
RAPID RESULTS IN
10
MINS
QuickVue
At-Home
OTC COVID-19 Test
USER
INSTRUCTIONS
For Emergency Use Authorization (EUA) only.
In vitro
diagnostic use only.
Store at Room Temperature,
59
°
F to 86
°
F (15
°
C to 30
°
C)

Wash Your hands
Before you start testing, wash your
hands or use hand sanitizer.
Make sure your hands are dry
before starting.

STEP 1:
Check Your Test Kit
Locate the kit components:
It is recommended gloves
(not provided) also be used
during testing.
Tubes
Tube Holder
Swabs
Watch or Timer
(not included)
Test Strips
10
2
ITEM
0569105 (05/17)
IVD
CONTENTS:
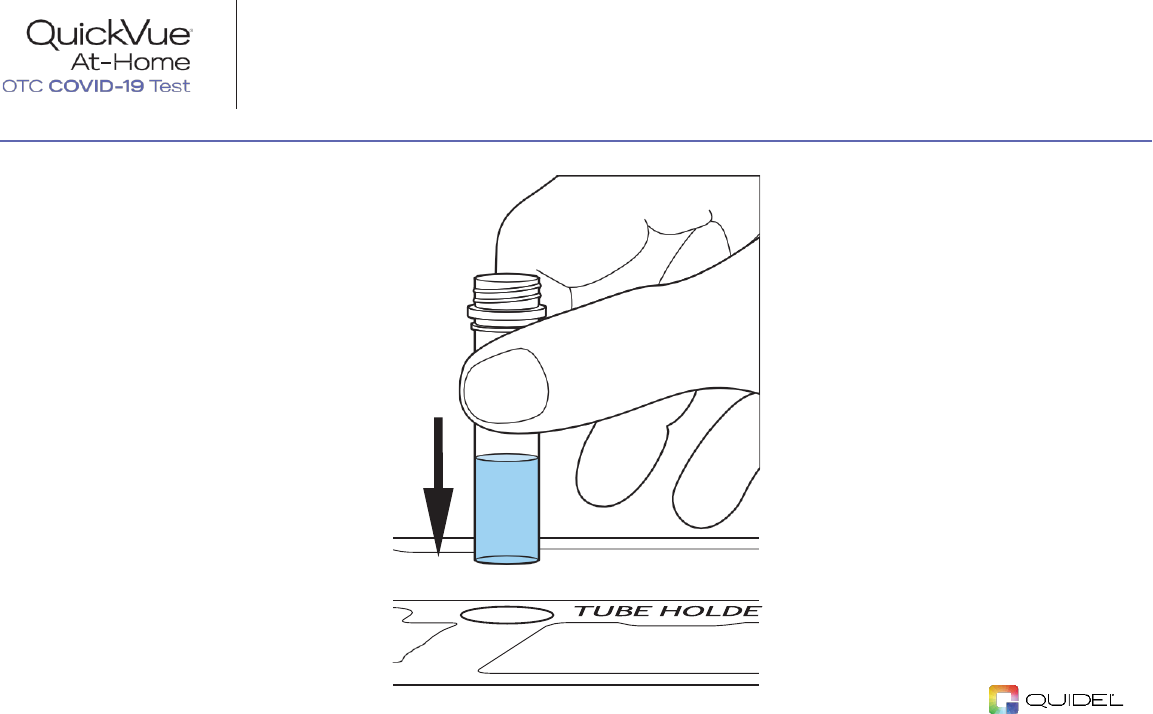
STEP 2:
Place Tube in the Tube Holder
Remove cap from one
TUBE
and
place it in the
TUBE HOLDER
.
NOTE:
Use of gloves
is recommended.

STEP 3:
Swab the Nostrils
Remove the
SWAB
from
its wrapper, being
careful not to touch the
SWAB
head.
Gently insert the
SWAB
½ to ¾ of an inch into the nostril, depending on
the size of the person’s nose. Firmly rub the
SWAB
in a circular motion
around the inside wall of
EACH NOSTRIL at least 4 times
.
Be sure to rub BOTH nostrils with the SAME SWAB.
NOTE:
If you are swabbing others,
please wear a face mask. With
children, you may not need
to insert the swab as far
into the nostril. For very
young children, you may
need another person to
steady the child’s head
while swabbing.
NOTE:
Failure to swab
properly may cause
false negative results.
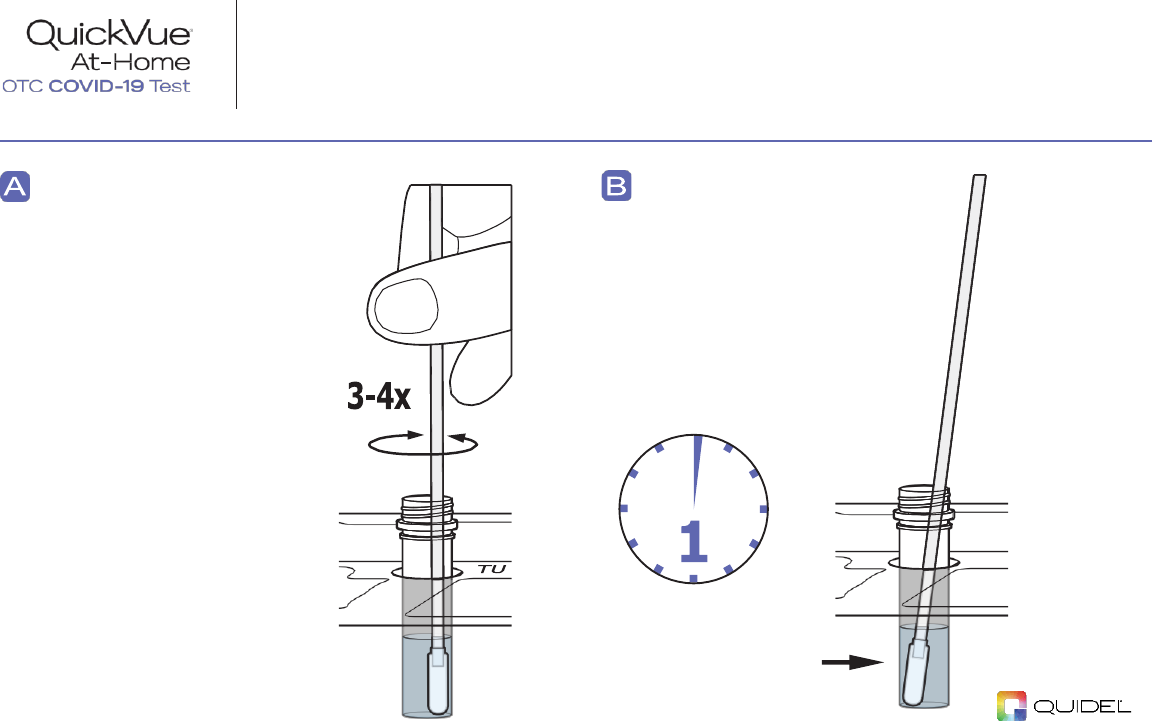
STEP 4:
Place Swab in the Tube
Immediately place the
SWAB
into the liquid
inside the
TUBE
, and
ensure it is touching the
bottom. Stir 3-4 times.
Leave the swab in the
solution for
ONE MINUTE
.
NOTE:
If the swab is in
the solution for more
than 10-minutes it
should not be used.
Leave swab in
at least
1 minute!
TU

Dispose of swab
in the trash.
STEP 5:
Remove Swab from the Tube
After
ONE MINUTE
,
remove the swab from the
TUBE
by rubbing the
swab head against the
inside wall of the tube to
squeeze out as much
liquid as possible.

STEP 6:
Open the Test Strip
Open the
TEST
STRIP
pouch carefully at the slit
and hold the
TEST STRIP
as
indicated.
Tear here
LOT
IVD
CONTENTS:
One (1) Test Strip
Quidel Corporation
San Diego, CA USA
201621
2022
-
10
-
13
1465000 QV SARS
EXP
ITEM
0569105 (05/17)
Hold here
Do not touch here

STEP 7:
Place Test Strip in the Tube
Place the
TEST STRIP
into the
TUBE
with the arrows
pointing down. Leave the strip in the
TUBE
for a
FULL TEN MINUTES
– do not handle or remove.
Leave strip
in
at least
10 minutes!
Arrows pointing down
10

STEP 8:
Remove Test Strip from the Tube
At
TEN MINUTES
, remove the
TEST STRIP
from the
TUBE
and place on the test strip outline in Steps 10-12. Ensure the
TEST STRIP
is on a flat surface in good lighting.
NOTE:
The test is intended to be read at 10 minutes.
If the test is read before this or is read more than
5 minutes after the indicated read time, results
may be inaccurate (false negative, false positive,
or invalid) and the test should be repeated.

STEP 9:
Check Your Results
There are three types of results possible.
1.
Check for a Positive Result
2.
Check for a Negative Result
3.
Check for an Invalid Result
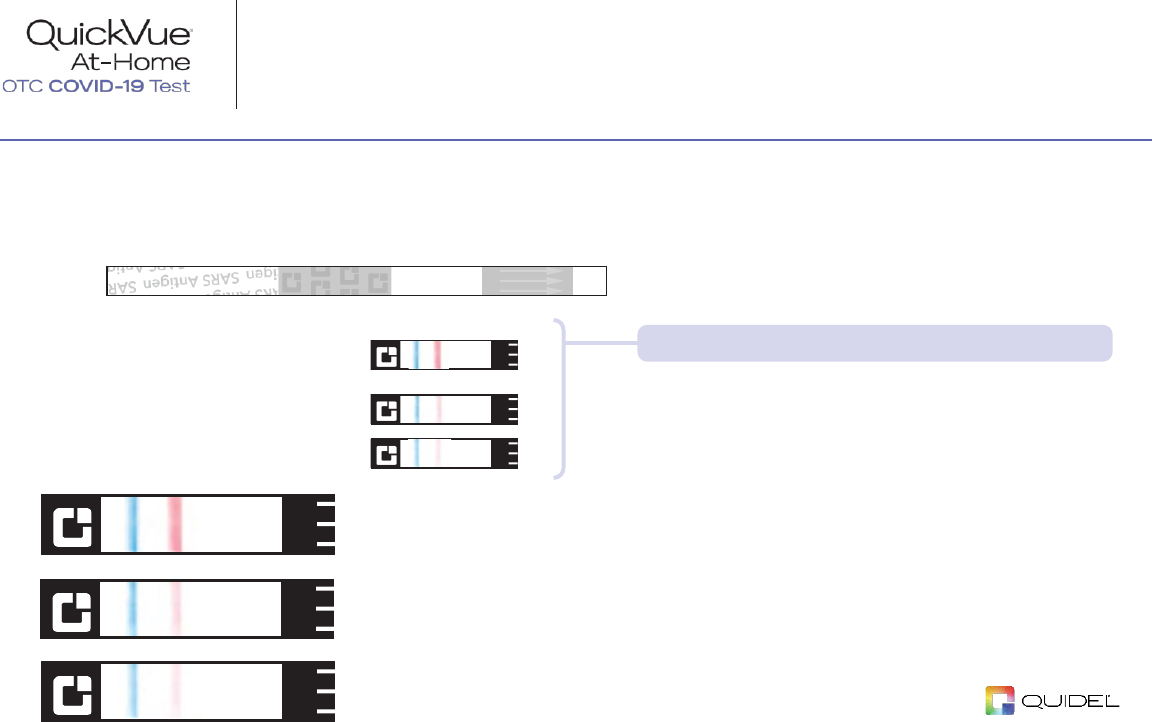
STEP 10:
Check for a Positive COVID-19 Result
Place the
TEST STRIP
on the test strip outline below
and compare with test result examples shown.
Results shown at 2x.
Positive Result
A
POSITIVE
result must show
BOTH
a
BLUE
line and a
PINK
line just below the
BLUE
line.
Look closely! Even a very faint, pink Test Line
and a blue Control Line is a POSITIVE result.
Pink shading or a line near the labels on the
strip is not considered a positive result.

Positive COVID-19 Result
A positive test result means that proteins from the virus that causes COVID-19 were
found in your sample and it is very likely you have COVID-19 and it is important to be
under the care of your healthcare provider. It is also likely that you may be placed in
isolation to avoid spreading the virus to others. There is a very small chance that this
test can give you a positive test result that is wrong (false positive). If you test positive
with the QuickVue At-Home OTC COVID-19 Test you should self-isolate and seek
follow-up care with your healthcare provider as additional testing may be necessary.
Your healthcare provider will work with you to determine how best to care for you
based on your test result along with your medical history, and your symptoms.

Negative Result
STEP 11:
Check for a Negative COVID-19 Result
Place the
TEST STRIP
on the test strip outline below
and compare with test result examples shown.
Results shown at 2x.
A
NEGATIVE
result will show a
BLUE
line but
NO
PINK
line.

Negative COVID-19 Result
A negative test result means that proteins from the virus that causes COVID-19 were not
found in your sample.
It is possible for this test to give a negative result that is incorrect (false negative) in some
people with COVID-19. This means that you could possibly still have COVID-19 even
though the test is negative. If you test negative and continue to experience COVID-19
like symptoms of fever, cough and/or shortness of breath you should seek follow up care
with your healthcare provider. Your healthcare provider will consider the test result
together with all other aspects of your medical history (such as symptoms, possible
exposures, and geographical location of places you have recently traveled) in deciding
how to care for you. For example, your healthcare provider may suggest you need
another test to determine if you have contracted the virus causing COVID-19.
It is important that you work with your healthcare provider to help you
understand the next steps you should take.

STEP 12:
Check for an Invalid COVID-19 Result
Place the
TEST STRIP
on the test strip outline below
and compare with test result examples shown.
Results shown at 2x.
Invalid Result
If there is
NO LINE
, or if there is
ONLY
a
PINK
line,
the test is
INVALID
and you should repeat the
steps starting at the beginning using a new
TUBE
,
SWAB
, and
TEST STRIP
.

Invalid COVID-19 Result
If at 10 minutes, the blue Control Line does not appear, even if any shade of
pink to-red Test Line appears, the result is invalid. If the Test Result is invalid,
a new swab should be collected, and the test should be performed again
with a new pre-filled tube and Test Strip.
If the second QuickVue At-Home OTC COVID-19 Test is also INVALID,
call 833-QUICKVUE (833-784-2588) for assistance.

STEP 13:
Dispose Used Test in the Trash
All used test components should be
disposed of in your household waste.

Wash Your hands
After completing all steps, wash hands
or use hand sanitizer.

The QuickVue At-Home OTC COVID-19 Test is
for FDA Emergency Use Authorization (EUA) Only
For
In Vitro
Diagnostic (IVD) Use.
■
This product has not been FDA cleared or approved but has been authorized by FDA under an EUA.
■
This product has been authorized only for the detection of proteins from SARS-CoV-2,
not for any other viruses or pathogens.
■
The emergency use of this product is only authorized for the duration of the declaration that circumstances
exist justifying the authorization of emergency use of IVDs for detection and/or diagnosis of COVID-19
under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1),
unless the declaration is terminated or authorization is revoked sooner.
■
For more information on EUAs go here:
https://www.fda.gov/emergency-preparednessand-response/
mcm-legal-regulatory-and-policy-framework/emergency-use-authorization
■
For the most up to date information on COVID-19, please visit: www.cdc.gov/COVID19
■
For detailed instructions, please visit
www.quickvueathome.com

Warnings, Precautions
and Safety Information
The QuickVue At-Home OTC COVID-19 Test is intended for the qualitative detection of the
nucleocapsid protein antigen from SARS-CoV-2 from individuals within 6-days of symptom onset or
individuals without symptoms or other epidemiological reasons to suspect COVID-19 infection when
tested twice over three days with at least 24 hours (and no more than 48 hours) between tests.
Read the written instructions fully before starting the test procedure
To ensure correct results, you must follow the instructions
Keep test kit and materials out of the reach of children and pets before and after use
Wear safety mask or other face covering when collecting swabs from children or others
Use of personal protection materials such as gloves are recommended
Do not open the materials until ready for use. If the test strip is open for an hour or longer,
invalid
test results may occur.
Improper swab collection may result in incorrectly negative (false negative) results
The test is intended to be read at 10 minutes. If the test is read before this or is read more than 5
minutes after the indicated read time, results may be inaccurate and the test should be repeated.
Do not use a test kit that is expired
Do not touch the swab head when handling the swab
Avoid exposure of your skin, eyes, nose, or mouth to the solution in the tube.

Hazardous Ingredients
for Liquid Reagent
Chemical Name/CAS
Harms (GHS Code) for each ingredient
Concentration
Sodium Phosphate Monobasic Monohydrate/10049-21-5
Causes skin
irritation
(H315)
Causes serious eye irritation (H319)
May cause respiratory irritation (H335)
0.7%
Sodium Phosphate Dibasic Anhydrous/7558-79-4
Causes serious eye damage (H318)
Causes serious eye irritation (H319)
0.7%
C12-14-Alkyldimethyl-betaines/66455-29-6
Causes severe skin burns and eye damage (H314)
Causes serious eye damage (H318)
Causes skin
irritation
(H315)
Causes serious eye irritation (H319)
0.03%
ProClin
®
300
Harmful if swallowed (H302)
Harmful if inhaled (H332)
Causes severe skin burns and eye damage (H314)
May cause an allergic skin reaction (H317)
0.03%
EDTA Tetrasodium Salt/64-02-8
Harmful if swallowed (H302)
Causes serious eye damage (H318)
Causes serious eye irritation (H319)
Harmful if inhaled (H332)
May cause respiratory irritation (H335)
May cause damage to organs (H371), single exposure
0.2%
■
The solution in the tube contains hazardous ingredients (see table above). If the
solution contacts the skin or eye, flush with plenty of water. If irritation persists, seek
medical advice. https://www.poison.org/contact-us or 1-800-222-1222

Intended Use
The QuickVue At-Home OTC COVID-19 Test is a lateral flow assay intended for the qualitative detection of nucleocapsid protein antigen from SARS-CoV-2. This test
is authorized for non-prescription home use with self-collected direct anterior nasal (nares) swab samples from individuals aged 14 years or older with symptoms
of COVID-19 within the first six days of symptom onset. This test is also authorized for non-prescription home use with adult-collected anterior nasal swab samples
from individuals aged 2 years or older with symptoms of COVID-19 within the first six days of symptom onset. This test is also authorized for non-prescription home
use with self-collected anterior nasal (nares) swab samples from individuals aged 14 years or older, or adult collected anterior nasal swab samples from individuals
aged 2 years or older, with or without symptoms or other epidemiological reasons to suspect COVID-19 infection when tested twice over three days with at least 24
hours (and no more than 48 hours) between tests.
The QuickVue At-Home OTC COVID-19 Test does not differentiate between SARS-CoV and SARS-CoV-2.
Results are for the identification of SARS-CoV-2 nucleocapsid protein antigen. Antigen is generally detectable in anterior nasal specimens during the acute phase of
infection. Positive results indicate the presence of viral antigens, but clinical correlation with past medical history and other diagnostic information is necessary to
determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses and the agent detected may not be the definite
cause of disease. Individuals who test positive with the QuickVue At-Home OTC COVID-19 Test should self-isolate and seek follow-up care with their physician or
healthcare provider as additional testing may be necessary.
Negative results should be treated as presumptive, do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient
management decisions, including infection control decisions. Negative results should be considered in the context of an individual’s recent exposures, history and
the presence of clinical signs and symptoms consistent with COVID-19, and confirmed with a molecular assay, if necessary, for patient management. For serial
testing programs, additional confirmatory testing with a molecular test for negative results may be necessary, if there is a high likelihood of SARS-CoV-2 infection,
such as in an individual with as a close contact with COVID-19 or with suspected exposure to COVID-19 or in communities with high prevalence of infection.
Additional confirmatory testing with a molecular test for positive results may also be necessary, if there is a low likelihood of SARS-CoV-2 infection, such as in
individuals without known exposures to SARS-CoV-2 or residing in communities with low prevalence of infection.
Persons who test negative and continue to experience COVID-19 like symptoms of fever, cough and/or shortness of breath may still have SARS-CoV-2 infection and
should seek follow up care with their physician or healthcare provider.
All results should be provided to a healthcare provider for public health reporting.
The QuickVue At-Home OTC COVID-19 Test is intended for non-prescription self-use and/or, as applicable for an adult lay user
testing another person aged 2 years or older in a non-laboratory setting. The QuickVue At-Home OTC COVID-19 Test is only for use
under the Food and Drug Administration’s Emergency Use Authorization.

What do I need to know about
Results from Serial Testing?
If your first or second test is positive, then proteins from the virus that causes COVID-19
has been found in your sample and you likely have COVID-19.
If you test positive with the
QuickVue At-Home OTC COVID-19 Test, you should self-isolate and seek follow-up care with
your healthcare provider to determine the next steps you should take. You may need
additional testing, depending on your personal health history and other factors.
If your first test is negative, you should test again in 24-48 hours. If both your first and second tests
are negative, you may not have COVID-19, however, you should follow-up with your
healthcare
provider if you are at high risk for COVID-19 infection.
If you test negative and
continue to
experience COVID-19 like symptoms of fever, cough and/or shortness of breath you should
seek follow up care with your healthcare provider. For example, your healthcare provider may
suggest you need another test to determine if you have contracted the virus causing COVID-19.
It is important that you work with your healthcare provider to help you understand the next
steps you should take.

Frequently Asked Questions
Will this test hurt?
No, the nasal swab is not sharp and it should not hurt. Sometimes the
swab can feel slightly uncomfortable. If you feel pain, please stop the test
and seek advice from a healthcare provider.
What are the known and potential risks and
benefits of this test?
Potential risks include:
■
Possible discomfort during sample collection.
■
Possible incorrect test results (see Results section).
Potential benefits include:
■
The results, along with other information, can help your healthcare
provider make informed recommendations about your care.
■
The results of this test may help limit the spread of COVID-19 to
your family and others in your community.
What is Serial Testing?
COVID-19 Serial testing is when one person tests themselves multiple
times for COVID-19 on a routine basis, such as every day or every other
day. By testing more frequently, you may detect COVID-19 more quickly
and reduce spread of infection. Serial testing (i.e. testing every day or
every other day) is more likely to detect COVID-19, especially when you
do not have any symptoms.
What is the difference between
an antigen and molecular test?
An antigen test, such as the QuickVue At-Home OTC COVID-19
Test, detects proteins from the virus. Molecular tests detect
genetic material from the virus. Antigen tests are very specific for
the virus, but not as sensitive as molecular tests. This means that a
positive result is highly accurate, but a negative result does not
rule out infection. If your test result is negative, you should
discuss with your healthcare provider on whether an additional
test is necessary and if you should continue isolating
at home.
There is a higher chance of false negative results with
antigen tests than with laboratory-based molecular tests.
This means that there is a higher chance this test will give
you a negative result when you have COVID-19.
How Accurate is this Test?
Based on the interim results of a clinical study where the
QuickVue At-Home OTC COVID-19 Test was compared to an
FDA
authorized molecular SARS-CoV-2 test, QuickVue
At-Home OTC COVID-19 Test correctly identified 83.5% of positive
specimens and 99.2% of negative specimens.
The performance of this test is still being studied in patients without signs
and symptoms of respiratory infection and
for
serial
screening.
Performance may differ in these populations

QuickVue
At-Home
OTC COVID-19 Test
RAPID RESULTS IN
10
MINS
A second test should be obtained with at least 24 hours
(and no more than 48 hours) between tests.
Please notify your Healthcare provider of the
results of your QuickVue At-Home OTC COVID-19 Test.
ASSISTANCE
If the test does not perform as expected,
call 833-QUICKVUE (833-784-2588).
Quidel Corporation
10165 McKellar Court, San Diego, CA 92121 USA
quidel.com
1479704EN00 (09/22)
USER
INSTRUCTIONS
