring the patient.
Like other codes describing caregiver training services, these proposed new codes would reflect
the training furnished to a caregiver, in tandem with the diagnostic and treatment services
furnished directly to the patient, in strategies and specific activities to assist the patient to carry
out the treatment plan. We believe that CTS may be reasonable and necessary when they are
integral to a patient's overall treatment and furnished after the treatment plan is established. The
CTS themselves need to be congruent with the treatment plan and designed to effectuate the
desired patient outcomes. Direct care training for caregivers of Medicare beneficiaries should be
directly relevant to the person-centered treatment plan for the patient in order for the services to
be considered reasonable and necessary under the Medicare program. We believe that since CTS
may be integral to a patient’s overall treatment and furnished after the treatment plan is
established, these services should be included in the definition of primary care services for
purposes of beneficiary assignment in support of the Shared Savings Program's goal to promote
coordinated, high quality care to an ACO's assigned beneficiaries. In the CY 2024 PFS final rule
(88 FR 79168 through 79169), we finalized the inclusion of other caregiver training services
(CPT codes 96202, 96203, 97550, 97551, and 97552) in the definition of primary care services
used for purposes of assignment in the Shared Savings Program. These new caregiver training
services codes (HCPCS GCTD1, GCTD2, and GCTD3) are similar to the caregiver training
services currently included in the Shared Savings Program definition of primary care services in
that these codes allow treating practitioners to report the training furnished to a caregiver, in
tandem with the diagnostic and treatment services furnished directly to the patient, in strategies
and specific activities to assist the patient to carry out the treatment plan. We also believe the
services billed under these codes reflect the types of services we expect primary care providers to
provide in order to improve care coordination and care management.
● Individual Behavior Management/Modification Caregiver Training Services (HCPCS
codes GCTB1 and GCTB2): GCTB1 (Caregiver training in behavior management/modification
for caregiver(s) of a patient with a mental or physical health diagnosis, administered by
physician or other qualified health care professional (without the patient present), face-to-face;
initial 30 minutes) and GCTB2 (Caregiver training in behavior management/modification for
caregiver(s) of a patient with a mental or physical health diagnosis, administered by physician
or other qualified health care professional (without the patient present), face-to-face; each
additional 15 minutes (List separately in addition to code for primary service) (Use GCTB2 in
conjunction with GCTB1)). In section II.E of this proposed rule, we are proposing to establish
new coding and payment for caregiver behavior management and modification training that
could be furnished to the caregiver(s) of an individual patient. Behavior
management/modification training for caregivers of Medicare beneficiaries should be directly
relevant to the person-centered treatment plan for the patient in order for the services to be
considered reasonable and necessary under the Medicare program. Each training activity should
be clearly identified and documented in the treatment plan. All other policies and procedures
surrounding CPT 96202 and 96203 will also apply to these services (88 FR 78914-78920). Refer
to section II.E. of this proposed rule for detailed, technical discussion regarding the proposed
description, payment and utilization of this HCPCS code.
We believe that, since CTS may be reasonable and necessary when they are integral to a
patient’s overall treatment and furnished after the treatment plan is established especially in the
case of medical treatment scenarios where assistance by the caregiver receiving the CTS is
necessary to ensure a successful treatment outcome for the patient, (for example when the patient
cannot follow through with the treatment plan for themselves), these services should be included
in the definition of primary care services for purposes of beneficiary assignment in support of the
Shared Savings Program's goal to promote coordinated, high quality care to an ACO's assigned
beneficiaries. In the CY 2024 PFS final rule (88 FR 79168 through 79169), we finalized the
inclusion of other caregiver training services (CPT codes 96202, 96203, 97550, 97551, and
97552) in the definition of primary care services used for purposes of assignment in the Shared
Savings Program. These new caregiver training services codes (HCPCS codes GCTD1, GCTD2,
GCTD3, GCTB1, and GCTB2) are similar to the caregiver training services currently included in
the Shared Savings Program definition of primary care services in that these codes allow treating
practitioners to report the training furnished to a caregiver, in tandem with the diagnostic and
treatment services furnished directly to the patient, in strategies and specific activities to assist
the patient to carry out the treatment plan, which is integral to care coordination. We also believe
the services billed under these codes reflect the types of services we expect primary care
providers to provide in order to improve care coordination and care management.
As part of this revised definition of primary care services used for assigning beneficiaries
under § 425.402, we propose to incorporate a provision in § 425.400(c)(1)(ix)(C), specifying that
the primary care service codes for purposes of assigning beneficiaries include a CPT code
identified by CMS that directly replaces a CPT code specified in § 425.400(c)(1)(ix)(A) or a
HCPCS code specified in § 425.400(c)(1)(ix)(B), when the assignment window or expanded
assignment window (as defined in § 425.20) for a benchmark or performance year includes any
day on or after the effective date of the replacement code for payment purposes under FFS
Medicare.
We seek comment on these proposed changes to the definition of primary care services
used for assigning beneficiaries under § 425.400(c)(1)(ix) to Shared Savings Program ACOs for
the performance year starting on January 1, 2025, and subsequent performance years. We also
welcome comments on any other existing HCPCS or CPT codes and new HCPCS or CPT codes
proposed elsewhere in this proposed rule that we should consider adding to the definition of
primary care services for purposes of assignment in future rulemaking.
b. Proposed Revisions to Criteria for ACO Models to Waive Shared Savings Program Statutory
Requirements Giving Precedence for Assignment based on Beneficiary Voluntary Alignment
(1) Background
Section 50331 of the Bipartisan Budget Act of 2018 amended section 1899(c) of the Act
to add a new paragraph (2)(B) that requires the Secretary, for performance year 2018 and each
subsequent performance year, to permit a Medicare FFS beneficiary to voluntarily identify an
ACO professional as the primary care provider of the beneficiary for purposes of assigning such
beneficiary to an ACO. A voluntary identification by a Medicare FFS beneficiary under this
provision supersedes any claims-based assignment. In earlier rulemaking (81 FR 80501 through
80510 and 83 FR 59959 through 59964) CMS finalized modifications to the Shared Savings
Program regulations at § 425.402(e) to implement the statutory requirements governing
voluntary alignment.
In the November 2018 final rule (83 FR 59959 through 59964), we finalized changes to
the beneficiary voluntary alignment policies (refer to § 425.402(e)) to revise the requirements
previously established for the voluntary alignment process. We explained that it could be
appropriate, in limited circumstances, to align a beneficiary to an entity participating in certain
specialty and disease-specific CMS Innovation Center models to test a new system of payment
and service delivery that CMS believes would lead to better health outcomes for Medicare
beneficiaries while lowering costs to Medicare Parts A and B. Additionally, we explained that it
could be difficult for the CMS Innovation Center to conduct a viable test of a specialty or
disease-specific model, if we were to require that beneficiaries who have previously designated
an ACO professional as their primary clinician remain assigned to the Shared Savings Program
ACO under all circumstances. We applied this exception for the Comprehensive ESRD Care
(CEC) model, which assigned beneficiaries to entities participating in the model through the
beneficiaries’ first treatment at a participating dialysis facility.
Currently, under § 425.402(e)(2)(ii)(D), we would not assign a beneficiary who has
voluntarily identified a Shared Savings Program ACO professional to a Shared Savings Program
ACO when the beneficiary is also eligible for claims-based assignment to an entity participating
in a model tested or expanded under section 1115A of the Act under which claims-based
assignment is based solely on claims for services other than primary care services and for which
there has been a determination by the Secretary that a waiver under section 1115A(d)(1) of the
Act of the requirement in section 1899(c)(2)(B) of the Act is necessary solely for purposes of
testing the model.
(2) Proposed Revisions
Since finalization of this limited exception to the Shared Savings Program’s voluntary
alignment policy, disease-specific CMS Innovation Center models have been developed that use
claims for both primary care services and services other than primary care in determining claims-
based assignment to entities participating in these models. We believe it would be appropriate to
propose to broaden this limited exception and allow a voluntarily aligned Shared Savings
Program beneficiary to be claims-based assigned to an entity participating in a disease- or
condition-specific CMS Innovation Center model when that model uses claims-based assignment
that is based on primary care and/or other services. Disease- or condition-specific CMS
Innovation Center models are designed to support condition management, coordination, and
services for patients that have a specific disease or condition that often requires coordination of
care across specialties and settings. For example, the CMS Innovation Center has tested disease-
and condition-based episode payment models, such as those focused on oncology and kidney

disease.
464
Doing so would help beneficiaries with certain diseases or conditions benefit from the
focused attention and care coordination related to the disease or condition that an entity
participating in such a model could provide. We would identify models for which the exception
would apply in our Shared Savings and Losses and Assignment Methodology and Quality
Performance Specifications document, which is located on the Shared Savings Program website,
https://www.cms.gov/medicare/payment/fee-for-service-providers/shared-savings-program-ssp-
acos. This proposed expanded exception would be applicable to beneficiaries assigned to entities
participating in CMS Innovation Center models under which assignment is based solely on (1)
claims for primary care and/or other services related to treatment of one or more specific
diseases or conditions targeted by the model, or (2) claims for services other than primary care
services, when the Secretary has determined that a waiver is necessary solely for purposes of
testing the model.
An example of a CMS Innovation Center model whose assigned beneficiaries may be
impacted by the proposed expanded exception is the Kidney Care Choices (KCC) model,
465
which is designed to help health care providers reduce the cost and improve the quality of care
for patients with late-stage chronic kidney disease and ESRD. The KCC model builds on the
previous CEC model
466
by adding strong financial incentives for health care providers to manage
the care for Medicare beneficiaries with chronic kidney disease (CKD) stage 4 and ESRD, to
delay the onset of dialysis and to incentivize kidney transplantation. Under the CEC model, the
CMS Innovation Center worked with groups of health care providers, dialysis facilities, and
other suppliers involved in the care of ESRD beneficiaries to improve the coordination and
quality of care that these individuals received. We determined that an ESRD beneficiary, who
was otherwise eligible for assignment to an entity participating in the CEC model, could benefit
464
Refer to Innovation Models website:
https://www.cms.gov/priorities/innovation/models#views=models&cat=disease-specific%20&%20episode-
based%20models.
465
Refer to https://www.cms.gov/priorities/innovation/innovation-models/kidney-care-choices-kcc-model.
466
Refer to https://www.cms.gov/priorities/innovation/innovation-models/comprehensive-esrd-care.

from the focused attention on and increased care coordination for their ESRD available under the
CEC model. As described above, we created a narrow exception to the general policy that a
beneficiary who had voluntarily aligned to a Shared Savings Program ACO professional would
supersede their alignment to a CMS Innovation Center model. Specifically, we did not assign a
beneficiary to the ACO when the beneficiary was also eligible for alignment to an entity
participating in the CEC model.
KCC is more complex than CEC and is designed to capture multiple care relationships
and uses a mix of E/M codes for alignment of beneficiaries with CKD and managing clinician
Monthly Capitation Payments for aligning ESRD beneficiaries. The existing exception is not
applicable to KCC in part because claims for primary care and other services related to the
treatment of one or more specific diseases or conditions targeted by the model (chronic kidney
disease (CKD) stage 4 and ESRD) are considered as part of the model’s beneficiary alignment
methodology, which takes into consideration where a beneficiary receives the majority of their
kidney care as well as the beneficiary’s diagnosis of CKD stages 4 or ESRD receiving
maintenance dialysis. KCC’s alignment methodology could align beneficiaries receiving primary
care services that are also considered for Shared Savings Program assignment if furnished and
billed under one of the HCPCS/CPT codes included in § 425.400(c) by ACO professionals who
are primary care physicians, physicians with one of the primary specialty designations in §
425.402(c), NPs, PAs, and/or CNSs. We note that outpatient/office E/M services are included in
§ 425.400(c) and that nephrology is one of the primary specialty designations under § 425.402(c)
so we anticipate that, if this proposal is finalized, most, if not all, beneficiaries who voluntarily
align to a physician that participates in a Shared Savings Program ACO and meet the KCC
alignment criteria would be claims-based align to the KCC model, assuming there is a
determination by the Secretary that waiver of the requirement in section 1899(c)(2)(B) of the Act
is necessary solely for purposes of testing the model.
We propose expanding upon current Shared Savings Program regulations to broaden the
existing exception to the program’s voluntary alignment policy, which would allow the
exception to apply to beneficiaries assigned to entities in a CMS Innovation Center model under
which claims-based assignment is based solely on (1) claims for primary care and/or other
services related to treatment of one or more specific diseases or conditions targeted by the model,
or (2) claims for services other than primary care services, and for which there has been a
determination by the Secretary that waiver of the requirement in section 1899(c)(2)(B) of the Act
is necessary for purposes of testing the model. Under the proposed revisions, if a beneficiary
voluntarily aligns to a Shared Savings Program ACO under § 425.402(e), we would not assign
the beneficiary to that Shared Savings Program ACO when the beneficiary is also eligible for
claims-based assignment to an entity participating in a model tested or expanded under section
1115A of the Act under which claims-based assignment is based solely on (1) claims for primary
care and/or other services related to treatment of one or more specific diseases or conditions
targeted by the model or (2) claims for services other than primary care service, and for which
there has been a determination by the Secretary that waiver of the requirement in section
1899(c)(2)(B) of the Act is necessary for purposes of testing the model. We would not supersede
voluntary alignment for CMS Innovation Center models that are not designed to target a specific
disease or condition, such as ACO REACH. While ACO REACH contains design features for
organizations serving high needs beneficiaries, it is designed more broadly, and not for
beneficiaries with a specific disease or condition. Such models do not target a specific disease or
condition. Therefore, a beneficiary’s claims-based assignment to an entity participating in such a
model would not supersede their voluntary alignment to a Shared Savings Program ACO under
our proposal.
For example, under the KCC model, alignment is based on where a beneficiary receives
the majority of their nephrology services and/or dialysis management services. Claims for those
kidney care services could include claims for services that, under the Shared Savings Program’s
claims-based assignment policies, would lead a beneficiary to be assigned to a Shared Savings
Program ACO. Since under the KCC model, claims-based assignment is based solely on claims
for primary care and/or other services (kidney care services) related to the treatment of one or
more specific diseases or conditions targeted by the model (chronic kidney disease (CKD) stage
4 and ESRD), our proposed exception would apply and a beneficiary who voluntarily aligned to
a Shared Savings Program ACO and who received kidney care services from an entity
participating in the KCC model would nonetheless be claims-based assigned to the KCC model,
if there is a determination by the Secretary that waiver of the requirement in section
1899(c)(2)(B) of the Act is necessary solely for purposes of testing the KCC model. This
proposed expansion of the voluntary alignment exception would support assignment of
beneficiaries to entities participating in CMS Innovation Center models, which would reduce
barriers for the CMS Innovation Center to conduct viable tests of disease-or condition-specific
models and thereby improve access to high-quality, value-based specialty care, such as that
provided by an entity participating in a model focused on diabetes care or care provided by
specific specialists, such as cardiologists or gastroenterologists.
This proposal would also support CMS’s goals of improving patient care, lowering costs,
and better aligning payment systems to promote patient-centered practices through accountable
and value-based care. We believe that specific subpopulations of Medicare beneficiaries who are
otherwise eligible for assignment to an entity participating in a disease or condition-specific
CMS Innovation Center model, but who may not be captured by § 425.402(e)(2)(ii)(D) because
their models consider primary care services for purposes of assignment, could benefit from the
focused attention and increased care coordination offered by an entity participating in a disease
or condition-specific model. Application of this exception would require a determination from
the Secretary to waive the voluntary alignment provision.
Under this proposal, if a beneficiary designated an ACO professional participating in a
Shared Savings Program ACO as the physician or practitioner they consider responsible for

coordinating their overall care (that is, their primary clinician), but the beneficiary is also eligible
for assignment to an entity participating in a model tested or expanded under section 1115A of
the Act under which claims-based assignment is based solely on (1) claims for primary care
and/or other services related to treatment of one or more specific diseases or conditions targeted
by the model, or (2) claims for services other than primary care services, and for which there has
been a determination that a waiver of the requirement in section 1899(c)(2)(B) of the Act is
necessary solely for purposes of testing the model, the CMS Innovation Center or its designee
would notify the beneficiary of their assignment to an entity participating in the model.
Additionally, although such a beneficiary may still voluntarily identify an ACO professional
participating in a Shared Savings Program ACO as their primary clinician and seek care from
any clinician, the beneficiary would not be assigned to a Shared Savings Program ACO even if
the designated primary clinician is an ACO professional in a Shared Savings Program ACO.
For PY 2024, there are approximately 152,000 beneficiaries with a primary clinician
selection who is a Shared Savings Program ACO professional as defined at § 425.20, and
approximately 83,000 are voluntarily aligned to a Shared Savings Program ACO after meeting
all the assignment eligibility criteria as described at § 425.401(a). Overall, this represents an
exceedingly small share of the overall Shared Savings Program assigned beneficiary population,
currently 10.8 million
467
beneficiaries. Additionally, simulating our proposed §
425.402(e)(2)(ii)(D) using PY 2024 data, less than 1 percent (703) of beneficiaries who are
voluntarily aligned to a Shared Savings Program ACO would instead be claims-based assigned
to an entity participating in a CMS Innovation Center model.
The benefit of allowing beneficiaries who voluntarily align to a Shared Savings Program
ACO to be claims-based assigned to an entity participating in a CMS Innovation Center tailored
to the needs of their specific disease or condition far outweighs any cost to the Shared Savings
Program. The impact of assigning these beneficiaries to a CMS Innovation Center model
467
Refer to https://www.cms.gov/files/document/2024-shared-savings-program-fast-facts.pdf.
notwithstanding their voluntary designation would be minimal because so few beneficiaries
would be impacted by this proposed expansion of the exception (for PY 2024, less than 1 percent
of all beneficiaries who voluntarily align to a Shared Savings Program ACO). As explained
earlier in this section, this proposal would enable us to better test CMS Innovation Center models
and ultimately improve health outcomes for Medicare beneficiaries with the specific diseases and
conditions targeted by CMS Innovation Center models. We also recognize the importance of
continuing to allow beneficiaries to voluntarily identify an ACO professional as their primary
clinician for purposes of assignment to a Shared Savings Program ACO, and we reiterate that,
based on PY 2024 data, this proposal would impact very few beneficiaries who voluntarily align
to Shared Savings Program ACOs (less than .01 percent of all such beneficiaries). Beneficiaries
who voluntarily align to a Shared Savings Program ACO but are, pursuant to our proposal,
ultimately claims-based assigned to an entity participating in a CMS Innovation Center model
would be notified of this in accordance with the CMS Innovation Center model’s participation
agreement. We propose to apply these modifications to our policies under the Shared Savings
Program regarding voluntary alignment beginning for performance year 2025, and subsequent
performance years. We propose to incorporate these new requirements into new regulations at §
425.402(e)(2)(iii). We solicit comments on this proposal.
Accordingly, since the new proposed provisions § 425.402(e)(2)(iii) will supersede the
existing provisions at § 425.402(e)(2)(ii) for performance year 2025 and subsequent performance
years, we propose to revise the introductory text at § 425.402(e)(2)(ii) to designate that
provision’s applicability for performance years starting on January 1, 2019, through 2024.
4. Quality Performance Standard & Other Reporting Requirements
a. Background
Section 1899(b)(3)(C) of the Act states that the Secretary shall establish quality
performance standards to assess the quality of care furnished by ACOs and seek to improve the
quality of care furnished by ACOs over time by specifying higher standards, new measures, or
both for purposes of assessing such quality of care. As we stated in the November 2011 final rule
establishing the Shared Savings Program (76 FR 67872), our principal goal in selecting quality
measures for ACOs has been to identify measures of success in the delivery of high-quality
health care at the individual and population levels. In the November 2011 final rule, we
established a quality measure set spanning four domains: patient experience of care and
wherever practicable, caregiver experience of care, care coordination/patient safety, preventative
health, and at-risk population (76 FR 67872 through 67891). We have subsequently updated the
measures that comprise the quality performance measure set for the Shared Savings Program
through rulemaking in the CY 2015, 2016, 2017, 2019, 2023, and 2024 PFS final rules (79 FR
67907 through 67921, 80 FR 71263 through 71268, 81 FR 80484 through 80489, 83 FR 59708
through 59715, 87 FR 69860 through 69863, and 88 FR 79112 through 79114, respectively).
b. Proposal to Require Shared Savings Program ACOs to Report the Alternative Payment Model
(APM) Performance Pathway (APP) Plus Quality Measure Set
(1) Background
In the CY 2021 PFS final rule, we finalized modifications to the Shared Savings Program
quality reporting requirements and quality performance standard for performance year 2021 and
subsequent performance years (85 FR 84720 through 84743). For performance year 2021 and
subsequent years, ACOs are required to report quality data via the APP codified at § 414.1367.
Pursuant to policies finalized under the CY 2022 and CY 2023 PFS (86 FR 65685; 87 FR
69858), to meet the quality performance standard under the Shared Savings Program through
performance year 2024, ACOs must report the ten CMS Web Interface measures or the three
electronic clinical quality measures (eCQMs)/Merit-based Incentive Payment System (MIPS)
clinical quality measures (CQMs), and administer the Consumer Assessment of Healthcare
Providers and Systems (CAHPS) for MIPS survey. In performance year 2025 and subsequent
performance years, ACOs must report the three eCQMs/MIPS CQMs and administer the CAHPS
for MIPS survey. In the CY 2024 PFS final rule, we established the Medicare Clinical Quality
Measures for Accountable Care Organizations Participating in the Medicare Shared Savings
Program (Medicare CQMs) as a new collection type for Shared Savings Program ACOs
reporting on the Medicare CQMs within the APP quality measure set for performance year 2024
and subsequent performance years (88 FR 79107). Shared Savings Program ACOs have the
option to report on Medicare CQMs, which are reported on an ACO’s eligible Medicare fee-for-
service beneficiaries, instead of the ACO’s all payer/all patient population. Medicare CQMs are
aligned with MIPS standards for data completeness, measure benchmarking, and scoring (88 FR
79099 and 88 FR 79108). In the CY 2024 PFS final rule, we stated that Medicare CQMs would
serve as a transition collection type to help some ACOs build the infrastructure, skills,
knowledge, and expertise necessary to report all payer/all patient eCQMs/MIPS CQMs and
support ACOs in the transition to digital quality measure reporting (88 FR 79097 through
79098).
Since the CY 2021 PFS final rule was issued, ACOs and other interested parties have
continued to express concerns about requiring ACOs to report all payer/all patient eCQMs/MIPS
CQMs due to the cost of purchasing and implementing a system wide infrastructure to aggregate
data from multiple ACO participant taxpayer identification numbers (TINs) and varying
electronic health record (EHR) systems (86 FR 65257). In the CY 2022 PFS final rule,
commenters supported our acknowledgement of the complexity of the transition to all payer/all
patient eCQMs/MIPS CQMs (86 FR 65259). In public comments on the CY 2023 PFS proposed
rule, some commenters expressed multiple concerns regarding the requirement to report all
payer/all patient eCQMs/MIPS CQMs beginning in performance year 2025, such as issues
related to meeting all payer data requirements, data completeness requirements, data aggregation
and deduplication issues, and interoperability issues among different EHRs (87 FR 69837).
ACOs face continued difficulties in aggregating data on the three all payer/all patient
eCQMs/MIPS CQMs that are part of the existing APP quality measure set. The Shared Savings
Program continues to receive feedback from ACOs and other stakeholders about the difficulties

with reporting on the three all payer/all patient eCQMs/MIPS CQMs and meeting data
management requirements given their muti-practice/multi EHR structure. Additionally, we
continue to receive feedback on the challenges of aggregating data due to the health information
technology (IT) infrastructure in use by ACOs and the current state of interoperability. Building
on our goal to provide technical support to ACOs and help ACOs build the skills necessary to
aggregate and match patient data to report all payer/all patient eCQMs/MIPS CQMs, in
December 2022, we hosted a webinar to support ACOs in the transition to reporting all payer/all
patient eCQMs/MIPS CQMs and released a guidance document on the topic. Resources from the
‘‘Reporting MIPS CQMs and eCQMs in the APM Performance Pathway’’ webinar are available
at https://youtu.be/LDrpoGnnRQs. The guidance document, entitled ‘‘Medicare Shared Savings
Program: Reporting MIPS CQMs and eCQMs in the Alternative Payment Model Performance
Pathway (APP)’’ is available in the Quality Payment Program Resource Library at https://qpp-
cm-prod-
content.s3.amazonaws.com/uploads/2179/APP%20Guidance%20Document%20for%20ACOs.pd
f. Over the past 2 years, we have learned that there are complexities and hurdles concerning
ACOs adopting the all payer/all patient collection types; as a result, the widespread adoption of
the all payer/all patient collection types require further time and support. For example, our
internal data indicate that in performance year 2021, 12 out of 475 ACOs reported eCQMs/MIPS
CQMs under the APP, while 37 out of 482 ACOs reported eCQMs/MIPS CQMs in performance
year 2022.
468
Submission data for performance year 2023 indicate that 73 out of 456 ACOs
reported eCQMs/MIPS CQMs under the APP. Further, we have come to understand that
additional maturation processes are needed to support large, complex organizations like ACOs
that participate in the Shared Savings Program to fully and equitably participate in the all
payer/all patient collection types.
CMS’ goal, as stated in the CY 2024 PFS final rule, is to support ACOs in the adoption
468
Counts based on internal analysis of ACOs’ quality reporting in performance years 2021 and 2022.

of all payer/all patient measures (88 FR 79098). In that rule, we described our intention to
monitor the reporting of quality data utilizing the Medicare CQM collection type, which would
include assessing if any Medicare CQMs qualify as topped out as described at
§ 414.1380(b)(1)(iv) (88 FR 79098). We also noted that, “[s]eparately, we may specify higher
standards, new measures, or both—up to and including proposing to sunset the Medicare CQM
collection type in future rulemaking—to ensure that Medicare CQMs conform to the intent of
section 1899(b)(3)(C) of the Act and the priorities established in the CMS National Quality
Strategy” (88 FR 79098).
Under the goals of the CMS National Quality Strategy to improve the quality and safety
of healthcare for everyone, CMS is implementing a building-block approach and aligning the
measures used to establish the Shared Savings Program quality performance standard with the
Universal Foundation of quality measures and streamlining quality measures across CMS quality
programs for measuring primary care clinician performance in the adult and pediatric
populations.
469
In the CY 2024 PFS proposed rule, we stated that “we intend to propose future
policies aligning the APP measure set for Shared Savings Program ACOs with the quality
measures under the ‘Universal Foundation’ beginning in performance year 2025” (88 FR 52423).
A few commenters were supportive of aligning the APP quality measure set with the Universal
Foundation measures, while other commenters were opposed. Several commenters urged CMS
to first test measures before making them required and scored measures for ACOs. Commenters
were concerned about balancing the alignment of the Universal Foundation measures with efforts
to reduce administrative burden, potential growth in the number of measures ACOs would have
to report, and implementing multiple major changes to the APP quality measure set in
performance year 2025. In the CY 2024 PFS final rule, we stated that we will take the comments
469
Centers for Medicare & Medicaid Services (2024). CMS National Quality Strategy. Accessed June 24, 2024.
https://www.cms.gov/medicare/quality/meaningful-measures-initiative/cms-quality-strategy.
under consideration in future rulemaking, as we evaluate the impact of aligning the APP quality
measure set with the Universal Foundation measures (88 FR 79114).
(2) Proposed Revisions
(a) Proposal to Require Shared Savings Program ACOs to Report the APP Plus Quality Measure
Set
To further advance Medicare’s overall value-based care strategy and maintain alignment
within and across CMS’ quality programs, in section IV.A.4.c.(2) of this proposed rule, we are
proposing to create the APP Plus quality measure set to align with the Adult Universal
Foundation measures. Out of the ten Adult Universal Foundation measures, five of the measures
are already in the APP quality measure set for performance year 2025 under policies finalized in
the CY 2024 PFS final rule (88 FR 79112 through 79113). There is one measure - Clinician and
Clinician Group Risk-standardized Hospital Admission Rates for Patients with Multiple Chronic
Conditions (Measure # 484) - in the APP quality measure set that is not an Adult Universal
Foundation measure, resulting in a total of six measures that are in the APP quality measure set.
Under the proposed approach, the APP Plus quality measure set would incrementally
grow to comprise of eleven measures, consisting of the six measures in the existing APP quality
measure set and five newly proposed measures from the Adult Universal Foundation measure set
that would be incrementally incorporated into the APP Plus quality measure set over
performance years 2025 through 2028. The proposed new measures and the timeline for
incorporating the measures into the APP Plus quality measure set are described in section
IV.A.4.c.(3) of this proposed rule and below. The proposal in section IV.A.4.c.(2) is to make the
APP Plus quality measure set an optional measure set for APP reporters. For performance year
2025 and subsequent performance years, we are proposing to require Shared Savings Program
ACOs to report the APP Plus quality measure set as proposed in section III.G.4.b.(2)(a) of this
proposed rule. Consequently, the APP quality measure set would no longer be available for
reporting by Shared Savings Program ACOs beginning in performance year 2025. Our proposal

would align the quality measures that Shared Savings Program ACOs would be required to
report with the quality measures under the Adult Universal Foundation measure set
incrementally beginning in performance year 2025.
Creating alignment with the Adult Universal Foundation would better align the quality
measures reported by Shared Savings Program ACOs with the Medicaid Core Sets and the
Marketplace Quality Rating System, which have previously adopted the quality measures in the
Universal Foundation.
470
As discussed in section IV.A.4.c.(2) of this proposed rule, alignment of
quality measures across CMS programs allows practitioners to better focus their quality efforts,
reduce administrative burden, and drive digital transformation and stratification of a focused
quality measure set to assess impact on disparities.
471
Our proposed alignment with the Adult
Universal Foundation would also better align the quality measures reported by Shared Savings
Program ACOs with the Value in Primary Care MIPS Value Pathway (MVP), which contains the
same Universal Foundation measures. This may create a smoother transition for clinicians from
MIPS to the Shared Savings Program. Alignment would allow clinicians to leverage their
familiarity and experience with the Adult Universal Foundation quality measures among primary
care clinicians participating in this MVP as they transition to reporting the APP Plus quality
measure set in the Shared Savings Program. Experience and familiarity with the same quality
measures, redesigned care processes, and quality improvement activities that are commonplace
in ACOs would streamline the pathway for clinicians to join ACOs in the future and is consistent
with our goal to have all beneficiaries in an accountable care relationship by 2030.
Section 1899(b)(3)(C) of the Act requires CMS to seek to improve the quality of care
furnished by ACOs over time by specifying higher standards, new measures, or both for
purposes of assessing such quality of care. In the November 2011 final rule, we finalized 33
470
Jacobs D, Schreiber M, Seshamani M, Tsai D, Fowler E, Fleisher L. Aligning Quality Measures across CMS –
The Universal Foundation. New England Journal of Medicine, February 1, 2023, available at
https://www.nejm.org/doi/full/10.1056/NEJMp2215539.
471
Jacobs D, et al., Update On The Medicare Value-Based Care Strategy: Alignment, Growth, Equity. Health
Affairs Forefront (March 14, 2024), available at https://www.healthaffairs.org/content/forefront/update-medicare-
value-based-care-strategy-alignment-growth-equity.
quality measures for use in establishing the quality performance standard measure set for ACOs:
including 22 measures that were actively reported by ACOs via the Group Practice Reporting
Option (GPRO) Web Interface (76 FR 67889). As we stated in the November 2011 final rule
establishing the Shared Savings Program, our principal goal in selecting quality measures for
ACOs has been to identify measures of success in the delivery of high-quality health care at the
individual and population levels, with a focus on outcomes (76 FR 67872). As we sought to
improve the quality of care furnished by ACOs over time, we have subsequently updated this
measure set through rulemaking in the CY 2015, 2016, 2017, 2019, and 2023 PFS final rules (79
FR 67907 through 67921, 80 FR 71263 through 71268, 81 FR 80484 through 80489, 83 FR
59707 through 59715, and 87 FR 69860 through 69763, respectively). We have also sometimes
increased the number of measures reported by ACOs through rulemaking. For example, in the
CY 2016 PFS final rule, we increased the Shared Savings Program quality measure set from 33
total measures to 34 total measures (80 FR 71265). In the CY 2016 PFS final rule, we noted that
since the November 2011 Shared Savings Program final rule, we have continued to review the
quality measures used for the Shared Savings Program to ensure that they are up to date with
current clinical practice and aligned with other CMS quality reporting programs (80 FR 71264).
Also, through rulemaking, we sometimes reduced the number of measures reported by ACOs.
For example, in the CY 2019 PFS final rule, we finalized policies which reduced the Shared
Savings Program quality performance measure set to 23 measures in PY 2019 (83 FR 59715). In
developing our proposals in the CY 2019 PFS final rule, we stated that we considered the
agency’s efforts to streamline quality measures, reduce regulatory burden, and promote
innovation as part of broader CMS initiatives (83 FR 59711). In the CY 2021 PFS final rule, we
again reduced the total number of measures that ACOs must report (85 FR 84733). Specifically,
through the adoption of the APP quality measure set, we reduced the total number of measures
from 23 to either 6 or 13 measures (depending on the ACO’s chosen reporting option) for PY
2021 (85 FR 84723).

Our proposal to adopt the APP Plus quality measure set for ACOs that participate in the
Shared Savings Program would increase the number of measures reported by ACOs that
currently report the eCQM/MIPS CQM measure set from three measures in performance year
2024 to five measures in performance year 2025. For ACOs that reported the CMS Web
Interface measures, our proposal to adopt the APP Plus quality measure set would decrease the
number of measures reported from ten measures in performance year 2024 to eight measures in
performance year 2025. While we acknowledge that the increase in the number of measures for
ACOs that currently report the eCQM/MIPS CQM measure set may be an increased burden for
those ACOs, we are using a phased-in approach to expand the APP Plus quality measure set
between performance years 2025 and 2028, which should help to minimize the impact of
increased burden associated with reporting additional measures. The option for ACOs to report
Medicare CQMs, which are MIPS CQMs that are reported on an ACO’s fee-for-service
population, may also alleviate the reporting burden for ACOs that report Medicare CQMs by
focusing an ACO’s patient matching and data aggregation efforts only on an ACO’s eligible
Medicare fee-for-service population. Additionally, we believe that the benefits of scoring an
increased number of measures may offset the increased burden that some ACOs may face in
adopting the additional measures. For example, as the number of measures in the measure set
increases, the individual weight of each measure on the ACO’s quality performance score
decreases. Each measure in a six-measure set would account for roughly 16.67 percent of an
ACO’s MIPS Quality performance category score while each measure in an eight-measure set
would account for 12.5 percent of an ACO’s MIPS Quality performance category score. The
scoring of more measures, in concert with the scoring policies proposed in sections
IV.A.4.f.(1)(b)(iii) and IV.A.4.f.(1)(c)(i) of this proposed rule, may result in improved quality
performance scores for the ACOs as each individual measure carries less weight.
The proposed APP Plus quality measure sets for Shared Savings Program ACOs for
performance year 2025, performance years 2026 and 2027, and performance year 2028 and

subsequent performance years are displayed in Tables 34, 35, and 36, respectively. Under our
proposal, there would be eight measures (five eCQMs/Medicare CQMs, two administrative
claims measures, and the CAHPS for MIPS Survey measure) in the APP Plus quality measure set
for Shared Savings Program ACOs in performance year 2025 (Table 34), nine measures (six
eCQMs/Medicare CQMs, two administrative claims measures, and the CAHPS for MIPS Survey
measure) in performance years 2026 and 2027 (Table 35), and eleven measures (eight
eCQMs/Medicare CQMs, two administrative claims measures, and the CAHPS for MIPS Survey
measure) in performance years 2028 and subsequent performance years (Table 36). We intend to
update the APP Plus quality measure set as new measures are added to or removed from the
Adult Universal Foundation measure set in the future.
(b) Proposed Collection Types Available for Shared Savings Program ACOs Reporting the APP
Plus Quality Measure Set
We are proposing to streamline the collection types available for Shared Savings Program
ACOs reporting the APP Plus quality measure set to the eCQM and Medicare CQM collection
types for performance year 2025 and subsequent performance years. We believe that our
proposal to establish the APP Plus quality measure set to align with the Adult Universal
Foundation measure set should also aim to prioritize the eCQM collection type—the gold
standard collection type that underlies the Digital Quality Measurement (dQM) Strategic
Roadmap (available at
https://ecqi.healthit.gov/sites/default/files/CMSdQMStrategicRoadmap_032822.pdf)—and use
Medicare CQMs as the transition step on our building-block approach for ACOs’ progress to
adopt digital quality measurement. We are also seeking to reduce reporting burden on ACOs by
using a phased-in approach to expand the APP Plus quality measure set between performance
years 2025 and 2028. We will also continue to provide the Medicare CQM reporting option as
ACOs increase their experience and overcome their challenges with reporting all payer/all
patient measures. As discussed more fully below, we are proposing not to include the MIPS

CQM collection type for Shared Savings Program ACOs reporting the APP Plus quality measure
set to focus ACOs’ efforts on the implementation of the APP Plus quality measure set, while
continuing to encourage the adoption of eCQMs. We believe that our proposed approach would
recognize the investments ACOs have made to report eCQMs and their benefits (that is, more
efficient data collection, real time provider feedback, and less burden through the use of digital
data) and allow ACOs that have invested in eCQMs to continue on that track and align with long
term goals of digital quality measurement.
472
Since Medicare CQMs are MIPS CQMs that are reported on an ACO’s eligible Medicare
fee-for-service population, ACOs that have invested in the infrastructure to report MIPS CQMs
would be able to report Medicare CQMs on a subset of their all payer/all patient population.
Furthermore, as noted in the CY 2024 PFS final rule, Medicare CQMs address ACO concerns
related to the difficulty of matching and aggregating patient data across multiple EHR systems
(88 FR 79106). Medicare CQMs also provide a transition path and alternative for ACOs that
have difficulty reporting patient data by limiting the beneficiaries for which an ACO must match
and aggregate data to only the ACO’s eligible Medicare fee-for-service beneficiaries, instead of
their all payer/all patient population (88 FR 79106). As a logical next step in the reporting of
digital quality measures, this population is larger than the sample currently used in the CMS Web
Interface, but not as large as the all payer/all patient population that must be reported for an
eCQM or MIPS CQM (88 FR 79106).
We aim to fully transition to digital quality measurement in CMS quality reporting and
value-based purchasing programs, and we are working to convert current eCQMs to the Fast
Healthcare Interoperability Resources (FHIR) standard (86 FR 65379). Including eCQMs as a
collection type for Shared Savings Program ACOs reporting the APP Plus quality measure set
aligns with our goal to transition to digital quality measurement including the alignment and
472
Centers for Medicare & Medicaid Services (2023). Electronic Clinical Quality Measure Basics (eCQM 101).
Accessed June 24, 2024. https://ecqi.healthit.gov/sites/default/files/eCQM-Basics-508.pdf.

development of FHIR standards and tools for eCQM reporting in the dQM Strategic Roadmap.
There are numerous benefits to using eCQMs, including their use of electronic standards that
reduce the burden of manual extraction and reporting for measured entities, their use of clinical
data to assess the outcomes of treatment by measured entities, and their fostering of access to
real-time data for point of care quality improvement and decision support.
473
Furthermore,
eCQMs align with the Meaningful Measures Framework 2.0 goal of improving quality reporting
efficiency by transitioning to digital quality measures.
474
A recent study noted the resource
intensity of quality reporting, underscoring the high cost of claims-based measures relative to
others and recommended that policy makers shift to electronic metrics to “optimize resources
spent in the overall pursuit of higher quality.”
475
For these reasons, and to continue encouraging
ACOs on their progress to adopt digital quality measurement, we are not modifying the
availability of eCQMs as a collection type for ACOs that reported the APP quality measure set
by including eCQMs as a collection type in the APP Plus quality measure set in performance
year 2025 and subsequent performance years. In section III.G.7.e. of this proposed rule, we seek
comment on a higher risk, higher reward track for Shared Savings Program ACOs participating
in the ENHANCED track. In this request for information, we seek comment on questions
relevant to our long-term goals of supporting ACOs in their transition to reporting all payer/all
patient quality measures: How should a revised ENHANCED track with higher risk and potential
reward also require additional accountability for quality? Should ACOs in this revised track be
required to report all payer/all patient quality measures?
In the CY 2024 PFS final rule, we stated that “Medicare CQMs are intended to serve as a
transition to all payer/all patient reporting and not as a permanent collection type. We
acknowledge that ACOs are at different stages of readiness to adopt all payer/all patient
473
eCQI Resource Center (2024). Get Started with eCQMs. https://ecqi.healthit.gov/ecqms.
474
Centers for Medicare & Medicaid Services (2024). Meaningful Measures 2.0: Moving to Measure Prioritization
and Modernization. https://www.cms.gov/medicare/quality/meaningful-measures-initiative/meaningful-measures-20.
475
Saraswathula, A., et al., The Volume and Cost of Quality Metric Reporting. JAMA (June 6, 2023), available at
https://jamanetwork.com/journals/jama/fullarticle/2805705.

measures, and we intend for Medicare CQMs to be available to ACOs during their transition to
all payer/all patient reporting.” (88 FR 79106). In the CY 2024 PFS final rule, we also stated that
“[w]e expect that the sunsetting of the Medicare CQM collection type may be paced with the
uptake of FHIR Application Programming Interface (API) technology, but this will be assessed
on industry readiness and CMS requirements” (88 FR 79106). Specifically, we anticipate that the
increased use of FHIR API technology will facilitate ACOs’ reporting of eCQMs and thus
increase their uptake of them. Future advancements in FHIR API technology and its uptake
among Shared Savings Program ACOs may accelerate our future plans to sunset Medicare
CQMs. As discussed earlier in this section, we are proposing to streamline the collection types
available for Shared Savings Program ACOs reporting the APP Plus quality measure set to the
eCQM and Medicare CQM collection types for performance year 2025 and subsequent
performance years and use Medicare CQMs as the transition step on our building-block approach
for ACOs’ progress to adopt digital quality measurement. As we continue to support ACOs in
fully and equitably participating in all payer/all patient collection types with our proposed
creation of the APP Plus quality measure set, our commitment to monitor ACOs’ reporting of
quality data using Medicare CQMs and to assess their appropriateness as a collection type
remains the same.
As we stated in the CY 2024 PFS final rule, ACOs that include or are composed solely of
FQHCs or RHCs must report quality data on behalf of the FQHCs or RHCs that participate in the
ACO. To clarify, while FQHCs and RHCs that provide services that are billed exclusively under
FQHC or RHC payment methodologies are exempt from reporting traditional MIPS, FQHCs and
RHCs that participate in APMs, such as the Shared Savings Program, are considered APM Entity
groups as described at § 414.1370 (88 FR 79099). If our proposal is finalized, FQHCs and RHCs
that participate in Shared Savings Program ACOs would have to report the APP Plus quality
measure set through their ACO for performance year 2025 and subsequent performance years.
We seek comment on all of the proposals described in this section.

(3) Proposed Changes to Regulation Text
As discussed in section III.G.4.b.(2)(a) of this proposed rule, for performance year 2025
and subsequent performance years, we are proposing to require Shared Savings Program ACOs
to report the APP Plus quality measure set as proposed in section IV.A.4.c.(3) of this proposed
rule. The APP Plus quality measure set would comprise of eleven measures, consisting of six
measures from the APP quality measure set and five newly proposed measures from the Adult
Universal Foundation measure set that would be incrementally incorporated into the APP Plus
quality measure set over performance years 2025 through 2028. We are also proposing to focus
the collection types available to Shared Savings Program ACOs for reporting the APP Plus
quality measure set to eCQMs and Medicare CQMs. We refer readers to sections IV.A.4.c,
IV.A.4.e.(1)(b)(i), IV.A.4.f.(1)(b)(iii), and IV.A.4.f.(1)(c)(i). of this proposed rule for changes to
the regulation text at 42 CFR part 414. We are proposing conforming changes to the regulation
text at 42 CFR part 425 as described below in this section. We seek comment on our proposed
regulation text changes.
● We are proposing to sunset the requirement that ACOs must submit quality data via
the APP to satisfactorily report on behalf of the eligible clinicians who bill under the TIN of an
ACO participant for purposes of the MIPS Quality performance category of the Quality Payment
Program, and to revise § 425.508(b) to indicate that the requirement will be applicable for
performance years beginning in 2021 - 2024. We are also proposing to replace the phrase
“Alternative Payment Model Performance Pathway (APP)” with the phrase “APM Performance
Pathway (APP)” to conform with the phrase used at § 414.1367.
● We are adding a new paragraph (c) at § 425.508 to establish that, for performance
years beginning on or after January 1, 2025, ACOs must submit quality data via the APM
Performance Pathway (APP) on the quality measures contained in the APP Plus quality measure
set established under § 414.1367 to satisfactorily report on behalf of the eligible clinicians who
bill under the TIN of an ACO participant for purposes of the MIPS Quality performance
category of the Quality Payment Program.
● We are proposing to revise the section heading at § 425.510 to “Application of the
APM Performance Pathway (APP) quality measure set or the APP Plus quality measure set (as
applicable) to Shared Savings Program ACOs for performance years beginning on or after
January 1, 2021.”
● We are proposing to sunset the requirement that ACOs must report quality data on the
APP quality measure set according to the method of submission established by CMS and to
revise § 425.510(b). We are adding a new paragraph (b)(1) at § 425.510 to indicate that the
requirement will be applicable for performance years beginning in 2021 - 2024.
● We are adding a new paragraph (b)(2) at § 425.510 to establish that, for performance
years beginning on or after January 1, 2025, ACOs must report quality data on the APP Plus
quality measure set established under § 414.1367, according to the submission method
established by CMS.
● We are revising § 425.512(a)(2)(iii) to establish that, for performance year 2025 and
subsequent performance years, an ACO in the first performance year of the ACO’s first
agreement period under the Shared Savings Program will meet the quality performance standard
if the ACO reports the APP Plus quality measure set and meets the data completeness
requirement on all eCQMs/Medicare CQMs, and the CAHPS for MIPS survey (except as
specified in § 414.1380(b)(1)(vii)(B)), and receives a MIPS Quality performance category score
for the applicable performance year.
● We are revising the introductory paragraph (a)(5)(i) to § 425.512 to read as follows:
“Except as specified in paragraphs (a)(2) and (7) of this section, CMS designates the quality
performance standard as:”.
● We are revising the introductory paragraph (a)(5)(i)(A) to read as follows: “For
performance year 2024, the ACO reporting quality data on the APP quality measure set
established under § 414.1367 of this subchapter, according to the method of submission
established by CMS and –”.
● We are revising the introductory paragraph (a)(5)(i)(B) to read as follows: “For
performance year 2025 and subsequent performance years, the ACO reporting quality data on the
APP Plus quality measure set established under § 414.1367 of this subchapter, according to the
method of submission established by CMS and –”.
● We are adding a new paragraph (a)(5)(ii)(A) to § 425.512 to indicate that an ACO will
meet the alternative quality performance standard for performance year 2024 if the ACO reports
quality data on the APP quality measure set established under § 414.1367 according to the
method of submission established by CMS and achieves a quality performance score equivalent
to or higher than the 10
th
percentile of the performance benchmark on at least one of the four
outcome measures in the APP quality measure set.
● We are adding a new paragraph (a)(5)(ii)(B) to § 425.512 to establish that an ACO
will meet the alternative quality performance standard for performance year 2025 and subsequent
years if the ACO reports the quality data on the APP Plus quality measure set established under §
414.1367 according to the method of submission established by CMS and achieves a quality
performance score equivalent to or higher than the 10th percentile of the performance benchmark
on at least one of the four outcome measures in the APP Plus quality measure set.
● We are revising § 425.512(a)(5)(iii)(B) to indicate that for performance year 2025 and
subsequent performance years, an ACO will not meet the quality performance standard or the
alternative quality performance standard if the ACO does not report any of the eCQMs/Medicare
CQMs in the APP Plus quality measure set and does not administer a CAHPS for MIPS survey
(except as specified in § 414.1380(b)(1)(vii)(B)).
● We are revising § 425.512(a)(7) introductory text and (a)(7)(i) and adding new
paragraphs (a)(7)(i)(A) and (B) to indicate for performance year 2024, CMS will use the higher
of the ACO’s health equity adjusted Quality performance category score or the equivalent of the
40
th
percentile MIPS Quality performance category score when an ACO reports all of the
required measures, meeting the data completeness requirement for each measure in the APP
quality measure set and receiving a MIPS Quality performance category score and the ACO
meets either of the following:
++ The ACO’s total available measure achievement points used to calculate the ACO’s
MIPS Quality performance category score are reduced under § 414.1380(b)(1)(vii)(A).
++ At least one of the eCQMs/MIPS CQMs/Medicare CQMs does not have a benchmark
as described at § 414.1380(b)(1)(i)(A).
● We are revising § 425.512(a)(7)(ii) and adding new paragraphs (a)(7)(ii)(A) and (B) to
indicate for performance year 2025 and subsequent performance years, an ACO will receive the
higher of the ACO’s health equity adjusted quality performance category score or the equivalent
of the 40
th
percentile MIPS Quality performance category score when an ACO reports all of the
required measures in the APP Plus quality measure set, meeting the data completeness
requirement for each measure in the APP Plus quality measure set, and receiving a MIPS Quality
performance category score, and the ACO meets either of the following:
++ The ACO’s total available measure achievement points used to calculate the ACO’s
MIPS Quality performance category score are reduced under § 414.1380(b)(1)(vii)(A).
++ At least one of the eCQMs/ Medicare CQMs does not have a benchmark as described
at § 414.1380(b)(1)(i)(A).
● We are revising § 425.512(b)(1) and (2) and (b)(4)(i) by removing the phrase “APP
measure set” and replacing with the phrase “APP quality measure set” to align naming
conventions for the two quality measure sets within the APP: the APP quality measure set and
the APP Plus quality measure set.
● We are revising § 425.512(b)(1) to update a renumbered cross reference.
● We are revising the heading for § 425.512(b)(2) by removing the phrase “and
subsequent performance years.”
● We are renumbering the current paragraph (b)(3) of § 425.512 to paragraph (b)(4) and
revising the cross references therein to reflect this renumbering.
● We are adding a new paragraph (b)(3) to § 425.512 to establish for performance year
2025 and subsequent performance years that for an ACO that reports all of the eCQMs/Medicare
CQMs in the APP Plus quality measure set, meeting the data completeness requirement for all of
the eCQMs/Medicare CQMs, and administers the CAHPS for MIPS survey (except as specified
in § 414.1380(b)(1)(vii)(B)), CMS calculates the ACO’s health equity adjusted quality
performance score as the sum of the ACO’s MIPS Quality performance category score for all
measures in the APP Plus quality measure set and the ACO’s health equity adjustment bonus
points. The sum of these values may not exceed 100 percent.
● We are renumbering the current paragraph (b)(4) of § 425.512 to paragraph (b)(5) and
revising the cross references therein to reflect this renumbering.
● We are revising renumbered § 415.512(b)(5)(iv) to add reference to new paragraph
(c)(3)(iv).
● We are revising § 425.512(c)(3) introductory text by removing the phrase “via the
APP” and adding in its place the phrase “on the APP quality measure set or APP Plus quality
measure set (as applicable)”.
● We are revising § 425.512(c)(3)(iii) by removing the phrase “and subsequent
performance years” after “For performance year 2024”.
● We are adding new paragraph (c)(3)(iv) to § 425.512 to establish for performance year
2025 and subsequent performance years, if CMS determines the ACO meets the requirements of
the Extreme and Uncontrollable Circumstances policy and the ACO reports the APP Plus quality
measure set, meets the data completeness requirement, and receives a MIPS Quality performance
category score, CMS will calculate the ACO’s quality score as the higher of the ACO’s health
equity adjusted quality performance score or the equivalent of the 40th percentile MIPS Quality
performance category score across all MIPS Quality performance category scores, excluding
entities/providers eligible for facility-based scoring, for the relevant performance year.
c. Proposed Changes to the Methodology for Calculating the MIPS Quality Performance
Category Score for Shared Savings Program ACOs Reporting the APP Plus Quality Measure Set
(1) Background
Consistent with the authority to establish the quality reporting and other reporting
requirements for the Medicare Shared Savings Program set forth in section 1899(b)(3) of the Act
and the statutory requirements for the Quality Payment Program set forth in section 1848(q) and
(r) of the Act for MIPS and section 1833(z) of the Act for Advanced APMs, since the Shared
Savings Program’s alignment with the APP in performance year 2021, MIPS eligible clinicians
identified on the Participation List or Affiliated Practitioner List of an APM Entity participating
in a MIPS APM – including ACOs that participate in the Medicare Shared Savings Program –
that report data via the APP have been scored according to the APP scoring methodology
described at § 414.1367. The MIPS Quality performance category score is calculated according
to the APP scoring methodology at § 414.1367(c)(1) (85 FR 84864). Under the waiver authority
at section 1115A(d)(1) of the Act for CMS Innovation Center APMs and at section 1899(f) of the
Act for the Medicare Shared Savings Program, the Cost performance category weight is zero
percent as described at § 414.1367(c)(2) (85 FR 84864) for MIPS eligible clinicians that report
via the APP. As noted in section 1848(q)(5)(C)(ii) of the Act, a MIPS eligible clinician in an
APM for a performance period automatically earns a minimum score of one half of the highest
potential score for the MIPS Improvement activities category for their participation in an APM
for the performance period. These baseline scores are automatically applied to the MIPS
Improvement activities performance category score for MIPS eligible clinician in an APM –
including ACOs that participate in the Medicare Shared Savings Program – that report via the
APP as described at § 414.1367(c)(3) (85 FR 84865). The Promoting Interoperability
performance category under the APP is reported and calculated in the same manner described at
§ 414.1375 (85 FR 84865).
As described in the CY 2021 PFS final rule, we waived the requirement to weight each
MIPS performance category as described in section 1848(q)(5)(E) of the Act using the waiver
authority in section 1899(f) of the Act for Medicare Shared Savings Program for MIPS eligible
clinicians that report via the APP – including ACOs that participate in the Medicare Shared
Savings Program (85 FR 84865). The performance category weights used to calculate the final
score for a MIPS eligible clinician who is scored through the APP at § 414.1367(d)(1) are:
● Quality: 50 percent.
● Cost: 0 percent.
● Improvement Activities: 20 percent.
● Promoting Interoperability: 30 percent.
Additionally, in the CY 2021 PFS final rule, we also stated that under the authority provided in
section 1848(q)(5)(F) of the Act, it may become necessary to reweight one or more performance
categories (85 FR 84866). As described at § 414.1367(d)(2), if CMS determines, in accordance
with § 414.1380(c)(2), that a different scoring weight should be assigned to the Quality or
Promoting Interoperability performance category, CMS will redistribute the performance
category weights as follows:
● If CMS reweights the Quality performance category to 0 percent: Promoting
Interoperability performance category is reweighted to 75 percent, and Improvement activities
performance category is reweighted to 25 percent.
● If CMS reweights the Promoting Interoperability performance category to 0 percent:
Quality performance category is reweighted to 75 percent, and Improvement activities
performance category is reweighted to 25 percent.
Lastly, as codified at § 414.1367(e), final scoring for APM participants reporting to MIPS
through the APP – including ACOs that participate in the Medicare Shared Savings Program –
would follow the same methodology as established for MIPS generally at § 414.1380 (85 FR
84866).
In performance year 2024, ACOs are scored on either the three eCQMs/MIPS

CQMs/Medicare CQMs or the ten CMS Web Interface measures, the CAHPS for MIPS survey,
and two administrative claims-based measures. Under this methodology, an ACO’s MIPS
Quality performance category score is calculated according to MIPS scoring rules for the Quality
performance category established at § 414.1380(b)(1) with exceptions for (1) measures that do
not have a benchmark or do not meet the case minimum requirement and (2) measures that are
identified as topped out. Specifically, each submitted measure that does not have a benchmark or
does not meet the case minimum requirement is excluded from an ACO’s total measure
achievement points (numerator) and total available measure achievement points (denominator).
Additionally, any measure that is identified as topped out is not subject to the scoring cap
described at § 414.1380(b)(1)(iv). Under current APP scoring rules, each required measure of the
APP quality measure set that is not submitted by an ACO via the APP receives zero measure
achievement points.
(2) Proposed Revisions
(a) Proposal to Establish the Data Submission Criteria for the APP Plus Quality Measure Set
As discussed in section IV.A.4.e.(1)(b)(i) of this proposed rule, for the APP Plus quality
measure set, we are proposing that Shared Savings Program ACOs that report the APP Plus
quality measure set and MIPS eligible clinicians, groups, and APM Entities that choose to report
the APP Plus quality measure set, will be required to report on all measures in the APP Plus
quality measure set, as applicable. Specifically, in § 414.1335(b), we are proposing to establish
the data submission criteria for the APP Plus quality measure set, which would require the
reporting of all measures within the APP Plus quality measure set, except for administrative
claims-based quality measures.
476
The MIPS Quality performance category score is calculated according to the APP scoring
476
As described at § 414.1325(a)(2)(i), there are no data submission requirements for administrative claims-based
quality measures as performance on such measures is calculated by CMS using administrative claims data, which
includes claims submitted with dates of service during the applicable performance period that are processed no later
than 60 days following the close of the applicable performance period.

methodology at § 414.1367(c)(1) (85 FR 84864 through 85 FR 84865). As such, an ACO’s
MIPS Quality performance category score is calculated according to MIPS scoring rules for the
Quality performance category established at § 414.1380(b)(1) with exceptions for (1) measures
that do not have a benchmark or do not meet the case minimum requirement and (2) measures
that are identified as topped out. Consistent with our proposal described above, under §
414.1380(b)(1), for performance year 2025 and subsequent performance years, ACOs would be
scored on all required measures in the APP Plus quality measure set.
We are proposing that the policies related to MIPS performance category scoring in the
APP at § 414.1367(c) would apply to Shared Savings Program ACOs that report the APP Plus
quality measure set for the purpose of meeting the Shared Savings Program’s quality
performance standard.
477
Specifically, we are proposing that the APP scoring policies at §
414.1367(c)(1) for the calculation of the ACO’s MIPS Quality performance category, §
414.1367(c)(2) for the calculation of an ACO’s MIPS Cost performance category, §
414.1367(c)(3) for the calculation of an ACO’s MIPS Improvement activities performance
category, and § 414.1367(c)(4) for the calculation of an ACO’s MIPS Promoting Interoperability
performance category would apply to ACOs that report the APP Plus quality measure set in
performance year 2025 and subsequent performance years. Additionally, we are proposing that §
414.1367(d) for the performance category weights and § 414.1367(e) for the calculation of the
final score would apply to Shared Savings Program ACOs that report the APP Plus quality
measure set in performance year 2025 and subsequent performance years.
If our proposals are finalized, then in performance year 2025, ACOs would be scored on
the required eight measures in the APP Plus quality measure set: five eCQMs/Medicare CQMs,
the CAHPS for MIPS survey, and two administrative claims-based measures. In performance
years 2026 and 2027, ACOs would be scored on the required nine measures: six
477
This discussion describes standards under the APP, which are applicable to APM Entities. We refer throughout to
ACOs in lieu of APM Entities as we are discussing the application of APP standards to ACOs participating in the
Shared Savings Program, and thus ACOs are the sole relevant type of APM Entity.

eCQMs/Medicare CQMs, the CAHPS for MIPS survey, and two administrative claims-based
measures. In performance year 2028 and subsequent performance years, ACOs would be scored
on the required eleven measures: eight eCQMs/Medicare CQMs, the CAHPS for MIPS survey,
and two administrative claims-based measures. We refer readers to Tables 34, 35, and 36 in
Section III.G.4.f of this proposed rule for additional detail on the required measures in each
performance year.
We refer readers to section IV.A.4.e.(1)(b)(i) of this proposed rule for a discussion of our
proposal to establish the data submission criteria for the APP Plus quality measure set,
specifically the proposal to require the reporting of all measures within the APP Plus quality
measure set.
(b) Proposal to Establish a Complex Organization Adjustment for Virtual Groups and APM
Entities
To account for the organizational complexities faced by Virtual Groups and APM
Entities, including Shared Savings Program ACOs, when reporting eCQMs, in section
IV.A.4.f.(1)(b)(iii) of this proposed rule, we are proposing to establish a Complex Organization
Adjustment beginning in the CY 2025 performance period/2027 MIPS payment year. A Virtual
Group and an APM Entity would receive one measure achievement point for each submitted
eCQM that meets the case minimum requirement at § 414.1380(b)(1)(iii) and the data
completeness requirement at § 414.1340. Each reported eCQM may not score more than 10
measure achievement points and the total achievement points (numerator) may not exceed the
total available measure achievement points (denominator) for the quality performance category.
The Complex Organization Adjustment for a Virtual Group or APM Entity may not exceed 10
percent of the total available measure achievement points in the quality performance category.
The adjustment would be added for each measure submitted at the individual measure level.
Since Shared Savings Program ACOs are APM Entities, this proposal would be
applicable to Shared Savings Program ACOs reporting the APP Plus quality measure set

beginning in performance year 2025. We refer readers to section IV.A.4.f.(1)(b)(iii) of this
proposed rule for discussion of our proposal to establish the Complex Organization Adjustment
for Virtual Groups and APM Entities
As the Adult Universal Foundation measures are phased into the APP Plus quality
measure set, ACOs that participate in the Shared Savings Program would be required to report on
a larger measure set relative to other eCQM reporters. Under our proposal as described in section
III.G.4.f of this proposed rule, the APP Plus quality measure set for Shared Savings Program
ACOs would include eight measures (five eCQMs/Medicare CQMs, two administrative claims
measures, and the CAHPS for MIPS Survey measure) in performance year 2025 (Table 34); nine
measures (six eCQMs/Medicare CQMs, two administrative claims measures, and the CAHPS for
MIPS Survey measure) in performance years 2026 and 2027 (Table 35); and eleven measures
(eight eCQMs/Medicare CQMs, two administrative claims measures, and the CAHPS for MIPS
Survey measure) in performance years 2028 and subsequent performance years (Table 36).
(c) Proposal to Score Shared Savings Program ACOs Reporting Medicare CQMs using Flat
Benchmarks
In the CY 2024 PFS final rule, we finalized our proposal to establish new benchmarks for
scoring ACOs on the Medicare CQMs under MIPS in alignment with MIPS benchmarking
policies (88 FR 79110). As historical Medicare CQM data would not be available, we finalized
that for performance years 2024 and 2025, we will score Medicare CQMs using performance
period benchmarks. We also finalized that, for performance year 2026 and subsequent
performance years, when baseline period data are available to establish historical benchmarks in
a manner that is consistent with the MIPS benchmarking policies at § 414.1380(b)(1)(ii), we will
score Medicare CQMs using historical benchmarks.
A few commenters noted in our proposal in the CY 2024 PFS proposed rule (88 FR
79109-79110) their concern about ACOs being compared only to other ACOs that report
Medicare CQMs since the Medicare CQMs would be available only to Shared Savings Program

ACOs. One commenter stated their preference to have their quality performance compared to all
other participants on these measures, while another commenter stated that CMS should stop
measuring ACOs against each other and instead measure ACOs on a national standard so that all
ACOs can pass and do not lose out on savings due to arbitrary quality decile cut points. In our
response to these comments, we stated that given that benchmarks are specific to each collection
type and that we proposed to establish Medicare CQMs as a new collection type for only Shared
Savings Program ACOs, only ACO data will be available to benchmark Medicare CQMs.
Additionally, the health equity adjustment would be applicable to Medicare CQMs for purposes
of determining shared savings payments/losses. The application of the health equity adjustment
would help improve performance when ACOs deliver high quality care to underserved patient
populations. For these reasons, it is appropriate to establish benchmarks for Medicare CQMs that
are consistent with MIPS benchmarking policies. ACOs that prefer to be compared to clinicians
at large may do so by reporting eCQMs or MIPS CQMs, for which CMS calculates a benchmark
using data reported by MIPS eligible clinicians reporting under the chosen collection type.
In performance year 2022, ACOs had a higher average performance on quality measures
they were required to report in order to share in savings compared to other similarly sized
clinician groups not in the Shared Savings Program.
478
This includes statistically significant
higher performance for quality measures related to diabetes and blood pressure control; breast
cancer and colorectal cancer screening; tobacco screening and smoking cessation; and depression
screening and follow-up.
479
In shifting to Medicare CQMs, ACO performance would be
benchmarked against other ACOs only reporting Medicare CQMs. Since ACOs are high
performers relative to comparably sized MIPS groups, benchmarking Medicare CQMs using
only ACO data would lower some ACOs’ MIPS measure achievement points on those measures.
478
Centers for Medicare & Medicaid Services (2023). Medicare Shared Savings Program Saves Medicare More
Than $1.8 Billion in 2022 and Continues to Deliver High-quality Care. [Press release].
https://www.cms.gov/newsroom/press-releases/medicare-shared-savings-program-saves-medicare-more-18-billion-
2022-and-continues-deliver-high.
479
Id.
In other words, high-performing ACOs could earn lower measure achievement points relative to
comparable MIPS groups because the Medicare CQM benchmarking pool is comprised of
higher-than-average performance data–in effect, creating a “tournament approach” to scoring
Medicare CQMs wherein ACOs must compete with other ACOs to earn measure achievement
points. This could be particularly disadvantageous for ACOs that serve a high proportion of
underserved populations because, while ACOs that report eCQMs and/or Medicare CQMs and
serve a high proportion of underserved populations are eligible for health equity adjustment
points, ACOs must score in the top or middle thirds of ACO measure performers to earn health
equity adjustment points.
As described in section III.G.4.b.(2)(b) of this proposed rule, for performance year 2025
and subsequent performance years, we are proposing to streamline the collection types available
for Shared Savings Program ACOs reporting the APP Plus quality measure set to the eCQM and
Medicare CQM collection types. Therefore, as discussed in section IV.A.4.f.(1)(c)(i) of this
proposed rule, we are proposing to add § 414.1380(b)(1)(ii)(F) to state that beginning in the CY
2025 performance period/2027 MIPS payment year, measures of the Medicare CQM collection
type would be scored using flat benchmarks for their first two performance periods in MIPS. Our
proposal in section IV.A.4.f.(1)(c)(i) of this proposed rule would expand the use of flat
benchmarks to Medicare CQMs in their first two performance periods in MIPS. The use of flat
benchmarks would allow ACOs with high scores to earn maximum or near maximum
achievement points while allowing room for quality improvement and rewarding that
improvement in subsequent years. Use of flat benchmarks also helps to ensure that ACOs with
high quality performance on a measure are not penalized as low performers. As discussed in
section IV.A.4.f.(1)(c)(i) of this proposed rule, we are proposing to add § 414.1380(b)(1)(ii)(F)
to incorporate this proposal. The use of historical benchmarks, when data are available, is
consistent with MIPS benchmarking policies at § 414.1380(b)(1)(ii), allow ACOs to know
benchmarks prior to start of the performance year, and create opportunities for improvement.

Table 30 lists the Medicare CQMs in the APP Plus quality measure set that would be
eligible for flat benchmarks in performance year 2025 through performance year 2029 under our
proposal.
TABLE 30: Medicare CQMs Eligible for Flat Benchmarks in Performance Year
2025 through 2029
Performance Year
Quality #
2025
001, 134, 236, 112, 113
2026
112, 113, 305
2027
305
2028
487, 493
2029
487, 493
A quality performance benchmark is the performance rate an ACO must achieve to earn
the corresponding quality points for each measure. Flat benchmarks assign a performance rate
range to each decile. In flat benchmarks for non-inverse measures, any performance rate at or
above 90 percent would be in the top decile; any performance rate between 80 percent and 89.99
percent would be in the second highest decile, and so on. For inverse measures, this would be
reversed – any performance rate at or below 10 percent would be in the top decile; any
performance rate between 10.01 percent and 20 percent would be in the second highest decile,
and so on. The number of measure achievement points received for each measure is determined
based on the applicable benchmark decile category and the percentile distribution.
For non-inverse measures, better quality performance is indicated by a higher
performance rate. For example, Quality #: 001 Controlling High Blood Pressure is a non-inverse
measure that measures the percentage of patients 18 - 85 years of age who had a diagnosis of
hypertension and whose blood pressure was adequately controlled (< 140/90 mmHg) during the
measurement period. Better quality performance on this measure is demonstrated by having a
higher percentage of patients whose blood pressure was adequately controlled. Table 31 lists the
flat benchmarks for a non-inverse Medicare CQM under our proposal described in section
IV.A.4.f.(1)(c)(i) of this proposed rule.

TABLE 31: Flat Benchmarks for a Non-Inverse Medicare CQM in its First Two
Performance Periods in MIPS in Performance Year 2025 and Subsequent Years
Decile
Performance Rate Range
1
< 10.00
2
10.00 – 19.99
3
20.00 – 29.99
4
30.00 – 39.99
5
40.00 – 49.99
6
50.00 – 59.99
7
60.00 – 69.99
8
70.00 – 79.99
9
80.00 – 89.99
10
>= 90.00
For example, if an ACO reports a non-inverse Medicare CQM in its first two
performance periods in MIPS in performance year 2025 and earns a performance rate of 55.25
percent, then the ACO would score in the 6
th
decile on that measure.
For inverse measures, better quality performance is indicated by a lower performance
rate. This is reflected in flat benchmark such that lower quality performance rates are found in
higher deciles. For example, Quality #: 001 Diabetes: Hemoglobin A1c (HbA1c) Poor Control is
an inverse quality measure that measures the percentage of patients 18-75 years of age with
diabetes who had hemoglobin A1c > 9.0 percent during the measurement period. Better quality
performance on this measure is demonstrated by having a lower percentage of patients whose
HbA1c was > 9.0 percent. Table 32 lists the flat benchmarks for an inverse Medicare CQM
under our proposal described in section IV.A.4.f.(1)(c)(i) of this proposed rule.
TABLE 32: Flat Benchmarks for an Inverse Medicare CQM in its First Two Performance
Periods in MIPS in Performance Year 2025 and Subsequent Years
Decile
Performance Rate Range
1
99.00 – 90.01
2
90.00 – 80.01
3
80.00 – 70.01
4
70.00 – 60.01
5
60.00 – 50.01
6
50.00 – 40.01
7
40.00 – 30.01
8
30.00 – 20.01
9
20.00 – 10.01
10
<= 10.00
For example, if an ACO reports an inverse Medicare CQM in its first two performance
periods in MIPS in performance year 2025 and earns a performance rate of 12.25 percent, then
the ACO would score in the 9
th
decile on that measure. In performance year 2025, Quality #: 001
Diabetes: Hemoglobin A1c (HbA1c) Poor Control is the only inverse Medicare CQM measure.
There are scoring scenarios in which ACOs would earn higher measure achievement
points under flat benchmarks compared to those they would earn under performance period
benchmarks. Most notable are scenarios in which ACOs have a tight distribution of performance
rates on a measure. For example, a non-inverse measure for which a performance rate of 90.00
percent is in the 8
th
decile. In this example, an ACO that reported a performance rate of 90.00
percent would be scored in the 8
th
decile when the hypothetical performance period benchmark is
applied. Using the flat benchmarks described in Table 31 of this proposed rule, an ACO that
reported a performance rate of 90.00 percent would be scored in the 10
th
decile, resulting in
greater measure achievement points than under the hypothetical performance period benchmarks
described in this example. For more details on the calculation of measure achievement points, we
refer readers to the “APM Performance Pathway (APP) Toolkit” which is updated for each
performance year and posted in the QPP Resource Library.
We seek comment on our proposal to score ACOs reporting Medicare CQMs using flat
benchmarks in performance year 2025 and subsequent performance years.
(3) Proposed Changes to Regulation Text
As discussed in sections III.G.4.c.(2)(a), III.G.4.c.(2)(b), and III.G.4.c.(2)(c) of this
proposed rule, we are proposing to establish scoring rules to calculate the MIPS Quality
performance category score for ACOs reporting the APP Plus quality measure set for
performance year 2025 and subsequent performance years. We believe that these proposed
scoring rules would incentivize the reporting of eCQMs in the APP Plus quality measure set
while continuing to support ACOs that report Medicare CQMs as they build the infrastructure,
skills, knowledge, and expertise necessary to aggregate patient data to report digital quality
measures. We refer readers to sections IV.A.4.e.(1)(b)(i), IV.A.4.f.(1)(b)(iii), and
IV.A.4.f.(1)(c)(i) of this proposed rule for changes to the regulation text at 42 CFR part 414.
d. Proposal to Extend the eCQM Reporting Incentive for Meeting the Shared Savings Program
Quality Performance Standard
(1) Background
In the CY 2023 PFS final rule, we extended the incentive for reporting eCQMs/MIPS
CQMs through performance year 2024 to align with the timeline for sunsetting of the CMS Web
Interface reporting option and to allow ACOs an additional year to gauge their performance on
the eCQMs/MIPS CQMs before full reporting of the measures are required beginning in
performance year 2025 (87 FR 69836 through 69838). We originally adopted this incentive in
the CY 2022 PFS final rule to encourage ACOs to begin the transition to eCQM/MIPS CQM
reporting in performance years 2022 and 2023 (86 FR 65269). We finalized an update to the
incentive for performance year 2024 such that:
● If an ACO reports the three eCQMs/ MIPS CQMs, meets the data completeness
requirement at § 414.1340 and the case minimum requirement at § 414.1380 for all three
eCQMs/MIPS CQMs, and:
● Achieves a quality performance score equivalent to or higher than the 10th percentile
of the performance benchmark on at least one of the four outcome measures in the APP measure
set and;
● Achieves a quality performance score equivalent to or higher than the 40th percentile
of the performance benchmark on at least one of the remaining five measures in the APP
measure set, the ACO will meet the quality performance standard used to determine eligibility
for shared savings and to avoid maximum shared losses, if applicable.
We received a few comments on our proposal in the CY 2023 PFS proposed rule to
extend the incentive for reporting eCQMs/MIPS CQMs through performance year 2024
suggesting that we extend the incentive beyond 2024 to facilitate the national shift towards
eCQMs. In our response in the CY 2023 PFS final rule (87 FR 69836), we stated that “We are
not extending the incentive beyond performance year 2024 at this time because this policy is
intended to align with the timeline for sunsetting of the CMS Web Interface reporting option at
the end of performance year 2024. We will continue to monitor the impact of this policy as we
gain more experience with ACOs reporting eCQMs/MIPS CQMs and may revisit the policy in
future rulemaking.”
(2) Proposed Revisions
We are committed to continuing to support ACOs in the transition to all payer/all patient
eCQMs and digital quality measurement reporting. As described in section III.G.4.b.(2)(a) of this
proposed rule, for performance year 2025 and subsequent performance years, we are proposing
to require Shared Savings Program ACOs to report the APP Plus quality measure set as proposed
in section IV.A.4.c.(3) of this proposed rule. The APP Plus quality measure set would
incrementally grow to comprise of eleven measures, consisting of six measures from the APP
quality measure set and five newly proposed measures from the Adult Universal Foundation
measure set that would be incrementally incorporated into the APP Plus quality measure set over
performance years 2025 through 2028. We are also proposing to focus the collection types
available to Shared Savings Program ACOs for reporting the APP Plus quality measure set to all
payer/all patient eCQMs and Medicare CQMs (while not adding the MIPS CQM as an available
collection type for Shared Savings Program ACOs under the APP Plus quality measure set).
The Shared Savings Program continues to hear from ACOs and other stakeholders about
the challenges with reporting on all payer/all patient measures and meeting data management
requirements given their muti-practice/multi EHR structure, the challenges to aggregate data
with the health IT infrastructure in use by ACOs and current state of interoperability. Shared
Savings Program quality reporting data over the past two performance years indicate that ACOs
have been slow to report eCQMs. In performance year 2021, 5 of 475 ACOs reported eCQMs
under the APP. In performance year 2022, among ACOs that reported quality data under the

APP, 24 out of 482 reported eCQMs with 7 of these ACOs reporting a combination of eCQMs
and MIPS CQMs.
480
We encourage ACOs, especially those ACOs serving large, underserved
populations, to leverage interoperability and digital data more fully and to more quickly
transition to eCQMs. As such, we are proposing to extend the eCQM reporting incentive to
performance year 2025 and subsequent performance years to support ACOs in meeting the
Shared Savings Program quality performance standard for sharing in savings at the maximum
rate under its track.
Specifically, we are proposing that for performance year 2025 and subsequent
performance years, an ACO will meet the quality performance standard used to determine
eligibility for maximum shared savings and to avoid maximum shared losses, if applicable:
● If the ACO reports all of the eCQMs in the APP Plus quality measure set applicable
for a performance year, meeting the data completeness requirement at § 414.1340 for all eCQMs,
and;
● Achieves a quality performance score equivalent to or higher than the 10th percentile
of the performance benchmark on at least one of the four outcome measures in the APP Plus
quality measure set, and;
● Achieves a quality performance score equivalent to or higher than the 40th percentile
of the performance benchmark on at least one of the remaining measures in the APP Plus quality
measure set.
The eCQM reporting incentive would apply only to those ACOs that report all of the
eCQMs in the APP Plus quality measure set applicable for a performance year and meet the data
completeness requirement for all of the eCQMs. The reporting incentive would not apply to
ACOs that report a combination of eCQMs/Medicare CQMs or report only Medicare CQMs. We
will further assess the need for the eCQM reporting incentive in the future as ACOs continue the
transition to adopting eCQMs and may make refinements as needed in future rulemaking. The
480
Counts based on internal analysis of ACOs’ quality reporting in performance year 2022 and 2021.
proposed APP Plus quality measure set for Shared Savings Program ACOs for performance year
2025, performance years 2026 and 2027, and performance year 2028 and subsequent
performance years are displayed in Tables 34, 35, and 36, respectively. We included the measure
type in these tables for each measure in the APP Plus quality measure set to provide ACOs with
a list of the outcome measures for purposes of qualifying for the eCQM reporting incentive.
(3) Proposed Changes to Regulation Text
We are proposing to add paragraphs (a)(5)(i)(B)(1) and (2) to § 425.512 to incorporate
our proposal to extend the eCQM reporting incentive to performance year 2025 and subsequent
performance years into the regulation text.
We seek comment on our proposal to extend the eCQM reporting incentive to
performance year 2025 and subsequent performance years.
e. Summary of Proposals
In Table 33 of this proposed rule, we summarize the proposed changes to the regulation
at § 425.512(a)(5) to reflect the changes we are proposing to the quality reporting requirements
and quality performance standard for performance year 2025 and subsequent performance years.

TABLE 33: Proposed APP Plus Quality Measure Set Reporting Requirements and Quality
Performance Standard for Shared Savings ACOs for Performance Year 2025 and
Subsequent Performance Years
Performance Year 2025
Performance Years 2026
and 2027
Performance Year 2028
and Subsequent
Performance Years
Shared Savings
Program ACO
Quality Reporting
Requirements
ACOs are required to report
the 5 eCQMs/Medicare
CQMs in the APP Plus
quality measure set and
administer the CAHPS for
MIPS survey. CMS will
calculate 2 claims-based
measures.
ACOs are required to report
6 eCQMs/Medicare CQMs
in the APP Plus quality
measure set and administer
the CAHPS for MIPS
survey. CMS will calculate
2 claims-based measures.
ACOs are required to report
8 eCQMs/Medicare CQMs
in the APP Plus quality
measure set and administer
the CAHPS for MIPS
survey. CMS will calculate
2 claims-based measures.
Shared Savings
Program ACO
Quality
Performance
Standard and
Alternative Quality
Performance
Standard
Quality performance
standard used to
determine eligibility for
maximum shared savings
and to avoid maximum
shared losses, if
applicable:
1. For ACOs that report
eCQMs/Medicare CQMs
and serve a high proportion
of underserved
beneficiaries, achieving a
health equity adjusted
quality performance score
that is equivalent to or
higher than the 40th
percentile across all MIPS
Quality performance
category scores, excluding
entities/providers eligible
for facility-based scoring, or
2. Reporting the 5 eCQMs
in the APP Plus quality
measure set, meeting the
data completeness
requirement at § 414.1340
for all 5 eCQMs, and
achieving a quality
performance score
equivalent to or higher than
the 10th percentile of the
performance benchmark on
at least 1 of the 4 outcome
measures in the APP Plus
quality measure set and a
quality performance score
equivalent to or higher than
the 40th percentile of the
performance benchmark on
at least 1 of the remaining 7
measures in the APP Plus
quality measure set.
Alternative quality
performance standard
used to determine shared
Quality performance
standard used to
determine eligibility for
maximum shared savings
and to avoid maximum
shared losses, if
applicable:
1. For ACOs that report
eCQMs/Medicare CQMs
and serve a high proportion
of underserved
beneficiaries, achieving a
health equity adjusted
quality performance score
that is equivalent to or
higher than the 40th
percentile across all MIPS
Quality performance
category scores, excluding
entities/providers eligible
for facility-based scoring, or
2. Reporting the 6 eCQMs
in the APP Plus quality
measure set, meeting the
data completeness
requirement at § 414.1340
for all 6 eCQMs, and
achieving a quality
performance score
equivalent to or higher than
the 10th percentile of the
performance benchmark on
at least 1 of the 4 outcome
measures in the APP Plus
quality measure set and a
quality performance score
equivalent to or higher than
the 40th percentile of the
performance benchmark on
at least 1 of the remaining 8
measures in the APP Plus
quality measure set.
Alternative quality
performance standard
used to determine shared
Quality performance
standard used to
determine eligibility for
maximum shared savings
and to avoid maximum
shared losses, if
applicable:
1. For ACOs that report
eCQMs/Medicare CQMs
and serve a high proportion
of underserved
beneficiaries, achieving a
health equity adjusted
quality performance score
that is equivalent to or
higher than the 40th
percentile across all MIPS
Quality performance
category scores, excluding
entities/providers eligible
for facility-based scoring, or
2. Reporting the 8 eCQMs
in the APP Plus quality
measure set, meeting the
data completeness
requirement at § 414.1340
for all 8 eCQMs, and
achieving a quality
performance score
equivalent to or higher than
the 10th percentile of the
performance benchmark on
at least 1 of the 4 outcome
measures in the APP Plus
quality measure set and a
quality performance score
equivalent to or higher than
the 40th percentile of the
performance benchmark on
at least 1 of the remaining
10 measures in the APP
Plus quality measure set.
Alternative quality
performance standard
used to determine shared

Performance Year 2025
Performance Years 2026
and 2027
Performance Year 2028
and Subsequent
Performance Years
savings using the sliding
scale methodology:
An ACO that fails to meet
the criteria above but meets
the alternative quality
performance standard by
achieving a quality
performance score
equivalent to or higher than
the 10th percentile of the
performance benchmark on
at least 1of the 4 outcome
measures in the APP Plus
quality measure set would
share in savings (if
otherwise eligible) at a
lower rate that is scaled by
the ACO’s health equity
adjusted quality
performance score.
If an ACO (1) does not
report any of the 5 eCQMs
/Medicare CQMs in the
APP Plus quality measure
set and (2) does not
administer a CAHPS for
MIPS survey, the ACO will
not meet the quality
performance standard or the
alternative quality
performance standard. This
ACO will be ineligible to
share savings and will owe
maximum shared losses, if
applicable.
savings using the sliding
scale methodology:
An ACO that fails to meet
the criteria above but meets
the alternative quality
performance standard by
achieving a quality
performance score
equivalent to or higher than
the 10th percentile of the
performance benchmark on
at least 1 of the 4 outcome
measures in the APP Plus
quality measure set would
share in savings (if
otherwise eligible) at a
lower rate that is scaled by
the ACO’s health equity
adjusted quality
performance score.
If an ACO (1) does not
report any of the 6 eCQMs
/Medicare CQMs in the
APP Plus quality measure
set and (2) does not
administer a CAHPS for
MIPS survey, the ACO will
not meet the quality
performance standard or the
alternative quality
performance standard. This
ACO will be ineligible to
share savings and will owe
maximum shared losses, if
applicable.
savings using the sliding
scale methodology:
An ACO that fails to meet
the criteria above but meets
the alternative quality
performance standard by
achieving a quality
performance score
equivalent to or higher than
the 10th percentile of the
performance benchmark on
at least 1 of the 4 outcome
measures in the APP Plus
quality measure set would
share in savings (if
otherwise eligible) at a
lower rate that is scaled by
the ACO’s health equity
adjusted quality
performance score.
If an ACO (1) does not
report any of the 8 eCQMs
/Medicare CQMs in the
APP Plus quality measure
set and (2) does not
administer a CAHPS for
MIPS survey, the ACO will
not meet the quality
performance standard or the
alternative quality
performance standard. This
ACO will be ineligible to
share savings and will owe
maximum shared losses, if
applicable.
f. Proposed APP Plus Quality Measure Set
(1) Background
The APP quality measure set for performance year 2024 and subsequent performance
years was finalized in the CY 2024 PFS final rule (88 FR 79112 through 79114). In that final
rule, for performance year 2024 and subsequent performance years, we also finalized the
addition to the APP quality measure set of the Medicare CQM collection type for Diabetes:
Hemoglobin A1c (HbA1c) Poor Control (Quality #: 001), Preventive Care and Screening:
Screening for Depression and Follow-up Plan (Quality #: 134), and Controlling High Blood
Pressure (Quality #: 236).

(2) Proposed Revisions
As described in section III.G.4.b.(2)(a) of this proposed rule, for performance year 2025
and subsequent performance years, we are proposing to require Shared Savings Program ACOs
to report the APP Plus quality measure set as proposed in section IV.A.4.c.(3) of this proposed
rule. The APP Plus quality measure set would comprise of eleven measures, consisting of six
measures from the APP quality measure set and five newly proposed measures from the Adult
Universal Foundation measure set that would be incrementally incorporated into the APP Plus
quality measure set over performance years 2025 through 2028. We are also proposing to focus
the collection types available to Shared Savings Program ACOs for reporting the APP Plus
quality measure set to all payer/all patient eCQMs and Medicare CQMs.
The proposed APP Plus quality measure set for Shared Savings Program ACOs for
performance year 2025, performance years 2026 and 2027, and performance year 2028 and
subsequent performance years are displayed in Tables 34, 35, and 36, respectively. In these
tables, we also included the measure type for each measure in the APP Plus quality measure set
to provide ACOs with a list of the outcome measures for purposes of qualifying for the eCQM
reporting incentive, as described in section III.G.4.d. of this proposed rule. This information is
also relevant to the alternative quality performance standard under which ACOs that fail to meet
the quality performance standard to qualify for the maximum sharing rate, but that achieve a
quality performance score equivalent to or higher than the 10
th
percentile of the performance
benchmark on at least one of the four outcome measures in the APP Plus quality measure set,
may be eligible to share in savings on a sliding scale, as discussed in the current §
425.512(a)(4)(ii).

TABLE 34: Measures Included in the APP Plus Quality Measure Set for Shared
Savings Program ACOs for Performance Year 2025
Quality #
Measure Title
Collection Type
Submitter Type
Meaningful
Measures 2.0
Area
Measure Type
321
CAHPS for MIPS
CAHPS for
MIPS Survey
Third Party
Intermediary
Person-
Centered Care
Patient
Engagement/Experience
479
Hospital-Wide, 30-day, All-
Cause Unplanned
Readmission (HWR) Rate
for MIPS Eligible Clinician
Groups
Administrative
Claims
N/A
Affordability
and Efficiency
Outcome^
484
Clinician and Clinician
Group Risk-standardized
Hospital Admission Rates
for Patients with Multiple
Chronic Conditions
Administrative
Claims
N/A
Affordability
and Efficiency
Outcome^
001
Diabetes: Hemoglobin A1c
(HbA1c) Poor Control
eCQM/Medicare
CQM
APM Entity/Third
Party Intermediary
Chronic
Conditions
Intermediate Outcome^
134
Preventive Care and
Screening: Screening for
Depression and Follow-up
Plan
eCQM/Medicare
CQM
APM Entity/Third
Party Intermediary
Behavioral
Health
Process
236
Controlling High Blood
Pressure
eCQM/Medicare
CQM
APM Entity/Third
Party Intermediary
Chronic
Conditions
Intermediate Outcome^
113
Colorectal Cancer Screening
eCQM/Medicare
CQM
APM Entity/Third
Party Intermediary
Wellness and
Prevention
Process
112
Breast Cancer Screening
eCQM/Medicare
CQM
APM Entity/Third
Party Intermediary
Wellness and
Prevention
Process
^ Indicates this is an outcome measure for purposes of qualifying for the eCQM reporting incentive and the
alternative quality performance standard.

TABLE 35: Measures Included in the APP Plus Quality Measure Set for Shared Savings
Program ACOs for Performance Years 2026 and 2027
Quality #
Measure Title
Collection Type
Submitter
Type
Meaningful
Measures 2.0
Area
Measure Type
321
CAHPS for MIPS
CAHPS for MIPS
Survey
Third Party
Intermediary
Person-
Centered Care
Patient
Engagement/E
xperience
479
Hospital-Wide, 30-
day, All-Cause
Unplanned
Readmission
(HWR) Rate for
MIPS Eligible
Clinician Groups
Administrative
Claims
N/A
Affordability
and Efficiency
Outcome
^
484
Clinician and
Clinician Group
Risk-standardized
Hospital
Admission Rates
for Patients with
Multiple Chronic
Conditions
Administrative
Claims
N/A
Affordability
and Efficiency
Outcome
^
001
Diabetes:
Hemoglobin A1c
(HbA1c) Poor
Control
eCQM/Medicare
CQM/
APM
Entity/Third
Party
Intermediary
Chronic
Conditions
Intermediate
Outcome^
134
Preventive Care
and Screening:
Screening for
Depression and
Follow-up Plan
eCQM/Medicare
CQM
APM
Entity/Third
Party
Intermediary
Behavioral
Health
Process
236
Controlling High
Blood Pressure
eCQM/Medicare
CQM
APM
Entity/Third
Party
Intermediary
Chronic
Conditions
Intermediate
Outcome^
113
Colorectal Cancer
Screening
eCQM/Medicare
CQM
APM
Entity/Third
Party
Intermediary
Wellness and
Prevention
Process
112
Breast Cancer
Screening
eCQM/Medicare
CQM
APM
Entity/Third
Party
Intermediary
Wellness and
Prevention
Process
305
Initiation and
Engagement of
Substance Use
Disorder Treatment
eCQM/Medicare
CQM
APM
Entity/Third
Party
Intermediary
Behavioral
health
Process
^ Indicates this is an outcome measure for purposes of qualifying for the eCQM reporting incentive and the
alternative quality performance standard.

TABLE 36: Measures Included in the APP Plus Quality Measure Set for Shared Savings
Program ACOs for Performance Year 2028 and Subsequent Performance Years
Quality #
Measure Title
Collection Type
Submitter
Type
Meaningful
Measures
2.0 Area
Measure Type
321
CAHPS for MIPS
CAHPS for
MIPS Survey
Third Party
Intermediary
Person-
Centered
Care
Patient
Engagement/Experience
479
Hospital-Wide, 30-
day, All-Cause
Unplanned
Readmission
(HWR) Rate for
MIPS Eligible
Clinician Groups
Administrative
Claims
N/A
Affordability
and
Efficiency
Outcome
^
484
Clinician and
Clinician Group
Risk-standardized
Hospital
Admission Rates
for Patients with
Multiple Chronic
Conditions
Administrative
Claims
N/A
Affordability
and
Efficiency
Outcome
^
001
Diabetes:
Hemoglobin A1c
(HbA1c) Poor
Control
eCQM/Medicare
CQM
APM
Entity/Third
Party
Intermediary
Chronic
Conditions
Intermediate Outcome^
134
Preventive Care
and Screening:
Screening for
Depression and
Follow-up Plan
eCQM/Medicare
CQM
APM
Entity/Third
Party
Intermediary
Behavioral
Health
Process
236
Controlling High
Blood Pressure
eCQM/Medicare
CQM
APM
Entity/Third
Party
Intermediary
Chronic
Conditions
Intermediate Outcome^
113
Colorectal Cancer
Screening
eCQM/Medicare
CQM
APM
Entity/Third
Party
Intermediary
Wellness and
Prevention
Process
112
Breast Cancer
Screening
eCQM/Medicare
CQM
APM
Entity/Third
Party
Intermediary
Wellness and
Prevention
Process
305
Initiation and
Engagement of
Substance Use
Disorder
Treatment
eCQM/Medicare
CQM
APM
Entity/Third
Party
Intermediary
Behavioral
health
Process
487
Screening for
Social Drivers of
Health
eCQM/Medicare
CQM
APM
Entity/Third
Party
Intermediary
Equity
Process
493
Adult
Immunization
Status
eCQM/Medicare
CQM
APM
Entity/Third
Party
Intermediary
Wellness and
Prevention
Process
^ Indicates this is an outcome measure for purposes of qualifying for the eCQM reporting incentive and the
alternative quality performance standard.

g. Survey Modes for the Administration of the Consumer Assessment of Healthcare Providers
and Systems (CAHPS) for MIPS Survey Request for Information
We seek public comment on the potential expansion of the survey modes of the CAHPS
for MIPS Survey from a mail-phone protocol to a web-mail-phone protocol. During the 2023
CAHPS for MIPS Web Mode Field Test,
481
adding the web-based survey mode to the current
mail-phone protocol of CAHPS for MIPS survey administration resulted in an increased
response rate. Additional information on the CAHPS for MIPS Survey Request for Information
is available in section IV.A.4.e.(1)(e) of this proposed rule.
5. Providing the Option of Prepaid Shared Savings
a. Background
In the CY 2023 PFS final rule (87 FR 69782 through 69805), CMS finalized a new
payment option for eligible Shared Savings Program ACOs entering agreement periods
beginning on or after January 1, 2024, to receive advance shared savings payments. This
payment option is referred to as advance investment payment (AIP) and the payments
themselves are referred to as advance investment payments.
These payments are intended to improve the quality and efficiency of items and services
furnished to Medicare beneficiaries by reducing the barriers to participation in the Shared
Savings Program by supporting investments in increased staffing, healthcare infrastructure, and
the provision of accountable care for underserved beneficiaries. Accordingly, advance
investment payments must be spent on one of the following categories: increased staffing,
healthcare infrastructure, and the provision of accountable care for underserved beneficiaries,
which may include addressing social determinants of health (42 CFR 425.630(e)(1)).
Advance investment payments are only available to ACOs newly entering the Shared
Savings Program in their first agreement period (§ 425.630(b)(1)). Many commentors on the CY
481
https://qpp-cm-prod-
content.s3.amazonaws.com/uploads/2893/2023_CAHPS_for_MIPS_WebMode_Field_Test.pdf.

2023 PFS final rule (87 FR 69782 through 69805) suggested that CMS should expand access to
advance investment payments by expanding the eligibility criteria to include currently
participating ACOs as well as high revenue ACOs. While we do not believe that it is appropriate
to expand the eligibility criteria for advance investment payments at this time, as CMS still needs
time to assess the impact of the new payment option, there is persuasive evidence that investment
in staffing, healthcare infrastructure, and accountable care for underserved beneficiaries could be
valuable for all ACOs, not just those that are new to the program. Investment in care
coordination for beneficiaries reduces costs and improve the quality of care received.
482,483,484
Investment in health information technology can be leveraged to empower individuals, address
patients’ full range of health needs, promote healthy behaviors, and facilitate better health
outcomes for individuals, families, and communities.
485
Additionally, there is evidence that
investment in services not currently covered by Medicare may improve beneficiary health and
482
Breckenridge ED, Kite B, Wells R, Sunbury TM. Population Health Management. Effect of Patient Care
Coordination on Hospital Encounters and Related Costs. September 26, 2019. Available at
https://doi.org/10.1089/pop.2018.0176.
483
Elliott MN, Adams JL, Klein DJ, et al. Journal of General Internal Medicine. Patient-Reported Care Coordination
is Associated with Better Performance on Clinical Care Measures. September 20, 2021. Available at
https://link.springer.com/article/10.1007/s11606-021-07122-8.
484
Figueroa JF, Feyman Y, Zhou X, et al. Hospital-level care coordination strategies associated with better patient
experience. BMJ Quality & Safety. April 4, 2018. Available at https://qualitysafety.bmj.com/content/27/10/844.
485
The Office of the National Coordinator for Health Information Technology. 2020-2025 Federal Health IT
Strategic Plan. Available at https://www.healthit.gov/sites/default/files/page/2020-
10/Federal%20Health%20IT%20Strategic%20Plan_2020_2025.pdf.

reduce avoidable health care utilization costs over time, including coverage of dental
486,487,488
hearing
489,490
and vision
491
care.
Furthermore, we have come to understand that, for beneficiaries, the benefits of
receiving services from providers associated with ACOs – such as improvements in
quality and coordinated care – may not be immediately apparent. By encouraging ACOs
to invest in new services that beneficiaries otherwise would not receive, like hearing,
vision and dental services, the benefits of receiving care from providers who are part of
an ACO would become more tangible. This would encourage beneficiaries to receive
care from providers participating in an ACO and may ultimately result in improved
quality and efficiency of care for beneficiaries.
For ACOs that are currently participating in the Shared Savings Program and that
reinvest their earned shared savings payments in activities that reduce costs and improve quality
of care, it could be more valuable to gain access to those shared savings payments early in and/or
throughout each performance year, instead of waiting months after the end of each performance
year when any earned shared savings payments are distributed. Currently, CMS completes the
financial reconciliation calculations for each ACO during the summer after the end of each
performance year, which allows time for claims runout and other necessary data to become
available. CMS compares the updated historical benchmark to an ACO’s assigned beneficiaries’
486
Schenkein HA, Loos BG. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases.
Journal of Clinical Periodontology. April 30, 2013. Available at https://doi.org/10.1111/jcpe.12060.
487
Teeuw WJ, Gerdes VE, Loos BG. Effect of periodontal treatment on glycemic control of diabetic patients: a
systematic review and meta-analysis. Diabetes Care. February 2010. Available at
https://pubmed.ncbi.nlm.nih.gov/20103557/.
488
Allareddy V, Rampa S, Lee MK, Allareddy V, Nalliah RP. Hospital-based emergency department visits
involving dental conditions: Profile and predictors of poor outcomes and resource utilization. The Journal of the
American Dental Association. November 19, 2014. Available at https://doi.org/10.14219/jada.2014.7.
489
Choi JS, Adams ME, Crimmins EM, Lin FR, Ailshire JA. Association between hearing aid use and mortality in
adults with hearing loss in the USA: a mortality follow-up study of a cross-sectional cohort. The Lancet Healthy
Longevity. January 3, 2024. Available at https://doi.org/10.1016/S2666-7568(23)00232-5.
490
Reed NS, Altan A, Deal JA, et al. Trends in Health Care Costs and Utilization Associated with Untreated
Hearing Loss Over 10 Years. JAMA Otolaryngology - Head and Neck Surgery. November 8, 2018. Available at
https://jamanetwork.com/journals/jamaotolaryngology/fullarticle/2714049.
491
Lipton BJ, Decker SL. The effect of health insurance coverage on medical care utilization and health outcomes:
Evidence from Medicaid adult vision benefits. Journal of Health Economics. November 11, 2015. Available at
https://doi.org/10.1016/j.jhealeco.2015.10.006.

per capita expenditures during the performance year to determine whether the ACO may share in
savings or losses, if owed. CMS then notifies the ACO in writing regarding whether the ACO
qualifies for a shared savings payment, and if so, the amount of the payment due. These
payments are generally distributed to ACOs in the early fall following the end of each
performance year. This is the sole payment CMS makes to an ACO in the Shared Savings
Program and generally an ACO’s sole source of revenue. Distributing prepaid shared savings
during a performance year would allow ACOs to invest these payments in additional services for
assigned beneficiaries, staffing, and healthcare infrastructure earlier and reap the benefits from
that investment earlier.
The CMS Innovation Center tested a number of strategies for providing more
experienced ACOs with advances of funding during each performance year. One of the
innovations was the infrastructure payments available in the Next Generation ACO model, a
CMS Innovation Center model that was intended for more experienced ACOs.
492
Most Next
Generation ACOs (82 percent) that participated in the Next Generation ACO model in 2018 had
prior experience as Medicare ACOs before starting in the model, and the majority (56 percent)
previously participated in the Shared Savings Program.
493
ACOs selecting the infrastructure
payment option received $6 per assigned beneficiary per month to support ACO Activities,
which was later recouped during financial settlement following each performance year. The
model defined ACO Activities as activities related to promoting accountability for the quality,
cost, and overall care for the population of beneficiaries assigned to the Next Generation ACO,
including managing, and coordinating care; encouraging investment in healthcare infrastructure
and redesigned care processes for high quality and efficient service delivery; or carrying out any
other obligation or duty of the ACO under the terms of the Next Generation ACO model.
492
Refer to “Next Generation ACO Model” available at https://www.cms.gov/priorities/innovation/innovation-
models/next-generation-aco-model.
493
NORC at the University of Chicago. Next Generation Accountable Care Organization Model Third Evaluation
Report. September 2020. Available at https://www.cms.gov/priorities/innovation/data-and-reports/2020/nextgenaco-
thirdevalrpt-fullreport.

Examples of these activities included, but were not limited to, providing direct patient care in a
manner that reduces costs and improves quality; promoting evidence-based medicine and patient
engagement; reporting on quality and cost measures; coordinating care, such as through the use
of telehealth, remote patient monitoring, and other enabling technologies; establishing and
improving clinical and administrative systems for the ACO; meeting the quality performance
standards; evaluating health needs; communicating clinical knowledge and evidence-based
medicine; and developing standards for beneficiary access and communication, including
beneficiary access to medical records. In interviews performed as part of the CMS Innovation
Center’s evaluation of the model, Next Generation ACO leaders described using these funds to
support upfront operating costs and healthcare infrastructure and clinical process enhancements
such as new staff, health information technology, data analytic capacity, population health
management, or care coordination.
494
Despite these ACOs’ prior experience as Medicare ACOs and the meaningful
investments many had made in their own healthcare infrastructure and providers, they still found
value in access to funding during the performance year. Almost all Next Generation ACOs used
the funds to develop workflows informed by data analytics and clinical staff input. Most Next
Generation ACOs also reported using the funds to support care management, such as acquiring
tools and developing healthcare infrastructure to support care coordination. Leaders from many
Next Generation ACOs described how the payments facilitated new processes for seamless
patient care handoffs between health care providers, enabled the creation of better workflows for
scheduling follow-up visits, and supported provision of screenings and assessments. Data from a
clinician survey suggested that the payments were likely helpful in improving the delivery or
coordination of care, with 63 percent of providers agreeing that additional resources to support
494
NORC at the University of Chicago. Evaluation of the Next Generation Accountable Care Organization
(NGACO) Model – Final Report. January 2024. Available at https://www.cms.gov/priorities/innovation/data-and-
reports/2024/nextgenaco-sixthevalrpt.

practice changes made their day-to-day work easier.
495
Separately, the ACO Investment Model
(AIM), a model run by the CMS Innovation Center which informed development of the advance
investment payments, gave participating ACOs upfront and quarterly funding to spend on ACO
start-up costs. These ACOs primarily invested in staffing and healthcare infrastructure including
care management, ACO administration, health IT and data analysis,
496
and these ACOs
generated an estimated net aggregate reduction in spending by Medicare of $381.5 million after
accounting for Medicare’s payment of AIM funds and participating ACOs’ earned shared
savings.
497
Section 1899(i)(3) of the Act authorizes the Secretary to use other payment models
instead of the one-sided model described in section 1899(d) of the Act so long as the Secretary
determines that the other payment model will improve the quality and efficiency of items and
services furnished to Medicare beneficiaries without additional program expenditures. We are
interested in building on experience from the Next Generation ACO model, and we agree, in
part, with comments on the CY 2023 PFS final rule that encouraged CMS to expand AIP to
additional ACOs. While we do not believe it is appropriate to expand the eligibility criteria for
AIPs at this time as explained earlier in this section, we agree with commenters that additional
ACOs could benefit from expanded access to performance year funding that encourages
investment in staffing, healthcare infrastructure, and additional services for beneficiaries. Prepaid
shared savings would be required to be spent at least partially on direct beneficiary services,
improving the quality of care beneficiaries receive.
Consequently, under the authority provided to the Secretary by section 1899(i)(3) of the
Act, we are proposing to provide prepaid shared savings to certain ACOs that meet the eligibility
495
NORC at the University of Chicago. Next Generation Accountable Care Organization (NGACO) Model
Evaluation Third Evaluation Report. 2020. Available at https://www.cms.gov/priorities/innovation/data-and-
reports/2020/nextgenaco-thirdevalrpt-fullreport.
496
Abt Associates, Evaluation of the Accountable Care Organization Investment Model, AIM Implementation and
Impacts over Two Performance Years (September 2019), page 55. Available at
https://www.cms.gov/priorities/innovation/aim-second-annrpt.pdf.
497
Abt Associates, Evaluation of the Accountable Care Organization Investment Model, Final Report (September
2020), page 39. Available at https://innovation.cms.gov/data-and-reports/2020/aim-final-annrpt.
criteria described in section III.G.5.b of this proposed rule. Such payments would be made
pursuant to the standards we propose to establish in new § 425.640. This new payment option
would provide prepaid shared savings to ACOs with a history of earning shared savings while
participating in the Shared Savings Program. These payments would be distributed on a quarterly
basis and would be recouped from shared savings CMS determines the ACO to have earned
during the annual financial reconciliation cycle. Prepaid shared savings would be the advance
payment of shared savings that are expected to be earned by the ACO and are covered under the
Shared Savings Distribution Waiver (76 FR 66726). If the ACO does not earn sufficient shared
savings to offset the advanced payment of shared savings during the applicable performance
year, CMS may withhold or terminate the ACO’s prepaid shared savings under proposed §
425.640(h)(1)(iii).
We have determined that the other payment model CMS has adopted under section
1899(i)(3) of the Act would continue to improve the quality and efficiency of care should this
proposal be finalized. Section 1899(i)(3)(A) of the Act requires CMS determine that the other
payment model will improve the quality and efficiency of items furnished under the Medicare
program. Based on the evidence for direct beneficiary services noted above, our experience
administering the Shared Savings Program, and the CMS Innovation Center’s experience with
the ACO Investment Model and infrastructure payments in the Next Generation ACO model, we
have determined that allowing ACOs access to funding earlier than currently available, in the
form of prepaid shared savings, would allow ACOs to more rapidly achieve the benefits of
investing in staffing, healthcare infrastructure, and direct beneficiary services. Improvement in
these areas would improve the quality and efficiency of beneficiary care therefore meeting the
standard of section 1899(i)(3)(A) of the Act. As we explained earlier in this section, ACOs have
expenditures throughout the PY, particularly when implementing care coordination and
beneficiary management strategies, and having access to their shared savings early can help
ensure the ACO has adequate funding to perform these services throughout the year.
Section 1899(i)(3)(B) of the Act requires CMS to determine that prepaid shared savings,
when implemented in combination with existing modifications made to the Shared Savings
Program payment model specified in section 1899(d) of the Act, will not result in additional
program expenditures. The addition of prepaid shared savings meets this standard in part because
the eligibility criteria for prepaid shared savings have been selected to only permit ACOs that
CMS estimates are most likely to earn shared savings to receive payments. Additionally, any
payments the ACO receives under this proposal must be repaid to CMS, and CMS would be
protected by the ACOs’ repayment mechanisms in the event that an ACO does not earn shared
savings or cannot otherwise repay the amount owed to CMS. Based on this design, we estimate
that there would be no additional program expenditures stemming from the implementation of
prepaid shared savings under this proposal. Please review section VII of this proposed rule for a
more complete discussion of the financial impact of the Shared Savings Program payment
model, including the findings necessary to demonstrate compliance with section 1899(i)(3)(B) of
the Act.
We intend to periodically reassess whether a payment model established under section
1899(i)(3) of the Act, including the payment of prepaid shared savings, continues to improve the
quality and efficiency of items and services furnished to Medicare beneficiaries without resulting
in additional program expenditures. If we determine that the payment model no longer satisfies
the requirements of section 1899(i)(3) of the Act (for example if the payment model results in net
program costs), we would undertake additional notice and comment rulemaking to adjust our
payment methodology to assure continued compliance with the statutory requirements.
b. Eligibility
To ensure that prepaid shared savings are provided only to ACOs that are well-positioned
to use prepaid shared savings to improve the quality and efficiency of care to their assigned
beneficiaries while minimizing the risk of an ACO being unable to repay prepaid shared savings,
we propose to limit the availability of prepaid shared savings to those ACOs that have a track
record of success in the Shared Savings Program. This approach is also consistent with our
compliance with section 1899(i)(3)(B) of the Act as such ACOs are most likely to be able to
repay the upfront funding through earned shared savings.
We propose to establish the eligibility criteria for prepaid shared savings in § 425.640(b).
CMS must determine that an ACO meets all of the following criteria for the ACO to be eligible
to receive prepaid shared savings during an agreement period:
● The ACO is a renewing ACO as defined under § 425.20 entering an agreement period
beginning on January 1, 2026, or in subsequent years.
● The ACO must have received a shared savings payment for the most recent
performance year that:
(A) Occurred prior to the agreement period for which the ACO has applied to receive
prepaid shared savings; and
(B) CMS has conducted financial reconciliation.
● The ACO must have a positive prior savings adjustment as calculated per § 425.658 at
application disposition for the agreement period in which they would receive prepaid shared
savings.
● The ACO does not have any outstanding shared losses or advance investment
payments that have not yet been repaid to CMS after reconciliation for the most recent
performance year for which CMS completed financial reconciliation.
● If the ACO received prepaid shared savings in the current agreement period or a prior
agreement period, the ACO must have fully repaid the amount of prepaid shared savings
received through the most recent performance year for which CMS has completed financial
reconciliation.
● The ACO is participating in Levels C-E of the BASIC track or the ENHANCED track
during the agreement period in which they would receive prepaid shared savings.
● The ACO has in place an adequate repayment mechanism in accordance with §
425.204(f) that can be used to recoup outstanding prepaid shared savings.
● During the agreement period immediately preceding the agreement period in which the
ACO would receive prepaid shared savings, the ACO:
(A) Met the quality performance standard as specified under § 425.512; and
(B) Has not been determined by CMS to have avoided at-risk beneficiaries as specified
under § 425.316(b)(2).
These eligibility criteria have been selected to allow only ACOs with a record of meeting
the quality performance standard, not avoiding at risk beneficiaries, and recent success in earning
shared savings to receive prepaid shared savings. This is for the protection of both CMS and the
ACOs, as CMS does not want to overestimate an ACO’s ability to earn future shared savings and
burden an ACO with debt that they would not be able to repay. Our experience administering the
Shared Savings Program leads us to determine that ACOs with prior success in the program –
that is, ACOs with a record of meeting the quality performance standard, not avoiding at risk
beneficiaries, and recent success in earning shared savings – are well positioned to identify
beneficiary needs and invest prepaid shared savings to improve beneficiary care and are
therefore most likely to benefit from prepaid shared savings. These ACOs would also be
reasonably confident they will be able to repay CMS through their earned shared savings and
would therefore be comfortable spending the funding they receive. Accordingly, CMS would
only permit ACOs that are currently participating in the Shared Savings Program, that have
earned shared savings in the most recent performance year for which financial reconciliation has
been completed, and that have a positive prior savings adjustment at application disposition to
receive prepaid shared savings, as they have the history of success that provides CMS with a
more reasoned expectation that they will earn shared savings in the future. New ACOs would not
be eligible for prepaid shared savings, as they do not have a recent performance history we can
use to estimate future performance. Many new ACOs are eligible to receive advance investment
payments, which are not available to ACOs currently participating in the Shared Savings
Program. Advance investment payments are more tailored to the needs of a new ACO as there is
more flexibility in the use of funding and advance investment payments do not need to be repaid
in the event that the ACO does not earn shared savings.
Additionally, ACOs that did not meet the quality performance standard as specified under
§ 425.512 or were subject to a pre-termination action from CMS after determining an ACO
avoided at-risk beneficiaries as specified under § 425.316(b)(2) in the agreement period
preceding the agreement period in which they would receive prepaid shared savings would be
prohibited from participating in the prepaid shared savings payment option, as these compliance
issues can prevent an ACO from earning shared savings that would be used to repay the prepaid
shared savings.
CMS also proposes to limit participation in the prepaid shared savings payment option to
ACOs that have fully repaid all shared losses they may owe and any advance investment
payments they may have received in a prior agreement period, and to ACOs that participate in a
two-sided risk track (Levels C-E of the BASIC track or the ENHANCED track), as these tracks
require a repayment mechanism in accordance with § 425.204(f), which could be used to recoup
prepaid shared savings. CMS also proposes these criteria in part to limit participation to ACOs
that are most likely to be able to repay any prepaid shared savings they receive. Similarly, if the
ACO received prepaid shared savings in a current or previous agreement period, they must have
fully repaid the amount of prepaid shared savings received though the most recent performance
year for which CMS has completed financial reconciliation before they would be able to renew
their participation in prepaid shared savings for another agreement period. For example, if an
ACO is in the fifth year of its 5-year agreement period during which they have been receiving
prepaid shared savings and is in the process of renewing for a new agreement period, CMS
would ensure they have fully repaid the prepaid shared savings received from the first four
performance years of their current agreement period through earned shared savings before they
are approved to receive prepaid shared savings in a new agreement period. As CMS intends to
provide prepaid shared savings to ACOs if they improve and maintain performance and continue
to see success in the program on an annual basis, ACOs that are not initially eligible would have
the option to participate in the prepaid shared savings payment option in future years if they
demonstrate a more recent history of success in the program and meet the other eligibility
criteria. These criteria also provide an additional incentive for ACOs to improve their
performance in the program. CMS would also continue to review the eligibility criteria over time
and may expand eligibility in future years if we determine that doing so is in the interests of the
Shared Savings Program, participating ACOs, and their beneficiaries, and all requirements under
section 1899(i)(3) of the Act are satisfied. Additionally, to standardize timelines for payment,
spending, and recoupment of prepaid shared savings, ACOs would only be eligible for prepaid
shared savings if they renew or early renew to begin a new agreement period. The proposed
policies for the calculation, spending and recoupment of prepaid shared savings allow for up to
five years for ACOs to receive, spend and repay the funding through earned shared savings. We
propose to create a new paragraph in § 425.100(e) to establish that an ACO may receive prepaid
shared savings if it meets the criteria under § 425.640(b). We propose in § 425.640(b) to specify
the eligibility criteria for an ACO to receive prepaid shared savings. We seek comments on these
proposals.
c. Application Procedure & Contents
We propose to establish the process for an ACO to apply for prepaid shared savings in §
425.640(c). Specifically, we propose that an ACO must submit to CMS supplemental application
information sufficient for CMS to determine whether the ACO is eligible to receive prepaid
shared savings. The application cycle for prepaid shared savings would be conducted as part of,
and in conjunction with, the Shared Savings Program application process under § 425.202, with
instructions and timelines published on the Shared Savings Program website. We propose the
initial application cycle to apply for prepaid shared savings would be for a January 1, 2026, start
date. We intend to provide further information regarding the process, including the application
contents and specific requirements such as the deadline for submitting applications and all
supplemental application information that would be required, through subregulatory guidance.
The prepaid shared savings application procedure would also include a process by which CMS
provides an applicant with feedback and an opportunity to clarify or revise their application.
CMS would provide preliminary information to the applicant ACO about its eligibility to
receive prepaid shared savings during the Phase 1 application cycle requests for information, and
a final determination about its eligibility to receive prepaid shared savings at the time of final
application dispositions. For example, for ACOs applying in 2025 for an agreement period
beginning in 2026, we would provide preliminary information identifying whether an ACO is
likely to earn shared savings in the 2024 performance year and have a positive prior savings
adjustment as calculated per § 425.658 at application disposition.
We propose at § 425.640(d)(1) that an ACO would be required to submit a spend plan as
part of its application for prepaid shared savings. We propose that the plan must describe how
the ACO would spend the prepaid shared savings during the first performance year of the
agreement period during which the ACO would receive prepaid shared savings, including the
breakdown of how the funding would be spent consistent with the allowable uses as described in
section III.G.5.d of this proposed rule and information about: (1) direct beneficiary services that
would be provided to ACO beneficiaries; and (2) investments that would be made in the ACO
with prepaid shared savings. ACOs must also include their communication strategy for
informing both CMS and any impacted beneficiaries if the ACO will no longer be providing any
direct beneficiary services (as described in section III.G.5.d of this proposed rule) that had
previously been provided by the ACO using prepaid shared savings. This communication
strategy must include when and how the ACO intends to notify CMS and the impacted
beneficiaries as well as any available alternatives for impacted beneficiaries to access similar
services. ACOs would be able to limit the distribution of direct beneficiary services to subgroups
of assigned beneficiaries including those with specific medical conditions or specific
socioeconomic needs. ACOs would be required to attest that they will not discriminate on the
basis of race, color, religion, sex, national origin, disability, or age with respect to their use of
prepaid shared savings. ACOs would have flexibility to alter their use of prepaid shared savings
from their submitted spend plans during each performance year but would be required to ensure
than any changes to proposed spending aligns with the restrictions on spending discussed in
section III.G.5.d of this proposed rule. CMS would review mid-year changes of the use of
prepaid shared savings at the end of each performance year. CMS would also be able to review
an ACO's spend plan at any time and require the ACO to modify its spend plan to comply with
the requirements of § 425.640(d) and (i).
As discussed in greater detail in section III.G.5.h of this proposed rule, we would reserve
the right to withhold or terminate an ACO’s ability to receive the prepaid shared savings if it is
not in compliance with the requirements of the Shared Savings Program codified in part 425 of
our regulations, pursuant to proposed § 425.640(h)(1)(i). In addition, by certifying their
application under § 425.202(a)(2), the ACO certifies that the information contained in the
application, including information related to the intended use of prepaid shared savings, is
accurate, complete, and truthful.
We propose at § 425.640(d) that we would review the information submitted in the
ACO’s prepaid shared savings application to determine whether an ACO meets the criteria for
prepaid shared savings and would approve or deny the application accordingly. We would
review the ACO’s Shared Savings Program renewal application simultaneously with the prepaid
shared savings application.
As discussed in section III.G.5.g of this proposed rule, we are also proposing to update
our public reporting requirements under § 425.308 by adding new paragraph (b)(10) to require
an ACO to publicly report its spend plan. We propose to require that the ACO post on its
dedicated public reporting web page: (1) the total amount of prepaid shared savings received
from CMS for each performance year; (2) the ACO’s spend plan; and (3) an itemization of how
the prepaid shared savings were actually spent during each performance year, including
expenditure categories, the dollar amounts spent on the various categories, information about
which groups of beneficiaries received direct beneficiary services that were purchased with
prepaid shared savings and investments that were made in the ACO with prepaid shared savings,
how these direct beneficiary services were provided to beneficiaries and how the direct
beneficiary services and investments supported the care of beneficiaries, any changes to the
spend plan as submitted under § 425.640(d)(2) (if applicable), and such other information as may
be specified by CMS. Additionally, we are proposing that the ACO would be required to report
the same information to CMS under § 425.640(i) to facilitate efficient monitoring. This would
help ensure that CMS efficiently obtains information in a consistent manner from all ACOs
receiving prepaid shared savings and thereby support CMS’s monitoring and analysis of the use
of prepaid shared savings. CMS will also make this data publicly available through a public use
file. Further, we expect to use the submitted data as the template that ACOs can use to populate
their public reporting webpage early in each performance year to minimize administrative burden
for ACOs. We also intend to use the information submitted to CMS to generate a public use file
that can be used to quickly review the use of prepaid shared savings across all participating
ACOs.
We propose to add § 425.640(c) and (d) to establish standards for the contents of an
application to be determined eligible for prepaid shared savings as well as the procedures for
filing such an application. We seek comments on these proposals.
d. Allowable and Prohibited Uses of Prepaid Shared Savings
We propose in § 425.640(e) to specify how an ACO may use prepaid shared savings.
Similar to advance investment payments, prepaid shared savings are intended to improve quality
and efficiency of items and services furnished to Medicare beneficiaries. We recognize that there
are many ways to do this, and that the most effective ways would vary by ACO. Our proposal
intends to provide ACOs with flexibility to use payments consistent with broad allowable uses.
However, as prepaid shared savings are only available to ACOs that are currently successfully
participating in the Shared Savings Program, we intend to place restrictions on the amount of
total annual prepaid shared savings that can be spent on each category of spending. Financially
successful ACOs are likely to have already made significant investments in staffing and
healthcare infrastructure, as they are necessary for the functioning of an ACO, and we intend to
encourage ACOs receiving prepaid shared savings to invest in direct beneficiary services that are
not already offered by the ACO. Direct beneficiary services like vision, hearing and dental, and
other services that have a reasonable expectation of improving or maintaining the health or
overall function of ACO beneficiaries, have the potential to further improve beneficiary
outcomes, reduce costs, and improve beneficiary engagement and willingness to receive care
from a provider affiliated with an ACO. However, staffing and healthcare infrastructure are still
important expenses that can have positive impacts on healthcare costs, ACO efficiency, the
quality of beneficiary care, regardless of an ACO’s experience in the Shared Savings Program.
Accordingly, we also intend to allow ACOs to use some of their prepaid shared savings to invest
in these areas. For each performance year, ACOs would be permitted to use up to 50 percent of
their estimated annual prepaid shared savings on staffing and healthcare infrastructure and up to
100 percent of their estimated annual prepaid shared savings on direct beneficiary services.
ACOs must use a minimum of 50 percent of their prepaid shared savings on direct beneficiary
services.
We note that an ACO may use prepaid shared savings for staffing, healthcare
infrastructure and direct beneficiary services in a manner that complies with the beneficiary
incentives provision at § 425.304(a), (b), and newly proposed (d) as discussed in section III.G.5.i
of this proposed rule, and all other applicable laws and regulations. Permitted uses for “staffing
and healthcare infrastructure” include but are not limited to the following:
● Staffing. Examples could include, but are not limited to, hiring physicians, physicians’
assistants, nurse practitioners, clinical nurse specialists, nutrition professionals, case managers,
licensed clinical social workers, community health workers, patient navigators, health equity
officers, psychiatrists, clinical psychologists, therapists, mental health counselors, licensed
professional counselors, substance use counselors, peer support specialists, and other behavioral
health clinicians, or staff education.
● Healthcare Infrastructure: Examples could include, but are not limited to, investments
in or improvements to existing case or practice management systems, clinical data registries,
electronic quality reporting, health information exchange participation, certified electronic health
record technology (CEHRT), health IT to support behavioral health or dental services, IT-
enabled screening tools, closed-loop referral tools, audiovisual interpreter technology, or practice
physical accessibility improvements. Investments can be made for individual ACO
providers/suppliers (as defined in § 425.20) or ACO wide.
● Direct beneficiary services include in-kind items or services provided to an ACO
beneficiary that are not otherwise covered by Traditional Medicare but have a reasonable
expectation of improving or maintaining the health or overall function of ACO beneficiaries.
Direct beneficiary services can also include cost sharing support including the reduction of
beneficiary copay or deductibles for Traditional Medicare beneficiaries. In advance of the
application deadline for agreement periods beginning on January 1, 2026, we intend to release
sub regulatory guidance with more specific information about permitted uses of funding for
direct beneficiary services. Permitted uses for direct beneficiary services could include, but are
not limited to the following: beneficiary meals, nutrition support, tenancy support and sustaining
services, housing assistance, utility support, caregiver support services, services to address social
isolation, home visits, transportation services, home or environmental modifications like air
conditioners, bathroom safety devices, personal emergency response systems or medical alert
systems, and vision, hearing or dental care directly provided by ACO providers/suppliers (as
defined in § 425.20) or covered under a health insurance plan purchased by the ACO on behalf
of the beneficiary. While some of these services are covered in some form by Traditional
Medicare, prepaid shared savings funding reserved for direct beneficiary services would only be
permitted to be used for those services if the version of the service offered by the ACO is not
currently covered by Traditional Medicare and there is a reasonable expectation that those
services will improve or maintain the health or overall function of ACO beneficiaries. For
example, some types of home visits are covered by Traditional Medicare, but an ACO would be
able to extend the number of home visits offered to beneficiaries beyond the number covered by
Traditional Medicare with prepaid shared savings. Direct beneficiary services would also include
cost-sharing support, including the reduction of beneficiary copay or deductibles for Traditional
Medicare beneficiaries for Part B primary care services. ACOs would be able to provide cost-
sharing support for primary care services (as defined in § 425.20) with respect to which
coinsurance applies under Part B.
As discussed in section III.G.5.i of this proposed rule, if this rulemaking is finalized,
CMS expects to make a determination that the anti-kickback statute safe harbor for CMS-
sponsored model patient incentives (§ 1001.952(ii)(2)) is available to protect direct beneficiary
services that are made in compliance with this policy and the conditions for use of the
antikickback statute safe harbor set out at § 1001.952(ii)(2). As noted earlier in this preamble,
ACOs that wish to provide direct beneficiary services beneficiaries through prepaid shared
savings will need to submit a spend plan with information including the groups of beneficiaries
they intend to provide with direct beneficiary services, how the direct beneficiary services will
be provided to beneficiaries and how such services support the care of beneficiaries, and attest
that they will not discriminate on the basis of race, color, religion, sex, national origin, disability,
or age with respect to how they propose to spend prepaid shared savings. As proposed, ACOs
will also be required to report their actual use of prepaid shared savings after the end of each
performance year, including which groups of beneficiaries received direct beneficiary services,
how such services were provided to beneficiaries, and how these services supported the care of
beneficiaries.
Many direct beneficiary services may be provided by staff working for an ACO or its
participating providers or suppliers. If a staff member is hired or directed to provide these
services, ACOs may use dollars designated for direct beneficiary services to cover the percentage
of their salary that aligns with the percentage of time the staff member spends providing direct
beneficiary services that are not otherwise covered by Traditional Medicare. This funding may
also be used to contract with a community-based organization (CBO) or other external entity to
pay their staff to provide direct beneficiary services. Additionally, ACOs should take care to
ensure that a direct beneficiary service that is provided to a beneficiary does not impact other
Federal, state, or local means-tested benefits a beneficiary is already receiving and should
provide beneficiaries with any necessary documentation regarding their receipt of the direct
beneficiary service. If CMS finalizes this proposal, we would include additional information in
our sub regulatory guidance regarding the approved uses for direct beneficiary services and
potential impacts on beneficiary eligibility for other Federal means tested programs.
We propose in § 425.640(e)(2) that an ACO may not use prepaid shared savings for any
expense other than those allowed under paragraph (e)(1). Prohibited uses of prepaid shared
savings would include management company or parent company profit, performance bonuses,
provision of medical services covered by Traditional Medicare, cash or cash equivalent
payments to patients, and items or activities unrelated to the management and operations of an
ACO or care of beneficiaries. Similar to advance investment payments, prepaid shared savings
are intended to help an ACO put care processes in place to directly care for the unique needs of
the ACO’s beneficiary population, it is not intended to solely increase profits or to be spent on
items unrelated to the management and operations of the ACO or the beneficiaries it serves.
Additionally, we propose that an ACO participating in Levels C-E of the BASIC track or the
ENHANCED track may not use any prepaid shared savings to pay back any shared losses that it
would have incurred as specified in a written notice from CMS under § 425.605(e)(2) or
§ 425.610(h)(2), respectively.

To the extent that an ACO is addressing unmet social needs, including food insecurity
and transportation problems, through direct beneficiary services, we encourage ACOs to
coordinate with a community-based organization to provide these services. As explained in the
CY 2023 PFS proposed rule (87 FR 46102), where we refer to CBO, we mean public or private
not-for-profit entities that provide specific services to the community or targeted populations in
the community to address the health and social needs of those populations. They may include
community-action agencies, housing agencies, area agencies on aging, or other non-profits that
apply for grants to perform social services. They may receive grants from other agencies in the
U.S. Department of Health and Human Services, including Federal grants administered by the
Center for Disease Control and Prevention (CDC), Administration for Children and Families
(ACF), Administration for Community Living (ACL), or other Federal or State funded grants to
provide social services.
Generally, such organizations know the populations they serve and their communities and
may have the infrastructure or systems in place to help coordinate supportive services that
address social determinants of health or serve as a source from which ACOs can receive
information regarding community needs. Since CBOs have developed such an expertise, we
believe it would be impactful for ACOs in the delivery of high-quality direct beneficiary services
to contract with CBOs in the provision of these services. CMS further encourages ACOs to work
with community care hubs, which are community-focused entities supporting a network of CBOs
that provide services addressing health-related social needs and centralize administrative
functions and operational infrastructure. Working directly with a community care hub can help
connect the ACO with multiple smaller CBOs in the provision of direct beneficiary services. If
an ACO works with a CBO to provide these types of services and this is reflected in its plan to
address the needs of its population, we would consider them to be in compliance with the
requirement at § 425.112(b)(2)(iii)(A), which requires an ACO to, in its plan to address the needs

of its population, describe how it intends to partner with community stakeholders to improve the
health of its population.
We also propose in § 425.640(f)(6) to allow ACOs receiving prepaid shared savings to
request a smaller quarterly payment amount from CMS. For example, if an ACO is eligible for a
maximum quarterly prepaid shared savings amount of one million dollars, we would estimate
their annual prepaid shared savings to be four million dollars. This allows the ACO to spend up
to two million dollars on staffing and healthcare infrastructure and up to their full $4 million
payment amount on direct beneficiary services. However, the ACO may request a lower
quarterly payment of $500,000 that results in the ACO only receiving two million dollars over
the full performance year. This would also reduce the amount the ACO can spend on staffing and
healthcare infrastructure as an ACO may not spend more than 50 percent of the prepaid shared
savings they receive on staffing and healthcare infrastructure. In the event that CMS stops or
reduces an ACOs quarterly payments during the performance year below the quarterly payment
amount previously requested by the ACO, the reduction does not impact the total maximum
amount they are permitted to spend on each category of allowable uses identified at the start of
each year, as it would not be appropriate to subject ACOs to mid-year spend plan changes when
they may have entered into contracting or other arrangements with staff or suppliers which could
impact continuity of care. We would monitor how ACOs are spending these funds and, as
necessary, revisit these guidelines in future rulemaking if changes are required.
We seek comment on these proposals.
e. Calculation of Prepaid Shared Savings
As noted in section III.G.5.a of this proposed rule, we have determined that prepaid
shared savings would not result in additional program expenditures. While ACOs would be
required to repay the prepaid shared savings they receive through earned shared savings, it is also
important for CMS to avoid paying ACOs an amount of prepaid shared savings that they are
unlikely to be able to repay through earned shared savings. While prepaid shared savings would
be helpful in providing successful ACOs with additional cash flow that would encourage their
investment in activities that could potentially reduce ACOs’ costs and improve the quality of
care that ACOs provide to their beneficiaries, overpaying ACOs might result in a level of
outstanding debt for some ACOs that could disrupt their operations and potentially their
participation in the Shared Savings Program as well as generate unnecessary financial risk for
CMS. Our proposed policies on the calculation and distribution of the prepaid shared savings
payments are intended to balance the benefit for the ACOs of receiving funding earlier with the
risk of overpayment both for CMS and the ACO, while helping to ensure that prepaid shared
savings do not result in additional program expenditures.
We are proposing a new § 425.640(f) to provide an ACO that CMS determines meets the
eligibility criteria described in section III.G.5.b of this proposed rule with a prepaid shared
savings payment for each quarter of an agreement period that they are determined to be eligible
for prepaid shared savings equal to the maximum quarterly payment amount calculated pursuant
to the methodology outlined in § 425.640(f)(2) (as further explained elsewhere in this section),
the ACO elects to receive a lesser amount as described in § 425.640(f)(6) (as further explained in
section III.G.5.d. of this proposed rule) or the payment is withheld or terminated pursuant to §
425.640(h). If an ACO’s quarterly payment is withheld or terminated (as further explained in
section III.G.5.f.(2) of this proposed rule), we would not provide ACOs with additional or catch-
up payments if quarterly payments of prepaid shared savings are later resumed. We propose that
under new § 425.640(f), CMS would notify in writing each ACO of its determination of the
amount of prepaid shared savings. The notice would inform the ACO of its right to request
reconsideration review in accordance with the procedures specified in subpart I of our
regulations. If CMS does not make any prepaid shared savings payment, the notice would
specify the reason(s) why and inform the ACO of its right to request reconsideration review in
accordance with the standards specified in subpart I of our regulations. Thus, prior to each
quarterly payment, we propose to provide the ACO with the notice described above in the form
of a report that shows our calculation of the ACO's quarterly prepaid shared savings amount. We
propose to coincide the timing of these notices with the timing of existing report packages sent to
ACOs for informational purposes, in December (after initial assignment prior to a given
performance year), May (after quarter 1 assignment for a given performance year), and August
(after quarter 2 assignment for a given performance year). Accordingly, notice regarding the first
and second quarterly payments that an eligible ACO would receive in a given performance year
would be provided in December of the immediately preceding year. Subsequent notices
regarding the third and fourth quarterly payments that an eligible ACO would receive in a given
performance year would then be provided in May and August, respectively, of that performance
year.
We are also proposing a new § 425.640(f)(2) to specify the calculation of an ACO’s
maximum quarterly prepaid shared savings payment. To calculate this payment, we propose
calculating a prepaid shared savings multiplier, adjusting it by several factors explained later in
this section, and then multiplying one-fourth of the adjusted multiplier by an ACO’s assigned
beneficiary person years. We propose to calculate the prepaid shared savings multiplier as the
simple average of per capita savings or losses generated by the ACO during the two most recent
performance years that have been financially reconciled at the time of the ACO’s renewal
application disposition, which constitute benchmark year (BY) 1 and BY2 of the agreement
period in which the ACO may receive prepaid shared savings (“current agreement period,”
hereafter). That is, we would exclude BY3 from the calculation of an ACO’s average per capita
savings or losses because the performance year that constitutes BY3 of the ACO’s current
agreement period would not have been financially reconciled at the time of the ACO’s
application disposition. Accordingly, the per capita savings for each performance year would be
determined as the quotient of the ACO’s total updated benchmark expenditures minus total
performance year expenditures divided by performance year assigned beneficiary person years.
For purposes of calculating the simple average of per capita savings or losses generated by the
ACO during the two most recent performance years that have been financially reconciled, we
would use all savings generated during each of the 2 performance years in the prepaid shared
savings multiplier, not just savings that met or exceeded the ACO’s minimum savings rate
(MSR) for that prior performance year.
Under new § 425.640(f)(2)(iii), we propose to apply a proration factor to the prepaid
shared savings multiplier to account for situations where an ACO's assigned beneficiary
population is larger in BY1 and BY2 when calculated using the ACO's certified ACO participant
list and assignment methodology for a given performance year within the current agreement
period, as compared to the ACO's assigned beneficiary population when the ACO was reconciled
for the performance years that constitute BY1 and BY2 of the current agreement period.
Mathematically, to apply this proration factor we would calculate the ratio between: (1) the
ACO's average assigned beneficiary person years for the 2 performance years that constitute
BY1 and BY2 for the ACO's current agreement period (regardless of whether these performance
years occurred over one or multiple prior agreement periods, which would occur if the ACO
early renews immediately before the current agreement period) and (2) the average assigned
beneficiary person years in BY1 and BY2 for the ACO's current agreement period calculated
using the ACO's certified ACO participant list and assignment methodology for a given
performance year within the current agreement period. Increases in the size of the ACO’s
assigned beneficiary population during the current agreement period would therefore result in a
ratio less than 1, while decreases in the assigned beneficiary population would result in a ratio
greater than 1. This ratio would be capped at 1 to avoid increasing the adjusted prepaid shared
savings multiplier if the average number of beneficiaries assigned to the ACO across the 2
benchmark years of its current agreement period is lower than the average number of
beneficiaries assigned during the 2 performance years that constitute BY1 and BY2. Prorating
for growth in assignment would ensure that the prepaid shared savings amount does not exceed
the amount of cumulative savings generated by the ACO during the performance years that
constitute BY1 and BY2 for its current agreement period.
It is necessary to calculate a proration factor at the start of the ACO's current agreement
period to account for several possible circumstances in which the ACO's assigned beneficiary
population may be different in BY1 and BY2 when calculated using the ACO's certified ACO
participant list and assignment methodology for a given performance year within the current
agreement period, as compared to the ACO's assigned beneficiary population when the ACO was
reconciled for the performance years that constitute BY1 and BY2 of the current agreement
period. Specifically, changes in the size of the ACO’s assigned beneficiary population at the start
of the ACO’s current agreement period could be due to the addition and removal of ACO
participants or ACO providers/suppliers in accordance with § 425.118(b), a change to the ACO's
beneficiary assignment methodology selection under § 425.226(a)(1), or changes to the
beneficiary assignment methodology specified in 42 CFR part 425, subpart E.
Additionally, these circumstances could potentially arise after the start of the ACO's
current agreement period. In turn, changes in the size of the ACO’s assigned beneficiary
population could potentially occur throughout the course of the current agreement period.
Therefore, we propose in new § 425.640(f)(3)(ii) that for the second and each subsequent
performance year during the term of the current agreement period, we would redetermine this
proration factor.
In addition to pro-rating the prepaid shared savings multiplier, we also propose to adjust
it in two ways. First, under new § 425.640(f)(2)(iv), we would apply a sharing rate scaling factor
of 1/2 (or 50 percent). This sharing rate scaling factor would be similar to the scaling factor we
apply under § 425.658(c)(1)(i) when calculating the prior savings adjustment, applicable to
agreement periods beginning on or after January 1, 2024, as finalized in the CY 2023 final rule
(refer to 87 FR 69899 through 69915). As with the prior savings adjustment calculation, it is
important to consider a measure of the sharing rate used in determining the shared savings
payment the ACO earned in the applicable performance years under the agreement period
immediately before it would receive prepaid shared savings. Consistent with the prior savings
adjustment scaling factor, 50 percent represents an appropriate multiplier in this context because
it represents a middle ground between the maximum sharing rate of 75 percent under the
ENHANCED track and the lower sharing rates available under the BASIC track.
Second, under new § 425.640(f)(2)(v)(A), we would apply a financial risk scaling factor
equal to 2/3. The purpose of the financial risk scaling factor would be to mitigate financial risk to
the Medicare Trust Funds and to ACOs by reducing the possibility that an ACO’s prepaid shared
savings payments exceed the ACO’s actual earned shared savings. The rationale for a financial
risk scaling factor of this magnitude is that it enables us to account for a scenario in which an
ACO earned zero per capita savings in the performance year that constitutes BY3 of the current
agreement period, which is necessarily excluded from the calculation of an ACO’s average per
capita savings or losses for purposes of the prepaid shared savings multiplier because, as
mentioned previously, the performance year that constitutes BY3 of the ACO’s current
agreement period would not have been financially reconciled at the time of the ACO’s
application disposition. Thus, by multiplying an ACO’s average per capita savings or losses
across BY1 and BY2 by a financial risk scaling factor equal to 2/3, we are imposing a downward
reduction on the prepaid shared savings multiplier by assuming that it would have been possible,
in principle, for an ACO to have not earned any per capita savings in the performance year that
constitutes BY3 of the current agreement period. By doing so, we are reducing the probability of
distributing excessive prepaid shared savings. As discussed previously, it is important to avoid
distribution of excessive prepaid shared savings because doing so could result in several
undesirable outcomes, such as ACOs accruing debt to CMS that they are unable to repay, which
could disruption the ACOs’ operations and participation in the Shared Savings Program.
Consistent with calculations of the prior savings adjustment (refer to § 425.658), the
positive regional adjustment (refer to § 425.656), and the proposed health equity benchmark
adjustment (refer to section III.G.7.b of this proposed rule), we propose under new §
425.640(f)(2)(v)(B), to cap the pro-rated, adjusted prepaid shared savings multiplier at 5 percent
of national per capita FFS expenditures for Parts A and B services in order to ensure that the
amount of prepaid shared savings that an ACO receives does not exceed an amount that the ACO
is able to repay through earned shared savings. Specifically, we propose to calculate the cap as 5
percent of national per capita FFS expenditures for Parts A and B services in BY2 for assignable
beneficiaries identified for the 12-month calendar year corresponding to BY2. Consequently,
under new § 425.640(f)(2)(v), the pro-rated, adjusted, and capped prepaid shared savings
multiplier that would ultimately be used to calculate a given maximum quarterly prepaid shared
savings payment would be equal to the lesser of (A) the pro-rated, adjusted prepaid shared
savings multiplier or (B) 5 percent of national per capita FFS expenditures for Parts A and B
services in BY2 for assignable beneficiaries.
To calculate a given maximum quarterly prepaid shared savings payment, we propose
under new § 425.640(f)(4), to multiply one-fourth of the pro-rated, adjusted, and capped prepaid
shared savings multiplier (to account for four quarterly payments) by the ACO’s assigned
beneficiary person years for the latest available assignment list for a given performance year
within the current agreement period. Varying the maximum quarterly payment to reflect the
latest available assigned beneficiary person years is similar to how we calculate the AIP
quarterly payment calculation (refer to § 425.630(f), CY 2023 PFS final rule (87 FR 69797)), for
which we use the latest available assignment list to calculate the quarterly advance investment
payment amount. We propose to use the latest available beneficiary assigned person years for the
maximum quarterly prepaid shared savings payment because an ACO’s assigned beneficiary
person years change over the course of a performance year and over the course of an agreement
period. Because later assignment lists more closely reflect the final assignment list that would be
used for calculating shared savings and losses for a given performance year within the current
agreement period, later assignment lists are more likely than earlier assignment lists to facilitate
calculation of quarterly prepaid shared savings payment amounts that closely align with the
earned shared savings or losses that an ACO actually generates in the contemporaneous
performance year. Using the latest available assigned beneficiary person years mitigates a
financial risk that an ACO experiencing declining person years over the course of a performance
year could receive excessive prepaid shared savings. As mentioned previously, overpaying
prepaid shared savings could result in ACOs accruing a level of debt to CMS that they are unable
to repay through earned shared savings which could, in turn, disrupt ACOs’ operations and
participation in the Shared Savings Program.
We propose to use assigned beneficiary person year values that CMS provides to ACOs
in annual and quarterly informational reports. For ACOs under preliminary prospective
assignment with retrospective reconciliation, Medicare assigns beneficiaries in a preliminary
manner at the beginning of a performance year based on the most recent data available (§
425.400(a)(2)(i)). Assignment is updated quarterly based on the most recent 12 or 24 months of
data, as applicable, under the methodology described in §§ 425.402 and 425.404 (§
425.400(a)(2)(ii)). ACOs under preliminary prospective assignment with retrospective
reconciliation receive an assigned beneficiary person years value based on the most recent 12 or
24 months of data, as applicable, in annual and quarterly informational reports. For ACOs under
prospective assignment, Medicare FFS beneficiaries are prospectively assigned to an ACO at the
beginning of each benchmark or performance year based on the beneficiary's use of primary care
services in the most recent 12 or 24 months, as applicable, for which data are available, using the
assignment methodology described in §§ 425.402 and 425.404 (§ 425.400(a)(3)(i)). Each
quarter, CMS excludes any prospectively assigned beneficiaries that meet the exclusion criteria
under § 425.401(b). ACOs under prospective assignment receive a year-to-date assigned
beneficiary person years value with each quarterly report package. For ACOs under prospective
assignment, we would annualize the quarterly year-to-date assigned beneficiary person years
values for use in the maximum quarterly prepaid shared savings payment calculation. For
example, a year-to-date person years value of 1,500 with quarter 1 informational reports would
be annualized by multiplying 1,500 by 4. A year-to-date person years value of 3,000 with quarter
2 information reports would be annualized by multiplying 3,000 by 2.
We further propose to account for circumstances when an ACO was not reconciled for
the performance year that constitutes BY1 in the calculation of average per capita prior savings
and the proration factor. For instance, ACOs that renew their agreement periods early or are re-
entering may not be reconciled for one or more of the years preceding the start of their current
agreement period depending upon the timing of the expiration or termination of their prior
agreement period and the start of their current agreement period. We propose under new §
425.640(f)(2)(i), that if an ACO was not reconciled during one of the 2 performance years that
constitute BY1 or BY2 of its current agreement period, the ACO would receive zero savings or
losses for the BY corresponding to the performance year that was not financially reconciled in
the calculation of the prepaid shared savings multiplier. CMS has no way to determine whether
the ACO would have generated savings or losses during a performance year for which it was not
reconciled. We believe this is appropriate because it enables us to obtain a more conservative
prediction of the ACO’s financial performance for a given performance year within the current
agreement period than we would be able to obtain if we were to exclude the BY corresponding to
the performance year that was not financially reconciled from the calculation of the prepaid
shared savings multiplier. Excluding this year entirely from the calculation of average per capita
prior savings would unduly increase the weight on the other year included in the prepaid shared
savings multiplier calculation. This would be problematic in a case where the ACO’s financial
performance in the BY corresponding to the performance year that was financially reconciled is
atypically high because it would upwardly bias the prediction of the ACO’s financial
performance for a given performance year within the current agreement period. Thus, by
imputing zero savings or losses for a BY corresponding to a performance year that was not
financially reconciled in the calculation of the prepaid shared savings multiplier, we are reducing
the probability of overpredicting the financial performance of the ACO for a given performance
year within the current agreement period and, in turn, the probability of distributing excessive
prepaid shared savings. As mentioned previously, excessive distribution of prepaid shared
savings could result in several undesirable outcomes, such as ACOs accruing debt to CMS that
they are unable to repay, which could disrupt the ACOs’ operations and participation in the
Shared Savings Program.
In contrast, we determined that it would also be appropriate to exclude a year for which
the ACO was not reconciled when calculating the proration factor. The purpose of the proration
factor is to account for situations where an ACO's assigned beneficiary population calculated at
financial reconciliation for the 2 performance years that constitute BY1 and BY2 of the ACO's
current agreement period (numerator) is smaller than the ACO's assigned beneficiary population
identified for those same years using the ACO's certified ACO participant list and assignment
methodology for a given performance year within the current agreement period (denominator). If
an ACO was not reconciled for one of the 2 performance years that constitute BY1 and BY2 of
the current agreement period, it would naturally have zero assigned beneficiary person years
determined at financial reconciliation for such year, which would factor into the numerator of the
proration factor if such year was considered. However, the ACO would have positive beneficiary
counts in the 2 performance years that constitute BY1 and BY2 of the current agreement period
generated using the ACO's certified ACO participant list and assignment methodology for a
given performance year within the current agreement period, which would factor into the
denominator of the proration factor if such year was considered. Thus, if the numerator and the
denominator were both calculated as averages over 2 years, incorporating a year for which the
ACO was not reconciled in the calculation of the proration factor would artificially decrease the
proration factor and lead to a smaller pro-rated average per capita prior savings for the ACO.
Alternatively, if the numerator were calculated in a manner that excludes a performance year for
which the ACO was not reconciled (that is, calculated in a manner that includes only the year for
which the ACO was reconciled from among the 2 performance years that constitute BY1 and
BY2 of the current agreement period) and the denominator was calculated as an average that
included both of the 2 performance years that constitute BY1 and BY2 of the current agreement
period, then the direction of the impact on the proration factor would depend on whether the
number of assigned beneficiaries calculated using an ACO’s current certified ACO participant
list and assignment methodology in the benchmark year for which the ACO was not reconciled
exceeds the number of assigned beneficiaries in the other benchmark year, and by how much.
Therefore, we see no compelling reason to include a performance year immediately preceding
the start of an ACO's current agreement period for which the ACO was not reconciled in the
numerator or the denominator of the proration factor. Excluding such a year would ensure that
the proration factor compares average person years determined for prior performance years at
financial reconciliation (numerator) to average person years for those performance years
determined using the ACO's current certified ACO participant list and assignment methodology
(denominator) across a consistent set of years preceding the start of the ACO's current agreement
period.
We also propose to account for certain circumstances where there could be changes to the
values used in calculating the prepaid shared savings multiplier as a result of issuance of a
revised initial determination of financial performance under § 425.315.
To account for these situations and for the need to recalculate the proration factor as
described elsewhere in this section, we propose to specify in new § 425.640(f)(3) when CMS
would recalculate the prepaid shared savings multiplier during the current agreement period. For
the first performance year in the current agreement period, the ACO's prepaid shared savings
multiplier would be recalculated for changes in per capita shared savings or losses for the
performance years that constitute BY1 or BY2 and that are used in the calculation of the prepaid
shared savings multiplier as a result of issuance of a revised initial determination under §
425.315. For the second and each subsequent performance year during the term of the current
agreement period, the ACO's prepaid shared savings multiplier would be recalculated due to
redetermining the proration factor for the addition and removal of ACO participants or ACO
providers/suppliers in accordance with § 425.118(b), for a change to the ACO's beneficiary
assignment methodology selection under § 425.226(a)(1), for a change to the beneficiary
assignment methodology specified in subpart E of this part, and for changes in per capita shared
savings or losses for the performance years that constitute BY1 or BY2 and that are used in the
calculation of the prepaid shared savings multiplier as a result of issuance of a revised initial
determination under § 425.315.
The specific computations involved in arriving at the maximum prepaid shared savings
payment amount for a given ACO in a given quarter are described below.
● Step 1: Calculate a prepaid shared savings multiplier as the average per capita savings
across the performance years that constitute BY1 and BY2 of the ACO’s current agreement
period. First, calculate the total per capita savings amount for each applicable performance year
by subtracting assigned beneficiary expenditures from total benchmark expenditures and divide
the difference by assigned beneficiary person years. Then, sum the resulting quotients and divide
by 2. The per capita savings or losses would be set to zero for a performance year if the ACO
was not reconciled for the performance year.
● Step 2: Apply a proration factor to the prepaid shared savings multiplier calculated in
Step 1. The proration factor is equal to the ratio of the ACO's average assigned beneficiary
person years for the 2 performance years that constitute BY1 and BY2 for the ACO's current
agreement period (regardless of whether these performance years occurred over one or multiple
prior agreement periods) and the ACO’s average assigned beneficiary person years in BY1 and
BY2 for the ACO’s current agreement period calculated using the ACO’s certified ACO
participant list and assignment methodology for a given performance year within the current
agreement period, capped at one. If the ACO was not reconciled for the performance year that
constitutes BY1, the person years from that year (or years) would be excluded from the averages
in the numerator and the denominator of this ratio. This ratio would be redetermined for each
performance year during the agreement period in the event of any changes to the number of
average person years in the benchmark years as a result of changes to the ACO's certified ACO
participant list, a change to the ACO's beneficiary assignment methodology selection under §
425.226(a)(1), or changes to the beneficiary assignment methodology specified in 42 CFR part
425, subpart E.
● Step 3: Adjust the pro-rated prepaid shared savings multiplier calculated in Step 2.
First, apply a shared savings scaling factor by multiplying the pro-rated prepaid shared savings
multiplier by 0.50. Then, multiply the resulting value by 2/3 to apply a financial risk scaling
factor.
● Step 4: Cap the pro-rated, adjusted prepaid shared savings multiplier at 5 percent of
national per capita FFS expenditures for Parts A and B services in BY2 for assignable
beneficiaries identified for the 12-month calendar year corresponding to BY2.
● Step 5: Multiply one-fourth of the pro-rated, adjusted, and capped prepaid shared
savings multiplier by the assigned beneficiary person years derived from the ACO’s latest
available assignment list. The resulting product would serve as the ACO’s total maximum
prepaid shared savings payment for the applicable quarter. As discussed previously, an ACO’s
latest available assignment list is updated quarterly. For ACOs under preliminary prospective
assignment with retrospective reconciliation, assignment is updated quarterly based on the most
recent 12 or 24 months of data, as applicable, under the methodology described in §§ 425.402
and 425.404 (§ 425.400(a)(2)(ii)). For ACOs under prospective assignment, assignment is
updated quarterly to exclude any prospectively assigned beneficiaries that meet the exclusion
criteria under § 425.401(b) (§ 425.401(b)). Thus, consistent with the methodology that we apply
in the case of advance investment payments, quarterly variations in an ACO’s assignment list
would translate to variations in the maximum quarterly total prepaid shared savings payments
that an ACO may receive in any given quarter, in order to help ensure that the payments

accurately reflect the attributes of the ACO’s assigned beneficiary population throughout the
current agreement period.
Table 37 presents a hypothetical example to demonstrate how the prepaid shared savings
calculation would work in practice.
TABLE 37: Calculation of Maximum Quarterly Prepaid Shared Savings Payment
Step 1: Calculate
prepaid shared
savings multiplier
Per capita savings generated in the two performance years that constitute BY1 and BY2
for the ACO’s current agreement period beginning January 1, 2022
PY 2019: $350
PY 2020: $400
Multiplier: Simple average of the per capita savings across BY1 and BY2
($350 + $400) / 2 = $375
Step 2: Pro-rate
the prepaid shared
savings multiplier
Assigned person years from the performance years that constitute BY1 and BY2 for the
ACO’s current agreement period beginning January 1, 2022:
PY 2019: 6,000
PY 2020: 7,000
Assigned person years for BY1 and BY2 of current agreement period (determined using
certified ACO participant list for the current performance year of PY 2022):
BY 2019: 8,000
BY 2020: 7,500
Proration factor: Ratio between the ACO’s average person years in the performance years
that constitute BY1 and BY2 and the average person years in BY1 and BY2, excluding
years for which the ACO was not reconciled, capped at 1.
Apply the proration factor to the prepaid shared savings multiplier: [(6,000 + 7,000)/2] /
[(8,000 + 7,500)/2] x $375 = $314.52
Step 3: Adjust the
pro-rated prepaid
shared savings
multiplier for
financial risk and
sharing rate
Shared savings scaling factor: (0.5)
Financial risk scaling factor: (2/3)
Apply the shared savings scaling factor and the financial risk scaling factor to the pro-
rated prepaid shared savings multiplier: $314.52 x (0.5) x (2/3) = $104.84
Step 4: Cap the
pro-rated,
adjusted prepaid
shared savings
multiplier
National assignable per capita FFS expenditures for assignable beneficiaries in BY2:
$10,000
Cap: 5 percent of national assignable per capita FFS expenditures for assignable
beneficiaries in BY2
0.05 * $10,000 = $500
Step 5: Determine
the maximum
prepaid shared
savings payments
for the applicable
quarter
Assigned beneficiary person years derived from the ACO’s latest available assignment
list: 8,500.
Total prepaid shared savings payments for the applicable quarter: Product of one-fourth of
the pro-rated, adjusted, capped prepaid shared savings multiplier and the assigned
beneficiary person years derived from the ACO’s latest available assignment list.
($104.84/4) x 8,500 = $222,785
The ACO’s maximum quarterly prepaid shared savings payments would set a ceiling on
the amount of quarterly prepaid shared savings that an ACO could receive from CMS for each
quarter. ACOs will be able to request to receive an amount of funding under this maximum
amount. Prior to each performance year, ACOs would notify CMS of the amount of prepaid
shared savings they want to receive in the first quarter under the maximum quarterly prepaid
shared savings amount and the first quarterly payment will be used to determine the total amount
of prepaid shared savings the ACO will use to budget for that performance year. We are
proposing in new § 425.640(f)(5) that for the purposes of determining the amount of prepaid
shared savings permitted to be allocated to the uses specified in § 425.640(e), the estimated
annual prepaid shared savings amount can be calculated by multiplying the first quarterly
payment amount the ACO would receive in each performance year by four. This allows the ACO
to calculate the total amount of funding they are permitted to spend on each allowable use at the
start of each performance year. If an ACO’s maximum quarterly payments decrease over the
performance year and result in the ACO receiving less than the estimated annual prepaid shared
savings amount, the ACO would not be subject to compliance actions solely because it spent
more than 50 percent of the actual annual amount of prepaid shared savings it received during
that PY on staffing and healthcare infrastructure, as long as it did not spend more than 50 percent
of the originally estimated annual prepaid shared savings amount on staffing and healthcare
infrastructure. For example, if an ACO is eligible for a maximum quarterly prepaid shared
savings payment of $300,000 for quarter 1 of a performance year, but only wishes to receive
$250,000 for quarter 1 of a performance year, their estimated annual prepaid shared savings
amount would be $1,000,000. This allows the ACO to spend up to $500,000 on staffing and
healthcare infrastructure, or up to the full amount of $1,000,000 on direct beneficiary services. If
an ACO has a reduction in assigned beneficiaries and is only eligible for a maximum quarterly
prepaid shared savings payment of $200,000 for quarters 2, 3 and 4, this results in an actual total
of $850,000 in received prepaid shared savings for the performance year. However, the ACO
would still be permitted to spend up to $500,000 on staffing and healthcare infrastructure in that
performance year, as that is 50 percent of the original estimated amount and we do not want to
change budget maximums retroactively for an ACO.
f. Duration, Frequency and Withholding or Termination of Prepaid Shared Savings Payments
(1) Duration and Frequency
CMS anticipates that the vast majority of ACOs receiving prepaid shared savings would
fully repay the amount they receive of prepaid shared savings from their earned shared savings
on an annual basis. This would allow CMS to distribute prepaid shared savings to ACOs
continually, throughout an agreement period in which the ACO is deemed eligible to participate,
without withholding prepaid shared savings pursuant to § 425.640(h). We are proposing at §
425.640(f)(1) that ACOs would receive quarterly prepaid shared savings payments for the
entirety of the ACO’s agreement period unless withheld or terminated pursuant to § 425.640(h).
However, we are also proposing at § 425.640(h)(3) that if CMS withholds or terminates a
quarterly payment pursuant to paragraph (h), the ACO would not receive additional or catch-up
payments if quarterly prepaid shared savings payments are later resumed. As discussed later in
this section, prepaid shared savings payments would generally be withheld from ACOs when
CMS has information that the ACO may not generate sufficient earned shared savings to repay
the prepaid shared savings in current or future performance years or has other Shared Savings
Program compliance issues. Once prepaid shared savings payments are withheld, if an ACO
earns shared savings in a future year, then prepaid shared savings can resume at the time of the
next scheduled quarterly payment, but catch-up payments would not be provided. This protects
CMS from distributing payments that the ACOs may not be able to repay and the ACOs from
accumulating more debt than they can repay through earned shared savings. An ACO would be
notified if CMS is willing to resume prepaid shared savings payments, and would have the
ability to elect to resume payments as well as select the payment amount they would like to
receive under the maximum quarterly payment, if desired.
(2) Withholding and Termination
In order to ensure orderly administration of the Shared Savings Program, including
protection of the Medicare Trust Funds, we intend to monitor the performance of ACOs
receiving prepaid shared savings and propose that we may withhold or terminate quarterly
prepaid shared savings payments under a variety of specified circumstances. Many of the
circumstances under which we propose that CMS may withhold to terminate the payments
directly relate to circumstances under which CMS would be concerned that the ACO has not or
will not meet the standards for the use prepaid shared savings, such as an ACO’s failure to
comply with the requirements of proposed § 425.640. Other circumstances would address
situations where it becomes apparent that the ACO’s is likely to lack the ability to repay prepaid
shared savings to CMS. For example, we propose that CMS may withhold or terminate the
payments if CMS predicts that the ACO will not generate sufficient earned shared savings to
repay the prepaid shared savings in future performance years or has other Shared Savings
Program compliance issues. These predictions would be based on a rolling 12-month window of
beneficiary claims data or year-to-date beneficiary claims data, depending on whether an ACO
selects prospective Assignment or preliminary prospective assignment with retrospective
reconciliation. We propose that CMS may also withhold quarterly payments if an ACO fails to
earn enough shared savings in a performance year to fully repay the prepaid shared savings the
ACO received during that performance year, in order to avoid the ACO accruing debt they would
be unable to repay. As noted earlier in this section, an ACO would be notified if CMS
determines the ACO is sufficiently likely to earn additional shared savings such that CMS could
resume prepaid shared savings payments, in which case the ACO would have the ability to elect
to resume payments and select the payment amount they would like to receive. Additionally,
while unspent funds received for a performance year must be reallocated in the spend plan for
the ACO’s next performance year as noted in § 425.640(e)(3), if an ACO fails to spend a
majority of the prepaid shared savings they receive in a performance year, CMS may withhold
future quarterly payments until the ACO spends the funding they have already received and
reports this spending to CMS through an updated spend plan. An ACO may also request that
CMS withhold their future quarterly payments until the ACO is ready for payments to resume.

ACOs that elect to have CMS withhold their prepaid shared savings payments would have the
ability to later elect to resume payments as well as select the payment amount they would like to
receive. If an ACO has unspent funding at the end of their agreement period, that funding must
be repaid to CMS pursuant to proposed § 425.640(e)(3).
Accordingly, we propose at § 425.640(h)(1) that CMS may withhold or terminate prepaid
shared savings during an agreement period if:
● The ACO fails to comply with any of the prepaid shared savings requirements of §
425.640;
● The ACO meets any of the grounds for ACO termination set forth in § 425.218(b);
498
● The ACO fails to earn sufficient shared savings from a performance year to repay the
prepaid shared savings they received during that performance year;
● CMS determines that the ACO is not expected to earn shared savings in a performance
year during the agreement period in which the ACO received prepaid shared savings, based on a
rolling 12-month window of beneficiary claims data or year-to-date beneficiary claims data;
● The ACO falls below 5,000 assigned beneficiaries;
● The ACO fails to spend the majority of prepaid shared savings they receive in a
performance year; or
● The ACO requests that CMS withhold a future quarterly payment
Additionally, we propose at § 425.640(h)(2) that CMS must terminate an ACO’s prepaid
shared savings during an agreement period if:
● The ACO fails to maintain an adequate repayment mechanism in accordance with §
425.204(f); or
498
Under §§ 425.216 and 425.218, CMS can terminate an ACO’s participation agreement or take pre-termination
actions (such as requesting a corrective action plan) if CMS determines that an ACO is not in compliance with the
requirements of Part 425 of our regulations.
● The ACO fails to meet the quality performance standard as specified under § 425.512
or is subject to a pre-termination action after CMS determined the ACO avoided at-risk
beneficiaries as specified under § 425.316(b)(2).
We further propose under § 425.640(h)(4) that CMS may immediately terminate an
ACO’s prepaid shared savings under § 425.640(h)(1) and (2) without taking any of the pre-
termination actions set forth in § 425.216.
In general, if an ACO is complying with the Shared Savings Program and prepaid shared
savings requirements but is not achieving, or is not predicted to achieve, success in earning
shared savings, CMS may withhold payments while the ACO works to improve their financial
performance. For example, if an ACO is eligible to receive quarterly prepaid shared savings
payments in an agreement period beginning in 2026 but does not earn shared savings during
2025 reconciliation that occurs in mid-2026, the ACO’s quarterly payments would be withheld
until the ACO earns shared savings in a future performance year reconciliation. Similar to our
rationale for the eligibility requirement described at § 425.640(b)(2), we believe that recent past
performance in earning shared savings provides information on the ACO’s potential to earn
future shared savings, and we would not distribute prepaid shared savings to ACOs that have not
earned sufficient shared savings in their most recent reconciled performance year to repay the
prepaid shared savings they received during that performance year.
Additionally, if CMS, through its financial monitoring of ACOs, predicts that an ACO
would not earn shared savings in its current performance year, quarterly prepaid shared savings
may be withheld until the ACO generates earned shared savings in the future. We expect that
immediate termination of prepaid shared savings during an agreement period, without a
possibility of resumption of payments during that agreement period, would be invoked only in
cases of serious noncompliance with the requirements of § 425.640, including deliberately
spending prepaid shared savings on a prohibited use, or when the ACO’s actions or inaction
poses a risk of harm to beneficiaries or negatively affects their access to care. We seek comment
on these proposals.
g. Monitoring ACO Eligibility for and use of Prepaid Shared Savings
To provide CMS with a clear indication of how ACOs intend to spend prepaid shared
savings, help provide adequate protection to the Medicare Trust Funds, and prevent funds from
being misdirected or appropriated for activities that do not fall within the parameters set forth
within proposed § 425.640(e), we propose in § 425.316(f)(1) to monitor ACOs receiving prepaid
shared savings for compliance with § 425.640(e) and to determine whether it would be
appropriate to withhold or terminate an ACO’s prepaid shared savings under § 425.640(h)(1) and
(h)(2). For the first performance year of the current agreement period, we would monitor the
ACO’s use of prepaid shared savings by comparing the anticipated spending as set forth in the
spend plan submitted with an ACO’s application against the actual spending as reported by the
ACO, including any expenditures not identified in the spend plan. ACOs would be required to
submit a revised spend plan with updated anticipated spending annually, as well as annually
report their actual expenditures to CMS and on their public reporting webpage as noted in §§
425.308(b)(10) and 425.640(i), and we would similarly monitor the ACO’s use of prepaid shared
savings during the current agreement period using the updated spend plan and those reports. The
reported annual spending must include any expenditures of prepaid shared savings on items not
identified in the spend plan. In the event that an ACO uses prepaid shared savings for uses not
permitted by § 425.640(e), CMS would require them to reallocate the funding to a permitted use
and may take compliance action as specified in §§ 425.216, 425.218 or withhold or terminate
payments as specified in proposed § 425.640(h)(1).
Similar to the policy for advance investment payments (§ 425.630), we additionally
believe that transparency of information in the healthcare sector facilitates more informed patient
choice and offers incentives and feedback that help improve the quality and lower the cost of
care and improve oversight with respect to program integrity. As CMS has discussed in previous
final rules, improved transparency supports a number of program requirements. In particular,
increased transparency is consistent with and supports the requirement under section
1899(b)(2)(A) of the Act for an ACO to be willing to “become accountable for the quality, cost,
and overall care” of the Medicare beneficiaries assigned to it. Therefore, we believe it is
desirable and consistent with section 1899(b)(2)(A) of the Act for several aspects of an ACO’s
use of prepaid shared savings to be available to the public. Making this information available
would provide both Medicare beneficiaries and the general public with insight into the use of
prepaid shared savings by an ACO.
Accordingly, we are proposing to modify § 425.308 to require that an ACO publicly
report information annually regarding prepaid shared savings on its public reporting webpage.
Specifically, under proposed new § 425.308(b)(10), we are proposing that, for each performance
year, an ACO would be required to report (in a standardized format specified by CMS) its spend
plan for each performance year, the total amount of prepaid shared savings received, and an
itemization of how any prepaid shared savings were actually spent during each year, including
expenditure categories, the dollar amounts spent on the various categories, information about
which beneficiaries received direct beneficiary services that were purchased with prepaid shared
savings and investments that were made in the ACO with prepaid shared savings, any changes to
the spend plan as submitted under § 425.640(d)(1), and such other information as may be
specified by CMS. We propose that this itemization would include expenditures not identified or
anticipated in the ACO’s submitted spend plan, and any amounts remaining unspent. We are also
proposing at § 425.640(i) that ACOs also be required to report this information directly to CMS.
Under this proposal, if CMS determined that an ACO used prepaid shared savings for a
prohibited use under proposed § 425.640(e)(2), allocated over 50 percent of their annual
maximum prepaid shared savings on staffing and healthcare infrastructure as proposed at §
425.640(e)(1)(i), or failed to spend at least 50 percent of the annual maximum prepaid shared
savings on direct beneficiary services, CMS would require the ACO to reallocate the funding in
compliance with § 425.640(e) and submit an updated spend plan demonstrating the reallocation
by a deadline specified by CMS and may withhold or terminate the ACO’s receipt of prepaid
shared savings under proposed § 425.640(h)(1). CMS could also take compliance action as
specified in §§ 425.216 and 425.218. If an ACO fails to reallocate prepaid shared savings it
received by a deadline specified by CMS, the ACO must repay all prepaid shared savings it
received and may be subject to compliance action as specified in §§ 425.216 and 425.218. CMS
would provide written notification to the ACO of the amount due and the ACO must pay such
amount no later than 90 days after the receipt of such notification.
Additionally, we note that under existing § 425.314, ACOs would be required to retain
and provide CMS with access to adequate books, contracts, records, and other evidence to ensure
that CMS has the information necessary to conduct appropriate monitoring and oversight of
ACOs’ use of prepaid shared savings (for example, invoices, receipts, and other supporting
documentation of prepaid shared savings disbursements). To protect the Shared Savings Program
and the Medicare Trust Funds, we would reserve the right under §§ 425.314 and 425.316(a) to
audit and monitor ACO compliance with Shared Savings Program requirements, including with
respect to prepaid shared savings. We would conduct audits as necessary to monitor and assess
an ACO’s use of prepaid shared savings and compliance with program requirements related to
such payments. We seek comment on these proposals.
h. Recoupment of Prepaid Shared Savings
CMS anticipates that a vast majority of ACOs receiving prepaid shared savings would
fully repay the amount they receive prepaid shared savings from their earned shared savings on
an annual basis. However, as prepaid shared savings are an advance of the shared savings
payments an ACO is expected to earn, we propose to recoup prepaid shared savings from ACOs
that are unable to fully repay prepaid shared savings through their earned shared savings. This
approach will also help ensure that prepaid shared savings would not result in additional
expenditures for the Shared Savings Program, as required by section 1899(i)(3)(B) of the Act.

We are proposing to add a new § 425.640(g)(1) to recoup prepaid shared savings from
earned shared savings, as defined in § 425.20, in each performance year. If there are insufficient
shared savings to recoup the prepaid shared savings made to an ACO for a performance year, we
would hold paying future prepaid shared savings payments and carry forward the remaining
balance owed to subsequent performance year(s) in which the ACO achieves shared savings.
Under new § 425.640(g)(2), we propose that in circumstances where the amount of
shared savings earned by the ACO is revised upward by CMS for any reason, we would reduce
the redetermined amount of shared savings by the amount of prepaid shared savings made to the
ACO as of the date of the redetermination. If the amount of shared savings earned by the ACO is
revised downward by CMS for any reason, we propose that the ACO would not receive a refund
of any portion of the prepaid shared savings previously recouped or otherwise repaid, and any
prepaid shared savings that are now outstanding due to the revision in earned shared savings
must be repaid to CMS upon request.
We propose under § 425.640(g)(3) that if an ACO has an outstanding balance of prepaid
shared savings after the calculation of shared savings or losses for the final performance year of
an agreement period in which an ACO receives prepaid shared savings, the ACO must repay any
outstanding amount of prepaid shared savings it received in full upon request from CMS. CMS
would provide written notification to the ACO of the amount due and the ACO must pay such
amount no later than 90 days after the receipt of notification. If an ACO fails to repay any
outstanding amount of prepaid shared savings within 90 days of the notification, CMS would
recoup that amount from the ACO’s repayment mechanism established under § 425.204(f).
For example, if an ACO received $300,000 in prepaid shared savings payments and
earned shared savings of $500,000 for the first performance year, we would recoup $300,000 in
prepaid shared savings payments and make $200,000 in reconciliation shared savings payments
to the ACO. Alternatively, if an ACO received $300,000 in prepaid shared savings and earned
shared savings of $200,000 for the first performance year, we would recoup only $200,000 in

prepaid shared savings payment and not make a reconciliation shared savings payment to the
ACO. The ACO would have future prepaid shared savings payments placed on hold, and the
outstanding balance of $100,000 would be carried forward, to be recouped in a future
performance year in which the ACO achieves shared savings. Under a third scenario, if the ACO
does not earn sufficient shared savings in all 5 performance years of its agreement period, CMS
would recoup the outstanding balance directly from the ACO under new § 425.640(g)(3). If the
ACO fails to repay the funding to CMS, we would recoup the outstanding balance from the
ACO’s repayment mechanism.
Under the new § 425.640(g)(4), we propose that if an ACO or CMS terminates its
participation agreement during the agreement period in which it received prepaid shared savings,
the ACO must repay all outstanding prepaid shared savings received in full. In such a case, CMS
would provide written notification to the ACO of the amount due and the ACO must pay such
amount no later than 90 days after the receipt of notification. If an ACO fails to repay any
outstanding amount of prepaid shared savings within 90 days of the notification, CMS would
recoup that amount from the ACO’s repayment mechanism established under § 425.204(f). We
also propose edits to § 425.204(f) replacing the phrase “shared losses” with “shared losses and
any prepaid shared savings” in several paragraphs to clarify that CMS would be able to recoup
outstanding prepaid shared savings from an ACO’s repayment mechanism. If the ACO
terminates its participation agreement early in order to renew under a new participation
agreement, CMS may also recover the amount owed by reducing the amount of any future shared
savings the ACO may be eligible to receive.
In the event the ACO enters into proceedings relating to bankruptcy, whether voluntary
or involuntary, we are proposing under § 425.630(g)(5) that the ACO must provide written
notice of the bankruptcy to CMS and to the U.S. Attorney's Office in the district where the
bankruptcy was filed, unless final payment for the agreement period has been made by either
CMS or the administrative or judicial review proceedings relating to any payments under the
Shared Savings Program have been fully and finally resolved. The notice of bankruptcy must be
sent by certified mail no later than 5 days after the petition has been filed and must contain a
copy of the filed bankruptcy petition (including its docket number). The notice to CMS must be
addressed to the CMS Office of Financial Management at 7500 Security Boulevard, Mailstop
C3-01-24, Baltimore, MD 21244, or such other address as may be specified on the CMS website
for purposes of receiving such notices.
i. OIG Safe Harbor Authority
CMS expects to make a determination, if this rulemaking is finalized, that the anti-
kickback statute safe harbor for CMS-sponsored model patient incentives (§ 1001.952(ii)(2)) is
available to protect patient incentives that may be permitted under the final rule, if issued.
Specifically, we expect to determine that the CMS-sponsored models safe harbor would be
available to protect direct beneficiary services provided to beneficiaries through the prepaid
shared savings payment option.
We propose to add a new paragraph (d) to § 425.304 that notes that CMS has determined
that the Federal anti-kickback statute safe harbor for CMS-sponsored model patient incentives
(42 CFR 1001.952(ii)(2)) is available to protect remuneration furnished in the prepaid shared
savings program option of the Shared Savings Program in the form of direct beneficiary services
that meets all safe harbor requirements set forth in § 1001.952(ii)(2).
We are seeking comment on all aspects of our proposals.
6. Advance Investment Payment Policies
a. Proposal to Allow ACOs Receiving Advance Investment Payments to Voluntarily Terminate
Payments while Continuing Participation in the Shared Savings Program
Beginning January 1, 2024, CMS implemented a new payment option in the Shared
Savings Program, advance investment payments (AIP), and codified AIP requirements at §
425.630. In the CY 2023 PFS final rule (87 FR 69803 through FR 69805), we discussed policies
for termination of advance investment payments from ACOs whose participation agreements are
terminated for noncompliance with certain requirements and finalized a recoupment policy in
which all advance investment payments must be repaid to CMS within 90 days from the date
CMS provided the ACO whose participation agreement was terminated with written notice of the
amount due. These regulations are codified at § 425.630(g) and (h).
Currently, there are no regulations that account for an ACO that seeks to voluntarily
terminate receipt of advance investment payments from CMS, but that wishes to remain in the
Shared Savings Program for the rest of their agreement period. While we expect advance
investment payment terminations to be an uncommon occurrence, since advance investment
payments are a voluntary payment option, ACOs should be able to decline further participation.
To accommodate voluntary terminations of advance investment payments for ACOs that wish to
continue participating in the Shared Savings Program, CMS proposes to modify program
regulations at § 425.630(g) and (h). CMS proposes to allow ACOs who wish to voluntarily
terminate receipt of advance investment payments to do so and remain in the Shared Savings
Program. An ACO may have justified business reasons for terminating receipt of advance
investment payments (such as an ACO’s desire to enter a CMS Innovation Center model whose
eligibility criteria exclude ACOs that receive AIP). CMS wishes to amend its termination
policies to account for such a scenario. It is the best interest of the Medicare Trust Funds and the
Shared Savings Program to allow continued program participation by ACOs that terminate
receipt of advance investment payments, especially among ACOs and ACO participants in, or
that serve, underserved communities. Therefore, CMS proposes new regulations effective
January 1, 2025, to allow ACOs to voluntarily terminate receipt of advance investment payments
while remaining in the Shared Savings Program. Under this proposal, CMS would develop an
advance investment payment voluntary termination notification process to allow ACOs to
voluntarily terminate receipt of these payments. If this proposal is finalized, CMS would issue
guidance regarding this process to participating Shared Savings Program ACOs shortly after
publication of the CY 2025 PFS final rule.
We propose to update § 425.630(g) to state that if an ACO opts to voluntarily terminate
from the advance investment payment option, they would be required to return any outstanding
advance investment payments to CMS. Upon an ACO notifying CMS that it wants to terminate
from the advance investment payment option, CMS would then provide a written notification to
the ACO of the total amount of recoupment due. CMS would then require the ACO to repay the
amount due no later than 90 days after the receipt of such notification. This aligns with how
CMS recoups advance investment payments from ACOs whose advance investment payments
are involuntarily terminated due to failure to comply with advance investment payment
eligibility requirements under § 425.316(e)(3) and with the repayment requirements under §
425.630(g)(4), if an ACO chooses to terminate from the Shared Savings Program.
ACOs that terminate from the advance investment payment option would no longer be
monitored for their appropriate use of advance investment payments once the payments are
repaid to CMS. As such, ACOs that terminate would no longer be subject to annual reporting
requirements for their spend plans once the payments are repaid to CMS. This proposal would
allow an ACO additional flexibility to determine its best payment and participation options,
making it easier for an ACO receiving advance investment payments to continue their
participation in the Shared Savings Program long-term. As noted in the CY 2023 PFS final rule
(87 FR 69784), advance investment payments were designed to assist ACOs that face difficulty
funding the start-up costs for forming ACOs, caring for beneficiaries in underserved
communities, and achieving long term success in the Shared Savings Program. Allowing these
ACOs more flexibility would have the effect of supporting continued Shared Savings Program
participation among these ACOs, including those serving rural and underserved populations.
CMS proposes to update § 425.630(g)(5) to state that if an ACO notifies CMS that it no
longer wants to participate in the advance investment payment option but does want to continue
its participation in the Shared Savings Program, the ACO must repay all outstanding advance
investment payments it received. CMS would provide written notice to the ACO of the amount

due and the ACO must pay such amount no later than 90 days after the receipt of such
notification.
Additionally, CMS proposes conforming revisions to § 425.630(h) to clarify that ACOs
can voluntarily terminate from the advance investment payment option. Specifically, CMS
proposes to add a paragraph (h)(1)(iv) to read “Voluntarily terminates payments of advance
investment payments but continues its participation in the Shared Savings Program.” CMS also
proposed conforming revisions to § 425.630(h)(1)(ii) and (iii). We seek comment on these
proposals. If finalized, the proposed changes would be effective beginning January 1, 2025.
b. Proposal to Recoup Advance Investment Payments when CMS Terminates the Participation
Agreement of an ACO
Under current advance investment payment recoupment regulations, there is no clear
pathway for CMS to recoup outstanding advance investment payments if CMS terminates an
ACO’s participation agreement in accordance with § 425.218(b). To address this and reduce the
risk to the Trust Fund, CMS proposes to add new § 425.630(g)(6) to require ACOs to repay any
outstanding advance investment payments in the event that CMS terminates the ACO’s Shared
Savings Program participation agreement.
Upon the termination of their Shared Savings Program participation agreement, the
ACO’s advance investment payments would cease immediately under § 425.630(h)(1)(ii). CMS
would provide the ACO with written notification of the total amount due for the full recoupment
of advance investment payments, and the ACO must pay such amount within 90 days after the
receipt of such notification. This approach aligns with how CMS recoups advance investment
payments for ACOs under § 425.630(g)(4) if an ACO receiving advance investment payments
chooses to voluntarily terminate from the Shared Savings Program. This proposal protects CMS
from not being able to recoup outstanding advance investment payments in the event CMS
terminates an ACO’s participation agreement in accordance with § 425.218(b).
Specifically, CMS proposes to add § 425.630(g)(6) to state that if CMS terminates the

participation agreement of an ACO that has an outstanding balance of advance investment
payments owed to CMS, the ACO must repay any outstanding advance investment payments it
received. CMS would provide written notification to the ACO of the amount due and the ACO
must pay such amount no later than 90 days after the receipt of such notification. If an ACO fails
to fully repay the advance investment payments they received, CMS would carry forward any
remaining balance owed to subsequent performance year(s) in which the ACO achieves shared
savings, including in any performance year(s) in a subsequent agreement period.
We also propose conforming edits to § 425.630(g)(3) to remove the phrase “paragraph
(g)(4) of this section” and adding in its place the phrase “paragraphs (g)(4) through (g)(6) of this
section.” This would allow CMS to recoup more than the amount of shared savings earned by an
ACO in a particular performance year in the event that an ACO or CMS terminates an ACO
from the advance investment payment option or the Shared Savings Program as a whole. This
proposal also would require CMS to renumber regulations at § 425.630(g). Therefore, we
propose a conforming change to redesignate § 425.630(g)(5) as § 425.630(g)(7). We seek
comment on these proposals. If finalized, these proposals would be effective beginning January
1, 2025.
7. Financial Methodology
a. Overview
In this section of this proposed rule, we are proposing modifications to the financial
methodologies used under the Shared Savings Program. The modifications we propose would
encourage participation in the program by removing barriers for ACOs serving underserved
communities
499
as well as provide greater specificity and clarity on how CMS would perform
certain financial calculations in the Shared Savings Program. Specifically, we are proposing to
499
As described in the CMS Framework for Health Equity and consistent with Executive Order 13985 on Advancing
Racial Equity and Support for Underserved Communities Through the Federal Government (86 FR 7009), the term
“underserved communities” refers to populations sharing a particular characteristic, including geographic
communities that have been systematically denied a full opportunity to participate in aspects of economic, social,
and civic life, as exemplified in the definition of “equity.”
create a health equity benchmark adjustment, to potentially provide an upward adjustment to an
ACO’s historical benchmark based on the proportion of beneficiaries they serve who are dually
eligible or enrolled in the Medicare Part D low-income subsidy (LIS). We are also proposing to
establish a calculation methodology to account for the impact of improper payments in
recalculating expenditures and payment amounts used in Shared Savings Program financial
calculations, upon reopening a payment determination pursuant to § 425.315(a). We are
proposing to establish an approach to identify significant, anomalous, and highly suspect
(“SAHS”) billing activity in CY 2024 or subsequent calendar years. We are proposing to specify
how we would exclude payment amounts from expenditure and revenue calculations for the
relevant calendar year for which the SAHS billing activity is identified as well as from historical
benchmarks used to reconcile the ACO for a performance year corresponding to the calendar
year for which the SAHS billing activity was identified to mitigate the impact of SAHS billing
activity. We seek comment on a financial model that would allow for higher risk and potential
reward than currently available under the ENHANCED track while still meeting the
requirements for use of our authority under section 1899(i)(3) of the Act, among other
considerations for the financial model design. We are also proposing certain modifications for
clarity and consistency in provisions of the Shared Savings Program regulations on calculation of
the ACO risk score growth cap in risk adjusting the benchmark each performance year and the
regional risk score growth cap in calculating the regional component of the three-way blended
benchmark update factor.
b. Health Equity Benchmark Adjustment
(1) Background
(a) Summary of Statutory and Regulatory Background on Adjusting the Historical Benchmark
Section 1899(d)(1)(B)(ii) of the Act addresses how ACO benchmarks are to be
established, updated, and reset at the start of each agreement period under the Shared Savings
Program. This provision specifies that the Secretary shall estimate a benchmark for each
agreement period for each ACO using the most recent available 3 years of per beneficiary
expenditures for Parts A and B services for Medicare FFS beneficiaries assigned to the ACO.
The benchmark shall be reset at the start of each agreement period. Section 1899(d)(1)(B)(ii) of
the Act also provides the Secretary with discretion to adjust the historical benchmark by “such
other factors as the Secretary determines appropriate.” Pursuant to this authority, over time we
have adopted a variety of methods to adjust the historical benchmark to meet certain policy
goals.
Benchmarking policies applicable to all ACOs in agreement periods beginning on
January 1, 2024, and in subsequent years, are specified in § 425.652. We refer readers to
discussions of the benchmark calculations in earlier rulemaking for details on the development of
the current policies (see November 2011 final rule, 76 FR 67909 through 67927; June 2015 final
rule, 80 FR 32785 through 32796; June 2016 final rule, 81 FR 37953 through 37991; December
2018 final rule, 83 FR 68005 through 68030; CY 2023 PFS final rule, 87 FR 69875 through
69928; and CY 2024 PFS final rule, 88 FR 79174 through 79208).
In the CY 2023 PFS final rule, we adopted policies to modify the regional adjustment
under § 425.656 (refer to 87 FR 69915 through 69923) and to reinstate a prior savings
adjustment under § 425.658 (refer to 87 FR 69898 through 69915). The modifications to the
regional adjustment are designed to limit the impact of negative regional adjustments on ACO
historical benchmarks and further incentivize program participation among ACOs serving high-
cost beneficiaries. In the CY 2024 PFS final rule (refer to 88 FR 79185 through 79196), we
modified the regional adjustment policy further to prevent any ACO from receiving an
adjustment that would cause its benchmark to be lower than it would have been in the absence of
a regional adjustment. The prior savings adjustment policy was developed such that a renewing
or re-entering ACO may be eligible to receive an adjustment to its benchmark to account for
savings generated in performance years that correspond to the benchmark years of its new
agreement period. In the CY 2024 PFS final rule (refer to 88 FR 79196 through 79200), we

modified the prior savings adjustment policy further to account for the following: a change in
savings earned by the ACO in a benchmark year due to compliance action taken to address
avoidance of at-risk beneficiaries or a change in the amount of savings or losses for a benchmark
year as a result of a reopening of a prior determination of ACO shared savings or shared losses
and the issuance of a revised initial determination under § 425.315.
(b) Methodology for Determining the Applicability of a Regional Adjustment or Prior Savings
Adjustment to the ACO’s Historical Benchmark, for Agreement Periods Beginning on or After
January 1, 2024
Under the benchmarking methodology for agreement periods beginning on January 1,
2024, and in subsequent years, CMS calculates two adjustments to the historical benchmark, a
regional adjustment (refer to § 425.656) and a prior savings adjustment (refer to § 425.658). We
determine which adjustment is applied to the benchmark, either the regional adjustment, prior
savings adjustment, or no adjustment (refer to § 425.652(a)(8) and (c)).
Under the current methodology, the adjustment that will apply in the establishment of
benchmarks for ACOs entering an agreement period beginning on January 1, 2024, and in
subsequent years, is calculated as follows:
● Step 1: Calculate capped regional adjustment expressed as a single dollar value as
specified in § 425.656. CMS calculates the regional adjustment to the historical benchmark
based on the ACO’s regional service area expenditures, making separate calculations for the
following populations of beneficiaries: ESRD, disabled, aged/dual eligible Medicare and
Medicaid beneficiaries, and aged/non-dual eligible Medicare and Medicaid beneficiaries.
++ Under § 425.656(c)(3), CMS caps the per capita dollar amount for each Medicare
enrollment type at a dollar amount equal to a percentage of national per capita expenditures for
Parts A and B services under the original Medicare fee-for-service (FFS) program in BY3 for
assignable beneficiaries in that enrollment type identified for the 12-month calendar year
corresponding to BY3 using data from the CMS Office of the Actuary.

-- Under § 425.656(c)(3)(i), for positive adjustments, the per capita dollar amount for a
Medicare enrollment type is capped at 5 percent of the national per capita expenditure amount
for the enrollment type for BY3.
-- Under § 425.656(c)(3)(ii), for negative adjustments, the per capita dollar amount for a
Medicare enrollment type is capped at negative 1.5 percent of the national per capita expenditure
amount for the enrollment type for BY3.
++ Under § 425.656(d)(1), CMS expresses the regional adjustment as a single value by
taking a person year
500
weighted average of the Medicare enrollment type-specific regional
adjustment values.
● Step 2: For eligible ACOs, calculate the capped prior savings adjustment as specified
in § 425.658. Under § 425.658(c)(1), CMS calculates an adjustment to the historical benchmark
to account for savings generated in the 3 years prior to the start of the ACO's current agreement
period for renewing or re-entering ACOs that were reconciled for one or more performance years
in the Shared Savings Program during this period.
● Step 3: Determine the final adjustment to the benchmark, as specified in
§ 425.652(a)(8). Compare the regional adjustment in accordance with § 425.656 and the prior
savings adjustment in accordance with § 425.658.
++ Under § 425.652(a)(8)(ii), if an ACO is not eligible to receive a prior savings
adjustment under § 425.658(b)(3)(i), and the regional adjustment, expressed as a single value as
described in § 425.656(d), is positive, the ACO will receive an adjustment to its benchmark
equal to the positive regional adjustment amount. The adjustment will be calculated as described
in § 425.656(c) and applied separately to the following populations of beneficiaries: ESRD,
500
To calculate person years: We sum the number of Shared Savings Program-eligible months (beneficiaries are
only assigned a monthly enrollment status for months in which they are alive on 1
st
of the month, enrolled in both
Parts A and B, and not enrolled in a Medicare Group Health Plan for the month) for each assigned beneficiary for
each Medicare enrollment type; we then divide this number by 12 (the number of months in a calendar year). Refer
to the Medicare Shared Savings Program, Shared Savings and Losses and Assignment Methodology Specifications
(version #11, January 2023), available at https://www.cms.gov/files/document/medicare-shared-savings-program-
shared-savings-and-losses-and-assignment-methodology-specifications.pdf-2 (Section 3.1 Calculating ACO-
Assigned Beneficiary Expenditures).

disabled, aged/dual eligible Medicare and Medicaid beneficiaries, and aged/non-dual eligible
Medicare and Medicaid beneficiaries. Under § 425.652(a)(8)(iii), if an ACO is not eligible to
receive a prior savings adjustment under § 425.658(b)(3)(i), and the regional adjustment,
expressed as a single value as described in § 425.656(d), is negative or zero, the ACO will not
receive an adjustment to its benchmark.
++ Under § 425.652(a)(8)(iv), if an ACO is eligible to receive a prior savings adjustment
and the regional adjustment, expressed as a single value as described in § 425.656(d), is positive,
the ACO will receive an adjustment to its benchmark equal to the higher of the following:
-- Under § 425.652(a)(8)(iv)(A), the positive regional adjustment amount. The
adjustment will be calculated as described in § 425.656(c) and applied separately to the
following populations of beneficiaries: ESRD, disabled, aged/dual eligible Medicare and
Medicaid beneficiaries, and aged/non-dual eligible Medicare and Medicaid beneficiaries.
-- Under § 425.652(a)(8)(iv)(B), the prior savings adjustment. The adjustment will be
calculated as described in § 425.658(c) and applied as a flat dollar amount to the following
populations of beneficiaries: ESRD, disabled, aged/dual eligible Medicare and Medicaid
beneficiaries, and aged/non-dual eligible Medicare and Medicaid beneficiaries.
++ Under § 425.652(a)(8)(v), if an ACO is eligible to receive a prior savings adjustment
and the regional adjustment, expressed as a single value as described in § 425.656(d), is negative
or zero, the ACO will receive an adjustment to its benchmark equal to the prior savings
adjustment. The adjustment will be calculated as described in § 425.658(c) and applied as a flat
dollar amount to the following populations of beneficiaries: ESRD, disabled, aged/dual eligible
Medicare and Medicaid beneficiaries, and aged/non-dual eligible Medicare and Medicaid
beneficiaries.
(c) Background on Incorporating Health Equity Data within the Shared Savings Program
Development of a health equity benchmark adjustment builds upon Shared Savings
Program policies finalized in the CY 2023 and CY 2024 PFS final rules to advance health equity,
including the establishment of the health equity adjustment to an ACO’s MIPS quality
performance category score (applicable to all ACOs beginning with performance year 2023) (87
FR 69838 through 69857 and 88 FR 79114 through 79117); the availability of advance
investment payments to eligible new, low revenue ACOs entering a new agreement period
beginning on January 1, 2024, and in subsequent years (87 FR 69782 through 69805 and 88 FR
79208 through 79216); as well as changes to the benchmarking methodology aimed to facilitate
participation by ACOs serving medically complex or underserved beneficiaries (87 FR 69915
through 69924 and 88 FR 79185 through 79195).
Further, in a Request for Information in the CY 2023 PFS final rule (87 FR 69977
through 69979), we discussed addressing health equity through benchmarking and summarized
related comments. In the CY 2023 PFS final rule (87 FR 69978), we explained our interest in
considering how direct modification of benchmarks to account for existing inequities in care can
be used to advance health equity. The vast majority of commenters expressed support for
exploring methodologies to address health equity via benchmarking changes. Specifically, many
of these commenters noted that benchmark adjustments could be an effective tool to redirect
resources to ACOs serving underserved communities. Multiple commenters commented
specifically on the health equity benchmark adjustment approach utilized by the ACO Realizing
Equity, Access, and Community Health (REACH) Model. Several of these commenters
expressed support for using a similar methodology in implementing a health equity benchmark
adjustment in the Shared Savings Program. In response, we stated that we will consider these
comments in the development of policies for future rulemaking. Based on our experience with
adjustments under the current benchmarking methodology, our experience establishing policies
to advance health equity in the Shared Savings Program, and the support received for addressing
health equity through benchmarking in response to the Request for Information, we have
determined that it would be timely to implement a health equity benchmark adjustment (HEBA)
into the Shared Savings Program’s benchmarking methodology. Implementing a HEBA would

ensure benchmarks continue to serve as a reasonable baseline when ACOs serve high
proportions of beneficiaries who are members of underserved communities and incentivize
ACOs to provide coordinated care to beneficiaries who are members of underserved
communities.
A health equity benchmark adjustment is likely to encourage more participation in the
Shared Savings Program by ACOs that serve beneficiaries who are members of rural and
underserved communities by allowing them to participate with potentially higher benchmarks.
That, in turn, would increase the likelihood that they could earn shared savings and increase the
amount of those shared savings payments and reduce the financial barriers to forming ACOs that
providers who serve underserved communities face. Benchmarks based on historically observed
spending could be set too low if they are based on the spending of a population of underserved
communities. An ACO serving such communities could be harmed financially if they are
successful at improving access to high-value care during the performance period. Additionally,
the Congressional Budget Office (CBO) recently reported high start-up costs for providers in
rural and underserved communities as a barrier to forming ACOs.
501
These providers may want
to participate in ACOs but are disincentivized due to steep start-up costs.
A health equity benchmark adjustment would also encourage currently participating
ACOs to attract more beneficiaries who are members of underserved communities and remain in
the Shared Savings Program. Direct increases to benchmarks for ACOs serving higher
proportions of beneficiaries who are members of underserved communities would grant
additional financial resources to health care providers accountable for the care of these
populations and may work to offset historical patterns of underspending that influence
benchmark calculations.
501
Congressional Budget Office (CBO), “Medicare Accountable Care Organizations: Past Performance and Future
Directions,” April 2024, available at https://www.cbo.gov/system/files/2024-04/59879-Medicare-ACOs.pdf.

The ACO REACH Model incorporates a HEBA to test a way to address historical health
inequities within CMS ACO initiatives, with the intent of incentivizing ACOs to seek out and
form relationships with beneficiaries who are members of underserved communities. The
adjustment is intended to mitigate the disincentive for ACOs to serve underserved communities
by accounting for historically suppressed spending levels for these populations. It is a critical
step towards enabling ACOs to serve underserved communities in a manner that reflects their
health needs.
502
Likewise, the Shared Savings Program aims to design a health equity benchmark
adjustment that achieves those same goals while aligning the program’s benchmarking policies
and health equity initiatives. The HEBA proposal is informed by CMS’ initial experience with
the ACO REACH Model, which includes a HEBA, that has been associated with increased
participation in ACOs by safety net providers.
503
Increasing access to providers participating in
ACOs in rural and other underserved areas remains a priority for CMS to help address inequities
in ACO participation and grow accountable care.
(2) Proposed Revisions
Relying on our authority under section 1899(d)(1)(B)(ii) of the Act, we are proposing a
HEBA applicable to ACOs in agreement periods beginning on January 1, 2025, and in
subsequent years. The proposed HEBA would offer a third method of upwardly adjusting an
ACO’s historical benchmark, in addition to the existing regional adjustment and prior savings
adjustment. This upward adjustment to the historical benchmark is designed to benefit ACOs
serving larger proportions of beneficiaries from underserved communities and receiving lower
regional adjustments (§ 425.656) or lower prior savings adjustments (§ 425.658) or receiving
neither adjustment. Under proposed § 425.652(a)(8)(ii), an ACO would receive the highest of the
502
Centers for Medicare & Medicaid Services, “ACO Realizing Equity, Access, and Community Health (REACH)
Model Finance-Focused Frequently Asked Questions” (Version 1, April 2022), available at
https://www.cms.gov/priorities/innovation/media/document/aco-reach-finfaqs.
503
See Rawal P, Seyoum S, Fowler E. “Advancing Health Equity Through Value-Based Care: CMS Innovation
Center Update”, Health Affairs Forefront, June 4, 2024. DOI: 10.1377/forefront.20240603.385559. Available at
https://www.healthaffairs.org/content/forefront/advancing-health-equity-through-value-based-care-cms-innovation-
center-update.
positive adjustments for which it is eligible, either the regional adjustment, prior savings
adjustment, or health equity benchmark adjustment. If an ACO is not eligible to receive a prior
savings adjustment or a HEBA, and the regional adjustment, expressed as a single value, is
negative or zero, then the ACO would not receive an adjustment to its benchmark.
By increasing the likelihood that an ACO would earn shared savings and by potentially
increasing the amount of shared savings earned, the HEBA is meant to provide a greater
financial incentive for ACOs to serve more beneficiaries from underserved communities and to
encourage ACOs already serving higher proportions of beneficiaries from underserved
communities to enter and remain in the Shared Savings Program. Practices that serve large
proportions of beneficiaries who are members of underserved communities that may otherwise
see financial risk in joining the program may be incentivized to form an ACO and join the
program with a health equity benchmark adjustment policy in place. In addition, currently
participating ACOs that may otherwise see risk in attracting additional beneficiaries from
underserved communities to their ACOs may be incentivized to do so with a health equity
benchmark adjustment policy in place. We note that, if finalized, the proposed prepaid shared
savings option (see section III.G.5 of this proposed rule) would operate synergistically with the
proposed HEBA, in that ACOs that have been successful in earning shared savings while serving
larger proportions of beneficiaries from underserved communities would in subsequent years
have additional capabilities through prepaid shared savings to address the unmet health-related
social needs of the beneficiaries they serve and may have higher benchmarks due to the HEBA.
We propose to calculate the HEBA as the multiplicative product of the HEBA scaler and
the proportion of the ACO's assigned beneficiaries who are enrolled in the Medicare Part D LIS
or dually eligible for Medicare and Medicaid. We propose to calculate the HEBA scaler as a
measure of the difference between the following two per-capita dollar values:
● 5 percent of national per capita expenditures for Parts A and B services under the
original Medicare FFS program in BY3 for assignable beneficiaries identified for the 12-month
calendar year corresponding to BY3 using data from the CMS Office of the Actuary, expressed
as a single value by taking a person year weighted average of the Medicare enrollment type-
specific values: ESRD, disabled, aged/dually eligible for Medicare and Medicaid, and aged/non-
dually eligible for Medicare and Medicaid, and
● the higher of the regional adjustment expressed as a single value, the prior savings
adjustment, or no adjustment, in the case where the regional adjustment is negative and the ACO
is not eligible for the prior savings adjustment.
This approach would ensure that the value of the HEBA itself cannot exceed 5 percent of
national assignable per capita expenditures expressed as a single value using the ACO’s BY3
enrollment proportions, similar to the cap applied to the regional adjustment under §
425.656(c)(3) and the cap applied to the prior savings adjustment under § 425.658(c)(1)(ii).
For this proposed health equity benchmark adjustment, we propose to identify
beneficiaries from underserved communities as those who are enrolled in the Medicare Part D
LIS or dually eligible for Medicare and Medicaid. Furthermore, we propose to determine the
proportion of the ACO's assigned beneficiaries who are enrolled in the Medicare Part D LIS or
dually eligible for Medicare and Medicaid using the ACO’s performance year assigned
population. Because a higher proportion of assigned beneficiaries who are enrolled in Medicare
Part D LIS or dually eligible would result in a higher HEBA, using the performance year
assigned population is expected to incentivize ACOs to provide coordinated care to beneficiaries
who are members of underserved communities while accounting for changes in the ACO’s
population over the agreement period.
We propose to provide ACOs with a preliminary calculation of the HEBA near the start
of their agreement period when final historical benchmarks are determined, using the ACO’s
BY3 assigned population in this preliminary calculation of the proportion of the ACO's assigned
beneficiaries who are enrolled in the Medicare Part D LIS or dually eligible for Medicare and
Medicaid. We would then update the calculation when the ACO's historical benchmark is

updated at the time of financial reconciliation for the performance year to reflect the ACO’s
performance year-assigned population in the calculation of the proportion of the ACO's assigned
beneficiaries who are enrolled in the Medicare Part D LIS or dually eligible for Medicare and
Medicaid.
We propose that ACOs with a proportion of assigned beneficiaries who are enrolled in
the Medicare Part D LIS or dually eligible for Medicare and Medicaid of less than 20 percent
would be ineligible for a HEBA.
504
We believe that imposing this threshold of 20 percent would
reinforce that the HEBA is intended for ACOs serving higher proportions of beneficiaries who
are members of underserved communities. Based on data from 2022, the average proportion of
ACO-assigned beneficiaries enrolled in the Medicare Part D LIS or dually eligible for Medicare
and Medicaid was roughly 15 percent. Thus, ACOs meeting the threshold of 20 percent are
serving a larger-than-average proportion of beneficiaries from underserved communities. Absent
such a threshold, an ACO with a lower than average regional adjustment or prior savings
adjustment (and therefore a larger HEBA scaler) that is providing care for relatively few
beneficiaries from underserved communities may receive a sizable HEBA, which would reward
the ACO despite it not serving a significant proportion of beneficiaries from underserved
communities. This would not support the purpose of the HEBA, which is to provide a greater
financial incentive for ACOs to serve more beneficiaries from communities and encourage
practices already serving higher proportions of beneficiaries from underserved communities to
enter and/or remain in the Shared Savings Program.
Under this proposed approach, simulation analysis based on 456 ACOs using historical
benchmark data from 2023 indicates that 20 ACOs would receive a HEBA greater than either the
prior savings adjustment or regional adjustment. With the HEBA applied, the average increase to
504
The health equity adjustment to an ACO’s MIPS quality performance category score (87 FR 69838 through
69857 and 88 FR 79114 through 79117) has established a similar 20 percent threshold. ACOs with an underserved
multiplier of less than 20 percent are not eligible to receive a health equity adjustment (§ 425.512(b)).

historical benchmarks among these 20 ACOs would be $230 per capita, which corresponds to an
increase of 1.57 percent to their historical benchmarks on average.
Tables 38 through 40 present hypothetical examples to demonstrate how the HEBA would work
in practice.
TABLE 38: ACO with a HEBA Greater Than the Regional Adjustment and Prior Savings
Adjustment
Calculation Step
Description of Calculation and Example
Step 1: Calculate Proportion
of Assigned Beneficiaries Who
Are Enrolled in Medicare Part
D LIS or Dually Eligible for
Medicare and Medicaid
Proportion of PY-assigned beneficiaries enrolled in Medicare Part D LIS or
dually eligible for Medicare and Medicaid: 0.60
Step 2: Calculate HEBA Scaler
5 percent of the national per capita expenditures for assignable beneficiaries
in BY3 expressed as a single value: $600
Prior savings adjustment: $200
Regional adjustment expressed as single value: $100
Difference between 5 percent of the national per capita expenditures for
assignable beneficiaries in BY3 expressed as a single value and the higher of
prior savings adjustment and regional adjustment expressed as a single value:
$600 – higher of $200 or $100 = $400
Step 3: Calculate HEBA
Product of the proportion of assigned beneficiaries who are enrolled in the
Medicare Part D LIS or dually eligible for Medicare and Medicaid and the
HEBA Scaler:
0.60 x $400 = $240
Step 4: Determine Final
Adjustment to Benchmark
Highest of regional adjustment expressed as a single value, prior savings
adjustment, or HEBA:
Highest of $200, $100, or $240 = $240
Per capita historical benchmark expenditures by enrollment type after
adjustment:
ESRD: $92,000 + $240 = $92,240
Disabled: $13,000 + $240 = $13,240
Aged/dual: $19,000 + $240 = $19,240
Aged/non-dual: $10,000 + $240 = $10,240

TABLE 39: ACO with a HEBA Less Than the Regional Adjustment and Prior Savings
Adjustment
Calculation Step
Description of Calculation and Example
Step 1: Calculate
Proportion of
Assigned Beneficiaries
Who Are Enrolled in
Medicare Part D LIS
or Dually Eligible for
Medicare and
Medicaid
Proportion of PY-assigned beneficiaries enrolled in Medicare Part D LIS or dually
eligible for Medicare and Medicaid: 0.25
Step 2: Calculate
HEBA Scaler
5 percent of the national per capita expenditures for assignable beneficiaries in BY3
expressed as a single value: $600
Prior savings adjustment: $200
Regional adjustment expressed as single value: $300
Difference between 5 percent of the national per capita expenditures for assignable
beneficiaries in BY3 expressed as a single value and the higher of prior savings
adjustment and regional adjustment expressed as a single value:
$600 – higher of $200 or $300 = $300
Step 3: Calculate
HEBA
Product of the proportion of assigned beneficiaries who are enrolled in the Medicare
Part D LIS or dually eligible for Medicare and Medicaid and the HEBA Scaler:
0.25 x $300 = $75
Step 4: Determine
Final Adjustment to
Benchmark
Highest of regional adjustment expressed as a single value, prior savings adjustment,
or HEBA:
Highest of $200, $300, or $75 = $300
Per capita historical benchmark expenditures by enrollment type after adjustment:
ESRD: $92,000 + $300 = $92,300
Disabled: $13,000 + $300 = $13,300
Aged/dual: $19,000 + $300 = $19,300
Aged/non-dual: $10,000 + $300 = $10,300

TABLE 40: ACO Ineligible for the HEBA
Calculation Step
Description of Calculation and Example
Step 1: Calculate Proportion
of Assigned Beneficiaries
Who Are Enrolled in the
Medicare Part D LIS or
Dually Eligible for Medicare
and Medicaid
Proportion of PY-assigned beneficiaries enrolled in Medicare Part D LIS or
dually eligible for Medicare and Medicaid: 0.10
Step 2: Calculate HEBA
Scaler
5 percent of the national per capita expenditures for assignable beneficiaries in
BY3 expressed as a single value: $600
Prior savings adjustment: $200
Regional adjustment expressed as single value: $300
Difference between 5 percent of the national per capita expenditures for
assignable beneficiaries in BY3 expressed as a single value and the higher of
prior savings adjustment and regional adjustment expressed as a single value:
$600 – higher of $200 or $300 = $300
Step 3: Calculate HEBA
Step not applicable as ACO has a proportion of assigned beneficiaries who are
enrolled in the Medicare Part D LIS or dually eligible for Medicare and
Medicaid less than 0.20 and is ineligible for a HEBA as a result.
Step 4: Determine Final
Adjustment to Benchmark
Higher of regional adjustment expressed as a single value or prior savings
adjustment:
Higher of $200 or $300 = $300
Per capita historical benchmark expenditures by enrollment type after
adjustment:
ESRD: $92,000 + $300 = $92,300
Disabled: $13,000 + $300 = $13,300
Aged/dual: $19,000 + $300 = $19,300
Aged/non-dual: $10,000 + $300 = $10,300
We propose to implement the changes described in this section through revisions to
§ 425.652 and the addition of § 425.662. Specifically, within § 425.652, which sets forth the
methodology for establishing, adjusting, and updating the benchmark for agreement periods
beginning on January 1, 2024, and in subsequent years, we propose revisions to § 425.652(a)(8).
As proposed, this revised provision would describe how we would determine and apply the
adjustment to an ACO’s benchmark, if any, based on a comparison of the ACO’s regional
adjustment expressed as a single value, prior savings adjustment, and the proposed health equity
benchmark adjustment. Furthermore, we propose to amend § 425.652 by redesignating
paragraphs (a)(9)(v) and (vi) as paragraphs (a)(9)(vi) and (vii), respectively, and to specify in a
new paragraph (a)(9)(v) the adjustments made to the health equity benchmark adjustment for the
first performance year during the term of the agreement period and in the second and each
subsequent performance year during the term of the ACO’s agreement period, if applicable. We
also propose conforming changes in newly redesignated § 425.652(a)(9)(vi), specifying that
CMS redetermines the adjustment to benchmark in accordance with § 425.652(a)(8), to list the
HEBA along with the regional adjustment and prior savings adjustment. In the proposed new
section of the regulation at § 425.662 we describe the calculation of the HEBA. We also propose
to make conforming changes to § 425.658(d), which describes the applicability of the prior
savings adjustment, to include consideration of the HEBA in addition to the regional adjustment,
in determining the adjustment (if any) that would be applied to the ACO’s benchmark. We seek
comment on these proposals.
In combination with the proportion of ACO-assigned beneficiaries who are enrolled in
the Medicare Part D LIS or are dually eligible for Medicare and Medicaid, we are seeking
comment on the use of the Area Deprivation Index (ADI) to identify beneficiaries from
underserved communities for purposes of determining eligibility for and the amount of any
health equity benchmark adjustment. For example, similar to how the ADI is used in the
underserved multiplier as part of the calculation of the health equity adjustment to an ACO’s
MIPS Quality performance category score (87 FR 69838 through 69857 and 88 FR 79114
through 79117), we are considering taking the higher of either the proportion of the ACO’s
assigned beneficiaries residing in a census block group with an ADI national percentile rank of at
least 85 or the proportion of the ACO's assigned beneficiaries who are enrolled in the Medicare
Part D LIS or dually eligible for Medicare and Medicaid to determine eligibility for and the
amount of any health equity benchmark adjustment. CMS will explore how best to incorporate
geographic parameters into Shared Savings Program benchmark adjustments, informed by the
current use of the ADI in other health equity provisions of the Shared Savings Program. CMS
will also consider learnings from the Innovation Center’s ACO REACH Model, which is testing
the use of the ADI as a component of the model’s HEBA. By considering the ADI in addition to
the proportion of ACO-assigned beneficiaries who are enrolled in the Medicare Part D LIS or are

dually eligible for Medicare and Medicaid, the HEBA would more closely align with existing
Shared Savings Program policies to advance health equity, such as the health equity adjustment
to an ACO’s MIPS Quality performance category score (87 FR 69838 through 69857 and 88 FR
79114 through 79117) and the calculation of the amount of quarterly advance investment
payments made available to eligible new, low revenue ACOs (87 FR 69782 through 69805 and
88 FR 79208 through 79216).
Additionally, recent analyses have found that the ADI weights 2 variables (median home
value and median income) higher relative to the weights associated with the other 15 variables in
the index, which may have limited contributions in determining the ADI. In many indexes,
variables are standardized to the same range for ease of comparison, prior to incorporation into
the index. The ADI does not standardize its variables; median home value and median income
are measured on their local area dollar-value scales, which are larger than the scales on which the
other variables are measured. Some researchers have reported that, without standardization, the
ADI overemphasizes the 2 variables (median home value and median income), a finding that
may underscore the importance of using standardized values.
505,506,507
We seek comment on
considering the ADI for purposes of determining eligibility for and the amount of any health
equity benchmark adjustment, and related factors including the calculation of the ADI.
c. Reopening ACO Payment Determinations
505
See Hannan, EL, et al. The Neighborhood Atlas Area Deprivation Index For Measuring Socioeconomic Status:
An Overemphasis On Home Value. Health Affairs, vol. 42, no. 5 (May 2023): 702-709. Available at
https://www.healthaffairs.org/doi/full/10.1377/hlthaff.2022.01406.
506
See Rehkopf, DH, and Phillips, RL, Jr. The Neighborhood Atlas Area Deprivation Index And Recommendations
For Area-Based Deprivation Measures. Health Affairs, vol. 42, no. 5 (May 2023): 710-711. Available at
https://www.healthaffairs.org/doi/full/10.1377/hlthaff.2023.00282.
507
See Petterson, S. Deciphering the Neighborhood Atlas Area Deprivation Index: the consequences of not
standardizing. Health Affairs Scholar, volume 1, issue no. 5 (November 2023), qxad063; Available at
https://academic.oup.com/healthaffairsscholar/article/1/5/qxad063/7342005.

(1) Background
(a) Statutory Background on Shared Savings Program Financial Calculations
Section 1899(d)(1)(B)(ii) of the Act provides for the calculation and update of ACO
benchmarks under the Shared Savings Program. This provision specifies that the Secretary shall
estimate a benchmark for each agreement period for each ACO using the most recent available 3
years of per beneficiary expenditures for Parts A and B services for Medicare FFS beneficiaries
assigned to the ACO. Such benchmark shall be adjusted for beneficiary characteristics and such
other factors as the Secretary determines appropriate and updated by the projected absolute
amount of growth in national per capita expenditures for Parts A and B services under the
original Medicare FFS program, as estimated by the Secretary. Further, an ACO’s benchmark
must be reset at the start of each agreement period. Section 1899(d)(1)(B)(i) of the Act specifies
that, in each year of the agreement period, an ACO is eligible to receive payment for shared
savings only if the estimated average per capita Medicare expenditures under the ACO for
Medicare FFS beneficiaries for Parts A and B services, adjusted for beneficiary characteristics, is
at least the percent specified by the Secretary below the applicable benchmark under section
1899(d)(1)(B)(ii) of the Act.
Section 1899(i)(3) of the Act authorizes the Secretary to use other payment models, if the
Secretary determines it is appropriate, and if the Secretary determines that doing so would
improve the quality and efficiency of items and services furnished under Title XVIII and the
alternative methodology would result in program expenditures equal to or lower than those that
would result under the statutory payment model. As discussed in earlier rulemaking, we have
used the authority under section 1899(i)(3) of the Act to adopt alternative policies to the
provisions of section 1899(d)(1)(B) of the Act for updating the historical benchmark
508
and
508
Such as using only assignable beneficiaries instead of all Medicare FFS beneficiaries in calculating the
benchmark update based on national FFS expenditures (81 FR 37985 through 37989), calculating the benchmark
update using factors based on regional FFS expenditures (81 FR 37977 through 37981), calculating the benchmark
update using a blend of national and regional expenditure growth rates (83 FR 68027 through 68030), removing
payment amounts for episodes of care for treatment of COVID-19 from expenditures used to calculate the

calculating performance year expenditures,
509
among other factors.
510
We have also used our
authority under section 1899(i)(3) of the Act to establish the Shared Savings Program's two-sided
payment models,
511
and to mitigate shared losses owed by ACOs affected by extreme and
uncontrollable circumstances during PY 2017 and subsequent performance years.
512
(b) Background on Shared Savings Program Reopening Policy and Financial Calculation
Methodology
Under § 425.315(a)(1), if CMS determines that the amount of shared savings due to the
ACO or the amount of shared losses owed by the ACO has been calculated in error CMS may
reopen the initial determination or a final agency determination under subpart I and issue a
revised initial determination: (i) at any time in the case of fraud or similar fault as defined in
§ 405.902;
513
or (ii) not later than 4 years after the date of the notification to the ACO of the
initial determination of savings or losses for the relevant performance year, for good cause.
In accordance with § 425.315(a)(2), good cause may be established when (i) there is new
and material evidence that was not available or known at the time of the payment determination
and may result in a different conclusion, or (ii) the evidence that was considered in making the
benchmark update (85 FR 27577 through 27582), and calculating the benchmark update using an Accountable Care
Prospective Trend / national-regional three-way blended update factor (87 FR 69881 through 69898).
509
Such as excluding indirect medical education and disproportionate share hospital payments from ACO
performance year expenditures (76 FR 67920 through 67922), determining shared savings and shared losses for the
6-month performance years (or performance period) in 2019 using expenditures for the entire CY 2019 and then
pro-rating these amounts to reflect the shorter performance year or performance period (83 FR 59949 through
59951, 83 FR 67950 through 67956), removing payment amounts for episodes of care for treatment of COVID-19
from performance year expenditures (85 FR 27577 through 27582), and the exclusion of the supplemental payment
for IHS/Tribal hospitals and Puerto Rico hospitals from performance year expenditures (87 FR 69954 through
69956).
510
Such as allowing for advance investment payments (87 FR 69782 through 69805), and expansion of the criteria
for certain low revenue ACOs participating in the BASIC track to qualify for shared savings in the event the ACO
does not meet the MSR as required under section 1899(d)(1)(B)(i) of the Act (87 FR 69946 through 69952).
511
See earlier rulemaking establishing two-sided models: Track 2 (76 FR 67904 through 67909), Track 3
(subsequently renamed the ENHANCED track) (80 FR 32771 and 32772), and the BASIC track (83 FR 67834
through 67841). We also used our authority under section 1899(i)(3) of the Act to remove payment amounts for
episodes of care for treatment of COVID-19 from ACO participants’ Medicare FFS revenue used to determine the
loss sharing limit in the two-sided models of the BASIC track (85 FR 27577 through 27582).
512
See earlier rulemaking establishing policies for mitigating shared losses owed by ACOs affected by extreme and
uncontrollable circumstances (82 FR 60916 and 60917, 83 FR 59974 through 59977).
513
As defined in § 405.902, “similar fault” means to obtain, retain, convert, seek, or receive Medicare funds to
which a person knows or should reasonably be expected to know that he or she or another for whose benefit
Medicare funds are obtained, retained, converted, sought, or received is not legally entitled. This includes, but is not
limited to, a failure to demonstrate that he or she filed a proper claim as defined in 42 CFR part 411.
payment determination clearly shows on its face that an obvious error was made at the time of
the payment determination. Section 425.315(a)(3) specifies that a change of legal interpretation
or policy by CMS in a regulation, CMS ruling or CMS general instruction, whether made in
response to judicial precedent or otherwise, is not a basis for reopening a payment determination
under the Shared Savings Program regulations. CMS has sole discretion to determine whether
good cause exists for reopening a payment determination (§ 425.315(a)(4)).
We first adopted a reopening policy in the November 2011 final rule, where we finalized
at § 425.314(a)(4) a provision reserving the right for CMS to reopen the initial determination and
issue a revised initial determination, if as a result of any inspection, evaluation, or audit, it is
determined that the amount of shared savings due to the ACO or amount of shared losses owed
by the ACO has been calculated in error (see 76 FR 67957 through 67958, and 67982). In the
June 2016 final rule, we revised the Shared Savings Program regulations, including to remove
the provision in § 425.314(a)(4), and further specify the reopening policy in a new section of the
regulation at § 425.315 (81 FR 37997 through 38002, and 38013 through 38014). We
subsequently revised § 425.315 to apply the policies on reopening determinations to payment
determinations for a 6-month performance year or 6-month performance period during CY 2019
(refer to the November 2018 final rule, 83 FR 59958 and 60092, and the December 2018 final
rule, 83 FR 67955 through 67967), and to ACOs participating in the BASIC track (refer to the
December 2018 final rule, 83 FR 67842 and 68068). In the CY 2023 PFS final rule, we clarified
the circumstances in which CMS would exercise discretion to reopen the initial determination of
an ACO’s financial performance for good cause to correct errors in the determination of MIPS
Quality performance category scores that affect the determination of whether an ACO is eligible
for shared savings, the amount of shared savings due to the ACO, or the amount of shared losses
owed by the ACO (see 87 FR 69868 through 69869).
Most recently, in the CY 2024 PFS final rule, we finalized an approach to recalculating
the prior savings adjustment for changes in values used in benchmark calculations due to
compliance action taken to address avoidance of at-risk beneficiaries, or as a result of the
issuance of a revised initial determination of financial performance for a previous performance
year following a reopening of ACO shared savings and shared losses calculations (88 FR 79195
through 79200). In the CY 2024 PFS final rule, we also discussed a proposed timing cutoff such
that changes to savings or losses for a benchmark year that were finalized after notification to the
ACO of the initial determination of shared savings or shared losses for a given performance year
would be reflected in the adjusted benchmark applied to any subsequent performance year during
the relevant agreement period but would not be retroactively applied to completed performance
years in the agreement period (88 FR 79198 through 79200). We stated that we believed it would
be appropriate to consider new information that could impact the prior savings adjustment up to
the point at which an ACO receives its initial determination. However, we also noted that we
would continue to consider the complexities surrounding reopening initial determinations for
multiple prior performance years throughout the program’s benchmarking and financial
reconciliation methodologies and may address this issue in future rulemaking (88 FR 79199). We
refer readers to these discussions in past rulemaking for additional details.
In our earlier rulemaking, we did not discuss the specific methodology that would be
employed for recalculating an ACO’s shared savings or shared losses in the event of a reopening
in order to issue a revised initial determination. As additional background, in the following
discussion, we summarize the general approach to identification and use of payment amounts
from Medicare FFS Parts A and B FFS claims and certain other payment amounts in Shared
Savings Program calculations.
Under the Shared Savings Program, providers and suppliers continue to bill for services
furnished to Medicare beneficiaries and receive FFS payments under traditional Medicare. CMS
uses payment amounts for Parts A and B FFS claims for calculating benchmark and performance
year expenditures and determining benchmark update factors as specified in the Shared Savings
Program regulations in subpart G. These operations typically require the determination of

expenditures for Parts A and B services under the original Medicare FFS program for a specified
population of Medicare FFS beneficiaries or the Medicare Parts A and B FFS revenue of ACO
participants. The Medicare FFS beneficiary population for which expenditures are determined
may differ depending on the specific program operation being performed and may reflect
expenditures for the ACO's assigned beneficiaries, assignable beneficiaries, or all Medicare FFS
beneficiaries. The applicable Medicare FFS beneficiary population is specified in the regulations
governing each program operation.
In calculating expenditures for Medicare FFS beneficiaries used in Shared Savings
Program calculations, CMS uses payment amounts included on Parts A and B FFS claims with
dates of service in the relevant benchmark or performance year, allowing for a 3-month claims
run out, as follows: claim payment amounts identified for inpatient, Skilled Nursing Facility
(SNF), outpatient, Home Health Agency (HHA), and hospice claims at any provider; and line
item payment amounts identified for carrier (including physician/supplier Part B) and Durable
Medical Equipment, Prosthetics, Orthotics & Supplies (DMEPOS) claims. For both Parts A and
B claims, CMS excludes payments on denied claims or line items from the calculation, for
claims or line items with dates of service within the relevant benchmark year or performance
year, processed before the end of the 3-month claims run out period. In calculating expenditure
amounts for Medicare FFS beneficiaries under the Shared Savings Program, CMS makes certain
adjustments,
514
which if applicable, exclude indirect medical education (IME) and
disproportionate share hospital (DSH) payments, and the supplemental payment for IHS/Tribal
hospitals and Puerto Rico hospitals, and take into consideration individually beneficiary
identifiable final payments made under a demonstration, pilot or time limited program. We also
account for certain population-based payments or other similarly structured payments made
under other Medicare shared savings initiatives, specifically the Pioneer ACO Model, Next
514
The Shared Savings Program’s financial models and benchmarking policies, among other program policies, have
changed over time as described in earlier rulemaking (refer to section III.G.1.b. of this proposed rule), and as
outlined in the provisions of subpart G.

Generation ACO Model, Vermont All-Payer ACO Model, and ACO REACH Model (as
applicable). Population-based payments are a per-beneficiary per month payment amount
intended to replace some or all of the FFS payments with prospective monthly payment.
515
The Shared Savings Program’s existing financial methodology does not fully account for
actions taken to protect the integrity of the Medicare program, or address the impact of improper
payments, including improper payments resulting from fraud or similar fault on program
calculations. For instance, demanded overpayment determinations resulting in adjusted claim or
line item payment amounts after the 3-month claims run out period, or aggregate amounts that
are not linked to specific claims or line items, are not accounted for in Shared Savings Program
expenditure calculations. Additionally, under the existing financial methodology for the Shared
Savings Program, we lack a means to account for improper payment amounts identified in a
settlement agreement between a provider or supplier and the Government or a court’s judgment,
including pursuant to conduct by individuals or entities performing functions or services related
to an ACO’s activities. Under the proposed approach described in section III.G.7.c.(2).(c) of this
proposed rule, the term “improper payment” for purposes of the Shared Savings Program would
include an amount associated with a demanded overpayment determination and certain amounts
identified in a settlement agreement or judgment that have the potential to impact program
financial calculations. Since January 2023, we have evaluated several cases where such improper
payments may have impacted one or more reconciled performance years for an ACO under the
Shared Savings Program, including cases where ACOs reported concerns about alleged fraud or
similar fault to CMS. It is thus timely and appropriate to undertake notice and comment
rulemaking to establish a calculation methodology to account for the impact of improper
payments in recalculating expenditures and payment amounts used in Shared Savings Program
515
See for example, Medicare Shared Savings Program, Shared Savings and Losses, Assignment and Quality
Performance Standard Methodology Specifications (Version 11, January 2023), available at
https://www.cms.gov/files/document/medicare-shared-savings-program-shared-savings-and-losses-and-assignment-
methodology-specifications.pdf-2 (refer to Section 3.1 Calculating ACO-Assigned Beneficiary Expenditures).
financial calculations, upon reopening a payment determination pursuant to § 425.315(a); to
describe factors that we may consider in exercising our discretion to reopen an ACO’s payment
determination under which we apply the proposed methodology to recalculate the ACO’s
financial performance; and to propose to establish a process by which an ACO could request a
reopening of an initial determination of shared savings or shared losses. Our experience
reviewing several cases supported the development of our proposed revisions to Shared Savings
Program policies.
(2) Proposed Revisions
This section of this proposed rule includes a proposed change to the provision specifying
CMS’ discretion to reopen payment determinations under § 425.315(a)(4) (as described in
section III.G.7.c.(2).(a) of this proposed rule). We discuss and seek comment on the
circumstances in which we would exercise our discretion to reopen a payment determination and
issue a revised initial determination to account for the impact of identified improper payments on
Shared Savings Program calculations (as described in section III.G.7.c.(2).(b) of this proposed
rule). We propose modifications to the Shared Savings Program regulations to specify a
calculation methodology to account for the impact of identified improper payments in
recalculating expenditures and payment amounts used in Shared Savings Program financial
calculations, upon reopening a payment determination pursuant to § 425.315(a) (as described in
section III.G.7.c.(2).(c) of this proposed rule). We also propose certain adjustments to Shared
Savings Program benchmark calculations to account for the impact of identified improper
payments, in the event a performance year for which we issue a revised initial determination
becomes a benchmark year of an ACO’s current agreement period, and when CMS has not yet
issued an initial determination for a performance year of the ACO’s current agreement period (as
described in section III.G.7.c.(2).(d) of this proposed rule). Lastly, we propose a process for
ACOs to request that CMS reopen a payment determination (as described in section
III.G.7.c.(2).(e) of this proposed rule), and briefly discuss the role of ACOs in preventing and
reporting Medicare fraud (as described in section III.G.7.c.(2).(f) of this proposed rule). Our
specific proposals are discussed in detail in the following sections.
We propose that the policy changes discussed in this section of this proposed rule would
be effective January 1, 2025, unless specified otherwise. Should the proposed policies be
finalized, the policies would apply to reopening requests made on or after January 1, 2025. As
described in section III.G.7.c.(2).(e) of this proposed rule, we propose to establish a process by
which an ACO may request a reopening review. If finalized, we anticipate continuing to evaluate
previously received reopening requests for performance years for which initial determinations
were issued prior to January 1, 2025, consistent with the timeframes specified under
§ 425.315(a)(1). We anticipate consistently applying the recalculation methodology to account
for the impact of improper payments, described in section III.G.7.c.(2).(c) of this proposed rule,
if finalized, in recalculating expenditures and payment amounts used in Shared Savings Program
financial calculations, upon reopening a payment determination pursuant to § 425.315(a).
(a) Proposed Change to Provision Specifying CMS’ Discretion to Reopen Payment
Determinations
In earlier rulemaking we explained that CMS would have discretion to reopen a payment
determination for fraud or similar fault, or good cause, as reflected in the provisions in
§ 425.315(a)(1) and (4). The latter provision expressly provides that CMS has sole discretion to
determine whether good cause exists for reopening a payment determination. In the June 2016
final rule, in restating the discussion of the proposal from the February 2016 proposed rule, we
explained that CMS would have discretion to reopen a payment determination at any time in the
case of fraud or ‘‘similar fault,’’ as defined in § 405.902 (81 FR 37998).
We continue to believe that it is important to maintain CMS’ sole discretion in
determining whether to reopen a payment determination. We also believe it is important to
preserve CMS’ flexibility in determining whether reopening is warranted to address the impact
of fraud or similar fault on Shared Savings Program calculations, in particular given the potential
for various actions to be taken by CMS, law enforcement agencies and courts in response to
fraud or similar fault. Thus, we are proposing revisions to § 425.315(a)(4) to make clear CMS’
discretion to determine whether to reopen a payment determination applies in the case of fraud or
similar fault, as well as to determining whether good cause exists to reopen a payment
determination.
(b) Considerations for Reopening a Payment Determination to Account for Improper Payments
In the discussion that follows we describe factors CMS may consider to inform our
decision of whether to reopen an initial determination of an ACO’s financial performance
pursuant to § 425.315(a)(1)(i) or (ii) to account for the impact of improper payments that affect
the determination of whether an ACO is eligible for shared savings or liable for shared losses,
and the amount of shared savings due to the ACO or the amount of shared losses owed by the
ACO. We welcome comments on these considerations. We also anticipate revisiting these
considerations as we gain experience with processing ACO reopening requests as described in
section III.G.7.c.(2).(e) of this proposed rule, reopening payment determinations and applying
the calculation methodology described in section III.G.7.c.(2).(c) of this proposed rule and
applying the benchmark adjustment described in section III.G.7.c.(2).(d) of this proposed rule. If
appropriate, we may revisit these considerations for exercising our discretion to reopen payment
determinations in future notice and comment rulemaking.
As an initial matter, the Shared Savings Program would need to identify improper
payments that have the potential to impact program financial calculations. The Shared Savings
Program depends on input from the CMS Center for Program Integrity (CPI) and law
enforcement agencies (including the Department of Justice) to identify and quantify improper
payments potentially impacting expenditures used in program calculations that are not otherwise
accounted for in Shared Savings Program expenditure calculations as described in this section of
this proposed rule. This could include: (1) certain demanded overpayment determinations, such
as demanded overpayment amounts that result in adjusted claim or line item payment amounts

associated with dates of service during a performance year or benchmark year, where the
adjustment occurs after the 3-month claims run out period, and demanded extrapolated
overpayment amounts which are aggregate amounts that are not linked to specific claims or line
items and are not currently accounted for in Shared Savings Program expenditures;
516
and (2)
improper payments resulting from conduct by individuals or entities performing functions or
services related to an ACO’s activities as identified in certain settlement agreements or
judgments. Refer to section III.G.7.c.(2).(c) of this proposed rule for discussion of considerations
for identifying these amounts. Further, as discussed in greater detail in section III.G.7.c.(2).(e) of
this proposed rule, ACOs can play an important role in identifying for CMS improper payments
that may impact Shared Savings Program calculations. ACO reopening requests submitted to
CMS may be another means by which the Shared Savings Program becomes aware of improper
payments impacting ACO financial calculations; however, CMS would retain discretion over
whether to reopen payment determinations after reviewing information provided in such
requests.
Second, we anticipate needing to perform an initial analysis of whether the improper
payments would warrant reopening the ACO’s payment determination. This analysis may
include a number of factors, such as whether the improper payments meet the requirements for
reopening for fraud or similar fault in accordance with § 425.315(a)(1)(i), or for good cause in
accordance with § 425.315(a)(1)(ii) and (a)(2). A variety of circumstances could lead CMS, law
enforcement agencies or courts to determine whether good cause exists or whether fraud or
similar fault has occurred. The timelines associated with the related investigations, and the
potential for various actions to be taken in response, can make it challenging to identify a one-
size-fits-all approach to addressing the impact of improper payments on Shared Savings Program
516
For additional information on overpayment procedures and overpayment estimation, see, for example, Medicare
Program Integrity Manual, Chapter 8 – Administrative Actions and Sanctions and Statistical Sampling for
Overpayment Estimation, available at https://www.cms.gov/regulations-and-
guidance/guidance/manuals/downloads/pim83c08.pdf.

calculations. We note that once we are notified of potential improper payments impacting Shared
Savings Program calculations, it may take months or years to determine the actual amount of any
improper payments impacting an ACO’s payment determination, particularly if we are awaiting
the conclusion of program integrity and law enforcement investigations, among other possible
determinations about the related conduct of providers or suppliers. Additionally, administrative
action and judicial action leading to the identification of improper payments may be subject to
appeal, and ultimately the amount of the improper payments may be redetermined or otherwise
amended.
517
It would further protract the timeline for considering use of improper payments in
recalculating ACO financial performance results to await the outcome of any appeal of an
improper payment.
Since there could be a variety of reasons for which CMS seeks to recoup an overpayment
amount from a provider or supplier, there are many possible circumstances that could warrant
reopening under § 425.315. As an example, we may consider a combination of factors in
evaluating whether demanded overpayment determinations would be the basis for reopening for
fraud or similar fault under § 425.315(a)(1)(i).
518
For instance, we may consider whether there is
“reliable evidence” (as defined according to § 405.902, which means evidence that is relevant,
credible, and material) of similar fault to warrant reopening a Shared Savings Program payment
determination.
519
For purposes of the Shared Savings Program’s reopening policy, we may find
517
For instance, a provider receiving an initial demand letter for an overpayment may appeal the overpayment by
requesting a redetermination, among other actions. See for example, CMS, MLN Fact Sheet, “Medicare
Overpayments” (MLN006379 October 2023), available at https://www.cms.gov/outreach-and-education/medicare-
learning-network-mln/mlnproducts/downloads/overpaymentbrochure508-09.pdf. The Medicare Parts A and B
appeals process includes multiple levels of appeal. See for example, CMS, MLN Booklet, “Medicare Parts A & B
Appeals Process” (MLN006562 November 2023), available at https://www.cms.gov/files/document/mln006562-
medicare-parts-b-appeals-process.pdf.
518
While this example presumes reopening for fraud or similar fault, there may be additional considerations and
complexities around reopening for good cause.
519
This approach may continue to maintain a degree of alignment between reopening policies under the Shared
Savings Program and other Medicare policies. In the February 2016 proposed rule, in which we proposed amending
the Shared Savings Program’s reopening policy, we referred to the longstanding policy in the Medicare program that
a determination may be reopened at any time if it was procured by fraud or similar fault, and as an example referred
to 42 CFR 405.980(b)(3) (see 81 FR 5855). In accordance with § 405.980(b)(3), a contractor may reopen an initial
determination or redetermination on its own motion at any time if there exists reliable evidence as defined in
§ 405.902 that the initial determination was procured by fraud or similar fault as defined in § 405.902.
there is reliable evidence of similar fault when a demanded overpayment determination was
issued to a provider or supplier for which CMS has revoked or deactivated their Medicare billing
privileges, or for which there is a closed law enforcement investigation, among other possible
factors. Although demanded overpayment determinations are subject to appeal, we believe use of
these amounts in reopening and recalculating an ACO’s financial performance under the Shared
Savings Program would allow us to more timely address the impact of improper payments on
Shared Savings Program calculations, rather than waiting to consider the outcome of any
possible appeal of the amounts (as discussed in section III.G.7.c.(2).(c) of this proposed rule).
As part of our initial analysis to evaluate whether to reopen an ACO’s initial
determination, we may also consider the significance of the improper payments to an ACO’s
financial calculations by estimating the financial impact of improper payments on an ACO’s
payment determination. If we estimate that the improper payments have impacted the dollar
amount of earned shared savings, or the amount of shared losses that the ACO owes or has paid
to CMS, we anticipate reopening an ACO’s payment determination. When determining whether
to reopen an ACO’s payment determination, we anticipate considering a combination of factors
including:
● The dollar value of improper payments and the number of claims or line items
impacted (if applicable).
● How any related impact on performance year expenditures may compare to the impact
on the ACO’s updated historical benchmark (which could include considering the impact on
benchmark year expenditures and factors used to establish, adjust and update the benchmark). In
particular, we may consider whether comparing performance year expenditures to the updated
benchmark expenditures used in financial reconciliation, once adjusted to account for the
estimated impact of the improper payments, would result in a significant change in the amount of
shared savings paid to or shared losses owed by the ACO. For purposes of this analysis we may
consider the following:

++ The MSR/MLR applicable to the ACO for the relevant performance year.
++ Whether the ACO met or exceeded the applicable MSR/MLR with the initial
determination.
++ Whether accounting for improper payments would cause a change in the ACO’s
financial performance compared to its performance under the initial determination, including:
-- Causing an ACO to meet or exceed its MSR/MLR when it did not do so under its
initial determination, or no longer meet or exceed the relevant threshold when it did so under its
initial determination.
-- Causing an ACO that shared savings or owed losses under the initial determination to
share in either a higher or lower amount of savings or losses (respectively).
-- Causing an ACO to continue to generate savings or losses less than the MSR/MLR
threshold, as it did under its initial determination, and therefore the ACO would remain ineligible
for shared savings, except in cases where certain low revenue ACOs participating in the BASIC
track may qualify for a shared savings payment in accordance with § 425.605(h), and would not
be held liable for shared losses.
We note that the existing reopening authority at § 425.315 and the proposed financial
methodology to address improper payments in such a reopening are not intended to address
particular instances of low-value improper payments which, in an individual case may be to the
benefit of either the ACO or CMS and in the aggregate are likely have a de minimis net effect on
program expenditures in the long run.
520
CMS would be highly unlikely to reopen in such cases
under § 425.315. We believe that considering the significance of the potential impact of the
improper payments on the ACO’s payment determination, in deciding whether to reopen the
payment determination, is a key component of striking a balance between improving the
accuracy of the calculations and ACOs’ and CMS’ interest in administrative finality of payment
determinations. We discuss related concerns and considerations elsewhere in this section of this
520
See, for example, 81 FR 38000 and 38001.

proposed rule. Therefore, we would seek to reopen an ACO’s payment determination only in
cases where the impact of improper payments warrants disrupting the initial determination.
Consider, for example, that in our initial determination we found that an ACO generated
savings below its minimum savings rate (MSR) and, therefore, did not qualify for a shared
savings payment according to the policies for determining the ACO’s eligibility for shared
savings applicable to its agreement period under the Shared Savings Program.
521
If, based on an
initial analysis, we estimate that the ACO’s savings, though higher once adjusted to remove
improper payments from performance year expenditure calculations, would still fall below the
minimum savings rate, it would not be necessary to reopen an ACO’s payment determination
because the ACO would still not qualify for a shared savings payment. Under such
circumstances, we would not reopen the initial determination or proceed with the recalculations
described in section III.G.7.c.(2).(c) of this proposed rule. We anticipate that this particular type
of situation could occur in cases where the improper payments at issue are relatively small and
the differential between an ACO’s generated shared savings and minimum savings rate as
calculated in the initial determination is relatively large such that recalculating the amounts
would not produce a different outcome to the payment determination.
It is also possible that improper payments would have no impact on Shared Savings
Program financial calculations as they may consist of claims or payment amounts that were not
used in reaching the initial determination of the ACO’s financial performance. For instance, if a
demanded overpayment determination was for a payment amount on a claim with a HCPCS or
CPT code identified as having significant, anomalous, and highly suspect billing activity, and
therefore the payment amount was excluded from certain financial calculations used in
determining the ACO’s financial performance under the proposed adjustment discussed in
section III.G.7.d of this proposed rule, we would not include this amount as part of a reopening
521
This example assumes a one-sided model ACO with an MSR based on the number of beneficiaries assigned to
the ACO, or a two-sided model ACO with an MSR/MLR greater than zero.
for the same performance year. As another example, if the demanded overpayment determination
was for a claim or line item that was initially paid after the end of the 3-month claims run out
period, we would not take into account through the reopening process a payment amount that
was not included in Shared Savings Program calculations to begin with. We anticipate improper
payments identified in these circumstances would not merit reopening the ACO’s initial
determination.
A number of steps would follow after CMS has decided to reopen the initial
determination. We would recalculate the ACO’s financial performance for a performance year
by applying the methodology as described in section III.G.7.c.(2).(c) of this proposed rule. With
this recalculation we would determine the amount of shared savings payment the ACO may be
eligible to receive or the amount of shared losses the ACO may owe for the performance year
after accounting for the impact of the improper payments. We would issue a revised initial
determination to the ACO with the recalculated payment determination for the performance year.
We would notify the ACO of savings and losses in accordance with § 425.604(f), § 425.605(e),
§ 425.606(h), § 425.609(e), or § 425.610(h) (as applicable). Depending on the outcome of the
recalculation as specified in the revised initial determination, we would engage in payment
activities and recoupment activities, as needed. As explained in earlier rulemaking, we anticipate
considering ways to minimize program disruptions for ACOs that could result from one or more
reopenings (see for example, 81 FR 38001 through 38002; see also, 87 FR 69868 through
69872). CMS may require considerable time after deciding to reopen an initial determination
before it can complete the process described above for a variety of reasons. For example,
additional time may be necessary for CMS or other agencies to ascertain the precise amount of
improper payments that affected the initial determination.
In reopening a payment determination, we note that improper payments may impact
either performance year expenditures, the ACO’s updated historical benchmark used in
determining the ACO’s financial performance (including calculation of benchmark expenditures

and factors used to establish, adjust and update the ACO’s historical benchmark), or both. The
recalculation of the ACO’s financial performance may have varying effects on the ACO’s
payment determination for the performance year. In some scenarios, the recalculation may
change the determination of whether the ACO earned shared savings or owes shared losses, or
may change the amount of any shared savings earned or shared losses owed. It is also possible
that we may observe there is no impact on the amount of shared savings earned or amount of
shared losses owed by the ACO, once we have performed the recalculation of the ACO’s
financial performance.
Under the Shared Savings Program’s benchmarking methodology, there are potential
interactions between performance of an ACO under the program for a performance year during
an agreement period and resetting the ACO’s benchmark for a subsequent agreement period.
Specifically, an ACO’s performance year may correspond to a benchmark year of its subsequent
agreement period, such that improper payments impacting expenditures for Medicare FFS
beneficiaries used to determine performance year expenditures may similarly impact
expenditures for the same period used to establish the ACO’s historical benchmark. For instance,
for ACOs that have participated in the Shared Savings Program over multiple agreement periods,
improper payments may impact the amount of a prior savings adjustment to the historical
benchmark (if applicable).
522
We discussed the complexity around some related interactions in
CY 2024 PFS rulemaking, in regard to recalculating the prior savings adjustment, as described in
section III.G.7.c.(1).(b) of this proposed rule (see 88 FR 79198 through 79200). We note that
reopenings at any time for fraud or similar fault could extend to any prior performance year of
the Shared Savings Program. Since Shared Savings Program policies have changed over time, in
522
Refer to § 425.658 specifying calculation of the prior savings adjustment applicable to ACOs in agreement
periods beginning on January 1, 2024, and in subsequent years. Refer to §425.603(b)(2) specifying an additional
adjustment is made to the historical benchmark to account for the average per capita amount of savings generated
during the ACO’s previous agreement period, implemented for renewing ACOs entering a second agreement period
in 2016. See the discussion in the CY 2023 PFS final rule, in which we finalized the prior savings adjustment
applicable for agreement periods beginning on January 1, 2024, and in subsequent years, and provided background
on, and a description of, the prior savings adjustment that applied to certain ACOs in an earlier agreement period (87
FR 69898 through 69915).
performing the recalculation we would apply the relevant financial model and benchmarking
policy for the ACO for that performance year, in accordance with the applicable provisions of
subpart G.
Third, we are considering limiting the instances in which we reopen an initial
determination to account for improper payments, pursuant to § 425.315(a), to strike a balance
between improving the accuracy of the calculations and ACOs’ and CMS’ interest in
administrative finality of payment determinations. In rulemaking for the Shared Savings Program
during 2016, we considered factors for balancing the need to reopen and correct Shared Savings
Program payment determinations with the need for administrative finality, which has
implications for both ACOs and CMS (81 FR 5853 through 5858, and 81 FR 37997 through
38002). Some of these factors were discussed more generally, in the February 2016 proposed
rule, with respect to our consideration of options for further developing our reopening policy
(see, for example, 81 FR 5854 and 5855). We explained that an approach of correcting even very
minor errors might result in significant operational burdens for ACOs and CMS, including
multiple financial reconciliation re-runs and off-cycle payment/recoupment activities that could
have the potential for significant and unintended operational consequences, and could jeopardize
the certainty of performance results for both ACOs and CMS. We explained our concern that a
relatively broad scope and extended timeframe for reopening could introduce financial
uncertainty that could limit an ACO’s ability to invest in additional improvements to increase
quality and efficiency of care. This uncertainty could also limit an ACO’s ability to get a clean
opinion from its financial auditors and/or to obtain funds from lenders or investors.
We remain especially concerned about the potential for financial uncertainty resulting
from a broad scope and extended timeframe for reopening for ACOs and CMS, particularly if
correcting minor errors resulting from improper payments. We are concerned that reopening
payment determinations for minor issues impacting calculations for one or several performance
years of an ACO’s earlier agreement period could in turn disrupt the administrative finality of
calculations for multiple performances years, in one or more subsequent agreement period, if the
impacted year(s) become benchmark year(s) used in resetting the ACO’s historical benchmark.
We also note that since an ACO’s performance can vary from year to year (in terms of whether
the ACO generates savings or losses and is eligible for shared savings or owes shared losses), it
is possible for there to be a mixed effect across reopening payment determinations for multiple
performance years. If the recalculation of financial performance identifies relatively small
changes in the amount of shared savings or shared losses, it could be possible for these changes
to balance out over a span of multiple performance years. This raises further questions about the
utility of reopening payment determinations versus maintaining administrative finality of initial
determinations.
A relatively straight-forward case would be to reopen a single performance year that we
identify as having been impacted by improper payments. When a performance year for which we
issue a revised initial determination becomes a benchmark year of an ACO’s subsequent
agreement period, whether we reopen an ACO’s payment determination to account for the
impact of improper payments in Shared Savings Program calculations would differ depending on
whether or not we have issued an initial determination for a performance year of the ACO’s
subsequent agreement period. If the subsequent agreement period is the ACO’s current
agreement period, and CMS has not yet issued an initial determination for a performance year
within the current agreement period, we would account for the impact of improper payments on
future financial calculations pursuant to the proposed benchmark adjustment specified in
modifications to §§ 425.601(a)(9) and 425.652(a)(9). See section III.G.7.c.(2).(d) of this
proposed rule for a discussion of our proposals related to modifying these provisions.
CMS’ decision to reopen an initial determination for a performance year is independent
of a determination by CMS to reopen an initial determination for any other performance year,
including in cases where multiple performance years are impacted by the same improper
payments, whether within the ACO’s current agreement period, or a past agreement period. In
these circumstances, we would need to potentially consider reopening initial determinations for
multiple performance years, which may span multiple agreement periods, in cases where an
ACO has continued its participation in the Shared Savings Program over time. Therefore, we are
considering applying a combination of the following factors in determining whether to reopen an
initial determination: (1) consideration of the timing of reopening and recalculating the payment
determination for a performance year, and the timing of financial reconciliation for one or more
performance year of a subsequent agreement period that includes the affected period as a
benchmark year, and (2) consideration of whether the improper payments result from conduct of
individuals or entities performing functions or services related to the ACO’s activities.
Regarding the timing for reopening, we may consider whether a performance year that is
being reopened corresponds to a benchmark year of an ACO’s subsequent agreement period. We
may consider whether we have completed financial reconciliation for a subsequent performance
year, using a benchmark that is impacted by the same improper payments that were accounted
for in reopening a payment determination for a performance year corresponding to a benchmark
year.
We expect ACOs continuing their participation over multiple agreement periods in the
Shared Savings Program have a heightened interest in administrative finality of payment
determinations, which would provide greater financial certainty to the continued operation of
ACOs and progress towards meeting the program’s goals. In such cases, we believe (1)
reopening payment determinations for a performance year to account for the impact of improper
payments remains important to improving the accuracy of the Shared Savings Program’s
calculations, and (2) maintaining the administrative finality of subsequent payment
determinations, if the same improper payments impact a benchmark year of an ACO’s
subsequent agreement period, could provide ACOs greater financial certainty with respect to
their participation which may outweigh the benefits of reopening the calculations. Maintaining
administrative finality of the payment determinations for these subsequent performance years
may be warranted in cases where the improper payments are not a result of the conduct of
individuals or entities within the ACO. On the other hand, in cases where improper payments
impacting Shared Savings Program calculations results from conduct by individuals or entities
within the ACO, CMS’ interest in addressing program integrity concerns would warrant
reopening all affected payment determinations. In these cases, if left unaddressed, ACOs, ACO
participants and ACO providers/suppliers, among others, may have incentives to continue to
engage in conduct, which could include fraud or similar fault, in a way that could improve the
ACO’s performance under the Shared Savings Program.
Although not expressly stated in § 425.315, we note that improper payments that are the
basis of a reopening may result from the conduct of individuals or entities including but not
limited to: (1) conduct of an ACO, ACO participant, ACO provider/supplier, ACO professional,
or other individuals or entities performing functions or services related to the ACO's activities; or
(2) conduct of a provider or supplier, or other individuals or entities outside the ACO. For
purposes of the discussion within this section of this proposed rule, we refer to the former as
improper payments originating “inside the ACO”, and the latter as improper payments
originating “outside the ACO”.
To follow is a brief summary of the approach we are considering for differentiating
between cases where improper payments originate inside the ACO versus outside the ACO. If
we identify a single performance year for which we have issued an initial determination that has
been impacted by improper payments, we would seek to reopen the payment determination if the
improper payments originated either inside the ACO or outside the ACO.
When a performance year for which we issue a revised initial determination becomes a
benchmark year of an ACO’s subsequent agreement period, we would consider whether to
reopen each initial determination for a subsequent performance year that is impacted. We are
considering taking the following approach as one means to operate reopenings in an equitable
and manageable manner:
● In cases where improper payments originated outside the ACO: Generally, we would
not seek to reopen payment determinations for any performance year of the ACO’s subsequent
agreement period in order to mitigate the extent to which we disrupt the administrative finality of
payment determinations for ACOs when the improper payments impacting Shared Savings
Program calculations originate outside the ACO. However, we may consider reopening the initial
determination for the performance year upon the ACO’s request for a reopening if the improper
payments are anticipated to result in significant adjustment to the ACO’s initial determination
upon recalculation.
● In cases where improper payments originated inside the ACO: As a means to address
our program integrity concerns, we would reopen the payment determination for any
performance year of the ACO’s subsequent agreement period issued prior to the revised initial
determination for the performance year corresponding to the benchmark year impacted by
improper payments originating inside the ACO, if the improper payments are anticipated to
result in significant adjustment to the ACO’s initial determination upon recalculation. We
believe this approach would guard against circumstances where an ACO may benefit from
improper payments remaining in its benchmark calculations that result from conduct by
individuals or entities performing functions or services related to the ACO’s activities.
We seek comment on the factors we have described in this section of this proposed rule,
that may inform our decision of whether to reopen an initial determination of an ACO’s financial
performance to account for the impact of improper payments. In particular, we seek comment on
the approach we outlined for conducting initial analysis of whether the improper payments
would warrant reopening the ACO’s payment determination. We also seek comment on
approaches to, and considerations in connection with, balancing the need for accuracy in
payment calculations with the need for administrative finality in payment determinations.
(c) Methodology for Recalculating Expenditures to Account for Improper Payments
We propose to establish a financial calculation methodology that may be used to account
for the impact of improper payments on Shared Savings Program financial calculations, upon
reopening a payment determination pursuant to § 425.315(a). We propose to add to subpart G a
new section of the Shared Savings Program regulation at § 425.674 specifying provisions on
accounting for the impact of improper payments on Shared Savings Program financial
calculations.
As a general rule, we propose to specify in paragraph (a) of § 425.674, that upon the
reopening of an initial determination pursuant to § 425.315(a)(4), CMS will use the methodology
set forth in § 425.674 to account for the impact of improper payments when: (1) determining
savings or losses for the relevant performance year in accordance with § 425.315 in order to
issue a revised initial determination, and (2) adjusting the benchmark by recalculating
benchmark year expenditures in the event that we recalculate a payment determination and issue
a revised initial determination for the corresponding performance year in a prior agreement
period (as discussed in section III.G.7.c.(2).(d) of this proposed rule).
We propose to specify in paragraph (b) of § 425.674 that for the purpose of the Shared
Savings Program, “improper payment” includes: (1) an amount associated with a demanded
overpayment determination, and (2) an amount identified in a settlement agreement or judgment,
pursuant to conduct of individuals or entities performing functions or services related to an
ACO's activities, less any penalties or damages.
We propose to establish a methodology under § 425.674 under which we would adjust
Medicare Parts A and B FFS expenditure values used in certain Shared Savings Program
financial calculations to account for a per capita amount of improper payments for an identified
population used in calculating performance year or benchmark year expenditures, and in
calculating county-level FFS expenditures used in factors based on regional expenditures.
We propose to specify under § 425.674 a generalized approach to calculating the per
capita amounts of improper payments that accounts for the fact that improper payments may be
associated with specific claims or line items, or may be aggregate amounts. A number of factors
informed our consideration of this approach. For one, we considered the need to establish a
calculation methodology to account for demanded overpayment determinations that result in
adjustments to payment amounts associated with claims and line items used in Shared Savings
Program calculations, such as the denial of claims or line items that occur after the 3-month
claims run out period, or in an aggregate amount, such as based on extrapolated overpayment
demands that do not result in adjustments to claim or line item payment amounts. Medicare Parts
A and B FFS claim adjustments for overpayments would be reflected in current Shared Savings
Program expenditure calculations if processed before the end of the 3-month claims run out
period but are not included in calculations if processed after the 3-month claims run out period.
Regarding the latter, the amounts of the claims adjusted overpayments can be identified for
Medicare FFS beneficiaries, and can be aggregated across a population of Medicare FFS
beneficiaries that is the basis for certain Shared Savings Program calculations. Additionally,
aggregate amounts of demanded overpayment determinations, such as extrapolated overpayment
demands, may be used to identify the amount of improper payments for a large set of claims for
a particular provider or supplier and a certain time period, since error rates are extrapolated and
applied to a universe of claims rather than individual claims. In these cases, an aggregate amount
of a demanded overpayment determination is attributable to a provider or supplier and would
have to be further prorated to determine its relevance to a particular population of Medicare FFS
beneficiaries that is the basis for certain Shared Savings Program calculations.
Second, we considered the need for the calculation methodology to account for improper
payments resulting from conduct by an ACO, ACO participant, ACO provider/supplier, ACO
professional, or other individuals or entities performing functions or services related to the
ACO's activities identified in certain settlements, or judgments. With respect to the Shared
Savings Program calculations, we anticipate that a key focus would be on improper payments
pursuant to conduct of individuals or entities performing functions or services related to an
ACO’s activities as identified in certain False Claims Act (31 U.S.C. 3729 et seq.) settlement
agreements, or judgments. In considering the amount of improper payments that are relevant to
Shared Savings Program calculations, we would exclude the amount of any penalties or damages
included in the settlement or judgment. In addition, we may seek to attribute an aggregate
improper payment amount to a provider or supplier that is specified within a settlement
agreement, or judgment, across a population of Medicare FFS beneficiaries that is the basis for
the applicable Shared Savings Program calculation.
Further, we anticipate there may be circumstances that warrant adjustment to payment
amounts used in Shared Savings Program calculations, at the claims level, instead of or in
addition to accounting for the amount of demanded overpayment determinations or an aggregate
amount in a settlement agreement or judgment. For instance, in analyzing improper payments
impacting Shared Savings Program calculations, we may conclude that a provider’s or supplier’s
billings for a particular HCPCS or CPT code for a population of Medicare FFS beneficiaries
resulted in inaccuracies in payment amounts used in Shared Savings Program calculations. We
propose that we may address these circumstances by decreasing or entirely removing the value of
HCPCS or CPT code payment amounts for certain claims or line items used in Shared Savings
Program calculations, in reopening and recalculating the ACO’s payment determination. We
anticipate using all information available to us from an investigation, settlement agreement, or
judgment to determine the correct payment amount or level of billing. This could include
considering the nature of the remedy in the case and how any related amount would be applied in
the proposed methodology to account for improper payments impacting Shared Savings Program
financial calculations. In particular, we would consider if it would be a more precise adjustment
to Shared Savings Program financial calculations to adjust the claim or line item payment
amounts, instead of or in addition to accounting for the amount of demanded overpayment

determinations or an aggregate amount in a settlement agreement or judgment (if applicable). For
instance, in cases where an investigation, settlement agreement, or judgement has determined
inaccurate use of a higher paying code
523
that is reflected in payment amounts used in Shared
Savings Program calculations, we may identify use of a code with lower reimbursement within a
HCPCS or CPT code category that would result in a more precise adjustment to the ACO’s
payment determination.
We propose to specify in paragraphs (c) and (d) of § 425.674 the general approach for
adjusting Medicare Parts A and B FFS expenditures for improper payments, according to the
following steps:
● Step 1 - Identify calculation for adjustment: Identify each Shared Savings Program
expenditure calculation for a performance year or benchmark year, as calculated according to the
standard methodology described in subpart G and expressed as a per capita dollar amount, that
would be adjusted for the impact of improper payments (as proposed in § 425.674(c)(1)).
● Step 2 - Determine the relevant population for adjustment: Determine each specific
population of Medicare FFS beneficiaries used to calculate the expenditure amount identified in
Step 1, expressed as person years (as proposed in § 425.674(c)(2)). The populations relevant for
a specific expenditure calculation may include:
++ The population of beneficiaries assigned to the ACO for calculating the ACO’s
performance year or benchmark year expenditures.
++ The population of assignable beneficiaries in each county in the ACO’s regional
service area for calculating county-level expenditures.
++ The national population of assignable beneficiaries for calculating national assignable
expenditures.
523
See, for example, CMS, Medicare Claims Processing Manual Chapter 23 - Fee Schedule Administration and
Coding Requirements, section 20.9.5 “Adjustments”, available at https://www.cms.gov/regulations-and-
guidance/guidance/manuals/downloads/clm104c23.pdf (explaining that if the wrong, higher paying code is paid on
the first of multiple claims submitted, A/B MACs processing Medicare Part B claims pay the subsequent claim(s)
and initiate recovery action on the previously paid claim(s)).
++ The national population of Medicare FFS beneficiaries for calculating national
expenditures.
● Step 3 - Determine per capita amount of improper payments attributable to the
relevant population: Determine the per capita amount of improper payments for the performance
year or benchmark year included in the per capita Medicare Parts A and B FFS expenditure
amount for a population identified in Step 2 (as proposed in § 425.674(c)(3)). We may use one or
more of the following approaches to determine the per capita amount of improper payments, for
all providers or suppliers with improper payments, that would be used to adjust the expenditure
calculations identified in Step 1 (as proposed in § 425.674(d)):
++ Step 3(i): Calculate aggregate improper payments attributable to a population
identified in Step 2 for each provider or supplier that had improper payments.
-- For improper payments associated with specific claims, we would do the following:
(A) For improper payments to a provider or supplier that correspond to payment amounts
on claims or line items that were used in a Shared Savings Program calculation identified in Step
1, and subsequently adjusted after the 3-month claims run out period, we would sum the
improper payment amounts across all such claims or line items with dates of service during the
period used to calculate performance year or benchmark year expenditures, for a population
identified in Step 2.
To allow for this approach, we propose to adjust Shared Savings Program expenditure
calculations to reflect adjustments occurring after the original 3-month claims run out period for
claim or line item payment amounts associated with improper payments. We would not capture
payments or payment adjustments occurring outside the original 3-month claims run out period
for claims or line items unrelated to improper payments.
(B) In the event that CMS determines it is necessary to account for the impact of
improper payments on Shared Savings Program financial calculations by adjusting the payment
amounts for a specific HCPCS or CPT code billed by the provider or supplier for the population
identified in Step 2, we would do the following: identify the applicable claims or line items with
dates of service during the period used to calculate performance year or benchmark year
expenditures processed before the end of the applicable 3-month claims run out period, and sum
the claim or line item payment amounts on the claims or line items identified; and if applicable,
multiply the resulting sum by a scaling factor to compute the payment differential between the
HCPCS or CPT code that was improperly billed and a CMS-identified alternate code. We would
apply a scaling factor in cases where it is determined that the provider or supplier did not bill the
correct code for a particular service. In cases where we determine it is appropriate to remove
payments for the billed HCPCS or CPT code in their entirety, we would not apply a scaling
factor.
-- For aggregate improper payment amounts that are not linked to specific claims or line
items, we would calculate the amount attributable to the population identified in Step 2 by
applying a proration factor to the aggregate improper payment amount identified for that
provider or supplier. We would calculate the proration factor as follows:
(A) The denominator of the proration factor would be total Medicare Parts A and B claim
or line item payment amounts to the provider or supplier for all FFS beneficiaries on claims of
specified claim types for the time period associated with the aggregate improper payment
amount identified for the provider or supplier that were made before the end of the applicable 3-
month claims run out period.
(B) The numerator of the proration factor would be the portion of the total from the
denominator that CMS determines is attributable to the population identified in Step 2 with dates
of service during the period used to calculate expenditures for the applicable performance year or
benchmark year.
Under this proposed approach, if an aggregate amount of improper payment is associated
with claims activity that spans multiple calendar years, we would account for this in the proration
factor by expanding the time period used to compute payments for the denominator to include
the relevant years. For example, if the aggregate amount of improper payments was associated
with claims activity in 2021 and 2022, we would include in the denominator payments on claims
or line items with dates of service in 2021 (made before the end of March 2022) and on claims or
line items with dates of service in 2022 (made before the end of March 2023). If we were
adjusting PY 2022 expenditures for an ACO’s assigned population, the numerator of the
proration factor would be the portion of the denominator that is attributable to the ACO’s
assigned population during CY 2022.
++ Step 3(ii): Sum the amounts calculated under Step 3(i) attributable to the population
identified in Step 2 across providers or suppliers that had identified improper payments.
++ Step 3(iii): Take the lesser of the following two values:
-- The sum from Step 3(ii); or
-- Total Medicare Parts A and B claim or line item payment amounts to all providers or
suppliers that had improper payments for the population identified in Step 2 on claims of
specified claim types with dates of service within the performance year or benchmark year made
before the end of the applicable 3-month claims run out period.
The purpose of taking the lesser of two values in this step is to ensure that the improper
payment amount that we attribute to a given population cannot be greater than the total amount
of payments for the providers or suppliers at issue that was included in the original expenditure
calculation for that population.
++ Step 3(iv): Express the lesser-of-amount from Step 3(iii) as a per capita value by
dividing by the total beneficiary person years in the population identified in Step 2 for the
applicable performance year or the benchmark year.
● Step 4 – Subtract per capita improper payment amount from original expenditures:
From the expenditure calculation identified in Step 1 for the population identified in Step 2,
subtract the per capita amount calculated in Step 3(iv) for each of the following populations of
beneficiaries: ESRD, disabled, aged/dual eligible Medicare and Medicaid beneficiaries, and
aged/non-dual eligible Medicare and Medicaid beneficiaries (as proposed in § 425.674(c)(4)).
● Step 5 – Determine adjusted regional expenditures: If applicable, we would do the
following to adjust regional expenditures for improper payments (as proposed in
§ 425.674(c)(5)):
++ Step 5(i): Adjust county-level FFS expenditures determined in Step 4, for each
county in the ACO’s regional service area, for severity and case mix of assignable beneficiaries
in the county using prospective HCC risk scores. This calculation would be for each of the
following populations of beneficiaries based on Medicare enrollment type: ESRD, disabled,
aged/dual eligible Medicare and Medicaid beneficiaries, and aged/non-dual eligible Medicare
and Medicaid beneficiaries. We note that under this approach CMS would not adjust the risk
scores used to calculate risk adjusted county-level FFS expenditures.
++ Step 5(ii): Weight the risk-adjusted county-level FFS expenditures determined in
Step 5(i) according to the ACO’s proportion of assigned beneficiaries in the county, determined
in accordance with § 425.601(d)(1), § 425.603(f)(1), or § 425.654(b)(1), as applicable, for each
of the populations of beneficiaries by Medicare enrollment type.
++ Step 5(iii): Aggregate the values determined in Step 5(ii) for each of the populations
of beneficiaries (by Medicare enrollment type) across all counties within the ACO’s regional
service area.
To illustrate how the proposed calculation methodology would be applied, we can
consider the following hypothetical example in which CMS confirmed that two suppliers, NPI 1
and NPI 2, received improper payments from Medicare during calendar year 2022. Specifically,
CMS identified $8 million in demanded overpayment determinations for NPI 1 which resulted in
CMS adjusting payment amounts after the 3-month claims run out period for PY 2022 on claims
or line items with dates of service during the performance year, and CMS identified an aggregate
extrapolated overpayment demand amount of $30 million for NPI 2. Assume that in this
example, CMS determines that reopening the ACO’s PY 2022 initial determination is warranted,
and CMS recalculates that ACO’s financial performance using the proposed methodology to
account for improper payments. To recalculate the ACO’s financial performance for PY 2022,
we would identify three separate expenditure calculations that need to be recalculated to
determine the impact on an ACO’s earned performance payment or owed shared losses: (1) PY
2022 expenditures for the ACO’s assigned beneficiaries; (2) PY 2022 expenditures for
assignable beneficiaries in the ACO’s regional service area; and (3) PY 2022 expenditures for
national assignable beneficiaries. For this example, in Table 41 we outline the steps and
calculations for recalculating expenditures for beneficiaries assigned to the ACO for PY 2022. In
Table 42 we outline how PY 2022 expenditures for assignable beneficiaries in the ACO’s
regional service area and PY 2022 expenditures for national assignable beneficiaries,
recalculated to account for improper payments, would be incorporated into the blended national-
regional benchmark update factor. In Table 43 we outline how an ACO’s financial performance
may be recalculated after accounting for improper payments in PY 2022 expenditures for the
ACO’s assigned beneficiaries, and using the recalculated blended national-regional benchmark
update factor.

TABLE 41: Hypothetical Example of Steps for Recalculating ACO Assigned Beneficiary
Expenditures Using Proposed Methodology to Account for Improper Payments
Amount
Steps 1 and 2: Identify calculation and relevant population for adjustment
ACO PY assigned beneficiary expenditures by enrollment type (per capita) [A]
ESRD
$80,000
Disabled
$11,000
Aged/dual
$15,000
Aged/non-dual
$12,000
ACO PY total assigned beneficiary person years [B]
20,000
Step 3: Determine per capita amount of improper payments attributable to the relevant population
Aggregate improper payments attributable to the ACO’s assigned beneficiaries for NPI
1 (identified at the claim or line item level)
Total aggregate improper payments for NPI 1 [C]
$8,000,000
Aggregate improper payments for NPI 1 attributable to the ACO’s assigned
beneficiaries [D]
$200,000
Aggregate improper payments attributable to the ACO’s assigned beneficiaries for NPI
2 (identified at the NPI level)
Total aggregate improper payments for NPI 2 [E]
$30,000,000
Total Medicare Parts A and B claim or line item payment amounts to NPI 2 for the
ACO’s assigned beneficiaries for PY (a portion of row [G]) [F]
$4,800,000
Total Medicare Parts A and B claim or line item payment amounts to NPI 2 for all
Medicare FFS beneficiaries, on claims of specified claim types for the time
period associated with improper payment amount, made before the end of the 3-
month claims run out period for PY [G]
$80,000,000
Proration factor [H] = [F] / [G]
0.06
Aggregate improper payments attributable to the ACO’s assigned beneficiaries [I]
= [E] x [H]
$1,800,000
Sum of improper payments attributable to the ACO’s assigned beneficiaries for NPI 1
and NP1 2 [J] = [D] + [I]
$2,000,000
Total Medicare Parts A and B claim or line item payment amounts to NPI 1 and NPI 2
for the ACO’s assigned beneficiaries made before the end of the 3-month claims run
out period for PY [K]
$5,200,000
Total aggregate improper payments attributable to the ACO’s assigned beneficiaries
[L] = Lesser of [J] and [K]
$2,000,000
Per capita improper payments attributable to the ACO’s assigned beneficiaries [M] =
[L] / [B]
$100
Step 4: Subtract per capita improper payment amount from original expenditures
Adjusted ACO PY assigned beneficiary expenditures by enrollment type (per capita)
[N] = [A] – [M]
ESRD
$79,900
Disabled
$10,900
Aged/dual
$14,900
Aged/non-dual
$11,900
In Step 1, we identify expenditures for the ACO’s assigned beneficiaries in PY 2022 as
the calculation to be recalculated. In Step 2, we identify the ACO’s assigned beneficiaries in PY
2022 as the population relevant for this expenditure calculation. In Step 3, we determine the per
capita amount of improper payments that is attributable to the ACO’s assigned beneficiaries. For
NPI 1, we identify that $200,000 of the NPI’s total aggregate improper payments were on claims
for the ACO’s assigned beneficiaries (row [D]). Because improper payments for NPI 2 were
identified at the NPI level and thus are not tied to individual claims, we need to apply a proration
factor to calculate the share of the total aggregate improper payments, $30 million (row [E]), that
is attributable to the ACO’s assigned beneficiaries. We calculate this proration factor as the total
Medicare Parts A and B claim or line item payment amounts made to NPI 2 for the ACO’s
assigned beneficiaries for PY 2022 ($4.8 million, row [F]), divided by the total Medicare Parts A
and B claim or line item payment amounts made to NPI 2 for all Medicare FFS beneficiaries
($80 million, row [G]); this results in a proration factor of 0.06 (row [H]), which when applied to
NPI 2’s total aggregate improper payments results in $1.8 million in aggregate improper
payments attributable to the ACO’s assigned beneficiaries (row [I]). Summing across NPI 1 and
NPI 2, we calculate $2 million in total aggregate improper payments attributable to the ACO’s
assigned beneficiaries for PY 2022 (row [J]). We then compare this sum (row [J]) with total
Medicare Parts A and B claim or line item payment amounts to the two suppliers for the ACO’s
assigned beneficiaries for PY 2022 (row [K]) and take the lesser of the two values (row [L]). We
then express this lesser-of value in per capita terms by dividing by the ACO’s total assigned
beneficiary person years for PY 2022, 20,000, arriving at a $100 per capita improper payment
amount attributable to the ACO’s assigned beneficiaries (row [M]). Finally, in Step 4, we
subtract the $100 per capita improper payment amount from the original PY 2022 per capita
expenditure amount for the ACO’s assigned beneficiaries used to make the initial payment
determination, conducting this adjustment by enrollment type (row [N]).
We note that subtracting the same per capita improper payment amount ($100 in this
example) from the expenditure calculation for each enrollment type population implicitly
assumes that improper payments attributable to the overall population are distributed in
proportion to the four enrollment types (ESRD, disabled, aged/dual eligible, aged/non-dual
eligible). For example, if the aged/non-dual eligible population represents 82 percent of an
ACO’s overall assigned population for the performance year, we are assuming that 82 percent of
improper payments attributable to the ACO’s entire assigned population are associated with

aged/non-dual eligible beneficiaries. We believe that this is a reasonable assumption as we
expect that, in most cases, improper payments are unlikely to be associated with a particular
enrollment type as defined by the Shared Savings Program and used in program financial
calculations.
524
This also allows for a standard approach across the potential variety of reopening
scenarios, lending greater transparency and simplicity to the proposed methodology.
We would follow the same overall methodology to account for the impact of improper
payments in recalculating PY 2022 expenditures for assignable beneficiaries in the ACO’s
regional service area and for national assignable beneficiaries. These amounts are calculated for
the following populations of beneficiaries, by Medicare enrollment type: ESRD, disabled,
aged/dual eligible Medicare and Medicaid beneficiaries, and aged/non-dual eligible Medicare
and Medicaid beneficiaries. We would then use these adjusted expenditure calculations as new
inputs along with other original calculations that were not adjusted for the impact of improper
payments (such as the ACO’s historical benchmark for PY 2022) to recalculate the ACO’s
financial performance for PY 2022, following the standard financial methodology described in
§ 425.605 (for ACOs participating in the BASIC track) or § 425.610 (for ACOs participating in
the ENHANCED track), as applicable.
In Table 42, we expand upon the hypothetical example described in Table 41 and
summarize how we would calculate national and regional update factors following the
methodology specified in § 425.652(b)(2) but using the adjusted regional and national
expenditures for the performance year for each enrollment type in place of the original values.
Because benchmark update factors are calculated by enrollment type under the standard financial
methodology, they would also be recalculated by enrollment type when using the adjusted
524
For criteria used to identify the four Medicare enrollment types, refer to the Medicare Shared Savings Program,
Shared Savings and Losses, Assignment and Quality Performance Standard Methodology Specifications (version
#11, January 2023), available at https://www.cms.gov/files/document/medicare-shared-savings-program-shared-
savings-and-losses-and-assignment-methodology-specifications.pdf-2 (Appendix E: Identifying Medicare
Enrollment Type).

national and regional expenditures. However, for brevity, we describe only the recalculation of
the update factors for the aged/non-dual eligible population in Table 42.
In this continued hypothetical example, we used the proposed methodology to account
for the impact of improper payments in recalculating national and regional per capita
expenditures in the performance year, resulting in adjusted expenditures of $11,609 (row [A])
and $11,210 (row [C]), respectively. Dividing these PY values by the original BY3 national and
regional per capita expenditures ($10,977, row [A], and $10,900, row [C], respectively), we
recalculate the national update factor (1.058, row [B]) and regional update factor (1.028, row
[D]). In this example, there is a $1 difference between the original and recalculated national per
capita expenditure amount. The resulting value for the recalculated national update factor, shown
rounded to the third decimal place, remains the same as the original value, but there would be a
difference in the values if additional precision was shown. We then blend these adjusted update
factors using the original national and regional weights (0.250, row [E], and 0.750, row [F],
respectively). As shown in row [G], accounting for improper payments in PY 2022 causes the
blended benchmark update factor to decrease from 1.042 to 1.036.
TABLE 42: Hypothetical Example of How the Blended National-Regional Benchmark
Update Factor for the Aged/Non-Dual Eligible Enrollment Type May Be Recalculated
After Accounting for Improper Payments
BY3
PY (Original)
PY (Adjusted)
National per capita expenditures [A]
$10,977
$11,610
$11,609
National update factor [B] = [A]
PY
/ [A]
BY3
1.058
1.058
Regional per capita expenditures [C]
$10,900
$11,300
$11,210
Regional update factor [D] = [C]
PY
/ [C]
BY3
1.037
1.028
National weight [E]
0.250
0.250
Regional weight [F]
0.750
0.750
National-regional blended update factor [G] = [B] x [E] +
[D] x [F]
1.042
1.036
Table 43 summarizes how recalculated blended update factor to account for improper
payments, based on adjusted national and regional expenditures for PY 2022, would be used with
other original calculations and adjusted PY expenditures for ACO assigned beneficiaries to
recalculate the ACO’s financial performance for PY 2022. Applying the blended update factor
(row [B]) to the original historical benchmark values by enrollment type (row [A]), we
recalculate the updated benchmark values by enrollment type (row [C]) that account for
improper payments occurring in PY 2022. The adjusted updated benchmark values (row [C]) and
adjusted PY expenditures for ACO assigned beneficiaries by enrollment type (row [D]), also
described in Table 41, are multiplied by original PY assigned beneficiary proportions by
enrollment type (row [E]), and summed across enrollment types to recalculate the per capita
updated benchmark (row [F]) and per capita ACO PY assigned beneficiary expenditures (row
[G]). We then express these per capita quantities as the total updated benchmark amount (row
[I]) and the total ACO PY assigned beneficiary expenditures amount (row [J]) by multiplying the
per capita dollar amount by the ACO’s total assigned beneficiary person years for PY 2022 (row
[H]). The recalculated total updated benchmark (row [I]) can then be used to recalculate the
minimum savings rate / minimum loss rate dollar threshold (row [L]). We subtract the
recalculated total ACO PY assigned beneficiary expenditures (row [J]) from the recalculated
total updated benchmark (row [I]) to determine if the ACO has gross savings or gross losses.
Under this example, the recalculation indicates the ACO has total gross savings (row [K]).
Finally, because the recalculated total gross savings (row [K]) is greater than the recalculated
minimum savings rate dollar threshold, we recalculate the ACO’s shared savings (row [N]) by
multiplying the total gross savings (row [K]) by the original sharing rate (row [M]).
The result of these calculations is an adjusted shared savings amount of $17,355,000
(before accounting for sequestration), compared to an original amount of $16,950,000. Thus,
while adjustments for improper payments reduced the ACO’s PY assigned beneficiary
expenditures by $2 million, the impact on the ACO’s recalculated shared savings is only
$405,000 due to the impact of improper payments on the expenditures for assignable
beneficiaries that factor into the ACO’s recalculated updated benchmark for PY 2022.

TABLE 43: Hypothetical Example of How an ACO’s Financial Performance May Be
Recalculated After Accounting for Improper Payments
Original
Adjusted
Adjusted
Minus
Original
Historical benchmark by enrollment type (risk adjusted, per
capita) [A]
ESRD
$89,200
Disabled
$12,700
Aged/dual
$17,700
Aged/non-dual
$12,200
National-regional blended update factor by enrollment type
[B]
ESRD
1.007
1.006
-0.001
Disabled
1.028
1.022
-0.006
Aged/dual
1.043
1.039
-0.004
Aged/non-dual
1.042
1.036
-0.006
Updated benchmark by enrollment type (per capita) [C] =
[A] x [B]
ESRD
$89,824
$89,735
-$89
Disabled
$13,056
$12,979
-$77
Aged/dual
$18,461
$18,390
-$71
Aged/non-dual
$12,712
$12,639
-$73
ACO PY assigned beneficiary expenditures by enrollment
type (per capita) [D]
ESRD
$80,000
$79,900
-$100
Disabled
$11,000
$10,900
-$100
Aged/dual
$15,000
$14,900
-$100
Aged/non-dual
$12,000
$11,900
-$100
ACO PY assigned beneficiary proportions by enrollment
type [E]
ESRD
0.010
Disabled
0.100
Aged/dual
0.070
Aged/non-dual
0.820
Updated benchmark (per capita) [F] = Sum of [C] x [E]
$13,920
$13,847
-$73
ACO PY assigned beneficiary expenditures (per capita) [G]
= Sum of [D] x [E]
$12,790
$12,690
-$100
ACO PY total assigned beneficiary person years [H]
20,000
Total updated benchmark [I] = [F] x [H]
$278,400,000
$276,940,000
-$1,460,000
Total ACO PY assigned beneficiary expenditures [J] = [G] x
[H]
$255,800,000
$253,800,000
-$2,000,000
Total updated benchmark expenditures minus Total ACO PY
assigned beneficiary expenditures [K] = [I] – [J] (example
showing gross savings)
$22,600,000
$23,140,000
$540,000
Minimum savings rate / Minimum loss rate in dollars [L] =
0.02 x [I]
$5,568,000
$5,538,800
-$29,200
Sharing rate [M]
75%
Shared savings [N] = [K] x [M]
$16,950,000
$17,355,000
$405,000
Under the proposed financial methodology, accounting for the impact of improper
payments on expenditures could increase or decrease an ACO’s amount of shared savings or
shared losses. As demonstrated in the hypothetical example, the direction of changes to an
ACO’s shared savings or shared losses would depend on the differential impact of improper
payments on the ACO’s assigned beneficiary expenditures compared to the impact on
expenditures for assignable beneficiaries used to determine the national and regional updates to
the ACO’s benchmark. In this example, the reduction in ACO PY assigned beneficiary
expenditures due to the adjustment for improper payments was larger than the reduction to the
updated benchmark stemming from adjustments to PY national and regional expenditures,
ultimately causing the ACO to see an increase in both gross savings and shared savings. Other
ACOs for which the reduction in ACO PY assigned beneficiary expenditures is greater than the
reduction to the updated benchmark, may switch from earning no shared savings to earning
shared savings or may see a reduction in shared losses owed. However, if accounting for
improper payments results in relatively larger reductions to the expenditures for assignable
beneficiaries in the ACO’s regional service area or in the national assignable population, and
relatively smaller reductions to the ACO’s PY assigned beneficiary expenditures, the ACO might
observe a reduction in shared savings or increase in shared losses, or potentially cause the ACO
to switch from earning shared savings to not earning any shared savings or to owing shared
losses.
As we propose in section III.G.7.c.(2).(d) of this proposed rule, if the reopened PY
becomes a BY for a subsequent agreement period, CMS would adjust the historical benchmark
to be used for any PY in that subsequent agreement period that has not yet been reconciled.
Accounting for improper payments as it affects the ACO’s benchmark could then result in
changes to the ACO’s shared savings or shared losses for a future performance year that differ in
direction compared to the change in shared savings or shared losses observed with the initial
reopening that affected PY expenditures. That is, following the example from Table 43,
accounting for improper payments occurring in calendar year 2022 might result in the ACO
earning greater shared savings (or smaller shared losses) for PY 2022 (because the reduction in
ACO PY assigned beneficiary expenditures outweighs the reduction in national and regional
expenditures used to update the benchmark), but may result in smaller shared savings (or greater
shared losses) for future performance years for which CY 2022 becomes a benchmark year
(because the adjustment for improper payments in BY 2022 causes a reduction in the overall
benchmark with no corresponding reduction to ACO PY expenditures).
As we described in section III.G.7.c.(2).(b) of this proposed rule, administrative action
and judicial action leading to the identification of improper payments may be subject to appeal,
and ultimately the amount of the improper payments may be redetermined or otherwise
amended. We acknowledge the potential inaccuracy in using amounts of improper payments that
may be reversed, in whole or in part, in recalculating an ACO’s financial performance. However,
waiting for each possible appeal to be raised and resolved with respect to improper payments
could delay our ability to reach a determination of whether to reopen an ACO’s payment
determination, identify the amounts of improper payments to be used in the recalculation, or
both. We considered whether to account for the possibility that the improper payment amounts
would be appealed, and the amount redetermined, as part the proposed methodology, but are not
proposing a related approach at this time. For instance, we considered whether to apply an
adjustment factor as part of the methodology, that would reduce the amount of improper
payments by a percentage, to account for the rate at which the amounts could change, and to base
this rate on statistics gathered on the outcomes of Medicare Parts A and B administrative appeals
processes. Given that the proposed approach, if finalized, would be the initial use of improper
payment amounts in Shared Savings Program calculation, we note our intent to monitor for the
impact of appeals on the amounts of improper payments that may be used in reopenings under
the Shared Savings Program. We may revisit our approach in future notice and comment
rulemaking, after we gain additional experience with using improper payment amounts in Shared
Savings Program calculations.
We propose to use our authority under section 1899(d)(1)(B)(ii) of the Act to calculate
benchmark year expenditures using the proposed methodology to account for the impact of
improper payments. This provision authorizes the Secretary to adjust the benchmark for
beneficiary characteristics and “such other factors as the Secretary determines appropriate”.
When reopening an initial determination for a performance year pursuant to § 425.315, we
consider it appropriate to account for the impact of improper payments on expenditures used to
establish the ACO’s historical benchmark, consistent with our proposal.
We propose to use our authority under section 1899(i)(3) of the Act to use the proposed
methodology to account for the impact of improper payments in calculating performance year
expenditures and calculating the historical benchmark update factors. CMS may only adopt an
alternative payment methodology pursuant to section 1899(i)(3) of the Act if we determine that
the alternative payment methodology will improve the quality and efficiency of items and
services furnished to Medicare beneficiaries, without resulting in additional program
expenditures.
The proposed adjustments would remove improper payments from the performance year
expenditures and factors used to calculate updated historical benchmarks, among other financial
calculations, that resulted in inaccuracies in an ACO’s payment determination, including the
amount of shared savings CMS paid an ACO or the amount of shared losses owed to CMS by an
ACO participating under a two-sided model. These policies improve the accuracy of financial
calculations by which ACOs are held accountable for the cost and quality of care for their
assigned beneficiary populations.
Addressing the impact of improper payments on ACO payment determinations could
serve as a mechanism to bolster program integrity. ACO accountability for the total cost of care
can deter fraud, waste, and abuse that is otherwise under the control of ACO participants.
Additionally, ACOs have unique insight into Medicare Part A, B, and D claims data for their
assigned beneficiary populations from monthly claim and claim line level data ACOs receive
from CMS for care coordination and quality improvement. This vantage point makes ACOs
uniquely situated to observe trends in expenditures and utilization patterns, including by
providers and suppliers that are not participating in the ACO. Further establishing policies to
specify the approach to excluding improper payments from Shared Savings Program calculations
could encourage ACOs to report to CMS and the Department of Health and Human Services
Office of Inspector General (HHS-OIG) potential fraud and abuse within the Medicare program.
Addressing improper payments in the Medicare program would protect the accuracy, fairness,
and integrity of Shared Savings Program financial calculations, and lead to greater beneficiary
protections, and protection of the Trust Funds.
Accounting for the impact of improper payments in financial calculations promotes
continued integrity and fairness of Shared Savings Program payment determinations and may in
turn bolster ACO participation in the Shared Savings Program. Policies that improve the
accuracy of the payment calculations could provide greater certainty to organizations considering
entering or continuing their participation in the Shared Savings Program and thereby lead to
more robust and sustained participation by ACOs in the Shared Savings Program. This, in turn,
means that these organizations would continue working towards meeting the Shared Savings
Program’s goals of lowering growth in Medicare FFS expenditures and improving the quality of
care furnished to Medicare beneficiaries.
As described in the Regulatory Impact Analysis (section VII. of this proposed rule), we
believe accounting for the impact of improper payments on performance year expenditures and
factors used to calculate updated historical benchmarks would not result in an increase in
spending beyond the expenditures that would otherwise occur under the statutory payment
methodology in section 1899(d) of the Act. As we also discuss elsewhere in this section of this
proposed rule, across an ACO’s reconciliations where improper payments impact performance
year or BY expenditures, the overall net impact of using the proposed methodology on the
ACO’s aggregate shared savings or shared losses across those reconciliations could be positive
or negative and would depend on the circumstances of a given reopening scenario.
We will continue to reexamine this projection in the future to ensure that the requirement
under section 1899(i)(3)(B) of the Act that an alternative payment model not result in additional
program expenditures continues to be satisfied. In the event that we later determine that the
payment model established under section 1899(i)(3) of the Act no longer meets this requirement,
we would undertake additional notice and comment rulemaking to make adjustments to the
payment model to assure continued compliance with the statutory requirements.
(d) Adjusting Historical Benchmarks to Account for the Impact of Improper Payments
CMS adjusts an ACO’s historical benchmark annually, during the term of the ACO’s
agreement period, to account for certain changes, as specified in the Shared Savings Program
regulations. The related adjustment is specified under § 425.601(a)(9), for the benchmarking
methodology applicable to agreement periods beginning on or after July 1, 2019, and before
January 1, 2024, and under § 425.652(a)(9), for the benchmarking methodology applicable to
agreement periods beginning on January 1, 2024, and in subsequent years. As finalized with the
CY 2024 PFS final rule (88 FR 79195 through 79200), § 425.652(a)(9) introductory text was
amended to specify, among other changes, that for each performance year during the term of the
agreement period, the ACO’s benchmark is adjusted for changes in values used in benchmark
calculations as a result of issuance of a revised initial determination under § 425.315 (among
other factors). Similar language is not currently included in § 425.601(a)(9) introductory text.
We propose to use our authority under section 1899(d)(1)(B)(ii) of the Act to adjust the
benchmark to account for the impact of improper payments, in the event CMS recalculates a
payment determination and issues a revised initial determination for a performance year in a
prior agreement period that corresponds to a benchmark year of the ACO’s current agreement
period. We propose to adjust an ACO’s historical benchmark for use in reaching an initial
determination of financial performance for a performance year, in cases where an ACO has a
benchmark year that corresponds to a performance year for which we issued a revised initial
determination. In such a case, we would apply the same methodology to recalculate the ACO’s
BY expenditures as used in recalculating the expenditures for the corresponding performance
year, as part of a reopening. Under this proposed approach, we would be able to improve the
accuracy of the benchmark year calculations used in reaching an initial determination for a
performance year, by addressing the impact of previously identified improper payments on the
expenditure calculations. Such an adjustment to the benchmark expenditures would appropriately
calculate the ACO’s historical benchmark that might otherwise be under- or over-stated due to
improper payments.
Expanding upon the example illustrated in Table 43, if we have issued a revised initial
determination for PY 2022 in December 2025, for an ACO that renewed to continue its
participation under a new agreement period beginning on January 1, 2025, our proposed policy
would enable us to use the same methodology for calculating BY 2022 expenditures for PY
2025, in reaching the initial determination for PY 2025.
We propose to amend §§ 425.601(a)(9) and 425.652(a)(9) to specify this proposed
adjustment to the historical benchmark. We propose to revise § 425.601(a)(9) introductory text
to further specify that for the second and each subsequent performance year during the term of
the agreement period, the ACO’s benchmark would be adjusted for changes in values used in
benchmark calculations as a result of issuance of a revised initial determination under § 425.315.
We also propose to add a new paragraph (a)(9)(iii) to § 425.601 and to add a new paragraph
(a)(9)(viii) to § 425.652, each specifying that we would recalculate benchmark year expenditures
to account for the impact of improper payments, for the benchmark year corresponding to a
performance year for which CMS issued a revised initial determination under § 425.315. In
recalculating expenditures for the benchmark year, CMS would apply the same calculation
methodology applied in recalculating expenditures for the corresponding performance year, in
accordance with the proposed new section of the regulation at § 425.674.
(e) ACO Reopening Requests
The following discussion of requesting and conducting a reopening pertains to reopening a
payment determination for good cause or for fraud or similar fault, unless specified otherwise.
We propose to establish a process at § 425.315(b) by which an ACO may request a
reopening of an initial determination, or a final agency determination under subpart I, of shared
savings or shared losses. Although an ACO’s submission of a reopening request is optional, we
propose to require that the ACO’s request be in a form and manner specified by CMS. Further,
we propose that the timing of the ACO’s reopening request must be consistent with the
timeframes specified in § 425.315(a)(1)(i) and (ii), respectively, either (i) at any time in the case
of fraud or similar fault, or (ii) not later than 4 years after the date of the notification to the ACO
of the initial determination of savings or losses for the relevant performance year for good cause.
We anticipate providing additional information on the reopening request process for ACOs
through guidance, including the form and manner in which CMS must receive a reopening
request.
CMS will need to receive sufficient, detailed information from ACOs to evaluate an
ACO’s reopening request. For instance, in the case of a reopening request in connection with
improper payments, or fraud or similar fault potentially impacting the ACO’s financial
calculations, receiving detailed information about the issue, including the following information,
would aid in our analysis of the ACO’s request:
● ACO identifier(s) (also referred to as “ACO ID”) and Legal Business Name(s).
● Identity of the provider or supplier for which there may be improper payment(s), or
that may be suspected of fraud or similar fault, including name, NPI or Provider Transaction
Access Number (PTAN), TIN, or other identifier.
● Time period during which potentially impacted claims were submitted or improper
conduct occurred.
● Short description of the improper payment, alleged fraud or similar fault, and how it
was identified, including information such as any specific claim type codes and HCPCS or CPT
codes.
● Evidence of financial impact on the ACO’s shared savings or shared losses
calculation, such as any analysis supporting the calculation of financial impact to the ACO and a
list of beneficiaries assigned to the ACO for whom claims were submitted by the provider or
supplier suspected of fraud or similar fault, or for which expenditures may be impacted by
improper payments.
As we describe elsewhere in this section of this proposed rule, a recalculation of shared
savings and shared losses to account for improper payments could result in a variety of
outcomes. An ACO should weigh these potential outcomes when considering whether to submit
a reopening request.
We acknowledge that the proposed process for requesting a reopening, whether for good
cause or for fraud or similar fault, would represent a new process. Therefore, we seek comments
and suggestions on the form and manner in which CMS should receive these requests. We also
seek comment on approaches to ensuring that ACOs submit reopening requests with sufficient
information to allow CMS to identify and evaluate the impact on Shared Savings Program
financial performance.
The following steps illustrate how we may conduct review of an ACO’s request to reopen
a payment determination to account for the impact of improper payments:
● Upon receiving an ACO’s reopening request, CMS would evaluate this request, and
ask the requesting ACO to provide supplemental information if needed.
● We would work with CPI and law enforcement agencies to identify, validate and
quantify improper payments potentially impacting expenditures used in program calculations.
We note that identification of improper payments may be contingent on the conclusion of an
investigation that is underway.
● We may conduct initial analysis to consider the basis for reopening the ACO’s
payment determination under § 425.315(a), and the significance of the improper payments to an
ACO’s financial calculations under the Shared Savings Program (as described in section
III.G.7.c.(2).(b) of this proposed rule):
++ If we find that the potential improper payment does not meet CMS’ standards for
reopening the payment determination, we anticipate notifying the ACO of our decision.
++ If we reach a determination to reopen the ACO’s payment determination for a
performance year:
-- We would recalculate expenditures to account for improper payments using the
methodology proposed in section III.G.7.c.(2).(c) of this proposed rule, recalculate the ACO’s
shared savings and shared losses, issue a revised initial determination, and engage in payment
activities and recoupment activities, as needed.
-- During the recalculation period CMS would also identify whether the relevant
performance year is also serving as a benchmark year for the ACO’s current agreement period
and prepare to adjust the ACO’s benchmark year expenditures to account for the revised initial
determination (once issued), as discussed in section III.G.7.c.(2).(d) of this proposed rule.
In the event that improper payments identified in analyzing an ACO’s reopening request
have the potential to impact the payment determinations of one or more other ACOs, we
anticipate only determining whether to reopen the payment determination for an ACO that
submitted the reopening request. More generally, we may initiate analysis of the impact of
improper payments on Shared Savings Program financial calculations, and potentially reopen the
payment determination for one or more ACOs absent an ACO’s request for reopening. For
instance, in learning of improper payments that may potentially impact Shared Savings Program
calculations for multiple ACOs, including through the reopening request process, we may seek to
reopen payment determinations where improper payments are anticipated to result in significant
adjustments to ACOs’ initial determinations upon recalculation. We also anticipate initiating
analysis of the impact of improper payments on an ACO’s payment determination upon learning
of improper payments originating inside the ACO that may potentially impact Shared Savings
Program calculations, and may reopen the ACO’s payment determination, as needed, to address
program integrity concerns.
We anticipate that our review and analysis of reopening requests could occur over a
protracted period of time during which we may be able to provide little additional information to
the ACO until we have reached our decision. We would aim to conduct a reopening such that the
timing of any issuance of a revised initial determination aligns with the timeframe for when
CMS typically completes annual performance year financial reconciliation and payment and
recoupment. However, because investigations into improper payments, including considering
whether there is reliable evidence of fraud or similar fault, may involve varying degrees of
complexity and scale, and because the application of our proposed methodology for calculating
expenditures relies on information that may result from such investigations, among other sources
of information, CMS may not always be able to conduct a reopening within a specific timeframe
after an ACO submits a reopening request. As reflected in the discussions within this section of
this proposed rule, the process for analyzing an ACO’s reopening request, reaching a decision on
whether to reopen the initial determination, recalculating the ACO’s payment determination, and
issuing a revised initial determination, may occur over a period of months or potentially years,
and may have impacts on future agreement periods. In cases where CMS and law enforcement
officials may have investigations underway, CMS must refrain from providing details to ACOs,
and other individuals or entities, of pending actions to protect the integrity of those
investigations. Therefore, we may be limited in the information we can communicate to an ACO
about our consideration of the ACO’s reopening request.
We seek comment on the aforementioned considerations for how we could conduct
review of an ACO’s request to reopen a payment determination to account for the impact of
improper payments. More generally, as we gain additional experience with ACOs’ submission of
reopening requests, including the volume of the requests, and nature of the requests, we may
revisit the reopening request process, as needed, in future notice and comment rulemaking.
(f) Preventing and Reporting Medicare Fraud
ACOs can help prevent fraud and abuse within the Medicare program or in other Federal
health care programs. Program integrity requirements for the Shared Savings Program include
the requirement under §425.300 that the ACO must have a compliance plan. Among other
required elements, an ACO’s compliance plan must include a method for employees or
contractors of the ACO, ACO participants, ACO providers/suppliers, and other individuals or
entities performing functions or services related to ACO activities to anonymously report
suspected problems related to the ACO to the compliance officer (§ 425.300(a)(3)). ACOs’
compliance plans must also include a requirement for the ACO to report probable violations of
law to an appropriate law enforcement agency (§ 425.300(a)(5)). (Refer to the November 2011
final rule, 76 FR 67951 and 67952.)
ACOs are encouraged to report potential fraud or abuse by submitting a complaint to the
CMS Center for Program Integrity (CPI), Fraud Investigations Group (FIG), Division of
Provider Investigations (DPI) at [email protected]. ACOs can also report potential fraud
or abuse by submitting a complaint to the HHS-OIG website, https://oig.hhs.gov/fraud/report-
fraud/, HHS-OIG hotline at 1-800-HHS-TIPS (1-800-447-8477), TTY at 1-800-377-4950, by fax
at 1-800-223-8164, or by mailing to: Office of Inspector General ATTN: OIG HOTLINE
OPERATIONS P.O. Box 23489 Washington, DC 20026. ACOs suspecting healthcare fraud,
waste, or abuse are encouraged to visit the CMS Center for Program Integrity’s webpage on
Reporting Fraud, at https://www.cms.gov/medicare/medicaid-coordination/center-program-
integrity/reporting-fraud for more information. More generally, anyone suspecting healthcare
fraud, waste or abuse is encouraged to report it to CMS or HHS-OIG.
In the absence of a reopening request submitted by an ACO in the form and manner
specified by CMS, as discussed in section III.G.7.c.(2).(e) of this proposed rule, the reporting of
potential fraud or abuse to CPI or HHS-OIG does not itself constitute a reopening request under
the Shared Savings Program.
We seek comment on the proposals, and considerations, described in this section of this
proposed rule, including: considerations for reopening a payment determination to account for
improper payments; the proposed changes to the regulations to specify (1) CMS’ discretion to
reopen payment determinations, (2) a calculation methodology to account for the impact of
improper payments in recalculating expenditures and payment amounts used in Shared Savings
Program financial calculations upon reopening a payment determination pursuant to § 425.315,
(3) an adjustment to the historical benchmark to account for the impact of improper payments,
and (4) a process for ACOs to request reopening of a payment determination. We also welcome
comments on considerations in connection with ACOs’ potential role in preventing and reporting
Medicare fraud.
d. Mitigating the Impact of Significant, Anomalous, and Highly Suspect Billing Activity on
Shared Savings Program Financial Calculations in Calendar Year 2024 or Subsequent Calendar
Years
(1) Background
(a) Statutory Background on Shared Savings Program Financial Calculations
Section 1899 of the Social Security Act (the Act) (42 U.S.C. 1395jjj), as added by section
3022 of the Patient Protection and Affordable Care Act (Pub. L. 111-148, enacted March 23,
2010), establishes the general requirements for payments to participating ACOs in the Shared
Savings Program. Specifically, section 1899(d)(1)(A) of the Act provides that providers of
services and suppliers participating in an ACO will continue to receive payment under the
original Medicare fee-for-service program under Medicare Parts A and B in the same manner as
they would otherwise be made. However, section 1899(d)(1)(A) of the Act also provides for an
ACO to receive payment for shared savings provided that the ACO meets both the quality
performance standards established by the Secretary and demonstrates that it has achieved savings
against a benchmark of expected average per capita Medicare FFS expenditures. Additionally,
section 1899(i) of the Act authorizes the Secretary to use other payment models in place of the
one-sided model described in section 1899(d) of the Act. This provision authorizes the Secretary
to select a partial capitation model or any other payment model that the Secretary determines will
improve the quality and efficiency of items and services furnished to Medicare beneficiaries
without additional program expenditures. We have used our authority under section 1899(i)(3) of
the Act to establish the Shared Savings Program’s two-sided payment model (see for example,
80 FR 32771 and 32772, and 83 FR 67834 through 67841) and to mitigate shared losses owed by
ACOs affected by extreme and uncontrollable circumstances during PY 2017 and subsequent
performance years (82 FR 60916 and 60917, 83 FR 59974 through 59977), among other uses of
this authority described elsewhere in this proposed rule.
Section 1899(d)(1)(B)(i) of the Act specifies that, in each year of the agreement period,
an ACO is eligible to receive payment for shared savings only if the estimated average per capita
Medicare expenditures under the ACO for Medicare FFS beneficiaries for Parts A and B
services, adjusted for beneficiary characteristics, is at least the percent specified by the Secretary
below the applicable benchmark under section 1899(d)(1)(B)(ii) of the Act. Section
1899(d)(1)(B)(ii) of the Act addresses how ACO benchmarks are to be established and updated
under the Shared Savings Program. This provision specifies that the Secretary shall estimate a
benchmark for each agreement period for each ACO using the most recent available 3 years of
per beneficiary expenditures for Parts A and B services for Medicare FFS beneficiaries assigned
to the ACO. This benchmark shall be adjusted for beneficiary characteristics and such other
factors as the Secretary determines appropriate and updated by the projected absolute amount of
growth in national per capita expenditures for Parts A and B services under the original Medicare
FFS program, as estimated by the Secretary.
In past rulemaking, we have used our authority under sections 1899(d)(1)(B)(ii) and
1899(i)(3) of the Act to establish adjustments to the benchmark and program expenditure

calculations, respectively, to exclude certain Medicare Parts A and B payments. In the November
2011 final rule (76 FR 67920 through 67922), we adopted an alternate payment methodology
that excluded Indirect Medical Education (IME) and Disproportionate Share Hospital (DSH)
payments from ACO benchmark and performance year expenditures due to concerns that the
inclusion of these amounts would incentivize ACOs to avoid referring patients to the types of
providers that receive these payments. In the CY 2023 PFS final rule (87 FR 69954 through
69956), we excluded new supplemental payments to Indian Health Service/Tribal hospitals and
hospitals located in Puerto Rico consistent with our longstanding policy to exclude IME, DSH
and uncompensated care payments from ACOs’ assigned and assignable beneficiary expenditure
calculations. In the May 8, 2020 COVID-19 IFC (85 FR 27577 through 27582), we established a
methodology to adjust Shared Savings Program financial calculations to account for the COVID-
19 Public Health Emergency. Specifically, we established a methodology that would exclude all
Medicare Parts A and B FFS payment amounts for a beneficiary’s episode of care for treatment
of COVID-19 to prevent distortion to, among other calculations, an ACO’s benchmark and
program expenditure calculations.
(b) Background on Significant, Anomalous, and Highly Suspect Billing Activity
Recently, ACOs and other interested parties have raised concerns about an increase in
billing to Medicare for selected intermittent urinary catheter supplies on Durable Medical
Equipment, Prosthetics, Orthotics & Supplies (DMEPOS) claims in CY 2023, alleging that the
increase in payments represents fraudulent activity (the “alleged conduct”). The observed
DMEPOS billing volume for intermittent urinary catheters in CY 2023 represents significant,
anomalous, and highly suspect (SAHS) billing activity.
525
525
SAHS billing activity may appear in claims for items and services rendered to beneficiaries assigned to an ACO
as well as for beneficiaries who are not assigned to an ACO. Such activity may be caused by providers and suppliers
who participate in an ACO and who do not participate in an ACO. This discussion is primarily focused on SAHS
billing activity performed by providers and suppliers that do not participate in ACOs billing items and services for
beneficiaries who are assigned to ACOs or who are in the assignable population used in national and regional factors
used in Shared Savings Program calculations.

Generally, a level of billing for a given HCPCS or CPT code is considered SAHS billing
activity when a given HCPCS or CPT code exhibits a level of billing that represents a significant
claims increase either in the volume or dollars (for example, dollar volume significantly above prior
year, or claims volume beyond expectations) with national or regional impact (for example, not
only impacting one or few ACOs) and represents a deviation from historical utilization trends
that is unexpected and is not clearly attributable to reasonably explained changes in policy or the
supply or demand for covered items or services. The billing level is significant and represents
billing activity that would cause significantly inaccurate and inequitable payments and
repayment obligations in the Shared Savings Program if not addressed.
Current Shared Savings Program regulations, codified at 42 CFR part 425, do not provide
a basis for CMS to adjust program expenditure or revenue calculations to remove the impact of
SAHS billing activity such as that arising from the alleged conduct in advance of issuing an
initial determination. As discussed in section III.G.7.c of this proposed rule, CMS may reopen an
initial determination or a final agency determination and issue a revised initial determination at
any time in the case of fraud or similar fault, and not later than 4 years after the date of the
notification to the ACO of the initial determination of savings or losses for the relevant
performance year for good cause (§ 425.315). This does not allow for CMS to address SAHS
billing activity, which must be addressed prior to conducting financial reconciliation, which is an
initial determination, to prevent significant inequity and inaccurate payment determinations.
We are concerned that such SAHS billing activity, should it occur in CY 2024 or later,
would inflate Medicare Parts A and B payment amounts and affect Shared Savings Program
calculations, including:
● Performance year reconciliation calculations, including expenditures for each ACO’s
assigned beneficiaries for the calendar year that has SAHS billing activity, the national-regional
blended update factor used to update the benchmark for ACOs beginning an agreement period
before January 1, 2024 (refer to § 425.601(b)), the three-way blended update factor used to

update the benchmark for ACOs beginning an agreement period on January 1, 2024 and in
subsequent years (refer to § 425.652(b)), and factors based on ACO participant revenue to
determine the loss recoupment limits for ACOs participating under two-sided models of the
BASIC track (Levels C, D, E) (refer to § 425.605(d)).
● Historical benchmark calculations for establishing the benchmark for ACOs beginning
new agreement periods on January 1, 2025, or in subsequent years with a benchmark year that
has SAHS billing activity (refer to § 425.652(a)).
● Factors used in the application cycle for ACOs applying to enter a new agreement
period beginning two years after the SAHS billing activity occurred, and the change request
cycle for ACOs continuing their participation in the program, including data used to determine
an ACO’s eligibility for Advance Investment Payments under § 425.630(b) or for the CMS
Innovation Center’s new ACO Primary Care Flex Model (ACO PC Flex Model) based on ACO
revenue status (high revenue or low revenue), and to determine repayment mechanism amounts
for ACOs entering, or continuing in, two-sided models (refer to § 425.204(f)).
The accuracy of the Shared Savings Program’s determination of an ACO’s financial performance
(through a process referred to as financial reconciliation) in terms of the ACO’s eligibility for
and amount of a shared savings payment or liability for shared losses, depends on the accuracy
of claims data. Absent CMS action, SAHS billing activity would affect performance year
financial reconciliation program-wide rather than being limited to ACOs that have assigned
beneficiaries directly impacted by the issue. For instance:
● An ACO with assigned beneficiaries impacted by the SAHS billing activity will see an
increase in performance year expenditures, reducing the ACO’s shared savings or increasing the
amount of shared losses owed by the ACO. The impact on the ACO’s performance may be
partially mitigated if the SAHS billing activity also increases the ACO’s regional service area
expenditures and the national expenditures used to calculate the two-way national-regional
blended benchmark update factor.

● An ACO with assigned beneficiary expenditures and regional service area
expenditures with little or no impact from the SAHS billing activity will receive a relatively
higher benchmark update under the national-regional blended update factors used in performance
year reconciliation, and therefore, may appear to perform better as a result of the national impact
of the SAHS billing activity, resulting in higher earned performance payments or lower or no
losses for the ACO.
Unaddressed, SAHS billing activity in a given calendar year can distort the historical
benchmarks for an ACO in an agreement period that have the calendar year as a benchmark year
and the accuracy of any future financial reconciliation performed against those benchmarks.
Similarly, inaccurate revenue and expenditure calculations based on data from a calendar year
affected by SAHS billing activity may affect an ACO’s revenue status and the amount of funds
an ACO in a two-sided model must secure as a repayment mechanism, one of the program’s
important safeguards for protecting the Medicare Trust Funds. Absent CMS action, SAHS billing
activity likely would significantly impact shared savings and losses calculations for the
performance year affected by SAHS billing activity, and for future performance years that have
benchmark years affected by SAHS billing activity. Under these circumstances, some ACOs
would likely experience adverse impacts (for example, lower or no shared savings or higher
shared losses) while other ACOs would experience windfall gains (for example, higher shared
savings or lower or no shared losses).
Failing to address SAHS billing activity would jeopardize the integrity of the Shared
Savings Program. There are 480 ACOs in the Shared Savings Program with over 608,000 health
care providers who care for 10.8 million assigned FFS beneficiaries.
526
In PY 2022, the most
recent year for which data is available, savings achieved by ACOs relative to benchmarks
amounted to $4.3 billion, of which ACOs received shared savings payments totaling $2.5 billion,
526
Refer to CMS, Shared Savings Program Fast Facts—As of January 1, 2024, available at
https://www.cms.gov/files/document/2024-shared-savings-program-fast-facts.pdf.

and Medicare retained $1.8 billion in savings.
527
ACOs are held accountable for 100 percent of
total Medicare Parts A and B expenditures for their assigned beneficiary populations (with
limited exceptions). This incentivizes ACOs to generate savings for the Medicare program as
they have the opportunity to share in those savings if certain requirements are met. It also
discourages the ACO from generating unnecessary expenditures for Medicare as they may be
required to repay those amounts to CMS. Accountable care arrangements such as this cannot
function if the ACO may be held responsible for all SAHS billing activity that is outside of their
control. Holding an ACO accountable for substantial losses due to SAHS billing activity is not
only inequitable but will dramatically increase the level of risk associated with participation,
making the Shared Savings Program unattractive.
In a separate proposed rule entitled “Medicare Program: Mitigating the Impact of
Significant, Anomalous, and Highly Suspect Billing Activity on Medicare Shared Savings
Program Financial Calculations in Calendar Year 2023” (89 FR 55168, July 3, 2024) (referred to
herein as the SAHS billing activity proposed rule), we have proposed an approach to address the
SAHS billing activity identified for CY 2023 to protect the accuracy, fairness, and integrity of
Shared Savings Program financial calculations. Specifically, we have proposed to exclude
payment amounts for two HCPCS codes (A4352 (Intermittent urinary catheter; Coude (curved)
tip, with or without coating (Teflon, silicone, silicone elastomeric, or hydrophilic, etc.), each)
and A4353 (Intermittent urinary catheter, with insertion supplies)) on DMEPOS claims
submitted by any supplier from expenditure and revenue calculations used for: assessing
performance year (PY) 2023 financial performance of Shared Savings Program ACOs,
establishing benchmarks for ACOs starting agreement periods in 2024, 2025, and 2026, and
calculating factors used to determine revenue status and repayment mechanism amounts in the
application and change request cycle for ACOs applying to enter a new agreement period
527
Refer to CMS, Shared Savings Program Performance Year Financial and Quality Results, 2022, available at
https://data.cms.gov/medicare-shared-savings-program/performance-year-financial-and-quality-results/data.
beginning on January 1, 2025, or continue their participation in the program in PY 2025,
respectively. There will be a 30-day public comment period on the SAHS billing activity
proposed rule so as to minimize the negative impact on ACOs of a delay in the issuance of PY
2023 initial financial determinations and disbursement of performance payments, as well as to
other program milestones, necessitated by the calculation of amounts under the financial
methodology proposed in the SAHS billing activity proposed rule.
(2) Proposed Revisions
It is important to establish a policy that would allow CMS to proactively make similar
adjustments for future calendar years, should new SAHS billing activity be identified. In general,
we anticipate that billing activity that meets the high bar to be considered significant, anomalous,
and highly suspect billing activity will be a rare occurrence. This is evidenced by the program’s
history. The SAHS billing activity surrounding selected catheter codes in 2023 is the first
occasion we have had in the program’s 12-year history to consider this issue. We propose that
we would notify ACOs and ACO applicants of our determinations to remove any codes and the
aggregate per capita dollar amount of the codes removed as part of the annual financial
reconciliation process. While we anticipate future occurrences of the scope and magnitude
observed for urinary catheters in CY 2023 to be rare, having a permanent policy in place would:
● Allow CMS to move quickly to make adjustments to financial calculations without
having to engage in additional rulemaking, ensuring timely issuance of initial determinations of
savings and losses and disbursement of earned performance payments;
● Provide ACOs with greater certainty that they will not be held accountable for SAHS
billing activity that is out of their control, promote integrity and fairness and ensure accuracy of
program calculations;
● Limit requests to reopen initial determinations, thus reducing burden for ACOs and
CMS.
In this proposed rule we are therefore proposing an approach by which we would adjust Shared
Savings Program calculations to mitigate the impacts of SAHS billing activity occurring in CY
2024 or subsequent calendar years.
(a) Identifying Significant, Anomalous, and Highly Suspect Billing Activity
We propose that CMS would have the sole discretion to identify cases of SAHS billing
activity for a particular calendar year that would warrant the adjustment of Shared Savings
Program financial calculations. We anticipate routinely examining billing trends identified by
CMS and other relevant information that had been raised through complaints by ACOs or other
interested parties to the HHS-OIG or to the CMS CPI. We would seek to identify and monitor
any codes that would potentially trigger the adjustment policy by meeting the high bar for
removal under the criteria used to determine SAHS billing activity. Shortly after the start of a
calendar year CMS would make a final determination as to which codes, if any, warrant
adjustments for the previous calendar year. For example, in early CY 2026 CMS would make a
final determination of whether any codes met the high bar for removal under the criteria used to
determine SAHS billing activity in CY 2025, allowing time for the adjustments to be
incorporated in forthcoming calculations.
CMS must retain sole discretion to identify cases of SAHS billing activity because we
cannot anticipate what SAHS billing activity we may encounter in the future that may warrant
adjustments to the program’s financial calculations. We are also concerned about balancing
adjustments for billing activity that rises to the level of SAHS versus removing payment amounts
associated with billing activity due to inefficiencies that are within the ACO’s control.
Depending on the frequency of the use of this authority and the occurrence of SAHS billing
activity, and thus the experience we develop in this area, we would consider proposing to codify
criteria to identify SAHS billing activity in the future through additional rulemaking.
Nonetheless, we believe that CMS should retain sole discretion to determine whether SAHS
billing activity occurred on a case-by-case basis at this time.
We anticipate considering multiple criteria in determining whether SAHS billing activity
warrants removal of the corresponding billing codes from Shared Savings Program financial
calculations. These criteria include:
● The observed increase in claims for a HCPCS or CPT code year-to-year meets the
definition of significant, anomalous, and highly suspect billing activity, as defined elsewhere in
this section of this proposed rule;
● The observed billing activity has national or regional impact or significance, such as:
++ Involves a Medicare provider or supplier, a beneficiary population and/or states with
claims activity that that significantly impacts national or regional expenditure values or trends;
++ Warrants adjustment (all or partial) to national Medicare expenditure trend
calculations used in payment (for example, United States Per Capita Cost) and/or Federal budget
forecast calculations;
++ Warrants removal from national and regional growth rates used to update ACO
historical benchmarks;
● If no action is taken there would be an imbalance between ACO performance year and
historical benchmark year expenditures;
● Use of payment amounts associated with the SAHS billing activity could result in
payment inaccuracies that produce significantly inaccurate and inequitable payment
determinations in the Shared Savings Program (including the amount of shared savings or shared
losses), due to factors beyond the control of ACOs; and
● The claims in question may be disproportionately represented by Medicare providers
or suppliers whose Medicare enrollment status has been revoked.
We anticipate utilizing this authority only in rare and extreme cases where a number of
the criteria are satisfied. We anticipate we would consider the extent to which the billing activity
meets each criterion when developing a holistic assessment of the billing activity’s impact on the
Shared Savings Program.

The extent of the geographic impact of the SAHS billing activity in question is relevant
given that the proposed policy would entail adjustments program-wide. One consideration for
determining whether the billing activity has national or regional significance would be if the
pattern warrants an adjustment to or special assumption for calculating official Medicare
expenditure trends (such as the United States Per Capita Cost (USPCC) or Federal budget
forecasts) due to the activity’s significant, anomalous, and highly suspect nature. For example,
the 2024 Medicare Trustees Report noted a significant increase in suspected fraudulent spending
on certain intermittent catheters in 2023.
528
The DME projections in the report include the
assumption that this suspected fraud will be addressed during 2024.
529
Billing activity in the
Medicare FFS program at a scale warranting a special assumption for calculating the USPCC or
Federal budget forecasts has per se national or regional significance, and thus would likely rise
to the high bar of warranting adjustment to Shared Savings Program expenditure and revenue
calculations.
We would seek to assess whether the billing activity creates an imbalance between ACO
performance year and historical benchmark year expenditures. This assessment could involve
considering whether the increase in billing activity was at such scale that it causes the difference
between performance year and benchmark year expenditures for an ACO’s assigned beneficiary
population for the claim type affected by the billing activity (for example, DMEPOS) to be
substantially larger than differences for other claim types.
We would also consider whether the billing activity, and any inaccurate or inequitable
payment determinations that could result from using the related payment amounts, was outside of
Shared Savings Program ACOs’ ability to reasonably control. Most commonly, this would entail
examining whether the Medicare providers or suppliers billing the codes in question are ACO
528
The Boards of Trustees, Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Funds,
“2024 Annual Report of the Boards of Trustees of the Federal Hospital Insurance and Federal Supplementary
Medical Insurance Trust Funds”, p. 136, available at https://www.cms.gov/oact/tr/2024.
529
Ibid.
providers or suppliers. Generally, we would be more likely to apply the proposed policy if the
SAHS billing activity were outside of the ACO’s control as the program may otherwise lack a
means to control the growth of such amounts.
Finally, we would consider whether billing activity were disproportionately represented
by Medicare providers or suppliers whose Medicare enrollment status has been revoked. Such a
circumstance would provide further evidence that the billing activity surrounding these codes
was highly suspect. We seek comment on the processes and criteria described.
(b) Adjustments to Shared Savings Program Calculations
In the event that CMS identifies one or more HCPCS or CPT codes with SAHS billing
activity in CY 2024 or a subsequent calendar year that warrant adjustment, we propose to
exclude all Medicare Parts A and B payment amounts associated with the identified codes on
specified claim types submitted by any provider or supplier from expenditure and revenue
calculations for the relevant calendar year for which the SAHS billing activity is identified. For
example, if CMS identifies one or more codes with SAHS billing activity in CY 2025 that
warrant adjustment, we would exclude payments for those codes for both calculations where CY
2025 is the performance year and in calculations where CY 2025 is a benchmark year for ACOs
in agreement periods beginning in 2026, 2027, and 2028.
We propose that we would also adjust the 3 most recent years prior to the start of the
ACO’s agreement period used in establishing the historical benchmark that is used to reconcile
the ACO for a performance year corresponding to the calendar year for which the SAHS billing
activity was identified. In the example where CMS identified SAHS billing activity for 2025, we
would adjust benchmark expenditures (ACO, national, and regional) for 2019, 2020, and 2021,
for an ACO that began an agreement period in 2022 (for which PY 2025 is the fourth
performance year in its agreement period) and would adjust benchmark expenditures (ACO,
national, and regional) for 2022, 2023, and 2024 for an ACO that began its agreement period in
2025 (for which PY 2025 is the first performance year in its agreement period). Note that in
computing benchmark expenditures for 2023 for this second ACO, because 2023 is a benchmark
year, CMS would also exclude payments for the catheter claims with SAHS billing activity in
2023, as proposed in the SAHS billing activity proposed rule, if finalized.
Our proposal to adjust an ACO’s historical benchmark to exclude Medicare Parts A and
B FFS payment amounts associated with the HCPCS or CPT codes displaying SAHS billing
activity during a performance year would achieve greater consistency between the benchmark
period and the performance year, given that we are excluding all payments on specified claim
types for the selected codes from performance year calculations, including payments that would
have been made in the absence of any SAHS billing activity. This helps to ensure a balance
between the benchmark and the performance year such that an ACO is not unfairly benefitting
from a benchmark that includes certain expenditures that are excluded from the performance
year. Under our proposal, we would identify any codes warranting adjustment at the start of the
next calendar year and our operational schedule would accommodate the additional calculations
required. Therefore, we anticipate we would be able to compute adjusted historical benchmarks
for the affected reconciliation with minimal, if any, delays to the typical timeline for issuing
initial determinations.
We propose that we would provide the historical benchmark that has been adjusted to
exclude payment amounts for HCPSC or CPT codes associated with SAHS billing activity
occurring in the performance year being reconciled to ACOs as part of their financial
reconciliation settlement package for the performance year, as opposed to providing a separate
new historical benchmark report in advance of settlement. This approach is consistent with what
we have done for rare past occasions where we computed revised benchmarks immediately prior
to reconciliation to correct for late-breaking data issues. Consistent with existing operational
practice, in calculating these adjusted benchmarks, we would recompute ACO expenditures
using beneficiary assignment data that was generated during the performance year being
reconciled for all ACOs. For example, if computing adjusted historical benchmarks for PY 2025

to exclude claim payments for codes with SAHS billing activity during the performance year, we
would use beneficiary assignment data generated during CY 2025. Although the benchmark year
assignment data generated during the performance year being reconciled would be based on the
same ACO participant list, assignment methodology selection under § 425.226(a)(1), and
assignment methodology under subpart E of Part 425 of the regulations as used in calculating the
ACO’s most recent prior benchmark, other factors, such as more recent Medicare beneficiary
eligibility data along with the ACOs included in the claims-based assignment competition, could
differ and impact an ACO’s assigned population. We considered whether to provide ACOs with
their adjusted benchmark at the time we announce our determination of SAHS billing activity for
a given calendar year (anticipated to occur near the start of the next calendar year), however we
concluded this would delay other important program milestones, such as the issuance of
preliminary and adjusted historical benchmarks for the new performance year.
When the calendar year with SAHS billing activity becomes a benchmark year, we are
proposing adjustments to calculations for the calendar year itself, and not for other years in the
benchmark period, or the performance years that will be reconciled against those benchmarks.
Thus, in the example where we identified codes with SAHS billing activity in CY 2025, in
establishing or resetting the benchmark for an ACO entering an agreement period in 2026, we
would exclude payments for the relevant codes identified for CY 2025 from BY 2025
calculations and, if our proposed policy in the SAHS billing activity proposed rule is finalized,
would remove payments for the specified catheter codes from BY 2023 calculations. We would
not exclude the catheter codes identified as having SAHS billing activity in BY 2023 or the
codes identified for CY 2025 from either BY 2024 calculations or calculations for PY 2026 or
any subsequent performance years in the same agreement period.
530
530
This assumes these same codes were not identified as having SAHS billing activity in CY 2024 or CY 2026 or
later years.
Specifically, we are proposing to adjust the following Shared Savings Program
calculations, as applicable, to exclude all Medicare Parts A and B payment amounts associated
with a HCPCS or CPT code on claims for the specified claim types displaying SAHS billing
activity:
● Calculation of Medicare Parts A and B FFS expenditures for an ACO’s assigned
beneficiaries for all purposes including the following: Establishing, adjusting, updating, and
resetting the ACO’s historical benchmark and determining performance year expenditures.
● Calculation of FFS expenditures for assignable beneficiaries as used in determining
county-level FFS expenditures and national Medicare FFS expenditures, including the following
calculations:
++ Determining average county FFS expenditures based on expenditures for the
assignable population of beneficiaries in each county in the ACO’s regional service area
according to §§ 425.601(c) and 425.654(a) for purposes of calculating the ACO’s regional FFS
expenditures.
++ Determining the 99th percentile of national Medicare FFS expenditures for
assignable beneficiaries for purposes of the following:
-- Truncating assigned beneficiary expenditures used in calculating benchmark
expenditures under §§ 425.601(a)(4) and 425.652(a)(4), and performance year expenditures
under §§ 425.605(a)(3) and 425.610(a)(4).
-- Truncating expenditures for assignable beneficiaries in each county for purposes of
determining county FFS expenditures according to §§ 425.601(c)(3) and 425.654(a)(3).
-- Truncating expenditures for assignable beneficiaries for purposes of determining
truncated national per capita FFS expenditures for purposes of calculating the Accountable Care
Prospective Trend (ACPT) according to § 425.660(b)(3).
++ Determining truncated national per capita expenditures FFS per capita expenditures
for assignable beneficiaries for purposes of calculating the ACPT according to § 425.660(b)(3).

++ Determining national per capita expenditures for Parts A and B services under the
original Medicare FFS program for assignable beneficiaries for purposes of capping the regional
adjustment to the ACO's historical benchmark according to §§ 425.601(a)(8)(ii)(C) and
425.656(c)(3), capping the prior savings adjustment according to § 425.658(c)(1)(ii), capping the
prepaid shared savings multiplier according to § 425.640(f)(2)(v), and calculating the proposed
HEBA scaler according to § 425.662(b)(2).
++ Determining national growth rates that are used as part of the blended growth rates
used to trend forward BY1 and BY2 expenditures to BY3 according to §§ 425.601(a)(5)(ii) and
425.652(a)(5)(ii) and as part of the blended growth rates used to update the benchmark according
to §§ 425.601(b)(2) and 425.652(b)(2)(i).
● Calculation of Medicare Parts A and B FFS revenue of ACO participants for purposes
of calculating the ACO’s loss recoupment limit under the BASIC track as specified in §
425.605(d).
● Calculation of total Medicare Parts A and B FFS revenue of ACO participants and
total Medicare Parts A and B FFS expenditures for the ACO's assigned beneficiaries for
purposes of identifying whether an ACO is a high revenue ACO or low revenue ACO, as defined
under § 425.20, determining an ACO's eligibility to receive advance investment payments
according to § 425.630, and determining whether an a ACO qualifies for a shared savings
payment under § 425.605(h).
● Calculation or recalculation of the amount of the ACO’s repayment mechanism
arrangement according to § 425.204(f)(4).
This approach would recognize that SAHS billing activity has the potential to impact an
ACO’s savings and loss determination for both the performance year when the SAHS billing
activity occurred and future performance years for which the affected year is a benchmark year.
Making adjustments when the affected period represents a performance year or benchmark year
is consistent with our approach for the exclusion of payment amounts for episodes of care for

treatment of COVID-19 that we established in the May 8, 2020 COVID-19 IFC (85 FR 27577
through 27581).
The listed calculations reflect the same set of calculations that CMS adjusts for a
beneficiary’s episode of care for treatment of COVID-19, specified at § 425.611(c), as amended
by the CY 2021 PFS final rule (85 FR 85044), the CY 2023 PFS final rule (87 FR 70241), and
the CY 2024 PFS final rule (88 FR 79548), with a few exceptions. First, § 425.611(c) includes
certain provisions that are not relevant for the proposed policy.
531
Second, the proposed policy
includes calculations related to truncated national per capita expenditures used in determining the
ACPT as described in § 425.660(b)(3) that are not included in § 425.611(c),
532
as well as
references to other new or proposed calculations that do not rely on expenditures from a period
of time overlapping the Public Health Emergency for COVID-19 for the United States which
was in effect from January 27, 2020, through May 11, 2023 (capping the proposed prepaid
shared savings multiplier (§ 425.640(f)(2)(v)), calculating the proposed HEBA scaler (§
425.662(b)(2)), and determining whether an ACO that does not meet its minimum savings
requirement qualifies for a shared savings payment (§ 425.605(h)). We propose to adjust
calculations used for the ACPT to mitigate the impact of any SAHS billing activity identified for
CY 2024 or subsequent calendar years. Specifically, in projecting growth rates at the start of an
agreement period according to § 425.660, we would make an adjustment to the growth rates to
mitigate the impact that any known SAHS billing activity have on spending growth projections.
531
This includes provisions under §§ 425.600, 425.602, 425.603, 425.604, and 425.606 which are not relevant for
the proposed policy because they are not applicable to PY 2024 or later performance years or for agreement periods
where CY 2024 or later years are benchmark years. These provisions are relevant for the COVID-19 episode
exclusion policy under § 425.611 because they are applicable to performance or benchmark years that overlap with
the COVID-19 PHE.
532
When establishing the ACPT in the CY 2023 PFS final rule, we noted that the first ACPT release would be
published in 2024 for agreement periods beginning on January 1, 2024, and would provide a projected annualized
growth rate (or rates) relative to the 2023 benchmark year (BY3). We noted further that to the extent that Medicare
projections made at that time (2024) anticipated lingering effects from the COVID-19 pandemic then they would be
reflected in the ACPT (see 87 FR 69894) and we opted not to amend § 425.611 to include adjustments of ACPT-
related calculations. However, it is appropriate to propose making adjustments to ACPT-related calculations in this
proposed rule.
We believe it is unlikely that fixed growth rates projected at the start of agreement
periods beginning in earlier years may also need mitigation from a code displaying SAHS billing
activity. For example, if CMS identifies a HCPCS or CPT code displaying SAHS billing activity
in CY 2025, the projected growth rate from 2023 to 2025 – which will be used to update the
historical benchmark for PY 2025 financial reconciliation for ACOs that began an agreement
period on January 1, 2024 – would likely have assumed typical billing patterns for the code in
CY 2025. Additionally, the projected growth rate from BY 2024 to PY 2025 – which will be
used to update the historical benchmark for PY 2025 financial reconciliation for ACOs that
began an agreement period on January 1, 2025 – would likely also have assumed typical billing
patterns for the code in CY 2025 given the projections were finalized early in CY 2025.
However, if we determine a bias exists due to differences between adjustments to the
projected growth rates for the ACPT and other Shared Savings Program calculations, we could
rely on our current policy under § 425.652(b)(4)(ii) to reduce the weight of the ACPT in the
three-way blend. We propose that we would use our discretion to reduce the weight of the ACPT
rather than recalculate the growth rates that had been projected at the start of agreement periods
starting in earlier years, as we believe it is important to maintain the policy that the projected
growth rates remain fixed for the ACO’s agreement period. In the CY 2023 PFS final rule (refer
to 87 FR 69886 through 69898) we finalized our proposal to establish the ACPT at the outset of
an agreement period, based on one or more annualized growth rates. We explained that we will
not adjust the ACPT due to external factors such as geographic price changes, efficiency
discounts, or other retrospective updates occurring during the performance years throughout the
agreement period. In response to commenters concern that CMS might adjust the ACPT
downward during the agreement period, we stated that we will not adjust the ACPT projections
over the course of the agreement period (87 FR 69897). However, we acknowledged that a
variety of circumstances could cause actual expenditure trends to significantly deviate from
projections. If unforeseen circumstances occur during an ACO's agreement period we retained
flexibility to reduce the impact of the prospectively determined ACPT portion of the three-way
blend when necessary to mitigate unforeseen circumstances. We explained that we anticipate
determining, on an ad hoc basis, whether an unforeseen circumstance warrants adjustment of the
weight placed on the ACPT component of the three-way blend by considering whether it has a
material impact across the entire Shared Savings Program. If we determine that expenditure
growth has differed significantly from projections made at the start of the agreement period due
to unforeseen circumstances, such as an economic recession, pandemic, or other factors, a
reduction in the weight placed on the ACPT may be considered.
To summarize, we propose that when projecting growth rates used for the ACPT at the
beginning of an agreement period, we would make an adjustment to mitigate the impact of any
known SAHS billing activity on spending growth projections. Additionally, in accordance with
§ 425.660(a), CMS would not adjust the ACPT projections over the course of the agreement
period to account for SAHS billing activity later identified. Rather, CMS may use its discretion
to reduce the weight of the ACPT in the three-way blend in accordance with § 425.652(b)(4)(ii)
if CMS determines that the SAHS billing activity represents an unforeseen circumstance that
warrants a reduction to the weight.
The direction and magnitude of the impact of the proposed adjustments may vary by
ACO. However, by making these adjustments, we would be helping to ensure that no ACOs are
held accountable, and financially penalized for SAHS billing activity that was outside their direct
control while also protecting the Trust Funds from other ACOs potentially receiving windfall
gains.
For this proposal, we rely on our authority under section 1899(d)(1)(B)(ii) of the Act.
Section 1899(d)(1)(B)(ii) of the Act authorizes the Secretary to adjust the benchmark for
beneficiary characteristics and such other factors as the Secretary determines appropriate. Here,
we are proposing to adjust the benchmark in order to remove payments for HCPCS or CPT codes
identified as exhibiting SAHS billing activity in CY 2024 or subsequent calendar years from the

determination of benchmark expenditures when the calendar year serves as a benchmark year or
from the determination of benchmark expenditures that will be used to reconcile the calendar
year when it serves as a performance year.
We propose to use our authority under section 1899(i)(3) of the Act to remove payment
amounts for HCPCS or CPT codes identified as exhibiting SAHS billing activity in CY 2024 or
subsequent calendar years from the following calculations: (1) performance year expenditures;
(2) updates to the historical benchmark; and (3) ACO participants’ Medicare FFS revenue used
for multiple purposes across the Shared Savings Program, including determinations of loss
sharing limits in the two-sided models of the BASIC track,
533
determinations of eligibility for
advance investment payments,
534
and expanded criteria for certain low revenue ACOs
participating in the BASIC track to qualify for shared savings in the event the ACO does not
meet the minimum savings rate.
535
Section 1899(i)(3) of the Act requires that we determine that
the alternative payment methodology adopted under that provision would improve the quality
and efficiency of items and services furnished to Medicare beneficiaries, without resulting in
additional program expenditures. The adjustments we are proposing herein, which would remove
payment amounts for codes with identified SAHS billing activity from the specified Shared
Savings Program calculations as proposed in § 425.672(c) and (e), would capture and remove
from program calculations expenditures that are outside of an ACO's control, but that could
significantly affect the ACO's performance under the program. In particular, failing to remove
these payments would likely create highly variable savings and loss results for individual ACOs
that happen to have over-representation or under-representation of SAHS billing activity for the
selected codes among their assigned beneficiary populations.
As described in the Regulatory Impact Analysis (section VII. of this proposed rule),
excluding payment amounts for the selected codes from the specified calculations are not
533
Refer to § 425.605(d)(1)(iii)(D), (d)(1)(iv)(D), and (d)(1)(v)(D) for BASIC track Levels C, D and E, respectively.
534
Refer to § 425.630(b).
535
Refer to § 425.605(h).
expected to result in an increase in spending beyond the expenditures that would otherwise occur
under the statutory payment methodology in section 1899(d) of the Act. Further, these
adjustments to our calculations to remove payment amounts for these codes would promote
continued integrity and fairness and improve the accuracy of Shared Savings Program financial
calculations. As a result, we expect these policies would support ACOs continued participation
in the Shared Savings Program and the program’s goals of lowering growth in Medicare FFS
expenditures and improving the quality of care furnished to Medicare beneficiaries.
Based on these considerations, and as specified in the Regulatory Impact Analysis
(section VII. of this proposed rule), we have determined that adjusting certain Shared Savings
Program calculations to remove payment amounts for selected codes, in the event we determine
SAHS billing activity occurs in CY 2024 or subsequent calendar years, from the calculation of
performance year expenditures, updates to the historical benchmark, and ACO participants'
Medicare FFS revenue used for multiple purposes across the Shared Savings Program, meets the
requirements for use of our authority under section 1899(i)(3) of the Act when incorporated into
the existing other payment model we have established pursuant to that section.
The changes that we are proposing in this section of this proposed rule would apply to
address the impact of SAHS billing activity identified in CY 2024 or subsequent calendar years,
and thus would apply to ACOs currently participating in PY 2024. Therefore, if finalized, these
changes to policies applicable for PY 2024 would constitute retroactive rulemaking. Section
1871(e)(1)(A)(ii) of the Act permits a substantive change in regulations, manual instructions,
interpretive rules, statements of policy, or guidelines of general applicability under Title XVIII
of the Act to be applied retroactively to items and services furnished before the effective date of
the change if the failure to apply the change retroactively would be contrary to the public
interest.
Failing to apply the proposed changes retroactively to PY 2024 would be contrary to the
public interest because it would unfairly punish Shared Savings Program ACOs by forcing them

to unexpectedly assume a substantial magnitude of financial risk for costs outside of their control
and not previously contemplated in the Shared Savings Program, undermining both the
sustainability of the Shared Savings Program and the public’s faith in CMS as a fair partner, in
the event we determine SAHS billing activity impacts CY 2024. We did not fully contemplate
the potential for SAHS billing activity outside of an ACO’s control when the Shared Savings
Program was established.
536
For this reason, the Shared Savings Program financial methodology
and the procedures we have utilized in the past did not provide a means to adequately account for
instances of SAHS billing activity outside of an ACO’s control, and thereby the related financial
risk is assumed entirely by ACOs. We view this outcome as particularly inequitable to ACOs
because they have no direct means of controlling such costs. Unlike Medicare Advantage
organizations, ACOs are not responsible for processing claims for their assigned beneficiaries
and otherwise have no means of causing the denial of such claims. CMS thus cannot reasonably
have expected ACOs to have intended to assume responsibility for all instances of SAHS billing
activity outside of an ACO’s control when they joined the Shared Savings Program. For these
reasons, it would be contrary to the public interest for CMS to fail to apply a policy mitigating
this issue retroactively.
We did not foresee the acute need to address SAHS billing activity impacting CY 2023,
and the need for the related policy proposal for addressing SAHS billing activity in CY 2024 or
subsequent calendar years, with sufficient time in advance of the start of PY 2024 to undertake
notice and comment rulemaking earlier, and to avoid retroactive rulemaking. More specifically,
we were only able to determine that the increase in billing on HCPCS codes A4352 and A4353 in
CY 2023 represented SAHS billing activity after the calendar year ended. To identify that the
billing activity in CY 2023 was significant, anomalous, and highly suspect, CMS reviewed actual
536
See, for example, 76 FR 67948 through 67950. Such approaches were more focused on policies to support
monitoring of ACO performance and ensuring program integrity.

billing levels after the calendar year closed and services furnished in CY 2023 had occurred and
the billing level could then be compared to billing levels observed in prior calendar years.
We seek comment on our proposal to apply the policy retroactively to PY 2024,
including whether failing to apply the policy retroactively would be contrary to the public
interest and how it would affect ACOs and their ability to participate in the Shared Savings
Program.
We are proposing a new § 425.672 to describe adjustments CMS could make to Shared
Savings Program calculations to mitigate the impact of SAHS billing activity for CY 2024 or
subsequent calendar years. We propose that § 425.672(b) specify that CMS, at its sole discretion,
may determine that the billing of specified HCPCS or CPT codes represents SAHS billing
activity in calendar year 2024 or subsequent calendar years that warrants adjustment to
calculations made under this part. We propose under § 425.672(c) to specify the Shared Savings
Program calculations for which CMS would exclude all Medicare Parts A and B FFS payment
amounts for the specified claim types associated with a HCPCS or CPT code identified in §
425.672(b) when an adjustment to the calculation is appropriate in light of the SAHS billing
activity. The calculations specified in § 425.672(c) include all potentially relevant financial
calculation provisions, including those covering the financial benchmarking methodologies
(including the proposed HEBA scaler at § 425.662(b)(2)) and those covering calculation of
shared savings and losses. We propose in § 425.672(d) that for calendar year 2024 or subsequent
calendar years,
537
CMS would adjust Shared Savings Program calculations for SAHS billing
activity identified under § 425.672(b) for the calendar year when it is either a performance year
or a benchmark year, as well as the 3 most recent years prior to the start of the ACO’s agreement
period used in establishing the historical benchmark, when such a benchmark is used to reconcile
537
We note that by anchoring this policy on the calendar year, this proposed provision differs from many other
program regulations that are applicable for a given performance year or for agreement periods beginning on a given
date or within a given range. However, we believe this approach is appropriate for this policy as (1) we would adjust
expenditures for the affected calendar year both when it is a performance year and when it is a benchmark year and
(2) it ties the policy to the period for which the SAHS billing activity was identified much in the way the policy for
COVID-19 episodes of care specified in § 425.611 is tied to the related public health emergency.
the ACO for a performance year adjusted for SAHS billing activity. We propose to specify under
§ 425.672(e) that we would also make adjustments for any calendar year corresponding to BY3
in projecting per capita growth in Medicare Parts A and B FFS expenditures according to
§425.660(b)(1) for purposes of calculating the ACPT for agreement periods beginning on
January 1, 2024, and in subsequent years. Additionally, we are proposing conforming revisions
to §§ 425.601(a)(9) and 425.652(a)(9), as well as proposing new paragraphs at §§
425.601(a)(9)(iv) and 425.652(a)(9)(ix) to include adjustments for SAHS billing activity as one
of the reasons that CMS would adjust an ACO’s benchmark during the term of its agreement
period. While we expect that the identification of SAHS billing activity that triggers these
proposed policies will be rare, we believe that, if finalized, these policies will allow us to
proactively ensure the accuracy of program calculations and provide greater certainty for ACOs
and the Trust Funds.
We seek comment on these proposals.
e. Seeking Comment on Establishing Higher Risk and Potential Reward under the ENHANCED
Track
(1) Background
As described in the CY 2024 PFS final rule (88 FR 79223), CMS has considered a higher
risk Shared Savings Program track under which the shared savings/loss rate would be
somewhere between 80 percent and 100 percent (that is, a rate higher than that currently offered
under the ENHANCED track) and that builds on the experience of the Next Generation ACO
(NGACO) and ACO REACH Models. A higher risk track would offer ACOs increased
incentives to generate savings, which would help improve care delivery by promoting
innovations in the delivery of high-quality care that is more patient-centered. In other words, by
increasing sharing rates for ACOs, ACOs will be better incentivized to develop innovations in
the delivery of high-quality care and, therefore, improve the care they offer to their beneficiaries.
A revised ENHANCED track could be implemented in accordance with section 1899(i)(3) of the
Act, provided the Secretary determines that such other payment model enhances the quality and
efficiency of items and services furnished under the Medicare program and does not result in
program expenditures greater than those that would result under the statutory payment model.
In the CY 2024 PFS final rule (88 FR 79223), we summarized public comments received
in response to our Request for Information (RFI) regarding a potential track within the Shared
Savings Program with higher risk than the current ENHANCED track. For a full summary of the
comments submitted in response to our comment solicitation, we refer readers to the relevant
discussion in the CY 2024 PFS final rule (88 FR 79225 through 79227). Commenters were
broadly supportive of such an approach and referenced existing policies under the ACO REACH
Model, and the NGACO Model. Some commenters suggested features of such a track that would
serve to encourage more participation in the Shared Savings Program and help ACOs deliver
more person-centered care to beneficiaries in Traditional Medicare. These features included
prospective payments, full sharing rates (a sharing rate of 100 percent, similar to the Global Risk
Sharing Option in the ACO REACH Model) as well as a benchmark discount rate (a reduction of
the benchmark by a predetermined percentage) to protect the Medicare Trust Funds.
A higher risk sharing arrangement could incentivize participating ACOs to improve
performance in the program as they would receive a greater share of any gross savings. That
improved performance may, in turn, result in reduced healthcare costs for Medicare and more
effective, efficient care for beneficiaries. In addition, higher risk sharing could incentivize ACOs
to develop new care delivery strategies in order to improve their financial performance, such as a
focus on specialty care integration and reduced care fragmentation. Offering a higher risk sharing
track may also help CMS reach our goal of having all beneficiaries in the traditional Medicare
program in a care relationship with a health care provider who is accountable for the costs and
quality of their care by 2030 by encouraging currently participating ACOs to continue
participation in the Shared Savings Program, as well as encourage ACOs not participating in the
Shared Savings Program to join as a result of increased potential reward.

A recent CBO report
538
proposed that higher sharing rates might incentivize providers to
decrease spending as they would stand to gain a larger portion of the savings generated. While in
the short term this might diminish CMS savings, the report postulates that this would increase
participation in the Shared Savings Program and provide a means for CMS to manage long-term
healthcare spending growth. The report also highlights the necessity of striking a delicate
balance: devising financial incentives enticing enough for ACOs to participate actively in the
Shared Savings Program, while ensuring that such participation leads to savings for the Medicare
program.
We seek comment on a participation option that would allow for higher risk and reward
than currently available under the ENHANCED track. A participation option of this type would
replace the existing ENHANCED track in order to avoid the self-selection issues that would
occur if a higher risk track were to be included alongside the ENHANCED track. If both
participation options were made available to ACOs, we have concerns that only the highest-
performing ACOs would self-select into the higher of the two risk tracks. While we included an
RFI on the topic in CY 2024 PFS rulemaking, we are concerned that ACOs did not have enough
detailed information to appropriately weigh the tradeoffs associated with a higher risk/reward
option than the current ENHANCED track, and that the additional information we have
generated since then will allow ACOs and other interested parties to provide more forthright and
helpful feedback. CMS is interested in public comments on the design of a higher risk option
within the Shared Savings Program that could be enacted under our authority granted by section
1899(i)(3) of the Act and that would encourage ACOs to participate actively in the Shared
Savings Program while ensuring that such participation leads to savings for the Medicare
program.
(a) Current ENHANCED Track
538
For more details, please refer to Congressional Budget Office (CBO), “Medicare Accountable Care
Organizations: Past Performance and Future Directions”, April 2024, available at
https://www.cbo.gov/system/files/2024-04/59879-Medicare-ACOs.pdf.

Currently, under the Shared Savings Program, ACOs may enter participation agreements
under the ENHANCED track. The ENHANCED track is a two-sided model that represents the
highest level of risk and potential reward currently offered under the Shared Savings Program.
The rules governing the participation options available to ACOs and the progression from lower
to higher risk for ACOs entering the program are described in § 425.600 of the regulations. To
qualify for a shared savings payment, an ACO must meet a minimum savings rate (MSR)
requirement, meet the quality performance standard or alternative quality performance standard
established under § 425.512, and otherwise maintain its eligibility to participate in the Shared
Savings Program under 42 CFR part 425, subpart B (§§ 425.100 through 425.118). For ACOs
meeting the applicable quality performance standard established under § 425.512(a)(2) or
(a)(5)(i) (for PY 2024 and subsequent performance years), the final shared savings rate is equal
to the maximum sharing rate of 75 percent, or savings at a rate of 75 percent multiplied by the
ACO’s health equity adjusted quality performance score if the ACO meets the alternative quality
performance standard at § 425.512(a)(5)(ii). CMS computes an ACO's shared savings payment
by applying the final sharing rate to the ACO's savings on a first dollar basis (meaning the final
sharing rate is applied to the ACO's full total savings amount), with the payment subject to a cap
that is equal to 20 percent of the updated benchmark (§ 425.610(e)(2)).
ACOs that operate under a two-sided model and have losses that meet or exceed a
minimum loss rate (MLR) must share losses with the Medicare program (§ 425.100(c)). Once
this MLR is met or exceeded, the ACO will share in losses at a rate determined according to the
ACO's track/level of participation, up to a loss recoupment limit (also referred to as the loss
sharing limit) (§ 425.605(d); § 425.610(f), (g)). In determining shared losses, ACOs participating
in the ENHANCED track are subject to losses at a rate determined using a sliding scale based on
ACO’s health equity adjusted quality performance score, if the applicable quality performance
standard established in § 425.512(a)(2) or (a)(5)(i) or the alternative quality performance
standard at § 425.512(a)(5)(ii) is met; with minimum shared loss rate of 40 percent and

maximum of 75 percent. If the ACO fails to meet the applicable quality performance standard
established in § 425.512 or the alternative quality performance standard, the ACO is subject to
1st dollar losses at a rate of 75 percent (§ 425.610(f)(4)(ii)). Shared losses are subject to a cap
that is equal to 15 percent of updated benchmark (§ 425.610(g)).
CMS adjusts historical benchmark expenditures by Medicare enrollment type by a
percentage of the difference between the average per capita expenditure amount for the ACO’s
regional service area and the ACO’s historical benchmark amount (referred to herein as the
“regional adjustment”) (§ 425.652(a)(8)). The weights used in the regional adjustment
calculation are determined in accordance with § 425.656(e) and are dependent on whether the
ACO has lower or higher spending compared to the ACO's regional service area and the
agreement period for which the ACO is subject to the regional adjustment. The first time that an
ACO's benchmark is adjusted based on the ACO's regional service area expenditures, CMS
calculates the regional adjustment using either 35 percent of the difference between the average
per capita amount of expenditures for the ACO's regional service area and the average per capita
amount of the ACO's initial or rebased historical benchmark, if the ACO is determined to have
lower spending than the ACO's regional service area (§ 425.656(e)(1)(i)); or 15 percent of the
difference between the average per capita amount of expenditures for the ACO's regional service
area and the average per capita amount of the ACO's initial or rebased historical benchmark, if
the ACO is determined to have higher spending than the ACO's regional service area (§
425.656(e)(1)(ii)). The second time that an ACO's benchmark is adjusted based on the ACO's
regional service area expenditures, CMS calculates the regional adjustment using either the 50
percent of the difference between the average per capita amount of expenditures for the ACO's
regional service area and the average per capita amount of the ACO's rebased historical
benchmark if the ACO is determined to have lower spending than the ACO's regional service
area (§ 425.656(e)(2)(i)); or 25 percent of the difference between the average per capita amount
of expenditures for the ACO's regional service area and the average per capita amount of the

ACO's rebased historical benchmark if the ACO is determined to have higher spending than the
ACO's regional service area (§ 425.656(e)(2)(ii)). The third time that an ACO's benchmark is
adjusted based on the ACO's regional service area expenditures, CMS calculates the regional
adjustment using the 50 percent of the difference between the average per capita amount of
expenditures for the ACO's regional service area and the average per capita amount of the ACO's
rebased historical benchmark if the ACO is determined to have lower spending than the ACO's
regional service area (§ 425.656(e)(3)(i)); or the 35 percent of the difference between the average
per capita amount of expenditures for the ACO's regional service area and the average per capita
amount of the ACO's rebased historical benchmark if the ACO is determined to have higher
spending than the ACO's regional service area (§ 425.656(e)(3)(ii)). The fourth or subsequent
time that an ACO's benchmark is adjusted based on the ACO's regional service area
expenditures, CMS calculates the regional adjustment to the historical benchmark using 50
percent of the difference between the average per capita expenditures for the ACO's regional
service area and the average per capita amount of the ACO's rebased historical benchmark (§
425.656(e)(4)). Among the ACOs participating in PY 2024, 78 percent of BASIC track ACOs
(176 of 227) received a positive regional adjustment, whereas 95 percent (155 of 163) of ACOs
in the ENHANCED track received a positive regional adjustment. A positive regional adjustment
indicates that their expenditures were less than that of their regional service area. For ACOs
receiving a positive regional adjustment, the average regional adjustment amount was 2.21
percent ($237) of historical benchmark expenditures.
As of January 1, 2024, 43 percent (207 of 480) Shared Savings Program ACOs are
participating under the ENHANCED track. Under Shared Savings Program policies, all ACOs
participating in a two-sided model can select a symmetrical MSR and MLR which applies for the
duration of its agreement period (§ 425.605(b)(2); § 425.610(b)(1)). Among ACOs participating
in the ENHANCED track for PY 2024, 61 percent (126 of 207) have selected an MSR/MLR of
0.5 percent or greater while 39 percent (81 of 207) have selected an MSR/MLR of 0.0 percent.
Among ACOs that participated in the ENHANCED track for PY 2022, 38 percent (55 of 146)
generated gross savings between zero and 5 percent of their updated benchmark expenditures,
and 12 percent (17 of 146) generated gross savings of 10 percent or more of their benchmark
expenditures.
(b) Other CMS Innovation Center Models
In the NGACO Model, NGACOs were offered the choice between two risk
arrangements, partial risk or full risk. Under both arrangements, the NGACO was responsible for
100 percent of performance year expenditures for services rendered to the NGACO's aligned
beneficiaries. Under the partial risk arrangement, the NGACO could receive or owe up to 80
percent of savings/losses, whereas under the full risk arrangement, the NGACO could receive or
owe up to 100 percent of savings/losses. To mitigate the ACO's risk of large shared losses, as
well as to protect the Medicare Trust Funds against paying out excessive shared savings,
NGACOs were required to choose a cap on gross savings/losses. The cap, expressed as a
percentage of the benchmark, ranged from 5 percent to 15 percent. The risk arrangement chosen
by the NGACO (80 or 100 percent) was applied to gross savings or losses after the application of
the cap. In PYs 1–3, a discount was applied to the NGACO's benchmark that was set at a
standard 3 percent, with various adjustments, that allowed the final discount to vary from 0.5
percent to 4.5 percent. In PYs 4–6, a discount of 0.5 percent was applied to the benchmark under
the partial risk arrangement, and a discount of 1.25 was applied to the benchmark under the full
risk arrangement. The purpose of the discount was to increase the likelihood that any savings
achieved by the NGACOs participating in the model would also result in savings for the
Medicare Program. The NGACO Model evaluation found that while NGACOs reduced gross
Medicare Parts A and B expenditures relative to a comparison group of similar fee-for-service
Medicare beneficiaries in their markets, they did not generate savings to the Medicare Trust
Funds. ACOs that elected a risk cap greater than 5 percent and participated in model population-
based payment mechanisms achieved greater declines in spending, suggesting that the

combination of risk and payment flows is impactful. Spending reductions grew larger almost
every year, reflecting a combination of NGACOs’ improvements in infrastructure and clinical
processes, exit by poorer-performing NGACOs, and the COVID-19 pandemic. While the
NGACO Model reduced spending in Medicare Parts A and B, CMS paid back these reductions
in the form of shared savings payments to ACOs. These results highlight the need to balance the
tradeoff between incentivizing participation in higher levels of risk and reward, in alternative
payment models such as the Shared Savings Program and ACO models tested by the Innovation
Center, and reducing the risk of loss to the Medicare Trust Funds.
Under the ACO REACH Model, REACH ACOs are offered the choice of participating
under the Global or the Professional Risk Sharing Options. As in the NGACO Model, under both
risk sharing options, the REACH ACO is responsible for 100 percent of performance year
expenditures for services rendered to aligned beneficiaries. Because ACOs electing the Global
Risk Sharing Option retain up to 100 percent of the savings/losses on all savings up to 25 percent
of their benchmark, with reduced sharing rates for savings exceeding 25 percent of their
benchmark, a discount is applied to the benchmark to ensure savings are also generated for CMS.
For ACOs in the Global Risk Sharing Option, the benchmark is reduced by a fixed percentage
based on the performance year.
539
The discount rate for PYs 2021 and 2022 was 2 percent, for
PYs 2023 and 2024 is 3 percent, and for PYs 2025 and 2026 will be above 3.5 percent. The
benchmark for ACOs participating in the Professional Risk Sharing Option does not include this
discount, and these ACOs are only eligible to retain 50 percent of savings or owe 50 percent of
any losses.
Preliminary evaluation results of the first 2 performance years of the Global and
Professional Direct Contracting Model, before its transition to the ACO REACH Model, suggest
that participating ACOs had mixed results in gross spending but consistent, significant increases
539
For more details, refer to CMS, ACO Realizing Equity, Access, and Community Health (REACH) Model,
PY2023 Financial Settlement Overview, available at https://innovation.cms.gov/media/document/aco-reach-py2023-
fncl-settlement (see Table 4: Schedule of Discounts by Risk Arrangement).
in net spending relative to a comparison group of similar FFS Medicare beneficiaries in their
markets, which included beneficiaries assigned to ACOs participating in the Shared Savings
Program. Standard ACOs, comprised of organizations that generally have experience serving
Medicare FFS beneficiaries, increased gross spending. Standard ACOs also reduced acute care
spending and utilization but comparison providers had larger reductions in acute care spending
and utilization. Increased spending among Standard ACOs was concentrated among the
integrated delivery system/hospital system ACOs in the model. High Needs ACOs that serve
Medicare FFS beneficiaries with complex needs, including dually eligible beneficiaries,
decreased gross spending. High Needs ACOs comprised of organizations that have not
traditionally provided services to Medicare FFS beneficiaries favorably reduced acute and post-
acute care utilization and spending. New Entrant ACOs had declines in gross spending but these
declines were similar to those of providers within their same markets. Standard and New Entrant
ACOs showed statistically significant improvement on at least one quality measure. These
interim evaluation results are mixed, and additional analyses and years of experience with the
Model will inform which features of ACO REACH could drive continued growth and innovation
in the Shared Savings Program and the focus of future Innovation Center ACO models.
(2) Considerations for Incorporating Higher Risk and Potential Reward Under the ENHANCED
Track
As we explained in the CY 2024 PFS final rule (88 FR 79223 through 79225), when
considering a higher risk track, CMS would need to balance the incentives for ACOs to transition
to higher levels of risk and potential reward and increase ACO participation in the Shared
Savings Program and in two-sided risk tracks, all while ensuring sufficient financial safeguards
to protect against inappropriately large shared losses for ACOs coordinating and improving
quality of care for high-cost beneficiaries. Considerations must also be directed towards
safeguarding the Medicare Trust Funds and ensuring that CMS satisfies any statutory
requirements under section 1899(i)(3) of the Act.
A revised ENHANCED track could be implemented in accordance with section
1899(i)(3) of the Act, provided the Secretary determines that such other payment model
enhances the quality and efficiency of items and services furnished under the Medicare program
and does not result in program expenditures greater than those that would result under the
statutory payment model. Increasing the sharing rate in the ENHANCED track may need to be
accompanied by other modifications to prevent spending from increasing and possibly
jeopardizing compliance with section 1899(i)(3) of the Act. One factor to consider is selective
participation with regard to which ACOs would choose to participate in a higher risk track, if
offered. For example, Shared Savings Program ACOs that have a history of high levels of earned
shared savings or have received a favorable high regional adjustment to their benchmark may be
more likely than other ACOs to switch to the higher risk track upon renewing or early renewing
their participation in the program so they can receive additional benefit from the higher levels of
potential reward offered in a higher risk track. This could result in increased spending on the part
of CMS which may jeopardize compliance with section 1899(i)(3) of the Act. If a higher risk
track were to be offered in the Shared Savings Program in the future, CMS would consider
replacing the existing ENHANCED track in order to prevent further selective participation and
maintain the balance between increased participation and compliance with applicable statutory
requirements.
We are seeking comment on the following potential features of a revised ENHANCED
track:
(a) Benchmark Discount Rate
Both the NGACO Model and the Global Risk Sharing Option of the ACO REACH
Model feature a discount rate that is applied to benchmarks. The discount rate serves to protect
the Medicare Trust Funds by reducing benchmarks and thereby improves the likelihood of
achieving savings for the Medicare program for risk tracks that can feature up to 100 percent
shared savings rates, such as the Global Risk Sharing Option in the ACO REACH Model. A
discount would be applied to an ACO’s updated historical benchmark before gross savings/losses
are calculated, which increases the likelihood of savings for CMS and the Medicare program. If
an ACO were to participate in a potential higher risk track and potentially share in 100 percent of
gross savings, this discount would serve as the primary means for CMS to capture savings from
ACOs participating in this option, as in the absence of a discount any and all gross savings would
go to ACOs in the form of a shared savings payment. For example, consider an ACO with an
updated benchmark of $10,000 and mean per-capita performance year expenditures of $9,500.
Applying a discount rate of 1 percent to the benchmark would reduce the ACO’s benchmark to
$9,900. Gross savings would then be calculated based on the discounted benchmark, and the
ACO’s shared saving rate would be applied to the savings, provided these savings met or
exceeded the ACO’s selected MSR.
A discount to the benchmark could also include a guardrail policy similar to the guardrail
implemented in the three-way blended update factor that was finalized in the CY 2023 PFS final
rule (87 FR 69881). Under such an approach, if an ACO were to be liable for shared losses after
discounting the benchmark, then gross savings or losses would be recalculated using a
benchmark without the discount. However, if the ACO were to generate gross savings in excess
of their MSR under the benchmark without the discount, they would still not be considered
eligible to share in savings. This approach would help ensure that CMS shares in any savings
generated by ACOs participating in a potential revised ENHANCED track while also not
increasing downside risk for ACOs that may be liable for shared losses.
We seek comment on what rate would be appropriate for a discount to the benchmark
that would protect the Medicare Trust Funds while providing an adequate incentive for ACOs to
participate in a potential revised ENHANCED track. We also seek comment on whether the
model features described in following subsections might replace a discount to the benchmark
while balancing financial incentives for ACOs and risk to CMS. Additionally, we also seek
comments from interested parties, including ACOs, on the discount to the benchmark and what

level of discount would be acceptable to ACOs participating in the Shared Savings Program, as
well as what would be considered too high of a discount.
(b) Tapered Sharing Arrangements
Currently in the ENHANCED track, ACOs can receive a shared savings payment of up to
20 percent of their updated benchmark (once the MSR is met or exceeded) (§ 425.610(e)(2)) or
be liable for losses not to exceed 15 percent of their updated benchmark (once the MLR is met or
exceeded) (§ 425.610(g)). Alternatively, CMS could set up marginal savings bands or risk
corridors under which shared savings or losses rates would vary with the amount of gross
savings or losses. As gross savings/losses increase, the ACO will retain a progressively smaller
portion of the total savings or will be responsible for a progressively smaller portion of the total
losses. For example, consider hypothetical marginal savings bands shown in Table 44. Under this
arrangement, an ACO would share in all savings up to 10 percent of their updated benchmark at
a rate of 100 percent. For savings between 10 to 15 percent, the ACO would share in 60 percent
of savings and CMS would retain the remaining 40 percent. For savings between 15 to 20
percent, the ACO would share in 40 percent of savings and CMS would retain the remaining 60
percent. In case of losses, ACOs would be responsible for 50-100 percent of the losses,
depending on the ACO’s quality performance score.
TABLE 44: Hypothetical Marginal Shared Savings Bands
Gross savings as % of benchmark
Shared Savings/Loss Rate
1
0-10%
100%
10-15%
60%
15-20%
40%
>20%
0%
Losses
50% - 100%
2
¹ Percentage of savings or losses retained by the ACO.
2
Shared Loss Rate would depend on an ACO’s quality performance, similar to § 425.610(f)(4).
We seek comment on whether the hypothetical marginal shared savings bands shown in
Table 44 represent an appropriate tapering schedule that would provide sufficient incentive for

an ACO to participate in a potential revised ENHANCED track, as well as whether the tapering
schedule should begin with lower shared savings rates and feature increasing rates as an ACO
generates greater amounts of savings. We also seek comment on whether a potential tapering
schedule should be symmetrical with respect to shared loss rates. Finally, we seek comment on
whether marginal shared savings bands provide the right incentives to ACOs relative to the fixed
savings rate in the current ENHANCED track.
(c) MSR/MLR
We are considering the option for all ACOs under a revised ENHANCED track to be
subject to a symmetric MSR/MLR of 0 percent. This would increase many ACOs’ exposure to
both positive savings and negative risk. While this approach would guarantee that any ACO
generating savings would share in those savings (provided they meet the quality performance
standard established under § 425.512 and otherwise maintain their eligibility to participate in the
Shared Savings Program), ACOs with performance year expenditures greater than their historical
benchmark would be liable for those losses due to the 0 percent MLR. We are seeking comment
on whether a potential revised ENHANCED track should retain the existing symmetric
MSR/MLR selection options that currently exist for ACOs in a two-sided risk model under §
425.610(b)(1).
(d) Cap On Regional Adjustment Weight
We are seeking comment on adjusting the weights used to calculate the regional
adjustment amounts under § 425.656(e) for ACOs in the revised ENHANCED track. This may
take the form of applying a cap of 35 percent to all the weights used to calculate regional
adjustment amounts. This would impact any ACOs in a second or subsequent agreement period
subject to a regional adjustment if their historical benchmark spending is lower than their
regional service area. If the cap were to apply to an ACO with lower spending than their regional
service area, then this would result in a decreased regional adjustment to that ACO’s historical
benchmark. Overall, this feature would reduce the cost to CMS associated with high regional
adjustments by reducing an ACO’s historical benchmark in the event that an ACO in a second or
subsequent agreement period receives a large positive regional adjustment, which may decrease
the need for higher benchmark discount rates or lower tapered shared savings rates that are less
favorable to ACOs and limit incentives for ACOs to transition from the BASIC track to the
revised ENHANCED track. This feature may also increase the relative impact of the prior
savings adjustment and the health equity benchmark adjustment proposed in section III.G.7.b. of
this proposed rule. We seek comment on whether further reductions to or the removal of the
regional adjustment to the historical benchmark would be appropriate as part of a potential
revised ENHANCED track. We also seek comment on whether maintaining the regional
adjustment in its current state would warrant further changes to the revised ENHANCED track
features described above, including, but not limited to, a discount to the benchmark or lower
tapered shared savings rates.
(e) Payment Mechanisms
We are seeking comments on alternative payment mechanisms the Innovation Center has
tested and their ability to help transform care delivery and improve health outcomes for ACOs
participating in the Shared Savings Program. These payment mechanisms test whether
alternative payment flows (that is, those other than fee for service reimbursement) facilitate
better investment in infrastructure and care coordination and encourage innovative downstream
payment arrangements that can improve health outcomes for Medicare beneficiaries. The
alternative payment mechanisms on which we seek comments are described below:
● Infrastructure Payments: Under these arrangements, CMS makes a payment to the
ACO, in addition to FFS reimbursement to the providers and suppliers participating in the ACO,
that is unrelated to claims. Infrastructure payments have been distributed either as a lump sum or
per beneficiary per month payments. Infrastructure payments are recouped during the payment
reconciliation process.
● Population-Based Payment, All-Inclusive Population-Based Payment, or Advance

Payment Option: In this arrangement, CMS provides a percentage of FFS reimbursement to the
ACO in the form of a monthly payment to support ongoing ACO activities and provide the ACO
flexibility in the types of arrangements it enters into with provider/suppliers. The ACO and
providers with whom it has a written business arrangement determine percentage reductions to
the base FFS payments to the providers interested in this payment arrangement. Providers
participating in this option have their FFS payments reduced by the agreed upon percentage,
which range from 1-100 percent. CMS pays the projected total annual amount taken out of the
base FFS rates to the ACO in monthly payments. At the end of each performance year, the
amount of payment paid to ACOs participating in this type of payment option is reconciled
against the reductions actually made to claims payments to providers participating in these
arrangements, linking the amount of these payments directly to utilization and FFS payment.
● Capitation: The ACO REACH Model
540
tests two capitation payment options--
Primary Care Capitation and Total Care Capitation.
The Primary Care Capitation Payment is the payment for primary care services provided
to aligned REACH beneficiaries by all Participant Providers and those Preferred Providers who
have selected Primary Care Capitation Payment. In Primary Care Capitation, a per beneficiary,
per month capitated payment is provided to an ACO for its aligned beneficiaries for the primary
care services provided by the ACO’s Participant Providers and its Preferred Providers who have
opted to participate in Primary Care Capitation Payment. The Primary Care Capitation payment
amount is generally equal to seven percent of the estimated total cost of care for the ACO’s
aligned population (that is, the risk adjusted, trended, and regionally blended benchmark).
The Primary Care Capitation payment includes two components, Base Primary Care
Capitation and Enhanced Primary Care Capitation. The Base Primary Care Capitation amount is
540
Refer to the ACO REACH Model Request for Applications, available at
https://www.cms.gov/priorities/innovation/media/document/aco-reach-rfa, and the ACO REACH Model PY2024
Participant and Preferred Provider Management Guide, available at https://www.cms.gov/files/document/aco-reach-
py24-part-pref-provider-mgmt-guide.pdf.
intended to cover primary care services furnished to aligned beneficiaries by Participant
Providers and those Preferred Providers who have agreed to participate in Primary Care
Capitation Payment that are thus subject to fee reductions under Primary Care Capitation
Payment. The Enhanced Primary Care Capitation amount, which will be recouped by CMS in
full during final financial settlement, is intended to enable ACOs to make upfront investments in
infrastructure, technology, tools, and resources to support increased access to primary care,
provision of care, and care coordination. The Primary Care Capitation Payment is expected to
encourage greater flexibility in payment and innovative primary care service delivery as a means
of improving the quality and cost effectiveness of care overall.
In Total Care Capitation, a per-beneficiary, per month capitated payment is provided to
an ACO for all Medicare Part A and Part B services provided to aligned beneficiaries by the
ACO’s Participant Providers and its Preferred Providers who have opted to participate in Total
Care Capitation payment. The Total Care Capitation payment amount reflects the estimated total
cost of care for the ACO’s aligned population (that is, the risk adjusted, trended, and regionally
blended benchmark) and is only available to ACOs participating in the Global risk option.
Participant Providers and those Preferred Providers that have elected to participate in the ACO’s
selected capitation payment mechanism continue to submit claims to CMS for services provided
to aligned beneficiaries. The CMS FFS claims processing system reduces claims payment
amounts according to the payment reduction arrangements with their providers. More details on
ACO REACH Model’s capitation payment mechanisms are available here:
https://www.cms.gov/files/document/aco-reach-py24-financial-ops-capitation-and-payment-
mechanisms.pdf.
Additionally, we are interested in feedback on the following questions related to
implementation of a revised ENHANCED track with higher risk and potential reward, as well as
comments that could inform changes to the Shared Savings Program and future Innovation
Center ACO models:
1. What would the option of a revised ENHANCED track allow an ACO to do that they are
unable to do currently?
2. How would higher downside risk impact an ACO’s care delivery strategies, including
advanced primary care, behavioral health, specialty integration, and integration with community-
based organizations to improve health outcomes or advance health equity?
3. How does higher downside risk impact an ACO’s downstream provider arrangements to
further advance incentives to reduce delivery of low value services and the total cost of care, and
to increase savings performance?
4. What types of organizations, including but not limited to ACOs and providers, are interested
in a higher risk and reward option in the Shared Savings Program?
5. What additional flexibilities or features (for example, benefit enhancements, advance
payments, capitation payments, etc.) would ACOs in a revised ENHANCED track with higher
risk and potential reward want CMS to offer to help them be successful in improving the quality
of care and reducing costs?
6. How should a revised ENHANCED track with higher risk and potential reward also require
additional accountability for quality? Should ACOs in this revised track be required to report all
payer/all patient quality measures?
7. Should a revised ENHANCED track with higher risk and potential reward require ACOs with
earned shared savings to share savings with beneficiaries or spend a flat dollar amount or a
certain percentage on beneficiaries in the form of items or services not covered by original
Medicare (for example, meals, dental, vision, hearing, or Part B cost-sharing reductions)?
8. How should CMS consider the discount, sharing rate, and risk corridors or marginal savings
bands in the design of a higher risk option that can realize savings for Medicare? Are there
special considerations that CMS should bear in mind when thinking through such features for
different types of ACOs (for example, low revenue, high revenue, health system-based, safety
net, etc.)?

9. How might we improve beneficiary assignment and are there different considerations for
different types of ACOs (for example, low revenue, high revenue, health system-based, safety
net, etc.)?
10. What other features should CMS consider in designing financial benchmarks that balance
prospectivity and accuracy, and that can lead to savings for both ACOs and Medicare? How
might administratively set benchmarks achieve these goals and what considerations should we
bear in mind if we test administrative benchmarking?
11. We are interested in ways to increase participation by healthcare providers and suppliers in
the Shared Savings Program and future Innovation Center ACO models, including how an ACO
model requiring provider participation or stronger participation incentives might be designed.
f. Proposed Technical Change for Consistency in Financial Calculations
(1) Background
For the benchmarking methodology applicable to agreement periods beginning on
January 1, 2024, and in subsequent years, we cap ACO prospective hierarchical condition
category (HCC) risk score growth between BY3 and the performance year (as finalized in the
CY 2023 PFS final rule, refer to 87 FR 69932 through 69946), as well as prospective HCC risk
score growth in an ACO’s regional service area between BY3 and the performance year (as
finalized in the CY 2024 PFS final rule, refer to 88 FR 79174 through 79185).The policy to cap
ACO prospective HCC risk score growth between BY3 and the performance year relied on our
authority granted by section 1899(d)(1)(B)(ii) of the Act to adjust the benchmark for beneficiary
characteristics and such other factors as the Secretary determines appropriate (see 87 FR 69934).
The policy to cap prospective HCC risk score growth in an ACO’s regional service area between
BY3 and the performance year by applying an adjustment factor in calculating the regional
component of the three-way blended benchmark update factor required use of our statutory
authority under section 1899(i)(3) of the Act (see 88 FR 79182 and 79183).

The current regulations describe how we cap ACO prospective HCC risk score growth at
§§ 425.605(a)(1) and 425.610(a)(2). As specified, positive adjustments in prospective HCC risk
scores are subject to a cap equal to the ACO's aggregate growth in demographic risk scores
between BY3 and the performance year (positive or negative) plus 3 percentage points. The cap
applies to prospective HCC risk score growth for any Medicare enrollment type only if the
ACO's aggregate growth in prospective HCC risk scores between BY3 and the performance year
across all of the Medicare enrollment type exceeds this cap. Growth in an ACO’s risk scores by
enrollment type is expressed as the ratio of the ACO’s performance year risk score for that
enrollment type to the ACO’s BY3 risk score for that enrollment type. The aggregate growth in
demographic and prospective HCC risk scores risk scores is calculated by taking a weighted
average of the risk ratio for demographic risk scores or prospective HCC risk scores, as
applicable, for each Medicare enrollment type using specified weights.
The current regulations further describe how we cap prospective HCC risk score growth
in the ACO’s regional service area at § 425.655. As specified, CMS determines aggregate
growth in regional prospective HCC and demographic risk scores by calculating growth in
prospective HCC and demographic risk scores between BY3 and the performance year for each
Medicare enrollment type, where growth in an ACO’s regional risk score by enrollment type is
expressed as the ratio of the performance year regional risk score for a Medicare enrollment type
to the BY3 regional risk score for that enrollment type. We then calculate aggregate risk score
growth by taking a weighted average of the regional prospective HCC or demographic risk
ratios, as applicable, across the four Medicare enrollment types, using specified weights. We
next determine the cap on regional risk score growth (refer to § 425.655(e)),
541
and then
determine if the ACO’s regional risk score growth is subject to a cap and apply a regional risk
541
To determine the cap on regional risk score growth, we calculate the non-market share adjusted cap on the
ACO’s regional risk score growth as the sum of the aggregate growth in regional demographic risk scores and 3
percentage points, then adjust the cap to reflect the ACO’s aggregate market share.

score growth cap adjustment factor for each Medicare enrollment type, as applicable (refer to §
425.655(f)).
542
When describing how we will cap prospective HCC risk score growth in the ACO’s
regional service area in the CY 2024 PFS final rule, we included a footnote (see 88 FR 79178)
that indicated that the weights to be used to compute aggregate risk score growth for this
calculation are the same as the weights to be used when calculating weighted average ACO
prospective HCC and demographic risk ratios under the risk adjustment methodology for
capping ACO risk score growth adopted in the CY 2023 PFS final rule and codified in §§
425.605(a)(1)(ii)(C) and 425.610(a)(2)(ii)(C). That is, it was our intention to use the same
weights in both the regional risk score growth cap calculation and the ACO risk score growth cap
calculation. However, in codifying the methodology for the regional risk score growth cap in the
new section of the regulations, § 425.655, we inadvertently introduced a discrepancy.
In §§ 425.605(a)(1)(ii)(C) and 425.610(a)(2)(ii)(C), where we codified how we will
calculate aggregate risk score growth used in determining the cap to apply to ACO prospective
HCC risk score growth, we describe the weight applied to the growth in demographic or
prospective HCC risk scores for each Medicare enrollment type as equal to the product of the
historical benchmark expenditures for that enrollment type and the performance year person
years for that enrollment type. In § 425.655(d)(2), where we codified how we will calculate
aggregate risk score growth used in determining the cap to apply to regional prospective HCC
risks score growth, we describe the weight applied to the growth in demographic or prospective
HCC risk scores for each Medicare enrollment type as equal to product of the ACO’s regionally
adjusted historical benchmark expenditures (emphasis added) for that enrollment type and the
ACO’s performance year assigned beneficiary person years for that enrollment type.
542
If the aggregate regional prospective HCC risk score growth does not exceed the cap on regional risk score
growth, the ACO's regional risk score growth is not subject to the cap. For these ACOs we set the risk score growth
cap adjustment factor equal to 1 for each Medicare enrollment type. If the aggregate regional prospective HCC risk
score growth exceeds the market share adjusted cap, the ACO's regional risk score growth is subject to the cap. For
these ACOs we next determine whether the cap on regional risk score growth applies for each Medicare enrollment
type.
The regulations at §§ 425.605(a)(1)(ii)(C) and 425.610(a)(2)(ii)(C) provide that we will
use the ACO’s historical benchmark expenditures in calculating the weights used to cap ACO
risk score growth. By contrast, the regulations at § 425.655(d)(2) provide that we will use an
ACO’s regionally adjusted historical expenditures in calculating the weights used in the
calculation of regional risk score growth cap. As written, the regulations text at § 425.655(d)(2)
is inconsistent with the language used in in §§ 425.605(a)(1)(ii)(C) and 425.610(a)(2)(ii)(C)
despite the fact that we indicated in the CY 2024 PFS final rule that we would use the same
weights in both calculations. Additionally, it is unclear how we would apply the calculation
described at § 425.655(d)(2) in practice. As we describe in section III.G.7.b. of this proposed
rule, for agreement periods beginning on January 1, 2024, and in subsequent years, in computing
an ACO's historical benchmark, CMS determines the per capita Parts A and B fee-for-service
expenditures for beneficiaries that would have been assigned to the ACO in any of the 3 most
recent years prior to the start of the agreement period using the ACO participant TINs identified
before the start of the agreement period as required under § 425.118(a) and the beneficiary
assignment methodology selected by the ACO for the first performance year of the agreement
period as required under § 425.400(a)(4)(ii). An ACO’s historical benchmark may then be
subject to a regional adjustment (refer to § 425.656), a prior savings adjustment (refer to §
425.658), or no adjustment (refer to § 425.652(a)(8) and (c)). This methodology, based on
policies finalized in the CY 2023 and CY 2024 PFS final rules, under which an ACO may
receive a prior savings adjustment, a regional adjustment, and or no adjustment at all, differs
from the methodology that was in effect for ACOs in an agreement period beginning on or after
July 1, 2019, but before January 1, 2024, under which all ACO historical benchmarks
incorporated a regional adjustment (see § 425.601). Furthermore, in section III.G.7.b. of this
proposed rule we are proposing to add a third type of adjustment that could be applied to an
ACO’s historical benchmark, the health equity benchmark adjustment. If the health equity
benchmark adjustment is finalized as proposed, an ACO may receive a regional adjustment, a
prior savings adjustment, a health equity benchmark adjustment, or no adjustment to its historical
benchmark.
(2) Proposed Revisions
As we described in a footnote in the CY 2024 PFS final rule (see 88 FR 79178), it was
our intention at the time to use the same weights to calculate the cap for prospective HCC risk
score growth in an ACO’s regional service area as the weights used to calculate the cap on
prospective HCC risk score growth for the ACO. We continue to believe that the same weights
should apply to both calculations. However, the regulation text language is not currently aligned
among the relevant provisions or with the preamble discussion and may also create confusion
with respect to how CMS will compute the weights used in setting the caps on ACO and regional
prospective HCC risk score growth, given that some ACOs will receive a regional adjustment to
their benchmarks, some will receive a prior savings or, if finalized, a health equity benchmark
adjustment, and some will receive no adjustment at all.
To address these issues, we are proposing technical changes to the regulation text at §§
425.605(a)(1)(ii)(C), 425.610(a)(2)(ii)(C), and 425.655(d)(2) to align the language describing the
calculation of the weights that will be used to compute aggregate risk score growth across the
three provisions and to clarify that the weight applied to the growth in ACO and regional risk
scores for each Medicare enrollment type, respectively, would be equal to the product of the
ACO's historical benchmark expenditures, adjusted in accordance with § 425.652(a)(8), for that
enrollment type and the ACO's performance year assigned beneficiary person years for that
enrollment type. That is, we would use the ACO’s historical benchmark expenditures that would
have already been adjusted to reflect a prior savings adjustment, a regional adjustment, a health
equity benchmark adjustment, if finalized, or no adjustment. Aligning the description of the
weight calculation across the three provisions would address the discrepancy that exists between
the current regulation text and the preamble discussion in the CY 2024 PFS final rule.
Additionally, providing additional detail in the description of the weight calculation, namely by
indicating that we will use an ACO’s historical benchmark expenditures adjusted in accordance
with § 425.652(a)(8), clarifies how we will operationalize the calculation which we believe is
important, especially given the proposed health equity benchmark adjustment, which, if
finalized, would add greater complexity to this historical benchmark calculation.
The technical changes that we are proposing in this section of this proposed rule relate to
benchmark calculations for ACOs in agreement periods beginning on or after January 1, 2024.
Although we will not implement the proposed methodologies for the first time until summer
2025 when we reconcile PY 2024, these policies, if finalized, would constitute retroactive
rulemaking because they are the standards under which we will score ACOs that are currently
participating in agreement periods that began on January 1, 2024, for PY 2024. Section
1871(e)(1)(A)(ii) of the Act permits a substantive change in regulations, manual instructions,
interpretive rules, statements of policy, or guidelines of general applicability under Title XVIII
of the Act to be applied retroactively to items and services furnished before the effective date of
the change if the failure to apply the change retroactively would be contrary to the public
interest. Here, we are proposing a technical change that would align the regulation text with our
stated intention as described in previous rulemaking. The current regulation text, in combination
with related discussion in the CY 2024 PFS final rule, fails to provide sufficient clarity with
regard to how CMS will calculate the weights used to calculate aggregate ACO or regional risk
score growth. While the discussion in the CY 2024 PFS final rule indicates that the same weights
should be use in both calculations, the related regulation text does not make this clear and,
furthermore, could raise questions for how CMS will perform calculations given that not all
ACO historical benchmarks will include a regional adjustment. Failure to apply the proposed
changes to our regulations at §§ 425.605(a)(1)(ii)(C), 425.610(a)(2)(ii)(C), and 425.655(d)(2)
retroactively would be contrary to the public interest because it creates unintended ambiguity in
the standard CMS will use when calculating risk score growth. Such ambiguity may make it
difficult for ACOs and other interested parties to understand how CMS will perform these

calculations or be interpreted to suggest that CMS would calculate risk score growth in a
different manner, which was not the agency’s intention.
We seek comment on these proposals.
8. Beneficiary Notification Requirements
a. Proposal to Modify the Requirements for When ACOs Must Provide the Beneficiary
Information Follow-up Communication
Under § 425.312(a), ACOs are required to notify beneficiaries about the ACO’s
participation in the Shared Savings Program, the beneficiary’s ability to decline claims data
sharing, and the beneficiary’s ability to select a provider for the purposes of voluntary alignment.
In the CY 2023 PFS final rule (87 FR 69961), CMS added the beneficiary information follow up
communication requirement under § 425.312(a)(2)(v), which requires an additional follow up
with a beneficiary who has received the beneficiary notification. In the CY 2023 PFS final rule
(87 FR 69960 through 69963), CMS noted that the follow up communication promotes
transparency and empowers beneficiaries to make an informed decision in choosing a primary
care physician and how they share their health data. The beneficiary information follow-up
communication affords the opportunity for additional direct engagement between the beneficiary
and the ACO, or ACO participant, and provides a chance for a meaningful dialog between the
patient and provider about the coordination of their care, the benefits of receiving care from an
ACO provider/supplier (as defined in § 425.20), the organizational operations of the ACO, and
how data is used to improve care and report quality outcomes.
Currently, under § 425.312(a)(2)(v)(A), “The follow-up communication must occur no
later than the earlier of the beneficiary's next primary care service visit or 180 days from the date
the standardized written notice was provided.” Regulations at § 425.312(a)(2)(v)(B) require
ACOs to document the beneficiary information follow-up communication in a system of record
supported by the ACO and to make the information available to CMS upon request.
Since CMS implemented the beneficiary information follow-up communication
requirement, we have received feedback from ACOs that requiring the follow-up communication
no later than the earlier of the beneficiary's next primary care service visit or 180 days from the
date the standardized written notice was provided is difficult for ACOs to operationalize as they
do not always know when the beneficiary’s next primary care service will be and in some cases
it can be very soon after the beneficiary receives the original beneficiary notification.
In order to address this issue and the burden it creates, CMS is proposing to remove the
requirement that ACOs must provide this follow up at the beneficiary’s next primary care visit.
Specifically, we propose to modify § 425.312(a)(2)(v)(A) to read “The follow-up
communication must occur no later than 180 days from the date the standardized written notice
was provided.” This would provide ACOs with more flexibility to implement their strategy for
following up with beneficiaries after they receive the beneficiary notice, while still providing the
opportunity for a meaningful dialog between a beneficiary and their provider. We seek comment
on this proposal. If finalized, this proposal would be effective beginning January 1, 2025.
b. Limit the Distribution of the Beneficiary Notification to Beneficiaries Likely to be Assigned
for ACOs under Preliminary Prospective Assignment with Retrospective Reconciliation
ACOs that select preliminary prospective assignment with retrospective reconciliation are
assigned beneficiaries in a preliminary manner and before the start of the performance year.
Beneficiary assignment for these ACOs is then updated quarterly based on the most recent 12 or
24 months of data, as applicable. This assignment methodology is codified at § 425.400(a)(2).
We are proposing to limit the distribution of the beneficiary notification under §
425.312(a)(2)(iii) to beneficiaries who are more likely be assigned to ACOs that select
preliminary prospective assignment with retrospective reconciliation, when compared to the
population of beneficiaries who must receive the beneficiary notification under current §
425.312(a)(2)(iii). Please note that this is not a proposal to modify the Shared Savings Program’s
assignment methodology.

Currently, ACOs that select preliminary prospective assignment with retrospective
reconciliation are required to send a beneficiary notice to “each fee-for-service beneficiary”
under § 425.312(a)(2)(iii). Under § 425.312(a)(2)(iii), the standardized written notice must be
furnished to “all fee-for-service beneficiaries prior to or at the first primary care service visit
during the first performance year in which the beneficiary receives a primary care service from
an ACO participant.” This can result in ACOs sending notices each year to beneficiaries who
may not ultimately be assigned to the ACO, as there are “fee-for-service beneficiar[ies]” to
whom ACOs must send notices under § 425.312(a)(2)(iii) and who are not eligible to be assigned
to those ACOs for a variety of reasons. This policy was intended to ensure that all beneficiaries
who receive a primary care visit from a ACO provider/supplier receive the beneficiary notice.
However, we have heard feedback from ACOs that this creates confusion for the beneficiary and
unnecessary administrative work for the ACO.
To reduce burden on ACOs and confusion for beneficiaries, we are proposing to update
the beneficiary notice requirement for ACOs that select preliminary prospective assignment with
retrospective reconciliation to focus on beneficiaries that are likely to be assigned to the ACO.
These beneficiaries are those who received at least one primary care service during the
assignment window or applicable expanded window for assignment (as defined in § 425.20)
from a physician who is an ACO professional in the ACO and who is a primary care physician as
defined under § 425.20 or who has one of the primary specialty designations included in §
425.402(c), a FQHC or RHC that is part of the ACO, or an ACO professional in the ACO whom
the beneficiary designated as responsible for coordinating their overall care under § 425.402(c).
This proposal would reduce the burden of sending the beneficiary notice to all “fee for
service beneficiar[ies],” including those who ultimately would not be eligible to be assigned to
ACOs that select preliminary prospective assignment with retrospective reconciliation.
Specifically, we propose to modify § 425.312(a)(2)(iii) to state in the case of an ACO that has
selected preliminary prospective assignment with retrospective reconciliation, the beneficiary
notice must be provided by the ACO or ACO participant to each beneficiary who received at
least one primary care service during the assignment window or applicable expanded window for
assignment (as defined in § 425.20) from a physician who is an ACO professional in the ACO
and who is a primary care physician as defined under § 425.20 or who has one of the primary
specialty designations included in § 425.402(c), a FQHC or RHC that is part of the ACO, or an
ACO professional in the ACO whom the beneficiary designated as responsible for coordinating
their overall care under § 425.402(e). Each such beneficiary must receive a standardized written
notice at least once during an agreement period in the form and manner specified by CMS. The
standardized written notice must be furnished to all of these beneficiaries prior to or at the first
primary care service visit during the first performance year in which the beneficiary receives a
primary care service from an ACO participant.
For ACOs that select prospective assignment, beneficiaries are prospectively assigned to
the ACO at the beginning of each benchmark or performance year based on the beneficiary's use
of primary care services in the most recent 12 or 24 months, as applicable, for which data are
available, using the assignment methodology described in §§ 425.402 and 425.404. See §
425.400(a)(3)(i). Beneficiaries that are prospectively assigned to an ACO under §
425.400(a)(3)(i) remain assigned to the ACO at the end of the benchmark or performance year
unless they meet any of the exclusion criteria under § 425.401(b). See § 425.400(a)(3)(ii). We
note that ACOs that select prospective assignment are subject to § 425.312(a)(2)(iv). Under this
regulation, ACOs that select prospective assignment are required to furnish the beneficiary
notice to all prospectively assigned beneficiaries once during an agreement period.
We seek comment on this proposal. If finalized, this proposed change would be effective
beginning on January 1, 2025.
H. Medicare Part B Payment for Preventive Services (§§ 410.10, 410.57, 410.64, 410.152)
1. Part B Preventive Vaccines and their Administration
a. Statutory Background
Under section 1861(s)(10) of the Act, Medicare Part B covers both the vaccine and
vaccine administration for the specified preventive vaccines – pneumococcal, influenza, hepatitis
B and COVID-19 vaccines. Section 1861(s)(10)(B) of the Act specifies that the hepatitis B
vaccine and its administration is only covered for those who are at high or intermediate risk of
contracting hepatitis B, as defined at § 410.63. Under sections 1833(a)(1)(B) and (b)(1) of the
Act, respectively, there is no applicable beneficiary coinsurance, and the annual Part B
deductible does not apply for these vaccines or the services to administer them. Per section
1842(o)(1)(A)(iv) of the Act, payment for these vaccines is based on 95 percent of the Average
Wholesale Price (AWP) for the vaccine product, except when furnished in the settings for which
payment is based on reasonable cost, such as a hospital outpatient department (HOPD), rural
health clinic (RHC), or federally qualified health center (FQHC). Some other preventive
vaccines, such as the zoster vaccine for the prevention of shingles, are not specified for Medicare
Part B coverage under section 1861(s)(10) of the Act and are instead covered under Medicare
Part D.
b. Pneumococcal, Influenza and Hepatitis B Vaccine Administration
In the CY 2022 PFS final rule (86 FR 65185), we finalized a uniform payment rate of $30
for the administration of a pneumococcal, influenza or hepatitis B vaccine covered under the
Medicare Part B preventive vaccine benefit. We explained that since payment policies for the
administration of the preventive vaccines described under section 1861(s)(10) of the Act are
independent of the PFS, these payment rates will be updated as necessary, independent of the
valuation of any specific codes under the PFS. (Please see COVID-19 vaccine administration
payment information in the next section.) The CY 2022 PFS final rule (86 FR 65180 through
65182) provides a detailed discussion on the history of the valuation of the three Level II
Healthcare Common Procedure Coding System (HCPCS) codes, G0008, G0009, and G0010,
which describe the services to administer an influenza, pneumococcal, and hepatitis B vaccine,
respectively.

In the CY 2023 PFS final rule (87 FR 69984), we finalized a policy to annually update
the payment amount for the administration of Part B preventive vaccines based upon the
percentage increase in the Medicare Economic Index (MEI). Additionally, we finalized the use
of the PFS Geographical Adjustment Factor (GAF) to adjust the payment amount to reflect cost
differences for the geographic locality based upon the fee schedule area where the preventive
vaccine is administered. These adjustments and updates apply to HCPCS codes G0008, G0009,
G0010.
These adjustments and updates also apply to Current Procedural Terminology (CPT)
code 90480 (Immunization administration by intramuscular injection of severe acute respiratory
syndrome coronavirus 2 (SARSCoV-2) (coronavirus disease [COVID-19]) vaccine, single dose)
that describe the service to administer COVID-19 vaccines and HCPCS code M0201
(Administration of pneumococcal, influenza, hepatitis b, and/or covid-19 vaccine inside a
patient's home; reported only once per individual home per date of service when such vaccine
administration(s) are performed at the patient's home), discussed below in section III.H.1.c and
III.H.1.d, respectively, of this proposed rule.
The current payment rates for G0008, G0009, and G0010, as finalized in the CY 2024
PFS final rule, can be found on the CMS Vaccine Pricing website under the “Seasonal Flu
Vaccines” tab, and then under the heading “Locality-Adjusted Payment Rates.”
543
The payment
rates for these services, with the annual update applied for CY 2025, will be made available at
the time of publication of the CY 2025 PFS final rule. The current forecast of the increase in the
MEI for CY 2025 is 3.6 percent based on the 2017-based MEI. We note that the CY 2025 MEI
increase factor for the final rule will be based on historical data through the 2nd quarter of 2024.
543
https://www.cms.gov/medicare/payment/fee-for-service-providers/part-b-drugs/average-drug-sales-
price/vaccine-pricing, under the tab “Seasonal Flu Vaccines”, and then under the header “Locality-Adjusted
Payment Rates.”
Tables 45 and 46 in section III.H.1.f. of this proposed rule provide the CY 2025 projected
payment rates for G0008, G0009, and G0010.
c. COVID-19 Vaccine Administration
In the CY 2022 PFS final rule (86 FR 65181 and 65182), we provided a detailed history
regarding the determinations of initial payment rates for the administration of COVID-19
vaccines, and an explanation of how the payment policy evolved to a rate of $40 per dose. For
CY 2022, we maintained the payment policy for the administration of COVID-19 vaccines and
stated that while we believe it is appropriate to establish a single, consistent payment rate for the
administration of all four Part B preventive vaccines in the long term, we will pay a higher, $40
payment rate for administration of COVID-19 vaccines in the short term, while pandemic
conditions persisted (86 FR 65185).
In the CY 2023 PFS final rule (87 FR 69988 through 69993), we stated that due to timing
distinctions between a PHE declared under section 319 of the Public Health Service (PHS) Act
and an Emergency Use Authorization (EUA) declaration under section 564 of the Federal Food,
Drug, and Cosmetic Act (FD&C Act), we reconsidered the policies finalized in the CY 2022 PFS
final rule in light of our goal to promote broad and timely access to COVID-19 vaccines. We
explained that our goal would be better served if our policies with respect to payment for
administration of these products, as addressed in the November 6, 2020 COVID-19 IFC (85 FR
71142) and CY 2022 PFS final rule (85 FR 18250), continue until the EUA declaration for drugs
and biological products with respect to COVID-19 is terminated. Therefore, we finalized that we
would maintain the current payment rate of $40 per dose for the administration of COVID-19
vaccines through the end of the calendar year in which the March 27, 2020 EUA declaration
under section 564 of the FD&C Act (EUA declaration) for drugs and biological products ends.
Effective January 1 of the year following the year in which the EUA declaration ends, the
COVID-19 vaccine administration payment would be set at a rate to align with the payment rate
for the administration of other Part B preventive vaccines, that is, approximately $30 per dose.

As mentioned above, we also finalized that, beginning January 1, 2023, we would annually
update the payment amount for the administration of all Part B preventive vaccines based upon
the percentage increase in the MEI, and that we would use the PFS GAF to adjust the payment
amount to reflect cost differences for the geographic locality based upon the fee schedule area
where the vaccine is administered.
On September 11, 2023, the Food and Drug Administration (FDA) announced its
recommendation to shift to a monovalent severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2) (coronavirus disease 2019 [COVID-19]) vaccine that targets the predominant
XBB lineage virus strain for the 2023-2024 vaccine administration season.
544
In anticipation of
this recommendation, in August 2023, the CPT Editorial Panel approved five new monovalent
COVID-19 vaccine product codes for Pfizer and Moderna vaccines. In addition, they approved a
new vaccine administration code (90480) for reporting the administration of any COVID-19
vaccine for any patient (pediatric or adult), replacing all previously approved specific vaccine
administration codes. All previously approved COVID-19 vaccine product and vaccine
administration codes were deleted from the CPT code set effective November 1, 2023, except for
product code 91304, which represents the Novavax COVID-19 vaccine product and remains
active.
545
The current payment rate for CPT code 90480 is available on the CMS COVID-19
Vaccine Pricing website, under “COVID-19 Vaccines & Monoclonal Antibodies”.
546
The
payment rate for this service, with the annual update applied for CY 2025, will be made available
at the time of publication of the CY 2025 PFS final rule. As noted above, the current forecast of
the increase in the MEI for CY 2025 is 3.6 percent based on the 2017-based MEI, however, the
544
https://www.fda.gov/news-events/press-announcements/fda-takes-action-updated-mrna-covid-19-vaccines-better-
protect-against-currently-circulating.
545
CPT® Assistant Special Edition: August Update / Volume 33 / 2023. https://www.ama-assn.org/system/files/cpt-
assistant-guide-coronavirus-august-2023-updated.pdf.
546
https://www.cms.gov/medicare/payment/fee-for-service-providers/part-b-drugs/average-drug-sales-
price/vaccine-pricing, under “COVID-19 Vaccines & Monoclonal Antibodies".
CY 2025 MEI increase factor for the final rule will be based on historical data through the 2nd
quarter of 2024.
Due to the uncertainty surrounding the future of the EUA declaration for drugs and
biological products for COVID–19, Tables 45 and 46, at the end of section III.H.1.f. of this
proposed rule, reflect the potential alternative payment amounts for Part B preventive vaccine
administration for CY 2025. Table 45 displays the CY 2025 Part B payment rates for preventive
vaccine administration if the EUA declaration continues into CY 2025, and Table 46 displays the
CY 2025 Part B payment rates for preventive vaccine administration if the EUA declaration ends
on or before December 31, 2024.
d. In-Home Additional Payment for Administration of Preventive Vaccines
In the CY 2022 PFS final rule (86 FR 65187 and 65190), we provide a detailed
discussion on the payment policy for COVID-19 vaccine administration in the home. In
summary, providers and suppliers that administer a COVID-19 vaccine in the home, under
certain circumstances, could bill Medicare for one of the existing COVID-19 vaccine
administration CPT codes along with HCPCS code M0201 (COVID-19 vaccine administration
inside a patient’s home; reported only once per individual home per date of service when only
COVID-19 vaccine administration is performed at the patient’s home). For CY 2022, we
continued to make an additional payment when a COVID–19 vaccine was administered in a
beneficiary’s home under certain circumstances and stated that we would make this payment
until the end of the year in which the PHE expires.
In the CY 2023 PFS final rule (87 FR 69984 through 69986), we discussed that we had
received many comments and requests from interested parties that the in-home add-on payment
be applied more broadly to all preventive vaccines. Commenters also expressed concerns that
discontinuation of the in-home additional payment would negatively impact access to the
COVID-19 vaccine for underserved homebound beneficiaries. Therefore, we continued the
policy of making an additional payment when a COVID–19 vaccine is administered in a
beneficiary’s home, under the certain circumstances for the duration of CY 2023. We explained
that we were continuing the policy of additional payment for at-home COVID-19 vaccinations
for another year in order to provide us time to track utilization and trends associated with its use,
in order to inform the Part B preventive vaccine policy on payments for in-home vaccine
administration for CY 2024. In addition, for CY 2023 we updated the payment amount by the
CY 2023 MEI percentage increase and adjusted for geographic cost differences as we do the
payment for the preventive vaccine administration service, that is, based upon the fee schedule
area where the COVID-19 vaccine is administered, by using the PFS GAF (87 FR 69986).
In the CY 2024 PFS final rule (88 FR 79235 through 79237), we discussed the policy for
the in-home additional payment for COVID–19 vaccine administration under the Part B
preventive vaccine benefit for CY 2024 and subsequent years. We maintained the payment
policy for COVID-19 vaccine administration and extended the additional payment to the
administration of the other three preventive vaccines included in the Part B preventive vaccine
benefit—the pneumococcal, influenza, and hepatitis B vaccines. As described at § 410.152(h)(3),
effective January 1, 2024, the payment amount for the in-home administration of all four
vaccines is identical, that is, Medicare Part B pays the same additional payment amount to
providers and suppliers that administer a pneumococcal, influenza, hepatitis B, or COVID-19
vaccine in the home. This additional payment amount is annually updated using the percentage
increase in the MEI and is adjusted to reflect geographic cost variations with the PFS GAF.
We stated that the in-home additional payment is limited to one payment per home visit,
even if multiple vaccines are administered during the same home visit. We noted that every
vaccine dose that is furnished during a home visit still receives its own unique vaccine
administration payment. The additional payment for in-home Part B vaccine administration is
only made if certain circumstances are met, as outlined at § 410.152(h)(3)(iii). Providers and
suppliers that administer one of the Part B preventive vaccines in the home, under those
circumstances, can bill Medicare for one of the existing Part B vaccine administration CPT codes

along with HCPCS code M0201 (Administration of pneumococcal, influenza, hepatitis b, and/or
covid-19 vaccine inside a patient's home; reported only once per individual home per date of
service when such vaccine administration(s) are performed at the patient's home) (88 FR 79235
through 79237).
The current payment rate for M0201 can be found on the CMS Vaccine Pricing website
under “COVID-19 Vaccines & Monoclonal Antibodies”.
547
The M0201 payment rate with the
annual update applied for CY 2025 will be made available at the time of publication of the CY
2025 PFS final rule. The current forecast of the increase in the MEI for CY 2025 is 3.6 percent
based on the 2017-based MEI. We note that the CY 2025 MEI increase factor for the final rule
will be based on historical data through the 2
nd
quarter of 2024. Tables 45 and 46 in section
III.H.1.f. of this proposed rule provide the CY 2025 projected payment rate for M0201.
e. COVID-19 Monoclonal Antibodies and their Administration
In CY 2023 PFS final rule (87 FR 69987 through 69993), we discussed that all COVID-
19 monoclonal antibody products and their administration are covered and paid for under the
Part B preventive vaccine benefit through the end of year in which the Secretary terminates the
EUA declaration for drugs and biological products with respect to COVID-19. In addition, we
explained that, under the authority provided by section 3713 of the CARES Act, we have
established specific coding and payment rates for the COVID-19 vaccine, as well COVID-19
monoclonal antibodies and their administration, through technical direction to Medicare
Administrative Contractors (MACs) and information posted publicly on the CMS website (87 FR
69987).
In the CY 2023 PFS final rule, we also established a policy to continue coverage and
payment for monoclonal antibodies that are used for pre-exposure prophylaxis (PrEP) of
COVID-19 under the Part B preventive vaccine benefit if they meet applicable coverage
547
https://www.cms.gov/medicare/payment/fee-for-service-providers/part-b-drugs/average-drug-sales-
price/vaccine-pricing, under “COVID-19 Vaccines & Monoclonal Antibodies”.
requirements (87 FR 69992). We explained that we will continue to pay for these products and
their administration even after the EUA declaration for drugs and biological products is
terminated, so long as after the EUA declaration is terminated, such products have market
authorization. Additionally, we established that payments for the administration of monoclonal
antibodies that are used for PrEP of COVID-19 would be adjusted for geographic cost variations
using the PFS GAF. In the CY 2024 PFS rule (88 FR 79239 through 79240), we codified these
policies in regulations at §§ 410.10(l) and 410.57(c).
In CY 2024 PFS final rule (88 FR 79239 through 79240), we noted that we did not
finalize any payment regulations regarding monoclonal antibodies for PrEP of COVID-19, since
at the time of the publication of the CY 2024 PFS final rule, there were no COVID-19
monoclonal antibodies approved or authorized for use against the dominant strains of COVID-19
in the United States. We stated that if a new monoclonal antibody for PrEP of COVID-19
became authorized for use, we would use the authority provided by section 3713 of the CARES
Act, as discussed in the CY 2023 PFS final rule (87 FR 69987), to establish specific coding and
payment rates for the administration of that product through technical direction to MACs and
information posted publicly on the CMS website. We explained that we would subsequently
propose coding and payment rates for the administration of that product via rulemaking.
We also noted that, for the purposes of the in-home additional payment discussed above
in section III.H.1.d. of this proposed rule, that additional payment is not applicable to the
administration of monoclonal antibodies for PrEP of COVID-19. For monoclonal antibodies for
PrEP of COVID-19, we set the coding and payment rates for the administration of COVID-19
monoclonal antibodies in the home (when applicable) to be higher than those in other health care
settings, and therefore such amounts already account for the higher costs of administering the
product in the home.

On March 22, 2024, the FDA issued an EUA for Pemgarda (pemivibart) injection, for
intravenous use.
548
Pemgarda is a monoclonal antibody product indicated for use for pre-
exposure prophylaxis to help prevent COVID-19 in adults and children 12 years of age and older
who weigh at least 88 pounds (40 kg) who:
● Are not currently infected with SARS-CoV-2 and who have not been known to be
exposed to someone who is infected with SARS-CoV-2 and
● Have moderate-to-severe immune compromise because of a medical condition or
because they receive medicines or treatments that suppress the immune system and they are
unlikely to have an adequate response to COVID-19 vaccination.
Therefore, under the authority provided by section 3713 of the CARES Act, we
established specific coding and payment rates for the administration of Pemgarda through
technical direction to MACs and information posted publicly on the CMS website. Since
Pemgarda is used for pre-exposure prophylaxis of COVID-19, which CMS is covering under the
Part B preventive vaccine benefit even after the EUA declaration for drugs and biological
products is terminated (so long as such products still have market authorization), we plan to
propose long-term coding and payment rates for the administration of this product in future
rulemaking. The current payment rates for Pemgarda and its administration can be found on the
CMS Vaccine Pricing website under “COVID-19 Vaccines & Monoclonal Antibodies”.
549
These payment rates are also listed below in Tables 45 and 46.
More information on our coding and payment policies for COVID-19 monoclonal
antibodies is available at https://www.cms.gov/monoclonal.
f. Summary of Payment Amounts for CY 2025
Due to the uncertainty surrounding the future of the EUA declaration for drugs and
biological products for COVID-19, we are including Tables 45 and 46, which summarize
548
https://www.fda.gov/media/177068/download?attachment.
549
https://www.cms.gov/medicare/payment/fee-for-service-providers/part-b-drugs/average-drug-sales-
price/vaccine-pricing, under “COVID-19 Vaccines & Monoclonal Antibodies”.
Medicare Part B the potential alternative preventive vaccine administration payment amounts at
the time of the publication of this proposed rule. If the EUA declaration continues to be in effect
on January 1, 2025, we propose that the payment rates in Table 45 will apply. If the EUA
declaration is terminated before January 1, 2025, we propose that the payment rates in Table 45
will apply.
For CY 2025, the proposed growth rate of the 2017-based MEI is estimated to be 3.6
percent, based on the IHS Global, Inc. (IGI) first quarter 2024 forecast with historical data
through fourth quarter 2023. Therefore, Table 45 represents our CY 2024 payment rates for the
listed items, multiplied by 1.036. We propose that if more recent data are subsequently available
(for example, a more recent estimate of the MEI percentage increase), we would use such data, if
appropriate, to determine the CY 2025 MEI percentage increase in the CY 2025 PFS final rule;
we would apply that updated MEI percentage increase to the rates found in the Tables 45 and 46
where applicable.

TABLE 45: CY 2025 Part B Payments for Preventive Vaccine Administration
if the EUA Declaration for Drugs and Biologicals with Respect to COVID-19 Continues
into CY 2025
Category of Part B Product
Administration
Part B Payment Amount
(Unadjusted)
Annual
Update
6
Geographic
Adjustment
Influenza,
Pneumococcal,
Hepatitis B Vaccines
1,4
$33.74
MEI
GAF
COVID-19 Vaccine
2,4
$44.99
MEI
GAF
In-Home Additional Payment for
Part B Vaccine Administration
(M0201)
4
$39.94
MEI
GAF
COVID-19 Monoclonal Antibodies
(for Treatment or Post-Exposure
Prophylaxis)
3,4,5
N/A
N/A
N/A
COVID-19 Monoclonal Antibodies
(for Pre-Exposure Prophylaxis)
3,4
Intravenous Infusion: Health Care
Setting
$450
N/A
GAF
1
HCPCS Codes G0008, G0009, G0010.
2
CPT code 90480.
3
https://www.cms.gov/monoclonal.
4
Beneficiary coinsurance and deductible are not applicable.
5
As of the issuance of the CY 2025 PFS proposed rule, there are no monoclonal antibodies approved or authorized
for the treatment or for post-exposure prophylaxis of COVID-19
6
The proposed CY 2025 percentage increase of the 2017-based MEI is 3.6 percent based on IGI’s first quarter of
2024 forecast with historical data through the 4
th
quarter of 2023.
TABLE 46: Part B Payments for Preventive Vaccine Administration Beginning January 1,
2025, if the EUA Declaration for Drugs and Biologicals with Respect to COVID 19 is
Terminated on or Before December 31, 2024
Category of Part B Product
Administration
Part B Payment Amount
(Unadjusted)
Annual
Update
6
Geographic
Adjustment
Influenza,
Pneumococcal,
Hepatitis B
1,4
$33.74
MEI
GAF
COVID-19
2,4
$33.74
MEI
GAF
In-Home Additional Payment for
Part B Vaccine Administration
(M0201)
4
$39.94
MEI
GAF
COVID-19 Monoclonal Antibodies
(for Treatment or Post-Exposure
Prophylaxis)
3
Medicare payment under the applicable payment system
COVID-19
Monoclonal Antibodies (for Pre-
Exposure Prophylaxis)
4,5
TBD
5
N/A
GAF
1
HCPCS Codes G0008, G0009, G0010.
2
CPT code 90480
3
Payment is in accordance with the applicable payment system of the setting in which the product is administered.
Beneficiary coinsurance and deductible are applicable.
4
Beneficiary coinsurance and deductible are not applicable.
5
Please see section III.H.1.e. of this proposed rule.
6
The proposed CY 2025 percentage increase of the 2017-based MEI is 3.6 percent, based on IGI’s first quarter of
2024 forecast with historical data through the 4th quarter of 2023.

2. Revised Payment Policies for Hepatitis B Vaccine Administration
In section III.M of this proposed rule, CMS proposes to improve access and utilization of
hepatitis B vaccines by expanding the list of individuals who are at high or intermediate risk of
contracting hepatitis B at § 410.63. Specifically, CMS proposes to expand coverage of hepatitis
B vaccinations by revising § 410.63(a)(2), Intermediate Risk Groups, by adding a new paragraph
(a)(2)(iv) to include individuals who have not previously received a completed hepatitis B
vaccination series and individuals whose previous vaccination history is unknown. CMS believes
that this proposed coverage change will help protect Medicare beneficiaries from acquiring
hepatitis B infection, contribute to eliminating viral hepatitis as a public health threat in the
United States, and is in the best interest of the Medicare program and its beneficiaries. Below,
we discuss how the proposal to expand coverage may impact Part B payment policy for hepatitis
B vaccines and administration.
a. Background
Section 2323 of the Deficit Reduction Act of 1984 (Pub. L. 98-369) amended section
1861(s)(10) of the Act by adding subparagraph (B) to provide Medicare Part B coverage for the
hepatitis B vaccine and its administration for those individuals who are at high or intermediate
risk of contracting hepatitis B. The statute required the Secretary to determine, by regulations,
criteria for identifying individuals who are at high or intermediate risk of contracting hepatitis B.
In addition, section 2323 of the Deficit Reduction Act of 1984 added section 1833(k) of the Act,
which states that the Secretary may provide, instead of the amount of payment otherwise
provided under Part B for the hepatitis B vaccine and its administration, for payment of such an
amount or amounts as reasonably reflects the general cost of efficiently providing such services.
In the June 4, 1990 Federal Register, CMS issued a final rule to implement section 2323
of the Deficit Reduction Act of 1984 and the coverage provisions were codified in regulation at §
410.63(a) (55 FR 22785). In the preamble to 1990 rule, we stated that, “[f]or Medicare payment
purposes, the hepatitis B vaccine may be administered—upon the order of a doctor of medicine

or osteopathy—by qualified staff of home health agencies, skilled nursing facilities, ESRD
facilities, hospital outpatient departments, HMO’s, persons recognized under the ‘incident to
physician’s services’ provision of the law (section 1861(s)(2)(A) of the Act), as well as doctors
of medicine and osteopathy.” This policy is included in the Medicare Claims Processing Manual,
Chapter 18, section 10.1.3.
In the CY 2013 PFS final rule (77 FR 69363), CMS amended the regulations at
§410.63(a) to include those diagnosed with diabetes mellitus in the list of groups at high risk of
contracting hepatitis B. In the November 6, 2020 COVID-19 IFC (85 FR 71145), in preamble
discussions surrounding the implementation of coverage and payment for the COVID-19
vaccine, we mentioned the unique coverage and payment requirements related the hepatitis B
vaccine under Part B. We noted that, unlike pneumococcal, influenza and COVID-19 vaccines,
hepatitis B vaccines require an assessment of a patient's risk of contracting hepatitis B. Because,
hepatitis B vaccinations claims needed a physician's order, they could not be roster billed by
mass immunizers. More information on the current physician’s order policy for the
administration of hepatitis B vaccines in Part B can be found in the Medicare Benefit Policy
Manual, Chapter 15, Section 50.4.4.2.
b. Revisions to Payment Policies for Hepatitis B Vaccinations
As discussed above, section III.M of this proposed rule revises § 410.63(a) to provide
coverage under Part B for hepatitis B vaccines and their administration for an expanded range of
Medicare enrollees. We explain that Medicare coverage of hepatitis B vaccination is outdated in
light of recent information about the risks of contracting hepatitis B, and that current research
indicates that individuals who remain unvaccinated against hepatitis B are at intermediate risk of
contracting hepatitis B virus. Under the new proposal, an assessment of an individual’s
vaccination status could now be made without the clinical expertise of a physician. Thus, if the
rule is finalized, we would remove our policy in the manual that the administration of a Part B
hepatitis B vaccine be preceded by a doctor’s order. If the new definition at § 410.63(a) is

finalized as proposed, then a doctor’s order would no longer be necessary for the administration
of a hepatitis B vaccine under Part B.
If the physician’s order requirement is eliminated, then we would also change our
procedures to allow mass immunizers to use the roster billing process to submit Medicare Part B
claims for hepatitis B vaccines and their administration.
Currently, instructions regarding hepatitis B vaccine administration under part B are
contained in CMS manual guidance. If changes to § 410.63(a) are finalized, then we would make
corresponding changes to guidance in the Medicare Benefit Policy Manual and Medicare Claims
Processing Manual. More information on roster billing is available on the CMS webpage at
https://www.cms.gov/roster-billing.
We note that the current payment rates for HCPCS code G0010, “Administration of
hepatitis b vaccine,” as finalized in the CY 2024 PFS final rule, can be found on the CMS
Vaccine Pricing website under “Seasonal Flu Vaccines”.
550
The payment rates for G0010, with
the annual update applied for CY 2025, will be made available at the time of publication of the
CY 2025 PFS final rule. Tables 45 and 46 in section III.H.1.f. of this proposed rule provide the
CY 2025 projected payment rates for G0010. More information on other policies related to the
administration of G0010 can be found in the section preceding this one (section III.H.1. of this
proposed rule), and proposed revisions to payment policies for the administration of G0010 in
RHCs and FQHCs can be found in the section immediately below (section III.H.2.c. of this
proposed rule).
c. Revisions to Payment Policies for Hepatitis B Vaccinations in Rural Health Clinics (RHC) and
Federally Qualified Health Centers (FQHC)
When section 2323 of the Deficit Reduction Act of 1984 added section 1861(s)(10)(B) to
the Act to add Medicare Part B coverage for the hepatitis B vaccine and its administration, it
550
https://www.cms.gov/medicare/payment/fee-for-service-providers/part-b-drugs/average-drug-sales-
price/vaccine-pricing, under “Seasonal Flu Vaccines”; see links to the relevant year under “Locality-Adjusted
Payment Rates.”
limited that coverage to certain settings. In RHCs and FQHCs, the law specified at section
1833(a)(3)(A) of the Act that the vaccines mentioned at section 1861(s)(10)(A) of the Act –
namely, pneumococcal and influenza (and later, COVID-19) vaccines – are not included in the
all-inclusive payment rate for an RHC or FQHC visit, but are reimbursed as a separate payment.
Pneumococcal, influenza and COVID-19 vaccines and their administration are paid at 100
percent of reasonable cost when administered in an RHC or FQHC, in accordance with section
1833(a)(1)(B) of the Act. By contrast, hepatitis B vaccines and the cost of administration are
included in the capitated payment for an RHC or FQHC visit. RHCs and FQHC visits are
generally paid at 80 percent of reasonable costs, and thus, they are subject to coinsurance for
Medicare Part B enrollees. The Deficit Reduction Act of 1984 also added section 1833(k) to the
Act, which states that, for hepatitis B vaccines and their administration as described at section
1861(s)(10)(B), the Secretary may provide, instead of the amount of payment otherwise dictated
in statute, for payment that “reasonably reflects the general cost of efficiently providing such
services.”
In CY 2011 PFS final rule (75 FR 73418), we addressed the issue of coinsurance for
hepatitis B vaccines and their administration in FQHCs. The CY 2011 PFS final rule, which
implemented the expansion of preventive services in Medicare as mandated by the ACA, stated
that effective January 1, 2011, Part B coinsurance on hepatitis B vaccinations was waived, as the
vaccine and its administration were deemed “preventive services” per section 1861(ddd)(3)(A)
of the Act as cross-referenced to section 1861(ww)(2) of the Act. (More information on
preventive services is provided immediately below at section III.H.3. of this proposed rule). The
CY 2011 PFS final rule codified this FQHC policy in regulation at § 405.2449. In the CY 2014
FQHC PPS final rule (79 FR 25474), at § 405.2410(b), we codified regulations regarding
coinsurance in RHCs and FQHCs which exempt from coinsurance "preventive services for
which Medicare pays 100 percent under § 410.152(l) of this chapter”, which explicitly includes
the hepatitis B vaccine. In the CY 2016 PFS final rule (80 FR 71088), we clarified that these

waivers of cost-sharing (both coinsurance and deductible) for preventive services applied to
RHCs as well, and we subsequently clarified in subregulatory guidance that these waivers apply
to the administration of hepatitis B vaccines in RHC and FQHCs.
551
We note that FQHC services
are always exempt from the Part B deductible, per section 1833(b)(4) of the Act.
Even though hepatitis B vaccines and their administration are deemed preventive services
for which coinsurance (and deductible in RHCs) is waived, hepatitis B vaccines are still
currently paid differently than other Part B vaccines in RHCs and FQHCs. Due to the statutory
differences explained above, pneumococcal, influenza and COVID-19 vaccines and their
administration are paid at 100 of reasonable cost in RHCs and FQHCs – that is, they are paid
separately from the FQHC PPS or the RHC All-Inclusive Rate (AIR) methodology – while
hepatitis B vaccines and their administration are paid as part of the FQHCs PPS or the RHC AIR,
which means that they are paid through changes to the facilities’ capitated rate.
In light of the proposal to expand coverage for hepatitis B vaccination in section III.M. of
this proposed rule, we propose to use the aforementioned authority at section 1833(k) of the Act
to align payment for hepatitis B vaccinations in RHCs and FQHCs with the payment for
pneumococcal, influenza and COVID-19 vaccinations in those settings. That is, we propose to
pay for hepatitis B vaccines and their administration in RHCs and FQHCs at 100 percent of
reasonable cost, separate from the FQHCs PPS and the RHC AIR methodology, for all
populations identified for coverage at § 410.63(a). As is the case for pneumococcal, influenza
and COVID-19 vaccine administration, under this proposal, a hepatitis B vaccine administration
would not be considered an RHC or FQHC visit. If this policy is finalized, then effective January
551
Updates were made to Chapter 13, section 220.1 of Medicare Benefit Policy Manual via Change Request 9864,
R2186CP, December 9, 2016, “Rural Health Clinic (RHC) and Federally Qualified Health Center (FQHC)
Updates”: https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/Downloads/R230BP.pdf.
Updates were also made to Chapter 9, section 60.3 of the Medicare Claims Processing Manual via Change Request
9397, R3434CP, December 31, 2015, “Reorganization of Chapter 9”: https://www.cms.gov/Regulations-and-
Guidance/Guidance/Transmittals/Downloads/R3434CP.pdf.
1, 2025, RHCs and FQHCs would bill for Part B hepatitis B vaccines in the same manner as they
currently bill for pneumococcal, influenza and COVID-19 vaccines, that is, on their cost report.
We note that we proposed above, in section III.B.5 of this proposed rule, to allow for billing and
payment of all Part B preventive vaccines and their administration at the time of service in RHCs
and FQHCs, with annual reconciliation on the facilities’ cost reports. That policy, if finalized as
proposed, would be effective for dates of service on or after July 1, 2025. Both proposals
together support our goal of streamlining payment for all Part B vaccines across Part B settings
of care. We believe that streamlining Part B vaccine and vaccine administration payments among
care settings aligns with the stated goals of section 1833(k) of the Act, since those payment
policy changes will allow for increased efficiency in Part B claims processing on both the part of
the RHCs and FQHCs and on the part of CMS. We also believe that the increased efficiency will
promote vaccine access, and thus health equity in general, in RHCs and FQHCs that already
serve vulnerable populations.
To implement this proposal regarding payment for hepatitis B vaccines and their
administration in RHCs and FQHCs, we also propose to amend the regulations at §
405.2466(b)(1)(iv), to add hepatitis B vaccines to the list of vaccines covered in RHCs and
FQHCs at 100 percent of reasonable cost. Upon the publication of the CY 2025 PFS final rule, if
revisions to §405.2466(b)(1)(iv) are finalized as proposed, we would make corresponding
changes to guidance in the Medicare Benefit Policy Manual, Chapter 13 and Medicare Claims
Processing Manual, Chapter 9. If finalized, we also plan to facilitate the necessary operational
systems updates needed to implement these changes.
d. Regulations Concerning Hepatitis B Vaccines and their Administration
Listed below are several Medicare Part B regulations that mention the hepatitis B vaccine
and refer to § 410.63(a) for a definition of hepatitis B vaccine coverage. Since we are proposing
to revise § 410.63(a) in section III.M. of this proposed rule, we do not believe additional
regulation text changes are needed to conform to the coverage proposal, as the update to the
definition at § 410.63(a) will apply to the use of the definition in these regulations:
● Section 410.10(p).
● Section 410.57(d).
● Section 411.15(e)(3) and (k)(5).
● Section 414.707(a)(2)(iii).
● Section 414.904(e)(1).
In addition, we note that there are no conforming regulation text changes needed to the
payment regulations at § 410.152, paragraphs (h) and (l)(1), to conform to the coverage proposal.
3. Payment for Drugs Covered as Additional Preventive Services (§410.152)
a. Statutory Background
Section 101 of the Medicare Improvements for Patients and Providers Act (MIPPA) of
2008 (Pub. L. 110-275) added section 1861(ddd)(1) and (2) of the Act to effectuate
“improvements to coverage of preventive services” in the Medicare program. Under section
1861(ddd)(1) of the Act, Medicare Part B covers “additional preventive services” that identify
medical conditions or risk factors and that the Secretary determines are reasonable and necessary
for: (A) the prevention or early detection of an illness or disability; (B) that are recommended
with a grade of A or B by the United States Preventive Services Task Force; and (C) that are
appropriate for individuals entitled to benefits under Part A or enrolled under Part B. Section
1861(ddd)(2) of the Act states that, in making determinations under section 1861(ddd)(1) of the
Act, the Secretary should use the process for making National Coverage Determinations (NCD)
in the Medicare program.
Section 101 of MIPPA also added section 1833(a)(1)(W) of the Act, which provides
requirements for payment of additional preventive services. Section 1833(a)(1)(W)(i) establishes
requirements for payment of additional preventive services that are clinical diagnostic laboratory
tests, and section 1833(a)(1)(W)(ii) establishes requirements for payment of all other services.
Section 1833(a)(1)(W)(ii) (as amended by section 4104 of the Affordable Care Act (Pub. L. 111-
148) requires that the amount paid for the provision of all other additional preventive services is
100 percent of the lesser of the actual charge for the service, or the amount determined under a
fee schedule established by the Secretary for purposes of this subparagraph.
We note that “additional preventive services” are a subset of “preventive services” under
Medicare Part B, per section 1861(ddd)(3) and 1861(ww)(2)(O) of the Act, respectively. Section
1833(b)(1) of the Act states that the annual Part B deductible does not apply to preventive
services, and section 1833(a)(1)(Y) of the Act waives coinsurance for preventive services that
are recommended with a grade of A or B by the USPSTF for any indication or population.
Based on all the above statutory authorities, there is no cost-sharing under Part B for additional
preventive services for Medicare enrollees, that is, there is no applicable beneficiary coinsurance
or deductible for these services.
The term “preventive services” is defined at § 410.2, and coverage for “additional
preventive services” is delineated at § 410.64. At § 410.152(l), we list the Part B preventive
services that are paid at 100 percent of the Medicare payment amount, that is, for which zero
coinsurance is charged. There, at § 410.152(l)(11), we include “additional preventive services
identified for coverage through the national coverage determination (NCD) process”. At §
410.160(b), we list the Part B services that are not subject to the Part B annual deductible and do
not count toward meeting that deductible, and “additional preventive services identified for
coverage through the national coverage determination (NCD) process” is included there at §
410.160(b)(13).
The payment authority under section 1833(a)(1)(W)(ii) of the Act has not been utilized to
date because CMS has not yet covered any additional preventive service that would require use
of that payment authority. While CMS currently covers certain screenings and therapies as
additional preventive services under the section 1861(ddd) of the Act, those screenings and
therapies are currently paid under the existing PFS fee schedule for physician services.
Furthermore, the Medicare Diabetes Prevention Program, described at section III.E of this
proposed rule, uses the section 1833(a)(1)(W)(ii) authority to waive the coinsurance and
deductible as described above, but its payment policy is based on separate authorities under the
model.
Specifically, we note that CMS has not yet covered or paid for any drugs or biologicals
(hereinafter, referred to as drugs) under the benefit category of additional preventive services.
This was highlighted when CMS released a Proposed NCD for Pre-Exposure Prophylaxis (PrEP)
for Human Immunodeficiency Virus (HIV) Infection Prevention on July 12, 2023. This proposed
NCD announced CMS’ intention to cover and pay for those drugs under section 1861(ddd) of the
Act’s additional preventive services authority, and a decision on the NCD is forthcoming. We
note that CMS covers and pays for Part B vaccines, which are also considered preventive
services under sections 1861(ddd)(3) and 1861(ww)(2)(A) of the Act, but they have unique
payment rates specified in statute at section 1842(o)(1)(A)(iv) of the Act (for more information,
see above at section III.H.1.a. of this proposed rule).
b. Proposed Fee Schedule for Drugs Covered as Additional Preventive Services (DCAPS)
As discussed above, the authority at section 1833(a)(1)(W)(ii) of the Act provides for
payment for additional preventive services, including drugs. This authority differs from the
authority used to pay drugs that are separately paid as drugs and biologicals under other Part B
payment authorities. Specifically, payment for most drugs separately payable under Part B is
authorized at section 1833(a)(1)(S) of the Act and outlined at section 1842(o)(1)(C) of the Act,
and those payments are generally made according to the Average Sales Price (ASP)
methodology that is described at section 1847A of the Act. In addition, because drugs covered as
additional preventive services (hereinafter, DCAPS; we will use the term “DCAPS drugs” for the
ease of the reader) are not described in section 1842(o)(1)(C) of the Act, provisions under section
1847A of the Act would not apply, including requirements for manufacturers to report ASP to
CMS on a quarterly basis (see sections 1847A(f) and 1927(b)(3)(A)(iii) of the Act). When
manufacturers are not required to report the manufacturer’s ASP for a drug, they may do so
voluntarily, but the availability of voluntarily reported ASP data cannot be guaranteed, and the
data may not reflect all available NDCs for the drug. However, we emphasize that DCAPS drugs
that are also covered under Part B for non-preventive indications (that is, are also used for
diagnosis or treatment) would be subject to ASP reporting requirements.
Above, we mentioned that section 1833(a)(1)(W)(ii) of the Act requires that the amount
paid for the provision of additional preventive services is 100 percent of the lesser of the actual
charge for the service, or the amount determined under a fee schedule established by the
Secretary for purposes of this subparagraph. For purposes of this policy, we refer to the amount
determined under the fee schedule as the payment limit, which we discuss in detail below.
We are proposing a fee schedule for DCAPS drugs that uses existing Part B drug pricing
mechanisms, because we believe that it is preferable to set all drug payment limits under Part B,
including those for DCAPS, as consistently as possible. Accordingly, we propose that the
payment limit for a DCAPS drug be determined using the methodology described in section
1847A of the Act (also referred to as ASP methodology), or, if ASP data is not available for a
particular drug, to use an alternative pricing mechanism, as described below. We propose to
update the fee schedule quarterly, on the same schedule as the ASP pricing file, which is updated
each calendar quarter.
(1) Payment Limit Based on Section 1847A of the Act
To determine the payment limit for the applicable billing and payment code for a DCAPS
drug under the fee schedule, we propose to apply ASP methodology described in section 1847A
of the Act when ASP data is available for the drug. We believe the use of ASP data would be
preferable for determining the payment limit for DCAPS drug billing and payment codes for two
reasons. First, this approach would determine the payment limit for these drugs in the same way
as the payment limit is usually determined for other drugs that are separately payable under Part
B, when possible. This would include the application of payment limit calculations for multiple

source drugs, single source drugs and biologicals, and biosimilar biological products, as is done
for products under section 1847A of the Act, for each applicable billing and payment code.
Second, because section 1847A(c)(3) of the Act requires that calculation of the manufacturer's
ASP for an NDC must include volume discounts, prompt pay discounts, cash discounts, free
goods that are contingent on any purchase requirement, chargebacks, and rebates (other than
rebates under the Medicaid drug rebate program, discounts under the 340B Program, and rebates
under the Part B and Part D Medicare inflation rebate program), this would set a payment limit
that would likely better reflect acquisition cost of the drug than list prices in available compendia
(such as Wholesale Acquisition Cost (WAC)).
We propose that CMS would determine the payment limit for DCAPS drugs as the
amount that would result from application of ASP methodology in section 1847A of the Act only
if ASP data for the drug is available for a given quarter (that is, positive manufacturer’s ASP data
is reported by the drug manufacturer, as explained in section III.A.2 of this proposed rule). We
propose that if ASP data is available for a DCAPS drug, the payment limit would be the amount
described in section 1847A(b) of the Act, which is usually 106 percent of ASP.
(2) Payment Limit Based on National Average Drug Acquisition Cost (NADAC) Pricing
If ASP data for a DCAPS drug (as described in the previous section) is not available (as
defined in the prior paragraph), we propose to determine the payment limit for the applicable
billing and payment code using the most recently published amount for the drug in Medicaid's
National Average Drug Acquisition Cost (NADAC) survey (OMB control number
0938-1041).
552
When using NADAC data, we propose to determine the payment limit per billing
unit, which would be an average of NADAC prices for all NDCs for the drug. If a drug is
available in generic and brand formulations, we propose all NDCs will be averaged together to
determine the payment limit.
552
https://www.medicaid.gov/medicaid/prescription-drugs/retail-price-survey/index.html.

Since the timing of ASP reporting and publishing has a two-quarter lag (for example,
payment limits calculated using data reported from the first quarter of sales become effective two
quarters later), we propose that “most recently published” for purposes of this policy means the
most recently updated NADAC survey available 30 days after the close of the quarter for which
ASP data would have been reported if it were available.
553
For example, in the calculation of the
payment limit for dates of service in the third calendar quarter, if NADAC is used to determine
the payment limit, CMS would use the most recent NADAC survey update available on the 30
th
day after the close of the first calendar quarter to determine the payment limit for the third
quarter.
The NADAC survey provides a national drug pricing benchmark for certain drugs that is
adequately comprehensive to serve as the first alternative pricing source in the case that ASP
data is not available. CMS conducts surveys of retail community pharmacy prices to develop the
NADAC pricing benchmark in the annual NADAC pricing file. The pricing benchmark is
reflective of the prices paid by retail community pharmacies to acquire prescription and over-the-
counter covered outpatient drugs. NADAC data is publicly available and it can be accessed at
https://data.medicaid.gov/nadac.
In the CY 2020 PFS final rule (84 FR 62655), we similarly finalized the use of NADAC
pricing as a pricing alternative for oral drugs under the Part B Opioid Treatment Program (OTP)
benefit when ASP data is not available. There, we stated that “[s]urvey data on invoice prices
provide the closest pricing metric to ASP that we are aware of.” Because the previous statement
continues to be true, it is an appropriate alternative in the pricing framework for DCAPS drugs
when ASP data is not available.
(3) Payment Limit Based on the Federal Supply Schedule (FSS)
Since NADAC pricing is only available for drugs typically dispensed through retail
community pharmacies, there could be circumstances in which ASP and NADAC data are not
553
42 CFR 414.804(a)(5).

available for DCAPS drugs. Therefore, if both ASP and NADAC pricing data are not available
for a DCAPS drug, we propose to use the most recently published and listed prices for
pharmaceutical products in the Federal Supply Schedule (FSS) to calculate the payment limit for
the applicable billing and payment code. In the same manner as discussed in the previous
section, we propose that “most recently published” for purposes of this policy means the most
recently updated FSS survey available 30 days after the close of the quarter for which ASP data
would have been reported if it were available.
554
For example, in the calculation of the payment
limit for dates of service in the third calendar quarter, if FSS is used to determine the payment
limit, CMS would use the most recent FSS update available on the 30
th
day after the close of the
first calendar quarter to determine the payment limit for the third quarter. When using the FSS,
we would calculate the average price per billing unit (as described in the billing and payment
code for the drug) for all NDCs listed for a drug.
Drug pricing information from the Veterans Affairs’ (VA’s) FSS pharmaceutical pricing
database is publicly available at the NDC level and published at
https://www.va.gov/opal/nac/fss/pharmPrices.asp. We propose to use FSS data when ASP and
NADAC data are not available because FSS data is one of the few existing options for drug
pricing that includes a wide variety of drug formulations, including both self-administered drugs
typically dispensed through retail community pharmacies and drugs administered incident to a
physician’s service. We believe that using FSS data to calculate the payment limit for DCAPS
drugs is preferable to instructing MACs to determine DCAPS drug payment limits according to
invoice (as discussed below), because invoice-based pricing requires MACs to manually process
claims and is therefore burdensome to the MACs.
(4) Invoice Pricing
554
42 CFR 414.804(a)(5).
Finally, if ASP, NADAC, and FSS pricing are not available for a particular drug covered
as an additional preventive service, then MACs will determine the payment for that drug
according to invoice. Since one of the three above pricing mechanisms should be available in
nearly all cases, we expect that invoice pricing would be necessary only in rare situations.
Specifically, we believe that invoice pricing would likely only be necessary for new drugs before
pricing data is available.
To summarize, we propose to establish a fee schedule using the following pricing
mechanisms to determine the payment limit for DCAPS drugs under Part B, which would be
updated quarterly:
(1) If ASP data is available for the DCAPS drug, the payment limit would be determined
based on the methodology under section 1847A(b) of the Act (usually 106 percent of ASP);
(2) If ASP data is not available, the payment limit would be calculated using NADAC
prices for the drug;
(3) If ASP data and NADAC prices are not available, the payment limit would be
calculated using the FSS prices for the drug; and
(4) If ASP data, NADAC prices, and FSS prices are not available, payment limit would
be the invoice price determined by the MAC.
We are proposing to amend § 410.152 by adding paragraph (o) to establish the fee
schedule and the pricing methodologies used to determine the payment limit for DCAPS drugs
under Part B. In addition, to highlight that coinsurance does not apply to DCAPS drugs, we
propose to publish the payment limits for DCAPS drugs along with other separately payable Part
B drugs on the ASP pricing file.
We invite public comment on the proposed fee schedule for drugs paid as additional
preventive services.
c. Payment for Supplying and Administration of Drugs under the Additional Preventive Services
Benefit
As explained above, DCAPS drugs are subject to payment under section
1833(a)(1)(W)(ii) of the Act. Because the fee schedule authorized under such section has not yet
been established, and since DCAPS drugs are not covered by Part B under the same authority as
other separately payable Part B drugs that would provide for administration or supplying fees,
there is no existing policy regarding payment for the administration of DCAPS drugs or the
supplying of DCAPS drugs by suppliers and providers. In a similar manner to the DCAPS drug
pricing mechanisms described above, we propose administration and supplying fees for DCAPS
drugs that mirror existing policies under the PFS and Part B drug payment. We anticipate that an
NCD that adds drugs to the additional preventive services benefit would include coverage for the
supplying or administration of the drug, as appropriate, and those fees would therefore be
considered payment for additional preventive services as well. Therefore, we are proposing
payment limits for the supply and administration of DCAPS drugs to be included on the DCAPS
fee schedule. As stated above, section 1833(a)(1)(W)(ii) of the Act requires that the amount paid
for the provision of additional preventive services is 100 percent of the lesser of the actual charge
for the service, or the amount determined under a fee schedule established by the Secretary for
purposes of this subparagraph. That is, the amount paid for the administration or supplying of the
DCAPS drug will be the lesser of either the actual charge for the service or the payment limit.
For drugs that are supplied by a pharmacy, we propose that the fee schedule include a
payment limit for a supplying fee that is similar to the supplying fee for other Part B-covered
drugs dispensed from a pharmacy, to allow for consistency among similar payments in Part B.
These other groups of drugs covered under Part B include immunosuppressives, oral anti-cancer,
and oral anti-emetic drugs, and supplying fees for these drugs are described at 42 CFR part 414,
subpart L (§§ 414.1000 and 414.1001). Generally, Medicare pays $24 for the first prescription of
one of these drugs supplied by a pharmacy in a 30-day period, and pays $16 for each subsequent

prescription, after the first one, supplied in that 30-day period.
555
We propose similar payment
limits for supplying fees for DCAPS drugs. Specifically, we propose that CMS will establish
payment limit of $24 to a pharmacy for the first DCAPS prescription that the pharmacy supplies
to a beneficiary in a 30-day period, and a payment limit of $16 to a pharmacy for all subsequent
DCAPS prescriptions that the pharmacy supplies to a beneficiary in that 30-day period. We are
proposing that the same fees would apply regardless of the number of days’ supply that is
dispensed.
As discussed in section III.A.4.c of this proposed rule, we intend to further study the
supplying fees for certain drugs paid under Part B (for example, immunosuppressive drugs) and
are not proposing to make any changes to the supplying fee amounts at this time (meaning the
current 30-day supplying fees would apply to any amount of days’ supply). The dispensing and
supplying fees under Part B (§ 414.1001) have been shown to be higher than dispensing fees
paid in the commercial market.
556
So, until additional study is done regarding input costs for
dispensing drugs billed to Medicare Part B and subsequent notice-and-comment rulemaking can
be done, if appropriate, in response to such information, we aim to continue the current fee
schedule for such Part B drugs regardless of the days’ supply dispensed. Therefore, we propose
to use the same approach for payment limits that are paid to pharmacies that supply DCAPS
prescriptions.
For drugs that are administered by a physician or a non-physician practitioner, we
propose that the fee schedule include a payment limit for such administration that aligns with the
administration fee for other drugs provided as incident to physician services, as paid according to
the PFS. To operationalize this, we propose that CMS determine the payment limit for
administration of a DCAPS drug provided incident to a physician service via a crosswalk to an
555
https://www.cms.gov/regulations-and-guidance/guidance/manuals/downloads/clm104c17.pdf.
556
https://www.pcmanet.org/rx-research-corner/mandating-pharmacy-reimbursement-increase-
spending/08/31/2021/#:~:text=The%20average%20dispensing%20fee%20in,the%20state's%20Medicaid%20FFS%
20rate.
existing, corresponding drug administration code under the PFS. Exact details on coding and
corresponding crosswalks would be included on the published fee schedule once DCAPS drugs
are finalized for coverage via the NCD process. The fee schedule would be published quarterly
on the CMS website and implemented in the Medicare claims processing systems.
No cost sharing would apply for the administration or supplying of DCAPS drugs,
because we are proposing that such administration or supplying would be considered an
additional preventive service, and as explained above, there is no cost-sharing for any additional
preventive services under section 1833(a)(1)(W) of the Act. We propose to codify these policies
at the newly added § 410.152(o).
We note that with regard to the July 12, 2023 Proposed NCD for Pre-Exposure
Prophylaxis (PrEP) for Human Immunodeficiency Virus (HIV) Infection Prevention, in section
II.E.4.b. of this proposed rule, in item 37, we propose national rates for HCPCS code G0012
(Injection of pre-exposure prophylaxis (PrEP) drug for HIV prevention, under skin or into
muscle) that are crosswalked from CPT code 96372 (Therapeutic, prophylactic, or diagnostic
injection (specify substance or drug); subcutaneous or intramuscular). Please see that section for
more information.
d. Payment for Drugs Covered as Additional Preventive Services in RHCs and FQHCs
Above, we mentioned that section 4104 of the ACA amended payment for additional
preventive services, to increase payment to the lesser of 100 percent of charges, or the amount
determined under a fee schedule established by the Secretary, per Section 1833(a)(1)(W)(ii).
This change waived coinsurance for additional preventive services. Section 4104 of the ACA
also removed several other barriers to access to preventive services in Medicare. Specifically,
section 4104 of the ACA amended section 1833 of the Act to waive the deductible for preventive
services at section 1833(b)(1) of the Act, and to waive coinsurance for preventive services that
are recommended with a grade of A or B by the USPSTF for any indication or population by
adding section 1833(a)(1)(Y) of the Act. We also mentioned above that “additional preventive

services” are a subset of “preventive services” under Medicare Part B, per section 1861(ddd)(3)
and 1861(ww)(2)(O) of the Act, respectively.
In the CY 2011 PFS final rule, we interpreted the above waivers of cost-sharing for
preventive services to apply to FQHCs (75 FR 73417); we note that FQHC services were already
exempt from the Part B deductible, per section 1833(b)(4) of the Act. The CY 2011 PFS final
rule codified this FQHC policy in regulation at § 405.2449 (75 FR 73613), and in sub-regulatory
guidance, we clarified that these waivers of cost-sharing for preventive services applied to RHCs
as well.
557
In the CY 2014 FQHC PPS final rule (79 FR 25474), at § 405.2410(b), we codified
regulations regarding coinsurance in RHCs and FQHCs, “[E]xcept for preventive services for
which Medicare pays 100 percent under § 410.152(l) of this chapter.” In the CY 2016 PFS final
rule (80 FR 71088), we clarified explicitly that these waivers of cost-sharing (that is, both
coinsurance and deductible) for preventive services applied to RHCs.
In the previous sections of III.H.3. of this proposed rule, we discussed drugs covered as
additional preventive services (henceforth “DCAPS drugs,” for the ease of the reader). In this
section, we are clarifying that drugs covered as additional preventive services, and any
accompanying administration and supplying fees, are not subject to cost-sharing in RHCs and
FQHCs. Since DCAPS drugs and the services to administer and supply them are all considered
additional preventive services, as explained in the previous section, they are paid at 100 percent
of the Medicare payment amount in RHCs and FQHCs per §§ 405.2410 and 410.152(l) and they
are paid on a claim-by-claim basis.
In addition, we are proposing that DCAPS drugs, when administered and supplied in an
RHC or FQHC, as well as any administration and supply fee for those drugs, would be paid
according to the fee schedule payment limits described above at section III.H.3.b. of this
proposed rule. Since regulations at § 405.2460 allow the payment limitations set out in Part 410
557
Change Request 7208, R2186CP, 03/28/2011 Waiver of Coinsurance and Deductible for Preventive Services in
Rural Health Clinics (RHCs), Section 4104 of Affordable Care Act (ACA): https://www.cms.gov/regulations-and-
guidance/guidance/transmittals/downloads/r2186cp.pdf.
to apply to payment for services provided by RHCs and FQHCs, we believe it is consistent with
our current RHC and FQHC payment policies to apply the proposed DCAPS fee schedule
payment limits, as discussed above, to those same DCAPS drugs when furnished in an RHC or
FQHC. Those payment limits are described earlier in section III.H.3.b., and if finalized as
proposed, they would be codified at § 410.152(o)(1). We propose to codify this RHC/FQHC
DCAPS policy in regulation as well, at a new § 405.2464(h).
We welcome comments on these proposals.
I. Medicare Prescription Drug Inflation Rebate Program
1. Background
a. Overview of the Medicare Prescription Drug Inflation Rebate Program
The Inflation Reduction Act of 2022 (IRA) (Pub. L. 117–169, enacted August 16, 2022)
established new requirements under which drug manufacturers must pay inflation rebates if they
raise their prices for certain Part B and Part D drugs faster than the rate of inflation. Drug
manufacturers are required to pay rebates to Medicare if prices for certain Part B drugs increase
faster than the rate of inflation for a calendar quarter beginning with the first quarter of 2023;
drug manufacturers are required to pay rebates to Medicare if prices for certain Part D drugs
increase faster than the rate of inflation over a 12-month period, starting with the 12-month
period that began October 1, 2022.
Section 11101 of the IRA amended section 1847A of the Act by adding a new
subsection (i), which establishes a requirement for drug manufacturers to pay rebates into the
Medicare Prescription Drug Account in the Federal Supplementary Medical Insurance Trust
Fund for Part B rebatable drugs if the specified amount exceeds the inflation-adjusted payment
amount, which is calculated as set forth in section 1847A(i)(3)(C) of the Act. The IRA also
provides for an adjustment to the beneficiary coinsurance amount in cases where the price of a
Part B rebatable drug increases faster than the rate of inflation such that the beneficiary
coinsurance is calculated based on the lower inflation-adjusted payment amount instead of the
applicable payment amount. Section 1847A(i)(2) of the Act defines a “Part B rebatable drug,” in
part, as a single source drug or biological product (as defined in section 1847A(c)(6)(D) of the
Act), including a biosimilar biological product (as defined in section 1847A(c)(6)(H) of the Act),
but excluding a qualifying biosimilar biological product (as defined in section
1847A(b)(8)(B)(iii) of the Act) for which payment is made under Part B.
Section 11102 of the IRA added section 1860D-14B of the Act, which requires drug
manufacturers to pay rebates into the Medicare Prescription Drug Account in the Federal
Supplementary Medical Insurance Trust Fund for each 12-month applicable period, starting with
the applicable period beginning on October 1, 2022, for Part D rebatable drugs if the annual
manufacturer price (AnMP) of such drug exceeds the inflation-adjusted payment amount.
Section 1860D-14B(g)(1)(A) of the Act defines a “Part D rebatable drug,” in part, as a drug or
biological described at section 1860D-14B(g)(1)(C) of the Act that is a “covered Part D drug” as
that term is defined in section 1860D-2(e) of the Act. The definition of a Part D rebatable drug
includes drugs approved under a new drug application under section 505(c) of the Federal Food,
Drug, and Cosmetic (FD&C) Act (that is, brand name drugs), generic drugs approved under
section 505(j) of the FD&C Act that meet certain statutory criteria (that is, sole source generic
drugs), and biologicals licensed under section 351 of the Public Health Service Act (PHS),
including biosimilars.
Under the IRA, certain statutory requirements vary for implementation of the Medicare
Part B Drug Inflation Rebate Program and the Medicare Part D Drug Inflation Rebate Program.
For example, section 1847A(i) of the Act requires CMS to calculate Part B drug inflation rebates
for a calendar quarter, whereas section 1860D-14B of the Act requires CMS to calculate Part D
drug inflation rebates for each 12-month applicable period. With respect to invoicing
manufacturers for the rebate amount owed, under section 1847A(i)(1) of the Act, CMS must
report rebate amounts to each manufacturer of a Part B rebatable drug no later than 6 months
after the end of each calendar quarter, except that for calendar quarters beginning in 2023 and

2024, CMS has until September 30, 2025, to invoice manufacturer for rebates. In contrast, under
section 1860D-14B(a) of the Act, CMS must report rebate amounts to each manufacturer of a
Part D rebatable drug no later than 9 months after the end of each applicable period, except that
for the first two applicable periods (that is, October 1, 2022, to September 30, 2023, and
October 1, 2023, to September 30, 2024), CMS has until December 31, 2025, to invoice
manufacturers for Part D inflation rebates. Additionally, there are statutory differences in the
inputs used to calculate the rebate amounts for Part B and Part D. As a result, CMS is proposing
to use different methodologies to calculate inflation rebates for Part B rebatable drugs and Part D
rebatable drugs. However, CMS has attempted to align policies across the Medicare Part B Drug
Inflation Rebate Program and Medicare Part D Drug Inflation Rebate Program to the extent
possible.
b. Summary of Proposed Policies for the Medicare Prescription Drug Inflation Rebate Program
CMS is proposing to codify policies established in the revised guidance for the Medicare
Part B Drug Inflation Rebate Program and the Medicare Part D Drug Inflation Rebate Program
558
(collectively referred to as the “Medicare Prescription Drug Inflation Rebate Program”) in
regulatory text. Specifically, CMS is proposing to codify with limited modification policies set
forth in guidance for the Medicare Prescription Drug Inflation Rebate Program by adding new
parts 427 and 428 to title 42, chapter IV of the Code of Federal Regulations for Part B and
Part D, respectively, and welcomes comments on these proposals.
In addition, CMS is proposing new policies for the Medicare Part B Drug Inflation
Rebate Program as follows:
558
Medicare Part B Drug Inflation Rebate Revised Guidance: https://www.cms.gov/files/document/medicare-part-b-
inflation-rebate-program-revised-guidance.pdf; Medicare Part D Drug Inflation Rebate Revised Guidance:
https://www.cms.gov/files/document/medicare-part-d-inflation-rebate-program-revised-guidance.pdf (collectively
referred to as the “revised guidance”). These revised guidance documents, published December 14, 2023,
implemented policies relating to the Medicare Prescription Drug Inflation Rebate Program for 2022, 2023, and
2024. CMS also published guidance on the use of the 340B modifier to report separately payable Part B drugs and
biologicals acquired under the 340B program (Revised Part B Inflation Rebate Guidance: Use of the 340B
Modifier, https://www.cms.gov/files/document/revised-part-b-inflation-rebate-340b-modifier-guidance.pdf).
● Proposed § 427.201(b) provides that CMS would compare the payment amount in the
quarterly pricing files published by CMS to the inflation-adjusted payment amount for a given
quarter when determining whether the criteria for a coinsurance adjustment are met.
● Proposed § 427.302(c)(3) provides that for a Part B rebatable drug first approved or
licensed by the FDA on or before December 1, 2020 but with a first marketed date after
December 1, 2020, the payment amount benchmark quarter for such drug is the third full
calendar quarter after the drug’s first marketed date. Proposed § 427.302(c)(4) further provides
that for a Part B rebatable drug that was billed under a NOC code during the calendar quarter
beginning July 1, 2021, or the third full calendar quarter after such drug’s first marketed date,
whichever is later, the payment amount benchmark quarter is the third full calendar quarter after
the drug is assigned a billing and payment code other than a NOC code.
● Proposed § 427.303(b)(1)(i) provides that CMS would remove 340B units for
professional claims with dates of service during 2024 (in addition to 2023) submitted by
Medicare suppliers that are listed by the Health Resources and Services Administration (HRSA)
340B Office of Pharmacy Affairs Information System as participating in the 340B Program, by
using National Provider Identifiers and/or Medicare Provider numbers to identify these suppliers
and the claims submitted with such identifiers.
● Proposed § 427.303(b)(5) provides that CMS would remove units of refundable
single-dose container or single-use package drugs subject to discarded drug refunds, from the
calculation of rebate amounts, generally in the reconciliation process.
● Proposed § 427.501 describes CMS’ method and process for reconciliation of a rebate
amount for a Part B rebatable drug, including the circumstances that may trigger such a
reconciliation.
● Proposed § 427.600 establishes a civil money penalty process in accordance with
section 1847A(i)(7) of the Act to address when a manufacturer of a Part B rebatable drug fails to
pay the rebate amount in full by the payment deadline for such drug for such applicable calendar
quarter.
● Proposed § 427.10 provides that, were any provision of part 427 to be held invalid or
unenforceable by its terms, or as applied to any person or circumstance, such provisions would
be severable from part 427 and the invalidity or unenforceability would not affect the remainder
thereof or any other part of this subchapter or the application of such provision to other persons
not similarly situated or to other, dissimilar circumstances.
CMS also is proposing new policies for the Medicare Part D Drug Inflation Rebate
Program as follows:
● Proposed § 428.202(c)(3) provides that if a Part D rebatable drug first approved or
licensed by the FDA on or before October 1, 2021, does not have AMP data reported under
section 1927(b)(3) of the Act for any quarters during the period beginning on January 1, 2021
and ending on September 30, 2021, CMS would identify the payment amount benchmark period
as the first calendar year, which would be no earlier than calendar year 2021, in which such drug
has at least 1 quarter of AMP reported. Proposed § 428.202(c)(4) further provides that for a Part
D rebatable drug first approved or licensed after October 1, 2021 (that is, a subsequently
approved drug), for which there are no quarters during the first calendar year beginning after the
drug’s first marketed date for which AMP has been reported under section 1927(b)(3), the
payment amount benchmark period would be the first calendar year in which such drug has at
least 1 quarter of AMP reported. CMS is also soliciting comments on alternative policies to
address certain instances in which AMP are not reported for certain NDC-9s of a Part D
rebatable drug.
● Proposed § 428.203(b)(2) provides that, for claims with dates of service on or after
January 1, 2026, and with respect to an applicable period, CMS would exclude from the total
number of units used to calculate the total rebate amount for a Part D rebatable drug those units
of the Part D rebatable drug for which a manufacturer provided a discount under the 340B
Program. To determine the total number of such units for which a manufacturer provided a
discount under the 340B Program, CMS would use data reflecting the total number of units of a
Part D rebatable drug for which a discount was provided under the 340B Program and that were
dispensed during the applicable period. CMS may apply adjustment(s) to these data as needed.
CMS is also soliciting comments on alternative policies for collecting and using 340B data to
calculate rebate amounts for Part D rebatable drugs.
● Proposed § 428.401 describes CMS’ method and process for reconciliation of a rebate
amount for a Part D rebatable drug, including the circumstances that may trigger such a
reconciliation.
● Proposed § 428.500 establishes a civil money penalty process in accordance with
section 1860D-14B(e) of the Act to address when a manufacturer of a Part D rebatable drug fails
to pay the rebate amount in full by the payment deadline for such drug for such applicable
period.● Proposed § 428.10 provides that, were any provision of part 428 to be held invalid or
unenforceable by its terms, or as applied to any person or circumstance, such provisions would
be severable from this part and the invalidity or unenforceability would not affect the remainder
thereof or any other part of this subchapter or the application of such provision to other persons
not similarly situated or to other, dissimilar circumstances.
Unless otherwise specified, CMS proposes that the provisions herein would apply, with
respect to Part B rebatable drugs, for all calendar quarters beginning with January 1, 2023, and
with respect to Part D rebatable drugs, for all applicable periods beginning with October 1, 2022.
The IRA directs the Secretary to calculate rebate amounts for Part B rebatable drugs beginning
on January 1, 2023, and Part D rebatable drugs beginning on October 1, 2022, using pricing data
from past periods of time, including benchmark data from periods prior to the statute’s
enactment. In some cases, the time periods during which prices are subject to rebates began as
early as several weeks after the IRA was enacted. In recognition of this timing, section
1860D-14B(h) of the Act specifically requires CMS to use program instruction to implement the
Medicare Part D Drug Inflation Rebate Program for 2022, 2023, and 2024. Similarly, the
existing provision at section 1847A(c)(5)(C) of the Act, provides authority for CMS to
implement the Medicare Part B Drug Inflation Rebate Program using program instruction or
other guidance. In addition, sections 1847A(i)(1)(C) and 1860D-14B(a)(3) of the Act, as added
by the IRA, permit the Secretary to delay the issuance of Rebate Reports for certain initial
calendar quarters and applicable periods until 2025.
Section 1871(e)(1)(A) of the Act provides that a substantive change in regulations,
manual instructions, interpretative rules, statements of policy, or guidelines of general
applicability under Title XVIII of the Act may not apply retroactively unless the Secretary has
determined that such retroactive application is necessary to comply with statutory requirements
or that failure to apply such policies retroactively would be contrary to the public interest. To the
extent any proposed provisions in this section III.I of this proposed rule are considered to apply
retroactively, CMS has determined that such retroactive application would be both necessary to
establish policies to implement the statutory requirements that CMS perform various calculations
that involve pricing activities from prior periods and also consistent with the statutory provisions
expressly allowing the agency to delay the issuance of rebate reports for initial applicable periods
until 2025. In addition, such retroactive application would be in the public interest because it
would ensure that the proposed regulations address the same time periods and manufacturer
pricing conduct Congress sought to address in the IRA and would promote consistency and
continuity in program implementation.
c. Timeline of Key Dates for the Medicare Prescription Drug Inflation Rebate Program

As sections 1847A(i)(2)(C) and 1860D-14B(a)(3) of the Act allow for delayed reporting and
invoicing of rebates amounts for applicable calendar quarters in 2023 and 2024 for Part B
rebatable drugs and the first two applicable periods for Part D rebatable drugs, Figures 1 and 2
provide example timelines for how rebates will be calculated for applicable calendar quarters and
one applicable period in calendar year 2025. The figures also depict how the rebate period and
components of the rebate calculation may shift based on the marketing and approval dates for a
rebatable drug or biological product.
FIGURE 1: Summary of Data Timelines for Part B Drug Inflation Rebate Provisions for
Calendar Year 2025
a
a
This graphic is an illustrative example of how rebates for quarters in calendar year 2025 will be calculated.
Note: A drug will be included on the six full calendar quarters (denoted with the numbers in the figure) after the
day the drug was first marketed or the first quarter of 2023, whichever is later. The Rebate Period CPI-U is the
greater of the benchmark period CPI-U or the CPI-U of the first month of the quarter two quarters prior to the
rebate period.
FIGURE 2: Summary of Data Timelines for Part D Drug Inflation Rebate Provisions for
Calendar Year 2025
a
a
This graphic is an illustrative example of how rebates for one applicable period including months in
calendar year 2025 will be calculated.
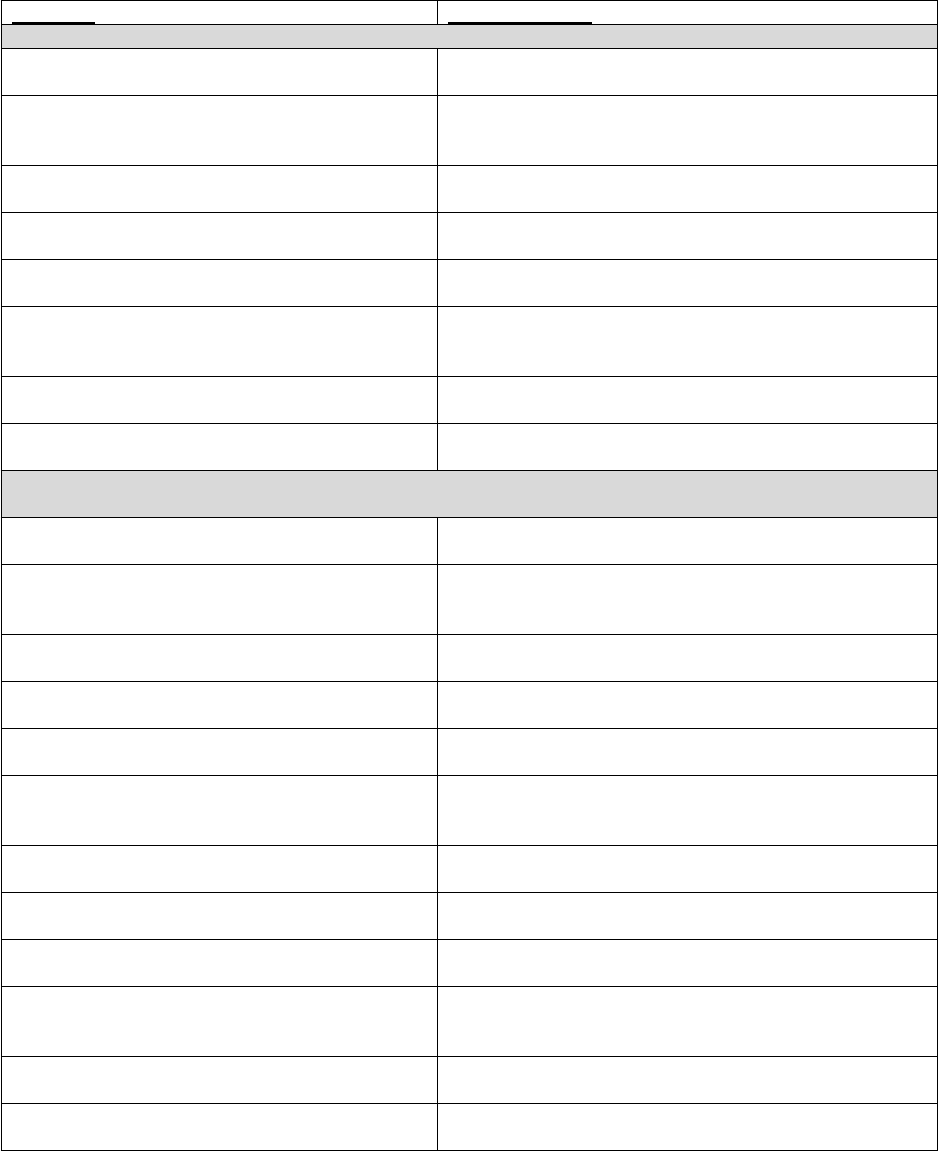
Table 47 describes a summary timeline for inflation rebate amount reports and deadlines
for applicable calendar quarters in calendar year 2025 and thereafter for Part B rebates and for
the Part D rebate applicable period beginning on October 1, 2024, and applicable periods
thereafter.
TABLE 47: Summary of Part B and D Drug Inflation Rebate Amount Reports and
Deadlines
a
Milestone
Timing/Deadline
Part B Rebate – CMS must invoice manufacturers not later than 6 months after each calendar quarter
Preliminary Rebate Report sent to Manufacturers
Not later than 5 months after the end of the calendar
quarter
Manufacturer Reviews
Manufacturer Suggestion of Error must be submitted to
CMS not later than 10 calendar days following receipt of
the Preliminary Rebate Report
Rebate Report sent to Manufacturers
Not later than 6 months after the end of the calendar
quarter
Manufacturer Rebate Amount Due (if applicable)
Not later than 30 calendar days after receipt of the Rebate
Report
Preliminary Reconciliation Rebate Report sent to
Manufacturers
Not later than 11 months after receipt of the Rebate Report
Manufacturer Reviews
Manufacturer Suggestion of Error must be submitted to
CMS not later than 10 calendar days following receipt of
the Preliminary Reconciliation Rebate Report
Reconciliation Rebate Report sent to
Manufacturers
Not later than 12 months after receipt of the Rebate Report
Manufacturer Reconciled Rebate Amount Due (if
any)
Not later than 30 calendar days after receipt of the
Reconciliation Rebate Report
Part D Rebate – CMS must invoice manufacturers not later than 9 months after the end of each applicable
period
Preliminary Rebate Report sent to Manufacturers
Not later than 8 months after the end of the applicable
period
Manufacturer Reviews
Manufacturer Suggestion of Error must be submitted to
CMS not later than 10 calendar days following receipt of
the Preliminary Rebate Report
Rebate Report sent to Manufacturers
Not later than 9 months after the end of the applicable
period
Manufacturer Rebate Amount Due (if applicable)
Not later than 30 calendar days after receipt of the Rebate
Report
First Reconciliation Preliminary Rebate Report sent
to Manufacturers
Not later than 11 months after the receipt of the Rebate
Report
Manufacturer Reviews
Manufacturer Suggestion of Error must be submitted to
CMS not later than 10 calendar days following receipt of
the First Reconciliation Preliminary Rebate Report
First Reconciliation Rebate Report sent to
Manufacturers
Not later than 12 months after the receipt of the Rebate
Report
Manufacturer Reconciled Rebate Amount Due (if
any)
Not later than 30 calendar days after receipt of the First
Reconciliation Rebate Report
Second Reconciliation Preliminary Rebate Report
sent to Manufacturers
Not later than 35 months after the receipt of the Rebate
Report
Manufacturer Reviews
Manufacturer Suggestion of Error should be submitted to
CMS not later than 10 calendar days following receipt of
the Second Reconciliation Preliminary Rebate Report
Second Reconciliation Rebate Report sent to
Manufacturers
Not later than 36 months after the receipt of the Rebate
Report
Manufacturer Reconciled Rebate Amount Due (if
any)
Not later than 30 calendar days after receipt of the Second
Reconciliation Rebate Report
a
The months referred to in these timelines represent calendar months. This means, for example, that if a
Preliminary Rebate Report is issued on August 15, 2027, the Rebate Report could be issued up until
September 30, 2027.
2. Medicare Part B Drug Rebates for Single Source Drugs and Biological Products with Prices
that Increase Faster than the Rate of Inflation
a. Definitions (§ 427.20)
In this proposed rule, CMS proposes to codify the definitions of terms consistent with the
meanings given in section 1847A(i) of the Act or established in the revised Medicare Part B
Drug Inflation Rebate Guidance, as applicable, as well as new definitions based on policies
detailed in this proposed rule.
At proposed § 427.20, CMS is proposing that the following terms in section 1847A of the
Act are defined:
● “Benchmark period CPI-U”.
● “Biosimilar biological product”.
● “Inflation-adjusted payment amount”.
● “Part B rebatable drug”.
● “Payment amount benchmark quarter”.
● “Payment amount in the payment amount benchmark quarter”.
● “Rebate period CPI-U”.
● “Single source drug or biological product”.
● “Specified amount”.
● “Subsequently approved drug”.
● “Unit”.
Further, in § 427.20 of this proposed rule, CMS proposes to codify definitions established
in the revised Medicare Part B Drug Inflation Rebate Guidance and new definitions based on
policies detailed in this proposed rule for the following terms:
● “Allowed charges”.

● “Applicable calendar quarter”.
● “Applicable threshold”.
● “Average sales price (ASP)”.
● “Billing and payment code”.
● “Billing unit”.
● “CPI-U”.
559
● “FDA application”.
● “Final action claim”.
● “First marketed date”.
● “Grouped billing and payment code”.
● “Manufacturer”.
● “National Drug Code” (NDC).
● “Not Otherwise Classified (NOC) code”.
b. Determination of Part B Rebatable Drugs (§§ 427.100 through 427.101)
i. Definitions
In proposed § 427.100, CMS proposes to define the following terms applicable to
proposed subpart B (§§ 427.100 through 427.101):
● “EUA Declaration”.
● “Individual who uses such a drug or biological”.
ii. Identification of Part B Rebatable Drugs
Section 1847A(i)(2) of the Act defines a “Part B rebatable drug,” in part, as a single
source drug or biological product (as defined in section 1847A(c)(6)(D)), including a biosimilar
biological product (as defined in section 1847A(c)(6)(H)), but excluding a qualifying biosimilar
biological product (as defined in section 1847A(b)(8)(B)(iii)), for which payment is made under
559
These data are referenced to 1982-84=100—that is, the average of pricing data for the 36 months from 1982
through 1984 serve as the basis for the index and are assigned a value of 100. These data are not seasonally adjusted.

Part B. The definitions for a biosimilar biological product and a qualifying biosimilar biological
product are codified in § 414.902.
In § 427.101, CMS proposes to codify the policies established in section 30.1 of the
revised Medicare Part B Drug Inflation Rebate Guidance to identify Part B rebatable drugs by
(1) identifying the applicable billing and payment code for each single source drug or biological
product, including biosimilar biological products, for which payment is made under Part B and
(2) excluding any billing and payment code corresponding to a drug or biological product in
excluded product categories or that have average total allowed charges below an applicable
threshold, to be codified in proposed § 427.101(b) and (c), respectively.
560
iii. Excluded Product Categories
Section 1847A(i)(2)(A) of the Act excludes qualifying biosimilar biological products (as
defined in section 1847A(b)(8)(B)(iii) of the Act) from the definition of a Part B rebatable drug.
As such, in § 427.101(b)(1) CMS proposes to codify the policy established in section 30.2 of the
revised Medicare Part B Drug Inflation Rebate Guidance to exclude such products from the
definition of a Part B rebatable drug and not subject them to Part B inflation rebates.
Section 1847A(i)(2) of the Act defines a Part B rebatable drug as a “single source drug or
biological as defined in section 1847A(c)(6)(D) of the Act” which requires that a single source
drug not be a multiple source drug. CMS has interpreted section 1847A(c)(6)(C)(ii) of the Act to
mean that single source drugs or biological products are treated as multiple source drugs if they
were within the same billing and payment code as of October 1, 2003. Accordingly, in
§ 427.101(b)(2), we are proposing to codify the existing policy established in section 30.1 of the
revised Medicare Part B Drug Inflation Rebate Guidance to exclude drugs and biological
products described in section 1847A(c)(6)(C)(ii) of the Act from the definition of a Part B
rebatable drug and not subject them to Part B inflation rebates.
560
For more information on HCPCS codes and how they are applied, see “HEALTHCARE COMMON
PROCEDURE CODING SYSTEM (HCPCS) LEVEL II CODING PROCEDURESHCPCS” at
https://www.cms.gov/medicare/coding/medhcpcsgeninfo/downloads/2018-11-30-hcpcs-level2-coding-procedure.pdf.

For drugs and biological products that are billed using a HCPCS code that represents a
NOC code, CMS has a process to determine the allowed payment amount for such billing and
payment codes; however, current Medicare claims data do not allow CMS to determine the
average total allowed charges for such drug or biological product for a year per individual that
uses such a drug or biological product or to identify units billed. CMS must perform these steps
to determine if a drug or biological product is a Part B rebatable drug. Therefore, in
§ 427.101(b)(3), we are proposing to codify the policy in section 30.1 of the revised Medicare
Part B Drug Inflation Rebate Guidance to exclude drugs and biological products that are billed
using a billing and payment code that represents a NOC code drug or biological product or
claims for such drugs and biological products when no other billing and payment code is
applicable. CMS notes that few Part B drugs and biological products are billed with such codes
and the quarterly process for updating billing and payment codes, including establishing new
billing and payment codes, provides an existing mechanism for CMS to minimize the number of
Part B rebatable drugs that are billed with such codes. As discussed in §§ 90.2 and 90.3 in
Chapter 17 of the Medicare Claims Processing Manual, CMS believes NOC codes are generally
used to bill Medicare for new-to-market, FDA-approved drug products until a specific billing
and payment code is assigned; and so, CMS expects that the impact of this exclusion will be
limited.
561
Consistent with section 303(h) of the Medicare Prescription Drug, Improvement, and
Modernization Act of 2003, radiopharmaceutical drugs and biologicals are not paid under section
1847A of the Act. Manufacturers of radiopharmaceuticals are therefore not required to report
ASP under section 1927(b)(3) of the Act and are not otherwise required to report ASP data to
CMS for separately payable radiopharmaceuticals. In addition, different payment methodologies
across the outpatient setting result in data variations that could inappropriately trigger an
inflation rebate amount due to methodological differences in reimbursement. Therefore, although
561
See: https://www.cms.gov/regulations-and-guidance/guidance/manuals/downloads/clm104c17.pdf.

some radiopharmaceuticals may appear on quarterly pricing files, in § 427.101(b)(4) CMS
proposes to codify the revised Medicare Part B Drug Inflation Rebate Guidance policy (as
described in section 30.1) that excludes separately payable radiopharmaceuticals for the purposes
of identifying Part B rebatable drugs. Additionally, CMS proposes to codify the existing policy
not to subject these units to the inflation-adjusted beneficiary coinsurance at § 427.201(c) and
described further in the following section of this preamble.
562
CMS aims to create a consistent coding and payment approach for the suite of products
currently referred to as skin substitutes as stated in section 30.1 of revised Medicare Part B Drug
Inflation Rebate Guidance. In the CY 2024 PFS proposed rule, CMS solicited comments on
potential changes to payment for skin substitutes. In the CY 2024 PFS final rule, CMS
acknowledged the comments received in response to this solicitation and stated that CMS would
take these comments into consideration for future rulemaking.
563
In § 427.101(b)(5) CMS
proposes to codify existing policy to exclude cellular- and tissue-based products that would aid
wound healing, currently referred to as skin substitutes, for the purposes of identifying Part B
rebatable drugs. In addition, CMS proposes not to subject these products to the beneficiary
coinsurance adjustment at § 427.201(c).
Section 1847A(i)(2)(A) of the Act excludes from the definition of a Part B rebatable drug
a drug or biological if, as determined by the Secretary, the average total allowed charges for such
drug or biological product under Part B for a year per individual who uses such a drug or
biological product are less than $100. Section 1847A(i)(2)(B) of the Act provides that the $100
amount for 2023 will be increased for 2024 and subsequent years by the percentage change in the
CPI-U for the 12-month period ending with June of the previous year, rounded to the nearest
multiple of $10. Therefore, in § 427.101(b)(6) CMS proposes to codify the policy established in
562
In this proposed rule, CMS also is proposing to clarify how radiopharmaceuticals are paid for in the physician’s
office and to codify these policies in regulation. Specifically, CMS proposes to clarify that for radiopharmaceuticals
furnished in a setting other than the hospital outpatient department, MACs can determine payment limits for
radiopharmaceuticals based on any methodology in place on or prior to November 2003.
563
See 88 FR 78818, November 16, 2023 (https://www.federalregister.gov/public-inspection/2023-24184/medicare-
and-medicaid-programs-calendar-year-2024-payment-policies-under-the-physician-fee-schedule).
revised Medicare Part B Drug Inflation Rebate Guidance to exclude from the definition of a
Part B rebatable drug those drugs and biologicals for which the Part B average total allowed
charges for a year per individual who uses such drug or biological is below the applicable
threshold.
Section 1847A(i)(2)(A)(ii) of the Act excludes vaccines described in subparagraph (A) or
(B) of section 1861(s)(10) of the Act from the definition of a Part B rebatable drug. Such
vaccines include the pneumococcal vaccine, the influenza vaccine, the COVID-19 vaccine; and
the hepatitis B vaccine when furnished to an individual who is at high or intermediate risk of
contracting hepatitis B (as determined by the Secretary under regulations). As such, in
§ 427.101(b)(7), CMS proposes to codify the existing policy established in section 30.3 of the
revised Medicare Part B Drug Inflation Rebate Guidance to exclude vaccines described in
subparagraph (A) or (B) of section 1861(s)(10) of the Act from the definition of a Part B
rebatable drug and not subject them to Part B inflation rebates. In addition, with respect to
monoclonal antibodies used for treatment or post-exposure prophylaxis of COVID-19, which are
covered and paid for under section 1861(s)(10) of the Act, CMS proposes to exclude these
products from the definition of Part B rebatable drugs for applicable quarters through the end of
the calendar year in which the EUA declaration under section 564 of the FD&C Act for drugs
and biological products is terminated. With respect to monoclonal antibodies that are used for
pre-exposure prophylaxis of COVID-19 that are covered and paid for under section 1861(s)(10)
of the Act, CMS proposes to exclude these products from the definition of Part B rebatable drug
for applicable calendar quarters even after the year in which the EUA Declaration ends, as long
as these products have an FDA-approved application or license after the EUA Declaration is
terminated.
Finally, Part B drugs approved under an Abbreviated New Drug Application (ANDA)
submitted under 505(j) of the FD&C Act do not meet the definition of “single source drug or
biological product,” as defined under section 1847A(c)(6)(D) of the Act, and thus, are not Part B
rebatable drugs. We propose to codify this exclusion at § 427.101(b)(8).
iv. Drugs and Biological Products with Average Total Allowed Charges Below the Applicable
Threshold
Pursuant to section 1847A(i)(2) of the Act, drugs and biological products, for which the
average total allowed charges for such drug or biological under Part B for a year per individual
who uses such drug or biological are below the applicable threshold, as determined by the
Secretary, are excluded from the definition of Part B rebatable drugs. As explained in
section 30.2 of the revised Medicare Part B Drug Inflation Rebate Guidance, CMS uses the term
“applicable threshold” to mean $100 for all four calendar quarters in 2023. For all four calendar
quarters in 2024, the applicable threshold will be $100 as increased in accordance with section
1847A(i)(2)(B) of the Act. For calendar quarters in 2025 and beyond, the applicable threshold
will be equal to the unrounded applicable threshold calculated for the prior calendar year,
increased by the percentage increase in the CPI-U for the 12-month period ending with June of
the previous year.
In § 427.101(c), CMS is proposing to codify policies from the revised Medicare Part B
Drug Inflation Rebate Guidance to exclude these drugs from the definition of a Part B rebatable
drug. To do so, in accordance with the statute, for each applicable calendar quarter, CMS
proposes to identify drugs and biological products with Part B average total allowed charges for
a year per individual that uses such a drug or biological product below the applicable threshold.
In § 427.101(c)(1), CMS is proposing that to identify the average total allowed charges
for a year per individual, for each Part B rebatable drug, CMS would:
● For single source drugs and biological products assigned to only one billing and
payment code, sum the allowed charges from final action claims greater than $0 and divide the
summed amount by the number of individuals who use such a drug or biological.
● For single source drugs and biological products assigned to more than one billing and
payment code, sum the allowed charges from final action claims greater than $0 for all billing
and payment codes and divide the summed amount by the number of individuals who use such a
drug or biological.
CMS may move a drug or biological product from a grouped billing and payment code to
a unique billing and payment code in instances where the drug is either approved through the
pathway established under section 505(b)(2) of the FD&C Act (hereinafter “section 505(b)(2)
drug products”) that CMS initially assigned to the same billing and payment code as its reference
drug for a period of time, or the drug was previously a multiple source drug but is now a single
source drug that was moved to its own billing and payment code. There may be instances where
a single source drug or biological product was previously crosswalked to a grouped billing and
payment code (other than a NOC code) during the full year. In such instances, CMS proposes to
calculate the average total allowed charges per individual per year for the drug using allowed
charges and the number of individuals who used the drug or biological product based on claims
for the previously grouped billing and payment code during the year. Such instances would apply
to section 505(b)(2) drug products, drugs that were previously multiple source drugs where all
other drugs under the same billing and payment code were discontinued (applicable only if the
sole remaining product was not approved under an ANDA), and to any other situations where a
drug was previously in a grouped billing and payment code (other than a NOC code).
Finally, there may be instances where a single source drug or biological product was
initially billed under a grouped billing and payment code (other than a NOC code) and was later
billed under a unique billing and payment code for some of the year. In such instances, CMS
proposes to calculate the average total allowed charges per individual for a year by: summing
the total allowed charges billed under the unique billing and payment code for the drug with
dates of service on or after the Medicare effective date for this unique billing and payment code
and identifying the individuals on those claims; summing the total allowed charges on claims

billed under the previously grouped billing and payment code and identifying the individuals
with claims prior to the unique billing and payment code’s effective date; and then summing the
total allowed charges under both billing and payment codes across the full year and dividing by
the total number of individuals (de-duplicated for those individuals identified under both the
previously grouped billing and payment code and the unique billing and payment code). If the
average total allowed charges for a year per individual who uses such drug or biological product
are less than the applicable threshold, CMS proposes to exclude the billing and payment code for
that calendar quarter. CMS welcomes comment on the proposed implementation of the exclusion
for drugs and biologicals with average total allowed charges below the applicable threshold.
CMS proposes in § 427.101(c)(2) to calculate the applicable threshold as follows:
● For applicable calendar quarters in 2023, the applicable threshold is equal to $100.
● For applicable calendar quarters in 2024, the applicable threshold is equal to $100
increased by the percentage increase in the CPI-U for the 12-month period ending with June of
2023.
● For applicable calendar quarters in each subsequent calendar year, the applicable
threshold is equal to the unrounded applicable threshold calculated for the prior calendar year
increased by the percentage increase in the CPI-U for the 12-month period ending with June of
the previous year.
● If the resulting amount from these calculations is not a multiple of $10, CMS will
round that amount to the nearest multiple of $10.
564
Accordingly, the formula to determine the applicable threshold for calendar quarters in
2024 is $100 multiplied by (CPI-U for June 2023 divided by CPI-U for June 2022) (apply
rounding to the nearest multiple of $10). To illustrate, the 2024 threshold is: 100 x
564
CMS will round any amount less than $5 over a multiple of $10 down to that multiple of $10, and any amount $5
or more over a multiple of $10 up to the next multiple of $10.
(305.109/296.311) = 102.969178 (which rounds down to $100 after applying CMS rounding) so
the threshold for calendar quarters in 2024 = $100.
For the purposes of this calculation, CMS proposes that “a year” means the 4 consecutive
calendar quarters beginning 6 calendar quarters before the applicable calendar quarter. CMS also
proposes using final action claims from the Medicare fee-for-service claims repository to identify
claims where separate payment was allowed for the applicable HCPCS code for dates of service
within a year. Drugs and biological products that do not meet the applicable threshold are not
considered Part B rebatable drugs. For example, for the calendar quarter beginning July 1, 2025,
CMS would use available final action Medicare Part B claims with dates of service beginning
January 1, 2024, and ending December 31, 2024, because January 1, 2024, is the beginning of
the calendar quarter that is 6 quarters before the applicable calendar quarter beginning on
July 1, 2025.
In § 427.101(c)(3), CMS proposes to codify the policies and methodological steps as
described in section 30.2 of the revised Medicare Part B Drug Inflation Rebate Guidance for
excluding drugs and biological products with average total allowed charges below the applicable
threshold at the billing and payment code level. For each applicable calendar quarter, CMS
would identify the applicable billing and payment codes for drugs and biological products with
average total allowed charges for a year per individual less than the applicable threshold and
exclude such drugs and biological products from the definition of Part B rebatable drug in
accordance with proposed § 427.101(b)(6). When a single source drug or biological product with
average total allowed charges below the applicable threshold is assigned to a unique billing and
payment code, CMS would exclude the assigned billing and payment code for the applicable
calendar quarter. There also may be instances where a single source drug or biological product is
assigned to more than one billing and payment code during a year and the average total allowed
charges for a year per individual that uses such drug or biological product are less than the
applicable threshold. In such instances, CMS proposes to exclude all assigned billing and
payment codes for such single source drug or biological product for that applicable calendar
quarter.
c. Inflation-Adjusted Beneficiary Coinsurance Adjustment and Adjusted Medicare Payment for
Part B Rebatable Drugs with Price Increases Faster than Inflation (§§ 427.200 through 427.201)
Section 1847A(i)(5) of the Act requires that for Part B rebatable drugs, as defined in
section 1847A(i)(2)(A) of the Act, furnished on or after April 1, 2023, in quarters in which the
payment amount described in section 1847A(i)(3)(A)(ii)(I) of the Act (or, in the case of selected
drugs described under section 1192(c) of the Act, the payment amount described in section
1847A(b)(1)(B) of the Act), exceeds the inflation-adjusted payment amount determined in
accordance with section 1847A(i)(3)(C) of the Act, the coinsurance will be 20 percent of the
inflation-adjusted payment amount for such quarter (hereafter, the inflation-adjusted coinsurance
amount). This inflation-adjusted coinsurance amount is applied as a percent, as determined by
the Secretary, to the payment amount that would otherwise apply for such calendar quarter in
accordance with section 1847A(b)(1)(B) or (C) of the Act, as applicable, including in the case of
a selected drug. In the CY 2024 Hospital Outpatient Prospective Payment System (OPPS) final
rule and the CY 2024 PFS final rule, CMS codified this inflation-adjusted coinsurance amount at
§§ 419.41(e), 410.152(m), and 489.30(b)(6), respectively.
Beginning with the April 2023 quarterly pricing files, the applicable beneficiary
coinsurance percentage is shown for each HCPCS code in the pricing files that are posted on the
CMS website. For example, the ASP Pricing files are posted at
https://www.cms.gov/medicare/payment/part-b-drugs/asp-pricing-files. The applicable
beneficiary coinsurance percentage for certain drugs and biologicals used predominantly in the
hospital outpatient setting are listed in the Hospital Outpatient Prospective Payment System
(OPPS) Addenda A and B, which can be found at
https://www.cms.gov/medicare/payment/prospective-payment-systems/hospital-
outpatient/addendum-a-b-updates. The applicable beneficiary coinsurance percentage for certain
drugs and biologicals used predominantly in the ambulatory surgical center setting are listed in
the ASC Addendum, which can be found at
https://www.cms.gov/medicare/payment/prospective-payment-systems/ambulatory-surgical-
center-asc/asc-payment-rates-addenda. The percentage is expressed as two digits with three
decimal places, for example, 18.760. If an adjusted beneficiary coinsurance does not apply, the
percentage would show as 20.000.
Section 11101(b) of the IRA amended section 1833(a)(1) of the Act by adding a new
subparagraph (EE), which requires that if the payment amount under section
1847A(i)(3)(A)(ii)(I) of the Act or, in the case of a selected drug, the payment amount described
in section 1847A(b)(1)(B) of the Act, for that drug exceeds the inflation-adjusted payment
amount for a Part B rebatable drug, the Part B payment amount would, subject to the Part B
deductible and sequestration, equal the difference between the payment limit and the
inflation-adjusted coinsurance amount. Consistent with the clarification in section 40 of the
revised Medicare Part B Drug Inflation Rebate Guidance and with the application of
sequestration in the context of Medicare payment and beneficiary coinsurance in general, we
note that the calculation to determine the applicable beneficiary coinsurance amount would not
be adjusted for sequestration. CMS codified the Medicare payment for Part B rebatable drugs in
the CY 2024 PFS final rule by adding new paragraph (m) to § 410.152.
In this proposed rule, CMS proposes to adopt new provisions at §§ 427.200 and 427.201
to codify the policies regarding the computation of the inflation-adjusted beneficiary
coinsurance, defined in § 427.200, for Part B rebatable drugs as required by section 1847A(i)(5)
of the Act. This proposed new provision includes references to the existing provisions at
§§ 410.152(m), 419.41(e), and 489.30(b)(6). CMS further proposes at § 427.201(c) that any
category of products that is excluded from the identification of Part B rebatable drugs at
§ 427.101(b) is not subject to the inflation-adjusted beneficiary coinsurance. Examples of these

excluded products include separately payable radiopharmaceuticals, skin substitute products, and
qualifying biosimilar biological products.
Additionally, CMS proposes at § 427.201(b) that CMS will use the published payment
amount in quarterly pricing files
565,566,567
to determine if a Part B rebatable drug should have an
adjusted beneficiary coinsurance equal to 20 percent of the inflation-adjusted payment amount as
described in section 1847A(i)(3)(C) for a calendar quarter. This proposed approach deviates
from the rebate calculation approach proposed in § 427.302, which relies on the specified
amount defined at § 427.20 even when the specified amount and the published payment amount
in quarterly pricing files differ. The approach proposed at § 427.201(b) would be used only to
determine whether there should be a coinsurance adjustment and would not impact the
applicability or calculation of inflation rebates. CMS believes this approach is consistent with the
statutory language and appropriately reflects the differences in the statutory text of section
1847A(i)(5) of the Act, which sets forth the payment amount that is used to determine whether
coinsurance should be adjusted, and section 1847A(i)(3)(A) of the Act, which sets forth the
“specified amount” used to determine rebate amounts.
CMS’ intent with this proposed policy is to hold beneficiaries harmless in situations
where the payment amount is calculated differently from the specified amount. Though the
payment amount is generally based on the same provisions as the specified amount, there may be
situations where the payment amount is updated or adjusted under other provisions of 1847A of
the Act, such as when ASP data are not available under section 1847A(c)(5)(B). For example, if
the specified amount is very low due to negative ASP data and the payment amount is updated
using other available data resulting in a payment amount that exceeds the inflation-adjusted
payment amount, beneficiaries would not receive the benefit of adjusted coinsurance. There may
565
See: https://www.cms.gov/medicare/payment/part-b-drugs/asp-pricing-files.
566
See: https://www.cms.gov/medicare/payment/prospective-payment-systems/hospital-outpatient/addendum-a-b-
updates.
567
See: https://www.cms.gov/medicare/payment/prospective-payment-systems/ambulatory-surgical-center-asc/asc-
payment-rates-addenda.
also be situations where the payment amount is lower than the inflation-adjusted payment
amount, but the specified amount is higher than the inflation-adjusted payment amount. In such a
situation, if the “specified amount” was used as the comparator to determine whether
coinsurance should be adjusted, beneficiaries would pay a coinsurance higher than 20 percent,
because 20 percent of the inflation-adjusted payment amount would be higher than 20 percent of
the payment amount. As such, we propose to codify at § 427.201(b) that we will compare the
published payment amount in the quarterly pricing files published by CMS to determine whether
a coinsurance adjustment applies. This policy would provide an adjusted beneficiary coinsurance
amount only when the payment amount for a Part B rebatable drug exceeds the inflation-adjusted
payment amount in a given quarter.
CMS believes this approach is valid and gives effect to the differing statutory language in
sections 1847A(i)(3)(A), 1847A(i)(5), and 1833(a)(1)(EE) of the Act, which sets forth the
coinsurance adjustment for Part B rebatable drugs. Unlike the “specified amount” in section
1847A(i)(3)(A), sections 1847A(i)(5) and 1833(a)(1)(EE) both refer to a “payment amount.”
Fundamentally, a payment amount cannot be a negative number; if the specified amount and
payment amount were the same amount, it would result in situations where the payment amount
at section 1833(a)(1)(EE) was a negative number. Rather, we believe that the term “payment
amount” in both sections 1847A(i)(5) and 1833(a)(1)(EE) is most naturally read to include the
amount, as updated and adjusted for the purposes of providing payment to providers, that CMS
publishes as the payment amount in quarterly pricing files; and that section 1833(a)(1)(EE)
operates to adjust the percentage of such payment amount. Furthermore, section 1847A(i)(5)(B)
of the Act provides the Secretary with discretion to apply the adjusted coinsurance percentage to
the payment amount that would otherwise apply under section 1847A(b)(1)(B) or (C) of the Act.
Lastly, sections 1847A(i)(8)(D) and (E) of the Act preclude administrative and judicial review of
the computation of the adjusted coinsurance and amounts paid to the provider under section
1833(a)(1)(EE).
In summary, we are proposing CMS will use the payment amount in quarterly pricing
files to determine if a Part B rebatable drug should have an adjusted beneficiary coinsurance, the
calculation to determine the adjusted Medicare payment (if applicable) will not be adjusted for
sequestration, and drugs excluded from the identification of Part B rebatable drugs will not be
subject to the inflation-adjusted beneficiary coinsurance. CMS invites comment on these
proposals.
d. Determination of the Rebate Amount for Part B Rebatable Drugs (§§ 427.300 through
427.304)
i. Definitions
In proposed § 427.300, CMS proposes to define the following terms applicable to
subpart D (§§ 427.300 through 427.304):
● “340B Program”.
● “Refundable single-use dose container or single-use package drug”.
ii. Calculation of the Total Part B Rebate Amount To Be Paid by Manufacturers
Section 1847A(i)(3) of the Act specifies the calculation of the rebate amount for a Part B
rebatable drug assigned to a billing and payment code for an applicable calendar quarter for
which a manufacturer must pay a rebate. CMS proposes to codify the rebate calculation, as
established in revised Medicare Part B Drug Inflation Rebate Guidance, as the estimated amount
is equal to the product of the total number of billing units determined in accordance with section
1847A(i)(3)(B) of the Act (proposed at § 427.303) and the amount (if any) by which the
specified amount (proposed at § 427.302(b)) exceeds the inflation-adjusted payment amount
determined in accordance with section 1847A(i)(3)(C) of the Act (proposed at § 427.302(g)) for
the drug or biological product for an applicable calendar quarter. The Part B drug inflation rebate
amount calculated in accordance with this subpart is subject to adjustment based on any
reductions in accordance with subpart E of this part or any reconciliations in accordance with
subpart F of the part.
Because Part B rebatable drugs are single source drugs or biologicals, they typically will
have one manufacturer. However, a Part B rebatable drug could have more than one
manufacturer. For example, a Part B rebatable drug could be produced by one or more
manufacturer(s) that is a repackager or relabeler. Multiple manufacturers of a rebatable drug also
could occur in the case of one or more authorized generic products that are marketed under the
same FDA-approval as the original FDA applicant. In such instances, all the NDCs for the drug
typically are assigned to the same billing and payment code(s), and each manufacturer is
responsible for reporting ASP data to CMS. When calculating the rebate owed by manufacturers
for a rebatable drug that has more than one manufacturer, CMS proposes to codify the policy
from section 50.13 of the revised Medicare Part B Drug Inflation Rebate Guidance to multiply
the total rebate amount calculated for the billing and payment code by the following quotient:
(Sum of the individual manufacturer’s billing units sold during the applicable calendar
quarter for all NDCs of the manufacturer assigned to the billing and payment code, as reported in
the ASP data submissions) divided by (Sum of all manufacturers’ total billing units sold during
the applicable calendar quarter for all NDCs of the Part B rebatable drug assigned to the billing
and payment code, as reported in the ASP data submissions)
CMS welcomes comment on this calculation approach.
Based on further review, CMS has observed that there are several instances where there
are multiple manufacturers in a billing and payment code and the ASP data, including the
number of units sold, for all or some manufacturers’ NDCs within a billing and payment code
may be negative, zero, or missing. To enable CMS to calculate the respective rebate amounts
attributable to each manufacturer when the ASP units are negative, zero, or missing, CMS is
soliciting comments on the new proposed policies outlined below and any other alternative
options.
(1) Scenarios in which All NDCs Within a Billing and Payment Code Have Negative, Zero, or
Missing ASP Units
If there are NDCs of multiple manufacturers in a billing and payment code, to determine
the respective rebate amount when the manufacturer-reported ASP units for all NDCs are either
negative, zero, or missing but there is a positive rebate amount calculated for the Part B rebatable
drug, CMS proposes to: (1) apportion a $0 rebate amount when the reported units for all NDCs
are missing for NDCs not marketed or sold during the applicable calendar quarter, negative,
and/or zero; and (2) equally apportion a positive rebate amount to each NDC with missing units
when the NDCs were marketed or sold during the applicable calendar quarter. If the NDCs
within a billing and payment code have a mix of negative units, zero units, missing units for
NDCs marketed or sold during the applicable calendar quarter, or missing units for NDCs not
marketed or sold during the applicable calendar quarter, CMS will apportion a $0 rebate amount
to the NDCs with missing units that are not marketed or sold during the applicable calendar
quarter, NDCs with negative units, and NDCs with zero units, and will equally apportion the
positive rebate amount across all NDCs with missing units that are marketed or sold during the
applicable quarter. CMS understands that this approach would treat missing units for NDCs not
marketed or sold during the applicable calendar quarter, negative units, and zero units as
representing zero sales, and we welcome comments on the extent to which this approach could
potentially exclude from rebate liability a manufacturer of a drug that did have sales in that
quarter (for example, if negative units represent price concessions). In addition, CMS welcomes
comments on the extent to which, in a scenario with a billing and payment code with multiple
manufacturers, a single manufacturer with missing ASP units could assume full rebate liability
for the entire billing and payment code if the manufacturer’s NDCs have missing units and are
marketed or sold during the applicable calendar quarter.
CMS also considered several alternative policies for attributing rebate amounts to each
respective manufacturer in this scenario, including: (1) using the reported ASP units from the
calendar quarter prior to the applicable calendar quarter; (2) using an average of units sold based
on sales data for several calendar quarters prior to the applicable calendar quarter (for example,
an average of the previous four calendar quarters); and (3) validation of ASP data based on
review of AMP data in combination with one of the aforementioned alternative proposed policies
to determine inflation rebate amounts. However, CMS has observed that ASP units are often
negative, zero, or missing for several quarters in a four-quarter lookback, so including additional
quarters may not necessarily yield additional data that could be used to apportion inflation rebate
amounts (and could complicate the calculation of an average by introducing a mix of zero,
negative, missing, and positive units within a single NDC). In addition, the AMP validation of
ASP sales could add another layer of complexity and potential bias as AMP data represent only
sales to retail community pharmacies, and ASP data represent all sales of a drug. CMS welcomes
comments on these alternatives.
(2) Scenarios in which Some (But Not All) NDCs Have Negative, Zero, or Missing ASP Units
When some manufacturers’ NDCs within a billing and payment code report negative,
zero, or missing ASP units, CMS proposes to: (1) treat any NDCs with missing units that are not
marketed or sold during the applicable calendar quarter, negative units, or zero units as not
having any sales for the applicable calendar quarter and apportion a $0 rebate amount to them;
(2) treat NDCs with missing units that are marketed or sold during the applicable calendar
quarter as though they had the same units as that of the NDC with the lowest positive units; and
(3) apportion rebate amounts across NDCs with missing units that are marketed or sold during
the applicable calendar quarter and NDCs with positive units based on the share of ASP units
sold in accordance with the policy outlined in section 50.13 of the revised Medicare Part B Drug
Inflation Rebate Guidance. CMS welcomes comments on the extent to which, in a scenario
where NDCs of multiple manufacturers are assigned to the same billing and payment code, a
single manufacturer that accounts for all positive ASP units could potentially be responsible for
the full rebate amount for the entire billing and payment code.
CMS also considered proposing other alternative policies for attributing rebate amounts
to each respective manufacturer in this scenario, including: (1) review of historical ASP data to
identify the most recent calendar quarter with positive ASP units for any of the NDCs with
negative, zero, or missing units in the applicable calendar quarter and allocation of financial
responsibility across NDCs with positive ASP units in that quarter (excluding NDCs without
positive units in that quarter); (2) using an average of units sold based on sales data for several
calendar quarters prior to the applicable quarter (for example, an average of the previous four
calendar quarters); (3) apportionment of rebates based on units at the NDC-9 level rather than the
NDC-11 level; and (4) apportionment of rebates to only those manufacturers within a HCPCS
code that reported positive ASP units for the applicable calendar quarter.
CMS elected to not propose use of a historical lookback approach (under options 1 and 2)
since ASP units are often negative, zero, or missing for the most recent calendar quarter and/or
over several quarters in a four-quarter lookback period, and so including additional quarters may
not necessarily yield additional data that could be used to apportion inflation rebate amounts (and
could complicate the calculation of an average by introducing a mix of zero, negative, missing,
and positive units within a single NDC). CMS also understands that a historical lookback
approach could create outliers that could affect the resulting allocation. When evaluating
option 3, CMS observed that ASP units are often negative, zero, or missing for several calendar
quarters when aggregating units sold at the NDC-9 level. Consequently, this approach may not
necessarily yield additional data that could be used to apportion inflation rebate amounts and
doing so would differ from our general policy on using NDC-11s as set forth in the revised
guidance. Finally, CMS decided not to propose apportioning the full rebate amount to only those
manufacturers that reported positive ASP units within a billing and payment code under option 4,
as we questioned whether that policy could inadvertently disfavor manufacturers that reported
units while benefiting manufacturers that did not report ASP data. We continue to evaluate these
alternative policy approaches for apportioning rebate liability and may adopt changes to this
proposed policy in the final rule.
CMS reminds manufacturers of their reporting obligations under sections 1847A(f)(2)
and 1927(b) of the Act and that failure to provide timely information may result in penalties as
detailed in sections 1847A(d)(4)(B) and (C) and 1927(b)(3)(C)(i) of the Act.
CMS welcomes comments on these proposed approaches as well as alternative policy
options on how CMS could apportion rebate amounts among multiple manufacturers’ NDCs that
reported negative, zero, and/or missing units for NDCs.
iii. Calculation of the Per Unit Part B Drug Rebate Amount
(1) Identification of the Specified Amount for the Applicable Calendar Quarter
In the calculation of the rebate amount for a Part B rebatable drug, CMS is statutorily
required to compare the inflation-adjusted payment amount to the specified amount, which is the
amount set forth in section 1847A(i)(3)(A)(ii)(I) of the Act. Statute also requires CMS to impose
an inflation rebate if the specified amount exceeds the inflation-adjusted payment amount. CMS
proposes to codify at § 427.302(a) the policy established in revised Medicare Part B Drug
Inflation Rebate Guidance to calculate the Part B per unit rebate amount for the applicable
calendar quarter by determining the amount by which the specified amount exceeds the
inflation-adjusted payment amount, after accounting for exclusions under § 427.303(b). CMS
proposes to codify the current operational steps for calculating Part B inflation rebates as
described in section 50 of the revised Medicare Part B Drug Inflation Rebate Guidance.
In § 427.302(b), CMS proposes to codify the policy established in section 50.2 of the
revised Medicare Part B Drug Inflation Rebate Guidance on how to calculate the specified
amount for the applicable calendar quarter. The “specified amount” refers to the amount
specified in section 1847A(i)(3)(A)(ii)(I)(aa) or (bb) of the Act, as applicable. In general, section
1847A(i)(3)(A)(ii)(I)(aa) and (bb) of the Act cross-reference provisions governing quarterly
payment limits for single source drugs and biological products that are typically, but not always,
reflected in the quarterly pricing files. Specifically, the specified amount for single source drugs
and biological products is 106 percent of the amount determined under section 1847A(b)(4) of
the Act—that is, the lesser of ASP or WAC—for the applicable calendar quarter. For biosimilar
biological products, the specified amount is the payment amount under section 1847A(b)(1)(C)
of the Act, which is based on 100 percent of the ASP for the biosimilar biological product plus
6 percent of the lesser of ASP or WAC for the reference biological product.
In § 427.302(b)(1), CMS proposes that the first applicable calendar quarter for a Part B
rebatable drug will be the earliest applicable calendar quarter that follows the payment amount
benchmark quarter identified in § 427.302(c)(1) through (6).
Additionally, for the purposes of determining the rebate amount for a Part B rebatable
drug, based on further consideration of data availability in specific circumstances, CMS proposes
to clarify the policy established in section 50 of the revised Medicare Part B Drug Inflation
Rebate Guidance and use the most updated price information reported by manufacturers,
determined in accordance with section 1847A(i)(3)(A)(ii)(I)(aa) or (bb) of the Act as applicable,
as the specified amount for the applicable calendar quarter for each HCPCS code identified in
accordance with § 427.101. That is, CMS would use the most updated price information reported
by manufacturers to compare whether 106 percent of WAC or 106 percent of ASP is less, and
would use the lower value for the specified amount. In circumstances in which all NDCs in the
HCPCS code have neither manufacturer-reported ASP nor WAC price data available for the
applicable calendar quarter, CMS proposes to use WAC price data from other public sources, if
available, to calculate 106 percent of WAC, which will serve as the specified amount. CMS
proposes to adopt this approach regardless of whether there is a price substitution for Medicare’s
payment during the quarter or whether other policies cause the published payment limit to differ
from the specified amount. In circumstances in which negative or zero manufacturer ASP data is
reported for all NDCs for a given quarter, that negative or zero ASP amount would be used to
compare 106 percent of WAC to 106 percent of ASP to determine the lower value for use as the
specified amount. CMS believes these proposals on treatment of missing pricing data and
treatment of pricing differences between reported prices and the published payment limit for a
billing and payment code will further clarify the application of the specified amount in the
calendar quarter are consistent with the requirements set forth in section 1847A(i)(3)(A)(ii)(I) of
the Act. CMS solicits comments on this policy.
(2) Identification of the Payment Amount Benchmark Quarter
In § 427.302(c), CMS proposes to codify policies from section 50.3 of the revised
Medicare Part B Drug Inflation Rebate Guidance to identify the applicable payment amount
benchmark quarter. Specifically, for drugs first approved or licensed by the FDA on or before
December 1, 2020, and with a first marketed date on or before December 1, 2020, the payment
amount benchmark quarter would be the calendar quarter beginning July 1, 2021. For
subsequently approved drugs—that is, drugs approved or licensed by the FDA after
December 1, 2020—the payment amount benchmark quarter would be the third full calendar
quarter after a drug’s first marketed date. Additionally, there may be cases where a drug was first
approved or licensed on or before December 1, 2020, but with a first marketed date after
December 1, 2020, and the drug lacks ASP or WAC data to calculate the payment amount for the
applicable calendar quarter beginning July 1, 2021. Under the policy applicable to drugs
approved or licensed and with a first marketed date before December 1, 2020, such drugs would
not have data to calculate the payment amount in the payment amount benchmark quarter. In
these cases, CMS proposes to treat such drugs in the same manner as it would treat subsequently
approved drugs and identify the payment amount benchmark quarter as the third full calendar
quarter after a drug’s first marketed date. CMS solicits comments on this policy proposal and
specifically on our proposal to treat drugs approved or licensed on or before December 1, 2020,
but with a first marketed date after December 1, 2020 as subsequently approved drugs.
For Part B rebatable drugs that were billed under a NOC code during the payment
amount benchmark quarter, CMS stated in the revised Medicare Part B Drug Inflation Rebate
Guidance that it would use the third full quarter after a drug was assigned a unique HCPCS code
as the payment amount benchmark quarter. In this rulemaking, CMS proposes to determine the
payment amount benchmark quarter as follows: For a Part B rebatable drug that was billed
under a NOC code during the calendar quarter beginning July 1, 2021, or the third full calendar
quarter after such drug’s first marketed date, whichever is later, CMS proposes that the payment
amount benchmark quarter be the third full calendar quarter after the Part B rebatable drug is
assigned a billing and payment code other than a NOC code. CMS solicits comments on these
proposals.
CMS continues to consider whether there is a need to identify additional or modified
methodologies to appropriately determine the payment amount benchmark quarter for products
with insufficient pricing data in the payment amount benchmark quarter or that otherwise do not
fall squarely into the categories otherwise described in § 427.302(c) and in a manner that enables
the calculation of rebate amounts consistent with section 1847A(i)(3) of the Act.
CMS has determined that ASP data are the most appropriate for identifying (1) the day
on which the drug was first marketed and (2) which calendar quarter is the third full calendar
quarter thereafter as the payment amount benchmark quarter for drugs first approved or licensed
by the FDA after December 1, 2020, or licensed on or before December 1, 2020, but with a first
marketed date after December 1, 2020. CMS also has determined that it is most appropriate and
administratively feasible to identify the first marketed date as the date of first sale of any
NDC-11 within a billing and payment code among all products and package sizes under the same
FDA application.
Additionally, CMS believes ASP data are accurate and reliable because manufacturers
attest to the accuracy of their submitted data and have the ability to update these data quarterly.
Therefore, in § 427.302(c), CMS proposes to codify existing policy from the revised Medicare
Part B Drug Inflation Rebate Guidance on the identification of the payment amount benchmark
quarter for each Part B rebatable drug. CMS would use the earliest first marketed date of any
NDC ever marketed under any FDA application under which any NDCs that have ever been
assigned to the billing and payment code for that Part B rebatable drug as of the applicable
calendar quarter have ever been marketed. The earliest first marketed date would apply to all
NDCs within a billing and payment code and to all products and package sizes marketed under
the same FDA approved application. If the original NDC on which the first marketed date is
based is terminated, the first marketed date for the associated billing and payment code would
remain the same. By defining the first marketed date for the Part B rebatable drug at the level of
the product’s FDA approval, CMS would retain the same first marketed date for the billing and
payment code even if the NDCs and/or billing and payment codes used to bill for the Part B
rebatable drug change over time. In addition, when the date of first sale is missing from ASP
data, CMS proposes to identify the first marketed date from alternative public sources, such as
the National Institutes of Health’s DailyMed.
Table 48 in this section provides an example, for illustration purposes only, of the
application of first marketed date based on the earliest date of first sale of any NDC ever
marketed under any NDA or BLA under which any NDCs that have ever been assigned to the
billing and payment code as of the applicable calendar quarter have ever been marketed. In the
example, NDC1 (marketed under NDA 000000) is first sold on January 15, 2022, and NDC2
(also marketed under NDA 000000) is first sold on October 15, 2023. Both NDCs are marketed
under an NDA associated with HCPCS code X0000. The first marketed date for HCPCS code
X0000 would be January 15, 2022, because that date is the earliest date of first sale for any NDC
that has ever been associated with an NDA or BLA within that HCPCS code as of the calendar
quarter. If NDC2 were subsequently assigned to a new HCPCS code Y0000, the first marketed
date for HCPCS Y0000 would similarly be January 15, 2022. In cases when NDCs that are
marketed under different NDA/BLAs are assigned to the same HCPCS code, using the example
in the table in this section, NDC3 (marketed under NDA 111111) was first sold on
November 1, 2024, and first billed under HCPCS Y0000, and the first marketed date for
HCPCS Y0000 would remain January 15, 2022, as noted, given that HCPCS Y0000 includes
NDC2, associated with NDA 000000. NDC3 was later assigned to a new HCPCS code Z0000.

Following CMS’ approach, the first marketed date for HCPCS code Z0000 is November 1, 2024,
because that is the earliest date of first sale for any NDC ever marketed under NDA 111111,
which is the only NDA ever associated with Z0000 as of the calendar quarter.
TABLE 48: Example of Application of First Marketed Date at the FDA Approval Level
Calendar
Quarter
HCPCS
Code
NDC
FDA Application
Number
Date of
First Sale
for NDC
HCPCS
Code
Effective
Date
Date of
First Sale
for Any
NDC in
NDA/BLA
First Marketed
Date
2023 Q2
X0000
NDC1
000000
1/15/2022
4/1/2023
1/15/2022
1/15/2022
2023 Q3
X0000
NDC1
000000
1/15/2022
4/1/2023
1/15/2022
1/15/2022
NDC2
000000
10/15/2023
4/1/2023
1/15/2022
2023 Q4
X0000
NDC1
000000
1/15/2022
4/1/2023
1/15/2022
1/15/2022
NDC2
000000
10/15/2023
4/1/2023
1/15/2022
2024 Q1
X0000
NDC2
000000
10/15/2023
4/1/2023
1/15/2022
1/15/2022
2024 Q2
X0000
NDC2
000000
10/15/2023
4/1/2023
1/15/2022
1/15/2022
2024 Q3
Y0000
NDC2
000000
10/15/2023
7/1/2024
1/15/2022
1/15/2022
2024 Q4
Y0000
NDC2
000000
10/15/2023
7/1/2024
1/15/2022
1/15/2022
NDC3
111111
11/1/2024
7/1/2024
11/1/2024
2025 Q1
Y0000
NDC2
000000
10/15/2023
7/1/2024
1/15/2022
1/15/2022
Z0000
NDC3
111111
11/1/2024
1/1/2025
11/1/2024
11/1/2024
(3) Identification of Payment Amount in the Payment Amount Benchmark Quarter
Section 1847A(i)(3)(C) of the Act specifies use of the “payment amount for the billing
and payment code for such drug in the payment amount benchmark quarter” (“payment amount
in the payment amount benchmark quarter”) in the determination of the inflation-adjusted
payment amount. While the specified amount and the payment amount in the payment amount
benchmark quarter are similar, the statutory requirements for determining these two amounts
differ. The specified amount for a Part B rebatable drug, as set forth in section
1847A(i)(3)(A)(ii)(I) of the Act, is based on item (aa) (for example, lesser of ASP+6 percent or
WAC+6 percent) or (bb) (that is, 100 percent of the ASP for the biosimilar biological product
plus 6 percent of the lesser of ASP or WAC for the reference biological product). The payment
amount in the payment amount benchmark quarter under section 1847A(i)(3)(C)(i) is based on
various provisions within section 1847A of the Act (for example, the lesser of 106 percent ASP
or WAC, WAC+3 percent, and price substitutions). To identify the payment amount in the
payment amount benchmark quarter for the Part B rebatable drug by billing and payment code,

in § 427.302(d), CMS proposes to codify the policies established in section 50.4 of the revised
Medicare Part B Drug Inflation Rebate Guidance. CMS would use the published payment limit
(as available) for the billing and payment code for the applicable payment amount benchmark
quarter determined in accordance with section 1847A of the Act. If a published payment limit is
not available for the applicable payment amount benchmark quarters, CMS would use the lower
of 106 percent of manufacturer-reported ASP or 106 percent of manufacturer-reported WAC. If
neither a published payment limit nor manufacturer-reported ASP or WAC data are available,
CMS would use WAC data from other public sources to calculate 106 percent of WAC, which,
solely for the purposes of identifying the payment amount in the payment amount benchmark
quarter, CMS would consider to be the payment amount for the payment amount benchmark
quarter. Table 49 and Figure 3 illustrate the specified amount and payment amount in the
payment amount benchmark quarter.
TABLE 49: Comparison of Specified Amount and Payment Amount in the Payment
Amount Benchmark Quarter
Specified Amount
Payment Amount in the Payment Amount
Benchmark Quarter
Purpose in Rebate
Calculation
Pricing Methodology
Under
1847A(i)(3)(A)(ii)(I)
Purpose in Rebate
Calculation
Pricing Methodology
Under 1847A(i)(3)(C)(i)
Part B amount described
under
1847A(i)(3)(A)(ii)(I) for
the calendar quarter in
which a rebate may be
assessed
• Lesser of ASP+6% or
WAC+6%
• In the case of a
biosimilar biological
product, 100% of ASP for
the biosimilar biological
product + 6% of the lesser
of ASP or WAC for the
reference biological
product
Part B published payment
limit for the payment
amount benchmark
quarter, which is
generally the quarter
beginning July 1, 2021
• Various Part B pricing
provisions consistent with
section 1847A of the Act
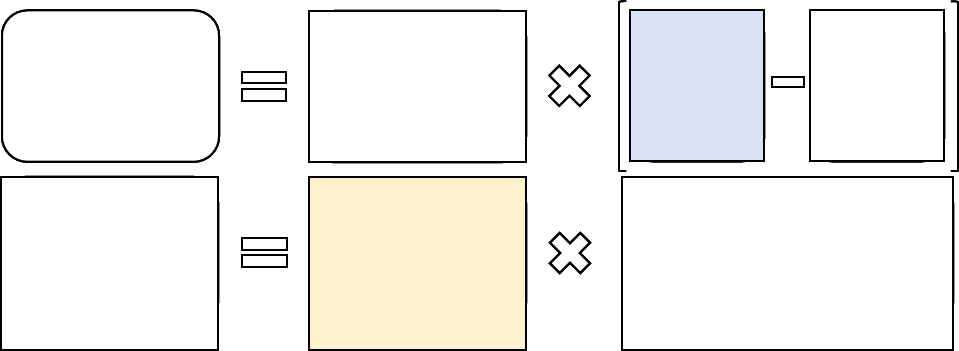
FIGURE 3: Use of the Specified Amount and the Payment Amount in the Benchmark
Quarter in Rebate Calculations
* See the next section for information about identification and calculation of the inflation-adjusted payment amount.
CMS notes that there may be situations when a Part B rebatable drug was previously
billed under a grouped billing and payment code during the benchmark quarter and later billed
under a unique billing and payment code, such as certain section 505(b)(2) drug products and
single source drugs that were previously multiple source drugs. For example, a multiple source
drug approved under an NDA may become a single source drug if all other therapeutically
equivalent drugs are no longer marketed and the now-single source NDA is later shifted into a
separately payable code. To identify the payment amount in the payment amount benchmark
quarter for such drugs, CMS proposes to codify policy established in section 50.4 of the
Medicare Part B Drug Inflation Rebate Guidance and identify the grouped billing and payment
code payment limit used by CMS for the payment amount in the payment amount benchmark
quarter and use that payment limit for the benchmark quarter.
Finally, consistent with the policy established in section 50.4 of the revised Medicare
Part B Drug Inflation Rebate Guidance, CMS would not apply a sequestration reduction to the
payment amount in the payment amount benchmark quarter as part of the methodology to
calculate a Part B inflation rebate amount.
(4) Identification of the Benchmark Period CPI-U
Rebate Amount
Rebatable Units in
the Rebate Quarter
Inflation-
Adjusted
Payment
Amount
Specified
Amount
Rebate Amount
Rebatable Units in
the Rebate Quarter
Inflation-
Adjusted
Payment
Amount
Specified
Amount
Rebate Amount
Rebatable Units in
the Rebate Quarter
Inflation-
Adjusted
Payment
Amount
Specified
Amount
Rebate Amount
Rebatable Units in
the Rebate Quarter
Inflation-
Adjusted
Payment
Amount
Specified
Amount
Billing Units in the
Applicable Calendar
Quarter
Inflation-
Adjusted
Payment
Amount
Specified
Amount
Inflation-Adjusted
Payment Amount*
Payment Amount
in the Benchmark
Quarter
Rebate Period CPI-U divided
by Benchmark Period CPI-U
Inflation-Adjusted
Payment Amount*
Payment Amount
in the Benchmark
Quarter
Rebate Period CPI-U divided
by Benchmark Period CPI-U
Inflation-Adjusted
Payment Amount*
Payment Amount
in the Benchmark
Quarter
Rebate Period CPI-U divided
by Benchmark Period CPI-U
Inflation-Adjusted
Payment Amount*
Payment Amount
in the Benchmark
Quarter
Rebate Period CPI-U divided
by Benchmark Period CPI-U
Inflation-Adjusted
Payment Amount*
Payment Amount in
the Payment Amount
Benchmark Quarter
Rebate Period CPI-U divided by
Benchmark Period CPI-U

For each Part B rebatable drug by HCPCS code, the statute requires CMS to identify the
applicable benchmark period CPIU. In accordance with section 1847A(i)(3)(E) of the Act, the
benchmark period CPI-U for drugs first approved or licensed by the FDA on or before
December 1, 2020, and with a first marketed date on or before December 1, 2020, is the CPI-U
for January 2021, which is 261.582.
568
CMS proposes to codify in § 427.302(e) policies
established in section 50.5 of the revised Medicare Part B Drug Inflation Rebate Guidance.
Specifically, the benchmark period CPI-U for drugs first approved or licensed on or before
December 1, 2020, with a first marketed date after December 1, 2020, would be the CPI-U for
the first month of the third full calendar quarter after a drug’s first marketed date. Additionally,
CMS proposes to codify policies in revised guidance that the benchmark period CPI-U for
subsequently approved drugs would be the first month of the first full calendar quarter after a
drug’s first marketed date in accordance with section 1847A(i)(4)(A) of the Act. Furthermore,
CMS proposes to determine the benchmark period CPI-U for certain drugs previously billed
under NOC codes as follows: For a Part B rebatable drug that was billed under a NOC code
during the calendar quarter beginning July 1, 2021, or the third full calendar quarter after such
drug’s first marketed date, whichever is later, CMS proposes that the benchmark period CPI-U
would be first month of the third full calendar quarter after the drug is assigned a billing and
payment code other than a NOC code. CMS solicits comments on these proposals.
(5) Identification of the Rebate Period CPI-U
As specified in section 1847A(i)(3)(F) of the Act, in § 427.302(f), CMS proposes to
codify the policy described in section 50.6 of the revised Medicare Part B Drug Inflation Rebate
Guidance, that the rebate period CPI-U means the greater of the benchmark period CPI-U index
level and the CPI
-
U index level for the first month of the calendar quarter that is 2 calendar
quarters prior to the applicable calendar quarter in which the Part B rebatable drug is furnished.
CMS will retrieve the CPI-U index level information from bls.gov.
568
CMS retrieved the January 2021 CPI-U from bls.gov on March 22, 2024.
(6) Determination of the Inflation-Adjusted Payment Amount
Section 1847A(i)(3)(C) of the Act specifies the determination of the inflation-adjusted
payment amount. In § 427.302(g), CMS proposes to codify the policy established in section 50.7
of revised Medicare Part B Drug Inflation Rebate Guidance for determining the
inflation-adjusted payment amount in accordance with this section of the Act. For each
applicable calendar quarter and for each Part B rebatable drug by billing and payment code,
CMS proposes to use the payment amount in the payment amount benchmark quarter (per
§ 427.302(d)), benchmark period CPI-U (per § 427.302(e)), and rebate period CPI-U (per
§ 427.302(f)) to identify the inflation-adjusted payment amount. Specifically, CMS would
calculate the inflation-adjusted payment amount by dividing the rebate period CPI-U by the
benchmark period CPI-U and then multiplying the quotient by the payment amount in the
payment amount benchmark quarter.
iv. Determination of Total Number of Billing Units
For calendar quarters starting on or after January 1, 2023, CMS proposes in § 427.303 to
codify policies established in section 50.8 of the revised Medicare Part B Drug Inflation Rebate
Guidance to determine the number of billing units for each Part B rebatable drug by HCPCS
code. Section 1847A(i)(3)(B) describes the total number of billing units of Part B rebatable drugs
that should be included in the rebate calculation. These billing units include the number of billing
units for the HCPCS code of the Part B rebatable drug furnished during the relevant calendar
quarter minus billing units of drugs with respect to which the manufacturer provides a discount
under the 340B Program, billing units with respect to which the manufacturer could have paid a
Medicaid rebate, and billing units that are packaged into the payment amount for an item or
service and are not separately payable. CMS further proposes codifying policy set forth in
revised Medicare Part B Drug Inflation Rebate Guidance in § 427.303 to exclude billing units
when a drug is no longer a Part B rebatable drug.
After identifying Part B rebatable drugs by HCPCS code (in accordance with policy
proposed in §§ 427.10, 427.20, and 427.100 through 427.101) using final action claims in the
CMS Medicare fee-for-service claims repository, CMS proposes to codify existing policy in the
revised Medicare Part B Drug Inflation Rebate Guidance in § 427.303 to determine the total
number of billing units for each HCPCS code as follows. CMS identifies claim lines for such
HCPCS code for dates of service in the calendar quarter, excludes billing units in claim specified
in section 1847A(i)(3)(B)(ii) of the Act, as applicable, and sums the number of billing units in
the remaining claim lines for which Medicare payment was allowed and greater than zero.
Including billing units where Medicare payment was allowed would ensure that billing units for
which Medicare and some beneficiaries have financial liability would be counted in the total
number of billing units.
CMS proposes to codify the policy in the revised Medicare Part B Drug Inflation Rebate
Guidance in § 427.303 and will perform this process at least 3 months after the end of a calendar
quarter to allow time for claims to be submitted, processed, and finalized. Subpart F of this
proposed rule describes the proposed rebate process, including reports of rebate amounts,
suggestion of error, and restatements. CMS welcomes comment on the following policies and
comments on any additional units that should be excluded from the rebate amount calculation.
(1) Units of Drugs Acquired Through the 340B Program
Section 1847A(i)(3)(B)(ii)(I) of the Act specifically excludes billing units of drugs for
which the manufacturer provides a discount under the 340B Program from the billing units of
drugs for which a manufacturer may otherwise have a Part B inflation rebate liability. CMS
proposes codifying the policy described in section 50.8.1 of the revised Medicare Part B Drug
Inflation Rebate Guidance in § 427.303 to remove separately payable billing units in claim lines
that are billed with the “JG” or “TB” modifiers from identified final action claim lines.
On December 20, 2022, CMS issued program guidance that requires all 340B covered
entities to include the “JG” or “TB” modifier, as applicable, on separately payable claim lines for

drugs acquired through the 340B Program with dates of service beginning no later than
January 1, 2024.
569
CMS proposes to codify this requirement in § 427.303(b)(1)(ii). Furthermore,
on November 22, 2023, in the CY 2024 OPPS final rule (88 FR 81791 through 81792), CMS
finalized a policy to utilize a single 340B modifier (“TB”), requiring hospitals that currently
report the “JG” modifier to use the “TB” modifier beginning January 1, 2025. As described in
the final rule, in CY 2024, these hospitals can choose to continue to use the “JG” modifier or
choose to transition to the use of “TB” modifier during that year. On December 14, 2023, CMS
updated the December 20, 2022 guidance titled “Part B Inflation Rebate Guidance: Use of the
340B Modifiers” to align with the updated single modifier requirement.
570
CMS proposes in § 427.303(b)(1)(i) to exclude separately payable billing units in claim
lines for professional claims with dates of service during 2023 from suppliers that are covered
entities listed by the HRSA 340B Office of Pharmacy Affairs Information System as
participating in the 340B Program. CMS will use National Provider Identifier numbers and/or
Medicare Provider Numbers to identify these suppliers and the claims submitted with such
identifiers. CMS proposes to continue this approach for professional claims with dates of service
during 2024. Consistent with the CMS updated 340B modifier guidance, CMS proposes in
§ 427.303(b)(1)(ii) excluding separately payable billing units in claim lines for institutional
providers with the “JG” and “TB” modifiers from identified final action claims with dates of
service through December 31, 2024. CMS proposes to codify policies established in
section 50.8.1 of the revised Medicare Part B Drug Inflation Rebate Guidance in
§ 427.303(b)(1)(iii) by excluding separately payable billing units in claim lines with the “TB”
modifier from identified final action claims with dates of service on or after January 1, 2025.
CMS is proposing to use these modifiers to identify and exclude billing units for which a
discount was acquired under the 340B Program because the “TB” modifier is an existing
569
See: https://www.cms.gov/files/document/part-b-inflation-rebate-guidance340b-modifierfinal.pdf.
570
See: https://www.cms.gov/files/document/revised-part-b-inflation-rebate-340b-modifier-guidance.pdf.
mechanism used to identify drugs acquired through the 340B Program and familiar to most 340B
covered entities paid under the OPPS.
(2) Units with a Rebate under Section 1927 of the Social Security Act
To receive payment under Medicaid for covered outpatient drugs, manufacturers must
participate in the Medicaid Drug Rebate Program (that is, have a drug rebate agreement in effect
with the Secretary of HHS) and are required to report certain pricing and drug product
information and pay Medicaid drug rebates for covered outpatient drugs furnished and paid for
under the Medicaid State plan. States invoice manufacturers no later than 60 days after the end of
each calendar quarter on the number of units of each dosage form and strength of each covered
outpatient drug furnished and paid for under the State plan. This invoice includes units of
covered outpatient drugs that are furnished to dually eligible beneficiaries when the claim for the
drug is paid for by Medicare Part B and the beneficiary’s cost sharing is covered by Medicaid.
To determine unit counts for rebate calculations, at this time, in § 427.303(b)(2), CMS proposes
codifying our policy described in revised Medicare Part B Drug Inflation Rebate Guidance in
section 50.8.2 to exclude billing units from claims with dates of service during a month within a
calendar quarter when the Medicare beneficiary has Medicaid coverage that may provide
cost-sharing assistance. These are Qualified Medicare Beneficiary (QMB) Plus, Specified
Low-Income Medicare Beneficiary (SLMB) Plus, QMB-only beneficiaries, and other full dually
eligible beneficiaries. CMS further proposes codifying the policy in revised guidance that billing
units for Part B rebatable drugs furnished to Medicare beneficiaries with Medicaid coverage that
does not include cost-sharing assistance (that is, SLMB Only, Qualified Disabled and Working
Individuals (QDWI), and Qualifying Individuals (QI) beneficiaries) be included in rebate
calculations. CMS would identify the months for which a beneficiary has Medicaid coverage
with cost-sharing assistance using available information (for example the State MMA File of
dually eligible beneficiaries) at the time the rebate amount is being calculated for a calendar
quarter. CMS proposes codifying this policy as manufacturers pay rebates through the Medicaid
Drug Rebate Program on units of covered outpatient drugs that are furnished to dually eligible
beneficiaries when the claim for the drug is paid for by Medicare Part B and the beneficiary’s
cost sharing is covered by Medicaid.
CMS also considered excluding all units furnished to dually eligible individuals but is not
proposing this alternative as it would result in the over exclusion of units.
(3) Units that Are Packaged into the Payment Amount for an Item or Service and Are Not
Separately Payable
As described earlier in this section, CMS proposes codifying our policy in section 50.8.3
of revised Medicare Part B Drug Inflation Rebate Guidance and only include claim lines with a
Medicare allowed amount greater than zero. Because CMS proposes in § 427.303(b)(3)
identifying billing units for separately payable claim lines for Part B rebatable drugs only, no
further action would be necessary to exclude billing units that are packaged into the payment
amount for an item or service and are not separately payable, such as drugs for which payment is
packaged under the OPPS, or the Ambulatory Surgical Center (ASC) payment system, or those
furnished in the Federally qualified health centers (FQHC) or rural health clinics (RHC) setting.
CMS notes that claim lines for drugs for which payment is bundled under the End-Stage Renal
Disease (ESRD) prospective payment system would not have a Medicare allowed amount that is
greater than zero and such units would therefore be excluded.
In accordance with policies established in the CY 2024 OPPS/ASC final rule and
codified in regulatory text at 88 FR 81540, CMS would except biosimilar biological products
from the OPPS threshold packaging policy when their reference biological products are
separately paid. This means that CMS would pay separately for these biosimilar biological
products even if their per-day cost is below the threshold packaging policy. Because units of
these biosimilar biological products are not packaged into the payment amount for an item or
service and are separately payable, they would be included in the Part B inflation rebate
calculation if they are not qualifying biosimilar biological products.

(4) Units When a Drug is No Longer a Part B Rebatable Drug
As described in section 1847A(i)(2) of the Act, multiple source drugs are not Part B
rebatable drugs. A single source drug that is a Part B rebatable drug could become a multiple
source drug at the start of or during a calendar quarter. In such cases, in § 427.303(b)(4), CMS
proposes codifying policy in section 50.8.4 of the revised Medicare Part B Drug Inflation Rebate
Guidance to identify the first marketed date, as described in § 427.20, of a drug product that is
rated as therapeutically equivalent to such a drug under FDA’s most recent publication of
Approved Drug Products with Therapeutic Equivalence Evaluations (commonly known as the
FDA Orange Book
571
) and determine whether the drug is no longer a Part B rebatable drug. In
§ 427.303(b)(4), CMS proposes to exclude billing units of such drug furnished on and after the
first day of the calendar month in which the therapeutically equivalent drug was first sold or
marketed during the applicable calendar quarter. CMS further proposes codifying policy that it
may consult with the FDA for technical assistance in instances where there is ambiguity as to
whether a new product is therapeutically equivalent. Units furnished on or after the calendar
month of the first marketed date would be excluded from the units identified in accordance with
§ 427.303(b)(4)(iii).
(5) Operational Considerations Related to the Inclusion of Units Furnished to Beneficiaries Who
Are Enrolled in Medicare Advantage (MA) Plans
Section 1847A(i) of the Act requires the manufacturer of a Part B rebatable drug to pay a
rebate that, generally, is calculated based on the total number of billing units of that drug that
were furnished in a calendar quarter, multiplied by the excess specified amount for the drug over
a statutorily defined inflation-adjusted payment amount. The inclusion in this calculation of
billing units of drugs that are furnished to Medicare beneficiaries who are enrolled in MA plans
poses significant operational complexities. At this time, CMS is not proposing to establish a
571
Accessible via https://www.fda.gov/drugs/drug-approvals-and-databases/approved-drug-products-therapeutic-
equivalence-evaluations-orange-book.

policy on treatment of MA units in the calculation of Part B inflation rebates due to operational
considerations, but may establish policy on this issue in future rulemaking. CMS solicits
comments on this approach.
(6) Units Subject to Discarded Drug Refunds
In § 427.303(b)(5), CMS proposes a policy addressing the interaction between Part B
inflation rebates and billing units of discarded drugs. Under the Infrastructure Investment and
Jobs Act of 2021, section 90004, manufacturers are required to provide a refund to CMS for
certain discarded amounts from separately payable single-dose container or single-use package
drugs beginning January 1, 2023. To implement the discarded drugs refund provision of the
Infrastructure Investment and Jobs Act of 2021, in the CY 2023 PFS final rule (87 FR 69711
through 69719), CMS finalized the requirement that providers and suppliers use the “JW” claim
modifier for all separately payable drugs with discarded amounts of drugs from a single-dose
container or from a single-use package Part B claims that bill for drugs and biological products
to report discarded amounts. CMS also finalized a requirement for providers and suppliers to use
the “JZ” modifier on claims that bill for drugs from single-dose containers that are separately
payable under Medicare Part B when there are no discarded amounts to attest that no amount of
drug was discarded and eligible for payment.
572
As of October 1, 2023, claims for drugs from
single-dose containers that do not use the modifiers as appropriate may be returned until claims
are properly resubmitted.
Although section 1847A(i)(3)(B)(ii) of the Act does not require that billing units of
discarded drugs be excluded from Part B inflation rebates, CMS is proposing to exclude billing
units of discarded drugs that are subject to discarded drug refunds from Part B inflation rebates.
CMS believes not applying Part B inflation rebates to billing units of discarded drugs for which a
refund is owed would balance fairness for manufacturers that owe refunds for billing units of
discarded drugs with the need to fulfill the requirements of section 11101 of the IRA.
572
See 87 FR 2512, November 18, 2022 (https://www.federalregister.gov/d/2022-23873/p-2512).
As new policy not established in section 50.8.6 of the revised Medicare Part B Drug
Inflation Rebate Guidance, CMS proposes to exclude billing units of a refundable single-dose
container or single-use package drug as defined in § 414.902 (hereinafter referred to as
“refundable drug”) subject to discarded drug refunds, from the calculation of rebate amounts
during the reconciliation process except for calendar quarters in calendar year 2023. In the
CY 2024 PFS final rule (codified in § 414.940), CMS finalized policy to send annual refund
reports for discarded drug refunds for the 4 quarters of a calendar year at or around the time it
sends Part B Inflation Rebate Reports for the first quarter of the following calendar year.
Therefore, CMS invoices manufacturers for discarded drug refunds on an annual basis but CMS
is required to invoice manufacturers for Part B inflation rebates on a quarterly basis.
Under the timeline for processing discarded drug refunds, data to determine which billing
units of discarded drugs are subject to discarded drug refunds will generally not be available
until after CMS issues the Rebate Report to the manufacturer. Due to these data limitations,
CMS proposes to include all discarded billing units, including units of a refundable drug subject
to the discarded drug refund (as defined in § 414.940), in the calculation of billing units for the
Preliminary Rebate Report and the Rebate Report. CMS proposes to use data available during
the reconciliation process to exclude billing units of discarded drugs that are subject to discarded
drug refunds from the calculation of the rebate amount.
For calendar quarters in calendar year 2023, CMS proposes to exclude billing units of a
refundable drug subject to discarded drug refunds from the calculation of the rebate amount
before CMS issues the Rebate Report to the manufacturer. As permitted by section
1847A(i)(1)(C) of the Act, CMS is delaying reporting of rebate information required by section
1847A(i)(1)(A) for calendar quarters in calendar years 2023 and 2024 until no later than
September 30, 2025. Under this timeline for calendar quarters in calendar year 2023, CMS will
have data available regarding which billing units are subject to discarded drug refunds when
CMS sends the Preliminary Rebate Report and Rebate Report in 2025 for calendar quarters in
calendar year 2023 and can exclude these billing units from the calculation of the rebate amount
in these reports.
CMS solicits comments on the proposed approach to excluding billing units of a
refundable drug subject to discarded drug refunds, from the calculation of Part B inflation rebate
amounts during the reconciliation process, except for calendar quarters in calendar year 2023.
v. Adjustments for Changes to Billing and Payment Codes
Changes to billing and payment codes, including new code assignments and dose
description changes, may occur.
When a new billing and payment code is assigned for a Part B rebatable drug and the
code dose description, which determines that amount of drug in each billing unit, remains the
same, CMS proposes to codify in § 427.304(b) the existing policy set forth in revised Medicare
Part B Drug Inflation Rebate Guidance to use the benchmark quarter’s payment amount, the
payment amount benchmark quarter, and the benchmark quarter CPI-U of the prior billing and
payment code to calculate the per unit Part B rebate amount. For example, a single source drug
or biological product may be assigned a new billing and payment code if it was initially assigned
to a billing and payment code with other products and then later assigned a unique billing and
payment code. In this situation, a multiple source drug in an NDA may become a single source
drug if all its other therapeutically equivalent drugs are discontinued and the now-single source
NDA is later shifted into a separately payable code.
When a Part B rebatable drug’s code dose description changes, CMS proposes to codify
in § 427.304(a) policies established in section 50.9 of the revised Medicare Part B Drug Inflation
Rebate Guidance and apply a conversion factor within the rebate calculation, when applicable.
For example, a billing and payment code dose description that determines the amount of drug in
each billing unit could be changed from 10mg to 5mg. If a billing and payment code dose
description changes from 10mg to 5mg, the payment amount in the payment amount benchmark
quarter for such drug was $200 based on 10mg, and the rebate period payment amount is based
on 5mg, then CMS would apply a conversion factor of 0.5 to the payment amount in the payment
amount benchmark quarter (yielding $100). In this example, the conversion factor would be
based on the ratio of the current billing unit description to the prior billing unit description (5mg
/ 10mg = 0.5). In addition, to ensure consistency in how CMS is calculating a rebate when a
billing and payment code’s dose description changes, CMS proposes to apply a conversion factor
before applying the percentage by which the rebate period CPI-U for the calendar quarter
exceeds the benchmark period CPI-U to determine the inflation-adjusted payment amount.
In situations where a new billing and payment code is assigned for a Part B rebatable
drug and the code dose description changes, CMS would apply a conversion factor, as
appropriate, and use the benchmark quarter’s payment amount, the payment amount benchmark
quarter, and the benchmark quarter CPI-U of the prior billing and payment code to calculate the
per unit Part B rebate amount—consistent with the policy in revised guidance that CMS is
proposing to codify in § 427.304(a) and (b).
To apply the provisions in section 1847A(i) of the Act appropriately, CMS also proposes
in § 427.304(c) to codify existing policy to maintain a crosswalk between such changes or codes.
CMS solicits comment on these proposals.
e. Reducing the Rebate Amount for Part B Rebatable Drugs in Shortage and When There Is a
Severe Supply Chain Disruption (§§ 427.400 through 427.402)
Section 1847A(i)(3)(G) of the Act requires the Secretary to reduce or waive the rebate
amount owed by a manufacturer for a Part B rebatable drug with respect to a calendar quarter in
two cases: (1) when a Part B rebatable drug is described as currently in shortage on a shortage
list in effect under section 506E of the FD&C Act at any point during the applicable period; and
(2) when CMS determines there is a severe supply chain disruption during the applicable quarter
for a Part B rebatable biosimilar biological product, such as a disruption caused by a natural
disaster or other unique or unexpected event. The statute does not describe how CMS should
reduce or waive inflation rebates in each of these cases.

To implement the statutory requirement under section 1847A(i)(3)(G), CMS proposes to
codify in subpart E of part 427 existing policies described in sections 50.10, 50.11, and 50.12 of
the revised Medicare Part B Drug Inflation Rebate Guidance to reduce the total rebate amount
owed by a manufacturer in each of these cases, as summarized in Table 50 and discussed later in
this section.
TABLE 50: Determination of Rebate Reduction Amount for Part B Rebatable
Drugs
As proposed, the rebate amount owed would not be fully waived in either of the cases
previously described. CMS believes the proposed rebate reduction policies balance providing
appropriate financial relief for manufacturers in certain circumstances, including when there is a
severe supply disruption resulting from exogenous circumstances outside of a manufacturer’s
control, while not incentivizing manufacturers to delay taking appropriate steps to resolve a drug
shortage or severe supply chain disruption to avoid an obligation to pay rebates. CMS will
continue to evaluate these policies and may update them in future years. CMS underscores that
most shortages involve multiple source generic drugs,
573
which are not Part B rebatable drugs
and thus are not subject to Part B drug inflation rebates. CMS solicits comments on the proposed
approach.
i. Definitions
573
See: https://www.iqvia.com/insights/the-iqvia-institute/reports-and-publications/reports/drug-shortages-in-the-
us2023.
Drug Shortage
Severe Supply Chain Disruption
Duration of Reduction
Indefinite for as long as drug is “currently in shortage”
Four calendar quarters; manufacturer
may request an extension for four
additional quarters for up to eight
calendar quarters total
Percent Reduction
Part B rebatable drug
other than a plasma-
derived product
Part B rebatable plasma-
derived product
Part B rebatable biosimilar biological
product
First four consecutive
calendar quarters
25%
75%
75%
Second four consecutive
calendar quarters
10%
50%
75%
Subsequent calendar
quarters
2%
25%
Not applicable

CMS proposes in § 427.400 to define the following terms applicable to proposed
subpart E (§§ 427.400 through 427.402)—
● “Drug shortage” or “shortage”.
● “Plasma-derived product”.
Proposed § 427.400 also would codify definitions established in the revised Medicare
Part B Drug Inflation Rebate Guidance for the following terms:
● “Currently in shortage”.
● “Natural disaster”.
● “Other unique or unexpected event”.
● “Severe supply chain disruption”.
ii. Reducing the Rebate Amount for Part B Rebatable Drugs Currently in Shortage
In proposed § 427.401, CMS proposes to codify the policy established in section 50.11 of
the revised Medicare Part B Drug Inflation Rebate Guidance whereby CMS would reduce the
total rebate amount for a Part B rebatable drug that is currently in shortage based on the length of
time the drug is in shortage during a calendar quarter and decrease the amount of the reduction
over time. CMS intends to use the shortage lists maintained by the FDA Center for Biologics
Evaluation and Research (CBER) and Center for Drug Evaluation and Research (CDER) to
determine whether a Part B rebatable drug is currently in shortage
574
during a calendar quarter.
CMS will not consider an NDC-10 in the status of “to be discontinued,” “discontinued,” or
“resolved” to be “currently in shortage.” At this time, CMS intends to provide the same
reduction in the rebate amount for Part B rebatable drugs currently in shortage regardless of the
cause of the shortage.
CMS will not provide a full waiver of the rebate amount for drugs currently in shortage
on an FDA shortage list, as providing a full waiver of the rebate amount could further incentivize
574
For the purposes of this proposed rule, CMS uses the term “currently in shortage” to refer to Part B rebatable
drugs that are in the status of “currently in shortage” on the CDER shortage list, as well as biological products listed
on CBER’s current shortages list.

manufacturers to delay taking appropriate steps that may resolve a shortage more expeditiously
simply to maintain having the drug listed on FDA’s drug shortage list to avoid an obligation to
pay rebates for an extended period. Further, in a report analyzing the root causes of drug
shortages between 2013 and 2017, FDA found that more than 60 percent of drug shortages were
the result of manufacturing or product quality issues, and providing a full waiver of the rebate
amount in situations that may be within a manufacturer’s control could be perceived as
rewarding manufacturers for poor quality management.
575
CMS would be responsible for monitoring the status of a Part B rebatable drug on an
FDA shortage list, and manufacturers would not need to submit any information to CMS to be
eligible for a reduction of the rebate amount for a Part B rebatable drug that is currently in
shortage.
To calculate the reduced total rebate amount for a Part B rebatable drug, in § 427.401(b),
CMS proposes the following formula:
Reduced Total Rebate Amount = total rebate amount multiplied by (1 minus applicable
percent reduction) multiplied by (percentage of time drug was currently in shortage during the
calendar quarter) added to the total rebate amount multiplied by (1 minus percentage of time
drug was currently in shortage during the calendar quarter)
For the purpose of this formula, for a Part B rebatable drug that is a plasma-derived
product, in § 427.401(b)(2)(i), CMS proposes an applicable percent reduction of 75 percent for
the first 4 consecutive calendar quarters such Part B rebatable drug is currently in shortage,
50 percent for the second 4 consecutive calendar quarters, and 25 percent for each subsequent
calendar quarter. For a Part B rebatable drug (including a biosimilar biological product) that is
not a plasma-derived product, in § 427.401(b)(2)(ii), CMS proposes an applicable percent
reduction of 25 percent for the first 4 consecutive calendar quarters such Part B rebatable drug is
575
See: https://www.fda.gov/media/131130/download?attachment#page=33.
currently in shortage, 10 percent for the second 4 consecutive calendar quarters, and 2 percent
for each subsequent calendar quarter.
Because drugs and biologicals on the FDA shortage lists are maintained at the NDC-10
level, and Part B drug inflation rebates are calculated at the HCPCS level, CMS proposes in
§ 427.401(c) that if any NDC-10 assigned to the HCPCS code(s) is currently in shortage, CMS
would apply the rebate reduction to all of the NDCs under the relevant HCPCS code(s). CMS
will closely monitor market data for the Part B rebatable drugs for which the rebate is reduced to
ensure the integrity of the application of the rebate reduction policy.
CMS intends to provide a reduction in the rebate amount for as long as a Part B rebatable
drug is currently in shortage. CMS believes the rebate reduction should be proportional to the
time the drug is currently in shortage and decrease over time to balance providing financial relief
to manufacturers experiencing a drug shortage while not incentivizing manufacturers to delay
taking appropriate steps to resolve a shortage simply to maintain having the drug listed on an
FDA shortage list to avoid an obligation to pay rebates for an extended period.
To determine the percentage of time a Part B rebatable drug was currently in shortage
during the calendar quarter, as proposed in § 427.401(b)(3), CMS would count the number of
days such drug is currently in shortage in a calendar quarter and divide by the total number of
days in that calendar quarter.
In § 427.401(b)(2), CMS proposes codifying the policy set forth in section 50.11 of the
revised Medicare Part B Drug Inflation Rebate Guidance to apply a greater applicable percent
reduction for plasma-derived products than non-plasma derived products because the former rely
on a variable supply of donated blood plasma that can impact downstream production and
therefore hamper the ability to promptly resolve a shortage.
When the status of a Part B rebatable drug changes from currently in shortage to resolved
during a calendar quarter and then changes to currently in shortage during one or more of the
subsequent three calendar quarters, CMS would apply the shortage reduction as if there was a

continuous shortage beginning with the quarter in which the drug has re-entered a shortage and
move to the percent reduction applicable for the second four consecutive quarters. (In this
scenario, once this drug enters its fifth quarter of shortage from the first quarter in which it was
listed as currently in shortage, the applicable percent reduction would be 50 percent for the fifth
through eighth calendar quarters for a Part B rebatable drug that is a plasma-derived product and
10 percent for a Part B rebatable drug that is not a plasma-derived product.) When the status of a
Part B rebatable drug changes from currently in shortage to resolved and either remains in the
status of resolved or is removed from the list for at least 4 full consecutive calendar quarters and
then subsequently reemerges on a shortage list, CMS would treat the subsequent shortage as a
new shortage and would apply the applicable percent reduction for the first 4 consecutive
calendar quarters.
iii. Reducing the Rebate Amount for Part B Rebatable Biosimilar Biological Products
When There is a Severe Supply Chain Disruption
In § 427.402 of this proposed rule, CMS proposes to codify the policy established in
section 50.12 of the revised Medicare Part B Drug Inflation Rebate Guidance for rebate
reductions when CMS determines there is a severe supply chain disruption during a calendar
quarter. As proposed in § 427.402(b)(1), CMS would provide a time-limited standard reduction
of 75 percent in the total rebate amount for a Part B rebatable biosimilar biological product when
CMS determines there is a severe supply chain disruption during the calendar quarter, such as
that caused by a natural disaster or other unique or unexpected event. To receive a rebate
reduction in accordance with proposed § 427.402(b)(1), the manufacturer would have to submit
to CMS a rebate reduction request
576
that meets the eligibility requirements in proposed
§ 427.402(c). A rebate reduction request should specify each NDC-11 and HCPCS code to which
the request applies and if CMS grants a manufacturer’s request for an NDC-11, CMS proposes in
576
The rebate reduction request forms are currently going through the Paperwork Reduction Act approval process
under the document identifier CMS–10858. The proposed collection was published for a 30-day comment period in
the June 3, 2024 Federal Register (89 FR 47563).

§ 427.402(b)(3) that the rebate reduction would apply to all the NDC-11s under the relevant
HCPCS code. CMS has proposed additional submission requirements for rebate reduction
requests in the information collection request under OMB control number: 0938-NEW
(CMS-10858).
As proposed in § 427.402(c)(4), CMS would grant a reduction in the rebate amount owed
if a manufacturer of an eligible drug submits to CMS a request in writing demonstrating supply
chain disruption has occurred during the calendar quarter, (2) the severe supply chain disruption
directly affects the manufacturer itself, a supplier of an ingredient or packaging, a contract
manufacturer,
577
or a method of shipping or distribution that the manufacturer uses in a
significant capacity to make or distribute the Part B rebatable biosimilar biological product, and
(3) the severe supply chain disruption was caused by a natural disaster or other unique or
unexpected event. As proposed in § 427.402(c)(2), for a natural disaster or other unique or
unexpected event occurring on or after August 2, 2024, that the manufacturer believes caused a
severe supply chain disruption, the manufacturer must submit the rebate reduction request within
60 calendar days from the first day that the natural disaster or other unique or unexpected event
occurred or began in order for CMS to consider a rebate reduction.
CMS believes that severe supply chain disruptions generally take time to resolve and for
purposes of this proposed rule, CMS proposes in § 427.402(a) to codify the policy established in
section 50.12 of the revised Medicare Part B Drug Inflation Rebate Guidance whereby a
determination that a severe supply chain disruption has occurred would be deemed to disrupt the
supply chain for the quarter in which the event occurred and the three subsequent calendar
quarters. If the manufacturer makes a timely request that includes all the supporting
documentation and CMS determines, based on its review of the reduction request and supporting
577
A contract manufacturer is a party that performs one or more manufacturing operations on behalf of a
manufacturer(s) of active pharmaceutical ingredients (APIs), drug substances, in-process materials, finished drug
products, including biological products, and combination products. See “Contract Manufacturing Arrangements for
Drugs: Quality Agreements Guidance for Industry,” November 2016: https://www.fda.gov/media/86193/download.
documentation, that a reduction should be granted, CMS would reduce the total rebate amount
owed by a manufacturer by 75 percent for the calendar quarter in which the event that caused the
severe supply chain disruption occurred or began, or the following calendar quarter if the request
is submitted less than 60 calendar days before the end of a calendar quarter, and the three
calendar quarters thereafter.
CMS proposes in § 427.402(c)(5) that if the manufacturer believes a severe supply chain
disruption continues into a fifth consecutive calendar quarter after the start of the natural disaster
or other unique or unexpected event, the manufacturer may request a reduction of the rebate
amount for the fifth through eighth calendar quarters by submitting a rebate reduction extension
request to CMS along with any new supporting documentation. CMS has proposed additional
submission requirements for rebate reduction extension requests in the information collection
request under OMB control number: 0938-NEW (CMS-10858). As proposed in
§ 427.402(c)(5)(ii), a rebate reduction extension request and any new supporting documentation
must be submitted at least 60 calendar days before the start of the fifth calendar quarter in order
for CMS to consider a rebate reduction extension.
If the manufacturer submits a complete and timely extension request, and CMS
determines that the information submitted warrants an extension of the rebate reduction, the total
rebate amount would be reduced by 75 percent for the fifth through eighth calendar quarters for
that manufacturer’s Part B rebatable biosimilar biological product, in accordance with proposed
§ 427.402(b)(2).
Consistent with the policy established in section 50.12 of the revised Medicare Part B
Drug Inflation Rebate Guidance, a manufacturer may receive only one extension of the rebate
reduction per Part B rebatable biosimilar biological product per CMS determination of a severe
supply chain disruption, as proposed in § 427.402(c)(5). Said differently, the severe supply chain
disruption rebate reduction would be limited to 8 consecutive calendar quarters total per Part B
rebatable biosimilar biological product per CMS determination of a severe supply chain
disruption.
As proposed in § 427.402(b)(4)(i), if the manufacturer believes there are multiple events
causing severe supply chain disruptions during the same four calendar quarters for the same
Part B rebatable biosimilar biological product and submits multiple rebate reduction requests for
the same product, CMS will grant no more than one rebate reduction for that Part B rebatable
biosimilar biological product for those 4 consecutive calendar quarters. For example, if the
manufacturer of a Part B rebatable biosimilar biological product is granted a severe supply chain
disruption rebate reduction request for its product due to a natural disaster that occurred in
January 2025 and then experiences a second severe supply chain disruption caused by a second,
distinct natural disaster in July 2025, CMS would not grant the second rebate reduction request.
That is, the manufacturer would receive the 75 percent reduction for four calendar quarters for
the severe supply chain disruption caused by the first natural disaster but would not receive a
reduction for the second natural disaster. However, if the second natural disaster exacerbated the
severe supply chain disruption caused by the first natural disaster, the manufacturer may reflect
such circumstances in its request for an extension of the rebate reduction for the fifth through
eighth calendar quarters.
As proposed in § 427.402(b)(4)(ii), if CMS grants a severe supply chain disruption rebate
reduction request for a Part B rebatable biosimilar biological product, and the product appears as
currently in shortage during one of the same four calendar quarter(s) as for which the severe
supply chain disruption reduction was granted, CMS would apply the 75 percent reduction to the
four calendar quarters for which the severe supply disruption request was granted and would not
grant any additional reduction for the shortage status during those quarters. For any subsequent
calendar quarters that the Part B rebatable biosimilar biological product appears as currently in
shortage, CMS would reduce the rebate amount in accordance with the drug shortages reduction
proposed in § 427.401, starting with the highest reduction (that is, 75 percent for a
plasma-derived product and 25 percent for a Part B rebatable drug that is not a plasma-derived
product). For example, if CMS grants a severe supply chain disruption rebate reduction request
for a Part B rebatable biosimilar biological product that was submitted on February 15, 2024, and
that product is currently in shortage from December 15, 2024 until May 15, 2025, CMS would
apply a 75 percent reduction in the total rebate amount to all four calendar quarters in 2024, and
then would apply the shortages reduction as proposed in § 427.401, beginning with a reduction
of 25 percent for a Part B rebatable biosimilar biological product or 75 percent in the case of a
plasma-derived product that is a Part B rebatable biosimilar biological product for the first two
calendar quarters of 2025.
As proposed in § 427.402(b)(4)(iii), if a Part B rebatable biosimilar biological product
that is currently in shortage experiences a severe supply chain disruption, the manufacturer may
submit a request for a severe supply chain disruption rebate reduction. If CMS grants the rebate
reduction request, the rebate amount would be reduced by 75 percent for the duration of
4 consecutive calendar quarters (that is, the calendar quarter in which the event that caused the
severe supply chain disruption occurred and the three calendar quarters thereafter), and CMS
would not grant any additional reduction under § 427.401 for the currently in shortage status
during those 4 calendar quarters. If CMS receives the request and all supporting documentation
describing the natural disaster or other unique or unexpected event causing the severe supply
chain disruption less than 60 days before the end of a calendar quarter, CMS would apply the
75 percent rebate reduction to the next calendar quarter and to the three subsequent calendar
quarters thereafter. For example, if a Part B rebatable biosimilar biological product that is
currently in shortage in the calendar quarter beginning October 1, 2024, is granted a severe
supply chain disruption rebate reduction request as a result of a natural disaster that occurs on
October 20, 2024, CMS would apply a 75 percent reduction in the rebate amount for the duration
of the calendar quarter in which the natural disaster occurred and the three subsequent calendar
quarters thereafter (that is, October 1, 2024, to September 30, 2025). In this same example, if the
natural disaster instead occurs on November 20, 2024, CMS would apply the shortages reduction
proposed in § 427.401 for the calendar quarter beginning October 1, 2024, and ending on
December 31, 2024, and then a 75 percent reduction under the severe supply chain disruptions
policy to the next calendar quarter and the three subsequent calendar quarters thereafter (that is,
January 1, 2025 to December 31, 2025).
In proposed § 427.402(c)(6), CMS proposes to review rebate reduction requests and
rebate reduction extension requests within 60 calendar days of receipt of all documentation, if
feasible, beginning with the calendar quarter that begins on October 1, 2024. CMS will begin
accepting rebate reduction requests and rebate reduction extension requests upon completion of
the Paperwork Reduction Act (PRA) process, including for severe supply chain disruptions
caused by a natural disaster or other unique or unexpected event that occurred on or after
January 1, 2023, prior to completion of the PRA process. If a manufacturer’s rebate reduction
request does not meet the criteria in proposed § 427.402(c)(4) or if the rebate reduction request is
incomplete or untimely based on the requirements in proposed § 427.402(c), CMS would deny
the request. CMS also proposes that if a manufacturer’s rebate reduction extension request does
not meet the criteria in proposed § 427.402(c)(5), is incomplete or untimely based on the
requirements in proposed § 427.402(c)(5), or if a reduction under proposed § 427.402(b)(1) was
not provided for such Part B rebatable biosimilar biological product, CMS would deny the rebate
reduction extension request. As proposed in § 427.402(c)(6)(iii), CMS’ decisions to deny a
request would be final and not be subject to an appeals process.
As proposed at § 427.402(c)(7), CMS would keep confidential, to the extent allowable
under law, any requests for a rebate reduction, including supporting documentation. CMS
proposes that information provided as part of a severe supply chain disruption rebate reduction
request that the submitter indicates is a trade secret or confidential commercial or financial
information would be protected from disclosure if CMS determines the information meets the
requirements set forth under Exemptions 3 and/or 4 of the Freedom of Information Act (FOIA).
In addition to the protections under the FOIA for trade secrets and commercial or financial
information obtained from a person that is privileged or confidential, the Trade Secrets Act at
18 U.S.C. 1905 requires executive branch employees to protect such information. CMS would
protect confidential and proprietary information as required by applicable law.
f. Reports of Rebate Amounts, Reconciliation, Suggestion of Error, and Payments (§§ 427.500
through 427.505)
Section 1847A(i)(1)(A) of the Act requires the Secretary to provide a report to each
manufacturer of a Part B rebatable drug with the following information not later than 6 months
after the end of an applicable calendar quarter: (1) the total number of billing units for each
Part B rebatable drug; (2) the amount, if any, of the excess average sales price increase (the
amount by which the specified amount exceeds the inflation-adjusted payment amount as
calculated in proposed § 427.301(g)) for an applicable calendar quarter; and (3) the rebate
amount for the Part B rebatable drug. In compliance with section 1847A(i)(1)(B) of the Act,
manufacturers of a Part B rebatable drug must provide a rebate for each Part B rebatable drug no
later than 30 calendar days after the receipt of the information provided by the Secretary in
section 1847A(i)(1)(A) of the Act.
To fulfill this statutory requirement, CMS is proposing to provide a Preliminary Rebate
Report followed by a Rebate Report, as described in proposed § 427.501(b) and (c), to all
manufacturers of a Part B rebatable drug, even if the amount due is $0; all rebate amounts would
be subject to reconciliation as proposed in § 427.501(d). As proposed in § 427.501(d)(4), CMS
does not intend to send notice to manufacturers for drugs that are not considered rebatable
pursuant to proposed § 427.20.
Additionally, to address the completeness and accuracy of the rebate amount, CMS
proposes to conduct one regular reconciliation to determine whether the rebate amount should be
adjusted due to updated claims and payment data used in the calculation of such rebate amount
(specified in proposed § 427.501(b)(1)) to occur 12 months after the issuance of the Rebate
Report. The reporting process for reconciliation will be the same process described for the
original Rebate Report, with payment due for any outstanding rebate amount 30 days after
receipt of a report with a reconciled rebate amount. In addition to regular reconciliation, CMS
proposes a process to conduct reconciliation of the rebate amount as needed to correct agency
error and when CMS determines that the information used by CMS to calculate a rebate amount
was inaccurate due to manufacturer misreporting.
CMS believes conducting a reconciliation for the Part B Rebate Program is important in
ensuring the accuracy of the rebate amount and for programmatic alignment with the Part D
Rebate Program.
i. Definitions
In proposed § 427.500, CMS proposes the following term applicable to proposed
subpart F (§§ 427.500 through 427.505):
● “Date of receipt”.
For example, if CMS issues a Rebate Report through the method and process described in
§ 427.504 on June 30, 2026, then July 1, 2026, will be the date of receipt and day one of the
30-calendar-day payment period.
ii. Reports of Rebate Amounts and Suggestion of Error
Consistent with the process specified in section 60 of the revised Medicare Part B Drug
Inflation Rebate Guidance involving preliminary and final reports, CMS proposes to codify a
multi-step process to provide a manufacturer as defined in § 427.20 with the rebate information
specified in section 1847A(i)(1)(A) of the Act. CMS considered the following factors in
determining a method and process for providing the rebate information: meeting statutorily
provided deadlines in section 1847A(i) of the Act (for example, dates by which to provide the
rebate amount to the manufacturer); the operational time to acquire the relevant information
specified in the proposed part 427; the operational time to calculate the rebate amount specified
in subpart D of the proposed part 427; clarity of the information provided as well as potential
burden on manufacturers; and how to ensure accuracy of the rebate amount.
CMS proposes at proposed § 427.501 the use of an initial Preliminary Rebate Report and
a subsequent Rebate Report, with an opportunity for manufacturers to identify certain
mathematical errors (see proposed § 427.503 and discussed in further detail later in this section)
and one regular reconciliation of the rebate amount to account for data revisions 12 months after
the Rebate Report is provided. CMS proposes at proposed § 427.501(d)(1), to conduct a
reconciliation 12 months after issuance of the subsequent Rebate Report specified in
§ 427.501(c) to include any restatements that have occurred in the drug pricing data and claims
billing data reported to CMS and used in the rebate calculation specified in subpart D of the part.
CMS proposes in proposed § 427.501 that the multi-step reporting process for providing
rebate information to a manufacturer would include: (1) an initial report, which CMS proposes
to entitle the “Preliminary Rebate Report” in proposed § 427.501(b) and (2) a second report,
which CMS proposes to entitle the “Rebate Report” in proposed § 427.501(c). The Rebate
Report would serve as the invoice for the rebate amount due, if any, for each NDC that has been
assigned to a billing and payment code for a product determined to be a Part B rebatable drug for
the applicable calendar quarter, as specified in proposed § 427.101. Manufacturers of Part B
rebatable drugs would receive a Rebate Report for their rebatable drugs even if the amount due is
$0. CMS proposes at proposed § 427.501(d)(1) a regular reconciliation of the rebate amount to
occur 12 months after issuance of the subsequent Rebate Report specified in proposed
§ 427.501(c).
As the first step in the reporting process, as proposed in proposed § 427.501(b) and
consistent with section 60 of the revised Medicare Part B Drug Inflation Rebate Guidance, CMS
would provide the manufacturer of a Part B rebatable drug with the preliminary rebate amount
through a Preliminary Rebate Report that is provided to each manufacturer of a Part B rebatable
drug at least 1 month prior to the issuance of the Rebate Report specified in proposed
§ 427.501(c) for an applicable calendar quarter (that is, at least 5 months after the end of the
applicable calendar quarter). To facilitate manufacturer understanding of the Preliminary Rebate
Report, CMS is proposing in proposed § 427.501(b)(1) that the Preliminary Rebate Report would
include the following information: the NDC(s) and billing and payment code for the Part B
rebatable drug as specified in proposed § 427.20, the total number of billing units as specified in
proposed § 427.303; the payment amount in the payment amount benchmark quarter as specified
in proposed § 427.302(d); the applicable calendar quarter specified amount as specified in
proposed § 427.302(b); the applicable benchmark period and rebate period CPI-Us as specified
in proposed § 427.302(e) and (f); the inflation-adjusted payment amount as specified in proposed
§ 427.302(g); the amount, if any, by which the specified amount as described in proposed
§ 427.302(b) exceeds the inflation-adjusted payment amount as described in proposed
§ 427.302(g) for the Part B rebatable drug for the applicable calendar quarter as determined
under proposed § 427.302; any applied reduction as described in proposed §§ 427.401 and
427.402; and the rebate amount due as specified in proposed § 427.301(a).
When determining what information should be included on rebate reports, CMS
considered the statutory requirements outlined in section 1847A(i)(1)(A) of the Act to determine
which data elements are necessary to review the Preliminary Rebate Report for error (described
later in this section) and to protect proprietary information. In response to comments on the
initial Medicare Part B Drug Inflation Rebate Guidance, CMS has proposed to disclose data
elements as suggested by interested parties that are not enumerated in the statute, such as the
applicable benchmark period and rebate period CPI-Us. CMS acknowledges requests from
interested parties to provide additional data elements such as claims-level data at the NDC-11
level, that are not included in this proposal. CMS considered these requests in development of
this proposed rule but does not believe it necessary to provide further information to fulfill its
statutory obligation and believes that the potential benefit to manufacturers of additional data are
outweighed by the administrative burdens additional reporting would impose to the agency. The
elements listed above provide sufficient information for a manufacturer review the Preliminary
Rebate Report for mathematical error, while protecting proprietary information, and these
elements are operationally feasible for CMS to provide. CMS believes the elements listed in
proposed § 427.501(b)(1) satisfy these considerations.
By structuring the Rebate Report process to include a Preliminary Rebate Report before
the Rebate Report, CMS is able to provide manufacturers with an opportunity to review the
Preliminary Rebate Report before the rebate amount is invoiced via the Rebate Report. While
CMS is not required to provide a preliminary report, CMS seeks to facilitate manufacturer
understanding of the report and believes it would be beneficial for manufacturers to review the
report for mathematical errors that can be corrected before invoicing via the Rebate Report.
Further, a Preliminary Rebate Report would provide additional notice to manufacturers regarding
whether they may owe a rebate amount.
In proposed § 427.503, CMS proposes a process in which a manufacturer may suggest to
CMS that the manufacturer believes the Preliminary Rebate Report includes a mathematical error
within 10 calendar days after the date of receipt of the Preliminary Rebate Report. For example,
if the Preliminary Rebate Report is provided on May 31, 2026, then June 1, 2026, would be the
date of receipt and, therefore, day one of the 10-calendar-day period to submit a Suggestion of
Error. In this example, Suggestions of Error would be due by 11:59 p.m. PT on June 10, 2026.
CMS reviewed comments on the 10-day Suggestion of Error period submitted in response to the
initial Medicare Part B Drug Inflation Rebate Guidance, many of which suggested that
manufacturers receive at least 30 days to review the Preliminary Rebate Report. CMS considered
a 10-day, 15-day, and 30-day Suggestion of Error period and believes a 10-calendar-day period
(see proposed § 427.503(c)) is sufficient after considering the volume of the data to be provided
to manufacturers, the narrow scope of items that may be identified as a Suggestion of Error, and
the operational time necessary for CMS to provide a Rebate Report within 6 months of the end
of the applicable calendar quarter as required under section 1847A(i)(1)(A) of the Act. However,
CMS proposes in proposed § 427.502(c)(1)(ii) to expand the Suggestion of Error period to
30 calendar days for the Preliminary Rebate Report for calendar year 2023 and calendar
year 2024. This extended Suggestion of Error period will provide additional time and flexibility
during the first invoicing cycle of the Part B Rebate Program.
Section 1847A(i)(8) of the Act precludes administrative or judicial review on the
determination of units, whether a drug is a Part B rebatable drug, and the calculation of the
rebate amount (see proposed § 427.503(a)(1)). Therefore, the Suggestion of Error process will be
limited to mathematical steps involved in determining the rebate amount and the elements
precluded from administrative or judicial review will not be considered in-scope for the
Suggestion of Error process. Additionally, CMS is not providing an administrative dispute
resolution process. CMS intends to consider all in-scope submissions under the Suggestion of
Error process as specified in proposed § 427.503(a) (for example, suggestions regarding a
mathematical error). CMS does not intend to review suggestions of error that are out-of-scope or
submissions for a rebatable drug with an amount due of $0.
As the second step in the reporting process, CMS proposes in proposed § 427.501(c) to
provide the rebate amount to the manufacturer through the Rebate Report no later than 6 months
after the end of the applicable calendar quarter. As proposed in § 427.501(c)(1), the Rebate
Report would include the same data elements as the Preliminary Rebate Report (specified in
§ 427.501(b)(1)) and include any recalculations based on CMS acceptance of a manufacturer’s
Suggestion of Error from proposed § 427.503, or any CMS-determined recalculations from
proposed § 427.501(d)(2), if applicable. Manufacturers must pay the rebate amount within
30 calendar days from the date of receipt of the Rebate Report (see proposed § 427.505(a)). For
example, if the Rebate Report is provided on June 30, 2026, then July 1, 2026, would be the date
of receipt and therefore day one of the 30-calendar-day payment period; payment would be due
no later than 11:59 p.m. PT on July 30, 2026.
In proposed §§ 427.504 and 427.505, CMS proposes that it will establish a standard
method and process to issue Rebate Reports and accept manufacturer rebate payments. This
method and process may include an online portal administered by a CMS contractor which
would provide manufacturers with access to their Rebate Reports, submit Suggestions of Error,
and pay a rebate amount due. CMS intends to provide technical instructions separate from this
rulemaking to manufacturers of Part B rebatable drugs regarding how to access Rebate Reports
and how to receive notifications alerting the manufacturer when information is available. CMS
also intends to issue reminder notices to manufacturers regarding the due date of rebate
payments. In proposed § 427.504(a), CMS notes that the manufacturer that may access Rebate
Reports and make applicable rebate amount payments is the manufacturer responsible for paying
a rebate and, as stated above, CMS proposes to identify the manufacturer that is responsible for
paying a rebate using the same approach used for reporting ASP and Medicaid Drug Rebate
Program data.
iii. Reconciliation of a Rebate Amount
As discussed in section 60 of the revised Medicare Part B Drug Inflation Rebate
Guidance, CMS considered options for establishing a standardized method and process at regular
intervals to determinate any appropriate adjustments to the rebate amount for a Part B rebatable
drug for an applicable calendar quarter to account for revised information as well as options for
recalculation based on CMS identifying an agency error or determining manufacturer data was
misreported. While the provisions in 1847A(i) of the Act do not expressly provide for
reconciliation in the Medicare Part B Drug Inflation Rebate Program, CMS has determined that a
process for reconciling the rebate amount for updated information is necessary and appropriate to
promote the accuracy of the rebate amount for each drug for each applicable calendar quarter.
CMS is proposing policies for reconciliation, including with respect to enforcement of payment
of any reconciled rebate amount, consistent with both the statutory framework for the Part B
Inflation Rebate Program and the express authority in sections 1102 and 1871 of the Act to adopt
regulations for the proper administration of the Medicare Prescription Drug Inflation Rebate
Program.
As proposed at § 427.501(d), CMS believes that it is necessary and appropriate for CMS
to recalculate the rebate amount for an applicable calendar quarter at a regular interval to include
updated information about key data elements included in the calculation of the rebate amount.
These data elements as set forth in proposed § 427.501(d)(1)(i) include: total units; the payment
amount in the payment amount quarter; and any applied reductions as described in proposed
§§ 427.401 and 427.402. Updating these calculation inputs at a regular reconciliation interval
will result in a rebate amount that more fully reflects the majority of shifts in the underlying data
following additional time for claims run-out, which refers to the maturation of claims in the
claims processing system. Because the information accessed represents the claims’ status in the
claims processing system at that moment in time, additional claims run-out may yield different
information, either because more claims with dates of service during the applicable calendar
quarter were finalized and added to the claims processing system or because the status of the
existing claims changed. CMS refers to “X months of run-out” as the period between the end of
the applicable calendar quarter and the date when CMS accesses information about the claims;
for example, “3 months of run-out” means that claims data are accessed for claims with service
dates during an applicable calendar quarter 3 months after the end of such applicable calendar
quarter. Conducting a reconciliation of the rebate amount with additional claims run-out will
improve the accuracy of the rebate amount. Additionally, reconciliation of payment amounts is
consistent with the approach to the calculation of payment amounts in other CMS programs
(such as the Coverage Gap Discount Program) that provide for a reconciliation period.
CMS notes that the reconciliation of a rebate amount, whether the regular reconciliation
proposed at § 427.501(d)(1) or a discretionary reconciliation proposed at § 427.501(d)(2)
discussed further below, will not create a separately payable and distinct rebate amount. Rather,
reconciliation updates the prior rebate amount owed to CMS, if any, by a manufacturer of a Part

B rebatable drug so that the rebate amount ultimately reflects a more precise calculation of the
rebate amount, as required by section 1847A(i) of the Act, to account for shifts in the underlying
data following additional time for claims run-out after the Rebate Report is issued as well as
subsequently identified data integrity issues. Moreover, because the reconciled rebate amount is
an adjustment to the prior rebate amount, CMS proposes at § 427.501(d)(1)(i)(F) for the report of
a reconciled rebate amount to also identify the difference between the rebate amount due as
specified on the Rebate Report set forth in § 427.501(c) and the reconciled rebate amount. CMS
would only collect the net rebate amount due, if any, upon reconciliation, so as to prevent any
duplicate payments. CMS also proposes to refund any overpayment made by a manufacturer, as
determined during reconciliation, as discussed in proposed § 427.505(c).
Additionally, as CMS suggested in section 60 of the revised Medicare Part B Drug
Inflation Rebate Guidance, CMS considered multiple options for establishing a standardized
method and process to occur at regular intervals to determine any appropriate adjustment to the
rebate amount for a Part B rebatable drug for an applicable calendar quarter to account for
revised information prior to adopting the proposal described here with for reconciliation of the
Part B inflation rebate amount at 12 months. CMS considerations included the length of time
needed to capture relevant changes to data inputs for recalculation, whether the timing should
align with the reconciliation of Part D rebate amounts, and manufacturer burden. Specifically,
CMS considered the average time span needed to ensure submission of the majority of revisions
from claims run-out periods for Part B,
578
and how such unit revisions compare to the Part D
plan unit revisions specified in section 1860D-14B(b)(6) of the Act. CMS also considered the
average time span needed to ensure the majority of Part B claims submitted would already be
adjudicated and determined to be final action claims, CMS’ policies related to the frequency of
ASP restatements, the reporting timeline for refunds on discarded drug units, and reporting
578
See the CCW White Paper: Medicare Claims Maturity,
https://www2.ccwdata.org/documents/10280/19002256/medicare-claims-maturity.pdf.
timelines for 340B claims and claims for beneficiaries dually eligible for Medicare and
Medicaid. Without a reconciliation process, the Part B rebate amount would include units of
discarded drugs on which manufacturers potentially owe a refund, thereby potentially requiring
manufacturers to pay both a discarded drug refund and a rebate amount on certain units of a Part
B rebatable drug due to the timing of revisions to discarded drug units discussed in further detail
in section II.2.c.iv of this proposed rule.
CMS believes a longer period of claims run-out (at least 12 months of run-out time in the
proposed approach) would ensure that CMS more fully accounts for capturing of revised units.
CMS considered that penalties associated with failure to submit timely and accurate ASP data
(specified in § 414.806(b)) encourage timely submission of ASP data with the submission
timeline in accordance with § 414.804(a)(5) when considering the completeness of 12 months of
claims run-out. While CMS considered a longer period until a revision is completed, such as the
36-month period provided by the Medicaid Drug Rebate Program (MDRP) for AMP
restatements in § 447.510(d)(3), CMS believes that a 12-month reconciliation period is
appropriate for the Part B rebate program because of requirements to submit timely and accurate
ASP data (specified in § 414.806(b)), and it provides sufficient time to capture the majority of
updates to the data specified in proposed § 427.301 while closing out (except for the proposed
circumstances in proposed § 427.501(d)(2) regarding CMS’ identification of mathematical errors
or manufacturer misreporting) the calculation of the rebate amount for a Part B rebatable drug
for an applicable calendar quarter within a reasonable time period after the Rebate Report is
issued. While CMS proposes a 12- and 36-month reconciliation period in the Medicare Part D
Drug Inflation Rebate Program, due largely to the 36-month restatement period provided for
MDRP AMP restatements (specified in § 447.510(d)(3)), CMS does not believe a second or
longer restatement process is needed for Part B rebatable drugs because, as described previously,
the ASP and claims run-out periods correspond with sufficient claims run-out and ASP
restatement timing for Part B (particularly when considering penalties associated with failure to
submit timely and accurate ASP data (specified in § 414.806(b)).
Further, in considering whether consistency across CMS programs is critical, CMS
believes that consideration for the completeness of data, as discussed above, should be
prioritized over consistency across program timelines. That is, when examining timelines from
other CMS programs that collect data contributing to calculation of the rebate amount, CMS
prioritized that, to the extent feasible, completeness and accuracy of the data elements
contributing to the calculation of the rebate amount rather than prioritizing consistency among
the data collection and reconciliation timelines themselves. Finally, CMS believes that a
restatement of each data element included in proposed § 427.501(d) to reconcile the rebate
amount provided in the Rebate Report in proposed § 427.501(c) and drugs acquired through the
340B Program as proposed in § 427.303(b)(1)(i) is appropriate to capture an updated rebate
amount and is in line with other CMS programs that provide for a reconciliation period,
including ASP restatements (see § 414.806). While some data points may not change, CMS
would review the data to determine if there are any updates in the data and use the updated data
in the reconciliation to provide a reconciled rebate amount to the manufacturer.
Based on these considerations, similar to the multi-step process for the Rebate Report
proposed in § 427.501(b) and (c), in summary, CMS proposes a multi-step process to provide
each manufacturer of a Part B rebatable drug with a reconciled rebate amount on a regular basis.
At the 12 month reconciliation, CMS proposes a reconciliation process will include: (1) a
preliminary reconciliation of the rebate amount, which CMS would provide to manufacturers of
Part B rebatable drugs as proposed in § 427.501(d)(1) and (2) a reconciled rebate amount, which
CMS would provide to manufacturers of a Part B rebatable drug as proposed in
§ 427.501(d)(1)(ii). CMS also proposes to apply the Suggestion of Error process specified in
proposed § 427.503 to the preliminary reconciliation.
In detail, first, as specified in proposed § 427.501(d)(1) and similar to the Preliminary
Rebate Report process proposed in § 427.501(b), CMS proposes to provide the manufacturer
with information about the preliminary reconciliation of the rebate amount at least 1 month prior
to the issuance of the reconciled rebate amount (see proposed § 427.501(d)(1)) to each
manufacturer of a Part B rebatable drug for an applicable calendar quarter. CMS proposes in
§ 427.501(d)(1) that the preliminary reconciliation would include, at a minimum, the same
information outlined for the Rebate Report and the following updated information, if applicable:
updated total number of rebatable units as specified in proposed § 427.303; the payment amount
in the payment amount benchmark quarter, if any inputs are restated within the reconciliation
run-out period, as specified in proposed § 427.302(d); applicable calendar quarter specified
amount (defined in § 427.302(b)), if any inputs are restated within the reconciliation run-out
period; the excess amount by which the specified amount exceeds the inflation-adjusted payment
amount, if any inputs are restated within the reconciliation run-out period, as specified in
§ 427.302; the reconciled total rebate amount calculated in accordance with § 427.301; and the
difference between the total rebate amount due as specified on the Rebate Report set forth in
proposed § 427.501(d)(1)(i)).
In proposed § 427.503(a), similar to the Suggestion of Error process proposed for the
Preliminary Rebate Report in proposed § 427.501(a), within 10 calendar days after date of
receipt of the information about the preliminary reconciliation of the rebate amount, CMS
proposes that a manufacturer may suggest to CMS that the manufacturer believes the preliminary
reconciled rebate amount contains a mathematical error. CMS believes a 10-calendar-day period
is sufficient due to the same considerations of data volume, the narrow set of reviewable items,
and the operational time period necessary for CMS to complete the process to publish the
reconciled rebate amount. The preclusions in section 1847A(i)(8) of the Act on administrative
and judicial review apply to the reconciliation process.
Second, in detail, CMS proposes in § 427.501(d) to provide the reconciled rebate amount
to the manufacturer 12 months after the Rebate Report was issued for an applicable calendar
quarter. As proposed in § 427.501(d)(1)(i), the information in the report for the reconciled rebate
amount would include the same data elements as provided in the information provided to the
manufacturer of a Part B rebatable drug regarding the preliminary reconciliation of a rebate
amount (specified in proposed § 427.501(d)(1)) and include any recalculations based on CMS
acceptance of a manufacturer’s Suggestion of Error from proposed § 427.503. A reconciliation
of the rebate amount may result in an increase, decrease, or no change to the rebate amount,
compared to the Rebate Report for an applicable calendar quarter (see proposed
§ 427.501(d)(3)).
Additionally, as CMS suggested in section 60 of the revised Medicare Part B Drug
Inflation Rebate Guidance, CMS considered options for establishing circumstances where a
recalculation of the rebate amount may be appropriate for an applicable calendar quarter after
issuing the Rebate Report and/or a reconciled rebate amount based on CMS identifying an error
or CMS determining that the information used by CMS to calculate a rebate amount was
inaccurate due to false reporting or similar fault by the manufacturer (for example, manufacturer
pricing or product data under section 1927(b)(3) of the Act). CMS also considered potential time
limits for revisions and whether certain circumstances, such as instances of false reporting,
should be exempt from such time limits.
Based on these considerations, CMS believes that, to capture an accurate rebate amount
and consistent with reconciliations of pricing data otherwise submitted to CMS that provide for
revisions when necessary due to errors, including mathematical errors, and manufacturer
misreporting, certain circumstances may merit a recalculation of the rebate amount separate from
the 12-month reconciliation proposed in § 427.501(d)(1). Specifically, CMS proposes in
§ 427.501(d)(2) that CMS may recalculate a rebate amount, when CMS identifies either: (1) an
agency error such as a mathematical error or an error in the information specified in a Rebate
Report as described in proposed § 427.501(c) or report of a reconciled rebate amount as
described in proposed § 427.501(d)(1) including reporting system or coding errors, or (2) CMS
determining that information used to calculate the rebate amount was inaccurate due to
manufacturer misreporting. Examples of agency errors could include CMS incorrectly assigning
a billing or payment code or incorrectly calculating the billing units per package, or the
mechanism that provides a Rebate Report to the manufacturer or the Rebate Report incorrectly
displays a rebate amount. Examples of manufacturer misreporting could include instances in
which the manufacturer has made a correction to previously submitted data as well as instances
in which the individual or entity reporting data or information to CMS on behalf of the
manufacturer knows or should know is inaccurate or misleading (for example, inaccurate ASP
data as specified in § 414.806). This does not include standard restatements to ASP or other data
outside of the standard process of issuing the reconciled rebate amount. In addition to
manufacturer-initiated corrections, CMS may become aware of manufacturer misreporting based
on fact finding and conclusions of enforcement authorities, for example, the HHS Office of
Inspector General, the CMS Center for Program Integrity, or the Department of Justice. In a
situation where an error or manufacturer misreporting is identified prior to the 12-month
reconciliation of the rebate amount proposed in § 427.501(d)(1), CMS may choose to include a
correction based on the circumstances proposed in § 427.501(d)(2) concurrently with the
12-month reconciliation. When CMS reconciles data due to an instance of agency error or
manufacturer misreporting, CMS proposes that the agency would limit the scope of the
reconciliation to the specific information that is the basis for the reconciliation and not update or
otherwise revise any other data elements in the Rebate Report (specified in proposed
§ 427.501(c)) or the report of the reconciled rebate amount (specified in proposed
§ 427.501(d)(1)) unless the correction directly impacts additional data fields. For example, CMS
believes corrections to an ASP quarterly file may not change the specified amount for the
applicable calendar quarter.
In addition, because reconciling a rebate amount imposes substantial administrative
burden on CMS to reprocess the rebate amount, retest the reporting system, and reissue a rebate
report, CMS proposes in § 427.501(d)(2) that it may exercise discretion not to initiate recalculate
the rebate amount in these situations which are outside of the regular reconciliation process
proposed in § 427.501(d)(1).
CMS proposes that for a recalculation due to agency error, the error must be identified
within 3 years of the date of receipt of the reconciled rebate amount for the applicable calendar
quarter (see proposed § 427.501(d)(2)(i)). Identification means that CMS has knowledge of the
error; CMS does not need to have completed its revision of the impacted data or determined if
the revision impacts the rebate amount within the 3-year period. CMS would timely complete
these steps and determine, when the reconciliation does impact the rebate amount, whether the
reconciliation must be included in a discretionary revision or within an upcoming reconciled
rebate amount for an applicable calendar quarter. CMS believes that a 3-year period dating from
the issuance of a reconciliation aligns broadly with the timeframe in which most manufacturers
provide Part B ASP restatements.
CMS proposes in § 427.501(d)(2)(ii) that for a circumstance in which a manufacturer
misreports data, CMS is not bound by the 3-year time limit for revision of the rebate amount. For
example, if a determination is made that a manufacturer misreported ASP data, then CMS may
recalculate the rebate amount owed for a Part B rebatable drug. CMS requests comments on the
proposals related to manufacturer misreporting.
CMS proposes in § 427.505(a)(1) that upon receipt of the reconciled rebate amount
manufacturers must pay the rebate within 30 calendar days from the date of receipt of the
reconciled rebate amount. A 30-day payment deadline aligns with the payment period set forth in
statute at section 1847A(i)(1)(B) of the Act. As specified in proposed § 427.504, CMS would use
the same method and process for issuing Rebate Reports and submission of payments for reports
with a reconciled rebate amount. CMS would provide notice to manufacturers when a report with
a reconciled rebate amount, which will include the information proposed in § 427.501(d), is
available for the manufacturer’s Part B rebatable drugs. CMS proposes in § 427.505(c) that if a
refund is owed to a manufacturer based on a reconciled rebate amount, CMS would initiate the
process to issue such a refund within 60 days from the date of receipt of the reconciled rebate
amount (proposed in § 427.501(d)). CMS will issue additional information on this method and
process through additional program communications.
iv. Rebate Report for Applicable Calendar Quarters in CY 2023 and CY 2024
Section 1847A(i)(1)(C) of the Act provides the CMS with the option to delay sending the
information required by section 1847A(i)(1)(A) for applicable calendar quarters in calendar
years 2023 and 2024 until not later than September 30, 2025. In § 427.502, consistent with
section 60.2 of the revised Medicare Part B Drug Inflation Rebate Guidance, CMS proposes
consolidating the Preliminary Rebate Reports and Rebate Reports for CYs 2023 and 2024 into
two reports: one report for the four applicable calendar quarters in CY 2023 and one report for
the four applicable calendar quarters in CY 2024. This approach allows for 12 months of claims
run-out for each applicable calendar quarter in CY 2023 and at least 3 months of claims run-out
for each applicable calendar quarter in CY 2024. For these combined reports, CMS proposes in
§ 427.502 to provide an extended 30 calendar day Suggestion of Error period for the Preliminary
Rebate Report.
CMS intends to send a reconciled rebate amount for the four applicable calendar quarters
in CY 2024 9 months after the Rebate Report, to allow for 12 months of claims run-out for each
applicable calendar quarter; CMS does not intend to conduct reconciliation for the four
applicable calendar quarters in CY 2023 since the Rebate Report would already reflect
12 months of claims run-out (see proposed § 427.502(b)). This approach aligns claims and
payment data run-out with the run-out used during a regular reconciliation cycle. The Suggestion
of Error period for the report containing the reconciled rebate amount for applicable calendar
quarters in CY 2024 would be 10 calendar days.
This approach also minimizes the number of reports issued to manufacturers as a result of
the delay in reporting and simplifies payment procedures, thereby minimizing manufacturer
burden. Starting with the first applicable calendar quarter of CY 2025, reporting would begin a
standard cadence and follow the procedures otherwise proposed in subpart F of this part 427.
CMS proposes that manufacturers that do not pay the Medicare Part B inflation rebate
amount owed for a Part B rebatable drug within 30 calendar days of receiving a Rebate Report,
including reports containing a reconciled rebate amount, may be subject to a civil money penalty
of 125 percent of the rebate amount, as applicable, for such drug for the applicable calendar
quarter. The civil money penalty is in addition to the rebate amount.
g. Enforcement of Manufacturer Payment of Rebate Amounts (§ 427.600)
Section 1847A(i)(7) of the Act gives CMS the authority to impose a civil money penalty
equal to at least 125 percent of the rebate amount for each drug for each applicable calendar
quarter on a manufacturer that fails to pay the rebate amount for each rebatable Part B drug.
Subpart G would implement this section of the Act and establish the procedures for determining
and collecting a civil money penalty.
In accordance with section 1847A(i)(1)(B) and proposed § 427.505(a), manufacturers
must provide to CMS a rebate amount owed within 30 calendar days of receipt of the Rebate
Report containing the rebate amount due. As described in proposed § 427.600(a), CMS is
proposing that it may impose a civil money penalty when a manufacturer fails to pay the rebate
amount in full by the payment deadlines proposed in § 427.505(a). This means a manufacturer
may be subject to a civil money penalty if the manufacturer fails to pay the full the rebate
amount as invoiced in the Rebate Report or any reconciled rebate amount that is greater than the
amount invoiced in the Rebate Report. More specifically, a manufacturer could be subject to a
civil money penalty when a manufacturer fails to pay a rebate amount due by any payment
deadline proposed in § 427.505(a)(1) and (2) for: (1) a Rebate Report specified in proposed
§ 427.501(c); (2) a reconciled rebate amount greater than the rebate amount reflected in the
Rebate Report specified in proposed § 427.501(d); or (3) a Rebate Report and a reconciled rebate
amount greater than the amount reflected in the Rebate Report, if applicable, for the applicable
calendar quarters in calendar years 2023 and 2024 as specified in proposed § 427.502. As
discussed earlier in subpart e.iii, CMS notes that the reconciled rebate amount is not a separately
payable and distinct rebate amount. Rather, the reconciled rebate amount is an update to the
rebate amount owed to CMS by a manufacturer of a Part B rebatable drug.
Civil money penalties are a point-in-time penalty tied to the rebate amount due at the
applicable payment deadline, which occurs 30 days after the date of receipt of a Rebate Report.
In proposed § 427.600(b), CMS proposes to establish the methodology for determining the
amount of the civil money penalty as equal to 125 percent of the rebate amount for such drug for
such applicable calendar quarter, and that this penalty would be due in addition to the rebate
amount due. That is, a manufacturer would be responsible for paying the full rebate amount due
in addition to any civil money penalty imposed because of late payment. While CMS has the
statutory authority to impose a civil money penalty greater than 125 percent of the rebate amount
in the Part B Rebate Program under section 1847(A)(i)(7), CMS is proposing a penalty amount
of 125 percent of the rebate amount to align with the penalty amount in the Part D Inflation
Rebate Program. CMS is proposing this approach to civil money penalties based on section
1847A(i)(1)(B) of the Act, which establishes a requirement by the manufacturer to provide CMS
with a rebate not later than 30 days after receipt from CMS of the report on the amount of the
excess average sales price increase. CMS believes that the ability to assess civil money penalties
is necessary in all circumstances where a payment is due for a rebate amount to CMS to ensure
compliance with the rebate program’s requirements. The civil money penalty would be
calculated based on the outstanding rebate amount due at the payment deadline, which is defined
in proposed § 427.505(a) as 30 calendar days after the date of receipt of a Rebate Report
containing any rebate amount due; once a civil money penalty is assessed due to a late payment,
the penalty would remain in effect even if the manufacturer pays the outstanding amount as the
penalty is initiated due to a missed payment deadline. Because the payment deadline is clearly
defined in section 1847A(i)(1)(B) of the Act, any late payments of a rebate amount due,
including late payment of any reconciled rebate amounts greater than the amount reflected in the
Rebate Report, would be considered a violation potentially subject to a civil money penalty. Any
civil money penalty would be assessed before the next reconciliation process.
CMS is proposing in § 427.600(b)(2) that civil money penalties may be calculated at
several points in time associated with missing a payment deadline for the rebate amount due
reflected in the Rebate Report or missing a payment deadline associated with any rebate amount
determined after a reconciliation to be greater than the amount invoiced in the Rebate Report. As
these separate events can result in distinct assessments of civil money penalties, this means that
CMS would not modify a civil money penalty from a prior missed payment deadline based on
changes to the rebate amount due following reconciliation, including scenarios where the rebate
amount is reduced following reconciliation. However, in the event that the rebate amount due on
a Rebate Report was not paid and a civil money penalty was issued for violation of the payment
deadline, CMS would not issue a second civil money penalty on a reconciled rebate amount if
reconciliation decreased the rebate amount stated on the Rebate Report. CMS believes that
enforcing this requirement after each payment deadline, regardless of what rebate amount a
manufacturer may or may not owe at a future payment deadline, is necessary to maintain the
integrity of the program and consistency of the implementation of the program. Further, CMS is
proposing this approach to ensure an enforcement approach that is operationally feasible and
applied consistently in all cases.
As an example of this approach in practice, CMS presents a scenario where the rebate
amount due on the Rebate Report is $100. Following reconciliation 12 months after the Rebate
Report was issued, CMS calculates a reconciled rebate amount for the applicable calendar
quarter of $120 (an increase of $20 from the rebate amount identified in the Rebate Report due to
updated claims run-out and payment data). Under this scenario, in the event the manufacturer
does not pay the $100 rebate amount owed within the 30-day deadline following receipt of the
Rebate Report, a civil money penalty for $125 ($100 x 1.25) could be assessed against the
manufacturer due to their failure to meet the payment deadline. If the manufacturer pays the
$100 before the reconciliation is completed, and then timely pays the $20 due within the 30-day
payment deadline following the reconciliation 12 months after the Rebate Report or does not pay
the $100 before the reconciliation is completed but timely pays the $120 due within the 30 day
payment deadline following reconciliation 12 months after the Rebate Report, no further civil
money penalty would be assessed.
Alternatively, in the event the manufacturer pays the $100 rebate amount due within the
30-day deadline following receipt of the Rebate Report but fails to meet the payment deadline
for the net $20 rebate amount due following reconciliation, a civil money penalty of $25 ($20 x
1.25) could be assessed against the manufacturer due to their failure to meet the payment
deadline for the updated rebate amount due following reconciliation. Finally, under this scenario
in the event the manufacturer fails to meet any payment deadline throughout the full
reconciliation cycle of this rebate amount; that is, the deadline is missed for the $100 amount due
stated in the Rebate Report, and the $20 net rebate amount due following reconciliation, CMS
may assess a separate civil money penalty on the rebate amount due at each of these missed
deadlines. In this example, violations of each of these payment deadlines would result in a
penalty of $125 ($100 x 1.25), followed by a penalty of $25 ($20 x 1.25), each of which would
be assessed following the manufacturer’s failure to meet the related payment deadline for the
outstanding rebate amount due.
In an alternative possible scenario, consider the following. The rebate amount due on the
Rebate Report is $100. Following reconciliation 12 months after the Rebate Report was issued,
CMS calculates a reconciled rebate amount owed for the applicable period of $80 (a decrease of
$20 from the rebate amount identified in the Rebate Report). In this scenario, if a manufacturer
does not pay the $100 by the payment deadline for the rebate amount due in the Rebate Report, a
civil money penalty for $125 ($100 x 1.25) may be assessed against the manufacturer due to its
failure to meet the payment deadline for the rebate amount due identified in the Rebate Report.
This civil money penalty is not affected if the manufacturer pays the rebate amount once it is
past the deadline, nor is it impacted by the reconciled rebate amount, because at the payment
deadline missed by the manufacturer, the manufacturer owed a rebate of $100 to CMS and that
rebate amount was not paid timely. As noted previously, under this scenario, given that there is
no additional rebate amount due upon reconciliation compared to the rebate amount stated on the
Rebate Report, there would not be a civil money penalty assessed on the reconciled rebate
amount.
Further, note that payment of any civil money penalty does not obviate the requirement
for the manufacturer to pay any outstanding rebate amount due, including any rebate amount due
following a reconciliation. Therefore, paying a civil money penalty does not satisfy the
obligation to pay the underlying rebate amount on which the civil money penalty is calculated. In
addition, CMS is evaluating all available options to ensure manufacturers’ timely compliance
with their rebate payment obligations, including, without limitation, potential recovery
approaches and enforcement actions. For example, CMS may refer manufacturers to the
Department of Justice, Department of the Treasury, and/or the Department of Health and Human
Services Office of Inspector General for further review and investigation.
In proposed § 427.600(c), CMS proposes that if CMS makes a determination to impose a
civil money penalty on a manufacturer for violation of a payment deadline, CMS would send a
written notice of the decision to impose a civil money penalty that includes a description of the
basis for the determination, the basis for the penalty, the amount of the penalty, the date the
penalty is due, the manufacturer’s right to a hearing, and information about where to file the
request for a hearing. To ensure a consistent approach to civil money penalties, CMS proposes
applying existing appeal procedures for civil money penalties in 42 CFR section 423, subpart T
of this title to manufacturers appealing a civil money penalty imposed under the Medicare Part B
Drug Inflation Rebate Program. CMS has utilized this appeals process many years for civil
money penalty determinations affecting MA organizations and Part D sponsors. CMS therefore
proposes to use this well-established process for civil money penalty appeals from manufacturers
that do not make inflation rebate payments by the payment deadline. CMS also proposes in
§ 427.600(e)(1) that the scope of appeals is limited to: (1) CMS determinations relating to
whether the rebate payment was made by the payment deadline; and (2) the calculation of the
penalty amount. Section 1847A(i)(8) of the Act precludes judicial review of specific data inputs
or calculations related to the underlying Rebate Report and reconciliation; therefore, such data
and calculations are not appealable through this process.
Section 1847A(i)(7) of the Act states that the provisions of section 1128A of the Act
(except subsections (a) and (b)) apply to civil money penalties under this subpart to the same
extent that they apply to a civil money penalty or procedure under section 1128A(a) of the Act.
CMS proposes to codify this requirement in proposed § 427.600(f). In alignment with the
procedure outlined in section 1128A of the Act, CMS proposes in § 427.600(d) that collection of
the civil money penalty would follow expiration of the timeframe for requesting an appeal,
which is 60 calendar days from the civil money penalty determination in cases where the
manufacturer did not request an appeal. In cases where a manufacturer requests a hearing and the
decision to impose the civil money penalty is upheld, CMS would initiate collection of the civil
money penalty once the administrative decision is final. CMS is seeking comment on proposals
related to the violations of payment deadlines and issuance of a civil money penalty.
CMS proposes in § 427.600(g) that in the event that a manufacturer declares bankruptcy,
as described in title 11 of the United States Code, and as a result of the bankruptcy, fails to pay
either the full rebate amount owed or the total sum of civil money penalties imposed, the
government reserves the right to file a proof of claim with the bankruptcy court to recover the
unpaid rebate amount and/or civil monetary penalties owed by the manufacturer.
h. Severability (§ 427.10)
In proposed § 427.10, we propose that, were any provision of part 427 to be held invalid
or unenforceable by its terms, or as applied to any person or circumstance, such provisions
would be severable from part 427 and the invalidity or unenforceability would not affect the
remainder thereof or any other part of this subchapter or the application of such provision to
other persons not similarly situated or to other, dissimilar circumstances. While the provisions in
part 427 are intended to present a comprehensive approach to implementing the Medicare Part B
Drug Inflation Rebate Program, we intend that each of them is a distinct, severable provision, as
proposed, and would not affect similar provisions in the Medicare Part D Drug Inflation Rebate
Program. Through this rulemaking, the Part B drug inflation rebate proposals are intended to
operate independently of each other, even if each serves the same general purpose or policy goal.
For example, CMS intends that the proposed policies related to reducing the rebate amount for
Part B rebatable drugs currently in shortage and when there is a severe supply chain disruption
(§§ 427.401 and 427.402) are distinct and severable from the proposals related to the
determination of Part B rebatable drugs subject to rebates (§ 427.101). As another example,
CMS intends that the proposed policy for using the payment limit for purposes of calculating the
beneficiary coinsurance adjustment (§ 427.201(b)) is distinct and severable from the proposals to
use the specified amount for purposes of the Part B rebate calculation (§ 427.301). Even where
one provision makes reference to a second provision, the preamble and the regulatory text clarify
the intent of the agency that the two provisions would be severable if one provision were to be
invalidated in whole or in part. For example, CMS would still be able to calculate drugs and
biological products with average total allowed charges below the applicable threshold as
described in § 427.101(c)(1), for exclusion from inflation rebate calculations, even if the
provision to apply the applicable threshold at the billing and payment code level is deemed
invalid (§ 427.101(c)(3)). We welcome comments on this severability policy.
3. Medicare Part D Drug Rebates for Drugs, Biologicals, and Sole Source Generic Drugs with
Prices that Increase Faster than the Rate of Inflation

a. Definitions (§ 428.20)
In this proposed rule, CMS proposes to codify definitions of terms with meanings given
in section 1860D-14B of the Act and established in the revised Medicare Part D Drug Inflation
Rebate Guidance, as well as new definitions based on policies detailed in this proposed rule.
At proposed § 428.20, CMS is proposing that the following terms in section 1860D-14B
of the Act are defined:
● “Annual manufacturer price (AnMP)”.
● “Applicable period”.
● “Applicable period Consumer Price Index for All Urban Consumers (CPI-U)”.
● “Benchmark period CPI-U”.
● “Part D rebatable drug”.
● “Payment amount benchmark period”.
● “Unit”.
Further, in § 428.20 of this proposed rule, CMS proposes to codify definitions established
in the revised Medicare Part D Drug Inflation Rebate Guidance and new definitions based on
policies detailed in this proposed rule for the following terms:
● “Applicable threshold”.
● “Average manufacturer price (AMP)”.
● “Benchmark period manufacturer price”.
● “Covered Part D drug”.
● “CPI-U”.
579
● “First marketed date”.
● “Inflation-adjusted payment amount”.
579
These data are referenced to 1982-84=100—that is, the average of pricing data for the 36 months from 1982
through 1984 serve as the basis for the index and are assigned a value of 100. These data are not seasonally adjusted.
● “Manufacturer”. CMS intends that manufacturer identification in the Medicare
Prescription Drug Inflation Rebate Program, inclusive of communications and rebate liability,
will be consistent with the policies and practices adopted under § 447.502 for purposes of
manufacturer obligations under the Medicaid Drug Rebate Program. We believe this approach
will provide clarity and allow for consistency in the agency’s treatment of financial transactions,
including in the contexts of debt collection, bankruptcy, and changes in ownership. We welcome
feedback on this proposed approach and whether there are alternative approaches that may better
achieve the agency’s goals for application of rebate liability and collection of rebate amount,
including whether additional policies and/or a Medicare Prescription Drug Inflation Rebate
Program agreement are needed to clarify financial accountability for rebate amounts in situations
where there are changes in ownership of a manufacturer or of a rebatable drug.
● “National Drug Code (NDC)”.
● “Subsequently approved drug”.
b. Determination of Part D Rebatable Drugs (§§ 428.100 through 428.101)
i. Definitions
In proposed § 428.100, CMS proposes to define the following terms applicable to
subpart B (§§ 428.100 through 428.101):
● “Individual who uses such a drug or biological”.
● “Gross covered prescription drug costs”.
ii. Identification of Part D Rebatable Drugs
Section 1860D-14B(g)(1)(A) of the Act defines a “Part D rebatable drug,” in part, as a
drug or biological described at section 1860D-14B(g)(1)(C) that is a “covered Part D drug” as
that term is defined in section 1860D-2(e) of the Act. A drug or biological described in section
1860D-14B(g)(1)(C) means a drug or biological that, as of the first day of the applicable period
involved, is: (1) a drug approved under an NDA under section 505(c) of the FD&C Act (that is,
a brand name drug); (2) a drug approved under an ANDA under section 505(j) of the FD&C Act

that meets the criteria in section 1860D-14B(g)(1)(C)(ii) (that is, a generic drug that meets
certain sole source criteria); or (3) a biological licensed under section 351 of the PHS Act (that
is, a biological product, including a biosimilar).
In § 428.101(a), CMS proposes to identify a Part D rebatable drug
580
for each applicable
period by determining which covered Part D drugs, as defined in section 1860D-2(e) of the Act,
meet the requirements in section 1860D-14B(g)(1)(C) of the Act (that is, are brand name drugs
approved under an NDA, biologicals licensed under a biologics license application (BLA), or
generic drugs approved under an ANDA). As noted, a Part D rebatable drug must meet the
requirements in section 1860D-14B(g)(1)(C) of the Act as of the first day of the applicable
period.
To evaluate whether a generic drug approved under an ANDA meets all the criteria in
section 1860D-14B(g)(1)(C)(ii) of the Act, CMS proposes in § 428.101(a)(3) to codify the policy
established in section 30 of the revised Medicare Part D Drug Inflation Rebate Guidance
whereby CMS would use specified FDA resources such as the “Approved Drug Products with
Therapeutic Equivalence Evaluations” (commonly known as the Orange Book)
581
and NDC
Directory to determine whether a generic drug meets the definition of a Part D rebatable drug. In
§ 428.101(a)(3)(i) and (ii), CMS proposes to clarify the policy established in revised Medicare
Part D Drug Inflation Rebate Guidance by adding that, for purposes of § 428.101, CMS
considers historical information from NDC Directory files, such as discontinued, delisted, and
expired listings, provided by FDA to CMS or published by FDA on its website to be included in
the NDC Directory. As proposed in § 428.101(a)(3)(iii), to determine whether the manufacturer
of the generic drug is a first applicant during the 180-day exclusivity period, or whether the
manufacturer of the generic drug is a first approved applicant for a competitive generic drug
580
For purposes of this proposed rule, CMS uses the term “Part D rebatable drug” to refer to the dosage form and
strength with respect to such drug for which Part D drug inflation rebates are calculated.
581
FDA Orange Book: https://www.fda.gov/drugs/drug-approvals-and-databases/approved-drug-products-
therapeuticequivalence-evaluations-orange-book.
therapy, CMS will refer to FDA website resources such as the Orange Book and may consult
with FDA for technical assistance as needed. CMS will determine whether a generic drug that is
a covered Part D drug meets the definition of a Part D rebatable drug based on the status of the
drug on the first day of the applicable period.
While generic drugs that do not meet the sole source criteria in section
1860D-14B(g)(1)(C)(ii) of the Act (that is, multiple source generic drugs) are excluded from the
definition of a Part D rebatable drug, CMS understands that a generic drug may meet the
definition of a Part D rebatable drug on the first day of an applicable period and then cease to
meet such definition later in the applicable period if, for example, the FDA approves another
therapeutically equivalent generic drug under a 505(j) ANDA and that drug is marketed during
such applicable period.
iii. Drugs and Biologicals with Average Annual Total Cost Under Part D Below the Applicable
Threshold
Under section 1860D-14B(g)(1)(B) of the Act, a drug or biological is excluded from the
definition of a Part D rebatable drug if the “average annual total cost” under Part D for such
period per individual who uses such a drug or biological product is less than $100 per year, as
determined by the Secretary using the most recent data available, or, if data are not available, as
estimated by the Secretary. The statute provides that the $100 annual amount for the applicable
period beginning October 1, 2022, is to be increased by percentage changes in the CPI-U for
subsequent applicable periods. In § 428.101(b), CMS proposes to codify the policy established in
section 30.2 of the revised Medicare Part D Drug Inflation Rebate Guidance for determining the
applicable threshold and excluding from the definition of a Part D rebatable drug, and thus Part
D drug inflation rebates, drugs and biologicals for which the average annual total cost under Part
D for such applicable period per individual who uses such drug or biological product is below
that applicable threshold.
As described in the revised Medicare Part D Drug Inflation Rebate Guidance, CMS
intends to calculate the average annual total cost based on gross covered drug costs for the Part D
rebatable drug at the NDC-9 level. CMS will divide the gross covered drug costs for the drug by
the number of unique Part D beneficiaries as described above that were dispensed the drug in
that applicable period. For this calculation, CMS will use PDE data with gross covered drug
costs greater than zero that are available for the drug with dates of service during that applicable
period. Drugs that are determined to have average annual total costs under Part D of less than
$100 per individual using such drug per year, adjusted by changes in the CPI-U, will be excluded
from Part D drug inflation rebate calculations for the applicable period in question.
c. Determination of the Rebate Amount for Part D Rebatable Drugs (§§ 428.200 through
428.204)
i. Definitions
In proposed § 428.200, CMS proposes to define the following terms applicable to
subpart C (§§ 428.200 through 428.204):
● “340B Program”.
● “Line extension”.
● “New formulation”.
● “Oral solid dosage form”.
ii. Calculation of the Total Rebate Amount To Be Paid by Manufacturers
Under section 1860D-14B(b)(1) of the Act, the Part D drug inflation rebate for each
Part D rebatable drug and applicable period, subject to certain considerations, is the estimated
amount that is equal to the product of: (1) the amount, if any, by which the annual manufacturer
price (AnMP) for such Part D rebatable drug for the applicable period exceeds the
inflation-adjusted payment amount for the Part D rebatable drug for the applicable period, and
(2) the total number of units of the Part D rebatable drug dispensed under Part D and covered and
paid by Part D plan sponsors during the applicable period. To calculate the Part D drug inflation
rebate consistent with section 1860D-14B(b)(1) of the Act, CMS proposes in § 428.201(a)(1) to
codify the calculation methodology described in section 40 of the revised Medicare Part D Drug
Inflation Rebate Guidance, which provides that the total Part D drug inflation rebate amount is
equal to the per unit Part D drug inflation rebate amount, as determined under proposed
§ 428.202(a), multiplied by the total number of units of a Part D rebatable drug dispensed under
Part D and covered by Part D plan sponsors, as determined in accordance with proposed
§ 428.203. CMS proposes in § 428.201(a)(2) that the total Part D drug inflation rebate amount
for a Part D rebatable drug that is a line extension of a Part D rebatable drug that is an oral solid
dosage form is equal to the amount specified in proposed § 428.204. The Part D drug inflation
rebate amount calculated in accordance with this subpart is subject to adjustment based on any
reductions in accordance with subpart D of this part or any reconciliations in accordance with
subpart E of the part.
In § 428.201(b), CMS proposes to exclude from the calculation performed under
subpart C drugs and biologicals that meet the definition of a Part D rebatable drug, but which are
missing AMP data for the entire duration of the applicable period because, for the reasons
specified below, there were no quarters during that period in which their manufacturers were
required to report AMP data under section 1927(b)(3) of the Act. The calculations for the rebate
amount set forth in section 1860D-14B(b) of the Act contemplate use of AMP and unit data
reported by manufacturers under section 1927 of the Act. Similarly, section 1860D-14B(d) of the
Act indicates CMS should use, for purposes of carrying out the Medicare Part D Drug Inflation
Rebate Program, information submitted by manufacturers under section 1927(b)(3) of the Act.
Section 1927 requires manufacturers that participate in the Medicaid Drug Rebate Program
(MDRP) to enter into agreements with the HHS Secretary and submit price and drug product
information to CMS for each covered outpatient drug (COD), as defined in sections
1927(k)(2)-(4) of the Act and in § 447.502.
Not every drug that satisfies the definition of a Part D rebatable drug may be marketed by
a manufacturer that has an MDRP agreement in effect with the Secretary during the applicable
period. Similarly, there may be limited instances in which a drug or biological satisfies the
definition of a Part D rebatable drug but is not a COD under the MDRP. As a result, information
may not be reported under section 1927(b)(3) of the Act for all Part D rebatable drugs, and thus
may not be available to CMS for purposes of calculating Part D drug inflation rebates under
section 1860D-14B of the Act. Said differently, in limited cases where a Part D rebatable drug is
marketed by a manufacturer that does not have an obligation to report pricing and drug product
data under section 1927(b)(3) of the Act for the reasons noted, the manufacturer does not
currently report information needed for CMS to be able to calculate Part D drug inflation rebates.
Due to this operational issue, CMS proposes in § 428.201(b) to codify the policy
established in section 30.1 of the revised Medicare Part D Drug Inflation Rebate Guidance
whereby CMS would exclude from Part D drug inflation rebate calculations drugs and
biologicals that meet the definition of a Part D rebatable drug but for which the manufacturer
does not have an MDRP agreement in effect with the HHS Secretary under section 1927 of the
Act at any point during the applicable period, or the Part D rebatable drug is one that does not
meet the definition of a COD. This would effectively exclude from rebate calculations Part D
rebatable drugs for which there is missing AMP data for the entire duration of the applicable
period for the sole reason that there were no quarters during that period in which the
manufacturer was required to report AMP data under section 1927(b)(3) of the Act. In either of
these situations, a manufacturer does not have an obligation to report pricing and drug product
data under section 1927(b)(3) of the Act and thus the information required to calculate Part D
drug inflation rebates for these drugs is not available to CMS. If a manufacturer is required to
report AMP under section 1927(b)(3) of the Act for any part of the applicable period for a drug
or biological that meets the definition of a Part D rebatable drug, CMS would not exclude such
drug or biological from Part D drug inflation rebate calculations. We also clarify that the
proposed exclusion at § 428.201(b) relates only to the calculation of the rebate amount and does
not affect the determination of whether a drug or biological meets the definition of a Part D
rebatable drug. When performing the reconciliation described at § 428.401(d), CMS would
reexamine whether the manufacturer was required to report AMP for any part of the applicable
period for the Part D rebatable drug; if at reconciliation the manufacturer was required to report
AMP for any part of the applicable period, CMS would calculate a Part D rebate amount for this
Part D rebatable drug. CMS intends to monitor how these exclusions from the Part D drug
inflation rebate calculation may impact manufacturer behavior and may revisit this exclusion in
the future.
In the initial Medicare Part D Drug Inflation Rebate Guidance, CMS solicited comments
on the proposed approach and alternative approaches. CMS continues to be interested in
comments on this topic and welcomes additional comments on this approach and alternative
approaches—specifically, how CMS should address the situations in which the manufacturer of a
Part D rebatable drug does not have an MDRP agreement in effect for any part of the applicable
period or when a Part D rebatable drug may be excluded from the definition of a COD and
manufacturers may not be required to report pricing and drug product information under section
1927(b)(3) of the Act.
iii. Calculation of the Per Unit Part D Drug Rebate Amount
To calculate the total rebate amount in accordance with proposed § 428.201(a), CMS will
first calculate the per unit Part D drug rebate amount as proposed in § 428.202. As described in
the revised Medicare Part D Drug Inflation Rebate Guidance and provided in proposed
§ 428.202(a), CMS will calculate the per unit Part D drug inflation rebate amount by determining
the amount by which the AnMP for a Part D rebatable drug exceeds the inflation-adjusted
payment amount for such drug for the applicable period. To determine the per unit Part D
inflation rebate amount for a Part D rebatable drug, CMS must calculate the AnMP for the drug,
identify the payment amount benchmark period and calculate the benchmark period

manufacturer price for the drug, identify the benchmark period CPI-U, and calculate the
inflation-adjusted payment amount for the drug.
(1) Calculation of the AnMP for the Applicable Period
To determine the AnMP for a Part D rebatable drug and applicable period, CMS proposes
in § 428.202(b) to codify the policy described in the revised Medicare Part D Drug Inflation
Rebate Guidance whereby CMS would use the AMP reported by a manufacturer to the Medicaid
Drug Programs system under sections 1927(b)(3)(A)(i) and (ii) of the Act for each calendar
quarter of the applicable period, as well as the units reported by a manufacturer under section
1927(b)(3)(A)(iv) of the Act for each month of the applicable period. The manufacturer-reported
AMP units represent the total units of a drug sold by the manufacturer each month to retail
community pharmacy and wholesaler purchasers as described under section 1927(k)(1)(A) of the
Act. Manufacturers may include under certain circumstances non-retail community pharmacy
sales units in the calculation of their AMPs for 5i drugs.
582
As specified in section 1860D-14B(b)(2) of the Act, the AnMP for a Part D rebatable
drug for an applicable period is equal to the sum of the products of (1) the AMP for the Part D
rebatable drug reported for each calendar quarter of the applicable period, and (2) the total units
of the Part D rebatable drug reported for each of the corresponding calendar quarters of the
applicable period divided by the total units of the Part D rebatable drug reported for the
4 calendar quarters in the applicable period. The following formula illustrates how CMS would
calculate the AnMP for a Part D rebatable drug as proposed in § 428.202(b):
(AMP for calendar quarter beginning October) multiplied by (sum of monthly units for
October calendar quarter divided by total units for 12-month applicable period) +
582
5i drugs are CODs that are inhaled, infused, instilled, implanted, or injected. Manufacturers are instructed to
calculate the AMP for 5i drugs that are not generally dispensed through a retail community pharmacy using the
methodology described at § 447.504(d) and (e). Section 447.507(b)(1) provides that a 5i drug is not generally
dispensed through a retail community pharmacy if 70 percent or more of the sales (based on units at the NDC-9
level) of the 5i drug, were to entities other than retail community pharmacies or wholesalers for drugs distributed to
retail community pharmacies.
(AMP for calendar quarter beginning January) multiplied by (sum of monthly units for
January calendar quarter divided by total units for 12-month applicable period) +
(AMP for calendar quarter beginning April) multiplied by (sum of monthly units for April
calendar quarter divided by total units for 12-month applicable period) +
(AMP for calendar quarter beginning July) multiplied by (sum of monthly units for July
calendar quarter divided by total units for 12-month applicable period)
In § 428.202(b)(2), CMS proposes that the first applicable period for a Part D rebatable
drug will be the earliest applicable period that follows the payment amount benchmark period
identified in proposed § 428.202(c)(1) through (4). For a Part D rebatable drug first approved or
licensed on or before October 1, 2021, with a payment amount benchmark period identified in
§ 428.202(c)(1), the first applicable period would begin on October 1, 2022 and end on
September 30, 2023. For a Part D rebatable drug first approved or licensed on or before
October 1, 2021 with a payment amount benchmark period identified in § 428.202(c)(3), or a
subsequently approved drug with a payment amount benchmark period identified in
§ 428.202(c)(2) or (4), the first applicable period would begin on October 1 of the year following
the payment amount benchmark period identified in proposed § 428.202(c)(2) through (4). As
described below, CMS is soliciting comments on alternative policies for determining the
payment amount benchmark period in certain instances of missing AMP. In the case of a Part D
rebatable drug that was previously a selected drug as described in proposed § 428.202(c)(5) for
which the payment amount benchmark period is reset as the last calendar year of the price
applicability period for such drug, the earliest applicable period that follows the reset payment
amount benchmark period would begin on October 1 of the year following the payment amount
benchmark period identified in proposed § 428.202(c)(5). The date that CMS will use to
determine when a drug is first approved or licensed is the FDA approval date that the
manufacturer reports to the Medicaid Drug Programs system under section 1927(b)(3)(A)(v) of
the Act.
(2) Identification of the Payment Amount Benchmark Period
Consistent with section 1860D-14B(g)(3) of the Act and as described in sections 40.2.2
and 40.3 of the revised Medicare Part D Drug Inflation Rebate Guidance, CMS proposes in
§ 428.202(c)(1) that for a drug first approved or licensed by the FDA on or before
October 1, 2021, the payment amount benchmark period is the period beginning on
January 1, 2021 and ending on September 30, 2021. For a subsequently approved drug, CMS
proposes in § 428.202(c)(2) that the payment amount benchmark period would be the first
calendar year beginning after the drug’s first marketed date, as specified under section
1860D-14B(b)(5)(A) of the Act. To identify the payment amount benchmark period for a Part D
rebatable drug, CMS would use the FDA approval date or the first marketed date reported under
section 1927(b)(3)(A)(v) of the Act, as applicable.
(a) Proposal to Establish a Payment Amount Benchmark Period in Certain Instances of Missing
AMP
Section 1860D-14B of the Act does not expressly address how CMS should calculate the
benchmark period manufacturer price for a Part D rebatable drug when a manufacturer has not
reported AMP during the payment amount benchmark period identified by statute. For example,
as described in the revised Medicare Part D Drug Inflation Rebate Guidance, while section
1860D-14B(g)(3) of the Act contemplates that drugs first approved or licensed by the FDA on or
before October 1, 2021, would have a payment amount benchmark period of January 1, 2021,
through September 30, 2021, the statute does not address circumstances in which such drugs are
not marketed until after October 1, 2021, and thus lack AMP from January 1, 2021, through
September 30, 2021, to calculate the benchmark period manufacturer price. In response to
comments, CMS stated in section 40.1.2 of the revised Medicare Part D Drug Inflation Rebate
Guidance that Part D rebatable drugs first approved or licensed on or before October 1, 2021,
that were not marketed until after that date and thus did not have AMP in the statutorily defined
payment amount benchmark period (that is, January 1, 2021, through September 30, 2021)
would be treated in the same manner as subsequently approved drugs for purposes of
establishing the payment amount benchmark period, benchmark period CPI-U, first applicable
period, and first applicable period CPI-U. In the revised guidance, CMS also stated that it
intended to address this policy in future rulemaking and would solicit comments on this policy at
that time.
Based on further review, CMS has observed that a number of NDC-9s of Part D rebatable
drugs approved on or before October 1, 2021, do not have AMP reported in the period of
January 1, 2021, through September 30, 2021, and a number of NDC-9s of subsequently
approved drugs do not have AMP reported in the first calendar year beginning after the drug’s
first marketed date. To enable CMS to calculate the benchmark period manufacturer price and
inflation rebate amounts for these NDC-9s, CMS is proposing in § 428.202(c)(3) that for a Part
D rebatable drug first approved or licensed on or before October 1, 2021, for which there are no
quarters during the period beginning on January 1, 2021, and ending on September 30, 2021, for
which AMP has been reported under section 1927(b)(3) of the Act, CMS would identify the
payment amount benchmark period as the first calendar year, which would be no earlier than
calendar year 2021, in which such drug has at least 1 quarter of AMP reported. Said differently,
to identify the payment amount benchmark period for the purpose of calculating the benchmark
period manufacturer price for a Part D rebatable drug first approved or licensed on or before
October 1, 2021, CMS would first look to the period from January 1, 2021, to
September 30, 2021 and if no AMP was reported to the MDRP for that 3-quarter period, CMS
would then identify the payment amount benchmark period as the first calendar year no earlier
than calendar year 2021 in which such drug has at least 1 quarter of AMP reported. Similarly, in
proposed § 428.202(c)(4), CMS proposes that for a subsequently approved drug for which there
are no quarters during the first calendar year beginning after the drug’s first marketed date for
which AMP has been reported under section 1927(b)(3), the payment amount benchmark period
would be the first calendar year in which such drug has at least 1 quarter of AMP reported. To
identify the payment amount benchmark period for the purpose of calculating the benchmark
period manufacturer price for a subsequently approved drug, CMS would look to the first
calendar year beginning after the drug’s first marketed date and if no AMP was reported to the
MDRP for such NDC-9 for that 4-quarter period, CMS would then identify the payment amount
benchmark period as the first calendar year in which such drug has at least 1 quarter of AMP
reported. This approach (or the alternative approaches described below), if finalized, would
replace the policy in the revised Medicare Part D Drug Inflation Rebate Guidance to treat Part D
rebatable drugs first approved or licensed on or before October 1, 2021, that were not marketed
until after that date in the same manner as subsequently approved drugs. As proposed in
§ 428.202(b)(2), the first applicable period for such drug would begin on October 1 of the year
following the payment amount benchmark period. This policy would apply to Part D rebatable
drugs first approved or licensed on or before October 1, 2021, drugs first approved or licensed on
or before October 1, 2021, but not marketed until after that date, as well as subsequently
approved drugs.
As an example of how CMS would identify the payment amount benchmark period as
proposed under § 428.202(c)(3), if a Part D rebatable drug that was first approved or licensed by
the FDA on July 7, 2021, and has a first marketed date of September 15, 2021, does not have
AMP reported in the period beginning January 1, 2021 and ending September 30, 2021, but does
have AMP reported for the second calendar quarter of 2022, CMS would identify the payment
amount benchmark period for such drug as calendar year 2022 (that is, January 1, 2022, through
December 31, 2022). In this example, the benchmark period CPI-U would be the CPI-U for
January 2022, the first applicable period would be the applicable period beginning
October 1, 2023, and ending September 30, 2024, and the applicable period CPI-U would be the
CPI-U for October 2023. Similarly, as an example of how CMS would identify the payment
amount benchmark period as proposed under § 428.202(c)(4), if a subsequently approved drug
with a first marketed date of December 15, 2021, does not have AMP reported for any quarters

in calendar year 2022 (that is, the first calendar year after the drug’s first marketed date) but does
have AMP reported for the first calendar quarter of 2023, CMS would identify the payment
amount benchmark period as calendar year 2023 (that is, January 1, 2023, through
December 31, 2023). In this example, the benchmark period CPI-U would be the CPI-U for
January 2023, the first applicable period for this drug would be the applicable period beginning
October 1, 2024, and ending September 30, 2025, and the applicable period CPI-U would be the
CPI-U for October 2024.
CMS is soliciting comments on this approach, as well as the alternatives described below.
(b) Comment Solicitation on Alternatives Considered for Calculating the Benchmark Period
Manufacturer Price When AMP Is Missing
CMS is aware that one reason why a manufacturer may not report AMP for any quarters
of a payment amount benchmark period described in § 428.202(c)(1) or (2), as applicable, is that
a manufacturer may acquire a Part D rebatable drug from another manufacturer and, due to that
acquisition and the use of a new labeler code, obtain a new NDC-9 for that Part D rebatable drug.
In this instance, the NDC-9 of the selling manufacturer and the NDC-9 of the buying
manufacturer belong to the same dosage form and strength and therefore the same Part D
rebatable drug. Although the buying manufacturer may not have AMP for the new NDC-9 to
report to the Medicaid Drug Programs system for the Part D rebatable drug’s payment amount
benchmark period described in § 428.202(c)(1) or (c)(2), the buying manufacturer is required by
the MDRP to report for the new NDC-9 the base date AMP associated with the dosage form and
strength to which the new NDC-9 belongs.
583
This base date AMP is equal to the quarterly AMP
that a manufacturer reports as described in § 447.509(a)(7)(ii)(B). There also may be instances
outside of the acquisition context in which a new NDC-9 for an existing dosage form and
strength is reported to the MDRP. To prevent a manufacturer from resetting the payment amount
583
Medicaid Drug Rebate Program Notice, Release No. 90 (April 18, 2014). https://www.medicaid.gov/medicaid-
chip-program-information/by-topics/prescription-drugs/downloads/rx-releases/mfr-releases/mfr-rel-090.pdf.

benchmark period and therefore the benchmark period manufacturer price by obtaining a new
NDC-9 for the Part D rebatable drug, CMS stated in section 40.2.2 of the revised Medicare Part
D Drug Inflation Rebate Guidance that it will use the benchmark period manufacturer price of
the earliest NDC-9 of the Part D rebatable drug.
After further consideration of this policy and the data that are available to CMS in the
Medicaid Drug Programs system, CMS does not believe that calculating the benchmark period
manufacturer price using the three or four quarters, as applicable, of AMP reported in the
payment amount benchmark period described in proposed § 428.202(c)(1) or (2) of the earliest
NDC-9 of the Part D rebatable drug is operationally feasible at this time. Although the buying
manufacturer is required by the MDRP to report for the new NDC-9 the base date AMP
associated with the earliest NDC-9 of the dosage form and strength, and to report the first
marketed date associated with the earliest NDC-9 of the dosage form and strength as the first
marketed date for the new NDC-9,
584
the buying manufacturer is not required to report which
NDC-9 was used to determine the base date AMP and first marketed date. CMS may therefore
lack the information necessary to identify the earliest NDC-9 of the Part D rebatable drug for
purposes of determining the benchmark period manufacturer price to be used in calculating the
inflation rebate amount.
CMS understands that statutory provisions at section 1860D-14B of the Act require that
CMS establish the payment amount benchmark period at the dosage form and strength level, and
that resetting the payment amount benchmark period for a new NDC-9 of an existing Part D
rebatable drug may not fully align with this directive. Simultaneously, and as described above in
this section III.I.3.c.2.b, CMS understands there may be a gap in the AMP data available to
584
See 88 FR 34238, 34257 (May 26, 2023). Section 1927(c)(2)(A)(ii)(II) of the Act expressly provides that the
base date AMP quarter, with respect to a dosage form and strength of a drug, is established “without regard to
whether or not the drug has been sold or transferred to an entity, including a division or subsidiary of the
manufacturer…” See also the data field definition of market date: “For S, I, and N drugs marketed under an FDA-
approved application (for example, BLA, NDA, ANDA), the earliest date the drug was first marketed under the
application number by any labeler.” https://www.medicaid.gov/sites/default/files/medicaid-chip-program-
information/by-topics/prescription-drugs/downloads/recordspecficationanddefinitions.pdf.
calculate the benchmark period manufacturer price at the dosage form and strength level for
certain drugs. To enable CMS to calculate the benchmark period manufacturer price when a new
NDC-9 of an existing Part D rebatable drug is reported to the MDRP and that NDC-9 lacks AMP
data for the time period described in proposed § 428.202(c)(1) or (2), CMS is soliciting
comments on alternative policy options that are described in more detail below.
First, CMS is soliciting comments on a modified version of the policy described in
section 40.1.2 of the revised Medicare Part D Drug Inflation Rebate Guidance. Under this
modified policy, if a new NDC-9 of an existing Part D rebatable drug is reported to the MDRP,
CMS would calculate the benchmark period manufacturer price for such NDC-9 using the base
date AMP reported by a manufacturer under section 1927(b)(3) of the Act for such Part D
rebatable drug, if such base date AMP was reported for a calendar quarter that overlaps with the
time period described in proposed § 428.202(c)(1) or (2), as applicable for that Part D rebatable
drug. CMS believes this modified policy would be operationally feasible because CMS would
calculate the benchmark period manufacturer price using the base date AMP that is reported with
the new NDC-9; CMS therefore would not need to identify the earliest NDC-9 of the Part D
rebatable drug. Under this policy, CMS would only use the base date AMP to calculate the
benchmark period manufacturer price if the base date AMP was associated with a calendar
quarter that overlapped with the time period described in proposed § 428.202(c)(1) or (2), as
applicable for that Part D rebatable drug. If CMS were to adopt this alternative approach, CMS
would expect to operationalize it through conforming changes to proposed § 428.202(c) and
other applicable proposed regulatory text.
CMS notes that, if CMS were to finalize this alternative approach, CMS would be unable
to use this approach to calculate the benchmark period manufacturer price in the case of a new
NDC-9 of an existing Part D rebatable drug with base date AMP that does not overlap with the
time period described in proposed § 428.202(c)(1) or (2). In such instances, CMS would either
establish a future payment amount benchmark period using an approach similar to that described
in proposed § 428.202(c)(3) and (4) or apply one of the other alternative policies described
below.
Another alternative CMS is considering is to require manufacturers of Part D rebatable
drugs to submit to CMS AMP data for the time period identified under proposed § 428.202(c)(1)
or (2) in cases where the manufacturer did not report AMP to the MDRP under section
1927(b)(3) of the Act for such period but AMP data are available either for the NDC-9 or for
another NDC-9 within the same dosage form and strength. For example, if the quarter for which
a manufacturer reports base date AMP for a new NDC-9 of an existing dosage form and strength
does not overlap with the time period identified under proposed § 428.202(c)(1) or (2), but the
earliest NDC-9 of the dosage form and strength that served as the basis for the base date AMP
has AMP data available during any quarter of that time period, CMS would require
manufacturers to report such AMP data. For a Part D rebatable drug with a payment amount
benchmark period identified under proposed § 428.202(c)(1), a manufacturer would be required
to submit to CMS AMP data for the calendar quarters in the period beginning January 1, 2021,
and ending on September 30, 2021, to the extent such drug was first marketed before
September 30, 2021. For a subsequently approved drug with a payment amount benchmark
period identified under proposed § 428.202(c)(2), a manufacturer would be required to submit to
CMS AMP data for the first calendar year beginning after the drug’s first marketed date. CMS
acknowledges the intersection between a potential reporting requirement under the Medicare
Part D Drug Inflation Rebate Program for manufacturers to provide AMP data and existing AMP
data reporting requirements for manufacturers under the MDRP. Should CMS pursue this option,
CMS would explore using existing AMP reporting processes for the MDRP to operationalize any
new AMP reporting requirement. This approach of requiring manufacturers to report such
information would be consistent with CMS’ understanding of the provisions of section 1860D-
14B of the Act requiring CMS to establish the payment amount benchmark period at the dosage
form and strength level, and with CMS’ authority under sections 1102(a) and 1871(a)(1) of the
Act to make rules and regulations as necessary for the efficient administration of programs,
including the Medicare Part D Drug Inflation Rebate Program. CMS welcomes comments on the
method by which CMS could collect such information, the timing of the potential collection and
deadlines, and whether information reported by manufacturers should be taken into account for
purposes of compiling the Rebate Reports for a Part D rebatable drug or instead only be included
in the reconciliation processes specified in proposed § 428.401(d) and described later in this
proposed rule.
CMS is also considering an alternative policy whereby CMS would calculate the
benchmark period manufacturer price for a new NDC-9 of an existing Part D rebatable drug that
lacks AMP data for the time period described in § 428.202(c)(1) or (2) using a reasonable proxy
metric. CMS welcomes comments on potential proxy metrics CMS could use to calculate the
benchmark period manufacturer price for a new NDC-9 of an existing dosage form and strength
for which no AMP data are reported for such periods.
These alternative policy options are intended to achieve the same goal as the policy
described in section 40.2.2 of the revised Medicare Part D Drug Inflation Rebate Guidance (that
is, to disincentivize a manufacturer from resetting its payment amount benchmark period by
obtaining a new NDC-9 for an existing Part D rebatable drug). CMS also welcomes comments
on the alternative policy described in the revised Medicare Part D Drug Inflation Rebate
Guidance whereby CMS would treat drugs first approved or licensed on or before
October 1, 2021, that were not marketed until after that date in the same manner as subsequently
approved drugs for purposes of establishing the payment amount benchmark period, benchmark
period CPI-U, first applicable period, and first applicable period CPI-U. CMS is soliciting
comments on these alternatives and may adopt one or more of such alternatives in the final rule
based on comments received. Additionally, CMS is seeking comments on other policies that
CMS should consider to prevent manufacturers from inappropriately resetting the payment
amount benchmark period by obtaining a new NDC-9 for an existing Part D rebatable drug.
Under CMS’ proposed policy to identify a payment amount benchmark period in certain
instances of missing AMP as proposed in § 428.202(c)(3) and (4) and each of these alternatives
considered, CMS would consider any restatements to the AMP data used to calculate the
benchmark period manufacturer price during reconciliation, as specified in proposed
§ 428.401(d) and described later in this proposed rule. Furthermore, CMS would also monitor
the extent to which manufacturers obtain a new NDC-9 for the same Part D rebatable drug in a
manner that could result in inappropriately resetting the payment amount benchmark period or
otherwise affect the calculation of the benchmark period manufacturer price. CMS reminds
manufacturers of their reporting obligations under section 1927(b) of the Act and § 447.510 of
this title and that failure to provide timely information may result in penalties as detailed in
section 1927(b)(3)(C)(i) of the Act.
If finalized, CMS would apply the policies described above to rebate calculations
beginning with the applicable period that began on October 1, 2022. CMS has determined that,
consistent with the policy described in section III.I.1, in order to calculate inflation rebates for
Part D rebatable drugs that do not have AMP or other pricing data available under section
1927(b)(3) on which to base the benchmark period manufacturer price, CMS’ proposed policy
must apply for applicable periods beginning with the applicable period that began on
October 1, 2022.
(c) Identification of the Payment Amount Benchmark Period for a Part D Rebatable Drug No
Longer Considered To Be a Selected Drug
In proposed § 428.202(c)(5), CMS proposes to codify policies described in section 40.2.2
of the revised Medicare Part D Drug Inflation Rebate Guidance relating to the identification of
the payment amount benchmark period for a selected drug (as defined in section 1192(c) of the
Act) with respect to a price applicability period (as defined in section 1191(b)(2) of the Act) in
the case such Part D rebatable drug is no longer considered to be a selected drug. The Medicare
Part D Drug Inflation Rebate Program applies to selected drugs notwithstanding the status of the
drug as a selected drug. However, the calculation of certain components of the rebate amount
formula for selected drugs depends upon whether the selected drug has reached the end of its
price applicability period and is no longer considered to be a selected drug under section 1192(c)
of the Act. Specifically, section 1860D-14B(b)(5)(C) of the Act specifies a different payment
amount benchmark period and benchmark period CPI-U for a Part D rebatable drug in the case
such drug is no longer considered to be a selected drug under section 1192(c) of the Act, for each
applicable period beginning after the price applicability period with respect to such drug.
Accordingly, in such a case where a Part D rebatable drug is no longer a selected drug, CMS
proposes in § 428.202(c)(5) that the payment amount benchmark period will be reset as the last
calendar year of such price applicability period for such selected drug.
(3) Calculation of the Benchmark Period Manufacturer Price
CMS proposes in § 428.202(d) that, subject to proposed § 428.202(g), to determine the
benchmark period manufacturer price for a Part D rebatable drug, CMS would use the AMP
reported by a manufacturer to the Medicaid Drug Programs system under sections
1927(b)(3)(A)(i) and (ii) of the Act for each calendar quarter of the payment amount benchmark
period, as identified in accordance with proposed § 428.202(c), as well as the units reported by a
manufacturer under section 1927(b)(3)(A)(iv) of the Act for each month of such payment
amount benchmark period. For a Part D rebatable drug first approved or licensed on or before
October 1, 2021, section 1860D-14B(b)(4) of the Act specifies that the benchmark period
manufacturer price is the sum of the products of (1) the AMP for the Part D rebatable drug
reported for each calendar quarter of the payment amount benchmark period (that is,
January 1, 2021, through September 30, 2021), and (2) the total units reported for each of the
corresponding calendar quarters of the payment amount benchmark period divided by the total
units of the Part D rebatable drug reported for the 3 calendar quarters in the payment amount
benchmark period. The following formula illustrates how CMS would calculate the benchmark

period manufacturer price for a Part D rebatable drug with a payment amount benchmark period
identified under proposed § 428.202(c)(1), as proposed in § 428.202(d)(1):
(AMP for calendar quarter beginning January 2021) multiplied by (sum of monthly AMP
units for January 2021 calendar quarter divided by sum of the units reported for the
3 quarters of the payment amount benchmark period) +
(AMP for calendar quarter beginning April 2021) multiplied by (sum of monthly AMP
units for April 2021 calendar quarter divided by sum of the units reported for the
3 quarters of the payment amount benchmark period) +
(AMP for calendar quarter beginning July 2021) multiplied by (sum of monthly AMP
units for July 2021 calendar quarter divided by sum of the units reported for the 3 quarters
of the payment amount benchmark period)
For a Part D rebatable drug with a payment amount benchmark period identified under
proposed § 428.202(c)(2) through (5), the following formula illustrates how CMS would
calculate the benchmark period manufacturer price for a Part D rebatable drug as proposed in
§ 428.202(d)(2):
(AMP for calendar quarter beginning January) multiplied by (sum of monthly AMP units
for January calendar quarter divided by sum of the monthly units reported for the
4 quarters of the payment amount benchmark period) +
(AMP for calendar quarter beginning April) multiplied by (sum of monthly AMP units
for April calendar quarter divided by sum of the monthly units reported for the 4 quarters
of the payment amount benchmark period) +
(AMP for calendar quarter beginning July) multiplied by (sum of monthly AMP units for
July calendar quarter divided by sum of the monthly units reported for the 4 quarters of
the payment amount benchmark period) +
(AMP for calendar quarter beginning October) multiplied by (sum of monthly AMP units
for October calendar quarter divided by sum of the monthly units reported for the
4 quarters of the payment amount benchmark period)
(4) Identification of the Benchmark Period CPI-U
To calculate the inflation-adjusted payment amount in accordance with section
1860D-14B(b)(3), CMS must identify the benchmark period CPI-U. As described in the revised
Medicare Part D Drug Inflation Rebate Guidance and in accordance with section 1860D-
14B(g)(4), CMS proposes in § 428.202(e)(1) that the benchmark period CPI-U for a Part D
rebatable drug first approved or licensed by the FDA on or before October 1, 2021, would be the
CPI-U for January 2021. For a subsequently approved drug, CMS proposes in § 428.202(e)(2)
that the benchmark period CPI-U would be the CPI-U for January of the first calendar year
beginning after the drug’s first marketed date, as required under section 1860D-14B(b)(5)(A).
As described earlier in this proposed rule, CMS has observed that a number of NDC-9s of
Part D rebatable drugs approved or licensed on or before October 1, 2021, do not have AMP
reported in the period beginning January 1, 2021, and ending September 30, 2021, and a number
of NDC-9s of subsequently approved drugs do not have AMP reported in the first calendar year
following the drug’s first marketed date. To enable CMS to calculate the benchmark period
manufacturer price and inflation rebate amounts for these NDC-9s, CMS is proposing in
§ 428.202(c)(3) and (4) to identify the payment amount benchmark period for such NDC-9s as
the first calendar year, which would be no earlier than calendar year 2021, in which such drug
has at least 1 quarter of AMP data reported. As previously discussed, CMS is soliciting
comments on alternative methodologies to identify the payment amount benchmark period and
calculate the benchmark period manufacturer price to address certain instances in which AMP
has not been reported and, if any such alternatives are adopted, CMS may adopt conforming
modifications to proposed § 428.202(e). To identify the benchmark period CPI-U for an NDC-9
described in proposed § 428.202(c)(3), CMS further proposes in § 428.202(e)(3) that for a Part D
rebatable drug first approved on or before October 1, 2021, for which there are no quarters
during the period beginning on January 1, 2021, and ending on September 30, 2021, for which
AMP has been reported to the MDRP, the benchmark period CPI-U would be the CPI-U for
January of the calendar year in which such drug has at least 1 quarter of AMP reported. CMS
proposes in § 428.202(e)(4) that for a subsequently approved drug for which there are no
quarters during the first calendar year beginning after the drug’s first marketed date for which
AMP has been reported to the MDRP, the benchmark period CPI-U is the CPI-U for January of
the calendar year in which such drug has at least 1 quarter of AMP reported.
As discussed previously, the Medicare Part D Drug Inflation Rebate Program applies to
selected drugs notwithstanding the status of the drug as a selected drug. However, the calculation
of certain components of the applicable rebate amount formula for selected drugs depends upon
whether the selected drug has reached the end of its price applicability period and is no longer
considered to be a selected drug under section 1192(c) of the Act. In accordance with section
1860D-14B(b)(5)(C) of the Act, in such a case where a Part D rebatable drug is no longer a
selected drug, CMS proposes in § 428.202(e)(5) that the benchmark period CPI-U will be the
CPI-U for January of the last calendar year of such price applicability period.
(5) Calculation of the Inflation-Adjusted Payment Amount
As specified in section 1860D-14B(b)(3) of the Act and described in section 40.2.3 of the
revised Medicare Part D Drug Inflation Rebate Guidance, the inflation-adjusted payment amount
with respect to a Part D rebatable drug and applicable period is the benchmark period
manufacturer price increased by the percentage by which the applicable period CPI-U exceeds
the benchmark period CPI-U. CMS proposes in § 428.202(f) to calculate the inflation-adjusted
payment amount for a Part D rebatable drug by dividing the applicable period CPI-U by the
benchmark period CPI-U and then multiplying the quotient by the benchmark period
manufacturer price. The following formula illustrates how CMS would calculate the
inflation-adjusted payment amount for a Part D rebatable drug as proposed in § 428.202(f):
(Benchmark period manufacturer price) multiplied by (applicable period CPI-U divided
by benchmark period CPI-U)
As described earlier in this proposed rule and proposed in § 428.202(a), CMS will use the
inflation-adjusted payment amount to calculate the per unit Part D drug inflation rebate amount
by determining the amount by which the AnMP for a Part D rebatable drug exceeds the
inflation-adjusted payment amount for a Part D rebatable drug for an applicable period.
(6) Situations in which Manufacturers Do Not Report Units under Section 1927(b)(3)(A)(iv)
Section 1860D-14B of the Act generally requires CMS to determine the per unit Part D
drug inflation rebate amount using the monthly units reported by manufacturers to the Medicaid
Drug Programs system under section 1927(b)(3)(A)(iv) of the Act. CMS understands it is
possible that a manufacturer may not have sales or monthly units of a COD to report to the
Medicaid Drug Programs system for a calendar quarter because, for example, there may be a
temporary interruption in sales of the COD, or there may be no sales immediately after the drug
is first approved or licensed by the FDA. CMS proposes in § 428.202(g)(1) to codify the policy
described in section 40.1.2 of the revised Medicare Part D Drug Inflation Rebate Guidance,
whereby in cases where there are 1 or more quarter(s) in the payment amount benchmark period
or applicable period for which a manufacturer has not reported units under section
1927(b)(3)(A)(iv) of the Act but has reported AMP under sections 1927(b)(3)(A)(i) and (ii) of
the Act, CMS would calculate the benchmark period manufacturer price or AnMP, as applicable,
using data only from quarter(s) with units. That is, quarter(s) in the payment amount benchmark
period or applicable period for which a manufacturer has not reported units under section
1927(b)(3)(A)(iv) of the Act would be excluded from the calculation. CMS proposes in
§ 428.202(g)(2) to codify the policy described in section 40.1.2 of the revised guidance whereby
if there are no quarters of the payment amount benchmark period or applicable period for which
a manufacturer has reported units under section 1927(b)(3)(A)(iv) of the Act, but the
manufacturer has reported AMP under sections 1927(b)(3)(A)(i) and (ii) of the Act for at least

1 quarter of such period, CMS would use the average of the AMP over the calendar quarters of
the payment amount benchmark period or applicable period for which AMP is reported to
calculate the benchmark period manufacturer price or AnMP, respectively.
iv. Determination of the Total Number of Units Dispensed Under Part D
In § 428.203(a), CMS proposes to codify the existing policy established in the revised
Medicare Part D Drug Inflation Rebate Guidance whereby CMS would determine the total
number of units of each Part D rebatable drug dispensed under Part D and covered by Part D
sponsors based on information reported to CMS by Part D plan sponsors on the Part D PDE
records for the 12-month applicable period. More specifically, CMS would determine the total
number of units from the Quantity Dispensed field on the PDE record for each Part D rebatable
drug with gross covered prescription drug costs greater than zero. Because the PDE record does
not provide the unit type used to determine Quantity Dispensed, CMS proposes in
§ 428.203(a)(2) that CMS would crosswalk the information from the PDE record to a drug
database that provides the unit type for an NDC, such as Medi-Span or the FDA’s
Comprehensive NDC Structured Product Labeling (SPL) Data Element (NSDE) file, matching
on the NDC of the Part D rebatable drug. CMS understands that in limited instances, the unit
type obtained from such drug databases may not match the AMP unit type reported by
manufacturers to the Medicaid Drug Programs system, and in these cases, CMS would convert
the total units reported on the PDE record to the AMP units reported to the Medicaid Drug
Program system.
CMS conducts a thorough review of PDE records, which includes the identification of
outliers in the quantity dispensed field of PDE records, as part of the Part D payment
reconciliation process that occurs between CMS and plan sponsors each year.
585
CMS intends to
rely on this payment reconciliation process, through which Part D plan sponsors have an
opportunity to correct PDE records flagged by CMS as containing potential outliers, to resolve
585
See https://www.hhs.gov/guidance/document/pde-analysis-process-withheld-and-invoiced-outlier-pdes.
outliers that would otherwise impact the Part D drug inflation rebate amount calculated under
proposed § 428.201(a). Because PDE records are not updated to reflect the resolution of outliers
identified through the Part D payment reconciliation process for a given calendar year until after
CMS plans to send Rebate Reports for the applicable period (capturing data that include the first
three quarters of that calendar year), the Rebate Report will not reflect the resolution of unit
outliers identified through the Part D payment reconciliation process. However, because CMS
intends to conduct a reconciliation of the rebate amount with additional PDE run-out (as
proposed in § 428.401(d) and described later in this proposed rule), the reconciled rebate
amounts will reflect the resolution of any unit outliers corrected by Part D plan sponsors through
the Part D payment reconciliation process. CMS does not intend to conduct separate outlier
analysis of PDE for the purposes of the Medicare Part D Drug Inflation Rebate Program, but
CMS did consider several adjustments to reduce the effect of outliers not resolved through the
Part D payment reconciliation process, including removal of PDE records that were identified by
CMS as having potential outlier quantity dispensed fields but were neither corrected nor verified
by Part D plan sponsors, removal of the quantity dispensed field for certain records at or above a
certain statistically derived threshold, and imputing quantity dispensed values for such records.
CMS is soliciting comments on this proposed approach to rely on CMS’ existing review of PDE
records, as well as on the adjustments considered to reduce the effect of outliers not resolved
through the Part D payment reconciliation process.
As proposed in § 428.203(b), CMS would remove from the total number of units any
units of a generic drug dispensed on or after the date that such generic drug no longer meets the
definition of a Part D rebatable drug, as well as units acquired through the 340B Program, as
described in section III.I.3.c of this proposed rule. CMS invites comments on any additional units
that should be excluded from the rebate amount calculation.
(1) Removal of Units When a Generic Drug Is No Longer a Part D Rebatable Drug
In § 428.203(b)(1), CMS proposes to codify the policy established in section 40.2.8 of the
revised Medicare Part D Drug Inflation Rebate Guidance to exclude from the rebate calculation
any units of a generic drug dispensed on or after the date that such generic drug no longer meets
the definition of a Part D rebatable drug. To determine whether a generic drug that meets the
definition of a Part D rebatable drug on the first day of an applicable period ceases to meet such
definition later in the applicable period, CMS will use the most recent version of the
downloadable FDA Orange Book to identify whether FDA has approved a 505(j) ANDA for a
drug that is rated as therapeutically equivalent to such generic drug. If CMS determines that FDA
has approved such a therapeutically equivalent drug under a 505(j) ANDA, CMS will then use
the NDC Directory, including historical information from NDC Directory files such as
discontinued, delisted, and expired listings provided by FDA or published on the FDA website to
determine the marketing status of such therapeutically equivalent drug and to determine whether,
during the applicable period, the therapeutically equivalent drug was marketed. Similarly, CMS
will use the NDC Directory to identify whether the reference listed drug, or an authorized
generic of the reference listed drug was marketed during the applicable period. CMS will
exclude from the rebate calculation any units dispensed on or after the first day of the calendar
month that a generic drug no longer meets the definition of a Part D rebatable drug. CMS intends
to apply this unit exclusion at the month level and would exclude all units of a generic drug that
ceases to meet the definition of a Part D rebatable drug beginning with the first day of the first
month when a therapeutically equivalent drug approved under a 505(j) ANDA is marketed based
on the marketing start date in the NDC Directory or when the reference listed drug, or an
authorized generic of the reference listed drug is marketed based on the marketing start date in
the NDC Directory. CMS proposes to apply this exclusion each calendar month because the
Orange Book downloadable data files are updated monthly.
(2) Exclusion of 340B Acquired Units from Part D Rebatable Drug Requirements
Section 1860D-14B(b)(1)(B) of the Act requires that beginning with plan year 2026,
CMS shall exclude from the total number of units for a Part D rebatable drug, with respect to an
applicable period, those units for which a manufacturer provides a discount under the 340B
Program. Because this requirement starts after the first quarter of the applicable period that
begins on October 1, 2025, the exclusion of 340B units would only apply for the last three
quarters of this applicable period. That is, CMS would exclude 340B units starting on
January 1, 2026.
Data on which units dispensed under Part D and covered by Part D plan sponsors were
purchased under the 340B Program is unavailable under the data sources specified at section
1860D-14B(d) of the Act (that is, information submitted by manufacturers, States, and Part D
plan sponsors), and CMS does not currently have access to this data through other means. CMS
understands that the 340B status of a Part D drug is usually not known by the dispenser at the
point-of-sale, and that 340B covered entities (hereinafter “covered entities”) typically identify
the 340B status of a Part D drug retrospectively. Because the covered entity and CMS do not
exchange dispensed Part D drug information confirming the 340B status of a Part D rebatable
drug, CMS is unable to identify 340B units at the claim-level at this time. For these reasons, CMS
believes it necessary to establish an estimation methodology to remove 340B units from the total
number of units for a Part D rebatable drug, as described in this section.
(a) Estimation Methodology to Remove 340B Units from Rebate Calculations
To fulfill the statutory requirement to remove 340B units from rebate calculations
beginning on January 1, 2026, CMS is proposing in § 428.203(b)(2) a new policy to remove units
from the total number of units dispensed of a Part D rebatable drug for each applicable period
based on a calculated percentage that reflects the portion of 340B purchasing relative to total sales.
CMS proposes the percentage (hereinafter, “estimation percentage”) to equal the total number of
units purchased by covered entities under the 340B Program for an NDC-9, divided by the total

units sold of that NDC-9. An example calculation for a Part D rebatable drug for a given applicable
period is shown below for illustrative purposes:
Total number of units dispensed under Part D determined under § 428.203(a), minus the
units determined under § 428.203(b)(1): 1,000
Estimation percentage:
Total number of units purchased by covered entities under the 340B Program:
5,000
Total units sold: 50,000
5,000 divided by 50,000 = 10 percent
340B units excluded under § 428.204(b)(2): 10 percent multiplied by 1,000 = 100
The proposed estimation policy is consistent with CMS’ authority under
section 1860D-14B(b)(1)(B) of the Act and sections 1102(a) and 1871(a)(1) of the Act, the latter
of which provide the authority to make rules and regulations as necessary for the efficient
administration of programs, including the Medicare Part D Drug Inflation Rebate Program.
Because the statutory requirement to remove 340B units from rebate calculations does not begin
until January 1, 2026, for the applicable year that begins on October 1, 2025, CMS proposes to
apply the estimation percentage only to those units associated with claims with dates of service in
the last three quarters of the applicable period (that is, January 1, 2026, through
September 30, 2026).
To identify the numerator of the estimation percentage (that is, the total number of units
purchased under the 340B Program for an NDC-9), CMS proposes to use data from HRSA’s
Prime Vendor Program (PVP). Certain supply chain entities report 340B unit data to the PVP at
the NDC-11 level, and based on the data received, CMS would aggregate these data at the
NDC-9 level
586
to identify the total number of 340B units of a Part D rebatable drug that covered
586
NDC-9 and NDC-11 numbers are identical except for two numbers in NDC-11s that indicate package size.
Because of this, NDC-11 is more granular than NDC-9, and multiple NDC-11 numbers can aggregate under a single
NDC-9 number.
entities purchased in a given time period. CMS proposes to work with HRSA to obtain the
necessary data from the PVP. CMS understands that there are limitations of using the PVP data,
including that some covered entities may choose not to participate in the PVP, and CMS would not
have access to 340B purchases reported by supply chain entities for this share of covered entities.
Further, certain 340B purchases may not be reported to the PVP if those purchases were made
through alternative distribution models such as a covered entity purchasing directly from a
manufacturer, certain specialty distribution channel purchases, or drugs that receive a 340B
rebate under the Ryan White HIV/AIDS Program’s AIDS Drug Assistance Program. CMS is
soliciting comments on what other data sources may be available to calculate the numerator of the
estimation percentage. CMS is also soliciting comments on how it could account for potential
underreporting of 340B units if data are not available on certain 340B purchases, such as those
described above, that may not be reported to the PVP.
To identify the denominator of the estimation percentage (that is, the total units sold of an
NDC-9), CMS proposes to use existing manufacturer reporting under the Medicaid Drug Rebate
Program (MDRP) of unit sales. Specifically, CMS proposes to use the total number of units that
are used to calculate the monthly AMP and which manufacturers are required to report to CMS for
each covered outpatient drug (COD) in accordance with section 1927(b)(3)(A)(iv) of the Act. CMS
believes that using these unit data to calculate an estimation percentage is consistent with the use of
these same data to calculate the AnMP in proposed § 428.202(b) and the benchmark period
manufacturer price in proposed § 428.202(d).
CMS recognizes the importance of ensuring that the numerator and denominator of the
proposed estimation percentage reflect the same time period of sales for units dispensed in the
same settings. CMS acknowledges that the proposed data source for the numerator (PVP data)
reflects purchases by covered entities that dispense or administer 340B-eligible drugs in retail
community pharmacies and in outpatient settings. The proposed data source for the denominator
(unit sales used to calculate AMP) represents, in accordance with the definition of AMP at section
1927(k)(1) of the Act, (1) manufacturer sales to wholesalers for drugs distributed to retail
community pharmacies, and (2) manufacturer sales to retail community pharmacies that purchase
drugs directly from the manufacturer. Therefore, the numerator of the proposed estimation
percentage represents 340B units dispensed in multiple settings, whereas the denominator
represents units typically dispensed only in the retail community pharmacy setting. CMS welcomes
evidence demonstrating how 340B dispensing rates differ between the retail community pharmacy
setting versus multiple settings and may consider adjusting the estimation percentage to reflect
variation between the percentage of 340B units dispensed in multiple settings (that is, retail
community pharmacies and outpatient settings) and the percentage of 340B units dispensed in only
the retail community pharmacy setting. The proposed regulatory text at § 428.203(b)(2) would be
subject to any such adjustment factor that may be adopted.
CMS also recognizes that the proposed estimation percentage represents the total number
of 340B units dispensed as a proportion of total units dispensed, irrespective of insurance/payor
type. CMS is soliciting comments on whether the agency should further adjust the percentage of
340B units dispensed to the general population to estimate the percentage of 340B units dispensed
to Part D beneficiaries for claims with dates of service on or after January 1, 2026, including
comments on how the percentage of 340B units dispensed to the general population compares with
the percentage of 340B units dispensed to Part D beneficiaries. CMS welcomes evidence that
demonstrates how these percentages differ. CMS will consider this information in developing its
final policies and may consider adjusting the estimation percentage to reflect variation between the
percentage of 340B units dispensed to Part D beneficiaries and the percentage of 340B units
dispensed to the general population. The proposed regulatory text at § 428.203(b)(2) would be
subject to any such adjustment factor that may be adopted. CMS is also soliciting comments on
whether there are other circumstances for which CMS should apply an adjustment factor to the
estimation percentage.
CMS considered using alternative data sources to calculate the estimation percentage. To
identify the total number of units purchased under the 340B Program to use in the numerator of
the estimation percentage, CMS considered requiring other entities throughout the
pharmaceutical supply chain, including manufacturers, to report these data to CMS. An
advantage of this approach is that manufacturers could provide data directly on total 340B units
sold; in other words, this data would capture the limited 340B sales that the PVP data does not
capture. A disadvantage of this approach is that not all manufacturers of Part D rebatable drugs
may have existing mechanisms for tracking 340B sales for Medicare Part D, which could
necessitate that new tracking and reporting mechanisms be created. At this time, CMS is not
proposing this alternative because CMS would prefer to rely on data that are already reported to
the PVP, as using these data would help to minimize reporting burdens and may result in cleaner
and more accurate data due to the quality checks performed on the PVP data for purposes of
compliance with the 340B Program. For example, audit and price integrity checks are performed
on the PVP data to ensure the distributors submit and code the data correctly.
To identify the total units sold to use in the denominator of the estimation percentage,
CMS similarly considered establishing a new requirement for other entities throughout the
pharmaceutical supply chain, including manufacturers, to report these data to CMS. An
advantage of this approach is that the denominator would represent sales that are ultimately
dispensed in retail community pharmacy settings and in outpatient settings (whereas, as
mentioned previously, unit reporting under the MDRP represents units typically dispensed only
in the retail community pharmacy setting). A disadvantage of this approach is that it could
necessitate that new tracking and reporting mechanisms be created. At this time, CMS is not
proposing this alternative as CMS believes that relying upon existing manufacturer reporting of
unit sales reported with AMP under the MDRP would be preferable to a new reporting option and
would help minimize reporting burden. Further, the use of unit sales reported with AMP may
provide cleaner and more accurate data than establishing a new manufacturer reporting

requirement since manufacturers must certify their AMP reporting, in accordance with
§ 447.510(e), and are subject to civil money penalties for false or inaccurate reporting, in
accordance with section 1927(b)(3)(B) of the Act. CMS also considered using data on unit sales
available in a nationally representative and commercially available database but one
disadvantage of this option would be that CMS would be unable to audit the quality of data
available through such a database.
(b) Comment Solicitation on a Medicare Part D Claims Data Repository
In the initial Medicare Part D Drug Inflation Rebate Guidance, CMS solicited comments
on the best mechanism to identify 340B units dispensed under Part D.
587
CMS discussed
requiring the dispensing entity to include a 340B claims indicator on the Part D drug claim to be
included in PDE records. Many commenters disagreed that the PDE record was the most
accurate way to identify 340B discounts for Part D drugs. A few commenters highlighted the
operational challenges, administrative burden, and potential for increased dispensing fees and
reimbursement issues with both point-of-sale modifiers and retrospective 340B identifiers. In
addition, a wide array of interested parties recommended that CMS create a mechanism through
which covered entities would retrospectively submit data to CMS identifying 340B claims
dispensed under Part D. Interested parties urged that this mechanism allow covered entities to
submit these data directly to CMS, rather than through claims that dispensers submit via Part D
plan sponsors.
In response to this feedback from interested parties, CMS is soliciting comments on
establishing a Medicare Part D claims data repository (hereinafter, “repository”) in a future year
of the Medicare Part D Drug Inflation Rebate Program to comply with the requirement under
section 1860D-14B(1)(B) of the Act that CMS shall exclude from the total number of units for a
Part D rebatable drug those units for which a manufacturer provides a discount under the 340B
Program. This approach would require that covered entities submit certain data elements from
587
See: https://www.cms.gov/files/document/medicare-part-d-inflation-rebate-program-initial-guidance.pdf.
340B-identified Part D claims to the repository. CMS solicits comments on such a requirement
later in this section.
As described later in this section, a repository could receive data elements submitted by
covered entities from 340B-identified claims for all drugs covered under Medicare Part D billed
to Medicare. As requested by interested parties in comments on the initial Medicare Part D Drug
Inflation Rebate Guidance, the repository could allow covered entities to submit these data
directly to CMS (or a contractor), rather than through claims that dispensers submit to Part D
plan sponsors. CMS could consider all data elements received by the repository to be associated
with 340B-identified claims; that is, the repository would not further verify the 340B status of a
claim but rather would serve solely to store these data. Under this process, CMS could require an
attestation from covered entities that the data elements from all claims submitted to the
repository are from verified 340B claims. CMS is exploring approaches to confirming
completeness and accuracy of the submission, and CMS is soliciting comments on methods to
review and ensure the accuracy of reported data. CMS could then match the stored data elements
to PDE records for each Part D rebatable drug dispensed during the applicable period. Units
associated with PDE records that match to data elements stored in the repository could be
considered those for which the manufacturer provides a discount under the 340B Program and
therefore removed from the total number of units used to calculate the total rebate amount.
(c) Comment Solicitation on Requiring Covered Entities to Submit 340B Claims Data to the
Repository
CMS is soliciting comments on using its authority under section 1860D-14B(b)(1)(B) of
the Act, as well as its authorities under sections 1102(a) and 1871(a)(1) of the Act, to require
covered entities to enroll in a repository and submit certain data elements from 340B-identified
claims for all covered Part D drugs billed to Medicare to this repository. CMS understands
covered entities typically contract with 340B third-party administrators (340B TPAs) to
determine 340B eligibility of claims using data submitted by covered entities and their contract

pharmacies.
588
CMS welcomes comments on whether or how, to the extent a covered entity uses
a 340B TPA, CMS could require or encourage TPAs to submit certain data elements to the
repository on behalf of that covered entity.
Requiring covered entities to submit data elements from 340B-identified Part D claims to
the repository could allow CMS to receive data directly from the entities that participate in the
340B Program to identify 340B units to exclude from Part D drug inflation rebate calculations
without intermediary entities needing to develop processes to capture these data and relay it to
CMS. CMS is considering requiring covered entities to submit the following data elements from
Part D claims for covered Part D drugs that are purchased under the 340B Program and
dispensed to Medicare Part D beneficiaries: (1) Date of Service (that is, the date the prescription
was filled by the pharmacy); (2) Prescription or Service Reference Number; (3) Fill Number
(that is, the code indicating whether the prescription is an original or a refill; if a refill, the code
indicates the refill number); and (4) Dispensing Pharmacy NPI. CMS believes that these would
be the minimum data elements required to match claims and remove 340B units from Part D
drug inflation rebate calculations. CMS is soliciting comments from interested parties on this list
of data elements and whether these data elements would be accessible to covered entities to
submit to CMS.
(d) Comment Solicitation on Timing Requirements for Potential Submissions to a Medicare Part
D Claims Data Repository
CMS is soliciting comments on requiring covered entities to submit the fields specified
by CMS to the repository within 3 months of the end of a given calendar quarter. For example,
for claims with dates of service between October 1, 2027, through December 31, 2027, covered
entities would be required to submit data elements from 340B-identified claims to CMS no later
than March 31, 2028. The 340B units identified from these quarterly submissions could be
588
Covered entities may elect to dispense 340B drugs to patients through contract pharmacy services, an
arrangement in which the covered entity enters a contract with the pharmacy to provide pharmacy services.
removed from the total number of units and total rebate amount specified in the Preliminary
Rebate Report and Rebate Report detailed in proposed § 428.401(b) and (c), respectively.
In accordance with the proposed regulation at § 428.401(d) to reconcile the rebate
amount in the case of revised information, including a reconciliation of the total number of units
detailed at proposed § 428.401, CMS is soliciting comments on providing covered entities with
additional time to submit data to reflect a revision to the 340B determination of claims with dates
of service throughout an applicable period. A revision could come in one of two forms: (1)
resubmission of data for a claim that the covered entity previously submitted to a repository in
error or with errors in the requested data fields, or (2) new submission of data for a claim that the
covered entity had previously determined was not purchased under the 340B Program, but later
identified was purchased under such program. For the first type of revision, CMS is soliciting
comments on requiring that the covered entity resubmit the data from such claim using a field to
indicate that such data should be removed from the repository’s dataset of 340B-identified
claims; if applicable, the covered entity could resubmit the claim with the correct information.
CMS is soliciting comments on the process and timing for covered entities to submit this revised
data to the repository after the end of the applicable period. Updates to the total number of units
and total rebate amount based on this revised information from covered entities would be
reflected in the reconciliation process detailed at proposed § 428.401(d).
CMS is soliciting comments from interested parties on the feasibility of the proposed
quarterly reporting timeline for covered entities to submit data elements from Part D 340B
claims, as well as the additional time to submit data to reflect a revision to the 340B
determination of claims.
(e) Alternative Policy Considered: 340B Claims Identifier
As described in section 40.2.7 of the initial Medicare Part D Drug Inflation Rebate
Guidance, CMS considered requiring that a 340B indicator be included on the PDE record at the
time of dispense to identify drugs purchased under the 340B Program that were dispensed under

Medicare Part D. As described in the “Summary of Public Comments on the Initial Medicare
Part D Drug Inflation Rebates Memorandum and CMS’ Responses” in the revised Medicare Part
D Drug Inflation Rebate Guidance, many commenters—including covered entities, pharmacies,
Part D plan sponsors, and pharmacy benefit managers—disagreed that the PDE record would be
the most accurate way to identify 340B discounts for Part D drugs. A few commenters
highlighted the operational challenges, administrative burden, and potential for increased
dispensing fees and reimbursement issues with 340B claim identifiers. After further
consideration of comments received in response to the initial guidance and of the process
through which a claim is determined to have 340B status, CMS is no longer pursuing this policy
at this time but may consider it in future rulemaking.
v. Treatment of New Formulations of Part D Rebatable Drugs
Section 1860D-14B(b)(5)(B)(i) of the Act requires CMS to determine a formula for the
rebate amount and the inflation-adjusted payment amount for a Part D rebatable drug that is a
line extension of a Part D rebatable drug that is an oral solid dosage form for an applicable
period that is consistent with the formula applied under section 1927(c)(2)(C) of the Act for
determining a rebate obligation for a rebate period under such section. Section 1927(c)(2)(C) of
the Act provides for an alternative rebate calculation for line extension drugs under the MDRP,
and CMS issued guidance on how this calculation is performed for these purposes.
589
Section 1860D-14B(b)(5)(B)(ii) of the Act further states that for a Part D rebatable drug,
the term line extension means, “a new formulation of the drug, such as extended release
formulation, but does not include an abuse-deterrent formulation of the drug (as determined by
the Secretary), regardless of whether such abuse-deterrent formulation is an extended release
formulation.” This language is identical to the definition of “line extension” in section
1927(c)(2)(C) of the Act. Regulatory definitions of “line extension” and “new formulation” for
589
See: https://www.medicaid.gov/medicaid/prescription-drugs/medicaid-drug-rebate-program/unit-rebate-
calculation/unit-rebate-amount-calculation-for-line-extension-drugs-with-example/index.html.

the MDRP were adopted through rulemaking
590
and can be found at § 447.502. In alignment
with CMS’ policy in section 40.4 of the revised Medicare Part D Drug Inflation Rebate
Guidance, CMS proposes in § 428.200 to adopt the definitions of “line extension” and “new
formulation” at § 447.502 of this title for the purposes of identifying new formulations of Part D
rebatable drugs.
In proposed § 428.204, CMS would determine the total rebate amount to be paid by
manufacturers by taking the greater of (1) the total rebate amount calculated in proposed
§ 428.201(a) for the applicable period for the Part D rebatable drug that is a line extension, or (2)
the alternative total rebate amount. This proposal is a modification to policy established in
revised Medicare Part D Drug Inflation Rebate Guidance. While the revised guidance stated that
CMS would compare the per unit rebate amount to the alternative per unit rebate amount, as
proposed in § 428.204, CMS would compare the total rebate amount calculated in proposed
§ 428.201(a) to the alternative total rebate amount, which CMS believes is consistent with the
existing regulations for new formulations at § 447.509(a)(4). CMS further proposes in § 428.204
to codify the policy described in section 40.4 of the revised guidance to calculate the alternative
inflation rebate amount for a Part D rebatable drug that is a line extension consistent with the
formula applied under section 1927(c)(2)(C) of the Act. That is, CMS would determine an
inflation rebate amount ratio for the initial drug identified by the manufacturer in accordance
with § 447.509(a)(4)(i)(B) by dividing the inflation rebate amount for that initial drug for the
applicable period by the AnMP for that initial drug for the applicable period, as calculated under
proposed § 428.202(b).
To identify the initial drug for the line extension, CMS would use information from the
Medicaid Drug Program system and identify line extensions based on manufacturer reporting of
drugs as line extensions and related pricing and product data in that system. CMS notes that
Medicaid rebates are calculated quarterly, and a different initial drug may be identified in
590
See: 85 FR 87000, 87101 (December 31, 2020).
different quarters by the manufacturer for a particular line extension drug. Part D drug inflation
rebates are calculated based on a 12-month applicable period, meaning there may be instances
where a Part D rebatable line extension drug has multiple potential initial drugs during the
applicable period that could be used for the alternative inflation rebate amount calculation. In
such situations, for consistency, CMS will use the initial drug identified by the manufacturer in
the last quarter of the Part D inflation rebate applicable period to identify the initial drug for the
line extension drug alternative inflation rebate calculation. If an initial drug was not identified in
the last quarter for a drug that is a line extension, CMS will use the initial drug identified for a
quarter most recently in that applicable period to identify the initial drug for the line extension
drug alternative inflation rebate calculation.
d. Reducing the Rebate Amount for Part D Rebatable Drugs in Shortage and When There Is a
Severe Supply Chain Disruption or Likely Shortage (§§ 428.300 through 428.303)
Section 1860D-14B(b)(1)(C) of the Act requires the Secretary to reduce or waive the
rebate amount owed by a manufacturer for a Part D rebatable drug with respect to an applicable
period in three distinct cases: (1) when a Part D rebatable drug is described as currently in
shortage on a shortage list in effect under section 506E of the FD&C Act at any point during the
applicable period; (2) when CMS determines there is a severe supply chain disruption during the
applicable period for a generic Part D rebatable drug or biosimilar, such as a disruption caused
by a natural disaster or other unique or unexpected event; and (3) when CMS determines that
without such a reduction or waiver, a generic Part D rebatable drug is likely to be described as in
shortage on such shortage list during a subsequent applicable period. The statute does not
describe how CMS should reduce or waive inflation rebates.
To implement the statutory requirement under section 1860D-14B(b)(1)(C), CMS
proposes to codify in subpart D of part 428 existing policies described in sections 40.5, 40.5.1,
40.5.2, and 40.5.3 of the revised Medicare Part D Drug Inflation Rebate Guidance to reduce the
total rebate amount owed by a manufacturer in each of these three cases, as summarized in
Table 51 and discussed later in this section.

TABLE 51: Determination of Rebate Reduction Amount for Part D Rebatable
Drugs
As proposed, CMS would not fully waive the rebate amount owed in any case. CMS
believes the proposed rebate reduction policies balance providing appropriate financial relief for
manufacturers in certain circumstances, including when there is a severe supply disruption
resulting from exogenous circumstances outside of a manufacturer’s control, while not
incentivizing manufacturers to delay taking appropriate steps to resolve a drug shortage or severe
supply chain disruption, or maintain a situation in which a generic would be at risk of shortage to
avoid an obligation to pay rebates. CMS will continue to evaluate these policies and may update
them in future years. CMS underscores that most shortages involve multiple source generic
drugs,
591
which are not Part D rebatable drugs and thus are not subject to Part D drug inflation
rebates. CMS solicits comments on the proposed approach.
i. Definitions
CMS proposes in § 428.300 to define the following terms applicable to subpart D
(§§ 428.300 through 428.303):
● “Biosimilar”.
● “Drug shortage” or “shortage”.
591
See: https://www.iqvia.com/insights/the-iqvia-institute/reports-and-publications/reports/drug-shortages-in-the-
us2023.
Drug Shortage
Severe Supply Chain
Disruption
Likely to be in
Shortage
Duration of
Reduction
Indefinite for as long as drug is “currently in
shortage”
One applicable period; manufacturer may
request an extension for an additional
applicable period for up to two applicable
periods total
Percent Reduction
Part D rebatable
drug other than a
plasma-derived
product or generic
Part D rebatable
drug
Part D rebatable
plasma-derived
product or generic
Part D rebatable drug
Part D rebatable
biosimilar or generic
Part D rebatable drug
Generic Part D
rebatable drug
First applicable
period
25%
75%
75%
75%
Second applicable
period
10%
50%
75%
75%
Subsequent applicable
periods
2%
25%
Not applicable
Not applicable

● “Generic Part D rebatable drug”.
● “Likely to be in shortage”.
● “Plasma-derived product”.
Proposed § 428.300 also would codify definitions established in the revised Medicare Part D
Drug Inflation Rebate Guidance for the following terms:
● “Currently in shortage”.
● “Natural disaster”.
● “Other unique or unexpected event”.
● “Severe supply chain disruption”.
ii. Reducing the Rebate Amount for Part D Rebatable Drugs Currently in Shortage
In proposed § 428.301, CMS proposes to codify the policy established in section 40.5.1
of the revised Medicare Part D Drug Inflation Rebate Guidance whereby CMS would reduce the
total rebate amount for a Part D rebatable drug that is currently in shortage based on the length of
time the drug is in shortage during an applicable period and decrease the amount of the reduction
over time. CMS intends to use the shortage lists maintained by the FDA Center for Biologics
Evaluation and Research (CBER) and Center for Drug Evaluation and Research (CDER) to
determine whether a Part D rebatable drug is currently in shortage
592
during an applicable period.
CMS will not consider an NDC-10 in the status of “to be discontinued,” “discontinued,” or
“resolved” to be “currently in shortage.” At this time, CMS intends to provide the same
reduction in the rebate amount for Part D rebatable drugs currently in shortage regardless of the
cause of the shortage.
CMS will not provide a full waiver of the rebate amount for drugs currently in shortage
on an FDA shortage list, as providing a full waiver of the rebate amount could further incentivize
manufacturers to delay taking appropriate steps that may resolve a shortage more expeditiously
592
For the purposes of this proposed rule, CMS uses the term “currently in shortage” to refer to Part D rebatable
drugs that are in the status of “currently in shortage” on the CDER shortage list, as well as biological products listed
on CBER’s current shortages list.

simply to maintain having the drug listed on FDA’s drug shortage list to avoid an obligation to
pay rebates for an extended period. Further, in a report analyzing the root causes of drug
shortages between 2013 and 2017, FDA found that more than 60 percent of drug shortages were
the result of manufacturing or product quality issues, and providing a full waiver of the rebate
amount in situations that may be within a manufacturer’s control could be perceived as
rewarding manufacturers for poor quality management.
593
CMS would be responsible for monitoring the status of a Part D rebatable drug on an
FDA shortage list, and manufacturers would not need to submit any information to CMS to be
eligible for a reduction of the rebate amount for a Part D rebatable drug that is currently in
shortage.
To calculate the reduced total rebate amount for a Part D rebatable drug, in
§ 428.301(b)(1), CMS proposes the following formula:
Reduced Total Rebate Amount = the total rebate amount multiplied by (1 minus
applicable percent reduction) multiplied by (percentage of time drug was currently in shortage
during the applicable period) added to the total rebate amount multiplied by (1 minus percentage
of time drug was currently in shortage during the applicable period)
For the purpose of this formula, for a Part D rebatable drug that is a generic drug or a
plasma-derived product, in § 428.301(b)(2)(i), CMS proposes an applicable percent reduction of
75 percent for the first applicable period such Part D rebatable drug is currently in shortage,
50 percent for the second applicable period, and 25 percent for each subsequent applicable
period. For a Part D rebatable drug (including a biosimilar) that is not a generic drug or a
plasma-derived product, in § 428.301(b)(2)(ii), CMS proposes an applicable percent reduction of
25 percent for the first applicable period such Part D rebatable drug is currently in shortage,
10 percent for the second applicable period, and 2 percent for each subsequent applicable period.
593
See: https://www.fda.gov/media/131130/download?attachment#page=33.
Because drugs and biologicals on the FDA shortage lists are maintained at the NDC-10
level, and Part D drug inflation rebates are calculated at the NDC-9 level, CMS proposes in
§ 428.301(c) that if any NDC-10 for a Part D rebatable drug is currently in shortage, CMS would
apply the rebate reduction to the entire Part D rebatable drug at the NDC-9 level. CMS will
closely monitor market data for the Part D rebatable drugs for which the rebate is reduced to
ensure the integrity of the application of the rebate reduction policy.
CMS intends to provide a reduction in the rebate amount for as long as a Part D rebatable
drug is currently in shortage. CMS believes the rebate reduction should be proportional to the
time the drug is currently in shortage and decrease over time to balance providing financial relief
to manufacturers experiencing a drug shortage while not incentivizing manufacturers to delay
taking appropriate steps to resolve a shortage simply to maintain having the drug listed on an
FDA shortage list to avoid an obligation to pay rebates for an extended period.
To determine the percentage of time a Part D rebatable drug was currently in shortage
during the applicable period, as proposed in § 428.301(b)(3), CMS would count the number of
days such drug is currently in shortage in an applicable period and divide by the total number of
days in that applicable period.
In proposed § 428.301(b)(2), CMS proposes codifying the policy set forth in section
40.5.1 of the revised Medicare Part D Drug Inflation Rebate Guidance to apply a greater
applicable percent reduction for generic Part D rebatable drugs, which, by definition, are sole
source generic drugs, compared to brand-name drugs and biologicals, including biosimilars.
CMS understands that generic drugs are often low-margin products whose prices are tied to the
marginal cost of production and thus are vulnerable to potential market exit and shortage when
input costs increase. CMS notes that the Medicare Part D Drug Inflation Rebate Program does
not apply to multiple source generic drugs, which are the generic drugs most likely to be in

shortage.
594
CMS proposes also applying a greater applicable percent reduction for
plasma-derived products than non-plasma derived products because the former rely on a variable
supply of donated blood plasma that can impact downstream production and therefore hamper
the ability to promptly resolve a shortage.
When the status of a Part D rebatable drug changes from currently in shortage to
“resolved” and either remains in the status of “resolved” or is removed from the list, and then
reemerges on the list in the status of currently in shortage in the next applicable period, CMS
would apply the shortage reduction as if there was a continuous shortage and move to the
applicable percent reduction for the second applicable period. (In this scenario, the applicable
percent reduction would be 50 percent for the second applicable period for a generic Part D
rebatable drug or plasma-derived product and 10 percent for a Part D rebatable drug that is not a
generic drug or plasma-derived product.) When the status of a Part D rebatable drug changes
from currently in shortage to “resolved” and either remains in the status of “resolved” or is
removed from the list for at least one applicable period, and then subsequently reemerges on a
shortage list, the subsequent shortage would be treated as a new shortage. In such case, the
applicable percent reduction for the first applicable period in which the drug reemerges on the
shortage list would be 75 percent for a generic Part D rebatable drug or plasma-derived product
and 50 percent for a Part D rebatable drug that is not a generic or plasma-derived product.
iii. Reducing the Rebate Amount for Generic Part D Rebatable Drugs and Biosimilars When
There Is a Severe Supply Chain Disruption
In § 428.302 of this proposed rule, CMS proposes to codify existing policy established in
section 40.5.2 of the revised Medicare Part D Drug Inflation Rebate Guidance for rebate
reductions when CMS determines there is a severe supply chain disruption during an applicable
period. As proposed in § 428.302(b)(1), CMS would provide a time-limited standard reduction of
594
See: https://www.iqvia.com/insights/the-iqvia-institute/reports-and-publications/reports/drug-shortages-in-the-
us2023.

75 percent in the total rebate amount for a generic Part D rebatable drug or biosimilar when CMS
determines there is a severe supply chain disruption during the applicable period, such as that
caused by a natural disaster or other unique or unexpected event. To receive a rebate reduction in
accordance with proposed § 428.302(b)(1), the manufacturer would have to submit to CMS a
rebate reduction request
595
that meets the eligibility requirements proposed in § 428.302(c). A
rebate reduction request should specify each NDC-11 to which the request applies, and if CMS
grants a manufacturer’s severe supply chain disruption rebate reduction request for an NDC-11,
CMS proposes in § 428.302(b)(3) that the rebate reduction would apply to the entire generic Part
D rebatable drug or biosimilar at the NDC-9 level. CMS has proposed additional submission
requirements for rebate reduction requests in the information collection request under OMB
control number: 0938-NEW (CMS-10858).
As proposed in § 428.302(c)(4), CMS would grant a reduction in the rebate amount owed
if a manufacturer of an eligible drug submits to CMS a request in writing demonstrating that (1)
a severe supply chain disruption has occurred during the applicable period, (2) the severe supply
chain disruption directly affects the manufacturer itself, a supplier of an ingredient or packaging,
a contract manufacturer,
596
or a method of shipping or distribution that the manufacturer uses in a
significant capacity to make or distribute the generic Part D rebatable drug or biosimilar, and (3)
the severe supply chain disruption was caused by a natural disaster or other unique or unexpected
event. CMS will begin accepting rebate reduction requests and rebate reduction extension
requests upon completion of the Paperwork Reduction Act (PRA) process, including for severe
supply chain disruptions caused by a natural disaster or other unique or unexpected event that
occurred on or after October 1, 2022, but before to completion of the PRA process. As proposed
595
The rebate reduction request forms are currently going through the Paperwork Reduction Act approval process
under the document identifier CMS-10858. The proposed collection was published for a 30-day comment period in
the June 3, 2024 Federal Register (89 FR 47563).
596
A contract manufacturer is a party that performs one or more manufacturing operations on behalf of a
manufacturer(s) of active pharmaceutical ingredients (APIs), drug substances, in-process materials, finished drug
products, including biological products, and combination products. See “Contract Manufacturing Arrangements for
Drugs: Quality Agreements Guidance for Industry,” November 2016: https://www.fda.gov/media/86193/download.
in § 428.302(c)(2), for a natural disaster or other unique or unexpected event occurring on or
after August 2, 2024, that the manufacturer believes caused a severe supply chain disruption, the
manufacturer must submit the rebate reduction request within 60 calendar days from the first day
that the natural disaster or other unique or unexpected event occurred or began in order for CMS
to consider a rebate reduction.
If the manufacturer makes a timely request that includes all the supporting
documentation, and CMS determines, based on its review of the reduction request and
supporting documentation, that a reduction should be granted, CMS would reduce the total
rebate amount owed by a manufacturer by 75 percent for the manufacturer’s generic Part D
rebatable drug or biosimilar for the applicable period in which the event that caused the severe
supply chain disruption occurred or began or, the following applicable period if the request is
submitted less than 60 calendar days before the end of an applicable period. CMS acknowledges
that the 60-day advance submission requirement may pose a challenge to timing of the rebate
reduction when the severe supply chain disruption-causing event occurs late in one applicable
period, and the request is not submitted until the next applicable period. In such circumstances,
CMS would apply a rebate reduction to an applicable period based on the timing of the natural
disaster or other unique or unexpected event causing a severe supply chain disruption and the
timing of the submission of the request and may adjust the timing of the application of the rebate
reduction as appropriate to meet the invoicing deadlines specified in statute and subpart E of
proposed part 428.
CMS proposes in § 428.302(c)(5) that if a manufacturer believes severe supply chain
disruption continues into a second, consecutive applicable period after the start of the natural
disaster or other unique or unexpected event, the manufacturer may request a reduction of the
total rebate amount for that second applicable period by submitting a rebate reduction extension
request to CMS, along with any new supporting documentation. CMS has proposed additional
submission requirements for rebate reduction extension requests in the information collection
request under OMB control number: 0938-NEW (CMS-10858). As proposed in
§ 428.302(c)(5)(ii), a rebate reduction extension request and any new supporting documentation
must be submitted at least 60 calendar days before the start of that second applicable period in
order for CMS to consider a rebate reduction extension, except for when the initial request is
made less than 60 calendar days before the end of an applicable period such that the initial rebate
reduction applied to the next applicable period rather than the applicable period in which the
event that caused the severe supply chain disruption occurred or began. In these cases, the rebate
reduction extension request must be submitted at least 60 calendar days prior to the end of the
applicable period in which the initial reduction applied.
If the manufacturer submits a complete and timely extension request, and CMS
determines that the information submitted warrants an extension of the rebate reduction, the total
rebate amount would be reduced by 75 percent for a second consecutive applicable period for
that manufacturer’s generic Part D rebatable drug or biosimilar in accordance with proposed
§ 428.302(b)(2).
Consistent with the policy established in section 40.5.2 of the revised Medicare Part D
Drug Inflation Rebate Guidance, a manufacturer may receive only one extension of the rebate
reduction per generic Part D rebatable drug or biosimilar per CMS determination of a severe
supply chain disruption, as proposed in § 428.302(c)(5). Said differently, CMS would limit the
severe supply chain disruption rebate reduction to two consecutive applicable periods total per
generic Part D rebatable drug or biosimilar per CMS determination of a severe supply chain
disruption.
As proposed in § 428.302(b)(4)(i), if the manufacturer believes there are multiple events
causing severe supply chain disruptions during the same applicable period for the same generic
Part D rebatable drug or biosimilar and submits multiple rebate reduction requests for the same
generic drug or biosimilar, CMS will grant no more than one rebate reduction for that generic
drug or biosimilar for the applicable period. For example, if the manufacturer of a generic Part D
rebatable drug or biosimilar is granted a severe supply chain disruption rebate reduction request
for its product due to a natural disaster that occurred in January 2025 and then experiences a
second severe supply chain disruption caused by a second, distinct natural disaster in July 2025,
CMS would not grant the second rebate reduction request. That is, the manufacturer would
receive the 75 percent reduction for one applicable period for the severe supply chain disruption
caused by the first natural disaster but would not receive a rebate reduction for the second natural
disaster. However, if the second natural disaster exacerbated the severe supply chain disruption
caused by the first natural disaster, the manufacturer may reflect such circumstances in its
request for an extension of the rebate reduction for a second applicable period.
As proposed in § 428.302(b)(4)(ii), if CMS grants a severe supply chain disruption rebate
reduction request for a generic Part D rebatable drug or biosimilar, and the drug or biosimilar
appears as currently in shortage during the same applicable period as the one for which the
severe supply chain disruption reduction request was granted, CMS would apply the 75 percent
reduction to the entire applicable period for which the severe supply disruption request was
granted and would not grant any additional reduction for the shortage status during that
applicable period. For any subsequent applicable periods that the generic Part D rebatable drug
or biosimilar appears as currently in shortage, CMS would reduce the total rebate amount in
accordance with the drug shortages reduction proposed in § 428.301, starting with the highest
reduction (that is, 75 percent for a generic Part D rebatable drug or plasma-derived product and
25 percent for a Part D rebatable drug that is not a generic drug or plasma-derived product). For
example, if CMS grants a severe supply chain disruption rebate reduction request for a generic
Part D rebatable drug or biosimilar that was submitted on November 15, 2024, and that generic
Part D rebatable drug or biosimilar is currently in shortage from September 15, 2025, until
May 15, 2026, CMS would apply a 75 percent reduction in the total rebate amount for the
duration of the applicable period for which the severe supply chain disruption rebate reduction
request was granted (that is, October 1, 2024, to September 30, 2025), and then would apply the
shortages reduction as proposed in § 428.301, beginning with a reduction of 25 percent for a
biosimilar or 75 percent for a generic Part D rebatable drug or plasma-derived product that is a
biosimilar for the applicable period beginning October 1, 2025.
As proposed in § 428.302(b)(4)(iii), if a generic Part D rebatable drug or biosimilar that
is currently in shortage experiences a severe supply chain disruption, the manufacturer may
submit a severe supply chain disruption rebate reduction request. If CMS grants the rebate
reduction request, the rebate amount would be reduced by 75 percent for the applicable period,
and CMS would not grant any additional reduction under proposed § 428.301 for the currently in
shortage status during that applicable period. For example, if a generic Part D rebatable drug or
biosimilar that is currently in shortage in the applicable period beginning October 1, 2024 is
granted a severe supply chain disruption rebate reduction request as a result of a natural disaster
that occurs on April 5, 2025, CMS would apply a 75 percent reduction in the rebate amount for
the duration of the applicable period in which the natural disaster occurred (that is,
October 1, 2024, to September 30, 2025). In this same example, if the natural disaster instead
occurs on September 5, 2025, CMS would apply the shortages reduction proposed in § 428.301
for the duration of the applicable period beginning October 1, 2024 (that is, October 1, 2024, to
September 30, 2025), and then a 75 percent reduction under the severe supply chain disruption
policy to the next applicable period beginning October 1, 2025 (that is, October 1, 2025, to
September 30, 2026).
In § 428.302(c)(6), CMS proposes to review rebate reduction requests and rebate
reduction extension requests within 60 calendar days of receipt of all documentation, if feasible,
beginning with the applicable period that begins on October 1, 2024. If a manufacturer’s rebate
reduction request does not meet the criteria in proposed § 428.302(c)(4) or if the rebate reduction
request is incomplete or untimely based on the requirements in proposed § 428.302(c), CMS
would deny the request. CMS also proposes that if a manufacturer’s rebate reduction extension
request does not meet the criteria in proposed § 428.302(c)(5), is incomplete or untimely based

on the requirements in proposed § 428.302(c)(5), or if a reduction under proposed
§ 428.302(b)(1) was not provided for such generic Part D rebatable drug or biosimilar, CMS
would deny the rebate reduction extension request. As proposed in § 428.302(c)(6)(iii), CMS’
decisions to deny a request would be final and not be subject to an appeals process.
As proposed at § 428.302(c)(7), CMS would keep confidential, to the extent allowable
under law, any requests for a rebate reduction, including supporting documentation. CMS
proposes that information provided as part of a severe supply chain disruption rebate reduction
request that the submitter indicates is a trade secret or confidential commercial or financial
information would be protected from disclosure if CMS determines the information meets the
requirements set forth under Exemptions 3 or 4 of the Freedom of Information Act (FOIA). In
addition to the protections under the FOIA for trade secrets and commercial or financial
information obtained from a person that is privileged or confidential, the Trade Secrets Act at
18 U.S.C. 1905 requires executive branch employees to protect such information. CMS would
protect confidential and proprietary information as required by applicable law.
iv. Reducing the Rebate Amount for Generic Part D Rebatable Drugs Likely To Be in Shortage
In § 428.303 of this proposed rule, CMS proposes to codify existing policy established in
section 40.5.3 of the revised Medicare Part D Drug Inflation Rebate Guidance for rebate
reductions when a generic Part D rebatable drug is likely to be in shortage, as defined in
proposed § 428.300. As proposed in § 428.303(b)(1), CMS would provide a time-limited
standard reduction of 75 percent in the total rebate amount for a generic Part D rebatable drug
when CMS determines that the generic Part D rebatable drug is likely to be in shortage. To
receive a rebate reduction in accordance with proposed § 428.303(b)(1), the manufacturer would
have to submit to CMS a rebate reduction request
597
that meets the eligibility requirements
proposed in § 428.303(c). A rebate reduction request should specify each NDC-11 to which the
597
The rebate reduction request forms are currently going through the Paperwork Reduction Act approval process
under the document identifier CMS–10858. The proposed collection was published for a 30-day comment period in
the June 3, 2024 Federal Register (89 FR 47563).
request applies and if CMS grants a manufacturer’s likely to be in shortage rebate reduction
request for an NDC-11, CMS proposes in § 428.303(b)(3) that the rebate reduction would apply
to the entire generic Part D rebatable drug at the NDC-9 level. CMS has proposed additional
submission requirements for rebate reduction requests in the information collection request under
OMB control number: 0938-NEW (CMS-10858).
As proposed in § 428.303(c)(4), CMS would grant a reduction in the rebate amount owed
if a manufacturer of an eligible drug submits to CMS a request in writing demonstrating that (1)
the generic Part D rebatable drug is likely to be in shortage, (2) the manufacturer is taking
actions to avoid the potential drug shortage, and (3) the reduction of the rebate amount would
reduce the likelihood of the drug appearing on an FDA shortage list. As proposed in
§ 428.303(c)(2), a manufacturer must submit the rebate reduction request before the start of the
next applicable period in which the manufacturer believes the generic Part D rebatable drug is
likely to be in shortage in order for CMS to consider a rebate reduction.
If the manufacturer makes a timely request that includes all the supporting
documentation, and CMS determines, based on its review of the reduction request and
supporting documentation, that a reduction should be granted, CMS would reduce the total
rebate amount owed by a manufacturer by 75 percent for the manufacturer’s generic Part D
rebatable drug for the applicable period in which the request was submitted or the following
applicable period, depending on the timing of the submission of the request.
CMS proposes in § 428.303(c)(5) that if a manufacturer believes the potential drug
shortage continues for a second, consecutive applicable period, the manufacturer may request a
reduction of the total rebate amount for that second applicable period by submitting a rebate
reduction extension request to CMS, along with any new supporting documentation. CMS has
proposed additional submission requirements for rebate reduction extension requests in the
information collection request under OMB control number: 0938-NEW (CMS-10858). As
proposed in § 428.303(c)(5)(ii), a rebate reduction extension request and any new supporting
documentation must be submitted at least 60 calendar days before the start of the second
applicable period in which the manufacturer believes the generic Part D rebatable drug is likely
to be in shortage in order for CMS to consider a rebate reduction extension.
If the manufacturer submits a complete and timely extension request, and CMS
determines that the information submitted warrants an extension of the rebate reduction, the total
rebate amount would be reduced by 75 percent for a second consecutive applicable period for
that manufacturer’s generic Part D rebatable drug in accordance with proposed § 428.303(b)(2).
Consistent with the policies established in section 40.5.3 of the revised Medicare Part D
Drug Inflation Rebate Guidance, a manufacturer may receive only one extension of the rebate
reduction per generic Part D rebatable drug per CMS determination of likelihood of shortage, as
proposed in § 428.303(c)(5). Said differently, CMS would limit the likely to be in shortage
rebate reduction to two consecutive applicable periods total per generic Part D rebatable drug per
CMS determination of likelihood of shortage.
As proposed in § 428.303(b)(4), if CMS grants a rebate reduction request for a generic
Part D rebatable drug that is likely to be in shortage, and the drug appears as currently in
shortage during the same applicable period as the one for which the likely to be in shortage
reduction request was granted, CMS would apply the 75 percent reduction to the entire
applicable period for which the likely to be in shortage request was granted and would not grant
any additional reduction for the shortage status during that applicable period. For any subsequent
applicable periods that the generic Part D rebatable drug appears as currently in shortage, CMS
would reduce the total rebate amount in accordance with the drug shortages reduction proposed
in § 428.301, starting with the highest reduction (that is, 75 percent for a generic Part D rebatable
drug). For example, if CMS grants a likely to be in shortage rebate reduction request for a
generic Part D rebatable drug that was submitted on August 15, 2024, and that generic Part D
rebatable drug is currently in shortage from September 15, 2025, until May 15, 2026, CMS
would apply a 75 percent reduction in the total rebate amount for the duration of the applicable
period for which the likely to be in shortage rebate reduction request was granted (that is,
October 1, 2024, to September 30, 2025), and then would apply the shortages reduction as
proposed in § 428.301, beginning with a reduction of 75 percent for a generic Part D rebatable
drug for the applicable period beginning October 1, 2025.
If the manufacturer of a generic Part D rebatable drug that is currently in shortage
believes such generic drug is likely to continue to be in shortage in the next applicable period,
the manufacturer may submit a likely to be in shortage rebate reduction request to CMS. If the
request meets the criteria described in proposed in § 428.303(c)(4), CMS would reduce the total
rebate amount owed by a manufacturer by 75 percent for the manufacturer’s generic Part D
rebatable drug. Consistent with the evaluation criteria proposed in § 428.303(c)(4), CMS does
not intend to consider a generic Part D rebatable drug as likely to be in shortage based solely
upon the drug being currently in shortage. However, if the manufacturer believes there are
circumstances that may exacerbate the current shortage such that without the reduction the
generic Part D rebatable drug is likely to be in shortage in the next applicable period, the
manufacturer may reflect such circumstances in its rebate reduction request. For example, if a
generic Part D rebatable drug is currently in shortage during the applicable period beginning
October 1, 2023 because the manufacturer had trouble meeting demand for the drug and then in
August 2024, the manufacturer faces difficulties securing the API for such drug and believes this
may worsen the shortage situation and result in the generic Part D rebatable drug being currently
in shortage in the next applicable period, the manufacturer may submit a likely to be in shortage
rebate reduction request to CMS providing information on the severity of the likely shortage.
CMS welcomes comments on this approach. In § 428.303(c)(6), CMS proposes to review rebate
reduction requests and rebate reduction extension requests within 60 calendar days of receipt of
all documentation, if feasible, beginning with the applicable period that begins on
October 1, 2024. If a manufacturer’s rebate reduction request does not meet the criteria in
proposed § 428.303(c)(4) or if the rebate reduction request is incomplete or untimely based on
the requirements in proposed § 428.303(c), CMS would deny the request. CMS also proposes
that if a manufacturer’s rebate reduction extension request does not meet the criteria in proposed
§ 428.303(c)(5), is incomplete or untimely based on the requirements in proposed
§ 428.303(c)(5), or if a reduction under proposed § 428.303(b)(1) was not provided for such
generic Part D rebatable drug, CMS would deny the rebate reduction extension request. As
proposed in § 428.303(c)(6)(iii), CMS’ decisions to deny a request would be final and not be
subject to an appeals process.
As proposed at § 428.303(c)(7), CMS would keep confidential, to the extent allowable
under law, any requests for a rebate reduction, including supporting documentation. CMS
proposes that information provided as part of a likely to be in shortage rebate reduction request
that the submitter indicates is a trade secret or confidential commercial or financial information
would be protected from disclosure if CMS determines the information meets the requirements
set forth under Exemptions 3 or 4 of FOIA. In addition to the protections under the FOIA for
trade secrets and commercial or financial information obtained from a person that is privileged or
confidential, the Trade Secrets Act at 18 U.S.C. 1905 requires executive branch employees to
protect such information. CMS would protect confidential and proprietary information as
required by applicable law.
e. Reports of Rebate Amounts, Reconciliation, Suggestion of Error, and Payments (§§ 428.400
through 428.405)
Section 1860D-14B(a)(1) of the Act requires the Secretary to report to each manufacturer
of a Part D rebatable drug the following information not later than 9 months after the end of the
applicable period: (1) the amount, if any, of the excess AnMP increase described in section
1860D-14B(b)(1)(A)(ii) for each Part D rebatable drug and (2) the rebate amount for each Part D
rebatable drug. In compliance with section 1860D-14B(a)(2) of the Act, the manufacturer of a
Part D rebatable drug must provide a rebate for each Part D rebatable drug no later than
30 calendar days after the receipt of the information provided by the Secretary in section
1860D-14B(a)(1) of the Act.
To fulfill this statutory requirement, CMS proposes to send a Preliminary Rebate Report
followed by a Rebate Report, as described in proposed § 428.401(b) and (c), to all manufacturers
of a Part D rebatable drug, even if the amount due is $0; all rebate amounts would be subject to
reconciliation as proposed in § 428.401(d). As proposed in § 428.401(b), CMS does not intend to
send notice to manufacturers for drugs that are not considered rebatable pursuant to proposed
§ 428.20.
Additionally, section 1860D-14B(b)(6) states that CMS shall provide a method and
process under which CMS adjusts the calculation of the rebate amount for a Part D rebatable
drug for an applicable period if CMS determines such an adjustment is necessary based on
revisions to the number of units of a rebatable covered Part D drug dispensed submitted by a
PDP sponsor of a prescription drug plan or an MA organization offering an MA-PD plan. The
statute also specifies that CMS must reconcile any underpayments in the rebate amount paid by
the manufacturer of the applicable Part D rebatable drug due to such an adjustment and
underpayments must be paid no later than 30 days from the date of receipt of information from
CMS about the adjustment. To fulfill this statutory obligation and to address the completeness
and accuracy of the rebate amount, CMS proposes to conduct regular reconciliations at two
points in time to determine whether the rebate amount must be adjusted due to updated claims
and payment data used in the calculation of such rebate amount (specified in proposed
§ 428.401(d)(1)): (1) 12 months after the issuance of the Rebate Report, and (2) 36 months after
the issuance of the Rebate Report. The reporting process for each reconciliation will be the same
process described for the original Rebate Report, with payment due for any outstanding rebate
amount 30 days after receipt of a report with a reconciled rebate amount. In addition to regular
reconciliations, CMS proposes a process to conduct reconciliations of the rebate amount as
needed to correct agency error and when CMS determines that the information used by CMS to
calculate a rebate amount was inaccurate due to manufacturer misreporting.
i. Definitions
In proposed § 428.400, CMS proposes to define the following term applicable to
subpart E (§§ 428.400 through 428.405):
● “Date of receipt”.
For example, if CMS issues a Rebate Report through the method and process described in
proposed § 428.404 on June 30, 2026, then July 1, 2026, will be the date of receipt and day one
of the 30-calendar-day payment period.
ii. Reports of Rebate Amounts and Suggestion of Error
Consistent with the process specified in section 50 of the revised Medicare Part D Drug
Inflation Rebate Guidance involving preliminary and final reports, CMS proposes to codify a
multi-step process to provide a manufacturer as defined in proposed § 428.20 with the rebate
information specified under section 1860D-14B(a) of the Act. CMS considered the following
factors in determining a method and process for providing the rebate information: meeting
statutorily provided deadlines in section 1860D-14B(a) of the Act (for example, dates by which
to provide the rebate amount owed to the manufacturer); the operational time to acquire the
relevant information specified in proposed part 428; the operational time to calculate the rebate
amount specified in subparts B and C of proposed part 428; clarity of the information provided
as well as potential burden on manufacturers; and how to ensure the accuracy of the rebate
amount.
CMS proposes at proposed § 428.401 the use of an initial Preliminary Rebate Report and
a subsequent Rebate Report, with an opportunity for manufacturers to identify certain
mathematical errors (see proposed § 428.403 and discussed in further detail later in this section)
and two regular reconciliations of the rebate amount to account for updates to claims and
payment data at 12 months and 36 months after the Rebate Report is issued as described in
proposed § 428.401(d).
CMS proposes in proposed § 428.401 that the multi-step reporting process for providing
rebate information to a manufacturer would include: (1) an initial report, which CMS proposes
to entitle the “Preliminary Rebate Report” in proposed § 428.401(b) and (2) a second report,
which CMS proposes to entitle the “Rebate Report” in proposed § 428.401(c). The Rebate
Report would serve as the invoice for the rebate amount due, if any, for each product determined
to be a Part D rebatable drug for the applicable period, as specified in proposed § 428.101.
Manufacturers of Part D rebatable drugs would receive a Rebate Report for their rebatable drugs
even if the amount due is $0. CMS proposes at proposed § 428.401(d)(1) two regular
reconciliations of the rebate amount to occur 12 months and 36 months after issuance of the
subsequent Rebate Report specified in proposed § 428.401(c), which would include any
restatements that have occurred in the drug pricing data and claims billing data reported to CMS
and used in the rebate calculation specified in subpart C of this part.
As the first step in the reporting process, as proposed in proposed § 428.401(b) and
consistent with section 50 of the revised Medicare Part D Drug Inflation Rebate Guidance, CMS
would provide each manufacturer of a Part D rebatable drug with the preliminary rebate amount
through a Preliminary Rebate Report at least 1 month prior to the issuance of the Rebate Report
specified in proposed § 428.401(c) for an applicable period (that is, approximately 8 months after
the end of the applicable period unless otherwise specified). To facilitate manufacturer
understanding of the Preliminary Rebate Report, CMS is proposing in proposed § 428.401(b)(1)
that the Preliminary Rebate Report would include the following information: the NDC(s) for the
Part D rebatable drug as defined under proposed § 428.20; the total number of units for the Part
D rebatable drug for the applicable period as determined under proposed § 428.203 (which
would remove units when a generic drug is no longer a Part D rebatable drug in proposed
§ 428.203(b)(1) and would exclude units acquired through the 340B Program in proposed
§ 428.203(b)(2)); the benchmark period manufacturer price as described in proposed
§ 428.202(d); the AnMP for the Part D rebatable drug for the applicable period as determined in
proposed § 428.202(b); the applicable benchmark period and applicable period CPI-Us as
identified in proposed §§ 428.202(e) and 428.20; the inflation-adjusted payment amount as
specified in proposed § 428.202(f); the amount, if any, of the excess AnMP for the Part D
rebatable drug for the applicable period as determined under proposed § 428.202(a); any applied
reductions as described in proposed §§ 428.301, 428.302, and 428.303; and the rebate amount
due as specified in proposed § 428.201(a). As proposed under proposed § 428.204, in cases
where a Part D rebatable drug is a line extension, CMS proposes to include the same elements
described above in the Preliminary Rebate Report as well as: the NDC for the initial drug; the
inflation rebate amount ratio for the initial drug; and the alternative rebate amount (see proposed
§ 428.401(b)(2)).
When determining what information should be included on rebate reports, CMS
considered the statutory requirements outlined in section 1860D-14B(a)(1) of the Act to
determine which data elements are necessary to review the Preliminary Rebate Report for error
(described later in this section) and to protect proprietary information. In response to comments
on the initial Medicare Part D Drug Inflation Rebate Guidance, CMS has proposed to disclose
data elements as suggested by interested parties that are not enumerated in the statute, such as
NDCs for Part D rebatable drugs and the applicable period CPI-U. CMS acknowledges requests
from interested parties to provide additional data elements including claims-level data such as
days’ supply, fill number, and prescription ID number on rebate reports that are not included in
this proposal. CMS considered these requests in development of this proposed rule but does not
believe it necessary to provide this further information to fulfill its statutory obligation and
believes that the potential benefit to manufacturers of additional data are outweighed by the
administrative burdens additional reporting would impose to the agency. The elements listed
above provide sufficient information for a manufacturer to review the Preliminary Rebate Report
for mathematical error, while protecting proprietary information, and these elements are
operationally feasible for CMS to provide. In proposed § 428.203(b)(2)(i)(A) and (B), CMS
would exclude 340B units beginning with January 1, 2026, which is the second calendar quarter
in the applicable period starting October 1, 2025, and beyond (as discussed in further detail in
section III.I.3.f of this proposed rule). This exclusion applies to all Preliminary Rebate Reports,
Rebate Reports, and reconciliations of a rebate amount that include the applicable period starting
with October 1, 2025, and beyond with claims for service dates on or after January 1, 2026. As
such, 340B units would not be excluded from the Rebate Reports for the applicable periods
beginning October 1, 2022, October 1, 2023, and October 1, 2024, as discussed in proposed
§ 428.402.
By structuring the Rebate Report process to include a Preliminary Rebate Report before
the Rebate Report, CMS is able to provide manufacturers with an opportunity to review the
Preliminary Rebate Report before the rebate amount is invoiced via the Rebate Report. While
CMS is not required to provide a preliminary report, CMS seeks to facilitate manufacturer
understanding of the Rebate Report and believes it would be beneficial for manufacturers to
review the report for mathematical errors that could be corrected before invoicing via the Rebate
Report. Further, a Preliminary Rebate Report would provide additional notice to manufacturers
regarding whether they may owe a rebate amount.
In proposed § 428.403, CMS proposes a process in which a manufacturer may suggest to
CMS that the manufacturer believes the Preliminary Rebate Report includes a mathematical error
within 10 calendar days after the date of receipt of the Preliminary Rebate Report. For example,
if the Preliminary Rebate Report is provided on May 31, 2026, then June 1, 2026, would be the
date of receipt and, therefore, day one of the 10-calendar-day period to submit a Suggestion of
Error; the Suggestion of Error would be due at 11:59 p.m. PT on June 10, 2026, in this example.
CMS reviewed comments on the 10-day Suggestion of Error period submitted in response to the
initial Medicare Part D Drug Inflation Rebate Guidance, many of which suggested that
manufacturers receive at least 30 days to review the Preliminary Rebate Report. CMS considered
a 10-day, 15-day, and 30-day Suggestion of Error period and believes a 10-calendar-day period
as (see proposed § 428.403(c)) is sufficient after considering the volume of the data to be
provided to manufacturers, the narrow scope of items that may be identified as a Suggestion of
Error, and the operational time necessary for CMS to provide a Rebate Report within 9 months
of the end of the applicable period as required under section 1860D-14B(a)(1) of the Act.
However, CMS proposes in § 428.402(c)(1)(i) to expand the Suggestion of Error period to
30 calendar days for the Preliminary Rebate Reports for the first two applicable periods
(beginning October 1, 2022, and October 1, 2023). This extended Suggestion of Error period will
provide additional time and flexibility during the first invoicing cycle of the Medicare Part D
Drug Inflation Rebate Program.
Section 1860D-14B(f) of the Act precludes administrative or judicial review on the
determination of units, whether a drug is a Part D rebatable drug, and the calculation of the
rebate amount (see proposed § 428.403(a)(1)). Therefore, the Suggestion of Error process will be
limited to mathematical steps involved in determining the rebate amount and the elements
precluded from administrative or judicial review will not be considered in-scope for the
Suggestion of Error process. Additionally, CMS is not providing an administrative dispute
resolution process. CMS intends to consider all in-scope submissions under the Suggestion of
Error process (for example, suggestions regarding a mathematical error) as described in proposed
§ 428.403(a). CMS does not intend to review suggestions of error that are out-of-scope or
submissions for a rebatable drug with an amount due of $0.
As the second step in the reporting process, CMS proposes in proposed § 428.401(c) to
provide the rebate amount to the manufacturer through the Rebate Report no later than 9 months
after the end of the applicable period. As proposed in proposed§ 428.401(c)(1), the Rebate
Report would include the same data elements as the Preliminary Rebate Report (specified in
proposed § 428.401(b)(1)) and include any recalculations based on CMS acceptance of a
manufacturer’s Suggestion of Error from proposed § 428.403, or any CMS-determined
recalculations from proposed § 428.401(d)(2), if applicable. Manufacturers must pay the rebate
amount within 30 calendar days from the date of receipt of the Rebate Report (see proposed
§ 428.405(a)). For example, if the Rebate Report is provided on June 30, 2026, then July 1, 2026,
would be the date of receipt and therefore day one of the 30-calendar-day payment period;
payment would be due no later than 11:59 p.m. PT on July 30, 2026.
In proposed §§ 428.404 and 428.405, CMS proposes that it will establish a standard
method and process to issue Rebate Reports and accept manufacturer rebate payments. This
method and process may include an online portal administered by a CMS contractor which
would provide manufacturers with access to their Rebate Report, the ability to submit a
Suggestions of Error, and pay a rebate amount due. CMS intends to provide technical
instructions separate from this rulemaking to manufacturers of Part D rebatable drugs regarding
how to access Rebate Reports and how to receive notifications alerting the manufacturer when
information is available. CMS also intends to issue reminder notices to manufacturers regarding
the due date of rebate payments. In proposed § 428.404(a), CMS notes that the manufacturer that
may access Rebate Reports and make applicable rebate amount payments is the manufacturer
responsible for paying a rebate, and as stated above, CMS proposes to identify the manufacturer
that is responsible for paying a rebate using the same approach used for reporting AMP data.
iii. Reconciliation of a Rebate Amount
As discussed in section 50 of the revised Medicare Part D Drug Inflation Rebate
Guidance, CMS considered options consistent with section 1860D-14B(b)(6) of the Act to
establish a method and process to determine adjustment to the rebate amount in the case of a Part
D plan sponsor submitting revisions to the number of units of a part D rebatable drug. As is also
discussed in section 50, CMS considered options for establishing a standardized method and
process at regular intervals to determinate any appropriate adjustments to the rebate amount for a
Part D rebatable drug for an applicable period to account for additional revised information as
well as options for recalculation based on CMS identifying an agency error or determining
manufacturer data was misreported. CMS is proposing policies for reconciliation, including with
respect to enforcement of payment of any reconciled rebate amount, consistent with both the
statutory framework for the Part D Drug Inflation Rebate Program and the express authority in
sections 1102 and 1871 of the Act to adopt regulations for the proper administration of the
Medicare Prescription Drug Inflation Rebate Program.
As proposed at § 428.401(d), CMS believes that it is necessary and appropriate for CMS
to recalculate the rebate amount for an applicable period at regular intervals to include updated
information about key data elements included in the calculation of the rebate amount, not limited
to those data described in section 1860D-14B(b)(6) of the Act. These data elements as set forth
in proposed § 428.401(d)(1)(i) include: total units; the benchmark period manufacturer price; the
payment amount in the payment amount benchmark period; the AnMP; and updated data on line
extension calculations. Updating these calculation inputs at regular reconciliation intervals will
result in a rebate amount that more fully reflects the majority of shifts in the underlying data
following additional time for claims run-out, which refers to the maturation of PDE records in
CMS’ internal PDE database. Because the information extracted represents the PDE records’
status in CMS’ internal PDE database at that moment in time, additional run-out may yield
different information, either because more PDE records with dispensing dates during the
applicable period were finalized and added to the database or because the status of the existing
PDE records changed. CMS refers to “X months of run-out” as the period between the end of the
applicable period and the date when CMS accesses information about the PDE records; for
example, “3 months of run-out” means that PDE records are accessed for PDE records with
dispensing dates during an applicable period 3 months after the end of such applicable period.
Conducting a reconciliation of the rebate amount with additional claims run-out will improve the
accuracy of the rebate amount. Additionally, reconciliation of payment amounts is consistent
with the approach to the calculation of the payment amounts in other CMS programs (such as the
Coverage Gap Discount Program) that provide for a reconciliation period.
CMS notes that the reconciliation of a rebate amount, whether during a reconciliation
proposed at § 428.401(d)(1) or a discretionary reconciliation proposed at § 428.401(d)(2)
discussed further below, will not create a separately payable and distinct rebate amount. Rather,
reconciliation updates the prior rebate amount owed to CMS, if any, by a manufacturer of a Part
D rebatable drug so that the rebate amount ultimately reflects a more precise calculation of the
rebate amount, as required by section 1860D-14B(a)(1) of the Act, to account for shifts in the
underlying data following additional time for claims run-out after the Rebate Report is issued as
well as subsequently identified data integrity issues. Moreover, because the reconciled rebate
amount is an adjustment of the prior rebate amount, CMS proposes at § 428.405(a)(1) for a
report of a reconciled rebate amount to also identify the difference between the rebate amount
due as specified on the Rebate Report set forth in proposed § 428.401(c) and the reconciled
rebate amount. CMS would only collect the net rebate amount due, if any, upon reconciliation,
so as to prevent any duplicate payments. CMS also proposes to refund any overpayment made by
a manufacturer, as determined during reconciliation, as discussed in proposed § 428.405(b).
Additionally, as CMS suggested in section 50 of the revised Medicare Part D Drug
Inflation Rebate Guidance, CMS considered multiple options for establishing a standardized
method and process to occur at regular intervals to determine an appropriate adjustment to the
rebate amount for a Part D rebatable drug for an applicable period to account for revised
information prior to adopting the proposal described here with two proposed regular
reconciliations of the Part D inflation rebate amount. CMS considerations included the length of
time needed to capture relevant changes to data inputs for recalculation, whether the timing
should align with the reconciliation of Part B rebate amounts, and manufacturer burden.
Specifically, CMS considered the average time span needed to ensure submission of the majority
of Part D plan unit revisions specified in section 1860D-14B(b)(6) of the Act, and the average
time span needed for the submission of the majority of manufacturer restatements of AMP data.
CMS also considered the 36-month period provided by MDRP for AMP restatements as
described in § 447.510(d)(3) of this title and whether consistency among program reconciliation
timelines is beneficial. CMS believes a longer period of claims run-out (at least 12 and
36 months of run-out time in the proposed approach) would ensure that CMS more fully
accounts for capturing of revised units. Further, the first reconciliation would be performed to
include at least 13 months of claims run-out for the applicable period and would be issued
12 months after the Rebate Report for the same applicable period. The second reconciliation
would include 37 months of claims run-out for the applicable period and would be issued
36 months after the Rebate Report for the same applicable period. The first reconciliation, issued
12 months after the Rebate Report, would provide sufficient time to capture the majority of
updates to the data specified in proposed § 428.401(b)(1). The second reconciliation, to be issued
36 months after the Rebate Report, is sufficient to capture the remainder of the run-out for
MDRP AMP restatements (that do not require CMS review in § 447.510) while also closing out
the calculation of the rebate amount for a Part D rebatable drug for an applicable period within a
reasonable time period after the Rebate Report is issued (except for the circumstances in
proposed § 428.401(d)(2) regarding CMS’ identification of mathematical errors or manufacturer
misreporting).
Further, in considering whether consistency across CMS programs is critical, CMS
believes that consideration for the completeness of data, as discussed above, should be
prioritized over consistency across program timelines. That is, when examining timelines from
other CMS programs that collect data contributing to calculation of the rebate amount, CMS
prioritized that, to the extent feasible, completeness and accuracy of the data elements
contributing to the calculation of the rebate amount rather than prioritizing consistency among
the data collection and reconciliation timelines themselves. Finally, CMS believes that solely
updating total units without updating other elements of the rebate calculation would lead to an
inaccurate rebate amount, and therefore proposes to update additional calculation inputs as
described in proposed § 428.401(d)(1)(i)(A) through (F). CMS believes that a restatement of
each data element included in proposed § 428.401(d)(1) to reconcile the rebate amount provided
in the Rebate Report in proposed § 428.401(c) is appropriate to capture an updated rebate
amount and is in line with other CMS programs that provide for a reconciliation period. While
some data points may not change, CMS would review the data to determine if there are any
updates in the data and use the updated data in the reconciliation to provide a reconciled rebate
amount to the manufacturer.
Based on these considerations, similar to the multi-step process for the Rebate Report
proposed in § 428.401(b) and (c), CMS proposes a multi-step process to provide each
manufacturer of a Part D rebatable drug with a reconciled rebate amount on a regular basis. At
both the 12 month reconciliation point and the 36 month reconciliation point, CMS proposes a
reconciliation process that will include: (1) a preliminary reconciliation of the rebate amount,
which CMS would provide to manufacturers of Part D rebatable drugs as proposed in
§ 428.401(d)(1)(i) and (d)(2) a reconciled rebate amount, which CMS would provide to
manufacturers of a Part D rebatable drug as proposed in § 428.401(d)(1)(ii). CMS also proposes
to apply the Suggestion of Error process specified in proposed § 428.403 to each preliminary
reconciliation.
In detail, first, as specified in proposed § 428.401(d) and similar to the Preliminary
Rebate Report process proposed in § 428.401(b), for each reconciliation CMS proposes to
provide the manufacturer with information about the preliminary reconciliation of the rebate
amount at least 1 month prior to the issuance of the reconciled rebate amount (see proposed
§ 428.401(d)) to each manufacturer of a Part D rebatable drug for an applicable period. CMS
proposes in § 428.401(d)(1) that the preliminary reconciliation would include, at a minimum, the
same information outlined for the Rebate Report and the following updated information, if
applicable: updated total number of rebatable units, including updates submitted by a PDP or
MA-PD plan sponsor and updates to 340B units (as applicable to the dates of service and
applicable periods specified in proposed § 428.203(b)(2)(i)(A) and (B)), or units otherwise
excluded as specified in proposed § 428.203(b); the benchmark period manufacturer price if any
inputs are restated within the reconciliation run-out period as specified in proposed § 428.202(d);
the AnMP if any inputs are restated within the reconciliation run-out period as specified in
proposed § 428.202(b); the excess amount by which the AnMP exceeds the inflation-adjusted
payment amount for the applicable period as specified in proposed § 428.202(a), using the most
recent AMP (if any inputs are restated within the reconciliation run-out period); updated data on
line extension calculations, including the initial drug identified in accordance with
§ 447.509(a)(4)(iii)(B), the inflation rebate amount ratio, and the alternative total rebate amount
as set forth in proposed § 428.204 if any inputs are restated within the reconciliation run-out
period; the reconciled rebate amount as set forth in proposed § 428.201(a); and the difference
between the total rebate amount due as specified on the Rebate Report set forth in proposed
§ 428.201(a) and the reconciled rebate amount as set forth in proposed § 428.201(a). CMS also
notes that changes to status of 5i drugs (defined at § 447.507) are captured through AMP
restatements.
In proposed § 428.403(a), similar to the Suggestion of Error process proposed for the
Preliminary Rebate Report in proposed § 428.401(b), within 10 calendar days after date of
receipt of the information about the preliminary reconciliation of the rebate amount, CMS
proposes that a manufacturer may suggest to CMS that the manufacturer believes the preliminary
reconciliation of the rebate amount contains a mathematical error. CMS believes a
10-calendar-day period is sufficient due to the same considerations of data volume, the narrow
set of in-scope items for review, and the operational time necessary for CMS to publish the
reconciled rebate amount. The preclusions in section 1860D-14B(f) of the Act on administrative
and judicial review apply to the reconciliation process.
Second, in detail, CMS proposes in § 428.401(d)(1)(ii) to provide a reconciled rebate
amount to the manufacturer within 12 months and 36 months after the Rebate Report was issued
for each applicable period. As proposed in § 428.401(d)(1)(ii), the information in the report for a
reconciled rebate amount would include the same data elements as provided in the information
provided to the manufacturer of a Part D rebatable drug regarding the preliminary reconciliation
of a rebate amount(specified in proposed § 428.401(d)(1)(i)) and would include any
recalculations based on CMS acceptance of a manufacturer’s Suggestion of Error from proposed
§ 428.403. A reconciliation of the rebate amount may result in an increase, decrease, or no
change to the rebate amount, compared to the Rebate Report for an applicable period or a
previous reconciliation in the case of reconciliation conducted 36 months after issuance of the
Rebate Report (see proposed § 428.401(d)(3)).
Additionally, as suggested in section 50 the revised Medicare Part D Drug Inflation
Rebate Guidance, CMS considered options for establishing circumstances where a recalculation
of the rebate amount may be appropriate for an applicable period after issuing the Rebate Report
and/or a reconciled rebate amount based on CMS identifying an error or CMS determining that
the information used by CMS to calculate a rebate amount was inaccurate due to false reporting
or similar fault by the manufacturer. CMS also considered potential time limits for revisions and
whether certain circumstances, such as instances of false reporting, should be exempt from such
time limits.
Based on these considerations, CMS believes that, to capture an accurate rebate amount
and consistent with reconciliations of pricing data submitted to CMS that provide for revisions
when necessary due to errors, including mathematical errors, and manufacturer misreporting,
certain circumstances merit reconciliation of the rebate amount separate from the 12-month and
36-month reconciliations proposed at § 428.401(d)(1). Specifically, CMS proposes in
§ 428.401(d)(2) that CMS may reconcile a rebate amount of an issued Rebate Report when CMS
identifies either: (1) an agency error such as a mathematical error or an error in the information
specified in a Rebate Report as described in proposed § 428.401(c) or report of a reconciled
rebate amount as described in proposed § 428.401(d), including reporting system or coding
errors; or (2) CMS determines that information used to calculate the rebate amount was
inaccurate due to manufacturer misreporting. Examples of agency errors could include CMS
incorrectly calculating the billing units per Part D rebatable drug or the mechanism that provides
a Rebate Report to the manufacturer or the Rebate Report incorrectly displays a rebate amount.
Examples of manufacturer misreporting could include instances in which the manufacturer has
made a correction to previously submitted data as well as instances in which the reporting
individual or entity reporting data or information to CMS on behalf of the manufacturer knows
or should know is inaccurate or misleading (for example, inaccurate manufacturer pricing or
product data under section 1927(b)(3) of the Act). This does not include standard restatements to
AMP or other data outside of the standard process of issuing the reconciled rebate amount. In
addition to manufacturer-initiated corrections, CMS may become aware of manufacturer
misreporting based on fact finding and conclusions of enforcement authorities, for example, the
HHS Office of Inspector General, the CMS Center for Program Integrity, or the Department of
Justice. In a situation where an error or manufacturer misreporting is identified prior to the 12- or
36-month reconciliation of the rebate amount proposed in § 428.401(d)(1), CMS may choose to
include a correction based on the circumstances proposed in § 428.401(d)(2) concurrently with
the 12- or 36-month reconciliation. When CMS reconciles data due to an instance of agency
error or manufacturer misreporting, CMS proposes that the agency would limit the scope of the
reconciliation to the specific information that is the basis for the reconciliation and not update or
otherwise revise any other data elements in the Rebate Report (specified in proposed
§ 428.401(c)) or the report of the reconciled rebate amount (specified in proposed § 428.401(d))
unless the correction directly impacts additional data fields. For example, corrections to an AMP
file may not change the AnMP for the applicable period.
In addition, because reconciling a rebate amount imposes substantial administrative
burden on CMS to reprocess the rebate amount, retest the reporting system, and reissue a Rebate
Report, CMS proposes in § 428.401(d)(2) that it may exercise discretion not to initiate
recalculation of the rebate amount in these situations which are outside of the regular
reconciliation process proposed in § 428.401(d)(1).
CMS proposes that for a recalculation due to an agency error, the error must be identified
within 5 years of the date of receipt of the Rebate Report for the applicable period (see proposed
§ 428.401(d)(2)(i)). Identification means that CMS has knowledge of the error; CMS does not
need to have completed its revision of the impacted data or determined if the revision impacts the
rebate amount within the 5-year period. CMS would timely complete these steps and determine,
when reconciliation does impact the rebate amount, whether the reconciliation must be included
in a discretionary revision or within an upcoming reconciled rebate amount for the applicable
period. CMS proposes 5 years for Part D (as opposed to the 3-year limit proposed for Part B) to
account for the additional time of the second reconciliation for Part D rebatable drugs to be
conducted at 36-months proposed in § 428.401(d)(1). CMS believes that a 5-year period dating
from the issuance of the Rebate Report allows for sufficient time to include AMP restatements in
the MDRP while also placing a reasonable time limit on potential discretionary reconciliations,
after which a manufacturer of a Part D rebatable drug would not receive additional Rebate
Reports for the applicable period.
CMS proposes in § 428.401(d)(2)(ii) that for a circumstance in which a manufacturer
misreports data, CMS is not bound by the 5-year time limit for revision of the rebate amount. For
example, if a determination is made that a manufacturer misreported AMP data, which affected
the calculation of the AnMP, then CMS may recalculate the rebate amount owed for a Part D
rebatable drug. CMS requests comments on the proposals related to manufacturer misreporting.
CMS proposes in § 428.405(a)(1) that upon receipt of a reconciled rebate amount,
manufacturers must pay that reconciled rebate amount within 30 calendar days from the date of
receipt of the reconciled rebate amount. A 30-day payment deadline aligns with the payment
period set forth in statute at section 1860D-14B(b)(6) of the Act. As specified in proposed
§ 428.404, CMS would use the same method and process for issuing Rebate Reports and
submission of payments for reports with a reconciled rebate amount. CMS would provide notice
to manufacturers for reports with a reconciled rebate amount. CMS proposes in § 428.405(b) that
if a refund is owed to a manufacturer based on a reconciled rebate amount, CMS would initiate
the process to issue such refund within 60 days from the date of receipt of the reconciled rebate
amount (proposed in § 428.401(d)). CMS will issue additional information on this method and
process through additional program communications.
iv. Rebate Reports for the Applicable Periods Beginning October 1, 2022, and October 1, 2023
Section 1860D-14B(a)(3) of the Act provides the CMS with the option to delay sending
the information required by section 1860D-14B(a)(1) for the applicable periods beginning
October 1, 2022, and October 1, 2023, until not later than December 31, 2025. In § 428.402,
consistent with section 50.2 of the revised Medicare Part D Drug Inflation Rebate Guidance,
CMS proposes to issue a Preliminary Rebate Report for each applicable period followed by
issuance of the Rebate Report for each applicable period no later than December 31, 2025. For
these reports, CMS proposes in § 428.402 to provide an extended 30 calendar day Suggestion of
Error period for these Preliminary Rebate Reports.
Because this approach provides for 13 months of claims run-out for the Rebate Report for
the applicable period beginning October 1, 2022, CMS intends to conduct a single reconciliation
21 months after issuance of the Rebate Report for this applicable period (see proposed
§ 428.402(c)(1)(ii)). As specified in § 428.402(c)(2)(ii), for the applicable period beginning
October 1, 2023, the rebate amount would be reconciled twice, in alignment with the
reconciliation process discussed previously. The first reconciliation would occur 9 months after
issuance of the Rebate Report to include 13 months of claims run-out and payment data; the
second reconciliation would occur 24 months after the first reconciliation and would include
37 months of claims run-out and payment data. This approach aligns claims and payment data
run-out with the run-out used during a regular invoicing cycle. The Suggestion of Error period
would be 10 calendar days for the reconciliations of the rebate amount for the applicable periods
beginning October 1, 2022, and the applicable period beginning October 1, 2023.
This approach also minimizes the number of reports issued to manufacturers as a result of
the delay in reporting and simplifies payment procedures, thereby minimizing manufacturer
burden. Starting with the applicable period beginning October 1, 2024, reporting would begin a
standard cadence and follow the procedures otherwise proposed in subpart E of this part 428.
CMS proposes that manufacturers that do not pay the Medicare Part D drug inflation
rebate amount owed for a Part D rebatable drug within 30 calendar days of receiving a Rebate
Report, including reports containing a reconciled rebate amount, may be subject to a civil money
penalty of 125 percent of the rebate amount, as applicable, for such drug for the applicable
periods. The civil money penalty is in addition to the rebate amount.
f. Enforcement of Manufacturer Payment of Rebate Amounts (§ 428.500)
Section 1860D-14B(e) of the Act gives CMS the authority to impose a civil money
penalty equal to 125 percent of the rebate amount for each drug for each applicable period on a
manufacturer that fails to pay the rebate amount, for each dosage form and strength for each
rebatable drug. Subpart F would implement this section of the Act and establish the procedures
for determining and collecting a civil money penalty.
In accordance with sections 1860D-14B(a)(2) and 1860D-14B(b) of the Act and
proposed § 428.405(a), manufacturers must provide to CMS a rebate amount owed within
30 calendar days of receipt of the rebate amount due. As described in proposed § 428.500(a),
CMS is proposing it may impose a civil money penalty when a manufacturer fails to pay the
rebate amount in full by the payment deadlines in proposed § 428.405(a). This means a
manufacturer may be subject to a civil money penalty if the manufacturer fails to pay the full
rebate amount as invoiced in the Rebate Report or any reconciled rebate amount that is greater
than the amount invoiced in the Rebate Report. More specifically, a manufacturer could be
subject to a civil money penalty when a manufacturer fails to pay a rebate amount due by any
payment deadline proposed in § 428.405(a)(1), for: (1) a Rebate Report specified in proposed
§ 428.401(c); (2) a reconciled rebate amount greater than the amount reflected in the Rebate
Report specified in proposed § 428.401(d); or (3) a Rebate Report and a reconciled rebate
amount greater than the amount reflected in the Rebate Report, if applicable, for the applicable
periods beginning October 1, 2022, and October 1, 2023 specified in proposed § 428.402. As
discussed earlier in section III.I.3.e, CMS notes that the reconciled or corrected rebate amount is
not a separately payable and distinct rebate amount. Rather, the reconciled rebate amount is an
update to the rebate amount owed to CMS by a manufacturer of a Part D rebatable drug.
Civil money penalties are a point-in-time penalty tied to the rebate amount due at the
applicable payment deadline, which occurs 30 days after the date of receipt of a Rebate Report.
In proposed § 428.500(b), CMS proposes to establish the methodology for determining the
amount of the civil money penalty as equal to 125 percent of the rebate amount for such drug for
such applicable period, and that this penalty would be due in addition to the rebate amount due.
That is, a manufacturer would be responsible for paying the full rebate amount due in addition to
any civil money penalty imposed because of late payment. CMS is proposing this approach to
civil money penalties based on section 1860D-14B(a)(2) of the Act, which establishes a
requirement by the manufacturer to provide CMS with a rebate not later than 30 days after
receipt from CMS of the report on the amount of the excess annual manufacturer price increase.
CMS believes that the ability to assess civil money penalties is necessary in all circumstances
where a payment is due for a rebate amount to CMS to ensure compliance with the rebate
program’s requirements. The civil money penalty would be calculated based on the outstanding
rebate amount due at the payment deadline, which is defined in proposed § 428.405(a)(1) as
30 calendar days after the date of receipt of a Rebate Report containing any rebate amount due;
once a civil money penalty is assessed due to a late payment, the penalty would remain in effect
even if the manufacturer pays the outstanding rebate amount as the penalty is initiated due to a
missed payment deadline. Because the payment deadline is clearly defined in section 1860D-
14B(a)(2) of the Act, any late payments of a rebate amount due, including late payment of any
reconciled rebate amounts greater than the amount reflected in the Rebate Report, would be
considered a violation potentially subject to a civil money penalty. Any civil money penalty
would be assessed before the next 12- or 36-month reconciliation.
CMS is proposing in § 428.500(b) that civil money penalties may be calculated at several
points in time associated with missing a payment deadline for the rebate amount due reflected in
the Rebate Report or missing a payment deadline associated with any rebate amount determined
after a reconciliation to be greater than the amount invoiced in the Rebate Report. As these
separate events can result in distinct assessments of civil money penalties, this means that CMS
would not modify a civil money penalty from a prior missed payment deadline based on changes
to the rebate amount due following reconciliation, including scenarios where the rebate amount
is reduced following reconciliation. However, in the event that the rebate amount due on a
Rebate Report was not paid and a civil money penalty was issued for violation of the payment
deadline, CMS would not issue a second civil money penalty on a reconciled rebate amount if
reconciliation decreased the rebate amount stated on the Rebate Report. CMS believes that
enforcing this requirement after each payment deadline, regardless of what rebate amount a
manufacturer may or may not owe at a future payment deadline, is necessary to maintain the
integrity of the program and consistency of the implementation of the program. Further, CMS is
proposing this approach to ensure an enforcement approach that is operationally feasible and
applied consistently in all cases.
For examples of how this approach to civil money penalties will work in practice, see
section III.I.2.g of this preamble. CMS is proposing that civil money penalties will function in
the same way for both the Part B and Part D rebate programs. Given that the Part D rebate
program has two proposed regular reconciliations, payment would be due no later than 30 days
after issuance of a report of a reconciled rebate amount for each reconciliation under Part D.
Further, note that payment of any civil money penalty does not obviate the requirement
for the manufacturer to pay any outstanding rebate amount due, including any rebate amount due
following a reconciliation. Therefore, paying a civil money penalty does not satisfy the
obligation to pay the underlying rebate amount on which the civil money penalty is calculated. In
addition, CMS is evaluating all available options to ensure manufacturers’ timely compliance
with their rebate payment obligations, including, without limitation, potential recovery
approaches and enforcement actions. For example, CMS may refer manufacturers to the
Department of Justice, Department of the Treasury, and/or the Department of Health and Human
Services Office of Inspector General for further review and investigation.
In proposed § 428.500(c), CMS proposes that if CMS makes a determination to impose a
civil money penalty on a manufacturer for violation of a payment deadline, CMS would send a
written notice of the decision to impose a civil money penalty that includes a description of the
basis for the determination, the basis for the penalty, the amount of the penalty, the date the
penalty is due, the manufacturer’s right to a hearing, and information about where to file the
request for a hearing. To ensure a consistent approach to civil money penalties, CMS proposes
applying existing appeal procedures for civil money penalties in 42 C.F.R. section 423, subpart T
of this title to manufacturers appealing a civil money penalty imposed under the Medicare Part D
Drug Inflation Rebate Program. CMS has utilized this appeals process for many years for civil
money penalty determinations affecting MA organizations and Part D sponsors. CMS therefore
proposes to use this well-established process for civil money penalty appeals from manufacturers
that do not make inflation rebate payments by the payment deadline. CMS also proposes in
§ 428.500(e)(1) that the scope of appeals is limited to: (1) CMS determinations relating to
whether the rebate payment was made by the payment deadline; and (2) the calculation of the
penalty amount. Section 1860D-14B(f) of the Act precludes judicial review of specific data
inputs or calculations related to the underlying Rebate Report and reconciliation; therefore, such
data and calculations are not appealable through this process.
Section 1860D-14B(e) of the Act states that the provisions of section 1128A of the Act
(except subsections (a) and (b)) apply to civil money penalties under this subpart to the same
extent that they apply to a civil money penalty or procedure under section 1128A(a) of the Act.
CMS proposes to codify this requirement in proposed § 428.500(f). In alignment with the
procedure outlined in section 1128A of the Act, CMS proposes in § 428.500(d) that collection of
the civil money penalty would follow expiration of the timeframe for requesting an appeal,
which is 60 calendar days from the civil money penalty determination in cases where the
manufacturer did not request an appeal. In cases where a manufacturer requests a hearing and the
decision to impose the civil money penalty is upheld, CMS would initiate collection of the civil
money penalty once the administrative decision is final. CMS is seeking comment on proposals
related to the violations of payment deadlines and issuance of a civil money penalty.
CMS proposes in § 428.500(g) that in the event that a manufacturer declares bankruptcy,
as described in title 11 of the United States Code, and as a result of the bankruptcy, fails to pay
either the full rebate amount owed or the total sum of civil monetary penalties imposed, the
government reserves the right to file a proof of claim with the bankruptcy court to recover the
unpaid rebate amount and/or civil monetary penalties owed by the manufacturer.
g. Severability (§ 428.10)
Proposed § 428.10 provides that, were any provision of part 428 to be held invalid or
unenforceable by its terms, or as applied to any person or circumstance, such provisions would
be severable from this part and the invalidity or unenforceability would not affect the remainder
thereof or any other part of this subchapter or the application of such provision to other persons
not similarly situated or to other, dissimilar circumstances. While the provisions in part 428 are
intended to present a comprehensive approach to implementing the Medicare Part D Drug
Inflation Rebate Program, we intend that each of them is a distinct, severable provision, as

proposed, and would not affect similar provisions in the Medicare Part B Drug Inflation Rebate
Program. Through this rulemaking, the Part D drug inflation rebate proposals are intended to
operate independently of each other, even if each serves the same general purpose or policy goal.
For example, CMS intends that the policies CMS is proposing related to exclusion of units
acquired through the 340B Program (proposed § 428.203(b)(2)) are distinct and severable from
the proposals related to Determination of Part D Rebatable Drugs (proposed §§ 428.100 and
428.101). Even where one provision makes reference to a second provision, the preamble and the
regulatory text clarify the intent of the agency that the two provisions would be severable if one
provision were to be invalidated in whole or in part. For example, CMS would still be able to
calculate a Part D drug inflation rebate even if the provision to identify the payment amount
benchmark period for a Part D rebatable drug as the first calendar year in which such drug has at
least 1 quarter of AMP in certain instances of missing AMP is deemed invalid (proposed
§ 428.202(c)(3) and (4)). CMS welcomes comments on this severability policy.
J. Request for Information: Building upon the MIPS Value Pathways (MVPs) Framework to
Improve Ambulatory Specialty Care
This request for information seeks input from the public regarding the design of a future
ambulatory specialty model. Responses to this request for information may be used to inform
potential future rulemaking and other policy development.
1. Background
Medicare beneficiaries’ care is becoming more fragmented as they are increasingly
seeing (1) more specialists and (2) specialists more often, while the number of visits with their
primary care clinician remains relatively constant.
598,599
A primary care team must now
598
Barnett ML, Bitton A, Souza J, Landon BE. Trends in Outpatient Care for Medicare Beneficiaries and
Implications for Primary Care, 2000 to 2019 [published correction appears in Ann Intern Med. 2022 Oct;175(10):
1492]. Ann Intern Med. 2021;174(12): 1658-1665. doi: 10.7326/M21-1523.
599
Timmins L, Urato C, Kern LM, Ghosh A, Rich E. Primary Care Redesign and Care Fragmentation Among
Medicare Beneficiaries. The American Journal of Managed Care, March 2022, Volume 28, Issue 3.

coordinate with more specialists than ever before
600
to achieve continuity in beneficiary care.
Medicare beneficiaries with chronic conditions, in particular, are at high risk of excess
emergency department visits due to fragmented care.
601
In 2021, the Innovation Center announced a strategic refresh with a vision for a health
care system that achieves equitable outcomes through high-quality, affordable, person
centered-care. This includes a bold goal of having 100 percent of Medicare fee-for-service (FFS)
beneficiaries and the vast majority of Medicaid beneficiaries in an accountable care relationship
by 2030.
602
To expand accountable care among specialists and to drive more person-centered
care thereby improving the quality, clinical outcomes, and affordability of healthcare for
beneficiaries, the Innovation Center has created a comprehensive specialty strategy to test
models and innovations. This strategy includes: (1) enhancing transparency of specialist data
and performance measures; (2) maintaining momentum established by episode payment models
by extending the Bundled Payments for Care Improvement Advanced (BPCI Advanced) Model,
launching a new model focusing on beneficiaries with cancer, and testing a new mandatory acute
episode payment model; (3) creating financial incentives to improve coordination and
collaboration between primary care and specialty care in both advanced primary care models and
in condition-based models; and (4) creating additional financial incentives for specialists to
affiliate with population-based models and move to value-based care.
603,604
As part of this strategy, we are considering a model design that would increase the
engagement of specialists in value-based payment and encourage specialty care provider
600
Center for Medicare and Medicaid Services. The CMS Innovation Center’s Strategy to Support Person-centered,
Value-based Specialty Care. November 7, 2022. https: //www.cms.gov/blog/cms-innovation-centers-strategy-
support-person-centered-value-based-specialty-care.
601
Kern, LM, Seirup, JK. Fragmented Ambulatory Care and Subsequent Healthcare Utilization Among Medicare
Beneficiaries. The American Journal of Managed Care. 2018;24(9):e278-e284.
602
Centers for Medicare and Medicaid Services. Driving Health System Transformation - A Strategy for the CMS
Innovation Center’s Second Decade. October 2021. https://www.cms.gov/priorities/innovation/strategic-direction-
whitepaper.
603
Center for Medicare and Medicaid Services. The CMS Innovation Center’s Strategy to Support Person-centered,
Value-based Specialty Care. November 7, 2022. https: //www.cms.gov/blog/cms-innovation-centers-strategy-
support-person-centered-value-based-specialty-care.
604
"The CMS Innovation Center’s Strategy to Support Person-Centered, Value-Based Specialty Care: 2024
Update." Health Affairs Forefront, April 2, 2024. DOI: 10.1377/forefront.20240328.868596.
engagement with primary care providers and beneficiaries. Specifically, we are currently
exploring developing a model for specialists in ambulatory settings that would leverage the
Merit-based Incentive Payment System (MIPS) Value Pathways, or MVP, framework. As
currently envisioned, participants under this model would not receive a MIPS payment
adjustment. Instead, a model participant would receive a payment adjustment based on (1) a set
of clinically relevant MVP measures that they are required to report and (2) comparing the
participant’s final score against a limited pool of clinicians (other model participants of their
same specialty type and clinical profile, who are also required to report on those same clinically
relevant MVP measures). Currently, under MIPS, performance and the subsequent payment
adjustment are based on a range of measures voluntarily reported by clinicians, who receive a
final score based on the submitted measures. A clinician’s performance is assessed against a pool
of all clinicians, regardless of specialty type or the services they provide. We expect that a more
targeted approach where clinicians are evaluated (1) on a set of relevant performance measures
they are required to report, and (2) among clinicians furnishing similar sets of services, would
produce scores and subsequent payment adjustments that are more reflective of clinician
performance. A more targeted approach to measurement would also offer more insight into how
clinical decisions and processes, such as care coordination, affect patient outcomes. We believe
this insight is necessary to support and incentivize accountable care, increasing beneficiary
access to coordinated specialty care. Furthermore, equipped with more specialty-relevant
performance information, we expect clinicians would be more likely to invest resources in
pursuit of better outcomes, reducing the incidence of poor outcomes arising from care
fragmentation, ultimately resulting in better care for patients.
MVPs are a reporting option under MIPS, which is one of the two primary ways a
clinician may participate in the CMS Quality Payment Program. The Quality Payment Program,
which commenced on January 1, 2017, was established pursuant to the Medicare Access and
CHIP Reauthorization Act of 2015 (Pub. L. 114–10, April 16, 2015). The Quality Payment
Program rewards the delivery of high-quality patient care through two avenues: Advanced
Alternative Payment Models (Advanced APMs) and MIPS for eligible clinicians or groups under
the Physician Fee Schedule (81 FR 77008). CMS assesses performance on measures and
activities in four performance categories to determine each MIPS eligible clinician's performance
under MIPS: quality; cost; clinical practice improvement activities; and Promoting
Interoperability (42 CFR 414.1330, 414.1350, 414.1355, and 414.1375).
In response to concerns made by interested parties that MIPS requirements are confusing,
burdensome, and that it is difficult to choose measures from the several hundred MIPS and
QCDR quality measures that are meaningful to their practices and have a direct benefit to
patients, CMS developed the MVP reporting option to create a simplified MIPS clinician
experience, improve value, reduce burden, and better inform patient choice in selecting clinicians
(86 FR 65376). MVPs provide MIPS eligible clinicians with a more cohesive subset of measures
and activities related to a specific specialty or condition (86 FR 65420 through 65427). MVPs
are developed in coordination with interested parties through an established process in which
clinician and patient perspectives are incorporated (85 FR 84850). The use of MVPs can create
more meaningful performance data, reduce complexity of the MIPS program for clinicians, and
lower the burden on participating clinicians. MIPS eligible clinicians have been able to report on
MVPs beginning with the CY 2023 MIPS performance period.
Like MIPS eligible clinicians participating in traditional MIPS, those who report MVPs
receive an adjustment to their Medicare Part B fee-for-service payments 2 years after the
corresponding MIPS performance period based on a total score calculated from reported
measures and activities across the 4 MIPS performance categories (see §§ 414.1365,
414.1405(e), and 414.1305). MVPs are designed to cover a range of medical conditions, care
settings, and clinician types, including primary care providers and specialists. For the 2024
performance year, 16 MVPs are reportable, allowing for a range of specialties to report a
streamlined set of measures most applicable to services they provide (88 FR 79978 through
80047). For information on the MVPs reportable for the 2024 performance period we refer
readers to sections IV.A.4 and Appendix 3 of this proposed rule.
Currently, 5 MVPs have quality measures including patient-reported outcome measures
and chronic condition episode-based cost measures, which could be the foundation for assessing
the value of care provided to chronic care patients. We believe coordination between primary
and specialty care is particularly critical to the ongoing management of chronic conditions with
beneficiaries not only for reasons of quality and cost, but also in understanding beneficiary goals,
expectations, and experiences with care.
CMS conducts ongoing MVP development through engagement with interested parties
with the long-term goal of sunsetting traditional MIPS reporting and making MVP reporting
mandatory for MIPS eligible clinicians in the future (86 FR 65395 and 87 FR 70040). For more
information on the long-term goal of sunsetting traditional MIPS reporting, see section IV.A.3.b.
of this proposed rule. For more information on CY 2025 MVP reporting option proposals under
the MIPS program, see section IV.A.4 of this proposed rule.
Using the MVP framework as the foundation for a model has many benefits. First, the
MVP framework advances value-based care by narrowing the available measure set based upon
clinician specialty, medical condition, or patient population, which allows for meaningful
comparisons to be made across providers and relevant feedback to be available to participants on
their performance, strengthening the foundation for accountability in specialty care. The MVPs
provide a framework for reporting a cohesive set of measures and activities focused on the
clinician’s performance in rendering care for their specialty or clinical condition.
Second, the payment methodology for the model built on MVPs could address concerns
interested parties have raised about the MIPS program. For instance, CMS has heard from
interested parties that the current range of Medicare Part B payment adjustments resulting from
MIPS participation may be insufficient to encourage meaningful specialty care transformation
that results in increased integration between primary and specialty care. The model could test
ways to enhance existing incentives, allowing for more specific comparisons to be made between
clinicians of the same type who are providing similar services to patients.
Third, such a model could reach a broad range of clinicians of various specialty types that
have limited opportunity to participate in Advanced APMs. There are 16 MVPs for the 2024
performance year spanning numerous specialties, and CMS is proposing additional MVPs for the
2025 performance year with the goal of creating MVPs that would be relevant to the practices of
80 percent of MIPS eligible clinicians. Using an existing framework that is agnostic to specialty
type, as opposed to creating multiple unique models that are each narrowly defined by a
condition or specialty, would allow the Innovation Center to take a more inclusive and unified
approach to increasing specialist engagement in value-based payment.
While CMS continues to develop more MVPs for additional health conditions and
specialties, an ambulatory specialty model leveraging the MVP framework could focus on a
subset of published MVPs in the initial years of implementation, with the goal of increasing the
number of MVPs, and thus the range of health conditions or specialty areas, included in the
model over time. Using specific MVPs as the basis for a model would, in part, require that
selected MVPs cover a sufficient volume of clinicians, address chronic conditions with high
Medicare expenditures, align with existing Innovation Center models (for example, the Making
Care Primary model), and present an opportunity to strengthen the integration between specialty
care and primary care.
We are soliciting comments on several parameters of a potential model, including
considering mandatory participation of relevant specialty care providers to overcome challenges
such as selection bias and participant attrition, and to ensure the model is reaching a
representative group of providers and beneficiaries to facilitate scaling of the model test. If CMS
were to propose a mandatory specialty model, it would be done via notice and comment
rulemaking. We expect this ambulatory specialty model would be implemented no earlier than
2026, ensuring participants have sufficient time to prepare for the model.
2. Solicitation of Public Comments
The Innovation Center is releasing this request for information (RFI) to gather feedback
on testing a new model design to improve clinical outcomes and reduce or maintain Medicare
spending. We request feedback on the design of a future ambulatory specialty model, specifically
on the following--
● Participant definition;
● MVP performance assessment;
● Payment methodology;
● Care delivery and incentives for partnerships with accountable care entities and
integration with primary care;
● Health information technology and data sharing;
● Health equity; and
● Multi-payer alignment.
Whenever possible, respondents are requested to draw their responses from objective,
empirical, and actionable evidence and to cite this evidence within their responses.
a. Participant Definition
A key component of this potential model is to define the Medicare Part B clinicians that
could participate in the model. Participants in an ambulatory specialty model using the MVP
framework could be limited to MIPS eligible clinicians (see § 414.1305) with specific
ambulatory-based specialties for a specific clinical focus area(s). The model design must also
consider whether and how clinicians participating in other APMs and Advanced APMs (see §§
414.1305 and 414.1415) would participate in the model.
To select participants, an ambulatory specialty model would need to determine which
clinicians are specialists and sub-specialists practicing in the specified clinical areas prioritized in
the model. This process may be complex as a single specialty often comprises several sub-
specialties, all of which have specific clinical areas of expertise and practice. For example, the

specialty of cardiology has subspecialties, including general cardiology, interventional
cardiology, electrophysiology, among others; and interventional cardiologists treat a wide range
of acute and chronic cardiovascular conditions and may receive advanced training in the
endovascular treatment of specific conditions, including coronary artery disease and peripheral
artery disease. The MVP framework could provide a natural starting point to identifying
participants within a given specialty or sub-specialty. Each MVP provides measures and
activities for a range of specialties and sub-specialties in a clinical area or health topic, allowing
for specialties and sub-specialists to select an applicable MVP and report the measures and
activities most relevant to them.
MVP currently policies do not require specialists to report specific MVPs but encourage
individuals or groups that are MIPS eligible clinicians to elect to be an MVP Participant and
report an MVP that is clinically relevant to them. Beginning with the CY 2026 performance
period, an MVP Participant is assessed on an MVP in accordance with § 414.1365 as an
individual MIPS eligible clinician,
605
a single specialty group,
606
subgroup,
607
or APM entity
608
(see § 414.1305, “MVP participant”). CMS has encouraged MIPS eligible clinicians that are
multispecialty groups to participate in subgroup reporting to allow the group to report measures
more relevant to each of the group’s constituent specialties. Multispecialty groups that choose to
report an MVP will be required to form subgroups for that purpose beginning in the CY 2026
performance period (§ 414.1305, “MVP participant”).
605
A MIPS eligible clinician who is an individual and elects to report 1 MVP relevant to the clinician’s clinical
practice area(s).
606
A single specialty group that is a MIPS eligible clinician and reports 1 MVP. Single specialty group means a
group that consists of one specialty type as determined by CMS using Medicare Part B claims (see § 414.1305). A
groups means a single TIN with two or more clinicians (including at least one MIPS eligible clinician), as identified
by their individual NPI, who have reassigned their billing rights to the TIN (Id.).
607
Subgroups means a subset of a group which contains at least one MIPS eligible clinicians and is identified by a
combination of the group TIN, subgroup identifier, and each eligible clinician’s NPIs (see § 414.1305). Each
subgroup consists of 2 or more clinicians from a group that is a MIPS eligible clinician. Multiple subgroups can
form from a single group, which may be either a single specialty group or a multispecialty group. Each subgroup
reports 1 MVP. An individual eligible clinician (as represented by a TIN-NPI combination) may register for no more
than one subgroup within a group's TIN. (see § 414.1318(a)(3)).
608
An APM entity is a participant in an APM or other payer arrangement through a direct agreement with CMS,
other payer, or through Federal or State law or regulation. An APM Entity can report and be scored on multiple
MVPs that are of clinical interest or relevance (see § 414.1305).

Within an ambulatory specialty model, identification of a given specialty or sub-
specialty—including whether an individual would be selected to participate in the model at an
individual, group, or subgroup level based on TIN and NPI combinations—would need to rely on
data sources to which CMS already has access. Further, approaches to participant identification
would need to appropriately identify non-physician clinician types (for example, physician
assistants or nurse practitioners) that may practice within a specific specialty or sub-specialty or
both but may not be categorized as practicing within a specific specialty within existing data
systems.
As multispecialty groups reporting MVPs in the 2026 MIPS performance period must
divide into subgroups, CMS finalized a process by which it will determine whether a MIPS
eligible clinician that is a group practice is a single specialty group or multispecialty group. CMS
finalized that it will use Medicare Part B claims data to determine a group's specialty type based
on the strong alignment between the Medicare Provider Enrollment, Chain, and Ownership
System (PECOS) and Medicare Part B claims data sources, and historical use of claims data to
identify a clinician or practice’s specialty (87 FR 70038 through 70040).
Given the different circumstances and challenges faced by clinicians working in specific
contexts, an ambulatory specialty model could consider additional characteristics of clinicians or
practices in addition to the identification of participants based on specialty or sub-specialty.
609
Additional characteristics that could be considered in participant selection in a model could be
related to factors documented to be associated with disparities in access to specialty care and
disparities in specialty-sensitive outcomes. Such factors could include being a solo clinician,
609
For example, special status designations for MIPS eligible clinicians confer additional flexibilities in
performance category reporting requirements and category reweighting within final score calculations. For the 2024
performance period, several provider designations can receive additional flexibilities in MVP reporting. Ambulatory
Surgical Center (ASC)-based, hospital-based, non-patient facing, and small practice-based (15 or fewer clinicians)
providers are eligible for automatic reweighting of the Promoting Interoperability performance category to 0
percent, with the category weight redistributed to other performance categories. Small practice-based participants
are also eligible for additional scoring flexibilities in the quality performance category (see § 414.1380). MVP
policies for the CY 2024 performance period state that special status determination is determined at the group level
for subgroup participants (see § 414.1365).
practicing within a rural area, serving a higher proportion of Medicare and Medicaid dually
eligible beneficiaries and patients receiving Medicare Part D low-income subsidies, operating
within a designated Health Provider Shortage Area, among others. For further discussion on
additional health equity considerations for the model, see section III.J.2.f. of this proposed rule,
Health Equity.
Participant identification approaches would also need to consider the heterogeneity of
existing specialty group practices as the variation in clinical practice across different specialties
has historically presented challenges for specialists to engage more deeply in value-based care.
Assuming an ambulatory specialty model would initially focus on a subset of specialists
and eventually incorporate additional specialties over time, CMS would need to identify
specialists for the prioritized clinical areas before the model begins. CMS seeks feedback on the
following questions:
(1) How should CMS identify single specialty and multispecialty groups while
accounting for regular clinician turnover? Which data sources and methodology should CMS
use to consider identifying specialists and sub-specialties that could potentially participate in an
ambulatory specialty model?
(2) Should CMS consider different identification approaches to identify individual
clinician specialist type versus practice- or group-level specialty types? If so, how?
(3) Are there certain characteristics of clinicians or practices or both that may warrant
additional policy flexibilities or exemption from participation in a mandatory ambulatory
specialty model? What flexibilities should CMS consider for these participants?
(4) How should CMS collect unbiased comparison group data on quality and costs for
evaluation purposes? Would mandating a control group to report MVPs be appropriate for model
evaluation?
(5) How can CMS support a multispecialty group’s ability to successfully participate in
MIPS and the model if a portion of its clinicians are reporting separate measures pursuant to the

model? What steps could CMS take to reduce any added administrative burden that might arise
from such separate reporting?
b. MVP Performance Assessment
CMS adjusts future Medicare Part B payments to MIPS eligible clinicians based on the
assessment of measures and activities
610
reported across the 4 MIPS performance categories:
Quality (including Population Health), Cost, Improvement Activities, and Promoting
Interoperability (see §§ 414.1380 and 414.1405). MVP Participants are assessed in the same
manner (see § 414.1365(d)). Scoring policies and procedures are standardized across MVPs (see
§ 414.1365(d)(3)). Resulting performance category scores are weighted as defined in regulations
(see §§ 414.1365(e)(1) and 414.1380(c)) and are aggregated to compute an overall final MVP
score, which ranges from 0 to 100 points.
Quality – Each MVP defines a set of reportable quality measures relevant to the specialty
or medical condition that provide meaningful and actionable results. Quality measure sets are
designed to cover a range of process and outcomes measures that are applicable to a range of
clinicians working within a given specialty or medical condition. As of the CY 2024
performance period, MVP Participants must report at least 4 quality measures, including at least
1 outcome measure or 1 measure designated “high priority” if an outcome measure is not
available within an MVP (see §§ 414.1365(c)(1) and 414.1305). Individual quality measures
must also meet case minimums to be scored (see § 414.1380(b)(1)). Please refer to section
IV.A.4.c.(1). of this proposed rule for proposed quality measure reporting and scoring policies
for the CY 2025 performance period. MVP participants are also scored on population health
administrative claims-based measures, which are factored into the MVP participant’s overall
quality performance category score (see § 414.1365(c)(4)(ii)) (please refer to sections
IV.A.4.c.(1).(a). and IV.A.4.c.(1).(b). of this proposed rule for proposed population health
610
Pursuant to section 1848(q)(2)(D) of the Act, CMS selects measures for inclusion in the MIPS program through
notice and comment rulemaking. To date, CMS has also elected to subject each MIPS measure to the pre-
rulemaking process pursuant to section 1848(q)(2)(D)(ix) of the Act.

measure reporting and scoring policies for the CY 2025 performance period).
611
The population
health measures help indicate the quality of a population or cohort’s overall health and well-
being, such as, access to care, clinical outcomes, coordination of care and community services,
health behaviors, preventive care and screening, health equity, or utilization of health services
(see § 414.1305).
Cost – Each MVP identifies cost measures that are relevant and applicable to the MVP
topic or medical condition. Given the availability of applicable cost measures, the number of
reportable cost measures varies across MVPs. An MVP may include the episode-based cost
measures (EBCM) that are relevant to MVP topic, total per capita cost (TPCC) measure, and
Medicare Spending Per Beneficiary Clinician (MSPB Clinician) measure. Cost measures are
calculated by CMS using administrative claims data, and CMS scores MVP participants on all
cost measures included in the MVP that they select and report (see § 414.1365(d)(3)(ii)). Please
refer to section IV.A.4.c.(2). of this proposed rule for proposed cost measure reporting and
scoring policies for the CY 2025 performance period/2027 MIPS payment year.
Improvement Activities – Improvement activities (IAs) are activities that relevant MIPS
eligible clinician, organizations and other relevant interested parties identify as improving
clinical practice or care delivery and that are likely to result in improved outcomes. (see §
414.1305). Improvement activities may cover multiple improvement domains, including
expanded patient access, population management, care coordination, beneficiary engagement,
patient safety and practice assessment, health equity, emergency response and preparedness, and
behavioral and mental health (see § 414.1355(c)). MVP participants must report IAs included in
a given MVP while meeting overall IA reporting requirements for the performance period.
611
For the CY 2024 performance period, the two population health measures from which participants could select to
be scored are: Hospital-Wide, 30-day, All-Cause Unplanned Readmission Rate for the MIPS Groups and Clinician,
or Clinician Group Risk-standardized Hospital Admission Rate for Patients with Multiple Chronic Conditions. If the
participant does not meet the case minimum for either measure, then the population health measure is excluded from
scoring within the quality performance category.
Please refer to section IV.A.4.c.(3). of this proposed rule for proposed IA reporting and scoring
policies for the CY 2025 performance period.
Promoting Interoperability – The Promoting Interoperability requirements of MVPs are
intended to emphasize the electronic exchange of information using certified electronic health
record technology (CEHRT), patient access to health information, exchange of information
among clinicians, and the systematic collection, analysis, and interpretation of health care data.
As of the CY 2024 performance period/2026 MIPS payment year, MVP participants are required
to report the entire MIPS Promoting Interoperability measure set, which includes measures
related to use of CEHRT for e-prescribing, health information exchange, public health and
clinical data exchange, and clinician-to-patient exchange, as well as required attestations, as
specified by CMS (see §§ 414.1365(c)(4)(i) and 414.1375(b)). Please also refer to section
IV.A.4.c.(4). and Table 62 for required objectives and measures for the MIPS Promoting
Interoperability performance category for the CY 2025 performance period/2027 MIPS payment
year.
Operating within the MVP measures framework, an ambulatory specialty model would
collect data on measures across quality, cost, and improvement activities performance categories
that would be clinically relevant to specialties or sub-specialties or both within an MVP topic
specified by the model. As discussed in III.J.1. of this proposed rule, the model could use a
subset of measures and activities included in an MVP that are most clinically relevant to the
model’s clinical topics and specialties. A clinically focused measure and activity set could allow
CMS to set performance standards that are most clinically relevant to sub-specialties within an
MVP topic under the model.
CMS requests the following feedback related to incorporating measures and activities
from MVP performance categories in a possible ambulatory specialty model:
(6) If CMS were to reduce the number of measures and activities in an MVP for
clinicians participating in the model to those measures and activities most relevant to a specified

specialty or subspecialty, how should CMS select the measures and activities? Consider the
following prioritization approaches: (a) measures with a performance gap; (b) measures with
meaningful benchmarks that can be applied; (c) measures that are reliable in the model context
given the expected sample size; (d) measures that are evidence-based and either strongly linked
to outcomes or an outcome measure; (e) measures that capture an adequate number and
representativeness of the clinicians intended by a possible ambulatory specialty model; (f)
measures that drive specialty integration with primary care and meaningful involvement with
accountable entities. Are there other measure selection principles that should be prioritized when
narrowing measuring in an MVP?
(7) Are there specific measure focus areas or objectives that should be prioritized across
MVPs (such as equity, population health measures, or patient-reported outcome-based
performance measures (PRO-PMs) and patient-reported experience measures)?
(8) To support improvements in primary and specialty care integration, an ambulatory
specialty model could initially focus on specialty types eligible to become rostered specialty care
partner clinicians in the MCP model, which include general cardiologists and physical medicine
and rehabilitation clinicians. Accordingly, which measures within the Advancing Care for Heart
Disease MVP and the Rehabilitative Support for Musculoskeletal Care MVP might be subset to
apply to general cardiology and physical medicine and rehabilitation, respectively?
(9) Similar to how other Innovation Center models may test new measures during their
implementation (for example, the Comprehensive Joint Replacement model (80 FR 73358
through 73382 and 86 FR 23543 through 23549) and the Guiding an Improved Dementia
Experience (GUIDE) Model
612
), what role could an ambulatory specialty model have in testing
potential new measures, such as relevant PRO-PMs, by gathering data for consideration in future
MVP measure sets?
612
https://www.cms.gov/files/document/guide-rfa.pdf.
(10) What kinds of strategies could be tested to obtain patient and family feedback on
how they experience care coordination between primary care and specialty care for the clinical
focus areas of the model?
(11) What types of peer engagement would specialists consider valuable to enhance their
performance within a given sub-specialty or clinical topic?
c. Payment Methodology
MIPS final scores are used to determine payment adjustments for future Medicare Part B
payments during the payment year that occurs 2 calendar years following the MIPS performance
period (for example, scores from the CY 2021 MIPS performance period resulted in Part B
Medicare payment adjustments for the 2023 calendar year) (see § 414.1305). CMS scores a
MIPS eligible clinician’s performance on measures and activities in accordance with section
1848(q)(5) of the Act and regulations at §§ 414.1317, 414.1365, 414.1367, and 414.1380, as
applicable to determine a final score for each MIPS eligible clinician.
In accordance with section 1848(q)(6) of the Act and § 414.1405(b), CMS compares each
MIPS eligible clinician’s final score against the performance threshold established for that MIPS
payment year and against one another in a single comparison pool to determine whether each
MIPS eligible clinician will receive a positive, negative, or neutral payment adjustment. CMS
calculates MIPS payment adjustment factors in accordance with regulations at § 414.1405.
Scores equal to the defined performance threshold receive a neutral (0 percent) payment
adjustment. Scores falling below one-quarter of the performance threshold receive a negative
adjustment of minus 9 percent, while scores between one-quarter of the performance threshold
and the performance threshold receive a negative payment adjustment less than 0 percent and up
to minus 9 percent based on a linear sliding scale. Scores above the performance threshold can
receive positive payment adjustments greater than 0 percent and up to positive 9 percent based
on a linear sliding scale. Depending on the range of scores within a given performance period, a
scaling factor (ranging from 0 to 3) is applied to positive adjustments to retain budget neutrality.

For the CY 2022 performance period/2024 MIPS payment year, over 624,000 clinicians
received MIPS payment adjustments to their Medicare Part B payments based on participation in
traditional MIPS: 14 percent of MIPS eligible clinicians received negative MIPS payment
adjustments, 7 percent received neutral MIPS payment adjustments, and 79 percent received
positive MIPS payment adjustments with a maximum positive payment adjustment of 8.26
percent.
613
MVPs were not a reporting option for the CY 2022 performance period/2024 MIPS
payment year.
As discussed in III.J.1. of this proposed rule, an ambulatory specialty model allow for
more specific comparisons between clinicians of the same type who are providing similar
services to patients. Comparing clinicians that provide a similar type of specialty care to
determine future Medicare Part B payment adjustments could lead to payment adjustments that
are more reflective of the range of performance of similar clinicians caring for beneficiaries
within a given clinical topic area. Such comparisons could create financial incentives that drive
quality improvement and care transformation within a given clinical topic and specialty. Further,
a more targeted approach to performance measurement and assessment, including enhanced data
on clinical and financial performance relative to similar clinicians, could incentivize shared
accountability for care and lead to increased beneficiary access to coordinated specialty care.
In considering an ambulatory specialty model that leverages the MVP framework to
increase specialist engagement in value-based care and transform specialist care delivery, CMS
is requesting feedback on the following questions:
(12) How could a model for applicable specialists improve the comparison of similar
specialists to determine future Medicare Part B payment adjustments?
613
QPP 2022 Participation Results At-a-Glance https://qpp-cm-prod-
content.s3.amazonaws.com/uploads/2816/QPP-2022-Participation-and-Performance-Results-At-A-Glance.pdf.
Note that for the CY 2022 performance period, MIPS participants were eligible for an additional MIPS payment
adjustment factor for exceptional performance (see § 414.1405).
(13) What range of upside and downside risk (as measured by the range of possible
payment adjustments to future Medicare Part B claims) could incentivize increased and
meaningful participation of specialists in APMs, care transformation, and strengthened
integration between primary and specialty care?
(14) What model design features should CMS consider in designing an ambulatory
specialty care model that increases risk over time to potentially qualify the model for Advanced
APM (AAPM) status under the Quality Payment Program (see § 414.1415)?
d. Care Delivery and Incentives for Partnerships with Accountable Care Entities and Integration
with Primary Care
CMS is exploring how an ambulatory specialty model could encourage model
participants to better engage with primary care providers engaged in care coordination activities
in the MCP model, Shared Savings Program, and other current and future accountable care
models. By promoting coordination and partnership between specialists and these accountable
entities, CMS seeks to extend opportunities for specialists to deliver comprehensive longitudinal
care and improve the quality of care provided by both parties. For example, CMS could test the
inclusion of new improvement activities in model MVPs that are fulfilled through care
coordination activities with an accountable care entity or its participant clinicians. As CMS
considers opportunities to encourage specialist delivery of comprehensive longitudinal care, an
underlying priority is care delivery from the beneficiary’s perspective. To address the issue of
highly fragmented care delivery to the beneficiary, CMS recognizes the need for meaningful
engagement among those who deliver care and seeks to create incentives for partnerships
between specialist clinicians, accountable care entities, and primary care clinicians. To help us
ensure these accountable entities reduce fragmentation and provide patients with the highest
value care, we seek input on the following questions:
(15) Are there model design features not discussed here that would incentivize primary
care and specialty care providers to improve how beneficiaries experience care coordination?

(16) How can CMS best encourage specialist clinicians and accountable care entities
collaborate to establish clear care pathways and protocols that optimize patient outcomes while
ensuring efficient resource utilization?
(17) How may CMS identify specialists who are most engaged in care management, care
coordination, and care improvement activities with an accountable care entity?
(18) In what ways can the model define clear expectations and performance metrics for
specialists, beyond what exists in the current MVP measure sets, to foster a collaborative
environment with ACOs and primary care clinicians to enhance healthcare outcomes and reduce
costs? What levers, such as the MIPS’s Improvement Activities, could be used to support
participants to close the care loop back to accountable care entities or primary care or both?
(19) What characteristics should CMS consider in the design of this model to account for
variations between ACOs, such as whether the ACO is physician-owned versus hospital-owned
(or a low revenue ACO versus a high revenue ACO), whether or not an ACO identifies as an
integrated delivery system (IDS), and differences in regional healthcare landscapes and local
dynamics? What other characteristics should we consider?
(20) How can the model proactively address concerns related to increased consolidation,
ensuring that integration efforts do not lead to reduced competition and potential negative
impacts on healthcare quality and costs?
(21) How might risk categorization of ACOs influence the design of incentive structures
of model participants engaging with ACOs, and what adjustments might be necessary to
accommodate different risk levels?
e. Health Information Technology and Data Sharing
The Quality Payment Program releases publicly the QPP Experience Report Public Use
Files (with companion methodology and data dictionary documentation), which allow users to
review details concerning participation and performance information in the MIPS program
during each performance period. These files cover eligibility and participation, performance

categories, and final score and payment adjustments, with details at the TIN/NPI level on each of
the performance categories for the previous performance period. Data can be sorted by variables
like clinician type, practice size, scores, and payment adjustments. Clinicians and authorized
representatives of practices, virtual groups, and APM Entities (including Shared Savings
Program ACOs) also have access to summaries of data that they have submitted and that CMS
has collected on their behalf. This includes performance category-level scores and weights,
bonus points, measure-level performance data and scores, activity-level scores, payment
adjustment information, and patient-level reports. Clinicians frequently request more timely and
expansive data feedback through MIPS to help guide continuous improvement efforts. CMS
requests the following feedback on health information technology and data sharing:
(22) What specific issues should CMS consider when determining whether additional
requirements and objectives may be necessary beyond those currently specified in the MVP
framework around the use of health IT by specialists participating in a potential model?
(23) What investments in health IT or information exchange would be most beneficial to
helping specialists succeed in such a model?
(24) What is your experience with the integration of health IT systems? Please highlight
any inoperability issues or opportunities for seamless data exchange between different systems,
such as electronic health records (EHRs) and telehealth platforms.
(25) How should CMS structure the model and any health IT and data sharing
requirements to align with, build upon, and otherwise, leverage advances in Federal
interoperability policy (for example, USCDI and USCDI+ or FHIR; TEFCA)?
(26) What data or metrics or both are important to clinicians in terms of monitoring
performance and improving patient outcomes? What data or metrics or both should CMS share
publicly to help inform beneficiaries of clinician performance?
(27) What additional resources or support mechanisms could CMS provide to help
clinicians make sense of the data, enhancing the data’s usability, effectiveness, and frequency of

updates, so that clinicians acquire actionable insights for improving patient care and experience?
And to enable data-driven referrals?
(28) What supports can this new model provide for decreasing burden of data collection
and measure reporting?
f. Health Equity
Disparities in access to ambulatory specialty care and chronic condition outcomes are
well-documented, such as racial/ethnic and geographic disparities in rates of ischemic heart
disease (IHD) among Medicare fee-for-service beneficiaries.
614
For example, Medicare fee-for-
service beneficiaries identifying as American Indian/Alaska Native (28 percent) had the highest
age-standardized prevalence of IHD and Asian/Pacific Islander (22 percent) beneficiaries had the
lowest prevalence, compared to the IHD prevalence among White (27 percent), Black/African
American (26 percent), and Hispanic (25 percent) beneficiaries in 2021.
615
Medicare fee-for-
service beneficiaries with complex chronic conditions in rural areas have also been shown to
have comparable access to primary care physicians but significantly lower access to ambulatory
specialists and higher rates of avoidable hospitalizations for chronic conditions compared to
similar beneficiaries in urban areas.
616
Engaging specialists in value-based care to create more
integrated and efficient care provides an opportunity to expand access to specialty care for
historically underserved beneficiaries and advance health equity.
Consistent with President Biden's Executive Order 13985 on “Advancing Racial Equity
and Support for Underserved Communities Through the Federal Government” (86 FR 7009) and
Executive Order 14091 on “Further Advancing Racial Equity and Support for Underserved
614
Centers for Medicare & Medicaid Services. Ischemic Heart Disease Disparities in Medicare Fee-for-Service
Beneficiaries Data Snapshot. April 2021. https: //www.cms.gov/About-CMS/Agency-
Information/OMH/Downloads/OMH_Dwnld-DataSnashot-Ischemic-Heart-Disease.pdf.
615
Centers for Medicare & Medicaid Services. Ischemic Heart Disease Disparities in Medicare Fee-for-Service
Beneficiaries Data Snapshot. April 2021. https: //www.cms.gov/About-CMS/Agency-
Information/OMH/Downloads/OMH_Dwnld-DataSnashot-Ischemic-Heart-Disease.pdf.
616
Johnston KJ, Wen H, Joynt Maddox KE. Lack Of Access to Specialists Associated with Mortality and
Preventable Hospitalizations of Rural Medicare Beneficiaries. Health Aff (Millwood). 2019;38(12): 1993-2002. doi:
10.1377/hlthaff.2019.00838.

Communities Through the Federal Government” (88 FR 10825), CMS has made advancing
health equity
617
the first pillar in its Strategic Plan. Health equity is also one of the five objectives
in the Innovation Center’s 2021 Strategy Refresh. Improving access to high-quality, patient-
centered care is a goal for the Innovation Center, and ensuring historically underserved
beneficiaries are adequately represented in value-based care models may help reduce inequities
when designed with the proper incentives.
The Innovation Center is also committed to prioritizing the unique needs of clinicians
that care for a large proportion of underserved populations, such as creating flexibilities
clinicians may need to be successful in models. To advance health equity goals within payment
models, the Innovation Center has implemented multiple equity-focused strategies within
existing models, including, but not limited to development of participant health equity plans,
reporting of aggregated health-related social need (HRSN) screening,
618
reporting and analysis of
attributed beneficiary sociodemographic data to support identification and monitoring of health
disparities, and payment adjustments.
Within the MVP framework, several MVPs include equity-focused measures within the
quality and improvement activities performance categories, such as screening for HRSNs or
engaging community resources to address HRSNs. MVPs also provide additional reporting and
scoring flexibilities for certain special status designations, many of which may include clinicians
caring for historically underserved beneficiaries. There may be additional clinician- and practice-
level characteristics, including the characteristics of beneficiaries served, that would be
important to account for within an ambulatory specialty model to support clinicians in
617
We define health equity as the attainment of the highest level of health for all people, where everyone has a fair
and just opportunity to attain their optimal health regardless of race, ethnicity, disability, sexual orientation, gender
identity, socioeconomic status, geography, preferred language, and other factors that affect access to care and health
outcomes.
618
Health-related social needs are adverse social conditions that negatively impact a person’s health or healthcare
such as lack of access to transportation for appointments. See A Guide to Using the Accountable Health
Communities Health-Related Social Needs Screening Tools: Promising Practices and Key Insights. CMS. (Updated
Aug. 2022). https://www.cms.gov/priorities/innovation/media/document/ahcm-screeningtool-companion;
Accountable Health Communities Health-Related Social Needs Screening Tool.

identifying and working to improve disparities related to access to specialty care and associated
outcomes.
To understand the potential health equity impacts of a new ambulatory specialty model
and to help ensure the goals laid out in the CMS Strategic Plan and the Innovation Center
Strategy Refresh are met, we request feedback on the following questions:
(29) Similar to how other Innovation Center models may offer financial and technical
supports to certain qualifying clinicians (for example, safety net clinicians) as part of a model’s
health equity strategy (for example, the GUIDE model
619
), how might CMS support the
participation of clinicians in an ambulatory specialty model that may serve a higher proportion of
underserved patients (for example, small practices or clinicians in rural areas)?
(30) How could an ambulatory specialty model support participant efforts to identify
health disparities within their practices, identify actionable equity goals, and design and
implement strategies to improve identified disparities?
(31) How could an ambulatory specialty focused model work synergistically with other
primary care focused models to improve health disparities?
(32) How could an ambulatory specialty model encourage clinicians to collect and use
HRSN screening and follow-up data collected on patients attributed to the model?
(33) How can measure stratification among patient subgroups or use of composite health
equity measures improve how participants identifies and quantifies potential disparities in care
and outcomes related to ambulatory specialty care?
g. Multi-payer Alignment
Given the Innovation Center’s strategic goal to make multi-payer alignment available in
all new models by 2030
620
, we are seeking feedback on how to best promote multi-payer
619
Center for Medicare and Medicaid Services. Guiding an Improved Dementia Experience (GUIDE) Model.
https://www.cms.gov/priorities/innovation/innovation-models/guide
.
620
Centers for Medicare and Medicaid Services. Driving Health System Transformation - A Strategy for the CMS
Innovation Center’s Second Decade. October 2021. https://www.cms.gov/priorities/innovation/strategic-direction-
whitepaper.

alignment between a potential ambulatory model, established models and programs, and payers
to achieve sustainable system-wide transformation. The increased engagement in value-based
care proposed in the model could support other payers like Medicare Advantage, Medicaid, and
commercial plans in achieving their goals around value-based care activities by providing an
onramp for specialists inexperienced with this form of payment. Also, leveraging existing design
principles from MIPS promotes directional alignment as they adhere to the broader CMS
National Quality Strategy, including an effort to move toward digital quality measurement,
621
and plans to employ a “Universal Foundation” of quality measures to create greater consistency
in quality reporting across payers.
622
We request feedback on the following questions:
(34) Are there opportunities to reduce clinician burden between this model, other CMMI
models, and beyond through multi-payer alignment, in areas such as performance measurement,
quality measurement, and data/reporting requirements?
(35) How could this model align with value-based care approaches in the Medicare
Advantage, Medicaid, and commercial payer space that focus on specialty integration? What
model components and payment incentives can be aligned with other payers to support
improvement goals?
(36) How can CMS align with other payer approaches to equity and disparity reduction?
This could include alignment on definitions, methods, and requirements for equity-related data
collection, etc.
(37) What technical assistance can CMS provide to support alignment and reduce
burden?
K. Expand Colorectal Cancer Screening
621
Centers for Medicare and Medicaid Services. dQMs - Digital Quality Measures. dQM Strategic Roadmap.
622
Jacobs DB, Schreiber M, Seshamani M, Tsai D, Fowler E, Fleisher LA. Aligning Quality Measures across CMS
– The Universal Foundation. 2023. New England Journal of Medicine, 388 (9), 776-779. DOI:
10.1056/NEJMp2215539.

Medicare coverage for colorectal cancer (CRC) screening tests under Part B are described
in statutes (sections 1861(s)(2)(R), 1861(pp), 1862(a)(1)(H) and 1834(d) of the Social Security
Act (the Act)), regulation (42 CFR 410.37), and National Coverage Determination (NCD)
(Section 210.3 of the Medicare National Coverage Determinations Manual). The statute and
regulations expressly authorize the Secretary to add other tests and procedures (and
modifications to tests and procedures) for colorectal cancer screening with such frequency and
payment limits as the Secretary finds appropriate based on consultation with appropriate
organizations. (Section 1861(pp)(1)(D) of the Act; § 410.37(a)(1)(v)) We are proposing to
exercise our authority at section 1861(pp)(1)(D) of the Act to update and expand coverage for
CRC screening by:
● Removing coverage for the barium enema procedure in regulations at § 410.37,
● Adding coverage for the computed tomography colonography (CTC) procedure in
regulations at § 410.37, and
● Expanding a “complete colorectal cancer screening” in § 410.37(k) to include a follow-
on screening colonoscopy after a Medicare covered blood-based biomarker CRC screening test
(described and authorized in NCD 210.3).
1. Background
The Center for Disease Control and Prevention (CDC) describes CRC as “a disease in
which cells in the colon or rectum grow out of control… Sometimes abnormal growths, called
polyps, form in the colon or rectum. Over time, some polyps may turn into cancer. Screening
tests can find polyps so they can be removed before turning into cancer. Screening also helps
find colorectal cancer at an early stage, when treatment works best.”
623
The National Cancer
Institute reports that CRC is the fourth most common type of cancer and estimates that the
United States experienced 153,020 new cases and 52,550 new deaths from CRC in 2023. In
addition, the rate of new cases and new deaths from CRC is more common in men than women
623
CDC Website: https://www.cdc.gov/cancer/colorectal/basic_info/what-is-colorectal-cancer.htm.

and significantly greater for those of African American and Non-Hispanic American Indian/
Alaska Native descent compared to all races.
624
At § 410.37(a)(4), we define the barium enema procedure as a screening double contrast
barium enema of the entire colorectum (including a physician's interpretation of the results of the
procedure); or in the case of an individual whose attending physician decides that he or she
cannot tolerate a screening double contrast barium enema, a screening single contrast barium
enema of the entire colorectum (including a physician's interpretation of the results of the
procedure). The CDC describes CTC, (also called a virtual colonoscopy), as “a screening test
that uses X-rays and computers to produce images of the entire colon, which are displayed on a
computer screen for the doctor to analyze.”
625
The U.S. Preventative Services Task Force (USPSTF) included CTC as a CRC screening
method in their June 2016 revised Final Recommendation Statement which included the topline
recommendations “[t]he USPSTF recommends the (CRC screening) service. There is high
certainty that the net benefit is substantial (Grade A)” and “[t]he USPSTF recommends the (CRC
screening) service. There is high certainty that the net benefit is moderate, or there is moderate
certainty that the net benefit is moderate to substantial)(Grade B)”.
626
The USPSTF cautioned,
“[t]here is insufficient evidence about the potential harms of associated extracolonic findings,
which are common.”
The USPSTF further wrote, “[t]here are numerous screening tests to detect
early-stage colorectal cancer, including stool-based tests (gFOBT, FIT, and FIT-DNA), direct
visualization tests (flexible sigmoidoscopy, alone or combined with FIT; colonoscopy; and CT
colonography), and serology tests (SEPT9 DNA test). The USPSTF found no head-to-head
studies demonstrating that any of these screening strategies are more effective than others,
although they have varying levels of evidence supporting their effectiveness, as well as different
624
NCI Website: https://seer.cancer.gov/statfacts/html/colorect.html.
625
CDC Website: https://www.cdc.gov/cancer/colorectal/basic_info/screening/tests.htm.
626
USPSTF June 2016 Revised Final Recommendation Statement
https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening-june-2016.

strengths and limitations.”
627
The USPSTF again included CTC as a CRC screening method in
the most recent May 2021 revised Final Recommendation Statement, which included the topline
recommendations “[t]he USPSTF recommends screening for colorectal cancer in all
adults aged 50 to 75 years(Grade A)” and “[t]he USPSTF recommends screening for colorectal
cancer in adults aged 45 to 49 years (Grade B)”.
628
CMS describes our consultations with
additional organizations and our review of clinical guidelines later in our proposal.
2. Statutory Authority
Section 4104 of the Balanced Budget Act of 1997 (Pub. L. 105-33) authorized the benefit
colorectal cancer screening tests under Medicare Part B. Section 1861(s)(2)(R) of the Act
includes CRC screening tests in the definition of medical and other health services that fall
within the scope of Medicare Part B benefits described in section 1832(a)(1) of the Act. Section
1861(pp) of the Act defines colorectal cancer screening tests and specifically names the
following tests:
● Screening fecal-occult blood test;
● Screening flexible sigmoidoscopy; and
● Screening colonoscopy.
Section 1861(pp)(1)(D) of the Act also authorizes the Secretary to include in the
definition of CRC screening tests other tests or procedures and modifications to the tests and
procedures described under this subsection, with such frequency and payment limits as the
Secretary determines appropriate, in consultation with appropriate organizations.
3. Regulatory and NCD Authority
In the CY 1998 PFS final rule (62 FR 59048), after consulting with appropriate
organizations, we finalized regulations to cover barium enema procedures for CRC screening in
627
USPSTF June 2016 Revised Final Recommendation Statement
https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening-june-2016.
628
USPSTF January 2021 Revised Final Recommendation Statement
https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening.

§ 410.37. Barium enema screening examinations have to be ordered by the beneficiary’s
attending physician (§ 410.37(h)). Currently, the regulations cover barium enemas as a CRC
screening tests subject to frequency limitations and whether or not the individual was at high risk
for colorectal cancer. As described in the CY 1998 PFS final rule (62 FR 59048), CMS consulted
with a number of appropriate organizations such as the American Cancer Society, American
College of Physicians, American Gastroenterological Association and U.S. Preventive Services
Task Force (USPSTF) and the decision to cover the barium enema procedure was based on the
prevailing clinical guidelines and recommendations at the time. In the CY 2023 PFS final rule
(87 FR 69404), we lowered the age limit for barium enema procedures for CRC screening to age
45 at § 410.37(i)(1).
In May 2009, we established a non-coverage policy for CTC in NCD 210.3 CTC
Screening Tests. We noted in the Final Decision Memorandum, “there is insufficient evidence
on the test characteristics and performance of screening CT colonography in Medicare aged
individuals and that the evidence is not sufficient to conclude that screening CT colonography
improves health benefits for asymptomatic, average risk Medicare beneficiaries.”
629
At that time,
the October 2008 USPSTF revised Final Recommendation Statement read, “[t]he USPSTF
concludes that the evidence is insufficient to assess the benefits and harms of computed
tomographic colonography and fecal DNA testing as screening modalities for colorectal cancer.
(Grade I)”
630
As described in the Final Decision Memo, guidelines from Professional Societies
were mixed. A joint guideline from the American Cancer Society, the U.S. Multi-Society Task
Force on Colorectal Cancer, and the American College of Radiology concluded “[i]n terms of
detection of colon cancer and advanced neoplasia, which is the primary goal of screening for
629
National Coverage Analysis CAG-00396N Screening Computed Tomography Colonography (CTC) for
Colorectal Cancer on Medicare Coverage Database (https://www.cms.gov/medicare-coverage-database/view/ncacal-
decision-
memo.aspx?proposed=N&NCAId=220&NcaName=Screening+Computed+Tomography+Colonography+(CTC)+f
or+Colorectal+Cancer),).
630
USPSTF October 2008 Final Recommendation Statement:
https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening-2008.

CRC and adenomatous polyps, recent data suggest CTC is comparable to Optical Colonoscopy
for the detection of cancer and polyps of significant size when state-of-the-art techniques are
applied.”
631
The American Gastroenterological Association issued the following
recommendation statement in 2008, “[t]he AGA does not endorse CT colonography as a first-
line colon cancer screening test. While AGA supports CT colonography as a screening option,
colonoscopy is the definitive test for colorectal cancer screening and prevention. Colonoscopy is
the only test that can both detect cancer at an early curable stage and prevent cancer by removing
pre-cancerous polyps. At this time, while CT colonography may be another technology for
colorectal cancer screening, many questions about CT colonography remain to be answered.”
632
The American Society for Gastrointestinal Endoscopy published guidelines in 2006 that
concluded “virtual colonoscopy is an evolving technique and is not currently recommended as
the primary method of screening for CRC.”
633
In the 2023 PFS final rule (87 FR 69404) we expanded the regulatory definition of CRC
Screening to include a complete colorectal cancer screening, which includes a follow-on
screening colonoscopy after a Medicare covered non-invasive stool-based colorectal cancer
screening test returns a positive result. (§ 410.37(k)) Although CMS had previously viewed a
colonoscopy after a positive non-invasive stool-based CRC screening test to be a diagnostic
colonoscopy, the clinical recommendations and guidance of medical professional societies and
screening experts have since evolved for stool-based colorectal cancer screening due to the
631
National Coverage Analysis CAG-00396N Screening Computed Tomography Colonography (CTC) for
Colorectal Cancer on Medicare Coverage Database: https://www.cms.gov/medicare-coverage-database/view/ncacal-
decision-
memo.aspx?proposed=N&NCAId=220&NcaName=Screening+Computed+Tomography+Colonography+(CTC)+f
or+Colorectal+Cancer).
632
National Coverage Analysis CAG-00396N Screening Computed Tomography Colonography (CTC) for
Colorectal Cancer on Medicare Coverage Database: https://www.cms.gov/medicare-coverage-database/view/ncacal-
decision-
memo.aspx?proposed=N&NCAId=220&NcaName=Screening+Computed+Tomography+Colonography+(CTC)+f
or+Colorectal+Cancer).
633
National Coverage Analysis CAG-00396N Screening Computed Tomography Colonography (CTC) for
Colorectal Cancer on Medicare Coverage Database: https://www.cms.gov/medicare-coverage-database/view/ncacal-
decision-
memo.aspx?proposed=N&NCAId=220&NcaName=Screening+Computed+Tomography+Colonography+(CTC)+f
or+Colorectal+Cancer).
relative number of false positive results, low follow-up colonoscopy rates and patient access
barriers. Published evidence highlighted that individuals who did not get a follow-up
colonoscopy were about twice as likely to die of colorectal cancer compared to individuals who
had one. Since the overall goal of programmatic cancer screening using any CRC screening test
is to prevent cancer, allowing for early detection and treatment and reducing cancer mortality,
the follow-up colonoscopy was found to be integral with non-invasive stool-based CRC
screening, since improvements in health outcomes would not be possible without the follow-up
colonoscopy. Our goal was that the patient and their healthcare professional make the most
appropriate choice in CRC screening, which included considerations of the risks, burdens and
barriers presented with an invasive screening colonoscopy in a clinical setting as their first step.
We went on to describe that CRC screening presents a unique scenario where there are
significant differences between screening stool-based tests and screening colonoscopy tests in
terms of invasiveness and burdens to the patient and healthcare system. We recognized there are
several advantages to choosing a non-invasive stool-based CRC screening test as a first step
compared to a screening colonoscopy, including relative ease of administering the test and
potentially reducing the experience of unnecessary burdensome preparation and invasive
procedures.
We noted in preamble of the CY 2023 PFS final rule (87 FR 69404) that many
commenters asked that CMS further expand our approach of a complete colorectal cancer
screening. Many requested that we remove the text “stool-based” from our proposed regulatory
text at § 410.37(k), resulting in a complete CRC screening including a follow-on screening
colonoscopy after a Medicare covered non-invasive screening test. Many commenters requested
that a complete CRC screening include a screening colonoscopy after a positive result from a
blood-based biomarker test, as well as a stool-based test. We responded to these public
comments by writing that “we disagree with the commenters that requested a further expansion
of a complete colorectal cancer screening that would include additional first step tests beyond a
non-invasive stool-based test. We believe the stool-based tests are unique to other CRC
screening tests in terms of their non-invasiveness, the fact that stool-based tests can be
implemented by the patient at home and mailed into the lab, the absence of bowel preparation
and anesthesia and the comparatively lighter burden and mitigated potential for over servicing of
the patient and the healthcare system.” We further wrote, “[w]e agree that blood-based
biomarker CRC screening tests have significant potential and we expanded coverage to include
them in the reconsidered NCD 210.3, effective January 2021.” We also recognized that blood-
based biomarker CRC screening tests continue to be an emerging and quickly evolving
technology. However, we also noted that, as of September 2022, no blood-based Biomarker tests
for CRC screening had achieved the coverage requirements of NCD 210.3 and that the May 2021
USPSTF revised Final Recommendation Statement did not include serum tests.
In the CY 2023 PFS final rule (87 FR 69404) we also established regulatory text at §
410.37(k) that the frequency limitations described for screening colonoscopy shall not apply in
the instance of a follow-on screening colonoscopy test. We wrote that we aimed to avoid
disruption to the existing conditions of coverage and payment for CRC screening for this unique
scenario and continuum of screening.
4. Proposed Revisions
We propose to exercise our authority in section 1861(pp)(1)(D) of the Act to remove
coverage for the barium enema procedure from CRC screening in regulations at § 410.37. CMS
has consulted with appropriate organizations and has heard that, while the barium enema
procedure was reasonable and necessary for CRC screening when it was initially covered in the
CY 1998 PFS final rule (62 FR 59048), circumstances have changed. The organizations have
expressed that barium enema procedures no longer meet modern clinical standards, are no longer
recommended in clinical guidelines, and would not be an appropriate CRC screening test given
the advancement of alternatives such as Stool-based tests, Colonoscopies, and CT Colonography.
In developing our proposal, we also considered that June 2016 and the May 2021 USPSTF

revised Final Recommendation Statements did not include the barium enema procedure as a
CRC screening method in their revised Final Recommendation Statements.
634,635
We also
considered the 2017 U.S. Multi-Society Task Force of Colorectal Cancer (MSTF)
recommendation statement, which reads, “CT colonography has replaced double-contrast barium
enema as the test of choice for colorectal imaging for nearly all indications. CT colonography is
more effective than barium enema and better tolerated.”
636
The 2018 American Cancer Society
(ACS) Colorectal Cancer Screening for Average-Risk Adults Guideline Update also reads,
“double-contrast barium enema is no longer included as an acceptable screening option.”
637
During the CY 2023 PFS, CMS received a joint public comment from the American
College of Gastroenterology (ACG), American Gastroenterological Association (AGA) and the
American Society for Gastrointestinal Endoscopy (ASGE)
638
that brought to our attention that
barium enema is not a recommended CRC screening modality in guidance from the USPSTF or
the U.S. Multi-Society Task Force on Colorectal Cancer. The public comment went on to note
that while the Barium Enema procedure once was considered a CRC screening modality and has
been included in guidelines in the past, barium enema is no longer included in any recent CRC
guidelines and is rarely performed today as it is considered inadequate for the exclusion of CRC.
They urged CMS to remove Barium Enema as a covered CRC screening test for all individuals.
An internal claims analysis indicates that Medicare only paid claims for barium enema for CRC
screening for 72 beneficiaries in CY 2022.
A 2016 study titled “[n]ew era of colorectal cancer screening”, states, “double-contrast
barium enema (DCBE) is a non-invasive radiological test, which provides a complete evaluation
of the large intestine. The sensitivity and specificity of barium enema for polyps of any size is
634
USPSTF June 2016 Revised Final Recommendation Statement,
https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening-june-2016.
635
USPSTF January 2021 Revised Final Recommendation Statement,
https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening.
636
Am J Gastroenterol 2017; 112:1016–1030; doi: 10.1038/ajg.2017.174; published online 6 June 2017.
637
doi: 10.3322/caac.21457. Available online at cacancerjournal.com.
638
CY 2023 PFS Public Comment CMS-2022-0113-21851_attachment_1.

38 percent and 86 percent, respectively. One study comparing barium enema to CTC and
colonoscopy showed that DCBE has the lowest sensitivity and specificity with sensitivity of
41 percent for lesions ≥ 6 mm and sensitivity and specificity of 48 and 90 percent respectively
for lesions ≥ 10 mm. These results are consistent with a meta-analysis comparing the
performance of barium enema to that of CTC showing CTC is more sensitive and more specific
than barium enema for large polyps (≥ 10 mm) and small polyps (6-9 mm) in average-risk and
high-risk populations. In the United States, CTC has largely replaced DCBE as a radiographic
option for CRC screening.”
639
In light of the new evidence and our consultations with appropriate organizations, we are
proposing to remove barium enema as a colorectal screening test under 42 CFR 410.37(a)(1)(iv).
We look forward to further consultation with the public and appropriate organizations through
the public comment period of this proposed rule. We invite public comment on this proposal to
remove all references to barium enemas in § 410.37.
We also propose to exercise our authority in section 1861(pp)(1)(D) of the Act to add
coverage for the CTC procedure for CRC screening in regulations at § 410.37. If finalized, we
will also address and revise the current non-coverage policy for CTC in NCD 210.3. In
developing our proposal to expand coverage for the CTC procedure, we consulted with
appropriate organizations and considered a number of potential benefits, risks and tradeoffs
described in guidelines and recommendations by professional societies and government bodies.
In developing the proposed rule, we considered that the USPSTF included the CTC
procedure as a CRC screening method in their June 2016 and May 2021 revised Final
Recommendation Statements.
640,641
In terms of benefits, the USPSTF wrote in their May 2021
revised Final Recommendation Statement, that CTC usually allows for greater colon
639
El Zoghbi M, Cummings LC. New era of colorectal cancer screening. World J Gastrointest Endosc. 2016 Mar
10;8(5):252-8. doi: 10.4253/wjge.v8.i5.252. PMID: 26981176; PMCID: PMC4781905.
640
USPSTF June 2016 Revised Final Recommendation Statement,
https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening-june-2016.
641
USPSTF January 2021 Revised Final Recommendation Statement,
https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening.

visualization compared to Flexible Sigmoidoscopy. In terms of risks and tradeoffs, USPSTF
noted that CTC, like Colonoscopy and flexible sigmoidoscopy, requires the burden of bowel
preparation. The USPSTF wrote “[u]nlike Colonoscopy and Flexible Sigmoidoscopy, CTC may
reveal extracolonic findings that require additional workup, which could lead to other potential
benefits or harms.” The USPSTF went on to state, “[h]arms from CT colonography are
uncommon (19 studies; n = 90 133), and the reported radiation dose for CT colonography ranges
from 0.8 to 5.3 mSv (compared with an average annual background radiation dose of 3.0 mSv
per person in the U.S.). Accurate estimates of rates of serious harms from colonoscopy following
abnormal CTC results are not available.” Regarding extracolonic findings, the USPSTF wrote,
“[e]xtracolonic findings on CTC are common. Based on 27 studies that included 48,235
participants, 1.3 percent to 11.4 percent of examinations identified extracolonic findings that
required workup. Three percent or less of individuals with extracolonic findings required
definitive medical or surgical treatment for an incidental finding. A few studies suggest that
extracolonic findings may be more common in older age groups. Long-term clinical follow-up of
extracolonic findings was reported in few studies, making it difficult to know whether it
represents a benefit or harm of CT colonography.” The USPSTF recommends screening CTC
frequency of every 5 years.
642
In a study titled “Incidental Extracolonic Findings on CT Colonography: The Impending
Deluge and Its Implications”, Lincoln L. Berland, MD, describes extracolonic findings as
findings on CTC that have potential deleterious health effects and are asymptomatic,
unsuspected, and unrelated to the colon. The study goes on to state, “as CT image quality has
improved, there has been an increase in the frequency of detecting "incidental findings," defined
as findings that are unrelated to the clinical indication for the imaging examination performed.
These ‘incidentalomas,’ as they are also called, often confound physicians and patients with how
642
USPSTF January 2021 Revised Final Recommendation Statement,
https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening.

to manage them. Although it is known that most incidental findings are likely benign and often
have little or no clinical significance, the inclination to evaluate them is often driven by
physician and patient unwillingness to accept uncertainty, even given the rare possibility of an
important diagnosis.”
643
The potential for extracolonic findings, both clinically significant and
insignificant, is an important tradeoff to be considered by the patient and clinician when
considering CTC as a CRC Screening option.
We also consider the 2018 ACS Colorectal Cancer Screening for Average-Risk Adults
Guideline Update, which includes the CTC procedure with their recommended tests and
procedures for CRC Screening.
644
In terms of benefits, the ACS guideline describes CTC
Sensitivity and specificity for cancer and advanced adenomas comparable to colonoscopy, longer
recommended screening intervals compared to stool-based tests, and no need for sedation
(compared to colonoscopy). In terms of risks and tradeoffs, the ACS guideline notes incidental
extracolonic findings may require workup (with unclear benefit-burden balance), exposure to
low-dose radiation and requires full bowel cleansing. The ACS guidelines recommended
screening CTC frequency of every 5 years.
We also consider the United States Multi-Society Task Force (MSTF) of Colorectal
Cancer, which represents the American College of Gastroenterology, the American
Gastroenterological Association, and The American Society for Gastrointestinal Endoscopy,
2017 Colorectal Cancer Screening recommendations
645
, which include CTC as a “Tier 2”
procedure alongside FIT-fecal DNA and Flexible Sigmoidoscopy. The recommendation states
that “CRC screening tests are ranked in 3 tiers based on performance features, costs, and
practical considerations. The first-tier tests are colonoscopy every 10 years and annual fecal
immunochemical test (FIT). Colonoscopy and FIT are recommended as the cornerstones of
643
Lincoln L. Berland, Incidental Extracolonic Findings on CT Colonography: The Impending Deluge and Its
Implications, Journal of the American College of Radiology, Volume 6, Issue 1, 2009, Pages 14-20, ISSN 1546-
1440, https://doi.org/10.1016/j.jacr.2008.06.018.
644
doi: 10.3322/caac.21457. Available online at cacancerjournal.com.
645
Am J Gastroenterol 2017; 112:1016–1030; doi: 10.1038/ajg.2017.174; published online 6 June 2017.

screening regardless of how screening is offered. Thus, in a sequential approach based on
colonoscopy offered first, FIT should be offered to patients who decline colonoscopy.
Colonoscopy and FIT are recommended as tests of choice when multiple options are presented as
alternatives. A risk-stratified approach is also appropriate, with FIT screening in populations
with an estimated low prevalence of advanced neoplasia and colonoscopy screening in high
prevalence populations. The second-tier tests include CT colonography every 5 years, the FIT-
fecal DNA test every 3 years, and flexible sigmoidoscopy every 5 to 10 years. These tests are
appropriate screening tests, but each has disadvantages relative to the tier 1 tests.” In terms of
benefits of CTC, the MSTF describes lower risk of perforation compared with colonoscopy and
write, “CT colonography appeals to a niche of patients who are willing to undergo bowel
preparation and are concerned about the risks of colonoscopy.” In terms of risks and tradeoffs,
the MSTF describe the requirement for bowel preparation, extracolonic findings, inferior
sensitivity compared to other screening tests and radiation exposure. The MSTF writes,
“[e]vidence that CT colonography reduces CRC incidence or mortality is lacking.”
We also consider the online resource RadiologyInfo,
646
which is an online public
information resource developed by health care professionals in collaboration with patients.
RadiologyInfo is sponsored by the Radiological Society of North America (RSNA) and the
American College of Radiology (ACR). In terms of benefits of CTC, RadiologyInfo described
CTC as less invasive than a Colonoscopy, though for CTC a small tube is inserted into the
rectum to allow for inflation with carbon dioxide or air. In addition, CTC does not require
sedation (and transportation accommodations) and carries less risk of bowel perforation
compared to Colonoscopy. In addition, CTC can identify precancerous polyps that may not be
detected by stool-based and blood-based tests. CTC may be a less burdensome first option for
patients who are medically fragile or have complex or unusual anatomy. In terms of risks and
tradeoffs, RadiologyInfo describes a very small risk of perforated bowel (during inflation), a
646
RadiologyInfo Website: https://www.radiologyinfo.org/.

small risk of secondary cancer due to radiation exposure and it being not recommended for
individuals who are pregnant. RadiologyInfo reports that CTC applies a patient radiation
exposure similar to Barium Enema at 6 millisieverts (mSv), which is greater than other
preventive screenings, such as CT Lung Cancer Screening at 1.5mSv and screening digital
mammography at 0.21 mSv.
647
After considering the above recommendations and guidelines from appropriate
organizations, we believe CTC to be reasonable and necessary as CRC screening test, especially
for patients and clinicians who seek a direct visualization procedure as a first step in CRC
screening that is less invasive and less burdensome on the patient and healthcare system
compared to Screening Colonoscopy. Our goal is that the patient and their clinician make the
most appropriate choice in CRC screening, which includes considerations of the risks, burdens
and tradeoffs for each covered test or procedure. We expect that clinicians who order CTC for
CRC Screening will educate their patients on risks and context of radiation exposure and
potential extracolonic findings. A shared decision-making tool is not mandated but may be
helpful for clinicians and patients to weigh their options for CRC screening.
We propose to add CTC as a covered CRC screening test at § 410.37. We propose to
describe in regulatory text that CTC means a test that uses X-rays and computers to produce
images of the entire colon (including image processing and a physician’s interpretation of the
results of the procedure). We also propose to codify in regulatory text that Medicare Part B pays
for a screening computed tomography colonography if it is ordered in writing by the
beneficiary's attending physician, physician assistant, nurse practitioner, or clinical nurse
specialist. We also propose the following limitations of coverage for CTC:
● In the case of an individual age 45 or over who is not at high risk of colorectal cancer,
payment may be made for a screening computed tomography colonography performed after at
least 59 months have passed following the month in which the last screening computed
647
https://www.radiologyinfo.org/en/info/safety-xray.
tomography colonography or 47 months have passed following the month in which the last
screening flexible sigmoidoscopy or screening colonoscopy was performed.
● In the case of an individual who is at high risk for colorectal cancer, payment may be
made for a screening computed tomography colonography performed after at least 23 months
have passed following the month in which the last screening computed tomography
colonography or the last screening colonoscopy was performed.
Congress has eliminated Part B coinsurance (section 1833(a)(1)(Y) of the Act, §
410.152(l)(5)) and deductibles (section1833(b)(1) of the Act) for covered prevention services
recommended with a grade of A or B by the USPSTF. As described earlier in our proposal, the
USPSTF included CTC as a screening method in their May 2021 revised Final Recommendation
Statement on CRC screening (Grade A). Thus, if our proposal is finalized, CTC will require no
Part B coinsurance nor deductible when furnished as a CRC screening procedure. We clarify that
CTC will continue to require Part B coinsurance and deductible when furnished as a diagnostic
or other non-preventive/ screening procedure.
We look forward to further consultation with the public and appropriate organizations
through the public comment period of this proposed rule. We invite public comment on this
proposal.
We also propose to exercise our authority in section 1861(pp)(1)(D) of the Act to expand
our approach to a “complete CRC screening” finalized in § 410.37(k). We propose to add a
Medicare covered Blood-based Biomarker CRC screening test (described and authorized in NCD
210.3) alongside the Medicare covered non-invasive stool-based CRC screening test within our
approach of a “complete CRC screening.”
Our goal is for the patient and their healthcare professional to make the most appropriate
choice in CRC screening, which include considerations of the risks, burdens and barriers
presented with an invasive screening colonoscopy in a clinical setting as their first step. CRC
screening presents a unique scenario where there are significant differences between screening

stool-based tests and direct visualization procedures such as colonoscopy, flexible
sigmoidoscopy and CTC tests in terms of invasiveness and burdens to the patient and healthcare
system. We recognize there are several advantages to choosing a non-invasive CRC screening
test as a first step compared to a screening colonoscopy, including relative ease of administering
the test and potentially reducing the experience of burdensome preparation and invasive
procedures. Since the CY 2023 PFS final rule we have heard from many interested parties,
including a number of professional societies, that Medicare covered blood-based biomarker tests
would be appropriately placed alongside covered non-invasive stool-based tests within a
complete colorectal cancer screening context. We have reconsidered our position that Medicare
covered blood-based biomarker tests would not belong alongside covered non-invasive stool-
based tests within our approach to a complete CRC screening. We consider that some patients
may consider a blood test less uncomfortable than administering a stool-based test, especially if
the blood draw is concurrent to a routine blood draw for other covered routine bloodwork. We
have also heard that some patients may prefer a non-invasive test as their first step but view the
stool sample collection process for stool-based tests as a meaningful barrier.
648
We also consider
that a blood test may be more accessible to many patients in rural and underserved communities
than facilities that furnish screening colonoscopies, flexible sigmoidoscopies and CTC.
NCD 210.3 requires that Blood-based Biomarker Tests for CRC screening must have
Food and Drug Administration (FDA) market authorization with an indication for colorectal
cancer screening; and proven test performance characteristics for a blood-based screening test
with both sensitivity greater than or equal to 74 percent and specificity greater than or equal to
90 percent in the detection of colorectal cancer compared to the recognized standard (accepted as
colonoscopy at this time), as minimal threshold levels, based on the pivotal studies included in
the FDA labeling. We have heard from interested parties that Blood-based Biomarker tests for
648
Kolata, Gina. “A Blood Test Shows Promise for Early Colan Cancer Detection” The New York Times, March
13, 2024.
CRC screening may achieve the coverage requirements described in NCD 210.3 within the near
term and thereafter quickly become adopted as a non-invasive option within the healthcare
system and patient community. Given our existing coverage policy for Blood-based Biomarker
Tests for CRC Screening (NCD 210.3), we believe our proposal is appropriately proactive,
provides for consistent regulatory treatment between blood and stool-based tests, and will ready
our regulatory policies for the quickly evolving state of medical technology in methods for CRC
screening. We note that while blood-based biomarker tests were not included as a screening
method within the May 2021 USPSTF revised Final Recommendation Statement on CRC
Screening, they do not require beneficiary cost sharing (coinsurance and deductible) because
blood-based biomarker tests will be paid under the Clinical Laboratory Fee Schedule (CLFS).
For additional information, see the CMS website at https://www.cms.gov/medicare/payment/fee-
schedules/clinical-laboratory-fee-schedule-clfs.
We propose to revise the regulatory text describing a complete CRC screening at §
410.37(k) to state that colorectal cancer screening tests include a follow-on screening
colonoscopy after a Medicare covered non-invasive stool-based colorectal cancer screening test
or a Medicare covered blood-based biomarker CRC screening test returns a positive result. We
also propose to revise the regulatory text at § 410.37(k) to state the instance of the follow-on
colonoscopy in the context of a complete colorectal cancer screening shall not apply to the
frequency limitations for colorectal cancer screenings. We believe this statement in regulatory
text is clearer and recognizes, outside the context of a complete colorectal cancer screening, the
instance of a screening colonoscopy is factored into the calculation of frequency limitations of
other covered CRC screening tests and procedures in addition to a subsequent screening
colonoscopy.
We look forward to further consultation with the public and appropriate organizations
through the public comment period of this proposed rule. We invite public comment on this
proposal.

6. Summary
In summary, we are proposing to exercise our authority at section 1861(pp)(1)(D) of the
Act update and expand coverage for CRC screening by (1) removing coverage for the barium
enema procedure for CRC screening; (2) adding coverage of the CTC procedure for CRC
screening; and (3) expanding our approach to a “Complete CRC Screening” to include a covered
blood-based biomarker test alongside a covered non-invasive stool-based test.
Our proposal to update and expand CRC screening aligns with the administration’s
strategic pillar to advance health equity by addressing the health disparities that underlie our
health system. In addition, our proposal supports Executive Order 13985 by advancing racial
equity and support for underserved communities in the Medicare program. We believe our
proposal will directly advance health equity by promoting access and removing barriers for much
needed cancer prevention and early detection within rural communities and communities of color
that are especially impacted by the incidence of CRC. Our proposal to expand colorectal cancer
screening directly supports the Administration’s Cancer Moonshot Goal of reducing the deadly
impact of cancer and improving patient experiences in the diagnosis, treatment, and survival of
cancer.
649
Our proposal is also supportive of the Administration’s Proclamation of March as
National Colorectal Cancer Awareness Month in 2024, which includes the statement, “As a
country, we have made impressive progress in the struggle to end cancer over the past several
decades due to advancements in prevention, early-detection measures, and new medicines and
therapies. Despite remarkable breakthroughs, every year, more Americans are diagnosed with
cancer under the age of 50. Earlier detection and improved treatment of colorectal cancer
continue to be critical goals of medical research. Further progress is also needed to improve
outcomes for those who are disproportionately impacted by this disease — including Americans
over the age of 45, Native Americans, Black Americans, and people with a family history of
649
https://www.whitehouse.gov/cancermoonshot/.

colorectal cancer. There is still more work to be done to ensure more Americans can prevent,
detect, treat, and survive colorectal cancer.”
650
L. Requirements for Electronic Prescribing for Controlled Substances for a Covered Part D
Drug under a Prescription Drug Plan or an MA-PD Plan
1. Previous Regulatory Action
Section 2003 of the Substance Use-Disorder Prevention that Promotes Opioid Recovery
and Treatment for Patients and Communities (SUPPORT) Act (Pub. L. 115-271, October 24,
2018) generally mandates that the prescribing of a Schedule II, III, IV, or V controlled substance
under Medicare Part D be done electronically in accordance with an electronic prescription drug
program beginning January 1, 2021, subject to any exceptions, which HHS may specify. In the
CY 2021, CY 2022, CY 2023, and CY 2024 PFS final rules, we finalized policies for the CMS
Electronic Prescribing for Controlled Substances (EPCS) Program requirements specified in
section 2003 of the SUPPORT Act. We refer readers to 85 FR 84802 through 84807, 86 FR
65361 through 65370, 87 FR 70008 through 70014, and 88 FR 79285 through 79292 for the
details of those finalized policies. Specifically, in the CY 2021 PFS final rule, we established a
requirement that all prescribers conduct electronic prescribing of Schedule II, III, IV, and V
controlled substances covered under the Medicare prescription drug program, subject to any
exceptions, which HHS may specify, using the NCPDP SCRIPT standard version 2017071 with
an effective date of January 1, 2021, and a compliance date of January 1, 2022 (85 FR 84807).
In the CY 2022 PFS final rule, we finalized a policy to require prescribers to electronically
prescribe at least 70 percent of their Schedule II, III, IV, and V controlled substances that are
Part D drugs, except in cases where an exception or waiver applies (86 FR 65366); and finalized
multiple proposals related to the classes of exceptions specified by section 2003 of the
650
https://www.whitehouse.gov/briefing-room/presidential-actions/2024/02/29/proclamation-on-national-
colorectal-cancer-awareness-month-2024/.
SUPPORT Act (86 FR 65366 through 65369). We also extended the earliest date of compliance
actions to no earlier than January 1, 2023 (86 FR 65364). For prescribers who do not meet the
compliance threshold based on prescriptions written for a beneficiary in a long-term care (LTC)
facility, we extended the earliest date of compliance actions to no earlier than January 1, 2025
(86 FR 65364 and 65365). We also finalized our proposal to limit compliance actions with
respect to compliance through December 31, 2023, to a non-compliance notice (86 FR 65370).
In the CY 2023 PFS final rule (87 FR 70012 through 70013), we extended the non-
compliance action of sending notices to non-compliant prescribers, which we had finalized for
the CY 2023 CMS EPCS Program implementation year (January 1, 2023, through December 31,
2023), to the CY 2024 Program implementation year (January 1, 2024, through December 31,
2024). We also finalized a change to the data sources used to identify the geographic location of
prescribers for purposes of the recognized emergency exception at § 423.160(a)(5)(iii) (87 FR
70011 through 70012) and finalized our proposal to use the Prescription Drug Event (PDE) data
from the current evaluated year instead of the preceding year when CMS determines whether a
prescriber qualifies for an exception based on issuing 100 or fewer Part D controlled substance
prescriptions per calendar year (87 FR 70009 through 70011).
In the CY 2024 PFS final rule (88 FR 79285 through 79287), we identified certain terms
that we will use in the CMS EPCS Program and clarified that, by virtue of the cross reference in
§ 423.160(a)(5) to “the applicable standards in paragraph (b) of this section,” which refers to the
standards in § 423.160(b), the CMS EPCS Program will automatically adopt the electronic
prescribing standards at § 423.160(b) as they are updated. Additionally, we finalized our
proposals to remove the same entity exception from the CMS EPCS Program and to add “subject
to the exemption in paragraph (a)(3)(iii) of this section” to § 423.160(a)(5) (88 FR 79287
through 79288). As a result, prescriptions that are prescribed and dispensed within the same legal
entity are included in CMS EPCS Program compliance calculations as part of the 70 percent
compliance threshold at § 423.160(a)(5), and prescribers are not exempt from the requirement to
prescribe electronically at least 70 percent of their Schedule II through V controlled substances
that are Part D drugs – but such prescriptions have to meet the applicable standards in §
423.160(b) subject to the exemption in § 423.160(a)(3)(iii). We also finalized a policy to count
only the unique prescriptions in the measurement year for the purposes of CMS EPCS Program
compliance threshold calculations (88 FR 79288). Furthermore, for the exceptions that we
moved to § 423.160(a)(5)(ii) and (iii), we modified the exceptions to permit prescribers to apply
for waivers in times of an emergency and disaster and to limit the emergencies or disasters that
will trigger the recognized emergency exception. We also modified the duration of both
exceptions and established timing requirements for submitting a waiver application (88 FR
79288 through 79291). Lastly, we stated that we will send notices of non-compliance for each
measurement year a prescriber is non-compliant and will provide educational opportunities to
support prescribers in becoming compliant (88 FR 79291 through 79292).
2. Timeline for Including Prescriptions Written for Beneficiaries in Long-term Care (LTC)
Facilities in CMS EPCS Program Compliance Calculation
a. Background
In the CY 2021 PFS final rule (85 FR 84807), we adopted the requirement for all
Schedule II, III, IV, and V controlled substances for covered Part D drugs prescribed
electronically to be prescribed using the applicable standards in § 423.160(b), including the
NCPDP SCRIPT standard version 2017071. In the CY 2022 PFS final rule (86 FR 65364), we
finalized a policy to extend the date on or after which we will pursue compliance actions against
prescribers based on Part D controlled substance prescriptions those prescribers write for
beneficiaries in long-term care (LTC) facilities to January 1, 2025. We acknowledged that
prescribers who work in LTC facilities or who provide care to residents in LTC facilities faced
technological barriers that other prescribers did not face. One such barrier was that the NCPDP
SCRIPT standard version 2017071 lacked appropriate guidance for EPCS in LTC facilities. We
also noted that NCPDP was in the process of creating a new version of the SCRIPT standard that
would be better suited for use by prescribers serving LTC facilities, which would allow willing
partners to enable three-way communication between the prescriber, LTC facility, and pharmacy
to bridge any outstanding gaps that impede use of the NCPDP SCRIPT standard version
2017071 for EPCS in the LTC setting (86 FR 65364).
We received public comments on the CY 2022 PFS proposed rule requesting that we
exempt prescribers writing Part D controlled substance prescriptions for beneficiaries in LTC
facilities from having to conduct EPCS until after NCPDP SCRIPT standard version 2022011
was adopted. In response to those comments, in the CY 2022 PFS final rule, we noted that our
intent when extending the date on or after which we will pursue compliance actions against
prescribers based on Part D controlled substance prescriptions those prescribers write for
beneficiaries in LTC facilities was to strike a balance between being responsive to stakeholder
concerns surrounding the increased implementation barriers faced by LTC facilities, while at the
same time helping to ensure that these facilities eventually implement, and receive the benefits of
EPCS (86 FR 65364). Furthermore, we noted that we were not persuaded to further delay
commencing compliance actions to await publication of the NCPDP SCRIPT standard version
2022011. We acknowledged that three-way communication is not as seamless in the NCPDP
SCRIPT standard version 2017071 as it may be in upcoming versions. We also stated that three-
way communication is still possible with some modifications to EPCS, and therefore, we did not
believe it would be appropriate to adopt a further delay on this basis alone (86 FR 65364).
In the 2024 PFS final rule (88 FR 79286 through 79287), we clarified that based on the
existing regulatory text at § 423.160(a)(5), the CMS EPCS Program will automatically adopt the
electronic prescribing standards at § 423.160(b) as they are updated. We noted that in the
“Medicare Program; Contract Year 2024 Policy and Technical Changes to the Medicare
Advantage Program, Medicare Prescription Drug Benefit Program, Medicare Cost Plan Program,
Medicare Parts A, B, C, and D Overpayment Provisions of the Affordable Care Act and
Programs of All-Inclusive Care for the Elderly; Health Information Technology Standards and
Implementation Specifications” proposed rule (CY 2024 Medicare Advantage and Part D Policy
and Technical Changes proposed rule) (87 FR 79550), we proposed to update provisions related
to e-prescribing standards at § 423.160(b), including, after a transition period, requiring the
NCPDP SCRIPT standard version 2022011 proposed for adoption at 45 CFR 170.205(b) and
retiring NCPDP SCRIPT standard version 2017071 by January 1, 2025.
Although we did not propose any policy changes regarding the NCPDP SCRIPT standard
version in the CY 2024 PFS proposed rule (88 FR 52532), we received public comments
requesting clarification on when the new NCPDP SCRIPT standard version would be adopted
and the implications for measuring EPCS compliance in LTC. In response to those comments, in
the CY 2024 PFS final rule (88 FR 79286), we acknowledged that we had not finalized our
proposal regarding the NCPDP SCRIPT standard version 2022011 that was proposed in the CY
2024 Medicare Advantage and Part D Policy and Technical Changes proposed rule. We also
acknowledged that some prescribers prescribing for beneficiaries in LTC facilities have adopted
EPCS, but that others have waited for the standard to be updated (88 FR 79286 through 79287).
We noted that if the requirement to use an updated version of the NCPDP SCRIPT standard is
finalized for a date after January 1, 2025, we may explore whether a waiver is appropriate for
prescribers who are not compliant solely as a result of prescriptions they have written for
beneficiaries in LTC facilities or we may revisit the compliance start date, if needed, through
future rulemaking (88 FR 79287).
In the “Medicare Program; Medicare Prescription Drug Benefit Program; Health
Information Technology Standards and Implementation Specifications” final rule (89 FR 51242
through 51247), which appeared in the June 17, 2024 Federal Register (hereinafter referred to
as the June 2024 Part D and Health IT Standards final rule), we finalized at § 423.160(b)(1) the
requirement that Part D sponsors, prescribers and dispensers, when electronically transmitting
prescriptions and prescription-related information for covered Part D drugs for Part D eligible
individuals, must comply with a standard in 45 CFR 170.205(b). Taken in conjunction with the
standards and expiration date adopted by the Office of the National Coordinator for Health
Information Technology (ONC), as described in the June 2024 Part D and Health IT Standards
final rule (89 FR 51258 through 51259), § 423.160(b)(1) will require use of NCPDP SCRIPT
standard version 2023011, which ONC is adopting at 45 CFR 170.205(b)(2), beginning January
1, 2028, and retire use of NCPDP SCRIPT standard version 2017071, which ONC previously
adopted at 45 CFR 170.205(b)(1) and to which it is applying an expiration date of January 1,
2028. ONC finalized January 1, 2028, as the expiration date for NCPDP SCRIPT standard
version 2017071 instead of January 1, 2027, in consideration of public comments requesting that
the date be delayed. As a result of these policies being finalized, the NCPDP SCRIPT standard
version 2023011 will be required for the CMS EPCS Program by January 1, 2028. As both
NCPDP SCRIPT standard version 2017071 and NCPDP SCRIPT standard version 2023011 will
be adopted at 45 CFR 170.205(b) and unexpired as of the effective date of the June 2024 Part D
and Health IT Standards final rule, entities subject to the requirement at § 423.160(b)(1) may use
either version of the NCPDP SCRIPT standard during the transition period beginning July 17,
2024, the effective date of the June 2024 Part D and Health IT Standards final rule, and ending
December 31, 2027, which is the last day before NCPDP SCRIPT standard version 2017071 will
expire for the purposes of HHS use.
b. Barriers to Electronic Prescribing of Controlled Substances for Beneficiaries in LTC and the
Role of Three-Way Communication in the NCPDP SCRIPT Standard
We understand the challenges of conducting EPCS in the LTC setting to be
multifactorial. The specific challenges include prescribers being responsible for covering
multiple LTC facilities, each with different electronic health record (EHR) systems; reliance on
LTC nursing staff to communicate prescriptions to the pharmacy on behalf of the prescriber; and
with respect to NCPDP SCRIPT standard version 2017071, lack of three-way (or multi-party)
communication between the prescriber, the LTC facility, and the pharmacy.

When conducting EPCS using the NCPDP SCRIPT standard version 2017071,
prescribers can submit prescriptions electronically to the pharmacy, but the prescriber must
subsequently contact the LTC facility separately to give an order for the medication so the LTC
facility can administer the medication to the patient as prescribed. In cases where EPCS is being
conducted and the prescriber has not communicated a separate order to the LTC facility, the
pharmacy may deliver a prescription to the LTC facility and the facility staff has no record of the
order. Then the LTC facility staff must contact the prescriber for an order to be able to
administer the drug to the patient.
To conduct EPCS without having to separately communicate an order to the LTC facility,
prescribers can use a web portal to enter an order in the LTC facility’s EHR and then, if the EHR
supports the necessary EPCS capability,
651
the prescription can be transferred to the pharmacy.
However, not all LTC facilities have EHRs with this functionality. Additionally, each LTC
facility may have its own web portal, making the number of portals and credentials overly
burdensome for prescribers who treat patients who reside in multiple different LTC facilities.
After providing an order to the LTC facility, prescribers often rely on LTC facility nursing staff
to relay verbal prescription orders to pharmacies as permitted under 21 CFR 1306.03(b) and
1306.21(a).
NCPDP SCRIPT standard version 2023011 permits three-way communication that would
better facilitate LTC workflows in a way that NCPDP SCRIPT standard version 2017071 does
not. In comments NCPDP submitted in response to the CY 2025 Medicare Advantage and Part
D Policy and Technical Changes proposed rule, NCPDP confirmed that it attempted to create
guidance on three-way communication using the NCPDP SCRIPT standard version 2017071, but
651
According to the Drug Enforcement Administration (DEA), for an electronic prescribing system to be used to
transmit controlled substance prescriptions, a third party must audit the electronic prescribing application for
compliance with the requirements of 21 CFR part 1311, or a certifying organization whose certification process has
been approved by DEA must verify and certify that the application meets the requirements of 21 CFR part 1311. See
https://www.deadiversion.usdoj.gov/ecomm/thirdparty.html.

it was not realistic in that version of the standard.
652
In NCPDP SCRIPT standard version
2023011, through use of a MessageIndicatorFlag, an RxFill transaction may be sent as a copy to
inform or synchronize systems.
653
Through use of this functionality, a prescriber can
electronically send a controlled substance prescription (including for a covered Part D drug) to a
pharmacy, and the pharmacy can use the MessageIndicatorFlag in an RxFill transaction when
dispensing the prescription to inform the LTC facility of the medication order. This functionality
streamlines prescribers’ workflows and ensures that the LTC facility responsible for providing
the controlled substance to the patient is aware of the order.
c. Timeframe for Including Prescriptions Written for Beneficiaries in LTC in the CMS EPCS
Program Compliance Calculation
We received multiple public comments in response to the proposal in section III.B.4. of
the CY 2025 Medicare Advantage and Part D Policy and Technical Changes proposed rule (88
FR 78489) to require NCPDP SCRIPT standard version 2023011 and retire NCPDP SCRIPT
standard version 2017071, requesting that we reconsider the current January 1, 2025, compliance
date for when we will include prescriptions written for covered Part D drugs for Part D eligible
individuals in a LTC facility in the CMS EPCS Program compliance calculation. Commenters
requested that we align the CMS EPCS Program compliance date for prescriptions written for
beneficiaries in LTC with the date that NCPDP SCRIPT standard 2023011 will be required. In
the June 2024 Part D and Health IT Standards final rule, we indicated that we would consider a
change to the CMS EPCS Program compliance date for LTC through the annual Medicare
Physician Fee Schedule rulemaking process (89 FR 51247).
652
https://standards.ncpdp.org/Standards/media/pdf/Correspondence/2024/NCPDP-Letter-to-CMS-regarding-
CMS-4205-P.pdf.
653
National Council for Prescription Drug Programs (NCPDP) SCRIPT Standard, Implementation Guide,
Version 2023011. Approval Date for American National Standards Institute (ANSI): January 17, 2023., April
2023. NCPDP SCRIPT standard implementation guides are available to NCPDP members for free and to
non-members for a fee at https://standards.ncpdp.org/Access-to-Standards.aspx.
In this proposed rule, we are proposing to revise § 423.160(a)(5) to state that
prescriptions written for a beneficiary in a LTC facility would not be included in determining
compliance until January 1, 2028, and that compliance actions against prescribers who do not
meet the compliance threshold based on prescriptions written for a beneficiary in a LTC facility
would commence on or after January 1, 2028. We do not otherwise propose to revise the text of
§ 423.160(a)(5).
As of the effective date of the June 2024 Part D and Health IT Standards final rule, July
17, 2024, Part D sponsors, prescribers and dispensers, when electronically transmitting
prescriptions and prescription-related information for covered Part D drugs for Part D eligible
individuals, may use NCPDP SCRIPT standard version 2023011. However, as discussed, there
will be a transition period where both NCPDP SCRIPT standard version 2023011 and NCPDP
SCRIPT standard version 2017071 can be used. ONC finalized an expiration date for NCPDP
SCRIPT standard version 2017071 of January 1, 2028 (rather than January 1, 2027, as proposed),
in part due to commenters’ concern about implementing the new standard in LTC facilities (89
FR 51247).
We recognize the administrative burden prescribers could potentially face when
implementing EPCS for prescriptions written for covered Part D drugs for Part D eligible
individuals in LTC facilities using NCPDP SCRIPT standard version 2017071, particularly with
the lack of guidance. We also believe that even though prescribers can use NCPDP SCRIPT
standard version 2023011 as of July 17, 2024, it may not be feasible to have electronic
prescribing systems configured to NCPDP SCRIPT standard version 2023011 by January 1,
2025, the current date by which prescriptions written for covered Part D drugs for Part D eligible
individuals in LTC facilities would be included in the CMS EPCS Program compliance threshold
calculation. By delaying the inclusion of prescriptions written for covered Part D drugs for Part
D eligible individuals in LTC facilities in the CMS EPCS Program compliance threshold
calculation to January 1, 2028, we would be aligning CMS EPCS Program compliance
calculations to the date by which the NCPDP SCRIPT standard version 2017071 is retired and
the new NCPDP SCRIPT standard version 2023011 is required for prescribers when
electronically transmitting prescriptions and prescription-related information for covered Part D
drugs for Part D eligible individuals. We believe doing so would provide sufficient time for
prescribers and pharmacies to adopt the new standard. Moreover, LTC facilities will need to
configure their EHR systems to be able to receive the MessageIndicatorFlag from the pharmacy,
indicating that the prescription has been filled, and establish the necessary policies or operations
to convert such a message into an order for the patient in the LTC facility.
We considered an alternative where we would permit prescribers to apply for a waiver for
circumstances beyond their control rather than modify the date to include prescriptions for
beneficiaries in LTC in the compliance threshold calculation. In 2022, approximately 4.7 percent
(4.5 million) of Part D Schedule II, III, IV, and V controlled substance prescriptions were written
for beneficiaries in LTC facilities, with roughly 52 percent (2.4 million) of them not meeting the
CMS EPCS Program standards for e-prescribing. If we kept the existing start date of January 1,
2025, as in the current regulatory text at § 423.160(a)(5) for the CMS EPCS Program, we
estimate at least 6,800 additional prescribers would become non-compliant. These estimates are
prior to considering emergency and disaster exceptions and waivers, which could reduce these
numbers. If we do not extend the current date by which prescriptions written for covered Part D
drugs for Part D eligible individuals in LTC facilities would be included in the CMS EPCS
Program compliance threshold calculation, then starting with the CY 2025 measurement year,
thousands of prescribers may become non-compliant, and those prescribers would potentially
apply for a waiver. We would expect that by the CY 2028 measurement year, many of these
prescribers would be compliant and would not need to apply for a waiver because beginning
January 1, 2028, NCPDP SCRIPT standard version 2023011 will be the required standard for
prescribing and dispensing Part D drugs to Part D eligible individuals and commenters have
indicated that this version of the standard will facilitate EPCS in LTC. We remind prescribers

that the CMS EPCS Program compliance rate is calculated using the Prescription Origin Code
data element in the PDE record (88 FR 79287), and the PDE is a record of the prescription
dispensing event.
654
We believe that the three-way communication in the NCPDP SCRIPT
standard version 2023011 improves communication of the controlled substance prescription as a
medication order to the LTC facility’s EHR when the pharmacy fills the prescription, but we
seek comment on how the NCPDP SCRIPT standard version 2023011 will improve prescribers’
ability to conduct EPCS to the pharmacy dispensing the prescription for individuals in LTC
facilities.
Should we finalize our proposal, we encourage prescribers who write Schedule II, III, IV,
or V controlled substance prescriptions for covered Part D drugs for Part D eligible individuals
in LTC facilities to use the additional time to prepare for when such prescriptions for
beneficiaries in LTC facilities would be included in the CMS EPCS Program compliance
threshold calculation by working to adopt the new standard or investing in technology necessary
to conduct EPCS.
We seek comment on our proposals to extend the date after which prescriptions for
covered Part D drugs for Part D eligible individuals in LTC facilities would be included in our
CMS EPCS Program compliance threshold calculation from January 1, 2025, to January 1, 2028,
and that related non-compliance actions would commence on or after January 1, 2028. We
additionally seek comment on how NCPDP SCRIPT standard version 2023011 is expected to
improve prescribers’ ability to conduct EPCS to pharmacies dispensing covered Part D drugs to
Part D eligible individuals in LTC facilities.
M. Expand Hepatitis B Vaccine Coverage
654
CMS Memorandum. “Updated Instructions: Requirements for Submitting Prescription Drug Event Data (PDE).”
April 27, 2006. Available from:
https://www.csscoperations.com/internet/csscw3_files.nsf/F/CSSCPDEGuidance.pdf/$FILE/PDEGuidance.pdf.

Hepatitis B vaccines are currently covered as a Medicare Part B benefit under section
1861(s)(10)(B) of the Act. Medicare beneficiaries who are at high or intermediate risk of
contracting hepatitis B can receive hepatitis B vaccines, with no cost to the beneficiary. The
statute expressly authorizes the Secretary to determine who is at high or intermediate risk of
contracting hepatitis B by issuing regulations. The Secretary, through past rulemaking, defined
high and intermediate risk groups for hepatitis B vaccine at 42 CFR 410.63. This definition was
last updated in the CY 2013 PFS final rule (77 FR 69363). Beneficiaries with coverage under
Medicare Part D whose level of risk falls outside high or intermediate may have their vaccine
covered under the Part D benefit.
655
Medicare coverage of hepatitis B vaccination is outdated in light of more recent
information about the risks of contracting hepatitis B. As explained in more detail in this section,
we are proposing to improve access and utilization of hepatitis B vaccines by expanding the list
of individuals who are at high or intermediate risk of contracting hepatitis B in §410.63(a).
1. Background
Hepatitis B is a vaccine-preventable liver disease caused by the hepatitis B virus.
656
The
vaccine consists of a series of typically 2-3 doses depending on the formulation delivered at
various intervals.
657
Hepatitis B virus is transmitted when body fluid (blood, semen, or other)
from a person infected with the virus enters the body of someone who is uninfected.
658
This can
happen through sexual contact; sharing needles, syringes, or other drug-injection equipment;
transmission from the gestational parent to baby during pregnancy or at birth; direct contact with
655
Sayed, BA, Finegold, K, Ashok, K, Schutz, S, De Lew, N, Sheingold, S, Sommers, BD. Inflation Reduction Act
Research Series: Medicare Part D Enrollee Savings from Elimination of Vaccine Cost-Sharing. (Issue Brief No. HP-
2023-05). Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human
Services. September 2023. Retrieved from
https://aspe.hhs.gov/sites/default/files/documents/407d41b6534e7af6702eb280b3945d00/aspe-ira-vaccine-part-
d.pdf.
656
CDC, 2023. Hepatitis B surveillance 2021. Retrieved from
https://www.cdc.gov/hepatitis/statistics/2021surveillance/hepatitis-b.htm.
657
CDC. Viral hepatitis. FAQ for health professionals. Atlanta, GA: U.S. HHS, CDC; 2022. Retrieved from
https://www.cdc.gov/hepatitis/hbv/hbvfaq.htm.
658
CDC, 2023. Hepatitis B surveillance 2021. Retrieved from
https://www.cdc.gov/hepatitis/statistics/2021surveillance/hepatitis-b.htm.

blood or open sores; or sharing contaminated items such as toothbrushes, razors or medical
equipment (such as a glucose monitor) of a person who has hepatitis B.
659
Hepatitis B can be an
acute, short-term illness and it can develop into a long-term, chronic infection. Chronic hepatitis
B can lead to serious health problems, including cirrhosis, liver cancer, and death. Treatments for
hepatitis B are available but no cure exists. There are currently an estimated 2.4 million
individuals in the U.S. living with hepatitis B virus and an estimated 20,000 new infections every
year.
660
Acute hepatitis B infections among adults leads to chronic hepatitis B disease in an
estimated 2 – 6 percent of cases.
661
Rates of reported cases of acute hepatitis B have steadily
increased among persons aged 40–49, 50–59 years, and 60 years and older from 2015–2019.
662
In 2020, rates declined in all adult age groups. In 2021, rates among all age groups remain stable
or declined compared to 2020. The highest rates were among persons 40–49 years (1.6 cases per
100,000 population) and 50–59 years (1.0 case per 100,000 population). The rates for people
aged 60 years and older were 0.5 cases per 100,000 population.
Hepatitis B vaccines are safe and effective in preventing hepatitis B virus.
663
The number
of reported hepatitis B cases has declined substantially since the vaccine was introduced in 1982,
which was achieved through incremental expansion of groups for whom the vaccine was
recommended. However, vaccination coverage among adults has been deficient and further
reduction in hepatitis B infections in the U.S. has stalled. Approximately 34 percent of adults
659
CDC. 2024. Viral Hepatitis FAQs for the public. Retrieved from https://www.cdc.gov/hepatitis/hbv/bfaq.htm.
660
Conners EE, Panagiotakopoulos L, Hofmeister MG, et al. Screening and testing for hepatitis B virus infection:
CDC recommendations – United States, 2023. MMWR Recomm Rep. 2023;72(1):1-25. Retrieved from
https://www.cdc.gov/mmwr/volumes/72/rr/rr7201a1.htm.
661
Weng, M., Doshani, M., Khan, M., Frey, S., et al. Universal hepatitis B vaccination in adults aged 19 – 59 years:
Updated recommendations of the Advisory Committee on Immunization Practices – United States, 2022. MMWR,
April 1, 2022, Vol 71(13);477–483.
662
CDC. Viral hepatitis. 2021 viral hepatitis surveillance report. Atlanta, GA: U.S. HHS, CDC; 2023. Retrieved
from https://www.cdc.gov/hepatitis/statistics/2021surveillance/hepatitis-b/figure-2.4.htm.
663
Weng, M., et al. 2022. Universal hepatitis B vaccination.

aged ≥19 years have been vaccinated against hepatitis B.
664
Furthermore, an estimated 20 percent
of adults aged ≥60 years have been vaccinated against hepatitis B.
Since 2011, rates of reported cases of acute hepatitis B decreased among children and
adolescents aged 0–19 years and persons aged 20–29 years.
665
The Centers for Disease Control
and Prevention (CDC) states that this is due, in part, because of the childhood hepatitis B vaccine
recommendations that were first implemented in 1991. The Advisory Group for Immunization
Practices (ACIP) is a group of medical and public health experts that develops recommendations
on how to use vaccines to control diseases in the U.S. and the CDC updates the U.S. adult and
childhood immunization schedules consistent with ACIP recommendations.
666
As the cohort of
persons vaccinated as children have grown older, rates of acute hepatitis B among persons aged
30–39 years began to consistently decrease beginning in 2015.
667
Conversely, rates of reported
cases of acute hepatitis B have steadily increased among persons aged 40–49, 50–59 years, and
60 years and older from 2015–2019 (see Table 52). Overall, the rate of acute hepatitis B cases
increased 11 percent from 2014 (0.9 per 100,000) to 2018 (1.0 per 100,000).
668
Injection drug
use and sexual transmission are known risk factors associated with rising acute hepatitis B cases.
For example, acute hepatitis B infections increased 114 percent from 2006 to 2013 in three states
affected by the opioid epidemic (Kentucky, Tennessee, and West Virginia).
669
TABLE 52: Rates of Reported Acute Hepatitis B Virus Infection, by Age Group – United
States
Age (years)
2015
2016
2017
2018
2019
0–19
0.0
0.0
0.0
0.0
0.0
664
CDC. 2023. Vaccination Coverage among Adults in the United States, National Health Interview Survey, 2021.
Retrieved from https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/pubs-resources/vaccination-
coverage-adults-
2021.html#:~:text=Hepatitis%20B%20vaccination%20coverage%20in,and%20Other%20(40.2%25)%20adults.
665
CDC. Viral hepatitis. 2021 viral hepatitis surveillance report. Atlanta, GA: U.S. HHS, CDC; 2023. Retrieved
from https://www.cdc.gov/hepatitis/statistics/2021surveillance/hepatitis-b/figure-2.4.htm.
666
CDC. ACIP. Retrieved from https://www.cdc.gov/vaccines/acip/index.html.
667
CDC. Viral hepatitis. 2021 viral hepatitis surveillance report. Atlanta, GA: U.S. HHS, CDC; 2023. Retrieved
from https://www.cdc.gov/hepatitis/statistics/2021surveillance/hepatitis-b/figure-2.4.htm.
668
CDC 2020. Viral hepatitis surveillance report 2018 – Hepatitis B. Retrieved from
https://www.cdc.gov/hepatitis/statistics/2018surveillance/HepB.htm.
669
HHS. 2016. Viral Hepatitis in the United States: Data and Trends. Retrieved from
https://www.hhs.gov/hepatitis/learn-about-viral-hepatitis/data-and-trends/index.html.

20–29
0.8
0.6
0.6
0.6
0.5
30–39
2.6
2.4
2.3
2.0
1.8
40–49
2.4
2.2
2.5
2.6
2.7
50–59
1.4
1.5
1.6
1.6
1.6
≥60
0.5
0.5
0.6
0.6
0.6
Source: CDC, National Notifiable Diseases Surveillance System.
* Rates per 100,000 population. Beginning in 2021, single-race population estimates are used
for rate calculations. For prior years, bridged-race population estimates are used.
† Reported confirmed cases. For the case definition, see
https://ndc.services.cdc.gov/conditions/hepatitis-b-acute/.
2. Statutory Authority
Section 1861(s)(10)(B) of the Act provides a benefit category under Part B for hepatitis B
vaccine and its administration, furnished to an individual who is at high or intermediate risk of
contracting hepatitis. The statute expressly authorizes the Secretary to determine who is at high
or intermediate risk of contracting hepatitis B for coverage of the hepatitis B vaccine.
3. Regulation
Medicare Part B pays for the hepatitis B vaccine as defined in § 410.63(a), which
describes individuals who are at high or intermediate risk of contracting hepatitis and covered for
hepatitis B vaccinations. In the CY 2013 PFS final rule (77 FR 69363), CMS expanded the
definition of individuals at risk of contracting hepatitis B, citing updated ACIP recommendations
about increased risk for diabetes patients to support the change. The ACIP stated that the
hepatitis B outbreaks were associated with adults with diabetes receiving assisted blood glucose
monitoring.
670
Today, the regulations are outdated as these risk categories have been shown
ineffective and are no longer the focus of how the medical community discusses hepatitis B
infection and prevention. In 2019, risk behavior and exposure data were missing for 37 percent
of case reports (1,183 of 3,192) of acute hepatitis B infections received by CDC.
671
ACIP also
cited a large national survey of family medicine and internal medicine physicians assessing
670
CDC. 2011. Use of Hepatitis B Vaccination for Adults with Diabetes Mellitus: Recommendations of the
Advisory Committee on Immunization Practices (ACIP). MMWR. 60(50);1709-1711. Retrieved from
https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6050a4.htm#:~:text=Based%20on%20the%20Work%20Group,
made%20(recommendation%20category%20A).
671
Weng, M., et al. 2022. Universal hepatitis B vaccination.

barriers to adult hepatitis B vaccination and found that 68% cited patients’ non-disclosure of risk
factors.
672
4. Proposed Regulatory Revisions
Since 1991, hepatitis B vaccination has been recommended by ACIP and the CDC for
infants at birth, completing the vaccination series by 16 months of age.
673
This is important
because in the U.S., the age cohorts who have received the completed series have low to no risk
of contracting the hepatitis B virus, as evidenced by the rate of zero acute hepatitis B virus
infections for the 0 – 19 age group.
674
The infant and childhood recommendations were not in
place for most of today’s adults which is evidenced by no other age group reaching a rate of zero
acute hepatitis B virus infections. Given this information, we consider the population of people
who have completed the vaccination series to be at low risk of contracting the hepatitis B virus.
Individuals who remain unvaccinated against hepatitis B are at intermediate risk, at minimum, of
contracting hepatitis B virus.
We conclude that anyone who is not fully vaccinated to be at intermediate risk of
contracting the hepatitis B virus as their risk would be above zero. Additionally, rates of reported
cases of acute hepatitis B steadily increased among age groups 40 and over between 2015 and
2019, with stabilizing or declining rates between 2020 and 2021, which may be due to the
COVID-19 pandemic.
675
While it is encouraging to see declining rates, these populations remain
at intermediate risk given their reported cases remained above zero. Therefore, we propose to
revise § 410.63(a)(2), Intermediate Risk Groups, by adding a new paragraph (a)(iv) to include
individuals who have not previously received a completed hepatitis B vaccination series or
672
Daley MF, Hennessey KA, Weinbaum CM, et al. Physician practices regarding adult hepatitis B vaccination: a
national survey. Am J Prev Med 2009;36:491–6. PMID:19362798 https://doi.org/10.1016/j. amepre.2009.01.037.
673
CDC, 2024. Vaccine safety: Hepatitis B vaccines. Retrieved from
https://www.cdc.gov/vaccinesafety/vaccines/hepatitis-b-
vaccine.html#:~:text=CDC%20recommends%20hepatitis%20B%20vaccine,not%20yet%20gotten%20the%20vacci
ne.
674
CDC. Viral hepatitis. 2021 viral hepatitis surveillance report. Atlanta, GA: U.S. HHS, CDC; 2023. Retrieved
from https://www.cdc.gov/hepatitis/statistics/2021surveillance/hepatitis-b/figure-2.4.htm.
675
CDC, 2023. Hepatitis B surveillance 2021. Retrieved from
https://www.cdc.gov/hepatitis/statistics/2021surveillance/hepatitis-b.htm.

whose vaccination history is unknown. We include the latter group in this proposal because the
CDC has stated that it is not harmful to receive either extra doses or a repeat vaccination
series.
676
This will allow these individuals to receive a covered vaccination series when medical
history may not be available. Also, the CDC states that screening for hepatitis B virus is not a
requirement for vaccination, and in settings where screening is not feasible, vaccination of
persons recommended to receive the vaccine should continue.
We note that § 410.63(a)(3) provides an exception to individuals considered intermediate
or high risk of contracting hepatitis B. This includes individuals who have undergone a
prevaccination screening and have been found to be currently positive for antibodies to hepatitis
B. We propose that this exception apply to the proposed § 410.63(a)(2)(iv) because individuals
with previous infection would not benefit from the vaccine. However, it should be noted that the
CDC states that it is not harmful to vaccinate people who are immune to hepatitis B virus
because of current or previous infection or vaccination, nor does it increase the risk for adverse
events.
677
5. Summary
This proposal will help protect Medicare beneficiaries from acquiring hepatitis B
infection, contribute to eliminating viral hepatitis as a public health threat in the United States
and is in the best interest of the Medicare program and its beneficiaries. We look forward to
receiving public comment on these proposals.
N. Low Titer O+ Whole Blood Transfusion Therapy During Ground Ambulance Transport
1. Ambulance Fee Schedule Background
676
CDC. Viral hepatitis. FAQ for health professionals. Atlanta, GA: U.S. HHS, CDC; 2022. Retrieved from
https://www.cdc.gov/hepatitis/hbv/hbvfaq.htm.
677
CDC. Viral hepatitis. FAQ for health professionals. Atlanta, GA: U.S. HHS, CDC; 2022. Retrieved from
https://www.cdc.gov/hepatitis/hbv/hbvfaq.htm.
Section 1861(s)(7) of the Act establishes an ambulance service as a Medicare Part B
service where the use of other methods of transportation is contraindicated by the individual’s
condition, but only to the extent provided in regulations. Our regulations relating to coverage for
ambulance services are set forth at 42 CFR part 410, subpart B. Since April 1, 2002, payment
for ambulance services has been made under the ambulance fee schedule (AFS), which the
Secretary established, as required by section 1834(l) of the Act, in 42 CFR part 414, subpart H.
Payment for an ambulance service is made at the lesser of the actual billed amount or the AFS
amount, which consists of a base rate for the level of service, a separate payment for mileage to
the nearest appropriate facility, a geographic adjustment factor (GAF), and other applicable
adjustment factors as set forth at section 1834(l) of the Act and § 414.610 of the regulations. In
accordance with section 1834(l)(3) of the Act and § 414.610(f), the AFS rates are adjusted
annually based on an inflation factor. The AFS also incorporates two permanent add-on
payments in § 414.610(c)(5)(i) and three temporary add-on payments in § 414.610(c)(1)(ii) and
(c)(5)(ii) to the base rate and/or mileage rate.
2. Low Titer O+ Whole Blood Transfusion Therapy During Ground Ambulance Transport
Under the AFS, Medicare Part B covers seven levels of service for ground (including
water) ambulance transports and two levels of service for air ambulance transports. The levels of
service for ground ambulance transports include basic life support (emergency); basic life
support (non-emergency); advanced life support, level 1 (ALS1) (emergency); ALS1 (non-
emergency); advanced life support, level 2 (ALS2); paramedic intercept; and specialty care
transport (§410.40(c)). Definitions for the levels of service can be found at § 414.605 and in the
Medicare Benefit Policy Manual, Chapter 10, Ambulance Services, section 30.1.1, Definition of
Ground Ambulance Services.
At § 414.605, ALS2 is defined as either transportation by ground ambulance vehicle,
medically necessary supplies and services, and the administration of at least three medications by
intravenous push/bolus or by continuous infusion, excluding crystalloid, hypotonic, isotonic, and

hypertonic solutions (Dextrose, Normal Saline, Ringer's Lactate); or transportation, medically
necessary supplies and services, and the provision of at least one of the following ALS
procedures: (1) Manual defibrillation/cardioversion; (2) Endotracheal intubation; (3) Central
venous line; (4) Cardiac pacing; (5) Chest decompression; (6) Surgical airway; (7) Intraosseous
line. These procedures must be performed by ALS personnel trained to the level of the
emergency medical technician-intermediate (EMT-Intermediate) or paramedic (§ 414.605).
According to the 2020 National Association of State Emergency Medical Services
Organizations Assessment (NASEMSO), there are approximately 11,450 ground EMS agencies
that provide 9-1-1 response with transport to an acute care hospital.
678
The administration of low
titer O+ whole blood transfusions, otherwise referred to as whole blood transfusion therapy
(WBT), began in 2017 when two Emergency Medical Services (EMS) systems in Texas began
providing WBT to patients in hemorrhagic shock during ambulance transports. Prior to this, use
of blood products in the treatment of hemorrhagic shock in the form of blood component therapy
was available only in the hospital setting and by one EMS system. Low titer O+ whole blood
contains low levels of antibodies that patients of any blood type can receive and is provided in
EMS settings to significantly increase these patients’ chances of survival.
By September 2023, over 121 EMS systems in the United States were using blood
products in the form of either WBT, packed red blood cells (PRBCs), plasma, or a combination
of PRBCs and plasma.
679
Seventy percent of these systems were using WBT.
680
As of March
2024, 147 (1.2 percent of the EMS systems in the United States) now carry whole blood
products, with 200 or more systems anticipated to provide some form of blood product
678
National Association of State EMS Officials. 2020 National Emergency Medical Services Assessment 2020.
Table 3, p 27. Available from: www./https://nasemso.org/. Accessed May 1, 2024.
679
Krohmer J. Chairman, steering committee of the Prehospital Blood Transfusion Initiative Coalition. Virtual
Meeting April 23, 2024.
680
Levy MJ, Garfinkel EM, May R, et al. Implementation of a prehospital whole blood program: Lessons. J Am
Coll Emerg Physicians Open. 2024;5: e13142. https://doi.org/10.1002/emp2.13142.

transfusion by the end of 2024.
681
Today, nearly 60 percent of those 147 EMS systems carry low
titer O+ whole blood, with the remainder utilizing other blood products.
682
EMS systems that administer WBT and other blood products (PRBCs and plasma)
generally utilize it for patients suffering hemorrhagic shock stemming from traumatic injury,
though it may also be indicated in certain non-traumatic medical conditions such as hemorrhagic
shock from a gastrointestinal bleed.
683
Traditional resuscitation protocol for massive hemorrhage
from trauma and other medical conditions such as gastrointestinal bleeding consists of crystalloid
fluids and blood component transfusions, which consist of a balanced portion of PBRCs,
platelets, and fresh frozen plasma.
684
During the conflicts in Iraq and Afghanistan, use of this traditional protocol was difficult
due to the austere combat environment and limited availability of blood components, which often
necessitated the use of fresh whole blood (FWB) in traumatic resuscitation.
685
Data collected
related to these conflicts demonstrated improvements in survival rate and reductions in
transfusion requirements for military casualties in hemorrhagic shock who received FWB versus
those receiving traditional blood component transfusion and spurred research and interest in the
use of WBT in civilian trauma.
686
Additional data demonstrating an improvement in 24-hour and
681
Levy MJ, Garfinkel EM, May ER, et al. Implementation of a prehospital whole blood program: Lessons. learned.
J Am Coll Emerg Physicians Open. 2024;5: Apr; 5(2): e13142. https://doi.org/10.1002/emp2.13142. Krohmer J.
Chairman, steering committee of the Prehospital Blood Transfusion Initiative Coalition. Virtual Meeting April 23,
2024.
682
Ibid.
683
Ibid.
Braverman MA, Smith A, Ciaraglia AV, et al. The regional whole blood program in San Antonio, TX: A 3-year
update on prehospital and in-hospital transfusion practices for traumatic and non-traumatic hemorrhage.
Transfusion. 2022; 62: S80-S89.
684
Young PP, Cotton BA, Goodnough LT. Massive Transfusion Protocols for Patients with Substantial
Hemorrhage. Transfusion Medicine Reviews. 2011, Vol 25(4). 293-303.
Washington State Department of Health Office of Community Health Systems Emergency Medical Services and
Trauma Section. Trauma Clinical Guideline: Massive Transfusion for Trauma.
685
Nessen SC, Eastridge BJ, Cronk D, et al. Fresh whole blood use by forward surgical teams in Afghanistan is
associated with improved survival compared to component therapy without platelets. Transfusion. 2013;53: 107S-
13S.
686
Spinella PC, Perkins GJ, Grathwohl KW, Beekley AC, Holcomb J. Warm Fresh Whole Blood is Independently
Associated with Improved Survival for Patients with Combat-Related Traumatic Injuries. J Trauma. 2009 April;
66(4 Suppl): S69–S76. doi:10.1097/TA.0b013e31819d85fb. Nessen SC, Eastridge BJ, Cronk D, et al. Fresh whole
blood use by forward surgical teams in Afghanistan is associated with improved survival compared to component
therapy without platelets. Transfusion. 2013;53: 107S-13S.

30-day survival rate among medically evacuated combat casualties in Afghanistan who received
prehospital transfusion encouraged research and interest in these techniques for possible
deployment by EMS services.
687
In the treatment of civilian patients with hemorrhagic shock from trauma, studies have
demonstrated that WBT provides a substantial survival benefit versus traditional component
therapy,
688
especially when provided early in the prehospital and hospital settings.
689
One study
found WBT increased the survival of such patients by as much 60 percent and reduced the need
for additional blood products in the 24-hour period following the initial transfusion by 7
percent.
690
Another study noted that there was a significant increase in the 24-hour and 30-day
survival rate in patients suffering from severe hemorrhage requiring a large transfusion
volume.
691
Patients suffering from hemorrhagic shock require stabilization in the field and rapid
transport to an acute care hospital to treat the source of hemorrhage.
692
Individuals who are
experiencing hemorrhagic shock primarily due to blood loss may require WBT as their only
resuscitative treatment. Each unit of whole blood takes 5-8 minutes to transfuse.
693
Depending
Gurney J, Staudt A, Cap A, Shackleford A, et al. Improved Survival in Critically Injured Combat Casualties Treated
with Fresh Whole Blood by Forward Surgical Teams in Afghanistan. Transfusion. 2020;60; S180-S188.
687
Shackelford SA, del Junco DJ, Powell-Dunford N, Mazuchowski EL, et al. Association of Prehospital Blood
Product Transfusion During Medical Evacuation of Combat Casualties in Afghanistan with Acute and 30-Day
Survival. JAMA. 2017; 318(16):1581-1591.
688
Hazelton JP, Ssentongo AE, Oh JS, et al. Use of Cold-Stored Whole Blood is Associated with Improved
Mortality in Hemostatic Resuscitation of Major Bleeding. A Multicenter Study. 2022. Annals of Surgery. Vol
276(4). 579-88.
689
b. Torres CM, Kent A, Scantling D, et al. Association of Whole Blood With Survival Among Patients Presenting
With Severe Hemorrhage in US and Canadian Adult Civilian Trauma Centers. JAMA Surg. 2023;158(5):532-540.
doi: 10.1001/jamasurg.2022.6978.
Brill JB, Tang B, Hatton G, Mueck KM, et al. Impact of incorporating whole blood into hemorrhagic shock
resuscitation: Analysis of 1,377 consecutive trauma patients receiving emergency-release uncrossmatched blood
products. J Am Coll Surg. 2022;234(4):408-418.
Guyette FX, Sperry JL, Peitzman AB, et al. Prehospital blood product and crystalloid resuscitation in the severely
injured patient: a secondary analysis of the prehospital air medical plasma trial. Ann Surg. 2021;273:358-364.
690
Ibid.
691
Ibid.
692
Centers for Disease Control and Prevention. Guidelines for field triage of injured patients. MMWR. 2009;58
(RR-1):1–34.
693
Vitberg D. Assistant Medical Director. District of Columbia Fire and EMS Department. Zoom meeting. February
20, 2024. Bank EA. Assistant Chief of EMS. Co-Chair of the South East Regional Advisory Council Trauma
Committee. Phone conversation, May 10, 2024.

on the time needed to transport and clinical need, patients generally receive 1-2 units of WBT
during ground transport.
694
While there may be variance between jurisdictions, the protocols for many EMS systems
currently providing WBT are designed for patients who require complex management at the
advanced life support level, demonstrating suspicion of blood loss along with evidence of
physiologic shock as indicated by parameters such as low blood pressure, an elevated pulse rate,
or slow capillary refill.
695
Other relevant factors may include an elevated lactate level, an EtCO2
waveform capnography reading < 25 as surrogate for elevated lactate, a shock index (heart
rate/systolic blood pressure) >1, and, where appropriate and consistent with protocol,
authorization by online or other medical authority.
696
We believe that many ground ambulance transports providing WBT already qualify for
ALS2 payment, since patients requiring such transfusions are generally critically injured or ill
and often suffering from cardio-respiratory failure and/or shock, and therefore are likely to
receive one or more procedures currently listed as ALS procedures in the definition of ALS2,
with endotracheal intubation, chest decompression, and/or placement of a central venous line or
an intraosseous line the most probable to be seen in these circumstances. Patients requiring
WBT are typically suffering from hemorrhagic shock, for which the usual course of treatment
includes airway stabilization, control of the hemorrhagic source, and stabilization of blood
pressure using crystalloid infusion and the provision of WBT or other blood product treatments
when available, but not necessarily the administration of advanced cardiac life support
medications.
697
Consequently, we do not believe it is likely that most patients who may require
694
Krohmer J. Chairman, steering committee of the Prehospital Blood Transfusion Initiative Coalition. Virtual
Meeting April 23, 2024.
695
Mark H. Yazer, Philip C. Spinella, Eric A. Bank, Jeremy W. Cannon, Nancy M. Dunbar, John B. Holcomb,
Bryon P. Jackson, Donald Jenkins, Michael Levy, Paul E. Pepe,Jason L. Sperry, James R. Stubbs & Christopher J.
Winckler (2022) THOR-AABB Working Party Recommendations for a Prehospital Blood Product Transfusion
Program, Prehospital Emergency Care, 26:6, 863-875.
Ibid., https://miemss.org/home/Clinicians/Whole-Blood.
696
Ibid.
697
Prehospital Hemorrhage Control and Treatment by Clinicians: A Joint Position Statement. Ann Emerg Med.
2023;82:e1-e8.

WBT would trigger the other pathway to qualify as ALS2, the administration of at least three
medications by intravenous push/bolus or by continuous infusion, excluding crystalloid,
hypotonic, isotonic, and hypertonic solutions (Dextrose, Normal Saline, Ringer's Lactate).
However, not all ground ambulance transports providing WBT may already qualify for
ALS2 payment. An ambulance transport would not qualify for ALS2 payment where a patient
received only WBT during a ground ambulance transport, and not one or more other services
that, either by themselves or in combination, presently qualify as ALS2. We believe WBT
should independently qualify as an ALS2 procedure because the administration of WBT and
handling of low titer O+ whole blood require a complex level of care beyond ALS1 for which
EMS providers and suppliers at the EMT-Intermediate or paramedic level require additional
training. In addition, WBT requires specialized equipment such as a blood warmer and rapid
infuser.
698
While there is no established national training protocol, many systems follow the
guidelines of the Association for the Advancement of Blood and Biotherapies (AABB), which
requires additional training that is 4 hours in length for paramedics and 6 hours in length for
EMS supervisory staff.
699
Medicare’s requirements for ambulance staffing at 42 CFR 410.41(b)
include compliance with state and local laws, which here would establish appropriate training
requirements with respect to WBT administration.
Therefore, we believe it is appropriate to modify the definition of ALS2 to account for
the instances where patients are administered WBT but do not otherwise qualify for ALS2
payment. Of note, we do not have the authority to provide an additional payment, such as an add-
on payment for the administration of WBT under the AFS.
698
Pokorny DM, Braverman MA, Edmundson PM, et al. The use of prehospital blood products in the resuscitation
of trauma patients; a review of prehospital transfusion practices and a description of our regional whole blood
program in San Antionio, TX. ISBT science series, 2018-08, Vol, 14(3), p 332-42.
Floccare D. Air Medical Director, State of Maryland. E-mail communication. May 14,2024
Krohmer J. Chairman, steering committee of the Prehospital Blood Transfusion Initiative Coalition. Virtual Meeting
April 23, 2024.
699
Bank EA. Assistant Chief of EMS. Co-Chair of the South East Regional Advisory Council Trauma Committee.
E-mail correspondence and phone conversation, May 10, 2024.

We propose to modify the definition of ALS2 at § 414.605 by adding the administration
of low titer O+ whole blood transfusion to the current list of seven ALS2 procedures as a new
number 8. We would also reflect this change in the Medicare Benefit Policy Manual, Chapter
10, Ambulance Services, section 30.1.1, Definition of Ground Ambulance Services. Under this
proposal, a ground ambulance transport that provides WBT would itself constitute an ALS2-
level transport.
We are aware that some established EMS systems may already provide WBT to treat
patients in hemorrhagic shock, while other jurisdictions, including those in rural areas, will not
and often will rely on alternative blood product treatments such as PRBCs and plasma. The
availability of WBT in rural areas is a complex and multifactorial issue. Fluctuating stock of the
“raw product” (blood donations) along with local healthcare demands for blood products
(PRBCs, platelets, plasma, etc.) affect the availability of WBT. Other issues in rural areas
include the logistical challenges and the costs involved in acquiring fresh units of WBT and
returning any unused units to a supplier.
700
The training, administration and monitoring is the same for these alternative blood
product treatments as it is for WBT. While we are not including alternative blood product
treatments in our proposal, we are seeking comment on whether we should add alternative blood
product treatments to the list of ALS2 procedures. We invite comments on this proposal to add
the administration of low titer O+ whole blood transfusion as an ALS2 procedure and comments
on whether we should add alternative blood product treatments such as the administration of
PRBCs or plasma.
O. Medicare Parts A and B Overpayment Provisions of the Affordable Care Act
700
Apelseth TO, Strandenes G. Kristofferson K, Hagen KG. How do I implement a whole blood–based blood
preparedness program in a small rural hospital? Transfusion. 2020. Vol 60(12) 2793-2800.
Schaefer R, Bank EA, Krohmer J, Haskell A, et al. Removing the Barriers to Prehospital Blood: A Roadmap to
Success. Journal of Trauma and Acute Care Surgery. 2024 (manuscript). Currently awaiting publication.
1. Executive Summary
In the proposed rule titled “Medicare Program; Contract Year 2024 Policy and Technical
Changes to the Medicare Advantage Program, Medicare Prescription Drug Benefit Program,
Medicare Cost Plan Program, Medicare Parts A, B, C, and D Overpayment Provisions of the
Affordable Care Act and Programs of All-Inclusive Care for the Elderly; Health Information
Technology Standards and Implementation Specifications”, which appeared in the December 27,
2022 Federal Register, we proposed to amend our regulations regarding the standard for an
“identified overpayment” under Medicare Parts A, B, C, and D to align the regulations with the
statutory language in section 1128J(d)(4)(A) of the Act, which provides that the terms
“knowing” and “knowingly” have the meaning given those terms in the Federal False Claims Act
(the False Claims Act) at 31 U.S.C. 3729(b)(1)(A) (87 FR 79452). We refer to that rule as the
“December 2022 Overpayment Proposed Rule.” In the December 2022 Overpayment Proposed
Rule, we proposed to remove the existing “reasonable diligence” standard and adopt by reference
the False Claims Act definition of “knowing” and “knowingly” as set forth at 31 U.S.C.
3729(b)(1)(A).
We have not yet finalized our proposals with respect to overpayments under Medicare
Parts A and B in the December 2022 Overpayment Proposed Rule. Instead, after considering the
public comments we received in connection with the December 2022 Overpayments Proposed
Rule, we are retaining the Parts A and B proposals published in the December 2022
Overpayment Proposed Rule and we are now making additional proposals to revise existing
regulations at § 401.305(b) regarding the deadline for reporting and returning overpayments.
2. Provisions of the Proposed Regulation (Preamble)
Section 6402(a) of the Patient Protection and Affordable Care Act (Pub. L. 111-148), as
amended by the Health Care and Education Reconciliation Act of 2010 (Pub. L. 111-152)
(collectively known as the Affordable Care Act), established section 1128J(d) of the Act.
Section 1128J(d)(1) of the Act requires a person who has received an overpayment to report and
return the overpayment to the Secretary, the State, an intermediary, a carrier, or a contractor, as
appropriate, and to notify the Secretary, State, intermediary, carrier or contractor to which the
overpayment was returned in writing of the reason for the overpayment. Section 1128J(d)(4)(B)
of the Act defines the term “overpayment” as any funds that a person receives or retains under
title XVIII or XIX to which the person, after applicable reconciliation, is not entitled under such
title. For purposes of Medicare Parts A and B, section 1128J(d)(4)(C) of the Act defines the
term “person” to include providers and suppliers as those terms are defined in the Act. Section
1128J(d)(4)(C) of the Act also defines the term “person,” for purposes of Medicare Parts C and
D, to also include a Medicare Advantage organization (MAO) (as defined in section 1859(a)(1)
of the Act) and a Part D Plan (PDP) sponsor (as defined in section 1860D-41(a)(13) of the Act).
Section 1128J(d)(2) of the Act requires that an overpayment be reported and returned by
the later of: (1) the date which is 60 days after the date on which the overpayment was identified;
or (2) the date any corresponding cost report is due, if applicable. Section 1128J(d)(3) of the Act
specifies that any overpayment retained by a person after the deadline for reporting and returning
an overpayment is an obligation (as defined in 31 U.S.C. 3729(b)(3)) for purposes of the False
Claims Act, 31 U.S.C. 3729.
Section 1128J(d)(4)(A) of the Act provides that the terms “knowing” and “knowingly”
have the meaning given those terms in the False Claims Act at 31 U.S.C. 3729(b)(1)(A). The
False Claims Act (31 U.S.C. 3729(b)(1)(A)) defines the terms “knowing” and “knowingly” to
include information about which a person “has actual knowledge,” “acts in deliberate ignorance
of the truth or falsity of the information,” or “acts in reckless disregard of the truth or falsity of
the information.”
a. Regulations Promulgated Under Section 1128J(d) of the Act
On May 23, 2014, CMS published a final rule titled “Medicare Program; Contract Year
2015 Policy and Technical Changes to the Medicare Advantage and the Medicare Prescription
Drug Benefit Programs” (79 FR 29844) (hereinafter referred to as the “Parts C and D
Overpayment Final Rule”), which provided, among other things, that an MAO or PDP sponsor
has identified an overpayment when the MAO or PDP sponsor has determined, or should have
determined through the exercise of reasonable diligence, that the MAO or PDP sponsor has
received an overpayment.
On February 12, 2016, we published a final rule titled “Medicare Program; Reporting and
Returning of Overpayments” (81 FR 7654) (hereinafter referred to as the “Parts A and B
Overpayment Final Rule”), which provided, among other things, that a provider or supplier has
identified an overpayment when the provider or supplier has determined, or should have
determined through the exercise of reasonable diligence, that the provider or supplier has
received an overpayment and quantified the amount of the overpayment.
In the December 2022 Overpayment Proposed Rule, we proposed to amend the existing
regulations for Medicare Parts A and B, as well as Parts C and D, regarding the standard for an
“identified overpayment” to align the regulations with the statutory language in section
1128J(d)(4)(A) of the Act. If finalized, these regulations would assign the meaning of the terms
“knowing” and “knowingly” in the False Claims Act at 31 U.S.C. 3729(b)(1)(A) to our
regulations for purposes of Medicare overpayments. Specifically, in the December 2022
Overpayment Proposed Rule, we proposed to remove the existing “reasonable diligence”
standard and adopt by reference the False Claims Act definition of “knowing” and “knowingly”
as set forth at 31 U.S.C. 3729(b)(1)(A). We continue to review the comments we received on the
December 2022 Overpayment Proposed Rule, and we plan to respond both to those comments
and the comments we receive on our new proposals when we publish the CY 2025 Physician Fee
Schedule final rule.
b. Relevant Litigation
In UnitedHealthcare Insurance Co. v. Azar, a group of MAOs challenged the Parts C and
D Overpayment Final Rule, and the District Court held, in relevant part, that by requiring MAOs
to use “reasonable diligence” in searching for and identifying overpayments, CMS impermissibly
established False Claims Act liability for mere negligence. UnitedHealthcare Ins. Co. v. Azar,
330 F. Supp. 3d 173, 191 (D.D.C. 2018), rev’d in part on other grounds sub nom.
UnitedHealthcare Ins. Co. v. Becerra, 16 F.4
th
867 (D.C. Cir. 2021), cert. denied, 142 S. Ct.
2851 (U.S. June 21, 2022) (No. 21-1140). The District Court noted that “(t)he False Claims
Act—which the ACA refers to for enforcement, see 42 U.S.C. 1320a-7k(d)(3)—imposes liability
for erroneous (‘false’) claims for payment submitted to the government that are submitted
‘knowingly’ … a term of art defined in the FCA to include false information about which a
person ‘has actual knowledge,’ ‘acts in deliberate ignorance of the truth or falsity of the
information,’ or ‘acts in reckless disregard of the truth or falsity of the information.’” Id. at 190.
Although the court’s ruling applied only to Medicare Part C, to provide for consistency in
Medicare regulations related to reporting and returning overpayments, in the December 2022
Overpayment Proposed Rule, we proposed to amend the regulations at current § 401.305(a)(2) to
remove the reference to “reasonable diligence” and replace it with language incorporating the
terminology of section 1128J(d)(4)(A) of the Act by ascribing the terms “knowing” and
“knowingly” the same meaning given those terms in the False Claims Act at 31 U.S.C.
3729(b)(1)(A). See UnitedHealthcare, 330 F. Supp. 3d at 191 (finding that this language would
be consistent with a 2000 agency rule, the False Claims Act, and the Affordable Care Act’s
reference to the False Claims Act).
c. Provisions of Proposed Regulations
In addition to our earlier proposals, which remain under consideration, we make the
following new proposals.
Existing § 401.305(b)(1) specifies when a person who has received an overpayment must
report and return an overpayment. We propose to amend this paragraph to reference revised
§ 401.305(b)(2), as well as to reference newly-proposed § 401.305(b)(3).
Existing § 401.305(b)(2) specifies the circumstances under which the deadline for
returning overpayments will be suspended. Overpayments must be reported no later than the date
which is 60 days after the date on which the overpayment was identified or the date any
corresponding cost report is due, if applicable. However, the deadline for returning a reported
overpayment will be suspended under specified circumstances, including the acknowledgement
of receipt of a submission to the OIG Self-Disclosure Protocol or the CMS Voluntary Self-
Referral Disclosure Protocol, or under specified conditions if a person requests an extended
repayment schedule as defined in § 401.603. We are proposing a technical modification to the
introductory language in § 401.305(b)(2) to acknowledge that this section might be applicable
after the suspension described in new § 401.305(b)(3) is complete.
New proposed § 401.305(b)(3) would specify the circumstances under which the deadline
for reporting and returning overpayments would be suspended to allow time for providers to
investigate and calculate overpayments. Proposed § 401.305(b)(3)(i) provides that the deadline
to report and return an overpayment would be suspended if: (1) a person has identified an
overpayment but has not yet completed a good-faith investigation to determine the existence of
related overpayments that may arise from the same or similar cause or reason as the initially
identified overpayment; and (2) the person conducts a timely, good-faith investigation to
determine whether related overpayments exist. Proposed § 401.305(b)(3)(ii) provides that, if the
conditions for proposed § 401.305(b)(3)(i) are met, the deadline for reporting and returning the
initially identified overpayment and related overpayments that arise from the same or similar
cause or reason as the initially identified overpayment will remain suspended until the earlier of
the date that the investigation of related overpayments has concluded and the aggregate amount
of the initially identified overpayments and related overpayments is calculated, or the date that is
180 days after the date on which the initial identified overpayment was identified.
The following example may elucidate a hypothetical circumstance. Assume that, on day
1, a person identifies an overpayment arising from a physician’s failure to properly document the
medical record to support the coding of a specific claim, and the person has reason to believe that
this may be a common practice of the physician, so there could be more affected claims. At this
point, the person has up to 180 days to conduct and conclude a good faith investigation to
determine whether related overpayments that arise from the same or similar cause or reason as
the initially identified overpayment exist. If the person does NOT conduct an investigation, or
the investigation is not timely or not conducted in good faith, the identified overpayment must be
reported and returned by day 60. If the person does conduct a timely, good faith investigation,
suspension of the report and return obligation under § 401.305(b)(3) begins on day 1. The
suspension ends when the investigation is concluded and the initially identified overpayment and
related overpayments, if any, are calculated, or by day 180, whichever is earlier. The
overpayment must be reported and returned within 60 days after either completion of the
investigation or day 180, whichever is earlier. However, the suspensions described in §
401.305(b)(2) may also be applicable. For example, if the person is reporting the overpayment
to the OIG Self-Disclosure Protocol, as provided for in § 401.305(b)(2) the overpayment return
requirement may be further suspended in accordance with that provision.
We make these proposals because many of the comments that we received on the
December 2022 Overpayment Proposed Rule expressed concern that we proposed to remove the
term “quantified” from the original regulatory text. We believe that our proposals, especially
proposed § 401.305(b)(3)(ii)(A), would address this concern. Other commenters expressed
concern that the December 2022 Overpayment Proposed Rule proposals removed a perceived 6-
month time period to investigate all overpayments that was referenced in an example in the
preamble to the original 2016 Parts A and B Overpayment Rule. The December 2022
Overpayment Proposed Rule was silent on this point and did not remove this time period to
investigate overpayments. We understand the importance of allowing time to investigate and
calculate overpayments. Therefore, we propose to codify this allowance into regulation at
proposed § 401.305(b)(3)(ii).
We solicit comment on these proposals.

IV. Updates to the Quality Payment Program
A. CY 2025 Modifications to the Quality Payment Program
1. Executive Summary
a. Overview
This section of this proposed rule sets forth proposed changes to the Quality Payment
Program starting January 1, 2025, except as otherwise noted for specific provisions. We
continue to move the Quality Payment Program forward, including focusing more on alignment
and new options for clinicians to participate in a more meaningful way, to achieve continuous
improvement in the quality of health care services provided to Medicare beneficiaries and other
patients through the Quality Payment Program’s Merit-based Incentive Payment System (MIPS)
and Advanced Alternative Payment Models (APMs) for the CY 2025 performance period/2027
MIPS payment year.
Authorized by the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA)
(Pub. L. 114-10, April 16, 2015), the Quality Payment Program is a value-based payment
program, by which the Medicare program rewards clinicians who provide high-value, high-
quality care to their patients in a cost-efficient manner. There are two ways for clinicians who
provide services under the Medicare program to participate in the Quality Payment Program:
MIPS and Advanced APMs. The statutory requirements for the Quality Payment Program are set
forth in section 1848(q) and (r) of the Act for MIPS and section 1833(z) of the Act for Advanced
APMs.
For the MIPS participation track, MIPS eligible clinicians (defined in § 414.1305)
701
are
subject to a MIPS payment adjustment (positive, negative, or neutral) based on their performance
in four performance categories: cost, quality, improvement activities, and Promoting
Interoperability. We assess each MIPS eligible clinician’s total performance according to
701
We note that the term MIPS eligible clinician is defined in § 414.1305 as including a group of at least one MIPS
eligible clinician billing under a single tax identification number. We refer readers to our policies governing group
reporting and scoring under MIPS as set forth in § 414.1310(e).
established performance standards with respect to the applicable measures and activities
specified in each of these four performance categories during a performance period to compute a
final composite performance score (a “final score” as defined at § 414.1305). In calculating the
final score, we must apply different weights for the four performance categories, subject to
certain exceptions, as set forth in section 1848(q)(5) of the Act and at § 414.1380. Unless we
assign a different scoring weight pursuant to these exceptions, for CY 2025 performance
period/2027 MIPS payment year, the scoring weights are as follows: 30 percent for the quality
performance category; 30 percent for the cost performance category; 15 percent for the
improvement activities performance category; and 25 percent for the Promoting Interoperability
performance category.
Once calculated, each MIPS eligible clinician’s final score is compared to the
performance threshold established in prior rulemaking for that performance period to calculate
the MIPS payment adjustment factor as specified in section 1848(q)(6) of the Act, such that the
MIPS eligible clinician will receive in the applicable MIPS payment year: (1) a positive
adjustment, if their final score exceeds the performance threshold; (2) a neutral adjustment, if
their final score meets the performance threshold; or (3) a negative adjustment, if their final score
is below the performance threshold. In calculating the MIPS payment adjustment factor for a
MIPS eligible clinician, CMS accounts for scaling factor and budget neutrality requirements, as
further specified in section 1848(q)(6) of the Act. CMS then applies the MIPS payment
adjustment factor to amounts otherwise paid under Part B with respect to covered professional
services for the MIPS eligible clinician for the applicable MIPS payment year such that their
payments for such covered professional services are increased, decreased, or not adjusted based
on the MIPS eligible clinician’s final score relative to the performance threshold.
Section 1848(q) of the Act sets forth other requirements applicable to MIPS, including
opportunities for feedback and targeted review and public reporting of MIPS eligible clinicians’
performance. Section 1848(r) of the Act sets forth more specific requirements for development
of measures for the cost performance category under MIPS.
For the Advanced APM track, if an eligible clinician participates in an Advanced APM
and achieves Qualifying APM Participant (QP) or Partial QP status, they are excluded from the
MIPS reporting requirements and payment adjustment (though eligible clinicians who are Partial
QPs may elect to be subject to the MIPS reporting requirements and payment adjustment).
Eligible clinicians who are QPs for the CY 2024 performance year receive a 1.88 percent APM
Incentive Payment in the 2026 payment year. Beginning with the CY 2024 performance year
(payment year 2026), QPs will also receive a higher PFS payment rate (calculated using the
differentially higher “qualifying APM conversion factor”) than non-QPs. QPs will continue to be
excluded from MIPS reporting and payment adjustments for the applicable year.
We plan to continue developing policies for the Quality Payment Program that more
effectively reward high-quality of care for patients and increase opportunities for Advanced
APM participation. We are continuing to develop new MIPS Value Pathways (MVPs) to allow
for a more cohesive participation experience by connecting activities and measures from the four
MIPS performance categories that are relevant to a specialty, medical condition, or a particular
population.
As we move into the eighth year of the Quality Payment Program, we are proposing the
updates set forth in this section of this proposed rule, encouraging continued improvement in
clinicians’ performance with each performance year and driving improved quality of health care
through payment policy.
b. Summary of Major Proposals
(1) Transforming the Quality Payment Program
Our National Quality Strategy (https://www.cms.gov/medicare/quality/meaningful-
measures-initiative/cms-quality-strategy) addresses the urgent need to advance towards a more
equitable, safe, and outcomes-based health care system for all individuals. We have a

corresponding cohesive value-based care strategy for Medicare along three main pillars:
Alignment, Growth, and Equity.
702
We continue to focus on transforming health care delivery
703
and our 2030 goal to have all traditional Medicare beneficiaries in an accountable care
relationship with their health care provider. In pursuit of this vision, we are driving higher value
care, supporting Advanced APM participation, increasing alignment to reduce burden, and
promoting health equity. We are exploring new care delivery and payment models; for example,
we are considering an ambulatory care model that would connect payment to performance for
specialists in the ambulatory setting to increase the number of specialists who deliver
longitudinal care in an accountable manner and to support greater integration between specialty
and primary care. This potential model would utilize MVPs as a foundation for assessing
specialist performance (refer to section III.J of this proposed rule). We are proposing in section
II.G.2 of this proposed rule to make payment for advanced primary care management (APCM)
services furnished by a physician or other qualified health care professional who is responsible
for all primary care (for example, physicians and non-physician practitioners, including nurse
practitioners, physician assistants, certified nurse-midwives and clinical nurse specialists), and
serve as the continuing focal point for all needed health care services during a calendar month.
This proposed payment would incorporate several specific, existing care management and
communication technology-based services into a bundle and include a performance
measurements requirement that could be met by reporting the Value in Primary Care MVP by
clinicians billing for APCM services. We are proposing that billing practitioners who are not
MIPS eligible clinicians (as defined at 42 CFR 414.1305) would not have to report the MVP in
order to furnish and bill for APCM services.
702
Update On The Medicare Value-Based Care Strategy: Alignment, Growth, Equity, Health Affairs Forefront,
March 14, 2024. https://www.healthaffairs.org/content/forefront/update-medicare-value-based-care-strategy-
alignment-growth-equity.
703
Quality in Motion, Acting on the CMS National Quality Strategy, April 2024.
https://www.cms.gov/files/document/quality-motion-cms-national-quality-strategy.pdf.

Separately, we are implementing MVPs and subgroup reporting option to allow clinicians
to report on a cohesive set of measures that more directly reflect their clinical practice. MVPs
allow for more clinically relevant performance measurement, engage more specialists in
performance measurement, and help reduce barriers to APM participation. While traditional
MIPS continues to be a submission option, we intend to move to full MVP adoption and to
sunset traditional MIPS in the future.
In section IV.A.3.d of this proposed rule, we discuss a Request for Information (RFI) that
addresses how we can best achieve full MVP adoption and subgroup participation as we plan to
move forward with sunsetting traditional MIPS and advancing the three pillars and the National
Quality Strategy. We seek feedback on clinician readiness to report MVPs, how we should
ensure there are applicable MVPs for all clinicians, and what parameters, including logistical and
regulatory policies, are needed for multispecialty groups to place clinicians into subgroups for
reporting an MVP relevant to the scope of care provided.
(a) MIPS Value Pathways Development and Maintenance
In an effort to promote high-quality, safe, and equitable care and to implement the vision
outlined in the CMS National Quality Strategy, we are proposing six new MVPs around the
following topics: Complete Ophthalmologic Care, Dermatological Care, Gastroenterology Care,
Optimal Care for Patients with Urologic Conditions, Pulmonology Care and Surgical Care.
Complete Ophthalmologic Care, Dermatological Care, Gastroenterology Care, Optimal Care for
Patients with Urologic Conditions, Pulmonology Care, and Surgical Care.
We are also proposing to modify the MVP maintenance webinar process to provide more
flexibility on how we communicate submitted maintenance recommendations prior to proposing
them formally in rulemaking (refer to section IV.A.4.a of this proposed rule).
Lastly, we are proposing MVP maintenance updates to our MVP inventory that are in
alignment with the MVP development criteria, and in consideration of the feedback from
interested parties we have received through the maintenance process.
(b) MVP Scoring
We are proposing to update the scoring of population health measures in MVPs by using
the highest score of all available population health measures, and we are proposing to remove the
requirement for MVP Participants to select a population health measure at the time of MVP
registration. We are also proposing to modify the MVP scoring policies at § 414.1365(d)(3)(ii)
with respect to the cost performance category to refer to, and therefore align with, our
methodology for scoring cost measures at § 414.1380(b)(2) under our traditional MIPS policies..
Additionally, we are proposing to align MVP scoring with traditional MIPS policies by removing
references to high- and medium-weighted improvement activities in MVPs. We are proposing to
update MVP scoring to assign 40 points for each improvement activity to provide full credit for
the improvement activities performance category for MVP Participants who report one
improvement activity. For the MVP Promoting Interoperability performance category, we are
proposing to modify our policy at § 414.1365(c)(4)(i)(A), requiring a subgroup to submit the
affiliated group’s data for the performance category, by removing references to specific
performance periods/MIPS payment years, thereby permitting subgroups to report data for this
category in this manner for the CY 2025 performance period/2027 MIPS payment year and
beyond.
(c) APM Performance Pathway
We are proposing to create within the APM Performance Pathway (APP) the APP Plus
quality measure set beginning with the CY 2025 performance period/2027 MIPS payment year
to align with the Universal Foundation measures under the CMS National Quality Strategy. We
are not proposing to modify the existing APP quality measure set, which already includes five of
the ten Universal Foundation measures. Instead, we are proposing to establish the APP Plus
quality measure set as a second, optional measure set that would be comprised of all of the
measures in the existing APP quality measure set and would additionally incrementally adopt the
remaining five Universal Foundation measures from the CY 2025 performance period/2027
MIPS payment year through the CY 2028 performance period/2030 MIPS payment year. Under
this proposal, a MIPS eligible clinician, group, or APM Entity that reports the APP may choose
to report either the APP quality measure set or the APP Plus quality measure set.
(d) Data Submission for the Performance Categories
We are proposing to adopt minimum criteria for a qualifying data submission for a MIPS
performance period for the quality, improvement activities, and Promoting Interoperability
performance categories, which we propose to codify at § 414.1325(a)(1)(i) through (iii).
Specifically, we are proposing that a qualifying data submission must include numerator and
denominator data for at least one MIPS quality measure from the final list of MIPS quality
measures for the quality performance category and include a response of “yes” for at least one
activity in the MIPS improvement activities Inventory for the improvement activities
performance category. For the Promoting Interoperability performance category, we are
proposing a qualifying data submission must include: (1) performance data, including any claim
of an applicable exclusion, for the measures in each objective, as specified by CMS; (2) required
attestation statements, as specified by CMS; (3) CMS EHR Certification ID (CEHRT ID) from
the Certified Health IT Product List (CHPL); and (4) the start date and end date for the
applicable performance period as set forth in § 414.1320.
We are also proposing to codify our existing policies governing our treatment of multiple
data submissions received for the quality and improvement activities performance categories at §
414.1325(f)(1). We are also proposing to modify our policy governing our treatment of multiple
data submissions received for the Promoting Interoperability performance category, which we
propose to codify at § 414.1325(f)(2). Specifically, for the quality and improvement activities
performance categories, we are proposing that for multiple data submissions received from
submitters in multiple organizations, CMS will calculate a score for each submission received
and assign the highest of the scores. For multiple data submissions received from a submitter in
the same organization, CMS will score the most recent submission. For the Promoting
Interoperability performance category, we are proposing to modify our policy so that, for
multiple data submissions received, CMS would calculate a score for each data submission
received and assign the highest of the scores.
(e) MIPS Performance Category Measures and Activities
(i) Quality Performance Category
We are proposing to establish the data submission criteria for the Alternative Payment
Model (APM) Performance Pathway (APP) quality measure set; maintain the data completeness
criteria threshold to at least 75 percent for the CY 2027 and CY 2028 performance periods/2029
and 2030 MIPS payment years; establish a measure set inventory of 196 MIPS quality measures,
of which 193 are available in traditional MIPS and 3 are available only for utilization in MVPs;
and codify previously established criteria pertaining to the removal of MIPS quality measures.
Additionally, in section IV.A.4.e.(1)(e) of this proposed rule, there are two quality-related RFIs.
The first RFI pertains to the potential expansion of the survey modes for the administration of the
Consumer Assessment of Healthcare Providers and Systems (CAHPS) for MIPS Survey,
particularly expanding the survey modes from a mail-phone protocol to a web-mail-phone
protocol. The second RFI pertains to the potential development of a set of guiding principles that
would be utilized for the selection and implementation of Patient-Reported Outcome Measures
(PROMs) and Patient-Reported Outcome Performance Measures (PRO–PMs).
(ii) Cost Performance Category
We are proposing to add six new episode-based measures to the cost performance
category beginning with the CY 2025 performance period/2027 MIPS payment year: Chronic
Kidney Disease, End-Stage Renal Disease, Kidney Transplant Management, Prostate Cancer,
Rheumatoid Arthritis, and Respiratory Infection Hospitalization. We are also proposing
modifications to two existing episode-based cost measures so that their specifications reflect re-
evaluated versions: Cataract Removal with Intraocular Lens (IOL) Implantation (currently titled
Routine Cataract Removal with IOL Implantation) and Inpatient (IP) Percutaneous Coronary
Intervention (PCI) (currently titled ST-Elevation Myocardial Infarction (STEMI) PCI). We are
proposing to adopt a 20-episode case minimum for the six new episode-based cost measures.
We are also proposing to maintain the existing case minimums for the two measures we are
proposing to modify in this rulemaking, which are a 20-episode case minimum for the IP PCI
measure and a 10-episode case minimum for the Cataract Removal with IOL Implantation
measure. Additionally, we are proposing to update the operational list of care episode and patient
condition groups and codes to reflect these new and modified measures that we are proposing.
Lastly, we are proposing to adopt criteria to specify objective bases for the removal of any cost
measures from the MIPS cost performance category, which we are also proposing to codify at §
414.1350(e).
(iii) Improvement Activities Performance Category
As part of our regular maintenance of the improvement activities Inventory, we are
proposing to add two new, modify two existing, and remove eight existing improvement
activities. The new activities help fill gaps we have identified in the Inventory while the
modified and removed activities will ensure that it includes only the most meaningful activities
that have a clear path to clinical practice improvement. In addition, we are proposing two
changes to the traditional MIPS improvement activities reporting and scoring policies for the CY
2025 performance period/2027 MIPS payment year: to eliminate the weighting of activities and
to reduce the number of activities to which clinicians are required to attest to achieve a score in
the improvement activities performance category. Lastly, we are proposing to codify seven
improvement activity removal factors to establish criteria used to identify activities for potential
removal or modification.
(iv) Promoting Interoperability Performance Category
We do not have any proposals for the Promoting Interoperability performance category.
We are seeking public comment on a RFI regarding the Public Health Reporting and Data
Exchange Objective.

(f) MIPS Final Scoring Methodology
(i) Scoring the Quality Performance Category
We are proposing to implement defined topped out benchmarks for topped out measures
in specialty sets affected by limited measure choice. Many specialty sets have not had the
measure development that was envisioned early on the program. As a result, we have retained
topped out measures to ensure there are applicable measures. To accommodate for this limited
measure choice and the rising performance threshold, we are proposing to remove the 7-point
topped out measure score cap for clinicians reporting measures included in certain specialty
measures and implement a benchmarking strategy for affected measures that would ensure
clinicians with limited measure choice are not unfairly penalized. Additionally, we are proposing
to apply a Complex Organization Adjustment for virtual groups and APM Entities (including
SSP ACOs) reporting eCQMs. We are also proposing to score Medicare CQMs using flat
benchmarks for their first 2 years in the program consistent with the Shared Saving Program’s
policies.
(ii) Scoring the Cost Performance Category
We are proposing to modify our methodology for scoring measures for the cost
performance category beginning with the CY 2024 performance period/2026 MIPS payment
year. Additionally, we are proposing to adopt a new cost measure exclusion policy beginning
with the CY 2025 performance period/2027 MIPS payment year.
(g) MIPS Payment Adjustments
We are proposing to establish the mean as the methodology for determining the
performance threshold for the CY 2025 performance period/2027 MIPS payment year through
the CY 2027 performance period/2029 MIPS payment year. To determine the performance
threshold for the CY 2025 performance period/2027 MIPS payment year, we are proposing that
we will use the mean of the final scores from the CY 2017 performance period/2019 MIPS
payment year. Based on the mean final score from that prior period, we are proposing to

establish a performance threshold of 75 points for the CY 2025 performance period/2027 MIPS
payment year.
(h) Calculating the Final Score
We are proposing to adopt a new reweighting policy at § 414.1380(c)(2)(i)(A)(10) and
(c)(2)(i)(C)(12). Specifically, we are proposing that, beginning with the CY 2024 performance
period/2026 MIPS payment year, we may reweight one or more of the performance categories
(specifically, quality, improvement activities, or Promoting Interoperability) where we
determine, based on information submitted to us on or before November 1st of the year
preceding the relevant MIPS payment year, that data for a MIPS eligible clinician are
inaccessible or unable to be submitted due to circumstances outside of the control of the clinician
because the MIPS eligible clinician delegated submission of the data to their third party
intermediary, evidenced by a written agreement between the MIPS eligible clinician and third
party intermediary, and the third party intermediary did not submit the data for the performance
category(ies) on behalf of the MIPS eligible clinician in accordance with applicable deadlines.
We also are proposing that, to determine whether to apply reweighting to the affected
performance category(ies), we will consider: whether the MIPS eligible clinician knew or had
reason to know of the issue with its third party intermediary’s submission of the clinician’s data
for the performance category(ies); whether the MIPS eligible clinician took reasonable efforts to
correct the issue; and whether the issue between the MIPS eligible clinician and their third party
intermediary caused no data to be submitted for the performance category(ies) in accordance
with applicable deadlines.
(i) Third Party Intermediaries
We are proposing to add a requirement that CMS-approved survey vendors must provide
information on the cost of their services beginning with the CY 2026 performance period/2028
MIPS payment year. These costs will only be applicable to the CAHPS for MIPS Survey
measure. If this proposal is finalized, the CAHPS for MIPS Survey Vendor Participation Form
and the CAHPS for MIPS Survey Minimum Business Requirements in the QPP Resource
Library would be updated to detail the required survey vendor cost information.
(2) Advanced APM Proposals
(a) Overview of the APM Incentive
Eligible clinicians who meet threshold levels of participation in Advanced APMs to
become QPs (or partial QPs) are excluded from MIPS reporting requirements and payment
adjustments. We assess the level of participation in Advanced APMs based on payment amounts
or patient counts as provided in our regulation at § 414.1425 using the threshold percentages
specified in § 414.1430. The threshold percentages are calculated using the ratio of attributed
beneficiaries to attribution-eligible beneficiaries. A beneficiary is considered attribution-eligible
if they meet the six criteria specified in the definition of “attribution-eligible beneficiary” under §
414.1305 of our regulations. We are proposing to modify the sixth criterion under the definition
of “attribution-eligible beneficiary.” Specifically, we are proposing to include as attribution-
eligible any beneficiary who has received a covered professional service furnished by the eligible
clinician (identified by their National Provider Identifier (NPI)) for the purpose of making QP
determinations. We are also proposing to amend § 414.1430 to reflect the statutory QP and
Partial QP threshold percentages for both the payment amount and patient count methods under
the Medicare Option and the All-Payer Option with respect to payment year 2026 (performance
year 2024) in accordance with amendments made by the CAA, 2024. Relatedly, we are
proposing to amend § 414.1450 to reflect the statutory APM Incentive Payment amount for the
2026 payment year (performance year 2024) of 1.88 percent of the eligible clinician’s estimated
aggregate payments for covered professional services in accordance with amendments made by
the CAA, 2024.
2. Definitions
At § 414.1305, we are proposing to revise the definition of the following term:
● Attribution-eligible beneficiary

These terms and definitions are discussed in detail in the relevant sections of this proposed rule.
3. Transforming the Quality Payment Program
a. Vision and Strategy Overview
Medicare plays a lead role in transitioning the health care system away from fee-for-
service payment, which incentivizes the quantity of care, toward value-based payment, which
incentivizes higher-quality care and smarter spending. We continue to focus on transforming
health care delivery and our 2030 goal to have all traditional Medicare beneficiaries in an
accountable care relationship with their health care provider. Under accountable care, a person-
centered care team is responsible for improving quality of care, care coordination, and health
outcomes for a defined group of individuals, reducing care fragmentation, and avoiding
unnecessary costs for individuals and the health system. We continue to pursue driving higher
value care, supporting Advanced APM participation, increasing alignment to reduce burden, and
promoting health equity. As stated previously (85 FR 50279 and 50284, 86 FR 65394 through
65396), we envision a full transition to MVP reporting and sunset of traditional MIPS to support
movement towards value-based payment. Through this Request for Information (RFI) we seek
feedback on policies to support full implementation of MVPs.
We have a cohesive value-based care strategy for Medicare based on three pillars:
Alignment, Growth, and Equity.
704
These pillars are inter-related and address the following:
● Aligning key aspects of value-based arrangements across Medicare can help set the
stage for broader synchronization of our health system and move health care providers to higher
levels of delivery system transformation.
● Growth of accountable care relationships in both traditional Medicare and Medicare
Advantage can improve quality and increase savings for Medicare beneficiaries by promoting
innovative care delivery that better provides whole-person care.
704
Update On The Medicare Value-Based Care Strategy: Alignment, Growth, Equity, Health Affairs Forefront,
March 14, 2024. https://www.healthaffairs.org/content/forefront/update-medicare-value-based-care-strategy-
alignment-growth-equity.

● The design of value-based arrangements in Medicare can be an important tool for
advancing health equity by encouraging the movement of care upstream to address the health-
related social needs and disparities that can lead to/exacerbate poor health outcomes.
We intend to continue our efforts to align the Quality Payment Program with the three
pillars and broader CMS initiatives. One of our CMS National Quality Strategy goals is to
improve quality and health outcomes across the health care journey through the implementation
of a “Universal Foundation” of impactful measures across all our quality and value-based
programs.
705,706,707
Adoption of the Universal Foundation will focus clinician attention on
specific quality measures, reduce burden, help identify disparities in care, prioritize development
of interoperable digital quality measures, allow for cross-comparisons across programs, and help
identify measurement gaps. We have identified adult and pediatric measures for the Universal
Foundation to be used across CMS programs and populations to the extent they are applicable.
For example, we finalized in the CY 2024 PFS final rule our proposal to consolidate the
previously finalized Promoting Wellness and Optimizing Chronic Disease Management MVPs
into a single consolidated Value in Primary Care MVP which aligns with the adult Universal
Foundation core set of quality measures (88 FR 80042 through 80047). In section IV.A.4.c.(2) of
this proposed rule we are proposing the creation of a new Alternative Payment Model (APM)
Performance Pathway (APP) Plus quality measure set that incrementally adopts all ten of the
Adult Universal Foundation measures starting in the CY 2025 performance period/2027 MIPS
payment year. In section IV.A.4.e.(1)(b)(i) of this proposed rule, we are proposing MIPS eligible
clinicians, groups, and APM Entities have the option to report such measures starting the 2025
performance period/2027 MIPS payment year. We are launching the Making Care Primary
705
CMS National Quality Strategy. (Centers for Medicare & Medicaid Services, April
2022). https://www.cms.gov/files/document/cms-national-quality-strategy-fact-sheet-april-2022.pdf.
706
The CMS National Quality Strategy: A Person-Centered Approach to Improving Quality. Centers for Medicare &
Medicaid Services, June 2022). The CMS National Quality Strategy: A Person-Centered Approach to Improving
Quality | CMS (https://www.cms.gov/blog/cms-national-quality-strategy-person-centered-approach-improving-
quality#_ftn4).
707
Quality in Motion, Acting on the CMS National Quality Strategy, April 2024.
https://www.cms.gov/files/document/quality-motion-cms-national-quality-strategy.pdf.

(MCP) Model in July 2024 to increase the number of primary care providers in value-based care
and beneficiaries in accountable care relationships in eight States.
708,709
The MCP model supports
care integration including expected strengthening of primary care clinicians’ connections with
specialists while using evidence-based behavioral health screening and evaluation to improve
patient care coordination. This will include implementation of new model-specific billing codes
that we expect to result in greater use of e-consults and expanded ongoing communication
among clinicians with a shared patient. The quality performance measures included in the MCP
model reflect our Universal Foundation work to streamline measures across programs and test
new and innovative measures. We are proposing in section II.G.2. of this proposed rule to adopt
specific coding and payment policies for advanced primary care management (APCM) services
for use by practitioners who are providing services under this specific model of “advanced
primary care”, beginning January 1, 2025. These services would be furnished under the direction
of a physician or other qualified health care professional who is responsible for all primary care
(e.g., physicians and non-physician practitioner, including nurse practitioner, physician assistant,
certified nurse midwife and clinical nurse specialist), and serve as the continuing focal point for
all needed health care services, during a calendar month. We are proposing three new APCM
codes that would recognize the resources involved in furnishing ongoing, beneficiary-centered
care management services under the broad model of advanced primary care without paying for
each activity separately while allowing for flexibility in addressing patient needs. APCM
payment would incorporate several specific, existing care management and communication
technology-based services into a bundle and include performance measurements requirements
that could be met by reporting the Value in Primary Care MVP beginning in the CY 2025
performance period/2027 MIPS payment year. Billing practitioners who are not MIPS eligible
clinicians (as defined at 42 CFR 414.1305) would not have to report the MVP in order to furnish
708
https://www.cms.gov/priorities/innovation/innovation-models/making-care-primary.
709
The CMS Innovation Center’s Strategy to Support High-quality Primary Care | CMS,
https://www.cms.gov/blog/cms-innovation-centers-strategy-support-high-quality-primary-care.

and bill for APCM services. The Value in Primary Care MVP contains the Adult Universal
Foundation quality measure set, which is consistent with the National Quality Strategy goal of
using the Universal Foundation measures across as many programs as is feasible.
710
The Shared
Savings Program, Innovation Center, and MIPS continue to collaborate strategically to develop
and elaborate on the long-term vision and implementation policies for the Quality Payment
Program.
This RFI addresses how we can achieve full MVP adoption and subgroup participation as
we move toward the sunsetting of traditional MIPS and advancing the three pillars and the
National Quality Strategy. We intend to obtain more meaningful comparable performance data
and to drive higher value care through MVPs and to provide as much transparency as possible as
we believe about the timing for sunsetting traditional MIPS (86 FR 39356). Specifically, we are
seeking feedback on clinician readiness to report MVPs, how we should ensure there are
applicable MVPs for all clinicians, including the option of creation of broadly applicable
MVP(s), and what guidance/parameters are needed for multispecialty groups to place clinicians
into subgroups for reporting an MVP relevant to the scope of care provided.
b. The Role of MVPs in Transforming MIPS
(1) Overview
We are moving towards full implementation of MVPs, which are a key component of
transforming MIPS. MVPs connect the MIPS performance categories to measure quality and
patient experience of care linked to related costs of care, encourage improvement of care,
simplify MIPS, and assure accessible health care performance data and interoperability. We are
implementing MVPs in MIPS to encourage clinicians to report on measures that are directly
relevant to their clinical practice and connect the performance categories to better measure the
value of care and support care improvements (86 FR 39351). MVPs may make it easier for MIPS
710
https://www.cms.gov/medicare/quality/cms-national-quality-strategy/aligning-quality-measures-across-cms-
universal-foundation.

eligible clinicians to select the measures and activities that are most relevant to their practice.
Rather than selecting individual measures and activities from large inventories to report under
potentially siloed MIPS performance categories under traditional MIPS, eligible clinicians who
submit an MVP will have a simplified process of selecting from a smaller, cohesive set of
measures and activities focused on the clinician’s performance in rendering care for a specialty
or clinical condition. MVPs provide a pathway to improve value, reduce burden, help patients
compare clinician performance to inform patient choice, and reduce barriers to movement into
Advanced APMs (86 FR 65391).
In the CY 2024 PFS final rule, we discussed the potential of developing policies to raise
the bar on quality and support continuous improvement for clinicians who consistently perform
well in MIPS (88 FR 79322). At this time, we are not proposing any changes related to the
continuous improvement based on feedback received. We are mindful of changes clinicians are
making to move towards MVP reporting.
MVPs support the measurement and improvement of specialty and primary care practice,
through a portfolio of MVPs that address care and clinician conditions of importance to our
patients. MVPs align the quality performance category, cost performance category, and
improvement activities performance category and a foundational layer of the Promoting
Interoperability performance category and population health measures. MVPs encourage the
measurement of performance on a cohesive set of measures and activities focused on the
clinician’s performance, and over time, improvement in performance in rendering care for
clinical conditions or patient populations. Widescale adoption of MVPs, using a standardized
connected set of measures and activities for a specialty or medical condition, may generate
important and meaningful information for patients to be able to compare performance of
clinicians on the same or similar sets of measures. As we have greater MVP adoption and
subgroup reporting we expect to obtain an expanded set of clinical specialty performance data
that will be meaningful to patients as they choose clinicians.
(2) Furthering MVP Adoption
We continue to incrementally develop and maintain MVPs that are relevant and
meaningful for all clinicians who participate in MIPS to support a full transition to MVPs. We
finalized five new MVPs in the CY 2024 PFS final rule (88 FR 79322 through 79323) and
finalized modifications to previously finalized MVPs (88 FR 79323). We refer readers to section
IV.A.4.a.(1) of this proposed rule for the discussion of six additional proposed MVPs addressing
a range of clinical conditions and specialty care. As we increase the inventory of MVPs, a greater
percentage of MIPS eligible clinicians may report MVPs. Based on our internal data the
proposed addition of six MVPs, if finalized, would allow approximately 80 percent of specialties
participating in the program to submit applicable MVPs.
In the CY 2022 PFS final rule, we finalized that voluntary reporting of MVPs would start
in the CY 2023 performance period/2025 MIPS payment year and to implement MVPs through a
gradual process that allows MVP Participants and third-party intermediaries time to adapt to
changes in policy, requirements, and programming updates that would need to occur in
technology systems (86 FR 65394 through 65396). We support a gradual movement to MVPs.
Existing flexibility allows organizations and clinicians to determine whether they are ready to
make the transition to MVP reporting, and time to familiarize themselves with MVP policies and
prepare to report MVPs that are relevant to their practice (86 FR 65396). Promisingly, based on
internal data, over 750 groups and clinicians registered to report MVPs for the CY 2023
performance period/2025 MIPS payment year. We are interested in learning from early MVP
Participants to understand lessons learned and any barriers that they encountered or overcame to
enable MVP submission. We are taking steps to encourage MVP adoption and expect additional
MIPS eligible clinicians to voluntarily begin reporting MVPs before the eventual sunset of
traditional MIPS. To engage clinicians in MVP adoption we provide MVP webinars and
outreach, and our qpp.cms.gov webpage provides information for clinicians related to reporting

MVPs, our MVP inventory and MVP development.
711
We have increased our inventory of MVPs
annually and we engage interested parties in MVP development, including a 45-day comment
period for public input on MVP candidates each year.
712
We are collaborating with the CMS Innovation Center as new payment models are
explored and developed that may incorporate specialist or clinical condition-based MVP
reporting. See section III.J. of this proposed rule for a Request For Information which seeks input
regarding the design of a future ambulatory specialty model, which may leverage MVPs as a
foundation for increasing specialty care provider engagement in value-based payment.
(3) Sunset of Traditional MIPS
We have discussed in previous PFS rules our intention to fully transition to MVPs and
sunset traditional MIPS (85 FR 50279 and 50284, 86 FR 65394 through 65396). Continuing to
maintain the traditional MIPS submission option may impair MVP adoption by clinicians who
have an available MVP. Slow adoption may delay the benefits of MVPs which will simplify
MIPS and improve comparable clinician performance data that helps to drive value and inform
clinician selection by patients. We solicited public comments in the CY 2022 PFS proposed rule
on the length of time MVP reporting should be voluntary and the timing for when we should
sunset traditional MIPS (86 FR 39356). Responding interested parties supported MVP goals and
a transparent, gradual transition to MVPs with voluntary MVP reporting, with adequate time to
prepare for reporting an MVP. Clinicians may take advantage of voluntary MVP reporting now
to gain experience. We have not proposed a target year to sunset traditional MIPS; however, it is
critical to develop a plan to sunset traditional MIPS for the awareness of all interested parties
(such as MVP Participants, third-party intermediaries, and health systems) so they may plan their
work accordingly to coincide with this timeline.
711
MIPS Value Pathways (MVPs) (cms.gov), https://qpp.cms.gov/mips/mips-value-pathways.
712
MVP Candidate Development & Submission. https://qpp.cms.gov/mips/mips-value-pathways/submit-candidate.
We are developing the timeline for the full transition to MVPs and are seeking feedback
on clinician readiness for MVP reporting and MIPS policies needed to sunset traditional MIPS in
the CY 2029 performance period/2031 MIPS payment year. This timeline would ensure MVPs
may be voluntarily reported during a period of 6 to 7 years while traditional MIPS is available,
allowing clinicians time to prepare for MVP reporting and to engage in the development of the
MVP inventory. As we plan for the full transition to MVPs, we appreciate that we must ensure
that any MIPS policies that require rulemaking to sunset traditional MIPS are proposed and
finalized, and that adequate prior notice is provided to clinicians who may need to update their
systems and work processes to report MVPs. We continue to assess remaining MVP gaps that
must be filled and to confirm participation options for MIPS eligible clinicians. We anticipate
that we may be ready to fully transition to MVPs by the CY 2029 performance period/2031
MIPS payment year.
Robust MVP availability and clinician coverage would be a precursor to sunsetting
traditional MIPS. Ideally, there would be MVPs applicable to a diverse set of specialties, patient
populations, and clinical conditions; and we are working towards this. We acknowledge that,
even with a robust inventory of MVPs, there may be some clinicians who cannot submit an
applicable MVP, as currently structured, due to lack of measures to build a respective MVP or
lack of measure case counts or specialization that does not allow reporting of MVP quality
measures and calculation of a cost measure.
While we are expanding our MVP portfolio, we are hampered by the limited number of
available quality and cost measures for certain patient populations, clinical conditions, and
specialties. We refer readers to section IV.A.4.a.(1) of this proposed rule which discusses MVP
development and measure gap areas. We recognize that resources for measure development are
limited, and measure development must be prioritized to move forward with quality and cost
measurement for the greatest impact. We are collaborating with measure developers and
providing transparency on measure gaps and the limitations around quality and cost measure
development.
(4) Potential Path Forward
We are aiming to have sufficient MVPs to meet the goals of the MVP Framework, allow
reporting by all clinicians in MIPS, sunset traditional MIPS, and transition fully to MVPs.
Additionally, in the CY 2026 performance period/2028 MIPS payment year, when subgroup
reporting becomes mandatory for multispecialty groups that participate in MIPS as MVP
Participants (§ 414.1305; 86 FR 65394 through 65397), we want to ensure there are opportunities
for specialists within the multispecialty group to submit MVPs. This will allow us to better
assess the scope of care provided by the group and provide a wider scope of clinician
performance data to patients. While we endeavor to create a robust set of MVPs allowing for
participation by all MIPS eligible clinicians as we sunset traditional MIPS in the future, we
acknowledge there are constraints, including measure availability, hindering attainment of this
goal. We refer readers to section IV.A.4.a.(1). of this proposed rule for a discussion of measure
gaps and MVP development. We continue to explore different ways to increase MVP coverage
for as many MIPS eligible clinicians as possible. The current MIPS quality and cost measure
inventories contain gaps which affect both traditional MIPS and MVPs, with MVPs requiring
fewer measures to meet requirements. Therefore, we are examining approaches to developing
MVPs to ensure that all MIPS eligible clinicians have MVPs to report.
Currently, MVPs include performance measures and activities that are broadly
applicable across specialties in addition to quality measures that are specialty/subspecialty
specific. When subspecialty specific or more focused measures are implemented, they are
considered for addition to the corresponding MVP. As stated earlier, we have made progress
with developing MVPs for the majority of specialties and priority clinical conditions. However,
we are exploring options of how MVPs can be further developed to facilitate greater reporting
rates for clinicians with fewer measures available for their specialty. We refer readers to the 2025

MVP Needs and Priorities document,
713
which identifies measurement gaps and specialties with
limited quality measures, and often limited outcomes and high priority measures, and cost
measures.
We are considering approaches that would assist in making MVPs available to all MIPS
eligible clinicians, however, all approaches to be more inclusive of clinicians are hindered by the
existing gaps in quality and cost measures. We seek comment on approaches that include
expanding finalized MVPs to include more specialties or subspecialties related to a care
condition; developing new, broader MVPs with a different emphasis from current MVPs focused
on a single specialty or clinical condition; and developing MVPs for non-patient facing MIPS
eligible clinicians.
● Expand previously finalized MVPs to include different specialties included in care
delivery for patient population. The applicability of an MVP could be expanded through
embedding measures and activities specific to additional specialties in a relevant MVP, which
may allow a broader array of clinicians to report the MVP. For example, in future program years,
we may be interested in expanding the Advancing Cancer Care MVP to include measures related
to non-patient facing MIPS eligible clinicians that would support care to cancer patients, such as
pathologists. A benefit of this approach would be the expansion of the clinician specialties who
could report a given MVP, without increasing the number of stand-alone MVPs. However, the
addition of too many measures and activities could undermine the goal of having a smaller,
cohesive set of measures and activities in MVPs.
● Expand previously finalized MVPs to include subspecialties. The applicability of an
MVP could be increased by broadening the measures and activities to include additional
subspecialties. For example, we do not anticipate the development of MVPs for each surgical
subspecialty or type of surgical procedure, but rather maintain broader surgical MVPs inclusive
713
2024 MVP Needs and Priorities. https://qpp-cm-prod-
content.s3.amazonaws.com/uploads/1803/MIPS%20Value%20Pathways%20(MVPs)%20Development%20Resource
s.zip.
of surgeries that are clinically related yet support multiple subspecialties and/or procedures
within the surgery specialty. As an example, lumbar surgical measures may be included within
MVPs for orthopedic surgeons and neurosurgeons but would not comprise a standalone MVP
solely focused on lumbar surgery. Conversely, an Improving Spine Care MVP could be
developed that encompasses multiple procedures and aspects of care that could support use by
multiple specialties, such as orthopedic surgeons and neurosurgeons. Similar to an approach that
expands MVPs to include additional specialties, this option adheres to our goal of having a
meaningful set of MVPs.
● Develop MVPs based on Multiple Specialty Measure Sets. For specialties that
currently do not have MVP coverage, we could develop an MVP applicable to multiple
conditions or specialties that currently do not have MVP coverage. This approach would serve as
a bridge until new applicable measures are developed that allow for creation of individual MVPs
for clinicians without an MVP specific to their specialty, patient populations served, or the
primary conditions treated. For example, we do not currently have an MVP for many non-patient
facing clinicians, but we could develop an MVP geared towards these multiple specialty types as
a temporary bridge until more meaningful options can be finalized. The benefit could be
allowing clinicians early experience in MVPs until we have additional specifically specific
measures to create more robust and meaningful MVPs. However, this approach may replicate
traditional MIPS and fall short of our MVP vision and goals of a cohesive set of measures and
activities; as clinicians’ performance would still be based upon the various measures and
activities chosen. Clinicians might repeatedly choose unlinked measures with high performance
levels rather than addressing performance areas in need of improvement and may also lesson the
comparability of performance measures reported by clinicians providing similar services.
● Develop MVPs based on Cross-Cutting and Broadly Applicable Measures. We may
consider building an MVP which applies to multiple specialty types by leveraging frequently
reported cross-cutting or broadly applicable measures that can be reported by MIPS eligible

clinicians who currently do not have MVPs specific to their scope of care. Cross-cutting quality
measures address important health issues with clinical quality actions that can be completed by a
multitude of clinician types, such as screening and cessation intervention for tobacco use,
screening for high blood pressure and follow-up and screening for social drivers of health.
Broadly applicable quality measures can be submitted by multiple specialties, including
Screening for Depression and Follow-up Plan; Preventive Care and Screening: Unhealthy
Alcohol Use: Screening & Brief Counseling, and Use of High-Risk Medications in Older Adults.
Population-based cost measures, such as the Total Per Capital Cost (TPCC) and Medicare
Spending per Beneficiary (MSPB) measures, could be used in such an MVP. A benefit of
developing new MVPs would be to create a bridge allowing for early experience in MVPs for
clinicians that currently have no other MVP reporting options. MVP development would need to
include examination of approaches to add additional quality and cost measures to MIPS. We
acknowledge this is a temporary step towards either a more robust and meaningful standalone
MVP or expansion of an existing MVP once specialty specific measures are developed and
finalized for use in MIPS. We also have concerns that the MVP would be duplicative in nature to
the Value in Primary Care MVP that represents concepts related to promoting wellness and
appropriate chronic disease management, which is of critical importance in driving positive
health outcomes. However, the broader, cross-cutting MVP does not appear to solve the concerns
of all specialties identified in our 2024 MVPs Needs and Priorities
714
interested in submitting
measures and activities related to their specialties. Additionally, if we developed an MVP that
relied on entirely cross-cutting and broadly applicable measures, we may need policies to
discourage clinicians from choosing this broad MVP when there is a more specifically applicable
MVP available. We may explore using claims-based data to ascertain services provided and
714
2024 MVP Needs and Priorities. https://qpp-cm-prod-
content.s3.amazonaws.com/uploads/1803/MIPS%20Value%20Pathways%20(MVPs)%20Development%20Resource
s.zip.
whether a clinical condition or specialty specific MVP better matches the type of care delivered
or if a bridge MVP submission fits, potentially within an auditing activity or tying payment to
MVP selection.
● Develop MVPs for Non-Patient Facing MIPS Eligible Clinicians. We are considering
exploring statutory flexibilities that could help address future challenges related to a lack of an
applicable MVP for some clinicians. Any flexibilities we explore must support our overall MIPS
goals. Reweighting a performance category, for example, would mean that the quality
performance category or cost performance category could be reweighted in an MVP, and this
would not support performance measurement to drive value or provide comparable information
for patient use in selecting clinicians or care teams.
We believe one such flexibility exists at section 1848(q)(2)(C)(iv) of the Act, which provides
that we must consider the circumstances of professional types that typically furnish services that
do not involve face-to-face interaction with a patient. We refer to these clinicians as non-patient
facing MIPS eligible clinicians as defined at § 414.1305. Section 1848(q)(2)(C)(iv) of the Act
further provides that, to the extent feasible and appropriate, we take into account such
circumstances and apply to such non-patient facing MIPS eligible clinicians “alternative
measures or activities that fulfill the goals of the applicable performance category.” In doing so,
section 1848(q)(2)(C)(iv)(II) of the Act requires that we consult with non-patient facing MIPS
eligible clinicians of such professional types or subcategories. We note that measure gaps for
some non-patient facing MIPS eligible clinicians, for example diagnostic radiologists and
pathologists, present challenges in developing a respective MVP. We refer readers to section
IV.A.4.a.(1) of this proposed rule for discussion of MVP portfolio development and specialties
with measure gaps. We are interested in exploring alternative measures and activities that would
allow us to measure the performance of non-patient facing MIPS eligible clinicians.
As we explore various approaches to enable all MIPS eligible clinicians to report MVPs,
we seek comment on options, such as expanding previously finalized MVPs, developing more

global MVPs with broadly applicable measures as an interim bridge for those clinicians with too
few specialty-specific quality measures and the potential use of statutory flexibilities, as we
move to full MVP adoption and sunsetting traditional MIPS.
(5) Consideration of MVP Policies and Requirements
A component of sunsetting traditional MIPS is examining requirements and policy that
may require revision to ensure a smooth transition into MVP reporting. For example, we intend
to assess our current policy which does not allow virtual groups and opt-in
715
reporters to submit
an MVP (§ 414.1305). We want to understand additional barriers preventing clinicians from
reporting MVPs, and when possible, examine MVP policies that might need to be revised to
remove the barriers. We may undertake these policy proposals in future rulemaking. We are
interested in feedback on potential changes required as part of an effort to accommodate
clinicians with few applicable and available quality and cost measures, who currently do not
have an MVP available.
We have established a policy which states that, starting in the CY 2026 performance
period/2028 MIPS payment year, multispecialty groups must form subgroups to report MVPs
(86 FR 65394 through 65397). This policy will enable a subset of clinicians in a group to submit
measures and activities in an MVP relevant to the scope of care provided by the subgroup and
will lead to a more comprehensive evaluation of services provided by groups. As described in
section IV.A.3.d. of this proposed rule, we are interested in feedback on how to balance the MVP
requirement for multispecialty groups to form multiple subgroups with the ability of the program
to evaluate specialty care and for patients to have meaningful information to use when making
care decisions.
c. Mandatory Subgroup Reporting Requirement
(1) Overview
715
Entities that exceed one or two, but not all three elements of the low-volume threshold defined at 42 CFR
414.1305 can opt-in to participate in MIPS (§414.1310(b)(1)(iii) and (b)(2)).
We have established a voluntary subgroup participation option for clinicians choosing to
report an MVP beginning in the CY 2023 performance period/2025 MIPS payment year. We
have also finalized a mandatory subgroup reporting requirement for multispecialty groups
choosing to report as an MVP Participant beginning in the CY 2026 performance period/2028
MIPS payment year (§ 414.1305; 86 FR 65394 through 65397). Beginning with the CY 2026
performance period/2028 MIPS payment year, a single specialty group may continue to submit
data for an MVP at the group level, and a multispecialty group must form subgroups to report an
MVP. Under the existing subgroup reporting policies, a group could place clinicians providing
similar scope of care into one or more subgroups for reporting a relevant MVP. The remaining
clinicians under the group TIN not part of a subgroup could participate as individuals for
reporting an MVP or traditional MIPS. The entire group TIN (including the clinicians that are
part of the subgroup) could also submit data as a group in traditional MIPS.
Subgroup participation for MVP reporting will allow group practices to comprehensively
capture the diverse range of services provided by the clinicians under a group TIN. We anticipate
subgroup reporting will also provide clinician information to patients related to a specific
condition or care needs. Subgroup reporting will provide specialist information to primary care
clinicians allowing relevant patient referrals. We finalized the implementation of the subgroup
reporting option to address public comments received that the existing group reporting
requirements in MIPS allow a group to report on broadly applicable quality measures that are not
representative of the scope of care provided and do not allow reporting of quality measures by
only a portion of the clinicians under the group TIN.
(2) Specialty Composition of a Group
We have previously established definitions for a single specialty group and a
multispecialty group at § 414.1305 (87 FR 70038 through 70040). Specifically, we stated that a
single specialty group means a group as defined at § 414.1305 that consists of one specialty type
as determined by CMS using Medicare Part B claims. A multispecialty group means a group as
defined at § 414.1305 that consists of two or more specialty types as determined by CMS using
Medicare Part B claims. MIPS eligible clinicians can check their eligibility status (individual,
group, virtual group, APM Entity, opt-in) on the QPP Participation Status Tool
(https://qpp.cms.gov/participation-lookup) to determine their MIPS participation options and
whether they are subject to MIPS payment adjustment for the applicable performance period.
Currently, the QPP Participation Status Tool does not provide information on group specialty
designations informing a group whether CMS considers its TIN is composed of a single or
multiple specialties. In the CY 2023 PFS final rule (87 FR 70039), we discussed that CMS
utilizes different data sources to identify a clinician’s specialty. For purposes of public reporting,
we rely on PECOS as the primary data source, and for purposes of MIPS eligibility
determination, we use both PECOS and claims data. Additionally, we use the information on
Medicare Part B claims to identify clinician specialty when attributing some of the measures in
the cost and quality performance categories. Based on CMS analysis of clinician specialty
identification using Medicare Part B claims data and PECOS data, we found a variance rate of
less than one percent between the two data sources and finalized the use of Medicare Part B
claims data as the best data source to use to determine a group’s specialty type or types for
purposes of participation in MVPs. We refer readers to the CY 2023 PFS final rule (87 FR 70038
through 70040) for additional details on using the Medicare Part B Claims data to determine a
group’s specialty composition.
We also recognize there are additional nuances to consider for using the Medicare Part B
claims data to appropriately implement the current definitions of a single specialty and
multispecialty group. We acknowledge a multidisciplinary group practice could consist of
clinicians across multiple specialties to provide team-based care in just one clinical area. For
example, a group consisting of internists, geriatricians, family medicine practitioners, physician
assistants, and nurse practitioners could be focused on providing primary care services to
patients, which could be reflected as multiple specialties in Medicare Part B claims. Beginning in
the CY 2026 performance period/2028 MIPS payment year, such a group TIN will be considered
a multispecialty group using the definition described under § 414.1305. The internists,
geriatricians, and family medicine practitioners could inappropriately be identified as unique
specialties resulting in the TIN having clinicians with more than one primary specialty
designation. Based on our previously established definition of a multispecialty group, this TIN
will be required to form subgroups to participate in MVPs even though all the clinicians under
the group TIN provide primary care services. This may result in multiple subgroups under the
same group TIN submitting data for the same MVP, which would yield redundant quality
measure reporting. Based on the scope of care provided, all the clinicians under the TIN could
choose to report the measures and activities in the Value in Primary Care MVP. Additional
scenarios that we would like to consider include instances when a primary specialty designation
of certain clinician types does not accurately reflect the scope of care provided. The physician
assistants and nurse practitioners that are part of a cardiology group would potentially be
involved in care relevant to stroke, heart failure, and other cardiac related conditions. The
physician assistants and nurse practitioners that are part of a large group practice could be
involved in a wide array of services. In both these instances, the primary specialty designation of
a physician assistant or nurse practitioner would only be representative of their education
credentials, and not the scope of care provided.
One approach we could consider is providing flexibility for a group practice to determine
and inform CMS of their specialty composition. We anticipate that allowing a group TIN to
indicate if they are a single specialty or multispecialty group could mitigate a few of the above
concerns. We use the above example of a group consisting of internists, geriatricians, family
medicine practitioners, physician assistants, and nurse practitioners focused on providing
primary care services to patients. By allowing a group TIN to identify the group’s specialty
composition, the group TIN could identify as a single specialty group and report the measures
and activities in the Promoting Wellness MVP as a group. Providing the ability for groups to
determine their specialty composition would provide the opportunity for groups to determine the
MVP participation option that is consistent with the scope of care. We anticipate it would
eliminate the need for duplicative reporting, support the team-based care approach, and allow
groups to comprehensively categorize the care provided by the clinicians in a group.
(3) Establishing Limits on the Composition of a Subgroup
We envision a future state in the Quality Payment Program where specific and highly
meaningful clinician information is available for patients to determine their care choices while
allowing clinicians to receive targeted feedback relevant to the scope of care provided. We
anticipate a future state where a multispecialty group would only participate as subgroups for
reporting MVPs. We have stated that one of the long-term goals of MVP and subgroup reporting
is to encourage team-based care and therefore, have not established any restrictions on subgroup
formation. Specifically, we do not expect a subgroup to include only clinicians from a single
specialty as it might be construed as discouraging team-based care. We are also concerned that
the absence of limitations on subgroup formation would result in a subgroup mimicking a
multispecialty group in traditional MIPS. A subgroup of 100 clinicians under a group TIN could
report on an MVP where the quality measures and activities in the MVP are applicable only to a
subset of clinicians in the subgroup.
We are considering placing some limits on the composition of a subgroup to make
subgroups meaningful and meet the end goals of MVP reporting. We also believe that a
multispecialty group TIN could use the subgroup formation criteria as a guidance for placing
clinicians into subgroups relevant to the scope of care provided. We recognize that any potential
subgroup composition criteria should not interfere with the team-based approach for care
provided by clinicians in multispecialty groups. We are considering whether we could establish
limits on the number of clinicians per subgroup based on the size of the overall group TIN. For a
multispecialty group TIN with less than 100 clinicians, should we consider limiting the
maximum size of a subgroup to 50 clinicians? Similarly for a multispecialty group TIN with
100+ clinicians, should we consider placing limits on the minimum size of a subgroup? We are
interested in approaches that would encourage multispecialty groups to report more than one
MVP.
We are also considering whether we could utilize the information on Medicare claims
data to potentially create subgroup composition restrictions. For example, we could identify the
care provided by the clinicians in a group based on the specific procedures or services performed
by the clinician. We are considering analyzing subgroups based on the volume of services billed
by the clinicians in a group practice for a specific medical condition (for example, heart failure,
joint replacement, etc.) or a specific procedure (beta blocker therapy, stent placement, hip
and/knee surgery, etc.). We anticipate this approach would serve as a guidance for clinicians
such as family practitioners, physician assistants, internists, etc. who provide a diverse range of
services across specialty types.
(4) Mandatory Subgroup Reporting for Small Practice TINs
At § 414.1305, we define a small practice to mean a TIN consisting of 15 or fewer
eligible clinicians during the MIPS determination period. For a group of 15 or fewer clinicians,
participation at the group or subgroup level for MVP reporting would provide similar
performance information to clinicians and patients. Therefore, we do not anticipate additional
benefits for mandating clinicians in a small practice TIN to split into multiple subgroups for
reporting an MVP. One approach we could consider is making an exception for multispecialty
small practice TINs from the mandatory subgroup reporting requirement for multispecialty
practices beginning in the CY 2026 performance period/2028 MIPS payment year. This would
alleviate the additional burden for small practice TINs to form subgroups and continue to
participate as a group for reporting an MVP. A multispecialty small practice could voluntarily
participate as subgroups for comprehensively capturing the diverse range of services provided by
the group.
d. RFI Questions
We request public comment on specifically the following questions:
● For those clinicians who submitted an MVP for the CY 2023 performance period/2025
MIPS payment year, what practice level barriers did you overcome to successfully submit an
MVP? How did you overcome any stated barriers? For those who did not submit an MVP, what
key barriers impacted your decision to continue to report traditional MIPS?
For those clinicians who participated as a subgroup for reporting an MVP for the CY
2023 performance period/2025 MIPS payment year, we are interested to hear the technological
barriers, if any, that impacted the ability to successfully submit subgroup level data. We are also
interested to hear feedback from groups on any technical issues with de-aggregating data
(specifically, the eCQM quality measure data) at the subgroup level.
● What does meaningful MIPS participation look like for clinicians who in the future
with the sunset of traditional MIPS may not have an applicable MVP, e.g., clinician types
without an MVP due to having less than four applicable quality performance measures and less
than one cost measure identified in the 2025 MVP Needs and Priorities. Should CMS consider
developing a more global MVP with broadly applicable measures as an interim bridge for those
clinicians with too few specialty-specific quality measures, knowing that the measures may not
be as highly relevant to the clinicians’ scope of care as we would like? Should flexibilities or
alternative policies such as non-patient facing clinician policy changes be considered for
clinicians with limited performance measures that allow them to participate in MIPS?
● As subgroup participation becomes mandatory for multispecialty groups reporting an
MVP beginning in CY 2026, how can we balance the increase in burden for groups while
allowing comprehensive reporting on the diverse range of services provided by the clinicians in a
group? For example, should we consider limiting the number of subgroups that a group must
form based on group size and composition?
Are there alternative approaches we could consider for setting limits on the minimum
and maximum number of subgroups per group TIN?
● Could we consider establishing a process during MVP registration for groups to self-
identify if the group is considered a single specialty or multispecialty group? Are there any
barriers that CMS should be aware of if we established a process for a group practice to identify
the overall specialty composition of a group TIN?
● Is it meaningful for a small practice to form subgroups? What additional performance
information would it provide to patients and clinicians?
● Are there additional approaches we should consider for providing guidance to groups
on appropriately placing clinicians into subgroups based on the scope of care provided?
Please note, this is an RFI only.
In accordance with the implementing regulations of the Paperwork Reduction Act of
1995 (PRA), specifically 5 CFR 1320.3(h)(4), this general solicitation is exempt from the PRA.
Facts or opinions submitted in response to general solicitations of comments from the public,
published in the Federal Register or other publications, regardless of the form or format thereof,
provided that no person is required to supply specific information pertaining to the commenter,
other than that necessary for self-identification, as a condition of the agency's full consideration,
are not generally considered information collections and therefore not subject to the PRA.
Respondents are encouraged to provide complete but concise responses. This RFI is
issued solely for information and planning purposes; it does not constitute a Request for Proposal
(RFP), applications, proposal abstracts, or quotations. This RFI does not commit the U.S.
Government to contract for any supplies or services or make a grant award. Further, CMS is not
seeking proposals through this RFI and will not accept unsolicited proposals. Responders are
advised that the U.S. Government will not pay for any information or administrative costs
incurred in response to this RFI; all costs associated with responding to this RFI will be solely at
the interested party’s expense. Not responding to this RFI does not preclude participation in any
future procurement, if conducted. It is the responsibility of the potential responders to monitor
this RFI announcement for additional information pertaining to this request. Please note that
CMS will not respond to questions about the policy issues raised in this RFI. CMS may or may
not choose to contact individual responders. Such communications would only serve to further
clarify written responses. Contractor support personnel may be used to review RFI responses.
Responses to this notice are not offers and cannot be accepted by the U.S. Government to form a
binding contract or issue a grant. Information obtained as a result of this RFI may be used by the
U.S. Government for program planning on a non-attribution basis. Respondents should not
include any information that might be considered proprietary or confidential. This RFI should
not be construed as a commitment or authorization to incur cost for which reimbursement would
be required or sought. All submissions become U.S. Government property and will not be
returned. CMS may publicly post the comments received, or a summary thereof.
a. CY 2025 MVP Development and Maintenance
(1) Development of New MIPS Value Pathways (MVPs)
In the CY 2023 PFS final rule (87 FR 70035 through 70037), we finalized modifications
to the MVP development process to broaden opportunities for the general public to provide
feedback on new candidate MVPs prior to the notice and comment rulemaking process. We refer
readers to the Quality Payment Program website to review the public feedback we received for
each 2025 MVP candidate (https://qpp.cms.gov/mips/candidate-feedback).
Through our development processes for new MVPs (85 FR 84849 through 84856, 87 FR
70035 through 70037), we aim to gradually develop new MVPs that are relevant and meaningful
for all MIPS eligible clinicians. In this proposed rule, we are proposing the inclusion of six new
MVPs titled:
● Complete Ophthalmologic Care;
● Dermatological Care;
● Gastroenterology Care;
● Optimal Care for Patients with Urologic Conditions;
● Pulmonology Care; and
● Surgical Care.
We refer readers to Appendix 3 in this proposed rule for a detailed description of each proposed
new MVP. With the proposed addition of the six new MVPs, we estimate approximately 80
percent of specialties will have applicable MVPs available for reporting.
Although our intended goal has been to offer MVPs for all specialties and subspecialties
during the transition from traditional MIPS to full MVP implementation (84 FR 40732 through
40740), we acknowledge our existing portfolio of quality and cost measures may not be
applicable to all specialties and/or subspecialties. For quality measures, while most specialties
and subspecialties can report on broadly applicable quality measures to meet the reporting
requirements for the quality performance category within an MVP, some
specialties/subspecialties do not have sufficient robust quality measures that are specific to their
scope of care. Thus, we continue to explore options for overcoming challenges to develop MVPs
for those specialties/subspecialties with limited quality measures.
For cost measures, while most specialties have at least one applicable episode-based cost
measure and/or population-based cost measure, these measures may not encompass the full array
of care that could be covered by a given specialty and, in some instances, some
specialties/subspecialties may not have an applicable cost measure. For example, the following
specialties have limited cost measures available and applicable based on the current MIPS cost
measure inventory:
● Diagnostic Radiology;
● Interventional Radiology;
● Optometry;
● Pathology;
● Radiation Oncology; and
● Speech Language Pathology.
Additionally, some specialties have one or more applicable cost measures, but
subspecialists may not be captured under these measures. In the case of the Melanoma Resection
measure, it applies to individual MIPS eligible clinicians, groups, and subgroups that perform a
sufficient number of melanoma excision procedures to meet the measure’s case minimum.
Although this measure is applicable to many dermatologists, whether a dermatologist is scored
on this measure depends on multiple factors, including whether they submit claims on, and are
attributed a sufficient number of qualifying melanoma excision procedures (minimum of 10
cases as specified under § 414.1350(c)(4)) to receive a score on this cost measure as set forth in §
414.1380(b)(2). While there are existing policies to reweight the cost performance category for
individual, groups, and subgroups of MIPS eligible clinicians that cannot be scored on cost
measures in accordance with § 414.1380(b)(2), an MVP cannot be developed for a
specialty/subspecialty if there is not at least one applicable cost measure, as finalized in the CY
2021 PFS final rule (85 FR 84472). The intent of MVPs is to assess MIPS eligible clinicians,
groups, and subgroups across all performance categories, and additional cost measures would
support this intent.
We use prioritization criteria from the CY 2022 PFS final rule (86 FR 65456) to
determine which cost measures to develop:
● Clinical coherence of measure concept (to ensure valid comparisons across clinicians).
● Impact and importance to MIPS (including cost coverage, clinician coverage, and
patient coverage).
● Opportunity for performance improvement.
● Alignment with quality measures and improvement activities to ensure meaningful
assessments of value.
The CY 2022 PFS final rule (86 FR 65457) also established the following standards for
cost measure construction:
● Measures must assign services that accurately capture the role of attributed clinicians.
● Measures must have clear, ex ante attribution to clinicians.
● Measures must be based on episode definitions that have clinical face validity and are
consistent with practice standards.
● Measures' construction methodology must be readily understandable to clinicians.
● Measures must hold clinicians accountable for only the costs they can reasonably
influence.
● Measures must convey clear information on how clinicians can alter their practice to
improve measured performance.
● Measures must demonstrate variation to help distinguish quality of care across
individual clinicians.
● Measure specifications must allow for consistent calculation and reproducibility using
Medicare claims data.
As of the CY 2024 performance period/2026 MIPS payment year, we have developed
and implemented 29 MIPS cost measures, which reflect the prioritization criteria and input from
interested parties about potential clinical topics, measure scope, clinically related services, and
potential challenges or barriers to measurement. This is a substantial achievement in building out
the cost measure portfolio since MIPS began with only two population-based cost measures, the
Total Per Capita Cost (TPCC) measure and the Medicare Spending Per Beneficiary (MSPB)
measure.
However, there are still MIPS eligible clinicians that do not have cost measures that
apply to the major aspects of their care practice. For example, there are specialties or clinical
topics where clinically coherent measure concepts have not yet been identified or where impact
in terms of cost, clinician, or patient coverage is lower than that of other measure concepts that
were prioritized for development. Therefore, we continue to encourage interested parties to
utilize our established pre-rulemaking processes, such as the Call for Measures
(https://mmshub.cms.gov/measure-lifecycle/measure-implementation/pre-rulemaking/overview),
to develop and submit candidate quality and cost measures relevant to their specialty.
Furthermore, we continue to develop MVPs based on needs and priorities, as described in the
MVP Needs and Priorities document (https://qpp-cm-prod-
content.s3.amazonaws.com/uploads/1803/MIPS%20Value%20Pathways%20(MVPs)%20Develo
pment%20Resources.zip). In section IV.A.3. of this proposed rule, we discuss our request for
information on Transforming the Quality Payment Program and solicit feedback on challenges to
adopting MVPs and a potential path forward for developing MVPs for MIPS eligible clinicians
with limited measures.
(2) MVP Maintenance Process
In the CY 2023 PFS final rule (87 FR 70037), we finalized a modification to the annual
maintenance process for MVPs previously finalized in the CY 2022 PFS final rule (86 FR
65410). We communicated that if we identified any potentially feasible and appropriate
submitted maintenance recommendations, we would host a public facing webinar open to
interested parties and the general public through which they could offer their feedback on the
potential maintenance updates we have identified.
In this proposed rule, because we have had a low volume of submitted maintenance
recommendations in past years, we are proposing to modify the MVP maintenance webinar
process to provide us more flexibility in how we communicate submitted maintenance
recommendations prior to proposing them formally in rulemaking. Allowing flexibility in
communicating recommendations through alternative webinar formats or other public
communication channels would offer similar opportunities for public review and feedback as a
live public webinar. For example, in lieu of a live webinar, we could choose to communicate
submitted maintenance recommendations via a pre-recorded webinar, which will encourage
interested parties to submit their feedback on the submitted recommendations in writing by email
before maintenance updates are formally proposed in rulemaking. It is important to reiterate this
public webinar process supports our commitment to consider interested parties’ feedback when
determining which maintenance updates are appropriate for inclusion in formal notice and
comment rulemaking. We request comment on this proposal.
(3) MVP Maintenance Updates to Previously Finalized MVPs
Between the CY 2022 PFS final rule (86 FR 65998 through 66031) and the CY 2023 PFS
final rule (87 FR 70037), we finalized 12 MVPs available for reporting beginning with the CY
2023 performance period/2025 MIPS payment year:
● Adopting Best Practices and Promoting Patient Safety within Emergency Medicine;
● Advancing Cancer Care;
● Advancing Care for Heart Disease;
● Advancing Rheumatology Patient Care;
● Coordinating Stroke Care to Promote Prevention and Cultivate Positive Outcomes;
● Improving Care for Lower Extremity Joint Repair;
● Optimizing Chronic Disease Management;
● Optimal Care for Kidney Health;
● Optimal Care for Neurological Conditions;
● Patient Safety and Support of Positive Experiences with Anesthesia;
● Promoting Wellness; and
● Supportive Care for Cognitive-Based Neurological Conditions.
In the CY 2024 PFS final rule (88 FR 79978 through 80047), we consolidated Promoting
Wellness and Optimizing Chronic Disease Management MVPs into a single primary care MVP
titled “Value in Primary Care MVP” as well as finalized five additional MVPs available for
reporting beginning with the CY 2024 performance period/2026 MIPS payment year:
● Focusing on Women’s Health;
● Prevention and Treatment of Infectious Disorders Including Hepatitis C and Human
Immunodeficiency Virus (HIV);
● Quality Care for the Treatment of Ear, Nose, and Throat Disorders;
● Quality Care in Mental Health and Substance Use Disorder; and
● Rehabilitative Support for Musculoskeletal Care.
In this proposed rule, we are proposing modifications to all 16 MVPs with the addition
and removal of measures and improvement activities based on the MVP development criteria (85
FR 84849 through 84854). Through these modifications, we can expand upon the clinical
concepts, advance health equity, address maintenance requests from the public, and remove
measures and activities that would either be finalized for removal from their respective MIPS
Inventory or replaced by more robust measures. In addition, through the MVP maintenance
process, we are proposing to consolidate the previously finalized Optimal Care for Patients with
Episodic Neurological Conditions MVP and the Supportive Care for
Neurodegenerative Conditions MVP into a single consolidated neurological MVP titled Quality
Care for Patients with Neurological Conditions MVP. We refer readers to Appendix 3 of this
proposed rule for the proposed modifications and detailed descriptions to the previously finalized
MVPs.
b. MVP Requirements and Scoring
In the CY 2022 PFS final rule (86 FR 65411 through 65415), we finalized policies for
MVP reporting requirements, including subgroup requirements, which took effect beginning in
the CY 2023 performance period/2025 MIPS payment year, at § 414.1365(c)(1) through (4). We
noted that MVP reporting requirements are based on the reporting requirements of traditional
MIPS but have some differences, such as reporting fewer measures, to reduce MVP reporting
burden and allow for measurement that is more meaningful by requiring clinicians to report on
measures and activities that comprehensively reflect an episode of care or clinical condition (86
FR 65411).
In the CY 2022 PFS final rule, we finalized policies for MVP scoring that took effect
beginning in the CY 2023 performance period/2025 MIPS payment year. We refer readers to 86
FR 65419 through 65427 for the details of those finalized policies. We previously finalized at
§ 414.1365(d)(2) that, unless otherwise indicated in § 414.1365(d), the performance standards
described at § 414.1380(a)(1)(i) through (iv) apply to the measures and activities included in the
MVP (86 FR 65419 through 65421). We noted that in general, we have adopted the scoring
policies from traditional MIPS for MVP Participants unless there is a compelling reason to adopt
a different policy to further the goals of the MVP framework (86 FR 65419). In this proposed
rule, we are proposing to update the registration process and scoring policies for population
health measures in the quality performance category, clarify the alignment between scoring cost
measures in MVPs and traditional MIPS, update requirements and scoring policies for
improvement activities in the improvement activities performance category, and update the
requirements for subgroup reporting in the Promoting Interoperability performance category.
We refer readers to section IV.A.4.d. of this proposed rule for proposed policies on data
submission requirements; IV.A.4.e.(1)(c)(i) of this proposed rule for proposed policies on the
data completeness threshold, IV.A.4.f.(1)(b) of this proposed rule for proposed policies on
scoring of topped out measures, and scoring virtual groups and APM Entities (including SSP
ACOs) in the quality performance category; IV.A.4.f.(1)(d)(ii)(B) of this proposed rule for
proposed benchmarking policies for scoring the cost performance category; IV.A.4.e.(3)(b)(iv)
of this proposed rule for proposed policies for requirements and scoring that remove medium-
and high-weighting from improvement activities in the improvement activities performance
category; and IV.A.4.e.(4) of this proposed rule for current requirements and a Request for
Information (RFI) for the Promoting Interoperability performance category.
(1) Quality Performance Category in MVPs
(a) Background on Population Health Administrative Claims-Based Measures
In the CY 2021 PFS final rule, we discussed the inclusion of population health measures
as a part of the foundational layer of MVPs, to improve patient outcomes, reduce reporting
burden and costs, and better align with clinician quality improvement efforts (85 FR 84856 and
84857). In the CY 2022 PFS final rule we defined a population health measure as a quality
measure that indicates the quality of a population or cohort's overall health and well-being, such
as, access to care, clinical outcomes, coordination of care and community services, health
behaviors, preventive care and screening, health equity, or utilization of health services (86 FR
65408). We also discussed in the CY 2022 PFS final rule the importance of currently adopted
population health measures, noting that they capture outcomes important to patients and thus
provide meaningful information to clinicians so they can improve their practice, and discussed
the use of population health measures as the foundational layer in MVPs to ensure that important
areas of measurement are reflected within all MVPs (86 FR 65408).
We finalized in the CY 2022 PFS final rule (86 FR 65414) at § 414.1365(c)(4)(ii) that an
MVP Participant is scored on one population health measure in accordance with
§ 414.1365(d)(1). Since the MVP population health measures are administrative claims-based,
they do not require data submission from clinicians and do not contribute to reporting burden. To
track which population health measure an MVP Participant intends to report, we finalized in the
CY 2022 PFS final rule (86 FR 65417) at § 414.1365(b)(2)(i) that MVP Participants are required
to select one population health measure at the time of MVP registration.
(b) Proposal to Use the Highest Score of All Available Population Health Measures
In the CY 2022 PFS final rule (86 FR 65421 and 65422) we finalized scoring rules for
population health measures in MVPs. We finalized at § 414.1365(d)(3)(i)(A) that, except as
provided in paragraph (d)(3)(i)(A)(1), each selected population health measure that does not
have a benchmark or meet the case minimum requirement is excluded from the MVP
Participant’s total measure achievement points and total available measure achievement points.
In cases where an MVP Participant selects a population health measure that cannot be scored
because it does not have a benchmark or meet the case minimum requirement, we do not score
any other population health measures that may be applicable and available.
Population health measures are included in the MVP foundational layer because they
capture outcomes important to patients (that is, hospitalizations for acute illness) and thus
provide meaningful information to clinicians so they can improve their practice (86 FR 65408).
Under the current policy, we cannot score an MVP Participant on a population health measure if
the MVP Participant selects a measure at registration that lacks a benchmark or if their case
volume does not meet the case minimum requirement for the selected measure, even if another
measure is applicable and available. In the CY 2022 PFS final rule (86 FR 65414) we discussed
calculating each population health measure and applying the higher score to the quality score,
however, we ultimately proposed and finalized the current policy to score only one selected
population health measure to mitigate concerns from interested parties that not all population
health measures are applicable to all specialties (86 FR 65414). We now realize that at the time
of registration, an MVP Participant will not be able to determine if they will have enough cases
to meet the case minimum required for scoring the selected population health measure and will
not know, in advance, how the measure will score compared to a benchmark. Requiring an MVP
participant to select the population health measure to be scored at the time of registration may
penalize an MVP Participant who selects a population health measure at MVP registration.
To increase the likelihood that a population health measure can be scored, we had
considered several options, including calculating the population health measure score by using
an average score of all population health measures that have a benchmark and meet the case
minimum requirement and using the score of the population health measure with the highest
number of cases in order to score the population health measure that represents the most care
provided by an MVP Participant. However, we determined these approaches could result in a
lower score for an MVP Participant that did not correlate to the MVP Participant’s performance.
We also considered whether an MVP Participant could select a population health measure at the
time of data submission when all other measures are reported. However, population health
measures are calculated by CMS using administrative claims-based data and therefore do not
require data submission from clinicians, and administrative claims-based data is not available for
CMS calculation until at least 60 days after the end of the reporting period. Therefore, the MVP
Participant would not know whether they would meet the case minimum requirement for the
selected population health measure at the time of data submission.
Because population health measures in the MVP capture outcomes important to patients
(that is, for example, hospitalizations for acute illness) and thus, provide meaningful information
to clinicians so they can improve their practice, we want to avoid scenarios where MVP
Participants may inadvertently select a measure that cannot be scored. As described for
traditional MIPS at § 414.1380(b)(1)(i), CMS calculates all administrative claims-based quality
measures and scores the clinician on each measure for which there is a benchmark and the
clinician meets the case minimum requirement. Calculating all population health measures in
MVPs would more closely align with the policy to calculate all administrative claims-based
quality measures. Additionally, we have developed MVPs with a smaller, more cohesive set of
measures and streamlined reporting requirements. A policy to take the highest population health
score would increase the likelihood that an MVP Participant is scored on a population health
measure and would ensure that MVP Participants receive the highest possible population health
score that correlates to their performance.
We are proposing to revise § 414.1365(d)(3)(i)(A) to state that for the CY 2023 through
2024 performance periods/2025 through 2026 MIPS payment years, MVP Participants will be
scored on the selected population health measure and beginning in the CY 2025 performance
period/2027 MIPS payment year, we would use the highest score of all available population
health measures. If no population health measure has a benchmark or meets the case minimum
requirement, then the population health measure is excluded from the MVP Participant’s total
measure achievement points and total available measure achievement points. To apply this
policy to subgroups reporting an MVP, we also propose to update § 414.1365(d)(3)(i)(A)(1) to
provide that for the CY 2023 through 2024 performance periods/2025 through 2026 MIPS
payment years, subgroups will be scored on the selected population health measure based on its
affiliated group score, if available, and beginning in the CY 2025 performance period/2027
MIPS payment year, a subgroup is scored on the highest scoring of all available population
health measures based on its affiliated group score, if available. If the subgroup's affiliated group
score is not available, each such measure is excluded from the subgroup's total measure
achievement points and total available measure achievement points.
We are also proposing to remove the requirement for an MVP Participant to select a
population health measure at the time of MVP registration. If we implement our proposal to
calculate each population health measure for an MVP Participant and use the participant’s
highest score for population health measures in MVPs, there would be no need for the MVP
Participant to select a measure during registration. We propose to revise § 414.1365(b)(2)(i) to
provide that for the CY 2024 performance period/2026 MIPS payment year, each MVP
Participant must select an MVP, one population health measure included in the MVP, and any
outcomes-based administrative claims-based measure on which the MVP Participant intends to
be scored. Beginning in the CY 2025 performance period/2027 MIPS payment year, each MVP
Participant must select an MVP and any outcomes-based administrative claims-based measure
on which the MVP Participant intends to be scored. We seek comment on these proposals.
(2) Cost Performance Category in MVPs
In the CY 2022 PFS final rule, we finalized at § 414.1365(d)(3)(ii) to use the
methodology established at § 414.1380(b)(2)(i) through (v) to score the cost performance
category for MVPs using the cost measures included in the MVP that MVP Participants select
and report. The finalized policies at § 414.1380(b)(2) score cost measures based on achievement
and improvement when the case minimum specified under § 414.1350(c) is met or exceeded and
CMS has determined a benchmark (86 FR 65422 and 65423). We discussed in the CY 2022 PFS
final rule that aligning MVP scoring policies with existing MIPS scoring policies balances the
statutory requirements and goals of the program with ease of use, stability, and meaningfulness
to MIPS eligible clinicians (86 FR 65419). We refer readers to section IV.A.4.f.(1)(d)(ii)(B) of
this proposed rule for proposals to modify the cost performance category’s scoring methodology.
To ensure alignment between MVP and MIPS scoring policies, it is important that MVP
cost performance category scoring policies refer to the MIPS policy on how cost measures are
scored. We remind readers that cost measures are scored based on the clinician's performance on
the measure during the performance period compared to the measure's benchmark, as stated at §
414.1380(b)(2). Currently, § 414.1365(d)(3)(ii) provides that the cost performance category
score is calculated for an MVP Participant using the methodology at § 414.1380(b)(2)(i) through
(v) and the cost measures included in the MVP that they select and report. To ensure continued
alignment, we propose to modify § 414.1365(d)(3)(ii) to replace the reference to §
414.1380(b)(2)(i) through (v) with a broader reference to the cost performance category scoring
policies at § 414.1380(b)(2).
We also propose to similarly revise § 414.1365(d)(3)(ii)(A). This regulation currently
provides that a subgroup is scored on each cost measure included in the MVP that it selects and
reports based on its affiliated group score for each such measure, if available. In addition, §
414.1365(d)(3)(ii)(A) provides that, if the subgroup's affiliated group score is not available for a
measure, the measure is excluded from the subgroup's total measure achievement points and total
available measure achievement points, as described under § 414.1380(b)(2)(i) through (v). We
propose to modify § 414.1365(d)(3)(ii)(A) to replace the reference to § 414.1380(b)(2)(i)
through (v) with a broader reference to the cost performance category scoring policies at §
414.1380(b)(2).
We seek comment on these proposals.
(3) Improvement Activities Performance Category in MVPs
The improvement activities performance category should provide clinicians with an
opportunity to select from a subset of improvement activities within an MVP that are relevant to
the clinical topic. In the CY 2022 final PFS rule (86 FR 65412 and 64513) we finalized at
§ 414.1365(c)(3), that an MVP Participant who reports an MVP must report one of the
following: two medium-weighted improvement activities; one high-weighted improvement
activity; or participation in a certified or recognized patient-centered medical home (PCMH) or
comparable specialty practice as described at § 414.1380(b)(3)(ii). We established that MVP
Participants submitting MVPs would report fewer improvement activities than eligible clinicians
reporting traditional MIPS to support MVP adoption.
Additionally, in the CY 2022 final PFS rule (86 FR 65423 and 65424) we finalized at
§ 414.1365(d)(3)(iii) that the improvement activities performance category score for MVP
Participants is calculated based on the submission of high- and medium-weighted improvement
activities. We finalized that MVP Participants would receive 20 points for each medium-
weighted improvement activity and 40 points for each high-weighted improvement activity
required under § 414.1360 on which data is submitted in accordance with § 414.1325 or for
participation in a certified or recognized patient-centered medical home (PCMH) or comparable
specialty practice, as described at § 414.1380(b)(3)(ii). Therefore, to receive a score of 40 points,
or full credit, an MVP Participant would be required to submit one high-weighted improvement
activity, or two medium-weighted improvement activities included in the MVP. We stated that
these requirements would provide an incentive for reporting MVPs, since fewer improvement
activities are required to receive a full score for the improvement activities category in an MVP
compared to traditional MIPS (86 FR 65423).
We refer readers to section IV.A.4.e.(3)(b)(iv) of this proposed rule for proposals to
remove the medium- and high-weighting for improvement activities in traditional MIPS starting
in the CY 2025 performance period/2027 MIPS payment year. We now propose to align MVP
policies with the traditional MIPS proposal regarding the weighting of improvement activities
and to reduce the number of improvement activities an MVP Participant must submit for an
MVP. Maintaining a lower reporting burden would encourage reporting MVPs. We finalized that
incentives for reporting MVPs, including reduced reporting requirements, allow MVP
Participants to report on a smaller, more cohesive subset of measures and activities that are
relevant to a given clinical topic, condition, or episode of care (86 FR 65419 and 65420).
Therefore, starting in the CY 2025 performance period/2027 MIPS payment year, MVP
Participants would be required to submit one improvement activity to achieve 40 points, or full
credit, whereas in traditional MIPS clinicians would be required to submit two improvement
activities to achieve full credit for the improvement activities performance category. We propose
to update reporting requirements and scoring rules related to the improvement activities
performance category for MVPs accordingly.
We propose to revise § 414.1365(c)(3) to reflect reporting requirements for the CY 2023
and 2024 performance periods/2024 through 2026 MIPS payment years and the reporting
requirements beginning in the CY 2025 performance period/2027 MIPS payment year. The
revisions proposed at § 414.1365(c)(3)(i) introductory text and additions proposed at paragraphs
(c)(3)(i)(A) through (C) would require that an MVP Participant who reports an MVP, in the CY
2023 through 2024 performance periods/2025 through 2026 MIPS payment years, report one of
the following: two medium-weighted improvement activities; one high-weighted improvement
activity; or participation in a certified or recognized patient-centered medical home (PCMH) or
comparable specialty practice as described at § 414.1380(b)(3)(ii). Additionally, we propose at
§ 414.1365(c)(3)(ii) introductory text and (c)(3)(ii)(A) and (B), beginning in the CY 2025
performance period/2027 MIPS payment year an MVP Participant who reports an MVP must
report either one improvement activity or participation in a certified or recognized patient-
centered medical home (PCMH), or comparable specialty practice as described at
§ 414.1380(b)(3)(ii). We seek comment on the proposals.
We also propose to align MVP scoring with proposed modifications to traditional MIPS
scoring that would remove the reference to high- and medium-weighted improvement activities
for scoring and assign 40 points for each improvement activity submitted by MVP Participants.
We propose at § 414.1365(d)(3)(iii) that in the CY 2023 through 2024 performance periods/2025
through 2026 MIPS payment years, the improvement activities performance category score is
calculated based on the submission of high- and medium-weighted improvement activities. MVP
Participants would receive 20 points for each medium-weighted improvement activity and 40
points for each high-weighted improvement activity required under § 414.1360 on which data is
submitted in accordance with § 414.1325 or for participation in a certified or recognized patient-
centered medical home (PCMH) or comparable specialty practice, as described at
§ 414.1380(b)(3)(ii). Beginning in the CY 2025 performance period/2027 MIPS payment year,
MVP Participants would receive 40 points for each improvement activity that is submitted or
participation in a certified or recognized patient-centered medical home (PCMH) or comparable
specialty practice. We seek comment on this proposal.
(4) Promoting Interoperability Performance Category in MVPs
In the CY 2022 PFS final rule, we finalized at § 414.1365(c)(4)(i) that an MVP
Participant is required to meet the Promoting Interoperability performance category’s reporting
requirements. We also finalized at § 414.1365(c)(4)(i)(A) the requirements for a subgroup
participating in MVP reporting (86 FR 65413 and 65414). Specifically, we stated that for the CY
2023 and 2024 MIPS performance periods/2025 and 2026 MIPS payment years, an MVP
Participant that is a subgroup is required to submit its affiliated group’s data for the Promoting
Interoperability performance category. The submission of the affiliated group’s data will be on
the subgroup’s behalf. If the affiliated group chooses to report as a group for the Promoting
Interoperability performance category, the group will still be required to submit its own data
separately and in accordance with the reporting rules for groups. We refer readers to the CY
2022 PFS final rule for additional details (86 FR 65413 and 65414).
We acknowledge the existing language under § 414.1365(c)(4)(i)(A) establishes the
requirement for a subgroup to submit its affiliated group’s data for the Promoting
Interoperability performance category in the foundational layer of an MVP for only the CY 2023
and 2024 performance periods/2025 and 2027 MIPS payment years. In the CY 2022 PFS final
rule, we stated our intent to assess the performance of clinicians participating in subgroups in the
Promoting Interoperability performance category using subgroup level data to the extent that it is
operationally feasible (86 FR 39371 and 39372). However, as discussed in the CY 2022 PFS
final rule (86 FR 39371), we heard from interested parties through the MVP Town Hall (85 FR
84846), that some clinicians would need additional time to resolve operational challenges,
including challenges related to configuration of EHR systems for reporting Promoting
Interoperability data at the subgroup level. We recognize that clinicians and interested parties
may need additional time to resolve the technical challenges related to configuration of EHR
systems for capturing and submitting data at the subgroup level. We propose that this subgroup
reporting policy for the Promoting Interoperability performance category in the MVP they select
apply beyond the CY 2023 performance period/2025 MIPS payment year and CY 2024
performance period/2026 MIPS payment years currently specified at § 414.1365(c)(4)(i)(A).
Specifically, we propose to modify § 414.1365(c)(4)(i)(A) by removing the references to the
specific performance periods/MIPS payment years and provide that an MVP Participant that is a
subgroup is required to submit its affiliated group's data for the Promoting Interoperability
performance category. The proposed change would allow a subgroup to continue to submit the
affiliated group’s data for the MVP Promoting Interoperability performance category for the CY
2025 performance period/2027 MIPS payment year and beyond. We note CMS will continue to
monitor the operational challenges with the EHR systems and reassess whether subgroups should
be required to submit subgroup level performance data for the Promoting Interoperability
performance category.
We request public comment on this proposal.
We refer readers to section V.B.8. of this proposed rule for discussion on the burden
estimates for these proposals.
c. APM Performance Pathway
(1) Overview
In the CY 2021 PFS final rule (85 FR 84859 through 84866), we finalized the APM
Performance Pathway (APP) at § 414.1367 beginning in CY 2021 performance period/2023
MIPS payment year, which was designed as a reporting and scoring pathway available only to
MIPS APM participants in order to provide a predictable and consistent MIPS reporting option
to reduce reporting burden for, and encourage continued APM participation, by these
clinicians. We also established that, beginning with the Shared Savings Program performance
year 2021 (CY 2021 performance period/2023 MIPS payment year), ACOs were required to
report quality data for purposes of the Shared Savings Program via the APP (42 CFR
425.512(a)(3); 85 FR 84722).
In that same rule, we finalized a quality measure set (85 FR 84860 and 84861) for
purposes of quality performance category scoring for the APP. For those MIPS eligible
clinicians, groups, or APM Entities for whom a given measure is unavailable due to the size of
the available patient population or who are otherwise unable to meet the minimum case threshold
for a measure, we established that such measure would be removed from the quality performance
category score for such MIPS eligible clinician, group, or APM Entity (85 FR 84861). The
complete existing APP quality measure set is shown in Table 53. As indicated in Table 53, the
current APP quality measure set includes six quality measures, of which five also are Universal
Foundation measures. Further, for MIPS eligible clinicians, groups, and APM Entities reporting
through the APP, we established that we would not apply the quality measure scoring cap at
§ 414.1380(b)(1)(iv) in the event that a measure in the APP quality measure set is determined to
be topped out. Because the APP quality measure set is fixed, we noted that it would not be
appropriate to limit the maximum quality performance category score available to APP reporters.
Should an APP quality measure be determined to be topped out, we would at that time consider
amending the APP quality measure set through future rulemaking, if appropriate.

TABLE 53: Existing APM Performance Pathway Quality Measure Set
Quality #
Measure Title
Collection Type
Submitter Type
Meaningful
Measures 2.0
Area
Measure
Type
Universal
Foundation
Measure
321
CAHPS for MIPS
CAHPS for MIPS
Survey
Third Party
Intermediary
Patient’s
Experience
Patient
Engagement/
Experience
Yes
:001
Diabetes:
Hemoglobin
A1c (HbA1c) Poor
Control
eCQM/MIPS
CQM (all APP
reporters)
Web Interface/
Medicare CQM
(SSP ACOs only)
MIPS Eligible
Clinician
Representative of
a Practice
APM Entity
Third Party
Intermediary
Mgt. of
Chronic
Conditions
Intermediate
Outcome
Yes
134
Preventive Care
and Screening:
Screening for
Depression and
Follow-up Plan
eCQM/MIPS
CQM (all APP
reporters)
Web Interface/
Medicare CQM
(SSP ACOs only)
MIPS Eligible
Clinician
Representative of
a Practice
APM Entity
Third Party
Intermediary
Treatment of
Mental Health
Process
Yes
236
Controlling High
Blood Pressure
eCQM/MIPS
CQM (all APP
reporters)
Web Interface/
Medicare CQM
(SSP ACOs only)
MIPS Eligible
Clinician
Representative of
a Practice
APM Entity
Third Party
Intermediary
Mgt. of
Chronic
Conditions
Intermediate
Outcome
Yes
479
Hospital-Wide, 30-
day, All-Cause
Unplanned
Readmission
(HWR) Rate for
MIPS Eligible
MIPS
Clinician Groups
Administrative
Claims
N/A
Admissions &
Readmissions
Outcome
Yes
484
Clinician and
Clinician Group
Risk-standardized
Hospital
Admission Rates
for Patients with
Multiple Chronic
Conditions
Administrative
Claims
N/A
Admissions &
Readmissions
Outcome
No
We stated when finalizing the APP that the goal of the APP quality measure set is not
necessarily to reflect the specific quality goals of clinicians within their respective APMs, but
rather to reduce the burden of reporting on quality measures twice: once to MIPS and once to
their APMs. We believed that by using this broadly applicable population-health-based measure
set, we would enable MIPS APM participants to focus more of their energy and attention on the

quality measures being reported through their APMs, while relying on a consistent measure set
within the APP from one year to the next (85 FR 84862).
We also finalized the Web Interface measure set for the CY 2021 MIPS performance
period within the APP for Shared Savings Program ACOs only (85 FR 84720 through 84723),
and in the CY 2022 PFS final rule, extended this collection type through CY 2024 (86 FR
65429). In the CY 2024 PFS final rule, we established the Medicare Clinical Quality Measure for
Accountable Care Organizations Participating in the Medicare Shared Savings Program
(Medicare CQM) collection type in the APP quality measure set and finalized that the Medicare
CQM collection type would be available to only ACOs participating in the Shared Savings
Program. Beginning with the 2024 performance year, ACOs in the Shared Savings Program have
the option to report the Medicare CQM under the APP on only “beneficiaries eligible for
Medicare CQMs as defined at § 425.20, instead of their all payer/all patient population” (88 FR
79329).
(2) Establishment of the APP Plus Quality Measure Set to Align with the Universal Foundation
Under the goals of the CMS National Quality Strategy to improve the quality and safety
of healthcare for everyone,
716
CMS is implementing a building-block approach to streamline
quality measures across CMS quality programs for measuring primary care clinician
performance in the adult and pediatric populations by leveraging the Universal Foundation of
quality measures. The Universal Foundation of quality measures focuses clinicians’ attention on
measures that are meaningful for the health of broad segments of the population; reduces
provider burden by streamlining and aligning measures; advances equity with the use of
measures that will help CMS recognize and track disparities in care among and within
populations; aids the transition from manual reporting of quality measures to seamless, automatic
digital reporting; and permits comparisons among various quality and value-based care programs
716
https://www.cms.gov/medicare/quality/meaningful-measures-initiative/cms-quality-strategy.

to help the Agency better understand what drives quality improvement and what does not.
717
The
Universal Foundation, which identifies a set of key quality measures for use where relevant
throughout CMS programs, is already reflected in the Medicaid Core Sets and the Marketplace
Quality Rating System.
718
In addition, in the CY 2024 PFS final rule (88 FR 79321 and 80043),
CMS consolidated the previously finalized Promoting Wellness and Optimizing Chronic Disease
Management MIPS Value Pathways (MVPs) into a single consolidated primary care MVP
(Value in Primary Care MVP) that aligns with the adult Universal Foundation quality measures.
In the Announcement of CY 2024 Medicare Advantage (MA) Capitation Rates and Part C and D
Payment Policies, we also solicited comment on adding the Universal Foundation measures to
Medicare Advantage and the Part D Star Ratings Program. We noted that we would take these
comments into consideration in the future, and that any additional measures added to the Star
Ratings Program would need to be added through rulemaking.
719
Alignment of quality measures
across CMS programs allows practitioners to better focus their quality efforts, reduces
administrative burden, and drives digital transformation and stratification of a focused quality
measure set to assess impact on disparities.
720
To further advance Medicare’s overall value-based care strategy, which emphasizes
preventive care and primary care and to promote greater alignment within and across CMS’s
quality programs, we are proposing to create the APP Plus quality measure set within the APP
specifically to incorporate all of the Adult Universal Foundation measures. Five of the ten adult
Universal Foundation measures already are represented in the existing APP quality measure set
for the CY 2025 performance period/2027 MIPS payment year under policies finalized in the
717
Jacobs D, Schreiber M, Seshamani M, Tsai D, Fowler E, Fleisher L. Aligning Quality Measures across CMS –
The Universal Foundation. New England Journal of Medicine, March 2, 2023, available at
https://www.nejm.org/doi/full/10.1056/NEJMp2215539.
718
"Update On The Medicare Value-Based Care Strategy: Alignment, Growth, Equity", Health Affairs Forefront,
March 14, 2024. DOI: 10.1377/forefront.20240311.141546.
719
Centers for Medicare and Medicaid Services (2023). Announcement of Calendar Year (CY) 2024 Medicare
Advantage (MA) Capitation Rates and Part C and Part D Payment Policies. Retrieved March 22, 2024 from
Announcement of Calendar Year (CY) 2024 Medicare Advantage (MA) Capitation Rates and Part C and Part D
Payment Policies (https://www.cms.gov/files/document/2024-announcement-pdf.pdf).
720
"Update On The Medicare Value-Based Care Strategy: Alignment, Growth, Equity", Health Affairs Forefront,
March 14, 2024. DOI: 10.1377/forefront.20240311.141546.
CY 2024 PFS final rule (88 FR 79113 through 79114). The Universal Foundation measures
included in the APP quality measure set are listed in Table 53. The inclusion of half of the
measures in the Universal Foundation in the existing APP quality measure set and the
recognition that a significant number of current and potential users of the APP—those clinicians
participating in MIPS APMs—practice in primary and preventive care areas that are relevant to
the Universal Foundation make the APP a meaningful addition to CMS’s efforts at quality
alignment by bringing in MIPS reporting by MIPS APM participants and in turn by providing
feedback in the form of their MIPS quality score to those participants as they also continue to
work towards advancing the care they provide within the context of their respective MIPS
APMs.
We note that we are not proposing to modify the existing APP quality measure set or the
overall framework for the APP as a reporting and scoring pathway. For example, the APP will
continue to be available to MIPS eligible clinicians, groups, and APM Entities participating in
MIPS APMs, meaning that only these clinician types will be able to report and be scored on the
APP Plus quality measure set. We are proposing that, within the APP, the APP Plus quality
measure set would be a second measure set distinct from the existing APP quality measure set
that MIPS eligible clinicians identified on the Participation List or Affiliated Practitioner List of
an APM Entity participating in a MIPS APM may optionally choose to report. Under the
proposal, when an applicable MIPS eligible clinician, group, or APM Entity chooses to report
the APP beginning in the CY 2025 performance period/2027 MIPS payment year, they will then
choose whether to report the APP quality measure set or the APP Plus quality measure set. For
the CY 2025 performance period/2027 MIPS payment year, the APP Plus quality measure set
would include the current APP quality measures and two additional quality measures from the
Adult Universal Foundation measure set. The measure set would incrementally add the
remaining three Adult Universal Foundation measures by the CY 2028 performance period/2030
MIPS payment year. Specifically, we are proposing to adopt one new quality measure beginning
with the CY 2026 performance period/2028 MIPS payment year, and two new quality measures
beginning with the CY 2028 performance period/2030 MIPS payment year.
We are proposing to revise § 414.1367(c)(1) such that each MIPS eligible clinician,
group, or APM Entity APM that elects to report the APP would choose to report either the APP
quality measure set or the APP Plus quality measure set. A MIPS eligible clinician, group, or
APM Entity that chooses to report the APP Plus quality measure set for a performance period
would be required to report all available measures in the APP Plus quality measure set for that
performance period and would be scored on all such measures. For example, with respect to the
CY 2026 performance period/2028 MIPS payment year, a MIPS eligible clinician, group, or
APM Entity that chooses to report the APP Plus quality measure set would be required to report
all nine MIPS quality measures (to the extent applicable and available): the nine measures are the
six measures incorporated from the APP quality measure set and the three additional Universal
Foundation measures we are proposing to incrementally adopt in the APP Plus quality measure
set in the CY 2025 and 2026 performance periods/2027 and 2028 MIPS payment years. The
clinician would also be scored on all nine of these measures.
The proposal would incrementally incorporate into the APP Plus measure set the
Universal Foundation measures that are not already included in the APP measure set beginning
in the CY 2025 performance period/2027 MIPS payment year. The Universal Foundation
measure set aligns quality measures used across CMS programs and initiatives and is relevant to
a significant subset of the clinicians who are eligible to report the APP. The APP Plus quality
measure set will allow MIPS eligible clinicians, groups, and APM Entities eligible to report the
APP to report Universal Foundation quality measures, which are used across CMS programs and
initiatives.

The APP Plus quality measure set would be separate from the APP quality measure set
and would be optional for a MIPS eligible clinician, group, or APM Entity to report.
721
Although
we want to promote greater familiarity with the Universal Foundation measures and to encourage
clinicians to use the Universal Foundation measures through their MIPS participation, it is
important to continue to allow the APP to serve its original purpose of offering a streamlined,
stable reporting and scoring pathway for MIPS APM participants, who are already performing
practice transformation and are reporting and being scored on quality measures within their
APMs. Further, we recognize that while the Adult Universal Foundation quality measures are
relevant to a significant portion of clinicians who are eligible to report the APP, they are not
relevant for all such clinicians. For example, there are specialists for whom few, if any, of these
measures may be relevant, and we do not wish to effectively exclude these clinicians from
accessing the benefits of the APP when they otherwise are eligible. Moreover, we recognize that
as CMS continues to evolve APM offerings for specialists, there may be more clinicians in the
future who are participating in MIPS APMs and would therefore be eligible for the APP, which
could shift the proportion of clinicians for whom the Universal Foundation measures are relevant
as compared to today. For these reasons, we believe it is important to maintain the existing APP
quality measure set and to continue to offer it as an option alongside the proposed APP Plus
quality measure set.
For the reasons specified above, we are proposing to amend § 414.1367(c)(1) to establish
the APP Plus quality measure set and provide MIPS eligible clinicians, groups, and APM
Entities the option to report the APP quality measure set or the APP Plus quality measure set
beginning with the CY 2025 performance period/2027 MIPS payment year. We request
comment on this proposal.
721
That said, we note that the Shared Savings Program is proposing in section [B-G pages].4.(2).a to require that an
ACO report the APP Plus quality measure set starting with PY 2025.
(3) Measures Proposed for Use in the APP Quality Measure Set and APP Plus Quality Measure
Sets
In the CY 2021 PFS final rule, we adopted the current APP quality measure set (85 FR
84860 and 84861). Table 53 contains the current APP quality measure set. We are not proposing
any changes to the existing APP quality measure set for the CY 2025 performance period/2027
MIPS payment year or successive years.
We are proposing a phased approach to establish the APP Plus quality measure set over
four years. By the CY 2028 performance period/2030 MIPS payment year, the APP Plus quality
measure set would consist of the measures currently contained in the APP quality measure set
and five additional quality measures from the Universal Foundation measure set. We are
proposing to phase in these new measures over time to allow for both the eCQM and, for Shared
Savings ACOs, Medicare CQM collection types to be developed and become available.
Specifically, we are proposing that the APP Plus quality measure set will consist of the six
measures currently contained in the APP quality measure set and the following five new
measures, which will be added incrementally:
● Beginning with the CY 2025 performance period/2027 MIPS payment year and
subsequent performance periods: The Breast Cancer Screening (Quality #: 112) and Colorectal
Cancer Screening (Quality #: 113) measures. These measures are currently available as eCQMs,
MIPS CQMs, and Medicare Part B Claims measures. If this proposal is finalized, we would
make the Medicare CQM collection type available for these measures prior to the start of
performance year 2025 only for Shared Savings Program ACOs.
● Beginning with the CY 2026 performance period/MIPS payment year 2028 and
continuing for subsequent performance periods: The Initiation and Engagement of Substance
Use Disorder Treatment (Quality #: 305) measure. This measure is currently available as an
eCQM. If this proposal is finalized, we would make the Medicare CQM collection type available

for this measure prior to the start of performance year 2026 and only for Shared Savings Program
ACOs.
● Beginning with the CY 2028 performance period/2030 MIPS payment year and
continuing for subsequent performance periods: The Screening for Social Drivers of Health
(Quality #: 487) and Adult Immunization Status (Quality #: 493) measures. These measures are
currently available as MIPS CQMs, but are not currently available as eCQMs or Medicare
CQMs. Because developing eCQM specifications typically takes three years, we are proposing to
add these measures to the APP Plus quality measure set in the CY 2028 performance
period/2030 MIPS payment year. If this proposal is finalized, we would make these measures
available prior to the start of CY 2028 performance period/2030 MIPS payment year to report as
eCQMs and, for Shared Savings Program ACOs only, Medicare CQMs.
As discussed above, we intend to incorporate the Adult Universal Foundation measures in the
APP Plus quality measure set. We note that the additional Universal Foundation measures that
we propose to include in the APP Plus quality measure set align with national condition-specific
initiatives and CMS priorities. Below, we briefly discuss each new Universal Foundation
measure that would be added to the APP Plus quality measure set and that is not already included
in the APP quality measure set: Breast Cancer Screening and Colorectal Cancer Screening
Measures.
Our proposed addition of the Breast Cancer Screening (Quality #: 112) and Colorectal
Cancer Screening (Quality #: 113) measures to the APP Plus quality measure set for CY 2025
performance period and subsequent performance periods aligns with the President and First
Lady’s Cancer Moonshot initiative, of which a key objective is to “make sure everyone has
access to cancer screenings—so more Americans can catch cancer early, when outcomes are
best.”
722
Breast cancer and colorectal cancer are two of the most common types of cancers,
722
The White House (n.d.). The President and First Lady’s Cancer Moonshot. Accessed March 28, 2024.
https://www.whitehouse.gov/cancermoonshot/.

accounting for an estimated 23 percent of all new cancer diagnoses in the United States in
2023.
723
Because the risk of developing these types of cancers increases with age, the Breast
Cancer Screening measure focuses on mammogram screening for breast cancer every 24 months
starting at age 50 and the Colorectal Cancer Screening measure focuses on appropriate screening
for colorectal cancer once per performance period, also starting at age 50. Additionally, the
February 2024 preliminary measure specifications for the eCQM version of Colorectal Cancer
Screening lower the starting age for screenings to 45, an update that aligns with United States
Preventive Services Task Force recommendation that colorectal cancer screening begin at age 45
to reduce risk of death.
724
(ii) Initiation and Engagement of Substance Use Disorder Treatment Measure
An estimated 48.7 million Americans aged 12 or older (17.3 percent of the population)
were classified as having had a substance use disorder (SUD) in the past year in 2022.
725
These
individuals are at an increased risk for having major medical conditions, injury, overdose, and
death.
726
Outcomes for individuals with SUDs are improved through early and regular
treatment.
727
The Initiation and Engagement of Substance Use Disorder
Treatment (Quality #: 305) measure ensures patients 13 years of age and older with a new SUD
episode have the initiation of intervention or medication within 14 days of the new SUD episode
or engage in ongoing treatment, including two additional interventions or short-term
723
Siegel, R. L., Miller, K. D., Wagle, N. S., & Jemal, A. (2023). Cancer statistics, 2023. CA: a cancer journal for
clinicians, 73(1), 17–48. https://doi.org/10.3322/caac.21763.
724
eCQI Resource Center (2023). Colorectal Cancer Screening. Accessed March 29, 2024.
https://ecqi.healthit.gov/ecqm/ec/2024/cms0130v12?compare=2024to2023.
United States Preventative Task Force (2021). Final Recommendation on Screening for Colorectal Cancer.
https://www.uspreventiveservicestaskforce.org/uspstf/sites/default/files/file/supporting_documents/colorectal-
cancer-screening-final-rec-bulletin.pdf.
725
Substance Abuse and Mental Health Services Administration. (2023). Key substance use and mental health
indicators in the United States: Results from the 2022 National Survey on Drug Use and Health (HHS Publication
No. PEP23-07-01-006, NSDUH Series H-58). Center for Behavioral Health Statistics and Quality, Substance Abuse
and Mental Health Services Administration. https://www.samhsa.gov/data/report/2022-nsduh-annual-national-
report.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5291754/.
726
Bahorik, A.L., D.D. Satre, A.H. Kline-Simon, C.M. Weisner, C.L. Campbell. 2017. “Alcohol, Cannabis, and
Opioid Use Disorders, and Disease Burden in an Integrated Health Care System.” J Addiction Medicine 11(1),3–9.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5291754/.
727
Kampman, K., K. Freedman. 2020. “American Society of Addiction Medicine (ASAM) National Practice
Guideline for the Treatment of Opioid Use Disorder: 2020 Focused Update.” Journal of Addiction Medicine 14, no.
2S: 1–91, https://doi.org/10.1097/ADM.0000000000000633.

medications, or one long-term medication within 34 days of the initiation of treatment. This
measure also supports CMS efforts to reduce deaths related to opioid overdoses, which have
significantly increased in recent years,
728
and the CMS Behavioral Health Strategy.
729
(iii) Screening for Social Drivers of Health Measure
In the CY 2023 PFS proposed rule, we sought comment on the potential future inclusion
of the Social Drivers of Health measure in the APP quality measure set (87 FR 46154 through
46155). While the majority of commenters were generally supportive of adding the Screening
for Social Drivers of Health measure, several raised concerns related to the undue burden on
collection, cost and resources of implementation, and holding providers accountable for the
collection of data which could be beyond their scope or ability. Some supportive commenters
appreciated that the Screening for Social Drivers of Health measure could drive the
standardization of measures that examine social drivers of health in Federal health care quality
and payment systems, and that this would ultimately drive the health of our patients and our
Nation, maximize the use of limited Government resources to support vulnerable patients, and
achieve quality improvement and equity in health outcomes. Commenters further stated that the
Screening for Social Drivers of Health measure is crucial in recognizing the impact of Social
Drivers of Health-related issues on patients and providers, in laying the foundation to invest in
those communities, and in avoiding fragmentation and provider/patient burden by supporting
alignment across public and private quality and payment programs. Some commenters opposed
the addition of the measure and cautioned CMS to test it before it would be required. Other
opposed commenters voiced their concern about the undue burden on data collection among
patients and providers and the costs and resources associated with implementing new Social
Drivers of Health measures, and that gathering Social Driver of Health measure data would lead
728
National Institute on Drug Abuse (2023). Drug Overdose Deaths. Accessed March 28, 2024.
https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates.
729
Centers for Medicare and Medicaid Services (2024). CMS Behavioral Health Strategy. Accessed April 19, 2024.
https://www.cms.gov/cms-behavioral-health-strategy.

to holding providers accountable for addressing social needs of patients that is beyond a
provider’s scope or ability.
We believe that the benefits of adding the measure to the APP Plus quality measure set
outweigh these concerns. For example, while the challenges and concerns noted previously in
this section associated with implementing screening for social drivers of health are voiced by
family medicine clinicians, social workers, and clinical staff, including the potential negative
impact screening could have on the patient-clinician relationship, screening for social drivers of
health uncovers patient needs, allows clinicians to provide their patients with resources or
referrals, results in appropriately adapting patient care, and prioritizes patient safety.
730
The
addition of the Screening for Social Drivers of Health (Quality #: 487) measure also is consistent
with our priorities to advance health equity and move toward whole-person care throughout our
various programs, including the MIPS and the Hospital Inpatient Quality Reporting (HICR)
programs. This measure addresses five social and economic determinants—namely, food
insecurity, housing instability, transportation needs, utility difficulties, and interpersonal
safety
731
—that are central to the Health Equity strategic plan pillar
(https://www.cms.gov/pillar/health-equity) and have been identified as both a measurement
priority and a performance gap among CMS programs.
The movement to address socioeconomic, environmental, and behavioral health factors
(referred to as drivers of health) has gained traction after a study estimated that only 20 percent
of a person’s health outcomes are linked to their medical care with the remaining 80 percent
attributable to drivers of health.
732
Because of the strong relationship between Social Drivers of
730
Porterfield, L., Jan, Q. H., Jones, F., Cao, T., Davis, L., Guillot-Wright, S., & Walcher, C. M. (2024). Family
Medicine Team Perspectives on Screening for Health-Related Social Needs. Journal of the American Board of
Family Medicine: JABFM, jabfm.2023.230167R3. Advance online publication.
https://doi.org/10.3122/jabfm.2023.230167R3.
731
https://qpp.cms.gov/docs/QPP_quality_measure_specifications/CQM-
Measures/2023_Measure_487_MIPSCQM.pdf.
732
Hood, C. M., K. P. Gennuso, G. R. Swain, and B. B. Catlin. 2016. County health rankings: Relationships
between determinant factors and health outcomes. American Journal of Preventive Medicine 50(2):129-
135. https://doi.org/10.1016/j.amepre.2015.08.024.

Health and physical health outcome, screening for Social Drivers of Health would support the
goals of improving health outcomes by providing clinicians with a more comprehensive
understanding of each patient’s circumstances to inform clinical decision making and ensure
high-quality care.
In addition, many of these drivers of health are not only linked to poorer health, but
disproportionately impact communities of color and underserved populations. Through
screening, once per performance period, of patients 18 years and older for food insecurity,
housing instability, transportation needs, utility difficulties, and interpersonal safety, screening
for Social Drivers of Health and appropriate referrals can potentially improve health outcomes
and reduce health disparities. As we indicated when we proposed to adopt Screening for Social
Drivers of Health in MIPS in the CY 2023 PFS proposed rule, we believe that consistently
addressing drivers of health will have two significant benefits. First, because drivers of health
disproportionately impact individuals and communities that are disadvantaged and/or
underserved by the healthcare system, the promotion of screening for these factors would support
clinician practices and health systems in actualizing an expressed commitment to address
disparities in care, implementing associated equity measures to track progress, and improving
overall health equity.
733
Second, patient-level driver of health data through screening is essential
in the long-term to encourage meaningful collaboration among clinicians and community-based
organizations, and implement and evaluate related innovations in healthcare and social service
delivery. (87 FR 46280)
(iv) Adult Immunization Status Measure
The Adult Immunization Status measure (Quality #: 493) ensures that adults are up to
date with the recommended routine vaccines: influenza; tetanus and diphtheria (Td) or tetanus,
diphtheria and acellular pertussis (Tdap); zoster; and pneumococcal. This robust measure
733
American Hospital Association. (December, 2020). Health Equity, Diversity & Inclusion Measures for Hospitals
and Health System Dashboards. Available at
https://ifdhe.aha.org/system/files/media/file/2020/12/ifdhe_inclusion_dashboard.pdf.

supports the comprehensive evaluation of compliance with recommended adult immunizations
that improve quality care and prevent disease.
(v) Maintaining the Use of the Clinician and Clinician Group Risk-standardized Hospital
Admission Rates for Patients with Multiple Chronic Conditions Measure in the APP Quality
Measure Set and Including It in the APP Plus Quality Measure Set
We note that Clinician and Clinician Group Risk-standardized Hospital Admission Rates
for Patients with Multiple Chronic Conditions (Quality # 484) is an administrative claims-based
measure that is in the APP quality measure set for the MIPS CY performance period 2025/2027
payment year under policies finalized in the CY 2024 PFS final rule (88 FR 79113 and 79114)
but is not one of the ten Adult Universal Foundation measures. Our proposal would continue to
maintain this measure in the APP quality measure set, and therefore also include it in APP Plus
quality measure set, for the CY 2025 performance period/2027 MIPS payment year and
subsequent performance periods. We continue to believe that hospital admission rates are an
effective marker of ambulatory care quality. As noted in our rationale for adopting the measure
in the measure specifications, “Hospital admissions from the outpatient setting reflect a
deterioration in patients’ clinical status and as such reflect an outcome that is meaningful to both
patients and providers.
734
Patients receiving optimal, coordinated high-quality care should use
fewer inpatient services than patients receiving fragmented, low-quality care. Thus, high
population rates of hospitalization may signal poor quality of care or inefficiency in health
system performance. Furthermore, these effects may be exacerbated in disadvantaged areas.
735
Patients with multiple chronic conditions are at high risk for hospital admission, often for
734
Centers for Medicare and Medicaid Services – Quality Payment Program (2023). Measure information for the
Multiple Chronic Care Conditions (MCC) Risk-standardized Hospital Submission Rate for Patients for the Merit-
based incentive Payment System (MIPS) Groups, Performance Year (PY)2023 MCC Measure Code Specifications,
Retrieved March 22, 2024 from 2023 Clinician and Clinician Group Risk-standardized Hospital Admission Rates
for Patients with Multiple Chronic Conditions -– QPP. https://qpp-cm-prod-
content.s3.amazonaws.com/uploads/2202/2023%20MIPS%20Multiple%20Chronic%20Conditions%20Measure%20
Specifications.zip.
735
Jencks, S. F., et al. (2019). "Safety-Net Hospitals, Neighborhood Disadvantage, and Readmissions Under
Maryland's All-Payer Program: An Observational Study." Ann Intern Med. doi: 10.7326/M16-2671.

potentially preventable causes, such as exacerbation of pulmonary disease.”
736
Maintaining this
measure in the APP quality measure set and, as a consequence, including it in the APP Plus
quality measure set also is consistent with our previously stated goals in the CY 2021 PFS final
rule to align the APP with the Meaningful Measures framework, an initiative to remove lower
value quality measures across CMS programs while keeping measures that have less burden and
are the most meaningful with the greatest impact on patient outcomes. This measure supports the
framework’s goals as it is identified among the highest priorities for quality measurement and
improvement while also reducing burden, promoting alignment, moving payment toward value,
and identifying key quality performance metrics for consumers (85 FR 84726).
(vi) The APP and APP Plus Quality Measure Sets Beginning with the CY 2025 Performance
Period/2027 MIPS Payment Year
Table 54 identifies the measures in the Adult Universal Foundation measure set,
crosswalks them to corresponding MIPS measures, and lists the proposed timeline for their
incorporation into the APP Plus quality measure set between the CY 2025 and 2028 performance
periods/2027 and 2030 MIPS payment years as they become available for both the eCQM and
Medicare CQM collection types. We note that Clinician and Clinician Group Risk-standardized
Hospital Admission Rates for Patients with Multiple Chronic Conditions (Quality # 484) is not
one of the ten Adult Universal Foundation measures and is not listed in Table 19; however, we
are maintaining reporting of this measure in the APP quality measure set, and, as such, also
proposing to include it in the APP Plus quality measure set for the CY 2025 performance
period/2027 MIPS payment year and subsequent performance periods.
736
Abernathy, K., Zhang, J., Mauldin, P., Moran, W., Abernathy, M., Brownfield, E., & Davis, K. (2016). Acute
Care Utilization in Patients With Concurrent Mental Health and Complex Chronic Medical Conditions. Journal of
primary care & community health, 7(4), 226–233. https://doi.org/10.1177/2150131916656155.

TABLE 54: Alignment of the APP Plus Measure Set with the Adult Universal Foundation
Measure Set
a
Quality #
Identification Number
and Name
Measure Title
Domain
b
Performance
Period Measure
Added to the
APP Plus
Measure Set
321
158 (varies by program):
Consumer Assessment of
Healthcare Providers and
Systems overall rating
measures
CAHPS for MIPS
Person-centered care
2025
479
561 or 44: Plan all-cause
readmissions or all-cause
hospital readmissions
Hospital-Wide, 30-day, All-Cause
Unplanned Readmission (HWR) Rate for
MIPS Eligible Clinician Groups
Seamless care
coordination
2025
001
204: Hemoglobin A1c
poor control (>9%)
Diabetes: Hemoglobin A1c (HbA1c)
Poor Control
Chronic conditions
2025
134
672: Screening for
depression and follow-up
plan
Preventive Care and Screening:
Screening for Depression and Follow-up
Plan
Behavioral health
2025
236
167: Controlling high
blood pressure
Controlling High Blood Pressure
Chronic conditions
2025
112
93: Breast cancer
screening
Breast Cancer Screening
Wellness and
prevention
2025
113
139: Colorectal cancer
screening
Colorectal Cancer Screening
Wellness and
prevention
2025
305
394: Initiation and
engagement of substance
use disorder treatment
Initiation and Engagement of Substance
Use Disorder Treatment
Behavioral health
2026
487
Identification number
undetermined: Screening
for social drivers of
health
Screening for Social Drivers of Health
Equity
2028
493
26: Adult immunization
status
Adult Immunization Status
Wellness and
prevention
2028
a
Jacobs D, Schreiber M, Seshamani M, Tsai D, Fowler E, Fleisher L. Aligning Quality Measures across CMS – The
Universal Foundation. New England Journal of Medicine, March 2, 2023, available at
https://www.nejm.org/doi/full/10.1056/NEJMp2215539. Domains are from Meaningful Measures 2.0. Identification
numbers are CMS Measures Inventory Tool measure family identification numbers; names reflect the descriptions
associated with those numbers.
b
Domains are from Meaningful Measures 2.0. Identification numbers are CMS Measures Inventory Tool measure
family identification numbers; names reflect the descriptions associated with those numbers.
We refer readers to Table 53 for the APP quality measure set for the CY 2025
performance period/2027 MIPS payment year and subsequent years. The proposed APP Plus
quality measures for the CY 2025 performance period, the CY 2026 and 2027 performance
periods, and the CY 2028 performance period and subsequent performance periods are displayed
in Tables 55, 56, and 57, respectively. Under our proposal, there would be eight measures in the
APP Plus quality measure set in the CY 2025 performance period (Table 55), nine measures in
the CY 2026 and 2027 performance periods (Table 56), and eleven measures in the CY 2028 and

subsequent performance periods (Table 57). We refer readers to Appendix 1 of this proposed
rule for additional measure specification information.
TABLE 55: APP Plus Quality Measure Set for the CY 2025 Performance Period
Quality #
Measure Title
Collection Type
Submitter Type
Meaningful
Measures 2.0
Area
Measure Type
321
CAHPS for MIPS
CAHPS for MIPS
Survey
Third Party Intermediary
Patient’s
Experience
Patient
Engagement/
Experience
001
Diabetes:
Hemoglobin
A1c (HbA1c) Poor
Control
eCQM/MIPS CQM/Part
B Claims (all APP
reporters)
Medicare CQM (SSP
ACOs only)
MIPS Eligible Clinician
Representative of a
Practice
APM Entity
Third Party Intermediary
Mgt. of Chronic
Conditions
Intermediate
Outcome
134
Preventive Care and
Screening:
Screening for
Depression and
Follow-up Plan
eCQM/MIPS CQM/Part
B Claims (all APP
reporters)
Medicare CQM (SSP
ACOs only)
MIPS Eligible Clinician
Representative of a
Practice
APM Entity
Third Party Intermediary
Treatment of
Mental Health
Process
236
Controlling High
Blood Pressure
eCQM/MIPS CQM/Part
B Claims (all APP
reporters)
Medicare CQM (SSP
ACOs only)
MIPS Eligible Clinician
Representative of a
Practice
APM Entity
Third Party Intermediary
Mgt. of Chronic
Conditions
Intermediate
Outcome
479
Hospital-Wide, 30-
day, All-Cause
Unplanned
Readmission
(HWR) Rate for
MIPS Eligible
MIPS
Clinician Groups
Administrative Claims
N/A
Admissions &
Readmissions
Outcome
484
Clinician and
Clinician Group
Risk-standardized
Hospital Admission
Rates for Patients
with Multiple
Chronic Conditions
Administrative Claims
N/A
Admissions &
Readmissions
Outcome
113
Colorectal Cancer
Screening
eCQM/MIPS CQM/Part
B Claims (all APP
reporters)
Medicare CQM (SSP
ACOs only)
MIPS Eligible Clinician
Representative of a
Practice
APM Entity
Third Party Intermediary
Wellness and
Prevention
Process
112
Breast Cancer
Screening
eCQM/MIPS CQM/Part
B Claims (all APP
reporters)
Medicare CQM (SSP
ACOs only)
MIPS Eligible Clinician
Representative of a
Practice
APM Entity
Third Party Intermediary
Wellness and
Prevention
Process

TABLE 56: APP Plus Quality Measure Set for the CY 2026 and 2027 Performance Periods
Quality #
Measure Title
Collection Type
Submitter Type
Meaningful
Measures 2.0
Area
Measure Type
321
CAHPS for MIPS
CAHPS for MIPS
Survey
Third Party Intermediary
Patient’s
Experience
Patient
Engagement/
Experience
001
Diabetes:
Hemoglobin
A1c (HbA1c) Poor
Control
eCQM/MIPS
CQM/Part B Claims
(all APP reporters)
Medicare CQM (SSP
ACOs only)
MIPS Eligible Clinician
Representative of a
Practice
APM Entity
Third Party Intermediary
Mgt. of Chronic
Conditions
Intermediate
Outcome
134
Preventive Care and
Screening:
Screening for
Depression and
Follow-up Plan
eCQM/MIPS
CQM/Part B Claims
(all APP reporters)
Medicare CQM (SSP
ACOs only)
MIPS Eligible Clinician
Representative of a
Practice
APM Entity
Third Party Intermediary
Treatment of
Mental Health
Process
236
Controlling High
Blood Pressure
eCQM/MIPS
CQM/Part B Claims
(all APP reporters)
Medicare CQM (SSP
ACOs only)
MIPS Eligible Clinician
Representative of a
Practice
APM Entity
Third Party Intermediary
Mgt. of Chronic
Conditions
Intermediate
Outcome
479
Hospital-Wide, 30-
day, All-Cause
Unplanned
Readmission
(HWR) Rate for
MIPS Eligible
MIPS
Clinician Groups
Administrative Claims
N/A
Admissions &
Readmissions
Outcome
484
Clinician and
Clinician Group
Risk-standardized
Hospital Admission
Rates for Patients
with Multiple
Chronic Conditions
Administrative Claims
N/A
Admissions &
Readmissions
Outcome
113
Colorectal Cancer
Screening
eCQM/MIPS
CQM/Part B Claims
(all APP reporters)
Medicare CQM (SSP
ACOs only)
MIPS Eligible Clinician
Representative of a
Practice
APM Entity
Third Party Intermediary
Wellness and
Prevention
Process
112
Breast Cancer
Screening
eCQM/MIPS
CQM/Part B Claims
(all APP reporters)
Medicare CQM (SSP
ACOs only)
MIPS Eligible Clinician
Representative of a
Practice
APM Entity
Third Party Intermediary
Wellness and
Prevention
Process
305
Initiation and
Engagement of
Substance Use
Disorder Treatment
eCQM (all APP
reporters)
Medicare CQM (SSP
ACOs only)
MIPS Eligible Clinician
Representative of a
Practice
APM Entity
Third Party Intermediary
Behavioral
health
Process
TABLE 57: APP Plus Quality Measure Set for the CY 2028 Performance Period and
Subsequent Performance Periods
Quality #
Measure Title
Collection Type
Submitter Type
Meaningful
Measures 2.0
Area
Measure
Type
321
CAHPS for MIPS
CAHPS for MIPS
Survey
Third Party Intermediary
Patient’s
Experience
Patient
Engagement/

Quality #
Measure Title
Collection Type
Submitter Type
Meaningful
Measures 2.0
Area
Measure
Type
Experience
001
Diabetes:
Hemoglobin
A1c (HbA1c) Poor
Control
eCQM/MIPS
CQM/Part B Claims
(all APP reporters)
Medicare CQM (SSP
ACOs only)
MIPS Eligible Clinician
Representative of a
Practice
APM Entity
Third Party Intermediary
Mgt. of Chronic
Conditions
Intermediate
Outcome
134
Preventive Care and
Screening:
Screening for
Depression and
Follow-up Plan
eCQM/MIPS
CQM/Part B Claims
(all APP reporters)
Medicare CQM (SSP
ACOs only)
MIPS Eligible Clinician
Representative of a
Practice
APM Entity
Third Party Intermediary
Treatment of
Mental Health
Process
236
Controlling High
Blood Pressure
eCQM/MIPS
CQM/Part B Claims
(all APP reporters)
Medicare CQM (SSP
ACOs only)
MIPS Eligible Clinician
Representative of a
Practice
APM Entity
Third Party Intermediary
Mgt. of Chronic
Conditions
Intermediate
Outcome
479
Hospital-Wide, 30-
day, All-Cause
Unplanned
Readmission
(HWR) Rate for
MIPS Eligible
MIPS
Clinician Groups
Administrative Claims
N/A
Admissions &
Readmissions
Outcome
484
Clinician and
Clinician Group
Risk-standardized
Hospital Admission
Rates for Patients
with Multiple
Chronic Conditions
Administrative Claims
N/A
Admissions &
Readmissions
Outcome
113
Colorectal Cancer
Screening
eCQM/MIPS
CQM/Part B Claims
(all APP reporters)
Medicare CQM (SSP
ACOs only)
MIPS Eligible Clinician
Representative of a
Practice
APM Entity
Third Party Intermediary
Wellness and
Prevention
Process
112
Breast Cancer
Screening
eCQM/MIPS
CQM/Part B Claims
(all APP reporters)
Medicare CQM (SSP
ACOs only)
MIPS Eligible Clinician
Representative of a
Practice
APM Entity
Third Party Intermediary
Wellness and
Prevention
Process
305
Initiation and
Engagement of
Substance Use
Disorder Treatment
eCQM (all APP
reporters)
Medicare CQM (SSP
ACOs only)
MIPS Eligible Clinician
Representative of a
Practice
APM Entity
Third Party Intermediary
Behavioral
health
Process
487
Screening for
Social Drivers of
Health
eCQM/MIPS CQM (all
APP reporters)
Medicare CQM (SSP
ACOs only)
MIPS Eligible Clinician
Representative of a
Practice
APM Entity
Third Party Intermediary
Equity
Process
493
Adult Immunization
Status
eCQM/MIPS CQM (all
APP reporters)
Medicare CQM (SSP
ACOs only)
MIPS Eligible Clinician
Representative of a
Practice
APM Entity
Third Party Intermediary
Wellness and
Prevention
Process
Under our proposal, scoring for the APP quality performance category scoring
methodology at § 414.1367(c)(1) would continue to be performed in accordance with
§ 414.1380(b)(1). For the APP quality measure set, this means that the scoring methodology
would not change. For the APP Plus quality measure set, we are proposing to calculate the MIPS
quality performance category score for a MIPS eligible clinician, group, or APM Entity that
chooses to report the APP Plus quality measure set via the APP by summing the scores for all of
the measures, as applicable, included in the APP Plus quality measure set for a given year.
Scoring clinicians on all measures, as applicable, in the APP Plus quality measure set will
promote the best, safest, and most equitable care and provide a comprehensive assessment of the
performance of those who choose to report the measure set.
Because we are proposing that a MIPS eligible clinician, group, or APM Entity that
chooses to report the APP Plus quality measure set would be scored on all of the measures in that
set, we are also proposing a conforming change to MIPS data submission requirements in
§ 414.1335(b) to require that a MIPS eligible clinician, group, or APM Entity that reports the
APP Plus quality measure set via the APP would be required to report on all measures included
in the APP Plus quality measure set, except for administrative claims-based measures, which are
calculated using data from claims submissions. We solicit comment on this proposal. For further
discussion on the data submission proposal for the APP Plus quality measure set, see section
IV.A.4.e.(1)(b) of this proposed rule.
d. Data Submission for the Performance Categories
(1) Overview
For previously established policies relevant to data submission for the MIPS performance
categories, we refer readers to § 414.1325 and the CY 2017 Quality Payment Program final rule
(81 FR 77087 through 77097), CY 2018 Quality Payment Program final rule (82 FR 53619
through 53626), CY 2023 PFS final rule (86 FR 65438 through 65441) and CY 2024 PFS final
rule (88 FR 79330 through 79332). Specifically, we finalized at § 414.1325(a)(1) that individual
MIPS eligible clinicians, groups, virtual groups, subgroups, and Advanced Payment Model
(APM) Entities must submit data on measures and activities for the quality, improvement
activities, and Promoting Interoperability performance categories in accordance with § 414.1325.
We note, that under the current policies described at § 414.1325(a)(2), there are no data
submission requirements for the cost performance category or administrative claims-based
quality measures.
In this section, we are proposing to adopt minimum criteria for a qualifying data
submission for a MIPS performance period for the quality, improvement activities, and
Promoting Interoperability performance categories, which we propose to codify at §
414.1325(a)(1)(i) through (iii). We are also proposing to codify our existing policies governing
our treatment of multiple data submissions received for the quality and improvement activities
performance categories at § 414.1325(f)(1). We are also proposing to modify our policy
governing our treatment of multiple data submissions received for the Promoting Interoperability
performance category, which we propose to codify at § 414.1325(f)(2).
Proposals in this section of this proposed rule are intended to eliminate certain issues with
the scoring of an unintended data submission affecting payment adjustments for individual MIPS
eligible clinicians, groups, virtual groups, subgroups, and APM Entities. We are proposing these
changes to be effective beginning with the CY 2024 performance period/2026 MIPS payment
year for the data submission period in CY 2025. Additionally, we refer readers to section
V.B.8.e. of this proposed rule for discussion on the burden estimates for these proposals.
(2) Proposed Minimum Criteria for a Qualifying Data Submission for the MIPS Quality,
Improvement Activities, and Promoting Interoperability Performance Categories
(a) Background
CMS uses the data submitted by (or on behalf of) individual MIPS eligible clinicians,
groups, virtual groups, subgroups, or APM Entities in the quality, improvement activities, and
Promoting Interoperability performance categories to assess their performance on the measures
and activities in these three categories and to determine their MIPS payment adjustments. Under
the previously established data submission policies at § 414.1325, individual MIPS eligible
clinicians, groups, virtual groups, subgroups, and APM Entities generally submit data on
measures and activities for the quality, improvement activities, and Promoting Interoperability
performance categories in accordance with the data submission deadlines at § 414.1325(e)(1).
Currently, we consider any submission of data received for a MIPS performance category during
the designated data submission period for a MIPS performance period in accordance with §
414.1325(e)(1) to be a data submission for the corresponding MIPS performance period and
assign a score for the submission.
For the quality and improvement activities performance categories, under the current
reweighting policies at § 414.1380(c)(2)(i)(A)(6) through (8) for an extreme and uncontrollable
circumstance (EUC) or other type of exception based on certain circumstances, we score any
data submitted by (or on behalf of) a MIPS eligible clinician with an approved reweighting
application. This includes MIPS eligible clinicians with an approved application-based EUC
reweighting or an approved reweighting for a clinician identified in a CMS-designated region
affected by an automatic EUC event. Under this current policy, in the event that a MIPS eligible
clinician submits any data for the quality or improvement activities performance category, such
submission overrides the approved reweighting for the applicable performance category and we
will score the performance categories for which data was submitted, and include the performance
category scores in the MIPS eligible clinician’s final score as otherwise provided in §
414.1380(c).
Similarly, for the Promoting Interoperability performance category, under the current
reweighting policies at § 414.1380(c)(2)(i)(C) for a significant hardship or other type of
exception based on certain circumstances, we score any data submitted by (or on behalf of) a
MIPS eligible clinician with an approved reweighting application, except as provided in
§ 414.1380(c)(2)(i)(C)(10) and (11). Under this current policy, in the event that a MIPS eligible
clinician submits any data for the Promoting Interoperability performance category, such
submission overrides the approved reweighting for the performance category and we will score
the Promoting Interoperability performance category and include the category score in the MIPS
eligible clinician’s final score as otherwise provided in § 414.1380(c).
We have received inquiries from MIPS eligible clinicians that highlight unintended
consequences associated with our current data submission requirements. Several MIPS eligible
clinicians have notified us that there have been instances where they unintentionally submitted
non-scorable data for a MIPS performance category, which overrode an approved reweighting or
a previously scorable data submission for the MIPS quality, improvement activities, or
Promoting Interoperability performance categories. Data submissions without any scorable data
(non-scorable data submissions) generally only include limited data that cannot be scored such as
a practice ID, date, activity ID, measure ID, or CMS Electronic Health Record (EHR)
Certification ID (CEHRT ID). MIPS eligible clinicians have also notified us that, in some
instances, the data submission overriding the prior approved reweighting or prior scorable
submission was performed by a third party intermediary or a practice representative.
The MIPS eligible clinician, group, virtual group, subgroup, APM Entity, or third party
intermediary acting on behalf of a MIPS eligible clinician, group, virtual group, subgroup, APM
Entity, as applicable, that submits data on measures and activities under MIPS is defined at §
414.1305 as the submitter type.
The mechanism by which a submitter type submits data to CMS (including, as applicable:
Direct, log in and upload, log in and attest, Medicare Part B claims, and the CMS Web Interface)
is defined at § 414.1305 as the submission type. The direct submission type allows users to
transmit data through a computer-to-computer interaction, such as an API. The log in and upload
submission type allows users to upload and submit data in the form and manner specified by
CMS with a set of authenticated credentials. The log in and attest submission type allows users
to manually attest that certain measures and activities were performed in the form and manner
specified by CMS with a set of authenticated credentials. We refer readers to § 414.1325(b) and
(c) for available data submission types individual MIPS eligible clinicians, groups, virtual
groups, subgroups, and APM Entities may utilize to submit data for the quality, improvement
activities, and Promoting Interoperability performance categories.
To submit data, a submitter must gain access to the Quality Payment Program website
(https://qpp.cms.gov/login) for submitting or viewing data for the associated individual MIPS
eligible clinician, group, subgroup, virtual group, or APM Entity. We refer readers to the Quality
Payment Program Resource Library (https://qpp-cm-prod-
content.s3.amazonaws.com/uploads/335/QPP%20Access%20User%20Guide.zip) for additional
information on the MIPS data submission process and obtaining access to submit data during the
designated submission period under § 414.1325(e)(1).
After gaining access to the Quality Payment Program website for the associated
individual MIPS eligible clinician, group, subgroup, virtual group, or APM entity, a submitter
can navigate to the “Eligibility and Reporting” tab and view whether there is any reweighting
applied for one or more of the MIPS performance categories for the associated individual MIPS
eligible clinician, group, virtual group, subgroup, or APM Entity. In addition, at the time of
submission, the system generates warnings to the submitter (for all the available submission
types) if there is an existing approved reweighting for the performance category in which the
data is being submitted or an existing data submission for an individual MIPS eligible clinician,
group, virtual group, subgroup, or APM Entity. For example, if a group has an approved
reweighting for the Promoting Interoperability performance category, the system alerts the
submitter prior to completing the data submission with a message stating: “This Action Will
Impact Your Category Weights. Currently, Promoting Interoperability does not count towards
your final score. By choosing to report Promoting Interoperability data, your score for this
category will be included in your final score. This action cannot be undone.” The submitter must
check the “Yes, I agree” box prior to confirming the data submission in the performance
category. We refer readers to the Quality Payment Program Resource Library
(https://qpp.cms.gov/resources/resource-library) for additional details on the process to submit
MIPS data for MIPS eligible clinicians, groups, virtual groups, subgroups, and APM Entities.
Under the current process, we assign a score for any submission received from an
individual MIPS eligible clinician, group, virtual group, subgroup or APM Entity for a
performance period during the designated MIPS submission period regardless of whether the
submission included data on the MIPS measures and activities. We implemented the process to
recognize any data submitted as an extension of the policy that submission of any data overrides
reweighting of the MIPS performance categories as described at § 414.1380(c)(2). We assign a
score for submissions with data on MIPS measures and activities, and also for submissions that
only include non-scorable data, such that they do not include any data that allows us to measure a
clinician’s performance on the applicable measures and activities. For example, if we receive a
submission for a MIPS performance category without any measure or activity data (for example,
without numerator and denominator data for any quality measures, without a response of “yes”
for any improvement activities, without a “yes” or “no” response for an attestation, or responses
for the required objectives and associated measures and attestation statements for the Promoting
Interoperability performance category), and the data submission includes only non-scorable data
(such as the practice ID, measure ID and TIN/NPI information), we assign a zero score for the
applicable MIPS performance category in the event we do not receive a subsequent submission
with measure or activity data.
Despite implementing these system warnings to alert the submitter of a potential impact
of their entry on the reweighting status or existing data submission, we continue to receive non-
scorable data submissions, which override an approved reweighting, or a previously scored data
submission, for the MIPS quality, improvement activities, or Promoting Interoperability
performance categories. To help address the unintentional overriding of an existing scorable
data submission or an approved reweighting for the MIPS performance categories, we are

proposing a narrower set of minimum criteria of what would qualify as a data submission under
our existing policies. We note that we are not proposing to change our existing policies to assign
a score for a data submission (meeting the proposed narrower minimum criteria) for the
applicable MIPS performance categories, including our policy governing data submissions from
a third party intermediary, even if the submission overrides an approved reweighting or a prior
scorable submission for the MIPS eligible clinician, group, virtual group, subgroup, or APM
Entity.
We have identified that we could potentially avoid submissions without any scorable data
on MIPS measures or activities from overriding previously approved reweighting or a prior
submission for the MIPS performance categories if we require a submission to include certain
data on measures or activities in the MIPS quality, improvement activities, or Promoting
Interoperability performance categories in order to assign a score. Therefore, we are proposing to
adopt minimum criteria for what we would consider to be a qualifying data submission for which
CMS can assign a score.
Specifically, we are proposing to consider a submission valid and scorable (including,
potentially, a score of zero) for the applicable MIPS performance category only if the data
submission includes: numerator and denominator data for at least one MIPS quality measure in
the quality performance category; a response of “yes” for at least one improvement activity in
the improvement activities performance category; and all required elements to report objectives
and associated measures and attestation statements for the Promoting Interoperability
performance category.
737
We discuss the details of these proposed data submission criteria for
each performance category in sections IV.A.4.d.(2)(b), IV.A.4.d.(2)(c), and IV.A.4.d.(2)(d) of
this proposed rule.
737
Attestation is one possible way to for MIPS eligible clinicians participating in APMs to earn credit in the
improvement activities performance category but is not required to earn credit. Consistent with our regulation at §
414.1380(b)(3)(i), we automatically award 50 percent credit for the improvement activities performance category to
MIPS eligible clinicians participating in APMs when they attest to having completed an improvement activity or
submit data for the quality or Promoting Interoperability performance categories. We are not proposing to change
this.
We note that we are not proposing any changes to the existing scoring or reweighting
policies described under § 414.1380 for the MIPS performance categories in this section of this
proposed rule. If the MIPS eligible clinician, group, virtual group, subgroup, or APM Entity
does not have an approved reweighting for one or more of the MIPS performance categories and
we do not receive a data submission for a performance category that has not been reweighted, we
will assign a score of zero for the applicable performance category.
(b) Quality Performance Category
We refer readers to §§ 414.1325 and 414.1330 through 414.1340 and the CY 2017
Quality Payment program final rule (81 FR 77097 through 77162) and CY 2018 Quality
Payment Program final rule (82 FR 53626 through 53641), the CY 2019 PFS final rule (83 FR
59754 through 59765), CY 2020 PFS final rule (84 FR 63949 through 62959), CY 2021 PFS
final rule (85 FR 84866 through 84877), CY 2022 PFS final rule (86 FR 65431 through 65445),
CY 2023 PFS final rule (87 FR 70047 through 70057), and CY 2024 PFS final rule (88 FR
79329 through 79338) for a description of previously established policies related to the quality
performance category. The data submitted from the final list of MIPS quality measures are used
to assess the performance of an individual MIPS eligible clinician, group, virtual group,
subgroup, or APM Entity for the quality performance category, to contribute to their overall
score, and to help determine the payment adjustment for MIPS eligible clinicians.
We are proposing that a data submission in the quality performance category must
include numerator and denominator data for at least one quality measure from the list of MIPS
quality measures to be assigned a score in the quality performance category. Under the current
policies described at § 414.1325, we finalized data submission types for MIPS eligible clinicians,
groups, virtual groups, subgroups, and APM Entities. In the CY 2018 Quality Payment Program
final rule (82 FR 53780), we stated that we will determine a quality performance category
percent score whenever a MIPS eligible clinician has submitted at least one quality measure. As
described previously in this section of this proposed rule, we currently assign a score for any data
submitted for the MIPS performance categories and have implemented operational measures to
limit unintentional overriding of an approved reweighting or existing scorable data submitted for
a MIPS performance category. However, we continue to receive unintentional submissions
without data that can be scored resulting in the overriding of an approved reweighting
application or a prior data submission that can be scored for the quality performance category.
We note that this proposal does not include any changes to the current scoring policies described
under § 414.1380(b)(1) for the quality performance category. Therefore, we will still assign a
score of zero for the quality performance category if an individual MIPS eligible clinician,
group, virtual group, subgroup, or APM Entity does not submit at least one available quality
measure unless the performance category has been reweighted as defined at § 414.1380(c)(2).
We propose to specify what we consider to be a data submission at § 414.1325(a)(1)(i) to
state that, for the quality performance category, a data submission must include numerator and
denominator data for at least one MIPS quality measure from the final list of MIPS quality
measures. We anticipate the proposed change would potentially avoid unintentional overriding of
an approved reweighting or a prior data submission for the quality performance category due to
submissions without any quality measure data. We are not proposing any changes to the data
submission requirements, data submission criteria, data completeness criteria, and scoring for the
quality performance category described under §§ 414.1325, 414.1335, 414.1440, and
414.1380(b)(1) respectively. We request public comments on this proposal.
(c) Improvement Activities Performance Category
We refer readers to §§ 414.1355 and 414.1360 and the CY 2017 Quality Payment
Program final rule (81 FR 77177 and 77178), CY 2018 Quality Payment Program final rule (82
FR 53648 through 53661), CY 2019 PFS final rule (83 FR 59776 and 59777), CY 2020 PFS
final rule (84 FR 62980 through 62990), CY 2022 PFS final rule (86 FR 65462) and the CY
2024 PFS final rule ( 88 FR 79328) for a description of previously established policies related to
the improvement activities performance category.
We previously finalized at § 414.1360(a)(2) that MIPS eligible clinicians, groups, virtual
groups, or subgroups must submit a yes response for each improvement activity that is
performed for at least a continuous 90-day period during the applicable performance period to
receive points in the improvement activities performance category described under
§414.1360(b)(3). We currently assign a score for any submission or attestation received in the
improvement activities performance category via the submission types described under §
414.1325(a)(1) regardless of whether the submission or attestation included a yes response or
not. In the event of a submission without yes responses, we currently assign a score of zero.
Data submitted in the improvement activities performance category is used to assess the
performance of an individual MIPS eligible clinician, group, virtual group, subgroup, or APM
Entity on the attestation or data submission for the improvement activities and to determine the
payment adjustment for MIPS eligible clinicians. We are proposing to specify for clinicians
what we consider to be a data submission and that we would score a data submission only if the
submission includes a response of “yes” for at least one improvement activity included in the
improvement activities inventory for the MIPS performance period. We anticipate the proposed
change would potentially avoid unintentional overriding of an approved reweighting or a prior
data submission for the improvement activities performance category due to submissions or
attestations without a response of “yes” for any of the improvement activities.
We propose to specify what we consider to be a data submission at § 414.1325(a)(1)(ii)
to state that for the improvement activities performance category, a data submission must include
a response of “yes” for at least one activity in the MIPS improvement activities inventory. We
note that we are not proposing any changes to the data submission criteria and scoring for the
improvement activities performance category described under §§ 414.1360 and 414.1380(b)(3)
respectively. We request public comments on this proposal.
(d) Promoting Interoperability Performance Category
We refer readers to § 414.1375 for our previously established policies regarding reporting
for the Promoting Interoperability performance category. We also refer readers to § 414.1305 for
the definition of attestation, § 414.1325 for data submission requirements, and § 414.1380(b)(4)
for Promoting Interoperability performance category scoring. We refer readers to §
414.1380(c)(2)(i)(C) for our previously finalized policies regarding scoring of data submission in
the Promoting Interoperability performance category after an approved reweighting for the
performance category. We also refer readers to the CY 2017 Quality Payment Program final rule
(81 FR 77199 through 77245), CY 2018 Quality Payment Program final rule (82 FR 53663
through 53688), CY 2019, CY 2021, CY 2022, CY 2023, and CY 2024 PFS final rules (83 FR
59785 through 59820, 84 FR 62991 through 63006, 85 FR 84886 through 84895, 86 FR 65466
through 65490, 87 FR 70060 through 70087, and 88 FR 79351 through 79365, respectively) for a
description of previously established policies related to the Promoting Interoperability
performance category.
We currently consider any information received for the Promoting Interoperability
performance category in the Quality Payment Program Submission environment a data
submission and assign a performance category score based on the submission. We assign a score
of zero for incomplete submissions in the Promoting Interoperability performance category, for
example, submissions that include only a date and CMS CEHRT ID without any data that can be
scored with respect to the required objectives, measures, or attestations, as specified by CMS.
Under § 414.1375, if we receive a complete data submission for the Promoting Interoperability
performance category with responses included for all the required Promoting Interoperability
objectives, associated measures, and attestation statements as specified by CMS and utilizing the
CEHRT (meeting the definition at §414.1305) as required, we score the data submission under
our established scoring policies for the performance category.
We previously finalized at § 414.1380(c)(2)(i)(C) that, if a MIPS eligible clinician with
an approved reweighting for the Promoting Interoperability performance category submits data,

they will be scored in this performance category and the reweighting will not be applied, except
as provided in § 414.1380(c)(2)(i)(C)(10) and (11). We also included in the educational materials
available on the Quality Payment Program resource library (https://qpp-cm-prod
content.s3.amazonaws.com/uploads/2602/2023MIPSSubmissionGuide.pdf) that a MIPS eligible
clinician will be scored in this performance category if they attest to any data, such as selecting
§performance period dates or responding to attestation statements during the submission period.
As set forth under § 414.1380(c)(2)(i)(C), submission of any data for the Promoting
Interoperability performance category overrides reweighting, including reweighting due an
approved significant hardship exception and automatic reweighting for clinicians that are
Ambulatory Surgical Center (ASC)-based, hospital-based, non-patient facing, and small
practices. Similarly, under § 414.1380(c)(2)(i)(A)(4)(iii), submission of any data also overrides
our automatic reweighting of the Promoting Interoperability performance category for clinical
social workers.
738
Furthermore, to earn a performance category score for the Promoting Interoperability
performance category, we established at §414.1375 that, for the performance period established
at § 414.1320, individual MIPS eligible clinicians, groups, virtual groups, subgroups, or APM
Entities must use CEHRT as defined at § 414.1305, report on objectives and associated measures
as specified by CMS, and submit attestations as specified by CMS. Under § 414.1325(b) and (c),
individual MIPS eligible clinicians, groups, virtual groups, subgroups and APM entities (or
authorized representatives submitting on their behalf) can submit data for the Promoting
Interoperability performance category using the direct, login and attest, or login and upload
submission types. Specifically, to submit data for the Promoting Interoperability performance
category, individual MIPS eligible clinicians, groups, virtual groups, subgroups and APM
entities (or authorized representatives submitting on their behalf) must use CEHRT as required
738
We note that this automatic reweighting policy for clinical social workers only applies through the CY 2024
performance period/2026 MIPS payment year.
(meeting the definition at § 414.1305) for the continuous 180-day performance period (§
414.1320(i)) to report the applicable objectives, measures, and attestations. We refer readers to
section IV.A.4.e.(4) of this proposed rule for additional details on CEHRT requirements
(including ONC health IT certification criteria set forth under 45 CFR 170.315) and objectives,
measures, and attestations required for the Promoting Interoperability performance category.
Currently, we receive submissions in the Promoting Interoperability performance
category without completed responses for all the required objectives, measures, and attestations.
For example, if a submission for the Promoting Interoperability performance category includes
only a date, practice ID, and/or a CEHRT ID, or the submission does not include all of the
required objectives, measures, and attestations, then we consider these to be incomplete data
submissions. Currently, an incomplete data submission would void an approved reweighting of
the Promoting Interoperability performance category in accordance with § 414.1380(c)(2)(i)(C).
As discussed in this section of this proposed rule, we believe that we should not consider data
submissions for the Promoting Interoperability performance category if the submission is
incomplete, and does not include all necessary required data. We are proposing that the
minimum criteria for a qualifying data submission for the Promoting Interoperability
performance category must include all required reporting elements for the performance category,
as specified below.
We considered whether CMS should accept incomplete submissions for the Promoting
Interoperability performance category. If CEHRT is utilized as required to collect and report
measure data and submit attestation statements and other requirements, it would generally result
in only complete submissions for the Promoting Interoperability performance category. We
recognize that some of the measures in the Promoting Interoperability performance category
(such as the SAFER Guides measure and security risk analysis) do not directly require the use of
CEHRT, whereas some measures (such as e-prescribing) directly require the use of CEHRT.
However, all the requirements for the Promoting Interoperability performance category are
directly related to a MIPS eligible clinician demonstrating that whether they are a meaningful
user of CEHRT in accordance with sections 1848(q)(2)(A)(iv), (B)(iv) and 1848(o)(2)(A) of the
Act. Further, section 1848(o)(2)(A) requires that all requirements set forth therein (meaningful
use of CEHRT, electronic exchange of health information, and reporting on clinical quality and
other measures using CEHRT) be met for a MIPS eligible clinician to be treated as a meaningful
EHR user for the applicable performance period. Therefore, accepting an incomplete data
submission for the Promoting Interoperability performance category would be counterintuitive to
a MIPS eligible clinician demonstrating whether they are a meaningful user of CEHRT in
accordance with sections 1848(q)(2)(A)(iv), (B)(iv) and 1848(o)(2)(A) of the Act.
We are proposing to adopt minimum criteria for what we would consider a qualifying
data submission for the Promoting Interoperability performance category only if the submission
includes all of the required reporting elements for the category, including data on all required
measures (including any claim of an applicable exclusion), required attestation statements, the
CEHRT ID, and the start and end date for the applicable performance period. This proposal
would clarify what counts as a data submission for MIPS eligible clinicians and it would
potentially avoid partial data submissions from overriding an approved reweighting or a
previously scored submission for the Promoting Interoperability performance category.
Specifically, we propose to specify minimum criteria as a qualifying data submission for
the Promoting Interoperability performance category at § 414.1325(a)(1)(iii) to provide that a
data submission must include all of the following elements:
● Performance data, including any claim of an applicable exclusion, for the measures in
each objective, as specified by CMS;
● Required attestation statements, as specified by CMS;
● CMS EHR Certification ID (CEHRT ID) from the Certified Health IT Product List
(CHPL); and
● The start date and end date for the applicable performance period as set forth in §
414.1320.
As discussed previously, we are not proposing any changes to the existing scoring or
reweighting policies described under § 414.1380 for the MIPS performance categories in this
section of this proposed rule. If the MIPS eligible clinician, group, virtual group, subgroup, or
APM Entity does not have an approved reweighting for one or more of the MIPS performance
categories and we do not receive a data submission meeting the proposed minimum criteria for a
performance category that has not been reweighted, we will assign a score of zero for the
applicable performance category. If we receive a qualifying data submission meeting the
proposed minimum criteria for reporting, then we will review the data submission and score the
Promoting Interoperability performance category in accordance with our applicable scoring
policies.
We refer readers to section IV.A.4.e.(4) of this proposed rule for additional details on the
reporting requirements and scoring of the objectives, measures, and attestations the Promoting
Interoperability performance category.
We request public comments on this proposal.
(3) Treatment of Multiple Data Submissions
(a) Background
Under the current policies described at § 414.1325(d), individual MIPS eligible
clinicians, groups, virtual groups, subgroups, and APM Entities may submit their MIPS data
using multiple data submission types for any performance category in accordance with §
414.1325(a)(1), as applicable; provided, however, that the individual MIPS eligible clinician,
group, virtual group, subgroup, or APM Entity uses the same identifier for all performance
categories and all data submissions. We established the policy to offer flexibility for individual
MIPS eligible clinicians, groups, virtual groups, subgroups, and APM Entities with reporting, as
it provides more options for submission of data for the applicable performance categories. We
refer readers to the CY 2017 and 2018 Quality Payment Program final rules (81 FR 77094 and
77095 and 82 FR 53619 through 53626, respectively) for additional details on the use of multiple
data submission mechanisms for any MIPS performance category.
As discussed in this section of this proposed rule, at § 414.1305, we define a submitter
type as a MIPS eligible clinician, group, virtual group, subgroup, APM Entity, or third party
intermediary acting on behalf of a MIPS eligible clinician, group, virtual group, subgroup, APM
Entity, as applicable, that submits data on measures and activities under MIPS. During a
submission period, a submitter associated with an organization (for example, registry, practice
administrator, or EHR vendor) could submit data for a MIPS eligible clinician, group, subgroup,
virtual group, or APM Entity. If needed, the submitter could also review and correct the data
submission resulting in multiple data submissions for the MIPS performance categories.
Additionally, there could be instances when a submitter unintentionally submits data multiple
times. There could also be instances when we receive data for a MIPS eligible clinician, group,
subgroup, virtual group, or an APM Entity from multiple organizations. For example, both a
registry and a QCDR could submit MIPS data on behalf of a group practice for a performance
period. Individual MIPS eligible clinicians, groups, practice representatives, and third party
intermediaries benefit from the flexibility to submit data multiple times as it provides
opportunities to correct errors in a prior submission and allows clinicians to submit data from
multiple sources (qualified registry and group submission) to increase their chances to provide
the most clinically relevant data.
For the quality, improvement activities, and Promoting Interoperability performance
categories, there is an established policy governing our treatment of multiple data submissions
received for a performance period; additional guidance on how we process and score multiple
submissions received in the MIPS performance categories via educational and outreach materials
is available on the Quality Payment Program Resource Library
(https://qpp.cms.gov/resources/resource-library). However, we have not codified this policy in
prior rules. In this section, we are proposing to codify at § 414.1325(f)(1) our existing policies
governing our treatment of multiple data submissions received for the quality and improvement
activities performance categories. We are also proposing to modify our policy governing our
treatment of multiple data submissions received for the Promoting Interoperability performance
category, which we also propose to codify at § 414.1325(f)(2).
(b) Quality and Improvement Activities Performance Categories
In the CY 2018 Quality Payment Program final rule (82 FR 53619 through 53626), we
discussed scoring policies for multiple submissions received in the MIPS performance
categories. Specifically, we stated that if an individual MIPS eligible clinician or group submits
the same measure through two different mechanisms, each submission would be calculated and
scored separately and that we do not have the ability to aggregate data on the same measure
across submission mechanisms. We would only count the submission that gives the clinician the
higher score, thereby avoiding double counting (82 FR 53620). We refer readers to CY 2019
PFS final rule (83 FR59747 through 59749) for our discussion of previously finalized policies
related to the use of the term “submission mechanism.”
Under the existing process for the quality and improvement performance categories, if we
receive multiple submissions for an individual clinician, group, subgroup, or virtual group from
submitters from separate organizations (for example, registry, practice administrator, or EHR
vendor), we score each submission and assign the highest of the scores for the performance
category. If we receive multiple submissions for an individual clinician, group, subgroup, or
virtual group from a submitter or submitters from the same organization, we will use the most
recent submission. For example, if a qualified registry submits improvement activities for a
group on Tuesday and a practice administrator submits improvement activities data for the same
group on Wednesday, we will score all the data submissions and assign the highest of the scores.
If the practice administrator from a group practice submits improvement activities data for the
group on Tuesday and either the practice administrator or another submitter employed by the
group practice submits improvement activities data for the group again on Wednesday, we will
score only the data submission received on Wednesday because a new data submission received
from the same organization on Wednesday will override the prior data submission on Tuesday.
To codify the existing process for multiple data submissions for the quality and improvement
activities performance categories, we are proposing to add at § 414.1325(f)(1) that for multiple
data submissions received in the quality and improvement activities performance categories in
accordance with paragraphs (a)(1)(i) and (ii) for an individual MIPS eligible clinician, group,
subgroup, or virtual group from submitters in multiple organizations (for example, qualified
registry, practice administrator, or EHR vendor), CMS will calculate and score each submission
received and assign the highest of the scores. We are also proposing to modify our policy
governing our treatment of multiple data submissions for the quality and improvement activities
performance category received for an individual MIPS eligible clinician, group, subgroup, or
virtual group from one or multiple submitters in the same organization and score the most recent
submission. We request public comments on this proposal.
(c) Promoting Interoperability Performance Category
For the Promoting Interoperability performance category, we explained in the educational
materials published on the Quality Payment Program Resource Library (https://qpp-cm-prod-
content.s3.amazonaws.com/uploads/2602/2023MIPSSubmissionGuide.pdf) that any data
submitted through multiple submission types or multiple submissions submitted through the
same submission type will result in a score of zero for the Promoting Interoperability
performance category. Additionally, we recommended using a single submission type (file
upload, API, or attestation by an individual MIPS eligible clinician, group, virtual group,
subgroup or a third party intermediary) to submit data for the Promoting Interoperability
performance category. As discussed in section IV.A.4.d.(2)(d) of this proposed rule, the
utilization of the CEHRT should not generate conflicting data for measures and objectives in the
Promoting Interoperability performance category. However, we have received inquiries from
MIPS eligible clinicians that were impacted by the existing process to assign a score of zero for
multiple submissions in the Promoting Interoperability performance category. Specifically, we
identified scenarios when a complete submission from an individual MIPS eligible clinician or
group followed by an incomplete submission resulted in a score of zero, either overriding a
previous score greater than zero or voiding an approved reweighting for the performance
category.
On this basis, we propose to amend our policy for treatment of multiple data submissions
for the Promoting Interoperability performance category. We are proposing that, for multiple
data submissions received, CMS would calculate a score for each data submission received and
assign the highest of the scores. We also are proposing to codify this proposal at
§ 414.1325(f)(2).
We believe this proposal is consistent with our existing policy for treatment of multiple
data submissions received in the quality and improvement activities performance categories, as
discussed previously. Implementing a similar policy for allowing multiple data submissions in
the Promoting Interoperability performance category may provide flexibility for individual MIPS
eligible clinicians, groups, virtual groups, subgroups, and APM Entities to fix errors in a prior
data submission. Additionally, we recognize there may be instances when a practice switches
EHR vendors during a performance period, potentially resulting in separate data submissions for
the Promoting Interoperability performance category. This proposed policy also aligns with our
intent to maintain consistency in data submission requirements across all MIPS performance
categories, to the extent possible, as it significantly reduces the complexity for MIPS eligible
clinicians participating in MIPS.
We request public comments on this proposal.
f. MIPS Performance Category Measures and Activities
(1) Quality Performance Category
(a) Background
Section 1848(q)(1)(A)(i) and (ii) of the Act requires the Secretary to develop a
methodology for assessing the total performance of each MIPS eligible clinician according to
certain specified performance standards and, using such methodology, to provide for a final
score for each MIPS eligible clinician. Section 1848(q)(2)(A)(i) of the Act provides that the
Secretary must use the quality performance category in determining each MIPS eligible
clinician's final score, and section 1848(q)(2)(B)(i) of the Act describes the measures that must
be specified under the quality performance category.
We refer readers to §§ 414.1330 through 414.1340 and the CY 2017 and CY 2018
Quality Payment Program final rules (81 FR 77097 through 77162 and 82 FR 53626 through
53641, respectively), and the CY 2019, CY 2020, CY 2021, CY 2022, CY 2023, and CY 2024
PFS final rules (83 FR 59754 through 59765, 84 FR 63949 through 62959, 85 FR 84866 through
84877, 86 FR 65431 through 65445, 87 FR 70047 through 70055, and 88 FR 79329 through
79338, respectively) for a description of previously established policies and statutory basis for
policies regarding the quality performance category.
In this proposed rule, we are proposing to:
● Establish the data submission criteria for the Alternative Payment Model (APM)
Performance Pathway (APP) quality measure set.
● Maintain the data completeness criteria threshold of at least 75 percent for the CY
2027 and CY 2028 performance periods/2029 and 2030 MIPS payment years.
● Codify previously established criteria pertaining to the removal of MIPS quality
measures.
● Modify the MIPS quality measure set as described in Appendix 1 of this proposed
rule, including the addition of new measures, updates to specialty sets, removal of existing
measures, and substantive changes to existing measures.
(b) Data Submission Criteria
(i) Data Submission Criteria for the Quality Performance Category
In the CY 2021 PFS final rule (85 FR 84859 through 84866), we established the APP in
§ 414.1367 as an available reporting option starting with the CY 2021 performance period/2023
MIPS payment year, which was designed to provide a predictable and consistent MIPS reporting
option to reduce reporting burden and encourage continued APM participation. Additionally, we
finalized a quality measure set (85 FR 84860 through 84861) for purposes of the quality
performance category scoring for the APP.
The APP and the APP quality measure set were designed to reduce the reporting burden
and create new scoring opportunities for MIPS APMs by having a stable, streamlined pathway
for reporting and scoring in MIPS while recognizing the reporting burden and performance
scoring that MIPS eligible clinicians, groups, and APM Entities already experience in their
respective MIPS APMs. We believed that using a broadly applicable population health-based
measure set would enable MIPS APM participants to focus on the quality measures being
reported through their APMs, while relying on a consistent measure set within the APP from
year to year. (85 FR 84862).
In section IV.A.4.c.(3) of this proposed rule, we are proposing to create a second quality
measure set as an available option under the APP, specifically the APP Plus quality measure set,
which is a set of measures that are included in the Adult Universal Foundation measure set. Of
the ten Adult Universal Foundation measures, five of the measures are already included in the
APP quality measure set for the CY 2025 performance period/2027 MIPS payment year (88 FR
79113 through 79114). The APP Plus quality measure set would initially consist of all the
measures currently within the APP quality measure set (five Adult Universal Foundation
measures and a separate quality measure) plus two additional measures from the Adult Universal
Foundation measure set. The set would incrementally add the remaining three Adult Universal
Foundation measures by the CY 2028 performance period/2030 MIPS payment year. (We refer
readers to section IV.A.4.c.(3) of this proposed rule for further discussion regarding the APP

Plus quality measure set.) Aligning the APP Plus quality measure set with the Adult Universal
Foundation measure set serves to advance Medicare’s overall value-based care strategy and
maintain alignment within and across CMS’s quality programs. The alignment of quality
measures across CMS programs allows clinicians to better focus their quality efforts, reduce
administrative burden, and drive digital transformation and stratification of a focused quality
measure set to assess the impact on disparities.
739
For the APP Plus quality measure set, we are proposing in § 414.1335(b) to require MIPS
eligible clinicians, groups, and APM Entities, including Medicare Shared Saving Program
Accountable Care Organizations (ACOs), to report on all measures in the APP Plus quality
measure set (with the exception of the administrative claims-based quality measures
automatically calculated by CMS) for the applicable performance period. As discussed further in
sections IV.A.4.c.(3) of this proposed rule, the APP Plus quality measure set would be optional
for MIPS eligible clinicians, groups, and APM Entities (not including Medicare Shared Savings
Program ACOs) meeting the reporting requirements under the APP starting with the CY 2025
performance period/2027 MIPS payment year. However, for a Medicare Shared Savings
Program ACOs, they would be required to report the APP Plus quality measure set to meet the
reporting requirements of the Medicare Shared Savings Program’s quality performance standard
as discussed in section IV.A.4.c.(2) of this proposed rule. Under the proposal in § 414.1335(b),
the requirement to report all measures within the APP Plus quality measure set (with the
exception of the administrative claims-based quality measures automatically calculated by CMS)
would be the same regardless of whether a MIPS eligible clinicians, group or APM Entity is
reporting the APP Plus quality measure set on a mandatory or optional basis. We are proposing
conforming amendments in § 414.1335(a).
Having Medicare Shared Savings Program ACOs use the APP Plus quality measure set
739
Update On The Medicare Value-Based Care Strategy: Alignment, Growth, Equity", Health Affairs Forefront,
March 14, 2024. DOI: 10.1377/forefront.20240311.141546.

allows for the comprehensive incorporation of the quality measures in the Adult Universal
Foundation measure set by the CY 2028 performance period/2030 MIPS payment year and the
better alignment of the quality measures reported by Medicare Shared Savings Program ACOs
with the Medicaid Core Sets and the Marketplace Quality Rating System, which have previously
adopted the quality measures in the Adult Universal Foundation.
740
Also, the alignment with the
Adult Universal Foundation would better align the quality measures reported by Medicare
Shared Savings Program ACOs with the Value in Primary Care MIPS Value Pathway (MVP).
Alignment would allow clinicians to leverage their familiarity and experience with the Adult
Universal Foundation quality measures among primary care clinicians participating in this MVP
as they transition to reporting the APP Plus quality measure set in the Medicare Shared Savings
Program. Experience and familiarity with the same quality measures, redesigned care processes,
and quality improvement activities that are commonplace in ACOs would streamline the
pathway for clinicians to join ACOs in the future, which is consistent with our goal to have all
beneficiaries in an accountable care relationship by 2030. The Medicare Shared Savings
Program has the authority under section 1899(b)(3)(C) of the Act to seek to improve the quality
of care furnished by ACOs over time by specifying higher standards, new measures, or both for
purposes of assessing such quality of care.
Lastly, we note that the existing reporting requirements and scoring policies established
in § 414.1367(c)(1) continue to be applicable to the APP quality measure set. Similarly, the
existing scoring policies established in § 414.1367(c)(1) would be applicable to the APP Plus
quality measure set. As discussed in more detail in section IV.A.4.c.(3) of this proposed rule we
are proposing to require that all measures within the APP Plus quality measure set be reported
(with the exception of the administrative claims-based quality measures that are automatically
calculated by CMS), and all measures in the APP Plus quality measure set would be scored,
740
Jacobs D, Schreiber M, Seshamani M, Tsai D, Fowler E, Fleisher L. Aligning Quality Measures across CMS –
The Universal Foundation. New England Journal of Medicine, March 2, 2023, available at
https://www.nejm.org/doi/full/10.1056/NEJMp2215539.
unless a measure does not have a benchmark or meet the case minimum requirements. If a
measure within the APP Plus quality measure set does not have a benchmark or meet the case
minimum requirements, the measure would still be required to be reported in order to meet the
reporting requirements of the APP and for the measure to be excluded from scored (such
measure would not contribute to the quality performance category score as long as the measure is
reported). If such a measure is not reported, then the measure would fail to meet the reporting
requirements of the APP and as a result, it would receive 0 achievement points.
We are seeking public comment on the proposal to establish the data submission criteria
for the APP Plus quality measure set, specifically the proposal to require the reporting of all
measures within the APP Plus quality measure set (with the exception of the administrative
claims-based quality measures automatically calculated by CMS).
(c) Data Completeness Criteria
(i) Data Completeness Criteria for the Quality Performance Category
As described in the CY 2017 Quality Payment Program final rule (81 FR 77125 through
77126), to ensure that data submitted on quality measures are complete enough to accurately
assess each MIPS eligible clinician’s quality performance, we established a data completeness
requirement. Section 1848(q)(5)(H) of the Act provides that analysis of the quality performance
category may include quality measure data from other payers, specifically, data submitted by
MIPS eligible clinicians with respect to items and services furnished to individuals who are not
entitled to benefits under Part A or enrolled under Part B of Medicare. For the CY 2017
performance period/2019 MIPS payment year (first year of the implementation of MIPS), we
established the data completeness criteria threshold to reflect a threshold of at least 50 percent
(81 FR 77125). The data completeness criteria threshold means the following: an individual
MIPS eligible clinician, group, virtual group, or APM Entity submitting measure data on
qualified clinical data registry (QCDR) measures, MIPS clinical quality measures (CQMs), or
electronic clinical quality measures (eCQMs) must submit data on at least a specific percent (that

is, 50 percent as specified above and 60 percent, 70 percent, and 75 percent as specified in the
following paragraphs) of their patients that meet the measure’s denominator criteria, regardless
of payer; an individual MIPS eligible clinician, group, virtual group, or APM Entity submitting
quality measure data on Medicare Part B claims measures must submit data on at least a
specified percent (i.e., 50 percent as specified above and 60 percent, 70 percent, and 75 percent
as specified in the following paragraphs) of their Medicare Part B patients seen during the
corresponding performance period; and an APM Entity, specifically a Medicare Shared Savings
ACO that meets the reporting requirements under the APP, submitting quality measure data on
Medicare CQMs must submit data on at least a specified percent (that is, 70 percent and 75
percent as specified in the following paragraphs) of the APM Entity's applicable beneficiaries
eligible for the Medicare CQM, as defined at § 425.20, who meet the measure’s denominator
criteria.
In the CY 2017 and CY 2018 Quality Payment Program final rules and the CY 2020 PFS
final rule, we noted that we would increase the data completeness criteria threshold over time (81
FR 77121, 82 FR 53632, and 84 FR 62951). We increased the data completeness criteria
threshold from at least 50 percent to at least 60 percent for the CY 2018 performance
period/2020 MIPS payment year (81 FR 77125 and 82 FR 53633) and maintained a threshold of
at least 60 percent for the CY 2019 performance period/2021 MIPS payment year (82 FR 53633
and 53634). For the CY 2020 performance period/2022 MIPS payment year, we increased the
data completeness criteria threshold from at least 60 percent to at least 70 percent (84 FR 62952).
We maintained data completeness criteria threshold of at least 70 percent for the CY 2021, CY
2022, and CY 2023 performance periods/2023, 2024, and 2025 MIPS payment years (86 FR
65435 through 65438). For the CY 2024 and CY 2025 performance periods/2026 and 2027
MIPS payment years, we increased the data completeness criteria threshold from at least 70
percent to at least 75 percent (87 FR 70049 through 70052). Lastly, we maintained the data

completeness criteria threshold of at least 75 percent for the CY 2026 performance period/2028
MIPS payment year (88 FR 79334 through 79337).
We continue to believe that it is important to incrementally increase the data
completeness criteria threshold as MIPS eligible clinicians, groups, virtual groups, subgroups,
and Alternative Payment Model (APM) Entities gain experience with MIPS. The incorporation
of higher data completeness criteria thresholds in future years ensures a more accurate
assessment of a MIPS eligible clinician’s performance on quality measures and prevents
selection bias to the extent possible (81 FR 77120, 82 FR 53632, 83 FR 59758, 86 FR 65436, 87
FR 70049, and 88 FR 79334). In order to improve compliance with the data completeness
threshold, we have encouraged all MIPS eligible clinicians to perform the quality actions
associated with the quality measures on their patients (82 FR 53632, 86 FR 65436, 87 FR 70049,
and 88 FR 79334) such that all applicable cases may be used when calculating a measure. The
data submitted for each measure is expected to be representative of the individual MIPS eligible
clinician, group, or virtual group’s overall performance for that measure.
Increasing the data completeness criteria threshold provides for a more accurate
assessment of performance. We want to ensure that an appropriate, yet achievable, data
completeness criteria threshold is applied to all eligible clinicians participating in MIPS. Based
on our analysis of data completeness rates from data submission for the CY 2017 performance
period,
741
it is generally feasible for eligible clinicians and groups to achieve a higher data
completeness criteria threshold without jeopardizing their ability to successfully participate and
perform well in MIPS. Our approach for increasing the data completeness criteria threshold
slowly and incrementally over time enhances the ability for individual MIPS eligible clinicians,
groups, virtual groups, subgroups, and APM Entities to meet the data completeness criteria
threshold as it increases and consequently, enables successful participation under MIPS. Thus, a
741
As described in the CY 2020 PFS final rule (84 FR 62951), the average data completeness rates were as follows:
for individual eligible clinicians, it was 76.14; for groups, it was 85.27; and for small practices, it was 74.76.
data completeness criteria threshold of less than 100 percent may reduce clinician burden and
accommodate operational issues that may arise during data collection during the initial years of
the program (82 FR 53632, 86 FR 65436, 87 FR 70049, and 88 FR 79334).
As MIPS eligible clinicians, groups, virtual groups, and APM Entities have gained
experience participating in MIPS, particularly meeting the data completeness criteria threshold
over the last 8 years (from the CY 2017 performance period to the CY 2024 performance
period), such experience has prepared MIPS eligible clinicians, groups, virtual groups, subgroups
(participation option available starting with the CY 2024 performance period), and APM Entities
to meet incremental increases in the data completeness criteria threshold. We have maintained a
data completeness criteria threshold of at least 70 percent for 4 years from the CY 2020
performance period through the CY 2023 performance period and as a result, individual MIPS
eligible clinicians, groups, virtual groups, and APM Entities had 4 years of a maintained data
completeness criteria threshold of at least 70 percent before transitioning to an increased data
completeness criteria threshold of at least 75 percent starting with the CY 2024 performance
period. We believed that maintaining the data completeness criteria threshold of at least 70
percent for 4 years provided adequate time for individual MIPS eligible clinicians, groups,
virtual groups, and APM Entities to adjust to the increase that went into effect at the onset of the
COVID-19 public health emergency and account for the implications the COVID-19 pandemic
had on the healthcare system.
As we assess the timeframe for a potential future increase to the data completeness
criteria threshold, we have determined that maintaining the data completeness criteria threshold
of at least 75 percent for a total of 5 years would provide sufficient time for MIPS eligible
clinicians, groups, virtual groups, subgroups, and APM Entities to adjust to the data
completeness criteria threshold of at least 75 percent. In response to the proposal in the CY 2023
PFS proposed rule to increase the data completeness criteria threshold to at least 80 percent
starting with the CY 2026 performance period/2028 MIPS payment year, interested parties
indicated in the public comments that increasing the data completeness threshold from 75 to 80
percent within two years of increasing the threshold from 70 to 75 percent would present various
challenges such as the following, which would make it more difficult to meet the data
completeness criteria threshold: increased burden (in particular, disproportionately increase
burden for smaller and rural practices due to limited resources and staff, and some practices that
are continuing to recover from the COVID-19 Public Health Emergency); and exacerbated
technical and interoperability challenges pertaining to data aggregation across multiple EHRs,
systems (utilizing different registries, and EHR developers and vendors), and sites (including
multiple TINs participating in the Medicare Shared Savings Program as an ACO) (88 FR 79337).
We accept these concerns, and we thus believe that MIPS eligible clinicians, groups, virtual
groups, subgroups, and APM Entities require more time to adjust and prepare for an increase.
We have implemented the data completeness criteria threshold of at least 75 percent for 2 years.
Specifically, we previously established that for the CY 2024 performance period through the CY
2026 performance period/2026 MIPS payment year through the 2028 MIPS payment year, we
would establish and maintain the data completeness threshold of at least 75 percent (87 FR
70049 through 70052, 88 FR 79334 through 79337). Therefore, for the CY 2027 and CY 2028
performance periods/2029 and 2030 MIPS payment years, we are proposing to maintain the data
completeness criteria threshold of at least 75 percent. In establishing data completeness criteria
thresholds in advance of an applicable performance period, it is advantageous to delineate the
expectations for MIPS eligible clinicians, groups, virtual groups, subgroups, and APM Entities as
it provides sufficient notice of the expectation and subsequently, allows such MIPS eligible
clinicians, groups, virtual groups, subgroups, and APM Entities to prepare for a potential
increase in future years.
In this proposed rule, we are proposing to maintain the data completeness criteria
threshold of at least 75 percent for 2 additional years. Specifically, in § 414.1340(a), we are

proposing the following data completeness criteria thresholds pertaining to QCDR measures,
MIPS CQMs, and eCQMs:
● At paragraph (a)(4), for the CY 2027 and CY 2028 performance periods/2029 and
2030 MIPS payment years, a MIPS eligible clinician, group, virtual group, subgroup, and APM
Entity submitting quality measures data on QCDR measures, MIPS CQMs, or eCQMs must
submit data on at least 75 percent of the MIPS eligible clinician, group, virtual group, subgroup,
or APM Entity’s patients that meet the measure’s denominator criteria, regardless of payer.
Similarly, in § 414.1340(b), respectively, we are proposing the following data
completeness criteria thresholds pertaining to Medicare Part B claims measures:
● At paragraph (b)(4), for the CY 2027 and CY 2028 performance periods/2029 and
2030 MIPS payment years, a MIPS eligible clinician, group, virtual group, subgroup, and APM
Entity submitting quality measures data on Medicare Part B claims measures must submit data
on at least 75 percent of the MIPS eligible clinician, group, virtual group, subgroup, or APM
Entity's patients seen during the corresponding performance period to which the measure applies.
Additionally, in § 414.1340(d), respectively, we are proposing the following data
completeness criteria thresholds pertaining to Medicare CQMs:
● At paragraph (d)(1), for the CY 2027 and CY 2028 performance periods/2029 and
2030 MIPS payment years, an APM Entity, specifically a Medicare Shared Savings Program
ACO that meets the reporting requirements under the APP, submitting quality measure data on
Medicare CQMs must submit data on at least 75 percent of the APM Entity's applicable
beneficiaries eligible for the Medicare CQM, as defined at § 425.20, who meet the measure’s
denominator criteria.
Lastly, for the data completeness criteria pertaining to the quality performance category,
we are proposing a conforming amendment to recognize that an APM Entity, specifically a
Medicare Shared Savings Program ACO that meets the reporting requirements under the APP,
must meet the data completeness criteria requirements established at § 414.1340(d)(1).
We are seeking public comment on these proposals.
(d) Selection of Quality Measures
(i) Addition of New Quality Measures
(A) Pre-Rulemaking Process
Prior to introducing a new MIPS quality measure in a proposed rule, CMS receives
public input on measures through the pre-rulemaking process (referred to as the Pre-Rulemaking
Measure Review (PRMR)) established in accordance with section 1890A of the Act. Although
section 1848(q)(2)(D)(viii) of the Act provides that the pre-rulemaking process under section
1890A of the Act is not required to apply to the selection of MIPS quality measures, we have
found that the pre-rulemaking process provides a comprehensive review of measures from multi-
stakeholder workgroups and have accordingly elected for such measures to be reviewed utilizing
the PRMR process (87 FR 70048). Pursuant to the established PRMR process (additional
information regarding the PRMR process is available at https://p4qm.org/PRMR), CMS has
contracted with a Consensus-Based Entity (CBE), which is responsible for convening a multi-
stakeholder panel comprised of clinicians, patients, measure experts, and health information
technology specialists to provide input on measures CMS is considering for use in Medicare.
The pre-rulemaking process begins with CMS’s publication of measures under
consideration for use in Medicare (the MUC List). Each measure on the MUC List is reviewed
by one of several committees convened by the PQM for the purpose of providing multi-
stakeholder input to the Secretary. The PRMR process includes opportunities for public
comment through a 21-day public comment period, as well as public listening sessions. The
PQM posts the compiled comments and listening session inputs received during the public
comment period and the listening sessions within 5 days of the close of the public comment
period. More details regarding the PRMR process may be found in the PQM Guidebook of
Policies and Procedures for Pre-Rulemaking Measure Review and Measure Set Review.

The final vote of a multistakeholder committee convened by the CBE may result in the
following disposition of a measure: recommended, recommended with conditions, do not
recommend, or no consensus. A “no consensus” recommendation signals continued
disagreement among the committee despite being presented with perspectives from public
comment, committee member feedback and discussion, and highlights the multi-faceted
assessments of quality measures. Quality measures that are considered for potential
implementation in MIPS starting with the CY 2025 performance period were included on the
2023 Measures Under Consideration (MUC) List (available at
https://mmshub.cms.gov/sites/default/files/2023-MUC-List.xlsx). The new MIPS quality
measures proposed are described in Table Group A of Appendix 1 of this proposed rule. There
may be cases in which the CBE does not recommend for a measure to move forward to the
rulemaking process and eventual implementation due to a measure not being endorsed by the
CBE or other CBE, but we go forth with proposing a measure. We note that section
1848(q)(2)(D)(iii)(v)(III) of the Act does not preclude the Secretary from proposing and
implementing measures that are not endorsed by a CBE as long as the measure is evidence-
based.
(ii) Removal of Quality Measures
In this proposed rule, we are codifying previously established criteria for the removal of
MIPS quality measures from the MIPS quality measure inventory. In the CY 2017 Quality
Payment Program final rule (81 FR 77136 through 77137), we established the following criteria
for measure removal to include: If the Secretary determines that the MIPS quality measure is no
longer meaningful, such as MIPS quality measures that are topped out; and, if a measure steward
is no longer able to maintain the quality measure. In the CY 2019 PFS final rule (83 FR 59763),
we expanded the criteria for measure removal to include MIPS quality measures that reached an
extremely topped out status (for example, a measure with an average mean performance within
the 98th to 100th percentile range); the MIPS quality measure may be proposed for removal in
the next rulemaking cycle, regardless of whether or not it is in the midst of the topped-out
measure lifecycle, due to the extremely high and unvarying performance where meaningful
distinctions and improvement in performance can no longer be made, after taking into account
any other relevant factors.
Also, in the CY 2019 PFS final rule (83 FR 59764), we established other criteria for
measure removal, specifically MIPS quality measures that are: duplicative; not maintained or
updated to reflect current clinical guidelines, which are not reflective of a clinician’s scope of
practice; and low-bar, standard of care process measures. As described in the CY 2019 PFS final
rule (83 FR 59765), we established an approach to incrementally remove process measures
where prior to removal, consideration will be given to, but will not be limited to the following:
● Whether the removal of the process measure impacts the number of measures available
for a specific specialty.
● Whether the MIPS quality measure addresses a priority area highlighted in the
Measure Development Plan: https://www.cms.gov/Medicare/Quality-Payment-
Program/Measure-Development/Measuredevelopment.html.
● Whether the MIPS quality measure promotes positive outcomes in patients.
● Considerations and evaluation of the measure’s performance data.
● Whether the MIPS quality measure is designated as high priority or not.
● Whether the MIPS quality measure has reached extremely topped out status within the
98th to 100th percentile range, due to the extremely high and unvarying performance where
meaningful distinctions and improvement in performance can no longer be made.
Lastly, in the CY 2020 PFS final rule (84 FR 62958 through 62959), we expanded the
criteria for measure removal to include MIPS quality measures that do not meet case minimum
and reporting volumes required for benchmarking after being in the program for 2 consecutive
CY performance periods and not available for MIPS quality reporting by or on behalf of all
MIPS eligible clinicians. For MIPS quality measures that do not meet case minimum and
reporting volumes required for benchmarking after being in the program for 2 consecutive CY
performance periods, we noted that we will factor in other considerations (such as, but not
limited to: The robustness of the measure; whether it addresses a measurement gap; if the
measure is a patient-reported outcome; and consideration of the MIPS quality measure in
developing MVPs) prior to determining whether to remove the MIPS quality measure.
We are proposing to codify the aforementioned criteria established for the removal of
MIPS quality measures from the MIPS quality measure inventory in § 414.1330(c), respectively.
(iii) Inventory of Quality Measures
Section 1848(q)(2)(D)(i) of the Act requires the Secretary, through notice and comment
rulemaking, to establish an annual final list of quality measures from which MIPS eligible
clinicians may choose for the purpose of assessment under MIPS. Section 1848(q)(2)(D)(i)(II) of
the Act requires that the Secretary annually update the list by removing measures from the list, as
appropriate; adding new measures to the list, as appropriate; and determining whether measures
that have undergone substantive changes should be included on the updated list.
Previously finalized MIPS quality measures can be found in the CY 2024 PFS final rule
(88 FR 79556 through 79964), CY 2023 PFS final rule (87 FR 70250 through 70633), CY 2022
PFS final rule (86 FR 65687 through 65968), CY 2021 PFS final rule (85 FR 85045 through
85377), CY 2020 PFS final rule (84 FR 63205 through 63513), CY 2019 PFS final rule (83 FR
60097 through 60285), CY 2018 Quality Payment Program final rule (82 FR 53966 through
54174), and CY 2017 Quality Payment Program final rule (81 FR 77558 through 77816). We
are proposing changes to the MIPS quality measure inventory, as set forth in Appendix 1 of this
proposed rule, including the following: the addition of new measures; updates to specialty sets
(i.e., creation of new specialty sets; addition and/or removal of measures; and substantive
changes to existing measures within specialty sets); removal of existing measures; and
substantive changes to existing measures. For the CY 2025 performance period, we are
proposing an inventory of 196 MIPS quality measures.

The new MIPS quality measures that we are proposing to include in MIPS for the CY
2025 performance period and future years can be found in Table Group A of Appendix 1 of this
proposed rule. For the CY 2025 performance period, we are proposing 9 new MIPS quality
measures, which includes 5 high priority measures, of which 2 are also patient-reported outcome
measures.
On January 3, 2024, we announced that we would be accepting recommendations for
potential new specialty measure sets or revisions to existing specialty measure sets for year 9
(CY 2017 performance period/2019 MIPS payment year through CY 2025 performance
period/2027 MIPS payment year) of MIPS under the Quality Payment Program.
742
The
recommendations we received were based on the MIPS quality measures finalized in the CY
2024 PFS final rule and the 2023 MUC List; the recommendations include the addition or
removal of current MIPS quality measures from existing specialty sets, and/or the creation of
new specialty sets. All specialty set recommendations submitted for consideration were assessed
and vetted, and as a result, the recommendations that we agree with are proposed in this
proposed rule. We are proposing the addition of a new specialty set and additionally proposing
modifications to existing specialty sets as described in Table Group B of Appendix 1 of this
proposed rule. Modifications to specialty sets include the addition of new measures and/or
existing measures within the MIPS quality measure inventory, removal of measures, and/or
substantive changes to previously finalized measures (we refer readers to Table Group D of
Appendix 1 in this proposed rule). Specialty and subspecialty sets are not inclusive of every
specialty or subspecialty. We develop and maintain specialty measure sets to assist MIPS
eligible clinicians with selecting quality measures that are most relevant to their scope of
practice.
742
Message to the Quality Payment Program listserv on January 3, 2024, entitled “The Centers for Medicare &
Medicaid Services (CMS) is Soliciting Stakeholder Recommendations for Potential Consideration of New Specialty
Measure Sets and/or Revisions to the Existing Specialty Measure Sets for the 2025 Performance Year of the Merit-
based Incentive Payment System (MIPS).”
In addition to establishing new individual MIPS quality measures, modifying existing
specialty sets, and creating new specialty sets as described in Tables Group A and Group B of
Appendix 1 of this proposed rule, we refer readers to Table Group C of Appendix 1 of this
proposed rule for a list of MIPS quality measures proposed for removal and applicable rationale
for each measure. We have previously specified certain criteria that will be used when we are
considering the removal of a measure (81 FR 77136 and 77137; 83 FR 59763 through 59765; 84
FR 62957 through 62959); and such criteria is outlined in the proposed § 414.1330(c) (as further
discussed in section IV.A.4.e.(1)(d)(ii) of this proposed rule). For the CY 2025 performance
period, we are proposing to remove 11 MIPS quality measures based on the previously
established criteria. Of the 11 MIPS quality measures proposed for removal, 2 MIPS quality
measures are duplicative to a proposed new MIPS quality measure; 3 MIPS quality measures are
duplicative of current measures; 1 MIPS quality measure has reached the topped out lifecycle; 2
MIPS quality measures are extremely topped out; 1 MIPS quality measure is no longer
owned/maintained; and 2 MIPS quality measures have limited adoption and consequently, have
not been able to establish benchmarks to provide a meaningful impact to quality improvement.
We have continuously communicated to interested parties our desire to reduce the number of
process measures within the MIPS quality measure set (see, for example, 83 FR 59763 through
59765). Seven of the MIPS quality measures proposed for removal are process measures that
would not provide granular information related to disparities. The proposal to remove the MIPS
quality measures described in Table Group C of Appendix 1 of this proposed rule would lead to
a more parsimonious inventory of meaningful, robust measures in the program, and that our
approach to removing measures should occur through an iterative process that includes an annual
review of the MIPS quality measures to determine whether they meet our removal criteria.
Also, we are proposing substantive changes to several MIPS quality measures, which can
be found in Table Group D of Appendix 1 of this proposed rule. We have previously established
criteria that would apply when we are considering making substantive changes to a quality
measure (81 FR 77137, and 86 FR 65441 through 65442). We are proposing substantive changes
to 66 MIPS quality measures, which includes 2 MIPS quality measures previously retained for
utilization only in MVPs (we refer readers to Table Group DD of Appendix 1 of this proposed
rule for such measures). On an annual basis, we review the established MIPS quality measure
inventory to consider updates to the measures. Possible updates to measures may be minor or
substantive. The aforementioned proposed inventory of 196 MIPS quality measures includes
193 MIPS quality measure available for utilization in traditional MIPS and MVPs, and 3 MIPS
quality measures available only for utilization in MVPs (as finalized in the CY 2024 PFS final
rule (88 FR 79897 through 77902)). In the CY 2024 PFS final rule, we removed the following 3
MIPS quality measures from traditional MIPS, but retained for utilization in MVPs: Quality
#112: Breast Cancer Screening; Quality #113: Colorectal Cancer Screening; and Quality #128:
Preventive Care and Screening: Body Mass Index (BMI) Screening and Follow-Up Plan (88 FR
79338 and 79897 through 79902). We note that some MIPS quality measures available in
traditional MIPS and/or MVPs are measures adopted by the Medicare Shared Savings Program
for utilization under the APP, specifically the APP quality measure set and the newly established
APP Plus quality measure set, as proposed in section IV.A.4.c.(3) of this proposed rule. For the
MIPS quality measures available in the APP quality measure set and APP Plus quality measure
set for the CY 2025 performance period, we refer readers to section IV.A.4.c.(1) and section
IV.A.4.c.(3) of this proposed rule.
Lastly, it should be noted that in this proposed rule, we are proposing a substantive
change to the following administrative claims measure, Quality #492: Risk-Standardized Acute
Cardiovascular-Related Hospital Admission Rates for Patients with Heart Failure under the
Merit-based Incentive Payment System (we refer readers to Table Group D of Appendix 1 of this
proposed rule), that would be applied retroactively starting with the CY 2023 performance
period/2025 MIPS payment year. In the CY 2023 PFS final rule, we inadvertently specified the
measure was availability at the individual clinician level. The inclusion of the availability of the
measure at the individual clinician level is a misrepresentation and erroneously conveys to MIPS
eligible clinicians reporting at the individual clinician level that the measure is available to meet
the minimum required number of measures to report under traditional MIPS or an MVP. The
measure was tested and developed for implementation at the group, virtual group, subgroup via
an MVP, and APM Entity levels. Thus, the measure is limited to groups, virtual groups,
subgroups via an MVP, and APM Entities participating in MIPS. We believe that a failure to
apply this substantive change retroactively would be contrary to the public interest.
Prior to the finalization of this measure as a new measure available within the MIPS
quality measure inventory in the CY 2023 PFS final rule, the measure was initially proposed as a
new measure in the CY 2022 PFS proposed rule. Based on the public comments received in
response to the initial proposal of this measure in the CY 2022 PFS proposed rule, there were
concerns regarding the attribution of certain patients to clinicians, particularly the risk
adjustment for clinicians with higher caseloads of patients with more complicated or severe heart
failure. As a result, the measure was not finalized as part of the CY 2022 PFS final rule;
however, we noted that we would continue to consider how to implement condition-specific
measures such as this measure under MIPS (86 FR 65692 through 65694).
In the CY 2023 PFS proposed rule, we re-proposed this measure, which mitigated the
concerns regarding the attribution of such patients to clinicians by excluding patients at advanced
stages of heart failure and requiring that a group, virtual group, subgroup via an MVP, and APM
Entity to include at least 1 cardiologist (and a 21-patient case minimum); and subsequently, the
measure was finalized in the CY 2023 PFS final rule (87 FR 70266 through 70271). The intent
of the measure is for assessment of performance to be conducted at the group, virtual group,
subgroup via an MVP, and APM Entity levels. The measure was not tested, developed, or
implemented at the individual clinician level. In order for this measure to be available at the
individual clinician level, the measure would need to be tested at the individual clinician level to
establish validity, reliability, and risk adjustments at the individual clinician level. It is not
appropriate for the measure to be available at the individual clinician level without further
testing. Consequently, any assessment of data for this measure at the individual clinician level
would produce invalid and unreliable results. By retroactively applying the substantive change
to this measure (modifying the measure to remove the individual clinician level as an option)
effective starting with the CY 2023 performance period/2025 MIPS payment year, the level of
reporting available for the measure would align with the intent, implementation, and
operationalization of the measure, and clarify that the measure is not available at the individual
clinician level.
We are seeking public comment on the proposals to modify the quality performance
category measure inventory, a set of 196 MIPS quality measures for the CY 2025 performance
period, which includes the following:
● Implementation of 9 new MIPS quality measures: 5 high priority measures, of which 2
are also patient-reported outcome measures;
● Removal of 11 MIPS quality measures: 2 MIPS quality measure are duplicative to a
proposed new quality measure; 3 MIPS quality measures are duplicative to current quality
measures; 1 MIPS quality measure has reached the topped-out lifecycle; 2 MIPS quality
measures are extremely topped out; 1 MIPS quality measure is no longer owned/maintained; and
2 MIPS quality measures have limited adoption and consequently, have not been able to
establish benchmarks to provide a meaningful impact to quality improvement; and
● Substantive changes to 66 MIPS quality measures.
We refer readers to Table Groups A through DD of Appendix 1 of this proposed rule for
the proposed modifications to the MIPS quality measure inventory for the CY 2025 performance
period.
(e) Quality Performance Category Requests for Information
In this proposed rule, we are seeking public comment on the following two requests for
information (RFIs) regarding to the quality performance category (see sections IV.A.4.e.(1)(e)(i)
and IV.A.4.e.(1)(e)(ii) of this proposed rule). In accordance with the implementation of
regulations pertaining to the Paperwork Reduction Act of 1995 (PRA), specifically 5 CFR
1320.3(h)(4), the general solicitation of public comments for the two RFIs are exempt from the
PRA. Facts or opinions submitted in response to general solicitations of public comments
published in the Federal Register or other publications, regardless of the form or format thereof,
provided that no person is required to supply specific information pertaining to themself, other
than that which is necessary for self-identification, as a condition of the agency's full
consideration, are not generally considered information collections and therefore not subject to
the PRA.
Respondents are encouraged to provide complete, but concise responses. The following
RFIs are issued solely for information and planning purposes; they do not constitute a Request
for Proposal (RFP), applications, proposal abstracts, or quotations. The RFIs do not commit the
U.S. Government to contract for any supplies or services or make a grant award. Further, CMS is
not seeking proposals through the following RFIs and will not accept unsolicited proposals.
Respondents are advised that the U.S. Government will not pay for any information or
administrative costs incurred in response to the RFIs; all costs associated with responding to the
RFIs will be solely at the interested party’s expense. Not responding to the RFIs do not preclude
participation in any future procurement, if conducted. It is the responsibility of the potential
respondents to monitor these RFI announcements for additional information pertaining to the
requests. Please note that CMS will not respond to questions about the policy issues raised in the
RFIs. CMS may or may not choose to contact individual respondents. Such communications
would only serve to further clarify written responses. Contractor support personnel may be used
to review responses for each RFI. Responses to these notices are not offers and cannot be
accepted by the U.S. Government to form a binding contract or issue a grant. Information
obtained as a result of the RFIs may be used by the U.S. Government for program planning on a
non-attribution basis. Respondents should not include any information that might be considered

proprietary or confidential. The following RFIs should not be construed as a commitment or
authorization to incur cost for which reimbursement would be required or sought. All
submissions become U.S. Government property and will not be returned. CMS may publicly post
the comments received, or a summary thereof.
(i) Survey Modes for the Administration of the Consumer Assessment of Healthcare Providers
and Systems (CAHPS) for MIPS Survey Request for Information
We are seeking public comment on the potential expansion of the survey modes of the
CAHPS for MIPS Survey from a mail-phone protocol to a web-mail-phone protocol. The current
protocol is to administer the survey first through the mail and then by phone interview with non-
respondents. The expansion to the protocol would include an initial administration of the survey
by web, followed by mail, and then by phone. During the 2023 CAHPS for MIPS Web Mode
Field Test,
743
adding the web-based survey mode to the current mail-phone protocol of CAHPS
for MIPS survey administration resulted in an increased response rate. Surveys for the field test
were administered to a random sample of 8,613 survey-eligible patients from 20 Medicare
Shared Savings Program ACOs between March 6, 2023, and May 31, 2023, and were compared
to survey data collected from the CY 2022 performance period CAHPS for MIPS Survey for the
same Medicare Shared Savings Program ACOs. In total, 3,638 patients completed a survey
during the field test, resulting in a 43 percent response rate for the web-mail-phone protocol
compared to 28 percent for the mail-phone protocol from the CY 2022 performance period
CAHPS for MIPS Survey. The percentage of patients with an email address varied by ACO,
ranging from 40 percent to 88 percent, and 38 percent of sampled patients had an email address.
Evidence from the 2023 field test indicates that the use of the web-mail-phone protocol results in
a higher response rate. Analysis of this field test data revealed that 43 percent of surveys
completed via the web-based survey mode came from patients who did not have an email
743
Centers for Medicare & Medicaid Services. (June 2024). 2023 CAHPS for MIPS Web Mode Field Test. Available at
https://qpp-cm-prod-content.s3.amazonaws.com/uploads/2893/2023_CAHPS_for_MIPS_WebMode_Field_Test.pdf.
address but responded by entering a URL and patient-specific PIN code printed in either the pre-
notification letter or web invitation letter. The web-mail-phone protocol also achieved higher
response rates among patients of Latino or Hispanic ethnicity; no statistically significant
differences in response rates were found by other demographic characteristics, including gender,
age, or race. Analysis of the 2023 field test data also showed that adding the web-based survey
mode reduced the response by mail from 93 percent during the CY 2022 performance period to
73 percent during the field test timeframe.
Given the potential for an increased response rate by expanding to a web-mail-phone
protocol, we are seeking public comment on the following:
● Would the increase in survey response rates (as shown from the results of the 2023
CAHPS field test), outweigh a possible increase in the cost of survey administration that would
be associated with a three-mode survey protocol (web-mail-phone) compared to the current two-
mode survey protocol (mail-phone)?
● Would providing email addresses to vendors be feasible for groups, virtual groups,
subgroups, and APM Entities (including Medicare Shared Savings Program ACOs)?
(ii) Guiding Principles for Patient-Reported Outcome Measures in Federal Models, and Quality
Reporting and Payment Programs Request for Information
We are committed to elevating the patient voice in healthcare. One critical approach to
elevating the patient voice that is aligned with the CMS National Quality Strategy and strategy of
the CMS Innovation Center is to incorporate more Patient-Reported Outcome Measures
(PROMs) and Patient-Reported Outcome Performance Measures (PRO–PMs) in CMS quality
reporting and payment programs and CMS Innovation Center Models.
CMS had defined a patient-reported outcome (PRO) as any report of the status of a
patient’s health condition or health behavior coming directly from the patient without

interpretation of the patient’s response by a clinician or anyone else.
744
PROs are critical for the
support of person-centered care, as they provide information from the patient or caregivers
perspective and offer important information to improve patient-clinician communication,
decision-making, and care delivery. PROMs are structured tools used to collect data on PROs,
tested for validity and reliability in the population of interest. PRO-PMs aggregate information
collected using PROMs into a reliable, valid measure of performance at the measured entity level
(for example, clinician or health system). Often these measures have established benchmarks for
assessing risk-adjusted outcomes.
A potential path forward is the development of an accessible and unified database of
PROMs/PRO-PMs used in programs and payment systems in health care by Federal, State-
based, and commercial payers, and healthcare systems. This database would identify the
measure steward and include information on the specifications of the measure. The PROMs in
this database could serve as a resource for the subsequent development of PRO-PMs.
Separately, considerations for a data infrastructure that allows PROMs and PRO-PMs to
be integrated into clinical workflow with minimal cost and administrative burden, with data
seamlessly shared across different healthcare settings and systems is important. Although we
recognize there may be important reasons for not restricting PROMs/PRO-PMs to a strictly
defined data infrastructure, we seek to avoid the evolution of multiple PROM/PRO-PM
repositories that may inhibit the development of these measures and potentially impose
additional costs on clinicians and healthcare systems.
One currently existing, unified, non-proprietary PROM repository is the Patient-Reported
Outcomes Measurement Information System
®
(PROMIS
®
) developed and funded by the
National Institutes of Health (NIH). PROMIS tools assess patient-reported health status for
physical, social, and mental wellbeing. NIH supported the development of PROMIS, and in 2018
744
Centers for Medicare & Medicaid Services. (December, 2023). Patient Reported Outcome Measures. Available at
https://mmshub.cms.gov/sites/default/files/Patient-Reported-Outcome-Measures.pdf.
PROMIS was made available to the public through a platform known as HealthMeasures
(www.healthmeasures.net). Northwestern University stewards HealthMeasures and oversees
proprietary and copyright issues, as well as scientific work using PROMIS developed data
elements and measures. HealthMeasures also provides several PRO-PMs for PROMIS available
at https://www.healthmeasures.net/implement-healthmeasures/evaluate-quality-of-
care/healthmeasures-pro-pms. As noted previously, this repository of PROMs and PRO-PMs
could be used in conjunction with a data infrastructure that integrates PROMs and PRO-PMs into
clinical workflow and is able to be shared across multiple healthcare environments.
As we move forward with including more PROMs and PRO-PMs in CMS quality
reporting and payment programs and CMS Innovation Center Models, it is important to develop
a set of guiding principles and considerations for the selection and implementation of PROMs or
PRO-PMs. An illustrative set of guiding principles may include:
● Data Infrastructure – Measures implemented in programs should, to the extent
possible, use existing data systems for data collection and reporting to minimize the
administrative burden associated with collecting PROMs and deriving PRO-PMs. The PROMs
and PRO-PMs should be deployed in a data infrastructure that supports necessary
interoperability standards to support sharing across providers, practices, hospitals, health
systems, health plans and States.
● Measure Testing – The PROMs have been psychometrically tested and undergone
rigorous reliability and validity testing (fully developed PRO-PMs have also been tested for
scientific acceptability including reliability as a quality measure for the accountable entity).
Moreover, PROMs have been tested as digital measures that can be collected and reported
electronically, making their adaptation as electronic Patient Reported Outcomes (ePROs)
feasible.
● Feasible Clinical Implementation – Measures that will be used for clinical decision
making can be integrated into the clinical workflow with minimal cost and administrative
burden. Further, the data can be seamlessly shared across different healthcare settings and payer
systems. The measures can be expected to have meaningful participation and response rates to
draw conclusions from measure score results (based on anticipated sample size from
participating providers).
● Accessible – The measures are easily accessible to clinicians and care teams, without
the creation of additional administrative or significant financial burdens, or the requirement for
additional resources.
● Patient Engagement – The measures have been tested to show that the concept is
meaningful to patients. Additionally, there is a validated feedback loop on how their patient-
reported data is used to drive performance improvement. and improvement in individual patient
outcomes/experience.
● Equity – Measures selection considers how measurement may ameliorate or
exacerbate disparities including but not limited to considerations for language concordance and
response rates. Measures may be stratified by subgroup to better understand disparities in
response rates and outcomes.
Through this RFI, we are seeking comment on principles related to data infrastructure,
selection, feasible implementation, and patient engagement of PROMs and PRO-PMs. We
request feedback from the public regarding the overarching principles and considerations.
Specifically we are seeking input on the following areas:
● Are the aforementioned guiding principles outlined comprehensive or are there
additional guiding principles and considerations we should consider for the selection, and
implementation of PROM and PRO-PMs?
● How can CMS accelerate the development of PRO-PMs and advance them more
rapidly into use? How can CMS support PRO-PM development while balancing the goal of
accessible PRO-PMs with the additional time and resources required to construct PROMs and
PRO-PMs from a PROM?
● How should CMS balance the use of broad PRO-PMs that might be applicable across
multiple clinical contexts compared to condition-specific PROMs and PRO-PMs measures that
can be more tailored to a given clinical situation but lead to a greater number of tools in use
across measures and health care providers?
● How can CMS support making PROMs broadly accessible without limiting both
innovation and resources committed to developing new tools? Are there other examples (beside
PROMIS) of currently existing PROMs/PRO-PMs repositories that make their tools widely
available to clinicians and healthcare systems?
(2) Cost Performance Category
Section 1848(q)(2)(A) of the Act includes resource use as a performance category under
MIPS. We refer to this performance category as the cost performance category. As required by
sections 1848(q)(2) and (5) of the Act, the four performance categories of MIPS are used in
determining the MIPS final score for each MIPS eligible clinician. In general, MIPS eligible
clinicians are evaluated under all four of the MIPS performance categories, including the cost
performance category.
We are proposing to add six new episode-based measures to the cost performance
category beginning with the CY 2025 performance period/2027 MIPS payment year. These six
measures include:
● Chronic Kidney Disease (CKD), which assesses MIPS eligible clinicians on the risk-
adjusted and specialty-adjusted cost to Medicare for patients who receive care to manage and
treat CKD stages 4 and 5;
● End-Stage Renal Disease (ESRD), which assesses MIPS eligible clinicians on the risk-
adjusted and specialty-adjusted cost to Medicare for patients who receive medical care to
manage ESRD;
● Kidney Transplant Management, which assesses MIPS eligible clinicians on the risk
adjusted and specialty-adjusted cost to Medicare for ongoing kidney transplant-related care and
management starting at least 90 days after transplant surgery;
● Prostate Cancer, which assesses MIPS eligible clinicians on the risk-adjusted and
specialty-adjusted cost to Medicare for the management and treatment of prostate cancer;
● Rheumatoid Arthritis, which assesses MIPS eligible clinicians on the risk-adjusted and
specialty-adjusted cost to Medicare for the management and treatment of rheumatoid arthritis;
and
● Respiratory Infection Hospitalization, which assesses MIPS eligible clinicians on the
risk-adjusted cost to Medicare for the inpatient treatment of respiratory infection.
We are proposing modifications to two existing episode-based measures so that their
specifications reflect reevaluated versions beginning with the CY 2025 performance period/2027
MIPS payment year. These two measures are:
● Cataract Removal with Intraocular Lens (IOL) Implantation, which assesses MIPS
eligible clinicians on the risk adjusted cost to Medicare for cataract removal procedures; and
● Inpatient (IP) Percutaneous Coronary Intervention (PCI), which assesses MIPS eligible
clinicians on the risk-adjusted cost to Medicare for the inpatient PCI treatment of patients who
present with a cardiac event.
We are proposing that MIPS eligible clinicians must be attributed a minimum of 20 cases
for each of the proposed six new measures. In addition, we are proposing to maintain the existing
case minimums for the two measures we are proposing to modify in this rulemaking, which are a
20-episode case minimum for the IP PCI measure and a 10-episode case minimum for the
Cataract Removal with IOL Implantation measure. We are also proposing to update the
operational list of care episode and patient condition groups and codes to reflect these new and
modified measures we are proposing.
Finally, we are proposing to adopt criteria to specify objective bases for the removal of
any cost measures from the MIPS cost performance category, which we are also proposing to
codify at § 414.1350(e).
For a description of the statutory authority for and existing policies pertaining to the cost
performance category, we refer readers to § 414.1350 and the CY 2017 Quality Payment
Program final rule (81 FR 77162 through 77177), CY 2018 Quality Payment Program final rule
(82 FR 53641 through 53648), CY 2019 PFS final rule (83 FR 59765 through 59776), CY 2020
PFS final rule (84 FR 62959 through 62979), CY 2021 PFS final rule (85 FR 84877 through
84881), CY 2022 PFS final rule (86 FR 65445 through 65461), CY 2023 PFS final rule (87 FR
70055 through 70057), and CY 2024 PFS final rule (88 FR 79339 through 79349).
More details on the proposals in this section, which we invite comments on, are provided
in section IV.A.4.e.(2)(a) through section IV.A.4.e.(2)(d) of this proposed rule. We also refer
readers to section V.B.8.k. of this proposed rule for discussion on the burden estimates for these
proposals.
(a) Proposed Updates to MIPS Episode-Based Measure Inventory
(i) Background on Episode-Based Measure Development and Use
Under § 414.1350(a), we specify cost measures for a performance period to assess the
performance of MIPS eligible clinicians on the cost performance category. There are currently
29 cost measures in the cost performance category for the CY 2024 performance period/2026
MIPS payment year, comprising of 27 episode-based measures covering a range of conditions
and procedures and two population-based measures. We worked with the measure development
contractor to identify the proposed six new episode-based measures through empirical analyses
and public comment. These measures cover clinical topics and MIPS eligible clinicians
practicing in certain specialties for whom there are currently limited or no applicable cost
measures. As such, these measures would help fill gaps in the cost performance category’s
measure set.
In addition, these measures would support the transition from traditional MIPS to MVPs
by allowing new MVPs to be created and enhancing existing MVPs. Further, the addition of
these measures would address interested parties’ feedback about the need for more clinically
refined episode-based measures in the cost performance category. The measures would also
increase the cost coverage of care episode and patient condition groups, moving closer towards
the statutory goal of covering 50 percent of expenditures under Medicare Parts A and B, as
specified under section 1848(r)(2)(i)(I) of the Act.
At a high level, episode-based measures represent the cost to the Medicare Program and
beneficiaries for the items and services furnished to Medicare beneficiaries during an episode.
They aim to compare MIPS eligible clinicians on the basis of the cost of care that is clinically
related to treatment and management of a Medicare beneficiary and provided during the
episode’s timeframe. Specifically, for such measures, we define and measure the cost of care for
the episode based on the allowed amounts on Medicare claims, which include both Medicare
trust fund payments and any applicable beneficiary deductible and coinsurance amounts. The
cost of care for these measures includes amounts paid under Medicare Parts A and B, and, on a
case-by-case basis, Medicare Part D that have been standardized to remove price variation from
non-clinical factors. The Parts A and B payment standardization methodology and the Part D
payment standardization methodology are available at https://resdac.org/articles/cms-price-
payment-standardization-overview. Information about how the Part D standardization
methodology incorporates rebates into standardized amounts is available at
https://www.cms.gov/files/document/2023-part-d-rebate-methodology.pdf. We refer the readers
to section IV.A.4.e.(2)(a)(iii) of this proposed rule for more information on the six episode-based
measures we are proposing.
In this proposed rule, we provide detail about the six new measures that we are proposing
to adopt in the cost performance category beginning with the CY 2025 performance period/2027
MIPS payment year. In section IV.A.4.e.(2)(a)(ii) of this proposed rule, we summarize the
timeline for development of these proposed measures, including engagement activities
undertaken by the measure development contractor. In section IV.A.4.e.(2)(a)(iii) of this
proposed rule, we summarize the proposed new measures that would be included in the cost
performance category beginning with the CY 2025 performance period/2027 MIPS payment
year. In section IV.A.4.e.(2)(a)(viii) of this proposed rule, we discuss the pre-rulemaking review
process these measures underwent. In section IV.A.4.e.(2)(b) of this proposed rule, we discuss
our proposal that MIPS eligible clinicians must be attributed a minimum of 20 cases for each of
these proposed measures to be assessed and scored on such measure.
(ii) Overview of Measure Development Process for New Episode-Based Measures
In this section, we describe the development process for the six proposed episode-based
measures.
Development of episode-based measures for the cost performance category must comply
with section 1848(q)(2)(B)(ii) of the Act, which provides that measures and activities specified
for a performance period include the measurement of resource use (cost) as provided under
section 1848(p)(3) of the Act, using the methodology under section 1848(r) of the Act, to
collaborate with physicians, practitioners, and other interested parties, as appropriate. Section
1848(p)(3) of the Act provides that costs shall be evaluated, to the extent practicable, based on a
composite of appropriate measures of costs established by the Secretary as further specified
therein. We note that the measure development contractor uses a “wave” approach to indicate
cycles of measure development where clinical expert panels convene to select episode groups to
develop into cost measures and to provide input on the measures’ specifications. All six of the
proposed measures have been developed with extensive engagement from interested parties,
including clinicians, persons with lived experience, and the general public. The term “persons
with lived experience,” as used in this section IV.A.4.e.(2) of this proposed rule, refers to
persons and family of persons who have experienced these conditions or diseases. Our approach
to engagement is outlined in the CY 2018 Quality Payment Program final rule (82 FR 53644 and
53645), the CY 2019 PFS final rule (83 FR 59767 through 59769), the CY 2022 PFS proposed
rule (86 FR 39396 and 39397), and the CY 2024 PFS proposed rule (88 FR 52568 through
52576). These processes have been refined over time to incorporate feedback from interested
parties, such as to extend the development timeline from 12 months in Wave 2 to 18 months in
Waves 3 and 4, and to integrate conversations between persons with lived experience and
clinical experts.
We began development of the six episode-based measures across several of these waves:
● The Prostate Cancer, Rheumatoid Arthritis, and Kidney Transplant Management
measures were initiated in Wave 5 in 2022 and followed the typical 18-month process.
● The CKD and ESRD measures were initiated in 2021 as part of an off-cycle
development process and were incorporated into Wave 5 in 2022 to be completed along with the
other three episode-based measures (that is, Prostate Cancer, Rheumatoid Arthritis, and Kidney
Transplant Management).
● The Respiratory Infection Hospitalization measure is a revised version of the Simple
Pneumonia with Hospitalization measure that was initially developed in Wave 1 in 2018. The
Simple Pneumonia with Hospitalization measure was adopted in MIPS in the CY 2019 PFS final
rule (83 FR 59767 through 59773) and then removed from use in MIPS in the CY 2024 PFS final
rule (88 FR 79348 and 79349). The measure underwent a comprehensive reevaluation process
from 2022 - 2023 to identify and incorporate refinements based on public feedback, clinical
input, and empiric testing. Unlike the other five new measures we are proposing, this measure
did not undergo further development as part of Wave 5. Because the Simple Pneumonia with
Hospitalization measure is no longer in use in MIPS, we are proposing the Respiratory Infection
Hospitalization measure as a new measure, and not as modifications to an existing measure.
More information on the comprehensive reevaluation process that this measure underwent is
described in sections IV.A.4.e.(2)(a)(iv) and IV.A.4.e.(2)(a)(v) of this proposed rule.
This section of this proposed rule will provide more details on the development process
for the measures that were developed during Wave 5 (CKD, ESRD, Kidney Transplant
Management, Prostate Cancer, and Rheumatoid Arthritis measures). We began developing the
CKD and ESRD measures in 2021 as priority clinical areas by convening a clinician expert
workgroup and gathering feedback from persons with lived experience. However, the clinician
expert workgroup recommended that we develop a Kidney Transplant Management measure to
fully capture the care continuum of kidney disease care before continuing to move forward with
the CKD and ESRD measures. As a result, we halted measure development on the CKD and
ESRD measures until 2022, while we gathered public feedback on the development of a Kidney
Transplant Management measure, and later incorporated the CKD and ESRD measures into the
Wave 5 development process.
The episode-based measures that were developed in the Wave 5 cycle of measure
development (CKD, ESRD, Kidney Transplant Management, Prostate Cancer, and Rheumatoid
Arthritis measures) underwent an 18-month development process. As a first step, the measure
development contractor held a public comment period from February to April 2022 to gather
feedback on which clinical areas to prioritize for measure development. The public comment
period solicited feedback on the importance and feasibility of prioritizing kidney transplant
management, prostate cancer, and rheumatoid arthritis, among other clinical areas, for measure
development. During the public comment period, the measure development contractor received
32 comments on the candidate episode groups for development in Wave 5. We used this
feedback, in conjunction with empirical testing by the measure development contractor, to
inform our decision to develop measures for three specific clinical areas – prostate cancer,
rheumatoid arthritis, and kidney transplant management – into episode-based measures. The
summary of the public comments is available in this document
https://www.cms.gov/files/document/wave-5-public-comment-summary-report.pdf. We selected
these clinical areas for measure development for several reasons including: representation of
high priority clinical areas, potential to improve clinician performance or patient outcomes,
potential to capture high costs of care paid by the Medicare Program, and ability to fill
measurement gaps in the MIPS cost performance category and MVPs.
Following our decision to develop measures for prostate cancer, rheumatoid arthritis, and
kidney transplant management, in addition to continuing to develop the CKD and ESRD
measures, the measure development contractor convened four clinician expert panels, comprised
of a total of 45 members, affiliated with 36 organizations and specialty societies. The CKD and
ESRD measures shared a clinician expert panel due to the close clinical overlap of these
diseases. Each panel also incorporated the perspective of persons with lived experience; their
input is collected via structured focus groups, interviews, or surveys, and then summarized and
presented to the clinical expert panels.
Then, the measure development contractor held a national field testing period from
January 17, 2023, to February 14, 2023 for the Wave 5 measures (CKD, ESRD, Kidney
Transplant Management, Prostate Cancer, and Rheumatoid Arthritis measures). During this field
testing period, individual MIPS eligible clinicians and groups meeting a minimum threshold of
20 episodes for each measure could review field test reports and an episode-level file with
detailed information to understand the types of services that comprise a large or small share of
their episode costs. Supplemental materials, such as testing information on measures, a
Frequently Asked Questions document, and mock field test reports were posted publicly for
interested parties’ review. The measure development contractor gathered feedback via survey
and a summary of this feedback from the field testing period is available at
https://www.cms.gov/files/document/2023-field-testing-feedback-summary-report.pdf.
The measure development contractor also has a standing technical expert panel (TEP),
which is referred to as the Physician Cost Measures and Patient Relationship Codes (PCMP)
TEP. The PCMP TEP is composed of 20 members from different clinical areas, academia,
health care and hospital administration, and persons with lived experience, which provides
overarching input on cross-measure topics, such as testing approaches and methodology. For
example, the PCMP TEP discussed general challenges in developing chronic condition episode-
based measures and ways that the chronic condition framework can address those challenges,
provided feedback on the attribution rules (that is, the algorithms and the types of codes used in
each algorithm) that would demonstrate a relationship between a clinician group and a patient
with a chronic condition(s), and discussed service assignment, risk adjustment, and exclusions,
which support the overall development of episode-based measures, including, but not limited to
those episode-based measures being proposed in this rulemaking. This input helped inform the
specifications for the chronic condition episode-based measure framework, which serves as the
framework for the chronic condition episode-based measures (that is, Prostate Cancer,
Rheumatoid Arthritis, CKD, ESRD, and Kidney Transplant Management episode-based
measures) developed in Wave 5 and being proposed in this proposed rule.
More information about the measure development and interested parties’ engagement
process for the six episode-based measures we are proposing for adoption in the cost
performance category is available in materials on the QPP Cost Measure Information page at
https://www.cms.gov/medicare/quality/value-based-programs/cost-measures. Summaries of the
public comment period and clinician expert workgroup meetings organized by the measure
development contractor are also available on the QPP Cost Measure Information page at
https://www.cms.gov/medicare/quality/value-based-programs/cost-measures. Descriptions of the
methods through which the measure development contractor gathered expert input during
measure development and other interested parties’ engagement activities is available in the
“2024 MIPS Summary of Cost Measures” document at
https://www.cms.gov/files/document/2024-mips-summary-costs-measures.pdf.
(iii) Description of Six New Episode-Based Measures Proposed for Adoption Beginning with the
CY 2025 Performance Period/2027 MIPS Payment Year
In this section of this proposed rule, we discuss the six new episode-based measures,
which we propose to add to the cost performance category beginning with the CY 2025
performance period/2027 MIPS payment year.
In conjunction with our measure development contractor, we developed these measures
with consideration of the common standards that are described in the CY 2022 PFS final rule (86
FR 65455 through 65459) to ensure consistency across episode-based measures being developed.
Specifically, the CY 2022 PFS final rule requires that any episode-based measure for the cost
performance category include the following: (1) episode definition based on trigger codes that
determine the patient cohort; (2) attribution; (3) service assignment; (4) exclusions; and (5) risk
adjustment. The six new episode-based measures we are proposing meet all requirements
described in CY 2022 PFS final rule, including these features. We provide more information on
the specific requirements for each of the episode-based measures later in this section of this
rulemaking.
Generally, for all episode-based measures, we exclude episodes where costs cannot be
fairly compared to the costs for the whole cohort in the episode-based measure. These
exclusions, like other features of each episode-based measure, are developed with extensive
clinician and interested parties’ engagement. We have specified exclusions for all six proposed
episode-based measures.
We also apply a risk adjustment model to all episode-based measures in the cost
performance category. The model includes standard risk adjustors that are applied to all episode-
based measures (for example, CMS Hierarchical Condition Category [HCC] variables,
comorbidities, age brackets, disability status, ESRD status), and measure-specific risk adjustors
(for example, for the Rheumatoid Arthritis episode-based measure, rheumatoid arthritis severity,
fractures, frailty, and cognitive status). We assess the risk adjustment model at the level of each
stratification to ensure that only like patients are compared to each other. The risk adjustment
model we use in development of the cost performance category’s episode-based measures is

described in detail in CY 2019 PFS final rule (83 FR 59767 through 59773). As mentioned
previously in this section, all six proposed episode-based measures have been risk adjusted in
accordance with this model.
More information on the episode-based measure development requirements, which were
outlined so that external interested parties could develop measures in the future, are available in
the Blueprint for the CMS Measures Management System
(https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/MMS-
Blueprint) and the Meaningful Measures Framework (https://www.cms.gov/Medicare/Quality-
Initiatives-Patient-Assessment-Instruments/QualityInitiativesGenInfo/MMF/General-info-Sub-
Page).
The episode-based measures that we are proposing for adoption beginning with the CY
2025 performance period/2027 MIPS payment year are set forth in Table 58.
TABLE 58: Proposed Episode-Based Measures Beginning with CY 2025 Performance
Period/2027 MIPS Payment Year
Measure Name
Episode Type
Chronic Kidney Disease (CKD)
Chronic condition
End-Stage Renal Disease (ESRD)
Chronic condition
Kidney Transplant Management
Chronic condition
Prostate Cancer
Chronic condition
Rheumatoid Arthritis
Chronic condition
Respiratory Infection Hospitalization
Acute inpatient medical condition
The five chronic condition episode-based measures assess outpatient treatment and
ongoing management of the following chronic conditions: CKD, ESRD, kidney transplant
management, prostate cancer, and rheumatoid arthritis. These measures assess the costs of
services related to these conditions, such as physician services, imaging or diagnostic services,
emergency room care or hospitalizations, medications, or other services related to ongoing
management or post-acute care. The measure construction for these proposed measures follows
the approach described in the CY 2022 PFS final rule (86 FR 65445 through 65461), which also
includes detailed discussion of the attribution methodology and examples of how episodes are
attributed.
To briefly summarize, the attribution methodology that identifies a clinician-patient care
relationship for these chronic condition episode-based measures is slightly different at the
clinician group and individual MIPS eligible clinician levels, to reflect that care provided at the
clinician group and individual MIPS eligible clinician levels, respectively. At a high level, these
proposed chronic condition episode-based measures attribute episodes to the clinician group that
renders services that constitute a trigger event, which is identified by the occurrence of two
claims billed in close proximity by the same clinician group. Both claims must have a diagnosis
code indicating the same chronic condition related to the specific episode-based measure. For
example, for the Prostate Cancer measure, both claims of the trigger event must have a diagnosis
indicating prostate cancer. The services that trigger an event for these chronic condition episode-
based measures are identified first by Evaluation and Management (E/M) codes for outpatient
services, and then by a second claim with either another E/M code for outpatient services or a
condition-related Current Procedural Terminology (CPT)/Healthcare Common Procedure
Coding System (HCPCS) code (CPT/HCPCS) related to the treatment or management of the
chronic condition. Note, some measures also use additional CPT/HCPS codes with a relevant
diagnosis as the initial trigger service. The trigger event opens a year-long attribution window
from the date of the initial E/M outpatient service, during which the same clinician group could
reasonably be considered responsible for managing the patient’s chronic condition. If we see
evidence that the relationship is ongoing, represented by another E/M or condition-related
procedure code that we refer to as the reaffirming claim, then this window can be extended.
For individual MIPS eligible clinicians, we would attribute episodes to each individual
MIPS eligible clinician within an attributed clinician group that renders at least 30 percent of
trigger or reaffirming codes on Part B Physician/Supplier claim lines during the episode, such as
office visits or diagnostic services. We also apply conditions to ensure the MIPS eligible
clinicians to whom the episode is attributed are reasonably responsible for the management of
the patient’s chronic condition. Specifically, the MIPS eligible clinician must have provided
condition-related care to this patient prior to or on the episode start date.
Additionally, for some measures, we use provider-level prescription billing patterns to
ensure that we are capturing the MIPS eligible clinicians directly involved in providing ongoing
chronic care management. Specifically, for some measures, the MIPS eligible clinician must also
have prescribed at least two prescriptions claimed under Medicare Part D and/or Medicare Part B
related to the management of the condition for two different patients during the measurement
period, plus a one-year lookback period. These conditions, which we use to attribute a cost
measure to MIPS eligible clinicians, also apply to the attribution methodology at the clinician
group level. Specifically, the clinician group would always meet the first condition by
construction (that is, there would always be an individual MIPS eligible clinician under the
clinician group that has provided care to the patient prior to or on the episode start date).
However, the clinician group must have at least one potentially attributable MIPS eligible
clinician under it who meets the second condition.
More information about the chronic condition episode-based measures attribution
methodology, including a one-page summary and a Frequently Asked Questions (FAQ)
document, is available at https://www.cms.gov/files/zip/mips-chrcondition-episode-based-cost-
measures-attribution-methodology-2023-zip.zip. More general information about the overall
chronic condition cost measure framework is available at
https://www.cms.gov/files/document/chronic-condition-cost-measure-framework-poster.pdf.
The Respiratory Infection Hospitalization measure is an acute inpatient medical condition
episode-based measure, which focuses on the inpatient treatment of respiratory infection and is
attributed to clinicians and clinician groups treating a patient during the hospitalization. It
includes the cost of services related to the inpatient treatment of a respiratory infection, such as
initial inpatient services, subsequent outpatient physician visits, and emergency room care or
hospitalizations for related complications. As noted in section IV.A.4.e.(2)(a)(ii) of this proposed

rule, the Respiratory Infection Hospitalization measure is the reevaluated version of the Simple
Pneumonia with Hospitalization measure, adopted for MIPS in CY 2019 PFS final rule (83 FR
59767 through 59773) and removed from MIPS in the CY 2024 PFS final rule (88 FR 79348 and
79349). We developed the Simple Pneumonia with Hospitalization acute inpatient medical
condition measure in accordance with the previously established framework for episode-based
measures, which we described in detail in the CY 2019 PFS final rule (83 FR 59769 through
59771). The Simple Pneumonia with Hospitalization measure was reevaluated following the
process described in sections IV.A.4.e.(2)(a)(iv), IV.A.4.e.(2)(a)(v), and IV.A.4.e.(2)(a)(viii) of
this proposed rule. We are proposing the Respiratory Infection Hospitalization measure, the
reevaluated version of the Simple Pneumonia with Hospitalization measure, because we continue
to believe it is important to capture pneumonia and other respiratory infections, as pneumonia is
a leading infectious cause of hospitalization and death among adults in the United States.
745
This
new, revised measure addresses the concerns with the previous version of the measure by
expanding the patient cohort to include beneficiaries hospitalized for pneumonia and related
respiratory infections, reflecting the coding changes as described in the CY 2024 PFS final rule
(88 FR 79348 and 79349). The revised measure also incorporates feedback we received from
interested parties about appropriate risk adjustment and exclusions during the reevaluation
process of the prior Simple Pneumonia with Hospitalization measure.
The specifications for all six episode-based measures we are proposing for adoption in
this rulemaking are available at https://www.cms.gov/medicare/quality/value-based-
programs/cost-measures. The specifications documents for each measure consist of a methods
document that describes the steps for constructing the measure and a measure codes list file that
contains the medical codes used in that methodology. First, the methods document provides
detailed methodology describing each step to construct the measure, including: identifying
745
Regunath, Hariharan, and Yuji Oba. “Community-Acquired Pneumonia.” National Library of Information,
August 8, 2022. https://www.ncbi.nlm.nih.gov/books/NBK430749.
patients receiving care; defining an episode-based measure; attributing episodes to MIPS eligible
clinicians and clinician groups; assigning costs; defining exclusions; risk adjusting; and
calculating measure score. Second, the measure codes list file contains the codes used in the
measure specifications, including the episode triggers, attribution, stratification, assigned items
and services, exclusions, and risk adjustors.
More information about the episode-based measures is available in the measure
justification forms, which were posted to support Pre-rulemaking Measure Review (PRMR)
discussions. These documents provide a comprehensive characterization of the measures, their
justification, and testing results of the measures’ specifications at this time. These documents are
available through the QPP Cost Measure Information page at
https://www.cms.gov/medicare/quality/value-based-programs/cost-measures.
(iv) Background on Episode-Based Measure Reevaluation and Maintenance
In this section, we describe the measure maintenance process we use, in conjunction with
our measure development contractor, to monitor, evaluate, and potentially modify cost measures.
Using this maintenance process, we holistically reviewed, and evaluated whether to modify or
take other actions with respect to, certain measures in the cost performance category. As a result
of this process, we propose to modify two episode-based measures currently in use (the Routine
Cataract Removal with Intraocular Lens (IOL) Implantation and ST-Elevation Myocardial
Infarction (STEMI) Percutaneous Coronary Intervention (PCI) measures), and propose the
reevaluated Respiratory Infection Hospitalization as a new measure, replacing the Simple
Pneumonia with Hospitalization measure previously removed from the cost performance
category.
The measure development contractor routinely conducts three types of measure
maintenance: measure monitoring, annual measure specifications updates, and comprehensive
reevaluation. Comprehensive reevaluation occurs every 3 years after a measure is implemented
in MIPS. The purpose of comprehensive reevaluation is to ensure that measures continue to meet
criteria for importance, scientific acceptability, and usability in line with the CMS Measures
Management System Blueprint (https://mmshub.cms.gov/blueprint-measure-lifecycle-overview).
In this process, we holistically review the measure, seek public comment, and consider whether
any changes need to be made to measure specifications. A new version of the measure may be
considered for implementation in MIPS in future years, after undergoing the pre-rulemaking and
the notice-and-comment rulemaking processes. For more information on recent reevaluation
efforts, please refer to the documents under the “Wave 1 cost measure comprehensive
reevaluation (2022-2023)” section of the QPP Cost Measure Information page at
https://www.cms.gov/medicare/quality/value-based-programs/cost-measures/prior.
For the purpose of assessing performance of MIPS eligible clinicians in the cost
performance category, we finalized, in the CY 2019 PFS final rule (83 FR 59767 through
59773), the Routine Cataract Removal with Intraocular Lens (IOL) Implantation, ST-Elevation
Myocardial Infarction (STEMI) Percutaneous Coronary Intervention (PCI), and Simple
Pneumonia with Hospitalization measures to be included in MIPS beginning with the CY 2019
performance period/2021 MIPS payment year. In this section, we are proposing to modify the
Routine Cataract Removal with IOL Implantation and STEMI PCI measures based on input from
interested parties from prior public comment periods and recommendations from Clinician
Expert Workgroups.
We also propose to modify the measure titles. Routine Cataract Removal with Intraocular
Lens (IOL) Implantation would be revised to Cataract Removal with Intraocular Lens (IOL)
Implantation to reflect substantive changes to the measure exclusions. ST-Elevation Myocardial
Infarction (STEMI) Percutaneous Coronary Intervention (PCI) would be revised to Inpatient (IP)
Percutaneous Coronary Intervention (PCI) to reflect the inclusion of patients with a non-STEMI
PCI diagnosis and patients with a PCI diagnosis but neither STEMI nor non-STEMI diagnoses.
The episode-based measures that we propose to modify beginning with the CY 2025
performance period/2027 MIPS payment year are listed in the Table 59.

TABLE 59: Proposed Reevaluated Episode-Based Measure Modifications Beginning with
CY 2025 Performance Period/2027 MIPS Payment Year
Original Measure Name
Revised Measure Name
Episode Type
Routine Cataract Removal with
Intraocular Lens (IOL)
Implantation
Cataract Removal with
Intraocular Lens (IOL)
Implantation
Procedural
ST-Elevation Myocardial
Infarction (STEMI) Percutaneous
Coronary Intervention (PCI)
Inpatient (IP) Percutaneous
Coronary Intervention (PCI)
Acute inpatient medical
condition
(v) Overview of Measure Reevaluation Process for Episode-Based Measures
As part of the reevaluation process, we worked with the measure development contractor
to gather feedback from interested parties on potential refinements to the measures. The measure
development contractor worked with a standing TEP, Clinician Expert Workgroups, and held
two public comment periods.
Initially, a public comment period was held from February to May 2022 to gather
feedback on which of the Wave 1 measures implemented in MIPS in 2019 that the measure
developer should prioritize reevaluating. A summary of public comments from the 2022 public
comment period can be found in the Wave 1 Comprehensive Reevaluation Public Comment
Summary Report at https://www.cms.gov/files/document/wave-one-public-comment-summary-
report.pdf.
The measure development contractor then convened the TEP for a meeting in August
2022 to provide input on the comprehensive reevaluation process for all Wave 1 episode-based
measures. In October 2022, the measure development contractor convened the Cataract Removal
with IOL Implantation Clinician Expert Workgroup, the IP PCI Clinician Expert Workgroup,
and the Respiratory Infection Hospitalization Clinician Expert Workgroup, to provide detailed
clinical input on measure specifications for each reevaluated measure. This input was informed
by empiric testing conducted by the measure development contractor. The workgroup
discussions from October 2022 can be found in the Summary of Wave 1 Comprehensive
Reevaluation Workgroup meetings at https://www.cms.gov/files/zip/summary-wave-1-
comprehensive-reevaluation-workgroup-meetings.zip.

After this, a second public comment period was held in February 2023 to gather feedback
on the draft revisions the measure developer made to the Cataract Removal with IOL
Implantation, IP PCI, and Respiratory Infection Hospitalization measures. A summary of public
comments from the 2023 public comment period can be found in the 2023 Revised Cost Measure
Feedback Period Summary Report at https://www.cms.gov/files/document/2023-revised-cost-
measure-feedback-period-summary-report.pdf.
The measure-specific expert workgroups then reviewed the feedback received through
public comments and provided input on proposed decisions regarding the measure specifications.
The workgroup discussions from Spring 2023 are available in the Summary of Wave 1 Post-
Feedback Period Workgroup meetings at https://www.cms.gov/files/zip/summary-wave-1-post-
feedback-period-pfr-workgroup-meetings-23.zip. After the proposed specifications for each
measure were finalized, we submitted the three revised measures (Cataract Removal with IOL
Implantation, IP PCI, and Respiratory Infection with Hospitalization measures) to the 2023 MUC
list and underwent review by the PRMR Clinician Committee and Clinician Committee
Recommendation Group, which reviewed the measures for use in MIPS. We provide more
information about this discussion in section IV.A.4.e.(2)(a)(viii) of this proposed rule.
(vi) Description of Proposed Modifications to the Routine Cataract Removal with Intraocular
Lens (IOL) Implantation Measure with the newly titled Cataract Removal with IOL Implantation
Measure
We finalized the Routine Cataract Removal with Intraocular Lens (IOL) Implantation
cost measure in the CY 2019 PFS final rule (83 FR 59767 through 59773) for use in MIPS as an
important measure of clinician cost performance, as cataract surgery is the most common
surgical procedure in the United States.
746
We continue to believe that the existing measure is
appropriate to use in MIPS. When we finalized this measure for use in MIPS, we noted that as
746
Pershing, S., D. E. Morrison, and T. Hernandez-Boussard. “Cataract Surgery Complications and Revisit Rates
among Three States.” [In eng]. Am J Ophthalmol 171 (Nov 2016): 130-38.
with all the cost measures, we would maintain this measure and update its specifications as
appropriate (83 FR 59766 and 59767). As a part of our routine measure maintenance, we
reevaluated the Routine Cataract Removal with IOL Implantation measure with consideration of
input from interested parties and testing. This feedback from interested parties included:
● The patient cohort for the measure should be expanded, while still accounting for
patient heterogeneity, to expand clinician and beneficiary coverage.
● The measure should account for patients with ocular conditions that impact case
complexity to account for patient heterogeneity.
● Certain Medicare Part B medication costs related to cataract procedures should be
included in the measure, while Medicare Part D medication costs should remain excluded to
better account for more clinically-related medication costs.
● The measure should more broadly capture services associated with cataract removal.
In response to this feedback, CMS and the measure development contractor conducted
additional empirical analyses and discussed potential modifications with the measure-specific
expert workgroup. Summaries of these discussions are available here:
https://www.cms.gov/medicare/quality/value-based-programs/cost-measures/prior. Based on
these testing results and the clinician expert input, we are proposing to replace the Routine
Cataract Removal with IOL Implantation with the revised Cataract Removal with IOL
Implantation cost measure. The modifications that we are proposing to incorporate into the
revised Cataract Removal with IOL Implantation measure and our rationale is outlined as
follows.
First, we propose to modify this cost measure by expanding the patient cohort based on
changes to the exclusion criteria. Testing has shown that many episodes excluded due to ocular
conditions had similar cost profiles, compared to episodes included in the measure, and
represented a significant portion of triggered episodes. The measure-specific expert workgroup
discussed the appropriateness of the original exclusion criteria and recommended potential
revisions. The proposed revised measure includes patients with certain previously excluded
ocular conditions, such as glaucoma and macular degeneration, in the measure cohort because of
their similar cost profiles. In response to expanding the measure cohort, we also propose updates
to the risk adjustment model to risk adjust for ocular conditions that are no longer excluded but
may still impact case complexity and episode costs. We believe that these changes are
appropriate as they further account for patient heterogeneity in the more clinically diverse patient
cohort. However, the proposed revised measure continues to exclude episodes for patients with
significant ocular conditions impacting surgical complication rate or visual outcomes because
testing did not suggest they had similar enough cost profiles for any expected cost differences to
be accounted for through risk adjustment.
Second, we propose to modify this cost measure’s service assignment specifications in
two ways, to include: (1) certain clinically-related telehealth services, pre-operative testing,
emergency department (ED) visits for ocular complaints, and postoperative durable medical
equipment (DME); and (2) certain additional clinically-related Medicare Part B medication costs
that were not initially included in the measure. The previous version of the measure included a
smaller subset of these services. However, testing showed that additional clinically-related
services within these categories occur during Cataract Removal episodes and exclusion of these
services from the measure could result in failure to capture important costs. We are proposing
to include the additional services because we believe this change would retain the original intent
of the measure while capturing a more complete picture of cost performance variation.
Additionally, we are proposing to expand the types of Part B medications assigned to the
measure because we believe it would be appropriate to use similar service assignment rules for
all clinically-related Part B medications.
Further details about the revised Cataract Removal with IOL Implantation measure are
available in the measure specifications documents, which are available at
https://www.cms.gov/medicare/quality/value-based-programs/cost-measures.

(vii) Description of the Proposed Modifications to the ST-Elevation Myocardial Infarction
(STEMI) with Percutaneous Coronary Intervention (PCI) measure with the newly titled Inpatient
(IP) Percutaneous Coronary Intervention (PCI) Measure
Similar to the Cataract Removal with IOL Implantation measure, we finalized the ST-
Elevation Myocardial Infarction (STEMI) Percutaneous Coronary Intervention (PCI) measure in
the CY 2019 PFS final rule (83 FR 59767 through 59773) for use in MIPS as an important
measurement of clinician cost performance, due to the high frequency and cost of PCI
procedures in the United States. PCI is one of the most common procedures for cardiac events
(AMIs) and is associated with $10 billion in health care costs annually.
747
We continue to believe
that the existing measure is appropriate for use in MIPS. However, when we finalized this
measure for use in MIPS, we noted that, as with all the cost measures, we would maintain this
measure and update its specifications as appropriate (83 FR 59766 and 59767). As a part of our
routine measure maintenance, we reevaluated the STEMI PCI measure with consideration of
input from interested parties and testing. This feedback from interested parties included:
● The patient cohort for the measure should be expanded, while still accounting for
patient heterogeneity to increase clinician coverage and capture more clinically-related services.
● The measure should account for patients with cardiac arrest and patients who smoke
tobacco to reduce factors outside of clinician control that influence measure performance.
In response to this feedback, CMS and the measure development contractor conducted
additional empirical analyses and discussed potential modifications with the measure-specific
Clinician Expert Workgroup. Summaries of these discussions are available here:
https://www.cms.gov/medicare/quality/value-based-programs/cost-measures/prior. Based on
these testing results and the clinician expert input, CMS is proposing to replace the STEMI PCI
747
Amin, Amit P., Patterson, Mark, House, John A., Giersiefen, Helmut, Spertus, John A., Baklanov, Dmitri V.,
Chhatriwalla, Adnan K., Safley, David M., Cohen, David J., Rao, Sunil V., Marso, Steven., "Cost Associated With
Access Site and Same-Day Discharge Among Medicare Beneficiaries Undergoing Percutaneous Coronary
Intervention: An Evaluation of the Current Percutaneous Coronary intervention Care Pathways in the United States,"
JACC Cardiovasc Interv 10, no. 4 (2017): 342-351.
measure with the revised IP PCI measure. The modifications that we are proposing to
incorporate into the revised IP PCI measure and our rationale is outlined as follows.
First, we propose to modify this cost measure by expanding the patient cohort based on
changes to the triggering logic. The previous version of the measure narrowly defined a subset of
STEMI PCI patients to promote homogeneity of the patient cohort. However, testing
demonstrated that PCI episodes with and without STEMI appear to have similar cost profiles and
involve similar clinician types. Therefore, it is appropriate to expand the patient cohort in the
proposed revised measure to include episodes beyond those with STEMI diagnoses, such as PCI
for non-STEMI diagnoses and PCI without either STEMI or non-STEMI diagnoses. As such, we
would no longer use ICD-10 diagnosis information to restrict assessment of costs under this
measure to only inpatient PCI procedures with a STEMI diagnosis. This change would increase
the number of clinicians and beneficiaries for whom this cost measure would be applicable.
Second, we are proposing to modify this cost measure to include additional sub-groups to
stratify the patient cohort based on diagnosis to account for variations in cost and treatment
pathways for inpatient procedures. While there are overall similarities between the diagnosis for
inpatient PCI episodes (that is, STEMI, non-STEMI, and other inpatient PCI episodes), there are
still expected differences in observed costs between these cohorts. This modification would
allow us to assess variation in clinician cost performance rather than expected cost differences
due to patient diagnoses. We believe this is appropriate as testing shows differences in observed
episode costs among STEMI, non-STEMI, and other inpatient PCI episodes are neutralized via
sub-grouping and risk adjustment.
Third, we are proposing that the proposed revised measure excludes episodes with
cardiac arrest and risk adjusts for patients with a history of tobacco use to further address
heterogeneity in the patient cohort, as these cases can result in more complex treatment and
higher observed costs for reasons outside of the control of the attributed clinician. This was
supported by testing on the expanded patient cohort.
Further detail about the revised IP PCI measure is included in the measure specifications
documents, which are available at https://www.cms.gov/medicare/quality/value-based-
programs/cost-measures.
(viii) Pre-Rulemaking Measure Review Process
After these extensive measure development and refinement activities, we included the six
proposed new episode-based measures and two modified episode-based measures on our 2023
Measures Under Consideration (MUC) List (available for download at
https://mmshub.cms.gov/sites/default/files/2023-MUC-List.xlsx) to be considered for potential
use in MIPS. The measures then underwent PRMR during the 2023 – 2024 review cycle. This
process involved reviews by the PRMR Clinician Committee advisory and recommendation
workgroups, as well as two public comment periods. More details on the PRMR process are
available at https://p4qm.org/PRMR.
Although we may pursue endorsement by the consensus-based entity (CBE), contracted
in accordance with section 1890 of the Act, for the proposed measures at a later time, we are not
required to use only CBE endorsed measures in MIPS. We emphasize that cost measures
undergo extensive review and testing before they are implemented in MIPS. As described in
section IV.A.4.e.(2)(a)(ii), the proposed new and revised cost measures in this proposed rule
were vetted by clinicians, specialty societies, persons with lived experience, consensus panels,
and the public at large without going through the CBE endorsement process. Cost measures are
developed through a rigorous process with a Clinician Expert Workgroup providing detailed
input on each aspect of the specifications, reviewing iterative empirical testing, and considering
the input from persons with lived experience. The cost measures undergo field testing during the
development process where MIPS eligible clinicians can download feedback reports to review
their performance before measure implementation, and share input with the developer to help
finalize the measure specifications. In addition, the proposed new and revised cost measures in
this proposed rule were reviewed through the pre-rulemaking process described in this section of
this proposed rule. We continue to believe in the strength of the episode-based measures,
proposed for adoption and modification in this rulemaking, based on valid and reliable testing
results and extensive review from interested parties as part of the measure development and
implementation process.
The PRMR Clinician Committee and Clinician Committee Recommendation Group
reviewed the measure information, a preliminary analysis of the measures and their testing
information developed by the CBE, and public comments. The PRMR Clinician Committee
Recommendation Group met in January 2024 to discuss the measures in more detail and vote on
their recommendations for the appropriateness of these measures’ use in MIPS. The final result
from the committee’s vote can be: Recommend, Recommend with conditions, Do not
recommend, or Consensus not reached. Consensus not reached signals continued disagreement
among the committee despite being presented with perspectives from public comment,
committee member feedback and discussion, and highlights the multi-faceted assessments of
measures. More details regarding the CBE PRMR voting procedures may be found in Chapter 4
of the Guidebook of Policies and Procedures for Pre-Rulemaking Measure Review and Measure
Set Review at https://p4qm.org/sites/default/files/2023-09/Guidebook-of-Policies-and-
Procedures-for-Pre-Rulemaking-Measure-Review-%28PRMR%29-and-Measure-Set-Review-
%28MSR%29-Final_0.pdf.
The PRMR had mixed recommendations for the episode-based measures we are
proposing to adopt and modify (in section IV.A.4.e.(2)(a)(ix)) in this rulemaking.
The PRMR Clinician Committee Recommendation Group voted to “recommend with
conditions” for the revised Cataract Removal with IOL Implantation and IP PCI measures. For
the Cataract Removal with IOL Implantation measure, while the committee did not provide or
explain its conditions, they generally suggested further examination of how implementation of
cost measures may impact patient outcomes. We plan to examine patient outcomes in more detail
as part of routine measure monitoring. For the IP PCI measure, the committee recommended
with the two conditions: CBE endorsement and additional testing to gather performance data
over a longer period. As previously discussed, CBE endorsement is not required for measures to
be adopted and used in MIPS. We plan to address these conditions by monitoring the IP PCI
measure’s performance over time.
The PRMR Clinician Committee Recommendation Group did not reach consensus on the
CKD, ESRD, Kidney Transplant Management, Prostate Cancer, and Respiratory Infection
Hospitalization measures. Overall, there was varied levels of support of the measures across the
PRMR Clinician Committee Recommendation Group. The main concerns raised were related to
scientific acceptability (for example, testing results suggesting low reliability for the CKD,
ESRD and Kidney Transplant Management measures), appropriateness of risk adjustment, and
potential unintended consequences. We recognize the Recommendation Group’s concerns, but
emphasize the tradeoffs between accuracy and reliability, as described in the CY 2022 PFS final
rule (86 FR 65453 through 65456). However, we did work with the measure development
contractor to conduct additional testing to address these concerns. We identified non-substantive
updates to existing outlier exclusions applied to cost measures that would address concerns about
measure reliability for the CKD, ESRD, and Kidney Transplant Management measures. These
adjusted outlier exclusions substantially improve reliability and are reflected in Table 60 of this
proposed rule. We note that all the proposed new and revised measures meet the 0.4 mean
reliability threshold that CMS typically applies for measures’ use in MIPS. The
Recommendation Group also expressed a strong interest in the measures undergoing CBE
endorsement. As noted above, we may pursue CBE endorsement of these measures in the future,
but it is not a requirement for measures to be adopted and used in MIPS.
The voting results of the PRMR Clinician Committee Recommendation Group for the
Rheumatoid Arthritis measure were “do not recommend.” The PRMR final recommendations
state that the Recommendation Group supported the measure concept, but ultimately had
reservations about its use in MIPS and concerns about measure performance. These concerns

aligned with the concerns the Recommendation Group raised about cost measures overall, such
as concerns around how cost measures impact patient outcomes. More information on the PRMR
recommendations is available in the PRMR 2023 Final Recommendation Report:
https://p4qm.org/sites/default/files/2024-02/PRMR-2023-MUC-Recommendations-Report-Final-
.pdf.
We acknowledge the feedback from the Recommendation Group regarding the
Rheumatoid Arthritis measure, but we disagree that the measure should not be used in MIPS. We
agree with the Recommendation Group’s feedback regarding the importance of this measure to
assess and address wide variation in cost performance for rheumatoid arthritis treatment and
management. Literature states that rheumatoid arthritis is highly prevalent and is most common
among older adults.
748,749
Given this high prevalence, rheumatoid arthritis is costly to the
healthcare system and individual patients, though costs vary based on treatment regimen.
750
Over
the past decade, Medicare costs associated with disease-modifying treatments have increased
substantially.
751
Additionally, certain treatment choices for Rheumatoid Arthritis are associated
with increased costs due to adverse outcomes associated with these choices, suggesting room for
improvement in cost performance and patient care. For example, chronic glucocorticoid use
among rheumatoid arthritis patients is associated with higher health care costs due to increased
occurrence of adverse events (e.g., developing diabetes or osteoporosis, cardiovascular events
748
Centers for Disease Control and Prevention, “Rheumatoid Arthritis (RA),” 2020,
https://www.cdc.gov/arthritis/basics/rheumatoid-arthritis.html.
749
Hunter TM, Boytsov NN, Zhang X, Schroeder K, Michaud K, Araujo AB. Prevalence of rheumatoid arthritis in
the United States adult population in healthcare claims databases, 2004-2014. Rheumatol Int. 2017;37(9):1551-1557.
doi:10.1007/s00296-017-3726-1.
750
Hunter et al., Prevalence of rheumatoid arthritis.
751
Stolshek BS, Wade S, Mutebi A, De AP, Wade RL, Yeaw J. Two-year adherence and costs for biologic therapy
for rheumatoid arthritis. Am J Manag Care. 2018;24(8 Spec No.): SP315-SP321.

such as thrombotic stroke, myocardial infarction, or death).
752,753,754
We believe that the cost of
these adverse events are important to assess in terms of Medicare spending and impact on the
patient population. In line with these findings, the Recommendation Group did express support
for the intent of this measure from a clinician and patient perspective.
The Recommendation Group stated that public comments submitted during the PRMR
process suggest the Rheumatoid Arthritis measure does not have support by certain
rheumatology associations. However, the comments received during PRMR did not include
comments from a major rheumatologic association that has called for the development of an
episode-based measure for rheumatologists. Members of this organization were also involved in
the development of the Rheumatoid Arthritis measure through the participation in the
Rheumatoid Arthritis Clinician Expert Workgroup. As described in section IV.A.4.e.(2)(a)(ii) of
this proposed rule, the Rheumatoid Arthritis episode-based cost measure was developed with
extensive engagement from interested parties (that is, clinicians who treat this disease, persons
with lived experience with Rheumatoid Arthritis, and the general public) through several
mechanisms including a TEP, public comment periods, a clinician expert workgroup, person and
family engagement opportunities, and national field testing. During the public comment period
for determining which measures to prioritize for development, we received feedback from
rheumatology professional societies that they supported the development of a Rheumatoid
Arthritis measure, as the current MIPS cost measure inventory does not include cost measures
specific to care typically provided by rheumatologists. Additionally, the Rheumatoid Arthritis
752
Black, R.J. et al., “A Survey of Glucocorticoid Adverse Effects and Benefits in Rheumatic Diseases: The Patient
Perspective,” Journal of Clinical Rheumatology 23, no. 8 (December 2017): 416-420,
https://doi.org/10.1097/rhu.0000000000000585.
753
Wilson, J.C. et al., “Incidence and Risk of Glucocorticoid-Associated Adverse Effects in Patients With
Rheumatoid Arthritis,” Arthritis Care & Research, 71, no. 4, (April 2019): 498-511,
https://doi.org/10.1002/acr.23611.
754
Best, J.H. et al., “Association Between Glucocorticoid Exposure and Healthcare Expenditures for Potential
Glucocorticoid-related Adverse Events in Patients with Rheumatoid Arthritis,” Journal of Rheumatology 45, no. 3
(March 2018): 320-328, https://doi.org/10.3899/jrheum.170418.
Clinician Expert Workgroup that advised on the measure specifications included representatives
from 11 professional societies, including key rheumatology societies.
The Recommendation Group also expressed concerns with the scientific acceptability of
the Rheumatoid Arthritis measure. However, national field testing indicates the measure is valid
and has high reliability at the group level, with a mean reliability of 0.74, and has moderate
reliability at the individual level with a mean reliability of 0.52. At both the group and individual
levels, the measure passes the 0.4 reliability threshold we typically apply for a cost measure’s
use in MIPS, as discussed in CY 2022 PFS final rule (86 FR 65453 through 65455).
Additionally, several commenters expressed concerns with the Rheumatoid Arthritis measure,
such as the measure could hold clinicians accountable for costs that they cannot control (for
example, medications), the risk adjustment methodology may not account for differences in the
patient population, and the measure is not tied directly to patient outcomes. Testing also
indicates that the cost measure reflects the cost related to treatment choices and the cost of
adverse outcomes resulting from care and that the risk adjustment model is working as intended.
There has not been evidence to suggest that this cost measure would impact patient outcomes
negatively. More information is available in the Measure Justification Form available for
download at https://www.cms.gov/files/zip/2023-wave-5-measure-justification-forms-zip.zip. The
Recommendation Group also acknowledged that the measure is already specified to adjust for
expected differences in medication costs based on whether a patient is enrolled in Medicare Part
D (that is, stratifying the measure by Part D enrollment), furthering supporting the scientific
acceptability of this measure.
The Recommendation Group suggested the measure should undergo endorsement review
and receive CBE endorsement. As discussed previously, we may pursue CBE endorsement of
these measures in the future, but CBE endorsement is not a requirement for measures to be
adopted and used in MIPS.
We are continuing to propose this measure despite the “do not recommend” vote from the
PRMR committee because we continue to believe in the use of this measure based on the empiric
testing results discussed in this section, including indications of moderate to high reliability, and
because of the importance of capturing this clinical area of care. We originally selected this
measure for development because of its high impact in terms of patient population, clinician
coverage, and Medicare spending, and the opportunity to build a chronic condition measure that
would address a condition not captured by other episode-based measures in the MIPS cost
performance category. We believe that there is a strong rationale for including this measure in
the cost performance category for these same reasons.
(ix) Proposal to Adopt Six Episode-Based Measures and Modify Two Episode-Based Measures
In consideration of the extensive development, reevaluation, and pre-rulemaking review
processes described in sections IV.A.4.e.(2)(a)(i) through IV.A.4.e.(2)(a)(viii), we are proposing
to make the following updates to the MIPS cost performance category:
We are proposing to adopt the six new episode-based measures in the cost performance
category, outlined in Table 58 and described in section IV.A.4.e.(2)(a)(iii) of this rulemaking,
beginning with the CY 2025 performance period/2027 MIPS payment year.
We are proposing to replace the original Routine Cataract Removal with Intraocular Lens
(IOL) Implantation with the revised Cataract Removal with Intraocular Lens (IOL) Implantation
measure, with the modifications described in section IV.A.4.e.(2)(a)(vi) of this rulemaking, in
the cost performance category beginning with the CY 2025 performance period/2027 MIPS
payment year.
We are proposing to replace the ST-Elevation Myocardial Infarction (STEMI)
Percutaneous Coronary Intervention (PCI) with the revised Inpatient (IP) Percutaneous Coronary
Intervention (PCI) measure, with the modifications described in section IV.A.4.e.(2)(a)(vii) of
this rulemaking, in the cost performance category beginning with the CY 2025 performance
period/2027 MIPS payment year.
We invite comments on the proposals in this section.
(b) Reliability and Case Minimum
In this section of this proposed rule, we discuss the case minima we are proposing for the
episode-based measures we are proposing to adopt and modify (in section IV.A.4.e.(2)(a)(ix)) in
this rulemaking.
Reliability is a metric that evaluates the extent that variation in a measure comes from
clinician performance (“signal”) rather than random variation (“noise”). Higher reliability
suggests that a measure is effectively capturing meaningful differences between clinicians’
performance. However, we continued to caution against using reliability as the sole metric to
evaluate a measure because of the tradeoffs between accuracy and reliability, and the role of
service assignment in reducing noise. These and other considerations are detailed in the CY 2022
PFS final rule (86 FR 65453 through 65455). We also noted that increasing case minima
necessarily reduces the number of clinicians who meet the case minimum for a given measure.
Because these are clinically refined measures, we aim to have as many MIPS eligible clinicians
as possible to be able to have their costs evaluated by them. Therefore, we considered that a
mean reliability of 0.4 represents moderate reliability because it accounts for these
considerations and is a sufficient threshold to ensure that the measure is performing as intended
when assessed in conjunction with other testing.
We previously established at § 414.1350(c)(5) a case minimum of 20 episodes for acute
inpatient medical condition episode-based measures and at § 414.1350(c)(4) a case minimum of
10 episodes for procedural episode-based measures in the CY 2019 PFS final rule (83 FR
59773 through 59774). We also established at § 414.1350(c)(6) a case minimum of 20 episodes
for chronic condition episode-based measures in the CY 2022 final rule (86 FR 65453 through
65455).
We examined the reliability of the eight episode-based measures (six new and two
modified) we are proposing in this rulemaking, and Table 60 presents the percentage of tax

identification numbers (TINs) and TIN/National Provider Identifiers (NPIs) that meet the 0.4
reliability threshold and the mean reliability for TINs and TIN/NPIs at our case minimum of 20
for each of the chronic condition and acute impatient medical condition episode-based measures.
At a 20-episode case minimum, the mean reliability for the measures exceeds 0.4 for both groups
and individual clinicians, and the majority of groups and individual clinicians meet the 0.4
reliability threshold. For the procedural measure, Cataract Removal with Intraocular Lens (IOL)
Implantation, we applied the case minimum of 10 episodes. At a 10-episode case minimum, the
mean reliability for the measure exceeds 0.4 for both groups and individual clinicians, and all
groups and individual clinicians meet the 0.4 reliability threshold.
TABLE 60: Percent of TINs and TIN/NPIs that Meet 0.4 Reliability Threshold and TIN
and TIN/NPI Mean Reliability
Measure
name
% TINs meeting
0.4 reliability
threshold
Mean reliability
for TINs
% TIN/NPIs
meeting 0.4
reliability
threshold
Mean reliability
for TIN/NPIs
Prostate Cancer
87.4%
0.68
84.1%
0.62
Rheumatoid Arthritis
95.0%
0.74
97.3%
0.76
Chronic Kidney Disease
88.8%
0.63
82.3%
0.57
End-Stage Renal Disease
92.5%
0.65
90.0%
0.59
Kidney Transplant
Management
91.2%
0.64
95.8%
0.68
Respiratory Infection
Hospitalization
100.0%
0.74
100.0%
0.53
Cataract Removal with
Intraocular Lens (IOL)
Implantation
100.0%
0.97
100.0%
0.96
Inpatient (IP) Percutaneous
Coronary Intervention (PCI)
100.0%
0.63
100.0%
0.52
We believe that calculating these proposed episode-based measures with these case
minimums would accurately and reliably assess the performance of clinicians and clinician group
practices. Therefore, we are proposing to adopt a case minimum of 20 episodes for the chronic
condition (CKD, ESRD, Kidney Transplant Management, Prostate Cancer, Rheumatoid
Arthritis) and acute inpatient medical condition (Respiratory Infection Hospitalization and IP
PCI) measures and a case minimum of 10 episodes for the procedural measure (Cataract
Removal with IOL Implantation) listed in Table 60. For the IP PCI and Cataract Removal with
IOL Implantation, these case minimums remain consistent with the case minimums for the
original measures (that is, STEMI PCI and Routine Cataract Removal with IOL Implantation)
that are currently in use. These proposals are also consistent with our regulation at §
414.1350(c)(4) through (6). We do not propose to modify these regulations establishing the case
minima for these types of cost measures.
We invite comments on the proposals in this section.
(c) Proposed Revisions to the Operational List of Care Episode and Patient Condition Groups
and Codes
We are proposing revisions to the operational list of care episode and patient condition
groups and codes to reflect the proposal of any new episode-based measures. This section of this
proposed rule provides context on the statutory requirements for care episode and patient
condition groups and proposes changes to the operational list.
Section 1848(r) of the Act specifies a series of steps and activities for the Secretary to
undertake to involve physicians, practitioners, and other interested parties in enhancing the
infrastructure for cost measurement, including for purposes of MIPS and APMs. Section
1848(r)(2) of the Act requires the development of care episode and patient condition groups, and
classification codes for such groups, and provides for care episode and patient condition groups
to account for a target of an estimated one-half of expenditures under Medicare Parts A and B
(with this target increasing over time as appropriate). Sections 1848(r)(2)(E) through (G) of the
Act require the Secretary to post on the CMS website a draft list of care episode and patient
condition groups and codes for solicitation of input from interested parties, and subsequently,
post an operational list of such groups and codes. Section 1848(r)(2)(H) of the Act requires that
not later than November 1 of each year (beginning with 2018), the Secretary shall, through
rulemaking, revise the operational list of care episode and patient condition codes as the
Secretary determines may be appropriate, and that these revisions may be based on experience,
new information developed under section 1848(n)(9)(A) of the Act, and input from physician
specialty societies and other interested parties.
For more information about past revisions to the operational list that we made as we
developed and proposed episode-based measures, we refer readers to the CY 2023 PFS final rule
(87 FR 70056 through 70057) and CY 2024 PFS final rule (88 FR 79348). The current
operational list and prior operational lists are available at the QPP Cost Measure Information
page at https://www.cms.gov/medicare/quality/value-based-programs/cost-measures/about.
Additionally, as required by section 1848(r)(2)(I) of the Act, information on resource use
(or cost) measures currently in use in MIPS, cost measures under development and the time-
frame for such development, potential future cost measure topics, a description of engagement
with interested parties, and the percent of expenditures under Medicare Parts A and B that are
covered by cost measures must be provided on the website of CMS not later than December 31
of each year.
In accordance with section 1848(r)(2)(H) of the Act, we are proposing to revise the
operational list beginning with the CY 2025 performance period/2027 MIPS payment year to
include six new care episode and patient condition groups, based on input from clinician
specialty societies and other interested parties, as discussed in section IV.A.4.e.(2)(a) of this
proposed rule. We propose including Respiratory Infection Hospitalization as a care episode
group and CKD, ESRD, Kidney Transplant Management, Prostate Cancer, and Rheumatoid
Arthritis as patient condition groups. These care episode and patient condition groups serve as
the basis for the six new episode-based measures that we are proposing in section
IV.A.4.e.(2)(a)(ix) of this proposed rule for the cost performance category. The codes that define
these six care episode and patient condition groups align with the trigger codes of the proposed
episode-based measures in section IV.A.4.e.(2)(a)(ix) of this proposed rule. As described in
section IV.A.4.e.(2)(a)(ii), these specifications are developed with extensive input from
interested parties.
Additionally, we propose to revise the care episode group codes listed to align with the
modifications proposed for Cataract Removal with Intraocular Lens (IOL) Implantation and
Inpatient (IP) Percutaneous Coronary Intervention (PCI) measures.
Our proposed revisions to the operational list are available on our QPP Cost Measure
Information page at https://www.cms.gov/medicare/quality/value-based-programs/cost-
measures/about.
We invite public comment on our proposals in this section.
(d) Proposed Removal Criteria for MIPS Cost Measures
Once adopted, cost measures are retained in the cost performance category measure
inventory, except when we specifically propose to remove a measure. We have identified a need
to establish and codify objective criteria that can be used to inform the removal of a cost measure
from the MIPS cost performance category. Specifically, when removing the Simple Pneumonia
with Hospitalization episode-based measure from the CY 2024 PFS final rule (88 FR 79348
through 79349), we confirmed that, unlike the MIPS quality performance category, the MIPS
cost performance category did not have clear guidelines for removing a measure established
through the notice-and-comment rulemaking process. Establishing such criteria would allow for
more consistency in our evaluation of the cost measures and our decision on whether to propose
that a cost measure be removed from the MIPS cost performance category.
Therefore, we are proposing to adopt the following factors that can be used to guide the
removal of a cost measure:
● Factor 1: It is not feasible to implement the measure specifications.
● Factor 2: A measure steward is no longer able to maintain the cost measure.
● Factor 3: The implementation costs or negative unintended consequences associated
with a cost measure outweigh the benefit of its continued use in the MIPS cost performance
category.
● Factor 4: The measure specifications do not reflect current clinical practice or
guidelines.
● Factor 5: The availability of a more applicable measure, including a measure that
applies across settings, applies across populations, or is more proximal in time to desired patient
outcomes for the particular topic.
We selected these factors because they outline instances that we anticipate based on
previous experience where a cost measure may not be appropriate to maintain in a program, but
not limited to these instances. We also worked to align these criteria with the MIPS quality
removal considerations and criteria outlined in the CY 2019 PFS final rule (83 FR 59763 through
59765) and CY 2020 PFS final rule (84 FR 62957 through 62959), where possible, and, in part,
the Hospital Value-Based Purchasing (HVBP) Program’s removal factors that are codified in our
regulations at 42 CFR 412.164(c)(3). We are proposing these specific criteria to encourage a
degree of alignment between existing measure removal policies within MIPS and across
Medicare programs, where appropriate, for cost measures.
There were some instances where certain removal criteria used by the MIPS quality
performance category and the HVBP Program would not be applicable for cost measures and, in
those instances, we are not proposing to adopt those factors. For example, we did not propose to
adopt the removal criterion for measures that do not reach appropriate reporting volumes. This is
because MIPS eligible clinicians do not separately report or submit data for cost measures.
Instead, we assess and score cost measures based on administrative claims data. In addition, our
existing scoring policies for the cost performance category at § 414.1380(b)(2) already address
situations where there are insufficient number of MIPS eligible clinicians meeting the case
minimum, such that the cost measure is not scored. If we were to determine that a cost measure
would be unlikely to be scored moving forwards due to insufficient number of MIPS eligible
clinicians meeting the case minimum, we believe that the first factor we are proposing (that is, it
is not feasible to implement the measure specifications) would be applicable for removal.
We note that these factors are criteria that would be used as guidance when considering
whether or not to propose to remove a measure, rather than firm requirements. Specifically, there
could be instances when a measure meets one or multiple measure removal factors, but would be
retained in the cost performance category regardless, provided that we determine that the benefit
of keeping the measure in the cost performance category would outweigh the benefit of
removing it. Prior to proposing a measure for removal in accordance with this policy, we would
carefully review the specifications of the cost measures by conducting necessary literature
reviews, empirical testing, or other information gathering.
Additionally, we propose to codify this proposed measure removal policy by amending
§ 414.1350 by adding the proposed cost removal criteria in paragraph (e). Specifically, we are
proposing at § 414.1350(e) that CMS may remove a cost measure from MIPS based on one or
more of the following factors, provided however CMS may retain a cost measure that meets one
or more of the following factors if CMS determines the benefit of retaining the measure
outweighs the benefit of removing it.
● It is not feasible to implement the measure specifications.
● A measure steward is no longer able to maintain the cost measure.
● The implementation costs or negative unintended consequences associated with a cost
measure outweigh the benefit of its continued use in the MIPS cost performance category.
● The measure specifications do not reflect current clinical practice or guidelines.
● The availability of a more applicable measure, including a measure that applies across
settings, applies across populations, or is more proximal in time to desired patient outcomes for
the particular topic.
We invite comments on this proposal.
(3) Improvement Activities Performance Category
(a) Background
For previous discussions on the general background of the improvement activities
performance category, we refer readers to the CY 2017 Quality Payment Program final rule (81
FR 77177 and 77178), the CY 2018 Quality Payment Program final rule (82 FR 53648 through
53661), the CY 2019 Physician Fee Schedule (PFS) final rule (83 FR 59776 and 59777), the CY
2020 PFS final rule (84 FR 62980 through 62990), CY 2021 PFS final rule (85 FR 84881
through 84886), the CY 2022 PFS final rule (86 FR 65462 through 65466), the CY 2023 PFS
final rule (87 FR 70057 through 70061), and the CY 2024 PFS final rule (88 FR 79350 and 88
FR 79351). We also refer readers to 42 CFR 414.1305 for the definitions of improvement
activities and attestation, § 414.1320 for standards establishing the performance period,
§ 414.1325 for the data submission requirements, § 414.1355 for standards related to the
improvement activity performance category generally, § 414.1360 for data submission criteria
for the improvement activity performance category, and § 414.1380(b)(3) for improvement
activities performance category scoring.
We are proposing two changes to the traditional Merit-based Incentive Payment System
(MIPS) and the MIPS Value Pathways (MVPs) improvement activities policies for the CY 2025
performance period/2027 MIPS payment year. We are also proposing at § 414.1355 to codify
the seven improvement activity removal factors, which were adopted in the CY 2020 PFS final
rule (FR 84 62988 through 62990) to establish criteria used to identify activities for potential
removal or modification. In addition, we are proposing changes to the improvement activities
Inventory for the CY 2025 performance period/2027 MIPS payment year and future years as
follows: adding two new improvement activities; modifying two existing improvement activities;
and removing eight previously adopted improvement activities.
(b) Improvement Activities Inventory
(i) Annual Call for Activities Background
In the CY 2017 Quality Payment Program final rule (81 FR 77190), for the transition
year of MIPS, we implemented the initial improvement activities Inventory consisting of
approximately 95 activities (81 FR 77817 through 77831). We took several steps to ensure the
Inventory was inclusive of activities in line with statutory and program requirements, including

conducting interviews with highly performing organizations of all sizes, conducting an
environmental scan to identify existing models, activities, or measures that met all or part of the
improvement activities performance category criteria, and reviewing comments received in the
CY 2016 PFS final rule with comment period (80 FR 71259) and in response to the MIPS and
Advanced Payment Models (APMs) Requests for Information (RFIs) in relation to the
improvement activities performance category.
For the CY 2018 performance period/2020 MIPS payment year, we provided an informal
process for submitting new improvement activities or modifications for potential inclusion in the
comprehensive improvement activities Inventory for the CY 2018 performance period/2020
MIPS payment year and future years through guidance.
755
In the CY 2018 Quality Payment
Program final rule (82 FR 53656 through 53659), for the CY 2019 performance period/2021
MIPS payment year and for future years, we finalized a formal Annual Call for Activities
process for the addition of possible new activities and possible modifications to current activities
in the improvement activities Inventory. An interested party must submit a nomination form
regarding a new activity or a modification to an existing improvement activity (OMB control #
0938-1314) available at www.qpp.cms.gov during the Annual Call for Activities.
(ii) Codification of Improvement Activity Removal Factors
In the CY 2018 Quality Payment Program proposed rule (82 FR 30056), we solicited
comments on the criteria that may be used to identify improvement activities for removal from
the improvement activities Inventory, citing that, over time, certain improvement activities
should be considered for removal to ensure the Inventory is robust and relevant. In the CY 2020
PFS final rule (84 FR 62568 through 63563), we established seven removal factors to identify
activities for potential removal or modification from the Inventory. In this proposed rule, we are
755
CMS, Annual Call for Measures and Activities: Fact Sheet, https://www.cms.gov/Medicare/Quality-IniCtiatives-Patient-
Assessment-Instruments/MMS/Downloads/Annual-Call-for-Measures-and-Activities-for-MIPS_Overview-Factsheet.pdf.
proposing to codify at § 414.1355 the following existing seven improvement activity removal
factors:
● Factor 1: Activity is duplicative of another activity.
● Factor 2: There is an alternative activity with a stronger relationship to quality care or
improvements in clinical practice.
● Factor 3: Activity does not align with current clinical guidelines or practice.
● Factor 4: Activity does not align with at least one meaningful measure area.
● Factor 5: Activity does not align with the quality, cost, or Promoting Interoperability
performance categories.
● Factor 6: There have been no attestations of the activity for 3 consecutive years.
● Factor 7: Activity is obsolete.
We note that these factors are criteria that are used as guidance in determining removal of
an activity, but its use is at CMS discretion. For example, there may be instances when an
activity meets one or multiple activity removal factors but may be retained in the improvement
activities performance category Inventory, because the benefit of retaining outweighs the benefit
of removing. We believe that codifying these removal factors would provide transparency and
alignment with removals of improvement activities from the Inventory. We solicit comment on
this proposal.
(iii) Changes to the Improvement Activities Inventory
In the CY 2018 Quality Payment Program final rule (82 FR 53660), we finalized that we
would establish improvement activities through notice-and-comment rulemaking. We refer
readers to Table H in the Appendix to the CY 2017 Quality Payment Program final rule (81 FR
77177 through 77199), Tables F and G in the Appendix to the CY 2018 Quality Payment
Program final rule (82 FR 54175 through 54229), Tables A and B in the Appendix 2 to the CY
2019 PFS final rule (83 FR 60286 through 60303), Tables A, B, and C in the Appendix 2 to the
CY 2020 PFS final rule (84 FR 63514 through 63538), Tables A, B, and C in the Appendix 2 to
the CY 2021 PFS final rule (85 FR 85370 through 85377), Tables A, B, and C in the Appendix 2
to the CY 2022 PFS final rule (86 FR 65969 through 65997), Tables A, B, and C in the
Appendix 2 to the CY 2023 PFS final rule (70633 through 70650), and Tables A, B, and C in the
Appendix 2 to the CY 2024 PFS final rule (88 FR 79965 through 79977) for our previously
finalized improvement activities Inventories. We also refer readers to the Quality Payment
Program website under Explore Measures and Activities at https://qpp.cms.gov/mips/explore-
measures?tab=improvementActivities&py=2024#measures for a complete list of the current
improvement activities. In the CY 2017 Quality Payment Program final rule (81 FR 77539), we
codified the definition of improvement activities at § 414.1305 to mean an activity that relevant
MIPS eligible clinicians, organizations, and other relevant interested parties identify as
improving clinical practice or care delivery and that the Secretary determines, when effectively
executed, is likely to result in improved outcomes.
We are proposing to add two new improvement activities, modify two existing
improvement activities, and remove eight previously adopted improvement activities for the CY
2025 performance period/2027 MIPS payment year and future years. We refer readers to
Appendix 2 of this proposed rule for more details.
In response to stakeholder feedback, we are making efforts to streamline the Inventory
over the coming rulemaking cycles to include only the most robust and clinically meaningful
improvement activities. The removal and modification of 10 total activities is an initial step
toward our goal of reducing the size of the Inventory and helping to ensure that it includes only
the most meaningful activities that have a clear path to clinical practice improvement, while the
two proposed new activities would help fill gaps we have identified in the Inventory.
Currently, there are 106 finalized activities in the improvement activities Inventory, all of
which are assigned either a high or medium weight. When each of the activities was added, their
requirements reflected clinical practice standards of the time, addressed areas of opportunity for
fostering clinical practice improvements, and aligned with national public health priorities and
CMS programs. The last seven performance periods have provided sufficient time for analysis
and review of submissions data to learn how MIPS eligible clinicians are choosing activities to
report each year. Over the last several performance years, we have observed that some activities
have not remained aligned with the latest updates to clinical practice standards, have not
incorporated the latest national priorities, and/or have activity requirements that are no longer
substantive enough to promote a sufficient level of clinical practice improvement in today’s
health care environment. Some activities are continuing to be highly utilized even though the
goals of the improvement activity have largely been achieved, with the completion of these
improvement activities contributing toward a final score without a corresponding result of
ongoing improvement. Therefore, we are proposing to remove several of these improvement
activities and to modify activities to better promote more meaningful clinical practice
improvement opportunities. In proposing activities for removal or modification, we are
considering the seven adopted improvement activity removal factors, discussed in the CY 2020
PFS final rule (84 FR 62568 through 63563) and in section IV.A.4.e.(3)(b)(ii) of this proposed
rule.
We refer readers to Table C of Appendix 2 of this proposed rule for details on the
proposal of these eight activity removals.
We are proposing two new improvement activities in the Population Management
subcategory. One new activity, IA_PM_XX, titled “Implementation of Protocols and Provision
of Resources to Increase Lung Cancer Screening Uptake” would allow MIPS eligible clinicians
to receive credit for establishing a process or procedure to increase rates of lung cancer
screening. While lung cancer is a leading cause of cancer-related deaths in the U.S., lung cancer
screening is under-utilized. This activity aims to increase this screening and improve associated
outcomes. Another activity, IA_PM_XX, titled “Save a Million Hearts: Standardization of
Approach to Screening and Treatment for Cardiovascular Disease Risk” would allow MIPS
eligible clinicians to receive credit for implementing a standardized, evidence-based
cardiovascular disease risk assessment and care management plan in their practices. This
activity proposal is informed by the results of the CMS Innovation Center Million Hearts Model,
which included initial atherosclerotic cardiovascular disease (ASCVD) assessment as well as
cardiovascular care management, which were shown to contribute to improved identification and
treatment of patients at risk for ASCVD and would expand on the work of this Model by (1)
increasing flexibility in requirements, allowing more clinician specialties to participate, along
with increased flexibility in risk assessment to fit the needs of attesting clinicians and their
patient populations; and (2) requiring the use of structured documentation of risk factors and
associated treatment plans with the aim of addressing all risk factors directly.
We are proposing two modifications to improvement activities focused on strengthening
the activities to better promote more meaningful clinical practice improvement. We are
proposing to modify IA_PM_XX (formerly IA-ERP_6), titled “Vaccine Achievement for
Practice Staff - COVID-19, Influenza, and Hepatitis B,” and its validation criteria to revise its
target goals, and to expand its focus and promote the vaccination of staff for COVID-19 as well
as Influenza and Hepatitis B. Adjusting the target goals for this activity would align with the
latest Centers for Disease Control and Prevention (CDC) recommendations, and feedback
received indicates that this could increase its utilization. Additionally, we are proposing to
expand the focus of this activity to include influenza and hepatitis B to highlight the importance
of staff vaccination for vaccine-preventable diseases prevalent today. We are also proposing to
change the activity’s subcategory, from Emergency Response & Preparedness to Population
Management, to emphasize that staff vaccination is a long-term strategy in reducing morbidity
and mortality rates for these diseases.
We are proposing to modify IA_BE_4, currently titled “Engagement of patients through
implementation of improvements in patient portal,” and its validation criteria to limit the activity
to new implementations of a patient/caregiver portal and encourage the measure’s adoption by
clinicians who do not currently utilize this health information exchange technology. We are

proposing to modify this activity’s name, description, and its validation criteria to better align
with current practices. This activity was originally created during a time of transition to EHRs to
encourage electronic information exchange. It has become standard practice to use patient
portals; therefore, the activity is no longer driving improvement among clinicians who have
already implemented patient portals.
We are separately proposing to remove eight existing activities, presented in Table 61.
TABLE 61: Improvement Activities Inventory: Proposed Removals
Proposed Removals Titles
Removal Criteria (Factor)
EPA_1 Provide 24/7 Access to MIPS
Eligible Clinicians or Groups Who
Have Real-Time Access to Patient's
Medical Record
Factor 7, activity is obsolete
PM_12 Population empanelment
Factor 7, activity is obsolete
CC_1 Implementation of use of
specialist reports back to referring
clinician or group to close referral loop
Factor 1, activity is duplicative; Factor
5, activity does not align with quality,
cost, or promoting interoperability
performance categories
CC_2 Implementation of
improvements that contribute to more
timely communication of test results
Factor 7, activity is obsolete
ERP_4 Implementation of a Personal
Protective Equipment (PPE) Plan
Factor 7, activity is obsolete
ERP_5 Implementation of a
Laboratory Preparedness Plan
Factor 7, activity is obsolete
BMH_8 Electronic Health Record
Enhancements for BH data capture
Factor 2, there is an alternative activity
with a stronger relationship to quality
care or improvements in clinical
practice
PSPA_27 Invasive Procedure or
Surgery Anticoagulation Medication
Management
Factor 1, activity is duplicative
We refer readers to Appendix 2 of this proposed rule for details on the proposed revisions
to the improvement activities Inventory.
(iv) Improvement Activity Scoring and Reporting Policies
We are proposing two scoring and reporting policy changes for the improvement
activities performance category effective for the CY 2025 performance period/2027 MIPS
payment year and subsequent years. First, we are proposing to eliminate the weighting of
improvement activities. This would simplify scoring of the category, as well as complement our
ongoing efforts to refine and improve the Inventory. In the CY 2017 Quality Payment Program
final rule (81 FR 77177 and 77178), we codified at 42 CFR 414.1380(b)(3) the scoring policies
for the improvement activities performance category. We established there that clinicians
(except for non-patient facing MIPS eligible clinicians, small practices, and practices located in
rural areas and geographic health professional shortage areas (HPSAs)) receive 10 points for
each medium-weighted improvement activity and 20 points for each high-weighted improvement
activity. Non-patient facing MIPS eligible clinicians, small practices, and practices located in
rural areas and geographic HPSAs receive 20 points for each medium-weighted improvement
activity and 40 points for each high-weighted improvement activity. We established a
differentially weighted model for the improvement activities performance category with two
categories, medium and high, to provide flexible scoring and because there are no nationally
recognized standards or definitions for these activities (81 FR 28210). Weights were assigned
based on the level of effort and resources needed to complete each activity, as well as alignment
with current national public health priorities and programs such as the Quality Innovation
Network-Quality Improvement Organization (QIN/QIO).
We have subsequently determined that the benefit to categorizing activities as high or
medium weighted has greatly diminished. Over the last several years of the Quality Payment
Program, we have made refinements and enhancements to the improvement activities Inventory
by adding new activities to incorporate newly identified opportunities for clinical improvement
and by modifying existing activities to support changes in practice standards, while also
eliminating activities that are duplicative or that no longer promote a sufficient level of clinical
improvement. In this and subsequent rulemaking cycles, we are focusing our efforts on
streamlining the Inventory to retain the highest priority activities that offer the strongest
promotion of clinical practice improvement. As the Inventory is streamlined, each retained
activity highlights a unique and vital aspect of clinical practice improvement, and therefore every
activity would be considered high priority.
Second, and related to the proposal in this section of this proposed rule, we are proposing
to further simplify improvement activity reporting requirements by reducing the number of
activities to which clinicians are required to attest to achieve a full score in the improvement
activities performance category. Currently, MIPS eligible clinicians are required to report two
high-weighted activities, four medium-weighted activities, or one high-weighted and two
medium-weighted activities while MVP participants are currently required to report one high-
weighted activity or two medium-weighted activities. We are proposing that MIPS eligible
clinicians who participate in traditional MIPS would be required to report two activities. In
addition, we are proposing that MIPS eligible clinicians who are categorized as small practice,
rural, in a provider-shortage area, or non-patient facing would now be required to report one
activity. We are proposing that these policies would be effective for the CY 2025 performance
period/2027 MIPS payment year and subsequent years.
We are also proposing that MVP participants would be required to report one activity. In
the CY 2022 PFS final rule (86 FR 65412 through 65413), we established that MVP Participants
submitting MVPs report fewer improvement activities than eligible clinicians reporting
traditional MIPS to incentivize and support MVP adoption. We continue to believe that reduced
reporting requirements are necessary to support the adoption of and reduce the burden for
implementation of MVPs.
We are proposing to lower the number of activities that MIPS eligible clinicians are
required to complete in order to obtain a full score to adjust for the ongoing reduction of
activities in the Inventory as well as to support eligible clinicians with simplified reporting as
they engage in fewer but more demanding activities. If the number of activities in the Inventory
is reduced after this and subsequent rulemaking, there would be fewer activities from which to
choose. However, the retained activities in the Inventory from which to choose would be the
highest priority activities that offer the strongest promotion of clinical practice improvement.
This proposal is also responsive to commenters who, in the past, have requested that the number
of required activities be reduced and that more activities be highly weighted (81 FR 77182). The
activity removals and modifications being proposed would result in an Inventory of activities
that are meaningful, timely, and rigorous. While decreasing the number of required activities
would simplify reporting, MIPS eligible clinicians would still be required to participate in
meaningful activities that yield significant practice improvement.
We are requesting comments on our proposals to remove weighting and to reduce the
number of activities to which clinicians are required to attest to achieve a full score in the
improvement activities performance category.
Specifically, we are requesting comments on our proposal to revise § 414.1380(b)(3) to
read that, beginning with the CY 2025 performance period/2027 MIPS payment year, MIPS
eligible clinicians (except for non-patient facing MIPS eligible clinicians, small practices, and
practices located in rural areas and geographic HPSAs) receive 20 points for each improvement
activity, while non-patient facing MIPS eligible clinicians, small practices, and practices located
in rural areas and geographic HPSAs receive 40 points for each improvement activity.
Therefore, to receive a score of 40 points, or full credit, MIPS eligible clinicians (except for non-
patient facing MIPS eligible clinicians, small practices, and practices located in rural areas and
geographic HPSAs) must report two improvement activities, while non-patient facing MIPS
eligible clinicians, small practices, and practices located in rural areas and geographic HPSAs
must report one improvement activity.
We are also requesting comment on our proposal to revise § 414.1365(c)(3) to state that,
beginning with the CY 2025 performance period/2027 MIPS payment year, MVP participants
receive 40 points for each improvement activity. Therefore, to receive a score of 40 points, or
full credit, MVP participants would be required to report one improvement activity.
(4) Promoting Interoperability Performance Category
(a) Background
Section 1848(q)(2)(A) of the Act includes the meaningful use of certified electronic
health record (EHR) technology (CEHRT) as a performance category under MIPS. We refer to
this performance category as the Promoting Interoperability performance category (and in past
rulemaking, we referred to it as the advancing care information performance category).
For our previously established policies regarding the Promoting Interoperability
performance category, we refer readers to our regulation at 42 CFR 414.1375, the CY 2017
Quality Payment Program final rule (81 FR 77199 through 77245), CY 2018 Quality Payment
Program final rule (82 FR 53663 through 53688), CY 2019 PFS final rule (83 FR 59785 through
59820), CY 2020 PFS final rule (84 FR 62991 through 63006), CY 2021 PFS final rule (85 FR
84886 through 84895), CY 2022 PFS final rule (86 FR 65466 through 65490), CY 2023 PFS
final rule (87 FR 70060 through 70087), and CY 2024 PFS final rule (88 FR 79308 through
79312 and 88 FR 79351 through 79365).
(b) Current Definition of CEHRT for the Quality Payment Program
In the CY 2024 Medicare Physician Fee Schedule (PFS) final rule (88 FR 79307 through
79312), we finalized revisions to the definition of CEHRT for the Quality Payment Program at
42 CFR 414.1305. In the CY 2024 PFS final rule (88 FR 79309 and 79310), we amended the
definition of CEHRT to be more flexible in response to changes proposed by the Office of the
National Coordinator for Health IT (ONC) in the “Health Data, Technology, and
Interoperability: Certification Program Updates, Algorithm Transparency, and Information
Sharing” (HTI–1) proposed rule (88 FR 23746 through 23917). Specifically, we amended our
definition of CEHRT at § 414.1305 to ensure references to ONC’s definition of Base EHR at 45
CFR 170.102 and its health IT certification criteria at 45 CFR 170.315 were responsive to any
changes ONC makes to its definition and criteria at any time. Instead of requiring that CEHRT
meet only the “2015 Edition Base EHR definition,” we added that it also may meet the
“subsequent Base EHR definition” as defined at 45 CFR 170.102. We also amended our
definition of CEHRT to provide that the CEHRT must be certified to the ONC health IT
certification criteria “as adopted and updated” in 45 CFR 170.315. This approach is consistent
with the policies subsequently finalized in the HTI–1 final rule (89 FR 1205 through 1210). For
additional background and information on this update, we refer readers to the discussion in the
CY 2024 PFS final rule on this topic (88 FR 79307 through 79312).
In consideration of the updates finalized in the CY 2024 PFS final rule and the HTI–1
final rule, we refer to “ONC health IT certification criteria” throughout this proposed rule where
we previously would have referred to “2015 Edition health IT certification criteria.” These
revisions to the definition of CEHRT in 42 CFR 414.1305 ensure that updates to the definition of
Base EHR in 45 CFR 170.102, and updates to applicable ONC health IT certification criteria in
45 CFR 170.315, are incorporated into the CEHRT definition without additional regulatory
action by CMS. For ease of reference, Table 65 sets forth the ONC health IT certification
criteria required to meet the Promoting Interoperability performance category objectives and
measures.
In the CY 2024 PFS final rule (88 FR 79408 through 79414), we also finalized changes
to the CEHRT definition at 42 CFR 414.1305 for Advanced APMs requiring use of EHR
technology certified under the ONC Health IT Certification Program that meets the ONC Base
EHR definition at 45 CFR 170.102 and any such ONC health IT certification criteria adopted or
updated in 45 CFR 170.315 that are determined applicable for the APM, for the year, considering
factors such as clinical practice area, promotion of interoperability, relevance to reporting on
applicable quality measures, clinical care delivery objectives of the APM, or any other factor
relevant to documenting and communicating clinical care to patients or their health care
providers in the APM. This CEHRT definition affords Advanced APMs the ability to tailor
additional CEHRT use requirements to those features or capabilities that are clinically relevant to
the APM and its participants, rather than referring to the same criteria associated with measures
in the Promoting Interoperability performance category of MIPS (88 FR 79413).
We highlight certain updates to ONC health IT certification criteria finalized in the ONC
HTI–1 final rule that impact certification criteria referenced under the CEHRT definition. ONC
adopted the certification criterion “Decision Support Interventions (DSI)” in 45 CFR
170.315(b)(11) to ultimately replace the “clinical decision support (CDS)” certification criterion
in 45 CFR 170.315(a)(9) included in the Base EHR definition (89 FR 1236). The finalized
“DSI” criterion ensures that Health IT Modules certified to 45 CFR 170.315(b)(11) must, among
other functions, enable a limited set of identified users to select (that is, activate) certain
evidence-based decision support interventions and Predictive DSI (as the latter term is defined in
45 CFR 170.102) (89 FR 1241) and support user access to specified “source attributes”—
categories of technical performance and quality information—for both evidence-based and
Predictive DSI (89 FR 1236). ONC further finalized that a Health IT Module may meet the Base
EHR definition by either being certified to the existing CDS certification criterion in 45
CFR 170.315(a)(9) or being certified to the revised DSI criterion in 45 CFR 170.315(b)(11), for
the period up to, and including, December 31, 2024. On and after January 1, 2025, ONC
finalized that a Health IT Module must be certified to the DSI certification criterion in 45
CFR 170.315(b)(11) in order to meet the Base EHR definition, and the adoption of the CDS
certification criterion in 45 CFR 170.315(a)(9) will expire on January 1, 2025 (89 FR 1281).
In the HTI-1 final rule, ONC also finalized other updates related to ONC health IT
certification criteria referenced in the CEHRT definition. ONC finalized January 1, 2026, as the
date when updates discussed below would take effect; accordingly, health IT developers must
update and provide certified Health IT Modules to their customers consistent with the
Maintenance of Certification requirements in 45 CFR 170.402(b)(3) by this date, including the
following updates:
• ONC updated the “Transmission to public health agencies—electronic case reporting”
criterion in 45 CFR 170.315(f)(5) to specify the use of consensus-based, industry-developed

electronic standards and implementation guides (IGs) to replace functional, descriptive
requirements in the existing criterion on and after January 1, 2026 (89 FR 1228).
• ONC adopted the United States Core Data for Interoperability (USCDI) version 3 in 45
CFR 170.213(b) and finalized that USCDI version 1 in 45 CFR 170.213(a) will expire on
January 1, 2026 (89 FR 1211 and 1223). This change impacts ONC health IT certification
criteria that reference the USCDI, including the “transitions of care” certification criteria in 45
CFR 170.315(b)(1)(iii)(A)(1) and (2), “clinical information reconciliation and incorporation—
Reconciliation” (45 CFR 170.315(b)(2)(iii)(D)(1) through (3)); and “view, download, and
transmit to 3rd party” (45 CFR 170.315(e)(1)(i)(A)(1)) (89 FR 1214).
• ONC updated the “demographics” certification criterion (45 CFR 170.315(a)(5)),
including renaming the criterion to “patient demographics and observations” (89 FR 1295 and
1296).
• ONC updated the “standardized API for patient and population services” certification
criterion in 45 CFR 170.315(g)(10) including finalizing references to newer versions of standards
referenced in the criterion, such as the US Core IG 6.1.0 (89 FR 1285) and the SMART App
Launch Implementation Guide Release 2.0.0 (89 FR 1292).
For complete information about the updates to ONC health IT certification criteria
finalized in the HTI–1 final rule, we refer readers to the text of the final rule (89 FR 1192) as
well as resources available on ONC’s website.
756
(c) Potential Future Update of the SAFER Guides Measure
(i) Background
ONC developed the Safety Assurance Factors for EHR Resilience Guides (SAFER
Guides) in 2014, and later updated in 2016. ONC provided the SAFER Guides, including the
High Priority Practices SAFER Guide, as a tool to help organizations at all levels conduct self-
756
For more information, see https://www.healthit.gov/topic/laws-regulation-and-policy/health-data-technology-
and-interoperability-certification-program.

assessments to optimize the safety and use of EHRs.
757
In the CY 2022 PFS final rule (86 FR
65475 through 65477), we adopted the SAFER Guides measure under the Protect Patient Health
Information objective beginning with the CY 2022 performance period/2024 MIPS payment
year. For the CY 2022 performance period/2024 MIPS payment year and the CY 2023
performance period/2025 MIPS payment year, MIPS eligible clinicians were required to attest to
whether they have conducted an annual self-assessment using the High Priority Practices SAFER
Guide
758
at any point during the calendar year in which the performance period occurs, with one
‘‘yes/no’’ attestation statement. MIPS eligible clinicians were not scored based on their answer
to the attestation, or their level of implementation of each of the practices. However, failure to
attest to this measure would result in earning a score of zero for the Promoting Interoperability
performance category for failing to meet the minimum reporting requirements.
In the CY 2024 PFS final rule (88 FR 79354 through 79356), we modified the SAFER
Guides measure. Beginning with the CY 2024 performance period/2026 MIPS payment year,
this modified measure requires MIPS eligible clinicians to conduct, and attest “yes” to having
completed, an annual self-assessment of their CEHRT, using the High Priority Practices SAFER
Guide. We remind readers that the SAFER Guides measure only requires completion of a self-
assessment and does not require MIPS eligible clinicians to implement fully each of the practices
identified in their self-assessment.
(ii) Status of Updates to SAFER Guides
As summarized in the CY 2024 PFS final rule (88 FR 79354 through 79356), we received
comments in response to our proposal to modify the SAFER Guides measure, including many
comments recommending that we collaborate with ONC to update the SAFER Guides, noting
that the SAFER Guides were last updated in 2016 (88 FR 59264). In response to these
comments, we noted that, while the current SAFER Guides reflect relevant and valuable
757
https://www.healthit.gov/topic/safety/safer-guides.
758
https://www.healthit.gov/sites/default/files/safer/guides/safer_high_priority_practices.pdf.

guidelines for safe practices with respect to current EHR systems, we would consider exploring
updates in collaboration with ONC. We reminded readers to visit the CMS resource library
website at https://www.cms.gov/regulations-guidance/promoting-interoperability/resource-
library and the ONC website at https://www.healthit.gov/topic/safety/safer-guides for resources
on the content and appropriate use of the SAFER Guides (88 FR 59262). We also noted that
future updates to the SAFER Guides would be provided with accompanying educational and
promotional materials to notify participants, in collaboration with ONC, when available (88 FR
59265).
In this proposed rule, we are seeking to make readers aware that efforts to update the
SAFER Guides are currently underway. We anticipate that updated versions of the SAFER
Guides may become available as early as CY 2025. We would consider proposing a change to
the SAFER Guides measure, as soon as feasible, potentially beginning in the CY 2026
performance period/2028 MIPS payment year to permit use of an updated versions of the
SAFER Guides at that time. We encourage MIPS eligible clinicians to become familiar with the
updated versions of the SAFER Guides when they become available and consider them as they
implement appropriate EHR safety practices.
(d) Modification of the Definition of Meaningful EHR User for Healthcare Providers That Have
Committed Information Blocking
The Department of Health and Human Services (HHS) final rule “21st Century Cures
Act: Establishment of Disincentives for Health Care Providers That Have Committed
Information Blocking” (hereafter referred to as the Disincentives final rule), was displayed for
public inspection by the Office of the Federal Register on June 26, 2024, and appeared in the
Federal Register on July 1, 2024.
759
The final rule implements the provision of the 21st Century
Cures Act specifying that a healthcare provider, determined by the HHS Office of the Inspector
759
88 FR 54662. Available at - https://www.govinfo.gov/content/pkg/FR-2024-07-01/pdf/2024-13793.pdf. See also
https://www.healthit.gov/topic/information-blocking#Disincentives.

General (OIG) to have committed information blocking, shall be referred to the appropriate
agency to be subject to appropriate disincentives set forth through notice and comment
rulemaking. In the Disincentives final rule, we finalized that a MIPS eligible clinician (other
than a qualified audiologist) will not be considered a meaningful EHR user in a performance
period if the OIG refers, during the calendar year of the performance period, a determination that
the MIPS eligible clinician committed information blocking as defined at 45 CFR 171.103.
Information blocking, in the case of a health care provider as defined in 45 CFR 171.102, is a
practice that is likely to interfere with the access, exchange, or use of electronic health
information, except as required by law or specified in an information blocking exception in 45
CFR part 171, subpart B, C, or D, and that the health care provider knows is unreasonable and is
likely to interfere with access, exchange, or use of electronic health information. Furthermore,
we finalized to revise the definition of “meaningful EHR User for MIPS” at § 414.1305 to state
that a MIPS eligible clinician (other than a qualified audiologist) is not a meaningful EHR user
for a performance period if the OIG refers a determination that the MIPS eligible clinician
committed information blocking, as defined at 45 CFR 171.103, during the calendar year of the
performance period. We also finalized amending the requirements at § 414.1375(b) to specify
that a MIPS eligible clinician must be a meaningful EHR user for MIPS (as defined at §
414.1305) to earn a score for MIPS Promoting Interoperability performance category. Under the
final policies, a MIPS eligible clinician that OIG determines has committed information blocking
would not be a meaningful EHR user, and therefore would be unable to earn a score (instead
earning a score of zero) for the Promoting Interoperability performance category.
Additional regulatory provisions were finalized at 45 CFR part 171, subpart J, related to
the application of disincentives.
We note that, as finalized, the revised definition of “meaningful EHR user for MIPS” at §
414.1305 and the revisions to § 414.1375(b) would become effective when the final rule takes
effect on July 31, 2024. For additional background and information, we refer readers to the
discussion in the “21st Century Cures Act: Establishment of Disincentives for Health Care
Providers That Have Committed Information Blocking” proposed rule (88 FR 74957 through
74962), as well as the Disincentives final rule.
(e) Future Goals of the Promoting Interoperability Performance Category
(i) Future Goals with Respect to Fast Healthcare Interoperability Resources® (FHIR) APIs for
Patient Access
In partnership with ONC, we envision a future where patients have timely, secure, and
easy access to their health information through the health application of their choice. We are
working with ONC to enable this type of access to health information by requiring the use of
APIs that utilize the Health Level Seven International® (HL7) FHIR standard. We work with
ONC and other Federal partners to improve timely and accurate data exchange, partner with
industry to enhance digital capabilities, advance adoption of FHIR, support enterprise
transformation efforts that increase our technological capabilities, and promote interoperability.
In the CY 2021 PFS proposed rule (85 FR 50303), we described our future vision for the
Promoting Interoperability performance category and stated that we will continue to consider
changes that support a variety of HHS goals, including supporting alignment with the 21st
Century Cures Act, advancing interoperability and the exchange of health information, and
promoting innovative uses of health IT. We also described plans to continue to align the
Promoting Interoperability performance category with policies finalized in the “21st Century
Cures Act: Interoperability, Information Blocking, and the ONC Health IT Certification
Program” final rule (85 FR 25642), including finalization of a new certification criterion for a
standards-based API using FHIR, among other health IT topics.
ONC finalized the HTI–1 final rule (89 FR 1192), effective March 11, 2024, to further
implement the 21st Century Cures Act, among other policy goals. ONC finalized revisions to
the “standardized API for patient and population services” certification criterion at §
170.315(g)(10). It also adopted the HL7 FHIR US Core Implementation Guide (IG) Standard for

Trial Use version 6.1.0 at § 170.215(b)(1)(ii), which provides the latest consensus-based
capabilities aligned with the USCDI version 3
760
data elements for FHIR APIs. The HTI–1 final
rule also created the Insights Condition and Maintenance of Certification requirements (Insights
Condition) within the ONC Health IT Certification Program to provide transparent reporting on
certified health IT (89 FR 1199). This Insights Condition will require developers of certified
health IT subject to the requirements to report on measures that provide information about the
use of specific certified health IT functionalities by end users. One such measure calculates the
number of unique individuals who access their electronic health information overall and by
different methods such as through a standardized API for patient and population services (89 FR
1313 and 1314).
By adopting these new and updated standards, implementation specifications,
certification criteria, and conditions of certification, provisions in the HTI–1 final rule advance
interoperability, improve transparency, and support the access, exchange, and use of electronic
health information. We aim to further advance the use of FHIR APIs through policies in the
Promoting Interoperability performance category to advance interoperability, encourage the
exchange of health information, and promote innovative uses of health IT. We also hope to gain
insights into the adoption and use of FHIR APIs by MIPS eligible clinicians due to the ONC
Health IT Certification Program’s Insights Condition. We believe maintaining our focus on
promoting interoperability, alignment, and simplification would reduce healthcare provider
burden while allowing flexibility to pursue innovative applications that improve care delivery.
For additional background and information, we refer readers to the discussion in the ONC HTI–1
final rule on this topic (89 FR 1192).
(ii) Improving Cybersecurity Practices
The Promoting Interoperability performance category encourages the advancement of
EHR safety by promoting appropriate cybersecurity practices through the Security Risk Analysis
760
https://www.healthit.gov/isa/united-states-core-data-interoperability-uscdi#uscdi-v3.

and SAFER Guides measures. On February 14, 2023, the National Institute of Standards and
Technology (NIST) published updated guidance for health care entities implementing
requirements of the Health Insurance Portability and Accountability of 1996 (HIPAA) Security
Rule (45 CFR part 160 and subparts A and C of 45 CFR part 164). The guidance, NIST SP 800-
66r2, provides information and resources to HIPAA-covered entities to improve their
cybersecurity risk practices.
761
We also wish to alert readers of additional HHS resources and
activities regarding cybersecurity best practices as recently summarized in an HHS strategy
document that provides an overview of HHS recommendations to help the health care sector
address cyber threats.
762
HHS has also recently published a website detailing recommended
cybersecurity performance goals.
763
We intend to consider how the Promoting Interoperability
performance can promote cybersecurity best practices for MIPS eligible clinicians in the future.
(iii) Improving Prior Authorization Processes
We recently released the CMS Interoperability and Prior Authorization final rule, which
appeared in the Federal Register on February 8, 2024 (89 FR 8758). The final rule aims to
enhance health information exchange and access to health records for patients, healthcare
providers, and payers, and improve prior authorization processes. In the final rule, we finalized
the addition of a new measure, the “Electronic Prior Authorization” measure, under the HIE
objective for the MIPS Promoting Interoperability performance category beginning with the CY
2027 performance period/2029 MIPS payment year (89 FR 8909 through 8927).
(f) Requirements for the Promoting Interoperability Performance Category for the CY 2025
Performance Period/2027 MIPS Payment Year
(i) Objectives and Measures for the CY 2025 Performance Period/2027 MIPS Payment Year
761
https://csrc.nist.gov/pubs/sp/800/66/r2/final.
762
https://aspr.hhs.gov/cyber/Documents/Health-Care-Sector-Cybersecurity-Dec2023-508.pdf.
763
https://hphcyber.hhs.gov/performance-goals.html.

For ease of reference, Table 62 lists the objectives and measures for the Promoting
Interoperability performance category required for the CY 2025 performance period/2027 MIPS
payment year as revised to reflect the policies we will finalize in the final rule.
TABLE 62: Objectives and Measures for the Promoting Interoperability
Performance Category for the CY 2025 Performance Period/2027 MIPS Payment Year
Objective
Measure
Numerator
Denominator
Exclusion
Electronic Prescribing:
Generate and transmit
permissible
prescriptions
electronically
e-Prescribing: At least
one permissible
prescription written by
the MIPS eligible
clinician is transmitted
electronically using
CEHRT.
Number of
prescriptions in the
denominator
generated and
transmitted
electronically using
CEHRT.
Number of
prescriptions written
for drugs requiring a
prescription in order
to be dispensed other
than controlled
substances during the
performance period;
or number of
prescriptions written
for drugs requiring a
prescription in order
to be dispensed
during the
performance period.
Any MIPS eligible clinician
who writes fewer than 100
permissible prescriptions
during the performance
period.
Electronic Prescribing
Query of PDMP: For
at least one Schedule
II opioid or Schedule
III or IV drug
electronically
prescribed using
CEHRT during the
performance period,
the MIPS eligible
clinician uses data
from CEHRT to
conduct a query of a
PDMP for prescription
drug history.
N/A (measure is
Yes/No and requires
an affirmative
attestation to fulfill)
N/A (measure is
Yes/No and requires
an affirmative
attestation to fulfill)
Any MIPS eligible clinician
who: 1. is unable to
electronically prescribe
Schedule II opioids and
Schedule III and IV drugs in
accordance with applicable
law during the performance
period; or 2. Any MIPS
eligible clinician who does
not electronically prescribe
any Schedule II opioids or
Schedule III or IV drugs
during the performance
period.
Health Information
Exchange: The MIPS
eligible clinician
provides a summary of
care record when
transitioning or
referring their patient
to another setting of
care, receives or
retrieves a summary of
care record upon the
receipt of a transition
or referral or upon the
first patient encounter
with a new patient,
and reconciles
summary of care
information from other
healthcare providers
into their EHR using
the functions of
CEHRT
Support Electronic
Referral Loops by
Sending Health
Information: For at
least one transition of
care or referral, the
MIPS eligible
clinician that
transitions or refers
their patient to another
setting of care or
healthcare provider (1)
creates a summary of
care using CEHRT;
and (2) electronically
exchanges the
summary of care
record.
Number of
transitions of care
and referrals in the
denominator where
the summary of care
record was created
using CEHRT and
exchanged
electronically
Number of transitions
of care and referrals
during the
performance period
for which the MIPS
eligible clinician was
the transferring or
referring clinician
Any MIPS eligible clinician
who transfers a patient to
another setting or refers a
patient fewer than 100 times
during the performance
period.

Objective
Measure
Numerator
Denominator
Exclusion
Health Information
Exchange
Support Electronic
Referral Loops by
Receiving and
Reconciling Health
Information: For at
least one electronic
summary of care
record received for
patient encounters
during the
performance period
for which a MIPS
eligible clinician was
the receiving party of
a transition of care or
referral, or for patient
encounters during the
performance period in
which the MIPS
eligible clinician has
never before
encountered the
patient, the MIPS
eligible clinician
conducts clinical
information
reconciliation for
medication, mediation
allergy, and current
problem list.
Number of
electronic summary
of care records in
the denominator for
which clinical
information
reconciliation is
completed using
CEHRT for the
following three
clinical information
sets: (1) Medication
– Review of the
patient's medication,
including the name,
dosage, frequency,
and route of each
medication; (2)
Medication allergy –
Review of the
patient's known
medication allergies;
and (3) Current
Problem List –
Review of the
patient’s current and
active diagnoses.
Number of electronic
summary of care
records received
using CEHRT for
patient encounters
during the
performance period
for which a MIPS
eligible clinician was
the receiving party of
a transition of care or
referral, and for
patient encounters
during the
performance period
in which the MIPS
eligible clinician has
never before
encountered the
patient.
Any MIPS eligible clinician
who receives transitions of
care or referrals or has
patient encounters in which
the MIPS eligible clinician
has never before
encountered the patient
fewer than 100 times during
the performance period.
Health Information
Exchange
HIE Bi-Directional
Exchange:
Statement 1: I
participate in an HIE
to enable secure, bi-
directional exchange
to occur for every
patient encounter,
transition or referral
and record stored or
maintained in the EHR
during the
performance period in
accordance with
applicable law and
policy.
Statement 2: The HIE
that I participate in is
capable of exchanging
information across a
broad network of
unaffiliated exchange
partners including
those using disparate
EHRs, and not
engaging in
exclusionary behavior
when determining
exchange partners.
Statement 3: I use the
functions of CEHRT
N/A (measure is
Yes/No and requires
an affirmative
attestation to fulfill)
N/A (measure is
Yes/No and requires
an affirmative
attestation to fulfill)
N/A

Objective
Measure
Numerator
Denominator
Exclusion
to support bi-
directional exchange
with an HIE.
Health Information
Exchange
Enabling Exchange
Under TEFCA MIPS
eligible clinicians
would attest to the
following:
● Participating as a
signatory to a
Framework
Agreement (as that
term is defined by the
Common Agreement
for Nationwide Health
Information
Interoperability as
published in the
Federal Register and
on ONC’s website) in
good standing (i.e. not
suspended) and
enabling secure, bi-
directional exchange
of information to
occur, in production,
for every patient
encounter, transition
or referral, and record
stored or maintained in
the EHR during the
performance period, in
accordance with
applicable law and
policy.
● Using the functions
of CEHRT to support
bi-directional
exchange of patient
information, in
production, under this
Framework
Agreement.
N/A (measure is
Yes/No and requires
an affirmative
attestation to fulfill)
N/A (measure is
Yes/No and requires
an affirmative
attestation to fulfill)
N/A
Provider to Patient
Exchange: The MIPS
eligible clinician
provides patients (or
patient-authorized
representative) with
timely electronic
access to their health
information.
Provide Patients
Electronic Access to
Their Health
Information: For at
least one unique
patient seen by the
MIPS eligible
clinician: 1. The
patient (or the patient-
authorized
representative) is
provided timely access
to view online,
download, and
transmit his or her
health information;
and 2. The MIPS
eligible clinician
Number of patients
in the denominator
(or patient
authorized
representative) who
are provided timely
access to health
information to view
online, download,
and transmit to a
third party and to
access using an
application of their
choice that is
configured meet the
technical
specifications of the
API in the MIPS
Number of unique
patients seen by the
MIPS eligible
clinician during the
performance period.
N/A

Objective
Measure
Numerator
Denominator
Exclusion
ensures the patient’s
health information is
available for the
patient (or patient-
authorized
representative) to
access using any
application of their
choice that is
configured to meet the
technical
specifications of the
Application
Programming
Interface (API) in the
MIPS eligible
clinician’s CEHRT.
eligible clinician’s
CEHRT.
Public Health and
Clinical Data
Exchange: The MIPS
eligible clinician is in
active engagement
with a public health
agency or clinical data
registry to submit
electronic public
health data in a
meaningful way using
CEHRT, except where
prohibited, and in
accordance with
applicable law and
practice.
Immunization Registry
Reporting: The MIPS
eligible clinician is in
active engagement
with a public health
agency to submit
immunization data and
receive immunization
forecasts and histories
from the public health
immunization
registry/immunization
information system
(IIS).
N/A (measure is
Yes/No and requires
an affirmative
attestation to fulfill)
N/A (measure is
Yes/No and requires
an affirmative
attestation to fulfill)
The MIPS eligible clinician:
1.does not administer any
immunizations to any of the
populations for which data
is collected by its
jurisdiction’s immunization
registry or immunization
information system during
the performance period; OR
2.operates in a jurisdiction
for which no immunization
registry or immunization
information system is
capable of accepting the
specific standards required
to meet the CEHRT
definition at the start of the
performance period; OR 3.
Operates in a jurisdiction
where no immunization
registry or immunization
information system has
declared readiness to
receive immunization data
as of 6 months prior to the
start of the performance
period.
Public Health and
Clinical Data
Exchange
Electronic Case
Reporting: The MIPS
eligible clinician is in
active engagement
with a public health
agency to
electronically submit
case reporting of
reportable conditions.
N/A (measure is
Yes/No and requires
an affirmative
attestation to fulfill)
N/A (measure is
Yes/No and requires
an affirmative
attestation to fulfill)
The MIPS eligible clinician:
1.Does not treat or diagnose
any reportable diseases for
which data is collected by
their jurisdiction’s
reportable disease system
during the performance
period; OR 2.operates in a
jurisdiction for which no
public health agency is
capable of receiving
electronic case reporting
data in the specific
standards required to meet
the CEHRT definition at the
start of the performance
period; OR 3. Operates in a

Objective
Measure
Numerator
Denominator
Exclusion
jurisdiction where no public
health agency has declared
readiness to receive
electronic case reporting
data as of 6 months prior to
the start of the performance
period:
Public Health and
Clinical Data
Exchange
Public Health Registry
Reporting: (bonus)
The MIPS eligible
clinician is in active
engagement with a
public health agency
to submit data to
public health
registries.
N/A (measure is
Yes/No and requires
an affirmative
attestation to fulfill)
N/A (measure is
Yes/No and requires
an affirmative
attestation to fulfill)
none
Public Health and
Clinical Data
Exchange
Clinical Data Registry
Reporting: (bonus)
The MIPS eligible
clinician is in active
engagement to submit
data to a clinical data
registry.
N/A (measure is
Yes/No and requires
an affirmative
attestation to fulfill)
N/A (measure is
Yes/No and requires
an affirmative
attestation to fulfill)
none
Public Health and
Clinical Data
Exchange
Syndromic
Surveillance
Reporting: (bonus)
The MIPS eligible
clinician is in active
engagement with a
public health agency
to submit syndromic
surveillance data from
an urgent care setting
N/A (measure is
Yes/No and requires
an affirmative
attestation to fulfill)
N/A (measure is
Yes/No and requires
an affirmative
attestation to fulfill)
none
Protect Patient Health
Information: Protect
electronic protected
health information
(ePHI) created or
maintained by the
CEHRT through the
implementation of
appropriate technical,
administrative, and
physical safeguards.
Security Risk
Assessment:
Conduct or review a
security risk analysis
in accordance with the
requirements in 45
CFR 164.308(a)(1),
including addressing
the security (to include
encryption) of ePHI
data created or
maintained by
certified electronic
health record
technology (CEHRT)
in accordance with
requirements in 45
CFR 164.312(a)(2)(iv)
and 45 CFR
164.306(d)(3),
implement security
updates as necessary,
and correct identified
security deficiencies
as part of the MIPS
eligible clinician’s risk
management process.
N/A (measure is
Yes/No and requires
an affirmative
attestation to fulfill)
N/A (measure is
Yes/No and requires
an affirmative
attestation to fulfill)
none

Objective
Measure
Numerator
Denominator
Exclusion
Protect Patient Health
Information
SAFER Guides
High Priority Practices
Guide: Conduct an
annual assessment of
the High Priority
Practices Guide
SAFER Guides
N/A (measure is
Yes/No and requires
an affirmative
attestation to fulfill)
N/A (measure is
Yes/No and requires
an affirmative
attestation to fulfill)
none
(ii) Scoring Methodology for the CY 2025 Performance Period/2027 MIPS Payment Year
Table 63 reflects the scoring methodology for the Promoting Interoperability
performance category for the CY 2025 performance period/2027 MIPS payment year.
TABLE 63: Scoring Methodology for the CY 2025 Performance Period/2027 MIPS
Payment Year
Objective
Measure
Maximum
Points
Required/Optional
e-Prescribing
10 points
Required
Electronic
Prescribing
Query of PDMP
10 points
Required
Support Electronic Referral Loops by Sending Health
Information
15 points
Support Electronic Referral Loops by Receiving and
Reconciling Health Information
15 points
-OR-
Health Information Exchange Bi-Directional Exchange
30 points
-OR-
Health Information
Exchange
Enabling Exchange under TEFCA
30 points
Required (MIPS
eligible clinician’s
choice of one of the
three reporting
options)
Provider to Patient
Exchange
Provide Patients Electronic Access to Their Health
Information
25 points
Required
Report the following two measures:
• Immunization Registry Reporting
• Electronic Case Reporting
25 points
Required
Public Health and
Clinical Data
Exchange
Report one of the following measures:
• Public Health Registry Reporting
• Clinical Data Registry Reporting
• Syndromic Surveillance Reporting
5 points (bonus)
Optional
Notes: The Security Risk Analysis measure and the SAFER Guides measure are required but will not be assigned
points. Failure to submit an affirmative (“yes”) attestation will result in a zero score for the Promoting
Interoperability performance category.
In addition, MIPS eligible clinicians must submit an affirmative (“yes”) attestation regarding ONC direct review,
and an affirmative (“yes”) attestation that they did not knowingly and willfully take action to limit or restrict the
compatibility or interoperability of CEHRT, as required by § 414.1375(b)(3).
(iii) Exclusion Redistribution
Many required measures have exclusions associated with them as shown in Table 62. If a
MIPS eligible clinician believes that an exclusion for a particular measure applies to them, they
may claim it when they submit their data. The maximum points available in Table 63 do not
include the points that will be redistributed if a MIPS eligible clinician claims an exclusion for a
specific measure. For ease of reference, Table 64 shows how points will be redistributed among

the objectives and measures specified for the Promoting Interoperability performance category
for the CY 2025 performance period/2027 MIPS payment year in the event a MIPS eligible
clinician claims an exclusion for a given measure.
TABLE 64: Exclusion Redistribution for CY 2025 Performance Period/2027 MIPS
Payment Year
Objective
Measure
Redistribution if exclusion is
claimed
e-Prescribing
10 points to HIE objective
Electronic Prescribing
Query of PDMP
10 points to e-Prescribing
measure
Support Electronic Referral Loops by Sending Health
Information
15 points to Provide Patients
Electronic Access to Their
Health Information measure
Support Electronic Referral Loops by Receiving and
Reconciling Health Information
15 points to the Support
Electronic Referral Loops by
Sending Health Information
measure
-OR-
Health Information Exchange Bi-Directional Exchange
No exclusion
-OR-
Health Information
Exchange
Enabling Exchange under TEFCA
No exclusion
Provider to Patient
Exchange
Provide Patients Electronic Access to Their Health Information
No exclusion
Public Health and
Clinical Data Exchange
Report the following two measures:
• Electronic Case Reporting
• Immunization Registry Reporting
If an exclusion is claimed for
both measures, 25 points are
redistributed to the Provide
Patients Electronic Access to
their Health Information
measure
Notes: The Security Risk Analysis measure and the SAFER Guides measure are required but will not be assigned points.
Failure to submit an affirmative (“yes”) attestation will result in a zero score for the Promoting Interoperability
performance category.
In addition, MIPS eligible clinicians must submit an affirmative (“yes”) attestation regarding ONC direct review, and an
affirmative (“yes”) attestation that they did not knowingly and willfully take action to limit or restrict the compatibility or
interoperability of CEHRT, as required by § 414.1375(b)(3).
(iv) ONC Health IT Certification Criteria
For ease of reference, Table 65 lists the objectives and measures for the Promoting
Interoperability performance category for the CY 2025 performance period/2027 MIPS payment
year and the associated ONC health IT certification criteria set forth at 45 CFR 170.315, as is
currently applicable. We refer readers to the CY 2024 PFS final rule (88 FR 79307 through
79312) for our discussion of and amendments to the definition of CEHRT at § 414.1305.

TABLE 65: Promoting Interoperability Performance Category Objectives and
Measures and ONC Health IT Certification Criteria
Objective
Measure
Certification Criteria (CY 2025 Performance
Period/2027 MIPS Payment Year) in Title 45 of the
CFR
e-Prescribing
§ 170.315(b)(3) Electronic prescribing
Electronic
Prescribing
Query of PDMP
§ 170.315(b)(3) Electronic prescribing
Support electronic referral loops by sending
health information
§ 170.315(b)(1) Transitions of care
§ 170.315(b)(1) Transitions of care
Health Information
Exchange
Support electronic referral loops by receiving
and reconciling health information
§ 170.315(b)(2) Clinical information reconciliation and
incorporation
Examples of certified health IT capabilities to support
the actions of this measure may include but are not
limited to technology certified to the following criteria:
§ 170.315(b)(1) Transitions of care
§ 170.315(b)(2) Clinical information reconciliation and
incorporation
§ 170.315(g)(7) Application access — patient selection
§ 170.315(g)(9) Application access — all data request
Health Information
Exchange
(alternative)
Health Information Exchange (HIE Bi-
Directional Exchange
§ 170.315(g)(10) Application access — standardized
API for patient and population services
Examples of certified health IT capabilities to support
the actions of this measure may include but are not
limited to technology certified to the following criteria:
§ 170.315(b)(1) Transitions of care
§ 170.315(b)(2) Clinical information reconciliation and
incorporation
§ 170.315(g)(7) Application access — patient selection
§ 170.315(g)(9) Application access — all data request
Health Information
Exchange
(alternative)
Enabling Exchange under TEFCA
§ 170.315(g)(10) Application access — standardized
API for patient and population services
§ 170.315(e)(1) View, download, and transmit to 3rd
party
§ 170.315(g)(7) Application access — patient selection
§ 170.315(g)(9) Application access — all data request
Provider to Patient
Exchange
Provide patients electronic access to their health
information
§ 170.315(g)(10) Application access — standardized
API for patient and population services
Immunization registry reporting
§ 170.315(f)(1) Transmission to immunization
registries
Syndromic surveillance reporting
§ 170.315(f)(2) Transmission to public health agencies
— syndromic surveillance
Electronic case reporting
§ 170.315(f)(5) Transmission to public health agencies
— electronic case reporting
§ 170.315(f)(6) Transmission to public health agencies
— antimicrobial use and resistance reporting
Public health registry reporting
§ 170.315(f)(7) Transmission to public health agencies
— health care surveys
Public Health and
Clinical Data
Exchange
Clinical data registry reporting
No 2015 health IT certification criteria at this time.
Security Risk Assessment
The requirements are a part of CEHRT specific to each
certification criterion.
Protect Patient
Health Information
Safety Assurance Factors for EHR Resilience
Guides (SAFER Guides)
No 2015 health IT certification criteria at this time.
(g) Request for Information (RFI) Regarding Public Health Reporting and Data Exchange
Please note, this is an RFI only. In accordance with the implementing regulations of the
Paperwork Reduction Act of 1995 (PRA), specifically 5 CFR 1320.3(h)(4), this general
solicitation is exempt from the PRA. Facts or opinions submitted in response to general
solicitations of comments from the public, published in the Federal Register or other
publications, regardless of the form or format thereof, provided that no person is required to
supply specific information pertaining to the commenter, other than that necessary for self-
identification, as a condition of the agency's full consideration, are not generally considered
information collections and therefore not subject to the PRA.
Respondents are encouraged to provide complete but concise responses. This RFI is
issued solely for information and planning purposes; it does not constitute a Request for Proposal
(RFP), applications, proposal abstracts, or quotations. This RFI does not commit the U.S.
Government to contract for any supplies or services or make a grant award. Further, CMS is not
seeking proposals through this RFI and will not accept unsolicited proposals. Responders are
advised that the U.S. Government will not pay for any information or administrative costs
incurred in response to this RFI; all costs associated with responding to this RFI will be solely at
the interested party’s expense. Not responding to this RFI does not preclude participation in any
future procurement, if conducted. It is the responsibility of the potential responders to monitor
this RFI announcement for additional information pertaining to this request. Please note that
CMS will not respond to questions about the policy issues raised in this RFI. CMS may or may
not choose to contact individual responders. Such communications would only serve to further
clarify written responses. Contractor support personnel may be used to review RFI responses.
Responses to this notice are not offers and cannot be accepted by the U.S. Government to form a
binding contract or issue a grant. Information obtained as a result of this RFI may be used by the
U.S. Government for program planning on a non-attribution basis. Respondents should not
include any information that might be considered proprietary or confidential. This RFI should
not be construed as a commitment or authorization to incur cost for which reimbursement would

be required or sought. All submissions become U.S. Government property and will not be
returned. CMS may publicly post the comments received, or a summary thereof.
(i) Background
The COVID-19 public health emergency (PHE) highlighted the interdependencies of
public health and healthcare, and the importance of timely, integrated, and interoperable data
exchange across the health ecosystem to protect the health and safety of patients, populations,
and the broader public. It also called attention to the distance between where we are as a nation,
and where we want to be, with the interoperability of data between healthcare providers and
Public Health Agencies (PHAs), especially in the event of a fast-evolving pandemic or other type
of PHE. While many jurisdictions were able to demonstrate the advantages of capabilities such
as electronic laboratory reporting for reportable conditions, surveillance systems to support case
investigations, immunization registries to track COVID-19 immunizations, and syndromic
surveillance data for situational awareness, exchange across jurisdictions remains inconsistent
and, in some cases, burdensome.
The Promoting Interoperability performance category plays an important role in
advancing the exchange of health information between PHAs and MIPS eligible clinicians, using
certified Health IT Modules that meet criteria and standards established under the ONC Health
IT Certification Program. Measures under the Public Health and Clinical Data Exchange
objective focus on a key set of exchange capabilities for healthcare providers that have evolved
over time to incorporate new priorities and technical approaches. In recent years, we have also
focused on expanding and strengthening the Public Health and Clinical Data Exchange objective
to further support the exchange of data that ultimately supports better patient and public health
outcomes.
Efforts across HHS to advance the public health information infrastructure offer
opportunities to further evolve the Promoting Interoperability performance category. In 2020,
Centers for Disease Control and Prevention (CDC) launched the Data Modernization Initiative

(DMI),
764
a multi-year, billion-plus dollar public health ecosystem initiative aimed at moving the
public health community from a siloed and brittle public health data system to connected,
resilient, adaptable, and sustainable “response-ready” systems capable of meeting present and
future health challenges. CDC's vision for public health data expands on modernization efforts
and focuses on critical components to advance data for public health action to equitably protect
health, safety and security.
765
To establish clear, near-term priorities and milestones that
complement that longer term vision and improve alignment of data modernization efforts at all
levels of public health and across partners, CDC released its first Public Health Data Strategy
(PHDS) in 2023 and recently updated it.
766
The PHDS outlines the data, technology, policy, and
administrative actions essential to exchange critical core data efficiently and securely across
healthcare and public health and sets ambitious goals against which CDC will measure progress
over the next two years.
In tandem with these efforts to chart a new strategic direction for improvements to the
nation’s public health infrastructure, evolving technical approaches are offering opportunities to
automate and expand information exchange between healthcare providers and PHAs. ONC is
exploring updates to existing health IT certification criteria that support current measures in the
Promoting Interoperability performance category’s Public Health and Clinical Data Exchange
objective, new criteria that incorporate modern approaches to exchange, support additional types
of information needed by PHAs, and criteria that focus on entities receiving public health data.
In the HTI–1 final rule, ONC finalized updates to the health IT certification criterion for
electronic case reporting in 45 CFR 170.315(f)(5) incorporating standards-based approaches to
existing functional requirements in accordance with the HL7 FHIR Electronic Case Report
(eCR) Implementation Guide (IG) or HL7 Clinical Document Architecture (CDA) Electronic
Initial Case Report (eICR) IG (89 FR 1226). ONC is also considering recent recommendations
764
https://www.cdc.gov/surveillance/data-modernization/index.html.
765
https://www.cdc.gov/ophdst/about/advancing-data-for-public-health-action.html.
766
https://www.cdc.gov/ophdst/public-health-data-strategy/index.html.

from Federal advisory committees that have focused on issues related to public health
interoperability. These include the Public Health Data Systems Task Force, which was charged
by the Health Information Technology Advisory Committee (HITAC) to inform ONC’s
continued collaborative work with CDC on improving public health data systems, and in support
of CDC’s greater DMI efforts. In November 2022, the Public Health Data Systems Task Force
issued recommendations to the HITAC,
767
which included a focus on new criteria for Health IT
modules that support public health use cases that aim to standardize technology that receives
information from healthcare providers. In addition, the CDC Advisory Committee to the
Director (ACD) Data and Surveillance Workgroup adopted a report on November 3, 2022, which
addressed standards for public health data systems and implementing a certification program for
public health IT as well as other issues.
768
We are working in partnership with the CDC and ONC to explore how the Promoting
Interoperability performance category could advance public health infrastructure through more
advanced use of health IT and data exchange standards. This RFI describes a series of goals and
principles for the Promoting Interoperability performance category’s Public Health and Clinical
Data Exchange objective, provides information about recommended updates to health IT
certification criteria under consideration that may impact MIPS eligible clinicians, and seeks
public comment on potential updates that could help achieve these goals.
(ii) Goals for Public Health Reporting
As we look toward the future of the Public Health and Clinical Data Exchange objective
of the Promoting Interoperability performance category, we believe decision-making and
prioritization about policy change should adhere to four goals:
767
See “Final Report of the Health Information Technology Advisory Committee on Public Health Data Systems”
https://www.healthit.gov/sites/default/files/page/2022-11/2022-11-
10_PHDS_TF_Recommendations_Report_Transmittal_Letter_508.pdf.
768
See “Data and Surveillance Workgroup Report,” CDC Advisory Committee to the Director (ACD) Data and
Surveillance Workgroup (DSW). www.cdc.gov/about/pdf/advisory/DSW-Recommendations-Report.pdf.
(1) The meaningful use of CEHRT enables continuous improvement in the quality,
timeliness, and completeness of public health data being reported;
(2) The meaningful use of CEHRT allows for flexibility to respond to new public health
threats and meet new data needs without requiring new and substantial regulatory and technical
development;
(3) The meaningful use of CEHRT supports mutual data sharing between public health
and healthcare providers; and
(4) Reporting burden on MIPS eligible clinicians is significantly reduced.
These goals inform the questions provided at the end of this RFI. We invite public
comment on these four goals.
(iii) Public Health in the ONC Health IT Certification Program
We continue to collaborate closely with ONC on policy changes in the ONC Health IT
Certification Program that either impact existing functionality reflected in the Promoting
Interoperability performance category’s measures or represent new capabilities for MIPS eligible
clinicians that could offer opportunities to achieve our goals for the Public Health and Clinical
Data Exchange objective in the Promoting Interoperability performance category. In this section,
we describe specific topics we are discussing through this collaboration with ONC.
(1) Making available new capabilities for exchanging data with PHAs using the FHIR standard.
Current public health certification related criteria at 45 CFR 170.315(f)(1) through (7)
generally reference HL7 version 2 and CDA standards that support single-patient, event-based
submission of data from healthcare providers to PHAs, such as electronic transmission of
laboratory results (HL7
®
Version 2.5.1 Implementation Guide for Electronic Laboratory
Reporting to Public Health, Release 1 with Errata and Clarifications) or electronic initial case
reports (HL7 CDA
®
R2 Implementation Guide: Public Health Case Report – the Electronic
Initial Case Report (eICR) Release 2). However, these standards may not adequately support
more complex data exchange use cases, such as bulk exchange of data for patients who received

a specific vaccine. Approaches using FHIR could more effectively support a wide-scale public
health response and reduce burden of implementation and maintenance for data exchange
between and among healthcare providers and PHAs.
Increased availability of FHIR-based APIs across systems used by PHAs and healthcare
providers could help to create an ecosystem where PHAs could use health IT to securely query
data directly from the source, in real time, based on an initial push of relevant data when needed.
Availability of a FHIR API in a healthcare provider’s certified health IT could enable a PHA to
query a MIPS eligible clinician’s CEHRT for data on any patient with a specific condition when
needed, avoiding the need for a MIPS eligible clinician to take action to submit additional
information.
As noted, ONC has already finalized an update to the electronic case reporting criterion
in 45 CFR 170.315(f)(5), which provides an option to implement the HL7 FHIR eCR IG as part
of a Health IT Module certified to the criterion (89 FR 1226). The Public Health Data Systems
Task Force report stated that “FHIR-based query may offer public health additional avenues to
meet the needs of case investigation to supplement electronic case reporting and emerging public
health threats” and that “FHIR may support a more focused and relevant response by providers
to meet public health queries.”
769
While FHIR specifications are not available for all the use cases currently supported in
the public health criteria at § 170.315(f)(1) through (7), ONC continues to evaluate standards
development activities around the use of FHIR for public health data exchange that could be
incorporated into existing or new certification criteria, such as replacing HL7 version 2 and CDA
exchange specifications with a FHIR approach over time.
(2) Expanding the scope of public health exchange supported by certified health IT capabilities.
769
See “Final Report of the Health Information Technology Advisory Committee on Public Health Data Systems,”
https://www.healthit.gov/sites/default/files/page/2022-11/2022-11-
10_PHDS_TF_Recommendations_Report_Transmittal_Letter_508.pdf.

Existing health IT certification criteria are linked to measures under the Promoting
Interoperability performance category, covering use cases from transmission to immunization
registries and syndromic surveillance, and electronic case reporting.
The Public Health Data Systems Task Force report recommended the addition of several
additional certification criteria reflecting exchange of information such as birth and death data,
the results of newborn screening services, and situational awareness. ONC is monitoring these
and other areas of importance to public health that are not reflected in the current certification
criteria.
(3) Introducing health IT certification criteria for systems that receive public health data.
To date, ONC health IT certification criteria have been designed with systems that send
data to PHAs in mind, particularly health IT systems used by healthcare providers, that exchange
data with PHAs. Misalignment between certified health IT products and technology and systems
used by PHAs has created challenges for both healthcare providers and PHAs, including reliance
on complex workflows to accommodate non-harmonized and variable data elements and
exchange standards. Inefficiencies associated with workarounds and custom processes can lead
to further reductions in data quality, completeness, consistency, and interoperability.
The HITAC Public Health Data Systems Task Force’s report includes a recommendation
that ONC establish certification criteria for public health technologies used by Public Health
Authorities in support of their responsibilities in exchanging data for public health purposes
including those defined in the existing (f) criteria.
770
By establishing minimum functional capabilities and exchange standards to both send
and receive public health data, health IT certification criteria could enhance interoperability
across healthcare providers and PHAs and provide a long-term mechanism for alignment as data
exchange matures over time. An expansion of the ONC Health IT Certification Program to focus
770
Public Health Data Systems Task Force, Recommendation 23, p. 11
https://www.healthit.gov/sites/default/files/page/2022-11/2022-11-
10_PHDS_TF_Recommendations_Report_Transmittal_Letter_508.pdf.
on the receiving side could also bolster CDC’s public health infrastructure modernization efforts,
described above, by helping PHAs to align with healthcare provider data sources using the same
certification criteria and standards, enabling these entities to move together on a common
timeline for updating technology requirements.
(iv) RFI Questions
Section 1848(q)(2)(B)(iv) of the Act requires that we must apply the requirements under
section 1848(o)(2) of the Act for our assessment of whether a MIPS eligible clinician is a
meaningful EHR user under the Promoting Interoperability performance category. Section
1848(o)(2)(A) of the Act sets forth three criteria for determining whether a MIPS eligible
clinician is a meaningful EHR user. One of these criteria at section 1848(o)(2)(A)(ii) of the Act
requires that the MIPS eligible clinician demonstrate to the satisfaction of the Secretary that
during the performance period their CEHRT is connected in a manner that provides, in
accordance with law and standards applicable to the exchange of information, for the electronic
exchange of health information to improve the quality of health care, such as promoting care
coordination. We request that commenters consider this criterion in responding to our questions
for this RFI.
(1) Questions for Goal #1: Quality, Timeliness, and Completeness of Public Health Reporting
The Promoting Interoperability performance category’s requirement that MIPS eligible
clinicians report the level of “active engagement” between the MIPS eligible clinician, and a
PHA, as well as the recently established one-year limitation in how long a MIPS eligible
clinician may spend in Pre-Production and Validation for measures under the Public Health and
Clinical Data Exchange objective, has provided a basis to broadly incentivize the exchange of
EHR data (87 FR 49339 through 49340). It has helped to identify barriers that prevent MIPS
eligible clinicians from moving from Pre-Production and Validation (Option 1) to Validated Data
Production (Option 2), and it has helped the development of solutions to overcome identified
barriers. However, because active engagement reporting only requires an attestation of whether
a MIPS eligible clinician is reporting production data or still in the process of validation, this
approach does not allow us to assess MIPS eligible clinicians on the comprehensiveness, quality,
or timeliness of the data they provide to PHAs.
We are considering whether alternatives to the “active engagement” approach for the
measures under the Public Health and Clinical Data Exchange objective could better allow us to
assess MIPS eligible clinician’s performance, meet the data needs of PHAs, and ultimately allow
us to incentivize increased performance in these areas. We are interested in how we could
recommend alternatives to the “active engagement” approach in the context of the evolving
technical infrastructure described above. We are also interested in the increasing focus on
leveraging FHIR-based data exchange for public health reporting. Finally, we are interested in
ensuring that any changes to the active engagement approach are implemented in a way that
takes advantage of opportunities to further automate reporting and minimize administrative
burden for MIPS eligible clinicians.
● To date, all the measures in the Public Health and Clinical Data Exchange objective
assess whether there is active engagement between a MIPS eligible clinician, but they do not
measure the level of performance the MIPS eligible clinician has achieved in sending
information. Specifically, we are seeking public comment on the following questions:
Should CMS shift to numerator/denominator reporting requirements for current and
future measures in the Public Health and Clinical Data Exchange objective? If so, should CMS
prioritize only certain measures for numerator/denominator reporting?
New technical approaches such as the use of FHIR APIs to support information
exchange with PHAs could enable PHAs to query healthcare provider systems directly, after an
initial trigger, rather than relying on a healthcare provider to take action to share information.
Healthcare providers having to take action to share information adds burden to the healthcare
providers and increases the time it takes for the PHA to receive the information. How could
performance be measured under approaches such as the use of FHIR APIs to support information
exchange with PHAs? Would numerator/denominator reporting be appropriate under such
approaches?
● Continued expansion of the measures under the Public Health and Clinical Data
Exchange objective to address different reporting use cases can incentivize MIPS eligible
clinicians to make more comprehensive information available to PHAs. We are seeking public
comment on the following questions:
++ Should CMS continue add measures under the Public Health and Clinical Data
Exchange objective to include additional system-specific requirements (for example, vital
records)? If so, which ones and why?
++ Should CMS create a new measure for each new type of data or use case added to
the Public Health and Clinical Data Exchange objective? What are the risks of including too
many measures under the objective?
++ Alternatively, should CMS explore ways to group data types and use cases under a
more limited set of Public Health and Clinical Data Exchange objective measure? If so, are there
specific scenarios where doing so would make sense? Anecdotal reports suggest that some
healthcare providers are attesting to active engagement with public health for the eCR measure if
they report cases for at least one notifiable condition (for example, COVID-19).
How can CMS incentivize more complete electronic case reporting to PHAs? For
example, should CMS update the measure to require healthcare providers to meet a certain
threshold for conditions reported?
++ What potential benefit versus burden trade-offs CMS should consider? How should
CMS account for varying levels of public health readiness and capacity for expanding conditions
reported electronically, such as in rural areas?
++ What additional levers besides the Promoting Interoperability performance category
should CMS explore to improve the completeness of reporting to public health? How should
CMS work with other partners to incentivize or require reporting?

(2) Questions for Goal #2: Flexibility and Adaptability of the Public Health Reporting
Enterprise
During the COVID-19 and Mpox PHEs, healthcare providers and PHAs often had to
quickly update their systems to report case, laboratory, and vaccination data related to these
novel pathogens and interventions devised in response to them. In this section, we are seeking
information about how the Promoting Interoperability performance category could improve the
ability for public health infrastructure
771
to quickly adapt to new threats. Specifically, we are
seeking public comment on the following questions:
● How can the Promoting Interoperability performance category support or incentivize
response ready reporting capabilities for healthcare providers? What, if any, challenges exist
around sharing data with PHAs?
● How can CMS and ONC work with EHR vendors to ensure that provider systems are
being continually updated to meet new data needs, such as those in rural areas?
(3) Questions for Goal #3: Increasing Bi-Directional Exchange with Public Health Agencies
The transition to, and use of more modern, flexible approaches and networks that support
data exchange between and across public health and healthcare is a key goal of HHS efforts to
modernize the public health information infrastructure. We are interested in ways that the
Promoting Interoperability performance category can support this transition. Specifically, we are
seeking public comment on the following questions:
● Both CDC’s ACD and ONC’s HITAC have recommended that CDC and ONC work
together to establish certification criteria for public health technologies used by PHAs and
implement a coordinated, phased approach to incentivize and eventually require their
adoption.
772
How, if at all, could the Promoting Interoperability performance category support or
incentivize PHA adoption of certified systems and technologies?
771
https://www.cdc.gov/infrastructure/pdfs/PHIC-Overview.pdf.
772
https://www.healthit.gov/sites/default/files/page/2023-03/2023-02
08_HITAC_Annual_Report_for_FY22_508_1.pdf.
● How can CMS use the Public Health and Clinical Data Exchange objective to
incentivize early adoption of FHIR-based APIs for public health data exchange?
● CMS previously finalized the Enabling Exchange under TEFCA measure under the
HIE objective for MIPS eligible clinicians to attest to engaging in health information exchange.
Should CMS introduce an additional measure to allow MIPS eligible clinicians to receive credit
for the HIE objective by exchanging public health data through participation in TEFCA?
(4) Questions for Goal #4: Significantly Reduce Reporting Burden for Healthcare Providers
We are committed to continuing to reduce reporting burden for healthcare providers, such
as in rural areas, as part of any updates to the Promoting Interoperability performance category
undertaken to support the priorities described above. Specifically, we are seeking public
comment on the following questions:
● Under the current Public Health and Clinical Data Exchange objective, which
measures, or other requirements result in the most administrative burden for MIPS eligible
clinicians?
● How can the Promoting Interoperability performance category balance robust Public
Health and Clinical Data Exchange objective requirements with our desire to reduce burden on
MIPS eligible clinicians?
● How can new technical approaches to data exchange with PHAs, such as the use of
FHIR APIs, reduce burden for MIPS eligible clinicians? What are potential barriers to achieving
burden reduction as these new approaches are implemented?
f. MIPS Final Score Methodology
(1) Performance Category Scores
(a) Background
Sections 1848(q)(1)(A)(i) and (ii) and (5)(A) of the Act provide, in relevant part, that the
Secretary shall develop a methodology for assessing the total performance of each MIPS eligible
clinician according to certain specified performance standards and, using such methodology,
provide for a final score for each MIPS eligible clinician. Section 1848(q)(6)(A) of the Act
specifies that, to then determine a MIPS payment adjustment factor for each MIPS eligible
clinician for an applicable MIPS payment year, we must compare the MIPS eligible clinician’s
final score for the given year to the performance threshold we established for that same year in
accordance with section 1848(q)(6)(D) of the Act. We refer readers to section IV.A.4.g.(2) of
this proposed rule for further discussion of the performance threshold, and our calculation of
MIPS payment adjustment factors, and our proposals with respect thereto.
Section 1848(q)(2)(A) of the Act provides that the Secretary must assess each MIPS
eligible clinician with respect to four performance categories in determining each MIPS eligible
clinician’s final score: quality, resource use (referred to as “cost”), clinical practice improvement
activities (referred to as “improvement activities”), and meaningful use of certified EHR
technology (referred to as “Promoting Interoperability”). Section 1848(q)(2)(B) of the Act
describes the measures and activities that must be specified under each performance category,
including the quality performance category and cost performance category. Section 1848(q)(3)
of the Act provides that we must establish performance standards with respect to the measures
and activities specified under the four performance categories for a performance period,
considering historical performance standards, improvement, and the opportunity for continued
improvement. To calculate a final score for each MIPS eligible clinician for the performance
period of an applicable MIPS payment year, section 1848(q)(5)(A) of the Act provides that we
must develop a methodology for assessing the total performance of each MIPS eligible clinician
according to the performance standards we have established with respect to applicable measures
and activities specified for each performance category, using a scoring scale of 0 to 100.
In calculating the final score, we must apply different weights for the four performance
categories, subject to certain exceptions, as set forth in section 1848(q)(5) of the Act and at
§ 414.1380. Unless we assign a different scoring weight pursuant to these exceptions, for the
CY 2025 performance period/2027 MIPS payment year, the scoring weights for each
performance category are as follows: 30 percent for the quality performance category; 30 percent
for the cost performance category; 15 percent for the improvement activities performance
category; and 25 percent for the Promoting Interoperability performance category.
For the CY 2025 performance period/2027 MIPS payment year, we propose to update our
scoring methodologies to respond to statutory requirements and impacts observed in
performance data. In this proposed rule, we are proposing to update our scoring policies
consistent with these goals. Specifically, we propose to—
● Establish defined topped out benchmarks for certain topped out measures for clinicians
impacted by limited measure choice;
● Establish Complex Organization Adjustment for eCQMs reported by Virtual Groups
and APM Entities.
● Score Medicare CQMs using flat benchmarks for their first two performance periods
in the program.
● Modify the benchmarking methodology for scoring measures in the cost performance
category;
● Adopt a new cost measure exclusion policy;
● Eliminate the weighting of activities in the improvement activities performance
category; and
● Reduce the number of activities to which clinicians are required to attest.
We do not propose any changes to scoring policies for the Promoting Interoperability
performance category.
We refer readers to section IV.A.4.e.(3)(b)(iv) for discussion of scoring proposals in the
Improvement Activities performance category.
We refer readers to section IV.A.4.f.(1)(d) for discussion of proposals for scoring the cost
performance category.
(b) Scoring the Quality Performance Category for the Following Collection Types: Medicare
Part B Claims Measures, eCQMs, MIPS CQMs, QCDR Measures, the CAHPS for MIPS Survey
Measure and Administrative Claims Measures
We refer readers to the CY 2017, CY 2018, and CY 2019 Quality Payment Program final
rules, the CY 2020, CY 2021, CY 2022, and CY 2023 PFS final rules, and § 414.1380(b)(1) for
our current policies regarding, among other things, quality measure benchmarks, calculating total
measure achievement points, calculating the quality performance category score, including
achievement and improvement points, and the small practice bonus (81 FR 77276 through
77308, 82 FR 53716 through 53748, 83 FR 59841 through 59855, 84 FR 63011 through 63018,
85 FR 84898 through 84913, 86 FR65490 through 65509, and 87 FR 70088 through 70091). In
the CY 2024 PFS final rule, we finalized updates to our scoring flexibilities policy at §
414.1380(b)(1)(vii)(A) (88 FR 79368 through 79369).
(i) Scoring for Topped Out Measures in Specialty Measure Sets with Limited Measure Choice
We refer readers to the CY 2017, CY 2018, and CY 2019 Quality Payment Program final
rules, the CY 2023 PFS final rule (81 FR 77282 through 77287, 82 FR 53721 through 53727, 83
FR 59761 through 59765, and 88 FR 70090 through 70091), and § 414.1380(b)(1)(iv) for our
established topped out measure scoring policies.
Topped out measures are measures for which measure performance is considered so high
and unvarying that meaningful distinctions and improvements in performance can no longer be
made (81 FR 77136). We define topped out measures in § 414.1305 differently for process
measures and non-process measures. A topped out process measure means a measure with a
median performance rate of 95 percent or higher. A topped out non-process measure means a
measure where the Truncated Coefficient of Variation is less than 0.01 and the 75
th
and 90
th
percentile are with 2 standard errors. For MIPS eligible clinicians electing to report on measures
where they expect to perform well, we anticipated many measures would have performance
distributions clustered near the top. (81 FR 77282). Section 1848(q)(3)(B) of the Act requires
that in establishing performance standards with respect to measures and activities, we consider,
among other things, the opportunity for continued improvement. Topped out measures do not
provide an opportunity for continued improvement nor, more broadly, do payment adjustments
based on topped out measures incentivize clinicians to improve their care. As a result, we
finalized policies to identify and cap the scoring potential of such measures. Additionally, we
established practices for the removal of such measures, such as establishing the topped out
measure lifecycle, to continue to drive quality improvement in areas where such improvement is
possible and necessary.
The topped out measure lifecycle is described in the CY 2018 PFS final rule (82 FR
53721 and 53727). We established at § 414.1380(b)(1)(iv)(B) that we would cap scoring for
topped out measures at 7 points in the second consecutive year that it is identified as topped out.
If a measure has been identified as topped out for 3 consecutive years after being originally
identified through the benchmarks, such measure may then be proposed for removal through
notice-and-comment rulemaking (83 FR 59761). This timeline, however, is not fixed. We noted
our concern where removal of topped out measures would leave clinicians with fewer than 6
applicable measures to report and that such removal in those instances would impact some
specialties more than others (82 FR 53721). e We stated that consideration for ensuring available
applicable measures would be made when considering measure removals (83 FR 59763).
The topped out scoring cap and the topped out measure lifecycle were established with
the intention to drive continued quality improvement by providing clinicians with the ability to
plan for optimal quality measurement and reporting and by providing measure developers time to
develop and submit alternative measures for use in the program (82 FR 53727). However, the
pace of measure development has not matched the rate at which topped out measures would
ideally be removed under the established topped out lifecycle policy. Since the CY 2017
performance period/2019 MIPS payment year, the MIPS final list of quality measures has
decreased from 271 to 198 including the removal of 34 topped out measures that had reached the
end of the topped out measure lifecycle.
We have received feedback from interested parties and independently verified that
clinicians reporting specialty sets in which there is high presence of topped out measures
receiving the 7-point cap are often facing both limited measure choice and limited scoring
opportunities. Analysis of data from the CY 2022 performance period/2024 MIPS payment year
showed that only 7 percent of quality measure submissions were for topped out measures.
However, of those submissions, clinicians representing five specialties accounted for 54 percent
of the submissions of topped out measures that contributed to the final score. When we analyzed
the data from the CY2022 performance period/2024 MIPS payment year, we found that
clinicians in these specialties were facing limited measure choice, with an overrepresentation of
topped out measures among their measure selection. Some such topped out measures have been
retained in the program to ensure specialists will have applicable measures in the absence of
more robust measure development.
We acknowledge that certain clinician specialists have limited measure choice and that
their opportunities to maximize their MIPS performance score may be particularly affected by
the current topped out measure scoring policy. We appreciate that, as the performance threshold
increases, it may become more difficult for these clinician specialists to maximize their MIPS
performance score and secure positive payment adjustments for reasons entirely outside of their
control, primarily the topped-out measure scoring cap. In order to determine a MIPS payment
adjustment factor for each MIPS eligible clinician for a year, we must compare the MIPS eligible
clinician’s final score for the given year to the performance threshold we established for that
same year in accordance with section 1848(q)(6)(D) of the Act. Section 1848(q)(6)(D)(i) of the
Act requires that we compute the performance threshold such that it is the mean or median (as
selected by the Secretary) of the final scores for all MIPS eligible clinicians with respect to a
“prior period” specified by the Secretary. In the CY 2024 PFS final rule, we finalized the
performance threshold at a score of 75 points for the CY 2024 performance period/2026 MIPS
payment year at § 414.1405(b)(9)(iii) (88 FR 79319). We are proposing in section
IV.A.4.g.(2)(c) to maintain the performance threshold at 75 points for the CY 2025 performance
year/2027 MIPS payment year. As the number of topped out measures a clinician reports
increases, a clinician who must report topped out measures will see their maximum potential
quality performance category score decrease and the clinician must score as close to perfect as
possible on the topped out measures to mitigate the effect of the 7-point cap on the clinician’s
final score. Affected clinicians face additional difficulty should they be subject to additional
scoring policies, including reweighting of performance categories and reporting quality measures
that lack benchmarks. Reweighting of the Promoting Interoperability, cost, or both performance
categories increases the weighting of the quality performance category in the final score from 30
percent to 55 or 85 percent.
We want to address scoring scenarios in which limited measure choice compels clinicians to
report topped out measures with scoring caps consistent with our desire to facilitate fairer scoring
of all specialties. For this reason, we are proposing that beginning with the CY 2025
performance period/2027 MIPS payment year CMS could remove the 7-point cap for certain
topped out measures that we would select based on evaluating the factors discussed below. This
would allow clinicians who practice in specialties impacted by limited measure choice to be
scored according to defined topped out measure benchmarks that do not cap scores at 7 measure
achievement points. Table 66 is an illustrative example of the defined topped out measure
benchmark.

TABLE 66: Example Defined Topped Out Measure Benchmark
Measure Achievement Points
Performance Rate
1-1.9
84-85.9%
2- 2.9
86-87.9%
3-3.9
88-89.9%
4-4.9
90-91.9%
5-5.9
92-93.9%
6-6.9
94-95.9%
7-7.9
96-97.9%
8-8.9
98-99.9%
10
100%
As discussed above, given that clinicians reporting specialty measure sets with limited
measure choice are disproportionately hindered by the 7-point topped out measure scoring cap,
we would, in accordance with the methodology discussed below, focus on identifying the topped
out measures within specialty measure sets which clinicians with limited measure choice report.
We propose to identify the measures for which we would apply the defined topped out measure
benchmark on a yearly basis. Measures receiving the defined topped out measure benchmarks
would be proposed and adopted through notice-and-comment rulemaking concurrent with our
adoption of the MIPS final list of quality measures.
This proposed performance standard would aim, for clinicians with limited measure
availability, to continue to require high performance, but would not cap scoring potential for
exceptional performers and would offer better scoring opportunities for those performing below
the median in the distribution than under our current policy. Under the proposed topped out
measure benchmarking methodology, those achieving high performance rates would be rewarded
for high performance. Scores between 9 – 9.9 were intentionally left out. We considered
inclusion of scores in the 9
th
decile, but ultimately excluded them to necessitate exceptional
clinical quality performance to achieve maximum scores. This approach would ameliorate the
challenge of reporting on measures with a scoring cap while maintaining a high performance
standard for topped out measures.
In addition to addressing the scoring limit of the cap, we are also proposing to address the
scoring limits caused by the heavily skewed distribution of topped out measures. Previously,
because median clinician performance was heavily skewed towards the top of distribution for
many topped out measures the second highest achievable decile after the 7th decile may be the
3
rd
or 4
th
decile. We therefore propose to specify a topped out measure benchmark that would set
an even performance standard. Such a benchmark policy would facilitate clinician efforts to
improve clinical quality among clinicians for whom improvement is still possible. The proposed
distribution would allow those performing at or above the 97
th
percentile to achieve a score of
7.5 measure achievement points or greater to reward high performance and encourage clinical
quality improvement for those who perform below the median.
We propose to conduct an analysis annually to determine which specialty measure sets
are impacted by limited measure choice and which measures should be subject to the scoring cap
exemption. Our analysis would evaluate all specialty measure sets by collection type to assess
the impact of limited measure choice taking into account the influence of several scoring
considerations including the number of capped topped out measures, the number of measures in
the specialty set without historical benchmarks, and the scoring potential to meet or exceed the
performance threshold. We would then consider each capped topped out measure in the
corresponding specialty measure sets on a case-by-case basis for application of the defined
topped out measure benchmark. Additionally, annual consideration of which measures would
have the defined topped out measure benchmark applied would take into account any changes to
the availability of applicable measures and changes in the topped out status of measures that
previously had the defined topped out measure applied. A measure would not have a defined
topped out measure benchmark applied until it was identified as topped out for 2 consecutive
performance periods, the point at which point the 7-point cap would be applied. If suppression of
a measure or removal of a benchmark impacts a measure scored according to the defined topped
out measure benchmark, it would not be proposed again for the application of the defined topped
out measure benchmark and the performance standard would return to the standard scoring
policy at § 414.1380(b)(1)(i).
Measures that are identified as topped out for 3 consecutive performance periods may
still be proposed for removal through notice-and-comment rulemaking and extremely topped out
measures, those with an average mean performance within the 98
th
to 100
th
percentile range, can
also still be proposed for removal in the next rulemaking cycle, regardless of whether or not they
are in the midst of the topped out measure lifecycle (83 FR 59763). If a measure that is scored
according to a defined topped out measure benchmark later shows extremely topped out status, it
will be subject to this policy. Any such measure removal would continue to occur through
notice-and-comment rulemaking. While we aim to be responsive to those facing limited measure
choice, we do not believe that measures with topped out performance have the same value in the
program as measures that are not topped out, and they should be scored accordingly in instances
where doing so does not unfairly limit a clinician’s scoring opportunity. We believe these
parameters identify those most impacted by limited measure choice while continuing to
encourage high clinical quality measure performance.
This proposal would remain consistent with our current topped out measure lifecycle,
program goals, and historical approaches to scoring scenarios with limited measure choice. In the
CY 2017 Quality Payment Program final rule, we exempted measures reported via the CMS
Web Interface from the 7-point measure cap. The CMS Web Interface requires that MIPS
eligible clinicians submitting via the CMS Web Interface must submit all measures included in
the CMS Web Interface (81 FR 77116). Their lack of ability to select alternative measures made
the application of the 7-point measure cap inappropriate. Instead, we finalized a proposal at §
414.1380(b)(1)(ii)(A) to use benchmarks from the corresponding year of the Shared Saving
Program as the Shared Savings Program incorporates a methodology for measures with high
performance into the benchmark (82 FR 53721). The defined topped out measure benchmark
similarly aims to score clinicians facing limited measure choice on topped out measures using a
methodology that adjusts for high performance.
We considered several policy options to address topped out measure scoring for
clinicians facing limited measure choice. These included removing all topped out measures at the
end of the topped out measure lifecycle, exempting all topped out measures in specialty measure
sets from application of the 7-point cap, applying a denominator reduction for those scoring 7 out
of 10 measure achievement points on topped out measures in specialty measure sets, and
adopting a new reweighting policy for the quality performance category for clinicians impacted
by limited measure choice that score below the performance threshold. These approaches would
not appropriately address the barriers to fair scoring posed by limited measure choice, nor would
they incentivize and reward improvement in clinical quality measure performance. Additionally,
these alternatives would introduce additional scoring complexity and in one case, require
clinicians’ additional submission of a reweighting application to access potential benefits. The
proposed approach of applying defined topped out measure benchmarks for certain topped out
measures selected in accordance with the methodology set forth above avoids the additional
complexity of the other approaches by building on historical and current quality measure scoring
policies to topped out measures that does not require additional steps to access and is applicable
as we transition to MVPs.
For the reasons stated above, we are proposing to add § 414.1380(b)(1)(iv)(C) to state that
beginning with the CY 2025 performance period/2027 MIPS payment year, measures impacted
by limited measure choice as specified in paragraph (b)(1)(ii)(E) are not subject to the 7 measure
achievement point cap specified in paragraph (b)(1)(iv)(B). We propose a conforming change to §
414.1380(b)(1)(iv)(B).
We also propose to add § 414.1380(b)(1)(ii)(E) to state that, beginning with the CY 2025
performance period/2027 MIPS payment year, CMS will publish a list in the Federal Register
of topped out measures determined to be impacted by limited measure choice. Measures included
in the list are scored from 1 to 10 measure achievement points according to defined topped out
measure benchmarks calculated from performance data in the baseline period in which a
performance rate in the 97
th
percentile corresponds to 7.5 measure achievement points.
Accordingly, we also propose to update § 414.1380(b)(1)(ii) to state that except as provided in
paragraphs (b)(1)(ii)(B) through (F), benchmarks will be based on performance by collection
type, from all available sources, including MIPS eligible clinicians and APMs, to the extent
feasible, during the applicable baseline or performance period. We are also proposing to make
conforming changes to this section to include a previous inadvertent omission of paragraph
(b)(1)(ii)(D) in addition the proposed new exceptions in paragraphs (b)(1)(ii)(E) and (F)
corresponding to policies discussed in sections IV.A.4.f.(1)(b)(i) and IV.A.4.f.(1)(c)(i)
respectively.
(ii) Proposed approach for determining topped out measures impacted by limited measure
choice and subject to the proposed defined topped out measure benchmark and the proposed list
of measures that would be subject to the defined topped out measure benchmark for the CY 2025
performance period/2027 MIPS Payment Year
Under this proposal, we would annually determine and publish a list of topped out
measures that would have the 7-point cap removed and be subject to the proposed defined topped
out measure benchmark. To identify which topped out measures would be added to the list, we
would review each specialty measure set by collection type and identify if the prevalence of
topped out measures within such a set hinders a clinician’s ability to successfully participate in
the MIPS quality performance category. To make such a determination, we would analyze the
ability of clinicians reporting the specialty measure sets under review to reasonably achieve
75 percent of available quality achievement points based upon the measures available to them
and program requirements. As stated, the analysis would be conducted for each specialty
measure set and would be further broken down by collection type. At the collection type level,
each measure would be assigned points based upon the current benchmarking data: new
measures receive 7 or 5 points based on year in the program, measures with benchmarks are
given points based upon the highest decile achievable with a less than perfect score (less than
100 percent or greater than 0 percent for inverse measures), and measures with no available
historic benchmark are given 0 points. All measure set points would be added together to get an
output of scoring potential; the Medicare Part B claims collection type measure sets have an
additional 6 points added to the output to account for the small practice bonus. The sum of
quality achievement points for each measure set would be compared to the analysis threshold,
which is 75 percent of available quality achievement points, based upon number of available
measures. Any measure sets that are not able to meet or exceed the threshold would be flagged as
‘at-risk.’ Additional factors that we would take into consideration would include whether or not:
● A measure within the specialty measure set is considered a cross cutting measure;
● A measure within the specialty measure set is a broadly applicable measure, which we
would consider to be a measure included in three or more specialty sets; and
● There are more than ten measures, by collection type, available in the specialty set. We
request comment on our proposal to score topped out measures impacted by limited measure
choice using specialty defined topped out measures including the proposed defined topped out
measure benchmark.
Table 67 contains the list of measures that meet the criteria specified above and for which
we are proposing to apply the defined topped out measure benchmark for the CY 2025
performance period/2027 MIPS payment year. Specialty sets impacted by limited measure
choice include Pathology, Anesthesiology, Diagnostic Radiology, and Radiation Oncology.

TABLE 67: Proposed topped out measures impacted by limited measure choice and subject
to defined topped out measure benchmark for the CY 2025 performance period/2027 MIPS
Payment Year by Specialty Set
Measure ID
Collection Type
Measure Title
143
eCQM, MIPS CQM
Oncology: Medical and Radiation – Pain
Intensity Quantified
249
Medicare Part B Claims, MIPS CQM
Barret’s Esophagus
250
Medicare Part B, MIPS CQM
Radical Prostatectomy Pathology
Reporting
360
MIPS CQM
Optimizing Patient Exposure to Ionizing
Radiation: Count of Potential High Dose
Radiation Imaging Studies: Computed
Tomography (CT) and Cardiac Nuclear
Medical Studies
364
MIPS CQM
Optimized Patient Exposure to Ionizing
Radiation: Appropriateness: Follow-up
CT imaging for Incidentally Detected
Pulmonary Nodules According to
Recommended Guidelines
395
Medicare Part B, MIPS CQM
Lung Cancer Reporting
(Biopsy/Cytology Specimens)
396
MIPS CQM
Lung Cancer Reporting (Resection
Specimens
397
Medicare Part B, MIPS CQM
Melanoma Reporting
405
MIPS CQM
Appropriate Follow-up Imaging for
Incidental Abdominal Lesions
406
MIPS CQM
Appropriate Follow-up Imaging for
Incidental Thyroid Nodules in Patients
424
MIPS CQM
Perioperative Temperature Management
430
MIPS CQM
Prevention of Post-Operative Nausea
and Vomiting (PONV) – Combination
Therapy
436
MIPS CQM
Radiation Consideration for Adult CT:
Utilization of Dose Lowering
Techniques
440
MIPS CQM
Skin Cancer: Biopsy Reporting Time –
Pathologist to Clinician
463
MIPS CQM
Prevention of Post-Operative (POV) –
Combination Therapy (Pediatrics)
477
MIPS CQM
Multimodal Pain Management
We request comment on the proposed approach that we would use each year to identify
the list of measures subject to the defined topped out measure benchmark, as well as the
proposed list of topped out measures impacted by limited measure choice and subject to defined
topped out measure benchmark for CY 2025 performance period/2027 MIPS Payment Year.
(iii) Complex Organization Adjustment for Virtual Groups and APM Entities

Section 1848(q)(5)(B)(ii)(I) of the Act requires the Secretary to encourage MIPS eligible
clinicians to report on applicable quality measures through the use of Certified Electronic Health
Record Technology (CEHRT) and qualified clinical data registries. Section 1848(q)(5)(B)(ii)(II)
of the Act provides that the Secretary shall treat such clinicians as satisfying the clinical quality
measures reporting requirement described in section 1848(o)(2)(A)(iii) of the Act if they report
such measures through the use of such EHR technology for a given performance period.
773
In the
CY 2017 Quality Payment Program final rule (81 FR 77297), we established the measure bonus
point and bonus cap for using CEHRT for end-to-end electronic reporting. We refer readers to §
414.1380(b)(1)(v)(B) for our previously finalized policies regarding measure bonus points for
end-to-end electronic reporting.
In the CY 2022 PFS final rule, we finalized our proposal to end measure bonus points for
end-to-end electronic reporting. We noted that as we move to MVPs we are simplifying scoring
by removing many of the transition policies that we established in the early years of the program
in order to develop a stronger MVP program and promote alignment between MIPS and MVPs.
As stated in previous rulemaking, we are working to develop ways to encourage the use of
CEHRT for electronic reporting without offering measure bonus points. We stated that we
believed that we could fulfill the statutory requirement at section 1848(q)(5)(B)(ii)(I) of the Act
to encourage the usage of CEHRT, through other means. Accordingly, over the past few years,
we have reduced the availability and limited who can submit data for the Medicare Part B claims
collection type to only small practices noting that the Medicare Part B claims collection type is
not an electronic means of submission.
In the current state, satisfying quality reporting requirements is not equally attainable for
each MIPS eligible clinician or entity in the Quality Payment Program (QPP). As the QPP and its
components (MIPS and Advanced APMs) has matured, reliance on and subsequent requirements
773
Section 1848(o)(2)(A)(iii) of the Act requires a meaningful EHR user to demonstrate to the satisfaction of the
Secretary that the eligible professional, among other things, has not knowingly and willfully taken action to limit or
restrict the compatibility or interoperability of the certified EHR technology.
necessitating the use of CEHRT have increased (88 FR 79331). The adoption of CEHRT has
been slower than anticipated, indicating additional support for quality reporting may further
support attainment of program goals while also addressing the observed and reported challenges
faced by complex entities and organizations working to meet new requirements.
Virtual Groups and APM Entities may experience technological barriers to electronic
report, including challenges aggregating patient data across multiple TINs, data deduplication,
and interoperability between different health IT/EHR systems. In the CY 2018 Quality Payment
Program final rule, commenters indicated that data aggregation across multiple TINs for Virtual
Groups would be burdensome for rural and small practices and that this burden could be
prohibitive for Virtual Groups’ successful participation in MIPS (82 FR 53610). Commenters
stated that the requirement for Virtual Groups to aggregate data across the Virtual Group could
be a potential barrier for Virtual Group participation and would not likely occur without error (82
FR 53610). Commenters noted that the potential penalty for failure to overcome technical
challenges in data aggregation was, at that time, a severe 5 percent decrease to the payment
adjustment for TINs that are already operating on small margins (82 FR 53610). Commenters
noted that the aggregation of data across various TINs and health IT systems may be logistically
difficult and complex, as groups and health IT systems have different ways of collecting and
storing data and stated that data aggregation across various systems for measures and activities
under each performance category may not be possible if qualified registries do not have the
option to assist Virtual Groups (82 FR 53610). Additionally, commenters stated that practices
already have an issue of not being able to deduplicate patient data across different health IT
systems/multiple EHRs and indicated that Virtual Groups need clear guidelines regarding how to
achieve accurate reporting (82 FR 53613).
Furthermore, commenters expressed concerns that registries supporting Virtual Group
reporting would be opening themselves to potential penalties as a result of technical challenges
in data aggregation across multiple EHR systems (82 FR 53610). The commenters indicated that
registries may not be able to support Virtual Group reporting due to legal and operational
complexity. Certain registries have internal data governance standards, including patient safety
organization requirements, that they must follow when contracting with single TIN participants
that may complicate their ability to support Virtual Group reporting due to necessary legal
agreements between solo practitioners and small groups within a Virtual Group (82 FR 53611).
Commenters requested that CMS provide guidance to registries regarding how to handle data
sharing among Virtual Groups with respect to patient safety organization requirements and
provide guidance regarding the expectations for registries supporting virtual group reporting (82
FR 53611).
APM Entities are organizations that participate in CMS’s various APMs, including the
Medicare Shared Savings Program and CMS Innovation Center models. APM Entities face
similar organizational challenges reporting eCQMs because of their complex structures, new and
innovative partnerships under their respective APMs, and utilization of multiple EHR
technologies (88 FR 79097). For ACOs in the Shared Savings Program, and ACOs and other
large organizations in CMS Innovation Center models, these issues are further exacerbated by
scale and patient volume, as discussed in more detail further in this section. In the CY 2023 PFS
proposed rule, commenters noted issues for ACOs participating in the Shared Savings Program
reporting all payer/all patient eCQMs/MIPS CQMs related to meeting all-payer data
requirements, data completeness requirements, and data aggregation (87 FR 69837). ACOs also
noted the financial burden of aggregating, deduplicating, and exporting eCQM data across
multiple TINs and EHRs (88 FR 79097). As summarized in the CY 2024 PFS final rule,
commenters noted that the current state of data standards and interoperability will not yet fully
enable Shared Saving Programs ACOs to meet the eCQM reporting requirements successfully
and encouraged CMS to continue working with providers to facilitate the transition to all-
payer/all-patient measures even as/if the provider or ACO chooses to report Medicare CQMs (88
FR 79107). In the CY 2024 PFS final rule (88 FR 79098), we stated that our long-term goal
continues to be to support Shared Savings Program ACOs in the adoption of all payer/all patient
measures and transition to digital quality measurement reporting. These challenges also can be
faced by other large APM Entities participating in CMS Innovation Center models.
Additionally, APM Entities in CMS Innovation Center models, regardless of size, are
participating in innovative payment and delivery designs through which they may forging new
partnerships among different providers and provider types to provide care to attributed
beneficiaries to meet the APM’s care delivery requirements. For example, the Making Care
Primary (MCP) Model includes several payment innovations to support participants in delivering
advanced primary care and aims to strengthen coordination between patients’ primary care
clinicians, specialists, social service providers, and behavioral health clinicians, ultimately
leading to chronic disease prevention, fewer emergency room visits, and better health outcomes.
The model will operate through three progressive tracks, with the first track being designed for
organizations with no prior value-based care experience. Additionally, the model includes State
partnerships and multi-payer alignment objectives. Participation in this model will involve
forming new relationships to provide whole-person care to beneficiaries, which is likely to
necessitate bridging data across multiple technologies and involve new and complex
administrative burdens in the provision of this advanced primary care.
Based on our assessment and understanding over the past 2 years, we have learned that
there are complexities and challenges for Virtual Groups and APM Entities in adopting all-
payer/all-patient collection types, and as a result, the widespread adoption of the all-payer/all
patient collection types requires further time and support. We have come to understand that
further support is needed for complex organizations. As an example, Shared Saving Program
ACOs provide a high volume of services, particularly those related to preventative screening
measures. An internal analysis of performance year 2022 submission data indicates that Shared
Savings Program ACOs reported on 33 times more denominator eligible patients for eCQM 001
– Diabetes: HbA1c Poor Control (>9%), 53 times more denominator eligible patients for eCQM
134 – Preventative Care and Screening: Screening for Depression and Follow-Up Plan, and 25
times more denominator eligible patients for eCQM 236 – Controlling High Blood Pressure than
other MIPS reporters. In performance year 2022, one ACO reported on over 700,000
denominator eligible beneficiaries for a single eCQM.
The requirement to aggregate patient data collected across multiple health records into a
single data stream before sending to CMS poses administrative challenges and the need for
additional resources for Virtual Groups and APM Entities, including Shared Savings Program
ACOs. Additionally, data deduplication is resource intensive and requires the development of
new workflows to ensure accuracy. Stakeholders have also noted that patient files exist in
multiple, disparate EHRs since each EHR system collects and stores data differently. This is
important as moving to reporting eCQMs requires building new processes to fill data gaps and
ensure data accuracy and causes participants often to customize workflows for data processing,
such as using Quality Reporting Document Architecture (QRDA) I (individual patient) and
QRDA III (measured entity’s aggregate) data submission approaches for quality reporting. EHR
vendors have also expressed concerns regarding the need for more time to develop new features
that can facilitate eCQM reporting processes. Some interested parties have also voiced concerns
that clinician specialty or patient population could yield lower quality scores when reporting
eCQMs and create resistance to switching to this collection type.
We noted in the CY 2024 PFS final rule that a few commenters agreed that Medicare
CQMs would address most of ACOs’ concerns regarding all payer/all patient reporting in the
Shared Saving Program, such as difficulties reporting for those ACOs with a higher proportion
of specialty practices or groups with multiple EHRs, beneficiaries with no primary care
relationship, and shouldering a greater burden when matching and deduplicating patient records
(88 FR 79101). Other commenters noted Medicare CQMs reduce concerns about specialists
reporting on primary care focused measures. Commenters shared that Medicare CQMs were
responsive to several key concerns raised by Shared Savings Program ACOs regarding feasibility
of implementing eCQMs/MIPS CQMs, including equity concerns (88 FR 79101). However, we
maintain that consistent with section 1848(q)(5)(B)(ii)(I) we support and encourage providers as
they perform any necessary bridging of data across multiple technologies, which can involve
new and complex administrative burdens.
To account for the organizational complexities faced by Virtual Groups and APM
Entities, including ACOs in the Shared Savings Program, we are proposing to establish a
Complex Organization Adjustment beginning in the CY 2025 performance period/2027 MIPS
Payment Year. Virtual Group and APM Entities would receive one measure achievement point
for each submitted eCQM that meets the data completeness at § 414.1380(b)(1)(iii) and case
minimum requirements at § 414.1340. Each reported eCQM may not receive more than 10
measure achievement points and the total achievement points (numerator) may not exceed the
total available measure achievement points (denominator) for the quality performance category.
The Complex Organization Adjustment for a Virtual Group or APM Entity may not exceed 10
percent of the total available measure achievement points in the quality performance category.
The adjustment would be added for each measure submitted at the individual measure level.
Adding one point for each eCQM would help complex organizations to overcome
barriers to reporting eCQMs while not masking overall quality performance. By limiting the
Complex Organization Adjustment to Virtual Groups and APM Entities, we can limit scoring
inflation and target this intervention to those facing challenges to eCQM implementation.
Moreover, while acknowledging the Complex Organization Adjustment is a recognition of
current challenges to eCQM reporting we believe that adoption of the Fast Healthcare
Interoperability Resources (FHIR) Application Programing Interface (API) would reduce or
eliminate the barriers posed by organizational complexities to eCQM reporting and will revisit
and end this Adjustment as uptake of FHIR API increases, requirements surrounding the use of
FHIR API are established, or other barriers posed by organizational complexity are otherwise
reduced. This Adjustment differs from the previous end-to-end electronic reporting bonus in that
it does not merely award measure achievement points for reporting but provides an adjustment
for clinicians facing complex organizational barriers for adopting the eCQM collection type.
We propose to add § 414.1380(b)(1)(vii)(C) to provide that, beginning in the CY 2025
performance period/2027 payment year, a Virtual Group and an APM Entity receives one
measure achievement point for each eCQM submitted that meets the case minimum requirement
at paragraph (b)(1)(iii) and the data completeness requirement at § 414.1340. Each measure may
not to exceed 10 measure achievement points. The total adjustment to the Virtual Group or APM
Entity’s quality performance category score under this paragraph may not exceed 10 percent of
the total available measure achievement points. Accordingly, we proposed to update
§ 414.1380(b)(1)(vii) to state a MIPS eligible clinician's quality performance category score is
the sum of all the measure achievement points assigned for the measures required for the quality
performance category criteria plus the measure bonus points in paragraph (b)(1)(v) and Complex
Organization Adjustment in paragraph (b)(1)(vii)(C). The sum is divided by the sum of total
available measure achievement points. The improvement percent score in paragraph (b)(1)(vi) is
added to that result. The quality performance category score cannot exceed 100 percentage
points.
We request comment on our proposal to implement a Complex Organization Adjustment
for Virtual Groups and APM Entities, including ACOs in the Shared Savings Program.
(c) Scoring the Quality Performance Category through MIPS for ACOs in the Shared Saving
Program.
(i) Proposal to Score for Shared Savings Program ACOs Reporting Medicare CQMs
using Flat Benchmarks
In section III.G.4.c.2.c of this proposed rule we are proposing to score Shared Savings
Program ACOs reporting Medicare CQMs in the APP Plus quality measure set using flat
benchmarks for their first two performance periods in MIPS. Consistent with this discussion, we
are proposing to add § 414.1380(b)(1)(ii)(F) to state that beginning in the CY 2025 performance
period/2027 MIPS payment year, measures of the Medicare CQM collection type would be
scored using flat benchmarks for their first two performance periods in MIPS. We request
comment on our proposal to score Medicare CQMs using flat benchmarks for their first two
performance periods in MIPS.
(d) Cost Performance Category Score
(i) Scoring the Cost Performance Category Background
As discussed previously, to calculate a final score for each MIPS eligible clinician for the
performance period of an applicable MIPS payment year, section 1848(q)(5)(A) of the Act
requires that we must develop a methodology for assessment of the total performance of each
MIPS eligible clinician, according to the performance standards we have established in
accordance with section 1848(q)(3) of the Act, with respect to applicable measures and activities
specified for each performance category. For the final score, we must use a scoring scale of 0 to
100.
We refer readers to § 414.1380(b)(2) for our policies regarding scoring for the cost
performance category and to previous rules where these policies were finalized, including the
CY 2017 Quality Payment Program final rule (81 FR 77308 through 77311), the CY 2018
Quality Payment Program final rule (82 FR 53748 through 53752), the CY 2019 PFS final rule
(83 FR 59856), the CY 2021 PFS final rule (85 FR 84877 through 84880), the CY 2022 PFS
final rule (86 FR 65507 through 65509), the CY 2023 PFS final rule (87 FR 70091 through
70093), and the CY 2024 PFS final rule (88 FR 79369 through 79373).
We are proposing to: (1) modify the benchmark methodology for scoring measures
specified for the cost performance category beginning with the CY 2024 performance
period/2026 MIPS payment year; and (2) adopt a new cost measure exclusion policy beginning
with the CY 2025 performance period/2027 MIPS payment year.
(ii) Benchmark Methodology for Scoring the Cost Performance Category
(A) Background on Methodology for Scoring the Cost Performance Category

Under § 414.1350(a), we specify cost measures for a performance period to assess the
performance of MIPS eligible clinicians on the cost performance category. Under §
414.1380(b)(2), we score each MIPS eligible clinician
774
on each cost measure attributed to them
in accordance with § 414.1350(b) so long as the MIPS eligible clinician meets the minimum case
volume specified under § 414.1350(c) to be scored on that cost measure. Cost performance
category measures are attributed to MIPS eligible clinicians through, and scored based on, claims
data; we do not require MIPS eligible clinicians to submit any additional data on cost measures
to CMS (§ 414.1325(a)). We have codified our cost performance category scoring policies at §
414.1380(b)(2).
Specifically, we finalized at § 414.1380(b)(2) that we will score each cost measure
attributed to a MIPS eligible clinician (meeting or exceeding the minimum case volume) by
assigning achievement points between one and ten based on the MIPS eligible clinician’s
performance on the cost measure during the performance period compared to the measure’s
benchmark. We award the achievement points (including partial points) based on which
benchmark decile range the MIPS eligible clinician’s performance on the measure is between.
The MIPS eligible clinician’s cost performance category score (to be added to the final score) is
the sum (not to exceed 100 percent) of: (1) the total number of achievement points earned by the
MIPS eligible clinician divided by the total number of available achievement points; and (2) the
cost improvement score, as determined under § 414.1380(b)(2)(iv) (§ 414.1380(b)(2)(iii)). We
will not calculate a cost performance category score if the MIPS eligible clinician is not
attributed any cost measures for the performance period because the MIPS eligible clinician has
not met the minimum case volume as specified under § 414.1350(c) for any of the cost measures
774
As noted previously, the term MIPS eligible clinician is defined in § 414.1305 as including a group of at least one
MIPS eligible clinician billing under a single tax identification number. A cost measure therefore may be attributed to
a group that includes at least one MIPS eligible clinician and the group may therefore be scored on the cost
performance category as a whole. We refer readers to our policies governing group reporting and scoring under MIPS
as set forth in § 414.1310(e).

or a benchmark has not been created for any of the cost measures that would otherwise be
attributed to the MIPS eligible clinician (§ 414.1380(b)(2)(v)).
As set forth in § 414.1380(b)(2)(i), we determine cost measure benchmark ranges based
on all MIPS eligible clinicians’ performance on each attributed cost measure during the
performance period. We determine a benchmark for a cost measure only if at least 20 MIPS
eligible clinicians are attributed and meet the minimum case volume for that measure, as
specified under § 414.1350(c). If we cannot determine a benchmark for a cost measure because
an insufficient number of MIPS eligible clinicians were attributed the measure (that is, less than
20 MIPS eligible clinicians meet the minimum case volume), then we will not assign any score
for the measure for any MIPS eligible clinician (§ 414.1380(b)(2)(i) and (v)). We refer readers to
our prior rulemakings, including the CY 2017 Quality Payment Program final rule (81 FR 77308
through 77311), for detailed discussion of our previously finalized policies for determining a
benchmark for each cost measure and then assignment of achievement points based on
comparison of a MIPS eligible clinician’s performance to that established benchmark.
Specifically, under our current scoring policy at § 414.1380(b)(2) and benchmark
methodology, MIPS eligible clinicians with the lowest average cost per episode or per
beneficiary would be in the top decile (Decile 10) and receive the highest number of available
achievement points (10). On the other end of the spectrum, MIPS eligible clinicians with the
highest average cost per episode or per beneficiary would be in the bottom decile (Decile 1) and
receive the lowest number of achievement points (1). More information about how average cost
per beneficiary or per episode are calculated and translated to MIPS achievement points is
available in the 2023 MIPS Cost User Guide.
775
Table 68 provides an example of using benchmark deciles along with partial achievement
points to assign achievement points for a sample cost measure under our current methodology.
775
https://qpp.cms.gov/resources/document/fac61617-20ef-4d31-9f0f-4a0e76620ca3.

The following formula is used to determine the number of partial points awarded to the MIPS
eligible clinician:
Benchmark Decile # + [(measure score, expressed as a dollar amount – bottom of
benchmark decile range) / (top of benchmark decile range – bottom of benchmark decile range)]
= Cost Measure Achievement Points.
776
TABLE 68: Example of Using Benchmark Deciles and Partial Points to Assign
Achievement Points for Performance on the Screening/Surveillance Colonoscopy Cost
Measure
Benchmark Decile
Cost per Episode
Percentile
Possible Points
Benchmark Decile 1
$1330.65-$1126.35
99
th
1.0–1.9
Benchmark Decile 2
$1126.34-$1062.93
90
th
2.0-2.9
Benchmark Decile 3
$1062.92-$1025.75
80
th
3.0-3.9
Benchmark Decile 4
$1025.74-$997.78
70
th
4.0-4.9
Benchmark Decile 5
$997.77-$969.73
60
th
5.0-5.9
Benchmark Decile 6
$969.72-$940.03
50
th
6.0-6.9
Benchmark Decile 7
$940.02-$904.83
40
th
7.0-7.9
Benchmark Decile 8
$904.82-$860.44
30
th
8.0-8.9
Benchmark Decile 9
$860.43-$779.69
20
th
9.0-9.9
Benchmark Decile 10
$779.68 and lower
10
th
10
In the CY 2021 PFS final rule (85 FR 84877 through 84880), we finalized at
§ 414.1350(d)(4) the weight of the cost performance category to be 20 percent of the MIPS final
score for the 2023 MIPS payment year and at § 414.1350(d)(5) the weight of the cost
performance category to be 30 percent of the MIPS final score for the 2024 MIPS payment year
and each subsequent MIPS payment year. We noted that such an approach would allow us to
reach the statutorily required weight of 30 percent by the 2024 MIPS payment year (see section
1848(q)(5)(E)(i)(II) of the Act) while reducing the impact of experiencing an increase in the
weight of the cost performance category too much in any one year and providing clinicians with
an eased gradual and incremental transition starting with the 2023 MIPS payment year.
Since, MIPS eligible clinicians have raised concerns about cost performance category
scoring having a negative impact on their final MIPS score. Multiple factors have likely
contributed to clinician concerns.
776
https://qpp-cm-prod-content.s3.amazonaws.com/uploads/2378/2023%20MIPS%20Cost%20User%20Guide.pdf.

First, there has been an increase in weight for the cost performance category over time.
Particularly, due to the COVID-19 Public Health Emergency (PHE) which ended on May 11,
2023, we reweighted the cost performance category’s score to zero percent of the final score for
many MIPS eligible clinicians. Specifically, we announced on April 6, 2020 that, due to the
COVID-19 PHE, we would apply our extreme and uncontrollable circumstances reweighting
policies described under § 414.1380(c)(2)(i) to MIPS eligible clinicians nationwide and extend
the deadline to submit an application for reweighting the quality, cost, improvement activities or
Promoting Interoperability reporting categories for the CY 2019 performance period/2021 MIPS
payment year (85 FR 19277 and 19278). Also, for the CY 2020 performance period/2022 MIPS
payment year and the CY 2021 performance period/2023 MIPS payment year, we extensively
applied our reweighting policies, described under § 414.1380(c)(2)(i), to MIPS eligible clinicians
nationwide due to the COVID-19 PHE.
777,778
As a result, the CY 2022 performance period/2024
MIPS payment year was the first MIPS payment year that the cost performance category score
generally constituted 30 percent of MIPS eligible clinicians’ final scores (section
1848(q)(5)(E)(i)(II) of the Act). Second, the number of cost measures has increased over time,
and therefore, more MIPS eligible clinicians are being measured on the cost performance
category and on new measures.
Additionally, based on our calculation of cost performance category scores for the CY
2022 performance period/2024 MIPS payment year, we observed lower scores for the cost
performance category than for the quality performance category, even though they each
generally constitute 30 percent of the final score. Recent analyses of CY 2022 performance
period/2024 MIPS payment year data have identified the unweighted mean cost performance
category score was 59 out of 100, while the unweighted mean score for the quality performance
category was 74 out of 100. We also note that the unweighted mean scores were 95 out of 100
777
https://qpp-cm-prod-content.s3.amazonaws.com/uploads/816/2020%20Cost%20Quick%20Start%20Guide.pdf.
778
https://qpp-cm-prod-
content.s3.amazonaws.com/uploads/1298/2021%20MIPS%20Cost%20Quick%20Start%20Guide.pdf.
for the improvement activities performance category and 94 out of 100 for the Promoting
Interoperability performance category.
There are several factors that may have contributed to a significantly lower score in the
cost performance category, compared to the other categories. First, measures in the cost
performance category are scored against a benchmark determined based on average performance
of all MIPS eligible clinicians during that same performance period (§ 414.1380(b)(2)
introductory text and (b)(2)(i)) rather than a benchmark determined based on historical data,
which is used, wherever possible, for non-administrative claims-based quality measures in the
quality performance category. Benchmarks determined based on historical data for the quality
performance category provide MIPS eligible clinicians with helpful performance targets in
advance of or during the performance period. Meanwhile, the performance period benchmarks
for the cost performance category do not provide MIPS eligible clinicians with information about
performance targets before or during the performance period. We believe that using benchmarks
based in the performance period is a better approach for the cost performance category than
using benchmarks based on historical data because different payment policies may apply during
the historical period than during the performance period, which may affect the cost of care for
patients treated by MIPS eligible clinicians.
Second, in traditional MIPS (compared to MVP reporting), MIPS eligible clinicians are
scored on each cost measure for which they meet the established case minimum and a
benchmark can be calculated. In the quality performance category, if a clinician reports more
than the required number of quality measures, we use the highest scored outcome measure and
then the next highest scored measures to reach a total of 6 sored quality measures to calculate the
clinician’s MIPS quality performance category score. The current cost benchmark methodology
uses a decile range based on linear percentile distributions and assigns 5.0 to 6.9 achievement
points to clinicians with cost measure scores within the 50th to 60th percentiles (Table 68).

For the example cost measure presented in Table 68, the cost measure median, the 50
th
percentile, is $969.72. If a MIPS eligible clinician’s average cost per episode for the measure is
$1,104 (about $135 above the median), the MIPS eligible clinician’s cost falls within Benchmark
Decile 2, for which the MIPS eligible clinician may receive between 2.0 and 2.9 achievement
points. We then use the following formula to determine the number of partial points awarded to
the MIPS eligible clinician:
Benchmark Decile # + [(measure score, expressed as a dollar amount – bottom of
benchmark decile range) / (top of benchmark decile range – bottom of benchmark decile range)]
= Cost Measure Achievement Points.
779
Based on this partial points calculation formula, the clinician would receive 0.3 partial
points, resulting in a cost measure score of 2.3 out of 10 achievement points for the
Screening/Surveillance Colonoscopy cost measure under this example.
This score may have the effect of lowering the MIPS eligible clinician’s final score, as
discussed previously. If the MIPS eligible clinician is only attributed and scored on this single
cost measure and does not receive a cost improvement score, then their score for the cost
performance category would be based on the cost measure’s score of 2.3 out of 10 achievement
points. Their score for the cost performance category would be 0.23 (2.3 / 10 = 0.23), equal to
the total number of achievement points earned by the MIPS eligible clinician divided by the total
number of available achievement points under § 414.1380(b)(2)(iii)(A). Based on the final score
calculation under § 414.1380(c), the contribution of the cost performance category score to the
final score for this MIPS eligible clinician would be equal to the cost performance category score
multiplied by the cost performance category weight (30 percent if the MIPS eligible clinician has
not received any reweighting) and would be 6.9 out of 30 (0.23 x 30 = 6.9), which would be 23
out of 100 points for the cost performance category’s contribution to the final score.
779
https://qpp-cm-prod-content.s3.amazonaws.com/uploads/2378/2023%20MIPS%20Cost%20User%20Guide.pdf.
To illustrate how this cost performance category score could lower the final score, if this
MIPS eligible clinician received perfect scores in each of the other three performance categories,
based on the final score calculation under § 414.1380(c) and the respective performance category
weights when all four performance categories are scored without reweighting, we would use the
formula as described below. For this example, we have not included the complex patient bonus.
MIPS Final score = [(60/60 × 30 for quality) + (2.3/10 × 30 for cost) + (40/40 × 15 for
improvement activities) + (100/100 × 25 for Promoting Interoperability)] × 100 = 76.9.
In this example, MIPS final score of 76.9 for the MIPS eligible clinician is just above the
2024 MIPS payment year performance threshold of 75. Therefore, under the current cost scoring
methodology, a MIPS eligible clinician scoring near the median on a cost measure would need to
score perfectly (or nearly perfectly) within the other three performance categories to receive a
final score slightly above the performance threshold and to avoid a negative payment adjustment.
The unweighted cost performance category score of 23 out of 100 noticeably lowers the MIPS
eligible clinician’s MIPS final score.
(B) Proposed Modification to Scoring Methodology for the Cost Performance Category
Beginning with CY 2024 Performance Period/2026 MIPS Payment Year
In light of the concerns identified with our current cost scoring policies, we are proposing
to modify the methodology for scoring the cost performance category beginning with the CY
2024 performance period/2026 MIPS payment year. The proposed cost scoring methodology
would be based on standard deviation, median, and an achievement point value that is derived
from the performance threshold. Specifically, for a MIPS eligible clinician whose average costs
attributed under a cost measure would be equal to the median cost for all MIPS eligible clinicians
attributed that measure, we would assign an achievement point value equal to 10 percent of the
performance threshold. For example, for the CY 2024 performance period/2026 MIPS payment
year, the median would have an achievement point value of 7.5, based on a performance
threshold of 75 as finalized at § 414.1405(b)(9)(iii). For each cost measure, the cut-offs for
benchmark ranges would be calculated based on standard deviations, expressed in dollars, from
the median.
The benchmark ranges, the median, and the performance threshold-derived achievement
point values aligned with the median would be dynamic and responsive to changes in average
costs per episode or per beneficiary assessed by cost measures and performance thresholds for
each CY performance period/MIPS payment year. The performance threshold-derived point
values could change based on the performance threshold established for each performance
period/MIPS payment year. The standard deviations from the median used to determine cutoffs
for benchmark ranges for each year would be reviewed for any necessary updates annually based
on performance across MIPS eligible clinicians within the cost performance category and the
performance threshold established for the performance period/MIPS payment year. We would
perform analyses when the performance threshold changes to set the benchmark ranges. To
determine the benchmark ranges, we would adhere to the following principles: (1) center the
majority of average costs per episode or per beneficiary around the performance threshold-
derived point value; (2) determine benchmark ranges according to the statistical distribution
curve of the average cost per episode or per beneficiary; and (3) distribution of achievement
points for cost measures should be reflective of overall program performance. We refer readers
to Table 69 for an example of how the proposed cost scoring methodology could be implemented
for a specific cost measure when the performance threshold is set to 75.
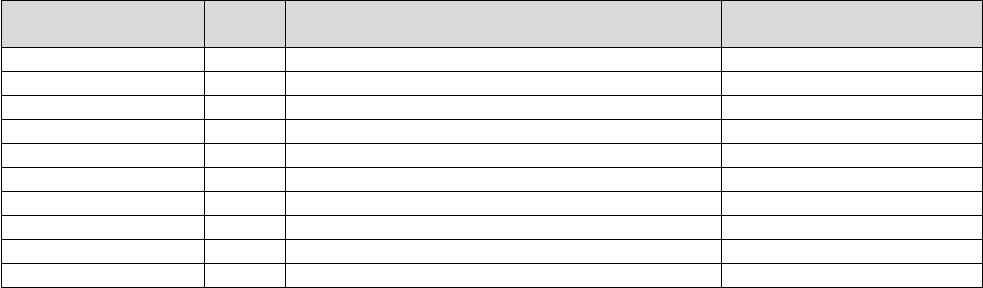
TABLE 69: Example of Implementation of the Proposed Cost Scoring Methodology for
Assignment of Achievement Points for Performance on the Screening/Surveillance
Colonoscopy cost measure
Benchmark Range
Points
Proposed Methodology for Bottom of Benchmark
Range ($)
Example Benchmark Ranges
Benchmark Range 1
1 - 1.9
Median cost + (2.75 x standard deviation)
$1341.93 - $1308.10
Benchmark Range 2
2 - 2.9
Median cost + (2.5 x standard deviation)
$1308.09 - $1.274.26
Benchmark Range 3
3 - 3.9
Median cost + (2.25 x standard deviation)
$1274.25 - $1240.43
Benchmark Range 4
4 - 4.9
Median cost + (2 x standard deviation)
$1240.42 - $1172.75
Benchmark Range 5
5 - 5.9
Median cost + (1.5 x standard deviation)
$1172.74 - $1105.08
Benchmark Range 6
6 – 6.9
Median cost + (1 standard deviation)
$1105.07 - $1037.40
Benchmark Range 7
7 - 7.9
Median cost + (0.5 x standard deviation)
$1037.39 - $902.05
Benchmark Range 8
8 - 8.9
Median cost - (0.5 x standard deviation)
$902.04 - $834.38
Benchmark Range 9
9 - 9.9
Median cost - (1 x standard deviation)
$834.37 - $766.70
Benchmark Range 10
10
Median cost - (1.5 x standard deviation)
$766.69 and below
Continuing with the example of the Screening/Surveillance Colonoscopy cost measure,
now presented in Table 69 as an example of implementation of the proposed cost scoring
methodology, the median (50th percentile) cost would remain $969.72. Under the proposed cost
scoring methodology, for the CY 2024 performance period/2026 MIPS payment year, a MIPS
eligible clinician with a cost per episode equal to the median cost of all cases attributed to all
MIPS eligible clinicians would receive 7.5 achievement points out of 10 possible achievement
points.
Using the same example as previously presented in section IV.A.4.f.(1)(d)(ii)(A) of this
proposed rule, we would apply the proposed cost scoring benchmark methodology as shown in
Table 69 to a MIPS eligible clinician with an average cost per episode for this measure that is
$1,104 (about $135 above the median). Based on the analysis of data in this example, the
standard deviation for the Screening/Surveillance Colonoscopy cost measure would be $135.35.
This value for the standard deviation would then be used to calculate the benchmark ranges in
Table 69 by plugging in this value for the standard deviation for each benchmark range. For
example, “Median cost + (1 x $135.35)” would be calculated for "Median cost + (1 standard
deviation)" for the bottom of Benchmark range 6. As shown with the example in Table 69,
under our proposed cost scoring methodology, the MIPS eligible clinician’s average cost per
episode of $1,104 would fall within Benchmark Range 6 for the Screening/Surveillance
Colonoscopy cost measure, for which the MIPS eligible clinician may receive between 6.0 and
6.9 achievement points.
Under our proposal to modify the cost performance category’s scoring methodology for
individual cost measures, we would continue to use our established formula to assign partial
achievement points:
Benchmark Range # + [(measure score, expressed as a dollar amount – bottom of
benchmark range) / (top of benchmark range – bottom of benchmark range)] = Cost Measure
Achievement Points.
As a result, using the example shown in Table 69, under our proposed cost scoring
methodology, the MIPS clinician would receive 6.02 cost measure achievement points (6 +
[($1,104 - $1,105.07) / ($1,037.40 - $1,105.07)] = 6.02). The assignment of 6.02 achievement
points under the proposed cost scoring methodology would be closer to the performance
threshold equivalent of 7.5 than the assignment of 2.3 achievement points under the current cost
scoring methodology, as discussed in our previous example in section IV.A.4.f.(1)(d)(ii)(A) of
this proposed rule.
In this example, the MIPS eligible clinician’s score for the cost performance category
would be 0.602 (6.02/10 = 0.602), equal to the total number of achievement points earned by the
MIPS eligible clinician divided by the total number of available achievement points under §
414.1380(b)(2)(iii)(A). Based on the final score calculation under § 414.1380(c), the contribution
of the cost performance category score to the final score for this MIPS eligible clinician would
be equal to the cost performance category score multiplied by the cost performance category
weight (30 percent if the MIPS eligible clinician has not received any reweighting) and would be
18.06 out of 30 (0.602 x 30 = 18.06) or 60.2 out of 100.
If this MIPS eligible clinician received perfect scores in each of the other three
performance categories, based on the final score calculation under § 414.1380(c) and the
respective performance category weights when all four performance categories are scored
without reweighting, we would use the formula as described below. For this example, we have
not included the complex patient bonus.
MIPS Final score = [(60/60 × 30 for quality) + (6.02/10 × 30 for cost) + (40/40 × 15 for
improvement activities) + (100/100 × 25 for Promoting Interoperability)] × 100 = 88.06.
This MIPS final score of 88.06 for the MIPS eligible clinician would be well above the
2024 MIPS payment year performance threshold of 75. The cost performance category score of
60.2 out of 100 would not noticeably lower the MIPS eligible clinician’s MIPS final score.
This proposed modification in our scoring methodology for cost measures would align
the assignment of achievement points for cost measures so that clinicians with costs near the
measure’s 50th percentile (median) would not receive a disproportionately low score. Based on
our analyses utilizing data from the CY 2022 performance period/2024 MIPS payment year, this
proposed methodology would increase the mean cost performance category score (unweighted)
for clinicians with a cost performance category score from 59 out of 100 to 71 out of 100 (an
increase of 11.9 points). Further, this proposed cost scoring methodology would increase the
means for each cost measure score by amounts ranging from 0.04 to 2.52 points. Our analysis
showed that, under our proposed methodology, the mean final score for MIPS eligible clinicians
assessed on at least one cost measure and receiving a cost performance category score would
increase by 3.89 points. Under our analysis, our proposed scoring methodology would not
negatively impact MIPS eligible clinicians whose average costs for a specific cost measure are
around the median.
Specifically, our analysis supports our intended goal for the proposed modification to the
scoring methodology: MIPS eligible clinicians who deliver care at an average cost near the
median costs for all MIPS eligible clinicians attributed the measure would receive scores at, or
very close to, the performance threshold-derived score. Additionally, this proposed modification
would address MIPS eligible clinicians’ concerns that cost measure scoring negatively impacts
their final scores more than other performance categories, including disparate negative effects for
MIPS eligible clinicians who are scored on the cost performance category compared to clinicians
not scored on the cost performance category.
To codify this proposed policy, we propose to modify § 414.1380(b)(2) to specify that
achievement points are awarded based on which benchmark range the MIPS eligible clinician's
performance on the measure is in. We also propose to specify that CMS assigns partial points
based on where the MIPS eligible clinician’s performance falls between the top and the bottom
of the benchmark ranges. The terms “decile” and “percentile distribution” are currently used in §
414.1380(b)(2) to describe the scoring methodology used to award achievement points and
assign partial points. However, under the proposed methodology, the term “decile” no longer
accurately describes how the benchmark ranges would be constructed. We believe the more
general term “benchmark range” accurately describes both the current and the proposed cost
scoring methodology, and therefore propose to modify § 414.1380(b)(2) to use “benchmark
range” in lieu of “decile” and “percentile distribution.” We are not proposing any modifications
to the remainder of the language currently at § 414.1380(b)(2), which provides that, for each cost
measure attributed to a MIPS eligible clinician, the clinician receives one to ten achievement
points based on the clinician’s performance on the measure during the performance period
compared to the measure’s benchmark.
We are also not proposing any modifications to the language currently at §
414.1380(b)(2)(i), generally governing if and how CMS determines a cost measure’s benchmark.
However, we are proposing to codify our current cost scoring policy, previously finalized in the
CY 2017 QPP final rule (81 FR 77308 through 77311), with modification by adding language at
§ 414.1380(b)(2)(i)(A). We propose to specify at § 414.1380(b)(2)(i)(A) that, for the 2019
through 2025 MIPS payment years, CMS determines cost measure benchmark ranges based on
linear percentile distributions.
We are also proposing to codify our proposed benchmarking methodology at §
414.1380(b)(2)(i)(B). We propose to specify at § 414.1380(b)(2)(i)(B) that, beginning with the
2026 MIPS payment year, for each cost measure, CMS determines 10 benchmark ranges based
on the median cost of all MIPS eligible clinicians attributed the measure, plus or minus standard
deviations and that CMS awards achievement points based on which benchmark range a MIPS
eligible clinician’s average cost for a cost measure corresponds. We also propose to codify at §
414.1380(b)(2)(i)(B) that, beginning with the 2026 MIPS payment year, CMS awards
achievement points equivalent to 10 percent of the performance threshold for a MIPS eligible
clinician whose average cost attributed under a cost measure is equal to the median cost for all
MIPS eligible clinicians attributed the measure.
We request public comments on this proposal to modify our scoring methodology for
measures specified under the cost performance category.
(iii) Proposed Adoption of Additional Cost Measure Exclusion Policy
(A) Background on Cost Measure Exclusion Policy
We refer readers to § 414.1380(b)(2)(v)(A) and the CY 2022 PFS final rule (86 FR 65507
through 65509) for our previously established policy for excluding a single cost measure from a
MIPS eligible clinician’s score for the cost performance category. As described at §
414.1380(b)(2)(v)(A), we established that, beginning with the 2024 MIPS payment year, if data
used to calculate a score for a cost measure are impacted by significant changes during the
performance period, such that calculating the cost measure score would lead to misleading or
inaccurate results, then the affected cost measure is excluded from the MIPS eligible clinician’s
or group’s cost performance category score. We also established at § 414.1380(b)(2)(v)(A) that
“significant changes” are changes external to the care provided, and that CMS determines may
lead to misleading or inaccurate results. We specified at § 414.1380(b)(2)(v)(A) that significant
changes include, but are not limited to, rapid or unprecedented changes to service utilization, and
will be empirically assessed by CMS to determine the extent to which the changes impact the
calculation of a cost measure score that reflects clinician performance.
As described in the CY 2022 PFS final rule (86 FR 65507 through 65509), we finalized
the policy at § 414.1380(b)(2)(v)(A) to provide scoring flexibility in instances where changes
during a performance period impede the effective measurement of cost. We identified that there
is a need for additional flexibility in calculating the scores for cost measures to account for the
impact of changing conditions that are beyond the control of individual MIPS eligible clinicians
and groups. We noted that this flexibility would allow us to ensure that clinicians are not
impacted negatively when performance is affected not due to the care provided, but due to
external factors. We noted that we would determine whether such external changes impede the
effective measurement of cost by considering factors including: The extent and duration of the
changes, and the conceptual and empirically tested relationship between the changes and each
measure’s ability to accurately capture clinician cost performance (86 FR 65508). Empirical
testing could include assessing whether there are rapid or unprecedented changes to patient case
volume or case mix, and the extent to which this could lead to misleading or inaccurate results
(86 FR 65508).
(B) Proposal to Permit Exclusion of a Cost Measure when Impacted by Errors and When
Significant Changes Occur Outside of the Performance Period
In the CY 2022 PFS final rule, for the quality performance category, we modified the
quality measure exclusion policy at § 414.1380(b)(1)(vii)(A) to change “significant changes” to
“significant changes or errors” (86 FR 65492) and to include the omission of codes or inclusion
of inactive or inaccurate codes to provide that for each measure submitted, if applicable, and
impacted by significant changes or errors prior to the applicable data submission deadline at
§ 414.1325(e), performance is based on data for 9 consecutive months of the applicable CY
performance period. Currently, for the cost performance category, we do not include “errors” in
addition to “significant changes” within our cost measure exclusion policy at
§ 414.1380(b)(2)(v)(A). To provide CMS with greater flexibilities to be responsive to any errors
or significant changes outside of the control of MIPS eligible clinicians that negatively impact
the ability of specific cost measure(s) to assess clinician performance, we are proposing to add a
new cost measure exclusion policy at § 414.1380(b)(2)(v)(B) similar to the quality measure
exclusion policy. Additionally, to further align our measure exclusion policies among the
performance categories, we propose to include “errors” for the cost performance category.
Specifically, we are proposing that, beginning with the 2027 MIPS payment year, if data used to
calculate a score for a cost measure are impacted by significant changes or errors affecting the
performance period, such that calculating the cost measure score would lead to misleading or
inaccurate results, then the affected cost measure is excluded from the MIPS eligible clinician's
or group's cost performance category score.
For purposes of this cost measure exclusion policy at § 414.1380(b)(2)(v)(B), we are
proposing to define “significant changes or errors” as changes or errors external to the care
provided, and that CMS determines may lead to misleading or inaccurate results that negatively
impact the measure’s ability to reliably assess performance. While we are proposing to include
“errors” within this policy for the cost performance category, as the quality performance
category already does, the list of what “significant changes or errors” includes would differ by
performance category to capture differences in how cost measures and quality measures are
calculated and measured. For instance, unlike quality measures for which MIPS eligible
clinicians generally must submit data to CMS to be assessed, cost measures are calculated by
CMS solely based on the review of administrative claims data and should not be impacted by
reporting errors. However, cost measures could be impacted by CMS calculation errors. Further,
under our proposed cost measure exclusion policy, errors would be external to the care provided,
and such that CMS determines may lead to misleading or inaccurate results that negatively
impact the measure’s ability to reliably assess performance. Under our proposed exclusion policy
for cost measures, significant changes or errors would include, but would not be limited to, rapid
or unprecedented changes to service utilization, the inadvertent omission of codes or inclusion of
codes, or changes to clinical guidelines or measure specifications. Additionally, these would not
automatically result in cost measure exclusion. Instead, we would determine whether there is a
negative impact from the significant change or error when deciding if a cost measure will be
excluded.
Specifically, before applying this cost measure exclusion policy, we are proposing that
CMS would empirically assess the affected cost measure to determine the extent to which the
changes or errors impact the calculation of a cost measure score such that calculating the cost
measure score would lead to misleading or inaccurate results that negatively impact the
measure’s ability to reliably assess performance. We believe that it is important to clarify that a
change or error would not automatically result in measure exclusion, but instead, that we would
need to determine whether there is a negative impact from the change or error that would affect
cost measure scoring.
Because significant changes or errors can have an ongoing impact on a measure beyond a
single performance period, we are proposing that the new cost measure exclusion policy at §
414.1380(b)(2)(v)(B) allow us to exclude cost measures when such changes and errors occur
outside of the performance period, but otherwise affect the performance period. For example, if a
cost measure is impacted by a coding change or guidance that requires substantive changes to a
measure, we may not be able to modify the measure within one performance period. In such
circumstances, we would want to exclude the cost measure for the affected performance periods
due to the ongoing impact on the measure. We would ensure that if data used to calculate a score
for a cost measure are impacted by significant changes or errors affecting one or more
performance periods delivering misleading or inaccurate results, then the affected cost measure
could be excluded from the MIPS eligible clinician's or group's cost performance category score.
We believe that the cost measure should be able to be excluded regardless of when we become
aware of the issue, when the significant change came into effect, or when the error first occurred.
Therefore, we are proposing language at § 414.1380(b)(2)(v)(B) to address data used to calculate
a score for a cost measure being impacted by significant changes and errors affecting a
performance period, even if they do not occur during the performance period.
We are proposing that this cost measure exclusion policy would be effective beginning
with the CY 2025 performance period/2027 MIPS payment year so this policy would be in place
as soon as feasible after the opportunity for notice-and-comment rulemaking.
This proposal would add language at § 414.1380(b)(2)(v)(B) to specify that, beginning
with the 2027 MIPS payment year, if data used to calculate a score for a cost measure are
impacted by significant changes or errors affecting the performance period, such that calculating
the cost measure score would lead to misleading or inaccurate results, then the affected cost
measure would be excluded from the MIPS eligible clinician’s or group’s cost performance
category score. We propose to specify that “significant changes or errors” are changes or errors
external to the care provided, and that CMS determines may lead to misleading or inaccurate
results that negatively impact the measure’s ability to reliable assess performance. We also
propose to specify that significant changes or errors would include, but are not limited to, rapid
or unprecedented changes to service utilization, the inadvertent omission of codes or inclusion of
codes, or changes to clinical guidelines or measure specifications. We propose that CMS would
empirically assess the affected cost measure to determine the extent to which the changes or
errors impact the calculation of a cost measure score such that calculating the cost measure score
would lead to misleading or inaccurate results that negatively impact the measure’s ability to
reliably assess performance.
We request public comments on this proposal.
g. MIPS Payment Adjustments
(1) Background
Section 1848(q)(6)(A) of the Act requires that we specify a MIPS payment adjustment
factor for each MIPS eligible clinician for a year. This MIPS payment adjustment factor is a
percentage determined by comparing the MIPS eligible clinician’s final score for the given year
to the performance threshold we established for that same year in accordance with section
1848(q)(6)(D) of the Act. The MIPS payment adjustment factors specified for a year must result
in differential payments such that MIPS eligible clinicians with final scores above the
performance threshold receive a positive MIPS payment adjustment factor, those with final
scores at the performance threshold receive a neutral MIPS payment adjustment factor, and those
with final scores below the performance threshold receive a negative MIPS payment adjustment
factor.
For previously established policies regarding our determination and application of MIPS
payment adjustment factors to each MIPS eligible clinician, we refer readers to the CY 2017
Quality Payment Program final rule (81 FR 77329 through 77343), CY 2018 Quality Payment
Program final rule (82 FR 53785 through 53799), CY 2019 PFS final rule (83 FR 59878 through
59894), CY 2020 PFS final rule (84 FR 63031 through 63045), CY 2021 PFS final rule (85 FR
84917 through 84926), CY 2022 PFS final rule (86 FR 65527 through 65537), CY 2023 PFS
final rule (87 FR 70096 through 70102), and CY 2024 PFS final rule (88 FR 79373 through
79380).
(2) Establishing the Performance Threshold
(a) Statutory Authority and Background
As discussed above, to determine a MIPS payment adjustment factor for each MIPS
eligible clinician for a year, we must compare the MIPS eligible clinician’s final score for the
given year to the performance threshold we established for that same year in accordance with
section 1848(q)(6)(D) of the Act. Section 1848(q)(6)(D)(i) of the Act requires that we compute
the performance threshold such that it is the mean or median (as selected by the Secretary) of the
final scores for all MIPS eligible clinicians with respect to a prior period specified by the
Secretary. Section 1848(q)(6)(D)(i) of the Act also provides that the Secretary may reassess the
selection of the mean or median every 3 years.
Sections 1848(q)(6)(D)(ii) through (iv) of the Act provided special rules, applicable only
for certain initial years of MIPS, for our computation and application of the performance
threshold for our determination of MIPS payment adjustment factors. These special rules are no
longer applicable for establishing the performance threshold for the CY 2025 performance
period/2027 MIPS payment year. We refer readers to the CY 2024 PFS proposed rule (88 FR
52596) for further information on these previously applicable requirements as they explain our
prior computations of the performance threshold.
In the CY 2022 PFS final rule (86 FR 65527 through 65532), we selected the mean as the
methodology for determining the performance threshold for the CY 2022 performance
period/2024 MIPS payment year through CY 2024 performance period/2026 MIPS payment
year. We also established in our regulation at § 414.1405(g) that, for each of the 2024, 2025, and
2026 MIPS payment years, the performance threshold would be the mean of the final scores for
all MIPS eligible clinicians from a prior period. As discussed under section IV.A.4.g.(2)(b) of
this proposed rule, we are proposing to continue using the mean as the methodology for
determining the performance threshold for the 2027, 2028, and 2029 MIPS payment years.
In the CY 2024 PFS final rule (88 FR 79373 through 79380), we established the
performance threshold for the CY 2024 performance period/2026 MIPS payment year by
calculating the mean of the final scores for all MIPS eligible clinicians using CY 2017
performance period/2019 MIPS payment year data. As further discussed under section
IV.A.4.g.(2)(c), we are proposing to continue using the mean of the final scores for all MIPS
eligible clinicians from the CY 2017 performance period/2019 MIPS payment year to establish
the performance threshold as 75 points for the CY 2025 performance period/2027 MIPS payment
year.
For further information on our current performance threshold policies, we refer readers to
the CY 2017 Quality Payment Program final rule (81 FR 77333 through 77338), CY 2018
Quality Payment Program final rule (82 FR 53787 through 53792), CY 2019 PFS final rule (83

FR 59879 through 59883), CY 2020 PFS final rule (84 FR 63031 through 63037), CY 2021 PFS
final rule (85 FR 84919 through 84923), CY 2022 PFS final rule (86 FR 65527 through 65532),
CY 2023 PFS final rule (87 FR 70096 through 70100), and CY 2024 PFS final rule (88 FR
79373 through 79380).
We codified the performance thresholds for each of the first 8 years of MIPS at
§ 414.1405(b)(4) through (9). These performance thresholds are shown in Table 71.
TABLE 71: Performance Thresholds for the CY 2017 Performance Period/2019 MIPS
Payment Year through the CY 2024 Performance Period/ 2026 MIPS Payment Years
MIPS
Performanc
e Period
2017
MIPS
Performa
nce
Period
2018
MIPS
Performa
nce
Period
2019
MIPS
Performa
nce
Period
2020
MIPS
Performa
nce
Period
2021
MIPS
Performa
nce
Period
2022
MIPS
Performa
nce
Period
2023
MIPS
Performa
nce
Period
2024
MIPS
Performa
nce
Period
Year of
MIPS
Year 1
Year 2
Year 3
Year 4
Year 5
Year 6
Year 7
Year 8
Performanc
e Threshold
3 points
15 points
30 points
45 points
60 points
75 points
75 Points
75 Points
Change
from prior
year
N/A
12 points
15 points
15 points
15 points
15 points
0 points
0 points
(b) Establishing the Performance Threshold Methodology for the 2027, 2028, and 2029 MIPS
Payment Years
Section 1848(q)(6)(D)(i) of the Act requires that we compute the performance threshold
such that it is the mean or median (as selected by the Secretary) of the final scores for all MIPS
eligible clinicians with respect to a prior period specified by the Secretary. That section also
provides that the Secretary may reassess the selection of the mean or median every three years.
In accordance with section 1848(q)(6)(D)(i) of the Act, we are proposing to continue using the
mean of the final scores for all MIPS eligible clinicians to compute the performance threshold
for the 2027, 2028, and 2029 MIPS payment years.
In the CY 2022 PFS final rule (86 FR 65527 through 65532), we selected the mean
(rather than the median) as the methodology for determining the performance threshold for the
2024, 2025, and 2026 MIPS payment years. For the CY 2019 performance period/CY 2021
MIPS payment year through CY 2021 performance period/2023 MIPS payment year, section
1848(q)(6)(D)(iv) of the Act required that we methodically increase the performance threshold
each year to “ensure a gradual and incremental transition” to the performance threshold we
estimated would be applicable in the CY 2022 performance period/2024 MIPS payment year.
Although sections 1848(q)(6)(D)(ii) through (iv) of the Act were no longer applicable for
establishing the performance threshold for the CY 2024 performance period/2026 MIPS payment
year, these previously applicable statutory requirements explained prior computations of the
performance threshold that impacted our policy considerations for establishing the performance
threshold for MIPS going forward. Based on our review of possible values for the CY 2022
performance period/2024 MIPS payment year, we believed that using the mean as our
methodology for setting the performance threshold for the CY 2022 performance period/2024
MIPS payment year through the CY 2024 performance period/2026 MIPS payment year would
continue the “gradual and incremental transition” that was previously required under section
1848(q)(6)(D)(iv) of the Act, as well as to provide consistency to our stakeholders. Therefore,
we finalized the proposal to use the mean as our methodology for setting the performance
threshold for that 3-year period. We also codified this methodology in our regulation at §
414.1405(g), providing that, for each of the 2024, 2025, and 2026 MIPS payment years, the
performance threshold would be the mean of the final scores for all MIPS eligible clinicians
from a prior period as specified.
At the time of this proposed rule, we have data available on MIPS eligible clinicians’
final scores from the CY 2017 performance period/2019 MIPS payment year through CY 2022
performance period/2024 MIPS payment year, as shown in Table 72. These values represent all
available computations of mean and median final scores for those performance periods/MIPS
payment years. As discussed previously, we may use either the mean or median of the final
scores from a prior period for computing the performance threshold for the next three years,
beginning with the CY 2025 performance period/2027 MIPS payment year. At the time of this

proposed rule, we do not have available MIPS eligible clinicians’ final scores from performance
periods after the CY 2022 performance period/2024 MIPS payment year, which may inform the
performance thresholds for the CY 2026 performance period/2028 MIPS payment year and CY
2027 performance period/2029 MIPS payment year. As provided in section 1848(q)(6)(D)(i) of
the Act, we must select whether we will use the mean or median of MIPS eligible clinicians’
final scores from a prior period, which we may reassess after three years. We assess these
selection options based on the data we currently have available.
TABLE 72: Possible Values for the 2027 MIPS Payment Year Performance Threshold
MIPS
Payment
Years
2019 MIPS
Payment
Year
2020 MIPS
Payment
Year
2021 MIPS
Payment
Year
2022 MIPS
Payment
Year
2023 MIPS
Payment
Year
2024 MIPS
Payment
Year
Mean
74.65
87.00
85.65
89.47
89.22
82.71
Median
89.71
99.63
92.32
96.82
97.22
85.17
As shown in Table 72, using the median final score gives a possible range of performance
thresholds from 85.17 points to 99.63 points (rounded to 85 points and 100 points, respectively).
Given our performance threshold of 75 points for the CY 2024 performance period/2026 MIPS
payment year, these values would result in an increase of 10 points to 25 points for the CY 2025
performance period/2027 MIPS payment year, and potentially the CY 2026 performance
period/2028 MIPS payment year and CY 2027 performance period/2029 MIPS payment year.
Selecting the median of final scores as our methodology would, at a minimum, result in a 13
percent increase in the performance threshold of 75 points, which we had established for the CY
2022 performance period/2024 MIPS payment year through the CY 2024 performance
period/2026 MIPS payment year. Further, as shown in Table 71, 75 points is the highest
performance threshold we have established for any MIPS payment year to date.
As shown in Table 72, using the mean final score as the methodology would yield a
possible range of performance thresholds from 74.65 points to 89.47 points (rounded to 75 points
and 89 points, respectively). Given our performance threshold of 75 points in the CY 2024
performance period/2026 MIPS payment year, these values would result in an increase of zero to

14 points for the CY 2025 performance period/2027 MIPS payment year, and potentially the CY
2026 performance period/2028 MIPS payment year and CY 2027 performance period/2029
MIPS payment year. Selecting the mean of final scores as our methodology would, at a
maximum, result in a 19 percent increase in the performance threshold of 75 points, which we
had established for the CY 2022 performance period/2024 MIPS payment year through the CY
2024 performance period/2026 MIPS payment year.
We believe that it is appropriate to incentivize performance improvement while also
ensuring that it is reflective of true clinician performance. Moreover, we believe that where
possible, it is important to offer stability and consistency for clinicians. After evaluating the
possible values for mean and median shown in Table 72 and our prior policies for consistently
selecting a performance threshold value of 75 points for the CY 2022 performance period/2024
MIPS payment year through the CY 2024 performance period/2026 MIPS payment year, we
believe that using the mean as our methodology for the 2027 through 2029 MIPS payment years
would offer the most consistent and predictable approach for MIPS eligible clinicians. On this
basis, we are proposing to continue using the mean of the final scores for all MIPS eligible
clinicians from a prior period as specified to compute the performance threshold for each of the
2027 through 2029 MIPS payment years.
We also are proposing to codify this proposal by amending our regulation text at §
414.1405. We propose to amend § 414.1405 by: (1) revising paragraph (g) to read only
“Performance Threshold Methodology”; (2) redesignating, with minor technical modification,
the substantive provision at paragraph (g) as a new paragraph (g)(1) to reflect the performance
threshold methodology we established and used to specify the performance threshold for the
2024, 2025 and 2026 MIPS payment years under § 414.1405(b)(9); and (3) adding paragraph
(g)(2) to provide that, for each of the 2027, 2028, and 2029 MIPS payment years, the
performance threshold is the mean of the final scores for all MIPS eligible clinicians from a prior
period as specified under § 414.1405(b)(10).
We request public comments on this proposal.
(c) Establishing the Performance Threshold for the CY 2025 Performance Period/2027 MIPS
Payment Year
We believe using the mean of 75 points from the CY 2017 performance period/2019
MIPS payment year continues to be the most appropriate option for establishing the performance
threshold for the CY 2025 performance period/2027 MIPS payment year for various reasons
described in this section, including: providing consistency for MIPS eligible clinicians while
allowing additional time for more recent data to become available, continuing to provide
opportunities for MIPS eligible clinicians to gain experience with cost measure scoring
(particularly if the methodology proposed in section IV.A.4.f.(1)(d)(ii)(B) of this proposed rule is
finalized), and ensuring that we do not inadvertently disadvantage certain clinician types, such as
small practices and solo practitioners, as we increase the performance threshold.
As shown in Table 72, we calculated the mean values for the CY 2017 performance
period/2019 MIPS payment year through the CY 2022 performance period/2024 MIPS payment
year, and we believe that the mean of 75 points from the CY 2017 performance period/CY 2019
MIPS payment year continues to be the most appropriate option that would provide stability for
MIPS eligible clinicians while still encouraging high quality of care. The final scores for the CY
2023 performance period/2025 MIPS payment year were not finalized in time for this proposed
rule and, therefore, the mean final score for the CY 2023 performance period/2025 MIPS
payment year was not included for consideration as a potential performance threshold value for
the CY 2025 performance period/2027 MIPS payment year.
Though we did consider the mean of 87 points from the CY 2018 performance
period/2020 MIPS payment year, we believe a substantial increase of 12 points could unfairly
impact clinicians as they continue to recover from the COVID-19 public health emergency
(PHE), which ended on May 11, 2023. We also considered using the means of the final scores
from the CY 2019 performance period/2021 MIPS payment year through the CY 2022

performance period/2024 MIPS payment year for establishing the CY 2025 performance
period/2027 MIPS payment year performance threshold. However, we decided they would not be
appropriate for measuring future clinician performance given the impact of the COVID-19 PHE
on data for MIPS, as described below.
Given issues with underlying data in prior periods due to the COVID-19 pandemic, we
believe that we should wait for more recent data that better reflects clinicians’ performance and
continue to rely on data from the CY 2017 performance period/2019 MIPS payment year, which
predated the COVID-19 PHE. Due to the timing of the COVID-19 PHE and our announcement
on March 22, 2020, extending the deadline for MIPS data submission,
780
we are still evaluating
the usability of data from the CY 2019 performance period/2021 MIPS payment year. While data
collection occurred during the CY 2019 performance period prior to the start of the COVID-19
PHE, data submission for the CY 2019 performance period (occurring during the first quarter of
CY 2020) was impacted. Specifically, in addition to extending the deadline for submitting MIPS
data, we announced on April 6, 2020, that, due to the COVID-19 PHE, we would apply our
extreme and uncontrollable circumstances reweighting policies described under §
414.1380(c)(2)(i) to MIPS eligible clinicians nationwide and extend the deadline to submit an
application for reweighting the quality, cost, improvement activities or Promoting
Interoperability reporting categories for the CY 2019 performance period/2021 MIPS payment
year (85 FR 19277 and 19278). These flexibilities for the submission of MIPS data occurring in
the first quarter of CY 2020 were intended to alleviate reporting burden on clinicians that were
responding to the onset of the COVID-19 pandemic. We believe the geographic differences of
COVID–19 incidence rates along with different impacts resulting from Federal, state, and local
laws and policy changes implemented in response to COVID–19 may have affected which MIPS
eligible clinicians were able to submit data for the CY 2019 performance period. This may have
780
https://www.cms.gov/newsroom/press-releases/cms-announces-relief-clinicians-providers-hospitals-and-
facilities-participating-quality-reporting.
led to final scores that were not wholly representative of performance for all MIPS eligible
clinicians. Also, for the CY 2020 performance period/2022 MIPS payment year and the CY 2021
performance period/2023 MIPS payment year, we extensively applied our reweighting policies,
described under § 414.1380(c)(2)(i), to MIPS eligible clinicians nationwide due to the COVID-
19 PHE. We believe these actions, particularly reweighting the performance categories, skewed
the final scores from those years such that they are not an appropriate indicator for future
performance of MIPS eligible clinicians. Specifically, we are concerned that the final scores
during the COVID-19 PHE reflect the performance of only MIPS eligible clinicians that may
have been less impacted by the pandemic, and do not accurately represent MIPS eligible
clinician performance overall during this period.
As discussed further in section IV.A.4.f.(1)(d)(ii) of this proposed rule, MIPS eligible
clinicians have expressed concern that the cost performance category scoring has a negative
impact on their MIPS final score. The CY 2022 performance period/2024 MIPS payment year
was the first MIPS payment year that the cost performance category score generally constituted
30 percent of MIPS eligible clinicians’ final scores (section 1848(q)(5)(E)(i)(II) of the Act). We
have observed lower category scores for the cost performance category as compared to the
quality performance category. In light of these concerns, which are supported by our analysis of
cost performance category scores as discussed in section IV.A.4.f.(1)(d)(ii) of this proposed rule,
we believe that maintaining a performance threshold of 75 points for the CY 2025 performance
period/2027 MIPS payment year would provide stability for MIPS eligible clinicians as they
become acquainted with the cost performance category (particularly if the scoring methodology
proposed in section IV.A.4.f.(1)(d)(ii)(B) of this proposed rule is finalized) without unfairly and
negatively impacting their final scores and MIPS payment adjustments. We also believe
maintaining the performance threshold at 75 points for the 2027 MIPS payment year would
provide us time to incorporate the impacts of this proposed cost performance category scoring
methodology (if finalized) as we establish future performance thresholds.

As discussed in section IV.A.4.f.(1)(d)(ii) of this proposed rule, we believe multiple
factors have likely contributed to MIPS eligible clinicians’ concerns, including increases in the
weight for the cost performance category over time (see section 1848(q)(5)(E)(i)(II) of the Act),
the number of cost measures, and the number of MIPS eligible clinicians that are being attributed
new cost measures and receiving a score for the cost performance category. This increase in
weight for the cost performance category over time has been particularly notable because, as
discussed previously, due to the application of our reweighting policies described under §
414.1380(c)(2)(i) for the COVID-19 PHE, many MIPS eligible clinicians were not scored on the
cost performance category for the CY 2019 performance period/2021 MIPS payment year
through the CY 2021 performance period/2023 MIPS payment year (85 FR 19277 through
19278).
781,782
We believe that our proposal to maintain a performance threshold of 75 points for
the CY 2025 performance period/2027 MIPS payment year may help alleviate some of MIPS
eligible clinicians’ concerns related to the cost performance category and its impact on their
MIPS final score.
We are also concerned that any increase in the performance threshold may inadvertently
and unfairly disadvantage certain clinician types, specifically small practices and solo
practitioners. As we stated in the CY 2024 PFS final rule, we want to consider the impacts of the
performance threshold and its related policies on small practices (88 FR 79377). We have
received feedback that many small practices and solo practitioners face challenges in their ability
to participate in MIPS, including the costs to implement and maintain certified electronic health
record (EHR) technology (CEHRT), staff and training costs, and limited staff capacity to manage
the complexity of the program. We have also heard that increases in the performance threshold
add administrative and financial burden for small practices that discourage their participation in
781
https://qpp-cm-prod-
content.s3.amazonaws.com/uploads/1198/2020%20MIPS%20Automatic%20EUC%20Fact%20Sheet.pdf.
782
https://qpp-cm-prod-
content.s3.amazonaws.com/uploads/1437/2021%20MIPS%20Automatic%20EUC%20Fact%20Sheet.pdf.
MIPS. Though we have several policies within MIPS that continue to support small and solo
practices, including scoring and reweighting policies, we are interested in understanding how to
best support small practices and enhance their ability to successfully participate in MIPS as
MIPS continues to evolve. As such, we have performed qualitative analysis through engagement
with small practices, third party intermediaries, and other interested parties to gather information
about the experience of small practices participating in the program. We are also planning to
reach out to small practices and solo practitioners in CY 2024 to gather additional information
about barriers for actively engaging with MIPS. On this basis, we believe establishing a
performance threshold of 75 points for the CY 2025 performance period/2027 MIPS payment
year would allow us time to gather additional data on the impacts of new policies on small and
rural practices, and to develop strategies to reduce barriers for small practices and solo
practitioners participating in MIPS.
We refer readers to the Regulatory Impact Analysis in section VII.E.17.d.(4) of this
proposed rule for an estimate of the percent of MIPS eligible clinicians that would receive a
negative payment adjustment for the CY 2025 performance period/2027 MIPS payment year if
the policies proposed in this proposed rule are finalized and the performance threshold is
established at 75 points.
As discussed in this section IV.A.4.g.(2)(c), we believe that maintaining a performance
threshold of 75 points allows additional time for more data to become available, continues to
provide opportunities for clinicians to become familiar with the cost measure scoring, and
ensures that we do not inadvertently disadvantage certain clinician types, such as small practices
and solo practitioners. Therefore, we are proposing to establish a performance threshold of 75
points for the CY 2025 performance period/2027 MIPS payment year based on the mean of
MIPS eligible clinicians’ final scores from the CY 2017 performance period/2019 MIPS
payment year, and to codify this performance threshold in our regulation by adding paragraphs at
§ 414.1405(b)(10) introductory text and (b)(10)(i).
We request public comments on this proposal.
(3) Example of Adjustment Factors
Figure 4 provides an illustrative example of how various final scores would be converted
to a MIPS payment adjustment factor using the statutory formula and based on our proposed
policies for the CY 2025 performance period/2027 MIPS payment year. In Figure 4, the
performance threshold is set at 75 points, as we have proposed in section IV.A.4.g.(2)(c) of this
proposed rule.
For purposes of determining the maximum and minimum range of potential MIPS
payment adjustment factors, section 1848(q)(6)(B) of the Act defines the applicable percentage
as 9 percent for the CY 2025 performance period/2027 MIPS payment year. The MIPS payment
adjustment factor is determined on a linear sliding scale from zero to 100, with zero being the
lowest possible score which receives the negative applicable percentage and resulting in the
lowest payment adjustment, and 100 being the highest possible score which receives the highest
positive applicable percentage and resulting in the highest payment adjustment.
However, there are two modifications to this linear sliding scale. First, as specified in
section 1848(q)(6)(A)(iv)(II) of the Act, there is an exception for a final score between zero and
one-fourth of the performance threshold (zero and 18.75 points based on the proposed
performance threshold of 75 points for the CY 2025 performance period/2027 MIPS payment
year). All MIPS eligible clinicians with a final score in this range will receive a negative MIPS
payment adjustment factor equal to 9 percent (the applicable percentage). Second, the linear
sliding scale for the positive MIPS payment adjustment factor is adjusted by the scaling factor,
which cannot be higher than 3.0, as required by section 1848(q)(6)(F)(i) of the Act.
If the scaling factor is greater than zero and less than or equal to 1.0, then the MIPS
payment adjustment factor for a final score of 100 will be less than or equal to 9 percent (the
applicable percentage). If the scaling factor is above 1.0 but is less than or equal to 3.0, then the
MIPS payment adjustment factor for a final score of 100 will be greater than 9 percent. Only

those MIPS eligible clinicians with a final score equal to 75 points (the performance threshold
proposed for the CY 2025 performance period/2027 MIPS payment year) will receive a neutral
MIPS payment adjustment.
Beginning with the CY 2023 performance period/2025 MIPS payment year, the
additional MIPS payment adjustment for exceptional performance described in section
1848(q)(6)(C) of the Act is no longer available. For this reason, Figure 4 does not illustrate an
additional adjustment factor for MIPS eligible clinicians with final scores at or above the
additional performance threshold described in section 1848(q)(6)(D)(ii) of the Act.
FIGURE 4: Illustrative Example of MIPS Payment Adjustment Factors Based on Final
Scores and Performance Threshold for the CY 2025 performance period/2027 MIPS
Payment Year
Note: The adjustment factor for final score values above the performance threshold is illustrative. For MIPS eligible
clinicians with a final score of 100, the adjustment factor will be 9 percent times a scaling factor greater than zero
and less than or equal to 3.0. The scaling factor is intended to ensure budget neutrality (BN) but cannot be higher
than 3.0. This example is illustrative as the actual payment adjustments may vary based on the distribution of final
scores for MIPS eligible clinicians.
Table 73 illustrates the changes in payment adjustment based on the final policies from
the CY 2024 PFS final rule (88 FR 52599 through 56001) for the CY 2024 performance
period/2026 MIPS payment year and the proposed policies for the CY 2025 performance
period/2027 MIPS payment year, as well as the applicable percent required by section
1848(q)(6)(B) of the Act.
TABLE 73: Illustration of Point System and Associated Adjustments Comparison between
the CY 2024 Performance Period/2026 MIPS Payment Year and the CY 2025 Performance
Period/2027 MIPS Payment Year
2024 Performance
Period
Final Score
Points
MIPS Adjustment for 2024
Performance Period
2025 Performance Period
Final Score
Points
MIPS Adjustment for
2025 Performance
Period
0.0-18.75
Negative 9%
0.0-18.75
Negative 9%
18.76-74.99
Negative MIPS payment
adjustment greater than
negative 9% and less than 0%
on a linear sliding scale
18.76-74.99
Negative MIPS payment
adjustment greater than
negative 9% and less than
0% on a linear sliding
scale
75.00
0% adjustment
75.00
0% adjustment
75.01-100
Positive MIPS payment
adjustment greater than 0% on
a linear sliding scale. The
linear sliding scale ranges from
greater than 0% to 9% for
scores from 75.01 to 100.00.
This sliding scale is multiplied
by a scaling factor greater than
zero but not exceeding 3.0 to
preserve budget neutrality.
75.01-100
Positive MIPS payment
adjustment greater than
0% on a linear sliding
scale. The linear sliding
scale ranges from greater
than 0% to 9% for scores
from 75.01 to 100.00.
This sliding scale is
multiplied by a scaling
factor greater than zero
but not exceeding 3.0 to
preserve budget
neutrality.
h. Review and Correction of MIPS Final Score – Feedback and Information to Improve
Performance
Under section 1848(q)(12)(A)(i) of the Act, we are required to provide MIPS eligible
clinicians with timely (such as quarterly) confidential feedback on their performance under the
quality and cost performance categories beginning July 1, 2017, and we have discretion to
provide such feedback regarding the improvement activities and Promoting Interoperability
performance categories. In the CY 2018 Quality Payment Program final rule (82 FR 53799
through 53801), we finalized that on an annual basis, beginning July 1, 2018, performance
feedback will be provided to MIPS eligible clinicians and groups for the quality and cost
performance categories, and if technically feasible, for the improvement activities and advancing
care information (now called the Promoting Interoperability) performance categories.
We made performance feedback available for the CY 2019 performance period/2021
MIPS payment year on August 5, 2020; for the CY 2020 performance period/2022 MIPS
payment year on August 2 and September 27, 2021; for the CY 2021 performance period/2023
MIPS payment year on August 22, 2022; and for the CY 2022 performance period/2024 MIPS
payment year on August 10, 2023. Although we aim to provide feedback for the CY 2023
performance period/2025 MIPS payment year on or around July 1, 2024, it is possible the release
date could be later depending on circumstances. We direct readers to qpp.cms.gov for more
information.
i. Calculating the Final Score
For a description of the statutory basis and our previously finalized policies for
calculating the final score for each MIPS eligible clinician, including performance category
weights and reweighting the performance categories, we refer readers to § 414.1380(c) and the
discussion in the CY 2017 and CY 2018 Quality Payment Program final rules, and the CY 2019,
CY 2020, CY 2021, CY 2022 and CY 2023 PFS final rules (81 FR 77319 through 77329, 82 FR
53769 through 53785, 83 FR 59868 through 59878, 84 FR 63020 through 63031, 85 FR 84908
through 84917, 86 FR 65509 through 65527, and 87 FR 70093 through 70096, respectively).
As described in more detail in the following sections, we propose to supplement our
current policies for reweighting one or more performance categories (that is, quality,
improvement activities, and Promoting Interoperability) to permit reweighting in circumstances
where we determine that data for a MIPS eligible clinician are inaccessible or unable to be
submitted due to circumstances outside of the control of the clinician because the MIPS eligible
clinician delegated submission of the data to their third party intermediary, evidenced by a
written agreement between the MIPS eligible clinician and third party intermediary, and the third
party intermediary did not submit the data for the performance category(ies) on behalf of the
MIPS eligible clinician in accordance with applicable deadlines.
We note that we are only proposing that this reweighting policy be available for the
quality, improvement activities, and Promoting Interoperability performance categories because
a MIPS eligible clinician may delegate data submission to a third party intermediary only with
respect to these three performance categories, and not the cost performance category. MIPS
eligible clinicians do not submit data separately for measures for the cost performance category;
we score cost measures based solely on Medicare claims data.
(1) Background
Section 1848(q)(5)(A) of the Act requires the Secretary to develop a methodology for
assessing the total performance of each MIPS eligible clinician according to the performance
standards for the applicable measures and activities for each performance category applicable to
such clinician for a performance period and, using the methodology, provide for a final score
(using a scoring scale of 0 to 100) for each MIPS eligible clinician for the performance period.
Additionally, section 1848(q)(5)(E) of the Act specifies how we must weigh the scores
for each performance category in our calculation of the MIPS eligible clinician’s final score. We
have codified these weights at § 414.1380(c)(1). Meanwhile, section 1848(q)(5)(F) of the Act
provides that, if there are not sufficient measures and activities applicable and available to each
type of MIPS eligible clinician involved, the Secretary shall assign different scoring weights
(including a weight of 0). We previously finalized at § 414.1380(c) that if a MIPS eligible
clinician is scored on fewer than two performance categories, they will receive a final score
equal to the performance threshold (81 FR 77326 through 77328 and 82 FR 53778 through
53779).
We also have finalized at § 414.1380(c)(2) several policies addressing on what basis we
may reweight one or more performance categories, and how those weights will be redistributed
to the remaining performance categories. For example, in the CY 2020 PFS final rule (84 FR
63023 through 63026), we finalized a reweighting policy at § 414.1380(c)(2)(i)(A)(9) and
(c)(2)(i)(C)(10) for the four MIPS performance categories. Under this policy, we may reweight

one or more of the performance categories for a MIPS eligible clinician if we determine, based
on information known to us prior to the beginning of the relevant MIPS payment year, that data
for a MIPS eligible clinician for the applicable performance category(ies) are inaccurate,
unusable, or otherwise compromised due to circumstances outside of the control of the clinician
and its agents. Under this policy, we are able to address circumstances where submitted data are
inaccurate, unusable, or otherwise compromised.
However, we have found this policy, and our other reweighting policies at §
414.1380(c)(2), do not address circumstances where data are inaccessible or unable to be
submitted due to circumstances outside of the control of the MIPS eligible clinician, particularly
where the clinician has delegated submission of the data to a third party intermediary and that
third party intermediary does not submit the data in accordance with applicable deadlines. In
accordance with our regulations governing third party intermediaries at § 414.1400(a)(3)(iv) and
(e)(1), we may take remedial action in the event a third party intermediary fails to meet the
criteria necessary for their approval as a third party intermediary, fails to comply with other
requirements applicable to third party intermediaries, has submitted a false certification, or
discontinues their services and do not assist MIPS eligible clinicians in connecting with a
different third party intermediary. However, our regulations do not address the impact of a third
party intermediary’s action or inaction resulting in failure to submit the MIPS eligible clinician’s
data as required, over which the MIPS eligible clinician has little to no control, on a MIPS
eligible clinician’s final score.
Currently, if we determine that data for a MIPS eligible clinician were not submitted
during the MIPS data submission period for reasons outside the clinician’s control, we assign the
clinician a score of zero for the performance category or categories for which data were not
submitted.
783
Because an excusable failure to submit data is not currently a basis for
783
As set forth in § 414.1325(a), data is only required to be submitted for certain measures and activities as specified
for certain performance categories. For example, MIPS eligible clinicians are not required to submit data for the cost

reweighting, the lack of data may reduce the MIPS eligible clinician’s final score and therefore
may reduce the clinician’s MIPS payment adjustment. However, we believe that reweighting of
the applicable performance categories may be appropriate in these rare cases as described in this
section IV.A.4.i. of this proposed rule.
Specifically, we believe that a MIPS eligible clinician should not be penalized in cases
where the MIPS eligible clinician enters into an agreement with a third party intermediary to
submit data on their behalf, and the data are not submitted due to reasons outside of the control
of the MIPS eligible clinician. While we encourage the impacted MIPS eligible clinician to take
steps to ensure data submission for subsequent years, by, for example, selecting an alternate third
party intermediary, there may be cases where there is insufficient time for the MIPS eligible
clinician to submit the data through an alternative mechanism in time for the data to be
considered for the relevant performance period. For instance, the MIPS eligible clinician may
become aware that their third party intermediary did not submit data on their behalf after the data
submission period closes. In these cases, we believe it is appropriate to provide relief to the
MIPS eligible clinician so that they are not unfairly penalized for their third party intermediary’s
inaction.
(2) Proposal to Adopt Reweighting Performance Category(ies) Policy When a Third Party
Intermediary Did Not Submit Data Due to Reasons Outside the MIPS Eligible Clinician’s
Control
We are proposing to adopt a new reweighting policy at § 414.1380(c)(2)(i)(A)(10) and
(c)(2)(i)(C)(12) to address this circumstance. Specifically, beginning with the CY 2024
performance period/2026 MIPS payment year, we are proposing that we may reweight one or
more of the quality, improvement activities, and Promoting Interoperability performance
categories where we determine, based on documentation submitted to us through a reweighting
performance category to receive a score for that category because cost measures are scored based on Medicare
claims data. We refer readers to our data submission requirements at § 414.1325 and our proposal to modify these
requirements in section IV.A.4.d. of this proposed rule.
request on or before November 1st of the year preceding the relevant MIPS payment year, that
data for a MIPS eligible clinician are inaccessible or unable to be submitted due to circumstances
outside of the control of the clinician because the MIPS eligible clinician delegated submission
of their data to a third party intermediary, evidenced by a written agreement between the MIPS
eligible clinician and the third party intermediary, and the third party intermediary did not submit
the data for the performance category(ies) on behalf of the MIPS eligible clinician in accordance
with applicable deadlines. We also are proposing that, to determine whether to apply reweighting
to the affected performance category(ies), we will consider: whether the MIPS eligible clinician
knew or had reason to know of the issue with its third party intermediary’s submission of the
clinician’s data for the performance category(ies); whether the MIPS eligible clinician took
reasonable efforts to correct the issue; and whether the issue between the MIPS eligible clinician
and their third party intermediary caused no data to be submitted for the performance
category(ies) in accordance with applicable deadlines. We believe these factors are necessary to
ensure we are only granting these requests in circumstances where MIPS eligible clinicians
would otherwise be unfairly penalized due to the actions or inactions of a third party
intermediary. MIPS eligible clinicians could request reweighting under this proposed policy in
circumstances where no data was submitted on their behalf by their third party intermediary
through the help desk at [email protected].
Under this proposed policy, MIPS eligible clinicians would be able to request
reweighting for each performance category for which their third party intermediary, to which the
MIPS eligible clinician delegated submission of their data, did not submit data for reasons
outside of the control of the MIPS eligible clinician. We note that we are only proposing that this
reweighting policy be available for the quality, improvement activities, and Promoting
Interoperability performance categories because a MIPS eligible clinician may delegate data
submission to a third party intermediary only with respect to these three performance categories,
and not the cost performance category. MIPS eligible clinicians do not submit data separately for
measures for the cost performance category; we score cost measures based solely on Medicare
claims data.
Under this proposed reweighting policy, the MIPS eligible clinician must submit
reweighting requests beginning with the close of a relevant performance period’s data
submission period, only after it is confirmed that no data has been submitted in accordance with
applicable deadlines. MIPS eligible clinicians would be able to submit reweighting requests on
or before November 1st of the year preceding the associated MIPS payment year in order to
allow time for CMS to re-calculate their final score and MIPS payment adjustment factor.
We would only approve reweighting requests with evidence of a written agreement
between the MIPS eligible clinician and a third party intermediary. Such written agreement must
provide that the MIPS eligible clinician delegated submission of their data to the third party
intermediary, and that the third party intermediary agreed to submit data on their behalf in
accordance with applicable deadlines, for the performance category or performance categories in
question.
We would review requests and make determinations to reweight based on our assessment
that data were not submitted outside the control of the MIPS eligible clinician. As discussed in
this section of this proposed rule, we are proposing that we would determine whether to apply
reweighting to the affected performance category(ies) under this policy based on our
consideration of the following criteria: whether the MIPS eligible clinician knew or had reason to
know of the issue with its third party intermediary’s submission of the clinician’s data for the
performance category(ies); whether the MIPS eligible clinician took reasonable efforts to correct
the issue; and whether the issue between the MIPS eligible clinician and their third party
intermediary caused no data to be submitted for the performance category(ies) in accordance
with applicable deadlines. These criteria would inform whether we would grant reweighting
requests under our proposed policy at § 414.1380(c)(2)(i)(A)(10) and (c)(2)(i)(C)(12).
Circumstances resulting in a clinician’s data being inaccessible or unable to be submitted that
would merit reweighting could include, but are not limited to, a critical systems failure, the third
party intermediary going out of business, the third party intermediary having collected data on a
MIPS eligible clinicians behalf and refusing to hand it over for the MIPS eligible clinician to
submit, or other circumstances CMS determines to be outside the control of the MIPS eligible
clinician.
This proposed reweighting policy is solely intended to mitigate the potentially adverse
financial impact of no data being submitted during the MIPS data submission period for one or
more performance categories on behalf of the MIPS eligible clinician due to the failure of a third
party intermediary to fulfill its contractual responsibilities. Our determination to grant a
reweighting request under this proposed policy does not indicate, and should not be interpreted
to suggest, that the third party intermediary could not be held liable for the failure to perform the
task as delegated, that is, to submit data on the performance category(ies) on behalf of the MIPS
eligible clinician. In these circumstances where we determine that a third party intermediary
failed to fulfill its agreement with the MIPS eligible clinician to submit their data, we believe it is
appropriate to give the MIPS eligible clinician the opportunity to request reweighting of the
affected performance category(ies), provided that all elements of our proposed policy are met.
We propose to apply reweighting only in cases when we receive documentation of a third
party intermediary failing to submit data on behalf of a MIPS eligible clinician demonstrating
that all elements of our proposed policy are met.
We propose that this policy would be effective beginning with CY 2024 performance
period/2026 MIPS payment year. If finalized, this policy change would become effective
prospectively, prior to the beginning of the data submission period for the CY 2024 performance
period/2026 MIPS payment year, which will occur January 2, 2025, through March 31, 2025. We
are proposing this effective date to provide relief to MIPS eligible clinicians, whose
circumstances meet all requirements set forth in this proposed reweighting policy, as soon as
feasible.
We propose to codify this new reweighting policy, including all proposed elements as
described in section IV.A.4.i.(2) of this proposed rule, at § 414.1380(c)(2)(i)(A)(10) for the
quality and improvement activities performance categories and at § 414.1380(c)(2)(i)(C)(12) for
the Promoting Interoperability performance category.
We request public comments on this proposal.
j. Third Party Intermediaries General Requirements
(1) Requirements for CMS-approved Survey Vendors
(a) Background
As codified at § 414.1305, a CMS-approved survey vendor means a survey vendor that is
approved by CMS for a particular performance period to administer the Consumer Assessment
of Healthcare Providers & Systems (CAHPS) for MIPS survey and to transmit survey measures
data to CMS.
We refer readers to § 414.1400(d), the CY 2017 Quality Payment Program final rule (81
FR 77386), the CY 2018 Quality Payment Program final rule (82 FR 53818 and 53819), the CY
2019 PFS final rule (83 FR 59907 and 59908), and the CY 2022 PFS final rule (86 FR 65538 and
65539) for previously finalized standards and criteria for CMS-approved survey vendors.
(b) Requirement to Submit Cost of Services
In the CY 2017 Quality Payment Program final rule (81 FR 77386 and 77387), we
established that CMS-approved survey vendors may transmit data collected from the CAHPS for
MIPS survey to CMS for use in MIPS. Section 414.1400(d)(2) requires that CMS-approved
survey vendors submit a survey vendor application to CMS in a form and manner specified by
CMS for each MIPS performance period for which it wishes to transmit such data. We
implemented this requirement through the Vendor Participation Form, which is available at
https://qpp.cms.gov/resources/resource-library.
The CY 2017 Quality Payment Program final rule contained requirements applicable to
other types of third party intermediaries, which varied based on whether the third party
intermediary was a Qualified Clinical Data Registry (QCDR) or qualified registry. For example,
we established requirements for QCDRs and qualified registries to “sign a document verifying
the QCDR's name, contact information, cost for MIPS eligible clinicians or groups to use the
QCDR or qualified registry” (81 FR 77368 and 77369; 81 FR 77385 and 77386). If QCDRs and
qualified registries do not provide this information, CMS may exclude them from MIPS in a
subsequent year. This requirement helps eligible clinicians determine which vendors to use prior
to registration and provides transparency on the cost of program participation. Currently, CMS-
approved survey vendors are not required to provide cost information even though other third
party intermediaries (QCDRs and qualified registries) are required to do so.
We are proposing under the current application submission requirement at §
414.1400(d)(2) that beginning with the CY 2026 performance period/2028 MIPS payment year,
a survey vendor must include on its application the range of costs of its third party intermediary
services. Ranges of cost estimates would vary based on different levels of service (i.e., number
of survey respondents, languages provided, etc.). With respect to a third party intermediary that
is solely a CMS-approved survey vendor, the publishable costs would be limited to the cost of
services related to the CAHPS for MIPS survey. CMS has received inquiries from MIPS
participants regarding survey vendor costs but has not been able to provide any specific
information in response to those requests. The cost information from survey vendors is not easily
available to the MIPS eligible clinicians who are considering contracting for services. Having
such information in the publicly-accessible QPP Resource Library (as part of the list of approved
vendors) would make it easier for MIPS eligible clinicians to contract for services and educate
themselves about the cost of using a third party intermediary survey vendor. In recent years,
some participants who registered for the CAHPS for MIPS survey later withdrew their
participation once they learned the costs of survey administration. Providing information on the
cost of CMS-approved CAHPS for MIPS survey vendor services may support MIPS participants
who are interested in the CAHPS for MIPS Survey but want to know what the costs of

administering the survey would be, thus allowing them to make more informed decisions about
whether to participate in the CAHPS for MIPS survey. This would also increase the consistency
in requirements across different types of third party intermediaries.
If this proposal is finalized, the CAHPS for MIPS Survey Vendor Participation Form
784
and the CAHPS for MIPS Survey Minimum Business Requirements
785
in the QPP Resource
Library would be updated to detail the required survey vendor cost estimate information. The
CAHPS for MIPS Survey Vendor Participation Form submitted by vendors seeking CMS
approval would be updated to include fields to report the cost information. The vendor-specific
cost information would then be published in the list of CAHPS for MIPS Survey Approved
Vendors which is also posted in the Resource Library. We refer readers to section V.B.8.c.(5) of
this proposed rule for discussion on the burden estimates for this proposal.
We request comment on this proposal.
k. Overview of QP Determinations and the APM Incentive
(1) Overview
The Quality Payment Program provides incentives for eligible clinicians to engage in
value-based, patient-centered care under Medicare Part B via MIPS and Advanced APMs. The
structure of the Quality Payment Program enables the Department to advance accountability and
encourage improvements in care. The Secretary has also adopted the closely related goal that all
people with Original Medicare be in an accountable care relationship by 2030, so that their needs
can be holistically assessed, and their care is coordinated within a broader total cost of care
system. Our vision for increased participation among clinicians in Advanced APMs is driven by
the belief that integrating individuals’ clinical needs across a spectrum of clinicians and settings
will improve patient care and population health.
784
2024 CAHPS for MIPS Survey Vendor Participation Form, March 25, 2024.
https://qpp.cms.gov/resources/document/6386fe4b-49b9-42c8-9a2b-a1149f7b142a.
785
2024 CAHPS for MIPS Survey Vendor Minimum Business Requirements, March 11, 2024.
https://qpp.cms.gov/resources/document/02e6e596-51de-4336-a6a6-ab63fc639dbc.
As we continue to make improvements to the Quality Payment Program, we seek to
develop, propose, and implement policies that encourage broad and meaningful clinician
participation, including by specialists, in Advanced APMs.
In the CY 2017 Quality Payment Program final rule (81 FR 77450 through 77457), we
finalized the payment amount method and patient count method for calculation of Threshold
Scores used for QP determinations under the Medicare option and codified these methods at
§ 414.1435(a) and (b), respectively. The payment amount method is based on payments for
Medicare Part B covered professional services, including certain supplemental service payments,
while the patient count method is based on numbers of patients. Both methods use the ratio of
“Attributed beneficiaries” to “Attribution-eligible beneficiaries”, as defined at § 415.1305.
An attributed beneficiary is a beneficiary attributed to the APM Entity under the terms of the
Advanced APM as indicated on the most recent available list of attributed beneficiaries at the
time of a QP determination. An attribution-eligible beneficiary is a beneficiary who:
● Is not enrolled in Medicare Advantage or a Medicare cost plan;
● Does not have Medicare as a secondary payer;
● Is enrolled in both Medicare Parts A and B;
● Is at least 18 years of age;
● Is a United States resident; and
● Has a minimum of:
++ One claim for E/M services furnished by an eligible clinician who is in the APM
Entity for any period during the QP Performance Period.
++ Or, for an Advanced APM that does not base attribution on E/M services and for
which attributed beneficiaries are not a subset of the attribution-eligible beneficiary population
based on the requirement to have at least one claim for E/M services furnished by an eligible
clinician who is in the APM Entity for any period during the QP Performance Period, the

attribution basis determined by CMS based upon the methodology the Advanced APM uses for
attribution, which may include a combination of E/M and/or other services.
In this section, we propose to modify the definition of “attribution-eligible beneficiary” to
include any beneficiary who has received a covered professional service (section 1833(z)(3)(A)
of the Act; 42 CFR 414.1305; section 1848(k)(3)(A) of the Act) furnished by the eligible
clinician (NPI) for whom we are making the QP determination. By no longer specifying
evaluation and management (E/M) services as the default attribution basis, we also would
eliminate the need to develop customized attribution bases for Advanced APMs that do not use
E/M services as the basis for attribution. Therefore, our proposal would standardize the
attribution methodology for QP determinations by making covered professional services the
basis for attribution across all Advanced APMs.
(2) Payment Amount and Patient Count Methods
The payment amount method for calculating threshold scores is based on payments for
Medicare Part B covered professional services, including certain supplemental service payments,
while the patient count method is based on numbers of patients. Both methods use the ratio of
“attributed beneficiaries” to “attribution-eligible beneficiaries,” as defined at § 415.1305.
786
Attributed beneficiaries are those who are attributed to the APM Entity (or individual
eligible clinician) under the terms of the Advanced APM as indicated on the most recent
available list of attributed beneficiaries at the time of a QP determination. Attribution-eligible
beneficiaries generally are those who, during the QP Performance Period, meet six criteria (listed
below) specified in the definition of that term at § 414.1305 and described in section
IV.A.4.m.(3) of this proposed rule.
When making QP determinations, we begin by calculating Threshold Scores using the
payment amount and patient count methodologies. These Threshold Scores are percentages
786
For technical information on the QP calculation methodology, see the “QP Methodology Fact Sheet” that we
publish annually, which can be found as part of the “2024 Learning Resources for QP Status and APM Incentive
Payment” materials on the Quality Payment Program Resource Library at qpp.cms.com.

based on the ratio of the payment amounts or patient counts for attributed beneficiaries to the
payment amounts or patient counts for attribution-eligible beneficiaries during the QP
performance period. If the Threshold Score (using either the payment amount or patient count
method) calculated at the APM Entity or individual eligible clinician level, as applicable, meets
or exceeds the relevant QP threshold described at § 414.1430(a), the relevant eligible clinician or
clinicians (either the individual eligible clinician or all those on the APM Entity’s Participation
List) achieve QP status for such year.
FIGURE 5: QP Determination Calculation
Attributed beneficiaries
Attribution-eligible beneficiaries
Our regulation at § 414.1435(b)(3) provides that a beneficiary may be counted only once
in the numerator and denominator for a single APM Entity group, and at § 414.1435(b)(4)
provides that a beneficiary may be counted multiple times in the numerator and denominator for
multiple different APM Entity groups. In the CY 2021 PFS final rule (85 FR 84951 through
84952), we amended § 414.1435(c)(1)(i) to specify that beneficiaries who have been
prospectively attributed to an APM Entity for a QP Performance Period will be excluded from
the attribution-eligible beneficiary count for any other APM Entity that is participating in an
APM where that beneficiary would be ineligible to be added to the APM Entity’s attributed
beneficiary list. This means that beneficiaries who have been attributed to one APM Entity and
are thus barred under the terms of an Advanced APM from attribution to another APM Entity are
removed from the denominator of both the payment amount method and patient count method in
QP Threshold Score calculations for the APM Entity to which they cannot be attributed. In other
words, we do not penalize an APM Entity in the QP Threshold Score calculation by including a
beneficiary in its denominator when the terms of an Advanced APM do not permit such
beneficiary to be attributed to such APM Entity.
(a) Attributed beneficiary:
An attributed beneficiary is a beneficiary attributed to the APM Entity under the terms of
the Advanced APM as indicated on the most recent available list of attributed beneficiaries at the
time of a QP determination. There may be beneficiaries on the most recent available list who do
not meet the criteria to be attribution-eligible beneficiaries because the QP performance period
does not align with the Advanced APM’s performance period or attribution period, or for other
reasons. There may also be cases where a beneficiary’s status changes, for example by enrolling
in a Medicare Advantage Plan. We exclude these beneficiaries from our Threshold Score
calculations because they do not meet criteria to be attribution-eligible beneficiaries. Although
APMs may have reconciliation processes in place to address changes in beneficiary status at
various intervals, those processes do not necessarily coincide with the timeframe of QP
determinations. Therefore, when calculating Threshold Scores for QP determinations, we
exclude from the list of attributed beneficiaries any beneficiaries who do not meet the criteria to
be attribution-eligible beneficiaries at that point in time.
(b) Attribution-eligible beneficiary:
Under our regulation at § 414.1305, we define an attribution-eligible beneficiary as a
beneficiary who:
● Is not enrolled in Medicare Advantage or a Medicare cost plan;
● Does not have Medicare as a secondary payer;
● Is enrolled in both Medicare Parts A and B;
● Is at least 18 years of age;
● Is a United States resident; and
● Has a minimum of one claim for E/M services furnished by an eligible clinician who is
in the APM Entity for any period during the QP Performance Period or, for an Advanced APM
that does not base attribution on E/M services and for which attributed beneficiaries are not a
subset of the attribution-eligible beneficiary population based on the requirement to have at least
one claim for E/M services furnished by an eligible clinician who is in the APM Entity for any
period during the QP Performance Period, the attribution basis determined by CMS based upon
the methodology the Advanced APM uses for attribution, which may include a combination of
E/M and/or other services.
Our stated intent when we finalized the definition of attribution-eligible
beneficiary (81 FR 77451 through 77452) was to have a definition that would, for purposes of
QP determinations, allow us to be consistent across Advanced APMs in how we consider the
population of beneficiaries served by an APM Entity. The criteria we used to define attribution-
eligible beneficiary were aligned with the attribution methodologies and rules for our then-
contemporaneous Advanced APMs. The first five criteria are conditions that are required for a
beneficiary to be attributed to any Advanced APM. The sixth criterion identifies beneficiaries
who have received certain services from an eligible clinician who is associated with an APM
Entity for any period during the QP Performance Period. We chose to refer to E/M services as
the primary basis for purposes of attribution-eligibility because many Advanced APMs use E/M
claims to attribute beneficiaries to their APM Entity groups. Over time, we have updated the list
of services that are considered to be E/M services for purposes of identifying attribution-eligible
beneficiaries and have published this list as part of the “2024 Learning Resources for QP Status
and APM Incentive Payment” materials on the Quality Payment Program Resource Library at
qpp.cms.gov.
We also included an exception in this sixth criterion to allow us to use an alternative
approach for Advanced APMs that do not base attribution on E/M services, and thus for which
attributed beneficiaries are not a subset of the attribution-eligible beneficiary population based on
the requirement to have at least one claim for an E/M service. To date, we have implemented this
alternative approach for four Advanced APMs:
● Bundled Payments for Care Improvement Advanced Model.
● Comprehensive Care for Joint Replacement Payment Model (CEHRT Track).
● Comprehensive ESRD Care Model (LDO arrangement and Non LDO Two Sided Risk
Arrangement).
● Maryland Total Cost of Care Model (Care Redesign Program).
We have published links to the methodologies we use to identify attribution-eligible
beneficiaries for these Advanced APMs in the “2024 Learning Resources for QP Status and
APM Incentive Payment” materials on the Quality Payment Program Resource Library at
qpp.cms.gov.
We adopted the general rule with flexibility to apply alternative methods for this criterion
to ensure that, for the Advanced APMs for which attribution is based on services other than E/M
services, the attributed beneficiary population is truly a subset of such Advanced APMs’
attribution-eligible beneficiary populations and, ultimately, so that our way of identifying
beneficiaries for purposes of Threshold Score calculations for QP determinations would be
appropriate for such Advanced APMs. That said, our thinking when we developed these
approaches was shaped by the form and nature of the Advanced APMs that existed at that time.
We believed that, by affording sufficient flexibility within the program, we could both foster
innovation in Advanced APMs and simplify our execution of the program. However, with our
more narrowly defined default approach to beneficiary attribution (relying on claims for E/M
services), we have increasingly needed to exercise the flexibility to identify an alternative
approach to attribution eligibility for Advanced APMs that fell into the exception, which meant
that we identified several individually-tailored ways of performing the attribution methodology
for specific Advanced APMs. We anticipate that Advanced APMs will continue to evolve and
use novel approaches to value-based care that may emphasize a broad range of covered
professional services, and in that event the application of our current regulations may result in
increased variability among the ways we define attribution-eligible when making QP
determinations, which frustrates our goal of a consistent approach.
Further, we recognize that primary care practitioners generally furnish a higher
proportion of E/M services than do specialists for the same beneficiary. The current reliance on
E/M services for attribution in our Threshold Score calculations means that primary care
practitioners may contribute more significantly to achieving QP status for an APM Entity group.
As such, our current policy may have inadvertently encouraged APM Entities to prefer primary
care practitioners over specialists in their Participation Lists.
As discussed in the CY 2024 PFS final rule (88 FR 79400 through 79406), we proposed
but did not finalize to revise the definition of “attribution-eligible beneficiary” at § 414.1305 to
use covered professional services as the basis for attribution for purposes of Threshold Score
calculations for all Advanced APMs regardless of the attribution methodology used under the
specific Advanced APMs in which the eligible clinician participates. We expressed concern that
the current policy to use E/M services as the default basis for attribution, and to use an
alternative approach for Advanced APMs that use a different attribution basis, could result in a
complex set of unique attribution approaches for various Advanced APMs.
After further consideration and consultation with interested parties, we believe it would
be appropriate to create a uniform basis for beneficiary attribution for purposes of Threshold
Score calculations across all Advanced APMs. We are once again proposing to modify the sixth
criterion of the definition of “attribution-eligible beneficiary” at § 414.1305 to include any
beneficiary who has received a covered professional service furnished by the eligible clinician
(NPI) for whom we are making the QP determination, beginning with the 2025 QP performance
period. By no longer specifying a claim for E/M services as the default attribution basis in the
sixth criterion, we also would eliminate the need to create unique attribution bases that are tied to
a specific Advanced APM’s attribution methodology. This would standardize the attribution
methodology by basing attribution on covered professional services across all Advanced APMs.
The proposal would address the issue discussed earlier in this section whereby, under our
current policy, beneficiary attribution for purposes of QP determinations in the default
methodology is contingent upon the beneficiary receiving an E/M service, and as a result,
beneficiaries who are actually furnished covered professional services by eligible clinicians on
an APM Entity’s Participation List are not attribution-eligible for purposes of the QP
determination if none of the services are E/M services. Under our proposal, we would consider
all covered professional services for purposes of attribution, and not solely the limited range of
E/M services currently used for attribution. As a result, we would be able to include as attributed
beneficiaries those who are receiving any services within the entire range of covered professional
services through the Advanced APM. We believe this proposal would result in a QP calculation
that, by including beneficiaries receiving any covered professional service, more accurately
reflects eligible clinicians’ actual participation in Advanced APMs and would be consistent
across all Advanced APMs. Further, we believe that the proposal better aligns the QP
determination methodology with the universe of services to which the Quality Payment Program
(MIPS and APMs) applies, noting that its provisions generally pertain to covered professional
services (for example, its financial incentives for both MIPS eligible clinicians and QPs are
applied to covered professional services payments).
We note that the proposal would not change the dates of service used for purposes of QP
determinations. As such, QP determinations at any given snapshot date (March 31, June 30, and
August 31, respectively) will be made by including all covered professional services furnished
during the QP Performance Period for January 1 through the applicable snapshot date.
We believe that this change would more appropriately recognize the Advanced APM
participation of the eligible clinicians for whom these determinations are being made. We further
believe that this proposal would simplify and streamline QP determinations and address the
challenges to Advanced APM participation reportedly faced by specialists who are less likely
than primary care practitioners to provide E/M services. Finally, we believe that the proposal
would standardize the approach to QP determinations across all Advanced APMs and conform
more closely with the scope of services that are subject to the Quality Payment Program. We
also acknowledge that, while this proposal would represent significant progress toward
rationalizing attribution for the broader range of Advanced APMs, our continued analysis
suggests there may be more work to be done in this area. We believe there still may be situations
in which the proposed change in attribution policy would limit QP determinations in certain
Advanced APMs, particularly in situations where an Advanced APM is focused on a limited set
of services. We recognize the need to provide, consistent with statutory requirements, equitable
opportunities to achieve QP status for participants in Advanced APMs that have different focus
areas, goals, scopes, and design features. Further, in the case of CMS Innovation Center models,
we recognize that there will be ongoing evolution and innovation in the model tests that are
Advanced APMs, including the development of new approaches to attribution that apply within
the models. We will continue to analyze these developments and issues with the goal to provide
for an equitable, rational, transparent, and meaningful methodology for QP determinations across
the full range of Advanced APMs.
We seek comment on this proposal to revise the sixth criterion of the definition of
“attribution-eligible beneficiary” at § 414.1305 to include a beneficiary who has at least one
claim for a covered professional service furnished by an eligible clinician who is on the
Participation List for the APM Entity (or by the individual eligible clinician, as applicable) at any
determination date during the QP Performance Period. We also invite comment more generally
on potential approaches we could consider to make QP determinations in the most equitable,
rational, transparent, and meaningful way for eligible clinicians across the broad range of
Advanced APMs, including Advanced APMs that are focused on a limited set of services.
(3) QP thresholds and Partial QP thresholds
Section 1833(z)(2) of the Act specifies the thresholds for the level of participation in
Advanced APMs required for an eligible clinician to become a QP for a year. The Medicare

Option, based on Part B payments for covered professional services or counts of patients
furnished covered professional services under Part B, has been applicable since payment year
2019 (performance period 2017). The All-Payer Combination Option, through which QP status is
calculated using the Medicare Option in addition to an eligible clinician's participation in Other
Payer Advanced APMs, has been applicable since payment year 2021 (performance period
2019). In the CY 2017 Quality Payment Program final rule (81 FR 77433 through 77439), we
finalized our policy for QP and Partial QP Thresholds for the Medicare Option as codified at
§ 414.1430(a) and for the All-Payer Combination Option at § 414.1430(b).
Section 304(a)(2) of Division G, Title I, Subtitle C, of the Consolidated Appropriations
Act, 2024 (CAA, 2024) (Pub. L. 118-42, March 9, 2024) amended section 1833(z)(2) of the Act
by extending for payment years 2025 and 2026 (performance periods 2023 and 2024) the
applicable payment amount and patient count thresholds for an eligible clinician to achieve QP
status. Specifically, section 304(a)(2) of the CAA, 2024, amended section 1833(z)(2) of the Act
to continue the QP payment amount thresholds that applied in payment year 2025 (performance
period 2023) to payment year 2026 (performance period 2024). Additionally, section 304(a)(2)
of the CAA, 2024, amended section 1833(z)(2) of the Act to require that, for payment year 2026,
the Secretary use the same percentage criteria for the QP patient count threshold that applied in
payment year 2022. As such, the Medicare Option QP thresholds for payment year 2026 will
remain at 50 percent for the payment amount method and 35 percent for the patient count
method. Section 304(b) of the CAA, 2024, also amended section 1848(q)(1)(C)(iii) of the Act to
extend through payment year 2026 the Partial QP thresholds that were established beginning for
payment year 2021 under the Medicare Option. Therefore, the Partial QP thresholds for payment
year 2026 (performance period 2024) will remain at 40 percent for the payment amount method
and 25 percent for the patient count method.
Under the All-Payer Combination Option, the QP thresholds for payment year
2026 (performance period 2025) will remain at 50 percent for the payment amount method and
35 percent for the patient count method. The Partial QP thresholds for payment year 2026
(performance period 2024) will continue at 40 percent for the payment amount method and
25 percent for the patient count method. To become a QP through the All-Payer Combination
Option, eligible clinicians must first meet certain minimum threshold percentages under the
Medicare Option. For payment year 2026 (performance period 2024), the minimum Medicare
Option threshold an eligible clinician must meet for the All-Payer Combination Option to
become a QP is 25 percent for the payment amount method or 20 percent for the patient count
method. For Partial QP status, the minimum Medicare Option threshold an eligible clinician
must meet for the All-Payer Combination Option is 20 percent for the payment amount method
or 10 percent for the patient count method.
To conform our regulation with the amendments made by the CAA, 2024, we propose to
amend § 414.1430 by revising paragraphs (a) and (b) to reflect the statutory QP and Partial
QP threshold percentages for both the payment amount and patient count under the Medicare
Option and the All-Payer Option with respect to payment year 2026 (performance period 2024)
The proposed revisions to § 414.1430(a) and (b) for the Medicare Option and All-Payer
Combination Option QP and Partial QP thresholds are as follows:
● Paragraph (a)(1)(v) to state that for 2026 the amount is 50 percent, and a new
paragraph (a)(1)(vi) to state that for 2027and later, the amount is 75 percent.
● Paragraph (a)(2)(v) to state that for 2026 the amount is 40 percent, and a new
paragraph (a)(2)(vi) to state that for 2027 and later, the amount is 50 percent.
● Paragraph (a)(3)(v) to state that for 2026 the amount is 35 percent, and a new
paragraph (a)(3)(vi) to state that for 2027 and later, the amount is 50 percent.
● Paragraph (a)(4)(v) to state that for 2026 the amount is 25 percent, and a new
paragraph (a)(4)(vi) to state that for 2027 and later, the amount is 35 percent.
● Paragraph (b)(1)(i)(A) to state that for 2021 through 2026 the amount is 50 percent,
and paragraph (b)(1)(i)(B) to state that for 2027 and later, the amount is 75 percent.
● Paragraph (b)(2)(i)(A) to state that for 2021 through 2026 the amount is 40 percent and
paragraph (b)(2)(i)(B) to state that for 2027 and later, the amount is 50 percent.
● Paragraph (b)(3)(i)(A) to state that for 2021 through 2026 the amount is 35 percent,
and paragraph (b)(3)(i)(B) to state that for 2027 and later, the amount is 50 percent.
● Paragraph (b)(4)(i)(A) to state that for 2021 through 2026 the amount is 25 percent,
and paragraph (b)(4)(i)(B) to state that for 2027 and later, the amount is 35 percent.

TABLE 74: QP Threshold Score Updates
(4) APM Incentive Payment
Prior to amendments made by section 304(a)(1) of the CAA, 2024, section 1833(z)(1) of
the Act provided for APM Incentive Payments for eligible clinicians who are QPs with respect to
a year in each payment year from 2019 through 2025. Specifically, for each of the specified
payment years, in addition to the amount of payment that would otherwise be made for covered
professional services furnished by an eligible clinician who is determined to be a QP for such
year, an additional lump sum APM Incentive Payment equal to 5 percent of the eligible
clinician’s estimated aggregate payment amounts for such covered professional services for the
preceding year (which we defined as the “base year”) in each payment year from 2019 through
Medicare Option - Payment Amount Method
QP Performance
Period / Payment
Year
2022/2024
(Percent)
2023/2025
(Percent)
2024/2026
(Percent)
2025/2027 and later
(Percent)
QP Payment
Amount Threshold
50
50
50
75
Partial QP Payment
Amount Threshold
40
40
40
50
Medicare Option - Patient Count Method
QP Performance
Period / Payment
Year
2022/2024
(Percent)
2023/2025
(Percent)
2024/2026
(Percent)
2025/2027 and later
(Percent)
QP Patient Count
Threshold
35
35
35
50
Partial QP Patient
Count Threshold
25
25
25
35
All-Payer Combination Option - Payment Amount Method
QP Performance
Period / Payment
Year
2022/2024
(Percent)
2023/2025
(Percent)
2024/2026
(Percent)
2025/2027 and later
(Percent)
QP Patient Count
Threshold
50
25
50
25
50
25
75
25
Partial QP Patient
Count Threshold
40
20
40
20
40
20
50
20
Total
Medicare
Minimum
Total
Medicare
Minimum
Total
Medicare
Minimum
Total
Medicare
Minimum
All-Payer Combination Option - Patient Count Method
QP Performance
Period / Payment
Year
2022/2024
(Percent)
2023/2025
(Percent)
2024/2026
(Percent)
2025/2027 and later
(Percent)
QP Patient Count
Threshold
35
20
35
20
35
20
50
20
Partial QP Patient
Count Threshold
25
10
25
10
25
10
35
10
Total
Medicare
Minimum
Total
Medicare
Minimum
Total
Medicare
Minimum
Total
Medicare
Minimum
2024, and 3.5 percent of such amounts in payment year 2025. Covered professional services is
defined at § 414.1305, with reference to the statutory definition at section 1848(k)(3) of the Act,
as services for which payment is made under, or based on, the PFS and which are furnished by
an eligible clinician (physician; practitioner as defined in section 1842(b)(18)(C) of the Act; PT,
OT, or speech-language pathologist; or qualified audiologist as defined under section
1861(ll)(4)(B) of the Act).
Section 304(a) of the CAA, 2024 amended section 1833(z)(1) of the Act to provide that
eligible clinicians who are QPs with respect to payment year 2026 (performance period 2024)
will receive an APM Incentive Payment equal to 1.88 percent of their estimated aggregate
payment amounts for Medicare Part B covered professional services in the preceding year. In
effect, this statutory change extends the APM Incentive Payment for one additional year, at 1.88
percent.
Accordingly, we propose to incorporate the change made by the CAA, 2024, by
amending the regulation text at § 414.1450 to add the payment year 2026 APM Incentive
Payment amount of 1.88 percent of covered professional services payments. We propose to
amend paragraph (b)(1) to state that the amount of the APM Incentive Payment for payment
years 2019 through 2024 is equal to 5 percent, for payment year 2025, 3.5 percent, and for
payment year 2026, 1.88 percent of the estimated aggregate payments for covered professional
services furnished during the calendar year immediately preceding the payment year.
Beginning with the 2026 payment year, which relates to the 2024 QP Performance
Period, section 1848(d)(1)(A) of the Act specifies that there shall be two separate PFS
conversion factors, one for items and services furnished by an eligible clinician who is a QP for
the year (the qualifying APM conversion factor), and the other for other items and services not
furnished by a QP (the nonqualifying APM conversion factor). Each conversion factor will be
equal to the conversion factor for the previous year multiplied by the applicable update for the
year specified in section 1848(d)(20) of the Act. The update specified for the qualifying APM
conversion factor for CY 2025 is 0.75 percent, while the update for the nonqualifying APM
conversion factor is 0.25 percent.
V. Collection of Information Requirements
Under the Paperwork Reduction Act of 1995 (PRA) (44 U.S.C. 3501 et seq.), we are
required to provide 60-day notice in the Federal Register and solicit public comment before a
“collection of information” requirement (as defined under 5 CFR 1320.3(c) of the PRA’s
implementing regulations) is submitted to the Office of Management and Budget (OMB) for
review and approval. To fairly evaluate whether a collection of information should be approved
by OMB, section 3506(c)(2)(A) of the PRA requires that we solicit comment on the following
issues:
● The need for the information collection and its usefulness in carrying out the proper
functions of our agency.
● The accuracy of our estimate of the information collection burden.
● The quality, utility, and clarity of the information to be collected.
● Recommendations to minimize the information collection burden on the affected
public, including automated collection techniques.
We are soliciting public comment (see section VI. of this proposed rule) on each of the
aforementioned issues for the following sections of this document that contain information
collection requirements (ICRs). Comments, if received, will be responded to within the
subsequent final rule.
A. Wage Estimates
To derive average costs, we used data from the U.S. Bureau of Labor Statistics’ (BLS)
May 2023 National Occupational Employment and Wage Estimates for all salary estimates
(https://bls.gov/oes/2023/may/oes_nat.htm). In this regard, Tables 75 and 76 presents BLS’
mean hourly wage, our estimated cost of fringe benefits and other indirect costs (calculated at

100 percent of salary), and our adjusted hourly wage. There are many sources of variance in the
average cost estimates, both because fringe benefits and other indirect costs vary significantly
from employer to employer, and because methods of estimating these costs vary widely from
study to study. Therefore, we believe that doubling the hourly wage to estimate total cost is a
reasonably accurate estimation method.
We note that the May 2023 BLS data does not include median hourly wage rates for a
number of the physician occupation types listed in Table 76; in these cases, the BLS identifies
that the median wage rate is equal to or greater than $115.00/hr or $239,200 per year. BLS data
for prior years, such as the May 2021 and May 2022 data, provide similar notes for median wage
rates for occupations that are above a given threshold ($100.00/hr or $208.000 per year for the
May 2021 BLS data (https://www.bls.gov/oes/2021/may/oes_nat.htm), and $115.00/hr or
$239,200 per year for the May 2022 BLS data (https://www.bls.gov/oes/2022/may/oes_nat.htm)).
Therefore, for consistency with previous years for estimating physician wage rates, we have
continued to use mean hourly wage rates across our wage estimates.
TABLE 75: National Occupational Employment and Wage Estimates
(Excluding Physicians)
Occupation Title
Occupation Code
Mean Hourly
Wage ($/hr)
Fringe Benefits
and Other
Indirect Costs
($/hr)
Adjusted Hourly
Wage ($/hr)
Billing and Posting Clerks
43-3021
22.66
22.66
45.32
Computer System Analysts
15-1211
53.27
53.27
106.54
Chief Executives
11-1011
124.47
124.47
248.94
Lawyers
23-1011
84.84
84.84
169.68
Licensed Practical and
Licensed Vocational Nurses
29-2061
29.23
29.23
58.46
Medical and Health Services
Managers
11-9111
64.64
64.64
129.28
Pharmacists
29-1051
64.81
64.81
129.62
For our purposes, BLS’ May 2023 National Occupational Employment and Wage
Estimates does not provide an occupation that we could use for “Physician” wage data. To
estimate a Physician’s costs, we are using an average conglomerate wage of $291.64/hr as
demonstrated below in Table 76.

TABLE 76: National Occupational Employment and Wage Estimates
(Physicians)
Occupation Title
Occupation Code
Mean Hourly
Wage ($/hr)
Fringe Benefits
and Other Indirect
Costs ($/hr)
Adjusted Hourly
Wage ($/hr)
Anesthesiologists
29-1211
163.21
163.21
326.42
Family Medicine
Physicians
29-1215
115.77
115.77
231.54
General Internal
Medicine Physicians
29-1216
118.01
118.01
236.02
Obstetricians and
Gynecologists
29-1218
133.97
133.97
267.94
Orthopedic Surgeons,
Except Pediatric
29-1242
181.85
181.85
363.7
Pediatric Surgeons
29-1243
216.02
216.02
432.04
Pediatricians, General
29-1221
98.97
98.97
197.94
Physicians, All Other
29-1229
119.54
119.54
239.08
Psychiatrists
29-1223
123.53
123.53
247.06
Surgeons
29-1240
167.74
167.74
335.48
Surgeons, All Other
29-1249
165.38
165.38
330.76
Total
3,207.98
Average Physician
Wage (3,207.98/11)
291.64
B. Proposed Information Collection Requirements (ICRs)
1. ICRs Regarding Clinical Laboratory Fee Schedule: Revised Data Reporting Period and
Phase-in of Payment Reductions (§ 414.504)
On November 17, 2023, section 502 of the Further Continuing Appropriations and Other
Extensions Act, 2024 (Pub. L. 118-22) (FCAOEA, 2024) was passed and delayed data reporting
requirements for CDLTs that are not ADLTs, and it also delayed the phase-in of payment
reductions under the CLFS from private payor rate implementation under section 1834A of the
Act. As described in section III.D of this proposed rule, under the Clinical Laboratory Fee
Schedule, “reporting entities” must report to CMS during a “data reporting period” “applicable
information” collected during a “data collection period” for their component “applicable
laboratories.” We are proposing to revise § 414.504(a)(1) to account for a delay in reporting until
January 1, 2025, through March 31, 2025. As stated in section 1834A(h)(2) of the Act, chapter
35 of title 44 U.S.C., which includes such provisions as the PRA, does not apply to information
collected under section 1834A of the Act. Consequently, we are not setting out any proposed
burden estimates under this section of this proposed rule. Please refer to section VII.XX. of this

proposed rule for a discussion of the impacts associated with the proposed changes described in
section III.D. of this proposed rule.
2. ICRs Regarding the Updates to the Medicare Diabetes Prevention Program
In section § 410.79(b), we are proposing to make conforming changes to our regulation
Conditions of Coverage to align with the 2024 Centers for Disease Control and Prevention
(CDC) Diabetes Prevention Recognition Program (DPRP) Standards.
787
We are proposing to
amend § 410.79(b) to add a new term for MDPP, “in-person with a distance learning
component.” The proposed “in-person with a distance learning component” code will reduce
administrative burden and allow MDPP suppliers to streamline data reporting to CDC because
they will only have to maintain one code if they are providing in-person and distance learning
delivery. To further align with 2024 CDC DPRP Standards, we are also adding the term
“combination with an online component” and revising the current “online” definition. We are
also clarifying in § 410.79(d)(1) that MDPP make-up sessions can only be furnished using
distance learning and in-person delivery modes, in alignment with the Extended flexibilities as
defined in the CY 2024 PFS final rule (88 FR 79528). We are also proposing to amend
§ 410.79(e)(3)(iii)(C) in response to comments that beneficiaries are unable to take a picture
while standing on their home scales due to risk of injury and physical health limitations (88 FR
79249). We are proposing revised language to specify that a beneficiary can self-report their
weight for an MDPP distance learning session by sending 2 (two) date-stamped photos: one with
their weight on the digital scale and one of the beneficiary visible in their home. Additionally, at
§ 414.84(c), to make it possible for Medicare Administrative Contractors (MACs) to process
claims for same day make-up sessions in MDPP, we are proposing that suppliers be required to
append an existing claim modifier (Current Procedural Terminology (CPT) Modifier 79) to any
claim for G9886 or G9887 to indicate a make-up session that was held on the same day as a
787
Centers for Disease Control and Prevention Diabetes Prevention Recognition Program. Standards and Operating
Procedures. Requirements for CDC Recognition. June 2024. https://nationaldppcsc.cdc.gov/s/article/DPRP-
Standards-and-Operating-Procedures.
regularly scheduled MDPP session. We are proposing to remove the MDPP bridge payment in
§ 414.84(a), (d), and (e). This payment is no longer necessary in MDPP’s CY 2024 fee for
service payment structure and could introduce the potential for fraud, waste, or abuse. Finally,
we are proposing to make minor edits throughout §§ 410.79, 424.205, and 414.84 to update
outdated references and align with previous rulemaking pertaining to MDPP terminology,
payment structure, and requirements. Section 1115A(d)(3) of the Act exempts Innovation Center
model tests and expansions, which include the MDPP expanded model, from the provisions of
the PRA. Accordingly, this collection of information section does not set out any burden for the
provisions, including the collection of weights, per the CY 2024 PFS final rule.
3. ICRs Regarding the Medicare Shared Savings Program
Section 1899(e) of the Act provides that chapter 35 of title 44 U.S.C., which includes
such provisions as the PRA, shall not apply to the Shared Savings Program. Accordingly, we are
not setting out proposed Shared Savings Program burden estimates under this section of the
preamble. Please refer to section VII.E.11. of this proposed rule for a discussion of the impacts
associated with the changes to the Shared Savings Program as described in section III.G. of this
proposed rule.
4. ICRs Regarding Rebate Reduction Requests Submitted Under Sections 11101 and 11102 of
the Inflation Reduction Act
The following proposed changes will be submitted to OMB for review under control
number 0938–NEW (CMS–10858).
As described in section III.I. of this proposed rule, to receive consideration for an
inflation rebate reduction for a specific rebatable drug when the manufacturer believes there is a
severe supply chain disruption or likely shortage, we are proposing that a manufacturer must
submit a rebate reduction request form along with supporting documentation. We are proposing
this because manufacturers hold some of the information and documentation that is needed to
determine whether the rebate amount for a Part B or Part D rebatable drug should be reduced due
to either a severe supply chain disruption or a likely shortage as required by sections
1847A(i)(3)(G)(ii), 1860D–14B(b)(1)(C)(ii), and 1860D–14B(b)(1)(C)(iii) of the Act.
In §§ 427.402(c)(4) and 428.302(c)(4), we are proposing the criteria that a Part B or Part
D rebatable drug would have to meet for CMS to grant a severe supply chain disruption rebate
reduction request. In §§ 427.402(c)(5) and 428.302(c)(5), we propose that if a manufacturer
believes a severe supply chain disruption continues into a fifth consecutive calendar quarter for a
Part B rebatable biosimilar biological product, or a second applicable period for a generic Part D
rebatable drug or biosimilar after the start of the natural disaster or other unique or unexpected
event, the manufacturer may request an extension of the rebate reduction one time by submitting
a rebate reduction extension request and supporting documentation. In § 428.303(c)(4), we
propose the criteria that a generic Part D rebatable drug would have to meet for CMS to grant a
rebate reduction request because the generic Part D rebatable drug is likely to be in shortage,
including the requirements for a one-time extension of a rebate reduction.
Outside of this proposed rule we have proposed additional submission requirements for
rebate reduction requests under OMB control number: 0938–NEW (CMS–10858). At this time,
the rebate reduction request forms are currently going through the PRA review and approval
process. The 30-day notice appeared in the Federal Register on June 3, 2024 (89 FR 47563).
We believe that few manufacturers will submit a rebate reduction request form due to the
statutory specifications regarding eligible rebatable drugs, as well as the policy criteria proposed
in this proposed rule. Using the wage rates in Table 75 of this proposed rule, we anticipate
collecting a total of 10 rebate reduction request forms per year. We estimate a total annual
burden of 310 hours (31 hrs per form * 10 forms) and total annual cost of $37,378 ((160 hrs x
$84.66/hr) + (80 hrs x $129.62/hr) + (50 hrs x $169.68/hr) + (20 hrs x $248.94/hr)).
Using the wage rates in Table 75 of this proposed rule, we also anticipate collecting a
total of 10 rebate reduction extension request forms per year, and a total annual burden estimate
of 310 hours (31 hr per form * 10 forms) at a cost of $37,378 ((160 hrs x $84.66/hr) + (80 hrs x
$129.62/hr) + (50 hrs x $169.68/hr) + (20 hrs x $248.94/hr)).
5. ICRs Regarding Medicare Parts A and B Overpayment Provisions of the Affordable Care Act
(§ 401.305(b)(1), (2), and (3))
Existing § 401.305(b)(1) specifies when a person who has received an overpayment must
report and return an overpayment. We propose to amend this regulation to reference revised
§ 401.305(b)(2) and new § 401.305(b)(3). We are proposing a technical modification to the
introductory language in § 401.305(b)(2) to acknowledge that this paragraph may be applicable
after the suspension described in new § 401.305(b)(3) is complete. New proposed §
401.305(b)(3) would identify the circumstances under which the deadline for reporting and
returning overpayments would be suspended to allow time for providers to investigate and
calculate overpayments.
The proposed amendments for Medicare Parts A and B are associated with OMB control
number 0938–1323 (CMS–10405); however, we are not making any revisions to the currently
approved requirements and burden under this control number since we cannot predict if there
will be any change to the number of overpayments identified or reported based on this
rulemaking’s proposed changes. We solicit comment on this assumption.
6. The Quality Payment Program (42 CFR part 414 and section IV of this proposed rule)
The following Quality Payment Program-specific ICRs reflect changes to our currently
approved burden to capture proposed policy changes in this CY 2025 proposed rule as well as
adjustments to the policies that have been previously finalized in the CY 2017 and CY 2018
Quality Payment Program final rules (81 FR 77008 and 82 FR 53568, respectively), and the
CY 2019, CY 2020, CY 2021, CY 2022, CY 2023, and CY 2024 Physician Fee Schedule (PFS)
final rules (83 FR 59452, 84 FR 62568, 85 FR 84472, 86 FR 64996, 87 FR 70131, and
88 FR 78818, respectively) due to revised assumptions based on updated data available at the
time of the publication of this proposed rule.
As described in the CY 2017 and CY 2018 Quality Payment Program final rules
(81 FR 77008 and 82 FR 53568, respectively), and the CY 2019, CY 2020, CY 2021, CY 2022,
CY 2023, and CY 2024 Physician Fee Schedule (PFS) final rules (83 FR 59452, 84 FR 62568,
85 FR 84472, 86 FR 64996, 87 FR 70131, and 88 FR 78818, respectively), we have used an
exhaustive approach for describing the burden associated with the MIPS requirements for the
Quality Payment Program. With the maturity and stabilization of the Quality Payment Program,
we believe there may be opportunities to present streamlined annual burden estimates to account
for changes, including changes due to proposed policies. We are soliciting public comment as
part of this proposed rule on our exhaustive approach to presenting burden estimates associated
with the Quality Payment Program.
a. Background
(1) ICRs Associated with Merit-based Incentive Payment System (MIPS) and Advanced
Alternative Payment Models (APMs)
In this section of this proposed rule, we discuss a series of ICRs associated with the
Quality Payment Program, including for MIPS and Advanced APMs. The following sections
describe the changes in the estimated burden for the information collections relevant to the
proposed revisions in the policies associated with the CY 2025 PFS proposed rule and proposed
revisions to our currently approved information requests for MIPS and Advanced APM ICRs.
The proposed changes will be submitted to OMB for review under control number 0938-1314
(CMS-10621). In the CY 2024 PFS final rule (88 FR 79446 and 79447), we provided updated
burden estimates for the information collections under the Consumer Assessment of Healthcare
Providers and Systems (CAHPS) for MIPS Survey PRA package. We have submitted these
burden estimates to OMB for approval under control number 0938-1222 (CMS-10450): the 60-
day notice appeared in the Federal Register on October 17, 2023 (88 FR 71573), while the 30-
day notice appeared on January 16, 2024 (89 FR 2622). The updated information collections for
the CAHPS for MIPS Survey discussed in section IV.A.4.j. of this proposed rule will be
submitted to OMB for review under control number 0938-1222 (CMS-10450) if the related
policy proposal is finalized in the CY 2025 PFS final rule, to be a requirement for CAHPS for
MIPS survey vendors beginning with the CY 2026 performance period/2028 MIPS payment
year. We note that we have received approval for the collection of information associated with
the virtual election process under OMB control number 0938-1343 (CMS-10652).
(a). Summary of Annual Quality Payment Program Burden Estimates
Table 77 summarizes this proposed rule’s total burden estimates for the Quality Payment
Program for the CY 2025 performance period/2027 MIPS payment year. We provide details of
the proposed policies, updated assumptions, and ICRs beginning in section V.B.8. of this
proposed rule.
In the CY 2024 PFS final rule (87 FR 70169), the total estimated burden for the CY 2024
performance period/2026 MIPS payment year was 724,212 hours at a cost of $81,322,556 (see
Table 77, row a). Accounting for updated wage rates and the subset of all Quality Payment
Program ICRs outlined in this proposed rule compared to the CY 2024 PFS final rule, the total
estimated annual burden of continuing policies and information set forth in the CY 2024 PFS
final rule into the CY 2025 performance period/2027 MIPS payment year is 727,906 hours at a
cost of $85,867,252 (see Table 77, row b). These represent an increase of 3,694 hours and an
increase of $4,544,696.
To understand the burden implications of the policies proposed in this rulemaking, we
provided an estimate of the total burden associated with continuing the policies and information
collections set forth in the CY 2024 PFS final rule into the CY 2025 performance period/2027
MIPS payment year. The estimated burden of 657,110 hours at a cost of $77,779,594 (see Table
77, row c) reflects the availability of more accurate data to account for all potential respondents
and submissions across all the performance categories and represents a decrease of 70,796 hours
and $8,087,658 (see Table 77, row d).

Our total proposed burden estimate for the CY 2025 performance period/2027 MIPS
payment year is 649,540 hours and $76,866,418 (see Table 77, row e), which represents a
decrease of 78,366 hours and $9,000,834 from the CY 2024 PFS final rule estimate with updated
wage rates and ICRs (see Table 77, row f). From these estimates, updated data and assumptions
not related to policies proposed in this proposed rule would reduce burden by 70,796 hours and
$8,087,658. We estimate that the policies proposed in this proposed rule would further reduce
burden by 7,570 hours (-78,366 hours − - 70,796 hours) and $913,176 (-$9,000,834 – -
$8,087,658) (see Table 77, row g) between this estimate and the total burden shown in Table 78
is the decrease in burden associated with impacts of the policies proposed for the CY 2025
performance period/2027 MIPS payment year.
TABLE 77: Summary of Burden Estimates and Requirements from the CY 2025
PFS Proposed Rule
Burden Estimate Description
Time (Hours)
Cost
Currently Approved Burden in CY 2024 PFS Final Rule (a)
724,212
$81,322,556
CY 2024 PFS Final Rule w/ Updated Wage Rates and ICRs (b)
727,906
$85,867,252
CY 2024 PFS Final Rule w/ Updated Data and Assumptions (c)
657,110
$77,779,594
Change in Burden Due to Updated Data and Assumptions
(d) = (c) – (b)
-70,796
-$8,087,658
CY 2025 PFS Proposed Rule Total Burden (e)
649,540
$76,866,418
Total Change in Burden (f) = (e) – (b)
-78,366
-$9,000,834
Change in Burden Associated with Policies (g) = (f) – (d)
-7,570
-$913,176

TABLE 78: Summary of Quality Payment Program Burden Estimates and Requirements
CMS-10621 (OMB 0938-1314)
Requirement
Currently
Approved
Responses
CMS-
1807-P
Responses
Change in
Responses
Currently
Approved
Total Time
(Hours)
CMS-
1807-P
Total
Time
(Hours)
Change
in Total
Time
(Hours)
§ 414.1400 Qualified Clinical
Data Registry (QCDR)
Simplified Self-Nomination and
other Requirements (see Tables
81 and 82)
44
39
-5
418
467
+49
§ 414.1400 QCDR Full Self-
Nomination and other
Requirements (see Tables 83 and
84)
12
17
+5
138
238
+100
§ 414.1400 Qualified Registry
Simplified Self-Nomination and
other Requirements (see Tables
85 and 86)
84
76
-8
42
38
-4
§ 414.1400 Qualified Registry
Full Self-Nomination and other
Requirements (see Tables 87 and
88)
27
30
+3
54
60
+6
§ 414.1400 Third Party
Intermediary Plan Audits (see
Tables 90 and 91)
126
89
-37
499
472
-27
Quality Payment Program
Identity Management
Application Process (see Tables
95 and 96)
6,500
6,237
-263
6,500
6,237
-263
§§ 414.1325 and 414.1335
Quality Performance Category:
Medicare Part B Claims
Collection Type (see Tables 97
and 98)
13,413
12,197
-1,216
190,465
173,197
-17,268
§§ 414.1325 and 414.1335
Quality Performance Category:
QCDR/MIPS Clinical Quality
Measure CQM Collection Type
(see Tables 99 and 100)
16,632
17,008
+376
151,068
154,484
+3,416
§§ 414.1325 and 414.1335
Quality Performance Category:
electronic Clinical Quality
Measure eCQM Collection Type
(see Tables 101 and 102)
28,714
27,179
-1,535
229,712
217,432
-12,280
§414.1365 MVP Registration
(see Tables 103 and 104)
9,585
6,285
-3,300
2,396
1,571
-825
MVP Quality Submission (see
Tables 105 and 106)
9,585
6,285
-3,300
61,662
40,193
-21,469
Call for Quality Measures (see
Tables 107 and 108)
31
16
-15
171
88
-83
§ 414.1380(c)(2) Reweighting
Applications for MIPS
Performance Categories (see
Tables 109 and 110)
29,227
3,297
-25,930
7,307
824
-6,483
§ 414.1375 Data Submission for
Promoting Interoperability
Performance Category (see
Tables 112 and 113)
25,990
18,609
-7,381
70,173
50,244
-19,929

Requirement
Currently
Approved
Responses
CMS-
1807-P
Responses
Change in
Responses
Currently
Approved
Total Time
(Hours)
CMS-
1807-P
Total
Time
(Hours)
Change
in Total
Time
(Hours)
§ 414.1360 Data Submission for
the Improvement Activities
Performance Category (see
Tables 115 and 116)
50,289
38,433
-11,856
4,174
3,190
-984
§§ 414.1310(b) and 414.1430
Partial Qualifying APM
Participant (QP) Elections (see
Tables 117 and 118)
287
18
-269
72
5
-67
§ 414.1445 Other Payer
Advanced APM Determinations:
Payer-Initiated Process (see
Tables 119 and 120)
15
10
-5
150
100
-50
§ 414.1445 Other Payer
Advanced APM Determinations:
Eligible Clinician-Initiated
Process (see Tables 121 and
122)
15
10
-5
150
100
-50
§ 414.1440 Submission of Data
for QP Determinations under the
All-Payer Combination Option
(see Tables 123 and 124)
551
120
-431
2,755
600
-2,155
TOTAL
191,127
135,955
-55,172
727,906
649,540
-78,366
Table 79 provides the reasons for changes in the estimated burden for the CY 2025
performance period/2027 MIPS payment year for information collections in the Quality Payment
Program segment of this proposed rule. We divided the reasons for our change in burden into
those related to proposed policies in the CY 2025 PFS proposed rule and those related to
adjustments in burden continued from the CY 2024 PFS final rule policies that reflect updated
data and revised methods.
TABLE 79: Reasons for Proposed Change in Burden Compared to The Currently
Approved CY 2024 Information Collection Burden
ICR Title
Changes in Burden Due to CY
2025 Proposed Rule Policies
Adjustments in Burden Continued
from CY 2024 PFS Final Rule
Policies Due to Revised Methods or
Updated Data
QCDR Simplified Self-
Nomination and other
Requirements (See Table 82)
None
Decrease in number of respondents and
increase in the number of hours per
respondent due to updated data.
Net increase in the total number of
hours due to updated data.

ICR Title
Changes in Burden Due to CY
2025 Proposed Rule Policies
Adjustments in Burden Continued
from CY 2024 PFS Final Rule
Policies Due to Revised Methods or
Updated Data
QCDR Full Self-Nomination and
other Requirements (See Table
84)
None
Increase in number of respondents and
increase in the number of hours per
respondent due to updated data.
Net increase in the total number of
hours due to updated assumptions for
the CY 2025 performance period/2027
MIPS payment year.
Qualified Registry Simplified Self-
Nomination and other
Requirements (See Table 86)
None
Decrease in number of respondents due
to updated data.
Qualified Registry Full Self-
Nomination and other
Requirements (See Table 88)
None
Increase in number of respondents due
to updated data.
Third Party Intermediary Plan
Audits (see Table 91)
None
Decrease in number of respondents due
to updated data.
Quality Payment Program Identity
Management Application Process
(see Table 96)
None
Decrease in number of respondents due
to updated data.
Quality Performance Category:
Medicare Part B Claims Collection
Type (see Table 98)
Decrease in number of
respondents due to the estimated
increase in the number of
respondents submitting for the
MVP quality performance
category via the claims
collection type due to the
proposed addition of 6 new
MVPs.
Decrease in the number of respondents
due to updated data.
Quality Performance Category:
QCDR/MIPS CQM Collection
Type (see Table 100)
Decrease in number of
respondents due to the estimated
increase in the number of
respondents submitting for the
MVP quality performance
category via the QCDR and
MIPS CQM collection type due
to proposed addition of new
MVPs.
Increase in the number of respondents
due to updated data.
Quality Performance Category:
eCQM Collection Type (see Table
102)
Decrease in number of
respondents due to the estimated
increase in the number of
respondents submitting for the
MVP quality performance
category via the eCQM
collection type due to proposed
addition of 6 new MVPs.
Decrease in the number of respondents
due to updated data.
MVP Registration (see Table 104)
Increase in number of
respondents due to proposed
addition of 6 new MVPs.
Decrease in the number of respondents
due to updated data.
MVP Quality Submission (see
Table 106)
Increase in number of
respondents due to proposed
addition of 6 new MVPs.
Decrease in the number of respondents
due to updated data.
Call for Quality Measures (see
Table 108)
None
Decrease in number of respondents due
to updated data.
Reweighting Applications for
MIPS Performance Categories (see
Table 110)
None
Decrease in number of respondents due
to updated data.

ICR Title
Changes in Burden Due to CY
2025 Proposed Rule Policies
Adjustments in Burden Continued
from CY 2024 PFS Final Rule
Policies Due to Revised Methods or
Updated Data
Data Submission for the Promoting
Interoperability Performance
Category (see Table 113)
None
Decrease in number of respondents due
to updated data.
Data Submission for the
Improvement Activities
Performance Category (see Table
116)
None.
Decrease in number of respondents due
to updated data.
Partial QP Elections (see Table
118)
None
Decrease in number of respondents due
to updated data.
Other Payer Advanced APM
Determinations: Payer-Initiated
Process (see Table 120)
None
Decrease in number of respondents due
to updated data.
Other Payer Advanced APM
Determinations: Eligible Clinician-
Initiated Process (see Table 122)
None
Decrease in number of respondents due
to updated data.
Submission of Data for QP
Determinations under the All-
Payer Combination Option (see
Table 124)
None
Decrease in number of respondents due
to updated data.
(2) Summary of Proposed Changes for the Quality Payment Program: MIPS
We have included the change in the estimated burden for the CY 2025 performance
period/2027 MIPS payment year due to the proposed policies and information collections in this
proposed rule. The proposed policies in this proposed rule impact the burden estimates for the
CY 2025 performance period/2027 MIPS payment year.
The following five MIPS ICRs show changes in burden due to the proposed policies in
this proposed rule: (1) Quality performance category data submission by Medicare Part B claims
collection type; (2) Quality performance category data submission by qualified clinical data
registry (QCDR) and MIPS clinical quality measure (CQM) collection type; (3) Quality
performance category data submission by electronic clinical quality measure (eCQM) collection
type; (4) MIPS Value Pathways (MVP) quality performance category submission, and (5) MVP
registration. In aggregate, we estimate the proposed policies would result in a net decrease in
burden of 7,570 hours and $913,176 for the CY 2025 performance period/2027 MIPS payment
year. The remaining changes to our currently approved burden estimates are proposed
adjustments due to the revised burden assumptions based on the updated data available at the
time of preparation of this proposed rule.
We are not proposing any changes or adjustments to the following ICRs: Registration for
virtual groups; CAHPS survey vendor requirements; CAHPS beneficiary burden; group
registration for the CAHPS survey; call for Improvement Activities; Open Authorization
(OAuth) credentialing and token request process; nomination of MVPs; subgroup registration;
and opt-out of performance data display on Compare Tools for voluntary participants. See
section V.B.8.a.(1). of this proposed rule for a summary of the ICRs, the overall burden
estimates, and a summary of the assumption and data changes affecting each ICR.
We incorporate submission data from CY 2022 performance period/2024 MIPS payment
year to calculate the total burden for data submission under the quality, Promoting
Interoperability, and improvement activities performance categories. The accuracy of our
estimates of the total burden for data submission for those performance categories may be
impacted by several primary factors. First, we are unable to predict with absolute certainty who
will be a Qualifying APM Participant (QP) for the CY 2025 performance period/2027 MIPS
payment year. Newly eligible clinician participants in Advanced APMs who become QPs will
be excluded from MIPS payment adjustments, and as such, are unlikely to report under MIPS;
while some current Advanced APM participants may end participation such that the APM
Entity’s eligible clinicians may not be QPs for a year based on § 414.1425(c)(5), and thus be
required to report under MIPS. Second, it is difficult to predict whether Partial QPs, who can
elect to report to MIPS, will choose to participate in the CY 2025 performance period/2027
MIPS payment year compared to the CY 2022 performance period/2024 MIPS payment year.
Therefore, the actual number of Advanced APM participants and how they elect to submit data
may differ from our estimates. However, we believe our estimates are the most appropriate
given the available data. We will continue to revise our estimates as updated data becomes
available.
In the CY 2024 PFS final rule, we finalized requirements to align the Shared Savings
Program’s certified electronic health record technology (CEHRT) requirements with MIPS
Promoting Interoperability (88 FR 79124 through 79131), codified at § 425.507(a) and (b). For
performance years beginning on or after January 1, 2025, unless otherwise excluded, an
Accountable Care Organization (ACO) participant, ACO provider/supplier, and (ACO)
professional that is a MIPS eligible clinician, Qualifying APM Participant (QP) and Partial
Qualifying APM Participant (Partial QP), regardless of track must report the MIPS Promoting
Interoperability measures and requirements to MIPS according to § 414(o) at the individual,
group, virtual group, or APM entity level, and earn a MIPS performance category score for the
MIPS Promoting Interoperability performance category at the individual, group, virtual group, or
APM entity level, barring an applicable exclusion defined at § 425.507(b). We refer readers to
the CY 2024 PFS final rule (88 FR 79124 through 79131) for details. We note we are not
updating burden estimates for the Promoting Interoperability performance category to reflect
requirements for ACO participants who are not MIPS eligible clinicians. Section 1899(e) of the
Act provides that chapter 35 of title 44 of the U.S.C., which includes such provisions as the PRA,
shall not apply to the Shared Savings Program. In section V.B.8.e.(7).of this proposed rule, we
provide our burden estimates for the Promoting Interoperability performance category for MIPS
eligible clinicians.
(3) Summary of Quality Payment Program Changes: Advanced APMs
For these ICRs, we are proposing adjustments to the currently approved burden estimates
for the CY 2025 performance period/2027 MIPS payment year based on updated submission
trends: Partial QP elections; Other Payer Advanced APM determinations: Payer-Initiated and
Eligible Clinician-initiated processes; and submission of Data for QP Determinations under the
All-Payer Combination Option.
(4) Framework for Understanding the Burden of MIPS Data Submission
Because of the wide range of information collection requirements under MIPS, Table 80
presents a framework for understanding how the organizations permitted or required to submit
data on behalf of clinicians vary across the types of data, and whether the clinician is a MIPS
eligible clinician or other eligible clinician voluntarily submitting data, MIPS APM participant,
or an Advanced APM participant. In Table 80, MIPS eligible clinicians and other clinicians
voluntarily submitting data to MIPS may submit data as individuals, groups, or virtual groups for
the quality, Promoting Interoperability, and improvement activities performance categories.
Note that virtual groups are subject to the same data submission requirements as groups, and
therefore, we will refer only to groups for the remainder of this section, unless otherwise noted.
Beginning with the CY 2023 performance period/2025 MIPS payment year, clinicians
could also participate as subgroups for reporting measures and activities in an MVP. We note
that the subgroup reporting option is not available for clinicians participating in traditional MIPS.
We finalized in the CY 2022 PFS final rule that for the CY 2023 and 2024 MIPS performance
periods/2025 and 2026 MIPS payment years, a subgroup reporting measures and activities in an
MVP will submit its affiliated group’s data for the Promoting Interoperability performance
category and in the scenario that a subgroup does not submit its affiliated group’s data, the
subgroup will receive a zero score for the Promoting Interoperability performance category (86
FR 65413 and 65414). As discussed in section IV.A.4.b.(1)(4). of this proposed rule, we are
proposing to clarify that we intend for the policy to apply beyond the CY 2023 and 2024
performance periods/2025 and 2026 MIPS payment years. We refer readers to section
V.B.8.g.(3). of this proposed rule for our burden estimates for the Promoting Interoperability
performance category.
Because MIPS eligible clinicians are not required to submit any additional information
for assessment under the cost performance category, the administrative claims data used to
calculate the scores for the cost performance category is not represented in Table 80.
For MIPS eligible clinicians participating in MIPS APMs, the organizations submitting
data on behalf of MIPS eligible clinicians will vary between performance categories and, in
some instances, between MIPS APMs. We previously finalized in the CY 2021 PFS final rule
that the APM Performance Pathway (APP) is available for clinicians who participate in a MIPS
APM for both ACO participants and non-ACO participants to submit quality data
(85 FR 84859 through 84866). Due to data limitations and our inability to determine who will
use the APP versus the traditional MIPS submission mechanism for the CY 2025 performance
period/2027 MIPS payment year, we assume Shared Savings Program ACO APM Entities will
submit quality data through the APP as required, and MIPS eligible clinicians in non-Shared
Savings Program ACO APM Entities will participate through traditional MIPS or MVPs,
submitting as an individual or group rather than as an APM Entity. Per section 1899(e) of the
Act, submissions received from eligible clinicians in ACOs are not included in burden estimates
for this proposed rule because quality data submissions to fulfill requirements of the Shared
Savings Program are not subject to the PRA. Accordingly, this burden is not included in Quality
Payment Program burden estimates.
In the CY 2021 PFS final rule (85 FR 84860 and 84861), we adopted the current APP
quality measure set. As discussed in section IV.A.4.c.(2). of this proposed rule, we are
proposing to create the APP Plus quality measure set that would allow for alignment of the APP
with the Adult Universal Foundation measures. Under this proposal, Shared Savings Program
ACOs would be required to report the APP Plus quality measure beginning with the CY 2025
performance period/2027 MIPS payment year. We are not proposing to modify the existing APP
quality measure set of six quality measures; instead, we are proposing to create the APP Plus
measure set that would be optional, with exception to the Shared Savings Program ACOs,
beginning in the CY 2025 performance period/2027 MIPS payment year. Under this proposal,
each MIPS eligible clinician, group, or APM Entity that elects to report the APP may choose to
report either the APP quality measure set or the APP Plus quality measure set. MIPS APM
participants may also elect to report via traditional MIPS or MVPs.
We are proposing to adopt five new quality measures for the APP Plus quality measure
set incrementally over several performance periods/MIPS payment years, as detailed in Tables
55, 56, and 57 in section IV.A.4.c.(3)(f): two new quality measures beginning with the CY 2025
performance period; one new quality measure beginning with the CY 2026 performance period,
and two new quality measures beginning with the CY 2028 performance period. We refer
readers to section IV.A.4.c.(3). of this proposed rule for additional details on the proposed
measures and collection types of the APP Plus quality measure set. As noted in section
IV.A.4.c.(3). of this proposed rule, we are proposing to establish that all measures in the APP
Plus quality measure set must be reported. As detailed in Table 53, the current APP quality
measure set consists of six measures: two administrative claims measures, the CAHPS for MIPS
survey, and three measures that MIPS eligible clinicians reporting the APP must actively report
to CMS via the Medicare CQM (Shared Savings Program ACOs only), eCQM, CQM, or Part B
claims collection types. MIPS eligible clinicians, groups, or APM Entities reporting the
proposed APP Plus quality measure set would report via the available collection types: eight
measures for the CY 2025 performance period/2027 MIPS payment year; nine measures for the
CY 2026 and 2027 performance periods/2028 and 2029 MIPS payment years; and eleven
measures for the CY 2028 performance period/2030 MIPS payment period.
The quality performance category burden for MIPS eligible clinicians who elect to report
the proposed APP Plus quality measure set varies compared to the current APP quality measure
set, traditional MIPs, and MVPs. We assume MIPS eligible clinicians incur no burden for
reporting the two administrative claims quality measures currently required under the APP
quality measure set, as similar to cost measures, we automatically calculate scores from
administrative claims reporting. Additionally, burden estimates for the CAHPS for MIPS
registration and patient reporting are provided in the CAHPS for MIPS PRA package under
OMB control number 0938-1222 (CMS-10450); we do not assume that MIPS eligible clinicians
incur additional reporting burden for reporting this measure under the current APP quality
measure set. Therefore, we assume that MIPS eligible clinicians reporting the proposed APP
Plus quality measure set would incur burden for actively submitting their quality performance
category data via the available collection types – eCQM, CQM/QCDR, and Medicare Part B
claims. We note, these assumptions for actively submitting to assess clinician reporting burden
may differ from MIPS scoring policy. This active submission of quality performance data would
include five of the eight quality measures for the CY 2025 performance period/2027 MIPS
payment year, six of the nine quality measures for the CY 2026 and 2027 performance
periods/2028 and 2029 MIPS payment years, and eight of the eleven measures for the CY 2028
performance period/2030 MIPS payment year. Continuing this burden comparison for MIPS
eligible clinicians reporting the proposed APP Plus quality measure set for the CY 2025
performance period/2027 MIPS payment year, clinicians would need to actively submit quality
performance category data for one less quality measure than clinicians participating in traditional
MIPS (six measures), two more quality measures than clinicians participating via the APP (three
measures), and one more quality measure than clinicians participating via MVPs (four
measures). For the CY 2026 and 2027 performance periods/2028 and 2029 MIPS payment
years, clinicians reporting the proposed APP Plus quality measure set would need to actively
submit quality performance category data for the same number of quality measures as clinicians
participating in traditional MIPS (six measures); they would need to report three more quality
measures than clinicians participating via the APP (three measures), and two more quality
measures than clinicians participating via MVPs (four measures). Beginning in the CY 2028
performance period/2030 MIPS payment year, clinicians reporting the proposed APP Plus
quality measure set would need to actively submit quality performance category data for two
more quality measures than clinicians reporting via traditional MIPS (six measures), five more
quality measures that clinicians participating via the APP (three measures), and four more
measures than clinicians participating via MVPs (four measures). For this comparison of MIPS
reporting requirements, we assume that clinicians reporting via traditional MIPS and MVPs will
report eCQM, CQM, and Part B Claims measures, and will not elect to report the CAHPS for
MIPS survey. As proposed in section IV.A.4.c.(2). of this proposed rule, all Shared Savings
Program ACOs would be required to report the APP Plus measure set for the CY 2025
performance period/2027 MIPS payment year. Per section 1899(e) of the Act, submissions
received from eligible clinicians in ACOs are not included in burden estimates for this proposed
rule because quality data submissions to fulfill requirements of the Shared Savings Program are
not subject to the PRA. As the APP Plus is a new measure set, and optional, with exception to
the Shared Savings Program ACOs, we are unable to estimate how many MIPS eligible
clinicians would submit quality measures via the APP Plus at this time via individual, group, or
non-Shared Savings Program ACO Entity reporting. We will update these estimates as
additional data are available. We refer readers to section VII.E.17.e.(2)(h). of this proposed rule
for additional discussion.
For the Promoting Interoperability performance category, group TINs may submit data
on behalf of eligible clinicians in MIPS APMs, or eligible clinicians in MIPS APMs may submit
data individually. Additionally, APM Entities may report the Promoting Interoperability
performance category at the APM Entity level beginning with the CY 2023 performance
period/2025 MIPS payment year (87 FR 70087 and 70088). In the CY 2017 Quality Payment
Program final rule (81 FR 30132), we codified at § 414.1380(b)(3)(i) that individual MIPS
eligible clinicians participating in APMs (as defined in section 1833(z)(3)(C) of the Act) for a
performance period will earn at least 50 percent for the improvement activities performance
category. We also stated that MIPS eligible clinicians participating in an APM for a
performance period may receive an improvement activity score higher than 50 percent
(81 FR 30132). To provide clarity for APM participants not scored under the APP in the CY
2024 PFS final rule (88 FR 79365 through 79367), we revised § 414.1380(b)(3)(i) to state that a
MIPS eligible clinician participating in an APM receives an improvement activities performance
category score of at least 50 percent if the MIPS eligible clinician reports a completed

improvement activity or submits data for the quality and Promoting Interoperability performance
categories.
MIPS eligible clinicians who attain Partial QP status may incur additional burden if they
elect to participate in MIPS, which is discussed in more detail in the CY 2018 Quality Payment
Program final rule (82 FR 53841 through 53844).
TABLE 80: Clinicians or Organizations Submitting MIPS Data on Behalf of Clinicians,
by Type of Data and Category of Clinician
Type of Data Submitted
Category of Clinician
Quality Performance Category
Individual clinician (MIPS eligible, voluntary, opt-in), group, virtual group,
subgroup, or APM Entity.
Subgroup reporting is only available for clinicians participating in MVP reporting.
Voluntary reporting and virtual group reporting are only available for clinicians
participating in traditional MIPS.
Opt-in reporting is only available for clinicians participating in traditional MIPS
and the APM Performance Pathway.
Promoting Interoperability
Performance Category
Individual clinician (MIPS eligible, voluntary, opt-in), group, virtual group,
subgroup, or APM Entity.
Each eligible clinician in an APM Entity could report data for the Promoting
Interoperability performance category at the individual level, or as part of their
group TIN, or under their APM Entity TIN. The burden estimates for this
proposed rule assume group TIN-level reporting.
Improvement Activities
Performance Category
Individual clinician (MIPS eligible, voluntary, opt-in), group, virtual group,
subgroup, or APM Entity.
For eligible clinicians in an APM Entity, the burden estimates for this proposed
rule assume individual or group TIN-level reporting.
Reweighting Applications for
extreme and uncontrollable
circumstances, significant
hardship, or other exceptions
Clinicians who submit an application may be eligible for a reweighting of the
approved performance category to zero percent under specific circumstances as
set forth in § 414.1380(c)(2), including, but not limited to, extreme and
uncontrollable circumstances, significant hardship, or another exception.
Certain types of MIPS eligible clinicians are automatically eligible reweighting
for the Promoting Interoperability performance category to zero percent as
described in § 414.1380(c)(2)(i)(C).
MVP and Subgroup
Registration
An MVP participant, as described at § 414.1305, electing to submit data for the
measures and activities in an MVP must register. Clinicians who choose to
participate as a subgroup for reporting an MVP must also register.
Partial QP Election
Eligible clinicians who attain Partial QP status and choose to participate in MIPS
must submit a partial QP election form.
Registration for the CAHPS
for MIPS Survey
Groups electing to use a CMS-approved survey vendor to administer the CAHPS
for MIPS Survey must register.
Virtual Group Registration
Virtual groups must register via email. Virtual group participation is limited to
MIPS eligible clinicians, specifically, solo practitioners who are MIPS eligible
and groups consisting of 10 eligible clinicians or fewer that have at least one
MIPS eligible clinician.
APM Performance Pathway
(APP)
Clinicians in MIPS APMs electing the APP. The burden estimates for this
proposed rule assume that Shared Savings ACO APM Entities will submit data
through the APP via the APP Plus measure set beginning in CY 2025
performance period/2027 MIPS payment year, and non-Shared Savings Program
ACO APM Entities will participate through traditional MIPS, submitting as an
individual or group rather than as an APM Entity.
The policies finalized in the CY 2017 and CY 2018 Quality Payment Program final rules
(81 FR 77008 and 82 FR 53568), the CY 2019, CY 2020, CY 2021, CY 2022, CY 2023, and
CY 2024 PFS final rules (83 FR 59452, 84 FR 62568, 85 FR 84472, 86 FR 64996, 87 FR 70131,
and 88 FR 78818), and continued in this proposed rule create some additional data collection
requirements not listed in Table 80. These additional data collections, some of which are
currently approved by OMB under the control number 0938-1314 (Quality Payment
Program/MIPS, CMS-10621) or pending renewal under 0938-1222 (CAHPS for MIPS, CMS-
10450), are as follows:
Additional ICRs related to MIPS third party intermediaries (see sections V.B.8.c. and
V.B.8.d. of this proposed rule):
● Self-nomination of new QCDRs (81 FR 77507 and 77508,
82 FR 53906 through 53908, and 83 FR 59998 through 60000) (OMB 0938-1314).
● Simplified self-nomination process of returning QCDRs (88 FR 79426 and 79427)
(OMB 0938-1314).
● Self-nomination of new qualified registries (81 FR 77507 and 77508,
82 FR 53906 through 53908, and 83 FR 59997 and 59998) (OMB 0938-1314),
● Simplified self-nomination process of returning qualified registries (88 FR 79429)
(OMB 0938-1314).
● Third party intermediary plan audits (87 FR 70140 through 70144) (OMB 0938-1314).
● Approval process for new and returning CAHPS for MIPS survey vendors
(82 FR 53908) (OMB 0938–1222).
● Open Authorization Credentialing and Token Request Process (OMB 0938-1314)
(85 FR 84969 and 84970).
Additional ICRs related to the data submission and the quality performance category (see
section V.B.8.e. of this proposed rule):
● CAHPS for MIPS Survey completion by beneficiaries (81 FR 77509,
82 FR 53916 and 53917, and 83 FR 60008 and 60009) (OMB 0938-1222).
● Quality Payment Program Identity Management Application Process (82 FR 53914 and
83 FR 60003 and 60004) (OMB 0938-1314).
Additional ICRs related to the Promoting Interoperability performance category (see
section V.B.8.g. of this proposed rule):
● Reweighting Applications for MIPS performance categories (82 FR 53918 and
83 FR 60011 and 60012) (OMB 0938-1314).
Additional ICRs related to call for new MIPS measures and activities (see sections
V.B.8.i., V.B.8.f., and V.B.8.j. of this proposed rule):
● Nomination of improvement activities (82 FR 53922 and 83 FR 60017 and 60018)
(OMB 0938-1314).
● Call for MIPS quality measures (83 FR 60010 and 60011) (OMB 0938-1314).
● Nomination of MVPs (85 FR 84990 through 84991) (OMB 0938-1314).
Additional ICRs related to MIPS (see section V.B.8.n. of this proposed rule):
● Opt out of performance data display on Compare Tools for voluntary reporters under
MIPS (82 FR 53924 and 53925 and 83 FR 60022) (OMB 0938-1314).
Additional ICRs related to APMs (see sections V.B.8.l. and V.B.8.m. of this proposed):
● Partial QP Election (81 FR 77512 and 77513, 82 FR 53922 and 53923, and
83 FR 60018 through 60019) (OMB 0938-1314).
● Other Payer Advanced APM determinations: Payer Initiated Process
(82 FR 53923 and 53924 and 83 FR 60019 through 60020) (OMB 0938-1314).
● Other Payer Advanced APM determinations: Eligible Clinician Initiated Process
(82 FR 53924 and 83 FR 60020) (OMB 0938-1314).
● Submission of Data for All-Payer QP Determinations (83 FR 60021) (OMB 0938-
1314).
b. ICRs Regarding the Virtual Group Election (§ 414.1315)
This rulemaking is not proposing any new or revised collection of information
requirements or burden related to the virtual group election. The virtual group election
requirements and burden are currently approved by OMB under control number 0938-1343
(CMS-10652). Consequently, we are not proposing any changes under that control number.
c. ICRs Regarding Third Party Intermediaries (§ 414.1400)
In sections V.B.8.c.(2)., V.B.8.c.(3)., V.B.8.c.(4)., and V.B.8.c.(5). of this proposed rule,
we identify proposed adjustments to the estimated burden for ICRs regarding third party
intermediaries that will be submitted to OMB for review under control number 0938-1314
(CMS-10621), as summarized in Tables 78 and 79. We note that these proposed adjustments to
estimated burden are due to the availability of updated data and related assumptions as identified
per ICR, rather than proposed policies or statute changes in section IV.A.4. of this proposed rule.
(1) Background
Under MIPS, the quality, Promoting Interoperability, and improvement activities
performance category data may be submitted via relevant third party intermediaries, such as
QCDRs and qualified registries. Data on the CAHPS for MIPS Survey, which counts as either
one quality performance category measure, or towards an improvement activity, can be
submitted via CMS-approved survey vendors. We refer readers to § 414.1400 for details on
third party intermediary requirements. Entities seeking approval to submit data on behalf of
clinicians as a QCDR, qualified registry, or survey vendor must complete a self-nomination
process annually. The processes for self-nomination of entities seeking approval as QCDRs and
qualified registries are similar with the exception that QCDRs have the option to nominate
QCDR measures for CMS consideration for the reporting of quality performance category data.
Therefore, the difference between the QCDR and qualified registry self-nomination is associated
with the preparation of QCDR measures for CMS consideration. As established in the CY 2024
PFS final rule (88 FR 79425), we continue to estimate burden separately for the simplified and
full self-nominations of QCDRs and qualified registries, to more accurately capture the distinct
number of estimated respondents and burden per self-nomination for the different processes. In
the CY 2024 PFS final rule (88 FR 79390 and 79391), we eliminated the category of health
information technology (IT) vendors from MIPS third party intermediaries beginning with the
CY 2025 performance period/2027 MIPS payment period.
(2) QCDR Self-Nomination Applications
We refer readers to the CY 2017 and CY 2018 Quality Payment Program final rules
(81 FR 77507 and 77508, and 82 FR 53906 through 53908, respectively), and the CY 2019,
CY 2020, CY 2021, CY 2022, CY 2023, and CY 2024 PFS final rules
(83 FR 59998 through 60000, 84 FR 63116 through 63121, 85 FR 84964 through 84969,
86 FR 65569 through 65573, 87 FR 70138 and 70139, and 88 FR 79426 through 79429
respectively) for our previously finalized requirements and estimated burden for self-nomination
of QCDRs and nomination of QCDR measures.
We are proposing to adjust burden for QCDR self-nomination applications based on
updated assumptions for the number of applications and QCDR measure submissions that we
expect to receive during the CY 2024 self-nomination period for the CY 2025 performance
period/2027 MIPS payment year. As discussed later in this section, we are proposing to adjust
our estimates for: (1) the number of QCDRs that will submit applications under the simplified
and full self-nomination processes; (2) the number of QCDR measures (existing or borrowed and
new measures) submitted by a QCDR; and (3) the average time required for a QCDR to submit
the QCDR measure information.
(a) Simplified Self-Nomination Process and Other Requirements
We estimate that 39 existing QCDRs will submit applications under the simplified self-
nomination process for the CY 2025 performance period/2027 MIPS payment year. We derive
this estimate from the number of applications received during the CY 2023 QCDR self-
nomination period for the CY 2024 performance period/2026 MIPS payment year, while
anticipating that: (1) some QCDRs may face remedial action or termination during the CY 2024
performance period/2026 MIPS payment year, and (2) some intermediaries may electively
discontinue participating as a QCDR. This estimate is a decrease of 5 respondents from the
currently approved estimate of 44 for the QCDR simplified self-nomination process
(88 FR 79426 and 79427).
In line with these assumptions and QCDR measure submission trends, we estimate that
simplified QCDR applicants will propose 14 QCDR measures, on average, for the CY 2025
performance period/2027 MIPS payment year, consisting of approximately 3 new QCDR
measures and 11 existing or borrowed QCDR measures per QCDR. This is an increase of 1 new
QCDR measure and 1 borrowed QCDR measure per QCDR from the currently approved
aggregated estimate of 12 QCDR measures in the CY 2024 PFS final rule (88 FR 79426). Due
to this change, we propose to adjust the estimated weighted average time required for each
QCDR to submit a QCDR measure from 0.75 hours to 0.82 hours [((3 new QCDR measures × 2
hr) + (11 existing or borrowed QCDR measures × 0.5 hr)) ÷ total # of QCDR measures (14)].
We are continuing our currently approved estimated response times to submit a new QCDR
measure (2 hr/response) and an existing or borrowed QCDR measure (0.5 hr/response). We are
also continuing our currently approved response time of 0.5 hours required for existing QCDRs
that do not submit QCDR measures under the simplified self-nomination process.
For existing QCDRs that submit QCDR measures as part of their self-nomination
application, we estimate that it will take 11.98 hours [0.5 hr for the simplified self-nomination
process + (14 QCDR measures × 0.82 hr/measure for QCDR measure submission)] for a QCDR
to submit an application under the simplified self-nomination process. We note that this
proposed change will result in an increase of 2.48 hours (11.98 hr adjusted estimate – 9.5 hr
currently approved estimate) for each applicant to complete the simplified QCDR self-
nomination process (88 FR 79426 and 79427).
From these assumptions, we estimate the total annual burden associated with a QCDR

self-nominating to be considered “qualified” to submit data on behalf of MIPS eligible clinicians.
As shown in Table 81, we assume that the staff involved in the simplified QCDR self-
nomination process will continue to be computer systems analysts or their equivalent, who have
an average adjusted labor rate of $106.54/hr. We estimate the burden per application will be
$1,276.35 (11.98 hr x $106.54/hr). In aggregate and inclusive of the 2.48 hr adjustment for the
CY 2025 performance period/2027 MIPS payment year, we estimate an annual burden of 467
hours (39 applications x 11.98 hr/application) at a cost of $49,778 (39 applications x
$1,276.35/application) for the simplified QCDR self-nomination process.
TABLE 81: Adjusted Burden for Simplified QCDR Self-Nomination and QCDR
Measure Submission
Burden and Respondent Descriptions
Burden Estimate
# of Simplified QCDR Self-Nomination Applications Submitted (a)
39
Annual Hours Per QCDR for Simplified Process (b)
11.98
Total Annual Hours for Self-Nomination (c) = (a) × (b)
467
Cost per Application at Labor Cost for Computer Systems Analyst at
$106.54/hr (d) = $106.54/hr × (b)
$1,276.35
Total Annual Cost (e) = (a) × (d)
$49,778
In Table 82, we illustrate the proposed net change in estimated burden for simplified
QCDR self-nomination and QCDR measure submission using the currently approved burden in
the CY 2024 PFS final rule (88 FR 79426 and 79427). In aggregate, using our currently
approved estimates for responses and time per response, the proposed decrease in the number of
simplified QCDR self-nomination applications and the increase in the estimated burden hours
per simplified QCDR self-nomination will result in a total annual adjustment of +49 hours at a
cost of +$5,244 for the CY 2025 performance period/2027 MIPS payment year.

TABLE 82: Change in Estimated Burden for Simplified QCDR Self-Nomination and
QCDR Measure Submission
Burden and Respondent Descriptions
Burden Estimate
Total Currently Approved Annual Time (hr) (a)
418
Total Annual Time (hr) for Respondents in CY 2025 PFS Proposed Rule
(b) (See Table 81, row (c))
467
Difference in Annual Hours (c) = (b) − (a)
+49
Total Currently Approved Annual Cost (d)
$44,534
Total Annual Cost for Respondents in CY 2025 PFS Proposed Rule (e)
(See Table 81, row (e))
$49,778
Difference in Annual Cost (f) = (e) − (d)
+$5,244
(b) Full QCDR Self-Nomination Process and Other Requirements
We estimate that 17 QCDRs will submit applications under the full self-nomination
process for the CY 2025 performance period/2027 MIPS payment year. We derive this estimate
from the number of applications received during the CY 2023 QCDR self- nomination period for
the CY 2024 performance period/2026 MIPS payment year, while anticipating that: (1) some
existing QCDRs may face remedial action and would need to complete the full self-nomination
process for the CY 2025 performance period/2027 MIPS payment year, and (2) several new
QCDR self-nominations. This estimate is an increase of 5 respondents (17 revised – 12 active
applications) for the QCDR full self-nomination process (88 FR 79427).
As estimated for the QCDR simplified self-nomination process in section V.B.8.c.(2)(a).
of this proposed rule, we estimate that QCDR applicants completing the full self-nomination
process will propose on average 14 QCDR measures for the CY 2025 performance period/2027
MIPS payment year, consisting of approximately 3 new QCDR measures and 11 existing or
borrowed QCDR measures per QCDR. This is an increase of 1 new QCDR measure and 1
borrowed QCDR measure per QCDR from the currently approved aggregated estimate of 12
QCDR measures in the CY 2024 PFS final rule (88 FR 79427). Due to this change, we also
propose to adjust the estimated weighted average time required for each QCDR to submit a
QCDR measure from 0.75 hours (10 existing QCDR measures × 0.5hr +2 new QCDR measures
× 2hr) ÷12 total QCDR measures) to 0.82 hours (11 existing QCDR measures x 0.5 hours + 3
new QCDR measures x 2 hours) ÷ 14 total QCDR measures). We are continuing our currently
approved time of 2.5 hours required for new QCDRs that do not submit QCDR measures under
the full self-nomination process, and our estimated per response times for a QCDR to submit a
new QCDR measure (2 hr/response) and an existing or borrowed QCDR measure (0.5
hr/response).
Due to the estimated (-5 applications = 44 active – 39 new estimate)increase in the
average number of existing or borrowed QCDR measures (+1) and new QCDR measures (+1)
submitted with the full self-nomination application, we estimate that it will take 13.98 hours [2.5
hr for the full self- nomination process + (14 QCDR measures × 0.82 hr/measure for QCDR
measure submission)] for a QCDR to submit an application under the full self-nomination
process. This proposed change will result in an overall increase of 2.48 hours (13.98 hr revised –
11.5 hr active) required for the full QCDR self-nomination application
(88 FR 79428 and 79429).
From these updated assumptions, we provide an estimate of the total annual burden
associated with a QCDR self-nominating to be considered “qualified” to submit data on behalf of
MIPS eligible clinicians. In Table 83, we assume that the staff involved in the full QCDR self-
nomination process will continue to be computer systems analysts or their equivalent who have
an average adjusted labor rate of $106.54/hr. We estimate the burden per application will be
$1,489.43 (13.98 hr x $106.54/hr). In aggregate, for the CY 2025 performance period/2027
MIPS payment year, we estimate an annual burden of 238 hours (17 applications x 13.98 hr) at a
cost of $25,320 (17 applications x $1,489.43/application) for the full QCDR self-nomination
process.

TABLE 83: Estimated Burden for Full QCDR Self-Nomination and QCDR
Measure Submission
Burden and Respondent Descriptions
Burden Estimate
# of Full QCDR Self-Nomination Applications Submitted (a)
17
Annual Hours Per QCDR for Full Process (b)
13.98
Total Annual Hours for Full Self-nomination (c) = (a) × (b)
238
Cost per Application at Labor Cost for Computer Systems Analyst
at $106.54/hr (d) = $106.54/hr × (b)
$1,489.43
Total Annual Cost (e) = (a) × (d)× (d)
$25,320
In Table 84, we use the currently approved burden as the baseline for calculating the
proposed net change in burden for the full QCDR self-nomination process (88 FR 79427). For
the CY 2025 performance period/2027 MIPS payment year, the proposed adjustment in the
number of applicants and time per applicant results in a total annual adjustment of +100 hours at
a cost of +$10,617 for the CY 2025 performance period/2027 MIPS payment year.
TABLE 84: Change in Estimated Burden for Full QCDR Self-Nomination and QCDR
Measure Submission
Burden and Respondent Descriptions
Burden Estimate
Total Currently Approved Annual Time (hr) (a)
138
Total Annual Time (hr) for Respondents in CY 2025 PFS Proposed Rule
(b) (See Table 83, row (c))
238
Difference in Annual Hours (c) = (b) − (a)
+100
Total Currently Approved Annual Cost (d)
$14,703
Total Annual Cost for Respondents in CY 2025 PFS Proposed Rule (e)
(See Table 83, row (e))
$25,320
Difference in Annual Cost (f) = (e) − (d)
+$10,617
(3) Qualified Registry Self-Nomination Process and Other Requirements
We refer readers to the CY 2017 and CY 2018 Quality Payment Program final rules
(81 FR 77507 and 77508, and 82 FR 53906 through 53908, respectively), and the CY 2019,
CY 2020, CY 2021, CY 2022, CY 2023, and CY 2024 PFS final rules (83 FR 59997 and 59998,
84 FR 63114 through 63116, 85 FR 84968 and 84969, 86 FR 65573 through 65576,
87 FR 70139 and 70140, and 88 FR 79429 and 79430 respectively) for our previously finalized
requirements and estimated burden for self-nomination of qualified registries.
In the CY 2024 PFS final rule (88 FR 79390 through 79391), we established the
elimination of the health IT vendor category amid MIPS third party intermediary beginning with
the CY 2025 performance period/2027 MIPS payment year. We believe that many third party
intermediaries serve as both health IT vendors and qualified registries for the purposes of
submitting data for MIPS eligible clinicians. As with our burden assumptions in the CY 2024
PFS final rule (88 FR 79425), we are not proposing any adjustments to the number of qualified
registries that will submit applications for the qualified registry self-nomination process during
the CY 2025 performance period/2027 MIPS payment year.
We are proposing to adjust burden for qualified registry self-nomination applications
based on the number of applications we expect to receive for the CY 2025 performance
period/2027 MIPS payment year. We note these proposed changes are not due to proposed
policies discussed in section IV.A. of this proposed rule.
(a) Simplified Qualified Registry Self-Nomination Process
For this proposed rule, we estimate that 76 qualified registries will complete the
simplified self-nomination process in CY 2025 performance period/2027 MIPS payment year.
We derive this estimate from the number of applications received during the CY 2023 qualified
registry self- nomination period for the CY 2024 performance period/2026 MIPS payment year,
while anticipating that: (1) some qualified registries may face remedial action or termination
during the CY 2024 performance period/2026 MIPS payment year, and (2) some intermediaries
may electively discontinue participating as a qualified registry. This estimate is a decrease of
eight applications from the currently approved estimate of 84 in the CY 2024 PFS final rule
(88 FR 79429). We note that we are continuing the currently approved time of 0.5 hours for the
simplified qualified registry self-nomination process.
Based on these assumptions, we estimate the total annual burden associated with a
qualified registry self-nominating to be considered “qualified” to submit data on behalf of MIPS
eligible clinicians. In Table 85, we assume that the staff involved in the simplified qualified
registry self-nomination process will continue to be computer systems analysts or their
equivalent, who have an average adjusted labor rate of $106.54/hr. We estimate the burden per
response will be $53.27 (0.5 hr x $106.54/hr) for the simplified self-nomination process. In
aggregate, we estimate that the annual burden for the simplified qualified registry self-
nomination process will be 38 hours (76 applications x 0.5 hr) at a cost of $4,049 (76
applications x $53.27/application) for the CY 2025 performance period/2027 MIPS payment
year.
TABLE 85: Estimated Burden for Simplified Qualified Registry Self-Nomination
Burden and Respondent Descriptions
Burden Estimate
# of Simplified Self-Nomination Applications Submitted (a)
76
Annual Hours Per Qualified Registry for Simplified Process (b)
0.5
Total Annual Hours for Simplified Self-Nomination (c) = (a) × (b)
38
Cost per Application at Labor Cost for Computer Systems Analyst at
$106.54/hr(d) = $106.54/hr × (b)
$53.27
Total Annual Cost (e) = (a)× (d)
$4,049
In Table 86, we illustrate the proposed net change in estimated burden for the simplified
qualified registry self-nomination using the currently approved burden in the CY 2024 PFS final
rule (88 FR 79429). In aggregate, using our proposed adjustment to the number of applications
results in a total annual change of minus 4 hours (42 hr active – 38 hr revised) and minus $426
($4,475 - $4,049) for the CY 2025 performance period/2027 MIPS payment year.
TABLE 86: Change in Estimated Burden for Simplified Qualified Registry Self-
Nomination
Burden and Respondent Descriptions
Burden Estimate
Total Currently Approved Annual Time (hr) (a)
42
Total Annual Time (hr) for Respondents in CY 2025 PFS Proposed Rule
(b) (See Table 85, row (c))
38
Difference in Annual Hours (c) = (b) − (a)
-4
Total Currently Approved Annual Cost (d)
$4,475
Total Annual Cost for Respondents in CY 2025 PFS Proposed Rule (e)
(See Table 85, row (e))
$4,049
Difference in Annual Cost (f) = (e) − (d)
-$426
(b) Full Qualified Registry Self-Nomination Process
We estimate that 30 qualified registries will submit applications under the full self-
nomination process for the CY 2025 performance period/2027 MIPS payment year. We derive
this estimate from the number of applications received during the CY 2023 qualified registry

self- nomination period for the CY 2024 performance period/2026 MIPS payment year, while
anticipating: (1) that some existing qualified registries may face remedial action during the
CY 2025 performance period/2026 MIPS payment year and may need to submit full self-
nomination applications, and (2) several new qualified registry applicants. This is an increase of
3 respondents from the currently approved estimate of 27 in the CY 2024 PFS final rule
(88 FR 79430). We note we are continuing our currently approved per response time estimate of
2 hours for the full qualified registry self-nomination process.
Based on the assumptions discussed in this section, we provide an estimate of the total
annual burden associated with a qualified registry self-nominating to be considered “qualified” to
submit data on MIPS eligible clinicians.
In Table 87, we assume the staff involved in the qualified registry self-nomination
process will continue to be computer systems analysts or their equivalent, who have an average
labor rate of $106.54/hr. We estimate the burden per response will be $213.08 (2 hr x 106.54/hr)
for the full qualified registry self-nomination process. In aggregate, for the CY 2025
performance period/2027 MIPS payment year, we estimate that the annual burden for the full
qualified registry self-nomination process will be 60 hours (30 applications x 2 hr) at a cost of
$6,392 (30 applications x $213.08/application).
TABLE 87: Estimated Burden for Full Qualified Registry Self-Nomination
Burden and Respondent Descriptions
Burden Estimate
# of Qualified Registry Full Self-Nomination Applications submitted (a)
30
Annual Hours Per Qualified Registry for Full Process (b)
2
Total Annual Hours for Full Self-Nomination (c) = (a) × (b)
60
Cost per Application at Labor Cost for Computer Systems Analyst at $106.54/hr
(d) = $106.54/hr × (b)
$213.08
Total Annual Cost (e) = (a) × (d)
$6,392
In Table 88, we illustrate the proposed net change in estimated burden for the full
qualified registry self-nomination process using the currently approved burden in the CY 2024
PFS final rule (88 FR 79429). In aggregate, the proposed adjustments result in a total annual
adjustment of +6 hours (+3 applications x 2 hr/response) at a cost of +$639 (3 applications x

$213.08/application) for the full qualified registry self-nomination process for the CY 2025
performance period/2027 MIPS payment year.
TABLE 88: Change in Estimated Burden for Full Qualified Registry Self-Nomination
Burden and Respondent Descriptions
Burden Estimate
Total Currently Approved Annual Time (hr) (a)
54
Total Annual Time (hr) for Respondents in CY 2025 PFS Proposed Rule
(b) (See Table 87, row (c))
60
Difference in Annual Hours (c) = (b) − (a)
+6
Total Currently Approved Annual Cost (d)
$5,753
Total Annual Cost for Respondents in CY 2025 PFS Proposed Rule (e)
(See Table 87, row (e))
$6,392
Difference in Annual Cost (f) = (e) − (d)
+$639
(4) Third Party Intermediary Plan Audits
The following proposed burden adjustments are due to the availability of updated data
and assumptions, rather than proposed policy changes.
(a) Targeted Audits
In the CY 2022 PFS final rule (86 FR 65547 and 65548), we finalized that beginning
with the CY 2021 performance period/CY 2023 MIPS payment year, the QCDR or qualified
registry must conduct targeted audits in accordance with requirements at § 414.1400(b)(3)(vi).
Consistent with our assumptions in the CY 2022 PFS, CY 2023 PFS, and CY 2024 PFS final
rules for the QCDRs (86 FR 65574, 87 FR 70141, and 88 FR 79431 and 79432 respectively) and
qualified registries (86 FR 65571, 87 FR 70141, and 88 FR 79431 and 79432 respectively) that
would submit the results of targeted audits, we estimate that the time required for a QCDR or
qualified registry to submit a targeted audit ranges between 5 and 10 hours for the simplified and
full self-nomination process, respectively. We assume the staff involved in submitting the
targeted audits will continue to be computer systems analysts or their equivalent, who have an
average labor rate of $106.54/hr.
Due to an increase in data issues among third party intermediaries during the CY 2023
performance period/2025 MIPS payment year, we estimate that 35 third party intermediaries will
submit targeted audits for the CY 2025 performance period/2027 MIPS payment year (see Table
89). We estimate that the cost for a QCDR or a qualified registry to submit a targeted audit will
range from $532.70 (5 hr x $106.54/hr) to $1,065.40 (10 hr x $106.54/hr). In aggregate for the
CY 2025 performance period/2027 MIPS payment year, we estimate an annual burden ranging
from 175 hours (35 responses × 5 hr/ audits × 5 hr/audit) and $18,645 (35 targeted audits x
$532.70/audit) to 350 hours (35 audits × 10 hr/audit) and $37,289 (35 targeted audits x
$1,065.40/audit) (see Table 90 for the cost per audit).
(b) Participation Plans
In the CY 2022 PFS final rule (86 FR 65546), we finalized requirements for approved
QCDRs and qualified registries that did not submit performance data and therefore will need to
submit a participation plan as part of their self-nomination process. We refer readers to
§ 414.1400(e) for to submit a participation plan as part of their self-nomination process. We
refer readers to § 414.1400(e) for additional details on policies for remedial action and
termination of third party intermediaries. In the CY 2020 PFS final rule (82 FR 63074), we
finalized requirements for QCDRs to submit a QCDR measure participation plan in instances
where a QCDR believes the low-reported QCDR measure that did not meet benchmarking
thresholds is still important and relevant to a specialist's practice. We refer readers to
§ 414.1400(b)(4)(iii)(B)(10) for additional details on policies for QCDR measure participation
plans.
Consistent with our assumptions in the CY 2024 PFS final rule (88 FR 79431), we
estimate that it will take 2 hours for a QCDR or qualified registry to submit a participation plan.
We assume the staff involved in submitting the participation plans will continue to be computer
systems analysts or their equivalent, who have an average labor rate of $106.54/hr.
As shown in Table 89, following additional review of the MIPS data submission reports,
we estimate that 28 third party intermediaries will submit self-nomination or QCDR measure
participation plans for the CY 2025 performance period/2027 MIPS payment year. This is a
decrease of 36 participation plans from the currently approved estimate of 64 (88 FR 79431).
Many QCDRs submitted a QCDR measure participation plan in their self-nomination for
CY 2024 performance period/2026 MIPS payment year, and therefore we anticipate limited
QCDR measure participation plan for QCDR self-nominations for the CY 2025 performance
period/2027 MIPS payment year.
In Table 90, we estimate that the cost for a QCDR or a qualified registry to submit a
participation plan is $213.08 (2 hr x $106.54/hr). In aggregate for the CY 2025 performance
period/2027 MIPS payment year, we estimate the total impact associated with QCDRs and
qualified registries to submit participation plans will be 56 hours (28 participation plans × 2
hr/plan) at a cost of $5,966 ((28 participation plans x $213.08/plan) (see Table 90 for the cost per
audit).
(c) Corrective Action Plans (CAPs)
In the CY 2017 Quality Payment Program final rule, we established the process for
corrective action plans (CAPs) (81 FR 77386 through 77389). We refer readers to
§ 414.1400(e)(1)(i) for third party intermediary requirements for submitting CAPs.
We have observed a decrease in the number of CAPs required from third party
intermediaries due to non-compliance with MIPS program requirements. Accordingly, we
estimate 20 third party intermediaries will submit CAPs for the CY 2025 performance
period/2027 MIPS payment year. This is a decrease of 4 respondents from the currently
approved estimate of 24 (88 FR 79431). We are continuing our currently approved estimate of 3
hours for a QCDR or qualified registry to submit a CAP. We also assume the staff involved in
submitting the CAP will continue to be computer systems analysts or their equivalent, who have
an average labor rate of $106.54/hr. In Table 90, we estimate that the cost for a QCDR or a
qualified registry to submit a CAP is $319.62 (3 hr x $106.54/hr). In aggregate for the CY 2025
performance period/2027 MIPS payment year, we estimate the total impact associated with
QCDRs and qualified registries to CAPs would be 60 hours (20 CAPs × 3 hr/plan) at a cost of
$6,392 (20 CAPs x $319.62/plan).
(d) Transition Plans
We established a policy at § 414.1400(a)(3)(iv) that states a condition of approval for the
third party intermediary is to agree that prior to discontinuing services to any MIPS eligible
individual clinician, group, virtual group, subgroup, or APM Entity during a performance period,
the third party intermediary must support the transition of such MIPS eligible clinician, group,
virtual group, subgroup, or APM Entity to an alternate third party intermediary, submitter type,
or, for any measure on which data has been collected, collection type according to a CMS
approved transition plan. In this proposed rule, we estimate that we will receive 6 transition
plans for the CY 2025 performance period/2027 MIPS payment year. This adjustment would
result in a decrease of 3 transition plans from the currently approved estimate of 9 transition
plans (88 FR 79431). We continue to estimate it will take 1 hour for a computer system analyst
or their equivalent at a labor rate of $106.54/hr to develop a transition plan on behalf of each
QCDR or qualified registry during the self-nomination period. However, we are unable to
estimate the burden for implementing the actions in the transition plan because the level of effort
may vary for each QCDR or qualified registry. In aggregate for the CY 2025 performance
period/2027 MIPS payment year, we estimate the impact associated with QCDRs and qualified
registries completing transition plans is 6 hours (6 transition plans × 1 hr/plan) at a cost of $639
(6 transition plans × $106.54/plan).
(e) Estimated Burden for Third Party Intermediary Plan Audits
In aggregate, as shown in Table 89, we assume that 89 third party intermediaries will
submit plan audits for the CY 2025 performance period/2027 MIPS payment year (35 targeted
audits, 28 participation plans, 20 CAPs, and 6 transition plans).

TABLE 89: Estimated Number of Respondents to Submit Plan Audits
Burden and Respondent Descriptions
# of Respondents
# of Targeted Audits (a)
35
# of Participation Plans (b)
28
# of Corrective Action Plans (CAPs) (c)
20
# of Transition Plans (d)
6
Total Respondents (e) = (a) + (b) + (c) + (d)
89
As shown in in Table 90, we assume that the staff involved in the submission of the plan
audits during the third party intermediary self-nomination process will continue to be computer
systems analysts or their equivalent, who have an average labor rate of $106.54/hr. For the
CY 2025 performance period/2027 MIPS payment year, in aggregate, the proposed estimated
annual burden for the submission of third party intermediary plan audits will range from 297
hours to 472 hours at a cost ranging from $31,642 and $50,286.
TABLE 90: Estimated Burden for Third Party Intermediary Plan Audits
Burden and Respondent Descriptions
Simplified
Process
Full
Process
# of Hours per Completion of Targeted Audit (a)
5
10
Total Annual Hours for Completion of 35 Targeted Audits (b)
175 (5 x 35)
350 (10 x
35)
# of Hours per Submission of Participation Plan (c)
2
2
Total Annual Hours for Submission of 28 Participation Plans (d)
56 (2 x 28)
56 (2 x 28)
# of Hours per Submission CAP (e)
3
3
Total Annual Hours for Submission of 20 CAPs (f)
60 (3 x 20)
60 (3 x 20)
# of Hours per Submission of Transition Plan (g)
1
1
Total Annual Hours for Submission of 6 Transition Plans (h)
6 (6 x 1)
6 (6 x 1)
Total Annual Hours for Submission of Plan Audits ((i) = (b) + (d) + (f) + (h)
297
472
Cost Per Targeted Audit (Computer Systems Analyst’s Labor Rate of $106.54/hr) (j) =
(a) × $106.54/hr
$532.70
$1,065.40
Cost Per Participation Plan (Computer Systems Analyst’s Labor Rate of $106.54/hr) (k)
= (c) × $106.54/hr
$213.08
$213.08
Cost per CAP (Computer Systems Analyst’s Labor Rate of $106.54/hr)) (l) = (e) ×
$106.54/hr
$319.62
$319.62
Cost per Transition Plan (Computer Systems Analyst’s Labor Rate of $106.54/hr (m) =
(g) × $106.54/hr
$106.54
$106.54
Total Cost of Targeted Audits (n) = (j) × 35
$18,645
$37,289
Total Cost of Participation Plans (o) = (k) × 28
$5,966
$5,966
Total Cost of CAPs (p) = (l) × 20
$6,392
$6,392
Total Cost of Transition Plans (q) = (m) × 6
$639
$639
Total Annual Cost (r) = (n) + (o) + (p) + (q) (simplified) and (r) = (n) + (o) + (p) +
(q) (full)
$31,642
$50,286
As shown in Table 91, for the CY 2025 performance period/2027 MIPS payment year,
the change in the number of respondents for third party intermediary plan audits results in an

adjustment of -57 hours at a cost of -$12,303 under the simplified self-nomination process and -
27 hours at a cost of -$2,878 under the full self-nomination process. We note for the purposes of
calculating estimated change in burden in Tables 77 through 79 of this proposed rule, we use
only estimated burden for the plan audits submitted under the full self-nomination process.
TABLE 91: Change in Estimated Burden for Third Party Intermediary Plan Audits
Burden and Respondent Descriptions
Simplified Process
Full Process
Total Currently Approved Annual Hours (a)
354
499
Total Annual Hours for Respondents in CY 2025 PFS Proposed Rule (b)
(See Table 90, row (i))
297
472
Difference in Annual Hours (c) = (b) − (a)
-57
-27
Total Currently Approved Annual Cost (d)
$43,945
$53,164
Total Annual Cost for Respondents in CY 2025 PFS Proposed Rule (e) (See
Table 90, row (r))
$31,642
$50,286
Difference in Annual Cost (f) = (e) − (d)
-$12,303
-$2,878
(5) Survey Vendor Requirements
The following proposed changes associated with CAHPS survey vendors to submit data
for eligible clinicians will be submitted to OMB for review under control number 0938-1222
(CMS-10450) if the policy proposal described in section IV.A.4.j. of this proposed rule is
finalized in the CY 2025 PFS final rule. We would plan to make the revised files available for
public review under the standard non-rule PRA process which includes the publication of 60-
and 30-day Federal Register notices.
We refer readers to § 414.1400(d) for the requirements for CMS-approved survey
vendors that may submit data on the CAHPS for MIPS Survey.
In section IV.A.4.j.(1)(b). of this proposed rule, we are proposing under the current
application submission requirement at § 414.1400(d)(2) that beginning with the CY 2026
performance period/2028 MIPS payment year, a survey vendor must include on its application
the range of cost of its third party intermediary services (cost estimates would vary based on the
level of services provided). With respect to a third party intermediary that is solely a CMS-
approved survey vendor, the publishable costs would be limited to the cost of services related to
the CAHPS for MIPS survey. If this proposal is finalized, we would update the CAHPS for
MIPS Survey Vendor Participation Form. We would also update the CAHPS for MIPS Survey
Minimum Business Requirements to detail the required survey vendor cost information. We
refer readers to section IV.A.4.j.(1). of this proposed rule for additional detail.
We anticipate that the proposed fields for cost information would request cost
information that is readily available to survey vendors. Therefore, we are not proposing any
adjustments in burden because we assume the additional cost requirement would not add
significant burden to the currently approved 10 hours per application first established in the
CY 2018 Quality Payment Program final rule (82 FR 30216). We also assume this change
would not affect survey vendor participation. We note that we proposed updates to our estimated
burden under control number 0938-1222 (CMS-10450) as discussed in the CY 2024 PFS final
rule (88 FR 79433 and 79434). The 60-day notice appeared in the Federal Register on October
17, 2023 (88 FR 71573), while the 30-day notice appeared on January 16, 2024 (89 FR 2622).
The status for OMB’s approval of the changes can be monitored at:
https://www.reginfo.gov/public/do/PRAOMBHistory?ombControlNumber=0938-1222. We are
not proposing any additional updates under this control number due to policies proposed in this
rulemaking. We refer readers to section VII.E.17.e(2)(a) of this proposed rule for additional
discussion.
The proposed change, if finalized, would result in updates to the CAHPS for MIPS
Survey Vendor Participation Form and the CAHPS for MIPS Survey Minimum Business
Requirements. The instrument and requirements guide are currently approved by OMB under
control number 0938-1222 (CMS-10450). The CAHPS for MIPS Survey Vendor Participation
Form and the CAHPS for MIPS Survey Minimum Business Requirements guide reflecting these
proposed changes will be updated if the policy proposal is finalized in the CY 2025 PFS final
rule as a requirement for CAHPS for MIPS survey vendors beginning in the CY 2026
performance period/2028 MIPS payment year. We will make the updated files available for
public review through a stand-alone non-rule Federal Register notice that is expected to appear
in the Federal Register in CY 2025 performance period/2027 MIPS payment period.
d. ICRs Regarding Open Authorization (OAuth) Credentialing and Token Request Process
This rulemaking is not proposing any new or revised collection of information
requirements or burden related to the OAuth credentialing and token request process for the
CY 2025 performance period/2027 MIPS payment year. The requirements and burden for the
OAuth credentialing and token request process are currently approved by OMB under control
number 0938–1314 (CMS–10621). Consequently, we are not proposing any changes to the
burden for the OAuth credentialing and token request process under that control number.
e. ICRs Regarding Quality Data Submission (§§ 414.1318, 414.1325, 414.1335, and 414.1365)
(1) Background
We refer readers to the CY 2017 and CY 2018 Quality Payment Program final rules
(81 FR 77502 and 77503 and 82 FR 53908 through 53912, respectively), the CY 2019, CY 2020,
CY 2021, CY 2022, CY 2023, and CY 2024 PFS final rules (83 FR 60000 through 60003,
84 FR 63121 through 63124, 85 FR 84970 through 84974, 86 FR 65576 through 65588,
87 FR 70145 through 70154, and 88 FR 79434 through 79442, respectively) for our previously
finalized estimated burden associated with data submission for the quality performance category.
Under our current policies, two groups of clinicians submit data for the quality
performance category under MIPS: those who submit data as MIPS eligible clinicians, and those
who submit data voluntarily but are not subject to MIPS payment adjustments. Clinicians are
ineligible for MIPS payment adjustments if they are newly enrolled to Medicare; are QPs; are
partial QPs who elect to not participate in MIPS; are not one of the clinician types included in the
definition for MIPS eligible clinician; or do not exceed the low-volume threshold as an
individual or as a group.
(2) Changes and Adjustments to Quality Performance Category Respondents
To determine QPs that are excluded from MIPS, we used the Advanced APM payment
and patient percentages from the APM Participant List for the final snapshot date for the
2022 QP Performance period. From this data, we calculated the QP determinations as described
in the Qualifying APM Participant (QP) definition at § 414.1305 for the CY 2025 performance
period/2027 MIPS payment year. Due to data limitations, we could not identify specific
clinicians who have not yet enrolled in Advanced APMs, but who may become QPs in the future
for the CY 2025 performance period/2027 MIPS payment year (and therefore will no longer
need to submit data to MIPS); hence, our model may underestimate or overestimate the number
of respondents.
In this proposed rule, we use submissions data from the CY 2022 performance
period/2024 MIPS payment year to estimate the number of respondents that will submit data for
the CY 2025 performance period/2027 MIPS payment year. We have adjusted the estimated
number of respondents that will submit data for the CY 2025 performance period/2027 MIPS
payment year from the currently approved estimates, as our burden estimates for the CY 2024
PFS final rule (88 FR 79434) did not incorporate all data updates in the CY 2024 PFS final rule
regulatory impact analysis model (88 FR 79503 through 79505) due to the timing of data
availability. These estimates in this proposed rule reflect updated performance category
submissions for the CY 2022 performance period/2024 MIPS payment year. We refer readers to
sections V.B.8.e., V.B.8.g.(3)., and V.B.8.h. of this proposed rule for additional details per
performance category and collection type.
We assume 100 percent of ACO APM Entities will submit quality data to CMS as
required under their models. While we do not believe there is additional quality reporting for
ACO APM entities, consistent with assumptions used in the CY 2021, CY 2022, CY 2023, and
CY 2024 PFS final rules (85 FR 84972, 86 FR 65567, 87 FR 70145, and 88 FR 79434,
respectively), we include all quality data voluntarily submitted by MIPS APM participants at the
individual or TIN-level in our respondent estimates. As stated in section V.B.8.a.(4). of this

proposed rule, we assume non-Shared Savings Program ACO APM Entities will participate
through traditional MIPS or MVPs and submit as an individual or group rather than as an entity.
To estimate who will be a MIPS APM participant in the CY 2025 performance period/2027
MIPS payment year, we used the Advanced APM payment and patient percentages from the
APM Participant List for the final snapshot date for the 2022 QP performance period. We
elected to use this data source because the overlap with the data submissions for the CY 2022
performance period/2024 MIPS payment year enabled the exclusion of Partial QPs that elected
to not participate in MIPS and required fewer assumptions as to who is a QP or not. Based on
this information, if we determine that a MIPS eligible clinician will not be scored as a MIPS
APM, then their reporting assumption is based on their reporting as a group or individual for the
CY 2022 performance period/2024 MIPS payment year.
Our burden estimates for the quality performance category do not include the burden for
the quality data that Shared Savings Program APM Entities submit to fulfill the requirements of
their APMs. The associated burden is excluded from this collection of information section but is
discussed in the regulatory impact analysis section of this proposed rule because sections 1899(e)
and 1115A(d)(3) of the Act (42 U.S.C. 1395jjj(e) and 1315a(d)(3), respectively) state that the
Shared Savings Program and the testing, evaluation, and expansion of Innovation Center models
tested under section 1115A of the Act (or section 3021 of the Affordable Care Act) are not
subject to the PRA.
788
For the CY 2025 performance period/2027 MIPS payment year, respondents would have
the option to submit quality performance category data via Medicare Part B claims, direct, and
log in and upload submission types. We estimate the burden for collecting data via collection
type: Medicare Part B claims, QCDR and MIPS CQMs, and eCQMs. We do not estimate burden
for administrative claims quality measures; similar to cost measures, we automatically calculate
788
Our estimates do reflect the burden on MIPS APM participants of submitting Promoting Interoperability or
improvement activities performance category data, which is outside the requirements of their APMs.

scores for individuals, groups, virtual groups, or APM Entities that meet requirements to be
scored on individual measures due to their administrative claims reporting. Additionally, we
capture the burden for clinicians who choose to submit via these collection types for the quality
performance category of MVPs. We believe that, while estimating burden by submission type
may be better aligned with the way clinicians participate with the Quality Payment Program, it is
more important to reduce confusion and enable greater transparency by maintaining consistency
with previous rulemaking. In the CY 2019 PFS final rule (83 FR 59752), we finalized proposals
to limit the Medicare Part B claims collection type to small practices beginning with the
CY 2019 performance period/2021 MIPS payment year and to allow clinicians in small practices
to report Medicare Part B claims as a group or as individuals.
Because MIPS eligible clinicians may submit data for multiple collection types for a
single performance category, the estimated numbers of individual clinicians and groups to collect
via the various collection types are not mutually exclusive and reflect the occurrence of
individual clinicians or groups that collected data via multiple collection types during the
CY 2022 performance period/2024 MIPS payment year. We captured the burden of any eligible
clinician that may have historically collected via multiple collection types, as we assume they
will continue to collect via multiple collection types and that our MIPS scoring methodology will
take the highest score where the same measure is submitted via multiple collection types.
We are not proposing changes to the quality performance category submission burden
due to the proposals discussed in section IV.A. of this proposed rule. We discuss below in this
section these proposed policies and our reasons for not changing the currently approved burden
for the relevant ICRs.
For the quality performance category, we are proposing to adopt minimum criteria for a
qualifying data submission for a MIPS performance period. Specifically, we are proposing to
specify what we consider to be a data submission at § 414.1325(a)(1)(i) to state that, for the
quality performance category, a data submission must include numerator and denominator data

for at least one MIPS quality measure from the final list of MIPS quality measures.
Additionally, we are proposing to codify our existing policies governing our treatment of
multiple data submissions received for the quality performance category at § 414.1325(f)(1). We
refer readers to sections IV.A.4.d.(2)(b) and IV.A.4.d.(3)(b) for details. These proposals, if
finalized, would not affect the requirements for MIPS eligible clinicians and groups that submit
data for the quality performance category. We assume these proposals, if finalized, would not
affect the number of quality submissions, as the intent is to eliminate certain issues with the
scoring of an unintended data submission affecting payment adjustments for individual MIPS
eligible clinicians, groups, virtual groups, subgroups, and APM Entities. Therefore, we are not
proposing any adjustments to our currently approved estimated burden for this ICR due to these
policy proposals.
As described in the CY 2017 Quality Payment Program final rule (81
FR 77125 and 77126), to ensure that data submitted on quality measures are complete enough to
accurately assess each MIPS eligible clinician’s quality performance, we established a data
completeness requirement. For the CY 2024 and CY 2025 performance periods/2026 and 2027
MIPS payment years, we increased the data completeness criteria threshold from at least 70
percent to at least 75 percent (87 FR 70049 through 70052). We maintained the data
completeness criteria threshold of at least 75 percent for the CY 2026 performance period/2028
MIPS payment year (88 FR 79334 through 79337). In section IV.A.4.e.(1)(c)(i). of this
proposed rule, we are proposing to maintain the data completeness criteria threshold of at least
75 percent for the CY 2027 and CY 2028 performance periods/2029 and 2030 MIPS payment
years. As this data completeness threshold proposal is consistent with the existing data
completeness criteria, this proposal will not affect burden for the applicable interested parties.
We refer readers to section IV.A.4.e.(1)(c)(i). of this proposed rule for additional information on
this proposal.
In this proposed rule, we are not proposing any new or revised collection of information
requirements or burden related to the submission of Medicare Part B claims data, QCDR and
MIPS CQMs, and eCQMs for the quality performance category. Several factors drive the
proposed changes and adjustments for the number of clinicians submitting quality data for MIPS
using each collection type. First, we incorporated the updated submission data available for the
CY 2022 performance period/2024 MIPS payment year as discussed in section V.B.8.e.(7)(a). of
this proposed rule. Second, our updated estimates for MVP participation impact the number of
estimated clinicians submitting quality data using each collection type. In section V.B.8.e.(7)(a)
of this rulemaking, we propose to adjust our estimates for the number of participants in
previously finalized MVPs, due to the availability of updated data. We then propose to update
this estimate to account for increased MVP participation due to the proposed addition of six new
MVPs. With this approach, any proposed adjustments to increase the number of MVP
participants will reduce the number of estimated submissions for each quality performance
category collection type via traditional MIPS. Similarly, any proposed decreases to the number
of MVP participants will increase the number of estimated submissions for each quality
performance category collection type via traditional MIPS. We refer readers to sections
V.B.8.e.(4)., V.B.8.e.(5)., and V.B.8.e.(6). of this proposed rule for estimated burden on each
quality performance category collection type.
Table 92 uses similar methods to estimate the number of MIPS eligible clinicians that
will submit data as individual clinicians via each collection type in the CY 2025 performance
period/2027 MIPS payment year. For the CY 2025 performance period/2027 MIPS payment
year, we estimate that approximately 13,522 clinicians will submit data as individuals using the
Medicare Part B claims collection type; approximately 12,056 clinicians will submit data as
individuals using MIPS CQM and QCDR collection type; and approximately 23,600 clinicians
will submit data as individuals using eCQMs collection type. Based on the availability of
updated data for the CY 2022 performance period/2024 MIPS payment year and updated

assumptions for MVP reporting as described in section V.B.8.e.(7)(a). of this proposed rule,
these are decreases of 1,216 and 1,657 respondents from the currently approved estimates of
13,413 and 22,897 for the Medicare Part B claims and eCQM collection types, respectively and
an increase of 168 respondents for MIPS CQM and QCDR collection type from the currently
approved estimate of 10,682.
TABLE 92: Estimated Number of Clinicians Submitting Quality Performance Category
Data as Individuals by Collection Type
Burden and Respondent Description
Medicare Part
B Claims
QCDR/MIPS
CQM
eCQM
Total
2025 MIPS Performance Period
(Excludes QPs) (a)
13,552
12,056
23,600
49,208
MVP Adjustment (10%) (b) = (a) × 0.10
-1,355
-1,206
-2,360
-4,921
2025 MIPS Performance Period
(Excludes QPs and Adjusted for MVP)
(c)= (a) – (b)
12,197
10,850
21,240
44,287
Currently Approved 2024 MIPS
Performance Period (Excludes QPs) (d)
13,413
10,682
22,897
46,992
Difference in # of Clinicians (e) = (c) –
(d)
-1,216
+168
-1,657
-2,705
Consistent with the policy finalized in the CY 2018 Quality Payment Program final rule,
we established that for MIPS eligible clinicians who collect measures via Medicare Part B
claims, MIPS CQM, eCQM, or QCDR collection types and submit more than the required
number of measures (82 FR 53735 and 54736), we will score the clinician on the required
measures with the highest assigned measure achievement points and thus, the same clinician may
be counted as a respondent for more than one collection type. Therefore, our columns in Table
92 are not mutually exclusive.
Table 93 provides our estimates for the number of groups or virtual groups that will
submit quality data on behalf of clinicians for each collection type in the CY 2025 performance
periods/2027 MIPS payment year. We assume clinicians who submitted quality data as groups
in the CY 2022 performance period/2024 MIPS payment year will continue to submit data for
the quality performance category either as groups, or virtual groups for the same collection types
for the CY 2025 performance period/2027 MIPS payment years. We used the same

methodology described in the CY 2022 PFS final rule (86 FR 65577) on our assumptions related
to the use of an alternate collection type for groups that submitted data via the CMS Web
Interface collection type for the CY 2022 performance period/2024 MIPS payment year.
As shown in Table 93, for the CY 2025 performance period/2027 MIPS payment year,
we estimate that 6,158 groups and virtual groups will submit data for the MIPS CQM and QCDR
collection type and 5,939 groups and virtual groups will submit for the eCQM collection type.
These are increases of 208 and 122 respondents from the currently approved estimates of 5,950,
and 5,817 for the groups and virtual groups that will submit data using MIPS CQM and QCDR,
and eCQM collection types, respectively.
TABLE 93: Estimated Number of Groups and Virtual Groups Submitting Quality
Performance Category Data by Collection Type
Burden and Respondent Description
Medicare
Part B Claims
QCDR/
MIPS CQM
eCQM
Total
2025 MIPS Performance Period (Excludes
QPs) (a) Prior to Adjustments
0
6,842
6,599
13,441
Adjustment for MVPs (10%) (b) = (a) × 0.10
0
-684
-660
-1,344
2025 MIPS Performance Period (Excludes
QPs and) Adjusted for MVP). (c) = (a) – (b)
0
6,158
5,939
12,097
Currently Approved 2024 MIPS Performance
Period (Excludes QPs) (d)
0
-5,950
-5,817
-11,767
Difference in # of Groups and Virtual
Groups (e) = (c) − (d)
0
+208
+122
+330
The burden associated with the submission of quality performance category data has
some limitations. We believe it is difficult to quantify the burden accurately because clinicians
and groups may have different processes for integrating quality data submission into their
practices’ workflows. Moreover, the time needed for a clinician to review quality measures and
other information, select measures applicable to their patients and the services they furnish, and
incorporate the use of quality measures into the practice workflows is expected to vary along
with the number of measures that are potentially applicable to a given clinician’s practice and by
the collection type. For example, clinicians submitting data via the Medicare Part B claims
collection type need to integrate the capture of quality data codes for each encounter whereas
clinicians submitting via the eCQM collection types may have quality measures automated as
part of their electronic health record (EHR) implementation.
We believe the burden associated with submitting quality measures data will vary
depending on the collection type selected by the clinician, group, or third party intermediary. As
such, we separately estimate the burden for clinicians, groups, and third party intermediaries to
submit quality measures data by the collection type used. For the purposes of our burden
estimates for the Medicare Part B claims, MIPS CQM and QCDR, and eCQM collection types,
we also assume that, on average, each clinician or group will submit 6 quality measures.
Additionally, as finalized in the CY 2022 PFS final rule (86 FR 65394 through 65397), group tax
identification numbers (TINs) could also choose to participate as subgroups for MVP reporting
beginning with the CY 2023 performance period/2025 MIPS payment year. We refer readers to
the CY 2022 PFS final rule for additional details on MVP quality reporting requirements
(86 FR 65411 and 65412).
In terms of the quality measures available for clinicians and groups to report for the
CY 2025 performance period/2027 MIPS payment year, we are proposing a measure set of 196
quality measures. The new MIPS quality measures proposed for inclusion in MIPS for the
CY 2025 performance period/2027 MIPS payment year and future years are found in Table
Group A of Appendix 1; MIPS quality measures with substantive changes can be found in Table
Group D of Appendix 1; and MIPS quality measures proposed for removal can be found in Table
Group C of Appendix 1. These measures are stratified by collection type in Table 94, as well as
counts of new, removed, and substantively changed measures. There are no changes to the
remaining measures not included in Appendix 1. We refer readers to Appendix 1 of this
proposed rule for additional information.

TABLE 94: Summary of Quality Measure Inventory Proposed for the CY 2025
Performance Period
Collection Type
# Measures
Proposed as
New*
# Measures
Proposed for
Removal*
# Measures
Proposed with
a Substantive
Change*
# Measures
Proposed for
CY 2025*
Medicare Part B Claims
0
-2
10
25
MIPS CQMs Specifications
+8
-11
60
170
eCQM Specifications
+1
-1
16
47
Survey – CSV
0
0
0
1
Administrative Claims
0
0
1
4
Total*
+9**
-11**
66
196***
*A measure may be specified under multiple collection types but is only counted once in the total.
**Note that one new measure and one measure removal included above were finalized in the CY 2024 PFS final
rule with a 1-year delay to the CY 2025 performance period/2027 MIPS payment year.
***Three of the 196 quality measures are only available in MVPs.
For the CY 2025 performance period/2027 MIPS payment year, we are proposing 196
measures, a net decrease of 2 quality measures compared to the currently approved estimate of
198 measures. Specifically, as discussed in section IV.A.4.e.(1)(d). of this rulemaking, we are
proposing to add 9 new MIPS quality measures, remove 11 MIPS quality measures, and make
substantive updates to 66 MIPS quality measures. We do not anticipate our provision to remove
these measures will increase or decrease the reporting burden on clinicians and groups as
respondents generally are still required to submit quality data for 6 measures in traditional MIPS
reporting or submit quality data for 4 measures in an MVP.
(3) Quality Payment Program Identity Management Application Process
This rulemaking does not propose any new or revised collection of information
requirements or burden related to the identity management application process. We are adjusting
our currently approved estimates based on updated data and assumptions. The proposed changes
will be submitted to OMB for review under control number 0938-1314 (CMS-10621).
In the CY 2023 and CY 2024 PFS final rules (87 FR 70148 and 88 FR 79437), we
estimated the number of eligible clinicians, groups, or third party intermediaries that register for
new accounts by applying a rolling average of the number of respondents registering for new
accounts. The Quality Payment Program requires users to utilize HCQIS Access Roles and
Profiles (HARP), a secure identity management portal, to log into the Quality Payment Program

portal. To assess the incremental change for new Quality Payment Program Identity
Management application registrations, we will assess the number of unique TINs associated with
new Quality Payment Program user accounts that signed into the Quality Payment Program
portal for the first time in a given year. Based on this approach and new data available from
March 2022 to February 2023, we propose to adjust our estimates from 6,500 to 6,237 for the
number of unique TINs accessing the Quality Payment Program portal for the first time in the
CY 2025 performance period/2027 MIPS payment year. This proposed adjustment will result in
a decrease of 263 registrations. We do not propose to adjust the currently approved estimated
time of 1 hour per response to obtain a new account. As shown in Table 95, it will take 1 hour at
$106.54/hr for a computer systems analyst (or their equivalent) to obtain a HARP account,
required to access the Quality Payment Program portal. In aggregate we estimate an annual
burden of 6,237 hours (6,237 registrations × 1 hr/registration) at a cost of $664,490
(6,237registrations × $106.54/registrations).
TABLE 95: Estimated Burden for Quality Payment Program Identity Management
Application Process
Burden and Respondent Descriptions
Burden Estimate
# of New TINs Completing the Identity Management Application Process (a)
6,237
Total Hours Per Application (b)
1
Total Annual Hours for Completing the Identity Management Application
Process (c) = (a) [x] (b)
6,237
Cost per Applicant at Computer Systems Analyst’s Labor Rate of
$106.54/hr(d) = $106.54/hr × (b)
$106.54
Total Annual Cost (e) = (a) × (d)
$664,490
In Table 96, we illustrate the proposed net change in estimated burden for the Quality
Payment Program Identity Management Application Process using the currently approved
burden in the CY 2023 PFS final rule (87 FR 70148 and 70149). In aggregate, using the
currently approved per response time estimate, the proposed decrease of respondents results in a
total annual adjustment of -263 hours (-263 responses × 1 hr/response) at a cost of -$28,020 (-
263 responses x $106.54/response) for the CY 2025 performance period/2027 MIPS payment
year.

TABLE 96: Change in Estimated Burden for Quality Payment Program Identity
Management Application Process
Burden and Respondent Descriptions
Burden Estimate
Total Currently Approved Annual Time (hr) (a)
6,500
Total Annual Time (hr) for Respondents in CY 2025 PFS Proposed
Rule(b) (See Table 95, row (c))
6,237
Difference in Annual Hours (c) = (b) − (a)
-263
Total Currently Approved Annual Cost (d)
$692,510
Total Annual Cost for Respondents in CY 2025 PFS Proposed Rule (e)
(See Table 95, row (e))
$664,490
Difference in Annual Cost (f) = (e) − (d)
-$28,020
(4) Quality Data Submission by Clinicians: Medicare Part B Claims-Based Collection Type
The following proposed changes will be submitted to OMB for review under control
number 0938-1314 (CMS-10621). In this section of this proposed rule, we propose updates to
the estimated burden for the Quality Data Submission by Individuals and Groups Using
Medicare Part B Claims-Based Collection Type that will be submitted to OMB for review under
control number 0938-1314 (CMS-10621). As noted in Table 79, the proposed change in burden
reflects adjustments for updated data and assumptions, and as well as the proposal for additional
MVPs as outlined in section V.B.8.e.(4). of this proposed rule. In Table 95, we identify the
changes in burden to this ICR due to the proposed policies.
We refer readers to the CY 2017 and CY 2018 Quality Payment Program final rules
(81 FR 77501 through 77504 and 82 FR 53912, respectively), the CY 2019, CY 2020, CY 2021,
CY 2022, CY 2023, and CY 2024 PFS final rules (83 FR 60004 and 60005,
84 FR 63124 through 63126, 85 FR 84975 and 84976, 86 FR 65582 through 65584,
87 FR 70149 through 70151, and 88 FR 79437 through 79439 respectively) for our previously
finalized requirements and burden for quality data submission via the Medicare Part B claims
collection type.
In this proposed rule, we are not proposing any new or revised collection of information
requirements related to the submission of Medicare Part B claims data for the quality
performance category. We refer readers to section V.B.8.e.(2). of this proposed rule for the
factors affecting the proposed changes and adjustments for each quality performance collection
type. We refer readers to Table 92 of this section for the estimated change in associated burden
for quality data submissions using Medicare Part B claims data related to MVP and subgroup
reporting in CY 2025 performance period/2027 MIPS payment year.
As noted in Table 92, based on updated data available for the CY 2022 performance
period/2024 MIPS payment year and updated MVP reporting assumptions, we estimate that
12,197 individual clinicians will collect and submit quality data via the Medicare Part B claims
collection type, a decrease of 1,216 from the currently approved estimate of 13,413
(88 FR 79437 and 79438).
In Table 97, consistent with our currently approved per response time figures and using
the updated wage rates in Table 75 of this proposed rule, we continue to estimate the burden of
quality data submission using Medicare Part B claims will range from 0.15 hours (9 minutes) at a
cost of $15.98 (0.15 hr x $106.54) for a computer systems analyst to 7.2 hours at a cost of
$767.09 (7.2 hr x $106.54/hr). The burden estimate also accounts for the effort to become
familiar with MIPS quality measure specifications.
Consistent with our currently approved per response time estimates and using the updated
wage rates in Table 75 of this proposed rule, we believe that the start-up cost for a clinician’s
practice to review measure specifications is 7 hours, consisting of 3 hours for a medical and
health services manager at $129.28/hr, 1 hour for a computer systems analyst at $106.54/hr, 1
hour for a Licensed Practical Nurse (LPN) at $58.46/hr, 1 hour for a billing and posting clerk at
$45.32/hr, and 1 hour for a physician at $291.64/hr.
In Table 97, considering both data submission and start-up requirements for our adjusted
number of clinicians, the estimated time (per clinician using the Medicare Part B claims
collection type) ranges from a minimum of 7.15 hours (0.15 hr + 7 hr) to a maximum of 14.2
hours (7.2 hr + 7 hr). In aggregate, the estimated total annual time for the CY 2025 performance
period/2027 MIPS payment year ranges from 87,209 hours (7.15 hr/response x 12,197
responses) to 173,197 hours (14.2 hr/response x 12,197 responses). The total annual cost for the

CY 2025 performance period/2027 MIPS payment year ranges from a minimum of $11,047,799
(12,197 responses x $905.78/response) to a maximum of $20,209,087 (12,197 responses x
$1,656.89/response). For purposes of calculating total burden associated with this proposed rule
as shown in Tables 77 through 79, only the maximum burden is used.
TABLE 97 : Estimated Burden for Quality Performance Category: Clinicians Using the
Medicare Part B Claims Collection Type
Burden and Respondent Descriptions
Minimum Burden
Estimate
Median Burden
Estimate
Maximum
Burden Estimate
# of Clinicians (a)
12,197
12,197
12,197
Hours Per Computer Systems Analyst to Submit
Quality Data (b)
0.15
1.05
7.2
# of Hours Medical and Health Services Manager
Review Measure Specifications (c)
3
3
3
# of Hours Computer Systems Analyst Review
Measure Specifications (d)
1
1
1
# of Hours LPN Review Measure Specifications
(e)
1
1
1
# of Hours Billing Clerk Review Measure
Specifications (f)
1
1
1
# of Hours Physician Review Measure
Specifications (g)
1
1
1
Annual Hours per Clinician (h) = (b) + (c) + (d) +
(e) + (f) + (g)
7.15
8.05
14.2
Total Annual Hours (i) = (a) × (h)
87,209
98,186
173,197
Cost to Submit Quality Data (Computer Systems
Analyst’s Labor Rate of $106.54/hr at varying
times) (j)
$15.98
$111.87
$767.09
Cost to Review Measure Specifications (Medical
and Health Services Manager's Labor Rate of
$129.28/hr for 3 hr) (k)
$387.84
$387.84
$387.84
Cost to Review Measure Specifications
(Computer Systems Analyst’s Labor Rate of
$106.54/hr for 1 hr) (l)
$106.54
$106.54
$106.54
Cost to Review Measure Specifications (LPN's
Labor Rate of $58.46/hr for 1 hr) (m)
$58.46
$58.46
$58.46
Cost to Review Measure Specifications (Billing
Clerk’s Labor Rate of $45.32/hr) (n)
$45.32
$45.32
$45.32
Cost to Review Measure Specifications
(Physician’s Labor Rate of $291.64/hr) (o)
$291.64
$291.64
$291.64
Total Annual Cost Per Clinician (p) = (j) + (k) +
(l) + (m) + (n) + (o)
$905.78
$1,001.67
$1,656.89
Total Annual Cost (q) = (a) × (p)
$11,047,799
$12,217,369
$20,209,087
In Table 98, we illustrate the net change in estimated burden for quality data submissions
from clinicians using the Medicare Part B Claims-based collection type using the currently
approved burden in the CY 2024 PFS final rule (88 FR 79438 and 79439). In aggregate, using
our currently approved per response time estimates, the decrease in number of responses from
13,413 to 12,197 (-1,216) (Table 92) will result in a total maximum adjustment of -17,268 hours

at a cost of -$2,014,779 for the CY 2025 performance period/2027 MIPS payment year. For
purposes of calculating total burden associated with this proposed rule as shown in Tables 77
through 79, only the maximum burden is used.
TABLE 98: Change in Estimated Burden for Quality Performance Category: Clinicians
Using the Medicare Part B Claims Collection Type
Burden and Respondent Descriptions
Burden Estimate
Total Currently Approved Annual Hours (a)
190,465
Total Annual Hours for Respondents in CY 2025 PFS Proposed Rule (b) (see Table 97,
row (i))
173,197
Difference in Annual Hours (c) = (b) − (a)
-17,268
Total Currently Approved Annual Cost (d)
$22,223,866
Total Annual Cost for Respondents in CY 2025 PFS Proposed Rule (e) (see Table 97,
row (q))
$20,209,087
Difference in Annual Cost (f) = (d) − (e)
-$2,014,779
(5) Quality Data Submission by Individuals and Groups Using MIPS CQM and QCDR
Collection Types
In this section, we identify proposed updates to the estimated burden for the Quality Data
Submission by Individuals and Groups Using MIPS CQM and QCDR Collection Types ICR that
will be submitted to OMB for review under control number 0938-1314 (CMS-10621). As noted
in Table 79, the proposed change in burden reflects adjustments for updated data and
assumptions, and as well as the proposal for additional MVPs as outlined in section V.B.8.e.(4).
of this proposed rule. In Table 146 (section VII.E.17.e.(1).), we identify the changes in burden to
this ICR due to the proposed policies.
We refer readers to the CY 2017 and CY 2018 Quality Payment Program final rules
(81 FR 77504 and 77505 and 82 FR 53912 and 53914, respectively), the CY 2019, CY 2020,
CY 2021, CY 2022, CY 2023, and CY 2024 PFS final rules (83 FR 60005 and 60006,
84 FR 63127 and 63128, 85 FR 84977 through 84979, 86 FR 65584 through 65586, and
87 FR 70151 through 70153, and 88 FR 79439 through 79441, respectively) for our previously
finalized requirements and burden for quality data submission via the MIPS CQM and QCDR
collection types. We refer readers to Table 105 for the estimated change in associated burden for
quality data submission using MIPS CQM and QCDR collection types related to MVP and
subgroup reporting in the CY 2025 performance period/2027 MIPS payment year. We refer
readers to section V.B.8.e.(2). of this proposed rule for the factors affecting the proposed
changes and adjustments for each quality performance collection type.
As noted in Tables 92 and 93, based on updated data available from the CY 2022
performance period/2024 MIPS payment year and updated MVP reporting assumptions, for the
CY 2025 performance period/2027 MIPS payment year, we estimate that 17,008 clinicians
(10,850 individuals and 6,158 groups and virtual groups) will submit quality data as individuals
or groups using MIPS CQM or QCDR collection types. This is an increase of 376 clinicians
from the currently approved estimate of 16,632 clinicians provided in the CY 2024 PFS final rule
(88 FR 79439 through 79441). Given the number of measures required for clinicians and groups
is the same, we expect the burden to be the same for each respondent collecting data via MIPS
CQM or QCDR, whether the clinician is participating in MIPS as an individual or group.
Under the MIPS CQM and QCDR collection types, the individual clinician or group may
either submit the quality measures data directly to us, log in and upload a file, or utilize a third
party intermediary to submit the data to us on the clinician’s or group’s behalf. We estimate that
the burden associated with the QCDR collection type is similar to the burden associated with the
MIPS CQM collection type; therefore, we discuss the burden for both collection types together.
For MIPS CQM and QCDR collection types, we estimate an additional time for respondents
(individual clinicians and groups) to become familiar with MIPS quality measure specifications
and, in some cases, specialty measure sets and QCDR measures. Therefore, we believe the
burden for an individual clinician or group to review measure specifications and submit quality
data is a total of 9 hours at a cost of $1,088.98 per response. This consists of 3 hours at
$106.54/hr for a computer systems analyst (or their equivalent) to submit quality data along with
2 hours at $129.28/hr for a medical and health services manager, 1 hour at $106.54/hr for a
computer systems analyst, 1 hour at $58.46/hr for a LPN, 1 hour at $45.32/hr for a billing clerk,
and 1 hour at $291.64/hr for a physician to review measure specifications. Additionally,
clinicians and groups who do not submit data directly will need to authorize or instruct the
qualified registry or QCDR to submit quality measures’ results and numerator and denominator
data on quality measures to us on their behalf. We estimate the time and effort associated with
authorizing or instructing the quality registry or QCDR to submit this data will be approximately
5 minutes (0.083 hr) at $106.54/hr for a computer systems analyst at a cost of $8.84 (0.083 hr x
$106.54/hr). Overall, we estimate 9.083 hr/response (3 hr + 2 hr + 1 hr + 1 hr + 1 hr + 1 hr +
0.083 hr) at a cost of $1,088.98/response [(3 hr x $106.54/hr) + (2 hr x $129.28/hr) + (1 hr x
$106.54/hr) + (1 hr x $58.46/hr) + (1 hr x $45.32/hr) + (1 hr x $291.64/hr) + (0.083 hr x
$106.54/hr)].
In Table 99, for the CY 2025 performance period/2027 MIPS payment year, in aggregate,
we estimate a burden of 154,484 hours (9.083 hr /response x 17,008 responses) at a cost of
$18,521,372 (17,008 responses x $1,088.98/response).

TABLE 99: Estimated Burden for Quality Performance Category: Clinicians
(Participating Individually or as Part of a Group) Using the MIPS CQM and QCDR
Collection Type
Burden and Respondent Descriptions
Burden Estimate
# of Clinicians Submitting as Individuals (a)
10,850
# of Groups Submitting via QCDR or MIPS CQM on Behalf of Individual Clinicians (b)
6,158
Total # of Respondents (c) = (a) + (b)
17,008
# of Hours Per Respondent to Report Quality Data (d)
3
# of Hours per Medical and Health Services Manager to Review Measure Specifications
(e)
2
# of Hours for Computer Systems Analyst to Review Measure Specifications (f)
1
# of Hours for LPN to Review Measure Specifications (g)
1
# of Hours for Billing Clerk to Review Measure Specifications (h)
1
# of Hours for Physician to Review Measure Specifications (i)
1
# of Hours Per Respondent to Authorize Qualified Registry to Report on Respondent’s
Behalf (j)
0.083
Annual Hours Per Respondent (k)= (d) + (e) + (f) + (g) + (h) + (i) + (j)
9.083
Total Annual Hours (l) = (c)×(k)
154,484
Cost Per Respondent to Submit Quality Data (at Computer Systems Analyst’s Labor
Rate of $106.54/hr) (m) = $106.54/hr × (d)
$319.62
Cost to Review Measure Specifications (at Medical and Health Services Manager's
Labor Rate of $129.28/hr) (n) = $129.28/hr × (e)
$258.56
Cost per Computer System’s Analyst Review of Measure Specifications (at Computer
Systems Analyst’s Labor Rate of $106.54/hr) (o) = $106.54/hr × (f)
$106.54
Cost per LPN to Review Measure Specifications (at LPN's Labor Rate of $58.46/hr) (p)
= $58.46/hr × (g)
$58.46
Cost per Billing Clerk to Review Measure Specifications at Clerk’s Labor Rate of
$45.32/hr) (q) = $45.32/hr × (h)
$45.32
Cost for Physician to Review Measure Specifications (at Physician’s Labor Rate of
$291.64/hr) (r) = $291.64/hr × (i)
$291.64
Cost for Respondent to Authorize Qualified Registry/QCDR to Report on Respondent's
Behalf (at Computer Systems Analyst’s Labor Rate of $106.54/hr) (s) = $106.54/hr × (j)
$8.84
Total Annual Cost Per Respondent (t) = (m) + (n) + (o) + (p) + (q) + (r) + (s)
$1,088.98
Total Annual Cost (u) = (c) × (t)
$18,521,372
In Table 100, we calculated the net change in estimated burden for quality performance
category submissions using the MIPS CQM and QCDR collection type by using the currently
approved burden in the CY 2024 PFS final rule (88 FR 79439 through 79441). In aggregate,
using the unchanged currently approved time per response estimate, the increase of 376
respondents from 16,632 to 17,008 for the CY 2025 performance period/2027 MIPS payment
year results in an increase of 3,416 hours at a cost of +$409,457.

TABLE 100: Change in Estimated Burden for Quality Performance Category: Clinicians
(Participating Individually or as Part of a Group) Using the MIPS CQM and QCDR
Collection Type
Burden and Respondent Descriptions
Burden
Estimate
Total Currently Approved Annual Hours (a)
151,068
Total Annual Hours for Respondents in CY 2025 PFS Proposed Rule (b) (see Table 99,
row (l))
154,484
Difference in Annual Hours (c) = (b) − (a)
+3,416
Total Currently Approved Annual Cost (d)
$18,111,915
Total Annual Cost for Respondents in CY 2025 PFS Proposed Rule (e) (see Table 99,
row (u))
$18,521,372
Difference in Annual Cost (f) = (e) − (d)
+$409,457
(6) Quality Data Submission by Clinicians and Groups: eCQM Collection Type
In this section, we identify proposed updates to the estimated burden for the Quality Data
Submission by Individuals and Groups Using eCQM Collection Type ICR that will be submitted
to OMB for review under control number 0938-1314 (CMS-10621). As noted in Table 79, this
proposed change in burden reflects adjustments for updated data and assumptions, and as well as
the proposal for additional MVPs as outlined in section V.B.8.e.(4). of this proposed rule. In
Table 146 (section VII.E.17.e.(1).of this proposed rule), we identify the changes in burden to this
ICR due to the proposed policies.
We refer readers to the CY 2017 and CY 2018 Quality Payment Program final rules
(81 FR 77505 and 77506 and 82 FR 53914 and 53915), CY 2019, CY 2020, CY 2021, the
CY 2022 PFS, the CY 2023, and the CY 2024 PFS final rule (83 FR 60006 and 60007,
84 FR 63128 through 63130, 85 FR 84979 and 84980, 86 FR 65586 through 65588,
87 FR 70153 and 70154, and 88 FR 79441 through 79443 respectively) for our previously
finalized requirements and burden for quality data submission via the eCQM collection types.
For the change in associated burden for quality data submission related to the provisions
introducing MVP and subgroup reporting beginning in the CY 2025 performance period/2027
MIPS payment year, we refer readers to Table 105. We refer readers to section V.B.8.e.(2). of
this proposed rule for the factors affecting the proposed changes and adjustments for each quality
performance collection type.
As noted in Tables 92 and 93, based on updated data available from the CY 2022
performance period/2024 MIPS payment year and updated MVP reporting assumptions, we
assume that 27,179 clinicians (21,240 individual clinicians and 5,939 groups and virtual groups)
will submit quality data using the eCQM collection type for the CY 2025 performance
period/2027 MIPS payment year. This is a decrease of 1,535 clinicians from the currently
approved estimate of 28,714 clinicians in the CY 2024 PFS final rule (88 FR 78818)). We
assume the burden to be the same for each respondent using the eCQM collection type, whether
the clinician is participating in MIPS as an individual or group.
Under the eCQM collection type, the individual clinician or group may either submit the
quality measures data directly to us from their eCQM, log in and upload a file, or utilize a third
party intermediary to derive data from their certified electronic health record technology
(CEHRT) and submit it to us on the clinician’s or group’s behalf.
To prepare for the eCQM collection type, the clinician or group must review the quality
measures on which we will be accepting MIPS data extracted from eCQMs, select the
appropriate quality measures, extract the necessary clinical data from their CEHRT, and submit
the necessary data to a QCDR/qualified registry to submit the data on behalf of the clinician or
group. We assume the burden for collecting quality measures data via eCQM is similar for
clinicians and groups who submit their data directly to us from their CEHRT and clinicians and
groups who use a QCDR or qualified registry to submit the data on their behalf. This includes
extracting the necessary clinical data from their CEHRT and submitting the necessary data to a
QCDR/qualified registry. We note that the CY 2024 PFS final rule eliminated the category of
health IT vendors for the Quality Payment Program beginning in the CY 2025 performance
period/2027 MIPS payment period (88 FR 79390 and 79391).
We estimate that it will take no more than 2 hours at $106.54/hr for a computer systems
analyst or their equivalent to submit the data file. The burden will also involve becoming
familiar with MIPS quality measure specifications. In this regard, we estimate it will take 6

hours for a clinician or group to review measure specifications. Of that time, we estimate 2
hours at $129.28/hr for a medical and health services manager, 1 hour at $291.64/hr for a
physician, 1 hour at $106.54/hr for a computer systems analyst, 1 hour at $58.46/hr for an LPN,
and 1 hour at $45.32/hr for a billing clerk. Overall, we estimate a cost of $973.60/response [(2 hr
x $106.54/hr) + (2 hr x $129.28/hr) + (1 hr x $106.54/hr) + (1 hr x $58.46/hr) + (1 hr x
$45.32/hr) + (1 hr x $4291.64/hr)].
In Table 101, for the CY 2025 performance period/2027 MIPS payment year, in
aggregate, we estimate a burden of 217,432 hours (8 hr x 27,179 responses) at a cost of
$26,461,474 (27,179 responses x $973.60/response).
TABLE 101: Estimated Burden for Quality Performance Category: Clinicians (Submitting
Individually or as Part of a Group) Using the eCQM Collection Type
Burden and Respondent Descriptions
Burden
Estimate
# of Clinicians Submitting as Individuals (a)
21,240
# of Groups Submitting via EHR on Behalf of Individual Clinicians (b)
5,939
Total # of Respondents (c)=(a)+(b)
27,179
# of Hours Per Respondent to Submit MIPS Quality Data File (d)
2
# of Hours Per Medical and Health Services Manager to Review Measure Specifications
(e)
2
# of Hours Per Computer Systems Analyst to Review Measure Specifications (f)
1
# of Hours Per LPN to Review Measure Specifications (g)
1
# of Hours Per Billing Clerk to Review Measure Specifications (h)
1
# of Hours Per Physician to Review Measure Specifications (i)
1
Annual Hours Per Respondent (j) = (d) + (e) + (f) + (g) + (h) + (i)
8
Total Annual Hours (k) = (c) × (j)
217,432
Cost Per Respondent to Submit Quality Data (at Computer Systems Analyst’s Labor Rate
of $106.54/hr) (l) = $106.54/hr × (d)
$213.08
Cost to Review Measure Specifications (at Medical and Health Services Manager's Labor
Rate of $129.28/hr) (m) = $129.28/hr × (e)
$258.56
Cost to Review Measure Specifications (at Computer System’s Analyst’s Labor Rate of
$106.54/hr) (n) = $106.54/hr × (f)
$106.54
Cost to Review Measure Specifications (at LPN's Labor Rate of $58.46/hr) (o) = $58.46/hr
× (g)
$58.46
Cost to Review Measure Specifications (at Clerk’s Labor Rate of $45.32/hr) (p) =
$45.32/hr) × (h)
$45.32
Cost to Review Measure Specifications (at Physician’s Labor Rate of $291.64/hr) (q) =
$291.64/hr × (i)
$291.64
Total Cost Per Respondent (r)=(l)+(m)+(n)+(o)+(p)+(q)
$973.60
Total Annual Cost (s) = (c) × (r)
$26,461,474
In Table 102, we illustrate the net change in burden for submissions in the quality
performance category using the eCQM collection type from the currently approved burden in the

CY 2024 PFS final rule (88 FR 79441 and 79442). In aggregate, using our currently approved
time per response burden estimate, the decrease of 1,535 respondents from 28,714 to 27,179 for
the CY 2025 performance period/2027 MIPS payment year results in a decrease of 12,280 hours
(1,535 responses x 8 hr/response) at a cost of -$1,494,476 (-1,535 response) at a cost of -
$1,494,476 (-1,535 responses x $973.60/response).
TABLE 102: Change in Estimated Burden for Quality Performance Category: Clinicians
(Participating Individually or as Part of a Group) Using the eCQM Collection Type
Burden and Respondent Descriptions
Burden
Estimate
Total Currently Approved Annual Hours (a)
229,712
Total Annual Hours for respondents in CY 2025 PFS Proposed Rule (b) (see Table 101,
row (k))
217,432
Difference in Annual Hours (c) = (b) − (a)
-12,280
Total Currently Approved Annual Cost (d)
$27,955,950
Total Annual Cost for Respondents in CY 2025 PFS Proposed Rule (e) (see Table 101,
row (s))
$26,461,474
Difference in Annual Cost (f) = (e) − (d)
-$1,494,476
(7) ICRs Regarding Burden for MVP Reporting
The following proposed changes will be submitted to OMB for review under control
number 0938–1314 (CMS–10621).
(a) Burden for MVP Reporting Requirements
In the CY 2022 PFS final rule, we finalized an option for clinicians choosing to report
MVPs to participate through subgroups beginning with the CY 2023 performance period/2025
MIPS payment year (86 FR 65392 through 65394). We refer readers to the CY 2022, CY 2023,
and CY 2024 PFS final rules for our previously finalized burden assumptions and requirements
for submission data for the MVP performance category, and for the estimated number of
clinicians participating as subgroups in the CY 2024 performance period/2026 MIPS payment
year (86 FR 65590 through 65592, 87 FR 70155, and 88 FR 79443).
As discussed in section II.G.2. of this proposed rule, we are proposing to make payment
for advanced primary care management(APCM) services furnished by a physician or other
qualified health care professional who is responsible for all primary care (for example,

physicians and non-physician practitioners, including nurse practitioners, physician assistants,
certified nurse-midwives and clinical nurse specialists), and serve as the continuing focal point
for all needed health care services during a calendar month. This proposed payment would
incorporate several specific, existing care management and communication technology-based
services into a bundle and require reporting the Value in Primary Care MVP by clinicians billing
for APCM services beginning in the CY 2025 performance period/2027 MIPS payment year.
We are proposing that billing practitioners who are not MIPS eligible clinicians (as defined at
§ 414.1305) would not be required to report the MVP in order to furnish and bill for APCM
services. Based on our approach for estimating MVP as a percentage of previous traditional
MIPS quality submissions discussed in section V.B.8.e.(7)(a)(i).of this proposed rule, we are
unable to determine how many additional clinicians or practices would report the Value in
Primary Care MVP for the CY 2025 performance period/2027 MIPS payment year above our
current MVP submission estimates. Similarly, we cannot assess what participation levels
clinicians or practices who may use these APCM codes, if finalized, have reported MIPS in the
past (for example, eligibility requirements and special statuses, participation at the individual,
group, virtual group, or Alternative Payment Model (APM) Entity level, or reporting via
traditional MIPS, the APM Performance Pathway (APP), or MVPs), or if they will be MIPS
eligible clinicians in future years. We refer readers to section II.G.2. of this proposed rule for
details on this proposal, and section VII.E.17.e.(2)(f) for additional discussion on burden impacts
to the Quality Payment Program.
In the CY 2024 PFS final rule (88 FR 79442), we conducted an analysis to calculate the
average quality measure submission rate for each newly proposed MVP for the CY 2024
performance period/2026 MIPS payment period, using measures submissions in the CY 2021
performance period/2023 MIPS payment year for clinicians with relevant clinical specialties for
each proposed MVP. The total of these average quality measure submissions for each MVP was
equivalent to about 2 percent of total quality measure submissions in the CY 2021 performance

period/2023 MIPS payment year. We added this incremental increase of 2 percentage points to
the previously approved estimate in the CY 2023 PFS final rule that 12 percent of clinicians who
participated in MIPS for the CY 2021 performance period/2023 MIPS payment year will submit
data for the quality performance category through MVP reporting in the CY 2023 performance
period/2025 MIPS payment year (88 FR 79443).
With updated submission data available for the CY 2022 performance period/2024 MIPs
payment year as discussed in section V.B.8.e.(3). of this proposed rule and updated quality
measure list revisions within the MVP inventory for the CY 2024 performance period/2026
MIPS payment year (88 FR 79978 through 80047), we conducted the analysis identified in the
preceding paragraph for the 16 MVPs approved for the CY 2024 performance period/2026 MIPS
payment year. The total of these average quality measure submissions for each approved MVP
was equivalent to 6 percent of the total quality measure submissions in the CY 2022 performance
period/2024 MIPS payment year. This is a decrease from the 14 percent estimate provided in the
CY 2024 PFS final rule (88 FR 79443).
As discussed in section IV.A.4.a.(1). of this proposed rule, we are proposing
modifications to the 16 MVPs approved for the CY 2024 performance period/2026 MIPS
payment year reporting with the addition and removal of measures and improvement activities
based on the MVP development criteria (85 FR 84849 through 84854). We are proposing to
consolidate the previously finalized Optimal Care for Patients with Episodic Neurological
Conditions MVP and Supportive Care for Neurodegenerative Conditions MVP into a
consolidated neurological MVP titled Quality Care for Patients with Neurological Conditions.
We are also proposing to add six (6) new MVPs to the MVP inventory. These proposals would
provide 21 MVPs for the CY 2025 performance period/2027 MIPS payment year.
For each newly proposed MVP, we similarly calculated the average quality measure
submission rate across the measures available in each MVP for the CY 2022 performance
period/2024 MIPS payment year. Using updated data available from the CY 2022 performance

period/2024 MIPS payment year, we calculated that the total of these average quality measure
submissions for each proposed MVP was equivalent to about 4 percent of total quality measure
submissions. We assume there would not be any changes to MVP submissions due to the
proposal to consolidate the measures in the Optimal Care for Patients with Episodic Neurological
Conditions MVP and Supportive Care for Neurodegenerative Conditions MVP into the Quality
Care for Patients with Neurological Conditions MVP. That is, we assume clinicians who would
have submitted the Optimal Care for Patients with Episodic Neurological Conditions MVP or the
Supportive Care for Neurodegenerative Conditions MVP would instead submit the Quality Care
for Patients with Neurological Conditions MVP. Therefore, we estimate the proposed changes to
the MVP inventory in this proposed rule will result in an additional 4 percent of MIPS clinicians
moving from traditional MIPS to MVP reporting.
Taking together the aforementioned analyses where we assessed the MVP participation
rate for the 16 established MVPs at 6 percent using updated quality measure submission data
from the CY 2022 performance period/2024 MIPS payment year, and the assessment that 4
percent of MIPS clinicians may move to the six proposed MVPs due to quality measure
submission trends for the CY 2022 performance period/2024 MIPS payment year, we estimate
that a total of 10 percent of the clinicians will participate in MVP reporting in the CY 2025
performance period/2027 MIPS payment year. This is a decrease of 4 percentage points from the
currently approved estimate of 14 percent in the CY 2024 PFS final rule (88 FR 79443). This
decrease reflects the updated analysis of MVP submissions for established MVPs (from 14
percent to 4 percent) to account for the latest available MIPS submission data, and the additional
6 percent of MIPS clinicians we believe may report the 6 newly proposed MVPs due to updated
quality measure submission data.
Continuing our approach used in the CY 2022, CY 2023, and CY 2024 PFS final rules
(86 FR 65589 and 65590, 87 FR 70156 and 701566, and 88 FR 79443 and 79444, respectively),
we assume that the number of MVP registrations will equal our estimated MVP quality

submissions. We note the MVP registration window for the 12 MVPs available for the CY 2023
performance period/2025 MIPS payment year closed on November 30, 2023. As noted in
section IV.A.3.b.(2). of this proposed rule, we received over 750 MVP registrations for the
CY 2023 performance period/2025 MIPS payment year. MIPS submission data for the CY2023
performance period/2025 MIPS payment year was unavailable while preparing these burden
estimates. We will reassess our approach as needed in future rules when both MVP registration
and submission data are available for the same performance period.
(i) Burden for MVP Registration: Individuals, Groups and APM Entities
We refer readers to the CY 2024 PFS final rule (88 FR 79443 and 79444) for our
previously finalized burden relevant to MVP registration for clinicians participating as an
individual and/or group for MVP reporting.
In the CY 2022 PFS final rule (86 FR 65414), we finalized at § 414.1365(c)(4)(ii) that an
MVP Participant is scored on one population health measure in accordance with
§ 414.1365(d)(1). Since the MVP population health measures are administrative claims-based,
they do not require data submission from clinicians and do not contribute to reporting burden.
To track which population health measure an MVP Participant intends to report, we finalized in
the CY 2022 PFS final rule (86 FR 65417) at § 414.1365(b)(2)(i) that MVP Participants are
required to select one population health measure at the time of MVP registration.
MVP Participants currently select one population measure during registration
(86 FR 65589), via a drop-down list. In section IV.A.4.b.(1)(b) of this proposed rule, we are
proposing to update the registration process and scoring policies for population health measures
in the quality performance category. We are proposing to revise § 414.1365(d)(3)(i)(A) to state
that for the CY 2023 through 2024 performance periods/2025 through 2026 MIPS payment
years, MVP Participants will be scored on the selected population health measure and beginning
in the CY 2025 performance period/2027 MIPS payment year, we would use the highest score of
all available population health measures. To apply this policy to subgroups reporting an MVP,
we also propose to update § 414.1365(d)(3)(i)(A)(1) to provide that for the CY 2023 through
2024 performance periods/2025 through 2026 MIPS payment years, subgroups will be scored on
the selected population health measure based on its affiliated group score, if available, and
beginning in the CY 2025 performance period/2027 MIPS payment year a subgroup is scored on
the highest score of all available population health measures based on its affiliated group score, if
available. We propose to revise § 414.1365(b)(2)(i) to provide that beginning in the CY 2025
performance period/2027 MIPS payment year, each MVP Participant must select an MVP and
any outcomes-based administrative claims-based measure on which the MVP Participant intends
to be scored. We refer readers to section IV.A.4.b.(1) of this rulemaking for details on this
proposal and scoring implications. This proposal would remove the requirement for the MVP
Participant to select a population health measure during MVP registration. We assume the
associated reduction in burden per application would be minimal. Therefore, we are not
adjusting the burden per MVP registration from the currently approved registration time of 15
minutes (0.25 hr) (88 FR 79443 and 79444).
As discussed, we estimate that approximately 10 percent of the clinicians that currently
participate in MIPS will submit data for the measures and activities in an MVP. For the
CY 2025 performance period/2027 MIPS payment year, we assume that the total number of
individual clinicians, groups, subgroups and APM Entities that will complete the MVP
registration process is 6,285. In Table 103, we estimate that it will take 1,571 hours (6,285
responses x 0.25 hr/response) at a cost of $167,)432 (6,285 registrations x $26.64/registration)
for individual clinicians, groups and APM Entities to register for MVP reporting in the CY 2025
performance period/2027 MIPS payment year.

TABLE 103: Estimated Burden for MVP Registration (Individuals, Groups, Subgroups,
and APM Entities)
Burden and Respondent Descriptions
Burden Estimate
Estimated # of Individual Clinicians, Groups, Subgroups and APM Entities
Registering (a)
6,285
Estimated Time Per Registration (hr) (b)
0.25
Estimated Total Annual Time for MVP Registration (c) = (a) × (b)
1,571
Computer Systems Analyst’s Labor Rate (d)
$106.54/hr
Estimated Cost Per Registration (e) = (d) × (b)
$26.64
Estimated Total Annual Cost for MVP Registration ((f) = (a) × (e)
$167,432
In Table 104, we illustrate the net change in burden for MVP registration using the
currently approved burden in the CY 2024 PFS final rule (88 FR 79443 and 79444). In
aggregate, for the CY 2025 performance period/2027 MIPS payment year, the change in the
number of respondents expected to register for MVP reporting from 9,585 to 6,285 will result in
a decrease of 3,300 responses. In aggregate, when combined with the currently approved per
response time estimate, this will result in a decrease of 825 hours (-3,300 responses x
0.25hr/response) at a cost of -$87,912 (-3,300 responses x $26.64/response).
TABLE 104: Change in Estimated Burden for MVP Registration (Individuals, Groups,
Subgroups, and APM Entities)
Burden and Respondent Descriptions
Burden Estimate
Total Currently Approved Annual Hours (a)
2,396
Total Annual Hours for Respondents in CY 2025 PFS Proposed Rule (b) (See
Table 103, row (c))
1,571
Difference in Annual Hours (c) = (b) – (a)
-825
Total Currently Approved Annual Cost (d)
$255,344
Total Annual Cost for Respondents in CY 2025 PFS Proposed Rule (e) (See Table
103, row (e))
$167,432
Difference in Annual Cost (f) = (e) − (d)
-$87,912
(ii) Burden for Subgroup Registration
We are not proposing any changes to our currently approved subgroup registration
burden (86 FR 65590) of 10 annual hours (20 responses at 0.5hr/response). As discussed in
section V.B.8.e.(7)(a) of this proposed rule, we assume the proposal to remove the selection of a
population health measure at the time of registration, as detailed in section IV.A.4.b.(1)(b). of
this proposed rule, will not significantly impact the currently approved burden for MVP

registration. We continue this assumption to subgroup registration. Therefore, we are
continuing our currently approved burden for subgroup registration time of 30 minutes (0.5 hr).
We expect clinician participation in subgroups will be relatively low for the CY 2025
performance period/2027 MIPS payment year due to the voluntary subgroup reporting option
and the additional burden involved for groups to organize clinicians into subgroups. Therefore,
we are not proposing any adjustments to our previously finalized estimate in the CY 2022 PFS
final rule (86 FR 65590) that 20 subgroups will participate in MVP reporting.
As identified in section IV.A.3.c.(1) of this proposed rule, we finalized a mandatory
subgroup reporting requirement for multispecialty groups choosing to report as an MVP
Participant beginning in the CY 2026 performance period/2028 MIPS payment year (§ 414.1305;
86 FR 65394 through 65397). Section IV.A.3.d. of this proposed rule includes a Request for
Information (RFI) to obtain feedback on what guidance/parameters are needed for multispecialty
groups to place clinicians into subgroups for reporting an MVP relevant to the scope of care
provided. Absent available submission data on MVP reporting as discussed in section
V.B.8.e.(7)(a). of this proposed rule, we are unable to estimate the effect of this established
policy on reporting for the CY 2026 performance period/2028 MIPS payment. We refer readers
to section VII.E.17.e.(2)(g). of this proposed rule for additional discussion on burden impacts of
this established policy to the Quality Payment Program.
The burden relevant to the subgroup registration requirement is currently approved by
OMB under control number 0938–1314 (CMS–10621). Since this rulemaking is not proposing
any new or revised subgroup registration requirements or burden, we are not proposing any
changes under that control number.
(iii) Burden for MVP Quality Performance Category Submission.
In the CY 2022 PFS final rule (86 FR 65411 through 65415), we finalized the reporting
requirements for the MVP quality performance category at § 414.1365(c)(1)(i).

In sections IV.A.4.d.(2)(b). and IV.A.4.d.(3).(b). of this proposed rule, we are proposing
to adopt minimum criteria for a qualifying data submission for a MIPS performance period for
the quality performance category and to codify our existing policies governing our treatment of
multiple submissions received for the quality performance category. In accordance with our
discussion of this policy proposal relevant to traditional MIPS quality reporting in section
V.B.8.e.(2). of this proposed rule, these proposed policies will not introduce new requirements to
submit data for the quality performance category of MVPs. Therefore, we are continuing our
currently approved per response time estimates for submitting the MVP quality performance
category data due to this proposal.
As described in section V.B.8.e.(7)(a) of this proposed rule, we estimate that 10 percent
of the clinicians who participated in MIPS for the CY 2022 performance period/2024 MIPS
payment year will submit data for the quality performance category of MVP in the CY 2025
performance period/2027 MIPS payment year. We also estimate there will be 20 subgroup
reporters in the CY 2025 performance period/2027 MIPS payment year. In Table 105, we
estimate that 3,020 clinicians and 10 subgroups will submit data using eCQMs collection type at
$644.93/response (see line q for eCQMs); 1,890 clinicians and 10 subgroups will submit data
using MIPS CQM and QCDR collection type at $716.31/response (see line q for CQM and
QCDRs); and 1,355 clinicians and 0 subgroups will submit data for the MVP quality
performance category using the Medicare Part B claims collection type at $1,101.24/response
(see line q for claims). For the CY 2025 performance period/2027 MIPS payment year, using
our currently approved per response time estimates for the clinicians and subgroups submitting
data for the MVP quality performance category, we estimate a burden of 16,059 hours [5.3 hr x
3,030 (3,020 +10) responses] at a cost of $1,954,138 (3,030 responses x $644.93/response) for
the eCQM collection type, 11,343 hours [5.97 hr x 1,900 (1,890 +10 responses)] at a cost of
$1,360,989 (1,900 responses x $716.31/response) for the MIPS CQM and QCDR collection type,

and 12,791 hours (9.44 hr x 1,355 clinician responses) at a cost of $1,492,180 (1,355 responses x
$1,101.24/response) for the Medicare Part B claims collection type.
TABLE 105: Estimated Burden for MVP Quality Performance Category
Submission
Burden and Respondent
Descriptions
eCQM Collection
Type
CQM and QCDR
Collection Type
Claims Collection
Type
# of Submissions from Pre-existing
collection types (a)
3,020
1,890
1,355
# of Subgroup Reporters (b)
10
10
0
Total MVP Participants (c) = (a) +
(b)
3,030
1,900
1,355
Hours Per Computer Systems
Analyst to Submit Quality Data (d)
1.33
2
4.8
# of Hours Medical and Health
Services Manager Review Measure
Specifications (e)
1.33
1.33
2
# of Hours Computer Systems
Analyst Review Measure
Specifications (f)
0.66
0.66
0.66
# of Hours LPN Review Measure
Specifications (g)
0.66
0.66
0.66
# of Hours Billing Clerk Review
Measure Specifications (h)
0.66
0.66
0.66
# of Hours Physician Review
Measure Specifications (i)
0.66
0.66
0.66
Annual Hours per Clinician
Submitting Data for MVPs (j) = (d) +
(e) + (f) + (g) + (h) + (i)
5.3
5.97
9.44
Total Annual Hours (k) = (c) × (j)
16,059
11,343
12,791
Cost to Submit Quality Data (at
Computer Systems Analyst’s Labor
Rate of $106.54/hr) (l) = $106.54/hr
× (d) varying times
$141.70
$213.08
$511.39
Cost to Review Measure
Specifications (at Medical and
Health Services Manager's Labor
Rate of $129.28/hr) (m) = $129.28/hr
× (e) varying times
$171.94
$171.94
$258.56
Cost to Review Measure
Specifications (at Computer Systems
Analyst’s Labor Rate of $106.54/hr)
(n) = $106.54/hr × (f)
$70.32
$70.32
$70.32
Cost to Review Measure
Specifications (at LPN's Labor Rate
of $58.46/hr) (o) = $58.46/hr × (g)
$38.58
$38.58
$38.58
Cost to Review Measure
Specifications (at Billing Clerk’s
Labor Rate of $45.32/hr) (p) =
$45.32/hr × (h)
$29.91
$29.91
$29.91
Cost to Review Measure
Specifications (at Physician’s Labor
Rate of $291.64/hr) (q) = $291.64/hr
× (i)
$192.48
$192.48
$192.48
Total Annual Cost Per Submission
(r) = (l) + (m) + (n) + (o) + (p) + (q)
$644.93
$716.31
$1,101.24
Total Cost (s) = (c) × (r)
$1,954,138
$1,360,989
$1,492,180

Table 106 illustrates the changes in estimated burden for clinicians who will submit the
MVP quality performance category utilizing the eCQM, MIPS CQM and QCDR, and Medicare
Part B claims collection types in the CY 2025 performance period/2027 MIPS payment year.
We note we used the currently approved burden in the CY 2024 PFS final rule
(88 FR 79444 through 79446) as the baseline to determine the net change in burden. In
aggregate, when combined with our currently approved per response time estimate, the decrease
in 3,300 respondents who will submit data for the MVP quality performance category will result
in a change of -8,766 hours and -$1,066,714 for the eCQM collection type, -4,877 hours and -
$585,225 for the CQM and QCDR collection type, and -7,826 hours and -$912,928 for the
Medicare Part B claims collection type.
TABLE 106: Change in Estimated Burden for MVP Quality Performance Category
Submission
Burden and Respondent Descriptions
eCQM
Collection Type
CQM and
QCDR
Collection Type
Claims
Collection Type
Total Currently Approved Annual Hours (a)
24,825
16,220
20,617
Total Annual Hours for Respondents in CY 2025
PFS Proposed Rule (b) (See Table 105, row (k))
16,059
11,343
12,791
Difference in Annual Hours (c) = (b) − (a)
-8,766
-4,877
-7,826
Total Currently Approved Annual Cost (d)
$3,020,852
$1,946,214
$2,405,108
Total Annual Cost for Respondents in CY 2025
PFS Proposed Rule (e) (See Table 105, row (s))
$1,954,138
$1,360,989
$1,492,180
Difference in Annual Cost (f) = (e) − (d)
-$1,066,714
-$585,225
-$912,928
(8) Beneficiary Responses to CAHPS for MIPS Survey
We are not proposing any new or revised collection of patient experience information
from survey respondents during the administration of the CAHPS for MIPS Survey for the
CY 2025 performance period/2027 MIPS payment year. We note that we proposed updates to
our estimated burden under control number 0938-1222 (CMS-10450) as discussed in the
CY 2024 PFS final rule (88 FR 79446 and 79447), which were submitted under the standard
non-rule PRA package. The 60-day notice for public comment for this PRA package appeared in
the Federal Register on October 17, 2023 (88 FR 71573), and the 30-day notice for public
comment appeared in the Federal Register on January 16, 2024 (89 FR 2622). Since this
rulemaking is not proposing any new or revised CAHPS for MIPS survey requirements or
burden for this ICR, we are not proposing any changes for this ICR under that control number.
(9) Group Registration for CAHPS for MIPS Survey
We are not proposing any new or revised collection of information requirements or
burden related to group registration for the CAHPS for MIPS Survey for the CY 2025
performance period/2027 MIPS payment year. We note that we proposed updates to our
estimated burden under control number 0938-1222 (CMS-10450) as discussed in the CY 2024
PFS final rule (88 FR 79447 and 79448), which were submitted under the standard non-rule PRA
package. The 60-day notice for this PRA package appeared in the Federal Register on
October 17, 2023 (88 FR 71573), while the 30-day notice appeared on January 16, 2024
(89 FR 2622). Since this rulemaking is not proposing any new or revised CAHPS for MIPS
survey requirements or burden for this ICR, we are not proposing any changes for this ICR under
that control number.
f. ICRs Regarding the Call for MIPS Quality Measures
In this section of this proposed rule, we identify proposed adjustments to the estimated
burden for the Call for MIPS Quality Measures ICR, that will be submitted to OMB for review
under control number 0938–1314 (CMS–10621). These proposed adjustments are summarized
in Tables 78 and 79. We are not proposing any new or revised collection of information
requirements related to the call for MIPS quality measures. However, based on quality measure
submissions received for CMS’ consideration during the 2023 MIPS Annual Call for Quality
Measures, we are adjusting our burden estimates for the CY 2025 performance period/2027
MIPS payment year. In the CY 2024 PFS proposed rule (88 FR 52662) and the CY 2024 PFS
final rule (88 FR 79448), we inadvertently noted we that we derived our estimates of 31
responses from submissions during the 2023 MIPS Annual Call for Quality Measures; we
derived these estimated response from data for the 2022 MIPS Annual Call for Quality
Measures. The proposed estimates in this proposed rule reflect submission changes from the

2022 and 2023 MIPS Annual Call for Quality Measures, and do not reflect proposed policies or
statute changes in this proposed rule.
In this proposed rule, we estimate that we will receive 16 quality measure submissions
during the 2025 MIPS Annual Call for Quality Measures, a decrease of 15 from the currently
approved number of quality measure submissions for consideration (88 FR 79448 and 79449).
We are not proposing any changes to the 5.5 hour (2.4 hr for practice administrator + 3.1 hr for
clinician) per response time estimate for quality measure submissions.
In Table 107, we estimate an annual burden of 88 hours (16 measure submissions × 5.5
hr/measure) at a cost of $19,430 (16 measure submissions x $1,214.35/submission for the
CY 2025 performance period/2027 MIPS payment year).
TABLE 107: Estimated Burden for Call for Quality Measures
Burden and Respondent Descriptions
Burden Estimate
# of New Quality Measures Submitted for Consideration (a)
16
# of Hours per Practice Administrator to Identify, Propose and Link Measure (b)
2.4
# of Hours per Clinician to Identify and Link Measure (c)
1.1
# of Hours per Clinician to Complete Peer Review Article Form (d)
2
Annual Hours Per Response (e) = (b) + (c) + (d)
5.5
Total Annual Hours (f)=(a)×(e)
88
Cost to Identify and Submit Measure (at Medical and Health Services Manager’s Labor Rate
of $129.28/hr) (g) = $129.28/hr × (b)
$310.27
Cost to Identify Quality Measure and Complete Peer Review Article Form (at Clinician’s
Labor Rate of $291.64/hr) (h) = $291.64/hr × [(c) + (d)]
$904.08
Total Annual Cost Per Submitted Measure (i) = (g) + (h)
$1,214.35
Total Annual Cost (j)=(a)×(i)
$19,430
In Table 108, we illustrate the net change in estimated burden for the call for quality
measures using the currently approved burden in the CY 2024 PFS final rule
(88 FR 79448 and 79449). In aggregate, the estimated decrease in the number of quality
measure submissions will result in an adjustment of -83 hours (-15 measure submissions x 5.5
hr/measure submission) at a cost of -$18,215 (-15 measure submissions x $1,214.35/measure
submission) for the CY 2025 performance period/2027 MIPS payment year.

TABLE 108: Change in Estimated Burden for Call for Quality Measures
Burden and Respondent Descriptions
Burden Estimate
Total Currently Approved Annual Hours for Respondents (a)
171
Total Annual Hours for Respondents in CY 2025 PFS Proposed
Rule (b) (See Table 107, row (f))
88
Difference in Annual Hours (c) = (b) − (a)
-83
Total Currently Approved Annual Cost for Respondents (d)
$37,645
Total Annual Cost for Respondents in CY 2025 PFS Proposed Rule
(e) (See Table 107, row (j))
$19,430
Difference in Annual Cost (f) = (e) − (d)
-$18,215
g. ICRs Regarding Promoting Interoperability Data (§§ 414.1375 and 414.1380)
(1) Background
For the CY 2025 performance period/2027 MIPS payment year, MIPS eligible clinicians,
groups, subgroups, and APM Entities can submit Promoting Interoperability performance
category data through direct log in and upload or log in and attest submission types. We note
that the log in and attest submission type is only available for the Promoting Interoperability
performance category and is not available for the quality performance category. With the
exception of submitters who elect to use the log in and attest submission type for the Promoting
Interoperability performance category, we anticipate that MIPS eligible individual clinicians,
groups, subgroups, and APM Entities will use the same data submission type for both the quality
and Promoting Interoperability performance categories and that the clinicians, practice managers,
and computer systems analysts involved in supporting the quality data submission will also
support the Promoting Interoperability data submission process. The following burden estimates
show only incremental hours required above and beyond the time already accounted for in the
quality data submission process. We note that this analysis assesses burden by performance
category and submission type and emphasizes that MIPS is a consolidated program. We
analyzed data submitted by MIPS eligible clinicians, groups, subgroups and APM Entities, and
assessed clinician performance based on all the four MIPS performance categories, as applicable.
(2) Reweighting Applications for MIPS Performance Categories
The following proposed changes will be submitted to OMB for review under control
number 0938-1314 (CMS-10621).
We refer readers to the CY 2017 Quality Payment Program final rule
(81 FR 77240 through 77243), CY 2018 Quality Payment Program final rule
(82 FR 53918 and 53919), and the CY 2019, CY 2020, CY 2021, CY 2022, CY 2023, and
CY 2024 PFS final rules (83 FR 60011 and 60012, 84 FR 63134 and 63135,
85 FR 84984 and 84985, 86 FR 65596 through 65598, 87 FR 70160 through 70162, and
88 FR 79449 and 97450, respectively) for our previously finalized requirements for, and our
analysis of the information collection and reporting burden associated with, reweighting
applications for the four MIPS performance categories.
As established in the CY 2017 and CY 2018 Quality Payment Program final rules, MIPS
eligible clinicians may submit an application requesting reweighting to zero percent for the
Promoting Interoperability, quality, cost, and/or improvement activities performance categories
under specific circumstances as set forth in § 414.1380(c)(2), including, but not limited to,
extreme and uncontrollable circumstances, significant hardship, or other exceptions
(81 FR 77240 through 77243, 82 FR 53680 through 53686, and 82 FR 53783 through 53785).
Respondents (MIPS eligible individual clinicians, groups, or APM Entities) who apply
for reweighting of the quality, cost, and/or improvement activities performance categories have
the option of applying for reweighting of the Promoting Interoperability performance category
on the same online form. We assume respondents applying for a reweighting of the Promoting
Interoperability performance category due to extreme and uncontrollable circumstances will also
request a reweighting of at least one of the other performance categories simultaneously and not
submit multiple reweighting applications.
In section IV.A.4.i.(2) of this proposed rule, we are proposing to adopt a new reweighting
policy at § 414.1380(c)(2)(i)(A)(10) and (c)(2)(i)(C)(12). Specifically, we are proposing that,
beginning with the CY 2024 performance period/2026 MIPS payment year, that we may

reweight one or more of the performance categories (that is, quality, improvement activities, or
Promoting Interoperability) where we determine, based on information submitted to us on or
before November 1st of the year preceding the relevant MIPS payment year, that data for a MIPS
eligible clinician are inaccessible or unable to be submitted due to circumstances outside of the
control of the clinician because the MIPS eligible clinician delegated submission of the data to
their third party intermediary, evidenced by a written agreement between the MIPS eligible
clinician and third party intermediary, and the third party intermediary did not submit the data for
the performance category(ies) on behalf of the MIPS eligible clinician in accordance with
applicable deadlines. Since MIPS eligible clinicians do not submit data separately for measures
in the cost performance category and we score cost measures based solely on Medicare claims
data, the proposed reweighting policy does not apply to the cost performance category. We also
are proposing that, to determine whether to apply reweighting to the affected performance
category(ies), we would consider: whether the MIPS eligible clinician knew or had reason to
know of the issue with its third party intermediary’s submission of the clinician’s data for the
performance category(ies); whether the MIPS eligible clinician took reasonable efforts to correct
the issue; and whether the issue between the MIPS eligible clinician and their third party
intermediary caused no data to be submitted for the performance category(ies) in accordance
with applicable deadlines. Because we believe these occurrences would be rare based on our
experience with related requests for reweighting, and the extent and source of documentation
provided to us for each event may vary considerably, we are not proposing any changes to our
currently approved burden estimates for this proposal. We refer readers to section
VII.E.17.e.(2)(e). of this proposed rule for additional discussion on these burden estimates.
Table 109 summarizes our analysis of the estimated burden, including for MIPS eligible
clinicians to apply for reweighting of one or more of the MIPS performance categories to zero
percent due to an extreme or controllable circumstance, significant hardship, or other exception
as provided in § 414.1380(c)(2)(i).

We are updating our burden estimates relevant to this ICR based on the number of
reweighting applications received for the CY 2023 performance period/2025 MIPS payment year
by January 2, 2024, that do not cite the public health emergency (PHE) for COVID-19 (PHE for
COVID-19) as the basis for reweighting. The Federal PHE for COVID-19 under section 319 of
the Public Health Service Act ended on May 11, 2023.
789
As a result of the end of the PHE,
MIPS eligible clinicians will no longer be able to submit a reweighting application citing
hardships from the PHE for COVID-19; therefore, we are excluding reweighting applications
citing the PHE for COVID-19 in our estimate for CY 2025 performance period/2027 MIPS
payment year reweighting applications. In this proposed rule, we estimate that we will receive a
total of 3,297 applications to request reweighting for any or all of the four MIPS performance
categories for the CY 2025 performance period/2027 MIPS payment year. Of the 3,297, we
estimate that 2,490 MIPS eligible clinicians or groups will submit a request that includes
reweighting the Promoting Interoperability performance category to zero percent due to a
significant hardship or other exception as provided in § 414.1380(c)(2)(i)(C). and we estimate
that 802 MIPS eligible clinicians or groups will submit a request to reweight one or more of the
MIPS performance categories as provided in § 414.1380(c)(2)(i). Additionally, we estimate 5
APM Entities will submit an extreme and uncontrollable circumstances exception application to
reweight one or more MIPS performance category for the CY 2025 performance period/2027
MIPS payment year. This adjustment, due to both updated data and the end of the PHE for
COVID-19, results in a decrease of 25,930 respondents compared to our currently approved
estimate of 29,227 respondents (88 FR 79449 and 79450). We note the currently approved
estimate included reweighting applications citing the PHE for COVID-19.
Consistent with our assumptions in the CY 2024 PFS final rule
(88 FR 79449 and 79450), we continue to estimate it will take 0.25 hours at $106.54/hr for a
computer system analyst to complete and submit the reweighting application. In Table 109, we
789
https://www.hhs.gov/coronavirus/covid-19-public-health-emergency/index.html.

estimate an annual burden of 824 hours (3,297 applications x 0.25 hr/application) at a cost of
$87,832 (3,297 applications × $26.64/application) for the CY 2025 performance period/2027
MIPS payment year.
TABLE 109: Estimated Burden for Reweighting Applications for MIPS Performance
Categories
Burden and Respondent Descriptions
Burden Estimate
# of Eligible Clinicians or Groups Applying Due to Significant Hardship and Other
Exceptions or Extreme and Uncontrollable Circumstances (a)
3,292
# APM Entities Requesting Extreme and Uncontrollable Circumstances Exception (b)
5
Total Applications Submitted (c)
3,297
Annual Hours Per Applicant per Application Submission (d)
0.25
Total Annual Hours (e) = (c) × (d)
824
Cost to Submit a Reweighting Application at Computer Systems Analyst’s Labor Rate of
$106.54/hr (f) = (d) × $106.54/hr (f)
$26.64
Total Annual Cost (g) = (c) × (f)
$87,832
In Table 110, we illustrate the net change in estimated burden for submission of
reweighting applications for MIPS performance categories using the currently approved burden
in the CY 2024 PFS final rule (88 FR 79449 and 79450). The adjustment in the estimated
number of respondents, from 29,227 to 3,297 respondents, results in a decrease of 25,930
respondents. In aggregate, using our currently approved per response time estimate, as shown in
Table 110, the decrease in 25,930 respondents results in an adjustment of -6,483 hours and -
$690,775 the CY 2025 performance period/2027 MIPS payment year.
TABLE 110: Change in Estimated Burden for Reweighting Applications for MIPS
Performance Categories
Burden and Respondent Descriptions
Burden Estimate
Total Currently Approved Annual Hours in CY 2024 PFS Final Rule (a)
7,307
Total Annual Hours for Respondents in CY 2025 PFS Proposed Rule (b) (See
Table 109, row (e))
824
Difference in Annual Hours (c) = (b) − (a)
-6,483
Total Currently Approved Annual Cost in CY 2024 PFS Final Rule (d)
$778,607
Total Annual Cost for Respondents in CY 2025 PFS Proposed Rule (e) (See
Table 109, row (g))
$87,832
Difference in Annual Cost (f) = (e) − (d)
-$690,775
(3) Submitting Promoting Interoperability Data

The following proposed changes relevant to the submission of Promoting Interoperability
data requirements and burden will be submitted to OMB for review under OMB under control
number 0938-1314 (CMS-10621).
We note that we adjusted the burden estimates from the currently approved CY 2024 PFS
final rule (88 FR 79451 through 79453) to incorporate updated available MIPS submission data
for the CY 2022 performance period/2024 MIPS payment year, as discussed in section
V.B.8.e.(3). of this proposed rule. We are not proposing changes based on the proposals
discussed in section IV.A. of this proposed rule. In the following paragraphs, we discuss these
proposed policies and our rationale for maintaining the currently approved burden for the
submission of Promoting Interoperability data requirements.
We refer readers to the CY 2017 and CY 2018 Quality Payment Program final rules
(81 FR 77509 through 77511, and 82 FR 53919 and 53920, respectively), and the CY 2019,
CY 2020, CY 2021, CY 2022, CY 2023, and CY 2024 PFS final rules (83 FR 60013 and 60014,
84 FR 63135 through 63137, 85 FR 84985 through 84987, 86 FR 65598 through 65600,
87 FR 70162 through 70164, and 88 FR 79451 through 79453, respectively) for our previously
finalized requirements and burden for submission of data for the Promoting Interoperability
performance category. We refer readers to § 414.1375 for our previously established policies
regarding reporting for the Promoting Interoperability performance category. We also refer
readers to § 414.1305 for the definition of attestation, § 414.1325 for data submission
requirements, and § 414.1380(b)(4) for Promoting Interoperability performance category
scoring. We refer readers to § 414.1380(c)(2)(i)(C) for our previously finalized policies
regarding scoring of data submission in the Promoting Interoperability performance category
after an approved reweighting for the performance category.
In section IV.A.4.d.(2)(d) of this proposed rule, we are proposing to adopt minimum
criteria for a qualifying data submission for the Promoting Interoperability performance category
at § 414.1325(a)(1)(iii). Currently, an incomplete data submission would void an approved
reweighting of the Promoting Interoperability performance category in accordance with §
414.1380(c)(2)(i)(C). This proposal would clarify what counts as a data submission for MIPS
eligible clinicians and it would potentially avoid partial data submissions from overriding an
approved reweighting or a previously scored submission for the Promoting Interoperability
performance category. Specifically, we propose that a qualifying data submission for the
Promoting Interoperability performance category must include all the following elements: (1)
performance data, including any claim of an applicable exclusion, for the measures in each
objective, as specified by CMS; (2) required attestation statements, as specified by CMS;
specified by CMS; (3) CMS EHR Certification ID (CEHRT ID) from the Certified Health IT
Product List (CHPL); and (4) the start date and end date for the applicable performance period as
set forth in § 414.1320. If we receive a qualifying data submission meeting the proposed
minimum criteria for reporting, then we will review the data submission and score the Promoting
Interoperability performance category in accordance with our applicable scoring policies. We
refer readers to section IV.A.4.d.(2)(d) of this proposed rule for additional background and
details on this proposal. We are not proposing any changes to the existing scoring or
reweighting policies described under § 414.1380 for the MIPS performance categories in this
section IV.A.4.d. Our current estimates assume that PI submissions include all requirements for
scoring; therefore, we are not adjusting our established per response time estimate.
In section IV.A.4.d.(3)(c). of this proposed rule, we are also proposing to modify our
policy governing our treatment of multiple data submissions received for the Promoting
Interoperability performance category, which we propose to codify at § 414.1325(f)(2).
Specifically, we are proposing that, in cases where CMS receives multiple submissions for the
Promoting Interoperability performance category, CMS would calculate a score for each data
submission received and assign the highest of the scores.
In our analysis of the information collection and reporting burden, we are not adjusting
our estimated number of respondents submitting Promoting Interoperability data. These

proposals intend to significantly reduce certain issues with the scoring of unintended data
submissions affecting MIPS payment adjustments for individual MIPS eligible clinicians,
groups, virtual groups, subgroups, and APM Entities. These two proposals may limit the
unintentional overriding of an approved reweighting or an existing scoreable submission. Our
currently approved per response estimates incorporate the required measures and attestations and
other required data elements identified in sections IV.A.4.d.(2)(d). and IV.A.4.d.(3)(c). of this
proposed rule.
In the CY 2022 PFS final rule, we finalized at § 414.1365(c)(4)(i) that an MVP
Participant is required to meet the Promoting Interoperability reporting requirements. We also
finalized at § 414.1365(c)(4)(i)(A) the requirements for a subgroup participating in MVP
reporting (86 FR 65413 and 65414). Specifically, we stated that for the CY 2023 and 2024
MIPS performance periods/2025 and 2026 MIPS payment years, an MVP Participant that is a
subgroup is required to submit its affiliated group’s data for the Promoting Interoperability
performance category. The submission of the affiliated group’s data will be on the subgroup’s
behalf. If the affiliated group chooses to report as a group for the Promoting Interoperability
performance category, the group will still be required to submit its own data separately and in
accordance with the reporting rules for groups. In section IV.A.4.b.(4). of this rulemaking, we
are proposing to modify § 414.1365(c)(4)(i)(A) by removing the references to the specific MIPS
performance periods/payment years to state that an MVP Participant that is a subgroup is
required to submit its affiliated group's data for the Promoting Interoperability performance
category. The proposed change would allow a subgroup to submit the affiliated group’s data for
the MVP Promoting Interoperability performance category for the CY 2025 performance
period/2027 MIPS payment year and beyond. As this proposal would not create new reporting
requirements, there are no burden implications for this proposal.
The Department of Health and Human Services (HHS) final rule, 21st Century Cures
Act: Establishment of Disincentives for Health Care Providers That Have Committed

Information Blocking (hereafter referred to as the Disincentives final rule) was released on June
24, 2024 (https://www.healthit.gov/topic/information-blocking#Disincentives). Section
IV.A.4.e.(4)(d). of this proposed rule summarizes several policies in the Disincentives final rule
under which a MIPS eligible clinician that the Office of Inspector General determines has
committed information blocking would not be a meaningful EHR user, and therefore would be
unable to earn a score (instead earning a score of zero) for the Promoting Interoperability
performance category. We note the Disincentives final rule described in section IV.A.4.e.(4)(d).
of this proposed rule would not create any additional reporting, recordkeeping, or third party
disclosure requirements. Consequently, we are not proposing any updates in this proposed rule.
In sections IV.A.4.e (4)(e)(iii) of this proposed rule, we note that we recently released the
CMS Interoperability and Prior Authorization final rule which appeared in the Federal Register
on February 8, 2024 (89 FR 8758). In the CMS Interoperability and Prior Authorization final
rule final rule, we finalized the addition of a new measure, the “Electronic Prior Authorization”
measure, under the Health Information Exchange (HIE) objective for the MIPS Promoting
Interoperability performance category beginning with the CY 2027 performance period/2029
MIPS payment year (89 FR 8909 through 8927). The burden estimate for MIPS clinicians to
report the “Electronic Prior Authorization measure” was provided in the CMS Interoperability
and Prior Authorization final rule (89 FR 8953 through 8956). In the CMS Interoperability and
Prior Authorization final rule final rule, we identified that this measure will be included in a PRA
package related to the CMS Interoperability and Prior Authorization final rule (89 FR 8946).
Consequently, we are not proposing any updates in this proposed rule.
Due to the availability of updated data on the Promoting Interoperability submissions
during the CY 2022 performance period/2024 MIPS payment year, we are adjusting our
currently approved estimated burden for the submission of data in Promoting Interoperability
performance category (88 FR 79451 and 79452). In Table 111, we estimate that a total number
of 18,609 respondents, consisting of 14,500 individual MIPS eligible clinicians, 4,089 groups

and virtual groups, and 20 subgroups will submit data for the Promoting Interoperability
performance category in the CY 2025 performance period/2027 MIPS payment year.
As noted in section V.B.8.a.(1)(a) of this proposed rule, we have not updated our Quality
Payment Program burden estimates to reflect MIPS Promoting Interoperability reporting
requirements of non-MIPS eligible clinicians due to requirements for the Shared Savings
Program. For MIPS eligible clinicians participating in an APM, we continue our assumption
from the CY 2023 PFS final rule (87 FR 70163) and CY 2024 PFS final rule (88 FR 79451) that
each MIPS eligible clinician in an APM Entity reports data for the Promoting Interoperability
performance category through either their group TIN or individual reporting in the CY 2019 PFS
final rule, we established that MIPS eligible clinicians who participate in the Shared Savings
Program are no longer limited to reporting for the Promoting Interoperability performance
category through their ACO participant TIN (83 FR 59822 and 59823). Burden estimates for
this proposed rule assume group TIN-level reporting as we believe this is the most reasonable
assumption for MIPS eligible clinicians in the Shared Savings Program, which requires that
ACOs include full TINs as ACO participants. Accordingly, we assume that any Promoting
Interoperability data submitted at the APM-Entity level adheres to APM or Shared Savings
Program requirements. Sections 1899 and 1115A of the Act (42 U.S.C. 1395jjj and 42 U.S.C.
1315a, respectively) state that the Shared Savings Program and the testing, evaluation, and
expansion of Innovation Center models are not subject to the PRA.
TABLE 111: Estimated Number of Respondents to Submit Promoting
Interoperability Performance Data
Burden and Respondent Descriptions
# of Respondents
# of Individual Clinicians to Submit Promoting Interoperability in CY 2025 Performance
Period (a)
14,500
# of Groups to Submit Promoting Interoperability in CY 2025 Performance Period (b)
4,089
# of Subgroups to Submit Promoting Interoperability in MVPs during the CY 2025
Performance Period (c)
20
Total Respondents in CY 2025 Performance Period (d) = (a) + (b) + (c)
18,609
Currently Approved Respondents (2024 PFS Final Rule) (e)
25,990
Difference in # of Respondents (f) = (d) – (e)
-7,381

As shown in Table 112, we are continuing our currently approved estimated time of 2.70
hours per response. Therefore, we estimate that it will result in a total burden of 50,244 hours
(18,609 respondents x 2.70 incremental hours for a computer analyst’s time above and beyond
the physician, medical and health services manager, and computer system’s analyst time required
to submit quality data) and $5,353,065 (18,609 responses × $287.66/response) to submit data for
the Promoting Interoperability performance category in the CY 2025 performance period/2027
MIPS payment year.
TABLE 112: Estimated Burden for Promoting Interoperability Performance Category
Data Submission
Burden and Respondent Description
Burden Estimate
Number of Individual Clinicians to Submit Promoting Interoperability (a)
14,500
Number of Groups to Submit Promoting Interoperability (b)
4,089
Number of Subgroups to Submit Promoting Interoperability (c)
20
Total Respondents (d) = (a) + (b) + (c)
18,609
Annual Hours Per Respondent (e)
2.70
Total Annual Hours (f) = (d) × (e)
50,244
Cost per Respondent to Submit Promoting Interoperability Data at Computer System
Analyst’s Labor Rate $106.54 (g) = (e) × $106.54/hr (g)
$287.66
Total Annual Cost (h) = (d) × (g)
$5,353,065
In Table 113, we illustrate the change in burden for clinicians to submit data in the
Promoting Interoperability performance category for the CY 2025 performance period/2027
MIPS payment year. In aggregate, we estimate that the decrease in the number of respondents
from 25,990 to 18,609 will result in an adjustment of -19,929 hours (-7,381 respondents x 2.70
hr/response) and -$2,123,218.
TABLE 113: Change in Estimated Burden for Promoting Interoperability Performance
Category Data Submission
Burden and Respondent Description
Burden Estimate
Total Currently Approved Annual Hours (a)
70,173
Total Annual Hours for Respondents in CY 2025 PFS Proposed Rule (b) (see
Table 112, row (f))
50,244
Difference in Annual Hours (c) = (b) − (a)
-19,929
Total Currently Approved Annual Cost (d)
$7,476,283
Total Annual Cost for Respondents in CY 2025 PFS Proposed Rule (e) (see
Table 112, row (h))
$5,353,065

Burden and Respondent Description
Burden Estimate
Difference in Annual Cost (f) = (e) − (d)
-$2,123,218
h. ICRs Regarding Improvement Activities Submission (§§ 414.1305, 414.1355, 414.1360, and
414.1365)
The following proposed changes will be submitted to OMB for review under control
number 0938–1314 (CMS–10621)
We are not proposing changes due to the improvement activities proposals discussed in
section IV.A. of this proposed rule. In the following paragraphs, we discuss these proposed
policies and our reasons for not changing the currently approved per response burden for
improvement activities submission.
In section IV.A.4.e.(3)(b)(iv) of this proposed rule, we are proposing two scoring and
reporting policy changes for the improvement activities performance category effective for the
CY 2025 performance period/2027 MIPS payment year and subsequent years. First, we are
proposing to eliminate the weighting of improvement activities. In the CY 2017 Quality
Payment Program final rule, we established a differentially weighted model for the improvement
activities performance category with two categories, medium and high, to provide flexible
scoring (81 FR 28210). In that rule (81 FR 77177 and 77178), we codified at § 414.1380(b)(3)
that clinicians (except for non-patient facing MIPS eligible clinicians, small practices, and
practices located in rural areas and geographic health professional shortage areas (HPSAs))
receive 10 points for each medium-weighted improvement activity and 20 points for each high-
weighted improvement activity. Non-patient facing MIPS eligible clinicians, small practices,
and practices located in rural areas and geographic HPSAs receive 20 points for each medium-
weighted improvement activity and 40 points for each high-weighted improvement activity.
Second, we are proposing to further simplify improvement activity reporting
requirements by reducing the number of activities to which clinicians are required to attest to
achieve a score in the improvement activities performance category, beginning in the CY 2025

performance period/2027 MIPS payment year. Currently, MIPS eligible clinicians are required
to report two high-weighted activities, four medium-weighted activities, or one high-weighted
and two medium-weighted activities while MVP participants are currently required to report one
high-weighted activity or two medium-weighted activities. We are proposing that MIPS eligible
clinicians who participate in traditional MIPS would be required to report two activities and
MVP participants would be required to report one activity to achieve 40 points, or full credit. In
addition, we are proposing that MIPS eligible clinicians who are categorized as small practice,
rural, in a provider-shortage area, or non-patient facing would now be required to report one
activity (for either traditional MIPS or MVPs).
We established our currently approved estimate that it will take a computer analyst 5
minutes to log in and manually attest that improvement activities were completed in the CY 2019
PFS final rule (83 FR 60016). We believe the proposed removal of weighting for improvement
activities will decrease burden for MIPS eligible clinicians who previously reported medium-
weighted activities. As MIPS eligible clinicians who previously only reported high-weighted
activities will have the same attestation requirements under this proposal, we are not proposing a
change to our currently estimated per response burden. We refer readers to section
VII.E.17.e.(2)(c). of this proposed rule where we discuss our impact analysis for this proposal.
In section IV.A.4.e.(3)(b)(iii) of this rulemaking, we are also proposing changes to the
improvement activities inventory for the CY 2025 performance period/2027 MIPS payment year
and future years as follows: adding two new improvement activities; modifying two existing
improvement activities; and removing eight previously adopted improvement activities. In the
CY 2023 PFS final rule (87 FR 70211) and the 2024 PFS final rule (88 FR 79519), we
anticipated that most clinicians performing improvement activities, to comply with existing
MIPS policies, will continue to perform the same activities because previously finalized
improvement activities continue to apply for the current and future years unless otherwise
modified per rulemaking (82 FR 54175). We believe this proposal will not significantly affect

burden because the majority of activities are not revised. We refer readers to section
VII.E.17.e.(2)(b) of this proposed rule where we discuss our impact analysis for this proposal,
and section VII.E.17.e.(2)(c) where we discuss the impact analysis for modifications to
modifications to improvement activities scoring and reporting policies. As discussed in section
IV.A.4.d.(2)(c) in this proposed rule, we previously finalized at § 414.1360(a)(2) that MIPS
eligible clinicians, groups, virtual groups, or subgroups must submit a yes response for each
improvement activity that is performed for at least a continuous 90-day period during the
applicable performance period to receive points in the improvement activities performance
category described under §414.1360(b)(3). We currently assign a score for any submission or
attestation received in the improvement activities performance category via the submission types
described under § 414.1325(a)(1) regardless of whether the submission or attestation included a
yes response or not. Several MIPS eligible clinicians have notified us that there have been
instances where they unintentionally submitted non-scorable data for a MIPS performance
category, which overrode an approved reweighting or a previously scorable data submission for a
performance categories. In the event of a submission without yes responses, we currently assign
a score of zero. In section IV.A.4.d.(2)(c). of this proposed rule, we are proposing to adopt
minimum criteria for a qualifying data submission for a performance period for the improvement
activities performance category. We are proposing to specify what we consider to be a data
submission at § 414.1325(a)(1)(ii) to state that for the improvement activities performance
category, a data submission must include a response of “yes” for at least one activity in the MIPS
improvement activities inventory. We anticipate the proposed change would potentially avoid
unintentional overriding of an approved reweighting or a prior data submission for the
improvement activities performance category due to submissions or attestations without a
response of “yes” for any of the improvement activities. We note that we are not proposing any
changes to the data submission criteria and scoring for the improvement activities performance
category described under §§ 414.1360 and 414.1380(b)(3) respectively.

Additionally, we are proposing to codify our existing policies governing multiple data
submissions received for the improvement activities performance category at § 414.1325(f)(1).
We refer readers to section IV.A.4.d.(3)(b) of this proposed rule for details. These proposals, if
finalized, would not affect the requirements for MIPS eligible clinicians and groups that submit
data for the improvement activities performance category. We assume these proposals would not
affect the number improvement activities submissions, as the intent is to eliminate certain issues
with the scoring of an unintended data submission affecting payment adjustments for individual
MIPS eligible clinicians, groups, virtual groups, subgroups, and APM Entities. Therefore, we
are not proposing any adjustments to our currently approved estimated burden due to these
policy proposals.
As shown in Table 114, we are adjusting the currently approved burden estimates
(88 FR 79454 and 79455) due to available updated submission data for the CY 2022
performance period/2024 MIPS payment year, as discussed in section V.B.8.e.(3). of this
proposed rule. As identified in Table 79, the proposed change in burden reflects adjustments for
updated data, rather than proposed requirements or statues in this proposed rule. We estimate
that a total of 38,433 respondents consisting of 29,017 individual clinicians, 9,396 groups and 20
subgroups will submit improvement activities during the CY 2025 performance period/2027
MIPS payment year. This adjustment represents a decrease of 11,856 respondents from the
currently approved estimate of 50,289 respondents in the CY 2024 PFS final rule
(88 FR 79454 and 79455). We did not include in our estimates clinicians who participated in an
APM Entity and are determined to be QPs for the CY 2022 performance period/2024 MIPS
payment year as we assume they are not required to submit improvement activities data.
TABLE 114: Estimated Number of Respondents to Submit Improvement Activities Data
Burden and Respondent Descriptions
Count
# of Clinicians to Participate in Improvement Activities Data Submission as During the
CY 2025 Performance Period (a)
29,017
# of Groups to Submit Improvement Activities on Behalf of During the CY 2025 Performance
Period (b)
9,396

Burden and Respondent Descriptions
Count
# of Subgroups to Submit Improvement Activities in MVPs During the CY 2025 Performance
Period (c)
20
Total # of Respondents (Groups, Subgroups, Virtual Groups, and Individual Clinicians)
to Submit Improvement Activities Data During the CY 2025 Performance Period (d) =
(a) + (b) + (c)
38,433
Total # of Currently Approved Respondents (Groups, Virtual Groups, and Individual
Clinicians) to Submit Improvement Activities Data (CY 2024 PFS Final Rule) (e)
50,289
Difference in # of Respondents (f) = (d) − (e)
-11,856
In Table 115, we continue to estimate that the time required per response per individual
or group is 5 minutes or 0.083 hours for a computer system analyst at a labor rate of $106.54/hr
to submit by logging in and manually attesting that certain activities were performed in the form
and manner specified by CMS with a set of authenticated credentials. Therefore, we estimate an
annual burden of 3,190 hours (38,433 respondents x 0.083 hr/response) at a cost of $339,748
(38,433 respondents x $8.84/response) for the CY 2025 performance period/2027 MIPS payment
year.
TABLE 115: Estimated Burden for Improvement Activities Data Submission
Burden and Respondent Descriptions
Burden Estimate
Total # of Respondents (Groups, Subgroups, Virtual Groups, and Individual Clinicians) to
Submit Improvement Activities Data on Behalf of Clinicians During the CY 2025
Performance Period (a)
38,433
Total Annual Hours Per Respondent (b)
0.083
Total Annual Hours (c) = (a) × (b)
3,190
Cost per Respondent to Submit Improvement Activities Data at Computer System Analyst’s
Labor Rate of $106.54/hr (d) = (b) × $106.54/hr
$8.84
Total Annual Cost (e) = (a) × (d)
$339,748
As shown in Table 116, using our unchanged currently approved per respondent burden
estimate, the decrease in the number of respondents results in an adjustment of -984 hours and -
$104,807 for the CY 2025 performance period/2027 MIPS payment year.
TABLE 116: Change in Estimated Burden for Improvement Activities Submission
Burden and Respondent Descriptions
Burden Estimate
Total Currently Approved Annual Hours (a)
4,174
Total Annual Hours for Respondents in CY 2025 PFS Proposed Rule (b) (See Table
115, row (c))
3,190
Difference in Annual Hours (c) = (b) − (a)
-984

Burden and Respondent Descriptions
Burden Estimate
Total Currently Approved Annual Cost (d)
$444,555
Total Annual Cost for Respondents in CY 2025 PFS Proposed Rule (e) (See Table 115,
row (e))
$339,748
Difference in Annual Cost (f) = (e) − (d)
-$104,807
i. ICRs Regarding the Nomination of Improvement Activities (§ 414.1360)
In this rulemaking, we are not proposing any new or revised collection of information
requirements or burden related to the Nomination of Improvement Activities for the CY 2025
performance period/2027 MIPS payment year. The requirements and burden associated with this
information collection are currently approved by OMB under control number 0938–1314 (CMS–
10621). Consequently, we are not proposing any changes to the Nomination of Improvement
Activities under that control number.
j. ICRs Regarding the Nomination of MVPs
In section IV.A.4.a.(2) of this proposed rule, we are proposing a modification to the MVP
maintenance webinar process previously finalized in the CY 2022 PFS final rule (86 FR 65410)
and modified in the CY 2023 PFS final rule (87 FR 70037). Specifically, we are proposing to
modify the MVP maintenance webinar process to provide us more flexibility in how we
communicate submitted maintenance recommendations prior to proposing them formally in
rulemaking. We believe this flexibility in communicating recommendations through alternative
webinar formats or other public communication channels would offer similar opportunities for
public review and feedback as a live public webinar. As this proposal would not require
additional steps to the MVP nomination process described in the CY 2021 PFS final rule
(85 FR 84990 and 84991), we are continuing our currently approved 12 hours per response
burden estimate for the CY 2025 performance period/2027 MIPS payment year. We refer
readers to section VII.E.17.e.(2)(d). of this proposed rule where we discuss our impact analysis
for this proposal.
The requirements and burden for the nomination of MVPs are currently approved by

OMB under control number 0938-1314 (CMS-10621). Since the proposed change does not set
out any new or revised requirements or burden outside of providing CMS with flexibility in how
we communicate, we are not proposing any changes to the nomination of MVPs under that
control number.
k. ICRs Regarding the Cost Performance Category (§ 414.1350)
The cost performance category relies on administrative claims data. The Medicare Parts
A and B claims submission process (OMB control number 0938-1197; CMS-1500 and CMS-
1490S) is used to collect data on cost measures from MIPS eligible clinicians. MIPS eligible
clinicians are not required to provide any documentation by CD or hardcopy. Moreover, the
following proposals in section IV.A.4.e.(2). of this proposed rule would not result in the need to
add or revise or delete any claims data fields: (1) add 6 new episode-based cost measures; (2)
modify 2 existing episode-based measures; (3) update the operational list of care episode and
patient condition groups and codes to reflect new and modified measures we are proposing; (4)
and adopt criteria to specify objective bases for the removal of any cost measures from the MIPS
cost performance category. Consequently, we are not proposing any changes under the
aforementioned control number. We refer readers to section IV.A.4.e.(2).for details on these
proposals.
l. ICRs Regarding Partial QP Elections (§§ 414.1310(b) and 414.1430)
We are not proposing any new or revised collection of information requirements or
related to the Partial QP Elections to participate in MIPS as a MIPS eligible clinician in the
CY 2025 performance period/2027 MIPS payment year. In this section of this proposed rule, we
identify proposed adjustments to the estimated burden for partial QP elections that will be
submitted to OMB for review under control number 0938–1314 (CMS–10621), as summarized
in Tables 78 and 79. These proposed adjustments to estimated burden for the CY 2025
performance period/2027 MIPS payment year are due to the availability of updated data, rather
than proposed policies or statute changes in section IV.A.4. of this proposed rule.

Based on the number of QP elections submitted for the CY 2023 performance
period/2025 MIPS payment year, we estimate in this proposed rule that we will receive a total of
18 partial QP elections from 18 APM respondents (representing 333 distinct national provider
identifiers (NPIs) and 363 distinct TIN/NPIs). We do not estimate any partial QP elections at the
eligible clinician level, as no individual eligible clinicians elected to report as partial QPs for the
CY 2023 performance period/2025 MIPS payment year. This estimate is a decrease of 269
submissions from the currently approved estimate of 287 (87 FR 70167 and 70168). We
continue to estimate it will take 0.25 hours for a computer system analyst or equivalent to
complete and submit the election process.
In Table 117, we estimate an annual burden of 5 hours (18 responses x 0.25 hr/response)
at a cost of $480 (18 responses x $26.64/response) for the CY 2025 performance period/2027
MIPS payment year.
TABLE 117: Estimated Burden for Partial QP Elections
Burden and Respondent Descriptions
Burden Estimate
Total # of Respondents Making Partial QP Election (18 APM Entities, 0 Eligible Clinicians)
(a)
18
Total Annual Hours per Other Payer Arrangement (b)
0.25
Total Annual Hours (c) = (a) × (b)
5
Cost per Respondent at Computer System Analyst’s Labor Rate of $106.54/hr (d) = (b) ×
$106.54/hr
$26.64
Total Annual Cost (e) = (a) × (d)
$480
In Table 118, we illustrate the net change in estimated burden for the Partial QP Election
Process using the currently approved burden in the CY 2023 PFS final rule
(87 FR 70167 and 70168). In aggregate, the estimated change in responses will result in an
adjustment of –67 hours and -$7,166 for the CY 2025 performance period/2027 MIPS payment
year.

TABLE 118: Change in Estimated Burden for Partial QP Elections
Burden and Respondent Descriptions
Burden Estimate
Total Currently Approved Annual Hours for Respondents (a)
72
Total Annual Hours for Respondents in CY 2025 PFS Proposed Rule (b) (See
Table 117, row (c))
5
Difference in Annual Hours (c) = (b) − (a)
-67
Total Currently Approved Annual Cost for Respondents (d)
$7,646
Total Annual Cost for Respondents in CY 2025 PFS Proposed Rule (e) (See
Table 117, row (e))
$480
Difference in Annual Cost (f) = (e) − (d)
-$7,166
m. ICRs Regarding Other Payer Advanced APM Determinations: Payer-Initiated Process
(§ 414.1445) and Eligible Clinician -Initiated Process (§ 414.1445)
In this section, we identify proposed adjustments to the estimate burden for ICRs
regarding other payer advanced APM determinations. These proposed adjustments, summarized
in Tables 78 and 79, reflect the availability of updated data rather than proposed policies or
statutory requirements, and will be submitted to OMB for review under control number 0938-
1314 (CMS-10621).
(1) Payer-Initiated Process (§ 414.1445)
We are not proposing any new or revised collection of information requirements related
to the Payer-Initiated Process for the CY 2025 performance period/2027 MIPS payment year.
Due to declining requests in recent years, we are adjusting our burden estimates for the CY 2025
performance period/2027 MIPS payment year. In this proposed rule, we estimate that we will
receive 10 submissions for the Payer-Initiated Process for the CY 2025 performance period/2027
MIPS payment year, a decrease of 5 submissions from the currently approved estimate of 15
responses (86 FR 65607 and 65608). We continue to estimate it will take 10 hours for a
computer system analyst per arrangement.
In Table 119, we estimate an annual burden of 100 hours (10 responses x 10 hr/response)
at a cost of $10,654 (10 responses x $1,065.40/response) for the CY 2025 performance
period/2027 MIPS payment year.

TABLE 119: Estimated Burden for Other Payer Advanced APM Identification
Determinations: Payer-Initiated Process
Burden and Respondent Descriptions
Burden Estimate
Total # of Other Payer Arrangements (2 Medicaid, 6 Medicare Advantage Organizations, 2
Remaining Other Payers) (a)
10
Total Annual Hours per Other Payer Arrangement (b)
10
Total Annual Hours (c) = (a) × (b)
100
Cost per Respondent at Computer System Analyst’s Labor Rate of $106.54/hr (d) = (b) ×
$106.54/hr
$
$1,065.40
Total Annual Cost (e) = (a) × (d)
$10,654
In Table 120, we illustrate the net change in estimated burden for the Payer-Initiated
Process using the currently approved responses in the CY 2022 PFS final rule
(86 FR 65606 and 65607). In aggregate, the estimated change in the number of responses will
result in an adjustment of –50 hours and -$5,327 for the CY 2025 performance period/2027
MIPS payment year.
TABLE 120: Change in Estimated Burden for Other Payer Advanced APM
Identification Determinations: Payer-Initiated Process
Burden and Respondent Descriptions
Burden Estimate
Total Currently Approved Annual Hours for Respondents (a)
150
Total Annual Hours for Respondents in CY 2025 PFS Proposed
Rule (b) (See Table 119, row (c))
100
Difference in Annual Hours (c) = (b) − (a)
-50
Total Currently Approved Annual Cost for Respondents (d)
$15,981
Total Annual Cost for Respondents in CY 2025 PFS Proposed Rule
(e) (See Table 119, row (e))
$10,654
Difference in Annual Cost (f) = (e) − (d)
-$5,327
(2) Eligible Clinician-Initiated Process (§ 414.1445)
We are not proposing any new or revised collection of information requirements related
to the Eligible Clinician-Initiated Process for the CY 2025 performance period/2027 MIPS
payment year. Due to declining requests in recent years, we are adjusting our burden estimates
for the CY 2025 performance period/2027 MIPS payment year. In this proposed rule, we
estimate that we will receive 10 submissions for the Eligible Clinician-Initiated Process for the
CY 2025 performance period/2027 MIPS payment year, a decrease of 5 submissions from the

currently approved estimate of 15 (86 FR 65607 and 65608). We continue to estimate it will
take 10 hours for a computer system analyst to complete and submit the payment arrangement.
In Table 121, we estimate an annual burden of 100 hours (10 responses x 10 hr/response)
at a cost of $10,654 (10 responses x $1,065.40/response) for the CY 2025 performance
period/2027 MIPS payment year.
TABLE 121: Estimated Burden for Other Payer Advanced APM Identification
Determinations: Eligible Clinician-Initiated Process
Burden and Respondent Descriptions
Burden Estimate
Total # of Other Eligible Clinician-Initiated Arrangements (a)
10
Total Annual Hours per Other Payer Arrangement (b)
10
Total Annual Hours (c) = (a) × (b)
100
Cost per Respondent at Computer System Analyst’s Labor Rate of $106.54/hr (d) = (b) ×
$106.54/hr
$1,065.40
Total Annual Cost (e) = (a) × (d)
$10,654
In Table 122, we illustrate the net change in estimated burden for the Eligible Clinician-
Initiated Process using the currently approved responses in the CY 2022 PFS final rule
(86 FR 65607 and 65608). In aggregate, the estimated change in the number of responses will
result in an adjustment of –50 hours and -$5,327 for the CY 2025 performance period/2027
MIPS payment year.
TABLE 122: Change in Estimated Burden for Other Payer Advanced APM
Identification Determinations: Clinician-Initiated Process
Burden and Respondent Descriptions
Burden Estimate
Total Currently Approved Annual Hours for Respondents (a)
150
Total Annual Hours for Respondents in CY 2025 PFS Proposed Rule (b) (See Table
121, row (c))
100
Difference in Annual Hours (c) = (b) − (a)
-50
Total Currently Approved Annual Cost for Respondents (d)
$15,981
Total Annual Cost for Respondents in CY 2025 PFS Proposed Rule (e) (See Table
121, row (e))
$10,654
Difference in Annual Cost (f) = (e) − (d)
-$5,327
(3) Submission of Data for QP Determinations under the All-Payer Combination Option
(§ 414.1440)

We are not proposing any new or revised collection of information requirements related
to the Submission of Data for QP Determinations under the All-Payer Combination Option for
the CY 2025 performance period/2027 MIPS payment year. In the past few years, we have
observed a significant drop in the number of submissions from payment arrangements from
outside parties. Therefore, we are adjusting our estimates downward for the CY 2025
performance period/2027 MIPS payment year. In this proposed rule, we estimate that 10 APM
Entities, 100 TINs, and 10 eligible clinicians will submit data for QP determinations under the
All-Payer Combination Option in CY 2025 performance period/2027 MIPS payment year. Our
aggregated estimate of 120 submissions is a decrease of 431 submissions from our currently
approved estimate of 551 in the CY 2020 PFS final rule (84 FR 63113 and 63114). We continue
to estimate it will take 5 hours for a medical or health systems to prepare and submit an
arrangement.
In Table 123, we estimate an annual burden of 600 hours (120 responses x 5 hr/response)
at a cost of $77,568 (120 responses x $646.40/response) for the CY 2025 performance
period/2027 MIPS payment year.
TABLE 123: Estimated Burden for the Submission of Data for All-Payer QP
Determinations
Burden and Respondent Descriptions
Burden Estimate
Total # of APM Entities Submitting Data for All-Payer QP Determinations (a)
10
Total # of TINs Submitting Data for All-Payer QP Determinations (b)
100
Total # of Eligible Clinicians Submitting Data for All-Payer QP Determinations (c)
10
Total # of Respondents (d) = (a) + (b) + (c)
120
Hours Per respondent QP Determinations (e)
5
Total Annual Hours (f) = (d) × (e)
600
Cost per Respondent at Medical and Health Services Manager Labor Rate of $129.28/hr (g)
= (e) × $129.28/hr
$646.40
Total Annual Cost (h) = (d) × (g)
$77,568
In Table 124, we illustrate the net change in estimated burden for the submission of data
for all-payer QP determinations using the currently approved responses in the CY 2020 PFS final
rule (84 FR 63113 and 63114). In aggregate, the estimated change in the number of responses

will result in an adjustment of –2,155 hours and -$278,598 for the CY 2025 performance
period/2027 MIPS payment year.
TABLE 124: Change in Estimated Burden for the Submission of Data for All-Payer QP
Determinations
Burden and Respondent Descriptions
Burden Estimate
Total Currently Approved Annual Hours for Respondents (a)
2,755
Total Annual Hours for Respondents in CY 2025 PFS Proposed Rule (b) (See Table
123, row (f))
600
Difference in Annual Hours (c) = (b) − (a)
-2,155
Total Currently Approved Annual Cost for Respondents (d)
$356,166
Total Annual Cost for Respondents in CY 2025 PFS Proposed Rule (e) (See Table
123, row (h))
$77,568
Difference in Annual Cost (f) = (e) − (d)
-$278,598
n. ICRs Regarding Voluntary Participants Election to Opt-Out of Performance Data Display on
Compare Tools (§ 414.1395)
This rulemaking is not proposing any new or revised collection of information
requirements or burden related to the election by voluntary participants to opt-out of public
reporting on Compare Tools for the CY 2025 performance period/2027 MIPS payment year.
The requirements and burden associated with this information collection are currently approved
by OMB under control number 0938-1314 (CMS-10621). Consequently, we are not proposing
any changes to the election of voluntary participants to opt-out of performance data display on
Compare Tools under that control number.
C. Summary of Proposed Annual Burden Estimates
Table 125 sets out the burden for this rulemaking’s proposed provisions that are subject
to the PRA. It does not score burden adjustments that are strictly based on updated data and are
unrelated to any of the proposed provisions.
TABLE 125: Annual Requirements and Burden Estimates
Section(s) Under Title 42 of the CFR
OMB
Control
Number
(CMS ID
No.)
No.
Respondents
Total Annual
Responses
Time per
Response
(hours)
Total Annual
Time
(hours)
Labor
Cost
($/hr)
Total Cost
($)
§§ 414.1318, 414.1325, 414.1335,
414.1360, 414.1365, 414.1375,
414.1380, 414.1400, 414.1430,
414.1440, and 414.1445
Quality Payment Program
0938-1314
(CMS-
10621)
57,247
(56,981
clinicians
and groups-
level
TINs;218
APMs, 10
payers, 218
third part
intermediarie
s or entities
responding to
calls for
nomination)
(35,357)
Varies
(78,366)
Varies
(9,000,834)
§§ 427.402(c)(4) and (5), 428.302(c)(4)
and (5), and 428.303(c)(4) and (5)
(Regarding Rebate Reduction Requests
Submitted Under Sections 11101 and
11102 of the Inflation Reduction Act)
0938-NEW
(CMS-
10858)
20
20
31
620
Varies
74,756
TOTAL
57,267
(35,337)
Varies
(77,746)
Varies
(8,926,078)
D. Submission of PRA-Related Comments
We have submitted a copy of this proposed rule to OMB for its review of the rule’s
information collection requirements. The requirements are not effective until they have been
approved by OMB.
To obtain copies of the supporting statement and any related forms for the proposed
collections discussed previously, please visit the CMS website at
https://www.cms.gov/regulations-and-guidance/legislation/paperworkreductionactof1995/pra-
listing, or call the Reports Clearance Office at 410-786-1326.
We invite public comments on these potential information collection requirements. If
you wish to comment, please submit your comments electronically as specified in the DATES
and ADDRESSES sections of this proposed rule and identify the rule (CMS-1807-P), the ICR’s
CFR citation, and OMB control number.
VI. Response to Comments
Because of the large number of public comments, we normally receive on Federal
Register documents, we are not able to acknowledge or respond to them individually. We will
consider all comments we receive by the date and time specified in the DATES section of this
preamble, and, when we proceed with a subsequent document, we will respond to the comments
in the preamble to that document.
VII. Regulatory Impact Analysis
A. Statement of Need
In this proposed rule, we are proposing payment and policy changes under the Medicare
PFS and changes to implement amendments made under the section 502 of the Further
Continuing Appropriations and Other Extensions Act, 2024 (Pub. L. 118-22) (FCAOEA,
2024). Our proposed policies in this rulemaking specifically address: changes to the PFS; and
other changes to Medicare Part B payment policies to ensure that payment systems are updated
to reflect changes in medical practice, the relative value of services, and changes in the statute;
updates and refinements to Medicare Shared Savings Program (Shared Savings Program)
requirements; updates to the Quality Payment Program (MIPS and Advanced APMs); changes to
payment policies for drugs and biologicals products paid under Medicare Part B, changes to the
Clinical Laboratory Fee Schedule requirements, other changes to Medicare Part B payment
policies for Rural Health Clinics and Federally Qualified Health Centers, the Medicare coverage
of opioid use disorder services furnished by opioid treatment programs and coverage and
payment for certain preventive services; updates to electronic prescribing for controlled
substances for a covered Part D drug under a prescription drug plan or an MA-PD plan (section
2003 of the SUPPORT Act); and proposed change to the regulations associated with the
Ambulance Fee Schedule. The policies reflect CMS’ stewardship of the Medicare program and
overarching policy objectives for ensuring equitable beneficiary access to appropriate and quality
medical care.
1. Statutory Provisions
a. Clinical Laboratory Fee Schedule (CLFS) – Proposed Revisions Consistent with Recent
Statutory Changes
In section III.F. of this proposed rule, we propose the conforming regulations text
changes for CLFS data reporting requirements due to the enactment of section 502 of the Further
Continuing Appropriations and Other Extensions Act, 2024 (Pub. L. 118-22) (FCAOEA, 2024).
For clinical diagnostic laboratory tests (CDLTs) that are not advanced diagnostic laboratory tests
(ADLTs), section 502(b) of the FCAOEA, 2024 delayed the next data reporting period by one
year. Instead of taking place from January 1, 2024, through March 31, 2024, data reporting will
now take place from January 1, 2025, through March 31, 2025, based on the original data
collection period of January 1, 2019, through June 30, 2019. Data reporting for these tests then
resumes on a 3-year cycle. Additionally, section 502(a) of the FCAOEA, 2024 amended the
statutory provisions for the phase-in of payment reductions resulting from private payor rate
implementation to specify that the applicable percent for CY 2024 is 0 percent, meaning that the
payment amount determined for a CDLT for CY 2024 shall not result in any reduction in
payment as compared to the payment amount for that test for CY 2023. Section 502(a) of the
FCAOEA, 2024 further amended the statutory phase-in provisions to provide that for CYs 2025
through 2027, the payment amount for a CDLT may not be reduced by more than 15 percent as
compared to the payment amount for that test established in the preceding year.
b. Medicare Prescription Drug Inflation Rebate Program
Section III.I. of this proposed rule proposes regulations to implement provisions of the
Inflation Reduction Act (IRA) that establish the Medicare Prescription Drug Inflation Rebate
Program. Section 11101 of the IRA adds new section 1847A(i) to the Act, which establishes a
requirement for manufacturers to pay Medicare Part B rebates for certain single source drugs and
biological products with prices that increase faster than the rate of inflation, beginning on
January 1, 2023. Section 11102 of the IRA adds new section 1860D-14B to the Act, which
established a requirement for manufacturers to pay Medicare Part D rebates for certain Part D
drugs and biological products with prices that increase faster than the rate of inflation, beginning
on October 1, 2022.
c. Requirement for Electronic Prescribing for Controlled Substances for a Covered Part D Drug
under a Prescription Drug Plan or an MA-PD Plan
In section III.L. of this rulemaking, we propose a change to the electronic prescribing for
controlled substances (EPCS) requirement specified in § 423.160(a)(5) (referred to as the CMS
EPCS Program). The provision in section III.L. of this proposed rule proposes to revise
§ 423.160(a)(5) to specify that prescriptions written for a beneficiary in a long-term care (LTC)
facility would not be included in determining CMS EPCS Program compliance until January 1,
2028, and that compliance actions against prescribers who do not meet the compliance threshold
based on prescriptions written for a beneficiary in a LTC facility would commence on or after
January 1, 2028.
d. Quality Payment Program
This proposed rule is also necessary to make changes to the Quality Payment Program to
move the program forward to focus more on measurement efforts, refine how clinicians would
be able to participate in a more meaningful way through the Merit-based Incentive Payment
System (MIPS) Value Pathways (MVPs), and highlight the value of participating in Advanced
Alternative Payment Models (APMs). Authorized by MACRA, the Quality Payment Program is
an incentive program that includes two participation tracks, MIPS and Advanced APMs. MIPS
eligible clinicians are subject to a MIPS payment adjustment based on their performance in four
performance categories: cost, quality, improvement activities, and Promoting Interoperability.
Currently, reporting for traditional MIPS is seen as siloed across the performance categories.
These policy proposals are intended to promote better quality reporting to improve patient health
outcomes by coordinating reporting for MIPS across performance categories and make changes
to scoring that would provide a better picture of clinicians’ performance.
2. Discretionary Provisions
a. Drugs and Biological Products Paid Under Medicare Part B
In Section III.A.1. of this proposed rule, as part of our continued implementation of

section 90004 of the Infrastructure Investment and Jobs Act (Pub. L. 117-58, November 15,
2021) (IIJA), which amended section 1847A of the Act to require manufacturers to provide a
refund to CMS for certain discarded amounts from a refundable single-dose container or single-
use package drug (hereinafter, refundable drug), we are proposing a change in how we would
identify certain drugs that are excluded from the definition of refundable drug for those which
payment has been made under Part B for fewer than 18 months; how we identify drugs from a
single-dose container; to require the JW modifier if a billing supplier is not administering a drug,
but there are discarded amounts during the preparation process before supplying the drug to the
patient; and we discuss an application received for increased applicable percentage.
In section III.A.2 of this proposed rule, we are proposing how payment limits would be
calculated when manufacturers report negative or zero ASP data to CMS. Generally, we are
proposing that negative and zero ASP data be considered “not available” under section
1847A(c)(5)(B) of the Act and that positive ASP data be considered available. In circumstances
in which negative or zero ASP data is reported for some, but not all National Drug Codes
(NDCs) associated with a billing and payment code for a drug, we are proposing to calculate the
payment limit using only NDCs with positive ASP data. In certain circumstances, we are
proposing to carryover the most recent positive ASP data for the drug to calculate a payment
limit when the manufacturer’s ASP is negative or zero. For biosimilars with negative or zero
ASP data for all NDCs, we are proposing to use positive ASP data from other biosimilars with
the same reference product, when available, to calculate the payment limit.
In section III.A.3. of this proposed rule, we are proposing to clarify how Medicare
Administrative Contractors (MACs) pay for radiopharmaceuticals that are furnished in the
physician’s office. We are proposing to codify in regulations at § 414.904(e)(6) that, for
radiopharmaceuticals furnished in a setting other than the hospital outpatient department, MACs
shall determine payment limits for radiopharmaceuticals based on any methodology used to
determine payment limits for radiopharmaceuticals in place on or prior to November 2003. Such
methodology may include, but is not limited to, the use of invoice-based pricing.
In section III.A.4. of this proposed rule, we are proposing policies to reduce barriers
faced by beneficiaries receiving immunosuppressive drugs under the Medicare Part B
immunosuppressive drug benefit. That is, we are proposing at § 410.30 to include orally and
enterally administered compounded formulations with active ingredients derived only from
FDA-approved drugs that have approved immunosuppressive indications or FDA-approved
drugs that have been determined by a MAC to be reasonable and necessary for specific purposes
in immunosuppressive treatment in the immunosuppressive drug benefit. In addition, we are
proposing changes regarding supplying fees and refills for immunosuppressive drugs. These
proposals include allowing payment of a supply fee for a prescription of a supply of up to 90
days and allowing prescriptions for immunosuppressive drugs to be refillable.
In section III.A.5. of this proposed rule, we are proposing to update § 410.63(b) to clarify
existing CMS policy that blood clotting factors must be self-administered to be considered
clotting factors for which the furnishing fee applies. Additionally, we are proposing to clarify at
§ 410.63(c) that the furnishing fee is only available to entities that furnish blood clotting factors,
unless the costs associated with furnishing the clotting factor are paid though another payment
system, including the PFS. That is, we are proposing to clarify through revisions to § 410.63 that
clotting factors (as specified in section 1861(s)(2)(I) of the Act) and those eligible to receive the
clotting factor furnishing fee (as specified in section 1842(o)(5) of the Act) are the same subset
of products.
b. RHCs and FQHCs
In section III.B.2. of this proposed rule, we are proposing several changes to the
furnishing of care coordination services in RHCs and FQHCs. We are proposing to require
RHCs and FQHCs to report the individual HCPCS codes that make up the general care
management HCPCS code G0511 and to utilize the same codes as those billing under the PFS.
RHCs and FQHCs would no longer be required to report the general care management HCPCS
code (G0511). We are also proposing to permit billing of the add-on codes associated with these
services. In addition, beginning in CY 2025, we are proposing to adopt the coding and policies
regarding Advanced Primary Care Management (APCM) services, as discussed in section II.G of
this proposed rule.
For all of the care coordination services, we are proposing to allow separate payment at
the national non-facility PFS payment rate when the individual code is on an RHC or FQHC
claim, either alone or with other payable services. Payment rates would be updated annually
based on the PFS amounts for these codes. We are also seeking comment on how we can
improve the transparency and predictability regarding which HCPCS codes are considered care
coordination services to automate processes downstream for RHCs and FQHCs.
In section III.B.3. of this proposed rule, we are proposing the policy to continue to adopt
the definition “immediate availability” as including real-time audio and visual interactive
telecommunications for the direct supervision of services and supplies furnished incident to a
physician’s service through December 31, 2025, for RHCs and FQHCs. We are also proposing,
on a temporary basis, to allow payment for non-behavioral health visits furnished via
telecommunication technology in a manner that would closely align with the payment
mechanisms mandated by statute through December 31, 2024, that is, RHCs and FQHCs would
continue to bill for RHC and FQHC services furnished using telecommunication technology
services by reporting HCPCS code G2025 on the claim through December 31, 2025. In addition,
we are proposing to continue to delay the in-person visit requirement for mental health services
furnished via communication technology by RHCs and FQHCs to beneficiaries in their homes
until January 1, 2026.
In section III.B.4. of this proposed rule, we discuss the implementation of section 4124 of
the CAA, 2023. Section 4124 of the CAA, 2023 established Medicare coverage for intensive
outpatient program (IOP) services furnished by a hospital to its outpatients, or by a community
mental health center, a RHC or a FQHC, as a distinct and organized intensive ambulatory
treatment service offering less than 24-hour daily care in a location other than an individual’s
home or inpatient or residential setting, effective January 1, 2024 (88 FR 81838). We are
proposing to provide a payment rate for 4 or more services per day in the RHC and FQHC
setting.
In section III.B.5. of this rulemaking, we are proposing to allow RHCs and FQHCs to bill
for Part B preventive vaccines and the administration at the time of service. We propose that
payments for these claims will be made according to Part B preventive vaccine payment rates in
other settings, to be annually reconciled with the facilities’ actual vaccine costs on their cost
reports. Due to the operational systems changes needed to facilitate payment through claims, we
propose that RHCs and FQHCs begin billing for preventive vaccines and their administration at
the time of service, for dates of service on or after July 1, 2025.
In section III.B.6. of this proposed rule, we discuss our proposal relating to RHC
productivity standards. We are proposing to remove productivity standards for RHCs.
In section III.B.7. of this proposed rule, we discuss proposals relating to the FQHC
market basket. We are proposing to rebase and revise the FQHC PPS market basket to reflect a
2022 base year.
In section III.B.8. of this proposed rule, we are clarifying that when RHCs and FQHCs
furnish dental services that align with the inextricably linked policies and operational
requirements in the physician setting, we would consider those services to be a qualifying visit
and the RHC would be paid at the RHC AIR and the FQHC would be paid under the FQHC PPS.
c. Modifications Related to Medicare Coverage for Opioid Use Disorder (OUD) Treatment
Services Furnished by Opioid Treatment Programs (OTPs)
In section III.F.2 of this proposed rule, we are proposing telecommunication flexibilities
related to periodic assessments and initiation of treatment with methadone. We are proposing to
allow periodic assessments to be furnished via audio-only communications when two-way audio-

video communications technology is not available to the beneficiary on a permanent basis, to the
extent that this flexibility is authorized by SAMHSA and DEA at the time the service is
furnished, and all other applicable requirements are met. We believe that making this current
flexibility permanent is appropriate, as it would allow a beneficiary to decide with their provider
the best modality for receiving care, and evidence has shown that audio-only visits produce
many of the same benefits as video-based visits.
790
Additionally, permanently extending the
flexibility to allow periodic assessments to be furnished via audio-only communications would
further contribute towards health equity, especially among Medicare beneficiaries who are from
underserved populations.
791
We are also proposing to allow OTPs to use audio-visual
telecommunications for initiation of treatment with methadone for any new patient who will be
treated by the OTP with methadone if the OTP determines that an adequate evaluation of the
patient can be accomplished via an audio-visual telehealth platform. We would allow the OTP
intake add-on code (HCPCS code G2076) to be paid for two-way audio-video communications
technology when it is billed for the initiation of treatment with methadone to the extent that the
use of audio-video telecommunications technology to initiate treatment with methadone is
authorized by DEA and SAMHSA at the time the service is furnished, and all other applicable
requirements are met. We believe this flexibility is needed to align with new policy amendments
finalized by SAMHSA for initiation of treatment with methadone at § 8.12(f)(2)(v)(A), and it
would help reduce barriers for many individuals beginning treatment with methadone who often
experience at least one barrier to accessing treatment (for example, reliable transportation, work
schedule conflicts, distance to treatment, etc.).
792
790
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9446840/.
791
https://pubmed.ncbi.nlm.nih.gov/33471458/; https://www.kff.org/medicare/issue-brief/medicare-and-telehealth-coverage-
and-use-during-the-covid-19-pandemic-and-options-for-the-future/ ; https://pubmed.ncbi.nlm.nih.gov/34534186/.
792
https://ascpjournal.biomedcentral.com/articles/10.1186/s13722-022-00316-3.

In section III.F.3 of this proposed rule, we are proposing payment updates to intake
activities (HCPCS code G2076) furnished by OTPs in response to recent regulatory reforms
finalized by SAMHSA at 42 CFR part 8 that aim to recognize more patient-centered and
evidence-based paradigms of care for OUD treatment (for example, harm reduction
interventions, recovery support services, etc.). Specifically, we are proposing to update the
payment for intake activities (HCPCS code G2076) to include payment for social determinants
of health risk assessments (HCPCS code G0136) in order to adequately reflect additional effort
for OTPs to identify a patient’s unmet health-related social needs (HRSNs), or the need and
interest for harm reduction interventions and recovery support services that are critical to the
treatment of an OUD. These would be consistent with new revisions to standards for initial
assessment service activities required by SAMHSA under § 8.12(f)(4)(i). CMS is further
requesting information to understand how OTPs currently coordinate care and make referrals to
community-based organizations (CBOs) that address unmet HRSNs, provide harm reduction
services, and/or offer recovery support services. Altogether, we believe these proposals are
necessary to help OTPs address key issues during initial assessments that may increase the risk
of a patient leaving OUD treatment prematurely or that pose as barriers to treatment engagement.
For example, patients with an OUD are more likely to have lower educational attainment, be
food insecure, encounter financial hardship, and housing instability, and they often report
financial and logistical barriers (for example, lack of access to transportation) as reasons for not
receiving treatment.
793
However, providers (including SUD treatment facilities) who coordinate
care with CBOs, including peer support organizations, housing agencies, and educational and
employment agencies, to address unmet HRSNs (for example, housing, transportation, etc.)
793
https://www.sciencedirect.com/science/article/pii/S1544319123000560?via%3Dihub.
https://www.sciencedirect.com/science/article/pii/S0749379722001040?via%3Dihub.

identified during assessments can positively influence health outcomes and better support a
patient’s engagement in SUD treatment.
794
Furthermore, in section III.F.4 of this proposed rule, we are proposing to establish
payment for new opioid agonist and antagonist medications that were recently approved by the
FDA. We would create a new add-on code to the bundled payment to reflect take-home supplies
for nalmefene hydrochloride (nalmefene) nasal spray (Opvee®), which is indicated for the
emergency treatment of known or suspected opioid overdose induced by natural or synthetic
opioids. The add-on code would include payment for a carton of two 2.7 mg nasal sprays of
nalmefene and overdose education furnished in conjunction with distributing nalmefene. We are
also proposing payment for a new extended-release injectable buprenorphine product (Brixadi®),
indicated to treat moderate to severe OUD and that comes in a weekly (8 mg, 16 mg, 24 mg, 32
mg) and monthly formulation (64 mg, 96 mg, and 128 mg). We would create a new weekly
bundled payment code (including both a non-drug and drug component) for weekly injectable
buprenorphine to reflect the weekly formulation of Brixadi®. In addition, we are proposing to
update payment for the drug component of the existing bundled payment under the Medicare
OTP benefit for monthly injectable buprenorphine (HCPCS G2069) in order to reflect payment
for the monthly formulation of Brixadi®. We believe these proposals are consistent with our
statutory authority under sections 1861(jjj)(1)(A) and 1834(w) of the Act, which allow the
Secretary to establish Medicare bundled payment for opioid agonist and antagonist treatment
medications that are approved by the FDA. These proposals would expand access to new opioid
agonist and antagonist medications that are important to help prevent additional opioid overdose
deaths, reduce illicit opioid use, and retain more individuals with an OUD in treatment.
795
794
https://www.commonwealthfund.org/sites/default/files/2022
09/ROI_calculator_evidence_review_2022_update_Sept_2022.pdf ;
https://aspe.hhs.gov/sites/default/files/private/pdf/260791/BestSUD.pdf.
795
https://www.cdc.gov/drugoverdose/pdf/pubs/2018-evidence-based-strategies.pdf.;
https://pubmed.ncbi.nlm.nih.gov/24247147/.
Lastly, in section III.F.5 of this proposed rule, we are clarifying a billing requirement that
an OUD diagnosis code is required on claims submitted under the Medicare OTP benefit for
OUD treatment services. This clarification is needed to ensure payments made to OTPs are in
alignment with statutory requirements under sections 1861(s)(2)(HH), 1861(jjj)(1), and 1834(w)
of the Act, which all specify that services paid to OTPs under Medicare Part B must be for the
treatment of opioid use disorder.
d. Medicare Shared Savings Program
In section III.G. of this proposed rule, we are proposing modifications to the Shared
Savings Program to further advance Medicare’s value-based care strategy of growth, alignment,
and equity, and to make changes that would allow for timely improvements to program policies
and operations.
The proposed changes to the Shared Savings Program include the following:
Proposed changes to the quality performance standard and other quality reporting
requirements, including to (1) require Shared Savings Program ACOs to report the APP Plus
quality measure set that would incrementally grow to comprise of 11 measures, consisting of the
6 measures in the existing APP quality measure set and 5 newly proposed measures from the
Adult Universal Foundation measure set that would be incrementally incorporated into the APP
Plus quality measure set over performance years 2025 through 2028, (2) focus the collection
types available to Shared Savings Program ACOs for reporting the APP Plus quality measure set
to all payer/all patient eCQMs and Medicare CQMs, (3) require Shared Savings Program ACOs
that report the APP Plus quality measure set, to report on all measures in the APP Plus quality
measure set, as applicable, (4) establish a Complex Organization Adjustment for Virtual Groups
and APM Entities, including Shared Savings Program ACOs, when reporting eCQMs, (5) score
Medicare CQMs using flat benchmarks in their first 2 performance periods in MIPS, and (6)
extend the eCQM reporting incentive in order to promote the adoption of eCQMs.
Proposed changes to establish a new “prepaid shared savings” option to assist eligible
ACOs, with a history of earning shared savings, with cash flow and encourage investments that
would provide additional services for beneficiaries, and proposed refinements to recently-
established advance investment payment policies.
Proposed modifications to the Shared Savings Program’s financial methodology
including to (1) ensure the benchmarking methodology includes sufficient incentive for ACOs
providing care to underserved communities to enter and remain in the program through the
application of a proposed health equity benchmark adjustment, (2) specify a calculation
methodology to account for the impact of improper payments in recalculating expenditures and
payment amounts used in Shared Savings Program financial calculations, upon reopening a
payment determination pursuant to § 425.315(a), (3) establish a methodology for excluding
payment amounts for HCPCS and CPT codes exhibiting significant, anomalous, and highly
suspect (SAHS) billing activity during CY 2024 or subsequent calendar years that warrant
adjustment, and (4) technical changes for consistency and clarity in provisions of the Shared
Savings Program regulations on financial calculations, to align and clarify the language we used
to describe weights applied to the growth in ACO and regional risk scores for each Medicare
enrollment type, as part of the calculation for capping ACO and regional risk score growth,
respectively.
Proposed changes to other programmatic areas, including: a proposal in connection with
the Shared Savings Program compliance requirements to permit continued participation by
ACOs whose number of assigned beneficiaries falls below 5,000 during their agreement period;
proposed updates to provisions of the Shared Savings Program regulations on application
procedures to reflect the latest approach Antitrust Agencies use to evaluate ACOs and enforce
antitrust measures; proposed updates to the beneficiary assignment methodology including to (1)
revise the definition of primary care services to align with payment policy proposals and include,
among other services for the purposes of beneficiary assignment, Safety Planning Interventions,

Post-Discharge Telephonic Follow-up Contacts Intervention, Virtual Check-in Services,
Advanced Primary Care Management Services, Cardiovascular Risk Assessment and Risk
Management Services, Interprofessional Consultation Services, Direct Care Caregiver Training
Services, and Individual Behavior Management/Modification Caregiver Training Services, and
(2) broaden the existing exception to the program’s voluntary alignment policy to allow for
additional beneficiaries to be claims-based assigned to entities participating in certain disease- or
condition-specific Innovation Center ACO models; and proposed modifications to the
beneficiary notification requirements.
e. Medicare Part B Payment for Preventive Services
Section III.H.1 of this proposed rule outlines the implementation of policies that impact
the payment amount for administration of preventive vaccines paid under the Part B vaccine
benefit, as well COVID-19 monoclonal antibodies and the in-home additional payment for Part
B vaccine administration. These provisions are necessary to provide stable payment for
preventive vaccine administration and related policies, and to allow predictability for providers
and suppliers to rely on for building and sustaining robust vaccination programs.
Section III.H.2 of this proposed rule addresses two items related to payment for hepatitis
B vaccine administration under Part B. In section III.M. of this proposed rule, we propose to
expand coverage of hepatitis B vaccinations by revising existing regulations. If that coverage
expansion of hepatitis B vaccines under Part B is finalized, we would clarify that a physician’s
order is no longer required for the administration of a hepatitis B vaccine in Part B, which will
facilitate roster billing by mass immunizers for hepatitis B vaccine administration. We are also
proposing that payment for hepatitis B vaccines and their administration be made at 100 percent
of reasonable cost in RHCs and FQHCs, separate from the FQHC PPS or the RHC All-Inclusive
Rate (AIR) methodology, to streamline payment for all Part B vaccines in those settings.
In section III.H.3. of this proposed rule, we are proposing a fee schedule for Drugs
Covered as Additional Preventive Services (DCAPS), per section 1833(a)(1)(W)(ii) of the Act.

We propose to determine payment limits for DCAPS drugs based on the ASP payment
methodology set forth under section 1847A of the Act if possible, and we propose alternative
payment mechanism for calculating payment limits for DCAPS drugs if ASP data is not
available. We also propose payment limits for supplying and administration fees for DCAPS
drugs that are similar to those fees for drugs paid under the ASP payment methodology set forth
under section 1847A of the Act. Finally, we propose to determine payment limits for DCAPS
drugs in RHCs and FQHCs, and any supply and administration fee, using this same fee schedule,
and to pay for DCAPS drugs and their administration on a claim-by-claim basis.
f. Expand Colorectal Cancer Screening
In section III.K. of this rulemaking, we propose to update and expand coverage for CRC
screening by (1) removing coverage for the barium enema procedure in regulations at § 410.37,
(2) adding coverage for the CTC procedure in regulations at § 410.37, and (3) expanding a
“complete colorectal cancer screening” in § 410.37(k) to include a follow-on screening
colonoscopy after a Medicare covered blood-based biomarker CRC screening test (described and
authorized in NCD 210.3) returns a positive result. The Center for Disease Control and
Prevention (CDC) describes CRC as “a disease in which cells in the colon or rectum grow out of
control… Sometimes abnormal growths, called polyps, form in the colon or rectum. Over time,
some polyps may turn into cancer. Screening tests can find polyps so they can be removed before
turning into cancer. Screening also helps find colorectal cancer at an early stage, when treatment
works best.”
796
The National Cancer Institute reports that CRC is the fourth most common type
of cancer and estimates that the United States experienced 153,020 new cases and 52,550 new
deaths from CRC in 2023. In addition, the rate of new cases and new deaths from CRC is more
common in men than women and significantly greater for those of African American and Non-
796
CDC website: https://www.cdc.gov/cancer/colorectal/basic_info/what-is-colorectal-cancer.htm.

Hispanic American Indian/ Alaska Native descent compared to all races.
797
g. Expand Hepatitis B Vaccine Coverage
In section III.M. of this rulemaking, we propose to expand Hepatitis B vaccine coverage
by revising our regulatory definition for intermediate risk groups by adding a new paragraph to
include individuals who have not previously received a completed hepatitis B vaccination series
or whose vaccination history is unknown (§ 410.63(a)(2)). Hepatitis B is a vaccine-preventable
liver disease caused by the hepatitis B virus.
798
The vaccine consists of a series of typically 3
doses delivered at various intervals.
799
Hepatitis B virus is transmitted when body fluid (blood,
semen, or other) from a person infected with the virus enters the body of someone who is
uninfected.
800
This can happen through sexual contact; sharing needles, syringes, or other drug-
injection equipment; transmission from the gestational parent to baby during pregnancy or at
birth; direct contact with blood or open sores; or sharing contaminated items such as
toothbrushes, razors or medical equipment (such as a glucose monitor) of a person who has
hepatitis B.
801
Hepatitis B can be an acute, short-term illness and it can develop into a long-term,
chronic infection. Chronic hepatitis B can lead to serious health problems, including cirrhosis,
liver cancer, and death. Treatments for hepatitis B are available but no cure exists. There are
currently an estimated 2.4 million individuals in the U.S. living with hepatitis B virus and an
estimated 20,000 new infections every year.
802
We believe our proposal will help protect
Medicare beneficiaries from acquiring hepatitis B infection, contribute to eliminating viral
hepatitis as a public health threat in the United States and is in the best interest of the Medicare
797
NCI Website: https://seer.cancer.gov/statfacts/html/colorect.html.
798
CDC, 2023. Hepatitis B surveillance 2021. Retrieved from
https://www.cdc.gov/hepatitis/statistics/2021surveillance/hepatitis-b.htm.
799
CDC. Viral hepatitis. FAQ for health professionals. Atlanta, GA: U.S. HHS, CDC; 2022. Retrieved from
https://www.cdc.gov/hepatitis/hbv/hbvfaq.htm.
800
CDC, 2023. Hepatitis B surveillance 2021. Retrieved from
https://www.cdc.gov/hepatitis/statistics/2021surveillance/hepatitis-b.htm.
801
CDC. 2024. Viral Hepatitis FAQs for the public. Retrieved from https://www.cdc.gov/hepatitis/hbv/bfaq.htm.
802
Conners EE, Panagiotakopoulos L, Hofmeister MG, et al. Screening and testing for hepatitis B virus infection:
CDC recommendations – United States, 2023. MMWR Recomm Rep. 2023;72(1):1-25. Retrieved from
https://www.cdc.gov/mmwr/volumes/72/rr/rr7201a1.htm.
program and its beneficiaries.
h. Medicare Parts A and B Overpayment Provisions of the Affordable Care Act (§ 401.305(b)(1)
thorugh (3))
Section III.O. of this proposed rule discusses existing § 401.305(b)(1), which specifies
when a person who has received an overpayment must report and return an overpayment. We
propose to amend this regulation to reference revised § 401.305(b)(2) and new § 401.305(b)(3).
We are proposing a technical modification to the introductory language in § 401.305(b)(2) to
acknowledge that this paragraph might be applicable after the suspension described in new
§ 401.305(b)(3) is complete. New proposed § 401.305(b)(3) would identify the circumstances
under which the deadline for reporting and returning overpayments would be suspended to allow
time for providers to investigate and calculate overpayments. We do not have a basis for
estimating the impact associated with this amendment. We solicit comment on the analysis and
conclusions provided in the RIA.
B. Overall Impact
We have examined the impacts of this proposed rule as required by Executive
Order 12866 on Regulatory Planning and Review (September 30, 1993), Executive Order 13563
on Improving Regulation and Regulatory Review (January 18, 2011), Executive Order 14094
entitled “Modernizing Regulatory Review” (April 6, 2023), the Regulatory Flexibility Act (RFA)
(September 19, 1980, Pub. L. 96-354), section 1102(b) of the Social Security Act, section 202 of
the Unfunded Mandates reform Act of 1995 (March 22, 1995; Pub. L. 104-4), and Executive
Order 13132 on Federalism (August 4, 1999).
Executive Orders 12866 and 13563 direct agencies to assess all costs and benefits of
available regulatory alternatives and, if regulation is necessary, to select regulatory approaches
that maximize net benefits (including potential economic, environmental, public health and
safety effects, distributive impacts, and equity). Executive Order 14094, entitled “Modernizing
Regulatory Review” (hereinafter, the Modernizing E.O.), amends section 3(f)(1) of Executive
Order 12866 (Regulatory Planning and Review). The amended section 3(f) of Executive Order
12866 defines a “significant regulatory action” as an action that is likely to result in a rule: (1)
having an annual effect on the economy of $200 million or more in any 1 year (adjusted every 3
years by the Administrator of the Office of Information and Regulatory Affairs (OIRA) for
changes in gross domestic product), or adversely affect in a material way the economy, a sector
of the economy, productivity, competition, jobs, the environment, public health or safety, or
State, local, territorial, or tribal governments or communities; (2) creating a serious inconsistency
or otherwise interfering with an action taken or planned by another agency; (3) materially
altering the budgetary impacts of entitlement grants, user fees, or loan programs or the rights and
obligations of recipients thereof; or (4) raise legal or policy issues for which centralized review
would meaningfully further the President’s priorities or the principles set forth in this Executive
order, as specifically authorized in a timely manner by the Administrator of OIRA in each case.
A regulatory impact analysis (RIA) must be prepared for major rules with significant
regulatory action/s and/or with significant effects as per section 3(f)(1) ($200 million or more in
any 1 year). Based on our estimates, OMB’s Office of Information and Regulatory Affairs has
determined this rulemaking is significant per section 3(f)(1)) as measured by the $200 million or
more in any 1 year. Accordingly, we have prepared an RIA that, to the best of our ability,
presents the costs and benefits of the rulemaking. The RFA requires agencies to analyze options
for regulatory relief of small entities. For purposes of the RFA, small entities include small
businesses, nonprofit organizations, and small governmental jurisdictions. Most hospitals,
practitioners, and most other providers and suppliers are small entities, either by nonprofit status
or by having annual revenues that qualify for small business status under the Small Business
Administration standards. (For details, see the SBA’s website at
https://www.sba.gov/document/support-table-size-standards (refer to the 620000 series).)
Individuals and States are not included in the definition of a small entity.
The RFA requires that we analyze regulatory options for small businesses and other
entities. We prepare a regulatory flexibility analysis unless we certify that a rule would not have
a significant economic impact on a substantial number of small entities. The analysis must
include a justification concerning the reason action is being taken, the kinds and number of small
entities the rule affects, and an explanation of any meaningful options that achieve the objectives
with less significant adverse economic impact on the small entities.
Approximately 95 percent of practitioners, other suppliers, and providers are considered
to be small entities, based upon the SBA standards. There are over 1 million physicians, other
practitioners, and medical suppliers that receive Medicare payment under the PFS. Because
many of the affected entities are small entities, the analysis and discussion provided in this
section, as well as elsewhere in this proposed rule is intended to comply with the RFA
requirements regarding significant impact on a substantial number of small entities.
In addition, section 1102(b) of the Act requires us to prepare an RIA if a rule may have a
significant impact on the operations of a substantial number of small rural hospitals. This
analysis must conform to the provisions of section 603 of the RFA. For purposes of section
1102(b) of the Act, we define a small rural hospital as a hospital that is located outside of a
Metropolitan Statistical Area for Medicare payment regulations and has fewer than 100 beds.
Medicare does not pay rural hospitals for their services under the PFS; rather, Medicare payment
is made under the PFS for physicians’ services, which can be furnished by physicians and NPPs
in a variety of settings, including rural hospitals. We did not prepare an analysis for section
1102(b) of the Act because we determined, and the Secretary certified, that this rulemaking will
not have a significant impact on the operations of a substantial number of small rural hospitals.
Section 202 of the Unfunded Mandates Reform Act of 1995 also requires that agencies
assess anticipated costs and benefits on State, local, or tribal governments or on the private sector
before issuing any rule whose mandates require spending in any 1 year of $100 million in 1995
dollars, updated annually for inflation. In 2024, that threshold is approximately $183 million.
This proposed rule will impose no mandates on State, local, or tribal governments or on the
private sector.
Executive Order 13132 establishes certain requirements that an agency must meet when it
issues a proposed rule (and subsequent final rule) that imposes substantial direct requirement
costs on State and local governments, preempts State law, or otherwise has federalism
implications. Since this rulemaking does not impose any costs on State or local governments, the
requirements of Executive Order 13132 are not applicable.
We prepared the following analysis, which, together with the information provided in the
rest of this proposed rule, meets all assessment requirements. The analysis explains the rationale
for and purposes of this proposed rule; details the costs and benefits of this rulemaking; analyzes
alternatives; and presents the measures we will use to minimize the burden on small entities. As
indicated elsewhere in this proposed rule, we discussed various changes to our regulations,
payments, or payment policies to ensure that our payment systems reflect changes in medical
practice and the relative value of services and to implement provisions of the statute. We
provide information for each policy change in the relevant sections of this proposed rule. We are
unaware of any relevant Federal rules that duplicate, overlap, or conflict with this proposed rule.
The relevant sections of this rulemaking describe significant alternatives we considered, if
applicable.
C. Changes in Relative Value Unit (RVU) Impacts
1. Resource-Based Work, PE, and MP RVUs
Section 1848(c)(2)(B)(ii)(II) of the Act requires that increases or decreases in RVUs may
not cause the amount of Medicare Part B expenditures for the year to differ by more than $20
million from what expenditures would have been in the absence of these changes. If this
threshold is exceeded, we make adjustments to preserve budget neutrality.
Our estimates of changes in Medicare expenditures for PFS services compared payment
rates for CY 2024 with payment rates for CY 2025 using CY 2023 Medicare utilization. The
payment impacts described in this proposed rule reflect averages by specialty based on Medicare
utilization. The payment impact for an individual practitioner could vary from the average and
will depend on the mix of services they furnish. The average percentage change in total
revenues will be less than the impact displayed here because practitioners and other entities
generally furnish services to both Medicare and non-Medicare patients. In addition, practitioners
and other entities may receive substantial Medicare revenues for services under other Medicare
payment systems. For instance, independent laboratories receive approximately 83 percent of
their Medicare revenues from clinical diagnostic laboratory tests that are paid under the Clinical
Laboratory Fee Schedule (CLFS). The PFS update adjustment factor for CY 2025, as specified in
section 1848(d)(19) of the Act, is 0.00 percent before applying other adjustments.
To calculate the estimated CY 2025 PFS conversion factor (CF), we took the CY 2024
conversion factor without the payment increase of 1.25 percent provided by the CAA, 2023 that
applied to services furnished from January 1, 2024 through March 8, 2024, and the 2.93 percent
payment increase provided by the CAA, 2024 that replaced the previous 1.25 percent increase
and applies to services furnished from March 9, 2024 through December 31, 2024 and multiplied
it by the budget neutrality adjustment required as described in the preceding paragraphs. We
estimate the CY 2025 PFS CF to be 32.3562 which reflects a 0.05 percent positive budget
neutrality adjustment required under section 1848(c)(2)(B)(ii)(II) of the Act, the 0.00 percent
update adjustment factor specified under section 1848(d)(19) of the Act, and the removal of the
temporary 2.93 percent payment increase for services furnished from March 9, 2024 through
December 31, 2024, as provided in the CAA, 2024. We estimate the CY 2025 anesthesia CF to
be 20.3340, reflecting the same overall PFS adjustments with the addition of anesthesia-specific
PE and MP adjustments.

TABLE 126: Calculation of the CY 2025 PFS Conversion Factor
CY 2024 Conversion Factor
33.2875
Conversion Factor without the CAA, 2024 (2.93 Percent
Increase for CY 2024)
32.3400
CY 2025 Statutory Update Factor
0.00 percent (1.0000)
CY 2025 RVU Budget Neutrality Adjustment
0.05 percent (1.0005)
CY 2025 Conversion Factor
32.3562
TABLE 127: Calculation of the CY 2025 Anesthesia Conversion Factor
CY 2024 National Average Anesthesia Conversion Factor
20.7739
Conversion Factor without the CAA, 2024 (2.93 Percent
Increase for CY 2024)
20.1826
CY 2025 Statutory Update Factor
0.00 percent (1.0000)
CY 2025 RVU Budget Neutrality Adjustment
0.05 percent (1.0005)
CY 2025 Anesthesia Fee Schedule Practice Expense and
Malpractice Adjustment
0.70 percent (1.0070)
CY 2025 Conversion Factor
20.3340
Table 128 shows the impact on PFS payment for physicians’ services based on the
proposed policies included this proposed rule. To the extent that there are year-to-year changes
in the volume and mix of services provided by practitioners, the actual impact on total Medicare
revenues will be different from those shown in Table 128 (CY 2025 PFS Estimated Impact on
Total Allowed Charges by Specialty). The following is an explanation of the information
represented in Table 128.
● Column A (Specialty): Identifies the specialty for which data are shown.
● Column B (Allowed Charges): The aggregate estimated PFS allowed charges for the
specialty based on CY 2023 utilization and CY 2024 rates. That is, allowed charges are the PFS
amounts for covered services and include coinsurance and deductibles (which are the financial
responsibility of the beneficiary). These amounts have been summed across all services
furnished by physicians, practitioners, and suppliers within a specialty to arrive at the total
allowed charges for the specialty.
● Column C (Impact of Work RVU Changes): This column shows the estimated CY
2025 impact on total allowed charges of the changes in the work RVUs, including the impact of
changes due to potentially misvalued codes.

● Column D (Impact of PE RVU Changes): This column shows the estimated CY 2025
impact on total allowed charges of the changes in the PE RVUs.
● Column E (Impact of MP RVU Changes): This column shows the estimated CY 2025
impact on total allowed charges of the changes in the MP RVUs.
● Column F (Combined Impact): This column shows the estimated CY 2025 combined
impact on total allowed charges of all the changes in the previous columns. Column F may not
equal the sum of columns C, D, and E due to rounding.
TABLE 128: CY 2025 PFS Estimated Impact on Total Allowed Charges by Specialty
(A)
Specialty
(B)
Allowed
Charges
(mil)
(C)
Impact
of Work
RVU
Changes
(D)
Impact
of PE
RVU
Changes
(E)
Impact
of MP
RVU
Changes
(F)
Combined
Impact
Allergy/Immunology
$207
0%
0%
0%
0%
Anesthesiology
$1,488
1%
1%
0%
2%
Audiologist
$70
0%
0%
0%
0%
Cardiac Surgery
$155
0%
0%
0%
0%
Cardiology
$5,748
0%
0%
0%
0%
Chiropractic
$616
0%
1%
0%
1%
Clinical Psychologist
$680
3%
1%
0%
3%
Clinical Social Worker
$794
3%
1%
0%
4%
Colon And Rectal Surgery
$143
0%
1%
0%
0%
Critical Care
$309
0%
0%
0%
1%
Dermatology
$3,717
0%
0%
0%
0%
Diagnostic Testing Facility
$875
0%
-2%
0%
-2%
Emergency Medicine
$2,240
0%
0%
0%
0%
Endocrinology
$491
0%
1%
0%
1%
Family Practice
$5,133
0%
0%
0%
1%
Gastroenterology
$1,372
0%
0%
0%
0%
General Practice
$341
0%
0%
0%
0%
General Surgery
$1,484
0%
0%
0%
0%
Geriatrics
$193
0%
1%
0%
1%
Hand Surgery
$251
-1%
0%
0%
-1%
Hematology/Oncology
$1,501
0%
0%
0%
0%
Independent Laboratory
$512
0%
0%
0%
0%
Infectious Disease
$513
0%
0%
0%
0%
Internal Medicine
$8,771
0%
0%
0%
1%
Interventional Pain Mgmt
$792
0%
0%
0%
0%
Interventional Radiology
$418
0%
-2%
0%
-2%
Multispecialty Clinic/Other Phys
$142
0%
0%
0%
0%
Nephrology
$1,571
0%
1%
0%
1%
Neurology
$1,252
0%
0%
0%
0%
Neurosurgery
$658
0%
0%
0%
0%
Nuclear Medicine
$47
0%
0%
0%
0%
Nurse Anes / Anes Asst
$987
0%
1%
0%
1%
Nurse Practitioner
$6,531
0%
0%
0%
0%
Obstetrics/Gynecology
$531
0%
0%
0%
-1%
Ophthalmology
$4,469
-1%
-1%
0%
-1%
Optometry
$1,280
0%
0%
0%
-1%

(A)
Specialty
(B)
Allowed
Charges
(mil)
(C)
Impact
of Work
RVU
Changes
(D)
Impact
of PE
RVU
Changes
(E)
Impact
of MP
RVU
Changes
(F)
Combined
Impact
Oral/Maxillofacial Surgery
$57
0%
0%
0%
0%
Orthopedic Surgery
$3,239
-1%
0%
0%
-1%
Other
$54
0%
0%
0%
0%
Otolaryngology
$1,095
0%
0%
0%
0%
Pathology
$1,090
0%
0%
0%
0%
Pediatrics
$51
0%
0%
0%
1%
Physical Medicine
$1,054
0%
0%
0%
0%
Physical/Occupational Therapy
$5,607
0%
0%
0%
0%
Physician Assistant
$3,472
0%
0%
0%
0%
Plastic Surgery
$280
0%
0%
0%
-1%
Podiatry
$1,780
0%
0%
0%
0%
Portable X-Ray Supplier
$69
0%
1%
0%
1%
Psychiatry
$795
1%
0%
0%
1%
Pulmonary Disease
$1,188
0%
0%
0%
1%
Radiation Oncology and Radiation Therapy
Centers
$1,458
0%
0%
0%
0%
Radiology
$4,273
0%
0%
0%
0%
Rheumatology
$496
0%
0%
0%
0%
Thoracic Surgery
$277
0%
0%
0%
0%
Urology
$1,532
0%
-1%
0%
-1%
Vascular Surgery
$937
0%
-2%
0%
-2%
Total
$106,413
0%
0%
0%
0%
* Column F may not equal the sum of columns C, D, and E due to rounding.
In recent years, we have received requests from interested parties to provide more
granular information that separates the specialty-specific impacts by site of service. These
interested parties have presented us with high-level information suggesting that Medicare
payment policies are directly responsible for consolidating privately owned physician practices
and freestanding supplier facilities into larger health systems. Their concerns highlight a need to
update the information under the PFS to account for current trends in healthcare delivery,
especially concerning independent versus facility-based practices. We published an RFI in the
CY 2023 PFS proposed rule to gather feedback on this issue and refer readers to the discussion
in the CY 2023 PFS final rule (87 FR 69429 through 69438). As part of our holistic review of
how best to update our data and offer interested parties additional information that addresses
some of the concerns raised, we have recently improved our current suite of public use files
(PUFs) by including a new file that shows estimated specialty payment impacts at a more
granular level, specifically by showing ranges of impact for practitioners within a specialty. This
file is available on the CMS website under downloads for the CY 2025 PFS proposed rule at
https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-
Federal-Regulation-Notices.html.
We provided an additional impact table for this rulemaking cycle that includes a
facility/non-facility breakout of payment changes. The following is an explanation of the
information represented in Table 129.
● Column A (Specialty): Identifies the specialty for which data are shown.
● Column B (Setting): Identifies the facility or nonfacility setting for which data are
shown.
● Column C (Allowed Charges): The aggregate estimated PFS allowed charges for the
specialty based on CY 2023 utilization and CY 2024 rates. That is, allowed charges are the PFS
amounts for covered services and include coinsurance and deductibles (which are the financial
responsibility of the beneficiary). These amounts have been summed across all services
furnished by physicians, practitioners, and suppliers within a specialty to arrive at the total
allowed charges for the specialty.
● Column D (Combined Impact): This column shows the estimated CY 2025 combined
impact on total allowed charges.

TABLE 129: CY 2025 PFS Estimated Impact on Total Allowed Charges by Setting
(A)
Specialty
(B)
Total: Non-
Facility/Facility
(C)
Allowed
Charges
(mil)
(D)
Combined
Impact
TOTAL
$207
0%
Non-Facility
$200
0%
Allergy/Immunology
Facility
$7
0%
TOTAL
$1,488
2%
Non-Facility
$297
0%
Anesthesiology
Facility
$1,191
2%
TOTAL
$70
0%
Non-Facility
$68
0%
Audiologist
Facility
$3
1%
TOTAL
$155
0%
Non-Facility
$28
-1%
Cardiac Surgery
Facility
$126
0%
TOTAL
$5,748
0%
Non-Facility
$3,622
0%
Cardiology
Facility
$2,126
0%
TOTAL
$616
1%
Non-Facility
$615
1%
Chiropractic
Facility
$1
1%
TOTAL
$680
3%
Non-Facility
$549
3%
Clinical Psychologist
Facility
$131
3%
TOTAL
$794
4%
Non-Facility
$670
4%
Clinical Social Worker
Facility
$124
4%
TOTAL
$143
0%
Non-Facility
$53
1%
Colon And Rectal Surgery
Facility
$90
0%
TOTAL
$309
1%
Non-Facility
$50
1%
Critical Care
Facility
$259
1%
TOTAL
$3,717
0%
Non-Facility
$3,580
0%
Dermatology
Facility
$137
0%
TOTAL
$875
-2%
Non-Facility
$874
-2%
Diagnostic Testing Facility
Facility
$1
0%
TOTAL
$2,240
0%
Non-Facility
$186
0%
Emergency Medicine
Facility
$2,054
0%

(A)
Specialty
(B)
Total: Non-
Facility/Facility
(C)
Allowed
Charges
(mil)
(D)
Combined
Impact
TOTAL
$491
1%
Non-Facility
$395
1%
Endocrinology
Facility
$96
0%
TOTAL
$5,133
1%
Non-Facility
$4,131
1%
Family Practice
Facility
$1,002
0%
TOTAL
$1,372
0%
Non-Facility
$505
1%
Gastroenterology
Facility
$868
0%
TOTAL
$341
0%
Non-Facility
$273
0%
General Practice
Facility
$68
0%
TOTAL
$1,484
0%
Non-Facility
$438
0%
General Surgery
Facility
$1,045
0%
TOTAL
$193
1%
Non-Facility
$125
1%
Geriatrics
Facility
$68
0%
TOTAL
$251
-1%
Non-Facility
$134
0%
Hand Surgery
Facility
$116
-3%
TOTAL
$1,501
0%
Non-Facility
$976
-1%
Hematology/Oncology
Facility
$525
0%
TOTAL
$512
0%
Non-Facility
$499
0%
Independent Laboratory
Facility
$13
0%
TOTAL
$513
0%
Non-Facility
$81
0%
Infectious Disease
Facility
$432
0%
TOTAL
$8,771
1%
Non-Facility
$4,373
1%
Internal Medicine
Facility
$4,397
0%
TOTAL
$792
0%
Non-Facility
$624
0%
Interventional Pain Mgmt
Facility
$168
1%
TOTAL
$418
-2%
Non-Facility
$259
-3%
Interventional Radiology
Facility
$159
1%
TOTAL
$142
0%
Multispecialty Clinic/Other Phys
Non-Facility
$71
0%

(A)
Specialty
(B)
Total: Non-
Facility/Facility
(C)
Allowed
Charges
(mil)
(D)
Combined
Impact
Facility
$71
0%
TOTAL
$1,571
1%
Non-Facility
$934
1%
Nephrology
Facility
$636
0%
TOTAL
$1,252
0%
Non-Facility
$808
0%
Neurology
Facility
$444
0%
TOTAL
$658
0%
Non-Facility
$115
0%
Neurosurgery
Facility
$543
0%
TOTAL
$47
0%
Non-Facility
$22
-1%
Nuclear Medicine
Facility
$24
1%
TOTAL
$987
1%
Non-Facility
$20
1%
Nurse Anes / Anes Asst
Facility
$968
1%
TOTAL
$6,531
0%
Non-Facility
$4,295
0%
Nurse Practitioner
Facility
$2,236
0%
TOTAL
$531
-1%
Non-Facility
$364
-1%
Obstetrics/Gynecology
Facility
$167
0%
TOTAL
$4,469
-1%
Non-Facility
$3,153
-2%
Ophthalmology
Facility
$1,315
-1%
TOTAL
$1,280
-1%
Non-Facility
$1,223
-1%
Optometry
Facility
$56
0%
TOTAL
$57
0%
Non-Facility
$46
0%
Oral/Maxillofacial Surgery
Facility
$11
1%
TOTAL
$3,239
-1%
Non-Facility
$1,424
0%
Orthopedic Surgery
Facility
$1,815
-2%
TOTAL
$54
0%
Non-Facility
$43
0%
Other
Facility
$11
1%
TOTAL
$1,095
0%
Non-Facility
$874
1%
Otolaryngology
Facility
$221
0%
Pathology
TOTAL
$1,090
0%

(A)
Specialty
(B)
Total: Non-
Facility/Facility
(C)
Allowed
Charges
(mil)
(D)
Combined
Impact
Non-Facility
$583
0%
Facility
$507
0%
TOTAL
$51
1%
Non-Facility
$32
0%
Pediatrics
Facility
$18
1%
TOTAL
$1,054
0%
Non-Facility
$520
0%
Physical Medicine
Facility
$534
0%
TOTAL
$5,607
0%
Non-Facility
$5,607
0%
Physical/Occupational Therapy
Facility
$
4%
TOTAL
$3,472
0%
Non-Facility
$2,389
0%
Physician Assistant
Facility
$1,083
0%
TOTAL
$280
-1%
Non-Facility
$127
-1%
Plastic Surgery
Facility
$153
-1%
TOTAL
$1,780
0%
Non-Facility
$1,586
0%
Podiatry
Facility
$195
0%
TOTAL
$69
1%
Non-Facility
$67
1%
Portable X-Ray Supplier
Facility
$3
1%
TOTAL
$795
1%
Non-Facility
$466
1%
Psychiatry
Facility
$329
1%
TOTAL
$1,188
1%
Non-Facility
$523
1%
Pulmonary Disease
Facility
$665
0%
TOTAL
$1,458
0%
Non-Facility
$1,002
-1%
Radiation Oncology and Radiation Therapy Centers
Facility
$457
2%
TOTAL
$4,273
0%
Non-Facility
$1,894
-1%
Radiology
Facility
$2,379
1%
TOTAL
$496
0%
Non-Facility
$446
0%
Rheumatology
Facility
$50
0%
TOTAL
$277
0%
Thoracic Surgery
Non-Facility
$57
-2%

(A)
Specialty
(B)
Total: Non-
Facility/Facility
(C)
Allowed
Charges
(mil)
(D)
Combined
Impact
Facility
$220
0%
TOTAL
$1,532
-1%
Non-Facility
$1,081
-1%
Urology
Facility
$451
1%
TOTAL
$937
-2%
Non-Facility
$676
-2%
Vascular Surgery
Facility
$261
0%
TOTAL
$85,081
0%
Non-Facility
$54,049
0%
TOTAL
Facility
$31,033
0%
2. CY 2025 PFS Impact Discussion
a. Changes in RVUs
The most widespread specialty-level impacts of the RVU changes are generally related to
the changes to RVUs for specific services resulting from the misvalued code initiative, including
RVUs for new and revised codes. The estimated impacts for some specialties, including clinical
social workers and clinical psychologists, geriatrics, anesthesiology, and nurse anesthetists,
psychiatry, and family practice, reflect increases relative to other specialties. These increases
can largely be attributed to the Year 4 update to clinical labor pricing and/or the proposed
adjustments to transfer of postoperative care for global surgical procedures. These increases are
also due to increases in values for particular services after considering the recommendations
from the American Medical Association’s (AMA) Relative Value Scale Update Committee
(RUC) and CMS review, and increased payments resulting from supply and equipment pricing
updates.
The estimated impacts for several specialties, including diagnostic testing facilities,
vascular surgery, interventional radiology, ophthalmology, and orthopedic surgery, reflect
decreases in payments relative to payment to other specialties, largely resulting from the
redistributive effects of the implementation of the Year 4 update to clinical labor pricing and/or
the proposed adjustments to transfer of postoperative care for global surgical procedures. The
services furnished by these specialties were negatively affected by the redistributive effects of
increases in work RVUs for other codes, and/or rely primarily on supply/equipment items for
their practice expense costs and, therefore, were affected negatively by the updated Year 4
clinical labor pricing under budget neutrality. These decreases are also due to the revaluation of
individual procedures based on reviews, including consideration of AMA RUC review and
recommendations, as well as decreases resulting from the continued phase-in implementation of
the previously finalized supply and equipment pricing updates. The estimated impacts also
reflect decreases due to the continued implementation of previously finalized code-level
reductions that are being phased in over several years. For independent laboratories, it is
important to note that these entities receive approximately 83 percent of their Medicare revenues
from services that are paid under the CLFS.
We often receive comments regarding the changes in RVUs displayed on the specialty
impact table (Table 128), including comments received in response to the valuations. We remind
interested parties that although the estimated impacts are displayed at the specialty level,
typically, the changes are driven by the valuation of a relatively small number of new and/or
potentially misvalued codes. The percentage changes in Table 128 are based upon aggregate
estimated PFS allowed charges summed across all services furnished by physicians,
practitioners, and suppliers within a specialty to arrive at the total allowed charges for the
specialty, and compared to the same summed total from the previous calendar year. Therefore,
they are averages and may not necessarily represent what is happening to the particular services
furnished by a single practitioner within any given specialty.
As discussed previously, we have reviewed our suite of public use files and have worked
on new ways to offer interested parties additional information that addresses concerns about the
lack of granularity in our impact tables. To illustrate how impacts can vary within specialties,
we created a public use file that models the expected percentage change in total RVUs per
practitioner. Using CY 2023 utilization data, Total RVUs change between -1 percent and 1
percent for more than 80 percent of practitioners, representing approximately 75 percent of the
changes in Total RVUs for all practitioners, with variation by specialty. Specialties, such as
gastroenterology, exhibit little variation in changes in total RVUs per practitioner. Table 128
(CY 2025 PFS Estimated Impact on Total Allowed Charges by Specialty) indicates an overall
change of 0 percent for this specialty, and the practitioner-level distribution shows that 98
percent of these practitioners will experience a change in Total RVUs between -1 percent and 1
percent. The specific service mix within a specialty may vary by practitioner, so individual
practitioners may experience different changes in total RVUs. For example, Table 128 indicates
a 1 percent increase in RVUs for the physical/occupational therapy specialty as a whole;
however, 24 percent of physical/occupational therapy specialty practitioners—representing over
21 percent of Total RVUs for the specialty—will experience a 1 percent or more increase in
Total RVUs. Meanwhile, 13 percent of physical/occupational therapy specialty practitioners will
experience 1 percent or more decreases in Total RVUs, and these practitioners account for 14
percent of Total RVUs for this specialty. We also note the code level RVU changes are available
in the Addendum B public use file that we make available with each rule (see
https://www.cms.gov/medicare/payment/prospective-payment-systems/hospital-
outpatient/addendum-a-b-updates).
The specialty impacts displayed in Table 128 reflect changes within the pool of total
RVUs. The specialty impacts table, therefore, includes any changes in spending that result from
finalized policies that are subject to the statutory budget neutrality requirement at section
1848(c)(2)(B)(ii)(II) (such as the updated proposals associated with the transfer of postoperative
care for global surgical procedures in CY 2025 or the clinical labor pricing update phase-in that
began in CY 2022) but does not include any changes in spending which result from finalized
policies that are not subject to the statutory budget neutrality adjustment, and therefore, have a
neutral impact across all specialties. The 2.50 and 2.93 percent temporary payment increases for
CY 2023 and CY 2024, respectively, are statutory changes that take place outside of BN, and
therefore, are not captured in the specialty impacts displayed in Table 128. Section 1848(t)(2)(C)
specifies that these temporary payment increases are not to be taken into account in determining
fee schedules for physicians’ services furnished in years after the respective increases end. As
such, these temporary increases are not subject to the PFS budget neutrality adjustment.
b. Impact
Column F of Table 128 displays the estimated CY 2025 impact on total allowed charges,
by specialty, of all the RVU changes. A table showing the estimated impact of all of the changes
on total payments for selected high volume procedures is available under “downloads” on the
CY 2025 PFS proposed rule website at https://www.cms.gov/Medicare/Medicare-Fee-for-
Service-Payment/PhysicianFeeSched/. We selected these procedures for the sake of illustration
from among the procedures most commonly furnished by a broad spectrum of specialties. The
change in both facility rates and nonfacility rates are shown. For an explanation of facility and
nonfacility PE, we refer readers to Addendum A on the CMS website at
https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/.
D. Impact of Changes Related to Telehealth Services
We are proposing to add several codes to the Medicare Telehealth Services List on a
provisional basis, including HCPCS codes G0011, G0013, G0248, GCTD1-3, and GCTB1-2,
and CPT Codes 97550, 97551, 97552, 96202, and 96203. We are proposing to maintain certain
Telecommunications technology-related flexibilities through 2025, including that we will
continue to use a definition of direct supervision that allows "immediate availability” of the
supervising practitioner using real-time audio and video interactive telecommunications. We are
also proposing to delay implementation of the telehealth frequency limitations for subsequent
nursing facility and inpatient hospital visits for an additional year, to include two-way, real-time
audio-only communication technology for any telehealth service furnished to a beneficiary in
their home, and to continue to permit the distant site practitioner to use their currently enrolled
practice location instead of their home address when providing telehealth services from their
home. While we note that certain other Medicare telehealth flexibilities related to the PHE for
COVID-19 are expiring, including the removal of statutory geographic and location limitations
for most Medicare telehealth services, the beneficiary's home continues to be a permissible
originating site for certain types of services including those furnished for the diagnosis,
evaluation, or treatment of a mental health disorder, including a Substance Use Disorder (SUD),
and for monthly End Stage Renal Disease (ESRD) related clinical assessments described in
section 1881(b)(3)(B). However, expiration of certain flexibilities for Medicare telehealth
services is not expected to impact broader utilization of these services because reasonable and
necessary services for the diagnosis or treatment of an illness or injury continue to be covered.
Please see our request for comment in section II.E. of this proposed rule with regard to what
impact, if any, the expiration of the current flexibilities would be expected to have on overall
service utilization for CY 2025. Despite the fact that some services will no longer be furnished
under telehealth, we expect that they will continue to be furnished in-person. We therefore
anticipate that our proposed provisions will result in continued utilization of services that can be
furnished as Medicare telehealth services during CY 2025 at levels comparable to observed
utilization of these services during CY 2024.
E. Other Provisions of the Regulation
1. Impact of Proposals for Medicare Parts A and B Payment for Dental Services Inextricably
Linked to Specific Covered Medical Services
In section II.J.2. of this proposed rule, we are proposing to add to the list in
§ 411.15(i)(3)(i) of clinical scenarios under which FFS Medicare payment may be made for
dental services inextricably linked to covered services to now include certain dental services
associated with dialysis services for beneficiaries with end-stage renal disease (ESRD).
Specifically, we are proposing that Medicare Parts A and B payment may be made for dental or
oral examination performed as part of a comprehensive workup prior to Medicare-covered
dialysis services when used in the treatment of ESRD; and medically necessary diagnostic and
treatment services to eliminate an oral or dental infection prior to, or contemporaneously with
covered dialysis services in the treatment of ESRD. By proposing to provide payment for these
dental services, we would respond to the interested parties’ request that we focus on the ESRD
patient population. We are also soliciting comment on the potential connection between dental
services and covered services used in the treatment of diabetes as well as covered services for
individuals with sickle cell disease, hemophilia, or autoimmune diseases receiving
immunosuppressive therapies. We do not anticipate any significant increase in utilization or
payment impact for additional dental services given the historically low utilization of these
therapies.
Based on the Renal Management Information System (REMIS) and Enrollment Data
Base (EDB) gathered from the Integrated Data Repository (IDR) we estimate Fee-For-Service
(FFS) Part B ESRD enrollment to have averaged roughly 240 thousand enrollees during CY
2023. Based on United States Renal Data System (USRDS) from the NIH, we estimate that
roughly 40,000 of these enrollees are on the kidney transplant waitlist in any given year and that
roughly 10,000 of these patients on the waitlist would typically receive a transplant. Since we
already include dental services associated with kidney transplant patients in § 411.15(i)(3)(i)(A)
as an example of services for which payment can be made for certain dental services, we
removed these patients from the estimate, which left roughly 30,000 FFS beneficiaries.
For a variety of reasons outlined previously, we have historically observed low FFS
dental utilization in instances when coverage could apply (<1 percent of potential users). FFS
dental billing patterns have shown a cost per covered utilizer of about $525 in recent years. To
illustrate the potential cost of the proposed payment for dental services inextricably linked to
dialysis services for beneficiaries with ESRD we applied three scenarios of utilization (0.1
percent, 1 percent, 3 percent) and cost per patient of approximately $525 to the 30,000 patients.
Under all of these scenarios the policy is projected to represent a negligible cost to the Medicare
program (<$1,000,000) in any given year.
Therefore, we do not anticipate a significant payment impact for these provisions. It is
important to note that there is some uncertainty in these take-up rate assumptions, but they are
consistent with the current utilization of dental services, including after the regulation changes
made in the CYs 2023 and 2024 PFS final rules. Additionally, given that our proposed addition
to the list of clinical scenarios under which payment may be made for dental services
inextricably linked to covered services is not a change in coverage or payment policy, the cost
impact of this proposal is negligible and therefore it is not necessary to adjust the conversion
factor under the PFS budget neutrality requirement.
2. Impact of Proposed Changes Related to Supervision of Outpatient Therapy Services in
Private Practices
As discussed in section II.H. of this proposed rule, we are proposing to change our
regulatory requirements for OTs and PTs who are enrolled as suppliers in Medicare as OTs and
PTs in private practice (OTPPs and PTPPs, respectively) to allow for general supervision of their
occupational therapy assistants (OTAs) and physical therapist assistants (PTAs) to the extent
permitted under State law. The requirement for OTPPs and PTPPs to provide direct supervision
of OTAs and PTAs, which has been in place since 2005, requires the OTPP/PTPP to be present
in the office suite or in the patient’s home, and immediately available to furnish assistance and
direction throughout the performance of the procedure performed by the OTA/PTA (or by an OT
or PT they are supervising who is not enrolled in Medicare as a supplier). In contrast, the
proposal to allow for general supervision would mean that the procedure is furnished under the
OTPP’s/PTPP’s overall direction and control, but the OTPP/PTPP need not be present in the
treatment location or immediately available.
Should we finalize this proposal, we believe that the change to allow for general
supervision of OTAs/PTAs by OTPPs and PTPPs will have a positive impact on patient access to

outpatient therapy services; and will align with the currently required general supervision of
PTAs/OTAs by PTs and OTs who work for Medicare institutional providers, such as
rehabilitation agencies, outpatient hospitals, and SNFs. It would also reflect the supervision
level specified in 44 State physical therapy practice acts
803
and all but one State occupational
therapy practice act.
3. Impacts of Proposed Changes Related to Advanced Primary Care Management Services
In section II.G.2 of this proposed rule, “Advanced Primary Care Management (APCM)
Services,” we are proposing to create three HCPCS codes to use for reporting the proposed
APCM services (HCPCS codes GPCM1, GPCM2, and GPCM3) to recognize the resources
involved in furnishing services using an advanced primary care delivery model under the PFS.
As described in sections II.G.2.b and II.G.2.c of this proposed rule, the proposed APCM services
incorporate elements of existing services with the understanding that some patients will require
more resources and some fewer based on variability in patient complexity and needs. As we
ordinarily do, we are proposing to base the PFS valuation for APCM codes on the resources
involved in furnishing the typical case of the service which may not necessarily reflect the actual
resources involved in furnishing every individual service. To value APCM, we compared the
service elements described by the proposed APCM codes to the values we have established for
the specific care management services and communication technology-based services (CTBS)
codes on which we modeled the proposed service elements of the APCM codes and which we
built into the service descriptors for GPCM1, GPCM2, and GPCM3 (see also Table 129 and
sections II.G.2.b. through II.G.2.d. of this proposed rule). Specifically, the proposed APCM
services incorporate elements of chronic care management (CPT codes 99487, 99489, 99490,
99491, 99439, 99437), principal care management (CPT codes 99424, 99425, 99426, 99427)),
transitional care management (CPT codes 99495 and 99496), interprofessional internet
803
Federation of State Boards of Physical Therapy Jurisdiction Licensure Reference Guide,
https://www.fsbpt.net/lrg/Home/SupervisionRequirementLevelsBySetting.
consultation furnished by treating/requesting practitioner (CPT code 99452), remote evaluation
of patient videos/images (HCPCS code G2250), virtual check-ins (HCPCS code G2251 and
G2252), and online digital E/M or e-visits (CPT codes 98970, 98971, 98972, 99421, 99422,
99423) into this new bundled PFS payment beginning for CY 2025.
As discussed throughout section II.G.2 of this proposed rule, we believe that the proposed
elements of APCM services reflect the comprehensive approach to care management involved in
care delivery using the advanced primary care model. This is a model of primary care that is
being integrated into current medical practice. As such, we believe that it would be appropriate
to use the current valuation and uptake of the codes on which we modeled the APCM codes to
inform our valuation of APCM services. Using Medicare FFS claims data and evidence from the
CMS Innovation Center’s testing of a series of advanced primary care models (see discussion in
section II.G.2.a.(1) of this proposed rule), we sought to understand how these different services
have been used historically and relate that information to the way we are thinking about the
service elements for APCM and the valuation of the three APCM code levels. As discussed in
section II.G.2.e. of this proposed rule, for Medicare beneficiaries who receive care management
services during a year, the non-complex CCM base code is billed on average for five months and
with three add-on codes during those five months. However, this does not account for the care
management services that are provided beyond one time-based billing interval and without
reaching the next; nor does it account for the resources involved in maintaining certain advanced
primary care practice capabilities and readiness, including patient population monitoring and
care needs assessment, to fully furnish and bill APCM services as is medically reasonable and
necessary for any individual patient during any calendar month. Finally, this does not account for
changes to utilization of APCM that may occur as a result of the billing and documentation
requirements for APCM services when compared to the current coding and payment for care
management and CTBS services.
We are estimating a utilization of approximately 300,000 claims for the proposed HCPCS
code GPCM1, 1.3 million claims for the proposed HCPCS code GPCM2, and 400,000 claims for
the proposed HCPCS code GPCM3, and are seeking comment on our assumptions. To estimate
utilization for GPCM1, we first calculated an eligible GPCM1 population by estimating the
number of Medicare beneficiaries without multiple chronic conditions who have an established
relationship with a primary care provider using Welcome to Medicare and Annual Wellness Visit
claims and estimating the uptake of APCM Level 1 based on average uptake of CCM/PCM/TCM
in CY 2022 claims data; then, we adjusted this estimate to account for increased frequency of
billing (multiplied by 12 to account for 12 months of assumed practitioner billing for the
proposed APCM service). To estimate utilization for GPCM2, we first calculated estimated
ratios to represent the average utilization of CCM/PCM/TCM services in the first year of policy
implementation compared to CY 2022 claims; then, we applied a reduced utilization ratio to CY
2022 claims for CPT codes 99490 and 99487 (10.4 percent) and multiplied by the eligible
GPCM2 population of Medicare beneficiaries with multiple chronic conditions who are non-
QMB; finally, as described for GPCM1, we adjusted this estimate to account for increased
frequency of billing (increase from an average of five months of CCM claims per beneficiary to
12 months of assumed practitioner billing for the proposed APCM service, or 237.3 percent). To
estimate utilization for GPCM3, we took the estimated number of GPCM2 claims for CPT codes
99490 and 99487 and multiplied by the eligible GPCM3 population of Medicare beneficiaries
with multiple chronic conditions who are QMB; again, we adjusted this estimate to account for
increased frequency of billing (same percentage applied to GPCM2).
We anticipate that these proposed coding and payment policies for APCM services would
result in slight reductions in utilization of existing care management and CTBS services during
CY 2025 when compared to observed utilization of these services during CY 2024. Specifically,
we are estimating an approximate 11.4 percent reduction in utilization from CY 2024 across the
20 service codes which are incorporated into the APCM services (see previously). The estimated
total net increase is approximately 700,000 claims, and we do not anticipate a significant
payment impact for these provisions. We believe that the cost impact of this proposal is
negligible and therefore it is not necessary to adjust the conversion factor under the PFS budget
neutrality requirement.
4. Impact of Proposed Changes Related to Strategies for Improving Global Surgery Payment
Accuracy
In section II.G.5 of this proposed rule, “Strategies for Improving Global Surgery Payment
Accuracy,” we discuss our current policy to require the use of modifiers to help us identify and
adjust payment for a global package when there is a formal transfer of care (that is, a written
agreement) between the practitioner who furnishes the procedure (proceduralist) and another
practitioner. The transfer of care modifiers identify and adjust the global package payment for
cases where the pre-operative, operative, and post-operative portions of the global package are
furnished by different practitioners. We are proposing to require the use of the appropriate
transfer of care modifier (modifier -54, -55, or -56) for all 90-day global surgical packages in any
case when a practitioner (or another in the same group practice) expects to furnish only a portion
of a global package (including but not limited to when there is a formal, documented transfer of
care as under current policy, or an informal, non-documented but expected, transfer of care. This
could result in more application of, and therefore more impact on spending from the appropriate
usage of the transfer of care modifiers (modifiers -54, -55, and -56).
As noted earlier, we are proposing to require the use of the existing modifier (-54) for all
90-day global surgical packages in any case when a practitioner (or group practice) expects to
furnish only the procedure portion of a global package (including when there is a formal,
documented transfer of care as under current policy). Since we believe that this will result in
expanded use of the transfer of care modifiers, which will have a corresponding effect on the
payment for the affected services, we have reflected this in our utilization estimates accordingly
(see download file for this proposed rule titled CY 2025 PFS final rule 2023 Utilization Data
Crosswalked to 2025 at https://www.cms.gov/medicare/payment/fee-schedules/physician/federal-
regulation-notices?DLSort=2&DLEntries=10&DLPage=1&DLSortDir=descending) and
anticipate more global surgical packages and post-op care to be billed separately using the
modifiers, which could have payment consequences for a selection of high-volume global
surgery codes. We assume that the same number of global surgery codes would be billed;
however, we anticipate more codes would be billed using the transfer of care modifiers. We do
not expect the utilization of separately billable post-operative E/M services would change.
Rather than modify our utilization estimates for these codes, our utilization estimate in this
proposal includes only 90-day high volume and/or high-cost procedure codes where reporting
post-operative visits with CPT code 99024 (Postoperative follow-up visit, normally included in
the surgical package, to indicate that an evaluation and management service was performed
during a postoperative period for a reason(s) related to the original procedure) is required. This
is a relatively small set of codes (approximately 180) versus the full range of approximately
4,000 global surgical codes; however, this subset of codes accounts for about 73 percent of total
Medicare 90-day procedure volume. The full list of affected codes is available in the file titled
“CY 2024 Analytic Crosswalk to CY 2025” on the CMS website under downloads for the CY
2025 PFS proposed rule at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-
Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices.html.
For this select list of global surgical codes, we are estimating that the transfer of care
modifier, modifier -54, will be employed 20 percent of the time. We believe that this is a
conservative estimate given the frequency with which these global surgical services involve a
transfer of postoperative care. RAND’s research has indicated that a post-operative transfer of
care is common for 90-day global surgical procedures but that these transfers of care are almost
never reported with the appropriate -54 or -55 modifiers. Then, for the 20 percent of cases where
we believe the transfer of care modifier will be employed, we are proposing to apply the
payment reduction associated with the modifier -54 for postoperative care and apply it to the
utilization estimate for the associated procedures billed using the transfer of care modifiers.
These percentages can be found in the PFS Relative Value Files under the columns labeled “pre
op, intra op, post op” at https://www.cms.gov/medicare/payment/fee-schedules/physician/pfs-
relative-value-files. For example, CPT code 27447 (Arthroplasty, knee, condyle and plateau;
medial AND lateral compartments with or without patella resurfacing (total knee arthroplasty))
is a high-volume knee replacement procedure where the postoperative portion of the total
payment is 21 percent. We are proposing that there will be a postoperative transfer of care 20
percent of the time for CPT code 27447, and in those 20 percent of cases, there will be a
corresponding 21 percent decrease in payment. This is reflected in a utilization crosswalk of
0.958 for CPT code 27447 as a result of this 4.2 percent reduction (20 percent times 21 percent)
to capture this estimated reduction in spending associated with our proposal to require the use of
these transfer of care modifiers.
We note that for purposes of estimating the utilization of the transfer of care modifiers,
our estimates include increased reporting of the transfer of care modifier for codes that are
subject to the RAND data collection exercise, with the exception of cases where the modifier is
already used 5% of the time or more. We recognize that this policy will apply more broadly and
seek comment on this.
We note that the impact of the conversion factor on this proposed reduction in spending
associated with this policy is redistributed across the PFS via an increase in the budget neutrality
adjustment to the conversion factor. We are soliciting comments from interested parties on our
proposed implementation of this postoperative transfer of care policy.
We are also proposing a new HCPCS code, GPOC1, to capture the additional practitioner
time and resources spent in providing follow up post-operative care by a practitioner who did not
perform the surgical procedure. Additionally, we expect the proposed global surgical add-on
code, HCPCS code GPOC1, will be billed during the post-operative period of 90 days following
the procedure. We expect that this code will be billed once during the global period when the
patient is seen for an office/outpatient (O/O) evaluation and management (E/M) visit that is
related to the recent surgical procedure. We believe that this code will be billed by a physician or
other practitioner (other than the proceduralist or another practitioner in the same practice) who
is seeing the patient for a visit during the post-operative period and did not furnish the surgical
procedure. We believe that there is additional time, resources, and complexity involved in the
first O/O E/M visit following a procedure that should be captured during the post-operative
period and may be billed in certain instances when a transfer of care modifier was not appended
to the claim.
We are estimating a utilization of approximately 40,000 total claims in the first year for
the proposed add-on code, HCPCS code GPOC1. We calculated this utilization estimate based
on claims data for procedure codes with a post-operative diagnosis code and an observed to
expected ratio (that is the ratio of visits that are included in the global surgical package compared
to the number of visits actually furnished) of less than 25 percent. We anticipate that uptake of
HCPCS code GPOC1 would be low initially, consistent with initial uptake of other new services
we have finalized under the PFS. We are seeking comment on these assumptions and welcome
input from the public.
5. Drugs and Biological Products Paid Under Medicare Part B
a. Requiring Manufacturers of Certain Single-Dose Container or Single-Use Package Drugs to
Provide Refunds with Respect to Discarded Amounts
Section 90004 of the Infrastructure Investment and Jobs Act (Pub. L. 117-58, November
15, 2021) amended section 1847A of the Act to require manufacturers to provide a refund to
CMS for certain discarded amounts from a refundable single-dose container or single-use
package drug. The refund amount is either as noted in section 1847A(b)(1)(B) of the Act in the
case of a single source drug or biological or as noted in section 1847A(b)(1)(C) of the Act in the
case of a biosimilar biological product, multiplied by the amount of discarded drug that exceeds
an applicable percentage, which is required to be at least 10 percent, of total charges (subject to
certain exclusions) for the drug in a given calendar quarter. In the CY 2023 and 2024 final rules,

we finalized several policies to implement the provision. These policies are described in section
III.A.1 of this proposed rule.
In section III.A.1 of this proposed rule, we are proposing additional policies for
implementing the provision including: a change in how we would identify certain drugs that are
excluded from the definition of refundable drug for those which payment has been made under
Part B for fewer than 18 months; how we identify drugs from a single-dose container; to require
the JW modifier if a billing supplier is not administering a drug, but there are discarded amounts
during the preparation process before supplying the drug to the patient; and we discuss an
application for increased applicable percentage (CMS 10835, OMB 0938-1435).
In the CY 2024 PFS final rule (88 FR 79485 through 79490), we analyzed JW modifier
data from 2021 as if the data represented dates of service on or after the effective date of section
90004 of the Infrastructure Act (that is, January 1, 2023).
804
Similar to our regulatory impact
analysis in the CY 2023 PFS final rule (87 FR 70187 through 70188), we used the 2021 JW
modifier data to estimate refund amounts as described in section 1847A(h)(3) of the Act. In this
proposed rule, we performed the same analysis on the 2022 JW modifier data. First, we
subtracted the percent units discarded by 10 percent (the applicable percentage for most
refundable drugs), except for drugs with an increased applicable percentage as described in
§ 414.940(d). We note that since the data indicating which drugs will have an increased
applicable percentage of 26 percent for the unique circumstances of rarely utilized orphan drugs
(§ 414.940(d)(5)) will not be available until the data is analyzed for the initial report, we entered
26 percent for orphan drugs furnished to fewer than 100 beneficiaries in CY 2022 based on data
on the CMS website.
805
Therefore, the drugs with increased applicable percentage under
§ 414.940(d)(5) may change each year based on claims data; it is applied in this analysis for
804
https://data.cms.gov/summary-statistics-on-use-and-payments/medicare-medicaid-spending-by-drug/medicare-
part-b-discarded-drug-units.
805
https://data.cms.gov/summary-statistics-on-use-and-payments/medicare-medicaid-spending-by-drug/medicare-
part-b-spending-by-drug

estimation purposes only. Then, we multiplied that percentage by the CY 2022 total allowed
amount to estimate the annual refund for a given billing and payment code. The quarterly refund
was estimated by dividing the annual estimate by 4. This analysis remains appropriate for this
proposed rule because we are applying the finalized policies from the CY 2023 and 2024 PFS
final rules to the most recent publicly available data for the JW modifier data from 2022.
Overall, according to data on the CMS website
806
for Medicare Part B discarded drug
units in the 2022 calendar year, Medicare paid over $800 million for discarded amounts of drugs
from a single-dose container or single-use package paid under Part B. In that year, there were 55
billing and payment codes with 10 percent or more discarded units based on JW modifier data.
Of these, 10 did not meet the definition of refundable single-dose container or single-use
package drug in section 1847A(h)(8) of the Act because they are not single source drugs or
biologicals; 5 were excluded from the definition of refundable single-dose container or single-
use package drug (as specified in section 1847A(h)(8)(B) of the Act) because they are identified
as radiopharmaceuticals or imaging agents in FDA-approved labeling; and 3 are products
referred to as skin substitutes, which were removed because we anticipate making changes to
coding and payment policies regarding those products in future rulemaking. After these
exclusions, there were 35 billing and payment codes that met the definition of refundable single-
dose container or single-use package drug. Of these, 29 codes have discarded units above the
relevant finalized applicable percentage, and 6 codes have discarded units that would fall below
increased applicable percentages finalized in this proposed rule.
We estimated refund amounts as described in section 1847A(h)(3) of the Act were
calculated based on this data by subtracting the percent units discarded by 10 percent (the
applicable percentage), except for drugs with higher applicable percentages finalized in the CY
2023 or 2024 final rules. Then, we multiplied the appropriate percentage by the CY 2022 total
806
https://data.cms.gov/summary-statistics-on-use-and-payments/medicare-medicaid-spending-by-drug/medicare-
part-b-discarded-drug-units.

allowed amount to estimate the annual refund for a given billing and payment code. The
quarterly refund was estimated by dividing the annual estimate by 4. Based on this data, there
would be approximately $98.7 million in refunds due from manufacturers for the calendar year
of 2022 ($24.7 million each calendar quarter).
There are several limitations to this analysis that could substantially affect the total
quarterly refund. Since new drugs are continually being approved, this estimate does not
consider newer drugs that will meet the definition of refundable single-dose container or single-
use package drug on or after the effective date of January 1, 2023. Since section
1847A(h)(8)(B)(iii) of the Act excludes drugs approved by FDA on or after November 15, 2021,
and for which payment has been made under Part B for fewer than 18 months from this
definition, we expect an impact on refund amounts after the 18-month exclusion has ended if the
drug otherwise meets the definition. We also noted that this estimate is based on CY 2022 data
for discarded drug amounts, which, for reasons discussed in the CY 2023 PFS final rule (87 FR
69716), we believe to be an underestimate due to the frequent omission of the JW modifier
Claims edits for both the JW and JZ modifiers will likely increase accurate reporting of
discarded drug amounts. Other substantial changes to this estimate may occur if a billing and
payment code no longer meets this definition. For example, if a generic version of one of these
drugs is marketed, the billing and payment code will become a multiple source drug code and
will no longer meet the definition of refundable single-dose container or single-use package
drug. Subsequently, the manufacturers will not be responsible for refunds under this provision.
There may be changes in the percent discarded units for a given refundable single-dose container
or single-use package drug if the manufacturer introduces additional vial sizes or modifies the
vial size to reduce the amount discarded. Lastly, since data from the CMS website only includes
billing and payment codes on the ASP drug pricing file
807
and implementation of section 90004
807
https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice.
of the Infrastructure Act is not restricted to billing and payment codes included on the file, there
may be other applicable data that was not assessed as part of this estimate.
b. Impacts Related to the Payment Limit Calculation When Manufacturers Report Negative or
Zero Average Sales
In section III.A.2 of this proposed rule, CMS is proposing how payment limits would be
calculated when manufacturers report negative or zero ASP data to CMS. We are proposing to
amend § 414.904(i) to reflect CMS’ approach to setting a payment limit for circumstances in
which negative or zero ASP data is reported by a manufacturer for a single source drug.
Specifically, we are proposing to codify that in cases where negative or zero ASP data is
reported for some, but not all, NDCs of a multiple source drug, we would calculate the payment
limit using the positive ASP data reported for the drug, except for the existing carryover policy
for multiple source drugs that we would apply when missing data results in a significant change
in the ASP payment limit. We are proposing to move this carryover policy for multiple source
drugs within § 414.904(i) to fit within the structure of the proposed new set of payment limit
methodologies. We are also proposing to codify that in the case of a multiple source drug for
which negative or zero ASP data is reported for all NDCs, we would set the payment limit using
the most recent available positive ASP data from a previous quarter until at least one NDC for
the drug has positive ASP data for a quarter.
We are proposing to codify that in cases where negative or zero ASP data is reported for
some, but not all, NDCs of a single source drug that is not a biosimilar, we would calculate the
payment limit using the positive ASP data reported for the drug. We are proposing to codify that
for single source drugs that are not biosimilars with all negative or zero ASP data for a given
quarter, the payment limit would be, until at least one NDC for the drug has positive
manufacturer ASP data for a quarter, the lesser of 106 percent of the volume-weighted average
of the most recent available positive manufacturer ASP data for at least one NDC from a
previous quarter and 106 percent of the wholesale acquisition cost, and we would use 106
percent of the lowest wholesale acquisition cost per billing unit if there is more than one
wholesale acquisition cost per billing unit.
We are also proposing to codify that in cases where negative or zero ASP data is reported
for some, but not all, NDCs of a biosimilar, we would calculate the payment limit using the
positive ASP data reported for the biosimilar. Lastly, we are proposing to codify two scenarios
when the manufacturer reports negative or zero ASP for all NDCs for a biosimilar for a given
quarter: (1) when positive ASP data is available for another biosimilar(s) with the same reference
product for the given quarter, we are proposing to set the payment limit equal to the sum of the
volume-weighted average of the positive ASP data from all other biosimilars referencing the
same reference product plus 6 percent (or 8 percent for a qualifying biosimilar biological) of the
amount determined under section 1847A(b)(4) of the Act for the reference biological product for
the given quarter; and (2) when either no other biosimilars have been approved for the same
reference product or no other biosimilars with the same reference product report positive ASP
data for the given quarter, we are proposing to set the payment limit equal to the sum of the
volume-weighted average of the most recent available positive ASP data from a previous quarter
plus 6 percent (or 8 percent for a qualifying biosimilar biological) of the amount determined
under section 1847A(b)(4) of the Act for the reference biological product for the given quarter.
With regard to estimating changes in expenditures for CY 2025, because drugs and
biologicals that report negative or zero ASP data vary by quarter and we cannot predict those that
will report such data, we used historic claims data to perform an illustrative analysis of how
program spending would have changed had the proposed policies been in place in CY 2023. In
the analysis, we identified single source and multiple source billing and payment codes
associated with negative or zero ASP data for which we published payment limits based on other
applicable pricing data (that is, the manufacturer or published wholesale acquisition cost) in the
four calendar quarters of 2023. For each such billing and payment code, we used claims data to
identify: (1) the number of allowed billing units in a calendar quarter (that is, the number of
billing units of a drug or biological paid for by Medicare); (2) the payment limit per billing unit
we applied to that drug or biological under our current policies; and (3) the payment limit per
billing unit our proposal would apply to the billing and payment code. We then subtracted the
product of the allowed billing units for the payment limit under current policies by the product of
the allowed billing units and the payment limit under our proposal, and the difference between
the two is what the difference between what Medicare spending on the billing and payment
codes would have been if our proposed payment limit methodologies were used in that calendar
quarter of 2023 and what Medicare actually spent. These data and net reductions (or increases) in
program spending are illustrated in Table 130.

TABLE 130: Theoretical Changes in Medicare Spending on Drugs and Biologicals with Negative or
Zero ASP Data Based on CY 2023 Claims Data
1Q2023
Allowed Billing
Units
Current Policy
(WAC+6)
Proposal
Δ Between Current and Proposal
J2770
15
$493.9695
$337.4653
$2,347.56
J1020
47,111
$4.6110
$1.4914
$146,966.53
J0600
406
$5,708.5940
$5,592.5623
$47,108.84
J0641
741,697
$0.3456
$0.2660
$58,985.13
J0720
116
$41.2552
$40.4146
$97.51
J1000
1,445
$29.6991
$26.4542
$4,688.84
J3300
360,762
$4.1851
$3.9035
$101,596.03
J9214
62
$32.5738
$31.8462
$45.11
J9302
601
$63.9572
$62.2336
$1,035.92
J9320
0
$370.1838
$332.9778
$0.00
Total
$362,871.47
2Q2023
Allowed Billing
Units
Current Policy
(WAC+6)
Proposal
Δ Between Current and Proposal
J1952
83,802
$98.4290
$88.7509
$811,044.34
J0285
79
$46.3220
$41.8181
$355.81
J0287
408
$11.1300
$10.2985
$339.24
J0600
435
$5,708.5940
$5,592.5623
$50,473.78
J1020
51,393
$4.6110
$3.1920
$72,927.54
J3300
381,643
$4.1850
$3.9035
$107,421.25
J9071
69,165
$3.8690
$3.5744
$20,374.38
J9214
31
$32.5740
$31.8462
$22.56
J9268
114
$2,585.4140
$2,320.1163
$30,243.93
J9320
0
$370.1840
$332.9778
$0.00
Total
3Q2023
Allowed Billing
Units
Current Policy
(WAC+6)
Proposal
Δ Between Current and Proposal
J0216
67
2.565
$2.23
$22.12
J0636
74,355
0.795
$0.76
$2,503.99
J9214
21
32.574
$31.85
$15.28
J0287
154
11.13
$10.30
$128.05
J1572
1,991
56.1164
$44.54
$23,049.18
J1738
242
3.321333333
$3.13
$46.94
J2360
13,633
16.536
$5.38
$152,117.66
J3300
342,992
4.268885
$3.90
$125,314.01
J7342
23
30.0192
$30.01
$0.25
J9046
42,252
48.54739429
$8.65
$1,685,649.00
J9198
406
40.28
$24.67
$6,330.93
J9296
3,417
9.655727059
$9.74
-$273.00
J9393
27,650
21.2
$3.07
$501,318.72
Total
$2,496,223.13

4Q2023
Allowed Billing
Units
Current Policy
(WAC+6)
Proposal
Δ Between Current and Proposal
J9071
140,721
3.869
$1.19
$377,386.29
J1000
1,003
32.6692
$30.68
$1,993.04
J1980
1,085
35.46442
$33.23
$2,421.54
J0595
272
2.54347
$5.05
-$681.42
J0893
12,457
2.12
$2.24
-$1,495.80
J9198
237
40.28
$24.67
$3,700.20
J9394
56,602
53
$1.65
$2,906,338.93
Total
$3,289,662.78
As illustrated in Table 131, the application of the proposed payment limit calculation
approaches would have reduced program spending for all but three drugs that reported negative
or zero ASP data in calendar quarters in 2023 and reduced spending by a total of $7,241,960.21
over the year.
We separately analyzed theoretical changes in program spending for one biosimilar
product (ZIEXTENZO®, Q5120) that has reported negative ASP data for all NDCs for three
consecutive quarters beginning with the second calendar quarter of 2022, and calculated the
payment limit under our proposed method for biosimilars with negative or zero ASP data and
changes in program spending for the three impacted quarters had our proposed payment
approach been applied. We also analyzed payment limits and theoretical changes in program
spending under two alternatives considered under the proposed rule. Under the first alternative,
we would include the ASP data and billing units sold of the reference biological for a given
quarter along with those of the other biosimilars in the volume-weighted average calculation.
Under the second alternative, we would set the payment limit for a given quarter using the
biosimilar’s most recent available positive ASP data and either 6 percent (or 8 percent for
qualifying biosimilar biologicals) of the amount determined under section 1847A(b)(4) of the
Act for the reference biological product (as defined in § 414.902) for the given quarter. The
calculated payment limits under the proposal and the two alternatives, as well as the estimate
reductions in program expenditures, are illustrated in Table 131.
TABLE 131: Theoretical Changes in Medicare Spending on Q5120 for 4Q 2023 through 2Q 2024

Ziextenzo
(Q5120)
Allowed
Billing
Units
Current Policy
(WAC+6)
Payment limit:
Proposal
Payment limit:
First
Alternative
Payment limit:
Second Alternative
4Q2023
2,299
$346.755
$118.369
$95.267
$29.24
1Q2024
1179
$346.755
$117.313
$86.053
$27.40
2Q2024
132
$346.755
$149.574
$127.623
$31.86
Spending Δ:
Proposal
Spending Δ:
First
Alternative
Spending Δ:
Second Alternative
4Q2023
$525,058.988
$578,171.42
$729,975.52
1Q2024
$270,512.618
$307,367.11
$376,513.91
2Q2024
$26,027.867
$28,925.38
$41,565.72
Total Savings
$821,599.473
$914,463.917
$1,148,055.145
We note that the spending change estimates reflect preliminary claims data. Providers
and suppliers have a 12-month period to submit Medicare Part B claims, including claims for
drugs payable under Part B, so a lag exists between the date of service when a drug is
administered and when the claim is submitted and adjudicated. Because of this lag in finalized
claims, there may also be a lag in available JW modifier data for any given date of service
quarter. An evaluation of July 2010 Medicare Part B claims in the Physician/Supplier-Carrier
setting showed that 91.68, 96.84, and 98.32, and 99.13 percent of claims were final at 3, 6, 9, and
12 months, respectively, following the date of service. At 24 and 48 months, 99.83 and 100
percent of the claims, respectively, were considered to be final. Therefore, for the allowed billing
units and estimated program expenditure reduction for the first 2 calendar quarters of CY 2024
are significantly lower than we would expect after claims mature for a full year. Over the 3
calendar quarters (4Q2023, 1Q2024, and 2Q2024), our proposed approach for calculating the
payment limit for biosimilars with only negative or zero ASP and the two alternative approaches
would have reduced program expenditures by at least $821,599.473, $914,463.917, and
$1,148,055.145, respectively.
After assessing the effect of applying the proposed alternative payment limit calculation
approaches to recent Medicare FFS claims experience over 2023 and 2024, we estimate an
average annual gross Part B effect of $10 million dollars in reduced program spending for 2025
and approximately $100 million over 2025 to 2034, as shown in Table 134. Historically we have
observed that negative or zero ASP pricing data may occasionally occur for a drug when it is
discontinued or substituted away for another product and assume this to occur in the future.
Moreover, given the infrequency of negative or zero ASP data, we do not expect in all years that
alternative pricing approaches will be necessary or to affect drugs with material levels of
utilization. Therefore, for a low estimate we project the policy to have a negligible effect on
program spending for the projection window. To illustrate a potential high impact estimate
scenario, the affected utilization from 2023 was doubled relative to the observed data. Please
note that the actual effect of the policy will be specific to the affected drugs in any given year
and considerations that affect their utilization and pricing, therefore actual experience may
deviate considerably from these projections.
c. Impacts Related to the Payment of Radiopharmaceuticals in the Physician Office
In section III.A.3. of this proposed rule, while we evaluate our broader policies in this
space for future rulemaking, we are proposing to codify in regulations at § 414.904(e)(6) that, for
radiopharmaceuticals furnished in a setting other than the hospital outpatient department, MACs
shall determine payment limits for radiopharmaceuticals based on any methodology used to
determine payment limits for radiopharmaceuticals in place on or prior to November 2003. Such
methodology may include, but is not limited to, the use of invoice-based pricing. The proposal
does not necessarily change the payment methodology in place for a MAC but rather clarifies
that any payment methodology that was being used by any MAC prior to the enactment of the
MMA can continue to be used by any MAC. Therefore, we believe that this proposal will have
no impact on Medicare spending.
d. Impacts Related to Immunosuppressive Therapy
In section III.A.4 of this proposed rule, we are proposing to modify regulations to include
orally or enterally administered compounded formulations of FDA-approved drugs that have
approved immunosuppressive indications, or that have been determined by a Medicare
Administrative Contractor (MAC) to be reasonable and necessary for specific purposes in
immunosuppressive treatment in the immunosuppressive drug benefit. In addition, we are
proposing two changes regarding supplies of immunosuppressive drugs to align with current
standards of practice and reduce barriers to medication adherence: to allow payment of a supply
fee for a prescription of a supply of up to 90 days and to allow prescriptions for these
immunosuppressive drugs to be refillable.
CMS has limited insight into how many patients who are currently prescribed
compounded immunosuppressive drugs would have their immunosuppressive medication paid
for under Part B if the proposed changes to the immunosuppressive drug benefit are finalized.
Medicare Part D claims data for CY 2023 indicates there were 2,662 prescriptions filled that year
for compounded immunosuppressive drugs that could have been administered through oral or
enteral routes (that is, that would likely be paid under Part B if the immunosuppressive drug
benefit revision is finalized as proposed). We estimate that this number of prescriptions
correlates to up to 2,000 Part D enrollees that were prescribed compounded immunosuppressive
drugs that would be covered if the proposal is finalized. However, we do not know how many
Part B beneficiaries currently have their compounded immunosuppressive drugs paid for by
means other than a Part D policy. And finally, and perhaps most importantly, compounded drugs
are priced by each A/B and DME MAC and have no estimable payment limit. Thus, we are
unable to estimate the cost shift from Part D and other plans to Part B that would result from a
finalization of the immunosuppressive drug benefit proposed policies, including allowing
payment of supply fees for prescriptions fills for supplies of up to 90 days and for
immunosuppressive drugs to be refillable.
e. Impacts Related to Clotting Factors
In section III.A.5. of this proposed rule, we are proposing to update § 410.63(b) to clarify
existing CMS policy that blood clotting factors must be self-administered to be considered
clotting factors for which the furnishing fee applies. Additionally, we are proposing to clarify at
§ 410.63(c) that the furnishing fee is only available to entities that furnish blood clotting factors,
unless the costs associated with furnishing the clotting factor are paid though another payment
system, including the PFS. That is, we are proposing to clarify through revisions to § 410.63 that
clotting factors (as specified in section 1861(s)(2)(I) of the Act) and those eligible to receive the
clotting factor furnishing fee (as specified in section 1842(o)(5) of the Act) are the same subset
of products. Accordingly, the clarification will not be adding a furnishing fee to any new
products. Therefore, we believe that this clarification will have no impact on Medicare spending.
6. Impacts related to Rural Health Clinics (RHCs) and Federally Qualified Health Centers
(FQHCs)
In section III.B.2. of this proposed rule, we are proposing to require RHCs and FQHCs to
bill the individual codes that make up the general care management HCPCS code, G0511.
Payment amounts for some services that comprise HCPCS code G0511 are less than the payment
amount for G0511 and if an RHC or FQHC mostly furnishes these services, they could see a
potential decline in payment. We are also proposing to allow RHCs and FQHCs to bill the add-
on codes for additional time spent once the minimum threshold of time was met to account for a
complete encounter. This could potentially offset any decrease in payments. In addition,
beginning in CY 2025, we are proposing to adopt the coding and policies regarding Advanced
Primary Care Management (APCM) services proposed under the PFS, as discussed in section
II.G of this proposed rule. In terms of estimated impacts to the Medicare program, we believe
that the proposals discussed in section III.B.2 of this proposed rule would have no impact on
Medicare spending.
In section III.B.3. of this proposed rule, we are proposing the policy to continue to adopt
the definition “immediate availability” as including real-time audio and visual interactive
telecommunications for the direct supervision of services and supplies furnished incident to a
physician’s service through December 31, 2025, for RHCs and FQHCs. We are also proposing,
on a temporary basis, to allow payment for non-behavioral health visits furnished via
telecommunication technology in a manner that would closely align with the payment
mechanisms mandated by statute through December 31, 2024, that is, RHCs and FQHCs would
continue to bill for RHC and FQHC services furnished using telecommunication technology
services by reporting HCPCS code G2025 on the claim through December 31, 2025. In addition,
we are proposing to continue to delay the in-person visit requirement for mental health services
furnished via communication technology by RHCs and FQHCs to beneficiaries in their homes
until January 1, 2026. We believe these RHC/FQHC proposals related to telecommunication
technology would have a negligible impact on Medicare spending.
In section III.B.4. of this proposed rule, we discuss the implementation of section 4124 of
the CAA, 2023. Section 4124 of the CAA, 2023 established Medicare coverage for intensive
outpatient (IOP) services furnished by a hospital to its outpatients, or by a community mental
health center, a RHC or a FQHC, as a distinct and organized intensive ambulatory treatment
service offering less than 24-hour daily care in a location other than an individual’s home or
inpatient or residential setting, effective January 1, 2024 (88 FR 81838). We are proposing to
provide a payment rate for 4 or more services per day in the RHC and FQHC setting. In terms of
impact, we believe that this proposal would have negligible impact on Medicare spending.
In section III.B.5. of this rulemaking, we are proposing to allow RHCs and FQHCs to bill for
Part B preventive vaccines and the administration at the time of service. We propose that
payments for these claims will initially be made according to Part B preventive vaccine payment
rates in other settings, but that they will be annually reconciled with the facilities’ actual vaccine
costs on their cost reports, which is current practice and statutorily mandated. Therefore, we
believe that this proposal would have no impact on Medicare spending.
In section III.B.6. of this proposed rule, we discuss our proposal relating to RHC
productivity standards. We are proposing to remove productivity standards for RHCs and
therefore believe that the proposal discussed in this section III.B.6. of this proposed rule would
have no impact on Medicare spending.
In section III.B.8. of this proposed rule, we are clarifying that when RHCs and FQHCs
furnish dental services that align with the inextricably linked policies and operational
requirements in the physician setting, we would consider those services to be a qualifying visit
and the RHC would be paid at the RHC AIR and the FQHC would be paid under the FQHC PPS.
We believe this clarification related to dental services furnished in RHCs and FQHCs would
have a negligible impact on Medicare spending. Even though this is a new benefit, it would only
cover dental services inextricably linked to specific medical services as described in section II.J.
of this proposed rule.
7. Changes in the RHC and FQHC CfCs: Provision of services (§ 491.9(a)(2) and (c)(2)(ii) and
(vi))
Provision of services (§ 491.9)
At § 491.9(a)(2), we are proposing to explicitly state that an RHC and FQHC must
provide primary care services and an RHC cannot be a rehabilitation agency or a facility that is
primarily for the care and treatment of mental diseases. We believe this proposal would result in
real, but difficult to estimate, long-term benefits to patients receiving services at RHCs and
FQHCs, as well as economic benefits to the clinic or center. Regarding the estimated impacts on
the Medicare program, the proposals discussed in section III.C.2 of this proposed rule would
have no impact on Medicare spending.
This proposed change would provide RHCs with additional flexibility to provide
outpatient specialty services on-site or hire additional providers with specialized expertise to
meet the needs of their community, including internal medicine, pediatrics, geriatrics, obstetrics
and gynecology, dermatology, cardiology, neurology, endocrinology, and ear, nose and throat.
As a result, RHCs would be able to improve access to care by serving more patients in
communities served by RHCs and FQHCs, including rural communities, and not requiring
patients to travel longer distances to receive specialty services. Patients could have access to
specialists within their own communities, improving overall access for Medicare beneficiaries.

Moreover, if this policy were to be finalized, CMS would no longer determine or enforce the
standard of RHCs “being primarily engaged in furnishing primary care services.”
808
which has
been enforced via the sub-regulatory guidance contained in the State Operations Manual
Appendix G – Guidance for Surveyors: Rural Health Clinics (RHCs). Resources that clinics are
currently using to evaluate if they are meeting this requirement could be devoted to other
administrative tasks. Therefore, we believe that there would be no burden imposed on RHCs or
FQHCs related to this proposal.
We are also proposing to remove hemoglobin and hematocrit (H&H) lab tests from the
list of specific tests RHCs must provide, as well as update the language regarding the primary
culturing requirement to reflect current standards of practice at § 491.9(c)(2)(ii) and (vi),
respectively. As discussed in section III.C.2.b of this proposed rule, RHCs report the H&H lab
requirement is particularly burdensome and costly for clinics due to purchasing and maintaining
the equipment, even if it is seldom or never used.
This proposed change would reduce the overall burden for RHCs by reducing the number
of diagnostic tests they must provide. RHCs would no longer be required to purchase or maintain
H&H lab test equipment or supplies, freeing up resources for other essential services. H&H lab
tests are most often ordered as part of a larger panel of labs that is not provided at the RHC.
When this is the case, patients will receive the H&H as part of that larger panel at an outside lab
that offers the larger panel of labs. These patients may be inconvenienced by having to travel to
another laboratory, but this limits the number of specimens they must provide for the laboratory
tests, reducing the number of times a patient’s veins must be accessed for blood draws. RHCs
report that when laboratory tests are ordered that are not provided by the RHC, such as a
comprehensive blood count (CBC), their patients are often sent to the nearest hospital that would
808
Centers for Medicare & Medicaid Services. (2020, February 21). State Operations Manual Appendix G -
Guidance for Surveyors: Rural Health Clinics (RHCs) (pp. 63-64). https://www.cms.gov/Regulations-and-
Guidance/Guidance/Manuals/downloads/som107ap_g_rhc.pdf.

have a full-service laboratory available to perform the test. A CBC test looks at a patient’s
overall health and can detect a wide range of conditions that the hemoglobin and hematocrit tests
cannot. This ensures comprehensive patient care and may result in a decreased need for follow-
up testing and decreased patient turnaround time.
809
Currently, there are approximately 5,462 Medicare-certified RHCs. Applying the most
recent data from 2021, 66 percent (3,605) of RHCs are designated as “provider-based,” which
are owned and operated as an integral part of a hospital, nursing home, or home health agency.
810
The remaining 34 percent (1,857) of RHCs are “independent clinics” and, though uncommon,
may be owned and/or operated by a healthcare system. Therefore, we assume that at most, half
of the independent clinics, for a total of 929 RHCs, would continue to provide H&H tests
because it is less likely that they are a part of a healthcare system. As a result, we assume that
4,534 (3,605 provider-based RHCs + 929 independent clinics) RHCs will continue to refer
patients to a fully certified laboratory rather than directly provide the H&H test. Because the
regulatory requirements at § 491.9 States that RHCs must provide H&H lab tests on-site, RHCs
must have and maintain the appropriate equipment to perform these tests, even if the equipment
is not utilized. There are variations in the H&H testing equipment RHCs may use; however, we
note that the average cost of an H&H meter or analyzer costs approximately $1,200 for the
system and is replaced on average every 3 years. The systems also have an approximate $100
annual maintenance fee. We estimate that over the next 3 years, 4,534 RHCs will each save
approximately $1,500. This would result in a total annual savings of $2,267,000 ((4,534 RHCs
that typically refer patients to a fully certified laboratory x $1,500)/3). After 3 years, the RHC
program would save a total of $6,801,000 (4,534 x $1,500).
8. Clinical Laboratory Fee Schedule
809
Mayo Foundation for Medical Education and Research. (2023, January 14). Complete blood count (CBC). Mayo
Clinic. https://www.mayoclinic.org/tests-procedures/complete-blood-count/about/pac-20384919.
810
Gale, J. A., Croll, Z., Croom, J., Munk, L., & Jonk, Y. (2022). Community Characteristics and Financial and
Operational Performance of Rural Health Clinics in the United States: A Chartbook. University of Southern Maine,
Muskie School of Public Service, Maine Rural Health Research Center.
https://digitalcommons.usm.maine.edu/cgi/viewcontent.cgi?article=1016&context=clinics.
In section III.D. of this proposed rule, we outline statutory revisions to the data reporting
period and phase-in of payment reductions under the CLFS. In accordance with section 502 of
the FCAOEA, 2024, we are proposing certain conforming changes to the data reporting and
payment requirements in our regulations at 42 CFR part 414, subpart G. Specifically, for CDLTs
that are not ADLTs, we are proposing to update certain definitions and revise § 414.504(a)(1) to
indicate that initially, data reporting begins January 1, 2017, and is required every 3 years
beginning January 2025. Section 502(b) of the FCAOEA, 2024 delays the next data reporting
period under the CLFS for CDLTs that are not ADLTs by 1 year, that is, it requires the next data
reporting period for these tests to take place during the period of January 1, 2025, through March
31, 2025. Subsequently, the next private payor rate-based CLFS update for these tests will be
effective January 1, 2026, instead of January 1, 2025. In addition, we are proposing conforming
changes to our requirements for the phase-in of payment reductions to reflect section 502(a) of
the FCAOEA, 2024. Specifically, we are proposing to revise § 414.507(d) to indicate that for
CY 2024, payment may not be reduced by more than 0.0 percent as compared to the amount
established for CY 2023, and for CYs 2025 through 2027, payment may not be reduced by more
than 15 percent as compared to the amount established for the preceding year.
We recognize that private payor rates for CDLTs paid on the CLFS and the volumes paid
at each rate for each test, which are used to determine the weighted medians of private payor
rates for the CLFS payment rates, have changed since the first data collection period (January 1,
2016, through June 30, 2016) and data reporting period (January 1, 2017, through March 31,
2017). In addition, as outlined in section III.D. of this proposed rule, in the CY 2019 PFS final
rule (83 FR 59671 through 59676), we amended the definition of applicable laboratory to include
hospital outreach laboratories that bill Medicare Part B using the CMS-1450 14x Type of Bill.
As such, the FCAOEA, 2024 amendments to the data reporting period will delay using updated
private payor rate data to set revised CLFS payment rates for CDLTs that are not ADLTs.
Due to unforeseen changes in private payor rates due to shifts in market-based pricing for

laboratory tests and the unpredictable nature of test volumes and their impact on calculating
updated CLFS payment rates based on the weighted median of private payor rates, it is uncertain
whether the delay in data reporting will result in a measurable budgetary impact. In other words,
to assess the impact of delayed reporting and subsequent implementation of updated CLFS rates,
we will need to calculate weighted medians of private payor rates based on new data and
compare the revised rates to the current rates. As such, we believe that we will only know the
impact of the delay in data reporting after collecting actual updated applicable information from
applicable laboratories and calculating the updated CLFS rates.
Regarding the proposed conforming changes to our requirements for the phase-in of
payment reductions, we note that for CYs 2025 through 2027, payment may not be reduced by
more than 15 percent as compared to the amount established for the preceding year. Based on
data reported in the 2017 data collection period, we estimate 14.8 percent (191) of tests on the
CLFS may be subject to the full 15 percent phase-in reduction in CY 2025.
9. Effects of Proposals Relating to the Medicare Diabetes Prevention Program Expanded Model
a. Effects on Beneficiaries
We are proposing to modify certain Medicare Diabetes Prevention Program (MDPP)
expanded model policies to: (1) align MDPP terminology and definitions with the proposed 2024
Centers for Disease Control and Prevention (CDC) Diabetes Prevention Recognition Program
(DPRP) Standard
811
definitions for “in-person with a distance learning component,”
“combination with an online component,” and “online”; (2) remove the MDPP bridge payment;
(3) provide a more effective option for a beneficiary to self-report their weight in an MDPP
distance learning session, by submitting 2 photos; (4) facilitate Medicare Administrative
Contractors (MACs) in processing claims for a MDPP make-up session held on the same day as
811
Centers for Disease Control and Prevention Diabetes Prevention Recognition Program. Standards and Operating
Procedures. Requirements for CDC Recognition. June 2024. https://nationaldppcsc.cdc.gov/s/article/DPRP-
Standards-and-Operating-Procedures.

a regularly scheduled session by requiring use of an existing HCPCS modifier; and (5) align
current rule language with previous rulemaking.
MDPP is a non-pharmacological behavioral intervention consisting of up to 22 sessions
using a CDC approved National Diabetes Prevention Program (National DPP) curriculum.
812
CDC administers a national quality assurance program recognizing eligible organizations that
furnish the National DPP through its evidence based DPRP Standards, which are updated every
three years. The 2024 CDC DPRP Standards replace the 2021 CDC DPRP Standards in June
2024.
813
The Calendar Year (CY) 2021 PFS final rule allowed virtual delivery of MDPP during the
COVID-19 Public Health Emergency (PHE) (85 FR 84830). Improvements to MDPP in the
Calendar Year 2024 final rule included a simplified payment structure to allow for fee-for-
service (FFS) payments for beneficiary attendance, while retaining the performance-based
payments for diabetes risk reduction (that is, weight loss) (88 FR 79241). This policy also
extended certain PHE flexibilities including the option to deliver some or all MDPP sessions via
distance learning, until December 31, 2027 (88 FR 79241). Another PHE flexibility extended
through December 31, 2027, at 42 CFR 410.79(e)(3)(iii), is for MDPP suppliers to obtain weight
measurements for beneficiaries using one of the following options: (1) via digital technology,
such as scales that transmit weights securely via wireless or cellular transmission; or (2) via self-
reported weight measurements from the at-home digital scale of the MDPP beneficiary.
The 2024 CDC DPRP Standards were proposed after the CY 2024 PFS was finalized. To
align with 2024 CDC DPRP Standards, we are proposing to update the MDPP definition for
“online” delivery to align with the proposed 2024 CDC DPRP definition. We are also proposing
to add terms and definitions for CDC’s new modalities including “in-person with a distance
812
https://www.cdc.gov/diabetes/prevention/resources/curriculum.html.
813
Centers for Disease Control and Prevention Diabetes Prevention Recognition Program. Standards and Operating
Procedures. Requirements for CDC Recognition. June 2024. https://nationaldppcsc.cdc.gov/s/article/DPRP-
Standards-and-Operating-Procedures.
learning component” and “combination with an online component” and to remove the existing
“combination” term and definition. Lastly, we are specifying that MDPP make-up sessions must
be provided in-person or via distance learning delivery, as required by the CY 2024 PFS final
rule.
4
Furthermore, the MDPP bridge payment (G9880), which is a payment made to the
subsequent supplier for the first session when a beneficiary switches MDPP suppliers, is no
longer necessary in MDPP’s CY 2024 FFS payment structure and may increase risk for fraud,
waste, or abuse. We are proposing to remove the bridge payment from the MDPP CY 2025 Fee
Schedule. In addition, we have identified a more effective option for a beneficiary to self-report
their weight in an MDPP distance learning session. We have also identified the need to require
suppliers to use Current Procedural Terminology (CPT) Modifier 79 to allow Medicare
Administrative Contractors (MACs) to identify a claim for an MDPP make-up session held on
the same day as a regularly scheduled session. Finally, we are proposing to align current rule
language with previous rulemaking pertaining to MDPP terminology, requirements, and payment
structure.
All the proposed changes for MDPP in CY 2025 are conforming or administrative and
expected to have a modest impact on beneficiaries’ access to MDPP services. Aligning with
2024 CDC DPRP Standards for MDPP delivery modes may help expand beneficiary access by
streamlining data submission for MDPP suppliers and increasing the number of MDPP eligible
organizations that enroll in Medicare as MDPP suppliers. Additionally, allowing for MDPP
make-up sessions to be scheduled on the same day as regularly scheduled sessions would
increase flexibility for both MDPP suppliers and beneficiaries and may help expand access for
beneficiaries with transportation and other scheduling issues that prevent scheduling sessions
more than one day a week or month. Increased flexibility in scheduling MDPP sessions may help
to address a lack of MDPP suppliers in certain communities and challenges related to beneficiary
logistics concerning course attendance.
Additionally, we are proposing to provide a more effective option to beneficiaries to self-
report weight for a MDPP distance learning session, by allowing beneficiaries to submit 2 (two)
photos to capture both the beneficiary weight on the digital scale and the beneficiary visible in
their home. Current MDPP supplier standards at § 424.205 require beneficiary weight to be
reported at each MDPP session attended. This proposed change would help to address concerns
voiced by MDPP suppliers who have reported that many of their beneficiaries are unable to take
a picture while standing on their home scales due to risk of injury and physical health limitations.
This new flexibility may promote more consistent collection of weight for MDPP sessions.
Lastly, we do not expect removing the MDPP bridge payment to have an impact on
beneficiary access. This payment for the first session attended with a new supplier when a
beneficiary switches MDPP suppliers is not necessary in MDPP’s CY 2024 FFS payment
structure that includes payment for every session attended and historically has been submitted by
few MDPP suppliers.
Overall, these modifications address MDPP supplier and beneficiary needs based upon
available monitoring and evaluation data received to date, feedback from Medicare Advantage
plans and existing MDPP suppliers, and feedback from beneficiary focus groups. The changes
are also in response to comments from interested parties made through public comments in
response to prior rulemaking.
b. Effects on the Market
We anticipate that the conforming and administrative changes proposed in this
rulemaking are likely to result in modest increases of MDPP suppliers and beneficiary access to
the set of MDPP services. We anticipate that this would assist in contributing to a reduction of
the incidence of diabetes among eligible Medicare beneficiaries. As of April 2024, there are
approximately 810 in-person organizations nationally that are eligible to become MDPP
suppliers based on their preliminary or full CDC Diabetes DPRP status. However, only 36

percent of these eligible in-person organizations are participating in MDPP.
814
Aligning with
CDC DPRP delivery modes, particularly adding the new “in-person with a distance learning
component” mode, is expected to help increase recruitment of new DPRP organization as MDPP
suppliers, who currently would need to obtain both in-person and distance learning CDC DPRP
recognition to deliver sessions via both modalities. Furthermore, only about one-third of MDPP
suppliers have submitted MDPP-related claims.
815
Our proposed change to remove the MDPP
bridge payment would help to further simplify the payment structure, which is expected to have a
positive impact on supplier claim submissions. While requiring MDPP suppliers to add a
modifier to indicate that a claim is for a MDPP same day make-up session does add complexity
because we have proposed to use an existing CPT modifier in use and recognized by the MACs,
we expect this addition to require minimal changes in claim processing systems. Additionally,
this modifier is only to be used for same day make-up sessions, which are only allowed once per
week, and, while an important tool to increase access for beneficiaries with barriers to
participation in MDPP, are not expected to be used for most session attendance claims. In
summary, we believe that having more flexibility in how the set of MDPP services are delivered
would make MDPP more accessible to beneficiaries, particularly those who live in rural areas or
in communities with gaps in MDPP supplier locations.
c. Payment for MDPP Services
Regulations at § 414.84 specify that MDPP suppliers may be eligible to receive payments
for furnishing MDPP services and meeting performance targets related to beneficiary weight loss
and attendance. We anticipate that the proposed change to the MDPP payment structure would
have minimal impact on total payment for MDPP services. A smaller proportion of MDPP
suppliers, only 9.8 percent since the start of the program through April 2024, have submitted
claims for the MDPP bridge payment, with an even smaller proportion of 2.7 percent having
814
Centers for Disease Control and Prevention. Diabetes Prevention Recognition Program Application. Registry of
All Recognized Organizations. https://dprp.cdc.gov/Registry.
815 Unpublished MDPP monitoring data. 2023.

received payment for a bridge payment claim. According to CDC DPRP data, less than 1 percent
of MDPP sessions are same day make-up sessions.
816
The total maximum payment per
beneficiary for MDPP of $768 would remain unchanged by our proposals.
d. Effects on the Medicare Program
(a) Estimated 10-Year Impact of MDPP
The changes proposed this year for implementation in the CY PFS 2025 are expected to
have no impact on Medicare spending.
10. Modifications Related to Medicare Coverage for Opioid Use Disorder (OUD) Treatment
Services Furnished by Opioid Treatment Programs (OTPs)
As outlined in section III.F.2 of this proposed rule, we are proposing to permanently
allow periodic assessments to be furnished via audio-only communication when two-way audio-
video communications technology is not available to the beneficiary, to the extent that it is
authorized by SAMHSA and DEA at the time the service is furnished and all other applicable
requirements are met. We are also proposing to allow the OTP intake add-on code to be
furnished via two-way audio-video communications technology when billed for the initiation of
treatment with methadone to the extent that the use of audio-video telecommunications
technology to initiate treatment with methadone is authorized by DEA and SAMHSA at the time
the service is furnished, an OTP determines that an adequate evaluation of the patient can be
accomplished via a via an audio-visual telehealth platform, and all other applicable requirements
are met. We believe the Part B cost impact of these flexibilities for the use of
telecommunications will be minimal because we do not expect that these flexibilities will
significantly increase the frequency with which medically necessary intake activities and
periodic assessments are furnished, and since the payment rate for these services will be the same
regardless of if an OTP furnishes these services via telecommunications or in-person.
816
Diabetes Prevention Recognition Program. Unpublished data. April 2024.
In section III.F.3 of this proposed rule, CMS is proposing to update the payment rate for
intake activities (HCPCS code G2076) by adding in the value of the non-facility rate for SDOH
risk assessments described by HCPCS code G0136 (Administration of a standardized, evidence-
based Social Determinants of Health Risk Assessment, 5–15 minutes, not more often than every 6
months). We believe updating the payment amount for intake activities with an addition of
HCPCS code G0136 would serve as a reasonable proxy to reflect the value and resources
required by new SAMHSA standards for initial assessment service activities at § 8.12(f)(4)(i)
that OTPs are required to provide, including an assessment to identify a patient’s unmet HRSNs
or the need for harm reduction intervention and recovery support services that are critical to the
treatment of an OUD. Currently, the CY 2024 payment rate for the intake add-on code (G0276)
is $201.73 and adding the value of a crosswalk to the CY 2024 non-facility rate of $18.66 would
result in a payment rate of approximately $220.39. The payment rate would also continue to be
updated annually by the percentage increase in the Medicare Economic Index (MEI) and the
Geographic Adjustment Factor (GAF) as codified in §410.67(d)(4)(ii) through (iii). According to
historical claims data for intake activities (HCPCS code G2076) furnished by OTPs from the
beginning of CY 2020 through the end of CY 2023, the number of claims for intake activities is
low. Due to low utilization of the intake activities add-on code (HCPCS code G2076), CMS
estimates that an increase in the add-on payment amount to HCPCS code G2076 by $18.66 per
claim would still result in a negligible cost to the Medicare program. Lastly, in section III.F.4 of
this proposed rule, we are proposing to establish payment for new opioid agonist and antagonist
medications that were recently approved by the FDA. We proposed to include a new add-on code
to the bundled payment for a new opioid overdose reversal product, nalmefene hydrochloride
nasal spray product (Opvee®), which includes one carton of two, 2.7 mg nasal sprays of
nalmefene. We proposed to price the drug component of this add-on code for nalmefene
according to the ASP payment methodology set forth in section 1847A, except that the payment
amount shall be ASP + 0. The non-drug component of this add-on code would also include
overdose education furnished in conjunction with nalmefene, and it would be updated annually
by the percentage increase in the MEI and GAFs consistent with other opioid antagonist
medications in § 410.67(d)(4)(ii) through (iii). We are limiting Medicare payment to OTPs for
nalmefene to one add-on code every 30 days, however, we will allow exceptions to this limit in
the case where the beneficiary overdoses and uses the initial supply of nalmefene dispensed by
the OTP, to the extent that it is medically reasonable and necessary to furnish additional doses of
nalmefene. We are also proposing payment for the weekly formulation of the new extended-
release injectable buprenorphine product Brixadi®, via a new weekly bundled payment code that
includes a drug and non-drug component. We proposed to price the drug component consistent
with our payment methodology for implantable and injectable medications codified
§ 410.67(d)(2)(i)(A), and we proposed to limit the payment amount to 100-percent of ASP and to
use a crosswalk to the weekly Brixadi® formulation described by HCPCS code J0577 (Injection,
buprenorphine extended release (brixadi), less than or equal to 7 days of therapy). The non-drug
component (individual and group therapy, SUD counseling, toxicology testing) would also
include administration of an injection based on CPT code 96372 (Therapeutic, prophylactic, or
diagnostic injection (specify substance or drug); subcutaneous or intramuscular); updated
annually by the percentage increase in MEI and GAFs. CMS is further proposing to update the
drug-component of the existing HCPCS code (G2069) for monthly injectable buprenorphine to
include the monthly formulation of Brixadi® based on a crosswalk to HCPCS code J0578
(Injection, buprenorphine extended release (brixadi), greater than 7 days and up to 28 days of
therapy). We proposed to continue to use the existing payment methodology for both the drug
and non-drug component of G2069 after averaging in payment for the monthly formulation of
Brixadi®. Since the proposed payment methodology for the new add-on code for Opvee®
(GOTP1), the weekly bundled payment for weekly Brixadi® (GOTP2), and the update to the
monthly bundled payment for injectable buprenorphine (HCPCS code G2069) is based on
comparable and existing drugs billed under the Medicare OTP benefit, and assuming an OTP
may provide these new drugs to a Medicare beneficiary in lieu of the comparable and existing
drugs under the Medicare OTP benefit (e.g, Opvee® instead of Narcan® or Kloxxado®, or the
weekly or monthly formulation of Brixadi® instead of Sublocade®), then CMS estimates the
financial impacts of these new drugs would be negligible.
11. Medicare Shared Savings Program
a. General Impacts
As of January 1, 2024, 10.8 million Medicare beneficiaries receive care from a health
care provider in one of the 480 ACOs participating in the Shared Savings Program, one of the
largest value-based care programs in the country. The Shared Savings Program proposed policies
in this proposed rule would advance Medicare’s value-based care strategy of growth, alignment,
and equity, with many proposals supporting more than one of these goals. The proposed policies
in this proposed rule are designed, in part, to further improve the quality of care furnished by
ACOs by revising the quality performance standard and reporting requirements, broaden
program participation particularly in underserved communities, and promote the continued integrity
and fairness of Shared Savings Program financial calculations.
As described in section III.G.7.b of this proposed rule, under the benchmarking
methodology for agreement periods beginning on January 1, 2024, and in subsequent years,
CMS calculates two adjustments in establishing the historical benchmark, a regional adjustment
(refer to § 425.656) and a prior savings adjustment (refer to § 425.658). We determine which
adjustment is applied to the benchmark, either the regional adjustment, prior savings adjustment,
or no adjustment (refer to § 425.652(a)(8) and (c)). One of the changes to the Shared Savings
Program financial methodology finalized with the CY 2024 PFS final rule (see 88 FR 79185
through 79195, see also 88 FR 79494 and 79495) was to mitigate the impact of the negative
regional adjustment on the benchmark for ACOs in agreement periods beginning on January 1,
2024, and in subsequent years. We explained our belief that this change would further encourage
continued participation among high-cost ACOs that serve medically complex beneficiaries by
eliminating the potential of a lower benchmark due to an overall negative regional adjustment,
and may also encourage ACOs serving such populations that may have otherwise been
discouraged from participating in the Shared Savings Program by the prospect of a lower
benchmark to join (see 88 FR 79188, see also 88 FR 79494 and 79495). Under this approach, an
ACO with an overall negative regional adjustment that was not eligible for a prior savings
adjustment would ultimately receive no adjustment, upward or downward, to its benchmark (see
§ 425.652(a)(8)(iii) and see also 88 FR 79190). The proposed Health Equity Benchmark
Adjustment (HEBA) described in section III.G.7.b of this proposed rule would add a third
avenue for ACOs to receive a positive adjustment to their historical benchmark, and would be
most impactful for new ACOs serving medically complex, high-cost populations in underserved
communities.
We combined an analysis of the impact of the HEBA on currently participating ACOs
with the projected impact of HEBA on new ACOs not yet participating in the Shared Savings
Program, to generate an overall impact estimate of the HEBA. The HEBA would likely have a
limited impact on currently participating ACOs. Only about 20 percent of the 456 ACOs
participating in performance year 2023 were estimated to have a proportion of assigned
beneficiaries who were enrolled in the Medicare Part D LIS or dually eligible for Medicare and
Medicaid equal to or greater than 20 percent. That 20 percent threshold would have to be met for
an ACO to be eligible for the HEBA under the HEBA proposal (as discussed in section III.G.7.b.
of this proposed rule, and specified in proposed § 425.662(b)(3)). Of that 20 percent of ACOs
participating in performance year 2023 that were estimated to have a proportion of assigned
beneficiaries who were enrolled in the Medicare Part D LIS or dually eligible for Medicare and
Medicaid equal to or greater than 20 percent, only about one quarter of such ACOs were
estimated to not already be eligible for a higher benchmark adjustment from either a positive
regional adjustment or a prior savings adjustment. That is, about 5 percent of ACOs participating
in performance year 2023 were estimated to benefit from the proposed HEBA policy at rebasing
(median effect about 1 percent increase to the benchmark), because an ACO would receive the
proposed HEBA only if the HEBA adjustment were higher than the existing “higher of”
adjustment method (see discussion in section III.G.7.b of this proposed rule, and proposed
§§ 425.652(a)(8)(ii)(B)(1) and 425.662(c)). The proposed HEBA is projected to increase
program spending for existing ACOs by about $140 million over 10 years.
The number of ACOs that would be incentivized to participate in the Shared Savings
Program by the HEBA is uncertain. Changes to the Shared Savings Program finalized in the CY
2023 CY 2024 PFS final rules were already projected to increase program participation among
ACOs with higher spending and provide more opportunities for improving care and reducing
spending (see 87 FR 70191 through 70196, and 88 FR 79494 and 79495). Therefore, we can
reasonably estimate that at least some new ACOs may join and succeed in the Shared Savings
Program regardless of the benefit afforded to them by the proposed HEBA (if finalized). Savings
to the Shared Savings Program are expected to grow to the extent that the HEBA were to cause
new, high-spending ACOs to participate – that is, ACOs serving populations with risk-adjusted
spending that is significantly higher than corresponding regional benchmark spending at
baseline. We project in the 2034 performance year, the proposed HEBA would likely increase
program participation by 25 additional ACOs (but our estimates of increased program
participation range from 0 to 100 additional ACOs), as compared to program participation today,
and on net increase Federal Medicare program savings by $460 million over the 2025-2034
period because of this new participation.
The estimated impact of the proposed HEBA, accounting for both its impact on currently
participating ACOs that are assumed to renew their participation in the Shared Savings Program
over the next 5 years and new ACOs that are expected to participate in the program for the first
time over the next 10 years, is shown in Table 132. Mean Shared Savings Program spending is
expected to be reduced by $320 million over the next ten years as a result of the HEBA, if
finalized. However, uncertainty regarding the number of high spending ACOs that would

participate in response to a HEBA, combined with uncertainty regarding high spending ACOs’
savings potential, results in a wide range of potential impacts in total over that 10 year period,
from $2.3 billion in net savings at the 10
th
percentile (that is, only 10 percent of stochastic trials
reduced Federal spending by a greater magnitude than $2.3 billion) to $1.2 billion in net
spending at the 90
th
percentile.
TABLE 132: Projected Impact of Proposed Health Equity Benchmark Adjustment ($ Millions;
Negative Values Represent Savings to the Program)
2025
2026
2027
2028
2029
2030
2031
2032
2033
2034
Total
Impact
Estimate
20
20
30
20
10
-20
-60
-100
-120
-120
-320
Estimate
Range:
Low
Estimate
(10
th
Percentile)
0
-20
-40
-90
-140
-240
-340
-420
-490
-540
-2,320
High
Estimate
(90
th
Percentile)
50
70
90
110
130
140
140
140
150
180
1,200
For the proposed calculation methodology to account for the impact of improper
payments in recalculating expenditures and payment amounts used in Shared Savings Program
financial calculations, upon reopening a payment determination pursuant to § 425.315(a)
(described in section III.G.7.c of this proposed rule), some ACOs would selectively elect to
request reopening for a prior performance year. As a result, we project at least some degree of
higher program spending for increased shared savings payments (or reduced loss recoupments)
in cases for which CMS decides to reopen the payment determination and issue a revised initial
determination to account for the impact of improper payments. However, because reopening
would not be limited to adjusting performance year and benchmark year expenditures for ACO
assigned beneficiaries but would also impact other potentially offsetting calculations including
regional and national expenditure trends used to update ACO benchmarks, and because, in
addition to the reopening, CMS would also adjust the historical benchmark calculated for a
potential subsequent agreement period, the frequency of requests, and the net impact of any

given request, are likely to be limited. The proposed reopening policy is projected to increase
program spending by $60 million in total over the 2025 to 2034 period, ranging from $30 million
at the 10
th
percentile to $90 million at the 90
th
percentile, as shown in Table 133.
TABLE 133: Projected Impact for Modifications to Specify the Calculation Methodology to
Account for Improper Payments in Recalculating Expenditures and Payment Amounts used in
Financial Calculations, Upon Reopening a Payment Determination ($ Millions; Negative Values
Represent Savings to the Program)
2025
2026
2027
2028
2029
2030
2031
2032
2033
2034
Total
Impact
Estimate
10
0
0
0
0
10
10
10
10
10
60
Estimate
Range:
Low
Estimate
(10
th
Percentile)
30
High
Estimate
(90
th
Percentile)
90
Note: Projections at the 10
th
and 90
th
percentile are shown in aggregate but not at the annual level because scenarios
contributing to the high estimate projection generally involve elevated spending in a limited number of years rather
than consistently higher spending across the projection period.
The proposed methodology for excluding payment amounts for HCPCS and CPT codes
exhibiting SAHS billing activity, as described in section III.G.7.d of this proposed rule, is
anticipated to be utilized only in rare and extreme cases where a number of criteria are satisfied,
including that the level of billing represents a significant claims increase representing a deviation
from historical utilization trends that is unexpected and not clearly attributable to reasonably
explained changes in policy or the supply or demand for covered items or services during a
limited time period. Even in cases where CMS may apply the adjustment to Shared Savings
Program calculations for SAHS billing activity (if finalized), for CY 2024 or subsequent
calendar years, there is no expectation that it would necessarily increase or decrease overall
shared savings or shared losses because the policy would be applied systematically across all
ACOs in the Shared Savings Program in a method that adjusts both performance year and
benchmark year expenditures for ACO assigned beneficiaries and regional and national
expenditures used in benchmark calculations. However, this policy would have the benefit of
reducing potential costs generated by selective reopening requests under the reopening proposal,
because it would prevent extreme cases of SAHS billing activity from injecting variation in the
distribution of ACO shared savings and loss calculations which could lead to an elevated number
of selective reopening requests from ACOs predicting that reopening would improve their
financial outcome. For this reason, without the proposal to adjust Shared Savings Program
calculations for SAHS billing activity during CY 2024 or subsequent calendar years, the
estimated impact shown in Table 133 would have included between $100 to $300 million in
additional projected spending from selective reopening requests.
We estimate that there would be no additional program expenditures stemming from the
implementation of the prepaid shared savings payment option, which we proposed to provide
eligible ACOs with additional cash flow to encourage their investment in activities that could
potentially reduce costs for the Medicare program and beneficiaries and improve the quality of
care furnished to their assigned beneficiaries. Any risk of higher program spending as a result of
finalization of our prepaid shared savings proposal would be fully mitigated by the fact that
eligibility would be limited to ACOs that CMS estimates are most likely to earn shared savings,
and any prepaid shared savings payments an ACO receives would have to be repaid to CMS.
CMS would be protected by the ACOs’ repayment mechanisms in the event that an ACO does
not earn shared savings or cannot otherwise repay the amount owed to CMS. On the other hand,
there is a high degree of uncertainty regarding whether (a) a meaningful number of ACOs would
choose this option given the requirements for how prepayments must be spent, and (b) the
potential impact (if any) that participation in this option would have on the cost of care.
As to this uncertainty, our analysis assumed that up to 30 ACOs would opt to receive
prepaid shared savings per year (with the probability distribution skewed toward zero
participants), with a 33 percent chance that ACOs receiving prepaid shared savings would
respond by reducing spending for assigned beneficiaries by between 0 to 2 percentage points
(with the probability distribution skewed toward zero impact) as compared to their current

spending on assigned beneficiaries.
817
This projection accounts for annual prepaid shared savings
(offset by eventual recoupments and/or repayments) of roughly $2 million per ACO participating
in the payment option. The associated impact on ACO spending was projected to be nominal.
Both at the mean and at the 90
th
percentile the projected net impacts on Medicare spending round
to zero. At the 10
th
percentile (the optimistic end of the range), we project small net savings of
$20 million in total to the Medicare Program over 10 years.
TABLE 134: Projected Impact for the Prepaid Shared Savings Option ($ Millions; Negative Values
Represent Savings to the Program)
2025
2026
2027
2028
2029
2030
2031
2032
2033
2034
Total
Impact
Estimate
0
0
0
0
0
0
0
0
0
0
0
Estimate
Range:
Low
Estimate
(10
th
Percentile)
-20
High
Estimate
(90
th
Percentile)
0
Note: Projections at the 10
th
and 90
th
percentiles are shown in aggregate but not at the annual level because scenarios
contributing to the $20 million savings at the 10
th
percentile are too small to round above zero in any given year.
The remaining proposed changes to the Shared Savings Program regulations are not
estimated to have an impact on program spending at the aggregate level. These proposed changes
include requiring Shared Savings Program ACOs to report the APP Plus quality measure set
beginning in performance year 2025, that would incrementally grow to comprise of 11 measures,
consisting of the 6 measures in the existing APP quality measure set and 5 newly proposed
measures from the Adult Universal Foundation measure set that would be incrementally
incorporated into the APP Plus quality measure set over performance years 2025 through 2028;
focusing the collection types available to Shared Savings Program ACOs for reporting the APP
Plus quality measure set to all payer/all patient eCQMs and Medicare CQMs; requiring Shared
817
The assumptions allow for a limited possibility that performance by ACOs receiving prepaid shared savings
could generate shared savings comparable to the savings generated by certain ACOs in the ACO Investment Model.
Abt Associates, Evaluation of the Accountable Care Organization Investment Model, Final Report (September
2020), available at https://www.cms.gov/priorities/innovation/data-and-reports/2020/aim-final-annrpt.

Savings Program ACOs that report the APP Plus quality measure set to report on all measures in
the APP Plus quality measure set, as applicable; establishing a Complex Organization
Adjustment for Virtual Groups and APM Entities, including Shared Savings Program ACOs,
when reporting eCQMs; scoring Medicare CQMs using flat benchmarks in their first 2
performance periods in MIPS; and promoting the adoption of eCQMs by extending the eCQM
reporting incentive. Additional proposed changes include permitting continued participation by
ACOs whose number of assigned beneficiaries falls below 5,000 during their agreement period;
ensuring clarity of provisions on application procedures; revisions to the definition of primary
care services under § 425.400(c) for purposes of beneficiary assignment; refining advance
investment payment policies; providing clarity and consistency in provisions of the Shared
Savings Program regulations on calculation of the ACO risk score growth cap in risk adjusting
the benchmark each performance year and the regional risk score growth cap in calculating the
regional component of the three-way blended benchmark update factor; and modifying
beneficiary notification requirements.
The combined impacts for all Shared Savings Program proposals are shown in Table 135.
TABLE 135: Projected Impact of Medicare Shared Savings Program Proposals (Individually
Shown in Tables 132, 133 and 134) ($ Millions; Negative Values Represent Savings to the Program)
2025
2026
2027
2028
2029
2030
2031
2032
2033
2034
Total
Impact
Estimate
30
20
30
20
10
-10
-50
-90
-110
-110
-260
Estimate
Range:
Low
Estimate
(10
th
Percentile)
10
-20
-40
-90
-140
-230
-330
-410
-480
-530
-2,260
High
Estimate
(90
th
Percentile)
60
70
90
110
130
150
150
150
160
190
1,260
b. Compliance with Requirements of Section 1899(i)(3) of the Act
Certain policies, including both existing policies and the proposed new policies described
in this proposed rule, rely upon the authority granted in section 1899(i)(3) of the Act to use other
payment models that the Secretary determines will improve the quality and efficiency of items
and services furnished under the Medicare program, and that do not result in program
expenditures greater than those that would result under the statutory payment model. The
following proposals require the use of our authority under section 1899(i) of the Act: the
proposal to allow ACOs to receive prepaid shared savings as described in section III.G.5 of this
proposed rule; the proposal to use a calculation methodology to account for the impact of
improper payments in recalculating expenditures and payment amounts for certain Shared
Savings Program financial calculations, upon reopening an ACO’s payment determination and
issuing a revised initial determination pursuant to § 425.315(a) as described in section III.G.7.c
of this proposed rule; the proposal to use a methodology for certain Shared Savings Program
calculations to mitigate the impact of SAHS billing activity occurring in CY 2024 or subsequent
calendar years, as described in section III.G.7.d of this proposed rule; and the proposed technical
changes to the provision describing how we calculate the weights applied when capping growth
in regional risk scores as part of the regional component of the three-way blended benchmark
update factor, as described in section III.G.7.f of this proposed rule. When considered together
these proposed changes to the Shared Savings Program’s payment methodology are expected to
improve the quality and efficiency of items and services furnished under the Medicare program
by improving the ability for ACOs to sustain effective participation particularly in serving
medically complex, high-cost populations in underserved communities, and promoting integrity
and fairness and ensuring the accuracy of Shared Savings Program financial calculations. These
proposed changes are not expected to result in a situation in which the payment methodology
under the Shared Savings Program, including all policies proposed to be adopted under the
authority of section 1899(i) of the Act, results in more spending under the program than would
have resulted under the statutory payment methodology in section 1899(d) of the Act.
In the CY 2023 PFS final rule, we estimated that the projected impact of the payment
methodology that incorporates all policies finalized by that final rule would result in $4.9 billion
in greater program savings compared to a hypothetical baseline payment methodology that
excluded the policies that required section 1899(i)(3) of the Act authority (see 87 FR 70195 and
70196). The marginal impact of the proposed changes in the CY 2024 PFS final rule were
estimated to lower net spending by $330 million over the subsequent ten-year period for all new
policies combined, including the cap an ACO’s regional service area risk score growth, the
addition of a new third step to the beneficiary assignment methodology, and the revised approach
to identify the assignable beneficiary population (88 FR 79496). The marginal impact of the
proposed changes in this proposed rule are estimated to lower net spending by an additional $260
million in total through 2034. Although the provisions in this proposed rule that require section
1899(i) of the Act authority are estimated to increase spending by only $60 million over 10
years, the cumulative impact of all policies (including those in this proposed rule) are estimated
to result in more than $4.9 billion in greater program savings compared to a hypothetical baseline
payment methodology that excludes the policies that require section 1899(i)(3) of the Act
authority. Therefore, we estimate that the implementation of the proposals made in this proposed
rule would not be greater than those that would result under the statutory payment model,
consistent with the requirements of section 1899(i)(3)(B) of the Act.
We will continue to reexamine this projection in the future to ensure that the requirement
under section 1899(i)(3)(B) of the Act that an alternative payment model not result in additional
program expenditures continues to be satisfied. Additional Shared Savings Program data
beginning to accumulate after the end of the COVID-19 public health emergency, along with
emerging information on the characteristics of new entrants in the Shared Savings Program for
agreement periods beginning on January 1, 2024 and January 1, 2025, are anticipated to
gradually improve our ability to reevaluate program impacts in a comprehensive fashion. In the
event that we later determine that the payment model that includes policies established under
section 1899(i)(3) of the Act no longer meets this requirement, we would undertake additional

notice and comment rulemaking to make adjustments to the payment model to assure continued
compliance with the statutory requirements.
12. Medicare Part B Payment for Preventive Services
In section III.H.2. of this proposed rule, based on the proposals in section III.M of this
proposed rule, we propose to clarify that a physician’s order is no longer required for the
administration of a hepatitis B vaccine in Part B, which would facilitate roster billing by mass
immunizers for hepatitis B vaccine administration. We also propose that payment for hepatitis B
vaccines and their administration be made at 100 of reasonable cost in RHCs and FQHCs,
separate from the FQHC PPS or the RHC All-Inclusive Rate (AIR) methodology, in order to
streamline payment for all Part B vaccines in those settings. We believe that Medicare spending
impacts from both of these proposals will be negligible, as hepatitis B vaccines are already
available to all Medicare enrollees under either Part B or Part D. While we believe that there will
be an uptake of hepatitis B vaccines under Part B as shifted from Part D, we believe that this
impact on the Part B program will be negligible for several reasons, including the fact that a
portion of current beneficiaries have already received the hepatitis B vaccine through either Part
B or Part D, and since a significant number of individuals will likely receive this vaccine by the
time they are Medicare age due to current CDC recommendations (please see section III.M of
this proposed rule for more information).
In section III.H.3. of this proposed rule, we propose a fee schedule for Drugs Covered as
Additional Preventive Services (DCAPS), per section 1833(a)(1)(W)(ii) of the Act. We also
propose payment limits for supply and administration fees for DCAPS drugs that are similar to
those fees for drugs paid under the ASP payment methodology set forth in section 1847A of the
Act, and we propose payment limits for DCAPS drugs and any supply and administration fees in
RHCs and FQHCs according to this same fee schedule. We believe impacts from these proposed
policies will be minimal as well. While no drugs are currently covered as DCAPS, DCAPS drugs

are likely to be covered under Part D before coverage under the Part B additional preventive
services benefit would commence.
13. Impact of Provisions for Medicare Prescription Drug Inflation Rebate Program
We are proposing regulations in section III.I. to codify existing policies established in
program guidance as well as revised and new policies to implement the Medicare Part B Drug
Inflation Rebate Program, including the requirement for manufacturers to pay rebates for certain
single source drugs and biological products with prices that increase faster than the rate of
inflation; criteria for the identification of Part B rebatable drugs; computation of the beneficiary
coinsurance adjustment for Part B rebatable drugs; determination of the rebate amount for Part B
rebatable drugs; reduction of the rebate amount for Part B rebatable drugs in shortage and when
there is a severe supply chain disruption; provision of reports to each manufacturer of a Part B
rebatable drug; and establishment of enforcement provisions via civil money penalties.
Additionally, we are proposing regulations in section III.I. to codify existing policies
established in program guidance as well as revised and new policies to implement the Medicare
Part D Drug Inflation Rebate Program, including the requirement for manufacturers to pay
rebates for certain Part D drugs and biological products; criteria for the identification of Part D
rebatable drugs; determination of the rebate amount for Part D rebatable drugs; reduction of the
rebate amount for shortages and when there is a severe supply chain disruption or likely
shortage; provision of reports to each manufacturer of a Part D rebatable drug; and establishment
of enforcement provisions via civil money penalties.
We do not expect these proposals to have a material impact on inflation rebates. The
majority of proposals codify existing guidance. Proposed new policies or changes to existing
policies in guidance are technical provisions that we do not expect to have a material impact on
the calculation of total rebates in aggregate.
As discussed in section III.I. of this proposed rule, for Part D drug inflation rebates, we
are proposing to implement section 1860D-14B (b)(1)(B) of the Act which requires the Secretary
to exclude 340B units from the total number of units used to calculate the total rebate amount
owed by a manufacturer, beginning on January 1, 2026. For the first three quarters of 2026, we
are proposing use an estimation policy that would remove a percentage of units from the total
number of units used to calculate the total rebate amount to remove 340B units from Part D drug
inflation rebate calculations. That percentage would be equal to the total number of units
purchased under the 340B Drug Pricing Program for an NDC-9, divided by the number of total
units sold of that NDC-9.
CMS does not currently have data on 340B claims for the Part D program or at the drug
level in general, which prevents CMS from quantifying the impact of this provision. While we
expect that the exclusion of 340B units from Part D inflation rebates will reduce the amount of
rebates collected through this program, the magnitude of this reduction is unknown due to the
lack of data on 340B claims for the Part D program. The use of a temporary estimation policy
will allow CMS to implement the exclusion of 340B units from Part D inflation rebates by
January 2026. It also alleviates the burden from interested parties to modify existing processes to
provide 340B claims information.
14. Expand Colorectal Cancer Screening
In section III.K. of this rulemaking we propose to update and expand coverage for CRC
screening by (1) removing coverage for the barium enema procedure in regulations at § 410.37,
(2) adding coverage for the CT colonography procedure in regulations at § 410.37, and (3)
expanding a “complete colorectal cancer screening” in § 410.37(k) to include a follow-on
screening colonoscopy after a Medicare covered blood-based biomarker CRC screening test
(described and authorized in NCD 210.3) returns a positive result.
We do not anticipate our proposal to remove coverage for the barium enema procedure to
result in a significant financial impact on the Medicare program. An internal claims analysis
found that Medicare Fee for Service only paid 62 claims for the screening barium enema
procedure in calendar year 2021 and only 72 claims for the screening barium enema procedure in

calendar year 2022.
We do not anticipate our proposal to add coverage for the CTC procedure for CRC
screening to result in a significant financial impact on the Medicare program. CTC could be an
appropriate option for patients and clinicians who seek a direct visualization procedure as a first
step in CRC screening that is less invasive and less burdensome on the patient (including those
who are medically fragile or have complex or usual anatomy) compared to Screening
Colonoscopy. We expect that patients will most often choose CTC as an alternative to
colonoscopy for CRC screening and that future increased utilization of CTC will be balanced, in
part, by avoided screening colonoscopies. Our goal is that the patient and their clinician make the
most appropriate choice in CRC screening, which includes considerations of the risks, burdens
and tradeoffs for each covered test or procedure. We expect that utilization of CTC for CRC
screening will be modest, especially considering that CTC requires bowel preparation and travel
to an outpatient clinical services site (similar to a colonoscopy) and also considering the
availability of non-invasive stool-based tests that can be administered at home and mailed to a
lab. A 2015 study titled “Medicare cost of colorectal cancer screening: CT colonography vs.
optical colonoscopy” concluded that CTC is 29 percent less expensive than colonoscopy
(accounting for related procedures) for the Medicare population in the base scenario. Although
the CTC cost advantage is increased or reduced under alternative scenarios, it is always
positive.
818
We do not anticipate our proposal for expanding a “complete colorectal cancer
screening” in § 410.37(k) to include a follow-on screening colonoscopy after a Medicare covered
blood-based biomarker CRC screening test returns a positive result to produce a significant
financial impact on the Medicare program. We expect that patients will choose either a stool-
based test or a blood-based biomarker test for a non-invasive first option in CRC screening and
818
Pyenson, B., Pickhardt, P.J., Sawhney, T.G. et al. Medicare cost of colorectal cancer screening: CT colonography
vs. optical colonoscopy. Abdom Imaging 40, 2966–2976 (2015). https://doi.org/10.1007/s00261-015-0538-1.
that patients who choose a blood-based biomarker test within the context of a complete
colorectal cancer screening under our proposal will be offset, in part, by the avoided utilization
of a stool-based test.
In conclusion, we anticipate that our proposal to update and expand coverage for CRC
screening will result in some additional utilization, but that additional utilization will be
balanced, in part or in whole, by avoided utilization of alternative types of tests as well as
benefits and savings resulting from increased prevention and early detection (allowing for less
invasive and more effective treatment).
15. Requirement for Electronic Prescribing for Controlled Substances for a Covered Part D
Drug under a Prescription Drug Plan or an MA-PD Plan
In section III.L of this proposed rule, we propose one update to the CMS EPCS Program.
We are proposing that prescriptions written for a beneficiary in a LTC facility would not be
included in determining compliance under the CMS EPCS Program until January 1, 2028, and
that compliance actions against prescribers who do not meet the compliance threshold based on
prescriptions written for a beneficiary in a LTC facility would commence on or after January 1,
2028. Without this provision, if we keep the existing date of January 1, 2025, as in the current
regulatory text at § 423.160(a)(5) for the CMS EPCS Program, we estimate at least 6,800
prescribers would become non-compliant due to CMS including prescriptions written for
beneficiaries in LTC in the CMS EPCS Program compliance threshold calculation. This
estimate is based on data from calendar year 2022 and is prior to considering emergency and
disaster exceptions and waivers, which could reduce these numbers. This proposal, should we
finalize it, would allow prescribers additional time to adopt the new e-prescribing standard,
NCPDP SCRIPT standard version 2023011, and utilize EPCS. Additionally, this proposal would
prevent an increased number of prescribers from potentially applying for a waiver for
circumstances beyond their control due to difficulty of reliably conducting EPCS for
beneficiaries in LTC facilities by the current deadline of January 1, 2025.

We do not believe this proposal would cause additional costs as we are only extending
the deadline by which we would include prescriptions written for patients in LTC facilities in the
CMS EPCS Program compliance threshold calculation and not modifying the requirement to
become compliant. We also note that beneficiaries in LTC facilities may not receive the full
benefits of EPCS, which we describe in the CY 2022 PFS final (86 FR 65362), until a later date,
but we believe the delay is necessary due to the logistical challenges of prescribers electronically
prescribing controlled substances prescriptions for beneficiaries in LTC facilities.
We seek public comments on our impact assumptions.
16. Expand Hepatitis B Vaccine Coverage
In section III.M. of this rulemaking, we propose to expand Hepatitis B vaccine coverage
by revising our regulatory definition for intermediate risk groups by adding a new paragraph to
include individuals who have not previously received a completed hepatitis B vaccination series
or whose vaccination history is unknown (§ 410.63(a)(2)).
Hepatitis B vaccine is currently covered under Medicare Part B for enrollees who are at
intermediate or high risk of contracting hepatitis B virus, and, for Part D enrollees who do not
fall into those categories the vaccine may be covered under Medicare Part D.
819
In 2021, about
51 million of 65 million Medicare beneficiaries were enrolled in Part D and 21,629 received the
vaccine. In 2019, Part B covered 300,000 doses of hepatitis B vaccine for beneficiaries who were
at high or intermediate risk for the disease.
820
Since the vaccine has been available for several
decades, we are not able to determine how many Medicare beneficiaries have already received
the vaccine.
819
Sayed, BA, Finegold, K, Ashok, K, Schutz, S, De Lew, N, Sheingold, S, Sommers, BD. Inflation Reduction Act
Research Series: Medicare Part D Enrollee Savings from Elimination of Vaccine Cost-Sharing. (Issue Brief No. HP-
2023-05). Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human
Services. September 2023. Retrieved from
https://aspe.hhs.gov/sites/default/files/documents/407d41b6534e7af6702eb280b3945d00/aspe-ira-vaccine-part-
d.pdf.
820
Medpac 2021. Report to the Congress: Medicare and the Health Care Delivery System. Chapter 7. Medicare
vaccine coverage and payment. Retrieved from https://www.medpac.gov/wp-
content/uploads/import_data/scrape_files/docs/default-source/default-document-
library/jun21_ch7_medpac_report_to_congress_sec.pdf.

Overall vaccination rates among adults, including older adults, are generally low.
821,822
A
Centers for Disease Control and Prevention (CDC) analysis of data from the National Health
Interview Survey found that fewer than half of all adults (less than 45 percent) received age-
appropriate recommended vaccinations in 2019.
823
An estimated 20 percent of adults aged ≥60
years have been vaccinated against hepatitis B; and approximately 34 percent of adults aged ≥19
years have been vaccinated against hepatitis B.
824
We do not anticipate our proposal to result in
significant economic impact on the Medicare program.
As of January 1, 2023, the Inflation Reduction Act (IRA) eliminated out-of-pocket costs
for vaccines covered under Medicare Part D that are recommended by the Advisory Committee
on Immunization Practices (ACIP).
825
Before the Inflation Reduction Act (IRA), beneficiaries
incurred out of pocket costs for Part D vaccines. While we would expect that after the IRA, more
beneficiaries would receive covered vaccines because of eliminating out of pocket costs, existing
research shows that cost-sharing is only one factor among other determinants. Trust in vaccines,
access to health care, health literacy, perceived risk, socio-demographic factors and awareness of
vaccine recommendations, all shape whether individuals obtain a recommended vaccine.
826
If the
number of people receiving the hepatitis B vaccine under Part D is any indication, we assume
that even by increasing access, there will not be immediate or significant change in the number
of covered hepatitis B vaccines paid under Medicare Part B. For these reasons, we do not
821
CDC. (2022, February 17). Vaccination Coverage among Adults in the United States, National Health Interview
Survey, 2019–2020. Centers for Disease Control and Prevention. Retrieved February 27, 2023, from
https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/pubs-resources/vaccination-coverage-adults-
2019-2020.html.
822
Gellin, B. G., Shen, A. K., Fish, R., Zettle, M. A., Uscher-Pines, L., & Ringel, J. S. (2016). The National Adult
Immunization Plan: Strengthening Adult Immunization Through Coordinated Action. American journal of
preventive medicine, 51(6), 1079–1083. https://doi.org/10.1016/j.amepre.2016.04.014.
823
CDC. (2022, February 17). Vaccination Coverage among Adults in the United States, National Health Interview
Survey, 2019–2020. Centers for Disease Control and Prevention. Retrieved February 27, 2023, from
https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/pubs-resources/vaccination-coverage-adults-
2019-2020.html.
824 CDC. 2023. Vaccination Coverage among Adults in the United States, National Health Interview Survey, 2021.
Retrieved from https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/pubs-resources/vaccination-
coverage-adults-
2021.html#:~:text=Hepatitis%20B%20vaccination%20coverage%20in,and%20Other%20(40.2%25)%20adults.
825
Sayed, BA, et al. 2023. Inflation Reduction Act Research Series
826
Sayed, BA, et al. 2023. Inflation Reduction Act Research Series
anticipate that expanding the definition of intermediate risk for hepatitis B vaccine will result in
a significant financial impact to the Medicare Program.
17. Low Titer O+ Whole Blood Transfusion Therapy During Ground Ambulance Transport
As discussed in section III.N of this proposed rule, we propose to modify the definition of
ALS2 at § 414.605 by adding the administration of low titer O+ whole blood transfusion therapy
(WBT) to the list of ALS2 procedures as a new number 8. We would also reflect this change in
the Medicare Benefit Policy Manual, Chapter 10, Ambulance Services, section 30.1.1, Definition
of Ground Ambulance Services. Under this proposal, a ground ambulance transport that
provides WBT would itself constitute an ALS2-level transport.
We believe that many ground ambulance transports providing WBT already qualify for
ALS2 payment, given that patients requiring such transfusions are generally critically injured or
ill and often suffering from cardio-respiratory failure and/or shock and are therefore likely to
receive one or more procedures currently listed as ALS procedures in the definition of ALS2,
such as endotracheal intubation, central venous line, chest decompression, and placement of an
intraosseous line. For impact analysis, for ground ambulance transports that provide WBT only
and currently do not qualify for ALS2 payment, we are assuming that these transports are
reported as ALS1 (advanced life support, level 1) emergencies.
In order to help identify the number of ground ambulance transports that could
potentially be affected by this proposal, we analyzed inpatient hospital claims related to multiple-
trauma that started with an ALS1 emergency ambulance transport and also included a blood
transfusion done in the hospital. The inpatient admissions were identified by DRG code “813”
and diagnosis code of “24,” the ambulance transport is identified by HCPCS “A0427,” and the
blood transfusion administered to these patients in the hospital setting is identified by the
presence of covered charges, patient liability amounts, and replacement units for blood.
Since payments vary for urban, rural, and super-rural ground ambulance transports, we
calculated the average Medicare payment amount for ALS2 (HCPCS A0433) and ALS1
(HCPCS A0427) over the last several years. The average payment differential over calendar
years 2019 and 2023 is estimated to be roughly $162 per transport. It is difficult to make an
assumption for the number of transports that will be impacted by this proposal, but the potential
number over the last several years, based on an analysis of actual experience, is very few. Even
if all of these ALS1 emergency transports shifted to being ALS2 transports, which is very
unlikely, the impact would be negligible.
18. Updates to the Quality Payment Program
In this section of this proposed rule, we estimated the overall and incremental impacts of
the Quality Payment Program policies. We estimated participation, final scores, and payment
adjustment for eligible clinicians participating through traditional MIPS, MVPs, and the
Advanced APMs. We also presented the incremental impacts to the number of expected
Qualified Participants (QPs) and associated APM Incentive Payments that result from our
policies relative to a baseline model that reflects the status quo in the absence of any
modifications to the previously finalized policies.
A. Overall MIPS Modeling Approach and Data Assessment
(1) MIPS Modeling Approach
For this proposed rule, we used a similar modeling approach as the CY 2024 PFS final
rule (88 FR 79504 through 79506). We created two MIPS RIA models: a baseline and proposed
policy model. Our baseline model includes previously finalized policies that will be in effect for
the CY 2024 performance period/2026 MIPS payment year in the absence of any of the newly
proposed policies in this proposed rule. Examples of previously finalized policies included in the
baseline model include: updated QP and partial QP thresholds, and the previously finalized list
of MVPs.
The proposed policies model builds off the baseline model and incorporates the MIPS
policies for the CY 2025 performance period/2027 MIPS payment year included in this proposed
rule. By comparing the baseline model to the proposed policies model, we are able to estimate
the incremental impact of the specific policies in this proposed rule.
Our modeling approach utilizes the same scoring engine that is used to determine MIPS
payment adjustments. This modeling approach enables our model to align as much as possible
with actual MIPS scoring and minimizes differences between our projections and policy
implementation. These limitations of our model are discussed later in this RIA.
(2) Data Used to Estimate Future MIPS Performance
In the CY 2024 PFS final rule (88 FR 79504), we explained our decision to use CY 2022
performance period submissions data. We noted that using CY 2022 performance data presents
the most current data and aligns our participation, final scoring, and payment adjustment analysis
around the same common data set. CY 2022 performance data were the most recently available
data in time for us to construct our simulation model for this proposed rule and for the same
reasons discussed in the CY 2024 PFS final rule (88 FR 79504), we are considering it to
construct the baseline and proposed policies model in this proposed rule. As more data becomes
available, we will assess the feasibility and validity of that data for use in RIA simulations.
b. APM Incentive Payments to QPs in Advanced APMs and Other Payer Advanced APMs
For payment years from 2019 through 2025, through the Medicare Option, eligible
clinicians who have a sufficient percentage of their Medicare Part B payments for covered
professional services or Medicare patients through Advanced APMs will be QPs for the
applicable QP Performance Period for a year and the corresponding payment year. In payment
years 2019 through 2024 these QPs will receive a lump-sum APM Incentive Payment equal to 5
percent of their estimated aggregate paid amounts for covered professional services furnished
during the calendar year immediately preceding the payment year. In payment year 2025, QPs
will receive a lump-sum APM Incentive Payment equal to 3.5 percent payment of their estimated
aggregate paid amounts for covered professional services furnished during CY 2024. Beginning
in payment year 2021, in addition to the Medicare Option, eligible clinicians may become QPs
through the All-Payer Combination Option. The All-Payer Combination Option allows eligible
clinicians to become QPs by meeting the QP payment amount or patient count threshold through
a pair of calculations that assess a combination of both Medicare Part B covered professional
services furnished or patients through Advanced APMs and services furnished or patients
through Other Payer Advanced APMs. Eligible clinicians who become QPs for a year are not
subject to MIPS reporting requirements and payment adjustments. Eligible clinicians who do not
become QPs but meet a lower threshold to become Partial QPs for the year may elect to report to
MIPS and, if they elect to report, will then be scored under MIPS and receive a MIPS payment
adjustment. Partial QPs are not eligible to receive the APM Incentive Payment.
If an eligible clinician does not attain either QP or Partial QP status, and is not excluded
from MIPS on another basis, the eligible clinician will be subject to the MIPS reporting
requirements and will receive the corresponding MIPS payment adjustment.
Beginning in payment year 2026, there are two separate PFS CFs—one for eligible
clinicians who are QPs for the year (the qualifying APM CF), and the other for all non-QP
eligible clinicians and other suppliers paid under the PFS (the non-qualifying APM CF). The
update to the qualifying APM CF for a year is 0.75 percent, while the update to the non-
qualifying APM CF for a year is 0.25 percent.
In addition, the thresholds to achieve QP status beginning in the 2025 QP Performance
Period will increase to 75 percent for the payment amount method, and 50 percent for the patient
count method. Overall, we estimated that for the 2025 QP Performance Period between 339,561
and 436,579 eligible clinicians will become QPs, and therefore be excluded from MIPS reporting
requirements and payment adjustments.
In section IV.A.4.k.(2) of this proposed rule, we propose to modify the definition of
“attribution-eligible beneficiary” to include any beneficiary who has received a covered
professional service furnished by the eligible clinician (NPI) for whom we are making the QP
determination. By no longer specifying evaluation and management (E/M) services as the default
attribution basis, we also would eliminate the need to develop customized attribution bases for
Advanced APMs that do not use E/M services as the basis for attribution. Therefore, our
proposal would standardize the attribution methodology for QP determinations by making
covered professional services the basis for attribution across all Advanced APMs.
We projected the number of eligible clinicians who will be QPs, and thus excluded from
MIPS, using several sources of information. First, the projections are anchored in the most
recently available public information on Advanced APMs. The projections reflect Advanced
APMs that will be operating during the 2025 QP Performance Period, as well as some Advanced
APMs anticipated to be operational during the 2025 QP Performance Period. The projections
also reflect an estimated number of eligible clinicians that will attain QP status through the All-
Payer Combination Option. The following APMs are expected to be Advanced APMs for the
2025 QP Performance Period:
● Bundled Payments for Care Improvement Advanced Model;
● ACO REACH Model (formerly Global and Professional Direct Contracting) Model;
● Kidney Care Choices Model (Comprehensive Kidney Care Contracting Options,
Professional Option and Global Option);
● Maryland Total Cost of Care Model (Care Redesign Program; Maryland Primary Care
Program);
● Medicare Shared Savings Program (Level E of the BASIC Track and the ENHANCED
Track); and
● Enhancing Oncology Model (EOM); and
● Primary Care First (PCF) Model.
We used the Participation Lists and Affiliated Practitioner Lists, as applicable, (see
§ 414.1425(a) for information on the APM Participant Lists and QP determinations) for the 2023
QP performance period third snapshot QP determination date to estimate the number of QPs,
total Part B paid amounts for covered professional services, and the aggregate total of APM
Incentive Payments for the 2025 QP Performance Period. We examined the extent to which
Advanced APM participants will meet the QP Thresholds of having at least 75 percent of their
Part B covered professional services or at least 50 percent of their Medicare beneficiaries
furnished Part B covered professional services through the APM Entity.
c. Estimated Number of MIPS Eligible Clinicians in the CY 2025 Performance Period/2027
MIPS Payment Year
(1) Initial Population of Clinicians Included in the RIA Baseline and Proposed Policies Models
For this proposed rule, we applied the same assumptions as in the CY 2024 PFS final rule
(88 FR 79505) to estimate our initial population of clinicians based on CY 2022 performance
period/2024 MIPS payment year data.
We used the same CY 2022 final reconciled eligibility determination file described in the
CY 2024 PFS final rule (88 FR 79505). This file reconciles eligibility from two determination
periods and aligns with the CY 2022 performance period submissions data on which we based
this model. Our analysis included 1,820,899 clinicians with PFS claims in this initial population.
This initial population of clinicians was used to determine eligibility using the methodology
described in the following sections.
(2) Estimated Number of MIPS Eligible Clinicians after Applying Eligibility Assumptions
(a) Methods and Assumptions Used to Estimate Eligibility
After identifying the clinician population with PFS claims we applied the same eligibility
assumptions and determination process described in the CY 2024 PFS final rule (88 FR 79505).
We are not proposing any modifications to MIPS eligibility requirements and the same eligibility
assumptions apply to both the baseline and final policies model.
For our RIA model, we established the “required eligibility” category, which means the
clinician exceeds the low-volume threshold in all 3 criteria and is subject to a MIPS payment
adjustment. We base this estimate on the CY 2022 performance period data described in this
section of this proposed rule, which includes the three low volume criteria. Within this category
we divide clinicians into two groups- clinicians who report data and clinicians who do not report
data.
Our next two eligibility assumptions concern clinicians and groups who may participate
in MIPS but are not required to participate. First, we estimate group eligibility. These are the
clinicians who have a group submission and their group exceeds the low-volume threshold in all
3 criteria. Next, we apply our opt-in eligibility assumptions. Individuals or groups who exceed
the low-volume threshold in 1 criterion but not all 3 may elect to opt-in. Based on the CY2022
data we determine which individuals opted-in to MIPS and for the purposes of our model
estimate that these clinicians will continue to opt-in to MIPS.
After applying the process outlined in this section of this proposed rule, we next estimate
the number of “Potentially MIPS Eligible” clinicians. These clinicians are not included in our
total number of MIPS eligible clinicians. These are clinicians who are not MIPS eligible
individually but who may either opt-in because they exceed the low volume threshold in at least
one criterion but not all three or who could report as part of a group which exceeds all three low
volume criteria.
Finally, we estimate the number of clinicians who are neither MIPS eligible nor
potentially MIPS eligible. First, we estimate the number of MIPS eligible clinicians who are
below all three low-volume criteria (both as an individual and as a group) again using the CY
2022 performance period data as described in this section of this proposed rule.
Next, we estimate the number of QPs (not MIPS eligible). In section VII.E.17.b. of this
proposed rule, we estimated a range of QPs. For the purposes of our RIA population, we
estimate a specific number of QPs. This is because it is necessary to establish a specific
population of clinicians to use to simulate the impacts of our final policies on participation, final
scores, and payment adjustments. Finally, we estimate the number of clinicians who are excluded
for other reasons including that they are a non-eligible clinician type or newly enrolled in
Medicare.

After applying these assumptions to our initial population, we estimate 686,645 MIPS
eligible clinicians with $5.5 billion in allowed charges. However, this number may be as high as
1,270,806 MIPS eligible clinicians and $7 billion allowed charges if all potentially MIPS
eligible
827
clinicians either opt-in or report as a group. This is an unlikely scenario but it
establishes the full range of possible MIPS eligible clinicians in our initial population.
(b) MIPS Eligibility Estimates
Eligibility among many clinicians is contingent on submission to MIPS as a group or
election to opt-in: therefore, we would not know the number of MIPS eligible clinicians who
submit until the submission period for the CY 2023 performance period is closed. For the
remaining analysis, we use the estimated population of 686,645 MIPS eligible clinicians
described in previously in this section of this proposed rule. Table 136 summarizes our
eligibility estimates for the proposed policies model after applying our assumptions discussed in
this section of this proposed rule.
827
We define potentially MIPS eligible clinicians as those clinicians who are not required to participate in 13MIPS
but may either opt-in or join a group that exceeds the low-volume threshold in all three criteria.

TABLE 136: Description of MIPS Eligibility Status for CY 2025 Performance Period/2027 MIPS
Payment Year Using the CY 2025 PFS Proposed Rule Assumptions**
Eligibility Status
Predicted Participation
Status in MIPS Among
Clinicians *
Number of
Clinicians
PFS allowed
charges ($ in
mil)**
MIPS Eligible Clinicians
MIPS eligible
(always subject to a MIPS payment adjustment because
individual clinicians exceed the low-volume threshold in all 3
criteria)
Reported to MIPS
105,843
$29,530
MIPS eligible
Did not Report to MIPS
40,813
$11,951
Group eligibility
(only subject to payment adjustment because clinicians' groups
exceed low-volume threshold in all 3 criteria)
Had a group submission
533,473
$13,108
Opt-In eligibility assumptions
(only subject to a positive, neutral, or negative adjustment
because the individual or group exceeds the low-volume
threshold in at least 1 criterion but not all 3, and they elect to
opt-in to MIPS)
Opted-in To MIPS
6,516
$350
Total Number of MIPS Eligible Clinicians and the
associated PFS allowed charges
686,645
$54,564
Not MIPS Eligible Clinicians
Potentially MIPS Eligible
(not subject to payment adjustment for non-participation; could
be eligible for one of two reasons: (1) meet group eligibility; or
(2) opt-in eligibility criteria)
Opt-in Eligible; Do not opt-in
178,216
$5,517
Potentially MIPS Eligible
Group Eligible; Did not
Report
405,945
$9,502
Below the low-volume threshold
(never subject to payment adjustment; both individual and group
is below all 3 low-volume threshold criteria)
Not applicable
129,806
$795
Excluded for other reasons
(Non-eligible clinician type, newly enrolled)
Not applicable
60,471
$501
Qualified Participant (QP)***
Not applicable
359,816
$17,602
Total Number of Clinicians Not MIPS Eligible
1,134,254
$33,916
Total Number of Clinicians (MIPS and Not MIPS Eligible)
1,820,899
$88,481
* Participation excludes facility-based clinicians who do not have scores in the 2022 MIPS submission data.
** Allowed charges estimated in 2022 dollars. Low-volume threshold is calculated using allowed charges. MIPS payment adjustments
are applied to the paid amount.
*** Our QP estimate differs from that reported in section VII.E.17.b of this proposed rule because, for purposes of establishing the
population used in our modeling, we estimate an absolute number of QPs rather than a range.
d. Modeling Approach and Methods for MIPs Value Pathways (MVPs) and Traditional MIPS
(1) Summary of Approach
In this proposed rule, we present several proposals which impact the measures and
activities, the performance category scores, final score calculation, and the MIPS payment
adjustment of MIPS eligible clinicians. We outline these changes in more detail in section
VII.E.17.d.(3). Of this proposed rule as we describe our methodology to estimate MIPS payment
adjustments for the CY 2025 performance period/2027 MIPS payment year. We then present the
impact of the proposed policies in the CY 2025 performance period/2027 MIPS payment year
and compare select metrics to the baseline model. By comparing the baseline model to the
policies model, we are able to estimate the incremental impact of the policies for the CY 2025
performance period/2027 MIPS payment year.
MIPS eligible clinician’s final scores are calculated based on the clinician’s performance
on measures and activities under the four MIPS performance categories: quality, cost,
improvement activities, and Promoting Interoperability. MIPS eligible clinicians can participate
in the four MIPS performance categories as an individual, group, virtual group, APM Entity,
clinicians participating in MIPS through the APM Performance Pathway (APP), or through an
MVP. MIPS APM participants can participate in the APP as an individual, group, virtual group,
APM Entity and are only scored on three MIPS performance categories: quality, improvement
activities, and Promoting Interoperability. Our simulation applies the proposed and previously
finalized policies to the existing MIPS scoring engine.
In the CY 2022 PFS final rule (86 FR 65394 through 65397), we finalized policies at
§ 414.1365 for implementing MIPS Value Pathways beginning in the CY 2023 performance
period/2025 MIPS payment year. We incorporate MVP participation and scoring rules in this
RIA where applicable as described in the following section.
(2) Methodology to Assess Impact for MIPS Value Pathways
(a) MVP Participant Assumptions
At § 414.1365(b), we require MVP Participants (which can be a group, individual,
subgroup, or APM entity) to register prior to submitting an MVP. We assessed whether to use
CY 2024 MVP registration data to estimate MVP participation but elected to again use the
approach described in the CY 2024 PFS final rule (88 FR 79507) for two reasons. First, we do
not presently have MVP scoring data, thus do not know the information of MVP registrants that
may submit MVP data to MIPS. Secondly, our model is based on CY 2022 performance data.
This data does not contain MVP scores and reconciliation between multiple years introduces
uncertainty and complexity into our model. As MVP scoring data becomes available in the
future, we will reassess our methodology for estimating MVP participation and final scores.
We assume for purposes of this model, that MVP Participants are MIPS eligible
individual clinicians or groups that submit the required MVP measures. For the baseline model,
we used the measures from the 16 MVPs finalized in the CY 2024 PFS final rule Appendix 3 (88
FR 79978 through 80047).
In section IV.A.4.a. and Appendix 3 of this proposed rule, we propose modifications to 7
existing MVPs and proposed 6 new MVPs. The 6 new proposed MVPs are:
● Complete Ophthalmologic Care
● Dermatological Care
● Gastroenterology Care
● Optimal Care for Patients with Urologic Conditions
● Pulmonology Care
● Surgical Care
For the proposed policies model, we incorporated the measure revisions for the existing
MVPs described in Appendix 3 of this proposed rule. Due to data availability, we are unable to
simulate scores for the following measures: 487, 488, 489, 490, 492, 493, 496, 497, 502, 503,
504, 505, ABG44, PIMSH13, UREQA10, 485, 486, 487 ,488, 489, 490, 492, 493, 495, 496, 497,
499, 500, 501, 502, 503, 504, 505, AAD16, AAD17, AAD18, ABG44, GIQIC26, IA_PM_XX,
IRIS61, MSK6, MSK7,MSK8,MSK9,MUC2023-141, MUC2023-161, MUC2023-162,
MUC2023-190, MUC2023-211, PIMSH13, UREQA10.
For these MVP Participants, we calculate both an MVP and a traditional MIPS score and
take the highest score consistent with the existing scoring hierarchy which was finalized in the
CY 2023 PFS final rule (86 FR 65537).
Our MVP Participant assumptions have limitations: the measure list used to simulate
MVP participation does not align completely with what is proposed in section IV.A.4.a. of this
proposed rule, we are not incorporating subgroups due to a lack of data, not all of the assumed
participants may elect to register for an MVP, and we may have additional clinicians or groups
register for an MVP. However, we believe this is a reasonable approach to simulate the impact of
MVPs and we sought comment on this assumption but did not receive any feedback.
(b) MVP Scoring Methods and Assumptions
We simulate an MVP score using the same data sources as we did for traditional MIPS.
We scored according to § 414.1365(d) and (e) using the MVP reporting requirements listed in
§ 414.1365(c) with one exception. We did not restrict the improvement activities to the activities
listed in the MVP inventory. We believed this would lower our estimated MVP score as
clinicians and groups were not required to select from a limited inventory in the CY 2022
performance period (upon which our model is based). Therefore, we scored any improvement
activities the MVP Participants submitted in 2022 as if those improvement activities are in the
MVP inventory. Additionally, in section IV.A.4.b.(1)(b) of this proposed rule, we proposed to
score all available population health measures for a clinician participating in an MVP and select
the highest scoring of those measures for use in determining their category score. We
incorporated this proposal into our simulation.
(3) Methodology to Assess Impact for Traditional MIPS
To estimate the impact of the policies on MIPS eligible clinicians, we generally used the
CY 2022 performance period’s data, including data submitted or calculated for the quality, cost,
improvement activities, and Promoting Interoperability performance categories.
We supplemented this information with the most recent data available for CAHPS for
MIPS and CAHPS for ACOs, administrative claims data for the new quality performance
category measures, and other data sets. We calculated a hypothetical final score for the CY 2025
performance period/2027 MIPS payment year for the baseline and policies scoring models for
each MIPS eligible clinician using score estimates for quality, cost, Promoting Interoperability,
and improvement activities performance categories, and the application of our final scoring
policies.
(a) Methodology to Estimate the Quality Performance Category Score
We used the CY 2024 PFS final rule final policies model as the starting point of our
baseline model. Since there are no previously finalized policies impacting the quality
performance category that were not already included in the CY 2024 PFS final rule policies
model, we did not make any modifications to the quality performance category and the baseline
model is identical to the CY 2024 PFS final rules policies model with respect to the quality
category.
Our proposed policies model incorporates the following proposals from this proposed
rule as outlined in section IV.A.4.e.(1) of this proposed rule:
In section IV.A.4.f.(1)(b)(i) of this proposed rule, to facilitate fairer scoring, we propose
to remove the scoring cap and change the benchmarking approach for certain topped out
measures applicable to clinicians facing both limited measure choice and limited scoring
opportunities. We did not simulate the addition of quality measures described in section
IV.A.4.e.(1)(d)(i) since we use existing quality measure data from the CY 2022 performance
period which does not include new measures. We do not simulate the removal of quality
measures described in section IV.A.4.e.(1)(d)(ii) since we cannot predict how clinician behavior
and measure selection would change in response.
(b) Methodology to Estimate the Cost Performance Category Score
We estimated the cost performance category score using a methodology similar to the
methodology described in the CY 2024 PFS final rule (88 FR 79508) for the baseline and the
proposed policies RIA models with the modifications described below.
For this proposed rule, the baseline policies RIA model used the same methodology as
the final policies RIA model in the CY 2024 PFS final rule (88 FR 79508). The proposed
policies RIA model incorporated and implemented the following changes:
● In section IV.A.4.e.(2)(a) of this proposed rule, we are proposing to adopt 6 new
episode-based cost measures and modify 2 existing episode-based cost measures . We
incorporated measure test data with the specifications for the new and modified measures.
● In section IV.A.4.f.(1)(d) of this proposed rule, we are proposing to modify our cost
scoring methodology. The median cost for a measure would be assigned achievement points
equal to 10 percent of the performance threshold (7.5 in the CY 2024 performance period/ CY
2026 payment year). The cut-offs for benchmark ranges would be calculated as standard
deviations from the median. This proposal is incorporated into our model based on the
specifications explained in section IV.A.4.f.(1)(d)(ii)(B) of this proposed rule.
(c) Methodology to Estimate the Promoting Interoperability Performance Category Score
We estimated Promoting Interoperability performance category score by using the same
methodology that we used in the CY 2024 PFS final rule (88 FR 79508). We did not incorporate
any changes to this category in our model. In section IV.A.4.e.(4)(f) of this proposed rule, we are
proposing minimum criteria for a qualifying data submission for the Promoting Interoperability
performance category. We conducted an analysis of this proposal and determined that the impact
on final scores and payment adjustments was negligible and therefore did not incorporate it into
our model.
(d) Methodology to Estimate the Improvement Activities Performance Category Score
For the baseline and policies model we used the same method to estimate the
improvement activities performance category score as described in the CY 2024 PFS final rule
(88 FR 79508) including alignment with the clarification provided regarding IA automatic
weighting for APM participants (88 FR 79366).
In section IV.A.4.e.(3)(b)(IV) of this proposed rule, we proposed to remove weighting of
improvement activities. We conducted an analysis of this proposal and determined that the
impact on final scores and payment adjustments was negligible and therefore did not incorporate
it into our model.
(e) Methodology to Estimate the Complex Patient Bonus Points
For the baseline and policies RIA model, we used the previously established method to
calculate the complex patient bonus as described in the CY 2022 PFS final rule (86 FR 64996).
(f) Methodology to Estimate the Final Score
We are not proposing any changes for how we calculated the MIPS final score. Our
baseline and policies RIA models assigned a final score for each TIN/NPI by multiplying each
estimated performance category score by the corresponding performance category weight,
adding the products together, multiplying the sum by 100 points, adding the complex patient
bonus, and capping at 100 points.
For both models, after adding any applicable bonus for complex patients, we reset any
final scores that exceeded 100 points to equal 100 points. For MIPS eligible clinicians who were
assigned a weight of zero percent for any performance category, we redistributed the weights
according to § 414.1380(c).
For the purposes of this model, if a MIPS eligible clinician was approved for reweighting
of one or more performance category to zero percent of their final score, and the category’s
weight redistributed to other performance category(ies), for the CY 2022 performance
period/2024 MIPS payment year (which was the data source used in our model) in accordance
with our reweighting policies under § 414.1380(c)(2), then we continue to apply that reweighting
in our model by assigning them a neutral score equal to the performance threshold if all
categories were reweighted or assigning the applicable weights to the categories which were
reweighted. Although it is unlikely (but possible) that the exact same clinicians would apply for
and receive reweighting in both the CY 2022 performance period/2024 MIPS payment year
(which our data is based on) and the CY 2025 performance period/2027 MIPS payment year
(which we are simulating), we believe that this assumption accurately reflects future clinician
behavior for two reasons. First, while the exact same clinicians may not receive reweighting 2
years in a row, we believe that this assumption allows us to quantify the impact of the
reweighting on a population level. In other words, even if the same clinicians do not apply for
and receive reweighting 2 years in a row, the absolute number of reweighting and the
characteristic of practices who receive reweighting is likely to remain similar. Secondly, if we
were to not assign reweighting to those clinicians, many of them would receive a very low final
score because they did not submit data for one or more performance categories during the year in
which they received reweighting. We do not believe that it is realistic to assume that, in the
absence of reweighting, those clinicians would continue to not submit data. For these reasons,
clinicians who received reweighting in the CY 2022 performance period/2024 MIPS payment
year also are approved for reweighting in the CY 2025 performance period/2027 MIPS payment
year. These clinicians are assigned a score of the performance threshold (75) in our model
because this corresponds with a neutral (0 percent) payment adjustment.
(g) Methodology to Estimate the MIPS Payment Adjustment
For the baseline and proposed policies RIA models, we applied the hierarchy as finalized
in the CY 2022 PFS final rule (86 FR 65536 through 65537) to determine which final score
should be used for the payment adjustment for each MIPS eligible clinician when more than one
final score is available. We then calculated the parameters of an exchange function in accordance
with the statutory requirements related to the linear sliding scale, budget neutrality, and
minimum and maximum adjustment percentages.
For the baseline model, we applied the performance threshold of 75 points finalized in
the CY 2024 PFS final rule (88 FR 79373). In section IV.A.4.g.(2)(c) of this proposed rule, we
are proposing to again set the performance threshold at 75. Therefore, for both the baseline and
final policies models we used a performance threshold of 75 to calculate the exchange function
used for MIPS payment adjustments. We note that the results of this exchange are not identical
between the baseline and final policies model. This is because the scaling factor used to
determine positive adjustments is dependent on the total dollar amount of negative payment
adjustments and those adjustments differ as final scores are not identical between both models.
For both the baseline and policies models, we used these resulting parameters to estimate the
positive or negative MIPS payment adjustment based on the estimated final score and the
allowed charges for covered professional services furnished by the MIPS eligible clinician.
(4) Simulation Results and Projected Impact to MIPS Eligible Clinicians
Based on the methodology described in the preceding sections we created a baseline and
proposed policies simulation. Using this simulation, we estimate the impact of the policies
proposed.
(a) Impact to Clinician Eligibility
In section VII.E.17.c.(2) of this proposed rule, we noted that we are not proposing any
modification to clinician eligibility and therefore there is no difference in the total number of
MIPS eligible clinicians between our models.
(b) Impact to Clinician’s Final Scores
Table 137 shows the median final score by practice size and the percentage of MIPS
eligible clinicians of each practice size with a positive or neutral or negative adjustment.

TABLE 137: CY 2025 Final Score Estimates by Practice Size
*Practice size is defined as the number of NPIs in a TIN
** The median final score includes clinicians who receive reweighting for all MIPS performance categories our
policies at § 414.1380(c)(2). These clinicians who have all performance categories reweighted are assigned a score
of 75 (neutral payment adjustment) in our model.
The median final score is 82.20 in our baseline model and 86.42 in our proposed policies
model. There is an increase in the number of clinicians receiving a positive payment adjustment
for all practice sizes and an increase in the median final score for all practice sizes except for
solo practitioners.
828
We project that 69.93 percent of MIPS eligible clinicians will receive a
positive adjustment in our baseline model and 77.51 percent of MIPS eligible clinicians will
receive a positive adjustment in our proposed policies model. This increase is largely due to our
proposed change to the cost scoring methodology discussed in section IV.A.4.f.(1)(d)(ii)(B) of
this proposed rule. Table 138 shows the median cost score for MIPS eligible clinicians who are
scored on the cost performance category for our baseline and final policies model. There is a
substantial difference in median cost scores between our two models. This is true across all
practice sizes. The median cost category score is 59.16 in our baseline model and 73.85 in our
proposed policies model.
828
See section VII.E.17.d.(b)(b)(i) of this proposed rule for a discussion of the performance of solo practitioners
specifically.
Practice
Size*
Total
Number
of MIPS
Eligible
Clinicians
Median
Final Score
Estimate**
Percent Eligible
Clinicians with
Positive Payment
Adjustment
Percent
Eligible
Clinicians
with Neutral
Payment
Adjustment
Percent Eligible
Clinicians with
Negative
Payment
Adjustment
Baseline
1) Solo
18,867
75.00
31.05%
22.00%
46.95%
2) 2-15
71,908
81.79
60.47%
14.97%
24.56%
3) 16-99
150,377
81.76
64.79%
10.32%
24.89%
4) 100+
445,493
82.80
74.84%
4.32%
20.84%
Overall
686,645
82.20
69.93%
7.23%
22.84%
Proposed Policies
1) Solo
18,867
75.00
32.41%
21.94%
45.65%
2) 2-15
71,908
86.02
64.29%
14.78%
20.93%
3) 16-99
150,377
85.87
72.41%
9.98%
17.61%
4) 100+
445,493
87.19
83.28%
4.13%
12.59%
Overall
686,545
86.42
77.51%
7.02%
15.47%

TABLE 138: CY 2025 Cost Score Estimates by Practice Size
Figure 6 shows the distribution of final scores for all MIPS eligible clinicians. Note that
there are a relatively large number of MIPS eligible clinicians with a final score of 75. As stated
in section VII.E.17.d.(3)(f) of this proposed rule MIPS eligible clinicians whom we approved for
reweighting of all MIPS performance categories in accordance with our reweighting policies at §
414.1380(c)(2) are assigned a final score of exactly the performance threshold (75). Overall, the
distribution is skewed to the right indicating that clinicians tend to receive final scores on the
higher end of the distribution with many final scores clustered near the performance threshold of
75. Our proposed policies have the effect of shifting final scores to the right. Many clinicians
with final scores just below the performance threshold in the baseline model see their scores
increased to a value just above the performance threshold in the proposed policies model.
Practice Size
MIPS Eligible
Clinicians
Receiving Cost
Score
Total Number
of MIPS
Eligible
Clinicians
Proportion of
MIPS Eligible
Clinicians
Receiving Cost
Score
Median Cost
Score Estimate
Baseline
1) Solo
7,380
18,867
39.12%
62.15
2) 2-15
32,476
71,908
45.16%
59.89
3) 16-99
65,428
150,377
43.51%
60.30
4) 100+
226,035
445,493
50.74%
59.02
Overall
331,319
686,645
48.25%
59.16
Proposed
Policies
1) Solo
7,636
18,867
40.47%
75.24
2) 2-15
33,242
71,908
46.23%
74.34
3) 16-99
66,311
150,377
44.10%
74.35
4) 100+
227,601
445,493
51.09%
73.78
Overall
334,790
686,545
48.76%
73.85

FIGURE 6: Count of MIPS Eligibles Clinicians by Final Score
(i) Impact to Small and Solo Practices
18,867 MIPS eligible clinicians or 2.7 percent of all MIPS eligible clinicians are solo
practitioners in both the baseline and final policies models. The median final score for solo
practitioners is exactly equal to the performance threshold in both the baseline and final policies
model although the portion of solo practitioners receiving a positive adjustment is higher in the
proposed policies model than in the baseline model. As stated in section VII.E.17.d.(3)(f) of this
proposed rule, clinicians receiving reweighting under our policies at § 414.1380(c)(2) are
assigned a final score exactly equal to the performance threshold if we approved for reweighting
of all MIPS performance categories.
Section VII.E.17.d.(3)(f) of this proposed rule, clinicians receiving reweighting under our
policies at § 414.1380(c)(2) are assigned a final score exactly equal to the performance threshold
if we approved for reweighting of all MIPS performance categories.
These practitioners have a lower median final score than other practice sizes. This is
largely due to the fact that many of these solo practitioners do not submit data to MIPS despite
being MIPS eligible clinicians. Our analysis indicates that 49.45 percent of solo practitioners
submit data to MIPS compared to 93.68 percent of all clinicians. The median final score in our
baseline and proposed policies model is 75.00 for all solo practitioners, but for solo practitioners
0
10,000
20,000
30,000
40,000
50,000
60,000
70,000
80,000
0-0.99
3-3.99
6-6.99
9-9.99
12-12.99
15-15.99
18-18.99
21-21.99
24-24.99
27-27.99
30-30.99
33-33.99
36-36.99
39-39.99
42-42.99
45-45.99
48-48.99
51-51.99
54-54.99
57-57.99
60-60.99
63-63.99
66-66.99
69-69.99
72-72.99
75-75.99
78-78.99
81-81.99
84-84.99
87-87.99
90-90.99
93-93.99
96-96.99
99-99.99
Eligible Clinicians - Proposed
Eligible Clinicians - Baseline
Count of MIPS Eligible Clinicians
Final Score
who submit data the median final score is 84.35 in the baseline and 87.03 in the proposed
policies model. These findings indicate that the lower final scores among solo practitioners is
likely due to not reporting data to MIPS. Figure 7 shows the distribution of final scores for solo
practitioners as a box plot. While the median final score is 75 in both models, the bottom quartile
increases from 17.52 to 22.33 between the baseline and proposed policies model. Figure 8 shows
the final score distribution for all practice sizes. The first quartile of final scores is 75 in the
baseline model and 77.45 in the proposed policies model. The range between Q1 and Q3 is
significantly narrower for all practice sizes than it is for solo practitioners. Figure 9 shows the
distribution of final scores for solo practitioners who submit data to MIPS. This distribution is
much closer to the distribution of final scores in the overall population with the first quartile at
73.45 in the baseline and 75.00 in the proposed policies model. This is similar to the median final
score for all practice sizes which is 86.42. This indicates that, while many solo practitioners do
not submit data to MIPS, those who do submit data perform similarly or better than the overall
population of MIPS eligible clinicians. This is further evidence that the main factor causing low
final scores among solo practitioners is the high proportion who do not submit data.

FIGURE 7: Distribution of Final Scores for Solo Practitioners

FIGURE 8: Distribution of Final Scores for All Practice Sizes

FIGURE 9: Distribution of Final Scores for Solo Practitioners who Submit Data
Small practices, defined as groups with a range between 2 and 15 clinicians, have a
median final score of 81.79 in the baseline and 86.02 in the proposed policies model. This is
similar but slightly lower than the median final score for all practice sizes of 86.42. Among
small practices who submit data the median final score is 89.81 in the proposed policies model
(and 86.83 in the baseline). This is significantly higher than the median final score for all
clinicians who submit data which is 87.53. This indicates that small practices perform similarly
to other practice sizes although a slightly larger proportion of small practices do not submit data.
Table 141 shows the percentage of clinicians by practice size who either do or do not submit data
to MIPS and the corresponding median final score. Note that the median final score for clinicians

who do not submit data is 75 for all practice sizes except for solo practitioners. This indicates
that many clinicians belonging to small, medium, or large practices (but not solo practitioners)
who do not submit data to MIPS have been approved for reweighting of all of their MIPS
performance categories under our policies at § 414.1380(c)(2). In contrast, many solo
practitioners who do not submit data do so despite not being eligible for application of our
reweighting policies or not applying for reweighting under those policies.
A large majority of all practice sizes except solo practitioners submit data to MIPS. It is
possible that the small percentage of MIPS eligible clinicians in those practice sizes who do not
submit data to MIPS are primarily MIPS eligible clinicians who have received reweighting under
our policies at § 414.1380(c)(2). It should be noted that median final scores increase for solo and
small practitioners between our baseline and proposed policies model indicating that the net
effect of our proposed policies is an increase in their final scores.
TABLE 139: Percentage of MIPS Eligible Clinicians who Submit Data and Median Final
Score
Percentage of
MIPS Eligible
Clinicians who
Submit Data (by
practice size)
Median Final Score of
MIPS Eligible Clinicians
who Submit Data
Median Final Score of
MIPS Eligible
Clinicians who Do not
submit data.
Baseline
1) Solo
49.39%
84.35
19.35
2) Small (2-15)
79.32%
86.83
75
3) Medium (16-99)
91.69%
83.42
75
4) Large(100+)
98.23%
83.15
75
Overall
93.47%
83.46
75
Proposed Policies
1) Solo
49.45%
87.03
22.89
2) Small (2-15)
79.46%
89.81
75
3) Medium (16-99)
92.03%
87.27
75
4) Large(100+)
98.41%
87.31
75
Overall
93.68%
87.53
75
(ii) Impact to Rural Providers
In our data we assign rural practitioners a special status. Analysis of this group of
clinicians indicates that their final scores are similar to the overall population of MIPS Eligible
Clinicians across all practice sizes. Table 140 shows the median final score and the percentage of

eligible clinicians with a positive or neutral or negative adjustment by practice size.
TABLE 140: CY 2025 Final Score Estimates by Practice Size for Rural Practitioners Only
The median final score for all rural practitioners is 81.29 in our baseline model and 85.41
in our proposed policies model. This is slightly lower than the median final score for all
practitioners which is 82.20 in our baseline model and 86.42 in our proposed policies model.
However, the median final score is identical for solo practitioners and higher for small and
medium practices. Large rural providers have a slightly lower median final score compared to
large practices generally. The lower overall median final scores for rural practitioners are driven
by large rural practices who perform slightly worse than other practice sizes and when compared
to large practices generally. It should be noted that median final scores increase for rural
providers of all practice sizes between our baseline and proposed policies model indicating that
the net effect of our proposed policies is an increase in their final scores.
(iii) Impact to Safety Net Providers
(a). Updated Definition of Safety Net Providers
In the CY 2023 PFS final rule (87 FR 70094), we finalized our complex patient bonus
methodology. This bonus is composed of two distinct calculations which are added together:
Medical Complexity and Social Risk. Medical Complexity is determined based on a MIPS
eligible clinicians Hierarchical Conditions Categories risk score and social risk is determined
Practice
Size
Total
Number
of MIPS
Eligible
Clinicians
Median
Final Score
Estimate
Percent Eligible
Clinicians with
Positive Payment
Adjustment
Percent
Eligible
Clinicians
with Neutral
Payment
Adjustment
Percent Eligible
Clinicians with
Negative
Payment
Adjustment
Baseline
1) Solo
2,694
75.00
34.89%
17.37%
47.74%
2) 2-15
11,760
83.89
65.94%
10.48%
23.58%
3) 16-99
30,444
85.05
71.38%
6.80%
21.82%
4) 100+
43,286
80.43
69.02%
5.08%
25.90%
Overall
88,184
81.29
68.38%
6.77%
24.85%
Proposed Policies
1) Solo
2,694
75.00
36.53%
17.33%
46.14%
2) 2-15
11,762
87.34
69.82%
10.42%
19.76%
3) 16-99
30,409
87.53
76.90%
6.37%
16.73%
4) 100+
43,281
84.27
81.78%
4.94%
13.28%
Overall
88,146
85.41
77.12%
6.54%
16.34%

based on the proportion of a MIPS eligible clinicians Medicare patient population who are dually
eligible for both Medicare and Medicaid.
In the CY 2024 PFS final rule (88 FR 79513), we compared the performance of clinicians
who received the complex patient bonus with our overall population. As we further developed
our model, we decided to adopt a more precise definition of safety net providers. We believe that
by narrowing our definition of safety net providers to the top 20 percent (80
th
percentile) of
social risk we can identify the providers who are caring for the largest proportion of low-income
or otherwise socially vulnerable individuals.
Table 143 shows the final score estimates for safety net providers under this new
definition. Safety net provers have higher median final scores than the overall population of
MIPS eligible clinicians across all practice sizes with the exception of small and solo
practitioners. When our analysis is restricted to providers who submit data to MIPS this
discrepancy disappears and small and solo safety net providers who submit data have higher
median final scores than the overall population of small and solo MIPS eligible clinicians who
submit data. However, only 43.65 percent of solo and 72.90 percent of small safety net providers
submit data compared to 49.45 percent and 79.46 percent of the overall population of solo and
small MIPS eligible clinicians respectively. These results are shown in Table 141. This indicates
that the lower scores among small and solo safety net practitioners is likely due to a larger
number of these practitioners not submitting data. It should be noted that median final scores
increase for solo and small safety net providers between our baseline and proposed policies
model indicating that the net effect of our proposed policies is an increase in their final scores.
TABLE 141: CY 2025 Final Score Estimates by Practice Size
for Safety Net Practitioners Only
Practice
Size
Total
Number
of MIPS
Eligible
Clinicians
Median
Final Score
Estimate
Percent Eligible
Clinicians with
Positive Payment
Adjustment
Percent
Eligible
Clinicians
with Neutral
Payment
Adjustment
Percent Eligible
Clinicians with
Negative
Payment
Adjustment
Baseline
1) Solo
5,347
62.31
26.95%
20.07%
52.98%

TABLE 142: CY 2025 Median Final Scores for Safety Net Practitioners who Submit Data
Practice
Size
Percent of
MIPS
Eligible
Clinicians
who
Submit
Data
Median Final
Score
Estimate
Percent Eligible
Clinicians with
Positive Payment
Adjustment
Percent
Eligible
Clinicians with
Neutral
Payment
Adjustment
Percent Eligible
Clinicians with
Negative Payment
Adjustment
Baseline
1) Solo
43.63%
86.12
61.77%
10.84%
27.39%
2) 2-15
72.90%
89.51
75.46%
7.06%
17.48%
3) 16-99
91.73%
86.54
72.78%
8.45%
18.77%
4) 100+
98.47%
86.18
84.88%
4.45%
10.67%
Overall
91.97%
86.37
80.48%
5.85%
13.67%
Proposed Policies
1) Solo
43.65%
89.06
63.45%
10.84%
25.71%
2) 2-15
72.94%
92.29
78.39%
7.04%
14.57%
3) 16-99
91.90%
89.53
76.67%
8.44%
14.89%
4) 100+
98.58%
89.31
88.35%
4.45%
7.20%
Overall
92.08%
89.57
83.99%
5.83%
10.18%
(c) Impact to MIPS Eligible Clinicians’ Payment Adjustments
We are not proposing an increase in the performance threshold in this proposed rule.
However, payment adjustments differ between the baseline and final policies model. This is
because our proposed policies increase final scores of MIPS eligible clinicians
829
and therefore a
larger proportion of MIPS eligible clinicians receive a final score greater than the performance
threshold and thus a positive payment adjustment. The parameters of the exchange function used
to determine payment adjustments depends on the final score distribution of MIPS eligible
clinicians. As the proportion of MIPS eligible clinicians receiving a negative payment
adjustment decreases the budget neutral funds available for redistribution also decrease. In the
829
This increase is largely due to the change in our cost performance category scoring policies discussed in section
IV.A.4.f.(1)(d)(ii)(B) of this proposed rule.
2) 2-15
14,202
80.84
55.01%
15.56%
29.43%
3) 16-99
37,246
85.12
66.77%
12.97%
20.26%
4) 100+
82,916
86.18
83.59%
5.64%
10.77%
Overall
139,711
85.35
74.04%
9.15%
16.81%
Proposed Policies
1) Solo
5,347
65.78
27.70%
20.05%
52.25%
2) 2-15
14,208
84.50
57.18%
15.52%
27.30%
3) 16-99
37,212
88.31
70.48%
12.79%
16.73%
4) 100+
82,839
89.23
87.11%
5.53%
7.36%
Overall
139,606
88.59
77.36%
9.03%
13.61%

baseline model we project redistributing $517 million and in the proposed policies model we
project redistributing $458 million. This decrease means that the scaling factor for positive
adjustments is reduced.
FIGURE 10: Payment Adjustment Function
We also report the median positive and negative payment adjustments by practice size in Table
143.
TABLE 143: CY 2025 Median Positive and Negative Payment Adjustment
Estimates By Practice Size
Practice Size
Median Positive Payment Adjustment*
Median Negative Payment
Adjustment*
Baseline
Solo (1)
2.06%
-9.00%
Small (2-15)
1.82%
-4.69%
Medium (16-99)
1.65%
-1.25%
Large (>99)
1.59%
-0.88%
Overall
1.65%
-1.10%
Proposed Policies Model
Solo (1)
1.55%
-6.42%
Small (2-15)
1.46%
-5.88%
Medium (16-99)
1.35%
-1.44%
Large (>99)
1.28%
-1.08%
Overall
1.31%
-1.48%
-10.00%
-8.00%
-6.00%
-4.00%
-2.00%
0.00%
2.00%
4.00%
0-0.99
3-3.99
6-6.99
9-9.99
12-12.99
15-15.99
18-18.99
21-21.99
24-24.99
27-27.99
30-30.99
33-33.99
36-36.99
39-39.99
42-42.99
45-45.99
48-48.99
51-51.99
54-54.99
57-57.99
60-60.99
63-63.99
66-66.99
69-69.99
72-72.99
75-75.99
78-78.99
81-81.99
84-84.99
87-87.99
90-90.99
93-93.99
96-96.99
99-99.99
Payment Adjustment (%) - Proposed
Payment Adjustment (%) - Baseline

*The median positive payment adjustment is defined as the medium payment adjustment among clinicians with a
final score above the performance threshold. The median negative adjustment is defined as the medium payment
adjustment among clinicians with a final score below the performance threshold. Neither median includes clinicians
with a final score equal the performance threshold.
For all practices sizes except for solo practitioners the median negative payment
adjustment increases in magnitude. This is because many MIPS eligible clinician’s final scores
are clustered near the performance threshold. An increase in median final scores will cause many
of those clinicians who have a minor negative adjustment to meet or exceed the performance
threshold and therefore be removed from the population of clinicians with a negative adjustment.
The remaining population of MIPS eligible clinicians with negative adjustments are more likely
to have negative payment adjustments higher in magnitude. In contrast to other practice sizes.
fewer solo practitioners with negative payment adjustments have a final score near the
performance threshold and an increase in the final score of these MIPS eligible clinicians will
reduce the size of their negative payment adjustment but is less likely to shift their final scores
above the performance threshold in the manner described earlier. As discussed in section
VII.E.17.d.(4)(b)(i) of this proposed rule, this is largely because many of these solo practitioners
do not submit data to MIPS despite being MIPS eligible clinicians. Our analysis indicates that
49.45 percent of solo practitioners submit data to MIPS in our proposed policies model
compared to 93.70 percent of all MIPS eligible clinicians. In Table 144, we report the
proportion of MIPS eligible clinicians who either did or did not submit data with the maximum
negative adjustment (-9 percent).
TABLE 144: CY 2024 CY 2024 Clinicians With The Maximum Negative Adjustment
Practice Size
Percent of Clinicians who Did NOT
Submit Data with Maximum
Negative Adjustment
Percent of Clinicians Who
Submit Data With Maximum
Negative Adjustment
Baseline (-9%)
Solo (1)
48.94%
2.90%
Small (2-15)
35.75%
0.84%
Medium (16-99)
17.88%
0.37%
Large (>99)
11.71%
0.12%
Overall
29.35%
0.28%
Proposed Policies Model (-9%)
Solo (1)
35.57%
2.26%

Across all practice sizes the proportion of clinicians who do not submit data who receive
the max negative payment adjustment decreased between the baseline and proposed policies
model. A larger proportion of solo practitioners (2.26 percent) who submit data receive the
maximum negative adjustment.
The median positive adjustment for solo practitioners is 1.55 percent which is higher than
the median positive adjustment for all practice sizes overall. This indicates that, while many solo
practitioners do not submit data to MIPS, those solo practitioners who do report data to MIPS
and receive a positive adjustment receive a similar median adjustment to other practice sizes.
e. Additional Impacts from Outside Payment Adjustments
(1) Burden Overall
In addition to policies affecting payment adjustments, we are proposing several policies
that have an impact on burden in the CY 2025 performance period/2027 MIPS payment year. In
section V.B.8. of this proposed rule, we outline estimates of the costs of data collection that
include both the effect of proposed policy updates and adjustments due to the use of updated data
sources. For each proposed provision included in this proposed rule that impacts our estimate of
collection burden, we summarize the incremental burden in Table 145. We also provide
proposed additional burden discussions that we are not able to quantify.
Practice Size
Percent of Clinicians who Did NOT
Submit Data with Maximum
Negative Adjustment
Percent of Clinicians Who
Submit Data With Maximum
Negative Adjustment
Small (2-15)
25.23%
0.72%
Medium (16-99)
12.52%
0.35%
Large (>99)
5.50%
0.10%
Overall
20.77%
0.24%

TABLE 145: Incremental Estimated Burden from Associated Proposed Policies
(Asterisks refer to paragraph directly following table)
Burden Description and Associated Proposed Provisions
Burden Hours
Burden Dollars
Total burden associated with the provision to continue the
policies and ICRs set forth in the CY 2024 PFS final rule into the
CY 2025 performance period/2027 MIPS payment year with
updated data and assumptions (as outlined in section V.B.8. of
this proposed rule).
657,110
$77,779,594
Burden change for MVP registration ICR due to the provision of
additional MVPs (as outlined in section V.B.8.e.(7)(a)(i). of this
proposed rule). *
+626
+$66,759
Burden change for Quality Data Submission by Clinicians:
Medicare Part B Claims-Based Collection Type ICR for
capturing reduced number of quality submissions due to the
provision of additional MVPs (as outlined in section V.B.8.e.(4).
of this proposed rule). *
-7,697
-$898,035
Burden change for Quality Data Submission by Clinicians:
CQM/QCDR Collection Type ICR for capturing reduced number
of quality submissions due to the provision of additional MVPs
(as outlined in section V.B.8.e.(5). of this proposed rule). *
-6,866
-$823,269
Burden change for Quality Data Submission by Clinicians:
eCQM Collection Type ICR for capturing reduced number of
quality submissions due to the provision of additional MVPs (as
outlined in section V.B.8.e.(6). of this proposed rule). *
-9,664
-$1,176,109
Burden change for MVP Quality Submission ICR submissions
due to the provision of additional MVPs (as outlined in section
V.B.8.e.(7)(a)(iii). of this proposed rule). *
+16,031
+$1,917,478
Total change in burden due to policy for CY 2025
-7,570
-$913,176
Total burden set forth in the CY 2025 PFS proposed rule
649,540
$76,866,418
* The total change in burden due to this proposed provision includes an increase in burden due to an anticipated increase in the
number of respondents that will participate in MVP reporting based on the proposed addition of 6 new MVPs. Therefore, there
will be a decrease in burden in the “Quality Data Submission: MIPS CQM and QCDR collection type,” “Quality Data
Submission: eCQM collection type,” and “Quality Data Submission: Claims collection type” ICRs due to respondents who
previously submitted MIPS through those collection types submitting data with reduced Quality submission requirements as a
MVP participant. Total change in burden also includes the increase in submission burden due to the increase in the number of
respondents for “MVP registration.” See section V.B.8.e.(2).of this proposed rule.
(2) Additional Impacts to Clinicians
(a) Impact on Third Party Intermediaries
In section IV.A.4.j.(1)(b). of this rulemaking, we are proposing that as part of the
Consumer Assessment of Healthcare Providers & Systems (CAHPS) vendor registration process,
in addition to the previously established registration requirements, CAHPS vendors would
provide information on the range of costs for their services beginning with the CY 2026
performance period/2028 MIPS payment year. We recognize that there may be additional
minimal burden associated with the proposed cost information requirement for the CAHPS
vendor registration. However, we assume that this information is brief and readily available to
vendors completing the registration process. We are unable to quantify the additional impact for
the proposed CAHPS vendor cost requirement.
(b) Modifications to the Improvement Activities Inventory
As discussed in section IV.A.4.e.(3)(b)(iii).of this proposed rule, we are proposing
changes to the improvement activities inventory for the CY 2025 performance period/2027 MIPS
payment year and future years as follows: adding two new improvement activities; modifying
two existing improvement activity; and removing eight previously adopted improvement
activities. We refer readers to Appendix 2 of this proposed rule for further details. We do not
expect these changes to the improvement activities inventory to affect our currently approved
information collection burden for the number of estimated respondents. Most of the
improvement activities in the Inventory remain unchanged for the CY 2025 performance
period/2027 MIPS payment year. We refer readers to section IV.A.4.e.(3)(b)(iii). of this
proposed rule for additional information on changes to the improvement activities Inventory.
(c) Modifications to Improvement Activities Scoring and Reporting Policies
As discussed in section IV.A.4.e.(3)(b)(iv)., we are proposing two scoring and reporting
policy changes for the improvement activities performance category effective for the CY 2025
performance period/2027 MIPS payment year and subsequent years. As noted in section
V.B.8.h., we established our currently approved estimate that it will take a computer analyst 5
minutes to log in and manually attest that improvement activities were completed in the CY 2019
PFS final rule (83 FR 60016). In the CY 2024 PFS final rule (88 FR79454 and 79455), this
estimate included scenarios where participants might submit 1, 2, 3, or 4 activities for the
improvement activities category, based on medium- or high-weighted activities and any
additional weighting scenarios such as for MIPs Value Pathways (MVP) Participants. We
believe this proposal would decrease burden for MIPS participants who previously reported
medium-weighted activities. As MIPS participants who previously only reported high weighted
activities would have the same attestation requirements under this proposal, we propose to

continue our currently approve estimates of 5 minutes to log in and attest that improvement
activities are completed. We expect reduced reporting burden for clinicians who previously
reported at least one medium-weighted activity; however, we are unable to estimate the
aggregated impact of this proposal given the current weighting and scoring rules that affect the
number of activities each clinician submits to receive full credit for the improvement activities
performance category.
(d) MVP Maintenance Process
In section VII.E.17.e.(2)(d). of this proposed rule, we are proposing a modification to the
MVP maintenance webinar process previously finalized in the CY 2022 PFS final rule (86 FR
65410) and modified in the CY 2023 PFS final rule (87 FR 70037). We communicated that if
we identified any potentially feasible and appropriate submitted maintenance recommendations,
we would host a public facing webinar open to interested parties and the general public through
which they could offer their feedback on the potential maintenance updates we have identified.
Due to the low volume of submitted maintenance recommendations in past years, we are
proposing to provide us more flexibility in how we communicate maintenance recommendations
prior to proposing them in rulemaking. Allowing flexibility in communicating recommendations
through alternative webinar formats or other public communication channels would offer similar
opportunities for public review and feedback as a live public webinar. For example, in lieu of a
live webinar, we could choose to communicate submitted maintenance recommendations via a
pre-recorded webinar, which will encourage interested parties to submit their feedback on the
submitted recommendations in writing by email before maintenance updates are formally
proposed in rulemaking.
As with the CY 2023 PFS final rule (87 FR 70210 through 70211), we acknowledge that
there is administrative burden associated with the monitoring and review of the candidate MVPs.
We are uncertain on the number of interested parties and members of the general public that will
submit their recommendations for potential revisions to established MVPs for an applicable
performance period. We are also uncertain if CMS will host a public webinar, webinar
alternative, or other communications based on the review of the recommendations. In summary,
we are unable to quantify the impact associated with the proposed changes to the MVP
development and maintenance process.
(e) Reweighting Performance Categories When Data is Not Submitted Due to Reasons Outside
the Clinician’s Control
In section IV.A.4.i.(2) of this rulemaking, we are proposing to adopt a new reweighting
policy at 42 CFR 414.1380(c)(2)(i)(A)(10) and (c)(2)(i)(C)(12). Specifically, we are proposing
that, beginning with the CY 2024 performance period/2026 MIPS payment year, we may
reweight one or more of the performance categories (specifically, quality, improvement
activities, or Promoting Interoperability), where we determine, based on information submitted
to us on or before November 1st of the year preceding the relevant MIPS payment year, that data
for a MIPS eligible clinician are inaccessible or unable to be submitted due to circumstances
outside of the control of the clinician because the MIPS eligible clinician delegated submission
of the data to their third party intermediary, evidenced by a written agreement between the MIPS
eligible clinician and third party intermediary, and the third party intermediary did not submit the
data for the performance category(ies) on behalf of the MIPS eligible clinician in accordance
with applicable deadlines. We also are proposing that, to determine whether to apply
reweighting to the affected performance category(ies), we will consider: whether the MIPS
eligible clinician knew or had reason to know of the issue with its third party intermediary’s
submission of the clinician’s data for the performance category(ies); whether the MIPS eligible
clinician took reasonable efforts to correct the issue; and whether the issue between the MIPS
eligible clinician and their third party intermediary caused no data to be submitted for the
performance category(ies) in accordance with applicable deadlines. More details on this
proposed reweighting policy are provided in section V.B.8.g.(2) of this proposed rule.

Because this is a new policy and we believe these occurrences would be rare based on our
experience, we are unable to estimate the number of clinicians, groups, or third party
intermediaries that may apply for reweighting based on this policy. Similarly, the extent and
source of documentation provided to us for each event may vary considerably. Therefore, we are
not proposing any changes to our currently approved burden estimates as a result of this
proposal.
(f) Advanced Primary Care Management
As discussed in section II.G.2. of this proposed rule, we are proposing to adopt specific
coding and payment policies for advanced primary care management (APCM) services for use
by practitioners who are providing services under this specific model of “advanced primary
care,” beginning January 1, 2025. These services would be furnished under the direction of a
physician or other qualified health care professional who is responsible for all primary care (e.g.,
physicians and non-physician practitioner, including nurse practitioner, physician assistant,
certified nurse midwife and clinical nurse specialist), and serve as the continuing focal point for
all needed health care services, during a calendar month. We are proposing three new APCM
codes that would recognize the resources involved in furnishing ongoing, beneficiary-centered
care management services under the broad model of advanced primary care without paying for
each activity separately while allowing for flexibility in addressing patient needs. APCM
payment would incorporate several specific, existing care management and communication
technology-based services into a bundle and require reporting the Value in Primary Care MVP
beginning in the CY 2025 performance period/2027 MIPS payment year. Billing practitioners
who are not MIPS eligible clinicians (as defined at 42 CFR 414.1305) would not be required to
report the MVP in order to furnish and bill for APCM services. As discussed in section
V.B.8.e.(7)(a) of this proposed rule, we are unable to estimate the effect of this proposal on MVP
submissions and registrations for the CY 2025 performance period/2027 MIPS payment year as
the codes are newly proposed. Specifically, we are unable to determine how many additional
clinicians or practices would submit the Value in Primary Care MVP measures for the CY 2025
performance period/2027 MIPS payment year above our current estimates. Similarly, we cannot
assess what participation levels clinicians or practices who might use these APCM codes, if
finalized, have reported MIPS in the past (for example, eligibility requirements and special
statuses, participation at the individual, group, virtual group, or Alternative Payment Model
(APM) Entity level, or reporting via traditional MIPS, the APM Performance Pathway (APP), or
MVPs), or if they will be MIPS eligible clinicians in future years. For MIPS eligible clinicians
who move from reporting traditional MIPS to MVPs, we expect a decrease in overall program
burden due to the reduced number of measures required for reporting the quality performance
category. We will update these assumptions for MVP quality performance category reporting
and MVP registration as more information is available.
(g) Mandatory Subgroup Registration
As summarized in section IV.A.3.c.(1). we established a voluntary subgroup participation
option for clinicians choosing to report an MVP beginning in the CY 2023 performance
period/2025 MIPS payment year. We finalized a mandatory subgroup reporting requirement for
multispecialty groups choosing to report as an MVP Participant beginning in the CY 2026
performance period/2028 MIPS payment year (§ 414.1305; 86 FR 65394 through 65397).
Beginning with the CY 2026 performance period/2028 MIPS payment year, a single specialty
group may continue to submit data for an MVP at the group level, and a multispecialty group
must form subgroups to report an MVP. Under the existing subgroup reporting policies, a group
could place clinicians providing similar scope of care into one or more subgroups for reporting a
relevant MVP. Each clinician may participate in one subgroup per tax identification number
(TIN). The remaining clinicians under the group TIN not part of a subgroup could participate as
individuals to report an MVP or traditional MIPS. The entire group TIN (including the clinicians
that are part of the subgroup) could also submit data as a group in traditional MIPS. We note
section IV.A.3.d. includes a Request for Information (RFI) to obtain feedback on what
guidance/parameters are needed for multispecialty groups to place clinicians into subgroups for
reporting an MVP relevant to the scope of care provided. Absent available submission data on
MVP and subgroup reporting as discussed in section V.B.8.e.(7)(a)(ii), we are unable to estimate
the effect of this established policy on reporting for the CY 2026 performance period/2028 MIPS
payment year.
(h) APM Performance Pathway Plus Quality Measure Set
In section IV.A.4.c.(2). of this proposed rule, we are proposing to establish the APP Plus
quality measure set beginning in the CY 2025 performance period/2027 MIPS payment year. As
described in section V.B.8.a.(4)., clinicians reporting the proposed APP Plus quality measure set
would report via one of the available collection types per measure: eight measures for the CY
2025 performance period/2027 MIPS payment year; nine measures for the CY 2026 and 2027
performance periods/2028 and 2029 MIPS payment years; and eleven measures for the CY 2028
performance period/2030 MIPS payment period. For the available collection types per measure,
please see Tables 55, 56, and 57 in section IV.A.4.c.(3)(f).
In section V.B.8.a.(4). of this proposed rule, we compare the quality performance
category burden for MIPS eligible clinicians who elect to report the proposed APP Plus quality
measure set compared to the current APP quality measure set, traditional MIPS, and MVPs. We
focus these analyses on quality measures required for MIPS eligible clinicians under the eCQM,
CQM/QCDR, and Medicare Part B claims collection types. We note, these assumptions for
actively submitting to assess clinician burden may differ from MIPS scoring policy. In that
comparison, we assume MIPS eligible clinicians incur no burden for reporting the two
administrative claims quality measures currently required under the APP quality measure set.
Additionally, burden estimates for the CAHPS for MIPS registration and patient reporting are
provided in the CAHPS for MIPS PRA package under OMB control number 0938-1222 (CMS-
10450); we do not assume that MIPS eligible clinicians incur additional reporting burden for
reporting the CAHPS for MIPS quality measure under the current APP quality measure set.
Therefore, MIPS eligible, clinicians reporting the proposed APP Plus quality measure set
beginning in the CY 2025 performance period/2027 MIPS payment year would need to actively
submit performance data for more quality measures than clinicians reporting via MVPs or the
APP. Compared to clinicians reporting via traditional MIPS, clinicians reporting the proposed
APP Plus quality measure set would actively submit performance data for fewer quality
measures for the CY 2025 performance period/2027 MIPS payment year; the same number of
quality measures for the CY 2026 and 2027 performance periods/2028 and 2029 MIPS payment
years, and more measures for the CY 2028 performance period/2030 MIPS payment year.
As noted in the CY 2021 PFS final rule, one goal of the APP quality measure set was to
reduce the reduce the burden of reporting quality measures twice; once to MIPS and once to their
APMs; therefore, clinicians reporting the APP and APP Plus who are require by their APMs to
submit the same measure sets incur limited additional burden (88 FR 84862). We assume that all
Shared Savings Program ACOs will report the APP via the APP Plus measure set for the CY
2025 performance period/2027 MIPS payment year. Per section 1899(e) of the Act, submissions
received from eligible clinicians in ACOs are not included in burden estimates for this proposed
rule because quality data submissions to fulfill requirements of the Shared Savings Program are
not subject to the PRA. As the APP Plus is a new and optional quality measure set for non-
Shared Savings Program ACOs with greater reporting burden than the current APP quality
measure set and APM specific requirements may vary, we are unable to estimate how many
individual MIPS eligible clinicians, groups, or APM Entities would submit quality measures via
the APP Plus at this time. Our burden estimates currently assume MIPS eligible clinicians in
non-Shared Savings ACO APM Entities will participate through traditional MIPS or MVPs,
submitting as an individual or group rather than as an APM Entity. We will update these
estimates and assumptions as additional data are available.
i. Assumptions & Limitations
In our MIPS eligible clinician assumptions, we assumed that clinicians who elected to
opt-in for the CY 2022 Quality Payment Program and submitted data will continue to elect to
opt-in for the CY 2025 performance period/2027 MIPS payment year.
As discussed in section V.B.8. of this proposed rule, we are unable to predict which
specific MIPS eligible clinicians would receive reweighting for one or more performance
categories under policies at § 414.1380(c)(2) in the CY 2025 performance period/ 2027 MIPS
payment rear. On this basis, we assume that those MIPS eligible clinicians for whom we
approved reweighting of one or more performance categories under our policies are
representative of the number and attributes of MIPS eligible clinicians who will receive
reweighting under these policies in the future.
In addition to the limitations described throughout the methodology sections, to the
extent that there are year-to-year changes in the data submission, volume, and mix of services
provided by MIPS eligible clinicians, the actual impact on total Medicare revenues will be
different from those shown in Table 137.
F. Alternatives Considered
This proposed rule contains a range of policies, including some proposals related to
specific statutory provisions. The preceding preamble provides descriptions of the statutory
provisions that are addressed, identifies those policies when we exercise agency discretion,
presents rationale for our policies, and, where relevant, alternatives that were considered. For
purposes of the payment impact on PFS services of the policies contained in this proposed rule,
we present above the estimated impact on total allowed charges by specialty.
1. Alternatives Considered Related to Strategies for Improving Global Surgery Payment
Accuracy
As discussed previously and in section II.G. of this proposed rule, beginning for services
furnished in 2025, we are proposing to broaden the applicability of the transfer of care modifiers
for the 90-day global packages. We are proposing to require the use of the appropriate transfer of

care modifier (modifier -54, -55, or -56) for all 90-day global surgical packages in any case when
a practitioner (or another within the same group practice) expects to furnish only a portion of a
global package (including but not limited to when there is a formal, documented transfer of care
as under current policy or an informal, non-documented but expected, transfer of care).
Practitioners billing for a global package procedure code with modifier -54 and other
practitioners in the same group practice as that practitioner would still be able to bill during the
global period for any separately identifiable E/M services they furnish to the patient that are
unrelated to the global package procedure. Additionally, we are proposing a global surgical add-
on code, HCPCS code GPOC1, which we expect will be billed during the postoperative period of
90 days following the procedure. We expect that this code will be billed once during that
timeframe when the patient is seen for an office/outpatient (O/O) evaluation and management
(E/M) visit that is related to the recent surgical procedure. We believe that this code will be
billed by a physician or practitioner who is seeing the patient for a visit during the post-operative
period and did not furnish the surgical procedure.
As we were developing this proposal, we analyzed a few different policy options to best
achieve our goal of improving the payment accuracy of the global packages. We considered
whether to propose to revalue the 10 and 90-day global packages on the PFS utilizing our
findings and data under the MACRA requirement to improve payment accuracy on the fee
schedule, however we are precluded from doing so under MACRA. We also considered
revaluing services specifically included in the RAND study,
830
which looked at claims for which
reporting of follow up visits was requested. We also considered proposing requiring separate
billing, which would result in separate payments for the procedures and post operative visits in
global packages, based on our current research and analysis of how practitioners may be
furnishing care described by global packages. We considered this alternative policy as an initial
830
Using Claims-Based Estimates of Post-Operative Visits to Revalue Procedures with 10- and 90-Day Global
Periods; Updated Results Using Calendar Year 2019 Data
step towards more accurately paying for global packages, specifically for services including high
utilization global packages discussed in the RAND study. We also considered proposing
revisions to all global surgical packages in a phased approach starting with the subset of
packages described above and gradually revising other global packages over time, to manage
payment predictability and stability within the PFS, rec.
2. Alternatives Considered Related to the Supervision of Outpatient Therapy Services in Private
Practices
As discussed in section II.H of this proposed rule, we are proposing to allow for the
general supervision of occupational therapy assistants (OTAs) and physical therapist assistants
(PTAs), by OT’s and PT’s in private practice (OTPPs and PTPPs, respectively) who are enrolled
as suppliers in Medicare. Currently, and since 2005, OTPPs and PTPPs are required to provide
direct supervision of their OTAs and PTAs which requires the OTPP/PTPP to be immediately
available to furnish assistance and direction throughout the performance of the procedure in the
office suite or in the patient’s home when Medicare patients are treated in order to bill for
therapy services furnished by their supervised OTAs and PTAs.
In developing our proposal to allow for general supervision in these private practice
settings, we considered the possibility of allowing for virtual direct supervision by the
OTPP/PTPP instead, as we have included OTPPs/PTPPs as “supervising practitioners” in the
application of our virtual direct supervision policy since October 6, 2021, which is now extended
through CY 2024. Due to the private practice direct supervision regulatory requirement, when
using virtual direct supervision, this means (per our clarification in the CY 2021 PFS final rule
(85 FR 84539)) that the OTPP or PTPP could meet the virtual direct supervision requirement by
being immediately available to engage via audio/video technology (excluding audio-only), and
would not require real-time presence or observation of the service via interactive audio and video
technology throughout the performance of the service.
However, if this alternative policy were selected, it would leave the direct supervision
requirement in place for OTPPs and PTPPs and they’d still have to be immediately available to
engage via audio/video technology and ensure their availability to do so. On the other hand, with
general supervision, the OTPP’s/PTPP’s physical or virtual presence is not required when the
OTA/PTA furnishes services, although the services continue to be furnished under their overall
direction and control allowing the OTPP/PTPP, for example, to provide an evaluative service in
the office while the OTA/PTA is off-site furnishing therapy services in a patient’s home.
3. Alternatives Considered for the Quality Payment Program
For purposes of the payment impact on the Quality Payment Program, we view the
performance threshold as a critical factor affecting the distribution of payment adjustments. In
section IV.A.4.g.(2)(c) of this proposed rule, we proposed to set the performance threshold to 75
points for the CY 2025 MIPS performance period/ CY 2027 MIPS payment year. Eighty-six (86)
is a possible alternative value (mean of the CY 2019 performance period/2021 MIPS payment
year) which we did not propose. To assess this alternative value, we ran a separate RIA model
with a performance threshold of 86. This model has the same mean and median final score as our
proposed policies RIA model since the alternative performance threshold which we are assessing
in this model does not change the final score. In our analysis of the alternative performance
threshold of 86, which we considered but did not propose, 55.98 percent of MIPS eligible
clinicians who submitted data would receive a negative payment adjustment in the baseline and
45.08 percent of MIPS eligible clinicians who submit data would receive a negative adjustment
in the proposed policies model.
We also reported the findings for the baseline RIA model which describes the impact for
the CY 2025 performance period/2027 MIPS payment year if this proposal is not finalized. The
baseline RIA model has a median final score of 82.20. We estimated that $517 million would be
redistributed based on the budget neutrality requirement in the baseline model. The baseline

includes a maximum payment adjustment of 2.98 percent and 22.84 percent of MIPS eligible
clinicians would receive a negative payment adjustment.
G. Impact on Beneficiaries
1. Medicare Shared Savings Program Provisions
As noted previously in this proposed rule, the proposed HEBA would mainly provide
upwards adjustments to benchmarks for – and likely draw increased participation from – new
ACOs with particular focus on coordinating care for beneficiaries in underserved communities.
New ACOs of this type are therefore projected to ultimately increase assignment to the Shared
Savings Program by roughly 500,000 beneficiaries per year, ranging from 50,000 to 1.0 million
at the low and high ends of this projection range.
ACOs have been found to perform better on certain patient-experience and performance
measures than physician groups participating in the MIPS. In performance year 2022, ACOs had
a higher average performance on quality measures they are required to report in order to share in
savings compared to other similarly sized clinician groups not in the Shared Savings Program.
831
This includes statistically significant higher performance for quality measures related to diabetes
and blood pressure control; breast cancer and colorectal cancer screening; tobacco screening and
smoking cessation; and depression screening and follow-up.
832
We anticipate that ACOs will
continue to improve the quality of care for their Medicare fee-for-service beneficiaries through
the reporting of Medicare CQMs, and that ACOs will continue to improve the quality of care for
their all payer/all patient population through the reporting of eCQMs.
Increased participation in the Shared Savings Program will extend ACO care
coordination and quality improvement to segments of the beneficiary population that potentially
have more to benefit from care management.
831
CMS, Press Release, “Medicare Shared Savings Program Saves Medicare More Than $1.8 Billion in 2022 and
Continues to Deliver High-quality Care” (August 24, 2023). Available at https://www.cms.gov/newsroom/press-
releases/medicare-shared-savings-program-saves-medicare-more-18-billion-2022-and-continues-deliver-high.
832
Id.
2. Quality Payment Program
There are several changes in this proposed rule that are expected to have a positive effect
on beneficiaries. In general, we believe that many of these changes, including the MVP and
subgroup provisions, if finalized, will lead to meaningful feedback to beneficiaries on the type
and scope of care provided by clinicians. Additionally, beneficiaries could use the publicly
reported information on clinician performance in subgroups to identify and choose clinicians in
multispecialty groups relevant to their care needs. Consequently, we anticipate the policies in
this proposed rule would improve the quality and value of care provided to Medicare
beneficiaries. For example, several of the new quality measures include patient-reported
outcome-based measures, which could be used to help patients make more informed decisions
about treatment options. Patient-reported outcome-based measures provide information on a
patient’s health status from the patient’s point of view and could also provide valuable insights
on factors such as quality of life, functional status, and overall disease experience, which would
not otherwise be available through routine clinical data collection. Patient-reported outcome-
based measured are factors frequently of interest to patients when making decisions about
treatment.
H. Estimating Regulatory Familiarization Costs
If regulations impose administrative costs on private entities, such as the time needed to
read and interpret this rulemaking, we should estimate the cost associated with regulatory
review. Due to the uncertainty involved with accurately quantifying the number of entities that
will review the rule, we assumed that the total number of unique commenters on this year’s
proposed rule will be the number of reviewers of last year’s proposed rule. We acknowledged
that this assumption may understate or overstate the costs of reviewing this rulemaking. It is
possible that not all commenters will review this year’s proposed rule in detail, and it is also
possible that some reviewers will choose not to comment on the proposed rule. For these
reasons we believe that the number of commenters will be a fair estimate of the number of
reviewers of this year’s proposed rule.
We also recognized that different types of entities are in many cases affected by mutually
exclusive sections of this proposed rule, and therefore for the purposes of our estimate we
assume that each reviewer reads approximately 50 percent of the rulemaking.
Using the wage information from the BLS for medical and health service managers
(Code 11-9111), we estimate that the cost of reviewing this rulemaking is $123.06, including
overhead and fringe benefits https://www.bls.gov/oes/current/oes_nat.htm. Assuming an average
reading speed, we estimate that it would take approximately 8.0 hours for the staff to review half
of this proposed rule. For each facility that reviews the rule, the estimated cost is $984.48 (8.0
hours x $123.06). Therefore, we estimated that the total cost of reviewing this regulation is
21,677,265 ($984.48 x 22,019 reviewers on this year’s proposed rule).
As for the Medicare Diabetes Prevention Program, given that we tried to align this
rulemaking as much as possible with the CDC DPRP Standards, there should be minimal
regulatory familiarization costs. This rulemaking impacts only enrolled MDPP suppliers and
eligible beneficiaries who have started the MDPP program or are interested in enrolling in
MDPP.
I. Accounting Statement
As required by OMB Circular A-4 (available at https://www.whitehouse.gov/wp-
content/uploads/legacy_drupal_files/omb/circulars/A4/a-4.pdf), in Tables 146 through 148
(Accounting Statements), we have prepared an accounting statement. This estimate includes
growth in incurred benefits from CY 2024 to CY 2025 based on the FY 2025 President’s Budget
baseline.

TABLE 146: Accounting Statement: Classification of Estimated Expenditures
CATEGORY
TRANSFERS
CY 2025 Annualized Monetized Transfers
Estimated decrease in expenditures of $1.8 billion for
PFS CF update.
From Whom To Whom?
Federal Government to physicians, other practitioners
and providers and suppliers who receive payment
under Medicare.
TABLE 147: Accounting Statement: Classification of Estimated Costs, Transfer, and
Savings
CATEGORY
TRANSFER
CY 2025 Annualized Monetized Transfers of
beneficiary cost coinsurance.
-$0.4 billion
From Whom to Whom?
Beneficiaries to Federal Government.
TABLE 148: Accounting Statement for Provisions for Medicare Shared Savings Program
(CYs 2025-2034) ($ Millions)
Category
Primary
Estimate
Minimum
Estimate
Maximum
Estimate
Source Citation
BENEFITS
Annualized monetized:
Discount rate: 2%
-$12.6 million
-$148.9 million
$92.6 million
Tables 132 through
134; summarized in
total in Table 135
Notes: Negative values reflect reduction in Federal net cost resulting from care management by ACOs. Estimates
may be a combination of benefits and transfers. To the extent that the incentives created by Medicare payments
change the amount of resources society uses in providing medical care, the more accurate categorization of effects
would be as costs (positive values) or benefits/cost savings (negative values), rather than as transfers.
J. Conclusion
The analysis in the previous sections, together with the remainder of this preamble,
provided an initial Regulatory Flexibility Analysis. The previous analysis, together with the
preceding portion of this preamble, provides an RIA. In accordance with the provisions of
Executive Order 12866, this regulation was reviewed by the Office of Management and Budget.
Chiquita Brooks-LaSure, Administrator of the Centers for Medicare & Medicaid
Services, approved this document on June 25, 2024.

List of Subjects
42 CFR Part 401
Claims, Freedom of information, Health facilities, Medicare, Privacy.
42 CFR Part 405
Administrative practice and procedure, Diseases, Health facilities, Health professions,
Medical devices, Medicare, Reporting and recordkeeping requirements, Rural areas, and X-rays.
42 CFR Part 410
Diseases, Health facilities, Health professions, Laboratories, Medicare, Reporting and
recordkeeping requirements, Rural areas, X-rays.
42 CFR Part 411
Diseases, Medicare, Reporting and recordkeeping requirements.
42 CFR Part 414
Administrative practice and procedure, Biologics, Diseases, Drugs, Health facilities,
Health professions, Medicare, Reporting and recordkeeping requirements.
42 CFR Part 423
Administrative practice and procedure, Emergency medical services, Health facilities,
Health maintenance organizations (HMO), Health professionals, Medicare, Penalties, Privacy,
Reporting and recordkeeping requirements.
42 CFR Part 424
Emergency medical services, Health facilities, Health professions, Medicare, Reporting
and recordkeeping requirements.
42 CFR Part 425
Administrative practice and procedure, Health facilities, Health professions, Medicare,
Reporting and recordkeeping requirements.
42 CFR Part 427

Administrative practice and procedure, Biologics, Inflation rebates, Medicare,
Prescription drugs.
42 CFR Part 428
Administrative practice and procedure, Biologics, Inflation rebates, Medicare,
Prescription drugs.
42 CFR Part 491
Grant programs-health, Health facilities, Medicaid, Medicare, Reporting and
recordkeeping requirements, Rural areas.

For the reasons set forth in the preamble, the Centers for Medicare & Medicaid Services
proposes to amend 42 CFR chapter IV as set forth below:
PART 401—GENERAL ADMINISTRATIVE REQUIREMENTS
1. The authority citation for part 401 is revised to read as follows:
Authority: 42 U.S.C. 1302, 1395hh, 1395w–5, and 1395kk-2.
2. Section 401.305 is amended by revising paragraphs (b)(1) introductory text and (b)(2)
introductory text and adding paragraph (b)(3) to read as follows:
§ 401.305 Requirements for reporting and returning of overpayments.
* * * * *
(b) * * *
(1) Except as provided in paragraphs (b)(2) and (3) of this section, a person who has
received an overpayment must report and return the overpayment by the later of either of the
following:
* * * * *
(2) The deadline for returning overpayments will be suspended (or will continue to be
suspended following the completion of a timely, good faith investigation in accordance with
paragraph (b)(3) of this section) when any of the following occurs:
* * * * *
(3)(i) The deadline for reporting and returning overpayments will be suspended when
both of the following occurs:
(A) A person has identified an overpayment but has not yet completed a good-faith
investigation to determine the existence of related overpayments that may arise from the same or
similar cause or reason as the initially identified overpayment; and
(B) The person conducts a timely, good-faith investigation to determine whether related
overpayments exist.
(ii) If the conditions of paragraph (b)(3)(i) of this section are satisfied, the deadline for

reporting and returning the initially identified overpayment and related overpayments that arise
from the same or similar cause or reason as the initially identified overpayment will remain
suspended until the earlier of:
(A) The date that the investigation of related overpayments has concluded and the
aggregate amount of the initially identified overpayments and related overpayments is
calculated; or
(B) The date that is 180 days after the date on which the initial identified overpayment
was identified.
* * * * *
PART 405-FEDERAL HEALTH INSURANCE FOR THE AGED AND DISABLED
3. The authority citation for part 405 continues to read as follows:
Authority: 42 U.S.C. 263a, 405(a), 1302, 1320b-12, 1395x, 1395y(a), 1395ff, 1395hh,
1395kk, 1395rr, and 1395ww(k).
4. Section 405.2410 is amended by revising paragraphs (c)(1) and (2) to read as follows:
§ 405.2410 Application of Part B deductible and coinsurance.
* * * * *
(c) * * *
(1) For RHCs, the coinsurance amount is determined as described in paragraph (b)(1) of
this section; or
(2) For FQHCs, the coinsurance amount is 20 percent of the lesser of—
(i) The FQHC's actual charge; or
(ii) The payment determined under § 405.2462(j)(2).
5. Section 405.2462 is amended by—
a. In paragraphs (f) heading, (f)(1) introductory text, (f)(2), and (g)(1)(ii), removing
“grandfathered” and adding in its place “historically excepted”; and
b. Revising and republishing paragraph (j).

The revisions and republications read as follows:
§ 405.2462 Payment for RHC and FQHC services.
* * * * *
(j) Payment amount for intensive outpatient services. (1) An RHC is paid the payment
rate determined under § 419.21(a) of this chapter for services described under § 410.44 of this
chapter. There are no adjustments to this rate.
(i) If the deductible has been fully met by the beneficiary prior to the RHC service,
Medicare pays eighty (80) percent of the payment amount determined under this paragraph
(j)(1).
(ii) If the deductible has not been fully met by the beneficiary prior to the RHC service,
Medicare pays eighty (80) percent of the difference between the remaining deductible and the
payment amount determined under this paragraph (j)(1); or
(iii) If the deductible has not been fully met by the beneficiary prior to the RHC service,
no payment is made to the RHC if the deductible is equal to or exceeds the payment amount
determined under this paragraph (j)(1).
(2) FQHCs are paid the payment rate determined under § 419.21(a) of this chapter for
services described under § 410.44 of this chapter. There are no adjustments to this rate, except
that historically excepted tribal FQHCs are paid pursuant to paragraph (j)(2)(ii) of this section.
(i) Medicare pays eighty (80) percent of the lesser of the FQHC's actual charge or the
payment rate determined under paragraph (j)(1)(ii) of this section; or
(ii) Medicare pays eighty (80) percent of the lesser of a historically excepted tribal
FQHC's actual charge or the amount described under paragraphs (f)(2) and (3) of this section.
(iii) No deductible is applicable to FQHC services.
6. Section 405.2463 is amended by—
a. Revising paragraph (b)(3) introductory text;

b. In paragraph (c)(4) introductory text, removing “grandfathered” and adding in its
place “historically excepted”.
The revision reads as follows:
§ 405.2463 What constitutes a visit.
* * * * *
(b) * * *
(3) Visit-Mental health. A mental health visit is a face-to-face encounter or an encounter
furnished using interactive, real-time, audio and video telecommunications technology or audio-
only interactions in cases where the patient is not capable of, or does not consent to, the use of
video technology for the purposes of diagnosis, evaluation or treatment of a mental health
disorder, including an in-person mental health service, beginning January 1, 2026, furnished
within 6 months prior to the furnishing of the telecommunications service and that an in-person
mental health service (without the use of telecommunications technology) must be provided at
least every 12 months while the beneficiary is receiving services furnished via
telecommunications technology for diagnosis, evaluation, or treatment of mental health
disorders, unless, for a particular 12-month period, the physician or practitioner and patient agree
that the risks and burdens outweigh the benefits associated with furnishing the in-person item or
service, and the practitioner documents the reasons for this decision in the patient's medical
record, between an RHC or FQHC patient and one of the following:
* * * * *
7. Section 405.2464 is amended by revising paragraphs (c) and (d) and adding paragraphs
(g) and (h) to read as follows:
§ 405.2464 Payment rate.
* * * * *
(c) Payment for care coordination services. RHCs and FQHCs are paid for the non-face-
to-face care management work involved in coordinating care.

(1) For Chronic Care Management (CCM) services furnished between January 1, 2016,
and December 31, 2017, payment to RHCs and FQHCs is based on the physician fee schedule
national non-facility payment rate.
(2) For psychiatric collaborative care model (CoCM) services furnished on or after
January 1, 2018, payment is based on the average of the national non-facility PFS payment rate
set for each psychiatric CoCM service and updated annually based on the PFS amounts.
(3) For CCM and general Behavioral Health Integration (BHI) services furnished
between January 1, 2018, and December 31, 2020, payment is based on the average of the
national non-facility PFS payment rate set for each CCM and general BHI service and updated
annually based on the PFS amounts.
(4) For CCM, general BHI, and Principal Care Management (PCM) services furnished
between January 1, 2021, and December 31, 2022, payment is based on the average of the
national non-facility PFS payment rate set for each CCM, general BHI, and PCM service and
updated annually based on the PFS amounts.
(5) For CCM, general BHI, PCM, Chronic Pain Management (CPM) services furnished
between January 1, 2023, and December 31, 2023, payment is based on the average of the
national non-facility PFS payment rate set for each CCM, general BHI, PCM and CPM service
and updated annually based on the PFS amounts.
(6) For CCM, general BHI, PCM, CPM, Remote Physiologic Monitoring (RPM),
Remote Therapeutic Monitoring (RTM), Community Health Integration (CHI), Principal Illness
Navigation (PIN), and PIN - Peer Support services furnished between January 1, 2024, and
December 31, 2024, the payment amount is based on a weighted average of each CCM, general
BHI, PCM, CPM, RPM, RTM, CHI, PIN, and PIN - Peer Support service using the most
recently available PFS utilization data.
(7) For CCM, general BHI, PCM, CPM, RPM, RTM, CHI, PIN, PIN - Peer Support, and
Advance Primary Care Management services furnished on or after January 1, 2025, payment is

based on the PFS national non-facility payment rate.
(d) Payment for FQHCs that are authorized to bill as historically excepted tribal FQHCs.
Historically excepted tribal FQHCs are paid at the outpatient per visit rate for Medicare as set
annually by the Indian Health Service for each beneficiary visit for covered services. There are
no adjustments to this rate.
* * * * *
(g) Payment for non-behavioral health telecommunication technology services. For an
encounter furnished using interactive, real-time, audio and video telecommunications technology
or for certain audio-only interactions in cases where the patient is not capable of, or does not
consent to, the use of video technology services that are not described in § 405.2463(b)(3),
payment to RHCs and FQHCs are subject to the national average payment rates for comparable
services under the physician fee schedule (PFS) and costs associated with these services shall not
be used in determining payments under the RHC all-inclusive rate or the FQHC prospective
payment system.
(h) Payment for drugs covered as additional preventive services (DCAPS). For drugs
covered as additional preventive services, as defined at § 410.64 of this subchapter, and for the
administration and supplying fees for those drugs, payment to RHCs or FQHCs is 100 percent of
the Medicare payment amount per § 405.2410(b) and § 410.152(l)(11) of this chapter, subject to
the payment limitations described at § 410.152(o) of this chapter.
8. Section 405.2466 is amended by revising paragraph (b)(1)(iv) to read as follows:
§ 405.2466 Annual reconciliation.
* * * * *
(b) * * *
(1) * * *
(iv) For RHCs and FQHCs, payment for pneumococcal, influenza, hepatitis B and
COVID-19 vaccine and their administration is 100 percent of Medicare reasonable cost.

* * * * *
§ 405.2469 [Amended]
9. Section 405.2469 is amended in paragraph (a)(2) by removing “grandfathered” and
adding in its place “historically excepted”.
PART 410—SUPPLEMENTARY MEDICAL INSURANCE (SMI) BENEFITS
10. The authority citation for part 410 continues to read as follows:
Authority: 42 U.S.C. 1302, 1395m, 1395hh, 1395rr, and 1395ddd.
11. Section 410.26 is amended by revising paragraph (a)(2) to read as follows:
§ 410.26 Services and supplies incident to a physician's professional services: Conditions.
(a) * * *
(2) Direct supervision means, except as provided in paragraphs (a)(2)(i) and (ii) of this
section, the level of supervision by the physician (or other practitioner) of auxiliary personnel as
defined in § 410.32(b)(3)(ii). For the following services furnished after December 31, 2025, the
presence of the physician (or other practitioner) required for direct supervision shall include
virtual presence through audio/video real-time communications technology (excluding audio-
only):
(i) Services furnished incident to a physician of other practitioner’s service provided by
auxiliary personnel employed by the billing practitioner and working under their direct
supervision and for which the underlying Healthcare Common Procedure Coding System
(HCPCS) code has been assigned a PC/TC indicator of ‘5’.
(ii) Office or other outpatient visits for the evaluation and management of an established
patient that may not require the presence of a physician or other qualified health care
practitioner.
* * * * *
12. Section 410.30 is amended by revising paragraph (a) to read as follows:
§ 410.30 Prescription drugs used in immunosuppressive therapy.
(a) Scope. Payment may be made for prescription drugs used in immunosuppressive
therapy that meet one of the following conditions:
(1) The drug has been approved for marketing by the FDA and —
(i) The approved labeling includes an indication for preventing or treating the rejection of
a transplanted organ or tissue; or
(ii) The approved labeling includes the indication for use in conjunction with
immunosuppressive drugs to prevent or treat rejection of a transplanted organ or tissue.
(2) The drug has been approved for marketing by FDA and determined by a Medicare
Administrative Contractor (MAC) (in accordance with part 421, subpart C, of this chapter), in
processing a Medicare claim, to be reasonable and necessary for the specific purpose of
preventing or treating the rejection of a patient's transplanted organ or tissue, or for use in
conjunction with immunosuppressive drugs for the purpose of preventing or treating the rejection
of a patient's transplanted organ or tissue. (In making these determinations, the MACs may
consider factors such as authoritative drug compendia, current medical literature, recognized
standards of medical practice, and professional medical publications.)
(3) The drug is a compounded formulation with active ingredients derived only from a
drug described in paragraph (a)(1) or (2) of this section and is orally or enterally administered.
* * * * *
13. Section 410.32 is amended by revising paragraph (b)(3)(ii) to read as follows:
§ 410.32 Diagnostic x-ray tests, diagnostic laboratory tests, and other diagnostic tests:
Conditions.
* * * * *
(b) * * *
(3) * * *
(ii) Direct supervision in the office setting means that the physician (or other supervising
practitioner) must be present in the office suite and immediately available to furnish assistance
and direction throughout the performance of the service. It does not mean that the physician (or
other supervising practitioner) must be present in the room when the service is performed.
Through December 31, 2025, the presence of the physician (or other practitioner) includes
virtual presence through audio/video real-time communications technology (excluding audio-
only).
* * * * *
14. Section 410.37 is amended by--
a. In paragraph (a)(1)(iv), removing the text “barium enemas” and adding in its place
“computed tomography colonography”;
b. Revising paragraph (a)(4);
c. In paragraph (e)(2), removing the text “barium enema” and adding in its place
“computed tomography colonography”;
d. In paragraph (g)(2), removing the text “barium enema” and adding in its place
“computed tomography colonography”;
e. Revising paragraphs (h), (i), and (k).
The revisions read as follows:
§ 410.37 Colorectal cancer screening tests: Conditions for and limitations on coverage.
* * * * *
(a) * * *
(4) Screening computed tomography colonography means a test that uses X-rays and
computers to produce images of the entire colon (including image processing and a physician’s
interpretation of the results of the procedure).
* * * * *
(h) Conditions for coverage of screening computed tomography colonography. Medicare
Part B pays for a screening computed tomography colonography if it is ordered in writing by the
beneficiary's attending physician who is a doctor of medicine or osteopathy (as defined in section
1861(r)(1) of the Act); or by a physician assistant, nurse practitioner, or clinical nurse specialist
(as defined in section 1861(aa)(5) of the Act).
(i) Limitations on coverage of screening computed tomography colonography. (1) In the
case of an individual age 45 or over who is not at high risk of colorectal cancer, payment may be
made for a screening computed tomography colonography performed after at least 59 months
have passed following the month in which the last screening computed tomography
colonography or 47 months have passed following the month in which the last screening flexible
sigmoidoscopy or screening colonoscopy was performed.
(2) In the case of an individual who is at high risk for colorectal cancer, payment may be
made for a screening computed tomography colonography performed after at least 23 months
have passed following the month in which the last screening computed tomography
colonography or the last screening colonoscopy was performed.
* * * * *
(k) A complete colorectal cancer screening. Effective January 1, 2025, colorectal cancer
screening tests include a follow-on screening colonoscopy after a Medicare covered non-invasive
stool-based colorectal cancer screening test or blood-based biomarker colorectal cancer
screening test returns a positive result. The instance of the follow-on screening colonoscopy in
the context of a complete colorectal cancer screening must not apply to the frequency limitations
for colorectal cancer screening.
15. Section 410.59 is amended by revising paragraphs (a)(3)(ii) and (c)(2) to read as
follows:
§ 410.59 Outpatient occupational therapy services: Conditions.
(a) * * *
(3) * * *
(ii) By, or under the general supervision (or as specified otherwise) of, an occupational
therapist in private practice as described in paragraph (c) of this section; or
* * * * *
(c) * * *
(2) Supervision of occupational therapy services. Except as otherwise provided in this
paragraph (c)(2), occupational therapy services are performed by, or under the general
supervision of, an occupational therapist in private practice. All services not performed
personally by the therapist must be performed by employees of the practice, generally supervised
by the therapist, and included in the fee for the therapist's services. Occupational therapy
services may be performed by an occupational therapy assistant under the general supervision of
the occupational therapist in private practice; services performed by an unenrolled occupational
therapist must be under the direct supervision of the occupational therapist.
* * * * *
16. Section 410.60 is amended by revising paragraphs (a)(3)(ii) and (c)(2) to read as
follows:
§ 410.60 Outpatient physical therapy services: Conditions.
(a) * * *
(3) * * *
(ii) By, or under the general supervision (or as specified otherwise) of, a physical
therapist in private practice as described in paragraph (c) of this section; or
* * * * *
(c) * * *
(2) Supervision of physical therapy services. Except as otherwise provided in this
paragraph (c)(2), physical therapy services are performed by, or under the general supervision of,
a physical therapist in private practice. All services not performed personally by the therapist
must be performed by employees of the practice, generally supervised by the therapist, and
included in the fee for the therapist's services. Physical therapy services may be performed by a
physical therapist assistant under the general supervision of the physical therapist in private
practice; services performed by an unenrolled physical therapist must be under the direct
supervision of the physical therapist.
* * * * *
17. Section 410.63 is amended by--
a. Revising paragraph (a) introductory text;
b. Removing the word “and” at the end of paragraph (a)(2)(ii);
c. Removing the period at the end of paragraph (a)(2)(iii) and adding in its place “; and”;
d. Adding paragraph (a)(2)(iv); and
e. Revising paragraphs (b) and (c)(1).
The revisions and addition read as follows:
§ 410.63 Hepatitis B vaccine and blood clotting factors: Conditions.
* * * * *
(a) Hepatitis B vaccine: Conditions. Effective January 1, 2025, hepatitis B vaccinations
are reasonable and necessary for the prevention of illness for those individuals who are at high or
intermediate risk of contracting hepatitis B as listed in paragraphs (a)(1) through (3) of this
section:
* * * * *
(2) * * *
(iv) Individuals who have not previously received a completed hepatitis B vaccination
series or whose previous vaccination history is unknown.
* * * * *
(b) Blood clotting factors: Conditions. Effective July 18, 1984, blood clotting factors that
are self-administered and control bleeding for hemophilia patients competent to use these factors
without medical or other supervision, and items related to the administration of those factors.
The amount of clotting factors covered under this provision is determined by the carrier based on
the historical utilization pattern or profile developed by the carrier for each patient, and based on
consideration of the need for a reasonable reserve supply to be kept in the home in the event of
emergency or unforeseen circumstance.
(c) * * *
(1) Effective January 1, 2005, a furnishing fee of $0.14 per unit of clotting factor is paid
to entities that furnish blood clotting factors, as described in paragraph (b) of this section, unless
the costs associated with furnishing the clotting factor are paid through another payment system,
for example, hospitals that furnish clotting factor to patients during a Part A covered inpatient
hospital stay, or practitioners that furnish clotting factor to patients in an outpatient setting and
are paid for under the Physician Fee Schedule.
* * * * *
18. Section 410.67 is amended in paragraph (b), in the definition of “Opioid use disorder
treatment service,” by revising paragraphs (vi) and (vii) to read as follows:
§ 410.67 Medicare coverage and payment of Opioid use disorder treatment services
furnished by Opioid treatment programs.
* * * * *
(b) * * *
Opioid use disorder treatment service * * *
(vi) Intake activities, including initial medical examination services required under §
8.12(f)(2) of this title and initial assessment services required under § 8.12(f)(4) of this title.
(A) For intake activities furnished via communications technology, the following
flexibilities apply:
(1) Services to initiate treatment with buprenorphine may be furnished via two-way
interactive audio-video communication technology, as clinically appropriate, and in compliance
with all applicable requirements. In cases where audio-video communications technology is not
available to the beneficiary, services to initiate treatment with buprenorphine may be furnished
using audio-only telephone calls if all other applicable requirements are met.
(2) Services to initiate treatment with methadone may be furnished via two-way
interactive audio-video communication technology, as clinically appropriate, and in compliance
with all applicable requirements, if an OTP determines that an adequate evaluation of the patient
can be accomplished through audio-video communication technology.
(B) [Reserved]
(vii) Periodic assessment services required under § 8.12(f)(4) of this title, that are
furnished during a face-to-face encounter, including services furnished via two-way interactive
audio-video communication technology, as clinically appropriate, and in compliance with all
applicable requirements. In cases where a beneficiary does not have access to two-way audio-
video communications technology, periodic assessments can be furnished using audio-only
telephone calls if all other applicable requirements are met.
* * * * *
19. Section 410.78 is amended by revising paragraph (a)(3) read as follows:
§ 410.78 Telehealth services.
(a) * * *
(3) Interactive telecommunications system means, except as otherwise provided in this
paragraph (a)(3), multimedia communications equipment that includes, at a minimum, audio and
video equipment permitting two-way, real-time interactive communication between the patient
and distant site physician or practitioner. Interactive telecommunications system may also
include two-way, real-time audio-only communication technology for any telehealth service
furnished to a patient in their home if the distant site physician or practitioner is technically
capable of using an interactive telecommunications system as defined in the previous sentence,
but the patient is not capable of, or does not consent to, the use of video technology. The
following modifiers must be appended to a claim for telehealth services furnished using two-
way, real-time audio-only communication technology to verify that the conditions set forth in the
prior sentence have been met:
(i) Current Procedural Terminology (CPT) modifier “93”; and
(ii) For rural health clinics (RHCs) and federally qualified health centers (FQHCs),
Medicare modifier “FQ”.
* * * * *
20. Section 410.79 is amended by—
a. In paragraph (b):
i. Removing the definition of “Combination delivery”;
ii. Adding the definitions of “Combination with an online component,” “In-person with a
distance learning component,” and “Online” in alphabetical order;
iii. Removing the definition of “Online delivery”; and
iv. Revising the definition of “Set of MDPP services”; and
b. Revising paragraphs (d)(1) introductory text, (e)(3)(iii)(C), (e)(3)(iv)(F)(3), and
(e)(3)(v)(F)(2).
The additions and revisions read as follows:
§ 410.79 Medicare Diabetes Prevention Program expanded model: Conditions of coverage.
* * * * *
(b) * * *
Combination with an online component refers to sessions that are delivered as a
combination of online (non-live) with in-person or distance learning.
* * * * *
In-person with a distance learning component refers to DPP sessions that are delivered in
person by trained Coaches where participants have the option of attending sessions via MDPP
distance learning.
* * * * *
Online means sessions that are delivered 100 percent through the internet via phone,
tablet, or laptop in an asynchronous (non-live) classroom where participants are experiencing the
content on their own time without a live (including non-artificial intelligence (AI)) Coach
teaching the content. These sessions must be furnished in a manner consistent with the DPRP
Standards for online sessions. Live Coach interaction must be offered to each participant during
weeks when the participant has engaged with content. E-mails and text messages can count
toward the requirement for live Coach interaction if there is bi-directional communication
between the Coach and participant. Chat bots and AI forums do not count as live Coach
interaction.
* * * * *
Set of MDPP services means the series of MDPP sessions, composed of core sessions and
core maintenance sessions, and subject to paragraph (c)(3) of this section offered over the course
of the MDPP services period.
* * * * *
(d) * * *
(1) An MDPP supplier may offer a make-up session to an MDPP beneficiary who missed
a regularly scheduled session. MDPP make-up sessions may only use in-person or distance
learning delivery. If an MDPP supplier offers one or more make-up sessions to an MDPP
beneficiary, each such session must be furnished in accordance with the following requirements:
* * * * *
(e) * * *
(3) * * *
(iii) * * *
(C) Self-reported weight measurements from the at-home digital scale of the MDPP
beneficiary. Self-reported weights must be obtained during live, synchronous online video
technology, such as video chatting or video conferencing, wherein the MDPP Coach observes
the beneficiary weighing themselves and views the weight indicated on the at-home digital scale,
or the MDPP supplier receives 2 (two) date-stamped photos or a video recording of the
beneficiary's weight, with the beneficiary visible on the scale, submitted by the MDPP
beneficiary to the MDPP supplier. Photo or video must clearly document the weight of the
MDPP beneficiary as it appears on their digital scale on the date associated with the billable
MDPP session. If choosing to submit 2 photos, one photo must show the beneficiary’s weight on
the digital scale, the second photo must show the beneficiary visible in their home, and both
photos must be date-stamped.
* * * * *
(iv) * * *
(F) * * *
(3) No more than 12 virtual sessions offered monthly during the ongoing maintenance
session intervals, months 13 through 24 for beneficiaries enrolled before January 1, 2022.
* * * * *
(v) * * *
(F) * * *
(2) For an MDPP beneficiary who began receiving the Set of MDPP services on or after
January 1, 2021, has suspended services during an applicable 1135 waiver event, the MDPP
supplier must use the baseline weight recorded at the beneficiary's first core session.
* * * * *
21. Section 410.152 is amended by adding paragraph (o) to read as follows:
§ 410.152 Amounts of payment.
* * * * *
(o) Amount of payment: Drugs covered as additional preventive services (DCAPS). For a
drug covered as an additional preventive service, as defined at § 410.64, payment must be made
as follows:
(1) Payment for a drug covered as an additional preventive service, per section
1861(a)(1)(W)(ii) of the Act and paragraphs (l)(11) of this section and § 410.160(b)(13), is 100
percent of the lesser of —
(i) The actual charge on the claim for program benefits; or
(ii) The amount determined under the fee schedule as described in paragraph (o)(3) of
this section.
(2) Payment for the supplying or administration of a drug covered as an additional
preventive service per section 1861(a)(1)(W)(ii) of the Act and paragraphs (l)(11) of this section
and § 410.160(b)(13), is 100 percent of the lesser of —
(i) The actual charge on the claim for program benefits; or
(ii) The amount determined under the fee schedule as described in paragraph (o)(4) of
this section.
(3) The payment limit for a drug covered as an additional preventive service, as defined
at § 410.64, appears on the DCAPS fee schedule and is determined as follows:
(i) If Average Sales Price (ASP) data is available for the drug, consistent with part 414,
subpart J, of this chapter, then the payment limit is determined using the methodology set forth in
section 1847A of the Act and according to the provisions in part 414, subpart K, of this chapter.
(ii) If ASP data is not available, then the payment limit is determined according to an
average of the most recently published National Average Drug Acquisition Cost (NADAC)
prices for the drug.
(iii) If ASP data and NADAC prices are not available, then the payment limit is
determined according to an average of the most recently published pharmaceutical pricing data
for the drug as included in the Federal Supply Schedule (FSS), as managed by the Department of
Veterans Affairs per 48 CFR part 38.
(iv) If ASP data, NADAC prices, and FSS pharmaceutical prices are not available, then
the payment limit is the invoice price determined by the MAC.
(4) The payment limits for supplying and administering a drug covered as an additional
preventive service, as defined at § 410.64, appear on the DCAPS fee schedule and are
determined as follows:
(i) For a drug that is supplied by a pharmacy, the payment limit for a supplying fee is as
follows:
(A) For the first prescription that the pharmacy provides to a beneficiary in a 30-day
period for a drug covered as an additional preventive service, $24.
(B) For all subsequent prescriptions that the pharmacy provides to a beneficiary in a 30-
day period for a drug covered as an additional preventive service, $16.
(ii) For a drug that is administered by a physician or a non-physician practitioner, the
payment limit for administration is set in accordance with part 414, subpart B, of this chapter.
This fee is not subject to the Part B deductible, per § 410.160(b)(13). This fee is equal to 100
percent of the Medicare payment amount established under the applicable payment methodology,
per paragraph (l)(11) of this section.
PART 411 - EXCLUSIONS FROM MEDICARE AND LIMITATIONS ON MEDICARE
PAYMENT
22. The authority citation for part 411 continues to read as follows:
Authority: 42 U.S.C. 1302, 1395w-101 through 1395w-152, 1395hh, and 1395nn.
23. Section 411.15 is amended by revising paragraph (i)(3)(i)(A) to read as follows:
§ 411.15 Particular services excluded from coverage.
* * * * *
(i) * * *
(3) * * *
(i) * * *
(A) Dental or oral examination performed as part of a comprehensive workup prior to,
and medically necessary diagnostic and treatment services to eliminate an oral or dental infection
prior to, or contemporaneously with, the following Medicare-covered services: organ transplant,
hematopoietic stem cell transplant, bone marrow transplant, cardiac valve replacement,
valvuloplasty procedures, chemotherapy when used in the treatment of cancer, chimeric antigen
receptor (CAR) T-cell therapy when used in the treatment of cancer, administration of high-dose
bone-modifying agents (antiresorptive therapy) when used in the treatment of cancer, and
dialysis services in the treatment of end stage renal disease.
* * * * *
PART 414--PAYMENT FOR PART B MEDICAL AND OTHER HEALTH SERVICES
24. The authority citation for part 414 continues to read as follows:
Authority: 42 U.S.C. 1302, 1395hh, and 1395rr(b)(l).
25. Section 414.84 is amended by—
a. In paragraph (a), removing the definition of “Bridge payment”;
b. Revising paragraphs (b)(1) introductory text and (b)(2) introductory text;
c. Adding paragraph (c)(4);
d. Removing paragraph (d);
e. Redesignating paragraph (e) as paragraph (d); and
f. Revising newly redesignated paragraph (d).
The revisions and addition read as follows:
§ 414.84 Payment for MDPP services.
* * * * *
(b) * * *
(1) Performance Goal 1: Achieves the required minimum 5-percent weight loss. CMS
makes a performance payment to an MDPP supplier for an MDPP beneficiary who achieves the
required minimum weight loss as measured in-person or during a distance learning session
during a core session or core maintenance session furnished by that supplier. The amount of this
performance payment is determined as follows:

* * * * *
(2) Performance Goal 2: Achieves 9-percent weight loss. CMS makes a performance
payment to an MDPP supplier for an MDPP beneficiary who achieves at least a 9-percent weight
loss as measured in-person or in a distance learning session during a core session or core
maintenance session furnished by that supplier. The amount of this performance payment is
determined as follows:
* * * * *
(c) * * *
(4) Current Procedural Terminology (CPT) Modifier 79 (repeat services by same
physician) must be appended to any claim for G9886 or G9887 to identify a MDPP make-up
session that was held on the same day as a regularly scheduled MDPP session.
(d) Updating performance payments and attendance payments. The performance
payments and attendance payments will be adjusted each calendar year by the percent change in
the Consumer Price Index for All Urban Consumers (CPI–U) (U.S. city average) for the 12-
month period ending June 30th of the year preceding the update year. The percent change update
will be calculated based on the level of precision of the index as published by the Bureau of
Labor Statistics (BLS) and applied based on one decimal place of precision. The annual MDPP
services payment update will be published by CMS transmittal.
26. Section 414.502 is amended by revising the definitions of “Data collection period”
and “Data reporting period” to read as follows:
§ 414.502 Definitions.
* * * * *
Data collection period is the 6 months from January 1 through June 30, during which
applicable information is collected and that precedes the data reporting period, except that for the
data reporting period of January 1, 2025, through March 31, 2025, the data collection period is
January 1, 2019, through June 30, 2019.

Data reporting period is the 3-month period, January 1 through March 31, during which
a reporting entity reports applicable information to CMS and that follows the preceding data
collection period, except that for the data collection period of January 1, 2019, through June 30,
2019, the data reporting period is January 1, 2025, through March 31, 2025.
* * * * *
§ 414.504 [Amended]
27. Section 414.504 is amended in paragraph (a)(1) by removing the reference “January
1, 2024” and adding in its place the reference “January 1, 2025”.
28. Section 414.507 is amended by revising paragraphs (d) introductory text and (d)(7)
and adding paragraph (d)(10) to read as follows:
§ 414.507 Payment for clinical diagnostic laboratory tests.
* * * * *
(d) Phase-in of payment reductions. For years 2018 through 2027, the payment rates
established under this section for each CDLT that is not a new ADLT or new CDLT, may not be
reduced by more than the following amounts for—
* * * * *
(7) 2024 – 0.0 percent of the payment rate established in 2023.
* * * * *
(10) 2027 - 15 percent of the payment rate established in 2026.
* * * * *
29. Section 414.605 is amended in the definition of “Advanced life support, level 2
(ALS2)” by adding paragraph (8) to read as follows:
§ 414.605 Definitions.
* * * * *
Advanced life support, level 2 (ALS2) * * *
(8) Administration of low titer O+ whole blood transfusion.
* * * * *
30. Section 414.902 is amended by revising the definition “Refundable single-dose
container or single-use package drug” to read as follows:
§ 414.902 Definitions.
* * * * *
Refundable single-dose container or single-use package drug, as used in this subpart:
(1) Means a single source drug or biological or a biosimilar biological product for which
payment is made under this part and is —
(i) Furnished from a single-dose container or single-use package based on Food and Drug
Administration (FDA)-approved labeling or product information; or
(ii) Furnished from an ampule for which product labeling does not have discard statement
or language indicating if the container is single-dose container, single-use package, multiple-
dose container, or single-patient-use container; or
(iii) Furnished from a container with a total labeled volume of 2 mL or less for which
product labeling does not have language indicating if the container is single-dose container,
single-use package, multiple-dose container, or single-patient-use container.
(2) And excludes—
(i) A drug that is a therapeutic radiopharmaceutical, a diagnostic radiopharmaceutical, or
an imaging agent as identified in the drug's FDA-approved labeling.
(ii) A drug for which the FDA-approved labeling for any National Drug Code assigned to
a billing and payment code of such drug requires filtration during the drug preparation process,
prior to dilution and administration and that any unused portion of such drug after the filtration
process be discarded after the completion of such filtration process.
(iii) A drug approved or licensed by the FDA on or after November 15, 2021, until the
last day of the sixth full quarter for which the drug has been marketed (as reported to CMS) for
the first National Drug Code assigned to the billing and payment code of such drug.
(iv) A drug approved or licensed by FDA on or after November 15, 2021 and for which
the date of first sale as reported to CMS does not adequately approximate the date of first
payment under Part B due to an applicable national coverage determination, until the last day of
the sixth full quarter for which the drug has been covered and paid under Medicare Part B for the
first National Drug Code assigned to the billing and payment code of such drug.
* * * * *
31. Section 414.904 is amended by adding paragraph (e)(6) and revising paragraph (i) to
read as follows:
§ 414.904 Average sales price as the basis for payment.
* * * * *
(e) * * *
(6) Radiopharmaceuticals furnished in settings other than the hospital outpatient
department. Medicare administrative contractors must determine payment limits for
radiopharmaceuticals based on any methodology used to determine payment limits for
radiopharmaceuticals in place on or prior to November 2003. Such methodology may include,
but is not limited to, the use of invoice-based pricing.
* * * * *
(i) Manufacturer’s average sales price (ASP) data not available prior to the publication
deadline for quarterly payment limits. For circumstances in which manufacturer’s ASP data is
not available prior to the publication deadline for quarterly payment limits as described in this
section, payment limit must be determined as follows:
(1) For a multiple source drug (as defined in § 414.902) —
(i) In circumstances in which negative or zero manufacturer’s ASP data is reported for
one or more, but not all, National Drug Codes (NDCs) associated with a billing and payment
code for that drug for a given quarter, the payment limit for the given quarter is calculated using
only NDCs for that drug with positive manufacturer’s ASP data, except in circumstances
described in paragraph (i)(1)(iii) of this section.
(ii) In circumstances in which negative or zero manufacturer’s ASP data is reported for
all NDCs associated with a billing and payment code for that drug for a given quarter, the
payment limit for the given quarter is calculated by carrying over all positive manufacturer’s
ASP data from the most recently available previous quarter with positive manufacturer’s ASP
data for at least one NDC until at least one NDC for the drug has positive manufacturer’s ASP
data for a quarter.
(iii) In circumstances in which manufacturer’s ASP data is not available and the
unavailability of the manufacturer’s ASP data results in a significant change in the ASP payment
limit compared to the previous quarter, the payment limit is calculated by carrying over the most
recent available ASP data for the individual NDC(s), adjusted by the weighted average of the
change in the manufacturer’s ASP data for the NDCs that were reported for both the most
recently available previous quarter and the current quarter.
(2) For a single source drug, excluding biosimilar biological products (both as defined in
§ 414.902) —
(i) In circumstances in which negative or zero manufacturer’s ASP data is reported for
one or more, but not all, NDCs associated with a billing and payment code for that drug for a
given quarter, the payment limit for the given quarter is calculated using only NDCs for that drug
with positive manufacturer’s ASP data.
(ii) In circumstances in which negative or zero manufacturer’s ASP data is reported for
all NDCs associated with a billing and payment code for that drug for a given quarter, the
payment limit for the given quarter is the lesser of the following until at least one NDC for the
drug has positive manufacturer’s ASP data for a quarter:
(A) 106 percent of the volume-weighted average of the most recent available positive
manufacturer’s ASP data from a previous quarter in which at least one NDC for the drug has
positive manufacturer’s ASP data for the quarter. If the payment limit from such quarter was
based on 106 percent of the wholesale acquisition cost because of the application of paragraph
(d)(1) of this section, that payment limit is carried over; or
(B) 106 percent of the wholesale acquisition cost. If there is more than one WAC per
billing unit for the drug, the payment limit is set using the lowest WAC per billing unit.
(3) For a biosimilar biological product (as defined in § 414.902) --
(i) In circumstances in which negative or zero manufacturer’s ASP data is reported for
one or more, but not all, NDCs for a given quarter, the payment limit for the given quarter is
calculated using only NDCs with positive manufacturer’s ASP data.
(ii) In circumstances in which negative or zero manufacturer’s ASP data is reported for
all NDCs for a given quarter, and other biosimilars referencing the same reference product report
positive ASP data for the given quarter, the payment limit for the given quarter is the sum of the
following until at least one NDC for the drug has positive manufacturer’s ASP data for a quarter:
(A) the volume-weighted average of the ASP data from all other biosimilars of the same
reference product; and
(B) Either:
(1) If the biosimilar is not a qualifying biosimilar (as both are defined at § 414.902), 6
percent of the amount determined under section 1847A(b)(4) of the Act for the reference
biological product (as defined in § 414.902) for the given quarter; or
(2) If the biosimilar is a qualifying biosimilar (as both are defined at § 414.902), 8
percent of the amount determined under section 1847A(b)(4) of the Act for the reference
biological product (as defined in § 414.902) for the given quarter.
(iii) In circumstances in which negative or zero manufacturer’s ASP data is reported for
all NDCs for a given quarter and either no other biosimilars have been approved for the same
reference product or no other biosimilars of the same reference product report positive
manufacturer’s ASP data for the given quarter, the payment limit for the given quarter is the sum
of the following until at least one NDC for the drug has positive manufacturer’s ASP data for a
quarter:
(A) The volume-weighted average of the most recent available positive manufacturer’s
ASP data from a previous quarter; and
(B) Either:
(1) If the biosimilar is not a qualifying biosimilar (as both are defined at § 414.902), 6
percent of the amount determined under section 1847A(b)(4) of the Act for the reference
biological product (as defined in § 414.902) for the given quarter; or
(2) If the biosimilar is a qualifying biosimilar (as both are defined at § 414.902), 8
percent of the amount determined under section 1847A(b)(4) of the Act for the reference
biological product (as defined in § 414.902) for the given quarter.
* * * * *
32. Section 414.1001 is amended by—
a. Revising paragraph (a);
b. Removing paragraph (b);
c. Redesignating paragraphs (c) and (d) as paragraphs (b) and (c), respectively;
d. In newly redesignated paragraph (b)(2), removing “(c)(1)” and adding in its place
“(b)(1)”.
The revision reads as follows:
§ 414.1001 Basis of payment.
(a) Supplying fees. Beginning in CY 2006—
(1) A supplying fee of $24 is paid to a pharmacy (no more often than once every 30 days)
for the first prescription of drugs and biologicals described in sections 1861(s)(2)(J),
1861(s)(2)(Q), and 1861(s)(2)(T) of the Act, that the pharmacy provided to a beneficiary, except
as provided in paragraph (a)(2) of this section.

(2) A supplying fee of $50 is paid to pharmacy for the initial supplied prescription of
drugs and biologicals described in section 1861(s)(2)(J) of the Act, that the pharmacy provided
to a patient during the first 30-day period following a transplant.
(3) A supplying fee of $16 is paid to a pharmacy (no more often than once every 30 days)
for each prescription following the first prescription (as specified in paragraphs (a)(1) and (2) of
this section) of drugs and biologicals described in sections 1861(s)(2)(J), 1861(s)(2)(Q), and
1861(s)(2)(T) of the Act, that the pharmacy provided to a beneficiary.
(4) A separate supplying fee is paid to a pharmacy for each prescription of drugs and
biologicals described in sections 1861(s)(2)(J), 1861(s)(2)(Q), and 1861(s)(2)(T) of the Act.
* * * * *
33. Section 414.1305 is amended in the definition of “Attribution-eligible beneficiary”
by revising paragraph (6) to read as follows:
§ 414.1305 Definitions.
* * * * *
Attribution-eligible beneficiary * * *
(6) Has a minimum of one claim for any covered professional service furnished by an
eligible clinician who is on the Participation List for an Advanced APM Entity at any
determination date during the QP Performance Period.
* * * * *
34. Section 414.1325 is amended by adding paragraphs (a)(1)(i) through (iii) and (f) to
read as follows:
§ 414.1325 Data submission requirements.
(a) * * *
(1) * * *
(i) For the quality performance category, a data submission must include numerator and
denominator data for at least one MIPS quality measure from the final list of MIPS quality
measures.
(ii) For the improvement activities performance category, a data submission must include
a response of “yes” for at least one activity in the MIPS improvement activities inventory.
(iii) For the Promoting Interoperability performance category, a data submission must
include all of the following elements:
(A) Performance data, including any claim of an applicable exclusion, for the measures in
each objective, as specified by CMS;
(B) Required attestation statements, as specified by CMS;
(C) CMS EHR Certification ID (CEHRT ID) from the Certified Health IT Product List
(CHPL); and
(D) The start date and end date for the applicable performance period as set forth in §
414.1320.
* * * * *
(f) Treatment of multiple data submissions. (1) For multiple data submissions received in
the quality and improvement activities performance categories in accordance with paragraphs
(a)(1)(i) and (ii) of this section for an individual MIPS eligible clinician, group, subgroup, or
virtual group from submitters in multiple organizations (for example, qualified registry, practice
administrator, or EHR vendor), CMS will calculate and score each submission received and
assign the highest of the scores. For multiple data submissions received for an individual MIPS
eligible clinician, group, subgroup, or virtual group from one or multiple submitters in the same
organization, CMS will score the most recent submission.
(2) For multiple data submissions received for the Promoting Interoperability
performance category, CMS will calculate a score for each data submission received and assign
the highest of the scores.
35. Section 414.1330 is amended by adding paragraph (c) to read as follows.
§ 414.1330 Quality performance category.
* * * * *
(c)(1) CMS uses the following criteria to determine the removal of a quality measure:
(i) If the Secretary determines that the quality measure is no longer meaningful, such as
measures that are topped out.
(ii) If a measure steward is no longer able to maintain the quality measure.
(iii) If the quality measure reached extremely topped out status.
(iv) If the quality measure does not meet case minimum and reporting volumes required
for benchmarking after being in the program for 2 consecutive CY performance periods.
(v) If the quality measure is duplicative.
(vi) If the quality measure is not updated to reflect current clinical guidelines, which are
not reflective of a clinician’s scope of practice.
(vii) If the quality measure is a process measure.
(viii) If the quality measure addresses a measurement gap.
(ix) If the quality measure is a patient-reported outcome.
(x) If the quality measure is not available for MIPS quality reporting by or on behalf of
all MIPS eligible clinicians.
(xi) The robustness of the quality measure.
(xii) Consideration of the quality measure in developing MIPS Value Pathways (MVPs).
(2) A quality measure that otherwise meets the criteria for removal in paragraph (c)(1) of
this section may nonetheless be retained based on the following considerations:
(i) Whether the removal of the process measure impacts the number of measures
available for a specific specialty.
(ii) Whether the quality measure addresses a priority area.
(iii) Whether the quality measure promotes positive outcomes in patients.

(iv) Whether the quality measure is designated as high priority or not.
(v) Whether the quality measure has reached extremely topped out status.
(vi) Evaluation of the quality measure’s performance data.
36. Section 414.1335 is amended by revising paragraph (a) introductory text and adding
paragraph (b) to read as follows.
§ 414.1335 Data submission criteria for the quality performance category.
(a) Criteria. Except as provided in paragraph (b) of this section, a MIPS eligible
clinician, group, virtual group, subgroup, or APM Entity must submit data on MIPS quality
measures in one of the following manners, as applicable:
* * * * *
(b) Special rule for the APM Performance Pathway (APP) Plus measure set. A MIPS
eligible clinician, group, or APM Entity that reports the APP Plus measure set via the APP must
report on all measures included in the APP Plus measure set, except for administrative claims-
based quality measures as provided in § 414.1325(a)(2)(i).
37. Section 414.1340 is amended by revising paragraphs (a)(4), (b)(4), (d) introductory
text, and (d)(1) to read as follows:
§ 414.1340 Data completeness criteria for the quality performance category.
(a) * * *
(4) At least 75 percent of the MIPS eligible clinician, group, virtual group, subgroup, and
APM Entity’s patients that meet the measure’s denominator criteria, regardless of payer for
MIPS payment years 2026, 2027, 2028, 2029, and 2030.
(b) * * *
(4) At least 75 percent of the applicable Medicare Part B patients seen during the
performance period to which the measure applies for MIPS payment years 2026, 2027, 2028,
2029, and 2030.
* * * * *

(d) APM Entities, specifically Medicare Shared Savings Program Accountable Care
Organizations that meet reporting requirements under the APP, submitting quality measure data
on Medicare CQMs must submit data on:
(1) At least 75 percent of the applicable beneficiaries eligible for the Medicare CQM, as
defined at § 425.20 of this chapter, who meet the measure’s denominator criteria for MIPS
payment years 2026, 2027, 2028, 2029, and 2030.
* * * * *
38. Section 414.1350 is amended by adding paragraph (e) to read as follows:
§ 414.1350 Cost performance category.
* * * * *
(e) Cost measure removal criteria. CMS may remove a cost measure from MIPS based
on one or more of the following factors, provided however CMS may retain a cost measure that
meets one or more of the following factors if CMS determines the benefit of retaining the
measure outweighs the benefit of removing it.
(1) It is not feasible to implement the measure specifications.
(2) A measure steward is no longer able to maintain the cost measure.
(3) The implementation costs or negative unintended consequences associated with a cost
measure outweigh the benefit of its continued use in the MIPS cost performance category.
(4) The measure specifications do not reflect current clinical practice or guidelines.
(5) The availability of a more applicable measure, including a measure that applies across
settings, applies across populations, or is more proximal in time to desired patient outcomes for
the particular topic.
39. Section 414.1355 is amended by adding paragraph (d) to read as follows:
§ 414.1355 Improvement activities performance category.
* * * * *
(d) CMS may remove an improvement activity from MIPS based on one or more of the
following factors, provided however CMS may retain an improvement activity that meets one or
more of the following factors if CMS determines the benefit of retaining the activity outweighs
the benefit of removing it:
(1) Factor 1: Activity is duplicative of another activity.
(2) Factor 2: There is an alternative activity with a stronger relationship to quality care
or improvements in clinical practice.
(3) Factor 3: Activity does not align with current clinical guidelines or practice.
(4) Factor 4: Activity does not align with at least one meaningful measures area.
(5) Factor 5: Activity does not align with the quality, cost, or Promoting Interoperability
performance categories.
(6) Factor 6: There have been no attestations of the activity for 3 consecutive years.
(7) Factor 7: Activity is obsolete.
40. Section 414.1365 is amended by revising paragraphs (b)(2)(i), (c)(3)(i) and (ii),
(c)(4)(i)(A), (d)(3)(i)(A) introductory text, (d)(3)(i)(A)(1), (d)(3)(ii) introductory text,
(d)(3)(ii)(A), and (d)(3)(iii) to read as follows:
§ 414.1365 MIPS Value Pathways.
* * * * *
(b) * * *
(2) * * *
(i) For the CY 2023 through 2024 performance periods/2025 through 2026 MIPS
payment years, each MVP Participant must select an MVP, one population health measure
included in the MVP, and any outcomes-based administrative claims measure on which the MVP
Participant intends to be scored. Beginning in the CY 2025 performance period/2027 MIPS
payment year, each MVP Participant must select an MVP and any outcomes-based
administrative claims measure on which the MVP Participant intends to be scored.
* * * * *
(c) * * *
(3) * * *
(i) For the CY 2023 and 2024 performance periods/2025 through 2026 MIPS payment
years:
(A) Two medium-weighted improvement activities.
(B) One high-weighted improvement activity.
(C) Participation in a certified or recognized patient-centered medical home (PCMH) or
comparable specialty practice, as described at § 414.1380(b)(3)(ii).
(ii) Beginning in the CY 2025 performance period/2027 MIPS payment year:
(A) One improvement activity.
(B) Participation in a certified or recognized patient-centered medical home (PCMH) or
comparable specialty practice, as described at § 414.1380(b)(3)(ii).
* * * * *
(4) * * *
(i) * * *
(A) An MVP Participant that is a subgroup is required to submit its affiliated group's data
for the Promoting Interoperability performance category.
* * * * *
(d) * * *
(3) * * *
(i) * * *
(A) Population health measures. Except as provided in paragraph (d)(3)(i)(A)(1) of this
section, for the CY 2023 through 2024 performance periods/2025 through 2026 MIPS payment
years, each selected population health measure that does not have a benchmark or meet the case
minimum requirement is excluded from the MVP participant's total measure achievement points
and total available measure achievement points. Beginning in the CY 2025 performance
period/2027 MIPS payment year, except as provided in paragraph (d)(3)(i)(A)(1), the highest
score of all applicable and available population health measures will be used. If no population
health measure has a benchmark or meet the case minimum requirement, each such measure is
excluded from the MVP participant's total measure achievement points and total available
measure achievement points.
(1) For the CY 2023 through 2024 performance periods/2025 through 2026 MIPS
payment years, a subgroup is scored on each selected population health measure based on its
affiliated group score, if available. Beginning in the CY 2025 performance period/2027 MIPS
payment year, a subgroup is scored on the highest scoring of all available population health
measures based on its affiliated group score, if available. If the subgroup's affiliated group score
is not available, each such measure is excluded from the subgroup's total measure achievement
points and total available measure achievement points.
* * * * *
(ii) Cost performance category. The cost performance category score is calculated for an
MVP Participant using the methodology at § 414.1380(b)(2) and the cost measures included in
the MVP that they select and report.
(A) A subgroup is scored on each cost measure included in the MVP that it selects and
reports based on its affiliated group score for each such measure, if available. If the subgroup's
affiliated group score is not available for a measure, the measure is excluded from the subgroup's
total measure achievement points and total available measure achievement points, as described
under § 414.1380(b)(2).
* * * * *
(iii) Improvement activities performance category. In the CY 2023 through 2024
performance periods/2025 through 2026 MIPS payment years, the improvement activities
performance category score is calculated based on the submission of high- and medium-weighted
improvement activities. MVP Participants will receive 20 points for each medium-weighted
improvement activity and 40 points for each high-weighted improvement activity required under
§ 414.1360 on which data is submitted in accordance with § 414.1325 or for participation in a
certified or recognized patient-centered medical home (PCMH) or comparable specialty practice,
as described at § 414.1380(b)(3)(ii). Beginning in the CY 2025 performance period/2027 MIPS
payment year, MVP Participants will receive 40 points for each improvement activity required
under § 414.1360 on which data is submitted in accordance with § 414.1325 or for participation
in a certified or recognized PCMH or comparable specialty practice, as described at §
414.1380(b)(3)(ii).
* * * * *
41. Section 414.1367 is amended by revising paragraph (c)(1) introductory text and
adding paragraph (c)(1)(iii) to read as follows:
§ 414.1367 APM performance pathway.
* * * * *
(c) * * *
(1) Quality. Except as provided in paragraphs (c)(1)(i) and (ii) of this section, the quality
performance category score is calculated for a MIPS eligible clinician, group, or APM Entity
group in accordance with § 414.1380(b)(1) based on the quality measure set applicable to the
MIPS eligible clinician, group, or APM Entity group under paragraph (c)(1)(iii) of this section
and established by CMS through rulemaking for a MIPS payment year.
* * * * *
(iii)(A) For performance periods beginning prior to CY 2025 and MIPS payment years
beginning prior to 2027, a MIPS eligible clinician, group, or APM Entity group must report the
APM Performance Pathway quality measure set.
(B) Beginning with the CY 2025 performance period/2027 MIPS payment year, a MIPS
eligible clinician, group, or APM Entity group may choose to report either the APM Performance
Pathway quality measure set or the APP Plus quality measure set.
* * * * *
42. Section 414.1380 is amended by —
a. Revising paragraph (b)(1)(ii) introductory text;
b. Adding paragraphs (b)(1)(ii)(E) and (F);
c. Revising paragraph (b)(1)(iv)(B);
d. Adding paragraph (b)(1)(iv)(C);
e. Revising paragraph (b)(1)(vii) introductory text;
f. Adding paragraph (b)(1)(vii)(C);
g. Revising paragraph (b)(2) introductory text;
h. Adding paragraphs (b)(2)(i)(A) and (B) and (b)(2)(v)(B);
i. Revising paragraph (b)(3) introductory text; and
j. Adding paragraphs (c)(2)(i)(A)(10) and (c)(2)(i)(C)(12).
The revisions and additions read as follows:
§ 414.1380 Scoring.
* * * * *
(b) * * *
(1) * * *
(ii) Benchmarks. Except as provided in paragraphs (b)(1)(ii)(B) through (F) of this
section, benchmarks will be based on performance by collection type, from all available sources,
including MIPS eligible clinicians and APMs, to the extent feasible, during the applicable
baseline or performance period.
* * * * *
(E) Beginning with the CY 2025 performance period/2027 MIPS payment year, CMS
will publish a list in the Federal Register of topped out measures determined to be impacted by
limited measure choice. Measures included on the list are scored from 1 to 10 measure
achievement points according to defined topped out measure benchmarks calculated from
performance data in the baseline period in which a performance rate in the 97
th
percentile
corresponds to 7.5 measure achievement points.
(F) Beginning in the CY 2025 performance period/2027 MIPS payment year, measures of
the Medicare CQM collection type use flat benchmarks for their first two performance periods in
MIPS.
* * * * *
(iv) * * *
(B) Beginning with the 2021 MIPS payment year, except as provided for in paragraph
(b)(1)(iv)(C) of this section, each measure (except for measures in the CMS Web Interface) for
which the benchmark for the applicable collection type is identified as topped out for 2 or more
consecutive years receives no more than 7 measure achievement points in the second consecutive
year it is identified as topped out, and beyond.
(C) Beginning with the CY 2025 performance period/2027 MIPS payment year, measures
impacted by limited measure choice as specified in paragraph (b)(1)(ii)(E) of this section are not
subject to the 7 measure achievement point cap specified in paragraph (b)(1)(iv)(B) of this
section.
* * * * *
(vii) Quality performance category score. A MIPS eligible clinician's quality
performance category score is the sum of all the measure achievement points assigned for the
measures required for the quality performance category criteria plus the measure bonus points in
paragraph (b)(1)(v) of this section and Complex Organization Adjustment in paragraph
(b)(1)(vii)(C) of this section. The sum is divided by the sum of total available measure
achievement points. The improvement percent score in paragraph (b)(1)(vi) of this section is
added to that result. The quality performance category score cannot exceed 100 percentage
points.
* * * * *
(C) Beginning in the CY 2025 performance period/2027 MIPS payment year, a Virtual
Group and an APM Entity receives one measure achievement point for each eCQM submitted
that meets the case minimum requirement at paragraph (b)(1)(iii) of this section and the data
completeness requirement at § 414.1340. Each measure may not exceed 10 measure achievement
points. The total adjustment to the Virtual Group or APM Entity’s quality performance category
score under this paragraph (b)(1)(vii)(C) may not exceed 10 percent of the total available
measure achievement points.
(2) Cost performance category. For each cost measure attributed to a MIPS eligible
clinician, the clinician receives one to ten achievement points based on the clinician's
performance on the measure during the performance period compared to the measure's
benchmark. Achievement points are awarded based on which benchmark range the MIPS
eligible clinician's performance on the measure is in. CMS assigns partial points based on where
the MIPS eligible clinician’s performance falls between the top and bottom of the benchmark
ranges.
(i)* * *
(A) For the 2019 through 2025 MIPS payment years, CMS determines cost measure
benchmark ranges based on linear percentile distributions.
(B) Beginning with the 2026 MIPS payment year, for each cost measure, CMS
determines 10 benchmark ranges based on the median cost of all MIPS eligible clinicians
attributed the measure, plus or minus standard deviations. CMS awards achievement points
based on which benchmark range a MIPS eligible clinician’s average cost for a cost measure
corresponds. Additionally, CMS awards achievement points equivalent to 10 percent of the
performance threshold for a MIPS eligible clinician whose average cost attributed under a cost
measure is equal to the median cost for all MIPS eligible clinicians attributed the measure.
* * * * *
(v) * * *
(B) Beginning with the 2027 MIPS payment year, if data used to calculate a score for a
cost measure are impacted by significant changes or errors affecting the performance period,
such that calculating the cost measure score would lead to misleading or inaccurate results, then
the affected cost measure is excluded from the MIPS eligible clinician's or group's cost
performance category score. For purposes of this paragraph (b)(2)(v)(B), “significant changes or
errors” are changes or errors external to the care provided, and that CMS determines may lead to
misleading or inaccurate results that negatively impact the measure’s ability to reliably assess
performance. Significant changes or errors include, but are not limited to, rapid or unprecedented
changes to service utilization, the inadvertent omission of codes or inclusion of codes, or changes
to clinical guidelines or measure specifications. CMS will empirically assess the affected cost
measure to determine the extent to which the changes or errors impact the calculation of a cost
measure score such that calculating the cost measure score would lead to misleading or
inaccurate results that negatively impact the measure’s ability to reliably assess performance.
(3) Improvement activities performance category. Subject to paragraphs (b)(3)(i) and (ii)
of this section, the improvement activities performance category score equals the total points for
all submitted improvement activities divided by 40 points, multiplied by 100 percent. In the CY
2023 through 2024 performance periods/2025 through 2026 MIPS payment years, MIPS eligible
clinicians (except for non-patient facing MIPS eligible clinicians, small practices, and practices
located in rural areas and geographic HPSAs) receive 10 points for each medium-weighted
improvement activity and 20 points for each high-weighted improvement activity required under
§ 414.1360 on which data is submitted in accordance with § 414.1325. Non-patient facing MIPS
eligible clinicians, small practices, and practices located in rural areas and geographic HPSAs
receive 20 points for each medium-weighted improvement activity and 40 points for each high-
weighted improvement activity required under § 414.1360 on which data is submitted in
accordance with § 414.1325. Beginning in the CY 2025 performance period/2027 MIPS payment
year, MIPS eligible clinicians (except for non-patient facing MIPS eligible clinicians, small
practices, and practices located in rural areas and geographic HPSAs) receive 20 points for each
improvement activity required under § 414.1360 on which data is submitted in accordance with §
414.1325. Non-patient facing MIPS eligible clinicians, small practices, and practices located in
rural areas and geographic HPSAs receive 40 points for each improvement activity required
under § 414.1360 on which data is submitted in accordance with § 414.1325.
* * * * *
(c) * * *
(2) * * *
(i) * * *
(A) * * *
(10) Beginning with the 2026 MIPS payment year, for the quality and improvement
activities performance categories, CMS determines, based on documentation provided to the
agency on or before November 1
st
of the year preceding the relevant MIPS payment year, that
data for a MIPS eligible clinician are inaccessible or unable to be submitted due to circumstances
outside of the control of the clinician because the MIPS eligible clinician delegated submission
of the data to their third party intermediary, evidenced by a written agreement between the MIPS
eligible clinician and third party intermediary, and the third party intermediary did not submit the
data for the performance category(ies) on behalf of the MIPS eligible clinician in accordance
with applicable deadlines. To determine whether to apply reweighting to the affected
performance category(ies), CMS will consider: whether the MIPS eligible clinician knew or had
reason to know of the issue with its third party intermediary’s submission of the clinician’s data
for the performance category(ies); whether the MIPS eligible clinician took reasonable efforts to
correct the issue; and whether the issue between the MIPS eligible clinician and their third party
intermediary caused no data to be submitted for the performance category(ies) in accordance
with applicable deadlines.
* * * * *
(C) * * *
(12) Beginning with the 2026 MIPS payment year, CMS determines, based on
documentation provided to the agency on or before November 1
st
of the year preceding the
relevant MIPS payment year, that data for a MIPS eligible clinician are inaccessible or unable to
be submitted due to circumstances outside of the control of the clinician because the MIPS
eligible clinician delegated submission of the data to their third party intermediary, evidenced by
a written agreement between the MIPS eligible clinician and third party intermediary, and the
third party intermediary did not submit the data for the performance category on behalf of the
MIPS eligible clinician in accordance with applicable deadlines. To determine whether to apply
reweighting to the Promoting Interoperability performance category, CMS will consider:
whether the MIPS eligible clinician knew or had reason to know of the issue with its third party
intermediary’s submission of the clinician’s data for the performance category; whether the
MIPS eligible clinician took reasonable efforts to correct the issue; and whether the issue
between the MIPS eligible clinician and their third party intermediary caused no data to be
submitted for the performance category in accordance with applicable deadlines.
* * * * *
43. Section 414.1405 is amended by adding paragraph (b)(10) and revising paragraph (g)
to read as follows:
§ 414.1405 Payment.
* * * * *
(b) * * *
(10) Pursuant to the methodology established at paragraph (g)(2) of this section:
(i) The performance threshold for the 2027 MIPS payment year is 75 points. The prior
period used to determine the performance threshold is the 2019 MIPS payment year.
(ii) [Reserved]
* * * * *

(g) Performance threshold methodology. (1) For each of the 2024, 2025, and 2026 MIPS
payment years, the performance threshold is the mean of the final scores for all MIPS eligible
clinicians from a prior period as specified under paragraph (b)(9) of this section.
(2) For each of the 2027, 2028, and 2029 MIPS payment years, the performance
threshold is the mean of the final scores for all MIPS eligible clinicians from a prior period as
specified under paragraph (b)(10) of this section.
44. Section 414.1430 is amended by—
a. Revising paragraph (a)(1)(v);
b. Adding paragraph (a)(1)(vi);
c. Revising paragraph (a)(2)(v);
d. Adding paragraph (a)(2)(vi);
e. Revising paragraph (a)(3)(v);
f. Adding paragraph (a)(3)(vi);
g. Revising paragraph (a)(4)(v);
h. Adding paragraph (a)(4)(vi); and
i. Revising paragraphs (b)(1)(i)(A) and (B), (b)(2)(i)(A) and (B), (b)(3)(i)(A) and (B),
and (b)(4)(i)(A) and (B).
The revisions and additions read as follows:
§ 414.1430 Qualifying APM participant determination: QP and partial QP thresholds.
(a) * * *
(1) * * *
(v) 2026: 50 percent.
(vi) 2027 and later: 75 percent.
(2) * * *
(v) 2026: 40 percent.
(vi) 2027 and later: 50 percent.
(3) * * *
(v) 2026: 35 percent.
(vi) 2027 and later: 50 percent.
(4) * * *
(v) 2026: 25 percent.
(vi) 2027 and later: 35 percent.
(b) * * *
(1) * * *
(i) * * *
(A) 2021 through 2026: 50 percent.
(B) 2027 and later: 75 percent.
* * * * *
(2) * * *
(i) * * *
(A) 2021 through 2026: 40 percent.
(B) 2027 and later: 50 percent.
* * * * *
(3) * * *
(i) * * *
(A) 2021 through 2026: 35 percent.
(B) 2027 and later: 50 percent.
* * * * *
(4) * * *
(i) * * *
(A) 2021 through 2026: 25 percent.
(B) 2027 and later: 35 percent.

* * * * *
45. Section 414.1450 is amended by revising paragraph (a)(1)(i) and the first sentence of
paragraph (b)(1) to read as follows:
§ 414.1450 APM incentive payment.
(a) * * *
(1) * * *
(i) For payment years 2019 through 2026, CMS makes a lump sum payment to QPs in
the amount described in paragraph (b) of this section in the manner described in paragraphs (d)
and (e) of this section.
* * * * *
(b) * * *
(1) For payment years 2019 through 2024, the amount of the APM Incentive Payment is
equal to 5 percent, with respect to payment year 2025, 3.5 percent, or with respect to payment
year 2026, 1.88 percent of the estimated aggregate payments for covered professional services as
defined in section 1848(k)(3)(A) of the Act furnished during the calendar year immediately
preceding the payment year. * * *
* * * * *
PART 423—VOLUNTARY MEDICARE PRESCRIPTION DRUG BENEFIT
46. The authority citation for part 423 continues to read as follows:
Authority: 42 U.S.C. 1302, 1306, 1395w–101 through 1395w–152, and 1395hh.
47. Section 423.160 is amended by revising paragraph (a)(5) introductory text to read as
follows:
§ 423.160 Standards for electronic prescribing.
(a) * * *
(5) Beginning on January 1, 2021, prescribers must, except in the circumstances
described in paragraphs (a)(5)(i) through (iii) of this section, conduct prescribing for at least 70
percent of their Schedule II, III, IV, and V controlled substances that are Part D drugs
electronically using the applicable standards in paragraph (b) of this section, subject to the
exemption in paragraph (a)(3)(iii) of this section. Prescriptions written for a beneficiary in a
long-term care facility will not be included in determining compliance until January 1, 2028.
Compliance actions against prescribers who do not meet the compliance threshold based on
prescriptions written for a beneficiary in a long-term care facility will commence on or after
January 1, 2028. Compliance actions against prescribers who do not meet the compliance
threshold based on other prescriptions will commence on or after January 1, 2023. Prescribers
will be exempt from this requirement in the following situations:
* * * * *
PART 424—CONDITIONS FOR MEDICARE PAYMENT
48. The authority citation for part 424 continues to read as follows:
Authority: 42 U.S.C. 1302 and 1395hh.
49. Section 424.24 is amended by revising paragraphs (c) heading, (c)(1)(i), and (c)(3)(ii)
and adding paragraph (c)(5) to read as follows:
§ 424.24 Requirements for medical and other health services furnished by providers under
Medicare Part B.
* * * * *
(c) Outpatient physical therapy, occupational therapy, and speech-language pathology
services—
(1) * * *
(i) The individual needs, or needed, physical therapy, occupational therapy, or speech-
language pathology services.
* * * * *
(3) * * *
(ii) If the plan of treatment is established by a physical therapist, occupational therapist,
or speech-language pathologist, the certification must be signed by a physician or by a nurse
practitioner, clinical nurse specialist, or physician assistant who has knowledge of the case,
except as specified in paragraph (c)(5) of this section.
* * * * *
(5) Treatment plan. If the plan of treatment is established by a physical therapist,
occupational therapist, or speech-language pathologist, and there is a written order or referral
from the individual’s physician, nurse practitioner (NP), physician assistant (PA), or clinical
nurse specialist (CNS) in the patient’s record and the therapist has documented evidence that the
plan of care has been delivered to the physician, NP, PA, or CNS within 30 days of completion
of the initial evaluation, the certification does not need to be signed by a physician, NP, CNS, or
PA who has knowledge of the case. If there is no written order or referral from the individual’s
physician, NP, CNS, or PA, in the patient’s record, the therapist must obtain the signature of the
physician, NP, PA, or CNS on the plan of treatment in accordance with paragraph (c)(3) of this
section. No references to an order or referral in this subsection shall be construed to require an
order or referral for outpatient physical therapy, occupational therapy, or speech-language
pathology services.
* * * * *
50. Section 424.205 is amended by revising paragraphs (c)(10), (f)(1)(ii), (f)(2)(i), and
(f)(5) to read as follows:
§ 424.205 Requirements for Medicare Diabetes Prevention Program suppliers.
* * * * *
(c) * * *
(10) Except as allowed under paragraph (d)(8) of this section, the MDPP supplier must
offer an MDPP beneficiary no fewer than all of the following:
(i) 16 in-person or distance learning core sessions no more frequently than weekly for the
first 6 months of the MDPP services period, which begins on the date of attendance at the first
such core session.
(ii) 1 in-person or distance learning core maintenance session each month during months
7 through 12 (6 months total) of the MDPP services period.
* * * * *
(f) * * *
(1) * * *
(ii) Basic beneficiary information for each MDPP beneficiary in attendance, including but
not limited to beneficiary name, Medicare Beneficiary Identifier (MBI), and age.
* * * * *
(2) * * *
(i) Documentation of the type of session (in-person or distance learning).
* * * * *
(5) The MDPP supplier's records must include an attestation from the MDPP supplier
that, as applicable, the MDPP beneficiary for which it is submitting a claim—
(i) Has achieved the required minimum 5-percent weight loss as measured in accordance
with § 410.79(e)(3)(iii) of this chapter during a core session or core maintenance session
furnished by that supplier, if the claim submitted is for a performance payment under §
414.84(b)(1) of this chapter.
(ii) Has achieved the required minimum 5-percent weight loss as measured in-person
during a core session or core maintenance session furnished by that supplier, if the claim
submitted is for a performance payment under § 414.84(b)(1) of this chapter.
(iii) Has achieved at least a 9-percent weight loss percentage as measured in accordance
with § 410.79(e)(3)(iii) of this chapter during a core session or core maintenance session
furnished by that supplier, if the claim submitted is for a performance payment under §
414.84(b)(2) of this chapter.
(iv) Has achieved at least a 9-percent weight loss percentage as measured in-person
during a core session or core maintenance session furnished by that supplier, if the claim
submitted is for a performance payment under § 414.84(b)(2) of this chapter.
* * * * *
PART 425—MEDICARE SHARED SAVINGS PROGRAM
51. The authority citation for part 425 continues to read as follows:
Authority: 42 U.S.C. 1302, 1306, 1395hh, and 1395jjj.
52. Section 425.100 is amended by adding paragraph (e) to read as follows:
§ 425.100 General.
* * * * *
(e) An ACO is eligible to receive prepaid shared savings if it meets the criteria under
§ 425.640(b).
53. Section 425.110 is amended by revising paragraph (b)(2) to read as follows:
§ 425.110 Number of ACO professionals and beneficiaries.
* * * * *
(b) * * *
(2) For performance years starting before January 1, 2025, if the ACO's assigned
population is not at least 5,000 by the end of the performance year specified by CMS in its
request for a corrective action plan (CAP), CMS terminates the participation agreement and the
ACO is not eligible to share in savings for that performance year.
* * * * *
54. Section 425.202 is amended by revising paragraph (a)(3) to read as follows:
§ 425.202 Application procedures.
(a) * * *
(3) An ACO that seeks to participate in the Shared Savings Program must agree
that CMS can share a copy of their application with the Antitrust Agencies.
* * * * *
55. Section 425.204 is amended by—
a. Revising paragraphs (f)(1) and (f)(3) introductory text;
b. In paragraphs (f)(3)(iv), (f)(4)(iv)(A), and (f)(6)(ii) introductory text, removing the
phrase “any shared losses incurred” and adding in its place the phrase “any shared losses
incurred and prepaid shared savings determined to be owed”;
c. In paragraphs (f)(5) and (f)(6)(iv)(A), removing the phrase “shared losses owed” and
adding in its place the phrase “shared losses owed or prepaid shared savings determined to be
owed”;
d. In paragraph (f)(6)(iii), removing the phrase “shared losses” and adding in its place
the phrase “shared losses or prepaid shared savings determined to be owed”; and
e. In paragraph (f)(6)(iv)(C), removing the phrase “owe any shared losses” and adding in
its place the phrase “owe any shared losses or prepaid shared savings”.
The revisions read as follows:
§ 425.204 Content of the application.
* * * * *
(f) * * *
(1) An ACO must have the ability to repay all shared losses for which it may be liable
under a two-sided model and any prepaid shared savings determined to be owed.
* * * * *
(3) An ACO that will participate under a two-sided model of the Shared Savings Program
must submit for CMS approval documentation that it is capable of repaying shared losses that it
may incur during its agreement period, including details supporting the adequacy of the
repayment mechanism. If the ACO will receive prepaid shared savings, the repayment
mechanism must also support repayment of prepaid shared savings in accordance with
§ 425.640.
* * * * *
56. Section 425.224 is amended by revising paragraph (a)(3) to read as follows:
§ 425.224 Application procedures for renewing ACOs and re-entering ACOs.
(a) * * *
(3) An ACO that seeks to enter a new participation agreement under the Shared
Savings Program must agree that CMS can share a copy of its application with the Antitrust
Agencies.
* * * * *
57. Section 425.304 is amended by adding paragraph (d) to read as follows:
§ 425.304 Beneficiary incentives.
* * * * *
(d) Application of the CMS-sponsored model patient incentives safe harbor. CMS has
determined that the Federal anti-kickback statute safe harbor for CMS-sponsored model patient
incentives (§ 1001.952(ii)(2) of this title) is available to protect remuneration furnished in the
prepaid shared savings option of the Shared Savings Program in the form of direct beneficiary
services that meets all safe harbor requirements set forth in § 1001.952(ii) of this title.
58. Section 425.308 is amended by adding paragraph (b)(10) to read as follows:
§ 425.308 Public reporting and transparency.
* * * * *
(b) * * *
(10) Information updated annually about the ACO’s use of prepaid shared savings under
§ 425.640, for each performance year, including the following:
(i) Total amount of any prepaid shared savings received from CMS.
(ii) The ACO’s spend plan.
(iii) An itemization of how prepaid shared savings were spent during the year, including
expenditure categories, the dollar amounts spent on the various categories, information about
which groups of beneficiaries received direct beneficiary services that were purchased with
prepaid shared savings and investments that were made in the ACO with prepaid shared savings,
how these direct beneficiary services were provided to beneficiaries and how the direct
beneficiary services and investments supported the care of beneficiaries, any changes to the
spend plan as submitted under § 425.640(d)(2) (if applicable), and such other information as may
be specified by CMS.
* * * * *
59. Section 425.312 is amended by revising paragraphs (a)(2)(iii) and (a)(2)(v)(A) to read
as follows:
§ 425.312 Beneficiary notifications.
(a) * * *
(2) * * *
(iii) In the case of an ACO that has selected preliminary prospective assignment with
retrospective reconciliation, by the ACO or ACO participant providing each beneficiary who
received at least one primary care service during the assignment window or applicable expanded
window for assignment (as defined in § 425.20) from a physician who is an ACO professional in
the ACO and who is a primary care physician as defined under § 425.20 or who has one of the
primary specialty designations included in § 425.402(c), a FQHC or RHC that is part of the
ACO, or an ACO professional in the ACO whom the beneficiary designated as responsible for
coordinating their overall care under § 425.402(e) with a standardized written notice at least once
during an agreement period in the form and manner specified by CMS. The standardized written
notice must be furnished to all of these beneficiaries prior to or at the first primary care service
visit during the first performance year in which the beneficiary receives a primary care service
from an ACO participant.
* * * * *
(v) * * *
(A) The follow-up communication must occur no later than 180 days from the date the
standardized written notice was provided.
* * * * *
60. Section 425.315 is amended by revising paragraph (a)(4) and adding paragraph (b) to
read as follows:
§ 425.315 Reopening determinations of ACO shared savings or shared losses to correct
financial reconciliation calculations.
(a) * * *
(4) CMS has the sole discretion to determine whether to reopen a payment determination
under this section.
(b) Reopening requests. An ACO may request a reopening in a form and manner
specified by CMS and consistent with the timeframes for a reopening specified in paragraphs
(a)(1)(i) and (ii) of this section.
61. Section 425.316 is amended by adding paragraph (f) to read as follows:
§ 425.316 Monitoring of ACOs.
* * * * *
(f) Monitoring ACO eligibility for and use of prepaid shared savings. (1) CMS monitors
an ACO that receives prepaid shared savings pursuant to § 425.640 to ensure ACO compliance
with § 425.640(e) and to determine whether it would be appropriate to withhold or terminate an
ACO’s prepaid shared savings under § 425.640(h).
(2) If CMS determines that an ACO receiving prepaid shared savings is using the funds
for a prohibited use under § 425.640(e)(2), fails to spend the funding in accordance with
§ 425.640(e)(1)(i) and (ii), or spends more than 50 percent of the estimated annual payment
amount on staffing and healthcare infrastructure CMS:
(i) Will require the ACO to reallocate the funding as permitted by § 425.640(e) and
submit an updated spend plan demonstrating the reallocation by a deadline specified by CMS.
(ii) May take compliance action as specified in §§ 425.216, 425.218, and 425.640(h)(1).
(3) If an ACO fails to reallocate prepaid shared savings it received as described in
paragraph (f)(2)(i) of this section by a deadline specified by CMS, the ACO must repay all
prepaid shared savings it received and may be subject to compliance action as specified in §§
425.216 and 425.218. CMS will provide written notification to the ACO of the amount due and
the ACO must pay such amount no later than 90 days after the receipt of such notification.
62. Section 425.400 is amended by revising paragraph (c)(1)(viii) introductory text and
adding paragraph (c)(1)(ix) to read as follows:
§ 425.400 General.
* * * * *
(c) * * *
(1) * * *
(viii) For the performance year starting on January 1, 2024, as follows:
* * * * *
(ix) For the performance year starting on January 1, 2025, and subsequent performance
years as follows:
(A) CPT codes:
(1) 96160 and 96161 (codes for administration of health risk assessment).
(2) 96202 and 96203 (codes for caregiver behavior management training).
(3) 97550, 97551, and 97552 (codes for caregiver training services).
(4) 99201 through 99215 (codes for office or other outpatient visit for the evaluation and
management of a patient).
(5) 99304 through 99318 (codes for professional services furnished in a nursing facility;
professional services or services reported on an FQHC or RHC claim identified by these codes
are excluded when furnished in a skilled nursing facility (SNF)).
(6) 99319 through 99340 (codes for patient domiciliary, rest home, or custodial care
visit).
(7) 99341 through 99350 (codes for evaluation and management services furnished in a
patient's home).
(8) 99354 and 99355 (add-on codes, for prolonged evaluation and management or
psychotherapy services beyond the typical service time of the primary procedure; when the base
code is also a primary care service code under this paragraph (c)(1)(ix)).
(9) 99406 and 99407 (codes for smoking and tobacco-use cessation counseling services).
(10) 99421, 99422, and 99423 (codes for online digital evaluation and management).
(11) 99424, 99425, 99426, and 99427 (codes for principal care management services).
(12) 99437, 99487, 99489, 99490 and 99491 (codes for chronic care management).
(13) 99439 (code for non-complex chronic care management).
(14) 99446, 99447, 99448, 99449, 99451, 99452 (codes for interprofessional consultation
services).
(15) 99483 (code for assessment of and care planning for patients with cognitive
impairment).
(16) 99484, 99492, 99493 and 99494 (codes for behavioral health integration services).
(17) 99495 and 99496 (codes for transitional care management services).
(18) 99497 and 99498 (codes for advance care planning; services identified by these
codes furnished in an inpatient setting are excluded).
(19) 9X091 (code for virtual check-in).
(B) HCPCS codes:
(1) G0019 and G0022 (codes for community health integration services).
(2) G0023 and G0024 (codes for principal illness navigation services).
(3) G0101 (code for cervical or vaginal cancer screening).
(4) G0136 (code for social determinants of health risk assessment services).
(5) G0317, G0318, and G2212 (codes for prolonged office or other outpatient visit for the
evaluation and management of a patient).
(6) G0402 (code for the Welcome to Medicare visit).
(7) G0438 and G0439 (codes for the annual wellness visits).
(8) G0442 (code for alcohol misuse screening service).
(9) G0443 (code for alcohol misuse counseling service).
(10) G0444 (code for annual depression screening service).
(11) G0463 (code for services furnished in electing teaching amendment (ETA)
hospitals).
(12) G0506 (code for chronic care management).
(13) G2010 (code for the remote evaluation of patient video/images).
(14) G2012 and G2252 (codes for virtual check-in).
(15) G2058 (code for non-complex chronic care management).
(16) G2064 and G2065 (codes for principal care management services).
(17) G2086, G2087, and G2088 (codes for office-based opioid use disorder services).
(18) G2211 (code for visit complexity inherent to evaluation and management services
add-on).
(19) G2214 (code for psychiatric collaborative care model).
(20) G3002 and G3003 (codes for chronic pain management).
(21) GCDRA and GCDRM (codes for cardiovascular risk assessment and risk
management services).
(22) GCTB1 and GCTB2 (codes for individual behavior management/modification
caregiver training services).
(23) GCTD1, GCTD2, and GCTD3 (codes for direct care caregiver training services).
(24) GFCI1 (code for post-discharge telephonic follow-up contacts intervention).
(25) GPCM1, GPCM2, and GPCM3 (codes for advanced primary care management
services).
(26) GSPI1 (code for safety planning interventions when the base code is also a primary
care service code under this paragraph (c)(1)(ix)).
(C) Primary care service codes include any CPT code identified by CMS that directly
replaces a CPT code specified in paragraph (c)(1)(ix)(A) of this section or a HCPCS code
specified in paragraph (c)(1)(ix)(B) of this section, when the assignment window or expanded
window for assignment (as defined in § 425.20) for a benchmark or performance year includes
any day on or after the effective date of the replacement code for payment purposes under FFS
Medicare.
* * * * *
63. Section 425.402 is amended by revising paragraph (e)(2)(ii) introductory text and
adding paragraph (e)(2)(iii) to read as follows:
§ 425.402 Basic assignment methodology.
* * * * *
(e) * * *
(2) * * *
(ii) For performance years starting on January 1, 2019, through 2024:
* * * * *
(iii) For performance year 2025 and subsequent performance years:
(A) The beneficiary meets the eligibility criteria established at § 425.401(a) and must not
be excluded by the criteria at § 425.401(b). The exclusion criteria at § 425.401(b) apply for
purposes of determining beneficiary eligibility for alignment to an ACO based on the
beneficiary's designation of an ACO professional as responsible for coordinating their overall
care under paragraph (e) of this section, regardless of the ACO's assignment methodology
selection under § 425.226(a)(1).
(B) The beneficiary must have designated an ACO professional as responsible for
coordinating their overall care.
(C) If a beneficiary has designated a provider or supplier outside the ACO as responsible
for coordinating their overall care, the beneficiary is not added under the assignment
methodology in paragraph (b) of this section to the ACO's list of assigned beneficiaries for a 12-
month performance year.
(D) The beneficiary is not assigned to an entity participating in a model tested or
expanded under section 1115A of the Act under which claims-based assignment is based solely
on:
(1) Claims for primary care and/or other services related to treatment of one or more
specific diseases or conditions targeted by the model; or
(2) Claims for services other than primary care services, and for which there has been a
determination by the Secretary that waiver of the requirement in section 1899(c)(2)(B) of the Act
is necessary solely for purposes of testing the model.
* * * * *
64. Section 425.508 is amended by revising paragraph (b) and adding paragraph (c) to
read as follows:
§ 425.508 Incorporating quality reporting requirements related to the Quality Payment
Program.
* * * * *
(b) For performance years beginning in 2021 – 2024. ACOs must submit the quality data
via the APM Performance Pathway (APP) established under § 414.1367 of this chapter to
satisfactorily report on behalf of the eligible clinicians who bill under the TIN of an ACO
participant for purposes of the MIPS Quality performance category of the Quality Payment
Program.
(c) For performance years beginning on or after January 1, 2025. ACOs must submit the
quality data via the APM Performance Pathway (APP) on the quality measures contained in the
APP Plus quality measure set established under § 414.1367 of this chapter to satisfactorily report
on behalf of the eligible clinicians who bill under the TIN of an ACO participant for purposes of
the MIPS Quality performance category of the Quality Payment Program.
65. Section 425.510 is amended by revising the section heading and paragraph (b) to read
as follows:
§ 425.510 Application of the APM Performance Pathway (APP) quality measure set or the
APP Plus quality measure set (as applicable) to Shared Savings Program ACOs for
performance years beginning on or after January 1, 2021.
* * * * *
(b) Quality reporting. (1) For performance years beginning in 2021 – 2024, ACOs must
report quality data on the APP quality measure set established under § 414.1367 of this chapter,
according to the method of submission established by CMS.
(2) For performance years beginning on or after January 1, 2025, ACOs must report
quality data on the APP Plus quality measure set established under § 414.1367 of this chapter,
according to the method of submission established by CMS.
* * * * *
66. Section 425.512 is amended by—
a. Revising paragraphs (a)(2)(iii), (a)(5)(i) introductory text, (a)(5)(i)(A) introductory
text, (a)(5)(i)(B), (a)(5)(ii), (a)(5)(iii)(B), and (a)(7);
b. Revising and republishing paragraph (b);
c. In paragraph (c)(3) introductory text, removing the phrase “via the APP” and adding
in its place the phrase “on the APP quality measure set or the APP Plus quality measure set (as
applicable)”;
d. In paragraph (c)(3)(iii), removing the phrase “and subsequent performance years”
after “For performance year 2024”; and
e. Adding paragraph (c)(3)(iv).
The revisions, republication, and addition read as follows:
§ 425.512 Determining the ACO quality performance standard for performance years
beginning on or after January 1, 2021.
(a) * * *
(2) * * *
(iii) For performance year 2025 and subsequent performance years, if the ACO reports
the APP Plus quality measure set and meets the data completeness requirement at § 414.1340 of
this subchapter on all eCQMs/Medicare CQMs, and the CAHPS for MIPS survey (except as
specified in § 414.1380(b)(1)(vii)(B) of this subchapter), and receives a MIPS Quality
performance category score under § 414.1380(b)(1) of this subchapter, for the applicable
performance year.
* * * * *
(5) * * *
(i) Except as specified in paragraphs (a)(2) and (7) of this section, CMS designates the
quality performance standard as:
(A) For performance year 2024, the ACO reporting quality data on the APP quality
measure set established under § 414.1367 of this subchapter, according to the method of
submission established by CMS and –
* * * * *
(B) For performance year 2025 and subsequent performance years, the ACO reporting
quality data on the APP Plus quality measure set established under § 414.1367 of this
subchapter, according to the method of submission established by CMS and –
(1) Achieving a health equity adjusted quality performance score that is equivalent to or
higher than the 40th percentile across all MIPS Quality performance category scores, excluding
entities/providers eligible for facility-based scoring; or
(2) If the ACO reports all of the eCQMs in the APP Plus quality measure set applicable
for a performance year, meeting the data completeness requirement at § 414.1340 of this
subchapter for all eCQMs, and achieving a quality performance score equivalent to or higher
than the 10th percentile of the performance benchmark on at least one of the four outcome
measures in the APP Plus quality measure set and a quality performance score equivalent to or
higher than the 40th percentile of the performance benchmark on at least one of the remaining
measures in the APP Plus quality measure set.
(ii) CMS designates an alternative quality performance standard for an ACO that does not
meet the criteria described in paragraph (a)(2) or (a)(5)(i) of this section as the following:
(A) For performance year 2024, the ACO reports quality data on the APP quality
measure set established under § 414.1367 of this subchapter according to the method of
submission established by CMS and achieves a quality performance score equivalent to or higher
than the 10th percentile of the performance benchmark on at least one of the four outcome
measures in the APP quality measure set.
(B) For performance year 2025 and subsequent performance years, the ACO reports
quality data on the APP Plus quality measure set established under § 414.1367 of this subchapter
according to the method of submission established by CMS and achieves a quality performance
score equivalent to or higher than the 10
th
percentile of the performance benchmark on at least
one of the four outcome measures in the APP Plus quality measure set.
(iii) * * *
(B) For performance year 2025 and subsequent performance years, the ACO does not
report any of the eCQMs/Medicare CQMs in the APP Plus quality measure set and does not
administer a CAHPS for MIPS survey (except as specified in § 414.1380(b)(1)(vii)(B) of this
subchapter).
* * * * *
(7) Facility-based scoring. CMS will use the higher of the ACO's health equity adjusted
quality performance score or the equivalent of the 40th percentile MIPS Quality performance
category score across all MIPS Quality performance category scores, excluding
entities/providers eligible for facility-based scoring, for the relevant performance year when –
(i) For performance year 2024, if an ACO reports all of the required measures, meeting
the data completeness requirement at § 414.1340 of this subchapter for each measure in the APP
quality measure set and receiving a MIPS Quality performance category score as described at §
414.1380(b)(1) of this subchapter and the ACO meets either of the following:
(A) The ACO’s total available measure achievement points used to calculate the ACO’s
MIPS Quality performance category score are reduced under § 414.1380(b)(1)(vii)(A) of this
subchapter.
(B) At least one of the eCQMs/MIPS CQMs/Medicare CQMs does not have a benchmark
as described at § 414.1380(b)(1)(i)(A) of this subchapter.
(ii) For performance year 2025 and subsequent performance years, if an ACO reports all
of the required measures in the APP Plus quality measure set, meeting the data completeness
requirement at § 414.1340 of this subchapter for each measure in the APP Plus quality measure
set, and receiving a MIPS Quality performance category score as described at § 414.1380(b)(1)
of this subchapter, for the relevant performance year, and the ACO meets either of the following:
(A) The ACO’s total available measure achievement points used to calculate the ACO’s
MIPS Quality performance category score are reduced under § 414.1380(b)(1)(vii)(A) of this
subchapter.
(B) At least one of the eCQMs/Medicare CQMs does not have a benchmark as described
at § 414.1380(b)(1)(i)(A) of this subchapter.
(b) Calculation of ACO's health equity adjusted quality performance score for
performance year 2023 and subsequent performance years--(1) For performance year 2023. For
an ACO that reports the three eCQMs/MIPS CQMs in the APP quality measure set, meeting the
data completeness requirement at § 414.1340 of this subchapter for all three eCQMs/MIPS
CQMs, and administers the CAHPS for MIPS survey, CMS calculates the ACO's health equity
adjusted quality performance score as the sum of the ACO's MIPS Quality performance category
score for all measures in the APP quality measure set and the ACO's health equity adjustment
bonus points calculated in accordance with paragraph (b)(4) of this section. The sum of these
values may not exceed 100 percent.
(2) For performance year 2024. For an ACO that reports the three eCQMs/MIPS
CQMs/Medicare CQMs in the APP quality measure set, meeting the data completeness
requirement at § 414.1340 of this subchapter for all three eCQMs/MIPS CQMs/Medicare CQMs,
and administers the CAHPS for MIPS survey (except as specified in § 414.1380(b)(1)(vii)(B) of
this subchapter), CMS calculates the ACO's health equity adjusted quality performance score as
the sum of the ACO's MIPS Quality performance category score for all measures in the APP
quality measure set and the ACO's health equity adjustment bonus points calculated in
accordance with paragraph (b)(4) of this section. The sum of these values may not exceed 100
percent.
(3) For performance year 2025 and subsequent performance years. For an ACO that
reports all of the eCQMs/Medicare CQMs in the APP Plus quality measure set, meeting the data
completeness requirement at § 414.1340 of this subchapter for all of the eCQMs/Medicare
CQMs, and administers the CAHPS for MIPS survey (except as specified in §
414.1380(b)(1)(vii)(B) of this subchapter), CMS calculates the ACO's health equity adjusted
quality performance score as the sum of the ACO's MIPS Quality performance category score
for all measures in the APP Plus quality measure set and the ACO's health equity adjustment
bonus points calculated in accordance with paragraph (b)(4) of this section. The sum of these
values may not exceed 100 percent.
(4) Calculation of ACO's health equity adjustment bonus points. CMS calculates the
ACO's health equity adjustment bonus points as follows:
(i) For each measure in the APP quality measure set, CMS groups an ACO's performance
into the top, middle, or bottom third of ACO measure performers by reporting mechanism.
(ii) CMS assigns values to the ACO for its performance on each measure as follows:
(A) Values of four, two, or zero for each measure for which the ACO's performance
places it in the top, middle, or bottom third of ACO measure performers, respectively.
(B) Values of zero for each measure that CMS does not evaluate because the measure is
unscored or the ACO does not meet the case minimum or the minimum sample size for the
measure.
(iii) CMS sums the values assigned to the ACO according to paragraph (b)(4)(ii) of this
section, to calculate the ACO's measure performance scaler.
(iv) CMS calculates an underserved multiplier for the ACO.
(A)(1) CMS determines the proportion ranging from zero to one of the ACO's assigned
beneficiary population for the performance year that is considered underserved based on the
highest of either of the following:
(i) The proportion of the ACO's assigned beneficiaries residing in a census block group
with an Area Deprivation Index (ADI) national percentile rank of at least 85. An ACO's assigned
beneficiaries without an available numeric ADI national percentile rank are excluded from the
calculation of the proportion of the ACO's assigned beneficiaries residing in a census block
group with an ADI national percentile rank of at least 85.
(ii) The proportion of the ACO's assigned beneficiaries who are enrolled in the Medicare
Part D low-income subsidy (LIS); or are dually eligible for Medicare and Medicaid.
(2) CMS calculates the proportions specified in paragraph (b)(4)(iv)(A)(1)(ii) of this
section as follows:
(i) For performance year 2023, the proportion of the ACO's assigned beneficiaries who
are enrolled in the Medicare Part D LIS or are dually eligible for Medicare and Medicaid divided
by the total number of the ACO's assigned beneficiaries' person years.
(ii) For performance year 2024 and subsequent performance years, the proportion of the
ACO's assigned beneficiaries with any months enrolled in LIS or dually eligible for Medicare
and Medicaid divided by the total number of the ACO's assigned beneficiaries.
(B) If the proportion determined in accordance with paragraph (b)(4)(iv)(A) of this
section is lower than 20 percent, the ACO is ineligible for health equity adjustment bonus points.
(v) Except as specified in paragraph (b)(4)(iv)(B) of this section, CMS calculates the
ACO's health equity adjustment bonus points as the product of the measure performance scaler
determined under paragraph (b)(4)(iii) of this section and the underserved multiplier determined
under paragraph (b)(4)(iv) of this section. If the product of these values is greater than 10, the
value of the ACO's health equity adjustment bonus points is set equal to 10.
(5) Use of ACO's health equity adjusted quality performance score. The ACO's health
equity adjusted quality performance score, determined in accordance with paragraphs (b)(1)
through (4) of this section, is used as follows:
(i) In determining whether the ACO meets the quality performance standard as specified
under paragraphs (a)(4)(i)(A), (a)(5)(i)(A)(1), (a)(5)(i)(B), and (a)(7) of this section.
(ii) In determining the final sharing rate for calculating shared savings payments under
the BASIC track in accordance with § 425.605(d), and under the ENHANCED track in
accordance with § 425.610(d), for an ACO that meets the alternative quality performance
standard by meeting the criteria specified in paragraph (a)(4)(ii) or (a)(5)(ii) of this section.
(iii) In determining the shared loss rate for calculating shared losses under the
ENHANCED track in accordance with § 425.610(f), for an ACO that meets the quality
performance standard established in paragraphs (a)(2), (a)(4)(i), and (a)(5)(i) of this section or
the alternative quality performance standard established in paragraph (a)(4)(ii) or (a)(5)(ii) of this
section.
(iv) In determining the quality performance score for an ACO affected by extreme and
uncontrollable circumstances as described in paragraphs (c)(3)(ii) through (iv) of this section.
(c) * * *
(3) * * *
(iv) For performance year 2025 and subsequent performance years, if the ACO reports
the APP Plus quality measure set and meets the data completeness requirement at § 414.1340 of
this subchapter and receives a MIPS Quality performance category score under § 414.1380(b)(1)
of this subchapter, CMS will use the higher of the ACO's health equity adjusted quality
performance score or the equivalent of the 40th percentile MIPS Quality performance category
score across all MIPS Quality performance category scores, excluding entities/providers eligible
for facility-based scoring, for the relevant performance year.
* * * * *
67. Section 425.601 is amended by revising paragraph (a)(9) introductory text and adding
paragraphs (a)(9)(iii) and (iv) to read as follows:
§ 425.601 Establishing, adjusting, and updating the benchmark for agreement periods
beginning on or after July 1, 2019, and before January 1, 2024.
(a) * * *
(9) For the second and each subsequent performance year during the term of the
agreement period, the ACO's benchmark is adjusted for the following, as applicable: For the
addition and removal of ACO participants or ACO providers/suppliers in accordance with
§ 425.118(b), for a change to the ACO’s beneficiary assignment methodology selection under
§ 425.226(a)(1), for a change to the beneficiary assignment methodology specified in subpart E
of this part, for changes in values used in benchmark calculations as a result of issuance of a
revised initial determination under § 425.315, and for changes in values used in benchmark
calculations as a result of the performance year being affected by significant, anomalous, and
highly suspect billing under § 425.672. To adjust the benchmark, CMS does the following:
* * * * *
(iii) Recalculates benchmark year expenditures to account for the impact of improper
payments, for the benchmark year corresponding to a performance year for which CMS issued a
revised initial determination under § 425.315. In recalculating expenditures for the benchmark
year, CMS applies the calculation methodology applied in recalculating expenditures for the
corresponding performance year in accordance with § 425.674.
(iv) Recalculates expenditures used in Shared Savings Program benchmark calculations
under this section, to exclude the same HCPCS or CPT codes identified as displaying significant,
anomalous, and highly suspect billing patterns in calculation of performance year expenditures,
in accordance with § 425.672.
* * * * *
68. Section 425.605 is amended by revising paragraph (a)(1)(ii)(C) to read as follows:
§ 425.605 Calculation of shared savings and losses under the BASIC track.
(a) * * *
(1) * * *
(ii) * * *
(C) The aggregate growth in demographic risk scores for purposes of paragraph
(a)(1)(ii)(A) of this section and the aggregate growth in prospective hierarchical condition
category (HCC) risk scores for purposes of paragraph (a)(1)(ii)(B) of this section is calculated by
taking a weighted average of the growth in demographic risk scores or prospective HCC risk
scores, as applicable, across the populations described in paragraph (a)(2) of this section. When
calculating the weighted average growth in demographic risk scores or prospective HCC risk
scores, as applicable, the weight applied to the growth in risk scores (expressed as a ratio of the
ACO's performance year risk score to the ACO's BY3 risk score) for each Medicare enrollment
type is equal to the product of the ACO’s historical benchmark expenditures, adjusted in
accordance with § 425.652(a)(8), for that enrollment type and the ACO’s performance year
assigned beneficiary person years for that enrollment type.
* * * * *
69. Section 425.610 is amended by revising paragraph (a)(2)(ii)(C) to read as follows:
§ 425.610 Calculation of shared savings and losses under the ENHANCED track.
(a) * * *
(2) * * *
(ii) * * *
(C) The aggregate growth in demographic risk scores for purposes of paragraph
(a)(2)(ii)(A) of this section and the aggregate growth in prospective HCC risk scores for
purposes of paragraph (a)(2)(ii)(B) of this section is calculated by taking a weighted average of
the growth in demographic risk scores or prospective HCC risk scores, as applicable, across the
populations described in paragraph (a)(3) of this section. When calculating the weighted average
growth in demographic risk scores or prospective HCC risk scores, as applicable, the weight
applied to the growth in risk scores (expressed as a ratio of the ACO's performance year risk
score to the ACO's BY3 risk score) for each Medicare enrollment type is equal to the product of
the ACO’s historical benchmark expenditures, adjusted in accordance with § 425.652(a)(8), for
that enrollment type and the ACO’s performance year assigned beneficiary person years for that
enrollment type.
* * * * *
70. Section 425.630 is amended by—
a. In paragraph (g)(3), removing the phrase “paragraphs (g)(4) of this section” and
adding in its place the phrase “paragraphs (g)(4) through (6) of this section”;
b. Redesignating paragraph (g)(5) as paragraph (g)(7);
c. Adding new paragraph (g)(5) and paragraph (g)(6);
d. In paragraph (h)(1)(ii), removing “or” at the end of the paragraph;
e. In paragraph (h)(1)(iii), removing the period at the end of paragraph and adding “; or”
in its place; and
f. Adding paragraph (h)(1)(iv).
The additions read as follows:
§ 425.630 Option to receive advance investment payments.
* * * * *
(g) * * *
(5) If an ACO notifies CMS that it no longer wants to participate in the advance
investment payment option but does want to continue its participation in the Shared Savings
Program, the ACO must repay all outstanding advance investment payments it received. CMS
will provide written notice to the ACO of the amount due and the ACO must pay such amount no
later than 90 days after the receipt of such notification.
(6) If CMS terminates the participation agreement of an ACO that has an outstanding
balance of advance investment payments owed to CMS, the ACO must repay any outstanding
advance investment payments it received. CMS will provide written notification to the ACO of
the amount due and the ACO must pay such amount no later than 90 days after the receipt of
such notification.
* * * * *
(h) * * *
(1) * * *
(iv) Voluntarily terminates payments of advance investment payments but continues its
participation in the Shared Savings Program.
* * * * *
71. Section 425.640 is added to read as follows:
§ 425.640 Option to receive prepaid shared savings.
(a) Purpose. Prepaid shared savings provide an additional cash flow option to ACOs with
a history of earning shared savings that will encourage their investment in activities that reduce
costs for the Medicare program and beneficiaries and improve the quality of care provided to
their assigned beneficiaries.
(b) Eligibility. An ACO is eligible to receive prepaid shared savings in an agreement
period as specified in this section if CMS determines that all of the following criteria are met:
(1) The ACO is a renewing ACO as defined under § 425.20 entering an agreement period
beginning on January 1, 2026, or in subsequent years.
(2) The ACO must have received a shared savings payment for the most recent
performance year that:
(i) Occurred prior to the agreement period for which the ACO has applied to receive
prepaid shared savings; and
(ii) CMS has conducted financial reconciliation.
(3) The ACO must have a positive prior savings adjustment for the agreement period for
which the ACO has applied to receive prepaid shared savings as calculated pursuant to §
425.658.
(4) The ACO does not have any outstanding shared losses or advance investment
payments that have not yet been repaid to CMS after reconciliation for the most recent
performance year for which CMS completed financial reconciliation.
(5) If the ACO received prepaid shared savings in the current agreement period or a prior
agreement period, the ACO must have fully repaid the amount of prepaid shared savings
received through the most recent performance year for which CMS has completed financial
reconciliation.
(6) The ACO is participating in Levels C through E of the BASIC track or the
ENHANCED track during the agreement period in which it would receive prepaid shared
savings.
(7) The ACO has in place an adequate repayment mechanism in accordance with §
425.204(f) that can be used to recoup outstanding prepaid shared savings.
(8) During the agreement period immediately preceding the agreement period in which
the ACO would receive prepaid shared savings, the ACO:
(i) Met the quality performance standard as specified under § 425.512; and
(ii) Has not been determined by CMS to have avoided at-risk beneficiaries as specified
under § 425.316(b)(2).
(c) Application procedure. To obtain a determination regarding whether an ACO may
receive prepaid shared savings, the ACO must submit to CMS a complete supplemental
application with its application to renew for a new agreement period in the Shared Savings
Program (submitted pursuant to § 425.202) in the form and manner and by a deadline specified
by CMS.
(d) Application contents and review--(1) General. An ACO must submit to CMS
supplemental application information sufficient for CMS to determine whether the ACO is
eligible to receive prepaid shared savings. In addition, the ACO must submit a proposed spend
plan as part of the supplemental application information.
(2) Spend plan. The ACO’s spend plan must:
(i) Describe how an ACO receiving prepaid shared savings will spend the payments
during the first performance year in which it will receive prepaid shared savings. The spend plan
must be updated annually for each performance year of the agreement period during which the
ACO receives prepaid shared savings.
(ii) Identify the categories of items and services that will be purchased and investments
that will be made in the ACO with prepaid shared savings (consistent with the allowable uses
under paragraph (e) of this section), the dollar amounts to be spent on such categories,
information about which groups of beneficiaries the ACO expects to receive direct beneficiary
services that will be purchased with prepaid shared savings, how direct beneficiary services will
be distributed to beneficiaries and how such services support the care of beneficiaries,
descriptions of the investments that will be made in the ACO with prepaid shared savings, and
such other information as may be specified by CMS.
(iii) Include an attestation that the ACO will not discriminate on the basis of race, color,
religion, sex, national origin, disability, or age with respect to their use of prepaid shared savings.
(iv) Include the ACO’s communication strategy for notifying CMS and any impacted
beneficiaries if an ACO will no longer be providing any direct beneficiary services that had
previously been provided by the ACO using prepaid shared savings.
(3) CMS review. CMS will review the supplemental application information to determine
whether an ACO meets the eligibility criteria and other requirements necessary to receive
prepaid shared savings and will approve or deny the ACO’s prepaid shared savings application
accordingly. CMS may review an ACO's spend plan at any time and require the ACO to modify
its spend plan to comply with the requirements of this paragraph (d) and paragraph (e) of this
section.
(e) Use and management of prepaid shared savings--(1) Allowable uses. An ACO must
use prepaid shared savings to improve the quality and efficiency of items and services furnished
to beneficiaries by investing in staffing, healthcare infrastructure, and direct beneficiary services.
Expenditures of prepaid shared savings must comply with paragraph (e)(2) of this section, the
beneficiary incentive provision at § 425.304(a), (b), and (d), and all other applicable laws and
regulations.
(i) An ACO may spend up to 50 percent of its estimated annual prepaid shared savings on
staffing and healthcare infrastructure in each performance year.
(ii) An ACO may spend up to 100 percent, but not less than 50 percent, of its estimated
annual prepaid shared savings on direct beneficiary services in each performance year.
(2) Prohibited uses. An ACO may not use prepaid shared savings for any expense other
than those allowed under paragraph (e)(1) of this section. Prohibited uses include the following –
(i) Management company or parent company profit;
(ii) Performance bonuses;
(iii) Provision of medical services covered by Medicare;
(iv) Cash or cash equivalent payments to patients;
(v) Items or activities unrelated to ACO operations or care of beneficiaries; and
(vi) In the case of an ACO participating in Levels C through E of the BASIC track or the
ENHANCED track, the repayment of any shared losses incurred as specified in a written notice
in accordance with § 425.605(e)(2) or § 425.610(h)(2), respectively.
(3) Duration for spending payments. An ACO must spend all prepaid shared savings in
the agreement period in which they are received. An ACO must repay to CMS any unspent funds
remaining at the end of each agreement period. Any unspent funds received for a performance
year must be reallocated in the spend plan for the ACO’s next performance year. When
reallocated in the spend plan for the next performance year, the total unspent funds in each
category must be reallocated within their originally indicated category specified in accordance
with paragraph (d)(2) of this section. If an ACO fails to spend a majority of the prepaid shared
savings they receive in a performance year, CMS may withhold future quarterly payments until
the ACO spends the funding they have already received and reports this spending to CMS
through an updated spend plan.
(f) Payment & payment methodology. An ACO determined eligible pursuant to paragraph
(b) of this section receives quarterly prepaid shared savings payments equal to the maximum
quarterly payment amount calculated pursuant to the methodology specified in paragraphs (f)(2)
through (4) of this section unless the ACO elects to receive a lesser amount pursuant to
paragraph (f)(6) of this section. CMS notifies in writing each ACO of its determination of the
amount of prepaid shared savings and the notice will inform the ACO of its right to request
reconsideration review in accordance with the procedures specified in subpart I of this part. If
CMS does not make any prepaid shared savings payment, the notice will specify the reason(s)
why and inform the ACO of its right to request reconsideration review in accordance with the
procedures specified in subpart I.
(1) Frequency of payments. An ACO will receive quarterly prepaid shared savings
payments for the entirety of the ACO's agreement period unless the payment is withheld or
terminated pursuant to paragraph (h) of this section. If an ACO’s quarterly payment is withheld
or terminated pursuant to paragraph (h), the ACO will not receive additional or catch-up
payments if quarterly prepaid shared savings payments are later resumed.
(2) Calculating the prepaid shared savings multiplier. (i) Calculate total per capita
savings or losses for each performance year that constitutes BY1 and BY2 of the agreement
period in which the ACO receives prepaid shared savings. Per capita savings or losses will be set
to zero for a performance year if the ACO was not reconciled for the performance year.
(ii) Take the simple average of the per capita savings or losses calculated in paragraph
(f)(2)(i) of this section, including values of zero, if applicable.
(iii) Apply a proration factor to account for any upward growth in the ACO's assigned
population in BY1 and BY2 of the agreement period in which the ACO receives prepaid shared
savings as compared to the size of the assigned population when the ACO was reconciled for the
corresponding performance years in its prior agreement period.
(iv) Adjust the pro-rated average per capita amount computed in paragraph (f)(2)(iii) of
this section by multiplying by 50 percent.
(v) The prepaid shared savings multiplier is the lesser of the following:
(A) Two-thirds of the pro-rated, adjusted average per capita amount computed in
paragraph (f)(2)(iv) of this section.
(B) 5 percent of national per capita expenditures for Parts A and B services under the
original Medicare fee-for-service program in BY2 for assignable beneficiaries identified for the
12-month calendar year corresponding to BY2 using data from the CMS Office of the Actuary
and expressed as a single value by taking a person-year weighted average of the Medicare
enrollment type-specific values.
(3) Recalculation of the prepaid shared savings multiplier during an agreement period.
For the first performance year during the term of the agreement period in which the ACO
receives prepaid shared savings, the ACO's prepaid shared savings multiplier is recalculated for
changes in per capita shared savings or losses for the performance years used in the calculation
of the prepaid shared savings multiplier as a result of issuance of a revised initial determination
under § 425.315. For the second and each subsequent performance year during the term of the
agreement period in which the ACO receives prepaid shared savings, the ACO's prepaid shared
savings multiplier is recalculated for the following, as applicable: For the addition and removal
of ACO participants or ACO providers/suppliers in accordance with § 425.118(b), for a change
to the ACO's beneficiary assignment methodology selection under § 425.226(a)(1), for a change
to the beneficiary assignment methodology specified in subpart E of this part, and for changes in
per capita shared savings or losses for the performance years used in the calculation of the
prepaid shared savings multiplier as a result of issuance of a revised initial determination under §
425.315. To recalculate the prepaid shared savings multiplier, CMS does the following:
(i) Takes into account changes to the ACO's savings or losses for a performance year for
either of the 2 years that constitute BY1 and BY2 of the agreement period for which the ACO
receives prepaid shared savings under paragraph (f)(2)(i) of this section, including values of
zero, if applicable, as a result of issuance of a revised initial determination under § 425.315,
when calculating the simple average of the per capita savings or losses calculated in paragraph
(f)(2)(ii) of this section.
(ii) Redetermines the proration factor used in calculating the prepaid shared savings
multiplier under paragraph (f)(2)(iii) of this section to account for changes in the ACO's assigned
beneficiary population in the benchmark years of the ACO's agreement period in which the ACO
receives prepaid shared savings due to the addition and removal of ACO participants or ACO
providers/suppliers in accordance with § 425.118(b), a change to the ACO's beneficiary
assignment methodology selection under § 425.226(a)(1), or changes to the beneficiary
assignment methodology under subpart E of this part.
(4) Calculating the maximum quarterly payment amount. For each quarter for each
performance year, the maximum quarterly prepaid shared savings amount is equal to the product
of one-fourth of the prepaid shared savings multiplier calculated in paragraph (f)(2)(v) of this
section or recalculated according to paragraph (f)(3) of this section and the ACO’s performance
year assigned beneficiary person years calculated from the ACO’s most recent assignment list.
(5) Estimated annual payment amount calculation methodology. For the purposes of
determining the amount of prepaid shared savings permitted to be allocated to the uses specified
in paragraph (e) of this section during each performance year, the estimated annual prepaid
shared savings amount can be calculated by multiplying the first quarterly payment amount the
ACO receives in each performance year by four. If an ACO’s maximum quarterly payments
decrease over the performance year, the ACO will not be subject to compliance action solely
because it spent more than 50 percent of the actual annual amount of prepaid shared savings it
received during that PY on staffing and healthcare infrastructure, as long as it did not spend more
than 50 percent of the originally estimated annual maximum prepaid shared savings amount on
staffing and healthcare infrastructure.
(6) ACO selection of quarterly payment amount. An ACO may request a smaller
quarterly payment amount from CMS in a form and manner and by a deadline specified by CMS.
(g) Recoupment and recovery of prepaid shared savings; notice of bankruptcy. (1) CMS
will recoup prepaid shared savings made to an ACO from any shared savings the ACO earns
until CMS has recouped in full the amount of prepaid shared savings made to the ACO. CMS
will carry forward any remaining balance owed to subsequent performance year(s) in which the
ACO achieves shared savings.
(2) If the amount of shared savings earned by the ACO is revised upward by CMS for
any reason, CMS will reduce the redetermined amount of shared savings by the amount of

prepaid shared savings made to the ACO as of the date of the redetermination. If the amount of
shared savings earned by the ACO is revised downward by CMS for any reason, the ACO will
not receive a refund of any portion of the prepaid shared savings previously recouped or
otherwise repaid, and any prepaid shared savings that are now outstanding due to the revision in
earned shared savings must be repaid to CMS upon request.
(3) If an ACO has an outstanding balance of prepaid shared savings after the calculation
of shared savings or losses for the final performance year of an agreement period in which an
ACO receives prepaid shared savings, the ACO must repay any outstanding amount of prepaid
shared savings it received in full upon request from CMS. CMS will provide written notification
to the ACO of the amount due and the ACO must pay such amount no later than 90 days after the
receipt of notification. If the ACO fails to repay any outstanding amount of prepaid shared
savings within 90 days of that written notification, CMS will recoup any outstanding balance of
prepaid shared savings from the ACO’s repayment mechanism established under § 425.204(f).
CMS may also recover any outstanding amount of prepaid shared savings owed by recouping
from any future shared savings the ACO may be eligible to receive in a subsequent agreement
period.
(4) Except as provided in paragraph (g)(4)(ii) of this section, if an ACO or CMS
terminates the ACOs participation agreement during the agreement period in which it received
prepaid shared savings, the ACO must repay all outstanding prepaid shared savings it received in
full upon request from CMS.
(i) CMS will provide written notification to the ACO of the amount due and the ACO
must pay such amount no later than 90 days after the receipt of notification. If the ACO fails to
repay within 90 days, CMS will recoup any outstanding balance from the ACO’s repayment
mechanism established under § 425.204(f).
(ii) If the ACO terminates its current participation agreement under § 425.220 and
immediately enters a new agreement period to continue its participation in the program, CMS
may recover the amount owed by recouping from any future shared savings the ACO may be
eligible to receive in subsequent agreement periods.
(5)(i) If an ACO has filed a bankruptcy petition, whether voluntary or involuntary, the
ACO must provide written notice of the bankruptcy to CMS and to the U.S. Attorney’s Office in
the district where the bankruptcy was filed, unless final payment for the agreement period has
been made by either CMS or the ACO and all administrative or judicial review proceedings
relating to any payments under the Shared Savings Program have been fully and finally resolved.
(ii) The notice of bankruptcy must be sent by certified mail no later than 5 days after the
petition has been filed and must contain a copy of the filed bankruptcy petition (including its
docket number). The notice to CMS must be addressed to the CMS Office of Financial
Management at 7500 Security Boulevard, Mailstop C3-01-24, Baltimore, MD 21244 or such
other address as may be specified on the CMS website for purposes of receiving such notices.
(h) Withholding or termination of prepaid shared savings--(1) General. Except as
provided in paragraph (h)(2) of this section, CMS may withhold or terminate an ACO’s prepaid
shared savings during an agreement period if–
(i) The ACO fails to comply with the requirements of this section;
(ii) The ACO meets any of the grounds for ACO termination set forth in § 425.218(b);
(iii) The ACO fails to earn sufficient shared savings in a performance year to repay the
prepaid shared savings they received during that performance year;
(iv) CMS determines that the ACO is not expected to earn shared savings in a
performance year during the agreement period in which the ACO received prepaid shared
savings based on a rolling 12-month window of beneficiary claims data or year to date
beneficiary claims data;
(v) The ACO falls below 5,000 assigned beneficiaries;
(vi) The ACO fails to spend the majority of prepaid shared savings they receive in a
performance year; or
(vii) The ACO requests that CMS withhold a future quarterly prepaid shared savings
payment.
(2) Eligibility sanction. CMS must terminate an ACO’s prepaid shared savings if—
(i) The ACO fails to maintain an adequate repayment mechanism in accordance with §
425.204(f); or
(ii) The ACO fails to meet the quality performance standard as specified under § 425.512
or is subject to a pre-termination action after CMS determined the ACO avoided at-risk
beneficiaries as specified under § 425.316(b)(2).
(3) No additional payments. If CMS withholds or terminates a quarterly payment
pursuant to this paragraph (h), the ACO will not receive additional or catch-up payments if
quarterly payments of prepaid shared savings are later resumed.
(4) No pre-termination actions. CMS may immediately terminate an ACO’s prepaid
shared savings under paragraphs (h)(1) and (2) of this section without taking any of the pre-
termination actions set forth in § 425.216.
(i) Reporting information on prepaid shared savings. The ACO must report information
on its receipt of and use of prepaid shared savings, as follows:
(1) The ACO must publicly report information about the ACO's use of prepaid shared
savings for each performance year, in accordance with § 425.308(b)(10).
(2) In a form and manner and by a deadline specified by CMS, the ACO must report to
CMS the same information it is required to publicly report under § 425.308(b)(10).
§ 425.650 [Amended]
72. Section 425.650 is amended in paragraph (a) by removing the reference “425.660”
and adding in its place the reference “425.662”.
73. Section 425.652 is amended by—
a. Revising paragraph (a)(8) and paragraph (a)(9) introductory text;
b. Redesignating paragraphs (a)(9)(v) and (vi) as paragraphs (a)(9)(vi) and (vii),
respectively;
c. Adding new paragraph (a)(9)(v);
d. Revising newly redesignated paragraph (a)(9)(vi); and
e. Adding paragraphs (a)(9)(viii) and (ix).
The revisions and additions read as follows:
§ 425.652 Establishing, adjusting, and updating the benchmark for agreement periods
beginning on January 1, 2024, and in subsequent years.
(a) * * *
(8) Adjusts the historical benchmark, if applicable:
(i) For agreement periods beginning on January 1, 2024, except as provided in paragraph
(a)(8)(i)(C) of this section, CMS adjusts the historical benchmark based on the ACO's regional
service area expenditures (as specified under § 425.656), or for savings generated by the ACO, if
any, in the 3 most recent years prior to the start of the agreement period (as specified under §
425.658). CMS does all of the following to determine the adjustment, if any, applied to the
historical benchmark:
(A) Computes the regional adjustment in accordance with § 425.656 and the prior savings
adjustment in accordance with § 425.658.
(B) If an ACO is not eligible to receive a prior savings adjustment under §
425.658(b)(3)(i), and the regional adjustment, expressed as a single value as described in §
425.656(d), is positive, the ACO will receive an adjustment to its benchmark equal to the
positive regional adjustment amount. The adjustment will be calculated as described in §
425.656(c) and applied separately to the following populations of beneficiaries: ESRD, disabled,
aged/dual eligible Medicare and Medicaid beneficiaries, and aged/non-dual eligible Medicare
and Medicaid beneficiaries.
(C) If an ACO is not eligible to receive a prior savings adjustment under §
425.658(b)(3)(i), and the regional adjustment, expressed as a single value as described in §
425.656(d), is negative or zero, the ACO will not receive an adjustment to its benchmark.
(D) If an ACO is eligible to receive a prior savings adjustment and the regional
adjustment, expressed as a single value as described in § 425.656(d), is positive, the ACO will
receive an adjustment to its benchmark equal to the higher of the following:
(1) The positive regional adjustment amount. The adjustment will be calculated as
described in § 425.656(c) and applied separately to the following populations of beneficiaries:
ESRD, disabled, aged/dual eligible Medicare and Medicaid beneficiaries, and aged/non-dual
eligible Medicare and Medicaid beneficiaries.
(2) The prior savings adjustment. The adjustment will be calculated as described in §
425.658(c) and applied as a flat dollar amount to the following populations of beneficiaries:
ESRD, disabled, aged/dual eligible Medicare and Medicaid beneficiaries, and aged/non-dual
eligible Medicare and Medicaid beneficiaries.
(E) If an ACO is eligible to receive a prior savings adjustment and the regional
adjustment, expressed as a single value as described in § 425.656(d), is negative or zero, the
ACO will receive an adjustment to its benchmark equal to the prior savings adjustment. The
adjustment will be calculated as described in § 425.658(c) and applied as a flat dollar amount to
the following populations of beneficiaries: ESRD, disabled, aged/dual eligible Medicare and
Medicaid beneficiaries, and aged/non-dual eligible Medicare and Medicaid beneficiaries.
(ii) For agreement periods beginning on January 1, 2025, and in subsequent years, except
as provided in paragraph (a)(8)(ii)(B)(2) of this section, CMS adjusts the historical benchmark
based on the ACO's regional service area expenditures (as specified under § 425.656), for
savings generated by the ACO, if any, in the 3 most recent years prior to the start of the
agreement period (as specified under § 425.658), or to account for the ACO serving higher
proportions of underserved beneficiaries (as specified in § 425.662). CMS does all of the
following to determine the adjustment, if any, applied to the historical benchmark:
(A) Computes the regional adjustment in accordance with § 425.656, the prior savings
adjustment in accordance with § 425.658, and the health equity benchmark adjustment (HEBA)
in accordance with § 425.662.
(B) Compares the regional adjustment, expressed as a single value as described in §
425.656(d), the per capita prior savings adjustment determined in § 425.658(c), if any, and the
HEBA determined in § 425.662(b), if any, to determine the adjustment applied to the historical
benchmark.
(1) The ACO will receive the highest of the positive adjustments for which it is eligible.
The adjustment will be calculated as described in § 425.656(c), § 425.658(c), or § 425.662(b),
respectively, and applied separately to the following populations of beneficiaries: ESRD,
disabled, aged/dual eligible Medicare and Medicaid beneficiaries, and aged/non-dual eligible
Medicare and Medicaid beneficiaries.
(2) If an ACO is not eligible to receive a prior savings adjustment under
§ 425.658(b)(3)(i) or the HEBA under § 425.662(b)(3), and the regional adjustment, expressed
as a single value as described in § 425.656(d), is negative or zero, the ACO will not receive an
adjustment to its benchmark.
(9) For the first performance year during the term of the agreement period, the ACO's
benchmark is adjusted for the following, as applicable: For changes in values used in benchmark
calculations in accordance with § 425.316(b)(2)(ii)(B) or (C) due to compliance action to address
avoidance of at-risk beneficiaries or as a result of issuance of a revised initial determination
under § 425.315, and for changes in values used in benchmark calculations as a result of the
performance year being affected by significant, anomalous, and highly suspect billing under
§ 425.672. For the second and each subsequent performance year during the term of the
agreement period, the ACO's benchmark is adjusted for the following, as applicable: For the
addition and removal of ACO participants or ACO providers/suppliers in accordance with §
425.118(b), for a change to the ACO's beneficiary assignment methodology selection under §
425.226(a)(1), for a change to the beneficiary assignment methodology specified in subpart E of
this part, for a change in the CMS-HCC risk adjustment methodology used to calculate
prospective HCC risk scores under § 425.659, for changes in values used in benchmark
calculations in accordance with § 425.316(b)(2)(ii)(B) or (C) due to compliance action to address
avoidance of at-risk beneficiaries or as a result of issuance of a revised initial determination
under § 425.315, and for changes in values used in benchmark calculations as a result of the
performance year being affected by significant, anomalous, and highly suspect billing under
§ 425.672. To adjust the benchmark, CMS does the following:
* * * * *
(v) Redetermines the HEBA scaler used in calculating the HEBA under § 425.662(b)(2)
to account for changes in the ACO’s regional adjustment or prior savings adjustment in
accordance with paragraphs (a)(9)(ii) through (iv) of this section.
(vi) In accordance with paragraph (a)(8) of this section, CMS redetermines the
adjustment to the historical benchmark based on the redetermined regional adjustment (as
specified under § 425.656), the prior savings adjustment (as specified under § 425.658), or the
HEBA (as specified under § 425.662) if applicable.
* * * * *
(viii) Recalculates benchmark year expenditures to account for the impact of improper
payments, for the benchmark year corresponding to a performance year for which CMS issued a
revised initial determination under § 425.315. In recalculating expenditures for the benchmark
year, CMS applies the calculation methodology applied in recalculating expenditures for the
corresponding performance year in accordance with § 425.674.
(ix) Recalculates expenditures used in Shared Savings Program benchmark calculations
under this section, and as applicable under §§ 425.654 through 425.662, to exclude the same
HCPCS or CPT codes identified as displaying significant, anomalous, and highly suspect billing
patterns in calculation of performance year expenditures, in accordance with § 425.672.
* * * * *
74. Section 425.655 is amended by revising paragraph (d)(2) to read as follows:
§ 425.655 Calculating the regional risk score growth cap adjustment factor.
* * * * *
(d) * * *
(2) Determines the aggregate growth in regional risk scores by calculating a weighted
average of the growth in regional prospective HCC risk scores or demographic risk scores, as
applicable, across the populations described in paragraph (d)(1) of this section. When calculating
the weighted average growth in prospective HCC risk scores or demographic risk scores, as
applicable, the weight applied to the growth in risk scores for each Medicare enrollment type is
equal to the product of the ACO's historical benchmark expenditures, adjusted in accordance
with § 425.652(a)(8), for that enrollment type and the ACO's performance year assigned
beneficiary person years for that enrollment type.
* * * * *
75. Section 425.658 by revising paragraph (d) to read as follows:
§ 425.658 Calculating the prior savings adjustment to the historical benchmark.
* * * * *
(d) Applicability of the prior savings adjustment. CMS compares the per capita prior
savings adjustment determined in paragraph (c)(1) of this section with the regional adjustment,
expressed as a single value as described in § 425.656(d), and the HEBA as determined in §
425.662(b), if any, to determine the adjustment, if any, that will be applied to the ACO's
benchmark in accordance with § 425.652(a)(8).
* * * * *
76. Section 425.662 is added to read as follows:
§ 425.662 Calculating the health equity adjustment to the historical benchmark.
(a) General. For agreement periods beginning on January 1, 2025, and in subsequent
years, CMS calculates a health equity adjustment to the historical benchmark (HEBA) to account
for ACOs serving higher proportions of underserved beneficiaries.
(b) Calculation of the health equity benchmark adjustment. To calculate the adjustment
described in paragraph (a) of this section, CMS does all of the following:
(1) Calculates the weighted average of the ACO’s third benchmark year (BY3) national
per capita expenditure amounts across the following populations of beneficiaries, where the
weights are the ACO's BY3 proportion of assigned beneficiaries for that enrollment type:
(i) ESRD.
(ii) Disabled.
(iii) Aged/dual eligible Medicare and Medicaid beneficiaries.
(iv) Aged/non-dual eligible Medicare and Medicaid beneficiaries.
(2) Calculates the HEBA scaler as the difference between 5 percent of the national per
capita expenditure amount, expressed as single value as calculated in paragraph (b)(1) of this
section, and the higher of: the regional adjustment, expressed as a single value as described in
§ 425.656(d); the per capita prior savings adjustment determined in § 425.658(c); or no
adjustment, in the case where the regional adjustment is negative and the ACO is not eligible for
the prior savings adjustment under § 425.658(b)(3)(i).
(3) Determines the ACO’s eligibility for the HEBA based on the proportion of the ACO's
assigned beneficiaries for the performance year who are enrolled in the Medicare Part D low-
income subsidy (LIS) or dually eligible for Medicare and Medicaid. An ACO is only eligible for
the HEBA if this proportion is greater than or equal to 20 percent. An ACO with a proportion
less than 20 percent is ineligible to receive a HEBA.
(4) Calculates the HEBA. If the ACO is eligible for the HEBA as determined in
paragraph (b)(3) of this section, the HEBA is equal to the product of the HEBA scaler calculated
in paragraph (b)(2) of this section and the proportion of the ACO's assigned beneficiaries for the
performance year who are enrolled in the Medicare Part D LIS or dually eligible for Medicare
and Medicaid.

(c) Applicability of the HEBA. CMS compares the HEBA determined in paragraph (b)(4)
of this section with the regional adjustment, expressed as a single value as described in §
425.656(d), and the per capita prior savings adjustment determined in § 425.658(c), if any, to
determine the adjustment, if any, that will be applied to the ACO's benchmark in accordance
with § 425.652(a)(8)(ii).
§§ 425.664 through 425.669 [Added and Reserved]
77. Sections 425.664 through 425.669 are added and reserved.
78. Section 425.672 is added to read as follows:
§ 425.672 Adjustments to mitigate the impact of significant, anomalous, and highly suspect
billing activity on Shared Savings Program financial calculations involving calendar year
2024 or subsequent calendar years.
(a) General. This section describes adjustments CMS may make to Shared Savings
Program calculations to mitigate the impact of significant, anomalous, and highly suspect billing
activity occurring in calendar year 2024 or subsequent calendar years.
(b) Significant, anomalous, and highly suspect billing activity for a HCPCS or CPT code
impacting Shared Savings Program calculations. CMS, at its sole discretion, may determine that
the billing of one or more specified HCPCS or CPT codes represents significant, anomalous, and
highly suspect billing activity for a calendar year that warrants adjustment to calculations made
under this part.
(c) Applicability of adjustments to performance year and benchmark year calculations.
Notwithstanding any other provision in this part, CMS adjusts the following Shared Savings
Program calculations, as applicable, to exclude all Medicare Parts A and B fee-for-service
payment amounts on claims for the specified claim types associated with a HCPCS or CPT code
identified pursuant to paragraph (b) of this section for the periods identified in paragraph (d) of
this section:
(1) Calculation of Medicare Parts A and B fee-for-service expenditures for an ACO’s
assigned beneficiaries for all purposes including the following: Establishing, adjusting, updating,
and resetting the ACO’s historical benchmark and determining performance year expenditures.
(2) Calculation of fee-for-service expenditures for assignable beneficiaries as used in
determining county-level fee-for-service expenditures and national Medicare fee-for-service
expenditures, including the following calculations:
(i) Determining average county fee-for-service expenditures based on expenditures for
the assignable population of beneficiaries in each county in the ACO’s regional service area
according to §§ 425.601(c) and 425.654(a) for purposes of calculating the ACO’s regional fee-
for-service expenditures.
(ii) Determining the 99
th
percentile of national Medicare fee-for-service expenditures for
assignable beneficiaries for purposes of the following:
(A) Truncating assigned beneficiary expenditures used in calculating benchmark
expenditures under §§ 425.601(a)(4) and 425.652(a)(4), and performance year expenditures
under §§ 425.605(a)(3) and 425.610(a)(4).
(B) Truncating expenditures for assignable beneficiaries in each county for purposes of
determining county fee-for-service expenditures according to §§ 425.601(c)(3) and
425.654(a)(3).
(C) Truncating expenditures for assignable beneficiaries for purposes of determining
truncated national per capita fee-for service expenditures for purposes of calculating the ACPT
according to § 425.660(b)(3).
(iii) Determining truncated national per capita fee-for-service Medicare expenditures for
assignable beneficiaries for purposes of calculating the ACPT according to § 425.660(b)(3).
(iv) Determining national per capita expenditures for Parts A and B services under the
original Medicare fee-for-service program for assignable beneficiaries for purposes of capping
the regional adjustment to the ACO's historical benchmark according to §§ 425.601(a)(8)(ii)(C)
and 425.656(c)(3), capping the prior savings adjustment according to § 425.658(c)(1)(ii),
capping the prepaid shared savings multiplier according to § 425.640(f)(2)(v), and calculating
the HEBA scaler according to § 425.662(b)(2).
(v) Determining national growth rates that are used as part of the blended growth rates
used to trend forward BY1 and BY2 expenditures to BY3 according to §§ 425.601(a)(5)(ii) and
425.652(a)(5)(ii) and as part of the blended growth rates used to update the benchmark according
to §§ 425.601(b)(2) and 425.652(b)(2)(i).
(3) Calculation of Medicare Parts A and B fee-for-service revenue of ACO participants
for purposes of calculating the ACO’s loss recoupment limit under the BASIC track as specified
in § 425.605(d).
(4) Calculation of total Medicare Parts A and B fee-for-service revenue of ACO
participants and total Medicare Parts A and B fee-for-service expenditures for the ACO's
assigned beneficiaries for purposes of identifying whether an ACO is a high revenue ACO or
low revenue ACO, as defined under § 425.20, determining an ACO's eligibility to receive
advance investment payments according to § 425.630, and determining whether an ACO
qualifies for a shared savings payment under § 425.605(h).
(5) Calculation or recalculation of the amount of the ACO’s repayment mechanism
arrangement according to § 425.204(f)(4).
(d) Periods of adjustment. CMS adjusts the Shared Savings Program calculations
identified in paragraph (c) of this section for significant, anomalous, and highly suspect billing
activity identified for calendar year 2024 or subsequent calendar years as follows:
(1) The calendar year for which the significant, anomalous, and highly suspect billing
activity was identified pursuant to paragraph (b) of this section, when it is either a performance
year or a benchmark year.
(2) The 3 most recent years prior to the start of the ACO’s agreement period used in
establishing the historical benchmark, when such a benchmark is used to reconcile the ACO for a
performance year adjusted in accordance with paragraph (d)(1) of this section.
(e) Adjustments for growth rates used in calculating the ACPT. In addition to adjustments
described in paragraph (c) of this section, CMS makes adjustments for payments associated with
a HCPCS or CPT code identified pursuant to paragraph (b) of this section for any calendar year
corresponding to BY3 in projecting per capita growth in Parts A and B fee-for-service
expenditures, according to § 425.660(b)(1), for purposes of calculating the ACPT for agreement
periods beginning on January 1, 2024, and in subsequent years.
79. Section 425.674 is added to read as follows:
§ 425.674 Accounting for the impact of improper payments on Shared Savings Program
financial calculations.
(a) General rule. Upon the reopening of an initial determination pursuant to
§ 425.315(a)(4), CMS will use the methodology specified in this section to account for the
impact of improper payments when:
(1) Determining savings or losses for the relevant performance year in accordance with
§ 425.315 in order to issue a revised initial determination.
(2) Adjusting the benchmark by recalculating benchmark year expenditures under
§§ 425.601(a)(9)(iii) and 425.652(a)(9)(viii) in the event that CMS recalculates a payment
determination and issues a revised initial determination for the corresponding performance year
in a prior agreement period, in accordance with paragraph (a)(1) of this section.
(b) Improper payment. For the purpose of this section, improper payment includes:
(1) An amount associated with a demanded overpayment determination.
(2) An amount identified in a settlement agreement or judgment, pursuant to conduct by
individuals or entities performing functions or services related to an ACO’s activities, less any
penalties or damages.
(c) Accounting for improper payments. To adjust Medicare Parts A and B fee-for-service
expenditures for improper payments CMS does the following:
(1) Identify each Shared Savings Program expenditure calculation for a performance year
or benchmark year, as calculated according to the standard methodology described in this subpart
and expressed as a per capita dollar amount, that will be adjusted for the impact of improper
payments.
(2) Determine each specific population of Medicare fee-for-service beneficiaries used to
calculate the expenditure amount identified in paragraph (c)(1) of this section. The populations
relevant for a specific expenditure calculation may include:
(i) The population of beneficiaries assigned to the ACO for calculating the ACO’s
performance year or benchmark year expenditures.
(ii) The population of assignable beneficiaries in each county in the ACO’s regional
service area for calculating county-level expenditures.
(iii) The national population of assignable beneficiaries for calculating national
assignable expenditures.
(iv) The national population of Medicare fee-for-service beneficiaries for calculating
national expenditures.
(3) Determine the per capita amount of improper payments for the performance year or
benchmark year included in the per capita Medicare Parts A and B fee-for-service expenditure
amount for a population identified in paragraph (c)(2) of this section in accordance with
paragraph (d) of this section for all providers or suppliers with identified improper payments.
(4) Subtract the per capita amount determined in paragraph (c)(3) of this section from the
expenditure calculation identified in paragraph (c)(1) of this section for the population identified
in paragraph (c)(2) of this section for each of the following populations of beneficiaries:
(i) ESRD.
(ii) Disabled.
(iii) Aged/dual eligible Medicare and Medicaid beneficiaries.
(iv) Aged/non-dual eligible Medicare and Medicaid beneficiaries.
(5) If applicable, CMS will do the following to adjust regional expenditures for improper
payments:
(i) Adjust county-level fee-for-service expenditures determined under paragraph (c)(4) of
this section, for each county in the ACO’s regional service area, for severity and case mix of
assignable beneficiaries in the county using prospective HCC risk scores. This calculation is
made for each of the populations of beneficiaries identified in paragraphs (c)(4)(i) through (iv) of
this section.
(ii) Weight the risk adjusted county-level fee-for-service expenditures determined under
paragraph (c)(5)(i) of this section according to the ACO’s proportion of assigned beneficiaries in
the county, determined in accordance with § 425.601(d)(1), § 425.603(f)(1), or § 425.654(b)(1),
as applicable, for each of the populations of beneficiaries identified in paragraphs (c)(4)(i)
through (iv) of this section.
(iii) Aggregate the values determined in paragraph (c)(5)(ii) of this section for each of the
populations of beneficiaries identified in paragraphs (c)(4)(i) through (iv) of this section across
all counties within the ACO’s regional service area.
(d) Determining the per capita amount of improper payments. CMS may use one or more
of the following approaches to determine the per capita amount that will be used to adjust
expenditure calculations identified in paragraph (c)(1) of this section:
(1) Calculate aggregate improper payments attributable to a population identified in
paragraph (c)(2) of this section for each provider or supplier that had improper payments.
(i) For improper payments associated with specific claims, CMS will do the following:
(A) For improper payments to a provider or supplier that correspond to payment amounts
on claims or line items that were used in a Shared Savings Program calculation identified in
paragraph (c)(1) of this section, and subsequently adjusted after the 3-month claims run out
period, CMS will sum the improper payment amounts across all such claims or line items with
dates of service during the period used to calculate performance year or benchmark year
expenditures for the population identified in paragraph (c)(2) of this section.
(B) In the event CMS determines it is necessary to account for the impact of improper
payments on Shared Savings Program financial calculations by adjusting the payment amounts
for a specific HCPCS or CPT code billed by the provider or supplier for the population identified
in paragraph (c)(2) of this section, CMS will do the following --
(1) Identify the applicable claims or line items with dates of service during the period
used to calculate performance year or benchmark year expenditures processed before the end of
the applicable 3-month claims run out period;
(2) Sum the claim or line item payment amounts, on the claims or line items identified in
paragraph (d)(1)(i)(B)(1) of this section; and
(3) If applicable, multiply the sum calculated in paragraph (d)(1)(i)(B)(2) of this section
by a scaling factor to compute the payment differential between the HCPCS or CPT code that
was improperly billed and a CMS-identified alternate code.
(ii) For aggregate improper payment amounts that are not linked to specific claims or line
items, CMS will calculate the amount attributable to the population identified in paragraph (c)(2)
of this section by applying a proration factor to the aggregate improper payment amount
identified for that provider or supplier. CMS calculates the proration factor as follows:
(A) The denominator of the proration factor is total Medicare Parts A and B claim or line
item payment amounts to the provider or supplier for all fee-for-service beneficiaries on claims
of specified claim types for the time period associated with the aggregate improper payment
amount identified for the provider or supplier that were made before the end of the applicable 3-
month claims run out period.
(B) The numerator of the proration factor is the portion of the total from the denominator,
in paragraph (d)(1)(ii)(A) of this section, that CMS determines is attributable to the population
identified in paragraph (c)(2) of this section with dates of service during the period used to
calculate expenditures for the applicable performance year or benchmark year.
(2) Sum the amounts calculated pursuant to paragraph (d)(1) of this section attributable to
a population identified in paragraph (c)(2) of this section across all providers or suppliers that
had identified improper payments.
(3) Take the lesser of the following two values—
(i) The sum from paragraph (d)(2) of this section; or
(ii) Total Medicare Parts A and B claim or line item payment amounts to all providers or
suppliers that had improper payments for the population identified in paragraph (c)(2) of this
section on claims of specified claim types with dates of service within the performance year or
benchmark year made before the end of the applicable 3-month claims run out period.
(4) Express the lesser-of amount from paragraph (d)(3) of this section as a per capita
value by dividing by the total beneficiary person years in the population identified in paragraph
(c)(2) of this section for the applicable performance year or the benchmark year.
80. Part 427 is added to read as follows:
PART 427--MEDICARE PART B DRUG INFLATION REBATE PROGRAM
Subpart A—General Provisions
Sec.
427.10 Basis and scope.
427.20 Definitions.
Subpart B—Determination of Part B Rebatable Drugs
427.100 Definitions.
427.101 Identification of Part B rebatable drugs.
Subpart C—Coinsurance Adjustment and Adjusted Medicare Payment for Part B Rebatable
Drugs with Price Increases Faster than Inflation
427.200 Definitions.
427.201Computation of beneficiary coinsurance and adjusted Medicare Payment for Part B
rebatable drugs with price increases faster than inflation.
Subpart D—Determination of the Rebate Amount for Part B Rebatable Drugs
427.300 Definitions.
427.301 Calculation of the total Part B rebate amount to be paid by manufacturers.
427.302 Calculation of the per unit Part B drug rebate amount.
427.303 Determination of total number of billing units.
427.304 Adjustments for changes to billing and payment codes.
Subpart E—Reducing the Rebate Amount for Part B Rebatable Drugs in Shortage and When
There Is a Severe Supply Chain Disruption
427.400 Definitions.
427.401 Reducing the rebate amount for Part B rebatable drugs currently in shortage.
427.402 Reducing the rebate amount for certain Part B rebatable drugs when there is a severe
supply chain disruption.
Subpart F—Reports of Rebate Amounts, Reconciliation, Suggestion of Error, and Payments
427.500 Definitions.
427.501 Rebate Reports and reconciliation.
427.502 Rebate Reports for applicable calendar quarters in calendar years 2023 and 2024.
427.503 Suggestion of Error.
427.504 Manufacturer access to Rebate Reports.
427.505 Deadline and process for payment of rebate amount.
Subpart G—Enforcement of Manufacturer Payment of Rebate Amounts
427.600 Civil money penalty notice and appeals procedures.
Authority: 42 U.S.C. 1395w-3a(i), 1302, and 1395hh.
Subpart A—General Provisions
§ 427.10 Basis and scope.
(a) Basis. This part implements section 1847A(i) of the Social Security Act (“the Act”).
(b) Scope. This part sets forth the requirements of the Medicare Part B Drug Inflation
Rebate Program, which requires, for each calendar quarter, manufacturers to pay rebates for
certain single source drugs and biological products with prices that increase faster than the rate
of inflation.
(c) Severability. Were any provision of this part to be held invalid or unenforceable by
its terms, or as applied to any person or circumstance, such provisions would be severable from
this part and the invalidity or unenforceability would not affect the remainder thereof or any
other part of this subchapter or the application of such provision to other persons not similarly
situated or to other, dissimilar circumstances.
§ 427.20 Definitions.
As used in this part, the following definitions apply:
Allowed charges means the amount that is inclusive of the beneficiary coinsurance and
Medicare payment for the covered Part B item or service.
Applicable calendar quarter means a calendar quarter (January 1 to March 31, April 1 to
June 30, July 1 to September 30, or October 1 to December 31) in which an inflation rebate is
applied, starting with January 1, 2023.
Applicable threshold means the amount calculated in accordance with § 427.101(c)(2).
Average sales price (ASP) means the manufacturer’s price for a quarter for a drug
represented by a particular 11-digit National Drug Code (NDC-11) calculated in accordance with
§ 414.804 of this chapter.
Benchmark period Consumer Price Index for All Urban Consumers (CPI-U) means the
CPI-U identified in § 427.302(e).
Billing and payment code means the specific code used to classify and report a drug or
biological for purposes of Medicare Part B payment. A Healthcare Common Procedure Coding
System (HCPCS) code, as established by CMS, is an example of a billing and payment code
used to describe a drug or biological and for which CMS publishes a payment amount.
Billing unit means the identifiable quantity of a drug or biological product associated
with a billing and payment code (for example, a HCPCS code), as established by CMS.
Biosimilar biological product has the meaning set forth in section 1847A(c)(6)(H) of the
Act.
CPI-U means the monthly Consumer Price Index for All Urban Consumers (United
States city average) index level for all items from the Bureau of Labor Statistics.
Food and Drug Administration (FDA) application means, for the purposes of calculating
the Part B rebate amount, a New Drug Application (NDA) or Biologics License Application
(BLA) approved by the FDA.
Final action claim means a non-rejected claim for which a Medicare payment has been
made, and for which all disputes and adjustments have been resolved.
First marketed date means the earliest date of first sale of any NDC-11 within a billing
and payment code among all products and package sizes under the same FDA application. The
first marketed date will be identified using ASP data reported by NDC-11 to CMS by a
manufacturer as required under sections 1927(b)(3)(A)(iii)(I) and 1847A(f)(2) of the Act, if
available.
Grouped billing and payment code, for the purposes of Part B rebate calculations, means
a billing and payment code, such as a HCPCS code, other than a Not Otherwise Classified
(NOC) code, that typically contains multiple drug products approved under multiple NDAs or
BLAs and may be inclusive of, but are not limited to, multiple source billing codes.
Inflation-adjusted payment amount means the amount calculated in § 427.302(g).
Manufacturer has the meaning set forth in section 1847A(c)(6)(A) of the Act.
National Drug Code (NDC) means the unique identifying prescription drug product
number that is listed with FDA identifying the product and package size and type.
Not Otherwise Classified (NOC) code means a billing and payment code, including an
unclassified, unspecified, or unlisted code, for drugs and biological products for which no
specific billing and payment code is assigned.
Part B rebatable drug means, subject to the exclusions described in § 427.101(b), a
single source drug or biological product, including a biosimilar biological product but excluding
a qualifying biosimilar biological product, for which payment is made under Part B.
Payment amount benchmark quarter means the calendar quarter identified in
§ 427.302(c).
Payment amount in the payment amount benchmark quarter means the amount identified
in § 427.302(d).
Rebate period CPI-U means the CPI-U identified in § 427.302(f).
Single source drug or biological product has the meaning set forth in section
1847A(c)(6)(D) of the Act.
Specified amount refers to the amount identified in § 427.302(b).
Subsequently approved drug means a drug first approved or licensed by the FDA after
December 1, 2020.
Unit means, with respect to a Part B rebatable drug, with respect to each National Drug
Code (including package size) associated with a drug or biological, the lowest identifiable
quantity (such as a capsule or tablet, milligram of molecules, or grams) of the drug or biological
that is dispensed, exclusive of any diluent without reference to volume measures pertaining to
liquids as reported under section 1847A(b)(2)(B) of the Act.
Subpart B—Determination of Part B Rebatable Drugs
§ 427.100 Definitions.
As used in this subpart, the following definitions apply:
EUA Declaration refers to the March 27, 2020, Emergency Use Authorization (EUA)
Declaration for Drugs and Biological Products under section 564 of the Food, Drug, and
Cosmetic (FD&C) Act.
Individual who uses such a drug or biological means a unique Medicare Part B
beneficiary who was furnished the Part B drug or biological that was covered under Part B
during the applicable calendar quarter, identified using final action claims data with dates of
service during the calendar year described in § 427.101(b)(6) and with allowed charges greater
than zero.
§ 427.101 Identification of Part B rebatable drugs.
(a) Determination of Part B rebatable drugs. (1) For each applicable calendar quarter,
CMS will:
(i) Identify single source drugs or biological products, including biosimilar biological
products, covered under Part B; and
(ii) Identify the applicable billing and payment code for each drug or biological product
identified in paragraph (a)(1)(i) of this section.
(2) For a drug or biological product identified under paragraph (a)(1) of this section,
CMS will determine whether the drug or biological product meets the exclusion criteria
described in paragraph (b) or (c) of this section as of the first day of the applicable calendar
quarter.
(3) To determine whether a drug or biological product is a Part B rebatable drug under
this section, CMS will use the most recent available data submitted to CMS by manufacturers
pursuant to section 1927(b)(3)(A)(iii) of the Act or section 1847A(f)(2), as applicable, and other
available data, including but not limited to information available at FDA.gov and information in
drug pricing compendia, as applicable.
(b) Excluded product categories. The following categories of products are not
considered Part B rebatable drugs:
(1) Qualifying biosimilar biological products. Biological products as defined under
section 1847A(b)(8)(B)(iii) of the Act.
(2) Products with historically excepted grouped billing and payment codes. Single
source drugs or biological products that were within the same billing and payment code as of
October 1, 2003, and which, as required under section 1847A(c)(6)(C)(ii) of the Act, are treated
as multiple source drugs.
(3) Products billed under a NOC code. A drug or biological product billed under a NOC
code.
(4) Radiopharmaceutical drugs and biological products. A separately payable
radiopharmaceutical drug or biological product not paid under section 1847A of the Act.
(5) Skin substitutes. A product included within the suite of cellular- and tissue-based
products that aid wound healing.
(6) Drugs with average total allowed charges under the applicable threshold. Drugs and
biological products for which the Medicare Part B average total allowed charges for a year per
individual that uses such drug or biological are below the applicable threshold, as described in
paragraph (c) of this section.
(7) Certain vaccines and other products. The following products:
(i) The vaccines described in section 1861(s)(10) of the Act, which includes the
influenza, pneumococcal, hepatitis B, and COVID-19 vaccines.
(ii) Monoclonal antibodies used for treatment or post-exposure prophylaxis of
COVID-19 that are covered and paid for under section 1861(s)(10) of the Act. This exclusion
will apply to applicable quarters until the end of the calendar year in which the EUA Declaration
ends.
(iii) Monoclonal antibodies that are used for pre-exposure prophylaxis of COVID-19 that
are covered and paid for under section 1861(s)(10) of the Act. This exclusion will apply to
applicable calendar quarters even after the year in which the EUA Declaration ends, as long as
after the EUA Declaration is terminated, these products have an FDA-approved application or
license.
(8) Generic drugs. Part B drugs approved under an Abbreviated New Drug Application
(ANDA) submitted under section 505(j) of the Federal Food, Drug, and Cosmetic Act (FD&C
Act).
(c) Drugs and biological products with average total allowed charges below the
applicable threshold. For each applicable calendar quarter, CMS will identify drugs and
biological products with Part B average total allowed charges for a year per individual that uses
such a drug or biological product that are below the applicable threshold in accordance with the
calculations described in this section. Such drugs and biological products are not considered Part
B rebatable drugs and will be excluded from the identification of Part B rebatable drugs in
paragraph (a) of this section.
(1) Average total allowed charges for a year per individual. For each drug or biological
that is identified in accordance with paragraph (a) of this section, CMS will calculate average
total allowed charges for a year per individual as follows:
(i) For single source drugs and biological products assigned to only one billing and
payment code, CMS will sum the allowed charges from final action claims greater than $0 and
divide the summed amount by the number of individuals who use such a drug or biological with
allowed charges for this billing and payment code.
(ii) For single source drugs and biological products assigned to more than one billing and
payment code, CMS will sum the allowed charges from final action claims greater than $0 for all
billing and payment codes and divide the summed amount by the number of individuals who use
such a drug or biological with allowed charges for these billing and payment codes.
(iii) For single source drugs and biological products previously crosswalked to a grouped
billing and payment code:
(A) If crosswalked to a grouped billing and payment code during the full year, CMS will
calculate the average total allowed charges per individual per year for the drug using allowed
charges and the number of individuals who used the drug or biological product based on claims
for the previously grouped billing and payment code during the year.
(B) If crosswalked to a grouped billing and payment code and later assigned to a unique
billing and payment code for part of the year, CMS will calculate average total allowed charges
per individual per year by:
(1) Summing the total allowed charges billed under the unique billing and payment code
for the drug with dates of service on or after the Medicare effective date for this unique billing
and payment code and identifying the individuals on those claims.
(2) Summing the total allowed charges on claims billed under the previously grouped
billing and payment code and identifying individuals with claims prior to the unique billing and
payment code’s effective date.
(3) Summing the total allowed charges as determined in paragraphs (c)(1)(iii)(B)(1) and
(2) of this section and dividing by the total number of individuals, de-duplicated for individuals
identified under paragraphs (c)(1)(iii)(B)(1) and (2).
(2) Applicable threshold. CMS will calculate the applicable threshold for an applicable
calendar quarter as follows:
(i) For applicable calendar quarters in 2023, the applicable threshold is equal to $100.
(ii) For applicable calendar quarters in 2024, the applicable threshold is equal to $100
increased by the percentage increase in the CPI-U for the 12-month period ending with June of
2023.
(iii) For applicable calendar quarters in each subsequent calendar year, the applicable
threshold is equal to the unrounded applicable threshold calculated for the prior calendar year
increased by the percentage increase in the CPI-U for the 12-month period ending with June of
the previous year.
(iv) If the resulting amount under paragraphs (c)(2)(i) through (iii) of this section is not a
multiple of $10, CMS will round that amount to the nearest multiple of $10.
(3) Application of the applicable threshold at the billing and payment code level. For
each applicable calendar quarter, CMS will apply the exclusion of drugs and biological products
identified in paragraph (c)(1) of this section, with average total allowed charges for a year per
individual less than the applicable threshold described in paragraph (c)(2) of this section, to
applicable billing and payment codes as follows:
(i) For single source drugs or biological products assigned to a unique billing and
payment code, CMS will exclude the assigned billing and payment code for an applicable
calendar quarter if the average total allowed charges for a year per individual are less than the
applicable threshold.
(ii) For a single source drug or biological product that is assigned to more than one
billing and payment code during a year, CMS will exclude all such assigned billing and payment
codes for an applicable calendar quarter.
(4) Definition of year. For purposes of the calculations described in this section, a year is
defined as the 4 consecutive calendar quarters beginning 6 calendar quarters before the
applicable calendar quarter. CMS will use final action claims from the Medicare fee-for-service
claims repository where separate payment was allowed for the applicable billing and payment
code for dates of service within a year to calculate Part B average total allowed charges for that
year.
Subpart C—Coinsurance Adjustment and Adjusted Medicare Payment for Part B
Rebatable Drugs with Price Increases Faster than Inflation
§ 427.200 Definitions.
As used in this subpart, inflation-adjusted beneficiary coinsurance means the coinsurance
adjustment as calculated in accordance with this subpart.
§ 427.201 Computation of beneficiary coinsurance and adjusted Medicare payment for
Part B rebatable drugs with price increases faster than inflation.
(a) Methodology. CMS must use the methodology set forth in this section to calculate the
inflation-adjusted beneficiary coinsurance and associated adjusted Medicare payment percentage
for Part B rebatable drugs as set forth in §§ 410.152(m), 419.41(e), and 489.30(b)(6) of this
chapter.
(b) Calculation of inflation-adjusted beneficiary coinsurance. To calculate the
inflation-adjusted beneficiary coinsurance for Part B rebatable drugs with respect to a calendar
quarter, CMS compares the payment amount, as described in paragraph (b)(3) of this section, to
the inflation-adjusted payment amount for the applicable calendar quarter.
(1) If the payment amount exceeds the inflation-adjusted payment amount, the inflation-
adjusted beneficiary coinsurance is calculated by multiplying the inflation-adjusted payment
amount by 0.20.
(2) If the inflation-adjusted payment amount does not exceed the payment amount, the
adjustment to the beneficiary coinsurance described in paragraph (b)(1) of this section is not
applied.
(3) CMS will use the published payment amount in quarterly pricing files published by
CMS as the payment amount in this determination.
(c) Exclusions. Any drug that is excluded from Part B rebatable drugs in accordance with
§ 427.101(b) is not subject to inflation-adjusted beneficiary coinsurance.
Subpart D—Determination of the Rebate Amount for Part B Rebatable Drugs
§ 427.300 Definitions.
As used in this subpart, the following definitions apply:
340B Program is the program under section 340B of the Public Health Service (PHS)
Act.
Refundable single-dose container or single-use package drug has the meaning set forth in
§ 414.902 of this chapter.
§ 427.301 Calculation of the total Part B rebate amount to be paid by manufacturers.
(a) Total rebate. Subject to paragraph (b) of this section, the total rebate amount to be
paid for a Part B rebatable drug, as identified under § 427.101, for an applicable calendar quarter
is equal to the product of the per unit Part B rebate amount of such drug, as determined under
§ 427.302, and the billing units of the Part B rebatable drug furnished during the applicable
calendar quarter, as identified in accordance with § 427.303. The rebate amount may be reduced
in accordance with subpart E of this part or adjusted in accordance with subpart F of this part.
(b) Apportionment of the Part B rebate amount. CMS will identify billing and payment
codes for which multiple manufacturers report ASP, in accordance with sections 1927(b)(3) and
1847A(f) of the Act, for NDCs assigned to the billing and payment code. CMS will calculate the
rebate amount owed by each manufacturer by:
(1) Determining the total billing units sold for each NDC assigned to the billing and
payment code, by multiplying the number of units reported by a manufacturer in ASP data
submissions at the NDC-11 package level by the number of billing units per NDC-11 reporting
unit.
(2) Summing the individual manufacturer’s total billing units sold during the applicable
calendar quarter (for all NDCs of the manufacturer assigned to the billing and payment code).
(3) Summing all manufacturers’ total billing units sold during the applicable calendar
quarter for all NDCs of the Part B rebatable drug assigned to the billing and payment code.
(4) Dividing the resulting amount from paragraph (b)(2) of this section by the resulting
amount from paragraph (b)(3) of this section.
(5) Multiplying the resulting amount from paragraph (b)(4) of this section by the total
rebate amount as determined under paragraph (a) of this section.
§ 427.302 Calculation of the per unit Part B drug rebate amount.
(a) Formula for calculating the per unit Part B rebate amount. CMS will calculate the
per unit Part B rebate amount for a Part B rebatable drug and applicable calendar quarter by
determining the amount by which the specified amount, as calculated in accordance with
paragraph (b) of this section, exceeds the inflation-adjusted payment amount, as calculated in
accordance with paragraph (g) of this section.
(b) Identification of the specified amount for the applicable calendar quarter. For each
applicable calendar quarter, subject to paragraph (b)(2) of this section, the specified amount is
equal to the amount determined in accordance with section 1847A(i)(3)(A)(ii)(I)(aa) or (bb) of
the Act, as applicable, for the calendar quarter.
(1) The first applicable calendar quarter for a Part B rebatable drug will be the earliest
applicable calendar quarter that follows the payment amount benchmark quarter identified in
paragraphs (c)(1) through (5) of this section.
(2) If all NDCs in the billing and payment code have neither manufacturer-reported ASP
nor Wholesale Acquisition Cost (WAC) price data available for the applicable calendar quarter,
CMS will use WAC price data from other public sources, if available, to calculate 106 percent of
WAC, which will serve as the specified amount.
(c) Identification of the payment amount benchmark quarter. For each Part B rebatable
drug, CMS will identify the applicable payment amount benchmark quarter using the earliest
first marketed date of any NDC ever marketed under any FDA application under which any
NDCs that have ever been assigned to the billing and payment code as of the applicable calendar
quarter have been marketed, as set forth in paragraphs (c)(1) through (3) of this section, as
applicable, subject to paragraphs (c)(4) and (5) of this section:
(1) For a Part B rebatable drug first approved or licensed by the FDA on or before
December 1, 2020, and with a first marketed date on or before December 1, 2020, the payment
amount benchmark quarter is the calendar quarter beginning July 1, 2021.
(2) For a Part B rebatable drug first approved or licensed by the FDA after
December 1, 2020, the payment amount benchmark quarter is the third full calendar quarter after
a drug’s first marketed date.
(3) For a Part B rebatable drug first approved or licensed by the FDA on or before
December 1, 2020, but with a first marketed date after December 1, 2020, the payment amount
benchmark quarter is the third full calendar quarter after a drug’s first marketed date.
(4) Notwithstanding paragraph (c)(3) of this section, for a Part B rebatable drug that was
billed under a NOC code during the calendar quarter beginning July 1, 2021, or the third full
calendar quarter after such drug’s first marketed date, whichever is later, the payment amount
benchmark quarter is the third full calendar quarter after the Part B rebatable drug is assigned a
billing and payment code other than a NOC code.
(5) For a Part B rebatable drug that is a selected drug (as defined in section 1192(c) of
the Act) with respect to a price applicability period (as defined in section 1191(b)(2) of the Act),
in the case such Part B rebatable drug is no longer considered to be a selected drug, for each
applicable quarter beginning after the price applicability period with respect to such drug, the
payment amount benchmark quarter is the calendar quarter beginning January 1 of the last year
during such price applicability period with respect to such selected drug.
(d) Identification of the payment amount in the payment amount benchmark quarter.
CMS will identify the payment amount in the payment amount benchmark quarter using the
published payment limit for the billing and payment code for the applicable payment amount
benchmark quarter identified in accordance with paragraph (c) of this section.
(1) For a Part B rebatable drug, subject to paragraphs (d)(1)(i) and (ii) of this section and
except as provided in paragraph (d)(2) of this section, CMS will identify the payment amount in
the payment amount benchmark quarter using the published payment limit for the billing and
payment code for the applicable payment amount benchmark quarter determined in accordance
with section 1847A of the Act.
(i) If a published payment limit is not available for the applicable payment amount
benchmark quarter, CMS will use the lower of 106 percent of manufacturer-reported ASP or
106 percent of manufacturer-reported WAC.
(ii) If neither a published payment limit nor manufacturer-reported ASP or WAC data
are available, CMS will use WAC data from other public sources to calculate 106 percent of
WAC, which, solely for the purposes of this section, CMS will consider to be the payment
amount for the payment amount benchmark quarter.
(2) For a Part B rebatable drug previously billed under a grouped billing and payment
code during the payment amount benchmark quarter and later billed under a unique billing and
payment code, CMS will use the grouped billing and payment code payment limit as the
payment amount in the payment amount benchmark quarter.
(e) Identification of the benchmark period CPI-U. For each Part B rebatable drug, CMS
will identify the applicable benchmark period CPI-U at the billing and payment code level as set
forth in paragraphs (e)(1) and (2) of this section, subject to paragraphs (e)(3) through (5) of this
section:
(1) For a Part B rebatable drug first approved or licensed by the FDA on or before
December 1, 2020, and with a first marketed date on or before December 1, 2020, the benchmark
period CPI-U is the CPI-U for January 2021.
(2) For a Part B rebatable drug first approved or licensed by the FDA after
December 1, 2020, the benchmark period CPI-U is the CPI-U for the first month of the first full
calendar quarter after a drug’s first marketed date.
(3) Notwithstanding paragraph (e)(2) of this section, for a Part B rebatable drug first
approved or licensed by FDA on or before December 1, 2020, and with a first marketed date
after December 1, 2020, the benchmark period CPI-U is the CPI-U for the first month of the
third full calendar quarter after a drug’s first marketed date.
(4) Notwithstanding paragraph (e)(3) of this section, for a Part B rebatable drug that was
billed under a NOC code during the calendar quarter beginning July 1, 2021, or the third full
calendar quarter after such drug’s first marketed date, whichever is later, the benchmark period
CPI-U is the CPI-U for the first month of the third full calendar quarter after the Part B rebatable
drug is assigned a billing and payment code other than a NOC code.
(5) Notwithstanding paragraph (e)(4) of this section, for a Part B rebatable drug that is a
selected drug (as defined in section 1192(c) of the Act) with respect to a price applicability
period (as defined in section 1191(b)(2) of the Act), in the case such Part B rebatable drug is no
longer considered to be a selected drug, the benchmark period CPI-U is the CPI-U for the July of
the year preceding the last year during such price applicability period.
(f) Identification of the rebate period CPI-U. For each Part B rebatable drug by billing
and payment code, CMS will identify and use the greater of the benchmark period CPI-U index
level or the CPI-U index level for the first month of the calendar quarter that is two calendar
quarters before the applicable calendar quarter in which the Part B rebatable drug is furnished.
(g) Determination of inflation-adjusted payment amount. For each applicable calendar
quarter and for each Part B rebatable drug by billing and payment code, CMS will calculate the
inflation-adjusted payment amount by dividing the rebate period CPI-U by the benchmark period
CPI-U and then multiplying the quotient by the payment amount in the payment amount
benchmark quarter, determined in accordance with paragraph (d) of this section.
§ 427.303 Determination of total number of billing units.
(a) General. For each Part B rebatable drug, CMS will determine the total number of
billing units of the billing and payment code subject to a rebate in the applicable calendar quarter
using final action Medicare fee-for-service claims for which Medicare payment was allowed and
greater than zero.
(b) Total billing units. Using final action claims in the Medicare fee-for-service claims
repository, at least 3 months after the end of the applicable calendar quarter, CMS will determine
the total number of billing units for a Part B rebatable drug in an applicable calendar quarter by
identifying separately payable claim lines for such billing and payment code for dates of service
in that applicable calendar quarter and excluding the following billing units in claim lines as
applicable:
(1) Billing units of drugs acquired through the 340B Program. CMS will exclude billing
units acquired under the 340B Program as identified through—
(i) Separately payable billing units in claim lines for professional claims with dates of
service during 2023 and 2024 from suppliers that are associated with covered entities listed by
the Health Resources and Services Administration (HRSA) 340B Office of Pharmacy Affairs
Information System as participating in the 340B Program. CMS will use National Provider
Identifiers and/or Medicare Provider Numbers to identify these suppliers and the claims
submitted with such identifiers;
(ii) Separately payable billing units in claim lines for institutional claims that are billed
with the “JG” or “TB” modifiers for claims with dates of service through December 31, 2024;
and
(iii) Separately payable billing units in claim lines billed with the “TB” modifier for
claims with dates of service on or after January 1, 2025.
(2) Billing units with a rebate under section 1927 of the Social Security Act. Subject to
paragraph (b)(2)(i) of this section, CMS will exclude billing units from claims with dates of
service during a month within an applicable calendar quarter if the units are furnished to a dually
eligible Medicare beneficiary who has Medicaid coverage that may provide cost-sharing
assistance.
(i) CMS will not exclude billing units from claims when the Medicare beneficiary has
Medicaid coverage that does not include cost-sharing assistance, including Specified
Low-Income Medicare Beneficiaries (SLMB), Qualified Disabled and Working Individuals
(QDWI), and Qualifying Individuals (QI) beneficiaries.
(ii) [Reserved]
(3) Billing units that are packaged into the payment amount for an item or service and
are not separately payable. CMS will exclude billing units that are packaged into the payment
amount for an item or service and are not separately payable.
(4) Billing units when a drug is no longer a Part B rebatable drug. In situations where a
Part B rebatable drug that is a single source drug becomes a multiple source drug during an
applicable calendar quarter, CMS will:
(i) Determine if such drug has become a multiple source drug by reviewing FDA’s most
recent publication of “Approved Drug Products with Therapeutic Equivalence Evaluations”
(commonly known as the Orange Book) for a drug that is that is rated as therapeutically
equivalent to such drug; and,
(ii) If a therapeutically equivalent drug is identified in accordance with paragraph
(b)(4)(i) of this section, determine if the therapeutically equivalent drug was sold or marketed
during the applicable calendar quarter; and
(iii) Exclude billing units of such drug furnished on and after the first day of the calendar
month in which the therapeutically equivalent drug was first sold or marketed during the
applicable calendar quarter.
(5) Billing units subject to discarded drug refunds. CMS will exclude billing units of
discarded refundable single-dose container or single-use package drugs for which a refund has
been paid in accordance with § 414.940 of this chapter from the calculation of rebate amounts.
For applicable calendar quarters beginning on or after January 1, 2024, these billing units will be
excluded as part of the reconciliation process described at § 427.501(d).
§ 427.304 Adjustments for changes to billing and payment codes.
(a) Changes in billing unit dose description. If there has been a change to the dose
description for a Part B rebatable drug (causing a new billing and payment code to be assigned),
CMS will calculate a conversion factor based on the ratio of the billing unit dose description for
the current billing and payment code to the billing unit dose description for the prior billing and
payment code. CMS will apply the conversion factor to the payment amount in the payment
amount benchmark quarter, as described in § 427.302(d), before applying the percentage by
which the rebate period CPI-U for the calendar quarter exceeds the benchmark period CPI-U.
(b) Instances when a new billing and payment code is assigned. If a new billing and
payment code is assigned for a Part B rebatable drug, CMS will use the payment amount in the
payment amount benchmark quarter, the payment amount benchmark quarter, and the
benchmark quarter CPI-U of the prior billing and payment code to calculate the per unit Part B
rebate amount under § 427.302.
(c) Documentation. CMS will maintain a crosswalk reflecting the changes in billing and
payment codes and dose descriptions as applicable.
Subpart E—Reducing the Rebate Amount for Part B Rebatable Drugs in Shortage and
When There Is a Severe Supply Chain Disruption
§ 427.400 Definitions.
As used in this subpart, the following definitions apply:
Currently in shortage means that at least one NDC-10 assigned to the billing and
payment code of a Part B rebatable drug with the status “currently in shortage” is on a shortage
list maintained by the FDA under section 506E of the FD&C Act.
Drug shortage or shortage means a period of time when the demand or projected demand
for the drug within the United States exceeds the supply of the drug (see section 506C(h)(2) of
the FD&C Act).
Natural disaster means any natural catastrophe, including, but not limited to any of the
following: hurricane, tornado, storm, high water, wind-driven water, tidal wave, tsunami,
earthquake, volcanic eruption, landslide, mudslide, snowstorm, or drought, or regardless of
cause, any fire, flood, or explosion.
Other unique or unexpected event means any exogenous, unpredictable event outside of a
manufacturer’s control, including, but not limited to, a geopolitical disruption, pandemic, or act
of terror.
Plasma-derived product means a licensed biological product that is derived from human
whole blood or plasma, as indicated on the approved product labeling.
Severe supply chain disruption means a change in production or distribution that is
reasonably likely to lead to a significant reduction in the U.S. supply of a Part B rebatable
biosimilar biological product by a manufacturer and significantly affects the ability of the
manufacturer of the biosimilar biological product to fill orders or meet expected demand for its
product in the United States for at least 90 days. This definition does not include interruptions in
manufacturing due to matters such as routine maintenance, manufacturing quality issues, or
insignificant changes made in the manufacturing process for the drug.
§ 427.401 Reducing the rebate amount for Part B rebatable drugs currently in shortage.
(a) General. As required under section 1847A(i)(3)(G)(i) of the Act, CMS will reduce
the total rebate amount calculated under § 427.301(a), if any is owed, for a Part B rebatable drug
that is currently in shortage, as defined in § 427.400, at any point during the applicable calendar
quarter.
(b) Calculation of the reduced rebate amount. (1) For each applicable calendar quarter
beginning on or after January 1, 2023, the reduced total rebate amount for a Part B rebatable
drug currently in shortage will be calculated using the following formula:
Equation 1 to Paragraph (b)(1)
Reduced Total Rebate Amount = the total rebate amount multiplied by (1 minus
applicable percent reduction) multiplied by (percentage of time drug was currently in shortage
during the applicable calendar quarter) added to the total rebate amount multiplied by (1 minus
percentage of time drug was currently in shortage during the applicable calendar quarter)
(2) For purposes of paragraph (b)(1) of this section, the applicable percent reduction is:
(i) For a Part B rebatable drug that is a plasma-derived product:
(A) 75 percent for the first 4 consecutive applicable calendar quarters such drug is
currently in shortage.
(B) 50 percent for the second 4 consecutive applicable calendar quarters such drug is
currently in shortage.
(C) 25 percent for each subsequent applicable calendar quarter such drug is currently in
shortage.
(ii) For a Part B rebatable drug that is not a plasma-derived product:
(A) 25 percent for the first 4 consecutive applicable calendar quarters such drug is
currently in shortage.
(B) 10 percent for the second 4 consecutive applicable calendar quarters such drug is
currently in shortage.
(C) 2 percent for each subsequent applicable calendar quarter such drug is currently in
shortage.
(3) For purposes of paragraph (b)(1) of this section, the percentage of time the drug is
currently in shortage during the applicable calendar quarter is equal to the number of days such
drug is currently in shortage in an applicable calendar quarter, divided by the total number of
days in the applicable calendar quarter.
(c) Application of reduction. CMS will apply a reduction of the rebate amount as
determined under paragraph (b) of this section to all the NDCs under the relevant billing and
payment code.
§ 427.402 Reducing the rebate amount for certain Part B rebatable drugs when there is a
severe supply chain disruption.
(a) General. As required under section 1847A(i)(3)(G)(ii) of the Act, CMS will reduce
the total rebate amount calculated under § 427.301(a), if any is owed, for a Part B rebatable
biosimilar biological product when CMS determines there is a severe supply chain disruption
during the applicable calendar quarter such as that caused by a natural disaster or other unique or
unexpected event.
(b) Calculation of the reduced rebate amount--(1) Initial reduction. If CMS determines
the criteria described in paragraph (c)(4) of this section are met, then CMS will reduce the total
rebate amount owed by the manufacturer for a Part B rebatable biosimilar biological product by
75 percent for the quarter in which the event occurred and the 3 subsequent applicable calendar
quarters.
(2) Extension of reduction. If CMS determines a severe supply chain disruption
continues into a fifth applicable calendar quarter as described in paragraph (c)(5) of this section,
then CMS will reduce the total rebate amount owed by the manufacturer for a Part B rebatable
biosimilar biological product by 75 percent for that fifth quarter and an additional 3 consecutive
applicable calendar quarters.
(3) Application of reduction. If CMS determines there is a severe supply chain disruption
for an NDC-11 assigned to a billing and payment code, CMS will apply any reduction of the
rebate amount as described in paragraphs (b)(1) and (2) of this section to all the NDCs under the
relevant billing and payment code.
(4) Limitation on rebate reductions. CMS will not apply multiple rebate reductions for
the same Part B rebatable drug and applicable calendar quarter.
(i) If a manufacturer believes there are multiple events causing severe supply chain
disruptions during the same 4 applicable calendar quarters for the same Part B rebatable
biosimilar biological product and submits multiple rebate reduction requests for the same
product, CMS will grant no more than 1 rebate reduction under paragraph (b)(1) or (2) of this
section for that product for those consecutive applicable calendar quarters.
(ii) If CMS grants a rebate reduction request under this section, and the Part B rebatable
biosimilar biological product subject to the reduction is currently in shortage during the same
four applicable calendar quarters as the ones for which the severe supply chain disruption
reduction request was granted, CMS will reduce the rebate amount as described in paragraph
(b)(1) of this section and will not grant a reduction under § 427.401 during those applicable
calendar quarters.
(iii) If a Part B rebatable biosimilar biological product that is currently in shortage
experiences a severe supply chain disruption, CMS will reduce the rebate amount as described in
paragraph (b)(1) of this section and will not grant a reduction under § 427.401 during those
applicable calendar quarters.
(c) Eligibility for a rebate reduction--(1) Eligible drug. Subject to paragraph (b)(3) of
this section, eligibility for a rebate reduction under this section is limited to Part B rebatable
biosimilar biological products for which a manufacturer submits a rebate reduction request under
this section.
(2) Timing. For a natural disaster or other unique or unexpected event occurring on or
after August 2, 2024, that the manufacturer believes caused a severe supply chain disruption, the
manufacturer must submit the rebate reduction request within 60 calendar days from the first day
that the natural disaster or other unique or unexpected event occurred or began in order to receive
consideration for a reduction in the rebate amount owed in accordance with paragraph (b)(1) of
this section.
(3) Required elements of a rebate reduction request. To receive consideration for a
reduction in the rebate amount owed in accordance with paragraph (b)(1) of this section, the
manufacturer must submit to CMS information and supporting documentation to substantiate the
evaluation criteria described in paragraph (c)(4) of this section. Such information and supporting
documentation include the following:
(i) Evidence that the severe supply chain disruption directly affects the manufacturer
itself, a supplier of an ingredient or packaging, a contract manufacturer, or a method of shipping
or distribution that the manufacturer uses to make or distribute the Part B rebatable biosimilar
biological product(s), such as a change in the production or distribution of the Part B rebatable
biosimilar biological product(s) that is reasonably likely to lead to a significant reduction in the
U.S. supply of product and significantly affects the manufacturer’s ability to fill orders or meet
expected demand for the Part B rebatable biosimilar biological product(s) for at least 90 days;
(ii) Information about when the manufacturer expects supply of the Part B rebatable
biosimilar biological product(s) to meet expected demand;
(iii) Evidence that the natural disaster or other unique or unexpected event caused the
severe supply chain disruption, including when the natural disaster or other unique or unexpected
event occurred or began occurring, and the expected or actual duration of the severe supply chain
disruption; and
(iv) Evidence of the manufacturer’s physical presence related to manufacturing the
Part B rebatable biosimilar biological product(s) in a geographic area where a natural disaster or
other unique or unexpected event occurred. If the manufacturer is not physically present in a
geographic area where a natural disaster or other unique or unexpected event occurred, but
believes there is a severe supply chain disruption caused by a natural disaster or other unique or
unexpected event that affects the manufacturer’s Part B rebatable biosimilar biological
product(s), the information and supporting documentation may include evidence of the impact of
the natural disaster or other unique or unexpected event on the supply chain of the Part B
rebatable drug or biosimilar, on a supplier of an ingredient or packaging, or method of shipping
or distribution that the manufacturer uses.
(4) Evaluation criteria. In accordance with paragraph (b)(1) of this section, CMS will
grant a reduction in the rebate amount owed if a manufacturer submits to CMS a request in
writing for an eligible drug, in accordance with the timing specified in paragraph (c)(2) of this
section, demonstrating that:
(i) A severe supply chain disruption has occurred during the applicable calendar quarter;
(ii) The severe supply chain disruption directly affects the manufacturer itself, a contract
manufacturer, a supplier of an ingredient or packaging, or a method of shipping or distribution
that the manufacturer uses in a significant capacity to make or distribute the Part B rebatable
biosimilar biological product; and
(iii) The severe supply chain disruption was caused by a natural disaster or other unique
or unexpected event.
(5) Rebate reduction extensions. If CMS determines that a Part B rebatable biosimilar
biological product that received a reduction of the rebate amount under paragraph (b)(1) of this
section continues to be affected by a severe supply chain disruption, CMS will grant a single
extension of the reduction for 4 additional consecutive applicable calendar quarters and reduce
the rebate amount owed in accordance with paragraph (b)(2) of this section.
(i) To receive consideration for a rebate reduction extension, a manufacturer must submit
a request with updated or new information and supporting documentation on why the Part B
rebatable biosimilar biological product continues to be affected by the severe supply chain
disruption during the fifth through eighth applicable calendar quarters.
(ii) A manufacturer must submit the rebate reduction extension request at least
60 calendar days before the start of the fifth applicable calendar quarter to receive consideration
for a reduction in the rebate amount owed, if any, in accordance with paragraph (b)(2) of this
section.
(6) Decision to grant or deny a request. CMS will review rebate reduction requests and
rebate reduction extension requests within 60 calendar days of receipt of all documentation, if
feasible, beginning with the applicable calendar quarter that begins on October 1, 2024.
(i) CMS will deny a rebate reduction request that does not meet the criteria in paragraph
(c)(4) of this section or that is incomplete or untimely based on the requirements of this
paragraph (c).
(ii) CMS will deny a rebate reduction extension request that does not meet the criteria in
paragraph (c)(5) of this section, that is incomplete or untimely based on the requirements of
paragraph (c)(5), or if a reduction under paragraph (b)(1) of this section was not provided for
such biosimilar biological product.
(iii) CMS’ decisions to deny a request are final and will not be subject to an appeals
process.
(7) Public disclosure of information. CMS will keep confidential, to the extent allowable
under law, any requests for a rebate reduction, including supporting documentation. Information
provided as part of a request for a rebate reduction that the submitter indicates is a trade secret or
confidential commercial or financial information will be protected from disclosure if CMS
determines the information meets the requirements set forth under Exemptions 3 and/or 4 in
5 U.S.C. 552.
Subpart F—Reports of Rebate Amounts, Reconciliation, Suggestion of Error, and
Payments
§ 427.500 Definitions.
As used in this subpart, date of receipt is the calendar day following the day on which a
report of a rebate amount (as set forth in §§ 427.501(b) through (d) and 427.502(c)) is made
available to the manufacturer of a Part B rebatable drug by CMS.
§ 427.501 Rebate Reports and reconciliation.
(a) General. This section applies to Part B rebatable drugs for all applicable calendar
quarters except as otherwise set forth in § 427.502 regarding the applicable calendar quarters in
calendar years 2023 and 2024.
(b) Preliminary Rebate Report. A Preliminary Rebate Report will be provided to each
manufacturer of a Part B rebatable drug at least 1 month prior to the issuance of the Rebate
Report as set forth in paragraph (c) of this section for an applicable calendar quarter.
(1) The Preliminary Rebate Report for each Part B rebatable drug will include the
following information:
(i) The NDC(s) and billing and payment codes for the Part B rebatable drug as defined
under § 427.20.
(ii) Total number of billing units as specified in § 427.303.
(iii) Payment amount in the payment amount benchmark quarter as specified in
§ 427.302(d).
(iv) Applicable calendar quarter specified amount as specified in § 427.302(b).
(v) Applicable benchmark period and rebate period CPI-Us as specified in § 427.302(e)
and (f).
(vi) Inflation-adjusted payment amount as specified in § 427.302(g).
(vii) The amount, if any, by which the specified amount as described in § 427.302(b)
exceeds the inflation-adjusted payment amount as described in § 427.302(g) for the Part B
rebatable drug for the applicable calendar quarter as determined under § 427.302.
(viii) Any applied reductions as described in §§ 427.401 and 427.402.
(ix) Rebate amount due as specified in § 427.301(a).
(2) [Reserved]
(c) Rebate Report. A Rebate Report will be provided to each manufacturer of a Part B
rebatable drug no later than 6 months after the end of each applicable calendar quarter.
(1) The Rebate Report will include the information specified in paragraph (b)(1) of this
section, with the inclusion of any revisions to such information resulting from CMS’ review of a
Suggestion of Error as set forth in § 427.503, if applicable, and any CMS-determined
recalculations from paragraph (d)(2) of this section.
(2) The Rebate Report is the invoice of a manufacturer’s rebate amount due as calculated
in § 427.301, if any, for a Part B rebatable drug for an applicable calendar quarter.
(d) Reconciliation of the rebate amount. CMS will perform reconciliation of a rebate
amount provided in a Rebate Report specified in paragraph (c) of this section for an applicable
calendar quarter in the following circumstances:
(1) Regular reconciliation. Except as otherwise described in § 427.502, CMS will
perform one regular reconciliation of the rebate amount within 12 months of the date of receipt
of the Rebate Report specified in paragraph (c) of this section for each applicable calendar
quarter in order to include revisions to the information used to calculate the rebate amount as
specified in paragraph (b)(1) of this section.
(i) Preliminary reconciliation. At least 1 month prior to the issuance of a report with the
reconciled rebate amount specified in paragraph (d)(1)(ii) of this section, CMS will conduct a
preliminary reconciliation of a rebate amount for an applicable calendar quarter specified in
paragraph (d)(3) of this section based on the information specified in this paragraph (b)(1)(i) and
paragraphs (d)(1)(ii) through (ix) of this section and provide the information specified in this
paragraph (b)(1)(i) and paragraphs (d)(1)(ii) through (ix) to the manufacturer of a Part B
rebatable drug for the applicable calendar quarter, if applicable:
(A) Updated total number of rebatable units, as specified in § 427.303.
(B) Payment amount in the payment amount benchmark quarter, as specified in
§ 427.302(d) if any inputs are restated within the reconciliation run-out period.
(C) Applicable calendar quarter specified amount as specified in § 427.302(b), if any
inputs are restated within the reconciliation run-out period.
(D) The excess amount by which the specified amount exceeds the inflation-adjusted
payment amount as specified in § 427.302, if any inputs are restated within the reconciliation
run-out period.
(E) Reconciled total rebate amount as specified in § 427.301(a).
(F) The difference between the total rebate amount due as specified on the Rebate Report
set forth in paragraph (c) of this section and the reconciled rebate amount as set forth in this
paragraph (d)(1)(i).
(ii) Report with reconciled rebate amount. With the inclusion of any additional revisions
to such information resulting from CMS’ review of a Suggestion of Error as set forth in
§ 427.503, if applicable, a report with the reconciled rebate amount will be provided to each
manufacturer of a Part B rebatable drug within 12 months after the issuance of the Rebate Report
described in paragraph (c) of this section.
(2) CMS identification of error and manufacturer misreporting. CMS may recalculate a
rebate amount and provide the manufacturer of a Part B rebatable drug a report with a reconciled
rebate amount when:
(i) CMS identifies an agency error in the information specified in paragraphs (c) and
(d)(1) of this section, including reporting system or coding errors, not later than 3 years from the
date of receipt by a manufacturer of a reconciled rebate amount for the applicable calendar
quarter; or
(ii) CMS determines at any time that the information used by CMS to calculate the
rebate amount was inaccurate due to manufacturer misreporting.
(3) Impact of reconciliation on rebate amount. A reconciliation as set forth in this
paragraph (d) could result in an increase, decrease, or no change to the rebate amount as
calculated in § 427.301 owed by a manufacturer for the applicable calendar quarter for the Part B
rebatable drug.
(i) A report with a reconciled rebate amount that is an increase to the rebate amount is
the invoice for such additional amount due on the manufacturer’s rebate amount as set forth in
§ 427.301 for a Part B rebatable drug for an applicable calendar quarter.
(ii) [Reserved]
(4) Drugs included in a reconciliation. A drug covered under Part B that does not meet
the requirements of a rebatable drug specified in subpart B for an applicable period will not be
included in a reconciliation under this paragraph (d).
§ 427.502 Rebate Reports for applicable calendar quarters in calendar years 2023 and
2024.
(a) Transition rule for reporting. Section 1847A(i)(1)(C) of the Act allows CMS to delay
the timeframe for reporting the information and rebate amount described in § 427.501(c) for
applicable calendar quarters in calendar years 2023 and 2024 until not later than
September 30, 2025.
(b) Rebate Report information for applicable calendar quarters in calendar years 2023
and 2024. The Rebate Reports for applicable calendar quarters in calendar years 2023 and 2024
will include the information described in § 427.501(b)(1).
(c) Rebate Report procedures for applicable calendar quarters in calendar years 2023 and
2024. Rebate amounts for the applicable calendar quarters in calendar year 2023 and 2024 will be
reported as follows:
(1) The four applicable calendar quarters in calendar year 2023 will be consolidated into
a single report and manufacturers will receive a single Preliminary Rebate Report followed by a
single Rebate Report.
(i) Discarded drug units for which a refund has been paid will be removed from the total
number of billing units in the single Preliminary Rebate Report for the applicable calendar quarters
in calendar year 2023.
(ii) For this single Preliminary Rebate Report for the applicable calendar quarters in
calendar year 2023, the Suggestion of Error period as described in § 427.503 will be 30 calendar
days.
(iii) No regular reconciliation of the rebate amount as described in § 427.501(d)(1) will be
conducted for the rebate amount in the single Rebate Report for the applicable calendar quarters in
calendar year 2023.
(2) The four applicable calendar quarters in calendar year 2024 will be consolidated into
a single report and manufacturers will receive a single Preliminary Rebate Report followed by a
single Rebate Report.
(i) For this single Preliminary Rebate Report for the applicable calendar quarters in
calendar year 2024, the Suggestion of Error period as described in § 427.503 will be 30 calendar
days.
(ii) Nine months after issuance of the single Rebate Report, CMS will perform one regular
reconciliation for the applicable calendar quarters in calendar year 2024 in order to include
revisions to the information used, specified in § 427.501(b)(1), to calculate the rebate amount.
Such reconciliation will be as described in § 427.501(d) inclusive of a preliminary reconciliation
and a report with the reconciled rebate amount.
(iii) The Suggestion of Error period for the preliminary reconciliation for the applicable
calendar quarters in calendar year 2024 will be 10 calendar days.
§ 427.503 Suggestion of Error.
(a) General. The manufacturer of a Part B rebatable drug may submit a Suggestion of
Error about the information in their Preliminary Rebate Report and the report detailing the
preliminary reconciliation of the rebate amount to CMS, for its discretionary consideration, if the
manufacturer believes that there is a mathematical error or errors to be corrected before the
Rebate Report or a subsequent reconciliation of the rebate amount, as applicable, is finalized.
(1) Section 1847A(i)(8) of the Act precludes administrative or judicial review on the
determination of units as set forth in § 427.303, the determination of whether a drug is a Part B
rebatable drug as set forth in§ 427.101, and the calculation of the rebate amount as set forth in
§ 427.301, inclusive of any reconciled rebate amount.
(2) [Reserved]
(b) Process of submission. Subject to the scope and timing requirements specified in
paragraphs (a) and (c) of this section, manufacturers may submit the Suggestion of Error and
provide supporting documentation (if applicable).
(c) Timing. Except as specified in § 427.502 for applicable calendar quarters in calendar
year 2023 and 2024, a manufacturer must submit its Suggestion of Error for the applicable
calendar quarter within 10 calendar days from the date of receipt of a Preliminary Rebate Report
or a preliminary reconciliation of a rebate amount using the method and process established by
CMS in paragraph (b) of this section.
(d) Notice. (1) CMS will include any revisions to the calculation of the rebate amount, if
determined necessary by CMS based on the Suggestion of Error submitted under this section
prior to issuance of the Rebate Report as set forth in § 427.501(c) or § 427.502(c) as well as any
report of reconciled rebate amount as set forth in § 427.501(d) or § 427.502(c)(2)(ii).
(2) CMS will notify the manufacturer whether CMS revised its calculation of the rebate
amount based on the Suggestion of Error.
§ 427.504 Manufacturer access to Rebate Reports.
(a) General. CMS will establish a method and process for a manufacturer of the Part B
rebatable drug to:
(1) Access the manufacturer’s Rebate Report as set forth in §§ 427.501 and 427.502,
including any report of reconciled rebate amount as set forth in §§ 427.501(d) and
427.502(c)(2)(ii);
(2) Submit a Suggestion of Error as set forth in §§ 427.502(c)(1)(ii) and (c)(2)(i) and
427.503; and
(3) Pay a rebate amount as set forth in § 427.505.
(b) [Reserved]
§ 427.505 Deadline and process for payment of rebate amount.
(a) Rebate amounts owed by a manufacturer. For a rebate amount owed by a
manufacturer, payment is due no later than 11:59 p.m. Pacific Time (PT) on the 30th calendar day
after the date of receipt of information regarding the rebate amount on—
(1) A Rebate Report specified in § 427.501(c) or § 427.502(c)(1) or (2); or
(2) A report of a reconciled rebate amount specified in § 427.501(d) or
§ 427.502(c)(2)(ii).
(b) Failure to pay a rebate amount. Failure to pay a rebate amount due timely and in full
may result in an enforcement action as described in subpart G of this part.
(c) Refund to the manufacturer. If a reconciled rebate amount for an applicable calendar
quarter as specified in § 427.501(d) or § 427.502(c)(2)(ii) is less than what the manufacturer paid
for that applicable calendar quarter, CMS will initiate the process to provide a refund equal to the
excess amount paid within 60 days of the date of receipt of the report with such reconciled rebate
amount.
Subpart G—Enforcement of Manufacturer Payment of Rebate Amounts
§ 427.600 Civil money penalty notice and appeals procedures.
(a) General. CMS may impose a civil money penalty on a manufacturer that fails to pay
the rebate amount set forth in § 427.301(a) on a Part B rebatable drug identified at § 427.101, by
the payment deadline as set forth in § 427.505(a) for such drug for such applicable calendar
quarter.
(b) Determination of the civil money penalty amount. CMS may impose a civil money
penalty for each failure by a manufacturer to provide an inflation rebate for an applicable
calendar quarter equal to 125 percent of the rebate amount determined in § 427.301(a).
(1) The civil money penalty is in addition to the rebate amount due.
(2) If a reconciled rebate amount as set forth in § 427.501(d) or § 427.502(c)(2)(ii)
results in an increase to the rebate amount due, a separate civil money penalty may be imposed
for the failure by a manufacturer to provide an inflation rebate for the applicable quarter for the
increase to the rebate amount due.
(c) Notice of imposition of civil money penalties. If CMS makes a determination to
impose a civil money penalty described in paragraph (b) of this section, CMS will send a written
notice of its decision to impose a civil money penalty to include the following:
(1) A description of the basis for the determination.
(2) The basis for the penalty.
(3) The amount of the penalty.
(4) The date the penalty is due.
(5) The manufacturer’s right to a hearing as specified in paragraph (e)(3) of this section.
(6) Information about where to file the request for a hearing.
(d) Collection. (1) A manufacturer must pay the civil money penalty in full within
60 calendar days after the date of the notice of imposition of a civil money penalty from CMS
under paragraph (c) of this section.
(2) In the event a manufacturer requests a hearing, pursuant to 42 CFR part 423,
subpart T, the manufacturer must pay the amount in full within 60 calendar days after the date of
a final decision by the Departmental Appeal Board, to uphold, in whole or in part, the civil
money penalty.
(3) If the 60
th
calendar day described in paragraphs (d)(1) and (2) of this section is a
weekend or a Federal holiday, then the timeframe is extended until the end of the next business
day.
(e) Appeal procedures for civil money penalties. Section 1128A(c)(2) of the Act provides
that CMS may not collect a civil money penalty until the affected party has had notice and the
opportunity for a hearing.
(1) Manufacturers may appeal the following determinations:
(i) A CMS determination that the rebate amount was not paid by the applicable payment
deadline as described in § 427.505.
(ii) The calculation of the amount of the civil money penalty.
(2) The notice provided to a manufacturer if CMS decides to impose a civil money
penalty is paragraph (c) of this section.
(3) A manufacturer has a right to a hearing following a decision by CMS to impose a
civil money penalty following the administrative appeal process and procedures established in
42 CFR part 423, subpart T.
(f) Other applicable provisions. The provisions of section 1128A of the Act (except
subsections (a) and (b) of section 1128A of the Act) apply to civil money penalties under this
section to the same extent that they apply to a civil money penalty or procedures under section
1128A of the Act.
(g) Bankruptcy. In the event that a manufacturer declares bankruptcy, as described in
title 11 of the United States Code, and as a result of the bankruptcy, fails to pay either the full
rebate amount owed or the total sum of civil money penalties imposed, the Government reserves
the right to file a proof of claim with the bankruptcy court to recover the unpaid amount of the
rebates and civil money penalties owed by the manufacturer.
81. Part 428 is added to read as follows:
PART 428—MEDICARE PART D DRUG INFLATION REBATE PROGRAM
Subpart A—General Provisions
Sec.
428.10 Basis and scope.
428.20 Definitions.
Subpart B—Determination of Part D Rebatable Drugs
428.100 Definitions.
428.101 Identification of Part D rebatable drugs.
Subpart C—Determination of the Rebate Amount for Part D Rebatable Drugs
428.200 Definitions.
428.201 Calculation of the total rebate amount to be paid by manufacturers.
428.202 Calculation of the per unit Part D drug rebate amount.
428.203 Determination of the total number of units dispensed under Part D.
428.204 Treatment of new formulations of Part D rebatable drugs.
Subpart D—Reducing the Rebate Amount for Part D Rebatable Drugs in Shortage and When
There Is a Severe Supply Chain Disruption or Likely Shortage
428.300 Definitions.
428.301 Reducing the rebate amount for Part D rebatable drugs currently in shortage.
428.302 Reducing the rebate amount for certain Part D rebatable drugs when there is a severe
supply chain disruption.
428.303 Reducing the rebate amount for generic Part D rebatable drugs likely to be in shortage.
Subpart E—Reports of Rebate Amounts, Reconciliation, Suggestion of Error, and Payments
428.400 Definitions.
428.401 Rebate Reports and reconciliation.
428.402 Rebate Reports for applicable periods beginning October 1, 2022, and October 1, 2023.
428.403 Suggestion of Error.
428.404 Manufacturer access to Rebate Reports.
428.405 Deadline and process for payment of rebate amount.
Subpart F—Enforcement of Manufacturer Payment of Rebate Amounts
428.500 Civil money penalty notice and appeals procedures.
Authority: 42 U.S.C. 1395w-114b, 1302, and 1395hh.
Subpart A—General Provisions
§ 428.10 Basis and scope.
(a) Basis. This part implements section 1860D-14B of the Social Security Act (“the
Act”).
(b) Scope. This part sets forth the requirements of the Medicare Part D Drug Inflation
Rebate Program, which requires, for each 12-month applicable period, manufacturers to pay
rebates for certain drugs and biological products with prices that increase faster than the rate of
inflation.
(c) Severability. Were any provision of this part to be held invalid or unenforceable by
its terms, or as applied to any person or circumstance, such provisions would be severable from
this part and the invalidity or unenforceability would not affect the remainder thereof or any
other part of this subchapter or the application of such provision to other persons not similarly
situated or to other, dissimilar circumstances.
§ 428.20 Definitions.
As used in this part, the following definitions apply:
Annual manufacturer price (AnMP) refers to the amount calculated in § 428.202(b).
Applicable period means a 12-month period beginning with October 1 of a year
(beginning with October 1, 2022).
Applicable period Consumer Price Index for All Urban Consumers (CPI-U) means, with
respect to an applicable period, the CPI-U for the first month of such applicable period (that is,
October).
Applicable threshold means the amount calculated in accordance with § 428.101(b)(2).
Average manufacturer price (AMP) means the average price paid to the manufacturer for
the drug by wholesalers for drugs distributed to retail community pharmacies and retail
community pharmacies that purchase drugs directly from the manufacturer, calculated in
accordance with § 447.504 of this chapter.
Benchmark period CPI-U means the CPI-U identified in § 428.202(e).
Benchmark period manufacturer price refers to the amount calculated in § 428.202(d).
Covered Part D Drug has the meaning set forth in section 1860D-2(e) of the Act and
§ 423.100 of this chapter.
CPI-U means the monthly Consumer Price Index for All Urban Consumers (United
States city average) index level for all items from the Bureau of Labor Statistics.
First marketed date means the date that a manufacturer is required to report for a Part D
rebatable drug as its “market date” under section 1927(b)(3)(A)(v) of the Act.
Inflation-adjusted payment amount means the amount calculated in § 428.202(f).
Manufacturer has the meaning set forth in section 1927(k)(5) of the Act.
National Drug Code (NDC) means the unique identifying prescription drug product
number that is listed with FDA identifying the product and package size and type.
Part D rebatable drug means, subject to the exclusion described in § 428.101(b), a drug
or biological that is a covered Part D drug that, as of the first day of the applicable period, is:
(1) A drug approved under a New Drug Application (NDA) under section 505(c) of the
Federal Food, Drug, and Cosmetic (FD&C) Act;
(2) A generic drug approved under an Abbreviated New Drug Application (ANDA)
under section 505(j) of the FD&C Act (“section 505(j) ANDA”), in the case where:
(i) The reference listed drug approved under an NDA under section 505(c) of the FD&C
Act, including any authorized generic drug as defined in section 505(t)(3) of the FD&C Act, is
not being marketed, as identified in the Food and Drug Administration’s (FDA) NDC Directory;
(ii) There is no other drug approved under section 505(j) of the FD&C Act that is rated
as therapeutically equivalent in FDA’s most recent publication of “Approved Drug Products with
Therapeutic Equivalence Evaluations” (commonly known as the Orange Book), and that is being
marketed, as identified in FDA’s NDC Directory;
(iii) The manufacturer is not a “first applicant” during the “180-day exclusivity period,”
as those terms are defined in section 505(j)(5)(B)(iv) of the FD&C Act; and
(iv) The manufacturer is not a “first approved applicant” for a competitive generic
therapy, as that term is defined in section 505(j)(5)(B)(v) of the FD&C Act; or
(3) A biological licensed under section 351 of the Public Health Service (PHS) Act,
including a biosimilar.
Payment amount benchmark period means the period identified in § 428.202(c).
Subsequently approved drug means a Part D rebatable drug first approved or licensed by
the FDA after October 1, 2021.
Unit means, with respect to a Part D rebatable drug, the lowest dispensable amount (such
as a capsule or tablet, milligram of molecules, or grams) of the Part D rebatable drug, as reported
under section 1927 of the Act.
Subpart B—Determination of Part D Rebatable Drugs
§ 428.100 Definitions.
As used in this subpart, the following definitions apply:
Individual who uses such a drug or biological means a unique Medicare Part D
beneficiary who was dispensed the Part D drug or biological that was covered by their Part D
plan sponsor during the applicable period, identified using Prescription Drug Event (PDE) data
with dates of service during the applicable period and with gross covered prescription drug costs
greater than zero.
Gross covered prescription drug costs has the meaning set forth in § 423.308 of this
chapter.
§ 428.101 Identification of Part D rebatable drugs.
(a) Determination of Part D rebatable drugs. (1) For each applicable period, CMS will
use PDE data to identify all covered Part D drugs.
(2) CMS will match the covered Part D drugs identified in the PDE data with application
numbers using FDA sources to determine whether each covered Part D drug is a drug or
biological approved under an NDA under section 505(c) of the FD&C Act, approved under an
ANDA under section 505(j) of the FD&C Act, or licensed under a Biologics License Application
(BLA) under section 351 of the PHS Act, as of the first day of the applicable period.
(3) For a covered Part D drug identified in the PDE that is approved under an ANDA
under section 505(j) of the FD&C Act, CMS will determine whether such drug meets the criteria
in section 1860D-14B(g)(1)(C)(ii) of the Act as of the first day of the applicable period as
follows:
(i) To determine whether the reference listed drug or an authorized generic of the
reference listed drug is being marketed, as required under section 1860D-14B(g)(1)(C)(ii)(I) of
the Act, CMS will use FDA’s NDC Directory, including historical information from NDC
Directory files such as discontinued, delisted, and expired listings, provided by the FDA or
published on the FDA website.
(ii) To determine whether another drug has been approved under an ANDA that is
therapeutically equivalent to the Part D rebatable drug identified in this paragraph (a)(3), CMS
will use FDA’s Orange Book. To determine if this therapeutically equivalent drug is being
marketed, as required under section 1860D-14B(g)(1)(C)(ii)(II) of the Act, CMS will use FDA’s
NDC Directory, including historical information from NDC Directory files, such as
discontinued, delisted, and expired listings, provided by the FDA or published on the FDA
website.
(iii) To determine whether the manufacturer of the drug identified in this paragraph
(a)(3) is a first applicant during the 180-day exclusivity period, or whether the manufacturer of
this drug is a first approved applicant for a competitive generic drug therapy, CMS will refer to
publicly available FDA sources such as the Orange Book and may consult with FDA for
technical assistance as needed.
(b) Drugs and biologicals with average annual total cost below the applicable threshold.
For each applicable period, CMS will identify drugs and biologicals with average annual total
costs under Part D for such applicable period, per individual who uses such drug or biological,
that are below the applicable threshold in accordance with the steps described in this paragraph
(b). Such drugs and biologicals are not considered Part D rebatable drugs and will be excluded
from the identification of Part D rebatable drugs in paragraph (a) of this section.
(1) Average annual total cost. For each drug or biological that is identified in accordance
with paragraph (a) of this section, CMS will calculate average annual total costs under Part D per
individual who uses such drug or biological by dividing the gross covered prescription drug costs
for the drug or biological by the number of individuals who use such drug or biological in the
applicable period.
(2) Applicable threshold. CMS will calculate the applicable threshold for an applicable
period as follows:
(i) For the applicable period beginning October 1, 2022, the applicable threshold is equal
to $100.
(ii) For the applicable period beginning October 1, 2023, the applicable threshold is
equal to $100 increased by the percentage increase in CPI-U for the 12-month period beginning
October 1, 2023.
(iii) For subsequent applicable periods, the applicable threshold is equal to the applicable
threshold for the prior applicable period increased by the percentage increase in the CPI-U for
the 12-month period beginning with October of the previous period.
(iv) If the resulting amount under paragraph (b)(2)(ii) or (iii) of this section is not a
multiple of $10, CMS will round that amount to the nearest multiple of $10.
Subpart C—Determination of the Rebate Amount for Part D Rebatable Drugs
§ 428.200 Definitions.
As used in this subpart, the following definitions apply:
340B Program is the program under section 340B of the PHS Act.
Line extension has the meaning set forth in § 447.502 of this chapter.
New formulation has the meaning set forth in § 447.502 of this chapter.
Oral solid dosage form has the meaning set forth in § 447.502 of this chapter.
§ 428.201 Calculation of the total rebate amount to be paid by manufacturers.
(a) Total rebate. (1) Subject to paragraph (b) of this section, the total rebate amount to be
paid by a manufacturer for a Part D rebatable drug, as identified under § 428.101, for an applicable
period is equal to:
(i) The product of the per unit Part D rebate amount of such drug, as determined under
§ 428.202(a), and the total number of units dispensed of such drug under Part D, as determined
under § 428.203; or
(ii) In the case of a Part D rebatable drug that is a line extension of a Part D rebatable
drug that is an oral solid dosage form, the amount specified in § 428.204.
(2) The rebate amount may be reduced in accordance with subpart D of this part or
adjusted in accordance with subpart E of this part.
(b) Drugs and biologicals excluded from Part D rebate calculations. CMS will exclude
from the Part D drug inflation rebate calculations described in this subpart—
(1) Drugs and biologicals that meet the definition of a Part D rebatable drug but whose
manufacturers do not have an agreement in effect with the HHS Secretary under section 1927 of
the Act at any point during the applicable period, as determined by CMS through consultation
with Medicaid Drug Rebate Program staff and review of the Medicaid Drug Programs system.
(2) Drugs and biologicals that meet the definition of a Part D rebatable drug but, for the
entire duration of the applicable period, are excluded from the definition of covered outpatient
drugs as defined in section 1927(k)(2)-(4) of the Act and § 447.502 of this chapter, as determined
by CMS through consultation with Medicaid Drug Rebate Program staff and review of the
Medicaid Drug Programs system.
§ 428.202 Calculation of the per unit Part D drug rebate amount.
(a) Formula for calculating the per unit Part D rebate amount. CMS will calculate the
per unit Part D drug inflation rebate amount for a Part D rebatable drug and applicable period by
determining the amount by which the AnMP for the Part D rebatable drug, as calculated in
accordance with paragraph (b) of this section, exceeds the inflation-adjusted payment amount, as
calculated in accordance with paragraph (f) of this section.
(b) Calculation of the AnMP for the applicable period. Subject to paragraph (g) of this
section, CMS will calculate the AnMP for a Part D rebatable drug using the AMP reported by a
manufacturer under sections 1927(b)(3)(A)(i) and (ii) of the Act for each calendar quarter of the
applicable period and units reported by a manufacturer under section 1927(b)(3)(A)(iv) of the
Act for each month of the applicable period.
(1) CMS will calculate the AnMP for a Part D rebatable drug as the sum of the following:
(i) The product of—
(A) The AMP for the Part D rebatable drug reported for the calendar quarter beginning
October of the applicable period; and
(B) The sum of the monthly units reported for the calendar quarter beginning October of
the applicable period divided by the sum of the monthly units reported for the 4 calendar quarters
in the applicable period.
(ii) The product of—
(A) The AMP for the Part D rebatable drug reported for the calendar quarter beginning
January of the applicable period; and
(B) The sum of the monthly units reported for the calendar quarter beginning January of
the applicable period divided by the sum of the monthly units reported for the 4 calendar quarters
in the applicable period.
(iii) The product of—
(A) The AMP for the Part D rebatable drug reported for the calendar quarter beginning
April of the applicable period; and
(B) The sum of the monthly units reported for the calendar quarter beginning April of the
applicable period divided by the sum of the monthly units reported for the 4 calendar quarters in
the applicable period.
(iv) The product of—
(A) The AMP for the Part D rebatable drug reported for the calendar quarter beginning
July of the applicable period; and
(B) The sum of the monthly units reported for the calendar quarter beginning July of the
applicable period divided by the sum of the monthly units reported for the 4 calendar quarters in
the applicable period.
(2) The first applicable period for a Part D rebatable drug will be the earliest applicable
period that follows the payment amount benchmark period identified in paragraphs (c)(1)
through (4) of this section.
(c) Identification of the payment amount benchmark period. For each Part D rebatable
drug, CMS will identify the payment amount benchmark period using the date of FDA approval
or licensure or the first marketed date as set forth in paragraphs (c)(1) and (2) of this section,
subject to paragraphs (c)(3) through (5) of this section:
(1) For a Part D rebatable drug first approved or licensed by the FDA on or before
October 1, 2021, the payment amount benchmark period is the period beginning on
January 1, 2021, and ending on September 30, 2021;
(2) For a subsequently approved drug, the payment amount benchmark period is the first
calendar year beginning after the drug’s first marketed date;
(3) Notwithstanding paragraph (c)(2) of this section, for a Part D rebatable drug first
approved or licensed by the FDA on or before October 1, 2021, for which there are no quarters
during the period beginning on January 1, 2021, and ending on September 30, 2021, for which
AMP has been reported under section 1927(b)(3) of the Act, the payment amount benchmark
period is the first calendar year no earlier than calendar year 2021 in which such drug has at least
1 quarter of AMP reported;
(4) Notwithstanding paragraph (c)(3) of this section, for a subsequently approved drug
for which there are no quarters during the first calendar year beginning after the drug’s first
marketed date for which AMP has been reported under section 1927(b)(3) of the Act, the
payment amount benchmark period is the first calendar year in which such drug has at least
1 quarter of AMP reported; and
(5) Notwithstanding paragraph (c)(4) of this section, for a Part D rebatable drug that is a
selected drug (as defined in section 1192(c) of the Act) with respect to a price applicability
period (as defined in section 1191(b)(2) of the Act), in the case such Part D rebatable drug is no
longer considered to be a selected drug, for each applicable period beginning after the price
applicability period with respect to such drug, the payment amount benchmark period is the last
calendar year of such price applicability period with respect to such selected drug.
(d) Calculation of benchmark period manufacturer price. Subject to paragraph (g) of this
section, CMS will calculate the benchmark period manufacturer price for a Part D rebatable drug
using the AMP reported by a manufacturer under sections 1927(b)(3)(A)(i) and (ii) of the Act for
each calendar quarter of the payment amount benchmark period and the monthly units reported by
a manufacturer under section 1927(b)(3)(A)(iv) of the Act during the payment amount benchmark
period.
(1) For a Part D rebatable drug with a payment amount benchmark period identified
under paragraph (c)(1) of this section, CMS will calculate the benchmark period manufacturer
price as the sum of the following:
(i) The product of—
(A) The AMP reported for the calendar quarter beginning January 2021; and
(B) The sum of the monthly units reported for the calendar quarter beginning
January 2021 divided by the sum of the monthly units reported for the 3 quarters of the payment
amount benchmark period.
(ii) The product of—
(A) The AMP reported for the calendar quarter beginning April 2021; and
(B) The sum of the monthly units reported for the calendar quarter beginning April 2021
divided by the sum of the monthly units reported for the 3 quarters of the payment amount
benchmark period.
(iii) The product of—
(A) The AMP reported for the calendar quarter beginning July 2021; and
(B) The sum of the monthly units reported for the calendar quarter beginning July 2021
divided by the sum of the units reported for the 3 quarters of the payment amount benchmark
period.
(2) For a Part D rebatable drug with a payment amount benchmark period identified
under paragraphs (c)(2) through (5) of this section, CMS will calculate the benchmark period
manufacturer price as the sum of the following:
(i) The product of—
(A) The AMP reported for the calendar quarter beginning January of the payment
amount benchmark period; and
(B) The sum of the monthly units reported for the calendar quarter beginning January of
the payment amount benchmark period divided by the sum of the monthly units reported for the
4 quarters of the payment amount benchmark period.
(ii) The product of—
(A) The AMP reported for the calendar quarter beginning April of the payment amount
benchmark period; and
(B) The sum of the monthly units reported for the calendar quarter beginning April of the
payment amount benchmark period divided by the sum of the monthly units reported for the
4 quarters of the payment amount benchmark period.
(iii) The product of—
(A) The AMP reported for the calendar quarter beginning July of the payment amount
benchmark period; and
(B) The sum of the monthly units reported for the calendar quarter beginning July of the
payment amount benchmark period divided by the sum of the monthly units reported for the
4 quarters of the payment amount benchmark period.
(iv) The product of—
(A) The AMP reported for the calendar quarter beginning in October of the payment
amount benchmark period; and
(B) The sum of the monthly units reported for the calendar quarter beginning October of
the payment amount benchmark period divided by the sum of the monthly units reported for the
4 quarters of the payment amount benchmark period.
(e) Identification of the benchmark period CPI-U. For each Part D rebatable drug, CMS
will identify the benchmark period CPI-U as set forth in paragraphs (e)(1) and (2) of this section,
subject to paragraphs (e)(3) through (5) of this section:
(1) For a Part D rebatable drug first approved or licensed by the FDA on or before
October 1, 2021, the benchmark period CPI-U is the CPI-U for January 2021.
(2) For a subsequently approved drug, the benchmark period CPI-U is the CPI-U for
January of the first calendar year beginning after a drug’s first marketed date.
(3) Notwithstanding paragraph (e)(2) of this section, for a Part D rebatable drug first
approved or licensed by the FDA on or before October 1, 2021, for which there are no quarters
during the period beginning on January 1, 2021, and ending on September 30, 2021, for which
AMP has been reported under section 1927(b)(3) of the Act, the benchmark period CPI-U is the
CPI-U for January of the payment amount benchmark period identified under paragraph (c)(3) of
this section.
(4) Notwithstanding paragraph (e)(3) of this section, for a subsequently approved drug
for which there are no quarters during the first calendar year beginning after the drug’s first
marketed date for which AMP has been reported under section 1927(b)(3) of the Act, the
benchmark period CPI-U is the CPI-U for January of the payment amount benchmark period
identified under paragraph (c)(4) of this section.
(5) Notwithstanding paragraph (e)(5) of this section, for a drug that is a selected drug (as
defined in section 1192(c) of the Act) with respect to a price applicability period (as defined in
section 1191(b)(2) of the Act), in the case such Part D rebatable drug is no longer considered to
be a selected drug, the benchmark period CPI-U is the CPI-U for January of the last calendar
year of such price applicability period.
(f) Calculation of inflation-adjusted payment amount. For an applicable period for each
Part D rebatable drug, CMS will calculate the inflation-adjusted payment amount by dividing the
applicable period CPI-U by the benchmark period CPI-U and then multiplying the quotient by the
benchmark period manufacturer price.
(g) Situations in which manufacturers do not report units under section 1927(b)(3)(A)(iv)
of the Act. For the purpose of calculating the AnMP in accordance with paragraph (b) of this
section and the benchmark period manufacturer price in accordance with paragraph (d) of this
section—
(1) If there is 1 or more quarter(s) in the payment amount benchmark period or
applicable period for which a manufacturer has not reported units under section
1927(b)(3)(A)(iv) of the Act but has reported AMP under sections 1927(b)(3)(A)(i) and (ii) of
the Act, CMS will calculate the benchmark period manufacturer price or AnMP, as applicable,
using data only from quarter(s) with units. Quarter(s) in the payment amount benchmark period
or applicable period for which a manufacturer has not reported units under
section 1927(b)(3)(A)(iv) of the Act will be excluded from the calculation.
(2) If there are no quarters of the payment amount benchmark period or applicable
period for which a manufacturer has reported units under section 1927(b)(3)(A)(iv) of the Act,
but the manufacturer but has reported AMP, under sections 1927(b)(3)(A)(i) and (ii) of the Act
for at least 1 quarter of such period, CMS will use the average of the AMP over the calendar
quarters of the payment amount benchmark period or applicable period for which AMP is
reported to calculate the benchmark period manufacturer price or AnMP, respectively.
§ 428.203 Determination of the total number of units dispensed under Part D.
(a) General. For each Part D rebatable drug, CMS will determine the total number of
units as follows:
(1) Use of PDE data to determine total units dispensed. To determine the total number of
units of each Part D rebatable drug dispensed under Part D and covered by Part D plan sponsors
during an applicable period, CMS will use the quantity dispensed reported on the PDE record for
each Part D rebatable drug with gross covered prescription drug costs greater than zero.
(2) Crosswalk to AMP units. CMS will crosswalk the information from the PDE record
to database(s) that includes the unit type (for example, each, capsule) for the Part D rebatable
drug, matching on the NDC of the Part D rebatable drug. If the unit type obtained from such
database does not match the AMP unit type reported by a manufacturer to the Medicaid Drug
Programs system, CMS will convert the total units reported on the PDE to the AMP units
reported.
(b) Removal of certain units. CMS will exclude certain units from the total number of
units dispensed of a Part D rebatable drug, with respect to an applicable period, as follows:
(1) Removal of units when a generic drug is no longer a Part D rebatable drug. To
determine whether a generic drug that meets the definition of a Part D rebatable drug on the first
day of an applicable period ceases to meet such definition later in the applicable period, CMS
will—
(i) Review FDA’s NDC Directory, including historical information from NDC Directory
files such as discontinued, delisted, and expired listings provided by the FDA or published on the
FDA website to determine whether the reference listed drug or an authorized generic of the
reference listed drug is being marketed;
(ii) Review the most recent version of the downloadable FDA Orange Book to determine
whether another drug has been approved under a section 505(j) ANDA that is therapeutically
equivalent to such generic drug. If CMS determines that FDA has approved such a
therapeutically equivalent drug under a section 505(j) ANDA, CMS will then: use the FDA’s
NDC Directory, including historical information from NDC Directory files such as discontinued,
delisted, and expired listings provided by the FDA or published on the FDA website to determine
the marketing status of such therapeutically equivalent drug and whether, during the applicable
period, the therapeutically equivalent drug was marketed; and
(iii) Exclude from the total number of units determined under paragraph (a) of this
section any units dispensed on or after the first day of the calendar month that a generic drug no
longer meets the definition of a Part D rebatable drug.
(2) Exclusion of units acquired through the 340B Program. (i) For the applicable period
beginning October 1, 2025, and subsequent applicable periods, CMS will exclude from the total
number of units determined under paragraph (a) of this section units for which a manufacturer
provided a discount under the 340B Program (“340B units”) as follows:
(A) For the applicable period beginning October 1, 2025, 340B units will be excluded
from the total number of units dispensed for claims with a date of service on or after
January 1, 2026.
(B) For the applicable period beginning October 1, 2026, and applicable periods
thereafter, 340B units will be excluded from the total number of units dispensed.
(ii) To determine the total number of such units for which a manufacturer provided a
discount under the 340B Program, CMS will use data reflecting the total number of units of a
Part D rebatable drug for which a discount was provided under the 340B Program and that were
dispensed during the applicable period.
§ 428.204 Treatment of new formulations of Part D rebatable drugs.
In the case of a Part D rebatable drug that is a line extension of a Part D rebatable drug
that is an oral solid dosage form, the rebate amount for an applicable period is equal to the
amount computed under § 428.201(a) for such new drug or, if greater, the alternative total rebate
amount. CMS will determine the alternative total rebate amount for such new formulations
according to the following:
(a) Identification of the initial drug. The initial drug that CMS will use to calculate the
inflation rebate amount ratio is the initial drug identified in accordance with
§ 447.509(a)(4)(iii)(B) of this chapter for the last quarter of the applicable period or, if an initial
drug was not identified in the last quarter, the initial drug identified for a quarter most recently in
that applicable period.
(b) Calculation of the inflation rebate amount ratio. The inflation rebate amount ratio is
equal to the per unit Part D drug inflation rebate amount for the initial drug, as calculated in
§ 428.202(a), divided by the AnMP for that initial drug for the applicable period.
(c) Calculation of the alternative total rebate amount. The alternative total rebate
amount is equal to the product of all of the following:
(1) The AnMP for the applicable period, as calculated in § 428.202(b), of the Part D
rebatable drug that is a line extension of a Part D rebatable drug that is an oral solid dosage form.
(2) The inflation rebate amount ratio as determined in paragraph (b) of this section.
(3) The total number of units dispensed under Part D identified in § 428.203.
Subpart D—Reducing the Rebate Amount for Part D Rebatable Drugs in Shortage and
When There Is a Severe Supply Chain Disruption or Likely Shortage
§ 428.300 Definitions.
As used in this subpart, the following definitions apply:
Biosimilar has the meaning set forth in section 351(i) of the PHS Act.
Currently in shortage means that at least one NDC-10 of a Part D rebatable drug with the
status “currently in shortage” is on a shortage list maintained by the FDA under section 506E of
the FD&C Act.
Drug shortage or shortage means a period of time when the demand or projected demand
for the drug within the United States exceeds the supply of the drug (see section 506C(h)(2) of
the FD&C Act).
Generic Part D rebatable drug means a generic drug approved under an ANDA under
section 505(j) of the FD&C Act that meets the sole source criteria specified in § 428.101(a)(3).
Likely to be in shortage means that a generic Part D rebatable drug is likely to be
described as currently in shortage during a subsequent applicable period without such rebate
reduction.
Natural disaster means any natural catastrophe, including, but not limited to any of the
following: hurricane, tornado, storm, high water, wind-driven water, tidal wave, tsunami,
earthquake, volcanic eruption, landslide, mudslide, snowstorm, or drought, or regardless of
cause, any fire, flood, or explosion.
Other unique or unexpected event means any exogenous, unpredictable event outside of a
manufacturer’s control, including, but not limited to, a geopolitical disruption, pandemic, or act
of terror.
Plasma-derived product means a licensed biological product that is derived from human
whole blood or plasma, as indicated on the approved product labeling.
Severe supply chain disruption means a change in production or distribution that is
reasonably likely to lead to a significant reduction in the U.S. supply of a generic Part D
rebatable drug or biosimilar by a manufacturer and significantly affects the ability of the
manufacturer of the generic drug or biosimilar to fill orders or meet expected demand for its
product in the United States for at least 90 days. This definition does not include interruptions in
manufacturing due to matters such as routine maintenance, manufacturing quality issues, or
insignificant changes made in the manufacturing process for the drug.
§ 428.301 Reducing the rebate amount for Part D rebatable drugs currently in shortage.
(a) General. As required under section 1860D-14B(b)(1)(C)(i) of the Act, CMS will
reduce the total rebate amount calculated under § 428.201(a), if any is owed, for a Part D
rebatable drug that is currently in shortage, as defined in § 428.300, at any point during the
applicable period.
(b) Calculation of the reduced rebate amount. (1) For each applicable period beginning
on or after October 1, 2022, the reduced rebate amount for a Part D rebatable drug currently in
shortage will be calculated using the following formula:
Equation 1 to Paragraph (b)(1)
Reduced Total Rebate Amount = the total rebate amount multiplied by (1 minus
applicable percent reduction) multiplied by (percentage of time drug was currently in shortage
during the applicable period) added to the total rebate amount multiplied by (1 minus percentage
of time drug was currently in shortage during the applicable period)
(2) For purposes of paragraph (b)(1) of this section, the applicable percent reduction is:
(i) For a Part D rebatable drug that is a generic drug or plasma-derived product:
(A) 75 percent for the first applicable period such drug is currently in shortage.
(B) 50 percent for the second applicable period such drug is currently in shortage.
(C) 25 percent for each subsequent period such drug is currently in shortage.
(ii) For a Part D rebatable drug that is not a generic drug or plasma-derived product:
(A) 25 percent for the first applicable period such drug is currently in shortage.
(B) 10 percent for the second applicable period such drug is currently in shortage.
(C) 2 percent for each subsequent applicable period such drug is currently in shortage.
(3) For purposes of paragraph (b)(1) of this section, the percentage of time the drug is
currently in shortage during the applicable period is equal to the number of days such drug is
currently in shortage in an applicable period, divided by the total number of days in the
applicable period.
(c) Application of reduction. CMS will apply a reduction of the rebate amount as
determined under paragraph (b) of this section to the Part D rebatable drug at the NDC-9 level.
§ 428.302 Reducing the rebate amount for certain Part D rebatable drugs when there is a
severe supply chain disruption.
(a) General. As required under section 1860D-14B(b)(1)(C)(ii) of the Act, CMS will
reduce the total rebate amount calculated under § 428.201(a), if any is owed, for a generic Part D
rebatable drug or biosimilar when CMS determines there is a severe supply chain disruption, as
defined in § 428.300, during the applicable period such as that caused by a natural disaster or
other unique or unexpected event.
(b) Calculation of the reduced rebate amount--(1) Initial reduction. If CMS determines
the criteria described in paragraph (c)(4) of this section are met, then CMS will reduce the total
rebate amount owed by the manufacturer for a generic Part D rebatable drug or biosimilar by
75 percent for an applicable period.
(2) Extension of reduction. If CMS determines a severe supply chain disruption
continues into a second consecutive applicable period as described in paragraph (c)(5) of this
section, then CMS will reduce the total rebate amount owed by the manufacturer for a generic
Part D rebatable drug or biosimilar by 75 percent for that second applicable period.
(3) Application of reduction. If CMS determines there is a severe supply chain disruption
for an NDC-11, CMS will apply any reduction of the rebate amount as described in paragraphs
(b)(1) and (2) of this section to a Part D rebatable drug at the NDC-9 level.
(4) Limitation on rebate reductions. CMS will not apply multiple rebate reductions for
the same Part D rebatable drug and applicable period.
(i) If a manufacturer believes there are multiple events causing severe supply chain
disruptions during the same applicable period for the same generic Part D rebatable drug or
biosimilar and submits multiple rebate reduction requests for the same drug or biosimilar, CMS
will grant no more than 1 rebate reduction under paragraph (b)(1) or (2) of this section for that
product for the applicable period.
(ii) If CMS grants a rebate reduction request under this section and the generic Part D
rebatable drug or biosimilar subject to the reduction is currently in shortage during the same
applicable period as the one for which the severe supply chain disruption reduction request was
granted, CMS will reduce the rebate amount as described in paragraph (b)(1) of this section and
will not grant a reduction under § 428.301 during that applicable period.
(iii) If a generic Part D rebatable drug or biosimilar that is currently in shortage
experiences a severe supply chain disruption, CMS will reduce the rebate amount as described in
paragraph (b)(1) of this section, and will not grant a reduction under § 428.301 during that
applicable period.
(c) Eligibility for a rebate reduction--(1) Eligible drug. Subject to paragraph (b)(3) of
this section, eligibility for a rebate reduction under this section is limited to Part D rebatable
drugs and biosimilars for which a manufacturer submits a rebate reduction request under this
section.
(2) Timing. For a natural disaster or other unique or unexpected event occurring on or
after August 2, 2024 that the manufacturer believes caused a severe supply chain disruption, the
manufacturer must submit the rebate reduction request within 60 calendar days from the first day
that the natural disaster or other unique or unexpected event occurred or began to receive
consideration for a reduction in the rebate amount owed in accordance with paragraph (b)(1) of
this section.
(3) Required elements of a rebate reduction request. To receive consideration for a
reduction in the rebate amount owed in accordance with paragraph (b)(1) of this section, the
manufacturer must submit to CMS information and supporting documentation to substantiate the
evaluation criteria described in paragraph (c)(4) of this section. Such information and supporting
documentation include the following:
(i) Evidence that the severe supply chain disruption directly affects the manufacturer
itself, a supplier of an ingredient or packaging, a contract manufacturer, or a method of shipping
or distribution that the manufacturer uses to make or distribute the generic Part D rebatable
drug(s) or biosimilar(s), such as a change in the production or distribution of the generic Part D
rebatable drug(s) or biosimilar(s) that is reasonably likely to lead to a significant reduction in the
U.S. supply of product and significantly affects the manufacturer’s ability to fill orders or meet
expected demand for the generic Part D rebatable drug(s) or biosimilar(s) for at least 90 days;
(ii) Information about when the manufacturer expects supply of the generic Part D
rebatable drug(s) or biosimilar(s) to meet expected demand;
(iii) Evidence that the natural disaster or other unique or unexpected event caused the
severe supply chain disruption, including when the natural disaster or other unique or unexpected
event occurred or began occurring, and the expected or actual duration of the severe supply chain
disruption; and
(iv) Evidence of the manufacturer’s physical presence related to manufacturing the
generic Part D rebatable drug(s) or biosimilar(s) in a geographic area where a natural disaster or
other unique or unexpected event occurred. If the manufacturer is not physically present in a
geographic area where a natural disaster or other unique or unexpected event occurred, but
believes there is a severe supply chain disruption caused by a natural disaster or other unique or
unexpected event that affects the manufacturer’s generic Part D rebatable drug(s) or
biosimilar(s), the information and supporting documentation may include evidence of the impact
of the natural disaster or other unique or unexpected event on the supply chain of the generic Part
D rebatable drug or biosimilar, on a supplier of an ingredient or packaging, or method of
shipping or distribution that the manufacturer uses.
(4) Evaluation criteria. In accordance with paragraph (b)(1) of this section, CMS will
grant a reduction in the rebate amount owed if a manufacturer submits to CMS a request in
writing for an eligible drug, in accordance with the timing specified in paragraph (c)(2) of this
section, demonstrating that:
(i) A severe supply chain disruption has occurred during the applicable period;
(ii) The severe supply chain disruption directly affects the manufacturer itself, a contract
manufacturer, a supplier of an ingredient or packaging, or a method of shipping or distribution
that the manufacturer uses in a significant capacity to make or distribute the generic Part D
rebatable drug or biosimilar; and
(iii) The severe supply chain disruption was caused by a natural disaster or other unique
or unexpected event.
(5) Rebate reduction extensions. If CMS determines that a generic Part D rebatable drug
or biosimilar that received a reduction of the rebate amount under paragraph (b)(1) of this section
continues to be affected by the severe supply chain disruption, CMS will grant a single extension
of the reduction for 1 additional consecutive applicable period and reduce the rebate amount
owed in accordance with paragraph (b)(2) of this section.
(i) To receive consideration for a rebate reduction extension, a manufacturer must submit
a request with updated or new information and supporting documentation on why the generic
Part D rebatable drug or biosimilar continues to be affected by the severe supply chain disruption
during the second applicable period.
(ii) A manufacturer must submit the rebate reduction extension request at least
60 calendar days before the start of the second consecutive applicable period to receive
consideration for a reduction in the rebate amount owed, if any, in accordance with paragraph
(b)(2) of this section, except for when the initial request is made less than 60 calendar days
before the end of an applicable period such that the initial rebate reduction is applied to the next
applicable period rather than the applicable period in which the event that caused the severe
supply chain disruption occurred or began. In these cases, the rebate reduction extension request
must be submitted at least 60 calendar days prior to the end of the applicable period in which the
initial reduction under paragraph (b)(1) of this section is applied.
(6) Decision to grant or deny a request. CMS will review rebate reduction requests and
rebate reduction extension requests within 60 calendar days of receipt of all documentation, if
feasible, beginning with the applicable period that begins on October 1, 2024.
(i) CMS will deny a rebate reduction request that does not meet the criteria in paragraph
(c)(4) of this section or that is incomplete or untimely based on the requirements of this
paragraph (c).
(ii) CMS will deny a rebate reduction extension request that does not meet the criteria in
paragraph (c)(5) of this section, that is incomplete or untimely based on the requirements of
paragraph (c)(5) of this section, or if a reduction under paragraph (b)(1) of this section was not
provided for such generic Part D rebatable drug or biosimilar.
(iii) CMS’ decisions to deny a request are final and will not be subject to an appeals
process.
(7) Public disclosure of information. CMS will keep confidential, to the extent allowable
under law, any requests for a rebate reduction, including supporting documentation. Information
provided as part of a request for a rebate reduction request that the submitter indicates is a trade
secret or confidential commercial or financial information will be protected from disclosure if
CMS determines the information meets the requirements set forth under Exemption 3 or
Exemption 4 in 5 U.S.C. 552.
§ 428.303 Reducing the rebate amount for generic Part D rebatable drugs likely to be in
shortage.
(a) General. As required under section 1860D-14B(b)(1)(C)(iii) of the Act, CMS will
reduce the total rebate amount calculated under § 428.201, if any is owed, for a generic Part D
rebatable drug when CMS determines that the generic Part D rebatable drug is likely to be in
shortage, as defined in § 428.300.
(b) Calculation of the reduced rebate amount—(1) Initial reduction. If CMS determines
the criteria described in paragraph (c)(4) of this section are met, then CMS will reduce the total
rebate amount owed by the manufacturer for a generic Part D rebatable drug by 75 percent for an
applicable period.
(2) Extension of reduction. If CMS determines the generic Part D rebatable drug is likely
to be in shortage in a second applicable period as described in paragraph (c)(5) of this section,
then CMS will reduce the total rebate amount owed by the manufacturer for a generic Part D
rebatable drug by 75 percent for a second consecutive applicable period.
(3) Application of reduction. If CMS determines that an NDC-11 is likely to be in
shortage, CMS will apply any reduction of the rebate amount as described in paragraphs (b)(1)
and (2) of this section to the generic Part D rebatable drug at the NDC-9 level.
(4) Limitation on rebate reductions. If CMS grants a rebate reduction request under this
section, and the generic Part D rebatable drug subject to the reduction is currently in shortage
during the same applicable period as the one for which the request was granted, CMS will reduce
the rebate amount as described in paragraph (b)(1) of this section and will not grant a reduction
under § 428.301 during that applicable period.
(c) Eligibility for a rebate reduction—(1) Eligible drug. Subject to paragraph (b)(3) of
this section, eligibility for a rebate reduction under this section is limited to generic Part D
rebatable drugs for which a manufacturer submits a rebate reduction request under this section.
(2) Timing. The manufacturer must submit the rebate reduction request before the start of
the next applicable period in which the manufacturer believes the generic Part D rebatable drug
is likely to be in shortage to receive consideration for a reduction in the rebate amount owed in
accordance with paragraph (b)(1) of this section.
(3) Required elements of a rebate reduction request. To receive consideration for a
reduction in the rebate amount owed in accordance with paragraph (b)(1) of this section, the
manufacturer must submit to CMS information and supporting documentation to substantiate the
evaluation criteria described in paragraph (c)(4) of this section. Such information and supporting
documentation include the following:
(i) Evidence that demonstrates a generic Part D rebatable drug is likely to be in shortage,
including anticipated cause(s) of the shortage and information about why the manufacturer
believes the generic Part D rebatable drug is likely to be in shortage; and
(ii) Evidence of the anticipated start date and duration of the potential drug shortage, the
actions the manufacturer is taking to avoid the potential drug shortage, and how the reduction of
the rebate amount would reduce the likelihood of the drug appearing on an FDA shortage list.
(4) Evaluation criteria. In accordance with paragraph (b)(1) of this section, CMS will
grant a reduction in the rebate amount owed if a manufacturer submits to CMS a request in
writing for an eligible drug, in accordance with the timing specified in paragraph (c)(2) of this
section, demonstrating that:
(i) The generic Part D rebatable drug is likely to be in shortage;
(ii) The manufacturer is taking actions to avoid the potential drug shortage; and
(iii) The reduction of the rebate amount would reduce the likelihood of the drug
appearing on an FDA shortage list.
(5) Rebate reduction extensions. If CMS determines that a generic Part D rebatable drug
that received a reduction of the rebate amount under paragraph (b)(1) of this section continues to
be affected by the potential drug shortage, CMS will grant a single extension of the reduction for
1 additional consecutive applicable period and reduce the rebate amount owed in accordance
with paragraph (b)(2) of this section.
(i) To receive consideration for a rebate reduction extension, a manufacturer must submit
a request with updated or new information and supporting documentation on why the generic
Part D rebatable drug continues to be affected by the potential drug shortage during the second
applicable period.
(ii) A manufacturer must submit the rebate reduction extension request at least
60 calendar days before the start of the second consecutive applicable period in which the
manufacturer believes the generic Part D rebatable drug is likely to be in shortage to receive
consideration for a reduction in the rebate amount owed, if any, in accordance with paragraph
(b)(2) of this section.
(6) Decision to grant or deny a request. CMS will review rebate reduction requests and
rebate reduction extension requests within 60 calendar days of receipt of all documentation, if
feasible, beginning with the applicable period that begins on October 1, 2024.
(i) CMS will deny a rebate reduction request that does not meet the criteria in paragraph
(c)(4) of this section or that is incomplete or untimely based on the requirements of this
paragraph (c).
(ii) CMS will deny a rebate reduction extension request that does not meet the criteria in
paragraph (c)(5) of this section, that is incomplete or untimely based on the requirements of
paragraph (c)(5) of this section, or if a reduction under paragraph (b)(1) of this section was not
provided for such generic Part D rebatable drug.
(iii) CMS’ decisions to deny a request are final and will not be subject to an appeals
process.
(7) Public disclosure of information. CMS will keep confidential, to the extent allowable
under law, any requests for a rebate reduction, including supporting documentation. Information
provided as part of a request for a rebate reduction that the submitter indicates is a trade secret or
confidential commercial or financial information will be protected from disclosure if CMS
determines the information meets the requirements set forth under Exemption 3 or Exemption 4
in 5 U.S.C. 552.
Subpart E—Reports of Rebate Amounts, Reconciliation, Suggestion of Error, and
Payments
§ 428.400 Definitions.
For the purposes of this subpart, date of receipt is the calendar day following the day in
which a report of a rebate amount (as set forth in §§ 428.401(b), (c), and (d) and 428.402(b) and
(c)) is made available to the manufacturer of a Part D rebatable drug by CMS.
§ 428.401 Rebate Reports and reconciliation.
(a) General. This section applies to Part D rebatable drugs for all applicable periods
except as otherwise set forth in § 428.402 for the applicable periods beginning October 1, 2022,
and October 1, 2023.
(b) Preliminary Rebate Report. A Preliminary Rebate Report will be provided to each
manufacturer of a Part D rebatable drug at least 1 month prior to the issuance of the Rebate
Report as set forth in paragraph (c) of this section for an applicable period.
(1) The Preliminary Rebate Report for each Part D rebatable drug will include the
following information:
(i) The NDC(s) for the Part D rebatable drug as defined under § 428.20;
(ii) The total number of units dispensed under Part D for the Part D rebatable drug for
the applicable period as determined under § 428.203;
(iii) The benchmark period manufacturer price as described in § 428.202(d).
(iv) The AnMP for the Part D rebatable drug for the applicable period as determined in
§ 428.202(b);
(v) The amount, if any, of the excess AnMP for the Part D rebatable drug for the
applicable period as set forth under § 428.202(a);
(vi) The benchmark period and applicable period CPI-Us as set forth in §§ 428.202(e)
and 428.20, respectively;
(vii) Inflation-adjusted payment amount as set forth in § 428.202(f);
(viii) Any applied reductions described in §§ 428.301, 428.302, and 428.303; and
(ix) Rebate amount due as set forth in § 428.201(a).
(2) If the Part D rebatable drug is a line extension, the Preliminary Rebate Report will
also include the following information described in § 428.204:
(i) The NDC for the initial drug;
(ii) The inflation rebate amount ratio for the initial drug; and
(iii) The alternative total rebate amount.
(c) Rebate Report. A Rebate Report will be provided to each manufacturer of a Part D
rebatable drug no later than 9 months after the end of each applicable period.
(1) The Rebate Report will include the information described in paragraphs (b)(1) and
(2) of this section, if applicable, with the inclusion of any revisions to such information resulting
from CMS’ review of a Suggestion of Error as set forth in § 428.403, if applicable, and any
CMS-determined recalculations from paragraph (d)(2) of this section.
(2) The Rebate Report is the invoice of a manufacturer’s rebate amount due as calculated
in § 428.201(a), if any, for a Part D rebatable drug for an applicable period.
(d) Reconciliation of the rebate amount. CMS will perform reconciliation of the rebate
amount provided in a Rebate Report specified in paragraph (c) of this section for an applicable
period in the following circumstances:
(1) Regular reconciliation. Except as otherwise described in § 428.402, CMS will
perform a reconciliation of the rebate amount within 12 months of the date of receipt of the
Rebate Report for an applicable period and a second reconciliation approximately 24 months
thereafter to include revisions to the information used to calculate the rebate amount as specified
in paragraph (c)(1) of this section.
(i) Preliminary reconciliation. At least 1 month prior to the issuance of a report with the
reconciled rebate amount for an applicable period specified in paragraph (d)(1)(ii) of this section,
CMS will conduct a preliminary reconciliation of the rebate amount for an applicable period
based on the information specified in paragraphs (d)(1)(i)(A) through (G) of this section, and
CMS will provide the information specified in paragraphs (d)(1)(i)(A) through (G) to the
manufacturer of a Part D rebatable drug for the applicable period, if applicable:
(A) Updated total number of rebatable units, including updates submitted by a
prescription drug plan (PDP) or Medicare Advantage Prescription Drug (MA-PD) plan sponsor
and updates to 340B units (as applicable to the dates of service and applicable periods specified
in § 428.203(b)(2)(i)(A) and (B)), or units otherwise excluded as specified in § 428.203(b);
(B) Inflation-adjusted payment amount as specified in § 428.202(f) if any inputs are
restated within the reconciliation run-out period;
(C) Updated benchmark period manufacturer price as specified in § 428.202(d);
(D) The excess amount by which the AnMP exceeds the inflation-adjusted payment
amount for the applicable period as specified in § 428.202(a), using the most recent AMP (if any
inputs are restated within the reconciliation run-out period);
(E) Updated data on line extension calculations, including the initial drug identified as
set forth in § 447.509(a)(4)(iii)(B) of this chapter, the inflation rebate amount ratio, and the
alternative total rebate amount as set forth in § 428.204 if any inputs are restated within the
reconciliation run-out period;
(F) Reconciled rebate amount as specified in § 428.201(a); and
(G) The difference between the total rebate amount due as specified on the Rebate
Report set forth in paragraph (c) of this section and the reconciled rebate amount as set forth in
this paragraph (d)(1)(i).
(ii) Report with a reconciled rebate amount. With the inclusion of any additional
revisions to such information resulting from CMS’ review of a Suggestion of Error as set forth in
§ 428.403, if applicable, a report with the reconciled rebate amount will be provided to each
manufacturer of a Part D rebatable drug within 12 months and 36 months after the issuance of
the Rebate Report described in paragraph (c) of this section.
(2) CMS identification of an error or manufacturer misreporting. CMS may recalculate a
rebate amount and provide the manufacturer of a Part D rebatable drug a report with a reconciled
rebate amount when:
(i) CMS identifies an error in the information specified in paragraphs (c) and (d)(1) of
this section, including reporting system or coding errors, not later than 5 years from the date of
receipt by a manufacturer of a reconciled rebate amount for the applicable period; or
(ii) CMS determines at any time that the information used by CMS to calculate the
rebate amount was inaccurate due to manufacturer misreporting.
(3) Impact of reconciliation on rebate amount. A reconciliation as set forth in this
paragraph (d) could result in an increase, decrease, or no change to the rebate amount as
calculated under § 428.201(a) owed by a manufacturer for the applicable period for the Part D
rebatable drug.
(i) A report with a reconciled rebate amount that is an increase to the rebate amount is
the invoice for such additional amount due on the manufacturer’s rebate amount as set forth in §
428.201 for a Part D rebatable drug for an applicable period.
(ii) [Reserved]
(4) Drugs included in a reconciliation. A drug covered under Part D that does not meet
the requirements of a rebatable drug specified in § 428.101 for an applicable period will not be
included in a reconciliation under this paragraph (d).
§ 428.402 Rebate Reports for applicable periods beginning October 1, 2022, and
October 1, 2023.
(a) Transition rule for reporting. Section 1860D-14B(a)(3) of the Act allows CMS to
delay the timeframe for reporting the information and rebate amount described in § 428.401 for
the applicable periods beginning October 1, 2022, and October 1, 2023, until not later than
December 31, 2025.
(b) Rebate Report information for applicable periods beginning October 1, 2022, and
October 1, 2023. The Rebate Reports for the applicable periods beginning October 1, 2022, and
October 1, 2023, will include the information described in § 428.401(b)(1).
(c) Rebate Report procedures for applicable periods beginning October 1, 2022, and
October 1, 2023. Rebate amounts for the applicable periods beginning October 1, 2022, and
October 1, 2023, will be reported as follows:
(1) The Rebate Report for the applicable period beginning October 1, 2022, will be
issued no later than December 31, 2025. The Preliminary Rebate Report for such applicable
period will be issued at least 1 month prior to the Rebate Report.
(i) For this single Preliminary Rebate Report for the applicable period, the Suggestion of
Error period as described in § 428.403 will be 30 calendar days.
(ii) The rebate amount will be reconciled 21 months after the Rebate Report specified in
paragraph (b)(1) of this section is issued to include the information specified in
§ 428.401(d)(1)(i)(A) through (G).
(iii) The Suggestion of Error period for the reconciliation described in paragraph
(b)(1)(ii) of this section will be 10 calendar days.
(2) The Rebate Report for the applicable period beginning October 1, 2023, will be
issued no later than December 31, 2025. The Preliminary Rebate Report for such applicable
period will be issued at least 1 month prior to the Rebate Report.
(i) For this single Preliminary Rebate Report for the applicable period, the Suggestion of
Error period as described in § 428.403 will be 30 calendar days.
(ii) The rebate amount will be reconciled 9 months after the Rebate Report and
33 months after the Rebate Report specified in paragraph (b)(2) of this section is issued to
include the information specified in § 428.401(d)(1)(i)(A) through (G).
§ 428.403 Suggestion of Error.
(a) General. Manufacturers of Part D rebatable drugs may submit a Suggestion of Error
about the information in their Preliminary Rebate Report and the report detailing the preliminary
reconciliation of the rebate amount to CMS, for its discretionary consideration, if the
manufacturer believes that there is a mathematical error or errors to be corrected before the
Rebate Report or a subsequent reconciliation, as applicable, is finalized.
(1) Section 1860D-14B(f) of the Act precludes administrative or judicial review on the
determination of units as set forth in § 428.203, the determination of whether a drug is a Part D
rebatable drug as set forth in § 428.101, and the calculation of the rebate amount as set forth in
§ 428.201(a) inclusive of any reconciled rebate amount.
(2) [Reserved]
(b) Process of submission. Subject to the scope and timing requirements specified in
paragraphs (a) and (c) of this section, manufacturers may submit the Suggestion of Error and
provide supporting documentation (if applicable).
(c) Timing. Except as specified in § 428.402 for the applicable periods beginning on
October 1, 2022, and October 1, 2023, a manufacturer must submit its Suggestion of Error for
the applicable period within 10 calendar days from the date of receipt of a Preliminary Rebate
Report or a preliminary reconciliation of a rebate amount using the method and process
established by CMS in paragraph (b) of this section.
(d) Notice. (1) CMS will include any revisions to the calculation of the rebate amount, if
determined necessary by CMS based on the Suggestion of Error submitted under this section
prior to issuance of the Rebate Report as set forth in § 428.401(c) or § 428.402(c) as well as any
report of a reconciled rebate amount as set forth in § 428.401(d) or § 428.402(c)(1)(ii) and
(c)(2)(ii).
(2) CMS will notify the manufacturer whether CMS revised its calculation of the rebate
amount based on the Suggestion of Error.
§ 428.404 Manufacturer access to Rebate Reports.
(a) General. CMS will establish a method and process for a manufacturer of the Part D
rebatable drug to:
(1) Access the Rebate Report as set forth in §§ 428.401 and 428.402, including any
report of a reconciled rebate amount as set forth in §§ 428.401 and 428.402;
(2) Submit a Suggestion of Error as set forth in §§ 428.402(c) and 428.403; and
(3) Pay a rebate amount as set forth in § 428.405.
(b) [Reserved]
§ 428.405 Deadline and process for payment of rebate amount.
(a) Rebate amounts owed by a manufacturer. For payment of a rebate amount owed by a
manufacturer:
(1) Upon receipt of a rebate amount, payment is due no later than 11:59 p.m. Pacific
Time (PT) on the 30 calendar days after the date of receipt of information regarding the rebate
amount on—
(i) A Rebate Report specified in § 428.401(c) or § 428.402; or
(ii) A report of a reconciled rebate amount specified in § 428.401(d) or § 428.402.
(2) Failure to pay a rebate amount due timely and in full may result in an enforcement
action as described in subpart F of this part.
(b) Refund to the manufacturer. If a reconciled rebate amount for an applicable period as
specified in § 428.401(d) or § 428.402 is less than what the manufacturer paid for that applicable
period, CMS will initiate the process to provide a refund equal to the excess amount paid within
60 days of the date of receipt of the report with such reconciled rebate amount.
Subpart F—Enforcement of Manufacturer Payment of Rebate Amounts
§ 428.500 Civil money penalty notice and appeals procedures.
(a) General. CMS may impose a civil money penalty on a manufacturer that fails to pay
the rebate amount set forth in § 428.201(a) on a Part D rebatable drug defined at § 428.20, by the
payment deadline as set forth in section § 428.405(a) for such drug for such applicable period.
(b) Determination of the civil money penalty amount. CMS may impose a civil money
penalty for each failure by a manufacturer to provide an applicable inflation rebate equal to
125 percent of the rebate amount determined in § 428.201(a).
(1) The civil money penalty is in addition to the rebate amount due.
(2) If a reconciled rebate amount as set forth in § 428.401(d) or § 428.402(c)(1)(ii) or
(c)(2)(ii) results in an increase to the rebate amount due, a separate civil money penalty may be
imposed for the failure by a manufacturer to provide an inflation rebate for the applicable period
for the increase to the rebate amount due.
(c) Notice of imposition of civil money penalties. If CMS makes a determination to
impose a civil money penalty described in paragraph (b) of this section, CMS will send a written
notice of its decision to impose a civil money penalty to include the following:
(1) A description of the basis for the determination.
(2) The basis for the penalty.
(3) The amount of the penalty.
(4) The date the penalty is due.
(5) The manufacturer’s right to a hearing as specified in paragraph (e) of this section.
(6) Information about where to file the request for a hearing.
(d) Collection. (1) A manufacturer must pay the civil money penalty in full within
60 calendar days after the date of the notice of imposition of a civil money penalty from CMS
under paragraph (c) of this section.
(2) In the event a manufacturer requests a hearing, pursuant to 42 CFR part 423,
subpart T, the manufacturer must pay the amount in full within 60 calendar days after the date of
a final decision by the Departmental Appeal Board, to uphold, in whole or in part, the civil
money penalty.
(3) If the 60
th
calendar day described in paragraphs (d)(1) and (2) of this section is a
weekend or a Federal holiday, then the timeframe is extended until the end of the next business
day.
(e) Appeal procedures for civil money penalties. Section 1128A(c)(2) of the Act provides
that CMS may not collect a civil money penalty until the affected party has had notice and the
opportunity for a hearing.
(1) Manufacturers may appeal the following determinations:
(i) A CMS determination that the rebate amount was not paid by the applicable payment
deadline as described in § 428.405.
(ii) The calculation of the amount of the civil money penalty.
(2) The notice provided to a manufacturer if CMS decides to impose a civil money
penalty is described in paragraph (c) of this section.
(3) A manufacturer has a right to a hearing following a decision by CMS to impose a
civil money penalty following the administrative appeal process and procedures established in
42 CFR part 423, subpart T.
(f) Other applicable provisions. The provisions of section 1128A of the Act (except
subsections (a) and (b) of section 1128A of the Act) apply to civil money penalties under this
section to the same extent that they apply to a civil money penalty or procedures under section
1128A of the Act.
(g) Bankruptcy. In the event that a manufacturer declares bankruptcy, as described in
title 11 of the United States Code, and as a result of the bankruptcy, fails to pay either the full
rebate amount owed or the total sum of civil money penalties imposed, the government reserves
the right to file a proof of claim with the bankruptcy court to recover the unpaid amount of the
rebates and civil money penalties owed by the manufacturer.
PART 491-CERTIFICATION OF CERTAIN HEALTH FACILITIES
82. The authority citation for part 491 continues to read as follows:
Authority: 42 U.S.C. 263a and 1302.
83. Section 491.9 by—
a. Adding paragraphs (a)(2)(i) and (ii);
b. Removing paragraph (c)(2)(ii);
c. Redesignating paragraphs (c)(2)(iii) through (vi) as paragraphs (c)(2)(ii) through (v),
respectively; and
d. Revising newly designated paragraph (c)(2)(v).
The additions and revisions read as follows:
§ 491.9 Provision of services.
(a) * * *
(2) * * *
(i) The clinic or center must provide primary care services.
(ii) The clinic is not a rehabilitation agency or a facility primarily for the care and
treatment of mental diseases.
* * * * *
(c) * * *
(2) * * *
(v) Collection of patient specimens for transmittal to a certified laboratory for culturing.
* * * * *

__________________________________
Xavier Becerra,
Secretary,
Department of Health and Human Services.

Note: The following Appendices will not appear in the Code of Federal Regulations.
APPENDIX 1: MIPS QUALITY MEASURES
NOTE: Except as otherwise noted in this proposed rule, previously finalized measures and specialty measure sets
will continue to apply for the CY 2025 performance period/2027 MIPS payment year and future years. Previously
finalized measures and specialty measure sets are in the CY 2017 through CY 2024 PFS final rules: 81 FR 77558
through 77816, 82 FR 53966 through 54174, 83 FR 60097 through 60285, 84 FR 63205 through 63513, 85 FR
85045 through 85369, 86 FR 65687 through 65968, 87 FR 70250 through 70633, and 88 FR 79556 through 79964.
In addition, electronic clinical quality measures (eCQMs) that are endorsed by a Consensus-Based Entity (CBE) are
shown in Table A of this Appendix as follows: CBE # / eCQM CBE #.
Table Group A: New MIPS Quality Measures Proposed for the CY 2025 Performance
Period/2027 MIPS Payment Year and Future Years
Note: In the CY 2024 PFS final rule, measure Q494: Excessive Radiation Dose or Inadequate Image Quality for
Diagnostic Computed Tomography (CT) in Adults (Clinician Level), was finalized with a 1-year delay to the CY
2025 performance period (88 FR 79556 through 79560) and does not have a new measure table in this proposed
rule.

A.1. Positive PD-L1 Biomarker Expression Test Result Prior to First-Line Immune Checkpoint Inhibitor Therapy
Category
Description
CBE # /
eCQM CBE #:
N/A / N/A
Quality #:
TBD
Description:
Percentage of patients, aged 18 years and older, with a diagnosis of metastatic non-small cell lung cancer
(NSCLC) or squamous cell carcinoma of head and neck (HNSCC) on first-line immune checkpoint inhibitor
(ICI) therapy, who had a positive PD-L1 biomarker expression test result prior to giving ICI therapy.
Measure Steward:
Society for Immunotherapy of Cancer (SITC)
Numerator:
Patients who had a positive PD-L1 biomarker expression test result prior to the initiation of first-line immune
checkpoint inhibitor therapy.
Denominator:
Patients aged 18 years and older with a diagnosis of metastatic non-small cell lung cancer (NSCLC) or squamous
cell carcinoma of head and neck (HNSCC) and on first-line immune checkpoint inhibitors without
chemotherapy.
Exclusions:
Patients with NSCLC with epidermal growth factor receptor (EGFR) mutations, ALK genomic tumor
aberrations, or other targetable genomic abnormalities with approved first-line targeted therapy, such as NSCLC
with ROS1 rearrangement, BRAF V600E mutation, NTRK 1/2/3 gene fusion, MET ex14 skipping mutation, and
RET rearrangement.
Measure Type:
Process
High Priority Measure:
Yes
Collection Type:
MIPS CQM Specifications
Measure-Specific Case
Minimum/Performance
Period:
N/A for this measure.
Rationale:
We are proposing this process measure because it addresses timely biomarker testing, which impacts treatment
decisions for patients with a diagnosis of metastatic non-small cell lung cancer or squamous cell carcinoma of the
head and neck. In addition, this measure aligns with CMS priorities of improving patient outcomes and safety,
while promoting efficacy of timely treatment. Appropriate intervention and timeliness of PD-L1 biomarker
expression testing prior to initiation of first-line treatment for metastatic non-small cell lung cancer or squamous
cell carcinoma of the head and neck can lead to improvements in mortality and morbidity rates.
1
Untimely
biomarker testing could lend to negative clinical implications or outcomes, including delayed care and treatment
and/or ineffective or incorrect prescribed therapies leading to chemotherapy toxicity, decreased quality of life,
and unnecessary healthcare costs.
2
3
Immunotherapy remains a new realm for oncology and health care clinicians; therefore, opportunities exist to
improve care provided to patients receiving immune checkpoint inhibitor therapy. In 2017, a survey conducted
by the Association of Community Cancer Centers (ACCC) indicated only 24 percent of respondents reported
they had a deep familiarity with checkpoint inhibitors, 32 percent with monoclonal antibody therapy, and only 17
percent with combination treatment regimens.
4
This measure is predicated on two evidence-based clinical guidelines that address the measure’s quality actions
of a positive PD-L1 biomarker expression test prior to giving first-line immune checkpoint inhibitor therapy in
the metastatic non-small cell lung cancer or squamous cell carcinoma of the head and neck population.
5
6
The
measure would enhance compliance with the clinical guidelines by incentivizing clinicians to address timely
biomarker testing, positively influencing treatment decisions and improving patient outcomes.
The Pre-Rulemaking Measure Review (PRMR) Clinician Recommendation Committee conditionally supported
this measure and requested additional testing examining measure performance and feasibility. This measure has
been fully developed and tested at the clinician level with high reliability based upon signal-to-noise scores, and
adequate face validity. As a part of the MERIT submission, the measure developer reported adequate reliability
testing of the individual data elements via Cohen’s kappa coefficient, which substantiates the feasibility for
implementation in MIPS. Testing of measure performance indicated a large gap in care for conducting timely
biomarker testing, with an average performance rate of 19.6 percent, which allows significant room for
improvement among clinicians treating this patient population and conducting the biomarker testing for this
measure. This measure is not currently CBE endorsed. Although CBE endorsement is preferred, it is still
recommended this measure be added to MIPS because it is an evidence-based measure, satisfying the
requirement set forth at section 1848(q)(2)(D)(v) of the Act, stating that any measure selected for inclusion in
MIPS that is not endorsed by a CBE shall have a focus that is evidenced-based. This measure aligns with the
National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines.
7
8
We recognize that not all measures within MIPS are applicable or appropriate for all clinicians due to the nuances
for each clinician specialization, scope of care, or regional location. However, this measure fills a gap in MIPS
quality measures for treatment of patients with non-small cell lung cancer and squamous cell carcinoma of the
head and neck. In addition, it would provide a specialty specific measure for the MIPS Oncology/Hematology
specialty set under Table B.27a of this Appendix. Furthermore, this measure could be added to the Advancing
Cancer Care MVP in the future and would fill a current quality measure inventory gap within the oncologic
clinical topic.
Note: Refer to the PRMR Clinician Recommendation Committee Spreadsheet of Final Recommendations to
CMS and HHS at https://p4qm.org/sites/default/files/2024-02/PRMR-Final-MUC-Recommendation-
Spreadsheet%20%283%29.xlsx.

1
Lim, C., Tsao, M. S., Le, L. W., Shepherd, F. A., Feld, R., Burkes, R. L., Liu, G., Kamel-Reid, S., Hwang, D., Tanguay, J.,
da Cunha Santos, G., & Leighl, N. B. (2015). Biomarker Testing and Time to Treatment Decision in Patients with
Advanced Nonsmall-cell Lung Cancer. Annals of Oncology: Official Journal of the European Society for Medical
Oncology, 26(7), 1415–1421. https://doi.org/10.1093/annonc/mdv208.
2
Pai, S., Blaisdell, D., Brodie, R., Carlson, R., Finnes, H., Galioto, M., Jensen, R. E., Valuck, T., Sepulveda, A. R., &
Kaufman, H. L. (2020). Defining Current Gaps in Quality Measures for Cancer Immunotherapy: Consensus Report
from the Society for Immunotherapy of Cancer (SITC) 2019 Quality Summit. Journal for Immunotherapy of
Cancer, 8(1), e000112. https://doi.org/10.1136/jitc-2019-000112.
3
See footnote Lim et al., 2015.
4
Association of Community Cancer Centers (ACCC). (2017-2018). Immuno-Oncology: Transforming the Delivery of
Cancer Care in the Community [White paper].
http://www.informz.net/ACCC/data/images/Attachments/2017%20IO%20White%20Paper.pdf.
5
Ettinger, D. S., Wood, D. E., Aisner, D. L., Akerley, W., Bauman, J. R., Bharat, A., Bruno, D. S., Chang, J. Y., Chirieac,
L. R., D'Amico, T. A., DeCamp, M., Dilling, T. J., Dowell, J., Gettinger, S., Grotz, T. E., Gubens, M. A., Hegde, A.,
Lackner, R. P., Lanuti, M., Lin, J., … Hughes, M. (2022). Non-Small Cell Lung Cancer, Version 3.2022, NCCN Clinical
Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network: JNCCN, 20(5), 497–530.
https://doi.org/10.6004/jnccn.2022.0025.
6
Pfister, D. G., Spencer, S., Adelstein, D., Adkins, D., Anzai, Y., Brizel, D. M., Bruce, J. Y., Busse, P. M., Caudell, J. J.,
Cmelak, A. J., Colevas, A. D., Eisele, D. W., Fenton, M., Foote, R. L., Galloway, T., Gillison, M. L., Haddad, R. I., Hicks,
W. L., Hitchcock, Y. J., Jimeno, A., … Darlow, S. D. (2020). Head and Neck Cancers, Version 2.2020, NCCN Clinical
Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network: JNCCN, 18(7), 873–898.
https://doi.org/10.6004/jnccn.2020.0031.
7
National Comprehensive Cancer Network (2021). NCCN Clinical Practice Guidelines in Oncology: Head and Neck
Cancer. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf.
8
National Comprehensive Cancer Network (2021). NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell
Lung Cancer. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.

A.2. Appropriate Germline Testing for Ovarian Cancer Patients
Category
Description
CBE # /
eCQM CBE #:
N/A / N/A
Quality #:
TBD
Description:
Percentage of patients aged 18 and older diagnosed with epithelial ovarian, fallopian tube, or primary peritoneal
cancer who undergo germline testing within 6 months of diagnosis.
Measure Steward:
American Society of Clinical Oncology
Numerator:
Patients who receive germline genetic testing for BRCA1 and BRCA2 (ideally within the context of a multigene
panel) or who have genetic counseling completed within 6 months of diagnosis.
Denominator:
All patients, aged 18 and older, with epithelial ovarian, fallopian tube, or primary peritoneal cancer newly
diagnosed between July 1st of the previous calendar year through June 30th of the current performance period
with two encounters during the performance period.
Exclusions:
Patients who have germline BRCA testing completed before diagnosis of epithelial ovarian, fallopian tube, or
primary peritoneal cancer.
Measure Type:
Process
High Priority Measure:
No
Collection Type:
MIPS CQM Specifications
Measure-Specific Case
Minimum/Performance
Period:
N/A for this measure

Category
Description
Rationale:
We are proposing this process measure because it addresses patients diagnosed with epithelial ovarian, fallopian
tube, or primary peritoneal cancer who undergo germline testing within 6 months of their diagnosis to guide the
most appropriate treatment. In addition, this measure addresses the CMS priority of promoting more personalized
diagnostic, predictive, prognostic, and therapeutic options for the patient. According to the American Cancer
Society, estimates indicate that there will be about 19,680 new cases of ovarian cancer diagnosed and an
estimated 12,740 women will die of the disease, in 2024 in the U.S.
9
“Knowledge about underlying molecular
alterations in ovarian cancer could allow for more personalized diagnostic, predictive, prognostic, and therapeutic
strategies for the patient but also have clinical implications for her family members.”
10
Despite current
recommendations for all women diagnosed with ovarian cancer to receive genetic testing, only approximately 30
percent of those women undergo any genetic testing.
11
Germline mutations in BRCA1 and BRCA2 have been identified in 13 to 15 percent of women diagnosed with
ovarian cancer, with somatic mutations found in an additional 7 percent of women. The high incidence of these
mutations, in conjunction with the advent of therapy targeting BRCA mutations, warrants testing in all
individuals diagnosed with ovarian cancer.
12
This testing serves multiple purposes, including determination of
appropriate and best treatment recommendations, risk of other cancers, and need for cascade testing of family
members. “Testing for germline mutations should be performed at the time of initial diagnosis. Presence of a
germline mutation in a woman with advanced cancer designates her as eligible for maintenance therapy with a
poly (ADP-ribose) polymerase (PARP) inhibitor (Olaparib) after response to initial chemotherapy.”
13
Although the FDA recently approved frontline maintenance therapy for patients independent of mutation status
following the publication of the ASCO evidence-based guidelines, emerging evidence is expected to indicate an
overall survival benefit in ovarian cancer patients with germline mutations, based upon prognostic information
from these patients. Germline mutations testing allows for more personalized therapeutic strategies, therefore,
ovarian cancer patients with germline mutations are expected to derive greater benefit from therapy, thereby
increasing the survival rate in this patient population.
14
Additionally, germline testing informs potential clinical
implications for the relatives of ovarian cancer patients with germline mutations who should be offered
individualized genetic risk evaluation, counseling, and genetic testing as reflected in Recommendation 1.5 in the
ASCO germline testing guidelines.
15
Furthermore, NCCN evidence-based guidelines
16
indicate all patients with
histologically confirmed ovarian, fallopian tube, or primary peritoneal cancer should undergo genetic risk
evaluation as well as germline and somatic testing if not previously performed due to germline and/or somatic
BRCA1 and BRCA2 statuses delineating future options for maintenance therapy.
This measure could be added to the Advancing Cancer Care MVP in the future and would fill a current quality
measure inventory gap within the oncologic clinical topic. In addition, it would provide a specialty specific
measure for the MIPS Oncology/Hematology specialty set under Table B.27a of this Appendix.
The PRMR Clinician Recommendation Committee conditionally supported this measure for rulemaking pending
endorsement of the measure by a CBE. Although CBE endorsement is preferred, it is still recommended this
measure be added to MIPS because it is an evidence-based measure, satisfying the requirement set forth at
section 1848(q)(2)(D)(v) of the Act, stating that any measure selected for inclusion in MIPS that is not endorsed
by a CBE shall have a focus that is evidenced-based. As mentioned above, this measure aligns with ASCO and
NCCN evidence-based clinical guidelines.
17
18
Note: Refer to the PRMR Clinician Recommendation Committee Spreadsheet of Final Recommendations to
CMS and HHS at https://p4qm.org/sites/default/files/2024-02/PRMR-Final-MUC-Recommendation-
Spreadsheet%20%283%29.xlsx.
A.3. Patient-Reported Pain Interference Following Chemotherapy among Adults with Breast Cancer
Category
Description
CBE # /
eCQM CBE #:
CBE 3718 / N/A
9
American Cancer Society (ACS). (2024). Key Statistics for Ovarian Cancer.
https://www.cancer.org/cancer/types/ovarian-cancer/about/key-
statistics.html#:~:text=The%20American%20Cancer%20Society%20estimates%20for%20ovarian%20cancer,About
%2012%2C740%20women%20will%20die%20from%20ovarian%20cancer.
10
Konstantinopoulos, P. A., Norquist, B., Lacchetti, C., Armstrong, D., Grisham, R. N., Goodfellow, P. J., Kohn, E. C.,
Levine, D. A., Liu, J. F., Lu, K. H., Sparacio, D., & Annunziata, C. M. (2020). Germline and Somatic Tumor Testing in
Epithelial Ovarian Cancer: ASCO Guideline. Journal of Clinical Oncology: Official Journal of the American Society of
Clinical Oncology, 38(11), 1222–1245. https://doi.org/10.1200/JCO.19.02960.
11
See footnote Konstantinopoulos et al., 2020.
12
See footnote Konstantinopoulos et al., 2020.
13
See footnote Konstantinopoulos et al., 2020.
14
See footnote Konstantinopoulos et al., 2020.
15
See footnote Konstantinopoulos et al., 2020.
16
NCCN. (2024). NCCN Guidelines: Detection, Prevention, and Risk Reduction.
https://www.nccn.org/guidelines/category_2.
17
See footnote Konstantinopoulos et al., 2020.
18
See footnote NCCN, 2024.

Category
Description
Quality #:
TBD
Description:
The PRO-PM will assess pain interference following chemotherapy administered with curative intent to adult
female patients with breast cancer.
Measure Steward:
Purchaser Business Group on Health
Numerator:
The mean of the patient-level PROMIS Pain Interference scores at the follow-up survey.
Denominator:
Adult patients with stages I-III female breast cancer receiving an initial chemotherapy regimen.
Exclusions:
● Patients on a therapeutic clinical trial
● Patients with recurrence/disease progression
● Patients who leave the practice during the follow-up period
● Patients who died during the follow-up period
Measure Type:
Patient-Reported Outcome-based Performance Measure (PRO-PM)
High Priority Measure:
Yes
Collection Type:
MIPS CQM Specifications
Measure-Specific Case
Minimum/Performance
Period:
N/A for this measure

Category
Description
Rationale:
We are proposing this measure because it addresses a CMS high priority as a PRO-PM and fills a gap in
providing the patient's experience of care related to breakthrough pain after chemotherapy for breast cancer
lending to performance improvement. Common persistent symptoms following chemotherapy include pain,
fatigue, and detriments to health-related quality of life. Data from this measure would provide insight into the
effectiveness of minimizing the persistent symptoms following treatment(s), thereby driving quality improvement
leading to practice changes and better patient outcomes.
This oncology PRO-PM’s conceptual development is grounded in the evidence-based premise that medical
oncologists who provide the highest quality care (including medical and non-medical support services) to patients
receiving curative-intent cytotoxic therapy can reduce longer-term symptom burden, thus improving a patient’s
transition into the cancer survivorship period.
19
20
21
Additionally, research suggests collecting and using patient-
reported symptoms during cancer care can improve patient outcomes, such as increased survival, reduced
symptom burden and improved patient experience.
22
23
Using a standardized symptom assessment process would
facilitate appropriate follow-up to ensure patient needs are addressed, while supporting and improving patient-
provider communication.
This measure could be added to the Advancing Cancer Care MVP in the future and would fill a current quality
measure inventory gap within the oncologic clinical topic. In addition, it would provide a specialty specific
measure for the MIPS Oncology/Hematology specialty set under Table B.27a of this Appendix. This would be
the first outcome specialty specific oncology measure to address the patient experience of care. There is potential
consideration for adding broader cancer diagnoses, such as colon and lung cancer, to this measure in the future.
The PRMR Clinician Recommendation Committee conditionally supported this measure for rulemaking with the
condition of implementation at the group level. This measure was endorsed by the CBE as CBE 3718. We are
proposing this measure for implementation at the individual clinician level in addition to the group level. As part
of the MERIT submission, testing was completed at the clinician level with a small sample size due to
accessibility to data. However, the measure steward estimated that measure score reliability given an average
sample size of 26 would yield a measure score reliability of 0.7. The measure was found during the MERIT
submission to be feasible at the clinician level and data element testing was completed showing exact agreement
values between 71.63 to 100 percent, with the lowest sensitivity variable being ‘recurrence,’ which would
correlate to a small subset of the patient population. The requirements for quality measure scoring include a case
minimum threshold; therefore, we are proposing to allow this measure for group and clinician level
implementation as it is an important concept and meets all testing and development criteria for MIPS quality
measures.
Note: Refer to the PRMR Clinician Recommendation Committee Spreadsheet of Final Recommendations to
CMS and HHS at https://p4qm.org/sites/default/files/2024-02/PRMR-Final-MUC-Recommendation-
Spreadsheet%20%283%29.xlsx.
19
NCCN. (2024). Clinical Practice Guidelines in Oncology, Cancer-Related Fatigue, Version 2.2024.
https://www.nccn.org/guidelines/guidelines-detail?category=3&id=1424.
20
Smith, T. G., Troeschel, A. N., Castro, K. M., Arora, N. K., Stein, K., Lipscomb, J., Brawley, O. W., McCabe, R. M.,
Clauser, S. B., & Ward, E. (2019). Perceptions of Patients With Breast and Colon Cancer of the Management of
Cancer-Related Pain, Fatigue, and Emotional Distress in Community Oncology. Journal of Clinical Oncology: Official
Journal of the American Society of Clinical Oncology, 37(19), 1666–1676. https://doi.org/10.1200/JCO.18.01579.
21
Bubis, L. D., Davis, L., Mahar, A., Barbera, L., Li, Q., Moody, L., Karanicolas, P., Sutradhar, R., & Coburn, N. G.
(2018). Symptom Burden in the First Year After Cancer Diagnosis: An Analysis of Patient-Reported
Outcomes. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 36(11), 1103–
1111. https://doi.org/10.1200/JCO.2017.76.0876.
22
Basch, E., Deal, A. M., Kris, M. G., Scher, H. I., Hudis, C. A., Sabbatini, P., Rogak, L., Bennett, A. V., Dueck, A. C.,
Atkinson, T. M., Chou, J. F., Dulko, D., Sit, L., Barz, A., Novotny, P., Fruscione, M., Sloan, J. A., & Schrag, D. (2016).
Symptom Monitoring With Patient-Reported Outcomes During Routine Cancer Treatment: A Randomized
Controlled Trial. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 34(6),
557–565. https://doi.org/10.1200/JCO.2015.63.0830.
23
Papageorgiou, L., Le Provost, J. B., Di Palma, M., Langlois, M., Salma, I., Lopes, M., Minvielle, E., Abbas, M., &
Scotté, F. (2024). Supportive Care Needs of Newly Diagnosed Cancer Patients in a Comprehensive Cancer Center:
Identifying Care Profiles and Future Perspectives. Cancers, 16(5), 1017. https://doi.org/10.3390/cancers16051017.

A.4. Patient-Reported Fatigue Following Chemotherapy among Adults with Breast Cancer
Category
Description
CBE # /
eCQM CBE #:
CBE 3720 / N/A
Quality #:
TBD
Description:
The PRO-PM will assess fatigue following chemotherapy administered with curative intent to adult female
patients with breast cancer.
Measure Steward:
Purchaser Business Group on Health
Numerator:
The mean of the patient-level PROMIS Fatigue scores at the follow-up survey.
Denominator:
Adult patients with stages I-III female breast cancer receiving an initial chemotherapy regimen.
Exclusions:
● Patients on a therapeutic clinical trial
● Patients with recurrence/disease progression
● Patients who leave the practice during the follow-up period
● Patients who died during the follow-up period
Measure Type:
Patient-Reported Outcome-based Performance Measure (PRO-PM)
High Priority Measure:
Yes
Collection Type:
MIPS CQM Specifications
Measure-Specific Case
Minimum/Performance
Period:
N/A for this measure

Category
Description
Rationale:
We are proposing this measure because it addresses a CMS high priority as a PRO-PM and fills a gap in
providing the patient reported symptom of fatigue experienced following chemotherapy for breast cancer, lending
to performance improvement. This measure is an important addition to MIPS for those patients diagnosed with
and receiving treatment for breast
cancer. Based on a recent study, stakeholder generated recommendations for
performance measures on how adults with cancer feel and function included depression/anxiety, pain, and fatigue
as top priorities.
24
This oncology PRO-PM’s conceptual development is grounded in the evidence-based premise that medical
oncologists who provide the highest quality care (including medical and non-medical support services) to patients
receiving curative-intent cytotoxic therapy can reduce longer-term symptom burden, thus improving a patient’s
transition into the cancer survivorship period.
25
26
27
Additionally, research suggests collecting and using patient-
reported symptoms during cancer care can improve patient outcomes, such as increased survival, reduced
symptom burden and improved patient experience.
28
Using a standardized symptom assessment process would
facilitate appropriate follow-up to ensure patient needs are addressed, while supporting and improving patient-
provider communication.
Evidence from one study indicated 30 to 50 percent of breast cancer patients reported not discussing, nor
receiving advice or desired help for three common symptoms including pain, fatigue, and emotional distress.
29
Fatigue was the most common symptom reported in the study (74 percent) with many patients reporting they
discussed the symptom (78 percent).
30
However, patients who experienced fatigue were least likely to report
receiving the desired help for it (40 percent).
31
Appropriate use of patient-reported outcome measures (PROMs),
which measure cancer symptoms and patients’ perceptions of their care, can improve the collection of clinically
actionable data, supporting clinical improvement in the treatment of common symptoms and the overall care of
patients with cancer, ultimately improving their quality of life.
32
This measure could be added to the Advancing Cancer Care MVP in the future and would fill a current quality
measure inventory gap within the oncologic clinical topic. In addition, it would provide a specialty specific
measure for the MIPS Oncology/Hematology specialty set under Table B.27a of this Appendix. This measure
would address the patient voice/experience of care for those with breast cancer with fatigue experienced
following chemotherapy. There is potential consideration for adding broader cancer diagnoses, such as colon and
lung cancer, to this measure in the future.
The PRMR Clinician Recommendation Committee did not reach consensus for this measure. This measure was
endorsed by the CBE as CBE 3720. While concerns were discussed regarding electronic health record
implementation, patient survey fatigue and low response impact, this measure is an important clinical topic for
oncology clinicians in conjunction with the pain PRO-PM. More specifically, the measure provides a means to
capture the patient voice while driving quality of care and improving patient outcomes. We are proposing this
measure for implementation at the individual clinician level in addition to the group level. Testing was completed
at the clinician level with a small sample size due to accessibility to data. The measure was found during the
MERIT submission process to be feasible at the clinician level and data element testing was completed showing
exact agreement values between 71.63 to 100 percent, with the lowest sensitivity variable being ‘recurrence,’
which would correlate to a small subset of the patient population. The requirements for quality measure scoring
include a case minimum threshold; therefore, we are proposing to allow this measure for group and clinician
level implementation as it is an important concept and meets all testing and development criteria for MIPS
quality measures.
Note: Refer to the PRMR Clinician Recommendation Committee Spreadsheet of Final Recommendations to
CMS and HHS at https://p4qm.org/sites/default/files/2024-02/PRMR-Final-MUC-Recommendation-
Spreadsheet%20%283%29.xlsx.
24
See footnote Smith et al., 2019 in Table A.3 of this Appendix.
25
See footnote Smith et al., 2019 in Table A.3 of this Appendix.
26
See footnote NCCN, 2024 in Table A.3 of this Appendix.
27
See footnote Bubis et al., 2018 in Table A.3 of this Appendix.
28
See footnote Basch et al., 2016 in Table A.3 of this Appendix.
29
See footnote Smith et al., 2019 in Table A.3 of this Appendix.
30
See footnote Smith et al., 2019 in Table A.3 of this Appendix.
31
See footnote Smith et al., 2019 in Table A.3 of this Appendix.
32
See footnote Smith et al., 2019 in Table A.3 of this Appendix.

A.5. Adult COVID-19 Vaccination Status
Category
Description
CBE # /
eCQM CBE #:
N/A / N/A
Quality #:
TBD
Description:
Percentage of patients aged 18 years and older seen for a visit during the performance period that are up-to-date
on their COVID-19 vaccinations as defined by Centers for Disease Control and Prevention (CDC)
recommendations on current vaccination.
Measure Steward:
Centers for Medicare & Medicaid Services
Numerator:
Patients that are up to date on their COVID-19 vaccinations as defined by CDC recommendations on current
vaccination as of the date of the encounter.
Denominator:
All patients aged 18 years and older seen for a visit during the performance period.
Exclusions:
Patient received hospice services any time during the performance period.
Measure Type:
Process
High Priority Measure:
No
Collection Type:
MIPS CQM Specifications
Measure-Specific Case
Minimum/Performance
Period:
N/A for this measure

Category
Description
Rationale:
We are proposing this process measure because it represents an important clinical topic following the recently
ended Public Health Emergency (PHE) for COVID-19. This process measure represents a CMS high priority
clinical topic and fills a gap in MIPS by addressing COVID-19 vaccination status for all patients and ensuring
clinician vaccination efforts at the point of care (for example, care for wellness and prevention against COVID-
19). Widespread vaccination to prevent a severe COVID-19 infection is critically important to stemming the
morbidity and mortality caused by this virus. The CDC reports millions of cases and deaths caused by COVID-
19.
33
In 2020 and 2021, COVID-19 was the third leading cause of death in the U.S., exceeded only by cancer and
heart disease.
34
The percent of the population reporting receipt of the updated 2023-24 COVID-19 vaccine is 13.1
percent (95 percent confidence interval: 12.5-13.7) for children and 22.5 percent (21.7-22.7) for adults 18 years
and older, including 41.5 percent (40.2-42.9) among adults age 65 years and older.
35
36
This recent data suggests a
considerable gap in care for patients in the measure population and allows clinicians the opportunity to positively
affect vaccination rates. Additionally, during measure testing, the measure developer found even the 75th
percentile of performance yielded only 58 percent of a clinician’s patient load being vaccinated with one booster.
The measure was initially submitted to the CY 2022 Call for Quality Measures and included on the Measures
under Consideration (MUC) list for review. During the 2022 cycle, the Measure Applications Partnership (MAP)
recommended not to support the measure and requested the measure be revised before resubmitting for
consideration to, among other things, include the most recent CDC recommendations for the numerator and use
the current CDC definition of “up-to-date” for assessment of the quality action for each denominator eligible
patient. These requests were addressed, and the measure was resubmitted to the CY 2023 Call for Quality
Measures. We acknowledge the recommendations for boosters have continued to evolve; however, the quality
action in this measure is aligned with current and potential future recommendations. Since December 2020, there
have been 19 ACIP recommendations relating to COVID-19 vaccination. On September 12, 2023, ACIP
recommended all persons aged 6 months and older receive an updated COVID-19 vaccine.
37
Updated COVID-19
vaccines are considered the 2023-2024 formula developed by the pharmaceutical and biotechnology companies
Moderna, Pfizer-BioNTech, and Novavax. Because this measure uses the CDC’s definition of “up-to-date,”
please refer to the CDC’s website
38
to confirm the current definition of “up-to-date.”
Based on clinical recommendations and systemic reviews, there is general agreement about the safety and
efficacy of the COVID-19 vaccine, preventing costly and potentially harmful hospitalizations.
39
40
41
While this
measure does not meet the definition of a fully developed measure as outlined in the Measures Management
System (MMS),
42
MIPS currently includes several quality measures that assess for vaccine administration, which
have been implemented for multiple years.
43
We request interested parties consider whether the measure is “beyond the measure concept phase of
development and [has] started testing, at a minimum, with strong encouragement and preference for measures
that have completed or are near completion of reliability and validity testing” when submitting a quality measure
for possible inclusion (83 FR 53636; 84 FR 62954). While we take under consideration whether a measure is
33
CDC. (2024). COVID Data Tracker. Atlanta, GA: U.S. Department of Health and Human Services.
https://covid.cdc.gov/covid-data-tracker.
34
Ahmad, F.B., Cisewski, J.A., Anderson, R.N. (2022). Provisional Mortality Data — United States, 2021. MMWR
Morb Mortal Wkly Rep 71,597-600. http://dx.doi.org/10.15585/mmwr.mm7117e1.
35
CDC. (2024). Vaccination Trends – Children. https://www.cdc.gov/respiratory-viruses/data-
research/dashboard/vaccination-trends-children.html.
36
CDC. (2024). Vaccination Trends – Adults. https://www.cdc.gov/respiratory-viruses/data-
research/dashboard/vaccination-trends-adults.html.
37
Centers for Disease Control and Prevention (CDC). (2023). Use of Updated COVID-19 Vaccines 2023–2024
Formula for Persons Aged ≥6 Months: Recommendations of the Advisory Committee on Immunization Practices —
United States. https://www.cdc.gov/mmwr/volumes/72/wr/mm7242e1.htm.
38
CDC. (2024). Stay Up to Date with COVID-19 Vaccines. https://www.cdc.gov/coronavirus/2019-
ncov/vaccines/stay-up-to-date.html.
39
Fitzpatrick, M. C., Moghadas, S. M., Pandey, A., & Galvani, A. P. (2022). Two Years of US COVID-19 Vaccines Have
Prevented Millions of Hospitalizations and Deaths. To the Point (blog), Commonwealth Fund. December 13.
https://doi.org/10.26099/whsf-fp90.
40
Polack, F. P., Thomas, S. J., Kitchin, N., Absalon, J., Gurtman, A., Lockhart, S., Perez, J. L., Pérez Marc, G., Moreira,
E. D., Zerbini, C., Bailey, R., Swanson, K. A., Roychoudhury, S., Koury, K., Li, P., Kalina, W. V., Cooper, D., Frenck, R.
W., Jr, Hammitt, L. L., Türeci, Ö., … C4591001 Clinical Trial Group. (2020). Safety and Efficacy of the BNT162b2
mRNA Covid-19 Vaccine. The New England Journal of Medicine, 383(27), 2603–2615.
https://doi.org/10.1056/NEJMoa2034577.
41
Graña, C., Ghosn, L., Evrenoglou, T., Jarde, A., Minozzi, S., ... & Boutron, I. (2022). Efficacy and Safety of COVID-19
Vaccines. Cochrane Database of Systematic Reviews, 2023(3).
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD015477/full.
42
CMS. (2023). Measure Implementation – Measure Selection. https://mmshub.cms.gov/measure-
lifecycle/measure-implementation/selection.
43
See 2024 MIPS Quality Measures List: https://qpp-cm-prod-
content.s3.amazonaws.com/uploads/2632/2024%20MIPS%20Quality%20Measures%20List.xlsx.

Category
Description
fully tested, it is not the only relevant standard for MIPS. Nevertheless, we believe this consideration reinforces
the importance of all clinicians actively addressing vaccination against the COVID-19 virus. According to the
World Health Organization (WHO), getting vaccinated is one of the most important steps an individual can take
to not only to protect oneself, but to help end the pandemic and stop the emergence of new variants.
44
The PRMR Clinician Recommendation Committee did not reach consensus on this measure for rulemaking,
expressing concerns about data collection for vaccines administered through off-site locations (for example,
pharmacies), as well as vaccine hesitancy that may vary across geography, political affiliation, ethnicity, and
income. The committee considered the measure’s importance to patients, with emphasis on those at higher risk
for complications from COVID-19. ACIP recommendations support the measure, all of which are evidence-
based.
45
This measure is not currently CBE endorsed. Although CBE endorsement is preferred, it is still
recommended this measure be added to MIPS because it is an evidence-based measure, satisfying the
requirement set forth at section 1848(q)(2)(D)(v) of the Act, stating that any measure selected for inclusion in
MIPS that is not endorsed by a CBE shall have a focus that is evidenced-based. The CDC COVID-19
Immunization Schedule, based on ACIP recommendations, can be referenced at the following webpage:
https://www.cdc.gov/vaccines/schedules/index.html. Clinical recommendations are in universal agreement that
COVID-19 vaccines are safe, effective, and may prevent costly and harmful hospitalizations.
The MIPS quality measure set includes several vaccine administration quality measures that have been
implemented for several years; however, such measures do not include the COVID-19 vaccination.
46
This
measure could also be considered for potential inclusion in the Primary Care MVP as it would fill a current
quality measure inventory gap within the vaccination clinical topic for primary care settings. This measure, along
with other activities, are a part of a larger Federal effort to promote and track vaccine uptake.
47
Since vaccine
uptake is partially driven by patients requesting the vaccine followed by clinicians administering it to eligible
patients, the patient/clinician relationship is a vital aspect for ensuring patients are vaccinated. This clinician-
level measure would provide useful information regarding the success of vaccination efforts at the point of care,
and, again, represents a priority topic to engage clinicians in quality improvements that drive positive health
outcomes for their patients.
We acknowledge this measure may not be selected by all clinicians, but this factor aligns with MIPS allowing for
clinician choice in choosing quality measures that best represent their scope of care. Clinicians are uniquely
positioned to encourage uptake of COVID-19 vaccination. As with all quality measures within MIPS, we
continue to monitor all updates to the clinical recommendations and guidelines and address changes as needed
utilizing the current annual revision cycle.
Note: Refer to the PRMR Clinician Recommendation Committee Spreadsheet of Final Recommendations to
CMS and HHS at https://p4qm.org/sites/default/files/2024-02/PRMR-Final-MUC-Recommendation-
Spreadsheet%20%283%29.xlsx.
44
World Health Organization. (2021, July). Vaccine Efficacy, Effectiveness and Protection.
https://www.who.int/news-room/feature-stories/detail/vaccine-efficacy-effectiveness-and-protection.
45
CDC. (2023, December 12). Vaccine Recommendations and Guidelines of the ACIP: COVID-19 ACIP Vaccine
Recommendations. https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html.
46
See 2024 MIPS Quality Measures List: https://qpp-cm-prod-
content.s3.amazonaws.com/uploads/2632/2024%20MIPS%20Quality%20Measures%20List.xlsx.
47
Department of Health & Human Services. Vaccines Federal Implementation Plan for the United States: 2021-
2025. https://www.hhs.gov/sites/default/files/vaccines-federal-implementation-plan-2021-2025.pdf.

A.6. Melanoma: Tracking and Evaluation of Recurrence
Category
Description
CBE # /
eCQM CBE #:
N/A / N/A
Quality #:
TBD
Description:
Percentage of patients who had an excisional surgery for melanoma or melanoma in situ with initial American
Joint Committee on Cancer (AJCC) staging of 0, I, or II, in the past 5 years in which the operating clinician
examines and/or diagnoses the patient for recurrence of melanoma.
Measure Steward:
American Academy of Dermatology
Numerator:
NUMERATOR CRITERIA 1: Documentation by the clinician who performed the surgery that an exam for
recurrence of melanoma was performed on the patient within the performance period.
NUMERATOR CRITERIA 2: All patients that were diagnosed with a recurrent melanoma in the current
performance period.
Denominator:
DENOMINATOR CRITERIA 1 & 2: All patients that the clinician has performed a type of excisional surgery
for melanoma or melanoma in situ in the past 5 years with an initial AJCC staging of 0, I, or II.
Exclusions:
Patients who died during the performance period
Measure Type:
Process
High Priority Measure:
Yes
Collection Type:
MIPS CQM Specifications
Measure-Specific Case
Minimum/Performance
Period:
N/A for this measure

Category
Description
Rationale:
We are proposing this measure because it evaluates the frequency of recurrence for melanoma along with the
type of recurrence after an excisional procedure, in addition to driving communication regarding the recurrence
status of melanoma patients. The measure also addresses a CMS high priority process measure for care
coordination on the clinical topic as miscommunication between the excising clinician and the clinician
continuing care has been identified as a gap. This measure allows for and promotes the development of a system
in which melanomas can be accurately tracked to increase the effectiveness of understanding melanoma
recurrence and follow-up care.
Melanoma recurrence requires precise evaluation. As indicated by one study, patients who have undergone
treatment of primary melanoma, early detection of a local recurrence has important implications. An isolated
local recurrence in a patient with favorable features can be treated with repeat wide local excision, with good
oncologic outcomes. For these patients, long-term prognosis is not adversely affected by the local recurrence if it
is detected and treated early, and 5-year survival continues to be a function of primary tumor thickness.
48
49
50
51
Regular clinical examination offers the highest diagnostic yield in detecting melanoma recurrences.
52
Common follow-up recommendations for all patients indicate the follow-up schedule is influenced by risk of
recurrence, prior primary melanoma, and family history of melanoma. It also includes other factors such as
atypical moles/dysplastic nevi.
53
For patients who present with stage I-II melanoma and are rendered free of
disease after initial treatment, recurrence rates are distributed as follows: approximately 15 to 20 percent are local
or in transit.
54
Data suggests the time between the risk of recurrence to reach its low plateau depends on the stage
of disease at first presentation.
55
In a retrospective study of patients who initially presented with stage I
melanoma (N = 1568), 80 percent of the 293 recurrences developed within the first 3 years, but some recurrences
(<8 percent) were detected 5 to 10 years after the initial treatment.
56
A prospective study found that for patients
with stage I or II at initial presentation, the risk of recurrence reached a low level by 4.4 years after initial
diagnosis.
57
Therefore, we are proposing this measure to incentivize clinicians who treat patients with melanoma
to perform these potentially lifesaving exams.
The PRMR Clinician Recommendation Committee did not reach consensus in recommending the measure for
inclusion in MIPS. The committee agreed with the importance of the measure’s intent. There was significant
concern raised about the burden of tracking and reporting, reliability of the measure and interpretation of testing
results, and the impact of lower reliability on clinician compensation. However, the measure was fully tested at
the clinician level and has been determined to be implementable in MIPS. The measure has been available for
MIPS reporting as a QCDR measure since 2022, which attests to its feasibility as no implementation issues have
been identified. This measure is not currently CBE endorsed. Although CBE endorsement is preferred, it is still
recommended this measure be added to MIPS because it is an evidence-based measure, satisfying the
requirement set forth at section 1848(q)(2)(D)(v) of the Act, stating that any measure selected for inclusion in
MIPS that is not endorsed by a CBE shall have a focus that is evidenced-based. This measure aligns with NCCN
guidelines.
This measure fills a gap in MIPS within the dermatologic clinical topic, as there are no other dermatology
measures related to the outcome of melanoma recurrence. There is potential to replace current structure measure
Q137: Melanoma: Continuity of Care with this more robust outcome measure as indicated in Table C.2 of this
Appendix. While the MIPS Dermatology specialty set includes 13 measures, only 6 are specialty specific. This
measure is relevant to specialty clinicians and could provide a new measure option for a specialty area that
currently encompasses many topped-out measures, while also potentially aiding in the development of a
meaningful MVP.
48
Rueth, N. M., Cromwell, K. D., & Cormier, J. N. (2015). Long-term Follow-up for Melanoma Patients: Is There Any
Evidence of a Benefit? Surgical Oncology Clinics of North America, 24(2), 359–377.
https://doi.org/10.1016/j.soc.2014.12.012.
49
Benvenuto-Andrade, C., Oseitutu, A., Agero, A. L., & Marghoob, A. A. (2005). Cutaneous Melanoma: Surveillance
of Patients for Recurrence and New Primary Melanomas. Dermatologic Therapy, 18(6), 423–435.
https://doi.org/10.1111/j.1529-8019.2005.00049.x.
50
Garbe, C., Paul, A., Kohler-Späth, H., Ellwanger, U., Stroebel, W., Schwarz, M., Schlagenhauff, B., Meier, F.,
Schittek, B., Blaheta, H. J., Blum, A., & Rassner, G. (2003). Prospective Evaluation of a Follow-up Schedule in
Cutaneous Melanoma Patients: Recommendations for an Effective Follow-up Strategy. Journal of Clinical Oncology:
Official Journal of the American Society of Clinical Oncology, 21(3), 520–529.
https://doi.org/10.1200/JCO.2003.01.091.
51
Rhodes A. R. (2006). Cutaneous Melanoma and Intervention Strategies to Reduce Tumor-related Mortality: What
we know, what we don't know, and what we think we know that isn't so. Dermatologic Therapy, 19(1), 50–69.
https://doi.org/10.1111/j.1529-8019.2005.00056.x.
52
See footnote Rueth et al., 2015.
53
Trotter, S. C., Sroa, N., Winkelmann, R. R., Olencki, T., & Bechtel, M. (2013). A Global Review of Melanoma
Follow-up Guidelines. The Journal of Clinical and Aesthetic Dermatology, 6(9), 18–26.
54
See footnote Trotter et al., 2013.
55
See footnote Trotter et al., 2013.
56
See footnote Trotter et al., 2013.
57
NCCN. NCCN Guidelines – Melanoma: Cutaneous. https://www.nccn.org/guidelines/guidelines-
detail?category=1&id=1492.

Category
Description
Note: Refer to the PRMR Clinician Recommendation Committee Spreadsheet of Final Recommendations to
CMS and HHS at https://p4qm.org/sites/default/files/2024-02/PRMR-Final-MUC-Recommendation-
Spreadsheet%20%283%29.xlsx.

A.7. First Year Standardized Waitlist Ratio (FYSWR)
Category
Description
CBE # /
eCQM CBE #:
N/A / N/A
Quality #:
TBD
Description:
The number of newly initiated patients on dialysis in a practitioner group who are under the age of 75 and were either listed on
the kidney or kidney-pancreas transplant waitlist or received a living donor transplant within the first year of initiating dialysis.
The practitioner group is inclusive of physicians and advanced practice providers. The measure is the ratio-observed number of
waitlist events in a practitioner group to its expected number of waitlist events. The measure uses the expected waitlist events
calculated from a Cox model, which is adjusted for age, patient comorbidities, and other risk factors at the time of dialysis.
Measure Steward:
Centers for Medicare & Medicaid Services
Numerator:
Numerator 1. Patients who initiated dialysis and had documentation of status at the end of the first year after initiating dialysis.
Numerator 2. The ratio of the observed number of waitlist events in a practitioner group to the model-based expected number of
waitlist events.
Denominator:
Denominator 1. Patients aged 75 years of age or less who have initiated dialysis during January 1st – December 31st of the
previous performance period.
Denominator 2. The denominator for the First Year Standardized Waitlist Ratio (FYSWR) is the total number of patients under
the age of 75 in the practitioner group according to each patient's treatment history for patients within the first year following
initiation of dialysis.
Exclusions:
Patients admitted to a skilled nursing facility (SNF).
Patients in hospice on their initiation of dialysis date or during the month of evaluation.
Patients that were on the kidney or kidney-pancreas waitlist prior to initiation of dialysis.
Patients who had a transplant prior to initiation of dialysis.
Measure Type:
Process
High Priority Measure:
No
Collection Type:
MIPS CQM Specifications
Measure-Specific Case
Minimum/Performance
Period:
If a dialysis practitioner group has fewer than 11 patients or 2 expected events, then the dialysis practitioner group is excluded
from reporting outcomes.

Category
Description
Rationale:
This measure was originally proposed in the CY 2024 PFS proposed rule (88 FR 52771 through 52772) but was not finalized in
the CY 2024 PFS final rule (88 FR 79565 through 88 FR 79566) due to implementation issues within MIPS regarding timing
and application of the risk adjustment methodology. We are again proposing this measure because it addresses a CMS high
priority clinical topic: patients with end-stage renal disease (ESRD). We indicated we would allow further refinement and
streamlining of the measure analytic for future MIPS implementation. The measure and measure analytic have been revised to
include two submission criteria, allowing for determination of data completeness and the full utilization of the risk-adjusted
model in the second submission criterion to create a continuous variable analytic, assessing the ratio of observed to expected
waitlist events.
ESRD affects nearly 786,000 Americans, and dialysis for ESRD patients represents a significant portion of annual Medicare
expenditures.
58
While dialysis is a treatment for ESRD, it is associated with increased mortality and lower quality of life for
ESRD patients when compared to kidney transplant.
59
This measure assesses whether patients who are in their first year of
dialysis, and are found to be an expected waitlist event based upon the Cox model, were placed on either the kidney or kidney-
pancreas transplant waitlist or received a living donor kidney transplant. Data submitted by the measure developer indicates a
performance gap for a process that can be directly linked to improved patient outcomes. This measure is separate from the other
transplant waitlist measure, proposed under Table A.8 of this Appendix, as it is limited to assessing the first year after initiation
of dialysis and the timely addition of those patients to the transplant waitlist—a crucial step in driving positive outcomes in the
patient population.
National and large regional studies provide strong empirical support for the association between processes within the clinical
scope and control of dialysis practitioners followed by subsequent patient transplant wait listing. For example, the clinical
assessments, provisions and/or referrals made by a dialysis practitioner are contributing factors for consideration in patient
transplant wait listing. In one large regional study conducted on facilities in the state of Georgia, a standardized dialysis facility
referral ratio was developed, adjusted for age, demographics, and comorbidities.
60
There was substantial variability across
dialysis facilities in referral rates, and a Spearman correlation performed between ranking on the referral ratio and dialysis
facility waitlist rates was highly significant (r=0.35, p<0.001).
61
A national study using registry data (United States Renal Data
System) from 2005-2007 examined the association between whether patients were informed about kidney transplantation based
on reporting on the Medical Evidence Form 2728 (https://www.cms.gov/Medicare/CMS-Forms/CMS-
Forms/Downloads/CMS2728.pdf) and subsequent access to kidney transplantation (wait listing or receipt of a live donor
transplant).
62
Approximately 30 percent of patients were uninformed about kidney transplantation, which was associated with
half the rate of access to transplantation compared to patients who were informed.
63
In a related survey study of 388
hemodialysis patients, whether provision of information about transplantation by nephrologists or dialysis staff occurred was
directly confirmed with patients.
64
The provision of such information was associated with a near threefold increase in likelihood
of wait listing.
65
The intent of this measure is to track the initial placement on the kidney or kidney-pancreas transplantation waitlist or receipt of
a living donor transplant within the first year after dialysis initiation, with the intended objective of improving the overall health
of patients on dialysis. Being waitlisted or receiving a living donor kidney transplant represents a desirable change in health
status for patients on dialysis, indicating achievement of a health condition conducive to kidney transplantation. Waitlisting is a
direct step in the process of transplantation which drives quality by progressing patients towards the goal of transplantation and
better health outcomes. Being waitlisted for kidney transplantation is the culmination of a variety of preceding preparatory
activities, which may include providing education to patients about the option(s) of transplantation, referral of patients to a
transplant center for evaluation, completion of the evaluation process, and optimizing the health of the patient while on dialysis.
These efforts depend heavily and, in many cases, primarily, on dialysis practitioner groups. Aspects that are not directly in the
clinician/groups control can be influenced through coordination of care, strong communication with transplant centers, and
advocacy for patients. All clinicians should be involved and actively work towards providing patients with high quality care
including ensuring placement on the transplant list as quickly as possible.
The PRMR (formerly the MAP), did not support this measure for rulemaking with the potential for mitigation to update the
measure and address the concern from the Renal Standing Committee regarding the evidence base and specifications, and thus
recommended this measure be resubmitted for endorsement by a CBE. Although CBE endorsement is preferred, it is still
recommended this measure be added to MIPS because it is an evidence-based measure, satisfying the requirement set forth at
section 1848(q)(2)(D)(v) of the Act, stating that any measure selected for inclusion in MIPS that is not endorsed by a CBE shall
have a focus that is evidenced-based. As discussed above, studies suggest a significant positive correlation between the clinician
activities and the addition of patients to a transplant waitlist, which are necessary for patients to receive the improved outcomes
58
National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). (2023). Kidney Disease Statistics for
the United States. https://www.niddk.nih.gov/health-information/health-statistics/kidney-disease.
59
Wouk N. (2021). End-Stage Renal Disease: Medical Management. American Family Physician, 104(5), 493–499.
60
Paul, S., Plantinga, L. C., Pastan, S. O., Gander, J. C., Mohan, S., & Patzer, R. E. (2018). Standardized
Transplantation Referral Ratio to Assess Performance of Transplant Referral among Dialysis Facilities. Clinical
Journal of the American Society of Nephrology, 13(2), 282-289. https://doi.org/10.2215/CJN.04690417.
61
See footnote Paul et al., 2018.
62
Kucirka, L. M., Grams, M. E., Balhara, K. S., Jaar, B. G., & Segev, D. L. (2012). Disparities in Provision of Transplant
Information Affect Access to Kidney Transplantation. American Journal of Transplantation, 12(2), 351-357.
https://doi.org/10.1111/j.1600-6143.2011.03865.x.
63
See footnote Kucirka et al., 2012.
64
Salter, M. L., Orandi, B., McAdams-DeMarco, M. A., Law, A., Meoni, L. A., Jaar, B. G., ... & Segev, D. L. (2014).
Patient-and Provider-Reported Information about Transplantation and Subsequent Waitlisting. Journal of the
American Society of Nephrology, 25(12), 2871-2877. https://doi.org/10.1681/ASN.2013121298.
65
See footnote Salter et al., 2014.

Category
Description
associated with kidney transplant.
Note: Refer to the 2022 MUC List-Final Recommendations to CMS and HHS at
https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fmmshub.cms.gov%2Fsites%2Fdefault%2Ffiles%2F2022-
MUC-List.xlsx&wdOrigin=BROWSELINK.

A.8. Percentage of Prevalent Patients Waitlisted (PPPW) and Percentage of Prevalent Patients Waitlisted in Active Status
(aPPPW)
Category
Description
CBE # /
eCQM CBE #:
N/A / N/A
Quality #:
TBD
Description:
The measure tracks dialysis patients who are under the age of 75 in a practitioner group and on the kidney or kidney-pancreas
transplant waitlist (all patients or patients in active status). This measure is a risk-adjusted percentage of waitlist events among
dialysis patients.
Measure Steward:
Centers for Medicare & Medicaid Services
Numerator:
Numerator 1: Percentage of Prevalent Patients Waitlisted (PPPW): Patients in the practitioner group’s denominator with
observed months on the waitlist for each month.
Numerator 2: Percentage of Prevalent Patients Waitlisted in Active (aPPPW): Patients in the practitioner group’s denominator
with observed months on the waitlist in active status for each month.
Denominator:
Denominator 1 and 2: All risk-adjusted patient-months for patients who are under the age of 75 in the reporting month and who
are assigned to a dialysis practitioner or practitioner group practice according to each patient’s treatment history on the last day
of each reporting month during the performance year.
Exclusions:
Patients who were admitted to a skilled nursing facility (SNF) during the month of evaluation were excluded from that month.
Patients who were admitted to a skilled nursing facility (SNF) within one year of dialysis initiation according to the CMS-2728
form.
Patients determined to be in hospice were excluded from month of evaluation and the remainder of reporting period.
Patients with dementia at any time prior to or during the month.
Measure Type:
Process
High Priority Measure:
No
Collection Type:
MIPS CQM Specifications
Measure-Specific Case
Minimum/Performance
Period:
If a dialysis practitioner group has fewer than 11 patients during the performance year, the dialysis practitioner group is
excluded from reporting outcomes.

Category
Description
Rationale:
This measure was originally proposed in the CY 2024 PFS proposed rule (88 FR 52773 through 52774) but was not finalized in
the CY 2024 PFS final rule (88 FR 79567 through 79568) due to implementation issues within MIPS regarding timing and
application of the risk adjustment methodology. We are again proposing this measure because it addresses a CMS high priority
clinical topic: patients with ESRD. We indicated we would allow further refinement and streamlining of the measure analytic
for future MIPS implementation. The measure, including component calculations, was revised to allow for a proportional
analytic to be used for the purposes of determining measure performance, while still incorporating the risk-adjusted model to
ensure the appropriate denominator eligible patient population for numerator assessment.
ESRD affects nearly 786,000 Americans, and dialysis for ESRD patients represents a significant portion of annual Medicare
expenditures.
66
While dialysis is a treatment for ESRD, it is associated with increased mortality and lower quality of life for
ESRD patients when compared to kidney transplant.
67
This measure would capture the adjusted count of patient months on the
kidney and kidney-pancreas transplant waitlists for all dialysis patients in a dialysis practitioner or group practice by assessing
patient status on the last day of each month during the reporting year, and those on the transplant waitlist in active status as of
the last day of the month during the reporting year. This process measure is directly linked to driving positive outcomes and
measure data indicates a performance gap.
Most ESRD patients have to wait for access to a deceased donor transplant, with the national median being roughly 4 years.
68
Maintenance of ‘active status’ on the transplant list requires ongoing collaboration between dialysis practitioners, transplant
centers, and transplant networks, thereby ensuring sustained suitability for a transplant while optimizing the health of patients.
69
This maintenance process is associated with higher transplantation rates and lowered mortality rates while on the waitlist.
70
In
addition, the maintenance of ‘active status’ is an important health equity issue. Research has found disparities in access to
kidney transplant by race.
71
Race-neutral efforts by clinicians to encourage maintenance of patients on the waitlist may reduce
such disparities while improving their performance on this measure.
72
This measure and the previously proposed measure under Table A.7, the First Year Standardized Waitlist Ratio measure, work
in tandem to assess initial and on-going care. This measure would assess monthly wait listing in active status of patients. It also
would evaluate and encourage maintenance of patients on the waitlist. This is an important area to which dialysis practitioners
can contribute through ensuring patients remain healthy and complete any ongoing testing activities required to remain active
on the waitlist. In contrast to this measure, the First Year Standardized Waitlist Ratio measure would focus solely on new wait
listings and living donor kidney transplants to incentivize early action, rather than ongoing maintenance on the waitlist, which
this measure assesses.
The PRMR conditionally supported this measure for rulemaking pending an update of the measure’s specifications to include
only the PPPW (CBE 3695) rate that was recommended for endorsement by the CBE’s Renal Standing Committee. Although
CBE endorsement is preferred, it is still recommended this measure be added to MIPS because it is an evidence-based measure,
satisfying the requirement set forth at section 1848(q)(2)(D)(v) of the Act, stating that any measure selected for inclusion in
MIPS that is not endorsed by a CBE shall have a focus that is evidenced-based. The CBE recommended endorsement for the
PPPW subset of this measure. It is important to include the aPPPW rate in this measure as well to capture patients in active
waitlist status, and the full scope of the transplant list with movement of patients between active and inactive status. The studies
cited above provide the evidentiary basis for the adoption of this measure. After review, it was determined that the testing
provided by the measure steward demonstrated statistically sufficient results for the reliability and validity of each of the
numerator actions, meeting requirements described within the CMS MMS Hub (https://mmshub.cms.gov/) regarding quality
measure testing.
Note: Refer to the 2022 MUC List-Final Recommendations to CMS and HHS at
https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fmmshub.cms.gov%2Fsites%2Fdefault%2Ffiles%2F2022-
MUC-List.xlsx&wdOrigin=BROWSELINK.
66
See footnote NIDDK, 2023.
67
See footnote Wouk, 2021.
68
Johansen, K. L., Chertow, G. M., Foley, R. N., Gilbertson, D. T., Herzog, C. A., Ishani, A., ... & Wetmore, J. B.
(2021). US Renal Data System 2020 Annual Data Report: Epidemiology of Kidney Disease in the United
States. American Journal of Kidney Diseases, 77(4), A7-A8. https://www.ajkd.org/article/S0272-6386(21)00024-
X/fulltext.
69
Grams, M. E., Massie, A. B., Schold, J. D., Chen, B. P., & Segev, D. L. (2013). Trends in the Inactive Kidney
Transplant Waitlist and Implications for Candidate Survival. American Journal of Transplantation, 13(4), 1012-1018.
https://onlinelibrary.wiley.com/doi/pdf/10.1111/ajt.12143.
70
See footnote Grams et al., 2013.
71
Kulkarni, S., Ladin, K., Haakinson, D., Greene, E., Li, L., & Deng, Y. (2019). Association of Racial Disparities with
Access to Kidney Transplant after the Implementation of the New Kidney Allocation System. JAMA surgery, 154(7),
618-625. https://jamanetwork.com/journals/jamasurgery/fullarticle/2729436.
72
See footnote Kulkarni et al., 2019.
Table Group B: New Specialty Measure Sets Proposed for Addition and Modifications to
Previously Finalized Specialty Measure Sets Proposed for the CY 2025 Performance
Period/2027 MIPS Payment Year and Future Years
We are proposing to add one new specialty measure set: Optometry. In the CY 2023 PFS final rule, we finalized a combined
Ophthalmology/Optometry specialty set (87 FR 70275 and 87 FR 70434 through 70439). Based on interested parties’ request, we
are proposing to revert this combined specialty set to “Ophthalmology” under Table B.28 of this Appendix with all measures
retained as finalized under the CY 2024 PFS final rule (88 FR 79777 through 79784). We are simultaneously proposing to create a
separate Optometry specialty set with a more limited number of quality measures based on differences in scope of practice to
ophthalmology. The Optometry specialty set is proposed for comment under Table B.29 of this Appendix.
We are proposing to modify the below previously finalized specialty measure sets based upon review of updates made to existing
quality measure specifications, the proposed addition of new measures for inclusion in MIPS, and feedback provided by specialty
societies. There may be instances where the quality measures within a specialty set remain static, but the individual measures
have proposed substantive changes in Table Group D of this Appendix. In the first column, existing measures with substantive
changes described in Table Group D of this Appendix are noted with an asterisk (*), core measures that align with Core Quality
Measure Collaborative (CQMC) core measure set(s) are noted with the symbol (§), and high priority measures are noted with an
exclamation point (!). The Indicator column includes a “high priority type” in parentheses after each high priority indicator (!) to
represent the regulatory definition of high priority measures. In addition, eCQMs that are endorsed by a CBE are shown in Table
Group B of this Appendix as follows: CBE # / eCQM CBE #.
Under § 414.1305, a high priority measure means an outcome (including intermediate-outcome and patient-reported outcome),
appropriate use, patient safety, efficiency, patient experience, care coordination, opioid, or health equity-related quality measure.
Further details of these types of measures may be found in the CMS Measures Management System Hub
(https://mmshub.cms.gov/).
Previously finalized measures that have no substantive changes are not open for comment under this proposed rule. We seek
comment on proposed additions and proposed removals under applicable specialty sets in Table Group B of this Appendix.
The following specialty sets have no measures added, no measures removed, and no substantive changes proposed for the CY
2025 performance period/2027 MIPS payment year: Anesthesiology and Dentistry.
The following specialty sets have no measures added and no measures removed, but have substantive changes proposed as
addressed under Table Group D: Audiology, Electrophysiology Cardiac Specialist, Certified Nurse Midwife, Chiropractic
Medicine, Diagnostic Radiology, General Surgery, Hospitalists, Mental/Behavioral Health and Psychiatry, Neurology,
Nutrition/Dietician, Ophthalmology, Orthopedic Surgery, Pathology, Pediatrics, Physical Medicine, Physical
Therapy/Occupational Therapy, Plastic Surgery, Podiatry, and Thoracic Surgery.
Note: In the CY 2024 PFS final rule, new measure Q494: Excessive Radiation Dose or Inadequate Image Quality for Diagnostic
Computed Tomography (CT) in Adults (Clinician Level) was finalized with a 1-year delay to the 2025 performance period (88
FR 79556 through 79560). As a result, measure Q436: Radiation Consideration for Adult CT: Utilization of Dose Lowering
Techniques was finalized for removal with a 1-year delay to the 2025 performance period (88 FR 79896). These decisions are
reflected within table B.10 Diagnostic Radiology specialty set of this Appendix.

B.1. Allergy/Immunology
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Allergy/Immunology specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a
measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Allergy/
Immunology specialty set.
B.1. Allergy/Immunology
PREVIOUSLY FINALIZED MEASURES IN THE ALLERGY/IMMUNOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS68
v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current Medications
in the Medical Record:
Percentage of visits for patients aged 18
years and older for which the eligible
clinician attests to documenting a list of
current medications using all immediate
resources available on the date of the
encounter.
Centers for
Medicare &
Medicaid
Services
§
N/A /
N/A
226
CMS13
8v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and Screening:
Tobacco Use: Screening and Cessation
Intervention:
Percentage of patients aged 12 years and
older who were screened for tobacco use
one or more times during the measurement
period AND who received tobacco
cessation intervention during the
measurement period or in the 6 months
prior to the measurement period if
identified as a tobacco user.
National
Committee
for Quality
Assurance
*
!
(Patient
Safety)
0022 /
N/A
238
CMS15
6v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Use of High-Risk Medications in Older
Adults:
Percentage of patients 65 years of age and
older who were ordered at least two high-
risk medications from the same drug class.
National
Committee
for Quality
Assurance
*
N/A /
N/A
317
CMS22
v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and Screening:
Screening for High Blood Pressure and
Follow-Up Documented:
Percentage of patient visits for patients
aged 18 years and older seen during the
measurement period who were screened
for high blood pressure AND a
recommended follow-up plan is
documented, as indicated, if blood
pressure is elevated or hypertensive.
Centers for
Medicare &
Medicaid
Services
*
!
(Appropriate
Use)
N/A /
N/A
331
N/A
MIPS CQM
Specifications
Process
Adult Sinusitis: Antibiotic Prescribed
for Acute Viral Sinusitis (Overuse):
Percentage of patients, aged 18 years and
older, with a diagnosis of acute viral
sinusitis who were prescribed an antibiotic
within 10 days after onset of symptoms.
American
Academy
of
Otolaryngo
logy –
Head and
Neck
Surgery
Foundation
!
(Appropriate
Use)
N/A /
N/A
332
N/A
MIPS CQM
Specifications
Process
Adult Sinusitis: Appropriate Choice of
Antibiotic: Amoxicillin With or
Without Clavulanate Prescribed for
Patients with Acute Bacterial Sinusitis
(Appropriate Use):
Percentage of patients aged 18 years and
older with a diagnosis of acute bacterial
sinusitis that were prescribed amoxicillin,
with or without clavulanate, as a first line
antibiotic at the time of diagnosis.
American
Academy
of
Otolaryngo
logy –
Head and
Neck
Surgery
Foundation

B.1. Allergy/Immunology
PREVIOUSLY FINALIZED MEASURES IN THE ALLERGY/IMMUNOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
§
!
(Outcome)
N/A /
N/A
338
CMS31
4v2
eCQM
Specifications,
MIPS CQM
Specifications
Outcome
HIV Viral Suppression:
Percentage of patients, regardless of age,
diagnosed with HIV prior to or during the
first 90 days of the performance period,
with an eligible encounter in the first 240
days of the performance period, whose
last HIV viral load test result was less than
200 copies/mL during the performance
period.
Health
Resources
and
Services
Administrat
ion
*
§
!
(Efficiency)
N/A /
N/A
340
N/A
MIPS CQM
Specifications
Process
HIV Medical Visit Frequency:
Percentage of patients, regardless of age
with a diagnosis of HIV who had at least
one medical visit in each 6-month period
of the 24-month measurement period, with
a minimum of 60 days between medical
visits.
Health
Resources
and
Services
Administrat
ion
*
!
(Care
Coordination)
N/A /
N/A
374
CMS50
v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Closing the Referral Loop: Receipt of
Specialist Report:
Percentage of patients with referrals,
regardless of age, for which the referring
clinician receives a report from the
clinician to whom the patient was referred.
Centers for
Medicare &
Medicaid
Services
!
(Outcome)
N/A /
N/A
398
N/A
MIPS CQM
Specifications
Outcome
Optimal Asthma Control:
Composite measure of the percentage of
pediatric and adult patients whose asthma
is well-controlled as demonstrated by one
of three age appropriate patient reported
outcome tools and not at risk for
exacerbation.
Minnesota
Community
Measureme
nt
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers of Health:
Percent of patients 18 years and older
screened for food insecurity, housing
instability, transportation needs, utility
difficulties, and interpersonal safety.
Centers for
Medicare &
Medicaid
Services
*
3620 /
N/A
493
N/A
MIPS CQM
Specifications
Process
Adult Immunization Status:
Percentage of patients 19 years of age and
older who are up-to-date on recommended
routine vaccines for influenza; tetanus and
diphtheria (Td) or tetanus, diphtheria and
acellular pertussis (Tdap); zoster; and
pneumococcal.
National
Committee
for Quality
Assurance
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community Service
Provider:
Percent of patients 18 years or older who
screen positive for one or more of the
following health related social needs
(HRSNs): food insecurity, housing
instability, transportation needs, utility
help needs, or interpersonal safety; and
had contact with a Community Service
Provider (CSP) for at least one of their
HRSNs within 60 days after screening.
OCHIN
*
!
(Outcome)
2483 /
N/A
503
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome
-Based
Performa
nce
Measure
Gains in Patient Activation Measure
(PAM®) Scores at 12 Months:
The Patient Activation Measure®
(PAM®) is a 10 – or 13 – item
questionnaire that assesses an individual´s
knowledge, skills and confidence for
managing their health and health care. The
measure assesses individuals on a 0-100
scale that converts to one of four levels of
activation, from low (1) to high (4). The
PAM® performance measure (PAM®-
PM) is the change in score on the PAM®
from baseline to follow-up measurement.
Insignia
Health,
LLC, a
wholly
owned
subsidiary
of Phreesia

B.1. Allergy/Immunology
MEASURES PROPOSED FOR ADDITION TO THE ALLERGY/IMMUNOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
N/A /
N/A
TBD
N/A
MIPS CQM
Specification
s
Process
Adult COVID-19
Vaccination
Status:
Percentage of
patients aged 18
years and older
seen for a visit
during the
performance
period that are up-
to-date on their
COVID-19
vaccinations as
defined by CDC
recommendations
on current
vaccination.
Centers for
Medicare &
Medicaid
Services
We are proposing to include
this measure in the Allergy/
Immunology specialty set as it
would be clinically relevant to
this clinician type. Widespread
vaccination against SARS-
CoV-2, the virus that causes
COVID-19, is critically
important to stemming the
morbidity and mortality caused
by this disease.
73
Clinicians are
uniquely positioned to
encourage uptake of COVID-
19 vaccination, and clinicians
are still a major driving force
in promoting patient
vaccination. The addition of
this quality measure in this
specialty set would help
strengthen compliance with
recommended COVID-19
vaccination, leading to
improvement in the quality of
patient care and prevention of
disease for the general
population. This quality
measure aligns with clinical
guidelines and the evidence-
based recommendations of the
ACIP, where there is general
agreement about the safety and
efficacy of the COVID-19
vaccine, preventing costly and
potentially harmful
hospitalizations.
74
Broadening
vaccination status awareness to
this clinician type is valuable
as it can help drive an increase
in the adult vaccination rates.
The COVID-19 vaccination
included within this measure
would reduce the prevalence of
severe diseases that may be
associated with hospitalization
and decrease overall health
care costs. The measure being
added to this specialty set
would be contingent on the
inclusion of applicable coding
by the time of the CY 2025
PFS final rule. In the event
appropriate coding is not
included in the final
specification, this measure
would not be finalized for
inclusion within this specialty
measure set. See Table A.5 of
this Appendix for rationale,
including clinical evidence
supporting the inclusion of this
measure in MIPS.
73
Ikeokwu, A. E., Lawrence, R., Osieme, E. D., Gidado, K. M., Guy, C., & Dolapo, O. (2023). Unveiling the Impact of
COVID-19 Vaccines: A Meta-Analysis of Survival Rates Among Patients in the United States Based on Vaccination
Status. Cureus, 15(8), e43282. https://doi.org/10.7759/cureus.43282.
74
See footnotes Fitzpatrick et al., 2022; Polack et al., 2020; and Graña et al., 2022 in Table A.5 of this Appendix.

B.2. Anesthesiology
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Anesthesiology specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a
measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, as applicable. This specialty set has no
proposed changes.
B.2. Anesthesiology
PREVIOUSLY FINALIZED MEASURES IN THE ANESTHESIOLOGY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure
Steward
!
(Outcome)
N/A /
N/A
404
N/A
MIPS CQM
Specifications
Intermediate
Outcome
Anesthesiology Smoking
Abstinence:
The percentage of current
smokers who abstain from
cigarettes prior to anesthesia on
the day of elective surgery or
procedure.
American Society
of
Anesthesiologists
!
(Outcome)
N/A /
N/A
424
N/A
MIPS CQM
Specifications
Outcome
Perioperative Temperature
Management: Percentage of
patients, regardless of age, who
undergo surgical or therapeutic
procedures under general or
neuraxial anesthesia of 60
minutes duration or longer for
whom at least one body
temperature greater than or equal
to 35.5 degrees Celsius (or 95.9
degrees Fahrenheit) was
achieved within the 30 minutes
immediately before or 15
minutes immediately after
anesthesia end time.
American Society
of
Anesthesiologists
!
(Patient
Safety)
N/A /
N/A
430
N/A
MIPS CQM
Specifications
Process
Prevention of Post-Operative
Nausea and Vomiting (PONV)
– Combination Therapy:
Percentage of patients, aged 18
years and older, who undergo a
procedure under an inhalational
general anesthetic, AND who
have three or more risk factors
for post-operative nausea and
vomiting (PONV), who receive
combination therapy consisting
of at least two prophylactic
pharmacologic anti-emetic
agents of different classes
preoperatively and/or
intraoperatively.
American
Society of
Anesthesiologists
!
(Patient
Safety)
N/A /
N/A
463
N/A
MIPS CQM
Specifications
Process
Prevention of Post-Operative
Vomiting (POV) –
Combination Therapy
(Pediatrics):
Percentage of patients aged 3
through 17 years, who undergo a
procedure under general
anesthesia in which an
inhalational anesthetic is used for
maintenance AND who have two
or more risk factors for post-
operative vomiting (POV), who
receive combination therapy
consisting of at least two
prophylactic pharmacologic anti-
emetic agents of different classes
preoperatively and/or
intraoperatively.
American
Society of
Anesthesiologists
!
(Opioid)
N/A /
N/A
477
N/A
MIPS CQM
Specifications
Process
Multimodal Pain Management:
Percentage of patients, aged 18
years and older, undergoing
selected surgical procedures that
were managed with multimodal
pain medicine.
American
Society of
Anesthesiologists
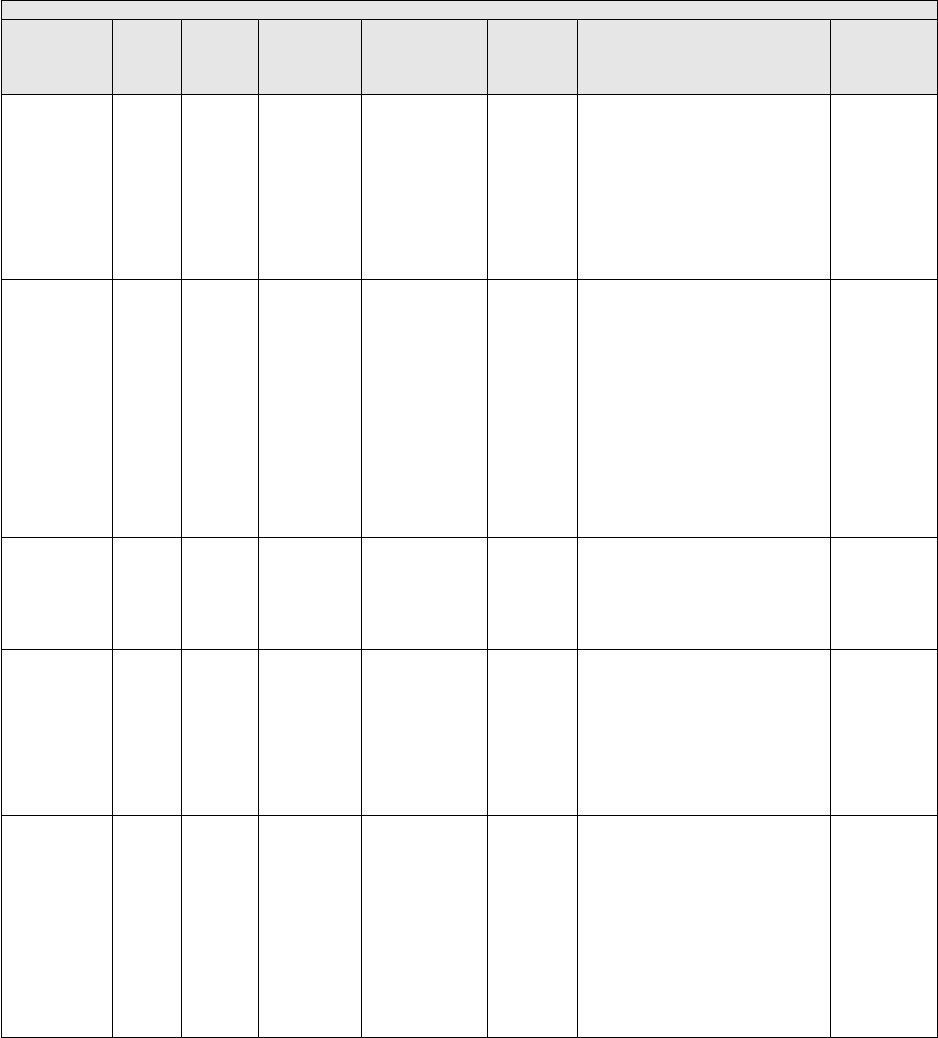
B.3. Audiology
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Audiology specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a measure
reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Audiology
specialty set that have proposed substantive changes in Table Group D of this Appendix as indicated by an * in the Indicator
column.
B.3. Audiology
PREVIOUSLY FINALIZED MEASURES IN THE AUDIOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure
Steward
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS68v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for patients
aged 18 years and older for which
the eligible clinician attests to
documenting a list of current
medications using all immediate
resources available on the date of
the encounter.
Centers for
Medicare &
Medicaid
Services
§
N/A /
N/A
134
CMS2v14
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and Screening:
Screening for Depression and
Follow-Up Plan:
Percentage of patients aged 12
years and older screened for
depression on the date of the
encounter or up to 14 days prior to
the date of the encounter using an
age-appropriate standardized
depression screening tool AND if
positive, a follow-up plan is
documented on the date of or up to
two days after the date of the
qualifying encounter.
Centers for
Medicare &
Medicaid
Services
*
!
(Care
Coordination)
0101 /
N/A
155
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Falls: Plan of Care:
Percentage of patients aged 65
years and older with a history of
falls who had a plan of care for falls
documented within 12 months.
National
Committee for
Quality
Assurance
*
!
(Patient
Safety)
N/A /
N/A
181
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Elder Maltreatment Screen and
Follow-Up Plan:
Percentage of patients aged 60
years and older with a documented
elder maltreatment screen using an
Elder Maltreatment Screening tool
on the date of encounter AND a
documented follow-up plan on the
date of the positive screen.
Centers for
Medicare &
Medicaid
Services
*
§
!
(Care
Coordination)
N/A /
N/A
182
N/A
MIPS CQM
Specifications
Process
Functional Outcome Assessment:
Percentage of visits for patients
aged 18 years and older with
documentation of a current
functional outcome assessment
using a standardized functional
outcome assessment tool on the
date of the encounter AND
documentation of a care plan based
on identified functional outcome
deficiencies within 2 days of the
date of the identified deficiencies.
Centers for
Medicare &
Medicaid
Services

B.3. Audiology
PREVIOUSLY FINALIZED MEASURES IN THE AUDIOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure
Steward
§
N/A /
N/A
226
CMS138v1
3
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and Screening:
Tobacco Use: Screening and
Cessation Intervention:
Percentage of patients aged 12
years and older who were screened
for tobacco use one or more times
during the measurement period
AND who received tobacco
cessation intervention during the
measurement period or in the 6
months prior to the measurement
period if identified as a tobacco
user.
National
Committee
for Quality
Assurance
!
(Care
Coordination)
NA /
NA
261
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Referral for Otologic Evaluation
for Patients with Acute or
Chronic Dizziness:
Percentage of patients aged birth
and older referred to a physician
(preferably a physician specially
trained in disorders of the ear) for
an otologic evaluation subsequent
to an audiologic evaluation after
presenting with acute or chronic
dizziness.
Audiology
Quality
Consortium
*
N/A /
N/A
317
CMS22v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and Screening:
Screening for High Blood
Pressure and Follow-Up
Documented: Percentage of patient
visits for patients aged 18 years and
older seen during the measurement
period who were screened for high
blood pressure AND a
recommended follow-up plan is
documented, as indicated, if blood
pressure is elevated or
hypertensive.
Centers for
Medicare &
Medicaid
Services
!
(Patient
Safety)
0101 /
N/A
318
CMS139v1
3
eCQM
Specifications
Process
Falls: Screening for Future Fall
Risk:
Percentage of patients 65 years of
age and older who were screened
for future fall risk during the
measurement period.
National
Committee
for Quality
Assurance
§
2152/
N/A
431
N/A
MIPS CQM
Specifications
Process
Preventive Care and Screening:
Unhealthy Alcohol Use:
Screening & Brief Counseling:
Percentage of patients aged 18
years and older who were screened
for unhealthy alcohol use using a
systematic screening method at
least once within the last 12 months
AND who received brief counseling
if identified as an unhealthy alcohol
user.
National
Committee
for Quality
Assurance
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers of
Health:
Percent of patients 18 years and
older screened for food insecurity,
housing instability, transportation
needs, utility difficulties, and
interpersonal safety.
Centers for
Medicare &
Medicaid
Services

B.3. Audiology
PREVIOUSLY FINALIZED MEASURES IN THE AUDIOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure
Steward
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years or older
who screen positive for one or more
of the following health related
social needs (HRSNs): food
insecurity, housing instability,
transportation needs, utility help
needs, or interpersonal safety; and
had contact with a Community
Service Provider (CSP) for at least
one of their HRSNs within 60 days
after screening.
OCHIN

B.4a. Cardiology
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Cardiology specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a measure
reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Cardiology
specialty set.
B.4a. Cardiology
PREVIOUSLY FINALIZED MEASURES IN THE CARDIOLOGY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure
Steward
§
0081 /
0081e
005
CMS13
5v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Heart Failure (HF):
Angiotensin-Converting
Enzyme (ACE) Inhibitor or
Angiotensin Receptor Blocker
(ARB) or Angiotensin
Receptor-Neprilysin Inhibitor
(ARNI) Therapy for Left
Ventricular Systolic
Dysfunction (LVSD):
Percentage of patients aged 18
years and older with a diagnosis
of heart failure (HF) with a
current or prior left ventricular
ejection fraction (LVEF) ≤ 40%
who were prescribed ACE
inhibitor or ARB or ARNI
therapy either within a 12-month
period when seen in the
outpatient setting OR at each
hospital discharge.
American Heart
Association
§
0067 /
N/A
006
N/A
MIPS CQM
Specifications
Process
Coronary Artery Disease
(CAD): Antiplatelet Therapy:
Percentage of patients aged 18
years and older with a diagnosis
of coronary artery disease (CAD)
seen within a 12-month period
who were prescribed aspirin or
clopidogrel.
American Heart
Association
§
0070 /
0070e
007
CMS14
5v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Coronary Artery Disease
(CAD): Beta-Blocker Therapy –
Prior Myocardial Infarction
(MI) or Left Ventricular
Systolic Dysfunction (LVEF ≤
40%):
Percentage of patients aged 18
years and older with a diagnosis
of coronary artery disease seen
within a 12-month period who
also have a prior MI or a current
or prior LVEF ≤ 40% who were
prescribed beta-blocker therapy.
American Heart
Association
§
0083 /
0083e
008
CMS14
4v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Heart Failure (HF): Beta-
Blocker Therapy for Left
Ventricular Systolic
Dysfunction (LVSD):
Percentage of patients aged 18
years and older with a diagnosis
of heart failure (HF) with a
current or prior left ventricular
ejection fraction (LVEF) ≤ 40%
who were prescribed beta-blocker
therapy either within a 12-month
period when seen in the
outpatient setting OR at each
hospital discharge.
American Heart
Association
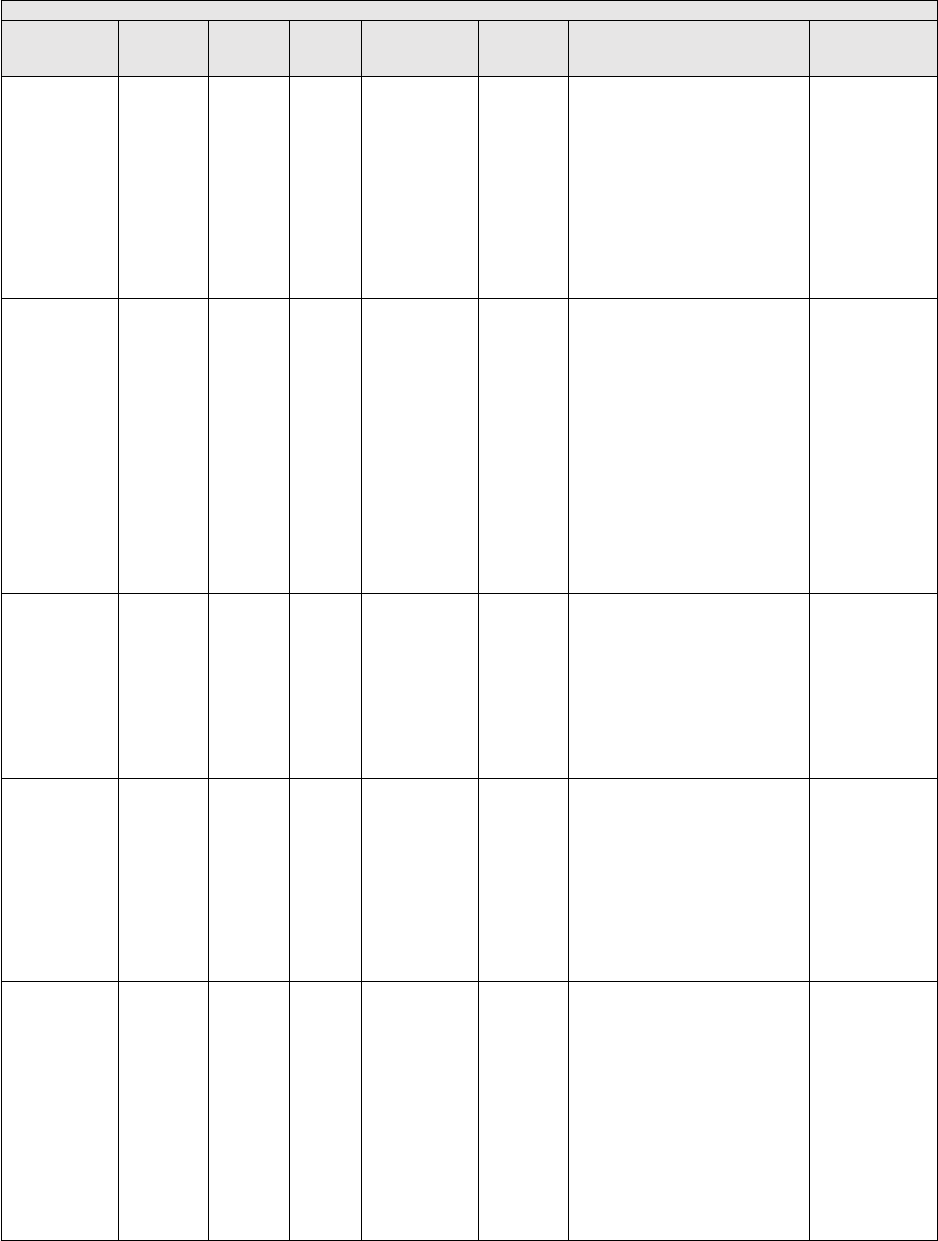
B.4a. Cardiology
PREVIOUSLY FINALIZED MEASURES IN THE CARDIOLOGY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure
Steward
*
!
(Care
Coordination)
0326
/ N/A
047
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Advance Care Plan:
Percentage of patients aged 65
years and older who have an
advance care plan or surrogate
decision maker documented in the
medical record or documentation
in the medical record that an
advance care plan was discussed
but the patient did not wish or
was not able to name a surrogate
decision maker or provide an
advance care plan.
National
Committee for
Quality
Assurance
§
0066 /
N/A
118
N/A
MIPS CQM
Specifications
Process
Coronary Artery Disease
(CAD): Angiotensin-Converting
Enzyme (ACE) Inhibitor or
Angiotensin Receptor Blocker
(ARB) Therapy – Diabetes or
Left Ventricular Systolic
Dysfunction (LVEF ≤ 40%):
Percentage of patients aged 18
years and older with a diagnosis
of coronary artery disease seen
within a 12-month period who
also have diabetes OR a current
or prior Left Ventricular Ejection
Fraction (LVEF) ≤ 40% who
were prescribed ACE inhibitor or
ARB therapy.
American
Heart
Association
*
§
!
(Patient
Safety)
N/A / N/A
130
CMS68
v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for patients
aged 18 years and older for which
the eligible clinician attests to
documenting a list of current
medications using all immediate
resources available on the date of
the encounter.
Centers for
Medicare &
Medicaid
Services
§
N/A / N/A
187
N/A
MIPS CQM
Specifications
Process
Stroke and Stroke
Rehabilitation: Thrombolytic
Therapy:
Percentage of patients aged 18
years and older with a diagnosis
of acute ischemic stroke who
arrive at the hospital within 3.5
hours of time last known well and
for whom IV thrombolytic
therapy was initiated within 4.5
hours of time last known well.
American Heart
Association
§
N/A / N/A
226
CMS13
8v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and Screening:
Tobacco Use: Screening and
Cessation Intervention:
Percentage of patients aged 12
years and older who were
screened for tobacco use one or
more times during the
measurement period AND who
received tobacco cessation
intervention during the
measurement period or in the 6
months prior to the measurement
period if identified as a tobacco
user.
National
Committee for
Quality
Assurance

B.4a. Cardiology
PREVIOUSLY FINALIZED MEASURES IN THE CARDIOLOGY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure
Steward
*
§
!
(Outcome)
N/A / N/A
236
CMS16
5v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Inter-
mediate
Outcome
Controlling High Blood
Pressure:
Percentage of patients 18-85
years of age who had a diagnosis
of essential hypertension starting
before and continuing into, or
starting during the first 6 months
of the measurement period, and
whose most recent blood pressure
was adequately controlled
(<140/90mmHg) during the
measurement period.
National
Committee for
Quality
Assurance
*
!
(Patient
Safety)
0022 /
N/A
238
CMS15
6v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Use of High-Risk Medications
in Older Adults:
Percentage of patients 65 years of
age and older who were ordered
at least two high-risk medications
from the same drug class.
National
Committee for
Quality
Assurance
!
(Care
Coordination)
0643 /
N/A
243
N/A
MIPS CQM
Specifications
Process
Cardiac Rehabilitation Patient
Referral from an Outpatient
Setting:
Percentage of patients evaluated
in an outpatient setting who
within the previous 12 months
have experienced an acute
myocardial infarction (MI),
coronary artery bypass graft
(CABG) surgery, a percutaneous
coronary intervention (PCI),
cardiac valve surgery, or cardiac
transplantation, or who have
chronic stable angina (CSA) and
have not already participated in
an early outpatient cardiac
rehabilitation/secondary
prevention (CR) program for the
qualifying event/diagnosis who
were referred to a CR program.
American Heart
Association
*
N/A /
N/A
317
CMS22
v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and Screening:
Screening for High Blood
Pressure and Follow-Up
Documented:
Percentage of patient visits for
patients aged 18 years and older
seen during the measurement
period who were screened for
high blood pressure AND a
recommended follow-up plan is
documented, as indicated, if
blood pressure is elevated or
hypertensive.
Centers for
Medicare &
Medicaid
Services
*
!
(Efficiency)
N/A / N/A
322
N/A
MIPS CQM
Specifications
Efficiency
Cardiac Stress Imaging Not
Meeting Appropriate Use
Criteria: Preoperative
Evaluation in Low-Risk
Surgery Patients:
Percentage of stress single-photon
emission computed tomography
(SPECT) myocardial perfusion
imaging (MPI), stress
echocardiogram (ECHO), cardiac
computed tomography
angiography (CCTA), or cardiac
magnetic resonance (CMR)
performed in low-risk surgery
patients 18 years or older for
preoperative evaluation during
the 12-month submission period.
American
College of
Cardiology
Foundation

B.4a. Cardiology
PREVIOUSLY FINALIZED MEASURES IN THE CARDIOLOGY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure
Steward
§
N/A / N/A
326
N/A
MIPS CQM
Specifications
Process
Atrial Fibrillation and Atrial
Flutter: Chronic
Anticoagulation Therapy:
Percentage of patients aged 18
years and older with atrial
fibrillation (AF) or atrial flutter
who were prescribed an FDA-
approved oral anticoagulant drug
for the prevention of
thromboembolism during the
measurement period.
American Heart
Association
*
!
(Outcome)
N/A / N/A
344
N/A
MIPS CQM
Specifications
Outcome
Rate of Carotid Artery Stenting
(CAS) for Asymptomatic
Patients, Without Major
Complications (Discharged to
Home by Post-Operative Day
#2):
Percent of asymptomatic patients
undergoing CAS who are
discharged to home no later than
post-operative day #2.
Society for
Vascular
Surgeons
*
!
(Care
Coordination)
N/A / N/A
374
CMS50
v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Closing the Referral Loop:
Receipt of Specialist Report:
Percentage of patients with
referrals, regardless of age, for
which the referring clinician
receives a report from the
clinician to whom the patient was
referred.
Centers for
Medicare &
Medicaid
Services
§
2152 /
N/A
431
N/A
MIPS CQM
Specifications
Process
Preventive Care and Screening:
Unhealthy Alcohol Use:
Screening & Brief Counseling:
Percentage of patients aged 18
years and older who were
screened for unhealthy alcohol
use using a systematic screening
method at least once within the
last 12 months AND who
received brief counseling if
identified as an unhealthy alcohol
user.
National
Committee for
Quality
Assurance
§
N/A / N/A
438
CMS34
7v8
eCQM
Specifications,
MIPS CQM
Specifications
Process
Statin Therapy for the
Prevention and Treatment of
Cardiovascular Disease:
Percentage of the following
patients - all considered at high
risk of cardiovascular events -
who were prescribed or were on
statin therapy during the
performance period:
•All patients who were previously
diagnosed with or currently have
a diagnosis of clinical
atherosclerotic cardiovascular
disease (ASCVD), including an
ASCVD procedure; OR
•Patients aged 20 to 75 years who
have ever had a low-density
lipoprotein cholesterol (LDL-C)
level ≥ 190 mg/dL or were
previously diagnosed with or
currently have an active diagnosis
of familial hypercholesterolemia;
OR;
•Patients aged 40 to 75 years with
a diagnosis of diabetes; OR;
•Patients aged 40 to 75 with a 10-
year ASCVD risk score of ≥ 20
percent.
Centers for
Medicare &
Medicaid
Services

B.4a. Cardiology
PREVIOUSLY FINALIZED MEASURES IN THE CARDIOLOGY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure
Steward
§
!
(Outcome)
N/A / N/A
441
N/A
MIPS CQM
Specifications
Intermedi
ate
Outcome
Ischemic Vascular Disease
(IVD) All or None Outcome
Measure (Optimal Control):
The IVD All-or-None Measure is
one outcome measure (optimal
control). The measure contains
four goals. All four goals within a
measure must be reached in order
to meet that measure. The
numerator for the all-or-none
measure should be collected from
the organization’s total IVD
denominator. All-or-None
Outcome Measure (Optimal
Control) – Using the IVD
denominator optimal results
include:
• Most recent blood pressure
(BP) measurement is less than or
equal to 140/90 mm Hg -- AND
• Most recent tobacco status is
Tobacco Free -- AND
• Daily Aspirin or Other
Antiplatelet Unless
Contraindicated -- AND
• Statin Use Unless
Contraindicated.
Wisconsin
Collaborative
for Healthcare
Quality
!
(Equity)
N/A / N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers of
Health:
Percent of patients 18 years and
older screened for food
insecurity, housing instability,
transportation needs, utility
difficulties, and interpersonal
safety.
Centers for
Medicare &
Medicaid
Services
*
3620 /
N/A
493
N/A
MIPS CQM
Specifications
Process
Adult Immunization Status:
Percentage of patients 19 years of
age and older who are up-to-date
on recommended routine vaccines
for influenza; tetanus and
diphtheria (Td) or tetanus,
diphtheria and acellular pertussis
(Tdap); zoster; and
pneumococcal.
National
Committee for
Quality
Assurance
*
!
(Equity)
N/A / N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years or
older who screen positive for one
or more of the following health
related social needs (HRSNs):
food insecurity, housing
instability, transportation needs,
utility help needs, or interpersonal
safety; and had contact with a
Community Service Provider
(CSP) for at least one of their
HRSNs within 60 days after
screening.
OCHIN

B.4a. Cardiology
PREVIOUSLY FINALIZED MEASURES IN THE CARDIOLOGY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure
Steward
*
!
(Outcome)
2483 /
N/A
503
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performan
ce
Measure
Gains in Patient Activation
Measure (PAM®) Scores at 12
Months:
The Patient Activation Measure®
(PAM®) is a 10 – or 13 – item
questionnaire that assesses an
individual´s knowledge, skills and
confidence for managing their
health and health care. The
measure assesses individuals on a
0-100 scale that converts to one
of four levels of activation, from
low (1) to high (4). The PAM®
performance measure (PAM®-
PM) is the change in score on the
PAM® from baseline to follow-
up measurement.
Insignia Health,
LLC, a wholly
owned
subsidiary of
Phreesia
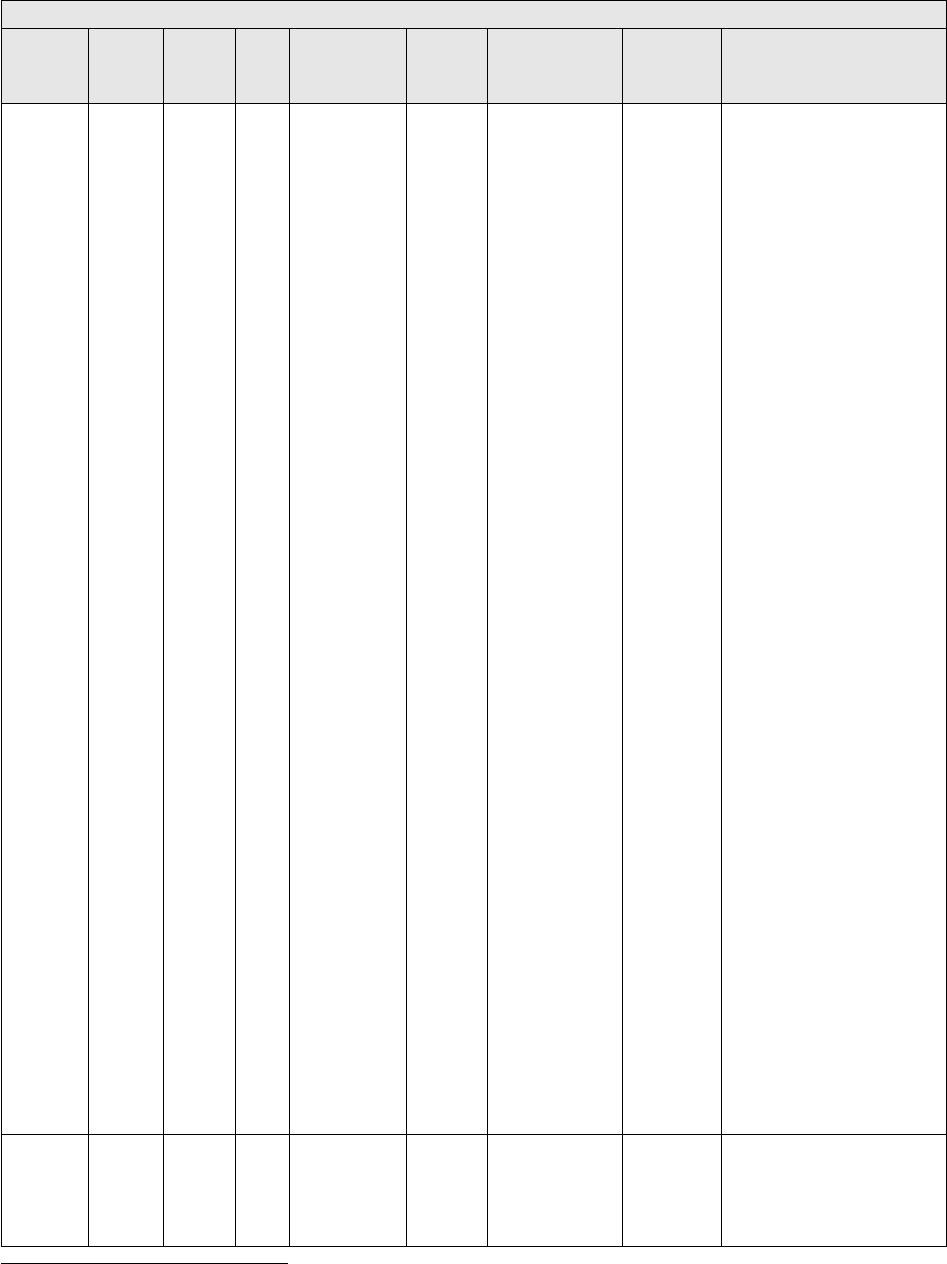
B.4a. Cardiology
MEASURES PROPOSED FOR ADDITION TO THE CARDIOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
!
(Outcome
)
3665 /
N/A
495
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome
-Based
Perform
ance
Measure
Ambulatory
Palliative Care
Patients'
Experience of
Feeling Heard
and
Understood:
The percentage
of top-box
responses among
patients aged 18
years and older
who had an
ambulatory
palliative care
visit and report
feeling heard and
understood by
their palliative
care clinician and
team within 2
months (60 days)
of the
ambulatory
palliative care
visit.
American
Academy
of Hospice
and
Palliative
Medicine
(AAHPM)
We are proposing to include
this measure in the Cardiology
specialty set as it would be
clinically relevant to this
clinician type. This PRO-PM
would help to fill a gap for
patients receiving palliative
care by capturing the patient’s
voice and experience of care by
assessing communication and
shared decision making with
the clinician. This is an
important patient-centered
measure that helps patients feel
heard and understood which
can effectively improve the
quality of care received and
outcomes for patients in
palliative care. Allowing
patients to feel heard and
understood adds an important
dimension to the care planning
for this unique patient
population commonly cared for
by clinicians in this specialty.
As more patients are living
longer with multiple
comorbidities, especially true
for the advanced heart disease
patient population, early
emergence of palliative care
into the overall care of cardiac
patients can notably improve
their quality of life, patient
satisfaction, and reduction in
symptoms.
75
This measure is
predicated on existing
guidelines and conceptual
models
76
and can facilitate and
improve effective patient-
clinician communication that
engenders trust,
acknowledgement, and a
whole-person orientation to the
care that is provided. Through
the benefits of enhanced
patient-provider
communication, this measure
would improve the quality of
care received and outcomes for
patients receiving palliative
care. The measure being added
to this specialty set would be
contingent on the inclusion of
applicable coding by the time
of the CY 2025 PFS final rule.
N/A /
N/A
TBD
N/A
MIPS CQM
Specifications
Process
Adult COVID-
19 Vaccination
Status:
Percentage of
patients aged 18
years and older
Centers for
Medicare &
Medicaid
Services
We are proposing to include
this measure in the Cardiology
specialty set as it would be
clinically relevant to this
clinician type. Widespread
vaccination against SARS-
75
Kilic, Y., Smer, A., & Goldstein, N. (2020). The Importance of Palliative Care in Cardiology: Differences Between
Countries. JACC. Case Reports, 2(2), 326–329. https://doi.org/10.1016/j.jaccas.2019.11.069.
76
National Consensus Project for Quality Palliative Care. (2018). Clinical Practice Guidelines for Quality Palliative
Care, 4th edition. https://www.nationalcoalitionhpc.org/ncp.

B.4a. Cardiology
MEASURES PROPOSED FOR ADDITION TO THE CARDIOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
seen for a visit
during the
performance
period that are
up-to-date on
their COVID-19
vaccinations as
defined by CDC
recommendation
s on current
vaccination.
CoV-2, the virus that causes
COVID-19, is critically
important to stemming the
morbidity and mortality caused
by this disease.
77
Clinicians are
uniquely positioned to
encourage uptake of COVID-
19 vaccination, and clinicians
are still a major driving force
in promoting patient
vaccination. The addition of
this quality measure in this
specialty set would help
strengthen compliance with
recommended COVID-19
vaccination, leading to
improvement in the quality of
patient care and prevention of
disease for the general
population. This quality
measure aligns with clinical
guidelines and the evidence-
based recommendations of the
ACIP, where there is general
agreement about the safety and
efficacy of the COVID-19
vaccine, preventing costly and
potentially harmful
hospitalizations.
78
Broadening
vaccination status awareness to
this clinician type is valuable
as it can help drive an increase
in the adult vaccination rates.
The COVID-19 vaccination
included within this measure
would reduce the prevalence of
severe diseases that may be
associated with hospitalization
and decrease overall health
care costs. The measure being
added to this specialty set
would be contingent on the
inclusion of applicable coding
by the time of the CY 2025
PFS final rule. In the event
appropriate coding is not
included in the final
specification, this measure
would not be finalized for
inclusion within this specialty
measure set. See Table A.5 of
this Appendix for rationale,
including clinical evidence
supporting the inclusion of this
measure in MIPS.
77
See footnote Ikeokwu et al., 2023 in Table B.1 of this Appendix.
78
See footnotes Fitzpatrick et al., 2022; Polack et al., 2020; and Graña et al., 2022 in Table A.5 of this Appendix.

B.4b. Electrophysiology Cardiac Specialist
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Electrophysiology Cardiac Specialist specialty set takes additional criteria into consideration, which includes, but is not
limited to: whether a measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types.
We may reassess the appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the
specialty set. Measure tables in this set include previously finalized measures we are maintaining within the set, measures
proposed to be added, and measures proposed for removal, as applicable. We request comment on the measures available in the
proposed Electrophysiology Cardiac Specialist specialty set that have proposed substantive changes in Table Group D of this
Appendix as indicated by an * in the Indicator column.
B.4b. Electrophysiology Cardiac Specialist
PREVIOUSLY FINALIZED MEASURES IN THE ELECTROPHYSIOLOGY CARDIAC SPECIALIST SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measur
e
Type
Measure Title
and Description
Measure Steward
§
!
(Outcome)
2474 /
N/A
392
N/A
MIPS CQM
Specifications
Outcome
Cardiac Tamponade and/or
Pericardiocentesis Following
Atrial Fibrillation Ablation:
Rate of cardiac tamponade and/or
pericardiocentesis following atrial
fibrillation ablation. This measure
is submitted as four rates stratified
by age and gender:
• Submission Age Criteria 1:
Females 18-64 years of age
• Submission Age Criteria 2: Males
18-64 years of age
• Submission Age Criteria 3:
Females 65 years of age and older
• Submission Age Criteria 4: Males
65 years of age and older
American College
of Cardiology
Foundation
*
!
(Outcome)
N/A /
N/A
393
N/A
MIPS CQM
Specifications
Outcome
Infection within 180 Days of
Cardiac Implantable Electronic
Device (CIED) Implantation,
Replacement, or Revision:
Infection rate following CIED
device implantation, replacement,
or revision.
American College
of Cardiology
Foundation

B.5. Certified Nurse Midwife
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Certified Nurse-Midwife specialty set takes additional criteria into consideration, which includes, but is not limited to:
whether a measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may
reassess the appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set.
Measure tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be
added, and measures proposed for removal, as applicable. We request comment on the measures available in the proposed
Certified Nurse Midwife specialty set that have proposed substantive changes in Table Group D of this Appendix as indicated by
an * in the Indicator column.
B.5. Certified Nurse-Midwife
PREVIOUSLY FINALIZED MEASURES IN THE CERTIFIED NURSE MIDWIFE SPECIALTY SET
Indicator
CBE
# /
eCQ
M
CBE
#
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Care
Coordination)
0326 /
N/A
047
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Advance Care Plan:
Percentage of patients aged 65
years and older who have an
advance care plan or surrogate
decision maker documented in the
medical record or documentation in
the medical record that an advance
care plan was discussed but the
patient did not wish or was not able
to name a surrogate decision maker
or provide an advance care plan.
National
Committee for
Quality Assurance
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS68
v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for patients
aged 18 years and older for which
the eligible clinician attests to
documenting a list of current
medications using all immediate
resources available on the date of
the encounter.
Centers for
Medicare &
Medicaid Services
§
N/A /
N/A
226
CMS13
8v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and Screening:
Tobacco Use: Screening and
Cessation Intervention:
Percentage of patients aged 12
years and older who were screened
for tobacco use one or more times
during the measurement period
AND who received tobacco
cessation intervention during the
measurement period or in the six
months prior to the measurement
period if identified as a tobacco
user.
National
Committee for
Quality Assurance
!
(Outcome)
N/A /
N/A
335
N/A
MIPS CQM
Specifications
Outcome
Maternity Care: Elective
Delivery (Without Medical
Indication) at < 39 Weeks
(Overuse):
Percentage of patients, regardless
of age, who gave birth during a 12-
month period, delivered a live
singleton at < 39 weeks of
gestation, and had elective
deliveries (without medical
indication) by cesarean birth or
induction of labor.
Centers for
Medicare &
Medicaid Services

B.5. Certified Nurse-Midwife
PREVIOUSLY FINALIZED MEASURES IN THE CERTIFIED NURSE MIDWIFE SPECIALTY SET
Indicator
CBE
# /
eCQ
M
CBE
#
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
§
!
(Care
Coordination)
N/A /
N/A
336
N/A
MIPS CQM
Specifications
Process
Maternity Care: Postpartum
Follow-up and Care
Coordination:
Percentage of patients, regardless
of age, who gave birth during a 12-
month period who were seen for
postpartum care before or at 12
weeks of giving birth and received
the following at a postpartum visit:
breast-feeding evaluation and
education, postpartum depression
screening, postpartum glucose
screening for gestational diabetes
patients, family and contraceptive
planning counseling, tobacco use
screening and cessation education,
healthy lifestyle behavioral advice,
and an immunization review and
update.
Centers for
Medicare &
Medicaid Services
§
2152 /
N/A
431
N/A
MIPS CQM
Specifications
Process
Preventive Care and Screening:
Unhealthy Alcohol Use: Screening
& Brief Counseling:
Percentage of patients aged 18
years and older who were screened
for unhealthy alcohol use using a
systematic screening method at
least once within the last 12 months
AND who received brief
counseling if identified as an
unhealthy alcohol user.
National
Committee for
Quality Assurance
§
N/A /
N/A
475
CMS34
9v7
eCQM
Specifications
Process
HIV Screening:
Percentage of patients aged 15-65
at the start of the measurement
period who were between 15-65
years old when tested for Human
Immunodeficiency Virus (HIV).
Centers for
Disease Control
and Prevention
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers of
Health:
Percent of patients 18 years and
older screened for food insecurity,
housing instability, transportation
needs, utility difficulties, and
interpersonal safety.
Centers for
Medicare &
Medicaid Services
N/A /
N/A
496
N/A
MIPS CQM
Specifications
Process
Cardiovascular Disease
(CVD) Risk Assessment
Measure – Proportion of
Pregnant/Postpartum Patients
that Receive CVD Risk
Assessment with a Standardized
Instrument: Percentage of
pregnant or postpartum patients
who received a cardiovascular
disease (CVD) risk assessment with
a standardized instrument.
University of
California, Irvine
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years or older
who screen positive for one or more
of the following health related
social needs (HRSNs): food
insecurity, housing instability,
transportation needs, utility help
needs, or interpersonal safety; and
had contact with a Community
Service Provider (CSP) for at least
1 of their HRSNs within 60 days
after screening.
OCHIN

B.5. Certified Nurse-Midwife
PREVIOUSLY FINALIZED MEASURES IN THE CERTIFIED NURSE MIDWIFE SPECIALTY SET
Indicator
CBE
# /
eCQ
M
CBE
#
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Outcome)
2483 /
N/A
503
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performan
ce
Measure
Gains in Patient Activation
Measure (PAM®) Scores at 12
Months:
The Patient Activation Measure®
(PAM®) is a 10 – or 13 – item
questionnaire that assesses an
individual´s knowledge, skills and
confidence for managing their
health and health care. The measure
assesses individuals on a 0-100
scale that converts to one of four
levels of activation, from low (1) to
high (4). The PAM® performance
measure (PAM®-PM) is the change
in score on the PAM® from
baseline to follow-up measurement.
Insignia Health,
LLC, a wholly
owned subsidiary
of Phreesia
!
(Safety)
N/A /
N/A
504
N/A
MIPS CQM
Specifications
Process
Initiation, Review, And/Or
Update To Suicide Safety Plan
For Individuals With Suicidal
Thoughts, Behavior, Or Suicide
Risk:
Percentage of adult aged 18 years
and older with suicidal ideation or
behavior symptoms (based on
results of a standardized assessment
tool or screening tool) or increased
suicide risk (based on the
clinician’s evaluation or clinician-
rating tool) for whom a suicide
safety plan is initiated, reviewed,
and/or updated in collaboration
between the patient and their
clinician.
American
Psychiatric
Association
*
!
(Outcome)
N/A /
N/A
505
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performan
ce
Measure
Reduction in Suicidal Ideation or
Behavior Symptoms:
The percentage of patients aged 18
and older with a mental and/or
substance use disorder AND
suicidal thoughts, behaviors or risk
symptoms who demonstrated a
reduction in suicidal ideation and/or
behavior symptoms based on
results from the Columbia-Suicide
Severity Rating Scale (C-SSRS)
‘Screen Version’ or ‘Since Last
Visit’ within 120 days after an
index assessment.
American
Psychiatric
Association
B.6. Chiropractic Medicine
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Chiropractic Medicine specialty set takes additional criteria into consideration, which includes, but is not limited to: whether
a measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess
the appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Chiropractic
Medicine specialty set that have proposed substantive changes in Table Group D of this Appendix as indicated by an * in the
Indicator column.

B.6. Chiropractic Medicine
PREVIOUSLY FINALIZED MEASURES IN THE CHIROPRACTIC MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure
Steward
*
§
!
(Care
Coordination
)
N/A /
N/A
182
N/A
MIPS CQM
Specifications
Process
Functional Outcome Assessment:
Percentage of visits for patients aged 18
years and older with documentation of a
current functional outcome assessment
using a standardized functional outcome
assessment tool on the date of the
encounter AND documentation of a care
plan based on identified functional
outcome deficiencies within 2 days of the
date of the identified deficiencies.
Centers for
Medicare &
Medicaid
Services
!
(Outcome)
N/A /
N/A
217
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Functional Status Change for Patients
with Knee Impairments:
A patient-reported outcome measure
(PROM) of risk-adjusted change in
functional status (FS) for patients 14
years+ with knee impairments. The
change in FS is assessed using the FOTO
Lower Extremity Physical Function
(LEPF) PROM. The measure is adjusted
to patient characteristics known to be
associated with FS outcomes (risk-
adjusted) and used as a performance
measure at the patient, individual
clinician, and clinic levels to assess
quality.
Focus on
Therapeutic
Outcomes,
Inc.
!
(Outcome)
N/A /
N/A
218
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Functional Status Change for Patients
with Hip Impairments:
A patient-reported outcome measure
(PROM) of risk-adjusted change in
functional status (FS) for patients 14
years+ with hip impairments. The change
in FS is assessed using the FOTO Lower
Extremity Physical Function (LEPF)
PROM. The measure is adjusted to patient
characteristics known to be associated
with FS outcomes (risk adjusted) and used
as a performance measure at the patient,
individual clinician, and clinic levels to
assess quality.
Focus on
Therapeutic
Outcomes,
Inc.
!
(Outcome)
N/A /
N/A
219
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Functional Status Change for Patients
with Lower Leg, Foot or Ankle
Impairments:
A patient-reported outcome measure
(PROM) of risk-adjusted change in
functional status (FS) for patients 14
years+ with foot, ankle or lower leg
impairments. The change in FS is assessed
using the FOTO Lower Extremity
Physical Function (LEPF) PROM. The
measure is adjusted to patient
characteristics known to be associated
with FS outcomes (risk-adjusted) and used
as a performance measure at the patient,
individual clinician, and clinic levels to
assess quality.
Focus on
Therapeutic
Outcomes,
Inc.
!
(Outcome)
N/A /
N/A
220
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Functional Status Change for Patients
with Low Back Impairments:
A patient-reported outcome measure
(PROM) of risk-adjusted change in
functional status (FS) for patients 14
years+ with low back impairments. The
change in FS is assessed using the FOTO
Low Back FS PROM. The measure is
adjusted to patient characteristics known
to be associated with FS outcomes (risk
adjusted) and used as a performance
measure at the patient, individual
clinician, and clinic levels to assess
quality.
Focus on
Therapeutic
Outcomes,
Inc.

B.6. Chiropractic Medicine
PREVIOUSLY FINALIZED MEASURES IN THE CHIROPRACTIC MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure
Steward
!
(Outcome)
N/A /
N/A
221
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Functional Status Change for Patients
with Shoulder Impairments:
A patient-reported outcome measure
(PROM) of risk-adjusted change in
functional status (FS) for patients 14
years+ with shoulder impairments. The
change in FS is assessed using the FOTO
Shoulder FS PROM. The measure is
adjusted to patient characteristics known
to be associated with FS outcomes (risk
adjusted) and used as a performance
measure at the patient, individual
clinician, and clinic levels to assess
quality.
Focus on
Therapeutic
Outcomes,
Inc.
!
(Outcome)
N/A /
N/A
222
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Functional Status Change for Patients
with Elbow, Wrist or Hand
Impairments:
A patient-reported outcome measure
(PROM) of risk-adjusted change in
functional status (FS) for patients 14
years+ with elbow, wrist, or hand
impairments. The change in FS is assessed
using the FOTO Elbow/Wrist/Hand FS
PROM. The measure is adjusted to patient
characteristics known to be associated
with FS outcomes (risk adjusted) and used
as a performance measure at the patient,
individual clinician, and clinic levels to
assess quality.
Focus on
Therapeutic
Outcomes,
Inc.
§
!
(Outcome)
N/A /
N/A
478
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Functional Status Change for Patients
with Neck Impairments:
A patient-reported outcome measure
(PROM) of risk-adjusted change in
functional status (FS) for patients 14
years+ with neck impairments. The
change in FS is assessed using the FOTO
Neck FS PROM. The measure is adjusted
to patient characteristics known to be
associated with FS outcomes (risk-
adjusted) and used as a performance
measure at the patient, individual
clinician, and clinic levels to assess
quality.
Focus on
Therapeutic
Outcomes,
Inc.
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers of Health:
Percent of patients 18 years and older
screened for food insecurity, housing
instability, transportation needs, utility
difficulties, and interpersonal safety.
Centers for
Medicare &
Medicaid
Services
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community Service
Provider:
Percent of patients 18 years or older who
screen positive for one or more of the
following health related social needs
(HRSNs): food insecurity, housing
instability, transportation needs, utility
help needs, or interpersonal safety; and
had contact with a Community Service
Provider (CSP) for at least 1 of their
HRSNs within 60 days after screening.
OCHIN

B.7. Clinical Social Work
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Clinical Social Work specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a
measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Clinical Social
Work specialty set.
B.7. Clinical Social Work
PREVIOUSLY FINALIZED MEASURES IN THE CLINICAL SOCIAL WORK SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Care
Coordination
)
0326 /
N/A
047
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Advance Care Plan:
Percentage of patients aged 65
years and older who have an
advance care plan or surrogate
decision maker documented in
the medical record or
documentation in the medical
record that an advance care plan
was discussed but the patient did
not wish or was not able to name
a surrogate decision maker or
provide an advance care plan.
National
Committee for
Quality Assurance
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS68
v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for patients
aged 18 years and older for
which the eligible clinician
attests to documenting a list of
current medications using all
immediate resources available on
the date of the encounter.
Centers for
Medicare &
Medicaid Services
§
N/A /
N/A
134
CMS2v
14
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Screening for
Depression and Follow-Up
Plan:
Percentage of patients aged 12
years and older screened for
depression on the date of the
encounter or up to 14 days prior
to the date of the encounter using
an age-appropriate standardized
depression screening tool AND if
positive, a follow-up plan is
documented on the date of or up
to two days after the date of the
qualifying encounter.
Centers for
Medicare &
Medicaid Services
*
!
(Patient
Safety)
N/A /
N/A
181
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Elder Maltreatment Screen
and Follow-Up Plan:
Percentage of patients aged 60
years and older with a
documented elder maltreatment
screen using an Elder
Maltreatment Screening tool on
the date of encounter AND a
documented follow-up plan on
the date of the positive screen.
Centers for
Medicare &
Medicaid Services

B.7. Clinical Social Work
PREVIOUSLY FINALIZED MEASURES IN THE CLINICAL SOCIAL WORK SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
§
N/A /
N/A
226
CMS13
8v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Tobacco Use:
Screening and Cessation
Intervention:
Percentage of patients aged 12
years and older who were
screened for tobacco use one or
more times during the
measurement period AND who
received tobacco cessation
intervention during the
measurement period or in the six
months prior to the measurement
period if identified as a tobacco
user.
National
Committee for
Quality Assurance
*
N/A /
2872e
281
CMS14
9v13
eCQM
Specifications
Process
Dementia: Cognitive
Assessment:
Percentage of patients, regardless
of age, with a diagnosis of
dementia for whom an
assessment of cognition is
performed and the results
reviewed at least once within a
12-month period.
American
Academy of
Neurology
*
N/A /
N/A
282
N/A
MIPS CQM
Specifications
Process
Dementia: Functional Status
Assessment:
Percentage of patients with
dementia for whom an
assessment of functional status
was performed at least once in
the last 12 months.
American
Academy of
Neurology/
American
Psychiatric
Association
*
!
(Patient
Safety)
N/A /
N/A
286
N/A
MIPS CQM
Specifications
Process
Dementia: Safety Concern
Screening and Follow-Up for
Patients with Dementia:
Percentage of patients with
dementia or their caregiver(s) for
whom there was a documented
safety concerns screening in two
domains of risk: 1)
dangerousness to self or others
and 2) environmental risks; and
if safety concerns screening was
positive in the last 12 months,
there was documentation of
mitigation recommendations,
including but not limited to
referral to other resources.
American
Psychiatric
Association/
American
Academy of
Neurology
*
!
(Care
Coordination
)
N/A /
N/A
288
N/A
MIPS CQM
Specifications
Process
Dementia: Education and
Support of Caregivers for
Patients with Dementia:
Percentage of patients with
dementia whose caregiver(s)
were provided with education on
dementia disease management
and health behavior changes
AND were referred to additional
resources for support in the last
12 months.
American
Academy of
Neurology /
American
Psychiatric
Association

B.7. Clinical Social Work
PREVIOUSLY FINALIZED MEASURES IN THE CLINICAL SOCIAL WORK SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Opioid)
N/A /
N/A
305
CMS13
7v13
eCQM
Specifications
Process
Initiation and Engagement of
Substance Use Disorder
Treatment:
Percentage of patients 13 years
of age and older with a new
substance use disorder (SUD)
episode who received the
following (Two rates are
reported):
a. Percentage of patients who
initiated treatment, including
either an intervention or
medication for the treatment of
SUD, within 14 days of the new
SUD episode.
b. Percentage of patients who
engaged in ongoing treatment,
including two additional
interventions or medication
treatment events for SUD, or one
long-acting medication event for
the treatment of SUD, within 34
days of the initiation.
National
Committee for
Quality Assurance
§
!
(Outcome)
0710 /
0710e
370
CMS15
9v13
eCQM
Specifications,
MIPS CQM
Specifications
Outcome
Depression Remission at
Twelve Months:
The percentage of adolescent
patients 12 to 17 years of age and
adult patients 18 years of age or
older with major depression or
dysthymia who reached
remission 12 months (+/- 60
days) after an index event date.
Minnesota
Community
Measurement
!
(Patient
Safety)
N/A /
N/A
382
CMS17
7v13
eCQM
Specifications
Process
Child and Adolescent Major
Depressive Disorder (MDD):
Suicide Risk Assessment:
Percentage of patient visits for
those patients aged 6 through 16
years at the start of the
measurement period with a
diagnosis of major depressive
disorder (MDD) with an
assessment for suicide risk.
Mathematica
*
§
!
(Outcome)
1879 /
N/A
383
N/A
MIPS CQM
Specifications
Intermediate
Outcome
Adherence to Antipsychotic
Medications for Individuals
with Schizophrenia:
Percentage of individuals at least
18 years of age as of the
beginning of the performance
period with schizophrenia or
schizoaffective disorder who had
at least two prescriptions filled
for any antipsychotic medication
and who had a Proportion of
Days Covered (PDC) of at least
0.8 for antipsychotic medications
during the performance period.
Centers for
Medicare &
Medicaid Services
§
2152 /
N/A
431
N/A
MIPS CQM
Specifications
Process
Preventive Care and Screening:
Unhealthy Alcohol Use:
Screening & Brief Counseling:
Percentage of patients aged 18
years and older who were
screened for unhealthy alcohol
use using a systematic screening
method at least once within the
last 12 months AND who
received brief counseling if
identified as an unhealthy alcohol
user.
National
Committee for
Quality Assurance

B.7. Clinical Social Work
PREVIOUSLY FINALIZED MEASURES IN THE CLINICAL SOCIAL WORK SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers of
Health:
Percent of patients 18 years and
older screened for food insecurity,
housing instability, transportation
needs, utility difficulties, and
interpersonal safety.
Centers for
Medicare &
Medicaid Services
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years or
older who screen positive for one
or more of the following health
related social needs (HRSNs):
food insecurity, housing
instability, transportation needs,
utility help needs, or
interpersonal safety; and had
contact with a Community
Service Provider (CSP) for at
least 1 of their HRSNs within 60
days after screening.
OCHIN
!
(Outcome)
N/A /
N/A
502
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Improvement or Maintenance
of Functioning for Individuals
with a Mental and/or
Substance Use Disorder:
The percentage of patients aged
18 and older with a mental and/or
substance use disorder who
demonstrated improvement or
maintenance of functioning
based on results from the 12-item
World Health Organization
Disability Assessment Schedule
(WHODAS 2.0) or Sheehan
Disability Scale (SDS) 30 to 180
days after an index assessment.
American
Psychiatric
Association
!
(Safety)
N/A /
N/A
504
N/A
MIPS CQM
Specifications
Process
Initiation, Review, And/Or
Update To Suicide Safety Plan
For Individuals With Suicidal
Thoughts, Behavior, Or
Suicide Risk: Percentage of
adult aged 18 years and older
with suicidal ideation or behavior
symptoms (based on results of a
standardized assessment tool or
screening tool) or increased
suicide risk (based on the
clinician’s evaluation or
clinician-rating tool) for whom a
suicide safety plan is initiated,
reviewed, and/or updated in
collaboration between the patient
and their clinician.
American
Psychiatric
Association
*
!
(Outcome)
N/A /
N/A
505
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Reduction in Suicidal Ideation
or Behavior Symptoms: The
percentage of patients aged 18
and older with a mental and/or
substance use disorder AND
suicidal thoughts, behaviors or
risk symptoms who demonstrated
a reduction in suicidal ideation
and/or behavior symptoms based
on results from the Columbia-
Suicide Severity Rating Scale (C-
SSRS) ‘Screen Version’ or
‘Since Last Visit’ within 120
days after an index assessment.
American
Psychiatric
Association

B.7. Clinical Social Work
MEASURES PROPOSED FOR ADDITION TO THE CLINICAL SOCIAL WORK SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
*
!
(Outcome
)
2483 /
N/A
503
N/A
MIPS
CQM
Specificati
ons
Patient-
Reported
Outcome-
Based
Performanc
e Measure
Gains in Patient
Activation
Measure (PAM
®) Scores at 12
Months:
The Patient
Activation
Measure® (PAM
®) is a 10- or 13-
item
questionnaire
that assesses an
individual's
knowledge, skills
and confidence
for managing
their health and
health care. The
measure assesses
individuals on a
0-100 scale that
converts to one
of four levels of
activation, from
low (1) to high
(4). The PAM®
performance
measure
(PAM®-PM) is
the change in
score on the
PAM® from
baseline to
follow-up
measurement.
Insignia
Health,
LLC, a
wholly
owned
subsidiary of
Phreesia
We are proposing to include
this measure in the Clinical
Social Work specialty set as it
would be clinically relevant to
this clinician type. The addition
of this measure to this specialty
set would be feasible given its
use through the continuum of
care and across different
clinical settings. This measure
addresses chronic conditions
and outcomes, both of which
are high priority areas for
measure consideration for
MIPS. It is utilized in research
within the U.S. and
internationally and has also
been shown to be valid and
reliable in different clinical
settings and under different
payment models.
79
The
measure being added to this
specialty set would be
contingent on the inclusion of
applicable coding by the time
of the CY 2025 PFS final rule.
79
Phreesia. (2024). Patient Activation Measure (PAM). https://www.phreesia.com/patient-activation-
measure/?utm_source=google&utm_%20medium=paid_%20search&utm_%20destinationmedium=mql_form&ut
m_campaign=payer_care_management_paid_search&utm_vendor=phreesia&utm_audience1=payer&utm_conte
nt=648172611574&utm_destinationco.

B.8. Dentistry
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Dentistry specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a measure
reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. This specialty set has no proposed changes.
B.8. Dentistry
PREVIOUSLY FINALIZED MEASURES IN THE DENTISTRY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Outcome)
N/A /
N/A
378
CMS
75v13
eCQM
Specifications
Outcome
Children Who Have Dental
Decay or Cavities:
Percentage of children, 1 - 20 years
of age at the start of the
measurement period, who have had
tooth decay or cavities during the
measurement period as determined
by a dentist.
Centers for Medicare
& Medicaid Services
N/A /
N/A
379
CMS
74v14
eCQM
Specifications
Process
Primary Caries Prevention
Intervention as Offered by
Dentists:
Percentage of children, 1 – 20 years
of age, who received two fluoride
varnish applications during the
measurement period as determined
by a dentist.
Centers for Medicare
& Medicaid Services

B.9. Dermatology
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Dermatology specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a measure
reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Dermatology
specialty set.
B.9. Dermatology
PREVIOUSLY FINALIZED MEASURES IN THE DERMATOLOGY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS68
v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for patients
aged 18 years and older for which
the eligible clinician attests to
documenting a list of current
medications using all immediate
resources available on the date of
the encounter.
Centers for
Medicare &
Medicaid Services
*
N/A/
N/A
176
N/A
MIPS CQM
Specifications
Process
Tuberculosis Screening Prior to
First Course of Biologic and/or
Immune Response Modifier
Therapy:
If a patient has been newly
prescribed a biologic and/or
immune response modifier that
includes a warning for potential
reactivation of a latent infection,
then the medical record should
indicate TB testing in the preceding
12-month period.
American College
of Rheumatology
§
N/A /
N/A
226
CMS13
8v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and Screening:
Tobacco Use: Screening and
Cessation Intervention:
Percentage of patients aged 12
years and older who were screened
for tobacco use one or more times
during the measurement period
AND who received tobacco
cessation intervention during the
measurement period or in the 6
months prior to the measurement
period if identified as a tobacco
user.
National
Committee for
Quality Assurance
*
N/A /
N/A
317
CMS22
v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and Screening:
Screening for High Blood
Pressure and Follow-Up
Documented:
Percentage of patient visits for
patients aged 18 years and older
seen during the measurement
period who were screened for high
blood pressure AND a
recommended follow-up plan is
documented, as indicated, if blood
pressure is elevated or
hypertensive.
Centers for
Medicare &
Medicaid Services
*
!
(Care
Coordinatio
n)
N/A /
N/A
374
CMS50
v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Closing the Referral Loop:
Receipt of Specialist Report:
Percentage of patients with
referrals, regardless of age, for
which the referring clinician
receives a report from the clinician
to whom the patient was referred.
Centers for
Medicare &
Medicaid Services

B.9. Dermatology
PREVIOUSLY FINALIZED MEASURES IN THE DERMATOLOGY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Outcome)
N/A /
N/A
410
N/A
MIPS CQM
Specifications
Outcome
Psoriasis: Clinical Response to
Systemic Medications:
Percentage of psoriasis vulgaris
patients receiving systemic
medication who meet minimal
physician-or patient- reported
disease activity levels. It is implied
that establishment and maintenance
of an established minimum level of
disease control as measured by
physician-and/or patient-reported
outcomes will increase patient
satisfaction with and adherence to
treatment.
American
Academy of
Dermatology
!
(Care
Coordinatio
n)
N/A /
N/A
440
N/A
MIPS CQM
Specifications
Process
Skin Cancer: Biopsy Reporting
Time – Pathologist to Clinician:
Percentage of biopsies with a
diagnosis of cutaneous basal cell
carcinoma (BCC) and squamous
cell carcinoma (SCC), or melanoma
(including in situ disease) in which
the pathologist communicates
results to the clinician within 7 days
from the time when the tissue
specimen was received by the
pathologist.
American
Academy of
Dermatology
*
!
(Outcome)
N/A /
N/A
485
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome
-Based
Performa
nce
Measure
Psoriasis – Improvement in
Patient-Reported Itch Severity:
The percentage of patients, aged 8
years and older, with a diagnosis of
psoriasis where at an initial (index)
visit have a patient reported itch
severity assessment performed,
score greater than or equal to four,
and who achieve a score reduction
of three or more points at a follow
up visit.
American
Academy of
Dermatology
*
!
(Outcome)
N/A /
N/A
486
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome
-Based
Performa
nce
Measure
Dermatitis – Improvement in
Patient-Reported Itch Severity:
The percentage of patients, aged 8
years and older, with a diagnosis of
dermatitis where at an initial
(index) visit have a patient reported
itch severity assessment performed,
score greater than or equal to 4, and
who achieve a score reduction of 3
or more points at a follow up visit.
American
Academy of
Dermatology
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers of
Health:
Percent of patients 18 years and
older screened for food insecurity,
housing instability, transportation
needs, utility difficulties, and
interpersonal safety.
Centers for
Medicare &
Medicaid Services
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years or older
who screen positive for one or more
of the following health related
social needs (HRSNs): food
insecurity, housing instability,
transportation needs, utility help
needs, or interpersonal safety; and
had contact with a Community
Service Provider (CSP) for at least
1 of their HRSNs within 60 days
after screening.
OCHIN

B.9. Dermatology
PREVIOUSLY FINALIZED MEASURES IN THE DERMATOLOGY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Outcome)
2483 /
N/A
503
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome
-Based
Performa
nce
Measure
Gains in Patient Activation
Measure (PAM®) Scores at 12
Months:
The Patient Activation Measure®
(PAM®) is a 10 – or 13 – item
questionnaire that assesses an
individual´s knowledge, skills and
confidence for managing their
health and health care. The measure
assesses individuals on a 0-100
scale that converts to one of four
levels of activation, from low (1) to
high (4). The PAM® performance
measure (PAM®-PM) is the change
in score on the PAM® from
baseline to follow-up measurement.
Insignia Health,
LLC, a wholly
owned subsidiary
of Phreesia

B.9. Dermatology
MEASURES PROPOSED FOR ADDITION TO THE DERMATOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quali
ty #
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
!
(Care
Coordinatio
n)
N/A /
N/A
TBD
N/A
MIPS
CQM
Specificati
ons
Process
Melanoma:
Tracking and
Evaluation of
Recurrence:
Percentage of
patients who had
an excisional
surgery for
melanoma or
melanoma in situ
with initial
American Joint
Committee on
Cancer (AJCC)
staging of 0, I, or
II in the past 5
years in which
the operating
provider
examines and/or
diagnoses the
patient for
recurrence of
melanoma.
American
Academy
of
Dermatol
ogy
We are proposing to include this
measure in the Dermatology
specialty set as it would be
clinically relevant to this clinician
type. Clinicians within this
specialty care for patients
diagnosed with melanoma and are
most likely to be the clinician to
evaluate the frequency of
melanoma recurrence following
excisional procedures for this
patient population.
80
This measure
addresses the CMS high priority
outcome for care coordination, as
a lack of communication has been
recognized between the excising
clinician and clinician continuing
care. This measure would allow
for the development of a system in
which melanomas can be
accurately tracked to increase the
understanding of the effectiveness
of care. The incorporation of this
measure in this specialty set
would help promote
communications between the
dermatologist treating the
melanoma and the clinicians
continuing care. Melanoma
recurrence is an outcome that
needs precise evaluation.
81
The
measure being added to this
specialty set would be contingent
on the inclusion of applicable
coding by the time of the CY
2025 PFS final rule. See Table
A.6 of this Appendix for rationale,
including clinical evidence
supporting the inclusion of this
measure in MIPS.
80
Rebecca, V. W., Sondak, V. K., & Smalley, K. S. (2012). A Brief History of Melanoma: From Mummies to
Mutations. Melanoma Research, 22(2), 114–122. https://doi.org/10.1097/CMR.0b013e328351fa4d.
81
Freeman, M., & Laks, S. (2019). Surveillance Imaging for Metastasis in High-Risk Melanoma: Importance in
Individualized Patient Care and Survivorship. Melanoma Management, 6(1), MMT12.
https://doi.org/10.2217/mmt-2019-0003.

B.9. Dermatology
PREVIOUSLY FINALIZED MEASURES PROPOSED FOR REMOVAL FROM THE DERMATOLOGY SPECIALTY SET
Note: In this proposed rule, we are proposing the removal of the following measure(s) below from this specific specialty measure set based
upon review of updates made to existing quality measure specifications, the proposed addition of new measures for inclusion in MIPS, and the
feedback provided by specialty societies.
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Removal
N/A /
N/A
137
N/A
MIPS CQM
Specifications
Structure
Melanoma: Continuity of
Care – Recall System:
Percentage of patients,
regardless of age, with a
current diagnosis of
melanoma or a history of
melanoma whose
information was entered, at
least once within a 12-
month period, into a recall
system that includes:
• A target date for the next
complete physical skin
exam, AND
• A process to follow up
with patients who either did
not make an appointment
within the specified
timeframe or who missed a
scheduled appointment.
American
Academy of
Dermatology
This measure is being
proposed for removal
beginning with the CY
2025 performance
period/2027 MIPS
payment year. See Table
Group C for rationale.

B.10. Diagnostic Radiology
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Diagnostic Radiology specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a
measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. As indicated in the Table Group B introduction, measure Q494 has been added to
the previously finalized measure set below and measure Q436 has been removed under this proposed rule as previously finalized
through the CY 2024 PFS final rule (88 FR 79556 through 79560 and 88 FR 79896). We request comment on the measures
available in the proposed Diagnostic Radiology specialty set that have proposed substantive changes in Table Group D of this
Appendix as indicated by an * in the Indicator column.
B.10. Diagnostic Radiology
PREVIOUSLY FINALIZED MEASURES IN THE DIAGNOSTIC RADIOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Patient
Safety)
N/A /
N/A
145
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Radiology: Exposure Dose
Indices Reported for
Procedures Using Fluoroscopy:
Final reports for procedures using
fluoroscopy that document
radiation exposure indices.
American College of
Radiology
*
!
(Appropriate
Use)
N/A /
N/A
360
N/A
MIPS CQM
Specifications
Process
Optimizing Patient Exposure to
Ionizing Radiation: Count of
Potential High Dose Radiation
Imaging Studies: Computed
Tomography (CT) and Cardiac
Nuclear Medicine Studies:
Percentage of computed
tomography (CT) and cardiac
nuclear medicine (myocardial
perfusion studies) imaging
reports for all patients, regardless
of age, that document a count of
known previous CT (any type of
CT) and cardiac nuclear medicine
(myocardial perfusion) studies
that the patient has received in
the 12-month period prior to the
current study.
American College of
Radiology
!
(Appropriate
Use)
N/A /
N/A
364
N/A
MIPS CQM
Specifications
Process
Optimizing Patient Exposure to
Ionizing Radiation:
Appropriateness: Follow-up
CT Imaging for Incidentally
Detected Pulmonary Nodules
According to Recommended
Guidelines:
Percentage of final reports for CT
imaging studies with a finding of
an incidental pulmonary nodule
for patients aged 35 years and
older that contain an impression
or conclusion that includes a
recommended interval and
modality for follow-up (e.g., type
of imaging or biopsy) or for no
follow-up, and source of
recommendations (e.g.,
guidelines such as Fleischner
Society, American Lung
Association, American College
of Chest Physicians).
American College of
Radiology

B.10. Diagnostic Radiology
PREVIOUSLY FINALIZED MEASURES IN THE DIAGNOSTIC RADIOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Appropriate
Use)
N/A /
N/A
405
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Appropriate Follow-up
Imaging for Incidental
Abdominal Lesions:
Percentage of final reports for
imaging studies for patients aged
18 years and older with one or
more of the following noted
incidentally with a specific
recommendation for no
follow‐up imaging recommended
based on radiological findings:
• Cystic renal lesion that is
simple appearing* (Bosniak I or
II)
• Adrenal lesion less than or
equal to 1.0 cm
• Adrenal lesion greater than 1.0
cm but less than or equal to 4.0
cm classified as likely benign or
diagnostic benign by unenhanced
CT or washout protocol CT, or
MRI with in- and opposed-phase
sequences or other equivalent
institutional imaging protocols
American College of
Radiology
!
(Appropriate
Use)
N/A /
N/A
406
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Appropriate Follow-Up
Imaging for Incidental Thyroid
Nodules in Patients:
Percentage of final reports for
computed tomography (CT), CT
angiography (CTA) or magnetic
resonance imaging (MRI) or
magnetic resonance angiogram
(MRA) studies of the chest or
neck for patients aged 18 years
and older with no known thyroid
disease with a thyroid nodule <
1.0 cm noted incidentally with
follow-up imaging
recommended.
American College of
Radiology

B.10. Diagnostic Radiology
PREVIOUSLY FINALIZED MEASURES IN THE DIAGNOSTIC RADIOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Outcome)
3633e,
3662e /
N/A
494
CMS10
56v2
eCQM
Specifications
Intermedi
ate
Outcome
Excessive Radiation Dose or
Inadequate Image Quality for
Diagnostic Computed
Tomography (CT) in Adults
(Clinician Level):
This measure provides a
standardized method for
monitoring the performance of
diagnostic CT to discourage
unnecessarily high radiation
doses, a risk factor for cancer,
while preserving image quality. It
is expressed as a percentage of
patients with CT exams that are
out-of-range based on having
either excessive radiation dose or
inadequate image quality relative
to evidence-based thresholds
based on the clinical indication
for the exam. All diagnostic CT
exams of specified anatomic sites
performed in inpatient, outpatient
and ambulatory care settings are
eligible. This measure is not
telehealth eligible. This eCQM
requires the use of additional
software to access primary data
elements stored within radiology
electronic health records and
translate them into data elements
that can be ingested by this
eCQM. Additional details are
included in the Guidance field.
Alara Imaging, Inc.
in collaboration with
the University of
California, San
Francisco (UCSF)

B.11. Emergency Medicine
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Emergency Medicine specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a
measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Emergency
Medicine specialty set.
B.11. Emergency Medicine
PREVIOUSLY FINALIZED MEASURES IN THE EMERGENCY MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure
Steward
§
!
(Appropriate
Use)
0069/
N/A
065
CMS15
4v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Appropriate Treatment for
Upper Respiratory Infection
(URI):
Percentage of episodes for patients
3 months of age and older with a
diagnosis of upper respiratory
infection (URI) that did not result in
an antibiotic order.
National
Committee for
Quality Assurance
§
!
(Appropriate
Use)
N/A /
N/A
066
CMS14
6v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Appropriate Testing for
Pharyngitis:
The percentage of episodes for
patients 3 years and older with a
diagnosis of pharyngitis that
resulted in an antibiotic order on or
within 3 days after the episode date
and a group A Streptococcus (Strep)
test in the 7-day period from three
days prior to the episode date
through three days after the episode
date.
National
Committee for
Quality Assurance
§
!
(Appropriate
Use)
0058 /
N/A
116
N/A
MIPS CQM
Specifications
Process
Avoidance of Antibiotic
Treatment for Acute
Bronchitis/Bronchiolitis:
The percentage of episodes for
patients ages 3 months and older
with a diagnosis of acute
bronchitis/bronchiolitis that did not
result in an antibiotic dispensing
event.
National
Committee for
Quality Assurance
§
N/A /
N/A
187
N/A
MIPS CQM
Specifications
Process
Stroke and Stroke Rehabilitation:
Thrombolytic Therapy:
Percentage of patients aged 18 years
and older with a diagnosis of acute
ischemic stroke who arrive at the
hospital within 3.5 hours of time
last known well and for whom IV
thrombolytic therapy was initiated
within 4.5 hours of time last known
well.
American Heart
Association
*
N/A /
N/A
317
CMS22
v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and Screening:
Screening for High Blood
Pressure and Follow-Up
Documented:
Percentage of patient visits for
patients aged 18 years and older
seen during the measurement period
who were screened for high blood
pressure AND a recommended
follow-up plan is documented, as
indicated, if blood pressure is
elevated or hypertensive.
Centers for
Medicare &
Medicaid Services
*
!
(Appropriate
Use)
N/A /
N/A
331
N/A
MIPS CQM
Specifications
Process
Adult Sinusitis: Antibiotic
Prescribed for Acute Viral
Sinusitis (Overuse):
Percentage of patients, aged 18
years and older, with a diagnosis of
acute viral sinusitis who were
prescribed an antibiotic within 10
days after onset of symptoms.
American
Academy of
Otolaryngology –
Head and Neck
Surgery
Foundation

B.11. Emergency Medicine
PREVIOUSLY FINALIZED MEASURES IN THE EMERGENCY MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure
Steward
!
(Appropriate
Use)
N/A /
N/A
332
N/A
MIPS CQM
Specifications
Process
Adult Sinusitis: Appropriate
Choice of Antibiotic: Amoxicillin
With or Without Clavulanate
Prescribed for Patients with
Acute Bacterial Sinusitis
(Appropriate Use):
Percentage of patients aged 18 years
and older with a diagnosis of acute
bacterial sinusitis that were
prescribed amoxicillin, with or
without clavulanate, as a first line
antibiotic at the time of diagnosis.
American
Academy of
Otolaryngology –
Head and Neck
Surgery
Foundation
!
(Efficiency)
N/A /
N/A
415
N/A
MIPS CQM
Specifications
Efficiency
Emergency Medicine: Emergency
Department Utilization of CT for
Minor Blunt Head Trauma for
Patients Aged 18 Years and
Older:
Percentage of emergency
department visits for patients aged
18 years and older who presented
with a minor blunt head trauma who
had a head CT for trauma ordered
by an emergency care provider who
have an indication for a head CT.
American College
of Emergency
Physicians
!
(Efficiency)
N/A /
N/A
416
N/A
MIPS CQM
Specifications
Efficiency
Emergency Medicine: Emergency
Department Utilization of CT for
Minor Blunt Head Trauma for
Patients Aged 2 through 17
Years:
Percentage of emergency
department visits for patients aged 2
through 17 years who presented
with a minor blunt head trauma who
had a head CT for trauma ordered
by an emergency care provider who
are classified as low risk according
to the Pediatric Emergency Care
Applied Research Network
(PECARN) prediction rules for
traumatic brain injury.
American College
of Emergency
Physicians
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers of
Health:
Percent of patients 18 years and
older screened for food insecurity,
housing instability, transportation
needs, utility difficulties, and
interpersonal safety.
Centers for
Medicare &
Medicaid Services
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years or older
who screen positive for one or more
of the following health related
social needs (HRSNs): food
insecurity, housing instability,
transportation needs, utility help
needs, or interpersonal safety; and
had contact with a Community
Service Provider (CSP) for at least
1 of their HRSNs within 60 days
after screening.
OCHIN

B.11. Emergency Medicine
MEASURES PROPOSED FOR ADDITION TO THE EMERGENCY MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
*
!
(Patient
Safety)
N/A /
N/A
181
N/A
Medicare
Part B
Claims
Measure
Specificati
ons, MIPS
CQM
Specificati
ons
Process
Elder
Maltreatment
Screen and
Follow-Up Plan:
Percentage of
patients aged 60
years and older
with a
documented
elder
maltreatment
screen using an
Elder
Maltreatment
Screening tool on
the date of
encounter AND a
documented
follow-up plan
on the date of the
positive screen.
Centers for
Medicare &
Medicaid
Services
We are proposing to include
this measure in the Emergency
Medicine specialty set as it
would be clinically relevant to
this clinician type. “Emergency
departments (EDs) are a
potentially important setting
for elder mistreatment
identification because they
provide care for a large number
of older adults who may be
elder mistreatment victims
especially given that the ED is
sometimes the only clinical
setting that the patient may
visit.”
82
The process of
standardized screening using
one or a combination of
validated assessment(s) and/or
instrument(s) should be done to
ensure that signs of abuse or
neglect are not overlooked.
82
Rosen, T., Platts-Mills, T. F., & Fulmer, T. (2020). Screening for Elder Mistreatment in Emergency Departments:
Current Progress and Recommendations for Next Steps. Journal of Elder Abuse & Neglect, 32(3), 295–315.
https://doi.org/10.1080/08946566.2020.1768997.

B.11. Emergency Medicine
PREVIOUSLY FINALIZED MEASURES PROPOSED FOR REMOVAL FROM THE EMERGENCY MEDICINE
SPECIALTY SET
Note: In this proposed rule, we are proposing the removal of the following measure(s) below from this specific specialty measure set based
upon review of updates made to existing quality measure specifications, the proposed addition of new measures for inclusion in MIPS, and the
feedback provided by specialty societies.
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Removal
N/A /
N/A
134
CMS2v
14
Medicare
Part B
Claims
Measure
Specification
s, eCQM
Specification
s, MIPS
CQM
Specification
s
Process
Preventive Care and
Screening: Screening for
Depression and Follow-Up
Plan:
Percentage of patients aged
12 years and older screened
for depression on the date of
the encounter or up to 14
days prior to the date of the
encounter using an age-
appropriate standardized
depression screening tool
AND if positive, a follow-up
plan is documented on the
date of or up to 2 days after
the date of the qualifying
encounter.
Centers for
Medicare &
Medicaid
Services
We are proposing to
remove this measure from
the Emergency Medicine
specialty set beginning
with the CY 2025
performance period/2027
MIPS payment year.
Complete emergency
medicine applicable coding
is not available within this
quality measure.
Therefore, this measure
has minimal eligibility for
this clinician type.
N/A /
N/A
254
N/A
MIPS CQM
Specification
s
Process
Ultrasound Determination
of Pregnancy Location for
Pregnant Patients with
Abdominal Pain:
Percentage of pregnant
female patients aged 14 to
50 who present to the
emergency department (ED)
with a chief complaint of
abdominal pain or vaginal
bleeding who receive a
trans-abdominal or trans-
vaginal ultrasound to
determine pregnancy
location.
American
College of
Emergency
Physicians
This measure is being
proposed for removal
beginning with the CY
2025 performance
period/2027 MIPS
payment year. See Table
Group C for rationale.

B.12. Endocrinology
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Endocrinology specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a
measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Endocrinology
specialty set.
B.12. Endocrinology
PREVIOUSLY FINALIZED MEASURES IN THE ENDOCRINOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
§
!
(Outcome)
0059 /
N/A
001
CMS12
2v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Intermediat
e Outcome
Diabetes: Hemoglobin A1c
(HbA1c) Poor Control
(>9%):
Percentage of patients 18-75
years of age with diabetes
who had hemoglobin A1c >
9.0% during the measurement
period.
National Committee
for Quality Assurance
0046 /
N/A
039
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Screening for Osteoporosis
for Women Aged 65-85 Years
of Age:
Percentage of women aged
65-85 years of age who ever
had a central dual-energy X-
ray absorptiometry (DXA) to
check for osteoporosis.
National Committee
for Quality Assurance
*
§
0055 /
N/A
117
CMS13
1v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Diabetes: Eye Exam:
Percentage of patients 18-75
years of age with diabetes and
an active diagnosis of
retinopathy in any part of the
measurement period who had
a retinal or dilated eye exam
by an eye care professional
during the measurement
period or diabetics with no
diagnosis of retinopathy in
any part of the measurement
period who had a retinal or
dilated eye exam by an eye
care professional during the
measurement period or in the
12 months prior to the
measurement period.
National Committee
for Quality Assurance
§
0066 /
N/A
118
N/A
MIPS CQM
Specifications
Process
Coronary Artery Disease
(CAD): Angiotensin-
Converting Enzyme (ACE)
Inhibitor or Angiotensin
Receptor Blocker (ARB)
Therapy – Diabetes or Left
Ventricular Systolic
Dysfunction (LVEF ≤ 40%):
Percentage of patients aged 18
years and older with a
diagnosis of coronary artery
disease seen within a 12-
month period who also have
diabetes OR a current or prior
Left Ventricular Ejection
Fraction (LVEF) ≤ 40% who
were prescribed ACE inhibitor
or ARB therapy.
American
Heart Association

B.12. Endocrinology
PREVIOUSLY FINALIZED MEASURES IN THE ENDOCRINOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
N/A /
N/A
126
N/A
MIPS CQM
Specifications
Process
Diabetes Mellitus: Diabetic
Foot and Ankle Care,
Peripheral Neuropathy –
Neurological Evaluation:
Percentage of patients aged 18
years and older with a
diagnosis of diabetes mellitus
who had a neurological
examination of their lower
extremities within 12 months.
American Podiatric
Medical Association
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS68
v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for
patients aged 18 years and
older for which the eligible
clinician attests to
documenting a list of current
medications using all
immediate resources available
on the date of the encounter.
Centers for Medicare
& Medicaid Services
§
N/A /
N/A
134
CMS2v
14
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Screening for
Depression and Follow-Up
Plan:
Percentage of patients aged 12
years and older screened for
depression on the date of the
encounter or up to 14 days
prior to the date of the
encounter using an age-
appropriate standardized
depression screening tool
AND if positive, a follow-up
plan is documented on the
date of or up to 2 days after
the date of the qualifying
encounter.
Centers for Medicare
& Medicaid Services
§
N/A /
N/A
226
CMS13
8v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Tobacco Use:
Screening and Cessation
Intervention:
Percentage of patients aged 12
years and older who were
screened for tobacco use one
or more times during the
measurement period AND
who received tobacco
cessation intervention during
the measurement period or in
the 6 months prior to the
measurement period if
identified as a tobacco user.
National Committee
for Quality Assurance
*
§
!
(Outcome)
N/A /
N/A
236
CMS16
5v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Intermediat
e Outcome
Controlling High Blood
Pressure:
Percentage of patients 18-85
years of age who had a
diagnosis of essential
hypertension starting before
and continuing into, or
starting during the first 6
months of the measurement
period, and whose most recent
blood pressure was adequately
controlled (<140/90mmHg)
during the measurement
period.
National Committee
for Quality Assurance

B.12. Endocrinology
PREVIOUSLY FINALIZED MEASURES IN THE ENDOCRINOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Care
Coordination)
N/A /
N/A
374
CMS50
v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Closing the Referral Loop:
Receipt of Specialist Report:
Percentage of patients with
referrals, regardless of age, for
which the referring clinician
receives a report from the
clinician to whom the patient
was referred.
Centers for Medicare
& Medicaid Services
*
0053 /
N/A
418
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Osteoporosis Management
in Women Who Had a
Fracture:
The percentage of women 50–
85 years of age who suffered a
fracture and who had either a
bone mineral density (BMD)
test or prescription for a drug
to treat osteoporosis in the 6
months after the fracture.
National Committee
for Quality Assurance
§
N/A /
N/A
438
CMS34
7v8
eCQM
Specifications,
MIPS CQM
Specifications
Process
Statin Therapy for the
Prevention and Treatment
of Cardiovascular
Disease:
Percentage of the following
patients – all considered at
high risk of cardiovascular
events – who were prescribed
or were on statin therapy
during the performance
period:
•All patients who were
previously diagnosed with or
currently have a diagnosis of
clinical atherosclerotic
cardiovascular disease
(ASCVD), including an
ASCVD procedure; OR
•Patients aged 20 to 75 years
who have ever had a low-
density lipoprotein cholesterol
(LDL-C) level ≥ 190 mg/dL
or were previously diagnosed
with or currently have an
active diagnosis of familial
hypercholesterolemia; OR
•Patients aged 40 to 75 years
with a diagnosis of diabetes;
OR
•Patients aged 40 to 75 with a
10-year ASCVD risk score of
≥ 20 percent.
Centers for Medicare
& Medicaid Services
*
N/A /
N/A
462
CMS64
5v8
eCQM
Specifications
Process
Bone Density Evaluation for
Patients with Prostate
Cancer and Receiving
Androgen Deprivation
Therapy:
Patients determined as having
prostate cancer who are
currently starting or
undergoing androgen
deprivation therapy (ADT),
for an anticipated period of 12
months or greater and who
receive an initial bone density
evaluation. The bone density
evaluation must be prior to the
start of ADT or within 3
months of the start of ADT.
Oregon Urology
Institute

B.12. Endocrinology
PREVIOUSLY FINALIZED MEASURES IN THE ENDOCRINOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers
of Health:
Percent of patients 18 years
and older screened for food
insecurity, housing instability,
transportation needs, utility
difficulties, and interpersonal
safety.
Centers for Medicare
& Medicaid Services
*
N/A /
N/A
488
CMS95
1v3
eCQM
Specifications,
MIPS CQM
Specifications
Process
Kidney Health Evaluation:
Percentage of patients aged
18-75 years with a diagnosis
of diabetes who received a
kidney health evaluation
defined by an Estimated
Glomerular Filtration Rate
(eGFR) AND Urine Albumin-
Creatinine Ratio (uACR)
within the measurement
period.
National Kidney
Foundation
*
3620 /
N/A
493
N/A
MIPS CQM
Specifications
Process
Adult Immunization Status:
Percentage of patients 19
years of age and older who are
up-to-date on recommended
routine vaccines for influenza;
tetanus and diphtheria (Td) or
tetanus, diphtheria and
acellular pertussis (Tdap);
zoster; and pneumococcal.
National Committee
for Quality Assurance
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years or
older who screen positive for
one or more of the following
health related social needs
(HRSNs): food insecurity,
housing instability,
transportation needs, utility
help needs, or interpersonal
safety; and had contact with a
Community Service Provider
(CSP) for at least 1 of their
HRSNs within 60 days after
screening.
OCHIN
*
!
(Outcome)
2483 /
N/A
503
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performan
ce
Measure
Gains in Patient Activation
Measure (PAM®) Scores at
12 Months:
The Patient Activation
Measure® (PAM®) is a 10 –
or 13 – item questionnaire that
assesses an individual´s
knowledge, skills and
confidence for managing their
health and health care. The
measure assesses individuals
on a 0-100 scale that converts
to one of four levels of
activation, from low (1) to
high (4). The PAM®
performance measure
(PAM®-PM) is the change in
score on the PAM® from
baseline to follow-up
measurement.
Insignia Health, LLC,
a wholly owned
subsidiary of Phreesia

B.12. Endocrinology
MEASURES PROPOSED FOR ADDITION TO THE ENDOCRINOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
N/A /
N/A
TBD
N/A
MIPS
CQM
Specificati
ons
Process
Adult COVID-
19 Vaccination
Status:
Percentage of
patients aged 18
years and older
seen for a visit
during the
performance
period that are
up-to-date on
their COVID-19
vaccinations as
defined by CDC
recommendation
s on current
vaccination.
Centers
for
Medicare
&
Medicaid
Services
We are proposing to include this
measure in the Endocrinology
specialty set as it would be
clinically relevant to this clinician
type. Widespread vaccination
against SARS-CoV-2, the virus
that causes COVID-19, is
critically important to stemming
the morbidity and mortality
caused by this disease.
83
Clinicians are uniquely positioned
to encourage uptake of COVID-
19 vaccination, and clinicians are
still a major driving force in
promoting patient vaccination.
The addition of this quality
measure in this specialty set
would help strengthen compliance
with recommended COVID-19
vaccination, leading to
improvement in the quality of
patient care and prevention of
disease for the general population.
This quality measure aligns with
clinical guidelines and the
evidence-based recommendations
of the ACIP, where there is
general agreement about the
safety and efficacy of the
COVID-19 vaccine, preventing
costly and potentially harmful
hospitalizations.
84
Broadening
vaccination status awareness to
this clinician type is valuable as it
can help drive an increase in the
adult vaccination rates. The
COVID-19 vaccination included
within this measure would reduce
the prevalence of severe diseases
that may be associated with
hospitalization and decrease
overall health care costs. The
measure being added to this
specialty set would be contingent
on the inclusion of applicable
coding by the time of the CY
2025 PFS final rule. In the event
appropriate coding is not included
in the final specification, this
measure would not be finalized
for inclusion within this specialty
measure set. See Table A.5 of this
Appendix for rationale, including
clinical evidence supporting the
inclusion of this measure in
MIPS.
83
See footnote Ikeokwu et al., 2023 in Table B.1 of this Appendix.
84
See footnotes Fitzpatrick et al., 2022; Polack et al., 2020; and Graña et al., 2022 in Table A.5 of this Appendix.

B.13. Family Medicine
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Family Medicine specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a
measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Family Medicine
specialty set.
B.13. Family Medicine
PREVIOUSLY FINALIZED MEASURES IN THE FAMILY MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
*
§
!
(Outcome)
0059 /
N/A
001
CMS1
22v13
Medicare Part B
Claims Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Intermediate
Outcome
Diabetes: Hemoglobin A1c
(HbA1c) Poor Control
(>9%):
Percentage of patients 18-75
years of age with diabetes who
had hemoglobin A1c > 9.0%
during the measurement
period.
National Committee
for Quality
Assurance
§
0081 /
0081e
005
CMS1
35v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Heart Failure (HF):
Angiotensin-Converting
Enzyme (ACE) Inhibitor or
Angiotensin Receptor
Blocker (ARB) or
Angiotensin Receptor-
Neprilysin Inhibitor (ARNI)
Therapy for Left Ventricular
Systolic Dysfunction
(LVSD):
Percentage of patients aged 18
years and older with a
diagnosis of heart failure (HF)
with a current or prior left
ventricular ejection fraction
(LVEF) ≤ 40% who were
prescribed ACE inhibitor or
ARB or ARNI therapy either
within a 12-month period
when seen in the outpatient
setting OR at each hospital
discharge.
American Heart
Association
§
0067 /
N/A
006
N/A
MIPS CQM
Specifications
Process
Coronary Artery Disease
(CAD): Antiplatelet
Therapy:
Percentage of patients aged 18
years and older with a
diagnosis of coronary artery
disease (CAD) seen within a
12-month period who were
prescribed aspirin or
clopidogrel.
American Heart
Association
§
0070 /
0070e
007
CMS1
45v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Coronary Artery Disease
(CAD): Beta-Blocker
Therapy – Prior Myocardial
Infarction (MI) or Left
Ventricular Systolic
Dysfunction (LVEF ≤ 40%):
Percentage of patients aged 18
years and older with a
diagnosis of coronary artery
disease seen within a 12-
month period who also have a
prior MI or a current or prior
LVEF ≤ 40% who were
prescribed beta-blocker
therapy.
American Heart
Association

B.13. Family Medicine
PREVIOUSLY FINALIZED MEASURES IN THE FAMILY MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
§
0083 /
0083e
008
CMS1
44v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Heart Failure (HF): Beta-
Blocker Therapy for Left
Ventricular Systolic
Dysfunction (LVSD):
Percentage of patients aged 18
years and older with a
diagnosis of heart failure (HF)
with a current or prior left
ventricular ejection fraction
(LVEF) ≤ 40% who were
prescribed beta-blocker
therapy either within a 12-
month period when seen in the
outpatient setting OR at each
hospital discharge.
American Heart
Association
*
N/A /
N/A
009
CMS1
28v13
eCQM
Specifications
Process
Antidepressant Medication
Management:
Percentage of patients 18 years
of age and older who were
treated with antidepressant
medication, had a diagnosis of
major depression, and who
remained on an antidepressant
medication treatment. Two
rates are reported.
A. Percentage of patients who
remained on an antidepressant
medication for at least 84 days
(12 weeks).
b. Percentage of patients who
remained on an antidepressant
medication for at least 180
days (6 months).
National
Committee for
Quality Assurance
!
(Care
Coordination)
N/A /
N/A
024
N/A
Medicare Part B
Claims Measure
Specifications,
MIPS CQM
Specifications
Process
Communication with the
Physician or Other Clinician
Managing On-Going Care
Post-Fracture for Men and
Women Aged 50 Years and
Older:
Percentage of patients aged 50
years and older treated for a
fracture with documentation of
communication, between the
physician treating the fracture
and the physician or other
clinician managing the
patient’s on-going care, that a
fracture occurred and that the
patient was or should be
considered for osteoporosis
treatment or testing. This
measure is submitted by the
physician who treats the
fracture and who therefore is
held accountable for the
communication.
National
Committee for
Quality Assurance
0046 /
N/A
039
N/A
Medicare Part B
Claims Measure
Specifications,
MIPS CQM
Specifications
Process
Screening for Osteoporosis for
Women Aged 65-85 Years of
Age:
Percentage of women aged 65-
85 years of age who ever had a
central dual-energy X-ray
absorptiometry (DXA) to
check for osteoporosis.
National
Committee for
Quality Assurance

B.13. Family Medicine
PREVIOUSLY FINALIZED MEASURES IN THE FAMILY MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Care
Coordination)
0326 /
N/A
047
N/A
Medicare Part B
Claims Measure
Specifications,
MIPS CQM
Specifications
Process
Advance Care Plan:
Percentage of patients aged 65
years and older who have an
advance care plan or surrogate
decision maker documented in
the medical record or
documentation in the medical
record that an advance care plan
was discussed but the patient did
not wish or was not able to name
a surrogate decision maker or
provide an advance care plan.
National
Committee for
Quality Assurance
N/A /
N/A
048
N/A
MIPS CQM
Specifications
Process
Urinary Incontinence:
Assessment of Presence or
Absence of Urinary
Incontinence in Women
Aged 65 Years and Older:
Percentage of female patients
aged 65 years and older who
were assessed for the presence
or absence of urinary
incontinence within 12 months.
National
Committee for
Quality Assurance
!
(Patient
Experience)
N/A /
N/A
050
N/A
MIPS CQM
Specifications
Process
Urinary Incontinence: Plan of
Care for Urinary Incontinence
in Women Aged 65 Years and
Older:
Percentage of female patients
aged 65 years and older with a
diagnosis of urinary
incontinence with a
documented plan of care for
urinary incontinence at least
once within 12 months.
National
Committee for
Quality Assurance
§
!
(Appropriate
Use)
0069 /
N/A
065
CMS1
54v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Appropriate Treatment for
Upper Respiratory Infection
(URI):
Percentage of episodes for
patients 3 months of age and
older with a diagnosis of upper
respiratory infection (URI) that
did not result in an antibiotic
order.
National
Committee for
Quality Assurance
§
!
(Appropriate
Use)
N/A /
N/A
066
CMS1
46v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Appropriate Testing for
Pharyngitis:
The percentage of episodes for
patients 3 years and older with a
diagnosis of pharyngitis that
resulted in an antibiotic order on
or within 3 days after the
episode date and a group A
Streptococcus (Strep) test in the
7-day period from three days
prior to the episode date through
three days after the episode date.
National
Committee for
Quality Assurance
§
!
(Appropriate
Use)
0058 /
N/A
116
N/A
MIPS CQM
Specifications
Process
Avoidance of Antibiotic
Treatment for Acute
Bronchitis/Bronchiolitis:
The percentage of episodes for
patients ages 3 months and
older with a diagnosis of acute
bronchitis/bronchiolitis that
did not result in an antibiotic
dispensing event.
National
Committee for
Quality Assurance

B.13. Family Medicine
PREVIOUSLY FINALIZED MEASURES IN THE FAMILY MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
*
§
0055 /
N/A
117
CMS1
31v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Diabetes: Eye Exam:
Percentage of patients 18-75
years of age with diabetes and
an active diagnosis of
retinopathy in any part of the
measurement period who had a
retinal or dilated eye exam by
an eye care professional during
the measurement period or
diabetics with no diagnosis of
retinopathy in any part of the
measurement period who had a
retinal or dilated eye exam by
an eye care professional during
the measurement period or in
the 12 months prior to the
measurement period.
National
Committee for
Quality Assurance
N/A /
N/A
126
N/A
MIPS CQM
Specifications
Process
Diabetes Mellitus: Diabetic
Foot and Ankle Care,
Peripheral Neuropathy –
Neurological Evaluation:
Percentage of patients aged 18
years and older with a
diagnosis of diabetes mellitus
who had a neurological
examination of their lower
extremities within 12 months.
American
Podiatric Medical
Association
*
§
!
(Patient Safety)
N/A /
N/A
130
CMS6
8v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for patients
aged 18 years and older for
which the eligible clinician
attests to documenting a list of
current medications using all
immediate resources available
on the date of the encounter.
Centers for
Medicare &
Medicaid Services
§
N/A /
N/A
134
CMS2v1
4
Medicare Part B
Claims Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Screening for
Depression and Follow-Up
Plan:
Percentage of patients aged 12
years and older screened for
depression on the date of the
encounter or up to 14 days
prior to the date of the
encounter using an age-
appropriate standardized
depression screening tool
AND if positive, a follow-up
plan is documented on the date
of or up to 2 days after the date
of the qualifying encounter.
Centers for
Medicare &
Medicaid Services
*
!
(Care
Coordination)
0101 /
N/A
155
N/A
Medicare Part B
Claims Measure
Specifications,
MIPS CQM
Specifications
Process
Falls: Plan of Care:
Percentage of patients aged 65
years and older with a history
of falls who had a plan of care
for falls documented within 12
months.
National
Committee for
Quality Assurance

B.13. Family Medicine
PREVIOUSLY FINALIZED MEASURES IN THE FAMILY MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
*
N/A /
N/A
176
N/A
MIPS CQM
Specifications
Process
Tuberculosis Screening Prior
to First Course of Biologic
and/or Immune Response
Modifier Therapy:
If a patient has been newly
prescribed a biologic and/or
immune response modifier that
includes a warning for
potential reactivation of a
latent infection, then the
medical record should indicate
TB testing in the preceding 12-
month period.
American College
of Rheumatology
*
!
(Patient
Safety)
N/A /
N/A
181
N/A
Medicare Part B
Claims Measure
Specifications,
MIPS CQM
Specifications
Process
Elder Maltreatment Screen
and Follow-Up Plan:
Percentage of patients aged 60
years and older with a
documented elder
maltreatment screen using an
Elder Maltreatment Screening
tool on the date of encounter
AND a documented follow-up
plan on the date of the positive
screen.
Centers for
Medicare &
Medicaid Services
*
§
!
(Care
Coordination)
N/A /
N/A
182
N/A
MIPS CQM
Specifications
Process
Functional Outcome
Assessment:
Percentage of visits for
patients aged 18 years and
older with documentation of a
current functional outcome
assessment using a
standardized functional
outcome assessment tool on
the date of the encounter AND
documentation of a care plan
based on identified functional
outcome deficiencies within 2
days of the date of the
identified deficiencies.
Centers for
Medicare &
Medicaid Services
*
§
!
(Outcome)
N/A /
N/A
236
CMS1
65v13
Medicare Part B
Claims Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Intermediate
Outcome
Controlling High Blood
Pressure:
Percentage of patients 18-85
years of age who had a
diagnosis of essential
hypertension starting before
and continuing into, or starting
during the first 6 months of the
measurement period, and
whose most recent blood
pressure was adequately
controlled (<140/90mmHg)
during the measurement
period.
National
Committee for
Quality Assurance
*
!
(Patient
Safety)
0022 /
N/A
238
CMS1
56v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Use of High-Risk
Medications in Older Adults:
Percentage of patients 65 years
of age and older who were
ordered at least two high-risk
medications from the same
drug class.
National
Committee for
Quality Assurance

B.13. Family Medicine
PREVIOUSLY FINALIZED MEASURES IN THE FAMILY MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Care
Coordination)
0643 /
N/A
243
N/A
MIPS CQM
Specifications
Process
Cardiac Rehabilitation
Patient Referral from an
Outpatient Setting:
Percentage of patients
evaluated in an outpatient
setting who within the
previous 12 months have
experienced an acute
myocardial infarction (MI),
coronary artery bypass graft
(CABG) surgery, a
percutaneous coronary
intervention (PCI), cardiac
valve surgery, or cardiac
transplantation, or who have
chronic stable angina (CSA)
and have not already
participated in an early
outpatient cardiac
rehabilitation/secondary
prevention (CR) program for
the qualifying event/diagnosis
who were referred to a CR
program.
American Heart
Association
!
(Opioid)
N/A /
N/A
305
CMS1
37v13
eCQM
Specifications
Process
Initiation and Engagement of
Substance Use Disorder
Treatment:
Percentage of patients 13 years
of age and older with a new
substance use disorder (SUD)
episode who received the
following (Two rates are
reported):
a. Percentage of patients who
initiated treatment, including
either an intervention or
medication for the treatment of
SUD, within 14 days of the
new SUD episode.
b. Percentage of patients who
engaged in ongoing treatment,
including two additional
interventions or medication
treatment events for SUD, or
one long-acting medication
event for the treatment of
SUD, within 34 days of the
initiation.
National
Committee for
Quality Assurance
§
N/A /
N/A
309
CMS1
24v13
eCQM
Specifications
Process
Cervical Cancer Screening:
Percentage of women 21-64
years of age who were
screened for cervical cancer
using either of the following
criteria:
• Women age 21-64 who had
cervical cytology performed
within the last 3 years
• Women age 30-64 who had
cervical human papillomavirus
(HPV) testing performed
within the last 5 years
National
Committee for
Quality Assurance
!
(Patient
Safety)
0101 /
N/A
318
CMS1
39v13
eCQM
Specifications
Process
Falls: Screening for Future
Fall Risk:
Percentage of patients 65 years
of age and older who were
screened for future fall risk
during the measurement
period.
National
Committee for
Quality Assurance

B.13. Family Medicine
PREVIOUSLY FINALIZED MEASURES IN THE FAMILY MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
§
!
(Patient
Experience)
0005 /
N/A
321
N/A
CMS-approved
Survey Vendor
Patient
Engagement
/Experience
CAHPS for MIPS
Clinician/Group Survey:
The Consumer Assessment of
Healthcare Providers and
Systems (CAHPS) for MIPS
Clinician/Group Survey is
comprised of 10 Summary
Survey Measures (SSMs) and
measures patient experience of
care within a group practice.
The CBE endorsement status
and endorsement id (if
applicable) for each SSM
utilized in this measure are as
follows:
• Getting Timely Care,
Appointments, and
Information; (Not endorsed by
CBE)
• How well Providers
Communicate; (Not endorsed
by CBE)
• Patient’s Rating of Provider;
(CBE endorsed # 0005)
• Access to Specialists; (Not
endorsed by CBE)
• Health Promotion and
Education; (Not endorsed by
CBE)
• Shared Decision-Making;
(Not endorsed by CBE)
• Health Status and Functional
Status; (Not endorsed by CBE)
• Courteous and Helpful Office
Staff; (CBE endorsed # 0005)
• Care Coordination; (Not
endorsed by CBE)
• Stewardship of Patient
Resources. (Not endorsed by
CBE)
Centers for
Medicare &
Medicaid Services
§
N/A /
N/A
326
N/A
MIPS CQM
Specifications
Process
Atrial Fibrillation and Atrial
Flutter: Chronic
Anticoagulation Therapy:
Percentage of patients aged 18
years and older with atrial
fibrillation (AF) or atrial
flutter who were prescribed an
FDA-approved oral
anticoagulant drug for the
prevention of
thromboembolism during the
measurement period.
American Heart
Association
*
!
(Appropriate
Use)
N/A /
N/A
331
N/A
MIPS CQM
Specifications
Process
Adult Sinusitis: Antibiotic
Prescribed for Acute Viral
Sinusitis (Overuse):
Percentage of patients, aged 18
years and older, with a
diagnosis of acute viral
sinusitis who were prescribed
an antibiotic within 10 days
after onset of symptoms.
American
Academy of
Otolaryngology –
Head and Neck
Surgery
Foundation

B.13. Family Medicine
PREVIOUSLY FINALIZED MEASURES IN THE FAMILY MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Appropriate
Use)
N/A /
N/A
332
N/A
MIPS CQM
Specifications
Process
Adult Sinusitis: Appropriate
Choice of Antibiotic:
Amoxicillin With or Without
Clavulanate Prescribed for
Patients with Acute Bacterial
Sinusitis (Appropriate Use):
Percentage of patients aged 18
years and older with a
diagnosis of acute bacterial
sinusitis that were prescribed
amoxicillin, with or without
clavulanate, as a first line
antibiotic at the time of
diagnosis.
American
Academy of
Otolaryngology –
Head and Neck
Surgery
Foundation
§
!
(Outcome)
N/A /
N/A
338
CMS3
14v2
eCQM
Specifications,
MIPS CQM
Specifications
Outcome
HIV Viral Suppression:
Percentage of patients,
regardless of age, diagnosed
with HIV prior to or during the
first 90 days of the
performance period, with an
eligible encounter in the first
240 days of the performance
period, whose last HIV viral
load test result was less than
200 copies/mL during the
performance period.
Health Resources
and Services
Administration
§
!
(Outcome)
0710 /
0710e
370
CMS1
59v13
eCQM
Specifications,
MIPS CQM
Specifications
Outcome
Depression Remission at
Twelve Months:
The percentage of adolescent
patients 12 to 17 years of age
and adult patients 18 years of
age or older with major
depression or dysthymia who
reached remission 12 months
(+/- 60 days) after an index
event date.
Minnesota
Community
Measurement
*
!
(Care
Coordination)
N/A /
N/A
374
CMS5
0v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Closing the Referral Loop:
Receipt of Specialist Report:
Percentage of patients with
referrals, regardless of age, for
which the referring clinician
receives a report from the
clinician to whom the patient
was referred.
Centers for
Medicare &
Medicaid Services
§
!
(Patient
Experience)
N/A /
N/A
377
CMS9
0v14
eCQM
Specifications
Process
Functional Status
Assessments for Heart
Failure:
Percentage of patients 18 years
of age and older with heart
failure who completed initial
and follow-up patient-reported
functional status assessments.
Centers for
Medicare &
Medicaid Services
*
§
!
(Outcome)
1879 /
N/A
383
N/A
MIPS CQM
Specifications
Intermediate
Outcome
Adherence to Antipsychotic
Medications for Individuals
with Schizophrenia:
Percentage of individuals at
least 18 years of age as of the
beginning of the performance
period with schizophrenia or
schizoaffective disorder who
had at least two prescriptions
filled for any antipsychotic
medication and who had a
Proportion of Days Covered
(PDC) of at least 0.8 for
antipsychotic medications
during the performance period.
Centers for
Medicare &
Medicaid Services

B.13. Family Medicine
PREVIOUSLY FINALIZED MEASURES IN THE FAMILY MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
N/A /
N/A
387
N/A
MIPS CQM
Specifications
Process
Annual Hepatitis C Virus
(HCV) Screening for
Patients who are Active
Injection Drug Users:
Percentage of patients,
regardless of age, who are
active injection drug users who
received screening for HCV
infection within the 12-month
reporting period.
American
Gastroenterologic
al Association
§
N/A /
N/A
394
N/A
MIPS CQM
Specifications
Process
Immunizations for
Adolescents:
The percentage of adolescents
13 years of age who had one
dose of meningococcal vaccine
(serogroups A, C, W, Y), one
tetanus, diphtheria toxoids and
acellular pertussis (Tdap)
vaccine, and have completed
the Human Papillomavirus
(HPV) vaccine series by their
13
th
birthday.
National
Committee for
Quality Assurance
!
(Outcome)
N/A /
N/A
398
N/A
MIPS CQM
Specifications
Outcome
Optimal Asthma Control:
Composite measure of the
percentage of pediatric and
adult patients whose asthma is
well-controlled as
demonstrated by one of three
age appropriate patient
reported outcome tools and not
at risk for exacerbation.
Minnesota
Community
Measurement
§
N/A /
N/A
400
N/A
MIPS CQM
Specifications
Process
One-Time Screening for
Hepatitis C Virus (HCV) and
Treatment Initiation:
Percentage of patients age >=
18 years have never been
tested for Hepatitis C Virus
(HCV) infection who receive
an HCV infection test AND
who have treatment initiated
within three months or who are
referred to a clinician who
treats HCV infection within
one month if tested positive for
HCV.
American
Gastroenterologic
al Association
§
N/A /
N/A
401
N/A
MIPS CQM
Specifications
Process
Hepatitis C: Screening for
Hepatocellular Carcinoma
(HCC) in Patients with
Cirrhosis:
Percentage of patients aged 18
years and older with a
diagnosis of chronic Hepatitis
C cirrhosis who underwent
imaging with either
ultrasound, contrast enhanced
CT or MRI for hepatocellular
carcinoma (HCC) at least once
within the 12-month
submission period.
American
Gastroenterologic
al Association
*
0053 /
N/A
418
N/A
Medicare Part B
Claims Measure
Specifications,
MIPS CQM
Specifications
Process
Osteoporosis Management in
Women Who Had a
Fracture:
The percentage of women 50–
85 years of age who suffered a
fracture and who had either a
bone mineral density (BMD)
test or prescription for a drug
to treat osteoporosis in the 6
months after the fracture.
National
Committee for
Quality Assurance

B.13. Family Medicine
PREVIOUSLY FINALIZED MEASURES IN THE FAMILY MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
§
2152 /
N/A
431
N/A
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Unhealthy
Alcohol Use: Screening &
Brief Counseling:
Percentage of patients aged 18
years and older who were
screened for unhealthy alcohol
use using a systematic
screening method at least once
within the last 12 months AND
who received brief counseling
if identified as an unhealthy
alcohol user.
National
Committee for
Quality Assurance
§
N/A /
N/A
438
CMS3
47v8
eCQM
Specifications,
MIPS CQM
Specifications
Process
Statin Therapy for the
Prevention and Treatment of
Cardiovascular Disease:
Percentage of the following
patients – all considered at
high risk of cardiovascular
events – who were prescribed
or were on statin therapy
during the performance period:
•All patients who were
previously diagnosed with or
currently have a diagnosis of
clinical atherosclerotic
cardiovascular disease
(ASCVD), including an
ASCVD procedure; OR
•Patients aged 20 to 75 years
who have ever had a low-
density lipoprotein cholesterol
(LDL-C) level ≥ 190 mg/dL or
were previously diagnosed
with or currently have an
active diagnosis of familial
hypercholesterolemia; OR
•Patients aged 40 to 75 years
with a diagnosis of diabetes;
OR
•Patients aged 40 to 75 with a
10-year ASCVD risk score of
≥ 20 percent.
Centers for
Medicare &
Medicaid Services

B.13. Family Medicine
PREVIOUSLY FINALIZED MEASURES IN THE FAMILY MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
§
!
(Outcome)
N/A /
N/A
441
N/A
MIPS CQM
Specifications
Intermediate
Outcome
Ischemic Vascular Disease
(IVD) All or None Outcome
Measure (Optimal Control):
The IVD All-or-None Measure
is one outcome measure
(optimal control). The measure
contains four goals. All four
goals within a measure must
be reached in order to meet
that measure. The numerator
for the all-or-none measure
should be collected from the
organization’s total IVD
denominator. All-or-None
Outcome Measure (Optimal
Control) – Using the IVD
denominator optimal results
include:
• Most recent blood pressure
(BP) measurement is less than
or equal to 140/90 mm Hg –
AND
• Most recent tobacco status is
Tobacco Free – AND
• Daily Aspirin or Other
Antiplatelet Unless
Contraindicated – AND
• Statin Use Unless
Contraindicated.
Wisconsin
Collaborative for
Healthcare Quality
§
!
(Appropriate
Use)
N/A /
N/A
443
N/A
MIPS CQM
Specifications
Process
Non-Recommended Cervical
Cancer Screening in
Adolescent Females:
The percentage of adolescent
females 16–20 years of age
who were screened
unnecessarily for cervical
cancer.
Centers for
Medicare &
Medicaid Services
*
!
(Appropriate
Use)
0657 /
N/A
464
N/A
MIPS CQM
Specifications
Process
Otitis Media with Effusion:
Systemic Antimicrobials –
Avoidance of Inappropriate
Use:
Percentage of patients aged 2
months through 12 years with
a diagnosis of OME who were
not prescribed systemic
antimicrobials.
American
Academy of
Otolaryngology –
Head and Neck
Surgery
Foundation
!
(Opioid)
N/A /
N/A
468
N/A
MIPS CQM
Specifications
Process
Continuity of
Pharmacotherapy for Opioid
Use Disorder (OUD):
Percentage of adults aged 18
years and older with
pharmacotherapy for opioid
use disorder (OUD) who have
at least 180 days of continuous
treatment.
University of
Southern
California
§
N/A /
N/A
475
CMS3
49v7
eCQM
Specifications
Process
HIV Screening:
Percentage of patients aged
15-65 at the start of the
measurement period who were
between 15-65 years old when
tested for Human
Immunodeficiency Virus
(HIV).
Centers for
Disease Control
and Prevention

B.13. Family Medicine
PREVIOUSLY FINALIZED MEASURES IN THE FAMILY MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Outcome)
N/A /
N/A
476
CMS7
71v6
eCQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Urinary Symptom Score
Change 6-12 Months After
Diagnosis of Benign Prostatic
Hyperplasia:
Percentage of patients with an
office visit within the
measurement period and with
a new diagnosis of clinically
significant Benign Prostatic
Hyperplasia who have
International Prostate
Symptoms Score (IPSS) or
American Urological
Association (AUA) Symptom
Index (SI) documented at time
of diagnosis and again 6-12
months later with an
improvement of 3 points.
Large Urology
Group Practice
Association and
Oregon Urology
Institute
!
(Outcome)
3568 /
N/A
483
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Person-Centered Primary
Care Measure Patient
Reported Outcome
Performance Measure
(PCPCM PRO-PM):
The Person-Centered Primary
Care Measure Patient Reported
Outcome Performance
Measure (PCPCM PRO-PM)
uses the PCPCM Patient
Reported Outcome Measure
(PROM) a comprehensive and
parsimonious set of 11 patient-
reported items – to assess the
broad scope of primary care.
Unlike other primary care
measures, the PCPCM PRO-
PM measures the high value
aspects of primary care based
on a patient’s relationship with
the clinician or practice.
The American
Board of Family
Medicine
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers
of Health:
Percent of patients 18 years
and older screened for food
insecurity, housing instability,
transportation needs, utility
difficulties, and interpersonal
safety.
Centers for
Medicare &
Medicaid Services
*
N/A /
N/A
488
CMS9
51v3
eCQM
Specifications,
MIPS CQM
Specifications
Process
Kidney Health Evaluation:
Percentage of patients aged
18-75 years with a diagnosis of
diabetes who received a
kidney health evaluation
defined by an Estimated
Glomerular Filtration Rate
(eGFR) AND Urine Albumin-
Creatinine Ratio (uACR)
within the measurement
period.
National Kidney
Foundation
*
3620 /
N/A
493
N/A
MIPS CQM
Specifications
Process
Adult Immunization Status:
Percentage of patients 19 years
of age and older who are up-
to-date on recommended
routine vaccines for influenza;
tetanus and diphtheria (Td) or
tetanus, diphtheria and
acellular pertussis (Tdap);
zoster; and pneumococcal.
National
Committee for
Quality Assurance

B.13. Family Medicine
PREVIOUSLY FINALIZED MEASURES IN THE FAMILY MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Outcome)
3665 /
N/A
495
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Ambulatory Palliative Care
Patients’ Experience of
Feeling Heard and
Understood: The percentage
of top-box responses among
patients aged 18 years and
older who had an ambulatory
palliative care visit and report
feeling heard and understood
by their palliative care
clinician and team within 2
months (60 days) of the
ambulatory palliative care
visit.
American
Academy of
Hospice and
Palliative
Medicine
(AAHPM)
*
N/A /
N/A
497
N/A
MIPS CQM
Specifications
Process
Preventive Care and
Wellness (composite):
Percentage of patients who
received age- and sex-
appropriate preventive
screenings and wellness
services. This measure is a
composite of seven component
measures that are based on
recommendations for
preventive care by the U.S.
Preventive Services Task
Force (USPSTF), ACIP),
American Association of
Clinical Endocrinology
(AACE), and American
College of Endocrinology
(ACE).
Centers for
Medicare and
Medicaid Services
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years or
older who screen positive for
one or more of the following
health related social needs
(HRSNs): food insecurity,
housing instability,
transportation needs, utility
help needs, or interpersonal
safety; and had contact with a
Community Service Provider
(CSP) for at least 1 of their
HRSNs within 60 days after
screening.
OCHIN
!
(Outcome)
N/A /
N/A
502
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Improvement or
Maintenance of Functioning
for Individuals with a
Mental and/or Substance Use
Disorder:
The percentage of patients
aged 18 and older with a
mental and/or substance use
disorder who demonstrated
improvement or maintenance
of functioning based on results
from the 12-item World Health
Organization Disability
Assessment Schedule
(WHODAS 2.0) or Sheehan
Disability Scale (SDS) 30 to
180 days after an index
assessment.
American
Psychiatric
Association

B.13. Family Medicine
PREVIOUSLY FINALIZED MEASURES IN THE FAMILY MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Outcome)
2483 /
N/A
503
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Gains in Patient Activation
Measure (PAM®) Scores at
12 Months:
The Patient Activation
Measure® (PAM®) is a 10 –
or 13 – item questionnaire that
assesses an individual´s
knowledge, skills and
confidence for managing their
health and health care. The
measure assesses individuals
on a 0-100 scale that converts
to one of four levels of
activation, from low (1) to
high (4). The PAM®
performance measure (PAM®-
PM) is the change in score on
the PAM® from baseline to
follow-up measurement.
Insignia Health,
LLC, a wholly
owned subsidiary
of Phreesia
!
(Safety)
N/A /
N/A
504
N/A
MIPS CQM
Specifications
Process
Initiation, Review, And/Or
Update To Suicide Safety
Plan For Individuals With
Suicidal Thoughts, Behavior,
Or Suicide Risk:
Percentage of adult aged 18
years and older with suicidal
ideation or behavior symptoms
(based on results of a
standardized assessment tool
or screening tool) or increased
suicide risk (based on the
clinician’s evaluation or
clinician-rating tool) for whom
a suicide safety plan is
initiated, reviewed, and/or
updated in collaboration
between the patient and their
clinician.
American
Psychiatric
Association
*
!
(Outcome)
N/A /
N/A
505
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Reduction in Suicidal
Ideation or Behavior
Symptoms: The percentage of
patients aged 18 and older with
a mental and/or substance use
disorder AND suicidal
thoughts, behaviors or risk
symptoms who demonstrated a
reduction in suicidal ideation
and/or behavior symptoms
based on results from the
Columbia-Suicide Severity
Rating Scale (C-SSRS)
‘Screen Version’ or ‘Since
Last Visit’ within 120 days
after an index assessment.
American
Psychiatric
Association

B.13. Family Medicine
MEASURES PROPOSED FOR ADDITION TO THE FAMILY MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
N/A /
N/A
TBD
N/A
MIPS
CQM
Specificati
ons
Process
Adult COVID-
19 Vaccination
Status:
Percentage of
patients aged 18
years and older
seen for a visit
during the
performance
period that are
up-to-date on
their COVID-19
vaccinations as
defined by CDC
recommendation
s on current
vaccination.
Centers for
Medicare &
Medicaid
Services
We are proposing to include
this measure in the Family
Medicine specialty set as it
would be clinically relevant to
this clinician type. Widespread
vaccination against SARS-
CoV-2, the virus that causes
COVID-19, is critically
important to stemming the
morbidity and mortality caused
by this disease.
85
Clinicians are
uniquely positioned to
encourage uptake of COVID-
19 vaccination, and clinicians
are still a major driving force
in promoting patient
vaccination. The addition of
this quality measure in this
specialty set would help
strengthen compliance with
recommended COVID-19
vaccination, leading to
improvement in the quality of
patient care and prevention of
disease for the general
population. This quality
measure aligns with clinical
guidelines and the evidence-
based recommendations of the
ACIP, where there is general
agreement about the safety and
efficacy of the COVID-19
vaccine, preventing costly and
potentially harmful
hospitalizations.
86
Broadening
vaccination status awareness to
this clinician type is valuable
as it can help drive an increase
in the adult vaccination rates.
The COVID-19 vaccination
included within this measure
would reduce the prevalence of
severe diseases that may be
associated with hospitalization
and decrease overall health
care costs. The measure being
added to this specialty set
would be contingent on the
inclusion of applicable coding
by the time of the CY 2025
PFS final rule. In the event
appropriate coding is not
included in the final
specification, this measure
would not be finalized for
inclusion within this specialty
measure set. See Table A.5 of
this Appendix for rationale,
including clinical evidence
supporting the inclusion of this
measure in MIPS.
85
See footnote Ikeokwu et al., 2023 in Table B.1 of this Appendix.
86
See footnotes Fitzpatrick et al., 2022; Polack et al., 2020; and Graña et al., 2022 in Table A.5 of this Appendix.

B.13. Family Medicine
PREVIOUSLY FINALIZED MEASURES PROPOSED FOR REMOVAL FROM THE FAMILY MEDICINE SPECIALTY
SET
Note: In this proposed rule, we are proposing the removal of the following measure(s) below from this specific specialty measure set based
upon review of updates made to existing quality measure specifications, the proposed addition of new measures for inclusion in MIPS, and the
feedback provided by specialty societies.
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Removal
N/A /
3475e
472
CMS24
9v7
eCQM
Specification
s
Process
Appropriate Use of DXA
Scans in Women Under 65
Years Who Do Not Meet
the Risk Factor Profile for
Osteoporotic Fracture:
Percentage of female
patients 50 to 64 years of
age without select risk
factors for osteoporotic
fracture who received an
order for a dual-energy x-
ray absorptiometry (DXA)
scan during the
measurement period.
Centers for
Medicare &
Medicaid
Services
This measure is being
proposed for removal
beginning with the CY
2025 performance
period/2027 MIPS
payment year. See Table
Group C for rationale.

B.14. Gastroenterology
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Gastroenterology specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a
measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Gastroenterology
specialty set.
B.14. Gastroenterology
PREVIOUSLY FINALIZED MEASURES IN THE GASTROENTEROLOGY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Care
Coordinatio
n)
0326 /
N/A
047
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Advance Care Plan:
Percentage of patients aged
65 years and older who have
an advance care plan or
surrogate decision maker
documented in the medical
record or documentation in
the medical record that an
advance care plan was
discussed but the patient did
not wish or was not able to
name a surrogate decision
maker or provide an advance
care plan.
National Committee
for Quality Assurance
*
§
!
(Patient
Safety)
N/A / N/A
130
CMS68v
14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for
patients aged 18 years and
older for which the eligible
clinician attests to
documenting a list of current
medications using all
immediate resources
available on the date of the
encounter.
Centers for Medicare
& Medicaid Services
*
§
!
(Care
Coordinatio
n)
N/A / N/A
185
N/A
MIPS CQM
Specifications
Process
Colonoscopy Interval for
Patients with a History of
Adenomatous Polyps –
Avoidance of Inappropriate
Use:
Percentage of patients aged
18 years and older receiving
a surveillance colonoscopy,
with a history of prior
adenomatous polyp(s) in
previous colonoscopy
findings, which had an
interval of 3 or more years
since their last colonoscopy.
American
Gastroenterological
Association
§
N/A / N/A
226
CMS138
v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Tobacco Use:
Screening and Cessation
Intervention:
Percentage of patients aged
12 years and older who were
screened for tobacco use one
or more times during the
measurement period AND
who received tobacco
cessation intervention during
the measurement period or in
the 6 months prior to the
measurement period if
identified as a tobacco user.
National Committee
for Quality Assurance

B.14. Gastroenterology
PREVIOUSLY FINALIZED MEASURES IN THE GASTROENTEROLOGY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
§
NA / N/A
275
N/A
MIPS CQM
Specifications
Process
Inflammatory Bowel
Disease (IBD): Assessment
of Hepatitis B Virus (HBV)
Status Before Initiating
Anti-TNF (Tumor Necrosis
Factor) Therapy:
Percentage of patients with a
diagnosis of inflammatory
bowel disease (IBD) who had
Hepatitis B Virus (HBV)
status assessed and results
interpreted prior to initiating
anti-TNF (tumor necrosis
factor) therapy.
American
Gastroenterological
Association
*
N/A / N/A
317
CMS22v
13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Screening for
High Blood Pressure and
Follow-Up Documented:
Percentage of patient visits
for patients aged 18 years
and older seen during the
measurement period who
were screened for high blood
pressure AND a
recommended follow-up plan
is documented, as indicated,
if blood pressure is elevated
or hypertensive.
Centers for Medicare
& Medicaid Services
*
§
!
(Care
Coordinatio
n)
0658 /
N/A
320
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Appropriate Follow-Up
Interval for Normal
Colonoscopy in Average
Risk Patients:
Percentage of patients aged
45 to 75 years of age
receiving a screening
colonoscopy without biopsy
or polypectomy who had a
recommended follow-up
interval of at least 10 years
for repeat colonoscopy
documented in their
colonoscopy report.
American
Gastroenterological
Association
*
!
(Care
Coordinatio
n)
N/A / N/A
374
CMS50v
13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Closing the Referral Loop:
Receipt of Specialist
Report:
Percentage of patients with
referrals, regardless of age,
for which the referring
clinician receives a report
from the clinician to whom
the patient was referred.
Centers for Medicare
& Medicaid Services
§
N/A / N/A
401
N/A
MIPS CQM
Specifications
Process
Hepatitis C: Screening for
Hepatocellular Carcinoma
(HCC) in Patients with
Cirrhosis:
Percentage of patients aged
18 years and older with a
diagnosis of chronic
Hepatitis C cirrhosis who
underwent imaging with
either ultrasound, contrast
enhanced CT or MRI for
hepatocellular carcinoma
(HCC) at least once within
the 12-month submission
period.
American
Gastroenterological
Association

B.14. Gastroenterology
PREVIOUSLY FINALIZED MEASURES IN THE GASTROENTEROLOGY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
§
2152 /
N/A
431
N/A
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Unhealthy
Alcohol Use: Screening &
Brief Counseling:
Percentage of patients aged
18 years and older who were
screened for unhealthy
alcohol use using a
systematic screening method
at least once within the last
12 months AND who
received brief counseling if
identified as an unhealthy
alcohol user.
National Committee
for Quality Assurance
!
(Equity
N/A / N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social
Drivers of Health:
Percent of patients 18 years
and older screened for food
insecurity, housing
instability, transportation
needs, utility difficulties, and
interpersonal safety.
Centers for Medicare
& Medicaid Services
*
!
(Equity)
N/A / N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years
or older who screen positive
for one or more of the
following health related
social needs (HRSNs): food
insecurity, housing
instability, transportation
needs, utility help needs, or
interpersonal safety; and had
contact with a Community
Service Provider (CSP) for at
least 1 of their HRSNs within
60 days after screening.
OCHIN
*
!
(Outcome)
2483 /
N/A
503
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performan
ce
Measure
Gains in Patient Activation
Measure (PAM®) Scores at
12 Months:
The Patient Activation
Measure® (PAM®) is a 10 –
or 13 – item questionnaire
that assesses an individual´s
knowledge, skills and
confidence for managing
their health and health care.
The measure assesses
individuals on a 0-100 scale
that converts to one of four
levels of activation, from low
(1) to high (4). The PAM®
performance measure
(PAM®-PM) is the change in
score on the PAM® from
baseline to follow-up
measurement.
Insignia Health, LLC,
a wholly owned
subsidiary of Phreesia

B.14. Gastroenterology
PREVIOUSLY FINALIZED MEASURES PROPOSED FOR REMOVAL FROM THE GASTROENTEROLOGY
SPECIALTY SET
Note: In this proposed rule, we are proposing the removal of the following measure(s) below from this specific specialty measure set based
upon review of updates made to existing quality measure specifications, the proposed addition of new measures for inclusion in MIPS, and the
feedback provided by specialty societies.
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure Steward
Rationale for
Removal
N/A /
N/A
439
N/A
MIPS CQM
Specifications
Efficiency
Age Appropriate
Screening Colonoscopy:
The percentage of
screening colonoscopies
performed in patients
greater than or equal to 86
years of age from January
1 to December 31.
American
Gastroenterological
Association
This measure is being
proposed for removal
beginning with the
CY 2025
performance
period/2027 MIPS
payment year. See
Table Group C for
rationale.

B.15. General Surgery
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the General Surgery specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a
measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed General Surgery
specialty set that have proposed substantive changes in Table Group D of this Appendix as indicated by an * in the Indicator
column.
B.15. General Surgery
PREVIOUSLY FINALIZED MEASURES IN THE GENERAL SURGERY SPECIALTY SET
Indicator
CBE
# /
eCQ
M
CBE
#
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Care
Coordination)
0326 /
N/A
047
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Advance Care Plan:
Percentage of patients aged 65
years and older who have an
advance care plan or surrogate
decision maker documented in
the medical record or
documentation in the medical
record that an advance care plan
was discussed but the patient
did not wish or was not able to
name a surrogate decision
maker or provide an advance
care plan.
National Committee
for Quality Assurance
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS68
v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for patients
aged 18 years and older for
which the eligible clinician
attests to documenting a list of
current medications using all
immediate resources available
on the date of the encounter.
Centers for Medicare
& Medicaid Services
§
N/A /
N/A
226
CMS13
8v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Tobacco Use:
Screening and Cessation
Intervention:
Percentage of patients aged 12
years and older who were
screened for tobacco use one or
more times during the
measurement period AND who
received tobacco cessation
intervention during the
measurement period or in the 6
months prior to the
measurement period if identified
as a tobacco user.
National Committee
for Quality Assurance
N/A /
N/A
264
N/A
MIPS CQM
Specifications
Process
Sentinel Lymph Node Biopsy
for Invasive Breast Cancer:
The percentage of clinically
node negative (clinical stage
T1N0M0 or T2N0M0) breast
cancer patients before or after
neoadjuvant systemic therapy,
who undergo a sentinel lymph
node (SLN) procedure.
American Society of
Breast Surgeons

B.15. General Surgery
PREVIOUSLY FINALIZED MEASURES IN THE GENERAL SURGERY SPECIALTY SET
Indicator
CBE
# /
eCQ
M
CBE
#
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
N/A /
N/A
317
CMS22
v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Screening for High
Blood Pressure and Follow-
Up Documented:
Percentage of patient visits for
patients aged 18 years and older
seen during the measurement
period who were screened for
high blood pressure AND a
recommended follow-up plan is
documented, as indicated, if
blood pressure is elevated or
hypertensive.
Centers for Medicare
& Medicaid Services
!
(Outcome)
N/A /
N/A
354
N/A
MIPS CQM
Specifications
Outcome
Anastomotic Leak
Intervention:
Percentage of patients aged 18
years and older who required an
anastomotic leak intervention
following gastric bypass or
colectomy surgery.
American College of
Surgeons
*
§
!
(Outcome)
N/A /
N/A
355
N/A
MIPS CQM
Specifications
Outcome
Unplanned Reoperation
within the 30-Day
Postoperative Period:
Percentage of patients aged 18
years and older who had any
unplanned reoperation within
the 30-day postoperative period.
American College of
Surgeons
!
(Outcome)
N/A /
N/A
356
N/A
MIPS CQM
Specifications
Outcome
Unplanned Hospital
Readmission within 30 Days
of Principal Procedure:
Percentage of patients aged 18
years and older who had an
unplanned hospital readmission
within 30 days of principal
procedure.
American College of
Surgeons
!
(Outcome)
N/A /
N/A
357
N/A
MIPS CQM
Specifications
Outcome
Surgical Site Infection (SSI):
Percentage of patients aged 18
years and older who had a
surgical site infection (SSI).
American College of
Surgeons
!
(Patient
Experience)
N/A /
N/A
358
N/A
MIPS CQM
Specifications
Process
Patient-Centered Surgical
Risk Assessment and
Communication:
Percentage of patients who
underwent a non-emergency
surgery who had their
personalized risks of
postoperative complications
assessed by their surgical team
prior to surgery using a clinical
data-based, patient-specific risk
calculator and who received
personal discussion of those
risks with the surgeon.
American College of
Surgeons
*
!
(Care
Coordination)
N/A /
N/A
374
CMS50
v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Closing the Referral Loop:
Receipt of Specialist Report:
Percentage of patients with
referrals, regardless of age, for
which the referring clinician
receives a report from the
clinician to whom the patient
was referred.
Centers for Medicare
& Medicaid Services

B.15. General Surgery
PREVIOUSLY FINALIZED MEASURES IN THE GENERAL SURGERY SPECIALTY SET
Indicator
CBE
# /
eCQ
M
CBE
#
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers
of Health:
Percent of patients 18 years and
older screened for food
insecurity, housing instability,
transportation needs, utility
difficulties, and interpersonal
safety.
Centers for Medicare
& Medicaid Services
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years or
older who screen positive for
one or more of the following
health related social needs
(HRSNs): food insecurity,
housing instability,
transportation needs, utility help
needs, or interpersonal safety;
and had contact with a
Community Service Provider
(CSP) for at least one of their
HRSNs within 60 days after
screening.
OCHIN

B.16. Geriatrics
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Geriatrics specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a measure
reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Geriatrics
specialty set.
B.16. Geriatrics
PREVIOUSLY FINALIZED MEASURES IN THE GERIATRICS SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
0046 /
N/A
039
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Screening for Osteoporosis
for Women Aged 65-85
Years of Age:
Percentage of women aged
65-85 years of age who ever
had a central dual-energy X-
ray absorptiometry (DXA) to
check for osteoporosis.
National Committee
for Quality Assurance
*
!
(Care
Coordination
)
0326 /
N/A
047
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Advance Care Plan:
Percentage of patients aged
65 years and older who have
an advance care plan or
surrogate decision maker
documented in the medical
record or documentation in
the medical record that an
advance care plan was
discussed but the patient did
not wish or was not able to
name a surrogate decision
maker or provide an advance
care plan.
National Committee
for Quality Assurance
N/A /
N/A
048
N/A
MIPS CQM
Specifications
Process
Urinary Incontinence:
Assessment of Presence or
Absence of Urinary
Incontinence in Women
Aged 65 Years and Older:
Percentage of female patients
aged 65 years and older who
were assessed for the
presence or absence of
urinary incontinence within
12 months.
National Committee
for Quality Assurance
!
(Patient
Experience)
N/A /
N/A
050
N/A
MIPS CQM
Specifications
Process
Urinary Incontinence: Plan
of Care for Urinary
Incontinence in Women
Aged 65 Years and Older:
Percentage of female patients
aged 65 years and older with
a diagnosis of urinary
incontinence with a
documented plan of care for
urinary incontinence at least
once within 12 months.
National Committee
for Quality Assurance
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS68v
14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for
patients aged 18 years and
older for which the eligible
clinician attests to
documenting a list of current
medications using all
immediate resources
available on the date of the
encounter.
Centers for Medicare &
Medicaid Services

B.16. Geriatrics
PREVIOUSLY FINALIZED MEASURES IN THE GERIATRICS SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
§
N/A /
N/A
134
CMS2v1
4
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Screening for
Depression and Follow-Up
Plan:
Percentage of patients aged
12 years and older screened
for depression on the date of
the encounter or up to 14
days prior to the date of the
encounter using an age-
appropriate standardized
depression screening tool
AND if positive, a follow-up
plan is documented on the
date of or up to 2days after
the date of the qualifying
encounter.
Centers for Medicare &
Medicaid Services
*
!
(Care
Coordination
)
0101 /
N/A
155
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Falls: Plan of Care:
Percentage of patients aged
65 years and older with a
history of falls who had a
plan of care for falls
documented within 12
months.
National Committee
for Quality Assurance
*
!
(Patient
Safety)
N/A /
N/A
181
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Elder Maltreatment Screen
and Follow-Up Plan:
Percentage of patients aged
60 years and older with a
documented elder
maltreatment screen using an
Elder Maltreatment
Screening tool on the date of
encounter AND a
documented follow-up plan
on the date of the positive
screen.
Centers for Medicare &
Medicaid Services
*
!
(Patient
Safety)
0022 /
N/A
238
CMS156
v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Use of High-Risk
Medications in Older
Adults:
Percentage of patients 65
years of age and older who
were ordered at least two
high-risk medications from
the same drug class.
National Committee
for Quality Assurance
*
N/A /
2872e
281
CMS149
v13
eCQM
Specifications
Process
Dementia: Cognitive
Assessment:
Percentage of patients,
regardless of age, with a
diagnosis of dementia for
whom an assessment of
cognition is performed and
the results reviewed at least
once within a 12-month
period.
American Academy of
Neurology
*
N/A /
N/A
282
N/A
MIPS CQM
Specifications
Process
Dementia: Functional
Status Assessment:
Percentage of patients with
dementia for whom an
assessment of functional
status was performed at least
once in the last 12 months.
American Academy of
Neurology/ American
Psychiatric Association

B.16. Geriatrics
PREVIOUSLY FINALIZED MEASURES IN THE GERIATRICS SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Patient
Safety)
N/A /
N/A
286
N/A
MIPS CQM
Specifications
Process
Dementia: Safety Concern
Screening and Follow-Up
for Patients with Dementia:
Percentage of patients with
dementia or their caregiver(s)
for whom there was a
documented safety concerns
screening in two domains of
risk: (1) dangerousness to
self or others and (2)
environmental risks; and if
safety concerns screening
was positive in the last 12
months, there was
documentation of mitigation
recommendations, including
but not limited to referral to
other resources.
American Psychiatric
Association/ American
Academy of Neurology
*
!
(Care
Coordination
)
N/A /
N/A
288
N/A
MIPS CQM
Specifications
Process
Dementia: Education and
Support of Caregivers for
Patients with Dementia:
Percentage of patients with
dementia whose caregiver(s)
were provided with education
on dementia disease
management and health
behavior changes AND were
referred to additional
resources for support in the
last 12 months.
American Academy of
Neurology / American
Psychiatric Association
!
(Patient
Safety)
0101 /
N/A
318
CMS139
v13
eCQM
Specifications
Process
Falls: Screening for Future
Fall Risk:
Percentage of patients 65
years of age and older who
were screened for future fall
risk during the measurement
period.
National Committee for
Quality Assurance
§
!
(Outcome)
0710 /
0710e
370
CMS159
v13
eCQM
Specifications,
MIPS CQM
Specifications
Outcome
Depression Remission at
Twelve Months:
The percentage of adolescent
patients 12 to 17 years of age
and adult patients 18 years of
age or older with major
depression or dysthymia who
reached remission 12 months
(+/- 60 days) after an index
event date.
Minnesota Community
Measurement
!
(Outcome)
N/A /
N/A
476
CMS771
v6
eCQM
Specifications
Patient-
Reported
Outcome-
Based
Performan
ce
Measure
Urinary Symptom Score
Change 6-12 Months After
Diagnosis of Benign
Prostatic Hyperplasia:
Percentage of patients with
an office visit within the
measurement period and with
a new diagnosis of clinically
significant Benign Prostatic
Hyperplasia who have
International Prostate
Symptoms Score (IPSS) or
American Urological
Association (AUA)
Symptom Index (SI)
documented at time of
diagnosis and again 6-12
months later with an
improvement of 3 points.
Large Urology Group
Practice Association
and Oregon Urology
Institute

B.16. Geriatrics
PREVIOUSLY FINALIZED MEASURES IN THE GERIATRICS SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Equity
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social
Drivers of Health:
Percent of patients 18 years
and older screened for food
insecurity, housing
instability, transportation
needs, utility difficulties, and
interpersonal safety.
Centers for Medicare &
Medicaid Services
*
N/A /
N/A
488
CMS951
v3
eCQM
Specifications,
MIPS CQM
Specifications
Process
Kidney Health Evaluation:
Percentage of patients aged
18-75 years with a diagnosis
of diabetes who received a
kidney health evaluation
defined by an Estimated
Glomerular Filtration Rate
(eGFR) AND Urine
Albumin-Creatinine Ratio
(uACR) within the
measurement period.
National Kidney
Foundation
1662/
N/A
489
N/A
MIPS CQM
Specifications
Process
Adult Kidney Disease:
Angiotensin Converting
Enzyme (ACE) Inhibitor or
Angiotensin Receptor
Blocker (ARB) Therapy:
Percentage of patients aged
18 years and older with a
diagnosis of chronic kidney
disease (CKD) (Stages 1-5,
not receiving Renal
Replacement Therapy
(RRT)) and proteinuria who
were prescribed ACE
inhibitor or ARB therapy
within a 12-month period.
Renal Physicians
Association
*
3620 /
N/A
493
N/A
MIPS CQM
Specifications
Process
Adult Immunization
Status:
Percentage of patients 19
years of age and older who
are up-to-date on
recommended routine
vaccines for influenza;
tetanus and diphtheria (Td)
or tetanus, diphtheria and
acellular pertussis (Tdap);
zoster; and pneumococcal.
National Committee
for Quality Assurance
*
NA /
N/A
497
N/A
MIPS CQM
Specifications
Process
Preventive Care and
Wellness (composite):
Percentage of patients who
received age- and sex-
appropriate preventive
screenings and wellness
services. This measure is a
composite of seven
component measures that are
based on recommendations
for preventive care by the
U.S. Preventive Services
Task Force (USPSTF),
ACIP, American Association
of Clinical Endocrinology
(AACE), and American
College of Endocrinology
(ACE).
Centers for Medicare
and Medicaid Services

B.16. Geriatrics
PREVIOUSLY FINALIZED MEASURES IN THE GERIATRICS SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years
or older who screen positive
for one or more of the
following health related
social needs (HRSNs): food
insecurity, housing
instability, transportation
needs, utility help needs, or
interpersonal safety; and had
contact with a Community
Service Provider (CSP) for at
least 1 of their HRSNs within
60 days after screening.
OCHIN

B.16. Geriatrics
MEASURES PROPOSED FOR ADDITION TO THE GERIATRICS SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And
Description
Measure
Steward
Rationale for Inclusion
!
(Outcome
)
3665 /
N/A
495
N/A
MIPS
CQM
Specificati
ons
Patient-
Reported
Outcome-
Based
Performance
Measure
Ambulatory
Palliative
Care Patients’
Experience of
Feeling Heard
and
Understood:
The percentage
of top-box
responses
among patients
aged 18 years
and older who
had an
ambulatory
palliative care
visit and report
feeling heard
and understood
by their
palliative care
clinician and
team within 2
months (60
days) of the
ambulatory
palliative care
visit.
America
n
Academy
of
Hospice
and
Palliative
Medicine
(AAHP
M)
We are proposing to include this
measure in the Geriatrics specialty
set as it would be clinically
relevant to this clinician type.
This PRO-PM would help to fill a
gap for patients receiving
palliative care by capturing the
patient’s voice and experience of
care by assessing communication
and shared decision making with
the clinician. This is an important
patient-centered measure that
helps patients feel heard and
understood which can effectively
improve the quality of care
received and outcomes for
patients in palliative care.
Allowing patients to feel heard
and understood adds an important
dimension to the care planning for
this unique patient population
commonly cared for by clinicians
in this specialty. As more patients
are living longer with multiple
comorbidities, especially true for
the advanced heart disease patient
population, early emergence of
palliative care into the overall
care of cardiac patients can
notably improve their quality of
life, patient satisfaction, and
reduction in symptoms.
87
This
measure is predicated on existing
guidelines and conceptual
models
88
and can facilitate and
improve effective patient-clinician
communication that engenders
trust, acknowledgement, and a
whole-person orientation to the
care that is provided. Through the
benefits of enhanced patient-
provider communication, this
measure would improve the
quality of care received and
outcomes for patients receiving
palliative care. The measure being
added to this specialty set would
be contingent on the inclusion of
applicable coding by the time of
the CY 2025 PFS final rule.
87
See footnote Kilic et al., 2020 in Table B.4a of this Appendix.
88
See footnote National Consensus Project for Quality Palliative Care, 2018 in Table B.4a of this Appendix.

B.16. Geriatrics
MEASURES PROPOSED FOR ADDITION TO THE GERIATRICS SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And
Description
Measure
Steward
Rationale for Inclusion
*
!
(Outcome
)
2483 /
N/A
503
N/A
MIPS
CQM
Specificati
ons
Patient-
Reported
Outcome-
Based
Performance
Measure
Gains in
Patient
Activation
Measure
(PAM®)
Scores at 12
Months:
The Patient
Activation
Measure®
(PAM®) is a
10 – or 13 –
item
questionnaire
that assesses an
individual´s
knowledge,
skills and
confidence for
managing their
health and
health care.
The measure
assesses
individuals on
a 0-100 scale
that converts to
one of four
levels of
activation,
from low (1) to
high (4). The
PAM®
performance
measure
(PAM®-PM) is
the change in
score on the
PAM® from
baseline to
follow-up
measurement.
Insignia
Health,
LLC, a
wholly
owned
subsidiar
y of
Phreesia
We are proposing to include this
measure in the Geriatrics specialty
set as it would be clinically
relevant to this clinician type. The
addition of this measure to this
specialty set would be feasible
given its use through the
continuum of care and across
different clinical settings. This
measure addresses chronic
conditions and outcomes, both of
which are high priority areas for
measure consideration for MIPS.
It is utilized in research within the
U.S. and internationally and has
also been shown to be valid and
reliable in different clinical
settings and under different
payment models.
89
The measure
being added to this specialty set
would be contingent on the
inclusion of applicable coding by
the time of the CY 2025 PFS final
rule.
89
See footnote Phreesia, 2024 in Table B.7 of this Appendix.

B.16. Geriatrics
MEASURES PROPOSED FOR ADDITION TO THE GERIATRICS SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And
Description
Measure
Steward
Rationale for Inclusion
N/A /
N/A
TBD
N/A
MIPS
CQM
Specificati
ons
Process
Adult
COVID-19
Vaccination
Status:
Percentage of
patients aged
18 years and
older seen for a
visit during the
performance
period that are
up-to-date on
their COVID-
19 vaccinations
as defined by
CDC
recommendatio
ns on current
vaccination.
Centers
for
Medicare
&
Medicaid
Services
We are proposing to include this
measure in the Geriatrics specialty
set as it would be clinically
relevant to this clinician type.
Widespread vaccination against
SARS-CoV-2, the virus that
causes COVID-19, is critically
important to stemming the
morbidity and mortality caused by
this disease.
90
Clinicians are
uniquely positioned to encourage
uptake of COVID-19 vaccination,
and clinicians are still a major
driving force in promoting patient
vaccination. The addition of this
quality measure in this specialty
set would help strengthen
compliance with recommended
COVID-19 vaccination, leading to
improvement in the quality of
patient care and prevention of
disease for the general population.
This quality measure aligns with
clinical guidelines and the
evidence-based recommendations
of the ACIP, where there is
general agreement about the
safety and efficacy of the
COVID-19 vaccine, preventing
costly and potentially harmful
hospitalizations.
91
Broadening
vaccination status awareness to
this clinician type is valuable as it
can help drive an increase in the
adult vaccination rates. The
COVID-19 vaccination included
within this measure would reduce
the prevalence of severe diseases
that may be associated with
hospitalization and decrease
overall health care costs. The
measure being added to this
specialty set would be contingent
on the inclusion of applicable
coding by the time of the CY
2025 PFS final rule. In the event
appropriate coding is not included
in the final specification, this
measure would not be finalized
for inclusion within this specialty
measure set. See Table A.5 of this
Appendix for rationale, including
clinical evidence supporting the
inclusion of this measure in
MIPS.
90
See footnote Ikeokwu et al., 2023 in Table B.1 of this Appendix.
91
See footnotes Fitzpatrick et al., 2022; Polack et al., 2020; and Graña et al., 2022 in Table A.5 of this Appendix.

B.17. Hospitalists
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Hospitalists specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a measure
reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on measures available in the proposed Hospitalists specialty
set that have proposed substantive changes in Table Group D of this Appendix as indicated by an * in the Indicator column.
B.17. Hospitalists
PREVIOUSLY FINALIZED MEASURES IN THE HOSPITALISTS SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
§
0081 /
0081e
005
CMS13
5v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Heart Failure (HF):
Angiotensin-Converting
Enzyme (ACE) Inhibitor or
Angiotensin Receptor
Blocker (ARB) or
Angiotensin Receptor-
Neprilysin Inhibitor (ARNI)
Therapy for Left
Ventricular Systolic
Dysfunction (LVSD):
Percentage of patients aged
18 years and older with a
diagnosis of heart failure
(HF) with a current or prior
left ventricular ejection
fraction (LVEF) ≤ 40% who
were prescribed ACE
inhibitor or ARB or ARNI
therapy either within a 12-
month period when seen in
the outpatient setting OR at
each hospital discharge.
American Heart
Association
§
0083 /
0083e
008
CMS14
4v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Heart Failure (HF): Beta-
Blocker Therapy for Left
Ventricular Systolic
Dysfunction (LVSD):
Percentage of patients aged
18 years and older with a
diagnosis of heart failure
(HF) with a current or prior
left ventricular ejection
fraction (LVEF) ≤ 40% who
were prescribed beta-blocker
therapy either within a 12-
month period when seen in
the outpatient setting OR at
each hospital discharge.
American Heart
Association
*
!
(Care
Coordination)
0326 /
N/A
047
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Advance Care Plan:
Percentage of patients aged 65
years and older who have an
advance care plan or surrogate
decision maker documented in
the medical record or
documentation in the medical
record that an advance care
plan was discussed but the
patient did not wish or was not
able to name a surrogate
decision maker or provide an
advance care plan.
National Committee
for Quality Assurance

B.17. Hospitalists
PREVIOUSLY FINALIZED MEASURES IN THE HOSPITALISTS SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS68
v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for
patients aged 18 years and
older for which the eligible
clinician attests to
documenting a list of current
medications using all
immediate resources available
on the date of the encounter.
Centers for Medicare &
Medicaid Services

B.18. Infectious Disease
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Infectious Disease specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a
measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Infectious Disease
specialty set.
B.18. Infectious Disease
PREVIOUSLY FINALIZED MEASURES IN THE INFECTIOUS DISEASE SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measur
e
Type
Measure Title
and Description
Measure Steward
§
!
(Appropria
te Use)
0069 /
N/A
065
CMS15
4v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Appropriate Treatment for
Upper Respiratory Infection
(URI):
Percentage of episodes for
patients 3 months of age and
older with a diagnosis of upper
respiratory infection (URI) that
did not result in an antibiotic
order.
National Committee for
Quality Assurance
§
!
(Appropria
te Use)
N/A /
N/A
066
CMS14
6v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Appropriate Testing for
Pharyngitis:
The percentage of episodes for
patients 3 years and older with a
diagnosis of pharyngitis that
resulted in an antibiotic order on
or within 3 days after the
episode date and a group A
Streptococcus (Strep) test in the
7-day period from three days
prior to the episode date through
3 days after the episode date.
National Committee for
Quality Assurance
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS68
v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for patients
aged 18 years and older for
which the eligible clinician
attests to documenting a list of
current medications using all
immediate resources available
on the date of the encounter.
Centers for Medicare &
Medicaid Services
*
N/A /
N/A
176
N/A
MIPS CQM
Specifications
Process
Tuberculosis Screening Prior
to First Course of Biologic
and/or Immune Response
Modifier Therapy:
If a patient has been newly
prescribed a biologic and/or
immune response modifier that
includes a warning for potential
reactivation of a latent infection,
then the medical record should
indicate TB testing in the
preceding 12-month period.
American College of
Rheumatology
§
N/A /
N/A
205
CMS11
88v2
eCQM
Specifications,
MIPS CQM
Specifications
Process
Sexually Transmitted
Infection (STI) Testing for
People with HIV:
Percentage of patients 13 years
of age and older with a diagnosis
of HIV who had tests for
syphilis, gonorrhea, and
chlamydia performed within the
performance period.
Health Resources and
Services Administration
§
N/A /
N/A
226
CMS13
8v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Tobacco Use:
Screening and Cessation
Intervention:
Percentage of patients aged 12
years and older who were
screened for tobacco use one or
more times during the
measurement period AND who
National Committee for
Quality Assurance

B.18. Infectious Disease
PREVIOUSLY FINALIZED MEASURES IN THE INFECTIOUS DISEASE SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measur
e
Type
Measure Title
and Description
Measure Steward
received tobacco cessation
intervention during the
measurement period or in the 6
months prior to the measurement
period if identified as a tobacco
user.
§
N/A /
N/A
240
CMS11
7v13
eCQM
Specifications
Process
Childhood Immunization
Status:
Percentage of children 2 years of
age who had four diphtheria,
tetanus and acellular pertussis
(DtaP); three polio (IPV), one
measles, mumps and rubella
(MMR); three or four H
influenza type B (HiB); three
hepatitis B (HepB); one chicken
pox (VZV); four pneumococcal
conjugate (PCV); one hepatitis
A (HepA); two or three rotavirus
(RV); and two influenza (flu)
vaccines by their second
birthday.
National Committee for
Quality Assurance
§
!
(Outcome)
NA /
N/A
338
CMS31
4v2
eCQM
Specifications,
MIPS CQM
Specifications
Outcome
HIV Viral Suppression:
Percentage of patients,
regardless of age, diagnosed
with HIV prior to or during the
first 90 days of the performance
period, with an eligible
encounter in the first 240 days of
the performance period, whose
last HIV viral load test result
was less than 200 copies/mL
during the performance period.
Health Resources and
Services Administration
*
§
!
(Efficiency
)
N/A /
N/A
340
N/A
MIPS CQM
Specifications
Process
HIV Medical Visit Frequency:
Percentage of patients,
regardless of age with a
diagnosis of HIV who had at
least one medical visit in each 6-
month period of the 24-month
measurement period, with a
minimum of 60 days between
medical visits.
Health Resources and
Services Administration
N/A /
N/A
387
N/A
MIPS CQM
Specifications
Process
Annual Hepatitis C Virus
(HCV) Screening for Patients
who are Active Injection Drug
Users:
Percentage of patients,
regardless of age, who are active
injection drug users who
received screening for HCV
infection within the 12-month
reporting period.
American
Gastroenterological
Association
§
N/A /
N/A
394
N/A
MIPS CQM
Specifications
Process
Immunizations for
Adolescents:
The percentage of adolescents
13 years of age who had one
dose of meningococcal vaccine
(serogroups A, C, W, Y), one
tetanus, diphtheria toxoids and
acellular pertussis (Tdap)
vaccine, and have completed the
Human Papillomavirus (HPV)
vaccine series by their 13
th
birthday.
National Committee for
Quality Assurance
§
N/A /
N/A
475
CMS34
9v7
eCQM
Specifications
Process
HIV Screening:
Percentage of patients aged 15-
65 at the start of the
measurement period who were
between 15-65 years old when
tested for Human
Centers for Disease
Control and Prevention
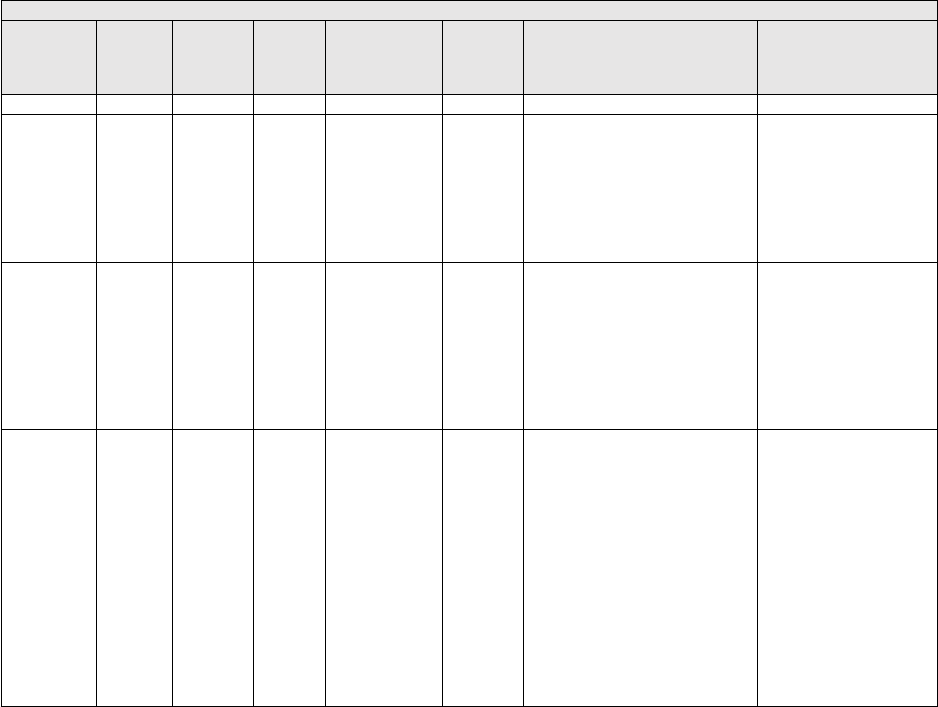
B.18. Infectious Disease
PREVIOUSLY FINALIZED MEASURES IN THE INFECTIOUS DISEASE SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measur
e
Type
Measure Title
and Description
Measure Steward
Immunodeficiency Virus (HIV).
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers of
Health:
Percent of patients 18 years and
older screened for food
insecurity, housing instability,
transportation needs, utility
difficulties, and interpersonal
safety.
Centers for Medicare &
Medicaid Services
*
3620 /
N/A
493
N/A
MIPS CQM
Specifications
Process
Adult Immunization Status:
Percentage of patients 19 years
of age and older who are up-to-
date on recommended routine
vaccines for influenza; tetanus
and diphtheria (Td) or tetanus,
diphtheria and acellular pertussis
(Tdap); zoster; and
pneumococcal.
National Committee for
Quality Assurance
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years or
older who screen positive for
one or more of the following
health related social needs
(HRSNs): food insecurity,
housing instability,
transportation needs, utility help
needs, or interpersonal safety;
and had contact with a
Community Service Provider
(CSP) for at least 1 of their
HRSNs within 60 days after
screening.
OCHIN

B.18. Infectious Disease
MEASURES PROPOSED FOR ADDITION TO THE INFECTIOUS DISEASE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
*
!
(Outcome
)
2483 /
N/A
503
N/A
MIPS
CQM
Specificati
ons
Patient-
Reported
Outcome-
Based
Performanc
e Measure
Gains in Patient
Activation
Measure
(PAM®) Scores
at 12 Months:
The Patient
Activation
Measure®
(PAM®) is a 10
– or 13 – item
questionnaire
that assesses an
individual´s
knowledge, skills
and confidence
for managing
their health and
health care. The
measure assesses
individuals on a
0-100 scale that
converts to one
of four levels of
activation, from
low (1) to high
(4). The PAM®
performance
measure
(PAM®-PM) is
the change in
score on the
PAM® from
baseline to
follow-up
measurement.
Insignia
Health,
LLC, a
wholly
owned
subsidiar
y of
Phreesia
We are proposing to include this
measure in the Infectious Disease
specialty set as it would be
clinically relevant to this clinician
type. The addition of this measure
to this specialty set would be
feasible given its use through the
continuum of care and across
different clinical settings. This
measure addresses chronic
conditions and outcomes, both of
which are high priority areas for
measure consideration for MIPS.
It is utilized in research within the
U.S. and internationally and has
also been shown to be valid and
reliable in different clinical
settings and under different
payment models.
92
The measure
being added to this specialty set
would be contingent on the
inclusion of applicable coding by
the time of the CY 2025 PFS final
rule.
92
See footnote Phreesia, 2024 in Table B.7 of this Appendix.

B.18. Infectious Disease
MEASURES PROPOSED FOR ADDITION TO THE INFECTIOUS DISEASE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
N/A /
N/A
TBD
N/A
MIPS
CQM
Specificati
ons
Process
Adult COVID-
19 Vaccination
Status:
Percentage of
patients aged 18
years and older
seen for a visit
during the
performance
period that are
up-to-date on
their COVID-19
vaccinations as
defined by CDC
recommendation
s on current
vaccination.
Centers
for
Medicare
&
Medicaid
Services
We are proposing to include this
measure in the Infectious Disease
specialty set as it would be
clinically relevant to this clinician
type. Widespread vaccination
against SARS-CoV-2, the virus
that causes COVID-19, is
critically important to stemming
the morbidity and mortality
caused by this disease.
93
Clinicians are uniquely positioned
to encourage uptake of COVID-
19 vaccination, and clinicians are
still a major driving force in
promoting patient vaccination.
The addition of this quality
measure in this specialty set
would help strengthen compliance
with recommended COVID-19
vaccination, leading to
improvement in the quality of
patient care and prevention of
disease for the general population.
This quality measure aligns with
clinical guidelines and the
evidence-based recommendations
of the ACIP, where there is
general agreement about the
safety and efficacy of the
COVID-19 vaccine, preventing
costly and potentially harmful
hospitalizations.
94
Broadening
vaccination status awareness to
this clinician type is valuable as it
can help drive an increase in the
adult vaccination rates. The
COVID-19 vaccination included
within this measure would reduce
the prevalence of severe diseases
that may be associated with
hospitalization and decrease
overall health care costs. The
measure being added to this
specialty set would be contingent
on the inclusion of applicable
coding by the time of the CY
2025 PFS final rule. In the event
appropriate coding is not included
in the final specification, this
measure would not be finalized
for inclusion within this specialty
measure set. See Table A.5 of this
Appendix for rationale, including
clinical evidence supporting the
inclusion of this measure in
MIPS.
93
See footnote Ikeokwu et al., 2023 in Table B.1 of this Appendix.
94
See footnotes Fitzpatrick et al., 2022; Polack et al., 2020; and Graña et al., 2022 in Table A.5 of this Appendix.

B.19. Internal Medicine
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Internal Medicine specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a
measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Internal Medicine
specialty set.
B.19. Internal Medicine
PREVIOUSLY FINALIZED MEASURES IN THE INTERNAL MEDICINE SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
*
§
!
(Outcome)
0059 / N/A
001
CMS122v
13
Medicare Part B
Claims Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Intermediate
Outcome
Diabetes: Hemoglobin A1c
(HbA1c) Poor Control
(>9%):
Percentage of patients 18-75
years of age with diabetes
who had hemoglobin A1c >
9.0% during the
measurement period.
National Committee for
Quality Assurance
§
0081 /
0081e
005
CMS135v
13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Heart Failure (HF):
Angiotensin-Converting
Enzyme (ACE) Inhibitor or
Angiotensin Receptor
Blocker (ARB) or
Angiotensin Receptor-
Neprilysin Inhibitor
(ARNI) Therapy for Left
Ventricular Systolic
Dysfunction (LVSD):
Percentage of patients aged
18 years and older with a
diagnosis of heart failure
(HF) with a current or prior
left ventricular ejection
fraction (LVEF) ≤ 40% who
were prescribed ACE
inhibitor or ARB or ARNI
therapy either within a 12-
month period when seen in
the outpatient setting OR at
each hospital discharge.
American Heart
Association
§
0067 / N/A
006
N/A
MIPS CQM
Specifications
Process
Coronary Artery Disease
(CAD): Antiplatelet
Therapy:
Percentage of patients aged
18 years and older with a
diagnosis of coronary artery
disease (CAD) seen within a
12-month period who were
prescribed aspirin or
clopidogrel.
American Heart
Association
§
0070 /
0070e
007
CMS145v
13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Coronary Artery Disease
(CAD): Beta-Blocker
Therapy – Prior
Myocardial Infarction
(MI) or Left Ventricular
Systolic Dysfunction
(LVEF ≤ 40%):
Percentage of patients aged
18 years and older with a
diagnosis of coronary artery
disease seen within a 12-
month period who also
have a prior MI or a current
or prior LVEF ≤ 40% who
were prescribed beta-
blocker therapy.
American Heart
Association

B.19. Internal Medicine
PREVIOUSLY FINALIZED MEASURES IN THE INTERNAL MEDICINE SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
§
0083 /
0083e
008
CMS144
v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Heart Failure (HF): Beta-
Blocker Therapy for Left
Ventricular Systolic
Dysfunction (LVSD):
Percentage of patients aged
18 years and older with a
diagnosis of heart failure
(HF) with a current or prior
left ventricular ejection
fraction (LVEF) ≤ 40%
who were prescribed beta-
blocker therapy either
within a 12-month period
when seen in the outpatient
setting OR at each hospital
discharge.
American Heart
Association
*
N/A / N/A
009
CMS128
v13
eCQM
Specifications
Process
Antidepressant
Medication Management:
Percentage of patients 18
years of age and older who
were treated with
antidepressant medication,
had a diagnosis of major
depression, and who
remained on an
antidepressant medication
treatment. Two rates are
reported.
a. Percentage of patients
who remained on an
antidepressant medication
for at least 84 days (12
weeks).
b. Percentage of patients
who remained on an
antidepressant medication
for at least 180 days (6
months).
National Committee
for Quality Assurance
!
(Care
Coordinat
ion)
N/A /
N/A
024
N/A
Medicare Part B
Claims Measure
Specifications,
MIPS CQM
Specifications
Process
Communication with the
Physician or Other
Clinician Managing On-
Going Care Post-Fracture
for Men and Women Aged
50 Years and Older:
Percentage of patients aged
50 years and older treated
for a fracture with
documentation of
communication, between
the physician treating the
fracture and the physician
or other clinician managing
the patient’s on-going care,
that a fracture occurred and
that the patient was or
should be considered for
osteoporosis treatment or
testing. This measure is
submitted by the physician
who treats the fracture and
who therefore is held
accountable for the
communication.
National Committee for
Quality Assurance

B.19. Internal Medicine
PREVIOUSLY FINALIZED MEASURES IN THE INTERNAL MEDICINE SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
0046 /
N/A
039
N/A
Medicare Part B
Claims Measure
Specifications,
MIPS CQM
Specifications
Process
Screening for
Osteoporosis for Women
Aged 65-85 Years of Age:
Percentage of women aged
65-85 years of age who
ever had a central dual-
energy X-ray
absorptiometry (DXA) to
check for osteoporosis.
National Committee for
Quality Assurance
*
!
(Care
Coordinat
ion)
0326 /
N/A
047
N/A
Medicare Part B
Claims Measure
Specifications,
MIPS CQM
Specifications
Process
Advance Care Plan:
Percentage of patients aged
65 years and older who
have an advance care plan
or surrogate decision maker
documented in the medical
record or documentation in
the medical record that an
advance care plan was
discussed but the patient
did not wish or was not
able to name a surrogate
decision maker or provide
an advance care plan.
National Committee
for Quality Assurance
N/A /
N/A
048
N/A
MIPS CQM
Specifications
Process
Urinary Incontinence:
Assessment of Presence or
Absence of Urinary
Incontinence in Women
Aged 65 Years and Older:
Percentage of female
patients aged 65 years and
older who were assessed
for the presence or absence
of urinary incontinence
within 12 months.
National Committee
for Quality Assurance
!
(Patient
Experienc
e)
N/A /
N/A
050
N/A
MIPS CQM
Specifications
Process
Urinary Incontinence: Plan
of Care for Urinary
Incontinence in Women
Aged 65 Years and Older:
Percentage of female
patients aged 65 years and
older with a diagnosis of
urinary incontinence with a
documented plan of care
for urinary incontinence at
least once within 12
months.
National Committee
for Quality Assurance
§
!
(Appropri
ate Use)
0058 /
N/A
116
N/A
MIPS CQM
Specifications
Process
Avoidance of Antibiotic
Treatment for Acute
Bronchitis/Bronchiolitis:
The percentage of episodes
for patients ages 3 months
and older with a diagnosis
of acute
bronchitis/bronchiolitis that
did not result in an
antibiotic dispensing event.
National Committee
for Quality Assurance

B.19. Internal Medicine
PREVIOUSLY FINALIZED MEASURES IN THE INTERNAL MEDICINE SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
*
§
0055 /
N/A
117
CMS131
v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Diabetes: Eye Exam:
Percentage of patients 18-
75 years of age with
diabetes and an active
diagnosis of retinopathy in
any part of the
measurement period who
had a retinal or dilated eye
exam by an eye care
professional during the
measurement period or
diabetics with no diagnosis
of retinopathy in any part of
the measurement period
who had a retinal or dilated
eye exam by an eye care
professional during the
measurement period or in
the 12 months prior to the
measurement period.
National Committee
for Quality Assurance
N/A / N/A
126
N/A
MIPS CQM
Specifications
Process
Diabetes Mellitus:
Diabetic Foot and Ankle
Care, Peripheral
Neuropathy –
Neurological Evaluation:
Percentage of patients aged
18 years and older with a
diagnosis of diabetes
mellitus who had a
neurological examination
of their lower extremities
within 12 months.
American Podiatric
Medical Association
*
§
!
(Patient
Safety)
N/A / N/A
130
CMS68v
14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of
Current Medications in
the Medical Record:
Percentage of visits for
patients aged 18 years and
older for which the eligible
clinician attests to
documenting a list of
current medications using
all immediate resources
available on the date of the
encounter.
Centers for Medicare
& Medicaid Services
§
N/A / N/A
134
CMS2v1
4
Medicare Part B
Claims Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Screening for
Depression and Follow-
Up Plan:
Percentage of patients aged
12 years and older screened
for depression on the date
of the encounter or up to 14
days prior to the date of the
encounter using an age-
appropriate standardized
depression screening tool
AND if positive, a follow-
up plan is documented on
the date of or up to two
days after the date of the
qualifying encounter.
Centers for Medicare
& Medicaid Services
*
!
(Care
Coordinat
ion)
0101 /
N/A
155
N/A
Medicare Part B
Claims Measure
Specifications,
MIPS CQM
Specifications
Process
Falls: Plan of Care:
Percentage of patients aged
65 years and older with a
history of falls who had a
plan of care for falls
documented within 12
months.
National Committee
for Quality Assurance

B.19. Internal Medicine
PREVIOUSLY FINALIZED MEASURES IN THE INTERNAL MEDICINE SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
*
N/A / N/A
176
N/A
MIPS CQM
Specifications
Process
Tuberculosis Screening
Prior to First Course of
Biologic and/or Immune
Response Modifier
Therapy:
If a patient has been newly
prescribed a biologic and/or
immune response modifier
that includes a warning for
potential reactivation of a
latent infection, then the
medical record should
indicate TB testing in the
preceding 12-month period.
American College of
Rheumatology
*
!
(Patient
Safety)
N/A / N/A
181
N/A
Medicare Part B
Claims Measure
Specifications,
MIPS CQM
Specifications
Process
Elder Maltreatment
Screen and Follow-Up
Plan:
Percentage of patients aged
60 years and older with a
documented elder
maltreatment screen using
an Elder Maltreatment
Screening tool on the date
of encounter AND a
documented follow-up plan
on the date of the positive
screen.
Centers for Medicare
& Medicaid Services
*
§
!
(Outcome
)
N/A / N/A
236
CMS165
v13
Medicare Part B
Claims Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Intermediate
Outcome
Controlling High Blood
Pressure:
Percentage of patients 18-
85 years of age who had a
diagnosis of essential
hypertension starting before
and continuing into, or
starting during the first six
months of the measurement
period, and whose most
recent blood pressure was
adequately controlled
(<140/90mmHg) during the
measurement period.
National Committee
for Quality Assurance
*
!
(Patient
Safety)
0022 /
N/A
238
CMS156
v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Use of High-Risk
Medications in Older
Adults:
Percentage of patients 65
years of age and older who
were ordered at least two
high-risk medications from
the same drug class.
National Committee
for Quality Assurance

B.19. Internal Medicine
PREVIOUSLY FINALIZED MEASURES IN THE INTERNAL MEDICINE SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Care
Coordinat
ion)
0643 /
N/A
243
N/A
MIPS CQM
Specifications
Process
Cardiac Rehabilitation
Patient Referral from an
Outpatient Setting:
Percentage of patients
evaluated in an outpatient
setting who within the
previous 12 months have
experienced an acute
myocardial infarction (MI),
coronary artery bypass graft
(CABG) surgery, a
percutaneous coronary
intervention (PCI), cardiac
valve surgery, or cardiac
transplantation, or who
have chronic stable angina
(CSA) and have not already
participated in an early
outpatient cardiac
rehabilitation/secondary
prevention (CR) program
for the qualifying
event/diagnosis who were
referred to a CR program.
American Heart
Association
*
N/A / N/A
277
N/A
MIPS CQM
Specifications
Process
Sleep Apnea: Severity
Assessment at Initial
Diagnosis:
Percentage of patients aged
18 years and older with a
diagnosis of obstructive
sleep apnea who had an
apnea hypopnea index
(AHI), a respiratory
disturbance index (RDI), or
a respiratory event index
(REI) documented or
measured within 2 months
of initial evaluation for
suspected obstructive sleep
apnea.
American Academy of
Sleep Medicine
N/A / N/A
279
N/A
MIPS CQM
Specifications
Process
Sleep Apnea: Assessment
of Adherence to
Obstructive Sleep Apnea
(OSA) Therapy:
Percentage of patients aged
18 years and older with a
diagnosis of obstructive
sleep apnea (OSA) that
were prescribed an
evidence-based therapy that
had documentation that
adherence to therapy was
assessed at least annually
through an objective
informatics system or
through self-reporting (if
objective reporting is not
available).
American Academy of
Sleep Medicine

B.19. Internal Medicine
PREVIOUSLY FINALIZED MEASURES IN THE INTERNAL MEDICINE SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Opioid)
N/A / N/A
305
CMS137
v13
eCQM
Specifications
Process
Initiation and
Engagement of Substance
Use Disorder Treatment:
Percentage of patients 13
years of age and older with
a new substance use
disorder (SUD) episode
who received the following
(Two rates are reported):
a. Percentage of patients
who initiated treatment,
including either an
intervention or medication
for the treatment of SUD,
within 14 days of the new
SUD episode.
b. Percentage of patients
who engaged in ongoing
treatment, including two
additional interventions or
medication treatment
events for SUD, or one
long-acting medication
event for the treatment of
SUD, within 34 days of the
initiation.
National Committee
for Quality Assurance
§
N/A / N/A
309
CMS124
v13
eCQM
Specifications
Process
Cervical Cancer
Screening:
Percentage of women 21-64
years of age who were
screened for cervical cancer
using either of the
following criteria:
• Women age 21-64 who
had cervical cytology
performed within the last 3
years
• Women age 30-64 who
had cervical human
papillomavirus (HPV)
testing performed within
the last 5 years
National Committee
for Quality Assurance
!
(Patient
Safety)
0101 /
N/A
318
CMS139
v13
eCQM
Specifications
Process
Falls: Screening for
Future Fall Risk:
Percentage of patients 65
years of age and older who
were screened for future
fall risk during the
measurement period.
National Committee
for Quality Assurance

B.19. Internal Medicine
PREVIOUSLY FINALIZED MEASURES IN THE INTERNAL MEDICINE SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
§
!
(Patient
Experienc
e)
0005 /
N/A
321
N/A
CMS-approved
Survey Vendor
Patient
Engagement/
Experience
CAHPS for MIPS
Clinician/Group Survey:
The Consumer Assessment
of Healthcare Providers and
Systems (CAHPS) for
MIPS Clinician/Group
Survey is comprised of 10
Summary Survey Measures
(SSMs) and measures
patient experience of care
within a group practice.
The CBE endorsement
status and endorsement id
(if applicable) for each
SSM utilized in this
measure are as follows:
• Getting Timely Care,
Appointments, and
Information; (Not endorsed
by CBE)
• How well Providers
Communicate; (Not
endorsed by CBE)
• Patient’s Rating of
Provider; (CBE endorsed #
0005)
• Access to Specialists;
(Not endorsed by CBE)
• Health Promotion and
Education; (Not endorsed
by CBE)
• Shared Decision-Making;
(Not endorsed by CBE)
• Health Status and
Functional Status; (Not
endorsed by CBE)
• Courteous and Helpful
Office Staff; (CBE
endorsed # 0005)
• Care Coordination; (Not
endorsed by CBE)
• Stewardship of Patient
Resources. (Not endorsed
by CBE)
Centers for Medicare &
Medicaid Services
§
N/A / N/A
326
N/A
MIPS CQM
Specifications
Process
Atrial Fibrillation and
Atrial Flutter: Chronic
Anticoagulation Therapy:
Percentage of patients aged
18 years and older with
atrial fibrillation (AF) or
atrial flutter who were
prescribed an FDA-
approved oral anticoagulant
drug for the prevention of
thromboembolism during
the measurement period.
American Heart
Association
*
!
(Appropri
ate Use)
N/A / N/A
331
N/A
MIPS CQM
Specifications
Process
Adult Sinusitis: Antibiotic
Prescribed for Acute
Viral Sinusitis (Overuse):
Percentage of patients, aged
18 years and older, with a
diagnosis of acute viral
sinusitis who were
prescribed an antibiotic
within 10 days after onset
of symptoms.
American Academy of
Otolaryngology –
Head and Neck
Surgery Foundation
B.19. Internal Medicine
PREVIOUSLY FINALIZED MEASURES IN THE INTERNAL MEDICINE SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Appropri
ate Use)
N/A / N/A
332
N/A
MIPS CQM
Specifications
Process
Adult Sinusitis:
Appropriate Choice of
Antibiotic: Amoxicillin
With or Without
Clavulanate Prescribed
for Patients with Acute
Bacterial Sinusitis
(Appropriate Use):
Percentage of patients aged
18 years and older with a
diagnosis of acute bacterial
sinusitis that were
prescribed amoxicillin, with
or without clavulanate, as a
first line antibiotic at the
time of diagnosis.
American Academy of
Otolaryngology –
Head and Neck
Surgery Foundation
§
!
(Outcome
)
N/A / N/A
338
CMS314
v2
eCQM
Specifications,
MIPS CQM
Specifications
Outcome
HIV Viral Suppression:
Percentage of patients,
regardless of age,
diagnosed with HIV prior
to or during the first 90
days of the performance
period, with an eligible
encounter in the first 240
days of the performance
period, whose last HIV
viral load test result was
less than 200 copies/mL
during the performance
period.
Health Resources and
Services
Administration
§
!
(Outcome)
0710 /
0710e
370
CMS159
v13
eCQM
Specifications,
MIPS CQM
Specifications
Outcome
Depression Remission at
Twelve Months:
The percentage of
adolescent patients 12 to 17
years of age and adult
patients 18 years of age or
older with major depression
or dysthymia who reached
remission 12 months (+/-
60 days) after an index
event date.
Minnesota
Community
Measurement
*
!
(Care
Coordinati
on)
N/A / N/A
374
CMS50v
13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Closing the Referral
Loop: Receipt of
Specialist Report:
Percentage of patients with
referrals, regardless of age,
for which the referring
clinician receives a report
from the clinician to whom
the patient was referred.
Centers for Medicare
& Medicaid Services
§
!
(Patient
Experienc
e)
N/A / N/A
377
CMS90v
14
eCQM
Specifications
Process
Functional Status
Assessments for Heart
Failure:
Percentage of patients 18
years of age and older with
heart failure who completed
initial and follow-up
patient-reported functional
status assessments.
Centers for Medicare
& Medicaid Services

B.19. Internal Medicine
PREVIOUSLY FINALIZED MEASURES IN THE INTERNAL MEDICINE SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
*
§
!
(Outcome
)
1879 /
N/A
383
N/A
MIPS CQM
Specifications
Intermediate
Outcome
Adherence to
Antipsychotic
Medications for
Individuals with
Schizophrenia:
Percentage of individuals at
least 18 years of age as of
the beginning of the
performance period with
schizophrenia or
schizoaffective disorder
who had at least two
prescriptions filled for any
antipsychotic medication
and who had a Proportion
of Days Covered (PDC) of
at least 0.8 for
antipsychotic medications
during the performance
period.
Centers for Medicare
& Medicaid Services
N/A / N/A
387
N/A
MIPS CQM
Specifications
Process
Annual Hepatitis C Virus
(HCV) Screening for
Patients who are Active
Injection Drug Users:
Percentage of patients,
regardless of age, who are
active injection drug users
who received screening for
HCV infection within the
12-month reporting period.
American
Gastroenterological
Association
!
(Outcome
)
N/A / N/A
398
N/A
MIPS CQM
Specifications
Outcome
Optimal Asthma Control:
Composite measure of the
percentage of pediatric and
adult patients whose asthma
is well-controlled as
demonstrated by one of
three age appropriate
patient reported outcome
tools and not at risk for
exacerbation.
Minnesota Community
Measurement
§
N/A / N/A
400
N/A
MIPS CQM
Specifications
Process
One-Time Screening for
Hepatitis C Virus (HCV)
and Treatment Initiation:
Percentage of patients age
>= 18 years have never
been tested for Hepatitis C
Virus (HCV) infection who
receive an HCV infection
test AND who have
treatment initiated within
three months or who are
referred to a clinician who
treats HCV infection within
one month if tested positive
for HCV.
American
Gastroenterological
Association
§
N/A / N/A
401
N/A
MIPS CQM
Specifications
Process
Hepatitis C: Screening for
Hepatocellular
Carcinoma (HCC) in
Patients with Cirrhosis:
Percentage of patients aged
18 years and older with a
diagnosis of chronic
Hepatitis C cirrhosis who
underwent imaging with
either ultrasound, contrast
enhanced CT or MRI for
hepatocellular carcinoma
(HCC) at least once within
the 12-month submission
period.
American
Gastroenterological
Association

B.19. Internal Medicine
PREVIOUSLY FINALIZED MEASURES IN THE INTERNAL MEDICINE SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
*
0053 /
N/A
418
N/A
Medicare Part B
Claims Measure
Specifications,
MIPS CQM
Specifications
Process
Osteoporosis
Management in Women
Who Had a Fracture:
The percentage of women
50–85 years of age who
suffered a fracture and who
had either a bone mineral
density (BMD) test or
prescription for a drug to
treat osteoporosis in the six
months after the fracture.
National Committee
for Quality Assurance
§
2152 /
N/A
431
N/A
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Unhealthy
Alcohol Use: Screening &
Brief Counseling:
Percentage of patients aged
18 years and older who
were screened for
unhealthy alcohol use using
a systematic screening
method at least once within
the last 12 months AND
who received brief
counseling if identified as
an unhealthy alcohol user.
National Committee
for Quality Assurance
§
N/A / N/A
438
CMS347
v8
eCQM
Specifications,
MIPS CQM
Specifications
Process
Statin Therapy for the
Prevention and
Treatment of
Cardiovascular Disease:
Percentage of the following
patients - all considered at
high risk of cardiovascular
events - who were
prescribed or were on statin
therapy during the
performance period:
• All patients who were
previously diagnosed with
or currently have a
diagnosis of clinical
atherosclerotic
cardiovascular disease
(ASCVD), including an
ASCVD procedure; OR
• Patients aged 20 to 75
years who have ever had a
low-density lipoprotein
cholesterol (LDL-C) level ≥
190 mg/dL or were
previously diagnosed with
or currently have an active
diagnosis of familial
hypercholesterolemia; OR
•Patients aged 40 to 75
years with a diagnosis of
diabetes; OR
• Patients aged 40 to 75
with a 10-year ASCVD risk
score of ≥ 20 percent.
Centers for Medicare
& Medicaid Services

B.19. Internal Medicine
PREVIOUSLY FINALIZED MEASURES IN THE INTERNAL MEDICINE SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
§
!
(Outcome
)
N/A / N/A
441
N/A
MIPS CQM
Specifications
Intermediate
Outcome
Ischemic Vascular
Disease (IVD) All or None
Outcome Measure
(Optimal Control):
The IVD All-or-None
Measure is one outcome
measure (optimal control).
The measure contains four
goals. All four goals within
a measure must be reached
in order to meet that
measure. The numerator for
the all-or-none measure
should be collected from
the organization’s total IVD
denominator. All-or-None
Outcome Measure (Optimal
Control) – Using the IVD
denominator optimal results
include:
• Most recent blood
pressure (BP) measurement
is less than or equal to
140/90 mm Hg -- AND
• Most recent tobacco status
is Tobacco Free -- AND
• Daily Aspirin or Other
Antiplatelet Unless
Contraindicated -- AND
• Statin Use Unless
Contraindicated.
Wisconsin
Collaborative for
Healthcare Quality
§
!
(Appropri
ate Use)
N/A / N/A
443
N/A
MIPS CQM
Specifications
Process
Non-Recommended
Cervical Cancer
Screening in Adolescent
Females:
The percentage of
adolescent females 16–20
years of age who were
screened unnecessarily for
cervical cancer.
Centers for Medicare
& Medicaid Services
!
(Opioid)
N/A / N/A
468
N/A
MIPS CQM
Specifications
Process
Continuity of
Pharmacotherapy for
Opioid Use Disorder
(OUD):
Percentage of adults aged
18 years and older with
pharmacotherapy for opioid
use disorder (OUD) who
have at least 180 days of
continuous treatment.
University of Southern
California
§
N/A / N/A
475
CMS349
v7
eCQM
Specifications
Process
HIV Screening:
Percentage of patients aged
15-65 at the start of the
measurement period who
were between 15-65 years
old when tested for Human
Immunodeficiency Virus
(HIV).
Centers for Disease
Control and Prevention

B.19. Internal Medicine
PREVIOUSLY FINALIZED MEASURES IN THE INTERNAL MEDICINE SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Outcome
)
N/A / N/A
476
CMS771
v6
eCQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Urinary Symptom Score
Change 6-12 Months
After Diagnosis of Benign
Prostatic Hyperplasia:
Percentage of patients with
an office visit within the
measurement period and
with a new diagnosis of
clinically significant
Benign Prostatic
Hyperplasia who have
International Prostate
Symptoms Score (IPSS) or
American Urological
Association (AUA)
Symptom Index (SI)
documented at time of
diagnosis and again 6-12
months later with an
improvement of 3 points.
Large Urology Group
Practice Association
and Oregon Urology
Institute
!
(Outcome
)
3568 /
N/A
483
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Person-Centered Primary
Care Measure Patient
Reported Outcome
Performance Measure
(PCPCM PRO-PM):
The Person-Centered
Primary Care Measure
Patient Reported Outcome
Performance Measure
(PCPCM PRO-PM) uses
the PCPCM Patient
Reported Outcome
Measure (PROM) a
comprehensive and
parsimonious set of 11
patient-reported items - to
assess the broad scope of
primary care. Unlike other
primary care measures, the
PCPCM PRO-PM
measures the high value
aspects of primary care
based on a patient’s
relationship with the
clinician or practice.
The American Board
of Family Medicine
!
(Equity)
N/A / N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social
Drivers of Health:
Percent of patients 18 years
and older screened for food
insecurity, housing
instability, transportation
needs, utility difficulties,
and interpersonal safety.
Centers for Medicare
& Medicaid Services
*
N/A / N/A
488
CMS951
v3
eCQM
Specifications,
MIPS CQM
Specifications
Process
Kidney Health
Evaluation:
Percentage of patients aged
18-75 years with a
diagnosis of diabetes who
received a kidney health
evaluation defined by an
Estimated Glomerular
Filtration Rate (eGFR)
AND Urine Albumin-
Creatinine Ratio (uACR)
within the measurement
period.
National Kidney
Foundation

B.19. Internal Medicine
PREVIOUSLY FINALIZED MEASURES IN THE INTERNAL MEDICINE SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
*
3620 /
N/A
493
N/A
MIPS CQM
Specifications
Process
Adult Immunization
Status:
Percentage of patients 19
years of age and older who
are up-to-date on
recommended routine
vaccines for influenza;
tetanus and diphtheria (Td)
or tetanus, diphtheria and
acellular pertussis (Tdap);
zoster; and pneumococcal.
National Committee
for Quality Assurance
!
(Outcome
)
3665 /
N/A
495
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Ambulatory Palliative
Care Patients’ Experience
of Feeling Heard and
Understood:
The percentage of top-box
responses among patients
aged 18 years and older
who had an ambulatory
palliative care visit and
report feeling heard and
understood by their
palliative care clinician and
team within 2 months (60
days) of the ambulatory
palliative care visit.
American Academy of
Hospice and Palliative
Medicine (AAHPM)
*
N/A / N/A
497
N/A
MIPS CQM
Specifications
Process
Preventive Care and
Wellness (composite):
Percentage of patients who
received age- and sex-
appropriate preventive
screenings and wellness
services. This measure is a
composite of seven
component measures that
are based on
recommendations for
preventive care by the U.S.
Preventive Services Task
Force (USPSTF), ACIP,
American Association of
Clinical Endocrinology
(AACE), and American
College of Endocrinology
(ACE).
Centers for Medicare
and Medicaid Services
*
!
(Equity)
N/A / N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to
Community Service
Provider:
Percent of patients 18 years
or older who screen
positive for one or more of
the following health related
social needs (HRSNs): food
insecurity, housing
instability, transportation
needs, utility help needs, or
interpersonal safety; and
had contact with a
Community Service
Provider (CSP) for at least
1 of their HRSNs within 60
days after screening.
OCHIN
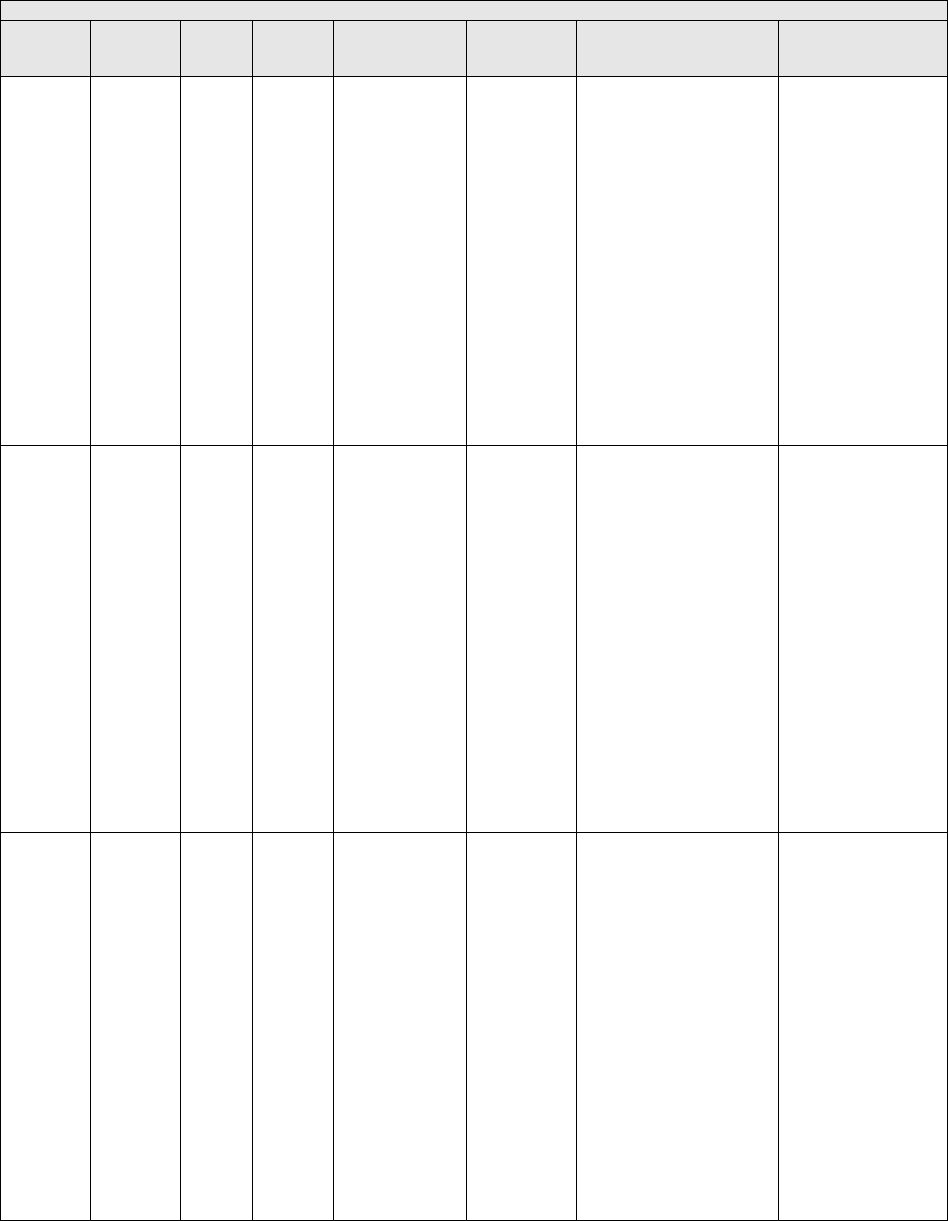
B.19. Internal Medicine
PREVIOUSLY FINALIZED MEASURES IN THE INTERNAL MEDICINE SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Outcome
)
N/A / N/A
502
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Improvement or
Maintenance of
Functioning for
Individuals with a Mental
and/or Substance Use
Disorder:
The percentage of patients
aged 18 and older with a
mental and/or substance use
disorder who demonstrated
improvement or
maintenance of functioning
based on results from the
12-item World Health
Organization Disability
Assessment Schedule
(WHODAS 2.0) or
Sheehan Disability Scale
(SDS) 30 to 180 days after
an index assessment.
American Psychiatric
Association
*
!
(Outcome
)
2483 /
N/A
503
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Gains in Patient
Activation Measure
(PAM®) Scores at 12
Months:
The Patient Activation
Measure® (PAM®) is a 10
– or 13 – item questionnaire
that assesses an individual´s
knowledge, skills and
confidence for managing
their health and health care.
The measure assesses
individuals on a 0-100 scale
that converts to one of four
levels of activation, from
low (1) to high (4). The
PAM® performance
measure (PAM®-PM) is
the change in score on the
PAM® from baseline to
follow-up measurement.
Insignia Health, LLC,
a wholly owned
subsidiary of Phreesia
!
(Safety)
N/A / N/A
504
N/A
MIPS CQM
Specifications
Process
Initiation, Review,
And/Or Update To
Suicide Safety Plan For
Individuals With Suicidal
Thoughts, Behavior, Or
Suicide Risk: Percentage
of adult aged 18 years and
older with suicidal ideation
or behavior symptoms
(based on results of a
standardized assessment
tool or screening tool) or
increased suicide risk
(based on the clinician's
evaluation or clinician-
rating tool) for whom a
suicide safety plan is
initiated, reviewed, and/or
updated in collaboration
between the patient and
their clinician.
American Psychiatric
Association

B.19. Internal Medicine
MEASURES PROPOSED FOR ADDITION TO THE INTERNAL MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
N/A /
N/A
TBD
N/A
MIPS
CQM
Specificati
ons
Process
Adult COVID-
19 Vaccination
Status:
Percentage of
patients aged 18
years and older
seen for a visit
during the
performance
period that are up
to date on their
COVID-19
vaccinations as
defined by CDC
recommendation
s on current
vaccination.
Centers
for
Medicare
&
Medicaid
Services
We are proposing to include this
measure in the Internal Medicine
specialty set as it would be
clinically relevant to this clinician
type. Widespread vaccination
against SARS-CoV-2, the virus
that causes COVID-19, is
critically important to stemming
the morbidity and mortality
caused by this disease.
95
Clinicians are uniquely positioned
to encourage uptake of COVID-
19 vaccination, and clinicians are
still a major driving force in
promoting patient vaccination.
The addition of this quality
measure in this specialty set
would help strengthen compliance
with recommended COVID-19
vaccination, leading to
improvement in the quality of
patient care and prevention of
disease for the general population.
This quality measure aligns with
clinical guidelines and the
evidence-based recommendations
of the (ACIP, where there is
general agreement about the
safety and efficacy of the
COVID-19 vaccine, preventing
costly and potentially harmful
hospitalizations.
96
Broadening
vaccination status awareness to
this clinician type is valuable as it
can help drive an increase in the
adult vaccination rates. The
COVID-19 vaccination included
within this measure would reduce
the prevalence of severe diseases
that may be associated with
hospitalization and decrease
overall health care costs. The
measure being added to this
specialty set would be contingent
on the inclusion of applicable
coding by the time of the CY
2025 PFS final rule. In the event
appropriate coding is not included
in the final specification, this
measure would not be finalized
for inclusion within this specialty
measure set. See Table A.5 of this
Appendix for rationale, including
clinical evidence supporting the
inclusion of this measure in
MIPS.
95
See footnote Ikeokwu et al., 2023 in Table B.1 of this Appendix.
96
See footnotes Fitzpatrick et al., 2022; Polack et al., 2020; and Graña et al., 2022 in Table A.5 of this Appendix.

B.19. Internal Medicine
PREVIOUSLY FINALIZED MEASURES PROPOSED FOR REMOVAL FROM THE INTERNAL MEDICINE
SPECIALTY SET
Note: In this proposed rule, we are proposing the removal of the following measure(s) below from this specific specialty measure set based
upon review of updates made to existing quality measure specifications, the proposed addition of new measures for inclusion in MIPS, and the
feedback provided by specialty societies.
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Removal
N/A /
3475e
472
CMS24
9v7
eCQM
Specification
s
Process
Appropriate Use of DXA
Scans in Women Under 65
Years Who Do Not Meet
the Risk Factor Profile for
Osteoporotic Fracture:
Percentage of female
patients 50 to 64 years of
age without select risk
factors for osteoporotic
fracture who received an
order for a dual-energy x-
ray absorptiometry (DXA)
scan during the
measurement period.
Centers for
Medicare &
Medicaid
Services
This measure is being
proposed for removal
beginning with the CY
2025 performance
period/2027 MIPS
payment year. See Table
Group C for rationale.

B.20. Interventional Radiology
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Interventional Radiology specialty set takes additional criteria into consideration, which includes, but is not limited to:
whether a measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may
reassess the appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set.
Measure tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be
added, and measures proposed for removal, as applicable. We request comment on the measures available in the proposed
Interventional Radiology specialty set.
B.20. Interventional Radiology
PREVIOUSLY FINALIZED MEASURES IN THE INTERVENTIONAL RADIOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Patient
Safety)
N/A /
N/A
145
N/A
Medicare
Part B
Claims
Measure
Specificatio
ns, MIPS
CQM
Specificatio
ns
Process
Radiology: Exposure Dose
Indices Reported for Procedures
Using Fluoroscopy:
Final reports for procedures using
fluoroscopy that document
radiation exposure indices.
American College of
Radiology
*!
(Care
Coordinatio
n)
N/A /
N/A
374
CMS
50v13
eCQM
Specificatio
ns, MIPS
CQM
Specificatio
ns
Process
Closing the Referral Loop:
Receipt of Specialist Report:
Percentage of patients with
referrals, regardless of age, for
which the referring clinician
receives a report from the clinician
to whom the patient was referred.
Centers for Medicare &
Medicaid Services
*!
(Outcome)
N/A /
N/A
413
N/A
MIPS
CQM
Specificatio
ns
Intermedi
ate
Outcome
Door to Puncture Time for
Endovascular Stroke Treatment:
Percentage of patients undergoing
endovascular stroke treatment who
have a door to puncture time of 90
minutes or less.
Society of
Interventional
Radiology
*!
(Outcome)
N/A /
N/A
420
N/A
MIPS
CQM
Specificatio
ns
Patient-
Reported
Outcome-
Based
Performan
ce
Measure
Varicose Vein Treatment with
Saphenous Ablation: Outcome
Survey:
Percentage of patients treated for
varicose veins (CEAP C2-S) who
are treated with saphenous ablation
(with or without adjunctive
tributary treatment) that report an
improvement on a disease specific
patient reported outcome survey
instrument after treatment.
Society of
Interventional
Radiology
N/A /
N/A
421
N/A
MIPS
CQM
Specificatio
ns
Process
Appropriate Assessment of
Retrievable Inferior Vena Cava
(IVC) Filters for Removal:
Percentage of patients in whom a
retrievable IVC filter is placed who,
within 3 months post-placement,
have a documented assessment for
the appropriateness of continued
filtration, device removal or the
inability to contact the patient with
at least two attempts.
Society of
Interventional
Radiology
!
(Patient
Safety)
N/A /
N/A
465
N/A
MIPS
CQM
Specificatio
ns
Process
Uterine Artery Embolization
Technique: Documentation of
Angiographic Endpoints and
Interrogation of Ovarian
Arteries:
The percentage of patients with
documentation of angiographic
endpoints of embolization AND the
documentation of embolization
strategies in the presence of
unilateral or bilateral absent uterine
arteries.
Society of
Interventional
Radiology

B.20. Interventional Radiology
PREVIOUSLY FINALIZED MEASURES IN THE INTERVENTIONAL RADIOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Equity)
N/A /
N/A
487
N/A
MIPS
CQM
Specificatio
ns
Process
Screening for Social Drivers of
Health:
Percent of patients 18 years and
older screened for food insecurity,
housing instability, transportation
needs, utility difficulties, and
interpersonal safety.
Centers for Medicare &
Medicaid Services
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS
CQM
Specificatio
ns
Process
Connection to Community
Service Provider:
Percent of patients 18 years or older
who screen positive for one or more
of the following health related
social needs (HRSNs): food
insecurity, housing instability,
transportation needs, utility help
needs, or interpersonal safety; and
had contact with a Community
Service Provider (CSP) for at least
1 of their HRSNs within 60 days
after screening.
OCHIN

B.20. Interventional Radiology
PREVIOUSLY FINALIZED MEASURES PROPOSED FOR REMOVAL FROM THE INTERVENTONAL RADIOLOGY
SPECIALTY SET
Note: In this proposed rule, we are proposing the removal of the following measure(s) below from this specific specialty measure set based
upon review of updates made to existing quality measure specifications, the proposed addition of new measures for inclusion in MIPS, and the
feedback provided by specialty societies.
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Removal
N/A /
N/A
409
N/A
MIPS CQM
Specification
s
Outcome
Clinical Outcome Post
Endovascular Stroke
Treatment: Percentage of
patients with a Modified
Rankin Score (mRS) score
of 0 to 2 at 90 days
following endovascular
stroke intervention.
Centers for
Medicare &
Medicaid
Services
This measure is being
proposed for removal
beginning with the CY
2025 performance
period/2027 MIPS
payment year. See Table
Group C for rationale.

B.21. Mental/Behavioral Health and Psychiatry
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Mental/Behavioral Health and Psychiatry specialty set takes additional criteria into consideration, which includes, but is not
limited to: whether a measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types.
We may reassess the appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the
specialty set. Measure tables in this set include previously finalized measures we are maintaining within the set, measures
proposed to be added, and measures proposed for removal, as applicable. We request comment on measures available in the
proposed Mental/Behavioral Health and Psychiatry specialty set that have proposed substantive changes in Table Group D of this
Appendix as indicated by an * in the Indicator column.
B.21. Mental/Behavioral Health and Psychiatry
PREVIOUSLY FINALIZED MEASURES IN THE MENTAL/BEHAVIORAL HEALTH AND PSYCHIATRY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measur
e
Type
Measure Title
and Description
Measure Steward
*
N/A /
N/A
009
CMS12
8v13
eCQM
Specifications
Process
Antidepressant Medication
Management:
Percentage of patients 18 years of
age and older who were treated
with antidepressant medication,
had a diagnosis of major
depression, and who remained on
an antidepressant medication
treatment. Two rates are reported.
a. Percentage of patients who
remained on an antidepressant
medication for at least 84 days
(12 weeks).
b. Percentage of patients who
remained on an antidepressant
medication for at least 180 days
(6 months).
National Committee
for Quality Assurance
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS68
v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for patients
aged 18 years and older for which
the eligible clinician attests to
documenting a list of current
medications using all immediate
resources available on the date of
the encounter.
Centers for Medicare
& Medicaid Services
§
N/A /
N/A
134
CMS2v
14
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and Screening:
Screening for Depression and
Follow-Up Plan:
Percentage of patients aged 12
years and older screened for
depression on the date of the
encounter or up to 14 days prior
to the date of the encounter using
an age-appropriate standardized
depression screening tool AND if
positive, a follow-up plan is
documented on the date of or up
to two days after the date of the
qualifying encounter.
Centers for Medicare
& Medicaid Services
*
!
(Patient
Safety)
N/A /
N/A
181
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Elder Maltreatment Screen and
Follow-Up Plan:
Percentage of patients aged 60
years and older with a
documented elder maltreatment
screen using an Elder
Maltreatment Screening tool on
the date of encounter AND a
documented follow-up plan on the
date of the positive screen.
Centers for Medicare
& Medicaid Services

B.21. Mental/Behavioral Health and Psychiatry
PREVIOUSLY FINALIZED MEASURES IN THE MENTAL/BEHAVIORAL HEALTH AND PSYCHIATRY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measur
e
Type
Measure Title
and Description
Measure Steward
§
N/A /
N/A
226
CMS13
8v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and Screening:
Tobacco Use: Screening and
Cessation Intervention:
Percentage of patients aged 12
years and older who were
screened for tobacco use one or
more times during the
measurement period AND who
received tobacco cessation
intervention during the
measurement period or in the six
months prior to the measurement
period if identified as a tobacco
user.
National Committee
for Quality Assurance
*
N/A /
2872e
281
CMS14
9v13
eCQM
Specifications
Process
Dementia: Cognitive
Assessment:
Percentage of patients, regardless
of age, with a diagnosis of
dementia for whom an assessment
of cognition is performed and the
results reviewed at least once
within a 12-month period.
American Academy of
Neurology
*
N/A /
N/A
282
N/A
MIPS CQM
Specifications
Process
Dementia: Functional Status
Assessment:
Percentage of patients with
dementia for whom an assessment
of functional status was
performed at least once in the last
12 months.
American Academy of
Neurology/ American
Psychiatric
Association
*
!
(Patient
Safety)
N/A /
N/A
286
N/A
MIPS CQM
Specifications
Process
Dementia: Safety Concern
Screening and Follow-Up for
Patients with Dementia:
Percentage of patients with
dementia or their caregiver(s) for
whom there was a documented
safety concerns screening in two
domains of risk: (1)
dangerousness to self or others
and (2) environmental risks; and
if safety concerns screening was
positive in the last 12 months,
there was documentation of
mitigation recommendations,
including but not limited to
referral to other resources.
American Psychiatric
Association/ American
Academy of
Neurology
*
!
(Care
Coordinatio
n)
N/A /
N/A
288
N/A
MIPS CQM
Specifications
Process
Dementia: Education and
Support of Caregivers for
Patients with Dementia:
Percentage of patients with
dementia whose caregiver(s) were
provided with education on
dementia disease management
and health behavior changes AND
were referred to additional
resources for support in the last
12 months.
American Academy of
Neurology / American
Psychiatric
Association

B.21. Mental/Behavioral Health and Psychiatry
PREVIOUSLY FINALIZED MEASURES IN THE MENTAL/BEHAVIORAL HEALTH AND PSYCHIATRY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measur
e
Type
Measure Title
and Description
Measure Steward
!
(Opioid)
N/A /
N/A
305
CMS13
7v13
eCQM
Specifications
Process
Initiation and Engagement of
Substance Use Disorder
Treatment:
Percentage of patients 13 years of
age and older with a new
substance use disorder (SUD)
episode who received the
following (Two rates are
reported):
a. Percentage of patients who
initiated treatment, including
either an intervention or
medication for the treatment of
SUD, within 14 days of the new
SUD episode.
b. Percentage of patients who
engaged in ongoing treatment,
including two additional
interventions or medication
treatment events for SUD, or one
long-acting medication event for
the treatment of SUD, within 34
days of the initiation.
National Committee
for Quality Assurance
*
N/A /
N/A
317
CMS22
v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and Screening:
Screening for High Blood
Pressure and Follow-Up
Documented:
Percentage of patient visits for
patients aged 18 years and older
seen during the measurement
period who were screened for
high blood pressure AND a
recommended follow-up plan is
documented, as indicated, if blood
pressure is elevated or
hypertensive.
Centers for Medicare
and Medicaid Services
§
N/A /
N/A
366
CMS13
6v14
eCQM
Specifications
Process
Follow-Up Care for Children
Prescribed ADHD Medication
(ADD):
Percentage of children 6-12 years
of age and newly prescribed a
medication for attention-
deficit/hyperactivity disorder
(ADHD) who had appropriate
follow-up care. Two rates are
reported.
(a) Percentage of children who
had one follow-up visit with a
practitioner with prescribing
authority during the 30-Day
Initiation Phase.
(b) Percentage of children who
remained on ADHD medication
for at least 210 days and who, in
addition to the visit in the
Initiation Phase, had at least two
additional follow-up visits with a
practitioner within 270 days (9
months) after the Initiation Phase
ended.
National Committee
for Quality Assurance
§
!
(Outcome)
0710 /
0710e
370
CMS15
9v13
eCQM
Specifications,
MIPS CQM
Specifications
Outcome
Depression Remission at Twelve
Months:
The percentage of adolescent
patients 12 to 17 years of age and
adult patients 18 years of age or
older with major depression or
dysthymia who reached remission
12 months (+/- 60 days) after an
index event date.
Minnesota Community
Measurement

B.21. Mental/Behavioral Health and Psychiatry
PREVIOUSLY FINALIZED MEASURES IN THE MENTAL/BEHAVIORAL HEALTH AND PSYCHIATRY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measur
e
Type
Measure Title
and Description
Measure Steward
!
(Patient
Safety)
N/A /
N/A
382
CMS17
7v13
eCQM
Specifications
Process
Child and Adolescent Major
Depressive Disorder (MDD):
Suicide Risk Assessment:
Percentage of patient visits for
those patients aged 6 through 16
years at the start of the
measurement period with a
diagnosis of major depressive
disorder (MDD) with an
assessment for suicide risk.
Mathematica
*
§
!
(Outcome)
1879 /
N/A
383
N/A
MIPS CQM
Specifications
Intermed
iate
Outcome
Adherence to Antipsychotic
Medications for Individuals
with Schizophrenia:
Percentage of individuals at least
18 years of age as of the
beginning of the performance
period with schizophrenia or
schizoaffective disorder who had
at least two prescriptions filled for
any antipsychotic medication and
who had a Proportion of Days
Covered (PDC) of at least 0.8 for
antipsychotic medications during
the performance period.
Centers for Medicare
& Medicaid Services
§
2152 /
N/A
431
N/A
MIPS CQM
Specifications
Process
Preventive Care and Screening:
Unhealthy Alcohol Use:
Screening & Brief Counseling:
Percentage of patients aged 18
years and older who were screened
for unhealthy alcohol use using a
systematic screening method at
least once within the last 12 months
AND who received brief
counseling if identified as an
unhealthy alcohol user.
National Committee
for Quality Assurance
!
(Opioid)
N/A /
N/A
468
N/A
MIPS CQM
Specifications
Process
Continuity of Pharmacotherapy
for Opioid Use Disorder (OUD):
Percentage of adults aged 18 years
and older with pharmacotherapy for
opioid use disorder (OUD) who
have at least 180 days of
continuous treatment.
University of Southern
California
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers of
Health:
Percent of patients 18 years and
older screened for food insecurity,
housing instability, transportation
needs, utility difficulties, and
interpersonal safety.
Centers for Medicare
& Medicaid Services
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years or
older who screen positive for one
or more of the following health
related social needs (HRSNs):
food insecurity, housing
instability, transportation needs,
utility help needs, or interpersonal
safety; and had contact with a
Community Service Provider
(CSP) for at least 1 of their
HRSNs within 60 days after
screening.
OCHIN

B.21. Mental/Behavioral Health and Psychiatry
PREVIOUSLY FINALIZED MEASURES IN THE MENTAL/BEHAVIORAL HEALTH AND PSYCHIATRY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measur
e
Type
Measure Title
and Description
Measure Steward
!
(Outcome)
N/A /
N/A
502
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performan
ce
Measure
Improvement or Maintenance
of Functioning for Individuals
with a Mental and/or Substance
Use Disorder:
The percentage of patients aged
18 and older with a mental and/or
substance use disorder who
demonstrated improvement or
maintenance of functioning based
on results from the 12-item World
Health Organization Disability
Assessment Schedule (WHODAS
2.0) or Sheehan Disability Scale
(SDS) 30 to 180 days after an
index assessment.
American Psychiatric
Association
!
(Safety)
N/A /
N/A
504
N/A
MIPS CQM
Specifications
Process
Initiation, Review, And/Or
Update To Suicide Safety Plan
For Individuals With Suicidal
Thoughts, Behavior, Or Suicide
Risk:
Percentage of adult aged 18 years
and older with suicidal ideation or
behavior symptoms (based on
results of a standardized
assessment tool or screening tool)
or increased suicide risk (based on
the clinician's evaluation or
clinician-rating tool) for whom a
suicide safety plan is initiated,
reviewed, and/or updated in
collaboration between the patient
and their clinician.
American Psychiatric
Association
*
!
(Outcome)
N/A /
N/A
505
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performan
ce
Measure
Reduction in Suicidal Ideation
or Behavior Symptoms:
The percentage of patients aged
18 and older with a mental and/or
substance use disorder AND
suicidal thoughts, behaviors or
risk symptoms who demonstrated
a reduction in suicidal ideation
and/or behavior symptoms based
on results from the Columbia-
Suicide Severity Rating Scale (C-
SSRS) ‘Screen Version’ or ‘Since
Last Visit’ within 120 days after
an index assessment.
American Psychiatric
Association
B.22. Nephrology
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Nephrology specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a measure
reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Nephrology
specialty set.

B.22. Nephrology
PREVIOUSLY FINALIZED MEASURES IN THE NEPHROLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
§
!
(Outcome)
0059 /
N/A
001
CMS12
2v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Intermediate
Outcome
Diabetes: Hemoglobin A1c
(HbA1c) Poor Control
(>9%):
Percentage of patients 18-75
years of age with diabetes who
had hemoglobin A1c > 9.0%
during the measurement
period.
National Committee
for Quality
Assurance
*
!
(Care
Coordination
)
0326 /
N/A
047
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Advance Care Plan:
Percentage of patients aged 65
years and older who have an
advance care plan or surrogate
decision maker documented in
the medical record or
documentation in the medical
record that an advance care
plan was discussed but the
patient did not wish or was not
able to name a surrogate
decision maker or provide an
advance care plan.
National Committee
for Quality
Assurance
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS68
v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for patients
aged 18 years and older for
which the eligible clinician
attests to documenting a list of
current medications using all
immediate resources available
on the date of the encounter.
Centers for Medicare
& Medicaid Services
*
§
!
(Care
Coordination
)
N/A /
N/A
182
N/A
MIPS CQM
Specifications
Process
Functional Outcome
Assessment:
Percentage of visits for patients
aged 18 years and older with
documentation of a current
functional outcome assessment
using a standardized functional
outcome assessment tool on the
date of the encounter AND
documentation of a care plan
based on identified functional
outcome deficiencies within
two days of the date of the
identified deficiencies.
Centers for Medicare
& Medicaid Services
§
N/A /
N/A
226
CMS13
8v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Tobacco Use:
Screening and Cessation
Intervention:
Percentage of patients aged 12
years and older who were
screened for tobacco use one or
more times during the
measurement period AND who
received tobacco cessation
intervention during the
measurement period or in the
six months prior to the
measurement period if
identified as a tobacco user.
National Committee
for Quality
Assurance

B.22. Nephrology
PREVIOUSLY FINALIZED MEASURES IN THE NEPHROLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
N/A /
N/A
317
CMS22
v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Screening for
High Blood Pressure and
Follow-Up Documented:
Percentage of patient visits for
patients aged 18 years and
older seen during the
measurement period who were
screened for high blood
pressure AND a recommended
follow-up plan is documented,
as indicated, if blood pressure
is elevated or hypertensive.
Centers for Medicare
& Medicaid Services
!
(Patient
Safety)
0101 /
N/A
318
CMS13
9v13
eCQM
Specifications
Process
Falls: Screening for Future
Fall Risk:
Percentage of patients 65 years
of age and older who were
screened for future fall risk
during the measurement
period.
National Committee
for Quality
Assurance
§
N/A /
N/A
400
N/A
MIPS CQM
Specifications
Process
One-Time Screening for
Hepatitis C Virus (HCV) and
Treatment Initiation:
Percentage of patients age >=
18 years have never been tested
for Hepatitis C Virus (HCV)
infection who receive an HCV
infection test AND who have
treatment initiated within three
months or who are referred to a
clinician who treats HCV
infection within one month if
tested positive for HCV.
American
Gastroenterological
Association
!
(Outcome)
N/A/
N/A
482
N/A
MIPS CQM
Specifications
Intermediate
Outcome
Hemodialysis Vascular
Access: Practitioner Level
Long-term Catheter Rate:
Percentage of adult
hemodialysis (HD) patient-
months using a catheter
continuously for three months
or longer for vascular access
attributable to an individual
practitioner or group practice.
Centers for Medicare
& Medicaid Services
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers
of Health:
Percent of patients 18 years
and older screened for food
insecurity, housing instability,
transportation needs, utility
difficulties, and interpersonal
safety.
Centers for Medicare
& Medicaid Services
*
N/A /
N/A
488
CMS95
1v3
eCQM
Specifications,
MIPS CQM
Specifications
Process
Kidney Health Evaluation:
Percentage of patients aged 18-
75 years with a diagnosis of
diabetes who received a kidney
health evaluation defined by an
Estimated Glomerular
Filtration Rate (eGFR) AND
Urine Albumin-Creatinine
Ratio (uACR) within the
measurement period.
National Kidney
Foundation

B.22. Nephrology
PREVIOUSLY FINALIZED MEASURES IN THE NEPHROLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
1662/
N/A
489
N/A
MIPS CQM
Specifications
Process
Adult Kidney Disease:
Angiotensin Converting
Enzyme (ACE) Inhibitor or
Angiotensin Receptor
Blocker (ARB) Therapy:
Percentage of patients aged 18
years and older with a
diagnosis of chronic kidney
disease (CKD) (Stages 1-5, not
receiving Renal Replacement
Therapy (RRT)) and
proteinuria who were
prescribed ACE inhibitor or
ARB therapy within a 12-
month period.
Renal Physicians
Association
*
3620 /
N/A
493
N/A
MIPS CQM
Specifications
Process
Adult Immunization Status:
Percentage of patients 19 years
of age and older who are up-to-
date on recommended routine
vaccines for influenza; tetanus
and diphtheria (Td) or tetanus,
diphtheria and acellular
pertussis (Tdap); zoster; and
pneumococcal.
National Committee
for Quality
Assurance
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years or
older who screen positive for
one or more of the following
health related social needs
(HRSNs): food insecurity,
housing instability,
transportation needs, utility
help needs, or interpersonal
safety; and had contact with a
Community Service Provider
(CSP) for at least 1 of their
HRSNs within 60 days after
screening.
OCHIN
*
!
(Outcome)
2483 /
N/A
503
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performanc
e Measure
Gains in Patient Activation
Measure (PAM®) Scores at
12 Months:
The Patient Activation
Measure® (PAM®) is a 10 –
or 13 – item questionnaire that
assesses an individual´s
knowledge, skills and
confidence for managing their
health and health care. The
measure assesses individuals
on a 0-100 scale that converts
to one of four levels of
activation, from low (1) to high
(4). The PAM® performance
measure (PAM®-PM) is the
change in score on the PAM®
from baseline to follow-up
measurement.
Insignia Health,
LLC, a wholly
owned subsidiary of
Phreesia

B.22. Nephrology
MEASURES PROPOSED FOR ADDITION TO THE NEPHROLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
!
(Outcome
)
3665 /
N/A
495
N/A
MIPS
CQM
Specificati
ons
Patient-
Reported
Outcome-
Based
Performanc
e Measure
Ambulatory
Palliative Care
Patients’
Experience of
Feeling Heard
and
Understood:
The percentage
of top-box
responses among
patients aged 18
years and older
who had an
ambulatory
palliative care
visit and report
feeling heard and
understood by
their palliative
care clinician and
team within 2
months (60 days)
of the
ambulatory
palliative care
visit.
American
Academy
of Hospice
and
Palliative
Medicine
(AAHPM)
We are proposing to include this
measure in the Nephrology
specialty set as it would be
clinically relevant to this
clinician type. This PRO-PM
would help to fill a gap for
patients receiving palliative care
by capturing the patient’s voice
and experience of care by
assessing communication and
shared decision making with the
clinician. This is an important
patient-centered measure that
helps patients feel heard and
understood which can
effectively improve the quality
of care received and outcomes
for patients in palliative care.
Allowing patients to feel heard
and understood adds an
important dimension to the care
planning for this unique patient
population commonly cared for
by clinicians in this specialty. As
more patients are living longer
with multiple comorbidities,
especially true for the advanced
heart disease patient population,
early emergence of palliative
care into the overall care of
cardiac patients can notably
improve their quality of life,
patient satisfaction, and
reduction in symptoms.
97
This
measure is predicated on
existing guidelines and
conceptual models
98
and can
facilitate and improve effective
patient-clinician communication
that engenders trust,
acknowledgement, and a whole-
person orientation to the care
that is provided. Through the
benefits of enhanced patient-
provider communication, this
measure would improve the
quality of care received and
outcomes for patients receiving
palliative care. The measure
being added to this specialty set
would be contingent on the
inclusion of applicable coding
by the time of the CY 2025 PFS
final rule.
N/A /
N/A
TBD
N/A
MIPS
CQM
Specificati
ons
Process
Adult COVID-
19 Vaccination
Status:
Percentage of
patients aged 18
years and older
seen for a visit
during the
performance
period that are up
Centers for
Medicare
&
Medicaid
Services
We are proposing to include this
measure in the Nephrology
specialty set as it would be
clinically relevant to this
clinician type. Widespread
vaccination against SARS-CoV-
2, the virus that causes COVID-
19, is critically important to
stemming the morbidity and
mortality caused by this
97
See footnote Kilic et al., 2020 in Table B.4a of this Appendix.
98
See footnote National Consensus Project for Quality Palliative Care, 2018 in Table B.4a of this Appendix.

B.22. Nephrology
MEASURES PROPOSED FOR ADDITION TO THE NEPHROLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
to date on their
COVID-19
vaccinations as
defined by CDC
recommendation
s on current
vaccination.
disease.
99
Clinicians are
uniquely positioned to
encourage uptake of COVID-19
vaccination, and clinicians are
still a major driving force in
promoting patient vaccination.
The addition of this quality
measure in this specialty set
would help strengthen
compliance with recommended
COVID-19 vaccination, leading
to improvement in the quality of
patient care and prevention of
disease for the general
population. This quality measure
aligns with clinical guidelines
and the evidence-based
recommendations of the ACIP,
where there is general agreement
about the safety and efficacy of
the COVID-19 vaccine,
preventing costly and potentially
harmful hospitalizations.
100
Broadening vaccination status
awareness to this clinician type
is valuable as it can help drive
an increase in the adult
vaccination rates. The COVID-
19 vaccination included within
this measure would reduce the
prevalence of severe diseases
that may be associated with
hospitalization and decrease
overall health care costs. The
measure being added to this
specialty set would be
contingent on the inclusion of
applicable coding by the time of
the CY 2025 PFS final rule. In
the event appropriate coding is
not included in the final
specification, this measure
would not be finalized for
inclusion within this specialty
measure set. See Table A.5 of
this Appendix for rationale,
including clinical evidence
supporting the inclusion of this
measure in MIPS.
99
See footnote Ikeokwu et al., 2023 in Table B.1 of this Appendix.
100
See footnotes Fitzpatrick et al., 2022; Polack et al., 2020; and Graña et al., 2022 in Table A.5 of this Appendix.

B.22. Nephrology
MEASURES PROPOSED FOR ADDITION TO THE NEPHROLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
N/A /
N/A
TBD
N/A
MIPS
CQM
Specificati
ons
Process
First Year
Standardized
Waitlist Ratio
(FYSWR):
The number of
incident (newly
initiated on
dialysis) patients
in a practitioner
(inclusive of
physicians and
advanced
practice
providers) group
who are under
the age of 75,
and were listed
on the kidney or
kidney-pancreas
transplant
waitlist or
received a living
donor transplant
within the first
year of initiating
dialysis. The
measure is
calculated to
compare the
observed number
of waitlist events
in a practitioner
group to its
expected number
of waitlist events.
The measure
uses the expected
waitlist events
calculated from a
Cox model,
adjusted for age,
patient
comorbidities,
and other risk
factors at
incidence of
dialysis.
Centers for
Medicare
&
Medicaid
Services
We are proposing to include this
measure in the Nephrology
specialty set as it would be
clinically relevant to this
clinician type. The measure’s
intended objective consists of
improving the overall health of
patients on dialysis, with
nephrologists at the forefront of
caring for this patient
population. Clinicians within
this specialty are responsible for
the education of patients about
the option of transplantation,
referral of patients to a
transplant center for evaluation,
completion of the evaluation
process, and optimizing the
health of the patient while on
dialysis. All clinicians should be
involved and actively work
towards providing patients with
high quality care including
ensuring placement on the
transplant list as quickly as
possible. The measure being
added to this specialty set would
be contingent on the inclusion of
applicable coding by the time of
the CY 2025 PFS final rule. See
Table A.7 of this Appendix for
rationale, including clinical
evidence supporting the
inclusion of this measure in
MIPS.

B.22. Nephrology
MEASURES PROPOSED FOR ADDITION TO THE NEPHROLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
N/A /
N/A
TBD
N/A
MIPS
CQM
Specificati
ons
Process
Percentage of
Prevalent
Patients
Waitlisted
(PPPW) and
Percentage of
Prevalent
Patients
Waitlisted in
Active Status
(aPPPW):
The percentage
of patients in
each dialysis
practitioner
group practice
who were on the
kidney or
kidney-pancreas
transplant
waitlist (all
patients or
patients in active
status). Results
are averaged
across patients
prevalent on the
last day of each
month during the
reporting year.
The measure is a
directly
standardized
percentage,
which is adjusted
for covariates
(e.g., age and
risk factors).
Centers for
Medicare
&
Medicaid
Services
We are proposing to include this
measure in the Nephrology
specialty set as would be
clinically relevant to this
clinician type. The maintenance
of end stage renal disease
patients on active status on the
waitlist is additionally important
given demonstrated disparities
and positive association with
subsequent transplantation.
These practices are important for
nephrologists who are at the
forefront of caring for this
patient population. This is an
important area to which dialysis
practitioners can contribute
through ensuring patients remain
healthy and complete any
ongoing testing activities
required to remain active on the
waitlist. The measure being
added to this specialty set would
be contingent on the inclusion of
applicable coding by the time of
the CY 2025 PFS final rule. See
Table A.8 of this Appendix for
rationale, including clinical
evidence supporting the
inclusion of this measure in
MIPS.

B.23. Neurology
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Neurology specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a measure
reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Neurology
specialty set that have proposed substantive changes in Table Group D of this Appendix as indicated by an * in the Indicator
column.
B.23. Neurology
PREVIOUSLY FINALIZED MEASURES IN THE NEUROLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Care
Coordination)
0326 /
N/A
047
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Advance Care Plan:
Percentage of patients aged 65
years and older who have an
advance care plan or surrogate
decision maker documented in
the medical record or
documentation in the medical
record that an advance care plan
was discussed but the patient did
not wish or was not able to name
a surrogate decision maker or
provide an advance care plan.
National
Committee for
Quality Assurance
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS68
v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for patients
aged 18 years and older for
which the eligible clinician
attests to documenting a list of
current medications using all
immediate resources available on
the date of the encounter.
Centers for
Medicare &
Medicaid Services
§
N/A /
N/A
134
CMS2v
14
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Screening for
Depression and Follow-Up
Plan:
Percentage of patients aged 12
years and older screened for
depression on the date of the
encounter or up to 14 days prior
to the date of the encounter using
an age-appropriate standardized
depression screening tool AND if
positive, a follow-up plan is
documented on the date of or up
to two days after the date of the
qualifying encounter.
Centers for
Medicare &
Medicaid Services
*
!
(Care
Coordination)
0101 /
N/A
155
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Falls: Plan of Care:
Percentage of patients aged 65
years and older with a history of
falls who had a plan of care for
falls documented within 12
months.
National
Committee for
Quality Assurance
*
!
(Patient
Safety)
N/A /
N/A
181
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Elder Maltreatment Screen
and Follow-Up Plan:
Percentage of patients aged 60
years and older with a
documented elder maltreatment
screen using an Elder
Maltreatment Screening tool on
the date of encounter AND a
documented follow-up plan on
the date of the positive screen.
Centers for
Medicare &
Medicaid Services

B.23. Neurology
PREVIOUSLY FINALIZED MEASURES IN THE NEUROLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
§
N/A /
N/A
226
CMS13
8v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Tobacco Use:
Screening and Cessation
Intervention:
Percentage of patients aged 12
years and older who were
screened for tobacco use one or
more times during the
measurement period AND who
received tobacco cessation
intervention during the
measurement period or in the six
months prior to the measurement
period if identified as a tobacco
user.
National
Committee for
Quality Assurance
N/A /
N/A
268
N/A
MIPS CQM
Specifications
Process
Epilepsy: Counseling for
Women of Childbearing
Potential with Epilepsy:
Percentage of all patients of
childbearing potential (12 years
and older) diagnosed with
epilepsy who were counseled at
least once a year about how
epilepsy and its treatment may
affect contraception and
pregnancy.
American
Academy of
Neurology
*
N/A /
N/A
277
N/A
MIPS CQM
Specifications
Process
Sleep Apnea: Severity
Assessment at Initial Diagnosis:
Percentage of patients aged 18
years and older with a diagnosis
of obstructive sleep apnea who
had an apnea hypopnea index
(AHI), a respiratory disturbance
index (RDI), or a respiratory
event index (REI) documented or
measured within 2 months of
initial evaluation for suspected
obstructive sleep apnea.
American
Academy of Sleep
Medicine
N/A /
N/A
279
N/A
MIPS CQM
Specifications
Process
Sleep Apnea: Assessment of
Adherence to Obstructive Sleep
Apnea (OSA) Therapy:
Percentage of patients aged 18
years and older with a diagnosis
of obstructive sleep apnea (OSA)
that were prescribed an evidence-
based therapy that had
documentation that adherence to
therapy was assessed at least
annually through an objective
informatics system or through
self-reporting (if objective
reporting is not available).
American
Academy of Sleep
Medicine
*
N/A /
2872e
281
CMS14
9v13
eCQM
Specifications
Process
Dementia: Cognitive
Assessment:
Percentage of patients, regardless
of age, with a diagnosis of
dementia for whom an
assessment of cognition is
performed and the results
reviewed at least once within a
12-month period.
American
Academy of
Neurology
*
N/A /
N/A
282
N/A
MIPS CQM
Specifications
Process
Dementia: Functional Status
Assessment:
Percentage of patients with
dementia for whom an
assessment of functional status
was performed at least once in
the last 12 months.
American
Academy of
Neurology/
American
Psychiatric
Association

B.23. Neurology
PREVIOUSLY FINALIZED MEASURES IN THE NEUROLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Patient
Safety)
N/A /
N/A
286
N/A
MIPS CQM
Specifications
Process
Dementia: Safety Concern
Screening and Follow-Up for
Patients with Dementia:
Percentage of patients with
dementia or their caregiver(s) for
whom there was a documented
safety concerns screening in two
domains of risk: (1)
dangerousness to self or others
and (2) environmental risks; and
if safety concerns screening was
positive in the last 12 months,
there was documentation of
mitigation recommendations,
including but not limited to
referral to other resources.
American
Psychiatric
Association/
American
Academy of
Neurology
*
!
(Care
Coordination)
N/A /
N/A
288
N/A
MIPS CQM
Specifications
Process
Dementia: Education and
Support of Caregivers for
Patients with Dementia:
Percentage of patients with
dementia whose caregiver(s)
were provided with education on
dementia disease management
and health behavior changes
AND were referred to additional
resources for support in the last
12 months.
American
Academy of
Neurology /
American
Psychiatric
Association
*
N/A /
N/A
290
N/A
MIPS CQM
Specifications
Process
Assessment of Mood Disorders
and Psychosis for Patients with
Parkinson’s Disease:
Percentage of all patients with a
diagnosis of Parkinson’s Disease
(PD) who were assessed for
depression, anxiety, apathy, AND
psychosis once during the
measurement period.
American
Academy of
Neurology
*
N/A /
N/A
291
N/A
MIPS CQM
Specifications
Process
Assessment of Cognitive
Impairment or Dysfunction for
Patients with Parkinson’s
Disease:
Percentage of all patients with a
diagnosis of Parkinson’s Disease
(PD) who were assessed for
cognitive impairment or
dysfunction once during the
measurement period.
American
Academy of
Neurology
*
!
(Care
Coordination)
N/A /
N/A
293
N/A
MIPS CQM
Specifications
Process
Rehabilitative Therapy
Referral for Patients with
Parkinson’s Disease:
Percentage of all patients with a
diagnosis of Parkinson’s Disease
(PD) who were referred to
physical, occupational, speech, or
recreational therapy once during
the measurement period.
American
Academy of
Neurology
*
N/A /
N/A
317
CMS22
v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Screening for High
Blood Pressure and Follow-Up
Documented:
Percentage of patient visits for
patients aged 18 years and older
seen during the measurement
period who were screened for
high blood pressure AND a
recommended follow-up plan is
documented, as indicated, if
blood pressure is elevated or
hypertensive.
Centers for
Medicare and
Medicaid Services

B.23. Neurology
PREVIOUSLY FINALIZED MEASURES IN THE NEUROLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Care
Coordination)
N/A /
N/A
374
CMS50
v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Closing the Referral Loop:
Receipt of Specialist Report:
Percentage of patients with
referrals, regardless of age, for
which the referring clinician
receives a report from the
clinician to whom the patient was
referred.
Centers for
Medicare &
Medicaid Services
*
!
(Patient
Experience)
N/A /
N/A
386
N/A
MIPS CQM
Specifications
Process
Amyotrophic Lateral Sclerosis
(ALS) Patient Care
Preferences:
Percentage of patients diagnosed
with Amyotrophic Lateral
Sclerosis (ALS) who were
offered assistance in planning for
end of life issues (e.g., advance
directives, invasive ventilation,
lawful physician-hastened death,
or hospice) or whose existing end
of life plan was reviewed or
updated at least once annually or
more frequency as clinically
indicated (i.e., rapid progression).
American
Academy of
Neurology
!
(Efficiency)
N/A /
N/A
419
N/A
MIPS CQM
Specifications
Process
Overuse of Imaging for the
Evaluation of Primary
Headache:
Percentage of patients for whom
imaging of the head (CT or MRI)
is obtained for the evaluation of
primary headache when clinical
indications are not present.
American
Academy of
Neurology
§
2152 /
N/A
431
N/A
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Unhealthy Alcohol
Use: Screening & Brief
Counseling:
Percentage of patients aged 18
years and older who were
screened for unhealthy alcohol
use using a systematic screening
method at least once within the
last 12 months AND who
received brief counseling if
identified as an unhealthy alcohol
user.
National
Committee for
Quality Assurance
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers of
Health:
Percent of patients 18 years and
older screened for food
insecurity, housing instability,
transportation needs, utility
difficulties, and interpersonal
safety.
Centers for
Medicare &
Medicaid Services
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years or
older who screen positive for one
or more of the following health
related social needs (HRSNs):
food insecurity, housing
instability, transportation needs,
utility help needs, or
interpersonal safety; and had
contact with a Community
Service Provider (CSP) for at
least 1 of their HRSNs within 60
days after screening.
OCHIN

B.23. Neurology
PREVIOUSLY FINALIZED MEASURES IN THE NEUROLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Outcome)
2483 /
N/A
503
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performan
ce
Measure
Gains in Patient Activation
Measure (PAM®) Scores at 12
Months:
The Patient Activation Measure®
(PAM®) is a 10 – or 13 – item
questionnaire that assesses an
individual´s knowledge, skills
and confidence for managing
their health and health care. The
measure assesses individuals on a
0-100 scale that converts to one
of four levels of activation, from
low (1) to high (4). The PAM®
performance measure (PAM®-
PM) is the change in score on the
PAM® from baseline to follow-
up measurement.
Insignia Health,
LLC, a wholly
owned subsidiary
of Phreesia

B.24. Neurosurgical
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Neurosurgical specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a
measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Neurosurgical
specialty set.
B.24. Neurosurgical
PREVIOUSLY FINALIZED MEASURES IN THE NEUROSURGICAL SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS68
v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for patients
aged 18 years and older for
which the eligible clinician
attests to documenting a list of
current medications using all
immediate resources available
on the date of the encounter.
Centers for Medicare
& Medicaid Services
§
N/A /
N/A
187
N/A
MIPS CQM
Specifications
Process
Stroke and Stroke
Rehabilitation:
Thrombolytic Therapy:
Percentage of patients aged 18
years and older with a
diagnosis of acute ischemic
stroke who arrive at the
hospital within 3.5 hours of
time last known well and for
whom IV thrombolytic therapy
was initiated within 4.5 hours
of time last known well.
American Heart
Association
§
N/A /
N/A
226
CMS13
8v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Tobacco Use:
Screening and Cessation
Intervention:
Percentage of patients aged 12
years and older who were
screened for tobacco use one or
more times during the
measurement period AND who
received tobacco cessation
intervention during the
measurement period or in the
six months prior to the
measurement period if
identified as a tobacco user.
National Committee
for Quality Assurance
*
!
(Outcome)
N/A /
N/A
344
N/A
MIPS CQM
Specifications
Outcome
Rate of Carotid Artery
Stenting (CAS) for
Asymptomatic Patients,
Without Major
Complications (Discharged to
Home by Post-Operative Day
#2):
Percent of asymptomatic
patients undergoing CAS who
are discharged to home no later
than post-operative day #2.
Society for Vascular
Surgeons
*
!
(Outcome)
N/A /
N/A
413
N/A
MIPS CQM
Specifications
Intermediat
e Outcome
Door to Puncture Time for
Endovascular Stroke
Treatment:
Percentage of patients
undergoing endovascular stroke
treatment who have a door to
puncture time of 90 minutes or
less.
Society of
Interventional
Radiology
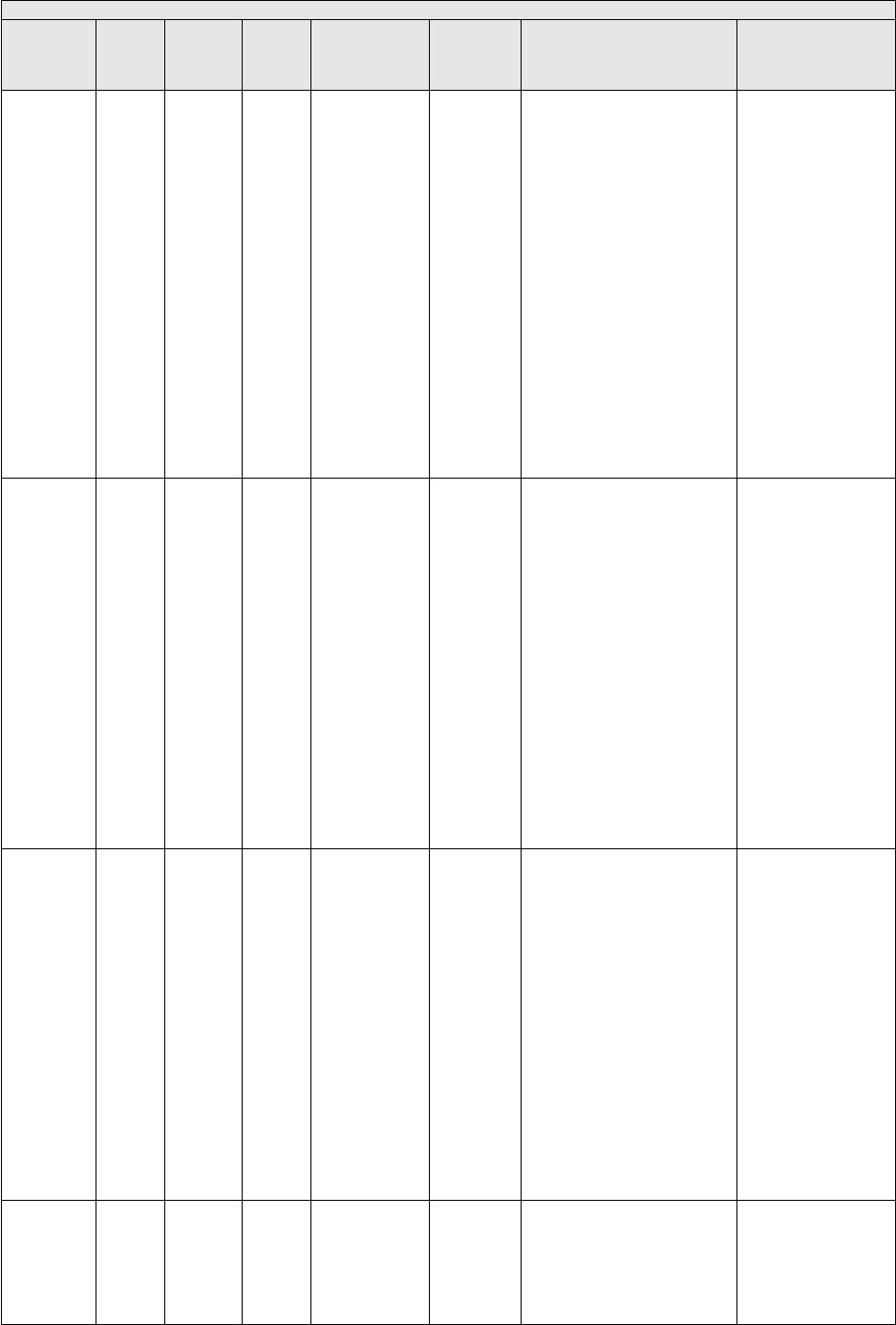
B.24. Neurosurgical
PREVIOUSLY FINALIZED MEASURES IN THE NEUROSURGICAL SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
§
!
(Outcome)
N/A /
N/A
459
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performan
ce Measure
Back Pain After Lumbar
Surgery:
For patients 18 years of age or
older who had a lumbar
discectomy/laminectomy or
fusion procedure, back pain is
rated by the patients as less
than or equal to 3.0 OR an
improvement of 5.0 points or
greater on the Visual Analog
Scale (VAS) Pain scale or a
numeric pain scale at three
months (6 to 20 weeks)
postoperatively for
discectomy/laminectomy or at
one year (9 to 15 months)
postoperatively for lumbar
fusion patients. Rates are
stratified by procedure type;
lumbar
discectomy/laminectomy or
fusion procedure.
Minnesota
Community
Measurement
§
!
(Outcome)
N/A /
N/A
461
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performan
ce Measure
Leg Pain After Lumbar
Surgery:
For patients 18 years of age or
older who had a lumbar
discectomy/laminectomy or
fusion procedure, leg pain is
rated by the patient as less than
or equal to 3.0 OR an
improvement of 5.0 points or
greater on the Visual Analog
Scale (VAS) Pain scale or a
numeric pain scale at three
months (6 to 20 weeks) for
discectomy/laminectomy or at
one year (9 to 15 months)
postoperatively for lumbar
fusion patients. Rates are
stratified by procedure type;
lumbar
discectomy/laminectomy or
fusion procedure.
Minnesota
Community
Measurement
§
!
(Outcome)
N/A /
N/A
471
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performan
ce Measure
Functional Status After
Lumbar Surgery:
For patients age 18 and older
who had lumbar
discectomy/laminectomy or
fusion procedure, functional
status is rated by the patient as
less than or equal to 22 OR an
improvement of 30 points or
greater on the Oswestry
Disability Index (ODI version
2.1a) at three months (6 to 20
weeks) postoperatively for
discectomy/laminectomy or at
one year (9 to 15 months)
postoperatively for lumbar
fusion patients. Rates are
stratified by procedure type;
lumbar discectomy or fusion
procedure.
Minnesota
Community
Measurement
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers
of Health:
Percent of patients 18 years and
older screened for food insecurity,
housing instability, transportation
needs, utility difficulties, and
interpersonal safety.
Centers for Medicare
& Medicaid Services

B.24. Neurosurgical
PREVIOUSLY FINALIZED MEASURES IN THE NEUROSURGICAL SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years or
older who screen positive for
one or more of the following
health related social needs
(HRSNs): food insecurity,
housing instability,
transportation needs, utility
help needs, or interpersonal
safety; and had contact with a
Community Service Provider
(CSP) for at least 1 of their
HRSNs within 60 days after
screening.
OCHIN

B.24. Neurosurgical
PREVIOUSLY FINALIZED MEASURES PROPOSED FOR REMOVAL FROM THE NEUROSURGICAL SPECIALTY
SET
Note: In this proposed rule, we are proposing the removal of the following measure(s) below from this specific specialty measure set based
upon review of updates made to existing quality measure specifications, the proposed addition of new measures for inclusion in MIPS, and the
feedback provided by specialty societies.
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Removal
NA /
NA
260
N/A
MIPS CQM
Specification
s
Outcome
Rate of Carotid
Endarterectomy (CEA) for
Asymptomatic Patients,
without Major
Complications (Discharged
to Home by Post-
Operative Day #2):
Percent of asymptomatic
patients undergoing Carotid
Endarterectomy (CEA) who
are discharged to home no
later than post-operative day
#2.
Society for
Vascular
Surgeons
This measure is being
proposed for removal
beginning with the CY
2025 performance
period/2027 MIPS
payment year. See Table
Group C for rationale.
N/A /
N/A
409
N/A
MIPS CQM
Specification
s
Outcome
Clinical Outcome Post
Endovascular Stroke
Treatment: Percentage of
patients with a Modified
Rankin Score (mRS) score
of 0 to 2 at 90 days
following endovascular
stroke intervention.
Centers for
Medicare &
Medicaid
Services
This measure is being
proposed for removal
beginning with the CY
2025 performance
period/2027 MIPS
payment year. See Table
Group C for rationale.

B.25. Nutrition/Dietician
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Nutrition/Dietician specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a
measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Nutrition/
Dietician specialty set that have proposed substantive changes in Table Group D of this Appendix as indicated by an * in the
Indicator column.
B.25. Nutrition/Dietician
PREVIOUSLY FINALIZED MEASURES IN THE NUTRITION/DIETICIAN SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality #
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
§
!
(Outcome)
0059 /
N/A
001
CMS12
2v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Intermediate
Outcome
Diabetes: Hemoglobin A1c
(HbA1c) Poor Control
(>9%):
Percentage of patients 18-75
years of age with diabetes
who had hemoglobin A1c >
9.0% during the
measurement period.
National
Committee for
Quality Assurance
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS68
v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the
Medical Record:
Percentage of visits for
patients aged 18 years and
older for which the eligible
clinician attests to
documenting a list of current
medications using all
immediate resources
available on the date of the
encounter.
Centers for
Medicare &
Medicaid Services
§
NA /
N/A
134
CMS2v
14
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Screening for
Depression and Follow-Up
Plan:
Percentage of patients aged
12 years and older screened
for depression on the date of
the encounter or up to 14
days prior to the date of the
encounter using an age-
appropriate standardized
depression screening tool
AND if positive, a follow-
up plan is documented on
the date of or up to two days
after the date of the
qualifying encounter.
Centers for
Medicare &
Medicaid Services
*
!
(Patient
Safety)
N/A /
N/A
181
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Elder Maltreatment
Screen and Follow-Up
Plan:
Percentage of patients aged
60 years and older with a
documented elder
maltreatment screen using
an Elder Maltreatment
Screening tool on the date of
encounter AND a
documented follow-up plan
on the date of the positive
screen.
Centers for
Medicare &
Medicaid Services
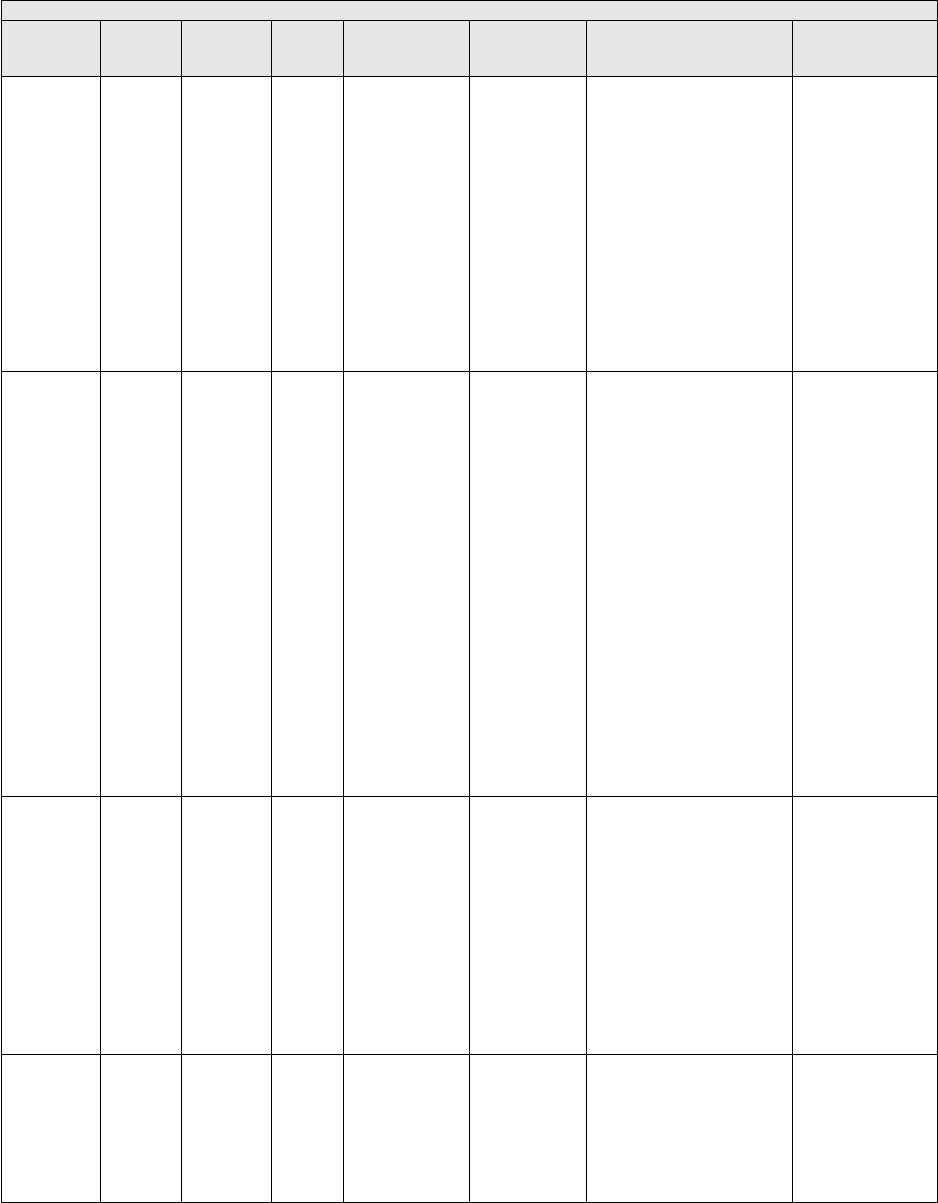
B.25. Nutrition/Dietician
PREVIOUSLY FINALIZED MEASURES IN THE NUTRITION/DIETICIAN SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality #
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
§
N/A /
N/A
226
CMS13
8v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Tobacco Use:
Screening and Cessation
Intervention:
Percentage of patients aged
12 years and older who were
screened for tobacco use one
or more times during the
measurement period AND
who received tobacco
cessation intervention
during the measurement
period or in the six months
prior to the measurement
period if identified as a
tobacco user.
National
Committee for
Quality Assurance
§
N/A /
N/A
239
CMS15
5v13
eCQM
Specifications
Process
Weight Assessment and
Counseling for Nutrition
and Physical Activity for
Children/Adolescents:
Percentage of patients 3-17
years of age who had an
outpatient visit with a
Primary Care Physician
(PCP) or
Obstetrician/Gynecologist
(OB/GYN) and who had
evidence of the following
during the measurement
period.
● Percentage of patients
with height, weight, and
body mass index (BMI)
percentile documentation
● Percentage of patients
with counseling for nutrition
● Percentage of patients
with counseling for physical
activity.
National
Committee for
Quality Assurance
§
2152 /
N/A
431
N/A
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Unhealthy
Alcohol Use: Screening &
Brief Counseling:
Percentage of patients aged
18 years and older who were
screened for unhealthy
alcohol use using a
systematic screening method
at least once within the last
12 months AND who
received brief counseling if
identified as an unhealthy
alcohol user.
National
Committee for
Quality Assurance
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social
Drivers of Health:
Percent of patients 18 years
and older screened for food
insecurity, housing
instability, transportation
needs, utility difficulties,
and interpersonal safety.
Centers for
Medicare &
Medicaid Services

B.25. Nutrition/Dietician
PREVIOUSLY FINALIZED MEASURES IN THE NUTRITION/DIETICIAN SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality #
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years
or older who screen positive
for one or more of the
following health related
social needs (HRSNs): food
insecurity, housing
instability, transportation
needs, utility help needs, or
interpersonal safety; and had
contact with a Community
Service Provider (CSP) for
at least 1 of their HRSNs
within 60 days after
screening.
OCHIN

B.26. Obstetrics/Gynecology
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Obstetrics/Gynecology specialty set takes additional criteria into consideration, which includes, but is not limited to: whether
a measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess
the appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Obstetrics/
Gynecology specialty set.
B.26. Obstetrics/Gynecology
PREVIOUSLY FINALIZED MEASURES IN THE OBSTETRICS/GYNECOLOGY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
0046 /
N/A
039
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Screening for Osteoporosis
for Women Aged 65-85
Years of Age:
Percentage of women aged
65-85 years of age who ever
had a central dual-energy X-
ray absorptiometry (DXA) to
check for osteoporosis.
National
Committee for
Quality Assurance
*
!
(Care
Coordinatio
n)
0326 /
N/A
047
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Advance Care Plan:
Percentage of patients aged
65 years and older who have
an advance care plan or
surrogate decision maker
documented in the medical
record or documentation in
the medical record that an
advance care plan was
discussed but the patient did
not wish or was not able to
name a surrogate decision
maker or provide an advance
care plan.
National
Committee for
Quality Assurance
N/A / N/A
048
N/A
MIPS CQM
Specifications
Process
Urinary Incontinence:
Assessment of Presence or
Absence of Urinary
Incontinence in Women
Aged 65 Years and Older:
Percentage of female patients
aged 65 years and older who
were assessed for the
presence or absence of
urinary incontinence within
12 months.
National
Committee for
Quality Assurance
!
(Patient
Experience)
N/A / N/A
050
N/A
MIPS CQM
Specifications
Process
Urinary Incontinence: Plan
of Care for Urinary
Incontinence in Women
Aged 65 Years and Older:
Percentage of female patients
aged 65 years and older with
a diagnosis of urinary
incontinence with a
documented plan of care for
urinary incontinence at least
once within 12 months.
National
Committee for
Quality Assurance
*
§
!
(Patient
Safety)
N/A / N/A
130
CMS68v1
4
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for
patients aged 18 years and
older for which the eligible
clinician attests to
documenting a list of current
medications using all
immediate resources
available on the date of the
encounter.
Centers for
Medicare &
Medicaid Services

B.26. Obstetrics/Gynecology
PREVIOUSLY FINALIZED MEASURES IN THE OBSTETRICS/GYNECOLOGY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
§
!
(Outcome)
N/A / N/A
236
CMS165v
13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Intermedi
ate
Outcome
Controlling High Blood
Pressure:
Percentage of patients 18-85
years of age who had a
diagnosis of essential
hypertension starting before
and continuing into, or
starting during the first six
months of the measurement
period, and whose most
recent blood pressure was
adequately controlled
(<140/90mmHg) during the
measurement period.
National
Committee for
Quality Assurance
§
N/A / N/A
309
CMS124v
13
eCQM
Specifications
Process
Cervical Cancer Screening:
Percentage of women 21-64
years of age who were
screened for cervical cancer
using either of the following
criteria:
• Women age 21-64 who had
cervical cytology performed
within the last 3 years
• Women age 30-64 who had
cervical human
papillomavirus (HPV) testing
performed within the last 5
years
National
Committee for
Quality Assurance
§
N/A / N/A
310
CMS153v
13
eCQM
Specifications
Process
Chlamydia Screening in
Women:
Percentage of women 16-24
years of age who were
identified as sexually active
and who had at least one test
for chlamydia during the
measurement period.
National
Committee for
Quality Assurance
!
(Outcome)
N/A / N/A
335
N/A
MIPS CQM
Specifications
Outcome
Maternity Care: Elective
Delivery (Without Medical
Indication) at < 39 Weeks
(Overuse):
Percentage of patients,
regardless of age, who gave
birth during a 12-month
period, delivered a live
singleton at < 39 weeks of
gestation, and had elective
deliveries (without medical
indication) by cesarean birth
or induction of labor.
Centers for
Medicare &
Medicaid Services
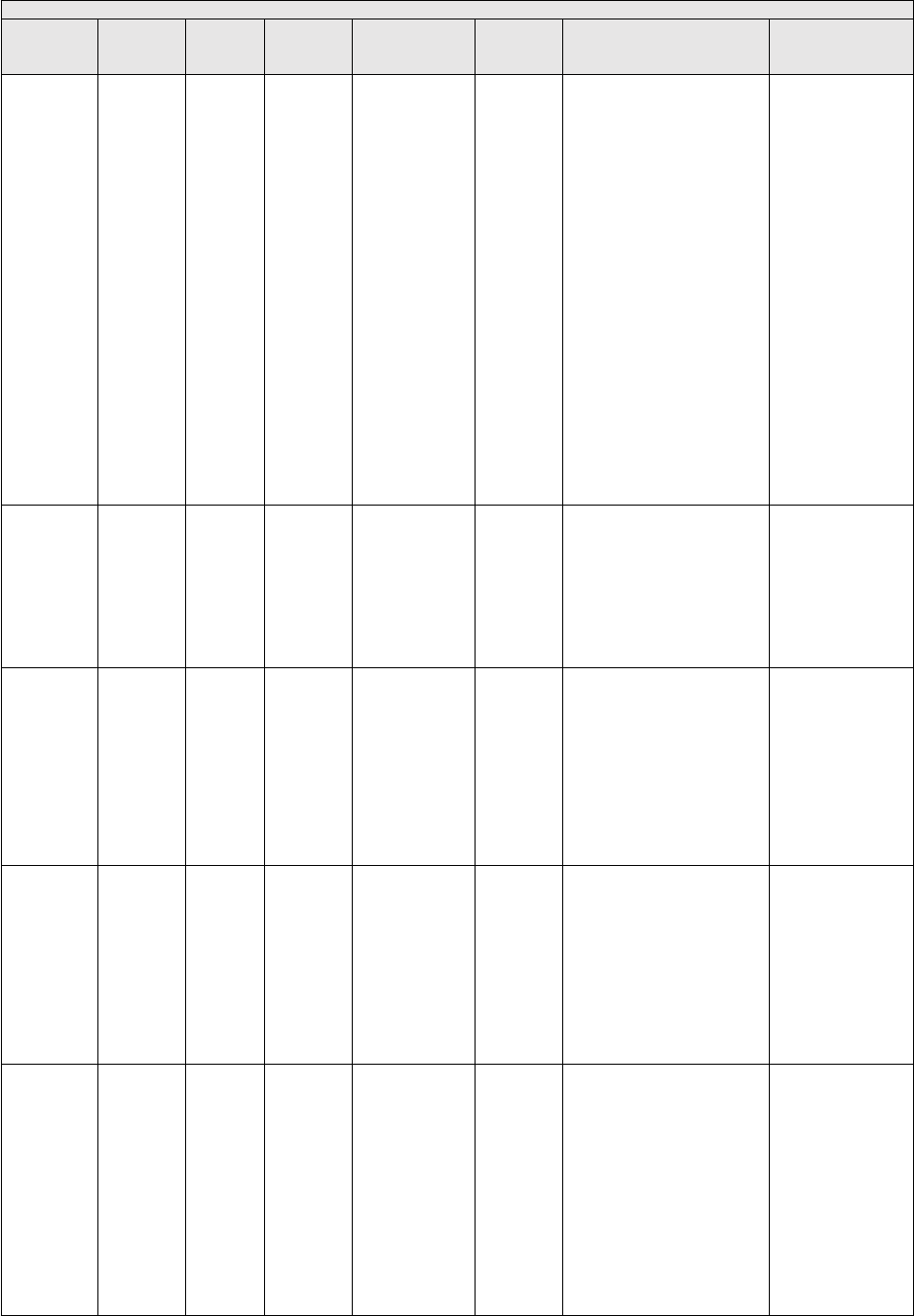
B.26. Obstetrics/Gynecology
PREVIOUSLY FINALIZED MEASURES IN THE OBSTETRICS/GYNECOLOGY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
§
!
(Care
Coordinatio
n)
N/A / N/A
336
N/A
MIPS CQM
Specifications
Process
Maternity Care:
Postpartum Follow-up and
Care Coordination:
Percentage of patients,
regardless of age, who gave
birth during a 12-month
period who were seen for
postpartum care before or at
12 weeks of giving birth and
received the following at a
postpartum visit: breast-
feeding evaluation and
education, postpartum
depression screening,
postpartum glucose screening
for gestational diabetes
patients, family and
contraceptive planning
counseling, tobacco use
screening and cessation
education, healthy lifestyle
behavioral advice, and an
immunization review and
update.
Centers for
Medicare &
Medicaid Services
*
!
(Care
Coordinatio
n)
N/A / N/A
374
CMS50v1
3
eCQM
Specifications,
MIPS CQM
Specifications
Process
Closing the Referral Loop:
Receipt of Specialist
Report:
Percentage of patients with
referrals, regardless of age,
for which the referring
clinician receives a report
from the clinician to whom
the patient was referred.
Centers for
Medicare &
Medicaid Services
*
0053 /
N/A
418
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Osteoporosis Management
in Women Who Had a
Fracture:
The percentage of women
50–85 years of age who
suffered a fracture and who
had either a bone mineral
density (BMD) test or
prescription for a drug to
treat osteoporosis in the six
months after the fracture.
National
Committee for
Quality Assurance
!
(Patient
Safety)
2063 /
N/A
422
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Performing Cystoscopy at
the Time of Hysterectomy
for Pelvic Organ Prolapse
to Detect Lower Urinary
Tract Injury:
Percentage of patients who
undergo cystoscopy to
evaluate for lower urinary
tract injury at the time of
hysterectomy for pelvic
organ prolapse.
American
Urogynecologic
Society
§
2152 /
N/A
431
N/A
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Unhealthy
Alcohol Use: Screening &
Brief Counseling:
Percentage of patients aged
18 years and older who were
screened for unhealthy
alcohol use using a
systematic screening method
at least once within the last
12 months AND who
received brief counseling if
identified as an unhealthy
alcohol user.
National
Committee for
Quality Assurance

B.26. Obstetrics/Gynecology
PREVIOUSLY FINALIZED MEASURES IN THE OBSTETRICS/GYNECOLOGY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Outcome)
N/A / N/A
432
N/A
MIPS CQM
Specifications
Outcome
Proportion of Patients
Sustaining a Bladder
Injury at the Time of any
Pelvic Organ Prolapse
Repair:
Percentage of patients
undergoing pelvic organ
prolapse repairs who sustain
an injury to the bladder
recognized either during or
within 30 days after surgery.
American
Urogynecologic
Society
§
!
(Appropriat
e Use)
N/A / N/A
443
N/A
MIPS CQM
Specifications
Process
Non-Recommended
Cervical Cancer Screening
in Adolescent Females:
The percentage of adolescent
females 16–20 years of age
who were screened
unnecessarily for cervical
cancer.
Centers for
Medicare &
Medicaid Services
*
!
(Care
Coordinatio
n)
N/A / N/A
448
N/A
MIPS CQM
Specifications
Process
Appropriate Workup Prior
to Endometrial Ablation:
Percentage of patients, aged
18 years and older, who
undergo endometrial
sampling or hysteroscopy
with biopsy and results are
documented before
undergoing an endometrial
ablation.
Centers for
Medicare &
Medicaid Services
§
N/A / N/A
475
CMS349v
7
eCQM
Specifications
Process
HIV Screening:
Percentage of patients aged
15-65 at the start of the
measurement period who
were between 15-65 years
old when tested for Human
Immunodeficiency Virus
(HIV).
Centers for Disease
Control and
Prevention
!
(Equity)
N/A / N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social
Drivers of Health:
Percent of patients 18 years
and older screened for food
insecurity, housing
instability, transportation
needs, utility difficulties, and
interpersonal safety.
Centers for
Medicare &
Medicaid Services
*
3620 /
N/A
493
N/A
MIPS CQM
Specifications
Process
Adult Immunization
Status:
Percentage of patients 19
years of age and older who
are up-to-date on
recommended routine
vaccines for influenza;
tetanus and diphtheria (Td)
or tetanus, diphtheria and
acellular pertussis (Tdap);
zoster; and pneumococcal.
National
Committee for
Quality Assurance
N/A / N/A
496
N/A
MIPS CQM
Specifications
Process
Cardiovascular Disease
(CVD) Risk Assessment
Measure - Proportion of
Pregnant/Postpartum
Patients that Receive CVD
Risk Assessment with a
Standardized Instrument:
Percentage of pregnant or
postpartum patients who
received a cardiovascular
disease (CVD) risk
assessment with a
standardized instrument.
University of
California, Irvine

B.26. Obstetrics/Gynecology
PREVIOUSLY FINALIZED MEASURES IN THE OBSTETRICS/GYNECOLOGY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
N/A / N/A
497
N/A
MIPS CQM
Specifications
Process
Preventive Care and
Wellness (composite):
Percentage of patients who
received age- and sex-
appropriate preventive
screenings and wellness
services. This measure is a
composite of seven
component measures that are
based on recommendations
for preventive care by the
U.S. Preventive Services
Task Force (USPSTF),ACIP,
American Association of
Clinical Endocrinology
(AACE), and American
College of Endocrinology
(ACE).
Centers for
Medicare and
Medicaid Services
*
!
(Equity)
N/A / N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years
or older who screen positive
for one or more of the
following health related
social needs (HRSNs): food
insecurity, housing
instability, transportation
needs, utility help needs, or
interpersonal safety; and had
contact with a Community
Service Provider (CSP) for at
least 1 of their HRSNs within
60 days after screening.
OCHIN
*
!
(Outcome)
2483 /
N/A
503
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performan
ce
Measure
Gains in Patient Activation
Measure (PAM®) Scores at
12 Months:
The Patient Activation
Measure® (PAM®) is a 10 –
or 13 – item questionnaire
that assesses an individual´s
knowledge, skills and
confidence for managing
their health and health care.
The measure assesses
individuals on a 0-100 scale
that converts to one of four
levels of activation, from low
(1) to high (4). The PAM®
performance measure
(PAM®-PM) is the change in
score on the PAM® from
baseline to follow-up
measurement.
Insignia Health,
LLC, a wholly
owned subsidiary
of Phreesia

B.26. Obstetrics/Gynecology
PREVIOUSLY FINALIZED MEASURES IN THE OBSTETRICS/GYNECOLOGY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Safety)
N/A / N/A
504
N/A
MIPS CQM
Specifications
Process
Initiation, Review, And/Or
Update To Suicide Safety
Plan For Individuals With
Suicidal Thoughts,
Behavior, Or Suicide Risk:
Percentage of adult aged 18
years and older with suicidal
ideation or behavior
symptoms (based on results
of a standardized assessment
tool or screening tool) or
increased suicide risk (based
on the clinician's evaluation
or clinician-rating tool) for
whom a suicide safety plan is
initiated, reviewed, and/or
updated in collaboration
between the patient and their
clinician.
American
Psychiatric
Association
*
!
(Outcome)
N/A / N/A
505
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performan
ce
Measure
Reduction in Suicidal
Ideation or Behavior
Symptoms:
The percentage of patients
aged 18 and older with a
mental and/or substance use
disorder AND suicidal
thoughts, behaviors or risk
symptoms who demonstrated
a reduction in suicidal
ideation and/or behavior
symptoms based on results
from the Columbia-Suicide
Severity Rating Scale (C-
SSRS) ‘Screen Version’ or
‘Since Last Visit’ within 120
days after an index
assessment.
American
Psychiatric
Association

B.26. Obstetrics/Gynecology
MEASURES PROPOSED FOR ADDITION TO THE OBSTRETRICS/GYNECOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measur
e
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
N/A /
N/A
TBD
N/A
MIPS
CQM
Specificati
ons
Process
Adult COVID-19
Vaccination
Status:
Percentage of
patients aged 18
years and older
seen for a visit
during the
performance
period that are up
to date on their
COVID-19
vaccinations as
defined by CDC
recommendations
on current
vaccination.
Centers for
Medicare &
Medicaid
Services
We are proposing to include this
measure in the Obstetrics/
Gynecology specialty set as it
would be clinically relevant to
this clinician type. Widespread
vaccination against SARS-CoV-
2, the virus that causes COVID-
19, is critically important to
stemming the morbidity and
mortality caused by this
disease.
101
Clinicians are
uniquely positioned to
encourage uptake of COVID-19
vaccination, and clinicians are
still a major driving force in
promoting patient vaccination.
The addition of this quality
measure in this specialty set
would help strengthen
compliance with recommended
COVID-19 vaccination, leading
to improvement in the quality of
patient care and prevention of
disease for the general
population. This quality measure
aligns with clinical guidelines
and the evidence-based
recommendations of the ACIP,
where there is general agreement
about the safety and efficacy of
the COVID-19 vaccine,
preventing costly and potentially
harmful hospitalizations.
102
Broadening vaccination status
awareness to this clinician type
is valuable as it can help drive
an increase in the adult
vaccination rates. The COVID-
19 vaccination included within
this measure would reduce the
prevalence of severe diseases
that may be associated with
hospitalization and decrease
overall health care costs. The
measure being added to this
specialty set would be
contingent on the inclusion of
applicable coding by the time of
the CY 2025 PFS final rule. In
the event appropriate coding is
not included in the final
specification, this measure
would not be finalized for
inclusion within this specialty
measure set. See Table A.5 of
this Appendix for rationale,
including clinical evidence
supporting the inclusion of this
measure in MIPS.
101
See footnote Ikeokwu et al., 2023 in Table B.1 of this Appendix.
102
See footnotes Fitzpatrick et al., 2022; Polack et al., 2020; and Graña et al., 2022 in Table A.5 of this Appendix.

B.26. Obstetrics/Gynecology
PREVIOUSLY FINALIZED MEASURES PROPOSED FOR REMOVAL FROM THE OBSTRETRICS/GYNECOLOGY
SPECIALTY SET
Note: In this proposed rule, we are proposing the removal of the following measure(s) below from this specific specialty measure set based
upon review of updates made to existing quality measure specifications, the proposed addition of new measures for inclusion in MIPS, and the
feedback provided by specialty societies.
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Removal
N/A /
N/A
433
N/A
MIPS CQM
Specification
s
Outcome
Proportion of Patients
Sustaining a Bowel Injury
at the time of any Pelvic
Organ Prolapse Repair:
Percentage of patients
undergoing surgical repair
of pelvic organ prolapse that
is complicated by a bowel
injury at the time of index
surgery that is recognized
intraoperatively or within 30
days after surgery.
American
Urogyneco-
logic Society
This measure is being
proposed for removal
beginning with the CY
2025 performance
period/2027 MIPS
payment year. See Table
Group C for rationale.
N/A /
3475e
472
CMS24
9v7
eCQM
Specification
s
Process
Appropriate Use of DXA
Scans in Women Under 65
Years Who Do Not Meet
the Risk Factor Profile for
Osteoporotic Fracture:
Percentage of female
patients 50 to 64 years of
age without select risk
factors for osteoporotic
fracture who received an
order for a dual-energy x-
ray absorptiometry (DXA)
scan during the
measurement period.
Centers for
Medicare &
Medicaid
Services
This measure is being
proposed for removal
beginning with the CY
2025 performance
period/2027 MIPS
payment year. See Table
Group C for rationale.

B.27a. Oncology/Hematology
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Oncology/Hematology specialty set takes additional criteria into consideration, which includes, but is not limited to: whether
a measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess
the appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed
Oncology/Hematology specialty set.
B.27a. Oncology/Hematology
PREVIOUSLY FINALIZED MEASURES IN THE ONCOLOGY/HEMATOLOGY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Care
Coordinati
on)
0326 /
N/A
047
N/A
Medicare Part B
Claims Measure
Specifications,
MIPS CQM
Specifications
Process
Advance Care Plan:
Percentage of patients aged 65
years and older who have an
advance care plan or surrogate
decision maker documented in
the medical record or
documentation in the medical
record that an advance care plan
was discussed but the patient did
not wish or was not able to name
a surrogate decision maker or
provide an advance care plan.
National
Committee for
Quality Assurance
§
!
(Appropria
te Use)
N/A /
N/A
102
CMS1
29v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Prostate Cancer: Avoidance of
Overuse of Bone Scan for
Staging Low Risk Prostate
Cancer Patients:
Percentage of patients,
regardless of age, with a
diagnosis of prostate cancer at
low (or very low) risk of
recurrence receiving interstitial
prostate brachytherapy, OR
external beam radiotherapy to
the prostate, OR radical
prostatectomy who did not have
a bone scan performed at any
time since diagnosis of prostate
cancer.
Centers for
Medicare &
Medicaid Services
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS6
8v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for patients
aged 18 years and older for which
the eligible clinician attests to
documenting a list of current
medications using all immediate
resources available on the date of
the encounter.
Centers for
Medicare &
Medicaid Services
§
N/A /
N/A
134
CMS2
v14
Medicare Part B
Claims Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Screening for
Depression and Follow-Up
Plan:
Percentage of patients aged 12
years and older screened for
depression on the date of the
encounter or up to 14 days prior
to the date of the encounter using
an age-appropriate standardized
depression screening tool AND
if positive, a follow-up plan is
documented on the date of or up
to two days after the date of the
qualifying encounter.
Centers for
Medicare &
Medicaid Services

B.27a. Oncology/Hematology
PREVIOUSLY FINALIZED MEASURES IN THE ONCOLOGY/HEMATOLOGY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
*
§
!
(Patient
Experience
)
0384 /
0384e
143
CMS1
57v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Oncology: Medical and
Radiation – Pain Intensity
Quantified:
Percentage of patient visits,
regardless of patient age, with a
diagnosis of cancer currently
receiving chemotherapy or
radiation therapy in which pain
intensity is quantified.
American Society
of Clinical
Oncology
§
N/A /
N/A
226
CMS1
38v13
Medicare Part B
Claims Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Tobacco Use:
Screening and Cessation
Intervention:
Percentage of patients aged 12
years and older who were
screened for tobacco use one or
more times during the
measurement period AND who
received tobacco cessation
intervention during the
measurement period or in the six
months prior to the measurement
period if identified as a tobacco
user.
National
Committee for
Quality Assurance
*
!
(Patient
Safety)
0022 /
N/A
238
CMS1
56v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Use of High-Risk Medications
in Older Adults:
Percentage of patients 65 years
of age and older who were
ordered at least two high-risk
medications from the same drug
class.
National
Committee for
Quality Assurance
§
N/A /
N/A
250
N/A
Medicare Part B
Claims Measure
Specifications,
MIPS CQM
Specifications
Process
Radical Prostatectomy
Pathology Reporting:
Percentage of radical
prostatectomy pathology reports
that include the pT category, the
pN category, the Gleason score
and a statement about margin
status.
College of
American
Pathologists
*
N/A /
N/A
317
CMS2
2v13
Medicare Part B
Claims Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Screening for High
Blood Pressure and Follow-Up
Documented:
Percentage of patient visits for
patients aged 18 years and older
seen during the measurement
period who were screened for
high blood pressure AND a
recommended follow-up plan is
documented, as indicated, if
blood pressure is elevated or
hypertensive.
Centers for
Medicare &
Medicaid Services

B.27a. Oncology/Hematology
PREVIOUSLY FINALIZED MEASURES IN THE ONCOLOGY/HEMATOLOGY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
§
!
(Patient
Experience
0005 /
N/A
321
N/A
CMS-approved
Survey Vendor
Patient
Engageme
nt/Experie
nce
CAHPS for MIPs
Clinician/Group Survey:
The Consumer Assessment of
Healthcare Providers and
Systems (CAHPS) for MIPS
Clinician/Group Survey is
comprised of 10 Summary
Survey Measures (SSMs) and
measures patient experience of
care within a group practice. The
CBE endorsement status and
endorsement id (if applicable)
for each SSM utilized in this
measure are as follows:
• Getting Timely Care,
Appointments, and Information;
(Not endorsed by CBE)
• How well Providers
Communicate; (Not endorsed by
CBE)
• Patient’s Rating of Provider;
(CBE endorsed # 0005)
• Access to Specialists; (Not
endorsed by CBE)
• Health Promotion and
Education; (Not endorsed by
CBE)
• Shared Decision-Making; (Not
endorsed by CBE)
• Health Status and Functional
Status; (Not endorsed by CBE)
• Courteous and Helpful Office
Staff; (CBE endorsed # 0005)
• Care Coordination; (Not
endorsed by CBE)
• Stewardship of Patient
Resources. (Not endorsed by
CBE).
Centers for
Medicare &
Medicaid Services
*
!
(Care
Coordinatio
n)
N/A /
N/A
374
CMS5
0v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Closing the Referral Loop:
Receipt of Specialist Report:
Percentage of patients with
referrals, regardless of age, for
which the referring clinician
receives a report from the
clinician to whom the patient
was referred.
Centers for
Medicare &
Medicaid Services
§
2152 /
N/A
431
N/A
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Unhealthy Alcohol
Use: Screening & Brief
Counseling:
Percentage of patients aged 18
years and older who were
screened for unhealthy alcohol
use using a systematic screening
method at least once within the
last 12 months AND who
received brief counseling if
identified as an unhealthy
alcohol user.
National
Committee for
Quality Assurance
*
§
!
(Appropria
te Use)
1858 /
N/A
450
N/A
MIPS CQM
Specifications
Process
Appropriate Treatment for
Patients with Stage I (T1c) –
III HER2 Positive Breast
Cancer:
Percentage of female patients
aged 18 to 70 with stage I (T1c)
– III HER2 positive breast
cancer for whom appropriate
treatment is initiated.
American Society
of Clinical
Oncology

B.27a. Oncology/Hematology
PREVIOUSLY FINALIZED MEASURES IN THE ONCOLOGY/HEMATOLOGY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
*
§
1859 /
N/A
451
N/A
MIPS CQM
Specifications
Process
RAS (KRAS and NRAS) Gene
Mutation Testing Performed
for Patients with Metastatic
Colorectal Cancer who receive
Anti-epidermal Growth Factor
Receptor (EGFR) Monoclonal
Antibody Therapy: Percentage
of adult patients (aged 18 or
over) with metastatic colorectal
cancer who receive anti-
epidermal growth factor receptor
monoclonal antibody therapy for
whom RAS (KRAS and NRAS)
gene mutation testing was
performed.
American Society
of Clinical
Oncology
§
!
(Appropria
te Use)
0210 /
N/A
453
N/A
MIPS CQM
Specifications
Process
Percentage of Patients Who
Died from Cancer Receiving
Systemic Cancer-Directed
Therapy in the Last 14 Days of
Life (lower score – better):
Percentage of patients who died
from cancer receiving systemic
cancer-directed therapy in the
last 14 days of life.
American Society
of Clinical
Oncology
§
!
(Appropriat
e Use)
0216 /
N/A
457
N/A
MIPS CQM
Specifications
Process
Percentage of Patients who
Died from Cancer Admitted to
Hospice for Less than 3 Days
(lower score – better):
Percentage of patients who died
from cancer and admitted to
hospice and spent less than 3
days there.
American Society
of Clinical
Oncology
*
N/A /
N/A
462
CMS6
45v8
eCQM
Specifications
Process
Bone Density Evaluation for
Patients with Prostate Cancer
and Receiving Androgen
Deprivation Therapy:
Patients determined as having
prostate cancer who are currently
starting or undergoing androgen
deprivation therapy (ADT), for
an anticipated period of 12
months or greater and who
receive an initial bone density
evaluation. The bone density
evaluation must be prior to the
start of ADT or within 3 months
of the start of ADT.
Oregon Urology
Institute
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers of
Health:
Percent of patients 18 years and
older screened for food
insecurity, housing instability,
transportation needs, utility
difficulties, and interpersonal
safety.
Centers for
Medicare &
Medicaid Services
*
N/A /
N/A
490
N/A
MIPS CQM
Specifications
Process
Appropriate Intervention of
Immune-Related Diarrhea
and/or Colitis in Patients
Treated with Immune
Checkpoint Inhibitors:
Percentage of patients, aged 18
years and older, with a diagnosis
of cancer, on immune checkpoint
inhibitor therapy, and grade 2 or
above diarrhea and/or grade 2 or
above colitis, who have immune
checkpoint inhibitor therapy held
and corticosteroids or
immunosuppressants prescribed
or administered.
Society for
Immunotherapy of
Cancer (SITC)

B.27a. Oncology/Hematology
PREVIOUSLY FINALIZED MEASURES IN THE ONCOLOGY/HEMATOLOGY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection Type
Measure
Type
Measure Title
and Description
Measure Steward
*
3620 /
N/A
493
N/A
MIPS CQM
Specifications
Process
Adult Immunization Status:
Percentage of patients 19 years
of age and older who are up-to-
date on recommended routine
vaccines for influenza; tetanus
and diphtheria (Td) or tetanus,
diphtheria and acellular pertussis
(Tdap); zoster; and
pneumococcal.
National
Committee for
Quality Assurance
!
(Outcome)
3665 /
N/A
495
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Ambulatory Palliative Care
Patients' Experience of Feeling
Heard and Understood:
The percentage of top-box
responses among patients aged
18 years and older who had an
ambulatory palliative care visit
and report feeling heard and
understood by their palliative
care clinician and team within 2
months (60 days) of the
ambulatory palliative care visit.
American
Academy of
Hospice and
Palliative
Medicine
(AAHPM)
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years or
older who screen positive for
one or more of the following
health related social needs
(HRSNs): food insecurity,
housing instability,
transportation needs, utility help
needs, or interpersonal safety;
and had contact with a
Community Service Provider
(CSP) for at least 1 of their
HRSNs within 60 days after
screening.
OCHIN
*
!
(Outcome)
2483 /
N/A
503
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performan
ce Measure
Gains in Patient Activation
Measure (PAM®) Scores at 12
Months:
The Patient Activation
Measure® (PAM®) is a 10 – or
13 – item questionnaire that
assesses an individual´s
knowledge, skills and confidence
for managing their health and
health care. The measure
assesses individuals on a 0-100
scale that converts to one of four
levels of activation, from low (1)
to high (4). The PAM®
performance measure (PAM®-
PM) is the change in score on
the PAM® from baseline to
follow-up measurement.
Insignia Health,
LLC, a wholly
owned subsidiary
of Phreesia

B.27a. Oncology/Hematology
MEASURES PROPOSED FOR ADDITION TO THE ONCOLOGY/HEMATOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
!
(Appropri
ate Use)
N/A /
N/A
TBD
N/A
MIPS
CQM
Specificati
ons
Process
Positive PD-L1
Biomarker
Expression Test
Result Prior to
First-Line
Immune
Checkpoint
Inhibitor
Therapy:
Percentage of
patients, aged 18
years and older,
with a diagnosis
of metastatic
non-small cell
lung cancer or
squamous cell
carcinoma of
head and neck on
first-line immune
checkpoint
inhibitor (ICI)
therapy, who had
a positive PD-L1
biomarker
expression test
result prior to
giving ICI
therapy.
Society
for
Immunot
herapy of
Cancer
(SITC)
We are proposing to include this
measure in the Oncology/
Hematology specialty set as it
would be clinically relevant to this
clinician type. Immunotherapy is
a rapidly developing and changing
subspecialty in the realm of
oncology, and this measure would
fill a gap within the oncologic
clinical topic. The incorporation
of this measure in this specialty
set would help promote
appropriate intervention and
timeliness of PD-L1 biomarker
expression testing prior to
initiation of first-line treatment for
the metastatic non-small cell lung
cancer or squamous cell
carcinoma of head and neck. This
timeliness of treatment initiation
can lead to improvements in
patient mortality and morbidity.
103
It’s important to address the
proper diagnosis of metastatic
non-small cell lung cancer or
squamous cell carcinoma that may
impact treatment decisions so that
appropriate treatment delivery is
not delayed, nor ineffective
therapies prescribed which could
both result in poor clinical
outcomes and unnecessary
healthcare costs.
104
See Table A.1
of this Appendix for rationale,
including clinical evidence
supporting the inclusion of this
measure in MIPS.
103
See footnote Pai et al., 2020 in Table A.1 of this Appendix
104
See footnotes Lim et al., 2015 and Pai et al., 2020 in Table A.1 of this Appendix.

B.27a. Oncology/Hematology
MEASURES PROPOSED FOR ADDITION TO THE ONCOLOGY/HEMATOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
N/A /
N/A
TBD
N/A
MIPS
CQM
Specificati
ons
Process
Appropriate
Germline
Testing for
Ovarian Cancer
Patients:
Percentage of
patients, aged 18
and older,
diagnosed with
epithelial
ovarian, fallopian
tube, or primary
peritoneal cancer
who undergo
germline testing
within 6 months
of diagnosis.
American
Society
of
Clinical
Oncology
We are proposing to include this
measure in the Oncology/
Hematology specialty set as it
would be clinically relevant to this
clinician type and would fill a gap
within the oncologic clinical
topic. This measure addresses
patients diagnosed with epithelial
ovarian, fallopian tube, or primary
peritoneal cancer who undergo
germline testing within 6 months
of their diagnosis and is
predicated on existing clinical
guidelines and recommendations.
It also addresses a CMS priority
that could allow for more
personalized diagnostic,
predictive, prognostic, and
therapeutic strategies for the
patient. Current recommendations
for all women diagnosed with
ovarian cancer is to receive
genetic testing, however, only
approximately 30 percent of
women undergo any genetic
testing.
105
The high incidence of
these mutations and the advent of
therapy targeted toward BRCA
mutations warrant testing in all
individuals diagnosed with
ovarian cancer for the purpose of
determining treatment
recommendations, risk of other
cancers, and need for cascade
testing of family members. See
Table A.2 of this Appendix for
rationale, including clinical
evidence supporting the inclusion
of this measure in MIPS.
105
See footnote Konstantinopoulos et al., 2020 in Table A.2 of this Appendix.

B.27a. Oncology/Hematology
MEASURES PROPOSED FOR ADDITION TO THE ONCOLOGY/HEMATOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
!
(Outcome
)
CBE
3718 /
N/A
TBD
N/A
MIPS
CQM
Specificati
ons
Patient-
Reported
Outcome-
based
Performanc
e Measure
(PRO-PM)
Patient-
Reported Pain
Interference
Following
Chemotherapy
among Adults
with Breast
Cancer: The
PRO-PM will
assess pain
interference
following
chemotherapy
administered
with curative
intent to adult
patients with
breast cancer.
Purchaser
Business
Group on
Health
We are proposing to include this
measure in the Oncology/
Hematology specialty set as it
would be clinically relevant to this
clinician type and would fill a gap
within the oncologic clinical
topic. This measure addresses a
CMS high priority as a patient-
reported outcome-based
performance quality measure
accounting for patient experience
of care for this patient population.
It is predicated on existing clinical
guidelines and
recommendations.
106
For the
breast cancer patient population,
it’s important to routinely assess
pain to properly identify barriers
to acceptable pain management
and to intervene appropriately,
which can improve the patient’s
health outcome and quality of
life.
107
See Table A.3 of this
Appendix for rationale, including
clinical evidence supporting the
inclusion of this measure in MIPS.
!
(Outcome
)
CBE
3720 /
N/A
TBD
N/A
MIPS
CQM
Specificati
ons
Patient-
Reported
Outcome-
based
Performanc
e Measure
(PRO-PM)
Patient-
Reported
Fatigue
Following
Chemotherapy
among Adults
with Breast
Cancer:
The PRO-PM
will assess
fatigue following
chemotherapy
administered
with curative
intent to adult
patients with
breast cancer.
Purchaser
Business
Group on
Health
We are proposing to include this
measure in the Oncology/
Hematology specialty set as it
would be clinically relevant to this
clinician type, addresses a CMS
high priority as a PRO-PM, and
would fill a gap within the
oncologic clinical topic. It takes
into consideration the patient
voice/experience of care for those
patients with breast cancer with
fatigue experienced following
chemotherapy. PRO assessment in
routine care remains
underutilized, and very few PRO-
PMs have been validated for the
cancer population. By taking into
consideration patient voice,
necessary interventions can be
completed to help improve their
quality of life during cancer
treatment. See Table A.4 of this
Appendix for rationale, including
clinical evidence supporting the
inclusion of this measure in MIPS.
106
Tegegn, H. K., & Gebreyohannes, E. A. (2017). Adequacy of Cancer Pain Management and Pain Interference
With Daily Functioning Among Patients Visiting the Oncology Ward of an Ethiopian University. Journal of Global
Oncology, 3(2). https://ascopubs.org/doi/10.1200/JGO.2017.009738.
107
See footnote Tegegn & Gebreyohannes, 2017.

B.27a. Oncology/Hematology
MEASURES PROPOSED FOR ADDITION TO THE ONCOLOGY/HEMATOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
N/A /
N/A
TBD
N/A
MIPS
CQM
Specificati
ons
Process
Adult COVID-
19 Vaccination
Status:
Percentage of
patients aged 18
years and older
seen for a visit
during the
performance
period that are up
to date on their
COVID-19
vaccinations as
defined by CDC
recommendation
s on current
vaccination.
Centers
for
Medicare
&
Medicaid
Services
We are proposing to include this
measure in the Oncology/
Hematology specialty set as it
would be clinically relevant to this
clinician type. Widespread
vaccination against SARS-CoV-2,
the virus that causes COVID-19,
is critically important to stemming
the morbidity and mortality
caused by this disease.
108
Clinicians are uniquely positioned
to encourage uptake of COVID-19
vaccination, and clinicians are still
a major driving force in
promoting patient vaccination.
The addition of this quality
measure in this specialty set
would help strengthen compliance
with recommended COVID-19
vaccination, leading to
improvement in the quality of
patient care and prevention of
disease for the general population.
This quality measure aligns with
clinical guidelines and the
evidence-based recommendations
of the ACIP, where there is
general agreement about the
safety and efficacy of the COVID-
19 vaccine, preventing costly and
potentially harmful
hospitalizations.
109
Broadening
vaccination status awareness to
this clinician type is valuable as it
can help drive an increase in the
adult vaccination rates. The
COVID-19 vaccination included
within this measure would reduce
the prevalence of severe diseases
that may be associated with
hospitalization and decrease
overall health care costs. The
measure being added to this
specialty set would be contingent
on the inclusion of applicable
coding by the time of the CY
2025 PFS final rule. In the event
appropriate coding is not included
in the final specification, this
measure would not be finalized
for inclusion within this specialty
measure set. See Table A.5 of this
Appendix for rationale, including
clinical evidence supporting the
inclusion of this measure in MIPS.
108
See footnote Ikeokwu et al., 2023 in Table B.1 of this Appendix.
109
See footnotes Fitzpatrick et al., 2022; Polack et al., 2020; and Graña et al., 2022 in Table A.5 of this Appendix.

B.27a. Oncology/Hematology
PREVIOUSLY FINALIZED MEASURES PROPOSED FOR REMOVAL FROM THE ONCOLOGY/HEMATOLOGY
SPECIALTY SET
Note: In this proposed rule, we are proposing the removal of the following measure(s) below from this specific specialty measure set based
upon review of updates made to existing quality measure specifications, the proposed addition of new measures for inclusion in MIPS, and the
feedback provided by specialty societies.
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Removal
0383 /
N/A
144
N/A
MIPS CQM
Specification
s
Process
Oncology: Medical and
Radiation – Plan of Care
for Pain:
Percentage of visits for
patients, regardless of age,
with a diagnosis of cancer
currently receiving
chemotherapy or radiation
therapy who report having
pain with a documented plan
of care to address pain.
American
Society of
Clinical
Oncology
This measure is being
proposed for removal
beginning with the CY
2025 performance
period/2027 MIPS
payment year. See Table
Group C for rationale.
1860 /
N/A
452
N/A
MIPS CQM
Specification
s
Process
Patients with Metastatic
Colorectal Cancer and
RAS (KRAS or NRAS)
Gene Mutation Spared
Treatment with Anti-
epidermal Growth Factor
Receptor (EGFR)
Monoclonal Antibodies:
Percentage of adult patients
(aged 18 or over) with
metastatic colorectal cancer
and RAS (KRAS or NRAS)
gene mutation spared
treatment with anti-EGFR
monoclonal antibodies.
American
Society of
Clinical
Oncology
This measure is being
proposed for removal
beginning with the CY
2025 performance
period/2027 MIPS
payment year. See Table
Group C for rationale.

B.27b. Radiation Oncology
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Radiation Oncology specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a
measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Radiation
Oncology specialty set.
B.27b. Radiation Oncology
PREVIOUSLY FINALIZED MEASURES IN THE RADIATION ONCOLOGY SPECIALTY SET
Indicator
CBE
# /
eCQ
M
CBE
#
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
§
!
(Appropriat
e Use)
N/A /
N/A
102
CMS
129v1
4
eCQM
Specifications,
MIPS CQM
Specifications
Process
Prostate Cancer: Avoidance of
Overuse of Bone Scan for
Staging Low Risk Prostate
Cancer Patients:
Percentage of patients, regardless
of age, with a diagnosis of
prostate cancer at low (or very
low) risk of recurrence receiving
interstitial prostate
brachytherapy, OR external beam
radiotherapy to the prostate, OR
radical prostatectomy who did
not have a bone scan performed
at any time since diagnosis of
prostate cancer.
Centers for Medicare &
Medicaid Services
*
§
!
(Patient
Experience)
0384 /
0384e
143
CMS
157v1
3
eCQM
Specifications,
MIPS CQM
Specifications
Process
Oncology: Medical and
Radiation – Pain Intensity
Quantified:
Percentage of patient visits,
regardless of patient age, with a
diagnosis of cancer currently
receiving chemotherapy or
radiation therapy in which pain
intensity is quantified.
American Society of
Clinical Oncology
§
N/A /
N/A
226
CMS
138v1
3
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and Screening:
Tobacco Use: Screening and
Cessation Intervention:
Percentage of patients aged 12
years and older who were
screened for tobacco use one or
more times during the
measurement period AND who
received tobacco cessation
intervention during the
measurement period or in the six
months prior to the measurement
period if identified as a tobacco
user.
National Committee for
Quality Assurance

B.27b. Radiation Oncology
PREVIOUSLY FINALIZED MEASURES PROPOSED FOR REMOVAL FROM THE RADIATION ONCOLOGY
SPECIALTY SET
Note: In this proposed rule, we are proposing the removal of the following measure(s) below from this specific specialty measure set based
upon review of updates made to existing quality measure specifications, the proposed addition of new measures for inclusion in MIPS, and the
feedback provided by specialty societies.
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Removal
0383 /
N/A
144
N/A
MIPS CQM
Specification
s
Process
Oncology: Medical and
Radiation – Plan of Care
for Pain:
Percentage of visits for
patients, regardless of age,
with a diagnosis of cancer
currently receiving
chemotherapy or radiation
therapy who report having
pain with a documented plan
of care to address pain.
American
Society of
Clinical
Oncology
This measure is being
proposed for removal
beginning with the CY
2025 performance
period/2027 MIPS
payment year. See Table
Group C for rationale.

B.28. Ophthalmology
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Ophthalmology specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a
measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Ophthalmology
specialty set that have proposed substantive changes in Table Group D of this Appendix as indicated by an * in the Indicator
column. As indicated in the Table Group B Introduction, we are proposing to rename this specialty set from “Ophthalmology/
Optometry” to “Ophthalmology” under this proposed rule. We are also proposing a new Optometry specialty set under Table
B.29 of this Appendix.
B.28. Ophthalmology
PREVIOUSLY FINALIZED MEASURES IN THE OPHTHALMOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
N/A /
0086e
012
CMS14
3v13
eCQM
Specifications
Process
Primary Open-Angle
Glaucoma (POAG):
Optic Nerve
Evaluation:
Percentage of patients
aged 18 years and older
with a diagnosis of
primary open-angle
glaucoma (POAG) who
have an optic nerve
head evaluation during
one or more visits
within 12 months.
American Academy of
Ophthalmology
*
!
(Care
Coordination)
N/A /
N/A
019
CMS14
2v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Diabetic Retinopathy:
Communication with
the Physician
Managing Ongoing
Diabetes Care:
Percentage of patients
aged 18 years and older
with a diagnosis of
diabetic retinopathy
who had a dilated
macular or fundus exam
performed with
documented
communication to the
physician who manages
the ongoing care of the
patient with diabetes
mellitus regarding the
findings of the macular
or fundus exam at least
once during the
performance period.
American Academy of
Ophthalmology

B.28. Ophthalmology
PREVIOUSLY FINALIZED MEASURES IN THE OPHTHALMOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
§
0055 /
N/A
117
CMS13
1v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Diabetes: Eye Exam:
Percentage of patients
18-75 years of age with
diabetes and an active
diagnosis of retinopathy
in any part of the
measurement period
who had a retinal or
dilated eye exam by an
eye care professional
during the measurement
period or diabetics with
no diagnosis of
retinopathy in any part
of the measurement
period who had a retinal
or dilated eye exam by
an eye care professional
during the measurement
period or in the 12
months prior to the
measurement period.
National Committee for
Quality Assurance
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS68
v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of
Current Medications
in the Medical Record:
Percentage of visits for
patients aged 18 years
and older for which the
eligible clinician attests
to documenting a list of
current medications
using all immediate
resources available on
the date of the
encounter.
Centers for Medicare &
Medicaid Services
!
(Outcome)
0563 /
N/A
141
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Outcome
Primary Open-Angle
Glaucoma (POAG):
Reduction of
Intraocular Pressure
(IOP) by 20% OR
Documentation of a
Plan of Care:
Percentage of patients
aged 18 years and older
with a diagnosis of
primary open-angle
glaucoma (POAG)
whose glaucoma
treatment has not failed
(the most recent IOP
was reduced by at least
20% from the pre-
intervention level) OR if
the most recent IOP was
not reduced by at least
20% from the pre-
intervention level, a
plan of care was
documented within the
12 month performance
period.
American Academy of
Ophthalmology

B.28. Ophthalmology
PREVIOUSLY FINALIZED MEASURES IN THE OPHTHALMOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Outcome)
0565 /
0565e
191
CMS13
3v13
eCQM
Specifications,
MIPS CQM
Specifications
Outcome
Cataracts: 20/40 or
Better Visual Acuity
within 90 Days
Following Cataract
Surgery:
Percentage of cataract
surgeries for patients aged
18 years and older with a
diagnosis of
uncomplicated cataract
and no significant ocular
conditions impacting the
visual outcome of surgery
and had best-corrected
visual acuity of 20/40 or
better (distance or near)
achieved in the operative
eye within 90 days
following the cataract
surgery.
American Academy of
Ophthalmology
§
N/A /
N/A
226
CMS13
8v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Tobacco
Use: Screening and
Cessation
Intervention:
Percentage of patients
aged 12 years and older
who were screened for
tobacco use one or more
times during the
measurement period
AND who received
tobacco cessation
intervention during the
measurement period or in
the six months prior to the
measurement period if
identified as a tobacco
user.
National Committee for
Quality Assurance
*
!
(Patient
Safety)
0022 /
N/A
238
CMS15
6v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Use of High-Risk
Medications in Older
Adults:
Percentage of patients
65 years of age and
older who were ordered
at least two high-risk
medications from the
same drug class.
National Committee for
Quality Assurance
!
(Outcome)
N/A /
N/A
303
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performanc
e Measure
Cataracts:
Improvement in
Patient’s Visual
Function within 90
Days Following
Cataract Surgery:
Percentage of patients
aged 18 years and older
who had cataract
surgery and had
improvement in visual
function achieved
within 90 days
following the cataract
surgery, based on
completing a pre-
operative and post-
operative visual
function survey.
American Academy of
Ophthalmology

B.28. Ophthalmology
PREVIOUSLY FINALIZED MEASURES IN THE OPHTHALMOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Patient
Experience)
N/A /
N/A
304
N/A
MIPS CQM
Specifications
Patient
Engagement/
Experience
Cataracts: Patient
Satisfaction within 90
Days Following
Cataract Surgery:
Percentage of patients
aged 18 years and older
who had cataract
surgery and were
satisfied with their care
within 90 days
following the cataract
surgery, based on
completion of the
Consumer Assessment
of Healthcare Providers
and Systems Surgical
Care Survey.
American Academy of
Ophthalmology
*
!
(Care
Coordination)
N/A /
N/A
374
CMS50
v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Closing the Referral
Loop: Receipt of
Specialist Report:
Percentage of patients
with referrals,
regardless of age, for
which the referring
clinician receives a
report from the clinician
to whom the patient was
referred.
Centers for Medicare &
Medicaid Services
*
!
(Outcome)
N/A /
N/A
384
N/A
MIPS CQM
Specifications
Outcome
Adult Primary
Rhegmatogenous
Retinal Detachment
Surgery: No Return to
the Operating Room
Within 90 Days of
Surgery:
Patients aged 18 years
and older who had
surgery for primary
rhegmatogenous retinal
detachment who did not
require a return to the
operating room within
90 days of surgery.
American Academy of
Ophthalmology
!
(Outcome)
N/A /
N/A
385
N/A
MIPS CQM
Specifications
Outcome
Adult Primary
Rhegmatogenous
Retinal Detachment
Surgery: Visual Acuity
Improvement Within
90 Days of Surgery:
Patients aged 18 years
and older who had
surgery for primary
rhegmatogenous retinal
detachment and
achieved an
improvement in their
visual acuity, from their
preoperative level,
within 90 days of
surgery in the operative
eye.
American Academy of
Ophthalmology

B.28. Ophthalmology
PREVIOUSLY FINALIZED MEASURES IN THE OPHTHALMOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Outcome)
N/A /
N/A
389
N/A
MIPS CQM
Specifications
Outcome
Cataract Surgery:
Difference Between
Planned and Final
Refraction:
Percentage of patients
aged 18 years and older
who had cataract
surgery performed and
who achieved a final
refraction within +/- 1.0
diopters of their planned
(target) refraction.
American Academy of
Ophthalmology
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social
Drivers of Health:
Percent of patients 18
years and older screened
for food insecurity,
housing instability,
transportation needs,
utility difficulties, and
interpersonal safety.
Centers for Medicare &
Medicaid Services
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to
Community Service
Provider:
Percent of patients 18
years or older who
screen positive for one
or more of the following
health related social
needs (HRSNs): food
insecurity, housing
instability,
transportation needs,
utility help needs, or
interpersonal safety; and
had contact with a
Community Service
Provider (CSP) for at
least 1 of their HRSNs
within 60 days after
screening.
OCHIN

B.28. Ophthalmology
PREVIOUSLY FINALIZED MEASURES IN THE OPHTHALMOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
N/A /
N/A
499
N/A
MIPS CQM
Specifications
Process
Appropriate Screening
and Plan of Care for
Elevated Intraocular
Pressure Following
Intravitreal or
Periocular Steroid
Therapy:
Percentage of patients
who had an intravitreal
or periocular
corticosteroid injection
(e.g., triamcinolone,
preservative-free
triamcinolone,
dexamethasone,
dexamethasone
intravitreal implant, or
fluocinolone intravitreal
implant) who, within
seven (7) weeks
following the date of
injection, are screened
for elevated intraocular
pressure (IOP) with
tonometry with
documented IOP =<25
mm Hg for injected eye
OR if the IOP was >25
mm Hg, a plan of care
was documented.
American Society of
Retina Specialists
*
N/A /
N/A
500
N/A
MIPS CQM
Specifications
Process
Acute Posterior
Vitreous Detachment
Appropriate
Examination and
Follow-up:
Percentage of patients
with a diagnosis of
acute posterior vitreous
detachment (PVD) in
either eye who were
appropriately evaluated
during the initial exam
and were re-evaluated
no later than 8 weeks.
American Society of
Retina Specialists
*
N/A /
N/A
501
N/A
MIPS CQM
Specifications
Process
Acute Posterior
Vitreous Detachment
and Acute Vitreous
Hemorrhage
Appropriate
Examination and
Follow-up:
Percentage of patients
with a diagnosis of
acute posterior vitreous
detachment (PVD) and
acute vitreous
hemorrhage in either
eye who were
appropriately evaluated
during the initial exam
and were re-evaluated
no later than 2 weeks.
American Society of
Retina Specialists

B.29. Optometry
As indicated in the introductory language of Table Group B of this Appendix to this proposed rule, we are proposing to add a
new Optometry specialty set. In addition to the considerations discussed in the introductory language of Table Group B, the
Optometry specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a measure
reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are proposing to add to this specialty set. We request comment on the
measures available in the proposed Optometry specialty set.
B.29. Optometry
MEASURES PROPOSED FOR ADDITION TO THE OPTOMETRY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure
Title
And
Description
Measure
Steward
Rationale for Inclusion
*
!
(Care
Coordinat
ion)
N/A /
N/A
019
CMS
142v1
3
eCQM
Specification
s, MIPS
CQM
Specification
s
Process
Diabetic
Retinopathy:
Communicati
on with the
Physician
Managing
Ongoing
Diabetes
Care:
Percentage of
patients aged
18 years and
older with a
diagnosis of
diabetic
retinopathy
who had a
dilated
macular or
fundus exam
performed
with
documented
communicatio
n to the
physician who
manages the
ongoing care
of the patient
with diabetes
mellitus
regarding the
findings of the
macular or
fundus exam
at least once
during the
performance
period.
American
Academy of
Ophthalmolog
y
We are proposing to include
this measure in the Optometry
specialty set as it would be
clinically relevant to this
clinician type. An
Optometrist is needed to
inform a primary care
clinician about a particular
patient's retinopathy severity,
possible diabetic macular
edema, or other ocular co-
morbidities. Retinopathy
serves as a strong predictor of
other serious medical
conditions such as heart
attack, stroke, kidney failure,
amputation, and others.
110
Without regular reporting
from the optometrist on this
issue, the primary care
clinician lacks valuable
information key to the overall
management of the patient.
This measure is essential to
patient safety and completes
the feedback essential for
treating a deadly, common
disease.
111
Better
communication between eye
specialists and primary care
clinicians can play a critical
role in patient care, as it’s an
important mechanism for
clinicians to communicate
with one another about a
patients’ disease symptoms,
adherence to care plan, and
treatment plans.
112
The
measure being added to this
specialty set would be
contingent on the inclusion of
applicable coding by the time
of the CY 2025 PFS final
rule.
110
Nag, S., Bilous, R., Kelly, W., Jones, S., Roper, N., & Connolly, V. (2007). All-cause and Cardiovascular Mortality in
Diabetic Subjects Increases Significantly with Reduced Estimated Glomerular Filtration Rate (eGFR): 10 Years' Data
from the South Tees Diabetes Mortality Study. Diabetic Medicine: A Journal of the British Diabetic
Association, 24(1), 10–17. https://doi.org/10.1111/j.1464-5491.2007.02023.x.
111
Reutens A. T. (2013). Epidemiology of Diabetic Kidney Disease. The Medical Clinics of North America, 97(1), 1–
18. https://doi.org/10.1016/j.mcna.2012.10.001.
112
Storey, P., and Haller, J. (2016). The Significance of Physician Communication in the Care of Patients With
Diabetes. Retina Today. https://retinatoday.com/articles/2016-sept/the-significance-of-physician-communication-
in-the-care-of-patients-with-diabetes.

B.29. Optometry
MEASURES PROPOSED FOR ADDITION TO THE OPTOMETRY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure
Title
And
Description
Measure
Steward
Rationale for Inclusion
*
§
0055 /
N/A
117
CMS
131v1
3
eCQM
Specification
s,
MIPS CQM
Specification
s
Process
Diabetes: Eye
Exam:
Percentage of
patients 18-75
years of age
with diabetes
and an active
diagnosis of
retinopathy in
any part of the
measurement
period who
had a retinal
or dilated eye
exam by an
eye care
professional
during the
measurement
period or
diabetics with
no diagnosis
of retinopathy
in any part of
the
measurement
period who
had a retinal
or dilated eye
exam by an
eye care
professional
during the
measurement
period or in
the 12 months
prior to the
measurement
period.
National
Committee for
Quality
Assurance
We are proposing to include
this measure in the Optometry
specialty set as it would be
clinically relevant to this
clinician type. This measure
could help accurately assess a
clinician’s ability to diagnose
and treat patients safely and
efficiently. We believe the
numerator options, give more
granular data can be captured
to discern between patients
with and without evidence of
retinopathy. This information
could give insight into those
patients with controlled blood
sugar and those with
uncontrolled blood sugar The
measure being added to this
specialty set would be
contingent on the inclusion of
applicable coding by the time
of the CY 2025 PFS final
rule.
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS
68v14
eCQM
Specification
s, MIPS
CQM
Specification
s
Process
Documentati
on of Current
Medications
in the
Medical
Record:
Percentage of
visits for
patients aged
18 years and
older for
which the
eligible
clinician
attests to
documenting a
list of current
medications
using all
immediate
resources
available on
the date of the
encounter.
Centers for
Medicare &
Medicaid
Services
We are proposing to include
this measure in the Optometry
specialty set as it is clinically
relevant to this clinician type.
Documentation of current
medications in the medical
record facilitates the process
of medication review and
reconciliation by the
clinician, which is necessary
for reducing ADEs and
promoting medication safety.
The need for clinician-to-
clinician coordination
regarding medication records,
and the existing gap in
implementation, is
highlighted in the American
Medical Association’s
Physician’s Role in
Medication Reconciliation,
which states that "critical
patient information, including
medical and medication
histories, current medications
the patient is receiving and
taking, and sources of
medications, is essential to
the delivery of safe medical

B.29. Optometry
MEASURES PROPOSED FOR ADDITION TO THE OPTOMETRY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure
Title
And
Description
Measure
Steward
Rationale for Inclusion
care.”
113
The measure being
added to this specialty set
would be contingent on the
inclusion of applicable coding
by the time of the CY 2025
PFS final rule.
§
N/A /
N/A
226
CMS
138v1
3
Medicare
Part B
Claims
Measure
Specification
s, eCQM
Specification
s, MIPS
CQM
Specification
s
Process
Preventive
Care and
Screening:
Tobacco Use:
Screening
and
Cessation
Intervention:
Percentage of
patients aged
12 years and
older who
were screened
for tobacco
use one or
more times
during the
measurement
period AND
who received
tobacco
cessation
intervention
during the
measurement
period or in
the six months
prior to the
measurement
period if
identified as a
tobacco user.
National
Committee for
Quality
Assurance
We are proposing to include
this measure in the Optometry
specialty set as it is clinically
relevant to this clinician type.
Tobacco use is the leading
preventable cause of disease,
disability, and death in the
U.S. cigarette smoking results
in more than 480,000
premature deaths each year
and accounts for
approximately 1 in every 5
deaths.
114
Due to the harmful
effect tobacco use can have
on patients’ health, we
believe that clinicians should
engage with their patients to
screen for tobacco use and, if
positive, provide tobacco
cessation counseling
annually. The measure being
added to this specialty set
would be contingent on the
inclusion of applicable coding
by the time of the CY 2025
PFS final rule.
*
!
(Patient
Safety)
0022 /
N/A
238
CMS
156v1
3
eCQM
Specification
s, MIPS
CQM
Specification
s
Process
Use of High-
Risk
Medications
in Older
Adults:
Percentage of
patients 65
years of age
and older who
were ordered
at least two
high-risk
medications
from the same
drug class.
National
Committee for
Quality
Assurance
We are proposing to include
this measure in the Optometry
specialty set as it is clinically
relevant to this clinician type.
Treating patients with high-
risk medications such as anti-
depressants or pain
medications, may be
associated with increased risk
of harm from drug side-
effects and toxicity.
115
Medication errors can occur
anywhere throughout the
many steps in the medication
management process, with
one of the most common
error sources being poor
interprofessional
communication resulting in
poor collaborative medication
113
American Medical Association. (2007). The Physician’s Role in Medication Reconciliation: Issues, Strategies, and
Safety Principles. Retrieved from https://brucelambert.soc.northwestern.edu//book_reviews/med-rec-
monograph.pdf.
114
CDC. (2023). Smoking and Tobacco Use – Adult Data.
https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm.
115
Zhan, C., Sangl, J., Bierman, A. S., Miller, M. R., Friedman, B., Wickizer, S. W., & Meyer, G. S. (2001). Potentially
Inappropriate Medication Use in the Community-Dwelling Elderly: Findings from the 1996 Medical Expenditure
Panel Survey. JAMA, 286(22), 2823–2829. https://doi.org/10.1001/jama.286.22.2823.

B.29. Optometry
MEASURES PROPOSED FOR ADDITION TO THE OPTOMETRY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure
Title
And
Description
Measure
Steward
Rationale for Inclusion
management.
116
The measure
being added to this specialty
set would be contingent on
the inclusion of applicable
coding by the time of the CY
2025 PFS final rule.
*
!
(Care
Coordinat
ion)
N/A /
N/A
374
CMS
50v13
eCQM
Specification
s, MIPS
CQM
Specification
s
Process
Closing the
Referral
Loop:
Receipt of
Specialist
Report:
Percentage of
patients with
referrals,
regardless of
age, for which
the referring
clinician
receives a
report from
the clinician to
whom the
patient was
referred.
Centers for
Medicare &
Medicaid
Services
We are proposing to include
this measure in the Optometry
specialty set as it is clinically
relevant to this clinician type.
Including this measure would
ensure patients referred to
Optometrist for a consultation
complete the encounter with a
consult report being returned
to the referring physician.
The measure being added to
this specialty set would be
contingent on the inclusion of
applicable coding by the time
of the CY 2025 PFS final
rule.
116
Pereira, F., Bieri, M., Del Rio Carral, M., Martins, M. M., & Verloo, H. (2022). Collaborative Medication
Management for Older Adults After Hospital Discharge: A Qualitative Descriptive Study. BMC Nursing, 21(1), 284.
https://doi.org/10.1186/s12912-022-01061-3.

B.30. Orthopedic Surgery
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Orthopedic Surgery specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a
measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Orthopedic
Surgery specialty set that have proposed substantive changes in Table Group D of this Appendix as indicated by an * in the
Indicator column.
B.30. Orthopedic Surgery
PREVIOUSLY FINALIZED MEASURES IN THE ORTHOPEDIC SURGERY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title and
Description
Measure Steward
!
(Care
Coordination
)
N/A /
N/A
024
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Communication with the
Physician or Other
Clinician Managing On-
Going Care Post-Fracture
for Men and Women Aged
50 Years and Older:
Percentage of patients aged
50 years and older treated
for a fracture with
documentation of
communication, between
the physician treating the
fracture and the physician
or other clinician managing
the patient’s on-going care,
that a fracture occurred and
that the patient was or
should be considered for
osteoporosis treatment or
testing. This measure is
submitted by the physician
who treats the fracture and
who therefore is held
accountable for the
communication.
National Committee for
Quality Assurance
*
!
(Care
Coordination
)
0326 /
N/A
047
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Advance Care Plan:
Percentage of patients aged
65 years and older who
have an advance care plan
or surrogate decision
maker documented in the
medical record or
documentation in the
medical record that an
advance care plan was
discussed but the patient
did not wish or was not
able to name a surrogate
decision maker or provide
an advance care plan.
National Committee for
Quality Assurance
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS68
v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of
Current Medications in
the Medical Record:
Percentage of visits for
patients aged 18 years and
older for which the eligible
clinician attests to
documenting a list of
current medications using
all immediate resources
available on the date of the
encounter.
Centers for Medicare &
Medicaid Services

B.30. Orthopedic Surgery
PREVIOUSLY FINALIZED MEASURES IN THE ORTHOPEDIC SURGERY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title and
Description
Measure Steward
§
N/A /
N/A
134
CMS2v
14
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Screening for
Depression and Follow-
Up Plan:
Percentage of patients aged
12 years and older
screened for depression on
the date of the encounter or
up to 14 days prior to the
date of the encounter using
an age-appropriate
standardized depression
screening tool AND if
positive, a follow-up plan
is documented on the date
of or up to two days after
the date of the qualifying
encounter.
Centers for Medicare &
Medicaid Services
*
!
(Care
Coordination
)
0101 /
N/A
155
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Falls: Plan of Care:
Percentage of patients aged
65 years and older with a
history of falls who had a
plan of care for falls
documented within 12
months.
National Committee for
Quality Assurance
*
N/A /
N/A
178
N/A
MIPS CQM
Specifications
Process
Rheumatoid Arthritis
(RA): Functional Status
Assessment:
Percentage of patients aged
18 years and older with a
diagnosis of rheumatoid
arthritis (RA) for whom a
functional status
assessment was performed
at least once during the
performance period.
American College of
Rheumatology
*
N/A /
N/A
180
N/A
MIPS CQM
Specifications
Process
Rheumatoid Arthritis
(RA): Glucocorticoid
Management:
Percentage of patients aged
18 years and older with a
diagnosis of rheumatoid
arthritis (RA) who have
been assessed for
glucocorticoid use and, for
those on prolonged doses
of prednisone > 5 mg daily
(or equivalent) with
improvement or no change
in disease activity,
documentation of
glucocorticoid
management plan during
the performance period.
American College of
Rheumatology

B.30. Orthopedic Surgery
PREVIOUSLY FINALIZED MEASURES IN THE ORTHOPEDIC SURGERY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title and
Description
Measure Steward
*
§
!
(Care
Coordination
)
N/A /
N/A
182
N/A
MIPS CQM
Specifications
Process
Functional Outcome
Assessment:
Percentage of visits for
patients aged 18 years and
older with documentation
of a current functional
outcome assessment using
a standardized functional
outcome assessment tool
on the date of the
encounter AND
documentation of a care
plan based on identified
functional outcome
deficiencies within two
days of the date of the
identified deficiencies.
Centers for Medicare &
Medicaid Services
!
(Outcome)
N/A /
N/A
217
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Functional Status
Change for Patients with
Knee Impairments:
A patient-reported
outcome measure (PROM)
of risk-adjusted change in
functional status (FS) for
patients 14 years+ with
knee impairments. The
change in FS is assessed
using the FOTO Lower
Extremity Physical
Function (LEPF) PROM.
The measure is adjusted to
patient characteristics
known to be associated
with FS outcomes (risk-
adjusted) and used as a
performance measure at
the patient, individual
clinician, and clinic levels
to assess quality.
Focus on Therapeutic
Outcomes, Inc.
!
(Outcome)
N/A /
N/A
218
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Functional Status
Change for Patients with
Hip Impairments:
A patient-reported
outcome measure (PROM)
of risk-adjusted change in
functional status (FS) for
patients 14 years+ with hip
impairments. The change
in FS is assessed using the
FOTO Lower Extremity
Physical Function (LEPF)
PROM. The measure is
adjusted to patient
characteristics known to be
associated with FS
outcomes (risk adjusted)
and used as a performance
measure at the patient,
individual clinician, and
clinic levels to assess
quality.
Focus on Therapeutic
Outcomes, Inc.
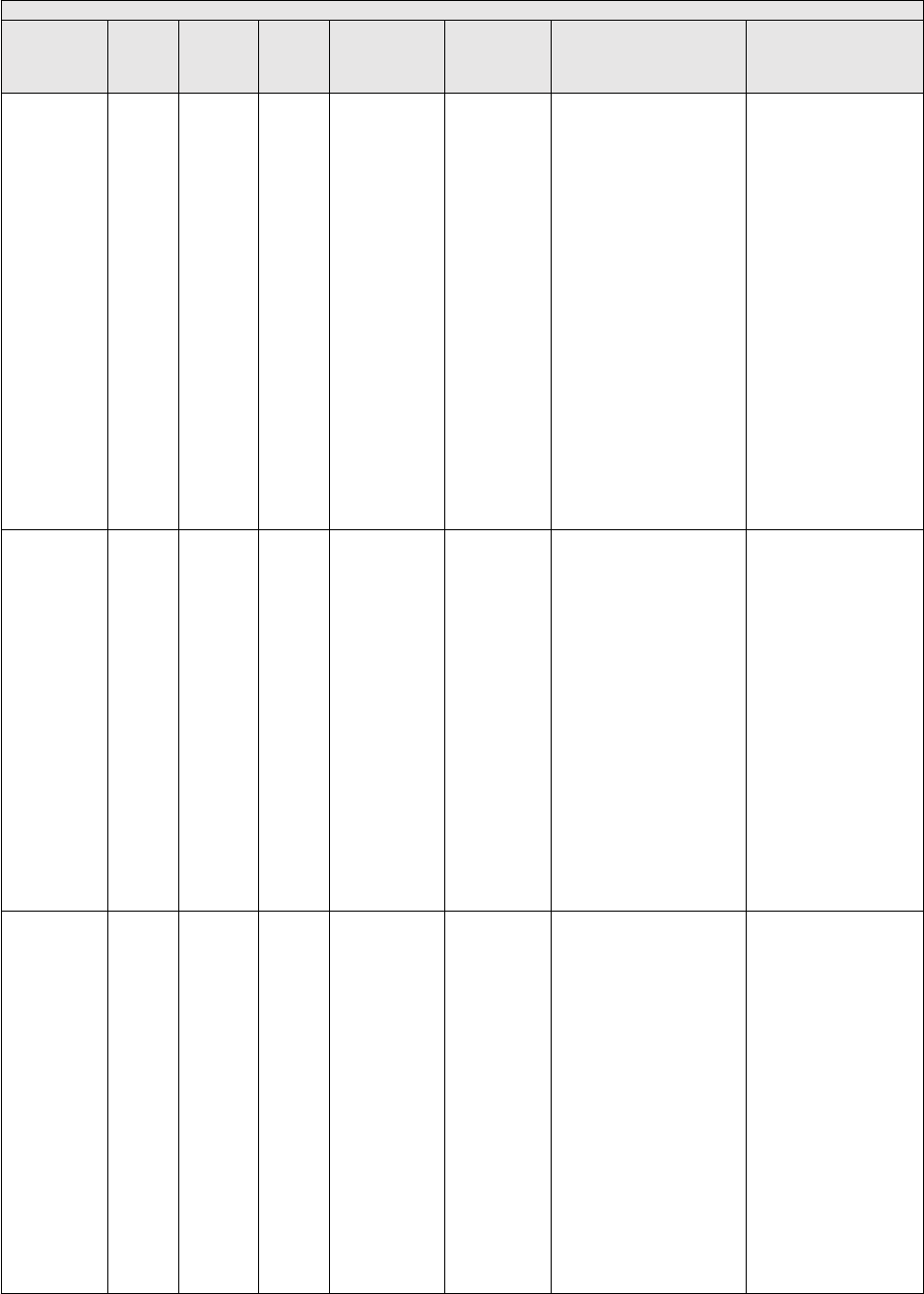
B.30. Orthopedic Surgery
PREVIOUSLY FINALIZED MEASURES IN THE ORTHOPEDIC SURGERY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title and
Description
Measure Steward
!
(Outcome)
N/A /
N/A
219
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Functional Status
Change for Patients with
Lower Leg, Foot or
Ankle Impairments:
A patient-reported
outcome measure (PROM)
of risk-adjusted change in
functional status (FS) for
patients 14 years+ with
foot, ankle or lower leg
impairments. The change
in FS is assessed using the
FOTO Lower Extremity
Physical Function (LEPF)
PROM. The measure is
adjusted to patient
characteristics known to be
associated with FS
outcomes (risk-adjusted)
and used as a performance
measure at the patient,
individual clinician, and
clinic levels to assess
quality.
Focus on Therapeutic
Outcomes, Inc.
!
(Outcome)
N/A /
N/A
220
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Functional Status
Change for Patients with
Low Back Impairments:
A patient-reported
outcome measure (PROM)
of risk-adjusted change in
functional status (FS) for
patients 14 years+ with
low back impairments. The
change in FS is assessed
using the FOTO Low Back
FS PROM. The measure is
adjusted to patient
characteristics known to be
associated with FS
outcomes (risk adjusted)
and used as a performance
measure at the patient,
individual clinician, and
clinic levels to assess
quality.
Focus on Therapeutic
Outcomes, Inc.
!
(Outcome)
N/A /
N/A
221
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Functional Status
Change for Patients with
Shoulder Impairments:
A patient-reported
outcome measure (PROM)
of risk-adjusted change in
functional status (FS) for
patients 14 years+ with
shoulder impairments. The
change in FS is assessed
using the FOTO Shoulder
FS PROM. The measure is
adjusted to patient
characteristics known to be
associated with FS
outcomes (risk adjusted)
and used as a performance
measure at the patient,
individual clinician, and
clinic levels to assess
quality.
Focus on Therapeutic
Outcomes, Inc.

B.30. Orthopedic Surgery
PREVIOUSLY FINALIZED MEASURES IN THE ORTHOPEDIC SURGERY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title and
Description
Measure Steward
!
(Outcome)
N/A /
N/A
222
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Functional Status
Change for Patients with
Elbow, Wrist or Hand
Impairments:
A patient-reported
outcome measure (PROM)
of risk-adjusted change in
functional status (FS) for
patients 14 years+ with
elbow, wrist, or hand
impairments. The change
in FS is assessed using the
FOTO Elbow/Wrist/Hand
FS PROM. The measure is
adjusted to patient
characteristics known to be
associated with FS
outcomes (risk adjusted)
and used as a performance
measure at the patient,
individual clinician, and
clinic levels to assess
quality.
Focus on Therapeutic
Outcomes, Inc.
§
N/A /
N/A
226
CMS13
8v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Tobacco Use:
Screening and Cessation
Intervention:
Percentage of patients aged
12 years and older who
were screened for tobacco
use one or more times
during the measurement
period AND who received
tobacco cessation
intervention during the
measurement period or in
the six months prior to the
measurement period if
identified as a tobacco
user.
National Committee for
Quality Assurance
*
N/A /
N/A
317
CMS22
v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Screening for
High Blood Pressure and
Follow-Up Documented:
Percentage of patient visits
for patients aged 18 years
and older seen during the
measurement period who
were screened for high
blood pressure AND a
recommended follow-up
plan is documented, as
indicated, if blood pressure
is elevated or hypertensive.
Centers for Medicare &
Medicaid Services
!
(Patient
Safety)
0101 /
N/A
318
CMS13
9v13
eCQM
Specifications
Process
Falls: Screening for
Future Fall Risk:
Percentage of patients 65
years of age and older who
were screened for future
fall risk during the
measurement period.
National Committee for
Quality Assurance

B.30. Orthopedic Surgery
PREVIOUSLY FINALIZED MEASURES IN THE ORTHOPEDIC SURGERY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title and
Description
Measure Steward
!
(Care
Coordination
)
N/A /
N/A
350
N/A
MIPS CQM
Specifications
Process
Total Knee or Hip
Replacement: Shared
Decision-Making: Trial of
Conservative (Non-
surgical) Therapy:
Percentage of patients
regardless of age
undergoing a total knee or
total hip replacement with
documented shared
decision- making with
discussion of conservative
(non-surgical) therapy
(e.g., non-steroidal anti-
inflammatory drug
(NSAIDs), analgesics,
weight loss, exercise,
injections) prior to the
procedure.
American Association
of Hip and Knee
Surgeons
!
(Patient
Safety)
N/A /
N/A
351
N/A
MIPS CQM
Specifications
Process
Total Knee or Hip
Replacement: Venous
Thromboembolic and
Cardiovascular Risk
Evaluation:
Percentage of patients
regardless of age undergoing
a total knee or total hip
replacement who are
evaluated for the presence or
absence of venous
thromboembolic and
cardiovascular risk factors
within 30 days prior to the
procedure (e.g., History of
Deep Vein Thrombosis
(DVT), Pulmonary
Embolism (PE), Myocardial
Infarction (MI), Arrhythmia
and Stroke).
American Association
of Hip and Knee
Surgeons
!
(Patient
Experience)
N/A /
N/A
358
N/A
MIPS CQM
Specifications
Process
Patient-Centered
Surgical Risk Assessment
and Communication:
Percentage of patients who
underwent a non-
emergency surgery who
had their personalized risks
of postoperative
complications assessed by
their surgical team prior to
surgery using a clinical
data-based, patient-specific
risk calculator and who
received personal
discussion of those risks
with the surgeon.
American College of
Surgeons
*
!
(Care
Coordination
)
N/A /
N/A
374
CMS50
v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Closing the Referral
Loop: Receipt of
Specialist Report:
Percentage of patients with
referrals, regardless of age,
for which the referring
clinician receives a report
from the clinician to whom
the patient was referred.
Centers for Medicare &
Medicaid Services

B.30. Orthopedic Surgery
PREVIOUSLY FINALIZED MEASURES IN THE ORTHOPEDIC SURGERY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title and
Description
Measure Steward
*
§
!
(Patient
Experience)
N/A /
N/A
376
CMS56
v13
eCQM
Specifications
Process
Functional Status
Assessment for Total Hip
Replacement:
Percentage of patients 19
years of age and older who
received an elective primary
total hip arthroplasty (THA)
and completed a functional
status assessment within 90
days prior to the surgery and
in the 300 – 425 days after
the surgery.
Centers for Medicare &
Medicaid Services
*
0053 /
N/A
418
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Osteoporosis
Management in Women
Who Had a Fracture:
The percentage of women
50–85 years of age who
suffered a fracture and who
had either a bone mineral
density (BMD) test or
prescription for a drug to
treat osteoporosis in the six
months after the fracture.
National Committee for
Quality Assurance
§
!
(Outcome)
N/A /
N/A
459
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Back Pain After Lumbar
Surgery:
For patients 18 years of
age or older who had a
lumbar
discectomy/laminectomy
or fusion procedure, back
pain is rated by the patients
as less than or equal to 3.0
OR an improvement of 5.0
points or greater on the
Visual Analog Scale
(VAS) Pain scale or a
numeric pain scale at three
months (6 to 20 weeks)
postoperatively for
discectomy/laminectomy
or at one year (9 to 15
months) postoperatively
for lumbar fusion patients.
Rates are stratified by
procedure type; lumbar
discectomy/laminectomy
or fusion procedure.
Minnesota
Community
Measurement
§
!
(Outcome)
N/A /
N/A
461
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Leg Pain After Lumbar
Surgery:
For patients 18 years of
age or older who had a
lumbar
discectomy/laminectomy
or fusion procedure, leg
pain is rated by the patient
as less than or equal to 3.0
OR an improvement of 5.0
points or greater on the
Visual Analog Scale
(VAS) Pain scale or a
numeric pain scale at three
months (6 to 20 weeks) for
discectomy/laminectomy
or at one year (9 to 15
months) postoperatively
for lumbar fusion patients.
Rates are stratified by
procedure type; lumbar
discectomy/laminectomy
or fusion procedure.
Minnesota
Community
Measurement

B.30. Orthopedic Surgery
PREVIOUSLY FINALIZED MEASURES IN THE ORTHOPEDIC SURGERY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title and
Description
Measure Steward
*
§
!
(Outcome)
N/A /
N/A
470
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Functional Status After
Primary Total Knee
Replacement:
For patients age 18 and older
who had a primary total
knee replacement procedure,
functional status is rated by
the patient as greater than or
equal to 37 on the Oxford
Knee Score (OKS) or a 71 or
greater on the KOOS, JR
tool at one year (9 to 15
months) postoperatively.
Minnesota Community
Measurement
§
!
(Outcome)
N/A /
N/A
471
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Functional Status After
Lumbar Surgery:
For patients age 18 and older
who had lumbar
discectomy/laminectomy or
fusion procedure, functional
status is rated by the patient
as less than or equal to 22
OR an improvement of 30
points or greater on the
Oswestry Disability Index
(ODI version 2.1a) at three
months (6 to 20 weeks)
postoperatively for
discectomy/laminectomy or
at one year (9 to 15 months)
postoperatively for lumbar
fusion patients. Rates are
stratified by procedure type;
lumbar discectomy or fusion
procedure.
Minnesota Community
Measurement
§
!
(Outcome)
N/A /
N/A
478
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Functional Status Change
for Patients with Neck
Impairments:
A patient-reported
outcome measure (PROM)
of risk-adjusted change in
functional status (FS) for
patients 14 years+ with
neck impairments. The
change in FS is assessed
using the FOTO Neck FS
PROM. The measure is
adjusted to patient
characteristics known to be
associated with FS
outcomes (risk-adjusted)
and used as a performance
measure at the patient,
individual clinician, and
clinic levels to assess
quality.
Focus on Therapeutic
Outcomes, Inc.

B.30. Orthopedic Surgery
PREVIOUSLY FINALIZED MEASURES IN THE ORTHOPEDIC SURGERY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title and
Description
Measure Steward
!
(Outcome)
3493 /
N/A
480
N/A
Administrativ
e Claims
Outcome
Risk-standardized
complication rate
(RSCR) following elective
primary total hip
arthroplasty (THA)
and/or total knee
arthroplasty (TKA) for
Merit-based Incentive
Payment System (MIPS):
This measure is a re-
specified version of the
measure, “Hospital-level
Risk-standardized
Complication rate (RSCR)
following Elective Primary
Total Hip Arthroplasty
(THA) and/or Total Knee
Arthroplasty (TKA)”
(National Quality Forum
1550), which was
developed for patients 65
years and older using
Medicare claims. This re-
specified measure
attributes outcomes to
Merit-based Incentive
Payment System
participating clinicians
and/or clinician groups
(“provider”) and assesses
each provider’s
complication rate, defined
as any one of the specified
complications occurring
from the date of index
admission to up to 90 days
post date of the index
procedure.
Centers for Medicare &
Medicaid Services
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social
Drivers of Health:
Percent of patients 18
years and older screened
for food insecurity,
housing instability,
transportation needs, utility
difficulties, and
interpersonal safety.
Centers for Medicare &
Medicaid Services
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to
Community Service
Provider:
Percent of patients 18
years or older who screen
positive for one or more of
the following health related
social needs (HRSNs):
food insecurity, housing
instability, transportation
needs, utility help needs, or
interpersonal safety; and
had contact with a
Community Service
Provider (CSP) for at least
1 of their HRSNs within
60 days after screening.
OCHIN

B.31. Otolaryngology
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Otolaryngology specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a
measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Otolaryngology
specialty set.
B.31. Otolaryngology
PREVIOUSLY FINALIZED MEASURES IN THE OTOLARYNGOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Care
Coordination)
0326 /
N/A
047
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Advance Care Plan:
Percentage of patients aged 65
years and older who have an
advance care plan or surrogate
decision maker documented in
the medical record or
documentation in the medical
record that an advance care
plan was discussed but the
patient did not wish or was not
able to name a surrogate
decision maker or provide an
advance care plan.
National Committee
for Quality
Assurance
§
!
(Appropriate
Use)
N/A /
N/A
066
CMS14
6v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Appropriate Testing for
Pharyngitis:
The percentage of episodes for
patients 3 years and older with
a diagnosis of pharyngitis that
resulted in an antibiotic order
on or within 3 days after the
episode date and a group A
Streptococcus (Strep) test in
the seven-day period from
three days prior to the episode
date through three days after
the episode date.
National Committee
for Quality
Assurance
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS68
v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for patients
aged 18 years and older for
which the eligible clinician
attests to documenting a list of
current medications using all
immediate resources available
on the date of the encounter.
Centers for Medicare
& Medicaid Services
*
!
(Care
Coordination)
0101 /
N/A
155
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Falls: Plan of Care:
Percentage of patients aged 65
years and older with a history
of falls who had a plan of care
for falls documented within 12
months.
National Committee
for Quality
Assurance
§
N/A /
N/A
226
CMS13
8v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Tobacco Use:
Screening and Cessation
Intervention:
Percentage of patients aged 12
years and older who were
screened for tobacco use one or
more times during the
measurement period AND who
received tobacco cessation
intervention during the
measurement period or in the
six months prior to the
measurement period if
identified as a tobacco user.
National Committee
for Quality
Assurance

B.31. Otolaryngology
PREVIOUSLY FINALIZED MEASURES IN THE OTOLARYNGOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Patient
Safety)
0022 /
N/A
238
CMS15
6v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Use of High-Risk
Medications in Older Adults:
Percentage of patients 65 years
of age and older who were
ordered at least two high-risk
medications from the same
drug class.
National Committee
for Quality
Assurance
*
N/A /
N/A
277
N/A
MIPS CQM
Specifications
Process
Sleep Apnea: Severity
Assessment at Initial
Diagnosis:
Percentage of patients aged 18
years and older with a
diagnosis of obstructive sleep
apnea who had an apnea
hypopnea index (AHI), a
respiratory disturbance index
(RDI), or a respiratory event
index (REI) documented or
measured within 2 months of
initial evaluation for suspected
obstructive sleep apnea.
American Academy
of Sleep Medicine
N/A /
N/A
279
N/A
MIPS CQM
Specifications
Process
Sleep Apnea: Assessment of
Adherence to Obstructive
Sleep Apnea (OSA) Therapy:
Percentage of patients aged 18
years and older with a
diagnosis of obstructive sleep
apnea (OSA) that were
prescribed an evidence-based
therapy that had documentation
that adherence to therapy was
assessed at least annually
through an objective
informatics system or through
self-reporting (if objective
reporting is not available).
American Academy
of Sleep Medicine
*
N/A /
N/A
317
CMS22
v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Screening for
High Blood Pressure and
Follow-Up Documented:
Percentage of patient visits for
patients aged 18 years and
older seen during the
measurement period who were
screened for high blood
pressure AND a recommended
follow-up plan is documented,
as indicated, if blood pressure
is elevated or hypertensive.
Centers for Medicare
& Medicaid Services
!
(Patient
Safety)
0101 /
N/A
318
CMS13
9v13
eCQM
Specifications
Process
Falls: Screening for Future
Fall Risk:
Percentage of patients 65 years
of age and older who were
screened for future fall risk
during the measurement
period.
National Committee
for Quality Assurance
*
!
(Appropriate
Use)
N/A /
N/A
331
N/A
MIPS CQM
Specifications
Process
Adult Sinusitis: Antibiotic
Prescribed for Acute Viral
Sinusitis (Overuse):
Percentage of patients, aged 18
years and older, with a
diagnosis of acute viral
sinusitis who were prescribed
an antibiotic within 10 days
after onset of symptoms.
American Academy
of
Otolaryngology-
Head and Neck
Surgery Foundation

B.31. Otolaryngology
PREVIOUSLY FINALIZED MEASURES IN THE OTOLARYNGOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Appropriate
Use)
N/A /
N/A
332
N/A
MIPS CQM
Specifications
Process
Adult Sinusitis: Appropriate
Choice of Antibiotic:
Amoxicillin With or Without
Clavulanate Prescribed for
Patients with Acute Bacterial
Sinusitis (Appropriate Use):
Percentage of patients aged 18
years and older with a
diagnosis of acute bacterial
sinusitis that were prescribed
amoxicillin, with or without
clavulanate, as a first line
antibiotic at the time of
diagnosis.
American
Academy of
Otolaryngology-
Head and Neck
Surgery Foundation
*
§
!
(Outcome)
N/A /
N/A
355
N/A
MIPS CQM
Specifications
Outcome
Unplanned Reoperation
within the 30-Day
Postoperative Period:
Percentage of patients aged 18
years and older who had any
unplanned reoperation within
the 30-day postoperative
period.
American College of
Surgeons
!
(Outcome)
N/A /
N/A
357
N/A
MIPS CQM
Specifications
Outcome
Surgical Site Infection (SSI):
Percentage of patients aged 18
years and older who had a
surgical site infection (SSI).
American College
of Surgeons
!
(Patient
Experience)
N/A /
N/A
358
N/A
MIPS CQM
Specifications
Process
Patient-Centered Surgical Risk
Assessment and
Communication:
Percentage of patients who
underwent a non-emergency
surgery who had their
personalized risks of
postoperative complications
assessed by their surgical team
prior to surgery using a clinical
data-based, patient-specific risk
calculator and who received
personal discussion of those
risks with the surgeon.
American
College
of Surgeons
*
!
(Care
Coordination)
N/A /
N/A
374
CMS50
v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Closing the Referral Loop:
Receipt of Specialist Report:
Percentage of patients with
referrals, regardless of age, for
which the referring clinician
receives a report from the
clinician to whom the patient
was referred.
Centers for Medicare &
Medicaid Services
!
(Outcome)
N/A /
N/A
398
N/A
MIPS CQM
Specifications
Outcome
Optimal Asthma Control:
Composite measure of the
percentage of pediatric and adult
patients whose asthma is well-
controlled as demonstrated by
one of three age appropriate
patient reported outcome tools
and not at risk for exacerbation.
Minnesota Community
Measurement
§
2152 /
N/A
431
N/A
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Unhealthy
Alcohol Use: Screening &
Brief Counseling:
Percentage of patients aged 18
years and older who were
screened for unhealthy alcohol
use using a systematic screening
method at least once within the
last 12 months AND who
received brief counseling if
identified as an unhealthy
alcohol user.
National Committee
for Quality
Assurance

B.31. Otolaryngology
PREVIOUSLY FINALIZED MEASURES IN THE OTOLARYNGOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Appropriate
Use)
0657 /
N/A
464
N/A
MIPS CQM
Specifications
Process
Otitis Media with Effusion:
Systemic Antimicrobials –
Avoidance of Inappropriate
Use:
Percentage of patients aged 2
months through 12 years with a
diagnosis of OME who were
not prescribed systemic
antimicrobials.
American Academy
of Otolaryngology –
Head and Neck
Surgery Foundation
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers
of Health: Percent of patients
18 years and older screened for
food insecurity, housing
instability, transportation
needs, utility difficulties, and
interpersonal safety.
Centers for Medicare
& Medicaid Services
*
3620 /
N/A
493
N/A
MIPS CQM
Specifications
Process
Adult Immunization Status:
Percentage of patients 19 years
of age and older who are up-to-
date on recommended routine
vaccines for influenza; tetanus
and diphtheria (Td) or tetanus,
diphtheria and acellular
pertussis (Tdap); zoster; and
pneumococcal.
National Committee
for Quality
Assurance
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years or
older who screen positive for
one or more of the following
health related social needs
(HRSNs): food insecurity,
housing instability,
transportation needs, utility
help needs, or interpersonal
safety; and had contact with a
Community Service Provider
(CSP) for at least 1 of their
HRSNs within 60 days after
screening.
OCHIN
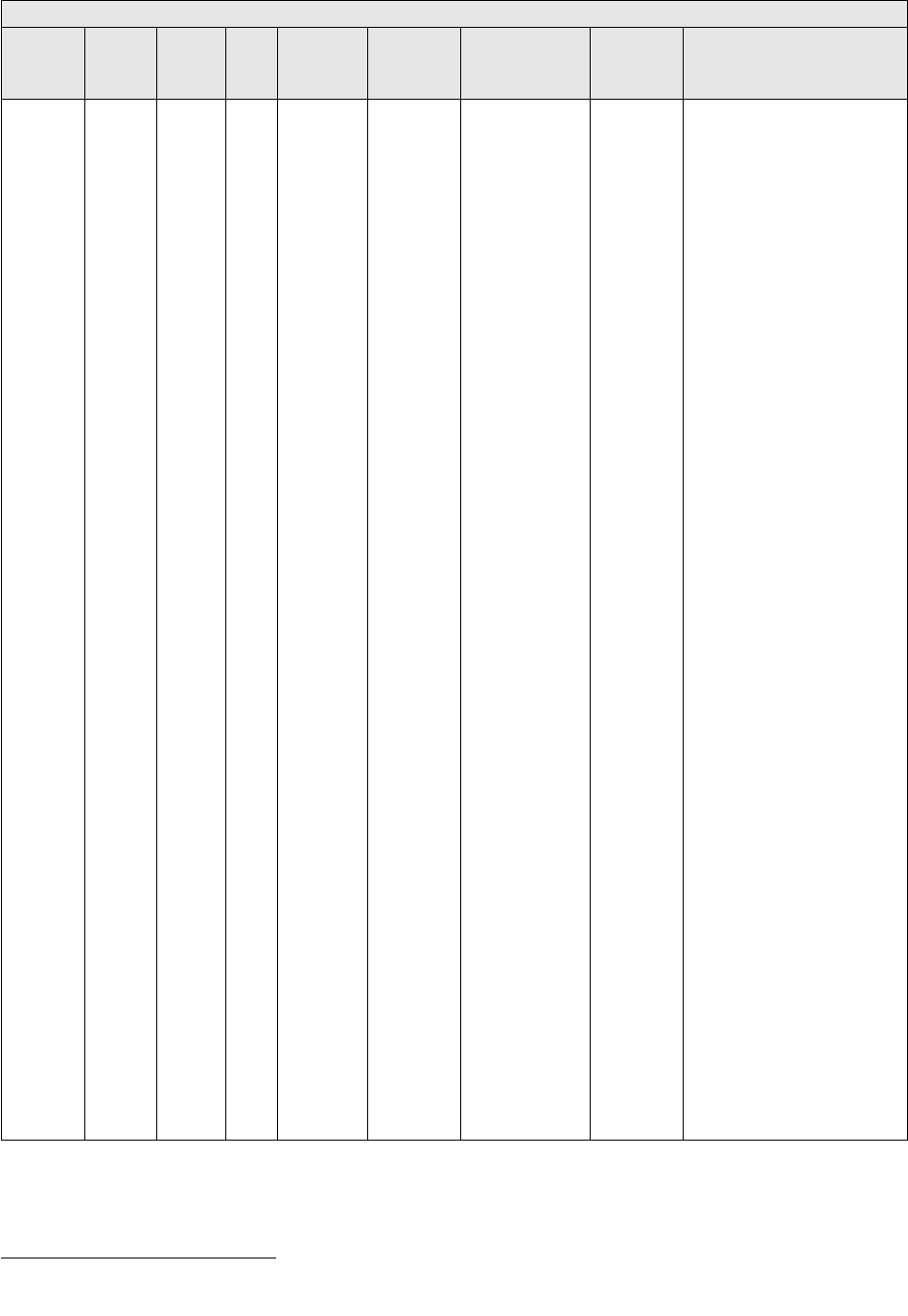
B.31. Otolaryngology
MEASURES PROPOSED FOR ADDITION TO THE OTOLARYNGOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
N/A /
N/A
TBD
N/A
MIPS
CQM
Specificati
ons
Process
Adult COVID-
19 Vaccination
Status:
Percentage of
patients aged 18
years and older
seen for a visit
during the
performance
period that are up
to date on their
COVID-19
vaccinations as
defined by CDC
recommendation
s on current
vaccination.
Centers for
Medicare
&
Medicaid
Services
We are proposing to include this
measure in the Otolaryngology
specialty set as it would be
clinically relevant to this
clinician type. Widespread
vaccination against SARS-CoV-
2, the virus that causes COVID-
19, is critically important to
stemming the morbidity and
mortality caused by this
disease.
117
Clinicians are
uniquely positioned to
encourage uptake of COVID-19
vaccination, and clinicians are
still a major driving force in
promoting patient vaccination.
The addition of this quality
measure in this specialty set
would help strengthen
compliance with recommended
COVID-19 vaccination, leading
to improvement in the quality of
patient care and prevention of
disease for the general
population. This quality measure
aligns with clinical guidelines
and the evidence-based
recommendations of the ACIP,
where there is general agreement
about the safety and efficacy of
the COVID-19 vaccine,
preventing costly and potentially
harmful hospitalizations.
118
Broadening vaccination status
awareness to this clinician type
is valuable as it can help drive
an increase in the adult
vaccination rates. The COVID-
19 vaccination included within
this measure would reduce the
prevalence of severe diseases
that may be associated with
hospitalization and decrease
overall health care costs. The
measure being added to this
specialty set would be
contingent on the inclusion of
applicable coding by the time of
the CY 2025 PFS final rule. In
the event appropriate coding is
not included in the final
specification, this measure
would not be finalized for
inclusion within this specialty
measure set. See Table A.5 of
this Appendix for rationale,
including clinical evidence
supporting the inclusion of this
measure in MIPS.
B.32. Pathology
117
See footnote Ikeokwu et al., 2023 in Table B.1 of this Appendix.
118
See footnotes Fitzpatrick et al., 2022; Polack et al., 2020; and Graña et al., 2022 in Table A.5 of this Appendix.

In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Pathology specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a measure
reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Pathology
specialty set that have proposed substantive changes in Table Group D of this Appendix as indicated by an * in the Indicator
column.
B.32. Pathology
PREVIOUSLY FINALIZED MEASURES IN THE PATHOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure
Steward
*
N/A /
N/A
249
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Barrett’s Esophagus:
Percentage of esophageal biopsy reports
that document the presence of Barrett’s
mucosa that also include a statement
about dysplasia.
College of
American
Pathologists
§
N/A /
N/A
250
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Radical Prostatectomy Pathology
Reporting:
Percentage of radical prostatectomy
pathology reports that include the pT
category, the pN category, the Gleason
score and a statement about margin
status.
College of
American
Pathologists
!
(Care
Coordination)
N/A /
N/A
395
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Lung Cancer Reporting
(Biopsy/Cytology Specimens):
Pathology reports based on lung biopsy
and/or cytology specimens with a
diagnosis of primary non-small cell lung
cancer classified into specific histologic
type following the International
Association for the Study of Lung
Cancer (IASLC) guidance or classified
as non-small cell lung cancer not
otherwise specified (NSCLC-NOS) with
an explanation included in the pathology
report.
College of
American
Pathologists
!
(Care
Coordination)
N/A /
N/A
396
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Lung Cancer Reporting (Resection
Specimens):
Pathology reports based on lung
resection specimens with a diagnosis of
primary lung carcinoma that include the
pT category, pN category and for non-
small cell lung cancer (NSCLC),
histologic type.
College of
American
Pathologists
!
(Care
Coordination)
N/A /
N/A
397
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Melanoma Reporting:
Pathology reports for primary malignant
cutaneous melanoma that include the pT
category, thickness, ulceration and
mitotic rate, peripheral and deep margin
status and presence or absence of
microsatellitosis for invasive tumors.
College of
American
Pathologists
!
(Care
Coordination)
N/A /
N/A
440
N/A
MIPS CQM
Specifications
Process
Skin Cancer: Biopsy Reporting Time –
Pathologist to Clinician:
Percentage of biopsies with a diagnosis
of cutaneous basal cell carcinoma (BCC)
and squamous cell carcinoma (SCC), or
melanoma (including in situ disease) in
which the pathologist communicates
results to the clinician within 7 days
from the time when the tissue specimen
was received by the pathologist.
American
Academy of
Dermatology
!
(Care
Coordination)
3661 /
N/A
491
N/A
MIPS CQM
Specifications
Process
Mismatch Repair (MMR) or
Microsatellite Instability (MSI)
Biomarker Testing Status:
College of
American
Pathologists

B.32. Pathology
PREVIOUSLY FINALIZED MEASURES IN THE PATHOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure
Steward
Percentage of surgical pathology reports
for primary colorectal, endometrial,
gastroesophageal or small bowel
carcinoma, biopsy or resection, that
contain impression or conclusion of or
recommendation for testing of mismatch
repair (MMR) by immunohistochemistry
(biomarkers MLH1, MSH2, MSH6, and
PMS2), or microsatellite instability
(MSI) by DNA-based testing status, or
both.

B.33. Pediatrics
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Pediatrics specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a measure
reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Pediatrics
specialty set that have proposed substantive changes in Table Group D of this Appendix as indicated by an * in the Indicator
column.
B.33. Pediatrics
PREVIOUSLY FINALIZED MEASURES IN THE PEDIATRICS SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
§
!
(Appropriate
Use)
0069 /
N/A
065
CMS15
4v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Appropriate Treatment for
Upper Respiratory Infection
(URI):
Percentage of episodes for
patients 3 months of age and
older with a diagnosis of upper
respiratory infection (URI) that
did not result in an antibiotic
order.
National Committee
for Quality Assurance
§
!
(Appropriate
Use)
N/A /
N/A
066
CMS14
6v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Appropriate Testing for
Pharyngitis:
The percentage of episodes for
patients 3 years and older with
a diagnosis of pharyngitis that
resulted in an antibiotic order
on or within 3 days after the
episode date and a group A
Streptococcus (Strep) test in
the seven-day period from
three days prior to the episode
date through three days after
the episode date.
National Committee
for Quality Assurance
§
!
(Appropriate
Use)
0058 /
N/A
116
N/A
MIPS CQM
Specifications
Process
Avoidance of Antibiotic
Treatment for Acute
Bronchitis/Bronchiolitis:
The percentage of episodes for
patients ages 3 months and
older with a diagnosis of acute
bronchitis/bronchiolitis that did
not result in an antibiotic
dispensing event.
National Committee
for Quality Assurance
§
N/A /
N/A
134
CMS2v
14
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Screening for
Depression and Follow-Up
Plan:
Percentage of patients aged 12
years and older screened for
depression on the date of the
encounter or up to 14 days
prior to the date of the
encounter using an age-
appropriate standardized
depression screening tool AND
if positive, a follow-up plan is
documented on the date of or
up to two days after the date of
the qualifying encounter.
Centers for Medicare
& Medicaid Services
§
N/A /
N/A
205
CMS11
88v2
eCQM
Specifications,
MIPS CQM
Specifications
Process
Sexually Transmitted
Infection (STI) Testing for
People with HIV:
Percentage of patients 13 years
of age and older with a
diagnosis of HIV who had tests
for syphilis, gonorrhea, and
chlamydia performed within
the performance period.
Health Resources and
Services
Administration

B.33. Pediatrics
PREVIOUSLY FINALIZED MEASURES IN THE PEDIATRICS SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
§
N/A /
N/A
226
CMS13
8v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Tobacco Use:
Screening and Cessation
Intervention: Percentage of
patients aged 12 years and
older who were screened for
tobacco use one or more times
during the measurement period
AND who received tobacco
cessation intervention during
the measurement period or in
the six months prior to the
measurement period if
identified as a tobacco user.
National Committee
for Quality Assurance
§
N/A /
N/A
239
CMS15
5v13
eCQM
Specifications
Process
Weight Assessment and
Counseling for Nutrition and
Physical Activity for
Children/Adolescents:
Percentage of patients 3-17
years of age who had an
outpatient visit with a Primary
Care Physician (PCP) or
Obstetrician/Gynecologist
(OB/GYN) and who had
evidence of the following
during the measurement
period.
• Percentage of patients with
height, weight, and body mass
index (BMI) percentile
documentation.
• Percentage of patients with
counseling for nutrition.
• Percentage of patients with
counseling for physical
activity.
National Committee
for Quality Assurance
§
N/A /
N/A
240
CMS11
7v13
eCQM
Specifications
Process
Childhood Immunization
Status:
Percentage of children 2 years
of age who had four diphtheria,
tetanus and acellular pertussis
(DtaP); three polio (IPV), one
measles, mumps and rubella
(MMR); three or four H
influenza type B (Hib); three
hepatitis B (HepB); one
chicken pox (VZV); four
pneumococcal conjugate
(PCV); one hepatitis A
(HepA); two or three rotavirus
(RV); and two influenza (flu)
vaccines by their second
birthday.
National Committee
for Quality Assurance

B.33. Pediatrics
PREVIOUSLY FINALIZED MEASURES IN THE PEDIATRICS SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Opioid)
N/A /
N/A
305
CMS13
7v13
eCQM
Specifications
Process
Initiation and Engagement of
Substance Use Disorder
Treatment:
Percentage of patients 13 years
of age and older with a new
substance use disorder (SUD)
episode who received the
following (Two rates are
reported):
a. Percentage of patients who
initiated treatment, including
either an intervention or
medication for the treatment of
SUD, within 14 days of the
new SUD episode.
b. Percentage of patients who
engaged in ongoing treatment,
including two additional
interventions or medication
treatment events for SUD, or
one long-acting medication
event for the treatment of SUD,
within 34 days of the initiation.
National Committee
for Quality Assurance
§
N/A /
N/A
310
CMS15
3v13
eCQM
Specifications
Process
Chlamydia Screening in
Women:
Percentage of women 16-24
years of age who were
identified as sexually active
and who had at least one test
for chlamydia during the
measurement period.
National Committee
for Quality Assurance
§
N/A /
N/A
366
CMS13
6v14
eCQM
Specifications
Process
Follow-Up Care for Children
Prescribed ADHD
Medication (ADD):
Percentage of children 6-12
years of age and newly
prescribed a medication for
attention-deficit/hyperactivity
disorder (ADHD) who had
appropriate follow-up care.
Two rates are reported.
(a) Percentage of children who
had one follow-up visit with a
practitioner with prescribing
authority during the 30-Day
Initiation Phase.
(b) Percentage of children who
remained on ADHD
medication for at least 210
days and who, in addition to
the visit in the Initiation Phase,
had at least two additional
follow-up visits with a
practitioner within 270 days (9
months) after the Initiation
Phase ended.
National Committee
for Quality Assurance
§
!
(Outcome)
0710 /
0710e
370
CMS15
9v13
eCQM
Specifications,
MIPS CQM
Specifications
Outcome
Depression Remission at
Twelve Months:
The percentage of adolescent
patients 12 to 17 years of age
and adult patients 18 years of
age or older with major
depression or dysthymia who
reached remission 12 months
(+/- 60 days) after an index
event date.
Minnesota Community
Measurement

B.33. Pediatrics
PREVIOUSLY FINALIZED MEASURES IN THE PEDIATRICS SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Patient
Safety)
N/A /
N/A
382
CMS17
7v13
eCQM
Specifications
Process
Child and Adolescent Major
Depressive Disorder (MDD):
Suicide Risk Assessment:
Percentage of patient visits for
those patients aged 6 through
16 years at the start of the
measurement period with a
diagnosis of major depressive
disorder (MDD) with an
assessment for suicide risk.
Mathematica
§
N/A /
N/A
394
N/A
MIPS CQM
Specifications
Process
Immunizations for
Adolescents:
The percentage of adolescents
13 years of age who had one
dose of meningococcal vaccine
(serogroups A, C, W, Y), one
tetanus, diphtheria toxoids and
acellular pertussis (Tdap)
vaccine, and have completed
the Human Papillomavirus
(HPV) vaccine series by their
13
th
birthday.
National Committee
for Quality Assurance
!
(Outcome)
N/A /
N/A
398
N/A
MIPS CQM
Specifications
Outcome
Optimal Asthma Control:
Composite measure of the
percentage of pediatric and
adult patients whose asthma is
well-controlled as
demonstrated by one of three
age appropriate patient
reported outcome tools and not
at risk for exacerbation.
Minnesota Community
Measurement
*
!
(Appropriate
Use)
0657 /
N/A
464
N/A
MIPS CQM
Specifications
Process
Otitis Media with Effusion:
Systemic Antimicrobials –
Avoidance of Inappropriate
Use:
Percentage of patients aged 2
months through 12 years with a
diagnosis of OME who were
not prescribed systemic
antimicrobials.
American Academy of
Otolaryngology –
Head and Neck
Surgery Foundation
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers
of Health:
Percent of patients 18 years
and older screened for food
insecurity, housing instability,
transportation needs, utility
difficulties, and interpersonal
safety.
Centers for Medicare
& Medicaid Services
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years or
older who screen positive for
one or more of the following
health related social needs
(HRSNs): food insecurity,
housing instability,
transportation needs, utility
help needs, or interpersonal
safety; and had contact with a
Community Service Provider
(CSP) for at least 1 of their
HRSNs within 60 days after
screening.
OCHIN

B.34. Physical Medicine
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Physical Medicine specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a
measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Physical Medicine
specialty set that have proposed substantive changes in Table Group D of this Appendix as indicated by an * in the Indicator
column.
B.34. Physical Medicine
PREVIOUSLY FINALIZED MEASURES IN THE PHYSICAL MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Care
Coordination)
0326 /
N/A
047
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Advance Care Plan:
Percentage of patients aged 65
years and older who have an
advance care plan or surrogate
decision maker documented in
the medical record or
documentation in the medical
record that an advance care
plan was discussed but the
patient did not wish or was
not able to name a surrogate
decision maker or provide an
advance care plan.
National Committee for
Quality Assurance
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS
68v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for
patients aged 18 years and
older for which the eligible
clinician attests to
documenting a list of current
medications using all
immediate resources available
on the date of the encounter.
Centers for Medicare &
Medicaid Services
*
!
(Care
Coordination)
0101 /
N/A
155
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Falls: Plan of Care:
Percentage of patients aged 65
years and older with a history
of falls who had a plan of care
for falls documented within
12 months.
National Committee for
Quality Assurance
*
§
!
(Care
Coordination)
N/A /
N/A
182
N/A
MIPS CQM
Specifications
Process
Functional Outcome
Assessment:
Percentage of visits for
patients aged 18 years and
older with documentation of a
current functional outcome
assessment using a
standardized functional
outcome assessment tool on
the date of the encounter
AND documentation of a care
plan based on identified
functional outcome
deficiencies within two days
of the date of the identified
deficiencies.
Centers for Medicare &
Medicaid Services

B.34. Physical Medicine
PREVIOUSLY FINALIZED MEASURES IN THE PHYSICAL MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
§
N/A /
N/A
226
CMS
138v1
3
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Tobacco Use:
Screening and Cessation
Intervention:
Percentage of patients aged 12
years and older who were
screened for tobacco use one
or more times during the
measurement period AND
who received tobacco
cessation intervention during
the measurement period or in
the six months prior to the
measurement period if
identified as a tobacco user.
National Committee for
Quality Assurance
*
N/A /
N/A
317
CMS
22v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Screening for
High Blood Pressure and
Follow-Up Documented:
Percentage of patient visits for
patients aged 18 years and
older seen during the
measurement period who
were screened for high blood
pressure AND a
recommended follow-up plan
is documented, as indicated, if
blood pressure is elevated or
hypertensive.
Centers for Medicare &
Medicaid Services
*
!
(Care
Coordination)
N/A /
N/A
374
CMS
50v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Closing the Referral Loop:
Receipt of Specialist Report:
Percentage of patients with
referrals, regardless of age, for
which the referring clinician
receives a report from the
clinician to whom the patient
was referred.
Centers for Medicare &
Medicaid Services
§
2152 /
N/A
431
N/A
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Unhealthy
Alcohol Use: Screening &
Brief Counseling:
Percentage of patients aged 18
years and older who were
screened for unhealthy
alcohol use using a systematic
screening method at least once
within the last 12 months
AND who received brief
counseling if identified as an
unhealthy alcohol user.
National Committee for
Quality Assurance
!
(Opioid)
N/A /
N/A
468
N/A
MIPS CQM
Specifications
Process
Continuity of
Pharmacotherapy for
Opioid Use Disorder (OUD):
Percentage of adults aged 18
years and older with
pharmacotherapy for opioid
use disorder (OUD) who have
at least 180 days of
continuous treatment.
University of Southern
California
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers
of Health:
Percent of patients 18 years
and older screened for food
insecurity, housing instability,
transportation needs, utility
difficulties, and interpersonal
safety.
Centers for Medicare &
Medicaid Services

B.34. Physical Medicine
PREVIOUSLY FINALIZED MEASURES IN THE PHYSICAL MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years or
older who screen positive for
one or more of the following
health related social needs
(HRSNs): food insecurity,
housing instability,
transportation needs, utility
help needs, or interpersonal
safety; and had contact with a
Community Service Provider
(CSP) for at least 1 of their
HRSNs within 60 days after
screening.
OCHIN

B.35. Physical Therapy/Occupational Therapy
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Physical Therapy/Occupational Therapy specialty set takes additional criteria into consideration, which includes, but is not
limited to: whether a measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types.
We may reassess the appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the
specialty set. Measure tables in this set include previously finalized measures we are maintaining within the set, measures
proposed to be added, and measures proposed for removal, as applicable. We request comment on the measures available in the
proposed Physical Therapy/Occupational Therapy specialty set that have proposed substantive changes in Table Group D of this
Appendix as indicated by an * in the Indicator column.
B.35. Physical Therapy/Occupational Therapy
PREVIOUSLY FINALIZED MEASURES IN THE PHYSICAL THERAPY/OCCUPATIONAL THERAPY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title and
Description
Measure Steward
N/A /
N/A
048
N/A
MIPS CQM
Specifications
Process
Urinary Incontinence:
Assessment of Presence or
Absence of Urinary
Incontinence in Women
Aged 65 Years and Older:
Percentage of female patients
aged 65 years and older who
were assessed for the presence
or absence of urinary
incontinence within 12
months.
National
Committee for
Quality Assurance
!
(Patient
Experience)
N/A /
N/A
050
N/A
MIPS CQM
Specifications
Process
Urinary Incontinence: Plan
of Care for Urinary
Incontinence in Women
Aged 65 Years and Older:
Percentage of female patients
aged 65 years and older with a
diagnosis of urinary
incontinence with a
documented plan of care for
urinary incontinence at least
once within 12 months.
National
Committee for
Quality Assurance
N/A /
N/A
126
N/A
MIPS CQM
Specifications
Process
Diabetes Mellitus: Diabetic
Foot and Ankle Care,
Peripheral Neuropathy –
Neurological Evaluation:
Percentage of patients aged 18
years and older with a
diagnosis of diabetes mellitus
who had a neurological
examination of their lower
extremities within 12 months.
American Podiatric
Medical
Association
N/A /
N/A
127
N/A
MIPS CQM
Specifications
Process
Diabetes Mellitus: Diabetic
Foot and Ankle Care, Ulcer
Prevention – Evaluation of
Footwear:
Percentage of patients aged 18
years and older with a
diagnosis of diabetes mellitus
who were evaluated for proper
footwear and sizing.
American Podiatric
Medical
Association
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS68
v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for
patients aged 18 years and
older for which the eligible
clinician attests to
documenting a list of current
medications using all
immediate resources available
on the date of the encounter.
Centers for
Medicare &
Medicaid Services

B.35. Physical Therapy/Occupational Therapy
PREVIOUSLY FINALIZED MEASURES IN THE PHYSICAL THERAPY/OCCUPATIONAL THERAPY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title and
Description
Measure Steward
§
N/A /
N/A
134
CMS2v
14
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Screening for
Depression and Follow-Up
Plan:
Percentage of patients aged 12
years and older screened for
depression on the date of the
encounter or up to 14 days
prior to the date of the
encounter using an age-
appropriate standardized
depression screening tool
AND if positive, a follow-up
plan is documented on the
date of or up to two days after
the date of the qualifying
encounter.
Centers for
Medicare &
Medicaid Services
*
!
(Care
Coordination)
0101 /
N/A
155
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Falls: Plan of Care:
Percentage of patients aged 65
years and older with a history
of falls who had a plan of care
for falls documented within
12 months.
National
Committee for
Quality Assurance
*
!
(Patient
Safety)
N/A /
N/A
181
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Elder Maltreatment Screen
and Follow-Up Plan:
Percentage of patients aged 60
years and older with a
documented elder
maltreatment screen using an
Elder Maltreatment Screening
tool on the date of encounter
AND a documented follow-up
plan on the date of the
positive screen.
Centers for
Medicare &
Medicaid Services
*
§
!
(Care
Coordination)
N/A /
N/A
182
N/A
MIPS CQM
Specifications
Process
Functional Outcome
Assessment:
Percentage of visits for
patients aged 18 years and
older with documentation of a
current functional outcome
assessment using a
standardized functional
outcome assessment tool on
the date of the encounter AND
documentation of a care plan
based on identified functional
outcome deficiencies within
two days of the date of the
identified deficiencies.
Centers for
Medicare &
Medicaid Services
!
(Outcome)
N/A /
N/A
217
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Functional Status Change
for Patients with Knee
Impairments:
A patient-reported outcome
measure (PROM) of risk-
adjusted change in functional
status (FS) for patients 14
years+ with knee
impairments. The change in
FS is assessed using the
FOTO Lower Extremity
Physical Function (LEPF)
PROM. The measure is
adjusted to patient
characteristics known to be
associated with FS outcomes
(risk-adjusted) and used as a
performance measure at the
patient, individual clinician,
and clinic levels to assess
quality.
Focus on
Therapeutic
Outcomes, Inc.

B.35. Physical Therapy/Occupational Therapy
PREVIOUSLY FINALIZED MEASURES IN THE PHYSICAL THERAPY/OCCUPATIONAL THERAPY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title and
Description
Measure Steward
!
(Outcome)
N/A /
N/A
218
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Functional Status Change
for Patients with Hip
Impairments:
A patient-reported outcome
measure (PROM) of risk-
adjusted change in functional
status (FS) for patients 14
years+ with hip impairments.
The change in FS is assessed
using the FOTO Lower
Extremity Physical Function
(LEPF) PROM. The measure
is adjusted to patient
characteristics known to be
associated with FS outcomes
(risk adjusted) and used as a
performance measure at the
patient, individual clinician,
and clinic levels to assess
quality.
Focus on
Therapeutic
Outcomes, Inc.
!
(Outcome)
N/A /
N/A
219
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Functional Status Change
for Patients with Lower Leg,
Foot or Ankle Impairments:
A patient-reported outcome
measure (PROM) of risk-
adjusted change in functional
status (FS) for patients 14
years+ with foot, ankle or
lower leg impairments. The
change in FS is assessed using
the FOTO Lower Extremity
Physical Function (LEPF)
PROM. The measure is
adjusted to patient
characteristics known to be
associated with FS outcomes
(risk-adjusted) and used as a
performance measure at the
patient, individual clinician,
and clinic levels to assess
quality.
Focus on
Therapeutic
Outcomes, Inc.
!
(Outcome)
N/A /
N/A
220
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Functional Status Change
for Patients with Low Back
Impairments:
A patient-reported outcome
measure (PROM) of risk-
adjusted change in functional
status (FS) for patients 14
years+ with low back
impairments. The change in
FS is assessed using the
FOTO Low Back FS PROM.
The measure is adjusted to
patient characteristics known
to be associated with FS
outcomes (risk adjusted) and
used as a performance
measure at the patient,
individual clinician, and clinic
levels to assess quality.
Focus on
Therapeutic
Outcomes, Inc.

B.35. Physical Therapy/Occupational Therapy
PREVIOUSLY FINALIZED MEASURES IN THE PHYSICAL THERAPY/OCCUPATIONAL THERAPY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title and
Description
Measure Steward
!
(Outcome)
N/A /
N/A
221
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Functional Status Change
for Patients with Shoulder
Impairments:
A patient-reported outcome
measure (PROM) of risk-
adjusted change in functional
status (FS) for patients 14
years+ with shoulder
impairments. The change in
FS is assessed using the
FOTO Shoulder FS PROM.
The measure is adjusted to
patient characteristics known
to be associated with FS
outcomes (risk adjusted) and
used as a performance
measure at the patient,
individual clinician, and clinic
levels to assess quality.
Focus on
Therapeutic
Outcomes, Inc.
!
(Outcome)
N/A /
N/A
222
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Functional Status Change
for Patients with Elbow,
Wrist or Hand
Impairments:
A patient-reported outcome
measure (PROM) of risk-
adjusted change in functional
status (FS) for patients 14
years+ with elbow, wrist, or
hand impairments. The
change in FS is assessed using
the FOTO Elbow/Wrist/Hand
FS PROM. The measure is
adjusted to patient
characteristics known to be
associated with FS outcomes
(risk adjusted) and used as a
performance measure at the
patient, individual clinician,
and clinic levels to assess
quality.
Focus on
Therapeutic
Outcomes, Inc.
§
N/A /
N/A
226
CMS13
8v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Tobacco Use:
Screening and Cessation
Intervention: Percentage of
patients aged 12 years and
older who were screened for
tobacco use one or more times
during the measurement
period AND who received
tobacco cessation intervention
during the measurement
period or in the six months
prior to the measurement
period if identified as a
tobacco user.
National
Committee for
Quality Assurance
*
N/A /
2872e
281
CMS14
9v13
eCQM
Specifications
Process
Dementia: Cognitive
Assessment: Percentage of
patients, regardless of age,
with a diagnosis of dementia
for whom an assessment of
cognition is performed and the
results reviewed at least once
within a 12-month period.
American Academy
of Neurology

B.35. Physical Therapy/Occupational Therapy
PREVIOUSLY FINALIZED MEASURES IN THE PHYSICAL THERAPY/OCCUPATIONAL THERAPY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title and
Description
Measure Steward
*
!
(Patient
Safety)
N/A /
N/A
286
N/A
MIPS CQM
Specifications
Process
Dementia: Safety Concern
Screening and Follow-Up
for Patients with Dementia:
Percentage of patients with
dementia or their caregiver(s)
for whom there was a
documented safety concerns
screening in two domains of
risk: (1) dangerousness to self
or others and (2)
environmental risks; and if
safety concerns screening was
positive in the last 12 months,
there was documentation of
mitigation recommendations,
including but not limited to
referral to other resources.
American
Psychiatric
Association/
American Academy
of Neurology
*
!
(Care
Coordination)
N/A /
N/A
288
N/A
MIPS CQM
Specifications
Process
Dementia: Education and
Support of Caregivers for
Patients with Dementia:
Percentage of patients with
dementia whose caregiver(s)
were provided with education
on dementia disease
management and health
behavior changes AND were
referred to additional
resources for support in the
last 12 months.
American Academy
of Neurology /
American
Psychiatric
Association
*
N/A /
N/A
291
N/A
MIPS CQM
Specifications
Process
Assessment of Cognitive
Impairment or Dysfunction
for Patients with
Parkinson’s Disease:
Percentage of all patients with
a diagnosis of Parkinson’s
Disease (PD) who were
assessed for cognitive
impairment or dysfunction
once during the measurement
period.
American Academy
of Neurology
!
(Patient
Safety)
0101 /
N/A
318
CMS13
9v13
eCQM
Specifications
Process
Falls: Screening for Future
Fall Risk:
Percentage of patients 65
years of age and older who
were screened for future fall
risk during the measurement
period.
National
Committee for
Quality Assurance
§
!
(Outcome)
N/A /
N/A
478
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Functional Status Change
for Patients with Neck
Impairments:
A patient-reported outcome
measure (PROM) of risk-
adjusted change in functional
status (FS) for patients 14
years+ with neck
impairments. The change in
FS is assessed using the
FOTO Neck FS PROM. The
measure is adjusted to patient
characteristics known to be
associated with FS outcomes
(risk-adjusted) and used as a
performance measure at the
patient, individual clinician,
and clinic levels to assess
quality.
Focus on
Therapeutic
Outcomes, Inc.

B.35. Physical Therapy/Occupational Therapy
PREVIOUSLY FINALIZED MEASURES IN THE PHYSICAL THERAPY/OCCUPATIONAL THERAPY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title and
Description
Measure Steward
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers
of Health:
Percent of patients 18 years
and older screened for food
insecurity, housing instability,
transportation needs, utility
difficulties, and interpersonal
safety.
Centers for
Medicare &
Medicaid Services
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years or
older who screen positive for
one or more of the following
health related social needs
(HRSNs): food insecurity,
housing instability,
transportation needs, utility
help needs, or interpersonal
safety; and had contact with a
Community Service Provider
(CSP) for at least 1 of their
HRSNs within 60 days after
screening.
OCHIN
!
(Outcome)
N/A /
N/A
502
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Improvement or
Maintenance of Functioning
for Individuals with a
Mental and/or Substance
Use Disorder:
The percentage of patients
aged 18 and older with a
mental and/or substance use
disorder who demonstrated
improvement or maintenance
of functioning based on
results from the 12-item
World Health Organization
Disability Assessment
Schedule (WHODAS 2.0) or
Sheehan Disability Scale
(SDS) 30 to 180 days after an
index assessment.
American
Psychiatric
Association
*
!
(Outcome)
2483 /
N/A
503
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Gains in Patient Activation
Measure (PAM®) Scores at
12 Months:
The Patient Activation
Measure® (PAM®) is a 10 –
or 13 – item questionnaire that
assesses an individual´s
knowledge, skills and
confidence for managing their
health and health care. The
measure assesses individuals
on a 0-100 scale that converts
to one of four levels of
activation, from low (1) to
high (4). The PAM®
performance measure
(PAM®-PM) is the change in
score on the PAM® from
baseline to follow-up
measurement.
Insignia Health,
LLC, a wholly
owned subsidiary
of Phreesia

B.36. Plastic Surgery
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Plastic Surgery specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a
measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Plastic Surgery
specialty set that have proposed substantive changes in Table Group D of this Appendix as indicated by an * in the Indicator
column.
B.36. Plastic Surgery
PREVIOUSLY FINALIZED MEASURES IN THE PLASTIC SURGERY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS68
v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for
patients aged 18 years and
older for which the eligible
clinician attests to
documenting a list of current
medications using all
immediate resources
available on the date of the
encounter.
Centers for Medicare &
Medicaid Services
§
N/A /
N/A
226
CMS13
8v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Tobacco Use:
Screening and Cessation
Intervention:
Percentage of patients aged
12 years and older who were
screened for tobacco use one
or more times during the
measurement period AND
who received tobacco
cessation intervention during
the measurement period or in
the six months prior to the
measurement period if
identified as a tobacco user.
National Committee for
Quality Assurance
*
N/A /
N/A
317
CMS22
v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Screening for
High Blood Pressure and
Follow-Up Documented:
Percentage of patient visits
for patients aged 18 years and
older seen during the
measurement period who
were screened for high blood
pressure AND a
recommended follow-up plan
is documented, as indicated,
if blood pressure is elevated
or hypertensive.
Centers for Medicare &
Medicaid Services
*
§
!
(Outcome)
N/A /
N/A
355
N/A
MIPS CQM
Specifications
Outcome
Unplanned Reoperation
within the 30-Day
Postoperative Period:
Percentage of patients aged
18 years and older who had
any unplanned reoperation
within the 30-day
postoperative period.
American College of
Surgeons

B.36. Plastic Surgery
PREVIOUSLY FINALIZED MEASURES IN THE PLASTIC SURGERY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Outcome)
N/A /
N/A
356
N/A
MIPS CQM
Specifications
Outcome
Unplanned Hospital
Readmission within 30
Days of Principal
Procedure:
Percentage of patients aged
18 years and older who had
an unplanned hospital
readmission within 30 days
of principal procedure.
American College of
Surgeons
!
(Outcome)
N/A /
N/A
357
N/A
MIPS CQM
Specifications
Outcome
Surgical Site Infection
(SSI):
Percentage of patients aged
18 years and older who had a
surgical site infection (SSI).
American College of
Surgeons
!
(Patient
Experience)
N/A /
N/A
358
N/A
MIPS CQM
Specifications
Process
Patient-Centered Surgical
Risk Assessment and
Communication: Percentage
of patients who underwent a
non-emergency surgery who
had their personalized risks
of postoperative
complications assessed by
their surgical team prior to
surgery using a clinical data-
based, patient-specific risk
calculator and who received
personal discussion of those
risks with the surgeon.
American College of
Surgeons
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social
Drivers of Health:
Percent of patients 18 years
and older screened for food
insecurity, housing
instability, transportation
needs, utility difficulties, and
interpersonal safety.
Centers for Medicare &
Medicaid Services
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years
or older who screen positive
for one or more of the
following health related
social needs (HRSNs): food
insecurity, housing
instability, transportation
needs, utility help needs, or
interpersonal safety; and had
contact with a Community
Service Provider (CSP) for at
least 1 of their HRSNs within
60 days after screening.
OCHIN

B.37. Podiatry
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Podiatry specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a measure
reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Podiatry specialty
set that have proposed substantive changes in Table Group D of this Appendix as indicated by an * in the Indicator column.
B.37. Podiatry
PREVIOUSLY FINALIZED MEASURES IN THE PODIATRY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
N/A /
N/A
126
N/A
MIPS CQM
Specifications
Process
Diabetes Mellitus: Diabetic
Foot and Ankle Care,
Peripheral Neuropathy –
Neurological Evaluation:
Percentage of patients aged 18
years and older with a
diagnosis of diabetes mellitus
who had a neurological
examination of their lower
extremities within 12 months.
American Podiatric
Medical Association
N/A /
N/A
127
N/A
MIPS CQM
Specifications
Process
Diabetes Mellitus: Diabetic
Foot and Ankle Care, Ulcer
Prevention – Evaluation of
Footwear:
Percentage of patients aged 18
years and older with a
diagnosis of diabetes mellitus
who were evaluated for proper
footwear and sizing.
American Podiatric
Medical Association
*
!
(Care
Coordination)
0101 /
N/A
155
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Falls: Plan of Care:
Percentage of patients aged 65
years and older with a history
of falls who had a plan of care
for falls documented within 12
months.
National Committee
for Quality Assurance
!
(Outcome)
N/A /
N/A
219
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performanc
e Measure
Functional Status Change
for Patients with Lower Leg,
Foot or Ankle Impairments:
A patient-reported outcome
measure (PROM) of risk-
adjusted change in functional
status (FS) for patients 14
years+ with foot, ankle or
lower leg impairments. The
change in FS is assessed using
the FOTO Lower Extremity
Physical Function (LEPF)
PROM. The measure is
adjusted to patient
characteristics known to be
associated with FS outcomes
(risk-adjusted) and used as a
performance measure at the
patient, individual clinician,
and clinic levels to assess
quality.
Focus on Therapeutic
Outcomes, Inc.

B.37. Podiatry
PREVIOUSLY FINALIZED MEASURES IN THE PODIATRY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
§
N/A /
N/A
226
CMS
138v1
3
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Tobacco Use:
Screening and Cessation
Intervention:
Percentage of patients aged 12
years and older who were
screened for tobacco use one
or more times during the
measurement period AND
who received tobacco
cessation intervention during
the measurement period or in
the six months prior to the
measurement period if
identified as a tobacco user.
National Committee
for Quality Assurance
*
N/A /
N/A
317
CMS
22v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Screening for
High Blood Pressure and
Follow-Up Documented:
Percentage of patient visits for
patients aged 18 years and
older seen during the
measurement period who were
screened for high blood
pressure AND a recommended
follow-up plan is documented,
as indicated, if blood pressure
is elevated or hypertensive.
Centers for Medicare &
Medicaid Services
!
(Patient
Safety)
0101 /
N/A
318
CMS
139v1
3
eCQM
Specifications
Process
Falls: Screening for Future
Fall Risk:
Percentage of patients 65 years
of age and older who were
screened for future fall risk
during the measurement
period.
National Committee
for Quality Assurance
!
(Patient
Experience)
N/A /
N/A
358
N/A
MIPS CQM
Specifications
Process
Patient-Centered Surgical
Risk Assessment and
Communication:
Percentage of patients who
underwent a non-emergency
surgery who had their
personalized risks of
postoperative complications
assessed by their surgical team
prior to surgery using a
clinical data-based, patient-
specific risk calculator and
who received personal
discussion of those risks with
the surgeon.
American College of
Surgeons
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers
of Health:
Percent of patients 18 years
and older screened for food
insecurity, housing instability,
transportation needs, utility
difficulties, and interpersonal
safety.
Centers for Medicare &
Medicaid Services

B.37. Podiatry
PREVIOUSLY FINALIZED MEASURES IN THE PODIATRY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years or
older who screen positive for
one or more of the following
health related social needs
(HRSNs): food insecurity,
housing instability,
transportation needs, utility
help needs, or interpersonal
safety; and had contact with a
Community Service Provider
(CSP) for at least 1 of their
HRSNs within 60 days after
screening.
OCHIN
*
!
(Outcome)
2483 /
N/A
503
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performan
ce
Measure
Gains in Patient Activation
Measure (PAM®) Scores at
12 Months:
The Patient Activation
Measure® (PAM®) is a 10 –
or 13 – item questionnaire that
assesses an individual´s
knowledge, skills and
confidence for managing their
health and health care. The
measure assesses individuals
on a 0-100 scale that converts
to one of four levels of
activation, from low (1) to
high (4). The PAM®
performance measure (PAM®-
PM) is the change in score on
the PAM® from baseline to
follow-up measurement.
Insignia Health, LLC, a
wholly owned
subsidiary of Phreesia

B.38. Preventive Medicine
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Preventive Medicine specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a
measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Preventive
Medicine specialty set.
B.38. Preventive Medicine
PREVIOUSLY FINALIZED MEASURES IN THE PREVENTIVE MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection Type
Measure
Type
Measure Title
and Description
Measure
Steward
*
§
!
(Outcome)
0059 /
N/A
001
CMS
122v1
3
Medicare Part B
Claims Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Intermediat
e Outcome
Diabetes: Hemoglobin A1c
(HbA1c) Poor Control (> 9%):
Percentage of patients 18-75
years of age with diabetes who
had hemoglobin A1c > 9.0%
during the measurement period.
National
Committee for
Quality
Assurance
!
(Care
Coordination)
N/A /
N/A
024
N/A
Medicare Part B
Claims Measure
Specifications,
MIPS CQM
Specifications
Process
Communication with the
Physician or Other Clinician
Managing On-Going Care Post-
Fracture for Men and Women
Aged 50 Years and Older:
Percentage of patients aged 50
years and older treated for a
fracture with documentation of
communication, between the
physician treating the fracture
and the physician or other
clinician managing the patient’s
on-going care, that a fracture
occurred and that the patient was
or should be considered for
osteoporosis treatment or testing.
This measure is submitted by the
physician who treats the fracture
and who therefore is held
accountable for the
communication.
National
Committee for
Quality
Assurance
0046 /
N/A
039
N/A
Medicare Part B
Claims Measure
Specifications,
MIPS CQM
Specifications
Process
Screening for Osteoporosis for
Women Aged 65-85 Years of
Age:
Percentage of women aged 65-85
years of age who ever had a
central dual-energy X-ray
absorptiometry (DXA) to check
for osteoporosis.
National
Committee for
Quality
Assurance
*
!
(Care
Coordination)
0326 /
N/A
047
N/A
Medicare Part B
Claims Measure
Specifications,
MIPS CQM
Specifications
Process
Advance Care Plan:
Percentage of patients aged 65
years and older who have an
advance care plan or surrogate
decision maker documented in
the medical record or
documentation in the medical
record that an advance care plan
was discussed but the patient did
not wish or was not able to name
a surrogate decision maker or
provide an advance care plan.
National
Committee for
Quality
Assurance
N/A /
N/A
048
N/A
MIPS CQM
Specifications
Process
Urinary Incontinence:
Assessment of Presence or
Absence of Urinary
Incontinence in Women Aged
65 Years and Older:
Percentage of female patients
aged 65 years and older who
were assessed for the presence or
absence of urinary incontinence
within 12 months.
National
Committee for
Quality
Assurance

B.38. Preventive Medicine
PREVIOUSLY FINALIZED MEASURES IN THE PREVENTIVE MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection Type
Measure
Type
Measure Title
and Description
Measure
Steward
§
!
(Appropriate
Use)
0058 /
N/A
116
N/A
MIPS CQM
Specifications
Process
Avoidance of Antibiotic
Treatment for Acute
Bronchitis/Bronchiolitis:
The percentage of episodes for
patients ages 3 months and older
with a diagnosis of acute
bronchitis/bronchiolitis that did
not result in an antibiotic
dispensing event.
National
Committee for
Quality
Assurance
N/A /
N/A
126
N/A
MIPS CQM
Specifications
Process
Diabetes Mellitus: Diabetic
Foot and Ankle Care,
Peripheral Neuropathy –
Neurological Evaluation:
Percentage of patients aged 18
years and older with a diagnosis
of diabetes mellitus who had a
neurological examination of their
lower extremities within 12
months.
American
Podiatric
Medical
Association
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS
68v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for patients
aged 18 years and older for which
the eligible clinician attests to
documenting a list of current
medications using all immediate
resources available on the date of
the encounter.
Centers for
Medicare &
Medicaid
Services
§
N/A /
N/A
134
CMS
2v14
Medicare Part B
Claims Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and Screening:
Screening for Depression and
Follow-Up Plan:
Percentage of patients aged 12
years and older screened for
depression on the date of the
encounter or up to 14 days prior
to the date of the encounter using
an age-appropriate standardized
depression screening tool AND if
positive, a follow-up plan is
documented on the date of or up
to two days after the date of the
qualifying encounter.
Centers for
Medicare &
Medicaid
Services
*
!
(Care
Coordination)
0101 /
N/A
155
N/A
Medicare Part B
Claims Measure
Specifications,
MIPS CQM
Specifications
Process
Falls: Plan of Care:
Percentage of patients aged 65
years and older with a history of
falls who had a plan of care for
falls documented within 12
months.
National
Committee for
Quality
Assurance
*
§
!
(Care
Coordination)
N/A /
N/A
182
N/A
MIPS CQM
Specifications
Process
Functional Outcome
Assessment:
Percentage of visits for patients
aged 18 years and older with
documentation of a current
functional outcome assessment
using a standardized functional
outcome assessment tool on the
date of the encounter AND
documentation of a care plan
based on identified functional
outcome deficiencies within two
days of the date of the identified
deficiencies.
Centers for
Medicare &
Medicaid
Services

B.38. Preventive Medicine
PREVIOUSLY FINALIZED MEASURES IN THE PREVENTIVE MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection Type
Measure
Type
Measure Title
and Description
Measure
Steward
!
(Care
Coordination)
0643 /
N/A
243
N/A
MIPS CQM
Specifications
Process
Cardiac Rehabilitation Patient
Referral from an Outpatient
Setting:
Percentage of patients evaluated
in an outpatient setting who
within the previous 12 months
have experienced an acute
myocardial infarction (MI),
coronary artery bypass graft
(CABG) surgery, a percutaneous
coronary intervention (PCI),
cardiac valve surgery, or cardiac
transplantation, or who have
chronic stable angina (CSA) and
have not already participated in
an early outpatient cardiac
rehabilitation/secondary
prevention (CR) program for the
qualifying event/diagnosis who
were referred to a CR program.
American Heart
Association
*
!
(Care
Coordination)
N/A /
N/A
374
CMS
50v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Closing the Referral Loop:
Receipt of Specialist Report:
Percentage of patients with
referrals, regardless of age, for
which the referring clinician
receives a report from the
clinician to whom the patient was
referred.
Centers for
Medicare &
Medicaid
Services
§
2152 /
N/A
431
N/A
MIPS CQM
Specifications
Process
Preventive Care and Screening:
Unhealthy Alcohol Use:
Screening & Brief Counseling:
Percentage of patients aged 18
years and older who were
screened for unhealthy alcohol
use using a systematic screening
method at least once within the
last 12 months AND who
received brief counseling if
identified as an unhealthy alcohol
user.
National
Committee for
Quality
Assurance
§
N/A /
N/A
438
CMS
347v8
eCQM
Specifications,
MIPS CQM
Specifications
Process
Statin Therapy for the
Prevention and Treatment of
Cardiovascular
Disease:
Percentage of the following
patients - all considered at high
risk of cardiovascular events -
who were prescribed or were on
statin therapy during the
performance period:
•All patients who were previously
diagnosed with or currently have
a diagnosis of clinical
atherosclerotic cardiovascular
disease (ASCVD), including an
ASCVD procedure; OR
•Patients aged 20 to 75 years who
have ever had a low-density
lipoprotein cholesterol (LDL-C)
level ≥ 190 mg/dL or were
previously diagnosed with or
currently have an active diagnosis
of familial hypercholesterolemia;
OR
•Patients aged 40 to 75 years with
a diagnosis of diabetes; OR
•Patients aged 40 to 75 with a 10-
year ASCVD risk score of ≥ 20
percent.
Centers for
Medicare &
Medicaid
Services

B.38. Preventive Medicine
PREVIOUSLY FINALIZED MEASURES IN THE PREVENTIVE MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection Type
Measure
Type
Measure Title
and Description
Measure
Steward
§
N/A /
N/A
475
CMS
349v7
eCQM
Specifications
Process
HIV Screening:
Percentage of patients aged 15-65
at the start of the measurement
period who were between 15-65
years old when tested for Human
Immunodeficiency Virus (HIV).
Centers for
Disease Control
and Prevention
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers of
Health:
Percent of patients 18 years and
older screened for food
insecurity, housing instability,
transportation needs, utility
difficulties, and interpersonal
safety.
Centers for
Medicare &
Medicaid
Services
*
N/A /
N/A
488
CMS
951v3
eCQM
Specifications,
MIPS CQM
Specifications
Process
Kidney Health Evaluation:
Percentage of patients aged 18-75
years with a diagnosis of diabetes
who received a kidney health
evaluation defined by an
Estimated Glomerular Filtration
Rate (eGFR) AND Urine
Albumin-Creatinine Ratio
(uACR) within the measurement
period.
National Kidney
Foundation
*
3620 /
N/A
493
N/A
MIPS CQM
Specifications
Process
Adult Immunization Status:
Percentage of patients 19 years of
age and older who are up-to-date
on recommended routine
vaccines for influenza; tetanus
and diphtheria (Td) or tetanus,
diphtheria and acellular pertussis
(Tdap); zoster; and
pneumococcal.
National
Committee for
Quality
Assurance
*
N/A /
N/A
497
N/A
MIPS CQM
Specifications
Process
Preventive Care and Wellness
(composite):
Percentage of patients who
received age- and sex-appropriate
preventive screenings and
wellness services. This measure
is a composite of seven
component measures that are
based on recommendations for
preventive care by the U.S.
Preventive Services Task Force
(USPSTF), ACIP, American
Association of Clinical
Endocrinology (AACE), and
American College of
Endocrinology (ACE).
Centers for
Medicare and
Medicaid
Services
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years or
older who screen positive for one
or more of the following health
related social needs (HRSNs):
food insecurity, housing
instability, transportation needs,
utility help needs, or
interpersonal safety; and had
contact with a Community
Service Provider (CSP) for at
least 1 of their HRSNs within 60
days after screening.
OCHIN

B.38. Preventive Medicine
PREVIOUSLY FINALIZED MEASURES IN THE PREVENTIVE MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection Type
Measure
Type
Measure Title
and Description
Measure
Steward
*
!
(Outcome)
2483 /
N/A
503
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performanc
e Measure
Gains in Patient Activation
Measure (PAM®) Scores at 12
Months:
The Patient Activation Measure®
(PAM®) is a 10 – or 13 – item
questionnaire that assesses an
individual´s knowledge, skills
and confidence for managing
their health and health care. The
measure assesses individuals on a
0-100 scale that converts to one
of four levels of activation, from
low (1) to high (4). The PAM®
performance measure (PAM®-
PM) is the change in score on the
PAM® from baseline to follow-
up measurement.
Insignia Health,
LLC, a wholly
owned
subsidiary of
Phreesia

B.38. Preventive Medicine
MEASURES PROPOSED FOR ADDITION TO THE PREVENTIVE MEDICINE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
N/A /
N/A
TBD
N/A
MIPS
CQM
Specificati
ons
Process
Adult COVID-
19 Vaccination
Status:
Percentage of
patients aged 18
years and older
seen for a visit
during the
performance
period that are up
to date on their
COVID-19
vaccinations as
defined by CDC
recommendation
s on current
vaccination.
Centers
for
Medicare
&
Medicaid
Services
We are proposing to include this
measure in the Preventive
Medicine specialty set as it would
be clinically relevant to this
clinician type. Widespread
vaccination against SARS-CoV-2,
the virus that causes COVID-19,
is critically important to stemming
the morbidity and mortality
caused by this disease.
119
Clinicians are uniquely positioned
to encourage uptake of COVID-
19 vaccination, and clinicians are
still a major driving force in
promoting patient vaccination.
The addition of this quality
measure in this specialty set
would help strengthen compliance
with recommended COVID-19
vaccination, leading to
improvement in the quality of
patient care and prevention of
disease for the general population.
This quality measure aligns with
clinical guidelines and the
evidence-based recommendations
of the ACIP, where there is
general agreement about the
safety and efficacy of the
COVID-19 vaccine, preventing
costly and potentially harmful
hospitalizations.
120
Broadening
vaccination status awareness to
this clinician type is valuable as it
can help drive an increase in the
adult vaccination rates. The
COVID-19 vaccination included
within this measure would reduce
the prevalence of severe diseases
that may be associated with
hospitalization and decrease
overall health care costs. The
measure being added to this
specialty set would be contingent
on the inclusion of applicable
coding by the time of the CY
2025 PFS final rule. In the event
appropriate coding is not included
in the final specification, this
measure would not be finalized
for inclusion within this specialty
measure set. See Table A.5 of this
Appendix for rationale, including
clinical evidence supporting the
inclusion of this measure in
MIPS.
119
See footnote Ikeokwu et al., 2023 in Table B.1 of this Appendix.
120
See footnotes Fitzpatrick et al., 2022; Polack et al., 2020; and Graña et al., 2022 in Table A.5 of this Appendix.

B.39. Pulmonology
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Pulmonology specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a measure
reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Pulmonology
specialty set.
B.39. Pulmonology
PREVIOUSLY FINALIZED MEASURES IN THE PULMONOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure
Steward
*
!
(Care
Coordination)
0326 /
N/A
047
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Advance Care Plan:
Percentage of patients aged 65
years and older who have an
advance care plan or surrogate
decision maker documented in the
medical record or documentation
in the medical record that an
advance care plan was discussed
but the patient did not wish or was
not able to name a surrogate
decision maker or provide an
advance care plan.
National
Committee for
Quality
Assurance
0102 /
N/A
052
N/A
MIPS CQM
Specifications
Process
Chronic Obstructive Pulmonary
Disease (COPD): Spirometry
Evaluation and Long-Acting
Inhaled Bronchodilator
Therapy:
Percentage of patients aged 18
years and older with a diagnosis of
COPD with a documented
FEV1/FVC < 70% measured by
spirometry, who are symptomatic
and were prescribed a long-acting
inhaled bronchodilator.
American
Thoracic
Society
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS68
v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for patients
aged 18 years and older for which
the eligible clinician attests to
documenting a list of current
medications using all immediate
resources available on the date of
the encounter.
Centers for
Medicare &
Medicaid
Services
§
N/A /
N/A
226
CMS13
8v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and Screening:
Tobacco Use: Screening and
Cessation Intervention:
Percentage of patients aged 12
years and older who were screened
for tobacco use one or more times
during the measurement period
AND who received tobacco
cessation intervention during the
measurement period or in the six
months prior to the measurement
period if identified as a tobacco
user.
National
Committee for
Quality
Assurance
*
§
!
(Outcome)
N/A /
N/A
236
CMS16
5v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Intermediate
Outcome
Controlling High Blood
Pressure:
Percentage of patients 18-85 years
of age who had a diagnosis of
essential hypertension starting
before and continuing into, or
starting during the first six months
of the measurement period, and
whose most recent blood pressure
was adequately controlled
(<140/90mmHg) during the
measurement period.
National
Committee for
Quality
Assurance

B.39. Pulmonology
PREVIOUSLY FINALIZED MEASURES IN THE PULMONOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure
Steward
*
!
(Patient
Safety)
0022 /
N/A
238
CMS15
6v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Use of High-Risk Medications in
Older Adults:
Percentage of patients 65 years of
age and older who were ordered at
least two high-risk medications
from the same drug class.
National
Committee for
Quality
Assurance
*
N/A /
N/A
277
N/A
MIPS CQM
Specifications
Process
Sleep Apnea: Severity
Assessment at Initial Diagnosis:
Percentage of patients aged 18
years and older with a diagnosis of
obstructive sleep apnea who had
an apnea hypopnea index (AHI), a
respiratory disturbance index
(RDI), or a respiratory event index
(REI) documented or measured
within 2 months of initial
evaluation for suspected
obstructive sleep apnea.
American
Academy of
Sleep
Medicine
N/A /
N/A
279
N/A
MIPS CQM
Specifications
Process
Sleep Apnea: Assessment of
Adherence to Obstructive Sleep
Apnea (OSA) Therapy:
Percentage of patients aged 18
years and older with a diagnosis of
obstructive sleep apnea (OSA) that
were prescribed an evidence-based
therapy that had documentation
that adherence to therapy was
assessed at least annually through
an objective informatics system or
through self-reporting (if objective
reporting is not available).
American
Academy of
Sleep
Medicine
*
!
(Care
Coordination)
N/A /
N/A
374
CMS50
v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Closing the Referral Loop:
Receipt of Specialist Report:
Percentage of patients with
referrals, regardless of age, for
which the referring clinician
receives a report from the clinician
to whom the patient was referred.
Centers for
Medicare &
Medicaid
Services
!
(Outcome)
N/A /
N/A
398
N/A
MIPS CQM
Specifications
Outcome
Optimal Asthma Control:
Composite measure of the
percentage of pediatric and adult
patients whose asthma is well-
controlled as demonstrated by one
of three age appropriate patient
reported outcome tools and not at
risk for exacerbation.
Minnesota
Community
Measurement
§
2152 /
N/A
431
N/A
MIPS CQM
Specifications
Process
Preventive Care and Screening:
Unhealthy Alcohol Use:
Screening & Brief Counseling:
Percentage of patients aged 18
years and older who were screened
for unhealthy alcohol use using a
systematic screening method at
least once within the last 12
months AND who received brief
counseling if identified as an
unhealthy alcohol user.
National
Committee for
Quality
Assurance
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers of
Health:
Percent of patients 18 years and
older screened for food insecurity,
housing instability, transportation
needs, utility difficulties, and
interpersonal safety.
Centers for
Medicare &
Medicaid
Services

B.39. Pulmonology
PREVIOUSLY FINALIZED MEASURES IN THE PULMONOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure
Steward
*
3620 /
N/A
493
N/A
MIPS CQM
Specifications
Process
Adult Immunization Status:
Percentage of patients 19 years of
age and older who are up-to-date
on recommended routine vaccines
for influenza; tetanus and
diphtheria (Td) or tetanus,
diphtheria and acellular pertussis
(Tdap); zoster; and pneumococcal.
National
Committee for
Quality
Assurance
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years or
older who screen positive for one
or more of the following health
related social needs (HRSNs):
food insecurity, housing
instability, transportation needs,
utility help needs, or interpersonal
safety; and had contact with a
Community Service Provider
(CSP) for at least 1 of their HRSNs
within 60 days after screening.
OCHIN
*
!
(Outcome)
2483 /
N/A
503
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Gains in Patient Activation
Measure (PAM®) Scores at 12
Months:
The Patient Activation Measure®
(PAM®) is a 10 – or 13 – item
questionnaire that assesses an
individual´s knowledge, skills and
confidence for managing their
health and health care. The
measure assesses individuals on a
0-100 scale that converts to one of
four levels of activation, from low
(1) to high (4). The PAM®
performance measure (PAM®-
PM) is the change in score on the
PAM® from baseline to follow-up
measurement.
Insignia
Health, LLC, a
wholly owned
subsidiary of
Phreesia

B.39. Pulmonology
MEASURES PROPOSED FOR ADDITION TO THE PULMONOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
N/A /
N/A
TBD
N/A
MIPS
CQM
Specificati
ons
Process
Adult COVID-
19 Vaccination
Status:
Percentage of
patients aged 18
years and older
seen for a visit
during the
performance
period that are up
to date on their
COVID-19
vaccinations as
defined by CDC
recommendation
s on current
vaccination.
Centers
for
Medicare
&
Medicaid
Services
We are proposing to include this
measure in the Pulmonology
Medicine specialty set as it would
be clinically relevant to this
clinician type. Widespread
vaccination against SARS-CoV-2,
the virus that causes COVID-19,
is critically important to stemming
the morbidity and mortality
caused by this disease.
121
Clinicians are uniquely positioned
to encourage uptake of COVID-
19 vaccination, and clinicians are
still a major driving force in
promoting patient vaccination.
The addition of this quality
measure in this specialty set
would help strengthen compliance
with recommended COVID-19
vaccination, leading to
improvement in the quality of
patient care and prevention of
disease for the general population.
This quality measure aligns with
clinical guidelines and the
evidence-based recommendations
of the ACIP, where there is
general agreement about the
safety and efficacy of the
COVID-19 vaccine, preventing
costly and potentially harmful
hospitalizations.
122
Broadening
vaccination status awareness to
this clinician type is valuable as it
can help drive an increase in the
adult vaccination rates. The
COVID-19 vaccination included
within this measure would reduce
the prevalence of severe diseases
that may be associated with
hospitalization and decrease
overall health care costs. The
measure being added to this
specialty set would be contingent
on the inclusion of applicable
coding by the time of the CY
2025 PFS final rule. In the event
appropriate coding is not included
in the final specification, this
measure would not be finalized
for inclusion within this specialty
measure set. See Table A.5 of this
Appendix for rationale, including
clinical evidence supporting the
inclusion of this measure in
MIPS.
B.40. Rheumatology
121
See footnote Ikeokwu et al., 2023 in Table B.1 of this Appendix.
122
See footnotes Fitzpatrick et al., 2022; Polack et al., 2020; and Graña et al., 2022 in Table A.5 of this Appendix.

In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Rheumatology specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a
measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Rheumatology
specialty set.
B.40. Rheumatology
PREVIOUSLY FINALIZED MEASURES IN THE RHEUMATOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title and
Description
Measure Steward
!
(Care
Coordination)
N/A /
N/A
024
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Communication with the
Physician or Other Clinician
Managing On-Going Care
Post-Fracture for Men and
Women Aged 50 Years and
Older:
Percentage of patients aged 50
years and older treated for a
fracture with documentation
of communication, between
the physician treating the
fracture and the physician or
other clinician managing the
patient’s on-going care, that a
fracture occurred and that the
patient was or should be
considered for osteoporosis
treatment or testing. This
measure is submitted by the
physician who treats the
fracture and who therefore is
held accountable for the
communication.
National Committee
for Quality Assurance
0046 /
N/A
039
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Screening for Osteoporosis
for Women Aged 65-85
Years of Age:
Percentage of women aged
65-85 years of age who ever
had a central dual-energy X-
ray absorptiometry (DXA) to
check for osteoporosis.
National Committee
for Quality Assurance
*
!
(Care
Coordination)
0326 /
N/A
047
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Advance Care Plan:
Percentage of patients aged 65
years and older who have an
advance care plan or surrogate
decision maker documented in
the medical record or
documentation in the medical
record that an advance care
plan was discussed but the
patient did not wish or was
not able to name a surrogate
decision maker or provide an
advance care plan.
National Committee
for Quality Assurance
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS68
v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for
patients aged 18 years and
older for which the eligible
clinician attests to
documenting a list of current
medications using all
immediate resources available
on the date of the encounter.
Centers for Medicare
& Medicaid Services

B.40. Rheumatology
PREVIOUSLY FINALIZED MEASURES IN THE RHEUMATOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title and
Description
Measure Steward
*
N/A /
N/A
176
N/A
MIPS CQM
Specifications
Process
Tuberculosis Screening
Prior to First Course of
Biologic and/or Immune
Response Modifier Therapy:
If a patient has been newly
prescribed a biologic and/or
immune response modifier
that includes a warning for
potential reactivation of a
latent infection, then the
medical record should indicate
TB testing in the preceding
12-month period.
American College of
Rheumatology
*
2523 /
N/A
177
N/A
MIPS CQM
Specifications
Process
Rheumatoid Arthritis (RA):
Periodic Assessment of
Disease Activity:
Percentage of patients aged 18
years and older with a
diagnosis of rheumatoid
arthritis (RA) who have an
assessment of disease activity
using an ACR-preferred RA
disease activity assessment
tool at ≥50% of encounters for
RA for each patient during the
performance period.
American College of
Rheumatology
*
N/A /
N/A
178
N/A
MIPS CQM
Specifications
Process
Rheumatoid Arthritis (RA):
Functional Status
Assessment:
Percentage of patients aged 18
years and older with a
diagnosis of rheumatoid
arthritis (RA) for whom a
functional status assessment
was performed at least once
during the performance
period.
American College of
Rheumatology
*
N/A /
N/A
180
N/A
MIPS CQM
Specifications
Process
Rheumatoid Arthritis (RA):
Glucocorticoid
Management:
Percentage of patients aged 18
years and older with a
diagnosis of rheumatoid
arthritis (RA) who have been
assessed for glucocorticoid
use and, for those on
prolonged doses of prednisone
> 5 mg daily (or equivalent)
with improvement or no
change in disease activity,
documentation of
glucocorticoid management
plan during the performance
period.
American College of
Rheumatology
§
N/A /
N/A
226
CMS13
8v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Tobacco Use:
Screening and Cessation
Intervention:
Percentage of patients aged 12
years and older who were
screened for tobacco use one
or more times during the
measurement period AND
who received tobacco
cessation intervention during
the measurement period or in
the six months prior to the
measurement period if
identified as a tobacco user.
National Committee
for Quality Assurance

B.40. Rheumatology
PREVIOUSLY FINALIZED MEASURES IN THE RHEUMATOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title and
Description
Measure Steward
*
§
!
(Outcome)
N/A /
N/A
236
CMS16
5v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Intermedi
ate
Outcome
Controlling High Blood
Pressure:
Percentage of patients 18-85
years of age who had a
diagnosis of essential
hypertension starting before
and continuing into or starting
during the first six months of
the measurement period, and
whose most recent blood
pressure was adequately
controlled (<140/90mmHg)
during the measurement
period.
National Committee
for Quality Assurance
*
!
(Patient
Safety)
0022 /
N/A
238
CMS15
6v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Use of High-Risk
Medications in Older
Adults:
Percentage of patients 65
years of age and older who
were ordered at least two
high-risk medications from
the same drug class.
National Committee
for Quality Assurance
*
N/A /
N/A
317
CMS22
v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Screening for
High Blood Pressure and
Follow-Up Documented:
Percentage of patient visits for
patients aged 18 years and
older seen during the
measurement period who were
screened for high blood
pressure AND a
recommended follow-up plan
is documented, as indicated, if
blood pressure is elevated or
hypertensive.
Centers for Medicare
& Medicaid Services
*
!
(Care
Coordination)
N/A /
N/A
374
CMS50
v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Closing the Referral Loop:
Receipt of Specialist Report:
Percentage of patients with
referrals, regardless of age, for
which the referring clinician
receives a report from the
clinician to whom the patient
was referred.
Centers for Medicare
& Medicaid Services
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers
of Health:
Percent of patients 18 years
and older screened for food
insecurity, housing instability,
transportation needs, utility
difficulties, and interpersonal
safety.
Centers for Medicare
& Medicaid Services
*
3620 /
N/A
493
N/A
MIPS CQM
Specifications
Process
Adult Immunization Status:
Percentage of patients 19
years of age and older who are
up-to-date on recommended
routine vaccines for influenza;
tetanus and diphtheria (Td) or
tetanus, diphtheria and
acellular pertussis (Tdap);
zoster; and pneumococcal.
National Committee
Quality Assurance
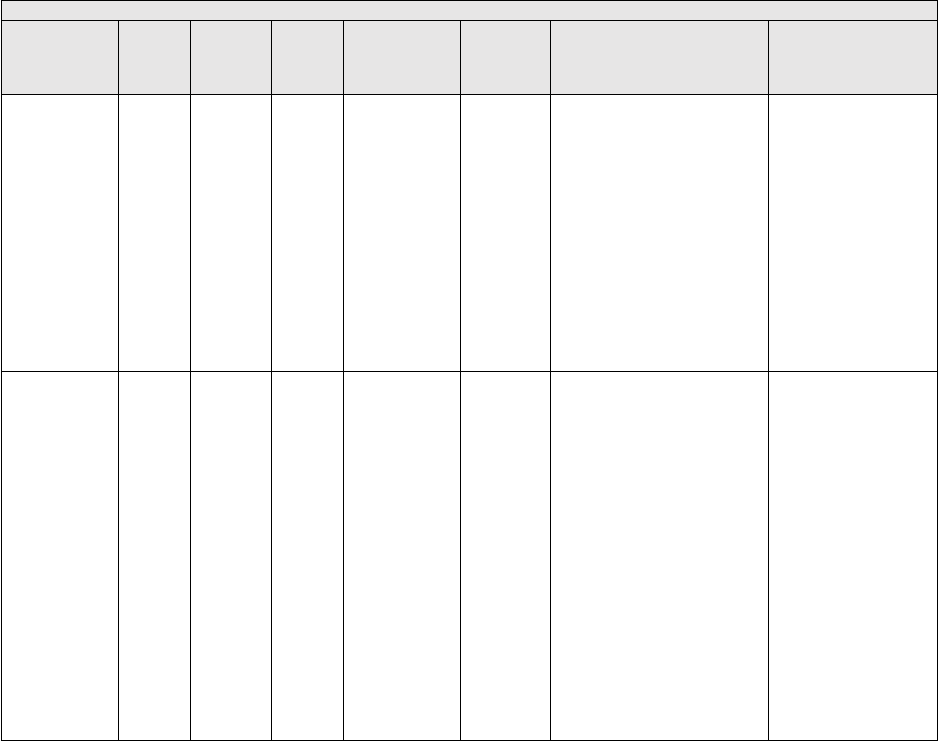
B.40. Rheumatology
PREVIOUSLY FINALIZED MEASURES IN THE RHEUMATOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title and
Description
Measure Steward
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years or
older who screen positive for
one or more of the following
health related social needs
(HRSNs): food insecurity,
housing instability,
transportation needs, utility
help needs, or interpersonal
safety; and had contact with a
Community Service Provider
(CSP) for at least 1 of their
HRSNs within 60 days after
screening.
OCHIN
*
!
(Outcome)
2483 /
N/A
503
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performan
ce
Measure
Gains in Patient Activation
Measure (PAM®) Scores at
12 Months:
The Patient Activation
Measure® (PAM®) is a 10 –
or 13 – item questionnaire that
assesses an individual´s
knowledge, skills and
confidence for managing their
health and health care. The
measure assesses individuals
on a 0-100 scale that converts
to one of four levels of
activation, from low (1) to
high (4). The PAM®
performance measure
(PAM®-PM) is the change in
score on the PAM® from
baseline to follow-up
measurement.
Insignia Health, LLC,
a wholly owned
subsidiary of Phreesia

B.40. Rheumatology
MEASURES PROPOSED FOR ADDITION TO THE RHEUMATOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
N/A /
N/A
TBD
N/A
MIPS
CQM
Specificati
ons
Process
Adult COVID-
19 Vaccination
Status:
Percentage of
patients aged 18
years and older
seen for a visit
during the
performance
period that are up
to date on their
COVID-19
vaccinations as
defined by CDC
recommendation
s on current
vaccination.
Centers
for
Medicare
&
Medicaid
Services
We are proposing to include this
measure in the Rheumatology
Medicine specialty set as it would
be clinically relevant to this
clinician type. Widespread
vaccination against SARS-CoV-2,
the virus that causes COVID-19,
is critically important to stemming
the morbidity and mortality
caused by this disease.
123
Clinicians are uniquely positioned
to encourage uptake of COVID-
19 vaccination, and clinicians are
still a major driving force in
promoting patient vaccination.
The addition of this quality
measure in this specialty set
would help strengthen compliance
with recommended COVID-19
vaccination, leading to
improvement in the quality of
patient care and prevention of
disease for the general population.
This quality measure aligns with
clinical guidelines and the
evidence-based recommendations
of the ACIP, where there is
general agreement about the
safety and efficacy of the
COVID-19 vaccine, preventing
costly and potentially harmful
hospitalizations.
124
Broadening
vaccination status awareness to
this clinician type is valuable as it
can help drive an increase in the
adult vaccination rates. The
COVID-19 vaccination included
within this measure would reduce
the prevalence of severe diseases
that may be associated with
hospitalization and decrease
overall health care costs. The
measure being added to this
specialty set would be contingent
on the inclusion of applicable
coding by the time of the CY
2025 PFS final rule. In the event
appropriate coding is not included
in the final specification, this
measure would not be finalized
for inclusion within this specialty
measure set. See Table A.5 of this
Appendix for rationale, including
clinical evidence supporting the
inclusion of this measure in
MIPS.
123
See footnote Ikeokwu et al., 2023 in Table B.1 of this Appendix.
124
See footnotes Fitzpatrick et al., 2022; Polack et al., 2020; and Graña et al., 2022 in Table A.5 of this Appendix.

B.41. Skilled Nursing Facility
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Skilled Nursing Facility specialty set takes additional criteria into consideration, which includes, but is not limited to: whether
a measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess
the appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Skilled Nursing
Facility specialty set.
B.41. Skilled Nursing Facility
PREVIOUSLY FINALIZED MEASURES IN THE SKILLED NURSING FACILITY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
§
0067 /
N/A
006
N/A
MIPS CQM
Specifications
Process
Coronary Artery Disease (CAD):
Antiplatelet Therapy:
Percentage of patients aged 18 years
and older with a diagnosis of
coronary artery disease (CAD) seen
within a 12-month period who were
prescribed aspirin or clopidogrel.
American Heart
Association
§
0070 /
0070e
007
CMS1
45v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Coronary Artery Disease (CAD):
Beta-Blocker Therapy – Prior
Myocardial Infarction (MI) or
Left Ventricular Systolic
Dysfunction (LVEF ≤ 40%):
Percentage of patients aged 18 years
and older with a diagnosis of
coronary artery disease seen within
a 12-month period who also have a
prior MI or a current or prior LVEF
≤ 40% who were prescribed beta-
blocker therapy.
American Heart
Association
§
0083 /
0083e
008
CMS1
44v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Heart Failure (HF): Beta-Blocker
Therapy for Left Ventricular
Systolic Dysfunction (LVSD):
Percentage of patients aged 18 years
and older with a diagnosis of heart
failure (HF) with a current or prior
left ventricular ejection fraction
(LVEF) ≤ 40% who were prescribed
beta-blocker therapy either within a
12-month period when seen in the
outpatient setting OR at each
hospital discharge.
American Heart
Association
*
!
(Care
Coordinat
ion)
0326 /
N/A
047
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Advance Care Plan:
Percentage of patients aged 65 years
and older who have an advance care
plan or surrogate decision maker
documented in the medical record
or documentation in the medical
record that an advance care plan
was discussed but the patient did not
wish or was not able to name a
surrogate decision maker or provide
an advance care plan.
National Committee
for Quality Assurance
§
0066 /
N/A
118
N/A
MIPS CQM
Specifications
Process
Coronary Artery Disease (CAD):
Angiotensin-Converting Enzyme
(ACE) Inhibitor or Angiotensin
Receptor Blocker (ARB) Therapy
- Diabetes or Left Ventricular
Systolic Dysfunction (LVEF ≤
40%):
Percentage of patients aged 18 years
and older with a diagnosis of
coronary artery disease seen within
a 12-month period who also have
diabetes OR a current or prior Left
Ventricular Ejection Fraction
(LVEF) ≤ 40% who were prescribed
ACE inhibitor or ARB therapy.
American Heart
Association

B.41. Skilled Nursing Facility
PREVIOUSLY FINALIZED MEASURES IN THE SKILLED NURSING FACILITY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Care
Coordinat
ion)
0101 /
N/A
155
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Falls: Plan of Care:
Percentage of patients aged 65 years
and older with a history of falls who
had a plan of care for falls
documented within 12 months.
National Committee
for Quality Assurance
*
!
(Patient
Safety)
N/A /
N/A
181
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Elder Maltreatment Screen and
Follow-Up Plan:
Percentage of patients aged 60 years
and older with a documented elder
maltreatment screen using an Elder
Maltreatment Screening tool on the
date of encounter AND a
documented follow-up plan on the
date of the positive screen.
Centers for Medicare
& Medicaid Services
*
!
(Patient
Safety)
0022 /
N/A
238
CMS1
56v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Use of High-Risk Medications in
Older Adults:
Percentage of patients 65 years of
age and older who were ordered at
least two high-risk medications
from the same drug class.
National Committee
for Quality
Assurance
*
N/A /
N/A
317
CMS2
2v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and Screening:
Screening for High Blood
Pressure and Follow-Up
Documented:
Percentage of patient visits for
patients aged 18 years and older
seen during the measurement period
who were screened for high blood
pressure AND a recommended
follow-up plan is documented, as
indicated, if blood pressure is
elevated or hypertensive.
Centers for
Medicare &
Medicaid Services
§
N/A /
N/A
326
N/A
MIPS CQM
Specifications
Process
Atrial Fibrillation and Atrial
Flutter: Chronic Anticoagulation
Therapy:
Percentage of patients aged 18 years
and older with atrial fibrillation
(AF) or atrial flutter who were
prescribed an FDA-approved oral
anticoagulant drug for the
prevention of thromboembolism
during the measurement period.
American Heart
Association
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers of
Health:
Percent of patients 18 years and
older screened for food insecurity,
housing instability, transportation
needs, utility difficulties, and
interpersonal safety.
Centers for
Medicare &
Medicaid Services
*
3620 /
N/A
493
N/A
MIPS CQM
Specifications
Process
Adult Immunization Status:
Percentage of patients 19 years of
age and older who are up-to-date on
recommended routine vaccines for
influenza; tetanus and diphtheria
(Td) or tetanus, diphtheria and
acellular pertussis (Tdap); zoster;
and pneumococcal.
National Committee
for Quality
Assurance

B.41. Skilled Nursing Facility
PREVIOUSLY FINALIZED MEASURES IN THE SKILLED NURSING FACILITY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years or older
who screen positive for one or more
of the following health related
social needs (HRSNs): food
insecurity, housing instability,
transportation needs, utility help
needs, or interpersonal safety; and
had contact with a Community
Service Provider (CSP) for at least 1
of their HRSNs within 60 days after
screening.
OCHIN

B.41. Skilled Nursing Facility
MEASURES PROPOSED FOR ADDITION TO THE SKILLED NURSING FACILITY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
N/A /
N/A
TBD
N/A
MIPS
CQM
Specificati
ons
Process
Adult COVID-
19 Vaccination
Status:
Percentage of
patients aged 18
years and older
seen for a visit
during the
performance
period that are up
to date on their
COVID-19
vaccinations as
defined by CDC
recommendation
s on current
vaccination.
Centers
for
Medicare
&
Medicaid
Services
We are proposing to include this
measure in the Skilled Nursing
Facility specialty set as it would
be clinically relevant to this
clinician type. Widespread
vaccination against SARS-CoV-2,
the virus that causes COVID-19,
is critically important to stemming
the morbidity and mortality
caused by this disease.
125
Clinicians are uniquely positioned
to encourage uptake of COVID-
19 vaccination, and clinicians are
still a major driving force in
promoting patient vaccination.
The addition of this quality
measure in this specialty set
would help strengthen compliance
with recommended COVID-19
vaccination, leading to
improvement in the quality of
patient care and prevention of
disease for the general population.
This quality measure aligns with
clinical guidelines and the
evidence-based recommendations
of the ACIP, where there is
general agreement about the
safety and efficacy of the
COVID-19 vaccine, preventing
costly and potentially harmful
hospitalizations.
126
Broadening
vaccination status awareness to
this clinician type is valuable as it
can help drive an increase in the
adult vaccination rates. The
COVID-19 vaccination included
within this measure would reduce
the prevalence of severe diseases
that may be associated with
hospitalization and decrease
overall health care costs. The
measure being added to this
specialty set would be contingent
on the inclusion of applicable
coding by the time of the CY
2025 PFS final rule. In the event
appropriate coding is not included
in the final specification, this
measure would not be finalized
for inclusion within this specialty
measure set. See Table A.5 of this
Appendix for rationale, including
clinical evidence supporting the
inclusion of this measure in
MIPS.
125
See footnote Ikeokwu et al., 2023 in Table B.1 of this Appendix.
126
See footnotes Fitzpatrick et al., 2022; Polack et al., 2020; and Graña et al., 2022 in Table A.5 of this Appendix.

B.42. Speech Language Pathology
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Speech Language Pathology specialty set takes additional criteria into consideration, which includes, but is not limited to:
whether a measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may
reassess the appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set.
Measure tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be
added, and measures proposed for removal, as applicable. We request comment on the measures available in the proposed Speech
Language Pathology specialty set.
B.42. Speech Language Pathology
PREVIOUSLY FINALIZED MEASURES IN THE SPEECH LANGUAGE PATHOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS68
v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for patients
aged 18 years and older for which
the eligible clinician attests to
documenting a list of current
medications using all immediate
resources available on the date of
the encounter.
Centers for Medicare
& Medicaid Services
§
N/A /
N/A
134
CMS2v
14
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and Screening:
Screening for Depression and
Follow-Up Plan:
Percentage of patients aged 12 years
and older screened for depression on
the date of the encounter or up to 14
days prior to the date of the
encounter using an age-appropriate
standardized depression screening
tool AND if positive, a follow-up
plan is documented on the date of or
up to two days after the date of the
qualifying encounter.
Centers for Medicare
& Medicaid Services
*
!
(Patient
Safety)
N/A /
N/A
181
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Elder Maltreatment Screen and
Follow-Up Plan:
Percentage of patients aged 60 years
and older with a documented elder
maltreatment screen using an Elder
Maltreatment Screening tool on the
date of encounter AND a
documented follow-up plan on the
date of the positive screen.
Centers for Medicare
& Medicaid Services
*
§
!
(Care
Coordinat
ion)
N/A /
N/A
182
N/A
MIPS CQM
Specifications
Process
Functional Outcome Assessment:
Percentage of visits for patients
aged 18 years and older with
documentation of a current
functional outcome assessment
using a standardized functional
outcome assessment tool on the date
of the encounter AND
documentation of a care plan based
on identified functional outcome
deficiencies within two days of the
date of the identified deficiencies.
Centers for Medicare
& Medicaid Services
§
N/A /
N/A
226
CMS13
8v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and Screening:
Tobacco Use: Screening and
Cessation Intervention:
Percentage of patients aged 12 years
and older who were screened for
tobacco use one or more times
during the measurement period
AND who received tobacco
cessation intervention during the
measurement period or in the six
months prior to the measurement
period if identified as a tobacco
user.
National Committee
for Quality Assurance

B.42. Speech Language Pathology
PREVIOUSLY FINALIZED MEASURES IN THE SPEECH LANGUAGE PATHOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
N/A /
N/A
291
N/A
MIPS CQM
Specifications
Process
Assessment of Cognitive
Impairment or Dysfunction for
Patients with Parkinson’s Disease:
Percentage of all patients with a
diagnosis of Parkinson’s Disease
(PD) who were assessed for
cognitive impairment or dysfunction
once during the measurement
period.
American Academy
of Neurology
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers of
Health:
Percent of patients 18 years and
older screened for food insecurity,
housing instability, transportation
needs, utility difficulties, and
interpersonal safety.
Centers for Medicare
& Medicaid Services
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community Service
Provider:
Percent of patients 18 years or older
who screen positive for one or more
of the following health related social
needs (HRSNs): food insecurity,
housing instability, transportation
needs, utility help needs, or
interpersonal safety; and had contact
with a Community Service Provider
(CSP) for at least 1 of their HRSNs
within 60 days after screening.
OCHIN

B.42. Speech Language Pathology
MEASURES PROPOSED FOR ADDITION TO THE SPEECH LANGUAGE PATHOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
*
N/A /
2872e
281
CM
S14
9v13
eCQM
Specificati
ons
Process
Dementia:
Cognitive
Assessment:
Percentage of
patients,
regardless of age,
with a diagnosis
of dementia for
whom an
assessment of
cognition is
performed and the
results reviewed
at least once
within a 12-month
period.
American
Academy
of
Neurology
We are proposing to include
this measure in the Speech
Language Pathology specialty
set as it would be clinically
relevant to this clinician type.
Speech language pathologists
(SLPs) utilize standardized
instruments with demonstrated
reliability for dementia
screening. These instruments
typically assess orientation to
time, place, and person.
127
Other tests (for example, story
recall/story retelling) assess
episodic memory and can be
useful for screening early
dementia.
128
If screening
reveals cognitive impairment,
the individual is referred to an
SLP for a comprehensive
evaluation of communicative
function. The measure being
added to this specialty set
would be contingent on the
inclusion of applicable coding
by the time of the CY 2025
PFS final rule.
*
N/A /
N/A
282
N/A
MIPS
CQM
Specificati
ons
Process
Dementia:
Functional
Status
Assessment:
Percentage of
patients with
dementia for
whom an
assessment of
functional status
was performed at
least once in the
last 12 months.
American
Academy
of
Neurology/
American
Psychiatric
Association
We are proposing to include
this measure in the Speech
Language Pathology specialty
set as it would be clinically
relevant to this clinician type.
SLPs play a critical role in
diagnosis and management of
dysphagia in patients with
dementia through
comprehensive assessment,
diet consistency modifications,
educating their caregiver on the
use of compensatory strategies,
prescribing exercise programs,
and referring them to other
professionals as needed. The
measure being added to this
specialty set would be
contingent on the inclusion of
applicable coding by the time
of the CY 2025 PFS final rule.
127
Rabin, L. A., Paré, N., Saykin, A. J., Brown, M. J., Wishart, H. A., Flashman, L. A., & Santulli, R. B. (2009).
Differential Memory Test Sensitivity for Diagnosing Amnestic Mild Cognitive Impairment and Predicting Conversion
to Alzheimer's Disease. Aging, Neuropsychology, and Cognition, 16(3), 357–
376. https://doi.org/10.1080/13825580902825220.
128
See footnote Rabin et al., 2009.

B.42. Speech Language Pathology
MEASURES PROPOSED FOR ADDITION TO THE SPEECH LANGUAGE PATHOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
*
!
(Patient
Safety)
N/A /
N/A
286
N/A
MIPS
CQM
Specificati
ons
Process
Dementia: Safety
Concern
Screening and
Follow-Up for
Patients with
Dementia:
Percentage of
patients with
dementia or their
caregiver(s) for
whom there was a
documented
safety concerns
screening in two
domains of risk:
(1) dangerousness
to self or others
and (2)
environmental
risks; and if safety
concerns
screening was
positive in the last
12 months, there
was
documentation of
mitigation
recommendations,
including but not
limited to referral
to other resources.
American
Psychiatric
Association
/ American
Academy
of
Neurology
We are proposing to include
this measure in the Speech
Language Pathology specialty
set as it would be clinically
relevant to this clinician type.
Dementia patients are at high
risk for safety concerns due to
underlying cognitive
communication impairment
which may impact judgment,
reasoning, and memory as well
as physical weakness resulting
from other medical
conditions.
129
SLPs work
directly with individuals with
dementia and their caregivers
to screen, assess, and establish
care plans to address these
issues through cognitive-
communication exercises, use
of compensatory strategies,
environmental modifications,
referrals to other professionals,
and providing caregiver
training. The measure being
added to this specialty set
would be contingent on the
inclusion of applicable coding
by the time of the CY 2025
PFS final rule.
*
!
(Care
Coordinat
ion)
N/A /
N/A
288
N/A
MIPS
CQM
Specificati
ons
Process
Dementia:
Education and
Support of
Caregivers for
Patients with
Dementia:
Percentage of
patients with
dementia whose
caregiver(s) were
provided with
education on
dementia disease
management and
health behavior
changes AND
were referred to
additional
resources for
support in the last
12 months.
American
Academy
of
Neurology /
American
Psychiatric
Association
We are proposing to include
this measure in the Speech
Language Pathology specialty
set as it would be clinically
relevant to this clinician type.
SLPs provide information
about the nature of dementia
and its course of progression.
SLPs make recommendations
for environmental
modifications, such as using
alarms and pill boxes for
medication reminders, visual
aids, and provide education on
communication strategies and
modifications to caregiver
behaviors to ensure the safety
of the patient and compliance
with the plan of care. The
measure being added to this
specialty set would be
contingent on the inclusion of
applicable coding by the time
of the CY 2025 PFS final rule.
129
Thyrian, J. R., Hertel, J., Wucherer, D., Eichler, T., Michalowsky, B., Dreier-Wolfgramm, A., Zwingmann, I.,
Kilimann, I., Teipel, S., & Hoffmann, W. (2017). Effectiveness and Safety of Dementia Care Management in Primary
Care: A Randomized Clinical Trial. JAMA Psychiatry, 74(10), 996–1004.
https://doi.org/10.1001/jamapsychiatry.2017.2124.

B.42. Speech Language Pathology
MEASURES PROPOSED FOR ADDITION TO THE SPEECH LANGUAGE PATHOLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
*
!
(Patient
Experienc
e)
N/A /
N/A
386
N/A
MIPS
CQM
Specificati
ons
Process
Amyotrophic
Lateral Sclerosis
(ALS) Patient
Care
Preferences:
Percentage of
patients diagnosed
with Amyotrophic
Lateral Sclerosis
(ALS) who were
offered assistance
in planning for
end of life issues
(e.g., advance
directives,
invasive
ventilation, lawful
physician-
hastened death, or
hospice) or whose
existing end of life
plan was reviewed
or updated at least
once annually or
more frequency as
clinically
indicated (i.e.,
rapid progression).
American
Academy
of
Neurology
We are proposing to include
this measure in the Speech
Language Pathology specialty
set as it would be clinically
relevant to this clinician type.
Over the course of the disease,
individuals with ALS exhibit
difficulty producing intelligible
speech to communicate basic
needs and wants.
130
SLPs are
trained to address
communication deficits via
exercise programs, use of
communication strategies, and
introducing augmentative and
alternative communication
(AAC) methods. The measure
being added to this specialty
set would be contingent on the
inclusion of applicable coding
by the time of the CY 2025
PFS final rule.
130
National Institute of Neurological Disorders and Stroke. (2024). Amyotrophic Lateral Sclerosis.
https://www.ninds.nih.gov/health-information/disorders/amyotrophic-lateral-sclerosis-als.

B.43. Thoracic Surgery
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Thoracic Surgery specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a
measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Thoracic Surgery
specialty set that have proposed substantive changes in Table Group D of this Appendix as indicated by an * in the Indicator
column.
B.43. Thoracic Surgery
PREVIOUSLY FINALIZED MEASURES IN THE THORACIC SURGERY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measur
e
Type
Measure Title
and Description
Measure Steward
*
!
(Care
Coordination)
0326 /
N/A
047
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Advance Care Plan:
Percentage of patients aged 65
years and older who have an
advance care plan or surrogate
decision maker documented in
the medical record or
documentation in the medical
record that an advance care
plan was discussed but the
patient did not wish or was not
able to name a surrogate
decision maker or provide an
advance care plan.
National Committee
for Quality
Assurance
*
§
!
(Patient
Safety)
N/A / N/A
130
CMS
68v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for patients
aged 18 years and older for
which the eligible clinician
attests to documenting a list of
current medications using all
immediate resources available
on the date of the encounter.
Centers for Medicare
& Medicaid Services
!
(Outcome)
0129 /
N/A
164
N/A
MIPS CQM
Specifications
Outcome
Coronary Artery Bypass
Graft (CABG): Prolonged
Intubation:
Percentage of patients aged 18
years and older undergoing
isolated CABG surgery who
require postoperative
intubation > 24 hours.
Society of Thoracic
Surgeons
!
(Outcome)
0114 /
N/A
167
N/A
MIPS CQM
Specifications
Outcome
Coronary Artery Bypass
Graft (CABG): Postoperative
Renal Failure:
Percentage of patients aged 18
years and older undergoing
isolated CABG surgery
(without pre-existing renal
failure) who develop
postoperative renal failure or
require dialysis.
Society of Thoracic
Surgeons
*
!
(Outcome)
0115 /
N/A
168
N/A
MIPS CQM
Specifications
Outcome
Coronary Artery Bypass
Graft (CABG): Surgical Re-
Exploration:
Percentage of patients aged 18
years and older undergoing
isolated CABG surgery who
require a return to the operating
room (OR) during the current
hospitalization for mediastinal
bleeding with or without
tamponade, graft occlusion,
valve dysfunction, or other
cardiac reason.
Society of Thoracic
Surgeons

B.43. Thoracic Surgery
PREVIOUSLY FINALIZED MEASURES IN THE THORACIC SURGERY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measur
e
Type
Measure Title
and Description
Measure Steward
§
N/A / N/A
226
CMS
138v1
3
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Tobacco Use:
Screening and Cessation
Intervention:
Percentage of patients aged 12
years and older who were
screened for tobacco use one or
more times during the
measurement period AND who
received tobacco cessation
intervention during the
measurement period or in the
six months prior to the
measurement period if
identified as a tobacco user.
National Committee
for Quality
Assurance
!
(Outcome)
N/A / N/A
356
N/A
MIPS CQM
Specifications
Outcome
Unplanned Hospital
Readmission within 30 Days
of Principal Procedure:
Percentage of patients aged 18
years and older who had an
unplanned hospital readmission
within 30 days of principal
procedure.
American College of
Surgeons
!
(Patient
Experience)
N/A / N/A
358
N/A
MIPS CQM
Specifications
Process
Patient-Centered Surgical
Risk Assessment and
Communication:
Percentage of patients who
underwent a non-emergency
surgery who had their
personalized risks of
postoperative complications
assessed by their surgical team
prior to surgery using a clinical
data-based, patient-specific risk
calculator and who received
personal discussion of those
risks with the surgeon.
American College of
Surgeons
*
!
(Care
Coordination)
N/A / N/A
374
CMS
50v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Closing the Referral Loop:
Receipt of Specialist Report:
Percentage of patients with
referrals, regardless of age, for
which the referring clinician
receives a report from the
clinician to whom the patient
was referred.
Centers for Medicare
& Medicaid Services
§
!
(Outcome)
0119 /
N/A
445
N/A
MIPS CQM
Specifications
Outcome
Risk-Adjusted Operative
Mortality for Coronary
Artery Bypass Graft
(CABG):
Percent of patients aged 18
years and older undergoing
isolated CABG who die,
including both all deaths
occurring during the
hospitalization in which the
CABG was performed, even if
after 30 days, and those deaths
occurring after discharge from
the hospital, but within 30 days
of the procedure.
Society of Thoracic
Surgeons
!
(Equity)
N/A / N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers
of Health:
Percent of patients 18 years
and older screened for food
insecurity, housing instability,
transportation needs, utility
difficulties, and interpersonal
safety.
Centers for Medicare
& Medicaid Services

B.43. Thoracic Surgery
PREVIOUSLY FINALIZED MEASURES IN THE THORACIC SURGERY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measur
e
Type
Measure Title
and Description
Measure Steward
*
!
(Equity)
N/A / N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years or
older who screen positive for
one or more of the following
health related social needs
(HRSNs): food insecurity,
housing instability,
transportation needs, utility
help needs, or interpersonal
safety; and had contact with a
Community Service Provider
(CSP) for at least 1 of their
HRSNs within 60 days after
screening.
OCHIN

B.44. Urgent Care
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Urgent Care specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a measure
reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Urgent Care
specialty set.
B.44. Urgent Care
PREVIOUSLY FINALIZED MEASURES IN THE URGENT CARE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
§
!
(Appropria
te Use)
0069 /
N/A
065
CMS15
4v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Appropriate Treatment for
Upper Respiratory Infection
(URI):
Percentage of episodes for
patients 3 months of age and
older with a diagnosis of upper
respiratory infection (URI) that
did not result in an antibiotic
order.
National Committee
for Quality Assurance
§
!
(Appropria
te Use)
N/A /
N/A
066
CMS14
6v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Appropriate Testing for
Pharyngitis:
The percentage of episodes for
patients 3 years and older with
a diagnosis of pharyngitis that
resulted in an antibiotic order
on or within 3 days after the
episode date and a group A
Streptococcus (Strep) test in the
seven-day period from three
days prior to the episode date
through three days after the
episode date.
National Committee
for Quality Assurance
§
!
(Appropria
te Use)
0058 /
N/A
116
N/A
MIPS CQM
Specifications
Process
Avoidance of Antibiotic
Treatment for Acute
Bronchitis/Bronchiolitis:
The percentage of episodes for
patients ages 3 months and
older with a diagnosis of acute
bronchitis/bronchiolitis that did
not result in an antibiotic
dispensing event.
National Committee
for Quality Assurance
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS68
v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for patients
aged 18 years and older for
which the eligible clinician
attests to documenting a list of
current medications using all
immediate resources available
on the date of the encounter.
Centers for Medicare
& Medicaid Services
§
N/A /
N/A
226
CMS13
8v13
Medicare Part B
Claims Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Tobacco Use:
Screening and Cessation
Intervention:
Percentage of patients aged 12
years and older who were
screened for tobacco use one or
more times during the
measurement period AND who
received tobacco cessation
intervention during the
measurement period or in the
six months prior to the
measurement period if
identified as a tobacco user.
National Committee
for Quality Assurance

B.44. Urgent Care
PREVIOUSLY FINALIZED MEASURES IN THE URGENT CARE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
N/A /
N/A
317
CMS22
v13
Medicare Part B
Claims Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Screening for
High Blood Pressure and
Follow-Up Documented:
Percentage of patient visits for
patients aged 18 years and
older seen during the
measurement period who were
screened for high blood
pressure AND a recommended
follow-up plan is documented,
as indicated, if blood pressure
is elevated or hypertensive.
Centers for Medicare
& Medicaid Services
*
!
(Appropria
te Use)
N/A /
N/A
331
N/A
MIPS CQM
Specifications
Process
Adult Sinusitis: Antibiotic
Prescribed for Acute Viral
Sinusitis (Overuse):
Percentage of patients, aged 18
years and older, with a
diagnosis of acute viral
sinusitis who were prescribed
an antibiotic within 10 days
after onset of symptoms.
American Academy of
Otolaryngology – Head
and Neck Surgery
Foundation
!
(Appropria
te Use)
N/A /
N/A
332
N/A
MIPS CQM
Specifications
Process
Adult Sinusitis: Appropriate
Choice of Antibiotic:
Amoxicillin With or Without
Clavulanate Prescribed for
Patients with Acute Bacterial
Sinusitis (Appropriate Use):
Percentage of patients aged 18
years and older with a
diagnosis of acute bacterial
sinusitis that were prescribed
amoxicillin, with or without
clavulanate, as a first line
antibiotic at the time of
diagnosis.
American Academy of
Otolaryngology – Head
and Neck Surgery
Foundation
§
2152 /
N/A
431
N/A
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Unhealthy Alcohol
Use: Screening & Brief
Counseling:
Percentage of patients aged 18
years and older who were
screened for unhealthy alcohol
use using a systematic
screening method at least once
within the last 12 months AND
who received brief counseling
if identified as an unhealthy
alcohol user.
National Committee
for Quality Assurance
*
!
(Appropria
te Use)
0657 /
N/A
464
N/A
MIPS CQM
Specifications
Process
Otitis Media with Effusion:
Systemic Antimicrobials –
Avoidance of Inappropriate
Use:
Percentage of patients aged 2
months through 12 years with a
diagnosis of OME who were
not prescribed systemic
antimicrobials.
American Academy of
Otolaryngology – Head
and Neck Surgery
Foundation
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers
of Health:
Percent of patients 18 years and
older screened for food
insecurity, housing instability,
transportation needs, utility
difficulties, and interpersonal
safety.
Centers for Medicare
& Medicaid Services

B.44. Urgent Care
PREVIOUSLY FINALIZED MEASURES IN THE URGENT CARE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years or
older who screen positive for
one or more of the following
health related social needs
(HRSNs): food insecurity,
housing instability,
transportation needs, utility
help needs, or interpersonal
safety; and had contact with a
Community Service Provider
(CSP) for at least 1 of their
HRSNs within 60 days after
screening.
OCHIN
B.44. Urgent Care
MEASURES PROPOSED FOR ADDITION TO THE URGENT CARE SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
*
N/A /
N/A
488
CM
S95
1v3
eCQM
Specificati
ons, MIPS
CQM
Specificati
ons
Process
Kidney Health
Evaluation:
Percentage of
patients aged 18-
75 years with a
diagnosis of
diabetes who
received a kidney
health evaluation
defined by an
Estimated
Glomerular
Filtration Rate
(eGFR) AND
Urine Albumin-
Creatinine Ratio
(uACR) within
the measurement
period.
National
Kidney
Foundation
We are proposing to include
this measure in the Urgent Care
specialty set as it would be
clinically relevant to this
clinician type. This measure
encourages an estimated
glomerular filtration rate
(eGFR) and urinary albumin-
to-creatinine ratio (uACR)
evaluation annually for patients
diagnosed with diabetes. The
measure aims for early
detection which can reduce
associated health risk of the co-
morbidities of diabetes and
CKD. Having an established
source of care is important in
this high-risk population,
however, in the general US
population, approximately 15
percent of adults do not have a
primary care physician.
131
According to the American
Diabetes Association annual
assessment of glomerular
filtration rate (GFR) in
adolescents, in addition to
adults, with diabetes is
necessary to appropriately
screen for early diabetic
nephropathy, and the
assessment of GFR is essential
to accurately diagnose diabetic
kidney disease early in the
disease process.
132
Including
this measure in this specialty
set could assist in capturing at-
risk patients who may lack a
primary care physician.
131
Toth-Manikowski, S. M., Hsu, J. Y., Fischer, M. J., Cohen, J. B., Lora, C. M., Tan, T. C., He, J., Greer, R. C., Weir, M.
R., Zhang, X., Schrauben, S. J., Saunders, M. R., Ricardo, A. C., Lash, J. P., & Chronic Renal Insufficiency Cohort
(CRIC) Study Investigators (2022). Emergency Department/Urgent Care as Usual Source of Care and Clinical
Outcomes in CKD: Findings From the Chronic Renal Insufficiency Cohort Study. Kidney Medicine, 4(4), 100424.
https://doi.org/10.1016/j.xkme.2022.100424.
132
Bjornstad, P., Cherney, D. Z., & Maahs, D. M. (2015). Update on Estimation of Kidney Function in Diabetic
Kidney Disease. Current Diabetes Reports, 15(9), 57. https://doi.org/10.1007/s11892-015-0633-2.

B.45. Urology
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Urology specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a measure
reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Urology specialty
set.
B.45. Urology
PREVIOUSLY FINALIZED MEASURES IN THE UROLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure
Steward
*
!
(Care
Coordination)
0326 /
N/A
047
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Advance Care Plan:
Percentage of patients aged 65
years and older who have an
advance care plan or surrogate
decision maker documented in
the medical record or
documentation in the medical
record that an advance care
plan was discussed but the
patient did not wish or was
not able to name a surrogate
decision maker or provide an
advance care plan.
National
Committee
for Quality
Assurance
N/A /
N/A
048
N/A
MIPS CQM
Specifications
Process
Urinary Incontinence:
Assessment of Presence or
Absence of Urinary
Incontinence in Women
Aged 65 Years and Older:
Percentage of female patients
aged 65 years and older who
were assessed for the presence
or absence of urinary
incontinence within 12
months.
National
Committee
for Quality
Assurance
!
(Patient
Experience)
N/A /
N/A
050
N/A
MIPS CQM
Specifications
Process
Urinary Incontinence: Plan of
Care for Urinary
Incontinence in Women Aged
65 Years and Older:
Percentage of female patients
aged 65 years and older with a
diagnosis of urinary
incontinence with a
documented plan of care for
urinary incontinence at least
once within 12 months.
National
Committee
for Quality
Assurance
§
!
(Appropriate
Use)
N/A /
N/A
102
CMS129v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Prostate Cancer: Avoidance
of Overuse of Bone Scan for
Staging Low Risk Prostate
Cancer Patients:
Percentage of patients,
regardless of age, with a
diagnosis of prostate cancer at
low (or very low) risk of
recurrence receiving interstitial
prostate brachytherapy, OR
external beam radiotherapy to
the prostate, OR radical
prostatectomy who did not have
a bone scan performed at any
time since diagnosis of prostate
cancer.
Centers for
Medicare &
Medicaid
Services

B.45. Urology
PREVIOUSLY FINALIZED MEASURES IN THE UROLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure
Steward
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS68v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for
patients aged 18 years and
older for which the eligible
clinician attests to
documenting a list of current
medications using all
immediate resources available
on the date of the encounter.
Centers for
Medicare &
Medicaid
Services
§
N/A /
N/A
134
CMS2v14
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Screening for
Depression and Follow-Up
Plan:
Percentage of patients aged 12
years and older screened for
depression on the date of the
encounter or up to 14 days
prior to the date of the
encounter using an age-
appropriate standardized
depression screening tool
AND if positive, a follow-up
plan is documented on the
date of or up to two days after
the date of the qualifying
encounter.
Centers for
Medicare &
Medicaid
Services
§
N/A /
N/A
226
CMS138v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Tobacco Use:
Screening and Cessation
Intervention:
Percentage of patients aged 12
years and older who were
screened for tobacco use one
or more times during the
measurement period AND
who received tobacco
cessation intervention during
the measurement period or in
the six months prior to the
measurement period if
identified as a tobacco user.
National
Committee for
Quality
Assurance
*
!
(Patient
Safety)
0022/
N/A
238
CMS156v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Use of High-Risk
Medications in Older
Adults:
Percentage of patients 65
years of age and older who
were ordered at least two
high-risk medications from
the same drug class.
National
Committee for
Quality
Assurance
*
N/A /
N/A
317
CMS22v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Screening for
High Blood Pressure and
Follow-Up Documented:
Percentage of patient visits for
patients aged 18 years and
older seen during the
measurement period who
were screened for high blood
pressure AND a
recommended follow-up plan
is documented, as indicated, if
blood pressure is elevated or
hypertensive.
Centers for
Medicare &
Medicaid
Services

B.45. Urology
PREVIOUSLY FINALIZED MEASURES IN THE UROLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure
Steward
§
!
(Patient
Experience)
0005/
N/A
321
N/A
CMS-approved
Survey Vendor
Patient
Engagement/
Experience
CAHPS for MIPS
Clinician/Group Survey:
The Consumer Assessment of
Healthcare Providers and
Systems (CAHPS) for MIPS
Clinician/Group Survey is
comprised of 10 Summary
Survey Measures (SSMs) and
measures patient experience
of care within a group
practice. The CBE
endorsement status and
endorsement id (if applicable)
for each SSM utilized in this
measure are as follows:
• Getting Timely Care,
Appointments, and
Information; (Not endorsed by
CBE)
• How well Providers
Communicate; (Not endorsed
by CBE)
• Patient’s Rating of Provider;
(CBE endorsed # 0005)
• Access to Specialists; (Not
endorsed by CBE)
• Health Promotion and
Education; (Not endorsed by
CBE)
• Shared Decision-Making;
(Not endorsed by CBE)
• Health Status and Functional
Status; (Not endorsed by
CBE)
• Courteous and Helpful
Office Staff; (CBE endorsed #
0005)
• Care Coordination; (Not
endorsed by CBE)
• Stewardship of Patient
Resources. (Not endorsed by
CBE)
Centers for
Medicare &
Medicaid
Services
!
(Patient
Experience)
N/A /
N/A
358
N/A
MIPS CQM
Specifications
Process
Patient-Centered Surgical
Risk Assessment and
Communication:
Percentage of patients who
underwent a non-emergency
surgery who had their
personalized risks of
postoperative complications
assessed by their surgical
team prior to surgery using a
clinical data-based, patient-
specific risk calculator and
who received personal
discussion of those risks with
the surgeon.
American
College of
Surgeons
*
!
(Care
Coordination)
N/A /
N/A
374
CMS50v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Closing the Referral Loop:
Receipt of Specialist Report:
Percentage of patients with
referrals, regardless of age, for
which the referring clinician
receives a report from the
clinician to whom the patient
was referred.
Centers for
Medicare &
Medicaid
Services
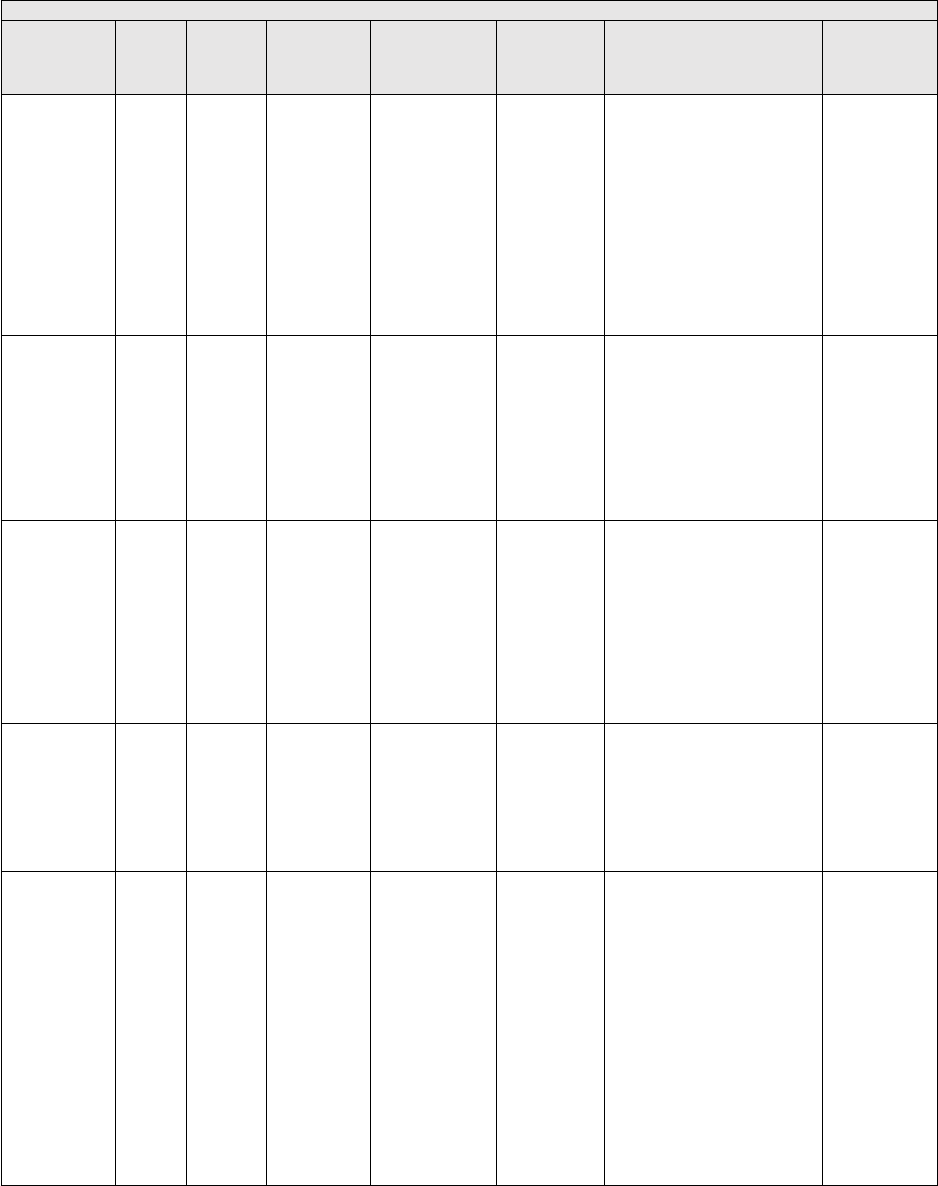
B.45. Urology
PREVIOUSLY FINALIZED MEASURES IN THE UROLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure
Steward
§
2152 /
N/A
431
N/A
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Unhealthy
Alcohol Use: Screening &
Brief Counseling:
Percentage of patients aged 18
years and older who were
screened for unhealthy
alcohol use using a systematic
screening method at least once
within the last 12 months
AND who received brief
counseling if identified as an
unhealthy alcohol user.
National
Committee
for Quality
Assurance
*
!
(Outcome)
N/A /
N/A
432
N/A
MIPS CQM
Specifications
Outcome
Proportion of Patients
Sustaining a Bladder Injury
at the Time of any Pelvic
Organ Prolapse Repair:
Percentage of patients
undergoing pelvic organ
prolapse repairs who sustain
an injury to the bladder
recognized either during or
within 30 days after surgery.
American
Urogyneco-
logic Society
§
!
(Appropriate
Use)
0210/
N/A
453
N/A
MIPS CQM
Specifications
Process
Percentage of Patients Who
Died from Cancer Receiving
Systemic Cancer-Directed
Therapy in the Last 14 Days
of Life (lower score –
better):
Percentage of patients who
died from cancer receiving
systemic cancer-directed
therapy in the last 14 days of
life.
American
Society of
Clinical
Oncology
§
!
(Appropriate
Use)
0216/
N/A
457
N/A
MIPS CQM
Specifications
Process
Percentage of Patients Who
Died from Cancer Admitted
to Hospice for Less than 3
days (lower score – better):
Percentage of patients who
died from cancer, and
admitted to hospice and spent
less than 3 days there.
American
Society of
Clinical
Oncology
*
N/A /
N/A
462
CMS645v8
eCQM
Specifications
Process
Bone Density Evaluation for
Patients with Prostate
Cancer and Receiving
Androgen Deprivation
Therapy:
Patients determined as having
prostate cancer who are
currently starting or
undergoing androgen
deprivation therapy (ADT),
for an anticipated period of 12
months or greater and who
receive an initial bone density
evaluation. The bone density
evaluation must be prior to the
start of ADT or within 3
months of the start of ADT.
Oregon
Urology
Institute

B.45. Urology
PREVIOUSLY FINALIZED MEASURES IN THE UROLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure
Steward
!
(Outcome)
N/A /
N/A
476
CMS771v6
eCQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Urinary Symptom Score
Change 6-12 Months After
Diagnosis of Benign
Prostatic Hyperplasia:
Percentage of patients with an
office visit within the
measurement period and with
a new diagnosis of clinically
significant Benign Prostatic
Hyperplasia who have
International Prostate
Symptoms Score (IPSS) or
American Urological
Association (AUA) Symptom
Index (SI) documented at time
of diagnosis and again 6-12
months later with an
improvement of 3 points.
Large
Urology
Group
Practice
Association
and Oregon
Urology
Institute
!
(Appropriate
Use)
N/A/
N/A
481
CMS646v5
eCQM
Specifications
Process
Intravesical Bacillus-
Calmette Guerin for Non-
Muscle Invasive Bladder
Cancer:
Percentage of patients initially
diagnosed with non-muscle
invasive bladder cancer and
who received intravesical
Bacillus-Calmette-Guerin
(BCG) within 6 months of
bladder cancer staging.
Oregon
Urology
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers
of Health:
Percent of patients 18 years
and older screened for food
insecurity, housing instability,
transportation needs, utility
difficulties, and interpersonal
safety.
Centers for
Medicare &
Medicaid
Services
*
N/A /
N/A
488
CMS951v3
eCQM
Specifications,
MIPS CQM
Specifications
Process
Kidney Health Evaluation:
Percentage of patients aged
18-75 years with a diagnosis
of diabetes who received a
kidney health evaluation
defined by an Estimated
Glomerular Filtration Rate
(eGFR) AND Urine Albumin-
Creatinine Ratio (uACR)
within the measurement
period.
National
Kidney
Foundation
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years or
older who screen positive for
one or more of the following
health related social needs
(HRSNs): food insecurity,
housing instability,
transportation needs, utility
help needs, or interpersonal
safety; and had contact with a
Community Service Provider
(CSP) for at least 1 of their
HRSNs within 60 days after
screening.
OCHIN

B.45. Urology
PREVIOUSLY FINALIZED MEASURES IN THE UROLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure
Steward
*
!
(Outcome)
2483 /
N/A
503
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Gains in Patient Activation
Measure (PAM®) Scores at
12 Months:
The Patient Activation
Measure® (PAM®) is a 10 –
or 13 – item questionnaire that
assesses an individual´s
knowledge, skills and
confidence for managing their
health and health care. The
measure assesses individuals
on a 0-100 scale that converts
to one of four levels of
activation, from low (1) to
high (4). The PAM®
performance measure
(PAM®-PM) is the change in
score on the PAM® from
baseline to follow-up
measurement.
Insignia
Health, LLC,
a wholly
owned
subsidiary of
Phreesia

B.45. Urology
MEASURES PROPOSED FOR ADDITION TO THE UROLOGY SPECIALTY SET
Indicator
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Inclusion
N/A /
N/A
TBD
N/A
MIPS
CQM
Specificati
ons
Process
Adult COVID-
19 Vaccination
Status:
Percentage of
patients aged 18
years and older
seen for a visit
during the
performance
period that are up
to date on their
COVID-19
vaccinations as
defined by CDC
recommendation
s on current
vaccination.
Centers
for
Medicare
&
Medicaid
Services
We are proposing to include this
measure in the Urology specialty
set as it would be clinically
relevant to this clinician type.
Widespread vaccination against
SARS-CoV-2, the virus that
causes COVID-19, is critically
important to stemming the
morbidity and mortality caused by
this disease.
133
Clinicians are
uniquely positioned to encourage
uptake of COVID-19 vaccination,
and clinicians are still a major
driving force in promoting patient
vaccination. The addition of this
quality measure in this specialty
set would help strengthen
compliance with recommended
COVID-19 vaccination, leading to
improvement in the quality of
patient care and prevention of
disease for the general population.
This quality measure aligns with
clinical guidelines and the
evidence-based recommendations
of the ACIP, where there is
general agreement about the
safety and efficacy of the
COVID-19 vaccine, preventing
costly and potentially harmful
hospitalizations.
134
Broadening
vaccination status awareness to
this clinician type is valuable as it
can help drive an increase in the
adult vaccination rates. The
COVID-19 vaccination included
within this measure would reduce
the prevalence of severe diseases
that may be associated with
hospitalization and decrease
overall health care costs. The
measure being added to this
specialty set would be contingent
on the inclusion of applicable
coding by the time of the CY
2025 PFS final rule. In the event
appropriate coding is not included
in the final specification, this
measure would not be finalized
for inclusion within this specialty
measure set. See Table A.5 of this
Appendix for rationale, including
clinical evidence supporting the
inclusion of this measure in
MIPS.
133
See footnote Ikeokwu et al., 2023 in Table B.1 of this Appendix.
134
See footnotes Fitzpatrick et al., 2022; Polack et al., 2020; and Graña et al., 2022 in Table A.5 of this Appendix.

B.45. Urology
PREVIOUSLY FINALIZED MEASURES PROPOSED FOR REMOVAL FROM THE UROLOGY SPECIALTY SET
Note: In this proposed rule, we are proposing the removal of the following measure(s) below from this specific specialty measure set based
upon review of updates made to existing quality measure specifications, the proposed addition of new measures for inclusion in MIPS, and the
feedback provided by specialty societies.
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Removal
N/A /
N/A
104
N/A
MIPS CQM
Specifications
Process
Prostate Cancer:
Combination Androgen
Deprivation Therapy for
High Risk or Very High
Risk Prostate Cancer:
Percentage of patients,
regardless of age, with a
diagnosis of prostate cancer
at high or very high risk of
recurrence receiving
external beam radiotherapy
to the prostate who were
prescribed androgen
deprivation therapy in
combination with external
beam radiotherapy to the
prostate.
American
Urological
Association
Education and
Research
This measure is being
proposed for removal
beginning with the CY
2025 performance
period/2027 MIPS
payment year. See Table
Group C for rationale.
N/A /
N/A
433
N/A
MIPS CQM
Specifications
Outcome
Proportion of Patients
Sustaining a Bowel Injury
at the time of any Pelvic
Organ Prolapse Repair:
Percentage of patients
undergoing surgical repair
of pelvic organ prolapse that
is complicated by a bowel
injury at the time of index
surgery that is recognized
intraoperatively or within 30
days after surgery.
American
Urogynecologic
Society
This measure is being
proposed for removal
beginning with the CY
2025 performance
period/2027 MIPS
payment year. See Table
Group C for rationale.
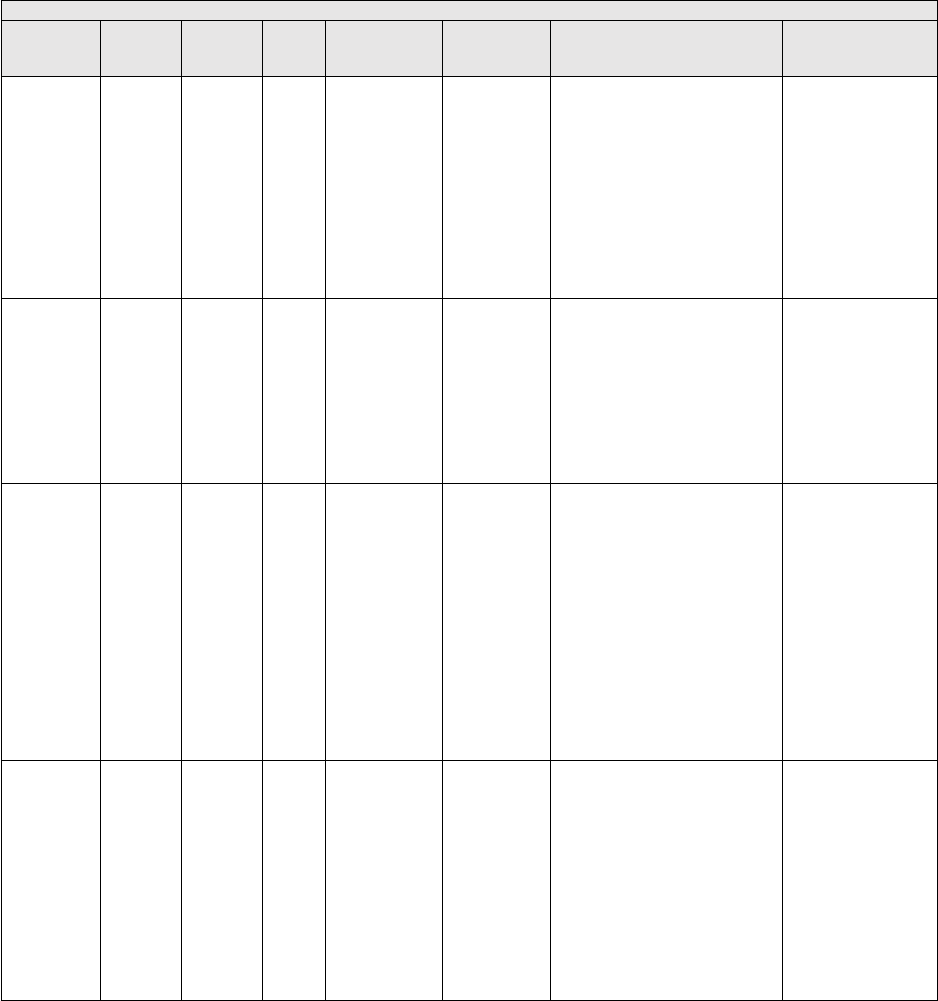
B.46. Vascular Surgery
In addition to the considerations discussed in the introductory language of Table Group B of this Appendix to this proposed rule,
the Vascular Surgery specialty set takes additional criteria into consideration, which includes, but is not limited to: whether a
measure reflects current clinical guidelines, and the coding of the measure includes relevant clinician types. We may reassess the
appropriateness of individual measures, on a case-by-case basis, to ensure appropriate inclusion in the specialty set. Measure
tables in this set include previously finalized measures we are maintaining within the set, measures proposed to be added, and
measures proposed for removal, as applicable. We request comment on the measures available in the proposed Vascular Surgery
specialty set.
B.46. Vascular Surgery
PREVIOUSLY FINALIZED MEASURES IN THE VASCULAR SURGERY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Care
Coordinatio
n)
0326 /
N/A
047
N/A
Medicare Part
B Claims
Measure
Specifications,
MIPS CQM
Specifications
Process
Advance Care Plan:
Percentage of patients aged 65
years and older who have an
advance care plan or surrogate
decision maker documented in
the medical record or
documentation in the medical
record that an advance care plan
was discussed but the patient did
not wish or was not able to
name a surrogate decision maker
or provide an advance care plan.
National Committee
for Quality
Assurance
*
§
!
(Patient
Safety)
N/A /
N/A
130
CMS
68v14
eCQM
Specifications,
MIPS CQM
Specifications
Process
Documentation of Current
Medications in the Medical
Record:
Percentage of visits for patients
aged 18 years and older for
which the eligible clinician
attests to documenting a list of
current medications using all
immediate resources available
on the date of the encounter.
Centers for Medicare
& Medicaid Services
§
N/A /
N/A
226
CMS
138v1
3
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Tobacco Use:
Screening and Cessation
Intervention:
Percentage of patients aged 12
years and older who were
screened for tobacco use one or
more times during the
measurement period AND who
received tobacco cessation
intervention during the
measurement period or in the six
months prior to the
measurement period if identified
as a tobacco user.
National Committee
for Quality
Assurance
*
§
!
(Outcome)
N/A /
N/A
236
CMS
165v1
3
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Intermediate
Outcome
Controlling High Blood
Pressure:
Percentage of patients 18-85
years of age who had a
diagnosis of essential
hypertension starting before and
continuing into, or starting
during the first six months of the
measurement period, and whose
most recent blood pressure was
adequately controlled
(<140/90mmHg) during the
measurement period.
National Committee
for Quality
Assurance

B.46. Vascular Surgery
PREVIOUSLY FINALIZED MEASURES IN THE VASCULAR SURGERY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
!
(Outcome)
N/A /
N/A
259
N/A
MIPS CQM
Specifications
Outcome
Rate of Endovascular
Aneurysm Repair (EVAR) of
Small or Moderate Non-
Ruptured Infrarenal
Abdominal Aortic Aneurysms
(AAA) without Major
Complications (Discharged to
Home by Post Operative Day
#2):
Percent of patients undergoing
endovascular repair of small or
moderate non-ruptured
infrarenal abdominal aortic
aneurysms (AAA) that do not
experience a major complication
(discharged to home no later
than post-operative day #2).
Society for Vascular
Surgeons
*
N/A /
N/A
317
CMS
22v13
Medicare Part
B Claims
Measure
Specifications,
eCQM
Specifications,
MIPS CQM
Specifications
Process
Preventive Care and
Screening: Screening for High
Blood Pressure and Follow-Up
Documented:
Percentage of patient visits for
patients aged 18 years and older
seen during the measurement
period who were screened for
high blood pressure AND a
recommended follow-up plan is
documented, as indicated, if
blood pressure is elevated or
hypertensive.
Centers for Medicare
& Medicaid Services
*
!
(Outcome)
N/A /
N/A
344
N/A
MIPS CQM
Specifications
Outcome
Rate of Carotid Artery
Stenting (CAS) for
Asymptomatic Patients,
Without Major Complications
(Discharged to Home by Post-
Operative Day #2):
Percent of asymptomatic
patients undergoing CAS who
are discharged to home no later
than post-operative day #2.
Society for Vascular
Surgeons
!
(Outcome)
N/A /
N/A
357
N/A
MIPS CQM
Specifications
Outcome
Surgical Site Infection (SSI):
Percentage of patients aged 18
years and older who had a
surgical site infection (SSI).
American College of
Surgeons
!
(Patient
Experience)
N/A /
N/A
358
N/A
MIPS CQM
Specifications
Process
Patient-Centered Surgical
Risk Assessment and
Communication:
Percentage of patients who
underwent a non-emergency
surgery who had their
personalized risks of
postoperative complications
assessed by their surgical team
prior to surgery using a clinical
data-based, patient-specific risk
calculator and who received
personal discussion of those
risks with the surgeon.
American College
of Surgeons
*
!
(Care
Coordinatio
n)
N/A /
N/A
374
CMS
50v13
eCQM
Specifications,
MIPS CQM
Specifications
Process
Closing the Referral Loop:
Receipt of Specialist Report:
Percentage of patients with
referrals, regardless of age, for
which the referring clinician
receives a report from the
clinician to whom the patient
was referred.
Centers for
Medicare &
Medicaid Services

B.46. Vascular Surgery
PREVIOUSLY FINALIZED MEASURES IN THE VASCULAR SURGERY SPECIALTY SET
Indicator
CBE # /
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
and Description
Measure Steward
*
!
(Outcome)
N/A /
N/A
420
N/A
MIPS CQM
Specifications
Patient-
Reported
Outcome-
Based
Performance
Measure
Varicose Vein Treatment with
Saphenous Ablation: Outcome
Survey:
Percentage of patients treated
for varicose veins (CEAP C2-S)
who are treated with saphenous
ablation (with or without
adjunctive tributary treatment)
that report an improvement on a
disease specific patient reported
outcome survey instrument after
treatment.
Society of
Interventional
Radiology
§
!
(Outcome)
N/A /
N/A
441
N/A
MIPS CQM
Specifications
Intermediate
Outcome
Ischemic Vascular Disease
(IVD) All or None Outcome
Measure (Optimal Control):
The IVD All-or-None Measure
is one outcome measure
(optimal control). The measure
contains four goals. All four
goals within a measure must be
reached in order to meet that
measure. The numerator for the
all-or-none measure should be
collected from the organization's
total IVD denominator. All-or-
None Outcome Measure
(Optimal Control) - Using the
IVD denominator optimal
results include:
• Most recent blood pressure
(BP) measurement is less than
or equal to 140/90 mm Hg --
AND
• Most recent tobacco status is
Tobacco Free -- AND
• Daily Aspirin or Other
Antiplatelet Unless
Contraindicated -- AND
• Statin Use Unless
Contraindicated.
Wisconsin
Collaborative for
Healthcare Quality
!
(Equity)
N/A /
N/A
487
N/A
MIPS CQM
Specifications
Process
Screening for Social Drivers of
Health:
Percent of patients 18 years and
older screened for food
insecurity, housing instability,
transportation needs, utility
difficulties, and interpersonal
safety.
Centers for
Medicare &
Medicaid Services
*
!
(Equity)
N/A /
N/A
498
N/A
MIPS CQM
Specifications
Process
Connection to Community
Service Provider:
Percent of patients 18 years or
older who screen positive for
one or more of the following
health related social needs
(HRSNs): food insecurity,
housing instability,
transportation needs, utility help
needs, or interpersonal safety;
and had contact with a
Community Service Provider
(CSP) for at least 1 of their
HRSNs within 60 days after
screening.
OCHIN

B.46. Vascular Surgery
PREVIOUSLY FINALIZED MEASURES PROPOSED FOR REMOVAL FROM THE VASCULAR SURGERY
SPECIALTY SET
Note: In this proposed rule, we are proposing the removal of the following measure(s) below from this specific specialty measure set based
upon review of updates made to existing quality measure specifications, the proposed addition of new measures for inclusion in MIPS, and the
feedback provided by specialty societies.
CBE #
/
eCQM
CBE #
Quality
#
CMS
eCQM
ID
Collection
Type
Measure
Type
Measure Title
And Description
Measure
Steward
Rationale for Removal
NA /
NA
260
N/A
MIPS CQM
Specification
s
Outcome
Rate of Carotid
Endarterectomy (CEA) for
Asymptomatic Patients,
without Major
Complications (Discharged
to Home by Post-
Operative Day #2):
Percent of asymptomatic
patients undergoing Carotid
Endarterectomy (CEA) who
are discharged to home no
later than post-operative day
#2.
Society for
Vascular
Surgeons
This measure is being
proposed for removal
beginning with the CY
2025 performance
period/2027 MIPS
payment year. See Table
Group C for rationale.
Table Group C: Previously Finalized Quality Measures Proposed for Removal for the CY
2025 Performance Period/2027 MIPS Payment Year and Future Years
In this proposed rule, we are proposing to remove 11 previously finalized MIPS quality measures for the CY 2025 performance
period/2027 MIPS payment year and future years which are discussed in detail in the removal tables below. We note measure
Q436: Radiation Consideration for Adult CT: Utilization of Dose Lowering Techniques was already finalized for removal with a
1-year delay to the CY 2025 performance period as noted in the Table Group B introduction (88 FR 79896); therefore, measure
Q436 does not have a removal table in this proposed rule and there are 10 removal tables under Table Group C.
The CY 2019 PFS final rule (83 FR 59763 through 59765) and CY 2020 PFS final rule (84 FR 62957 through 62959) discusses
our incremental approach to removing process measures.
Under our measure removal criteria, consideration is given to the following, but is not limited to:
• Whether the removal of the process measure impacts the number of measures available for a specific specialty.
• Whether the measure addresses a priority area highlighted in the Measure Development Plan at
https://www.cms.gov/Medicare/Quality-Payment-Program/Measure-Development/Measure-development.
• Whether the measure promotes positive outcomes in patients.
• Considerations and evaluation of the measure’s performance data.
• Whether the measure is designated as high priority or not.
• If they do not meet case minimum and reporting volumes required for benchmarking after being in the program
for two consecutive CY performance periods.
• After factoring in other considerations (such as, but not limited to: the robustness of the measure; whether it
addresses a measurement gap; if the measure is a patient-reported outcomes; consideration of the measure in
developing MVPs).
• If we determine the measure is not available for MIPS reporting by or on behalf of all MIPS eligible clinicians.
Further considerations are given in the evaluation of the measure’s performance data to determine whether there is or no longer is
variation in performance. As discussed in the CY 2019 PFS final rule (83 FR 59761 through 59763), an additional criterion we
use for the removal of measures includes extremely topped-out measures, which refers to measures topped out with an average
(mean) performance rate between 98-100 percent.
For a measure proposed for removal due to criteria relating to the benchmark and performance data, further information
regarding 2024 MIPS benchmarking data can be located at https://qpp.cms.gov/benchmarks.

C.1. Prostate Cancer: Combination Androgen Deprivation Therapy for High Risk or Very High Risk Prostate Cancer
Category
Description
CBE# / eCQM CBE #:
N/A / N/A
Quality #:
104
CMS eCQM ID:
N/A
Collection Type:
MIPS CQM Specifications
Measure Description:
Percentage of patients, regardless of age, with a diagnosis of prostate cancer at high or
very high risk of recurrence receiving external beam radiotherapy to the prostate who
were prescribed androgen deprivation therapy in combination with external beam
radiotherapy to the prostate.
Measure Steward:
American Urological Association Education and Research
High Priority Measure:
No
Measure Type:
Process
Rationale for Removal:
We are proposing the removal of this quality measure from MIPS (finalized in 81 FR
77558 through 77675) because the limited patient population as well as adoption of this
quality measure does not allow for the creation of a benchmark. For more information on
benchmarks, see the MIPS 2024 Quality Benchmarks User Guide
https://www.cms.gov/files/document/2024-quality-benchmarks-user-guide-scoring-
examples-pdf.pdf. The current 2024 MIPS benchmarking data is located at
https://qpp.cms.gov/benchmarks.
In the Circumstance the Measure Was
Retained:
There are no substantive changes or specialty set movement proposed for this measure. If
the measure is not finalized for removal in the CY 2025 PFS final rule, it would be added
back into the applicable previously finalized specialty set(s) under Table Group B of this
Appendix and the reason for its retention would be addressed under Table Group C of this
Appendix.
C.2. Melanoma: Continuity of Care – Recall System
Category
Description
CBE# / eCQM CBE #:
N/A / N/A
Quality #:
137
CMS eCQM ID:
N/A
Collection Type:
MIPS CQM Specifications
Measure Description:
Percentage of patients, regardless of age, with a current diagnosis of melanoma or a
history of melanoma whose information was entered, at least once within a 12 month
period, into a recall system that includes:
• A target date for the next complete physical skin exam, AND
• A process to follow up with patients who either did not make an appointment within the
specified timeframe or who missed a scheduled appointment.
Measure Steward:
American Academy of Dermatology
High Priority Measure:
Yes
Measure Type:
Structure
Rationale for Removal:
We are proposing the removal of this quality measure from MIPS (finalized in 81 FR
77558 through 77675) because this measure is duplicative of the Melanoma: Tracking and
Evaluation of Recurrence measure being proposed in Table A.6 of this Appendix.
Measure Q137 establishes a recall system linked to the process of notifying patients, with
a current diagnosis or history of melanoma, when their next physical exam is due, as well
as to follow up with patients who did not make an appointment within the specified
timeframe or who missed a scheduled appointment. It does not assess whether the patient
completed an annual skin exam nor if the process to follow up for those patients who
missed an appointment is effective. The new measure proposed in Table A.6 of this
Appendix is a process measure that not only assesses a significantly similar patient
population, but also assesses patients who had excisional surgery for melanoma or
melanoma in situ in the past 5 years for completion of an exam and/or diagnosed for
recurrence of melanoma. We believe the new measure provides a more meaningful impact
to quality improvement for this patient population.
In the Circumstance the Measure Was
Retained:
There are no substantive changes or specialty set movement proposed for this measure. If
the measure is not finalized for removal in the CY 2025 PFS final rule, it would be added
back into the applicable previously finalized specialty set(s) under Table Group B of this
Appendix and the reason for its retention would be addressed under Table Group C of this
Appendix.

C.3. Oncology: Medical and Radiation – Plan of Care for Pain
Category
Description
CBE# / eCQM CBE #:
0383 / N/A
Quality #:
144
CMS eCQM ID:
N/A
Collection Type:
MIPS CQM Specifications
Measure Description:
Percentage of visits for patients, regardless of age, with a diagnosis of cancer currently
receiving chemotherapy or radiation therapy who report having pain with a documented
plan of care to address pain.
Measure Steward:
American Society of Clinical Oncology
High Priority Measure:
Yes
Measure Type:
Process
Rationale for Removal:
We are proposing the removal of this quality measure from MIPS (finalized in 81 FR
77558 through 77675) because this measure is duplicative of measure Q143: Oncology:
Medical and Radiation – Pain Intensity Quantified. Measure Q143 specifically questions
cancer patients, currently receiving chemotherapy or radiation therapy, to quantify their
pain intensity at each visit using a standardized tool. This measure is more robust than
measure Q144 as it encourages the clinician to initiate a discussion regarding pain
intensity with the patient at every denominator eligible visit. Measure Q144 is narrower in
scope focusing on cancer patients identified with pain. To identify this patient population,
a clinician would still need to screen their patients. Measure Q143 promotes screening of a
broader patient population for pain, which still allows clinicians to administer care if pain
is present. Additionally, measure Q143 is available for reporting within the eCQM
Specifications collection type, allowing clinicians more measure options if reporting via
eCQMs.
In the Circumstance the Measure Was
Retained:
There are no substantive changes or specialty set movement proposed for this measure. If
the measure is not finalized for removal in the CY 2025 PFS final rule, it would be added
back into the applicable previously finalized specialty set(s) under Table Group B of this
Appendix and the reason for its retention would be addressed under Table Group C of this
Appendix.
C.4. Ultrasound Determination of Pregnancy Location for Pregnant Patients with Abdominal Pain
Category
Description
CBE# / eCQM CBE #:
N/A / N/A
Quality #:
254
CMS eCQM ID:
N/A
Collection Type:
MIPS CQM Specifications
Measure Description:
Percentage of pregnant female patients aged 14 to 50 who present to the emergency
department (ED) with a chief complaint of abdominal pain or vaginal bleeding who
receive a trans-abdominal or trans-vaginal ultrasound to determine pregnancy location.
Measure Steward:
American College of Emergency Physicians
High Priority Measure:
No
Measure Type:
Process
Rationale for Removal:
We are proposing the removal of this quality measure from MIPS (finalized in 81 FR
77558 through 77675) because this measure has reached the end of the topped-out
lifecycle (82 FR 53640) and has a limited opportunity to improve clinical outcomes.
Topped-out process measures are those with a median performance rate of 95 percent or
higher (81 FR 77286).
135
This measure’s continued topped-out status is based on the
current 2024 MIPS benchmarking data located at https://qpp.cms.gov/benchmarks, in
addition to previous years MIPS benchmarking data. For more information on
benchmarks, see the MIPS 2024 Quality Benchmarks User Guide at
https://www.cms.gov/files/document/2024-quality-benchmarks-user-guide-scoring-
examples-pdf.pdf.
In the Circumstance the Measure Was
Retained:
There are no substantive changes or specialty set movement proposed for this measure. If
the measure is not finalized for removal in the CY 2025 PFS final rule, it would be added
back into the applicable previously finalized specialty set(s) under Table Group B of this
Appendix and the reason for its retention would be addressed under Table Group C of this
Appendix.
C.5. Rate of Carotid Endarterectomy (CEA) for Asymptomatic Patients, without Major Complications (Discharged to
Home by Post-Operative Day #2):
Category
Description
CBE# / eCQM CBE #:
N/A / N/A
Quality #:
260
CMS eCQM ID:
N/A
Collection Type:
MIPS CQM Specifications
135
See the 2024 MIPS Call for Measures Fact Set: https://qpp-cm-prod-
content.s3.amazonaws.com/uploads/2711/2024-MIPS-Call-for-Measures-and-Activities.zip.

Category
Description
Measure Description:
Percent of asymptomatic patients undergoing Carotid Endarterectomy (CEA) who are
discharged to home no later than post-operative day #2.
Measure Steward:
Society for Vascular Surgeons
High Priority Measure:
Yes
Measure Type:
Outcome
Rationale for Removal:
We are proposing the removal of this quality measure from MIPS (finalized in 81 FR
77558 through 77675) because this measure would be duplicative of measure Q344: Rate
of Carotid Artery Stenting (CAS) for Asymptomatic Patients, Without Major
Complications (Discharged to Home by Post-Operative Day #2) if the proposed changes
to the measure are finalized. We are proposing in Table D.34 of this Appendix substantive
changes to measure Q344, which describe the inclusion of the measure concept
represented in Q260. Measure Q260 is focused on performing CEA on asymptomatic
patients who are at low risk for morbidity and therefore are expected to have very low
complication rates as indicated by patients being discharged to home by post-operative
day #2. As a result, we are proposing to maintain measure Q344 and remove measure
Q260 from MIPS.
In the Circumstance the Measure Was
Retained:
There are no substantive changes or specialty set movement proposed for this measure. If
the measure is not finalized for removal in the CY 2025 PFS final rule, it would be added
back into the applicable previously finalized specialty set(s) under Table Group B of this
Appendix and the reason for its retention would be addressed under Table Group C of this
Appendix.
C.6. Clinical Outcome Post Endovascular Stroke Treatment
Category
Description
CBE# / eCQM CBE #:
N/A / N/A
Quality #:
409
CMS eCQM ID:
N/A
Collection Type:
MIPS CQM Specifications
Measure Description:
Percentage of patients with a Modified Rankin Score (mRS) score of 0 to 2 at 90 days
following endovascular stroke intervention.
Measure Steward:
Society of Interventional Radiology
High Priority Measure:
Yes
Measure Type:
Outcome
Rationale for Removal:
We are proposing the removal of this quality measure from MIPS (finalized in 81 FR
77558 through 77675) at the measure steward’s request as it is no longer being maintained
for inclusion.
In the Circumstance the Measure Was
Retained:
There are no substantive changes or specialty set movement proposed for this measure. If
the measure is not finalized for removal in the CY 2025 PFS final rule, it would be added
back into the applicable previously finalized specialty set(s) under Table Group B of this
Appendix and the reason for its retention would be addressed under Table Group C of this
Appendix.

C.7. Proportion of Patients Sustaining a Bowel Injury at the time of any Pelvic Organ Prolapse Repair
Category
Description
CBE# / eCQM CBE #:
N/A / N/A
Quality #:
433
CMS eCQM ID:
N/A
Collection Type:
MIPS CQM Specifications
Measure Description:
Percentage of patients undergoing surgical repair of pelvic organ prolapse that is
complicated by a bowel injury at the time of index surgery that is recognized
intraoperatively or within 30 days after surgery.
Measure Steward:
American Urogynecologic Society
High Priority Measure:
Yes
Measure Type:
Outcome
Rationale for Removal:
We are proposing the removal of this quality measure from MIPS (finalized in 81 FR
77558 through 77675) because this measure would be duplicative of measure Q432:
Proportion of Patients Sustaining a Bladder Injury at the Time of any Pelvic Organ
Prolapse Repair, if the proposed changes to the measure are finalized. We are proposing in
Table D.46 of this Appendix substantive changes to measure Q432, which describe the
inclusion of the measure concept represented in measure Q433. In addition, we are
proposing to update the measure title and description for measure Q432 to include patients
who sustain a bowel injury at the time of any pelvic organ prolapse repair.
In the Circumstance the Measure Was
Retained:
There are no substantive changes or specialty set movement proposed for this measure. If
the measure is not finalized for removal in the CY 2025 PFS final rule, it would be added
back into the applicable previously finalized specialty set(s) under Table Group B of this
Appendix and the reason for its retention would be addressed under Table Group C of this
Appendix.
C.8. Age Appropriate Screening Colonoscopy
Category
Description
CBE# / eCQM CBE #:
N/A / N/A
Quality #:
439
CMS eCQM ID:
N/A
Collection Type:
MIPS CQM Specifications
Measure Description:
The percentage of screening colonoscopies performed in patients greater than or equal to
86 years of age from January 1 to December 31.
Measure Steward:
American Gastroenterological Association
High Priority Measure:
Yes
Measure Type:
Efficiency
Rationale for Removal:
We are proposing the removal of this quality measure from MIPS (finalized in 81 FR
77558 through 77675) because the quality action being measured has become standard of
care, based upon MIPS performance data, and thus has limited opportunity to improve
clinical outcomes. Performance on this measure is extremely high and unvarying, making
this measure extremely topped out as discussed in the CY 2019 PFS final rule (83 FR
59761 through 59763). The average performance for this inverse measure is 0.15 percent
for the MIPS CQM Specifications collection type. For an inverse measure, a lower
calculated performance rate indicates better clinical care or control. As such, the MIPS
CQM Specifications collection type is considered extremely topped out. The average
performance rate is based on the current 2024 MIPS benchmarking data located at
https://qpp.cms.gov/benchmarks. For more information on benchmarks, see the MIPS
2024 Quality Benchmarks User Guide at https://www.cms.gov/files/document/2024-
quality-benchmarks-user-guide-scoring-examples-pdf.pdf.
In the Circumstance the Measure Was
Retained:
There are no substantive changes or specialty set movement proposed for this measure. If
the measure is not finalized for removal in the CY 2025 PFS final rule, it would be added
back into the applicable previously finalized specialty set(s) under Table Group B of this
Appendix and the reason for its retention would be addressed under Table Group C of this
Appendix.

C.9. Patients with Metastatic Colorectal Cancer and RAS (KRAS or NRAS) Gene Mutation Spared Treatment with Anti-
epidermal Growth Factor Receptor (EGFR) Monoclonal Antibodies
Category
Description
CBE# / eCQM CBE #:
1860 / N/A
Quality #:
452
CMS eCQM ID:
N/A
Collection Type:
MIPS CQM Specifications
Measure Description:
Percentage of adult patients (aged 18 or over) with metastatic colorectal cancer and RAS
(KRAS or NRAS) gene mutation spared treatment with anti-EGFR monoclonal
antibodies.
Measure Steward:
American Society of Clinical Oncology
High Priority Measure:
Yes
Measure Type:
Process
Rationale for Removal:
We are proposing the removal of this quality measure from MIPS (finalized in 81 FR
77558 through 77675) because this measure is duplicative of measure Q451: RAS (KRAS
and NRAS) Gene Mutation Testing Performed for Patients with Metastatic Colorectal
Cancer who receive Anti-epidermal Growth Factor Receptor (EGFR) Monoclonal
Antibody Therapy. Measures Q451 and Q452 ask the same clinical question but approach
questioning from different clinical perspectives. Measure Q451 assesses patients who
have already received anti-EGFR monoclonal therapy to determine if they received RAS
gene mutation testing prior to initiation of therapy, while measure Q452 is a standard-of-
care measure, as well as the counter clinical perspective to measure Q451. Measure Q452
only evaluates for patients who received anti-EGFR monoclonal treatment. Therefore,
measure Q452 is a component of the quality action within measure Q451. The molecular
biomarkers for the evaluation of colorectal cancer guidelines
136
strongly recommend
“Patients with colorectal carcinoma being considered for anti-EGFR therapy must receive
RAS mutational testing,” which is the standard of care being evaluated by this measure.
Measure Q451 provides an opportunity for a retrospective review and has the potential to
improve clinical outcomes. For example, if the performance rates show there is a gap in
medical care or high incidence of initiating therapy without first testing for the RAS gene
mutation, clinical processes can be revised to improve practice. “Clinical care review is
the process of retrospectively examining potential errors or gaps in medical care, with a
goal of future practice improvement.”
137
In the Circumstance the Measure Was
Retained:
If the measure is not finalized for removal in the CY 2025 PFS final rule, we are
proposing to apply the following substantive changes to the measure specifications: (1)
add denominator instructions to clarify the denominator eligible timeframe for diagnosis
and (2) only patients who have been newly diagnosed with Stage IV colorectal cancer, or
patients who have distant metastases at the time of colon cancer diagnosis, are to be
included in the denominator of the measure to ensure the appropriate patient population is
assessed for the numerator action.
If the measure is not finalized for removal in the CY 2025 PFS final rule, it would be
added back into the applicable previously finalized specialty set(s) under Table Group B
and the reason for its retention would be addressed under Table Group C. The substantive
changes outlined above would be applied to the measure specifications.
136
Sepulveda, A. R., Hamilton, S. R., Allegra, C. J., Grody, W., Cushman-Vokoun, A. M., Funkhouser, W. K., Kopetz, S.
E., Lieu, C., Lindor, N. M., Minsky, B. D., Monzon, F. A., Sargent, D. J., Singh, V. M., Willis, J., Clark, J., Colasacco, C.,
Rumble, R. B., Temple-Smolkin, R., Ventura, C. B., & Nowak, J. A. (2017). Molecular Biomarkers for the Evaluation
of Colorectal Cancer: Guideline From the American Society for Clinical Pathology, College of American Pathologists,
Association for Molecular Pathology, and the American Society of Clinical Oncology. Journal of Clinical Oncology:
Official Journal of the American Society of Clinical Oncology, 35(13), 1453–1486.
https://doi.org/10.1200/JCO.2016.71.9807.
137
Walker, L. E., Nestler, D. M., Laack, T. A., Clements, C. M., Erwin, P. J., Scanlan-Hanson, L., & Bellolio, M. F.
(2018). Clinical Care Review Systems in Healthcare: A Systematic Review. International Journal of Emergency
Medicine, 11(1), 6. https://doi.org/10.1186/s12245-018-0166-y.

C.10. Appropriate Use of DXA Scans in Women Under 65 Years Who Do Not Meet the Risk Factor Profile for
Osteoporotic Fracture
Category
Description
CBE# / eCQM CBE #:
N/A / 3475e
Quality #:
472
CMS eCQM ID:
CMS249v7
Collection Type:
eCQM Specifications
Measure Description:
Percentage of female patients 50 to 64 years of age without select risk factors for
osteoporotic fracture who received an order for a dual-energy x-ray absorptiometry
(DXA) scan during the measurement period.
Measure Steward:
Centers for Medicare & Medicaid Services
High Priority Measure:
Yes
Measure Type:
Process
Rationale for Removal:
We are proposing the removal of this quality measure from MIPS (finalized in 83 FR
60104 through 60105) because the quality action being measured has become standard of
care, based upon MIPS performance data, and thus has limited opportunity to improve
clinical outcomes. Performance on this measure is extremely high and unvarying, making
this measure extremely topped out as discussed in the CY 2019 PFS final rule (83 FR
59761 through 59763). The average performance for this inverse measure is 1.1 percent
for the eCQM Specifications collection type. For an inverse measure, a lower calculated
performance rate indicates better clinical care or control. As such, the eCQM
Specifications collection type is considered extremely topped out. The average
performance rate is based on the current 2024 MIPS benchmarking data located at
https://qpp.cms.gov/benchmarks. For more information on benchmarks, see the MIPS
2024 Quality Benchmarks User Guide at https://www.cms.gov/files/document/2024-
quality-benchmarks-user-guide-scoring-examples-pdf.pdf.
In the Circumstance the Measure Was
Retained:
There are no substantive changes or specialty set movement proposed for this measure. If
the measure is not finalized for removal in the CY 2025 PFS final rule, it would be added
back into the applicable previously finalized specialty set(s) under Table Group B of this
Appendix and the reason for its retention would be addressed under Table Group C of this
Appendix.
Table Group D: Proposed Substantive Changes to Previously Finalized MIPS Quality
Measures for the CY 2025 Performance Period/2027 MIPS Payment Year and Future
Years
The D Tables within this proposed rule provide the substantive changes proposed for the MIPS quality measures in CY 2025. We
note that some MIPS quality measures available in traditional MIPS and MVPs are adopted by the Medicare Shared Savings
Program for utilization in the Alternative Payment Model (APM) Performance Pathway (APP) and/or APP Plus, as proposed in
section IV.A.4.c.(3) of this proposed rule. For such measures, the collection type applicable for purposes of the APP and/or APP
Plus (Medicare CQM for Accountable Care Organizations Participating in the Medicare Shared Savings Program (Medicare
CQM)) is also specified as a collection type available for such measures described in Table Group D.
The changes that are made to the denominator codes sets are generalizations of the revisions communicated from the measure
stewards to CMS. Additionally, International Classification of Diseases Tenth Edition (ICD-10) and Current Procedural
Terminology (CPT) codes that are identified as invalid for CY 2025 may not be identified within this proposed rule due to the
availability of these changes to the public. If coding revisions to the denominator are impacted due to the timing of 2025 CPT and
ICD-10 updates and assessment of these codes’ inclusion by the Measure Steward, these changes may be postponed until CY
2025. The 2025 Quality Measure Release Notes provide a comprehensive, detailed reference of exact code changes to the
denominators of the quality measures. The Quality Measure Release Notes are available for each of the collection types in the
Quality Payment Program website at https://qpp.cms.gov. In addition, eCQMs that are endorsed by a CBE are shown in Table D
of this Appendix as follows: CBE # / eCQM CBE #.
In addition to the proposed substantive changes, there may be changes to the coding utilized within the denominator that are not
considered substantive in nature, but they are important to communicate to interested parties. These changes align with the scope
of the current coding; however, though not substantive in nature, these changes would expand or contract the measure’s current
eligible population. Therefore, please refer to the current year measure specification and the 2025 Quality Measure Release Notes
or the eCQM Technical Release Notes once posted to review all coding changes to ensure correct implementation. Language has
also been added, to all applicable 2025 quality measure specifications, in the form of an ‘Instructions Note’ to clarify that
telehealth encounters are allowed for determination of denominator eligibility. Only where telehealth encounters previously were
not allowed as denominator eligible would the D table corresponding to a measure reflect an update to the denominator allowing
for telehealth encounters in the ‘Substantive Change’ cell.
The eCQM Technical Release Notes should also be carefully reviewed for revisions within the logic portion of the measure. In
addition to the proposed substantive changes, there may be revisions within the logic that are not considered substantive in
nature, however, it is important to review to ensure proper implementation of the measure. As not all systems and clinical
workflows are the same, it is important to review these changes in the context of a specific system and/or clinical workflow.

D.1. Diabetes: Hemoglobin A1c (HbA1c) Poor Control (>9%)
Category
Description
CBE # / eCQM CBE #:
0059 / N/A
Quality #:
001
CMS eCQM ID:
CMS122v13
Current Collection Type:
Medicare Part B Claims Measure Specifications/ eCQM Specifications/ MIPS CQM Specifications/
Medicare CQM Specifications (collection type available only in the APP and/or APP Plus)
Current Measure Description:
Percentage of patients 18-75 years of age with diabetes who had hemoglobin A1c > 9.0% during the
measurement period.
Substantive Change:
The measure title is revised from “Diabetes: Hemoglobin A1c (HbA1c) Poor Control (>9%)”
to: Diabetes: Glycemic Status Assessment Greater Than 9%.
The measure description is revised to read: For all collection types: Percentage of patients 18-75
years of age with diabetes who had a glycemic status assessment (hemoglobin A1c [HbA1c] or
glucose management indicator [GMI]) > 9.0% during the measurement period.
Updated denominator exclusion: For all collection types: Removed: specific encounter
requirements from the frailty/advanced illness exclusion.
The measure numerator is revised to read: For all collection types: Patients whose most recent
glycemic status assessment (HbA1c or GMI) (performed during the measurement period) is > 9.0%
or is missing, or was not performed during the measurement period.
Updated guidance: For the eCQM Specifications collection type: Revised: If the glycemic status
assessment (HbA1c or GMI) is in the medical record, the test can be used to determine numerator
compliance.
Added: Glycemic status assessment (HbA1c or GMI) must be reported as a percentage (%).
If multiple glycemic status assessments were recorded for a single date, use the lowest result.
Updated numerator instructions: For the MIPS CQM Specifications and the Medicare Part B
Claims Measure Specifications collection types: Added: GMI as an assessment option.
Updated numerator options: For the MIPS CQM Specifications and the Medicare Part B
Claims Measure Specifications collection types: Added: GMI as an assessment option.
Updated numerator note: For the MIPS CQM Specifications and the Medicare Part B Claims
Measure Specifications collection types: Added: If multiple glycemic status assessments were
recorded for a single date, use the lowest result.
Measure Steward:
National Committee for Quality Assurance
High Priority Measure:
Yes
Measure Type:
Intermediate Outcome
Rationale
We are proposing to revise this measure to include glucose management indicators (GMI) to broaden
the acceptable methods for monitoring the glycemic status of patients with diabetes. This measure
revision supports those clinicians that are using this monitoring method to ensure management of
persons with diabetes using continuous glucose monitoring.
138
We are also proposing to update the denominator exclusion by removing the requirement for patients
to have had at least one inpatient or two outpatient encounters to recognize a diagnosis of advanced
illness. The removal of this requirement within the denominator exclusion reduces the burden of
identifying applicable encounters, while still identifying patients with an indication of frailty. This is
particularly applicable for those patients seen outside of their reporting clinician’s medical record.
This revision would update the denominator exclusion to remove any patient 66 years of age and
older who has a diagnosis of advanced illness during the measurement period or the year prior from
the denominator of measure Q001. Decreasing complexity and burden of this element ensures
consistent implementation allowing for more comparable data.
138
ElSayed, N. A., Aleppo, G., Aroda, V. R., Bannuru, R. R., Brown, F. M., Bruemmer, D., Collins, B. S., Hilliard, M. E.,
Isaacs, D., Johnson, E. L., Kahan, S., Khunti, K., Leon, J., Lyons, S. K., Perry, M. L., Prahalad, P., Pratley, R. E., Seley, J.
J., Stanton, R. C., Gabbay, R. A., … on behalf of the American Diabetes Association (2023). 7. Diabetes Technology:
Standards of Care in Diabetes-2023. Diabetes Care, 46(Suppl 1), S111–S127. https://doi.org/10.2337/dc23-S007.

D.2. Anti-Depressant Medication Management
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
009
CMS eCQM ID:
CMS128v13
Current Collection Type:
eCQM Specifications
Current Measure Description:
Percentage of patients 18 years of age and older who were treated with antidepressant medication,
had a diagnosis of major depression, and who remained on an antidepressant medication treatment.
Two rates are reported.
a. Percentage of patients who remained on an antidepressant medication for at least 84 days (12
weeks).
b. Percentage of patients who remained on an antidepressant medication for at least 180 days (6
months).
Substantive Change:
The measure initial patient population is revised to read: Patients 18 years of age and older as of
the IPSD who were dispensed antidepressant medications during the Intake Period, and were
diagnosed with major depression 60 days prior to, or 60 days after the dispensing event and had a
visit 60 days prior to, or 60 days after the dispensing event.
Updated logic and logic definition: Revised: logic to base it on age and IPSD.
Measure Steward:
National Committee for Quality Assurance
High Priority Measure:
No
Measure Type:
Process
Rationale
We are proposing to revise the initial patient population look-back window to evaluate the age of the
patient when medication is dispensed during the intake period. The updated timeframe distinguishes
between a new prescription and a continuing prescription to ensure the measure logic is more
accurate in determining the antidepressant treatment has met the numerator’s quality action. We are
also proposing to update the logic to look at the age and IPSD so that the look-back window allows
all patients to be assessed for the exclusion criteria related to previous anti-depression medication.

D.3. Diabetic Retinopathy: Communication with the Physician Managing Ongoing Diabetes Care
Category
Description
CBE # / eCQM CBE #:
N/A/ N/A
Quality #:
019
CMS eCQM ID:
CMS142v13
Current Collection Type:
eCQM Specifications / MIPS CQM Specifications
Current Measure Description:
Percentage of patients aged 18 years and older with a diagnosis of diabetic retinopathy who had a
dilated macular or fundus exam performed with documented communication to the physician who
manages the ongoing care of the patient with diabetes mellitus regarding the findings of the macular
or fundus exam at least once during the performance period.
Substantive Change:
Modified collection type: eCQM Specifications collection type.
Updated Guidance: Revised: The communication of results, including the level of severity of
diabetic retinopathy and presence or absence of macular edema to the primary care physician
providing ongoing care of a patient's diabetes should be completed soon after the dilated exam is
performed.
Measure Steward:
American Academy of Ophthalmology
High Priority Measure:
Yes
Measure Type:
Process
Rationale
We are proposing to remove the MIPS CQM Specifications collection type as it has reached the end
of the topped-out lifecycle (82 FR 53640). The average performance rate and topped-out status is
based on the current MIPS benchmarking data located at https://qpp.cms.gov/benchmarks. However,
the benchmarking data continues to show a gap for the eCQM Specifications collection type and as
such, we are proposing to retain that collection type.
Additionally, we are proposing to revise the measure guidance to define required information to be
included within the report for the purposes of numerator compliance. This ensures all appropriate
information is being communicated for patient care coordination.

D.4. Advance Care Plan
Category
Description
CBE # / eCQM CBE #:
0326 / N/A
Quality #:
047
CMS eCQM ID:
N/A
Current Collection Type:
Medicare Part B Claims Spcifications, MIPS CQM Specifications
Current Measure Description:
Percentage of patients aged 65 years and older who have an advance care plan or surrogate decision
maker documented in the medical record or documentation in the medical record that an advance care
plan was discussed but the patient did not wish or was not able to name a surrogate decision maker or
provide an advance care plan.
Substantive Change:
Updated the denominator criteria: For the MIPS CQM Specifications and the Medicare Part B
Specifications collection types: Added: coding for neuropsychology.
Measure Steward:
National Committee for Quality Assurance
High Priority Measure:
Yes
Measure Type:
Process
Rationale
We are proposing to update denominator criteria to include coding for neuropsychology as this
measure is applicable to their scope of care.

D.5. Diabetes: Eye Exam
Category
Description
CBE # / eCQM CBE #:
0055 / N/A
Quality #:
117
CMS eCQM ID:
CMS131v13
Current Collection Type:
eCQM Specifications/ MIPS CQM Specifications
Current Measure Description:
Percentage of patients 18-75 years of age with diabetes and an active diagnosis of retinopathy in any
part of the measurement period who had a retinal or dilated eye exam by an eye care professional
during the measurement period or diabetics with no diagnosis of retinopathy in any part of the
measurement period who had a retinal or dilated eye exam by an eye care professional during the
measurement period or in the 12 months prior to the measurement period.
Substantive Change:
Updated denominator exclusion: For all collection types: Removed: specific encounter
requirements from the frailty/advanced illness exclusion.
Measure Steward:
National Committee for Quality Assurance
High Priority Measure:
No
Measure Type:
Process
Rationale
We are proposing to update the denominator exclusion by removing the requirement for patients to
have had at least one inpatient or two outpatient encounters to recognize a diagnosis of advanced
illness. The removal of this requirement within the denominator exclusion reduces the burden of
identifying applicable encounters, while still identifying patients with an indication of frailty. This is
particularly applicable for those patients seen outside of their reporting clinician’s medical record.
This revision would update the denominator exclusion to remove any patient 66 years of age and
older who has a diagnosis of advanced illness during the measurement period or the year prior from
the denominator of measure Q117. Decreasing complexity and burden of this element ensures
consistent implementation allowing for more comparable data.

D.6. Documentation of Current Medications in the Medical Record
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
130
CMS eCQM ID:
CMS68v14
Current Collection Type:
eCQM Specifications / MIPS CQM Specifications
Current Measure Description:
Percentage of visits for patients aged 18 years and older for which the eligible clinician attests to
documenting a list of current medications using all immediate resources available on the date of the
encounter.
Substantive Change:
The measure description is revised to read: For all collection types: Percentage of visits for which
the eligible clinician attests to documenting a list of current medications using all immediate
resources available on the date of the encounter.
Updated guidance: For the eCQM specifications collection type: Revised: allows for
documentation to be completed on the day of the encounter.
Updated initial patient population: For the eCQM specifications collection type: Removed: age
criteria.
Updated denominator: For the MIPS CQM Specifications collection type: Removed: age
criteria.
Updated denominator criteria: For the MIPS CQM Specifications collection type: Removed:
age criteria.
Added: coding for pediatric audiology services.
Updated numerator note: For the MIPS CQM Specifications collection type: Revised: allows
for documentation to be completed on the day of the encounter.
Measure Steward:
Centers for Medicare & Medicaid Services
High Priority Measure:
Yes
Measure Type:
Process
Rationale
We are proposing to update multiple components of the measure to remove the age criteria so that all
patients, regardless of age, are assessed for having a list of their current medications documented in
their medical record. This revision broadens the denominator, supporting assessment of current
medications for all patient age ranges. Assessing for missing information about the dosage, route, or
frequency of a medication supports clinical communication and may assist in avoiding patient
harm.
139
Additionally, we are proposing to update the measure to allow for the quality action to occur on the
day of the encounter rather than limiting to during the encounter. This allows for flexibility to align
with the clinician’s workflows and systems.
We are also proposing to update the denominator criteria to include coding for pediatric audiology
services as this measure is applicable to their scope of care.
139
Owen, M. C., Chang, N. M., Chong, D. H., & Vawdrey, D. K. (2011). Evaluation of Medication List Completeness,
Safety, and Annotations. AMIA Annual Symposium Proceedings. AMIA Symposium, 2011, 1055–1061.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3243276/.

D.7. Oncology: Medical and Radiation – Pain Intensity Quantified
Category
Description
CBE # / eCQM CBE #:
0384 / 0384e
Quality #:
143
CMS eCQM ID:
CMS157v13
Current Collection Type:
eCQM Specifications / MIPS CQM Specifications
Current Measure
Description:
Percentage of patient visits, regardless of patient age, with a diagnosis of cancer currently receiving
chemotherapy or radiation therapy in which pain intensity is quantified.
Substantive Change:
Updated initial patient population: For the eCQM Specifications collection type: Revised:
Population 1: All patient visits, regardless of patient age, with a diagnosis of cancer currently receiving
chemotherapy.
Population 2: All patient visits, regardless of patient age, with a diagnosis of cancer currently receiving
radiation therapy.
Measure Steward:
American Society of Clinical Oncology
High Priority Measure:
Yes
Measure Type:
Process
Rationale
We are proposing to revise this measure to split the initial patient population to delineate between patients
who are receiving radiation therapy and are assessed for pain intensity and their pain level quantified. This
additional patient population would allow clinicians reporting this measure the ability to distinguish their
performance between patients receiving chemotherapy and those receiving radiation services. It would also
align the measure across all collection types.

D.8. Falls: Plan of Care
Category
Description
CBE # / eCQM CBE #:
0101 / N/A
Quality #:
155
CMS eCQM ID:
N/A
Current Collection Type:
Medicare Part B Claims Measure Specifications / MIPS CQM Specifications
Current Measure Description:
Percentage of patients aged 65 years and older with a history of falls who had a plan of care for falls
documented within 12 months.
Substantive Change:
Modified collection type: MIPS CQM Specifications collection type.
Measure Steward:
National Committee for Quality Assurance
High Priority Measure:
Yes
Measure Type:
Process
Rationale
We are proposing to revise this measure by removing the Medicare Part B Claims Measure
Specifications collection type for this measure as it has reached the end of the topped-out lifecycle
(82 FR 53640). The average performance rate is based on the current 2024 MIPS benchmarking data
located at https://qpp.cms.gov/benchmarks.

D.9. Coronary Artery Bypass Graft (CABG): Surgical Re-Exploration
Category
Description
CBE # / eCQM CBE #:
0115 / N/A
Quality #:
168
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of patients aged 18 years and older undergoing isolated CABG surgery who require a
return to the operating room (OR) during the current hospitalization for mediastinal bleeding with or
without tamponade, graft occlusion, valve dysfunction, or other cardiac reason.
Substantive Change:
The measure description is revised to read: Percentage of patients aged 18 years and older
undergoing isolated CABG surgery who require a return to the operating room (OR) for mediastinal
bleeding with or without tamponade, unplanned coronary artery intervention (native vessel, graft or
both), valve dysfunction, aortic reintervention or other cardiac reason during the current
hospitalization.
The measure numerator is revised to read: Patients undergoing isolated CABG surgery who
require a return to the OR for mediastinal bleeding with or without tamponade, unplanned coronary
artery intervention (native vessel, graft or both), valve dysfunction, aortic reintervention or other
cardiac reason during the current hospitalization.
Updated numerator options: Added: unplanned coronary artery intervention (native vessel, graft,
or both) and aortic reintervention
Measure Steward:
Society of Thoracic Surgeons
High Priority Measure:
Yes
Measure Type:
Outcome
Rationale
We are proposing to revise this measure to broaden the scope of cardiac complications that may
require a return to the operating room following isolated CABG surgery. These revisions support the
measure to decrease surgical re-exploration following CABG surgery, which is a serious
complication and impacts length of stay, efficient use of resources, and increases risk for additional
injury and death.
140
Although rates of re-exploration after cardiac surgery have significantly declined
recently, it has been linked to much higher complications, mortality, and hospitalizations.
141
140
Tran, Z., Williamson, C., Hadaya, J., Verma, A., Sanaiha, Y., Chervu, N., Gandjian, M., & Benharash, P. (2022).
Trends and Outcomes of Surgical Reexploration After Cardiac Operations in the United States. The Annals of
Thoracic Surgery, 113(3), 783–792. https://doi.org/10.1016/j.athoracsur.2021.04.011.
141
See footnote Tran et al., 2022.

D.10. Tuberculosis Screening Prior to First Course of Biologic and/or Immune Response Modifier Therapy
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
176
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
If a patient has been newly prescribed a biologic and/or immune response modifier that includes a
warning for potential reactivation of a latent infection, then the medical record should indicate TB
testing in the preceding 12-month period.
Substantive Change:
Updated denominator instructions: Added: new biosimilar medications.
Measure Steward:
American College of Rheumatology
High Priority Measure:
No
Measure Type:
Process
Rationale
We are proposing to update the list of medications by adding new biosimilar medications to the
existing list in the denominator instructions. This expansion would provide an up-to-date list of
appropriate biologics and/or immune response modifiers that clinicians may have prescribed for their
patients, thereby ensuring that the right patients are identified in the denominator of this measure.
These targeted immunotherapies are associated with a high risk of progression to active TB infection,
therefore screening for latent TB prior to initiating first course therapy is highly recommended since
it has been shown to effectively reduce the incidence of progression in patients with latent TB.
142
142
CDC. (2020). Latent Tuberculosis Infection – A Guide for Primary Health Care Providers.
https://www.cdc.gov/tb/media/pdfs/Latent-TB-Infection-A-Guide-for-Primary-Health-Care-Providers.pdf.

D.11. Rheumatoid Arthritis (RA): Periodic Assessment of Disease Activity
Category
Description
CBE # / eCQM CBE #:
2523 / N/A
Quality #:
177
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of patients aged 18 years and older with a diagnosis of rheumatoid arthritis (RA) who
have an assessment of disease activity using an ACR-preferred RA disease activity assessment tool at
≥50% of encounters for RA for each patient during the measurement year.
Substantive Change:
The measure description is revised to read: Percentage of patients aged 18 years and older with
two or more diagnoses of rheumatoid arthritis (RA) at least 90 days apart who have an assessment of
disease activity using an ACR-preferred RA disease activity assessment tool at ≥50% of encounters
for RA for each patient during the performance period.
The measure denominator is revised to read: Patients aged 18 years and older with two or more
RA diagnoses documented at least 90 days apart with at least one encounter with an RA diagnosis
occurring during the performance period and an additional encounter with an RA diagnosis occurring
in the performance period or prior performance period.
Updated denominator definition: Added:
Encounter – An encounter during the performance period where one of the CPT or HCPCS codes
listed in the patient encounter criteria is used without a telehealth modifier (i.e., only non-telehealth
visits are to be considered for this measure).
Additional encounter - An additional encounter during the performance period or prior performance
period where one of the CPT or HCPCS codes listed in the patient encounter is used to confirm an
RA diagnosis with ICD-10-CM diagnosis codes as listed in the Denominator criteria.
Updated denominator criteria: Added: An additional encounter with an RA diagnosis during the
performance period or prior performance period that is at least 90 days before or after an encounter
with an RA diagnosis during the performance period.
Updated numerator definition: Revised: A result within the valid range of the selected tool
qualifies for meeting numerator performance as long as a result is captured at ≥50% of each patient’s
qualified encounters. If the result of a recorded disease activity assessment is outside the valid range
of scores for the tool (e.g., a CDAI score of 101 when the maximum possible score is 76.0) or is only
recorded as a disease activity level (e.g., low, moderate, or high) in place of a calculated numerical
score, this score should not be included in the count to meet the ≥50% requirement in the numerator.
Measure Steward:
American College of Rheumatology
High Priority Measure:
No
Measure Type:
Process
Rationale
We are proposing to revise the denominator to provide greater specificity on the timeframe and
criteria for denominator coding and how it should be captured to ensure the appropriate patients are
being identified for this measure. Diagnosing RA requires “a combination of physical exams, blood
tests for inflammatory markers, and imaging tests (like X-rays and MRIs), in addition to patient-
reported symptoms,” which can take multiple visits to determine an accurate assessment of RA
disease activity.
143
Therefore, this change would align more closely with the workflow of clinicians
and allows the assessment of a patient for RA disease activity during more than one clinical visit.
These changes support a comprehensive perspective of the patient’s disease activity level and should
promote optimal treatment outcomes.
We are proposing to revise the definition for ‘Assessment of Disease Activity’ to confirm that only
those assessments where the disease activity is correctly recorded are included in the numerator
quality action for the purposes of meeting performance. Not only do the results need to be within the
range of the selected validated qualifying tool, but also a calculated numeric score and not simply a
disease activity level. This ensures that all assessment information is correctly captured to drive
appropriate care.
143
Brody, B. (2020). The American College of Rheumatology Updated Its Approved Disease Activity Measures for
Rheumatoid Arthritis — Here’s What That Means. https://creakyjoints.org/living-with-
arthritis/symptoms/approved-disease-activity-measures-for-rheumatoid-arthritis/.

D.12. Rheumatoid Arthritis (RA): Functional Status Assessment
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
178
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of patients aged 18 years and older with a diagnosis of rheumatoid arthritis (RA) for
whom a functional status assessment was performed at least once within 12 months.
Substantive Change:
The measure description is revised to read: Percentage of patients aged 18 years and older with
two or more diagnoses of rheumatoid arthritis (RA) at least 90 days apart for whom a functional
status assessment was performed at least once during the performance period.
The measure denominator is revised to read: Patients aged 18 years and older with two or more
RA diagnoses documented at least 90 days apart with at least one encounter with an RA diagnosis
occurring during the performance period and an additional encounter with an RA diagnosis occurring
in the performance period or prior performance period.
Updated denominator definition: Added:
Encounter – An encounter during the performance period where one of the CPT or HCPCS codes
listed in the patient encounter criteria is used.
Additional encounter – An additional encounter during the performance period or prior performance
period where one of the CPT or HCPCS codes listed in the patient encounter is used to confirm an
RA diagnosis with ICD-10-CM diagnosis codes as listed in the Denominator criteria.
Updated denominator criteria: Added: an additional encounter with an RA diagnosis during the
performance period or prior performance period that is at least 90 days before or after an encounter
with an RA diagnosis during the performance period.
Measure Steward:
American College of Rheumatology
High Priority Measure:
No
Measure Type:
Process
Rationale
We are proposing to revise the denominator to provide greater specificity on the timeframe and
criteria for denominator coding and how it should be captured to ensure the appropriate patients are
being identified for this measure. Diagnosing RA requires “a combination of physical exams, blood
tests for inflammatory markers, and imaging tests (like X-rays and MRIs), in addition to patient-
reported symptoms,” which can take multiple visits to determine an accurate assessment of RA
disease activity.
144
Therefore, this change would align more closely with the workflow of clinicians
and allows assessment of a patient for RA disease activity during more than one clinical visit. These
changes support a comprehensive perspective of the patient’s disease activity level and should
promote optimal treatment outcomes.
144
See footnote Brody, 2020 in Table D.11 of this Appendix.

D.13. Rheumatoid Arthritis (RA): Glucocorticoid Management
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
180
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of patients aged 18 years and older with a diagnosis of rheumatoid arthritis (RA) who
have been assessed for glucocorticoid use and, for those on prolonged doses of prednisone > 5 mg
daily (or equivalent) with improvement or no change in disease activity, documentation of
glucocorticoid management plan within 12 months.
Substantive Change:
The measure description is revised to read: Percentage of patients aged 18 years and older with
two or more diagnoses of rheumatoid arthritis (RA) at least 90 days apart who have been assessed for
glucocorticoid use and, for those on prolonged doses of prednisone >5 mg daily (or equivalent) with
improvement or no change in disease activity, documentation of glucocorticoid management plan
during the performance period.
The measure denominator is revised to read: Patients aged 18 years and older with two or more
RA diagnoses documented at least 90 days apart with at least one encounter with an RA diagnosis
occurring during the performance period and an additional encounter with an RA diagnosis occurring
in the performance period or prior performance period.
Updated denominator definition: Added:
Encounter – An encounter during the performance period where one of the CPT or HCPCS codes
listed in the patient encounter criteria is used.
Additional encounter -– An additional encounter during the performance period or prior performance
period where one of the CPT or HCPCS codes listed in the patient encounter is used to confirm an
RA diagnosis with ICD-10-CM diagnosis codes as listed in the Denominator criteria.
Updated denominator criteria: Added: An additional encounter with an RA diagnosis during the
performance period or prior performance period that is at least 90 days before or after an encounter
with an RA diagnosis during the performance period.
Measure Steward:
American College of Rheumatology
High Priority Measure:
No
Measure Type:
Process
Rationale
We are proposing to revise the denominator to provide greater specificity on the timeframe and
criteria for denominator coding and how it should be captured to ensure the appropriate patients are
being identified for this measure. Diagnosing RA requires “a combination of physical exams, blood
tests for inflammatory markers, and imaging tests (like X-rays and MRIs), in addition to patient-
reported symptoms,” which can take multiple visits to determine an accurate assessment of RA
disease activity.
145
Therefore, this change would align more closely with the workflow of clinicians
and allows assessment of a patient for RA disease activity during more than one clinical visit. These
changes support a comprehensive perspective of the patient’s disease activity level and should
promote optimal treatment outcomes.
145
See footnote Brody, 2020 in Table D.11 of this Appendix.

D.14. Elder Maltreatment Screen and Follow-Up Plan
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
181
CMS eCQM ID:
N/A
Current Collection Type:
Medicare Part B Claims Measure Specifications / MIPS CQM Specifications
Current Measure Description:
Percentage of patients aged 60 years and older with a documented elder maltreatment screen using an
Elder Maltreatment Screening tool on the date of encounter AND a documented follow-up plan on
the date of the positive screen.
Substantive Change:
Updated denominator criteria: For all collection types: Added: coding for emergency
department.
Measure Steward:
Centers for Medicare & Medicaid Services
High Priority Measure:
Yes
Measure Type:
Process
Rationale
We are proposing to add encounter codes for emergency department (ED) visits to the denominator
criteria as the emergency department is an appropriate setting for screening for elder maltreatment.
“EDs are a potentially important setting for elder mistreatment identification because they provide
care for a large number of older adults who may be elder mistreatment victims” especially given that
the ED is sometimes the only clinical setting that the patient may visit.
146
Expanding the denominator
to include ED visits ensures a more complete denominator patient population and provides support
and delivery of interventions that could prevent actual abuse.
146
See footnote Rosen et al., 2020 in Table B.11 of this Appendix.

D.15. Functional Outcome Assessment
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
182
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of visits for patients aged 18 years and older with documentation of a current functional
outcome assessment using a standardized functional outcome assessment tool on the date of the
encounter AND documentation of a care plan based on identified functional outcome deficiencies
within two days of the date of the identified deficiencies.
Substantive Change:
Updated numerator definition: Revised:
Functional Outcome Assessment: Patient completed questionnaires designed to measure a patient's
limitations in performing the usual human tasks of living and to directly quantify functional and
behavioral symptoms. If a patient is unable to complete a questionnaire, a standardized clinical
assessment tool may be used to measure a patient's limitations.
Added: To Table 1. Definitions for Magnitude of Effects, Based on Mean Between-Group
Differences – Modified*: clarification that list of standardized tools is not exhaustive.
Measure Steward:
Centers for Medicare & Medicaid Services
High Priority Measure:
Yes
Measure Type:
Process
Rationale
We are proposing to update the numerator definition to clarify that clinicians may use a standardized
clinical assessment tool to measure a patient’s limitation if they are unable to complete a
questionnaire. This change would allow flexibility in performing the quality action and clarify that
the list of standardized tools named in the measure are only examples, not an exhaustive list. The
National Institute of Health indicates that it supports the use of standardized tools, stating they offer a
clinician-designed approach to promoting care standardization that accommodates patients' individual
differences, respects clinicians’ clinical judgement, and keeps pace with the rapid growth of medical
knowledge.
147
147
Farias, M., Jenkins, K., Lock, J., Rathod, R., Newburger, J., Bates, D. W., Safran, D. G., Friedman, K., & Greenberg,
J. (2013). Standardized Clinical Assessment and Management Plans (SCAMPs) Provide a Better Alternative to
Clinical Practice Guidelines. Health Affairs (Project Hope), 32(5), 911–920.
https://doi.org/10.1377/hlthaff.2012.0667.

D.16. Colonoscopy Interval for Patients with a History of Adenomatous Polyps – Avoidance of Inappropriate Use
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
185
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of patients aged 18 years and older receiving a surveillance colonoscopy, with a history of
prior adenomatous polyp(s) in previous colonoscopy findings, which had an interval of 3 or more
years since their last colonoscopy.
Substantive Change:
Updated denominator exception: Added: To the ‘Documentation of system reasons(s): patient
cannot provide precise date or details from previous colonoscopy.
Measure Steward:
American Gastroenterological Association
High Priority Measure:
Yes
Measure Type:
Process
Rationale
We are proposing to update the denominator exception to exclude patients that are unable to provide
the precise date or details from a previous colonoscopy. This addition would assist with data
collection when a patient with a history of adenomatous polyps is unable to provide information
regarding their last colonoscopy.
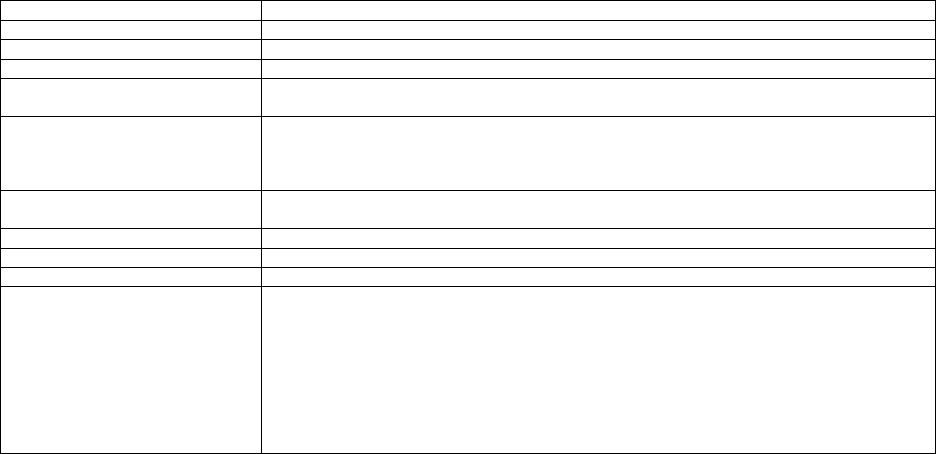
D.17. Controlling High Blood Pressure
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
236
CMS eCQM ID:
CMS165v13
Current Collection Type:
Medicare Part B Claims Measure Specifications / eCQM Specifications/ MIPS CQM Specifications/
Medicare CQM Specifications (collection type available only in the APP and/or APP Plus)
Current Measure Description:
Percentage of patients 18-85 years of age who had a diagnosis of essential hypertension starting
before and continuing into, or starting during the first six months of the measurement period, and
whose most recent blood pressure was adequately controlled (<140/90mmHg) during the
measurement period.
Substantive Change:
Updated denominator exclusion: For all collection types: Removed: specific encounter
requirements from the frailty/advanced illness exclusion.
Measure Steward:
National Committtee for Quality Assurance
High Priority Measure:
Yes
Measure Type:
Intermediate Outcome
Rationale
We are proposing to update the denominator exclusion by removing the requirement for patients to
have had at least one inpatient or two outpatient encounters to recognize a diagnosis of advanced
illness. The removal of this requirement within the denominator exclusion reduces the burden of
identifying applicable encounters, while still identifying patients with an indication of frailty. This is
particularly applicable for those patients seen outside of their reporting clinician’s medical record.
This revision would update the denominator exclusion to remove any patient 66 years of age and
older who has a diagnosis of advanced illness during the measurement period or the year prior from
the denominator of measure Q236. Decreasing complexity and burden of this element ensures
consistent implementation allowing for more comparable data.

D.18. Use of High-Risk Medications in Older Adults
Category
Description
CBE # / eCQM CBE #:
0022 / N/A
Quality #:
238
CMS eCQM ID:
CMS156v13
Current Collection Type:
eCQM Specifications / MIPS CQM Specifications
Current Measure Description:
Percentage of patients 65 years of age and older who were ordered at least two high-risk medications
from the same drug class.
Substantive Change:
Updated numerator definition: For the MIPS CQM Specifications collection type:
Numerator (Submission Criteria 1): Table 1 - High-Risk Medications at any Dose or Duration
Removed:
From Anticholinergics, first-generation antihistamines: Carbinoxamine, Clemastine,
Dexbrompheniramine, Dexchlorpheniramine, Pyrilamine
From Antispasmodics: Belladonna alkaloids, Methscopolamine, Propantheline
From Cardiovascular, alpha agonists, central: Methyldopa
From Cardiovascular, other: Disopyramide
From Central nervous system, antidepressants: Protriptyline Trimipramine
From Central nervous system, barbiturates: Amobarbital, Butabarbital, Pentobarbital, Secobarbital
From Central nervous system, vasodilators: Isoxsuprine
From Endocrine system, sulfonylureas, long-duration: Chlorpropamide
From Endocrine system, other: Megestrol
From Pain medications, other: Meperidine
Added: A row with one medication is considered a group (or drug class) of one; therefore, two
orders of that same medication are numerator compliant.
Added:
To Central nervous system, barbiturates: Primidone
Endocrine system, megestrol: Megestrol
Pain medications, meperidine: Meperidine
To Pain medications, other: Ketorolac, includes parenteral and oral
Table 3 – High-Risk Medications With Average Daily Dose Criteria
Removed:
Alpha agonists, central: Reserpine >0.1 mg per day
Numerator (Submission Criteria 2): Table 4 – High-Risk Medications
Removed:
From Benzodiazepines, long, short and intermediate acting: Flurazepam, Quazepam
Added:
To Benzodiazepines, long, short and intermediate acting: Clobazam
Measure Steward:
National Committee for Quality Assurance
High Priority Measure:
Yes
Measure Type:
Process
Rationale
We are proposing to update the list of high-risk medications to align with 2023 AGS Beers Criteria
148
and ensure that older adults are not prescribed inappropriate medications. This change would also
align with PY 2025 eCQM version (CMS156v13), which supports alignment between the collection
types. We are also proposing to add clarification that the medications found within a single row of
table one, within the specification, represent a unique group or drug class. Therefore, two orders from
that group or drug class would be consider numerator compliant for this measure, which has an
inverse analytic for the calculation of performance.
148
The 2023 American Geriatrics Society Beers Criteria Update Expert Panel. (2023). American Geriatrics Society
2023 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. Journal of the
American Geriatrics Society, 71(7), 2052-2081. https://doi.org/10.1111/jgs.18372.

D.19. Barrett’s Esophagus
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
249
CMS eCQM ID:
N/A
Current Collection Type:
Medicare Part B Claims Measure Specifications / MIPS CQM Specifications
Current Measure Description:
Percentage of esophageal biopsy reports that document the presence of Barrett’s mucosa that also
include a statement about dysplasia.
Substantive Change:
Updated denominator exception: For all collection types: Added: Specimen site other than
anatomic location of esophagus.
Updated denominator exclusion: For all collection types: Removed: Specimen site other than
anatomic location of esophagus.
Measure Steward:
College of American Pathologists
High Priority Measure:
No
Measure Type:
Process
Rationale
We are proposing to remove the denominator exclusion so that all patients with a diagnosis for
Barrett’s Esophagus are included in the denominator of the measure and are proposing to add a
denominator exception for specimen sites other than the anatomic location of the esophagus.
Revising the measure to include this element as a denominator exception would allow for an
automated capture of the relevant cases with less manual intervention reducing overall burden of
measure reporting.

D.20. Sleep Apnea: Severity Assessment at Initial Diagnosis
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
277
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of patients aged 18 years and older with a diagnosis of obstructive sleep apnea who had
an apnea hypopnea index (AHI), a respiratory disturbance index (RDI), or a respiratory event index
(REI) documented or measured within 2 months of initial evaluation for suspected obstructive sleep
apnea.
Substantive Change:
Updated denominator exception: Added: patients previously diagnosed with OSA and severity
assessed by another provider.
Measure Steward:
American Academy of Sleep Medicine
High Priority Measure:
No
Measure Type:
Process
Rationale
We are proposing to update the denominator exception by adding that patients previously diagnosed
with obstructive sleep apnea (OSA), with severity assessed by another clinician and documented
would not need to have the testing completed again. This denominator exceptions allows clinician
judgement as to whether repeat testing is needed, as we don’t want to promote overutilization. This
revision increases clarity of this element and ensures consistent implementation allowing for more
comparable data.

D.21. Dementia: Cognitive Assessment
Category
Description
CBE # / eCQM CBE #:
N/A / 2872e
Quality #:
281
CMS eCQM ID:
CMS149v13
Current Collection Type:
eCQM Specifications
Current Measure Description:
Percentage of patients, regardless of age, with a diagnosis of dementia for whom an assessment of
cognition is performed and the results reviewed at least once within a 12-month period.
Substantive Change:
Updated guidance: Added: The measure requires a diagnosis of dementia is present before the
routine assessment of cognition once in a 12-month period.
Measure Steward:
American Academy of Neurology
High Priority Measure:
No
Measure Type:
Process
Rationale
We are proposing to update the measure guidance to clarify that the diagnosis of dementia must be
present prior to the cognitive assessment. This would ensure that any cognitive assessments
performed prior to the dementia diagnosis but occurred during the 12-month lookback period would
not be attributed to this measure numerator.
149
This change would ensure consistency in the
abstraction of the measure’s numerator elements based upon a specific timeframe that support
rigorous data for the calculation of MIPS performance rates.
149
Office of the Assistance Secretary for Planning and Evaluation. (2016). Examining Models of Dementia Care:
Final Report. https://aspe.hhs.gov/reports/examining-models-dementia-care-final-report-0.

D.22. Dementia: Functional Status Assessment
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
282
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of patients with dementia for whom an assessment of functional status was performed at
least once in the last 12 months.
Substantive Change:
Updated denominator criteria: Added: coding for speech language pathology and nuclear
medicine.
Measure Steward:
American Academy of Neurology/American Psychiatric Association
High Priority Measure:
No
Measure Type:
Process
Rationale
We are proposing to add encounter codes for speech language pathologists and nuclear medicine to
the denominator criteria as these are appropriate specialties to identify changes in functional status in
patients diagnosed with dementia. Dementia, which can be caused by “different brain diseases,”
affects a person’s activities of daily living including, but not limited to eating and swallowing, and
speech.
150
150
American Speech-Language-Hearing Association (ASHA). Dementia.
https://www.asha.org/public/speech/disorders/dementia/.

D.23. Dementia: Safety Concern Screening and Follow-Up for Patients with Dementia
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
286
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of patients with dementia or their caregiver(s) for whom there was a documented safety
concerns screening in two domains of risk: 1) dangerousness to self or others and 2) environmental
risks; and if safety concerns screening was positive in the last 12 months, there was documentation of
mitigation recommendations, including but not limited to referral to other resources.
Substantive Change:
Updated denominator criteria: Added: coding for speech language pathology and nuclear
medicine.
Measure Steward:
American Academy of Neurology/American Psychiatric Association
High Priority Measure:
Yes
Measure Type:
Process
Rationale
We are proposing to update the denominator criteria to include coding for speech language pathology
and nuclear medicine as this measure is applicable to their scope of care. Dementia, which can be
caused by “different brain diseases,” affects a person’s activities of daily living including, but not
limited to eating and swallowing, and speech.
151
151
See footnote ASHA in Table D.22 of this Appendix.

D.24. Dementia: Education and Support of Caregivers for Patients with Dementia
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
288
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of patients with dementia whose caregiver(s) were provided with education on dementia
disease management and health behavior changes AND were referred to additional resources for
support in the last 12 months.
Substantive Change:
Updated denominator criteria: Added: coding for speech language pathology and nuclear
medicine.
Measure Steward:
American Academy of Neurology/American Psychiatric Association
High Priority Measure:
Yes
Measure Type:
Process
Rationale
We are proposing to update the denominator criteria to include coding for speech language pathology
and nuclear medicine as this measure is applicable to their scope of care. Dementia, which can be
caused by “different brain diseases,” affects a person’s activities of daily living including, but not
limited to eating and swallowing, and speech.
152
152
See footnote ASHA in Table D.22 of this Appendix.

D.25. Assessment of Mood Disorders and Psychosis for Patients with Parkinson’s Disease
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
290
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of all patients with a diagnosis of Parkinson’s Disease [PD] who were assessed for
depression, anxiety, apathy, AND psychosis once during the measurement period.
Substantive Change:
Updated denominator criteria: Added: coding for neuropsychology and behavioral health.
Measure Steward:
American Academy of Neurology
High Priority Measure:
No
Measure Type:
Process
Rationale
We are proposing to update the denominator criteria to include coding for neuropsychology and
behavioral health as it’s clinically appropriate for these clinician types to assess for depression,
anxiety, apathy, and psychosis.

D.26. Assessment of Cognitive Impairment or Dysfunction for Patients with Parkinson’s Disease
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
291
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of all patients with a diagnosis of Parkinson’s Disease [PD] who were assessed for
cognitive impairment or dysfunction once during the measurement period.
Substantive Change:
Updated denominator criteria: Added: coding for neuropsychology, behavioral health, and
physical and occupational therapy.
Measure Steward:
American Academy of Neurology
High Priority Measure:
No
Measure Type:
Process
Rationale
We are proposing to update the denominator criteria to include coding for neuropsychology,
behavioral health, and physical and occupational therapy as it’s clinically appropriate for these
clinician types to assess for cognitive impairment or dysfunction.

D.27. Rehabilitative Therapy Referral for Patients with Parkinson’s Disease
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
293
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of all patients with a diagnosis of Parkinson’s Disease who were referred to physical,
occupational, speech, or recreational therapy once during the measurement period.
Substantive Change:
Updated denominator criteria: Added: coding for neuropsychology, behavioral health, and speech
language pathology.
Measure Steward:
American Academy of Neurology
High Priority Measure:
Yes
Measure Type:
Process
Rationale
We are proposing to update the denominator criteria to include coding for neuropsychology,
behavioral health, and speech language pathology as this measure is applicable to their scope of care.

D.28. Preventive Care and Screening: Screening for High Blood Pressure and Follow-Up Documented
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
317
CMS eCQM ID:
CMS22v13
Current Collection Type:
Medicare Part B Claims Measure Specifications / eCQM Specifications / MIPS CQM Specifications
Current Measure Description:
Percentage of patient visits for patients aged 18 years and older seen during the measurement period
who were screened for high blood pressure AND a recommended follow-up plan is documented, as
indicated, if blood pressure is elevated or hypertensive.
Substantive Change:
Updated denominator criteria: For the MIPS CQM Specifications and the Medicare Part B
Claims Measure Specifications collection types: Added: coding for nutrition/dietitian clinician
type.
Updated numerator definition: For all collection types: Revised: intervals for rescreening for first
and second hypertensive BP readings.
Measure Steward:
Centers for Medicare & Medicaid Services
High Priority Measure:
No
Measure Type:
Process
Rationale
We are proposing to add encounter codes for nutrition therapy to the denominator criteria for the
MIPS CQM Specifications and the Medicare Part B Claims Measure Specifications collection types
as this is an appropriate setting to identify people who may have elevated blood pressure (BP)
readings. Nutritional approaches play a pivotal role in helping to reduce the risk of hypertension or
control blood pressure in people with hypertension.
153
We are proposing to remove the minimum timeframe for follow-up screenings for patients with
elevated BP readings for all collection types. This change allows clinician discretion to recommend a
follow-up plan based on the patient’s current health status. Additionally, this supports stability of this
measure component, as the frequency is each visit, within the specification over time, while still
maintaining consistency with the current guidelines.
154
153
Physicians Committee for Responsible Medicine. (2023). Nutrition Guide for Clinicians: Hypertension.
https://nutritionguide.pcrm.org/nutritionguide/view/Nutrition_Guide_for_Clinicians/1342053/all/Hypertension.
154
Whelton, P. K., Carey, R. M., Aronow, W. S., Casey, D. E., Jr, Collins, K. J., Dennison Himmelfarb, C., DePalma, S.
M., Gidding, S., Jamerson, K. A., Jones, D. W., MacLaughlin, E. J., Muntner, P., Ovbiagele, B., Smith, S. C., Jr,
Spencer, C. C., Stafford, R. S., Taler, S. J., Thomas, R. J., Williams, K. A., Sr, Williamson, J. D., … Wright, J. T., Jr
(2018). 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention,
Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of
Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Journal of the American College
of Cardiology, 71(19), e127–e248. https://doi.org/10.1016/j.jacc.2017.11.006.

D.29. Appropriate Follow-Up Interval for Normal Colonoscopy in Average Risk Patients
Category
Description
CBE # / eCQM CBE #:
0658 / N/A
Quality #:
320
CMS eCQM ID:
N/A
Current Collection Type:
Medicare Part B Claims Measure Specifications / MIPS CQM Specifications
Current Measure Description:
Percentage of patients aged 45 to 75 years of age receiving a screening colonoscopy without biopsy
or polypectomy who had a recommended follow-up interval of at least 10 years for repeat
colonoscopy documented in their colonoscopy report.
Substantive Change:
The measure description is revised to read: For all collection types: Percentage of patients aged
45 to 75 years of age receiving a screening colonoscopy without biopsy or polypectomy who had a
recommended follow-up interval of 10 years for repeat colonoscopy documented in their
colonoscopy report.
Updated numerator: For all collection types: Revised: Patients who had recommended follow-up
interval of 10 years for repeat colonoscopy documented in their colonoscopy report.
Updated numerator note: For all collection types: Added: To meet the numerator, patients with a
negative screening colonoscopy should have documentation that they received counseling or
instruction to have a follow-up or repeat colonoscopy in 10 years. A 6 month period before or after
10 years is considered within the recommended follow-up interval.
Updated numerator options: For all collection types: Revised:
Performance Met: Recommended follow-up interval for repeat colonoscopy of 10 years
documented in colonoscopy report and communicated with patient.
Denominator Exception: Documentation of medical reason(s) for not recommending a 10 year
follow-up interval (e.g., inadequate prep, familial or personal history of colonic polyps, patient had
no adenoma and age is ≥ 66 years old, or life expectancy < 10 years, other medical reasons)
Performance Not Met: A 10 year follow-up interval for colonoscopy not recommended, reason not
otherwise specified.
Measure Steward:
American Gastroenterological Association
High Priority Measure:
Yes
Measure Type:
Process
Rationale
We are proposing to update the measure description, numerator, and numerator options to ensure the
time frame for recommended follow up after a normal colonoscopy for the average risk patient
accurately reflects current guidelines which states “[n]ew observational and modeling studies of
colonoscopy confirm and strengthen the evidence base to support the conclusion that individuals with
normal colonoscopy are at lower-than-average risk for CRC. Based on this reduced risk, we
recommend CRC screening in average-risk individuals be repeated 10 years after a normal
examination complete to the cecum with bowel preparation adequate to detect polyps >5 mm in
size.”
155
Additionally, we are proposing to add a numerator note to indicate inclusion of
documentation that the patient has received counseling and instruction on when a repeat colonoscopy
should be scheduled. This change would ensure the patient is aware of clinical recommendations to
drive healthy outcomes.
155
Gupta, S., Lieberman, D., Anderson, J. C., Burke, C. A., Dominitz, J. A., Kaltenbach, T., Robertson, D. J., Shaukat,
A., Syngal, S., & Rex, D. K. (2020). Recommendations for Follow-Up After Colonoscopy and Polypectomy: A
Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Gastrointestinal Endoscopy, 91(3),
463–485.e5. https://doi.org/10.1016/j.gie.2020.01.014.

D.30. Cardiac Stress Imaging Not Meeting Appropriate Use Criteria: Preoperative Evaluation in Low-Risk Surgery
Patients
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
322
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of stress single-photon emission computed tomography (SPECT) myocardial perfusion
imaging (MPI), stress echocardiogram (ECHO), cardiac computed tomography angiography (CCTA),
or cardiac magnetic resonance (CMR) performed in low-risk surgery patients 18 years or older for
preoperative evaluation during the 12-month submission period.
Substantive Change:
The measure description is revised to read: Percentage of stress single-photon emission computed
tomography (SPECT) myocardial perfusion imaging (MPI), stress echocardiogram (ECHO),
multigated acquisition scan (MUGA), cardiac computed tomography angiography (CCTA), or
cardiac magnetic resonance (CMR) performed in low-risk surgery patients 18 years or older for
preoperative evaluation during the 12-month submission period.
Updated instructions: Added: multigated acquisition scan (MUGA).
Updated denominator: Added: multigated acquisition scan (MUGA).
Updated denominator criteria: Added: coding for multigated acquisition scan (MUGA).
Updated numerator: Added: multigated acquisition scan (MUGA).
Measure Steward:
American College of Cardiology Foundation
High Priority Measure:
Yes
Measure Type:
Efficiency
Rationale
We are proposing to update multiple components of the measure to add MUGA. Even though MUGA
scanning allows a clinician to evaluate many heart parameters and can be done while resting or under
stress, this diagnostic imaging test would ensure this type of imaging isn’t performed on low-risk
surgery patients since the risks of the procedure exceed the expected clinical benefit for the
denominator eligible patient population.
156
The MUGA scan is primarily used for assessing
myocardial function in patients on cardiotoxic chemotherapy.
157
156
Winchester, D. E., Maron, D. J., Blankstein, R., Chang, I. C., Kirtane, A. J., Kwong, R. Y., Pellikka, P. A., Prutkin, J.
M., Russell, R., & Sandhu, A. T. (2023). ACC/AHA/ASE/ASNC/ASPC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2023
Multimodality Appropriate Use Criteria for the Detection and Risk Assessment of Chronic Coronary
Disease. Journal of Cardiovascular Magnetic Resonance: Official Journal of the Society for Cardiovascular Magnetic
Resonance, 25(1), 58. https://doi.org/10.1186/s12968-023-00958-5.
157
Odak, M., & Kayani, W. T. (2023). MUGA Scan. In StatPearls. StatPearls Publishing.
https://www.ncbi.nlm.nih.gov/books/NBK564365/.

D.31. Adult Sinusitis: Antibiotic Prescribed for Acute Viral Sinusitis (Overuse)
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
331
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of patients, aged 18 years and older, with a diagnosis of acute viral sinusitis who were
prescribed an antibiotic within 10 days after onset of symptoms.
Substantive Change:
Updated the instructions: Revised: This measure is to be submitted once for each occurrence of
acute viral sinusitis (AVS) during the performance period. Each unique occurrence starts with the
onset of AVS symptoms and concludes with the resolution of AVS symptoms or after 90 days if a
resolution of AVS symptoms is not documented. If multiple encounters are documented within an
occurrence, Merit-based Incentive Payment System (MIPS) eligible clinicians should submit the most
recent encounter during that occurrence. A new occurrence of AVS cannot start until the previous
occurrence during the performance period has concluded.
Measure Steward:
American Academy of Otolaryngology – Head and Neck Surgery Foundation
High Priority Measure:
Yes
Measure Type:
Process
Rationale
We are proposing to update the measure instructions to clarify what constitutes an occurrence for the
purposes of this measure. This additional guidance would further clarify how patients are attributed to
the denominator of this measure for each eligible occurrence. This change would ensure consistency
in the abstraction of the measure’s elements that support rigorous data for the calculation of MIPS
performance rates.

D.32. Maternity Care: Postpartum Follow-up and Care Coordination
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
336
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of patients, regardless of age, who gave birth during a 12-month period who were seen for
postpartum care before or at 12 weeks of giving birth and received the following at a postpartum
visit: breast-feeding evaluation and education, postpartum depression screening, postpartum glucose
screening for gestational diabetes patients, family and contraceptive planning counseling, tobacco use
screening and cessation education, healthy lifestyle behavioral advice, and an immunization review
and update.
Substantive Change:
The measure description is revised to read: Percentage of patients, regardless of age, who gave
birth during a 12-month period who were seen for postpartum care before or at 12 weeks of giving
birth and received the following at a postpartum visit: breastfeeding evaluation and education,
postpartum depression screening, intimate partner violence screening, postpartum glucose screening
for gestational diabetes patients, family and contraceptive planning counseling, tobacco use screening
and cessation education, healthy lifestyle behavioral advice, and an immunization review and update.
Updated numerator: Added: intimate partner violence screening.
Updated numerator definition: Added: Intimate Partner Violence Screening – Patients who were
screened for intimate partner violence before or at 12 weeks postpartum. Questions may be asked
either directly by a health care provider or in the form of self-completed paper-or computer-
administered questionnaires, and results should be documented in the medical record. Intimate
partner violence screening should include a self-reported validated intimate partner violence
screening tool (e.g., Abuse Assessment Screen (AAS), Extended – Hurt, Insult, Threaten, Scream (E-
HITS), Humiliation, Afraid, Rape, Kick (HARK)).
Updated numerator instructions: Added: intimate partner violence screening.
Measure Steward:
Centers for Medicare & Medicaid Services
High Priority Measure:
Yes
Measure Type:
Process
Rationale
We are proposing to update multiple measure components to include intimate partner violence
screening. “The effects of intimate partner violence (IPV) on maternal and neonatal outcomes are
multifaceted and largely preventable.”
158
This type of abuse has maternal and neonatal consequences
on mental and physical health that could be far reaching beyond the perinatal period;
159
therefore,
intimate partner violence screening is being proposed for inclusion. This revision would support
clinicians currently screening and would encourage others to begin screening for this risk within this
patient population as IPV may escalate during the postpartum period.
160
The American College of
Obstetricians and Gynecologists recommend including screening for IPV at the postpartum checkup
to help identify patients experiencing IPV so that support may be offered to break this cycle, leading
to positive outcomes for both maternal and neonatal health.
161
158
Alhusen, J. L., Ray, E., Sharps, P., & Bullock, L. (2015). Intimate Partner Violence During Pregnancy: Maternal and
Neonatal Outcomes. Journal of Women's Health (2002), 24(1), 100–106. https://doi.org/10.1089/jwh.2014.4872.
159
Chisholm, C. A., Bullock, L., & Ferguson, J. E. J., 2nd (2017). Intimate Partner Violence and Pregnancy:
Epidemiology and Impact. American Journal of Obstetrics and Gynecology, 217(2), 141–144.
https://doi.org/10.1016/j.ajog.2017.05.042.
160
American College of Obstetricians and Gynecologists (ACOG). (2012). Committee on Health Care for
Underserved Women Opinion: Intimate Partner Violence. https://www.acog.org/-
/media/project/acog/acogorg/clinical/files/committee-opinion/articles/2012/02/intimate-partner-violence.pdf.
161
See footnote ACOG, 2012.

D.33. HIV Medical Visit Frequency
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
340
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of patients, regardless of age with a diagnosis of HIV who had at least one medical visit in
each 6 month period of the 24 month measurement period, with a minimum of 60 days between
medical visits.
Substantive Change:
Modified collection type: eCQM Specifications, MIPS CQM Specifications collection type.
The measure title is revised from “HIV Medical Visit Frequency” to: HIV Annual Retention in
Care.
The measure description is revised to read: Percentage of patients, regardless of age, with a
diagnosis of Human Immunodeficiency Virus (HIV) before or during the first 240 days of the
performance period who had at least two eligible encounters or at least one eligible encounter and
one HIV viral load test that were at least 90 days apart within the measurement period.
The measure denominator is revised to read: Patients, regardless of age, with a diagnosis of HIV
before or during the first 240 days of the performance period who had at least one eligible encounter
during the first 240 days of the performance period.
The measure denominator note is revised to read: Only patients with an eligible encounter in the
first 240 days are included in this measure to allow for sufficient time to complete a second eligible
encounter or viral load laboratory at least 90 days after the initial encounter during the performance
period.
Updated denominator criteria: Added: coding for telephone and home visit patient encounters.
Updated denominator exclusion: Removed: Patient died at any time during the 24‐month
measurement period.
The measure numerator is revised to read: Number of patients who had at least one eligible
encounter and one HIV viral load test at least 90 days apart during the performance period, or who
had at least two eligible encounters at least 90 days apart during the performance period.
Updated numerator note: Added: A patient would be included in the measure numerator if they
have either 1) two eligible encounters at least 90 days apart, or 2) one eligible encounter and one viral
load test at least 90 days apart from each other. The encounter or encounters that cause a patient to be
included in the numerator do not need to include the encounter that caused the patient to be included
in the denominator.
The measure numerator options are revised to read:
Performance Met: Patient had two eligible encounters at least 90 days apart or one eligible
encounter and one HIV viral load test at least 90 days apart
Performance Not Met: Patient did not have two eligible encounters at least 90 days apart or one
eligible encounter and one HIV viral load test at least 90 days apart
Measure Steward:
Health Resources and Services Administration
High Priority Measure:
Yes
Measure Type:
Process
Rationale
We are proposing to update the collection types available for this measure to include the eCQM
Specifications collection type to allow choice in submission method. We are also proposing to revise
multiple components of the measure allowing for improved quality outcome which is to engage
persons who are infected with HIV in regular HIV care that promote test-and-treat strategies.
162
In the event the proposed substantive change(s) are finalized, the substantive changes would not
allow for a direct comparison of performance data from prior years to performance data submitted
after the implementation of these substantive changes. As such, if the performance data submitted
meets the criteria for creation of a performance period benchmark, a new benchmark would be used
for scoring.
D.34. Rate of Carotid Artery Stenting (CAS) for Asymptomatic Patients, Without Major Complications (Discharged to
Home by Post-Operative Day #2)
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
344
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
162
Gardner, E. M., McLees, M. P., Steiner, J. F., Del Rio, C., & Burman, W. J. (2011). The Spectrum of Engagement in
HIV Care and its Relevance to Test-and-treat Strategies for Prevention of HIV Infection. Clinical Infectious Diseases:
An Official Publication of the Infectious Diseases Society of America, 52(6), 793–800.
https://doi.org/10.1093/cid/ciq243.

Category
Description
Current Measure Description:
Percent of asymptomatic patients undergoing CAS who are discharged to home no later than post-
operative day #2.
Substantive Change:
The measure title is revised from Rate of Carotid Artery Stenting (CAS) for Asymptomatic
Patients, Without Major Complications (Discharged to Home by Post-Operative Day #2) to:
Rate of Carotid Endarterectomy (CEA) or Carotid Artery Stenting (CAS) for Asymptomatic Patients,
Without Major Complications (Discharged to Home by Post-Operative Day #2)
The measure description is revised to read: Percent of asymptomatic patients undergoing Carotid
Endarterectomy (CEA) or Carotid Artery Stenting (CAS) without major complication who are
discharged to home no later than post-operative day #2.
Updated instructions: Added: CEA.
Updated denominator: Added: CEA.
Updated denominator criteria: Added: coding for carotid endarterectomy.
Updated numerator: Added: CEA.
Updated numerator definition: Added: procedure for CEA or CAS.
Updated numerator options: Added: CEA.
Measure Steward:
Society for Vascular Surgeons
High Priority Measure:
Yes
Measure Type:
Outcome
Rationale
We are proposing to revise this measure to include Carotid Endarterectomy (CEA) to assess for post-
operative complications for asymptomatic patients who had CEA. CEA for asymptomatic carotid
stenosis reduces the risk of ipsilateral stroke, and any stroke, by approximately 30% over 3 years.
163
Previously, assessment of outcomes for CEA procedures was a separate measure. As both procedures
are appropriate for treating asymptomatic carotid artery stenosis, these measures are being combined
so that a full picture of positive outcomes can be captured for this patient population.
164
Additionally,
by combining these measures the denominator eligible patient population may increase allowing for
more robust data for participating MIPS clinicians.
In the event the proposed substantive change(s) are finalized, the substantive changes would not
allow for a direct comparison of performance data from prior years to performance data submitted
after the implementation of these substantive changes. As such, if the performance data submitted
meets the criteria for creation of a performance period benchmark, a new benchmark would be used
for scoring.
163
Chambers, B. R., & Donnan, G. A. (2005). Carotid Endarterectomy for Asymptomatic Carotid Stenosis. The
Cochrane Database of Systematic Reviews, 2005(4), CD001923.
https://doi.org/10.1002/14651858.CD001923.pub2.
164
Wang, J., Bai, X., Wang, T., Dmytriw, A. A., Patel, A. B., & Jiao, L. (2022). Carotid Stenting Versus Endarterectomy
for Asymptomatic Carotid Artery Stenosis: A Systematic Review and Meta-Analysis. Stroke, 53(10), 3047–3054.
https://doi.org/10.1161/STROKEAHA.122.038994.

D.35. Unplanned Reoperation within the 30-Day Postoperative Period
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
355
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of patients aged 18 years and older who had any unplanned reoperation within the 30 day
postoperative period.
Substantive Change:
Updated denominator criteria: Revised: coding for fissurectomy, including sphincterotomy.
Measure Steward:
American College of Surgeons
High Priority Measure:
Yes
Measure Type:
Outcome
Rationale
We are proposing to update denominator criteria to revise coding for fissurectomy, including
sphincterotomy as this is an operative procedure that could have complications requiring unplanned
reoperation within 30 days postoperatively.

D.36. Optimizing Patient Exposure to Ionizing Radiation: Count of Potential High Dose Radiation Imaging Studies:
Computed Tomography (CT) and Cardiac Nuclear Medicine Studies
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
360
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of computed tomography (CT) and cardiac nuclear medicine (myocardial perfusion
studies) imaging reports for all patients, regardless of age, that document a count of known previous
CT (any type of CT) and cardiac nuclear medicine (myocardial perfusion) studies that the patient has
received in the 12-month period prior to the current study.
Substantive Change:
The measure description is revised to read: Percentage of computed tomography (CT) and cardiac
nuclear medicine (myocardial perfusion or infarct avid imaging) reports for all patients, regardless of
age, that document a count of known previous CT (any type of CT) and cardiac nuclear medicine
(myocardial perfusion or infarct avid imaging) studies that the patient has received in the 12-month
period prior to the current study.
The measure denominator is revised to read: All final reports for patients, regardless of age,
undergoing a CT or cardiac nuclear medicine (myocardial perfusion or infarct avid imaging)
procedure.
Updated denominator criteria: Added: coding for cardiology infarct imaging.
The measure numerator is revised to read: CT and cardiac nuclear medicine (myocardial perfusion
or infarct avid imaging) reports that document a count of known previous CT (any type of CT) and
cardiac nuclear medicine (myocardial perfusion or infarct avid imaging) studies that the patient has
received in the 12-month period prior to the current study.
Updated numerator instructions: Added: infarct avid imaging.
The measure numerator options are revised to read:
Performance Met: Count of previous CT (any type of CT) and cardiac nuclear medicine
(myocardial perfusion or infarct avid imaging) studies documented in the 12-month period prior to
the current study.
Performance Not Met: Count of previous CT and cardiac nuclear medicine (myocardial perfusion or
infarct avid imaging) studies not documented in the 12-month period prior to the current study,
reason not given.
Measure Steward:
American College of Radiology
High Priority Measure:
Yes
Measure Type:
Process
Rationale
We are proposing to revise multiple components of this measure to include infarct avid imaging in
the cardiac nuclear medicine imaging study that involves the use of radiation, including a radiotracer
or contrast agent and/or Imaging modalities, such as positron emission tomography (PET), single-
photon emission computed tomography (SPECT), or magnetic resonance imaging (MRI).
165
Given
that this imaging can expose a patient to potentially high doses of radiation,
166
including it in the
denominator of this measure requires the clinician to document a more complete count of previous
cardiac nuclear medicine imaging studies, increasing patient safety by preventing continued exposure
to radiation.
165
Enabnit, A. & Warren, A. (2023). Infarct Avid Imaging Study: Purpose, Procedure, and Applications.
https://www.dovemed.com/health-topics/focused-health-topics/infarct-avid-imaging-study-purpose-procedure-
and-applications.
166
Salah, H., Alkhorayef, M., Jambi, L., Almuwannis, M., & Sulieman, A. (2023). Radiation Dose to Patients and
Public Exposure in Cardiac Rest and Stress Single Photon Emission Computed Tomography Examinations. Radiation
Physics and Chemistry. https://doi.org/10.1016/j.radphyschem.2023.111383.
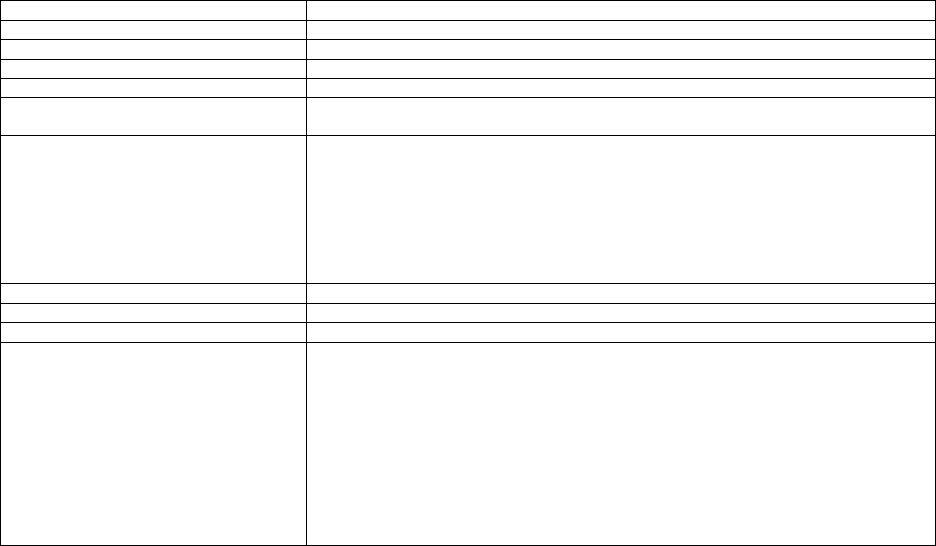
D.37. Closing the Referral Loop: Receipt of Specialist Report
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
374
CMS eCQM ID:
CMS50v13
Current Collection Type:
eCQM Specifications / MIPS CQM Specifications
Current Measure Description:
Percentage of patients with referrals, regardless of age, for which the referring clinician
receives a report from the clinician to whom the patient was referred.
Substantive Change:
Updated denominator criteria: For all collection types: Added: coding for psychology and
neuropsychology.
The measure numerator definition is revised to read: For the MIPS CQM Specifications
collection type: Revised: A written document prepared by the eligible clinician (and staff) to
whom the patient was referred and that accounts for their findings, provides summary of care
information about findings, diagnostics, assessments and/or plans of care, or states the patient
did not attend the appointment, and is provided to the referring eligible clinician.
Measure Steward:
Centers for Medicare & Medicaid Services
High Priority Measure:
Yes
Measure Type:
Process
Rationale
We are proposing to add psychology and neuropsychology encounter codes to the
denominator criteria as this measure is applicable to their scope of care. This expansion of the
denominator would allow more clinician types to submit the measure leading to an increase in
denominator eligibility, capturing a more complete patient population, as it is clinically
appropriate for these clinician types to complete the quality action for a patient referred to a
specialist.
We are proposing to revise the numerator definitions for the MIPS CQM Specifications
collection type to clarify which information should be included in the referring clinician’s
report to successfully close the referral loop. This revision increases clarity of this element and
ensures consistent implementation allowing for more comparable data.

D.38. Functional Status Assessment for Total Hip Replacement
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
376
CMS eCQM ID:
CMS56v13
Current Collection Type:
eCQM Specifications
Current Measure Description:
Percentage of patients 19 years of age and older who received an elective primary total hip
arthroplasty (THA) and completed a functional status assessment within 90 days prior to the surgery
and in the 300 – 425 days after the surgery.
Substantive Change:
Updated initial patient population: Revised: encounter timeframe from November of the year prior
to August of the year prior.
Measure Steward:
Centers for Medicare & Medicaid Services
High Priority Measure:
Yes
Measure Type:
Process
Rationale
We are proposing to revise initial patient population by changing the timing of the encounter to better
align with the post-surgical assessment timeframe of 300 to 425 days after the original THA surgery.
This would also harmonize the timeframe of the patient encounter with the administrative claims
measure “Hospital-Level Risk-Standardized Complication Rate (RSCR) Following Elective Primary
Total Hip Arthroplasty (THA) and/or Total Knee Arthroplasty (TKA)” available for Hospital
Inpatient Quality Reporting (88 FR 59067 through 59070), thereby aligning timing specificity and
patterns across similar measures. Measure alignment, in accordance with clinical recommendations,
guidelines, and best practices, allows for consistent and comparable data points, which leads to
actionable data to drive quality care, through understanding where the gaps in care are within a
patient population or along the continuum of care.

D.39. Adherence to Antipsychotic Medications For Individuals with Schizophrenia
Category
Description
CBE # / eCQM CBE #:
1879 / N/A
Quality #:
383
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of individuals at least 18 years of age as of the beginning of the performance period with
schizophrenia or schizoaffective disorder who had at least two prescriptions filled for any
antipsychotic medication and who had a Proportion of Days Covered (PDC) of at least 0.8 for
antipsychotic medications during the performance period.
Substantive Change:
Updated denominator note: Removed: distinction of typical versus atypical, 'days' supply' notation,
and medication HCPCS codes.
Updated denominator criteria: Removed: HCPCS coding from prescription criteria.
Measure Steward:
Centers for Medicare & Medicaid Services
High Priority Measure:
Yes
Measure Type:
Intermediate Outcome
Rationale
We are proposing to remove “days’ supply” and HCPCS (J codes) from the list of antipsychotic
medications in the measure. This revision would ensure inclusion for any duration of the listed long
acting injectables permitting the clinician, or site, to obtain days’ supply information from any
appropriate clinical or administrative source, thus aligning the calculation of PDC with all
appropriate medications listed in the measure. This revision reduces the burden of collecting this
aspect of the measure from medical records. Decreasing complexity and burden of this element
ensures consistent implementation allowing for more comparable data.
Finally, we are proposing to remove “typical” and “atypical” as part of the subheadings of the lists of
antipsychotic medications as this distinction does not apply to the list of oral and long-acting
injectable antipsychotic medications listed under each of the categories which include both typical
and atypical medications.
167
167
Cleveland Clinic. (2023). Antipsychotic Medications. https://my.clevelandclinic.org/health/treatments/24692-
antipsychotic-medications.

D.40. Adult Primary Rhegmatogenous Retinal Detachment Surgery: No Return to the Operating Room Within 90 Days
of Surgery
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
384
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Patients aged 18 years and older who had surgery for primary rhegmatogenous retinal detachment
who did not require a return to the operating room within 90 days of surgery.
Substantive Change:
Updated instructions: Revised: timeframe for when surgery for primary rhegmatogenous retinal
detachment must have occurred to meet the denominator criteria.
Updated numerator note: Added: For the purposes of meeting the numerator, complications are
only those related to the following procedures: 67107, 67108, 67110.
Measure Steward:
American Academy of Ophthalmology
High Priority Measure:
Yes
Measure Type:
Outcome
Rationale
We are proposing to update the instructions to clarify that surgery for primary rhegmatogenous
retinal detachment must occur between January 1
st
and September 30
th
of the performance period to
allow 90 days after the surgery to assess for the numerator action for patients who have surgeries
performed by September 30
th
. We are also proposing to add a numerator note to clarify that the
numerator is not required to capture only procedures covered by the three original procedure codes in
the denominator, but rather any procedure that is related to complications arising from the original
procedures listed.

D.41. Amyotrophic Lateral Sclerosis (ALS) Patient Care Preferences
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
386
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of patients diagnosed with Amyotrophic Lateral Sclerosis (ALS) who were offered
assistance in planning for end of life issues (e.g., advance directives, invasive ventilation, lawful
physician-hastened death, or hospice) or whose existing end of life plan was reviewed or updated at
least once annually or more frequency as clinically indicated (i.e., rapid progression)
Substantive Change:
Updated denominator criteria: Added: coding for speech language pathology.
Measure Steward:
American Academy of Neurology
High Priority Measure:
Yes
Measure Type:
Process
Rationale
We are proposing to update the denominator criteria to include coding for speech language pathology
and nuclear medicine as this measure is applicable to their scope of care.

D.42. Infection within 180 Days of Cardiac Implantable Electronic Device (CIED) Implantation, Replacement, or
Revision
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
393
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Infection rate following CIED device implantation, replacement, or revision.
Substantive Change:
Updated instructions: Added: A new device would be either the first device OR a device implanted
with new functionality.
Measure Steward:
American College of Cardiology Foundation
High Priority Measure:
Yes
Measure Type:
Outcome
Rationale
We are proposing to update the measure instructions to clarify the definition of a new device. This
change would ensure alignment and consistency in the abstraction of the measure’s elements that
support rigorous data for the calculation of MIPS performance rates.

D.43. Door to Puncture Time for Endovascular Stroke Treatment
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
413
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of patients undergoing endovascular stroke treatment who have a door to puncture time of
90 minutes or less.
Substantive Change:
Updated denominator exclusion: Added: Patients with secondary stroke within 5 days of the initial
procedure.
Measure Steward:
Society of Interventional Radiology
High Priority Measure:
Yes
Measure Type:
Intermediate Outcome
Rationale
We are proposing to add a denominator exclusion to remove patients with secondary stroke, such as
those which may occur with vasospasm in the setting of subarachnoid hemorrhage. Treatment for an
initial stroke is most effective when administered very shortly after the onset of symptoms.
168
However, the same is not the case for secondary stroke which is more challenging to manage as
patients with secondary stroke may already be on “blood thinners or aspirin, medication to control
cholesterol, or drugs to lower blood pressure” as a result of the initial stroke and “experience more
severe and long-lasting disability.”
169
This additional exclusion would more precisely reflect the time
to reperfusion for obtaining favorable outcomes in cerebral revascularization within the target patient
population.
168
Esenwa, C., & Gutierrez, J. (2015). Secondary Stroke Prevention: Challenges and Solutions. Vascular Health and
Risk Management, 11, 437–450. https://doi.org/10.2147/VHRM.S63791.
169
Medical News Today. (2023). Recurrent Strokes: What to Know.
https://www.medicalnewstoday.com/articles/recurrent-strokes.

D.44. Osteoporosis Management in Women Who Had a Fracture
Category
Description
CBE # / eCQM CBE #:
0053 / N/A
Quality #:
418
CMS eCQM ID:
N/A
Current Collection Type:
Medicare Part B Claims Measure Specifications / MIPS CQM Specifications
Current Measure Description:
The percentage of women 50–85 years of age who suffered a fracture and who had either a bone
mineral density (BMD) test or prescription for a drug to treat osteoporosis in the six months after the
fracture.
Substantive Change:
The measure description is revised to read: For all collection types: The percentage of women
50-85 years of age who suffered a fracture and who had either a bone mineral density (BMD) test or
prescription for a drug to treat osteoporosis in the 180 days after the fracture.
Updated instructions: For all collection types: Revised: from six months to 180 days.
Updated denominator exclusion: For all collection types: Removed: specific encounter
requirements from the frailty/advanced illness exclusion.
The measure numerator is revised to read: For all collection types: Patients who received either a
bone mineral density test or a prescription for a drug to treat osteoporosis in the 180 days after the
fracture.
Measure Steward:
National Committee for Quality Assurance
High Priority Measure:
No
Measure Type:
Process
Rationale
We are proposing to use both 6 months and 180 days within the measure denominator and numerator
to allow for the appropriate time anchors. In the denominator, 6 months is used so that the
measurement period to identify a fracture is always July 1 – June 30 (during the 6 months prior to the
performance period through June 30 of the performance period). The numerator action of
administration of a bone mineral density test or a prescription for a drug to treat osteoporosis within a
specific number of days (180 days after the date of a fracture) rather than months with varying
number of days (e.g., 28 – 31 days).
We are proposing to update the denominator exclusion by removing the requirement for patients to
have had at least one inpatient or two outpatient encounters to recognize a diagnosis of advanced
illness. The removal of this requirement within the denominator exclusion reduces the burden of
identifying applicable encounters, while still identifying patients with an indication of frailty. This is
particularly applicable for those patients seen outside of their reporting clinician’s medical record.
This revision would update the denominator exclusion to remove any patient 66-80 years of age and
older who has a diagnosis of advanced illness during the measurement period or the year prior from
the denominator of measure Q418. Decreasing complexity and burden of this element ensures
consistent implementation allowing for more comparable data.

D.45. Varicose Vein Treatment with Saphenous Ablation: Outcome Survey
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
420
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of patients treated for varicose veins (CEAP C2-S) who are treated with saphenous
ablation (with or without adjunctive tributary treatment) that report an improvement on a disease
specific patient reported outcome survey instrument after treatment.
Substantive Change:
Updated numerator definition: Added: Varicose Veins Symptom Questionnaire (VVSymQ) and
Venous Clinical Severity Score (VCSS).
Measure Steward:
Society of Interventional Radiology
High Priority Measure:
Yes
Measure Type:
Patient-Reported Outcome-Based Performance Measure
Rationale
We are proposing to revise the definition of an ‘Outcome Survey’ to include the VVSymQ and
VCSS. These outcome surveys measure improvement for saphenous vein ablation and would allow
clinicians more choices for meeting the numerator, which may encourage further adoption of this
measure.
170
170
Paty, J., Turner-Bowker, D. M., Elash, C. A., & Wright, D. (2016). The VVSymQ® Instrument: Use of a New
Patient-Reported Outcome Measure for Assessment of Varicose Vein Symptoms. Phlebology, 31(7), 481–488.
https://doi.org/10.1177/0268355515595193.

D.46. Proportion of Patients Sustaining a Bladder Injury at the Time of any Pelvic Organ Prolapse Repair
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
432
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of patients undergoing pelvic organ prolapse repairs who sustain an injury to the bladder
recognized either during or within 30 days after surgery.

Category
Description
Substantive Change:
The measure title is revised from Proportion of Patients Sustaining a Bladder Injury at the
Time of any Pelvic Organ Prolapse Repair to: Proportion of Patients Sustaining a Bladder or
Bowel Injury at the time of any Pelvic Organ Prolapse Repair.
The measure description is revised to read: Percentage of patients undergoing surgical repair of
pelvic organ prolapse that is complicated by a bladder or bowel injury at the time of index surgery
that is recognized intraoperatively or within 30 days after surgery.
The measure instructions are revised to read: This measure is to be submitted each time an
anterior, posterior, or apical prolapse repair surgery is performed from December 1st of the previous
performance period through November 30th of the current performance period. There is no diagnosis
associated with this measure. This measure may be submitted by Merit-based Incentive Payment
System (MIPS) eligible clinicians who perform the quality actions described in the measure based on
the services provided and the measure-specific denominator coding.
This measure will be calculated with 2 performance rates:
1) Percentage of patients undergoing prolapse repair who sustain a bladder injury that necessitates
repair either intraoperatively or within 30 days after surgery.
2) Percentage of patients undergoing prolapse repair who sustain a bowel injury that necessitates
repair either intraoperatively or within 30 days after surgery.
Submission of the two performance rates is required for this measure. A simple average, which is the
sum of the performance rates divided by the number of the performance rates will be used to
calculate performance.
THERE ARE TWO SUBMISSION CRITERIA FOR THIS MEASURE:
1) All patients undergoing anterior or apical pelvic organ prolapse (POP) surgery who sustain a
bladder injury.
2) All patients undergoing anterior, posterior, or apical pelvic organ prolapse (POP) surgery who
sustain a bowel injury.
This measure contains two submission criteria which together ensure that the proper evaluation and
treatment is provided for patients who undergo pelvic organ prolapse repair. Submission Criteria 1
evaluates whether patients sustained a bladder injury intraoperatively or within 30 days after surgery.
Submission Criteria 2 evaluates whether patients sustained a bowel injury intraoperatively or within
30 days after surgery. Patients who undergo a procedure that meets the denominator of both
submission criteria should be included in both and assessed for each clinical outcome.
Updated denominator: Added: SUBMISSION CRITERIA 2:
All patients undergoing anterior, posterior, or apical pelvic organ prolapse (POP) surgery.
Updated denominator criteria: Added: SUBMISSION CRITERIA 2:
All patients, regardless of age
AND
Patient procedure during the denominator identification period
WITHOUT
Telehealth Modifier
Updated definition: Added: SUBMISSION CRITERIA 1 & 2: Denominator identification period –
the twelve month period in which eligible patients have a procedure, which December 1st of the
previous performance period through November 30th of the current performance period.
Updated numerator:
Revised: SUBMISSION CRITERIA 1:
Percentage of patients undergoing prolapse repair who sustain a bladder injury that necessitates repair
either intraoperatively or within 30 days after surgery.
Added: SUBMISSION CRITERIA 2:
Percentage of patients undergoing prolapse repair who sustain a bowel injury that necessitates repair
either intraoperatively or within 30 days after surgery.
Update numerator instructions: Added: SUBMISSION CRITERIA 2:
INVERSE MEASURE – A lower calculated performance rate for this measure indicates better
clinical care or control. The “Performance Not Met” numerator option for this measure is the
representation of the better clinical quality or control. Submitting that numerator option will produce
a performance rate that trends closer to 0%, as quality increases. For inverse measures, a rate of
100% means all of the denominator eligible patients did not receive the appropriate care or were not
in proper control.
Updated numerator note: Added: SUBMISSION CRITERIA 2:
In order to meet the measure, bowel injury is sustained as a result of the prolapse surgery.
Updated numerator options: Added: SUBMISSION CRITERIA 2:
Performance Met: Patient sustained bowel injury at the time of surgery or discovered subsequently up
to 30 days post-surgery
OR
Denominator Exception: Documented medical reasons for not reporting bowel injury (e.g.
gynecologic or other pelvic malignancy documented, planned (e.g. not due to an unexpected bowel

Category
Description
injury) resection and/or re-anastomosis of bowel, or patient death from non-medical causes not
related to surgery, patient died during procedure without evidence of bowel injury)
OR
Performance Not Met:Patient did not sustain a bowel injury at the time of surgery nor discovered
subsequently up to 30 days post-surgery
Measure Steward:
American Urogynecologic Society
High Priority Measure:
Yes
Measure Type:
Outcome
Rationale
We are proposing to revise this measure to add a submission criteria and performance rate for
patients who retain a bowel injury at the time of any pelvic organ prolapse repair. We are proposing
to add submission criteria two to evaluate all patients undergoing anterior, posterior, or apical pelvic
organ prolapse surgery who sustain a bowel injury.
Previously, assessment of bowel injury outcomes for POP procedures was a separate measure. As
both measures are assessing for adverse outcomes following POP procedures, these measures are
being combined so that a full picture of adverse outcomes can be captured for this patient population.
Additionally, by combining these measures, the denominator eligible patient population may increase
allowing for more robust data for participating MIPS clinicians.
We are also proposing to add a definition for the denominator identification period of December 1st
of the previous performance period through November 30th of the current performance period.
In the event the proposed substantive change(s) are finalized, the substantive changes would not
allow for a direct comparison of performance data from prior years to performance data submitted
after the implementation of these substantive changes. As such, if the performance data submitted
meets the criteria for creation of a performance period benchmark, a new benchmark would be used
for scoring.

D.47. Appropriate Workup Prior to Endometrial Ablation
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
448
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of patients, aged 18 years and older, who undergo endometrial sampling or hysteroscopy
with biopsy and results are documented before undergoing an endometrial ablation.
Substantive Change:
Updated the instructions: Revised: submission of the measure to once per performance period.
Measure Steward:
Centers for Medicare & Medicaid Services
High Priority Measure:
Yes
Measure Type:
Process
Rationale
We are proposing to change the frequency of this measure from each time a procedure for
endometrial ablation is performed to once per performance period, as this is more aligned with the
clinical action being assessed for this measure.

D.48. Appropriate Treatment for Patients with Stage I (T1c) - III HER2 Positive Breast Cancer
Category
Description
CBE # / eCQM CBE #:
1858 / N/A
Quality #:
450
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of female patients aged 18 to 70 with stage I (T1c) - III HER2 positive breast cancer for
whom appropriate treatment is initiated.
Substantive Change:
The measure description is revised to read: Percentage of patients aged 18 to 70 with stage I (T1c)
– III HER2 positive breast cancer for whom appropriate treatment is initiated.
The measure denominator is revised to read: All breast cancer patients aged 18 to 70 with
pathologic stage I (T1c) – III HER2 positive breast cancer diagnosed between July 1st of the previous
performance period through June 30th of the current performance period.
Updated denominator instructions: Added: For the purposes of this measure, only pathologic
staging and HER-2 testing performed between July 1st of the previous performance period through
June 30th of the current performance period will be included in the denominator of this measure.
Updated denominator note: Added: This measure includes both female and male breast cancers.
While treatment recommendations for males have largely been extrapolated from results of clinical
trials focused on breast cancer in females, management of breast cancer in males is similar in overall
management to breast cancer in females. Consistent with guidance in NCCN guideline
recommendations for adjuvant systemic therapy, chemotherapy with/without HER2-targeted therapy
should be recommended for males with breast cancer according to guidelines for females with breast
cancer.
Updated denominator criteria: Revised: Patients age 18-70 years on date of encounter
Added: diagnosis codes for male breast cancer.
Added: Diagnosis of breast cancer between July 1st of the previous performance period through June
30th of the current performance period.
Updated numerator note: Added: The timeframe to identify the adjuvant treatment course is within
six months of breast cancer pathologic staging. To satisfy the numerator, both chemotherapy and
HER2-targeted therapy must occur within six months of pathologic staging.
Measure Steward:
American Society of Clinical Oncology
High Priority Measure:
Yes
Measure Type:
Process
Rationale
We are proposing to update multiple components of this measure to ensure that all patients diagnosed
with breast cancer are included in the measure’s denominator. While treatment recommendations for
males have largely been extrapolated from results of clinical trials focused on breast cancer in
females,
171
management of breast cancer in males is similar in overall management to breast cancer in
females. Consistent with guidance in NCCN recommendations for adjuvant systemic therapy,
chemotherapy with/without HER2-targeted therapy should be recommended for males with breast
cancer according to guidelines for females with breast cancer.
172
Additionally, revisions to the denominator definition would clarify the timeframe in which pathologic
staging and HER-2 testing should be performed for patient to be included in the denominator. We are
also proposing revisions to the numerator note to further clarify definition of and timeframe for when
adjuvant treatment, for the purposes of this measure, should occur relative to pathologic staging.
171
Hassett, M. J., Somerfield, M. R., & Giordano, S. H. (2020). Management of Male Breast Cancer: ASCO Guideline
Summary. JCO Oncology Practice, 16(8), e839–e843. https://doi.org/10.1200/JOP.19.00792.
172
Gradishar, W. J., Moran, M. S., Abraham, J., Abramson, V., Aft, R., Agnese, D., Allison, K. H., Anderson, B.,
Burstein, H. J., Chew, H., Dang, C., Elias, A. D., Giordano, S. H., Goetz, M. P., Goldstein, L. J., Hurvitz, S. A.,
Jankowitz, R. C., Javid, S. H., Krishnamurthy, J., Leitch, A. M., … Kumar, R. (2023). NCCN Guidelines® Insights: Breast
Cancer, Version 4.2023. Journal of the National Comprehensive Cancer Network: JNCCN, 21(6), 594–608.
https://doi.org/10.6004/jnccn.2023.0031.

D.49. RAS (KRAS and NRAS) Gene Mutation Testing Performed for Patients with Metastatic Colorectal Cancer who
Receive Anti-epidermal Growth Factor Receptor (EGFR) Monoclonal Antibody Therapy
Category
Description
CBE # / eCQM CBE #:
1859 / N/A
Quality #:
451
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of adult patients (aged 18 or over) with metastatic colorectal cancer who receive anti-
epidermal growth factor receptor monoclonal antibody therapy for whom RAS (KRAS and NRAS)
gene mutation testing was performed.
Substantive Change:
Updated denominator instructions: Added: The denominator of this measure is intended to capture
newly diagnosed stage IV patients or patients who have distant metastases at the time of colon cancer
diagnosis. For the purposes of this measure, the patient’s initial diagnosis may occur between
December 1 of the prior year through November 30 of the performance period, and anti-EGFR
monoclonal antibody therapy may occur between December 1 of the prior year through December 31
of the performance period.
Measure Steward:
American Society of Clinical Oncology
High Priority Measure:
No
Measure Type:
Process
Rationale
We are proposing to add denominator instructions to clarify that only patients who have been newly
diagnosed with Stage IV colorectal cancer or patients who have distant metastases at the time of
colon cancer diagnosis are to be captured in the denominator of the measure. This would ensure that
the appropriate patient population is assessed for the numerator action.

D.50. Bone Density Evaluation for Patients with Prostate Cancer and Receiving Androgen Deprivation Therapy
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
462
CMS eCQM ID:
CMS645v8
Current Collection Type:
eCQM Specifications
Current Measure Description:
Patients determined as having prostate cancer who are currently starting or undergoing androgen
deprivation therapy (ADT), for an anticipated period of 12 months or greater and who receive an
initial bone density evaluation. The bone density evaluation must be prior to the start of ADT or
within 3 months of the start of ADT.
Substantive Change:
The measure denominator is revised to read: Patients with a qualifying encounter in the
measurement period AND with a diagnosis of prostate cancer AND with an order for ADT or an
active medication of ADT with an intent for treatment greater than or equal to 12 months during the
measurement period AND order for ADT in 3 months before to 9 months after the start of the
measurement period.
Measure Steward:
Oregon Urology Institute
High Priority Measure:
No
Measure Type:
Process
Rationale
We are proposing to expand the denominator to include all patients regardless of gender. This
revision broadens the denominator population to capture all patients with a diagnosis of prostate
cancer who are receiving androgen deprivation therapy and ensure a bone density evaluation is
completed prior to the start of treatment. As significantly lower PSA screening rates were seen
among transgender individuals for ages 40-54 and 55-69, but higher rates within the age group 70-80
(P.ß<.ß.001 for all),
173
broadening the denominator would ensure these patient populations are being
screened in accordance with current clinical guidelines.
173
Premo, H., Gordee, A., Lee, H. J., Scales, C. D., Moul, J. W., & Peterson, A. (2023). Disparities in Prostate Cancer
Screening for Transgender Women: An Analysis of the MarketScan Database. Urology, 176, 237–242.
https://doi.org/10.1016/j.urology.2023.03.016.

D.51. Otitis Media with Effusion: Systemic Antimicrobials - Avoidance of Inappropriate Use
Category
Description
CBE # / eCQM CBE #:
0657 / N/A
Quality #:
464
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of patients aged 2 months through 12 years with a diagnosis of OME who were not
prescribed systemic antimicrobials.
Substantive Change:
The measure instructions are revised to read: This measure is to be submitted once for each
occurrence of otitis media with effusion (OME) in children seen during the performance period. Each
unique occurrence starts with the onset of OME symptoms and concludes with the resolution of OME
or after 90 days if a resolution of OME symptoms is not documented. If multiple encounters are
documented within an occurrence, Merit-based Incentive Payment System (MIPS) eligible clinicians
should submit the most recent encounter during that occurrence. A new occurrence of OME cannot
start until the previous occurrence during the performance period has concluded.
Measure Steward:
American Academy of Otolaryngology – Head and Neck Surgery Foundation
High Priority Measure:
Yes
Measure Type:
Process
Rationale
We are proposing to update the measure instructions to clarify what constitutes an occurrence for the
purposes of this measure. This additional guidance would further clarify how patients are attributed to
the denominator of this measure for each eligible occurrence. This change would ensure consistency
in the abstraction of the measure’s elements that support rigorous data for the calculation of MIPS
performance rates.

D.52. Functional Status After Primary Total Knee Replacement
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
470
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
For patients age 18 and older who had a primary total knee replacement procedure, functional status
is rated by the patient as greater than or equal to 37 on the Oxford Knee Score (OKS) or a 71 or
greater on the KOOS, JR. tool at one year (9 to 15 months) postoperatively.
Substantive Change:
Updated numerator note: Revised: list of situations that denote performance not met.
Measure Steward:
Minnesota Community Measurement
High Priority Measure:
Yes
Measure Type:
Patient-Reported Outcome-Based Performance Measure
Rationale
We are proposing to revise the numerator note by clarifying that if a tool other than the Oxford Knee
Score (OKS) or Knee injury/Osteoarthritis Outcome Score Joint Replacement (KOOS, JR.) is used to
assess a patient’s functional status for this measure, it would result in a performance not met. The
requirements for meeting this measure require use of the specific tools referenced in the measure
specification, as they have been tested, validated, and determined to be most appropriate for capturing
the numerator action.

D.53. Psoriasis – Improvement in Patient-Reported Itch Severity
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
485
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
The percentage of patients aged 8 years and older, with a diagnosis of psoriasis where at an initial
(index) visit have a patient-reported itch severity assessment performed, score greater than or equal to
4, and who achieve a score reduction of 3 or more points at a follow-up visit.
Substantive Change:
Updated denominator note: Added: The initial (index) visit assessment and the follow-up visit for
assessment must occur during the performance period. The initial (index) visit is the first encounter
with the patient during the performance period. Every visit after the initial (index) visit during the
performance period is a follow-up visit. An assessment should be completed at each visit.
Updated numerator instructions: Removed: If a patient has multiple follow-up visits within the
measurement period, the last (most recent) visit should be used.
Measure Steward:
American Academy of Dermatology
High Priority Measure:
Yes
Measure Type:
Patient-reported Outcome-based Performance Measure
Rationale
We are proposing to update the denominator note to clarify encounter timing by including language
outlining that a patient’s first visit during the measurement period is considered the initial (index)
encounter. Each visit after the initial (index) visit during the measurement period would be deemed a
follow-up visit used to determine the outcome of the measure. This change would ensure consistency
in the abstraction of the measure’s elements that support rigorous data for the calculation of MIPS
performance rates.

D.54. Dermatitis – Improvement in Patient-Reported Itch Severity
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
486
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
The percentage of patients aged 8 years and older, with a diagnosis of dermatitis where at an initial
(index) visit have a patient-reported itch severity assessment performed, score greater than or equal to
4, and who achieve a score reduction of 3 or more points at a follow-up visit.
Substantive Change:
Updated denominator note: Added: The initial (index) assessment and the follow-up visit for
assessment must occur during the performance period. The initial (index) visit is the first encounter
with the patient during the performance period. Every visit after the initial (index) visit during the
performance period is a follow-up visit. An assessment should be completed at each visit.
Updated numerator instructions: Removed: If a patient has multiple follow-up visits within the
measurement period, the last (most recent) visit should be used.
Measure Steward:
American Academy of Dermatology
High Priority Measure:
Yes
Measure Type:
Patient-Reported Outcome-based Performance Measure
Rationale
We are proposing to update the denominator note to clarify encounter timing by including language
outlining that a patient’s first visit during the measurement period is considered the initial (index)
encounter. Each visit after the initial (index) visit during the measurement period would be deemed a
follow-up visit used to determine the outcome of the measure. This change would ensure consistency
in the abstraction of the measure’s elements that support rigorous data for the calculation of MIPS
performance rates.

D.55. Kidney Health Evaluation
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
488
CMS eCQM ID:
CMS951v3
Current Collection Type:
eCQM Specifications / MIPS CQM Specifications
Current Measure Description:
Percentage of patients aged 18-75 years with a diagnosis of diabetes who received a kidney health
evaluation defined by an Estimated Glomerular Filtration Rate (eGFR) AND Urine Albumin-
Creatinine Ratio (uACR) within the measurement period
Substantive Change:
Updated description: For all collection types: Revised: patients aged 18-85 years.
Updated denominator: For all collection types: Revised: patients aged 18-85 years.
Updated denominator criteria: For the MIPS CQM Specifications collection type: Revised:
patients aged 18-85 years.
Measure Steward:
National Kidney Foundation
High Priority Measure:
No
Measure Type:
Process
Rationale
We are proposing to update the age from 18-75 to 18-85 years of age for denominator eligibility.
Based on information from National Institutes of Health states, “A high proportion of older CKD
patients are usually affected by multimorbidity, polypharmacy, frailty, functional and cognitive
impairment, and disability.”
174
“The benefits of preventing/slowing the progression of CKD has the
potential to impact different social and health domains, for example, reducing the need for long-term
care and the cost related to caregiving.”
175
Increasing the denominator age criteria support the
reporting of clinicians currently screening a broader elderly population for this preventable disease.
174
Corsonello, A., Freiberger, E., & Lattanzio, F. (2020). The Screening for Chronic Kidney Disease Among Older
People across Europe (SCOPE) Project: Findings from Cross-Sectional Analysis. BMC Geriatrics, 20(Suppl 1), 316.
https://doi.org/10.1186/s12877-020-01701-w.
175
See footnote Corsonello et al., 2020.

D.56. Appropriate Intervention of Immune-Related Diarrhea and/or Colitis in Patients Treated with Immune Checkpoint
Inhibitors
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
490
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of patients, aged 18 years and older, with a diagnosis of cancer, on immune checkpoint
inhibitor therapy, and grade 2 or above diarrhea and/or grade 2 or above colitis, who have immune
checkpoint inhibitor therapy held and corticosteroids or immunosuppressants prescribed or
administered.
Substantive Change:
Updated denominator definition: For the MIPS CQM Specifications collection type: Added:
immune checkpoint inhibitors Lag-3 inhibitor drug: Relatlimab; and Tremelimumab to CTLA-4
inhibitor drugs.
Measure Steward:
Society for Immunotherapy of Cancer (SITC)
High Priority Measure:
No
Measure Type:
Process
Rationale
We are proposing to update the denominator definition to add new Food and Drug Administration
(FDA) approved checkpoint inhibitors, Relatlimab and Tremelimumab. These inhibitors are clinically
appropriate to include in the denominator of this measure.
176
177
176
FDA. (2022). FDA Approves Tremelimumab in Combination with Durvalumab and Platinum-based
Chemotherapy for Metastatic Non-small Cell Lung Cancer. https://www.fda.gov/drugs/resources-information-
approved-drugs/fda-approves-tremelimumab-combination-durvalumab-and-platinum-based-chemotherapy-
metastatic-non.
177
FDA. (2022). FDA Approves Opdualag for Unresectable or Metastatic Melanoma.
https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-opdualag-unresectable-or-
metastatic-melanoma.

D.57. Risk-Standardized Acute Cardiovascular-Related Hospital Admission Rates for Patients with Heart Failure under
the Merit-based Incentive Payment System
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
492
CMS eCQM ID:
N/A
Current Collection Type:
Administrative Claims
Current Measure Description:
Annual risk-standardized rate of acute, unplanned cardiovascular-related admissions among Medicare
Fee-for-Service (FFS) patients aged 65 years and older with heart failure (HF) or cardiomyopathy.
Substantive Change:
Updated: Reporting Requirements: Removed: individual reporting.
Measure Steward:
Centers for Medicare & Medicaid Services
High Priority Measure:
Yes
Measure Type:
Outcome
Rationale
We are proposing a substantive change to this measure that would be applied retroactively starting
with the CY 2023 performance period/2025 MIPS payment year. In the CY 2023 PFS final rule, we
inadvertently specified the measure was availability at the individual clinician level. The inclusion of
the availability of the measure at the individual clinician level is a misrepresentation and erroneously
conveys to MIPS eligible clinicians reporting at the individual clinician level that the measure is
available to meet the minimum required number of measures to report under traditional MIPS or an
MVP. The measure was tested and developed for implementation at the group, virtual group,
subgroup via an MVP, and APM Entity levels. Thus, the measure is limited to groups, virtual groups,
subgroups via an MVP, and APM Entities participating in MIPS. We believe that a failure to apply
this substantive change retroactively would be contrary to the public interest.
Prior to the finalization of this measure as a new measure available within the MIPS quality measure
inventory in the CY 2023 PFS final rule, the measure was initially proposed as a new measure in the
CY 2022 PFS proposed rule. Based on the public comments received in response to the initial
proposal of this measure in the CY 2022 PFS proposed rule, there were concerns regarding the
attribution of certain patients to clinicians, particularly the risk adjustment for clinicians with higher
caseloads of patients with more complicated or severe heart failure. As a result, the measure was not
finalized as part of the CY 2022 PFS final rule; however, we noted that we would continue to
consider how to implement condition-specific measures such as this measure under MIPS (86 FR
65692 through 65694).
In the CY 2023 PFS proposed rule, we re-proposed this measure, which mitigated the concerns
regarding the attribution of such patients to clinicians by excluding patients at advanced stages of
heart failure and requiring that a group, virtual group, subgroup via an MVP, and APM Entity to
include at least 1 cardiologist (and a 21-patient case minimum); and subsequently, the measure was
finalized in the CY 2023 PFS final rule (87 FR 70266) through 70271). The intent of the measure is
for assessment of performance to be conducted at the group, virtual group, subgroup via an MVP, and
APM Entity levels. The measure was not tested, developed, or implemented at the individual
clinician level. In order for this measure to be available at the individual clinician level, the measure
would need to be tested at the individual clinician level to establish validity, reliability, and risk
adjustments at the individual clinician level. It is not appropriate for the measure to be available at the
individual clinician level without further testing. Consequently, any assessment of data for this
measure at the individual clinician level would produce invalid and unreliable results. By
retroactively applying the substantive change to this measure (modifying the measure to remove the
individual clinician level as an option) effective starting with the CY 2023 performance period/2025
MIPS payment year, the level of reporting available for the measure would align with the intent,
implementation, and operationalization of the measure, and clarify that the measure is not available at
the individual clinician level.

D.58. Adult Immunization Status
Category
Description
CBE # / eCQM CBE #:
3620 / N/A
Quality #:
493
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of patients 19 years of age and older who are up-to-date on recommended routine
vaccines for influenza; tetanus and diphtheria (Td) or tetanus, diphtheria and acellular pertussis
(Tdap); zoster; and pneumococcal.
Substantive Change:
Updated instructions: Revised: pneumococcal vaccine on or after their 19th birthday.
Updated numerator: Revised: Submission Criteria 4: pneumococcal vaccine on or after their 19th
birthday.
Measure Steward:
National Committee for Quality Assurance
High Priority Measure:
No
Measure Type:
Process
Rationale
We are proposing to update the measure instructions and numerator to lower the minimum age from
60 years of age to 19 years of age. This revision aligns with the updated ACIP pneumococcal
vaccination guidelines
178
that recommend administering conjugate vaccines to all adults with certain
underlying medical conditions.
In the CY 2023 PFS final rule, this measure was finalized, and it was noted that the scoring of the
measure would use a weighted average for the first 2 years of implementation; and starting with the
CY 2025 performance period/2027 MIPS payment year, the measure would be scored as an all-or-
none composite measure to ensure a more thorough assessment of a patient’s vaccination status (87
FR 70272 through 70274). However, based upon an analysis of preliminary data submitted for the
CY 2023 performance period, low measure adoption, and feedback received through Quality
Payment Program Service Now tickets regarding burden of implementation and ability to meet
performance on all four components, we are proposing to maintain the weighted average analytic for
the CY 2025 performance period and subsequent years as determined by CMS. The utilization of the
weighted average metric/analytic beyond 2 years would provide clinicians with more time to prepare
for the transition to a more stringent all-or-none metric/analytic, which would require a complete
vaccination history to meet numerator compliance.
178
Kobayashi, M., Pilishvili, T., Farrar, J. L., Leidner, A. J., Gierke, R., Prasad, N., Moro, P., Campos-Outcalt, D.,
Morgan, R. L., Long, S. S., Poehling, K. A., & Cohen, A. L. (2023). Pneumococcal Vaccine for Adults Aged ≥19 Years:
Recommendations of the Advisory Committee on Immunization Practices, United States, 2023. MMWR.
Recommendations and Reports: Morbidity and Mortality Weekly Report. Recommendations and Reports, 72(3), 1–
39. https://doi.org/10.15585/mmwr.rr7203a1.

D.59. Preventive Care and Wellness (composite)
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
497
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of patients who received age- and sex-appropriate preventive screenings and wellness
services. This measure is a composite of seven component measures that are based on
recommendations for preventive care by the U.S. Preventive Services Task Force (USPSTF),
Advisory Committee on Immunization Practices (ACIP), American Association of Clinical
Endocrinology (AACE), and American College of Endocrinology (ACE).
Substantive Change:
Updated instructions: Updated: Percentage of patients 65 years of age or older who received a
pneumococcal vaccination on or after their 19th birthday.
Updated denominator exclusion: Removed: (Submission Criteria 3 and Submission Criteria 4):
specific encounter requirements from the frailty/advanced illness exclusion.
Updated denominator criteria: Added: (Submission Criteria 7) coding for nutrition/dietitian
clinician type.
Updated numerator definition: Revised: intervals for rescreening for first and second hypertensive
BP readings.
Measure Steward:
Centers for Medicare & Medicaid Services
High Priority Measure:
No
Measure Type:
Process
Rationale
We are proposing to update the measure instructions for patients 65 years of age and older who have
received a pneumococcal vaccination on or after their 19
th
birthday. This update would allow for the
identification of patients 65 years of age and older who may have a clinical condition that would
make them a candidate to receive the pneumococcal vaccine prior to 65 years of age.
179
We are proposing to add encounter codes for nutrition therapy to the denominator criteria as this is an
appropriate setting to identify people who may have elevated blood pressure (BP) readings.
Nutritional approaches play a pivotal role in helping to reduce the risk of hypertension or control
blood pressure in people with hypertension.
180
We are proposing to remove the minimum timeframe for follow-up screenings for patients with
elevated BP readings. This change allows clinician discretion to recommend a follow-up plan based
on the patient’s current health status. Additionally, this supports stability of this measure component,
as the frequency is each visit, within the specification over time, while still maintaining consistency
with the current guidelines.
181
We are proposing to update the denominator exclusion by removing the requirement for patients to
have had at least one inpatient or two outpatient encounters to recognize a diagnosis of advanced
illness. The removal of this requirement within the denominator exclusion reduces the burden of
identifying applicable encounters, while still identifying patients with an indication of frailty. This is
particularly applicable for those patients seen outside of their reporting clinician’s medical record.
This revision would update the denominator exclusion to remove any patient 66 years of age and
older who has a diagnosis of advanced illness during the measurement period or the year prior from
the denominator for submission criteria 3 and submission criteria 4 of measure Q497.
179
See footnote Kobayashi et al., 2023 in Table D.58 of this Appendix.
180
See footnote Physicians Committee for Responsible Medicine, 2023 in Table D.28 of this Appendix.
181
See footnote Whelton et. al., 2018 in Table D.28 of this Appendix.

D.60. Connection to Community Service Provider
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
498
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percent of patients 18 years or older who screen positive for one or more of the following health
related social needs (HRSNs): food insecurity, housing instability, transportation needs, utility help
needs, or interpersonal safety; and had contact with a Community Service Provider (CSP) for at least
1 of their HRSNs within 60 days after screening.
Substantive Change:
Updated denominator criteria: Added: coding for Dentistry, Emergency Medicine, Inpatient,
Nuclear Medicine, Interventional Radiology, Psychiatry, Mental and Behavioral Health, Nephrology,
Nutrition, Dietician, Obstetrics/Gynecology, Ophthalmology, Otolaryngology, Physical
Therapy/Occupational Therapy, Home Care and Skilled Nursing.
Measure Steward:
OCHIN
High Priority Measure:
Yes
Measure Type:
Process
Rationale
We are proposing to update the denominator criteria to include coding for multiple patient services as
this measure is applicable to the scope of care associated with these services. Clinicians treating
patients within these services have an opportunity for screening for HRSNs and connecting with an
appropriate CSP. Addressing the social determinants is an important and emerging area of practice
that entails starting earlier and broadening the scope of interventions, thus making entire families and
communities healthier.
182
182
Andermann, A., & CLEAR Collaboration (2016). Taking Action on the Social Determinants of Health in Clinical
Practice: A Framework for Health Professionals. CMAJ: Canadian Medical Association Journal, 188(17-18), E474–
E483. https://doi.org/10.1503/cmaj.160177.

D.61. Acute Posterior Vitreous Detachment Appropriate Examination and Follow-up
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
500
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of patients with a diagnosis of acute posterior vitreous detachment (PVD) in either eye
who were appropriately evaluated during the initial exam and were re-evaluated no later than 8
weeks.
Substantive Change:
Updated denominator criteria: Added: acute PVD.
Removed: coding for non-acute disorders related to PVD.
Measure Steward:
American Society of Retina Specialists
High Priority Measure:
No
Measure Type:
Process
Rationale
We are proposing to revise the denominator criteria by removing diagnosis codes for non-acute PVD
and adding acute PVD within the criteria of the denominator. The ICD-10-CM PVD diagnosis codes
do not differentiate between acute and non-acute disorders of vitreous detachment; therefore, to
ensure the intended patient population is identified, the denominator criteria must be specific to acute
PVD.

D.62. Acute Posterior Vitreous Detachment and Acute Vitreous Hemorrhage Appropriate Examination and Follow-up
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
501
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of patients with a diagnosis of acute posterior vitreous detachment (PVD) and acute
vitreous hemorrhage in either eye who were appropriately evaluated during the initial exam and were
re-evaluated no later than 2 weeks
Substantive Change:
Updated denominator criteria: Removed: coding for non-acute disorders related to PVD.
Measure Steward:
American Society of Retina Specialists
High Priority Measure:
No
Measure Type:
Process
Rationale
We are proposing to revise the denominator criteria by removing diagnosis codes for non-acute PVD
and adding acute PVD within the criteria of the denominator. The ICD-10-CM PVD diagnosis codes
do not differentiate between acute and non-acute disorders of vitreous detachment; therefore, to
ensure the intended patient population is identified, the denominator criteria must be specific to acute
PVD.
D.63. Gains in Patient Activation Measure (PAM®) Scores at 12 Months
Category
Description
CBE # / eCQM CBE #:
2483 / N/A
Quality #:
503
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
The Patient Activation Measure® (PAM®) is a 10- or 13- item questionnaire that assesses an
individual's knowledge, skills and confidence for managing their health and health care. The measure
assesses individuals on a 0-100 scale that converts to one of four levels of activation, from low (1) to
high (4). The PAM® performance measure (PAM®-PM) is the change in score on the PAM® from
baseline to follow-up measurement.
Substantive Change:
Updated instructions: Revised: follow-up PAM® survey collected on at least 25% of all eligible
patients within 4 to 12 months of the baseline during the performance period.
The measure denominator is revised to read:
For Submission Criteria 1: Patients aged 14 years and older with at least two qualifying visits
during the performance period.
For Submission Criteria 2, 3 & 4: Patients aged 14 years and older with Performance Met for
Submission Criteria 1 who had a baseline PAM® score and a second score within 4 to 12 month of
baseline PAM® score and who were seen for a qualifying visit at least twice during the performance
period.
Updated denominator criteria: Revised: For Submission Criteria 2, 3, & 4: follow-up PAM®
survey collected on at least 25% of all eligible patients within 4 to 12 months of the baseline during
the performance period.
Updated denominator exception: Revised: For Submission Criteria 1: to remove patients with
excessive missing responses or patients who were not seen for the second PAM survey within 4
months of the baseline.
Updated denominator exclusion: Added: For ALL Submission Criteria: Patients who died during
the performance period.
Updated numerator: Revised: for ALL Submission Criteria: timing of follow-up PAM® survey to
occur 4 to 12 months after baseline.
Updated numerator definition: Added: For Submission Criteria 1: clarification as to what denotes
excessive missing responses for each PAM survey.
Measure Steward:
Insignia Health, LLC, a wholly owned subsidiary of Phreesia
High Priority Measure:
Yes
Measure Type:
Patient-Reported Outcome-Based Performance Measure
Rationale
We are proposing to revise multiple components of this measure to allow re-administration of the
PAM® survey no less than 4 months after the baseline survey is administered as opposed to 6
months. This change would allow a higher percentage of patients to complete the survey and be
included in the measure denominator.
In addition, we are proposing to lower the minimum performance threshold for collected follow-up
PAM® surveys from 50 percent to 25 percent and remove patients who were missing more than 3
responses on the PAM-10® surveys or more than 4 responses on the PAM-13® surveys. Revising the
threshold for the collected follow-up PAM® survey reduces clinician burden by lowering the number
of survey responses that need to be collected to meet the measure denominator. We are also
proposing to add a definition to clarify what denotes excessive for missing responses. Removing
patients with excessive missing responses allows for a more complete assessment of an individual's
knowledge, skills, and confidence for managing their health and health care.
We are also proposing to increase the number of qualifying visits to two during the performance
period and add an exclusion to remove patients who may have died during the performance period.
These revisions to the denominator ensure a patient is established within that clinician’s patient
population which supports the completion of the baseline and follow-up of the survey.

D.64. Initiation, Review, and/or Update to Suicide Safety Plan for Individuals with Suicidal Thoughts, Behavior, or
Suicide Risk
Category
Description
CBE # / eCQM CBE #:
N/A / N/A
Quality #:
504
CMS eCQM ID:
N/A
Current Collection Type:
MIPS CQM Specifications
Current Measure Description:
Percentage of adult aged 18 years and older with suicidal ideation or behavior symptoms (based on
results of a standardized assessment tool or screening tool) or increased suicide risk (based on the
clinician's evaluation or clinician-rating tool) for whom a suicide safety plan is initiated, reviewed,
and/or updated in collaboration between the patient and their clinician.
Substantive Change:
The measure description is revised to read: Percentage of patients aged 12 years and older with
suicidal ideation or behavior symptoms (based on results of a standardized assessment tool or
screening tool) or increased suicide risk (based on the clinician's evaluation or clinician-rating tool)
for whom a suicide safety plan is initiated, reviewed, and/or updated in collaboration between the
patient and their clinician.
The measure denominator is revised to read: For denominator submission criteria 1 & 2:
Patients aged 12 years and older with a mental and/or substance use disorder with suicidal ideation
and/or behavior symptoms or suicide risk at a clinical encounter during the denominator
identification period.
Measure Steward:
American Psychiatric Association
High Priority Measure:
Yes
Measure Type:
Process
Rationale
We are proposing to revise the age from 18 years and older to 12 years and older for denominator
eligibility as suicide risk assessment is an important part of child and adolescent mental health. As
noted in an American Academy of Pediatrics publication.
183
“Suicide is the second leading cause of
death for 10- to 24-year-olds in the United States and is a global public health issue, with a recent
declaration of a National State of Emergency in Children’s Mental Health by the American Academy
of Pediatrics, American Academy of Child and Adolescent Psychiatry, and Children’s Hospital
Association.”
184
183
Hua, L. L., Lee, J., Rahmandar, M. H., Sigel, E. J., Committee on Adolescence, & Council on Injury, Violence, and
Poison Prevention. (2024). Suicide and Suicide Risk in Adolescents. Pediatrics, 153(1), e2023064800.
https://doi.org/10.1542/peds.2023-064800.
184
See footnote Hua et al., 2024.
Table Group DD: Proposed Substantive Changes to Previously Finalized MIPS Quality
Measures Available Only for Use in Relevant MVPs for the CY 2025 Performance
Period/2027 MIPS Payment Year and Future Years
As finalized for the CY 2024 performance period/2026 MIPS payment year and future years, the following three MIPS quality
measures were retained for utilization in MVPs only while removed from traditional MIPS: Q112: Breast Cancer Screening,
Q113: Colorectal Cancer Screening, and Q128: Preventive Care and Screening: Body Mass Index (BMI) Screening and Follow-
Up Plan (88 FR 79897 through 79902). We note that some MIPS quality measures available only in MVPs are adopted by the
Medicare Shared Savings Program for utilization in the Alternative Payment Model (APM) Performance Pathway (APP) and/or
APP Plus, as proposed in section XX of this proposed rule. For such measures, the collection type applicable for purposes of the
APP and/or APP Plus (Medicare CQM for Accountable Care Organizations Participating in the Medicare Shared Savings
Program (Medicare CQM)) is also specified as a collection type available for such measures described in Table Group DD.
Table Group DD within this proposed rule provides substantive changes proposed for the CY 2025 performance period/2027
MIPS payment year for MIPS quality measures available only in a relevant MVP. Two of the aforementioned MIPS quality
measures, Q112 and Q113, have proposed substantive changes under Table Group DD. The changes that are made to the
denominator codes sets are generalizations of the revisions communicated from the measure stewards to CMS. Additionally,
International Classification of Diseases Tenth Edition (ICD-10) and Current Procedural Terminology (CPT) codes that are
identified as invalid for CY 2025 may not be identified within this proposed rule due to the availability of these changes to the
public. If coding revisions to the denominator are impacted due to the timing of 2025 CPT and ICD-10 updates and assessment of
these codes inclusion by the Measure Steward, these changes may be postponed until CY 2026. The 2025 Quality Measure
Release Notes provide a comprehensive, detailed reference of exact codes changes to the denominators of the quality
measures. The Quality Measure Release Notes are available for each of the collection types in the Quality Payment Program
website at https://qpp.cms.gov.
Note: Electronic clinical quality measures (eCQMs) that are endorsed by a CBE are shown in Table DD of this Appendix as
follows: CBE # / eCQM CBE #.
In addition to the proposed substantive changes, there may be changes to the coding utilized within the denominator that are not
considered substantive in nature, but they are important to communicate to interested parties. These changes align with the scope
of the current coding; however, though not substantive in nature, these changes would expand or contract the measure’s current
eligible patient population. Therefore, please refer to the current year measure specification and the 2025 Quality Measure
Release Notes or the eCQM Technical Release Notes once posted to review all coding changes to ensure correct implementation.
The eCQM Technical Release Notes should also be carefully reviewed for revisions within the logic portion of the measure. In
addition to the proposed substantive changes, there may be revisions within the logic that are not considered substantive in
nature, however, it is important to review to ensure proper implementation of the measure. As not all systems and clinical
workflows are the same, it is important to review these changes in the context of a specific system and/or clinical workflow.
We request comments on these substantive changes.
DD.1. Breast Cancer Screening
Category
Description
CBE # / eCQM CBE #:
2372 / N/A
Quality #:
112
CMS eCQM ID:
CMS125v13
Current Collection Type:
Medicare Part B Claims Measure Specifications / eCQM Specifications / MIPS CQM Specifications
Current Measure Description:
Percentage of women 40 - 74 years of age who had a mammogram to screen for breast cancer in the
27 months prior to the end of the measurement period.
Substantive Change:
Modified collection type: Medicare Part B Claims Measure Specifications, eCQM Specifications,
MIPS CQM Specifications, and Medicare CQM Specifications (collection type available only in the
APP and/or APP Plus).
Updated denominator exclusion: For all collection types: Removed: specific encounter
requirements from the frailty/advanced illness exclusion.
Measure Steward:
National Committee for Quality Assurance
High Priority Measure:
No
Measure Type:
Process
Rationale
We are proposing to update the denominator exclusion by removing the requirement for patients to
have had at least one inpatient or two outpatient encounters to recognize a diagnosis of advanced
illness. The removal of this requirement within the denominator exclusion reduces the burden of
identifying applicable encounters, while still identifying patients with an indication of frailty. This is
particularly applicable for those patients seen outside of their reporting clinician’s medical record.
This revision would update the denominator exclusion to remove any patient 66 years of age and
older who has a diagnosis of advanced illness during the measurement period or the year prior from
the denominator of measure Q112. Decreasing complexity and burden of this element ensures
consistent implementation allowing for more comparable data.

DD.2. Colorectal Cancer Screening
Category
Description
CBE # / eCQM CBE #:
0034 / N/A
Quality #:
113
CMS eCQM ID:
CMS130v13
Current Collection Type:
Medicare Part B Claims Measure Specifications / eCQM Specifications / MIPS CQM Specifications
Current Measure Description:
Percentage of patients 45-75 years of age who had appropriate screening for colorectal cancer.
Substantive Change:
Modified collection type: Medicare Part B Claims Measure Specifications, eCQM Specifications,
MIPS CQM Specifications, and Medicare CQM Specifications (collection type available only in the
APP and/or APP Plus)
Updated denominator exclusion: For all collection types: Removed: specific encounter
requirements from the frailty/advanced illness exclusion.
Measure Steward:
National Committee for Quality Assurance
High Priority Measure:
No
Measure Type:
Process
Rationale
We are proposing to update the denominator exclusion by removing the requirement for patients to
have had at least one inpatient or two outpatient encounters to recognize a diagnosis of advanced
illness. The removal of this requirement within the denominator exclusion reduces the burden of
identifying applicable encounters, while still identifying patients with an indication of frailty. This is
particularly applicable for those patients seen outside of their reporting clinician’s medical record.
This revision would update the denominator exclusion to remove any patient 66 years of age and
older who has a diagnosis of advanced illness during the measurement period or the year prior from
the denominator of measure Q113. Decreasing complexity and burden of this element ensures
consistent implementation allowing for more comparable data.

APPENDIX 2: IMPROVEMENT ACTIVITIES
NOTE: In this proposed rule, for the CY 2025 performance period/2027 MIPS payment year
and future years, we are proposing to add two new improvement activities, modify two
previously adopted improvement activities, and remove eight previously adopted improvement
activities. These proposals are discussed in section IV.A.4.e.(3)(b)(iii) of this proposed rule and
in more detail below. We request comment on our proposals.
TABLE A: New Improvement Activities
for the CY 2025 Performance Period/2027 MIPS Payment Year and for Future Years
New Improvement Activity
Proposed Activity
ID:
IA_PM_XX
Proposed
Subcategory:
Population Management
Proposed Activity
Title:
Implementation of Protocols and Provision of Resources to Increase Lung Cancer
Screening Uptake
Proposed Activity
Description:
Establish a process or procedure to increase rates of lung cancer screening through one or
more of the following interventions:
• Implementation of protocols that support enhanced documentation methods to
identify eligible patients for lung cancer screening.
++ Example: A practice could embed electronic health record (EHR)
prompts to flag insufficient patient smoking history (for example, total
pack-years) and increase practice awareness around patient eligibility for
screening
++ Example: A practice could implement documentation processes or
procedures (for example, retrospective chart review, lung cancer screening
eligibility questionnaire) to improve patient lung cancer screening eligibility
data in the medical record
• Development of a patient outreach and activation plan consisting of educational
materials and resources for patients at high-risk of lung cancer for improved
patient engagement and decision-making.
++ Example: Providers or clinic staff could provide culturally and
linguistically appropriate patient-directed educational or care navigation
materials related to lung cancer screening, eligibility criteria for low-dose
computed tomography (LDCT), and the purpose and benefits of screening
++ Example: Providers or clinic staff could provide tools to prepare patients
for shared decision-making (SDM) clinical encounters and promote
patient/provider communication on lung cancer screening decision-making
• Establishment of a navigation program to improve uptake and adherence of lung
cancer screening and increase rates of LDCT referral completion.
++ Example: A practice could designate and train existing clinic staff or
hire an additional staff member to counsel patients on the importance of
lung cancer screening and refer them to existing resources (for example,
transportation assistance, translator, financial services, appointment
scheduling) to support ability to obtain LDCT
++ Example: A practice could create a process to follow up with referred
patients via telephone reminders or virtual notifications (for example, email,
patient charts)
Rationale:
Lung cancer is a leading cause of cancer-related deaths (21%) in the U.S., more than
colon and breast cancers combined.
1,2
While there are established guidelines for the
targeted use of LDCT for eligible patients, including particular criteria for age and
smoking history
3,4
, national lung cancer screening (LCS) rates are estimated at only 6.5%
as of 2020, compared to 63% for colon and 64% for breast cancer screening in 2019.
5,6
Evidence demonstrates that screening for lung cancer can improve patient outcomes. The
implementation of protocols that support enhanced documentation methods to identify
eligible patients for LCS has been found to increase early-stage lung cancer detection, in

one study by 24 percent (Russel et al., 2022).
7
Other studies have also shown benefits
from enhancing documentation to allow for more appropriate LCS:
• Following a retrospective chart review of existing patient smoking history and
implementation of a notification system for primary care providers (PCPs), a
community hospital identified 82 percent of patients who were eligible for LCS
not yet referred for screening, and of that, 31 percent completed an LDCT
referral.
8
• Implementation of a clinical reminder system in an EHR to refer eligible patients
to an LCS program, increased the number of LDCTs performed from 54 percent
to 79 percent in one Veterans Affairs (VA) medical center.
9
• EHR prompts generating a notification for providers to order LDCT screening
for eligible patients, increased documentation of complete patient smoking
history by 31 percent.
10
• Updating patient pack-year data in the EHR through a patient questionnaire on
smoking history and implementation of an EHR screen assessing pack-year data
in alignment with evidence-based guidelines; development of a patient outreach
and activation plan consisting of educational materials and resources for patients
at high-risk of lung cancer for improved patient engagement and decision-
making: among patients from a tobacco quit-line utilizing a video-based LCS
decision aid, 50 percent reported feeling well-prepared to make screening
decisions than with traditional materials and 68 percent reported being clear
about their values related to the harms and benefits of screening. Additionally,
patients using the decision-aid were more knowledgeable about LCS than
participants using standard educational materials at each follow-up assessment.
11
• A web-based tool developed for SDM led to improved patient engagement,
knowledge of LDCT, and preparation for SDM discussions among veterans in a
primary care setting.
12
• A computer tailored decision-support tool developed in alignment with the U.S.
Preventive Services Task Force (USPSTF) LCS guidelines, increased LCS
knowledge scores (2.33 mean change) and patient-perceived self-efficacy and
benefits of LCS in a community-based clinic.
13
Evidence also demonstrates the outcomes-improvement potential of the establishment of
a navigation program to improve uptake and adherence of LCS and to increase rates of
LDCT referral completion:
• In a patient navigator-led program including patient outreach to determine LCS
eligibility, SDM discussions, and appointment-scheduling with patient’s PCPs,
23.5 percent of LDCTs were performed in the intervention group compared to
8.6 percent in the control group among high-risk current smokers across 5
community health centers.
14
• An oncology nurse navigator-led provider education program resulted in
improved provider knowledge of LDCT and documented tobacco cessation
discussions, as well as increased LDCT ordering for eligible patients.
15
• A nurse-practitioner-led LCS clinic observed a 60 percent increase in the total
number of LDCTs conducted and 85 percent of stakeholder participants noted
the clinic was effective at addressing barriers to LCS.
16
Exemplifying the importance of efforts seeking to increase LCS and follow up, guidelines
and recommendations on expanded LCS eligibility criteria and SDM
requirements by the Centers for Medicare & Medicaid Services (CMS) have recently
been implemented:
• In 2021, leading LCS clinical guideline developers, including USPSTF and the
AAFP lowered the starting age of LCS with LDCT from 55 to 50 years and
patient pack-year history from 30 to 20 years, resulting in a larger eligible
screening population.
17,3
• In 2022, under the Medicare National Coverage Determination, CMS expanded
LCS eligibility criteria for age and pack-year history for beneficiaries receiving
LDCT in alignment with clinical guidelines (for example, USPSTF, AAFP).
• In addition to the expanded eligibility criteria, CMS requires a counseling and
SDM visit to be appropriately documented in a patient’s medical record and be
inclusive of determination of beneficiary eligibility and the use of one or more
decision-aids.
18

There is also published evidence that cost-effectiveness of care can improve as a result of
expanded eligibility screening. Research has found downstream effects linked to early
detection and prevention in LCS to be associated with long-term cost-effectiveness in
LCS care delivery:
• In 2021, the USPSTF expanded screening recommendations to include
individuals at an earlier age of 50 from 55 years, and minimum cumulative
smoking exposure from 30 to 20 pack-years. An economic evaluation of this
guideline change indicated that the updated recommendations were cost
effective compared to the earlier recommendations, with a mean incremental
cost-effectiveness ratio of $72,564 per quality-adjusted life-year (QALY)
gained.
19
• One study demonstrates the cost-savings of earlier diagnosis of lung cancer. In a
review of patients with non-small cell lung cancer (NSCLC), it was revealed that
the total per-patient per-month health care costs after diagnosis were
significantly higher among those diagnosed at a Stage IV and lower among
those diagnosed at Stage I ($7,239 Stage I, $9,484 Stage II, $11,193 Stage IIIa,
$17,415 Stage IIIb, and $21,441 Stage IV).
20
• One modeling study of costs and outcomes associated with lung cancer found
that implementation of a patient navigation program is cost-effective for lung
cancer patients in Medicare, including that the program was cost-effective at a
probability of 0.91 at $100,000/QALY.
21
• Given the significant impact of lung cancer in the U.S.--and the effectiveness of
LCS and related interventions--as noted above, this activity has a high likelihood
of making a positive impact on outcomes for eligible clinicians’ patients.
New Improvement Activity
Proposed Activity
ID:
IA_PM_XX
Proposed
Subcategory:
Population Management
Proposed Activity
Title:
Save a Million Hearts: Standardization of Approach to Screening and Treatment for
Cardiovascular Disease Risk
Proposed Activity
Description:
Implement standardized, evidence-based cardiovascular disease risk assessment and care
management for a defined population in the clinician’s practice.
The clinician or clinician group will apply standardized risk assessment and care
management to a broad, clinician-defined patient population in the practice. The
population can be defined by 1) patient age and/or atherosclerotic cardiovascular disease
(ASCVD) risk factors; or 2) the constraints of the risk assessment tool (for example, the
American College of Cardiology (ACC)/American Heart Association (AHA) ASCVD
Risk Calculator is validated for patients over age 40).
The results of screening and the plan for treatment and follow up will be documented
using a standardized method in the patient’s medical record. Care management plan and
follow up intervals will be influenced by the degree of patient risk.
Cardiovascular care management should be defined by risk assessment and lead to the
development of individualized care plans with specific goals. Shared decision making
should be part of the development of every patient care plan.
Rationale:
Heart disease is the leading cause of death in the United States. Stroke is the fifth most
common cause of death in the United States.
22
This activity is informed by the results of
the CMS Innovation Center Million Hearts Model, which included initial ASCVD
assessment as well as cardiovascular care management.
23
The Million Hearts Model used
the ACC/AHA ASCVD Risk Calculator: ASCVD Risk Estimator (acc.org).
24
The proposed new activity supports improved identification and treatment of patients at
risk for ASCVD, and would expand on the work of the model in two ways: (1) increasing
flexibility in requirements, allowing more clinician specialties to participate, along with
increased flexibility in risk assessment to fit the needs of attesting clinicians and their
patient populations; (2) requiring the use of structured documentation of risk factors and
associated treatment plans with the aim of addressing all risk factors directly.

This activity accommodates the use of any evidence-based approach to risk evaluation
and patient care management that is implemented using standardized methods and across
an entire patient population. The ASCVD Risk Estimator used by the Million Hearts
Model is evidence based and simple to implement but may not be appropriate for every
practice scenario or patient population. There is strong published evidence validating the
ACC/AHA ASCVD Risk Calculator; describing optimal care for coronary artery disease
(including making comparisons to European Union best practices); and providing
guidelines for primary prevention of coronary artery disease.
25,26,27,28
1
American Cancer Society. (2021) Can Lung Cancer Be Found Early?, https://www.cancer.org/cancer/lung-
cancer/detection-diagnosis-staging/detection.html.
2
NIH National Cancer Institute. Cancer Stat Facts: Lung and Bronchus Cancer. (2022).
https://seer.cancer.gov/statfacts/html/lungb.html.
3
American Academy of Family Physicians (AAFP). AAFP Updates Recommendation on Lung Cancer Screening.
(2021). https://www.aafp.org/news/health-of-the-public/20210406lungcancer.html.
4
National Comprehensive Cancer Network. NCCN Guidelines for Patients. (2020) Lung Cancer Screening.
https://www.nccn.org/patients/guidelines/content/PDF/lung_screening-patient.pdf.
5
Fedewa, S. A., Bandi, P., Smith, R. A., Silvestri, G. A., & Jemal, A. (2022). Lung Cancer Screening Rates During the
COVID-19 Pandemic. Chest, 161(2), 586–589. https://doi.org/10.1016/j.chest.2021.07.030.
6
National Cancer Institute (2023). Lung Cancer Screening. https://progressreport.cancer.gov/detection/lung_cancer;
accessed May 2023, last updated March 2024.
7
Russel, C., McNeill, M. (2022). Improving Lung Cancer Screening Rates Through and Evidence-Based Electronic
Health Record Smoking History. Journal of nursing care quality, 37(3), 263–268.
https://doi.org/10.1097/NCQ.0000000000000623.
8
Sheppard, R., Joseph, J., Achi, S., Ayinla, R. (2021). Bridging the Gap to Improve the Rate of Lung Cancer
Screening in a Minority Population: A Quality Improvement Initiative in a Community Hospital. American
College of Chest Physicians. https://doi.org/10.1016/j.chest.2021.07.1474.
9
Miotke, L., York, A., Bowles, A., Lewis-Williams, F., BeckJ E. (2022). Quality Initiative to Improve Lung Cancer
Screening in a Veterans Affairs Medical Center. Journal of Clinical Oncology, 40:28_suppl, 108-108.
https://doi.org/10.1200/JCO.2022.40.28.
10
Puskoor, S., Mooneyham, J., Nguyen., J, Todd, R., Germanos G., and Surapaneni, R. (2022). Improving Lung
Cancer Screening in a Community Healthcare System by Electronic Health Record (EHR) Optimization.
Journal of Clinical Oncology, 40:28_suppl, 437-437. https://doi.org/10.1200/JCO.2022.40.28.
11
Volk, R. J., Lowenstein, L. M., Leal, V. B., Escoto, K. H., Cantor, S. B., Munden, R. F., Rabius, V. A., Bailey, L.,
Cinciripini, P. M., Lin, H., Housten, A. J., Luckett, P. G., Esparza, A., Godoy, M. C., & Bevers, T. B. (2020).
Effect of a Patient Decision Aid on Lung Cancer Screening Decision-Making by Persons Who Smoke: A
Randomized Clinical Trial. JAMA network open, 3(1), e1920362.
https://doi.org/10.1001/jamanetworkopen.2019.20362.
12
Schapira, M. M., Chhatre, S., Prigge, J. M., Meline, J., Kaminstein, D., Rodriguez, K. L., Fraenkel, L., Kravetz, J.
D., Whittle, J., Bastian, L. A., Vachani, A., Akers, S., Schrand, S., Ibarra, J. V., & Asan, O. (2022). A
Veteran-Centric Web-Based Decision Aid for Lung Cancer Screening: Usability Analysis. JMIR formative
research, 6(4), e29039. https://formative.jmir.org/2022/4/e29039.
13
Carter-Harris, L., Comer, R. S., Slaven Ii, J. E., Monahan, P. O., Vode, E., Hanna, N. H., Ceppa, D. P., & Rawl, S.
M. (2020). Computer-Tailored Decision Support Tool for Lung Cancer Screening: Community-Based Pilot
Randomized Controlled Trial. Journal of medical Internet research, 22(11), e17050.
https://pubmed.ncbi.nlm.nih.gov/33141096/.
14
Percac-Lima, S., Ashburner, J. M., Rigotti, N. A., Park, E. R., Chang, Y., Kuchukhidze, S., & Atlas, S. J. (2018).
Patient navigation for lung cancer screening among current smokers in community health centers a
randomized controlled trial. Cancer medicine, 7(3), 894–902. https://doi.org/10.1002/cam4.1297.
15
Watson, J., Broome, M. E., & Schneider, S. M. (2020). Low-Dose Computed Tomography: Effects of Oncology
Nurse Navigation on Lung Cancer Screening. Clinical journal of oncology nursing, 24(4), 421–429.
https://doi.org/10.1188/20.CJON.421-429.
16
Schlabach, T., King, T. S., Browning, K. K., & Kue, J. (2022). Nurse practitioner-led lung cancer screening clinic:
An evidence-based quality improvement evaluation. Worldviews on evidence-based nursing, 19(3), 227–234.
https://doi.org/10.1111/wvn.12578.
17
U.S. Preventive Services Task Force (USPSTF). Final Recommendation Statement Lung Cancer: Screening. (2021).
https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening.
18
Centers for Medicare and Medicaid Services (CMS). (2022). Screening for Lung Cancer with Low-Dose Computed
Tomography (LDCT). https://www.cms.gov/medicare-coverage-database/view/ncacal-
decisionmemo.aspx?proposed=N&ncaid=304#:~:text=Beneficiary%20eligibility%20cri
teria%3A,1%20pack%20%3D%2020%20cigarettes)%3B.
19
Toumazis, I., de Nijs, K., Cao, P., Bastani, M., Munshi, V., Ten Haaf, K., Jeon, J., Gazelle, G. S., Feuer, E. J., de
Koning, H. J., Meza, R., Kong, C. Y., Han, S. S., & Plevritis, S. K. (2021). Cost-effectiveness Evaluation of
the 2021 US Preventive Services Task Force Recommendation for Lung Cancer Screening. JAMA oncology,
7(12), 1833–1842. https://doi.org/10.1001/jamaoncol.2021.4942.
20
Gildea, T. R., DaCosta Byfield, S., Hogarth, D. K., Wilson, D. S., & Quinn, C. C. (2017). A retrospective analysis of
delays in the diagnosis of lung cancer and associated costs. ClinicoEconomics and outcomes research :
CEOR, 9, 261–269. https://doi.org/10.2147/CEOR.S132259.
21
Shih, Y. C., Chien, C. R., Moguel, R., Hernandez, M., Hajek, R. A., & Jones, L. A. (2016). Cost-Effectiveness
Analysis of a Capitated Patient Navigation Program for Medicare Beneficiaries with Lung Cancer. Health
services research, 51(2), 746–767. https://doi.org/10.1111/1475-6773.12333.
22
Centers for Disease Control and Prevention (n.d.). Leading Causes of Death. Retrieved from:
https://www.cms.gov/priorities/innovation/data-and-reports/2023/mhcvdrrm-finalannevalrpt-fg. Last
reviewed January 17, 2024.
23
American College of Cardiology (n.d.). Million Hearts Cardiovascular Disease Reduction Model (Million Hearts
Model). https://www.cms.gov/priorities/innovation/data-and-reports/2023/mhcvdrrm-finalannevalrpt-fg.
24
American College of Cardiology (n.d.). ASCVD Risk Estimator:
https://tools.acc.org/ldl/ascvd_risk_estimator/index.html#!/calulate/estimator/.
25
Muntner, P., Colantonio, L. D., Cushman, M., Goff, D. C., Howard, G., Howard, V. J., ... & Safford, M. M. (2014).
Validation of the atherosclerotic cardiovascular disease Pooled Cohort risk equations. Jama, 311(14), 1406-
1415.
26
Rodríguez-Ariza, C. D., Cabrera-Villamizar, A., Rodríguez-Pulido, A. L., Callegari, S., Ossa Rodríguez, N. A.,
Pinilla-Roncancio, M., ... & Sánchez-Vallejo, C. A. (2023). External validation of the ACC/AHA ASCVD
risk score in a Colombian population cohort. Scientific Reports, 13(1), 6139.
27
Reed, S. C., Dhir, N., &
Widmer, R. J. (2022, September). Optimal cardiovascular medical therapy: current guidelines and new
developments. In Baylor University Medical Center Proceedings (Vol. 35, No. 5, pp. 636-642). Taylor &
Francis.
28
Arnett, D. K., Blumenthal, R. S., Albert, M. A., Buroker, A. B., Goldberger, Z. D., Hahn, E. J., ... & Ziaeian, B.
(2019). 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the
American College of Cardiology/American Heart Association Task Force on Clinical Practice
Guidelines. Circulation, 140(11), e596-e646.

TABLE B: Changes to Previously Adopted Improvement Activities
for the CY 2025 Performance Period/2027 MIPS Payment Year and for Future Years
In this proposed rule, we are proposing to modify two previously finalized improvement
activities for the CY 2025 performance period/2027 MIPS payment year and future years.
These proposals are discussed in section IV.A.4.e.(3)(b)(iii) of this proposed rule and in more
detail below.
Current Improvement Activity
Current Activity ID:
IA_ERP_6
Current Subcategory:
Emergency Response & Preparedness
Current Activity Title:
COVID-19 Vaccine Achievement for Practice Staff
Current Activity
Description:
Demonstrate that the MIPS eligible clinician’s practice has maintained or achieved a
rate of 100 percent of office staff staying up to date with COVID vaccines according to
the Centers for Disease Control and Prevention.
29
Please note that those who are
determined to have a medical contraindication specified by CDC recommendations are
excluded from this activity.
Current Weighting:
Medium
Proposed Activity
ID:
IA_PM_XX
Proposed
Subcategory:
Population Management
Proposed Revised
Activity Title:
Vaccine Achievement for Practice Staff: COVID-19, Influenza, and Hepatitis B
Proposed Revised
Activity Description:
Demonstrate that the MIPS eligible clinician’s practice has achieved and/or maintained
a vaccination rate of 60 percent of clinical practice staff for COVID-19, and 80 percent
for influenza. Demonstrate vaccination, immunity, or non-responder status to hepatitis
B for 95 percent of clinical practice staff. Vaccination recommendations are from
Centers for Disease Control and Prevention; staff with contraindications to the
vaccinations, as determined by the CDC, are excluded from the requirements.
Vaccines and Immunizations | CDC.
Proposed Change and
Rationale:
Adjusting the target goals for this activity would align with the latest CDC
recommendations and feedback received indicates that the proposal could increase the
activity’s utilization. Additionally, we are expanding the focus of this activity to include
influenza and hepatitis B to highlight the importance of staff vaccination for vaccine-
preventable diseases prevalent today. We are also proposing a change in this activity’s
subcategory, from Emergency Response & Preparedness to Population Management, to
emphasize that staff vaccination is a long-term strategy in reducing morbidity and
mortality rates for these diseases.
Current Improvement Activity
Current Activity ID:
IA_BE_4
Current Subcategory:
Beneficiary Engagement
Current Activity Title:
Engagement of patients through implementation of improvements in patient portal
Current Activity
Description:
To receive credit for this activity, MIPS eligible clinicians must provide access to an
enhanced patient/caregiver portal that allows users (patients or caregivers and their
clinicians) to engage in bidirectional information exchange. The primary use of this
portal should be clinical and not administrative. Examples of the use of such a portal
include but are not limited to: brief patient reevaluation by messaging; communication
about test results and follow up; communication about medication adherence, side
effects, and refills; blood pressure management for a patient with hypertension; blood
sugar management for a patient with diabetes; or any relevant acute or chronic disease
management.
Current Weighting:
Medium
Proposed Activity
ID:
IA_BE_4
Proposed
Subcategory:
Beneficiary Engagement
Proposed Revised
Activity Title:
Engagement of Patients through Implementation of New Patient Portal

Proposed Revised
Activity Description:
To receive credit for this activity, MIPS eligible clinicians must implement and provide
access to a new patient/caregiver portal that allows users (patients or caregivers and
their clinicians) to engage in bidirectional information exchange. The primary use of
this portal should be clinical and not administrative. Examples of the use of such a
portal include, but are not limited to, the following: brief patient reevaluation by
messaging; communication about test results and follow up; communication about
medication adherence, side effects, and refills; blood pressure management for a patient
with hypertension; blood sugar management for a patient with diabetes; and/or any
relevant acute or chronic disease management.
Proposed Change and
Rationale:
We are proposing to modify this activity’s description and its validation criteria to
specify the implementation of a new patient/caregiver portal by clinicians who were not
previously using a patient portal. This activity was originally created during a time of
transition to EHRs to encourage electronic information exchange. It has become
standard practice to use patient portals; therefore, the activity is likely no longer driving
improvement among clinicians who have already implemented a patient portal. This
activity has been highly utilized year over year and continues to be in the top ten
activities reported. Limiting the activity to clinicians that implement new patient portals
in practices that previously did not use them would refocus the measure on its original
purpose and encourage clinicians who have previously implemented patient portals to
report other improvement activities that may offer meaningful opportunities for
improvement.
29
Centers for Disease Control and Prevention (2024). Stay Up to Date with COVID-19 Vaccines. Last Updated
April 25, 2024. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/stay-up-to-date.html

TABLE C: Improvement Activities Proposed for Removal
for the CY 2025 Performance Period/20267MIPS Payment Year and for Future Years
In this proposed rule, we are proposing to remove eight previously finalized improvement
activities beginning with the CY 2025 performance period/2027 MIPS payment year. These
proposals are discussed in section IV.A.4.e.(3)(b)(iii) of this proposed rule and in more detail
below; activity removal factors are discussed in the CY 2020 PFS final rule (84 FR 62568
through 63563).
Current Improvement Activity
Current Activity ID:
IA_ EPA_1
Current Subcategory:
Expanded Practice Access
Current Activity Title:
Provide 24/7 Access to MIPS Eligible Clinicians or Groups Who Have Real-Time
Access to Patient's Medical Record
Current Activity
Description:
Provide 24/7 access to MIPS eligible clinicians, groups, or care teams for advice about
urgent care (for example, MIPS eligible clinician and care team access to medical
record, cross-coverage with access to medical record, or protocol-driven nurse line with
access to medical record) that could include one or more of the following:
• Expanded hours in evenings and weekends with access to the patient medical
record for example, coordinate with small practices to provide alternate hour office
visits and urgent care);
• Use of alternatives to increase access to care team by MIPS eligible clinicians and
groups, such as e-visits, phone visits, group visits, home visits and alternate locations
(for example, senior centers and assisted living centers); and/or
• Provision of same-day or next-day access to a MIPS eligible clinician, group or
care team when needed for urgent care or transition management.
Current Weighting:
High
Removal Rationale:
We are proposing to remove this activity under removal factor seven, activity is
obsolete; this activity was created, in part, to incentivize utilization of EHRs to increase
access to clinicians in off hours and decrease emergency room (ER) visits. Today,
EHRs are highly utilized, and this activity has become standard of care. This is
supported by the fact that this activity continues to be in the top ten activities reported,
indicating that it is overutilized and that the clinical practice improvement promoted by
the activity has been achieved. We are proposing the removal of this activity in the
context of our regular review of the Inventory. In conducting this review recently, we
concluded that the goal of this activity has been widely achieved by eligible clinicians
and practices.
Current Improvement Activity
Current Activity ID:
IA_PM_12
Current Subcategory:
Population Management
Current Activity Title:
Population empanelment
Current Activity
Description:
Empanel (assign responsibility for) the total population, linking each patient to a MIPS
eligible clinician or group or care team.
Empanelment is a series of processes that assign each active patient to a MIPS eligible
clinician or group and/or care team, confirm assignment with patients and clinicians,
and use the resultant patient panels as a foundation for individual patient and population
health management.
Empanelment identifies the patients and population for whom the MIPS eligible
clinician or group and/or care team is responsible and is the foundation for the
relationship continuity between patient and MIPS eligible clinician or group /care team
that is at the heart of comprehensive primary care. Effective empanelment requires
identification of the “active population” of the practice: those patients who identify and
use your practice as a source for primary care. There are many ways to define “active
patients” operationally, but generally, the definition of “active patients” includes
patients who have sought care within the last 24 to 36 months, allowing inclusion of
younger patients who have minimal acute or preventive health care.
Current Weighting:
Medium

Removal Rationale:
We propose to remove this activity under removal factor seven, activity is obsolete; this
activity was designed in the early years of the MIPS program to highlight the
importance of patient population empanelment to drive patient-centered care and, over
time, to drive quality improvement. Empanelment is now more widely accepted and/or
used as an option to drive and/or measure comprehensive care, and this activity has no
requirement for implementation or improvement beyond the empanelment; therefore,
we are recommending its removal.
Current Improvement Activity
Current Activity ID:
IA_CC_1
Current Subcategory:
Care Coordination
Current Activity Title:
Implementation of use of specialist reports back to referring clinician or group to close
referral loop
Current Activity
Description:
Performance of regular practices that include providing specialist reports back to the
referring individual MIPS eligible clinician or group to close the referral loop or where
the referring individual MIPS eligible clinician or group initiates regular inquiries to
specialist for specialist reports which could be documented or noted in the EHR
technology.
Current Weighting:
Medium
Removal Rationale:
We propose to remove this activity under removal factor one, this activity is
duplicative, and factor five, this activity does not align with the quality, cost, or
Promoting Interoperability performance categories. This activity provides credit for
ensuring that consultation reports are communicated between an ordering and a
consulting provider, and this is now standard of care. Also, this concept is redundant
with some quality and Quality Clinical Data Registry (QCDR) measures, including
QM#374. Our recommendation for removal is supported by the fact that this activity
continues to be in the top ten activities reported, indicating that it is overutilized and
that the clinical practice improvement promoted by the activity has been achieved. We
are proposing the removal of this activity in the context of our regular review of the
Inventory. In conducting this review recently, we concluded that the goal of this activity
has been widely achieved by eligible clinicians and practices.
Current Improvement Activity
Current Activity ID:
IA_CC_2
Current Subcategory:
Care Coordination
Current Activity Title:
Implementation of improvements that contribute to more timely communication of test
results
Current Activity
Description:
Timely communication of test results defined as timely identification of abnormal test
results with timely follow-up.
Current Weighting:
Medium
Removal Rationale:
We propose to remove this activity under removal factor seven, activity is obsolete.
This activity was created, in part, to encourage strategies for timely communication and
to improve upon those strategies. This process has become widely used with the use of
EHRs and the adoption of patient portals, and is now standard of care. This is supported
by the fact that this activity continues to be in the top ten activities reported, indicating
that it is overutilized. We are proposing the removal of this activity in the context of our
regular review of the Inventory. In conducting this review recently, we concluded that
the goal of this activity has been widely achieved by eligible clinicians and practices.
Current Improvement Activity
Current Activity ID:
IA_ERP_4
Current Subcategory:
Emergency Response and Preparedness
Current Activity Title:
Implementation of a Personal Protective Equipment (PPE) Plan
Current Activity
Description:
Implement a plan to acquire, store, maintain, and replenish supplies of personal
protective equipment (PPE) for all clinicians or other staff who are in physical
proximity to patients.
In accordance with guidance from the Centers for Disease Control and Prevention
(CDC) the PPE plan should address:
• Conventional capacity: PPE controls that should be implemented in general
infection prevention and control plans in healthcare settings, including training in
proper PPE use.
• Contingency capacity: actions that may be used temporarily during periods of
expected PPE shortages.

• Crisis capacity: strategies that may need to be considered during periods of known
PPE shortages.
The PPE plan should address all of the following types of PPE:
• Standard precautions (for example, hand hygiene, prevention of needle-stick or
sharps injuries, safe waste management, cleaning and disinfection of the environment)
• Eye protection
• Gowns (including coveralls or aprons)
• Gloves
• Facemasks
• Respirators (including N95 respirators)
Current Weighting:
Medium
Removal Rationale:
We propose to remove this activity under removal factor seven, activity is obsolete;
since the COVID-19 pandemic, most clinicians are well prepared in PPE safety, as PPE
enhancements have been made throughout patient care settings. It is unlikely that this
activity will drive new improvements. We acknowledge the ongoing importance of
PPE, and will work to ensure that, going forward, improvement activities that support
continued enhancement and maintenance of PPE protocols are reflected in MIPS.
Current Improvement Activity
Current Activity ID:
IA_ERP_5
Current Subcategory:
Emergency Response and Preparedness
Current Activity Title:
Implementation of a Laboratory Preparedness Plan
Current Activity
Description:
Develop, implement, update, and maintain a preparedness plan for a laboratory intended
to support continued or expanded patient care during COVID-19 or another public
health emergency. The plan should address how the laboratory would maintain or
expand patient access to health care services to improve beneficiary health outcomes
and reduce healthcare disparities.
For laboratories without a preparedness plan, MIPS eligible clinicians would meet with
stakeholders, record minutes, and document a preparedness plan, as needed. The
laboratory must then implement the steps identified in the plan and maintain them.
For laboratories with existing preparedness plans, MIPS eligible clinicians should
review, revise, or update the plan as necessary to meet the needs of the current PHE,
implement new procedures, and maintain the plan.
Maintenance of the plan in this activity could include additional hazard assessments,
drills, training, and/or developing checklists to facilitate execution of the plan.
Participation in debriefings to evaluate the effectiveness of plans are additional
examples of engagement in this activity.
Current Weighting:
Medium
Removal Rationale:
We propose to remove this activity under removal factor seven, activity is obsolete;
since the COVID-19 pandemic, most clinicians are now well prepared in COVID-19-
related patient safety and laboratory-preparedness enhancements have been made
throughout patient care settings. It is unlikely that this activity will drive new
improvements. We acknowledge the ongoing importance of laboratory preparedness,
and will work to ensure that, going forward, improvement activities that support
continued enhancement and maintenance of lab preparedness protocols are reflected in
MIPS.
Current Improvement Activity
Current Activity ID:
IA_BMH_8
Current Subcategory:
Behavioral and Mental Health
Current Activity Title:
Electronic Health Record Enhancements for BH data capture
Current Activity
Description:
Enhancements to an electronic health record to capture additional data on behavioral
health (BH) populations and use that data for additional decision-making purposes (for
example, capture of additional BH data results in additional depression screening for at-
risk patient not previously identified).
Current Weighting:
Medium
Removal Rationale:
We propose to remove this activity under removal factor two, there is an alternative
activity with a stronger relationship to quality care or improvements in clinical practice.
This activity was created, in part, to assist in the transition from paper charts to EHRs.
While the use of EHRs is now highly prevalent and has become part of current basic
standards of care, there is still much progress to be made in terms of adoption and use

of EHRs and other health information technologies in a behavioral health context. This
activity, though, allows attestation with a low level of effort and with vague
requirements related to clinical outcomes. Because there are other, more potentially
impactful, behavioral health activities in the current Inventory, we are recommending
that this activity be removed. IA_BMH_7, Implementation of Integrated Patient
Centered Behavioral Health Model, which includes ‘use of a registry or health
information technology functionality to support active care management and outreach
to patients in treatment,’ is a strong alternative activity. We also intend, in future
rulemaking, to develop a new activity (or to modify an existing activity) to promote the
effective use of health information technologies in behavioral health.
Current Improvement Activity
Current Activity ID:
IA_PSPA_27
Current Subcategory:
Patient Safety and Practice Assessment
Current Activity Title:
Invasive Procedure or Surgery Anticoagulation Medication Management
Current Activity
Description:
For an anticoagulated patient undergoing a planned invasive procedure for which
interruption in anticoagulation is anticipated, including patients taking vitamin K
antagonists (warfarin), target specific oral anticoagulants (such as apixaban, dabigatran,
and rivaroxaban), and heparins/low molecular weight heparins, documentation,
including through the use of electronic tools, that the plan for anticoagulation
management in the periprocedural period was discussed with the patient and with the
clinician responsible for managing the patient’s anticoagulation. Elements of the plan
should include the following: discontinuation, resumption, and, if applicable, bridging,
laboratory monitoring, and management of concomitant antithrombotic medications
(such as antiplatelets and nonsteroidal anti-inflammatory drugs (NSAIDs)). An invasive
or surgical procedure is defined as a procedure in which skin or mucous membranes and
connective tissue are incised, or an instrument is introduced through a natural body
orifice.
Current Weighting:
Medium
Removal Rationale:
We propose to remove this activity under removal factor one, this activity is
duplicative. We recommend removal of this activity as its focus is duplicative with
IA_CC_15: PSH [Perioperative-Surgical Home] Care Coordination. IA_CC_15
requires coordination of patient care through the perioperative period and includes
anticoagulant management as one part of its requirements. This activity, IA_PSPA_27,
is more tightly focused in an area that is high risk and, therefore, is not likely changing
clinical practice widely. We acknowledge the ongoing importance of care coordination
and medication management as a patient safety goal, and will work to ensure that, going
forward, improvement activities that support enhancement and maintenance of
medication management activities (for example, anticoagulation) are reflected in MIPS.

APPENDIX 3: MVP INVENTORY
MVP Development: Background
In the CY 2021 PFS final rule (85 FR 84849 through 84854), the CY 2022 PFS final rule (86 FR 65998 through
66031), and the CY 2023 PFS final rule (87 FR 70210 through 70211) we finalized a set of criteria to use in the
development of MVPs, including MVP reporting requirements, MVP maintenance, and the selection of measures
and activities within an MVP.
This appendix contains two groups of proposed MVP tables: Group A: proposed new MVPs and Group B: proposed
modifications to previously finalized MVPs. Group A includes six new proposed MVPs. Group B includes 16
previously finalized MVPs with proposed modifications.
Each MVP includes measures and activities from the quality performance category, improvement activities
performance category, and the cost performance category relevant to the clinical theme of the MVP. Each MVP also
includes a foundational layer comprised of population health measures and Promoting Interoperability performance
category measures.
MVP Development: Performance Category Sources
The MVP tables contain a set of MIPS quality measures, QCDR measures (as applicable), improvement activities,
cost measures, and foundational measures based on clinical topics. For further reference, the sources of the measures
and activities in the MVP tables are as follows:
● Existing MIPS quality measures are in the 2024 MIPS Quality Measures List on the Quality Payment
Program website.
1017
See Appendix 1: MIPS Quality Measures of this proposed rule for any proposed additions
(Table Group A), proposed removals (Table Group B), or proposed modifications to existing quality measures
(Table Groups D and DD).
● Existing QCDR measures are based on the most recent publication of the 2024 QCDR Measure
Specification file, located on the Quality Payment Program website.
1018
We plan to modify the list of 2025 QCDR
measures around December 2024.
● Improvement activities are in the 2024 Improvement Activities Inventory and the 2024 MIPS Data
Validation Criteria, located on the Quality Payment Program website.
1019
See Appendix 2: Improvement Activities
of this proposed rule for any proposed additions (Table Group A), proposed modifications to existing improvement
activities (Table Group B), or proposed removals (Table Group C).
● Existing cost measures are in the 2024 Cost Measures Inventory.
1020
See section IV.A.4.e.(2) of this
proposed rule for any proposed removals, additions, or modifications to existing cost measures.
● For further details on the population health measures (attributed to the Quality Performance Category)
included in the foundational layer, see the CY 2022 PFS final rule (86 FR 65408 through 65409).
● Existing Promoting Interoperability measures adopted in prior rulemaking and included in the
foundational layer are located on the Quality Payment Program website.
1021
There were policy updates to the
Promoting Interoperability performance category but no proposed new, modifications, or removed Promoting
Interoperability measures for the CY 2025 performance period/2027 MIPS payment year (for policy updates on this
category see section IV.A.4.e. of this proposed rule.
MVP Development: Improvement Activity Policy Update and Global Inclusion of an Improvement Activity
• We are proposing to eliminate the weighting from improvement activities and provide full credit for the
improvement activities performance category when an MVP participant attests to one improvement activity
option within the selected MVP. See section IV.A.4.e.(3)(b)(iv)of this proposed rule for detailed
1017
See the 2024 MIPS Quality Measures List: https://qpp-cm-prod-
content.s3.amazonaws.com/uploads/2632/2024%20MIPS%20Quality%20Measures%20List.xlsx.
1018
See https://qpp-cm-prod-content.s3.amazonaws.com/uploads/2617/2024%20QCDR%20Measure%20Specifications.xlsx for
QCDR measures.
1019
See the 2024 Improvement Activities Inventory: https://qpp-cm-prod-
content.s3.amazonaws.com/uploads/2644/2024ImprovementActivitiesInv.zip and 2024 MIPS Data Validation Criteria:
https://qpp-cm-prod-content.s3.amazonaws.com/uploads/2666/2024MIPSDataValidationCriteria.zip.
for improvement activity details.
1020
See the 2024 Cost Measures Inventory: https://qpp.cms.gov/mips/explore-measures?tab=costMeasures&py=2024.
1021
See the 2024 Promoting Interoperability Measure Specifications: https://qpp-cm-prod-
content.s3.amazonaws.com/uploads/2223/2024%20MIPS%20Promoting%20Interoperability%20Measure%20Specifications.zip
for Promoting Interoperability measure details.

information regarding the proposed removal of weighting from improvement activities and for detailed
information regarding our proposed reporting requirement changes for improvement activities.
• We are proposing to modify IA_ERP_6: COVID-19 Vaccine Achievement for Practice Staff to expand the
focus and importance of vaccination status to drive improvement across the practice. We are proposing to add
the proposed modified IA_ERP_6 to all new and previously finalized MVPs because of the importance of
vaccination status in practice settings. See Appendix 2, Improvement Activities: Table B of this proposed rule
for detailed information regarding the proposed modifications to IA_ERP_6: COVID-19 Vaccine
Achievement for Practice Staff, including a proposed activity ID and title update.
MVP Table Symbol Information and Definitions
Please note the following symbols and definitions used within the MVP tables in the Group A and Group B tables
below:
● Quality measures, improvement activities, and cost measures proposed for addition to a previously
finalized MVP are identified with a plus sign (+) within the Group B MVP tables in this appendix.
● New quality measures, improvement activities, and cost measures proposed for inclusion in MIPS
beginning with the CY 2025 performance period/2027 MIPS payment year and future years are identified with a
caret symbol (^). See Appendix 1, MIPS Quality Measures: Table Group A of this proposed rule for further
information regarding new MIPS quality measures. See Appendix 2: Improvement Activities: Table A of this
proposed rule for further information regarding new improvement activities. See section IV.A.4.e.(2)(a)(iii)of this
proposed rule for further information regarding new cost measures.
● Existing measures and improvement activities with proposed revisions are identified with a single
asterisk (*). See Appendix 1, MIPS Quality Measures: Table Group D of this proposed rule for further information
regarding proposed revisions to MIPS quality measures. See Appendix 2: Improvement Activities: Table B of this
proposed rule for further information regarding proposed revisions to improvement activities. See section
IV.A.4.e.(2)(a)(vi) and IV.A.4.e.(2)(a)(vii)of this proposed rule for further information regarding proposed revisions
to cost measures. We intend to include existing measures or activities with proposed revisions in MVPs (as
applicable) regardless of whether the proposed revisions are finalized beginning with the CY 2025 performance
period/2027 MIPS payment year.
● Quality measures and improvement activities identified with a double asterisk (**) can only be submitted
when included in an MVP.
● Quality measures considered high priority (as defined in § 414.1305) are identified with a single
exclamation point (!) while outcome measures (as defined in § 414.1305) are identified with a double exclamation
point (!!). Further details of these types of measures are in the CMS Measures Management System Hub.
1022
● QCDR measures identified with a pound sign (#) indicate testing data is still pending and due on or
before September 1, 2024. We refer readers to the CY 2022 PFS final rule for additional details regarding
requirements for QCDR measures considered for an MVP (86 FR 65407 through 65408).
● Quality measures and improvement activities that include a health equity component are identified with a
tilde (~) within the MVP table.
● IA_PCMH: Electronic submission of Patient Centered Medical Home accreditation is identified with a
percent (%) to indicate attestation to this improvement activity provides full credit for the improvement activity
performance category within an MVP.
● Quality measure collection types are identified in parentheses after each quality measure title, and
improvement activity medium/high weight designations are identified in parentheses after each improvement
activity
Group A: New MVPs Proposed for the CY 2025 Performance Period/2027 MIPS Payment
Year and Future Years
A.1 Complete Ophthalmologic Care MVP
The proposed Complete Ophthalmologic Care MVP assesses meaningful outcomes in cataract, glaucoma, retinal detachment, and
broadly applicable ocular care. This MVP would be most applicable to clinicians who treat patients within the practice of
ophthalmology and optometry.
Quality Measures
We are proposing to include 18 MIPS quality measures and 6 QCDR measures within the quality performance category of this
MVP, which are specific to the clinical topic of ocular care by assessing ocular health and treatment of disorders attributed to
diabetes related disease, glaucoma, retinal detachment, and cataracts. We reviewed the MIPS quality measure inventory and
1022
See https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/Downloads/Blueprint.pdf.

considered feedback received during the 2025 MVP candidate feedback period to determine which quality measures best
represent the clinical topic of this MVP.
The following quality measures provide a meaningful and comprehensive assessment of the clinical care for clinicians who
specialize in ocular care:
• Q012: Primary Open-Angle Glaucoma (POAG): Optic Nerve Evaluation: This MIPS quality measure evaluates
changes in the optic nerve which define the progression and worsening of glaucoma disease status.
• Q019: Diabetic Retinopathy: Communication with the Physician Managing Ongoing Diabetes Care: This MIPS quality
measure focuses on the communication between the primary physician managing ongoing care and the physician
performing the dilated macular or fundus exam on patients with diabetic retinopathy.
• Q117: Diabetes: Eye Exam: This MIPS quality measure supports eye screening for diabetic retinal disease.
• Q141: Primary Open-Angle Glaucoma (POAG): Reduction of Intraocular Pressure (IOP) by 20% OR Documentation
of a Plan of Care: This MIPS quality measure focuses on glaucoma treatment and follow up, ensuring the IOP is within
a range at which visual field loss is unlikely to significantly reduce a patient’s health-related quality of life over their
lifetime.
• Q191: Cataracts: 20/40 or Better Visual Acuity within 90 Days Following Cataract Surgery: This MIPS quality
measure evaluates visual acuity as a surgical outcome following cataract surgery.
• Q303: Cataracts: Improvement in Patient’s Visual Function within 90 Days Following Cataract Surgery: This MIPS
quality measure looks for improvement in visual function following cataract surgery.
• Q304: Cataracts: Patient Satisfaction within 90 Days Following Cataract Surgery: This MIPS quality measure assesses
patient satisfaction following cataract surgery. Patient satisfaction is a valuable performance indicator for measuring
quality of care delivered by clinicians providing cataract surgery. Patient satisfaction is an assessment of the patient’s
experience with the care process delivered by the clinician and health care services.
• Q384: Adult Primary Rhegmatogenous Retinal Detachment Surgery: No Return to the Operating Room Within 90
Days of Surgery: This MIPS quality measure assesses for successful surgical procedures by evaluating if patients
required a return to the operation room or not.
• Q385: Adult Primary Rhegmatogenous Retinal Detachment Surgery: Visual Acuity Improvement Within 90 Days of
Surgery: This MIPS quality measure assesses for successful surgical procedures by evaluating if patients showed
improvement of visual acuity following surgery as compared to their preoperative level.
• Q389: Cataract Surgery: Difference Between Planned and Final Refraction: This MIPS quality measure evaluates
patients for achieving a final refraction within +/- 1.0 diopters of their planned (target) refraction following cataract
surgery.
• Q499: Appropriate Screening and Plan of Care for Elevated Intraocular Pressure Following Intravitreal or Periocular
Steroid Therapy: This MIPS quality measure focuses on patient safety and ensures appropriate screening and plan of
care for elevated intraocular pressure following treatment with intravitreal or periocular steroid.
• Q500: Acute Posterior Vitreous Detachment Appropriate Examination and Follow-up: This MIPS quality measure
evaluates patients following acute posterior vitreous detachment to ensure prompt and appropriate care to minimize
potential for complications.
• Q501: Acute Posterior Vitreous Detachment and Acute Vitreous Hemorrhage Appropriate Examination and Follow-up:
This MIPS quality measure evaluates patients following acute posterior vitreous detachment and acute vitreous
hemorrhage to ensure prompt and appropriate care to minimize potential for complications.
• IRIS2: Glaucoma – Intraocular Pressure Reduction: This QCDR measure focuses on glaucoma patients to assess
management of their IOP by evaluating if it is below a threshold level based on severity of their glaucoma.
• IRIS13: Diabetic Macular Edema – Loss of Visual Acuity: This QCDR measure evaluates outcomes of treatment for
diabetic macular edema by assessing for change in visual acuity after treatment.
• IRIS39: Intraocular Pressure Reduction Following Trabeculectomy or an Aqueous Shunt Procedure: This QCDR
measure assesses for successful treatment of patients with glaucoma who have undergone trabeculectomy or an
aqueous shunt procedure by evaluating for intraocular pressure reduction.
• IRIS54: Complications after Cataract Surgery: This QCDR measure assesses for successful cataract surgeries by
reviewing patients for complications within 90 days of the procedure.
• IRIS58: Improved Visual Acuity after Vitrectomy for Complications of Diabetic Retinopathy within 120 Days: This
QCDR measure assesses for successful vitrectomy procedure in patients with diabetic retinopathy by reviewing patients
for complications within 120 days of the procedure.
• IRIS61: Visual Acuity Improvement Following Cataract Surgery and Minimally Invasive Glaucoma Surgery: This
QCDR measure evaluates visual acuity as a surgical outcome following cataract surgery and minimally invasive
glaucoma surgery.
The following broadly applicable MIPS quality measures are relevant to clinicians who specialize in ocular care. The quality
measures below assess for age-specific screenings and the patients’ understanding of engagement in their healthcare:
• Q130: Documentation of Current Medications in the Medical Record: This MIPS quality measure bases performance
on clinicians documenting the list of current medications using all immediate resources for capture of this important
clinical topic.
•
Q226: Preventive Care and Screening: Tobacco Use: Screening and Cessation Intervention: This MIPS quality measure
ensures patients are screened for tobacco use and if screened positive receive tobacco cessation intervention.
•
Q374: Closing the Referral Loop: Receipt of Specialist Report: This MIPS quality measure is attributable to the
clinician referring the patient and ensures report receival from the referred to clinician, closing the communication
loop.

•
Q487: Screening for Social Drivers of Health: This MIPS quality measure ensures adults are screened for food
insecurity, housing instability, transportation needs, utility difficulties, and interpersonal safety.
• Q503: Gains in Patient Activation Measure (PAM®) Scores at 12 Months: This MIPS quality measure ensures capture
of the patient voice and experience of care related to the patient’s understanding and confidence in the clinician’s
ability to manage their health and be an active partner in the health care journey.
Improvement Activities
We reviewed the Improvement Activities Inventory and considered feedback received during the 2025 MVP candidate feedback
period to determine the set of improvement activities to include in this MVP. We are proposing to include 14 improvement
activities that reflect actions and processes undertaken by clinicians who specialize in ocular care, as well as activities that
promote patient engagement and patient-centeredness, health equity, shared decision making, and care coordination. These
improvement activities provide opportunities for clinicians, in collaboration with patients, to drive outcomes and improve quality
of care. The following improvement activities are proposed for inclusion in this MVP:
• IA_AHE_1: Enhance Engagement of Medicaid and Other Underserved Populations
• IA_AHE_9: Implement Food Insecurity and Nutrition Risk Identification and Treatment Protocols
• IA_BE_4: Engagement of patients through implementation of improvements in patient portal
• IA_BE_6: Regularly Assess Patient Experience of Care and Follow Up on Findings
• IA_BE_25: Drug Cost Transparency
• IA_CC_9: Implementation of practices/processes for developing regular individual care plans
• IA_CC_10: Care transition documentation practice improvements
• IA_CC_13: Practice improvements to align with OpenNotes principles
• IA_ERP_6: COVID-19 Vaccine Achievement for Practice Staff
• IA_MVP: Practice-Wide Quality Improvement in MIPS Value Pathways
• IA_PCMH: Electronic submission of Patient Centered Medical Home accreditation
• IA_PM_13: Chronic care and preventative care management for empaneled patients
• IA_PM_16: Implementation of medication management practice improvements
• IA_PSPA_7: Use of QCDR data for ongoing practice assessment and improvements
We are proposing to modify the IA_BE_4: Engagement of patients through implementation of improvements in patient portal
improvement activity, which includes a proposed activity title update. Please see Appendix 2, Improvement Activities: Table
Group B of this proposed rule for all proposed revisions to this activity.
Cost Measures
We are proposing to include one MIPS cost measure within the cost performance category of this MVP, which applies to the
clinical topic of ocular care. We reviewed the MIPS cost measure inventory and considered feedback received during the 2025
MVP candidate feedback period to determine the set of cost measures to include in this MVP. The following cost measure
provides a meaningful assessment of the clinical care for clinicians who specialize in ocular care, specifically cataract removal,
and aligns with other measures and activities within this MVP:
• Routine Cataract Removal with Intraocular Lens (IOL) Implantation: This MIPS episode-based cost measure assesses
costs associated with routine cataract removal. The addition of this measure aligns with included quality measures, such
as Q191: Cataracts: 20/40 or Better Visual Acuity within 90 Days Following Cataract Surgery and Q303: Cataracts:
Improvement in Patient’s Visual Function within 90 Days Following Cataract Surgery.
We are proposing to modify the Routine Cataract Removal with Intraocular Lens (IOL) Implantation cost measure, which
includes a proposed measure title update. Please see section IV.A.4.e(2)(a)(vi) of this proposed rule for all proposed revisions to
this measure.
Complete Ophthalmologic Care MVP Tables
As stated in the introduction of this appendix, we considered measures and improvement activities available within the MIPS
inventory and selected those we determined best fit the clinical concept of the proposed Complete Ophthalmologic Care MVP.
We request comment on the measures and activities included in this MVP.
Symbol Key:
Single asterisk (*): existing measures and improvement activities with proposed revisions
Double asterisk (**): measures and improvement activities only available when included in an MVP
Single exclamation point (!): high priority measures
Double exclamation point (!!): outcome measures
Tilde (~): measures and improvement activities that include a health equity component
Percent (%): attestation to IA_PCMH: Electronic submission of Patient Centered Medical Home accreditation provides full credit
for the improvement activity performance category
Pound sign (#): QCDR measures pending testing data
TABLE A.1a: Complete Ophthalmologic Care MVP Measures and Improvement Activities

Quality
Improvement Activities
Cost
Q012: Primary Open-Angle Glaucoma
(POAG): Optic Nerve Evaluation
(Collection Type: eCQM Specifications)
(*)(!) Q019: Diabetic Retinopathy:
Communication with the Physician Managing
Ongoing Diabetes Care
(Collection Type: eCQM Specifications,
MIPS CQMs Specifications)
(*) Q117: Diabetes: Eye Exam
(Collection Type: eCQM Specifications,
MIPS CQMs Specifications)
(*)(!) Q130: Documentation of Current
Medications in the Medical Record
(Collection Type: eCQM Specifications,
MIPS CQMs Specifications)
(!!) Q141: Primary Open-Angle Glaucoma
(POAG): Reduction of Intraocular Pressure
(IOP) by 20% OR Documentation of a Plan of
Care
(Collection Type: Medicare Part B Claims
Specifications, MIPS CQMs Specifications)
(!!) Q191: Cataracts: 20/40 or Better Visual
Acuity within 90 Days Following Cataract
Surgery
(Collection Type: eCQM Specifications,
MIPS CQMs Specifications)
Q226: Preventive Care and Screening:
Tobacco Use: Screening and Cessation
Intervention
(Collection Type: Medicare Part B Claims,
eCQM Specifications, MIPS CQMs
Specifications)
(!!) Q303: Cataracts: Improvement in
Patient’s Visual Function within 90 Days
Following Cataract Surgery
(Collection Type: MIPS CQMs
Specifications)
(!) Q304: Cataracts: Patient Satisfaction
within 90 Days Following Cataract Surgery
(Collection Type: MIPS CQMs
Specifications)
(*)(!) Q374: Closing the Referral Loop:
Receipt of Specialist Report
(Collection Type: eCQM Specifications,
MIPS CQMs Specifications)
(*)(!!) Q384: Adult Primary Rhegmatogenous
Retinal Detachment Surgery: No Return to
the Operating Room Within 90 Days of
Surgery
(Collection Type: MIPS CQMs
Specifications)
(!!) Q385: Adult Primary Rhegmatogenous
Retinal Detachment Surgery: Visual Acuity
Improvement Within 90 Days of Surgery
(Collection Type: MIPS CQMs
Specifications)
(!!) Q389: Cataract Surgery: Difference
Between Planned and Final Refraction
(Collection Type: MIPS CQMs
Specifications)
(~)(!) Q487: Screening for Social Drivers of
Health
(Collection Type: MIPS CQMs
(~) IA_AHE_1: Enhance Engagement of
Medicaid and Other Underserved Populations
(High)
(~) IA_AHE_9: Implement Food Insecurity
and Nutrition Risk Identification and
Treatment Protocols
(Medium)
(*) IA_BE_4: Engagement of patients
through implementation of improvements in
patient portal
(Medium)
IA_BE_6: Regularly Assess Patient
Experience of Care and Follow Up on
Findings
(High)
IA_BE_25: Drug Cost Transparency
(High)
(~) IA_CC_9: Implementation of
practices/processes for developing regular
individual care plans
(Medium)
(~) IA_CC_10: Care transition
documentation practice improvements
(Medium)
IA_CC_13: Practice improvements to align
with OpenNotes principles
(Medium)
(*) IA_ERP_6: COVID-19 Vaccine
Achievement for Practice Staff
(Medium)
(**) IA_MVP: Practice-Wide Quality
Improvement in MIPS Value Pathways
(High)
(%) IA_PCMH: Electronic submission of
Patient Centered Medical Home accreditation
IA_PM_13: Chronic care and preventative
care management for empaneled patients
(Medium)
IA_PM_16: Implementation of medication
management practice improvements
(Medium)
(~) IA_PSPA_7: Use of QCDR data for
ongoing practice assessment and
improvements
(Medium)
(*) Routine Cataract Removal with
Intraocular Lens (IOL) Implantation

Quality
Improvement Activities
Cost
Specifications)
Q499: Appropriate Screening and Plan of
Care for Elevated Intraocular Pressure
Following Intravitreal or Periocular Steroid
Therapy
(Collection Type: MIPS CQMs
Specifications)
(*) Q500: Acute Posterior Vitreous
Detachment Appropriate Examination and
Follow-up
(Collection Type: MIPS CQMs
Specifications)
(*) Q501: Acute Posterior Vitreous
Detachment and Acute Vitreous Hemorrhage
Appropriate Examination and Follow-up
(Collection Type: MIPS CQMs
Specifications)
(*)(!!) Q503: Gains in Patient Activation
Measure (PAM®) Scores at 12 Months
(Collection Type: MIPS CQMs
Specifications)
(!!)(#) IRIS2: Glaucoma – Intraocular
Pressure Reduction
(Collection Type: QCDR)
(!!)(#) IRIS13: Diabetic Macular Edema –
Loss of Visual Acuity
(Collection Type: QCDR)
(!!)(#) IRIS39: Intraocular Pressure
Reduction Following Trabeculectomy or an
Aqueous Shunt Procedure
(Collection Type: QCDR)
(!!)(#) IRIS54: Complications after Cataract
Surgery
(Collection Type: QCDR)
(!!)(#) IRIS58: Improved Visual Acuity after
Vitrectomy for Complications of Diabetic
Retinopathy within 120 Days
(Collection Type: QCDR)
(!!)(#) IRIS61: Visual Acuity Improvement
Following Cataract Surgery and Minimally
Invasive Glaucoma Surgery
(Collection Type: QCDR)
TABLE A.1b: Complete Ophthalmologic Care MVP Foundational Layer

Population Health Measures
Promoting Interoperability
(!!) Q479: Hospital-Wide, 30-Day, All-Cause Unplanned
Readmission (HWR) Rate for the Merit-Based Incentive Payment
Systems (MIPS) Groups
(Collection Type: Administrative Claims)
(!!) Q484: Clinician and Clinician Group Risk-standardized Hospital
Admission Rates for Patients with Multiple Chronic Conditions
(Collection Type: Administrative Claims)
Security Risk Analysis
High Priority Practices Safety Assurance Factors for EHR Resilience
Guide (SAFER Guide)
e-Prescribing
Query of Prescription Drug Monitoring Program (PDMP)
Provide Patients Electronic Access to Their Health Information
Support Electronic Referral Loops By Sending Health Information
AND
Support Electronic Referral Loops By Receiving and Reconciling
Health Information
OR
Health Information Exchange (HIE) Bi-Directional Exchange
OR
Enabling Exchange Under the Trusted Exchange Framework and
Common Agreement (TEFCA)
Immunization Registry Reporting
Syndromic Surveillance Reporting (Optional)
Electronic Case Reporting
Public Health Registry Reporting (Optional)
Clinical Data Registry Reporting (Optional)
Actions to Limit or Restrict Compatibility or Interoperability of
CEHRT
ONC Direct Review Attestation
A.2 Dermatological Care MVP
The proposed Dermatological Care MVP focuses on the clinical theme of providing treatment and management of dermatologic
care. This MVP would be most applicable to clinicians who treat patients within the practice of dermatology, including
nonphysician practitioners (NPPs) such as nurse practitioners and physician assistants.
Quality Measures
We are proposing to include 11 MIPS quality measures and 6 QCDR measure within the quality performance category of this
MVP, which are specific to the clinical topic of dermatology. We reviewed the MIPS quality measure inventory and considered
feedback received during the 2025 MVP candidate feedback period to determine which quality measures best represent the
clinical topic of this MVP.
The following quality measures provide a meaningful and comprehensive assessment of the clinical care for clinicians who
specialize in dermatology:
• Q176: Tuberculosis Screening Prior to First Course of Biologic and/or Immune Response Modifier Therapy: This
MIPS quality measure ensures TB testing is completed prior to the first course of biologic and/or immune response
modifier therapy.
• Q397: Melanoma Reporting: This MIPS quality measure assesses that the pathology report for primary malignant
cutaneous melanoma includes reporting identifiers needed for microsatellitosis of invasive tumors.
• Q410: Psoriasis: Clinical Response to Systemic Medications: This MIPS quality measure ensures patients with
psoriasis vulgaris who are being treated with systemic medications maintain disease control by evaluating documented
body surface assessments meet at least one of the specified benchmarks.
• Q440: Skin Cancer: Biopsy Reporting Time – Pathologist to Clinician: This MIPS quality measure ensures pathology
reports diagnosing carcinoma or melanoma are communicated from the Pathologist/Dermatopathologist within 7 days
from the Pathologist receiving the specimen.
• Q485: Psoriasis – Improvement in Patient-Reported Itch Severity: This MIPS quality measure evaluates patients with a
diagnosis of psoriasis for a reduction in itch severity of 3 or more points at follow up visits.
• Q486: Dermatitis – Improvement in Patient-Reported Itch Severity: This MIPS quality measure evaluates patients with
a diagnosis of dermatitis for a reduction in itch severity of 3 or more points at follow up visits.
• TBD: Melanoma: Tracking and Evaluation of Recurrence: This proposed MIPS quality measure ensures patients who
undergo excisional surgery for melanoma or melanoma in situ within the previous 5 years have documentation that an
exam for recurrence of melanoma was performed within the performance period and results captured.

• AAD6: Skin Cancer Biopsy Reporting Time – Clinician to Patient: This QCDR measure ensures timely communication
from the clinician to the patient when they have a positive finding for carcinoma, melanoma, or primary cutaneous
malignancies.
• AAD8: Chronic Skin Conditions: Patient Reported Quality-of-Life: This QCDR measure ensures a patient- reported
quality-of-life assessment is completed and recorded in the medical record with a plan of care at least once in the
performance period.
• AAD12: Melanoma: Appropriate Surgical Margins: This QCDR measure ensures the initial biopsy and surgical
margins are documented in the medical record and are in compliance with the minimum margin recommended in the
current National Comprehensive Cancer Network (NCCN) guideline.
• AAD16: Avoidance of Post-operative Systemic Antibiotics for Office-based Closures and Reconstruction After Skin:
This QCDR measure assesses for the appropriate use of post operative antibiotics for patients with skin cancer
undergoing an office-based closure or reconstruction procedure.
• AAD17: Continuation of Anticoagulation Therapy in the Office-based Setting for Closure and Reconstruction After
Skin Cancer Resection Procedures: This QCDR measure assesses the percentage of patients who had their
anticoagulation therapy continued prior to an in-office procedure for intermediate layer and/or complex linear closures
OR reconstruction after skin cancer resection performed.
• AAD18: Avoidance of Opioid Prescriptions for Closure and Reconstruction After Skin Cancer Resection: This QCDR
measure identifies the number of patients diagnosed with skin cancer who were prescribed an opioid/narcotic therapy
as a first line pain management option post-operative by the reconstructing surgeon.
The following broadly applicable MIPS quality measures are relevant to clinicians who specialize in dermatology. The quality
measures below assess for age-specific screenings, and follow-up actions for select measures:
• Q130: Documentation of Current Medications in the Medical Record: This MIPS quality measure bases performance
on clinicians documenting the list of current medications using all immediate resources for capture of this important
clinical topic.
•
Q226: Preventive Care and Screening: Tobacco Use: Screening and Cessation Intervention: This MIPS quality measure
ensures patients are screened for tobacco use and if screened positive receive tobacco cessation intervention.
•
Q487: Screening for Social Drivers of Health: This MIPS quality measure ensures adults are screened for food
insecurity, housing instability, transportation needs, utility difficulties, and interpersonal safety.
• Q503: Gains in Patient Activation Measure (PAM®) Scores at 12 Months: This MIPS quality measure ensures capture
of the patient voice and experience of care related to the patient’s understanding and confidence in the clinician’s
ability to manage their health and be an active partner in the health care journey.
Improvement Activities
We reviewed the Improvement Activities Inventory and considered feedback received during the 2025 MVP candidate feedback
period to determine the set of improvement activities to include in this MVP. We are proposing to include 11 improvement
activities that reflect actions and processes undertaken by clinicians who specialize in dermatology, as well as activities that
promote patient engagement and patient-centeredness, health equity, shared decision making, and care coordination. These
improvement activities provide opportunities for clinicians, in collaboration with patients, to drive outcomes and improve quality
of care. The following improvement activities are proposed for inclusion in this MVP:
• IA_AHE_1: Enhance Engagement of Medicaid and Other Underserved Populations
• IA_AHE_6: Provide Education Opportunities for New Clinicians
• IA_BE_4: Engagement of patients through implementation of improvements in patient portal
• IA_BE_6: Regularly Assess Patient Experience of Care and Follow Up on Findings
• IA_BE_15: Engagement of patients, family and caregivers in developing a plan of care
• IA_EPA_2: Use of telehealth services that expand practice access
• IA_ERP_6: COVID-19 Vaccine Achievement for Practice Staff
• IA_MVP: Practice-Wide Quality Improvement in MIPS Value Pathways
• IA_PCMH: Electronic submission of Patient Centered Medical Home accreditation
• IA_PM_16: Implementation of medication management practice improvements
• IA_PSPA_8: Use of Patient Safety Tools
We are proposing to modify the IA_BE_4: Engagement of patients through implementation of improvements in patient portal
improvement activity, which includes a proposed activity title update. Please see Appendix 2, Improvement Activities: Table
Group B of this proposed rule for all proposed revisions to this activity.
Cost Measures
We are proposing to include one MIPS cost measure within the cost performance category of this MVP, which applies to the
clinical topic of dermatology. We reviewed the MIPS cost measure inventory and considered feedback received during the 2025
MVP candidate feedback period to determine the set of cost measures to include in this MVP. The following cost measure
provides a meaningful assessment of the clinical care for clinicians who specialize in dermatology, specifically melanoma
resection, and aligns with other measures and activities within this MVP:
• Melanoma Resection: This MIPS episode-based cost measure assesses costs associated with excision procedures to
remove a cutaneous melanoma. The addition of this measure aligns with Q397: Melanoma Reporting and TBD:
Melanoma: Tracking and Evaluation of Recurrence.
Dermatological Care MVP Tables
As stated in the introduction of this appendix, we considered measures and improvement activities available within the MIPS
inventory and selected those we determined best fit the clinical concept of the proposed Dermatological Care MVP. We request
comment on the measures and activities included in this MVP.
Symbol Key:
Carat symbol (^): new proposed measures and improvement activities
Single asterisk (*): existing measures and improvement activities with proposed revisions
Double asterisk (**): measures and improvement activities only available when included in an MVP
Single exclamation point (!): high priority measures
Double exclamation point (!!): outcome measures
Tilde (~): measures and improvement activities that include a health equity component
Percent (%): attestation to IA_PCMH: Electronic submission of Patient Centered Medical Home accreditation provides full credit
for the improvement activity performance category
Pound sign (#): QCDR measures pending testing data

TABLE A.2a: Dermatological Care MVP Measures and Improvement Activities
Quality
Improvement Activities
Cost
(*)(!) Q130: Documentation of Current
Medications in the Medical Record
(Collection Type: eCQM Specifications,
MIPS CQMs Specifications)
(*) Q176: Tuberculosis Screening Prior to
First Course of Biologic and/or Immune
Response Modifier Therapy
(Collection Type: MIPS CQMs
Specifications)
Q226: Preventive Care and Screening:
Tobacco Use: Screening and Cessation
Intervention
(Collection Type: Medicare Part B Claims
Specifications, eCQM Specifications, MIPS
CQMs Specifications)
(!) Q397: Melanoma Reporting
(Collection Type: Medicare Part B Claims,
MIPS CQMs Specifications)
(!!) Q410: Psoriasis: Clinical Response to
Systemic Medications
(Collection Type: MIPS CQMs
Specifications)
(!) Q440: Skin Cancer: Biopsy Reporting
Time – Pathologist to Clinician
(Collection Type: MIPS CQMs
Specifications)
(*)(!!) Q485: Psoriasis – Improvement in
Patient-Reported Itch Severity
(Collection Type: MIPS CQMs
Specifications)
(*)(!!) Q486: Dermatitis – Improvement in
Patient-Reported Itch Severity
(Collection Type: MIPS CQMs
Specifications)
(~)(!) Q487: Screening for Social Drivers of
Health
(Collection Type: MIPS CQMs
Specifications)
(*)(!!) Q503: Gains in Patient Activation
Measure (PAM®) Scores at 12 Months
(Collection Type: MIPS CQMs
Specifications)
(^)(!) TBD: Melanoma: Tracking and
Evaluation of Recurrence
(Collection Type: MIPS CQMs
Specifications)
(!)(#) AAD6: Skin Cancer Biopsy Reporting
Time – Clinician to Patient
(Collection Type: QCDR)
(!) AAD8: Chronic Skin Conditions: Patient
Reported Quality-of-Life
(Collection Type: QCDR)
(!!)(#) AAD12: Melanoma: Appropriate
Surgical Margins
(Collection Type: QCDR)
(!)(#) AAD16: Avoidance of Post-operative
Systemic Antibiotics for Office-based
Closures and Reconstruction After Skin
Cancer Procedures
(Collection Type: QCDR)
(~) IA_AHE_1: Enhance Engagement of
Medicaid and Other Underserved Populations
(High)
(~) IA_AHE_6: Provide Education
Opportunities for New Clinicians
(High)
(*) IA_BE_4: Engagement of patients
through implementation of improvements in
patient portal
(Medium)
IA_BE_6: Regularly Assess Patient
Experience of Care and Follow Up on
Findings
(High)
IA_BE_15: Engagement of patients, family
and caregivers in developing a plan of care
(Medium)
IA_EPA_2: Use of telehealth services that
expand practice access
(Medium)
(*) IA_ERP_6: COVID-19 Vaccine
Achievement for Practice Staff
(Medium)
(**) IA_MVP: Practice-Wide Quality
Improvement in MIPS Value Pathways
(High)
(%) IA_PCMH: Electronic submission of
Patient Centered Medical Home accreditation
IA_PM_16: Implementation of medication
management practice improvements
(Medium)
IA_PSPA_8: Use of Patient Safety Tools
(Medium)
Melanoma Resection

Quality
Improvement Activities
Cost
(!)(#) AAD17: Continuation of
Anticoagulation Therapy in the Office-based
Setting for Closure and Reconstruction After
Skin Cancer Resection Procedures
(Collection Type: QCDR)
(!)(#) AAD18: Avoidance of Opioid
Prescriptions for Closure and Reconstruction
After Skin Cancer Resection
(Collection Type: QCDR)
TABLE A.2b: Dermatological Care MVP Foundational Layer
Population Health Measures
Promoting Interoperability
(!!) Q479: Hospital-Wide, 30-Day, All-Cause Unplanned
Readmission (HWR) Rate for the Merit-Based Incentive Payment
Systems (MIPS) Groups
(Collection Type: Administrative Claims)
(!!) Q484: Clinician and Clinician Group Risk-standardized Hospital
Admission Rates for Patients with Multiple Chronic Conditions
(Collection Type: Administrative Claims)
Security Risk Analysis
High Priority Practices Safety Assurance Factors for EHR Resilience
Guide (SAFER Guide)
e-Prescribing
Query of Prescription Drug Monitoring Program (PDMP)
Provide Patients Electronic Access to Their Health Information
Support Electronic Referral Loops By Sending Health Information
AND
Support Electronic Referral Loops By Receiving and Reconciling
Health Information
OR
Health Information Exchange (HIE) Bi-Directional Exchange
OR
Enabling Exchange Under the Trusted Exchange Framework and
Common Agreement (TEFCA)
Immunization Registry Reporting
Syndromic Surveillance Reporting (Optional)
Electronic Case Reporting
Public Health Registry Reporting (Optional)
Clinical Data Registry Reporting (Optional)
Actions to Limit or Restrict Compatibility or Interoperability of
CEHRT
ONC Direct Review Attestation

A.3 Gastroenterology Care MVP
The proposed Gastroenterology Care MVP focuses on the clinical theme of providing treatment and management of the digestive
system and the liver. This MVP would be most applicable to clinicians who treat patients within the practice of gastroenterology,
including nonphysician practitioners (NPPs) such as nurse practitioners and physician assistants.
Quality Measures
We are proposing to include 11 MIPS quality measures and 3 QCDR measures within the quality performance category of this
MVP, which are specific to the clinical topic of gastroenterology. We reviewed the MIPS quality measure inventory and
considered feedback received during the 2025 MVP candidate feedback period to determine which quality measures best
represent the clinical topic of this MVP.
The following quality measures provide a meaningful and comprehensive assessment of the clinical care for clinicians who
specialize in gastroenterology:
• Q113: Colorectal Cancer Screening: This MIPS quality measure ensures appropriate screening of patients for colorectal
cancer.
• Q185: Colonoscopy Interval for Patients with a History of Adenomatous Polyps - Avoidance of Inappropriate Use:
This MIPS quality measure ensures appropriate follow-up, an interval of 3 or more years, for patients with a history of
prior adenomatous polyp(s) in previous colonoscopy.
• Q275: Inflammatory Bowel Disease (IBD): Assessment of Hepatitis B Virus (HBV) Status Before Initiating Anti-TNF
(Tumor Necrosis Factor) Therapy: This MIPS quality measure requires patients with a diagnosis of inflammatory
bowel disease (IBD) have Hepatitis B Virus (HBV) status assessed prior to initiating anti-TNF (tumor necrosis factor)
therapy.
• Q320: Appropriate Follow-Up Interval for Normal Colonoscopy in Average Risk Patients: This MIPS quality measure
ensures appropriate follow-up for patients receiving a screening colonoscopy without biopsy or polypectomy.
• Q400: One-Time Screening for Hepatitis C Virus (HCV) and Treatment Initiation: This MIPS quality measure requires
patients have received a one-time screening for hepatitis C virus (HCV) infection as well as treatment initiation or
referral if screening is positive.
• Q401: Hepatitis C: Screening for Hepatocellular Carcinoma (HCC) in Patients with Cirrhosis: This MIPS quality
measure ensures patients with a diagnosis of chronic Hepatitis C cirrhosis have appropriate surveillance imaging for
hepatocellular carcinoma at least once during the performance period.
• GIQIC23: Appropriate follow-up interval based on pathology findings in screening colonoscopy: This QCDR measure
ensures appropriate follow-up consistent with US Multi-Society Task Force (USMSTF) recommendations based upon
pathology findings from screening colonoscopy with biopsy or polypectomy documented in colonoscopy report.
• GIQIC26: Screening Colonoscopy Adenoma Detection Rate: This QCDR measure evaluates patients who had a
screening colonoscopy and at least one conventional adenoma or colorectal cancer was detected.
• NHCR4: Repeat screening or surveillance colonoscopy recommended within one year due to inadequate bowel
preparation: This QCDR measure ensures patients with inadequate bowel prep receive a repeat screening or
surveillance colonoscopy or an alternate tier 1 or tier 2 colorectal cancer screening modality within one year.
The following broadly applicable MIPS quality measures are relevant to clinicians who specialize in gastroenterology. The
quality measures below assess for age-specific screenings, and follow-up actions for select measures:
• Q130: Documentation of Current Medications in the Medical Record: This MIPS quality measure bases performance
on clinicians documenting the list of current medications using all immediate resources for capture of this important
clinical topic.
•
Q226: Preventive Care and Screening: Tobacco Use: Screening and Cessation Intervention: This MIPS quality measure
ensures patients are screened for tobacco use and if screened positive receive tobacco cessation intervention.
•
Q374: Closing the Referral Loop: Receipt of Specialist Report: This MIPS quality measure is attributable to the
clinician referring the patient and ensures report receival from the referred to clinician, closing the communication
loop.
•
Q487: Screening for Social Drivers of Health: This MIPS quality measure ensures adults are screened for food
insecurity, housing instability, transportation needs, utility difficulties, and interpersonal safety.
• Q503: Gains in Patient Activation Measure (PAM®) Scores at 12 Months: This MIPS quality measure ensures capture
of the patient voice and experience of care related to the patient’s understanding and confidence in the clinician’s
ability to manage their health and be an active partner in the health care journey.

Improvement Activities
We reviewed the Improvement Activities Inventory and considered feedback received during the 2025 MVP candidate feedback
period to determine the set of improvement activities to include in this MVP. We are proposing to include 11 improvement
activities that reflect actions and processes undertaken by clinicians who specialize in gastroenterology, as well as activities that
promote patient engagement and patient-centeredness, health equity, shared decision making, and care coordination. These
improvement activities provide opportunities for clinicians, in collaboration with patients, to drive outcomes and improve quality
of care. The following improvement activities are proposed for inclusion in this MVP:
• IA_AHE_3: Promote Use of Patient-Reported Outcome Tools
• IA_AHE_6: Provide Education Opportunities for New Clinicians
• IA_AHE_9: Implement Food Insecurity and Nutrition Risk Identification and Treatment Protocols
• IA_BE_4: Engagement of patients through implementation of improvements in patient portal
• IA_CC_7: Regular training in care coordination
• IA_CC_9: Implementation of practices/processes for developing regular individual care plans
• IA_CC_10: Care transition documentation practice improvements
• IA_CC_13: Practice improvements to align with OpenNotes principles
• IA_ERP_6: COVID-19 Vaccine Achievement for Practice Staff
• IA_MVP: Practice-Wide Quality Improvement in MIPS Value Pathways
• IA_PCMH: Electronic submission of Patient Centered Medical Home accreditation
We are proposing to modify the IA_BE_4: Engagement of patients through implementation of improvements in patient portal
improvement activity. which includes a proposed activity title update. Please see Appendix 2, Improvement Activities: Table
Group B of this proposed rule for all proposed revisions to this activity.
Cost Measures
We are proposing to include two MIPS cost measures within the cost performance category of this MVP, which apply to the
clinical topic of gastroenterology. We reviewed the MIPS cost measure inventory and considered feedback received during the
2025 MVP candidate feedback period to determine the set of cost measures to include in this MVP. The following cost measures
provide a meaningful assessment of the clinical care for clinicians who specialize in gastroenterology, including colonoscopies
and broader gastroenterology care, and align with other measures and activities within this MVP:
• Screening/Surveillance Colonoscopy: This MIPS episode-based cost measure assesses costs associated with screening
or surveillance colonoscopy procedures. This measure aligns with quality measures such as Q113: Colorectal Cancer
Screening or Q320: Appropriate Follow-Up Interval for Normal Colonoscopy in Average Risk Patients.
• Total Per Capita Cost (TPCC): This MIPS cost measure assesses the overall cost of care delivered to a Medicare
patient with a focus on the primary care the patient receives from their providers. Gastroenterologists are included in
attribution for the TPCC measure as they may provide broad, ongoing care to their patients, which is in line with the
intent of the TPCC measure.
Gastroenterology Care MVP Tables
As stated in the introduction of this appendix, we considered measures and improvement activities available within the MIPS
inventory and selected those we determined best fit the clinical concept of the proposed Gastroenterology Care MVP. We request
comment on the measures and activities included in this MVP.
Symbol Key:
Single asterisk (*): existing measures and improvement activities with proposed revisions
Double asterisk (**): measures and improvement activities only available when included in an MVP
Single exclamation point (!): high priority measures
Double exclamation point (!!): outcome measures
Tilde (~): measures and improvement activities that include a health equity component
Percent (%): attestation to IA_PCMH: Electronic submission of Patient Centered Medical Home accreditation provides full credit
for the improvement activity performance category
Pound sign (#): QCDR measures pending testing data

TABLE A.3a: Gastroenterology Care MVP Measures and Improvement Activities
Quality
Improvement Activities
Cost
(*) Q113: Colorectal Cancer Screening
(Collection Type: Medicare Part B Claims
Specifications, eCQM Specifications, MIPS
CQMs Specifications)
(*)(!) Q130: Documentation of Current
Medications in the Medical Record
(Collection Type: eCQM Specifications,
MIPS CQMs Specifications)
(*)(!) Q185: Colonoscopy Interval for
Patients with a History of Adenomatous
Polyps - Avoidance of Inappropriate Use
(Collection Type: MIPS CQMs
Specifications)
Q226: Preventive Care and Screening:
Tobacco Use: Screening and Cessation
Intervention
(Collection Type: Medicare Part B Claims
Specifications, eCQM Specifications, MIPS
CQMs Specifications)
Q275: Inflammatory Bowel Disease (IBD):
Assessment of Hepatitis B Virus (HBV)
Status Before Initiating Anti-TNF (Tumor
Necrosis Factor) Therapy
(Collection Type: MIPS CQMs
Specifications)
(*)(!) Q320: Appropriate Follow-Up Interval
for Normal Colonoscopy in Average Risk
Patients
(Collection Type: Medicare Part B Claims
Specifications, MIPS CQMs Specifications)
(*)(!) Q374: Closing the Referral Loop:
Receipt of Specialist Report
(Collection Type: eCQM Specifications,
MIPS CQMs Specifications)
Q400: One-Time Screening for Hepatitis C
Virus (HCV) and Treatment Initiation
(Collection Type: MIPS CQMs
Specifications)
Q401: Hepatitis C: Screening for
Hepatocellular Carcinoma (HCC) in Patients
with Cirrhosis
(Collection Type: MIPS CQMs
Specifications)
(~)(!) Q487: Screening for Social Drivers of
Health
(Collection Type: MIPS CQMs
Specifications)
(*)(!!) Q503: Gains in Patient Activation
Measure (PAM®) Scores at 12 Months
(Collection Type: MIPS CQMs
Specifications)
(!) GIQIC23: Appropriate follow-up interval
based on pathology findings in screening
colonoscopy
(Collection Type: QCDR)
(!!)(#) GIQIC26: Screening Colonoscopy
Adenoma Detection Rate
(Collection Type: QCDR)
(!) NHCR4: Repeat screening or surveillance
colonoscopy recommended within one year
due to inadequate bowel preparation
(Collection Type: QCDR)
(~) IA_AHE_3: Promote use of Patient-
Reported Outcome Tools
(High)
(~) IA_AHE_6: Provide Education
Opportunities for New Clinicians
(High)
(~) IA_AHE_9: Implement Food Insecurity
and Nutrition Risk Identification and
Treatment Protocols
(Medium)
(*) IA_BE_4: Engagement of patients
through implementation of improvements in
patient portal
(Medium)
IA_CC_7: Regular training in care
coordination
(Medium)
(~) IA_CC_9: Implementation of
practices/processes for developing regular
individual care plans
(Medium)
(~) IA_CC_10: Care transition
documentation practice improvements
(Medium)
IA_CC_13: Practice improvements to align
with OpenNotes principles
(Medium)
(*) IA_ERP_6: COVID-19 Vaccine
Achievement for Practice Staff
(Medium)
(**) IA_MVP: Practice-Wide Quality
Improvement in MIPS Value Pathways
(High)
(%) IA_PCMH: Electronic submission of
Patient Centered Medical Home accreditation
Screening/Surveillance Colonoscopy
Total Per Capita Cost (TPCC)

TABLE A.3b: Gastroenterology Care MVP Foundational Layer
Population Health Measures
Promoting Interoperability
(!!) Q479: Hospital-Wide, 30-Day, All-Cause Unplanned
Readmission (HWR) Rate for the Merit-Based Incentive Payment
Systems (MIPS) Groups
(Collection Type: Administrative Claims)
(!!) Q484: Clinician and Clinician Group Risk-standardized Hospital
Admission Rates for Patients with Multiple Chronic Conditions
(Collection Type: Administrative Claims)
Security Risk Analysis
High Priority Practices Safety Assurance Factors for EHR Resilience
Guide (SAFER Guide)
e-Prescribing
Query of Prescription Drug Monitoring Program (PDMP)
Provide Patients Electronic Access to Their Health Information
Support Electronic Referral Loops By Sending Health Information
AND
Support Electronic Referral Loops By Receiving and Reconciling
Health Information
OR
Health Information Exchange (HIE) Bi-Directional Exchange
OR
Enabling Exchange Under the Trusted Exchange Framework and
Common Agreement (TEFCA)
Immunization Registry Reporting
Syndromic Surveillance Reporting (Optional)
Electronic Case Reporting
Public Health Registry Reporting (Optional)
Clinical Data Registry Reporting (Optional)
Actions to Limit or Restrict Compatibility or Interoperability of
CEHRT
ONC Direct Review Attestation
A.4 Optimal Care for Patients with Urologic Conditions MVP
The proposed Optimal Care for Patients with Urologic Conditions MVP focuses on assessing optimal care for patients treated for
a broad range of urologic conditions, including kidney stones, urinary incontinence, bladder cancer, and prostate cancer. This
MVP would be most applicable to clinicians who treat patients within the practice urology including general urologists, urology
oncologists, and sub-specialists focused on urology care for women, including nonphysician practitioners (NPPs) such as nurse
practitioners and physician assistants.
Quality Measures
We are proposing to include nine MIPS quality measures and five QCDR measure within the quality performance category of
this MVP, which are specific to the clinical topic of urology. We reviewed the MIPS quality measure inventory and considered
feedback received during the 2025 MVP candidate feedback period to determine which quality measures best represent the
clinical topic of this MVP.
The following quality measures provide a meaningful and comprehensive assessment of the clinical care for clinicians who
specialize in urology:
• Q050: Urinary Incontinence: Plan of Care for Urinary Incontinence in Women Aged 65 Years and Older: This MIPS
quality measure ensures patients have a documented plan of care for urinary incontinence at least once within 12
months.
• Q462: Bone Density Evaluation for Patients with Prostate Cancer and Receiving Androgen Deprivation Therapy: This
MIPS quality measure ensures patients with prostate cancer currently on or starting androgen deprivation therapy
(ADT), with an intent for treatment greater than or equal to 12 months, have a bone density evaluation prior to starting
or within 3 months after the start of ADT.
• Q476: Urinary Symptom Score Change 6-12 Months After Diagnosis of Benign Prostatic Hyperplasia: This MIPS
quality measure assesses for improvement in urinary symptoms for patients with a diagnosis of benign prostatic
hyperplasia based upon the International Prostate Symptoms Score (IPSS) or American Urological Association (AUA)
Symptom Index (SI).
• Q481: Intravesical Bacillus-Calmette Guerin for Non-muscle Invasive Bladder Cancer: This MIPS quality measure
ensures patients initially diagnosed with non-muscle invasive bladder cancer have treatment initiated within 6 months
of the cancer staging.

• AQUA8: Hospital Admissions or Infectious Complications Within 30 days of Prostate Biopsy: This QCDR measure
assesses the number of patients who have urinary retention, infection, or a new antibiotic prescription at least 24 hours
after and within 30 days of a prostate biopsy or inpatient consultation or require hospitalization within 30 days of
prostate biopsy.
• AQUA14: Stones: Repeat Shock Wave Lithotripsy (SWL) Within 6 Months of Initial Treatment: This QCDR measure
assesses the number of patients who had a repeat shock wave lithotripsy procedure within the 6 months of the initial
treatment.
• AQUA15: Stones: Urinalysis or Urine Culture Performed Before Surgical Stone Procedures: This QCDR measure
ensures patients have a urinalysis or culture within 14 days prior to surgical stone procedures.
• AQUA16: Non-Muscle Invasive Bladder Cancer: Repeat Transurethral Resection of Bladder Tumor (TURBT) for T1
disease: This QCDR measure assesses the number of patients who undergo a second TURBT within 6 weeks of the
initial procedure.
• MUSIC4: Prostate Cancer: Active Surveillance/Watchful Waiting for Newly Diagnosed Low-Risk Prostate Cancer
Patients: This QCDR measure ensures newly diagnosed low-risk prostate cancer patients are managed via active
surveillance or watchful waiting to maintain the patient’s quality of life.
The following broadly applicable MIPS quality measures are relevant to clinicians who specialize in urology. The measures
assess for age-specific screenings, and follow-up actions for select measures, in addition to recommended vaccinations:
• Q318: Falls: Screening for Future Fall Risk: This MIPS quality measure ensures patients are screened each
performance period for future fall risk.
• Q321: CAHPS for MIPS Clinician/Group Survey: This survey provides direct input from patients and their experience
regarding timely care, effective communication, shared decision making, care coordination, promotion of health and
education, completion of health status/functionality, and courtesy of office staff.
• Q358: Patient-Centered Surgical Risk Assessment and Communication: This MIPS quality measure ensures a
personalized surgical risk assessment is completed on each patient using a validated risk calculator or multi-
institutional clinical data prior to the surgery along with discussion of the identified risks with the surgeon.
•
Q487: Screening for Social Drivers of Health: This MIPS quality measure ensures adults are screened for food
insecurity, housing instability, transportation needs, utility difficulties, and interpersonal safety.
• Q503: Gains in Patient Activation Measure (PAM®) Scores at 12 Months: This MIPS quality measure ensures capture
of the patient voice and experience of care related to the patient’s understanding and confidence in the clinician’s
ability to manage their health and be an active partner in the health care journey.
Improvement Activities
We reviewed the Improvement Activities Inventory and considered feedback received during the 2025 MVP candidate feedback
period to determine the set of improvement activities to include in this MVP. We are proposing to include 17 improvement
activities that reflect actions and processes undertaken by clinicians who specialize in urology, as well as activities that promote
patient engagement and patient-centeredness, health equity, shared decision making, and care coordination. These improvement
activities provide opportunities for clinicians, in collaboration with patients, to drive outcomes and improve quality of care. The
following improvement activities are proposed for inclusion in this MVP:
• IA_AHE_3: Promote Use of Patient-Reported Outcome Tools
• IA_AHE_12: Practice Improvements that Engage Community Resources to Address Drivers of Health
• IA_BE_6: Regularly Assess Patient Experience of Care and Follow Up on Findings
• IA_BE_15: Engagement of patients, family and caregivers in developing a plan of care
• IA_CC_7: Regular training in care coordination
• IA_CC_13: Practice improvements to align with OpenNotes principles
• IA_CC_17: Patient Navigator Program
• IA_EPA_2: Use of telehealth services that expand practice access
• IA_ERP_6: COVID-19 Vaccine Achievement for Practice Staff
• IA_MVP: Practice-Wide Quality Improvement in MIPS Value Pathways
• IA_PCMH: Electronic submission of Patient Centered Medical Home accreditation
• IA_PM_17: Participation in Population Health Research
• IA_PM_21: Advance Care Planning
• IA_PSPA_7: Use of QCDR data for ongoing practice assessment and improvements
• IA_PSPA_12: Participation in private payer CPIA
• IA_PSPA_19: Implementation of formal quality improvement methods, practice changes or other practice
improvement processes
• IA_PSPA_21: Implementation of fall screening and assessment programs
Cost Measures
We are proposing to include three MIPS cost measures within the cost performance category of this MVP, which apply to the
clinical topic of urology. We reviewed the MIPS cost measure inventory and considered feedback received during the 2025 MVP
candidate feedback period to determine the set of cost measures to include in this MVP. The following cost measures provide a
meaningful assessment of the clinical care for clinicians who specialize in urology and align with other measures and activities
within this MVP:

• Medicare Spending Per Beneficiary (MSPB) Clinician: This MIPS cost measure applies to clinicians providing care in
inpatient hospitals, including those who treat patients with urology-related conditions or procedures.
• Renal or Ureteral Stone Surgical Treatment: This MIPS episode-based cost measure assesses costs associated with
surgical treatment for renal or ureteral stones. This also aligns with quality measures such as AQUA14: Stones: Repeat
Shock Wave Lithotripsy (SWL) Within 6 Months of Initial Treatment or AQUA15: Stones: Urinalysis or Urine
Culture Performed Before Surgical Stone Procedures.
• Prostate Cancer: This proposed MIPS episode-based cost measure would assess costs associated with prostate cancer.
This also aligns with quality measures such as Q462: Bone Density Evaluation for Patients with Prostate Cancer and
Receiving Androgen Deprivation Therapy or MUSIC4: Prostate Cancer: Active Surveillance/Watchful Waiting for
Newly Diagnosed Low-Risk Prostate Cancer Patients. We note that this is a new cost measure we are proposing to
adopt as described in section IV.A.4.e.(2)(a)(iii)of this proposed rule.
Optimal Care for Patients with Urologic Conditions MVP Tables
As stated in the introduction of this appendix, we considered measures and improvement activities available within the MIPS
inventory and selected those we determined best fit the clinical concept of the proposed Optimal Care for Patients with Urologic
Conditions MVP. We request comment on the measures and activities included in this MVP.
Symbol Key:
Carat symbol (^): new proposed measures and improvement activities
Single asterisk (*): existing measures and improvement activities with proposed revisions
Double asterisk (**): measures and improvement activities only available when included in an MVP
Single exclamation point (!): high priority measures
Double exclamation point (!!): outcome measures
Tilde (~): measures and improvement activities that include a health equity component
Percent (%): attestation to IA_PCMH: Electronic submission of Patient Centered Medical Home accreditation provides full credit
for the improvement activity performance category
TABLE A.4a: Optimal Care for Patients with Urologic Conditions MVP Measures and Improvement Activities
Quality
Improvement Activities
Cost
(!) Q050: Urinary Incontinence: Plan of Care
for Urinary Incontinence in Women Aged 65
Years and Older
(Collection Type: MIPS CQMs
Specifications)
(!) Q318: Falls: Screening for Future Fall
Risk
(Collection Type: eCQM Specifications)
(!) Q321: CAHPS for MIPS Clinician/Group
Survey
(Collection Type: CSV)
(!) Q358: Patient-Centered Surgical Risk
Assessment and Communication
(Collection Type: MIPS CQMs
Specifications)
(*) Q462: Bone Density Evaluation for
Patients with Prostate Cancer and Receiving
Androgen Deprivation Therapy
(Collection Type: eCQM Specifications)
(!!) Q476: Urinary Symptom Score Change
6-12 Months After Diagnosis of Benign
Prostatic Hyperplasia
(Collection Type: eCQM Specifications)
(!) Q481: Intravesical Bacillus-Calmette
Guerin for Non-muscle Invasive Bladder
Cancer
(Collection Type: eCQM Specifications)
(~)(!) Q487: Screening for Social Drivers of
Health
(Collection Type: MIPS CQMs
Specifications)
(*)(!!) Q503: Gains in Patient Activation
Measure (PAM®) Scores at 12 Months
(~) IA_AHE_3: Promote use of Patient-
Reported Outcome Tools
(High)
(~) IA_AHE_12: Practice Improvements that
Engage Community Resources to Address
Drivers of Health
(High)
IA_BE_6: Regularly Assess Patient
Experience of Care and Follow Up on
Findings
(High)
IA_BE_15: Engagement of patients, family
and caregivers in developing a plan of care
(Medium)
IA_CC_7: Regular training in care
coordination
(Medium)
IA_CC_13: Practice improvements to align
with OpenNotes principles
(Medium)
IA_CC_17: Patient Navigator Program
(High)
IA_EPA_2: Use of telehealth services that
expand practice access
(Medium)
(*) IA_ERP_6: COVID-19 Vaccine
Achievement for Practice Staff
(Medium)
(**) IA_MVP: Practice-Wide Quality
Improvement in MIPS Value Pathways
(High)
Renal or Ureteral Stone Surgical
Treatment
Medicare Spending Per Beneficiary
(MSPB) Clinician
(^) Prostate Cancer

Quality
Improvement Activities
Cost
(Collection Type: MIPS CQMs
Specifications)
(!!) AQUA8: Hospital Admissions or
Infectious Complications Within 30 days of
Prostate Biopsy
(Collection Type: QCDR)
(!!) AQUA14: Stones: Repeat Shock Wave
Lithotripsy (SWL) Within 6 Months of Initial
Treatment
(Collection Type: QCDR)
(!) AQUA15: Stones: Urinalysis or Urine
Culture Performed Before Surgical Stone
Procedures
(Collection Type: QCDR)
AQUA16: Non-Muscle Invasive Bladder
Cancer: Repeat Transurethral Resection of
Bladder Tumor (TURBT) for T1 disease
(Collection Type: QCDR)
(!) MUSIC4: Prostate Cancer: Active
Surveillance/Watchful Waiting for Newly
Diagnosed Low-Risk Prostate Cancer Patients
(Collection Type: QCDR)
(%) IA_PCMH: Electronic submission of
Patient Centered Medical Home accreditation
IA_PM_17: Participation in Population
Health Research
(Medium)
IA_PM_21: Advance Care Planning
(Medium)
(~) IA_PSPA_7: Use of QCDR data for
ongoing practice assessment and
improvements
(Medium)
IA_PSPA_12: Participation in private payer
CPIA
(Medium)
IA_PSPA_19: Implementation of formal
quality improvement methods, practice
changes or other practice improvement
processes
(Medium)
IA_PSPA_21: Implementation of fall
screening and assessment programs
(Medium)

TABLE A.4b: Optimal Care for Patients with Urologic Conditions MVP Foundational Layer
Population Health Measures
Promoting Interoperability
(!!) Q479: Hospital-Wide, 30-Day, All-Cause Unplanned
Readmission (HWR) Rate for the Merit-Based Incentive Payment
Systems (MIPS) Groups
(Collection Type: Administrative Claims)
(!!) Q484: Clinician and Clinician Group Risk-standardized Hospital
Admission Rates for Patients with Multiple Chronic Conditions
(Collection Type: Administrative Claims)
Security Risk Analysis
High Priority Practices Safety Assurance Factors for EHR Resilience
Guide (SAFER Guide)
e-Prescribing
Query of Prescription Drug Monitoring Program (PDMP)
Provide Patients Electronic Access to Their Health Information
Support Electronic Referral Loops By Sending Health Information
AND
Support Electronic Referral Loops By Receiving and Reconciling
Health Information
OR
Health Information Exchange (HIE) Bi-Directional Exchange
OR
Enabling Exchange Under the Trusted Exchange Framework and
Common Agreement (TEFCA)
Immunization Registry Reporting
Syndromic Surveillance Reporting (Optional)
Electronic Case Reporting
Public Health Registry Reporting (Optional)
Clinical Data Registry Reporting (Optional)
Actions to Limit or Restrict Compatibility or Interoperability of
CEHRT
ONC Direct Review Attestation
A.5 Pulmonology Care MVP
The proposed Pulmonology Care MVP focuses on assessing optimal care for patients treated for a broad range of pulmonology
conditions including COPD, asthma, sleep apnea, and general pulmonology. This MVP would be most applicable to clinicians
who treat patients within the practice of pulmonology and sleep medicine, including nonphysician practitioners (NPPs) such as
nurse practitioners, and physician assistants.
Quality Measures
We are proposing to include nine MIPS quality measures and one QCDR measure within the quality performance category of this
MVP, which are specific to the clinical topic of pulmonology. We reviewed the MIPS quality measure inventory and considered
feedback received during the 2025 MVP candidate feedback period to determine which quality measures best represent the
clinical topic of this MVP.
The following quality measures provide a meaningful and comprehensive assessment of the clinical care for clinicians who
specialize in pulmonology:
• Q052: Chronic Obstructive Pulmonary Disease (COPD): Spirometry Evaluation for Long-Acting Inhaled
Bronchodilator Therapy: This MIPS quality measure ensures adults 18 and older diagnosed with COPD have
spirometry results documented airflow obstruction confirming diagnosis, and have been prescribed a long-acting
bronchodilator to provide proper treatment if symptomatic.
• Q277: Sleep Apnea: Severity Assessment at Initial Diagnosis: This MIPS quality measure ensures adults diagnosed
with obstructive sleep apnea have an appropriate assessment completed and documented at the time of diagnosis or
measured within 2 months of initial evaluation for suspected obstructive sleep apnea.
• Q279: Sleep Apnea: Assessment of Adherence to Obstructive Sleep Apnea (OSA) Therapy: This MIPS quality
measure ensures patients with a diagnosis of obstructive sleep apnea (OSA) who were prescribed an evidence-based
therapy with documentation that adherence to the therapy was assessed annually.
• Q398: Optimal Asthma Control: This MIPS quality measure assesses pediatric and adult patients to ensure their asthma
is well-controlled as demonstrated by one of three age-appropriate patient reported outcome tools and not at risk for
exacerbation.
•
ACEP25: Tobacco Use: Screening and Cessation Intervention for Patients with Asthma and COPD: This QCDR
measure ensures patients with asthma or COPD seen in the emergency department receive tobacco cessation if screened
positive for tobacco use.

The following broadly applicable MIPS quality measures are relevant to clinicians who specialize in pulmonology care. The
measures assess for age-specific screenings, and follow-up actions for select measures, in addition to recommended vaccinations:
• Q047: Advance Care Plan: This MIPS quality measure assesses for medical record documentation of an advance care
plan or surrogate decisions maker.
•
Q128: Preventive Care and Screening: Body Mass Index (BMI) Screening and Follow-Up Plan: This MIPS quality
measure assesses patients for a BMI documented with a follow-up plan documented if their most recent documented
BMI was outside of normal parameters.
•
Q226: Preventive Care and Screening: Tobacco Use: Screening and Cessation Intervention: This MIPS quality measure
ensures patients are screened for tobacco use and if screened positive receive tobacco cessation intervention.
•
Q487: Screening for Social Drivers of Health: This MIPS quality measure ensures adults are screened for food
insecurity, housing instability, transportation needs, utility difficulties, and interpersonal safety.
• Q503: Gains in Patient Activation Measure (PAM®) Scores at 12 Months: This MIPS quality measure ensures capture
of the patient voice and experience of care related to the patient’s understanding and confidence in the clinician’s
ability to manage their health and be an active partner in the health care journey.
Improvement Activities
We reviewed the Improvement Activities Inventory and considered feedback received during the 2025 MVP candidate feedback
period to determine the set of improvement activities to include in this MVP. We are proposing to include 11 improvement
activities that reflect actions and processes undertaken by clinicians who specialize in pulmonology care, as well as activities that
promote patient engagement and patient-centeredness, health equity, shared decision making, and care coordination. These
improvement activities provide opportunities for clinicians, in collaboration with patients, to drive outcomes and improve quality
of care. The following improvement activities are proposed for inclusion in this MVP:
• IA_AHE_3: Promote Use of Patient-Reported Outcome Tools
• IA_AHE_9: Implement Food Insecurity and Nutrition Risk Identification and Treatment Protocols
• IA_AHE_12: Practice Improvements that Engage Community Resources to Address Drivers of Health
• IA_BE_23: Integration of patient coaching practices between visits
• IA_CC_9: Implementation of practices/processes for developing regular individual care plans
• IA_EPA_2: Use of telehealth services that expand practice access
• IA_ERP_6: COVID-19 Vaccine Achievement for Practice Staff
• IA_MVP: Practice-Wide Quality Improvement in MIPS Value Pathways
• IA_PCMH: Electronic submission of Patient Centered Medical Home accreditation
• IA_PM_13: Chronic care and preventative care management for empaneled patients
• IA_PM_16: Implementation of medication management practice improvements
Cost Measures
We are proposing to include two MIPS cost measures within the cost performance category of this MVP, which apply to the
clinical topic of pulmonology care. We reviewed the MIPS cost measure inventory and considered feedback received during the
2025 MVP candidate feedback period to determine the set of cost measures to include in this MVP. The following cost measures
provide a meaningful assessment of the clinical care for clinicians who specialize in pulmonology and align with other measures
and activities within this MVP:
• Inpatient Chronic Obstructive Pulmonary Disease (COPD) Exacerbation: This MIPS episode-based cost measure
assesses costs associated with inpatient treatment for an acute exacerbation of COPD. This also aligns with quality
measures such as Q052: Chronic Obstructive Pulmonary Disease (COPD): Spirometry Evaluation for Long-Acting
Inhaled Bronchodilator Therapy.
• Asthma/Chronic Obstructive Pulmonary Disease (COPD): This MIPS episode-based cost measure assesses costs
associated with medical care to manage and treat asthma or COPD. This also aligns with quality measures such as
Q398: Optimal Asthma Control or ACEP25: Tobacco Use: Screening and Cessation Intervention for Patients with
Asthma and COPD.
Pulmonology Care MVP Tables
As stated in the introduction of this appendix, we considered measures and improvement activities available within the MIPS
inventory and selected those we determined best fit the clinical concept of the proposed Pulmonology Care MVP. We request
comment on the measures and activities included in this MVP.
Symbol Key:
Single asterisk (*): existing measures and improvement activities with proposed revisions
Double asterisk (**): measures and improvement activities only available when included in an MVP
Single exclamation point (!): high priority measures
Double exclamation point (!!): outcome measures
Tilde (~): measures and improvement activities that include a health equity component
Percent (%): attestation to IA_PCMH: Electronic submission of Patient Centered Medical Home accreditation provides full credit
for the improvement activity performance category

TABLE A.5a: Pulmonology Care MVP Measures and Improvement Activities
Quality
Improvement Activities
Cost
(*)(!) Q047: Advance Care Plan
(Collection Type: Medicare Part B Claims,
MIPS CQMs Specifications)
Q052: Chronic Obstructive Pulmonary
Disease (COPD): Spirometry Evaluation and
Long-Acting Inhaled Bronchodilator Therapy
(Collection Type: MIPS CQMs
Specifications)
(**) Q128: Preventive Care and Screening:
Body Mass Index (BMI) Screening and
Follow-Up Plan
(Collection Type: Medicare Part B Claims
Specifications, eCQM Specifications, MIPS
CQMs Specifications)
Q226: Preventive Care and Screening:
Tobacco Use: Screening and Cessation
Intervention
(Collection Type: Medicare Part B Claims
Specifications, eCQM Specifications, MIPS
CQMs Specifications)
(*) Q277: Sleep Apnea: Severity Assessment
at Initial Diagnosis
(Collection Type: MIPS CQMs
Specifications)
Q279: Sleep Apnea: Assessment of
Adherence to Obstructive Sleep Apnea (OSA)
Therapy
(Collection Type: MIPS CQMs
Specifications)
(!!) Q398: Optimal Asthma Control
(Collection Type: MIPS CQMs
Specifications)
(~)(!) Q487: Screening for Social Drivers of
Health
(Collection Type: MIPS CQMs
Specifications)
(*)(!!) Q503: Gains in Patient Activation
Measure (PAM®) Scores at 12 Months
(Collection Type: MIPS CQMs
Specifications)
ACEP25: Tobacco Use: Screening and
Cessation Intervention for Patients with
Asthma and COPD
(Collection Type: QCDR)
(~) IA_AHE_3: Promote use of Patient-
Reported Outcome Tools
(High)
(~) IA_AHE_9: Implement Food Insecurity
and Nutrition Risk Identification and
Treatment Protocols
(Medium)
(~) IA_AHE_12: Practice Improvements that
Engage Community Resources to Address
Drivers of Health
(High)
IA_BE_23: Integration of patient coaching
practices between visits
(Medium)
(~) IA_CC_9: Implementation of
practices/processes for developing regular
individual care plans
(Medium)
IA_EPA_2: Use of telehealth services that
expand practice access
(Medium)
(*) IA_ERP_6: COVID-19 Vaccine
Achievement for Practice Staff
(Medium)
(**) IA_MVP: Practice-Wide Quality
Improvement in MIPS Value Pathways
(High)
(%) IA_PCMH: Electronic submission of
Patient Centered Medical Home accreditation
IA_PM_13: Chronic care and preventative
care management for empaneled patients
(Medium)
IA_PM_16: Implementation of medication
management practice improvements
(Medium)
Inpatient Chronic Obstructive Pulmonary
Disease (COPD) Exacerbation
Asthma/Chronic Obstructive Pulmonary
Disease (COPD)
TABLE A.5b: Pulmonology Care MVP Foundational Layer
Population Health Measures
Promoting Interoperability
(!!) Q479: Hospital-Wide, 30-Day, All-Cause Unplanned
Readmission (HWR) Rate for the Merit-Based Incentive Payment
Systems (MIPS) Groups
(Collection Type: Administrative Claims)
(!!) Q484: Clinician and Clinician Group Risk-standardized Hospital
Admission Rates for Patients with Multiple Chronic Conditions
(Collection Type: Administrative Claims)
Security Risk Analysis
High Priority Practices Safety Assurance Factors for EHR Resilience
Guide (SAFER Guide)
e-Prescribing
Query of Prescription Drug Monitoring Program (PDMP)
Provide Patients Electronic Access to Their Health Information
Support Electronic Referral Loops By Sending Health Information
AND
Support Electronic Referral Loops By Receiving and Reconciling
Health Information
OR
Health Information Exchange (HIE) Bi-Directional Exchange
OR
Enabling Exchange Under the Trusted Exchange Framework and
Common Agreement (TEFCA)
Immunization Registry Reporting
Syndromic Surveillance Reporting (Optional)
Electronic Case Reporting
Public Health Registry Reporting (Optional)
Clinical Data Registry Reporting (Optional)
Actions to Limit or Restrict Compatibility or Interoperability of
CEHRT
ONC Direct Review Attestation
A.6 Surgical Care MVP
The proposed Surgical Care MVP focuses on the clinical theme of surgery. This MVP would be most applicable to clinicians
who treat patients within the surgical settings of general surgery, neurosurgery, cardiothoracic surgery, anesthesiologists,
including nonphysician practitioners (NPPs) such as certified registered nurse anesthetists (CRNAs), nurse practitioners, and
physician assistants.
Quality Measures
We are proposing to include 15 MIPS quality measures within the quality performance category of this MVP, which are specific
to the clinical theme of surgery. We reviewed the MIPS quality measure inventory and considered feedback received during the
2025 MVP candidate feedback period to determine which quality measures best represent the clinical topic of this MVP.
The following quality measures provide a meaningful and comprehensive assessment of the clinical care for clinicians who
specialize in surgery:
• Q164: Coronary Artery Bypass Graft (CABG): Prolonged Intubation: This MIPS quality measure identifies patients
who undergo an isolated CABG and require intubation > 24 hours following exit from the operating room.
• Q167: Coronary Artery Bypass Graft (CABG): Postoperative Renal Failure: This MIPS quality measure assesses
patients for postoperative renal failure or who require dialysis after CABG.
• Q168: Coronary Artery Bypass Graft (CABG): Surgical Re-Exploration: This MIPS quality measure identifies patients
who return to the operating room for surgical re-exploration following an isolated CABG surgery during the current
hospitalization.
• Q264: Sentinel Lymph Node Biopsy for Invasive Breast Cancer: This MIPS quality measure assesses the percentage of
patients with a diagnosis of primary invasive breast cancer who undergo a sentinel lymph node procedure.
• Q354: Anastomotic Leak Intervention: This MIPS quality measure evaluates for anastomotic leak intervention
following gastric bypass or colectomy surgery.
• Q355: Unplanned Reoperation within the 30-Day Postoperative Period: This MIPS quality measure evaluates for an
unplanned reoperation within 30 days of a denominator eligible procedure.
• Q357: Surgical Site Infection (SSI): This MIPS quality measure evaluates for SSI within 30 days of a denominator
eligible procedure.

• Q358: Patient-Centered Surgical Risk Assessment and Communication: This MIPS quality measure ensures a
personalized surgical risk assessment is completed on each patient using a validated risk calculator or multi-
institutional clinical data prior to the surgery along with discussion of the identified risks with the surgeon.
• Q445: Risk-Adjusted Operative Mortality for Coronary Artery Bypass Graft (CABG): This MIPS quality measure
assesses patients undergoing isolated CABG for quality outcomes by identifying the percentage of patients who die,
including all deaths occurring during CABG hospitalization and deaths occurring after discharge up to 30 days post
CABG surgery.
• Q459: Back Pain After Lumbar Surgery: This MIPS quality measure evaluates patients for a decrease in back pain post
lumbar surgery based upon predetermined benchmarks.
• Q461: Leg Pain After Lumbar Surgery: This MIPS quality measure evaluates patients for a decrease in leg pain post
lumbar surgery based upon predetermined benchmarks.
• Q471: Functional Status After Lumbar Surgery: This MIPS quality measure evaluates patients for an increase in
functional status post lumbar surgery based upon predetermined benchmarks.
The following broadly applicable MIPS quality measures are relevant to clinicians who treat patients in surgical settings. The
measures assess for age-specific screenings, and follow-up actions for select measures, in addition to recommended vaccinations:
• Q047: Advance Care Plan: This MIPS quality measure assesses for medical record documentation of an advance care
plan or surrogate decisions maker.
• Q226: Preventive Care and Screening: Tobacco Use: Screening and Cessation Intervention: This MIPS quality measure
ensures patients are screened for tobacco use and if screened positive receive tobacco cessation intervention.
•
Q487: Screening for Social Drivers of Health: This MIPS quality measure ensures adults are screened for food
insecurity, housing instability, transportation needs, utility difficulties, and interpersonal safety.
Improvement Activities
We reviewed the Improvement Activities Inventory and considered feedback received during the 2025 MVP candidate feedback
period to determine the set of improvement activities to include in this MVP. We are proposing to include 12 improvement
activities that reflect actions and processes undertaken by surgical care clinicians, as well as activities that promote patient
engagement and patient-centeredness, health equity, shared decision making, and care coordination. These improvement
activities provide opportunities for clinicians, in collaboration with patients, to drive outcomes and improve quality of care. The
following improvement activities are proposed for inclusion in this MVP:
• IA_AHE_3: Promote Use of Patient-Reported Outcome Tools
• IA_AHE_9: Implement Food Insecurity and Nutrition Risk Identification and Treatment Protocols
• IA_BE_12: Use evidence-based decision aids to support shared decision-making
• IA_CC_15: PSH Care Coordination
• IA_CC_17: Patient Navigator Program
• IA_CC_18: Relationship-Centered Communication
• IA_ERP_6: COVID-19 Vaccine Achievement for Practice Staff
• IA_MVP: Practice-Wide Quality Improvement in MIPS Value Pathways
• IA_PCMH: Electronic submission of Patient Centered Medical Home accreditation
• IA_PM_11: Regular review practices in place on targeted patient population needs
• IA_PSPA_7: Use of QCDR data for ongoing practice assessment and improvements
• IA_PSPA_8: Use of Patient Safety Tools
Cost Measures
We are proposing to include six MIPS cost measures within the cost performance category of this MVP, which apply to the
clinical theme of surgical care. We reviewed the MIPS cost measure inventory and considered feedback received during the 2025
MVP candidate feedback period to determine the set of cost measures to include in this MVP. The following cost measures
provide a meaningful assessment of the clinical care for clinicians who specialize in surgical care and align with other measures
and activities within this MVP:
• Colon and Rectal Resection: This MIPS episode-based cost measure assesses costs associated with colon or rectal
resections for either benign or malignant indications.
• Femoral or Inguinal Hernia Repair: This MIPS episode-based cost measure assesses costs associated with surgical
procedures to repair a femoral or inguinal hernia.
• Lumbar Spine Fusion for Degenerative Disease, 1-3 Levels: This MIPS episode-based cost measure assesses costs
associated with surgery for lumbar spine fusion. This also aligns with quality measures such as Q471: Functional
Status After Lumbar Surgery or Q461: Leg Pain After Lumbar Surgery.
• Lumpectomy, Partial Mastectomy, Simple Mastectomy: This MIPS episode-based cost measure assesses costs
associated with partial or total mastectomy for breast cancer.
• Medicare Spending Per Beneficiary (MSPB) Clinician: This MIPS cost measure applies to clinicians providing care in
inpatient hospitals, including those who treat patients within the surgical settings of general surgery, neurosurgery, and
cardiothoracic surgery.
• Non-Emergent Coronary Artery Bypass Graft (CABG): This MIPS episode-based cost measure assesses costs
associated with CABG. This also aligns with quality measures such as Q164: Coronary Artery Bypass Graft (CABG):
Prolonged Intubation or Q168: Coronary Artery Bypass Graft (CABG): Surgical Re-Exploration.

Surgical Care MVP Tables
As stated in the introduction of this appendix, we considered measures and improvement activities available within the MIPS
inventory and selected those we determined best fit the clinical concept of the proposed Surgical Care MVP. We request
comment on the measures and activities included in this MVP.
Symbol Key:
Single asterisk (*): existing measures and improvement activities with proposed revisions
Double asterisk (**): measures and improvement activities only available when included in an MVP
Single exclamation point (!): high priority measures
Double exclamation point (!!): outcome measures
Tilde (~): measures and improvement activities that include a health equity component
Percent (%): attestation to IA_PCMH: Electronic submission of Patient Centered Medical Home accreditation provides full credit
for the improvement activity performance category
TABLE A.6a: Surgical Care MVP Measures and Improvement Activities
Quality
Improvement Activities
Cost
(*)(!) Q047: Advance Care Plan
(Collection Type: Medicare Part B Claims
Specifications, MIPS CQMs Specifications)
(!!) Q164: Coronary Artery Bypass Graft
(CABG): Prolonged Intubation
(Collection Type: MIPS CQMs
Specifications)
(!!) Q167: Coronary Artery Bypass Graft
(CABG): Postoperative Renal Failure
(Collection Type: MIPS CQMs
Specifications)
(*)(!!) Q168: Coronary Artery Bypass Graft
(CABG): Surgical Re-Exploration
(Collection Type: MIPS CQMs
Specifications)
Q226: Preventive Care and Screening:
Tobacco Use: Screening and Cessation
Intervention
(Collection Type: Medicare Part B Claims
Specifications, eCQM Specifications, MIPS
CQMs Specifications)
Q264: Sentinel Lymph Node Biopsy for
Invasive Breast Cancer
(Collection Type: MIPS CQMs
Specifications)
(!!) Q354: Anastomotic Leak Intervention
(Collection Type: MIPS CQMs
Specifications)
(*)(!!) Q355: Unplanned Reoperation within
the 30-Day Postoperative Period
(Collection Type: MIPS CQMs
Specifications)
(!!) Q357: Surgical Site Infection (SSI)
(Collection Type: MIPS CQMs
Specifications)
(!) Q358: Patient-Centered Surgical Risk
Assessment and Communication
(Collection Type: MIPS CQMs
Specifications)
(!!) Q445: Risk-Adjusted Operative Mortality
for Coronary Artery Bypass Graft (CABG)
(Collection Type: MIPS CQMs
Specifications)
(!!) Q459: Back Pain After Lumbar Surgery
(Collection Type: MIPS CQMs
Specifications)
(~) IA_AHE_3: Promote use of Patient-
Reported Outcome Tools
(High)
(~) IA_AHE_9: Implement Food Insecurity
and Nutrition Risk Identification and
Treatment Protocols
(Medium)
IA_BE_12: Use evidence-based decision aids
to support shared decision-making
(Medium)
IA_CC_15: PSH Care Coordination
(High)
IA_CC_17: Patient Navigator Program
(High)
IA_CC_18: Relationship-Centered
Communication
(Medium)
(*) IA_ERP_6: COVID-19 Vaccine
Achievement for Practice Staff
(Medium)
(**) IA_MVP: Practice-Wide Quality
Improvement in MIPS Value Pathways
(High)
(%) IA_PCMH: Electronic submission of
Patient Centered Medical Home accreditation
(~) IA_PM_11: Regular review practices in
place on targeted patient population needs
(Medium)
(~) IA_PSPA_7: Use of QCDR data for
ongoing practice assessment and
improvements
(Medium)
IA_PSPA_8: Use of Patient Safety Tools
(Medium)
Colon and Rectal Resection
Femoral or Inguinal Hernia Repair
Lumbar Spine Fusion for Degenerative
Disease, 1-3 Levels
Lumpectomy, Partial Mastectomy, Simple
Mastectomy
Medicare Spending Per Beneficiary
(MSPB) Clinician
Non-Emergent Coronary Artery Bypass
Graft (CABG)

Quality
Improvement Activities
Cost
(!!) Q461: Leg Pain After Lumbar Surgery
(Collection Type: MIPS CQMs
Specifications)
(!!) Q471: Functional Status After Lumbar
Surgery
(Collection Type: MIPS CQMs
Specifications)
(~)(!) Q487: Screening for Social Drivers of
Health
(Collection Type: MIPS CQMs
Specifications)

TABLE A.6b: Surgical Care MVP Foundational Layer
Population Health Measures
Promoting Interoperability
(!!) Q479: Hospital-Wide, 30-Day, All-Cause Unplanned
Readmission (HWR) Rate for the Merit-Based Incentive Payment
Systems (MIPS) Groups
(Collection Type: Administrative Claims)
(!!) Q484: Clinician and Clinician Group Risk-standardized Hospital
Admission Rates for Patients with Multiple Chronic Conditions
(Collection Type: Administrative Claims)
Security Risk Analysis
High Priority Practices Safety Assurance Factors for EHR Resilience
Guide (SAFER Guide)
e-Prescribing
Query of Prescription Drug Monitoring Program (PDMP)
Provide Patients Electronic Access to Their Health Information
Support Electronic Referral Loops By Sending Health Information
AND
Support Electronic Referral Loops By Receiving and Reconciling
Health Information
OR
Health Information Exchange (HIE) Bi-Directional Exchange
OR
Enabling Exchange Under the Trusted Exchange Framework and
Common Agreement (TEFCA)
Immunization Registry Reporting
Syndromic Surveillance Reporting (Optional)
Electronic Case Reporting
Public Health Registry Reporting (Optional)
Clinical Data Registry Reporting (Optional)
Actions to Limit or Restrict Compatibility or Interoperability of
CEHRT
ONC Direct Review Attestation

Group B: Modifications to Previously Finalized MVPs for the CY 2025 Performance
Period/2027 MIPS Payment Year and Future Years
B.1: Adopting Best Practices and Promoting Patient Safety within Emergency Medicine
MVP
The B.1 tables represent the measures and activities finalized within the Adopting Best Practices and Promoting Patient Safety
within Emergency Medicine MVP (88 FR 88029 through 80032) with modifications proposed for the CY 2025 performance
period/2027 MIPS payment year and future years. We request comment on the proposed modifications included in this MVP.
Quality Measures
We are proposing to modify the previously finalized Adopting Best Practices and Promoting Patient Safety within Emergency
Medicine MVP to remove one MIPS quality measure as it is a process measure and has become standard of care, based upon
MIPS performance data showing continued high performance.
• Q254: Ultrasound Determination of Pregnancy Location for Pregnant Patients with Abdominal Pain
Improvement Activities
For the reasons stated in the introduction of this appendix
1023
, we are proposing the following: add the proposed modified
IA_ERP_6 to all new and previously finalized MVPs because of the importance of vaccination status in practice settings; remove
the weights associated with the improvement activities contained in this MVP; and remove one improvement activity being
proposed for removal from MIPS:
• IA_CC_2: Implementation of improvements that contribute to more timely communication of test results
We are proposing to modify the IA_BE_4: Engagement of patients through implementation of improvements in patient portal
improvement activity, which includes a proposed activity title update. Please see Appendix 2, Improvement Activities: Table
Group B of this proposed rule for all proposed revisions to this activity.
Symbol Key:
Plus sign (+): proposed additions of MIPS quality measures, improvement activities, or cost measures
Single asterisk (*): existing measures and improvement activities with proposed revisions
Double asterisk (**): measures and improvement activities only available when included in an MVP
Single exclamation point (!): high priority measures
Double exclamation point (!!): outcome measures
Tilde (~): measures and improvement activities that include a health equity component
Percent (%): attestation to IA_PCMH: Electronic submission of Patient Centered Medical Home accreditation provides full credit
for the improvement activity performance category
1023
See Improvement Activity Policy Update and Global Inclusion of an Improvement Activity
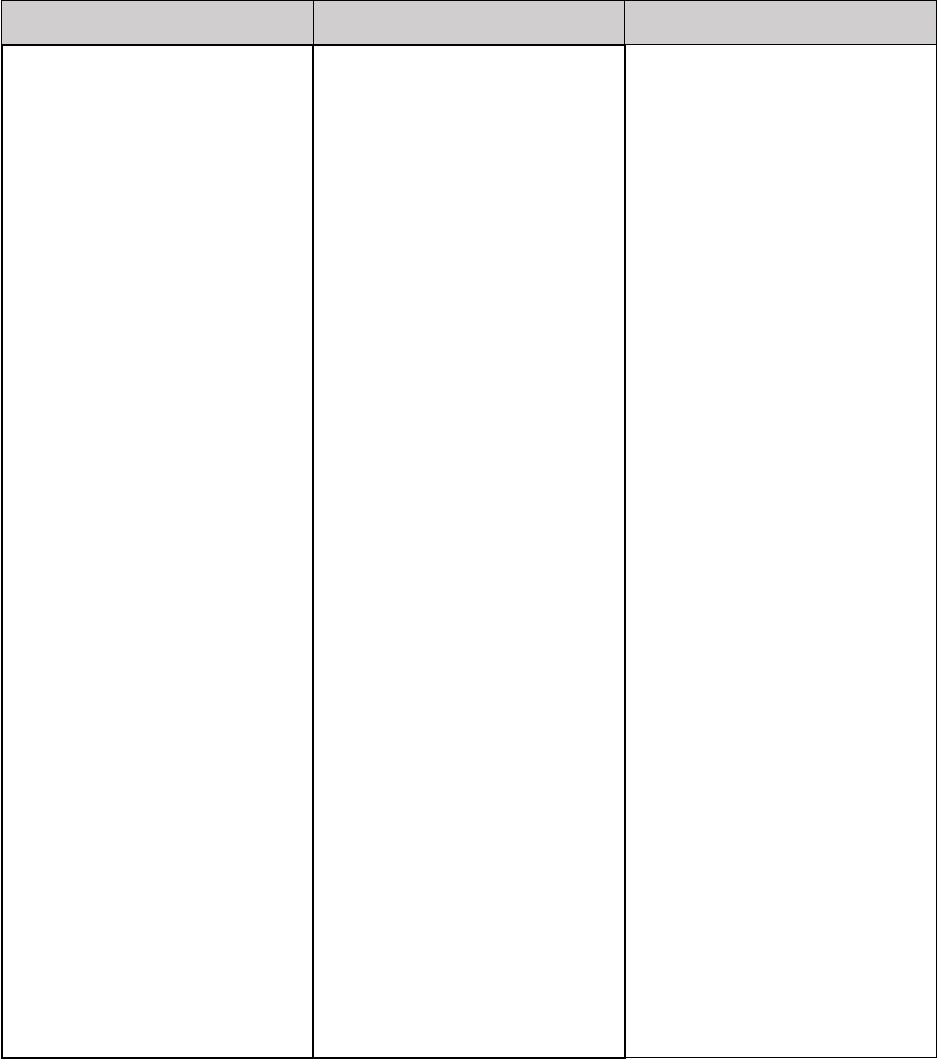
TABLE B.1a: Adopting Best Practices and Promoting Patient Safety within Emergency Medicine MVP Measures and
Improvement Activities
Quality
Improvement Activities
Cost
(!) Q065: Appropriate Treatment for Upper
Respiratory Infection (URI)
(Collection Type: eCQM Specifications,
MIPS CQMs Specifications)
(!) Q116: Avoidance of Antibiotic
Treatment for Acute Bronchitis/Bronchiolitis
(Collection Type: MIPS CQMs
Specifications)
(!) Q321: CAHPS for MIPs Clinician/Group
Survey
(Collection Type: CAHPS Survey Vendor)
(*)(!) Q331: Adult Sinusitis: Antibiotic
Prescribed for Acute Viral Sinusitis
(Overuse)
(Collection Type: MIPS CQMs
Specifications)
(!) Q415: Emergency Medicine: Emergency
Department Utilization of CT for Minor
Blunt Head Trauma for Patients Aged 18
Years and Older
(Collection Type: MIPS CQMs
Specifications)
(!) Q416: Emergency Medicine: Emergency
Department Utilization of CT for Minor
Blunt Head Trauma for Patients Aged 2
Through 17 Years
(Collection Type: MIPS CQMs
Specifications)
(~)(!) Q487: Screening for Social Drivers of
Health
(Collection Type: MIPS CQMs
Specifications)
(!!) ACEP50: ED Median Time from ED
arrival to ED departure for all Adult Patients
(Collection Type: QCDR)
(!) ACEP52: Appropriate Emergency
Department Utilization of Lumbar Spine
Imaging for Atraumatic Low Back Pain
(Collection Type: QCDR)
(!) ECPR46: Avoidance of Opiates for Low
Back Pain or Migraines
(Collection Type: QCDR)
(!) HCPR24: Appropriate Utilization of
Vancomycin for Cellulitis
(Collection Type: QCDR)
(~) IA_AHE_12: Practice Improvements
that Engage Community Resources to
Address Drivers of Health
(High)
(*) IA_BE_4:
Engagement of patients
through implementation of improvements in
patient portal
(Medium)
IA_BE_6: Regularly Assess Patient
Experience of Care and Follow Up on
Findings
(High)
IA_BMH_12: Promoting Clinician Well-
Being
(High)
(+)(*) IA_ERP_6: COVID-19 Vaccine
Achievement for Practice Staff
(Medium)
(**) IA_MVP: Practice-Wide Quality
Improvement in MIPS Value Pathways
(High)
(%) IA_PCMH: Electronic submission of
Patient Centered Medical Home
accreditation
IA_PSPA_1: Participation in an AHRQ-
listed patient safety organization
(Medium)
(~) IA_PSPA_7: Use of QCDR data for
ongoing practice assessment and
improvements
(Medium)
IA_PSPA_15: Implementation of an
Antimicrobial Stewardship Program (ASP)
(Medium)
Emergency Medicine

TABLE B.1b: Adopting Best Practices and Promoting Patient Safety within Emergency Medicine MVP Foundational
Layer
Population Health Measures
Promoting Interoperability
(!!) Q479: Hospital-Wide, 30-Day, All-Cause Unplanned
Readmission (HWR) Rate for the Merit-Based Incentive Payment
Systems (MIPS) Groups
(Collection Type: Administrative Claims)
(!!) Q484: Clinician and Clinician Group Risk-standardized Hospital
Admission Rates for Patients with Multiple Chronic Conditions
(Collection Type: Administrative Claims)
Security Risk Analysis
High Priority Practices Safety Assurance Factors for EHR Resilience
Guide (SAFER Guide)
e-Prescribing
Query of Prescription Drug Monitoring Program (PDMP)
Provide Patients Electronic Access to Their Health Information
Support Electronic Referral Loops By Sending Health Information
AND
Support Electronic Referral Loops By Receiving and Reconciling
Health Information
OR
Health Information Exchange (HIE) Bi-Directional Exchange
OR
Enabling Exchange Under the Trusted Exchange Framework and
Common Agreement (TEFCA)
Immunization Registry Reporting
Syndromic Surveillance Reporting (Optional)
Electronic Case Reporting
Public Health Registry Reporting (Optional)
Clinical Data Registry Reporting (Optional)
Actions to Limit or Restrict Compatibility or Interoperability of
CEHRT
ONC Direct Review Attestation
B.2: Advancing Cancer Care MVP
The B.2 tables represent the measures and activities finalized within the Advancing Cancer Care MVP (88 FR 80008 through
80011) with modifications proposed for the CY 2025 performance period/2027 MIPS payment year and future years. We request
comment on the proposed modifications included in this MVP.
Quality Measures
We are proposing to modify the previously finalized Advancing Cancer Care MVP within the quality performance category of
this MVP to include six additional MIPS quality measures and one additional QCDR measure that address appropriate cancer
care treatment. We reviewed the MIPS quality measure inventory and considered feedback received during the 2025 MVP
maintenance period to determine which quality measures to include in this MVP.
The following quality measures proposed within this MVP provide a meaningful and comprehensive assessment of the clinical
care for clinicians providing care to patients with cancer:
• Q102: Prostate Cancer: Avoidance of Overuse of Bone Scan for Staging Low Risk Prostate Cancer Patients: This MIPS
quality measure assesses overuse of bone scans for patients with prostate cancer with low incidence of recurrence
receiving treatment.
• TBD: Positive PD-L1 Biomarker Expression Test Result Prior to First-Line Immune Checkpoint Inhibitor Therapy:
This proposed MIPS quality measure ensures timely biomarker testing for patients with a diagnosis of metastatic non-
small cell lung cancer or squamous cell carcinoma of head and neck on first-line immune checkpoint inhibitor (ICI)
therapy.
• TBD: Appropriate Germline Testing for Ovarian Cancer Patients: This proposed MIPS quality measure assesses
patients with a diagnosed with epithelial ovarian, fallopian tube, or primary peritoneal cancer for completion germline
testing within 6 months of diagnosis.
• TBD: Patient-Reported Pain Interference Following Chemotherapy among Adults with Breast Cancer: This proposed
MIPS quality measure assesses pain interference following chemotherapy administered with curative intent to adult
patients with breast cancer.
•
TBD: Patient-Reported Fatigue Following Chemotherapy among Adults with Breast Cancer: This proposed MIPS
quality measure assess fatigue following chemotherapy administered with curative intent to adult patients with breast
cancer.

•
TBD: Utilization of Prophylactic GCSF for Cancer Patients Receiving Low-Risk Chemotherapy (inverse measure):
The intent of this QCDR measure is to assess prophylactic use of granulocyte colony stimulating factor (G-CSF) when
it is not indicated for low-risk chemotherapy and is not restricted to metastatic colorectal cancer.
In addition, we are proposing the following broadly applicable MIPS quality measure, which is relevant to patients receiving
cancer care and their experience of their health care treatment journey:
• Q495: Ambulatory Palliative Care Patients’ Experience of Feeling Heard and Understood: This MIPS quality measure
ensures palliative care clinicians and/or teams are actively engaged to ensure patients are understood in a significant
and empowering way.
We are proposing to modify the previously finalized Advancing Cancer Care MVP to remove two MIPS quality measures as they
are duplicative in concept to current MIPS quality measures and are being proposed for removal from MIPS:
• Q144: Oncology: Medical and Radiation - Plan of Care for Pain
• Q452: Patients with Metastatic Colorectal Cancer and RAS (KRAS or NRAS) Gene Mutation Spared Treatment with
Anti-epidermal Growth Factor Receptor (EGFR) Monoclonal Antibodies
We are also proposing to remove one QCDR measure which will be replaced by TBD: Utilization of Prophylactic GCSF for
Cancer Patients Receiving Low-Risk Chemotherapy (inverse measure), which has a broader denominator and is not restricted to
metastatic colorectal cancer.
• PIMSH2: Oncology: Utilization of GCSF in Metastatic Colorectal Cancer
Improvement Activities
For the reasons stated in the introduction of this appendix
1024
, we are proposing the following: add the proposed modified
IA_ERP_6 to all new and previously finalized MVPs because of the importance of vaccination status in practice settings; and add
an additional improvement activity that addresses maintenance requests from the public, as well as addresses priority areas
including food insecurity and the incorporation of patient voices into health care decision making:
• IA_AHE_9: Implement Food Insecurity and Nutrition Risk Identification and Treatment Protocols
For the reasons stated in the introduction of this appendix, we are proposing the following: remove the weights associated with
the improvement activities contained in this MVP; and remove three improvement activities being proposed for removal from
MIPS:
• IA_CC_1: Implementation of Use of Specialist Reports Back to Referring Clinician or Group to Close Referral Loop
• IA_EPA_1: Provide 24/7 Access to MIPS Eligible Clinicians or Groups Who Have Real-Time Access to Patient's
Medical Record
• IA_ERP_4: Implementation of a Personal Protective Equipment (PPE) Plan
We are proposing to modify the IA_BE_4: Engagement of patients through implementation of improvements in patient portal
improvement activity, which includes a proposed activity title update. Please see Appendix 2, Improvement Activities: Table
Group B of this proposed rule for all proposed revisions to this activity.
Cost Measures
We are proposing to add one MIPS cost measure within the cost performance category of this MVP, which applies to the clinical
topic of cancer care. We reviewed the MIPS cost measure inventory and considered feedback received from interested parties
through the MVP maintenance process to determine the cost measures to include in this MVP. The following cost measure
provides a meaningful assessment of the clinical care for clinicians who specialize in cancer care and aligns with other measures
and activities included within this MVP:
• Prostate Cancer: This proposed MIPS episode-based cost measure would assess costs associated with prostate cancer.
This also aligns with quality measures such as Q102: Prostate Cancer: Avoidance of Overuse of Bone Scan for Staging
Low Risk Prostate Cancer Patients. We note that this is a new cost measure we are proposing to adopt as described in
section IV.A.4.e.(2)(a)(iii)of this proposed rule.
Symbol Key:
Plus sign (+): proposed additions of MIPS quality measures, improvement activities, or cost measures
Carat symbol (^): new proposed measures and improvement activities
Single asterisk (*): existing measures and improvement activities with proposed revisions
Double asterisk (**): measures and improvement activities only available when included in an MVP
Single exclamation point (!): high priority measures
Double exclamation point (!!): outcome measures
Tilde (~): measures and improvement activities that include a health equity component
Percent (%): attestation to IA_PCMH: Electronic submission of Patient Centered Medical Home accreditation provides full credit
for the improvement activity performance category
TABLE B.2a: Advancing Cancer Care MVP Measures and Improvement Activities
1024
See MVP Development: Improvement Activity Policy Update and Global Inclusion of an Improvement Activity

Quality
Improvement Activities
Cost
(*)(!) Q047: Advance Care Plan
(Collection Type: Medicare Part B Claims
Measure Specifications, MIPS CQMs
Specifications)
(+)(!) Q102: Prostate Cancer: Avoidance of
Overuse of Bone Scan for Staging Low Risk
Prostate Cancer Patients
(Collection Type: eCQM Specifications,
MIPS CQMs Specifications)
Q134: Preventive Care and Screening:
Screening for Depression and Follow-Up
Plan
(Collection Type: Medicare Part B Claims
Measure Specifications, eCQM
Specifications, MIPS CQMs Specifications)
(*)(!) Q143: Oncology: Medical and
Radiation – Pain Intensity Quantified
(Collection Type: eCQM Specifications,
MIPS CQMs Specifications)
(!) Q321: CAHPS for MIPS Clinician/Group
Survey
(Collection Type: CAHPS Survey Vendor)
(*)(!) Q450: Appropriate Treatment for
Patients with Stage I (T1c) – III HER2
Positive Breast Cancer
(Collection Type: MIPS CQMs
Specifications)
(*) Q451: RAS (KRAS and NRAS) Gene
Mutation Testing Performed for Patients
with Metastatic Colorectal Cancer who
receive Anti-epidermal Growth Factor
Receptor (EGFR) Monoclonal Antibody
Therapy
(Collection Type: MIPS CQMs
Specifications)
(!) Q453: Percentage of Patients Who Died
from Cancer Receiving Systemic Cancer-
Directed Therapy in the Last 14 Days of Life
(lower score – better)
(Collection Type: MIPS CQMs
Specifications)
(!) Q457: Percentage of Patients Who Died
from Cancer Admitted to Hospice for Less
than 3 days (lower score – better)
(Collection Type: MIPS CQMs
Specifications)
(*) Q462: Bone Density Evaluation for
Patients with Prostate Cancer and Receiving
Androgen Deprivation Therapy
(Collection Type: eCQM Specifications)
(~)(!) Q487: Screening for Social Drivers of
Health
(Collection Type: MIPS CQMs
Specifications)
(*) Q490: Appropriate Intervention of
Immune-related Diarrhea and/or Colitis in
Patients Treated with Immune Checkpoint
Inhibitors
(Collection Type: MIPS CQMs
Specifications)
(+)(!!) Q495: Ambulatory Palliative Care
Patients’ Experience of Feeling Heard and
Understood
(+)(~) IA_AHE_9: Implement Food
Insecurity and Nutrition Risk Identification
and Treatment Protocols
(Medium)
(*) IA_BE_4:
Engagement of patients
through implementation of improvements in
patient portal
(Medium)
IA_BE_6: Regularly Assess Patient
Experience of Care and Follow Up on
Findings
(High)
IA_BE_15: Engagement of patients, family
and caregivers in developing a plan of care
(Medium)
IA_BE_24: Financial Navigation Program
(Medium)
IA_BMH_12: Promoting Clinician Well-
Being
(High)
IA_CC_13: Practice Improvements to align
with OpenNotes principles
(Medium)
IA_CC_17: Patient Navigator Program
(High)
IA_EPA_2: Use of telehealth services that
expand practice access
(Medium)
(+)(*) IA_ERP_6: COVID-19 Vaccine
Achievement for Practice Staff
(Medium)
(**) IA_MVP: Practice-Wide Quality
Improvement in MIPS Value Pathways
(High)
(%) IA_PCMH: Electronic submission of
Patient Centered Medical Home
accreditation
(~) IA_PM_14: Implementation of
methodologies for improvements in
longitudinal care management for high risk
patients
(Medium)
IA_PM_15: Implementation of episodic
care management practice improvements
(Medium)
IA_PM_16: Implementation of medication
management practice improvements
(Medium)
IA_PM_21: Advance Care Planning
(Medium)
IA_PSPA_13: Participation in Joint
Commission Evaluation Initiative
(Medium)
IA_PSPA_16: Use decision support—
ideally platform-agnostic, interoperable
clinical decision support (CDS) tools —and
standardized treatment protocols to manage
(^)(+) Prostate Cancer
Total Per Capita Cost (TPCC)

Quality
Improvement Activities
Cost
(Collection Type: MIPS CQMs
Specifications)
(*)(!!) Q503: Gains in Patient Activation
Measure (PAM®) Scores at 12 Months
(Collection Type: MIPS CQMs
Specifications)
(+)(^)(!) TBD: Positive PD-L1 Biomarker
Expression Test Result Prior to First-Line
Immune Checkpoint Inhibitor Therapy
(Collection Type: MIPS CQMs
Specifications)
(+)(^) TBD: Appropriate Germline Testing
for Ovarian Cancer Patients
(Collection Type: MIPS CQMs
Specifications)
(+)(^)(!!) TBD: Patient-Reported Pain
Interference Following Chemotherapy
among Adults with Breast Cancer
(Collection Type: MIPS CQMs
Specifications)
(+)(^)(!!) TBD: Patient-Reported Fatigue
Following Chemotherapy among Adults
with Breast Cancer
(Collection Type: MIPS CQMs
Specifications)
(!) PIMSH13: Oncology: Mutation testing
for Stage IV Lung Cancer Completed Prior
to start of Targeted Therapy
(Collection Type: QCDR)
(+)(!) TBD: Utilization of Prophylactic
GCSF for Cancer Patients Receiving Low-
Risk Chemotherapy (inverse measure)
(Collection Type: QCDR)
workflow on the care team to meet patient
needs
(Medium)
IA_PSPA_28: Completion of an Accredited
Safety or Quality Improvement Program
(Medium)

TABLE B.2b: Adopting Best Practices and Promoting Patient Safety within Emergency Medicine MVP Foundational
Layer
Population Health Measures
Promoting Interoperability
(!!) Q479: Hospital-Wide, 30-Day, All-Cause Unplanned
Readmission (HWR) Rate for the Merit-Based Incentive Payment
Systems (MIPS) Groups
(Collection Type: Administrative Claims)
(!!) Q484: Clinician and Clinician Group Risk-standardized Hospital
Admission Rates for Patients with Multiple Chronic Conditions
(Collection Type: Administrative Claims)
Security Risk Analysis
High Priority Practices Safety Assurance Factors for EHR Resilience
Guide (SAFER Guide)
e-Prescribing
Query of Prescription Drug Monitoring Program (PDMP)
Provide Patients Electronic Access to Their Health Information
Support Electronic Referral Loops By Sending Health Information
AND
Support Electronic Referral Loops By Receiving and Reconciling
Health Information
OR
Health Information Exchange (HIE) Bi-Directional Exchange
OR
Enabling Exchange Under the Trusted Exchange Framework and
Common Agreement (TEFCA)
Immunization Registry Reporting
Syndromic Surveillance Reporting (Optional)
Electronic Case Reporting
Public Health Registry Reporting (Optional)
Clinical Data Registry Reporting (Optional)
Actions to Limit or Restrict Compatibility or Interoperability of
CEHRT
ONC Direct Review Attestation
B.3: Advancing Care for Heart Disease MVP
The B.3 tables represent the measures and activities finalized within the Advancing Care for Heart Disease MVP (88 FR 80022
through 80025) with modifications proposed for the CY 2025 performance period/2027 MIPS payment year and future years. We
request comment on the proposed modifications included in this MVP.
Quality Measures
We are proposing to modify the previously finalized Advancing Care for Heart Disease MVP within the quality performance
category of this MVP to include one additional broadly applicable MIPS quality measure relevant to patients receiving care for
heart disease. We reviewed the quality measure inventory and considered feedback received during the 2025 MVP maintenance
period to determine which quality measures to include in this MVP.
• Q495: Ambulatory Palliative Care Patients’ Experience of Feeling Heard and Understood: This MIPS quality measure
ensures palliative care clinicians and/or teams empathize to ensure patients are understood in a significant and
empowering way.
Improvement Activities
For the reasons stated in the introduction of this appendix
1025
, we are proposing the following: add the proposed modified
IA_ERP_6 to all new and previously finalized MVPs because of the importance of vaccination status in practice settings; and
remove the weights associated with the improvement activities contained in this MVP.
Cost
We are not proposing to modify the MIPS cost measures included within the cost performance category of this previously
finalized Advancing Care for Heart Disease MVP by proposing to add or remove cost measures from the MVP.
However, we are proposing to modify the ST-Elevation Myocardial Infarction (STEMI) with Percutaneous Coronary
Intervention (PCI) cost measure, which includes a proposed measure title update. Please see section IV.A.4.e.(2)(a)(vii) of this
proposed rule for all proposed revisions to this cost measure.
1025
See MVP Development: Improvement Activity Policy Update and Global Inclusion of an Improvement Activity

Symbol Key:
Plus sign (+): proposed additions of MIPS quality measures, improvement activities, or cost measures
Single asterisk (*): existing measures and improvement activities with proposed revisions
Double asterisk (**): measures and improvement activities only available when included in an MVP
Single exclamation point (!): high priority measures
Double exclamation point (!!): outcome measures
Tilde (~): measures and improvement activities that include a health equity component
Percent (%): attestation to IA_PCMH: Electronic submission of Patient Centered Medical Home accreditation provides full credit
for the improvement activity performance category
TABLE B.3a: Advancing Care for Heart Disease MVP Measures and Improvement Activities
Quality
Improvement Activities
Cost
Q005: Heart Failure (HF): Angiotensin-
Converting Enzyme (ACE) Inhibitor or
Angiotensin Receptor Blocker (ARB) or
Angiotensin Receptor-Neprilysin Inhibitor
(ARNI) Therapy for Left Ventricular
Systolic Dysfunction (LVSD)
(Collection Type: eCQM Specifications,
MIPS CQMs Specifications)
Q006: Coronary Artery Disease (CAD):
Antiplatelet Therapy
(Collection Type: MIPS CQMs
Specifications)
Q007: Coronary Artery Disease (CAD):
Beta-Blocker Therapy – Prior Myocardial
Infarction (MI) or Left Ventricular Systolic
Dysfunction (LVEF ≤ 40%)
(Collection Type: eCQM Specifications,
MIPS CQMs Specifications)
Q008: Heart Failure (HF): Beta-Blocker
Therapy for Left Ventricular Systolic
Dysfunction (LVSD)
(Collection Type: eCQM Specifications,
MIPS CQMs Specifications)
(*)(!) Q047: Advance Care Plan
(Collection Type: Medicare Part B Claims
Measure Specifications, MIPS CQMs
Specifications)
Q118: Coronary Artery Disease (CAD):
Angiotensin-Converting Enzyme (ACE)
Inhibitor or Angiotensin Receptor Blocker
(ARB) Therapy - Diabetes or Left
Ventricular Systolic Dysfunction (LVEF
≤40%)
(Collection Type: MIPS CQMs
Specifications)
(**) Q128: Preventive Care and Screening:
Body Mass Index (BMI) Screening and
Follow-Up Plan
(Collection Type: Medicare Part B Claims
Measure Specifications, eCQM
Specifications, MIPS CQMs Specifications)
Q134: Preventive Care and Screening:
Screening for Depression and Follow-Up
Plan
(Collection Type: Medicare Part B Claims
Measure Specifications, eCQM
Specifications, MIPS CQMs Specifications)
(*)(!) Q238: Use of High-Risk Medications
in Older Adults
(Collection Type: eCQM Specifications,
MIPS CQMs Specifications)
(!) Q243: Cardiac Rehabilitation Patient
Referral from an Outpatient Setting
(~) IA_AHE_9: Implement Food Insecurity
and Nutrition Risk Identification and
Treatment Protocols
(Medium)
(~) IA_AHE_12: Practice Improvements
that Engage Community Resources to
Address Drivers of Health
(High)
IA_BE_6: Regularly Assess Patient
Experience of Care and Follow Up on
Findings
(High)
IA_BE_12: Use evidence-based decision
aids to support shared decision-making
(Medium)
IA_BE_15: Engagement of patients, family
and caregivers in developing a plan of care
(Medium)
IA_BE_24: Financial Navigation Program
(Medium)
IA_BE_25: Drug Cost Transparency
(High)
(~) IA_CC_9: Implementation of
practices/processes for developing regular
individual care plans
(Medium)
(+)(*) IA_ERP_6: COVID-19 Vaccine
Achievement for Practice Staff
(Medium)
(**) IA_MVP: Practice-Wide Quality
Improvement in MIPS Value Pathways
(High)
(%) IA_PCMH: Electronic submission of
Patient Centered Medical Home
accreditation
IA_PM_13: Chronic care and preventative
care management for empaneled patients
(Medium)
(~) IA_PM_14: Implementation of
methodologies for improvements in
longitudinal care management for high-risk
patients
(Medium)
IA_PSPA_4: Administration of the AHRQ
Survey of Patient Safety Culture
(Medium)
Elective Outpatient Percutaneous
Coronary Intervention (PCI)
Heart Failure
(*) ST-Elevation Myocardial Infarction
(STEMI) with Percutaneous Coronary
Intervention (PCI)
Medicare Spending Per Beneficiary
(MSPB) Clinician
Total Per Capita Cost (TPCC)

Quality
Improvement Activities
Cost
(Collection Type: MIPS CQMs
Specifications)
Q326: Atrial Fibrillation and Atrial Flutter:
Chronic Anticoagulation Therapy
(Collection Type: MIPS CQMs
Specifications)
(!) Q377: Functional Status Assessments for
Heart Failure
(Collection Type: eCQM Specifications)
(!!) Q392: Cardiac Tamponade and/or
Pericardiocentesis Following Atrial
Fibrillation Ablation
(Collection Type: MIPS CQMs
Specifications)
(*)(!!) Q393: Infection within 180 Days of
Cardiac Implantable Electronic Device
(CIED) Implantation, Replacement, or
Revision
(Collection Type: MIPS CQMs
Specifications)
(!!) Q441: Ischemic Vascular Disease (IVD)
All or None Outcome Measure (Optimal
Control)
(Collection Type: MIPS CQMs
Specifications)
(~)(!) Q487: Screening for Social Drivers of
Health
(Collection Type: MIPS CQMs
Specifications)
(*)(!!) Q492: Risk-Standardized Acute
Cardiovascular-Related Hospital Admission
Rates for Patients with Heart Failure under
the Merit-based Incentive Payment System
(Collection Type: Administrative Claims)
(+)(!!) Q495: Ambulatory Palliative Care
Patients’ Experience of Feeling Heard and
Understood
(Collection Type: MIPS CQMs
Specifications)
(*)(!!) Q503: Gains in Patient Activation
Measure (PAM®) Scores at 12 Months
(Collection Type: MIPS CQMs
Specifications)
(~) IA_PSPA_7: Use of QCDR data for
ongoing practice assessment and
improvements
(Medium)
TABLE B.3b: Advancing Care for Heart Disease MVP Foundational Layer

Population Health Measures
Promoting Interoperability
(!!) Q479: Hospital-Wide, 30-Day, All-Cause Unplanned
Readmission (HWR) Rate for the Merit-Based Incentive Payment
Systems (MIPS) Groups
(Collection Type: Administrative Claims)
(!!) Q484: Clinician and Clinician Group Risk-standardized Hospital
Admission Rates for Patients with Multiple Chronic Conditions
(Collection Type: Administrative Claims)
Security Risk Analysis
High Priority Practices Safety Assurance Factors for EHR Resilience
Guide (SAFER Guide)
e-Prescribing
Query of Prescription Drug Monitoring Program (PDMP)
Provide Patients Electronic Access to Their Health Information
Support Electronic Referral Loops By Sending Health Information
AND
Support Electronic Referral Loops By Receiving and Reconciling
Health Information
OR
Health Information Exchange (HIE) Bi-Directional Exchange
OR
Enabling Exchange Under the Trusted Exchange Framework and
Common Agreement (TEFCA)
Immunization Registry Reporting
Syndromic Surveillance Reporting (Optional)
Electronic Case Reporting
Public Health Registry Reporting (Optional)
Clinical Data Registry Reporting (Optional)
Actions to Limit or Restrict Compatibility or Interoperability of
CEHRT
ONC Direct Review Attestation
B.4: Advancing Rheumatology Patient Care MVP
The B.4 tables represent the measures and activities finalized within the Advancing Rheumatology Patient Care MVP (88 FR
80026 through 80029) with modifications proposed for the CY 2025 performance period/2027 MIPS payment year and future
years. We request comment on the proposed modifications included in this MVP.
Quality Measures
We are proposing to modify the previously finalized Advancing Rheumatology Patient Care MVP within the quality performance
category of this MVP to include one additional MIPS quality measure and two QCDR measures that are relevant to patients
receiving rheumatology care. We reviewed the MIPS quality measure inventory and considered feedback received during the
2025 MVP maintenance period to determine which quality measures to include in this MVP.
The following quality measures proposed within this MVP provide address appropriate clinical care for patients with
rheumatological conditions:
• Q039: Screening for Osteoporosis for Women Aged 65-85 Years of Age: This MIPS quality measure assesses women
who have ever received a dual-energy x-ray absorptiometry (DXA) test to evaluate for the disease osteoporosis.
• UREQA2: Ankylosing Spondylitis: Appropriate Pharmacologic Therapy: This QCDR measure assesses patients newly
diagnosed with ankylosing spondylitis for appropriate pharmacologic therapy by ensuring a course of NSAIDs is
prescribed before initiation of biologics during the first six months of treatment.
• UREQA9: Screening for Osteoporosis for Men Aged 70 Years and Older: This QCDR measure identifies male patients
who have ever had a central dual-energy X-ray absorptiometry (DXA) to screen for osteoporosis to identify
osteoporotic risk for fracture.
Improvement Activities
For the reasons stated in the introduction of this appendix
1026
, we are proposing the following: add the proposed modified
IA_ERP_6 to all new and previously finalized MVPs because of the importance of vaccination status in practice settings; remove
the weights associated with the improvement activities contained in this MVP; and remove one improvement activities being
proposed for removal from MIPS:
1026
See MVP Development: Improvement Activity Policy Update and Global Inclusion of an Improvement Activity

• IA_EPA_1: Provide 24/7 Access to MIPS Eligible Clinicians or Groups Who Have Real-Time Access to Patient's
Medical Record
We are proposing to modify the IA_BE_4: Engagement of patients through implementation of improvements in patient portal
improvement activity, which includes a proposed activity title update. Please see Appendix 2, Improvement Activities: Table
Group B of this proposed rule for all proposed revisions to this activity.
Cost Measures
We are proposing to add one MIPS cost measure within the cost performance category of this MVP, which applies to the clinical
topic of rheumatology care. We reviewed the MIPS cost measure inventory and considered feedback received from interested
parties through the MVP maintenance process to determine the cost measures to include in this MVP. The following cost
measure provides a meaningful assessment of the clinical care for clinicians who specialize in rheumatology care and aligns with
the other measures and activities included within this MVP:
• Rheumatoid Arthritis: This proposed MIPS episode-based cost measure would assess costs associated with medical
care to manage and treat rheumatoid arthritis. This also aligns with quality measures such as Q177: Rheumatoid
Arthritis (RA): Periodic Assessment of Disease Activity or Q178: Rheumatoid Arthritis (RA): Functional Status
Assessment. We note that this is a new cost measure we are proposing to adopt as described in section IV.A.4.e.(2)(a)
(iii) of this proposed rule.
Symbol Key:
Plus sign (+): proposed additions of MIPS quality measures, improvement activities, or cost measures
Carat symbol (^): new proposed measures and improvement activities
Single asterisk (*): existing measures and improvement activities with proposed revisions
Double asterisk (**): measures and improvement activities only available when included in an MVP
Single exclamation point (!): high priority measures
Double exclamation point (!!): outcome measures
Tilde (~): measures and improvement activities that include a health equity component
Percent (%): attestation to IA_PCMH: Electronic submission of Patient Centered Medical Home accreditation provides full credit
for the improvement activity performance category
TABLE B.4a: Advancing Rheumatology Patient Care MVP Measures and Improvement Activities
Quality
Improvement Activities
Cost
(+) Q039: Screening for Osteoporosis for
Women Aged 65-85 Years of Age
(Collection Type: Medicare Part B Claims
Measure Specifications, MIPS CQMs
Specification)
(*)(!) Q130: Documentation of Current
Medications in the Medical Record
(Collection Type: eCQM Specifications,
MIPS CQMs Specifications)
Q134: Preventive Care and Screening:
Screening for Depression and Follow-Up
Plan
(Collection Type: Medicare Part B Claims
Measure Specifications, eCQM
Specifications, MIPS CQMs Specifications)
(*) Q176: Tuberculosis Screening Prior to
First Course of Biologic and/or Immune
Response Modifier Therapy
(Collection Type: MIPS CQMs
Specifications)
(*) Q177: Rheumatoid Arthritis (RA):
Periodic Assessment of Disease Activity
(Collection Type: MIPS CQMs
Specifications)
(*) Q178: Rheumatoid Arthritis (RA):
Functional Status Assessment
(Collection Type: MIPS CQMs
Specifications)
(*) Q180: Rheumatoid Arthritis (RA):
Glucocorticoid Management
(Collection Type: MIPS CQMs
Specifications)
(~) IA_AHE_3: Promote use of Patient-
Reported Outcome Tools
(High)
(~) IA_BE_1: Use of certified EHR to
capture patient reported outcomes
(Medium)
(*) IA_BE_4: Engagement of patients
through implementation of improvements in
patient portal
(Medium)
IA_BE_6: Regularly Assess Patient
Experience of Care and Follow Up on
Findings
(High)
IA_BE_15: Engagement of patients, family
and caregivers in developing a plan of care
(Medium)
IA_BE_24: Financial Navigation Program
(Medium)
IA_BE_25: Drug Cost Transparency
(High)
IA_BMH_2: Tobacco use
(Medium)
IA_EPA_2: Use of telehealth services that
expand practice access
(Medium)
(+)(*) IA_ERP_6: COVID-19 Vaccine
Achievement for Practice Staff
(Medium)
(^)(+) Rheumatoid Arthritis
Total Per Capita Cost (TPCC)

Quality
Improvement Activities
Cost
(~)(!) Q487: Screening for Social Drivers of
Health
(Collection Type: MIPS CQMs
Specifications)
(*) Q493: Adult Immunization Status
(Collection Type: MIPS CQMs
Specifications)
(*)(!!) Q503: Gains in Patient Activation
Measure (PAM®) Scores at 12 Months
(Collection Type: MIPS CQMs
Specifications)
ACR12: Disease Activity Measurements for
Patients with PsA
(Collection Type: QCDR)
(!!) ACR14: Gout Serum Urate Target
(Collection Type: QCDR)
(!) ACR15: Safe Hydroxychloroquine
Dosing
(Collection Type: QCDR)
(+)(!) UREQA2: Ankylosing Spondylitis:
Appropriate Pharmacologic Therapy
(Collection Type: QCDR)
(+) UREQA9: Screening for Osteoporosis
for Men Aged 70 Years and Older
(Collection Type: QCDR)
(!!) UREQA10: Ankylosing Spondylitis:
Controlled Disease Or Improved Disease
Function
(Collection Type: QCDR)
(**) IA_MVP: Practice-Wide Quality
Improvement in MIPS Value Pathways
(High)
(%) IA_PCMH: Electronic submission of
Patient Centered Medical Home
accreditation
IA_PM_16: Implementation of medication
management practice improvements
(Medium)
IA_PSPA_28: Completion of an Accredited
Safety or Quality Improvement Program
(Medium)

TABLE B.4b: Advancing Rheumatology Patient Care MVP Foundational Layer
Population Health Measures
Promoting Interoperability
(!!) Q479: Hospital-Wide, 30-Day, All-Cause Unplanned
Readmission (HWR) Rate for the Merit-Based Incentive Payment
Systems (MIPS) Groups
(Collection Type: Administrative Claims)
(!!) Q484: Clinician and Clinician Group Risk-standardized Hospital
Admission Rates for Patients with Multiple Chronic Conditions
(Collection Type: Administrative Claims)
Security Risk Analysis
High Priority Practices Safety Assurance Factors for EHR Resilience
Guide (SAFER Guide)
e-Prescribing
Query of Prescription Drug Monitoring Program (PDMP)
Provide Patients Electronic Access to Their Health Information
Support Electronic Referral Loops By Sending Health Information
AND
Support Electronic Referral Loops By Receiving and Reconciling
Health Information
OR
Health Information Exchange (HIE) Bi-Directional Exchange
OR
Enabling Exchange Under the Trusted Exchange Framework and
Common Agreement (TEFCA)
Immunization Registry Reporting
Syndromic Surveillance Reporting (Optional)
Electronic Case Reporting
Public Health Registry Reporting (Optional)
Clinical Data Registry Reporting (Optional)
Actions to Limit or Restrict Compatibility or Interoperability of
CEHRT
ONC Direct Review Attestation
B.5: Coordinating Stroke Care to Promote Prevention and Cultivate Positive Outcomes
MVP
The B.5 tables represent the measures and activities finalized within the Coordinating Stroke Care to Promote Prevention and
Cultivate Positive Outcomes MVP (88 FR 80039 through 80041) with modifications proposed for the CY 2025 performance
period/2027 MIPS payment year and future years. We request comment on the proposed modifications included in this MVP.
Quality Measures
We are proposing to modify the previously finalized Coordinating Stroke Care to Promote Prevention and Cultivate Positive
Outcomes MVP to include one additional broadly applicable MIPS quality measure relevant to patients receiving stroke care and
their experience of their health care treatment journey. We reviewed the MIPS quality measure inventory and considered
feedback received during the 2025 MVP maintenance period to determine which quality measures to include in this MVP.
• Q495: Ambulatory Palliative Care Patients’ Experience of Feeling Heard and Understood: This MIPS quality measure
ensures palliative care clinicians and/or teams empathize to ensure patients are understood in a significant and
empowering way.
We are also proposing to modify the previously finalized Coordinating Stroke Care to Promote Prevention and Cultivate Positive
Outcomes MVP to remove one MIPS quality measure as this measure is proposed for removal from MIPS:
• Q409: Clinical Outcome Post Endovascular Stroke Treatment
We are proposing to modify the Q344: Rate of Carotid Artery Stenting (CAS) for Asymptomatic Patients, Without Major
Complications (Discharged to Home by Post-Operative Day #2) quality measure, which includes a proposed measure title update.
Please see Appendix 1: MIPS Quality Measures, Table Group D of this proposed rule for all proposed revisions to this measure.
Improvement Activities
For the reasons stated in the introduction of this appendix
1027
, we are proposing the following: add the proposed modified
IA_ERP_6 to all new and previously finalized MVPs because of the importance of vaccination status in practice settings; remove
the weights associated with the improvement activities contained in this MVP; and remove one improvement activity being
proposed for removal from MIPS:
1027
See MVP Development: Improvement Activity Policy Update and Global Inclusion of an Improvement Activity
• IA_CC_2: Implementation of improvements that contribute to more timely communication of test results
We are proposing to modify the IA_BE_4: Engagement of patients through implementation of improvements in patient portal
improvement activity, which includes a proposed activity title update. Please see Appendix 2, Improvement Activities: Table
Group B of this proposed rule for all proposed revisions to this activity.
Symbol Key:
Plus sign (+): proposed additions of MIPS quality measures, improvement activities, or cost measures
Single asterisk (*): existing measures and improvement activities with proposed revisions
Double asterisk (**): measures and improvement activities only available when included in an MVP
Single exclamation point (!): high priority measures
Double exclamation point (!!): outcome measures
Tilde (~): measures and improvement activities that include a health equity component
Percent (%): attestation to IA_PCMH: Electronic submission of Patient Centered Medical Home accreditation provides full credit
for the improvement activity performance category

TABLE B.5a: Coordinating Stroke Care to Promote Prevention and Cultivate Positive Outcomes MVP Measures and
Improvement Activities
Quality
Improvement Activities
Cost
(*)(!) Q047: Advance Care Plan
(Collection Type: Medicare Part B Claims
Measure Specifications, MIPS CQMs
Specifications)
Q187: Stroke and Stroke Rehabilitation:
Thrombolytic Therapy
(Collection Type: MIPS CQMs
Specifications)
(*)(!!) Q236: Controlling High Blood
Pressure
(Collection Type: Medicare Part B Claims
Measure Specifications, eCQM
Specifications, MIPS CQMs Specifications)
Q326: Atrial Fibrillation and Atrial Flutter:
Chronic Anticoagulation Therapy
(Collection Type: MIPS CQMs
Specifications)
(*)(!!) Q344: Rate of Carotid Artery
Stenting (CAS) for Asymptomatic Patients,
Without Major Complications (Discharged
to Home by Post-Operative Day #2)
(Collection Type: MIPS CQMs
Specifications)
(*)(!!) Q413: Door to Puncture Time for
Endovascular Stroke Treatment
(Collection Type: MIPS CQMs
Specifications)
Q438: Statin Therapy for the Prevention and
Treatment of Cardiovascular Disease
(Collection Type: eCQM Specifications,
MIPS CQMs Specifications)
(!!) Q441: Ischemic Vascular Disease (IVD)
All or None Outcome Measure (Optimal
Control)
(Collection Type: MIPS CQMs
Specifications)
(~)(!) Q487: Screening for Social Drivers of
Health
(Collection Type: MIPS CQMs
Specifications)
(+)(!!) Q495: Ambulatory Palliative Care
Patients’ Experience of Feeling Heard and
Understood
(Collection Type: MIPS CQMs
Specifications)
(~) IA_AHE_9: Implement Food Insecurity
and Nutrition Risk Identification and
Treatment Protocols
(Medium)
(~) IA_BE_1: Use of certified EHR to
capture patient reported outcomes
(Medium)
(*) IA_BE_4: Engagement of patients
through implementation of improvements in
patient portal
(Medium)
IA_BE_6: Regularly Assess Patient
Experience of Care and Follow Up on
Findings
(High)
IA_BE_24: Financial Navigation Program
(Medium)
(~) IA_BMH_15: Behavioral/Mental Health
and Substance Use Screening and Referral
for Older Adults
(High)
IA_CC_13: Practice improvements to align
with OpenNotes principles
(Medium)
IA_CC_17: Patient Navigator Program
(High)
(+)(*) IA_ERP_6: COVID-19 Vaccine
Achievement for Practice Staff
(Medium)
(**) IA_MVP: Practice-Wide Quality
Improvement in MIPS Value Pathways
(High)
(%) IA_PCMH: Electronic submission of
Patient Centered Medical Home
accreditation
IA_PM_13: Chronic care and preventative
care management for empaneled patients
(Medium)
IA_PM_15: Implementation of episodic
care management practice improvements
(Medium)
Intracranial Hemorrhage or Cerebral
Infarction

TABLE B.5b: Coordinating Stroke Care to Promote Prevention and Cultivate Positive Outcomes MVP Foundational
Layer
Population Health Measures
Promoting Interoperability
(!!) Q479: Hospital-Wide, 30-Day, All-Cause Unplanned
Readmission (HWR) Rate for the Merit-Based Incentive Payment
Systems (MIPS) Groups
(Collection Type: Administrative Claims)
(!!) Q484: Clinician and Clinician Group Risk-standardized Hospital
Admission Rates for Patients with Multiple Chronic Conditions
(Collection Type: Administrative Claims)
Security Risk Analysis
High Priority Practices Safety Assurance Factors for EHR Resilience
Guide (SAFER Guide)
e-Prescribing
Query of Prescription Drug Monitoring Program (PDMP)
Provide Patients Electronic Access to Their Health Information
Support Electronic Referral Loops By Sending Health Information
AND
Support Electronic Referral Loops By Receiving and Reconciling
Health Information
OR
Health Information Exchange (HIE) Bi-Directional Exchange
OR
Enabling Exchange Under the Trusted Exchange Framework and
Common Agreement (TEFCA)
Immunization Registry Reporting
Syndromic Surveillance Reporting (Optional)
Electronic Case Reporting
Public Health Registry Reporting (Optional)
Clinical Data Registry Reporting (Optional)
Actions to Limit or Restrict Compatibility or Interoperability of
CEHRT
ONC Direct Review Attestation
B.6: Focusing on Women’s Health MVP
The B.6 tables represent the measures and activities finalized within the Focusing on Women’s Health MVP (88 FR 79981
through 79986) with modifications proposed for the CY 2025 performance period/2027 MIPS payment year and future years. We
request comment on the proposed modifications included in this MVP.
Quality Measures
We are proposing to modify the previously finalized Focusing on Women’s Health MVP within the quality performance category
of this MVP to include to include one additional MIPS quality measure that is relevant to women’s health. We reviewed the
MIPS quality measure inventory and considered feedback received during the 2025 MVP maintenance period to determine which
quality measures to include in this MVP.
The following quality measure proposed within this MVP provides a meaningful and comprehensive assessment of the clinical
care for clinicians providing women’s health care to patients:
• Q039: Screening for Osteoporosis for Women Aged 65-85 Years of Age: This MIPS quality measure assesses women
who have ever received a dual-energy x-ray absorptiometry (DXA) test to evaluate for the disease osteoporosis.
We are also proposing to modify the previously finalized Focusing on Women’s Health MVP to remove one MIPS quality
measure as it is a process measure that has become standard of care, based on MIPS performance data as demonstrated by the
measure’s high performance in the PY2024 MIPS Historical Quality Benchmarks file, as well as previous year’s benchmark data,
and is being proposed for removal from MIPS:
• Q472: Appropriate Use of DXA Scans in Women Under 65 Years Who Do Not Meet the Risk Factor Profile for
Osteoporotic Fracture
We are proposing to modify the Q432: Proportion of Patients Sustaining a Bladder Injury at the Time of any Pelvic Organ
Prolapse Repair quality measure, which includes a proposed measure title update. Please see Appendix 1: MIPS Quality
Measures, Table Group D of this proposed rule for all proposed revisions to this measure.
Improvement Activities
For the reasons stated in the introduction of this appendix
1028
, we are proposing the following: add the modified proposed
IA_ERP_6 to all new and previously finalized MVPs because of the importance of vaccination status in practice settings; and
remove the weights associated with the improvement activities contained in this MVP.
We are proposing to modify the IA_BE_4: Engagement of patients through implementation of improvements in patient portal
improvement activity, which includes a proposed activity title update. Please see Appendix 2, Improvement Activities: Table
Group B of this proposed rule for all proposed revisions to this activity.
Symbol Key:
Plus sign (+): proposed additions of MIPS quality measures, improvement activities, or cost measures
Single asterisk (*): existing measures and improvement activities with proposed revisions
Double asterisk (**): measures and improvement activities only available when included in an MVP
Single exclamation point (!): high priority measures
Double exclamation point (!!): outcome measures
Tilde (~): measures and improvement activities that include a health equity component
Percent (%): attestation to IA_PCMH: Electronic submission of Patient Centered Medical Home accreditation provides full credit
for the improvement activity performance category
TABLE B.6a: Focusing on Women’s Health MVP Measures and Improvement Activities
Quality
Improvement Activities
Cost
(+) Q039: Screening for Osteoporosis for
Women Aged 65-85 Years of Age
(Collection Type: Medicare Part B Claims
Measure Specifications, MIPS CQMs
Specification)
Q048: Urinary Incontinence: Assessment of
Presence or Absence of Urinary Incontinence in
Women Aged 65 Years and Older
(Collection Type: MIPS CQMs Specifications)
(*)(**) Q112: Breast Cancer Screening
(Collection Type: Medicare Part B Claims
Measure Specifications, eCQM Specifications,
MIPS CQMs Specification)
Q134: Preventive Care and Screening:
Screening for Depression and Follow-Up Plan
(Collection Type: Medicare Part B Claims
Measure Specifications, eCQM Specifications,
MIPS CQMs Specification)
Q226: Preventive Care and Screening: Tobacco
Use: Screening and Cessation Intervention
(Collection Type: Medicare Part B Claims
Measure Specifications, eCQM Specifications,
MIPS CQMs Specification)
Q309: Cervical Cancer Screening
(Collection Type: eCQM Specifications)
Q310: Chlamydia Screening in Women
(Collection Type: eCQM Specifications)
(!!) Q335: Maternity Care: Elective Delivery
(Without Medical Indication) at < 39 Weeks
(Overuse)
(Collection Type: MIPS CQMs Specifications)
(*)(!) Q336: Maternity Care: Postpartum
Follow-up and Care Coordination
(Collection Type: MIPS CQMs Specifications)
Q400: One-Time Screening for Hepatitis C
Virus (HCV) and Treatment Initiation
(Collection Type: MIPS CQMs Specifications)
(!) Q422: Performing Cystoscopy at the Time
of Hysterectomy for Pelvic Organ
Prolapse to Detect Lower Urinary
Tract Injury
(~) IA_AHE_1: Enhance Engagement of
Medicaid and Other Underserved Populations
(High)
(~) IA_AHE_3: Promote use of Patient-
Reported Outcome Tools
(High)
(~) IA_AHE_9: Implement Food Insecurity
and Nutrition Risk Identification and
Treatment Protocols
(Medium)
(~) IA_AHE_12: Practice Improvements that
Engage Community Resources to Address
Drivers of Health
(High)
(*) IA_BE_4: Engagement of patients through
implementation of improvements in patient
portal
(Medium)
(~) IA_BE_16: Promote Self-management in
Usual Care
(Medium)
(~) IA_BMH_11: Implementation of a
Trauma-Informed Care (TIC) Approach to
Clinical Practice
(Medium)
(~) IA_BMH_14: Behavioral/Mental Health
and Substance Use Screening and Referral for
Pregnant and Postpartum Women
(High)
(~) IA_CC_9: Implementation of
practices/processes for developing regular
individual care plans
(Medium)
IA_EPA_2: Use of telehealth services that
expand practice access
(Medium)
(+)(*) IA_ERP_6: COVID-19 Vaccine
Achievement for Practice Staff
(Medium)
Medicare Spending Per Beneficiary
(MSPB) Clinician
Total Per Capita Cost (TPCC)
1028
See MVP Development: Improvement Activity Policy Update and Global Inclusion of an Improvement Activity

Quality
Improvement Activities
Cost
(Collection Type: Medicare Part B Claims
Measure Specifications, MIPS CQMs
Specifications)
Q431: Preventive Care and Screening:
Unhealthy Alcohol Use: Screening & Brief
Counseling
(Collection Type: MIPS CQMs Specifications)
(*)(!!) Q432: Proportion of Patients Sustaining
a Bladder Injury at the Time of any Pelvic
Organ Prolapse Repair
(Collection Type: MIPS CQMs Specifications)
(*)(!) Q448: Appropriate Workup Prior to
Endometrial Ablation
(Collection Type: MIPS CQMs Specifications)
Q475: HIV Screening
(Collection Type: eCQM Specifications)
(~)(!) Q487: Screening for Social Drivers of
Health
(Collection Type: MIPS CQMs Specifications)
(*) Q493: Adult Immunization Status
(Collection Type: MIPS CQMs Specifications)
Q496: Cardiovascular Disease (CVD) Risk
Assessment Measure - Proportion of
Pregnant/Postpartum Patients that Receive
CVD Risk Assessment with a Standardized
Instrument
(Collection Type: MIPS CQMs Specifications)
(!!) UREQA8: Vitamin D level: Effective
Control of Low Bone Mass/Osteopenia and
Osteoporosis: Therapeutic Level Of 25 OH
Vitamin D Level Achieved
(Collection Type: QCDR)
(**) IA_MVP: Practice-Wide Quality
Improvement in MIPS Value Pathways
(High)
(%) IA_PCMH: Electronic submission of
Patient Centered Medical Home accreditation
(~) IA_PM_6: Use of toolsets or other
resources to close healthcare disparities across
communities
(Medium)
(~) IA_PM_23: Use of Computable
Guidelines and Clinical Decision Support to
Improve Adherence for Cervical Cancer
Screening and Management Guidelines
(High)

TABLE B.6b: Focusing on Women’s Health MVP Foundational Layer
Population Health Measures
Promoting Interoperability
(!!) Q479: Hospital-Wide, 30-Day, All-Cause Unplanned
Readmission (HWR) Rate for the Merit-Based Incentive
Payment Systems (MIPS) Groups
(Collection Type: Administrative Claims)
(!!) Q484: Clinician and Clinician Group Risk-standardized
Hospital Admission Rates for Patients with Multiple Chronic
Conditions
(Collection Type: Administrative Claims)
Security Risk Analysis
High Priority Practices Safety Assurance Factors for EHR Resilience Guide
(SAFER Guide)
e-Prescribing
Query of Prescription Drug Monitoring Program (PDMP)
Provide Patients Electronic Access to Their Health Information
Support Electronic Referral Loops By Sending Health Information
AND
Support Electronic Referral Loops By Receiving and Reconciling Health
Information
OR
Health Information Exchange (HIE) Bi-Directional Exchange
OR
Enabling Exchange Under the Trusted Exchange Framework and Common
Agreement (TEFCA)
Immunization Registry Reporting
Syndromic Surveillance Reporting (Optional)
Electronic Case Reporting
Public Health Registry Reporting (Optional)
Clinical Data Registry Reporting (Optional)
Actions to Limit or Restrict Compatibility or Interoperability of CEHRT
ONC Direct Review Attestation

B.7: Improving Care for Lower Extremity Joint Repair MVP
The B.7 tables represent the measures and activities finalized within the Improving Care for Lower Extremity Joint Repair MVP
(88 FR 80033 through 80035) with modifications proposed for the CY 2025 performance period/2027 MIPS payment year and
future years. We request comment on the proposed modifications included in this MVP.
Improvement Activities
For the reasons stated in the introduction of this appendix
1029
, we are proposing the following: add the proposed modified
IA_ERP_6 to all new and previously finalized MVPs because of the importance of vaccination status in practice settings; and add
an additional improvement activity that addresses maintenance requests from the public, as well as addresses priority areas
including food insecurity and the incorporation of patient voices into health care decision making:
• IA_AHE_9: Implement Food Insecurity and Nutrition Risk Identification and Treatment Protocols
In addition, we are proposing the following: remove the weights associated with the improvement activities contained in this
MVP; and remove one improvement activity being proposed for removal from MIPS:
• IA_PSPA_27: Invasive Procedure or Surgery Anticoagulation Medication Management
Symbol Key:
Plus sign (+): proposed additions of MIPS quality measures, improvement activities, or cost measures
Single asterisk (*): existing measures and improvement activities with proposed revisions
Double asterisk (**): measures and improvement activities only available when included in an MVP
Single exclamation point (!): high priority measures
Double exclamation point (!!): outcome measures
Tilde (~): measures and improvement activities that include a health equity component
Percent (%): attestation to IA_PCMH: Electronic submission of Patient Centered Medical Home accreditation provides full credit
for the improvement activity performance category
1029
See MVP Development: Improvement Activity Policy Update and Global Inclusion of an Improvement Activity

TABLE B.7a: Improving Care for Lower Extremity Joint Repair MVP Measures and Improvement Activities
Quality
Improvement Activities
Cost
(!) Q024: Communication with the
Physician or Other Clinician Managing On-
Going Care Post-Fracture for Men and
Women Aged 50 Years and Older
(Collection Type: Medicare Part B Claims
Measure Specifications, MIPS CQMs
Specifications)
(**) Q128: Preventive Care and Screening:
Body Mass Index (BMI) Screening and
Follow-Up Plan
(Collection Type: Medicare Part B Claims
Measure Specifications, eCQM
Specifications, MIPS CQMs Specifications)
(!) Q350: Total Knee or Hip Replacement:
Shared Decision-Making: Trial of
Conservative (Non-surgical) Therapy
(Collection Type: MIPS CQMs
Specifications)
(!) Q351: Total Knee or Hip Replacement:
Venous Thromboembolic and
Cardiovascular Risk Evaluation
(Collection Type: MIPS CQMs
Specifications)
(*)(!) Q376: Functional Status Assessment
for Total Hip Replacement
(Collection Type: eCQM Specifications)
(*)(!!) Q470: Functional Status After
Primary Total Knee Replacement
(Collection Type: MIPS CQMs
Specifications)
(!!) Q480: Risk-standardized complication
rate (RSCR) following elective primary total
hip arthroplasty (THA) and/or total knee
arthroplasty (TKA) for Merit-based
Incentive Payment System (MIPS)
(Collection Type: Administrative Claims)
(~)(!) Q487: Screening for Social Drivers of
Health
(Collection Type: MIPS CQMs
Specifications)
(~) IA_AHE_3: Promote use of Patient-
Reported Outcome Tools
(High)
(+)(~) IA_AHE_9: Implement Food
Insecurity and Nutrition Risk Identification
and Treatment Protocols (Medium Weight)
(Medium)
IA_BE_6: Regularly Assess Patient
Experience of Care and Follow Up on
Findings
(High)
IA_BE_12 Use evidence-based decision
aids to support shared decision-making
(Medium)
IA_CC_7: Regular training in care
coordination
(Medium)
(~) IA_CC_9: Implementation of
practices/processes for developing regular
individual care plans
(Medium)
IA_CC_13: Practice improvements to align
with OpenNotes principles
(Medium)
IA_CC_15: PSH Care Coordination
(High)
(+)(*) IA_ERP_6: COVID-19 Vaccine
Achievement for Practice Staff
(Medium)
(**) IA_MVP: Practice-Wide Quality
Improvement in MIPS Value Pathways
(High)
(%) IA_PCMH: Electronic submission of
Patient Centered Medical Home
accreditation
(~) IA_PSPA_7: Use of QCDR data for
ongoing practice assessment and
improvements
(Medium)
(~) IA_PSPA_18: Measurement and
improvement at the practice and panel level
(Medium)
Elective Primary Hip Arthroplasty
Knee Arthroplasty

TABLE B.7b: Improving Care for Lower Extremity Joint Repair MVP Foundational Layer
Population Health Measures
Promoting Interoperability
(!!) Q479: Hospital-Wide, 30-Day, All-Cause Unplanned
Readmission (HWR) Rate for the Merit-Based Incentive Payment
Systems (MIPS) Groups
(Collection Type: Administrative Claims)
(!!) Q484: Clinician and Clinician Group Risk-standardized Hospital
Admission Rates for Patients with Multiple Chronic Conditions
(Collection Type: Administrative Claims)
Security Risk Analysis
High Priority Practices Safety Assurance Factors for EHR Resilience
Guide (SAFER Guide)
e-Prescribing
Query of Prescription Drug Monitoring Program (PDMP)
Provide Patients Electronic Access to Their Health Information
Support Electronic Referral Loops By Sending Health Information
AND
Support Electronic Referral Loops By Receiving and Reconciling
Health Information
OR
Health Information Exchange (HIE) Bi-Directional Exchange
OR
Enabling Exchange Under the Trusted Exchange Framework and
Common Agreement (TEFCA)
Immunization Registry Reporting
Syndromic Surveillance Reporting (Optional)
Electronic Case Reporting
Public Health Registry Reporting (Optional)
Clinical Data Registry Reporting (Optional)
Actions to Limit or Restrict Compatibility or Interoperability of
CEHRT
ONC Direct Review Attestation
B.8: Optimal Care for Kidney Health MVP
The B.8 tables represent the measures and activities finalized within the Optimal Care for Kidney Health MVP (88 FR 80012
through 80015) with modifications proposed for the CY 2025 performance period/2027 MIPS payment year and future years. We
request comment on the proposed modifications included in this MVP.
Quality Measures
We are proposing to modify the previously finalized Optimal Care for Kidney Health MVP within the quality performance
category of this MVP to include three additional broadly applicable MIPS quality measures that are relevant to patients receiving
care for kidney health. We reviewed the quality measure inventory and considered feedback received during the 2025 MVP
maintenance period to determine which quality measures to include in this MVP.
• Q495: Ambulatory Palliative Care Patients’ Experience of Feeling Heard and Understood: This MIPS quality measure
ensures palliative care clinicians and/or teams empathize to ensure patients are understood in a significant and
empowering way.
• TBD: First Year Standardized Waitlist Ratio (FYSWR): This MIPS quality measure measures number of incident
(newly initiated on dialysis) patients in a practitioner (inclusive of physicians and advanced practice providers) groups
who were listed on the kidney or kidney-pancreas transplant waitlist or received a living donor transplant within the
first year of initiating dialysis.
• TBD: Percentage of Prevalent Patients Waitlisted (PPPW) and Percentage of Prevalent Patients Waitlisted in Active
Status (aPPPW): This MIPS quality measure tracks dialysis patients who at a practitioner group practice who were on
the kidney or kidney-pancreas transplant waitlist (all patients or patients in active status).
We are proposing to modify the Q001: Diabetes: Hemoglobin A1c (HbA1c) Poor Control (>9%) quality measure, which includes
a proposed measure title update. Please see Appendix 1: MIPS Quality Measures, Table Group D of this proposed rule for all
proposed revisions to this measure.
Improvement Activities
For the reasons stated in the introduction of this appendix
1030
, we are proposing the following: add the proposed modified
IA_ERP_6 to all new and previously finalized MVPs because of the importance of vaccination status in practice settings; remove
1030
See MVP Development: Improvement Activity Policy Update and Global Inclusion of an Improvement Activity
the weights associated with the improvement activities contained in this MVP; and remove one improvement activity being
proposed for removal from MIPS:
• IA_CC_2: Implementation of improvements that contribute to more timely communication of test results
We are proposing to modify the IA_BE_4: Engagement of patients through implementation of improvements in patient portal
improvement activity, which includes a proposed activity title update. Please see Appendix 2, Improvement Activities: Table
Group B of this proposed rule for all proposed revisions to this activity.
Cost Measures
We are proposing to add three MIPS cost measures within the cost performance category of this MVP, which apply to the clinical
topic of kidney health. We reviewed the MIPS cost measure inventory and considered feedback received from interested parties
through the MVP maintenance process to determine the cost measures to include in this MVP. The following proposed new cost
measures provide a meaningful assessment of the clinical care for clinicians who specialize in kidney care and align with the
other measures and activities included within this MVP and are described in section IV.A.4.e(2)(a)(iii) of this proposed rule:
• Chronic Kidney Disease (CKD): This proposed MIPS episode-based cost measure would assess costs associated with
medical care to manage and treat stage 4 or 5 chronic kidney disease.
• End-Stage Renal Disease (ESRD): This proposed MIPS episode-based cost measure would assess costs associated with
medical care to manage ESRD.
• Kidney Transplant Management: This proposed MIPS episode-based cost measure would assess costs associated with
medical care related to kidney transplant, beginning no sooner than 90 days post-transplant.
Symbol Key:
Plus sign (+): proposed additions of MIPS quality measures, improvement activities, or cost measures
Carat symbol (^): new proposed measures and improvement activities
Single asterisk (*): existing measures and improvement activities with proposed revisions
Double asterisk (**): measures and improvement activities only available when included in an MVP
Single exclamation point (!): high priority measures
Double exclamation point (!!): outcome measures
Tilde (~): measures and improvement activities that include a health equity component
Percent (%): attestation to IA_PCMH: Electronic submission of Patient Centered Medical Home accreditation provides full credit
for the improvement activity performance category
TABLE B.8a: Optimal Care for Kidney Health MVP Measures and Improvement Activities
Quality
Improvement Activities
Cost
(*)(!!) Q001: Diabetes: Hemoglobin A1c
(HbA1c) Poor Control (>9%)
(Collection Type: Medicare Part B Claims
Measure Specifications, eCQM
Specifications, MIPS CQMs Specifications)
(*)(!) Q047: Advance Care Plan
(Collection Type: Medicare Part B Claims
Measure Specifications, MIPS CQMs
Specifications)
(*)(!) Q130: Documentation of Current
Medications in the Medical Record
(Collection Type: eCQM Specifications,
MIPS CQMs Specifications)
(*)(!!) Q236: Controlling High Blood
Pressure
(Collection Type: Medicare Part B Claims
Measure Specifications, eCQM
Specifications, MIPS CQMs Specifications)
(!!) Q482: Hemodialysis Vascular Access:
Practitioner Level Long-term Catheter Rate
(Collection Type: MIPS CQMs
Specifications)
(~)(!) Q487: Screening for Social Drivers of
Health
(Collection Type: MIPS CQMs
Specifications)
(*) Q488: Kidney Health Evaluation
(Collection Type: eCQM Specifications,
MIPS CQMs Specifications)
Q489: Adult Kidney Disease: Angiotensin
(~) IA_AHE_3: Promote use of Patient-
Reported Outcome Tools
(High)
(~) IA_AHE_9: Implement Food Insecurity
and Nutrition Risk Identification and
Treatment Protocols
(Medium)
(*) IA_BE_4: Engagement of patients
through implementation of improvements in
patient portal
(Medium)
IA_BE_6: Regularly Assess Patient
Experience of Care and Follow Up on
Findings
(High)
(~) IA_BE_14: Engage Patients and
Families to Guide Improvement in the
System of Care
(High)
IA_BE_15: Engagement of patients, family
and caregivers in developing a plan of care
(Medium)
(~) IA_BE_16: Promote Self-management
in Usual Care
(Medium)
IA_CC_13: Practice improvements to align
with OpenNotes principles
(Medium)
(+)(*) IA_ERP_6: COVID-19 Vaccine
Acute Kidney Injury Requiring New
Inpatient Dialysis (AKI)
(^)(+) Chronic Kidney Disease (CKD)
(^)(+) End-Stage Renal Disease (ESRD)
(^)(+) Kidney Transplant Management
Total Per Capita Cost (TPCC)

Quality
Improvement Activities
Cost
Converting Enzyme (ACE) Inhibitor or
Angiotensin Receptor Blocker (ARB)
Therapy
(Collection Type: MIPS CQMs
Specifications)
(*) Q493: Adult Immunization Status
(Collection Type: MIPS CQMs
Specifications)
(+)(!!) Q495: Ambulatory Palliative Care
Patients’ Experience of Feeling Heard and
Understood
(Collection Type: MIPS CQMs
Specifications)
(*)(!!) Q503: Gains in Patient Activation
Measure (PAM®) Scores at 12 Months
(Collection Type: MIPS CQMs
Specifications)
(+)(^) TBD: First Year Standardized
Waitlist Ratio (FYSWR)
(Collection Type: MIPS CQMs
Specifications)
(+)(^) TBD: Percentage of Prevalent
Patients Waitlisted (PPPW) and Percentage
of Prevalent Patients Waitlisted in Active
Status (aPPPW)
(Collection Type: MIPS CQMs
Specifications)
Achievement for Practice Staff
(Medium)
(**) IA_MVP: Practice-Wide Quality
Improvement in MIPS Value Pathways
(High)
(%) IA_PCMH: Electronic submission of
Patient Centered Medical Home
accreditation
(~) IA_PM_11: Regular review practices in
place on targeted patient population needs
(Medium)
IA_PM_13: Chronic care and preventative
care management for empaneled patients
(Medium)
IA_PM_16: Implementation of medication
management practice improvements
(Medium)
IA_PSPA_16: Use decision support—
ideally platform-agnostic, interoperable
clinical decision support (CDS) tools —and
standardized treatment protocols to manage
workflow on the care team to meet patient
needs
(Medium)

TABLE B.8b: Optimal Care for Kidney Health MVP Foundational Layer
Population Health Measures
Promoting Interoperability
(!!) Q479: Hospital-Wide, 30-Day, All-Cause Unplanned
Readmission (HWR) Rate for the Merit-Based Incentive Payment
Systems (MIPS) Groups
(Collection Type: Administrative Claims)
(!!) Q484: Clinician and Clinician Group Risk-standardized Hospital
Admission Rates for Patients with Multiple Chronic Conditions
(Collection Type: Administrative Claims)
Security Risk Analysis
High Priority Practices Safety Assurance Factors for EHR
Resilience Guide (SAFER Guide)
e-Prescribing
Query of Prescription Drug Monitoring Program (PDMP)
Provide Patients Electronic Access to Their Health Information
Support Electronic Referral Loops By Sending Health Information
AND
Support Electronic Referral Loops By Receiving and Reconciling
Health Information
OR
Health Information Exchange (HIE) Bi-Directional Exchange
OR
Enabling Exchange Under the Trusted Exchange Framework and
Common Agreement (TEFCA)
Immunization Registry Reporting
Syndromic Surveillance Reporting (Optional)
Electronic Case Reporting
Public Health Registry Reporting (Optional)
Clinical Data Registry Reporting (Optional)
Actions to Limit or Restrict Compatibility or Interoperability of
CEHRT
ONC Direct Review Attestation
B.9: Patient Safety and Support of Positive Experiences with Anesthesia MVP
The B.9 tables represent the measures and activities finalized within the Patient Safety and Support of Positive Experiences with
Anesthesia MVP (88 FR 80036 through 80038) with modifications proposed for the CY 2025 performance period/2027 MIPS
payment year and future years. We request comment on the proposed modifications included in this MVP.
Improvement Activities
For the reasons stated in the introduction of this appendix
1031
, we are proposing the following: add the proposed modified
IA_ERP_6 to all new and previously finalized MVPs because of the importance of vaccination status in practice settings; remove
the weights associated with the improvement activities contained in this MVP; and remove two improvement activities being
proposed for removal from MIPS:
• IA_CC_2: Implementation of improvements that contribute to more timely communication of test results
• IA_EPA_1: Provide 24/7 Access to MIPS Eligible Clinicians or Groups Who Have Real-Time Access to Patient's
Medical Record
Symbol Key:
Plus sign (+): proposed additions of MIPS quality measures, improvement activities, or cost measures
Single asterisk (*): existing measures and improvement activities with proposed revisions
Double asterisk (**): measures and improvement activities only available when included in an MVP
Single exclamation point (!): high priority measures
Double exclamation point (!!): outcome measures
Tilde (~): measures and improvement activities that include a health equity component
Percent (%): attestation to IA_PCMH: Electronic submission of Patient Centered Medical Home accreditation provides full credit
for the improvement activity performance category
TABLE B.9a: Patient Safety and Support of Positive Experiences with Anesthesia MVP Measures and Improvement
Activities
Quality
Improvement Activities
Cost
1031
See MVP Development: Improvement Activity Policy Update and Global Inclusion of an Improvement Activity
Quality
Improvement Activities
Cost
(!!) Q404: Anesthesiology Smoking
Abstinence
(Collection Type: MIPS CQMs
Specifications)
(!!) Q424: Perioperative Temperature
Management
(Collection Type: MIPS CQMs
Specifications)
(!) Q430: Prevention of Post-Operative
Nausea and Vomiting (PONV) –
Combination Therapy
(Collection Type: MIPS CQMs
Specifications)
(!) Q463: Prevention of Post-Operative
Vomiting (POV) – Combination Therapy
(Pediatrics)
(Collection Type: MIPS CQMs
Specifications)
(!) Q477: Multimodal Pain Management
(Collection Type: MIPS CQMs
Specifications)
(~)(!) Q487: Screening for Social Drivers of
Health
(Collection Type: MIPS CQMs
Specifications)
(!) ABG44: Low Flow Inhalational General
Anesthesia
(Collection Type: QCDR)
(!!) AQI48: Patient-Reported Experience
with Anesthesia
(Collection Type: QCDR)
(!!) EPREOP31: Intraoperative
Hypotension (IOH) among Non-Emergent
Noncardiac Surgical Cases
(Collection Type: QCDR)
IA_BE_6: Regularly Assess Patient
Experience of Care and Follow Up on
Findings
(High)
IA_BE_22: Improved practices that engage
patients pre-visit
(Medium)
IA_BMH_2: Tobacco use
(Medium)
IA_CC_15: PSH Care Coordination
(High)
IA_CC_19: Tracking of clinician’s
relationship to and responsibility for a
patient by reporting MACRA patient
relationship codes
(High)
(+)(*) IA_ERP_6: COVID-19 Vaccine
Achievement for Practice Staff
(Medium)
(**) IA_MVP: Practice-Wide Quality
Improvement in MIPS Value Pathways
(High)
(%) IA_PCMH: Electronic submission of
Patient Centered Medical Home
accreditation
IA_PSPA_1: Participation in an AHRQ-
listed patient safety organization
(Medium)
(~) IA_PSPA_7: Use of QCDR data for
ongoing practice assessment and
improvements
(Medium)
IA_PSPA_16: Use decision support—
ideally platform-agnostic, interoperable
clinical decision support (CDS) tools —and
standardized treatment protocols to manage
workflow on the care team to meet patient
needs
(Medium)
Medicare Spending Per Beneficiary
(MSPB) Clinician

TABLE B.9b: Patient Safety and Support of Positive Experiences with Anesthesia MVP Foundational Layer
Population Health Measures
Promoting Interoperability
(!!) Q479: Hospital-Wide, 30-Day, All-Cause Unplanned
Readmission (HWR) Rate for the Merit-Based Incentive Payment
Systems (MIPS) Groups
(Collection Type: Administrative Claims)
(!!) Q484: Clinician and Clinician Group Risk-standardized Hospital
Admission Rates for Patients with Multiple Chronic Conditions
(Collection Type: Administrative Claims)
Security Risk Analysis
High Priority Practices Safety Assurance Factors for EHR Resilience
Guide (SAFER Guide)
e-Prescribing
Query of Prescription Drug Monitoring Program (PDMP)
Provide Patients Electronic Access to Their Health Information
Support Electronic Referral Loops By Sending Health Information
AND
Support Electronic Referral Loops By Receiving and Reconciling
Health Information
OR
Health Information Exchange (HIE) Bi-Directional Exchange
OR
Enabling Exchange Under the Trusted Exchange Framework and
Common Agreement (TEFCA)
Immunization Registry Reporting
Syndromic Surveillance Reporting (Optional)
Electronic Case Reporting
Public Health Registry Reporting (Optional)
Clinical Data Registry Reporting (Optional)
Actions to Limit or Restrict Compatibility or Interoperability of
CEHRT
ONC Direct Review Attestation
B.10: Prevention and Treatment of Infectious Disorders Including Hepatitis C and HIV
MVP
The B.10 tables represent the measures and activities finalized within the Prevention and Treatment of Infectious Disorders
Including Hepatitis C and HIV MVP (88 FR 79991 through 79995) with modifications proposed for the CY 2025 performance
period/2027 MIPS payment year and future years. We request comment on the proposed modifications included in this MVP.
Quality Measures
We are not proposing to modify the previously finalized Prevention and Treatment of Infectious Disorders Including Hepatitis C
and HIV MVP within the quality performance category of this MVP by proposing to add or remove quality measures from the
MVP. However, we are proposing to modify the Q340: HIV Medical Visit Frequency quality measure, which includes a
proposed measure title update. Please see Appendix 1: MIPS Quality Measures, Table Group D of this proposed rule for all
proposed revisions to this measure.
Improvement Activities
For the reasons stated in the introduction of this appendix
1032
, we are proposing the following: add the proposed modified
IA_ERP_6 to all new and previously finalized MVPs because of the importance of vaccination status in practice settings; remove
the weights associated with the improvement activities contained in this MVP; and remove one improvement activity being
proposed for removal from MIPS:
• IA_EPA_1: Provide 24/7 Access to MIPS Eligible Clinicians or Groups who Have Real-Time Access to Patient's
Medical Record
We are proposing to modify the IA_BE_4: Engagement of patients through implementation of improvements in patient portal
improvement activity, which includes a proposed activity title update. Please see Appendix 2, Improvement Activities: Table
Group B of this proposed rule for all proposed revisions to this activity.
Symbol Key:
Plus sign (+): proposed additions of MIPS quality measures, improvement activities, or cost measures
Single asterisk (*): existing measures and improvement activities with proposed revisions
1032
See MVP Development: Improvement Activity Policy Update and Global Inclusion of an Improvement Activity
Double asterisk (**): measures and improvement activities only available when included in an MVP
Single exclamation point (!): high priority measures
Double exclamation point (!!): outcome measures
Tilde (~): measures and improvement activities that include a health equity component
Percent (%): attestation to IA_PCMH: Electronic submission of Patient Centered Medical Home accreditation provides full credit
for the improvement activity performance category

TABLE B.10a: Prevention and Treatment of Infectious Disorders Including Hepatitis C and HIV MVP Measures and
Improvement Activities
Quality
Improvement Activities
Cost
(!) Q065: Appropriate Treatment for Upper
Respiratory Infection (URI)
(Collection Type: eCQM Specifications,
MIPS CQMs Specifications)
(*)(!) Q130: Documentation of Current
Medications in the Medical Record
(Collection Type: eCQM Specifications,
MIPS CQMs Specification)
Q134: Preventive Care and Screening:
Screening for Depression and Follow-Up
Plan
(Collection Type: Medicare Part B Claims
Measure Specifications, eCQM
Specifications, MIPS CQMs Specification)
Q205: Sexually Transmitted Infection (STI)
Testing for People with HIV
(Collection Type: eCQM Specifications,
MIPS CQMs Specifications)
Q240: Childhood Immunization Status
(Collection Type: eCQM Specifications)
Q310: Chlamydia Screening in Women
(Collection Type: eCQM Specifications)
(!!) Q338: HIV Viral Suppression
(Collection Type: eCQM Specifications,
MIPS CQMs Specifications)
(*)(!) Q340: HIV Medical Visit Frequency
(Collection Type: MIPS CQMs
Specifications)
Q387: Annual Hepatitis C Virus (HCV)
Screening for Patients who are Active
Injection Drug Users
(Collection Type: MIPS CQMs
Specifications)
Q400: One-Time Screening for Hepatitis C
Virus (HCV) and Treatment Initiation
(Collection Type: MIPS CQMs
Specifications)
Q401: Hepatitis C: Screening for
Hepatocellular Carcinoma (HCC) in Patients
with Cirrhosis
(Collection Type: MIPS CQMs
Specifications)
Q475: HIV Screening
(Collection Type: eCQM Specifications)
(~)(!) Q487: Screening for Social Drivers of
Health
(Collection Type: MIPS CQMs
Specifications)
(*) Q493: Adult Immunization Status
(Collection Type: MIPS CQMs
Specifications)
(~) IA_AHE_1: Enhance Engagement of
Medicaid and Other Underserved
Populations
(High)
(~) IA_AHE_5: MIPS Eligible Clinician
Leadership in Clinical Trials or CBPR
(Medium)
(~) IA_AHE_12: Practice Improvements
that Engage Community Resources to
Address Drivers of Health
(High)
(*) IA_BE_4: Engagement of patients
through implementation of improvements in
patient portal
(Medium)
IA_BE_15: Engagement of patients, family
and caregivers in developing a plan of care
(Medium)
(+)(*) IA_ERP_6: COVID-19 Vaccine
Achievement for Practice Staff
(Medium)
(**) IA_MVP: Practice-Wide Quality
Improvement in MIPS Value Pathways
(High)
(%) IA_PCMH: Electronic submission of
Patient Centered Medical Home
accreditation
(~) IA_PM_6: Use of toolsets or other
resources to close healthcare disparities
across communities
(Medium)
(~) IA_PM_11: Regular review practices in
place on targeted patient population needs
(Medium)
(~) IA_PM_14: Implementation of
methodologies for improvements in
longitudinal care management for high risk
patients
(Medium)
(~) IA_PM_22: Improving Practice
Capacity for Human Immunodeficiency
Virus (HIV) Prevention Services
(Medium)
IA_PSPA_23: Completion of CDC Training
on Antibiotic Stewardship
(High)
IA_PSPA_32: Use of CDC Guideline for
Clinical Decision Support to Prescribe
Opioids for Chronic Pain via Clinical
Decision Support
(High)
Total Per Capita Cost (TPCC)
TABLE B.10b: Prevention and Treatment of Infectious Disorders Including Hepatitis C and HIV MVP Foundational
Layer
Population Health Measures
Promoting Interoperability
(!!) Q479: Hospital-Wide, 30-Day, All-Cause Unplanned
Readmission (HWR) Rate for the Merit-Based Incentive Payment
Systems (MIPS) Groups
(Collection Type: Administrative Claims)
Security Risk Analysis
High Priority Practices Safety Assurance Factors for EHR Resilience
Guide (SAFER Guide)

Population Health Measures
Promoting Interoperability
(!!) Q484: Clinician and Clinician Group Risk-standardized Hospital
Admission Rates for Patients with Multiple Chronic Conditions
(Collection Type: Administrative Claims)
e-Prescribing
Query of Prescription Drug Monitoring Program (PDMP)
Provide Patients Electronic Access to Their Health Information
Support Electronic Referral Loops By Sending Health Information
AND
Support Electronic Referral Loops By Receiving and Reconciling
Health Information
OR
Health Information Exchange (HIE) Bi-Directional Exchange
OR
Enabling Exchange Under the Trusted Exchange Framework and
Common Agreement (TEFCA)
Immunization Registry Reporting
Syndromic Surveillance Reporting (Optional)
Electronic Case Reporting
Public Health Registry Reporting (Optional)
Clinical Data Registry Reporting (Optional)
Actions to Limit or Restrict Compatibility or Interoperability of
CEHRT
ONC Direct Review Attestation
B.11: Quality Care for Patients with Neurological Conditions MVP
We are proposing to modify the previously finalized Optimal Care for Patients with Episodic Neurological Conditions and the
Supportive Care for Neurodegenerative Conditions MVPs into a single consolidated neurological MVP titled Quality Care for
Patients with Neurological Conditions. This modification is being proposed because the QCDR measures previously included
will no longer be available for use within both of the previously finalized MVPs. As such, due to the removal of these specialty
specific QCDR measures we believe the Episodic Neurological Conditions MVP no longer provides a meaningful representation
of the care provided by the clinicians identified for reporting this MVP. Therefore, we are proposing to combine the previously
finalized MVPs to ensure the availability of a robust and meaningful MVP capturing a broad scope of care provided by
neurologists. The B.11 tables represent the measures and activities finalized within the Optimal Care for Patients with Episodic
Neurological Conditions MVP (88 FR 80015 through 80018) and the Supportive Care for Neurodegenerative Conditions MVP
(88 FR 80019 through 80021) with modifications proposed for the CY 2025 performance period/2027 MIPS payment year and
future years. We request comment on the proposed modifications included in this MVP.
Quality Measures
We are proposing to modify the previously finalized neurology MVPs within the quality performance category of this MVP to
include two additional broadly applicable MIPS quality measures relevant to patients receiving care for neurodegenerative
disorders. We reviewed the MIPS quality measure inventory and considered feedback received during the 2024 MVP
maintenance period to determine which quality measures to include in this MVP.
• Q155: Falls: Plan of care: This MIPS quality measure ensures adult patients, with a history of falls, have a plan of care
for falls.
• Q495: Ambulatory Palliative Care Patients’ Experience of Feeling Heard and Understood: This MIPS quality measure
ensures palliative care clinicians and/or teams empathize to ensure patients are understood in a significant and
empowering way.
We are proposing to modify the previously finalized MVPs to remove six QCDR measures no longer being supported by Axon
Registry QCDR:
• AAN5: Treatment Prescribed for Acute Migraine Attack
• AAN9: Querying and Follow-Up About Symptoms of Autonomic Dysfunction for Patients with Parkinson’s Disease
• AAN22: Quality of Life Outcome for Patients with Neurologic Conditions
• AAN31: Acute Treatment Prescribed for Cluster Headache
• AAN32: Preventive Treatment Prescribed for Cluster Headache
• AAN34: Patient reported falls and plan of care
Improvement Activities

For the reasons stated in the introduction of this appendix
1033
, we are proposing the following: add the proposed modified
IA_ERP_6 to all new and previously finalized MVPs because of the importance of vaccination status in practice settings; remove
the weights associated with the improvement activities contained in this MVP; and remove three improvement activities being
proposed for removal from MIPS:
• IA_BMH_8: Electronic Health Record Enhancements for BH data capture
• IA_CC_1: Implementation of Use of Specialist Reports Back to Referring Clinician or Group to Close Referral Loop
• IA_EPA_1: Provide 24/7 Access to MIPS Eligible Clinicians or Groups Who Have Real-Time Access to Patient's
Medical Record
We are proposing to modify the IA_BE_4: Engagement of patients through implementation of improvements in patient portal
improvement activity, which includes a proposed activity title update. Please see Appendix 2, Improvement Activities: Table
Group B of this proposed rule for all proposed revisions to this activity.
Symbol Key:
Plus sign (+): proposed additions of MIPS quality measures, improvement activities, or cost measures
Single asterisk (*): existing measures and improvement activities with proposed revisions
Double asterisk (**): measures and improvement activities only available when included in an MVP
Single exclamation point (!): high priority measures
Double exclamation point (!!): outcome measures
Tilde (~): measures and improvement activities that include a health equity component
Percent (%): attestation to IA_PCMH: Electronic submission of Patient Centered Medical Home accreditation provides full credit
for the improvement activity performance category
TABLE B.11a: Quality Care for Patients with Neurological Conditions MVP Measures and Improvement Activities
Quality
Improvement Activities
Cost
(*)(!) Q047: Advance Care Plan
(Collection Type: Medicare Part B Claims
Measure Specifications, MIPS CQMs
Specifications)
(*)(!) Q130: Documentation of Current
Medications in the Medical Record
(Collection Type: eCQM Specifications,
MIPS CQMs Specifications)
(+)(*)(!) Q155: Falls: Plan of Care
(Collection Type: Medicare Part B Claims
Measure Specifications, MIPS CQMs
Specifications)
(*)(!) Q238: Use of High-Risk Medications
in Older Adults
(Collection Type: eCQM Specifications,
MIPS CQMs Specifications)
Q268: Epilepsy: Counseling for Women of
Childbearing Potential with Epilepsy
(Collection Type: MIPS CQMs
Specifications)
(*) Q281: Dementia: Cognitive Assessment
(Collection Type: eCQM Specifications)
(*) Q282: Dementia: Functional Status
Assessment
(Collection Type: MIPS CQMs
Specifications)
(*)(!) Q286: Dementia: Safety Concern
Screening and Follow-Up for Patients with
Dementia
(Collection Type: MIPS CQMs
Specifications)
(*)(!) Q288: Dementia: Education and
Support of Caregivers for Patients with
Dementia
(Collection Type: MIPS CQMs
Specifications)
(~) IA_AHE_3: Promote use of Patient-
Reported Outcome Tools
(High)
(*) IA_BE_4: Engagement of patients
through implementation of improvements in
patient portal
(Medium)
IA_BE_6: Regularly Assess Patient
Experience of Care and Follow Up on
Findings
(High)
(~) IA_BE_16: Promote Self-management
in Usual Care
(Medium)
IA_BE_24: Financial Navigation Program
(Medium)
IA_BMH_4: Depression screening
(Medium)
IA_EPA_2: Use of telehealth services that
expand practice access
(Medium)
(+)(*) IA_ERP_6: COVID-19 Vaccine
Achievement for Practice Staff
(Medium)
(**) IA_MVP: Practice-Wide Quality
Improvement in MIPS Value Pathways
(High)
(%) IA_PCMH: Electronic submission of
Patient Centered Medical Home
accreditation
(~) IA_PM_11: Regular review practices in
place on targeted patient population needs
(Medium)
Medicare Spending Per Beneficiary
(MSPB) Clinician
1033
See MVP Development: Improvement Activity Policy Update and Global Inclusion of an Improvement Activity

Quality
Improvement Activities
Cost
(*) Q290: Assessment of Mood Disorders
and Psychosis for Patients with Parkinson’s
Disease
(Collection Type: MIPS CQMs
Specifications)
(*) Q291: Assessment of Cognitive
Impairment or Dysfunction for Patients with
Parkinson’s Disease
(Collection Type: MIPS CQMs
Specifications)
(*)(!) Q293: Rehabilitative Therapy Referral
for Patients with Parkinson’s Disease
(Collection Type: MIPS CQMs
Specifications)
(*)(!) Q386: Amyotrophic Lateral Sclerosis
(ALS) Patient Care Preferences
(Collection Type: MIPS CQMs
Specifications)
(!) Q419: Overuse of Imaging for the
Evaluation of Primary Headache
(Collection Type: MIPS CQMs
Specifications)
(~)(!) Q487: Screening for Social Drivers of
Health
(Collection Type: MIPS CQMs
Specifications)
(+)(!!) Q495: Ambulatory Palliative Care
Patients’ Experience of Feeling Heard and
Understood
(Collection Type: MIPS CQMs
Specifications)
(*)(!!) Q503: Gains in Patient Activation
Measure (PAM®) Scores at 12 Months
(Collection Type: MIPS CQMs
Specifications)
IA_PM_16: Implementation of medication
management practice improvements
(Medium)
IA_PM_21: Advance Care Planning
(Medium)
IA_PSPA_21: Implementation of fall
screening and assessment programs
(Medium)

TABLE B.11b: Quality Care for Patients with Neurological Conditions MVP Foundational Layer
Population Health Measures
Promoting Interoperability
(!!) Q479: Hospital-Wide, 30-Day, All-Cause Unplanned
Readmission (HWR) Rate for the Merit-Based Incentive Payment
Systems (MIPS) Groups
(Collection Type: Administrative Claims)
(!!) Q484: Clinician and Clinician Group Risk-standardized Hospital
Admission Rates for Patients with Multiple Chronic Conditions
(Collection Type: Administrative Claims)
Security Risk Analysis
High Priority Practices Safety Assurance Factors for EHR Resilience
Guide (SAFER Guide)
e-Prescribing
Query of Prescription Drug Monitoring Program (PDMP)
Provide Patients Electronic Access to Their Health Information
Support Electronic Referral Loops By Sending Health Information
AND
Support Electronic Referral Loops By Receiving and Reconciling
Health Information
OR
Health Information Exchange (HIE) Bi-Directional Exchange
OR
Enabling Exchange Under the Trusted Exchange Framework and
Common Agreement (TEFCA)
Immunization Registry Reporting
Syndromic Surveillance Reporting (Optional)
Electronic Case Reporting
Public Health Registry Reporting (Optional)
Clinical Data Registry Reporting (Optional)
Actions to Limit or Restrict Compatibility or Interoperability of
CEHRT
ONC Direct Review Attestation
B.12: Quality Care for the Treatment of Ear, Nose, and Throat Disorders MVP
The B.12 tables represent the measures and activities finalized within the Quality Care for the Treatment of Ear, Nose, and
Throat Disorders MVP (88 FR 79986 through 79990) with modifications proposed for the CY 2025 performance period/2027
MIPS payment year and future years. We request comment on the proposed modifications included in this MVP.
Quality Measures
We are proposing to modify the previously finalized Quality Care for the Treatment of Ear, Nose, and Throat Disorders MVP
within the quality performance category of this MVP to remove two QCDR measures whose quality actions reflect a standard of
care based upon clinical guidelines recognized as best practices by health care clinicians. Based upon MIPS performance data,
AAO16 is high performing and AAO23 has had minimal variation in its historical benchmark. Though allergic rhinitis falls
within the spectrum of care otolaryngologists provide, the complexity of caring for the condition is typically low. Many non-
surgical clinician specialties, including primary care, treat allergic rhinitis regularly. Further, measure AAO23 requires the use of
medications that all are available over the counter. Removal of AAO23 will encourage use of other measures within the MVP
that represent the complexity of care otolaryngologists provide.
• AAO16: Age-Related Hearing Loss: Audiometric Evaluation
• AAO23: Allergic Rhinitis: Intranasal Corticosteroids or Oral Antihistamines
Improvement Activities
For the reasons stated in the introduction of this appendix
1034
, we are proposing the following: add the proposed modified
IA_ERP_6 to all new and previously finalized MVPs because of the importance of vaccination status in practice settings; remove
the weights associated with the improvement activities contained in this MVP; and remove two improvement activities being
proposed for removal from MIPS:
• IA_CC_1: Implementation of Use of Specialist Reports Back to Referring Clinician or Group to Close Referral Loop
• IA_EPA_1: Provide 24/7 Access to MIPS Eligible Clinicians or Groups Who Have Real-Time Access to Patient's
Medical Record
1034
See MVP Development: Improvement Activity Policy Update and Global Inclusion of an Improvement Activity
We are proposing to modify the IA_BE_4: Engagement of patients through implementation of improvements in patient portal
improvement activity, which includes a proposed activity title update. Please see Appendix 2, Improvement Activities: Table
Group B of this proposed rule for all proposed revisions to this activity.
Symbol Key:
Plus sign (+): proposed additions of MIPS quality measures, improvement activities, or cost measures
Single asterisk (*): existing measures and improvement activities with proposed revisions
Double asterisk (**): measures and improvement activities only available when included in an MVP
Single exclamation point (!): high priority measures
Double exclamation point (!!): outcome measures
Tilde (~): measures and improvement activities that include a health equity component
Percent (%): attestation to IA_PCMH: Electronic submission of Patient Centered Medical Home accreditation provides full credit
for the improvement activity performance category

TABLE B.12a: Quality Care for the Treatment of Ear, Nose, and Throat Disorders MVP Measures and Improvement
Activities
Quality
Improvement Activities
Cost
(**) Q128: Preventive Care and Screening:
Body Mass Index (BMI) Screening and
Follow-Up Plan
(Collection Type: Medicare Part B Claims
Measure Specifications, eCQM
Specifications, MIPS CQMs Specification)
Q226: Preventive Care and Screening:
Tobacco Use: Screening and Cessation
Intervention
(Collection Type: Medicare Part B Claims
Measure Specifications, eCQM
Specifications, MIPS CQMs Specification)
(*) Q277: Sleep Apnea: Severity Assessment
at Initial Diagnosis
(Collection Type: MIPS CQMs
Specifications)
(*)(!) Q331: Adult Sinusitis: Antibiotic
Prescribed for Acute Viral Sinusitis (Overuse)
(Collection Type: MIPS CQMs
Specifications)
(!) Q332: Adult Sinusitis: Appropriate Choice
of Antibiotic: Amoxicillin With or Without
Clavulanate Prescribed for Patients with
Acute Bacterial Sinusitis (Appropriate Use)
(Collection Type: MIPS CQMs
Specifications)
(*)(!!) Q355: Unplanned Reoperation within
the 30 Day Postoperative Period
(Collection Type: MIPS CQMs
Specifications)
(!!) Q357: Surgical Site Infection (SSI)
(Collection Type: MIPS CQMs
Specifications)
(~)(!) Q487: Screening for Social Drivers of
Health
(Collection Type: MIPS CQMs
Specifications)
AAO20: Tympanostomy Tubes:
Comprehensive Audiometric Evaluation
(Collection Type: QCDR)
AAO21: Otitis Media with Effusion (OME):
Comprehensive Audiometric Evaluation for
Chronic OME > or = 3 months
(Collection Type: QCDR)
(~) IA_AHE_3: Promote use of Patient-
Reported Outcome Tools
(High)
(~) IA_AHE_5: MIPS Eligible Clinician
Leadership in Clinical Trials or CBPR
(Medium)
(*) IA_BE_4: Engagement of patients
through implementation of improvements in
patient portal
(Medium)
IA_BE_15: Engagement of patients, family
and caregivers in developing a plan of care
(Medium)
IA_CC_13: Practice improvements to align
with OpenNotes principles
(Medium)
(+)(*) IA_ERP_6: COVID-19 Vaccine
Achievement for Practice Staff
(Medium)
(**) IA_MVP: Practice-Wide Quality
Improvement in MIPS Value Pathways
(High)
(%) IA_PCMH: Electronic submission of
Patient Centered Medical Home accreditation
IA_PM_16: Implementation of medication
management practice improvements
(Medium)
(~) IA_PSPA_7: Use of QCDR data for
ongoing practice assessment and
improvements
(Medium)
Medicare Spending Per Beneficiary
(MSPB) Clinician

TABLE B.12b: Quality Care for the Treatment of Ear, Nose, and Throat Disorders MVP Foundational Layer
Population Health Measures
Promoting Interoperability
(!!) Q479: Hospital-Wide, 30-Day, All-Cause Unplanned
Readmission (HWR) Rate for the Merit-Based Incentive Payment
Systems (MIPS) Groups
(Collection Type: Administrative Claims)
(!!) Q484: Clinician and Clinician Group Risk-standardized Hospital
Admission Rates for Patients with Multiple Chronic Conditions
(Collection Type: Administrative Claims)
Security Risk Analysis
High Priority Practices Safety Assurance Factors for EHR Resilience
Guide (SAFER Guide)
e-Prescribing
Query of Prescription Drug Monitoring Program (PDMP)
Provide Patients Electronic Access to Their Health Information
Support Electronic Referral Loops By Sending Health Information
AND
Support Electronic Referral Loops By Receiving and Reconciling
Health Information
OR
Health Information Exchange (HIE) Bi-Directional Exchange
OR
Enabling Exchange Under the Trusted Exchange Framework and
Common Agreement (TEFCA)
Immunization Registry Reporting
Syndromic Surveillance Reporting (Optional)
Electronic Case Reporting
Public Health Registry Reporting (Optional)
Clinical Data Registry Reporting (Optional)
Actions to Limit or Restrict Compatibility or Interoperability of
CEHRT
ONC Direct Review Attestation
B.13: Quality Care in Mental Health and Substance Use Disorders MVP
The B.13 tables represent the measures and activities finalized within the Quality Care in Mental Health and Substance Use
Disorders MVP (88 FR 79986 through 80001) with modifications proposed for the CY 2025 performance period/2027 MIPS
payment year and future years. We request comment on the proposed modifications included in this MVP.
Improvement Activities
For the reasons stated in the introduction of this appendix
1035
, we are proposing the following: add the proposed modified
IA_ERP_6 to all new and previously finalized MVPs because of the importance of vaccination status in practice settings; and
remove the weights associated with the improvement activities contained in this MVP.
Symbol Key:
Plus sign (+): proposed additions of MIPS quality measures, improvement activities, or cost measures
Single asterisk (*): existing measures and improvement activities with proposed revisions
Double asterisk (**): measures and improvement activities only available when included in an MVP
Single exclamation point (!): high priority measures
Double exclamation point (!!): outcome measures
Tilde (~): measures and improvement activities that include a health equity component
Percent (%): attestation to IA_PCMH: Electronic submission of Patient Centered Medical Home accreditation provides full credit
for the improvement activity performance category
1035
See MVP Development: Improvement Activity Policy Update and Global Inclusion of an Improvement Activity

TABLE B.13a: Quality Care in Mental Health and Substance Use Disorders MVP Measures and Improvement Activities
Quality
Improvement Activities
Cost
(*) Q009: Antidepressant Medication
Management
(Collection Type: eCQM Specifications)
Q134: Preventive Care and Screening:
Screening for Depression and Follow-Up
Plan
(Collection Type: Medicare Part B Claims
Measure Specifications, eCQM
Specifications, MIPS CQMs Specification)
(!) Q305: Initiation and Engagement of
Substance Use Disorder Treatment
(Collection Type: eCQM Specifications)
Q366: Follow-Up Care for Children
Prescribed ADHD Medication (ADD)
(Collection Type: eCQM Specifications)
(!!) Q370: Depression Remission at Twelve
Months
(Collection Type: eCQM Specifications,
MIPS CQMs Specification)
(!) Q382: Child and Adolescent Major
Depressive Disorder (MDD): Suicide Risk
Assessment
(Collection Type: eCQM Specifications)
(*)(!!) Q383: Adherence to Antipsychotic
Medications For Individuals with
Schizophrenia
(Collection Type: MIPS CQMs
Specifications)
(!) Q468: Continuity of Pharmacotherapy
for Opioid Use Disorder (OUD)
(Collection Type: MIPS CQMs
Specifications)
(~)(!) Q487: Screening for Social Drivers of
Health
(Collection Type: MIPS CQMs
Specifications)
(!!) Q502: Improvement or Maintenance of
Functioning for Individuals with a Mental
and/or Substance Use Disorder
(Collection Type: MIPS CQMs
Specifications)
(!) Q504: Initiation, Review, And/Or Update
To Suicide Safety Plan For Individuals With
Suicidal Thoughts, Behavior, Or Suicide
Risk
(Collection Type: MIPS CQMs
Specifications)
(*)(!!) Q505: Reduction in Suicidal Ideation
or Behavior Symptoms
(Collection Type: MIPS CQMs
Specifications)
(!!) MBHR2: Anxiety Response at 6-months
(Collection Type: QCDR)
(!!) MBHR7: Posttraumatic Stress Disorder
(PTSD) Outcome Assessment for Adults and
Children
(Collection Type: QCDR)
(~) IA_AHE_1: Enhance Engagement of
Medicaid and Other Underserved Populations
(High)
(~) IA_AHE_3: Promote use of Patient-
Reported Outcome Tools
(High)
(~) IA_AHE_5: MIPS Eligible Clinician
Leadership in Clinical Trials or CBPR
(Medium)
(~) IA_AHE_9: Implement Food Insecurity
and Nutrition Risk Identification and
Treatment Protocols
(Medium)
(~) IA_AHE_12: Practice Improvements that
Engage Community Resources to Address
Drivers of Health
(High)
IA_BE_12: Use evidence-based decision aids
to support shared decision-making.
(Medium)
(~) IA_BE_16: Promote Self-management in
Usual Care
(Medium)
IA_BE_23: Integration of patient coaching
practices between visits
(Medium)
IA_BMH_2: Tobacco use
(Medium)
IA_BMH_5: MDD prevention and treatment
interventions
(Medium)
(~) IA_BMH_7: Implementation of
Integrated Patient Centered Behavioral Health
Model
(High)
(~) IA_BMH_14: Behavioral/Mental Health
and Substance Use Screening and Referral for
Pregnant and Postpartum Women
(~) IA_BMH_15: Behavioral/Mental Health
and Substance Use Screening and Referral for
Older Adults
(High)
IA_EPA_2: Use of telehealth services that
expand practice access
(Medium)
(+)(*) IA_ERP_6: COVID-19 Vaccine
Achievement for Practice Staff
(Medium)
(**) IA_MVP: Practice-Wide Quality
Improvement in MIPS Value Pathways
(High)
(%) IA_PCMH: Electronic submission of
Patient Centered Medical Home accreditation
(~) IA_PM_6: Use of toolsets or other
resources to close healthcare disparities
across communities
(Medium)
Medicare Spending Per Beneficiary
(MSPB) Clinician
Depression
Psychoses and Related Conditions

Quality
Improvement Activities
Cost
IA_PSPA_32: Use of CDC Guideline for
Clinical Decision Support to Prescribe
Opioids for Chronic Pain via Clinical
Decision Support
(High)
TABLE B.13b: Quality Care in Mental Health and Substance Use Disorders MVP Foundational Layer
Population Health Measures
Promoting Interoperability
(!!) Q479: Hospital-Wide, 30-Day, All-Cause Unplanned
Readmission (HWR) Rate for the Merit-Based Incentive Payment
Systems (MIPS) Groups
(Collection Type: Administrative Claims)
(!!) Q484: Clinician and Clinician Group Risk-standardized Hospital
Admission Rates for Patients with Multiple Chronic Conditions
(Collection Type: Administrative Claims)
Security Risk Analysis
High Priority Practices Safety Assurance Factors for EHR Resilience
Guide (SAFER Guide)
e-Prescribing
Query of Prescription Drug Monitoring Program (PDMP)
Provide Patients Electronic Access to Their Health Information
Support Electronic Referral Loops By Sending Health Information
AND
Support Electronic Referral Loops By Receiving and Reconciling
Health Information
OR
Health Information Exchange (HIE) Bi-Directional Exchange
OR
Enabling Exchange Under the Trusted Exchange Framework and
Common Agreement (TEFCA)
Immunization Registry Reporting
Syndromic Surveillance Reporting (Optional)
Electronic Case Reporting
Public Health Registry Reporting (Optional)
Clinical Data Registry Reporting (Optional)
Actions to Limit or Restrict Compatibility or Interoperability of
CEHRT
ONC Direct Review Attestation
B.14: Rehabilitative Support for Musculoskeletal Care MVP
The B.14 tables represent the measures and activities finalized within the Rehabilitative Support for Musculoskeletal Care MVP
(88 FR 80002 through 80007) with modifications proposed for the CY 2025 performance period/2027 MIPS payment year and
future years. We request comment on the proposed modifications included in this MVP.
Quality Measures
We are proposing to modify the previously finalized Rehabilitative Support for Musculoskeletal Care MVP within the quality
performance category of this MVP to include one additional MIPS quality measure and four QCDR measures that are relevant to
patients receiving rehabilitative support for Musculoskeletal Care. We reviewed the MIPS quality measure inventory and
considered feedback received during the 2024 MVP maintenance period to determine which quality measures to include in this
MVP.
• Q050: Urinary Incontinence: Plan of Care for Urinary Incontinence in Women Aged 65 Years and Older: This MIPS
quality measure ensures that female patients with a diagnosis of urinary incontinence have a documented plan of care
regarding rehabilitative treatment for urinary incontinence at least once within 12 months. A rehabilitation plan of care
for these patients would address the musculoskeletal impairments of the pelvic floor related to urinary incontinence in
women.
•
MSK6: Patients Suffering From a Neck Injury who Improve Pain: This QCDR measure evaluates patients with a neck
injury for achieving the minimal clinically important difference (MCID) improvement in pain by the end of treatment.
•
MSK7: Patients Suffering From an Upper Extremity Injury who Improve Pain: This QCDR measure evaluates patients
with an upper extremity injury for achieving the minimal clinically important difference (MCID) improvement in pain
by the end of treatment.
•
MSK8: Patients Suffering From a Back Injury who Improve Pain: This QCDR measure evaluates patients with a back
injury for achieving the minimal clinically important difference (MCID) improvement in pain by the end of treatment.
•
MSK9: Patients Suffering From a Lower Extremity Injury who Improve Pain: This QCDR measure evaluates patients
with a lower extremity injury for achieving the minimal clinically important difference (MCID) improvement in pain
by the end of treatment.
Improvement Activities
For the reasons stated in the introduction of this appendix
1036
, we are proposing the following: add the proposed modified
IA_ERP_6 to all new and previously finalized MVPs because of the importance of vaccination status in practice settings; remove
the weights associated with the improvement activities contained in this MVP; and remove two improvement activities being
proposed for removal from MIPS:
• IA_CC_1: Implementation of Use of Specialist Reports Back to Referring Clinician or Group to Close Referral Loop
• IA_EPA_1: Provide 24/7 Access to MIPS Eligible Clinicians or Groups Who Have Real-Time Access to Patient's
Medical Record
Symbol Key:
Plus sign (+): proposed additions of MIPS quality measures, improvement activities, or cost measures
Single asterisk (*): existing measures and improvement activities with proposed revisions
Double asterisk (**): measures and improvement activities only available when included in an MVP
Single exclamation point (!): high priority measures
Double exclamation point (!!): outcome measures
Tilde (~): measures and improvement activities that include a health equity component
Percent (%): attestation to IA_PCMH: Electronic submission of Patient Centered Medical Home accreditation provides full credit
for the improvement activity performance category
Pound sign (#): QCDR measures pending testing data
TABLE B.14a: Rehabilitative Support for Musculoskeletal Care MVP Measures and Improvement Activities
Quality
Improvement Activities
Cost
(+)(!) Q050: Urinary Incontinence: Plan of
Care for Urinary Incontinence in Women Aged
65 Years and Older
(Collection Type: MIPS CQMs Specifications)
(**) Q128: Preventive Care and Screening:
Body Mass Index (BMI) Screening and
Follow-Up Plan
(Collection Type: Medicare Part B Claims
Measure Specifications, eCQM Specifications,
MIPS CQMs Specification)
(*)(!) Q155: Falls: Plan of Care
(Collection Type: Medicare Part B Claims
Measure Specifications, MIPS CQMs
Specification)
(!!) Q217: Functional Status Change for
Patients with Knee Impairments
(Collection Type: MIPS CQMs Specifications)
(!!) Q218: Functional Status Change for
Patients with Hip Impairments
(Collection Type: MIPS CQMs Specifications)
(!!) Q219: Functional Status Change with
Lower Leg, Foot or Ankle Impairments
(Collection Type: MIPS CQMs Specifications)
(!!) Q220: Functional Status Change for
Patients with Low Back Impairments
(Collection Type: MIPS CQMs Specifications)
(!!) Q221: Functional Status Change for
Patients with Shoulder Impairments
(Collection Type: MIPS CQMs Specifications)
(!!) Q222: Functional Status Change for
Patients with Elbow, Wrist or Hand
Impairments
(Collection Type: MIPS CQMs Specifications)
(!!) Q478: Functional Status Change for
(~) IA_AHE_3: Promote use of Patient-
Reported Outcome Tools
(High)
(~) IA_AHE_6: Provide Education
Opportunities for New Clinicians
(High)
(~) IA_AHE_9: Implement Food Insecurity
and Nutrition Risk Identification and
Treatment Protocols
(Medium)
(~) IA_AHE_12: Practice Improvements that
Engage Community Resources to Address
Drivers of Health
(High)
IA_BE_6: Regularly Assess Patient
Experience of Care and Follow Up on
Findings
(High)
IA_BMH_12: Promoting Clinician Well-
Being
(High)
(~) IA_BMH_15: Behavioral/Mental Health
and Substance Use Screening and Referral for
Older Adults
(High)
IA_CC_8: Implementation of documentation
improvements for practice/process
improvements
(Medium)
IA_CC_12: Care coordination agreements
that promote improvements in patient
tracking across settings
(Medium)
Low Back Pain
1036
See MVP Development: Improvement Activity Policy Update and Global Inclusion of an Improvement Activity

Quality
Improvement Activities
Cost
Patients with Neck Impairments
(Collection Type: MIPS CQMs Specifications)
(~)(!) Q487: Screening for Social Drivers of
Health
(Collection Type: MIPS CQMs Specifications)
(+)(!!)(#) MSK6: Patients Suffering From a
Neck Injury who Improve Pain
(Collection Type: QCDR)
(+)(!!)(#) MSK7: Patients Suffering From an
Upper Extremity Injury who Improve Pain
(Collection Type: QCDR)
(+)(!!)(#) MSK8: Patients Suffering From a
Back Injury who Improve Pain
(Collection Type: QCDR)
(+)(!!)(#) MSK9: Patients Suffering From a
Lower Extremity Injury who Improve Pain
(Collection Type: QCDR)
IA_EPA_2: Use of telehealth services that
expand practice access
(Medium)
(~) IA_EPA_3: Collection and use of patient
experience and satisfaction data on access
(Medium)
(+)(*) IA_ERP_6: COVID-19 Vaccine
Achievement for Practice Staff
(Medium)
(**) IA_MVP: Practice-Wide Quality
Improvement in MIPS Value Pathways
(High)
(%) IA_PCMH: Electronic submission of
Patient Centered Medical Home accreditation
IA_PSPA_16: Use decision support—ideally
platform-agnostic, interoperable clinical
decision support (CDS) tools —and
standardized treatment protocols to manage
workflow on the care team to meet patient
needs
(Medium)
IA_PSPA_21: Implementation of fall
screening and assessment programs
(Medium)

TABLE B.14b: Rehabilitative Support for Musculoskeletal Care MVP Foundational Layer
Population Health Measures
Promoting Interoperability
(!!) Q479: Hospital-Wide, 30-Day, All-Cause Unplanned Readmission
(HWR) Rate for the Merit-Based Incentive Payment Systems (MIPS)
Groups
(Collection Type: Administrative Claims)
(!!) Q484: Clinician and Clinician Group Risk-standardized Hospital
Admission Rates for Patients with Multiple Chronic Conditions
(Collection Type: Administrative Claims)
Security Risk Analysis
High Priority Practices Safety Assurance Factors for EHR
Resilience Guide (SAFER Guide)
e-Prescribing
Query of Prescription Drug Monitoring Program (PDMP)
Provide Patients Electronic Access to Their Health Information
Support Electronic Referral Loops By Sending Health Information
AND
Support Electronic Referral Loops By Receiving and Reconciling
Health Information
OR
Health Information Exchange (HIE) Bi-Directional Exchange
OR
Enabling Exchange Under the Trusted Exchange Framework and
Common Agreement (TEFCA)
Immunization Registry Reporting
Syndromic Surveillance Reporting (Optional)
Electronic Case Reporting
Public Health Registry Reporting (Optional)
Clinical Data Registry Reporting (Optional)
Actions to Limit or Restrict Compatibility or Interoperability of
CEHRT
ONC Direct Review Attestation
B.15: Value in Primary Care MVP
The B.15 tables represent the measures and activities finalized within the Value in Primary Care MVP (88 FR 80042 through
80047) with modifications proposed for the CY 2025 performance period/2027 MIPS payment year and future years. We request
comment on the proposed modifications included in this MVP.
Quality Measures
We are not proposing to modify the previously finalized Value in Primary Care MVP within the quality performance category of
this MVP by proposing to add or remove quality measures from the MVP. However, we are proposing to modify the Q001:
Diabetes: Hemoglobin A1c (HbA1c) Poor Control (>9%) quality measure, which includes a proposed measure title update.
Please see Appendix 1: MIPS Quality Measures, Table Group D of this proposed rule for all proposed revisions to this measure.
Improvement Activities
For the reasons stated in the introduction of this appendix
1037
, we are proposing the following: add the proposed modified
IA_ERP_6 to all new and previously finalized MVPs because of the importance of vaccination status in practice settings; and add
a proposed improvement activity that addresses risk for heart disease, the leading cause of death in the United States, as well as
risk for stroke, which is the fifth most common cause of death. Promoting the implementation of standardized, evidence-based
cardiovascular disease risk assessment and care management in the primary care setting has the potential to impact patient
outcomes sizably and positively. This new activity, IA_PM_XX, is based on the results of the CMS Innovation Center Million
Hearts Model:
• IA_PM_XX: Save a Million Hearts: Standardization of Approach to Screening and Treatment for Cardiovascular
Disease Risk
In addition, we are proposing the following: remove the weights associated with the improvement activities contained in this
MVP; and remove two improvement activities being proposed for removal from MIPS:
• IA_CC_2: Implementation of improvements that contribute to more timely communication of test results
• IA_EPA_1: Provide 24/7 Access to MIPS Eligible Clinicians or Groups Who Have Real-Time Access to Patient's
Medical Record
1037
See MVP Development: Improvement Activity Policy Update and Global Inclusion of an Improvement Activity

We are proposing to modify the IA_BE_4: Engagement of patients through implementation of improvements in patient portal
improvement activity, which includes a proposed activity title update. Please see Appendix 2, Improvement Activities: Table
Group B of this proposed rule for all proposed revisions to this activity.
Symbol Key:
Plus sign (+): proposed additions of MIPS quality measures, improvement activities, or cost measures
Carat symbol (^): new proposed measures and improvement activities
Single asterisk (*): existing measures and improvement activities with proposed revisions
Double asterisk (**): measures and improvement activities only available when included in an MVP
Single exclamation point (!): high priority measures
Double exclamation point (!!): outcome measures
Tilde (~): measures and improvement activities that include a health equity component
Percent (%): attestation to IA_PCMH: Electronic submission of Patient Centered Medical Home accreditation provides full credit
for the improvement activity performance category
TABLE B.15a: Value in Primary Care MVP Measures and Improvement Activities
Quality
Improvement Activities
Cost
(*)(!!) Q001: Diabetes: Hemoglobin A1c
(HbA1c) Poor Control (>9%)
(Collection Type: Medicare Part B Claims
Measure Specifications, eCQM
Specifications, MIPS CQMs Specifications)
(*)(!) Q047: Advance Care Plan
(Collection Type: Medicare Part B Claims
Measure Specifications, MIPS CQMs
Specifications)
Q134: Preventive Care and Screening:
Screening for Depression and Follow-Up
Plan
(Collection Type: Medicare Part B Claims
Measure Specifications, eCQM
Specifications, MIPS CQMs Specifications)
(*)(!!) Q236: Controlling High Blood
Pressure
(Collection Type: Medicare Part B Claims
Measure Specifications, eCQM
Specifications, MIPS CQMs Specifications)
(!) Q305: Initiation and Engagement of
Substance Use Disorder Treatment
(Collection Type: eCQM Specifications)
(!) Q321: CAHPS for MIPS Clinician/Group
Survey
(Collection Type: CAHPS Survey Vendor)
Q438: Statin Therapy for the Prevention and
Treatment of Cardiovascular Disease
(Collection Type: eCQM Specifications,
MIPS CQMs Specifications)
Q475: HIV Screening
(Collection Type: eCQM Specifications)
(!!) Q483: Person-Centered Primary Care
Measure Patient Reported Outcome
Performance Measure (PCPCM PRO-PM)
(Collection Type: MIPS CQMs
Specifications)
(~)(!) Q487: Screening for Social Drivers of
Health
(Collection Type: MIPS CQMs
Specifications)
(*) Q493: Adult Immunization Status
(Collection Type: MIPS CQMs
Specifications)
(*) Q497: Preventive Care and Wellness
(composite)
(~) IA_AHE_3: Promote use of Patient-
Reported Outcome Tools
(High)
(~) IA_AHE_9: Implement Food Insecurity
and Nutrition Risk Identification and
Treatment Protocols
(Medium)
(~) IA_AHE_12: Practice Improvements that
Engage Community Resources to Address
Drivers of Health
(High)
(*) IA_BE_4: Engagement of patients through
implementation of improvements in patient
portal
(Medium)
IA_BE_6: Regularly Assess Patient
Experience of Care and Follow Up on Findings
(High)
IA_BE_12: Use evidence-based decision aids
to support shared decision-making
(Medium)
IA_CC_13: Practice improvements to align
with OpenNotes principles
(Medium)
(+)(*) IA_ERP_6: COVID-19 Vaccine
Achievement for Practice Staff
(Medium)
(**) IA_MVP: Practice-Wide Quality
Improvement in MIPS Value Pathways
(High)
(%) IA_PCMH: Electronic submission of
Patient Centered Medical Home accreditation
(~) IA_PM_11: Regular review practices in
place on targeted patient population needs
(Medium)
IA_PM_13: Chronic care and preventative
care management for empaneled patient
(Medium)
IA_PM_16: Implementation of medication
management practice improvements
(Medium)
(~) IA_PM_22: Improving Practice Capacity
for Human Immunodeficiency Virus (HIV)
Prevention Services
Asthma/Chronic Obstructive
Pulmonary Disease (COPD)
Diabetes
Depression
Heart Failure
Total Per Capita Cost (TPCC)

Quality
Improvement Activities
Cost
(Collection Type: MIPS CQMs
Specifications)
(!) Q504: Initiation, Review, And/Or Update
To Suicide Safety Plan For Individuals With
Suicidal Thoughts, Behavior, Or Suicide
Risk
(Collection Type: MIPS CQMs
Specifications)
(Medium)
(~) IA_PM_23: Use of Computable Guidelines
and Clinical Decision Support to Improve
Adherence for Cervical Cancer Screening and
Management Guidelines
(High)
(^)(+) IA_PM_XX: Save a Million Hearts:
Standardization of Approach to Screening and
Treatment for Cardiovascular Disease Risk
(High)

TABLE B.15b: Value in Primary Care MVP Foundational Layer
Population Health Measures
Promoting Interoperability
(!!) Q479: Hospital-Wide, 30-Day, All-Cause Unplanned
Readmission (HWR) Rate for the Merit-Based Incentive Payment
Systems (MIPS) Groups
(Collection Type: Administrative Claims)
(!!) Q484: Clinician and Clinician Group Risk-standardized Hospital
Admission Rates for Patients with Multiple Chronic Conditions
(Collection Type: Administrative Claims)
Security Risk Analysis
High Priority Practices Safety Assurance Factors for EHR Resilience
Guide (SAFER Guide)
e-Prescribing
Query of Prescription Drug Monitoring Program (PDMP)
Provide Patients Electronic Access to Their Health Information
Support Electronic Referral Loops By Sending Health Information
AND
Support Electronic Referral Loops By Receiving and Reconciling
Health Information
OR
Health Information Exchange (HIE) Bi-Directional Exchange
OR
Enabling Exchange Under the Trusted Exchange Framework and
Common Agreement (TEFCA)
Immunization Registry Reporting
Syndromic Surveillance Reporting (Optional)
Electronic Case Reporting
Public Health Registry Reporting (Optional)
Clinical Data Registry Reporting (Optional)
Actions to Limit or Restrict Compatibility or Interoperability of
CEHRT
ONC Direct Review Attestation
[FR Doc. 2024-14828 Filed: 7/10/2024 4:15 pm; Publication Date: 7/31/2024]
