
Exact Sciences
Madison, WI USA
PATIENT GUIDE
www.cologuardtest.com
Rx Only
INDICATION FOR USE
Cologuard is intended for the qualitative detection of colorectal neoplasia
associated DNA markers and for the presence of occult hemoglobin in
human stool. A positive result may indicate the presence of colorectal cancer
(CRC) or advanced adenoma (AA) and should be followed by diagnostic
colonoscopy. Cologuard is indicated to screen adults of either sex,
45 years or older, who are at typical average-risk for CRC. Cologuard is not a
replacement for diagnostic colonoscopy or surveillance colonoscopy in high
risk individuals.
Cologuard® colorectal cancer screening test is a registered trademark of Exact Sciences Corporation.
LBL-10033rev7
To report any adverse effects from using this kit, or if you need assistance,
please call us at 1-844-870-8870. We are available 24 hours a day, 7 days a
week to answer your questions.
EN_LBL_10033_r7.indd 1 12/12/19 1:24 PM

Page 2
www.cologuardtest.com | Rx Only
TABLE OF CONTENTS
Contra-Indications ......................................................................................................................3
Cologuard Warnings and Precautions ............................................................................... 4
How the Cologuard Collection Kit Works ......................................................................... 6
What Does the Cologuard Test Result Mean? .................................................................7
What Is Inside the Cologuard Collection Kit? ................................................................. 8
Who Should Use Cologuard? ................................................................................................ 9
Caution ..........................................................................................................................................10
Risks ...............................................................................................................................................10
What Should I Know Before Using the Cologuard Collection Kit? ......................... 11
When Should I Not Use the Cologuard Collection Kit?.............................................. 12
How to Use the Cologuard Collection Kit ....................................................................... 13
Glossary .......................................................................................................................................28
Symbols Used on the Cologuard Collection Kit ...........................................................29
Clinical Study Results .............................................................................................................29
EN_LBL_10033_r7.indd 2 12/12/19 1:24 PM

www.cologuardtest.com | Rx Only
Page 3
CONTRA-INDICATIONS
Cologuard is intended for use with patients, age 45 years and older,
at average risk who are typical candidates for CRC screening.
Cologuard was not clinically evaluated for the following types of patients:
• Patients with a history of colorectal cancer, adenomas, or other related
cancers.
• Patients who have had a positive result from another colorectal cancer
screening method within the last 6 months.
• Patients who have been diagnosed with a condition that is associated
with high risk for colorectal cancer. These include but are not limited to:
– Inflammatory Bowel Disease (IBD)
– Chronic ulcerative colitis (CUC)
– Crohn’s disease
– Familial adenomatous polyposis (FAP)
– Family history of colorectal cancer
• Patients who have been diagnosed with a relevant familial (hereditary)
cancer syndrome, such as Hereditary non-polyposis colorectal cancer
syndrome (HNPCCC or Lynch Syndrome), Peutz-Jeghers Syndrome,
MYH-Associated Polyposis (MAP), Gardner’s syndrome, Turcot’s (or
Crail’s) syndrome, Cowden’s syndrome, Juvenile Polyposis, Cronkhite-
Canada syndrome, Neurofibromatosis, or Familial Hyperplastic Polyposis.
EN_LBL_10033_r7.indd 3 12/12/19 1:24 PM

Page 4
www.cologuardtest.com | Rx Only
COLOGUARD WARNINGS AND PRECAUTIONS
The performance of Cologuard has been established in a cross-sectional
study (i.e., single point in time). Programmatic performance of Cologuard (i.e.,
benefits and risks with repeated testing over an established period of time)
has not been studied. Performance has not been evaluated in adults who
have been previously tested with Cologuard. Non-inferiority or superiority
of Cologuard programmatic sensitivity as compared to other recommended
screening methods for CRC and AA has not been established.
• The clinical validation study was conducted in patients 50 years of age and
older. ACS Guidelines recommend screening begin at age 45. Cologuard
performance in patients ages 45-49 was estimated by sub-group analysis of
near-age groups.
• CRC screening guideline recommendations vary for persons over the age
of 75. The decision to screen persons over the age of 75 should be made on
an individualized basis in consultation with a healthcare provider. Cologuard
test results should be interpreted with caution in older patients as the rate of
false positive results increases with age.
• A negative Cologuard test result does not guarantee absence of cancer or
advanced adenoma. Patients with a negative Cologuard test result should be
advised to continue participating in a colorectal cancer screening program
with another recommended screening method. The screening interval for
this follow-up has not been established.
• Cologuard may produce false negative or false positive results. A false positive
result occurs when Cologuard produces a positive result, even though a
colonoscopy will not find cancer or precancerous polyps. A false negative
result occurs when Cologuard does not detect a precancerous polyp or
colorectal cancer even when a colonoscopy identifies the positive result.
EN_LBL_10033_r7.indd 4 12/12/19 1:24 PM

www.cologuardtest.com | Rx Only
Page 5
COLOGUARD WARNINGS AND PRECAUTIONS
• Patients should not provide a sample for Cologuard if they have
diarrhea or if they have blood in their urine or stool (e.g., from
bleeding hemorrhoids, bleeding cuts or wounds on their hands,
rectal bleeding, or menstruation).
• To ensure the integrity of the sample, the laboratory must receive
the patient specimen within 72 hours of collection. Initiate the
return process within a day of collecting your sample to allow
enough delivery time. Refer to the shipping instructions provided
in this box, or ask your prescriber, for more information.
• Patients should be advised of the caution listed in the Cologuard
Patient Guide. Patients should NOT drink the preservative liquid.
• The risks related to using the Cologuard Collection Kit are low,
with no serious adverse events reported among people in a
clinical trial. Patients should be careful when opening and closing
the lids to avoid the risk of hand strain.
EN_LBL_10033_r7.indd 5 12/12/19 1:24 PM

Page 6
www.cologuardtest.com | Rx Only
HOW THE COLOGUARD COLLECTION KIT WORKS
Your healthcare provider has ordered Cologuard to screen for colorectal
cancer and precancer.
Cologuard is a screening test that uses a stool sample (your bowel movement)
to detect colorectal cancer and precancer. Every day, your colon sheds cells
that line the colon. As part of this process, normal cells along with abnormal
cells from precancer or cancers are shed into the colon. Your stool picks up
those cells as it passes through your colon. Cologuard is designed to detect
the DNA and hemoglobin (blood) released from these abnormal cells in your
stool.
The Cologuard collection kit is used to collect your stool sample. After you
collect your stool sample following the instructions in this Patient Guide, the
collection kit will be delivered to a lab. The lab will test your stool sample and
send the results to your healthcare provider. Your provider will contact you
with the test results.
Note: You are not required to change your diet or medications to use
this screening test.
HOW DO I STORE THE COLOGUARD
COLLECTION KIT WHEN IT ARRIVES?
You can store your kit until you are ready to use it.
• Store at room temperature.
• Keep away from heat and direct sunlight.
• Keep out of the reach of children.
EN_LBL_10033_r7.indd 6 12/12/19 1:24 PM

www.cologuardtest.com | Rx Only
Page 7
WHAT DOES THE COLOGUARD TEST RESULT MEAN?
Your healthcare provider will talk with you about your results.
The test result can be Positive, Negative or No result obtained.
WHAT DOES A POSITIVE RESULT MEAN?
• A Positive result means the test detected abnormal DNA and/or blood
that could be caused by precancer or cancer in the colon.
• The test can also have a Positive result that is incorrect (false positive). This
means the test result is Positive, but no cancer or precancer is actually present.
• Any Positive result should be followed by a colonoscopy.
• Talk about your test result with your healthcare provider to find out if
additional testing is needed.
WHAT DOES A NEGATIVE RESULT MEAN?
• A Negative test result means the test did not detect abnormal DNA
and/or blood in the sample.
• The test can also have a Negative result that is incorrect (false negative).
This means the test result missed a potential cancer or precancer. For that
reason, it is recommended that you schedule regular screenings.
• Talk to your healthcare provider about your test result. Your provider will
recommend a screening schedule that is best for you.
WHAT DOES NO RESULT OBTAINED MEAN?
• A “No Result Obtained” means the test was not able to provide a result.
• If this happens, your healthcare provider will talk with you about the next steps.
For example, you may be asked to provide another stool sample to test.
EN_LBL_10033_r7.indd 7 12/12/19 1:24 PM

Page 8
www.cologuardtest.com | Rx Only
• The bottle of liquid contains a preservative (less than 10% EDTA in Tris buffered solution).
• The tube contains a 10% albumin in Tris buffered detergent solution with an antimicrobial agent.
WHAT IS INSIDE THE COLOGUARD
COLLECTION KIT?
EN_LBL_10033_r7.indd 8 12/12/19 1:24 PM

www.cologuardtest.com | Rx Only
Page 9
WHO SHOULD USE COLOGUARD?
Cologuard is intended for people who are typical candidates for colorectal
cancer screening, which include:
• Men and women 45 years or older
• At average risk for colorectal cancer
IS COLOGUARD RIGHT FOR YOU?
Talk with your healthcare provider about using Cologuard if any of the
following apply to you:
• A history of colorectal cancer, adenomas, or other related cancers.
• Had a positive result from another colorectal cancer screening method
within the last 6 months.
• Been diagnosed with a condition that is associated with high risk for
colorectal cancer. These include but are not limited to:
– Inflammatory Bowel Disease (IBD)
– Chronic ulcerative colitis (CUC)
– Crohn’s disease
– Familial adenomatous polyposis (FAP)
– Family history of colorectal cancer
• Been diagnosed with a relevant familial (hereditary) cancer syndrome,
such as Hereditary non-polyposis colorectal cancer syndrome (HNPCCC
or Lynch Syndrome), Peutz-Jeghers Syndrome, MYH-Associated
Polyposis (MAP), Gardner’s syndrome, Turcot’s (or Crail’s) syndrome,
Cowden’s syndrome, Juvenile Polyposis, Cronkhite-Canada syndrome,
Neurofibromatosis, Familial Hyperplastic Polyposis.
EN_LBL_10033_r7.indd 9 12/12/19 1:24 PM

Page 10
www.cologuardtest.com | Rx Only
CAUTION
Caution
• Do not drink the bottle of preservative liquid.
• Do not let the liquid touch your skin or eyes.
• If the liquid touches your skin or eyes, wash the area with water.
RISKS
The risks related to using the Cologuard collection kit are low. No serious
adverse events were reported among 10,023 people in a clinical trial.
• Opening or closing the lids of items in the kit may be difficult
for some people.
– Be careful when opening and closing the lids to avoid the risk
of hand strain.
– Close all sample containers tightly.
• Using this kit requires sitting down on the toilet and standing up
from the toilet.
– Have someone who can help you if needed.
• There is a chance that a stool sample sent to the lab may have no result.
– If this happens, you will be contacted. You may be asked to provide
another sample.
EN_LBL_10033_r7.indd 10 12/12/19 1:24 PM

www.cologuardtest.com | Rx Only
Page 11
WHAT SHOULD I KNOW BEFORE USING
THE COLOGUARD COLLECTION KIT?
Only remove the items you need
to collect your sample following
the steps in this guide.
• Leave the plastic bag inside the box – do not remove. The box, zippered
bag, and white tray inside the bag will be used to return your sample.
• Use the collection kit before the expiration date printed on the side of the
box.
• Your stool sample should be no larger than the bottle of liquid included
in the box.
• Avoid getting urine in the container when collecting your stool sample.
– Empty your bladder as much as possible, before collecting your stool
sample.
• Avoid getting toilet paper or other materials in the container when
collecting your stool sample.
EN_LBL_10033_r7.indd 11 12/12/19 1:24 PM

Page 12
www.cologuardtest.com | Rx Only
WHAT SHOULD I KNOW BEFORE USING
THE COLOGUARD COLLECTION KIT? CONTINUED
• The lab must receive your sample promptly.
– Collect a sample when you can return it within a day of collection. Make
sure that a Sunday or a holiday will not delay your return.
– Refer to your return instructions for detailed information on how to
return your kit.
• You are not required to change your diet or medications to use this
screening test.
When you’re ready to collect your sample with the Cologuard collection kit,
our video will help you through each step. Go to the following website for
more information:
www.cologuardtest.com/already-ordered-cologuard
WHEN SHOULD I NOT USE THE COLOGUARD
COLLECTION KIT?
Certain conditions may cause an incorrect test result or no result.
Do not use this kit to collect a stool sample if you have:
• Bleeding hemorrhoids
• Bleeding cuts or wounds on your hands
• Rectal bleeding
• Menstrual period
• Diarrhea (abnormal bowel/stool consistency)
EN_LBL_10033_r7.indd 12 12/12/19 1:24 PM

www.cologuardtest.com | Rx Only
Page 13
STEP 1: CHECK THE EXPIRATION DATE AND THE KIT
Check your kit to make sure the kit has not expired and you have
all the parts of the kit.
a. Locate the expiration date
on the outside of the box.
b. Use the kit before the
expiration date printed
on the outside of the box.
• If the date has passed, do not
collect your sample. Refer
to your return instructions;
use the contact information
provided to request a new kit.
EN_LBL_10033_r7.indd 13 12/12/19 1:24 PM

Page 14
www.cologuardtest.com | Rx Only
Leave the plastic bag and
white tray inside the box.
c. Check the items in your box and
make sure you have the following:
• Bracket
• Sample container
• Tube
• Bottle of Liquid (preservative)
• Sample labels
• Patient Guide Instructions
• How to Return Your Kit
If any items are missing from
your kit, do not use the kit.
Use the contact information
provided to request a new kit.
d. If all items are included in your
kit, your kit is ready to use.
Follow the next steps
when you feel ready to have
a bowel movement.
STEP 1 CONTINUED
EN_LBL_10033_r7.indd 14 12/12/19 1:24 PM

www.cologuardtest.com | Rx Only
Page 15
a. Decide the best time to collect
a stool sample.
• Follow these steps when
you feel ready to have
a bowel movement.
• Collect your sample on
a day when you can return
it promptly.
– Refer to your
return instructions.
• Do not collect a stool sample
if you have:
– Bleeding hemorrhoids
– Bleeding cuts or wounds on
your hands
– Rectal bleeding
– Menstrual period
– Diarrhea
STEP 2: PREPARE TO COLLECT THE STOOL SAMPLE
Follow these steps in your bathroom to set up the bracket
and sample container.
TIMING YOUR
COLLECTION
EN_LBL_10033_r7.indd 15 12/12/19 1:24 PM
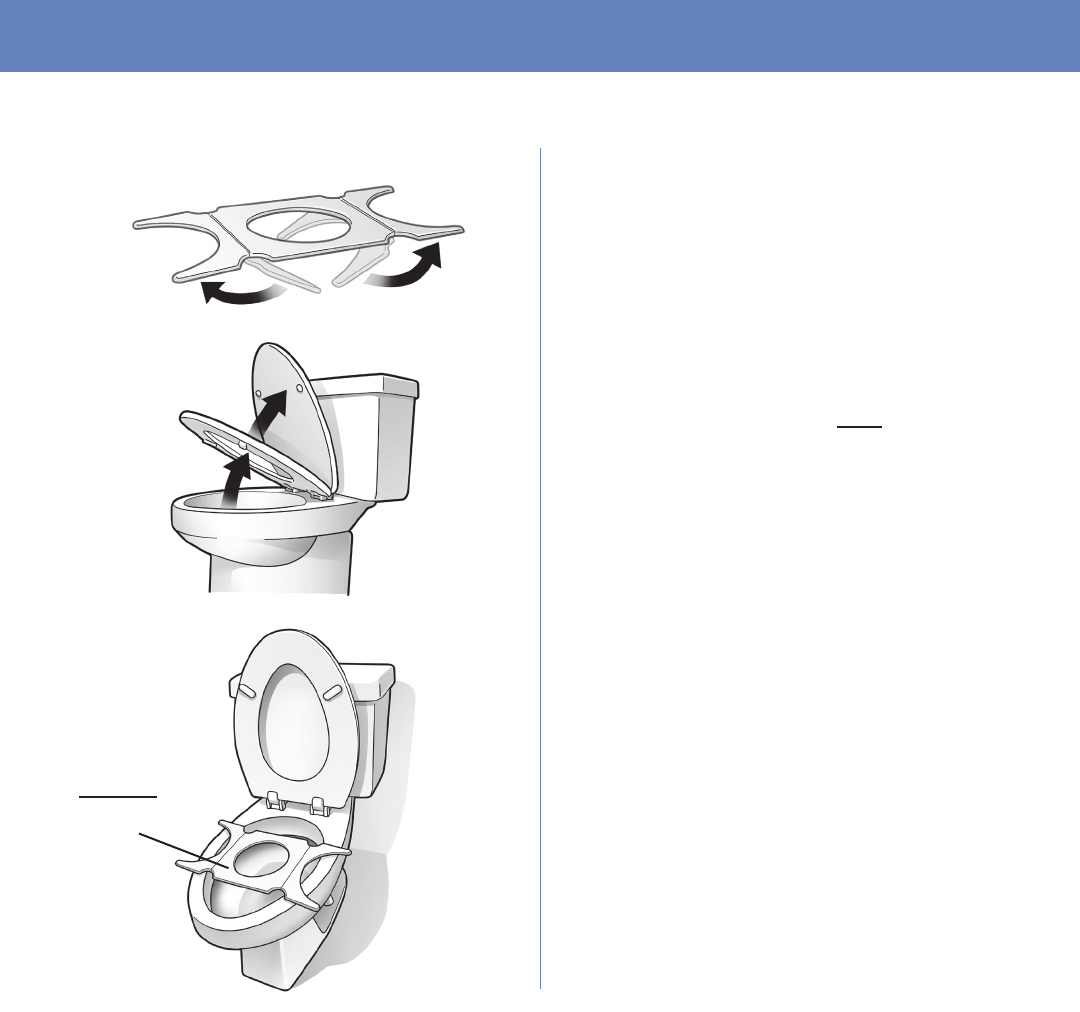
Page 16
www.cologuardtest.com | Rx Only
b.
c.
d.
b. Remove the bracket from
the box and unfold the sides
of the bracket.
c. Raise the toilet lid and seat.
d. Place the bracket on the toilet
as shown.
• Place the bracket toward
the back of the toilet.
Place
under
toilet seat
STEP 2 CONTINUED
EN_LBL_10033_r7.indd 16 12/12/19 1:24 PM

www.cologuardtest.com | Rx Only
Page 17
e. Lower the toilet seat onto
the bracket.
• The entire opening of the
bracket should be visible.
f. Lift the sample container out
of the box and place on a hard,
flat surface.
g. Turn the container lid and
unscrew it.
• If the lid sticks, try pushing
down or holding the container
between your knees to secure
it, while turning the lid.
h. Set the container lid aside.
i. Place the container into the top of
the bracket.
e.
g.
i.
Turn to
open
Place
into
bracket
EN_LBL_10033_r7.indd 17 12/12/19 1:24 PM

Page 18
www.cologuardtest.com | Rx Only
STEP 3: COLLECT THE STOOL SAMPLE
Make sure you have help if you have trouble sitting and standing
when using the toilet.
a. Sit on the toilet and have a
bowel movement in the sample
container.
• Your stool sample should be
no larger than the bottle of
liquid included in the box.
• Try to keep urine from
going into the container.
• Do not put toilet paper or
other items into the container.
b. When your bowel movement
is complete, stand up.
c. Lift the sample container
from the bracket and set
the container on a flat,
stable surface.
• Leave the container open.
a.
c.
Leave
container
open
EN_LBL_10033_r7.indd 18 12/12/19 1:24 PM

www.cologuardtest.com | Rx Only
Page 19
d. Remove the bracket from
the toilet.
• The bracket can be recycled
or thrown in the trash.
e. Finish using the bathroom
if needed.
• Follow the rest of the steps in
this guide immediately after
collecting your stool sample.
d.
e.
IMPORTANT: Complete
the next step before
you close the container.
NEXT PAGE
EN_LBL_10033_r7.indd 19 12/12/19 1:24 PM

Page 20
www.cologuardtest.com | Rx Only
STEP 4: SCRAPE THE STOOL SAMPLE
You must scrape the stool sample with the probe from the tube
to get another small sample for the Cologuard test.
a. Lift the tube out of the box.
b. Turn the white tube cap
and unscrew it.
c. Pull the probe from the tube.
• You may have to pull hard.
d. Scrape the surface of your
stool sample until the end
of the probe has stool on it.
• Your stool sample may look
different from the stool
sample pictured.
• You only need to cover the
grooves on the probe with
stool. DO NOT FILL TUBE
WITH STOOL.
b.
c.
d.
Pull up Grooves on
probe end
Scrape the
surface of the
stool to cover
only the grooves
on probe.
Turn to open
EN_LBL_10033_r7.indd 20 12/12/19 1:24 PM

www.cologuardtest.com | Rx Only
Page 21
e. Place the probe back into the
open end of the tube.
f. Turn the cap to close.
g. Set the tube aside.
Note: Do not place the tube into
the stool container.
e.
f.
Stool
on probe
Turn to
close
EN_LBL_10033_r7.indd 21 12/12/19 1:24 PM

Page 22
www.cologuardtest.com | Rx Only
STEP 5: PREPARE STOOL SAMPLE CONTAINER
FOR RETURN
The stool sample must have the preservative poured onto it to make sure
the lab can test it. Then, the container must be closed tightly for return.
a. Lift the bottle of liquid
preservative out of the box.
b. Hold the bottle and turn the cap
to unscrew it.
Caution
• Do not drink the liquid.
• If the liquid from the bottle
touches your skin or eyes,
wash with water.
c. Pour ALL the liquid in the bottle
into the container with the stool.
• The empty bottle and cap
can be recycled or thrown
in the trash.
b.
c.
Turn to
open
Use all
the liquid
EN_LBL_10033_r7.indd 22 12/12/19 1:24 PM

www.cologuardtest.com | Rx Only
Page 23
d. Hold the sample container on a
hard, flat surface.
e. Place the lid on top of
the container.
• Make sure the lid is straight
so it will close tightly.
f. Turn the lid to tighten until
it does not tighten anymore.
• Note: If the lid is not on
correctly, liquid could leak
out of the container.
e.
f.
Tight seal
Correct!
Lid with gap
Wrong:
loosen and
tighten again
Turn to
close tight
EN_LBL_10033_r7.indd 23 12/12/19 1:24 PM

Page 24
www.cologuardtest.com | Rx Only
a.
STEP 6: LABEL YOUR SAMPLES
You must label your samples so the lab can identify them. For best
results fill out the labels, as indicated below, before you apply them to the
containers. Use a ballpoint pen and write on top of a hard, flat surface.
a. Find the Sample Label Card
included in the kit.
b. Fill out both labels. Print
the information in this order:
• Your first name (as it appears
on the shipping label)
• Your last name
• Your birthdate (MM/DD/YY)
• The date you collected your
stool sample (MM/DD/YY)
• The time you collected your
stool sample; you must also
circle AM or PM
b.
EN_LBL_10033_r7.indd 24 12/12/19 1:24 PM

www.cologuardtest.com | Rx Only
Page 25
c. Peel the label away from the
paper.
d. Wrap one label around the tube.
• The label can cover the
existing label.
e. Place one label on the lid of the
sample container.
c.
d.
e.
EN_LBL_10033_r7.indd 25 12/12/19 1:24 PM

Page 26
www.cologuardtest.com | Rx Only
STEP 7: PREPARE YOUR SAMPLES FOR RETURN
For best results, prepare your box on a hard, flat surface.
When you start, the zippered bag should be inside the box.
The white tray for holding the container and tube should be inside the bag.
a. Place the tube and sample
container into the tray
inside the box.
b. Fold the bag and push extra air
out of the bag.
• Too much air in the bag will
make it hard to close the box.
c. Slide the zipper across the
top of the bag to close and
seal the bag.
d. Fold the bag until it fits inside
the box.
d.
a.
Zipper
Zippered
Bag
Sample
Container
Tube
Outer
Box
EN_LBL_10033_r7.indd 26 12/12/19 1:24 PM

www.cologuardtest.com | Rx Only
Page 27
e. Fold the smaller flaps over the
top of the box.
f. Fold the long flap over the two
smaller flaps.
• This is the white flap with the
blue writing on it.
g. Peel the paper backing off the
tape on the box lid.
• Discard paper backing
in garbage.
h. Fold the box top over the other
flaps and press down firmly to
seal the box.
i. Return your samples. Refer to your
return instructions.
e.
f.
g.
h.
Throw
away
Thr
o
w
EN_LBL_10033_r7.indd 27 12/12/19 1:24 PM

Page 28
www.cologuardtest.com | Rx Only
GLOSSARY
COLON:
A part of the human digestive system sometimes known as the large intestine.
COLORECTAL CANCER:
A disease of abnormal growths in the colon or rectum that, if left untreated,
may spread throughout the body. Colon cancer generally develops over a
number of years.
COLONOSCOPY:
A medical test where a flexible tube is placed into the colon, so the surface
of the colon can be seen by a camera. Instruments can be placed through the
tube to remove growths found on the colon wall.
DNA:
Deoxyribonucleic acid. Changes in the stool DNA (sDNA) can show your
healthcare provider if there is cancer or a possibility of cancer.
HEMOGLOBIN:
A compound from blood. Blood or hemoglobin in the stool that cannot be
seen by the naked eye is referred to as occult blood or occult hemoglobin.
NEOPLASIA:
A growth that is not normal.
PRECANCEROUS POLYP (ADENOMA):
A growth on the wall of the colon that may become cancer.
EN_LBL_10033_r7.indd 28 12/12/19 1:24 PM

www.cologuardtest.com | Rx Only
Page 29
SYMBOLS USED ON THE COLOGUARD COLLECTION KIT
Collection of harmonized symbols used on labeling for the kit.
CLINICAL STUDY RESULTS
Cologuard was studied in a large clinical trial to determine the effectiveness
of the test. The trial included more than 10,000 patients at 90 sites in the US
and Canada. This study included adults ages 50-84. Each person in the study
completed Cologuard and a fecal occult blood test before having a standard
colonoscopy. The main purpose of the study was to find out the performance of
Cologuard for finding cancer and precancer compared to a colonoscopy.
In the clinical study, Cologuard correctly detected 92% of colorectal cancers
and 42% of advanced adenomas in the study population that had disease. The
Cologuard test correctly gave a negative screening result for 87% of the study
subjects that did not have colorectal cancer or advanced adenomas. In other
words, 13% of people without cancer or precancer tested positive. The performance
of Cologuard in adults ages 45-49 is estimated based on this large study.
Lot
Number
Manufacturer
Storage
Temperature
Contains
Sufficient
for (n) Tests
Catalog
Number
In Vitro
Diagnostic Use
Expiration
Date
Caution
EN_LBL_10033_r7.indd 29 12/12/19 1:24 PM

www.cologuardtest.com | Rx Only
Cologuard
®
is a registered trademark of Exact Sciences Corporation.
Refer to www.exactsciences.com for additional information regarding
Exact Sciences intellectual property holdings and policies.
©2019 Exact Sciences Corporation. All rights reserved.
EXACT SCIENCES CORPORATION
441 Charmany Drive | Madison, WI 53719 | www.exactsciences.com
EN_LBL_10033_r7.indd 30 12/12/19 1:24 PM

www.cologuardtest.com | Rx Only
EN_LBL_10033_r7.indd 31 12/12/19 1:24 PM
