
1
CushingCC, etal. BMJ Open 2019;9:e030029. doi:10.1136/bmjopen-2019-030029
Open access
Responsive Asthma Care for Teens
(ReACT): development protocol for an
adaptive mobile health intervention for
adolescents with asthma
Christopher C Cushing,
1
David A Fedele,
2
Susana R Patton,
3
Elizabeth L McQuaid,
4
Joshua M Smyth,
5
Sreekala Prabhakaran,
6
Selina Gierer,
7
Natalie Koskela-Staples,
6
Adrian Ortega,
1
Kandace K Fleming,
8
Arthur M Nezu
9
To cite: CushingCC, FedeleDA,
PattonSR, etal. Responsive
Asthma Care for Teens (ReACT):
development protocol for
an adaptive mobile health
intervention for adolescents
with asthma. BMJ Open
2019;9:e030029. doi:10.1136/
bmjopen-2019-030029
► Prepublication history for
this paper is available online.
To view these les, please visit
the journal online (http:// dx. doi.
org/ 10. 1136/ bmjopen- 2019-
030029).
DAF and CCC contributed
equally and the author order
was determined by a coin ip.
Received 25 February 2019
Revised 28 June 2019
Accepted 1 July 2019
For numbered afliations see
end of article.
Correspondence to
DrChristopher CCushing;
christopher. cushing@ ku. edu
Protocol
© Author(s) (or their
employer(s)) 2019. Re-use
permitted under CC BY-NC. No
commercial re-use. See rights
and permissions. Published by
BMJ.
ABSTRACT
Introduction Asthma is a leading cause of youth
morbidity in the USA, affecting >8% of youth. Adherence
to inhaled corticosteroids (ICS) can prevent asthma-
related morbidity; however, the typical adolescent with
asthma takes fewer than 50% of their prescribed doses.
Adolescents are uniquely vulnerable to suboptimal asthma
self-management due to still-developing executive
functioning capabilities that may impede consistent self-
regulation and weaken attempts to use problem solving to
overcome barriers to ICS adherence.
Methods and analysis The aims of this project are to
improve adherence to ICS as an important step towards
better self-management among adolescents aged 13–17
years diagnosed with asthma by merging the efcacious
behaviour change strategies found in behavioural health
interventions with scalable, adaptive mobile health
(mHealth) technologies to create the Responsive Asthma
Care for Teens programme (ReACT). ReACT intervention
content will be developed through an iterative user-centred
design process that includes conducting (1) one-on-one
interviews with 20 teens with asthma; (2) crowdsourced
feedback from a nationally representative panel of 100
adolescents with asthma and (3) an advisory board of
youth with asthma, a paediatric pulmonologist and a
behavioural health expert. In tandem, we will work with
an existing technology vendor to programme ReACT
algorithms to allow for tailored intervention delivery.
We will conduct usability testing of an alpha version
of ReACT with a sample of 20 target users to assess
acceptability and usability of our mHealth intervention.
Participants will complete a 4-week run-in period to
monitor their adherence with all ReACT features turned
off. Subsequently, participants will complete a 4-week
intervention period with all ReACT features activated.
The study started in October 2018 and is scheduled to
conclude in late 2019.
Ethics and dissemination Institutional review board
approval was obtained at the University of Kansas and
the University of Florida. We will submit study ndings
for presentation at national research conferences that
are well attended by a mix of psychologists, allied health
professionals and physicians. We will publish study
ndings in peer-reviewed journals read by members of the
psychology, nursing and pulmonary communities.
INTRODUCTION
Asthma affects over 8% of youth and is a
leading cause of morbidity.
1 2
Some asthma
symptoms and healthcare utilisation could
be prevented via consistent engagement in
disease self-management behaviours (eg,
symptom recognition and monitoring, appro-
priate administration of medications).
2 3
Adherence to daily controller medications,
such as inhaled corticosteroids (ICS), is
central to control asthma and reduce
morbidity for youth with persistent asthma.
2
ICS adherence rates with adolescents are
often <50%,
4 5
placing them at significant
risk for reduced lung function, increased
morbidity and poor quality of life.
4 6
We
posit that the complexity of the asthma treat-
ment regimen coupled with still-developing
executive functioning and problem-solving
Strengths and limitations of this study
► Intervention content will be developed rst with
theory and evidence-based decision-making and
rened via an iterative, user-centred approach with
target users and key stakeholders.
► Adaptive algorithms will be programmed into an ex-
isting, patient-facing asthma management platform.
► Intervention usability and acceptability will be strin-
gently assessed prior to efcacy testing to allow for
modications and improvements.
► Although we will have data on patient preferences,
usability and acceptability, the current protocol is not
designed to evaluate the efcacy of the Responsive
Asthma Care for Teens (ReACT) programme; this
limitation will be addressed in a future randomised
controlled trial.
► While medical providers were involved in the de-
velopment of interviews, content and interpretation
of results, the current protocol will not incorporate
shared decision-making between patients and pro-
viders in the intervention given the focus of ReACT.
on September 4, 2024 by guest. Protected by copyright.http://bmjopen.bmj.com/BMJ Open: first published as 10.1136/bmjopen-2019-030029 on 20 August 2019. Downloaded from

2
CushingCC, etal. BMJ Open 2019;9:e030029. doi:10.1136/bmjopen-2019-030029
Open access
abilities makes adolescents uniquely vulnerable to subop-
timal asthma self-management through self-regulation
deficits.
7–11
The importance of self-regulation in asthma self-man-
agement is well-supported.
12–17
Because the self-regu-
lation abilities that are needed to successfully manage
one’s own asthma are linked to brain regions that are
still developing in adolescents (ie, frontal lobes respon-
sible for top-down control), external supports in the
form of parents or technology may be required to ensure
that dosing occurs as prescribed. Simply put, to develop
self-regulation, adolescents with chronic and persistent
asthma need to spend time thinking about their asthma,
medication and actively planning how to incorporate
adherence into their lives. Developmentally, adolescents’
executive functioning skills may not be ideally suited for
this task as evidenced by data indicating that adolescents
take their medication as prescribed on some occasions,
and nearly every adolescent misses some doses on some
occasions.
18 19
Similar to the problem of underdeveloped self-regu-
latory skill, it is common for adolescents with persistent
asthma to be met with barriers to ICS adherence that
they may not have the experience or cognitive develop-
ment to effectively problem-solve.
12 20
Indeed, intraper-
sonal factors such as attitudes (eg, motivation), feelings
(eg, mood, stress) and external factors such as the social
and family environment, have consistently been associ-
ated with asthma management behaviours and disease
outcomes.
13 21–26
Efficacious behavioural interventions
often attempt to actively reframe ICS adherence as central
to an adolescent’s self-concept (eg, better lung function
will allow you to pursue your interests). Once adolescents
see adherence as helping them reach their own personal
goals rather than as a chore, they are sufficiently moti-
vated to learn skills that can help ensure adherence to
ICS. To this end, adolescents are taught to identify intra-
personal and external barriers to adherence and prob-
lem-solve around those barriers. Beyond simply teaching
a formula for problem solving, many effective adherence
promotion programmes tailor intervention content to
personally salient barriers and help the adolescent iden-
tify and implement strategies specifically designed to
increase the chances of regimen success.
27–29
A persistent problem in this approach is the difficulty
of relying on infrequent face-to-face visits (eg, for clin-
ical care) to implement interventions. Smartphones are
habitually carried by >70% of adolescents
30
; as such,
mobile technology provides a readily available medium
to approximate the features of efficacious behavioural
health interventions
31 32
by leveraging passive monitoring,
data listening, preprogrammed algorithms and content
libraries focused on improving self-regulation and prob-
lem-solving skills.
33 34
Despite their potential, existing
mobile health (mHealth) interventions have been largely
developed without the benefit of behavioural theory,
use reminder-based approaches to behaviour change
and lack the kinds of tailored problem-solving training
that characterises efficacious in-person interventions
35–37
(for a systematic evaluation of behaviour change tech-
niques in current asthma self-management applications,
see Ramsey et al
37
). We believe that this, at least in part,
helps explain why very few existing mHealth interventions
for asthma medication adherence have yet to demon-
strate their efficacy beyond active controls.
38–40
There is a clear need for an mHealth intervention that
merges digital delivery modalities with the theory-based
behavioural framework and tailoring found in efficacious
in-person treatments. Recent technological advances
have catalysed the development of mobile-based interven-
tion platforms that deliver tailored support to individuals
in a timely fashion.
41 42
We propose to extend this work to
paediatric asthma by developing Responsive Asthma Care
for Teens (ReACT), an innovative adaptive mHealth inter-
vention that facilitates self-regulation by aiding adoles-
cents in self-monitoring, goal setting and problem solving
when adolescents’ adherence data indicate they need
additional support the most (figure 1). We will outline
how ReACT content will be developed, refined and tested
through an iterative user-centred design process guided
by theory and prior evidence.
METHODS AND ANALYSIS
Objectives
The aims of the study are: (1) conduct a hybrid user-cen-
tred and evidence-based design process comprising indi-
vidual interviews, crowdsourcing and advisory boards to
develop ReACT content and features; (2) test the accept-
ability and usability of ReACT in a sample of 20 adoles-
cents with persistent asthma. We hypothesise that ReACT
will be an acceptable and usable mHealth adherence
promotion intervention as determined by high accept-
ability and usability ratings and themes from think aloud
testing and qualitative one-on-one interviews with target
users.
Project overview
This multisite study will take place at the University of
Florida, University of Kansas and their affiliated clinics.
The study commenced in October 2018 and is anticipated
to be completed in late 2019. Features and intervention
content of ReACT will be developed concurrently. We will
work with a technology vendor to add functionality for
ReACT to an existing mHealth adherence monitoring
platform. We will use a strong theoretical framework and
prior evidence combined with a series of user-centred
design phases to determine what intervention content
should populate the ReACT system (design phase I).
Study team members and an advisory board will then
refine intervention content and complete preliminary
usability testing of ReACT (design phase II). Finally,
we will conduct acceptability and usability testing using
an alpha version of ReACT (see figure 2 for the study
timeline).
on September 4, 2024 by guest. Protected by copyright.http://bmjopen.bmj.com/BMJ Open: first published as 10.1136/bmjopen-2019-030029 on 20 August 2019. Downloaded from

3
CushingCC, etal. BMJ Open 2019;9:e030029. doi:10.1136/bmjopen-2019-030029
Open access
Core ReACT functionality
We will create ReACT by expanding the capabilities of an
existing Android and iOS compatible mobile phone app
that uses an integrated mobile sensor designed to fit onto
an asthma metered dose inhaler or Diskus. The sensor
works passively to sense when ICS or short-acting beta-ag-
onist medications are dispensed. Bluetooth is used to pass
the information to the app, which has a set of optional
features (eg, provide feedback about adherence) that can
be turned on as intervention parameters dictate. Figure 3
outlines an adolescent’s intervention experience through
ReACT. Building from theory and prior evidence, core
ReACT components in sequence are: (1) core education
on asthma management and skills training content on goal
setting and problem solving,
4 43
(2) conditional activation
of ReACT features when an adolescent is <80% adherent
to their ICS, (3) an evidence-based goal-setting algorithm
that shapes adherence to dosing recommendations over
time, (4) timely assessment of an adolescent’s barriers to
adherence and (5) delivery of tailored problem-solving
training based on recent and salient barriers. Determi-
nants of self-regulation will be integrated into the core
ReACT functionality to best scaffold support for adoles-
cents, as outlined below.
ReACT will begin with an orientation module within
the app that guides the users through the platform,
core educational and skills training modules and passive
monitoring of medications. Adolescents will complete an
asthma education module based on the National Heart,
Lung and Blood Institute guidelines
44
to ensure that they
have an understanding of the importance of medication
adherence as a means to avoid asthma-related impair-
ments. Adolescents will receive skills training on empir-
ically supported techniques consistent with our guiding
self-regulation theory. Goal-setting content will use a
Specific, Measurable, Attainable, Relevant, Time-Bound
goal framework. Problem-solving skills training will focus
on orienting to the problem, defining and formulating
the problem, generating alternative solutions, deciding
on a course of action and implementing a solution.
45 46
We will use engaging videos created by the team to deliver
content. Participants will complete the orientation
modules with study staff to encourage engagement and
provide assistance if necessary.
Figure 1 ReACT conceptual model. Black arrows represent mechanistic processes occurring during the ReACT
intervention period. Blue arrows indicate recursive processes happening repeatedly during the intervention period. ICS
adherence,adherence to inhaled corticosteroids; ReACT, Responsive Asthma Care for Teens.
Figure 2 Study timeline.
on September 4, 2024 by guest. Protected by copyright.http://bmjopen.bmj.com/BMJ Open: first published as 10.1136/bmjopen-2019-030029 on 20 August 2019. Downloaded from

4
CushingCC, etal. BMJ Open 2019;9:e030029. doi:10.1136/bmjopen-2019-030029
Open access
ReACT will use passive sensing to objectively monitor
rates of adolescent ICS adherence throughout the inter-
vention period. The use of the ICS adherence sensor
offloads the burden of self-monitoring such that adoles-
cents do not have to remember or calculate their adher-
ence. Active intervention elements in ReACT will activate
only when an adolescent falls below a clinically derived
80% adherence threshold
47
based on a 7-day rolling
average, thus reducing intervention fatigue.
42
If an
adolescent has <80% adherence to ICS based on the 7-day
rolling average, ReACT will prompt the adolescent to set
a goal for adherence in the next 7-day period, a strategy
with demonstrated efficacy in previous asthma adherence
interventions.
48
The algorithm will only allow goals that
are reasonable given performance over the past week
to avoid overly ambitious and unattainable goals. Each
evening, the adolescent will receive a feedback message
about how much their adherence that day moved them
towards their goal, additionally facilitating the self-reg-
ulatory process of observation. Furthermore, the condi-
tional activation of the ReACT features should support
self-regulatory skills, specifically judgement, by notifying
the adolescent when his or her adherence declines.
ReACT will identify contextual barriers to ICS adher-
ence by also initiating an assessment as soon as the 7-day
rolling average indicates that adherence is <80%. Specif-
ically, adolescents will be prompted via a push notifica-
tion to complete a brief electronic momentary assessment
(EMA) survey of barriers identified from our pilot data
and design phase I to identify what barrier the prob-
lem-solving content should be tailored to address. Partic-
ipants will answer brief questions in the app regarding
barriers that got in the way of taking their ICS followed by
a rank order item denoting which one to make the focus of
their problem-solving efforts. Once a barrier is identified,
ReACT will deliver problem-solving content in the app
that is tailored to that specific barrier. For instance, if an
adolescent has identified and chooses to work on stress as
their top barrier to ICS adherence, the tailored problem
solving will deliver structured problem-solving training
using a flexible system of branching that is tailored to
stress specifically. To execute this feature, ReACT will
have several content banks that use the same structure. To
illustrate the user experience, a possible problem-solving
intervention could include: (1) defining the problem:
participants will be presented with a standard message
reflecting the problem they identified in the EMA survey;
(2) setting a realistic, achievable goal: several goals will
be presented and the participant will choose one and
receive feedback on their choice; (3) generating multiple
solutions: the participant will be asked to choose from a
list of possible solutions for the goal that they selected
in step #2; (4) evaluating pros and cons: participants will
evaluate the pros and cons of multiple solutions listed in
step #3; (5) selecting a solution: participants will choose
the solution that may work the best; (6) making an action
plan: participants will select from a list of action plans
that correspond to the solution they have selected; (7)
evaluating the outcome: after a week participants will be
asked whether they implemented the solution selected
in step #6. The goal-setting and tailored problem-solving
process should enhance one’s reaction to suboptimal
adherence by improving self-efficacy and facilitating skills
to overcome their personalised adherence barriers.
Once an adolescent receives intervention content, ReACT
will prompt adolescents to complete a survey in the app 2 days
later to assess content use. If the participant responds that
they used the intervention strategy, ReACT will assess the
participant’s confidence in their ability to repeat the plan in
the future. If the participant has not used the strategy, they
will receive a supportive prompt with options to review the
content they saw last time, see the same content in a different
Figure 3 React participant ow. Diamonds indicate intervention decision rules. EMA, electronic momentary assessment; ICS,
inhaled corticosteroids; ReACT, Responsive Asthma Care for Teens; SABA, short-acting beta-agonist.
on September 4, 2024 by guest. Protected by copyright.http://bmjopen.bmj.com/BMJ Open: first published as 10.1136/bmjopen-2019-030029 on 20 August 2019. Downloaded from
5
CushingCC, etal. BMJ Open 2019;9:e030029. doi:10.1136/bmjopen-2019-030029
Open access
media format, review a different set of content on the same
topic or set a goal to use the strategies by a specific time in the
next 2 days. Another programme safeguard is to reduce over-
whelming participants with content; therefore, the tailored
problem-solving process (figure 3) will only run once in a
given 7-day window. This mirrors normative approaches for
in-person interventions during which only one or two new
concepts would be introduced each week.
Participants and recruitment
Participants
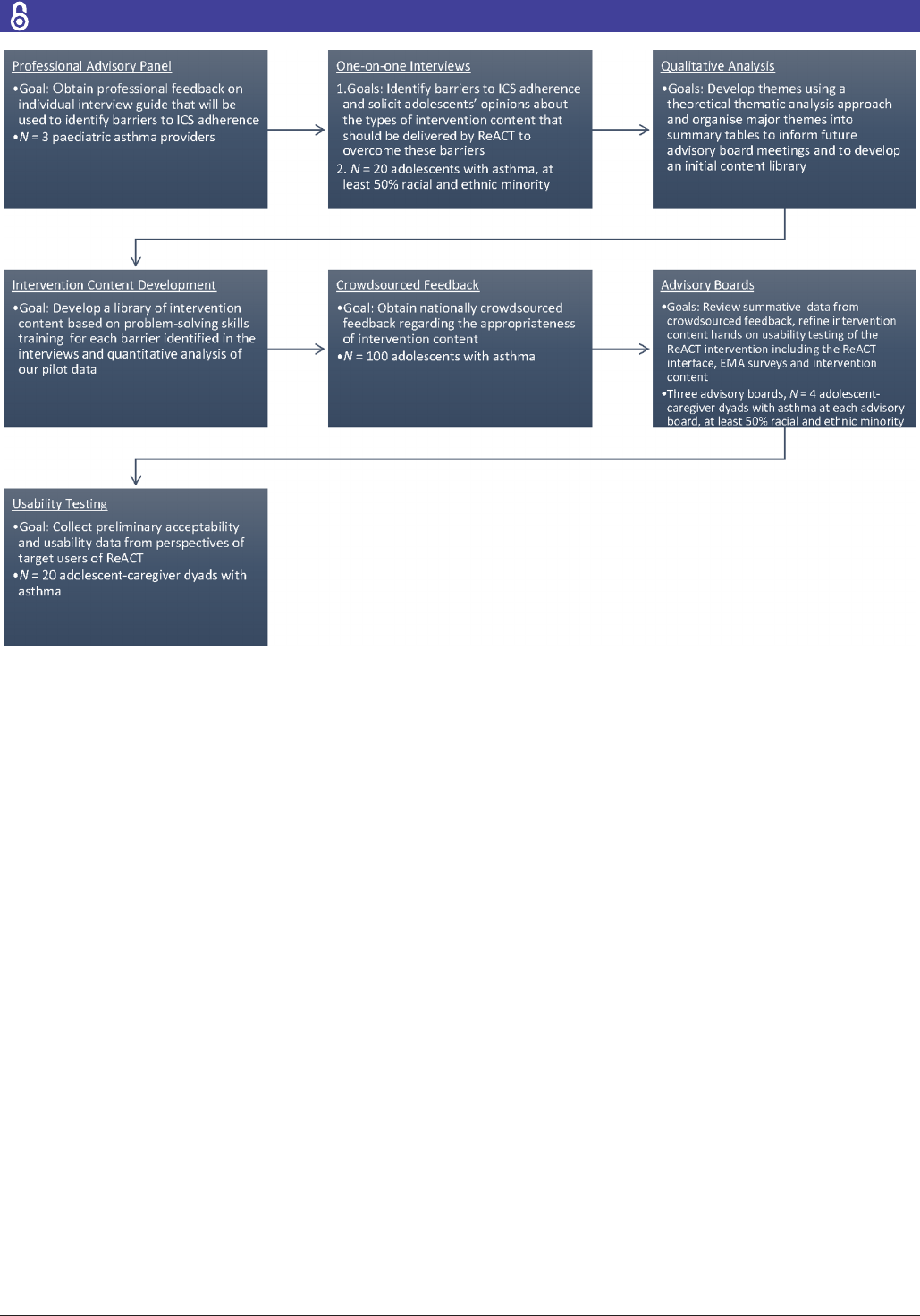
Participants will include four separate samples of adolescents
aged 13–17 years with asthma and their caregivers (figure 4).
Twenty adolescent-caregiver dyads will complete individual
interviews, 100 adolescents will provide crowdsourced feed-
back via a national online panel, 4 adolescent-caregiver dyads
will participate in advisory board meetings and 20 adoles-
cent-caregiver dyads will complete user testing of ReACT.
We intend that at least half of adolescent participants in the
interviews and advisory boards will be from racial and ethnic
minority groups.
Inclusion and exclusion criteria
For interviews, advisory boards and user testing, adoles-
cents must have a physician-verified diagnosis of current
asthma with persistent symptoms requiring regular ICS
use for ≥6 months. They must have a daily ICS or ICS/
LABA prescription that is sensor-compatible, and they
and their caregivers must speak and read English. Asthma
status and prescription information will be verified via
the electronic medical record. Families will be excluded
from the study if the adolescent is currently involved in
an asthma management intervention, has a comorbid
chronic health condition that may impact lung function
or has a significant cognitive impairment or develop-
mental delay that interferes with study completion. For
crowdsourced feedback, inclusion will be determined
at the level of a national online panel, which will screen
participants for persistent asthma.
Recruitment
Participants will be recruited (1) through university-affil-
iated clinics and (2) via flyers. Research and clinic staff
members with access to the electronic medical record will
identify eligible patients with upcoming medical appoint-
ments. During these appointments, providers with clin-
ical relationships with the eligible participants will first
approach the patients to determine their interest in
hearing more about the study. Then, in coordination with
the clinic staff, research staff will meet with interested
patients to provide a study overview, complete in-person
screening for eligibility and invite participation. In
the event that a family is unable to complete screening
Figure 4 ReACT ow of formative work. EMA, electronic momentary assessment; ICS, inhaled corticosteroids; ReACT,
Responsive Asthma Care for Teens.
on September 4, 2024 by guest. Protected by copyright.http://bmjopen.bmj.com/BMJ Open: first published as 10.1136/bmjopen-2019-030029 on 20 August 2019. Downloaded from

6
CushingCC, etal. BMJ Open 2019;9:e030029. doi:10.1136/bmjopen-2019-030029
Open access
during a clinic visit, research staff will request permission
for a member of the study team to contact patients for
screening using an institutional review board (IRB)-ap-
proved ‘consent-to-contact’ form. A member of the study
staff will then call interested participants to provide a study
overview and invite participation. In addition, IRB-ap-
proved flyers will be posted or made available in clinics,
community organisations, schools, physician offices and
common areas. Flyers will encourage families and nurses
to call our research office to learn about the project,
determine initial eligibility and if eligible, schedule an
in-person screening visit where informed consent will
be collected prior to study enrolment. Throughout all
phases of the project, participants will be incentivised and
compensated for their participation.
ReACT development
Patient and public involvement
Adolescents diagnosed with asthma, their caregivers and
paediatric asthma providers are involved in all stages of
ReACT development, as described below.
Design phase I: content development
We have developed a list of common barriers to ICS
adherence from our own pilot data and the extant paedi-
atric asthma literature. Our goal for design phase I is
to use individual interviews with adolescents diagnosed
with asthma to translate these barriers into terms easily
understood by adolescents, develop a final list of barriers
to adherence and subsequently develop a library of inter-
vention content to overcome adherence barriers that is
informed by self-regulation theory. Individual interview
participants and their caregivers will also complete asth-
ma-related measures for sample description purposes and
to obtain preliminary data on constructs of interest to the
project (table 1). Adolescent-caregiver dyads will receive
US$60 for their participation.
The study team will create an individual interview guide
that will be used to identify what barriers to adherence
are most salient to adolescents with asthma, and to solicit
their opinion about the types of intervention content that
they would prefer to receive when experiencing these
barriers. Prior to the start of individual interviews, at least
three paediatric asthma providers (eg, pulmonologists,
nurses) will provide feedback on the interview guide. All
interviews will be audio-recorded and conducted with
self-regulation theory in mind. If a component of self-reg-
ulation theory is not discussed, we will probe for content
in the omitted domain to facilitate development of inter-
vention content. Interviews will be transcribed and eval-
uated by the study team to inform digital intervention
content development (see ‘Data analysis plan’ section).
Research staff with experience developing digital
intervention content will leverage information gathered
during design phase I to develop a library of intervention
content for each barrier identified in the interviews and
quantitative analysis of our pilot data. We anticipate that
intervention content will include a combination of skills
training videos, brief text content, educational videos
and images. Delivery modality (SMS, app, etc) will be
discussed with the advisory board.
Design phase II: renement of content and preliminary usability
testing
In design phase 2, we will refine intervention content
generated in design phase I through (1) nationally crowd-
sourced feedback from adolescents with asthma and (2)
advisory board meetings. Preliminary usability testing and
iterative refinement will be conducted with our advisory
board meetings (described below).
Nationally crowdsourced feedback will be solicited
via an online panel and survey-technology provider.
Participants will be identified from panels of adoles-
cents who have agreed to participate in research. These
panels are accessed by our survey vendor through their
business-to-business partnerships. Participants will be
screened for persistent asthma using the following stan-
dard set of questions commonly used in epidemiological
trials (eg, National Health Interview Survey): (1) Have
you ever been told by a doctor, nurse or other health-
care professional that you have asthma? (2) Do you still
have asthma? Affirmative answers to both questions will
qualify an adolescent for participating.
49
Participants
will review intervention content and rate its appropriate-
ness using a dichotomous ‘yes’ (I like the message as it
is) or ‘no’ (change it to make it better) response choice.
They will receive US$15 for their participation. Content
receiving ≥60% ‘no’ votes will be discarded, and those
with ≤39% ‘no’ votes will be accepted as final interven-
tion content. Content with 40%–59% ‘no’ votes will be
revised or clarified while retaining any theoretically or
empirically derived concepts.
50
We will design surveys
to take no more than 30 min each. If necessary, we will
split the content into two surveys to keep the administra-
tion time <30 min. Although adolescent stakeholders will
be involved in developing ReACT intervention content
rated during crowdsourcing, we acknowledge that there
is a possibility that a higher than expected amount of
content will be viewed unfavourably during this phase. We
will review crowdsourcing feedback data from an initial
wave of 20 participants. In the event that >60% of content
is viewed unfavourably, we will pause crowdsourcing to
develop new intervention content.
An advisory board comprising adolescent-caregiver
dyads and study staff members will convene three times.
The first meeting will focus on reviewing summative data
and themes that emerged from crowdsourcing phase.
The second advisory board meeting will involve discus-
sion about methods to further refine intervention content
that received 40%–59% ‘no’ votes during crowdsourcing.
We will incorporate modified content that reaches group
consensus into applicable ReACT intervention content
libraries. Preliminary usability testing will take place
during the final advisory board meeting. Members will
conduct hands-on testing of ReACT alongside study staff.
We will use a ‘think aloud’ approach with members as
on September 4, 2024 by guest. Protected by copyright.http://bmjopen.bmj.com/BMJ Open: first published as 10.1136/bmjopen-2019-030029 on 20 August 2019. Downloaded from

7
CushingCC, etal. BMJ Open 2019;9:e030029. doi:10.1136/bmjopen-2019-030029
Open access
they explore components of the ReACT interface (eg,
layout, visual feedback), answer EMA questions and view
intervention content.
51
This process more closely approx-
imates actual use and will enable us to receive feedback
in real-time. The study team will transcribe participants’
commentary during testing for review. Results of the
‘think aloud’ testing will help to inform final design deci-
sions in ReACT.
51
For instance, whether content is deliv-
ered using SMS, in-app, push notification or some other
medium will be informed by user preferences. Advisory
board participants and their caregivers will also complete
asthma-related measures to pilot data collection proce-
dures and provide baseline descriptive statistics (table 1).
Adolescent-caregiver dyads will receive US$50 for each
advisory board meeting they attend.
Acceptability and usability testing of ReACT
In the final phase, adolescents will conduct user testing
with the alpha version of ReACT. The overarching
goal is to gather acceptability and usability data from
the perspectives of target users of ReACT. A sample of
20 participants will complete a 4-week run-in period to
Table 1 ReACT outcome measures
Outcome Measure Assessment schedule
Demographics A caregiver-report questionnaire assesses adolescent and family demographic
characteristics.
II, AB, UT
Asthma morbidity A caregiver-report questionnaire assesses frequency of asthma symptoms,
exacerbations, activity limitations, missed school days due to asthma, ED visits
and hospitalisations.
II, AB, UT
Medical information Medical chart review assesses prescribed ICS regimen and dosage. II, AB, UT
Asthma knowledge
and skills
The Asthma Child Knowledge and Skills Questionnaire,
12
a modied version
of the Children’s Asthma Knowledge Questionnaire,
59
is a 30-item adolescent-
report measure that assesses both asthma knowledge and self-assessment of
skills required for taking medication.
II, AB, UT
Asthma control The Asthma Control Test
60
is a 5-item, validated, adolescent-report
questionnaire that assesses asthma control in past 4 weeks.
II, AB, UT
Asthma
management
The Asthma Management Efcacy Questionnaire
61
is a 14-item, validated,
adolescent-report questionnaire that assesses asthma self-management
behaviours.
II, AB, UT
Asthma adherence The Medication Adherence Report Scale for Asthma
62
is a 10-item, validated,
adolescent-report measure of ICS adherence.
II, AB, UT
Self-regulation The Treatment Self-Regulation Questionnaire-Asthma
63
is a 15-item,
adolescent-report measure that assesses motivation for using controller
medication.
II, AB, UT
Stress The Adolescent Stress Questionnaire
64
Revised is a 58-item, validated,
adolescent-report questionnaire that assesses stressors in adolescence.
II, AB, UT
Social support The Social Support Questionnaire
65
is a 27-item, validated, adolescent-report
measure of social support.
II, AB, UT
Problem solving The Social Problem Solving Inventory-Revised: Short Form
66
is a 25-item,
validated, adolescent-report measure that assesses problem-solving orientation
and skills in everyday life.
II, AB, UT
Asthma-related
quality of life
PAQLQ
67
is a 23-item, validated, adolescent-report questionnaire that measures
extent of asthma impairment in quality of life.
II, AB, UT
Acceptability The ReAct Satisfaction Questionnaire is an 8-item modication of the Client
Satisfaction Questionnaire
56
that assesses overall participant satisfaction with
the ReACT intervention. Semi-structured interviews assess what adolescents
like and do not like about ReACT, its relevance and its perceived helpfulness
with medication adherence.
UT
Usability The Health Information Technology Usability Evaluation Scale
57
is a 20-item,
validated questionnaire that assesses perceived usefulness, impact on disease,
perceived ease of use and user control. Think aloud testing gathers stream of
consciousness data regarding thoughts and feelings of users as they complete
specied tasks. Semi-structured interviews assess the look and feel of ReACT,
ease of navigation and experience accessing intervention content.
UT
AB,advisory boards; ED, emergency department; ICS, inhaled corticosteroids; II, design phase I individual interviews;PAQLQ, Pediatric
Asthma Quality of Life Questionnaire; ReACT, Responsive Asthma Care for Teens; UT,user testing.
on September 4, 2024 by guest. Protected by copyright.http://bmjopen.bmj.com/BMJ Open: first published as 10.1136/bmjopen-2019-030029 on 20 August 2019. Downloaded from

8
CushingCC, etal. BMJ Open 2019;9:e030029. doi:10.1136/bmjopen-2019-030029
Open access
monitor their adherence with all ReACT features turned
off. Subsequently, study staff will meet with participants to
complete ReACT orientation. This visit will ensure that
participants are able to download and use ReACT, and
that relevant ReACT components (eg, asthma education
and skills training videos) are accessed before beginning.
Participants will complete asthma-related study question-
naires (table 1) and then begin a 4-week intervention
period with all ReACT features activated. Notably, accept-
ability and usability measures will be administered at a
final study visit at the conclusion of the 4-week interven-
tion period. Again, participants’ comments and sugges-
tions during the final study visit will be transcribed for
review.
Data analysis plan
Individual interviews
Study staff will enter transcribed files and expanded notes
into NVivo. We will code and aggregate interviews using
a theoretical thematic analysis approach to developing
themes.
52–54
Our theoretical thematic analysis approach
will use an a priori theoretical framework guided by
self-regulation theory, informed by advisory board meet-
ings. The investigators will mark comments identified to
represent discrete thoughts or themes using a semantic
analysis, and they will use an essential realist approach to
arrive at themes.
52
These patterns or themes will comprise
the initial set of categories. Research staff will then recode
the data using these categories and organise major themes
into summary tables to inform initial development of a
digital content library. Interviews will continue until no
new themes emerge in the data coding process (ie, satu-
ration).
55
After the coding process is complete, data will
be described descriptively.
ReACT acceptability
Acceptability of ReACT will be determined in two ways
during the usability testing phase. First, the ReACT Satis-
faction Questionnaire
56
will assess overall satisfaction,
perceptions regarding how helpful ReACT could be in
managing asthma and whether adolescents would recom-
mend ReACT to friends with asthma on a 4-point Likert
scale. An average rating of 3 (mostly satisfied) will be
considered a successful outcome. Second, the semi-struc-
tured interviews will solicit adolescent feedback about
ReACT. Our comprehensive interview guide will cover
a range of topics, including: (1) perceived usefulness of
ReACT; (2) how effective ReACT might be in changing
asthma self-management behaviours and (3) suggestions
on further refining ReACT (eg, incorporating other indi-
viduals). Qualitative data analysis will help determine
overall project success. The process for identifying themes
will be similar to the process from the earlier interview
phase, but in this case we will use an entirely de novo
process of identifying themes.
52
We will mark comments
identified to represent discrete thoughts or themes using
a semantic analysis, and we will use an essential realist
approach to arrive at themes.
52
In particular, we will be
attentive to themes that relate to the acceptability, useful-
ness and user experience of ReACT. Themes that indicate
that ReACT was perceived to be effective, appropriately
tailored and acceptable burden will be the criteria for
success.
ReACT usability
Usability will be determined in three ways. First, an
average rating of 3 (agree) on the Health Information
Technology Usability Evaluation Scale
57
will be a criterion
for success. Second, themes from think aloud testing that
suggest adolescents can navigate the ReACT interface
intuitively and with minimal difficulty will be markers of
success. Finally, our semi-structured interview will ask for
feedback regarding: (1) the layout of the ReACT inter-
face; (2) the navigation experience; (3) clarity of the
wording; (4) clarity of the video content and (5) ways to
improve the usability and content of ReACT. These data
will be used to inform future refinements of ReACT in
advance of a subsequent trial.
Ethics and dissemination
All research team members will complete certification
in topics related to the responsible conduct of research.
To minimise risk from research participation, potential
subjects will be fully informed regarding the purpose,
process and amount of time required for participation.
It is possible that research staff will identify an adolescent
whose asthma appears undertreated. Research staff will
review all cases with local medical personnel and facilitate
a referral for evaluation and appropriate medication if
indicated.
We plan to disseminate findings from the current
project to multiple audiences including the local medical
community and the broader scientific community via
local and national presentations at relevant conferences
and meetings. Beyond paediatric asthma, we also envision
that the ReACT infrastructure and design process can be
used to develop and test behaviour change interventions
in other disease populations. If successful, this would be
a significant step towards the 2016 National Institutes of
Health-Wide Strategic Plain goal of using mHealth to
‘enhance health promotion and disease prevention’.
58
Limitations
The current project is a pilot feasibility, acceptability and
usability study in a targeted sample (ie, adolescents with
chronic and persistent asthma), who also have a high need
for this type of intervention. As such, we will not be able
to contribute knowledge about the feasibility of ReACT
in all of the populations it might benefit (eg, adults with
chronic obstructive pulmonary disease). Moreover, the
current project is not powered to understand heteroge-
neity of outcomes across sex, socioeconomic status, race,
culture and literacy levels. The protocol will not incor-
porate shared decision-making between patients and
providers in the intervention given the focus of ReACT.
ReACT does not target all of the factors that might
on September 4, 2024 by guest. Protected by copyright.http://bmjopen.bmj.com/BMJ Open: first published as 10.1136/bmjopen-2019-030029 on 20 August 2019. Downloaded from

9
CushingCC, etal. BMJ Open 2019;9:e030029. doi:10.1136/bmjopen-2019-030029
Open access
influence adherence. Specifically, structural issues such
as inadequate insurance coverage will not be addressed
in the current protocol. At this stage, ReACT does not
involve providers at least in part because there are already
other commercial systems that do a good job of achieving
that function. The novelty of ReACT is to identify the
developmentally appropriate individual-level interven-
tions that can increase adherence.
Author afliations
1
Clinical Child Psychology Program and Schiefelbusch Institute for LifeSpan Studies,
University of Kansas
2
Department of Clinical & Health Psychology, University of Florida, Gainesville, FL
3
Department of Pediatrics, University of Kansas Medical Center, Kansas City,
Kansas, USA
4
Department of Psychiatry and Human Behavior, Brown University, Providence,
Rhode Island, USA
5
Pennsylvania State University, University Park, Pennsylvania, USA
6
Department of Pediatrics, University of Florida, Gainesville, Florida, USA
7
Division of Allergy and Immunology, University of Kansas Medical Center, Kansas
City, KS, USA
8
Life Span Institute, University of Kansas, Lawrence, Kansas, USA
9
Department of Psychology, Drexel University, Philadelphia, Pennsylvania, USA
Contributors DAF and CCC conceived the study. DAF, CCC, SPa, SG, ELM, SPr and
JMS developed the protocol. DAF, CCC, NK-S, AO, KKF and AMN were involved in
drafting of the article. Specically, DAF and CCC collaboratively wrote the text, NK-S
and AO edited the text, and created gures. KKF and AMN provided expertise and
section edits on the statistical approach and features of problem-solving therapy.
SPa and ELM provided expertise and writing about qualitative work. JMS provided
expertise and writing about adaptive interventions. All authors completed a critical
revision of the article and approved the nal text. DAF and CCC contributed equally
to the paper as joint rst authors (order determined by coin ip).
Funding This work was supported by the National Institutes of Health grant
number 1R56HL141394-01A1.
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval Institutional review board approval was obtained at the University
of Kansas and the University of Florida.
Provenance and peer review Not commissioned; externally peer reviewed.
Open access This is an open access article distributed in accordance with the
Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which
permits others to distribute, remix, adapt, build upon this work non-commercially,
and license their derivative works on different terms, provided the original work is
properly cited, appropriate credit is given, any changes made indicated, and the use
is non-commercial. See: http:// creativecommons. org/ licenses/ by- nc/ 4. 0/.
REFERENCES
1. Akinbami LJ, Simon AE, Rossen LM. Changing Trends in Asthma
Prevalence Among Children. Pediatrics 2016;137:e20152354.
2. Guidelines for the Diagnosis and Management of Asthma (EPR-3).
Bethesda, MD, 2007.
3. Guevara JP, Wolf FM, Grum CM, et al. Effects of educational
interventions for self management of asthma in children and
adolescents: systematic review and meta-analysis. BMJ
2003;326:1308–9.
4. Morton RW, Everard ML, Elphick HE. Adherence in childhood
asthma: the elephant in the room. Arch Dis Child 2014;99:949–53.
5. Bender BG. Nonadherence to asthma treatment: Getting unstuck. J
Allergy Clin Immunol Pract 2016;4:849–51.
6. Anderson WC, Szeer SJ. New and future strategies to improve
asthma control in children. J Allergy Clin Immunol 2015;136:848–59.
7. Drotar D, Bonner MS. Inuences on adherence to pediatric asthma
treatment: a review of correlates and predictors. J Dev Behav Pediatr
2009;30:574–82.
8. D'Zurilla TJ, Maydeu-Olivares A, Kant GL. Age and gender
differences in social problem-solving ability. Pers Individ Dif
1998;25:241–52.
9. Jaffee WB, D'Zurilla TJ. Adolescent problem solving, parent problem
solving, and externalizing behavior in adolescents. Behav Ther
2003;34:295–311.
10. Muscara F, Catroppa C, Anderson V. Social problem-solving skills
as a mediator between executive function and long-term social
outcome following paediatric traumatic brain injury. J Neuropsychol
2008;2:445–61.
11. McGee CL, Fryer SL, Bjorkquist OA, et al. Decits in social problem
solving in adolescents with prenatal exposure to alcohol. Am J Drug
Alcohol Abuse 2008;34:423–31.
12. Rhee H, Belyea MJ, Ciurzynski S, et al. Barriers to asthma self-
management in adolescents: Relationships to psychosocial factors.
Pediatr Pulmonol 2009;44:183–91.
13. Rhee H, Belyea MJ, Brasch J. Family support and asthma outcomes
in adolescents: barriers to adherence as a mediator. J Adolesc Health
2010;47:472–8.
14. Modi AC, Quittner AL. Barriers to treatment adherence for children
with cystic brosis and asthma: What gets in the way? J Pediatr
Psychol 2006;31:846–58.
15. Buston KM, Wood SF. Non-compliance amongst adolescents with
asthma: listening to what they tell us about self-management. Fam
Pract 2000;17:134–8.
16. Bender BG, Bender SE. Patient-identied barriers to asthma
treatment adherence: responses to interviews, focus groups, and
questionnaires. Immunol Allergy Clin North Am 2005;25:107–30.
17. Clark NM, Gong M, Kaciroti N. A model of self-regulation for control
of chronic disease. Health Educ Behav 2001;28:769–82.
18. Gray WN, Netz M, McConville A, et al. Medication adherence in
pediatric asthma: A systematic review of the literature. Pediatr
Pulmonol 2018;53:668–84.
19. Bender B, Zhang L. Negative affect, medication adherence, and
asthma control in children. J Allergy Clin Immunol 2008;122:490–5.
20. Modi AC, Quittner AL. Barriers to treatment adherence for children
with cystic brosis and asthma: what gets in the way? J Pediatr
Psychol 2006;31:846–58.
21. Riekert KA, Borrelli B, Bilderback A, et al. The development of
a motivational interviewing intervention to promote medication
adherence among inner-city, African-American adolescents with
asthma. Patient Educ Couns 2011;82:117–22.
22. Christiaanse ME, Lavigne JV, Lerner CV. Psychosocial aspects of
compliance in children and adolescents with asthma. J Dev Behav
Pediatr 1989;10:75–80.
23. Fiese BH, Wamboldt FS, Anbar RD. Family asthma management
routines: connections to medical adherence and quality of life. J
Pediatr 2005;146:171–6.
24. Penza-Clyve SM, Mansell C, McQuaid EL. Why don't children
take their asthma medications? A qualitative analysis of children's
perspectives on adherence. J Asthma 2004;41:189–97.
25. Leeman J, Crandell JL, Lee A, et al. Family functioning and the well-
being of children with chronic conditions: A meta-analysis. Res Nurs
Health 2016;39:229–43.
26. Wolf F, Guevara J, Grum C, et al. Educational interventions
for asthma in children. Cochrane Database Syst Rev
2010;1:CD000326.
27. Naar-King S, Ellis D, King PS, et al. Multisystemic therapy for high-
risk African American adolescents with asthma: a randomized clinical
trial. J Consult Clin Psychol 2014;82:536–45.
28. Nezu AM, Nezu CM, Perri MG. Problem solving to promote treatment
adherence. In: O’Donohue WT, Levensky ER, eds. Promoting
Treatment Adherence: A Practical Handbook for Health Care
Providers. New York: SAGE Publications, 2006:135–48.
29. Duncan CL, Hogan MB, Tien KJ, et al. Efcacy of a parent-youth
teamwork intervention to promote adherence in pediatric asthma. J
Pediatr Psychol 2013;38:617–28.
30. Lenhart A. Teens, Social Media & Technology Overview 2015.
2015 http://www. pewinternet. org/ 2015/ 04/ 09/ teens- social- media-
technology- 2015/
31. Ritterband LM, Gonder-Frederick LA, Cox DJ, et al. Internet
interventions: In review, in use, and into the future. Prof Psychol
2003;34:527–34.
32. Schueller SM, Muñoz RF, Mohr DC. Realizing the Potential
of Behavioral Intervention Technologies. Curr Dir Psychol Sci
2013;22:478–83.
33. Riley WT, Rivera DE, Atienza AA, et al. Health behavior models in the
age of mobile interventions: are our theories up to the task? Transl
Behav Med 2011;1:53–71.
34. Mohr DC, Schueller SM, Montague E, et al. The behavioral
intervention technology model: an integrated conceptual and
technological framework for eHealth and mHealth interventions. J
Med Internet Res 2014;16:e146.
on September 4, 2024 by guest. Protected by copyright.http://bmjopen.bmj.com/BMJ Open: first published as 10.1136/bmjopen-2019-030029 on 20 August 2019. Downloaded from

10
CushingCC, etal. BMJ Open 2019;9:e030029. doi:10.1136/bmjopen-2019-030029
Open access
35. Breland JY, Yeh VM, Yu J. Adherence to evidence-based guidelines
among diabetes self-management apps. Transl Behav Med
2013;3:277–86.
36. Fedele DA, Cushing CC, Fritz A, et al. Mobile health interventions for
improving health outcomes in youth: A meta-analysis. JAMA Pediatr
2017;171:461.
37. Ramsey RR, Caromody JK, Voorhees SE, et al. A systematic
evaluation of asthma management apps examining behavior change
techniques. J Allergy Clin Immunol Pract 2019.
38. Miller L, Schüz B, Walters J, et al. Mobile technology interventions
for asthma self-management: Systematic review and meta-Analysis.
JMIR Mhealth Uhealth 2017;5:e57.
39. T- jung Y, Joeng HY, Hill TD, et al. Using SMS to provide continuous
assessment and improve health outcomes for children with asthma.
IHI’ 12 Proceedings of the 2nd ACM SIGHIT International Health
Informatics Symposium. Miami, FL, 2012:621–30.
40. Chan AH, Stewart AW, Harrison J, et al. The effect of an electronic
monitoring device with audiovisual reminder function on adherence
to inhaled corticosteroids and school attendance in children
with asthma: a randomised controlled trial. Lancet Respir Med
2015;3:210–9.
41. Smyth JM, Heron KE. Is providing mobile interventions “just-in-
time” helpful? an experimental proof of concept study of just-in-
time intervention for stress management. IEEE Wireless Health
Conference. Bethesda, MD, 2016:89–95.
42. Nahum-Shani I, Smith SN, Spring BJ, et al. Just-in-Time Adaptive
Interventions (JITAIs) in mobile health: Key components and design
principles for ongoing health behavior support. Ann Behav Med
2018;52:446-462.
43. Klok T, Kaptein AA, Brand PLP. Non-adherence in children with
asthma reviewed: The need for improvement of asthma care and
medical education. Pediatr Allergy Immunol 2015;26:197–205.
44. National Asthma Education and Prevention Program. Expert Panel
Report 3 (EPR-3): Guidelines for the Diagnosis and Management of
Asthma-Summary Report 2007. J Allergy Clin Immunol 2007;120(5
Suppl):S94–138.
45. D’Zurilla TJ, Nezu AM, Therapy P-S. In: Dobson KS, ed. Handbook of
Cognitive-Behavioral Therapies. 3rd edn. New York, NY: The Guilford
Press, 2010:197–225.
46. Nezu AM, Nezu CM. Emotion-Centered Problem-Solving Therapy:
Treatment Guidlelines. New York: Springer Publishing, 2019.
47. Lasmar L, Camargos P, Champs NS, et al. Adherence rate to
inhaled corticosteroids and their impact on asthma control. Allergy
2009;64:784–9.
48. Otsuki M, Eakin MN, Rand CS, et al. Adherence feedback to improve
asthma outcomes among inner-city children: a randomized trial.
Pediatrics 2009;124:1513–21.
49. Centers for Disease Control and Prevention (CDC). CDC - Asthma -
National Health Interview Survey (NHIS) Data.
50. Woolford SJ, Barr KL, Derry HA, et al. OMG do not say LOL: obese
adolescents' perspectives on the content of text messages to
enhance weight loss efforts. Obesity 2011;19:2382–7.
51. Ben-Zeev D, Kaiser SM, Brenner CJ, et al. Development and
Usability Testing of FOCUS: A Smartphone System for Self-
Management of Schizophrenia. 2013.
52. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res
Psychol 2006;3:77–101.
53. Corbin J, Strauss AL. Basics of Qualitative Research: Techniques
and Procedures for Developing Grounded Theory. Thousand Oaks,
California: SAGE Publications, 2008.
54. Miles MB, Huberman AM, Saldaña J. Qualitative Data Analysis
: A Methods Sourcebook. 3rd edn. Thousand Oaks, CA: SAGE
Publicatitons, 2013.
55. Wu YP, Thompson D, Aroian KJ, et al. Commentary: Writing
and evaluating qualitative research reports. J Pediatr Psychol
2016;41:493–505.
56. Larsen DL, Attkisson CC, Hargreaves WA, et al. Assessment of
client/patient satisfaction: development of a general scale. Eval
Program Plann 1979;2:197–207.
57. Schnall R, Cho H, Liu J. Health Information Technology Usability
Evaluation Scale (Health-ITUES) for Usability Assessment of
Mobile Health Technology: Validation Study. JMIR Mhealth Uhealth
2018;6:e4.
58. Department of Health & Human Services. NIH-Wide Strategic Plan:
Fiscal Years 2016-2020. 2015. https://www. nih. gov/ sites/ default/
les/ about- nih/ strategic- plan- fy2016- 2020- 508. pdf [Accessed 18
Jan 2019].
59. Bartholomew LK, Gold RS, Parcel GS, et al. Watch, Discover, Think,
and Act: evaluation of computer-assisted instruction to improve
asthma self-management in inner-city children. Patient Educ Couns
2000;39(2-3):269–80.
60. Liu AH, Zeiger R, Sorkness C, et al. Development and cross-
sectional validation of the Childhood Asthma Control Test. J Allergy
Clin Immunol 2007;119:817–25.
61. Bursch B, Schwankovsky L, Gilbert J, et al. Construction and
validation of four childhood asthma self-management scales: parent
barriers, child and parent self-efcacy, and parent belief in treatment
efcacy. J Asthma 1999;36:115–28.
62. Cohen JL, Mann DM, Wisnivesky JP, et al. Assessing the validity
of self-reported medication adherence among inner-city asthmatic
adults: the Medication Adherence Report Scale for Asthma. Ann
Allergy Asthma Immunol 2009;103:325–31.
63. Levesque CS, Williams GC, Elliot D, et al. Validating the theoretical
structure of the Treatment Self-Regulation Questionnaire
(TSRQ) across three different health behaviors. Health Educ Res
2007;22:691–702.
64. Byrne DG, Davenport SC, Mazanov J. Proles of adolescent stress:
the development of the adolescent stress questionnaire (ASQ). J
Adolesc 2007;30:393–416.
65. Sarason IG, Levine HM, Basham RB, et al. Assessing social
support: The Social Support Questionnaire. J Pers Soc Psychol
1983;44:127–39.
66. D'Zurilla TJ, Nezu AM. Development and preliminary evaluation of the
Social Problem-Solving Inventory. Psychol Assess 1990;2:156–63.
67. Juniper EF, Guyatt GH, Feeny DH, et al. Measuring quality of life in
children with asthma. Qual Life Res 1996;5:35–46.
on September 4, 2024 by guest. Protected by copyright.http://bmjopen.bmj.com/BMJ Open: first published as 10.1136/bmjopen-2019-030029 on 20 August 2019. Downloaded from
